Abstract
Reports of environmental and human health impacts of per- and polyfluoroalkyl substances (PFAS) have greatly increased in the peer-reviewed literature. The goals of the present review are to assess the state of the science regarding toxicological effects of PFAS and to develop strategies for advancing knowledge on the health effects of this large family of chemicals. Currently, much of the toxicity data available for PFAS are for a handful of chemicals, primarily legacy PFAS such as perfluorooctanoic acid and perfluorooctane sulfonate. Epidemiological studies have revealed associations between exposure to specific PFAS and a variety of health effects, including altered immune and thyroid function, liver disease, lipid and insulin dysregulation, kidney disease, adverse reproductive and developmental outcomes, and cancer. Concordance with experimental animal data exists for many of these effects. However, information on modes of action and adverse outcome pathways must be expanded, and profound differences in PFAS toxicokinetic properties must be considered in understanding differences in responses between the sexes and among species and life stages. With many health effects noted for a relatively few example compounds and hundreds of other PFAS in commerce lacking toxicity data, more contemporary and high-throughput approaches such as read-across, molecular dynamics, and protein modeling are proposed to accelerate the development of toxicity information on emerging and legacy PFAS, individually and as mixtures. In addition, an appropriate degree of precaution, given what is already known from the PFAS examples noted, may be needed to protect human health.
Keywords: Per- and polyfluoroalkyl substances, Perfluorooctane sulfonate, Perfluorooctanoic acid, Persistent compounds, Contaminants of emerging concern
INTRODUCTION
Per- and polyfluoroalkyl substances (PFAS) are ubiquitous in environmental media because of their prolific use in a variety of industrial and consumer products and processes (Jian et al. 2018; Sunderland et al. 2019). Widespread human exposure to PFAS in water, food, and air coupled with the lengthy environmental persistence and biological half-lives of some PFAS have led to measurable PFAS in the blood of nearly the entire population in developed countries, with health effects reported globally (Kato et al. 2011; Khalil et al. 2016; Stubleski et al. 2016; Jian et al. 2018). Information needed to evaluate the potential risk of harm from PFAS includes the types of adverse health effects that might occur at environmentally relevant exposures, especially in sensitive life stages. Information is also needed regarding the mode(s) of action for PFAS toxicity, PFAS toxicokinetics in both humans and laboratory animal models, and dose-response relationships. Risk estimates can be used to inform public health exposure limits that will determine the need for exposure mitigation and environmental cleanup.
There are several challenges in obtaining the information needed to assess human health risk from the large number of PFAS with a wide range of structures and chemical properties (Buck et al. 2011; Wang Z et al. 2017; Organisation for Economic Co-operation Development 2018). Data on the identity, composition, and quantity of PFAS used in products and processes are often treated as confidential business information, hampering efforts to estimate exposure sources and routes. The Organisation for Economic Co-operation and Development’s (OECD’s) chemical inventory reports over 4000 substances that contain at least one perfluoroalkyl (–CnF2n–) moiety (Organisation for Economic Co-operation Development 2018), and the US Environmental Protection Agency (USEPA) has a curated list of over 8000 PFAS included, based on structure (US Environmental Protection Agency 2018) from the CompTox Chemicals Dashboard (Williams et al. 2017). The USEPA estimates that more than 600 PFAS are currently in commercial use (US Environmental Protection Agency 2019). Experimental studies of PFAS have been limited by funding and the availability of analytical standards, confounded by the prevalence of background contamination in laboratory materials, and challenged by physicochemical properties such as high surface activity that can interfere with and complicate measurements. Consequently, sufficient information to conduct quantitative risk assessment is currently available for only a relative few PFAS (Post 2020). Further, although typical human exposures involve various combinations of PFAS (Centers for Disease Control and Prevention 2017), only a few efforts address interactions of PFAS mixtures; and a well-founded, scientific basis on which to evaluate their combined toxic potential does not yet exist (Carr et al. 2013; Wolf et al. 2014; Zhou et al. 2017; Hoover et al. 2019; US Environmental Protection Agency 2020).
The Society of Environmental Toxicology and Chemistry (SETAC) North America held the focused topic meeting and workshop “Environmental Risk Assessment of PFAS” on 12 to 15 August 2019, covering a wide range of topics related to the characterization of health risks posed by PFAS. The overarching purpose of the meeting was to begin a scientific discussion on how best to approach studying, grouping, and regulating the large number of PFAS to which people and other species are potentially exposed (for charge questions and other details, see Johnson et al. 2020). We refer to these PFAS as “legacy” (those perfluoroalkyl acids for which there are accumulating health data but that may be phased out or decreased in use) and “emerging” (those which are being used as replacements, often with minimal health effects data). The objectives of the Human Health Toxicity section were to provide an assessment of the state of the science in understanding toxicological effects of PFAS and to explore and discuss strategies for advancing knowledge on the toxicity of individual and groups of PFAS.
CURRENT KNOWLEDGE OF PFAS TOXICITY IN HUMANS
Like other chemicals, PFAS are potentially capable of producing a wide range of adverse health effects depending on the circumstances of exposure (magnitude, duration, and route of exposures, etc.) and factors associated with the individuals exposed (e.g., age, sex, ethnicity, health status, and genetic predisposition). Aspects to consider when establishing the health effects of greatest concern are 1) effects for which evidence is the strongest (strength of evidence can come from consistency of effect across studies, strength of effect associations in epidemiological studies, and species concordance, as examples), and 2) effects for which potential impact is greatest (factors contributing to impact can include severity of effect, functional impairment, persistence, and specific age groups that are susceptible, as examples). Brief summaries of candidate PFAS health effects from human and experimental reports are provided in this section (Figure 1).
FIGURE 1:
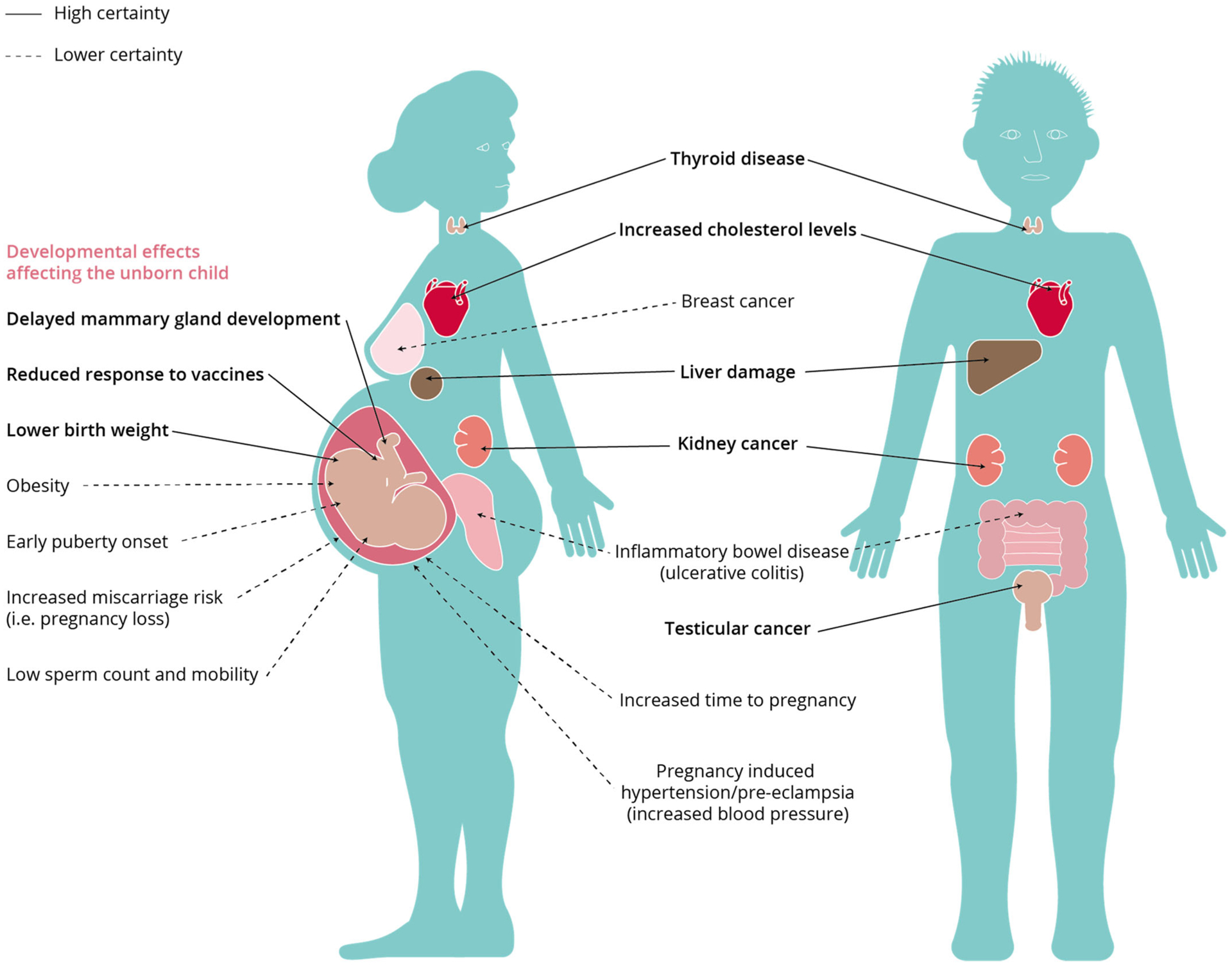
Effects of per- and polyfluoroalkyl substances on human health. Used with permission from European Environment Agency (2019). Original sources for this figure: National Toxicology Program (2016), C8 Science Panel (2012), IARC Working Group on the Evaluation of Carcinogenic Risks to Humans (2017), Barry et al. (2013), Fenton et al. (2009), and White et al. (2011b).
Immune function
Epidemiological studies have explored relationships between PFAS exposure and laboratory biomarkers of immunomodulation, such as vaccine responses. A doubling of perfluorooctane sulfonate (PFOS) in maternal serum was associated with a 39% (p < 0.001) reduction in diphtheria antibody concentration in children (age 5 yr), with increased odds of falling below clinically protective values against diphtheria and tetanus at age 7 yr. The authors noted that a “2-fold greater concentration of major PFCs [perfluorinated compounds] in child serum was associated with a difference of −49% (95% CI, −67% to −23%) in the overall antibody concentration” (Grandjean et al. 2012). Decreased immunological response persisted at age 13 yr (Grandjean et al. 2017). Adverse associations were also noted for responses to rubella, mumps, and Hemophilus influenza vaccinations in children and to vaccinations in adults (Granum et al. 2013; Looker et al. 2014; Stein et al. 2016; Abraham et al. 2020). In a single study, modest down-regulation of C-reactive protein response, a marker of human systemic inflammation, was also reported to be associated with perfluorooctanoic acid (PFOA) blood levels (Genser et al. 2015).
Disease outcomes linked with immunosuppression such as clinician-recorded diagnoses of childhood infections have also been associated with prenatal exposures to PFOS and perfluorohexane sulfonate (PFHxS) (Goudarzi et al. 2017). A pregnancy cohort study prospectively detected increased risk of airway and throat infections and diarrhea in children through age 10 yr, correlated with cord-blood PFAS measurements (Impinen et al. 2018, 2019). A recent review concluded that exposure to PFAS in infancy and childhood resulted in an immunosuppressive effect characterized by an increased incidence of atopic dermatitis and lower respiratory tract infections (Kvalem et al. 2020). Some of the immunological effects were sex-specific, but the authors cautioned that there were inconsistencies across studies (Kvalem et al. 2020). Overall, available data provide strong evidence that PFAS exposure can suppress the human immune response.
Population studies of immune hyperreactive diseases have resulted in mixed findings. Studies on childhood allergy and asthma outcomes have shown no association with PFAS (Impinen et al. 2018, 2019), whereas others have found substantial effects, including provocative evidence that subgroups of individuals not adequately immunized may be at an increased risk for disease a priori (Qin et al. 2017; Timmermann et al. 2017a). For example, a case-control study of Taiwanese children compared the first and fourth quartiles of serum measurements for 11 PFAS with asthma and other immune markers and reported confidence intervals well above 1.0 for PFOA and others (Qin et al. 2017). However, review articles concerning PFAS and childhood allergy and asthma offer nuanced, age- and sex-specific interpretations and advise against firm conclusions (Kvalem et al. 2020).
Chronic autoimmune outcomes, including thyroid disease (see section Thyroid function) and inflammatory bowel disease (IBD), have also been considered. A study in contaminated communities (n = 32 254) detected an association between both prevalence and incidence of ulcerative colitis (UC) and PFOA exposure (linear trend p = 0.0001 [Steenland et al. 2013]). A worker study (n = 3713) found a higher prevalence (p = 0.01) and incidence (p < 0.05) of UC with increasing log PFOA serum concentrations (Steenland et al. 2015). A case-control study of children and young adults from a background exposure community in Atlanta, Georgia, USA, also found higher serum PFOA levels in patients with UC (Steenland et al. 2018b). In contrast to PFOA-related associations in US populations, a study of a contaminated community in Sweden (n = 63 074) did not show a consistent association of IBD with any PFAS exposure (Xu et al. 2020b).
Recent, thorough reviews (National Toxicology Program 2016; DeWitt et al. 2019; Pachkowski et al. 2019) emphasize some key concepts: 1) there is concordance between animal studies and human epidemiological observations that PFAS modify the immune response, and 2) there are noted complexities in assuming dose-response continuums, including possible differences in life-stage vulnerability. Authors of these reviews note uncertainty about which outcome will be of most importance but agree that immunotoxicity should be included among sensitive human PFAS toxicity endpoints.
Thyroid function
The C8 Science Panelists concluded that there is a “probable link” of PFOA exposure to thyroid disease, with sex-specific outcomes in women (for hyperthyroid disease) versus men (hypothyroid disease) (C8 Science Panel 2012). Subsequent reviews drew attention to hypothyroid outcomes in women and children and to the possibility that populations with a priori circulating antithyroid peroxidase antibodies may be at additional risk (Coperchini et al. 2017). A broad childhood disease review noted “some evidence” that PFAS cause childhood hypothyroidism and characterized the number of studies as “limited” for childhood disease conclusions (Rappazzo et al. 2017). A meta-analysis of 12 child and adult studies that excluded populations with higher exposures noted that PFAS exposure is negatively associated with serum total thyroxine levels and that “PFAS could induce thyroid dysfunction and disease” (Lee and Choi 2017).
Human thyroid disease is mostly the result of an autoimmune response and is 5 to 10 times more prevalent in women than men (Tadic et al. 2018). Concerning PFAS and clinically diagnosed outcomes, women in the highest quartile of PFOA exposure (>5.7 ng/mL) reported clinical hypothyroid disease (odds ratio 2.2, 95% confidence interval [CI] 1.4–3.7) over 3 cycles of National Health and Nutrition Examination Survey (NHANES) data (1999–2006, n = 3974 adults), with similar findings in men (Melzer et al. 2010). The C8 Science Panel studies (median serum PFOA 26.1 ng/mL) found thyroid disease hazard ratios of 1.00, 1.24, 1.27, 1.36, and 1.37 across cumulative exposure quintiles in women (log-linear trend p = 0.03 [Winquist and Steenland 2014b]), with parallel hypothyroid findings in children aged 1 to 17 yr (Lopez-Espinosa et al. 2012). The Ronneby, Sweden, population experienced excess risk of thyroid disease in a discrete time period (1984–2005) among women (hazard ratio 1.29, 95% CI 1.05–1.57) that did not persist over time despite higher cumulative PFAS exposure (Andersson et al. 2019). The authors did not link exposure to hypothyroid outcome, noting a nonmonotonic dose-response relationship (Andersson et al. 2019).
Human population studies augment experimental data that PFAS interact with thyroid hormone binding proteins (Berg et al. 2015; Ren et al. 2016; Zhang J et al. 2016), one of several mechanisms by which PFAS can perturb feedback relationships between free thyroid hormone and the hypothalamic-pituitary-thyroid axis. Exposures to PFAS also interfere with thyroid peroxidase (TPO) enzyme activity in vitro (Song et al. 2012). Several PFAS studies have pursued this putative mechanism, finding that maternal and neonatal thyroid hormone outcomes were more readily detected in those with a priori abnormally high circulating anti-TPO antibodies (Webster et al. 2014, 2016). One case-control study investigated congenital hypothyroidism, a rare condition. Serum concentrations of PFOA (5.40 vs 2.12 ng/mL; p < 0.01), perfluorononanoic acid (PFNA; 1.93 vs 0.63 ng/mL; p < 0.001), perfluorodecanoic acid (PFDA; 0.52 vs 0.30 ng/mL; p < 0.005), and perfluoroundecanoic acid (0.98 vs 0.44 ng/mL; p < 0.005) were higher in the diagnosed newborns; and levels of several PFAS, including PFOA and PFHxS, were correlated with thyroid autoantibodies (Kim et al. 2016).
Thyroid disease is not the only concern. Clinicians are concerned about subclinically elevated thyroid-stimulating hormone (TSH) in early pregnancy because it may be associated with several possible adverse maternal and fetal outcomes (Forhead and Fowden 2014). This general concern has prompted numerous PFAS-exposure evaluations of corresponding TSH in maternal serum, cord blood, and newborns. A review of maternal and child biomarkers with PFAS exposure noted that higher TSH has been reported in 4 second-trimester studies (Ballesteros et al. 2017), but there are also conflicting findings. Studies measuring PFAS in the first trimester have also found associations between PFAS exposure and altered TSH levels in newborns, including nonmonotonic patterns of dose response that mirror the marked alterations of thyroid hormone levels during pregnancy (Inoue et al. 2019).
From the available studies, PFAS definitively alter human thyroid hormones and potentially contribute to thyroid auto-immunity but do not so far appear to be a cause of thyroid cancer (Barry et al. 2013; Vieira et al. 2013). Also, thyroid cancer is usually survived; thus, morbidity rather than mortality studies are useful.
Liver disease and cancer
The liver is a primary target organ for long-chain PFAS storage, and accompanying experimental evidence of toxicity includes hepatocyte fat infiltration, specific P450 (CYP) pathway induction, apoptosis, hepatocellular adenomas and carcinomas, and disrupted fatty acid trafficking that can be peroxisome proliferator-activated receptor alpha (PPARα)-dependent or -independent and present across species (Maestri et al. 2006; Cui et al. 2009; Wan et al. 2012; Huang et al. 2013; Perez et al. 2013; Filgo et al. 2015; Xu et al. 2016, 2020a; Yao et al. 2016; Zhang L et al. 2016b; Hui et al. 2017; Li et al. 2017a; Guillette et al. 2020; National Toxicology Program 2020a).
Population studies demonstrate significant associations of long-chain PFAS (>6 fluorinated carbons) exposure to higher liver enzymes, such as alanine aminotransferase in adults and adolescents (Sakr et al. 2007a; Gallo et al. 2012; Yamaguchi et al. 2013; Gleason et al. 2015; Attanasio 2019; Nian et al. 2019), including in longitudinal studies (Sakr et al. 2007b; Darrow et al. 2016). Following low-dose exposures, these associations may be more evident in obese participants (Lin et al. 2010; Gallo et al. 2012; Jain and Ducatman 2019e).
Based on experimental data (Martin et al. 2007; Wan et al. 2012; Wang et al. 2013; Das et al. 2017), nonalcoholic fatty liver disease (NAFLD) has been investigated as a clinical outcome of PFAS exposure mediating consistent population PFAS-altered liver enzyme findings. Studies with NAFLD cytokeratin C18 biomarkers have provided supportive evidence for PFAS inducing steatosis (Bassler et al. 2019). Metabolomic studies have been directed at potentially explanatory human glycerophosphocholine and fatty acid profiles (Kingsley et al. 2019; Salihovic et al. 2019; Wahlang et al. 2019). Processes which favor steatosis promote advanced liver disease including liver cancer in humans (Massoud and Charlton 2018; National Toxicology Program 2020a). Associations of PFAS with advanced human liver disease and liver cancer are technically hard to study for reasons including (and not limited to) lethality, selection of comparison populations, and alterations of excretion mechanics associated with disease states. In a clinic-based study, mostly obese (85%) children aged 7 to 19 yr with biopsy-proven NAFLD had more advanced disease associated with PFOS and PFHxS exposure as well as associations with lipid and amino acid pathways linked to NAFLD pathogenesis (Jin et al. 2020). However, an adult study reported that serum PFHxS was inversely associated with hepatic lobular inflammation in morbidly obese bariatric surgery patients (Rantakokko et al. 2015). A study of heavily exposed workers (n = 462, geometric mean serum PFOA of 4048 ng/mL) detected significantly increased incident mortality for cirrhosis (relative risk = 3.87, 95% CI 1.18–12.7) and liver cancer (relative risk = 6.69, 95% CI 1.71–26.2) compared to a regional population (Girardi and Merler 2019), whereas no PFAS association to cancer or advanced liver disease was reported in a 3M worker cohort or in the C8 Health study population (Lundin et al. 2009; Barry et al. 2013; Vieira et al. 2013).
Emerging animal toxicology and histology and human population data provide mechanistic clues that PFAS disrupt hepatic metabolism, leading to increased bile acid reuptake and lipid accumulation in liver (Salihovic et al. 2020; Schlezinger et al. 2020). A review of NAFLD and toxicant exposure concluded that PFAS are associated with early steatosis (“fatty liver”), the preclinical stage of NAFLD (Armstrong and Guo 2019).
Lipid and insulin dysregulation
Cross-sectional and longitudinal investigations indicate that PFAS increase serum total and low-density lipoprotein cholesterol in adults and children (Steenland et al. 2009; Frisbee et al. 2010; Nelson et al. 2010; Eriksen et al. 2013; Fisher et al. 2013; Fitz-Simon et al. 2013; Geiger et al. 2013; Fu et al. 2014; Starling et al. 2014; Winquist and Steenland 2014a; Skuladottir et al. 2015; Zeng et al. 2015; Koshy et al. 2017; Convertino et al. 2018; He et al. 2018; Seo et al. 2018; Dong et al. 2019; Lin et al. 2019; Li et al. 2020; Liu G et al. 2020), including clinically defined high cholesterol (Steenland et al. 2009; Winquist and Steenland 2014a; Lin et al. 2019). Studies of large populations, featuring wide exposure ranges, demonstrate that serum lipids rapidly increase beginning at background (1–10 ng/mL) serum concentration and then are followed by attenuating (“plateaued”) cholesterol measurements as (log-transformed) exposures to long-chain PFAS increase (Steenland et al. 2009; Frisbee et al. 2010; Li et al. 2020). These findings suggest partially saturable mechanisms; thus, the cholesterol dose response at pharmacologic or acutely toxic doses should be viewed with caution; associations can be missed or may be misleading when an environmental range of exposure is absent. At background exposure levels, residual associations may be more detectable in obese participants (Timmermann et al. 2014; Jain and Ducatman 2019d), a finding congruent with experimental PFAS outcomes in rodents fed “Western” or high-fat diets (Tan et al. 2013; Quist et al. 2015; Rebholz et al. 2016). Human gene expression pathways provide support for an interaction of obesity and PFAS exposures and suggest possible sex differences (Fletcher et al. 2013). A pharmacokinetic model predicts that approximately half of the PFOS-exposed population would experience a >20% rise in serum cholesterol (Chou and Lin 2020). Risk-assessment implications for low-PFAS dose increases in cholesterol have been noted (New Jersey Drinking Water Quality Institute Health Effects Subcommittee 2017; Li et al. 2020), and a review of population and toxicity data concluded that dyslipidemia is the strongest metabolic outcome of PFAS exposure (Sunderland et al. 2019).
Human PFAS lipid findings may be related to experimental findings of induced adipogenesis, impaired bile acid metabolism/synthesis, strongly decreased CYP7A1 enzyme activity, altered fatty acid transport, and intracellular lipid accumulation with steatosis, including in PPAR-α-null or PPAR-α-humanized animals (Guruge et al. 2006; Lau et al. 2007; Bijland et al. 2011; Bjork et al. 2011; Wang et al. 2014; Filgo et al. 2015; Das et al. 2017; Salihovic et al. 2019; Zhang et al. 2019; Behr et al. 2020a; Liu S et al. 2020b; Schlezinger et al. 2020). Independent of PFAS exposure, similar alterations in metabolic pathways have been related to disrupted fatty acid beta-oxidation and increased free cholesterol in toxicology studies (Perla et al. 2017).
Cross-sectional studies of diabetes outcomes can be misleading for reasons discussed in the renal section (see section Kidney disease, uric acid, and kidney cancer). Emerging longitudinal and diabetes clinical trial data indicate that PFAS may increase human insulin resistance, associated with dysregulated lipogenesis activity (Alderete et al. 2019; Lin et al. 2019). Longitudinal studies of clinically diagnosed diabetes patients have sometimes associated PFAS exposures with diabetes (Sun et al. 2018) or with small changes in glycemic markers (Cardenas et al. 2017); however, diabetes associations to date are not consistent (Karnes et al. 2014; Cardenas et al. 2017; Donat-Vargas et al. 2019). Future studies should consider whether PFAS may instigate autoimmune diabetic outcomes in humans, as shown in experimental studies (Bodin et al. 2016). Experimental data reveal that PFAS activate G protein-coupled receptor 40, a free fatty acid-regulated membrane receptor on islet ß cells, stimulating insulin secretion (Qin et al. 2020; Zhang L et al. 2020).
Kidney disease, uric acid, and kidney cancer
Extended human half-lives of long-chain PFAS are attributed to active renal tubular reabsorption. Of concern, legacy PFAS such as PFOA and PFOS are concentrated in renal tissues, and histopathologic, molecular, oxidative stress, and epigenetic studies provide evidence of potential nephrotoxicity (Wen et al. 2016; Stanifer et al. 2018; Sakuma et al. 2019; Rashid et al. 2020). In addition, the strong influence of kidney reabsorption on the extended half-lives of long-chain PFAS is consistent with both human protein binding and experimental PFAS excretion data.
Human studies have associated legacy PFAS exposure to diminished glomerular filtration and/or defined chronic kidney disease in adults and children (Shankar et al. 2011; Watkins et al. 2013; Kataria et al. 2015; Blake et al. 2018). However, this outcome may be due to reverse causation (Watkins et al. 2013; Dhingra et al. 2017). Some reviews of the available epidemiologic and toxicologic evidence suggest causative links between PFAS and diminished kidney function and chronic kidney disease (Stanifer et al. 2018; Ferrari et al. 2019); these authors also note several knowledge gaps and uncertainty about which proposed mechanisms of action are most important. A propensity score approach to NHANES data (Jain and Ducatman 2019c; Zhao et al. 2020) and a study with repeated PFAS and health measures over an 18-yr period (Blake et al. 2018) recently concluded that PFAS exposure likely causes diminished renal glomerular filtration.
Uric acid, a biomarker of increased risk for renal disease (Obermayr et al. 2008), is also consistently associated with PFAS exposure in adults and children (Steenland et al. 2010; Geiger et al. 2013; Gleason et al. 2015; Kataria et al. 2015; Qin et al. 2016; Zeng et al. 2019), including a visible dose-response curve that begins at or near historic background levels in human populations (Steenland et al. 2010; Zeng et al. 2019). Serum PFAS concentrations exhibit an inverted U-shaped pattern related to glomerular filtration, initially exhibiting a modest accumulation as glomerular filtration begins to decrease and then decreasing in advancing renal disease, likely due to failure of normal strong reabsorption mechanisms in moderate to severe kidney disease (Jain and Ducatman 2019c). This finding is more dramatic across stages of glomerular filtration when there is also albuminuria (Jain and Ducatman 2019b). Studies suggest that the association of PFAS to uric acid is not due to reverse causation and is underestimated because the failing kidney excretes long-chain PFAS but retains uric acid. An implication is that population outcomes that occur in the presence of either albuminuria or moderate to severe renal disease such as hypertension (Jain 2020) increasing presence of and uric acid (a biomarker of renal disease; Jain and Ducatman 2019a; Zeng et al. 2019) can be underestimated in cross-sectional studies; in other words, the link between these health outcomes and PFAS exposure is obscured in these studies because of enhanced PFAS excretion patterns in the presence of either albuminuria or moderate to severe kidney disease. Furthermore, the strong influence of renal reabsorption on the long half-lives of long chain PFAS is consistent with both human protein binding of PFAS and experimental PFAS excretion rates in high-dose rodent studies (Cheng and Ng 2017).
Kidney cancer diagnoses have been increasing since 1975, a finding that is partially independent of improved detection, with 5-yr cancer-specific survival of approximately 80% (Gandaglia et al. 2014). The C8 Health studies noted longitudinal (n = 32 254) increases of kidney cancer (hazard ratio = 1.10, 95% CI 0.98–1.24) and kidney cancer mortality (Steenland and Woskie 2012; Barry et al. 2013; Vieira et al. 2013). A review of 6 published studies found long-chain PFAS exposure associated with kidney cancer or kidney cancer mortality, with risks ranging from 1.07 to 12.8 (Stanifer et al. 2018). Subsequent preliminary data from the heavily exposed Veneto, Italy, population also suggest a significant increase in kidney cancer mortality with PFAS exposure (Mastrantonio et al. 2018). Evidence is accumulating for PFAS as a cause of chronic disease and kidney cancer. Study designs must consider the peculiar PFAS excretion mechanics involved in and associated with kidney disease.
Reproductive and developmental outcomes
Exposure to PFOA impairs human sperm motility and sperm penetration into viscous media (Sabovic et al. 2020; Yuan et al. 2020) and is longitudinally associated with lower sperm concentration and count and higher adjusted levels of luteinizing and follicle-stimulating hormones in young men (Joensen et al. 2009; Vested et al. 2013; Song et al. 2018). Serum concentrations of PFAS are also cross-sectionally associated with deleterious markers of semen quality (Louis et al. 2015; Pan et al. 2019).
Legacy and emerging PFAS have been found in follicular fluid (Kang et al. 2020). They appear to alter endometrial regulation such as progesterone activity in young women (Di Nisio et al. 2020b) and possibly menstrual cycle length (Lum et al. 2017). Associations with menarche and menopause may be substantially due to reverse causation because menstruation is a route by which women eliminate PFAS (Dhingra et al. 2017), partially explaining why men have higher PFAS levels than women in the same communities. Women on birth control and who do not menstruate or with poor cyclicity because of age, activity level, or disease may have elevated PFAS levels in comparison with menstruating women. Exposure to PFAS has been associated with endometriosis in the United States and in China (Louis et al. 2012; Campbell et al. 2016; Wang B et al. 2017a), but the specific PFAS associated with this effect vary among studies.
Time-to-pregnancy (fecundity) studies provide indirect evidence of changes in fertility. Methodologic considerations include maternal and paternal age, parity (which in turn affects serum PFAS), and health status. Among 1240 women in the Danish National Birth Cohort, PFOS exposure was associated with decreased fecundity (median serum PFOS 35.5 ng/mL; Fei et al. 2009). Reverse causation may explain this finding because it is duplicated in parous, but not among nonparous, women (Whitworth et al. 2012; Bach et al. 2015). Prospective odds of actual infertility in the Maternal-Infant Research on Environmental Chemicals cohort (n = 1743) at low-dose exposures were associated with PFOA (geometric mean 1.66 ng/mL; odds ratio = 1.31, 95% CI 1.11–1.53) and PFHxS (odds ratio = 1.27, 95% CI 1.09–1.48; Velez et al. 2015). The reported fertility rate improved following water filtration in a PFAS-contaminated community (incidence rate ratio 0.73, 95% CI 0.69–0.77 prior to filtration) along with measures of birth weight (Waterfield et al. 2020).
Per- and polyfluoroalkyl substances reliably move across the placenta and enter breast milk (Gyllenhammar et al. 2018; VanNoy et al. 2018); serum PFAS levels in young children generally exceed maternal serum concentrations (Fromme et al. 2010; Papadopoulou et al. 2016; Eryasa et al. 2019). Population studies provide evidence that breastfeeding duration and milk quantity are adversely affected by PFAS exposure (Romano et al. 2016; Timmermann et al. 2017b; Rosen et al. 2018).
A systematic review reported that PFOA exposure was associated with a small decrease in infant birth weight; the meta-analysis estimated that a 1-ng/mL increase in PFOA was associated with an approximately 19-g reduction (95% CI −29.8 to −7.9 g) in birth weight (Lam et al. 2014). The authors noted similarities in experimental studies (Johnson et al. 2014; Koustas et al. 2014) and concluded that there was “sufficient” human and corroborative toxicology evidence of a detrimental effect of PFOA on birth weight (Johnson et al. 2014; Koustas et al. 2014; Lam et al. 2014). However, another meta-subpopulation analysis, focused on early pregnancy or the time shortly before conception, detected only a small and nonsignificant association, which was less subject to bias (Steenland et al. 2018a). Different approaches to the possible confounding role of shifting glomerular filtration rates in pregnancy can affect interpretations; evidence suggests this consideration can, at most, only partially explain associations of PFAS exposure to decreased birth weight (Interstate Technology and Regulatory Council 2020; Wikstrom et al. 2020). A recent review of mostly prospective cohort studies (n = 24 studies) noted PFAS associated with altered fetal and postnatal growth measures, such as lower birth weight. Many (n = 22) of the relevant studies suggest developmental and childhood immunomodulatory effects, whereas 21 studies concerning neurodevelopment were inconclusive (Liew et al. 2018). The authors of the review noted methodologic challenges of developmental and newborn epidemiology, including consideration of critical exposure windows for developmental effects, the effects of breastfeeding and parity on maternal PFAS levels, and the variety of possible mechanistic explanations for growth outcomes, such as disruption of glucocorticoid and thyroid hormone metabolism in utero (Liew et al. 2018). Recent Faroe Island studies report that prenatal PFAS effects on thyroid hormone status do not support a causal relationship (Xiao et al. 2020).
Review articles suggest that prenatal exposure to PFOA may increase risk of subsequent childhood adiposity, noting that steroid hormones, retinoid X receptor, and other pathways may be contributing to this effect (Halldorsson et al. 2012; Hall and Greco 2019). Prospective evidence supports this relationship in adults with a high risk of diabetes (Cardenas et al. 2017). However, some well-performed community studies do not support this outcome in adults or children (Barry et al. 2014; Martinsson et al. 2020).
Based on several preliminary findings, supported by longitudinal follow-up studies (Stein et al. 2009; Savitz et al. 2012; Darrow et al. 2013; Avanasi et al. 2016a, 2016b), the C8 Science Panel concluded that PFOA is probably linked to pregnancy-induced hypertension or preeclampsia. Population-level evidence implicating additional PFAS having this effect has included studies with longitudinal designs (Huang et al. 2019; Wikstrom et al. 2019; Borghese et al. 2020). Experimental support includes PFAS effects on human trophoblast migration in vitro (Szilagyi et al. 2020) and recent evidence of PFOA and GenX (or hexafluoropropylene oxide dimer acid) effects on mouse placenta, as well as excessive gestational weight gain (Blake et al. 2020). However, a recent longitudinal study did not find an association of PFAS with pregnancy-associated hypertension (Huo et al. 2020).
The possibility that circulating PFAS may reduce bone mineral density has been investigated. Cross-sectional and practical trial associations have been found in adults (Lin et al. 2014; Hu et al. 2019; Di Nisio et al. 2020a), and there is emerging longitudinal evidence from a mother and child pair study indicating that children may also be affected (Cluett et al. 2019).
Testicular cancer diagnoses are increasing steadily, a trend unrelated to improved detection (Cheng et al. 2018; Park et al. 2018). Most patients diagnosed (>90%) will be cured and die of other causes; mortality studies therefore provide little help in understanding disease risk factors. The C8 Science Panel detected longitudinal evidence for increased testicular cancer risk (1.35, 95% CI 1.00–1.79) for cumulative PFOA exposure (Barry et al. 2013). There are ample supportive data of testicular damage following PFAS exposure, including strong evidence of endocrine disruption; but the cell-specific associations are different in humans (germ cell) than the outcomes in rodents (stromal).
Per- and polyfluoroalkyl substances have deleterious effects on conception, pregnancy, and infant development. The underlying birth weight data are mostly supportive, although the subsequent growth and adiposity literature is mixed. The most sensitive reproductive and developmental outcomes are a topic of ongoing discussion.
Outcomes replicated across populations, such as perfluorocarboxylate (PFCA) and perfluorosulfonate (PFSA) exposures associated with down-regulation of immune response; increases in cholesterol, liver enzymes, and uric acid; alterations in thyroid hormone binding proteins; growth deficits; and effects on breast milk and lactation, indicate priority areas for understanding mechanisms and health implications.
CURRENT KNOWLEDGE OF PFAS TOXICITY IN EXPERIMENTAL MODELS
Animal studies have focused most intensely on PFOA and PFOS, using laboratory rodents and, more recently, zebrafish as models. Perfluoroalkyl acids of varied carbon-chain lengths as well as a few replacement chemicals with ether linkages in the carbon backbone (such as GenX and 3H-perfluoro-3-[(3-methoxy-propoxy)propanoic acid], or ADONA) have also been examined, with outcome profiles thus far generally consistent with legacy chemicals. The varying extent of responses is likely related to toxicokinetic disposition (excretion or half-life) and relative potency and affinity of the individual chemical for binding to receptor proteins. Some PFAS (i.e., PFHxS, PFOA, and PFNA) have longer half-lives in mice than rats and typically much longer half-lives in humans (Table 1). These differences in elimination kinetics complicate the cross-species evaluation of toxicity. In addition, some PFAS (such as PFOA and PFNA) exhibit a profound sex difference in the rate of chemical elimination and bioaccumulation in the rat: females eliminate them much faster than males (Table 1). Sex differences in half-lives, although important, are much smaller in humans and have a different explanation. The mouse also typically has more limited sex-based PFAS elimination differences, making this species more amenable for extrapolation to humans, especially for mechanistic and toxicity evaluations.
TABLE 1:
Per- and polyfluoroalkyl substances serum half-life estimates in rat, mouse, monkey, and humans
| PFBS (C4) | PFHxS (C6) | PFOS (C8) | PFBA (C4) | PFHxA (C6) | PFHpA (C7) | PFOA (C8) | PFNA (C9) | PFDA (C10) | F-53B | GenX | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | |
| Rat | 0.6–4.0 h | 2.1–4.5 h | 1.8 d | 6.8 d | 62–71d | 38–41d | 1.0–1.8 h | 6–9 h | 0.4–0.6 h | 1.0–1.7 h | 1.2 h | 2.4 h | 2–4 h | 4–6 d | 1.4–6.4 d | 31–55 d | 59–75 d | 40–80 d | 8 h | 3 h | ||
| Mouse | 4.5 h | 5.8 h | 25–2 d | 28–3 d | 31–3 d | 36–4 d | 3 h | 12 h | ~1.2 h | ~1.6 h | 16 d | 22 d | 26–6 d | 34–6 d | 18 h | 20 h | ||||||
| Cynomolgus Monkey | 3.5 d | 4.0 d | 87 d | 141 d | 110 d | 132 d | 1.7 d | 2.4 h | 5.3 h | 30 d | 21 d | |||||||||||
| Human | 28 d | 5.3–8.5 yr | 3.4–5.0 yr | 3 d | 32 d | 1.2–2.5 yr | 2.1–3.8 yr | 2.5–4.3 yr | 15.3 yr | |||||||||||||
GenX = hexafluoropropylene oxide dimer acid; PFBA = perfluorobutanoic acid; PFBS = perfluorobutanesulfonic acid; PFDA = perfluorodecanoic acid; PFHpA = perfluoroheptanoic acid; PFHxA = perfluorohexanoic acid; PFHxS = perfluorohexane sulfonate; PFNA = perfluorononanoic acid; PFOA = perfluorooctanoic acid; PFOS = perfluorooctane sulfonate.
In general, human health effects associated with PFOA and PFOS exposure (described in section Current Knowledge of PFAS Toxicity in Humans) have also been reported in animal models: hepatic/lipid metabolic toxicity, developmental toxicity, immune suppression, tumor induction, endocrine disruption, and obesity. These findings are often derived from well-controlled laboratory experiments in more than one species using wide dose ranges that are often orders of magnitude higher than typical human exposure, to account for differences in half-life across species. Some of the phenotypic findings are supported by in vitro mechanistic investigation and/or molecular queries on target tissues. Our understanding of the toxicologic properties of PFAS other than PFOA and PFOS is notably less advanced and, in the case of emerging replacements and by-products, completely unexplored.
Hepatic and metabolic toxicity
In rodent studies, dose-dependent increases in liver weight, in hepatocellular hypertrophy associated with vacuole formation, and with or without increased peroxisome proliferation have been observed with a significant body burden of PFAS, especially for the most persistent and potent long-chain homologs. Hepatocyte proliferation, necrosis, and apoptosis are outcomes occurring at relatively low doses. This is also true for a new replacement chemical, GenX, which altered liver histopathology and function and increased apoptosis in mice and fish (Blake et al. 2020; Guillette et al. 2020). Correspondingly, transcriptional activation of mouse and, to a lesser extent, human PPARα-related genes in liver was detected in adult-exposed models; activation of other nuclear receptors such as PPARγ, constitutive androstane receptor (CAR), and pregnane X-receptor (PXR) has also been reported. These nuclear receptors, metabolic sensors that regulate lipid and glucose metabolism and transport and inflammation, tend to be more responsive in tissues of rodents than in humans (Wolf et al. 2012; Rosen et al. 2017). Recent work using developmental models reports that mitochondrial dysfunction is associated with hepatocellular hypertrophy in young adult mice (Quist et al., 2015) and that other fatty acid metabolism pathways are activated (Jones et al. 2003; Shabalina et al. 2016). Steatosis is also a common feature of PFAS chronic exposure in rodents. Exposure in rodent models typically decreases serum cholesterol, whereas elevations of circulating cholesterol levels have been reported in humans. The mode of action concerning serum cholesterol is debatable. For example, PFOA exposure increased liver weight, increased liver enzymes, and led to persistent histopathological changes (particularly damage to the bile duct) in livers of wild-type and PPARα-null rodent strains (reviewed in Division of Science and Research, New Jersey Department of Environmental Protection 2019). Many of these effects are reversible on cessation of PFAS exposure, and this observation has been interpreted by some as evidence of “adaptive” responses to exposure. However, this reversibility is irrelevant to ongoing environmental PFAS exposure (for instance, from drinking water) because exposure will persist until contamination is remediated. In summary, there is a strong confluence of animal toxicology and histology and human population data that PFAS disrupt hepatic metabolism and lead to lipid accumulation in liver, although the mechanism(s) is unclear. Effects on bile acid metabolism, mitochondrial perturbation, and cholestatic mechanisms deserve further investigation at human-relevant exposures.
Reproductive and developmental toxicity
Only a few reproductive toxicity studies of males and females are available, primarily focusing on long-chain PFAS. Profound developmental toxicity has been described following gestational and lactational exposure to PFOS, PFOA, and PFNA in mice (Thibodeaux et al. 2003; Lau et al. 2006; Das et al. 2015) and in mice and rats gestationally exposed to GenX (Conley et al. 2019; Blake et al. 2020). Neonatal morbidity and mortality were seen with exposure to high doses of legacy PFAS; growth deficits and developmental delays were noted in offspring exposed to lower doses. Evidence of lactation impairment was seen in mice at doses of 5 mg PFOA/kg body weight (White et al. 2007), leading to increased offspring mortality (Lau et al. 2006); recent studies have indicated a role of placental dysfunction in these adverse developmental outcomes (Blake et al. 2020). Deficits of mammary gland development were also observed in mice exposed to PFOA (doses of 1 mg/kg body wt and lower) during gestation, which persisted into adulthood, although these exposure levels did not alter body weight, lactational function, or neonatal growth of offspring (F1 or F2 mice; Macon et al. 2011; White et al. 2011b; Tucker et al. 2015). Systematic reviews support a relationship between in utero exposure to PFOA and PFOS and reduced fetal growth in animals and humans, and the relationship between PFOA and reduced fetal growth in mice was recently validated (Koustas et al. 2014; Blake et al. 2020). Also, PFAS are reported to have reproductive effects such as ovulation failure in mice (Zhang Y et al. 2020).
Immunotoxicity
A few long-chain PFAS (PFOS, PFOA, PFNA, and PFDA) have been shown to alter immune status in rodents and non-human primates. Effects are predominantly immunosuppressive and include reductions in thymus and spleen weights and associated immune cell populations, in numbers of circulating immune cells, in certain aspects of innate immunity (i.e., natural killer cell cytotoxicity), in infectious disease resistance, and in antibodies produced in response to an antigen (i.e., analogous to the vaccine response in humans). In their 2018 draft Toxicological Profile for Perfluoroalkyls, the US Agency for Toxic Substances and Disease Registry (ATSDR) noted changes to the aforementioned immune parameters observed in experimental rodents exposed to PFOA, PFOS, PFNA, PFHxS, PFDA, perfluorobutanesulfonic acid (PFBS), or perfluorobutanoic acid (PFBA; Agency for Toxic Substances and Disease Registry 2018). The US National Toxicology Program conducted a systematic review of the immunotoxicological literature for PFOA and PFOS and concluded that PFOA and PFOS were presumed to be immune hazards to humans based on a high level of evidence for suppression of antibody responses in experimental animals and a moderate level of evidence for suppression of antibody responses in humans (National Toxicology Program 2016). The ATSDR (Agency for Toxic Substances and Disease Registry 2018) also included a decreased antibody response to vaccines (PFOA, PFOS, PFHxS, and PFDA) and increased risk of asthma diagnosis (PFOA) among the list of adverse health effects in PFAS-exposed humans. Reduction in the antibody response to a vaccine, an adaptive immune function, is a well-accepted measure of immunotoxicity, is consistent with the mode of action for the effects of fatty acids on immune system function (Fritsche 2006), and is compelling evidence that the immune system is a sensitive target of PFAS.
Tumor induction
Per- and polyfluoroalkyl substances are not known to be directly mutagenic; PFOA, PFOS, and other tested PFAS show little or no evidence for induction of gene mutation, clastogenicity, or aneuploidy in vitro or in vivo by a direct mode of action (see EFSA Panel on Contaminants in the Food Chain [2020] for details). There is evidence that PFAS can induce DNA damage, such as strand breaks, and other genotoxic effects, secondary to oxidative stress (EFSA Panel on Contaminants in the Food Chain 2020). This occurs at concentrations or doses that are high relative to human environmental exposures to PFAS, and the mechanism is such that their dose-response will be sublinear. Hence, PFAS are unlikely to be of mutagenic concern in exposed populations.
In adult-exposed rodents and fish, PFOA and PFOS have been shown to induce tumors. Liver adenomas, pancreatic acinar cell tumors, and testicular Leydig cell adenomas have been detected in rats treated chronically with PFOA (IARC Working Group on the Evaluation of Carcinogenic Risks to Humans 2017) as well as its replacement, GenX (Caverly Rae et al. 2015). Following gestational and chronic exposure to PFOA, 58% of male rats demonstrated pancreatic tumors at the lowest dose administered (National Toxicology Program 2020b). This finding has spurred Minnesota and California policymakers to consider cancer as an endpoint in risk assessment, whereas the European Food Safety Authority (EFSA Panel on Contaminants in the Food Chain 2020) has the opinion that there is not adequate evidence for a link between exposure to PFAS and cancer risk in humans. This “tumor triad” profile has been associated with the PPARα-mediated molecular signaling pathway in rats exposed to high doses of PFAS. Consequently, liver tumors involving this mode of action are not considered relevant to humans at equivalent PFAS exposures (Post et al. 2017). The human relevance of PPARα-mediated pancreatic tumors in rodents remains to be determined. Liver lesions evident in PPARα-null mice exposed to PFOA during pregnancy and lactation (Filgo et al. 2015) suggest a non-PPARα-mediated liver response. Induction of liver tumors mediated by estrogen receptor (ER) activation has also been reported in fish (Tilton et al. 2008), and several non-PPARα-mediated hypotheses, including increased reactive oxygen species formation, oxidative stress, and mitochondrial dysfunction; decreased tumor cell surveillance by the immune system; and diminished gap junction cellular communication, are documented (IARC Working Group on the Evaluation of Carcinogenic Risks to Humans 2017; New Jersey Drinking Water Quality Institute Health Effects Subcommittee 2017).
Endocrine disruption
The primary evidence for the endocrine-disrupting potential of PFAS involves induction of hypothyroxinemia and reduction of serum testosterone in rats. An early review of PFAS endocrine-disrupting properties in humans concluded that the “thyroid may be one axis significantly affected by PFOA exposure while the animal toxicology literature is less certain due to technical issues” (White et al. 2011a).
The effects of PFAS on thyroid hormone status detected in animal studies differ from classical hypothyroidism, in that reduction of circulating total thyroxine is not accompanied by a compensatory increase of TSH. A possible mechanism for these effects may be related to the propensity of protein binding of legacy PFAS, which could lead to displaced total thyroxine binding to its carrier proteins (transthyretin and thyroxine-binding globulin). Human population studies augment animal data showing that PFAS interact with thyroid hormone binding proteins (Berg et al. 2015; Ren et al. 2016; Zhang J et al. 2016a), one of several mechanisms by which PFAS can perturb feedback relationships between free thyroid hormone available to cells (free total thyroxine) and the hypothalamic-pituitary axis. Some estrogenic effects of PFAS have also been illustrated by in vitro studies, although there is no evidence of direct transactivation of estrogen, androgen, or glucocorticoid receptors (Behr et al. 2018, 2020b).
The evidence for PFAS affecting ER signaling in humans and animals is mixed. Although studies have identified some PFAS as being without estrogenic activity (Behr et al. 2018; Borghoff et al. 2018; Gogola et al. 2019), others suggest an ability of PFAS to modulate or even activate ER-mediated effects (Benninghoff et al. 2010; Kjeldsen and Bonefeld-Jørgensen 2013; Wang et al. 2018; Bjerregaard-Olesen et al. 2019; Qiu et al. 2020), with some effects only observed in aquatic organisms (Wei et al. 2009; Chen et al. 2016, 2018). Microarray analyses of human primary hepatocytes confirmed that PFOA activated the ER pathway (Buhrke et al. 2015).
Neurotoxicity
Potential adverse effects of PFAS on the nervous system and functions have not been widely investigated. A few studies reported neurotoxicity of PFOS, PFHxS, and PFOA in cell culture systems (Slotkin et al. 2008), as well as altered behavioral responses (Goulding et al. 2017) and deficits in learning and memory ability in rodents (Viberg et al. 2013). In contrast, no significant developmental neurotoxic effects were seen from prenatal exposure to PFOS in USEPA guideline-based studies with rats (Butenhoff et al. 2009).
Obesity
Numerous cell-based assays in human and mouse pre-adipocytes and animal studies with and without high-fat diets have consistently shown that some PFAS have the potential to increase lipid production by adipocytes and fat pads (van Esterik et al. 2016). Exposure of pregnant mice to low doses of PFOA produced obesity in young adult female offspring (Hines et al. 2009; van Esterik et al. 2016), a finding that was re-capitulated in Danish women exposed in utero to PFOA (Halldorsson et al. 2012). Both PFOA and GenX increased weight gain of pregnant mice (Blake et al. 2020), an effect also seen in women during pregnancy (Ashley-Martin et al. 2016), although discordant results have been reported in other studies (Barry et al. 2014; Ngo et al. 2014). These apparently disparate findings in experimental models may be associated with differences among mouse strains examined, exposure periods, statistical methodology, and/or the rodent diets used.
There are specific differences in human and rodent health outcomes that deserve further investigation: 1) cholesterol metabolism, 2) thyroid effects, 3) mode of action for liver effects (different or same), and 4) kidney transporter or other mode of action leading to large differences in half-life. However, species concordance in the 6 human health effects discussed in the present review supports a weight of evidence for these effect for the handful of extensively studied PFAS.
Human health advisory and guidance values for a few PFAS have been issued to date by the USEPA, the ATSDR, several individual state environmental agencies or health departments, as well as regulatory agencies in Canada and Europe that are largely (but not exclusively) based on toxicological findings in animal models. However, risk-assessment scientists have not reached consensus in selecting a singular apical endpoint as the basis for a point of departure for assessments. Three toxicological features of PFAS that have been commonly highlighted, based on their sensitivity (low dose effect), strength of evidence (robust corroborating studies with mechanistic support for human relevance), and corresponding findings noted in epidemiological investigation, are hepatotoxicity (and alterations in lipid metabolism), developmental toxicity, and immunotoxicity. It should be noted that apical endpoints that drive risk assessments often differ among individual PFAS, perhaps highlighting the complexity of these chemicals and the family of PFAS, in general.
IMPORTANCE OF TOXICOKINETICS IN UNDERSTANDING PFAS TOXICITY
Species and sex differences
Few of the substantial number of structurally diverse PFAS have been tested for toxicological effects. Some available toxicological information has come from studies in animals, where marked species and (in rat) sex differences in half-life for some PFAS (Table 1) have been observed and the relevance to humans is uncertain. These differences are due to toxicokinetic and toxicodynamic factors. There are also differences in mean PFAS serum levels between men and women in the same communities. Children may have elevated serum levels compared to parents, even with the same exposures (Emmett et al. 2006; Daly et al. 2018; Graber et al. 2019), for reasons relating to transplacental transfer, breastfeeding, and body mass (Emmett et al. 2006; Daly et al. 2018; Graber et al. 2019; Blake et al. 2020). Transplacental transfer of PFAS confers a substantial burden to the newborn infant. Because the infant has a smaller overall mass and blood volume, PFAS are concentrated, increasing PFAS per volume (Koponen et al. 2018). In addition, transfer of PFAS is common through lactation, and the longer a child breastfeeds, the higher the body burden (Gyllenhammar et al. 2018; VanNoy et al. 2018).
Effects of comorbidity on PFAS toxicokinetics
Factors affecting renal function can influence PFAS toxicokinetics. As discussed, opposing types of causation should be considered. Human toxicokinetics appear to vary bidirectionally with changing renal function, leading to nonmonotonic dose-response relationships and, depending on the study goal, possibly to errors in estimating disease associations. As progress is made in the field of PFAS toxicokinetics, new chemistries may have different clearance factors and nuances that vary by PFAS group or structures, and that will need to be investigated to accurately model half-lives in different exposure subgroups.
Sources of information on toxicokinetics in humans: strengths and limitations of studies
Some PFAS half-life data in humans were obtained from retired industry workers, particularly those who worked with PFOS, PFOA, and PFHxS (Olsen et al. 2007). Since then, these estimates have been modified slightly or confirmed with longitudinal data and modeling from contaminated communities once uncontaminated water options were provided (Bartell et al. 2010; Li et al. 2018). Other contemporary PFAS estimates are derived from biomonitoring studies of production workers, blood donors, study participants, and/or occupationally exposed cohorts (Olsen et al. 2009, 2017; Russell et al. 2013; Zhang et al. 2013). Some caution must be taken in using these data because variables affecting PFAS clearance may not be taken into consideration (age, sex, menstruation, disease, and medication status) and may contribute to confounding.
The challenge in determining a reliable human half-life in these types of studies is that exposure does not end with a clean water source, retirement, or a change of job and that continued exposures vary over potential depuration periods. Model components may also vary in subclasses. Children (small blood volumes and a large fraction of exposures comes from drinking), pregnant women (large increase in blood volume and water intake), parous women (transfer to fetus and breast milk), and athletes (water intake elevated) are examples of subpopulations with expected variation in half-life compared to adult men (Post et al. 2017). There will be more human estimates of PFAS forthcoming that involve variations in half-life (Post et al. 2017). Realistic computational modeling can help, so long as it clearly characterizes exposures and applicable populations. The continued goal should be to provide predictive values for those PFAS lacking actual measurements, based on chemical structures and trusted physiological parameters.
Physiologically based pharmacokinetic/toxicokinetic modeling in different-aged populations
In the blood and other tissues, PFAS toxicokinetics are influenced by their interactions with proteins (Andersen et al. 2006; Katakura et al. 2007; Nakagawa et al. 2008; Weaver et al. 2009; Figure 2). Certain toxicokinetic features are saturable, and thus dosing in toxicokinetic studies is of profound importance. Studies of renal reabsorption mechanisms in mammals show that reduced activity of transporters such as organic anion transporting polypeptide 1a1, through inactivation (e.g., genetic manipulation, castration, treatment with estrogen) or by saturation at increasing doses, leads to substantial reductions in half-lives of PFOA and PFOS (Andersen et al. 2006; Nakagawa et al. 2008; Weaver et al. 2009; Yang et al. 2009).
FIGURE 2:
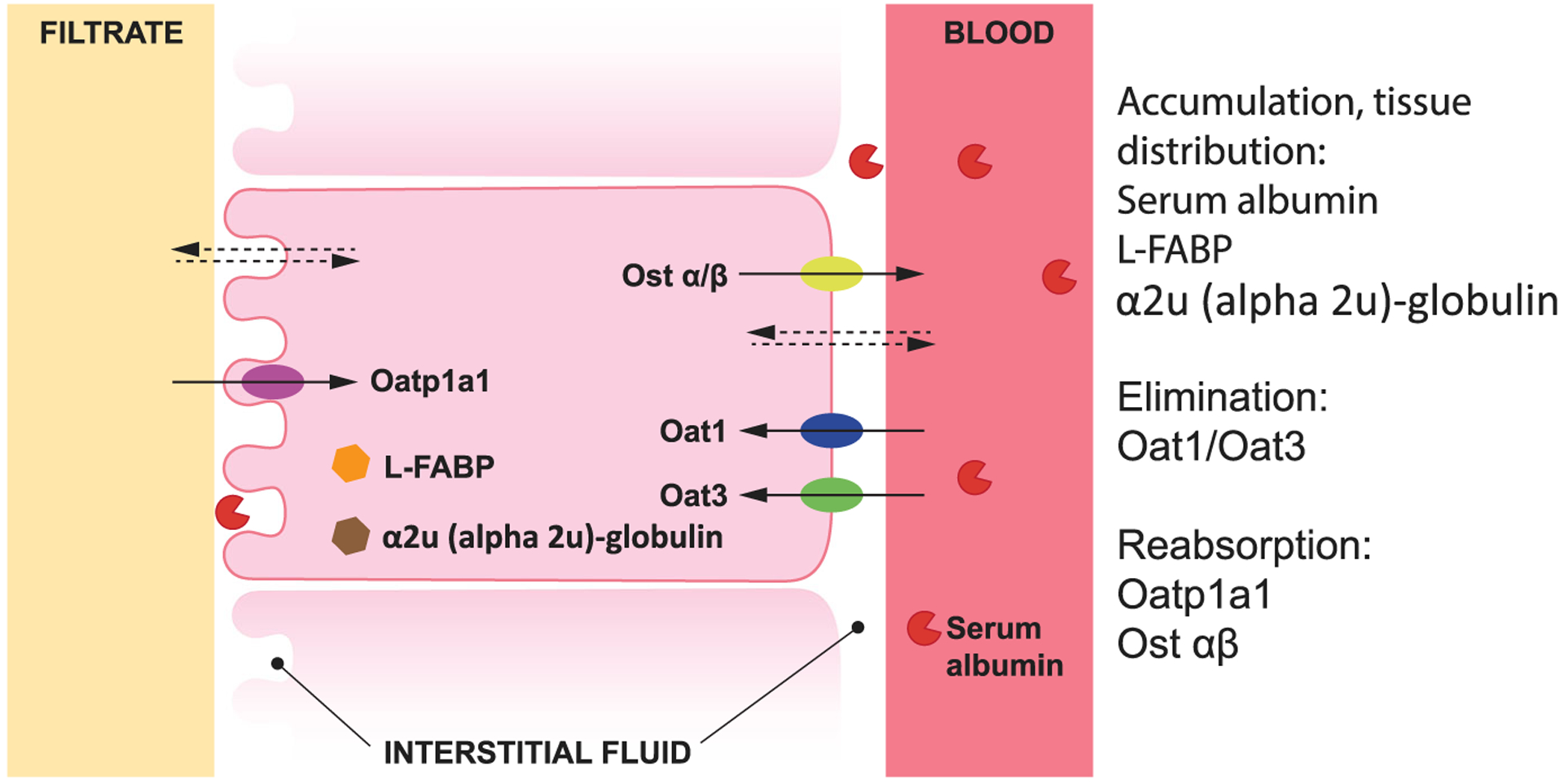
Example of proteins that are known to influence per- and polyfluoroalkyl substance toxicokinetics through binding (which affects tissue distribution and accumulation) and facilitation of membrane transport (which affects clearance and reabsorption). Illustrated for kidney and blood. L-FABP = liver fatty acid binding protein; Oat1 = organic anion transporting 1; Oatp1a1 = organic anion transporting polypeptide 1a1; Ost = organic solute transporter.
These protein-associated toxicokinetic processes were recently incorporated into a model for PFOA in the male Sprague-Dawley rat (Cheng and Ng 2017), which provides a useful platform to explore how changes in protein interactions might affect estimates of PFAS half-life (Figure 3). At high doses, it is typical to see clear biphasic behavior with rapid initial clearance, during which the serum half-life appears to be shorter especially at high enough doses that processes such as renal reabsorption are saturated, followed by a much longer tail (Figure 3A). In a similar fashion, the magnitude of internal dose and rate of serum clearance can be profoundly influenced by proteins known to bind PFAS, such as serum albumin (Figure 3B). Increasing and decreasing the extent of reabsorption in the kidney increases and decreases the serum half-life, respectively (Figure 3C). Finally, the effect of saturating reabsorption is magnified when the half-life is longer because of increased serum binding (Figure 3D). In this case, taking an initial slope to calculate the serum half-life at high doses would lead to a profound underestimation.
FIGURE 3:
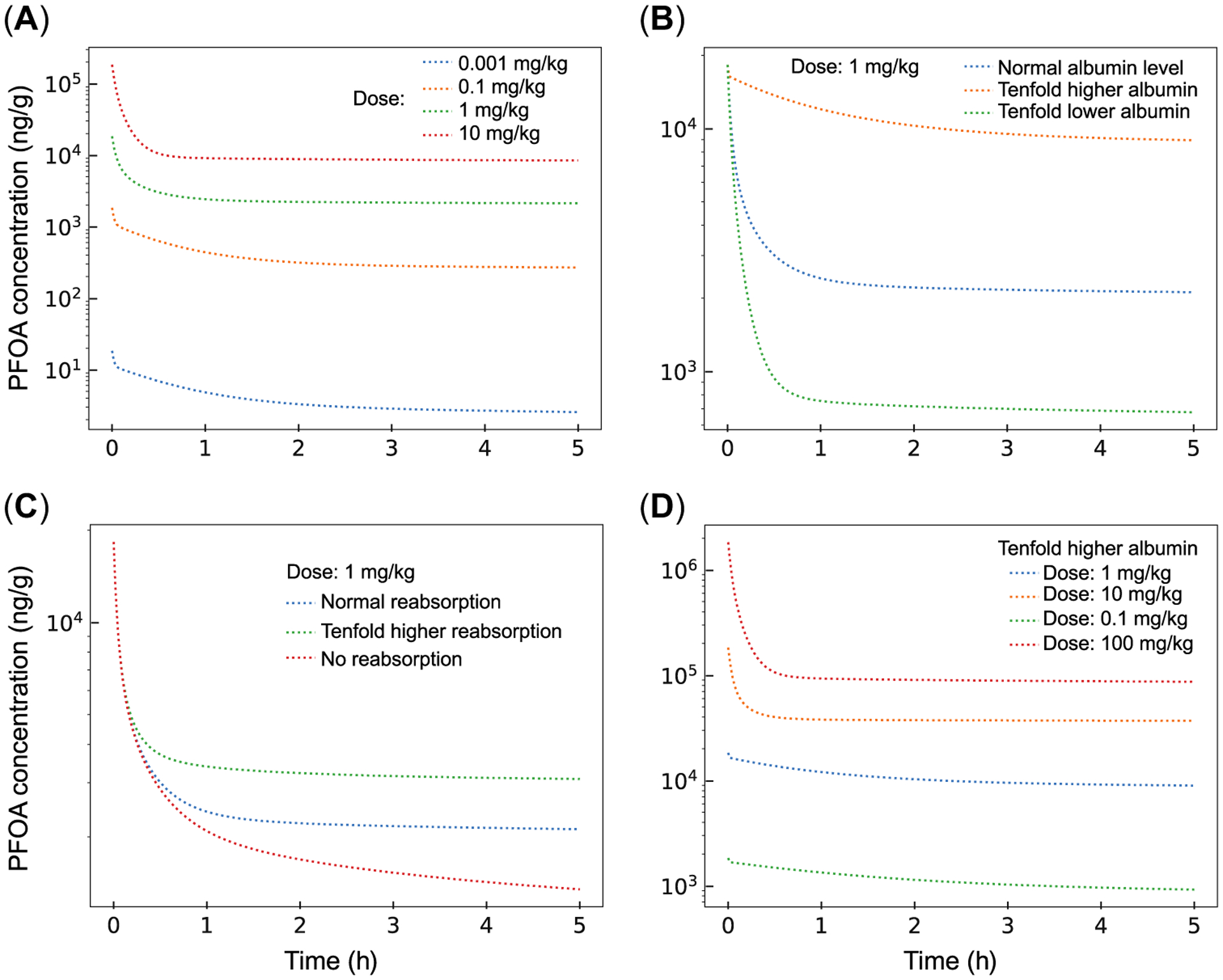
Simulations based on Cheng and Ng (2017), perfluorooctanoic acid (PFOA) toxicokinetic model for Sprague-Dawley rats. (A) Effect of dose on initial half-life. (B) Effect of higher and lower levels of serum albumin, which binds to PFOA, on serum clearance dynamics. (C) Effect of extent of reabsorption in kidney on serum half-life, based on organic anion transporting polypeptide 1a1 activity. (D) Effect of dose on elimination kinetics when half-life is longer because of higher albumin binding. Oat1 = organic anion transporting 1; Oat3 = organic anion transporting 3; Ost = organic solute transporter.
Differences in protein expression, circulating levels, and even protein type across populations, sex, and species could lead to important species and sex differences in PFAS biological half-lives (Han et al. 2012); such differences should be investigated and taken into account in the extrapolation to human equivalent doses. Because expression of proteins may change at different life stages, clearance factors and toxicokinetics may also change.
Given the large number of species-, sex-, and age-specific differences that have been observed, coupled with the lack of data for many PFAS, the parameterization of complex physiologically based toxicokinetic models remains a persistent challenge. Therefore, lower-resolution models (e.g., one-compartment or few-compartment models) may be more appropriate for species and settings where insufficient data are available for reasonably accurate parameterization. Alternatively, in silico and in vitro methods are under development that could aid in parameterization in the absence of in vivo data, as discussed in the section New approaches for developing PFAS toxicity information.
SO MANY PFAS, SO LITTLE TIME: ACCELERATING THE PACE OF DISCOVERY
Importance of determining mode of action and adverse outcome pathways
Information on modes of action and/or adverse outcome pathways (AOPs) is invaluable in 1) establishing human relevance of experimental evidence, 2) assessing causality in epidemiological studies, 3) applying “read-across” to PFAS for which there is little toxicological information, 4) assessing risks from mixtures, 5) guiding development and interpretation of new approach methodologies, 6) informing the development of biomarkers in epidemiologic investigation, and 7) identifying potentially vulnerable subpopulations and life stage-specific effects (Meek et al. 2014; LaLone et al. 2017). Verified modes of action and AOPs can inform risk assessment based on intermediate effects and enable development of new methodology-based approaches to assess PFAS safety (Meek et al. 2014).
Postulated modes of action/AOPs for PFAS
Mechanistic studies have been performed on only a few PFAS. These have been shown to activate a range of putative molecular initiating targets, among which are the nuclear receptors PPARα, PPARγ, PPARβ/δ, CAR, PXR, liver X receptor α, and ERα (Bijland et al. 2011; Bjork et al. 2011; Rosen et al. 2017; Li et al. 2019). However, modes of action verified by agreed procedures (World Health Organization 2020) have been established for few reported effects of PFAS, and those that have been interrogated involve activation of PPARα and, at higher doses, CAR as molecular initiating events (Klaunig et al. 2012; Rosen et al. 2017). Several AOPs involving these molecular targets are in various stages of development (Organisation for Economic Co-operation Development 2020), but few have been endorsed by the OECD following its agreed procedures (Organisation for Economic Co-operation Development 2017). Demonstration of receptor activation alone is insufficient to establish involvement of a mode of action or AOP in an observed effect, for which an overall weight-of-evidence approach is necessary (World Health Organization 2020).
Andersen et al. (2007) provide a useful, albeit dated, review of possible PFAS modes of action. Established modes of action are restricted largely to the liver and include species-specific hepatic hyperplasia and liver tumors (Butenhoff et al. 2012; Elcombe et al. 2012; Corton et al. 2018). Available studies on PFBS, PFHxS, perfluorohexanoic acid, PFNA and PFDA suggest that they share molecular targets with similar consequences, albeit with differences in potency, in part due to differences in their excretion and protein-interaction kinetics (Zeilmaker et al. 2018). However, studies in vitro have established intrinsic differences in potency among PFAS analogues. Potency in activating PPARα showed some relationship with PFAS chain length (Wolf et al. 2008). A mode of action or AOP provides a causal chain of key events between chemical exposure and outcome. The established modes of action for PFOS and PFOA provide a causal explanation for development of liver tumors observed in rodents on exposure to these compounds, through activation of PPARα, and the possible relevance to humans. However, this does not mean that other effects of PFAS are due to activation of PPARα or that other pathways might not lead to liver tumors in humans, such as secondary to the primary effect of steatosis.
Until recently, there has been little study of modes of action/AOPs for effects of PFAS other than hepatic outcomes in rodents, particularly for critical effects, such as immunosuppression and developmental toxicity, and from PFAS other than PFOS and PFOA (EFSA Panel on Contaminants in the Food Chain 2020; Temkin et al. 2020). The ability of various PFAS to interact with and modify lipid metabolism is, however, an intriguing hypothesis (Xu et al. 1999; Jones et al. 2003; Andersen et al. 2007; Tan et al. 2013; Pouwer et al. 2019). Other putative molecular initiating/key events for PFAS, in addition to nuclear receptor activation, include gap junctional inhibition to disrupt cell-cell communication, mitochondrial dysfunction, interference of protein binding, partitioning into lipid bilayers, oxidative stress, altered calcium homeostasis, and inappropriate activation of molecular signals controlling cell functions. Many of these effects are consistent with a nonspecific action of PFAS on the cellular lipid membrane (Spector and Yorek 1985; Bourre et al. 1989; Dodes Traian et al. 2012; Casares et al. 2019). However, these alternative events lack robust evidence to support a specific pathophysiological role in the multifaceted effects of PFAS. A better characterization of the modes of action/AOPs for PFAS toxicities remains an important area of future investigation, necessary to improve our understanding of PFAS impacts on human health.
At present, there is insufficient evidence to determine which of, and to what extent, these molecular interactions play a pathophysiological role in observed adverse outcomes of PFAS (Michigan PFAS Science Advisory Panel 2018). Hence, there is a need to integrate such mechanistic information into a weight-of-evidence framework, first by establishing the mode of action or AOP linking a proposed chain of key events to an adverse outcome and then by demonstrating that at human exposure levels of PFAS the established AOP or mode of action is causal in the adverse outcome observed. The substantial advantage offered by such an approach is the ability to read across from representative members of appropriate PFAS groupings, based on quantitative information from new approach methodologies and exposure estimates. Hence, better characterization of the modes of action/AOPs for PFAS toxicities remains a critical area of future investigation and will allow us to understand which adversely PFAS-modified pathways must be interrogated prior to new chemicals joining this class. Predicting PFAS activity in the body should be the goal prior to approving novel PFAS for use.
New approaches for developing PFAS toxicity information
When it comes to determining which PFAS should be prioritized for further testing, there are too many chemicals, even in one subclass, for traditional approaches. Numerous creative and high-throughput methodologies are being developed and tested to provide valuable data on PFAS with no toxicity data.
Collaborative approaches.
Problem formulation and approach must be guided by available equipment, funds, and technical staff, and important principles: 1) What biological activity and toxicology information can be generated in a responsive time frame? 2) Can this information be used to make public health decisions? 3) What are appropriate tools to bring to this problem (platforms, species/sex of cells used, metabolic competency of the model system, and data analysis)? 4) How do we organize, and what are the best mechanisms to report useful biological activity/toxicological information?
Developing “how” to evaluate potential health effects of new PFAS requires some thought to PFAS heterogeneity. Although subclass names have been suggested by several investigators (Buck et al. 2011; Wang Z et al. 2017; Sha et al. 2019), there is still disagreement on those groupings. In addition, half-lives and biological persistence are not predictable based on structure, and exposure routes may be complex. Given that traditional approaches to generate toxicity information are resource-intensive, new approach methodologies, which may include in vitro high-throughput toxicity screening and toxicokinetic testing, will be needed to inform further (in vivo) testing of PFAS.
One example of how agencies/institutes are collaborating to prioritize a list of PFAS needing further study is the REACT Program (Responsive Evaluation and Assessment of Chemical Toxicity). Scientists from the USEPA and the National Institute of Environmental Health Sciences (NIEHS) National Toxicology Program have joined forces to determine if read-across approaches would work. Essentially, they will use existing data for a data-rich substance (the source, e.g., PFOA or PFOS) as an anchor for a data-poor substance (the target, a novel PFAS), which is considered similar enough to the source substance to use the same data as a basis for the safety assessment. For example, the US National Toxicology Program 28-d PFAS or chronic PFOA data set (National Toxicology Program 2020c) could be used as an anchor. The goal is to group PFAS by biological activities and then use in vitro to in vivo extrapolation data and models to estimate oral equivalent exposures for PFAS. For example, multiple biological endpoints (Table 2) were chosen to generate data on 150 PFAS (Patlewicz et al. 2019), representing several structural subclasses for use in read-across.
TABLE 2:
Fit-for purpose assays proposed in the REACT program
| Endpoint of interest | Assay proposed |
|---|---|
| High-throughput transcriptomics | Metabolically competent human liver cells/MCF-7 (Tempo-Seq®) |
| Hepatotoxicity | 2D HepaRG® cells |
| Developmental toxicity | Zebrafish embryo assay |
| Developmental neurotoxicity | Multielectrode array in neonatal cortical cells and neurite outgrowth |
| Immunotoxicity | Cytokine alterations in human vascular endothelial cells (BioSeek®) |
| Hepatic clearance | Metabolic clearance in 50 donor-pooled hepatocyte suspensions |
| Plasma protein binding | Serum protein binding assay using human serum |
| Enterohepatic recirculation | Qualyst B-CLEAR® hepatocyte transporter assay |
| In vitro disposition | In vitro disposition in cell lines under study |
REACT = Responsive Evaluation and Assessment of Chemical Toxicity.
Selecting assays shown in Table 2 based on PFOA and PFOS health effects covers a broad range of biology. However, because of the structural diversity of PFAS, biological activity of subclasses of PFAS may be missed; but this can be addressed in 2 ways. First, using transcriptomics as a screen, similar and unique pathways altered by different PFAS can be identified. Second, structure-activity relationships may predict potentially missing biological activities. As an example, Leadscope model predictions conducted at the NIEHS predicted biology that was covered in assays already chosen for evaluation, which increased confidence in the approaches chosen. Because model predictions are only as robust as data sets from which they are generated, these outputs should be used to identify assays for screening efforts and not as synonymous with toxicities induced by PFAS. Ultimately, the REACT program aims to prioritize PFAS for additional targeted testing and follow-up with in vivo studies as needed.
Molecular dynamics and protein interactions.
Advances in computational tools, many developed for drug discovery, allow environmental and public health researchers to better anticipate some impacts of emerging contaminants even in the absence of substantial experimental data (Rabinowitz et al. 2008). For example, molecular docking and molecular dynamics to predict strengths of interactions between biomolecules and contaminants can be an in vitro screening tool for assessing legacy and emerging PFAS for bioaccumulation potential, to identify potential sites of toxic action (Salvalaglio et al. 2010; Ng and Hungerbuehler 2015; Cheng and Ng 2018; Li et al. 2019) and to gain insights into toxic mechanisms (Sheng et al. 2018). Relatively strong binding with particular proteins (e.g., serum albumin, liver fatty acid binding protein) has already proven useful in correlating PFAS structure with potential for bioaccumulation (Ng and Hungerbühler 2014; Cheng and Ng 2017). Tools including molecular docking and molecular dynamics can correlate relative binding affinities of emerging PFAS with these target proteins and subsequently compare with affinities of legacy chemicals with known bioaccumulation potentials, thus providing a first-tier rapid screening mechanism (Luebker et al. 2002; Cheng and Ng 2018).
The use of fluorinated substances in pharmaceutical products has led to an unexpected data source for discovery of structural features in PFAS associated with various types of bioactivity. These data were recently used to train machine learning models to predict potential bioactivity for thousands of untested PFAS (Cheng and Ng 2019). Classification approaches such as these serve as preliminary screening tools for identifying PFAS as a first step in a tiered assessment when detailed mechanistic information is not available.
Addressing mixtures.
Based on their potential for complex exposure patterns, PFAS are a mixtures issue. Communities with water-monitoring programs reporting PFAS concentrations demonstrated that they are exposed to mixtures of PFAS. This mixture may be from one or more point sources releasing multiple PFAS and/or PFAS by-products into the air and water, such as a Chemours plant in North Carolina, and suggest that exposures may be substantial (McCord and Strynar 2019). However, numerous other PFAS sources are known to impact community exposure to PFAS mixtures, such as landfill leachate, biosolids recycling, and aqueous film-forming foam contamination of drinking water sources, among others (Sunderland et al. 2019; Solo-Gabriele et al. 2020). Aqueous film-forming foam and other mixtures evident in drinking water, food packaging, health and beauty products, and food-based sources are often poorly characterized (Sunderland et al. 2019; Susmann et al. 2019).
Discussions on whether PFAS may be addressed using a relative potency framework or toxic equivalency factor approach are ongoing. Substances could be grouped by bioaccumulation and persistence (toxicokinetics), function (biology), molecular initiating events, with potency factors derived from several assays, or subclass (structural similarity).
SPECIAL CONSIDERATIONS IN FUTURE STUDY DESIGNS
Future epidemiological studies
Future human studies need to characterize immune outcomes including (and not limited to) immune effects from exposure in early pregnancy and possible roles of PFAS in initiating allergic and autoimmune processes, conditions for which a dose response is hard to predict. Interactions of immune pathways with liver and lipid toxicity deserve additional consideration.
Liver and lipid studies have reasonably characterized associations between PFAS and effects and should now address why and what to do about it. Characterization of possible a priori susceptibility, such as in the obese, is important. Human and animal lipid data suggest that future experimental studies should focus on mitochondrial toxicity, alterations in bile acid metabolism, cholestasis, and resultant steatosis. These outcomes are already known to be associated with altered serum lipids, liver enzymes, and uric acid in the human population regardless of PFAS (Cohen and Fisher 2013; Sattar et al. 2014; Arguello et al. 2015; Jensen et al. 2018).
Studies of human kidney markers related to PFAS exposures illustrate the importance of understanding physiology to inform study design choices and reasonable interpretations. These substances have complex excretion mechanics that vary with dose, state of the healthy or progressively diseased kidney, as well as a potentially additional causative effect on kidney disease outcome(s). Appropriate definition of biological and mechanistic targets and more precise investigation of PFAS subclasses will better inform study designs and research questions. For example, consistent reports of disrupted cholesterol metabolism should prompt mechanistic studies evaluating effects on steroid hormones that may influence cancer, fecundity, lactation, and developmental signals seen in human population data. More attention could be given to effects of PFAS on the hypothalamic-pituitary-gonadal axis and then reconsidered based on life stages.
The history of long-chain PFAS studies indicates that collaborative team approaches featuring clinical, epidemiologic, computational modeling, and laboratory toxicological expertise are needed. Future population designs and more sensitive analytical methodologies should address replacement chemicals, typically found as mixtures; study designs must account for shorter PFAS half-lives and unpredictable PFAS detection in exposed individuals/communities. Innovative use of biomarkers in specifically designated risk subpopulations (obesity, immune) will likely be important.
Sex differences, nonmonotonic dose responses, sensitive subpopulations
Although serum-level differences exist between men and women similarly exposed to individual PFAS, sex-dependent differences in half-life have not been reported in human populations for short-chain (PFBS, PFBA) or long-chain per-fluoroalkyl acids thus far (Li et al. 2017b). Perhaps the half-life differences between the sexes is similar to interindividual variability and cannot be detected above background, or studies deriving data sets used to model half-lives were not designed to detect sex differences (convenience sampling or workers were mostly male, etc.). However, sex-specific elimination half-lives are defined (Table 1) for some PFAS in rodent models. In addition, developmental exposure studies in experimental models have consistently shown effects at lower doses than adult-only exposures and should be given priority in testing replacement chemicals. In vitro and alternative models that capture developmental susceptibility are encouraged. In summary, care should be taken in testing replacement PFAS in rodent or alternative (cell-based or zebrafish, for example) models to consider 1) the possibilities of sex-based differences in elimination half-lives, 2) dose range used (to include human relevant exposures), 3) life stage represented in the model system, and 4) variability of the response to enable the use of data generated for risk assessment.
Future experimental model studies
Experimental rodent studies have been essential in confirming PFAS health effects (liver and thyroid disease, lipid homeostasis), even when effects were not identical to those in humans; in some cases, novel targets (mammary and immune changes) were identified in animals. Future animal, cell-based, and high-throughput toxicity screening should enhance transparency in reporting to include blinded dose allocation, reporting of all data, adherence to Animal Research Reporting In Vivo Experiments (ARRIVE) guidelines (Kilkenny et al. 2010), and dose ranges that approach human relevance (adjusted to reflect the differences in elimination between species and potentially chronic exposures) so that they suitably inform systematic reviews that may be used in chemical regulation.
Model selection for health effects evaluation is critical. An appropriate model should be sensitive, be susceptible to the outcome(s) of interest (obesity, immune), and produce outcomes that will inform human health effects. Alternative research models, such as transgenic mice, zebrafish, developmental models for most affected target tissues, and diet-challenged designs in susceptible rodent strains, will strengthen our knowledge of PFAS-related health effects. Validation of fish neurobehavior models to inform mammalian, including human, developmental responses is needed.
Finally, advanced human cell-based platforms—that have been validated for relevant outcomes in humans—will facilitate concurrent screening of larger numbers of PFAS, but bioavailability of PFAS in the culture system needs to be understood because binding to media proteins or labware, the instability of some PFAS in some vehicles, and altered metabolism may exist in some cases (Gaballah et al. 2020; Liberatore et al. 2020).
Future alternative approaches
One way to determine the toxicity of the large number of PFAS compounds currently used in commerce is to develop quantitative structure-activity relationships (QSAR). Such QSAR attempt to define relationships between a PFAS compound structure with a specific biological activity or response that identifies or is a biomarker for toxicity. Few data are available for receptor binding of PFAS, mainly limited to a few PFCAs and PFSAs; and even between carboxylates and sulfonates of similar chain length substantial differences have been observed (Cheng and Ng 2017, 2018). If there are substantial differences between perfluoroalkyl carboxylic and sulfonic acids, which differ only in their acid head group, construction of successful QSAR for the large and diverse class of all PFAS will be particularly challenging. Several QSAR may be developed, each predictive of toxicity of a distinct class or subclass of PFAS, based on a unique functional moiety or other feature. Although this brings additional challenges in finding sufficient data for QSAR training and validation, big data approaches, such as the recently developed machine learning models to predict PFAS bioactivity (Cheng and Ng 2019), show promise for advancing these computational approaches at the screening level.
For example, it may be determined by affinity for receptor-specific binding and nonspecific interactions with cellular membranes that the specific toxic effect exhibits a multiphasic dose response reflecting 2 potential modes of action. In addition, the critical effect may change with levels of PFAS exposure. Add to this that people are typically exposed to PFAS mixtures, each of which may have a different affinity for a binding site and ability to impact cellular membrane fluidity, and the potential to predict PFAS toxicity becomes extremely complicated. In the foreseeable future, we may be limited to assessing PFAS toxicity using high-throughput assays designed to inform regulators as to the relative toxicity of PFAS mixtures or compounds. Such approaches are suited to the use of artificial intelligence (i.e., machine learning approaches) that integrate data from multiple sources to identify bioaccumulation potential, relevant pathways triggered, protein binding affinities, and modes of action involved in the development of individual and mixture toxicity of PFAS.
The utility of any future approach to determining PFAS toxicity must consider tissue-specific modes of action. Such an approach may rely on molecular interactions with specific binding sites on enzymes/storage/transport proteins or the nonspecific ability to alter cell membrane fluidity by which membrane-bound protein activities are altered within a particular organ/system. Regardless of the mode of action, model, and/or simulation, the predictive result should be biologically plausible and represent dose-effect responses across species.
CONCLUSION
Future research on the health effects of replacement PFAS and mechanistic studies on legacy PFAS must apply “lessons learned” such as those highlighted in the present review. There are only a handful of PFAS with enough health effects data for use in decision-making, as evidenced by state-led standard setting. There are numerous health effects reported for those PFAS tested, which sets this family of chemicals apart from many others and elevates the need for precautionary action. With hundreds of PFAS lacking health effects data, translational research teams using innovative methodologies and carefully designed studies will be critical to our state of knowledge on PFAS-related health effects and our enhanced strategies for informing risk assessment of this large family of chemicals.
Acknowledgment—
We would like to express our gratitude to the presenters in the Human Health Toxicity plenary session at SETAC’s Focused Topic Meeting on Environmental Risk Assessment of PFAS for setting the stage for productive discussions that followed: S. Chang, M. DeVito, J. DeWitt, A. Ducatman, C. Lau, C. Ng, S. Roberts, and R. Thomas. We extend our appreciation to those who provided constructive comments in the breakout sessions and during the development of this document (L. Birnbaum, NIEHS; G. Post, New Jersey Department of Environmental Protection; C. Blystone, Division of the National Toxicology Program; J. Rogers, USEPA). An additional thank you to A. Owoc and S. Mantooth (NIEHS contractors) for their excellence in reference construction.
Footnotes
Publisher's Disclaimer: Disclaimer—The views expressed in this publication are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, the Department of Defense, the USEPA, the National Institutes of Health, or the US government. J.S. Smith, S.E. Fenton, and C. Lau are employees of the US government. Their work in the preparation of this publication was part of their official duties. Title 17, U.S.C., §105 provides that copyright protection under this title is not available for any work of the US government. Title 17, U.S.C., §1010 defines a US government work as a work prepared by a military service member or employee of the US government as part of that person’s official duties.
Data Availability Statement—Data, associated metadata, and calculation tools are available from the corresponding author (smroberts@ufl.edu).
REFERENCES
- Abraham K, Mielke H, Fromme H, Volkel W, Menzel J, Peiser M, Zepp F, Willich SN, Weikert C. 2020. Internal exposure to perfluoroalkyl substances (PFASs) and biological markers in 101 healthy 1-year-old children: Associations between levels of perfluorooctanoic acid (PFOA) and vaccine response. Arch Toxicol 94:2131–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry. 2018. Toxicological profile for perfluoroalkyls. US Department of Health and Human Services, Washington, DC. [cited 2020 July 13]. Available from: https://www.atsdr.cdc.gov/toxprofiles/tp200.pdf [Google Scholar]
- Alderete TL, Jin R, Walker DI, Valvi D, Chen Z, Jones DP, Peng C, Gilliland FD, Berhane K, Conti DV, Goran MI, Chatzi L. 2019. Perfluoroalkyl substances, metabolomic profiling, and alterations in glucose homeostasis among overweight and obese Hispanic children: A proof-of-concept analysis. Environ Int 126:445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen ME, Butenhoff JL, Chang S-C, Farrar DG, Kennedy GL Jr, Lau C, Olsen GW, Seed J, Wallace KB. 2007. Perfluoroalkyl acids and related chemistries—Toxicokinetics and modes of action. Toxicol Sci 102:3–14. [DOI] [PubMed] [Google Scholar]
- Andersen ME, Clewell HJ, Tan Y-M, Butenhoff JL, Olsen GW. 2006. Pharmacokinetic modeling of saturable, renal resorption of perfluoroalkylacids in monkeys—Probing the determinants of long plasma half-lives. Toxicology 227:156–164. [DOI] [PubMed] [Google Scholar]
- Andersson EM, Scott K, Xu Y, Li Y, Olsson DS, Fletcher T, Jakobsson K. 2019. High exposure to perfluorinated compounds in drinking water and thyroid disease. A cohort study from Ronneby, Sweden. Environ Res 176:108540. [DOI] [PubMed] [Google Scholar]
- Arguello G, Balboa E, Arrese M, Zanlungo S. 2015. Recent insights on the role of cholesterol in non-alcoholic fatty liver disease. Biochim Biophys Acta 1852:1765–1778. [DOI] [PubMed] [Google Scholar]
- Armstrong LE, Guo GL. 2019. Understanding environmental contaminants’ direct effects on non-alcoholic fatty liver disease progression. Curr Environ Health Rep 6:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley-Martin J, Dodds L, Arbuckle TE, Morisset AS, Fisher M, Bouchard MF, Shapiro GD, Ettinger AS, Monnier P, Dallaire R, Taback S, Fraser W. 2016. Maternal and neonatal levels of perfluoroalkyl substances in relation to gestational weight gain. Int J Environ Res Public Health 13:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attanasio R 2019. Sex differences in the association between perfluoroalkyl acids and liver function in US adolescents: Analyses of NHANES 2013–2016. Environ Pollut 254:113061. [DOI] [PubMed] [Google Scholar]
- Avanasi R, Shin HM, Vieira VM, Bartell SM. 2016a. Variability and epistemic uncertainty in water ingestion rates and pharmacokinetic parameters, and impact on the association between perfluorooctanoate and preeclampsia in the C8 Health Project population. Environ Res 146: 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avanasi R, Shin HM, Vieira VM, Savitz DA, Bartell SM. 2016b. Impact of exposure uncertainty on the association between perfluorooctanoate and preeclampsia in the C8 Health Project population. Environ Health Perspect 124:126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach CC, Liew Z, Bech BH, Nohr EA, Fei C, Bonefeld-Jorgensen EC, Henriksen TB, Olsen J. 2015. Perfluoroalkyl acids and time to pregnancy revisited: An update from the Danish National Birth Cohort. Environ Health 14:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros V, Costa O, Iniguez C, Fletcher T, Ballester F, Lopez-Espinosa MJ. 2017. Exposure to perfluoroalkyl substances and thyroid function in pregnant women and children: A systematic review of epidemiologic studies. Environ Int 99:15–28. [DOI] [PubMed] [Google Scholar]
- Barry V, Darrow LA, Klein M, Winquist A, Steenland K. 2014. Early life perfluorooctanoic acid (PFOA) exposure and overweight and obesity risk in adulthood in a community with elevated exposure. Environ Res 132:62–69. [DOI] [PubMed] [Google Scholar]
- Barry V, Winquist A, Steenland K. 2013. Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environ Health Perspect 121:1313–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartell SM, Calafat AM, Lyu C, Kato K, Ryan PB, Steenland K. 2010. Rate of decline in serum PFOA concentrations after granular activated carbon filtration at two public water systems in Ohio and West Virginia. Environ Health Perspect 118:222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassler J, Ducatman A, Elliott M, Wen S, Wahlang B, Barnett J, Cave MC. 2019. Environmental perfluoroalkyl acid exposures are associated with liver disease characterized by apoptosis and altered serum adipocytokines. Environ Pollut 247:1055–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behr AC, Kwiatkowski A, Stahlman M, Schmidt FF, Luckert C, Braeuning A, Buhrke T. 2020a. Impairment of bile acid metabolism by perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) in human HepaRG hepatoma cells. Arch Toxicol 94:1673–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behr A-C, Lichtenstein D, Braeuning A, Lampen A, Buhrke T. 2018. Perfluoroalkylated substances (PFAS) affect neither estrogen and androgen receptor activity nor steroidogenesis in human cells in vitro. Toxicol Lett 291:51–60. [DOI] [PubMed] [Google Scholar]
- Behr A-C, Plinsch C, Braeuning A, Buhrke T. 2020b. Activation of human nuclear receptors by perfluoroalkylated substances (PFAS). Toxicol In Vitro 62:104700. [DOI] [PubMed] [Google Scholar]
- Benninghoff AD, Bisson WH, Koch DC, Ehresman DJ, Kolluri SK, Williams DE. 2010. Estrogen-like activity of perfluoroalkyl acids in vivo and interaction with human and rainbow trout estrogen receptors in vitro. Toxicol Sci 120:42–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg V, Nost TH, Hansen S, Elverland A, Veyhe AS, Jorde R, Odland JO, Sandanger TM. 2015. Assessing the relationship between perfluoroalkyl substances, thyroid hormones and binding proteins in pregnant women; a longitudinal mixed effects approach. Environ Int 77:63–69. [DOI] [PubMed] [Google Scholar]
- Bijland S, Rensen PC, Pieterman EJ, Maas AC, van der Hoorn JW, van Erk MJ, Havekes LM, Willems van Dijk K, Chang SC, Ehresman DJ, Butenhoff JL, Princen HMG. 2011. Perfluoroalkyl sulfonates cause alkyl chain length-dependent hepatic steatosis and hypolipidemia mainly by impairing lipoprotein production in APOE*3-Leiden CETP mice. Toxicol Sci 123:290–303. [DOI] [PubMed] [Google Scholar]
- Bjerregaard-Olesen C, Bach CC, Long M, Wielsøe M, Bech BH, Henriksen TB, Olsen J, Bonefeld-Jørgensen EC. 2019. Associations of fetal growth outcomes with measures of the combined xenoestrogenic activity of maternal serum perfluorinated alkyl acids in Danish pregnant women. Environ Health Perspect 127:017006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JA, Butenhoff JL, Wallace KB. 2011. Multiplicity of nuclear receptor activation by PFOA and PFOS in primary human and rodent hepatocytes. Toxicology 288:8–17. [DOI] [PubMed] [Google Scholar]
- Blake BE, Cope HA, Hall SM, Keys RD, Mahler BW, McCord J, Scott B, Stapleton HM, Strynar MJ, Elmore SA, Fenton SE. 2020. Evaluation of maternal, embryo, and placental effects in CD-1 mice following gestational exposure to perfluorooctanoic acid (PFOA) or hexafluoropropylene oxide dimer acid (HFPO-DA or GenX). Environ Health Perspect 128:27006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake BE, Pinney SM, Hines EP, Fenton SE, Ferguson KK. 2018. Associations between longitudinal serum perfluoroalkyl substance (PFAS) levels and measures of thyroid hormone, kidney function, and body mass index in the Fernald Community Cohort. Environ Pollut 242:894–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodin J, Groeng EC, Andreassen M, Dirven H, Nygaard UC. 2016. Exposure to perfluoroundecanoic acid (PFUnDA) accelerates insulitis development in a mouse model of type 1 diabetes. Toxicol Rep 3:664–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese MM, Walker M, Helewa ME, Fraser WD, Arbuckle TE. 2020. Association of perfluoroalkyl substances with gestational hypertension and preeclampsia in the MIREC study. Environ Int 141:105789. [DOI] [PubMed] [Google Scholar]
- Borghoff SJ, Fitch S, Rager JE, Huggett D. 2018. A hypothesis-driven weight-of-evidence analysis to evaluate potential endocrine activity of perfluorohexanoic acid. Regul Toxicol Pharmacol 99:168–181. [DOI] [PubMed] [Google Scholar]
- Bourre J-M, Francois M, Youyou A, Dumont O, Piciotti M, Pascal G, Durand G. 1989. The Effects of dietary α-linolenic acid on the composition of nerve membranes, enzymatic activity, amplitude of electrophysiological parameters, resistance to poisons and performance of learning tasks in rats. J Nutr 119:1880–1892. [DOI] [PubMed] [Google Scholar]
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA, van Leeuwen SP. 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr Environ Assess Manag 7:513–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhrke T, Kruger E, Pevny S, Rossler M, Bitter K, Lampen A. 2015. Perfluorooctanoic acid (PFOA) affects distinct molecular signalling pathways in human primary hepatocytes. Toxicology 333:53–62. [DOI] [PubMed] [Google Scholar]
- Butenhoff JL, Chang SC, Olsen GW, Thomford PJ. 2012. Chronic dietary toxicity and carcinogenicity study with potassium perfluorooctanesulfonate in Sprague Dawley rats. Toxicology 293:1–15. [DOI] [PubMed] [Google Scholar]
- Butenhoff JL, Ehresman DJ, Chang SC, Parker GA, Stump DG. 2009. Gestational and lactational exposure to potassium perfluorooctanesulfonate (K+PFOS) in rats: Developmental neurotoxicity. Reprod Toxicol 27: 319–330. [DOI] [PubMed] [Google Scholar]
- C8 Science Panel. 2012. Probable Link Evaluation of Thyroid disease. [cited 2020 July 13]. Available from: http://www.c8sciencepanel.org/pdfs/Probable_Link_C8_Thyroid_30Jul2012.pdf
- Campbell S, Raza M, Pollack AZ. 2016. Perfluoroalkyl substances and endometriosis in US women in NHANES 2003–2006. Reprod Toxicol 65:230–235. [DOI] [PubMed] [Google Scholar]
- Cardenas A, Gold DR, Hauser R, Kleinman KP, Hivert MF, Calafat AM, Ye X, Webster TF, Horton ES, Oken E. 2017. Plasma concentrations of per- and polyfluoroalkyl substances at baseline and associations with glycemic indicators and diabetes incidence among high-risk adults in the Diabetes Prevention Program Trial. Environ Health Perspect 125: 107001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr CK, Watkins AM, Wolf CJ, Abbott BD, Lau C, Gennings C. 2013. Testing for departures from additivity in mixtures of perfluoroalkyl acids (PFAAs). Toxicology 306:169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casares D, Escribá PV, Rosselló CA. 2019. Membrane lipid composition: Effect on membrane and organelle structure, function and compartmentalization and therapeutic avenues. Int J Mol Sci 20:2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caverly Rae JM, Craig L, Slone TW, Frame SR, Buxton LW, Kennedy GL. 2015. Evaluation of chronic toxicity and carcinogenicity of ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate in Sprague-Dawley rats. Toxicol Rep 2:939–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2017. Per- and polyfluorinated substances (PFAS) factsheet. [cited 2020 May 19]. Available from: https://www.cdc.gov/biomonitoring/PFAS_FactSheet.html
- Chen J, Wang X, Ge X, Wang D, Wang T, Zhang L, Tanguay RL, Simonich M, Huang C, Dong Q. 2016. Chronic perfluorooctanesulphonic acid (PFOS) exposure produces estrogenic effects in zebrafish. Environ Pollut 218: 702–708. [DOI] [PubMed] [Google Scholar]
- Chen P, Wang Q, Chen M, Yang J, Wang R, Zhong W, Zhu L, Yang L. 2018. Antagonistic estrogenic effects displayed by bisphenol AF and perfluorooctanoic acid on zebrafish (Danio rerio) at an early developmental stage. Environ Sci Technol Lett 5:655–661. [Google Scholar]
- Cheng L, Albers P, Berney DM, Feldman DR, Daugaard G, Gilligan T, Looijenga LHJ. 2018. Testicular cancer. Nat Rev Dis Primers 4:29. [DOI] [PubMed] [Google Scholar]
- Cheng W, Ng CA. 2017. A permeability-limited physiologically based pharmacokinetic (PBPK) model for perfluorooctanoic acid (PFOA) in male rats. Environ Sci Technol 51:9930–9939. [DOI] [PubMed] [Google Scholar]
- Cheng W, Ng CA. 2018. Predicting relative protein affinity of novel per- and polyfluoroalkyl substances (PFASs) by an efficient molecular dynamics approach. Environ Sci Technol 52:7972–7980. [DOI] [PubMed] [Google Scholar]
- Cheng W, Ng CA. 2019. Using machine learning to classify bioactivity for 3486 per- and polyfluoroalkyl substances (PFASs) from the OECD list. Environ Sci Technol 53:13970–13980. [DOI] [PubMed] [Google Scholar]
- Chou WC, Lin Z. 2020. Probabilistic human health risk assessment of perfluorooctane sulfonate (PFOS) by integrating in vitro, in vivo toxicity, and human epidemiological studies using a Bayesian-based dose-response assessment coupled with physiologically based pharmacokinetic (PBPK) modeling approach. Environ Int 137:105581. [DOI] [PubMed] [Google Scholar]
- Cluett R, Seshasayee SM, Rokoff LB, Rifas-Shiman SL, Ye X, Calafat AM, Gold DR, Coull B, Gordon CM, Rosen CJ, Oken E, Sagiv SK, Fleisch AF. 2019. Per- and polyfluoroalkyl substance plasma concentrations and bone mineral density in midchildhood: A cross-sectional study (Project Viva, United States). Environ Health Perspect 127:87006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen DE, Fisher EA. 2013. Lipoprotein metabolism, dyslipidemia, and nonalcoholic fatty liver disease. Semin Liver Dis 33:380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley JM, Lambright CS, Evans N, Strynar MJ, McCord J, McIntyre BS, Travlos GS, Cardon MC, Medlock-Kakaley E, Hartig PC, Wilson VS, Gray LE Jr. 2019. Adverse maternal, fetal, and postnatal effects of hexafluoropropylene oxide dimer acid (GenX) from oral gestational exposure in Sprague-Dawley rats. Environ Health Perspect 127:37008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Convertino M, Church TR, Olsen GW, Liu Y, Doyle E, Elcombe CR, Barnett AL, Samuel LM, MacPherson IR, Evans TRJ. 2018. Stochastic pharmacokinetic-pharmacodynamic modeling for assessing the systemic health risk of perfluorooctanoate (PFOA). Toxicol Sci 163:293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coperchini F, Awwad O, Rotondi M, Santini F, Imbriani M, Chiovato L. 2017. Thyroid disruption by perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA). J Endocrinol Invest 40:105–121. [DOI] [PubMed] [Google Scholar]
- Corton JC, Peters JM, Klaunig JE. 2018. The PPARα-dependent rodent liver tumor response is not relevant to humans: Addressing misconceptions. Arch Toxicol 92:83–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Zhou QF, Liao CY, Fu JJ, Jiang GB. 2009. Studies on the toxicological effects of PFOA and PFOS on rats using histological observation and chemical analysis. Arch Environ Contam Toxicol 56:338–349. [DOI] [PubMed] [Google Scholar]
- Daly ER, Chan BP, Talbot EA, Nassif J, Bean C, Cavallo SJ, Metcalf E, Simone K, Woolf AD. 2018. Per- and polyfluoroalkyl substance (PFAS) exposure assessment in a community exposed to contaminated drinking water, New Hampshire, 2015. Int J Hyg Environ Health 221: 569–577. [DOI] [PubMed] [Google Scholar]
- Darrow LA, Groth AC, Winquist A, Shin HM, Bartell SM, Steenland K. 2016. Modeled perfluorooctanoic acid (PFOA) exposure and liver function in a mid-Ohio Valley community. Environ Health Perspect 124:1227–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow LA, Stein CR, Steenland K. 2013. Serum perfluorooctanoic acid and perfluorooctane sulfonate concentrations in relation to birth outcomes in the mid-Ohio Valley, 2005–2010. Environ Health Perspect 121:1207–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das KP, Grey BE, Rosen MB, Wood CR, Tatum-Gibbs KR, Zehr RD, Strynar MJ, Lindstrom AB, Lau C. 2015. Developmental toxicity of perfluorononanoic acid in mice. Reprod Toxicol 51:133–144. [DOI] [PubMed] [Google Scholar]
- Das KP, Wood CR, Lin MT, Starkov AA, Lau C, Wallace KB, Corton JC, Abbott BD. 2017. Perfluoroalkyl acids-induced liver steatosis: Effects on genes controlling lipid homeostasis. Toxicology 378:37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt JC, Blossom SJ, Schaider LA. 2019. Exposure to per-fluoroalkyl and polyfluoroalkyl substances leads to immunotoxicity: Epidemiological and toxicological evidence. J Expo Sci Environ Epidemiol 29:148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra R, Winquist A, Darrow LA, Klein M, Steenland K. 2017. A study of reverse causation: Examining the associations of perfluorooctanoic acid serum levels with two outcomes. Environ Health Perspect 125:416–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nisio A, De Rocco Ponce M, Giadone A, Rocca MS, Guidolin D, Foresta C. 2020a. Perfluoroalkyl substances and bone health in young men: A pilot study. Endocrine 67:678–684. [DOI] [PubMed] [Google Scholar]
- Di Nisio A, Rocca MS, Sabovic I, De Rocco Ponce M, Corsini C, Guidolin D, Zanon C, Acquasaliente L, Carosso AR, De Toni L, Foresta C. 2020b. Perfluorooctanoic acid alters progesterone activity in human endometrial cells and induces reproductive alterations in young women. Chemosphere 242:125208. [DOI] [PubMed] [Google Scholar]
- Division of Science and Research, New Jersey Department of Environmental Protection. 2019. Interim specific ground water criterion for perfluorooctanoic acid (PFOA, C8) (CAS #: 335-67-1; chemical structure: CF3(CF2)6COOH)*. Trenton, NJ, USA. [cited 2020 July 13]. Available from: https://www.nj.gov/dep/dsr/supportdocs/PFOA_TSD.pdf [Google Scholar]
- Dodes Traian MM, Cattoni DI, Levi V, Gonzalez Flecha FL. 2012. A two-stage model for lipid modulation of the activity of integral membrane proteins. PLoS One 7:e39255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donat-Vargas C, Bergdahl IA, Tornevi A, Wennberg M, Sommar J, Kiviranta H, Koponen J, Rolandsson O, Akesson A. 2019. Perfluoroalkyl substances and risk of type II diabetes: A prospective nested case-control study. Environ Int 123:390–398. [DOI] [PubMed] [Google Scholar]
- Dong Z, Wang H, Yu YY, Li YB, Naidu R, Liu Y. 2019. Using 2003–2014 U.S. NHANES data to determine the associations between per- and polyfluoroalkyl substances and cholesterol: Trend and implications. Ecotoxicol Environ Saf 173:461–468. [DOI] [PubMed] [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain. 2020. Public consultation on the draft scientific opinion on the risks to human health related to the presence of perfluoroalkyl substances in food. European Food Safety Authority, Parma, Italy. [cited 2020 September 15]. Available from: https://www.efsa.europa.eu/en/consultations/call/public-consultation-draft-scientific-opinion-risks-human-health [Google Scholar]
- Elcombe CR, Elcombe BM, Foster JR, Chang SC, Ehresman DJ, Butenhoff JL. 2012. Hepatocellular hypertrophy and cell proliferation in Sprague-Dawley rats from dietary exposure to potassium perfluorooctanesulfonate results from increased expression of xenosensor nuclear receptors PPARα and CAR/PXR. Toxicology 293:16–29. [DOI] [PubMed] [Google Scholar]
- Emmett EA, Shofer FS, Zhang H, Freeman D, Desai C, Shaw LM. 2006. Community exposure to perfluorooctanoate: Relationships between serum concentrations and exposure sources. J Occup Environ Med 48:759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen KT, Raaschou-Nielsen O, McLaughlin JK, Lipworth L, Tjonneland A, Overvad K, Sorensen M. 2013. Association between plasma PFOA and PFOS levels and total cholesterol in a middle-aged Danish population. PLoS One 8:e56969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eryasa B, Grandjean P, Nielsen F, Valvi D, Zmirou-Navier D, Sunderland E, Weihe P, Oulhote Y. 2019. Physico-chemical properties and gestational diabetes predict transplacental transfer and partitioning of perfluoroalkyl substances. Environ Int 130:104874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Environment Agency. 2019. Emerging chemical risks in Europe—“PFAS.” Copenhagen, Denmark. [cited 2020 July 13]. Available from: https://www.eea.europa.eu/themes/human/chemicals/emerging-chemical-risks-in-europe [Google Scholar]
- Fei C, McLaughlin JK, Lipworth L, Olsen J. 2009. Maternal levels of perfluorinated chemicals and subfecundity. Hum Reprod 24:1200–1205. [DOI] [PubMed] [Google Scholar]
- Fenton SE, Reiner JL, Nakayama SF, Delinsky AD, Stanko JP, Hines EP, White SS, Lindstrom AB, Strynar MJ, Petropoulou SE. 2009. Analysis of PFOA in dosed CD-1 mice. Part 2. Disposition of PFOA in tissues and fluids from pregnant and lactating mice and their pups. Reprod Toxicol 27:365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari F, Orlando A, Ricci Z, Ronco C. 2019. Persistent pollutants: Focus on perfluorinated compounds and kidney. Curr Opin Crit Care 25:539–549. [DOI] [PubMed] [Google Scholar]
- Filgo AJ, Quist EM, Hoenerhoff MJ, Brix AE, Kissling GE, Fenton SE. 2015. Perfluorooctanoic acid (PFOA)-induced liver lesions in two strains of mice following developmental exposures: PPARalpha is not required. Toxicol Pathol 43:558–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Arbuckle TE, Wade M, Haines DA. 2013. Do perfluoroalkyl substances affect metabolic function and plasma lipids? Analysis of the 2007–2009, Canadian Health Measures Survey (CHMS) cycle 1. Environ Res 121:95–103. [DOI] [PubMed] [Google Scholar]
- Fitz-Simon N, Fletcher T, Luster MI, Steenland K, Calafat AM, Kato K, Armstrong B. 2013. Reductions in serum lipids with a 4-year decline in serum perfluorooctanoic acid and perfluorooctanesulfonic acid. Epidemiology 24:569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher T, Galloway TS, Melzer D, Holcroft P, Cipelli R, Pilling LC, Mondal D, Luster M, Harries LW. 2013. Associations between PFOA, PFOS and changes in the expression of genes involved in cholesterol metabolism in humans. Environ Int 57–58:2–10. [DOI] [PubMed] [Google Scholar]
- Forhead AJ, Fowden AL. 2014. Thyroid hormones in fetal growth and prepartum maturation. J Endocrinol 221:R87–R103. [DOI] [PubMed] [Google Scholar]
- Frisbee SJ, Shankar A, Knox SS, Steenland K, Savitz DA, Fletcher T, Ducatman AM. 2010. Perfluorooctanoic acid, perfluorooctanesulfonate, and serum lipids in children and adolescents: Results from the C8 Health Project. Arch Pediatr Adolesc Med 164:860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche K 2006. Fatty acids as modulators of the immune response. Annu Rev Nutr 26:45–73. [DOI] [PubMed] [Google Scholar]
- Fromme H, Mosch C, Morovitz M, Alba-Alejandre I, Boehmer S, Kiranoglu M, Faber F, Hannibal I, Genzel-Boroviczeny O, Koletzko B, Volkel W. 2010. Pre- and postnatal exposure to perfluorinated compounds (PFCs). Environ Sci Technol 44:7123–7129. [DOI] [PubMed] [Google Scholar]
- Fu Y, Wang T, Fu Q, Wang P, Lu Y. 2014. Associations between serum concentrations of perfluoroalkyl acids and serum lipid levels in a Chinese population. Ecotoxicol Environ Saf 106:246–252. [DOI] [PubMed] [Google Scholar]
- Gaballah S, Swank A, Sobus JR, Howey XM, Schmid J, Catron T, McCord J, Hines E, Strynar M, Tal T. 2020. Evaluation of developmental toxicity, developmental neurotoxicity, and tissue dose in zebrafish exposed to GenX and other PFAS. Environ Health Perspect 128:47005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, Leonardi G, Genser B, Lopez-Espinosa MJ, Frisbee SJ, Karlsson L, Ducatman AM, Fletcher T. 2012. Serum perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS) concentrations and liver function biomarkers in a population with elevated PFOA exposure. Environ Health Perspect 120:655–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandaglia G, Ravi P, Abdollah F, Abd-El-Barr AE, Becker A, Popa I, Briganti A, Karakiewicz PI, Trinh QD, Jewett MA, Sun M. 2014. Contemporary incidence and mortality rates of kidney cancer in the United States. Can Urol Assoc J 8:247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger SD, Xiao J, Shankar A. 2013. Positive association between perfluoroalkyl chemicals and hyperuricemia in children. Am J Epidemiol 177:1255–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genser B, Teles CA, Barreto ML, Fischer JE. 2015. Within- and between-group regression for improving the robustness of causal claims in cross-sectional analysis. Environ Health 14:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardi P, Merler E. 2019. A mortality study on male subjects exposed to polyfluoroalkyl acids with high internal dose of perfluorooctanoic acid. Environ Res 179:108743. [DOI] [PubMed] [Google Scholar]
- Gleason JA, Post GB, Fagliano JA. 2015. Associations of perfluorinated chemical serum concentrations and biomarkers of liver function and uric acid in the US population (NHANES), 2007–2010. Environ Res 136:8–14. [DOI] [PubMed] [Google Scholar]
- Gogola J, Hoffmann M, Ptak A. 2019. Persistent endocrine-disrupting chemicals found in human follicular fluid stimulate the proliferation of granulosa tumor spheroids via GPR30 and IGF1R but not via the classic estrogen receptors. Chemosphere 217:100–110. [DOI] [PubMed] [Google Scholar]
- Goudarzi H, Miyashita C, Okada E, Kashino I, Chen CJ, Ito S, Araki A, Kobayashi S, Matsuura H, Kishi R. 2017. Prenatal exposure to perfluoroalkyl acids and prevalence of infectious diseases up to 4 years of age. Environ Int 104:132–138. [DOI] [PubMed] [Google Scholar]
- Goulding DR, White SS, McBride SJ, Fenton SE, Harry GJ. 2017. Gestational exposure to perfluorooctanoic acid (PFOA): Alterations in motor related behaviors. Neurotoxicology 58:110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber JM, Alexander C, Laumbach RJ, Black K, Strickland PO, Georgopoulos PG, Marshall EG, Shendell DG, Alderson D, Mi Z, Mascari M, Weisel CP. 2019. Per- and polyfluoroalkyl substances (PFAS) blood levels after contamination of a community water supply and comparison with 2013–2014 NHANES. J Expo Sci Environ Epidemiol 29:172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Andersen EW, Budtz-Jorgensen E, Nielsen F, Molbak K, Weihe P, Heilmann C. 2012. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA 307:391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Heilmann C, Weihe P, Nielsen F, Mogensen UB, Budtz-Jorgensen E. 2017. Serum vaccine antibody concentrations in adolescents exposed to perfluorinated compounds. Environ Health Perspect 125:077018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granum B, Haug LS, Namork E, Stolevik SB, Thomsen C, Aaberge IS, van Loveren H, Lovik M, Nygaard UC. 2013. Pre-natal exposure to perfluoroalkyl substances may be associated with altered vaccine antibody levels and immune-related health outcomes in early childhood. J Immunotoxicol 10:373–379. [DOI] [PubMed] [Google Scholar]
- Guillette TC, McCord J, Guillette M, Polera ME, Rachels KT, Morgeson C, Kotlarz N, Knappe DRU, Reading BJ, Strynar M, Belcher SM. 2020. Elevated levels of per- and polyfluoroalkyl substances in Cape Fear River striped bass (Morone saxatilis) are associated with biomarkers of altered immune and liver function. Environ Int 136:105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guruge KS, Yeung LW, Yamanaka N, Miyazaki S, Lam PK, Giesy JP, Jones PD, Yamashita N. 2006. Gene expression profiles in rat liver treated with perfluorooctanoic acid (PFOA). Toxicol Sci 89:93–107. [DOI] [PubMed] [Google Scholar]
- Gyllenhammar I, Benskin JP, Sandblom O, Berger U, Ahrens L, Lignell S, Wiberg K, Glynn A. 2018. Perfluoroalkyl acids (PFAAs) in serum from 2–4-month-old infants: Influence of maternal serum concentration, gestational age, breast-feeding, and contaminated drinking water. Environ Sci Technol 52:7101–7110. [DOI] [PubMed] [Google Scholar]
- Hall JM, Greco CW. 2019. Perturbation of nuclear hormone receptors by endocrine disrupting chemicals: Mechanisms and pathological consequences of exposure. Cells 9:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halldorsson TI, Rytter D, Haug LS, Bech BH, Danielsen I, Becher G, Henriksen TB, Olsen SF. 2012. Prenatal exposure to perfluorooctanoate and risk of overweight at 20 yr of age: A prospective cohort study. Environ Health Perspect 120:668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Nabb DL, Russell MH, Kennedy GL, Rickard RW. 2012. Renal elimination of perfluorocarboxylates (PFCAs). Chem Res Toxicol 25:35–46. [DOI] [PubMed] [Google Scholar]
- He X, Liu Y, Xu B, Gu L, Tang W. 2018. PFOA is associated with diabetes and metabolic alteration in US men: National Health and Nutrition Examination Survey 2003–2012. Sci Total Environ 625:566–574. [DOI] [PubMed] [Google Scholar]
- Hines EP, White SS, Stanko JP, Gibbs-Flournoy EA, Lau C, Fenton SE. 2009. Phenotypic dichotomy following developmental exposure to perfluorooctanoic acid (PFOA) in female CD-1 mice: Low doses induce elevated serum leptin and insulin, and overweight in mid-life. Mol Cell Endocrinol 304:97–105. [DOI] [PubMed] [Google Scholar]
- Hoover G, Kar S, Guffey S, Leszczynski J, Sepulveda MS. 2019. In vitro and in silico modeling of perfluoroalkyl substances mixture toxicity in an amphibian fibroblast cell line. Chemosphere 233:25–33. [DOI] [PubMed] [Google Scholar]
- Hu Y, Liu G, Rood J, Liang L, Bray GA, de Jonge L, Coull B, Furtado JD, Qi L, Grandjean P, Sun Q. 2019. Perfluoroalkyl substances and changes in bone mineral density: A prospective analysis in the POUNDS-LOST study. Environ Res 179:108775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Zhang J, Martin FL, Peng S, Tian M, Mu X, Shen H. 2013. Perfluorooctanoic acid induces apoptosis through the p53-dependent mitochondrial pathway in human hepatic cells: A proteomic study. Toxicol Lett 223:211–220. [DOI] [PubMed] [Google Scholar]
- Huang R, Chen Q, Zhang L, Luo K, Chen L, Zhao S, Feng L, Zhang J. 2019. Prenatal exposure to perfluoroalkyl and polyfluoroalkyl substances and the risk of hypertensive disorders of pregnancy. Environ Health 18:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui Z, Li R, Chen L. 2017. The impact of exposure to environmental contaminant on hepatocellular lipid metabolism. Gene 622:67–71. [DOI] [PubMed] [Google Scholar]
- Huo X, Huang R, Gan Y, Luo K, Aimuzi R, Nian M, Ao J, Feng L, Tian Y, Wang W, Ye W, Zhang J, Shanghai Birth Cohort. 2020. Perfluoroalkyl substances in early pregnancy and risk of hypertensive disorders of pregnancy: A prospective cohort study. Environ Int 138:105656. [DOI] [PubMed] [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 2017. Perfluorooctanoic acid. In Some Chemicals Used as Solvents and in Polymer Manufacture. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 110. International Agency for Research on Cancer, Lyon, France, pp 37–110. [PubMed] [Google Scholar]
- Impinen A, Longnecker MP, Nygaard UC, London SJ, Ferguson KK, Haug LS, Granum B. 2019. Maternal levels of perfluoroalkyl substances (PFASs) during pregnancy and childhood allergy and asthma related outcomes and infections in the Norwegian Mother and Child (MoBa) cohort. Environ Int 124:462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impinen A, Nygaard UC, Lodrup Carlsen KC, Mowinckel P, Carlsen KH, Haug LS, Granum B. 2018. Prenatal exposure to perfluoralkyl substances (PFASs) associated with respiratory tract infections but not allergy- and asthma-related health outcomes in childhood. Environ Res 160: 518–523. [DOI] [PubMed] [Google Scholar]
- Inoue K, Ritz B, Andersen SL, Ramlau-Hansen CH, Hoyer BB, Bech BH, Henriksen TB, Bonefeld-Jorgensen EC, Olsen J, Liew Z. 2019. Perfluoroalkyl substances and maternal thyroid hormones in early pregnancy; findings in the Danish National Birth Cohort. Environ Health Perspect 127:117002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Interstate Technology and Regulatory Council. 2020. Chemistry, terminology and acronyms. [cited 2020 May 19]. Available from: https://pfas-1.itrcweb.org/2-2-chemistry-terminology-and-acronyms/
- Jain RB. 2020. Variabilities in concentrations of selected perfluoroalkyl acids among normotensives and hypertensives across various stages of glomerular function. Arch Environ Occup Health 2020:1–11. [DOI] [PubMed] [Google Scholar]
- Jain RB, Ducatman A. 2019a. Dynamics of associations between perfluoroalkyl substances and uric acid across the various stages of glomerular function. Environ Sci Pollut Res Int 26:12425–12434. [DOI] [PubMed] [Google Scholar]
- Jain RB, Ducatman A. 2019b. Perfluoroalkyl acids serum concentrations and their relationship to biomarkers of renal failure: Serum and urine albumin, creatinine, and albumin creatinine ratios across the spectrum of glomerular function among US adults. Environ Res 174:143–151. [DOI] [PubMed] [Google Scholar]
- Jain RB, Ducatman A. 2019c. Perfluoroalkyl substances follow inverted U-shaped distributions across various stages of glomerular function: Implications for future research. Environ Res 169:476–482. [DOI] [PubMed] [Google Scholar]
- Jain RB, Ducatman A. 2019d. Roles of gender and obesity in defining correlations between perfluoroalkyl substances and lipid/lipoproteins. Sci Total Environ 653:74–81. [DOI] [PubMed] [Google Scholar]
- Jain RB, Ducatman A. 2019e. Selective associations of recent low concentrations of perfluoroalkyl substances with liver function biomarkers: NHANES 2011 to 2014 data on US adults aged ≥20 years. J Occup Environ Med 61:293–302. [DOI] [PubMed] [Google Scholar]
- Jensen T, Niwa K, Hisatome I, Kanbay M, Andres-Hernando A, Roncal-Jimenez CA, Sato Y, Garcia G, Ohno M, Lanaspa MA, Johnson RJ, Kuwabara M. 2018. Increased serum uric acid over five years is a risk factor for developing fatty liver. Sci Rep 8:11735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian JM, Chen D, Han FJ, Guo Y, Zeng L, Lu X, Wang F. 2018. A short review on human exposure to and tissue distribution of per- and polyfluoroalkyl substances (PFASs). Sci Total Environ 636:1058–1069. [DOI] [PubMed] [Google Scholar]
- Jin R, McConnell R, Catherine C, Xu S, Walker DI, Stratakis N, Jones DP, Miller GW, Peng C, Conti DV, Vos MB, Chatzi L. 2020. Perfluoroalkyl substances and severity of nonalcoholic fatty liver in children: An untargeted metabolomics approach. Environ Int 134:105220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joensen UN, Bossi R, Leffers H, Jensen AA, Skakkebaek NE, Jorgensen N. 2009. Do perfluoroalkyl compounds impair human semen quality? Environ Health Perspect 117:923–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MS, Buck RC, Cousins IT, Weis CP, Fenton SE. 2020. Estimating environmental hazard and risks from exposure to per- and polyfluoroalkyl substances (PFAS): Outcome of a SETAC focused topic meeting. Environ Toxicol Chem, in press. 10.1002/etc.4784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PI, Sutton P, Atchley DS, Koustas E, Lam J, Sen S, Robinson KA, Axelrad DA, Woodruff TJ. 2014. The Navigation Guide—Evidence-based medicine meets environmental health: Systematic review of human evidence for PFOA effects on fetal growth. Environ Health Perspect 122:1028–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PD, Hu W, De Coen W, Newsted JL, Giesy JP. 2003. Binding of perfluorinated fatty acids to serum proteins. Environ Toxicol Chem 22:2639–2649. [DOI] [PubMed] [Google Scholar]
- Kang Q, Gao F, Zhang X, Wang L, Liu J, Fu M, Zhang S, Wan Y, Shen H, Hu J. 2020. Nontargeted identification of per- and polyfluoroalkyl substances in human follicular fluid and their blood-follicle transfer. Environ Int 139:105686. [DOI] [PubMed] [Google Scholar]
- Karnes C, Winquist A, Steenland K. 2014. Incidence of type II diabetes in a cohort with substantial exposure to perfluorooctanoic acid. Environ Res 128:78–83. [DOI] [PubMed] [Google Scholar]
- Katakura M, Kudo N, Tsuda T, Hibino Y, Mitsumoto A, Kawashima Y. 2007. Rat organic anion transporter 3 and organic anion transporting polypeptide 1 mediate perfluorooctanoic acid transport. J Health Sci 53:77–83. [Google Scholar]
- Kataria A, Trachtman H, Malaga-Dieguez L, Trasande L. 2015. Association between perfluoroalkyl acids and kidney function in a cross-sectional study of adolescents. Environ Health 14:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM. 2011. Trends in exposure to polyfluoroalkyl chemicals in the U.S. population: 1999–2008. Environ Sci Technol 45:8037–8045. [DOI] [PubMed] [Google Scholar]
- Khalil N, Chen A, Lee M, Czerwinski SA, Ebert JR, DeWitt JC, Kannan K. 2016. Association of perfluoroalkyl substances, bone mineral density, and osteoporosis in the U.S. population in NHANES 2009–2010. Environ Health Perspect 124:81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. 2010. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol 8:e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Kim UJ, Kim HY, Choi SD, Oh JE. 2016. Perfluoroalkyl substances in serum from South Korean infants with congenital hypothyroidism and healthy infants—Its relationship with thyroid hormones. Environ Res 147:399–404. [DOI] [PubMed] [Google Scholar]
- Kingsley SL, Walker DI, Calafat AM, Chen A, Papandonatos GD, Xu Y, Jones DP, Lanphear BP, Pennell KD, Braun JM. 2019. Metabolomics of childhood exposure to perfluoroalkyl substances: A cross-sectional study. Metabolomics 15:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjeldsen LS, Bonefeld-Jørgensen EC. 2013. Perfluorinated compounds affect the function of sex hormone receptors. Environ Sci Pollut Res 20:8031–8044. [DOI] [PubMed] [Google Scholar]
- Klaunig JE, Hocevar BA, Kamendulis LM. 2012. Mode of action analysis of perfluorooctanoic acid (PFOA) tumorigenicity and human relevance. Reprod Toxicol 33:410–418. [DOI] [PubMed] [Google Scholar]
- Koponen J, Winkens K, Airaksinen R, Berger U, Vestergren R, Cousins IT, Karvonen AM, Pekkanen J, Kiviranta H. 2018. Longitudinal trends of per- and polyfluoroalkyl substances in children’s serum. Environ Int 121:591–599. [DOI] [PubMed] [Google Scholar]
- Koshy TT, Attina TM, Ghassabian A, Gilbert J, Burdine LK, Marmor M, Honda M, Chu DB, Han X, Shao Y, Kannan K, Urbina EM, Trasande L. 2017. Serum perfluoroalkyl substances and cardiometabolic consequences in adolescents exposed to the World Trade Center disaster and a matched comparison group. Environ Int 109:128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koustas E, Lam J, Sutton P, Johnson PI, Atchley DS, Sen S, Robinson KA, Axelrad DA, Woodruff TJ. 2014. The Navigation Guide—Evidence-based medicine meets environmental health: Systematic review of nonhuman evidence for PFOA effects on fetal growth. Environ Health Perspect 122:1015–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvalem HE, Nygaard UC, Lodrup Carlsen KC, Carlsen KH, Haug LS, Granum B. 2020. Perfluoroalkyl substances, airways infections, allergy and asthma related health outcomes—Implications of gender, exposure period and study design. Environ Int 134:105259. [DOI] [PubMed] [Google Scholar]
- LaLone CA, Ankley GT, Belanger SE, Embry MR, Hodges G, Knapen D, Munn S, Perkins EJ, Rudd MA, Villeneuve DL, Whelan M, Willett C, Zhang X, Hecker M. 2017. Advancing the adverse outcome pathway framework—An international horizon scanning approach. Environ Toxicol Chem 36:1411–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J, Koustas E, Sutton P, Johnson PI, Atchley DS, Sen S, Robinson KA, Axelrad DA, Woodruff TJ. 2014. The Navigation Guide—Evidence-based medicine meets environmental health: Integration of animal and human evidence for PFOA effects on fetal growth. Environ Health Perspect 122:1040–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. 2007. Perfluoroalkyl acids: A review of monitoring and toxicological findings. Toxicol Sci 99:366–394. [DOI] [PubMed] [Google Scholar]
- Lau C, Thibodeaux JR, Hanson RG, Narotsky MG, Rogers JM, Lindstrom AB, Strynar MJ. 2006. Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol Sci 90:510–518. [DOI] [PubMed] [Google Scholar]
- Lee JE, Choi K. 2017. Perfluoroalkyl substances exposure and thyroid hormones in humans: Epidemiological observations and implications. Ann Pediatr Endocrinol Metab 22:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-H, Ren X-M, Cao L-Y, Qin W-P, Guo L-H. 2019. Investigation of binding and activity of perfluoroalkyl substances to the human peroxisome proliferator-activated receptor β/δ. Environ Sci Process Impacts 21: 1908–1914. [DOI] [PubMed] [Google Scholar]
- Li K, Sun J, Yang J, Roberts SM, Zhang X, Cui X, Wei S, Ma LQ. 2017a. Molecular mechanisms of perfluorooctanoate-induced hepatocyte apoptosis in mice using proteomic techniques. Environ Sci Technol 51:11380–11389. [DOI] [PubMed] [Google Scholar]
- Li Y, Barregard L, Xu Y, Scott K, Pineda D, Lindh CH, Jakobsson K, Fletcher T. 2020. Associations between perfluoroalkyl substances and serum lipids in a Swedish adult population with contaminated drinking water. Environ Health 19:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Cheng Y, Xie Z, Zeng F. 2017b. Perfluorinated alkyl substances in serum of the southern Chinese general population and potential impact on thyroid hormones. Sci Rep 7:43380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Fletcher T, Mucs D, Scott K, Lindh CH, Tallving P, Jakobsson K. 2018. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup Environ Med 75:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberatore HK, Jackson SR, Strynar MJ, McCord JP. 2020. Solvent suitability for HFPO-DA (“GenX” parent acid) in toxicological studies. Environ Sci Technol Lett 7:477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew Z, Goudarzi H, Oulhote Y. 2018. Developmental exposures to perfluoroalkyl substances (PFASs): An update of associated health outcomes. Curr Environ Health Rep 5:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Lin LY, Chiang CK, Wang WJ, Su YN, Hung KY, Chen PC. 2010. Investigation of the associations between low-dose serum perfluorinated chemicals and liver enzymes in US adults. Am J Gastroenterol 105: 1354–1363. [DOI] [PubMed] [Google Scholar]
- Lin LY, Wen LL, Su TC, Chen PC, Lin CY. 2014. Negative association between serum perfluorooctane sulfate concentration and bone mineral density in US premenopausal women: NHANES, 2005–2008. J Clin Endocrinol Metab 99:2173–2180. [DOI] [PubMed] [Google Scholar]
- Lin PD, Cardenas A, Hauser R, Gold DR, Kleinman KP, Hivert MF, Fleisch AF, Calafat AM, Webster TF, Horton ES, Oken E. 2019. Per- and polyfluoroalkyl substances and blood lipid levels in pre-diabetic adults-longitudinal analysis of the diabetes prevention program outcomes study. Environ Int 129:343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Zhang B, Hu Y, Rood J, Liang L, Qi L, Bray GA, DeJonge L, Coull B, Grandjean P, Furtado JD, Sun Q. 2020. Associations of perfluoroalkyl substances with blood lipids and apolipoproteins in lipoprotein sub-species: The POUNDS-lost study. Environ Health 19:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Yang R, Yin N, Faiola F. 2020. The short-chain perfluorinated compounds PFBS, PFHxS, PFBA and PFHxA, disrupt human mesenchymal stem cell self-renewal and adipogenic differentiation. J Environ Sci 88:187–199. [DOI] [PubMed] [Google Scholar]
- Looker C, Luster MI, Calafat AM, Johnson VJ, Burleson GR, Burleson FG, Fletcher T. 2014. Influenza vaccine response in adults exposed to perfluorooctanoate and perfluorooctanesulfonate. Toxicol Sci 138:76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Espinosa MJ, Mondal D, Armstrong B, Bloom MS, Fletcher T. 2012. Thyroid function and perfluoroalkyl acids in children living near a chemical plant. Environ Health Perspect 120:1036–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis GM, Chen Z, Schisterman EF, Kim S, Sweeney AM, Sundaram R, Lynch CD, Gore-Langton RE, Barr DB. 2015. Perfluorochemicals and human semen quality: The LIFE study. Environ Health Perspect 123:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis GM, Peterson CM, Chen Z, Hediger ML, Croughan MS, Sundaram R, Stanford JB, Fujimoto VY, Varner MW, Giudice LC, Kennedy A, Sun L, Wu Q, Kannan K. 2012. Perfluorochemicals and endometriosis: The ENDO study. Epidemiology 23:799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebker DJ, Hansen KJ, Bass NM, Butenhoff JL, Seacat AM. 2002. Interactions of fluorochemicals with rat liver fatty acid-binding protein. Toxicology 176:175–185. [DOI] [PubMed] [Google Scholar]
- Lum KJ, Sundaram R, Barr DB, Louis TA, Buck, Louis GM. 2017. Perfluoroalkyl chemicals, menstrual cycle length, and fecundity: Findings from a prospective pregnancy study. Epidemiology 28:90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin JI, Alexander BH, Olsen GW, Church TR. 2009. Ammonium perfluorooctanoate production and occupational mortality. Epidemiology 20:921–928. [DOI] [PubMed] [Google Scholar]
- Macon MB, Villanueva LR, Tatum-Gibbs K, Zehr RD, Strynar MJ, Stanko JP, White SS, Helfant L, Fenton SE. 2011. Prenatal perfluorooctanoic acid exposure in CD-1 mice: Low-dose developmental effects and internal dosimetry. Toxicol Sci 122:134–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestri L, Negri S, Ferrari M, Ghittori S, Fabris F, Danesino P, Imbriani M. 2006. Determination of perfluorooctanoic acid and perfluorooctanesulfonate in human tissues by liquid chromatography/single quadrupole mass spectrometry. Rapid Commun Mass Spectrom 20:2728–2734. [DOI] [PubMed] [Google Scholar]
- Martin MT, Brennan RJ, Hu W, Ayanoglu E, Lau C, Ren H, Wood CR, Corton JC, Kavlock RJ, Dix DJ. 2007. Toxicogenomic study of triazole fungicides and perfluoroalkyl acids in rat livers predicts toxicity and categorizes chemicals based on mechanisms of toxicity. Toxicol Sci 97: 595–613. [DOI] [PubMed] [Google Scholar]
- Martinsson M, Nielsen C, Bjork J, Rylander L, Malmqvist E, Lindh C, Rignell-Hydbom A. 2020. Intrauterine exposure to perfluorinated compounds and overweight at age 4: A case-control study. PLoS One 15:e0230137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massoud O, Charlton M. 2018. Nonalcoholic fatty liver disease/nonalcoholic steatohepatitis and hepatocellular carcinoma. Clin Liver Dis 22:201–211. [DOI] [PubMed] [Google Scholar]
- Mastrantonio M, Bai E, Uccelli R, Cordiano V, Screpanti A, Crosignani P. 2018. Drinking water contamination from perfluoroalkyl substances (PFAS): An ecological mortality study in the Veneto region, Italy. Eur J Public Health 28:180–185. [DOI] [PubMed] [Google Scholar]
- McCord J, Strynar M. 2019. Identification of per- and polyfluoroalkyl substances in the Cape Fear River by high resolution mass spectrometry and nontargeted screening. Environ Sci Technol 53:4717–4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek ME, Boobis A, Cote I, Dellarco V, Fotakis G, Munn S, Seed J, Vickers C. 2014. New developments in the evolution and application of the WHO/IPCS framework on mode of action/species concordance analysis. J Appl Toxicol 34:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer D, Rice N, Depledge MH, Henley WE, Galloway TS. 2010. Association between serum perfluorooctanoic acid (PFOA) and thyroid disease in the U.S. National Health and Nutrition Examination Survey. Environ Health Perspect 118:686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michigan PFAS Science Advisory Panel. 2018. Scientific evidence and recommendations for managing PFAS contamination in Michigan. Lansing, MI, USA. [cited 2020 July 13]. Available from: https://www.michigan.gov/documents/pfasresponse/Science_Advisory_Board_Report_641294_7.pdf [Google Scholar]
- Nakagawa H, Hirata T, Terada T, Jutabha P, Miura D, Harada KH, Inoue K, Anzai N, Endou H, Inui K, Kanai Y, Koizumi A. 2008. Roles of organic anion transporters in the renal excretion of perfluorooctanoic acid. Basic Clin Pharmacol Toxicol 103:1–8. [DOI] [PubMed] [Google Scholar]
- National Toxicology Program. 2016. Immunotoxicity associated with exposure to perfluorooctanoic acid (PFOA) or perfluorooctane sulfonate (PFOS). US Department of Health and Human Services, Research Triangle Park, NC. [cited 2020 July 13]. Available from: https://ntp.niehs.nih.gov/ntp/ohat/pfoa_pfos/pfoa_pfosmonograph_508.pdf [Google Scholar]
- National Toxicology Program. 2020a. NTP technical report on the toxicology and carcinogenesis studies of perfluorooctanoic acid (CAS no. 335-67-1) administered in feed to Sprague Dawley (Hsd:Sprague Dawley® SD®) rats. Technical Report 598. US Department of Health and Human Services, Research Triangle Park, NC. [cited 2020 September 15]. Available from: https://ntp.niehs.nih.gov/ntp/about_ntp/trpanel/2019/december/tr598draft.pdf [Google Scholar]
- National Toxicology Program. 2020b. P08: Statistical analysis of primary tumors—Perfluorooctanoic acid. US Department of Health and Human Services, Research Triangle Park, NC. [cited 2020 September 15]. Available from: https://www.documentcloud.org/documents/6155302-Statistical-Analysis-Tumors.html [Google Scholar]
- National Toxicology Program. 2020c. Testing status of perfluorooctanoic acid (PFOA) M910070. US Department of Health and Human Services, Research Triangle Park, NC. [cited 2020 May 19]. Available from: https://ntp.niehs.nih.gov/go/ts-m910070 [Google Scholar]
- Nelson JW, Hatch EE, Webster TF. 2010. Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general U.S. population. Environ Health Perspect 118:197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New Jersey Drinking Water Quality Institute Health Effects Subcommittee. 2017. Health-based maximum contaminant level support document: Perfluorooctanoic acid (PFOA). Trenton, NJ, USA. [cited 2020 July 13]. Available from: https://www.state.nj.us/dep/watersupply/pdf/pfoa-appendixa.pdf [Google Scholar]
- Ng CA, Hungerbühler K. 2014. Bioaccumulation of perfluorinated alkyl acids: Observations and models. Environ Sci Technol 48:4637–4648. [DOI] [PubMed] [Google Scholar]
- Ng CA, Hungerbuehler K. 2015. Exploring the use of molecular docking to identify bioaccumulative perfluorinated alkyl acids (PFAAs). Environ Sci Technol 49:12306–12314. [DOI] [PubMed] [Google Scholar]
- Ngo HT, Hetland RB, Sabaredzovic A, Haug LS, Steffensen IL. 2014. In utero exposure to perfluorooctanoate (PFOA) or perfluorooctane sulfonate (PFOS) did not increase body weight or intestinal tumorigenesis in multiple intestinal neoplasia (Min/+) mice. Environ Res 132:251–263. [DOI] [PubMed] [Google Scholar]
- Nian M, Li QQ, Bloom M, Qian ZM, Syberg KM, Vaughn MG, Wang SQ, Wei Q, Zeeshan M, Gurram N, Chu C, Wang J, Tian Y-P, Hu L-W, Liu K-K, Yang B-Y, Liu R-Q, Feng D, Zeng X-W, Dong G-H. 2019. Liver function biomarkers disorder is associated with exposure to perfluoroalkyl acids in adults: Isomers of C8 Health Project in China. Environ Res 172:81–88. [DOI] [PubMed] [Google Scholar]
- Obermayr RP, Temml C, Gutjahr G, Knechtelsdorfer M, Oberbauer R, Klauser-Braun R. 2008. Elevated uric acid increases the risk for kidney disease. J Am Soc Nephrol 19:2407–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, Zobel LR. 2007. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 115:1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Chang SC, Noker PE, Gorman GS, Ehresman DJ, Lieder PH, Butenhoff JL. 2009. A comparison of the pharmacokinetics of perfluorobutanesulfonate (PFBS) in rats, monkeys, and humans. Toxicology 256:65–74. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Mair DC, Lange CC, Harrington LM, Church TR, Goldberg CL, Herron RM, Hanna H, Nobiletti JB, Rios JA, Reagan WK, Ley CA. 2017. Per- and polyfluoroalkyl substances (PFAS) in American Red Cross adult blood donors, 2000–2015. Environ Res 157:87–95. [DOI] [PubMed] [Google Scholar]
- Organisation for Economic Co-operation Development. 2017. Revised guidance document on developing and assessing adverse outcome pathways. Series on Testing and Assessment, No. 184. ENV/JM/MONO (2013)6 Paris, France. [cited 2020 July 13]. Available from: http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2013)6&doclanguage=en [Google Scholar]
- Organisation for Economic Co-operation Development. 2018. Toward a new comprehensive global database of per-and polyfluoroalkyl substances (PFASs): Summary report on updating the OECD 2007 list of per-and polyfluoroalkyl substances (PFASs). ENV/JM/MONO(2018)7. Series on Risk Management, No. 39 Paris, France. [cited 2020 July 13]. Available from: https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV-JM-MONO(2018)7&doclanguage=en [Google Scholar]
- Organisation for Economic Co-operation Development. 2020. AOPs. [cited 2020 May 19]. Available from: https://aopwiki.org/aops
- Pachkowski B, Post GB, Stern AH. 2019. The derivation of a reference dose (RfD) for perfluorooctane sulfonate (PFOS) based on immune suppression. Environ Res 171:452–469. [DOI] [PubMed] [Google Scholar]
- Pan Y, Cui Q, Wang J, Sheng N, Jing J, Yao B, Dai J. 2019. Profiles of emerging and legacy per-/polyfluoroalkyl substances in matched serum and semen samples: New implications for human semen quality. Environ Health Perspect 127:127005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulou E, Sabaredzovic A, Namork E, Nygaard UC, Granum B, Haug LS. 2016. Exposure of Norwegian toddlers to perfluoroalkyl substances (PFAS): The association with breastfeeding and maternal PFAS concentrations. Environ Int 94:687–694. [DOI] [PubMed] [Google Scholar]
- Park JS, Kim J, Elghiaty A, Ham WS. 2018. Recent global trends in testicular cancer incidence and mortality. Medicine 97:e12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlewicz G, Richard AM, Williams AJ, Grulke CM, Sams R, Lambert J, Noyes PD, DeVito MJ, Hines RN, Strynar M, Guiseppe-Elie A, Thomas RS. 2019. A chemical category-based prioritization approach for selecting 75 per- and polyfluoroalkyl substances (PFAS) for tiered toxicity and toxicokinetic testing. Environ Health Perspect 127: 014501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez F, Nadal M, Navarro-Ortega A, Fabrega F, Domingo JL, Barcelo D, Farre M. 2013. Accumulation of perfluoroalkyl substances in human tissues. Environ Int 59:354–362. [DOI] [PubMed] [Google Scholar]
- Perla FM, Prelati M, Lavorato M, Visicchio D, Anania C. 2017. The role of lipid and lipoprotein metabolism in non-alcoholic fatty liver disease. Children (Basel) 4:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post GB. 2020. Recent US state and federal drinking water guidelines for per- and polyfluoroalkyl substances (PFAS). Environ Toxicol Chem, in press. 10.1002/etc.4863 [DOI] [PubMed] [Google Scholar]
- Post GB, Gleason JA, Cooper KR. 2017. Key scientific issues in developing drinking water guidelines for perfluoroalkyl acids: Contaminants of emerging concern. PLoS Biol 15:e2002855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouwer MG, Pieterman EJ, Chang SC, Olsen GW, Caspers MPM, Verschuren L, Jukema JW, Princen HMG. 2019. Dose effects of ammonium perfluorooctanoate on lipoprotein metabolism in APOE*3-Leiden.CETP mice. Toxicol Sci 168:519–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin WP, Cao LY, Li CH, Guo LH, Colbourne J, Ren XM. 2020. Perfluoroalkyl substances stimulate insulin secretion by islet beta cells via G protein-coupled receptor 40. Environ Sci Technol 54:3428–3436. [DOI] [PubMed] [Google Scholar]
- Qin XD, Qian Z, Vaughn MG, Huang J, Ward P, Zeng XW, Zhou Y, Zhu Y, Yuan P, Li M, Bai Z, Paul G, Hao Y-T, Chen W, Chen P-C, Dong G-H, Lee YL. 2016. Positive associations of serum perfluoroalkyl substances with uric acid and hyperuricemia in children from Taiwan. Environ Pollut 212:519–524. [DOI] [PubMed] [Google Scholar]
- Qin XD, Qian ZM, Dharmage SC, Perret J, Geiger SD, Rigdon SE, Howard S, Zeng XW, Hu LW, Yang BY, Zhou Y, Li M, Xu S-L, Bao W-W, Zhang Y-Z, Yuan P, Wang J, Zhang C, Tian Y-P, Nian M, Xiao X, Chen W, Lee YL, Dong G-H. 2017. Association of perfluoroalkyl substances exposure with impaired lung function in children. Environ Res 155:15–21. [DOI] [PubMed] [Google Scholar]
- Qiu Z, Qu K, Luan F, Liu Y, Zhu Y, Yuan Y, Li H, Zhang H, Hai Y, Zhao C. 2020. Binding specificities of estrogen receptor with perfluorinated compounds: A cross species comparison. Environ Int 134:105284. [DOI] [PubMed] [Google Scholar]
- Quist EM, Filgo AJ, Cummings CA, Kissling GE, Hoenerhoff MJ, Fenton SE. 2015. Hepatic mitochondrial alteration in CD-1 mice associated with prenatal exposures to low doses of perfluorooctanoic acid (PFOA). Toxicol Pathol 43:546–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz JR, Goldsmith M-R, Little SB, Pasquinelli MA. 2008. Computational molecular modeling for evaluating the toxicity of environmental chemicals: Prioritizing bioassay requirements. Environ Health Perspect 116:573–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantakokko P, Mannisto V, Airaksinen R, Koponen J, Viluksela M, Kiviranta H, Pihlajamaki J. 2015. Persistent organic pollutants and non-alcoholic fatty liver disease in morbidly obese patients: A cohort study. Environ Health 14:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappazzo KM, Coffman E, Hines EP. 2017. Exposure to perfluorinated alkyl substances and health outcomes in children: A systematic review of the epidemiologic literature. Int J Environ Res Public Health 14:691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid F, Ramakrishnan A, Fields C, Irudayaraj J. 2020. Acute PFOA exposure promotes epigenomic alterations in mouse kidney tissues. Toxicol Rep 7:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebholz SL, Jones T, Herrick RL, Xie C, Calafat AM, Pinney SM, Woollett LA. 2016. Hypercholesterolemia with consumption of PFOA-laced Western diets is dependent on strain and sex of mice. Toxicol Rep 3:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren XM, Qin WP, Cao LY, Zhang J, Yang Y, Wan B, Guo LH. 2016. Binding interactions of perfluoroalkyl substances with thyroid hormone transport proteins and potential toxicological implications. Toxicology 366–367: 32–42. [DOI] [PubMed] [Google Scholar]
- Romano ME, Xu Y, Calafat AM, Yolton K, Chen A, Webster GM, Eliot MN, Howard CR, Lanphear BP, Braun JM. 2016. Maternal serum perfluoroalkyl substances during pregnancy and duration of breastfeeding. Environ Res 149:239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen EM, Brantsaeter AL, Carroll R, Haug L, Singer AB, Zhao S, Ferguson KK. 2018. Maternal plasma concentrations of per- and polyfluoroalkyl substances and breastfeeding duration in the Norwegian Mother and Child Cohort. Environ Epidemiol 2:e027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen MB, Das KP, Rooney J, Abbott B, Lau C, Corton JC. 2017. PPARalpha-independent transcriptional targets of perfluoroalkyl acids revealed by transcript profiling. Toxicology 387:95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell MH, Nilsson H, Buck RC. 2013. Elimination kinetics of perfluorohexanoic acid in humans and comparison with mouse, rat and monkey. Chemosphere 93:2419–2425. [DOI] [PubMed] [Google Scholar]
- Sabovic I, Cosci I, De Toni L, Ferramosca A, Stornaiuolo M, Di Nisio A, Dall’Acqua S, Garolla A, Foresta C. 2020. Perfluoro-octanoic acid impairs sperm motility through the alteration of plasma membrane. J Endocrinol Invest 43:641–652. [DOI] [PubMed] [Google Scholar]
- Sakr CJ, Kreckmann KH, Green JW, Gillies PJ, Reynolds JL, Leonard RC. 2007a. Cross-sectional study of lipids and liver enzymes related to a serum biomarker of exposure (ammonium perfluorooctanoate or APFO) as part of a general health survey in a cohort of occupationally exposed workers. J Occup Environ Med 49:1086–1096. [DOI] [PubMed] [Google Scholar]
- Sakr CJ, Leonard RC, Kreckmann KH, Slade MD, Cullen MR. 2007b. Longitudinal study of serum lipids and liver enzymes in workers with occupational exposure to ammonium perfluorooctanoate. J Occup Environ Med 49:872–879. [DOI] [PubMed] [Google Scholar]
- Sakuma A, Wasada Ochi H, Yoshioka M, Yamanaka N, Ikezawa M, Guruge KS. 2019. Changes in hepato-renal gene expression in microminipigs following a single exposure to a mixture of perfluoroalkyl acids. PLoS One 14:e0210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salihovic S, Dickens AM, Schoultz I, Fart F, Sinisalu L, Lindeman T, Halfvarson J, Oresic M, Hyotylainen T. 2020. Simultaneous determination of perfluoroalkyl substances and bile acids in human serum using ultra-high-performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 412:2251–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salihovic S, Fall T, Ganna A, Broeckling CD, Prenni JE, Hyotylainen T, Karrman A, Lind PM, Ingelsson E, Lind L. 2019. Identification of metabolic profiles associated with human exposure to perfluoroalkyl substances. J Expo Sci Environ Epidemiol 29:196–205. [DOI] [PubMed] [Google Scholar]
- Salvalaglio M, Muscionico I, Cavallotti C. 2010. Determination of energies and sites of binding of PFOA and PFOS to human serum albumin. J Phys Chem B 114:14860–14874. [DOI] [PubMed] [Google Scholar]
- Sattar N, Forrest E, Preiss D. 2014. Non-alcoholic fatty liver disease. BMJ 349:g4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz DA, Stein CR, Bartell SM, Elston B, Gong J, Shin HM, Wellenius GA. 2012. Perfluorooctanoic acid exposure and pregnancy outcome in a highly exposed community. Epidemiology 23:386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlezinger J, Puckett H, Oliver J, Nielsen G, Heiger-Bernays W, Webster T. 2020. Perfluorooctanoic acid activates multiple nuclear receptor pathways and skews expression of genes regulating cholesterol homeostasis in liver of humanized PPARα mice fed an American diet. Toxicol Appl Pharmacol 405:115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo SH, Son MH, Choi SD, Lee DH, Chang YS. 2018. Influence of exposure to perfluoroalkyl substances (PFASs) on the Korean general population: 10-year trend and health effects. Environ Int 113:149–161. [DOI] [PubMed] [Google Scholar]
- Sha B, Schymanski EL, Ruttkies C, Cousins IT, Wang Z. 2019. Exploring open cheminformatics approaches for categorizing per- and polyfluoroalkyl substances (PFASs). Environ Sci Process Impacts 21:1835–1851. [DOI] [PubMed] [Google Scholar]
- Shabalina IG, Kalinovich AV, Cannon B, Nedergaard J. 2016. Metabolically inert perfluorinated fatty acids directly activate uncoupling protein 1 in brown-fat mitochondria. Arch Toxicol 90:1117–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar A, Xiao J, Ducatman A. 2011. Perfluoroalkyl chemicals and chronic kidney disease in US adults. Am J Epidemiol 174:893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng N, Cui R, Wang J, Guo Y, Wang J, Dai J. 2018. Cytotoxicity of novel fluorinated alternatives to long-chain perfluoroalkyl substances to human liver cell line and their binding capacity to human liver fatty acid binding protein. Arch Toxicol 92:359–369. [DOI] [PubMed] [Google Scholar]
- Skuladottir M, Ramel A, Rytter D, Haug LS, Sabaredzovic A, Bech BH, Henriksen TB, Olsen SF, Halldorsson TI. 2015. Examining confounding by diet in the association between perfluoroalkyl acids and serum cholesterol in pregnancy. Environ Res 143:33–38. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, MacKillop EA, Melnick RL, Thayer KA, Seidler FJ. 2008. Developmental neurotoxicity of perfluorinated chemicals modeled in vitro. Environ Health Perspect 116:716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solo-Gabriele HM, Jones AS, Lindstrom AB, Lang JR. 2020. Waste type, incineration, and aeration are associated with per- and polyfluoroalkyl levels in landfill leachates. Waste Manag 107:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Kim YJ, Park YK, Ryu JC. 2012. Changes in thyroid peroxidase activity in response to various chemicals. J Environ Monit 14:2121–2126. [DOI] [PubMed] [Google Scholar]
- Song X, Tang S, Zhu H, Chen Z, Zang Z, Zhang Y, Niu X, Wang X, Yin H, Zeng F, He C. 2018. Biomonitoring PFAAs in blood and semen samples: Investigation of a potential link between PFAAs exposure and semen mobility in China. Environ Int 113:50–54. [DOI] [PubMed] [Google Scholar]
- Spector AA, Yorek MA. 1985. Membrane lipid composition and cellular function. J Lipid Res 26:1015–1035. [PubMed] [Google Scholar]
- Stanifer JW, Stapleton HM, Souma T, Wittmer A, Zhao X, Boulware LE. 2018. Perfluorinated chemicals as emerging environmental threats to kidney health: A scoping review. Clin J Am Soc Nephrol 13:1479–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starling AP, Engel SM, Whitworth KW, Richardson DB, Stuebe AM, Daniels JL, Haug LS, Eggesbo M, Becher G, Sabaredzovic A, Eggesbo M, Hoppin JA, Travlos GS, Wilson RE, Trogstad LI, Magnus P, Longnecker MP. 2014. Perfluoroalkyl substances and lipid concentrations in plasma during pregnancy among women in the Norwegian Mother and Child Cohort Study. Environ Int 62:104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Barry V, Savitz D. 2018a. Serum perfluorooctanoic acid and birthweight: An updated meta-analysis with bias analysis. Epidemiology 29:765–776. [DOI] [PubMed] [Google Scholar]
- Steenland K, Kugathasan S, Barr DB. 2018b. PFOA and ulcerative colitis. Environ Res 165:317–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Tinker S, Frisbee S, Ducatman A, Vaccarino V. 2009. Association of perfluorooctanoic acid and perfluorooctane sulfonate with serum lipids among adults living near a chemical plant. Am J Epidemiol 170:1268–1278. [DOI] [PubMed] [Google Scholar]
- Steenland K, Tinker S, Shankar A, Ducatman A. 2010. Association of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) with uric acid among adults with elevated community exposure to PFOA. Environ Health Perspect 118:229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Woskie S. 2012. Cohort mortality study of workers exposed to perfluorooctanoic acid. Am J Epidemiol 176:909–917. [DOI] [PubMed] [Google Scholar]
- Steenland K, Zhao L, Winquist A. 2015. A cohort incidence study of workers exposed to perfluorooctanoic acid (PFOA). Occup Environ Med 72:373–380. [DOI] [PubMed] [Google Scholar]
- Steenland K, Zhao L, Winquist A, Parks C. 2013. Ulcerative colitis and perfluorooctanoic acid (PFOA) in a highly exposed population of community residents and workers in the mid-Ohio Valley. Environ Health Perspect 121:900–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein CR, McGovern KJ, Pajak AM, Maglione PJ, Wolff MS. 2016. Perfluoroalkyl and polyfluoroalkyl substances and indicators of immune function in children aged 12–19 y: National Health and Nutrition Examination Survey. Pediatr Res 79:348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein CR, Savitz DA, Dougan M. 2009. Serum levels of perfluorooctanoic acid and perfluorooctane sulfonate and pregnancy outcome. Am J Epidemiol 170:837–846. [DOI] [PubMed] [Google Scholar]
- Stubleski J, Salihovic S, Lind L, Lind PM, van Bavel B, Karrman A. 2016. Changes in serum levels of perfluoroalkyl substances during a 10-year follow-up period in a large population-based cohort. Environ Int 95:86–92. [DOI] [PubMed] [Google Scholar]
- Sun Q, Zong G, Valvi D, Nielsen F, Coull B, Grandjean P. 2018. Plasma concentrations of perfluoroalkyl substances and risk of type 2 diabetes: A prospective investigation among U.S. women. Environ Health Perspect 126:037001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. 2019. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol 29:131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susmann HP, Schaider LA, Rodgers KM, Rudel RA. 2019. Dietary habits related to food packaging and population exposure to PFASs. Environ Health Perspect 127:107003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilagyi JT, Freedman AN, Kepper SL, Keshava AM, Bangma JT, Fry RC. 2020. Per- and polyfluoroalkyl substances (PFAS) differentially inhibit placental trophoblast migration and invasion in vitro. Toxicol Sci 175:210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadic M, Cuspidi C, Vasic D, Kerkhof PLM. 2018. Cardiovascular implications of diabetes, metabolic syndrome, thyroid disease, and cardiooncology in women. In Kerkhof PLM, Miller VM, eds, Sex-Specific Analysis of Cardiovascular Function. Springer International, Cham, Switzerland, pp 471–488. [Google Scholar]
- Tan X, Xie G, Sun X, Li Q, Zhong W, Qiao P, Sun X, Jia W, Zhou Z. 2013. High fat diet feeding exaggerates perfluorooctanoic acid-induced liver injury in mice via modulating multiple metabolic pathways. PLoS One 8:e61409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temkin AM, Hocevar BA, Andrews DQ, Naidenko OV, Kamendulis LM. 2020. Application of the key characteristics of carcinogens to per and polyfluoroalkyl substances. Int J Environ Res Public Health 17:1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Barbee BD, Richards JH, Butenhoff JL, Stevenson LA, Lau C. 2003. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. I: Maternal and prenatal evaluations. Toxicol Sci 74:369–381. [DOI] [PubMed] [Google Scholar]
- Tilton SC, Orner GA, Benninghoff AD, Carpenter HM, Hendricks JD, Pereira CB, Williams DE. 2008. Genomic profiling reveals an alternate mechanism for hepatic tumor promotion by perfluorooctanoic acid in rainbow trout. Environ Health Perspect 116:1047–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermann CA, Budtz-Jorgensen E, Jensen TK, Osuna CE, Petersen MS, Steuerwald U, Nielsen F, Poulsen LK, Weihe P, Grandjean P. 2017a. Association between perfluoroalkyl substance exposure and asthma and allergic disease in children as modified by MMR vaccination. J Immunotoxicol 14:39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermann CA, Rossing LI, Grontved A, Ried-Larsen M, Dalgard C, Andersen LB, Grandjean P, Nielsen F, Svendsen KD, Scheike T, Jensen TK. 2014. Adiposity and glycemic control in children exposed to perfluorinated compounds. J Clin Endocrinol Metab 99:E608–E614. [DOI] [PubMed] [Google Scholar]
- Timmermann CAG, Budtz-Jorgensen E, Petersen MS, Weihe P, Steuerwald U, Nielsen F, Jensen TK, Grandjean P. 2017b. Shorter duration of breastfeeding at elevated exposures to perfluoroalkyl substances. Reprod Toxicol 68:164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker DK, Macon MB, Strynar MJ, Dagnino S, Andersen E, Fenton SE. 2015. The mammary gland is a sensitive pubertal target in CD-1 and C57Bl/6 mice following perinatal perfluorooctanoic acid (PFOA) exposure. Reprod Toxicol 54:26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Environmental Protection Agency. 2018. PFAS structures in DSSTox. Washington, DC. [cited 2020 May 19]. Available from: https://comptox.epa.gov/dashboard/chemical_lists/PFASSTRUCT [Google Scholar]
- US Environmental Protection Agency. 2019. EPA’s per- and polyfluoroalkyl substances (PFAS) action plan. EPA 823R18004. Washington, DC. [cited 2020 July 13]. Available from: www.epa.gov/pfas [Google Scholar]
- US Environmental Protection Agency. 2020. Announcement of preliminary regulatory determinations for contaminants on the fourth drinking water contaminant candidate list. 85 FR 14098 Washington, DC. [cited 2020 September 15]. Available from: https://www.federalregister.gov/documents/2020/03/10/2020-04145/announcement-of-preliminary-regulatory-determinations-for-contaminants-on-the-fourth-drinking-water [Google Scholar]
- van Esterik JCJ, Sales LB, Dollé MET, Håkansson H, Herlin M, Legler J, van der Ven LTM. 2016. Programming of metabolic effects in C57BL/6JxFVB mice by in utero and lactational exposure to perfluorooctanoic acid. Arch Toxicol 90:701–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanNoy BN, Lam J, Zota AR. 2018. Breastfeeding as a predictor of serum concentrations of per- and polyfluorinated alkyl substances in reproductive-aged women and young children: A rapid systematic review. Curr Environ Health Rep 5:213–224. [DOI] [PubMed] [Google Scholar]
- Velez MP, Arbuckle TE, Fraser WD. 2015. Maternal exposure to perfluorinated chemicals and reduced fecundity: The MIREC study. Hum Reprod 30:701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vested A, Ramlau-Hansen CH, Olsen SF, Bonde JP, Kristensen SL, Halldorsson TI, Becher G, Haug LS, Ernst EH, Toft G. 2013. Associations of in utero exposure to perfluorinated alkyl acids with human semen quality and reproductive hormones in adult men. Environ Health Perspect 121:453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viberg H, Lee I, Eriksson P. 2013. Adult dose-dependent behavioral and cognitive disturbances after a single neonatal PFHxS dose. Toxicology 304:185–191. [DOI] [PubMed] [Google Scholar]
- Vieira VM, Hoffman K, Shin HM, Weinberg JM, Webster TF, Fletcher T. 2013. Perfluorooctanoic acid exposure and cancer outcomes in a contaminated community: A geographic analysis. Environ Health Perspect 121:318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlang B, Jin J, Beier JI, Hardesty JE, Daly EF, Schnegelberger RD, Falkner KC, Prough RA, Kirpich IA, Cave MC. 2019. Mechanisms of environmental contributions to fatty liver disease. Curr Environ Health Rep 6:80–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan HT, Zhao YG, Wei X, Hui KY, Giesy JP, Wong CK. 2012. PFOS-induced hepatic steatosis, the mechanistic actions on beta-oxidation and lipid transport. Biochim Biophys Acta 1820:1092–1101. [DOI] [PubMed] [Google Scholar]
- Wang B, Zhang R, Jin F, Lou H, Mao Y, Zhu W, Zhou W, Zhang P, Zhang J. 2017. Perfluoroalkyl substances and endometriosis-related infertility in Chinese women. Environ Int 102:207–212. [DOI] [PubMed] [Google Scholar]
- Wang L, Wang Y, Liang Y, Li J, Liu Y, Zhang J, Zhang A, Fu J, Jiang G. 2013. Specific accumulation of lipid droplets in hepatocyte nuclei of PFOA-exposed BALB/c mice. Sci Rep 3:2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wang Y, Liang Y, Li J, Liu Y, Zhang J, Zhang A, Fu J, Jiang G. 2014. PFOS induced lipid metabolism disturbances in BALB/c mice through inhibition of low density lipoproteins excretion. Sci Rep 4:4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Bai Y, Tang C, Cao X, Chang F, Chen L. 2018. Impact of perfluorooctane sulfonate on reproductive ability of female mice through suppression of estrogen receptor alpha-activated kisspeptin neurons. Toxicol Sci 165:475–486. [DOI] [PubMed] [Google Scholar]
- Wang Z, DeWitt JC, Higgins CP, Cousins IT. 2017. A never-ending story of per- and polyfluoroalkyl substances (PFASs)? Environ Sci Technol 51: 2508–2518. [DOI] [PubMed] [Google Scholar]
- Waterfield G, Rogers M, Grandjean P, Auffhammer M, Sunding D. 2020. Reducing exposure to high levels of perfluorinated compounds in drinking water improves reproductive outcomes: Evidence from an intervention in Minnesota. Environ Health 19:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins DJ, Josson J, Elston B, Bartell SM, Shin HM, Vieira VM, Savitz DA, Fletcher T, Wellenius GA. 2013. Exposure to perfluoroalkyl acids and markers of kidney function among children and adolescents living near a chemical plant. Environ Health Perspect 121:625–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver YM, Ehresman DJ, Butenhoff JL, Hagenbuch B. 2009. Roles of rat renal organic anion transporters in transporting perfluorinated carboxylates with different chain lengths. Toxicol Sci 113:305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster GM, Rauch SA, Marie NS, Mattman A, Lanphear BP, Venners SA. 2016. Cross-sectional associations of serum perfluoroalkyl acids and thyroid hormones in U.S. adults: Variation according to TPOAb and iodine status (NHANES 2007–2008). Environ Health Perspect 124:935–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster GM, Venners SA, Mattman A, Martin JW. 2014. Associations between perfluoroalkyl acids (PFASs) and maternal thyroid hormones in early pregnancy: A population-based cohort study. Environ Res 133: 338–347. [DOI] [PubMed] [Google Scholar]
- Wei Y, Dai J, Liu M, Wang J, Xu M, Zha J, Wang Z. 2009. Estrogen-like properties of perfluorooctanoic acid as revealed by expressing hepatic estrogen-responsive genes in rare minnows (Gobiocypris rarus). Environ Toxicol Chem 26:2440–2447. [DOI] [PubMed] [Google Scholar]
- Wen LL, Lin CY, Chou HC, Chang CC, Lo HY, Juan SH. 2016. Perfluorooctanesulfonate mediates renal tubular cell apoptosis through PPARgamma inactivation. PLoS One 11:e0155190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SS, Calafat AM, Kuklenyik Z, Villanueva L, Zehr RD, Helfant L, Strynar MJ, Lindstrom AB, Thibodeaux JR, Wood C, Fenton SE. 2007. Gestational PFOA exposure of mice is associated with altered mammary gland development in dams and female offspring. Toxicol Sci 96:133–144. [DOI] [PubMed] [Google Scholar]
- White SS, Fenton SE, Hines EP. 2011a. Endocrine disrupting properties of perfluorooctanoic acid. J Steroid Biochem Mol Biol 127:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SS, Stanko JP, Kato K, Calafat AM, Hines EP, Fenton SE. 2011b. Gestational and chronic low-dose PFOA exposures and mammary gland growth and differentiation in three generations of CD-1 mice. Environ Health Perspect 119:1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth KW, Haug LS, Baird DD, Becher G, Hoppin JA, Skjaerven R, Thomsen C, Eggesbo M, Travlos G, Wilson R, Longnecker MP. 2012. Perfluorinated compounds and subfecundity in pregnant women. Epidemiology 23:257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikstrom S, Lin PI, Lindh CH, Shu H, Bornehag CG. 2020. Maternal serum levels of perfluoroalkyl substances in early pregnancy and offspring birth weight. Pediatr Res 87:1093–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikstrom S, Lindh CH, Shu H, Bornehag CG. 2019. Early pregnancy serum levels of perfluoroalkyl substances and risk of preeclampsia in Swedish women. Sci Rep 9:9179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AJ, Grulke CM, Edwards J, McEachran AD, Mansouri K, Baker NC, Patlewicz G, Shah I, Wambaugh JF, Judson RS, Richard AM. 2017. The CompTox Chemistry Dashboard: A community data resource for environmental chemistry. J Cheminform 9:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winquist A, Steenland K. 2014a. Modeled PFOA exposure and coronary artery disease, hypertension, and high cholesterol in community and worker cohorts. Environ Health Perspect 122:1299–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winquist A, Steenland K. 2014b. Perfluorooctanoic acid exposure and thyroid disease in community and worker cohorts. Epidemiology 25:255–264. [DOI] [PubMed] [Google Scholar]
- Wolf CJ, Rider CV, Lau C, Abbott BD. 2014. Evaluating the additivity of perfluoroalkyl acids in binary combinations on peroxisome proliferator-activated receptor-alpha activation. Toxicology 316:43–54. [DOI] [PubMed] [Google Scholar]
- Wolf CJ, Schmid JE, Lau C, Abbott BD. 2012. Activation of mouse and human peroxisome proliferator-activated receptor-alpha (PPARα) by perfluoroalkyl acids (PFAAs): Further investigation of C4–C12 compounds. Reprod Toxicol 33:546–551. [DOI] [PubMed] [Google Scholar]
- Wolf CJ, Takacs ML, Schmid JE, Lau C, Abbott BD. 2008. Activation of mouse and human peroxisome proliferator-activated receptor alpha by perfluoroalkyl acids of different functional groups and chain lengths. Toxicol Sci 106:162–171. [DOI] [PubMed] [Google Scholar]
- World Health Organization. 2020. Mode of action framework (for cancer and non-cancer risk assessment). Geneva, Switzerland. [cited 2020 May 19]. Available from: https://www.who.int/ipcs/methods/harmonization/areas/cancer/en/ [Google Scholar]
- Xiao C, Grandjean P, Valvi D, Nielsen F, Jensen TK, Weihe P, Oulhote Y. 2020. Associations of exposure to perfluoroalkyl substances with thyroid hormone concentrations and birth size. J Clin Endocrinol Metab 105:735–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HE, Lambert MH, Montana VG, Parks DJ, Blanchard SG, Brown PJ, Sternbach DD, Lehmann JM, Wisely GB, Willson TM, Kliewer SA, Milburn MV. 1999. Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol Cell 3:397–403. [DOI] [PubMed] [Google Scholar]
- Xu J, Shimpi P, Armstrong L, Salter D, Slitt AL. 2016. PFOS induces adipogenesis and glucose uptake in association with activation of Nrf2 signaling pathway. Toxicol Appl Pharmacol 290:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Liu G, Li M, Huo M, Zong W, Liu R. 2020a. Probing the cell apoptosis pathway induced by perfluorooctanoic acid and perfluorooctane sulfonate at the subcellular and molecular levels. J Agric Food Chem 68: 633–641. [DOI] [PubMed] [Google Scholar]
- Xu Y, Li Y, Scott K, Lindh CH, Jakobsson K, Fletcher T, Ohlsson B, Andersson EM. 2020b. Inflammatory bowel disease and biomarkers of gut inflammation and permeability in a community with high exposure to perfluoroalkyl substances through drinking water. Environ Res 181:108923. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Arisawa K, Uemura H, Katsuura-Kamano S, Takami H, Sawachika F, Nakamoto M, Juta T, Toda E, Mori K, Hasegawa M, Tanto M, Shima M, Sumiyoshi Y, Morinaga K, Kodama K, Suzuki T, Nagai M, Satoh H. 2013. Consumption of seafood, serum liver enzymes, and blood levels of PFOS and PFOA in the Japanese population. J Occup Health 55:184–194. [DOI] [PubMed] [Google Scholar]
- Yang CH, Glover KP, Han X. 2009. Organic anion transporting polypeptide (Oatp) 1a1-mediated perfluorooctanoate transport and evidence for a renal reabsorption mechanism of Oatp1a1 in renal elimination of perfluorocarboxylates in rats. Toxicol Lett 190:163–171. [DOI] [PubMed] [Google Scholar]
- Yao X, Sha S, Wang Y, Sun X, Cao J, Kang J, Jiang L, Chen M, Ma Y. 2016. Perfluorooctane sulfonate induces autophagy-dependent apoptosis through spinster 1-mediated lysosomal-mitochondrial axis and impaired mitophagy. Toxicol Sci 153:198–211. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Ding X, Cheng Y, Kang H, Luo T, Zhang X, Kuang H, Chen Y, Zeng X, Zhang D. 2020. PFOA evokes extracellular Ca2+ influx and compromises progesterone-induced response in human sperm. Chemosphere 241: 125074. [DOI] [PubMed] [Google Scholar]
- Zeilmaker M, Fragki S, Verbruggen E, Bokkers B, Lijzen J. 2018. Mixture exposure to PFAS: A relative potency factor approach. RIVM-2018–0070. Rijksinstituut voor Volksgezondheid en Milieu, Bilthoven, Netherlands. [cited 2020 July 13]. Available from: 10.21945/rivm-2018-0070 [DOI] [Google Scholar]
- Zeng XW, Lodge CJ, Dharmage SC, Bloom MS, Yu Y, Yang M, Chu C, Li QQ, Hu LW, Liu KK, Yang B-Y, Dong G-H. 2019. Isomers of per- and polyfluoroalkyl substances and uric acid in adults: Isomers of C8 Health Project in China. Environ Int 133:105160. [DOI] [PubMed] [Google Scholar]
- Zeng XW, Qian Z, Emo B, Vaughn M, Bao J, Qin XD, Zhu Y, Li J, Lee YL, Dong GH. 2015. Association of polyfluoroalkyl chemical exposure with serum lipids in children. Sci Total Environ 512–513:364–370. [DOI] [PubMed] [Google Scholar]
- Zhang H, He J, Li N, Gao N, Du Q, Chen B, Chen F, Shan X, Ding Y, Zhu W, Wu Y, Tang J, Jia X. 2019. Lipid accumulation responses in the liver of Rana nigromaculata induced by perfluorooctanoic acid (PFOA). Ecotoxicol Environ Saf 167:29–35. [DOI] [PubMed] [Google Scholar]
- Zhang J, Begum A, Brannstrom K, Grundstrom C, Iakovleva I, Olofsson A, Sauer-Eriksson AE, Andersson PL. 2016. Structure-based virtual screening protocol for in silico identification of potential thyroid disrupting chemicals targeting transthyretin. Environ Sci Technol 50:11984–11993. [DOI] [PubMed] [Google Scholar]
- Zhang L, Duan X, Sun W, Sun H. 2020. Perfluorooctane sulfonate acute exposure stimulates insulin secretion via GPR40 pathway. Sci Total Environ 726:138498. [DOI] [PubMed] [Google Scholar]
- Zhang L, Krishnan P, Ehresman DJ, Smith PB, Dutta M, Bagley BD, Chang SC, Butenhoff JL, Patterson AD, Peters JM. 2016. Editor’s highlight: Perfluorooctane sulfonate-choline ion pair formation: A potential mechanism modulating hepatic steatosis and oxidative stress in mice. Toxicol Sci 153:186–197. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Beesoon S, Zhu L, Martin JW. 2013. Biomonitoring of perfluoroalkyl acids in human urine and estimates of biological half-life. Environ Sci Technol 47:10619–10627. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cao X, Chen L, Qin Y, Xu Y, Tian Y, Chen L. 2020. Exposure of female mice to perfluorooctanoic acid suppresses hypothalamic kisspeptin-reproductive endocrine system through enhanced hepatic fibroblast growth factor 21 synthesis, leading to ovulation failure and prolonged dioestrus. J Neuroendocrinol 32:e12848. [DOI] [PubMed] [Google Scholar]
- Zhao J, Hinton P, Chen J, Jiang J. 2020. Causal inference for the effect of environmental chemicals on chronic kidney disease. Comput Struct Biotechnol J 18:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Cheng W, Feng Y, Wei H, Liang F, Wang Y. 2017. Interactions between three typical endocrine-disrupting chemicals (EDCs) in binary mixtures exposure on myocardial differentiation of mouse embryonic stem cell. Chemosphere 178:378–383. [DOI] [PubMed] [Google Scholar]


