Abstract
Background
Gemcitabine is a frequently used chemotherapeutic agent but its effects on the immune system are incompletely understood. Recently, the randomized NVALT19-trial revealed that maintenance gemcitabine after first-line chemotherapy significantly prolonged progression-free survival (PFS) compared to best supportive care (BSC) in malignant mesothelioma. Whether these effects are paralleled by changes in circulating immune cell subsets is currently unknown. These analyses could offer improved mechanistic insights into the effects of gemcitabine on the host and guide development of effective combination therapies in mesothelioma.
Methods
We stained peripheral blood mononuclear cells (PBMCs) and myeloid-derived suppressor cells (MDSCs) at baseline and 3 weeks following start of gemcitabine or BSC treatment in a subgroup of mesothelioma patients included in the NVALT19-trial. In total, 24 paired samples including both MDSCs and PBMCs were included. We performed multicolour flow-cytometry to assess co-inhibitory and-stimulatory receptor- and cytokine expression and matched these parameters with PFS and OS.
Findings
Gemcitabine treatment was significantly associated with an increased NK-cell- and decreased T-regulatory cell proliferation whereas the opposite occurred in control patients. Furthermore, myeloid-derived suppressor cells (MDSCs) frequencies were lower in gemcitabine-treated patients and this correlated with increased T-cell proliferation following treatment. Whereas gemcitabine variably altered co-inhibitory receptor expression, co-stimulatory molecules including ICOS, CD28 and HLA-DR were uniformly increased across CD4+ T-helper, CD8+ T- and NK-cells. Although preliminary in nature, the increase in NK-cell proliferation and PD-1 expression in T cells following gemcitabine treatment was associated with improved PFS and OS.
Interpretation
Gemcitabine treatment was associated with widespread effects on circulating immune cells of mesothelioma patients with responding patients displaying increased NK-cell and PD-1 + T-cell proliferation. These exploratory data provide a platform for future on treatment-biomarker development and novel combination treatment strategies.
Keywords: Malignant mesothelioma, Lymphocytes, Myeloid-derived suppressor cells, Gemcitabine, Immunotherapy
1. Introduction
Advancesin the field of mesothelioma treatment have been limited with recent trials involving anti-PD-1 or anti-CTLA-4 monotherapy yielding no significant improvements in clinical outcomes [1, 2]. Therefore, the mainstay of treatment for mesothelioma remains platinum-pemetrexed doublet chemotherapy with a median overall survival ranging from 13 to 16 months with a persisting demand for novel effective treatments [3]. Recently, we reported results from the NVALT19-study, a randomized phase II open-label trial investigating the benefit of maintenance gemcitabine in mesothelioma patients who did not progress following first-line chemotherapy [4]. Gemcitabine significantly improved progression-free survival (PFS) compared to best-supportive care (BSC)-treated mesothelioma patients and was associated with a manageable toxicity. Overall survival, however, was only improved in a small group of patients warranting mechanistic analysis of why some benefitted and others did not.
Research in context.
Evidence before this study
Gemcitabine is a frequently used treatment in various types of cancer. Recently, gemcitabine was found to provide a progression free survival benefit as switch-maintenance therapy in patients with malignant mesothelioma (NVALT19). Gemcitabine has known direct anti-tumor effects but whether gemcitabine affects the immune system and whether this is associated with treatment efficacy is currently unknown.
Added value of this study
Gemcitabine treatment in mesothelioma patients was associated with an anti- to pro-inflammatory shift in circulating immune cell phenotype evidenced by decreased MDSC-frequencies and regulatory T-cell proliferation but increased T- and NK-cell activation. Further exploratory analyses revealed several immunological parameters correlate with improved clinical outcome indicating a possible role for the immune system in dictating gemcitabine efficacy.
Implications of all the available evidence
These pilot data provide a platform for future development of on-treatment biomarkers that predict improved patient outcome and should be further validated and explored in larger patient studies. Our findings provide early indications of possible synergy between gemcitabine and immunotherapy in mesothelioma.
Alt-text: Unlabelled box
The last decade has witnessed a surge in studies reporting immune-modifying functions of chemotherapy relying partially, or completely on elicited immune-mediated tumour destruction [5]. Chemotherapy-induced immune activation can be successively monitored in peripheral blood, with clinically responding patients exhibiting marked increases in immune-effector cell frequencies and phenotype depending on the type of agent investigated [6, 7]. Gemcitabine has previously been reported to decrease the frequencies of myeloid-derived suppressor cells (MDSCs) and T-regulatory (Treg) cells in humans and preclinical tumour models [8], [9], [10]. Furthermore, Albelda and colleagues found that the anti-tumour efficacy of gemcitabine was lost in nude mice lacking T cells underscoring their role in dictating tumour outcome [11]. The effects of gemcitabine on T- and NK-cell phenotype and proliferation in patients are currently unknown and could yield novel insights into the immunological mechanisms underlying the efficacy of gemcitabine.
We hypothesized that gemcitabine could improve antitumor immune responses by positively modulating cytotoxic T cells, regulatory T cells, and myeloid-derived suppressor cells and that these immunomodulatory effects could be detected in peripheral blood during treatment. This exploratory study paves the way for further in depth investigations of the mechanism of action of gemcitabine and its association with clinical response in mesothelioma, and potentially in other solid cancer types.
2. Methods
2.1. Trial design and study population
Blood samples obtained during the NVALT19-study were used to assess the effect of gemcitabine on PBMCs. The NVALT19-study was a multicenter, investigator-initiated, open-label, randomized, phase 2 trial, conducted in The Netherlands between 2014 and 2019, investigating the efficacy of switch maintenance gemcitabine in 130 malignant mesothelioma patients. Patients without progressive disease were included 21–42 days after having obtained 4–6 cycles of first-line platinum-pemetrexed chemotherapy. Gemcitabine was administered at day one and eight of every 21-day cycle at a dose of 1250 mg/m2 until disease progression (according to modified RECIST-criteria in pleural malignant mesothelioma [12]), unacceptable toxicity or death.
The NVALT19 study was conducted in agreement with the Declaration of Helsinki and according to the ICH Harmonized Tripartite Guideline on Good Clinical Practice. The NVALT19 study-protocol was approved by the central ethical committee and local institutional review boards (Reference number: METC19.0668, e-supplement). All patients provided written informed consent (Netherlands Trial Registry: NTR4132/NL3847) for the NVALT19 study and the current subgroup analyses. Further trial details have been published elsewhere [4].
The current study is a predefined exploratory analysis of a subgroup of patients included in the NVALT19 trial (see supplemental study protocol). Paired baseline and week three blood samples were collected from 46 malignant pleural mesothelioma patients of which 27 received gemcitabine and 19 BSC. For 12 patients, only PBMC data were available and in 10 patients only MDSCs were measured for varying reasons including insufficient sample quality and acquisition of samples beyond the predefined time range. Both PBMCs and MDSCs were available for 24 cases. A flowchart of how samples were selected for analysis is shown in figure S8. Patient groups had similar characteristics at baseline (Table S1).
To assess the T- and NK-cell phenotype, we constructed several comprehensive immune cell flow-cytometry panels including markers of proliferation, memory differentiation, co-stimulatory/-inhibitory receptors and cytokine production capacity (Table S2A). Also, myeloid cell subsets were investigated focusing on MDSCs with monocyte and dendritic cell (DC-)subsets being characterized in a subgroup of patients (Table S2B).
2.2. Peripheral blood processing
Peripheral blood samples were acquired at day one of cycle one (before the start of therapy; baseline) and at day one of cycle two (median 21 days in gemcitabine group (range: 19–42 days), median 22 days in the BSC group (range: 18–49 days)). Approximately 20 millilitres of blood were collected in EDTA tubes and transported to the laboratory facility within 4 h for immediate processing in order to preserve the MDSC-phenotype. PBMCs were isolated by density-gradient centrifugation using Ficoll-hypaque (GE Healthcare). A total of 1 × 106 cells were used for fresh flow cytometry staining of myeloid-derived suppressor cells (MDSC). The remaining cells were cryopreserved in 10% dimethylsulfoxide (Sigma-Aldrich), 40% FCS (Gibco) and RPMI (Invitrogen, Molecular Probes) for later reconstitution and analysis.
2.3. Flow cytometry
T- and NK-cell lymphocyte characterization was performed on PBMCs stored in liquid nitrogen following thawing and reconstitution in medium with FCS and FACS-staining buffer. For cytokine analysis, cells were first stimulated for 4 h in vitro at 37 °C using phorbol 12-myristate 13-acetate (PMA) and ionomycin (Sigma-Aldrich), supplemented with GolgiStop (BD Biosciences). In both instances, cells were first stained for membrane markers (Fig. S2) allowing for immune-cell subset identification, for 30 min at 4 °C, followed by fixation and permeabilization using the FoxP3 transcription factor-kit according to the manufacturer's instructions (Thermofisher Scientific). Subsequently, intracellular proteins were stained for 60 min at 4 °C after which cells were suspended in staining buffer and acquired on a LSR II flow cytometer (BD Biosciences). Flow cytometric analysis were performed using FlowJo software (v10, Tree Star Inc.).
2.4. Statistical analysis
All statistical analyses other than Kaplan-Meier curves (produced in R, statistical significance determined using a Log-Rank test and Cox proportional hazard regression analyses to estimate hazard ratios) were executed using Graphpad Prism software (version 8). For survival analyses within each subgroup, the unadjusted 95% CIs were reported. [13] Using the same software, heatmaps were constructed depicting mean changes in cell parameters, which were derived from the ratio of individual patient data from day 21 post start of treatment divided by baseline values. Paired non-parametric (Wilcoxon matched-pairs signed rank) tests were performed in order to calculate statistical significance of changes compared to baseline values. When continuous variables (e.g. magnitude of increase/decrease in MDSCs during therapy) were compared, non-parametric Spearman correlations were established yielding a Spearman Rho and corresponding p-value. In case of a Gaussian distribution of the data, a Pearson correlation coefficient was computed generating an r-squared (r2)- and p-value indicating statistical significance. Only, in case a paired sample was available, the samples were included in the analyses. Sensitivity analyses were performed demonstrating comparable clinical efficacy of gemcitabine in the immunomonitoring compared to the complete NVALT19 cohort (Hazard ratio (HR) of 0•62, 95% CI −1•487–0•137) similar to that observed in the entire NVALT19 cohort (HR 0•48; 95% CI, 0•33 to 0•71 Fig. S9).
3. Role of funding source
The Koningin Wilhelmina Fonds voor de Nederlandse Kankerbestrijding (KWF) had neither a role in the study design, data collection, analyses or data interpretation nor in the writing of the report. The Nederlandse Vereniging van Artsen voor Longziekten en Tuberculose (NVALT) Study Group staff had no role in the writing of the report. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit the publication.
4. Results
4.1. Gemcitabine differentially modulates proliferation of circulating lymphocyte subsets
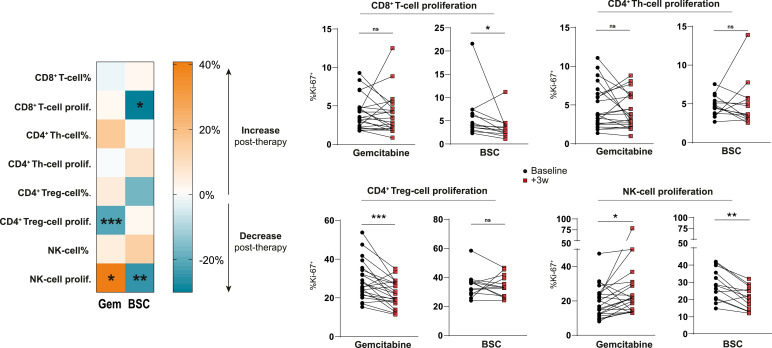
NK-cell proliferation significantly increased during gemcitabine treatment, whereas untreated patients exhibited a decrease in both CD8+ T-cells and natural killer (NK)-cell proliferation through time, (Fig. 1). Additionally, FoxP3+CD4+ T regulatory-cell (Treg) proliferation was strongly decreased in gemcitabine-treated patients compared to untreated patients. As FoxP3-expression marks a heterogeneous group of activated and regulatory T-cells, we further subdivided FoxP3+ cells based on the markers CD45RA and the magnitude of FoxP3-expression as described by Miyara et al. [14] (Fig. S1A). Using this distinction, we found that the proliferation of activated FoxP3-high Tregs (aTregs), previously described to be highly immune-suppressive, was decreased following gemcitabine. Similarly, FoxP3-expressing T-helper (Th) cell-proliferation was diminished following treatment (Fig. S1B). No statistically significant changes in T-cell frequencies (of total leukocytes) or T-cell memory subset distribution were noted in either patient group (Fig. S2). These findings illustrate that regulatory and non-regulatory lymphocyte subsets may be differentially affected by gemcitabine chemotherapy.
Fig. 1.
Gemcitabine differentially modulates proliferation in circulating lymphocyte subsets.
T-cell percentages subtypes of total (CD45+) leukocytes and proliferation determined by intracellular Ki-67-staining in peripheral blood of mesothelioma patients treated with or without gemcitabine at baseline, and after 3 weeks. A heatmap shows the mean changes per parameter compared to baseline values in both patient groups. Wilcoxon matched-pairs signed rank tests were performed to calculate statistical significance. A total of 35 patients were included in the analysis (n = 22 GEM; n = 13 BSC). Th = T-helper, Treg = regulatory T cell, NK = natural killer, BSC = best supportive care, ns = not significant, * = p<0.05, ** = p<0.01, *** = p<0.001.
4.2. Gemcitabine depletes MDSCs in mesothelioma correlating with improved T-cell proliferation
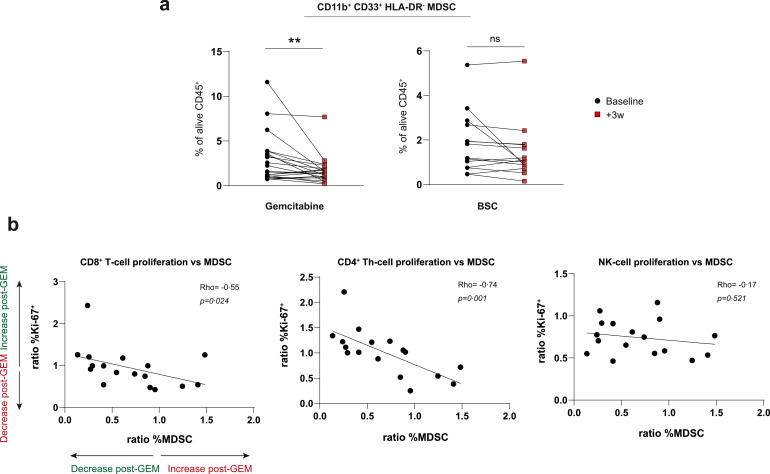
Gemcitabine has previously been reported to deplete MDSCs but whether this occurs in mesothelioma or affects T-cell proliferation in vivo remains largely unknown. We assessed CD11b+CD33+HLA-DR−MDSC frequencies by direct ex vivo measurement following ficoll-density gradient centrifugation and could confirm significant MDSC-reduction by gemcitabine in patients (Fig. 2A). The magnitude of MDSC-reduction significantly correlated with CD4+ T-helper and CD8+ T-cell but not NK-cell proliferation, strengthening results by others showing T-cell suppressive capacities of MDSCs in vitro (Fig. 2B) [15]. Other myeloid cell subsets available in a subset of patients showed less interference of gemcitabine, with only plasmacytoid dendritic cells (pDC) being significantly increased following treatment, which is in line with earlier data in pancreatic cancer patients (Fig. S3) [16]. These data show that the decreased MDSCs in peripheral blood of mesothelioma patients during gemcitabine therapy were paralleled by an increased T-cell proliferation.
Fig. 2.
The gemcitabine-associated decrease in MDSCs correlates with increased T-cell proliferation
a, changes in percentages of myeloid derived suppressor cells (MDSCs) following gemcitabine or best-supportive care (BSC). b, correlations of CD8+, CD4+ FoxP3 (Th)- and NK-lymphocyte proliferation (Ki67+) dynamics with changes in MDSC-frequencies in peripheral blood following gemcitabine (GEM) treatment. Wilcoxon matched-pairs signed rank tests were performed to calculate statistical significance. MDSCs were available for 35 patients (n = 21 GEM; n = 14 BSC). Spearman correlation coefficients were calculated and a Rho was generated for 17 gemcitabine-treated patients of whom matched T-cells and MDSCs were available. NK = natural killer, ns = not significant, ** = p<0.01.
4.3. Gemcitabine promotes an activated T-cell and NK-cell phenotype
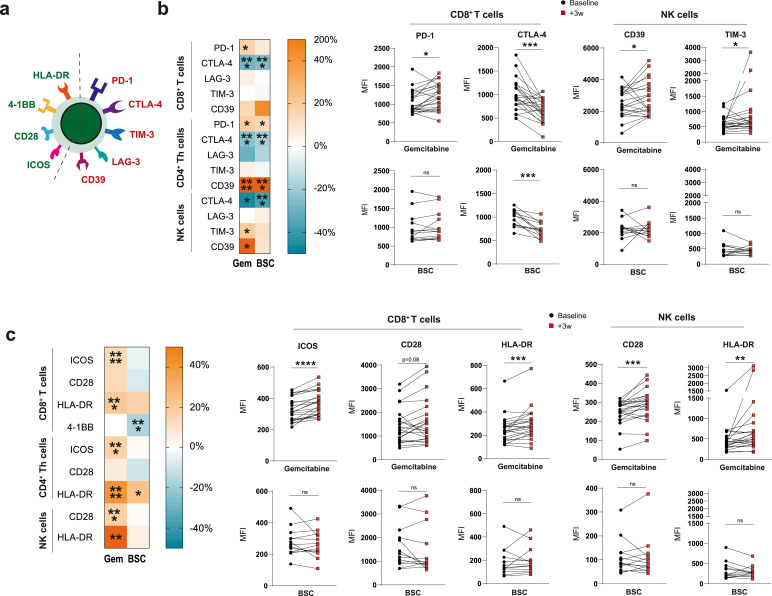
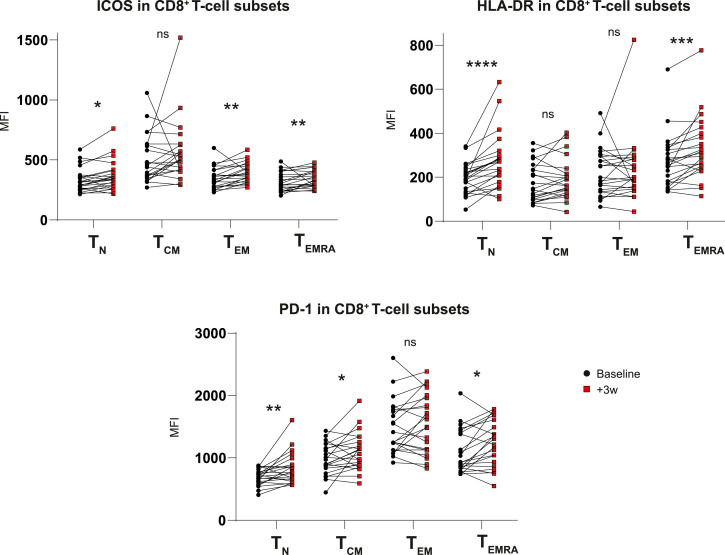
In order to complement our understanding of how gemcitabine alters T-cell phenotype and aid rational combination therapy selection, we analysed the expression of a variety of co-inhibitory and –stimulatory receptors on peripheral blood lymphocytes in our patient cohort (Fig. 3A). Percentages of receptor-positive cells mirrored median fluorescent intensity (MFI) values enabling MFI for further analysis (Fig. S4). Besides PD-1, which was significantly increased on CD8+T cells in gemcitabine-treated patients only, the majority of inhibitory receptors changed with similar dynamics in both patients groups, albeit more markedly following gemcitabine (Fig. 3B). In patients with a malignancy, NK cells have been reported to express several co-inhibitory receptors including TIM-3 which has been associated with increased NK-cell maturation but diminished functionality upon TIM-3-ligation [17]. NK cells expressed CTLA-4, LAG-3, TIM-3 and CD39, of which the latter two were significantly increased in the gemcitabine group but not in the control group (Fig. 3B). As the upregulation of co-inhibitory receptors is associated with both exhaustion and activation of lymphocytes, we assessed co-stimulatory receptor expression on T- and NK-cells to attempt to differentiate between these cellular states. As opposed to co-inhibitory receptors whose expression was heterogeneously altered following gemcitabine, co-stimulatory markers including ICOS, CD28 and HLA-DR were uniformly increased on both T- and NK cells in treated patients (Fig. 3C). Interestingly, these changes did not correlate with decreasing MDSC-frequencies nor were they related to the magnitude of Treg-proliferation which we found to be decreased following gemcitabine treatment earlier (Figure S5). These findings suggest that, whereas T-cell proliferation relates to MDSCs, the activation phenotype does not. T-cell activation in turn may result from direct gemcitabine-mediated modulation or indirectly via effects on tumour cells [5]. T-cell activation due to increased (tumour-derived) antigen recognition was deemed unlikely as gemcitabine did not affect or induce activation of effector-memory T cells specifically. Gemcitabine treatment rather increased activation marker expression across all memory subsets investigated, including naïve T-cells (Fig. 4).
Fig. 3.
Gemcitabine-treated patients display an activated lymphocyte phenotype in peripheral blood
a, co-stimulatory (green) and co-inhibitory (red) receptors assessed on lymphocyte surface in peripheral blood. b, heatmaps displaying mean percentage of change and paired analyses of co-inhibitory receptors C, and co-stimulatory receptor expression in response to gemcitabine or best-supportive care (BSC). Wilcoxon matched-pairs signed rank tests were performed to calculate statistical significance. A total of 35 patients were included in the analysis (n = 22 GEM; n = 13 BSC). Th = T-helper, Treg = regulatory T cell, NK = natural killer, BSC = best supportive care, PD-1 = programmed cell death protein 1, CTLA-4 = cytotoxic T-lymphocyte-associated protein 4, TIM-3 = T-cell immunoglobulin and mucin-domain containing-3, ICOS = inducible co-stimulatory molecule, HLA-DR = human-leukocyte antigen DR, MFI = mean fluorescent intensity, ns = not significant, * = p<0.05, ** = p<0.01, *** = p<0.001, **** = p<0.0001.
Fig. 4.
Co-stimulatory-molecule expression is increased independent of T-cell memory subset
Expression of ICOS, HLA-DR and PD-1 in different CD8+T cell stages of differentiation before and after gemcitabine administration. Wilcoxon matched-pairs signed rank tests were performed to calculate statistical significance. A total of 22 gemcitabine-treated patients was included in the analysis. PD-1 = programmed cell death protein 1, ICOS = inducible co-stimulatory molecule, HLA-DR = human-leukocyte antigen DR, Tn = naïve T cell, Tcm = central memory T cell, Tem = effector memory T cell, Temra = terminally differentiated T cell, MFI = mean fluorescent intensity, ns = not significant, * = p<0.05, ** = p<0.01, *** = p<0.001, **** = p<0.0001.
4.4. Gemcitabine does not alter cytokine expression by T cells
In addition to co-inhibitory and –stimulatory receptor expression on circulating T cells, we assessed cytokine- and granzyme-production capacity by stimulating PBMCs in vitro with PMA/ionomycin followed by intracellular staining. In contrast to aforementioned surface-markers, cytokine production did not statistically differ in treated and untreated patients although a trend towards increased expression was appreciated in gemcitabine-treated patients (Fig. S6A). Cytokine and granzyme-expression was found to be strongly coupled to specific memory T-cell subsets (Fig. S6B). Specifying cytokine expression (e.g. IFN-γ) to memory subsets yielded increased expression for high cytokine-producing subsets after gemcitabine although this did not reach statistical significance (Fig. S6C, data not shown).
4.5. Immune monitoring identifies lymphocyte parameters associated with response to gemcitabine
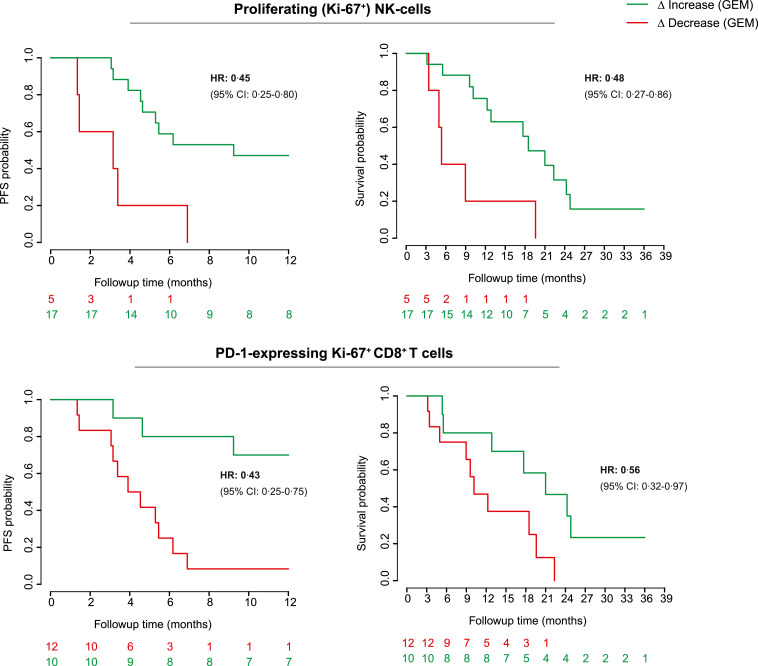
Although we detected increased markers of T- and NK-cell activation in gemcitabine-treated patients, the biological and clinical relevance of these findings remains unknown. Relating the investigated parameters to patient outcomes may also further define whether gemcitabine-mediated immune effects could potentially drive disease outcome. Furthermore, as OS was not significantly improved by gemcitabine in the intention to treat-population, biomarkers for patient stratification are warranted. We correlated the expression of key parameters, which were significantly altered by gemcitabine, to patient OS and PFS in both the gemcitabine-treated patients and the BSC-cohort, to detect which parameters indicated potential clinical benefit. We found that patients, showing an increase in NK-cell proliferation following gemcitabine, had a significantly better PFS and OS (HR for OS: 0•45, p = 0•01, HR PFS: 0•48, p = 0•01, Fig. 5). In addition, an increase in PD-1-expression on proliferating (but not total) CD8+ T cells was associated with improved clinical outcome (HR OS: 0•43, p<0•01, HR PFS: 0•56, p = 0•04). These parameters did not correlate with improved clinical outcome in BSC-treated patients, suggesting a gemcitabine-specific response (Fig. S7A). ICOS-expression in CD8+ T cells was near uniformly increased following gemcitabine except in one patient who coincidentally experienced progressive disease and death soon after treatment with gemcitabine (HR for increased vs. decreased ICOS-expression OS: 0•22, p = 0•03, HR PFS: 0•22, p = 0•03, Fig. S7B). The magnitude of ICOS-induction by gemcitabine further correlated with improved response to therapy albeit not significantly (Fig. S7C). These findings derived from a small exploratory cohort analysis suggest that key gemcitabine-induced immune effects might be associated with a survival benefit. This might help to better understand the interaction between and predict the efficacy of chemotherapy and immunotherapy.
Fig. 5.
Increases in NK- and PD-1 + T-cell proliferation following gemcitabine correlate with clinical outcome
Kaplan–Meier (KM-) curves showing differences in progression free- (PFS) and overall survival (OS) between patients exhibiting an increase (green) or decrease (red) of selected immune parameters following gemcitabine (GEM). 22 gemcitabine-treated patients were included in the analysis. Log-rank tests were applied. NK = natural killer, PD-1 = programmed cell death protein 1, HR = hazard ratio, CI = confidence interval.
5. Discussion
Using comprehensive immune monitoring of peripheral blood in mesothelioma patients treated with gemcitabine, we uncovered widespread myeloid and lymphoid immune modulation that might associate with treatment response. The alterations in T- and NK-cell proliferation and activation that were detected have not been previously reported for this kind of chemotherapy and tumour type. The increased PD-1 and ICOS-expression on lymphocytes following gemcitabine furthermore suggests that the combination of gemcitabine with immunotherapy using antagonistic and agonistic antibodies to PD-1 and ICOS, respectively, might be efficacious. These findings are of particular importance, as anti-PD-1 monotherapy was recently found to be ineffective in the majority of mesothelioma patients, emphasizing the demand for novel drug combinations to sensitize mesothelioma to immune-checkpoint inhibitors. [1] In line with our suggestions are recent data by Tallon de Lara et al., showing increased efficacy of gemcitabine-anti-PD-1 treatment in a preclinical mesothelioma model and radiographic responses in two mesothelioma patients following combination therapy [18]. Further randomized studies are needed to confirm whether these combinations are effective in larger patient cohorts. As gemcitabine is widely used for a variety of cancer including pancreatic cancer, breast cancer and non-small cell lung cancer (NSCLC), these findings could potentially be extrapolated to other tumour types as well.
The pleiotropic functions of gemcitabine on a wide variety of immune cells in vivo without functional in vitro data, preclude us from pinpointing which cellular mechanisms are mainly responsible for therapeutic benefit. Furthermore, we documented a significantly increased proliferation of NK-cells and decreased Treg-proliferation in gemcitabine-treated patients, while cell frequencies remained largely unaltered in peripheral blood. Although our method of cell isolation precludes proper enumeration of cells, several explanations could account for this discrepancy. First, as we assessed cellular states following the first cycle of chemotherapy, a change in proliferation would likely precede consequent changes in cell frequencies, which may become apparent at later time points. Secondly, whereas cellular state characterized by markers such as Ki-67, PD-1 and ICOS may offer a snapshot of underlying T-cell biology, circulating cell frequencies may not adequately reflect what happens in tumours. Although we did not have pre- and post-treatment tumour tissues, pre-clinical findings by others confirm increased NK-cell infiltration and decreased MDSC-frequencies into gemcitabine-treated tumours [19]. The fact that the investigated patients were only recently pre-treated with first-line chemotherapy could further influence circulating leukocyte frequencies. Whether this also accounts for the observed decrease in CD8+ T-cell proliferation in BSC-patients, or whether this is due to differences in time to disease progression, remains unknown. Furthermore, factors like concurrent infection or other comorbidities could have influenced the effects observed in our analysis. However, clinical and laboratory assessments were captured at baseline and after three weeks (at start of the second treatment cycle) and these did not reveal clear confounding factors. Moreover, by including a non-gemcitabine (BSC)-treated patient cohort first-line treatment induced alterations and confounding factors independent of treatment should have been comparable between both patients groups, increasing the likelihood that a real difference between the two groups was detected. Further investigations e.g. in pre-clinical disease models and functional in vitro studies in larger patients cohorts will be needed to formally establish causality of the observed immunological alteration in gemcitabine-treated mesothelioma patients.
As opposed to T-cell proliferation, which was tightly correlated to MDSC-frequencies, NK-cell proliferation and increased lymphocyte activation were not, suggesting that other mechanisms could be responsible for these shifts in phenotypes. Gemcitabine has recently been reported to increase tumour NKG2D-ligand expression in vitro [20], which could explain the increases in NK-cell proliferation and activation. In addition, gemcitabine has been found to increase tumour-antigenicity in mesothelioma mouse models by increasing antigen-presenting cell (APC) MHC-expression and promoting cross-presentation of tumour-antigens leading to increased tumour control [[21], [22], [23]]. Although we did not assess tumour-specific immune responses, we observed increased expression of activation markers in both naïve- and memory T-cell subsets, unlikely reflecting novel effector-T-cell clone induction. Whether this global change in T- and NK-cell activation resulted from direct effects of gemcitabine on lymphocytes, or from increased tumour adjuvanticity (e.g. by release of inflammatory mediators from dying tumour cells, as has been reported for gemcitabine) remains to be investigated [23].
Although gemcitabine did not uniformly or significantly increase circulating PD-1+/Ki-67+ CD8+ T cells (data not shown), an increase was associated with improved clinical outcome (Fig. 5) similar to recent reports identifying a similar population to associate with anti-PD-1 therapy response in NSCLC and melanoma [24, 25]. Although the exact nature of this phenotype remains to be identified, these cells showed significant overlap with tumour-infiltrating T cells in previous analyses, which, irrespective of the type of treatment, inferred durable tumour control [25] .
Our exploratory study design bears some important limitations. Although our sample size was limited with regard to the total NVALT19 population, we could show that our cohort was representative of the total study population with comparable clinical outcomes and patients characteristics (Table S1, Fig. S9). It is tempting to speculate whether the observed outliers also correlate with alternative clinical outcomes, as is shown for ICOS-expression which increased in all but one patient in response to gemcitabine, with that patient performing poorest (Fig. S7). The limited cohort size and absence of a validation group precludes formal conclusions to be made on the effects of gemcitabine on circulating immunity and therefore should be considered exploratory. Nonetheless, the results from this pilot study indicate immune-mediated anti-cancer efficacy of gemcitabine, warranting further research into these phenomenon in larger cohorts.
We report novel observations on circulating T- and NK-cells in gemcitabine-treated mesothelioma patients in a subset of patients from the NVALT-19 trial. These findings suggest preferential activation of anti-tumour immune cell populations and inhibition of Tregs and MDSCs. These pilot data, if validated in larger prospective cohorts, may provide a platform for future development of on-treatment biomarkers that predict improved patient outcome.
Data sharing
Qualified researchers may request access to anonymized individual patient level data by sending a request to the corresponding author. Data will be shared after approval of a proposal, with a signed data access agreement.
Declaration of Interests
JGA reports personal fees and non-financial support from MSD, personal fees from BMS, personal fees from Boehringer Ingelheim, personal fees from amphera, personal fees from Eli-Lilly, personal fees from Takeda, personal fees from Bayer, personal fees from Roche, personal fees from Astra Zeneca, all outside the submitted work; In addition, JGA has a patent on allogenic tumour cell lysate licensed to amphora (EP2938354A1), a patent combination immunotherapy in cancer (pending), and a patent biomarker for immunotherapy (pending) . PB participated in advisory boards of MSD and BMS and AstraZeneca for which the Netherlands Cancer Institute received a reimbursement, outside the submitted work. PB participated in advisory boards of Takeda. PB received financial support for an investigator-initiated trial from MSD and BMS. RC participated in advisory boards of MSD and Roche and received a speaker fee from Roche, Pfizer and BMS, outside the submitted work. JAB reports reimbursement from BMS and F Hoffmann-La Roche for the Netherlands Cancer Institute, and financial support for an investigator-initiated trial from MSD, outside the submitted work. CJG, VN, JS, FD, BB, GB, MJ, LK, JM, LL, CW, MVG, HV, MKL and ML declare no competing interests.
Acknowledgments
We thank the members of the NVALT19 Study Team for their many contributions in conducting the trial. We thank The Koningin Wilhelmina Fonds voor de Nederlandse Kankerbestrijding (KWF) and The Nederlandse Vereniging van Artsen voor Longziekten en Tuberculose (NVALT) Study Group for the funding support.
Footnotes
Funding: Dutch Cancer Society (KWF Kankerbestrijding) and the NVALT Study Group.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2020.103160.
Contributor Information
Floris Dammeijer, Email: f.dammeijer@erasmusmc.nl.
Joachim G. Aerts, Email: j.aerts@erasmusmc.nl.
Appendix. Supplementary materials
Fig. S1. Proliferation is decreased in activated Treg and Foxp3-expressing Th-cells following gemcitabine treatment
a, Representative flow cytometry plot of regulatory T cells (Tregs) gated according to Miyara et al. using CD45RA and FoxP3. Activated Tregs (aTregs) are the highest expressers of Ki-67, CD39 and ICOS on baseline as compared to resting Tregs (rTregs) or FoxP3-positive T-helper cells (bar graphs showing means with SEM). Wilcoxon matched-pairs signed rank tests were performed to calculate statistical significance. A total of 22 gemcitabine-treated patients was included in the analysis. b, Proliferation as determined by Ki-67 expression between the different FoxP3-expressing CD4+T-cell subsets. hi = high, int = intermediate, MFI = mean fluorescent intensity, ns = not significant, *** = p<0.001, **** = p<0.0001.
Fig. S2. Gemcitabine does not alter T-cell memory phenotype.
Representative dot plot of T-cell memory subsets (CD8+T-cells are shown) based on the membrane markers CD45RA and CCR7. A heatmap displays the mean changes in proportions of T-cell memory subsets following gemcitabine or best-supportive care (BSC). CCR = chemokine C-receptor, Tn = naïve T cell, Tcm = central memory T cell, Tem = effector memory T cell, Temra = terminally differentiated T cell, ns = not significant.
Fig. S3. Plasmacytoid DCs are increased in peripheral blood following gemcitabine.
Changes in monocyte- and dendritic cell (DC)-subsets following gemcitabine or best-supportive care (BSC) in a subset of patients from figure 2. Wilcoxon matched-pairs signed rank tests were performed to calculate statistical significance. (n = 11 GEM- and n = 12 BSC-treated patients included). cDC = conventional dendritic cell, pDC = plasmacytoid dendritic cell, ns = not significant, ** = p<0.01.
Fig. S4. Similar effects of gemcitabine on immune cells when expressed changes in percentage positive
Change in percentages of PD-1, CTLA-4 and ICOS on CD8+T cells following gemcitabine as opposed to MFI depicted in Fig. 3. A correlation is shown between PD-1 MFI and percentage positive in CD8+T cells (both gemcitabine and best-supportive care patients included). Wilcoxon matched-pairs signed rank tests were performed to calculate statistical significance. A total of 22 gemcitabine-treated patients was included in the analysis. Pearson correlation coefficient was calculated in case of MFI vs percentage PD-1 positive and a Pearson's r was generated. PD-1 = programmed cell death protein 1, CTLA-4 = cytotoxic T-lymphocyte-associated protein 4, ICOS = inducible co-stimulatory molecule, MFI = mean fluorescent intensity, ns = not significant, * = p<0.05, *** = p<0.001, **** = p<0.0001.
Fig. S5. No correlations between lymphocyte receptor expression and Treg proliferation or MDSCs
a, Correlations between the changes in PD-1/CTLA-4/ICOS MFI with changes in MDSCs and b, Treg-proliferation in peripheral blood following gemcitabine (GEM) treatment. Spearman correlation coefficients were calculated and a Rho was generated for 17 gemcitabine-treated patients of whom matched T-cells and MDSCs were available. MDSC = myeloid derived suppressor cell, Treg = regulatory T cell, PD-1 = programmed cell death protein 1, CTLA-4 = cytotoxic T-lymphocyte-associated protein 4, ICOS = inducible co-stimulatory molecule, MFI = mean fluorescent intensity
Fig. S6. Gemcitabine is not associated with increased cytokine or granzyme expression in lymphocytes
a, heatmap showing mean changes in cytokine/granzyme-B expression before and after gemcitabine (GEM) or best-supportive care (BSC) in different lymphocyte subsets. b, expression of intracellular effector molecules in different CD8+T-cell memory subsets on baseline. c, expression of IFN-γ by different CD8+T-cell memory subsets following gemcitabine. Wilcoxon matched-pairs signed rank tests were performed to calculate statistical significance. All included patients were used to compute b, whereas 22 gemcitabine-treated patients were included in c. GzmB = granzyme B, IFN-γ = interferon-gamma, IL-2 = interleukin 2, TNF-α = tumour-necrosis factor alpha, IL-10 = interleukin 10, NK = natural killer, Th = T-helper, Tn = naïve T cell, Tcm = central memory T cell, Tem = effector memory T cell, Temra = terminally differentiated T cell, ns = not significant.
Fig. S7. Parameters associated with clinical outcome in gemcitabine-treated patients are not detected in control patients
a-b, Kaplan–Meier (KM-) curves showing differences in progression free- (PFS) and overall survival (OS) between patients exhibiting an increase (green) or decrease (red) of selected immune parameters compared to baseline. Log-rank tests were used determine statistical significance. c, the median change in ICOS-expression in the total group (GEM + BSC) compared to baseline (+20%) is shown. NK = natural killer, PD-1 = programmed cell death protein 1, ICOS = inducible co-stimulatory molecule, HR = hazard ratio, CI = confidence interval.
Fig. S8. Flowchart illustrating inclusion of study samples for immunomonitoring
MDSC = myeloid-derived suppressor cell, BSC = best-supportive care.
Fig. S9. No differences in progression-free survival between immunomonitored patients and the complete NVALT19 cohort
Individual progression-free survival (PFS) KM-curves of all BSC- (blue) and gemcitabine (red) treated patients in the NVALT19-study (dotted line) those included in the immunomonitoring cohort (closed line) and not included (dashed line). The hazard ratio (HR) of the subgroup (0.62, 95% CI −1.487–0.137) is similar to that observed in the entire NVALT19 cohort (HR 0•48; 95% CI, 0•33–0•71; p = 0•0002).
Table S1. Patient and tumour characteristics of the included, and total NVALT19 cohort.
Table S2. Flow-cytometry panels used for the immune characterization studies.
In case of a; a backbone was constructed using the markers in the left column with additional co-inhibitory, -stimulatory and cytokine/granzyme markers being added in the individual panels. b, lymphocytes were excluded from myeloid cell analyses by selecting lineage (CD3, CD19 or CD56) negative cells.
References
- 1.Popat S., Curioni-Fontecedro A., Polydoropoulou V., Shah R., O'Brien M., Pope A. A multicentre randomized phase III trial comparing pembrolizumab (P) vs single agent chemotherapy (CT) for advanced pre-treated malignant pleural mesothelioma (MPM): results from the European Thoracic Oncology Platform (ETOP 9- 15) PROMISE-meso trial. Ann Oncol. 2019;30((Popat S.) doi: 10.1016/j.annonc.2020.09.009. Medicine, Royal Marsden Hospital, London, United Kingdom):v931. [DOI] [PubMed] [Google Scholar]
- 2.Maio M., Scherpereel A., Calabro L., Aerts J., Perez S.C., Bearz A. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): a multicentre, international, randomised, double-blind, placebo-controlled phase 2b trial. Lancet Oncol. 2017;19:e161–ee72. doi: 10.1016/S1470-2045(17)30446-1. [DOI] [PubMed] [Google Scholar]
- 3.Scherpereel A., Wallyn F., Albelda S.M., Munck C. Novel therapies for malignant pleural mesothelioma. Lancet Oncol. 2018;19(3):e161–e172. doi: 10.1016/S1470-2045(18)30100-1. [DOI] [PubMed] [Google Scholar]
- 4.de Gooijer C.J., van der Noort V., Stigt J.A., Baas P., Biesma B., Cornelissen R. Switch maintenance gemcitabine after first line chemotherapy in patients with malignant mesothelioma (NVALT19): an investigator-initiated randomised open label phase 2 trial. Lancet Respir Med. 2021 doi: 10.1016/S2213-2600(20)30362-3. [DOI] [PubMed] [Google Scholar]
- 5.Galluzzi L., Buque A., Kepp O., Zitvogel L., Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015;28(6):690–714. doi: 10.1016/j.ccell.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Aerts J., de Goeje P.L., Cornelissen R., Kaijen-Lambers M.E.H., Bezemer K., van der Leest C.H. Autologous dendritic cells pulsed with allogeneic tumor cell lysate in mesothelioma: from mouse to human. Clin Cancer Res. 2018;24(4):766–776. doi: 10.1158/1078-0432.CCR-17-2522. [DOI] [PubMed] [Google Scholar]
- 7.Zitvogel L., Kepp O., Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8(3):151–160. doi: 10.1038/nrclinonc.2010.223. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki E., Kapoor V., Jassar A.S., Kaiser L.R., Albelda S.M. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11(18):6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson E., Wenthe J., Irenaeus S., Loskog A., Ullenhag G. Gemcitabine reduces MDSCs, tregs and TGFbeta-1 while restoring the teff/treg ratio in patients with pancreatic cancer. J Transl Med. 2016;14(1):282. doi: 10.1186/s12967-016-1037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Homma Y., Taniguchi K., Nakazawa M., Matsuyama R., Mori R., Takeda K. Changes in the immune cell population and cell proliferation in peripheral blood after gemcitabine-based chemotherapy for pancreatic cancer. Clin Transl Oncol. 2014;16(3):330–335. doi: 10.1007/s12094-013-1079-0. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki E., Sun J., Kapoor V., Jassar A.S., Albelda S.M. Gemcitabine has significant immunomodulatory activity in murine tumor models independent of its cytotoxic effects. Cancer Biol Ther. 2007;6(6):880–885. doi: 10.4161/cbt.6.6.4090. [DOI] [PubMed] [Google Scholar]
- 12.Byrne M.J., Nowak A.K. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol. 2004;15(2):257–260. doi: 10.1093/annonc/mdh059. [DOI] [PubMed] [Google Scholar]
- 13.Rothman K.J. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46. [PubMed] [Google Scholar]
- 14.Miyara M., Yoshioka Y., Kitoh A., Shima T., Wing K., Niwa A. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30(6):899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Condamine T., Dominguez G.A., Youn J.I., Kossenkov A.V., Mony S., Alicea-Torres K. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci Immunol. 2016;1(2) doi: 10.1126/sciimmunol.aaf8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soeda A., Morita-Hoshi Y., Makiyama H., Morizane C., Ueno H., Ikeda M. Regular dose of gemcitabine induces an increase in CD14+ monocytes and CD11c+ dendritic cells in patients with advanced pancreatic cancer. Jpn J Clin Oncol. 2009;39(12):797–806. doi: 10.1093/jjco/hyp112. [DOI] [PubMed] [Google Scholar]
- 17.Ndhlovu L.C., Lopez-Verges S., Barbour J.D., Jones R.B., Jha A.R., Long B.R. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood. 2012;119(16):3734–3743. doi: 10.1182/blood-2011-11-392951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tallon de Lara P., Cecconi V., Hiltbrunner S., Yagita H., Friess M., Bode B. Gemcitabine synergizes with immune checkpoint inhibitors and overcomes resistance in a preclinical model and mesothelioma patients. Clin Cancer Res. 2018;24(24):6345–6354. doi: 10.1158/1078-0432.CCR-18-1231. [DOI] [PubMed] [Google Scholar]
- 19.Gurlevik E., Fleischmann-Mundt B., Brooks J., Demir I.E., Steiger K., Ribback S. Administration of gemcitabine after pancreatic tumor resection in mice induces an antitumor immune response mediated by natural killer cells. Gastroenterology. 2016;151(2):338–350. doi: 10.1053/j.gastro.2016.05.004. e7. [DOI] [PubMed] [Google Scholar]
- 20.Gravett A.M., Dalgleish A.G., Copier J. In vitro culture with gemcitabine augments death receptor and NKG2D ligand expression on tumour cells. Sci Rep. 2019;9(1):1544. doi: 10.1038/s41598-018-38190-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDonnell A.M., Lesterhuis W.J., Khong A., Nowak A.K., Lake R.A., Currie A.J. Tumor-infiltrating dendritic cells exhibit defective cross-presentation of tumor antigens, but is reversed by chemotherapy. Eur J Immunol. 2015;45(1):49–59. doi: 10.1002/eji.201444722. [DOI] [PubMed] [Google Scholar]
- 22.Nowak A.K., Lake R.A., Marzo A.L., Scott B., Heath W.R., Collins E.J. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol (Baltimore, Md : 1950) 2003;170(10):4905–4913. doi: 10.4049/jimmunol.170.10.4905. [DOI] [PubMed] [Google Scholar]
- 23.Liu W.M., Fowler D.W., Smith P., Dalgleish A.G. Pre-treatment with chemotherapy can enhance the antigenicity and immunogenicity of tumours by promoting adaptive immune responses. Br J Cancer. 2010;102(1):115–123. doi: 10.1038/sj.bjc.6605465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamphorst A.O., Pillai R.N., Yang S., Nasti T.H., Akondy R.S., Wieland A. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1–targeted therapy in lung cancer patients. Proc Natl Acad Sci. 2017;114(19):4993. doi: 10.1073/pnas.1705327114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang A.C., Postow M.A., Orlowski R.J., Mick R., Bengsch B., Manne S. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545(7652):60–65. doi: 10.1038/nature22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Proliferation is decreased in activated Treg and Foxp3-expressing Th-cells following gemcitabine treatment
a, Representative flow cytometry plot of regulatory T cells (Tregs) gated according to Miyara et al. using CD45RA and FoxP3. Activated Tregs (aTregs) are the highest expressers of Ki-67, CD39 and ICOS on baseline as compared to resting Tregs (rTregs) or FoxP3-positive T-helper cells (bar graphs showing means with SEM). Wilcoxon matched-pairs signed rank tests were performed to calculate statistical significance. A total of 22 gemcitabine-treated patients was included in the analysis. b, Proliferation as determined by Ki-67 expression between the different FoxP3-expressing CD4+T-cell subsets. hi = high, int = intermediate, MFI = mean fluorescent intensity, ns = not significant, *** = p<0.001, **** = p<0.0001.
Fig. S2. Gemcitabine does not alter T-cell memory phenotype.
Representative dot plot of T-cell memory subsets (CD8+T-cells are shown) based on the membrane markers CD45RA and CCR7. A heatmap displays the mean changes in proportions of T-cell memory subsets following gemcitabine or best-supportive care (BSC). CCR = chemokine C-receptor, Tn = naïve T cell, Tcm = central memory T cell, Tem = effector memory T cell, Temra = terminally differentiated T cell, ns = not significant.
Fig. S3. Plasmacytoid DCs are increased in peripheral blood following gemcitabine.
Changes in monocyte- and dendritic cell (DC)-subsets following gemcitabine or best-supportive care (BSC) in a subset of patients from figure 2. Wilcoxon matched-pairs signed rank tests were performed to calculate statistical significance. (n = 11 GEM- and n = 12 BSC-treated patients included). cDC = conventional dendritic cell, pDC = plasmacytoid dendritic cell, ns = not significant, ** = p<0.01.
Fig. S4. Similar effects of gemcitabine on immune cells when expressed changes in percentage positive
Change in percentages of PD-1, CTLA-4 and ICOS on CD8+T cells following gemcitabine as opposed to MFI depicted in Fig. 3. A correlation is shown between PD-1 MFI and percentage positive in CD8+T cells (both gemcitabine and best-supportive care patients included). Wilcoxon matched-pairs signed rank tests were performed to calculate statistical significance. A total of 22 gemcitabine-treated patients was included in the analysis. Pearson correlation coefficient was calculated in case of MFI vs percentage PD-1 positive and a Pearson's r was generated. PD-1 = programmed cell death protein 1, CTLA-4 = cytotoxic T-lymphocyte-associated protein 4, ICOS = inducible co-stimulatory molecule, MFI = mean fluorescent intensity, ns = not significant, * = p<0.05, *** = p<0.001, **** = p<0.0001.
Fig. S5. No correlations between lymphocyte receptor expression and Treg proliferation or MDSCs
a, Correlations between the changes in PD-1/CTLA-4/ICOS MFI with changes in MDSCs and b, Treg-proliferation in peripheral blood following gemcitabine (GEM) treatment. Spearman correlation coefficients were calculated and a Rho was generated for 17 gemcitabine-treated patients of whom matched T-cells and MDSCs were available. MDSC = myeloid derived suppressor cell, Treg = regulatory T cell, PD-1 = programmed cell death protein 1, CTLA-4 = cytotoxic T-lymphocyte-associated protein 4, ICOS = inducible co-stimulatory molecule, MFI = mean fluorescent intensity
Fig. S6. Gemcitabine is not associated with increased cytokine or granzyme expression in lymphocytes
a, heatmap showing mean changes in cytokine/granzyme-B expression before and after gemcitabine (GEM) or best-supportive care (BSC) in different lymphocyte subsets. b, expression of intracellular effector molecules in different CD8+T-cell memory subsets on baseline. c, expression of IFN-γ by different CD8+T-cell memory subsets following gemcitabine. Wilcoxon matched-pairs signed rank tests were performed to calculate statistical significance. All included patients were used to compute b, whereas 22 gemcitabine-treated patients were included in c. GzmB = granzyme B, IFN-γ = interferon-gamma, IL-2 = interleukin 2, TNF-α = tumour-necrosis factor alpha, IL-10 = interleukin 10, NK = natural killer, Th = T-helper, Tn = naïve T cell, Tcm = central memory T cell, Tem = effector memory T cell, Temra = terminally differentiated T cell, ns = not significant.
Fig. S7. Parameters associated with clinical outcome in gemcitabine-treated patients are not detected in control patients
a-b, Kaplan–Meier (KM-) curves showing differences in progression free- (PFS) and overall survival (OS) between patients exhibiting an increase (green) or decrease (red) of selected immune parameters compared to baseline. Log-rank tests were used determine statistical significance. c, the median change in ICOS-expression in the total group (GEM + BSC) compared to baseline (+20%) is shown. NK = natural killer, PD-1 = programmed cell death protein 1, ICOS = inducible co-stimulatory molecule, HR = hazard ratio, CI = confidence interval.
Fig. S8. Flowchart illustrating inclusion of study samples for immunomonitoring
MDSC = myeloid-derived suppressor cell, BSC = best-supportive care.
Fig. S9. No differences in progression-free survival between immunomonitored patients and the complete NVALT19 cohort
Individual progression-free survival (PFS) KM-curves of all BSC- (blue) and gemcitabine (red) treated patients in the NVALT19-study (dotted line) those included in the immunomonitoring cohort (closed line) and not included (dashed line). The hazard ratio (HR) of the subgroup (0.62, 95% CI −1.487–0.137) is similar to that observed in the entire NVALT19 cohort (HR 0•48; 95% CI, 0•33–0•71; p = 0•0002).
Table S1. Patient and tumour characteristics of the included, and total NVALT19 cohort.
Table S2. Flow-cytometry panels used for the immune characterization studies.
In case of a; a backbone was constructed using the markers in the left column with additional co-inhibitory, -stimulatory and cytokine/granzyme markers being added in the individual panels. b, lymphocytes were excluded from myeloid cell analyses by selecting lineage (CD3, CD19 or CD56) negative cells.