Key Points
Question
What are the benefits and harms associated with pharmacologic interventions for managing breathlessness in adults with advanced cancer?
Findings
This systematic review and meta-analysis of 19 studies (17 randomized clinical trials and 2 retrospective studies) found that opioids and anxiolytics were not associated with improved breathlessness in patients with advanced cancer within the limits of the identified studies, which mainly focused on exertional breathlessness for opioids. Data on harms were too limited to draw conclusions.
Meaning
Although the existing data on opioids and pharmacologic interventions do not show an association with improved breathlessness in advanced cancer, they may be considered in selected patients in the context of potential harms and the evidence of an association of nonpharmacologic interventions with improved breathlessness.
Abstract
Importance
Improved survival in patients with advanced cancer has increased the need for better understanding of how to manage common symptoms that they may experience, such as breathlessness.
Objective
To assess the benefits and harms associated with pharmacologic interventions for breathlessness in adults with advanced cancer.
Data Sources
PubMed, Embase, CINAHL, Web of Science, and the Cochrane Central Register of Controlled Trials were searched for studies published from database inception through May 31, 2020, using predefined eligibility criteria within a PICOTS (population, intervention, comparator, outcome, timing, setting) format.
Study Selection
Randomized clinical trials (RCTs), non-RCTs, and observational studies with a comparison group that evaluated benefits and/or harms and cohort studies that reported harms were selected.
Data Extraction and Synthesis
Two reviewers independently screened studies for eligibility, serially abstracted data, independently assessed risk of bias, and graded strength of evidence (SOE).
Main Outcomes and Measures
Benefits and harms of pharmacologic interventions were compared, focusing on breathlessness, anxiety, exercise capacity, and health-related quality of life. When possible, meta-analyses were conducted and standardized mean differences (SMDs) calculated.
Results
In this systematic review and meta-analysis, a total of 7729 unique citations were identified, of which 19 studies (17 RCTs and 2 retrospective studies) that included a total of 1424 patients assessed the benefits of medications for management of breathlessness in advanced cancer or reported harms. The most commonly reported type of cancer was lung cancer. Opioids were not associated with more effectiveness than placebo for improving breathlessness (SMD, −0.14; 95% CI, −0.47 to 0.18) or exercise capacity ( SMD, 0.06; 95% CI, −0.43 to 0.55) (SOE, moderate); most studies examined exertional breathlessness. Specific dose and/or route of administration of opioids did not differ in effectiveness for breathlessness (SMD, 0.15; 95% CI, −0.22 to 0.52) (SOE, low). Anxiolytics were not associated with more effectiveness than placebo for breathlessness or anxiety (reported mean between-group difference, −0.52; 95% CI, −1.045 to 0.005) (SOE, low). Evidence for other pharmacologic interventions was limited. Pharmacologic interventions demonstrated some harms compared with usual care, but dropout attributable to adverse events was minimal in these short-term studies (range 3.2%-16%).
Conclusions and Relevance
Evidence did not support the association of opioids or other pharmacologic interventions with improved breathlessness. Given that studies had many limitations, pharmacologic interventions should be considered in selected patients but need to be considered in the context of potential harms and evidence of an association of nonpharmacologic interventions with improved breathlessness.
This systematic review assesses the benefits and harms of pharmacologic interventions for breathlessness in adults with advanced cancer.
Introduction
In 2020, the American Cancer Society reported the largest 1-year decrease in cancer deaths, in large part because of emerging therapeutic developments that prolong time living with advanced cancer.1 These improvements in survival necessitate evidence on how to improve quality of life (QOL) and symptoms, such as dyspnea, among patients with advanced cancer.
Dyspnea, defined as the sensation of breathlessness, is frequent and debilitating in patients with advanced cancer,2 reducing QOL, functional status, and the ability to participate in desired activities.3 Oncology guidelines and reviews endorse the use of opioids and anxiolytics as interventions for symptomatic relief of breathlessness in patients with advanced cancer.4,5,6 However, these recommendations are driven by older data extrapolated from broad patient populations and from studies that excluded patients with cancer. Patients with cancer may respond differently to these agents because of their unique pathophysiologic mechanisms of breathlessness, concurrent opioid use for pain, and coexisting symptoms. Furthermore, the causes and management of breathlessness may differ at various phases of cancer and may depend on other factors, such as comorbidity, current treatments, and the patient’s life expectancy.
Since the release of previous guidelines and reviews,4,5,6 a number of studies7,8 have examined the efficacy of therapies for breathlessness in patients who have advanced cancer, and a recent large trial7 of specific opioid approaches in mixed populations with chronic breathlessness also did not find a benefit for opioids. Therefore, we conducted a systematic review and meta-analysis to examine the associations of pharmacologic options with improved breathlessness, anxiety, and physiologic outcomes in patients with advanced cancer.
Methods
This report comes from a broader systematic review that assesses nonpharmacologic and pharmacologic interventions. The full evidence report9 has additional details on the methods and other results. With input from a technical expert panel and representatives from the Agency for Healthcare Research and Quality (AHRQ), the American Society for Clinical Oncology, and the Patient-Centered Outcomes Research Institute, we developed a protocol that was posted on the AHRQ Effective Health Care Program’s website. We followed the AHRQ’s Methods Guide for Effectiveness and Comparative Effectiveness Reviews.10 This study followed the Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA) reporting guideline.11
Study Selection
We searched PubMed, Embase, CINAHL, Web of Science, and the Cochrane Central Register of Controlled Trials for articles published from database inception through May 31, 2020, using predefined eligibility criteria within a PICOTS (population, intervention, comparator, outcome, timing, setting) format. We included randomized clinical trials (RCTs), non-RCTs, and observational trials with a concurrent comparison group that enrolled adult patients with advanced cancer and assessed benefits and/or harms of pharmacologic interventions with the intent to alleviate breathlessness. We defined advanced cancer as cancer unlikely to be cured with treatment. We excluded studies with fewer than 10 patients enrolled in each arm and studies with mixed populations in which the percentage of patients with cancer was less than 50%. Key outcomes included in the review are breathlessness, anxiety, exercise capacity, health-related QOL (HRQOL), and physiologic outcomes (see eFigure 1 in the Supplement).
Two reviewers (J.L.F. and J.M.W.) independently screened titles, abstracts, and full text for inclusion. Differences between investigators were resolved through consensus adjudication. We used DistillerSR (Evidence Partners) to manage this process.
Risk of Bias and Strength of Evidence
Two reviewers (J.L.F. and J.M.W.) independently assessed the risk of bias (ROB) in studies using the Cochrane Risk of Bias Tool, version 2, for assessing the ROB of RCTs and the Cochrane Risk of Bias Assessment Tool for Non-Randomized Studies of Interventions tool. We graded the strength of evidence (SOE) using the grading scheme recommended by the Methods Guide for Effectiveness and Comparative Effectiveness Reviews.10 We applied evidence grades to the bodies of evidence about each comparison for the outcomes we classified during protocol development as the critical outcomes. We assessed the limitations to individual study quality (using individual ROB assessments), consistency, directness, precision, and reporting bias. We classified SOE into 4 grades: high, moderate, low, and insufficient (eTable 1 in the Supplement).
Statistical Analysis
We used standardized forms in Excel for data extraction (Microsoft Corp). Reviewers (J.L.F. and J.M.W.) extracted information on general study characteristics, participant characteristics, eligibility criteria, interventions, outcome measures, method of ascertainment, and results of each outcome, including measures of variability. One reviewer (J.L.F.) completed data abstraction, and another reviewer (J.M.W.) confirmed the first reviewer’s abstraction for completeness and accuracy.
We conducted meta-analyses for outcomes using a random-effects model with the DerSimonian and Laird method when there were at least 2 sufficiently homogeneous studies. Patient-reported outcomes and clinical scales were standardized by estimating the standardized mean difference (SMD) using the Cohen d method. For studies that did not include variability measures, the SD of change in mean was calculated using a correlation coefficient of 0.5, in accordance with methods provided in Fu et al.12 We used Cohen classification to categorize effect sizes as small, medium, or large.13 When studies reported harms categorically, we calculated relative risks (RRs).
We considered a 10-mm difference on a 100-mm visual analog scale as clinically significant for breathlessness, which corresponds to an SMD of 0.35.14 We used Stata statistical software, version 14 (StataCorp LLC) for all meta-analyses. We qualitatively summarized studies that were not amenable to pooling (eAppendix in the Supplement)
Results
A total of 7729 unique citations were identified, of which 18 studies (17 RCTs and 2 retrospective studies) that included a total of 1424 patients assessed the benefits of medications for management of breathlessness in advanced cancer or reported harms15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32 (eFigure 2 in the Supplement). The most commonly reported type of cancer was lung cancer. The number of participants in RCTs ranged from 10 to 432; the studies were published between 1993 and 2020. Nine RCTs were placebo controlled, and 8 RCTs and 2 retrospective studies compared results between drugs. Follow-up ranged from 1 minute to 28 days (eTable 2 in the Supplement).
Breathlessness
Table 1 summarizes the reported effects of the pharmacologic interventions in the 18 studies on breathlessness.12,13,14,15,19,20,21,22,23,24,25,26,27,28,29,30,31,32 Detailed information on ROB and SOE are presented in eTables 6 and 7 in the Supplement.
Table 1. Summary of Key Results for the Effects of Pharmacologic Interventions on Breathlessness in Patients With Advanced Cancer.
| Comparison | Evidence of difference | Strength of evidencea | No. of studies (No. analyzed) | Key findings | Conclusion |
|---|---|---|---|---|---|
| Placebo-controlled comparisons | |||||
| Opioids vs placebo | Equivalence | Moderate | 6 RCTs (N = 107); fentanyl vs placebo (4),16,17,18,19 hydromorphone (nebulized) vs hydromorphone (oral or subcutaneous) vs placebo (nebulized) (1),15 morphine vs placebo (1)20 | Pooled analysis with Charles et al15; saline vs nebulized hydromorphone comparison: SMD, −0.12; 95% CI, −0.45 to 0.20; I2 = 0.0%, P = .43; pooled analysis with Charles et al15: saline vs systemic hydromorphone comparison: SMD, −0.14; 95% CI: −0.47 to 0.18; I2 = 0.0%, P = .49 | Opioids were not more effective than placebo within the limits of the identified studies |
| Anxiolytics vs placebo | Equivalence | Low | 2 RCTs (N = 311); buspirone vs placebo (1),21 midazolam vs placebo (1)31 | Buspirone vs placebo: reported MBGD, −0.52; 95% CI, −1.045 to 0.005; midazolam vs placebo: no statistically significant difference between groups (P = .75) at 60 min; unable to calculate SMD, data presented as number of spray bottles rather than number of participants | Anxiolytics were no more effective than placebo |
| Corticosteroids vs placebo | No conclusion drawn | Insufficient | 1 RCT (N = 28); dexamethasone vs placebo (1)22 | Calculated SMD, −0.06; 95% CI, −0.70 to 0.58 | NA |
| Drug-drug comparisons | |||||
| Opioids vs opioids | Equivalence | Low | 7 RCTs (N = 132)15,23,24,27,28,30,32; subcutaneous vs sublingual morphine (1), subcutaneous vs nebulized morphine (1), high- vs low-dose sublingual fentanyl (1), low- vs high-dose opioids (drug unspecified) (1), hydromorphone (nebulized) vs hydromorphone (oral or subcutaneous) vs placebo (nebulized) (1), buccal fentanyl vs oral morphine (1), oral morphine hydrochloride vs oral morphine sulfate (1) | Pooled analysis: SMD, 0.15; 95% CI, −0.22 to 0.52; I2 = 4.8%, P = .37 | No difference in effectiveness between opioid doses or routes in improving breathlessness |
| Opioids vs anxiolytics | Equivalence | Low | 2 RCTs (N = 108)25,26; oral morphine vs oral midazolam (1), subcutaneous morphine vs subcutaneous midazolam vs combination (1) | For breathlessness intensity: 1 study found midazolam was more effective than morphine at 5 d (P < .001); another study found no significant differences between groups at 24 or 48 h; for categorical variable of percentage not experiencing breathlessness relief: calculated RR, 0.075; 95% CI, 0.004 to 1.270; calculated RR, 1.33; 95% CI, 1.02 to 1.75 | Opioids were not more effective than anxiolytics for improving breathlessness |
| Opioids vs corticosteroids vs bronchodilators | No conclusion drawn | Insufficient | 1 Retrospective cohort (N = 343)29; morphine vs methylprednisolone vs aminophylline (1) | Methylprednisolone vs aminophylline: calculated SMD, 0.41; 95% CI, 0.15 to 0.68; morphine vs aminophylline: calculated SMD, 1.18; 95% CI, 0.90 to 1.46; morphine vs methylprednisolone: calculated SMD, 0.76; 95% CI, 0.49 to 1.03 | NA |
Abbreviations: MBGD, mean between-group difference; NA, not applicable; RCT, randomized clinical trial; RR, relative risk; SMD, standardized mean difference.
Moderate strength indicates that further research may change the result; low strength indicates low confidence that the evidence reflects the true effect, and further research is very likely to change the result; and insufficient evidence indicates that evidence is unavailable or does not permit a conclusion.
Opioids vs Placebo
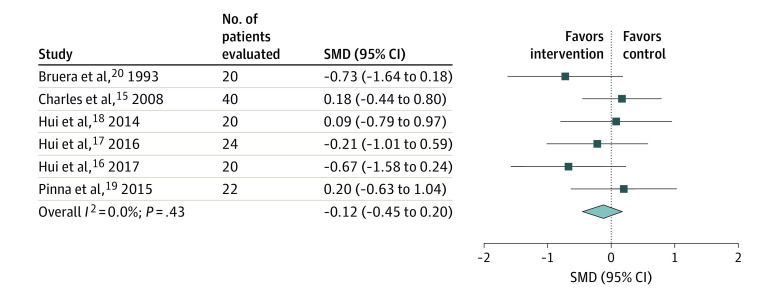
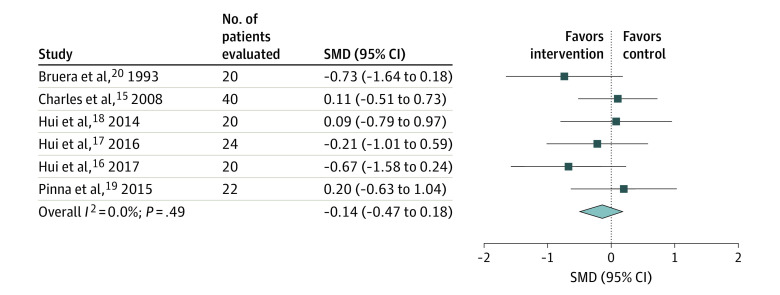
Six RCTs15,16,17,18,19,20 assessed the effect of opioids compared with placebo on breathlessness, and most of these studies examined exertional breathlessness. Four RCTs16,17,18,19 compared fentanyl products, 1 RCT15 evaluated hydromorphone, and 1 RCT20 evaluated subcutaneous morphine. We performed 2 meta-analyses to reflect the 2 placebo comparisons provided in 1 of the RCTs and equivalence with and without the nebulized arm15 (calculated SMD for saline vs nebulized hydromorphone comparison, −0.12; 95% CI, −0.45 to 0.20; I2 = 0.0%) (calculated SMD for saline vs systemic hydromorphone comparison, −0.14; 95% CI, −0.47 to 0.18; I2 = 0.0%) (Figure 115,16,17,18,19,20 and Figure 215,16,17,18,19,20). All studies reported an active placebo effect on within-group differences. On the basis of the overall pooled results from the meta-analysis, opioids were not more effective than placebo for improving breathlessness in patients with advanced cancer (SOE, moderate).
Figure 1. Meta-analysis of the Effects on Breathlessness in Randomized Clinical Trials Comparing Opioids With Placebo in Patients With Advanced Cancer (Including the Charles et al15 Saline vs Nebulized Hydromorphone Comparison).
SMD indicates standardized mean difference. The squares indicate the SMDs, the horizontal lines the 95% CIs, and the diamond the overall result.
Figure 2. Meta-analysis of the Effects on Breathlessness in Randomized Clinical Trials Comparing Opioids With Placebo in Patients With Advanced Cancer (Including the Charles et al15 Saline vs Systemic Hydromorphone Comparison).
SMD indicates standardized mean difference. The squares indicate the SMDs, the horizontal lines the 95% CIs, and the diamond the overall result.
Anxiolytics vs Placebo
Two RCTs21,31 assessed the effect of anxiolytics compared with placebo. One study,21 evaluating buspirone vs placebo, found no statistically significant difference between groups (reported mean between-group difference, −0.52; 95% CI, −1.045 to 0.005). The other RCT31 of intranasal midazolam vs placebo also found no statistically significant difference in breathlessness between groups. On the basis of the available evidence, anxiolytics were not more effective than placebo for the treatment of breathlessness (SOE, low). We did not perform a meta-analysis given the different mechanisms of actions for these anxiolytics.
Corticosteroids vs Placebo
One RCT22 of oral dexamethasone compared with placebo found no statistically significant effect in breathlessness (calculated SMD, −0.06; 95% CI, −0.70 to 0.58). Evidence was insufficient to evaluate the effectiveness of corticosteroids vs placebo for the treatment of breathlessness.
Opioids vs Opioids
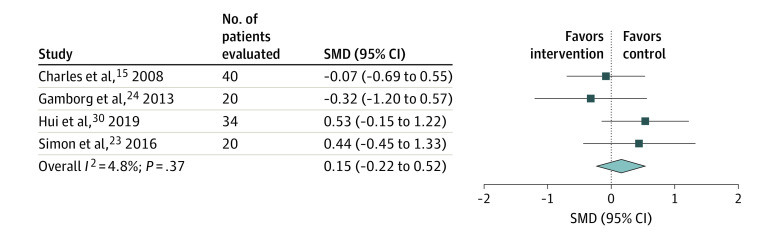
Seven RCTs15,23,24,27,28,30,32 compared the effect of different routes of administration or different doses of opioids for treatment of breathlessness in patients with advanced cancer. Meta-analysis of 4 of the RCTs15,23,24,30 found no difference between opioid doses or routes in treating breathlessness in patients with advanced cancer (calculated SMD, 0.15; 95% CI, −0.22 to 0.52; I2 = 4.8%) (Figure 3). Three of the RCTs27,28,32 were not included in the analysis because they reported results as median rather than mean, data were derived from figures, or not enough information was included for calculations. Two of these RCTs27,28 reported no statistically significant differences between groups, and 1 RCT32 reported a significant difference favoring morphine. We concluded that there were no differences between opioid doses or routes.
Figure 3. Meta-analysis of the Effects on Breathlessness in Randomized Clinical Trials Comparing Opioids With Opioids in Patients With Advanced Cancer.
SMD indicates standardized mean difference. The squares indicate the SMDs, the horizontal lines the 95% CIs, and the diamond the overall result.
Opioids vs Anxiolytics
Two RCTs25,26 evaluated the effect of midazolam compared with morphine or the combination of both drugs. One RCT25 found that midazolam was more effective at relieving breathlessness at 5 days, and another RCT26 found no statistically significant differences in breathlessness intensity at 24 or 48 hours. The combination morphine and midazolam group, however, had a statistically significantly higher percentage of patients reporting breathlessness relief than either agent alone at 24 hours, which was persistent compared with the midazolam-alone group at 48 hours. We concluded that opioids were not more effective than anxiolytics (midazolam) for improving breathlessness (SOE, low). Given the heterogeneity of patient populations, we did not conduct a meta-analysis.
Opioids vs Corticosteroids vs Bronchodilators
One single-center retrospective cohort study29 evaluated morphine compared with methylprednisolone or aminophylline. Specifically, methylprednisolone improved breathlessness more than aminophylline (calculated SMD, 0.41; 95% CI, 0.15-0.68), and morphine improved breathlessness more than either of the other agents (morphine vs aminophylline: calculated SMD, 1.18; 95% CI, 0.90-1.46; morphine vs methylprednisolone: calculated SMD, 0.76; 95% CI, 0.49-1.03). Given only 1 retrospective cohort study,29 we concluded that evidence was insufficient to evaluate the effectiveness of opioids vs corticosteroids vs bronchodilators for treatment of breathlessness.
Anxiety, Exercise Capacity, and HRQOL
Table 2 summarizes the effects of the pharmacologic interventions on anxiety, exercise capacity, and HRQOL. Detailed information on ROB and SOE are presented in eTables 6 and 7 in the Supplement. Only placebo-controlled comparisons are reported for these outcomes. Two RCTs21,31 of anxiolytics compared with placebo reported no difference in the treatment of anxiety in patients with advanced cancer. We concluded that anxiolytics were no more effective than placebo in treating anxiety (SOE, low). One RCT22 of oral dexamethasone compared with placebo in patients with advanced cancer found no difference on HRQOL using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire–Core Questionnaire. Three RCTs17,18,19 of fentanyl compared with placebo reported results in terms of distance in a 6-minute walk test. Meta-analysis of 3 RCTs16,17,18 found no differences in 6-minute walk distance (calculated SMD, 0.06; 95% CI, −0.43 to 0.55; I2 = 0.0%) (eFigure 3 in the Supplement). We concluded that opioids were not more effective than placebo for improving exercise capacity (SOE, moderate).
Table 2. Summary of Key Results for the Effects of Pharmacologic Interventions on Anxiety, Health-Related Quality of Life, and Exercise Capacity in Patients With Advanced Cancer.
| Comparison | Evidence of difference | Strength of evidencea | No. of studies (No. analyzed) | Key findings | Conclusion |
|---|---|---|---|---|---|
| Anxiolytics vs placebo for anxiety | Equivalence | Low | 2 RCTs (N = 311)21,31; buspirone vs placebo (1); intranasal midazolam vs placebo | Buspirone vs placebo: no statistically significant differences between groups; unable to calculate SMD; intranasal midazolam vs placebo: no difference between arms reported by authors | Anxiolytics were not more effective than placebo for improving anxiety |
| Corticosteroids vs placebo for health-related quality of life | No conclusion drawn | Insufficient | 1 RCT (N = 28)22; oral dexamethasone vs placebo | Calculated SMD, −0.06; 95% CI, −0.70 to 0.58 | NA |
| Opioids vs placebo for exercise capacity | Equivalence | Moderate | 3 RCTs (N = 77)17,18,19; fentanyl vs placebo | Pooled analysis of 3 studies: SMD, 0.06; 95% CI, −0.43 to 0.55; I2 = 0.0%, P = .90; fourth study reported no significant differences between groups; unable to calculate SMD, data reported as medium rather than mean | Opioids were not more effective than placebo for improving exercise capacity |
Abbreviations: NA, not applicable; RCT, randomized clinical trial; SMD, standardized mean difference.
Moderate strength indicates that further research may change the result; low strength indicates low confidence that the evidence reflects the true effect, and further research is very likely to change the result; and insufficient evidence indicates that evidence is unavailable or does not permit a conclusion.
Physiologic Outcomes
eTable 3 in the Supplement summarizes the physiologic outcomes in 12 studies. 15,17,18,19,20,23,24,25,26,27,28,29,30 No study reported any significant effects from any drugs on physiologic outcomes (eFigures 4–13 in the Supplement).
Harms and Dropout
Fourteen studies15,16,17,18,19,21,22,25,26,27,29,30,31,33 (12 RCTs15,16,17,18,19,21,22,25,26,27,30,31 and 2 retrospective studies29,33) addressed the adverse effects and dropout associated with pharmacologic interventions (eTable 4 in the Supplement). No study reported harms of opioids compared with other opioids or other opioid dosing. None of these studies reported on headaches or opioid use disorder.
Central Nervous System Effects
Three RCTs16,17,18 comparing opioids with placebo assessed central nervous system adverse effects. A meta-analysis found no statistically significant difference between fentanyl and placebo for dizziness (RR, 0.68; 95% CI, 0.15-3.11; I2 = 0.0%; P = .41) (eFigure 14 in the Supplement). A meta-analysis on 2 of the 3 RCTs to evaluate drowsiness found no statistically significant difference between fentanyl or placebo (RR, 0.38; 95% CI, 0.06-2.27; I2 = 0.0%; P = .70) (eFigure 15 in the Supplement). Finally, a meta-analysis on all 3 RCTs16,17,18 found no statistically significant difference in fatigue between groups (mean between-group difference: SMD, −0.15; 95% CI, −0.64 to 0.34; I2 = 0.0%; P = .67) (eFigure 16 in the Supplement). For central nervous system adverse effects, corticosteroids had lower rates of drowsiness compared with placebo or opioids, but results for dizziness were inconsistent.
Gastrointestinal Effects
For gastrointestinal adverse effects, opioids had higher rates of constipation compared with steroids (RR, 0.01; 95% CI, 0-0.15) for the methylprednisolone and aminophylline groups compared with morphine; studies that compared opioids with placebo were short term and did not report this adverse effect. Study reporting was insufficient to draw conclusions about nausea for any of the pharmacologic comparison groups.
Dropout
The rate of dropout attributable to adverse effects was reported in 5 studies.15,16,21,22,25 Adverse effects led to dropout in a small percentage of patients (range, 3.2%-16%) for all types of pharmacologic interventions (eTable 5 in the Supplement).
Discussion
This systematic review and meta-analysis found that opioids were not associated with more effectiveness than placebo and not more effective than other pharmacologic interventions for improving breathlessness for patients with advanced cancer. Anxiolytics were not associated with more effectiveness than placebo for these outcomes, with low SOE. Furthermore, there was limited or insufficient evidence that pharmacologic therapies impacted other outcomes, including anxiety, HRQOL, or exercise capacity.
Existing guidelines emphasize the use of opioids and benzodiazepines for management of breathlessness in patients with advanced cancer.4,5 The findings of the current study differ somewhat from previously published reviews,6,34 and several factors may contribute to these differences. The results of this review must be interpreted within the context of the included RCTs. First, this study includes 19 studies, 10 of which have been published since the Cochrane review and were not included in previous guidelines.16,17,18,19,21,22,23,29,30,31 Many of those more recent studies16,17,19,21,22,23 are randomized and placebo controlled and evaluate opiates for breathlessness, with most studies demonstrating improvement in the placebo groups. This active placebo effect may have contributed to the lack of benefit over placebo that was found in the current study.
Second, at least half of the studies evaluated in the Cochrane review were in populations other than cancer, whereas this review includes studies in which most patients had advanced cancer. This difference is important because breathlessness in patients with advanced cancer may have unique features and considerations. The patterns and physiologic mechanisms of breathlessness in patients with advanced cancer may be different from those without cancer and can be associated with the cancer itself or with treatments or adverse effects of treatments. Breathlessness in patients with cancer may also be associated with concurrent symptoms, such as anxiety, fatigue, or pain.35 Thus, it is important to focus on this patient population.
Third, the included studies differed widely in the patient populations (medical comorbidities, concurrent opioid or other supportive medications, concurrent interventions to relieve, or prognosis), outcomes evaluated, and measurement instruments used. For example, management of breathlessness in patients with a long-term prognosis compared with those at the end stages of advanced cancer may have different contributing factors and interventions for those factors. The heterogeneity limited the ability to synthesize results.
Fourth, accruing patients to these types of studies can be difficult. In clinical practice, it may be challenging to apply study results to real-world clinical practice, where numerous additional factors may be introduced and cannot all be controlled for. Collaborative group efforts and well-designed, real-world evidence data collection and studies may help to address some of these issues in complex patient populations in whom accrual can be challenging or potentially unethical, such as actively dying patients.
Fifth, determining how best to measure outcomes of interest can be challenging. Breathlessness may be measured at different time points and using different methods. The data included in the studies in this meta-analysis were reported before and after intervention, but interpretation outside the studied time points may be limited; longer-term studies are needed. Furthermore, breathlessness is a complex and subjective multidimensional patient experience, but the studies in this review used unidimensional scales. Consensus recommendations prefer multidimensional scales36; for example, the Personalized Dyspnea Intensity Goal37 can personalize outcome measurements in the context of patients’ expectations and may be more clinically relevant. Multidimensional measures could help improve the evidence of interventions for breathlessness.
In oncology, advanced disease is not synonymous with end of life and can include patients with long life expectancies, particularly in the era of improved survival and new therapeutic agents. For example, a patient with advanced breast cancer, prostate cancer, or lung cancer may have prolonged survival exceeding 5 years, despite having incurable disease. Recommendations for pharmacologic interventions need to be integrated within the context of life expectancy and improved survival for many advanced and incurable cancers.
Implementing the findings of this review may seem counter to previous guidelines and reviews. There is a role for pharmacologic agents, such as opioids and anxiolytics, in the treatment of patients with advanced cancer, but practitioners must account for many factors. Future studies should incorporate a multidimensional common measure of breathlessness. Evaluation of more real-world evidence on the efficacy of pharmacologic agents may help to better explain how and when to implement interventions and how to integrate them with other interventions, such as nonpharmacologic interventions, which have demonstrated evidence of the association with improved breathlessness.38 Future studies also need to incorporate life expectancy as the number of patients with advanced cancer who have long survival grows.
Limitations
This review has limitations, particularly in the context of individual study quality and the intrinsic limitations of the published literature. As previously mentioned, breathlessness is a subjective multidimensional phenomenon that can be difficult to capture with the unidimensional scales in this review. The RCTs reported different types of breathlessness (chronic, episodic, and exertional) in various settings (eg, outpatient clinics and palliative care units) with varied duration of follow-up. In addition, minimum clinically important differences that were defined in our analyses were extrapolated from studies that include broad patient populations, such as patients who have breathlessness from chronic obstructive pulmonary disease; these clinically important differences could be different in advanced cancer. Furthermore, most of the studies were small, highlighting the difficulty accruing patients for these types of studies, and had major limitations on risk of bias assessment, with missing or incomplete information about the randomization data and about outcomes measurements and data (eTable 6 in the Supplement). Treating crossover trial data similarly to parallel studies may have resulted in slightly wider CIs, but a corrected SE was imputed when possible to address this issue. In addition, studies used unidimensional scales for breathlessness rather than recommended multidimensional constructs, and many did not report important outcomes other than breathlessness. Reporting harms and adverse events was inconsistent among the studies, making it difficult to combine results. In addition, some studies that studied interventions aimed at relieving reversible causes of breathlessness, such as pleural effusions or anemia, were excluded, as were studies that targeted closely related symptoms, such as pain or cough.
Conclusions
This study found that pharmacologic interventions were not associated with more effectiveness than placebo for the management of breathlessness in patients with advanced cancer. Practitioners may need to reassess the role of pharmacologic agents, such as opioids and anxiolytics, in the management of breathlessness for patients with advanced cancer, taking into consideration many patient-related factors.
eAppendix. Methods Details
eTable 1. Definitions of the Grades of Overall Strength of Evidence
eTable 2. Characteristics of Included Studies
eTable 3. Summary of Findings for the Effects of Pharmacological Interventions on Physiologic Outcomes in Patients With Advanced Cancer
eTable 4. List of Studies Reporting Harms and Dropouts in Studies of Pharmacological Interventions for Breathlessness in Patients With Advanced Cancer
eTable 5. Rate of Dropouts Due to Adverse Effects of Pharmacological Interventions for Breathlessness in Patients With Advanced Cancer
eTable 6. Risk of Bias of Randomized Controlled Trials (A) and Observational Studies (B) That Evaluate the Effects of Pharmacologic Interventions
eTable 7. Strength of Evidence of Studies That Evaluate the Effects of Pharmacologic Interventions
eFigure 1. Analytic Framework for Evaluating Interventions for Breathlessness in Patients With Advanced Cancer
eFigure 2. Study Search and Preferred Reporting Items for Systematic Reviews and Meta-analyses Flowchart
eFigure 3. Meta-analysis of the Effects of Exercise Capacity Measures in Randomized Controlled Trials Comparing Opioids With Placebo In Patients With Advanced Cancer
eFigure 4. Meta-analysis of the Effects of Placebo vs Opioids on Blood Pressure in Patients With Advanced Cancer in Inpatient Hospice or Palliative Care Units
eFigure 5. Meta-analysis of the Effects of Placebo vs Opioids on Heart Rate in Patients With Advanced Cancer in Inpatient Hospice or Palliative Care Units (Charles, 2008 et al15 Saline vs Nebulized Hydromorphone Comparison)
eFigure 6. Meta-analysis of the Effects of Placebo vs Opioids on Heart Rate in Patients With Advanced Cancer in Inpatient Hospice or Palliative Care Units (Charles, 2008 et al15 Saline vs Systemic Hydromorphone Comparison)
eFigure 7. Meta-analysis of the Effects of Opioids vs Opioids on Heart Rate in Patients With Advanced Cancer in Inpatient Hospice or Palliative Care Units
eFigure 8. Meta-analysis of the Effects of Placebo vs Opioids on Oxygen Saturation in Patients With Advanced Cancer in Inpatient Hospice or Palliative Care Units (Charles, 2008 et al15 Saline vs Nebulized Hydromorphone Comparison)
eFigure 9. Meta-analysis of the Effects of Placebo vs Opioids on Oxygen Saturation in Patients With Advanced Cancer in Inpatient Hospice or Palliative Care Units (Charles, 2008 et al15 Saline Vs Systemic Hydromorphone Comparison)
eFigure 10. Meta-analysis of the Effects of Opioids vs Opioids on Oxygen Saturation in Patients With Advanced Cancer in Inpatient Hospice or Palliative Care Units
eFigure 11. Meta-analysis of the Effects of Placebo vs Opioids on Respiratory Rates in Patients With Advanced Cancer in Inpatient Hospice or Palliative Care Units (Charles, 2008 et al15 Saline vs Nebulized Hydromorphone Comparison)
eFigure 12. Meta-analysis of the Effects of Placebo vs Opioids on Respiratory Rates in Patients With Advanced Cancer in Inpatient Hospice or Palliative Care Units (Charles, 2008 et al15 Saline vs Systemic Hydromorphone Comparison)
eFigure 13. Meta-analysis of the Effects of Opioids vs Opioids on Respiratory Rates in Patients With Advanced Cancer in Inpatient Hospice or Palliative Care Units
eFigure 14. Meta-analysis of the Effects of Placebo vs Opioids on Dizziness Outcomes in Patients With Advanced Cancer in Inpatient Hospice or Palliative Care Units
eFigure 15. Meta-analysis of the Effects of Placebo vs Opioids on Drowsiness Outcomes in Patients With Advanced Cancer in Inpatient Hospice or Palliative Care Units
eFigure 16. Meta-analysis of the Effects of Placebo vs Opioids on Fatigue Outcomes in Patients With Advanced Cancer in Inpatient Hospice or Palliative Care Units
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 2.Walling AM, Weeks JC, Kahn KL, et al. Symptom prevalence in lung and colorectal cancer patients. J Pain Symptom Manage. 2015;49(2):192-202. doi: 10.1016/j.jpainsymman.2014.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson MJ, Yorke J, Hansen-Flaschen J, et al. Towards an expert consensus to delineate a clinical syndrome of chronic breathlessness. Eur Respir J. 2017;49(5):1602277. doi: 10.1183/13993003.02277-2016 [DOI] [PubMed] [Google Scholar]
- 4.Kloke M, Cherny N; ESMO Guidelines Committee . Treatment of dyspnoea in advanced cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2015;26(suppl 5):v169-v173. doi: 10.1093/annonc/mdv306 [DOI] [PubMed] [Google Scholar]
- 5.Levy M, Smith T, Alvarez-Perez A, et al. Palliative care, version 1.2016. J Natl Compr Canc Netw. 2016;14(1):82-113. doi: 10.6004/jnccn.2016.0009 [DOI] [PubMed] [Google Scholar]
- 6.Barnes H, McDonald J, Smallwood N, Manser R. Opioids for the palliation of refractory breathlessness in adults with advanced disease and terminal illness. Cochrane Database Syst Rev. 2016;3:CD011008. doi: 10.1002/14651858.CD011008.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferreira DH, Louw S, McCloud P, et al. ; Australian National Palliative Care Clinical Studies Collaborative (PaCCSC) . Controlled-release oxycodone vs. placebo in the treatment of chronic breathlessness—a multisite randomized placebo controlled trial. J Pain Symptom Manage. 2020;59(3):581-589. doi: 10.1016/j.jpainsymman.2019.10.017 [DOI] [PubMed] [Google Scholar]
- 8.Currow D, Louw S, McCloud P, et al. ; Australian National Palliative Care Clinical Studies Collaborative (PaCCSC) . Regular, sustained-release morphine for chronic breathlessness: a multicentre, double-blind, randomised, placebo-controlled trial. Thorax. 2020;75(1):50-56. doi: 10.1136/thoraxjnl-2019-213681 [DOI] [PubMed] [Google Scholar]
- 9.Dy SM, Gupta A, Waldfogel JM, et al. Interventions for Breathlessness in Patients With Advanced Cancer. AHRQ Comparative Effectiveness Reviews. Agency for Healthcare Research and Quality; 2020. doi: 10.23970/AHRQEPCCER232 [DOI] [PubMed] [Google Scholar]
- 10.Agency for Healthcare Research and Quality. Methods guide for effectiveness and comparative effectiveness reviews. Published February 26, 2015. AHRQ publication 10(14)-EHC063-EF. Updated December 15, 2017. Accessed September 20, 2019. https://effectivehealthcare.ahrq.gov/products/cer-methods-guide/overview
- 11.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65-94. doi: 10.7326/0003-4819-151-4-200908180-00136 [DOI] [PubMed] [Google Scholar]
- 12.Fu R, Vandermeer BW, Shamliyan TA, et al. Handling continuous outcomes in quantitative synthesis methods. In: Agency for Healthcare Research and Quality. Guide for Effectiveness and Comparative Effectiveness Reviews.2008. [PubMed] [Google Scholar]
- 13.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. L Erlbaum Associates. 1988. [Google Scholar]
- 14.Ries AL. Minimally clinically important difference for the UCSD Shortness of Breath Questionnaire, Borg Scale, and Visual Analog Scale. COPD. 2005;2(1):105-110. doi: 10.1081/COPD-200050655 [DOI] [PubMed] [Google Scholar]
- 15.Charles MA, Reymond L, Israel F. Relief of incident dyspnea in palliative cancer patients: a pilot, randomized, controlled trial comparing nebulized hydromorphone, systemic hydromorphone, and nebulized saline. J Pain Symptom Manage. 2008;36(1):29-38. doi: 10.1016/j.jpainsymman.2007.08.016 [DOI] [PubMed] [Google Scholar]
- 16.Hui D, Kilgore K, Frisbee-Hume S, et al. Effect of prophylactic fentanyl buccal tablet on episodic exertional dyspnea: a pilot double-blind randomized controlled trial. J Pain Symptom Manage. 2017;54(6):798-805. doi: 10.1016/j.jpainsymman.2017.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hui D, Kilgore K, Park M, Williams J, Liu D, Bruera E. Impact of prophylactic fentanyl pectin nasal spray on exercise-induced episodic dyspnea in cancer patients: a double-blind, randomized controlled trial. J Pain Symptom Manage. 2016;52(4):459-468, e451. doi: 10.1016/j.jpainsymman.2016.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hui D, Xu A, Frisbee-Hume S, et al. Effects of prophylactic subcutaneous fentanyl on exercise-induced breakthrough dyspnea in cancer patients: a preliminary double-blind, randomized, controlled trial. J Pain Symptom Manage. 2014;47(2):209-217. doi: 10.1016/j.jpainsymman.2013.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinna MA, Bruera E, Moralo MJ, Correas MA, Vargas RM. A randomized crossover clinical trial to evaluate the efficacy of oral transmucosal fentanyl citrate in the treatment of dyspnea on exertion in patients with advanced cancer. Am J Hosp Palliat Care. 2015;32(3):298-304. doi: 10.1177/1049909113513063 [DOI] [PubMed] [Google Scholar]
- 20.Bruera E, MacEachern T, Ripamonti C, Hanson J. Subcutaneous morphine for dyspnea in cancer patients. Ann Intern Med. 1993;119(9):906-907. doi: 10.7326/0003-4819-119-9-199311010-00007 [DOI] [PubMed] [Google Scholar]
- 21.Peoples AR, Bushunow PW, Garland SN, et al. Buspirone for management of dyspnea in cancer patients receiving chemotherapy: a randomized placebo-controlled URCC CCOP study. Support Care Cancer. 2016;24(3):1339-1347. doi: 10.1007/s00520-015-2903-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hui D, Kilgore K, Frisbee-Hume S, et al. Dexamethasone for dyspnea in cancer patients: a pilot double-blind, randomized, controlled trial. J Pain Symptom Manage. 2016;52(1):8-16, e11. doi: 10.1016/j.jpainsymman.2015.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simon ST, Kloke M, Alt-Epping B, et al. EffenDys-fentanyl buccal tablet for the relief of episodic breathlessness in patients with advanced cancer: a multicenter, open-label, randomized, morphine-controlled, crossover, phase II trial. J Pain Symptom Manage. 2016;52(5):617-625. doi: 10.1016/j.jpainsymman.2016.05.023 [DOI] [PubMed] [Google Scholar]
- 24.Gamborg H, Riis J, Christrup L, Krantz T. Effect of intraoral and subcutaneous morphine on dyspnea at rest in terminal patients with primary lung cancer or lung metastases. J Opioid Manag. 2013;9(4):269-274. doi: 10.5055/jom.2013.0168 [DOI] [PubMed] [Google Scholar]
- 25.Navigante AH, Castro MA, Cerchietti LC. Morphine versus midazolam as upfront therapy to control dyspnea perception in cancer patients while its underlying cause is sought or treated. J Pain Symptom Manage. 2010;39(5):820-830. doi: 10.1016/j.jpainsymman.2009.10.003 [DOI] [PubMed] [Google Scholar]
- 26.Navigante AH, Cerchietti LC, Castro MA, Lutteral MA, Cabalar ME. Midazolam as adjunct therapy to morphine in the alleviation of severe dyspnea perception in patients with advanced cancer. J Pain Symptom Manage. 2006;31(1):38-47. doi: 10.1016/j.jpainsymman.2005.06.009 [DOI] [PubMed] [Google Scholar]
- 27.Bruera E, Sala R, Spruyt O, Palmer JL, Zhang T, Willey J. Nebulized versus subcutaneous morphine for patients with cancer dyspnea: a preliminary study. J Pain Symptom Manage. 2005;29(6):613-618. doi: 10.1016/j.jpainsymman.2004.08.016 [DOI] [PubMed] [Google Scholar]
- 28.Allard P, Lamontagne C, Bernard P, Tremblay C. How effective are supplementary doses of opioids for dyspnea in terminally ill cancer patients? a randomized continuous sequential clinical trial. J Pain Symptom Manage. 1999;17(4):256-265. doi: 10.1016/S0885-3924(98)00157-2 [DOI] [PubMed] [Google Scholar]
- 29.Tian C, Wang JY, Wang ML, Jiang B, Zhang LL, Liu F. Morphine versus methylprednisolone or aminophylline for relieving dyspnea in patients with advanced cancer in China: a retrospective study. Springerplus. 2016;5(1):1945. doi: 10.1186/s40064-016-3651-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hui D, Hernandez F, Larsson L, et al. Prophylactic fentanyl sublingual spray for episodic exertional dyspnea in cancer patients: a pilot double-blind randomized controlled trial. J Pain Symptom Manage. 2019;58(4):605-613. doi: 10.1016/j.jpainsymman.2019.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hardy J, Randall C, Pinkerton E, Flatley C, Gibbons K, Allan S. A randomised, double-blind controlled trial of intranasal midazolam for the palliation of dyspnoea in patients with life-limiting disease. Support Care Cancer. 2016;24(7):3069-3076. doi: 10.1007/s00520-016-3125-2 [DOI] [PubMed] [Google Scholar]
- 32.Aabom B, Laier G, Christensen PL, Karlsson T, Jensen MB, Hedal B. Oral morphine drops for prompt relief of breathlessness in patients with advanced cancer—a randomized, double blinded, crossover trial of morphine sulfate oral drops vs. morphine hydrochloride drops with ethanol (red morphine drops). Support Care Cancer. 2020;28(7):3421-3428. doi: 10.1007/s00520-019-05116-1 [DOI] [PubMed] [Google Scholar]
- 33.Kawabata M, Kaneishi K. Continuous subcutaneous infusion of compound oxycodone for the relief of dyspnea in patients with terminally ill cancer: a retrospective study. Am J Hosp Palliat Care. 2013;30(3):305-311. doi: 10.1177/1049909112448924 [DOI] [PubMed] [Google Scholar]
- 34.Hui D, Maddocks M, Johnson MJ, et al; ESMO Guidelines Committee. Management of breathlessness in patients with cancer: ESMO Clinical Practice Guidelines. ESMO Open. 2020;5(6):e001038. doi: 10.1136/esmoopen-2020-001038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKenzie E, Zhang L, Chan S, et al. Symptom correlates of dyspnea in advanced cancer patients using the Edmonton Symptom Assessment System. Support Care Cancer. 2020;28(1):87-98. doi: 10.1007/s00520-019-04787-0 [DOI] [PubMed] [Google Scholar]
- 36.Mularski RA, Campbell ML, Asch SM, et al. A review of quality of care evaluation for the palliation of dyspnea. Am J Respir Crit Care Med. 2010;181(6):534-538. doi: 10.1164/rccm.200903-0462PP [DOI] [PubMed] [Google Scholar]
- 37.Mercadante S, Adile C, Aielli F, et al. Personalized goal for dyspnea and clinical response in advanced cancer patients. J Pain Symptom Manage. 2019;57(1):79-85. doi: 10.1016/j.jpainsymman.2018.10.492 [DOI] [PubMed] [Google Scholar]
- 38.Gupta A, Sedhom R, Sharma R, et al. Nonpharmacological interventions for managing breathlessness in patients with advanced cancer: a systematic review. JAMA Oncol. Published online November 19, 2020. doi: 10.1001/jamaoncol.2020.5184 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Methods Details
eTable 1. Definitions of the Grades of Overall Strength of Evidence
eTable 2. Characteristics of Included Studies
eTable 3. Summary of Findings for the Effects of Pharmacological Interventions on Physiologic Outcomes in Patients With Advanced Cancer
eTable 4. List of Studies Reporting Harms and Dropouts in Studies of Pharmacological Interventions for Breathlessness in Patients With Advanced Cancer
eTable 5. Rate of Dropouts Due to Adverse Effects of Pharmacological Interventions for Breathlessness in Patients With Advanced Cancer
eTable 6. Risk of Bias of Randomized Controlled Trials (A) and Observational Studies (B) That Evaluate the Effects of Pharmacologic Interventions
eTable 7. Strength of Evidence of Studies That Evaluate the Effects of Pharmacologic Interventions
eFigure 1. Analytic Framework for Evaluating Interventions for Breathlessness in Patients With Advanced Cancer
eFigure 2. Study Search and Preferred Reporting Items for Systematic Reviews and Meta-analyses Flowchart
eFigure 3. Meta-analysis of the Effects of Exercise Capacity Measures in Randomized Controlled Trials Comparing Opioids With Placebo In Patients With Advanced Cancer
eFigure 4. Meta-analysis of the Effects of Placebo vs Opioids on Blood Pressure in Patients With Advanced Cancer in Inpatient Hospice or Palliative Care Units
eFigure 5. Meta-analysis of the Effects of Placebo vs Opioids on Heart Rate in Patients With Advanced Cancer in Inpatient Hospice or Palliative Care Units (Charles, 2008 et al15 Saline vs Nebulized Hydromorphone Comparison)
eFigure 6. Meta-analysis of the Effects of Placebo vs Opioids on Heart Rate in Patients With Advanced Cancer in Inpatient Hospice or Palliative Care Units (Charles, 2008 et al15 Saline vs Systemic Hydromorphone Comparison)
eFigure 7. Meta-analysis of the Effects of Opioids vs Opioids on Heart Rate in Patients With Advanced Cancer in Inpatient Hospice or Palliative Care Units
eFigure 8. Meta-analysis of the Effects of Placebo vs Opioids on Oxygen Saturation in Patients With Advanced Cancer in Inpatient Hospice or Palliative Care Units (Charles, 2008 et al15 Saline vs Nebulized Hydromorphone Comparison)
eFigure 9. Meta-analysis of the Effects of Placebo vs Opioids on Oxygen Saturation in Patients With Advanced Cancer in Inpatient Hospice or Palliative Care Units (Charles, 2008 et al15 Saline Vs Systemic Hydromorphone Comparison)
eFigure 10. Meta-analysis of the Effects of Opioids vs Opioids on Oxygen Saturation in Patients With Advanced Cancer in Inpatient Hospice or Palliative Care Units
eFigure 11. Meta-analysis of the Effects of Placebo vs Opioids on Respiratory Rates in Patients With Advanced Cancer in Inpatient Hospice or Palliative Care Units (Charles, 2008 et al15 Saline vs Nebulized Hydromorphone Comparison)
eFigure 12. Meta-analysis of the Effects of Placebo vs Opioids on Respiratory Rates in Patients With Advanced Cancer in Inpatient Hospice or Palliative Care Units (Charles, 2008 et al15 Saline vs Systemic Hydromorphone Comparison)
eFigure 13. Meta-analysis of the Effects of Opioids vs Opioids on Respiratory Rates in Patients With Advanced Cancer in Inpatient Hospice or Palliative Care Units
eFigure 14. Meta-analysis of the Effects of Placebo vs Opioids on Dizziness Outcomes in Patients With Advanced Cancer in Inpatient Hospice or Palliative Care Units
eFigure 15. Meta-analysis of the Effects of Placebo vs Opioids on Drowsiness Outcomes in Patients With Advanced Cancer in Inpatient Hospice or Palliative Care Units
eFigure 16. Meta-analysis of the Effects of Placebo vs Opioids on Fatigue Outcomes in Patients With Advanced Cancer in Inpatient Hospice or Palliative Care Units