Key Points
Question
Is treatment with convalescent plasma associated with improved clinical outcomes?
Findings
In a meta-analysis of 4 peer-reviewed and published randomized clinical trials including 1060 patients with COVID-19 treated with convalescent plasma vs control, the risk ratio for mortality was 0.93 and after the addition of 6 unpublished randomized clinical trials and 10 722 patients, the risk ratio for mortality was 1.02; neither finding was statistically significant. No significant associations with benefit were shown for hospital length of stay, mechanical ventilation use, clinical improvement, or clinical deterioration.
Meaning
Among patients with COVID-19, treatment with convalescent plasma compared with control was not associated with improved survival or other positive clinical outcomes.
Abstract
Importance
Convalescent plasma is a proposed treatment for COVID-19.
Objective
To assess clinical outcomes with convalescent plasma treatment vs placebo or standard of care in peer-reviewed and preprint publications or press releases of randomized clinical trials (RCTs).
Data Sources
PubMed, the Cochrane COVID-19 trial registry, and the Living Overview of Evidence platform were searched until January 29, 2021.
Study Selection
The RCTs selected compared any type of convalescent plasma vs placebo or standard of care for patients with confirmed or suspected COVID-19 in any treatment setting.
Data Extraction and Synthesis
Two reviewers independently extracted data on relevant clinical outcomes, trial characteristics, and patient characteristics and used the Cochrane Risk of Bias Assessment Tool. The primary analysis included peer-reviewed publications of RCTs only, whereas the secondary analysis included all publicly available RCT data (peer-reviewed publications, preprints, and press releases). Inverse variance–weighted meta-analyses were conducted to summarize the treatment effects. The certainty of the evidence was assessed using the Grading of Recommendations Assessment, Development, and Evaluation.
Main Outcomes and Measures
All-cause mortality, length of hospital stay, clinical improvement, clinical deterioration, mechanical ventilation use, and serious adverse events.
Results
A total of 1060 patients from 4 peer-reviewed RCTs and 10 722 patients from 6 other publicly available RCTs were included. The summary risk ratio (RR) for all-cause mortality with convalescent plasma in the 4 peer-reviewed RCTs was 0.93 (95% CI, 0.63 to 1.38), the absolute risk difference was −1.21% (95% CI, −5.29% to 2.88%), and there was low certainty of the evidence due to imprecision. Across all 10 RCTs, the summary RR was 1.02 (95% CI, 0.92 to 1.12) and there was moderate certainty of the evidence due to inclusion of unpublished data. Among the peer-reviewed RCTs, the summary hazard ratio was 1.17 (95% CI, 0.07 to 20.34) for length of hospital stay, the summary RR was 0.76 (95% CI, 0.20 to 2.87) for mechanical ventilation use (the absolute risk difference for mechanical ventilation use was −2.56% [95% CI, −13.16% to 8.05%]), and there was low certainty of the evidence due to imprecision for both outcomes. Limited data on clinical improvement, clinical deterioration, and serious adverse events showed no significant differences.
Conclusions and Relevance
Treatment with convalescent plasma compared with placebo or standard of care was not significantly associated with a decrease in all-cause mortality or with any benefit for other clinical outcomes. The certainty of the evidence was low to moderate for all-cause mortality and low for other outcomes.
This meta-analysis of randomized trials reported in medical journals, preprint servers, and press releases estimates associations between convalescent plasma treatment and clinical outcomes (all-cause mortality, length of stay, clinical improvement, clinical deterioration, mechanical ventilation use, serious adverse events) in patients with COVID-19.
Introduction
Patients with COVID-19 have frequently been treated with convalescent plasma (ie, plasma from persons who have recovered from SARS-CoV-2 infection), but the clinical evidence of benefits or harms is limited.1 Preliminary reports indicating that convalescent plasma is well tolerated with low risk of adverse events2 led to Emergency Use Authorization in the US in August 2020.3 Despite the large number of clinical trials being conducted since the start of the pandemic, only a few have been published in peer-reviewed journals and some have posted preliminary results on preprint servers.
The Randomized Evaluation of COVID-19 Therapy (RECOVERY) platform trial is by far the largest clinical trial on COVID-19 treatments, and has provided important evidence for several promising treatments, including dexamethasone,4 hydroxychloroquine,5 lopinavir-ritonavir,6 and azithromycin.7 The part of the trial investigating treatment with convalescent plasma was halted based on the recommendation of the RECOVERY data monitoring committee. Communicated as a press release on January 15, 2021, the preliminary reported results based on data from 10 406 patients indicate no significant association of a benefit with convalescent plasma in reducing all-cause mortality compared with standard of care (risk ratio [RR], 1.04; 95% CI, 0.95-1.14).8
Given the previously reported clinical trials and this recent announcement,8 a systematic review and meta-analysis was conducted to summarize and assess all published evidence from randomized clinical trials (RCTs) on the association between treatment with convalescent plasma compared with standard of care or placebo on clinical outcomes in patients with COVID-19.
Methods
This review has been reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-analysis.9
Search Strategy and RCT Selection
Two reviewers (P.J. and C.A.) systematically searched PubMed (using peer-review of electronic search strategies10), the Cochrane COVID-19 trial registry, and the Living Overview of Evidence platform for all published RCTs as of January 29, 2021, aiming to assess the benefits and harms of convalescent plasma to treat patients with COVID-19. Search strategies were designed with terms related to convalescent plasma and COVID-19 along with standard RCT filters (eMethods in the Supplement).
In addition, we searched for press releases presenting results of RCTs assessing convalescent plasma. Peer-reviewed publications, preprints, and press releases were eligible for inclusion. There were no restrictions on language or geographic region.
The selected RCTs included patients with suspected or confirmed SARS-CoV-2 infection randomly allocated to receive convalescent plasma, placebo together with standard of care, or only standard of care. The RCTs were included regardless of the level of plasma titer (ie, low or high antibody titer) or health care setting. The RCTs aimed at preventing the occurrence of COVID-19 were excluded.
Outcomes
The outcomes were all-cause mortality at any time point, length of hospital stay, number of patients with clinical improvement or deterioration, number of patients requiring mechanical ventilation, and number of patients experiencing serious adverse events.
Data Extraction and Risk of Bias Assessment
We extracted the following information for each RCT: trial design characteristics (randomization procedure and blinding), descriptions of the experimental and control groups, baseline characteristics of the patients, eligibility criteria for plasma donors, and trial location. High antibody titer was defined in this meta-analysis as S-protein receptor-binding domain–specific IgG antibody titer of 1:640 or higher or serum neutralization titer of 1:40 or higher. For each outcome, we collected either the number of events for the convalescent plasma and control groups or the effect size and corresponding 95% CI (only hazard ratios [HRs] were consistently reported for length of hospital stay). Data on outcomes (F.E. and M.H.) and characteristics (A.M.S. and V.G.) were extracted independently by 2 reviewers.
For each RCT, 2 reviewers (A.M.S. and V.G.) independently assessed the risk of bias for all-cause mortality, mechanical ventilation use, and length of hospital stay using version 2 of the Cochrane Risk of Bias Assessment Tool (low risk, some concerns, or high risk of bias).11 We also used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE)12 to assess the certainty of the evidence for the summarized outcomes regarding the treatment effect of convalescent plasma on patients with COVID-19.
Disagreements among reviewers were discussed with a third reviewer (P.J.) until a consensus was reached.
Statistical Analyses
The primary analysis included only RCTs published in peer-reviewed journals. A secondary analysis included all the RCTs (peer-reviewed, preprints, and information from the press release for the RECOVERY trial).
For outcomes with available data (all-cause mortality, length of hospital stay, and mechanical ventilation use), we conducted meta-analyses to summarize the treatment effects using RRs and HRs when applicable. The treatment effects for clinical improvement, clinical deterioration, and serious adverse events were not summarized due to inconsistent definitions of these outcomes and insufficient reporting of relevant details. When possible (based on the available data), we also estimated and summarized the treatment effects across the RCTs on an absolute risk difference scale.
We conducted inverse variance–weighted random-effects meta-analyses using the Paule and Mandel τ2 estimator for heterogeneity.13 We applied the Hartung-Knapp adjustment14 to account for uncertainties due to large variations in sample size and in the number of outcome events across the RCTs. Heterogeneity across the RCTs was described using the I2 and τ2 metrics.15
We conducted sensitivity analyses to assess the robustness of the results using the following meta-analytic models: Sidik-Jonkman τ2 estimator (instead of the Paule and Mandel estimator), the profile likelihood model, and the inverse variance–weighted fixed-effects model.
All tests were 2-sided and statistical significance was based on the 95% CIs excluding the null. All analyses were conducted using R version 3.6.2 meta and metafor packages (R Foundation for Statistical Computing).
Results
A total of 4357 records were identified in databases, registries, and other sources. There were 4 RCTs published in peer-reviewed journals16,17,18,19 and 5 RCTs published as preprints20,21,22,23,24 that were included. In addition, press releases were identified for 2 RCTs (the RECOVERY trial8 and the Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia [REMAP-CAP]25) but only the reported results from the RECOVERY trial8 (NCT04381936) were included, stating 1873 deaths among 10 406 patients randomized (eFigure 1 in the Supplement).
Of the 10 included RCTs, 3 were conducted in India, 2 in Argentina, and 1 each in Bahrain, China, the Netherlands, Spain, and the UK (Table 1). Five RCTs were terminated early; 2 were terminated early due to futility (Convalescent Plasma as Therapy for Covid-19 Severe SARS-CoV-2 Disease [ConCOVID; NCT04342182]22 and RECOVERY [NCT04381936]8) and 3 were terminated early due to slow recruitment (Convalescent Plasma Therapy vs SOC for the Treatment of COVID-19 in Hospitalized Patients [ConPlas-19; NCT04345523],23 ChiCTR2000029757,19 and NCT04479163).16 There were 2 double-blind RCTs (NCT04479163 and Convalescent Plasma and Placebo for the Treatment of COVID-19 Severe Pneumonia [PlasmAr; NCT04383535]),18 whereas the other 8 were open-label RCTs.
Table 1. Characteristics of the 10 Trials.
| Trial registration No. (study acronym)a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ChiCTR 200002975719 |
NCT 0447916316 |
NCT 04383535 (PlasmAr)18 |
CTRI /2020/04/ 024775 (PLACID)17 |
NCT 04345523 (ConPlas-19)23 |
NCT 04346446 (ILBS-COVID-02)21 |
NCT 0435653420 |
NCT 04342182 (ConCOVID)22 |
CTRI /2020/05/ 025209 (PICP19)24 |
NCT 04381936 (RECOVERY)8 |
|
| Publication format | Journal | Journal | Journal | Journal | Preprint | Preprint | Preprint | Preprint | Preprint | Press release |
| Peer-reviewed | Yes | Yes | Yes | Yes | No | No | No | No | No | No |
| No. included | 103 | 160 | 333 | 464 | 81 | 29 | 40 | 86 | 80 | 10 406 |
| No. planned for inclusion | 200 | 210 | 333 | 452 | 278 | 40 | 40 | 426 | 80 | 20 000 |
| Setting | Hospitalized | Outpatient | Hospitalized | Hospitalized | Hospitalized | Hospitalized | Hospitalized | Hospitalized | Hospitalized | Hospitalized |
| Oxygen supplementation | All patients | None | Some patients | All patients | Some patients | All patients | All patients | Some patients | All patients | Some patients |
| Plasma titerb | High | High: >1:1000 | High: ≥1:800 (RBD) | No minimum | High: ≥1:80 neutralizing | No minimum | No minimum | Low: ≥1:400 RBD | Unclear | Unclear |
| Dose description | Single transfusion of 4-13 mL/kg | Single transfusion of 250 mL | Single transfusion of 5-10 mL/kg (minimum, 400 mL; maximum, 700 mL) | Two transfusions of 200 mL administered 24 h apart | Single transfusion of 250-300 mL | Two transfusions of 500 mL administered 24 h apart | Two transfusions of 200 mL administered 24 h apart | Single transfusion of 300 mLc | Two transfusions of 200 mL administered 24 h apart | Two transfusions of 275 mL (±75 mL) administered 24 h apart |
| Treatment since symptom onset | Any time | ≤72 h | Any time | Any time | ≤12 d | ≤3 d | ≤14 d | Any time | ≤14 d | Any time |
| Type of control | Standard of care | Placebo and standard of care | Placebo and standard of care | Standard of care | Standard of care | Placebo and standard of care | Standard of care | Standard of care | Standard of care | Standard of care |
Abbreviations: ConCOVID, Convalescent Plasma as Therapy for Covid-19 Severe SARS-CoV-2 Disease; ConPlas-19, Convalescent Plasma Therapy vs SOC for the Treatment of COVID-19 in Hospitalized Patients; PICP19, Passive Immunization With Convalescent Plasma in Severe COVID-19 Disease; PlasmAr, Convalescent Plasma and Placebo for the Treatment of COVID-19 Severe Pneumonia; RBD, receptor-binding domain; RECOVERY, Randomized Evaluation of COVID-19 Therapy.
Three of the trials did not have study acronyms (only trial registration numbers) and ILBS-COVID-02 and PLACID did not have expansions in the original publications.
High was defined in this meta-analysis as S-protein RBD–specific IgG antibody titer of 1:640 or higher or serum neutralization titer of 1:40 or higher.
The COVIDAR IgG test was used to determine the dose.
From the 4 RCTs published in peer-reviewed journals, there were 1060 patients (595 randomized to convalescent plasma and 465 to placebo together with standard of care or only standard of care). From the 5 RCTs published as preprints, there were 316 patients (155 randomized to convalescent plasma and 161 to placebo together with standard of care or only standard of care). From the RECOVERY trial, there were 10 406 patients (the number of patients randomized per group was not reported in the press release information).
Of the 10 RCTs, 9 included only patients with confirmed SARS-CoV-2 infection but the RECOVERY trial included those with either confirmed or suspected SARS-CoV-2 infection. Only 1 RCT included outpatients, 5 included inpatients requiring supplemental oxygen, and 4 included inpatients regardless of need for supplemental oxygen (Table 1). Patients were administered a single convalescent plasma transfusion in 5 of the RCTs and were administered 2 transfusions 24 hours apart in the other 5 RCTs (Table 1). Of the 10 RCTs, high plasma titer was used in 4, low titer was used in 1, a minimum plasma titer cutoff was not used in 3, and it was unclear in 2 (Table 1). Six RCTs used donated plasma from men, nulliparous women, or women testing negative for HLA antibodies (this type of description was not reported for 4 RCTs: RECOVERY [NCT04381936], NCT04479163, ChiCTR2000029757, and ConPlas-19 [NCT04345523]). Only 3 RCTs (PlasmAr [NCT04383535], NCT04356534, and PLACID [CTRI/2020/04/024775]) reported the COVID-19 severity of plasma donors.
Detailed information on patient characteristics were available for 9 of the 10 RCTs (Table 2). The mean age of patients was younger than 70 years and they were typically male (≤80%); these generalizations did not apply to NCT04479163. Comorbidities at randomization were common when reported in the trials and only 2 RCTs reported the concurrent treatments at randomization.
Table 2. Patient Baseline Characteristics in 9 Trials.
| Trial registration No. (study acronym)a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ChiCTR 200002975719 |
NCT0447916316 |
NCT04383535 (PlasmAr)18 |
CTRI/2020/04/ 024775 (PLACID)17 |
NCT04345523 (ConPlas-19)23 |
NCT04346446 (ILBS-COVID-02)21 |
NCT0435653420 |
NCT04342182 (ConCOVID)22 |
CTRI/2020/05/ 025209 (PICP19)24 |
||
| No. of patients randomized | ||||||||||
| Convalescent plasma group | 52 | 80 | 228 | 235 | 38 | 14 | 20 | 43 | 40 | |
| Control groupb | 51 | 80 | 105 | 229 | 43 | 15 | 20 | 43 | 40 | |
| Age, median (IQR), y | ||||||||||
| Convalescent plasma group | 70 (62-80) | 76.4 (8.7)c | 62.5 (53-72.5) | 52 (42-60) | 60.5 (46-74) | 48.1 (9.1)c | 52.6 (14.9)c | 61 (56-70) | NR | |
| Control groupb | 69 (63-76) | 77.9 (8.4)c | 62 (49-71) | 52 (41-60) | 58 (51-73) | 48.3 (10.8)c | 50.7 (12.5)c | 63 (55-77) | NR | |
| Sex, No. (%) | ||||||||||
| Convalescent plasma group | ||||||||||
| Male | 27 (52) | 26 (32) | 161 (71) | 177 (75) | 20 (53) | 11 (79) | 17 (85) | 29 (67) | 30 (75) | |
| Female | 25 (48) | 54 (68) | 67 (29) | 58 (25) | 18 (47) | 3 (21) | 3 (15) | 14 (33) | 10 (25) | |
| Control groupb | ||||||||||
| Male | 33 (65) | 34 (42) | 64 (61) | 177 (77) | 24 (56) | 11 (73) | 15 (75) | 33 (77) | 27 (67) | |
| Female | 18 (35) | 46 (58) | 41 (39) | 52 (23) | 19 (54) | 4 (27) | 5 (25) | 10 (23) | 13 (33) | |
| Type of mechanical ventilation use at randomization, No. (%)d | ||||||||||
| Invasive | ||||||||||
| Convalescent plasma group | 14 (28) | 0 | 0 | 0 | 0 | 0 | 0 | 5 (12) | 0 | |
| Control groupb | 11 (22) | 0 | 0 | 0 | 0 | 0 | 0 | 8 (19) | 0 | |
| Noninvasive | ||||||||||
| Convalescent plasma group | 21 (41) | 0 | 0 | 0 | 0 | 0 | 0 | NR | 0 | |
| Control groupb | 23 (46) | 0 | 0 | 0 | 0 | 0 | 0 | NR | 0 | |
| Comorbidities at randomization, No. (%) | ||||||||||
| Hypertension | ||||||||||
| Convalescent plasma group | 29 (56) | 62 (78) | 111 (49) | 92 (39) | 20 (53) | 0 | 5 (25) | 11 (26) | NR | |
| Control groupb | 27 (53) | 52 (65) | 48 (46) | 81 (35) | 12 (28) | 0 | 5 (25) | 11 (26) | NR | |
| Diabetes | ||||||||||
| Convalescent plasma group | 9 (17) | 23 (29) | 40 (18) | 113 (48) | 12 (32) | 0 | 7 (35) | 13 (30) | NR | |
| Control groupb | 12 (24) | 13 (16)e | 21 (20) | 87 (38) | 5 (12) | 0 | 9 (45) | 8 (19) | NR | |
| Cardiac disease | ||||||||||
| Convalescent plasma group | 14 (27) | 14 (18)e | 8 (4) | 15 (6) | 6 (16) | 0 | 2 (10) | 9 (21) | NR | |
| Control groupb | 12 (24) | 7 (9)e | 3 (3) | 17 (7) | 9 (21) | 0 | 2 (10) | 11 (26) | NR | |
| Pulmonary diseasef | ||||||||||
| Convalescent plasma group | NR | 5 (6) | 32 (14) | 8 (3) | 2 (5) | 0 | 3 (15) | 12 (28) | NR | |
| Control groupb | NR | 8 (10) | 7 (7) | 7 (3) | 8 (19) | 0 | 0 | 11 (26) | NR | |
| Chronic kidney failure | ||||||||||
| Convalescent plasma group | 2 (4) | 1 (1) | 10 (4) | 8 (3) | 2 (5) | 0 | 1 (5) | 1 (2) | NR | |
| Control groupb | 4 (8) | 3 (4)e | 4 (4) | 9 (4) | 2 (5) | 0 | 1 (5) | 6 (14) | NR | |
| Cancer | ||||||||||
| Convalescent plasma group | 3 (6) | 4 (5) | 27 (12) | 1 (<1) | NR | 0 | NR | 5 (12) | NR | |
| Control groupb | 0 | 2 (2) | 14 (14) | 0 | NR | 0 | NR | 3 (7) | NR | |
| Liver disease | ||||||||||
| Convalescent plasma group | 5 (10) | 0 | NR | 0 | NR | NR | 0 | 1 (2) | NR | |
| Control groupb | 5 (10) | 0 | NR | 0 | NR | NR | 0 | 0 | NR | |
| Risk factors at randomization, No. (%) | ||||||||||
| Smokerg | ||||||||||
| Convalescent plasma group | NR | 13 (16) | 107 (47) | 19 (8) | NR | NR | 0 | NR | NR | |
| Control groupb | NR | 10 (12) | 43 (41) | 18 (8) | NR | NR | 0 | NR | NR | |
| Body mass index >30h | ||||||||||
| Convalescent plasma group | NR | 4 (5) | 104 (46) | 16 (7) | NR | NR | NR | NR | NR | |
| Control groupb | NR | 8 (10)e | 52 (50) | 17 (7) | NR | NR | NR | NR | NR | |
Abbreviations: ConCOVID, Convalescent Plasma as Therapy for Covid-19 Severe SARS-CoV-2 Disease; ConPlas-19, Convalescent Plasma Therapy vs SOC for the Treatment of COVID-19 in Hospitalized Patients; NR, not reported; PICP19, Passive Immunization With Convalescent Plasma in Severe COVID-19 Disease; PlasmAr, Convalescent Plasma and Placebo for the Treatment of COVID-19 Severe Pneumonia.
Three of the trials did not have study acronyms (only trial registration numbers) and ILBS-COVID-02 and PLACID did not have expansions in the original publications. Only 3 trials reported concurrent treatments at randomization. Of 81 patients in ConPlas-19, 70 (86.4%) received hydroxychloroquine, 50 (61.7%) received azithromycin, and 46 received (56.8%) corticosteroids. Of 103 patients in ChiCTR2000029757, 85 (82.5%) received antiviral drugs, 77 (74.8%) received antibacterial or antibiotic drugs, and 37 (35.9%) received corticosteroids. Of 333 patients in PlasmAr, 9 (2.7%) received corticosteroids. Information on patient baseline characteristics were not available for the Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial because the results were communicated as a press release.
Placebo together with standard of care or only standard of care.
Reported as mean (SD).
Categorized according to what was reported in the trial reports.
The denominator was 79 patients.
Includes chronic obstructive pulmonary disease, asthma, and tuberculosis.
Includes current and former smokers.
Calculated as weight in kilograms divided by height in meters squared.
Risk of Bias
The risk of bias for mortality, length of hospital stay, and mechanical ventilation use was deemed low for 7 of the 10 RCTs. For 2 of the RCTs, the risk of bias was classified as having some concerns (NCT04356534 and ConPlas-19 [NCT04345523]) and for 1 RCT it was deemed high (Passive Immunization With Convalescent Plasma in Severe COVID-19 Disease [PICP19; CTRI/2020/05/025209]; Figure 1). Loss to follow-up was less than 10% when reported in 9 RCTs (data were unavailable for the RECOVERY trial).
Figure 1. Risk of Bias Assessments for the Outcomes of All-Cause Mortality, Length of Hospital Stay, and Mechanical Ventilation Use.
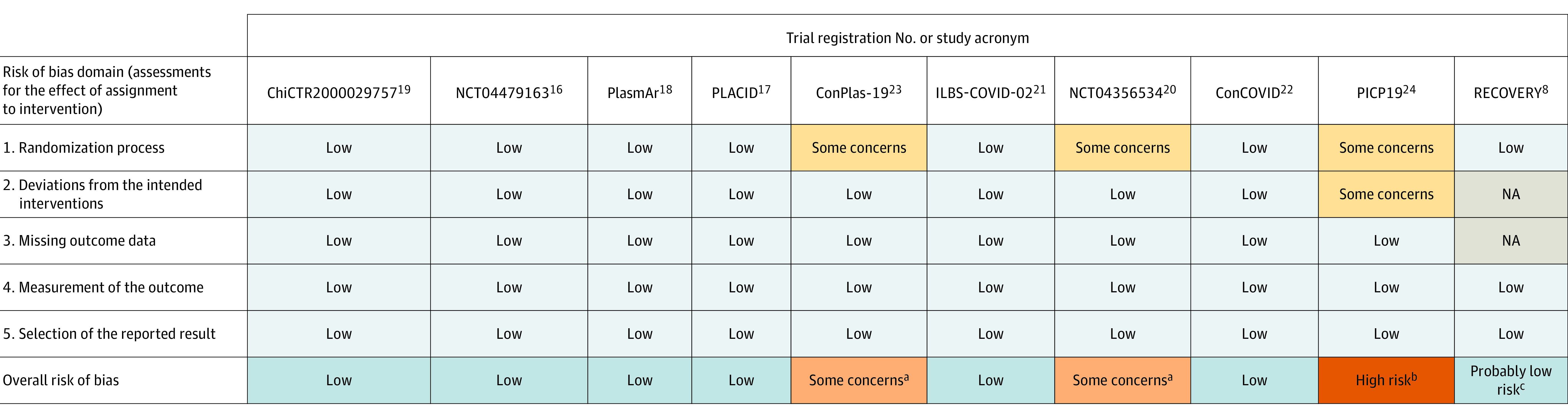
Three of the trials did not have study acronyms (only trial registration numbers) and ILBS-COVID-02 and PLACID did not have expansions in the original publications. ConCOVID indicates Convalescent Plasma as Therapy for Covid-19 Severe SARS-CoV-2 Disease; ConPlas-19, Convalescent Plasma Therapy vs SOC for the Treatment of COVID-19 in Hospitalized Patients; NA, not available; PICP19, Passive Immunization With Convalescent Plasma in Severe COVID-19 Disease; PlasmAr, Convalescent Plasma and Placebo for the Treatment of COVID-19 Severe Pneumonia; RECOVERY, Randomized Evaluation of COVID-19 Therapy.
aThere was no detailed information reported regarding (1) the randomization process or (2) the concealment of randomized assignment.
bThere was no detailed information reported regarding (1) the randomization process, (2) the concealment of randomized assignment, (3) the flow of patients through the trial, and (4) possible deviations from the intended interventions due to the open-label setting of the trial.
cThe results were communicated as a press release. The assessment of this trial considered the study protocol and publications reporting results from other treatment groups of the trial.4,5,6,26,27
The RECOVERY trial was deemed as having probably low risk of bias based on the trial protocol and published information for other treatments assessed by the trial (Figure 1).4,5,6,26,27
Data Availability
Mortality was assessed in all 10 RCTs and for 8 of the trials it was assessed between 15 to 30 days after randomization (1 RCT assessed mortality at 60 days and 1 RCT did not report length of follow-up; eTable 1 in the Supplement). Length of hospital stay was assessed in 7 RCTs; 3 used medians or means (1 published in a peer-reviewed journal and 2 published as preprints), 1 used HRs (published as a preprint), and 3 used both medians and HRs (2 published in peer-reviewed journals and 1 published as a preprint). The need for mechanical ventilation use was reported in 5 RCTs (3 peer-reviewed and 2 preprints). Data on clinical deterioration and clinical improvement were available in 5 RCTs (3 peer-reviewed and 2 preprints) and 3 RCTs reported data on serious adverse events (1 peer-reviewed and 2 preprints).
Association of Convalescent Plasma With Clinical Outcomes
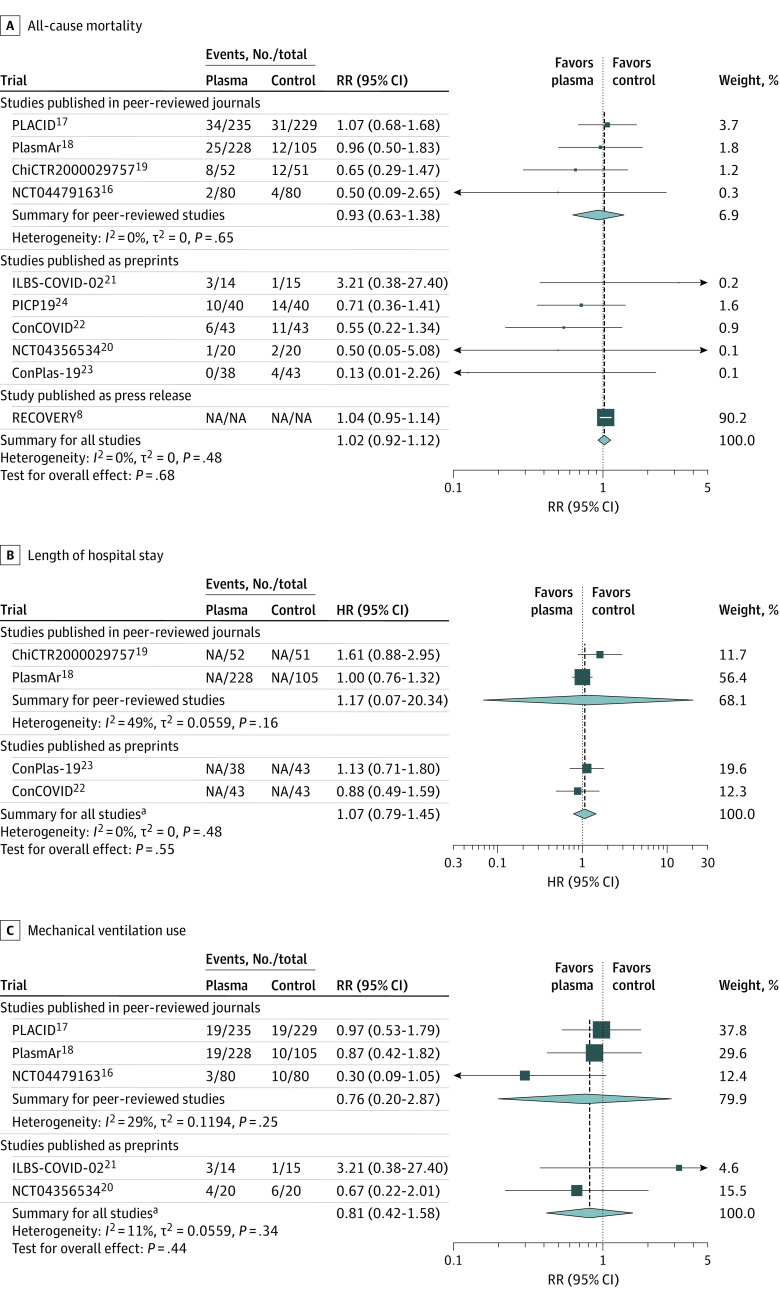
In the primary analysis including only peer-reviewed RCTs, the mortality in patients receiving convalescent plasma was 11.6% (69/595) and 12.7% (59/465) in control patients. The summary RR for all-cause mortality with convalescent plasma was 0.93 (95% CI, 0.63 to 1.38; P = .60) and the absolute risk difference was −1.21% (95% CI, −5.29% to 2.88%). There was no significant between-trial heterogeneity (I2 = 0%; τ2 = 0 [95% CI, 0 to 1.35]) (Figure 2A). In the RECOVERY trial, the reported 28-day mortality rates were 18% with convalescent plasma and 18% for usual care (control).
Figure 2. Association of Convalescent Plasma With All-Cause Mortality, Length of Hospital Stay, and Mechanical Ventilation Use in Peer-Reviewed Trials and Non–Peer-Reviewed Trials (Preprints and the RECOVERY Trial).
Three of the trials did not have study acronyms (only trial registration numbers) and ILBS-COVID-02 and PLACID did not have expansions in the original publications. Hartung-Knapp adjustment was used for the random-effects model and the Paule-Mandel estimator was used for τ2. The weight percentages correspond to the secondary analysis for all studies. ConCOVID indicates Convalescent Plasma as Therapy for Covid-19 Severe SARS-CoV-2 Disease; ConPlas-19, Convalescent Plasma Therapy vs SOC for the Treatment of COVID-19 in Hospitalized Patients; HR, hazard ratio; NA, not available; PICP19, Passive Immunization With Convalescent Plasma in Severe COVID-19 Disease; PlasmAr, Convalescent Plasma and Placebo for the Treatment of COVID-19 Severe Pneumonia; RECOVERY, Randomized Evaluation of COVID-19 Therapy; RR, risk ratio.
aIncludes only the studies shown that were published in peer-reviewed journals or as preprints.
Across the 10 RCTs, the summary RR for all-cause mortality with convalescent plasma was 1.02 (95% CI, 0.92 to 1.12]; P = .68). There was no significant between-trial heterogeneity (I2 = 0%; τ2 = 0 [95% CI, 0 to 0.86]). In this meta-analysis of the 10 RCTs for all-cause mortality, the RECOVERY trial accounted for 90.2% of the weight and 88.3% (10 406/11 782) of the patients (Figure 2). The results of the sensitivity analyses were consistent with the main results (eTable 2 in the Supplement).
The 4 peer-reviewed RCTs showed no significant associations between treatment with convalescent plasma and reductions in length of hospital stay (summary HR, 1.17 [95% CI, 0.07 to 20.34], P = .61 for analysis of 436 patients) or mechanical ventilation use (summary RR, 0.76 [95% CI, 0.20 to 2.87], P = .35 for analysis of 957 patients) (Figure 2). The absolute risk difference for mechanical ventilation use was −2.56% (95% CI, −13.16% to 8.05%). Similar results were observed for the peer-reviewed and preprint RCTs for length of hospital stay (HR, 1.07 [95% CI, 0.79 to 1.45], P = .87 for analysis of 603 patients) and for mechanical ventilation use (RR, 0.81 [95% CI, 0.42 to 1.58], P = .88 for analysis of 1026 patients; Figure 2). The absolute risk difference for mechanical ventilation use was −2.21% (95% CI, −8.94% to 4.51%) (eFigure 2 in the Supplement).
For clinical improvement and clinical deterioration, the RRs were not summarized across RCTs due to inconsistent definitions and insufficient reporting of relevant details for these outcomes (eTable 1 and eFigure 3 in the Supplement). Of the 5 RCTs (3 peer-reviewed and 2 preprints) that reported such data, none demonstrated statistically significant clinical deterioration or improvement in patients who received convalescent plasma compared with the control group and the 95% CIs were wide (eFigure 3 in the Supplement).
No meta-analysis was conducted on serious adverse events due to inconsistencies in the reporting. PlasmAr (NCT04383535), ConPlas-19 (NCT04345523), and ConCOVID (NCT04342182) were the RCTs that reported data on serious adverse events (eFigure 4 in the Supplement); 60 serious adverse events were reported for the 309 patients in the convalescent plasma groups and 26 serious adverse events were reported for the 191 patients in the control groups. Even though ConCOVID (NCT04342182) included all-cause mortality in its definition of serious adverse events and 17 patients died, only plasma-related serious adverse events were reported (with 0 events). Similarly, PLACID (CTRI/2020/04/024775) and NCT04356534 reported recording serious adverse events including all-cause mortality but no clear data were shown.
The Certainty of the Evidence
For the primary analysis that only included the 4 RCTs published in peer-reviewed journals, the certainty of the evidence (using GRADE) for mortality was low due to very serious imprecision concerns regarding the wide 95% CI for the summary RR, which would be compatible with substantial benefit or harm. For the secondary analysis that included all 10 RCTs (published in peer-reviewed journals, published as preprints, and the RECOVERY trial), the concern regarding imprecision was reduced and the certainty of the evidence was rated as moderate (eTable 3 in the Supplement).
For length of hospital stay and mechanical ventilation use, the certainty of the evidence was rated as low for peer-reviewed trials only and when considering all publicly available trials due to very serious imprecision concerns (wide 95% CIs for the summary RR estimates; eTable 3 in the Supplement).
Discussion
In this meta-analysis that included 4 RCTs published in peer-reviewed journals for the primary analysis and an additional 6 RCTs not published in peer-reviewed journals (5 preprints and 1 press release) for the secondary analysis, treatment with convalescent plasma compared with placebo in combination with standard of care or only standard of care was not significantly associated with a decrease in all-cause mortality or with any benefit for other clinical outcomes among patients with COVID-19.
The certainty of the evidence on all-cause mortality was low when only the peer-reviewed trials were included and then moderate when the evidence from the RCTs published as preprints and the RECOVERY trial was added. The evidence was largely dominated by the RECOVERY trial, which accounted for 90.2% of the weight in the meta-analysis, although the pooled results from the 4 peer-reviewed trials were similar. The results from the RECOVERY trial published as a press release warrant cautious interpretation until the trial results are fully analyzed and reported in a peer-reviewed journal.
There also was no significant association of convalescent plasma with benefits on other patient-relevant clinical outcomes, including reduction in the length of hospital stay or mechanical ventilation use; however, summarized sample sizes were considerably smaller (range, 603-1026 patients) than for all-cause mortality (11 782 patients). Data on clinical improvement or deterioration were limited and inconclusive due to the use of inconsistent definitions for the outcomes and insufficient reporting of the relevant details for these outcomes. Similarly, the safety of convalescent plasma regarding serious adverse events could not be reliably assessed because only 3 RCTs reported data and there were inconsistencies in the definitions used. Although it was identified during the literature search, the press release for the REMAP-CAP trial25 was not included because it did not present quantitative results. However, according to their reported preliminary analysis including 912 participants requiring intensive care unit support, treatment with convalescent plasma did not show a beneficial effect on the number of days requiring intensive support or on mortality. The REMAP-CAP preliminary findings are consistent with our summarized results and, given the relatively small sample size of REMAP-CAP compared with the RECOVERY trial,8 the data would likely not change our interpretation.
Difficulties in synthesizing evidence across COVID-19 trials because of heterogeneous outcome measures were anticipated by Zarin and Rosenfeld28 who identified 351 unique descriptions for outcome measures among 232 trials registered until June 2020, including 14 unique ordinal scales. Besides precluding a meaningful overview, unnecessary variation in outcome measures makes precise conclusions more challenging. To aid the development of uniform outcome measurement across trials, core outcome sets involving patients may be a fruitful way forward.29
Limitations
This study has several limitations. First, 3 of the 10 RCTs had some concerns or high risk of bias. However, those 3 RCTs only contributed to 1.8% of the weight of the meta-analysis on all-cause mortality, which was highly dominated by data from the RECOVERY trial. Although access to full publication of the results was not yet available, the mortality results from the RECOVERY trial appear likely to be at low risk of bias and without a specific reason to downgrade the certainty of evidence based on previously published treatment group results and the RECOVERY trial protocol.4,5,6,26,27
Second, the reporting of clinical outcomes, other than all-cause mortality, for RECOVERY was insufficient and inconsistent regarding the use of definitions and relevant details across its COVID-19 treatment trials.
Third, the data were too limited to perform meaningful subgroup analyses. The observations reported in the literature regarding a benefit with early high-titer plasma1 administration in observational studies call for further analyses based on individual patient data such as the Continuous Monitoring of Pooled International Trials of Convalescent Plasma for COVID-19 Hospitalized Patients (COMPILE) project.30
Fourth, except for 1 RCT with outpatients,16 all patients were hospitalized with or without oxygen supplementation, indicative of moderate to critical COVID-19. The generalizability of the results to patients with milder COVID-19 is unclear.
Fifth, the primary focus of this meta-analysis was on published RCTs. There are many ongoing trials (>100) assessing convalescent plasma that are at risk of being terminated early or never published, but a collaborative meta-analysis of all these data is underway.31
Conclusions
Treatment with convalescent plasma compared with placebo or standard of care was not significantly associated with a decrease in all-cause mortality or with any benefit for other clinical outcomes. The certainty of the evidence was low to moderate for all-cause mortality and low for other outcomes.
eMethods. Literature search strategies
eFigure 1. Flowchart of the literature search
eFigure 2. Forest plots with summary effect sizes per publication type for A) all-cause mortality, B) Length of hospitalization, and C) Mechanical ventilation requirement
eFigure 3. Association of convalescent plasma with A) study-defined clinical improvement and B) study-defined clinical deterioration for peer-reviewed and preprint trials (no pooling due to heterogeneity of outcome definitions)
eFigure 4. Serious adverse events reported in peer-reviewed and preprint trials (no pooling due to heterogeneity of outcome definitions)
eTable 1. Outcomes reported by trials
eTable 2. Sensitivity analyses
eTable 3. GRADE assessment
References
- 1.Mair-Jenkins J, Saavedra-Campos M, Baillie JK, et al. ; Convalescent Plasma Study Group . The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211(1):80-90. doi: 10.1093/infdis/jiu396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joyner MJ, Bruno KA, Klassen SA, et al. Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients. Mayo Clin Proc. 2020;95(9):1888-1897. doi: 10.1016/j.mayocp.2020.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US Food and Drug Administration . FDA issues Emergency Use Authorization for convalescent plasma as potential promising COVID-19 treatment, another achievement in administration’s fight against pandemic. Published August 24, 2020. Accessed January 27, 2021. https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-convalescent-plasma-potential-promising-covid-19-treatment
- 4.RECOVERY Collaborative Group . Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med. Published online July 17, 2020. doi: 10.1056/NEJMoa2021436 [DOI] [Google Scholar]
- 5.Horby P, Mafham M, Linsell L, et al. ; RECOVERY Collaborative Group . Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383(21):2030-2040. doi: 10.1056/NEJMoa2022926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horby PW, Mafham M, Bell JL, et al. Lopinavir–ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2020;396(10259):1345-1352. doi: 10.1016/S0140-6736(20)32013-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horby P, Lim WS, Emberson J, et al. Effect of dexamethasone in hospitalized patients with COVID-19—preliminary report. medRxiv. Published online June 22, 2020. doi: 10.1101/2020.06.22.20137273 [DOI]
- 8.RECOVERY Trial . Press release: RECOVERY trial closes recruitment to convalescent plasma treatment for patients hospitalised with COVID-19. Published January 15, 2021. Accessed January 27, 2021. https://www.recoverytrial.net/news/statement-from-the-recovery-trial-chief-investigators-15-january-2021-recovery-trial-closes-recruitment-to-convalescent-plasma-treatment-for-patients-hospitalised-with-covid-19
- 9.Page M, McKenzie J, Bossuyt P, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. MetaArXiv. Published online September 14, 2020. doi: 10.31222/osf.io/v7gm2 [DOI]
- 10.McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS: peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40-46. doi: 10.1016/j.jclinepi.2016.01.021 [DOI] [PubMed] [Google Scholar]
- 11.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 12.Guyatt GH, Oxman AD, Vist GE, et al. ; GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langan D, Higgins JPT, Simmonds M. Comparative performance of heterogeneity variance estimators in meta-analysis: a review of simulation studies. Res Synth Methods. 2017;8(2):181-198. doi: 10.1002/jrsm.1198 [DOI] [PubMed] [Google Scholar]
- 14.IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14:25. doi: 10.1186/1471-2288-14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539-1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 16.Libster R, Pérez Marc G, Wappner D, et al. ; Fundación INFANT–COVID-19 Group . Early high-titer plasma therapy to prevent severe Covid-19 in older adults. N Engl J Med. Published online January 6, 2021. doi: 10.1056/NEJMoa2033700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P; PLACID Trial Collaborators . Convalescent plasma in the management of moderate COVID-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ. 2020;371:m3939. doi: 10.1136/bmj.m3939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simonovich VA, Burgos Pratx LD, Scibona P, et al. ; PlasmAr Study Group . A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. Published online November 24, 2020. doi: 10.1056/NEJMoa2031304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324(5):460-470. doi: 10.1001/jama.2020.10044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.AlQahtani M, Abdulrahman A, Almadani A, et al. Randomized controlled trial of convalescent plasma therapy against standard therapy in patients with severe COVID-19 disease. medRxiv. Published online November 4, 2020. doi: 10.1101/2020.11.02.20224303 [DOI] [PMC free article] [PubMed]
- 21.Bajpai M, Kumar S, Maheshwari A, et al. Efficacy of convalescent plasma therapy compared to fresh frozen plasma in severely ill COVID-19 patients: a pilot randomized controlled trial. medRxiv. Published online October 27, 2020. doi: 10.1101/2020.10.25.20219337 [DOI]
- 22.Gharbharan A, Jordans CCE, Geurtsvankessel C, et al. Convalescent plasma for COVID-19: a randomized clinical trial. medRxiv. Published online July 3, 2020. doi: 10.1101/2020.07.01.20139857 [DOI]
- 23.Avendaño-Solà C, Ramos-Martinez A, Muñez-Rubio E, et al. Convalescent plasma for COVID-19: a multicenter, randomized clinical trial. medRxiv. Published online September 29, 2020. doi: 10.1101/2020.08.26.20182444 [DOI]
- 24.Ray Y, Paul SR, Bandopadhyay P, et al. Clinical and immunological benefits of convalescent plasma therapy in severe COVID-19: insights from a single center open label randomised control trial. medRxiv. Published online November 29, 2020. doi: 10.1101/2020.11.25.20237883 [DOI]
- 25.European Union Recover Project . Press release: REMAP-CAP: international trial of SARS-CoV-2 convalescent plasma pauses enrollment of critically ill COVID-19 patients. Published online January 11, 2021. Accessed February 1, 2021. https://www.recover-europe.eu/press-release-international-trial-of-sars-cov-2-convalescent-plasma-pauses-enrollment-of-critically-ill-covid-19-patients/
- 26.RECOVERY Trial . Randomised evalution of COVID-19 therapy (RECOVERY protocol). Accessed January 27, 2021. https://www.recoverytrial.net/files/recovery-protocol-v12-1-2020-12-16.pdf
- 27.Horby PW, Roddick A, Spata E, et al. Azithromycin in hospitalised patients with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. medRxiv. Published online December 14, 2020. doi: 10.1101/2020.12.10.20245944 [DOI]
- 28.Zarin DA, Rosenfeld S. Lack of harmonization of coronavirus disease ordinal scales. Clin Trials. Published online December 15, 2020. doi: 10.1177/1740774520972082 [DOI] [PubMed] [Google Scholar]
- 29.Marshall JC, Murthy S, Diaz J, et al. ; WHO Working Group on the Clinical Characterisation and Management of COVID-19 Infection . A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20(8):e192-e197. doi: 10.1016/S1473-3099(20)30483-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petkova E, Antman EM, Troxel AB. Pooling data from individual clinical trials in the COVID-19 era. JAMA. 2020;324(6):543-545. doi: 10.1001/jama.2020.13042 [DOI] [PubMed] [Google Scholar]
- 31.Janiaud P, Axfors C, Saccilotto R, Hemkens L, Schmitt A. COVID-evidence: a living database of trials on interventions for COVID-19. Published online April 1, 2020. Last updated August 19, 2020. Accessed February 17, 2021. https://osf.io/gehfx
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Literature search strategies
eFigure 1. Flowchart of the literature search
eFigure 2. Forest plots with summary effect sizes per publication type for A) all-cause mortality, B) Length of hospitalization, and C) Mechanical ventilation requirement
eFigure 3. Association of convalescent plasma with A) study-defined clinical improvement and B) study-defined clinical deterioration for peer-reviewed and preprint trials (no pooling due to heterogeneity of outcome definitions)
eFigure 4. Serious adverse events reported in peer-reviewed and preprint trials (no pooling due to heterogeneity of outcome definitions)
eTable 1. Outcomes reported by trials
eTable 2. Sensitivity analyses
eTable 3. GRADE assessment
Data Availability Statement
Mortality was assessed in all 10 RCTs and for 8 of the trials it was assessed between 15 to 30 days after randomization (1 RCT assessed mortality at 60 days and 1 RCT did not report length of follow-up; eTable 1 in the Supplement). Length of hospital stay was assessed in 7 RCTs; 3 used medians or means (1 published in a peer-reviewed journal and 2 published as preprints), 1 used HRs (published as a preprint), and 3 used both medians and HRs (2 published in peer-reviewed journals and 1 published as a preprint). The need for mechanical ventilation use was reported in 5 RCTs (3 peer-reviewed and 2 preprints). Data on clinical deterioration and clinical improvement were available in 5 RCTs (3 peer-reviewed and 2 preprints) and 3 RCTs reported data on serious adverse events (1 peer-reviewed and 2 preprints).