Abstract
Background
Given the risk of surgical site infection (SSI), the use of mesh in contaminated ventral hernia repair (VHR) is not standardized and still a clinical dilemma. This meta-analysis aimed to assess whether mesh use increased the risk of SSI in patients following VHR in contaminated field.
Methods
We performed a systematic review of published literature. Studies comparing the mesh repair and anatomic repair, the use of mesh in different Center for Disease Control and Prevention (CDC) wound classes and mesh repair with synthetic mesh or other type of meshes to treat complicated and contaminated VHR were considered for analysis. The main outcome was SSI incidence.
Results
Six studies compared mesh and suture repairs. No significant difference in SSI incidence was observed between patients with complicated VHR in the mesh and suture repair groups.
Five studies analyzed mesh repair in patients by field contamination level. There was no significant difference between the use of mesh in clean-contaminated, contaminated and dirty field versus clean wound class. Moreover, there was no significant difference between the use of mesh in clean-contaminated and contaminated cases.
Four studies compared mesh repair technique with synthetic mesh or other type of meshes were included. The incidence of SSI was significantly lower in the synthetic mesh group.
Conclusions
The use of mesh repair in the management of complicated VHR compared to suture repair is not associated with an increased incidence of SSI even in potentially contaminated fields.
Keywords: Ventral hernia, Contaminated field, Complicated hernia, Surgical site infection
Highlights
-
•
Mesh repair in contaminated ventral hernia was associated with similar surgical site infection rate compared with anatomic repair.
-
•
The use of mesh is not associated with an increased incidence of surgical site infection even in potentially contaminated fields.
-
•
The synthetic mesh is suitable for ventral hernia repair in contaminated settings.
1. Introduction
To date, guidelines for the use of mesh in the contaminated field for Ventral hernia repair (VHR) are not standardized and still a clinical dilemma. Classic surgical teaching “Thou shall not use synthetic mesh in emergent VHR or contaminated fields”(1). Implementation of prosthetic materials in this condition is considered contraindicated given the risk of postoperative infectious complications. Surgical site infection (SSI) after prosthetic VHR can be devastating and requiring complex debridement and mesh removal [2]. Lately, it has been demonstrated that mesh can be safely used in the settings of clean-contaminated and contaminated fields [3,4]. This conclusion has challenged the conventional dogma and then multiple reports on the use of mesh in contaminated VHR have been made. Several studies have clearly reported the safety of synthetic mesh repair in contaminated setting [[4], [5], [6]]. More recently, the introduction of biologic and biosynthetic meshes were suggested as an alternative for patients at high risk of developing surgical site complications. However, studies on this subject reached contradictory findings [[7], [8], [9]]. It is still a lack of evidence regarding the appropriate type of mesh repair for complicated VHR. Therefore, given the lack of high-quality evidence we conducted this systematic review and meta-analysis to determine whether or not mesh repair is associated with a higher risk of SSI than suture repair and investigate differences in SSI between Synthetic mesh and other types of meshes in patients following VHR in contaminated field.
2. Methods
2.1. Review design and registration
This review was performed according to the Cochrane Handbook of Systematic Reviews and Interventions, the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines and AMSTAR (Assessing the methodological quality of systematic reviews) guidelines [10]. It was prospectively registered on PROSPERO (ID: CRD42020173908).
2.2. Criteria of eligibility
Only trials meeting the following PICOs criteria [11] were allowed to be included.
P (patients): Patients with strangulated, incarcerated or contaminated ventral hernias were included. Ventral hernias included all hernias arising from a defect in the anterior abdominal that are epigastric, umbilical Spigelian and incisional hernias. An incarcerated hernia is a hernia in which the content has become irreducible while strangulation occurs when the blood supply to the contents of the hernia (omentum or bowel) is compromised [12]. We excluded patients referred for parastomal and groin hernia repair.
The degree of intraoperative contamination during the hernia repair was recorded according the Center for Disease Control and Prevention (CDC) [13] surgical wound classification as adopted by the World Society of Emergency Surgery (WSES) [12] and the European registry for abdominal wall hernias [14] (Annexe1).
I (Intervention) and C (Control): These include open VHR treated with any non-mesh repair or with mesh placed in the onlay, sublay, or underlay position.
O (outcome): The outcome assessed for this meta-analysis was SSI incidence. SSI was defined according to the standard criteria devised by CDC as an infection that occurs in the part of the body (abdominal wall) where the surgery took place and is further defined as superficial, deep, and organ space SSIs [15,16].
S (study type): Study type was randomized controlled trial (RCT), prospective, observational study, or retrospective cohort study. Studies comparing the mesh repair and suture repair, the use of mesh in different CDC wound classes and mesh repair with synthetic mesh or other type of meshes to treat VHR were considered for analysis.
2.3. Data sources and search strategy
An extensive electronic search of the relevant literature was performed by the authors on 31 December 2020. The Keywords used for the final search using the following databases: MEDLINE, the Cochrane Library, Scopus, Embase were “strangulated”,“incarcerated”,“acute”,“complicated”,“contaminated”,“mesh”,“prosthesis”,“ventral hernia”,“incisional hernia”,” abdominal wall hernia” and “surgical site infection”. Additionally, references from eligible articles and reviews on the topic not found in the literature search were reviewed. No language restrictions were applied.
2.4. Data extraction and quality assessment
Study selection: Two authors (MM and YBS) independently reviewed all abstracts. They assessed the full text of all studies that might meet the inclusion criteria and where disagreement occurred, resolution was reached by consulting a third reviewer (KH).
Risk of Bias and Quality Assessment: The quality of each enrolled study was evaluated independently by 2 authors (MM and YBS), following the criteria recommended in the Cochrane Handbook for Systematic Reviews of Interventions [11]. The quality of randomized controlled trials (RCTs) was assessed using the modified Jadad scale according a maximum of eight points (1 point each for randomization, blinding, withdrawals, dropouts, inclusion/exclusion criteria, adverse effects and statistical analysis). Studies with a score equal to or higher than 4 indicate high quality [17]. Quality analysis of non-randomized was conducted by using the Methodological Index for Non-Randomized Studies (MINORS) index [18]. The ideal global score is 24 for comparative studies. Non-randomized studies with a MINORS index higher than 12 for comparative studies were maintained for analysis. Disagreements were resolved by consulting a third senior author (KH).
Data collection: Data were extracted by one author (MM) with complete and independent verification by a second author (YBS). Any disagreement at the different stages or discrepancies in outcome extraction was resolved either by discussion and re-examination of the relevant study until consensus was achieved.
2.5. Quality of evidence
We evaluated the quality of evidence for each outcome using the GRADE approach with the help of the Grade Pro Software (https://gradepro.org/). The quality of evidence may be rated as high, moderate, low or very low.
2.6. Statistical analysis
Measure of effect size: The odds ratio (OR) was used as the statistical measure for dichotomous outcomes with 95% confidence intervals (CIs) estimated using the Mantel–Haenszel method. Random-effects model was used. All Results were presented in forest plots.
Assessment of heterogeneity: Between-study heterogeneity was assessed using the Cochrane Chi-square test (Q test) and the I2 test. If the P value for Cochrane's Q test was less than 0.1 or if the I2 statistic was greater than 50%, heterogeneity was considered to be significant. In cases of high heterogeneity >75%, the outlier article was removed. The Egger's test was performed and shown by the Funnel Plot. A new Forrest Plot was performed to evaluate the results. All Statistical analysis were carried out using the Review Manager 5.1 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008).
3. Results
3.1. Literature search results
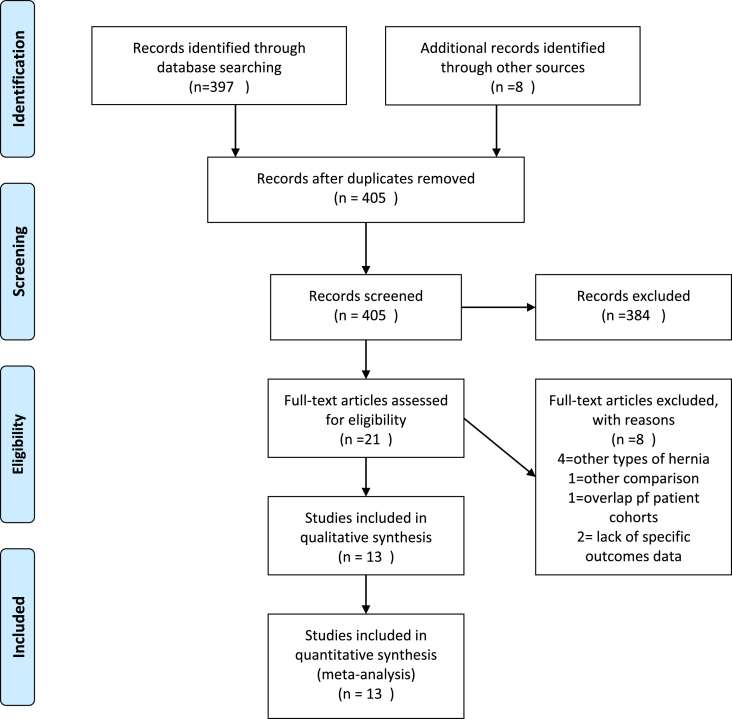
The electronic literature search identified 397 records. After verification of inclusion and exclusion criteria, 21 articles were assessed for eligibility. Of these, eight articles were not suitable for this review.
Four studies [4,[19], [20], [21]] enrolled other types of hernia, such as groin hernias or parastomal hernias. One study discussed different topics concerning wound closed [22]. Two studies exuded due to a lack of specific outcomes data [23,24].
Two studies [2,25] report an overlap of patient cohorts. Therefore, the recent study [25] was included.
Finally, 13 studies entered the meta-analysis model. Included studies were either RCT (n = 1) [26], prospective studies (n = 3) [1,6,27], retrospective studies (n = 5) [25,[28], [29], [30], [31]] and database reviews(n = 4) [16,[32], [33], [34]].
The full search process search and study selection history are presented in Fig. 1.
Fig. 1.
PRISMA flowchart.
3.2. Study characteristics
Studies and patients characteristics data are reported in Table 1. Table 2 presents the quality assessment of the included studies according to MINORS criteria and Modified Jadad scale.
Table 1.
Characteristics of the included studies.
| Author Table 1: Characteristics of the included studies |
Inclusion criteria | No. of Patients (%) | CDC wound Class (%) | SSI rate (%) | Intervention |
|---|---|---|---|---|---|
| Studies evaluating mesh or suture repair in complicated vrentral hernia | |||||
| Abdel-Baki 2007 [26] |
Complicated Paraumbilical hernia | M: 21(50) S: 21(50) |
I: M 18 S 18 II-III: M 3(14,3) S 3(14,3) |
M: 2 (9.5) S: 3 (14.3) |
M: On-lay monofilament polypropylene mesh repair S: Keel repair |
| Haskins 2013 [16] | Ventral Hernia hernia with or without resection of gangrenous bowel | M: 700 [29] S: 1749 (71) |
II: M 418 (30.8) S 939 (69.2) III: M 162 (27.6) S 425 (72.4) IV: M 120(23.8) S 385 (76.2) |
M: 91(13) S:197(11.2) |
Unclear |
| Bondre 2016 [32] | Complicated Ventral Hernia Umbilical(%): 267 [35] Incisional (%): 494 (65) |
M: SM:303 [40] BM:167 [22] S: 291 [38] |
I: SM 249 BM 86 S: 176 II: SM 50 BM 30 S: 90 III: SM 4 BM 37 S: 19 (6.5%) IV: BM 14 S: 6 (2.1%) |
S:44 (15.1) SM:54 (17.8) BM:35 [21] |
M: low-density and/or mid-density polypropylene repair (synthetic), and nonecross-linked biologic matrix repair (biologic) S: suture repair |
| Emile 2017 [6] | Ventral hernia with or without resection of gangrenous bowel Umbilical(%): 103 (84.4) Epigastric (%): 6 [5] Spigelian (%): 3 (2.4) Incisional (%): 10 (8.1) |
M: 66 (54) S: 56 (46) |
I: M 31 (47)M S 21 (37.5) II:M 33 (50) S 16 (28.5) III: M 2 [3] S 19 [34] |
M:5 (7.5) S: 3 (6.5) |
M: On-lay prosthetic polyprolene mesh repair S: Simple primary repair or Mayo's repair |
| Xourafas 2010 [30] |
Ventral Hernia with simultaneous Bowel Resection | M: 51 S: 126 |
Unclear | M:11(20) S: 6 (4.7) |
M: Polypropylene mesh in 74%, 10% Biological Mesh, 2% Absorbable Mesh, 2% Polyesyer Mesh, 6% other type of Mesh S: suture repair |
| Warren 2020 [25] | Contaminated Ventral Hernia | M: SM: 402 BSM:55 BM:38 S: 46 |
II: S: 15 (32.61) SM:212 (52.74) BSM:15 (27.27) BM:3 (7.89) III:S: 6 (13.04) SM:167 (41.54) BSM:22 [40] BM:19 (50) IV: S: 25(54.35) SM: 23 (5.72) BSM: 18(32.73) BM:16 (42.11) |
M: 89 [18] S:8 (17.4) |
Mesh position: Onlay 23 (4.25) Inlay 1 (0.18) Retromuscular 406 (75.05) Preperitoneal 29 (5.36) Intraperitoneal16 (2.96) |
| Studies evaluating mesh repair in different CDC wound classes | |||||
| Casas 2020 [31] | Abdominal wall repairs with polypropylene meshes in potentially contaminated fields | 69 | II: 33(47.8) III: 36(52.2) |
II: 3 [9] III:9 [25] |
Unclear |
| Bessa 2010 [27] | Ventral Hernia with or without resection of gangrenous bowel para-umbilical: 71 (88.75) epigastric 6 (7.5) incisional 3 (3.75) |
80 | I: 62 >I: 18 |
I:8 (15.6) >I: 1(3.4) |
M: On-lay prosthetic polyprolene mesh repair |
| Choi 2012 [33] |
Ventral Hernia hernia with or without resection of gangrenous bowel | ClassI: 29.931 (88) Class > I: 3901(12) |
I: 29.931 II: 3879 III: 22 |
II:1111(3.7) III:376 (9.6) |
Unclear |
| Carbonel 2013 [28] |
Clean-contaminated and contaminated Ventral hernia repair | 100 | II: 42 III: 58 |
II: 11 (26.1) III:20 (23.5) |
Polypropylene mesh in the retro-rectus position |
| Birolini 2019 [1] | Chronic mesh infection resulting from a previous hernia repair compared to a cohort of patients with clean ventral hernia repair. | Infected Mesh: 40(50) Clean control: 40 (50) |
I: 40(50) IV: 40(50) |
I:4 [10] IV:6 [15] |
The previous infected mesh removed entirely. -Monofilament polypropylene Mesh used in the onlay position |
| Studies evaluating different types of meshes | |||||
| Majumder 2016 [31] | ventral hernia repair in clean-contaminated/contaminated fields | SM:57 (45.2) BM:69 (54.8) |
II: BM: 41 (59.5) SM: 37 (64.9%) III: BM: 28 (40.5) SM: 20 (34.1) |
BM:22 (31.9) SM: 7 (12.3) |
Mesh position Sublay:BM 68 (98.6), SM 56 (98.2) Onlay: BM:0 (0.0); SM; 1 (1.8) Underlay: BM 1 (1.4); SM:0 (0.0) |
| Chamieh 2017 [29] | Ventral hernia in a Contaminated Field | SM:24 [41] BM:34 (59) |
II: SM: 10(41.7) BM: 17(50) III: SM: 8(33.3) BM: 10(9.4) IV: SM: 7(20.6) BM: 6(25) |
SM: 7(29.2) BM: 17 (50) |
Mesh location: Open Intraperitoneal Onlay Mesh:18 (53) Onlay: BM 6(17.6); SM 3(12.5) Retrorectus: BM 10(29.4); SM 21(87.5) |
M: Mesh repair; S: Suture repair, SM: Synthetic Mesh, BM: Biologic Mesh; BSM: Biosynthetic Mesh.
Table 2.
Characteristics of studies retained, in alphabetical order.
| First Author | Year of publication | Country of origin | Study period | No of patients | Type of study | Modified Jaded score | MINORS score |
|---|---|---|---|---|---|---|---|
| Abdel-Baki [26] | 2007 | Egypt | 2004–2005 | 42 | RCT | 5 | |
| Bessa [27] | 2012 | Egypt | 2004–2011 | 80 | Prospective | 19 | |
| Birolini [1] | 2019 | Brazil | 2012–2015 | 80 | Prospective cohorted | 18 | |
| Bondre [32] | 2015 | USA | 2010–2011 | 761 | Retro. database reviewsa | 16 | |
| Carbonel [28] | 2013 | USA | 2007–2013 | 100 | Retro | 16 | |
| Chamieh [29] | 2016 | USA | 2013–2015 | 58 | Retro | 16 | |
| Choi [33] | 2012 | USA | 2005–2010 | 33832 | Retro. database Reviewsb |
14 | |
| Emile [6] | 2017 | Egypt | 2014–2016 | 122 | Prospective | 18 | |
| Haskins [16] | 2016 | USA | 2005–2013 | 2449 | Retro. database Reviewsb |
14 | |
| Majumder [31] | 2016 | USA | 2009–2015 | 126 | Multicenter, retrospective | 16 | |
| Xourafas [30] | 2010 | Italy | 1992–2007 | 177 | Retro | 16 | |
| Warren [25] | 2020 | USA | 2007–2019 | 541 | Retro. database Reviewsc |
16 | |
| Casas [31] | 2020 | Argentina | 2012–2019 | 69 | Retro | 14 |
RCT: randomized control trial, Retro: Retrospective.
Ventral Hernia Outcomes Collaborative multicenter database.
National Surgical Quality Improvement Program (NSQIP).
tAmericas Hernia Society Quality Collaborative (AHSQC).
Prospectie study compared to a cohort.
3.3. The studies were published between 2007 and 2020
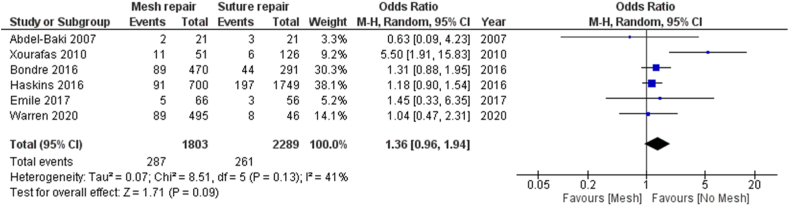
Six studies [6,16,25,26,30,32] with 4092 patients compared mesh and suture repairs.
Five studies [1,27,28,31,33] analyzed mesh repair in patients by field contamination level with a total of 34161 patients.
Four studies [25,29,32,34] comprising 1148 patients reported SSI, comparing mesh repair technique with synthetic mesh or other type of meshes.
3.4. Surgical site infection
3.4.1. Mesh repair versus suture repair
In six included studies, a total of 4092 patients underwent emergent VHR; 599 (14.6%) patients had CDC wound class I (225 in Mesh repair group and 374 in Suture repair group), 3493 (85.4%) patients had CDC wound classes II, III and IV (1419 in mesh group and 2074 in suture repair group).
Three studies [16,25,30] included only patients with CDC wound classes III and IV.
A total of 548 (13.4%) wound infections were reported (287 (17.7%) in the mesh repair group and 261 (10,7%) in suture repair group).
The wound infection rate was higher in the mesh repair group than in suture repair group, but the difference was not statistically significant (odds ratio [OR] = 1.36, 95% CI (0.96–1.94),p = 0.09. The Forest Plot of the Wound infection is shown in Fig. 2. Analysis of all studies for wound infection showed moderate heterogeneity (I2 = 41%).
Fig. 2.
Forest plot of comparison: 1 Mesh repair versus suture repair, outcome: wound infection.
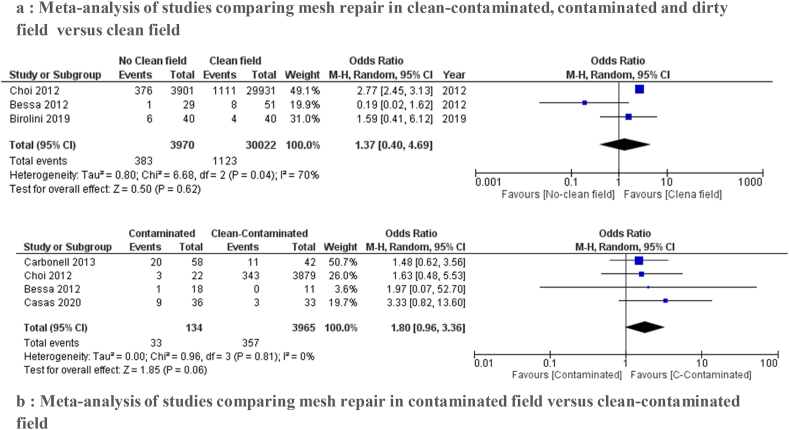
a: Meta-analysis of studies comparing mesh repair in clean-contaminated, contaminated and dirty field versus clean field.
b: Meta-analysis of studies comparing mesh repair in contaminated field versus clean-contaminated field.
The quality of the evidence was regarded as very low based on the GRADE approach.
3.4.2. Mesh repair in different CDC wound classes
Five studies [1,27,28,31,33] evaluated wound infection occurrence by field contamination. We performed two sets of analysis following the CDC wound classification:
3.4.2.1. CDC wound classes II, III and IV versus CDC wound class I
The purpose of three studies [1,27,33] was to establish whether the use of mesh is safe in clean-contaminated, contaminated and dirty field (CDC wound classes II, III and IV) with the clean wound class data (CDC wound class I).
Wound infection rates were 1.5% in contaminated field and 3.9% in clean wound class. A meta-analysis on these studies found a pooled OR of 1.37 (95% CI: 0.40–4.69) in the random effects model. We found no statistically significant mean difference between the two groups (p = 0.62).
There was a high heterogeneity between the studies (I2 = 70%).The results are shown in the forest plot in Fig. 3a.
Fig. 3.
Forest plot of comparison: Mesh repair in different Center for Disease Control and Prevention wound classes, outcome: wound infection.
3.4.2.2. CDC wound class II versus CDC wound class III
Four studies [27,28,31,33] compared the data for clean-contaminated (CDC wound class II) and contaminated cases (CDC wound class III).
The incidence of SSI was lower in the CDC wound class II than in the CDC wound class III. The analysis showed no statistically significant difference between the two groups (OR of 1.80 (95% CI: 0.96–3.136)) with low heterogeneity (I2 = 0%) (Fig. 3b).
The quality of evidence was very low.
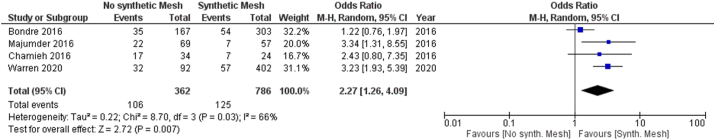
3.4.3. Type of the Mesh
Four studies [25,29,32,34] including 1148 patients reported SSI rate by type of the Mesh.
There were 786 (68.5%) patients in the synthetic mesh group versus 420 (31.5%) patients in the no synthetic Mesh group (Biologic Mesh and absorbable synthetic mesh).
Among these, 29.2% were clean cases, 36.1% were clean contaminated, 27.4% were contaminated and 7.3% infected.
Incidence of SSI was significantly higher in the no synthetic mesh group than the synthetic mesh group (106 (29.3%) versus 125 (15.9%) respectively, odds ratio [OR] = 2.27, 95% CI (1.26–4.09),p = 0.007). The I2 statistic was 66%, indicating moderate heterogeneity. The results are displayed in the forest plot in Fig. 4.
Fig. 4.
Forest plot of comparison: Mesh repair by type of Mesh, outcome: wound infection.
4. Discussion
Our study showed that the mesh repair in patients with complicated or contaminated VHR was associated with similar SSI rate compared with anatomic repair. Second, we have observed that the use of mesh repair in contaminated field could be safe and not associated with either major SSI.
The consensus conference on emergency repair of abdominal wall hernias of the World Society of Emergency Surgery supports our results. Prosthetic repair with a synthetic mesh is recommended in emergency VHR in clean surgical field and clean–contaminated surgical field (grade 1A recommendation) [12]. In all five included studies comparing mesh and suture repairs, the infection rates between the two groups were similar except the study of Xourafas [30].
Authors included 177 patients with concomitant bowel resection during VHR. SSI rate was significantly higher in the mesh repair group (21.6% versus 4.8%, [OR] = 5.0, 95% CI (1.74–14.33)) which might be the reason for the high heterogeneity in our analysis. After removing this study and performing a new analysis, heterogeneity was good (I = 0%).
Conversely, other included studies found that the use of mesh is safe with bowel resection and in contaminated field.
Carbonell el al [28] challenged the dogma that synthetic mesh is contraindicated in contaminated VHR. The authors reported 100 cases of contaminated VHR performed with synthetic mesh. The rate of SSI was 19% for 58 contaminated cases and mesh removal was indicated in just 4 cases. The meshes were not removed as a consequence of superficial or deep SSI (anastomotic leaks with extensive intraperitoneal soilage in 2 cases, colocutaneous fistula in one case and mucocutaneous disruption in one case. Their good outcomes led them to recommended prosthetic repair in all cases of abdominal wall hernia even in contaminated and infected fields.
Other studies [1,27,33] have reported similar success of prosthetic mesh repair in the management of complicated VH. None of 18 patients reported in the study of Bessa et al. [27] who had resection-anastomosis of necrosis small intestine developed SSI and authors concluded that there is no correlation between bowel resection and SSI. These studies disclaim the notion that the intestinal resection is considered as a contraindication for prosthetic mesh repair.
A meta-analysis on the risk factors for mesh related infections after hernia repair surgery estimated that emergency operation is a significant risk factor RR = 2.46 [1.56, 3.91], P < 0.001(35).
Xourafas et al.(30) and Nieuwenhuizen et al. [19] in two retrospective studies on the use of mesh in complicated hernias concluded that prosthetic mesh was a significant risk factor associated with the occurrence of SSI. Surely, there are cases of mesh-related complications in VHR. Carbonell(28) confirmed that the majority of mesh-related complications in open hernia repair are due to older mesh technology, such as microporous Meshs. Recently, macroporous polypropylene-based meshes can resist to bacterial adherence similar to biologic Meshes. The Blatnik et al. study [36] add further evidence to this conclusion. They suggest that this favorable resistance of the synthetic mesh to bacterial infections can be explained by the macroporous structure of the mesh. With pores of diameter larger than 70 μm, the macroporous, structure can clear a large percentage of bacterial infections as it allows contact of bacteria with granulocytes and macrophages (diameter of 15–20 μm).
Bury et al. [37] demonstrated that a synthetic implant even in a dirty field, does not affect the normal healing process and does not influence the persistence of bacterial peritonitis in an experimental model.
In the prospective study by Birolini et al. [1]., 40 patients underwent operations for complicated VHR with synthetic mesh in dirty-infected field (Class IV) compared to a cohort of 40 patients with clean ventral hernia repairs. Similar infection rates were reported in the clean and infected group (10% vs 15% respectively, p = 0.499). Mesh removal was required in just one patient in each group. They concluded that using of mesh in infected field presented similar SSI rate compared to clean repairs.
Several studies [4,38,39] analyze the outcomes of the use of synthetic mesh in abdominal wall repair in class IV wounds. SSI varies between 23 and 27%. Future implication of this result could be the use of synthetic meshes in emergency closures of laparotomies, even in dirty field [4].
Another issue of controversy is the use of bioprosthesis and biosynthetic mesh for complicated VHR.
Sevral reviews [9,40,41] and meta-analysis [7,42] on this subject reached contradictory conclusions. The WSES and the Ventral Hernia Working Group recommended the use of biological mesh in contaminated–dirty surgical field (grade 2C recommendation) [12].This solution is not easily available everywhere owing to their cost. In other words, 100 pieces of synthetic mesh cost the same as one biologic Mesh [6]. Totten et al. [43] demonstrated that the cost of biologic mesh for hernia repair is significantly higher than synthetic mesh considering the costs of surgical outcomes such as recurrence, postoperative complications and rehospitalisation.
Many comparative studies [2,29,34] have shown that the rate of SSI was comparable between the synthetic and biologic meshes. The systematic review of Primus [44] does not support the affirmation that the use of biologic mesh is better than synthetic mesh for repair of potentially contaminated hernias. Bellows concluded in his review of 60 studies that there is an insufficient level of high-quality evidence in the literature on the value of biological tissue in complex and contaminated incisional hernia repair [8].
The analysis of Atema [7] showed no superiority of biologic over synthetic mesh for contaminated VHR with comparable SSI rates. Furthermore, Rosen [45] reported that the appropriate mesh for contaminated VHR is still not clear. Because of their high-cost and poor long-term, the potential alternative to biologic mesh is biosynthetic absorbable materials. Sahoo et al. [2] evaluated the use of biosynthetic Mesh in contaminated VHR. Authors didn't observe any advantages of biosynthetic mesh over polypropylene Mesh and they reported that biosynthetic mesh usage appeared to increase significantly the rate of SSI (22.4% in the biosynthetic Mesh vs. 10.9% in the synthetic mesh, p = 0.03).
Therefore, there are any benefits of biosynthetic mesh over synthetic mesh in terms of SSI. Our meta-analysis showed that incidence of SSI was significantly higher in the no synthetic mesh group than the synthetic mesh group . There was a high level of heterogeneity among the studies according to this outcome (p=0,03 , I2=66%). This could be due to the including of multiple mesh products. This may alter the postoperative outcomes. While this prevented us from making a stronger level of recommendations.
Our study should be interpreted in view of certain limitations for a number of reasons. Most importantly, the review is limited by the low quality of included evidence. Only one RCT was included, whereas most reports were observational studies. Three studies had a prospective design and nine studies were retrospective cohort or retrodatabase reviews. These studies were at serious risk of bias related to outcome detection and assessor. The use or the type of the mesh were not blinded in the majority of studies. In addition, SSI rate between the different surgical wound classes might cause bias. Second, variation in the definition for SSI and the degree of contamination among the studies resulted in marked heterogeneity. Moreover there was substantial heterogeneity regarding the type and duration of prophylactic antibiotics, surgical techniques such as mesh position. Furthermore, it was not possible to perform an analysis for mesh position and SSI since most included studies didn't reported these data.
For all these reasons, the quality of evidence was very low for most outcomes (Table 3). This suggest that further studies and randomized trials might be helpful to still impact the results.
Table 3.
GRADE Summary of Findings (SoF) table.
| Outcome | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects |
|
|---|---|---|---|---|---|
| Risk with control | Risk with intervention | ||||
| Mesh repair versus suture repair | 4094 (6 observational studies) |
⨁◯◯◯ VERY LOWa |
OR 1.34 (0.96–1.86) | 114 per 1 000 | 33 more per 1 000 (4 fewer to 79 more) |
| Clean-contaminated and contaminated Vs clean wound class | 33992 (3 observational studies) |
⨁◯◯◯ VERY LOWa |
OR 1.37 (0.40–4.69) | 37 per 1 000 | 13 more per 1 000 (22 fewer to 117 more) |
| Clean-contaminated Versuss and contaminated wound class | 4099 (4 observational studies) |
⨁◯◯◯ VERY LOWa |
OR 1.80 (0.96–3.36) | 89 per 1 000 | 61 more per 1 000 (3 fewer to 159 more) |
| Type of the Mesh | 1148 (4 observational studies) |
⨁◯◯◯ VERY LOWa |
OR 2.27 (1.26–4.09) | 150 per 1 000 | 135 more per 1 000 (50 more to 239 more) |
CI: Confidence interval; OR: Odds ratio.
Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
In conclusion, although the reading of our findings may be restrained by the heterogeneity, methodological limitations and absence of a higher level of evidence, our results showed that the use of prosthetic mesh repair in the management of complicated VHR is not associated with an increased incidence of SSI even in potentially contaminated fields. Further randomized controlled trials are needed to define the best mesh choice for repairing ventral hernias in contaminated field.
Ethical approval
No Ethical Approval required as this research project is a systematic review of previous studies.
Consent
No Ethical Approval or consent required as this research project is a systematic review of previous studies.
Author contribution
MM, YBS: study design, data collection, data analysis, writing.
AM,GHK: data collection, data analysis.
ABD, SD,SS: data analysis, writing.
KH, CD: conceptualization, methodology.
MBM: conceptualization, supervision.
Registration of research studies
-
1.
Name of the registry: PROSPERO.
-
2.
Unique Identifying number or registration ID: CRD42020173908.
-
3.
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=173908.
Guarantor
Mohamed Maatouk.
Review registration
PROSPERO (ID: CRD42020173908).
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2021.02.019.
Annex. The surgical wound classification system of the Centers for Disease Control and Prevention as adopted by the World Society of Emergency Surgery (WSES) and the European registry for abdominal wall hernias
| Wound Class | CDC Definition | Example for VHR |
|---|---|---|
| Class I: Clean | These are uninfected operative wounds in which no inflammation is encountered and the respiratory, alimentary, genital, or uninfected urinary tracts are not entered | Intestinal incarceration and no signs of intestinal strangulation or concurrent bowel resection |
| Class II: Cleancontaminated | These are operative wounds in which the respiratory, alimentary, genital, or urinary tract is entered under controlled conditions and without unusual contamination | -Bowel lesion during adhesiolysis, without gross spillage of bowel content -Combined cholecystectomy and hernia repair -Bowel resection for incarceration -Presence of a colostomy |
| Class III: Contaminated | These include open, fresh, accidental wounds, operations with major breaks in sterile technique or gross spillage from the gastrointestinal tract, and incisions in which acute, nonpurulent inflammation is encountered | - Bowel necrosis or bowel lesion with gross spillage during intestinal resection -Enterocutaneous fistula |
| Class IV: Dirty | These include old traumatic wounds with retained devitalised tissue and those that involve existing clinical infection or perforated viscera. This definition suggests that the organisms causing post-operative infection were present in the operative field before the operation | -Peritonitis from bowel perforation -Presence of infected mesh |
CDC: centre for disease control,VHR: ventral Hernia Repair.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Birolini C., de Miranda J., Tanaka E., Utiyama E., Rasslan S., Birolini D. The use of synthetic mesh in contaminated and infected abdominal wall repairs: challenging the dogma—a long-term prospective clinical trial. Hernia. 2019:1–17. doi: 10.1007/s10029-019-02035-2. [DOI] [PubMed] [Google Scholar]
- 2.Sahoo S., Haskins I.N., Huang L.-C., Krpata D.M., Derwin K.A., Poulose B.K. Early wound morbidity after open ventral hernia repair with biosynthetic or polypropylene mesh. J. Am. Coll. Surg. 2017;225(4):472–480. e1. doi: 10.1016/j.jamcollsurg.2017.07.1067. [DOI] [PubMed] [Google Scholar]
- 3.Hentati H., Dougaz W., Dziri C. Mesh repair versus non-mesh repair for strangulated inguinal hernia: systematic review with meta-analysis. World J. Surg. 2014;38(11):2784–2790. doi: 10.1007/s00268-014-2710-0. [DOI] [PubMed] [Google Scholar]
- 4.Pandey H., Thakur D., Somashekar U., Kothari R., Agarwal P., Sharma D. Use of polypropylene mesh in contaminated and dirty strangulated hernias: short-term results. Hernia. 2018;22(6):1045–1050. doi: 10.1007/s10029-018-1811-3. [DOI] [PubMed] [Google Scholar]
- 5.Lin Y.-T., Weng T.-Y., Tam K.-W. Effectiveness and safety of mesh repair for incarcerated or strangulated hernias: a systematic review and meta-analysis. World J. Surg. 2020:1–9. doi: 10.1007/s00268-020-05430-4. [DOI] [PubMed] [Google Scholar]
- 6.Emile S.H., Elgendy H., Sakr A., Gado W.A., Abdelmawla A.A., Abdelnaby M. Outcomes following repair of incarcerated and strangulated ventral hernias with or without synthetic mesh. World J. Emerg. Surg. 2017;12(1):31. doi: 10.1186/s13017-017-0143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atema J.J., de Vries F.E., Boermeester M.A. Systematic review and meta-analysis of the repair of potentially contaminated and contaminated abdominal wall defects. Am. J. Surg. 2016;212(5):982–995. e1. doi: 10.1016/j.amjsurg.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Bellows C.F., Smith A., Malsbury J., Helton W.S. Repair of incisional hernias with biological prosthesis: a systematic review of current evidence. Am. J. Surg. 2013;205(1):85–101. doi: 10.1016/j.amjsurg.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 9.Sbitany H., Kwon E., Chern H., Finlayson E., Varma M.G., Hansen S.L. Outcomes analysis of biologic mesh use for abdominal wall reconstruction in clean-contaminated and contaminated ventral hernia repair. Ann. Plast. Surg. 2015;75(2):201–204. doi: 10.1097/SAP.0000000000000030. [DOI] [PubMed] [Google Scholar]
- 10.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann. Intern. Med. 2009;151(4) doi: 10.7326/0003-4819-151-4-200908180-00136. W-65-W-94. [DOI] [PubMed] [Google Scholar]
- 11.Higgins J.P., Thomas J., Chandler J., Cumpston M., Li T., Page M.J. John Wiley & Sons; 2019. Cochrane Handbook for Systematic Reviews of Interventions. [Google Scholar]
- 12.De Simone B., Birindelli A., Ansaloni L., Sartelli M., Coccolini F., Di Saverio S. Emergency repair of complicated abdominal wall hernias: WSES guidelines. Hernia. 2019:1–10. doi: 10.1007/s10029-019-02021-8. [DOI] [PubMed] [Google Scholar]
- 13.Garner J.S. CDC guideline for prevention of surgical wound infections, 1985. Infect. Contr. Hosp. Epidemiol. 1986;7(3):193–200. doi: 10.1017/s0195941700064080. [DOI] [PubMed] [Google Scholar]
- 14.Muysoms F., Campanelli G., Champault G., DeBeaux A., Dietz U., Jeekel J. EuraHS: the development of an international online platform for registration and outcome measurement of ventral abdominal wall hernia repair. Hernia. 2012;16(3):239–250. doi: 10.1007/s10029-012-0912-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Control CfD . vol. 11. 2018. Prevention. Surgical Site Infection (SSI) Event 2018 Web Site. Accessed January. [Google Scholar]
- 16.Haskins I., Horne C., Krpata D., Prabhu A., Tastaldi L., Perez A.J. A call for standardization of wound events reporting following ventral hernia repair. Hernia. 2018;22(5):729–736. doi: 10.1007/s10029-018-1748-6. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y., Zhou L., Liu X., Liu L., Wu Y., Zhao Z. The effectiveness of the problem-based learning teaching model for use in introductory Chinese undergraduate medical courses: a systematic review and meta-analysis. PloS One. 2015;10(3) doi: 10.1371/journal.pone.0120884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slim K., Nini E., Forestier D., Kwiatkowski F., Panis Y., Chipponi J. Methodological index for non‐randomized studies (MINORS): development and validation of a new instrument. ANZ J. Surg. 2003;73(9):712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 19.Nieuwenhuizen J., Van Ramshorst G., Ten Brinke J., de Wit T., van der Harst E., Hop W. The use of mesh in acute hernia: frequency and outcome in 99 cases. Hernia. 2011;15(3):297–300. doi: 10.1007/s10029-010-0779-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozbagriacik M., Bas G., Basak F., Sisik A., Acar A., Kudas I. Management of strangulated abdominal wall hernias with mesh; early results. Northern clin. Istanbul. 2015;2(1):26. doi: 10.14744/nci.2015.03522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venara A., Hubner M., Le Naoures P., Hamel J.-F., Hamy A., Demartines N. Surgery for incarcerated hernia: short-term outcome with or without mesh. Langenbeck's Arch. Surg. 2014;399(5):571–577. doi: 10.1007/s00423-014-1202-x. [DOI] [PubMed] [Google Scholar]
- 22.Zafar H., Zaidi M., Qadir I., Memon A.A. Emergency incisional hernia repair: a difficult problem waiting for a solution. Ann. Surg Innovat. Res. 2012;6(1):1–5. doi: 10.1186/1750-1164-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keogh K., Slater K. Comparison of biosynthetic versus synthetic mesh in clean and contaminated ventral hernia repairs. ANZ J. Surg. 2020;90(4):542–546. doi: 10.1111/ans.15587. [DOI] [PubMed] [Google Scholar]
- 24.Juul N., Henriksen N., Jensen K. Increased risk of postoperative complications with retromuscular mesh placement in emergency incisional hernia repair: a nationwide register-based cohort study. Scand. J. Surg. 2020 doi: 10.1177/1457496920966237. [DOI] [PubMed] [Google Scholar]
- 25.Warren J., Desai S.S., Boswell N.D., Hancock B.H., Abbad H., Ewing J.A. Safety and efficacy of synthetic mesh for ventral hernia repair in a contaminated field. J. Am. Coll. Surg. 2020;230(4):405–413. doi: 10.1016/j.jamcollsurg.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Abdel-Baki N., Bessa S., Abdel-Razek A. Comparison of prosthetic mesh repair and tissue repair in the emergency management of incarcerated para-umnbilical hernia: a prospective randomized study. Hernia. 2007;11(2):163–167. doi: 10.1007/s10029-007-0189-4. [DOI] [PubMed] [Google Scholar]
- 27.Bessa S., Abdel-Razek A. Results of prosthetic mesh repair in the emergency management of the acutely incarcerated and/or strangulated ventral hernias: a seven years study. Hernia. 2013;17(1):59–65. doi: 10.1007/s10029-012-0938-x. [DOI] [PubMed] [Google Scholar]
- 28.Carbonell A.M., Criss C.N., Cobb W.S., Novitsky Y.W., Rosen M.J. Outcomes of synthetic mesh in contaminated ventral hernia repairs. J. Am. Coll. Surg. 2013;217(6):991–998. doi: 10.1016/j.jamcollsurg.2013.07.382. [DOI] [PubMed] [Google Scholar]
- 29.Chamieh J., Tan W.H., Ramirez R., Nohra E., Apakama C., Symons W. Synthetic versus biologic mesh in single-stage repair of complex abdominal wall defects in a contaminated field. Surg. Infect. 2017;18(2):112–118. doi: 10.1089/sur.2016.106. [DOI] [PubMed] [Google Scholar]
- 30.Xourafas D., Lipsitz S.R., Negro P., Ashley S.W., Tavakkolizadeh A. Impact of mesh use on morbidity following ventral hernia repair with a simultaneous bowel resection. Arch. Surg. 2010;145(8):739–744. doi: 10.1001/archsurg.2010.144. [DOI] [PubMed] [Google Scholar]
- 31.Casas M.A., Dreifuss N.H., Schlottmann F., Sadava E.E. Safety and long-term outcomes after hernia repairs with synthetic mesh in contaminated fields. J. Gastrointest. Surg. 2020;24(12):2849–2851. doi: 10.1007/s11605-020-04743-y. [DOI] [PubMed] [Google Scholar]
- 32.Bondre I.L., Holihan J.L., Askenasy E.P., Greenberg J.A., Keith J.N., Martindale R.G. Suture, synthetic, or biologic in contaminated ventral hernia repair. J. Surg. Res. 2016;200(2):488–494. doi: 10.1016/j.jss.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 33.Choi J.J., Palaniappa N.C., Dallas K.B., Rudich T.B., Colon M.J., Divino C.M. Use of mesh during ventral hernia repair in clean-contaminated and contaminated cases: outcomes of 33,832 cases. Ann. Surg. 2012;255(1):176–180. doi: 10.1097/SLA.0b013e31822518e6. [DOI] [PubMed] [Google Scholar]
- 34.Majumder A., Winder J.S., Wen Y., Pauli E.M., Belyansky I., Novitsky Y.W. Comparative analysis of biologic versus synthetic mesh outcomes in contaminated hernia repairs. Surgery. 2016;160(4):828–838. doi: 10.1016/j.surg.2016.04.041. [DOI] [PubMed] [Google Scholar]
- 35.Mavros M.N., Athanasiou S., Alexiou V.G., Mitsikostas P.K., Peppas G., Falagas M.E. Risk factors for mesh-related infections after hernia repair surgery: a meta-analysis of cohort studies. World J. Surg. 2011;35(11):2389. doi: 10.1007/s00268-011-1266-5. [DOI] [PubMed] [Google Scholar]
- 36.Blatnik J.A., Krpata D.M., Jacobs M.R., Gao Y., Novitsky Y.W., Rosen M.J. In vivo analysis of the morphologic characteristics of synthetic mesh to resist MRSA adherence. J. Gastrointest. Surg. 2012;16(11):2139–2144. doi: 10.1007/s11605-012-1992-5. [DOI] [PubMed] [Google Scholar]
- 37.Bury K., Śmietański M., Justyna B., Gumiela P., Śmietańska A.I., Owczuk R. Effects of macroporous monofilament mesh on infection in a contaminated field. Langenbeck's Arch. Surg. 2014;399(7):873–877. doi: 10.1007/s00423-014-1225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Birolini C., De Miranda J., Utiyama E., Rasslan S. A retrospective review and observations over a 16-year clinical experience on the surgical treatment of chronic mesh infection. What about replacing a synthetic mesh on the infected surgical field? Hernia. 2015;19(2):239–246. doi: 10.1007/s10029-014-1225-9. [DOI] [PubMed] [Google Scholar]
- 39.Slater N.J., Bokkerink W.J., Konijn V., Bleichrodt R.P., van Goor H. Safety and durability of one-stage repair of abdominal wall defects with enteric fistulas. Ann. Surg. 2015;261(3):553–557. doi: 10.1097/SLA.0000000000000733. [DOI] [PubMed] [Google Scholar]
- 40.Kamarajah S., Chapman S., Glasbey J., Morton D., Smart N., Pinkney T. Systematic review of the stage of innovation of biological mesh for complex or contaminated abdominal wall closure. BJS Open. 2018;2(6):371–380. doi: 10.1002/bjs5.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El-Gazzaz G., Erem H., Aytac E., Salcedo L., Stocchi L., Kiran R. Risk of infection and hernia recurrence for patients undergoing ventral hernia repair with non-absorbable or biological mesh during open bowel procedures. Tech. Coloproctol. 2013;17(3):315–320. doi: 10.1007/s10151-012-0928-0. [DOI] [PubMed] [Google Scholar]
- 42.Lee L., Mata J., Landry T., Khwaja K.A., Vassiliou M.C., Fried G.M. A systematic review of synthetic and biologic materials for abdominal wall reinforcement in contaminated fields. Surg. Endosc. 2014;28(9):2531–2546. doi: 10.1007/s00464-014-3499-5. [DOI] [PubMed] [Google Scholar]
- 43.Totten C.F., Davenport D.L., Ward N.D., Roth J.S. Cost of ventral hernia repair using biologic or synthetic mesh. J. Surg. Res. 2016;203(2):459–465. doi: 10.1016/j.jss.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 44.Primus F.E., Harris H.W. A critical review of biologic mesh use in ventral hernia repairs under contaminated conditions. Hernia. 2013;17(1):21–30. doi: 10.1007/s10029-012-1037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosen M.J., Bauer J.J., Harmaty M., Carbonell A.M., Cobb W.S., Matthews B. Multicenter, prospective, longitudinal study of the recurrence, surgical site infection, and quality of life after contaminated ventral hernia repair using biosynthetic absorbable mesh: the COBRA study. Ann. Surg. 2017;265(1):205. doi: 10.1097/SLA.0000000000001601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.