Abstract
Background: Nitrate (NO3−)-rich beetroot (BR) juice supplementation has been shown to improve cardiovascular function via reduction to nitrite (NO2−) and then to the bioactive molecule nitric oxide (NO). However, limited research exists for the role of inorganic NO2− that is contained naturally within BR. Objective: As BR juice can naturally contain both NO3− and NO2− the objective of this study was to evaluate the individual effects of NO3− and NO2− consumed from BR on plasma [NO3−]/[NO2−] and their subsequent effects on various cardiovascular measures. Design: In four separate treatments, 11 healthy adults consumed 250 mL of BR containing one of the following: (i) high NO3−, low NO2− (HL; 572 mg NO3−, 32 mg NO2−); (ii) medium NO3−, medium NO2− (MM; 280 mg NO3−, 237 mg NO2−); (iii) low NO3−, medium NO2− (LM; 43 mg NO3−, 262 mg NO2−); (iv) placebo (PL; low NO3−, low NO2−: 8 mg NO3−, 5.8 mg NO2−). Plasma [NO3−]/[NO2−], blood pressure, heart rate, mean arterial pressure (MAP), cardiac output and stroke volume were measured at baseline and every hour or second hour for 6 h post-BR consumption. Outcomes: Ingestion of the HL and MM BR increased plasma [NO2−] and [NO3−] after 2 h, with both remaining elevated after 6 h (p < 0.05). LM increased plasma [NO3−] (p < 0.05) but did not increase plasma [NO2−] compared to PL (p = 0.177). MAP was lower following the consumption of HL at 4 h and LM at 6 h (p < 0.05). Conclusion: Inorganic NO3− consumption is the critical factor in elevating plasma [NO3−] and [NO2−]; however, both NO2− and NO3− show potential to reduce MAP. The known reduction of systolic blood pressure (SBP)/diastolic blood pressure (DBP) following NO3− supplementation was not observed, making it unclear if NO2− contributes to a reduction in SBP/DBP alongside NO3−.
Keywords: nitric oxide, cardiovascular disease, cardioprotective, blood pressure
1. Introduction
Cardiovascular disease (CVD) places a significant burden on the health system, accounting for approximately one-third of all deaths [1]. A major risk factor for the development of CVD is increased blood pressure (BP) or hypertension [2,3] which is often the target for CVD-based interventions. Epidemiological evidence suggests a diet rich in fruit and vegetables reduces BP and the subsequent risk of CVD [4,5,6,7]. This effect has been previously attributed to the abundance of antioxidants and vitamins which are present in fruit and vegetables [4,7]. However, other studies failed to see any cardioprotective effect of antioxidants and vitamins [8,9,10], thus prompting researchers to investigate potential cardioprotective effects of other compounds present in fruits and vegetables. While it is likely that the cardioprotective effect observed with fruit and vegetable intake is multifactorial, the consumption of high nitrate (NO3−) vegetables [11,12] has been shown to lead to the greatest reduction in BP compared to other vegetables [13,14]. As a result, there has been increased interest in the potential of NO3− derived from fruit and vegetables to lead to a reduction in BP [13].
Beetroot juice (BR) is used as a supplement because of its high inorganic nitrate (NO3−) content, a compound found naturally in vegetables and in processed meats, where it is used as a preservative [15]. Beetroot juice contains varying amounts of both inorganic NO3− and inorganic nitrite [16,17,18] which in the human body can reduce to the bioactive molecule nitric oxide (NO) through an exogenous pathway [19]. This exogenous pathway begins with the absorption of NO3− in the gastrointestinal tract, which is then distributed to various locations, including the mouth [16]. In the mouth NO3− is reduced to NO2− by anaerobic bacteria [20]. NO2− is then swallowed and re-enters the gastrointestinal tract; when it reaches the stomach, the acidic environment reduces NO2− to NO [21]. NO enters the circulation and is then able to lower BP by relaxing and dilating the endothelium [22,23,24].
Consistent with the exogenous pathway, multiple studies have reported a reduction of systolic BP (SBP; 5–22 mmHg) and diastolic BP (DBP; 2.4–18.3 mmHg) following supplementation with BR [20,25,26,27,28,29]. This effect has been shown to occur in a dose-dependent manner, with a larger dose of NO3− reducing BP to a greater extent than a smaller dose [25,29,30]. However, some studies have shown no change in either SBP or DBP following BR consumption [31,32]. Although the exact reason for the contradictory data is unclear, it may relate to dosage level—one of these studies [31] used a low NO3− dose (119 mg; 1.4 mmol/day) which was likely insufficient to result in a reduction in BP.
Interestingly, several studies support NO2− having a cardioprotective effect [33,34,35]. Specifically, in humans the oral consumption of NO2− via NaNO2− elicits an elevated plasma NO2− [36], which correlates with a reduction in BP [37,38,39]. When specifically investigating the cardioprotective effect of NaNO2− capsules (80 mg NO2− or 160 mg NO2−), an improvement in brachial artery flow was seen but, interestingly, no change in BP [40]. Studies in mice have shown that NO2− infusion led to a reduction in mean arterial pressure [41,42] and reversed endothelial dysfunction [43]. The cardioprotective effects of NO2− can be explained via the exogenous NO pathway, and the ability for NO2− to also produce the bioactive molecule NO [21,40]. While there is potential for NO2− to reduce BP via the exogenous NO pathway, to date there has been no consideration as to whether the NO2− in BR contributes to the observed reductions in BP.
Therefore, we determined the impact of different doses of NO3− and NO2− from BR on plasma levels of NO3− and NO2− and examined the effect of these different doses on cardiovascular measures. In addition, correlations between the plasma NO3− and NO2− levels and physiological outcomes were investigated.
2. Methods and Materials
2.1. Participants
Eleven healthy adults (18–50 years old) volunteered for this study (5 M; 6 F). All participants completed a health-screening questionnaire to determine eligibility. Inclusion criteria included healthy men and pre-menopausal women aged 18–50 years who were proficient in English and could attend the laboratory on multiple occasions. Those who regularly consumed NO3−-based dietary supplements, or were unable to participate in blood collection, or had a beetroot allergy, or were pregnant or had known health issues, e.g., hypertension or cardiovascular disease, were excluded. Prior to their involvement, the study was explained to all participants and each was provided with an information sheet. Participants were fully informed of any risks associated with the experiments before giving their informed written consent to participate in the investigation. The study was approved by the Massey University Human Ethics Committee (SOA 18/35).
2.2. Study Design and Procedures
Participants were required to visit the laboratory (18–20 °C, 40–60% relative humidity) on five occasions. During the first visit they were familiarised with the testing procedures and equipment, including an ultrasonic cardiac output monitor (USCOM1A; Uscom Ltd.; Sydney, Australia) and automated sphygmomanometer (deluxe HEM-7310; OMRON Healthcare CO. Ltd.; Kyoto, Japan). Height was measured using a stadiometer (Seca portable stadiometer, Amtech, New Zealand) and body mass using scales accurate to 0.1 kg (A & D Weighing, HV-200KGL, Adelaide, Australia).
For 24 h prior to visit 2, participants recorded their food and fluid intake and were asked to replicate these diets for each 24-h period prior to the remaining visits (3 to 5). Participants were instructed to arrive at the laboratory in a fasted state, and to have refrained from caffeine ingestion, strenuous exercise and alcohol for 24 h pre-trial. Each trial commenced in the morning at approximately the same time of day (9 a.m. +/− 1 h).
Each participant was randomly allocated the order which the supplemented beverages would be consumed in a double-blinded, randomized crossover design. Visits 2 to 5 were the experimental trials and each trial was separated by a one-week washout period to allow for complete normalization of nitrate levels in the body [44,45,46]. The drink was consumed within a 10-min period alongside a standardised, isocaloric breakfast with a macronutrient distribution of 15 g protein, 30 g carbohydrate and 10 g fat. Three hours post-supplement intake the participants received a standardised, isocaloric lunch with a macronutrient distribution of 25 g protein, 45 g carbohydrate and 16 g fat.
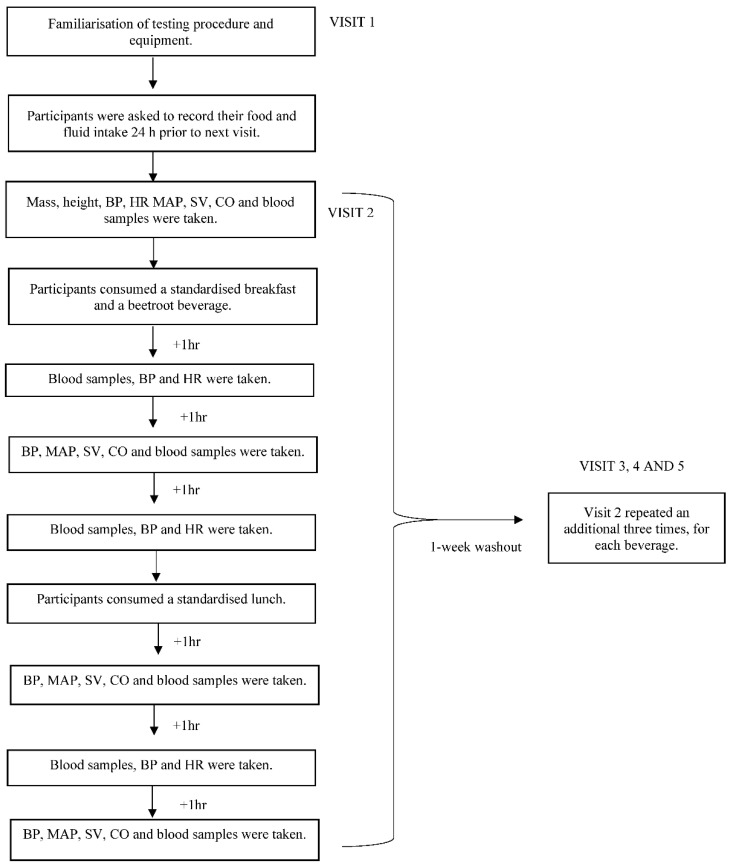
On experimental trial days, baseline measures of blood pressure and a resting blood sample were taken on arrival and prior to supplementation. The allocated drink and breakfast were consumed and the timer started immediately after the drink was finished. Blood samples, BP and heart rate (HR) were measured each hour and other hemodynamic measurements (stroke volume (SV), mean arterial pressure (MAP) and cardiac output (CO)) were carried out via the USCOM every second hour (0 h, +2 h, +4 h, +6 h). Participants remained in the laboratory for the duration of the trial and were able to complete office work between sampling periods. The study protocol is illustrated in Figure 1.
Figure 1.
Protocol of the experimental trials. BP, blood pressure; HR, heart rate; SV, stroke volume; MAP, mean arterial pressure; CO, cardiac output.
2.3. Intervention
For each of the experimental trials, participants consumed 250 mL of one of the four juices containing different levels of nitrate and nitrite (Table 1). Each drink was similar in both appearance and taste and was not perceived as significantly different (p > 0.05) when presented to a consumer sensory panel [47]. The high and medium doses of NO3− (572 mg and 280 mg, respectively) were chosen as these levels have been shown to be sufficient to elicit cardioprotective effects [29]. The chosen nitrite dose was the variable being tested—due to the low concentration of naturally occurring nitrite we were unable to produce a beverage containing >500 mg nitrite.
Table 1.
Nitrate and nitrite content of the drinks (per 250 mL) (as determined by high-performance liquid chromatography (HPLC)).
| Drink | Nitrate mg | Nitrite mg | Nitrate mmol | Nitrite mmol |
|---|---|---|---|---|
| High NO3−, low NO2− (HL) | 572 | 32 | 6.72 | 0.46 |
| Medium NO3−, medium NO2− (MM) | 280 | 237 | 3.29 | 3.43 |
| Low NO3−, medium NO2− (LM) | 43 | 262 | 0.51 | 3.79 |
| Low NO3−, low NO2− (PL) | 8 | 5.8 | 0.09 | 0.08 |
Nitrate (NO3−); Nitrite (NO2−).
Freshly prepared BR was blended with apple juice and water, and the flavour adjusted with a natural beetroot powder (low in NO3−) to produce two standardized drinks of a constant soluble solids concentration (11 °Brix). These drinks were each used as they were and further as the base drink for one other formulation. The drinks were pasteurized and poured into 250 mL plastic bottles, stored frozen and then thawed immediately prior to use. The placebo (PL) was made to a standardized low nitrate (8 mg/250 mL) and low nitrite (5.8 mg/250 mL) concentration which also formed the base of the high NO3−, low NO2− (HL) beverage. The low nitrate, medium nitrite (LM) beverage was standardized to contain 43 mg nitrate and 262 mg nitrite per 250 mL. This drink was also used as the base formulation for the medium nitrate and medium nitrite beverage (MM).
The medium nitrate and medium nitrite beverage (MM) was prepared immediately prior to consumption by replacing 30 mL of the LM beverage with 30 mL of a commercial BR with known NO3− and NO2− content (determined by high-performance liquid chromatography (HPLC)). This produced a 250 mL beverage of 11 ° Brix containing standardized medium nitrate (280 mg/250 mL) and medium nitrite (237 mg/250 mL). Similarly, to prepare the HL solution, 70 mL of the placebo drink was replaced with 70 mL of the commercial BR immediately prior to consumption to produce a 250 mL beverage of 11° Brix containing standardized high nitrate (572 mg/250 mL) and low nitrite (32 mg/250 mL). The final concentrations of both nitrate and nitrite within the drinks were determined using high-performance liquid chromatography.
2.4. Blood Measurements
Venous blood samples (6 mL) were taken via a cannula (where possible) or by venepuncture within the antecubital area and collected into heparinized tubes. Following cannula blood sampling, 5 mL of a noncoagulant saline solution was then injected through the cannula to prevent clotting and blockage of the line. Samples were mixed and immediately centrifuged (MF-50 Hanil Science Industrial, Gimposi, Korea) at 1300× g for 10 min, and the collected plasma was aliquoted and stored at −80 °C for later HPLC analysis of NO3− and NO2−.
2.5. HPLC Analysis
The quantification of NO3− and NO2− in beetroot juice was based on previously described methods [48,49]. Briefly, samples were centrifuged, filtered (0.45 µm), diluted and separated on a Gracesmart C-18 column (4.6 × 250 mm; 5 µm), using an isocratic mobile phase of 0.01 M Octylammonium orthophosphate (pH 3–3.5) at a flow rate of 0.8 mL/min (20 °C). Detection occurred at UV wavelengths of 193 nm for NO2− and 213 nm for NO3−. Quantification was achieved with external standard Na NO3− and Na NO2− solutions.
The quantification of NO2− in plasma was based on the method described by Li et al., [50]. The plasma samples were filtered using 10 kD cut-off filters (Amicon Ultra 2 10 KDa 2 mL Thermo LPN 00288739 UFC201024) which had each been washed four times with water. The determination of NO2− occurs through its derivatisation with 2,3-diaminonaphthalene (DAN) to yield the highly fluorescent 2,3-naphthotriazole (NAT). Briefly, NAT was separated at a flow rate of 1.3 mL/min (20 °C) on a Waters Spherisorb S5C8 column (4.6 × 250 mm; 5 µm) using a mobile phase of 15 mM sodium phosphate (pH 7.5) containing 50% methanol (0–5 min), sequentially followed by 100% Milli-Q water (5.1–7 min), 100% methanol (7.1–10 min) and 100% Milli-Q water (10.1–12 min) before returning to the initial buffer (12.1–17 min). Fluorescence was monitored with excitation at 375 nm and emission at 415 nm.
This same filtration process was utilized for plasma NO3− analysis. Following the filtration, the method previously described to quantify the NO3− within juice was used to quantify the NO3− within plasma following a suitable dilution.
2.6. Cardiovascular Measurements
Blood pressure and HR were measured via an automated sphygmomanometer (mean value of three measurements taken from the left arm [51]. The USCOM was used to measure velocity time integral (VIT), HR, SBP and DBP. Utilizing these measurements and various algorithms, the mean arterial pressure, stroke volume and cardiac output were obtained.
2.7. Statistical Analysis
Data were checked for normality using the Shapiro–Wilk test and variance using Levene’s test. Data were expressed as mean ± SD and all analyses were completed on raw data and delta-changed data to eliminate the effect of baseline differences. A p-value < 0.05 was considered to be statistically significant. For groups showing statistical differences, an effect size was calculated, with 0.2 being considered a ”small” effect size, 0.5 being considered a ”medium” effect size and 0.8 a “large” effect size [52]. All statistical analyses were completed using IBM SPSS package version 22 (IBM Corporation, Chicago, IL, USA).
Any differences between individual baseline values for all variables for both different drinks and different trial numbers were determined using one-way ANOVA. The main effect of drink, the main effect of time and the interaction effect of drink and time were determined for each variable using a repeated measures two-way ANOVA. To ensure accuracy, both the measured values (absolute value) and the change from baseline (delta-change) were analyzed. Additionally, to exclude the effect of lunch, analysis of each variable was completed taking into account pre-lunch time points (0 h to 3 h) along with the analysis on all time points (0 h to 6 h) for both forms of data. To limit confounders, this was repeated for the main effect of trial, the main effect of time and the interaction effect of trial and time. When a significant interaction was found, Holm–Bonferroni post-hoc tests were used to find out where differences lay.
Pearson’s correlation was used to investigate relationships between plasma [NO2−] and [NO3−] and cardiovascular measures; where 0.2 inferred a weak correlation, 0.5 inferred a moderate correlation and 0.8 inferred a strong correlation [53].
3. Results
3.1. Participants
All eleven participants completed the BP, HR and USCOM measures. Of the eleven, only eight participants completed all blood samples due to difficulties during the blood collection process. Participant characteristics are reported in Table 2.
Table 2.
Baseline characteristics of participants, Mean ± SD.
| Participant Characteristics | Total (n = 11) |
|---|---|
| Age (y) | 24 ± 5.7 |
| Height (cm) | 173 ± 8.9 |
| Body mass (kg) | 67.6 ± 13.3 |
| Plasma NO2− (μM) | 0.77 ± 0.086 * |
| Plasma NO3− (μM) | 36.74 ± 1.89 * |
| SBP (mmHg) | 111.36 ± 1.73 * |
| DBP (mmHg) | 70.83 ± 1.22 * |
Systolic Blood Pressure (SBP); Diastolic Blood Pressure (DBP). * Values are taken as a mean of eligible participants measured at 0 h for each trial.
3.1.1. Plasma NO2−
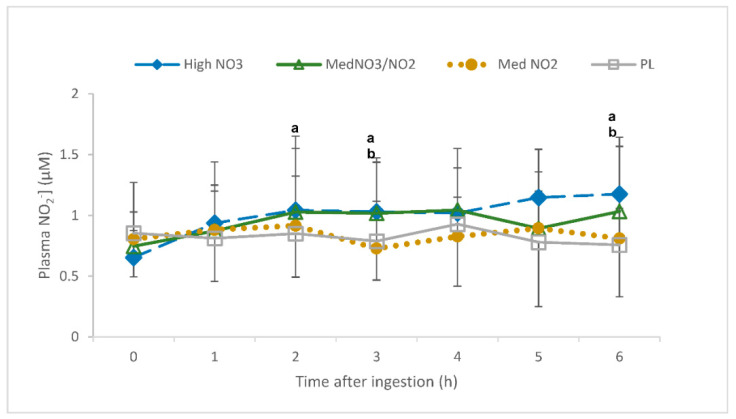
There was an interaction of drink and time for plasma [NO2−] (p < 0.001). Specifically, plasma [NO2−] was elevated in the high NO3− trial relative to the high NO2− (3 h and 6 h post-consumption) and PL (2 h and 6 h post-consumption; p < 0.05; Figure 2). PL and high NO2− consumption showed no increases in plasma [NO2−] at any time point (p > 0.05). The delta change values also showed an elevation in plasma [NO2−] following consumption of HL relative to PL and LM (3 h and 6 h; p < 0.05). Plasma [NO2−] had a higher increase in the MM trial relative to PL (3 h and 6 h) and LM (3 h; p < 0.05); however, it was still lower than HL at 6 h (p < 0.05) but not at 3 h (p = 0.16).
Figure 2.
Plasma nitrite concentration ([NO2−]) (uM) over a 6-h period following the ingestion of placebo (PL); high nitrate, low nitrite (HL); low nitrate, medium nitrite (LM); and medium nitrate and medium nitrite (MM) beverages. a Significant difference between HL and PL (p < 0.05). b Significant difference between HL and LM (p < 0.05).
3.1.2. Plasma NO3−
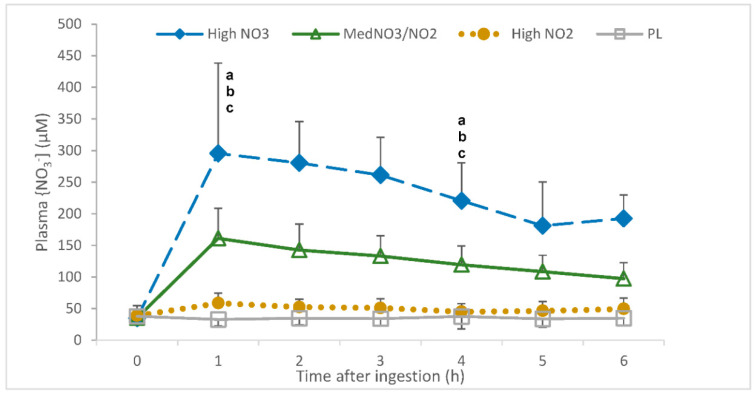
There was an interaction of drink and time for plasma [NO3−] (p < 0.001). Specifically, plasma [NO3−] was elevated in the HL, MM and LM trials relative to PL (2 h and 6 h post-consumption; p < 0.01; Figure 3). In all trials, time was shown to increase plasma [NO3−], rising from 1 h (p < 0.001) and plateauing at 5 h (5 h vs 6 h p = 0.80). The delta change values showed that the highest increase in plasma [NO3−] occurred following the consumption of HL (p < 0.001), with the second highest increase occurring following the consumption of MM (p < 0.001).
Figure 3.
Plasma nitrate concentration ([NO3−]) (uM) over a 6-h period following the ingestion of placebo (PL); high nitrate, low nitrite (HL); low nitrate, medium nitrite (LM); and medium nitrate and medium nitrite (MM) beverages. a Significant difference between HL and PL (p < 0.05). b Significant difference between HL and LM (p < 0.05). c Significant difference between HL and MM (p < 0.05).
3.2. Blood Pressure (BP)
There was no interaction of drink and time for SBP (p = 0.325). SBP was reduced in all trials (2 h, 3 h, 5 h and 6 h post-consumption; p < 0.001), with no difference occurring between trials (p = 0.783). These findings were consistent when analysing delta change and pre-lunch data.
There was a trend for an interaction of drink and time for absolute data and change in diastolic blood pressure (p = 0.059; p = 0.058); this trend was not present pre-lunch (p > 0.05). There was no difference in DBP between trials (p = 0.692) and no effect of time on DBP (p = 0.124).
3.3. Heart Rate
There was no interaction of drink and time for HR in absolute or delta change data (p > 0.05). However, there was an interaction of drink and time for pre-lunch HR. Specifically, HR was reduced in the PL trials relative to MM (3 h post-consumption, p < 0.05).
There was a main effect of time for HR (p < 0.05). Specifically, in all trials HR was reduced, 1 h, 4 h, 5 h and 6 h post-consumption (p < 0.05), with the greatest decrease in HR occurring at 3 h (p < 0.001).
3.4. USCOM Measures
There was no main effect of time or treatment or interaction of drink and time for CO or SV (all p > 0.05). These findings were consistent when analysing delta change and pre-lunch data.
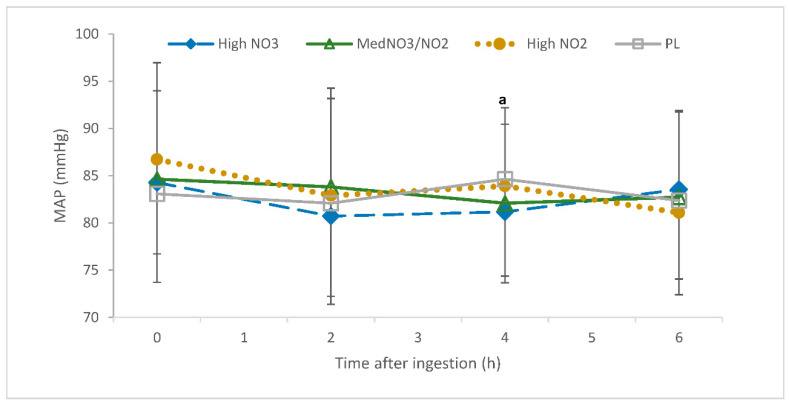
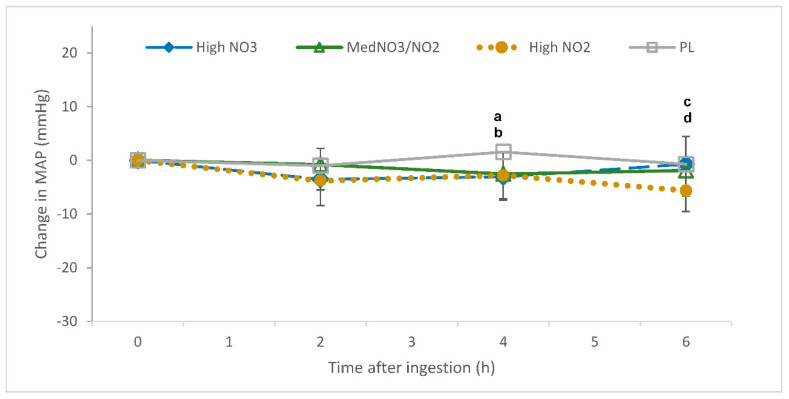
There was an interaction of drink and time for mean arterial pressure (p = 0.013). MAP was reduced in the HL trial relative to LM (4 h post-consumption; p < 0.05; Figure 4). The delta change values also showed an interaction of drink and time for MAP, which was reduced in the HL and MM trials relative to PL (4 h post-consumption; p < 0.05; Figure 5) and in the LM group relative to HL and PL (6 h post-consumption; p < 0.05; Figure 5).
Figure 4.
Mean arterial pressure (mmHg) over a 6-h period following the ingestion of placebo (PL); high nitrate, low nitrite (HL); low nitrate, medium nitrite (LM); and medium nitrate and medium nitrite (MM) beverages. a Significant difference between HL and LM p < 0.05).
Figure 5.
Change in mean arterial pressure (mmHg) over a 6-h period following the ingestion of placebo (PL); high nitrate, low nitrite (HL); low nitrate, medium nitrite (LM); and medium nitrate and medium nitrite (MM) beverages. a Significant difference between HL and PL (p < 0.05). b Significant difference between MM and PL (p < 0.05). c Significant difference between HL and LM (p < 0.05). d Significant difference between LM and PL (p < 0.05).
3.5. Correlations
Plasma [NO3−] showed a weak correlation with SBP at 6 h (r = 0.428; p < 0.006). Additionally, a weak negative correlation was also seen with the delta change data, where plasma [NO3−] correlated with DBP at 4 h (r = −0.364; p = 0.021).
4. Discussion
This study examined the effect of acute supplementation with beetroot juice rich in either NO3− or NO2−, or a combination of both, on plasma [NO3−], plasma [NO2−] and cardiovascular responses in normotensive adults. The main findings of this study demonstrate that: (1) NO3− consumption is the critical factor in elevating both plasma [NO3−] and [NO2−]; (2) NO3− ingestion reduces MAP 4 h post-consumption while NO2− reduces MAP 6 h post-consumption; (3) an increase in plasma [NO3−] leads to decreased DBP after 4 h.
4.1. Plasma [NO2−] and [NO3−]
This is the first study to directly evaluate the effects of NO2− present in BR on plasma [NO2−] and [NO3−]. Both HL and MM increased plasma [NO3−] and [NO2−] to a greater extent than LM and PL, implying that NO3− consumption is the critical factor for the rise in plasma [NO3−] and [NO2−]. Furthermore, HL caused a higher rise in both plasma [NO3−] and [NO2−] than MM, a change which is proportional to the [NO3−] within the drink. Both HL and MM drinks contained similar combined quantities of NO3− and NO2− (604 mg and 517 mg respectively), but different quantities of NO3− (572 mg; 280 mg respectively) and NO2− (32 mg; 237 mg respectively), leading to the conclusion that there is little effect of NO2− on plasma [NO3−] or [NO2−].
Following the consumption of HL, peak plasma [NO3−] occurred after 1 h, and peak plasma [NO2−] occurred after 2 h. These findings are consistent with studies in the literature which showed NO3−-rich BR led to peak plasma [NO3−] 1–1.5 h post-ingestion [54] and peak plasma [NO2−] 2.5–3 h post-ingestion [55]. Furthermore, the current study showed that following the consumption of LM, peak plasma [NO3−] occurred 1 h post-ingestion but with no increase in plasma [NO2−]. This result is inconsistent with previous studies, where the ingestion of NaNO2− capsules increased both plasma [NO3−] and [NO2−], leading to the conclusion that NO2− was 95–98% bioavailable (compared to the same IV dose) [36]. Although the source of supplement was different between our study and Hunault et al.’s [36], there were comparable doses of NO2− (193–253 mg vs. 262 mg). Although specific research comparing the effect of NO2− supplementation between BR and capsules does not exist, it has been suggested that synthetic nutrients delivered in capsules may not be used by the body in the same way as their natural counterparts [7].
Previous literature suggests that a reduction in BP following BR consumption (400–800 mg NO3−) is reliant on a rise in plasma [NO2−] (0.5–0.9 μmol/L) [37,38,39]. Our findings show for the first time that BR high in NO3− but low in NO2− (HL) led to the greatest rise in plasma [NO2−], and therefore NO3− consumption may be the critical factor in producing a reduction in BP following BR supplementation. Furthermore, when BR is stored at room temperature, NO3− begins to convert to NO2− [17]; hence when BR is left at room temperature for a period of time, it may not lead to the same increase in plasma [NO2−] and therefore may have a reduced ability to lower BP. The current study did not show a correlation between BP and plasma [NO2−] despite similar NO3− ingestion to past studies [26,29].
4.2. Blood Pressure
4.2.1. Systolic and Diastolic Blood Pressure
SBP and DBP decreased following the consumption of all drinks, including PL, suggesting that participants were becoming more relaxed over time. Interestingly, the reduction of SBP was not different between trials, leading to the conclusion that neither NO3− or NO2− had an influence on SBP. Similarly, participants’ DBP did not differ after consuming the different drinks; however, there was a trend for HL to reduce DBP more than the other drinks. This may be secondary to the young, healthy participants who volunteered for this study.
Very few studies show no reduction in either SBP or DBP following NO3− supplementation [31,32]. More commonly, it is concluded that NO3− supplementation (comparable doses to the present study) elicits a reduction in SBP [20,25,26,27,28,29,30,44,56] and DBP [25,27,28,29,30,57]. In studies where an effect was not observed, the presence of confounding factors preventing NO3− from reducing BP has been suggested. For example, Floyd et al. [32] suggested that an elevation in plasma [glucose] and [insulin] can prevent a reduction in BP in a younger population (27 ± 6.5 years) following potassium NO3− supplementation (2.4 g; 24 mmol NO3−). In the current study, 15 g of carbohydrate was consumed alongside the supplement, with a further 30 g consumed at lunch (+4 h) which may have prevented a reduction in BP due to the rise in [glucose] and [insulin]. However, the low baseline SBP of 113 mmHg in the participants in Floyd et al. [32] and in the current study (111 mmHg) may better explain the lack of effect. A metaregression, including 19 eligible randomised clinical trials investigating the effects of BR supplementation (316–860 mg NO3−/day) over 2–56 d, showed that a larger decrease in SBP occurred in participants with a higher baseline SBP measure [58]. Most previous studies which have shown BR consumption to have an effect on SBP had participants with baseline SBP measures ranging between 127–149 mmHg [20,27,28,29,30,31].
4.2.2. Cardiovascular Functions
The current study showed no change in HR, SV or CO following NO3−-rich BR consumption, which is consistent with previous literature [27,59]. The present study has further shown that consumption of NO2−-rich BR also had no effect on these variables. The cardioprotective effects observed in previous literature are likely mediated by the relaxation and dilation of the endothelium associated with NO production [21,60]. Additionally, the endogenous formation of NO, via l-arginine supplementation, has shown to limit noradrenaline production, depressing sympathetic activity and increasing the parasympathetic tone [61]. A main characteristic of the sympathetic drive is increased HR, CO and SV [62], implying that endogenous NO has the ability to reduce HR, CO and SV [63]. Exogenous NO3− has not been investigated, but the current study, along with previous literature, suggest that this does not have the same effect on noradrenaline and hence does not have the same effect on HR, CO and SV that endogenous NO3− does.
It has been concluded that NO3−-rich supplementation reduces BP, more significantly in those with a higher baseline BP. As the current study failed to show any BP-lowering effect associated with NO3−, it is unlikely that any effect of NO2− on BP would be shown. Further research into the potential effects of NO2− on SBP and DBP is required in a population with a higher baseline SBP.
4.2.3. Mean Arterial Pressure
NO3−, independent of dose (HL and MM), reduced MAP after 4 h, and NO2− (LM) reduced MAP after 6 h. Due to the direct conversion of NO2− to NO in the body, high NO2− was proposed to provide an early onset of cardioprotective effects compared to high NO3− [21,64]. Previous literature has shown a decrease in MAP (−8.2 ± 7.6 mmHg; −7 ± 1 mm Hg) following NO3−-rich BR consumption (300 mg; 450 mg) [65,66], specifically occurring 4 h post-supplementation [66], which is consistent with the current study. However, the delayed effect of NO2− on MAP in comparison to NO3− cannot be explained by the current literature and is not consistent with the exogenous NO pathway. Further studies are required to ensure that this is an accurate and replicable outcome.
Further research investigating the pharmacokinetics of the NO2− present in BR, and the subsequent correlations of both plasma [NO3−] and [NO2−], is warranted. The inconsistent findings of SBP and DBP associated with NO3−-rich BR in the current study indicate a need for further research to confirm the effect of NO2− present in BR. Future studies should include participants with a higher baseline SBP as this has been associated with a larger reduction in SBP.
5. Conclusions
Plasma [NO3−] increased in a dose-dependent manner following the ingestion of combined NO2− and NO3−, while plasma [NO2−] increased following the ingestion of NO3− only. There was a significantly higher increase of plasma [NO3−] following HL supplementation compared to MM supplementation. There was no effect of NO3− or NO2− ingestion on SBP, DBP, SV or CO compared to PL. MAP decreased following the consumption of HL, MM and LM; however, in comparison LM showed its effect 2 h later. Collectively, these results indicate that consumption of NO3− is the critical factor in elevating plasma [NO3−] and [NO2−], although the consumption of either NO3− or NO2− can reduce MAP. Further research is required in a population with a higher baseline BP so any effects of NO2− consumption on SBP/DBP can be observed.
Author Contributions
Conceptualization, K.R.-M., M.W. and A.A.; Data curation, M.C., E.M.J.; Formal analysis, K.R.-M., M.C. and A.A.; Investigation, E.M.J., M.C., K.R.-M., M.W. and A.A.; Methodology, E.M.J., M.C., K.R.-M., M.W. and A.A.; Project administration, K.R.-M. and A.A.; Supervision, K.R.-M. and A.A.; Writing—original draft, E.M.J.; Writing—review & editing, E.M.J., M.C., K.R.-M., M.W. and A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Massey University Human Ethics Committee (Southern A 18/35).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Heart Foundation NZ General Health Statistics in New Zealand. [(accessed on 1 December 2017)]; Available online: https://www.heartfoundation.org.nz/statistics-2.
- 2.Deb S., Dasgupta A. A Study on Risk Factors of Cardiovascular Diseases in an Urban Health Center of Kolkata. Indian J. Community Med. Off. Publ. Indian Assoc. Prev. Soc. Med. 2008;33:271. doi: 10.4103/0970-0218.43239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang W., Lee E.T., Fabsitz R.R., Devereux R., Best L., Welty T.K., Howard B.V. A longitudinal study of hypertension risk factors and their relation to cardiovascular disease: The strong heart study. Hypertension. 2006;47:403–409. doi: 10.1161/01.HYP.0000200710.29498.80. [DOI] [PubMed] [Google Scholar]
- 4.Borgi L., Muraki I., Satija A., Willett W.C., Rimm E.B., Forman J.P. Fruit and Vegetable Consumption and the Incidence of Hypertension in Three Prospective Cohort Studies. Hypertension. 2016;67:288–293. doi: 10.1161/HYPERTENSIONAHA.115.06497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Challa H.J., Uppaluri K.R. Dash Diet (Dietary Approaches to Stop Hypertension) StatPearls Publishing; Treasure Island, FL, USA: 2018. [Google Scholar]
- 6.Dauchet L., Kesse-Guyot E., Czernichow S., Bertrais S., Estaquio C., Péneau S., Vergnaud A.-C., Chat-Yung S., Castetbon K., Deschamps V. Dietary patterns and blood pressure change over 5-Y follow-up in the Su. Vi. max cohort. Am. J. Clin. Nutr. 2007;5:1650–1656. doi: 10.1093/ajcn/85.6.1650. [DOI] [PubMed] [Google Scholar]
- 7.Liu R.H. Health Benefits of Fruit and Vegetables Are from Additive and Synergistic Combinations of Phytochemicals. Am. J. Clin. Nutr. 2003;78:517S–520S. doi: 10.1093/ajcn/78.3.517S. [DOI] [PubMed] [Google Scholar]
- 8.Lee I.-M., Cook N.R., Gaziano J.M., Gordon D., Ridker P.M., Manson J.E., Hennekens C.H., Buring J.E. Vitamin E in the primary prevention of cardiovascular disease and cancer: The women’s health study: A randomized controlled trial. JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 9.Sesso H.D., Buring J.E., Christen W.G., Kurth T., Belanger C., MacFadyen J., Bubes V., Manson J.E., Glynn R.J., Gaziano J.M. Vitamins E and C in the prevention of cardiovascular disease in men: The physicians’ health study ii randomized controlled trial. JAMA. 2008;300:2123–2133. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waters D.D., Alderman E.L., Hsia J., Howard B.V., Cobb F.R., Rogers W.J., Ouyang P., Thompson P., Tardif J.C., Higginson L. Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women: A randomized controlled trial. JAMA. 2002;288:2432–2440. doi: 10.1001/jama.288.19.2432. [DOI] [PubMed] [Google Scholar]
- 11.Remington J., Winters K. Effectiveness of dietary inorganic nitrate in lowering blood pressure in hypertensive adults: A systematic review protocol. JBI Database Syst. Rev. Implement. Rep. 2017;15:2445–2452. doi: 10.11124/JBISRIR-2016-003307. [DOI] [PubMed] [Google Scholar]
- 12.Santamaria P. Nitrate in vegetables: Toxicity, content, intake and EC regulation. J. Sci. Food Agric. 2006;86:10–17. doi: 10.1002/jsfa.2351. [DOI] [Google Scholar]
- 13.Hord N.G., Tang Y., Bryan N.S. Food sources of nitrates and nitrites: The physiologic context for potential health benefits. Am. J. Clin. Nutr. 2009;90:1–10. doi: 10.3945/ajcn.2008.27131. [DOI] [PubMed] [Google Scholar]
- 14.Joshipura K.J., Ascherio A., Manson J.E., Stampfer M.J., Rimm E.B., Speizer F.E., Hennekens C.H., Spiegelman D., Willett W.C. Fruit and vegetable intake in relation to risk of ischemic stroke. JAMA. 1999;282:1233–1239. doi: 10.1001/jama.282.13.1233. [DOI] [PubMed] [Google Scholar]
- 15.Murphy M., Eliot K., Heuertz R., Weiss E. Whole beetroot consumption acutely improves running performance. J. Acad. Nutr. Diet. 2012;112:548–552. doi: 10.1016/j.jand.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Habermeyer M., Roth A., Guth S., Diel P., Engel K.H., Epe B., Fürst P., Heinz V., Humpf H.U., Joost H.G. Nitrate and nitrite in the diet: How to assess their benefit and risk for human health. Mol. Nutr. Food Res. 2015;59:106–128. doi: 10.1002/mnfr.201400286. [DOI] [PubMed] [Google Scholar]
- 17.Lee C., Shallenberger R., Downing D., Stoewsand G., Peck N. Nitrate and nitrite nitrogen in fresh, stored and processed table beets and spinach from different levels of field nitrogen fertilisation. J. Sci. Food Agric. 1971;2:90–92. doi: 10.1002/jsfa.2740220212. [DOI] [PubMed] [Google Scholar]
- 18.Lidder S., Webb A.J. Vascular effects of dietary nitrate (as Found in Green Leafy Vegetables and Beetroot) via the nitrate-nitrite-nitric oxide pathway. Br. J. Clin. Pharmacol. 2013;75:677–696. doi: 10.1111/j.1365-2125.2012.04420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carlström M., Lundberg J.O., Weitzberg E. Mechanisms underlying blood pressure reduction by dietary inorganic nitrate. Actual Physiol. 2018;224:e13080. doi: 10.1111/apha.13080. [DOI] [PubMed] [Google Scholar]
- 20.Bailey S.J., Winyard P., Vanhatalo A., Blackwell J.R., DiMenna F.J., Wilkerson D.P., Tarr J., Benjamin N., Jones A.M. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J. Appl. Physiol. 2009;107:1144–1155. doi: 10.1152/japplphysiol.00722.2009. [DOI] [PubMed] [Google Scholar]
- 21.Bailey S.J., Vanhatalo A., Winyard P.G., Jones A.M. The nitrate-nitrite-nitric oxide pathway: Its role in human exercise physiology. Eur. J. Sport Sci. 2012;12:309–320. doi: 10.1080/17461391.2011.635705. [DOI] [Google Scholar]
- 22.Bian K., Doursout M.F., Murad F. Vascular system: Role of nitric oxide in cardiovascular diseases. J. Clin. Hypertens. 2008;10:304–310. doi: 10.1111/j.1751-7176.2008.06632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Contreras F., Rivera M., Vasquez J., De la Parte M., Velasco M. Endothelial dysfunction in arterial hypertension. J. Hum. Hypertens. 2000;14:S20. doi: 10.1038/sj.jhh.1000982. [DOI] [PubMed] [Google Scholar]
- 24.d’El-Rei J., Cunha A.R., Trindade M., Neves M.F. Beneficial effects of dietary nitrate on endothelial function and blood pressure levels. Int. J. Hypertens. 2016;2016:6791519. doi: 10.1155/2016/6791519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hobbs D.A., Kaffa N., George T.W., Methven L., Lovegrove J.A. Blood pressure-lowering effects of beetroot juice and novel beetroot-enriched bread products in normotensive male subjects. Br. J. Nutr. 2012;108:2066–2074. doi: 10.1017/S0007114512000190. [DOI] [PubMed] [Google Scholar]
- 26.Jajja A., Sutyarjoko A., Lara J., Rennie K., Brandt K., Qadir O., Siervo M. Beetroot supplementation lowers daily systolic blood pressure in older, overweight subjects. Nutr. Res. 2014;34:868–875. doi: 10.1016/j.nutres.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Kapil V., Khambata R.S., Robertson A., Caulfield M.J., Ahluwalia A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: A randomized, phase 2, double-blind, placebo-controlled study. Hypertension. 2015;65:320–327. doi: 10.1161/HYPERTENSIONAHA.114.04675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanhatalo A., Bailey S.J., Blackwell J.R., DiMenna F.J., Pavey T.G., Wilkerson D.P., Benjamin N., Winyard P.G., Jones A.M. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;299:R1121–R1131. doi: 10.1152/ajpregu.00206.2010. [DOI] [PubMed] [Google Scholar]
- 29.Wylie L.J., Kelly J., Bailey S.J., Blackwell J.R., Skiba P.F., Winyard P.G., Jeukendrup A.E., Vanhatalo A., Jones A.M. Beetroot juice and exercise: Pharmacodynamic and dose-response relationships. J. Appl. Physiol. 2013;115:325–336. doi: 10.1152/japplphysiol.00372.2013. [DOI] [PubMed] [Google Scholar]
- 30.Kapil V., Milsom A.B., Okorie M., Maleki-Toyserkani S., Akram F., Rehman F., Arghandawi S., Pearl V., Benjamin N., Loukogeorgakis S. Inorganic nitrate supplementation lowers blood pressure in humans: Role for nitrite-derived NO. Hypertension. 2010;56:274–281. doi: 10.1161/HYPERTENSIONAHA.110.153536. [DOI] [PubMed] [Google Scholar]
- 31.Bondonno C.P., Liu A.H., Croft K.D., Ward N.C., Shinde S., Moodley Y., Lundberg J.O., Puddey I.B., Woodman R.J., Hodgson J.M. Absence of an effect of high nitrate intake from beetroot juice on blood pressure in treated hypertensive individuals: A randomized controlled trial. Am. J. Clin. Nutr. 2015;102:368–375. doi: 10.3945/ajcn.114.101188. [DOI] [PubMed] [Google Scholar]
- 32.Floyd C.N., Lidder S., Hunt J., Omar S.A., McNeill K., Webb A.J. Acute interaction between oral glucose (75 G as Lucozade) and inorganic nitrate: Decreased insulin clearance, but lack of blood pressure-lowering. Br. J. Clin. Pharmacol. 2019;85:1443–1453. doi: 10.1111/bcp.13913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brunton T.L. On the use of nitrite of amyl in angina pectoris. Lancet. 1867;90:97–98. doi: 10.1016/S0140-6736(02)51392-1. [DOI] [Google Scholar]
- 34.Reichert E.T., Mitchell S.W. The physiological action of potassium nitrite. Am. J. Med. Sci. 1880;156:158–180. doi: 10.1097/00000441-188007000-00011. [DOI] [Google Scholar]
- 35.Gladwin M.T., Shelhamer J.H., Schechter A.N., Pease-Fye M.E., Waclawiw M.A., Panza J.A., Ognibene F.P., Cannon R.O. Role of circulating nitrite and s-nitrosohemoglobin in the regulation of regional blood flow in humans. Proc. Natl. Acad. Sci. USA. 2000;97:11482–11487. doi: 10.1073/pnas.97.21.11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hunault C.C., van Velzen A.G., Sips A.J., Schothorst R.C., Meulenbelt J. Bioavailability of sodium nitrite from an aqueous solution in healthy adults. Toxicol. Lett. 2009;190:48–53. doi: 10.1016/j.toxlet.2009.06.865. [DOI] [PubMed] [Google Scholar]
- 37.Jonvik K.L., Nyakayiru J., Pinckaers P.J., Senden J.M., van Loon L.J., Verdijk L.B. Nitrate-rich vegetables increase plasma nitrate and nitrite concentrations and lower blood pressure in healthy adults–3. J. Nutr. 2016;146:986–993. doi: 10.3945/jn.116.229807. [DOI] [PubMed] [Google Scholar]
- 38.Ormesher L., Myers J.E., Chmiel C., Wareing M., Greenwood S.L., Tropea T., Lundberg J.O., Weitzberg E., Nihlen C., Sibley C.P. Effects of dietary nitrate supplementation, from beetroot juice, on blood pressure in hypertensive pregnant women: A randomised, double-blind, placebo-controlled feasibility trial. Nitric Oxide. 2018;80:37–44. doi: 10.1016/j.niox.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 39.Webb A.J., Patel N., Loukogeorgakis S., Okorie M., Aboud Z., Misra S., Rashid R., Miall P., Deanfield J., Benjamin N. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeVan A.E., Johnson L.C., Brooks F.A., Evans T.D., Justice J.N., Cruickshank-Quinn C., Reisdorph N., Bryan N.S., McQueen M.B., Santos-Parker J.R. Effects of sodium nitrite supplementation on vascular function and related small metabolite signatures in middle-aged and older adults. J. Appl. Physiol. 2015;120:416–425. doi: 10.1152/japplphysiol.00879.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duranski M.R., Greer J.J., Dejam A., Jaganmohan S., Hogg N., Langston W., Patel R.P., Yet S.-F., Wang X., Kevil C.G. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J. Clin. Investig. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rifkind J.M., Nagababu E., Barbiro-Michaely E., Ramasamy S., Pluta R.M., Mayevsky A. Nitrite infusion increases cerebral blood flow and decreases mean arterial blood pressure in rats: A role for red cell NO. Nitric Oxide. 2007;16:448–456. doi: 10.1016/j.niox.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 43.Sindler A.L., Fleenor B.S., Calvert J.W., Marshall K.D., Zigler M.L., Lefer D.J., Seals D.R. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell. 2011;10:429–437. doi: 10.1111/j.1474-9726.2011.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coles L.T., Clifton P.M. Effect of beetroot juice on lowering blood pressure in free-living, disease-free adults: A randomized, placebo-controlled trial. Nutr. J. 2012;11:106. doi: 10.1186/1475-2891-11-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cosby K., Partovi K.S., Crawford J.H., Patel R.P., Reiter C.D., Martyr S., Yang B.K., Waclawiw M.A., Zalos G., Xu X. Nitrite reduction to Nitric Oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003;9:1498. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 46.Lansley K.E., Winyard P.G., Bailey S.J., Vanhatalo A., Wilkerson D.P., Blackwell J.R., Gilchrist M., Benjamin N., Jones A.M. Acute dietary nitrate supplementation improves cycling time trial performance. Med. Sci. Sports Exerc. 2011;43:1125–1131. doi: 10.1249/MSS.0b013e31821597b4. [DOI] [PubMed] [Google Scholar]
- 47.Stanaway L., Rutherfurd-Markwick K., Page R., Wong M., Jirangrat W., Teh K.H., Ali A. Acute supplementation with Nitrate-rich beetroot juice causes a greater increase in plasma nitrite and reduction in blood pressure of older compared to younger adults. Nutrients. 2019;11:1683. doi: 10.3390/nu11071683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chou S.-S., Chung J.-C., Hwang D.-F. A high performance liquid chromatography method for determining nitrate and nitrite levels in vegetables. J. Food Drug Anal. 2003;11 doi: 10.38212/2224-6614.2702. [DOI] [Google Scholar]
- 49.Cheng C.F., Tsang C.W. Simultaneous determination of nitrite, nitrate and ascorbic acid in canned vegetable juices by reverse-phase ion-interaction HPLC. Food Addit. Contam. 1998;15:753–758. doi: 10.1080/02652039809374706. [DOI] [PubMed] [Google Scholar]
- 50.Li H., Meininger C.J., Wu G. Rapid determination of nitrite by reversed-phase high-performance liquid chromatography with fluorescence detection. J. Chromatogr. B Biomed. Sci. Appl. 2000;746:199–207. doi: 10.1016/S0378-4347(00)00328-5. [DOI] [PubMed] [Google Scholar]
- 51.Ogedegbe G., Pickering T. Principles and Techniques of Blood Pressure Measurement. Cardiol. Clin. 2010;28:571–586. doi: 10.1016/j.ccl.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Routledge Academic; New York, NY, USA: 1988. [Google Scholar]
- 53.Chan Y. Biostatistics 104: Correlational analysis. Singap. Med. J. 2003;44:614–619. [PubMed] [Google Scholar]
- 54.Clements W.T., Lee S.-R., Bloomer R.J. Nitrate Ingestion: A review of the health and physical performance effects. Nutrients. 2014;6:5224–5264. doi: 10.3390/nu6115224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McIlvenna L.C., Monaghan C., Liddle L., Fernandez B.O., Feelisch M., Muggeridge D.J., Easton C. Beetroot juice versus chard gel: A pharmacokinetic and pharmacodynamic comparison of nitrate bioavailability. Nitric Oxide. 2017;64:61–67. doi: 10.1016/j.niox.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 56.Stanaway L., Rutherfurd-Markwick K., Page R., Wong M., Jirangrat W., Teh K.H., Ali A. Does acute supplementation with nitrate-rich beetroot juice benefit older adults more than younger adults. Multidiscip. Digit. Publ. Inst. Proc. 2019;8:26. doi: 10.3390/proceedings2019008026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Larsen F.J., Ekblom B., Sahlin K., Lundberg J.O., Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. N. Engl. J. Med. 2006;355:2792–2793. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 58.Bahadoran Z., Mirmiran P., Kabir A., Azizi F., Ghasemi A. The nitrate-independent blood pressure–lowering effect of beetroot juice: A systematic review and meta-analysis. Adv. Nutr. 2017;8:830–838. doi: 10.3945/an.117.016717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oggioni C., Jakovljevic D., Klonizakis M., Ashor A., Ruddock A., Ranchordas M., Williams E., Siervo M. Dietary nitrate does not modify blood pressure and cardiac output at rest and during exercise in older adults: A randomised cross-over study. Int. J. Food Sci. Nutr. 2018;69:74–83. doi: 10.1080/09637486.2017.1328666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carlström M., Liu M., Yang T., Zollbrecht C., Huang L., Peleli M., Borniquel S., Kishikawa H., Hezel M., Persson A.E.G. Cross-Talk between nitrate-nitrite-no and no synthase pathways in control of vascular no homeostasis. Antioxid. Redox Signal. 2015;23:295–306. doi: 10.1089/ars.2013.5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee C.-W., Li D., Channon K.M., Paterson D.J. L-Arginine supplementation reduces cardiac noradrenergic neurotransmission in spontaneously hypertensive rats. J. Mol. Cell. Cardiol. 2009;47:149–155. doi: 10.1016/j.yjmcc.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.White D.W., Raven P.B. Autonomic neural control of heart rate during dynamic exercise: Revisited. J. Physiol. 2014;592:2491–2500. doi: 10.1113/jphysiol.2014.271858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thorin E., Thorin-Trescases N. Vascular endothelial ageing, heartbeat after heartbeat. Cardiovasc. Res. 2009;84:24–32. doi: 10.1093/cvr/cvp236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zand J., Lanza F., Garg H.K., Bryan N.S. All-Natural nitrite and nitrate containing dietary supplement promotes Nitric Oxide production and reduces triglycerides in humans. Nutr. Res. 2011;31:262–269. doi: 10.1016/j.nutres.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 65.Keen J.T., Levitt E.L., Hodges G.J., Wong B.J. Short-term dietary nitrate supplementation augments cutaneous vasodilatation and reduces mean arterial pressure in healthy humans. Microvasc. Res. 2015;98:48–53. doi: 10.1016/j.mvr.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 66.Kemmner S., Lorenz G., Wobst J., Kessler T., Wen M., Günthner R., Stock K., Heemann U., Burkhardt K., Baumann M. Dietary nitrate load lowers blood pressure and renal resistive index in patients with chronic kidney disease: A pilot study. Nitric Oxide. 2017;64:7–15. doi: 10.1016/j.niox.2017.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.