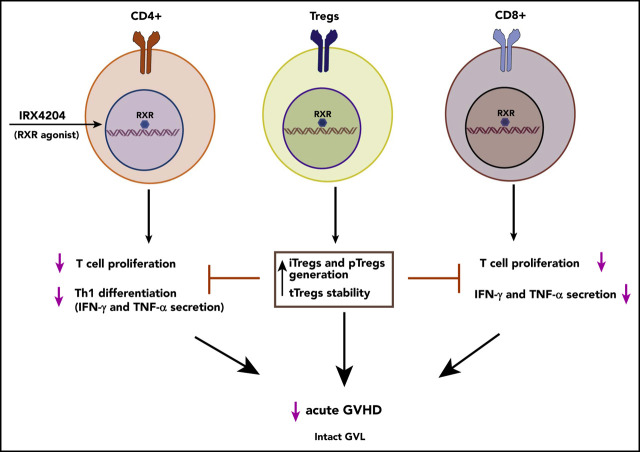
There is a Blood Commentary on this article in this issue.
Key Points
A novel RXR agonist attenuates acute GVHD, while retaining graft-versus-leukemia responses.
The RXR agonist enhances Treg generation and stabilizes Foxp3, even in a highly inflammatory environment.
Abstract
The nuclear receptor (NR) subclass, retinoid X receptors (RXRs), exert immunomodulatory functions that control inflammation and metabolism via homodimers and heterodimers, with several other NRs, including retinoic acid receptors. IRX4204 is a novel, highly specific RXR agonist in clinical trials that potently and selectively activates RXR homodimers, but not heterodimers. In this study, in vivo IRX4204 compared favorably with FK506 in abrogating acute graft-versus-host disease (GVHD), which was associated with inhibiting allogeneic donor T-cell proliferation, reducing T-helper 1 differentiation, and promoting regulatory T-cell (Treg) generation. Recipient IRX4204 treatment reduced intestinal injury and decreased IFN-γ and TNF-α serum levels. Transcriptional analysis of donor T cells isolated from intestines of GVHD mice treated with IRX4204 revealed significant decreases in transcripts regulating proinflammatory pathways. In vitro, inducible Treg differentiation from naive CD4+ T cells was enhanced by IRX4204. In vivo, IRX4204 increased the conversion of donor Foxp3− T cells into peripheral Foxp3+ Tregs in GVHD mice. Using Foxp3 lineage-tracer mice in which both the origin and current FoxP3 expression of Tregs can be tracked, we demonstrated that IRX4204 supports Treg stability. Despite favoring Tregs and reducing Th1 differentiation, IRX4204-treated recipients maintained graft-versus-leukemia responses against both leukemia and lymphoma cells. Notably, IRX4204 reduced in vitro human T-cell proliferation and enhanced Treg generation in mixed lymphocyte reaction cultures. Collectively, these beneficial effects indicate that targeting RXRs with IRX4204 could be a novel approach to preventing acute GVHD in the clinic.
Visual Abstract
Introduction
The success of allogeneic bone marrow hemapoietic stem cell transplantation (HSCT) is impeded by graft-versus-host disease (GVHD), which can cause transplant-related morbidity and mortality. The incidence of acute GVHD in patients ranges from 20% to 70%.1 Alloreactive donor T cells play a key role in mediating GVHD by mounting immune responses against histocompatibility-disparate recipient tissues.2 Strategies involving either depletion or immunosuppression of donor T cells can reduce GVHD risk, but, in some settings, such strategies may increase the risk of leukemia relapse and opportunistic infections.3,4 Immunomodulatory therapies that reduce GVHD without full loss of graft-versus-leukemia (GVL) responses are highly desirable.
Retinoid X receptors (RXRs) regulate numerous biological processes, including cellular proliferation, apoptosis, and metabolism as a result of their capacity to form heterodimers with several other nuclear receptors.5-7 Previous studies in mice reported that signaling of RXRα in T cells can negatively affect T-helper 1 (Th1) differentiation.8,9 In particular, retinoic acid (RA) signaling can regulate immune responses through engagement of RA receptor (RAR)-RXR heterodimers.10 Depending on the local milieu, RA signaling can elicit either anti-inflammatory or proinflammatory responses.10-12 For instance, in inflammatory settings such as murine acute GVHD, endogenous RA or exogenous RA administration worsens the disease.13-15 Using a genetic approach, we and others obtained proof of concept that inhibiting donor T-cell RA signaling attenuates acute GVHD.13,15 Therefore, targeting RXR may have therapeutic potential in the treatment of acute GVHD. However, selective RXR modulation, which has been challenging to achieve with pharmacological agents, is needed to retain the beneficial effects while averting the pleiotropic effects of RXR activation. IRX4204 is a novel, second-generation, highly specific RXR agonist16-19 that preferentially activates RXR homodimers rather than RAR-RXR heterodimers, with a 2000-fold higher affinity for RXR homodimers. In addition, IRX4204 does not activate RXR heterodimers with peroxisome proliferator-associated receptors, liver X receptor, or farnesoid X receptor, as those pathways are linked to unwanted side effects, even at physiological concentrations.16 A recent study reported that IRX4204 attenuates experimental autoimmune encephalomyelitis in mice by reducing the number and frequency of IL-17–producing CD4+ T cells.17 In the present study, we demonstrated that IRX4204, given at the time of transplantation, markedly ameliorated acute GVHD lethality, an effect dependent on generation of peripheral Tregs (pTregs) while maintaining alloreactivity without obliterating GVL responses.
Materials and methods
Mice
C57BL/6 (H-2b; termed B6), B6 CD45.1, and BALB/c (H-2d) mice were purchased from the National Cancer Institute Laboratory and Charles River Laboratories. B6 Thy1.1 and Scurfy female mice were purchased from The Jackson Laboratory. B6 luciferase-expressing transgenic mice (kindly provided by Robert S. Negrin), B6 Foxp3-GFP knockin (KI) mice (kindly provided by Vijay Kuchroo), and B6 (Rosa-RFPxFoxp3eGFP-Cre-ERT2) reporter mice20 (kindly provided by Laurence A. Turka) were bred at the University of Minnesota. Mice were housed in a specific-pathogen-free facility and used in procedures approved by the University of Minnesota Institutional Animal Care and Use Committee.
Bone marrow transplantation
Recipient mice were irradiated on day −1 with a lethal dose of 700 cGy for BALB/c and 1100 cGy for B6 mice. On day 0, recipients were given non–T-cell–depleted (TCD) bone marrow (BM), with or without allogeneic donor splenocytes or purified splenic T cells (purity, >95%) isolated using biotin-labeled anti-CD19 (1D3), CD45R (RA3-6B2), CD11b (M1/70), CD11c (N418), CD49b (DX5), NK1.1 (PK136), TCR γδ (GL3), and TER-119 (TER-119), followed by streptavidin RapidSpheres depletion with the EasySep magnet (Stemcell Technologies), as described previously.14 Weights were recorded biweekly for 30 days and then weekly for the remainder of the experiment.13
IRX4204 and FK506
The novel RXR agonist (Io Therapeutics) has been described previously.21 Drugs were prepared in phosphate-buffered saline (containing ∼4% dimethyl sulfoxide and 1% Tween-80), once a week and stored at 4°C. Bone marrow transplant recipients were given vehicle or IRX4204 daily at a dose rate of 200 μg per mouse intraperitoneally (IP) from day 0 to 56, unless otherwise indicated, in survival and GVL studies. FK506 (Selleckchem) was suspended in corn oil (Sigma) according to the manufacturer’s instructions and administered at 12 mg/kg per dose IP.22 In experiments comparing IRX4204 and FK506 efficacy, both drugs were administered daily either from day −3 to 11 or from day 3 to 17 after transplantation.
GVL experiments
The following B6 tumor cell lines and doses were used to study GVL responses: 3 × 104 C1498 firefly luciferase (C1498ff), a myeloid cell leukemia,23 and 104 B-cell lymphoma luciferase (TBL12 Luc; E-μ-MYC/BCRHEL).24 In brief, lethally irradiated B6 recipients were given TCD BM, with or without tumor cells and with or without T cells on the day of transplantation. The level of tumor burden was determined by injecting luciferin substrate (30 mg/mL, 0.1 mL per mouse; PerkinElmer) followed by imaging with the Xenogen IVIS imaging system.
Antibodies and flow cytometry
Treg isolation and suppression assay, an in vitro cell trace violet proliferation study, carboxyfluorescein succinimidyl ester (CFSE) staining, mixed-lymphocyte reactions (humans), inducible Treg (iTreg) generation, fluorescein isothiocyanate (FITC)-dextran assay, histopathology, lamina propria lymphocyte (LPL) isolation, bioluminescent imaging (BLI), RNA sequencing, transient transfection, receptor activation analysis for Nurr-1, peripheral Treg (pTreg) identification, BM chimeras, tamoxifen treatment, serum cytokine analysis, and statistical analyses are described in supplemental Methods (available on the Blood Web site).
Results
IRX4204 reduces intestinal damage and attenuates acute GVHD
The anti-inflammatory T-cell effects of IRX4204, such as reduction of IL-17– and IFN-γ–producing CD4+ T cells, prompted its testing in murine acute GVHD models.17 Lethally irradiated BALB/c recipients received transplants of donor B6 BM. Some cohorts received splenocytes (SPL; 5 × 106) and vehicle or IRX4204 given IP on days 0 to 56 or on days 0 to 100 (supplemental Figure 1A). Both schedules significantly improved survival, weights, and clinical parameters (supplemental Figure 1A) and had comparable long-term outcomes. Therefore, we focused on a daily schedule from day 0 to 56. Pooled data from 2 replicate experiments revealed a significant survival advantage to IRX4204- vs vehicle-treated recipients (Figure 1A).
Figure 1.
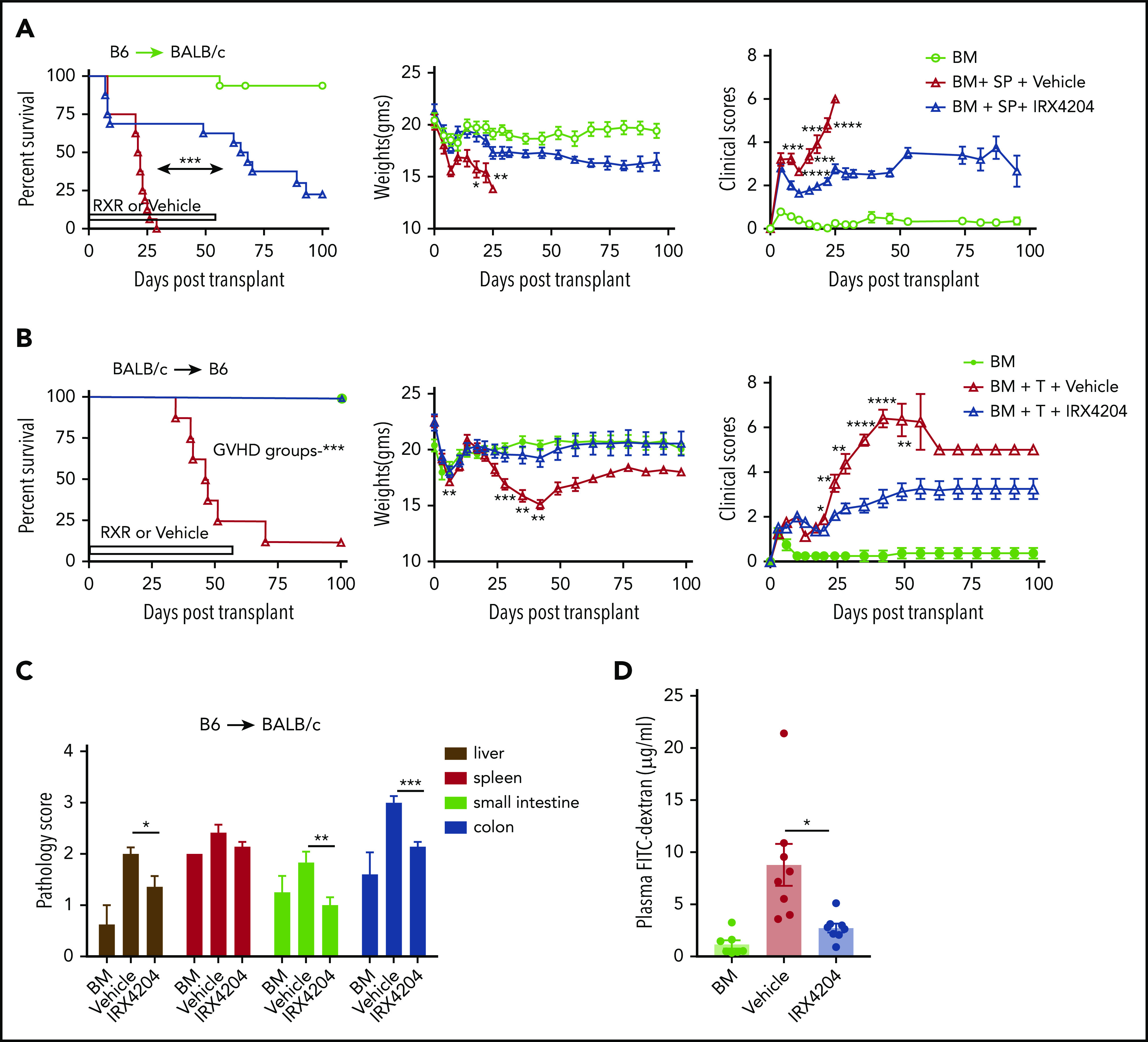
IRX4204 reduces intestinal damage and attenuates acute GVHD. (A) Survival, weights, and clinical scores of lethally irradiated BALB/c recipients of 107 B6 non-TCD BM cells, with or without 5 × 106 B6 splenocytes (SPL; n = 15−16 per group). Recipients were treated IP with either vehicle or IRX4204 from day 0 to 56 after transplantation. IRX4204-treated recipients survived longer than vehicle-treated recipients. Data are combined from 2 experiments. (B) Survival, weights, and clinical scores of lethally irradiated B6 recipients of 107 BALB/c NTCD BM cells, with or without 1.5 × 106 CD25- BALB/c T cells (n = 8/group). Recipients were treated with vehicle or IRX4204 from day 0 to 56. IRX4204-treated recipients survived longer than those receiving vehicle. (C) Histopathological scores of BALB/c recipients on day 7 after transplantation (n = 4-6 per group). (D) Plasma FITC-dextran concentration of BALB/c recipients on day 7 after transplantation (n = 7-8/group). *P < .05; **P < .01; ***P < .001; ****P < .0001.
Consistent with the beneficial effects of IRX4204 in the B6→BALB/c acute GVHD system, IRX4204 treatment of BALB/c→B6 recipients of BM plus purified T cells (1.5 × 106) was highly effective (Figure 1B). A shorter schedule given daily (day 0-42) or intermittently (day 0-21, then 4 days on and 3 days off through day 42) also significantly improved outcomes (supplemental Figure 1B). Next, we compared the efficacy of IRX4204 to one of the standard drugs used in GVHD prophylaxis. FK506 has been used widely to prevent GVHD.25 We compared the prophylactic and early treatment efficacy of IRX4204 vs FK506 in GVHD recipients by administering those drugs on days −3 to 11 or 3 to 17 after transplantation, respectively. Compared with FK506, IRX4204 significantly prolonged the survival of GVHD recipients in both regimens (supplemental Figure 1C). The data suggest that IRX4204 has favorable characteristics compared with FK506 in controlling acute GVHD at those doses, route, vehicles, and schedules. Except for the spleen, histopathology scores of day 7 GVHD target organs (liver and small and large intestine [SI and LI, respectively]) showed significant reductions in the IRX4204-treated cohort compared with the vehicle-treated one (Figure 1C). In GVHD mice, epithelial tight junctions are hindered, and the barrier integrity of the gastrointestinal tract is compromised. The loss of integrity permits microbes and bacterial lipopolysaccharides, among other immune stimuli, access to the host’s bloodstream.26 Using a nonabsorbable FITC-labeled dextran compound, we measured damage to the epithelial barrier by quantifying the amount of FITC-labeled dextran that leaked into the blood stream. On day 7 after transplantation, the IRX4204-treated recipients had significantly lower plasma levels of FITC dextran than those treated with vehicle, indicating less intestinal damage (Figure 1D).
IRX4204 amelioration of acute GVHD is associated with reduced donor T-cell expansion, Th1 differentiation, and proinflammatory cytokine production
Donor T-cell expansion is a prerequisite for GVHD initiation.27 We tested whether IRX4204 reduction in acute GVHD lethality is associated with a lower extent of allogeneic donor T-cell expansion in vivo. Lethally irradiated BALB/c recipients were infused with purified B6- luciferase transgenic (luc) donor T cells. BLI was used to determine the net expansion and homing of donor B6 luc T cells on day 6 after BMT. Whole-body BLI revealed that IRX4204-treated recipients tended to have lower signals than those of the vehicle controls (supplemental Figure 2A,C). Ex-vivo organ analysis showed reduced donor T cells and homing in major GVHD target organs (spleen and intestines) in IRX4204- vs vehicle-treated recipients (supplemental Figure 2B,D). No significant differences were seen in peripheral or mesenteric lymph nodes (MLNs), thymus, and liver (data not shown). IRX4204 directly repressed the proliferation of in vitro–activated mouse T cells (supplemental Figure 3A). We also observed that IRX4204 inhibited human CD4+ as well as CD8+ T-cell proliferation (supplemental Figure 3B) in allogeneic mixed-lymphocyte reaction cultures (supplemental Figure 3B). To determine whether the lower donor T-cell detection was related to lower proliferation, we labeled donor T cells with CFSE. Proliferation capacity and responder frequency of donor T cells were calculated as described previously.28 In spleen on day 6, the proliferation capacity of both CD4+ and CD8+ donor T cells and the responder frequency of CD8+ donor T cells were significantly reduced in recipients treated with IRX4204 vs vehicle (Figure 2A-B). As reduced proliferation may be caused by increased cell death, we used annexin-V staining to quantify donor T-cell apoptosis in IRX4204- vs vehicle-treated recipients. The percentages of annexin-V–stained donor CD4+ and CD8+ T cells were not significantly different between the groups (supplemental Figure 3C).
Figure 2.
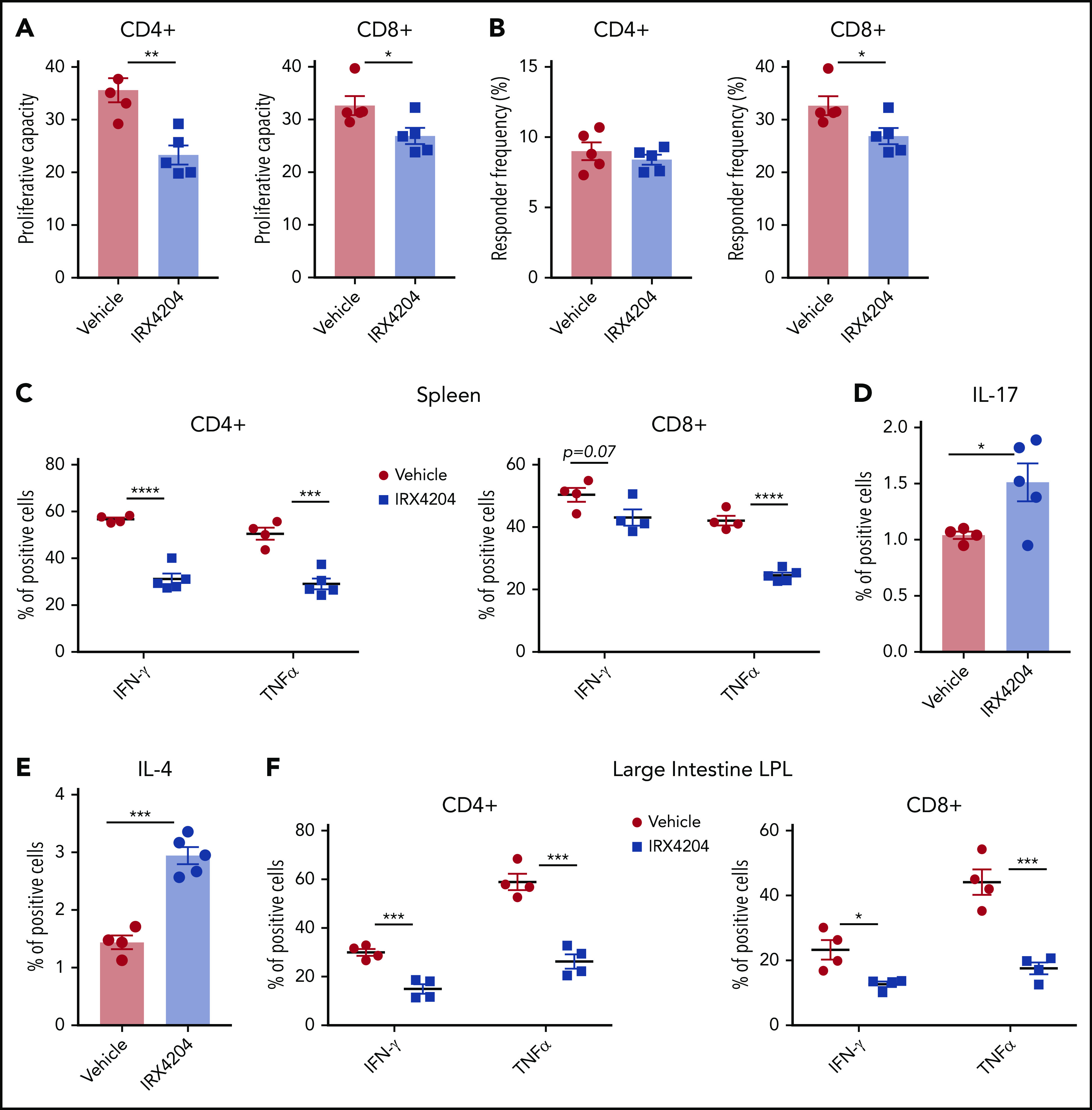
IRX4204 amelioration of acute GVHD is associated with reduced donor T-cell expansion and Th1 differentiation. (A-B) Lethally irradiated BALB/c recipients were infused with 107 B6 NTCD BM cells and 8.5 × 106 CFSE-labeled purified B6 T cells. Recipients were treated IP daily with either vehicle or IRX4204. Mice were euthanized on day 6 after transplantation to determine proliferation capacity (A) and responder frequency (B). (C-F) Lethally irradiated BALB/c recipients were infused with 107 B6 NTCD BM cells and 5 × 106 B6 splenocytes, with or without daily IP injections of IRX4204. The frequency of donor T cells expressing IFN-γ and TNF-α (C), IL-17 (D), and IL-4 (E) from spleen on day 7 and LI LPLs (F) on day 21 after transplantation was determined by intracellular staining. *P < .05; **P < .01; ***P < .001; ****P < .0001.
Because dampening RA signaling inhibits donor Th1 differentiation,13,14 we considered the possibility that IRX4204 skewed donor T cells from a proinflammatory to an anti-inflammatory phenotype in acute GVHD mice. To determine whether IRX4204 modulates Th1 differentiation in vivo, percentages of splenic IFN-γ– or TNF-α–producing donor Th1 cells were enumerated and found to be significantly lower on days 7 (Figure 2C) and 14 (CD4+, supplemental Figure 4A; CD8+, data not shown) in T cells isolated from IRX4204 vs vehicle-treated recipients. Consistent with these data, day 7 serum IFN-γ and TNF-α levels were significantly reduced in IRX4204- vs vehicle-treated recipients (supplemental Figure 4B-C). Unexpectedly, IRX4204-treated recipients had increased splenic IL-17–producing CD4+ T-cell frequency (Figure 2D). In contrast to mean splenic T-cell IFN-γ or TNF-α frequencies that ranged from 42% to 59% in controls, mean IL-17 frequencies were low at 1.0%, modestly increasing to only 1.5% with IRX4204 treatment. Moreover, IRX4204 increased the frequency of splenic IL-4–producing CD4+ T cells from 1.4% to 3% (Figure 2E), potentially offsetting the proinflammatory effects of IL-17.
Acute GVHD-induced gut injury is the primary cause of mortality, both in patients who undergo HSCT and in preclinical models.13,15,26 To determine whether these immunological perturbations in day 7 spleen and serum would be observed in the intestine during a time of acute GVHD injury, day 21 LI LPLs were isolated from GVHD mice treated with IRX4204 or vehicle. Compared with vehicle controls, day 13 and 21 LPLs of IRX4204-treated recipients had lower frequencies of IFN-γ- and TNF-α–producing donor T cells (supplemental Figure 4D; Figure 2F). These data indicate that the increased survival of IRX4204-treated recipients impairs donor T-cell proliferation and reduces proinflammatory cytokine levels.
The transcriptional profile of gut-localized donor T cells shows lower proinflammatory gene expression
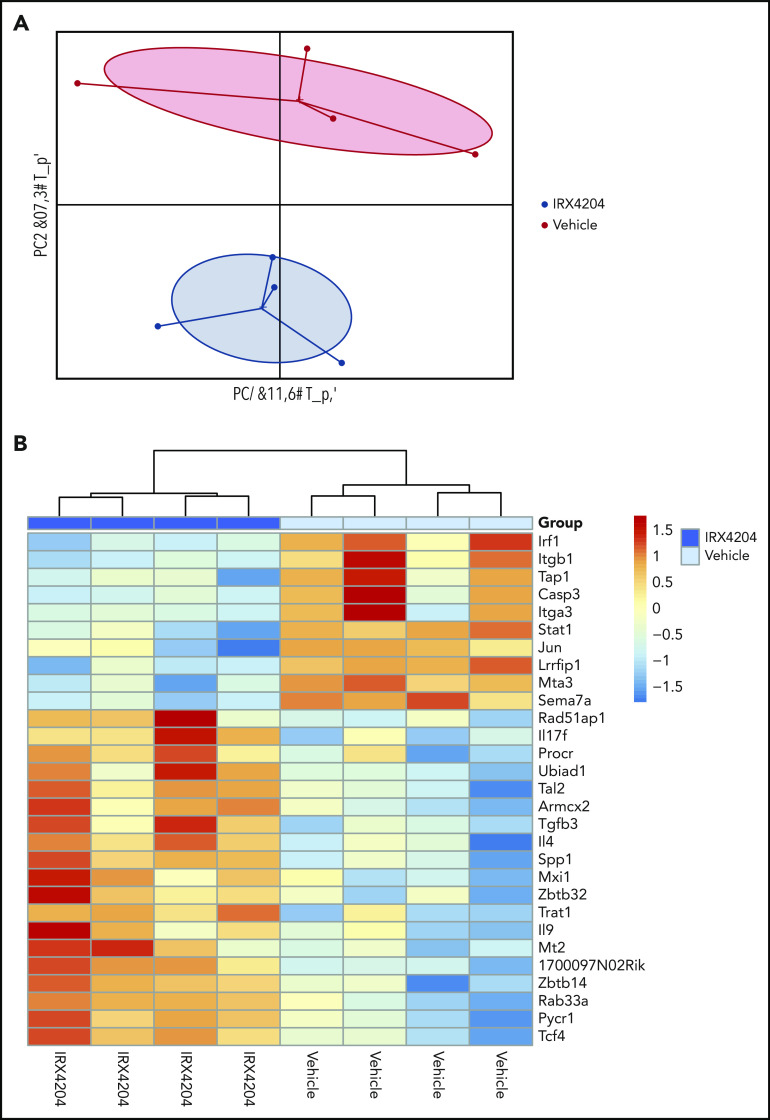
Because RA upregulates gut-homing receptors on T cells, we next sought to determine whether the IRX4204 attenuation of GVHD gut injury impairs the upregulation of gut-homing receptor (CCR9, α4β7, CXCR3) expression on donor T cells.29 Surprisingly, as compared with vehicle controls, the percentage of splenic donor T cells expressing CCR9 or α4β7 (only CD8+) was significantly higher in IRX4204-treated recipients, although CXCR3 expression was significantly lower (supplemental Figure 5). To begin to elucidate the mechanism by which IRX4204 reduces gut injury, despite enhancing donor T-cell expression of CCR9 and CD8 α4β7 gut-homing receptors, on day 18, we isolated SI donor T cells of treated recipients and analyzed their gene expression profiles with NanoString (Figure 3). Principal component analysis revealed a distinct signature between the 2 groups, suggesting that IRX4204 modulates the transcriptional profile of donor T cells. Significant decreases were observed in several proinflammatory transcripts including Sema7a, Stat1, Irf1; a proliferation transcript Jun; and cell migratory Itgb1, along with increased anti-inflammatory IL-4. Overall, these data suggest that IRX4204 treatment reduces the expression of proinflammatory transcripts in gut-infiltrating donor T cells (Figure 3).
Figure 3.
IRX4204 modulates the transcription profile of gut-localized donor T cells. BALB/c recipients were lethally irradiated and infused with 107 B6 NTCD CD45.2 BM cells and 1.5 × 106 B6 CD45.1+ CD4+ T cells. Recipients were treated IP with either vehicle or IRX4204 daily. On day 18 after transplantation, LP donor (CD45.1+) T cells were isolated from the SI of treated recipients. RNA was isolated from these LP donor T cells for NanoString gene expression analysis (n = 4/group). PCA plots (A) and heat maps (B) are shown.
IRX4204 promotes iTreg and pTreg generation
CD25-depleted donor T-cell grafts can accelerate GVHD lethality.30,31 Conversely, cotransferring Tregs with donor T cells can markedly suppress acute GVHD lethality.32,33 Therefore, we investigated whether the observed skewing of gut-infiltrating donor T cells toward an anti-inflammatory gene expression profile and increased survival in IRX4204 recipients is related to enhanced generation of pTregs. IRX4204-treated recipients had higher percentages of Tregs in spleen, MLNs, and LI LPLs than did vehicle-treated controls at 2 time points (days 7 and 14) after transplantation (Figure 4A-B). Absolute numbers of Tregs were also significantly higher in spleen and MLNs (supplemental Figure 6A), but not in LI LPLs (data not shown). Furthermore, the percentage of Tregs was significantly higher in the peripheral blood and spleen of IRX4204-treated recipients at day 6 after transplantation (supplemental Figure 6B-E). No differences were observed in the percentages of T-effector/-memory cells in the peripheral blood and spleens of GVHD recipients, except the percentage of splenic CD8+ cells that was significantly reduced in IRX4204 recipients (supplemental Figure 6F-G). These data indicate that IRX4204 directly or indirectly enhances Treg generation, expansion, and stability and/or reduces Treg cell death.
Figure 4.
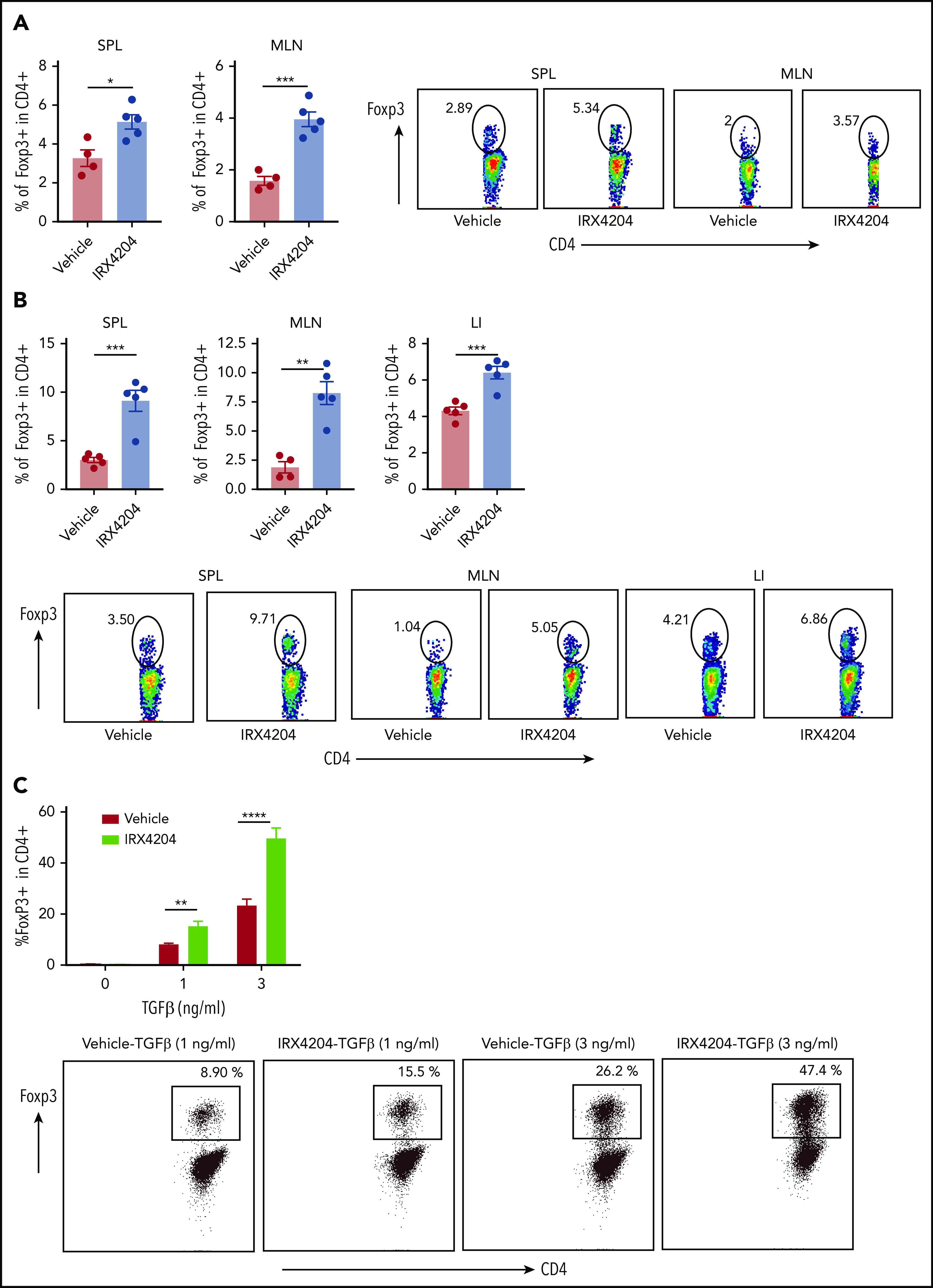
Increased frequency of Tregs in lymphoid and nonlymphoid tissues of IRX4204-treated recipients. (A-B) BALB/c recipients were lethally irradiated and infused with 107 B6 NTCD BM cells and 5 × 106 B6 splenocytes. Recipients were treated IP with either vehicle or IRX4204 daily. The frequency of donor Tregs and representative flow cytometry plots show Foxp3 expression in donor CD4+ cells on days 7 (spleen [SPL], mesenteric lymph nodes [MLN]) (A) and 14 (B) after transplantation. Data from an experiment with n = 5 per group per day. (C) Naive CD4+ T cells (CD4+ CD44loCD62Lhi) were activated with soluble anti-CD3, TGF-β, and irradiated T-cell–depleted syngeneic splenocytes, with or without 100 nM IRX4204. The percentage of Foxp3+ cells was determined on day 4 by flow cytometry. Data are representative of 2 experiments. *P < .05; **P < .01; ***P < .001; ****P < .0001.
Enhanced Treg percentages prompted us to test the effects of IRX4204 on Treg generation both in vitro and in vivo. To determine whether IRX4204 would support iTreg generation, purified naive CD4+ CD25− CD44low CD62Lhi T cells were exposed to IRX4204 (100 nM) in the presence of TGFβ and syngeneic irradiated TCD splenocytes loaded with the anti-CD3 monoclonal antibody. As shown in Figures 4C, IRX4204 enhanced iTreg generation as analyzed on day 4. We found that IRX4204 consistently enhanced human Tregs in mixed-lymphocyte reaction cultures (supplemental Figure 7A). Retroviral transduction of murine naive CD4+ T cells with Nurr-1 (Nr4a2) has been shown to induce suppressive Foxp3+ T cells, whereas CD4+ T-cell Nurr-1 deficiency impairs iTreg generation.34 Previous studies have reported that Nurr-1 can form heterodimers with RXRs that can be activated by RXR ligands.16,35 To examine whether IRX4204 can activate the RXR-Nurr-1 pathway, COS cells were transfected with an effector plasmid containing the ligand binding domain of Nurr-1 fused to the GAL4 DNA-binding domain (GAL-Nurr-1), together with a GAL4UAS-dependent luciferase reporter gene. Some cells were cotransfected with an effector plasmid expressing the ligand binding domain of RXRα (L-RXRα).36 In the presence of L-RXRα, luciferase expression was strongly activated whereas, in the absence of L-RXRα, only basal levels were observed (supplemental Figure 7B). Because L-RXR is unable to bind to DNA, these findings indicate that IRX4204 can robustly activate the RXRα/Nurr-1 heterodimers.
To test whether IRX4204 could increase pTregs in vivo, irradiated BALB/c mice received wild-type (WT) B6 BM and flow cytometry–purified (99%) Foxp3-GFP− donor T cells from Foxp3-GFP KI mice. Cohorts were treated with IRX4204 or vehicle from day 0 to 14. In IRX4204-treated recipients, there was a two- to threefold increase in the percentage of CD4 GFP+ (Foxp3+) donor T cells (pTregs) in spleens, MLNs, and SI (Figure 5A-D). The frequency of CD8 Tregs was also higher in MLNs, with a trend toward significance seen for SI (Figure 5A-C). The number of pTregs was also significantly higher in spleen and MLNs of IRX4204-treated recipients (supplemental Figure 7C). In SI LPLs, a statistical trend (P = .08) toward a higher absolute number of pTregs was seen with IRX4204 vs vehicle (supplemental Figure 7C). To further determine whether the reduced lethality in IRX4204-treated recipients depended on increased pTregs, congenic B6 WT mice were lethally irradiated and used as recipients for creating scurfy chimeras by infusing TCD donor BM cells from male B6 scurfy and congenic B6 WT mice. After 3 months of reconstitution, most of the T cells in the spleens of chimeras were derived from scurfy BM. Scurfy CD25− T cells isolated from chimeras were injected with WT BM into lethally irradiated BALB/c recipients treated with IRX4204 or vehicle. For comparison, a cohort of lethally irradiated BALB/c recipients were infused with B6 BM + CD25− WT T cells. Importantly, IRX4204 did not prevent acute GVHD in recipients given CD25− scurfy T cells, whereas GVHD lethality was significantly reduced when CD25− WT T cells were infused (Figure 5E-F). These data indicate that IRX4204 requires functional pTregs generated from donor T cells to mitigate GVHD.
Figure 5.
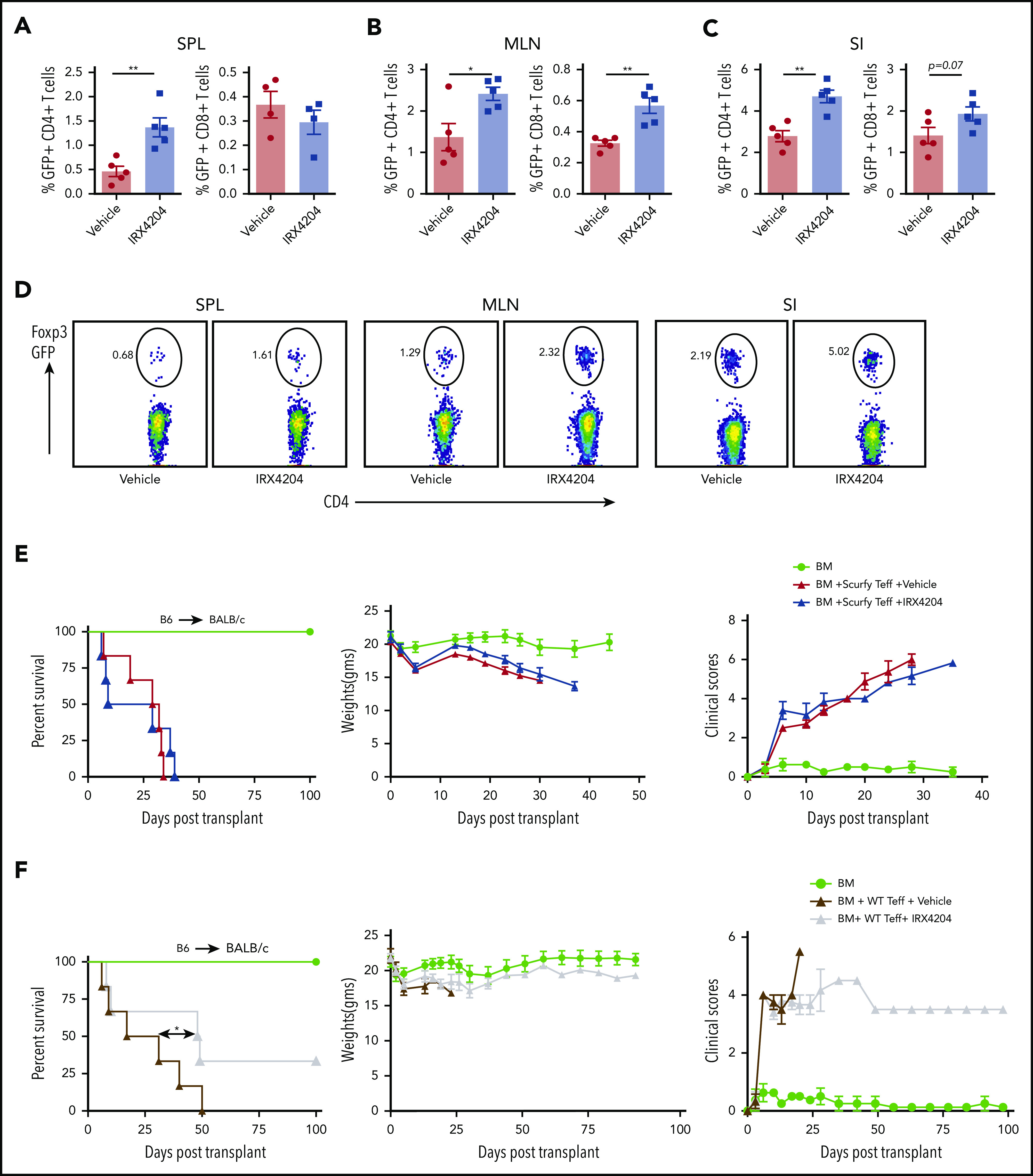
Effects of IRX4204 in acute GVHD depends on pTreg generation. (A-C) Lethally irradiated BALB/c recipients were infused with 107 B6 NTCD BM cells and 1 × 106 flow cytometry–sorted GFP− T cells from B6 Foxp3 GFP KI mice. Recipients were injected with either vehicle or IRX4204 IP daily. The frequency of GFP+ T cells (pTregs) was determined in recipient spleens (SPL; A), MLNs (B), and SI (C) 2 weeks after transplantation. (D) Representative flow cytometry plots showing Foxp3 expression in donor CD4+ cells from GVHD mouse SPL), MLN, and SI, treated with either vehicle or IRX4204. (E-F) Lethally irradiated BALB/c recipients were infused with 107 B6 NTCD BM cells and 1 × 106 purified B6 scurfy or WT CD25− T cells isolated from scurfy chimeras and WT mice, respectively. Recipients were injected daily IP with either vehicle or IRX4204. Survival plots, weights, and clinical scores of scurfy (E) and WT (F) are shown. One experiment was performed in n = 5-6 per group. *P < .05; **P < .01.
IRX4204 stabilizes Tregs but without increasing suppressor function
Nurr-1 has also been shown to sustain Treg stability by maintaining Foxp3 expression.34 We therefore evaluated whether IRX4204 can increase Treg stability in GVHD settings, by using FoxP3 lineage tracing in Rosa-RFP × Foxp3eGFP-Cre-ERT2 reporter mice.20 In this model, cells that once expressed Foxp3 but lost expression (ex-Tregs; RFP+ eGFP−) can be distinguished from cells currently expressing Foxp3 (RFP+ eGFP+). Lethally irradiated BALB/c recipients were infused with CD45.1+ WT BM, T cells, and CD45.2+ eGFP+ RFP+ flow cytometry–sorted Tregs (∼99% purified) and then recipients were treated with IRX4204 or vehicle. Compared with vehicle controls, IRX4204 treatment significantly reduced the percentage of ex-Tregs (eGFP−RFP+) in SI (Figure 6A) but not spleen (Figure 6B) on day 18 after transplantation. Tregs from IRX4204-treated recipients trended toward lower expression of the proinflammatory cytokines IFN-γ and IL-17, which may be associated with increased Treg stability (Figure 6C-D). These results indicate that IRX4204 can stabilize Tregs in a highly inflammatory environment.
Figure 6.
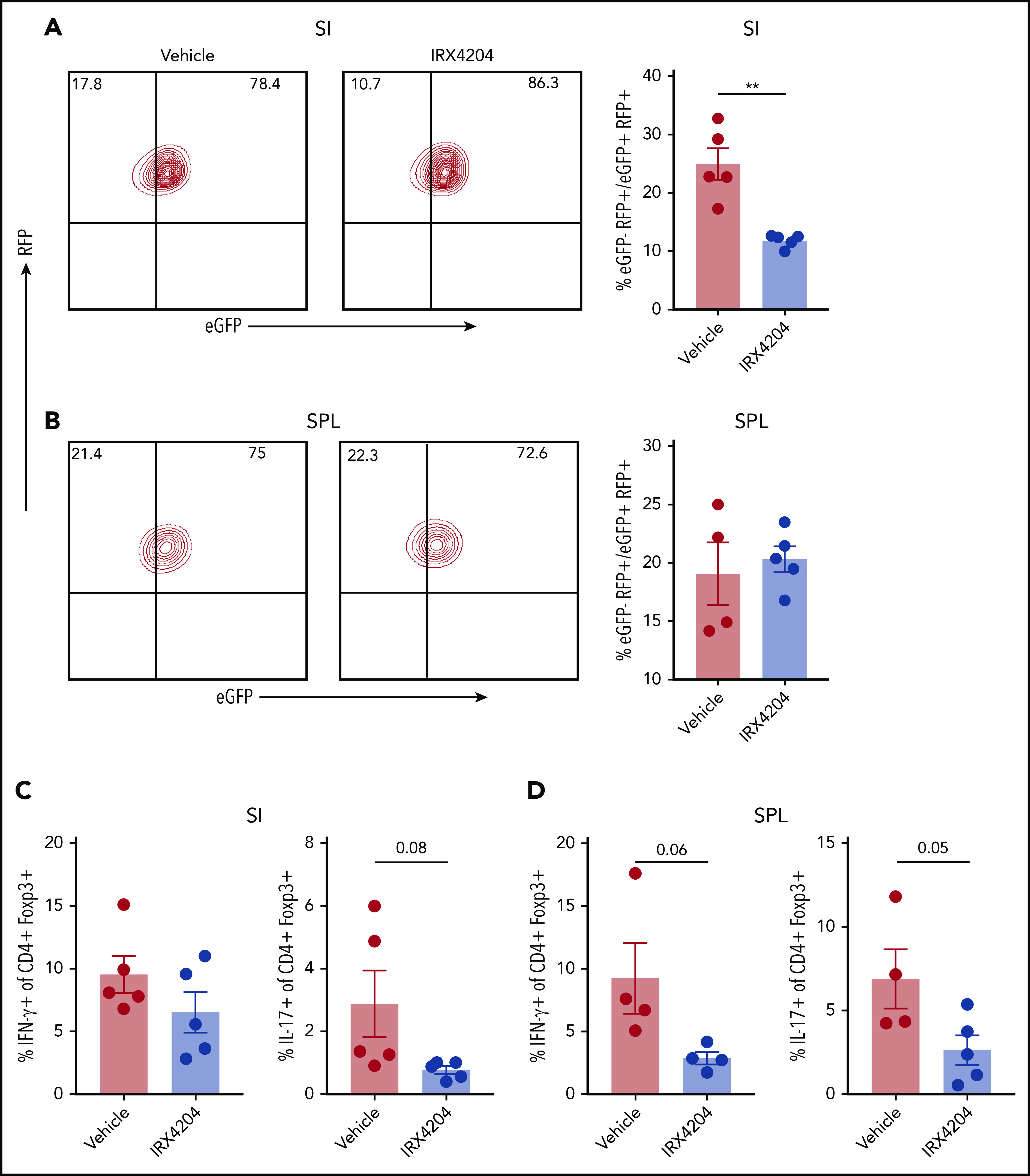
IRXR4204 stabilizes Tregs. (A-D) Lethally irradiated BALB/c recipients were infused with 107 CD45.1+ B6 NTCD BM cells, along with 0.9 × 106 CD45.1+ CD25- T cells and 0.45 × 106 flow cytometry–sorted RFP+ eGFP+ Tregs (A-B) from B6 CD45.2+ Rosa-RFP × Foxp3eGFP-Cre-ERT2 reporter mice. Recipients were treated with either vehicle or IRX4204. On day 18 after transplantation, the frequency of ex-Tregs (RFP+ eGFP−) was analyzed in SIs (A) and SPLs (B). (A-B) Representative lineage tracing of Tregs in vehicle- vs IRX4204-treated mice by flow cytometry. Cells depicted are gated on donor CD45.2+ CD4+ T cells. Bar graphs indicate the frequency of ex-Tregs. (C-D) SI LPLs and splenocytes and were restimulated with phorbol 12-myristate 13-acetate and ionomycin to determine the frequency of IFN-γ– and IL-17– producing donor CD45.2+ Tregs. One experiment was performed. **P < .01.
To test whether IRX4204 directly enhances Treg function, we treated freshly isolated Tregs with IRX4204 overnight before a standard suppression assay. IRX4204 led to a modest, albeit inconsistent, reduction in suppression of CD4+ T cells with even lesser effects on CD8+ T cells, the latter in only 1 of 3 Treg/T effector cell ratios tested (supplemental Figure 7D).
IRX4204 inhibition of acute GVHD did not abrogate GVL responses
To evaluate whether IRX4204 treatment compromises GVL responses, we used 2 different aggressive tumor models: C1498ff, an acute myeloid leukemia model,23 and TBL12, an E-μ-MYC/BCRHEL transgenic B-cell lymphoma model.24 Tumor cells were transduced with luciferase to track tumor growth by BLI.23,24 Recipients transplanted with BM+ C1498 tumor cells (3 × 104; day 0) had disseminated tumor growth and died of tumor burden by day 25 (Figure 7). Donor T cells (1.5 × 106) added to BM and tumor (BM+T+C1498) significantly restrained tumor growth. Vehicle-treated recipients died of acute GVHD without detectable BLI signal of tumor burden. Notably, the IRX4204 group of T-cell recipients had no tumor detectable by BLI and survived significantly longer than the other groups (Figure 7). Deaths were consistent with the tempo and magnitude of GVHD lethality. Cytolytic pathways are crucial to mediate GVL responses.3 The frequency of day 6 splenic donor T cells expressing granzyme B was significantly reduced in IRX4204 recipients compared with the control (CD4+: 11.6% vs 7%, P < .0001; CD8+: 25.9% vs 14.1%, P < .001). Similar to the C1498ff model, TBL12 was not detectable by BLI, whereas survival was prolonged, with 40% succumbing to GVHD (supplemental Figure 8). Together, these results suggest that treatment with IRX4204 does not eliminate GVL responses against acute myeloid leukemia and an aggressive B-cell lymphoma line.
Figure 7.
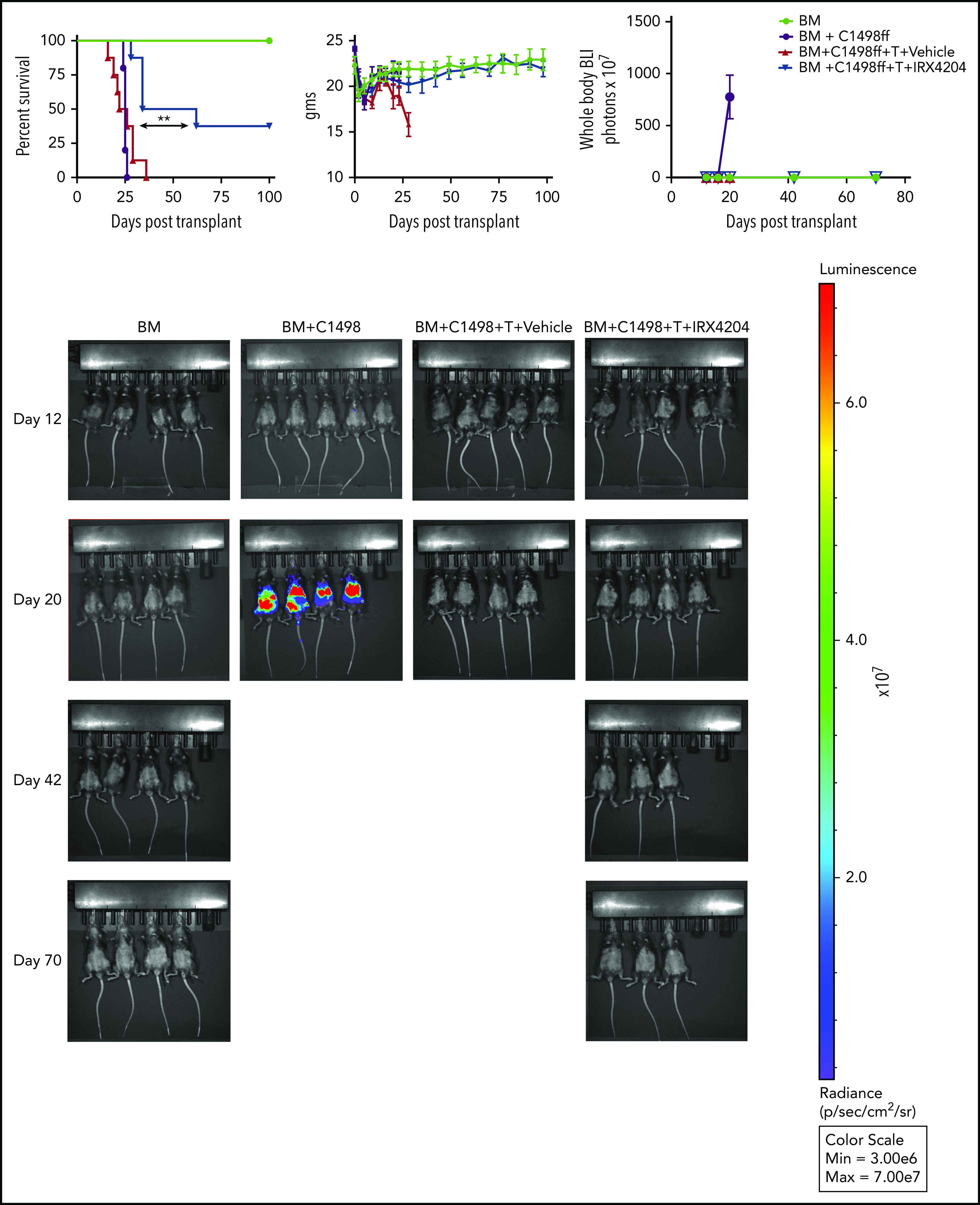
IRX4204 inhibition of acute GVHD did not abrogate GVL responses. Lethally irradiated B6 recipients were received 107 BALB/c T-cell–depleted BM, with or without B6 C1498ff (3 × 104), with or without 1.5 × 106 BALB/c CD25− T cells. Recipients were treated IP with either vehicle or IRX4204 from day 0 to 56 after transplantation. Survival, weights, and BLI graph are shown. Tumor growth was monitored with BLI on the days indicated. BLI images are shown (n = 5-8 per group). One experiment was performed. **P < .01.
Discussion
The amelioration of acute GVHD by the novel RXR agonist IRX4204 was associated with decreased donor T-cell proliferation, impaired Th1 differentiation, increased pTregs, and a preserved GVL effect. IRX4204 compared favorably to FK506 in preventing and controlling acute GVHD lethality under the conditions tested.
The differentiation of naive CD4+ T cells into Th1 (CD4+ IFN-γ+ cells) is an important factor in driving the pathogenesis and tissue damage of GVHD.2,37 TNF-α also aggravates acute GVHD and can lead to lethality.38 An earlier study reported that RA signaling is necessary for in vivo Th1 stability by supporting the expression of Th1 lineage-determining genes.39 IRX4204 is a potent activator of the Nurr-1 signaling pathway that has been shown to repress Th1 differentiation by an as yet unknown mechanism.34 Consistent with these findings, IRX4204-treated recipients had reduced percentages of IFN-γ− and TNF-α–secreting donor T cells and also levels of serum IFN-γ and TNF-α, which could lead to reduced tissue damage, as demonstrated by lower pathology scores and increased intestinal epithelial integrity. In addition, there was a twofold increase in the percentages of splenic CD4+ IL-4+ cells between the 2 groups, suggesting that IRX4204 treatment favors an anti-inflammatory phenotype.
We also found that donor T cells isolated from IRX4204-treated intestines exhibited an anti-inflammatory signature, with a significant increase in IL4 transcripts and reduced proinflammatory transcripts Stat1, Sema7a, Irf1, and Jun. Several transcripts are involved with proinflammatory cytokine production that can drive (eg, Stat1) or suppress (eg, IL-4) acute GVHD.40,41 Sema7a (CD108), the class 7 semaphorin, is expressed by activated T cells and has been shown to contribute to T-cell–mediated inflammation by promoting Th1/Th17 differentiation.42,43 Earlier studies reported that interferon-γ–induced transcription factor (Irf1) promotes Th1 differentiation by suppressing IL4 gene transcription.44,45 Irf1 affects Treg function by repressing Foxp3 expression.46 Jun heterodimerizes with activator protein-1 (AP-1) transcription factor, shown to enhance T-cell proliferation and survival.47 A previous study reported that the pharmacological blockade of AP-1 pathway attenuates acute GVHD by reducing Th1, Th17 differentiation while increasing Th2, Treg differentiation.48
Chemokine receptors and integrins participate in regulating the migration of T cells to GVHD target organs.49 CXCR3−/− donor T-cell infusion reduces gastrointestinal and liver GVHD and improves survival.50 IFN-γ–producing Th1 and effector CD8+ T cells preferentially express CXCR3.51 In the current study, IRX4204-treated recipients had a lower frequency of CXCR3-expressing donor T cells that may have been caused by the reduction in IFN-γ+–producing donor T cells. Furthermore, donor T cells isolated from IRX4204-treated intestines had a decrease in Itgb1 that would result in decreased T-cell migration to inflamed organs.52
We also observed that IRX4204 treatment significantly inhibited the proliferation of murine T cells both in vitro and in vivo. Similarly, IRX4204 inhibited the proliferation of human CD4+ and CD8+ T cells. The reduced proliferation in acute GVHD was not related to activation-induced cell death, as annexin-V staining of donor T cells was not significantly different between the 2 groups. Among other possibilities are increased pTreg generation and reduced systemic proinflammatory cytokines that may contribute to reduced proliferation of donor T cells in IRX4204-treated recipients.
Tregs from patients with acute GVHD have impaired stability.53 We found that IRX4204 treatment enhanced Treg generation and stability, but not function, potentially mediated by activation of the RXR-Nurr-1 pathway. Nurr-1 activated the Foxp3 promoter/enhancer to augment Foxp3 expression on CD4+ T cells, which maintains the suppressive capacity of Tregs.34 The beneficial effect of IRX4204 in GVHD depended on pTreg generation from donor T cells, as IRX4204 failed to attenuate GVHD in recipients that were infused with Foxp3-deficient scurfy donor T cells in contrast to coinfusion with WT donor T cells. By using GFP− T cells from Foxp3 GFP-KI mice, we demonstrated that IRX4204 potentiated pTreg generation. Although IRX4204 is capable of enhancing iTreg generation, the reduction of proinflammatory cytokine milieu may also have favored pTreg generation in those treated recipients, similar to what we observed when RA signaling is impaired in donor T cells.13 We31 and others54 have also shown that by infusing blocking antibodies for proinflammatory cytokines, pTregs are increased and acute GVHD reduced. We favor the hypothesis that the direct effects of IRX4204 on supporting pTreg generation and indirect effects of lowering proinflammatory cytokines, when taken together, provide an optimal environment to suppress GVHD.
In response to inflammatory milieu, Tregs can lose Foxp3 expression and convert into effector cells. Nurr-1 has been shown to confer Treg lineage stability by cooperating with Runx/CBFβ.34 In the present study, we found that IRX4204 treatment promoted Treg stability. Given its ability to maintain Treg stability, IRX4204 could be beneficial in clinical settings.
Gut injury plays a critical role in GVHD lethality by creating a proinflammatory environment, leading to the recruitment of donor T cells that exacerbates gut injury.26 Diminishing RA signaling in donor T cells attenuated acute GVHD associated with reduced gut-homing receptor expression.13 Interestingly, IRX4204 treatment upregulated CCR9 on donor CD4+, CD8+ T cells and α4β7 on donor CD8+ T cells. The reduced gut injury observed in IRX4204 recipients may have been attributable to altered proinflammatory gene expression, decreased CXCR3 expression on donor T cells, or enhanced Treg generation. Consistent with RA-induced gut homing of iTregs, IRX4204 upregulated gut-homing receptors on donor T cells. It will be interesting to test whether IRX4204 can improve the potency of Treg therapy in GVHD by treating ex vivo Tregs with IRX4204 for subsequent efficient homing of those Tregs to the inflamed gut.
Harnessing donor T-cell immune responses in promoting GVL and suppressing GVHD has been a significant challenge in HSCT. In the current study, despite increased pTregs in IRX4204-treated recipients, GVL responses against 2 aggressive tumor models were not lost at the tumor cell doses we tested. Data from previous studies have suggested that there is a threshold difference in donor T-cell responses that mediate GVHD vs those that mediate GVL.55,56 We surmised that increased Tregs, observed in the current study, did not appear to impair T-cell responses to levels lower than the GVL threshold.
In summary, IRX4204 exerts potent immunomodulatory effects by suppressing acute GVHD while preserving alloreactive responses against tumors. Currently, it is being tested in a clinical trial (www.clinicaltrials.gov #NCT02991651) as an anticancer agent and may be readily testable in patients who undergo HSCT. As IRX4204 enhances Treg generation in both mouse and human T cells, other clinical uses such as prevention of autoimmunity and organ graft rejection can be envisioned.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The authors thank Elizabeth Nowak for input.
This work was funded by National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases grants P01 AI056299 and R01 AI034495, and NIH/National Heart, Lung, and Blood Institute grants R01 HL56067 (B.R.B.) and R01 AI091627 (I.M.); Deutsche Forschungsgemeinschaft grants 872/4-1 and SFB1160 TP B09, Deutsche Krebshilfe grant 70113473, Jose-Carreras Leukemia Foundation grant DJCLS 01R/2019 (R.Z); a fellowship from the Canadian Institutes of Health Research (CIHR) (G.T.); and funding from the Leukemia and Lymphoma Society, the Children’s Cancer Research Fund, and the KidsFirst Fund (B.R.B.).
Conflict-of-interest disclosure: B.R.B. has received remuneration as an advisor to Kamon Pharmaceuticals, Inc, Five Prime Therapeutics Inc, Regeneron Pharmaceuticals, Magenta Therapeutics, and BlueRock Therapeutics and research support from Fate Therapeutics, RXi Pharmaceuticals, Alpine Immune Sciences Inc, and Abbvie Inc. B.R.B. is a cofounder of Tmunity. The remaining authors declare no competing financial interests.
Roshantha A. Chandraratna died on 8 January 2019.
Footnotes
Original data are available by e-mail request to the corresponding author.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: G.T. designed and performed research, provided and analyzed the data, and wrote the paper; C.W., M.J.O., M.L., A.S., K.A., C.M.-H., E.G.A., and K.L.H. performed experiments, and edited the paper; S.N.F. and C.B. analyzed the data; A.S.J. and B.B. performed experiments and provided the data; Y.R. provided TBL12 tumor cells and edited the paper; R.A.C. provided reagents and feedback; A.P.-M. performed histopathological analysis and edited the paper; K.P.M., G.R.H., R.Z., I.M., J.S.S., W.J.M., D.H.M., B.B., V.K., L.S.K., and R.J.N., provided advice and edited the paper; and B.R.B. designed, organized, and supervised the research and edited the paper.
Correspondence: Govindarajan Thangavelu, Department of Pediatrics, University of Minnesota, 420 Delaware St SE, MMC 109, Minneapolis, MN 55455; e-mail: than0023@umn.edu.
REFERENCES
- 1.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363(22):2091-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeiser R, Blazar BR. Acute Graft-versus-Host Disease - Biologic Process, Prevention, and Therapy. N Engl J Med. 2017;377(22):2167-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van den Brink MR, Burakoff SJ. Cytolytic pathways in haematopoietic stem-cell transplantation. Nat Rev Immunol. 2002;2(4):273-281. [DOI] [PubMed] [Google Scholar]
- 4.Anderson BE, Tang AL, Wang Y, et al. Enhancing alloreactivity does not restore GVHD induction but augments skin graft rejection by CD4+ effector memory T cells. Eur J Immunol. 2011;41(9):2782-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83(6):841-850. [DOI] [PubMed] [Google Scholar]
- 6.Szanto A, Narkar V, Shen Q, Uray IP, Davies PJ, Nagy L. Retinoid X receptors: X-ploring their (patho)physiological functions. Cell Death Differ. 2004;11(suppl 2):S126-S143. [DOI] [PubMed] [Google Scholar]
- 7.Evans RM, Mangelsdorf DJ. Nuclear Receptors, RXR, and the Big Bang. Cell. 2014;157(1):255-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du X, Tabeta K, Mann N, Crozat K, Mudd S, Beutler B. An essential role for Rxr alpha in the development of Th2 responses. Eur J Immunol. 2005;35(12):3414-3423. [DOI] [PubMed] [Google Scholar]
- 9.Stephensen CB, Borowsky AD, Lloyd KC. Disruption of Rxra gene in thymocytes and T lymphocytes modestly alters lymphocyte frequencies, proliferation, survival and T helper type 1/type 2 balance. Immunology. 2007;121(4):484-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo Y, Brown C, Ortiz C, Noelle RJ. Leukocyte homing, fate, and function are controlled by retinoic acid. Physiol Rev. 2015;95(1):125-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao S, Jin H, Korn T, et al. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J Immunol. 2008;181(4):2277-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larange A, Cheroutre H. Retinoic Acid and Retinoic Acid Receptors as Pleiotropic Modulators of the Immune System. Annu Rev Immunol. 2016;34(1):369-394. [DOI] [PubMed] [Google Scholar]
- 13.Aoyama K, Saha A, Tolar J, et al. Inhibiting retinoic acid signaling ameliorates graft-versus-host disease by modifying T-cell differentiation and intestinal migration. Blood. 2013;122(12):2125-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thangavelu G, Lee YC, Loschi M, et al. Dendritic Cell Expression of Retinal Aldehyde Dehydrogenase-2 Controls Graft-versus-Host Disease Lethality. J Immunol. 2019;202(9):2795-2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Dodge J, Komorowski R, Drobyski WR. A critical role for the retinoic acid signaling pathway in the pathophysiology of gastrointestinal graft-versus-host disease. Blood. 2013;121(19):3970-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Bi W, Zhao W, et al. Selective brain penetrable Nurr1 transactivator for treating Parkinson’s disease. Oncotarget. 2016;7(7):7469-7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandraratna RA, Noelle RJ, Nowak EC. Treatment with retinoid X receptor agonist IRX4204 ameliorates experimental autoimmune encephalomyelitis. Am J Transl Res. 2016;8(2):1016-1026. [PMC free article] [PubMed] [Google Scholar]
- 18.Balasubramanian S, Chandraratna RA, Eckert RL. Suppression of human pancreatic cancer cell proliferation by AGN194204, an RXR-selective retinoid. Carcinogenesis. 2004;25(8):1377-1385. [DOI] [PubMed] [Google Scholar]
- 19.Stephensen CB, Rasooly R, Jiang X, et al. Vitamin A enhances in vitro Th2 development via retinoid X receptor pathway. J Immunol. 2002;168(9):4495-4503. [DOI] [PubMed] [Google Scholar]
- 20.Fan MY, Low JS, Tanimine N, et al. Differential Roles of IL-2 Signaling in Developing versus Mature Tregs. Cell Rep. 2018;25(5):1204-1213 e1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vuligonda V, Thacher SM, Chandraratna RA. Enantioselective syntheses of potent retinoid X receptor ligands: differential biological activities of individual antipodes. J Med Chem. 2001;44(14):2298-2303. [DOI] [PubMed] [Google Scholar]
- 22.Blazar BR, Taylor PA, Fitzsimmons WE, Vallera DA. FK506 inhibits graft-versus-host disease and bone marrow graft rejection in murine recipients of MHC disparate donor grafts by interfering with mature peripheral T cell expansion post-transplantation. J Immunol. 1994;153(4):1836-1846. [PubMed] [Google Scholar]
- 23.Taylor PA, Ehrhardt MJ, Lees CJ, et al. Insights into the mechanism of FTY720 and compatibility with regulatory T cells for the inhibition of graft-versus-host disease (GVHD). Blood. 2007;110(9):3480-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Refaeli Y, Young RM, Turner BC, Duda J, Field KA, Bishop JM. The B cell antigen receptor and overexpression of MYC can cooperate in the genesis of B cell lymphomas. PLoS Biol. 2008;6(6):e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blazar BR, Weisdorf DJ, Defor T, et al. Phase 1/2 randomized, placebo-control trial of palifermin to prevent graft-versus-host disease (GVHD) after allogeneic hematopoietic stem cell transplantation (HSCT). Blood. 2006;108(9):3216-3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90(8):3204-3213. [PubMed] [Google Scholar]
- 27.Socié G, Blazar BR. Acute graft-versus-host disease: from the bench to the bedside. Blood. 2009;114(20):4327-4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wells AD, Gudmundsdottir H, Turka LA. Following the fate of individual T cells throughout activation and clonal expansion. Signals from T cell receptor and CD28 differentially regulate the induction and duration of a proliferative response. J Clin Invest. 1997;100(12):3173-3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21(4):527-538. [DOI] [PubMed] [Google Scholar]
- 30.Taylor PA, Noelle RJ, Blazar BR. CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J Exp Med. 2001;193(11):1311-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bucher C, Koch L, Vogtenhuber C, et al. IL-21 blockade reduces graft-versus-host disease mortality by supporting inducible T regulatory cell generation. Blood. 2009;114(26):5375-5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99(10):3493-3499. [DOI] [PubMed] [Google Scholar]
- 33.Pierini A, Colonna L, Alvarez M, et al. Donor Requirements for Regulatory T Cell Suppression of Murine Graft-versus-Host Disease. J Immunol. 2015;195(1):347-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sekiya T, Kashiwagi I, Inoue N, et al. The nuclear orphan receptor Nr4a2 induces Foxp3 and regulates differentiation of CD4+ T cells. Nat Commun. 2011;2(1):269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perlmann T, Jansson L. A novel pathway for vitamin A signaling mediated by RXR heterodimerization with NGFI-B and NURR1. Genes Dev. 1995;9(7):769-782. [DOI] [PubMed] [Google Scholar]
- 36.Forman BM, Umesono K, Chen J, Evans RM. Unique response pathways are established by allosteric interactions among nuclear hormone receptors. Cell. 1995;81(4):541-550. [DOI] [PubMed] [Google Scholar]
- 37.Yu Y, Wang D, Liu C, et al. Prevention of GVHD while sparing GVL effect by targeting Th1 and Th17 transcription factor T-bet and RORγt in mice. Blood. 2011;118(18):5011-5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooke KR, Hill GR, Crawford JM, et al. Tumor necrosis factor- alpha production to lipopolysaccharide stimulation by donor cells predicts the severity of experimental acute graft-versus-host disease. J Clin Invest. 1998;102(10):1882-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown CC, Esterhazy D, Sarde A, et al. Retinoic acid is essential for Th1 cell lineage stability and prevents transition to a Th17 cell program. Immunity. 2015;42(3):499-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma H, Lu C, Ziegler J, et al. Absence of Stat1 in donor CD4+ T cells promotes the expansion of Tregs and reduces graft-versus-host disease in mice. J Clin Invest. 2011;121(7):2554-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henden AS, Hill GR. Cytokines in Graft-versus-Host Disease. J Immunol. 2015;194(10):4604-4612. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki K, Okuno T, Yamamoto M, et al. Semaphorin 7A initiates T-cell-mediated inflammatory responses through alpha1beta1 integrin. Nature. 2007;446(7136):680-684. [DOI] [PubMed] [Google Scholar]
- 43.Xie J, Wang H. Semaphorin 7A as a potential immune regulator and promising therapeutic target in rheumatoid arthritis. Arthritis Res Ther. 2017;19(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kano S, Sato K, Morishita Y, et al. The contribution of transcription factor IRF1 to the interferon-gamma-interleukin 12 signaling axis and TH1 versus TH-17 differentiation of CD4+ T cells. Nat Immunol. 2008;9(1):34-41. [DOI] [PubMed] [Google Scholar]
- 45.Elser B, Lohoff M, Kock S, et al. IFN-gamma represses IL-4 expression via IRF-1 and IRF-2. Immunity. 2002;17(6):703-712. [DOI] [PubMed] [Google Scholar]
- 46.Fragale A, Gabriele L, Stellacci E, et al. IFN regulatory factor-1 negatively regulates CD4+ CD25+ regulatory T cell differentiation by repressing Foxp3 expression. J Immunol. 2008;181(3):1673-1682. [DOI] [PubMed] [Google Scholar]
- 47.Gazon H, Barbeau B, Mesnard JM, Peloponese JM Jr.. Hijacking of the AP-1 Signaling Pathway during Development of ATL. Front Microbiol. 2018;8:2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park MJ, Moon SJ, Lee SH, et al. Blocking activator protein 1 activity in donor cells reduces severity of acute graft-versus-host disease through reciprocal regulation of IL-17-producing T cells/regulatory T cells. Biol Blood Marrow Transplant. 2014;20(8):1112-1120. [DOI] [PubMed] [Google Scholar]
- 49.Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, Serody JS. Leukocyte migration and graft-versus-host disease. Blood. 2005;105(11):4191-4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duffner U, Lu B, Hildebrandt GC, et al. Role of CXCR3-induced donor T-cell migration in acute GVHD. Exp Hematol. 2003;31(10):897-902. [DOI] [PubMed] [Google Scholar]
- 51.Groom JR, Luster AD. CXCR3 in T cell function. Exp Cell Res. 2011;317(5):620-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bertoni A, Alabiso O, Galetto AS, Baldanzi G. Integrins in T Cell Physiology. Int J Mol Sci. 2018;19(2):E485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Su X, Wang Q, Guo W, et al. Loss of Lkb1 impairs Treg function and stability to aggravate graft-versus-host disease after bone marrow transplantation. Cell Mol Immunol. 2019;17:483-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen X, Das R, Komorowski R, et al. Blockade of interleukin-6 signaling augments regulatory T-cell reconstitution and attenuates the severity of graft-versus-host disease. Blood. 2009;114(4):891-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y, Sandy AR, Wang J, et al. Notch signaling is a critical regulator of allogeneic CD4+ T-cell responses mediating graft-versus-host disease. Blood. 2011;117(1):299-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Valenzuela JO, Iclozan C, Hossain MS, et al. PKCtheta is required for alloreactivity and GVHD but not for immune responses toward leukemia and infection in mice. J Clin Invest. 2009;119(12):3774-3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.