Abstract
Programmed cell-death ligand 1 (PD-L1) has been shown to induce potent T-cell mediated anti-tumoral immunity. The significance of PD-L1 expression in the prognosis of breast cancer (BC) remains controversial and its prevalence and prognostic value in breast cancer from Middle Eastern ethnicity is lacking. A total of 1003 unselected Middle Eastern breast cancers were analyzed for PD-L1 expression using immunohistochemistry. PD-L1 expression, seen in 32.8% (329/1003) of cases, was significantly associated with poor prognostic indicators such as younger patients, high-grade tumors, estrogen-receptor (ER)-negative, progesterone-receptor (PR)-negative, and triple-negative breast cancers (TNBC) as well as high Ki-67 index. We also found a significant association between PD-L1 expression and deficient mismatch repair protein expression. No association was found between PD-L1 expression and clinical outcome. However, on further subgroup analysis, PD-L1 expression was found to be an independent marker for favorable overall survival and recurrence-free survival in TNBC. In conclusion, we demonstrated strong association between PD-L1 and mismatch repair deficiency in Middle Eastern BC patients and that PD-L1 overexpression in tumor cells was an independent prognostic marker in TNBCs from Middle Eastern ethnicity. Overall, these findings might help in the development of more appropriate treatment strategies for BC in Middle Eastern population.
Keywords: PD-L1, breast cancer, triple negative breast cancer, prognosis
1. Introduction
Breast cancer (BC) incidence in Saudi Arabia is on the rise. A unique characteristic of breast cancer in this population is the relatively younger age of disease onset, where a large number of patients present with invasive ductal carcinoma before the age of 50 years [1]. Despite the advances in therapeutic modalities for BC, distant metastasis or recurrence have occurred in more than 50% of patients with invasive breast cancer, resulting in treatment failure [2,3]. Therefore, there is an urgent need to identify molecular biomarker targets that could help in introducing new therapeutic approaches to specific patient sub-groups.
Immunotherapy is one of the most encouraging finding of cancer therapy in recent years [4,5,6]. One of the most common mechanisms underlying immunotherapy is programmed cell-death protein-1 (PD-1) and programmed cell-death ligand-1 (PD-L1), which serve as immune checkpoints in the tumor microenvironment [7,8]. Upon activation, the PD-1/PD-L1 axis induces functional impairment of antigen-specific T-cells, thus shielding the tumor cells from T-cell mediated killing [9,10,11]. PD-1/PD-L1 pathway inhibitors have achieved great success in clinical trials for patients with various types of cancer, and have been approved for use in clinical practice for patients with several cancers, including triple-negative breast cancer (TNBC) [7,12,13,14,15].
PD-L1 expression has been reported as an important prognostic biomarker in multiple studies, although its prognostic significance varied according to tumor type [16,17,18,19]. In breast cancer, association of PD-L1 overexpression with prognosis has revealed conflicting data. While some reports have noted that overexpression of PD-L1 is associated with worse prognosis [20,21,22], others have found PD-L1 expression to be associated with favorable prognosis and correlated with longer disease-free survival, especially in TNBC patients [23,24,25,26]. Furthermore, the prevalence and the predictive role of PD-L1 expression in breast cancer from Middle Eastern ethnicity has not been explored previously.
Therefore, we conducted this study to evaluate PD-L1 expression on more than 1000 breast cancer tissues from Middle Eastern ethnicity, surgically removed in a single institute, and assessed the correlation of PD-L1 expression with several clinico-pathological and molecular markers. Furthermore, the effect of PD-L1 on clinical outcome was explored to determine its potential as a biomarker for Middle Eastern BC patients’ prognosis.
2. Materials and Methods
2.1. Patient Samples and Data Collection
One thousand and nine patients with breast cancer diagnosed between 1990 and 2011 were selected from the files of the King Faisal Specialist Hospital and Research Centre (KFSHRC). The patients included in this study had their diagnosis, treatment, and follow-up care in the Department of Surgical Oncology at KFSHRC. The histologic subtype of each breast tumor sample was determined according to World Health Organization (WHO) criteria. Detailed clinico-pathological data, including follow-up data, were noted from case records and summarized in Table 1. Waiver of consent was obtained for the study from the Institutional Review Board and Research Ethics Committee of KFSHRC under Project RAC# 2140 008 on breast cancer archival clinical samples.
Table 1.
Clinico-pathological variables for the patient cohort (n = 1009).
| Clinico-Pathologic Variables | n (%) |
|---|---|
| Age (years) | |
| ≤50 | 686 (68.0) |
| >50 | 323 (32.0) |
| Median (in years) | 45.0 |
| Range (IQR) ^ | 39.0–54.0 |
| Histological Type | |
| Infiltrating ductal carcinoma | 913 (90.5) |
| Infiltrating lobular carcinoma | 44 (4.4) |
| Mucinous carcinoma | 16 (1.6) |
| Others | 36 (3.5) |
| Tumor stage | |
| I | 91 (9.0) |
| II | 401 (39.7) |
| III | 379 (37.6) |
| IV | 91 (9.0) |
| Unknown | 47 (4.7) |
| Histologic grade | |
| Well-differentiated | 77 (7.6) |
| Moderately differentiated | 514 (50.9) |
| Poorly differentiated | 405 (40.2) |
| Unknown | 13 (1.3) |
| Estrogen receptor | |
| Positive | 662 (65.6) |
| Negative | 346 (34.3) |
| Unknown | 1 (0.1) |
| Progesterone receptor | |
| Positive | 579 (57.4) |
| Negative | 426 (42.2) |
| Unknown | 4 (0.4) |
| Her-2 neu | |
| Positive | 379 (37.6) |
| Negative | 628 (62.2) |
| Unknown | 2 (0.2) |
| Triple negative breast cancer | |
| Yes | 149 (14.8) |
| No | 852 (84.4) |
| Unknown | 8 (0.8) |
| Survival duration (in months) | |
| Median | 48.0 |
| Range (IQR) ^ | 26.0–74.0 |
^ IQR, inter quartile range.
2.2. Tissue Microarray (TMA) Construction
TMA construction was performed as described earlier [27]. Briefly, tissue cylinders with a diameter of 0.6 mm were punched from representative tumor regions of each donor tissue block and brought into a recipient paraffin block using a modified semiautomatic robotic precision instrument (Beecher Instruments, Woodland, WI). Two cores of breast cancer were arrayed from each case.
2.3. Immunohistochemistry (IHC) Staining and Evaluation
Standard protocol was followed for manual IHC staining. For antigen retrieval, Dako (Dako Denmark A/S, Glostrup, Denmark) Target Retrieval Solution pH 9.0 (Catalog number S2368) was used, and the slides were placed in a Pascal pressure cooker at 120 °C for 10 min. Primary antibody against PD-L1 (E1L3N, 1:50 dilution, pH 9.0, Cell Signaling Technology, Danvers, MA) was used. The Dako Envision Plus System kit was used as the secondary detection system with 3, 30-diaminobenzidine as chromogen. All slides were counterstained with hematoxylin, dehydrated, cleared, and mounted. Normal tissues of different organ systems were also included in the TMA to serve as positive controls. Negative control was performed by omission of the primary antibody. Only freshly cut slides were stained simultaneously to minimize the influence of slide aging and maximize reproducibility of the experiment. The slides were independently examined by two pathologists. If there was a discrepancy in the individual scores, both pathologists carried out a re-evaluation until a consensus was reached.
A membranous and/or cytoplasmic staining was observed. Only the membrane staining was considered for scoring. PD-L1 was scored as described previously [28]. Scoring was done for tumor cells only. Briefly, the proportion of positively stained cells were calculated as a percentage for each core and the scores were averaged across two tissue cores from the same tumor to yield a single percent staining score representing each cancer patient. For the purpose of statistical analysis, the scores were dichotomized. Cases showing expression level of ≥5% were classified as positive for PD-L1 and those with less than 5% as negative.
Staining and scoring of estrogen-receptor (ER), progesterone-receptor (PR), Her-2 neu, Ki-67, and mismatch repair proteins was performed as described previously [29,30,31].
2.4. Statistical Analysis
The associations between clinico-pathological variables and protein expression was performed using contingency table analysis and Chi-square tests. The Mantel–Cox log-rank test was used to evaluate overall survival and recurrence-free survival. Survival curves were generated using the Kaplan–Meier method. The Cox proportional hazards regression model was used for multivariate analysis. Age, histologic subtype, tumor grade, lymph node metastasis, and tumor stage were included as covariates, since they are well-known prognostic factors in breast cancer. Two-sided tests were used for statistical analyses with a limit of significance defined as p value < 0.05. Data analyses were performed using the JMP11.0 (SAS Institute, Inc., Cary, NC) software package.
3. Results
3.1. PD-L1 Expression in Breast Cancer and Its Clinico-Pathological Associations
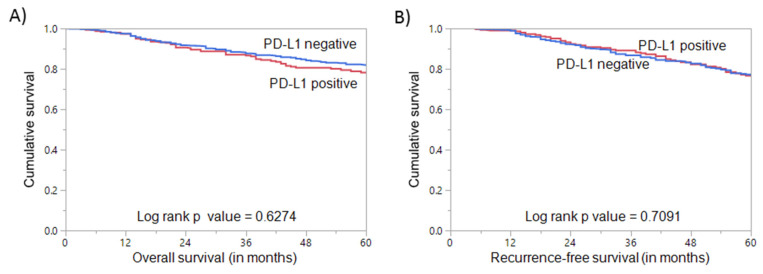
PD-L1 protein expression was analyzed immunohistochemically in 1009 BC samples. However, six cases were excluded due to missing tissue cores in the TMA. Hence, 1003 samples were included for further analysis. PD-L1 expression ranged from 0–100% (median = 0%). Using a cut-off of ≥5%, PD-L1 expression was noted in 32.8% (329/1009) of BC (Figure 1A,B) and found to be associated with adverse clinico-pathological parameters such as younger age (p = 0.0432), higher grade (p = 0.0025), ER-negative (p < 0.0001), PR-negative (p = 0.0001), and triple-negative (p = 0.0062) breast cancers, as well as a high proliferative index (Ki-67) (p < 0.0001). We also found a significant association between PD-L1 expression and deficient mismatch repair (dMMR) protein expression (p = 0.0009) (Table 2). However, no significant association was found between PD-L1 expression and overall survival (OS) (p = 0.6274) or recurrence-free survival (RFS) (0.7091) in the entire cohort (Figure 2).
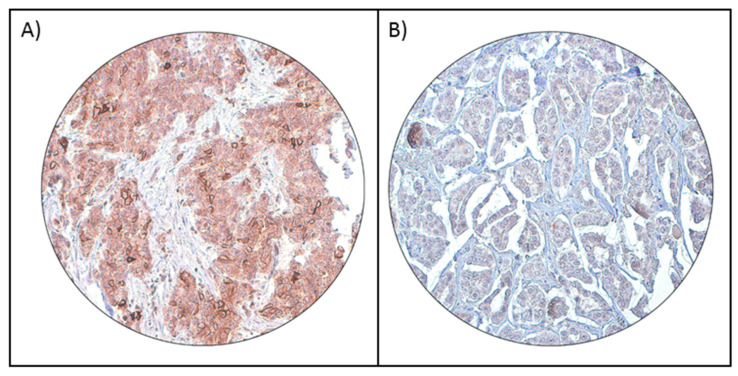
Figure 1.
Programmed cell-death ligand 1 (PD-L1) immunohistochemical staining in breast cancer tissue microarray (TMA). Representative examples of tumors showing (A) positive and (B) negative (right panel) membrane staining of PD-L1 (20 X/0.70 objective on an Olympus BX 51 microscope (Olympus America Inc, Center Valley, PA, USA)).
Table 2.
Correlation of PD-L1 protein expression with clinico-pathological parameters in breast cancer.
| Clinico-Pathologic Variables | Total | PD-L1 Positive | PD-L1 Negative | p Value | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Total Number of Cases | 1003 | 329 | 32.8 | 674 | 67.2 | ||
| Age Groups | |||||||
| ≤50 | 680 | 67.8 | 237 | 34.9 | 443 | 65.1 | 0.0432 * |
| >50 | 323 | 32.2 | 92 | 28.5 | 231 | 71.5 | |
| Histology | |||||||
| Infiltrating ductal carcinoma | 909 | 94.0 | 296 | 32.6 | 613 | 67.4 | 0.6176 |
| Infiltrating lobular carcinoma | 42 | 4.3 | 11 | 26.2 | 31 | 73.8 | |
| Mucinous carcinoma | 16 | 1.7 | 6 | 37.5 | 10 | 62.5 | |
| Histological Grade | |||||||
| Well-differentiated | 76 | 7.7 | 17 | 22.4 | 59 | 77.6 | 0.0025 * |
| Moderately differentiated | 511 | 51.5 | 151 | 29.6 | 360 | 70.4 | |
| Poorly differentiated | 404 | 40.8 | 155 | 38.4 | 249 | 61.6 | |
| pT | |||||||
| T1 | 213 | 22.1 | 68 | 31.9 | 145 | 68.1 | 0.8039 |
| T2 | 484 | 50.2 | 163 | 33.7 | 321 | 66.3 | |
| T3 | 143 | 14.8 | 42 | 29.4 | 101 | 70.6 | |
| T4 | 124 | 12.9 | 40 | 32.3 | 84 | 67.7 | |
| pN | |||||||
| N0 | 307 | 33.2 | 102 | 33.2 | 205 | 66.8 | 0.2121 |
| N1 | 297 | 32.1 | 102 | 34.3 | 195 | 65.7 | |
| N2 | 192 | 20.8 | 50 | 26.0 | 142 | 74.0 | |
| N3 | 128 | 13.9 | 44 | 34.4 | 84 | 65.6 | |
| pM | |||||||
| M0 | 808 | 89.9 | 265 | 32.8 | 543 | 67.2 | 0.2968 |
| M1 | 91 | 10.1 | 25 | 27.5 | 66 | 72.5 | |
| Tumor Stage | |||||||
| I | 91 | 9.5 | 33 | 36.3 | 58 | 63.7 | 0.6161 |
| II | 398 | 41.5 | 128 | 32.2 | 270 | 67.8 | |
| III | 378 | 39.5 | 126 | 33.3 | 252 | 66.7 | |
| IV | 91 | 9.5 | 25 | 27.5 | 66 | 72.5 | |
| Estrogen Receptor | |||||||
| Positive | 656 | 65.5 | 184 | 28.1 | 472 | 71.9 | <0.0001 * |
| Negative | 346 | 34.5 | 145 | 41.9 | 201 | 58.1 | |
| Progesterone Receptor | |||||||
| Positive | 575 | 57.6 | 161 | 28.0 | 414 | 72.0 | 0.0001 * |
| Negative | 424 | 42.4 | 168 | 39.6 | 256 | 60.4 | |
| Her-2 neu | |||||||
| Positive | 379 | 37.9 | 131 | 34.6 | 248 | 65.4 | 0.3729 |
| Negative | 622 | 62.1 | 198 | 31.8 | 424 | 68.2 | |
| Triple-Negative Breast Cancer | |||||||
| Yes | 149 | 15.0 | 64 | 43.0 | 85 | 57.0 | 0.0062 * |
| No | 846 | 85.0 | 265 | 31.3 | 581 | 68.7 | |
| Ki-67 | |||||||
| High | 630 | 64.1 | 238 | 37.8 | 392 | 62.2 | <0.0001 * |
| Low | 352 | 35.9 | 88 | 25.0 | 264 | 75.0 | |
| MMR Protein Expression | |||||||
| Deficient MMR | 33 | 3.3 | 20 | 60.6 | 13 | 39.4 | 0.0009 * |
| Proficient MMR | 970 | 96.7 | 309 | 31.9 | 661 | 68.1 | |
*, significant p value; MMR—mismatch repair.
Figure 2.
Survival analysis of PD-L1 protein expression in breast cancer. Kaplan–Meier survival plot showing no statistically significant difference between PD-L1 positive and negative tumors for (A) overall survival (p = 0.6274) and (B) recurrence-free survival (p = 0.7091).
3.2. PD-L1 Expression in Triple-Negative Breast Cancer
Since several previous studies have noted an association between PD-L1 expression and TNBC, we sought to analyze the clinico-pathological associations and prognostic impact of PD-L1 in this sub-group of BCs. PD-L1 over-expression was seen in 45.0% (67/149) of TNBCs and was significantly associated with lymph node metastasis (p = 0.0459) (Table 3). No associations were found with other clinico-pathological variables.
Table 3.
Correlation of PD-L1 protein expression with clinico-pathological parameters in triple-negative breast cancer.
| Clinico-Pathologic Variables | Total | PD-L1 Positive | PD-L1 Negative | p Value | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Total Number of Cases | 149 | 67 | 45.0 | 82 | 55.0 | ||
| Age Groups | |||||||
| ≤50 | 113 | 75.8 | 52 | 46.0 | 61 | 54.0 | 0.6471 |
| >50 | 36 | 24.2 | 15 | 41.7 | 21 | 58.3 | |
| Histology | |||||||
| Infiltrating Ductal Carcinoma | 139 | 98.6 | 61 | 43.9 | 78 | 56.1 | 0.0711 |
| Infiltrating Lobular Carcinoma | 2 | 1.4 | 2 | 100.0 | 0 | 0.0 | |
| Histological Grade | |||||||
| Moderately differentiated | 41 | 27.7 | 15 | 36.6 | 26 | 63.4 | 0.2225 |
| Poorly differentiated | 107 | 72.3 | 51 | 47.7 | 56 | 52.3 | |
| pT | |||||||
| T1 | 23 | 16.2 | 11 | 47.8 | 12 | 52.2 | 0.6633 |
| T2 | 75 | 52.8 | 33 | 44.0 | 42 | 56.0 | |
| T3 | 22 | 15.5 | 12 | 54.5 | 10 | 45.5 | |
| T4 | 22 | 15.5 | 8 | 36.4 | 14 | 63.6 | |
| pN | |||||||
| N0 | 59 | 44.0 | 22 | 37.3 | 37 | 62.7 | 0.0459 * |
| N1 | 40 | 29.8 | 25 | 62.5 | 15 | 37.5 | |
| N2 | 21 | 15.7 | 7 | 33.3 | 14 | 66.7 | |
| N3 | 14 | 10.5 | 5 | 35.7 | 9 | 64.3 | |
| pM | |||||||
| M0 | 114 | 83.8 | 53 | 46.5 | 61 | 53.5 | 0.1987 |
| M1 | 22 | 16.2 | 7 | 31.8 | 15 | 68.2 | |
| Tumor Stage | |||||||
| I | 13 | 9.3 | 8 | 61.5 | 5 | 38.5 | 0.3864 |
| II | 57 | 40.7 | 26 | 45.6 | 31 | 54.4 | |
| III | 48 | 34.3 | 21 | 43.8 | 27 | 56.2 | |
| IV | 22 | 15.7 | 7 | 31.8 | 15 | 68.2 | |
| Ki-67 | |||||||
| High | 137 | 91.9 | 61 | 44.5 | 76 | 55.5 | 0.7153 |
| Low | 12 | 8.1 | 6 | 50.0 | 6 | 50.0 | |
| MMR Protein Expression | |||||||
| Deficient MMR | 4 | 2.7 | 3 | 75.0 | 1 | 25.0 | 0.2159 |
| Proficient MMR | 145 | 97.3 | 64 | 44.1 | 81 | 55.9 | |
| Overall Survival | 83.1 | 62.3 | 0.0226 * | ||||
| Recurrence-Free Survival | 81.5 | 64.6 | 0.0169 * | ||||
*, significant p value; MMR—mismatch repair.
3.3. PD-L1 Expression and Clinical Outcome in Triple Negative Breast Cancer
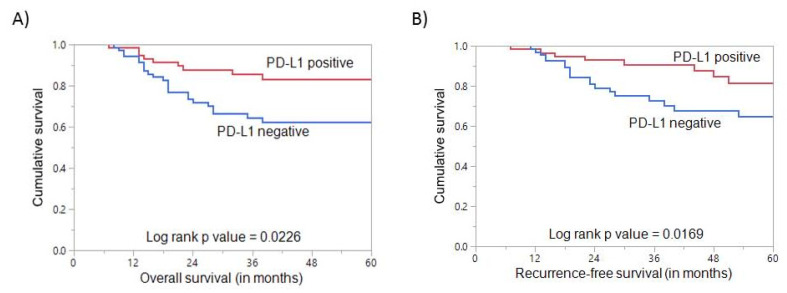
PD-L1 positive TNBCs were found to have a favorable impact on OS (p = 0.0226, Table 3, Figure 3A). On multivariate analysis, PD-L1 was an independent prognostic indicator of OS (HR = 0.28, 95% CI = 0.11–0.64, p = 0.0043) (Table 4). Patients with PD-L1 positive TNBCs were also found to have a favorable RFS (p = 0.0169, Table 3, Figure 3B). On multivariate analysis, PD-L1 expression was also an independent predictor of favorable RFS (HR = 0.31, 95% CI = 0.13–0.67, p = 0.0043) (Table 4).
Figure 3.
Survival analysis of PD-L1 protein expression in triple negative breast cancer. (A) Kaplan–Meier survival plot showing statistically significant good overall survival in PD-L1 positive tumors compared to PD-L1 negative (p = 0.0226) (B) Kaplan–Meier survival plot showing statistically significant good recurrence-free survival for PD-L1 positive tumors (p = 0.0169).
Table 4.
Univariate and multivariate analysis of clinico-pathological variables and PD-L1 expression using the Cox proportional hazard model for overall survival and recurrence-free survival in triple-negative breast cancer.
| Overall Survival | Recurrence-Free Survival | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||||
| Clinico-Pathological Variables | Number of Events per Covariate | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | Number of Events per Covariate | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value |
|
Age (years) >50 ( vs. ≤ 50) |
10 (vs. 27) | 0.73 (0.29–1.57) | 0.4519 | 0.27 (0.09–0.80) | 0.0175 * | 11 (vs. 28) | 0.74 (0.30–1.60) | 0.4821 | 0.35 (0.11–0.86) | 0.0355 * |
|
Histology IDC (vs. others) |
26 (vs. 11) | 0.48 (0.03–2.23) | 0.4706 | 0.81 (0.10–6.67) | 0.8426 | 28 (vs. 11) | 0.26 (0.01–1.27) | 0.1922 | 0.18 (0.01–1.03) | 0.1199 |
|
Grade 3 (vs. 1–2) |
25 (vs. 12) | 0.61 (0.31–1.27) | 0.1660 | 0.54 (0.25–1.18) | 0.1241 | 29 (vs. 10) | 1.02 (0.47–2.54) | 0.9716 | 0.68 (0.28–1.82) | 0.4119 |
|
Lymph Node Metastasis N1-3 (vs. N0) |
25 (vs. 12) | 3.34 (1.52–8.38) | 0.0050 * | 4.98 (1.95–12.75) | 0.0008 * | 24 (vs. 15) | 1.82 (0.92–3.79) | 0.0943 | 2.70 (1.31–5.87) | 0.0089 * |
|
Stage IV (vs. I–III) |
11 (vs. 26) | 3.91 (1.82–7.92) | 0.0002 * | 2.80 (1.21–6.45) | 0.0159 * | 10 (vs. 29) | 0.82 (0.20–2.33) | 0.7481 | 0.76 (0.18–2.23) | 0.6593 |
|
PD-L1 Positive (vs. Negative) |
11 (vs. 26) | 0.45 (0.21–0.89) | 0.0272 * | 0.28 (0.11–0.64) | 0.0043 * | 14 (vs. 25) | 0.42 (0.20–0.86) | 0.0205 * | 0.31 (0.13–0.67) | 0.0043 * |
*, significant p value.
4. Discussion
It has become widely recognized that the interaction of PD-1 and PD-L1 plays an important role in immune evasion by tumors, and PD-L1 expression in tumor tissue may be a good marker to predict the efficacy of anti-PD-L1 antibodies [32,33]. The importance of PD-L1 expression in tumor tissues as prognostic marker has not reached consensus, and data on its prognostic value in BC from Middle Eastern ethnicity is completely lacking.
In this study, we analyzed PD-L1 expression and its association with clinico-pathological characteristics. PD-L1 expression was seen in 32.8% (329/1003) of the BC cases, which is in alignment with reported PD-L1 positivity rate ranging from 17%–56% in existing literature [23,34,35,36,37]. Two of these five studies reported PD-L1 expression in whole tissue sections, whereas the other three studies analyzed PD-L1 expression in TMAs. This variation reported in PD-L1 expression can be attributed to sample size, variations in tissue preparation, use of different antibody clones, cut-off values and interpretation of IHC results. By analyzing clinico-pathological data, we found significant correlation between PD-L1 expression and higher grade (p = 0.0025), younger patients (p = 0.0432), higher Ki-67 index (>30%, p < 0.0001), hormone-receptor-negative (ER–PR) tumors (p = 0.0001), and TNBC (p = 0.0062). Despite the association with poor prognostic features observed in the overall cohort, we were unable to establish an association between tumor PD-L1 positivity and clinical outcome (OS and RFS). Importantly, PD-L1 positivity was significantly correlated with dMMR. Analogously, significant association between PD-L1 protein expression and dMMR was observed in a previous report [38]. This association is of important clinical relevance since dMMR seems to have strong connection with PD-L1 expression and PD-1/PD-L1 blockade therapy as evidenced by the fact that pembrolizumab was approved for many types of solid tumors with dMMR [39,40].
Upon further stratification, based on breast cancer subtype, PD-L1 expression as expected was higher in the TNBC than in other subtypes. This can be explained by the increased immunogenicity of TNBC which has been previously reported [41]. Most importantly, PD-L1 expression in TNBC subgroup was inversely associated with lymph node metastasis and significantly associated with favorable overall survival and better disease-free survival. This association with favorable patient outcome remains significant when considering all the factors using multivariate analysis. One explanation for the favorable prognosis could be due to a lower proportion of M1 patients and a higher fraction of stage I patients in the PD-L1 positive group. Recent studies have reported that PD-1, but not PD-L1, predicted good prognosis in TNBCs [42,43]. Additional studies also reported positive prognostic and predictive values of PD-L1 expression in immune and/or tumor cells in TNBC [25,26,44]. On the contrary, a study from China [45], which also analyzed PD-L1 expression in tumor cells alone, showed a worse prognosis in TNBC patients expressing PD-L1. In our study, we focused on PD-L1 expression in tumor cells only and were able to identify the prognostic and predictive role in TNBC patients. The association between PD-L1 expression and improved outcomes in TNBC can be partially explained by the fact that TNBC is immunologically active [46,47] or the increased chemosensitivity in immune-active TNBC [48,49].
While this study provides important information with potential impact in clinical practice about BC from Middle Eastern ethnicity, it has several limitations. First, the use of TMA, as many miss true protein expression due to intra-tumor heterogeneity, although we minimize this limitation by sampling from two representative areas in each tumor. Second, this study is retrospective, and a single-center study. Third, the majority (91%) of patients with advanced (Stage II or higher) BC were included, which might affect the influence of PD-L1 expression on prognosis. Lastly, immunohistochemical double staining with pan-cytokeratin could more accurately differentiate PD-L1 expression on tumor cells, thereby increasing the accuracy and precision of the PD-L1 measure.
5. Conclusions
In conclusion, our study shows that PD-L1 was an independent prognostic factor in the TNBC subgroup of BC but not in the overall cohort. Additionally, we demonstrated strong association between PD-L1 expression and mismatch repair deficiency in Middle Eastern BC patients. Overall, these findings might help in the development of more appropriate treatment strategies for BC in Middle Eastern population. Since results of PDL1 in TNBC have been contradictory, and this particular study is the first study in Middle eastern ethnicity, additional studies are needed to verify our study results.
Acknowledgments
The authors would like to thank Padmanaban Annaiyappanaidu and Felisa DeVera for their technical assistance.
Author Contributions
Conceptualization, K.S.A.-K. and A.K.S.; methodology, S.K.P. and A.K.S.; software, S.K.P.; validation, S.K.P., S.O.A., and L.O.G.; formal analysis, S.K.P. and A.K.S.; investigation, S.K.P., S.O.A., L.O.G., and S.M.A.; resources, F.A.-D., A.T., and D.A.; data curation, S.K.P. and S.M.A.; writing—K.S.A.-K.; writing—review and editing, S.K.P. and A.K.S.; visualization, S.K.P. and A.K.S.; supervision, K.S.A.-K. and A.K.S.; project administration, K.S.A.-K. and A.K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alrawaji A., Alshahrani Z., Alzahrani W., Alomran F., Almadouj A., Alshehri S., Alzahrani A., Bazarbashi S., Alhashmi H., Almutlaq H., et al. Cancer Incidence Report Saudi Arabia 2015. Saudi Cancer Registry, Saudi Health Council; Riyadh, Saudi Arabia: 2018. [Google Scholar]
- 2.Weigelt B., Peterse J.L., Veer L.J.V. Breast cancer metastasis: Markers and models. Nat. Rev. Cancer. 2005;5:591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 3.Colleoni M., Sun Z., Price K.N., Karlsson P., Forbes J.F., Thürlimann B., Gianni L., Castiglione M., Gelber R.D., Coates A.S., et al. Annual Hazard Rates of Recurrence for Breast Cancer During 24 Years of Follow-Up: Results from the International Breast Cancer Study Group Trials I to V. J. Clin. Oncol. 2016;34:927–935. doi: 10.1200/JCO.2015.62.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yasunaga M. Antibody therapeutics and immunoregulation in cancer and autoimmune disease. Semin. Cancer Biol. 2020;64:1–12. doi: 10.1016/j.semcancer.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Darvin P., Toor S.M., Nair V.S., Elkord E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018;50:1–11. doi: 10.1038/s12276-018-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Y. Cancer immunotherapy: Harnessing the immune system to battle cancer. J. Clin. Investig. 2015;125:3335–3337. doi: 10.1172/JCI83871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zou W., Wolchok J.D., Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 2016;8:328rv4. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J., Jiang C.C., Jin L., Zhang X.D. Regulation of PD-L1: A novel role of pro-survival signalling in cancer. Ann. Oncol. 2016;27:409–416. doi: 10.1093/annonc/mdv615. [DOI] [PubMed] [Google Scholar]
- 9.Ahmadzadeh M., Johnson L.A., Heemskerk B., Wunderlich J.R., Dudley M.E., White D.E., Rosenberg S.A. Tumor antigen–specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han Y., Liu D., Li L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020;10:727. [PMC free article] [PubMed] [Google Scholar]
- 11.He J., Hu Y., Hu M., Li B. Development of PD-1/PD-L1 Pathway in Tumor Immune Microenvironment and Treatment for Non-Small Cell Lung Cancer. Sci. Rep. 2015;5:srep13110. doi: 10.1038/srep13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Disis M.L., Taylor M.H., Kelly K., Beck J.T., Gordon M., Moore K.M., Patel M.R., Chaves J., Park H., Mita A.C. Efficacy and safety of avelumab for patients with recurrent or refractory ovarian cancer: Phase 1b results from the JAVELIN solid tumor trial. JAMA Oncol. 2019;5:393–401. doi: 10.1001/jamaoncol.2018.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheng X., Yan X., Chi Z., Si L., Cui C., Tang B., Li S., Mao L., Lian B., Wang X., et al. Axitinib in Combination with Toripalimab, a Humanized Immunoglobulin G4 Monoclonal Antibody Against Programmed Cell Death-1, in Patients with Metastatic Mucosal Melanoma: An Open-Label Phase IB Trial. J. Clin. Oncol. 2019;37:2987–2999. doi: 10.1200/JCO.19.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmid P., Adams S., Rugo H.S., Schneeweiss A., Barrios C.H., Iwata H., Diéras V., Hegg R., Im S.A., Shaw Wright G., et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018;379:2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 15.Dang T.O., Ogunniyi A., Barbee M.S., Drilon A. Pembrolizumab for the treatment of PD-L1 positive advanced or metastatic non-small cell lung cancer. Expert Rev. Anticancer. Ther. 2016;16:13–20. doi: 10.1586/14737140.2016.1123626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu P., Wu D., Li L., Chai Y., Huang J. PD-L1 and Survival in Solid Tumors: A Meta-Analysis. PLoS ONE. 2015;10:e0131403. doi: 10.1371/journal.pone.0131403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y., Liang L., Dai W., Cai G., Xu Y., Li X., Li Q., Cai S. Prognostic impact of programed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor infiltrating lymphocytes in colorectal cancer. Mol. Cancer. 2016;15:1–15. doi: 10.1186/s12943-016-0539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aust S., Felix S., Auer K., Bachmayr-Heyda A., Kenner L., Dekan S., Meier S.M., Gerner C., Grimm C., Pils D. Absence of PD-L1 on tumor cells is associated with reduced MHC I expression and PD-L1 expression increases in recurrent serous ovarian cancer. Sci. Rep. 2017;7:srep42929. doi: 10.1038/srep42929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu W., Hua Y., Qiu H., Hao J., Zou K., Li Z., Hu S., Guo P., Chen M., Sui S., et al. PD-L1 promotes tumor growth and progression by activating WIP and β-catenin signaling pathways and predicts poor prognosis in lung cancer. Cell Death Dis. 2020;11:1–16. doi: 10.1038/s41419-020-2701-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin T., Zeng Y.-D., Qin G., Xu F., Lu J.-B., Fang W.-F., Xue C., Zhan J.-H., Zhang X.-K., Zheng Q.-F., et al. High PD-L1 expression was associated with poor prognosis in 870 Chinese patients with breast cancer. Oncotarget. 2015;6:33972–33981. doi: 10.18632/oncotarget.5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muenst S., Schaerli A.R., Gao F., Däster S., Trella E., Droeser R.A., Muraro M.G., Zajac P., Zanetti R., Gillanders W.E., et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res. Treat. 2014;146:15–24. doi: 10.1007/s10549-014-2988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang W., Ran R., Shao B., Li H. Prognostic and clinicopathological value of PD-L1 expression in primary breast cancer: A meta-analysis. Breast Cancer Res. Treat. 2019;178:17–33. doi: 10.1007/s10549-019-05371-0. [DOI] [PubMed] [Google Scholar]
- 23.Baptista M.Z., Sarian L.O., Derchain S.F.M., Vassallo J., Vassallo J. Prognostic significance of PD-L1 and PD-L2 in breast cancer. Hum. Pathol. 2016;47:78–84. doi: 10.1016/j.humpath.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Botti G., Collina F., Scognamiglio G., Rao F., Peluso V., De Cecio R., Piezzo M., Landi G., De Laurentiis M., Cantile M., et al. Programmed Death Ligand 1 (PD-L1) Tumor Expression Is Associated with a Better Prognosis and Diabetic Disease in Triple Negative Breast Cancer Patients. Int. J. Mol. Sci. 2017;18:459. doi: 10.3390/ijms18020459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z.-Q., Milne K., DeRocher H., Webb J.R., Nelson B.H., Watson P.H. PD-L1 and intratumoral immune response in breast cancer. Oncotarget. 2017;8:51641–51651. doi: 10.18632/oncotarget.18305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrett M.T., Lenkiewicz E., Malasi S., Basu A., Yearley J., Annamalai L., McCullough A.E., Kosiorek H.E., Narang P., Sayres M.A.W., et al. The association of genomic lesions and PD-1/PD-L1 expression in resected triple-negative breast cancers. Breast Cancer Res. 2018;20:1–15. doi: 10.1186/s13058-018-1004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bavi P., Jehan Z., Atizado V., Al-Dossari H., Al-Dayel F., Tulbah A., Amr S.S., Sheikh S.S., Ezzat A., El-Solh H., et al. Prevalence of Fragile Histidine Triad Expression in Tumors from Saudi Arabia: A Tissue Microarray Analysis. Cancer Epidemiol. Biomark. Prev. 2006;15:1708–1718. doi: 10.1158/1055-9965.EPI-05-0972. [DOI] [PubMed] [Google Scholar]
- 28.Mesnage S.J.L., Auguste A., Genestie C., Dunant A., Pain E., Drusch F., Gouy S., Morice P., Bentivegna E., Lhomme C., et al. Neoadjuvant chemotherapy (NACT) increases immune infiltration and programmed death-ligand 1 (PD-L1) expression in epithelial ovarian cancer (EOC) Ann. Oncol. 2017;28:651–657. doi: 10.1093/annonc/mdw625. [DOI] [PubMed] [Google Scholar]
- 29.Siraj A.K., Beg S., Jehan Z., Prabhakaran S., Ahmed M., Hussain A.R., Al-Dayel F., Tulbah A., Ajarim D., Al-Kuraya K.S. ALK alteration is a frequent event in aggressive breast cancers. Breast Cancer Res. 2015;17:1–12. doi: 10.1186/s13058-015-0610-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beg S., Siraj A.K., Prabhakaran S., Jehan Z., Ajarim D., Al-Dayel F., Tulbah A., Al-Kuraya K.S. Loss of PTEN expression is associated with aggressive behavior and poor prognosis in Middle Eastern triple-negative breast cancer. Breast Cancer Res. Treat. 2015;151:541–553. doi: 10.1007/s10549-015-3430-3. [DOI] [PubMed] [Google Scholar]
- 31.Siraj A.K., Prabhakaran S., Bavi P., Bu R., Beg S., Al Hazmi M., Al-Rasheed M., Al-Assiri M., Sairafi R., Al-Dayel F., et al. Prevalence of Lynch syndrome in a Middle Eastern population with colorectal cancer. Cancer. 2015;121:1762–1771. doi: 10.1002/cncr.29288. [DOI] [PubMed] [Google Scholar]
- 32.Yi M., Jiao D., Xu H., Liu Q., Zhao W., Han X., Wu K. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol. Cancer. 2018;17:1–14. doi: 10.1186/s12943-018-0864-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis A.A., Patel V. The role of PD-L1 expression as a predictive biomarker: An analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J. Immunother. Cancer. 2019;7:1–8. doi: 10.1186/s40425-019-0768-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pelekanou V., Carvajal-Hausdorf D., Altan M., Wasserman B., Carvajal-Hausdorf C., Wimberly H., Brown J., Lannin D.R., Pusztai L., Rimm D.L. Effect of neoadjuvant chemotherapy on tumor-infiltrating lymphocytes and PD-L1 expression in breast cancer and its clinical significance. Breast Cancer Res. 2017;19:1–11. doi: 10.1186/s13058-017-0884-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y., Opyrchal M., Yao S., Peng X., Yan L., Jabbour H., Khoury T. The role of programmed death ligand-1 and tumor-infiltrating lymphocytes in breast cancer overexpressing HER2 gene. Breast Cancer Res. Treat. 2018;170:293–302. doi: 10.1007/s10549-018-4745-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Z., Dong P., Ren M., Song Y., Qian X., Yang Y., Li S., Zhang X., Liu F. PD-L1 Expression Is Associated with Tumor FOXP3+ Regulatory T-Cell Infiltration of Breast Cancer and Poor Prognosis of Patient. J. Cancer. 2016;7:784–793. doi: 10.7150/jca.14549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen S., Wang R.-X., Liu Y., Yang W.-T., Shao Z.-M. PD-L1 expression of the residual tumor serves as a prognostic marker in local advanced breast cancer after neoadjuvant chemotherapy. Int. J. Cancer. 2017;140:1384–1395. doi: 10.1002/ijc.30552. [DOI] [PubMed] [Google Scholar]
- 38.Mills A.M., Dill E.A., Moskaluk C.A., Dziegielewski J., Bullock T.N., Dillon P. The Relationship Between Mismatch Repair Deficiency and PD-L1 Expression in Breast Carcinoma. Am. J. Surg. Pathol. 2018;42:183–191. doi: 10.1097/PAS.0000000000000949. [DOI] [PubMed] [Google Scholar]
- 39.Lemery S., Keegan P., Pazdur R. First FDA Approval Agnostic of Cancer Site—When a Biomarker Defines the Indication. N. Engl. J. Med. 2017;377:1409–1412. doi: 10.1056/NEJMp1709968. [DOI] [PubMed] [Google Scholar]
- 40.Le D.T., Durham J.N., Smith K.N., Wang H., Bartlett B.R., Aulakh L.K., Lu S., Kemberling H., Wilt C., Luber B.S., et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mittendorf E.A., Philips A.V., Meric-Bernstam F., Qiao N., Wu Y., Harrington S., Su X., Wang Y., Gonzalez-Angulo A.M., Akcakanat A., et al. PD-L1 Expression in Triple-Negative Breast Cancer. Cancer Immunol. Res. 2014;2:361–370. doi: 10.1158/2326-6066.CIR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ren X., Wu H., Lu J., Zhang Y., Luo Y., Xu Q., Shen S., Liang Z. PD1 protein expression in tumor infiltrated lymphocytes rather than PDL1 in tumor cells predicts survival in triple-negative breast cancer. Cancer Biol. Ther. 2018;19:373–380. doi: 10.1080/15384047.2018.1423919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang C., Cao S., Li N., Jiang L., Sun T. PD-1 and PD-L1 correlated gene expression profiles and their association with clinical outcomes of breast cancer. Cancer Cell Int. 2019;19:1–9. doi: 10.1186/s12935-019-0955-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Emens L.A., Cruz C., Eder J.P., Braiteh F., Chung C., Tolaney S.M., Kuter I., Nanda R., Cassier P.A., Delord J.-P. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: A phase 1 study. JAMA Oncol. 2019;5:74–82. doi: 10.1001/jamaoncol.2018.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu X., Zhang Q., Wang D., Liu C., Han B., Yang J. Expression of PD-L1 Attenuates the Positive Impacts of High-level Tumor-infiltrating Lymphocytes on Prognosis of Triple-negative Breast Cancer. Cancer Biol. Ther. 2019;20:1105–1112. doi: 10.1080/15384047.2019.1595282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Desmedt C., Haibe-Kains B., Wirapati P., Buyse M., Larsimont D., Bontempi G., Delorenzi M., Piccart M., Sotiriou C. Biological Processes Associated with Breast Cancer Clinical Outcome Depend on the Molecular Subtypes. Clin. Cancer Res. 2008;14:5158–5165. doi: 10.1158/1078-0432.CCR-07-4756. [DOI] [PubMed] [Google Scholar]
- 47.Lehmann B.D., Jovanović B., Chen X., Estrada M.V., Johnson K.N., Shyr Y., Moses H.L., Sanders M.E., Pietenpol J.A. Refinement of Triple-Negative Breast Cancer Molecular Subtypes: Implications for Neoadjuvant Chemotherapy Selection. PLoS ONE. 2016;11:e0157368. doi: 10.1371/journal.pone.0157368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Denkert C., Von Minckwitz G., Darb-Esfahani S., Lederer B., Heppner B.I., Weber K.E., Budczies J., Huober J., Klauschen F., Furlanetto J., et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19:40–50. doi: 10.1016/S1470-2045(17)30904-X. [DOI] [PubMed] [Google Scholar]
- 49.Foukakis T., Lövrot J., Matikas A., Zerdes I., Lorent J., Tobin N., Suzuki C., Brage S.E., Carlsson L., Einbeigi Z., et al. Immune gene expression and response to chemotherapy in advanced breast cancer. Br. J. Cancer. 2018;118:480–488. doi: 10.1038/bjc.2017.446. [DOI] [PMC free article] [PubMed] [Google Scholar]