Summary
Ulcerative colitis (UC) is a chronic inflammatory bowel disease, characterized by relapsing and remitting colon mucosal inflammation. For patients suffering from UC, a higher risk of colon cancer has been widely recognized. Here, we found that Elf4−/− mice developed colon tumors with 3 cycles of dextran sulfate sodium salt (DSS) treatment alone. We further showed that ELF4 suppression was prevalent in both patients with UC and DSS-induced mice models, and this suppression was caused by promoter region methylation. ELF4, upon PARylation by PARP1, transcriptionally regulated multiple DNA damage repair machinery components. Consistently, ELF4 deficiency leads to more severe DNA damage both in vitro and in vivo. Oral administration of montmorillonite powder can prevent the reduction of ELF4 in DSS-induced colitis models and lower the risk of colon tumor development during azoxymethane (AOM) and DSS induced colitis-associated cancer (CAC). These data provided additional mechanism of CAC initiation and supported the “epigenetic priming model of tumor initiation”.
Subject areas: Biochemistry, Molecular Biology, Transcriptomics
Graphical abstract

Highlights
-
•
Elf4 expression is suppressed in both colitis and colitis-associated cancer (CAC).
-
•
Elf4 deficiency leads to increased hyper-susceptibility to colitis and CAC in mice
-
•
Elf4 promotes DNA damage repair upon PARylation by PARP1
-
•
Oral administration of montmorillonite lowers risk of CAC development
Biochemistry; Molecular Biology; Transcriptomics
Introduction
Ulcerative colitis (UC) is a subtype of inflammatory bowel disease (IBD). Currently, IBD has become a global disease with accelerating incidence in newly industrialized countries whose societies have become more westernized (Ng et al., 2018). UC is characterized by relapsing and remitting colon mucosal inflammation (Ungaro et al., 2017). It has been known for years that patients with UC face a higher risk of colon cancer (Eaden et al., 2001; Jess et al., 2012), namely, colitis-associated cancer (CAC). It should be noted that CAC is different in many ways from sporadic colorectal cancer, namely, CRC (Grivennikov and Cominelli, 2016). CAC serves as an excellent model for studying relation between inflammation and cancer (Grivennikov, 2013).
ELF4 belongs to the E-Twenty-Six (ETS) domain transcription factor family and is involved in a variety of biological processes, including immune response and cell development (Suico et al., 2017). Previously, we have shown that ELF4 is a critical transcription factor for the host antiviral response, during which ELF4 cooperates with nuclear factor-kB (NF-КB) to induce robust interferons and inflammatory cytokine production (You et al., 2013). A recent report indicates that ELF4 is a suppressor of Th17 polarization and plays a role in experimental autoimmune encephalomyelitis (Lee et al., 2014). UC is a chronic inflammatory colon disease with autoimmune roots (Conrad et al., 2014), and whether ELF4 plays a role in the pathogenesis of UC is unknown.
A hallmark of tumor initiation is the presence of genome alterations (Colotta et al., 2009; Fouad and Aanei, 2017). It is long recognized that in addition to direct genotoxic responses, suppression of DNA repair response is also a significant contributor to the accumulated genome alterations during chronic inflammation (Grivennikov, 2013; Sanford et al., 1997), although the mechanism underneath this observation is unknown. Recently, an additional “epigenetic priming” stage of tumor development is proposed (Vicente-Duenas et al., 2018). In this model, epigenetic priming could occur at the very early stage and reprogram normal cells for genome alteration accumulation. However, evidence supporting this model is largely lacking, and whether epigenetic priming includes suppression of DNA repair response has not been explored.
Here, we showed that suppression of ELF4 predisposes host with UC to CAC. We identified ELF4 as an important element keeping intestinal epithelial genome safe, which upon PARylation by PARP1 transcriptionally regulates multiple DNA damage response (DDR) machinery components in active UC. ELF4 deficiency leads to more severe DNA damages both in vitro and in vivo causing host cells prone to tumorigenesis, while ELF4 is epigenetically suppressed in both patients with CAC and mouse models. We further showed that oral administration of montmorillonite powder, a commonly used intestinal mucosal protective agent, can prevent the reduction of ELF4 in DSS-induced colitis models. Moreover, our retrospective study demonstrated that patients with CAC regularly on montmorillonite treatment in early UC phase showed significantly delayed CAC development. These findings identify ELF4 as a key target of epigenetic priming during colon cancer evolution. Furthermore, our data suggest that montmorillonite powder might serve as an ideal CAC prevention drug for patients with UC.
Results
ELF4 deficiency leads to hyper-susceptibility to CAC
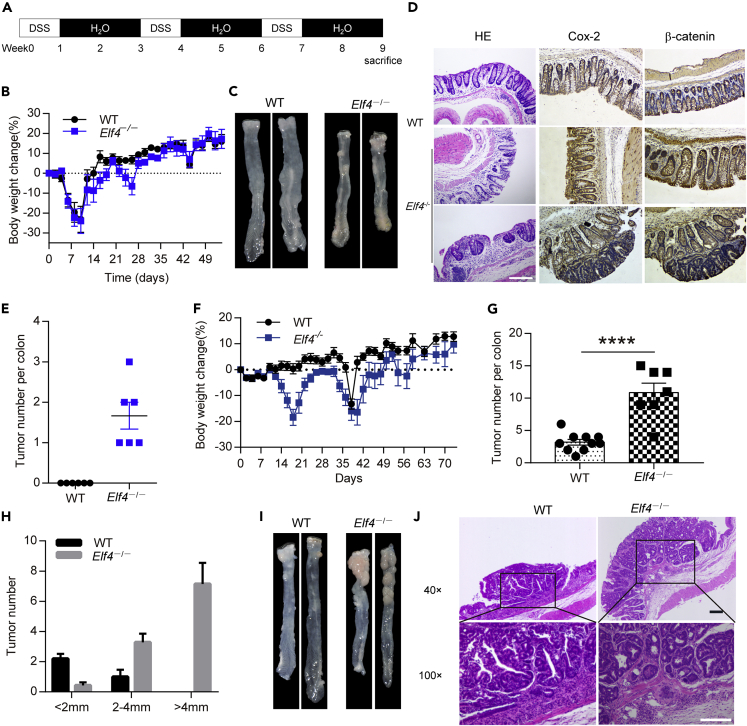
The tissue expression pattern of ELF4 showed the highest expression in the colon of mice (Figures S1A–S1C) and high expression in colon epithelia in humans (Figure S1D) (Uhlen et al., 2005). To examine the role of ELF4 in UC pathogenesis, we induced chronic colitis in wild-type (WT) and Elf4−/− mice with 3 cycles of 1.5% DSS treatment (Figure 1A). The body weight loss showed slight difference (Figure 1B). However, we surprisingly found all of Elf4−/− mice developed tumors in the colon, while WT mice only showed moderate dysplasia (Figures 1C–1E). It is noteworthy that induction of colon tumors by DSS alone usually requires sufficient duration (more than 5 cycles, about 100 days) (Gkouskou et al., 2016; Okayasu et al., 2002). The susceptibility of Elf4−/− mice to colon tumor development was also significant using standard AOM/DSS protocol (Figures 1F–1J), although there was no difference in terms of histopathology for developed tumors compared to WT mice (Figures S1E and S1F). These data indicate that ELF4 plays a pivotal role in the initiation of CAC.
Figure 1.
Elf4-deficient mice are hyper-susceptible to inflammation-driven colorectal cancer
(A) Schematic of DSS administration.
(B) Body weight change of indicated mice treated with low dose DSS (1.5%) protocol. WT, n = 6; Elf4−/−, n = 6, data are represented as mean ± standard error of mean (SEM).
(C) Representative pictures of colon tumors.
(D) Representative H&E-stained, immunohistochemical Cox-2 and β-catenin-stained images of colon sections, scale bar: 200 um.
(E) Number of tumors in the whole colon was counted. WT, n = 6; Elf4−/−, n = 6, data are represented as mean ± standard error of mean (SEM).
(F) Body weight change of indicated mice treated with standard AOM-DSS (1.5%) protocol. WT, n = 10; Elf4−/−, n = 9, data are represented as mean ± standard error of mean (SEM).
(G) Number of tumors in the whole colon was counted. WT, n = 10; Elf4−/−, n = 7. ∗∗∗∗P = 3.64 × 10−5. Unpaired two-sided Student’ s t test, data are represented as mean ± standard error of mean (SEM).
(H) Representative distribution of the average number of tumors per mouse. WT, n = 10; Elf4−/−, n = 7, data are represented as mean ± standard error of mean (SEM).
(I) Representative pictures of colon tumors.
(J) Representative H&E-stained images of colon sections, scale bar: 200 um.
See also Figure S1.
ELF4 suppression is prevalent in patients with UC and DSS-induced mice models
To determine the clinical relevance of ELF4, we collected endoscopic biopsy samples from patients with UC and healthy controls. Interestingly, we detected significant suppression of ELF4 at mRNA level (Figure 2A). To consolidate this finding, we mined the Gene Expression Omnibus database with web-based tool GEO2R (Table S1). We noticed that ELF4 was significantly decreased in the colon mucosa of patients with UC and active inflammation from two independent cohorts (Figures S2A and S2B). This deficiency of ELF4 was also found in non-dysplastic mucosa from patients with UC harboring remote neoplastic lesions (Figure S2C). In addition, we induced acute colitis with DSS in WT mice to mimic UC flare in patients and found similar suppression of ELF4 in mice colon mucosa with active inflammation (Figure 2B). A data set in the Gene Expression Omnibus (GEO) also supported this finding (Figure S2D). More interestingly, the distal colon with dysplasia and intramucosal adenocarcinoma also showed reduction of ELF4 in an AOM/DSS-induced CAC model (Figure S2E), which was further confirmed in our AOM/DSS CAC model (Figures 2C and 2D). Lastly, we found reduction of ELF4 in the cancer tissue of three patients with CAC (Figure 2E). These data suggested that ELF4 is an intriguing factor in inflammation-driven tumorigenesis and of great clinical relevance.
Figure 2.
ELF4 deficiency is prevalent in patients with UC and CAC and mice models
(A) Comparison of ELF4 mRNA level in colon mucosa between 9 healthy donors and 9 patients with active UC. ∗P = 0.0359. Unpaired two-sided Student’ s t test, data are represented as mean ± Standard Error of Mean (SEM).
(B) Comparison of Elf4 mRNA and protein level in colon mucosa between regular water drinking and DSS drinking wild-type mice. D, DSS drinking, n = 10; W, water drinking, n = 8. ∗∗∗∗P = 4.02 × 10−6. Unpaired two-sided Student’ s t test, data are represented as mean ± Standard Error of Mean (SEM).
(C) Comparison of Elf4 mRNA level between tumor adjacent and tumor tissue in wild-type mice treated with standard AOM/DSS protocol. n = 8. Tumor, tumor tissue; Adj, tumor adjacent tissue. ∗∗∗∗P = 2.79 × 10−7, paired Student's t-test.
(D) Comparison of Elf4 protein level between tumor adjacent and tumor tissue in wild-type mice treated with standard AOM/DSS protocol. Tu1, tu2, and tu3 were tumor tissues; adj1, adj2, and adj3 were tumor adjacent tissues.
(E) Comparison of ELF4 protein level between adjacent and cancer tissue in 3 patients with CAC. Tu1, tu2, and tu3 were tumor tissues; adj1, adj2, and adj3 were tumor adjacent tissues.
Intestinal epithelial ELF4 restrains colitis by supporting the function of ILC3s
The frequency and severity of UC flares is closely associated with CAC risk (Ekbom et al., 1990); we then reasoned that CAC predisposition might root in repeated colitis flare. Indeed, Elf4−/− mice showed poor survival (Figure 3A) and more severe body weight loss (Figure 3B) after being treated with an acute dose of DSS. Histologically, Elf4−/− mice showed more leukocytes infiltration, crypt elongation, and loss of goblet cells (Figures 3C and 3D). We previously found that ELF4 is essential for antiviral innate immunity (You et al., 2013). We thus investigated if deficiency of ELF4 in myeloid cells caused colon inflammation. We generated ELF4 conditional deficient mice by crossing Elf4fl/fl mice with Lyz2-Cre mice. Interestingly, Elf4fl/fl-Lyz2-Cre mice showed comparable weight loss and lethality to WT mice after DSS treatment (Figures 3E and 3F). Therefore, Elf4fl/fl-Villin-Cre mice were included to explore whether loss of colon epithelial ELF4 is responsible for increased inflammation (Figure S3A). We found that Elf4fl/fl-Villin-Cre mice showed similar phenotypes with Elf4−/− mice after DSS treatment (Figures 3G and 3H). It suggested that intestinal epithelium ELF4 but not myeloid cell ELF4 restrains colitis.
Figure 3.
Intestinal epithelium Elf4 is critical for multiple colon mucosa protecting factors
(A) Mortality of age- and sex-matched wild-type and Elf4−/− mice treated with standard DSS-induced acute colitis protocol. WT, n = 6; Elf4−/−, n = 5. ∗∗P = 0.0070, log-rank (Mantel-Cox) test.
(B) Body weight change of indicated mice. WT, n = 10; Elf4−/−, n = 10. Day 4, ∗∗∗∗P < 0.0001. Unpaired two-sided Student’ s t test, data are represented as mean ± standard error of mean (SEM).
(C) Representative H&E-stained images of colon sections, scale bar: 200 um.
(D) Histology score of inflammation at indicated time. WT, n = 10; Elf4−/−, n = 10. ∗∗P = 0.005. Unpaired two-sided Student’ s t test, data are represented as mean ± standard error of mean (SEM).
(E and F) Mortality (E) and body weight percent (F) of six-week-old male wild-type and Elf4fl/fl-Lyz2-Cre mice treated with 2% DSS for 7 days. WT, n = 7, Elf4fl/fl-Lyz2-Cre, n = 7. NS, not significant. Data are represented as mean ± standard error of mean (SEM).
(G) Mortality of six-week-old male wild-type and Elf4fl/fl-Villin-Cre mice treated with 1.5% DSS for 5 days. WT, n = 4, Elf4fl/fl-Villin-Cre, n = 7. ∗∗∗∗P < 0.0001, log-rank (Mantel-Cox) test.
(H) Body weight percent of six-week-old female wild-type and Elf4fl/fl-Villin-Cre mice treated with 2% DSS for 5 days. WT, n = 4, Elf4fl/fl-Villin-Cre, n = 3. ∗P = 0.0186. Unpaired two-sided Student’ s t test, data are represented as mean ± standard error of mean (SEM).
(I) Comparison of indicated genes between DSS-treated WT and Elf4−/− mice by RT-qPCR. WT, n = 5; Elf4−/−, n = 5. ∗P < 0.05. Unpaired two-sided Student’ s t test, data are represented as mean ± standard error of mean (SEM).
(J) Left, flow cytometry analyzing ILC3s (CD3−CD90+RORγt+) from the small intestine (upper) and large intestine (lower); right, frequency and the total number of ILC3s in lamina propria lymphocytes from the small intestine (upper) and large intestine (lower) (n = 8 per genotype). Data are represented as mean ± standard error of mean (SEM).
(K) Body weight change (left) and colon length (right) of indicated mice after being infected with C. rodentium (n = 6 per genotype). Day 7, day 8, day 9, day 10, ∗P < 0.05, ∗∗P = 0.005. Unpaired two-sided Student’ s t test, data are represented as mean ± standard error of mean (SEM).
(L) Left, flow cytometry analyzing IL-22-producing ILC3s (CD3−CD90+RORγt+IL-22+) from the small intestine; right, frequency and the total number of IL-22-producing ILC3s in lamina propria total ILC3s from the small intestine (n = 6 per genotype). Unpaired two-sided Student’ s t test, data are represented as mean ± standard error of mean (SEM).
(M) Left, flow cytometry analyzing IL-22-producing ILC3s (CD3−CD90+RORγt+IL-22+) from the large intestine; right, frequency and the total number of IL-22-producing ILC3s in lamina propria total ILC3s from the large intestine (n = 6 per genotype). Unpaired two-sided Student’ s t test, data are represented as mean ± standard error of mean (SEM).
(N) Comparison of indicated genes between C. rodentium infected WT and Elf4−/− mice by RT-qPCR (n = 6 per genotype). ∗P = 0.0407; ∗∗P = 0.0092. Unpaired two-sided Student’ s t test, data are represented as mean ± standard error of mean (SEM).
See also Figure S3.
The inflamed colons of WT and Elf4−/− mice were subjected to whole genome RNA sequencing. We found that Elf4 deficiency led to a significantly different transcriptional response to DSS treatment, as demonstrated by general transcriptome dissimilarity (Figure S3B). Among 16,788 detected genes, there are a total of 1980 genes upregulated in Elf4−/− mice and 2141 genes downregulated in Elf4−/− mice (Figure S3C). We also did whole-genome RNA sequencing of inflamed colons of WT and Elf4fl/fl-Villin-Cre mice treated with 2% DSS for 5 days. Gene set enrichment analysis showed pathways enriched in Elf4fl/fl-Villin-Cre mice mainly focus on inflammatory response, the activation and regulation of macrophages, the migration of monocytes, and cell chemotaxis (Figure S3D). Differentially expressed gene analysis showed that expression of certain mucin genes and regenerating gene (REG) was significantly lower in Elf4−/− mice. We then confirmed the difference using quantitative reverse transcription-PCR (RT-qPCR) (Figures S3E and 3I). Mucin and REG proteins play important roles in the pathogenesis of UC and CAC. For example, Muc2-deficient mice spontaneously develop colitis and adenomas, which develop into invasive adenocarcinomas (Heazlewood et al., 2008; Velcich et al., 2002). Reg3γ-deficient mice also spontaneously develop colitis (Loonen et al., 2014). Reg3β and Reg3γ are critical for intestinal microbiota homeostasis and tissue repair (Wells et al., 2017). Severely impaired mucin and REG protein expression led to the breakdown of colonic epithelial barrier, which exacerbated inflammation.
In the intestine, ILC3s play an important role in regulating tissue repair through their major effector IL-22 (Mielke et al., 2013; Sonnenberg and Artis, 2015). IL-22 signaling induces the generation of mucin and REG proteins to facilitate tissue repair. To further explore the barrier protection role of ELF4, we investigated the function of ILC3s in Elf4−/− mice. We found intestinal ILC3s were normally developed in Elf4−/− mice at the resting state (Figure 3J). We then challenged WT and Elf4−/− mice with C. rodentium to test the function of ILC3s. Elf4−/− mice were more susceptible to C. rodentium infection and showed more severe inflammation (Figure 3K). ILC3s from Elf4−/− mice showed smaller number and dampened capability to produce IL-22 in response to IL-23 stimulation compared to WT mice (Figures 3L and 3M). RT-qPCR results supported this finding (Figure 3N). Thus, impaired ILC3 function also contributed to the vulnerability of intestinal barrier of Elf4−/− mice. Collectively, these data suggested that the intestinal epithelial ELF4 was critical for mucin protein expression and ILC3 function.
ELF4 promotes DNA damage repair by upregulating DDR machinery components
As mentioned above, genomic mutation accumulation is a key step of tumorigenesis. It has been reported that ELF4 can promote the faster repair of damaged DNA in response to γ-irradiation (Sashida et al., 2011), so we focus on whether ELF4 suppression in the development of CAC will lead more DNA damage. Expression of a set of DNA damage response pathway genes was significantly lower in Elf4−/− mice compared to control mice (Figure 4A). These genes play essential roles in various DNA damage responses (DDR) (Table S2). To confirm this finding, we replicated the experiment with a larger sample number. Indeed, H2ax, Taok3, Nabp1, Nbn, Fbxo18, and Ints3 were all decreased in Elf4−/− mice (Figure 4B) and ELF4 knockdown cell lines (Figure S4A). The protein level of H2AX showed consistent decrease (Figures 4C and S4B).
Figure 4.
Elf4 is a master transcription regulator of DNA damage response.
(A) Heatmap representing selected DNA damage response genes of indicated mice.
(B) Comparison of indicated genes between DSS-treated WT and Elf4−/− mice by RT-qPCR. WT, n = 5; Elf4−/−, n = 5. ∗P = 0.016, ∗∗P = 0.0024, ∗∗∗P < 0.001, ∗∗∗∗P = 1.07 × 10−5. Unpaired two-sided Student’ s t test, data are represented as mean ± standard error of mean (SEM).
(C) Comparison of H2AX in colon mucosa between wild-type and Elf4−/− mice treated with DSS at day 6.
(D) Representative images of Comet assay in WT and ELF4−/− SW480 cells treated with H2O2 for indicated time.
(E) Representative immunostaining for DAPI (blue) and 8-oxo-dG (green) and co-staining (merge) in the colon of indicated mice, scale bar: 200 um.
(F and G) Luciferase assay of HEK 293T cells transfected with H2AX-luc plasmid and indicated expression plasmids. Data were pooled from three independent experiments; data are represented as mean ± standard error of mean (SEM).
(H) ChIP-qRT-PCR experiments showing Flag-ELF4 binding to indicated promoters in SW480 cells stably expressing ELF4 and control vector. hIFNB1 promoter serves as positive control; data are represented as mean ± standard error of mean (SEM).
(I) Immunoprecipitation of Flag-tagged full-length PARP1 and catalytic domain deleted PARP1. First lane is IgG control.
(J) Flag-tagged ELF4 was co-immunoprecipitated with HA-tagged PARP1.
(K) mRNA levels of H2AX and TAOK3 when PARP1 was knocked down with shRNA; data are represented as mean ± Standard Error of Mean (SEM).
Based on this observation and the hyper-susceptibility of Elf4−/− mice to CAC, we hypothesized that ELF4 might play an important role in keeping colon epithelial genome safe by regulating a spectrum of key components of DDR machinery. To further confirm this, the Elf4−/− cells were established in NCM460 and SW480, the former a normal human colon mucosal epithelial cell line (Moyer et al., 1996) and the latter derived from human colon cancer. Comet assays showed that there was more DNA damage in Elf4−/− SW480 cells (Figures 4D and S4C) and Elf4−/− NCM460 cells (Figures S4D and S4E) in response to H2O2 treatment compared to control groups. 8-oxo-dG (8-Oxo-7,8-dihydro-2′-deoxyguanosine) is an excellent marker for DNA damage produced by oxidants because it represents one of the major products generated by a wide array of treatments associated with oxidant damage (Roszkowski et al., 2011). We detected much more 8-oxo-dG in the colon epithelia of Elf4−/− mice treated with 1 cycle of DSS (Figures 4E and S4F), indicating impaired DDR in Elf4−/− mice.
Next, to test the possibility that ELF4 transcriptionally regulated these genes, we constructed promoter luciferase reporters of H2AX, NABP1, FBXO18, and TAOK3. Overexpression of ELF4 in HEK 293T showed that ELF4 activated these promoters in a dose-dependent manner (Figures 4F and S4G). To identify the critical domains of ELF4 required for these processes, we constructed a series of deletion and mutation mutants of ELF4. We found that the ETS domain, nuclear localization sequence region, and transactivation domain (amino acids 1–87) were all indispensable for induction of H2AX, FBXO18, TAOK3, and NABP1 expression (Figures 4G and S4G), similar to the case of IFNB1 induction (You et al., 2013). Two highly conserved arginines in the ETS domain are critical for DNA binding (Wei et al., 2010). Replacing these two arginines with alanines (ELF4-RRAA) also abrogated activation of H2AX, FBXO18, TAOK3, and NABP1 promoters (Figures 4G and S4G). Mining Elf4 ChIP-seq data with Cistrome Data Browser showed endogenous evidence that Elf4 indeed binded to the promoters of H2ax, Taok3, Fbxo18, Nabp1, Nbn, and Ints3 (Figure S4H) (Curina et al., 2017; Mei et al., 2017). We confirmed these bindings in SW480 cells stably expressing ELF4 by Chromatin immunoprecipitation (ChIP) coupled to quantitative PCR (ChIP-qPCR) assay (Figure 4H).
In an effort to identify ELF4-interacting partners, we found PARP1 is a potential candidate in a Flag-tagged ELF4 overexpression system by mass spectrometry (Table S3). PARP1 is a well-known DNA damage sensor (Ray Chaudhuri and Nussenzweig, 2017). We thus reasoned that PARP1/ELF4 DNA damage response axis might be the biological relevance of this interaction. We confirmed this interaction by co-immunoprecipitation (Figure 4I). Interestingly, we noticed a band shift of ELF4 by PARP1 overexpression, which was dependent on the catalytic domain of PARP1 (Figure 4I). We repeated this experiment in a dual overexpression system. The band of ELF4 was similarly shifted (Figure 4J). In addition, when we incubated the replicative lanes with anti-PAR antibody, we found bands of same size with main ELF4 and shifted ELF4, indicating ELF4 was PARylated by PARP1 (Figure 4J). Furthermore, knockdown of PARP1 led to similar reduction of H2AX and TAOK3 (Figure 4K). Thus, upon PARylation by PARP1, ELF4 might play an important role in keeping colon epithelial genome safe, and ELF4 deficiency predisposed host to mutagenesis and tumorigenesis.
Collectively, on the one hand, ELF4 was critical for mucin protein expression, which anatomically shielded colon epithelia and probably the colon stem cells from the actions of carcinogens (Bischoff et al., 2014). On the other hand, ELF4 supported the transcription of multiple DDR machinery components to ensure a competent DDR.
Suppression of ELF4 in CAC depends on its promoter methylation
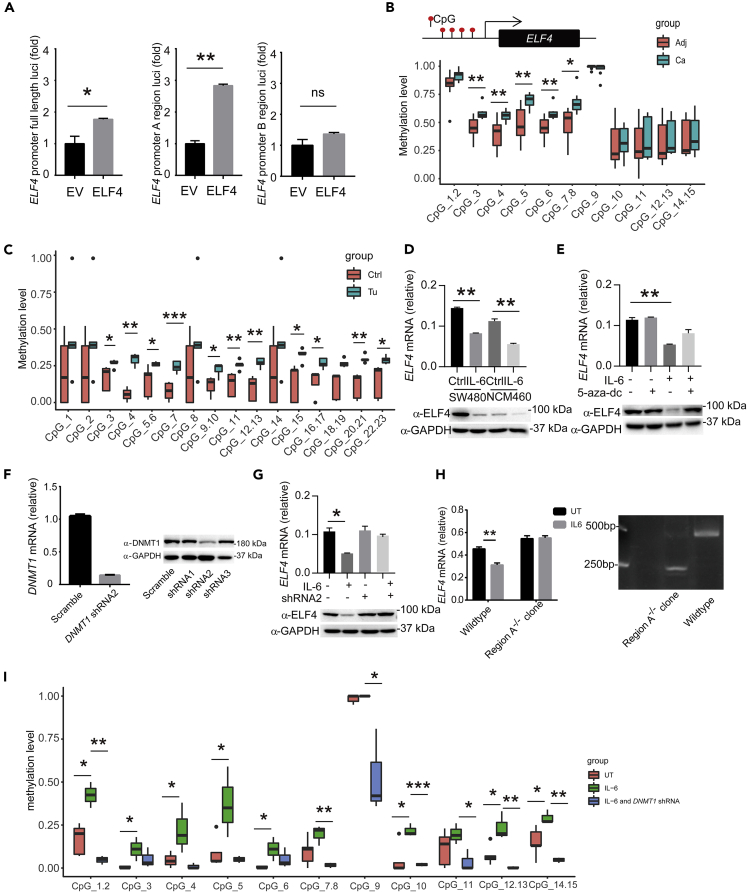
We next asked how ELF4 was suppressed in CAC. It is well recognized that tumorigenesis is a multi-step process marked by a series of genetic and epigenetic alterations (Hanahan and Weinberg, 2011). In this model, the driver mutation signals the beginning of the long evolution and selection process toward malignancy. Recently, the phenomenon of “trained immunity” indicates that cells can be transformed by epigenetic reprogramming (Netea et al., 2016). So, we hypothesized that before the genetic reprogramming stage, there was an epigenetic reprogramming stage, which created a mutation-prone environment. ELF4 suppression could be such an example. The ELF4 promoter region showed CpG island enrichment in human colon cells; we predicted and got two CpG regions (region A and region B) by MethPrimer (Figure S5A) (Akhtar-Zaidi et al., 2012; Li and Dahiya, 2002; Mei et al., 2017). In order to make sure which region is mainly responsible to transcriptional regulation of ELF4, we constructed luciferase reporters of ELF4 promoter region A, ELF4 promoter region B, and ELF4 promoter full length. Overexpression of ELF4 in SW480 (Figure 5A) and NCM460 (Figure S5B) showed region A is mainly responsible for ELF4 transcriptional regulation. In CAC tissue, ELF4 promoter region A methylation should be responsible for the more stable suppression (Figure 5B), and we also observed that the Elf4 promoter methylation level of tumor tissue induced by AOM-DSS protocol is higher than that of peripheral tissue (Figure 5C). IL-6 can induce promoter hypermethylation of tumor suppressor genes via DNMT1 (Li et al., 2012). We showed that IL-6 treatment suppressed ELF4 in NCM460 and SW480 cells (Figure 5D), and this suppression was also dependent on DNMT1 (Figures 5E–5G). To further investigate the mechanism of ELF4 suppression, we used clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated (Cas) protein 9 system to delete the region A of ELF4 promoter in SW480 cells (Figure S5C). As shown in Figure 5H, IL-6 treatment can only suppress ELF4 expression in WT SW480 cells, while the ELF4 suppression effect and ELF4 promoter methylation level increase of IL-6 treatment is abolished by DNMT1 shRNA treatment (Figures 5G and 5I). Thus, before any genetic changes, there is an epigenetic reprogramming stage preparing the cell for transformation by creating a mutation-prone transcriptional environment.
Figure 5.
ELF4 is epigenetically suppressed in CAC
(A) Luciferase assay of SW480 cells transfected with ELF4 promoter full length or truncation luciferase plasmid and indicated expression plasmids. ∗P = 0.0443, ∗∗P = 0.0016, ns, not significant. Unpaired two-sided Student’ s t test, data are represented as mean ± standard error of mean (SEM) Data were pooled from three independent experiments.
(B) Quantitative methylation analysis on each CpG site in a selected ELF4 promoter region in paired clinical samples from 8 patients with CAC. Methylation level 1 represents 100% methylated CpG dinucleotides on this site. Adj, cancer adjacent tissue; Ca, cancer tissue. ∗P = 0.0141, ∗∗P < 0.01. Unpaired two-sided Student’ s t test, data are represented as mean ± standard error of mean (SEM).
(C) Quantitative methylation analysis on each CpG site in a selected Elf4 promoter region in paired mice CAC samples. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P = 0.0009. Unpaired two-sided Student’ s t test, data are represented as mean ± standard error of mean (SEM).
(D) ELF4 mRNA (top) and protein (bottom) level change after IL-6 treatment in SW480 and NCM460 cells. ∗∗P < 0.01. Unpaired two-sided Student’ s t test, data are represented as mean ± standard error of mean (SEM).
(E) Analysis of DNMT1 inhibitor 5-aza-dc on IL-6 induced suppression of ELF4 in SW480 cells. ∗∗P = 0.0082. Unpaired two-sided Student’ s t test, data are represented as mean ± standard error of mean (SEM).
(F) Knockdown efficiency of DNMT1 shRNA measured by qPCR (left) and immunoblot (right), data are represented as mean ± standard error of mean (SEM).
(G) Effects of DNMT1 knockdown on IL-6 induced suppression of ELF4 in SW480 cells. ∗P = 0.0222. Unpaired two-sided Student’ s t test, data are represented as mean ± standard error of mean (SEM).
(H) Effects of region CpG1-11 deletion of ELF4 promoter on IL-6 induced ELF4 suppression in SW480 cells (left). Nucleic acid gel electrophoresis to identify the deletion of region CpG1-11 of ELF4 promoter (right). ∗∗P = 0.0043. Unpaired two-sided Student’ s t test, data are represented as mean ± standard error of mean (SEM).
(I) Quantitative methylation analysis on each CpG site in a selected ELF4 promoter region (region A) in untreated, IL-6 treated, or IL-6 and DNMT1 shRNA2-treated SW480 cells. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. Unpaired two-sided Student’ s t test, data are represented as mean ± standard error of mean (SEM).
See also Figure S5.
Oral administration of montmorillonite rescues ELF4 expression and lowers risk of CAC development
We next asked what can rescue Elf4 expression. Tissue damage and epithelium disruption is a major histopathologic feature in UC, which exposes mucosal immune cells directly to various stimuli, causing diverse cellular responses (Martini et al., 2017). Montmorillonite, a natural dioctahedron phyllosilicate, is a commonly used intestinal mucosal protective agent for diarrhea (Dupont and Vernisse, 2009; Gonzalez et al., 2004), which increases the resistance of the intestinal epithelium to toxic stimuli in humans (Dupont et al., 2009). We then hypothesized that montmorillonite could also protect the intestinal barrier during active colitis and prevent ELF4 suppression by reducing inflammation. We found that oral administration of montmorillonite powder prevented Elf4 suppression (Figure 6A) and significantly attenuated DNA damage in colon mucosa (Figures 6B and S6A). Histologically, intestinal structure of the montmorillonite powder treatment group is more complete compared with the control group (Figure S6B). The mRNA expression of Elf4 and Dnmt1 was negatively correlated (Figures S6C–S6E) after treatment with montmorillonite powder, and we did observe a decrease in the Elf4 promoter methylation level difference after montmorillonite powder treatment, although not statistically significant (Figure S6F).
Figure 6.
Montmorillonite restrains CAC through rescuing Elf4 expression
(A) Effects of montmorillonite powder on DSS induced suppression of Elf4, ∗∗∗∗P = 4.02 × 10−6, ns, not significant. Unpaired two-sided Student’ s t test.
(B) Representative immunostaining for DAPI (blue) and 8-oxo-dG (green) and co-staining (merge) in the colon of indicated mice. Scale bar: 200 um.
(C) Tumor number count of indicated group of mice treated with standard AOM-DSS protocol. Elf4fl/fl DSS, n = 5, Elf4fl/fl DSS plus montmorillonite powder, n = 4, Elf4fl/fl-Villin Cre DSS, n = 4, Elf4fl/fl-Villin Cre DSS plus montmorillonite, n = 5. ∗P < 0.05. Unpaired two-sided Student’ s t test, data are represented as mean ± standard error of mean (SEM).
(D) Eighteen patients with CAC were grouped as never taken montmorillonite powder (n = 12), occasionally taken montmorillonite powder (n = 3), and regularly taken Montmorillonite powder (n = 3). Comparisons of UC and CAC interval time duration among groups are shown. ∗∗∗P = 0.0003, ∗P = 0.0386. Unpaired two-sided Student’ s t test, data are represented as mean ± standard error of mean (SEM).
(E) Grading scores from the TNM staging system were compared. The T of TNM refers to the size and extent of the main tumor, data are represented as mean ± standard error of mean (SEM).
(F) Blood CA19-9 level before surgery. Mann-Whitney test, ∗P = 0.028. Unpaired two-sided Student’ s t test, data are represented as mean ± standard error of mean (SEM).
See also Figures S6 and S7 and Table S4.
To further test the cancer prevention potential of montmorillonite, we added montmorillonite powder treatment during CAC induction course by AOM/DSS in mice. As expected, Elf4fl/fl CAC mice treated with montmorillonite powder showed less colon tumor development, while Elf4fl/fl-Villin-Cre mice showed no response (Figure 6C). The hematoxylin and eosin (H&E) staining shows that tumors of Elf4fl/fl-Villin-Cre mice represent features of adenocarcinomas (Figure S7A). Moreover, we did a retrospective study of 18 patients with CAC from Peking University Cancer Hospital between 2013 and 2018 (Table S4), who were grouped as never taken montmorillonite powder (n = 12), occasionally taken montmorillonite powder (n = 3), and regularly taken Montmorillonite powder (n = 3). The result showed that 3 of them who were regularly on montmorillonite powder treatment in addition to use of anti-inflammation drug during UC activation showed delayed development of CAC (Figure 6D), although the tumor number and size grading showed no difference (Figure 6E). CA19-9, one of the cancer biomarker tested, was significantly lower in these three patients (Figure 6F). These data suggested that montmorillonite powder might be a good candidate drug for CAC prevention.
Discussion
IBD now affects between one in 200 and one in 300 people in developed countries (Ng et al., 2018). The prevalence in developing countries is still much lower than this, but the incidence is increasing rapidly. In China, for example, these conditions have changed from being rare to common (Zhao et al., 2013). Based on the large population China and other developing countries have, CAC could also be much more common in the next few decades. Etiologically, extensive studies have highlighted the importance of inflammation in the development of CAC (Grivennikov, 2013). In this study, we demonstrate that suppression of ELF4-mediated DDR also contributes to the development of CAC.
In sporadic CRC, tumors do not arise in the context of preceding inflammation, while in the case of CAC, clinically detectable IBD always precedes (sometimes by decades) tumor initiation. Inflammation indeed plays a significant role in CAC initiation and promotion (Terzic et al., 2010). In CAC, the hallmark of chronic inflammation is its ability to cause mutations. Colitis induces robust production of reactive oxygen species (ROS) and reactive nitrogen intermediates, which are highly reactive and mutagenic (Hussain et al., 2003; Meira et al., 2008). These oxidative DNA damages could lead to mutations of critical driver genes such as p53 in tumor cells and the inflamed but nondysplastic epithelium (Long et al., 2017). In mice, repeated DSS treatment can cause a low incidence of colonic adenomas (17), which is also thought as a result of inflammation-induced DNA damage (Meira et al., 2008). Our data showed that suppression of DDR is also an important contributor to colonic adenomas in colitis. Besides recruiting and activating nuclear complexes, PARP1 also exerts its effects by directly modifying protein activity and localization. Transcription factors such as Oct-1 and hnRNP K (heterogeneous nuclear ribonucleoprotein K) are known targets of PARP1, which, when ADP-ribosylated, are repelled from DNA, hence resulting in altered transcript expression profiles (Gagné et al., 2003; Nie et al., 1998). PARP1 sensed DNA damage and activated ELF4 through PARylation. ELF4 then bound to the promoters of multiple DNA repair genes, including H2AX, FBXO18, TAOK3, NBN, NABP1, and INTS3, and ensured potent transcription. ELF4 deficiency led to decreased expression of these genes and increased DNA damage in vivo and in vitro. H2AX upregulation and activation is an important signal to tell the cells that DNA damages occurred and initiated DNA repair process (Mah et al., 2010). With compromised downregulation of H2AX due to ELF4 downregulation, DNA damage repair machinery failed to repair genome efficiently, leading faster mutation accumulation, which predisposed cell to be cancerous. Nevertheless, we cannot rule out the possibility that ELF4 promotes DDR in additional transcription activity independent ways (Malewicz and Perlmann, 2014; Sashida et al., 2011), as a previous report showed that ELF4 translocated to DNA damage site in response to irradiation (Sashida et al., 2011).
Inflammation-induced mutagenesis can also cause inactivation or repression of mismatch repair genes, and ROS can directly oxidize and inactivate mismatch repair enzymes at protein level (Colotta et al., 2009; Hussain et al., 2003). However, ELF4 suppression is not genetic but epigenetic. Inflammation can also drive various epigenetic changes to silence genes during IBD and CAC. For example, DNA promoter methylation of tumor suppressor genes, such as RUNX3, MINT1, and SOCS3, has been reported in CAC (Emmett et al., 2017; Li et al., 2012). DNA methyltransferases Dnmt1 were shown to be upregulated and activated by inflammatory cytokines such as IL-6 highly produced in UC and CAC (Foran et al., 2010; Li et al., 2012).
CAC is the most feared long-term intestinal complication of IBD, including UC (Peyrin-Biroulet et al., 2012). Especially, patients with early-onset UC face higher risk of developing not only CAC but also various kinds of cancers (Olen et al., 2017). It is frustrating that despite of this, there is no effective way but surveillance colonoscopy with biopsies to counterstrike this risk (Yu et al., 2016). Our study now showed that oral administration of montmorillonite can prevent ELF4 downregulation in DSS-induced colitis and lower the risk of CAC in mice models and possibly in patients with UC. Although further study including prospective clinical study should be conducted, our data showed hope for safe, effective, and economical treatment in CAC prevention.
In summary, we have shown that Elf4−/− mice are hyper-susceptible to CAC, and patients with UC show ELF4 deficiency in the colon mucosa. ELF4 intrinsically regulates a set of DDR machinery components to keep the epithelial genome stable in response to various genotoxic stimulations. Meanwhile, ELF4 is critical for mucin and REG protein expression, which form a physical barrier to protect intestinal epithelia from mutagen attacks. ELF4 deficiency is an oncogenic hit and might be caused by a recently proposed process of epigenetic priming (Vicente-Duenas et al., 2018), which can be prevented by administration of montmorillonite powder, although further prospective clinical study should be done to fully explore its cancer prevention potential. These findings should inspire future clinical investigation and might change the paradigm of UC treatment.
Limitations of the study
The mechanisms of ELF4 suppression proposed in this manuscript are mostly based on preclinical animal model of UC and CAC, and further studies are needed to determine if those are clinically meaningful. The most exciting finding in this study is that the use of montmorillonite powder prevented ELF4 downregulation and delayed CAC development in some patients; however, the sample size is too small to propose a promising and novel cancer prevention strategy. ELF4 suppression is confirmed in patient cohorts from GEO but only in a small number of our patients. More well-cryopreserved clinical tissues should be collected for qPCR and western blot (WB) to confirm our finding in animal models.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Fuping You, Ph.D (fupingyou@hsc.pku.edu.cn).
Material availability
This study did not generate new unique reagents.
Data and code availability
The RNA-seq data that support the findings in this study have been deposited in the GEO database with the accession codes GSE121305 and GSE164355. Raw data from Figures 1, 2, 3, 4, 5, and 6 and Figures S1, S3, S4, S6, and S7 were deposited on Mendeley at [https://data.mendeley.com/datasets/f734b48h8h/draft?a=8e1387fe-44f3-4551-b7d6-df1cd3986c6f].
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank the patients for their understanding and cooperation. This work was supported by the National Key Research and Development Program of China (2016YFA0500302 to F.Y.) and the National Natural Science Foundation of China (31570891 and 31872736 to F.Y., as well as 81871160 to M.L.).
Author contributions
H.D. conceived the project; designed, executed, and interpreted the animal studies; analyzed data and wrote the manuscript. H.X. and TT.L. executed most animal and cellular studies. Y.L. and J.L. collected clinical samples and analyzed patient information. B.X. performed comet tail assays. J.X. and T.L. executed animal studies related to infection mice models. L.C. and S.L. executed mass spectrometry assays. S.L. and P.W. constructed ELF4 knockdown and knockout plasmids. D.W., Z.Z., and Y.L. helped with lentivirus packaging. X.G. critically discussed ILC data. A.W. and M.L. conceived the project, critically discussed data, provided reagents, and revised manuscript. F.Y. conceived the project, designed and interpreted the animal and cellular studies, and wrote the manuscript.
Declaration of interests
The authors declare no competing interests.
Published: March 19, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102169.
Contributor Information
Aiwen Wu, Email: drwuaw@sina.com.
Mo Li, Email: limo@hsc.pku.edu.cn.
Fuping You, Email: fupingyou@hsc.pku.edu.cn.
Supplemental information
References
- Akhtar-Zaidi B., Cowper-Sal-lari R., Corradin O., Saiakhova A., Bartels C.F., Balasubramanian D., Myeroff L., Lutterbaugh J., Jarrar A., Kalady M.F. Epigenomic enhancer profiling defines a signature of colon cancer. Science. 2012;336:736–739. doi: 10.1126/science.1217277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff S.C., Barbara G., Buurman W., Ockhuizen T., Schulzke J.D., Serino M., Tilg H., Watson A., Wells J.M. Intestinal permeability--a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. doi: 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colotta F., Allavena P., Sica A., Garlanda C., Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- Conrad K., Roggenbuck D., Laass M.W. Diagnosis and classification of ulcerative colitis. Autoimmun. Rev. 2014;13:463–466. doi: 10.1016/j.autrev.2014.01.028. [DOI] [PubMed] [Google Scholar]
- Curina A., Termanini A., Barozzi I., Prosperini E., Simonatto M., Polletti S., Silvola A., Soldi M., Austenaa L., Bonaldi T. High constitutive activity of a broad panel of housekeeping and tissue-specific cis-regulatory elements depends on a subset of ETS proteins. Genes Dev. 2017;31:399–412. doi: 10.1101/gad.293134.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont C., Foo J.L., Garnier P., Moore N., Mathiex-Fortunet H., Salazar-Lindo E., Peru, and Malaysia Diosmectite Study, G. Oral diosmectite reduces stool output and diarrhea duration in children with acute watery diarrhea. Clin. Gastroenterol. Hepatol. 2009;7:456–462. doi: 10.1016/j.cgh.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Dupont C., Vernisse B. Anti-diarrheal effects of diosmectite in the treatment of acute diarrhea in children: a review. Paediatr. Drugs. 2009;11:89–99. doi: 10.2165/00148581-200911020-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaden J.A., Abrams K.R., Mayberry J.F. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekbom A., Helmick C., Zack M., Adami H.O. Ulcerative colitis and colorectal cancer. A population-based study. New Engl. J. Med. 1990;323:1228–1233. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- Emmett R.A., Davidson K.L., Gould N.J., Arasaradnam R.P. DNA methylation patterns in ulcerative colitis-associated cancer: a systematic review. Epigenomics. 2017;9:1029–1042. doi: 10.2217/epi-2017-0025. [DOI] [PubMed] [Google Scholar]
- Foran E., Garrity-Park M.M., Mureau C., Newell J., Smyrk T.C., Limburg P.J., Egan L.J. Upregulation of DNA methyltransferase-mediated gene silencing, anchorage-independent growth, and migration of colon cancer cells by interleukin-6. Mol. Cancer Res. 2010;8:471–481. doi: 10.1158/1541-7786.MCR-09-0496. [DOI] [PubMed] [Google Scholar]
- Fouad Y.A., Aanei C. Revisiting the hallmarks of cancer. Am. J. Cancer Res. 2017;7:1016–1036. [PMC free article] [PubMed] [Google Scholar]
- Gagné J.P., Hunter J.M., Labrecque B., Chabot B., Poirier G.G. A proteomic approach to the identification of heterogeneous nuclear ribonucleoproteins as a new family of poly(ADP-ribose)-binding proteins. Biochem. J. 2003;371:331–340. doi: 10.1042/BJ20021675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gkouskou K.K., Ioannou M., Pavlopoulos G.A., Georgila K., Siganou A., Nikolaidis G., Kanellis D.C., Moore S., Papadakis K.A., Kardassis D. Apolipoprotein A-I inhibits experimental colitis and colitis-propelled carcinogenesis. Oncogene. 2016;35:2496–2505. doi: 10.1038/onc.2015.307. [DOI] [PubMed] [Google Scholar]
- Gonzalez R., de Medina F.S., Martinez-Augustin O., Nieto A., Galvez J., Risco S., Zarzuelo A. Anti-inflammatory effect of diosmectite in hapten-induced colitis in the rat. Br. J. Pharmacol. 2004;141:951–960. doi: 10.1038/sj.bjp.0705710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov S.I. Inflammation and colorectal cancer: colitis-associated neoplasia. Semin. Immunopathol. 2013;35:229–244. doi: 10.1007/s00281-012-0352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov S.I., Cominelli F. Colitis-associated and sporadic colon cancers: different diseases, different mutations? Gastroenterology. 2016;150:808–810. doi: 10.1053/j.gastro.2016.02.062. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Heazlewood C.K., Cook M.C., Eri R., Price G.R., Tauro S.B., Taupin D., Thornton D.J., Png C.W., Crockford T.L., Cornall R.J. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008;5:e54. doi: 10.1371/journal.pmed.0050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain S.P., Hofseth L.J., Harris C.C. Radical causes of cancer. Nat. Rev. Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- Jess T., Rungoe C., Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin. Gastroenterol. Hepatol. 2012;10:639–645. doi: 10.1016/j.cgh.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Lee P.H., Puppi M., Schluns K.S., Yu-Lee L.Y., Dong C., Lacorazza H.D. The transcription factor E74-like factor 4 suppresses differentiation of proliferating CD4+ T cells to the Th17 lineage. J. Immunol. 2014;192:178–188. doi: 10.4049/jimmunol.1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.C., Dahiya R. MethPrimer: designing primers for methylation PCRs. Bioinformatics. 2002;18:1427–1431. doi: 10.1093/bioinformatics/18.11.1427. [DOI] [PubMed] [Google Scholar]
- Li Y., Deuring J., Peppelenbosch M.P., Kuipers E.J., de Haar C., van der Woude C.J. IL-6-induced DNMT1 activity mediates SOCS3 promoter hypermethylation in ulcerative colitis-related colorectal cancer. Carcinogenesis. 2012;33:1889–1896. doi: 10.1093/carcin/bgs214. [DOI] [PubMed] [Google Scholar]
- Long A.G., Lundsmith E.T., Hamilton K.E. Inflammation and colorectal cancer. Curr. Colorectal Cancer Rep. 2017;13:341–351. doi: 10.1007/s11888-017-0373-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loonen L.M., Stolte E.H., Jaklofsky M.T., Meijerink M., Dekker J., van Baarlen P., Wells J.M. REG3gamma-deficient mice have altered mucus distribution and increased mucosal inflammatory responses to the microbiota and enteric pathogens in the ileum. Mucosal Immunol. 2014;7:939–947. doi: 10.1038/mi.2013.109. [DOI] [PubMed] [Google Scholar]
- Mah L.J., El-Osta A., Karagiannis T.C. gammaH2AX: a sensitive molecular marker of DNA damage and repair. Leukemia. 2010;24:679–686. doi: 10.1038/leu.2010.6. [DOI] [PubMed] [Google Scholar]
- Malewicz M., Perlmann T. Function of transcription factors at DNA lesions in DNA repair. Exp. Cell. Res. 2014;329:94–100. doi: 10.1016/j.yexcr.2014.08.032. [DOI] [PubMed] [Google Scholar]
- Martini E., Krug S.M., Siegmund B., Neurath M.F., Becker C. Mend your fences: the epithelial barrier and its relationship with mucosal immunity in inflammatory bowel disease. Cell Mol. Gastroenterol. Hepatol. 2017;4:33–46. doi: 10.1016/j.jcmgh.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei S., Qin Q., Wu Q., Sun H., Zheng R., Zang C., Zhu M., Wu J., Shi X., Taing L. Cistrome Data Browser: a data portal for ChIP-Seq and chromatin accessibility data in human and mouse. Nucleic Acids Res. 2017;45:D658–D662. doi: 10.1093/nar/gkw983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meira L.B., Bugni J.M., Green S.L., Lee C.W., Pang B., Borenshtein D., Rickman B.H., Rogers A.B., Moroski-Erkul C.A., McFaline J.L. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J. Clin. Invest. 2008;118:2516–2525. doi: 10.1172/JCI35073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke L.A., Jones S.A., Raverdeau M., Higgs R., Stefanska A., Groom J.R., Misiak A., Dungan L.S., Sutton C.E., Streubel G. Retinoic acid expression associates with enhanced IL-22 production by γδ T cells and innate lymphoid cells and attenuation of intestinal inflammation. J. Exp. Med. 2013;210:1117–1124. doi: 10.1084/jem.20121588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer M.P., Manzano L.A., Merriman R.L., Stauffer J.S., Tanzer L.R. NCM460, a normal human colon mucosal epithelial cell line. Vitro Cell Dev. Biol. Anim. 1996;32:315–317. doi: 10.1007/BF02722955. [DOI] [PubMed] [Google Scholar]
- Netea M.G., Joosten L.A., Latz E., Mills K.H., Natoli G., Stunnenberg H.G., O'Neill L.A., Xavier R.J. Trained immunity: a program of innate immune memory in health and disease. Science. 2016;352:aaf1098. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng S.C., Shi H.Y., Hamidi N., Underwood F.E., Tang W., Benchimol E.I., Panaccione R., Ghosh S., Wu J.C.Y., Chan F.K.L. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- Nie J., Sakamoto S., Song D., Qu Z., Ota K., Taniguchi T. Interaction of Oct-1 and automodification domain of poly(ADP-ribose) synthetase. FEBS Lett. 1998;424:27–32. doi: 10.1016/s0014-5793(98)00131-8. [DOI] [PubMed] [Google Scholar]
- Okayasu I., Yamada M., Mikami T., Yoshida T., Kanno J., Ohkusa T. Dysplasia and carcinoma development in a repeated dextran sulfate sodium-induced colitis model. J. Gastroenterol. Hepatol. 2002;17:1078–1083. doi: 10.1046/j.1440-1746.2002.02853.x. [DOI] [PubMed] [Google Scholar]
- Olen O., Askling J., Sachs M.C., Frumento P., Neovius M., Smedby K.E., Ekbom A., Malmborg P., Ludvigsson J.F. Childhood onset inflammatory bowel disease and risk of cancer: a Swedish nationwide cohort study 1964-2014. BMJ. 2017;358:j3951. doi: 10.1136/bmj.j3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrin-Biroulet L., Lepage C., Jooste V., Gueant J.L., Faivre J., Bouvier A.M. Colorectal cancer in inflammatory bowel diseases: a population-based study (1976-2008) Inflamm. Bowel Dis. 2012;18:2247–2251. doi: 10.1002/ibd.22935. [DOI] [PubMed] [Google Scholar]
- Ray Chaudhuri A., Nussenzweig A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat. Rev. Mol. Cell Biol. 2017;18:610–621. doi: 10.1038/nrm.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszkowski K., Jozwicki W., Blaszczyk P., Mucha-Malecka A., Siomek A. Oxidative damage DNA: 8-oxoGua and 8-oxodG as molecular markers of cancer. Med. Sci. Monit. 2011;17 doi: 10.12659/MSM.881805. CR329–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford K.K., Price F.M., Brodeur C., Makrauer F.L., Parshad R. Deficient DNA repair in chronic ulcerative colitis. Cancer Detect Prev. 1997;21:540–545. [PubMed] [Google Scholar]
- Sashida G., Bae N., Di Giandomenico S., Asai T., Gurvich N., Bazzoli E., Liu Y., Huang G., Zhao X., Menendez S. The mef/elf4 transcription factor fine tunes the DNA damage response. Cancer Res. 2011;71:4857–4865. doi: 10.1158/0008-5472.CAN-11-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg G.F., Artis D. Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nat. Med. 2015;21:698–708. doi: 10.1038/nm.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suico M.A., Shuto T., Kai H. Roles and regulations of the ETS transcription factor ELF4/MEF. J. Mol. Cell Biol. 2017;9:168–177. doi: 10.1093/jmcb/mjw051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzic J., Grivennikov S., Karin E., Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114 e2105. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- Uhlen M., Bjorling E., Agaton C., Szigyarto C.A., Amini B., Andersen E., Andersson A.C., Angelidou P., Asplund A., Asplund C. A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol. Cell Proteomics. 2005;4:1920–1932. doi: 10.1074/mcp.M500279-MCP200. [DOI] [PubMed] [Google Scholar]
- Ungaro R., Mehandru S., Allen P.B., Peyrin-Biroulet L., Colombel J.F. Ulcerative colitis. Lancet. 2017;389:1756–1770. doi: 10.1016/S0140-6736(16)32126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velcich A., Yang W., Heyer J., Fragale A., Nicholas C., Viani S., Kucherlapati R., Lipkin M., Yang K., Augenlicht L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- Vicente-Duenas C., Hauer J., Cobaleda C., Borkhardt A., Sanchez-Garcia I. Epigenetic priming in cancer initiation. Trends Cancer. 2018;4:408–417. doi: 10.1016/j.trecan.2018.04.007. [DOI] [PubMed] [Google Scholar]
- Wei G.H., Badis G., Berger M.F., Kivioja T., Palin K., Enge M., Bonke M., Jolma A., Varjosalo M., Gehrke A.R. Genome-wide analysis of ETS-family DNA-binding in vitro and in vivo. EMBO J. 2010;29:2147–2160. doi: 10.1038/emboj.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J.M., Brummer R.J., Derrien M., MacDonald T.T., Troost F., Cani P.D., Theodorou V., Dekker J., Meheust A., de Vos W.M. Homeostasis of the gut barrier and potential biomarkers. Am. J. Physiol. Gastrointest. Liver Physiol. 2017;312:G171–G193. doi: 10.1152/ajpgi.00048.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You F., Wang P., Yang L., Yang G., Zhao Y.O., Qian F., Walker W., Sutton R., Montgomery R., Lin R. ELF4 is critical for induction of type I interferon and the host antiviral response. Nat. Immunol. 2013;14:1237–1246. doi: 10.1038/ni.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.X., East J.E., Kaltenbach T. Surveillance of patients with inflammatory bowel disease. Best Pract. Res. Clin. Gastroenterol. 2016;30:949–958. doi: 10.1016/j.bpg.2016.10.014. [DOI] [PubMed] [Google Scholar]
- Zhao J., Ng S.C., Lei Y., Yi F., Li J., Yu L., Zou K., Dan Z., Dai M., Ding Y. First prospective, population-based inflammatory bowel disease incidence study in mainland of China: the emergence of "western" disease. Inflamm. Bowel Dis. 2013;19:1839–1845. doi: 10.1097/MIB.0b013e31828a6551. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA-seq data that support the findings in this study have been deposited in the GEO database with the accession codes GSE121305 and GSE164355. Raw data from Figures 1, 2, 3, 4, 5, and 6 and Figures S1, S3, S4, S6, and S7 were deposited on Mendeley at [https://data.mendeley.com/datasets/f734b48h8h/draft?a=8e1387fe-44f3-4551-b7d6-df1cd3986c6f].