Abstract
We present the robotic harvest of a pedicled omentum flap for reconstruction of a near-total anterior chest wall defect. The patient was a 68-year-old woman with recurrent secondary chest wall angiosarcoma after previous mastectomy and radiation therapy. She underwent neoadjuvant chemotherapy and radiation, followed by wide radical chest wall resection with a final defect size of 15×35 cm. A one-stage reconstruction was performed with an omentum flap harvested by robotic technique and split-thickness skin grafts from thigh donor sites. The patient healed with minimal complications. Our case supports more widespread application of robotics in plastic and reconstructive surgery.
Keywords: surgery, breast surgery, plastic and reconstructive surgery
Background
Closure of irradiated chest wall defects continue to present a challenge to the reconstructive surgeon. Omentum flaps have been shown to provide effective coverage and palliation for patients with locally recurrent breast cancer after wide radical resection.1–3 However, there is a paucity of literature regarding the use of robotic surgery in chest wall reconstruction. In this report, we present the use of the robotic da Vinci system (Intuitive Surgical, Sunnyvale, California, USA) to harvest a pedicled omentum flap for reconstruction of a near-total anterior chest wall defect after wide radical resection of a secondary angiosarcoma.
Case presentation
A 68-year-old woman presented to our institution with recurrent secondary chest wall angiosarcoma after previous mastectomy and radiation therapy. Six years prior, she was diagnosed with right invasive ductal carcinoma and treated with lumpectomy, right axillary dissection then adjuvant chemotherapy and radiation. Four years after the completion of radiation, she developed biopsy-proven secondary angiosarcoma on the right breast. She then underwent bilateral mastectomy with wide local excision (3 cm margin) of the angiosarcoma on the right and she was reconstructed with a right latissimus dorsi myocutaneous flap. At the time of resection, the angiosarcoma measured 10 cm and involved the right medial breast and nipple. Surgical margins were negative on final pathology. Five months later, she again had biopsy-proven recurrence of the angiosarcoma on the right chest, with no evidence of metastatic disease on imaging. At this time, she presented to our institution for treatment.
Treatment
She underwent neoadjuvant chemotherapy with paclitaxel, as well as 5000 cGy of radiation over 25 fractions (figure 1). One month after the completion of radiation, she underwent radical resection of the entire chest wall, including the right pectoralis major and minor muscles. A margin of 10 cm was drawn surrounding all biopsy-proven recurrent angiosarcoma sites (figure 2). The final defect measured 15×35 cm. Vacuum-assisted closure (VAC) (KCI USA, San Antonio, Texas, USA) therapy was applied for 4 weeks in an attempt to promote formation of granulation tissue (figure 3).
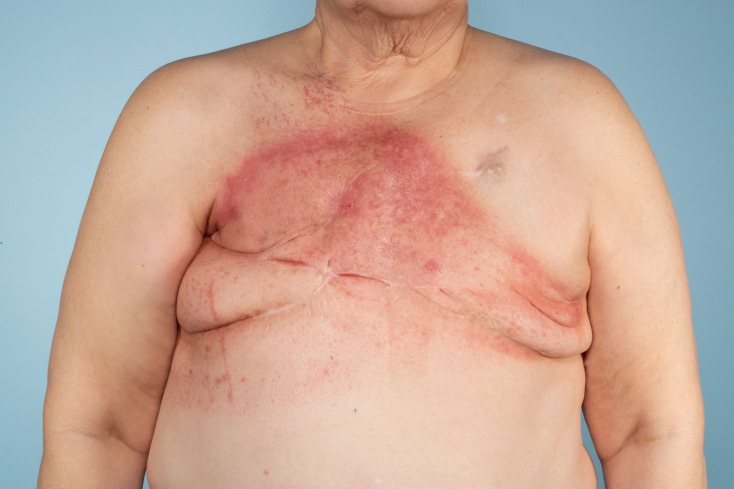
Figure 1.
Patient after completion of neoadjuvant chemotherapy and radiation for recurrent secondary angiosarcoma.
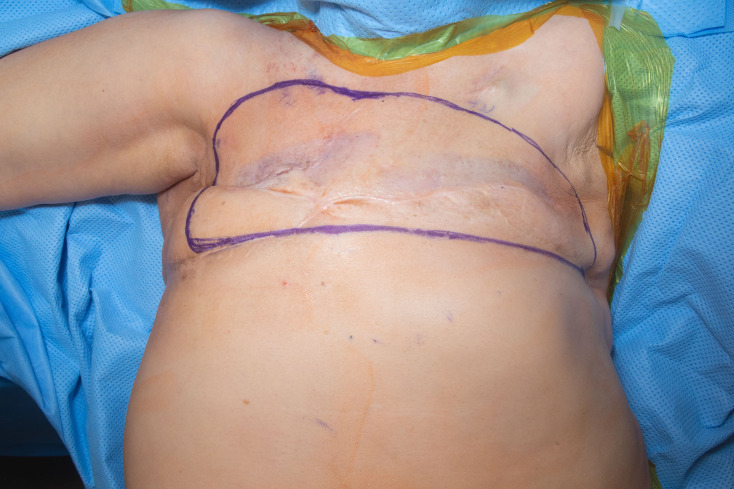
Figure 2.
Chest wall area marked for surgical resection.
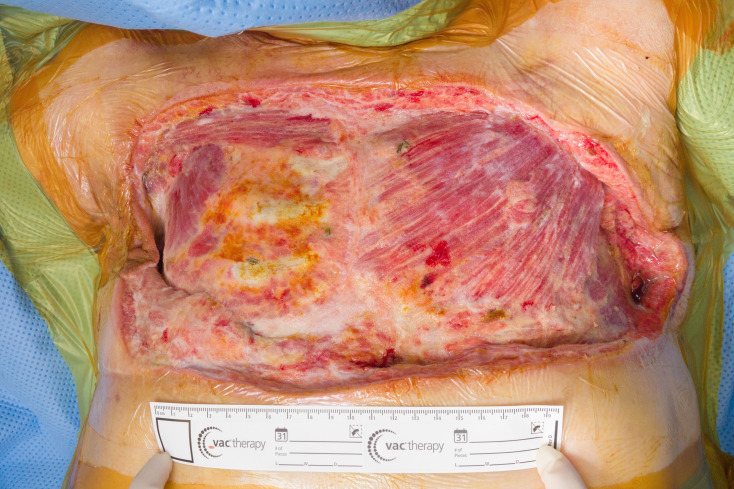
Figure 3.
Three weeks after surgical resection and vacuum-assisted closure therapy, showing extensive defect with rib exposure.
Since there was minimal progression of the wound with VAC therapy, a robotically harvested omentum flap was used to reconstruct the chest wall (figure 4). Four 7 mm robotic ports placed transversely in line with the umbilicus and the omentum was mobilised off of the transverse colon using a combination of electrocautery and Vessel Sealer to ensure haemostasis. The transverse colon was lifted using our accessory arm and the omentum freed from the hepatic flexure proximally up to the splenic flexure within the avascular plane. Along the greater curvature of the stomach, we continued to mobilise the omentum, taking care to preserve the vascular arcades along the gastroepiploic arch to ensure adequate perfusion of the pedicled flap. The left gastroepiploic artery was divided to allow for adequate mobilisation of the omentum since extensive coverage of the chest wall defect was required. A fasciotomy was made from the xiphoid extending 4 cm inferiorly, and the omentum was exteriorised and delivered to the chest wall defect, taking care not to twist or compress the omentum and ensuring its viability. The omentum flap was inset to the chest defect with interrupted absorbable sutures. Split-thickness skin grafts were taken from the bilateral thighs and meshed at a ratio of 1:1.5 and 1:2 for inset onto the omentum flap. VAC therapy was applied to the flap for 5 days postoperatively.
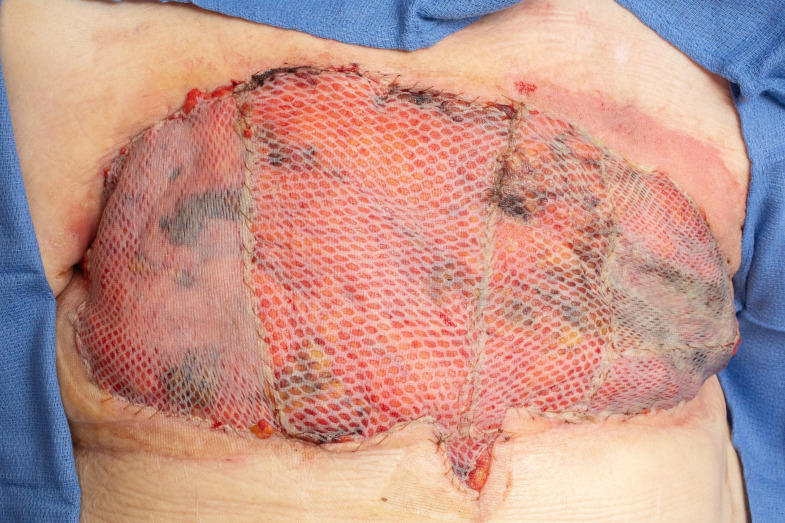
Figure 4.
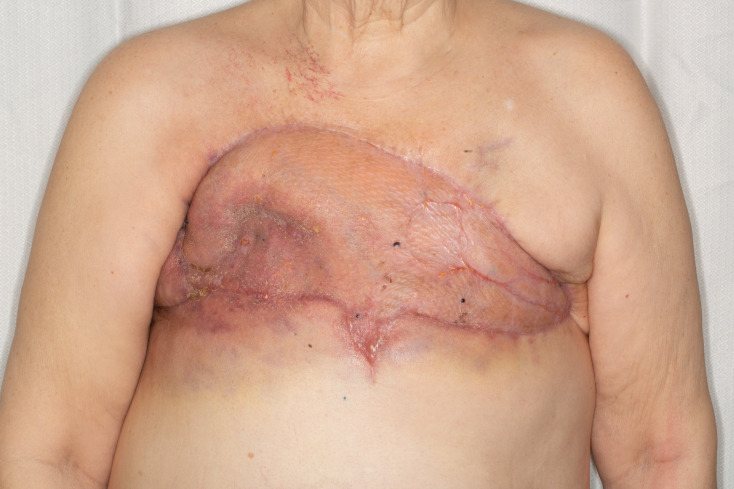
Chest wall defect reconstructed with omentum flap and split-thickness skin grafts. Flap is shown here after VAC takedown.
Outcome and follow-up
The patient’s postoperative course was complicated by sloughing of the skin graft on the left lateral chest wall, which was managed with petrolatum gauze and antibiotic ointment daily dressing changes. Two months postoperatively, her chest was healed (figure 5), but we noted two new areas of purple, blanching discoloration on the inferior chest wall. She was re-staged with imaging, which was consistent with metastatic recurrence in the right chest wall invading into the pleural space as well as new splenic, hepatic and osseous lesions. The patient elected to undergo hospice care at that time and subsequently expired 1 month later.
Figure 5.
Eleventh week postoperative result. The flap and skin grafts are healed.
Discussion
Large chest wall defects, especially in the setting of prior radiation, present a challenge to the reconstructive surgeon. Various flaps have been described for chest wall reconstruction, including latissimus dorsi, rectus abdominis pedicled and free flaps, as well as omentum flaps.4 In our patient, the latissimus dorsi flap had already been used. A left pectoralis turnover flap for coverage of the exposed ribs on the right chest wall could have been considered. However, this technique would have relied on the internal mammary perforators potentially impacted by previous surgical dissection during tumour excision or repeated chest wall radiation. Furthermore, there the pectoralis flap would not have provided as much bulk as other options which would have resulted in a significant contour deformity. A pedicled or free rectus abdominis flap would have been unlikely to provide closure for the entirety of the defect. The omentum flap brings well-vascularised, non-irradiated adipose tissue into the wound bed, providing potential benefits, such as neoangiogenesis and tissue regeneration.5 Thus, we proceeded with an omentum flap.
The omentum flap was first introduced for chest wall reconstruction after breast cancer excision in 1963.6 The flap’s angiogenic and immunologic effects in promotion of wound healing, especially in the irradiated settings, are well described.7–9 The omentum has been shown to provide effective coverage of extrathoracic and intrathoracic defects.10 11 Donor-site complications have been described, including abdominal wall infection, ventral hernia or gastrointestinal haemorrhage.12 Laparoscopic omental harvest was introduced to avoid the laparotomy incision previously necessary for flap transposition. However, little has been written regarding the robotic intra-abdominal flap harvest for chest wall reconstruction. To our knowledge, this is the first reported case of robotic omentum harvest for a near-total anterior chest wall soft tissue defect.
Robotic systems have been used in plastic surgery for harvest of latissimus dorsi and free rectus abdominis flaps.13 14 Robotic omentum mobilisation without pedicle dissection has also been reported in the urologic literature in the setting of ureterolysis.15 Recently, a case was reported of free omentum harvest for lower extremity reconstruction.16 Advantages of the robotic system over laparoscopy include elimination of tremor, three-dimensional visualisation of the surgical field and improved surgical ease with articulating instruments that better mimic the human hand.16 In addition, the improved visualisation provided by a dual camera system improves the identification and preservation of vasculature that is key to ensuring viability of the omental flap.
Our case report demonstrates the efficacy of robot use in intra-abdominal flap harvest for the reconstruction of an irradiated near-total anterior chest wall soft tissue defect. While laparoscopy’s long learning curve created excessive initial costs to the healthcare system during its adoption, robotic surgery has a shorter learning curve which can translate to relatively lower initial costs.17 We advocate for continued investigation of robotic applications in plastic and reconstructive surgery.
Learning points.
Closure of irradiated chest wall defects continues to present reconstructive challenges.
We presented the successful reconstruction of an irradiated near-total anterior chest wall soft tissue defect with a robotically harvested pedicled omentum flap.
Our case advocates for more widespread application of robotics in plastic and reconstructive surgery.
Footnotes
Contributors: MDN and SJD were involved in the conception of the project and data collection. SJD, BD and MDN, all contributed to the interpretation of results, drafting, revision and final approval of the manuscript. All authors have given final approval to the completed version and agree to the accuracy and integrity of the work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer-reviewed.
References
- 1.Henderson MA, Burt JD, Jenner D, et al. Radical surgery with omental flap for uncontrolled locally recurrent breast cancer. ANZ J Surg 2001;71:675–9. 10.1046/j.0004-8682.2001.02234.x [DOI] [PubMed] [Google Scholar]
- 2.Cheung KL, Willsher PC, Robertson JF, et al. Omental transposition flap for gross locally recurrent breast cancer. Aust N Z J Surg 1997;67:185–6. 10.1111/j.1445-2197.1997.tb01937.x [DOI] [PubMed] [Google Scholar]
- 3.Williams RJ, Fryatt IJ, Abbott WC, et al. Omental transposition in the treatment of locally advanced and recurrent breast cancer. Br J Surg 1989;76:559–63. 10.1002/bjs.1800760611 [DOI] [PubMed] [Google Scholar]
- 4.Villa MT, Chang DW. Muscle and omental flaps for chest wall reconstruction. Thorac Surg Clin 2010;20:543–50. 10.1016/j.thorsurg.2010.07.001 [DOI] [PubMed] [Google Scholar]
- 5.Di Nicola V. Omentum a powerful biological source in regenerative surgery. Regen Ther 2019;11:182–91. 10.1016/j.reth.2019.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.KIRICUTA I. [The use of the great omentum in the surgery of breast cancer]. Presse Med 1963;71:15–17. [PubMed] [Google Scholar]
- 7.Beelen RH. The greater omentum: physiology and immunological concepts. Neth J Surg 1991;43:145–9. [PubMed] [Google Scholar]
- 8.Williams RJ, White H. Transposition of the greater omentum in the prevention and treatment of radiation injury. Neth J Surg 1991;43:161–6. [PubMed] [Google Scholar]
- 9.Cartier R, Brunette I, Hashimoto K, et al. Angiogenic factor: a possible mechanism for neovascularization produced by omental pedicles. J Thorac Cardiovasc Surg 1990;99:264–8. 10.1016/S0022-5223(19)37010-2 [DOI] [PubMed] [Google Scholar]
- 10.Acarturk TO, Swartz WM, Luketich J, et al. Laparoscopically harvested omental flap for chest wall and intrathoracic reconstruction. Ann Plast Surg 2004;53:210–6. 10.1097/01.sap.0000116285.98328.f7 [DOI] [PubMed] [Google Scholar]
- 11.Zaha H, Inamine S. Laparoscopically harvested omental flap: results for 96 patients. Surg Endosc 2010;24:103–7. 10.1007/s00464-009-0533-0 [DOI] [PubMed] [Google Scholar]
- 12.Hultman CS, Carlson GW, Losken A, et al. Utility of the omentum in the reconstruction of complex extraperitoneal wounds and defects: donor-site complications in 135 patients from 1975 to 2000. Ann Surg 2002;235:782–95. 10.1097/00000658-200206000-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clemens MW, Kronowitz S, Selber JC. Robotic-Assisted latissimus dorsi harvest in delayed-immediate breast reconstruction. Semin Plast Surg 2014;28:020–5. 10.1055/s-0034-1368163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel NV, Pedersen JC. Robotic harvest of the rectus abdominis muscle: a preclinical investigation and case report. J Reconstr Microsurg 2012;28:477–80. 10.1055/s-0031-1287674 [DOI] [PubMed] [Google Scholar]
- 15.Mufarrij PW, Lipkin ME, Stifelman MD. Robot-Assisted ureterolysis, retroperitoneal biopsy, and omental wrap: pilot series for the treatment of idiopathic retroperitoneal fibrosis. J Endourol 2008;22:1669–76. 10.1089/end.2008.0034 [DOI] [PubMed] [Google Scholar]
- 16.Özkan Ömer, Özkan Özlenen, Çinpolat A, et al. Robotic harvesting of the omental flap: a case report and mini-review of the use of robots in reconstructive surgery. J Robot Surg 2019;13:539–43. 10.1007/s11701-019-00949-8 [DOI] [PubMed] [Google Scholar]
- 17.Saleh DB, Syed M, Kulendren D, et al. Plastic and reconstructive robotic microsurgery--a review of current practices. Ann Chir Plast Esthet 2015;60:305–12. 10.1016/j.anplas.2015.03.005 [DOI] [PubMed] [Google Scholar]