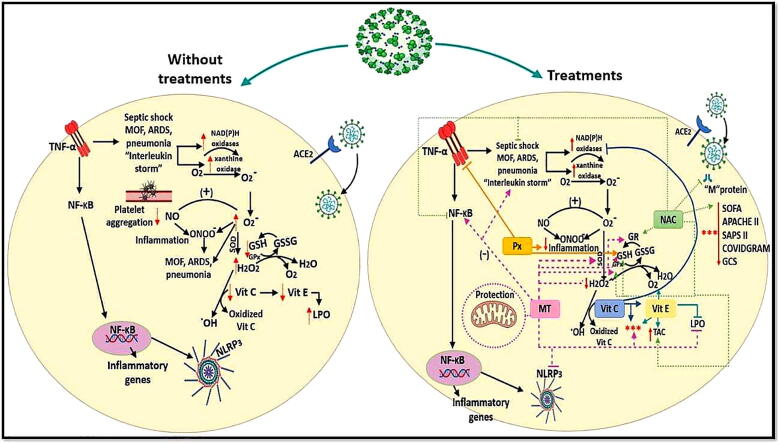
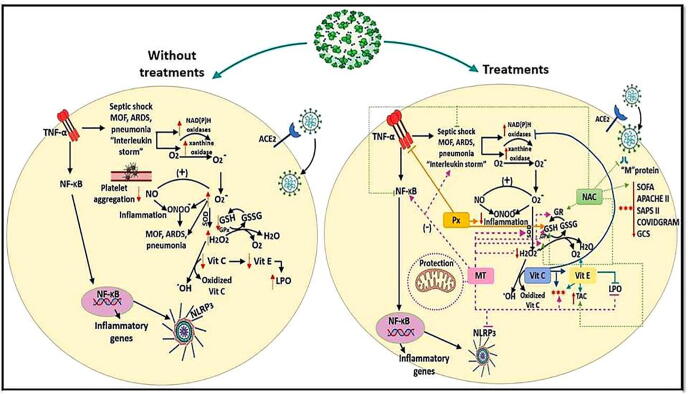
Graphical abstract
Keywords: SARS-CoV-2, Antioxidants, Sepsis, COVID-19, Pneumonia, Interleukin
Abstract
The type 2 coronavirus causes severe acute respiratory syndrome (SARS-CoV-2) and produces pneumonia with pulmonary alveolar collapse. In some cases it also causes sepsis and septic shock. There is no specific treatment for coronavirus disease 2019 (COVID-19). Vitamin C (Vit C), Vitamin E (Vit E), N-acetylcysteine (NAC) and Melatonin (MT) increase the intracellular content of GSH, kidnap free radicals and protect DNA, proteins in the cytosol and lipids in cell membranes. Pentoxifylline (Px) has anti-inflammatory activities. Here we evaluate the effect of Vit C, Vit E, NAC, and MT plus Px in COVID-19 patients with moderate and severe pneumonia. 110 patients of either sex were included. They were divided into five groups with 22 patients each. Group 1 received Vit C + Px, group 2 Vit E + Px, group 3 NAC + Px, group 4 MT + Px, and group 5 only Px. Oxidative stress (OS) markers such as lipid peroxidation (LPO) levels, total antioxidant capacity (TAC) and nitrites (NO2–) were evaluated in plasma. The antioxidant therapy improved the survival scores including the Sequential Organ Failure Assessment (SOFA), the Acute Physiology and chronic Health Evaluation II (Apache II), the Simplified Acute Physiology Score II (SAPS II), the Critical Illness Risk Score, Launched during COVID-19 crisis (COVIDGRAM) and the Glasgow Coma Scale (GCS). We found that LPO (p≤0.04) and inflammation markers such as interleukin-6 (IL-6, p≤ 0.01), C reactive protein (CRP, p ≤ 0.01) and procalcitonin (PCT, p ≤ 0.05) were elevated. TAC (p ≤ 0.03) and NO2– (p ≤ 0.04) found themselves diminished in diminished in COVID-19 patients upon admission to the hospital. The different antioxidants reversed this alteration at the end of the treatment. The treatment with antioxidant supplements such as Vit C, E, NAC, and MT plus Px could decelerate the aggressive and lethal development of COVID-19. Antioxidant therapy can be effective in this pandemia since it improves the survival scores including SOFA, Apache II, SAPS II, COVIDGRAM, GCS by lowering the LPO, IL-6, CRP, PCT and increasing systemic TAC and NO2–.
1. Introduction
Coronavirus disease 2019 (COVID-19) is caused by the type 2 coronavirus that causes severe acute respiratory syndrome (SARS-CoV-2) and results in pneumonia with different presentations ranging from mild to severe. Pneumonia is defined as a lung inflammation caused by bacterial or viral infections in which the air sacs fill with pus and may solidify. In patients with the severe form of the disease, pneumonia leads to pulmonary alveolar collapse with cessation of oxygen exchange. Patients also show dyspnea, (respiratory rate ≥ 30/min), decreased blood oxygen saturation (≤93%), reduced ratio of partial pressure of arterial oxygen to fraction of inspired oxygen (〈3 0 0) and/or presence of pulmonary infiltrates (>50%) within 24 to 48 h [1]. There may also be respiratory distress. Lymphopenia, lactate, creatinine, and kinase dehydrogenase levels are elevated, and there are increased concentrations of interleukins such as interleukin (IL) IL-1β, IL-5, IL-7, IL-8, IL-9, IL-10, IL-15, IL-12p70, fibroblast growth factors (FGF), granulocyte colony stimulating factor (GCSF), granulocyte macrophage colony stimulating factor (GMCSF), interferon γ (IFNγ), inducible protein 10 (IP10), monocyte chemoattractant protein 1 (MCP1), macrophage inflammatory protein 1A (MIP1A), macrophage inflammatory protein 1B (MIP1B), platelet derived growth factor (PDGF), tumor ecrosis factor-α (TNF-α) and vascular endothelial growth factor (VEGF). Alterations in the redox homeostasis in respiratory tract infected cells are one of the key events that are linked to infection and to inflammation [2].
Patients with moderate and severe pneumonia by COVID-19 may develop sepsis which is defined as life-threatening organ dysfunction caused by the host's inadequate response to infection. Septic shock is a consequence of sepsis and is associated with multiple organ failure (MOF) and high mortality [3]. Sepsis is the leading cause of mortality in intensive care units (ICU) worldwide, reaching up to 80% in patients with MOF [4]. In experimental models and in humans with septic shock, there is a high production of reactive species oxygen (ROS), and reactive nitrogen species (RNS, e g., nitric oxide (NO)) that can cause multiple organ failure (pulmonary, cardiac, neurological, and hepatic) [3]. During septic shock, there is an increase in cardiac output accompanied by a decrease in systemic vascular resistance caused by arterial dilation [5]. Excessive or prolonged reduction in vascular resistance produces vasopressor-resistant hypotension, which contributes to severe heart failure [5], [6]. There is no specific treatment for COVID-19. Most treatments are accompanied by measures that decrease the inflammatory condition known as the cytokine storm [7]. However, much less attention has been placed in the use of antioxidant therapy. ROS are produced by phagocytic leukocytes such as neutrophils, monocytes, macrophages, and eosinophils during invasion of the tissues by microorganisms, and may lead to cytotoxicity and cellular injury. ROS also participate the mechanism of inflammation by activating nuclear factor kB (NFkB) resulting in the transcription of cytokine producing genes that may be involved in the cytokine storm present in COVID-19 patients [1].
The use of antioxidant therapy for septic shock was proposed since Hippocrates, who used myrrha (Commiphora mukul, Commiphora myrrha) for therapeutic and anti-inflammatory medicinal purposes. The supplementation with antioxidants improves oxygenation rates, leads to higher levels of glutathione (GSH) and strengthens the immune response [3]. In addition, with its use, the hospital stay is reduced as well as the time of mechanical ventilation, the length of the ICU stays, multiple organ dysfunction rates, and the mortality rate in patients with acute lung injury (ALI) and reduce the sequential organ failure assessment score (SOFA). Among antioxidants Vitamin C (Vit C), Vitamin E (Vit E), N-acetylcysteine (NAC), melatonin (MT) and pentoxifylline (Px) have been proposed [3]. Therapies using these agents might reduce the effects of COVID-19 on several organs.
Vit C is a cofactor of multiple enzymes [8]. It inhibits the production of superoxide (O2–) and peroxynitrite (ONOO–) by inhibiting the O2– producing NADPH oxidase and the expression of the mRNA of the inducible nitric oxide synthase (iNOS) [9]. Furthermore, patients with sepsis that receiving Vit C, have a greater decrease in the SOFA score than placebo groups [10]. Vit E is considered as the most important lipophilic antioxidant in cell membranes, lipids, plasma, and red blood cells [11]. It protects cell membranes from lipid peroxidation (LPO) by ending the lipid chain reaction. It also functions as an O2– and hydroxyl radical (OH) scavenger. A study ex vivo that evaluated the effect of Vit E on the production of O2– in septic and non-septic patients in the ICU, showed that in the septic patients, the administration of Vit E significantly decreased the O2– overproduction through inactivation of the NADPH oxidase [12]. A study in humans with 7469 elderly men with community acquired pneumonia reported that this condition was lowered by treatment with Vit E [13]. Supplementation with nutrients that are a source of Vit E, controls nutritional deficiencies, overweight and promotes an adequate nutritional status in COVID-19 patients, improving the immune response and the antioxidant status during the infective phase.
NAC is a GSH precursor. It increases the activities of redox enzymes that employ GSH such as glutathione-S-transferase (GST), glutathione peroxidase (GPx), glutathione reductase (GR), thioredoxin reductase (TrxR) [14]. It also has a direct action on ROS, thus limiting oxidative lung injury [15], and may reduce the levels of IL-8, IL-6, and intercellular adhesion molecule (ICAM). The use of NAC in patients with septic shock was associated with a shorter time of mechanical ventilation and a shorter stay in the ICU [16].
MT sequesters ROS, thus protecting lipids in cell membranes, proteins in the cytosol, DNA, and mitochondria [17]. In vitro and in vivo studies have shown that it can prevent increases in LPO [18], preserve cellular membrane permeability by increasing its fluidity, and reduce levels of H2O2 in mitochondria and cytosol by restoring GSH homeostasis [19], through the stimulation of the activity of the γ-glutamyl cysteine synthase and glutathione synthase. These actions increase the intracellular synthesis of GSH [20]. MT may also stimulate antioxidant enzymes, such as catalase (CAT), super oxide dismutase (SOD) isoforms, GPx and GR [21]. SARS-CoV-2 infection leads to inflammation in the lungs by pyroptosis [22]. COVID-19 patients suffer from anxiety and lack of sleep and MT improves sleep habits, reduces anxiety, and stimulates immunity. It has therefore been proposed as an adjuvant therapy [23]. Pentoxifylline (Px), exerts several antioxidant and anti-inflammatory activities, such as the maintenance of GSH levels and mitochondrial viability, the inhibition of the production of TNF-α and the preservation of vascular endothelial functions [24]. In addition, a recent work by our group showed that the antioxidant therapy with Vit C, Vit E, NAC, and MT in patients with septic shock reduced MOF, the SOFA score, the time in ICU, oxidative stress (OS) and inflammation [3]. Therefore, the aim of this study was to evaluate the effect of the use of Vit C, Vit E, NAC, MT and Px on the outcome of patients with moderate and severe COVID-19 with and without sepsis.
2. Materials and methods
2.1. Description of the study population
This was an open, quasi-experimental, analytical, prospective, and longitudinal (before-after) study run between August 20 and September 20, 2020. The study population consisted of patients over 18 years of age who were admitted to the ICU of the CITIBANAMEX Center and who developed or not septic shock, secondary to moderate or severe pneumonia due to COVID-19. The diagnostic criteria for septic shock were based on the Sepsis-3 consensus [25]. Patients considered to have septic shock had to have an acute increase of at least 2 points in the SOFA score [26], lactate levels≥2 mmol/L and they had to be dependent on a vasopressor for at least 2 h before the time of enrollment. Exclusion from this study occurred when patients were younger than 18 years, were not able to grant an informed consent, refused to be included, if pregnant or breastfeeding or if they were under chronic use (last 6th months) or recent use of steroids, statins, or antioxidants. Patients were also excluded if there was any contraindication for the use of Vit C, Vit E, NAC, MT and Px.
The hospitalized patients that formed the general group, were classified as moderate or severe and this classification was decided according to their ventilatory status. Patients with the severe condition required invasive mechanical intubation according to the criteria of Berlin for acute respiratory distress syndrome (ARDS). The Berlin definition proposes 3 categories of ARDS based on the severity of hypoxemia: mild (200 mm Hg < Pao2/Fio2 ≤ 300 mm Hg), moderate (100 mm Hg < Pao2/Fio2 ≤ 200 mm Hg), and severe (Pao2/Fio2 ≤ 100 mm Hg), along with explicit criteria related to timing of the syndrome's onset, origin of edema, and the chest radiograph findings. The ARDS definition task force was considered [27].
Ethical approval was obtained from the local ethics committee on August 19th, 2020 (Control-9867/2020, register REG. CONBIOETICA-09-CEI-011-20160627). A written informed consent for enrollment or consent to use patient data was obtained from each patient or their legal surrogate. The protocol was registered (TRIAL REGISTRATION: ClinicalTrials.gov Identifier: NCT 04570254).
2.2. Collection samples to verify infection by SARS-CoV-2
In the study design, samples from patients and from personnel engaged in clinical and laboratory activities were collected. Paired saliva and nasopharyngeal swab samples were collected from 110 patients who were suspected to be infected by SARS-CoV-2. Samples were classified as positive for SARS-CoV-2 when both the N1 and N2 primer-probe sets were detected. The presence of the SARS-CoV-2 virus was evaluated for (COVID-19) using specific probes for the detection of the virus in conjunction with the real-time reverse transcriptase polymerase chain reaction technique (qRT-PCR). To evaluate organ dysfunction, the SOFA score (neurologic, respiratory, hemodynamic, hepatic, and hematologic) was calculated at admission and during all of the days of treatment [26].
2.3. Standard therapeutic management
The treatment applied during hospitalization was chosen according to standard maneuvers considering the requirements of each individual patient, the hemodynamic and electrolyte demands, and the ventilator demands. Treatment was initiated during the first hour after admission and before the recognition of the presence or absence of septic shock. Cultures were performed on admission before starting the administration of a broad-spectrum intravenous antibiotic. In this study, only one patient required management with antibiotics since the coexistence with pseudomonas infection was found and the patient was treated with Meropenem. In all patients, the use of hydroxychloroquine or antivirals was not considered, since they received individualized management according to the algorithm proposed [1].
Management with crystalloid solutions and/or albumin was considered depending on the hemodynamic status, by means of dynamic indicators. Use of vasopressors to maintain a media arterial pressure (MAP) ≥ 65 mmHg was given if necessary. Norepinephrine was the first option and/or vasopressin was used when there was a need to increase the MAP or reduce the dose of norepinephrine. Inotropics were administered in cases of myocardial dysfunction (dobutamine). Transfusion of blood packs were applied in case of a decrease in hemoglobin (<7.0 g/dl) in the absence of myocardial ischemia, severe hypoxemia, or severe bleeding. Mechanical ventilation with tidal volumes of 6 ml/kg was used in patients with ARDS. Plateau pressure was maintained at≤30 cmH2O, and alveolar conduction pressure of≤13 cmH2O. Positive end expiration pressure (PEEP) titration was managed by the use of the FiO2/PEEP (Fraction inspired of oxygen/Positive end expiration pressure) according to the table published by Ancukiewicz [28]. The treatment with anticoagulants was based on the Thachil guidelines [29]. The management with the prone position was necessary in patients with PaO2/FiO2 of≤150 [30].
In all patients, the standard therapeutic management with dexamethasone 8 mg i.v. each 24 h for 7 days was applied between day 1 and 21 of the onset of symptoms when not contraindicated. It was contraindicated when there was a requirement of O2>3 L, progressive requirement of = 2, PAO2/FiO2 ≤ 250 mmHg, use of O2 plus bilateral infiltrates in the radiography, use of O2 plus DHL ≤ 250U/L or ferritin ≥ 300 or DD ≥ 1000 ng/mL, CPK ≥ 2 times the upper normal value. The following conditions were not considered as contraindications or relative contraindications: glucose >250 mg/dL with hypoglycemic, hypokalemia < 3.3 meq, blood pressure > 155/95 mmHg with antihypertensive treatment, glaucoma, triglycerides > 500 mg/dL (start treatment), history of known peptic ulcer or bleeding from recent gastrointestinal tract, untreated or decompensated dementia or psychiatric illness, use of non-potassium sparing diuretics or use of inhaled B2 agonists. The next conditions were monitored at follow-up: pre-prandial capillary glucometer (7–13–19 hrs.) for 10 days, even in fasting patients, blood pressure per shift and basal potassium every 72 h.
2.4. Antioxidant therapy
To administer the antioxidant therapy, a medical management algorithm was used considering the presence of comorbidities in each patient; this algorithm was previously reported by our group [1]. The antioxidant was adjusted to each comorbid condition or to the presence of potential allergies or heart rhythm disorders due to each individual history. All antioxidants were orally administered or administered by a nasogastric tube, depending on the clinical status of the patient during the 5 days of the treatment. All data entry was monitored at the coordinating center, with site visits for source data verification.
2.5. Doses of the antioxidant therapy
Five treatments were used each in an independent group of twenty-two patients. Group 1; Vit C tablets of 1 g were administered every 12 h by oral route or naso-enteral tube for 5 days. Group 2; Vit E (α-tocopheryl acetate) capsules of 400 IU equivalent to 800 mg were administered every 12 h for 5 days. Group 3; effervescent tablets of NAC 600 mg were used every 12 h by the oral route or naso-enteral tube for 5 days. Group 4; MT of 5 mg (50 mg) (10 prolonged-release capsules) was given every 12 h by oral route or naso-enteral tube for 5 days. Group 5; Px tablets of 400 mg a dose every 12 h by oral route or naso-enteral tube for 5 days. Additionally, groups 1–4 received Px at the same concentration as in group 5.
2.6. Blood sample obtainment and storage
Blood samples were obtained from each patient that entered the draw, before initiation of the treatment and 48 h after the administration of the antioxidant. The blood samples were centrifuged for 20 min at 936 g and 4 °C. The plasma of the samples was placed in 3 or 4 aliquots and stored at −30 °C.
Laboratory tests were made in patients with COVID-19 to determine acute-phase reactants, hemoglobin, leukocytes, lymphocytes, platelets, creatinine, urea nitrogen, glucose, C-reactive protein (CRP), albumin, D-dimer, ferritin, fibrinogen, procalcitonin (PCT), interleukin-6 (IL-6) and oxygen saturation. Data from the patient’s medical history including demographic, prior illnesses to infection by SARS-CoV-2, test result for COVID-19, whether mechanical ventilation was used, and type of treatment given were used for the analysis of the results. Additionally, other biochemical variables were determined in plasma such as OS markers, lipid peroxidation (LPO), total antioxidant capacity (TAC), and nitrite (NO2–).
2.7. Oxidative stress markers
2.7.1. Nitrites
The NO2– levels in plasma were determined by the Griess reaction. 100 μl of plasma previously deproteinated with 0.5 N, NaOH and 10%, ZnSO4 were centrifuged at 1789 g for10 min. The supernatant was recovered and 200 µl of 1% sulfanilamide and 200 µl of 0.1% N-naphthyl-ethyl diamine were added. The total volume was adjusted to 1 ml. The calibration curve was obtained with solution KNO2 of 5–0.156 nM and the absorbance was measured at 540 nm.
2.7.2. Lipid peroxidation levels
50 µl CH3-OH with 4% BHT plus phosphate buffer pH 7.4 were added to 100 µl of plasma. The reaction tube was incubated to 100 °C for 1 h and centrifuged at 936 g at room temperature for 2 min. Then, the n-butanol phase was extracted, and the absorbance was measured at 532 nm [31].
2.7.3. Evaluation of total antioxidant capacity
100 μl of plasma were suspended in 1.5 ml of a reaction mixture prepared as follows: 300 mM acetate buffer pH 3.6, 20 mM hexahydrate of ferric chloride, and 10 mM of 2,4,6-Tris-2- pyridyl-s-triazine dissolved in 40 mM chlorhydric acid. These reactants were added in a relation of 10:1:1 v/v, respectively. After mixing, the samples were incubated at 37 °C for 15 min in the dark. The absorbance was measured at 593 nm [31].
2.7.4. Interleukin-6 concentration
IL-6 levels were measured in plasma samples by enzyme-linked immunosorbent assay (ELISA) using a commercial kit according to the manufacturer’s instructions (BioLegend, San Diego, CA, USA).
3. Statistical analysis
Continuous variables were expressed as the mean ± standard deviation or median with minimum and maximum, depending on their distribution. Categorical variables are expressed as frequencies and percentages. The normality of the variables was evaluated using the Shapiro-Wilk or Shapiro-France test, depending on the size of the sample. A graphical analysis of the distribution of the variables was also performed with histograms and/or stem and leaf graphs. We used non-parametric tests (Mann-Whitney test, Kruskal-Wallis t, depending on the particular case) to contrast variables without Gaussian distribution. The analysis of paired samples (before-after) was carried out with Friedman or Wilcoxon signed rank test depending on the distribution of the data. The Pearson correlation was used for the estimation of biomarkers. Statistical analyses were performed with SPSS version 26. The Sigma Plot 14 program (Jendel Corporation, 1986–2017) was used to generate the analysis and graphs of the OS markers and statistical significance was determined by the Mann-Whitney rank sum test followed by the normality test (Shapiro-Wilk). Differences were considered statistically significant when p ≤ 0.05.
4. Results
4.1. Demographic characteristics
A total of 110 patients were examined, of which 78 (71%) were men and 32 (29%) were women. Patients had an age range of 57.9 ± 12.8 years. In all patients, infection by SARS-CoV-2 was diagnosed through a CRP-test. The demographic characteristics of the patients are shown in Table 1. The average body mass index was 29.1 ± 4.1 kg/m2 with normal weight in 13 (12%) subjects, overweight in 52 (47%) and obesity in 45 (41%). Comorbid conditions prior to SARS-CoV-2 infection were, dyslipidemia 52 (47.3%), systemic arterial hypertension 44 (40%) diabetes mellitus 41 (37%), chronic obstructive lung disease 5 (4.5%), chronic kidney disease 4 (4%) and coronary heart disease 4 (3.6%). There were no patients with cancer, liver disease or previous autoimmune diseases or infection associated with bacteria and fungi. The main symptoms at the time of hospital admission were cough 74 (67%), fever 73 (66%) headache 51 (46.4%), anosmia 26 (24%), dysgeusia 23 (21%), conjunctivitis 10 (9%), diarrhea 14 (13%), arthralgia 39 (35.5%), myalgia 43 (39%), pharyngodynia 23 (21%) and rhinorrhea 16 (14.5%). Interventional treatment was warranted. 35 patients required invasive mechanical ventilation and in 75 required non-invasive techniques at the time of admission. The days required of invasive mechanical ventilation and length of stay in the ICU in patients with severe condition had a median of 13 days with a minimum of 2 and a maximum of 30 days. The total time of hospital stay had a median of 17 days with a minimum of 6 and a maximum of 36 days.
Table 1.
Demographic characteristics at admission of patients infected with COVID-19 Abbreviations: BMI = Body mass index, HR = Heart rate, MAP = Mean arterial pressure, HDL = High-density lipoproteins, LDL = Low-density lipoproteins, TB = Total bilirubin, DB = Direct bilirubin, IL = Interleukin, N/L = Neutrophil/lymphocyte, DM = Diabetes Mellitus, SAH = Systemic arterial hypertension, CD = Cardiovascular disease, COPD = Chronic obstructive pulmonary disease, ECKD = End-stage chronic kidney disease, FiO2 = Inspired fraction of oxygen, PCO2 = Partial pressure of carbon dioxide, SpO2 = Oxygen saturation.
| All Number and (%) | Moderate Number and (%) | Severe Number and (%) | p | |
|---|---|---|---|---|
| Women | 32 (29%) | 18 (31) | 14 (27) | NS |
| Men | 78 (71%) | 41 (69) | 37 (73) | NS |
| DM | 41 (37.3) | 17 (29) | 24 (47) | 0.05 |
| SAH | 44 (4) | 21 (36) | 23 (45) | NS |
| Dyslipidemia | 52 (47.3) | 32 (54) | 20 (39) | NS |
| COPD | 5 (4.5) | 2 (4) | 3 (6) | NS |
| CD | 2 (1.8) | 0 | 2 (4) | NS |
| ECKD | 4 (3.6) | 0 | 4 (8) | 0.04 |
| Norepinephrine | 21 (19) | 0 | 21 (41) | 0.0001 |
| Enteral nutrition | 45 (41) | 33 (56) | 12 (24) | 0.0001 |
| Deaths | 3 (2.7) | 0 | 3 (6) | 0.09 |
| Mean ± SE | ||||
| Age | 57.9 ± 12.8 | 54 ± 12.3 | 62 ± 12.3 | 0.002 |
| BMI (kg/m2) | 29.1 ± 4.1 | 29.3 ± 4.4 | 28.9 ± 3.8 | NS |
| Temperature (°C) | 36.6 ± 0.46 | 36.6 ± 0.48 | 36.6 ± 0.45 | NS |
| PaO2 (58.5–67.1 mmHg) | 84.7 ± 44.1 | 84.4 ± 37.9 | 85.02 ± 50.7 | NS |
| PCO2 (30.4–40 mmHg) | 31.7 ± 6.1 | 30.65 ± 4.3 | 33.04 ± 7.5 | 0.052 |
| PaO2/FiO2 (>164) | 149.7 ± 60.7 | 171.3 ± 55.1 | 125.4 ± 57.9 | 0.0001 |
| SpO2/FiO2 (>300) | 146.3 ± 54.5 | 162.5 ± 52.6 | 125.1 ± 51.3 | 0.0001 |
| HR (60–100 beats/min) | 80.8 ± 19.3 | 84.5 ± 17.6 | 76.4 ± 20.4 | 0.03 |
| MAP (70–105 mmHg) | 80.3 ± 11.7 | 81 ± 12 | 79 ± 12 | NS |
| Glucose (70–105 mg/dL) | 147.5 ± 68.1 | 141.9 ± 64.05 | 153.9 ± 72.5 | NS |
| Urea (<40 mg/dL) | 43.9 ± 38.3 | 33.02 ± 11.5 | 55.3 ± 51.3 | 0.005 |
| Ureic nitrogen (7–25 mg/dL) | 22 ± 16.40 | 18.7 ± 7.8 | 25.7 ± 21.9 | 0.04 |
| Total cholesterol (<200 mg/dL) | 139.4 ± 33.8 | 141.04 ± 32.2 | 137.6 ± 35.8 | NS |
| Triglycerides (<150 mg/dL) | 163.4 ± 88.09 | 174.7 ± 106 | 150.4 ± 58.4 | NS |
| HDL (23–92 mg/dL) | 32.3 ± 8.9 | 32.2 ± 9.9 | 32.5 ± 8.5 | NS |
| LDL (75–193 mg/dL) | 75.7 ± 25.5 | 76.8 ± 24 | 74.4 ± 27.2 | NS |
| DHL (140–271 U/L) | 290.4 ± 103 | 266.5 ± 95.1 | 317.3 ± 105.9 | 0.01 |
| TB (0.03–1 mg/dL) | 0.65 ± 0.30 | 0.59 ± 0.20 | 0.71 ± 0.39 | 0.05 |
| DB 0.03–0.18 mg/dL) | 0.19 ± 0.11 | 0.17 ± 0.10 | 0.22 ± 0.11 | 0.03 |
| Leukocytes (3.56–10.310^3/µL) | 10.2 ± 4.0 | 9.5 ± 3.5 | 11.1 ± 4.5 | 0.05 |
| Lymphocytes (0.99–3.2410^3/µL) | 0.91 ± 0.87 | 1.05 ± 1.1 | 0.74 ± 0.45 | 0.05 |
| Platelets (150000–50000010^3/µL) | 254.2 ± 89 | 260.8 ± 99.9 | 246.8 ± 87.2 | NS |
| (Median, min–max) | ||||
| Ferritin (11–307 ng/mL) | 586 (24.7–3373) | 579 (24.7–2354) | 59 7.7 (146–3373) | NS |
| IL-6 admission (pg/mL) | 43.2 (7.8–638.5) | 24.6 (7.8–626) | 75.4 (7.8–638) | 0.001 |
| Index N/L (>3) | 11 (1–106) | 10 (2–46) | 12 (1–106) | 0.05 |
| D-Dimer (0–0.24 µg/mL) | 740 (200–35,200) | 610 (200–35200) | 880 (210–34920) | 0.006 |
Thirty-one patients developed septic shock and were kept in the ICU. Of them, 23 were admitted with this condition and 8 were admitted without data of septic shock and developed this condition during their stay in the ICU. The survival rate was of 100% in patients with moderate symptoms that received ventilatory help with a mask and air flow and only three of them required invasive ventilatory help. In the group of patients with severe symptoms, all patients required mechanical ventilation since they arrived to the hospital and only three patients died. One of them developed an infection by pseudomonas. Of the other two patients who did not develop septic shock and died, one had end-stage chronic kidney failure and the other an acute myocardial infarction. The survival analysis did not show differences between the applied treatments and the Log-Rank did not show significance (p = 0.21).
Regarding adverse effects of the antioxidant therapy, only one patient had an allergic reaction to the antioxidant and was treated without presenting consequences. The indexes for organic and physiological severity, risk, thrombo-prophylaxis, and state of consciousness were evaluated in the experimental groups at the time of admission and throughout the in-hospital period and are shown in Table 2 according to the severity of the condition. The survival rate to 30 days and 90 days was of 90% as shown in Table 3.
Table 2.
Characteristics of the scores between initially seriously ill patients and patients who progressed to severity and those who were stable and persisted stable.
| All (median, min–max) | Moderate (median, min–max) | Severe (median, min–max) | p | |
|---|---|---|---|---|
| SOFA | 1 (0–8) | 0 (0–5) | 2 (0–8) | 0.001 |
| Apache II | 5 (3–14) | 5 (3–8) | 6 (4–14) | 0.001 |
| SAPS II | 27 (3–32) | 26 (3–31) | 28 (13–32) | 0.001 |
| COVIDGRAM | 116 (50–240) | 113 (50–162) | 120 (81–240) | 0.009 |
| Padua | 6 (5–7) | 5 (5–7) | 6 (5–7) | NS |
| GCS | 15 (12–15) | 15 (14–15) | 15 (12–15) | 0.001 |
Abbreviations: SOFA = Sequential Organ Failure Assessment, Apache = Acute Physiology and chronic Health Evaluation II, SAPS = Simplified Acute Physiology Score II, COVIDGRAM = Name of Critical Illness Risk Score, Launched during COVID-19 crisis, Padua = Name of prediction score for risk of venous thromboembolism, GCS = Glasgow Coma Scale.
Table 3.
Determination of inflammation markers, IL-6, CRP and PCT levels before (basal) and after antioxidant treatment in the group of COVID-19 patients, in the general group and divided into moderate and severe.
| General group 110 (100%) |
Moderate 59 (54%) |
Severe 51 (46%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Basal (Median, min–max) | Post treatment (Median, min–max) | P | Basal (Median, min–max) | Post treatment (Median, min–max) | P | Basal (Median, min–max) | Post treatment (Median, min–max) | P | |
| IL-6 (pg/ml) | |||||||||
| Vit C + Px | 22.4 (7.8–638) | 7.8 (7.8–547) | 0.01 | 13.5 (7.8–214.08) | 7.8 (7.8–10.6) | 0.02 | 57.89 (7.8–638) | 11.8 (7.8–547) | NS |
| Vit E + Px | 51.6 (7.8–395) | 7.8 (7.8–284.9) | 0.001 | 29.9 (7.8–177) | 7.8 (7.8–45.2) | 0.04 | 217 (7.8–637) | 7.8 (7.8–282) | 0.02 |
| NAC + Px | 77.9 (7.8–637) | 7.8 (7.8–282.08) | 0.004 | 20.5 (7.8–466) | 7.8 (7.8–108) | 0.06 | 37.6 (9.1–324.11) | 7.8 (7.8–406) | NS |
| MT + Px | 68.4 (7.8–626) | 7.8 (7.8–406) | 0.07 | 34.2 (7.8–293.2) | 7.8 (7.8–7.8) | 0.005 | 74.9 (9.5–395.6) | 8 (7.8–284.9) | 0.08 |
| Px | 68.4 (7.8–626) | 7.8 (7.8–640) | NS | 56.4 (7.8–626.8) | 7.8 (7.8–150) | 0.001 | 190.1 (23–315) | 198.6 (7.8–640.1) | NS |
| CRP (1–3 mg/L) | |||||||||
| Vit C + Px | 117 (20–494) | 44.6 (4.10–201.48) | 0.01 | 120.4 (20.1–494) | 37.6 (6.8–91.1) | 0.04 | 57.8 (7.8–638.5) | 11.8 (7.8–547.7) | NS |
| Vit E + Px | 165 (28.5–384.7) | 43.8 (1.8–263.5) | 0.01 | 143.4 (28.5–259.2) | 22.3 (1.8–110) | 0.02 | 217.4 (7.8–637) | 7.8 (7.8–282) | NS |
| NAC + Px | 144 (15.1–221) | 43.3 (7.3–148) | 0.001 | 146 (15.1–178) | 33.4 (7.3–148) | 0.06 | 37.6 (9.1–324.1) | 7.8 (7.8–406.9) | 0.01 |
| MT + Px | 145 (6.8–308) | 23.4 (3.3–357.9) | 0.004 | 152 (6.8–308) | 14.1 (3.3–57.3) | 0.005 | 74.9 (9,5–395.6) | 8 (7.8–284.9) | NS |
| Px | 141.6 (33.7–283) | 20.3 (2.09–191.77) | 0.0001 | 90.4 (33.7–252) | 12.5 (2.09–191.7) | 0.001 | 190.1 (9.5–395.6) | 8 (7.8–284.9) | 0.04 |
| PCT (ng/ml) | |||||||||
| Vit C + Px | 0.16 (0.03–2.04) | 0.07 (0.03–0.78) | 0.04 | 0.15 (0.03–0.41) | 0.04 (0.03–0.08) | 0.05 | 0.20 (0.09–2.04) | 0.12 (0.04–0.78) | NS |
| Vit E + Px | 0.33 (0.06–2.2) | 0.16 (0.01–0.50) | 0.004 | 0.40 (0.14–1.7) | 0.11 (0.01–0.50) | 0.04 | 0.33 (0.06–2.2) | 0.19 (0.04–0.39) | 0.04 |
| NAC + Px | 0.23 (0.08–11.4) | 0.12 (0.02–0.90) | 0.01 | 0.21 (0.10–0.90) | 0.07 (0.04–0.90) | NS | 0.28 (0.08–11.4) | 0.13 (0.02–0.32) | 0.01 |
| MT + Px | 0.40 (0.06–34.7) | 0.15 (0.01–8.3) | NS | 0.46 (0.0–1.23) | 0.08 (0.01–0.19) | 0.03 | 0.40 (0.09–34.7) | 0.29 (0.06–8.3) | NS |
| Px | 0.19 (0.03–1.35) | 0.06 (0.01–1.25) | NS | 0.12 (0.03–0.68) | 0.05 (0.01–0.21) | NS | 0.45 (0.12–1.35) | 0.80 (0.16–1.25) | NS |
Abbreviations: IL-6 = Interleukin-6, C reactive protein = CRP, Procalcitonin = PCT, Vit C = Vitamin C, Vit E = Vitamin E, NAC = N acetylcysteine, MT = Melatonin, Px = Pentoxifylline.
4.2. Oxidative stress markers in plasma
The LPO index showed a significant increase in the general group that included patients with moderate and severe symptoms when subjects were admitted to the hospital. After of the antioxidant therapy there was a decrease in LPO levels in the patients that received Vit E + Px, NAC + Px and MT + Px (p = 0.002, p = 0.004 and p < 0.001 respectively, Fig. 1A). However, when the patients were separated into groups separating the patients the moderate and severe symptoms, the LPO index in the group of patients with moderate symptoms showed a significant decrease with the Vit C + Px, NAC + Px and MT + Px treatments (p = 0.01, p = 0.04 and p = 0.01 respectively, Fig. 1B). In the group of patients with severe symptoms, the LPO index was decrease with the Vit E + Px and NAC + Px therapies (p = 0.02 and p = 0.05 respectively, Fig. 1C).
Fig. 1.
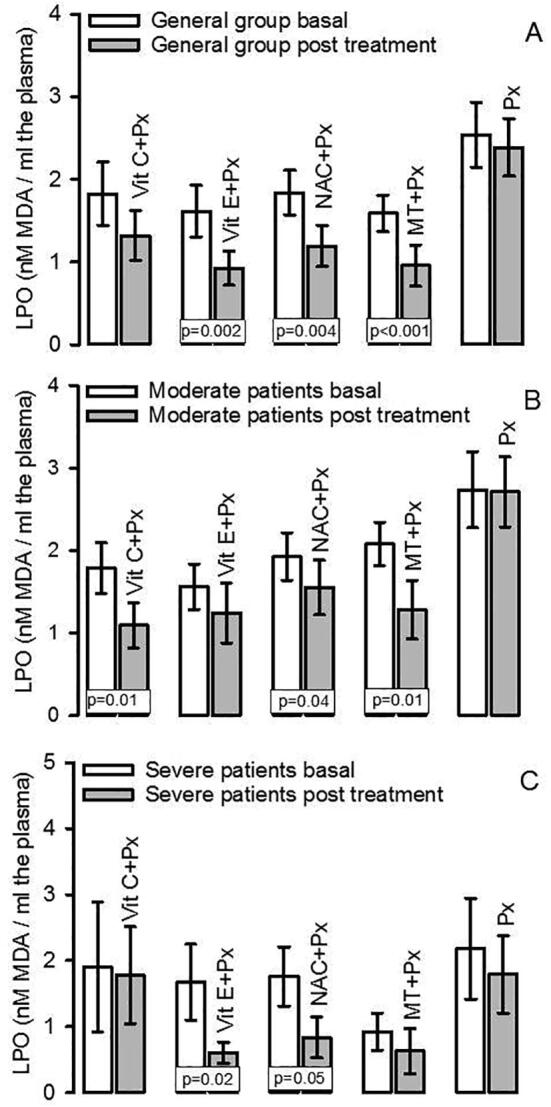
LPO index in the general group both in basal and posttreatment conditions (Panel A), Panel B shows the LPO index in moderate patients both in basal and post treatment conditions, and panel C shows the LPO index in severe patients both in basal and post treatment conditions.
The TAC showed a significant decrease in the general group that included moderate and severe patients when subjects were admitted to the hospital. After of the antioxidant therapy there were increases in the TAC levels in patients receiving Vit C + Px and NAC + Px (p = 0.02, and p = 0.03 respectively). However, there was a tendency to a decrease in the patients treated with Vit E + Px (p = 0.06, Fig. 2A). When patients were separated into groups of patients showing moderate and severe symptoms, the TAC in the patients with moderate symptoms showed a significant increase with the Vit C + Px treatment (p = 0.01, Fig. 2B). In the patients with severe symptoms the TAC was increase in the group treated with NAC + Px (p = 0.03, Fig. 2C).
Fig. 2.
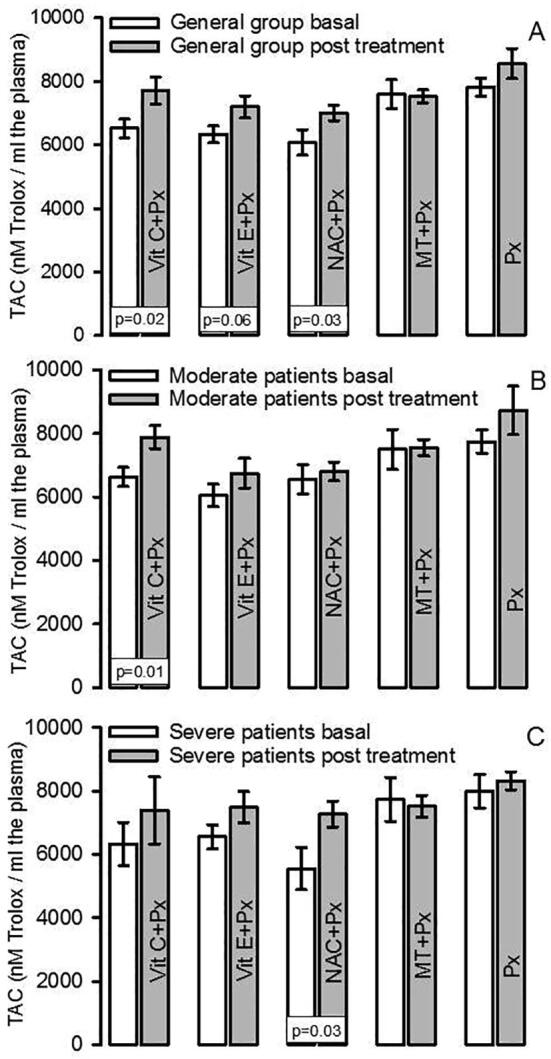
TAC index in the general group both in basal and post treatment conditions (panel A). Panel B shows the TAC index in moderate patients both in basal and post treatment conditions, and panel C shows the TAC index in severe patients both in basal and post treatment conditions.
The NO2– concentration showed significant decrease in the general group that included patients with moderate and severe symptoms when subjects were admitted to the hospital. After the antioxidant therapy there was an increase with the Vit C + Px, Vit E + Px, NAC + Px and MT + Px treatments (p < 0.001). However, in the Px group was (p = 0.02, Fig. 3A). When the patients were separated into the moderate and severe symptons groups, the NO2– in the group of patients with moderate symptoms showed a significant increase with the Vit C + Px, Vit E + Px, NAC + Px, MT + Px and Px treatments (p < 0.001, Fig. 3B). In the group patients with severe symptoms, the NO2– was increased with the Vit C + Px, Vit E + Px, NAC + Px and MT + Px treatments (p = 0.002, p < 0.001, p = 0.004 and p = 0.04 respectively, Fig. 3C).
Fig. 3.
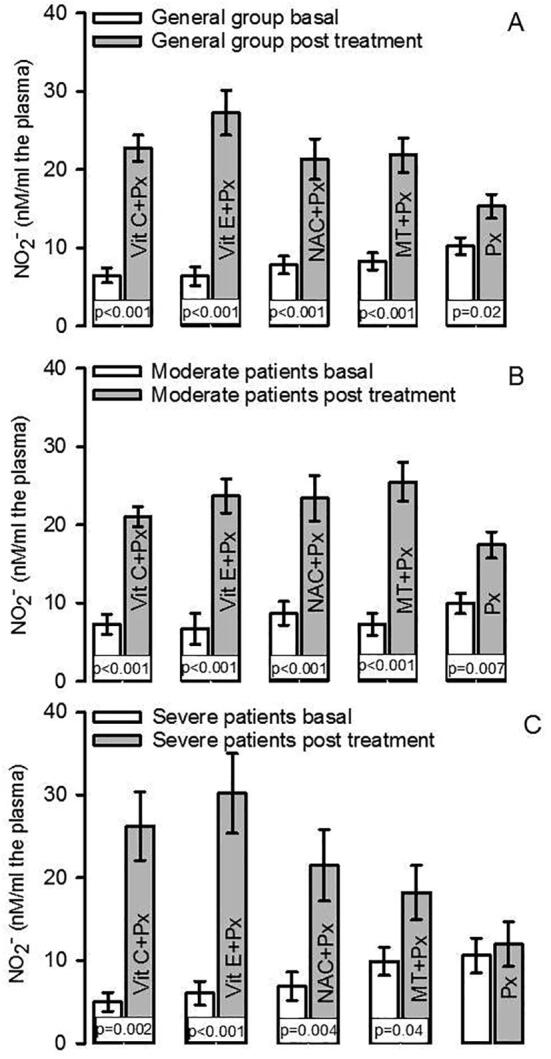
Plasma nitrite concentration in the general group both in basal and post treatment conditions (panel A), panel B shows the NO2– concentration in moderate patients both in basal and post treatment conditions. The panel C shows the NO2– concentration in severe patients both in basal and post treatment conditions.
Table 3 shows that the levels of IL-6 were significantly increased in the general patient group that included patients with moderate and severe symptoms when subjects were admitted to the hospital. After of the antioxidant treatment, there was a decrease in the IL-6 levels with the Vit C + Px, Vit E + Px, and NAC + Px treatments (p ≤ 0.01). In the MT + Px group there was only a tendency to a decrease (p = 0.07). However, when the patients were separated into groups including patients with moderate and severe symptoms, the IL-6 levels showed a significant decrease with all of the treatments (p = 0.02, p = 0.04, p = 0.005 and p = 0.001 respectively) except with the group treated only with NAC + Px group there was only a tendency to a decrease (p = 0.06), in group of patients with moderate symptoms. In the group of patients with severe symptoms, the IL-6 levels were decreased only with the Vit E + Px treatment (p = 0.02), and there was a tendency to a decrease with the MT + Px treatment (p = 0.08).
Table 3 also shows that the CRP levels were found significantly increased in the general patient group that included patients with moderate and severe symptoms when subjects were admitted to the hospital. After the antioxidant treatment there was a decrease with all of the treatments (p ≤ 0.01). In addition, when the patients were separated into groups of patients with moderate and severe symptoms, the CRP levels in the patients with moderate symptoms, showed a significant decrease with all of the treatments (p = 0.04, p = 0.02, p = 0.005 and p = 0.001 respectively) except for the patients receiving NAC + Px in whom only a tendency was observed (p = 0.06). In the patients with severe symptoms, the CRP levels were only decreased with the NAC + Px and Px treatments (p = 0.01 and p = 0.04 respectively).
In addition, the PCT levels showed a significant increase in the general patient group that included patients with moderate and severe symptoms when subjects were admitted to the hospital. After the antioxidant treatment the level of PCT was decreased with VitC + Px, Vit E + Px and NAC + Px (p = 0.04, p = 0.004 and 0.01 respectively). However, when the patients were separated into groups including subjects with moderate and severe symptoms, the CPR levels in the patients with moderate symptoms, showed a significant decrease with the Vit C + Px, Vit E + Px and MT + Px treatments (p = 0.05, p = 0.04 and p = 0.03 respectively). In the severe patients the CRP levels were decreased only with the Vit E + Px and NAC + Px treatments (p = 0.04 and p = 0.01 respectively, Table 3).
5. Discussion
There is still no vaccine against SARS-CoV-2, and despite the overwhelming information in the literature, the effects and interactions of multiple therapies that may be used against COVID-19 are still unknown. Therefore, there is not yet an effective therapy. A two-phase combined therapy strategy has proven to be the most effective measure for the treatment of the disease. The first phase aims to lower the viral load that is the source and origin of the chronic inflammatory condition leading to severe sepsis and MOF. The second phase reduces the septic condition, thereby reducing MOF, and the uncontrolled cytokine storm. The use of antioxidants might decrease the exacerbated inflammatory response without collateral effects. Since the two phases occur simultaneously, the therapeutic strategy should be selected avoiding subsequent collateral damage in patient with COVID-19. The aim of this study was to evaluate the use of antioxidants such as Vit C, Vit E, NAC, MT and Px (anti-inflammatory) in patients with moderate and severe infection by SARS-CoV-2. There is evidence that most people infected with SARS-CoV-2 (81%) have mild or uncomplicated forms of the disease [32]. However, some patients develop the serious form of the illness requiring oxygen therapy (14%) and approximately 5% need treatment in the ICU. Most critically ill patients require mechanical ventilation [33].
The most common condition in patients with severe COVID-19 is pneumonia. The death rate by severe pneumonia in China was from 15% [34]. In this COVID-19 pandemic, severe pneumonia and septic shock are the leading causes of morbidity and mortality in ICU around the world.
OS seems to play an important role in the pathophysiological mechanisms responsible for this disease and the use of antioxidant therapies could be effective. Our group has found that antioxidant therapy is useful in other conditions such as sepsis [3]. In chronic obstructive pulmonary disease (COPD), ALI, and ARDS, there is an increase in ROS [35], which is associated with a high release of pro-inflammatory mediators including IL-6, IL-8, and TNF-α by bronchial epithelial cells and alveolar macrophages [36]. ROS activate neutrophils and macrophages, resulting in destruction of the alveolar wall and collapse of small airways [37]. These changes induce endothelial damage, pulmonary capillary hyperpermeability, and pulmonary edema, resulting in impaired pulmonary gas exchange [38]. Furthermore, during severe sepsis, where there is life-threatening organ dysfunction caused by an inadequate host response to infection such as in ALI/ARDS, the cardiovascular system increases cardiac output and decreases peripheral resistance, adopting a hemodynamic profile that leads to arterial dilation. An excessive drop in peripheral resistance or its prolonged duration lead to progressive hypotension that is refractory to catecholamines can contribute to severe cardiovascular failure [39].
In different experimental animal models and in humans with severe septic shock, there is high production and release of O2– and ONOO– by different pathways that contributes to the failure of the lungs, heart, brain, and liver [40]. Although clinical data are limited, many viral diseases such as SARS-CoV lead to moderate and severe septic shock and increase ROS. This condition is associated with overexpression of iNOS, NADPH oxidases, cyclooxygenase 2 and xanthine oxidase which activate the transcription of factors such as NF-κB resulting in an exacerbated pro-inflammatory host response [1]. In addition, O2– and ONOO– participate as important mediators of a pro-inflammatory interleukin storm, which stimulates the production and release of more ROS. This interferes with the mitochondrial respiration and depletes ATP. Mitochondrial dysfunction is commonly induced in an environment of septic shock [41]. Our results show that the OS markers in plasma such as LPO and TAC were increase and decrease respectively, and inflammation markers such as the IL-6, CRP and PCT were increase in COVID-19 patients when they were admitted to the hospital. The use of the different antioxidants including Vit C + Px, Vit E + Px, NAC + Px and MT + Px, reversed these alterations at the end of the hospital stay. Although in the group that received only Px there were no significant changes in OS markers. IL-6 and CRP were decreased in these groups. This suggests an additive effect between Px which has anti-inflammatory properties and the antioxidant capacity caused by each of the antioxidants used in this study.
Vit C is a potent natural antioxidant that removes ROS which are over-produced in inflammation. Alveolar epithelial type II cells in the lung require high concentrations of intercellular Vit C (mM) to sustain their pivotal functions in innate immunity [42]. Low levels of Vit C are present in patients with septic shock [43]. This decrease is caused by an inadequate intake, acute or chronic consumption secondary to an increase of the OS and/or an increase in the loss of this vitamin. Critically ill patients have low levels of Vit C despite the administration of its daily requirements. In the CITRIS-ALI trial, an intravenous administration of vitamin C was recommended to reduce mortality [44]. There is also a decreased level of Vit C in severe MOF. However, there was a decrease in the SOFA score in a phase I study where the Vit C was administered in patients with severe sepsis. No adverse effects were reported. In the VITAMINS trial that studied ARDS, although there was not a significant difference in the SOFA score, there were decreased CRP levels and reduced mortality [45]. In another study with ARDS patients, there were decreased levels of CRP after treatment with Vit C [46]. In an in vivo mice model of sepsis, the administration of Vit C attenuated the elevation of the serum aminotransferase, TNF-α, cyclooxygenase 2 mRNAs and of LPO [47]. In another study, Vit C or Vit E, attenuated the increase in the CYP1A1 and CYP2E1 mRNA expression in the liver and decreased aminotransferase and the LPO levels in serum [48].
Vit C in plasma is incorporated into cells by a sodium dependent Vit C transporter. In parallel, dehydroascorbic acid, an oxidized form of Vit C, is taken up through glucose transporters. Since glucose competes with dehydroascorbic acid for the transporters, Vit C availability may be limited in cells when high blood sugar conditions are present. This might be a potential reason for the pathological severity of COVID-19 in diabetic patients [48].
Few studies have evaluated the antioxidant effect of Vit E as monotherapy and most have been carried out with its administration in conjunction with other antioxidants. An ex vivo study showed the effect of Vit E on the O2– production in septic and non-septic patients in the ICU and concluded that septic patients have significantly decreased levels of Vit E and an O2– overproduction. However, the administration of the combination of Vit E and simvastatin reduces this phenomenon through inactivation of NADPH oxidase [49]. In addition, there is a decrease in the incidence of pneumonia in old men who quit smoking after administration of Vit E [50]. Also, a recent study by our group demonstrated that treatment with Vit E reduces the PCT levels in serum in patients with severe sepsis [3]. The results of this study suggest that the combination of Vit E with Px reduces the LPO and increases TAC in COVID-19 patients. The treatment also has a synergic effect decreasing markers of inflammation such as IL-6, CRP, and PCT. This treatment can be used as an adjuvant without showing collateral effects.
MT is not an antiviral drug, but it may have indirect anti-viral actions due to its anti-inflammatory, antioxidant, and immune enhancing properties. MT levels are decrease in the pineal gland and in mitochondria and this decrease can contribute to elevate the replication of viruses and the severity of many viral infections [51]. In mice whose central nervous system is infected by viruses, MT decreases the viral load, and reduces paralysis and death [52]. In models of respiratory syncytial virus infections, MT down regulates acute lung oxidative injury, pro-inflammatory cytokine release and inflammatory cell recruitment [53]. MT also down-regulates NF-κB activation in T cells and lung tissue [54]. The anti- oxidant effect of MT is due to its anti-inflammatory and antioxidant actions by up-regulating anti-oxidative enzymes such as SOD, and down-regulating pro-oxidative enzymes including iNOS. MT also directly interacts with ROS as a free radical scavenger and induces up regulation of Nrf2 which is depleted in COVID-19 [55]. Furthermore, MT regulates the immune system and improves proliferation and maturation of natural killer cells, T and B lymphocytes, granulocytes and monocytes in the bone marrow and other tissues [56]. In a clinical trial of patients suffering of severe multiple sclerosis, MT administration reduced serum concentrations of TNF-α, IL-6, IL-1β and LPO [52]. A recent meta-analysis suggested that the supplementary use of MT significantly reduced TNF-α and IL-6 levels [57]. The results from the present study suggest that the use of MT as an adjuvant may effectively reduce the circulating cytokine levels and lower the pro-inflammatory cytokine storm and the OS present in COVID-19 patients.
Published reports also indicate that MT application may ameliorate septic shock via the NLRP3 pathway. MT prevents against sepsis-induced renal injury, septic cardiomyopathy, and liver injury [58]. Moreover, MT exerts neurological protection by reducing the cerebral inflammatory response, cerebral edema, and brain-blood barrier permeability [59]. In addition, deep sedation of the patients in the ICU is associated with increased long-term mortality, and MT application reduces the use of sedatives and the frequency of anxiety, agitation, and pain [59]. MT does not present adverse effects up to a dose of 1 g/day for a month, [60].
Another antioxidant that has protective actions in sepsis is NAC which is a precursor of GSH. Low levels of GSH are found in COVID-19 patients [61]. In animal models, in in vitro studies and in clinical trials, the use of NAC has beneficial effects. NAC administration reverses the enteropathogenic effects of the porcine coronavirus and of diarrhea viruses. It decreases levels of H2O2 in plasma and mucosa layers. NAC also inhibits the H5N1 infection in lung epithelial cells in vitro and the production of pro-inflammatory mediators [62]. NAC administration after sepsis induction by injecting LPS in rats, prevented the decrease in the mean arterial pressure, and diminished markers of organic injury such as BUN, creatinine, lactate dehydrogenase, creatine phosphokinase, ALT, AST TNF-α, IL-6 [63]. Similarly, NAC administration before injecting endotoxin to rats showed decreased lung NF-kB activation and diminished cytokine-induced neutrophil chemo attractant mRNA expression in lung tissue. This was associated with a diminished inflammatory response in the lungs [64]. Treatment with NAC increased the TAC in patients with sepsis [3]. NAC has also been used as a treatment in numerous pulmonary ailments including COPD and chronic bronchitis. It prevents COPD exacerbations and chronic bronchitis flare-ups, and this was also shown in a meta-analysis. NAC also exhibited promising results when used as an adjuvant treatment for idiopathic pulmonary fibrosis [65]. A recent review discussed the potential use of NAC for the treatment of COVID-19 [65]. NAC binds to Cys-145, the active site of the M protein, and thus inhibit the protease activity and viral replication [66]. This structural characteristic might render NAC as a potentially specific first-line drug for SARS-CoV-2.
NAC reduces the incidence of pneumonia, as well as the frequency and severity of influenza [67]. It also reduces levels of TNF-α and malondialdehyde and improves OS and modulates inflammation [68]. In patients with mild to moderate acute lung injury, intravenous treatment with NAC improves systemic oxygenation, reduces the need for ventilatory support, and also slightly decreased the mortality rate [69]. The effect of NAC in COVID-19 in a double-blind, randomized, placebo-controlled and unicentric trial, conducted in patients with the severe disease, having as a primary endpoint the need for mechanical ventilation was recently published. Time of mechanical ventilation, admission to ICU, time in ICU, and mortality were secondary endpoints of this paper and it was found that administration of NAC in high doses did not affect the evolution of severe form of COVID-19 [70]. The difference with our study resides in the studied populations, the doses of NAC and in the fact that the authors did not explain whether the therapy was an adjuvant to standard comprehensive management. Here we evaluated the clinical anti-inflammatory status and the OS deregulation, and our results are promising. Therefore, our results and others shown in the literature, suggest that NAC could contribute to the deceleration of the aggressive and lethal development of COVID-19 with the use of moderate doses [1], [71]. ARDS patients have decreased plasma concentrations of GSH in alveolar epithelial tissue and erythrocytes. In a randomized crossover study, patients who were given intravenous NAC, showed an increase in GSH levels. These individuals also exhibited a clinical response to the treatment with increased oxygen delivery, improved lung compliance, and resolution of pulmonary edema. In another randomized controlled trial in patients with community acquired pneumonia, NAC treatment in conjunction with conventional therapy decreased the inflammatory response [68]. ROS production increases IL-6 production and LPO resulting in cell damage. Early treatment with NAC + Px during COVID-19 may bypass the excessive inflammation and cell damage that leads to the severe form of the infection, since SARS-CoV-2 influences intracellular GSH levels by decreasing the function of intracellular Nrf2 [72].
On the other hand, the combination of Px with any of the antioxidants used in this study, decreased IL-6 and CRP concentrations. This suggests a synergic effect by decreasing inflammation markers. Px is a xanthine drug indicated in some severe cases of alcoholic hepatitis. It may act on the plasma membrane of red blood cells and render it more malleable, thus improving blood perfusion. Px exerts anti-inflammatory activities, decreasing the production of TNF-α and CRP. In a randomized controlled study in newborns the administration of Px was associated with reduced levels of TNF-α and CRP. It decreased the need for vasopressors, the duration of respiratory support, the antibiotic treatment, it shortened hospitalization, reduced the incidence of disseminated intravascular coagulopathy, of metabolic acidosis, and thrombocytopenia [73]. However, no difference in short-term morbidity was found between Px-treated and untreated septic infants [74]. In a meta-analysis, the administration of Px in septic newborns showed it to be effective in reducing all-cause mortality and length of hospital stay. A subgroup analysis demonstrated significantly reduced mortality in premature newborns and infants, newborns with proven sepsis, and infants with gram-negative sepsis [75]. This led to the conclusion that Px may represent a beneficial adjuvant therapy in COVID-19 sepsis. However, it is contraindicated in patients with recent or active cerebral or retinal hemorrhage, in coronary artery disease and in patients with impaired renal or hepatic function. In addition, production, and release from extra thyroidal sources of PCT into the circulation is enormously amplified during bacterial infections. It is actively sustained by enhanced concentrations of TNF-α and IL-6. Nevertheless, the synthesis of this biomarker is inhibited by INF-γ, whose concentration increases during viral infections. Therefore, PCT value would be expected to stay within the reference range in patients with non-complicated SARS-CoV-2 infection. Therefore, its substantial increase would reflect bacterial co-infection in patients developing the severe form of COVID-19 and could contribute to complicate the clinical picture [76]. However the results of this study suggest that the combination therapy with antioxidants is able to elevate the decreased levels of PCT.
An increase in circulating neutrophils enhances ROS release, LPO and reduces nitric oxide (NO) during obstructive sleep apnea and ARDS. This may cause an increase in platelet aggregation, elevated levels of adhesion molecules, endothelin and vascular endothelial growth that contributes to sepsis [77]. In ARDS there is a low local pH in the damaged capillaries which might help produce NO from NO2–. Even at a near neutral pH, NO2– can be reduced to NO in hypoxic conditions by multiple enzymes, such as deoxy hemoglobin from erythrocytes, blood xanthine oxidoreductase, or cytochrome oxidase from the mitochondrial respiratory chain. Lower or impaired NO metabolism is associated with the pathological severity of COVID-19. The NO synthesis by nitric oxide synthases requires O2, and in ARDS, this is due to hypoxia that is decreased. As a consequence, the NO oxidation products NO3– and/or NO2– in plasma would be expected to be low [78]. The results in this study show that NO2– was decreased in all groups from the entrance to the hospital and that the antioxidant therapy increased it. In the absence of blood circulation in the lungs there is LPO and OS due to the hypoxic condition. Furthermore, NADPH oxidase and iNOS in the endothelium are one of the main causes of oxidation in pulmonary ischemia. Immune cells such as macrophages and neutrophils can also contribute to oxidative damage in the lungs by the same enzymatic mechanism [79]. However, NO overproduction could be expected in sepsis by infection SARS-CoV-2 since it results from the activation of iNOS which contributes to the inflammation process. Nevertheless, our results show a decrease in the plasmatic levels of NO2–. The possible explanation to this may be through the hypoxic condition, in which the O2 concentration decreased and optimal concentrations of O2 are required for NO synthesis by the oxide nitric synthases. In addition, the NO which is synthetized might be oxidized by ROS to ONOO– and this nitrogen specie may contribute to the inflammatory process in COVID-19 patients [38].
In this study, when patients with severe pneumonia were only treated with Px there was a decrease in CRP, which confirms the participation of this drug in the control of inflammation. There was not a decrease in LPO, but an increase the TAC was found. There was also a lower average of days of stay in ventilation, of days in the ICU and of days of hospitalization (date not shown). We observed that Px had a better effect in patients with moderate pneumonia. It improved levels of inflammatory biomarkers in patients with moderate and severe pneumonia. The combination of Vit E + Px reduced IL-6 levels more than Px alone. There were no statistically relevant data in severe patients. However, CRP levels decreased with the use of NAC combined with Px and Px alone. On the other hand, the use of NAC and Vit E combined with Px produced better effects up-PCT levels. This suggests that the inflammatory state could have a better performance with the combination of two antioxidants and the use of Px, NAC and MT were the ones that showed the best performance. Therefore, we propose that this could be a further improvement to the therapy for patients with severe pneumonia due to COVID-19.
On the other hand, the decrease observed in the inflammatory state through the decrease in IL-6, CRP and PCT in most patients who did not develop septic shock in this study; suggest that the use of antioxidants or Px should be started early. The antioxidants proposed are Vit C, Vit E, NAC, MT, and Px and their preventive use should also be analyzed in the context of clinical trials. This therapy should also be evaluated as a means to stop the use of invasive intubation. The Fig. 4 resume the results the antioxidant therapy on the SARS-CoV-2 infection in COVID-19 patients.
Fig. 4.
Effect of different treatments for SARS-Cov-2 on antioxidants systems. Abbreviations: ACE2 = angiotensin converting enzyme 2, Apache II = acute physiology and chronic health evaluation II, ARDS = acute respiratory distress syndrome, COVIDGRAM = name of critical illness risk score, H2O2 = hydrogen peroxide, GCS = Glasgow Coma Scale, GPx = glutathione peroxidase, GR = glutathione reductase, GSH = glutathione, GSSG = oxidized glutathione, LPO = lipid peroxidation, MOF = multiple organ failure, MT = Melatonin, NAC = N-acetylcysteine, NF-κB = nuclear factor k-light-chain-enhancer of activated B cells, NLR3 = NLR family pyrin domain containing 3, NO = nitric oxide, O2- = superoxide anion, OH = hydroxyl radical, ONOO- = peroxynitrate, Px = Pentoxifylline, SAPS = Simplified Acute Physiology Score II, SOD = super oxide dismutase, SOFA = Sequential Organ Failure Assessment, TAC = total antioxidant capacity, TNF-α = factor de necrosis tumoral alfa, NLRP3 = NOD-like receptor protei 3 that activation of the inflammasome.
Finally, it is relevant to point out that we obtained only 2.7% mortality in this series and therefore the survival rate was high. Regarding the causes of death in 3 of our patients, it should be mentioned that there were in them other non-COVID-19 related factors that determined their fate. From their arrival to the hospital, these patients needed invasive ventilatory support and one of them was already in a terminal renal state. In a second patient there was a myocardial infarction and in the last one there was a co-existent infection with pseudomona together with SARS-CoV2. There is pulmonary micro thrombosis in lung alveoli in COVID-19 patients and fibrinotic thrombi have been found in echographic autopsies of pulmonary arterioles. These thrombi are different from those found in pulmonary embolia [80]. Intravascular pulmonary microthrombi are associated to the development of hypoxemia in the first stages of ARDS in COVID-19. Therefore, in patients with associated prothrombotic disorders as in these cases, the deterioration is imminent and may explain the poor response to any treatment. Therefore, deaths in this series were not related to complications of COVID-19 and this study generates the hypothesis that the treatment should be applied using the combination of Px with the antioxidants since it shows better clinical improvements in the regulation of biomarkers of inflammation and OS
6. Conclusion
This study confirms the presence of OS in COVID-19 patients. The results suggest that the treatment with antioxidant supplements such as Vit C, Vit E, NAC, and MT plus Px could contribute to the deceleration of the aggressive and lethal development COVID-19. There is evidence that antioxidants in moderate doses decrease inflammation and control of OS; therefore, their use as an adjuvant therapy to improve prognosis is confirmed. The antioxidant therapy can be effective in this pandemia since it improves all of the survival scores including SOFA, Apache II, SAPS II, COVIDGRAM, GCS by lowering the LPO, IL-6, CRP, PCT and increasing systemic TAC. The survival prognosis is >90%, in patients with pneumonia by COVID-19 which supports its use. There is still needed to evaluate the reproducibility of our findings in a similar treatment and context.
7. Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Author contributions
A.P.C., R.R.V.V., J.G.D.C., H.H.B., H.C.S., L.M.-C., G.A.E., F.H., O.G.-M., H.S.-O., treated and recruited the patients in the intensive care unit and collected all of the results, including the pretreatment and post treatment dates. M.E.S., V.G.-L., and I.P.-T., and designed the study and wrote the manuscript. V.G.-L. revised and structured the manuscript; I.P.-T., and M.E.S. made the laboratory determination, designed the tables, figures, and performed and planned the statistical analysis. R. M. made the IL-6 determination; L.M.P. designed and made the graphical abstract. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Conacyt # 312167.
Thank to Instituto Nacional de Cardiología “Ignacio Chávez” by the payment for the open access to this paper.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Israel Pérez-Torres, Email: pertorisr@yahoo.com.mx.
Maria Elena Soto, Email: mesoto50@hotmail.com.
References
- 1.Soto M.E., Guarner-Lans V., Soria-Castro E., Manzano-Pech L., Pérez-Torres I. Is antioxidant therapy a useful complementary measure for covid-19 treatment? An algorithm for its application. Medicina (Kaunas) 2020;56:386. doi: 10.3390/medicina56080386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khomich O.A., Kochetkov S.N., Bartosch B., Ivanov A.V. Redox Biology of respiratory viral infections. Viruses. 2018;10:392. doi: 10.3390/v10080392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aisa-Alvarez A., Soto M.E., Guarner-Lans V., Camarena-Alejo G., Franco-Granillo J., Martinez-Rodríguez E.A. Usefulness of antioxidants as adjuvant therapy for septic shock: a randomized clinical trial. Medicina (Kaunas) 2020;56:619. doi: 10.3390/medicina56110619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Timsit J.F., Perner A., Bakker J., Bassetti M., Benoit D., Cecconi M. Year in review in intensive care Medicine 2014: III. Severe infections, septic shock, health care associated infections, highly resistant bacteria, invasive fungal infections, severe viral infections, Ebola virus disease and paediatrics. Intensive Care Med. 2015;41:575–588. doi: 10.1007/s00134-015-3755-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kakihana Y., Ito T., Nakahara M., Yamaguchi K., Yasuda T. Sepsis induced myocardial dysfunction: pathophysiology and management. J Intensive Care. 2016;4:22. doi: 10.1186/s40560-016-0148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marshall J.C., Vincent J.L., Guyatt G., Angus D.C., Abraham E., Bernard G. Outcome measures for clinical research in sepsis: a report of the 2nd Cambridge Colloquium of the International Sepsis Forum. Crit Care Med. 2005;33:1708–1716. doi: 10.1097/01.ccm.0000174478.70338.03. [DOI] [PubMed] [Google Scholar]
- 7.Feng H., Yu D., Weina L. Coronavirus disease 2019 (COVID-19): what we know? J Med Virol. 2020;92:719–725. doi: 10.1002/jmv.25766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smirnoff N. Ascorbic acid metabolism and functions: a comparison of plants and mammals. Free Radic Biol Med. 2018;122:116–129. doi: 10.1016/j.freeradbiomed.2018.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pérez-Torres I., Manzano-Pech L., Rubio-Ruíz M.E., Soto M.E., Guarner-Lans V. Nitrosative stress and its association with cardiometabolic disorders. Molecules. 2020;25:2555. doi: 10.3390/molecules25112555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berger M.M., Oudemans-van S.H.M. Vitamin C supplementation in the critically ill patient. Curr Opin Clin Nutr Metab Care. 2015;18:193–201. doi: 10.1097/MCO.0000000000000148. [DOI] [PubMed] [Google Scholar]
- 11.Pérez-Torres I., Guarner-Lans V., Rubio-Ruiz M.E. Reductive stress in inflammation-associated diseases and the pro- effect of antioxidant agents. Int J Mol Sci. 2017;18:2098. doi: 10.3390/ijms18102098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lassnigg A., Punz A., Barker R., Keznickl P., Manhart N., Roth E. Influence of intravenous vitamin E supplementation in cardiac surgery on oxidative stress: a double-blinded, randomized, controlled study. Br J Anaesth. 2003;90:148–154. doi: 10.1093/bja/aeg042. [DOI] [PubMed] [Google Scholar]
- 13.Harri H. Vitamin E administration may decrease the incidence of pneumonia in elderly males. Clin Interv Aging. 2016;11:1379–1385. doi: 10.2147/CIA.S114515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zúñiga-Muñoz A.M., Pérez-Torres I., Guarner-Lans V., Núñez-Garrido E., Velázquez E.R., Huesca-Gómez C. Glutathione system participation in thoracic aneurysms from patients with Marfan syndrome. Vasa. 2017;46:177–186. doi: 10.1024/0301-1526/a000609. [DOI] [PubMed] [Google Scholar]
- 15.Rank N., Michel C., Haertel C., Lenhart A., Welte M., Meier-Hellmann A. N acetylcysteine increases liver blood flow and improves liver function in septic shock patients: Results of a prospective, randomized, double-blind study. Crit Care Med. 2000;28:3799–3807. doi: 10.1097/00003246-200012000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Paterson R.L., Galley H.F., Webster N.R. The effect of N-acetylcysteine on nuclear factor-kappa B activation, interleukin-6, interleukin-8, and intercellular adhesion molecule-1 expression in patients with sepsis. Crit Care Med. 2003;31:2574–2578. doi: 10.1097/01.CCM.0000089945.69588.18. [DOI] [PubMed] [Google Scholar]
- 17.Sánchez-Barceló E.J., Mediavilla M.D., Tan D.X., Reiter R.J. Clinical uses of melatonin: Evaluation of human trials. Curr Med Chem. 2010;17:2070–2095. doi: 10.2174/092986710791233689. [DOI] [PubMed] [Google Scholar]
- 18.D’Amato L.A., Mistraletti G., Longhi D., Piva I.R., Marrazzo F., Villa C. Melatonin blood values and total antioxidant capacity in critically ill patients. Crit Care. 2014;18:P436. [Google Scholar]
- 19.Lowes D.A., Almawash A.M., Webster N.R., Reid V.L., Galley H.F. Melatonin and structurally similar compounds have differing effects on inflammation and mitochondrial function in endothelial cells under conditions mimicking sepsis. Br J Anaesth. 2011;107:193–201. doi: 10.1093/bja/aer149. [DOI] [PubMed] [Google Scholar]
- 20.Urata Y., Honma S., Goto S., Todoroki S., Iida T., Cho S. Melatonin induces gamma-glutamylcysteine synthetase mediated by activator protein-1 in human vascular endothelial cells. Free Radic Biol Med. 1999;27:838–847. doi: 10.1016/s0891-5849(99)00131-8. [DOI] [PubMed] [Google Scholar]
- 21.Galley H.F., Lowes D.A., Allen L., Cameron G., Aucott L.S., Webster N.R. Melatonin as a potential therapy for sepsis: a phase I dose escalation study and an ex vivo whole blood model under conditions of sepsis. J Pineal Res. 2014;56:427–438. doi: 10.1111/jpi.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang M. Cell pyroptosis, a potential pathogenic mechanism of 2019-nCoV infection. SSRN. 2020;1–7:doi:10.2139/ssrn.3527420.
- 23.Shneider A., Kudriavtsev A., Vakhrusheva A. Can melatonin reduce the severity of COVID-19 pandemic? Int Rev Immunol. 2020;39:153–162. doi: 10.1080/08830185.2020.1756284. [DOI] [PubMed] [Google Scholar]
- 24.Yung-Ming C., Chao-Jung T., Kung-Yu H., Kwan-Dun W., Tsai T.-J. Inhibition by pentoxifylline of TNF-alpha-stimulated fractalkine production in vascular smooth muscle cells: evidence for mediation by NF-kappa B down-regulation. Br J Pharmacol. 2003;138:950–958. doi: 10.1038/sj.bjp.0705088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olson G., Andrew M.D. Diagnosis and treatment of adults with community-acquired pneumonia. JAMA. 2020;323:885–886. doi: 10.1001/jama.2019.21118. [DOI] [PubMed] [Google Scholar]
- 26.Lambden S., Laterre P.F., Levy M.M., Francois B. The SOFA score-development, utility, and challenges of accurate assessment in clinical trials. Crit Care. 2019;23:374. doi: 10.1186/s13054-019-2663-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 28.Brower R.G., Lanken P.N., MacIntyre N., Matthay M.A., Morris A., Marek A. National heart, lung, and blood institute ARDS clinical trials network higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 29.Thachil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guimarães H.P., Timerman S., Rodrigues R.D.R., Corrêa T.D., Schubert D.U.C., Freitas A.P. Position statement: cardiopulmonary resuscitation of patients with confirmed or suspected COVID-19-2020. Arq Bras Cardiol. 2020;114:1078–1087. doi: 10.36660/abc.20200548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manzano-Pech L.G., Caballero-Chacón S.C., Guarner-Lans V., Díaz-Díaz E., Moreno G.A., Pérez-Torres I. Effect of oophorosalpingo-hysterectomy on serum antioxidant enzymes in female dogs. Sci Rep. 2019;9:9674. doi: 10.1038/s41598-019-46204-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Narayanasamy K., Natarajan M., Ramachandran A., Wesley J., Thangaraj V., Etherajan T. Clinical outcomes among asymptomatic or mildly symptomatic COVID-19 patients in an isolation facility in Chennai. India. Am J Trop Med Hyg. 2020 doi: 10.4269/ajtmh.20-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen N., Zhou M., Dong X., Qu J., Gong F., Han T. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howe K.P., Clochesy J.M., Goldstein L.S., Owen H. Mechanical ventilation antioxidant trial. Am J Crit Care. 2015;24:440–445. doi: 10.4037/ajcc2015335. [DOI] [PubMed] [Google Scholar]
- 36.Cazzola M., Calzetta L., Facciolo F., Rogliani P., Matera M.G. Pharmacological investigation on the antioxidant and anti-inflammatory activity of N-acetylcysteine in an ex vivo model of COPD exacerbation. Respir Res. 2017;18:26. doi: 10.1186/s12931-016-0500-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh V.P., Aggarwal R., Singh S., Banik A., Ahmad T., Patnaik B.R. Metabolic syndrome is associated with increased oxo-nitrative stress and asthma-like changes in lungs. PLoS ONE. 2015;10:e0129850. doi: 10.1371/journal.pone.0129850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delgado-Roche L., Mesta F. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) Infection. Arch Med Res. 2020;51:384–387. doi: 10.1016/j.arcmed.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rivers E.P., McIntyre L., Morro D.C., Rivers K.K. Early and innovative interventions for severe sepsis and septic shock: taking advantage of a window of opportunity. CMAJ. 2005;173:1054–1065. doi: 10.1503/cmaj.050632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clapp B.R., Hingorani A.D., Kharbanda R.K., Mohamed-Ali V., Stephens J.W., Vallance P. Inflammation-induced endothelial dysfunction involves reduced nitric oxide bioavailability and increased oxidant stress. Cardiovasc Res. 2004;64:172–178. doi: 10.1016/j.cardiores.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 41.Pérez-Torres I., Soto M.E., Castrejón-Tellez V., Rubio-Ruiz M.E., Manzano-Pech L., Guarner-Lans V. Oxidative, reductive, and nitrosative stress effects on epigenetics and on posttranslational modification of enzymes in cardiometabolic diseases. Oxid Med Cell Longev. 2020;2020:8819719. doi: 10.1155/2020/8819719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ang A., Pullar J.M., Currie M.J., Vissers M.C.M. Vitamin C and immune cell function in inflammation and cancer. Biochem Soc Trans. 2018;46:1147–1159. doi: 10.1042/BST20180169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fowler A.A., Syed A.A., Knowlson S., Sculthorpe R., Farthing D., DeWilde C. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med. 2014;12:32. doi: 10.1186/1479-5876-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fowler A.A., Truwit J.D., Hite R.D., Morris P.E., DeWilde C., Priday A. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. JAMA. 2019;322:1261–1270. doi: 10.1001/jama.2019.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fujii T., Luethi N., Young P.J., Frei D.R., Eastwood G.M., French C.J. Effect of vitamin C, hydrocortisone, and thiamine vs hydrocortisone alone on time alive and free of vasopressor support among patients with septic shock: the Vitamins Randomized Clinical Trial. JAMA. 2020;323:423–431. doi: 10.1001/jama.2019.22176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boretti A., Banik B.K. Intravenous vitamin C for reduction of cytokines storm in acute respiratory distress syndrome. Pharma Nutr. 2020;12:100190. doi: 10.1016/j.phanu.2020.100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim J.-Y., Lee S.-M. Effect of ascorbic acid on hepatic vasoregulatory gene expression during polymicrobial sepsis. Life Sci. 2004;75:2015–2026. doi: 10.1016/j.lfs.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 48.Kim J.-Y., Lee S.-M. Vitamins C and E protect hepatic cytochrome P450 dysfunction induced by polymicrobial sepsis. Eur J Pharmacol. 2006;534:202–209. doi: 10.1016/j.ejphar.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 49.Maret G., Traber J., Stevens F. Vitamins C and E: beneficial effects from a mechanistic perspective. Free Radic Biol Med. 2011;51:1000–1013. doi: 10.1016/j.freeradbiomed.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hemilä H. Vitamin E administration may decrease the incidence of pneumonia in elderly males. Clin Interv Aging. 2016;11:1379–1385. doi: 10.2147/CIA.S114515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boga J.A., Coto-Montes A., Rosales-Corral S.A., Tan D.-X., Reiter R.J. Beneficial actions of melatonin in the management of viral infections: a new use for this “molecular handyman”? Rev Med Virol. 2012;22:323–338. doi: 10.1002/rmv.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ben-Nathan D., Maestroni G.J., Lustig S., Conti A. Protective effects of melatonin in mice infected with encephalitis viruses. Arch Virol. 1995;140:223–230. doi: 10.1007/BF01309858. [DOI] [PubMed] [Google Scholar]
- 53.Hardeland R. Melatonin and inflammation-story of a double-edged blade. J Pineal Res. 2018;65:e12525. doi: 10.1111/jpi.12525. [DOI] [PubMed] [Google Scholar]
- 54.Shang Y., Xu S.P., Wu Y., Jiang Y.X., Wu Z.Y., Yuan S.Y. Melatonin reduces acute lung injury in endotoxemic rats. Chin Med J. 2009;122:1388–1393. [PubMed] [Google Scholar]
- 55.Ahmadi Z., Ashrafizadeh M. Melatonin as a potential modulator of Nrf2. Fund Clin Pharmacol. 2020;34:11–19. doi: 10.1111/fcp.12498. [DOI] [PubMed] [Google Scholar]
- 56.Miller S.C., Pandi-Perumal S.R., Esquifino A.I., Cardinali D.P., Maestroni G.J.M. The role of melatonin in immuno-enhancement: potential application in cancer. Int J Exp Pathol. 2006;87:81–87. doi: 10.1111/j.0959-9673.2006.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zarezadeh M., Khorshidi M., Emami M., Janmohammadi P., Kord-Varkaneh H., Mousavi S.M. Melatonin supplementation and pro-inflammatory mediators: a systematic review and meta-analysis of clinical trials. Eur J Nutr. 2019;59:1803–1813. doi: 10.1007/s00394-019-02123-0. [DOI] [PubMed] [Google Scholar]
- 58.Chen J., Xia H., Zhang L., Zhang H., Wang D., Tao X. Protective effects of melatonin on sepsis-induced liver injury and dysregulation of gluconeogenesis in rats through activating SIRT1/STAT3 pathway. Biomed Pharmacother. 2019;117:109150. doi: 10.1016/j.biopha.2019.109150. [DOI] [PubMed] [Google Scholar]
- 59.Tordjman S., Chokron S., Delorme R., Charrier A., Bellissant E., Jaafari N. Melatonin: pharmacology, functions and therapeutic benefits. Curr Neuropharmacol. 2017;15:434–443. doi: 10.2174/1570159X14666161228122115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Andersen L.P.H., Gogenur I., Rosenberg J., Reiter R.J. The safety of melatonin in humans. Clin Drug Investig. 2016;36:169–175. doi: 10.1007/s40261-015-0368-5. [DOI] [PubMed] [Google Scholar]
- 61.Silvagno F., Vernone A., Pescarmona G.P. The Role of glutathione in protecting against the severe inflammatory response triggered by COVID-19. Antioxidants (Basel) 2020;9:624. doi: 10.3390/antiox9070624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Geiler J. N-acetyl-L-cysteine (NAC) inhibits virus replication and expression of pro-inflammatory molecules in A549 cells infected with highly pathogenic H5N1 influenza A virus. Biochem Pharmacol. 2010;79:413–420. doi: 10.1016/j.bcp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 63.Hsu B.G., Lee R.P., Yang F.L., Harn H.J., Chen H.I. Posttreatment with N-acetylcysteine ameliorates endotoxin shock-induced organ damage in conscious rats. Life Sci. 2006;79:2010–2016. doi: 10.1016/j.lfs.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 64.Prauchner C.A. Oxidative stress in sepsis: pathophysiological implications justifying antioxidant co-therapy. Burns. 2017;43:471–485. doi: 10.1016/j.burns.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 65.Nasi A., McArdle S., Gaudernack G., Westman G., Melief C., Rockberg J. Reactive oxygen species as an initiator of toxic innate immune responses in retort to SARS-CoV-2 in an ageing population, consider N-acetylcysteine as early therapeutic intervention. Toxicol Rep. 2020;7:768–771. doi: 10.1016/j.toxrep.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guthappa R. Molecular docking studies of N-acetylcysteine, zinc acetylcysteine and niclosamide on SARS Cov 2 protease and its comparison with hydroxychloroquine. Chemarxiv. 2020 doi: 10.26434/chemrxiv.12161493.v1. [DOI] [Google Scholar]
- 67.Sharafkhah M., Abdolrazaghnejad A., Zarinfar N., Mohammadbeigi A., Ali M.A., Abaszadeh S. Safety and efficacy of N-acetylcysteine for prophylaxis of ventilator-associated pneumonia: a randomized, double blind, placebo-controlled clinical trial. Med Gas Res. 2018;8:19–23. doi: 10.4103/2045-9912.229599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Q., Ju Y., Ma Y., Wang T. N-acetylcysteine improves oxidative stress and inflammatory response in patients with community acquired pneumonia a randomized controlled trial. Medicine. 2018;97:e13087. doi: 10.1097/MD.0000000000013087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suter P.M., Domenighetti G., Schaller M.D., Laverrière M.C., Ritz R., Perret C. N-Acetylcysteine enhances recovery from acute lung injury in man: a randomized, double-blind, placebo-controlled clinical study. Chest. 1994;105:190–194. doi: 10.1378/chest.105.1.190. [DOI] [PubMed] [Google Scholar]
- 70.Garcia de Alencar JC, Moreira CL, Müller AD, Chaves CE, Fukuhara MA, Aparecida da Silva E, et al. Covid Register Group Double-blind, randomized, placebo-controlled trial with N-acetylcysteine for treatment of severe acute respiratory syndrome caused by COVID-19. Clin Infect Dis 2020;ciaa1443. [DOI] [PMC free article] [PubMed]
- 71.Zhongcheng S., Puyo C.A. N-Acetylcysteine to combat COVID-19: an evidence review. Ther Clin Risk Manag. 2020;16:1047–1055. doi: 10.2147/TCRM.S273700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guloyan V., Oganesian B., Baghdasaryan N., Yeh C., Singh M., Guilford F. Glutathione supplementation as an adjunctive therapy in COVID-19. Antioxidants (Basel) 2020;9:914. doi: 10.3390/antiox9100914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shabaan A.E., Nasef N., Shouman B., Nour I., Mesbah A., Abdel-Hady H. Pentoxifylline therapy for late-onset sepsis in preterm infants: a randomized controlled trial. Pediatr Infect Dis J. 2015;34:E143–E148. doi: 10.1097/INF.0000000000000698. [DOI] [PubMed] [Google Scholar]
- 74.Speer E.M., Dowling D.J., Ozog L.S., Xu J., Yang Jie, Kennady G. Pentoxifylline inhibits the production of inflammatory cytokines mediated by TLR and inflammasome in human blood with greater efficacy and potency in neonates. Pediatric Res. 2017;81:806–816. doi: 10.1038/pr.2017.6. [DOI] [PubMed] [Google Scholar]
- 75.Salman S., Hibbert J., Page-Sharp M., Manning L., Simmer K., Doherty D.A. Effects of maturation and size on population pharmacokinetics of pentoxifylline and its metabolites in very preterm infants with suspected late-onset sepsis or necrotizing enterocolitis: a pilot study incorporating clinical outcomes. Br J Clin Pharmacol. 2019;85:147–159. doi: 10.1111/bcp.13775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kotula J.J., Moore W.S., Chopra A., Cies J.J. Association of procalcitonin value and bacterial coinfections in pediatric patients with viral lower respiratory tract infections admitted to the pediatric intensive care unit. J Pediatr Pharmacol Ther. 2018;23:466–472. doi: 10.5863/1551-6776-23.6.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaur R., Kaur M., Singh J. Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: molecular insights and therapeutic strategies. Cardiovasc Diabetol. 2018;17:121. doi: 10.1186/s12933-018-0763-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yamasaki H. Blood nitrate and nitrite modulating nitric oxide bioavailability: potential therapeutic functions in COVID-19. Nitric Oxide. 2020;103:29–30. doi: 10.1016/j.niox.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mittal M., Siddiqui M.R., Tran K., Reddy S.P., Malik A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20:1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dolhnikoff M., Duarte-Neto A.N., de Almeida Monteiro R.A., da Silva L.F.F., de Oliveira E.P., Saldiva P.H.N. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J Thromb Haemost. 2020;18:1517–1519. doi: 10.1111/jth.14844. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.