Abstract
Chitinases are a diverse group of enzymes having the ability to degrade chitin. Chitin is the second most abundant polysaccharide on earth, predominantly found in insect exoskeletons and fungal cell walls. In this study, we performed a genome-wide search for chitinase genes and identified a total of 49 chitinases in tea. These genes were categorized into 5 classes, where an expansion of class V chitinases has been observed in comparison to other plant species. Extensive loss of introns in 46% of the GH18 chitinases indicates that an evolutionary pressure is acting upon these genes to lose introns for rapid gene expression. The promoter upstream regions in 65% of the predicted chitinases contain methyl-jasmonate, salicylic acid and defense responsive cis-acting elements, which may further illustrate the possible role of chitinases in tea plant’s defense against various pests and pathogens. Differential expression analysis revealed that transcripts of two GH19 chitinases TEA028279 and TEA019397 got upregulated during three different fungal infections in tea. While GH19 chitinase TEA031377 showed an increase in transcript abundance in the two insect infested tea tissues. Semi-quantitative RT-PCR analysis revealed that five GH19 chitinases viz. TEA018892, TEA031484, TEA28279, TEA033470 and TEA031277 showed significant increase in expression in the tea plants challenged with a biotrophic pathogen Exobasidium vexans. The study endeavours in highlighting biotic stress responsive defensive role of chitinase genes in tea.
Keywords: Chitinase, Camellia sinensis, Differential gene expression, Genome wide identification, Biotic stress
Introduction
Chitin, a derivative of glucose and a natural homopolymer of N-acetylglucosamine, is a major component of fungal and some algal cell walls, exoskeletons of insects, crustaceans and some other invertebrates (Kasprzewska 2003; Li and Roseman 2004). Chitinases catalyze the hydrolytic breakdown of chitin in the β-1-4-glycosidic linkage of N-acetyl glucosamine (GlcNAc) polymers (Xu et al. 2016) and chitosan, the N-deacetylated derivative of chitin (Tanabe et al. 2000). Bacterial peptidoglycan, plant cell wall glycoproteins, arabinogalactan proteins, rhizobial nod factors etc. having GlcNAc in their structures are known to be substrates for chitinases (Grover 2012). Chitin hydrolysis mediated release of oligosaccharides activates pathogen associated molecular patterns (PAMP)-triggered immunity (PTI) in the host plants (Cao et al. 2019).
Chitinases play varied roles in plant growth and development, defense, frost tolerance and symbiotic associations including nodulation and mycorrhiza formation (Kasprzewska 2003; Collinge et al. 1993; Grover 2012). Understanding the role of chitinases in the plant defense mechanisms is important in formulating biotechnological methods of crop protection against pests and pathogens. Pathogenesis-related (PR) proteins are induced in response to different abiotic and biotic stresses in plants (Kasprzewska 2003). Chitinases are one of the important groups of PR protein induced in response to stresses in plants. Viral/fungal infection induces chitinase activity in the plants due to which they are considered as PR proteins in general. Various studies have shown the role of chitinases in the inhibition of fungal growth in plants (Schlumbaum et al. 1986; Roberts and Selitrennikoff 1988).
Based on the type of catalytic domains present, chitinases are divided into two categories of glycosyl hydrolases (GH) viz. GH18 and GH19 families. In general, chitinases are categorized into five classes based on their differences in gene sequences, amino acid composition and conserved motif distribution. Class I, II and IV chitinases contain the GH19 domain as the catalytic domain, whereas the GH18 domain is found to be present in the class III and V chitinases (Chen et al. 2018; Cao et al. 2019). Although class I and IV chitinases are structurally similar in the presence of the N-terminal conserved chitin-binding domain and the type of catalytic domain, but the domains in the class IV chitinases are shorter in length due to deletions in both of the domains. Class I and II chitinases share sequence homology but class II chitinases lack N-terminal chitin-binding domain. The chitin binding domain is also absent in class III and V chitinases. Class III chitinases possess lysozyme activity and are characterized by the presence of a C-terminal extension. They are mostly similar to fungal and viral chitinases. The presence or absence of the N-terminal domain responsible for substrate binding may be an important factor to determine its antifungal or defensive potential (Kasprzewska 2003). Class III and V chitinases are widely distributed in a range of organisms from fungi, viruses to higher plants, whereas class I, II and IV chitinases are reported from bacteria and also found in some higher plants (Grover 2012).
Plants receive signals about fungal attack through chitinases, which also play significant role in subsequent inhibition of the pathogen growth (Kasprzewska 2003; Wang et al. 2009). Apoplastic chitinases indirectly induce release of fungal elicitors in the host plant followed by vacuolar chitinases that come into action to ward off the pathogen (Kasprzewska 2003). The antifungal role of chitinases in plants has been well-established in many studies. Enhanced resistance to fungal and bacterial pathogens has been observed in transgenic plants overexpressing chitinase encoding genes (Singh et al. 2015; Dana et al. 2006; Grover 2012; Xu et al. 2016).
Tea being a perennial crop, is often subjected to biotic stress by a number of pathogens which is the major limiting factor in tea productivity. More than 300 species of fungi contribute to disease development in tea (Agnihothrudu 1964; Chen and Chen 1990). Foliar diseases are primarily responsible for degrading tea quality as well as crop loss (Baby et al. 1998). Anthracnose, grey blight, blister blight, red root, collar canker are some of the major tea diseases which pose a great threat to its agricultural output. Insects also create menace in tea production, although moderate jassids and thrips infestation in Darjeeling tea was reported to enhance its aroma and flavour (Gohain et al. 2012). Tea pathogens also alter the morpho-physiological, biochemical characteristics and normal metabolism of the plant (Borchetia et al. 2018). Lately, high throughput next-generation sequencing (NGS) has increased the feasibility for easy and efficient genome-wide assays even in organisms having complex genomes structure. Recently, availability of tea genome sequence has paved the ways for high throughput analysis of genomic data as well as large scale detection of phenotypic events in relation to genomic resources. In view of the potential of chitinases in eliciting defense responses and mediating plant immunity, we have carried out a genome wide identification, structure analysis and expression profiling of tea chitinase genes during five different biotic stress conditions. Our findings will help in dissecting important information regarding the role of chitinases in defense responses of the tea plant.
Materials and methods
Identification and characterization of tea chitinase genes
For the identification and extraction of chitinase sequences from the tea genome, we downloaded the peptide sequences of Camellia sinensis from a publicly available database, TPIA (Tea Plant Information Archive) (Xia et al. 2019) to construct a local protein database. The Hidden Markov Model (HMM) profiles of GH19 (PF00182) and GH18 (PF00704) were downloaded from the Pfam database (El-Gebali et al. 2019) and were used as queries to identify candidate chitinases in the tea genome through the HMMER 3.0 program (Finn et al. 2011) with an e-value cutoff of 1e-5. The extracted protein sequences were further confirmed for the presence of the catalytic domains by searching the identified sequences against the NCBI Conserved Domain Database (CDD). The other parameters like number of amino acids, molecular weight, pI value etc. have been obtained from the Protparam tool (Gasteiger et al. 2005). The sub-cellular locations of the predicted chitinases were obtained from the online tool FUEL-mLoc (Wan et al. 2017) keeping all the parameters to default. For signal peptide prediction, the online tool SignalP 4.1 server (Petersen et al. 2011) was used and the presence or absence of signal peptides was calculated using the C, S and Y values. The C, S, and Y-scores indicate cleavage site, signal peptide-ness and combined cleavage site predictions respectively (Bendtsen et al. 2004).
Prediction of gene structure and conserved motif analysis
The exon–intron structure of the predicted chitinases wereobtained from the online tool GSDS (Gene Structure Display Server) (Hu et al. 2015) by setting the parameters to default and using the coding and genomic sequences of chitinases downloaded from the TPIA database. To find out the conserved motifs, the online tool MEME (Bailey et al. 2009) was used with a maximum motif width of 300 and motif number of 10.
Chromosomal location and estimation of gene distribution
Specific chromosomal positions of the genes encoding chitinase proteins were determined by BLASTP search of the tea chitinase sequences against the tea genome available in TPIA (Xia et al. 2019). The genes were plotted separately onto the fifteen tea chromosomes according to their ascending order of physical position and finally displayed using MapChart (Voorrips 2002). Two separate MapChart figures were generated for GH18 and GH19 chitinase families.
Identification of putative cis-acting elements in the promoter regions of the chitinase genes of tea
For the prediction of cis-acting elements in the upstream of promoter region of the chitinases, we used the PlantCARE database (Higo et al. 1999; Lescot et al. 2002). 1500 bp upstream sequences of the chitinase genes were extracted from TPIA database by specifying the sequence length of 1500 bp upstream for each gene and then analysed for prediction of cis-acting elements using PlantCARE online tool.
Multiple sequence alignment and phylogenetic analysis
Multiple sequence alignment for the tea chitinases was performed using ClustalX version 2.1 (Thompson et al. 1997). GeneDoc version 2.7 was used for visualization of the aligned amino acid sequences (Nicholas and Nicholas 1997) (Supplementary file S1). Twenty four identified Arabidopsis chitinase protein sequences were downloaded from the TAIR (The Arabidopsis Information Resource) database and two separate trees for GH18 and GH19 families with Arabidopsis chitinases were constructed using the MEGA-X software (Kumar et al. 2018) through the Maximum Likelihood method with 1000 bootstrap replicates. The other parameters were set to default. For evaluating the phylogenetic relationship of tea chitinases with their orthologs in other plant species, we carried out a BLAST search in the Phytozome database (Goodstein et al. 2012) with chitinase protein sequence as query (TEA030241 and TEA019397) against all the available 64 plant species. The parameters were kept in default mode and the first BLAST hit for each plant species was considered for phylogenetic tree construction.
Estimation of Ka/Ks ratios of tea chitinases with their orthologs in Arabidopsis
To estimate the synonymous and non-synonymous substitution rates and Ka/Ks ratios, we followed a previously described methodology (Bordoloi et al. 2021). Briefly, the orthologs of tea chitinases in Arabidopsis genome were extracted from the Phytozome database (Goodstein et al. 2012) and for each pair of orthologous genes, the PAL2NAL tool (Suyama et al. 2006) was used to calculate the Ka and Ks values. Selection pressure on the orthologous gene pairs was analyzed by using the Ka/Ks ratios. The divergence time was calculated using the formula Ks/(2 × 6.5 × 10–9) × 106 (Wei et al. 2018).
Differential in silico gene expression analysis of the predicted chitinases during the biotic stress conditions
We used raw RNA-seq data of C. sinensis from five different biotic stress related bioprojects. The stress conditions include (i) inoculation of blister blight resistant and susceptible tea plants with spore suspension of E. vexans (PRJNA306068) (Jayaswall et al. 2016) (ii) inoculation of two tea varieties viz. Longjing and Zhenong with anthracnose disease causal organism Colletotrichum gloeosporioides (PRJNA493214) (Shi et al. 2019) (iii) infection of tea plants by Didymella segeticola (PRJNA528172) that causes leaf spot disease of tea (Yang et al. 2021) (iv) treatment of tea plants with the tea geometrid Ectropis oblique (PRJNA439206) (Yang et al. 2019) and (v) green leafhopper (PRJNA553681) infestation on tea (Zhao et al. 2020). The RNA-seq datasets were downloaded from Sequence Read Archive (SRA) database of NCBI and converted to FASTQ format by SRA toolkit. Details of all the sample files (Bioproject ID, SRR numbers, stress details etc.) have been tabulated in the supplementary file S2. Sample files including all replicates were checked for quality by FastQC program. The differential gene expression analysis was performed using HISAT2, StringTie (Pertea et al. 2016) and DESeq tools (Anders and Huber 2010). The expression heatmaps were generated using the online tool ClustVis (Metsalu and Vilo 2015).
Plant materials and stress treatments followed by semi-quantitative RT-PCR for expression analysis of chitinases in tea
Two year old tea saplings of a blister blight susceptible cultivar were inoculated with E. vexans spore suspension according to a previously described protocol (Bhorali et al. 2012; Jayaswall et al. 2016). Following the development of symptoms (Fig. 8) the uninfected and infected samples were collected and immediately frozen in liquid N2. Total RNA was isolated using the TRIzol reagent according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA). cDNA synthesis was carried out using the PrimeScriptTMRT reagent kit with gDNA eraser (TaKaRa). A total of 9 primer pairs (Supplementary file S3) were designed based on the expression profiling of chitinases from the SRA data analyzed. The semi-quantitative RT-PCR amplification was carried out according to a previously described protocol (Borah et al. 2019). We used GelQuant.NET software (Biochemlabsolutions, Wayne, PA, USA) to quantify the band intensities by taking GAPDH gene (TPIA accession id: TEA003029) as the reference gene (Xu et al. 2020). The statistical significance of differential expression of the chitinase genes was calculated by two way ANOVA followed by a bonferroni post-test (Borah et al. 2019) using Graph Pad Prism (GraphPad Software, La Jolla California USA).
Fig. 8.

E. vexans uninfected control (left) and infected (right) leaves of a susceptible cultivar
Results
Genome-wide identification and characterization of chitinases in tea
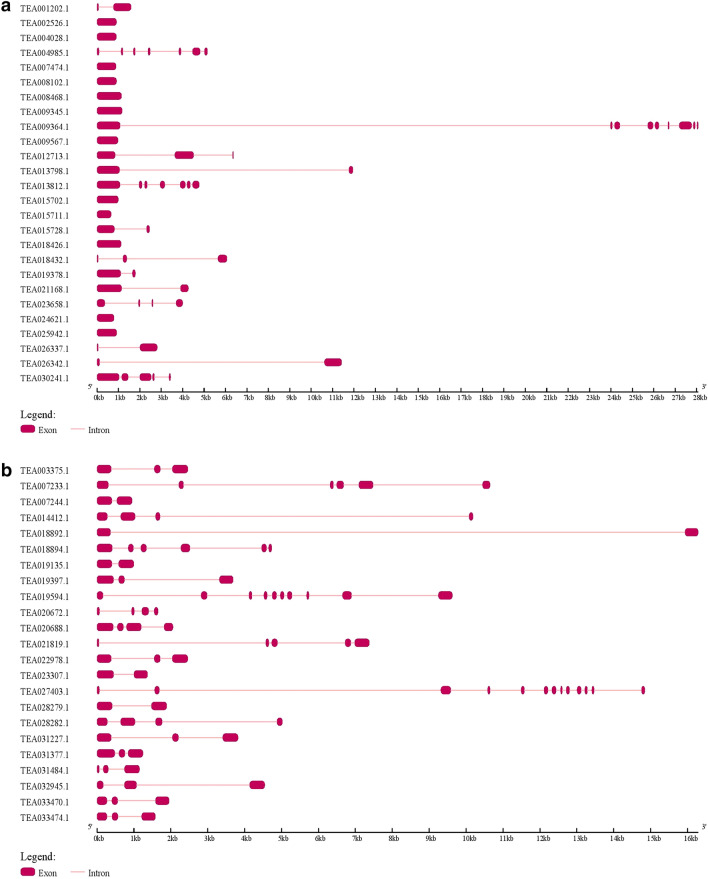
By using the HMM profiles of GH18 and GH19 domains to search against the tea peptide sequence database by the HMMER program, we were able to identify a total of 49 chitinases in the tea genome. Based on the presence of GH18 (PF00704) or GH19 (PF00182) domains, 26 chitinases were grouped under the GH18 category and 23 under the GH19 category. We categorized the chitinases to classes I, II, III, IV and V according to the classification criteria as previously described in Arabidopsis (Passarinho and de Vries 2002; Neuhaus et al. 1996). Additionally, we searched for chitinases class annotation in the NCBI CDD database and evaluated the evolutionary relationship of tea chitinases with Arabidopsis chitinases for further confirmation. We found 10 class I, 3 class II, 7 class III, 10 class IV and 19 class V chitinases in tea. As reported in Arabidopsis, presence of GH18 domain was seen in class III and V chitinases but with no N-terminal chitin binding domain. Class I, II and IV chitinases had the GH19 as catalytic domain. However, 7 class I and 3 class IV chitinases showed the lack of chitin binding domains in their structure. The predicted 49 chitinases showed pI values ranging from 4.48 (TEA031377, TEA020688) to 9.25 (TEA028282). The number of amino acids in the tea chitinases ranged from a minimum of 140 (TEA020672) to maximum of 862 (TEA009364) (Table 1). TEA009364 showed the highest molecular weight of 94.5 kDa and TEA020672 showed the lowest of 15.6 kDa. TEA009364, TEA030241 and TEA013712 were three longest chitinases and TEA020672, TEA018432 and TEA031484 were the shortest in terms of amino acid length. The gene structure prediction was done by the online GSDS tool and it was seen that in GH18 category the exons number ranged from 2 to 9 in 14 chitinases. Eight class V and 4 class III chitinases lacked any intron in their structure (Fig. 1a). In the GH19 category, twenty one chitinases contained 2–6 exons and the other two had 10 (TEA019594) and 13 (TEA027403) exons (Fig. 1b).
Table 1.
Classification of tea chitinases, their sub cellular localization and physicochemical characterization
| Sequence IDs | Catalytic domain | Class | Genome location | Strand | Signal peptide | No. of introns | No. of amino acids | Mol. Wt (Da) | pI value | Sub cellular location |
|---|---|---|---|---|---|---|---|---|---|---|
| TEA030241 | GH18 | V | Scaffold694: 2,541,323–2,544,756 | Positive | 1–24 | 4 | 668 | 73,575.49 | 5.71 | Vacuole |
| TEA009345 | GH18 | V | Scaffold237: 330,420–331,595 | Negative | 1–24 | 0 | 391 | 42,720.1 | 8.64 | Vacuole |
| TEA008468 | GH18 | V | Scaffold3800: 138,517–139,659 | Positive | 1–27 | 0 | 380 | 41,680.86 | 5.98 | Vacuole |
| TEA013798 | GH18 | V | Scaffold426: 2,037,530–2,049,481 | Positive | 1–24 | 2 | 412 | 45,178.63 | 4.58 | Vacuole |
| TEA021168 | GH18 | V | Scaffold2220: 968,697–972,962 | Negative | No | 1 | 504 | 55,175.29 | 5.33 | Vacuole |
| TEA009364 | GH18 | V | Scaffold237: 371,101–399,233 | Negative | 1–24 | 8 | 862 | 94,504.35 | 5.22 | Vacuole |
| TEA019378 | GH18 | V | Scaffold3284: 1,067,222–1,069,010 | Negative | No | 1 | 412 | 45,541.19 | 8.97 | Vacuole |
| TEA018426 | GH18 | V | Scaffold2844: 1,022,255–1,023,382 | Positive | No | 0 | 375 | 41,567.06 | 5.65 | Vacuole |
| TEA024621 | GH18 | V | Scaffold656: 1,478,471–1,479,265 | Positive | No | 0 | 264 | 29,238.88 | 6.52 | Vacuole |
| TEA013812 | GH18 | V | Scaffold426: 2,072,901–2,078,587 | Positive | 1–22 | 6 | 743 | 84,240.66 | 7.52 | Vacuole |
| TEA009567 | GH18 | V | Scaffold3646: 49,295–50,275 | Negative | 1–24 | 0 | 326 | 36,308.94 | 9.11 | Vacuole |
| TEA012713 | GH18 | V | Scaffold1770: 233,879–240,252 | Positive | 1–23 | 2 | 586 | 65,606.95 | 4.86 | Vacuole |
| TEA004028 | GH18 | III | Scaffold295: 3,977,575–3,978,477 | Positive | 1–27 | 0 | 300 | 32,043.36 | 5.33 | Vacuole |
| TEA007474 | GH18 | III | Scaffold1936: 113,649–114,533 | Positive | 1–21 | 0 | 294 | 31,216.59 | 6.8 | Vacuole |
| TEA026337 | GH18 | III | Scaffold1492: 1,339,465–1,342,275 | Positive | No | 1 | 286 | 30,500.17 | 5.17 | Vacuole |
| TEA026342 | GH18 | III | Scaffold1492: 1,321,896–1,333,320 | Positive | No | 1 | 307 | 32,716.7 | 4.9 | Vacuole |
| TEA002526 | GH18 | III | Scaffold1379: 756,280–757,188 | Negative | 1–27 | 0 | 302 | 32,560.66 | 8.42 | Vacuole |
| TEA008102 | GH18 | III | Scaffold979: 2,477,988–2,478,896 | Positive | 1–28 | 0 | 302 | 32,736.95 | 4.83 | Vacuole |
| TEA018432 | GH18 | V | Scaffold2844: 1,066,287–1,072,356 | Negative | No | 2 | 216 | 23,799.82 | 6.04 | Vacuole |
| TEA025942 | GH18 | V | Scaffold601: 111,028–111,945 | Negative | 1–26 | 0 | 305 | 33,984.91 | 4.68 | Vacuole |
| TEA015728 | GH18 | V | Scaffold3698: 607,367–609,821 | Positive | 1–25 | 1 | 317 | 35,222.69 | 6.04 | Vacuole |
| TEA015702 | GH18 | V | Scaffold3698: 632,009–633,001 | Positive | 1–25 | 0 | 330 | 36,838.47 | 6.1 | Vacuole |
| TEA001202 | GH18 | V | Scaffold2608: 101,213–102,803 | Negative | 1–25 | 1 | 297 | 33,007.48 | 5.3 | Vacuole |
| TEA004985 | GH18 | V | Scaffold2420: 396,093–401,252 | Positive | No | 0 | 313 | 35,811.67 | 6.7 | Vacuole |
| TEA015711 | GH18 | V | Scaffold3698: 640,085–640,744 | Positive | 1–25 | 0 | 219 | 24,307.49 | 5.84 | Vacuole |
| TEA023658 | GH18 | III | Scaffold1723: 750–4783 | Negative | 1–30 | 3 | 258 | 27,079.73 | 8.74 | Vacuole |
| TEA033470 | GH19 | I | Scaffold9306: 407,797–409,993 | Negative | 1–19 | 2 | 263 | 28,437.65 | 5.68 | Vacuole |
| TEA033474 | GH19 | I | Scaffold9306: 262,583–264,555 | Negative | 1–19 | 2 | 263 | 28,466.88 | 8.33 | Extracellular |
| TEA019397 | GH19 | I | Scaffold2615: 179,119–183,041 | Positive | 1–20 | 2 | 323 | 34,499.6 | 8.44 | Extracellular |
| TEA031377 | GH19 | I | Scaffold4098: 77,398–78,929 | Positive | 1–24 | 2 | 346 | 37,874.45 | 4.48 | Vacuole |
| TEA020688 | GH19 | I | Scaffold2762: 1,257,364–1,259,482 | Positive | 1–24 | 3 | 410 | 45,185.65 | 4.48 | Extracellular |
| TEA021819 | GH19 | I | Scaffold1713: 1,753,881–1,761,398 | Negative | No | 4 | 477 | 30,020.72 | 4.77 | Extracellular |
| TEA032945 | GH19 | I | Scaffold2938: 866,597–871,448 | Positive | No | 2 | 300 | 32,467.38 | 6.34 | Extracellular |
| TEA031227 | GH19 | II | Scaffold2248: 957,584–961,853 | Negative | 1–24 | 2 | 318 | 34,973.48 | 6.17 | Extracellular |
| TEA003375 | GH19 | II | Scaffold482: 2,215,824–2,218,946 | Positive | 1–22 | 2 | 320 | 35,183.8 | 5.68 | Extracellular |
| TEA022978 | GH19 | II | Scaffold1341: 3,985,577–3,988,923 | Positive | 1–22 | 2 | 320 | 35,195.85 | 5.68 | Extracellular |
| TEA028282 | GH19 | IV | Scaffold140: 706,451–711,515 | Negative | 1–24 | 3 | 328 | 36,090.63 | 9.25 | Cell wall/extracellular |
| TEA028279 | GH19 | IV | Scaffold140: 719,044–721,168 | Negative | 1–27 | 1 | 274 | 29,389.76 | 4.57 | Extracellular |
| TEA014412 | GH19 | IV | Scaffold41: 3,519,091–3,529,318 | Positive | 1–24 | 3 | 294 | 32,505.62 | 9.1 | Cell wall/extracellular |
| TEA031484 | GH19 | IV | Scaffold3112: 948,687–949,837 | Negative | No | 2 | 199 | 21,755.32 | 5.86 | Cell wall/extracellular |
| TEA018894 | GH19 | IV | Scaffold7100: 421,549–426,281 | Positive | 1–29 | 5 | 382 | 41,038.43 | 6.85 | Extracellular |
| TEA007244 | GH19 | IV | Scaffold3038: 1,131,354–1,132,544 | Negative | 1–27 | 1 | 270 | 28,549.9 | 4.73 | Cell wall/extracellular |
| TEA019135 | GH19 | IV | Scaffold1695: 5,054,879–5,056,202 | Positive | 1–27 | 1 | 270 | 28,962.48 | 4.96 | Cell wall/extracellular |
| TEA019594 | GH19 | I | Scaffold8657: 223,024–232,651 | Negative | No | 9 | 499 | 54,654.79 | 8.74 | Chloroplast |
| TEA007233 | GH19 | IV | Scaffold3038: 1,140,217–1,150,864 | Negative | 1–30 | 5 | 428 | 46,553.94 | 8.2 | Cell wall/extracellular |
| TEA018892 | GH19 | IV | Scaffold7100: 368,498–384,785 | Positive | 1–30 | 1 | 238 | 25,081.04 | 8.58 | Cell wall/extracellular |
| TEA023307 | GH19 | IV | Scaffold9042: 326,429–327,852 | Positive | 1–29 | 1 | 271 | 28,827.92 | 8.36 | Cell wall/extracellular |
| TEA020672 | GH19 | I | Scaffold1792: 714,685–716,337 | Negative | 1–21 | 3 | 140 | 15,620.02 | 4.84 | Extracellular |
| TEA027403 | GH19 | I | Scaffold844: 3,110,165–3,125,005 | Positive | No | 12 | 420 | 46,283.43 | 7.11 | Peroxisome |
Fig. 1.
Gene structure of predicted GH18 (a) and GH19 (b) chitinases of tea
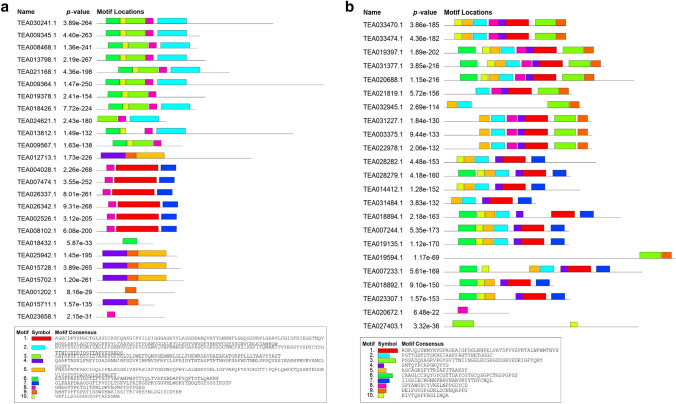
In order to evaluate the divergence among the chitinase genes, we have predicted conserved motifs and their distribution pattern among the genes. The existence of similar motifs and their identical distribution pattern among same classes of chitinases support the sequence similarity between members of same classes as well as their divergence from other classes of chitinases. The GH19 (Fig. 2b) chitinases contain more number of motifs in their sequences as compared to GH18 (Fig. 2a). The class III chitinases showed similar motif composition consisting of motifs 1, 7 and 8 except for TEA023658 which lacked motifs 1 and 7. TEA018432 had only one motif unlike the other members of class V, which is well justified by the fact that it is one of the shortest proteins among all the chitinases. However, no motifs were found in TEA004985. Among the GH19 chitinases, motif 1 is seen to be present in all of the proteins except four class I chitinases (TEA032945, TEA019594, TEA020672 and TEA027403) (Fig. 2b). The signal peptide prediction showed that not all chitinases contain signal peptides in their sequence (Table 1). Out of all 49 chitinases, 13 showed absence of signal peptide, out of which 6 were from class V, 4 from class I, 2 from class III and 1 from class IV. All the GH18 chitinases were predicted to localize in vacuoles although most of the GH19 chitinases were predicted to localize extracellularly (Table 1).
Fig. 2.
Prediction of different motifs by MEME in the GH18 (a) and GH19 (b) chitinases
Phylogenetic analysis of the predicted chitinases of tea
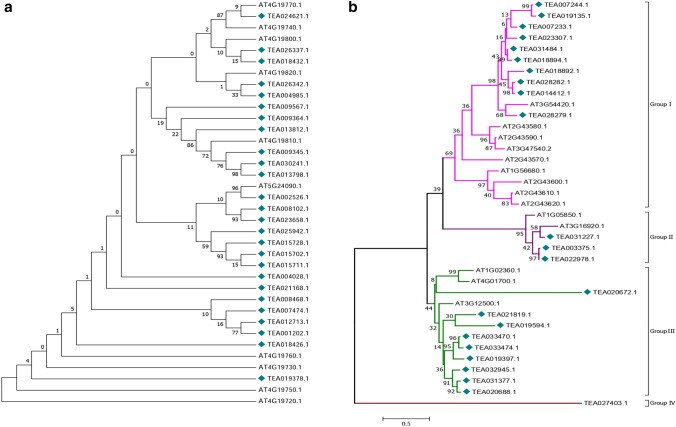
For better visualization and to avoid complexity, two separate phylogenetic trees each for GH18 (Fig. 3a) and GH19 (Fig. 3b) tea chitinases were constructed. As expected in the GH19 category, all class IV chitinases of tea formed one clade (Group I) along with the class IV chitinases of Arabidopsis. The three class II chitinases of tea formed a different group (Group II) along with one Arabidopsis class II chitinase (AT1G05850). The class I chitinases formed a 3rd group (Group III) with the single class I chitinase of Arabidopsis and the other two class II chitinases of Arabidopsis. This indistinct separation of class I and II chitinases of Arabidopsis and tea is in line with the hypothesis suggested by Hamel et al. (1997), which states that class II chitinases might have been derived from class I chitinases. TEA027403, a class I chitinase formed a separate clade (Group IV) suggesting its greater level of divergence from the other chitinases. However in the GH18 category no specific grouping could be done in the phylogenetic tree. The class III and V chitinases showed indistinct separation into clades, which might suggest that their evolutionary divergence is not class specific.
Fig. 3.
Phylogenetic tree of GH18 (a) and GH19 (b) tea chitinases with GH18 and GH19 chitinases of Arabidopsis
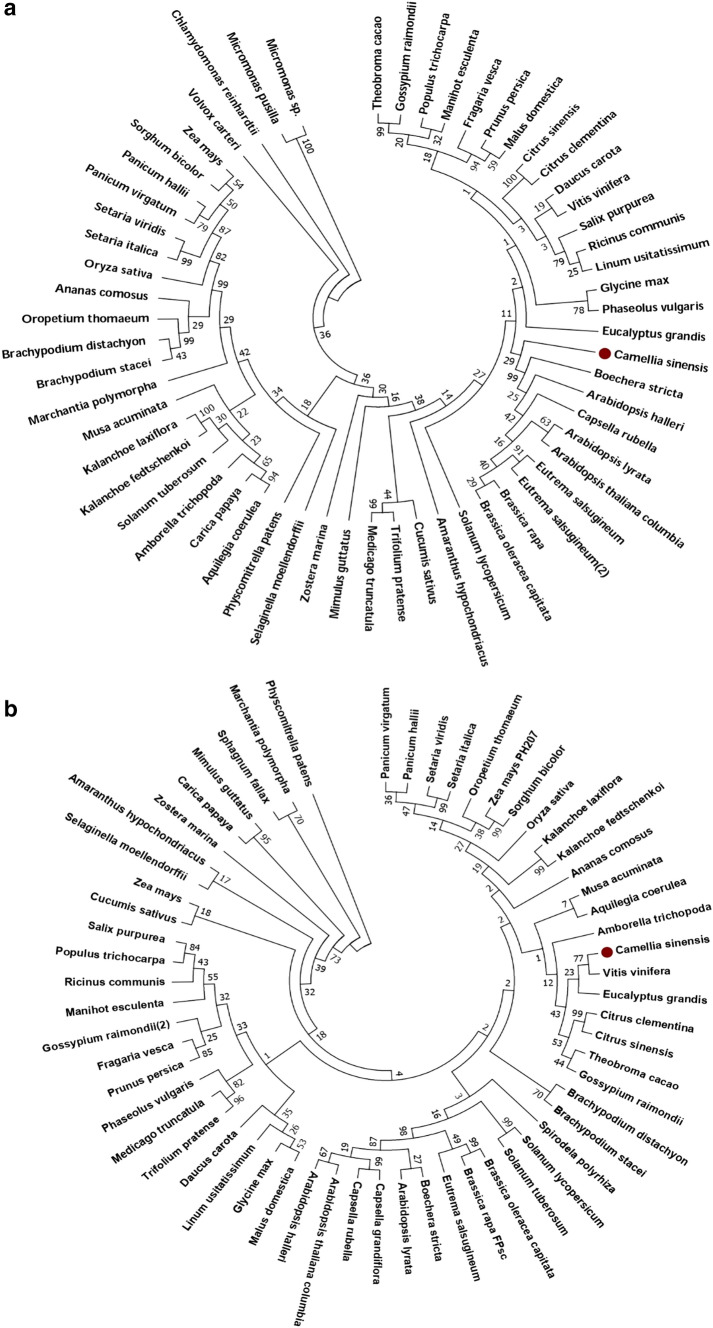
In order to estimate the phylogenetic relationship of the tea chitinases with other plant species, two phylogenetic trees were constructed each for GH18 (Fig. 4a) and GH19 (Fig. 4b) tea chitinases with their orthologs. For this we performed a BLAST search against all the 64 plant species of the Phytozome database, keeping one sequence each for GH18 (TEA030241) and GH19 (TEA019397) as query. The first hit for each plant species was selected for further analysis (Supplementary file S4.). The GH18 tea chitinase formed one clade with single branch and clustered together with its sister clade consisting of Boechera stricta, Arabidopsis thaliana var Columbia, Arabidopsis lyrata, Eutrema salsugineum, Brassica rapa, Brassica oleracea var Capitata and Capsella rubella. The GH19 chitinase of tea formed one small clade with Vitis vinifera and clustered together to form a bigger clade with those of Eucalyptus grandis, Citrus clementina, Citrus sinensis, Theobroma cacao, Gossypium raimondii, Musa acuminata, Aquilegia coerulea and Amborella trichopoda.
Fig. 4.
Evolutionary relationship of tea chitinase genes GH18 (a) and GH19 (b) with their orthologs in phytozome database
Calculation of divergence time and ratio of non-synonymous to synonymous substitution rates of tea chitinases with their orthologs in Arabidopsis
For estimating the divergence of chitinases of tea, we calculated the ratio of non-synonymous substitutions per site (Ka) to synonymous substitutions per site (Ks) for each pair of genes i.e., a chitinase with its ortholog in Arabidopsis which provided an idea about the selection pressure upon the gene pairs. We found that the Ka/Ks ratio was < 1 for all the gene pairs except TEA025942/AT4G19810 and TEA015702/AT4G19730, which suggests that the two gene pairs are under positive selection and other chitinase genes are under purifying process. The average divergence time of tea chitinases was found to be approximately 1.6 × 103 million years ago (Supplementary file S5).
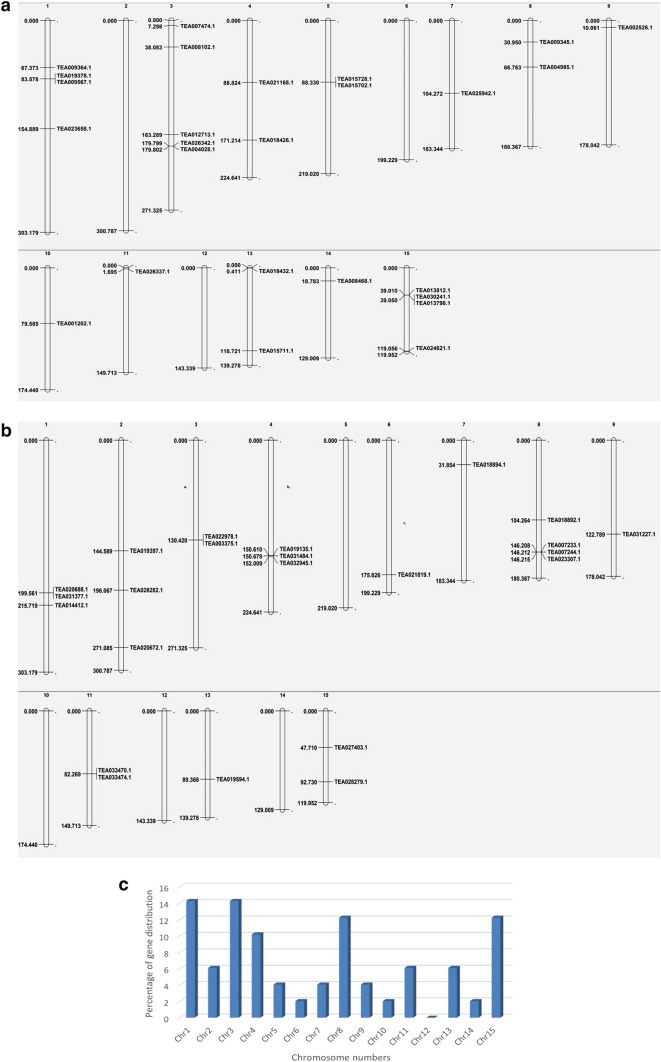
Chromosomal distribution of chitinases in the tea genome
All the 49 predicted chitinases were physically mapped to the tea chromosomes except chromosome number 12. Among all, chromosome number 1 and 3 contain the highest number of chitinases (14.28%), while chromosomes 6, 10 and 14 showed minimum gene distribution (2.04%) (Fig. 5). The distribution and density of the genes on the chromosomes are not uniform. Also, the results showed no correlation between length of chromosomes and chitinase gene distribution.
Fig. 5.
Distribution of chitinase genes on fifteen tea chromosomes. a Localization of GH18 chitinases on tea chromosomes, b localization of GH19 chitinases on tea chromosomes, c percentage of chitinase genes on tea chromosomes to show their distribution abundance. Chromosomal distances are given in Mbp
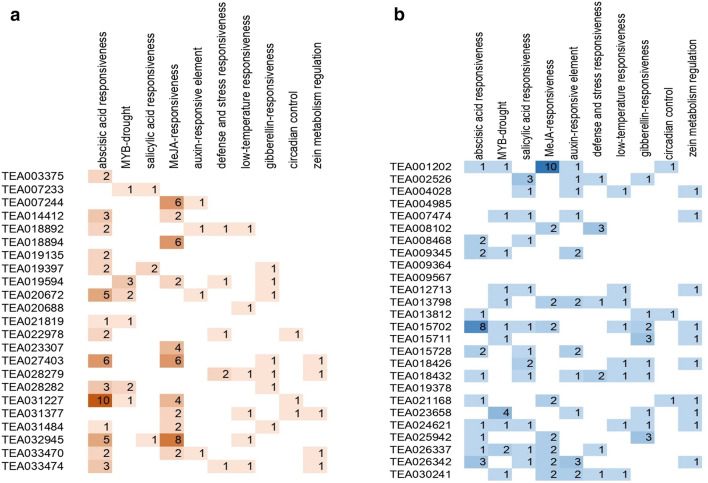
Prediction of cis-acting elements in the gene upstream regions
In order to determine the cis-acting elements that may regulate the expression patterns of tea chitinase genes, we extracted the sequences lying 1500 bp upstream of the chitinase genes and analyzed using the PlantCARE database. Cis-acting elements with more than 17 different functions have been found in upstream regions of predicted GH18 and GH19 tea chitinases. The elements were found to be involved in different abiotic, biotic as well as hormonal responses. Abiotic stress responsive elements were those involved in light, drought, and temperature responses. Many of them were also involved in methyl-jasmonate (Me-JA), salicylic acid (SA) and defense responses along with other hormonal responses like those of auxin, gibberellin, and abscisic acid. A few of them served as binding sites for MYB transcription factors for light and drought responses. As jasmonic acid and salicylic acid are involved in plant defense against biotic stresses (Caarls et al. 2017), presence of Me-JA, SA and defense and stress responsive elements in the promoters of these chitinases may suggest their potential involvement in biotic stress response in tea. Out of the 49 chitinases, 32 chitinases were found to contain at least one of the Me-JA, SA or defense and stress responsive elements in the upstream regions of their sequences (Fig. 6a and b). A class III chitinase, TEA026337 contains all of the three above-mentioned responsive elements. TEA001202 has the highest number (10) of MeJA responsive elements in its upstream of promoter region followed by TEA032945 (8) and TEA007244 (6). Other defense and stress responsive elements were found to occur in the upstream regions of many chitinases including 7 GH18 and 5 GH19 genes.
Fig. 6.
Figure showing frequency of occurrence of cis acting elements in the upstream of promoter sequences of chitinases under the functions (rows) specified a GH19 chitinases, b GH18 chitinases
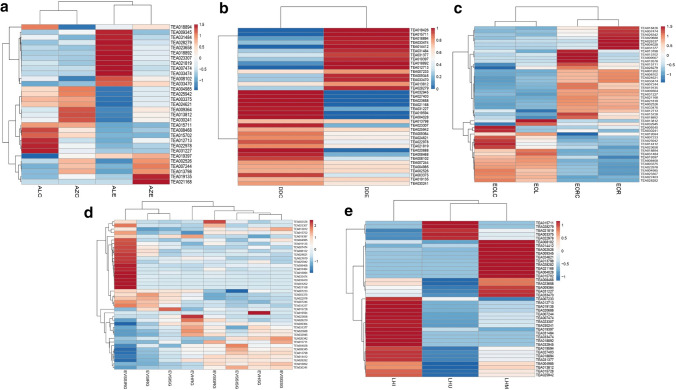
Differential expression analysis of chitinase genes in different biotic stress conditions
Gene expression analysis for the predicted 49 chitinase genes was carried out in five different biotic stress conditions in the tea plant as mentioned earlier. The chitinases showed diverse expression profiles among different experimental conditions and a number of differentially expressed transcripts were identified. TEA028279 displayed elevated expression in the C. gloeosporioides (Fig. 7a), D. segeticola (Fig. 7b) and E. vexans (Fig. 7d) infected tea tissues. This may imply that TEA028279 may be involved in fungal stress tolerance in the tea plant. In addition to this, upregulated expression of TEA028279 was observed in leaves infested by insect E. oblique (Fig. 7c). TEA031377 was upregulated in both E. oblique (Fig. 7c) and leafhopper infested tissues (Fig. 7e). TEA019397 showed a higher expression level in the C. gloeosporioides and D. segeticola infected tea samples. Expression levels of TEA019397 was also increased in the tea plant infected with E. vexans. TEA023307 was significantly downregulated in D. segeticola and E. oblique infected tea plants, while its increase in expression levels was observed in the other three conditions of stresses. Enhancement in transcript levels of TEA033470 and TEA033474 was seen in the leaf tissues treated with E. vexans and also in the leaves with anthracnose disease. TEA031484 showed increased expression in D. segeticola and E. oblique treated plants and in E. vexans resistant cultivar. Expression of TEA002526 was also increased in response to D. segeticola, E. oblique and leafhopper stressed plants. Moreover, expression levels of TEA018892 was increased in the susceptible variety infected with E. vexans, and also in the C. gloeosporioides and leafhopper challenged tea plants.
Fig. 7.
Expression profiling of tea chitinase genes in a C. gloeosporioides infected, b D. segeticola infected, c E. oblique infested, d E. vexans infected and e Leafhopper infested tea plants. Sample abbreviations: ALC: Anthracnose “Longjing43” variety control, AZC: Anthracnose “Zhenong 139” variety control, ALE: Anthracnose “Longjing 43” variety infected, AZE: Anthracnose “Zhenong 139” variety infected, DDE: D. segeticola infected tissue, DDC: D. segeticola control tissue, EOLC: E. oblique leaf control, EORC: E. oblique root control, EOL: E. oblique leaf infested, EOR: E. oblique root infested, EVSIRG: E. vexans spore inoculated tissue in resistant genotype, EVSISG: E. vexans spore inoculated tissue in susceptible genotype, EVSGRG: E. vexans spore germinated tissue in resistant genotype, EVSGSG: E. vexans spore germinated tissue in susceptible genotype, EVHRG: E. vexans haustorial development in resistant genotype, EVHSG: E. vexans haustorial development in susceptible genotype, EVSIRG: E. vexans sporulation and secondary infection in resistant genotype, EVSSISG: E. vexans sporulation and secondary infection in susceptible genotype, LHU: Leafhopper uninfested tissue, LHI: Leafhopper infested tissue, LHM: Leafhopper non-infested but mechanically wounded tissue
Expression of TEA003375 and TEA022978 was considerably reduced in the leafhopper infested and mechanically wounded tea samples as well as in tea geometrid infested tea leaf samples. Also, transcripts of TEA003375 and TEA022978 accumulated less in the C. gloeosporioides infected tea tissues of the Zhenong variety as compared to that of the control. It is worth mentioning that both of TEA003375 and TEA022978 are GH19 domain containing class II chitinase enzymes and they show similar physicochemical properties (Table 1). TEA031227 exhibited considerably less transcript accumulation in C. gloeosporioides infected tea tissues of both Longjing and Zhenong tea varieties and also in the geometrid infested experiments. Significant reduction in expression levels of TEA021819 was observed in the E. vexans susceptible tea varieties as compared to the resistant ones during different stages of pathogen invasion in the host. TEA021819 was also substantially downregulated in the mechanically wounded samples of the leafhopper experiment. In the E. oblique infested and D. segeticola infected tea plants, expression of TEA030241 was markedly reduced than that of the untreated plants. The differential expression results have been tabulated (Supplementary file S6.).
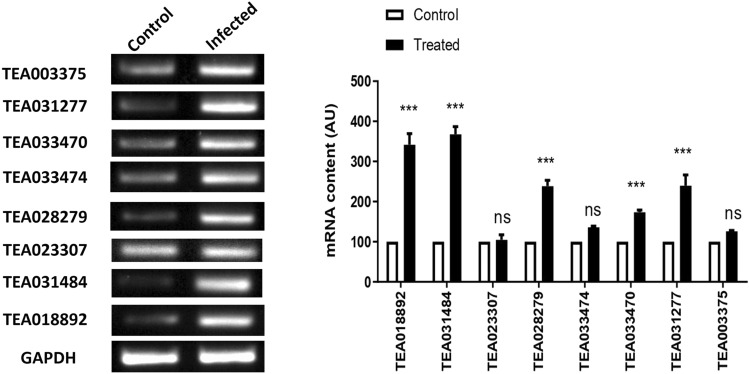
Semi-quantitative RT-PCR analysis of chitinase genes in the tea samples
Typical symptoms of blister blight disease were developed in the E.vexans inoculated leaves (Fig. 8). Nine chitinases (two class I, two class II and five class IV) were selected for semi-quantitative RT-PCR analysis in order to validate the differential expression data obtained from SRA data analysis. The results of semi-quantitative RT-PCR in samples (Fig. 9) are in line with the expression studies carried out from public datasets mentioned in previous section. However, we were unable to amplify TEA028282 in the samples analyzed. Though the exact reason for this could not be ascertained, but this might happen because of the use of different tea cultivar for the validation purpose. Expression of TEA018892, TEA031484, TEA028279, TEA033470 and TEA031227 genes were found to be significantly increased in infected samples compared to the control tissues (Fig. 7). TEA018892 and TEA031484 showed approximately 3.5 folds increase in expression in the infected tissues when compared with the healthy tissues. In the in silico expression analysis, we have seen that transcript accumulation of TEA018892 was upregulated to 2–5 folds in the susceptible genotype and TEA031484 showed 2–4 folds of upregulation in the resistant genotype. In semi-quantitative RT-PCR experiment TEA028279 and TEA031227 exhibited upregulation in the E. vexans infected samples whereas in in silico expression analysis both of these genes showed high levels of expression during late stage of pathogen invasion in the susceptible genotype. Expression of TEA033470 was upregulated in the E. vexans infected tissues as compared to the control. The change in expression of TEA023307, TEA033474 and TEA003375 in the E. vexans infected tissues were statistically insignificant. The statistical results can be found in the supplementary files (S7(a) and S7(b)).
Fig. 9.
The gel image and bar diagram represent the semi-quantitative PCR amplification of the selected chitinase genes in the control (uninfected) and E. vexans challenged tea tissues. The bars in the figure indicates mean ± SEM, n = 3. The p-values are from a 2 way ANOVA test, *** represents p < 0.0001, ns = not significant at p < 0.0001
Discussion
Tea plant is inevitably subjected to a number of pathogen attacks and diseases. Tea plant is a preferred host for many viral, bacterial, fungal pathogens and several insect pests which limits yield and quality. More than 300 species of fungus are found to infect tea plant. However, tea plants also have evolved defense mechanisms to counteract infections caused by pathogens (Mukhopadhyay et al. 2016). As mentioned earlier, chitinases are responsible for the degradation of chitin which is an important constituent of fungal cell wall and insect exoskeleton. Thus our basic objectives were to perform a genome-wide identification of tea chitinases, their characterization and expression analysis during biotic stress conditions.
Our study identified a total of 49 chitinases in the tea plant. In comparison with other plant species, the number of chitinases identified in tea was higher than those of previously identified chitinases in Solanum lycopersicum (43), Oryza sativa (37), Arabidopsis (24), Populus trichocarpa (37), Ammopiptanthus nanus (32) and Hevea brasiliensis (39) (Jiang et al. 2013; Misra 2015; Cao and Tan 2019). This may be due to the fact that tea being a monoculture crop has encountered a range of pests and pathogens, which might exert selection pressure to evolve more defense genes in tea than other plant species. However, in Eucalyptus grandis 67 chitinases were reported (Tobias et al. 2017). Out of the total 49 chitinases, 26 chitinases belong to the GH18 subfamily and 23 to the GH19 subfamily. Among them, 10, 3, 7, 10 and 19 chitinases were assigned to classes I, II, III, IV and V chitinases respectively. Previous studies in P. trichocarpa, A. nanus and H. brasiliensis showed that class III chitinases were significantly higher in number than the other classes. Surprisingly, in contrast to the previous studies, class V chitinases in tea were found to be more in number than those of previously studied plant species. Class V chitinases are reported to contain a C-terminal extension which is meant for vacuolar targeting and may contain chitin binding domain (Heitz et al. 1994). It is noteworthy that none of the class V chitinases of tea contain chitin binding domain. Presence of chitin binding domain in the Class I and IV chitinases is an important feature (Passarinho and Vries 2002), however, our study showed that chitin binding domains were missing in 7 class I and 4 class IV chitinases. Unlike the presence of chitin binding domain in class V chitinases of A. nanus (Cao et al. 2019), no chitin binding domains were present in class V chitinases of tea.
Interestingly, it was seen that there is an absence of introns in 46% of the GH18 chitinases as predicted from GSDS analysis. In contrast, presence of at least one intron in all of the GH19 chitinases has been found. The average number of introns in the chitinases of tea (2.18) was relatively lower as compared to previously identified chitinases of A. nanus (2.93) and Arabidopsis (4.38) (Jeffares et al. 2008; Cao et al. 2019). Loss of introns is an evolutionary phenomenon where genes exposed to stresses have a tendency to lose its introns in the long evolutionary run (Jeffares et al. 2008). Higher gene expression and presence of one/no introns in class V chitinase TEA021168 and class III chitinase TEA002526 may provide an evidence supporting the hypothesis.
Three class V chitinases viz. TEA025942, TEA015702 and TEA15728 are observed to form a single clade in the phylogenetic tree. This observation has been supported by domain analysis that has shown that their catalytic domains resemble the narbonin proteins which are the storage proteins found in seeds of Vicia narbonensis. Narbonin proteins are physiochemically similar to concanavalin B, which are close relatives of GH18 chitinases that have lost their enzymatic activity but retained their carbohydrate binding affinity (Nong et al. 1995; Passarinho and Vries 2002). The similar motif composition of the class I, II and IV chitinases provide evidence to the hypothesis that class I and IV chitinases have evolved from the class II chitinases and in the process of evolution, have gained the chitin binding domain (Araki and Torikata 1995).
The tea chitinases in different biotic stress conditions exhibit similarity in expression within same classes or subfamilies. The class IV chitinases form a single clade in the phylogenetic tree along with one Arabidopsis class IV chitinase (AT3G54420). Eight of the class IV tea chitinases viz. TEA007244, TEA019135, TEA023307, TEA031484, TEA018894, TEA018892, TEA028279 and TEA028282 of the clade display upregulation in their gene expression patterns in stressed tissues to many folds. Two of the class I chitinases TEA033470 and TEA033474 clustered into a small clade within the major clade of class I members and their expression was induced in tea tissues infected by C. gloeosporioides. TEA003375 and TEA022978 exhibiting similar physicochemical properties and gene expression patterns are also clustered in the same clade in the phylogenetic tree (Fig. 4b). These might suggest that evolutionarily related genes show less difference in their expression. It is noteworthy that majority of the class IV members were significantly upregulated during stress in the tea plant. This study suggests that class IV chitinases may be considered as potential candidates for future genetic manipulation experiments of tea.
Chitinases in plants have long been known to inhibit pathogen growth and spread which is supported by the fact that chitin is absent in plants and present in fungal cell walls (Bartnicki-Garcia 1968; Abeles et al. 1970). Since chitinases have the ability to hydrolyze chitin and to switch on the immune responses of the plant, it is expected to find stress responsive cis acting elements in the regions upstream of their promoter sequences. Salicylic acid (SA) is an indicator of stress and a well-known regulator of PR proteins induced in response to insects, bacterial, fungal and other biotrophic pathogens in plants (Broekgaarden et al. 2015; Xu et al. 2018). About 12 chitinases showed the presence of at least one SA-responsive cis acting element in the upstream regions of the genes. Jasmonic acid (JA) is another stress regulator that plays undeniable role in defense responses in plants against necrotrophic pathogens, especially insects (Woldemariam et al. 2018). Methyl jasmonate and other derivatives of JA accumulates in plants during feeding of sap sucking herbivores and insects (Walters 2011). A total of 18 (36%) chitinases contained at least one Me-JA responsive cis acting element in the region upstream of the gene. Overexpression of a class I chitinase gene (Singh et al. 2015), endo-1,3-beta-D-glucanase (Singh et al. 2018) and defensin gene (Singh et al. 2019) was seen to induce resistance against the biotrophic pathogen E. vexans in transgenic tea plants. β-1,3-glucanases are shown to provide resistance against a range of fungal pathogens in a number of transgenic plants (Sundaresha et al. 2010; Mondal et al. 2007; Mackintosh et al. 2007; Amian et al. 2011).
In conclusion, our study shows that many chitinases were induced in response to fungal infection and insect infestation in tea plant and hence it can be considered that chitinases are important for eliciting defense responses in tea plant. However, further research and validations are necessary to explicate the exact roles and mechanism of action of the genes and how they are regulated. To identify exactly what factors are responsible for regulating expression of stress-induced chitinases needs further investigation. Effect of transgenes on endogenous genes must be evaluated while opting transgenic approach for developing pathogen resistant plants.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to acknowledge Dr. Biswajit Bera, Director (Research), Tea Board of India for the valuable suggestions to conduct this study. We are thankful to DTRDC, Kurseong authority for providing tea saplings to conduct the experiments. We are thankful to DST, Govt. of India for providing DST-FIST support to the Department of Botany, Gauhati University, where this research work was carried out.
Funding
The research work was funded by ECR award granted to Niraj Agarwala by Science and Engineering Research Board (SERB), Govt. of India (ECR 000710/2017). This research work was partially supported by The Council of Scientific and Industrial Research (CSIR) and DST (Department of Science and Technology).
Data availability
All the SRA data samples were downloaded from NCBI SRA database (https://www.ncbi.nlm.nih.gov/sra/). The chitinase gene sequences of tea were downloaded from Tea Plant Information Archive (TPIA) database (http://tpia.teaplant.org/). The chitinase protein sequences of Arabidopsis were downloaded from The Arabidopsis Information Resource (TAIR) (https://www.arabidopsis.org/).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-021-00947-x.
References
- Abeles FB, Bosshart RP, Forrence LE, Habig WH. Preparation and purification of glucanase and chitinase from bean leaves. Plant Physiol. 1970;47:129–134. doi: 10.1104/pp.47.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnihothrudu V. Notes on fungi from north-east India—XXII some species of hypoxylon from Assam. Mycopathologia et Mycologia Applicata. 1964;23:111–117. doi: 10.1007/BF02049265. [DOI] [PubMed] [Google Scholar]
- Amian AA, Papenbrock J, Jacobsen HJ, Hassan F. Enhancing transgenic pea (Pisum sativum L.) resistance against fungal diseases through stacking of two antifungal genes (Chitinase and Glucanase) GM Crops. 2011;2:104–109. doi: 10.4161/gmcr.2.2.16125. [DOI] [PubMed] [Google Scholar]
- Anders S, Huber W. Differential expression analysis for sequence countdata. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki T, Torikata T. Structural classification of plant chitinases: two subclasses in class I and class II chitinases. Biosci Biotechnol Biochem. 1995;59:336–338. doi: 10.1271/bbb.59.336. [DOI] [PubMed] [Google Scholar]
- Baby UI, Ravichandran R, Ganesan V, Parthiban R, Sukumar S. Effect of blister blight disease on the biochemical and quality constituents of green leaf and CTC tea. Trop Agric. 1998;75(4):452–456. [Google Scholar]
- Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Noble WS. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37:W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartnicki-Garcia S. Cell wall chemistry, morphogenesis and taxonomy of fungi. Annu Rev Microbiol. 1968;22:87–108. doi: 10.1146/annurev.mi.22.100168.000511. [DOI] [PubMed] [Google Scholar]
- Bendtsen JD, Nielsen H, Heijne GV, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Bhorali P, Gohain B, Gupta S, et al. Molecular analysis and expression profiling of blister blight defense related genes in tea. Indian J Genet Plant Breed. 2012;72:226–233. [Google Scholar]
- Borah AK, Singh A, Yasmin R, et al. 1α, 25-dihydroxy Vitamin D3 containing fractions of Catharanthus roseus leaf aqueous extract inhibit preadipocyte differentiation and induce lipolysis in 3T3-L1 cells. BMC Complement Altern Med. 2019;19:338. doi: 10.1186/s12906-019-2754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchetia S, Handique G, Roy S, Wani SH (2018) Genomics approaches for biotic and abiotic stress improvement in tea. In: Stress physiology of tea in the face of climate change, pp 289–312
- Bordoloi KS, Dihingia P, Krishnatreya DB, Agarwala N. Genome-wide identification, characterization and expression analysis of the expansin gene family under drought stress in tea (Camellia sinensis L.) Plant Sci Today. 2021;8(1):32–44. [Google Scholar]
- Broekgaarden C, Caarls L, Vos IA, Pieterse CMJ, Wees SCMV. Ethylene: traffic controller on hormonal crossroads to defense. Plant Physiol. 2015;169:2371–2379. doi: 10.1104/pp.15.01020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caarls L, Does DVD, Hickman R, Jansen W, Verk MCV, Proietti S, Lorenzo O, Solano R, Pieterse CMJ, Wees SCMV. Assessing the role of ethylene response factor transcriptional repressors in salicylic acid-mediated suppression of Jasmonic acid-responsive genes. Plant Cell Physiol. 2017;58(2):266–278. doi: 10.1093/pcp/pcw187. [DOI] [PubMed] [Google Scholar]
- Cao J, Tan X. Comprehensive analysis of the chitinase family genes in tomato (Solanumlycopersicum) Plants. 2019;8:52. doi: 10.3390/plants8030052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S, Wang Y, Li Z, Shi X, Gao F, Zhou Y, Zhang G, Feng J. Genome-wide identification and expression analyses of the chitinases under cold and osmotic stress in Ammopiptanthus nanus. Genes. 2019;10:472. doi: 10.3390/genes10060472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZM, Chen XF. The diagnosis of tea diseases and their control. Shanghai: Shanghai Scientific and Technical Publishers; 1990. [Google Scholar]
- Chen J, Piao Y, Liu Y, Li X, Piao Z. Genome-wide identification and expression analysis of chitinase gene family in Brassica rapa reveals its role in clubroot resistance. Plant Sci. 2018;270:257–267. doi: 10.1016/j.plantsci.2018.02.017. [DOI] [PubMed] [Google Scholar]
- Collinge DB, Kragh KM, Mikkelsen JD, Nielsen KK, Rasmussen U, Vad K. Plant chitinases. Plant J. 1993;3:31–40. doi: 10.1046/j.1365-313x.1993.t01-1-00999.x. [DOI] [PubMed] [Google Scholar]
- Dana M, Pintor-Toro JA, Cubero B. Transgenic tobacco plants overexpressing chitinases of fungal origin show enhanced resistance to biotic and abiotic stress agents. Plant Physiol. 2006;142:722–730. doi: 10.1104/pp.106.086140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Gebali S, Mistry J, Bateman A, Eddy SR, Luciani A, Potter SC, Qureshi M, Richardson LJ, Salazar GA, Smart A, et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2019;47:D427–D432. doi: 10.1093/nar/gky995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RD, Clements J, Eddy SR. HMMER web server: interactive sequence similarity searching. Nucleic Acids Res. 2011;39:W29–W37. doi: 10.1093/nar/gkr367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, Bairoch A. Protein identification and analysis tools on the ExPASy server. In: Walker JM, Totowa NJ, editors. The proteomics protocols handbook. Totowa: Humana Press; 2005. [Google Scholar]
- Gohain B, Borchetia S, Bhorali P, Agarwal N, Bhuyan LP, Rahman A, Sakata K, Mizutani M, Shimizu B, Gurusubramaniam G, Ravindranath R, Kalita M, Hazarika M, Das S. Understanding Darjeeling tea flavour on a molecular basis. Plant Mol Biol. 2012;78(6):577–597. doi: 10.1007/s11103-012-9887-0. [DOI] [PubMed] [Google Scholar]
- Goodstein DM, Shu SQ, Howson R, Neupane R, Hayes RD, Fazo J, et al. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 2012;40:D1178–D1186. doi: 10.1093/nar/gkr944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover A. Plant chitinases: genetic diversity and physiological roles. Crit Rev Plant Sci. 2012;31:57–73. [Google Scholar]
- Hamel F, Boivin R, Tremblay C, Bellemare G. Structural and evolutionary relationships among chitinases of flowering plants. J Mol Evol. 1997;44:614–624. doi: 10.1007/pl00006184. [DOI] [PubMed] [Google Scholar]
- Heitz T, Segond S, Kauffmann S, Geoffroy P, Prasad V, Brunner F, Fritig B, Legrand M. Molecular characterization of a novel tobacco pathogenesis-related (PR) protein: a new plant chitinase/lysozyme. Mol Gen Genet. 1994;245:246–254. doi: 10.1007/BF00283273. [DOI] [PubMed] [Google Scholar]
- Higo K, Ugawa Y, Iwamoto M, Korenaga T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999;27:297–300. doi: 10.1093/nar/27.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Jin J, Guo AY, Zhang H, Luo J, Gao G. GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics. 2015;31:1296–1297. doi: 10.1093/bioinformatics/btu817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaswall K, Mahajan P, Singh G, et al. Transcriptome analysis reveals candidate genes involved in blister blight defense in tea (Camellia sinensis (L) Kuntze) Sci Rep. 2016;6:1–14. doi: 10.1038/srep30412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffares DC, Penkett CJ, Bähler J. Rapidly regulated genes are intron poor. Trends Genet. 2008;24:375–378. doi: 10.1016/j.tig.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Jiang C, Huang RF, Song JL, Huang MR, Xu LA. Genome wide analysis of the chitinase gene family in Populus trichocarpa. J Genet. 2013;92:121–125. doi: 10.1007/s12041-013-0222-6. [DOI] [PubMed] [Google Scholar]
- Kasprzewska A. Plant chitinases-regulation and function. Cell Mol Biol Lett. 2003;8:809–824. [PubMed] [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35(6):1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouzé P, Rombauts S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30:325–327. doi: 10.1093/nar/30.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Roseman S. Thechitinolytic cascade in Vibrios is regulated bychitin oligosaccharides and a two-component chitin catabolic sensor/kinase. Proc Natl Acad Sci USA. 2004;101:627–631. doi: 10.1073/pnas.0307645100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh C, Lewis J, Radmer L, Shin S, Heinen S, Smith L, Wyckoff M, Dill-Macky R, Evans C, Kravchenko S, Baldridge G, Zeyen R, Muehlbauer G. Overexpression of defense response genes in transgenic wheat enhances resistance to Fusarium head blight. Plant Cell Rep. 2007;26:479–488. doi: 10.1007/s00299-006-0265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsalu T, Vilo J. ClustVis: a web tool for visualizing clustering of multivariate data using principal component analysis and heatmap. Nucleic Acids Res. 2015;43:W566–W570. doi: 10.1093/nar/gkv468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra BB. Molecular evolution and functional divergence of chitinase gene family in Hevea brasiliensis genome. New York: The Winnower; 2015. [Google Scholar]
- Mondal K, Bhattacharya R, Koundal K, Chatterjee S. Transgenic Indian mustard (Brassica juncea) expressing tomato glucanase leads to arrested growth of Alternaria brassicae. Plant Cell Rep. 2007;26:247–252. doi: 10.1007/s00299-006-0241-3. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay M, Mondal TK, Chand PK. Biotechnological advances in tea (Camellia sinensis [L.] O. Kuntze): a review. Plant Cell Rep. 2016;35(2):255–287. doi: 10.1007/s00299-015-1884-8. [DOI] [PubMed] [Google Scholar]
- Neuhaus JM, Fritig B, Linthorst HJM, Meins F, Mikkelsen JD, Ryals J. A revised nomenclature for chitinase genes. Plant Mol Biol Rep. 1996;14:102–104. [Google Scholar]
- Nicholas KB, Nicholas Jr, HB (1997) GeneDoc: a tool for editing and annotating multiple sequence alignments
- Nong VH, Schlesier B, Bassüner R, Repik A, Hortsmann C, Müntz K. Narbonin, a novel 2S protein from Vicia narbonensis L. seed: cDNA, gene structure and developmentally regulated formation. Plant Mol Biol. 1995;28:61–72. doi: 10.1007/BF00042038. [DOI] [PubMed] [Google Scholar]
- Passarinho PA, de Vries SC. Arabidopsis chitinases: a genomic survey. Arab Book/Am Soc Plant Biol. 2002;1:e0023. doi: 10.1199/tab.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertea M, Kim D, Pertea GM, Leek JT, Salzberg SL. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat Protoc. 2016;11:1650–1667. doi: 10.1038/nprot.2016.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen TN, Brunak S, Von Heijne G, Nielsen H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8:785. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- Roberts WK, Selitrennikoff CP. Plant and bacterial chitinases differ in antifungal activity. J Gen Microbiol. 1988;134:169–176. [Google Scholar]
- Schlumbaum A, Mauch F, Vogeli U, Boller T. Plant chitinases are potent inhibitors of fungal growth. Nature. 1986;324:365–367. [Google Scholar]
- Shi YL, Sheng YY, Cai ZY, et al. Involvement of salicylic acid in anthracnose infection in tea plants revealed by transcriptome profiling. Int J Mol Sci. 2019;20(10):2439. doi: 10.3390/ijms20102439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh HR, Deka M, Das S. Enhanced resistance to blister blight in transgenic tea (Camellia sinensis [L.] O. Kuntze) by overexpression of class I chitinase gene from potato (Solanum tuberosum) Funct Integr Genom. 2015;15:461–480. doi: 10.1007/s10142-015-0436-1. [DOI] [PubMed] [Google Scholar]
- Singh HR, Hazarika P, Agarwala N, et al. Transgenic tea over-expressing Solanum tuberosum endo-1,3-beta-D-glucanase gene conferred resistance against blister blight disease. Plant Mol Biol Rep. 2018;36:107–122. [Google Scholar]
- Singh HR, Hazarika P, Deka M, Das S. Study of Agrobacterium-mediated cotransformation of tea for blister blight disease resistance. J Plant Biochem Biotech. 2019;29:24–35. [Google Scholar]
- Sundaresha S, Manoj Kumar A, Rohini S, Math S, Keshamma E, Chandrashekar S, Udayakumar M. Enhanced protection against two major fungal pathogens of groundnut, Cercospora arachidicola and Aspergillus flavus in transgenic groundnut over-expressing a tobacco β-1,3-glucanase. Eur J Plant Pathol. 2010;126:497–508. [Google Scholar]
- Suyama M, Torrents D, Bork P. PAL2NAL: robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res. 2006;34:W609–W612. doi: 10.1093/nar/gkl315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe T, Kawase T, Watanabe T, Uchida Y, Mitsutomi M. Enzymology and protein engineering purification and characterization of a 49-kDa chitinase from Streptomyces griseus HUT 6037. J Biosci Bioeng. 2000;89:27–32. doi: 10.1016/s1389-1723(00)88046-9. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias PA, Christie N, Naidoo S, Guest DI, Külheim C. Identification of the Eucalyptus grandischitinase gene family and expression characterization under different biotic stress challenges. Tree Physiol. 2017;37:565–582. doi: 10.1093/treephys/tpx010. [DOI] [PubMed] [Google Scholar]
- Voorrips RE. MapChart: software for the graphical presentation of linkage maps and QTLs. J Hered. 2002;93:77–78. doi: 10.1093/jhered/93.1.77. [DOI] [PubMed] [Google Scholar]
- Walters DR (2011) Plant defense-warding off attack by pathogens, herbivores, and parasitic plants
- Wan S, Mak MW, Kung SY. FUEL-mLoc: feature-unified prediction and explanation of multi-localization of cellular proteins in multiple organisms. Bioinformatics. 2017;33:749–750. doi: 10.1093/bioinformatics/btw717. [DOI] [PubMed] [Google Scholar]
- Wang J, Tian N, Huang X, Chen LY, Schlappi M, Xu ZQ. Tall fescue turf grass class I chitinase is activated by fungal elicitors, dehydration ethylene and mechanical wounding. Plant Mol Biol. 2009;27:305–314. [Google Scholar]
- Wei C, Yang H, Wang S, et al. Draft genome sequence of Camellia sinensis var.sinensis provides insights into the evolution of the tea genome and tea quality. PNAS. 2018;115(18):E4151–E4158. doi: 10.1073/pnas.1719622115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldemariam MG, Ahern K, Jander G, Tzin V. A role for 9-lipoxygenases in maize defense against insect herbivory. Plant Signal Behav. 2018;13(1):e1422462. doi: 10.1080/15592324.2017.1422462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia EH, Li FD, Tong W, et al. Tea plant information archive (TPIA): a comprehensive genomics and bioinformatics platform for tea plant. Plant Biotechnol J. 2019;17:1938–1953. doi: 10.1111/pbi.13111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Xu X, Tian L, Wang G, Zhang X, Wang X, Guo W. Discovery and identification of candidate genes from the chitinase gene family for Verticillium dahliae resistance in cotton. Sci Rep. 2016;6:29022. doi: 10.1038/srep29022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HX, Qian LX, Wang XW, Shao RX, Hong Y, Liu SS, Wang XW. A salivary effector enables whitefly to feed on host plants by eliciting salicylic acid-signaling pathway. PNAS. 2018;116(2):490–495. doi: 10.1073/pnas.1714990116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Dong Y, Yu Y, et al. Identification and evaluation of reliable reference genes for quantitative real-time PCR analysis in tea plants under differential biotic stresses. Sci Rep. 2020;10:2429. doi: 10.1038/s41598-020-59168-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Wang Y, Li L, Li F, He Y, Wu J, Wei C. Transcriptomic and phytochemical analyses reveal root-mediated resource-based defense response to leaf herbivory by Ectropis oblique in tea plant (Camellia sinensis) J Agric Food Chem. 2019;67:5465–5476. doi: 10.1021/acs.jafc.9b00195. [DOI] [PubMed] [Google Scholar]
- Yang R, Jiang SL, Li DX, et al. Integrated mRNA and small RNA sequencing for analyzing leaf spot pathogen Didymella segeticola and its host, tea (Camellia sinensis), during infection. Mol Plant Microbe In. 2021;34(1):127–130. doi: 10.1094/MPMI-07-20-0207-A. [DOI] [PubMed] [Google Scholar]
- Zhao X, Chen S, Wang S, Shan W, Wang X, Lin Y, et al. Defensive responses of tea plants (Camellia sinensis) against tea green leafhopper attack: a multi-omics study. Front Plant Sci. 2020;10:1705. doi: 10.3389/fpls.2019.01705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the SRA data samples were downloaded from NCBI SRA database (https://www.ncbi.nlm.nih.gov/sra/). The chitinase gene sequences of tea were downloaded from Tea Plant Information Archive (TPIA) database (http://tpia.teaplant.org/). The chitinase protein sequences of Arabidopsis were downloaded from The Arabidopsis Information Resource (TAIR) (https://www.arabidopsis.org/).