Abstract
Eukaryotes critically rely on endocytosis of autologous and heterologous material to maintain homeostasis and to proliferate. Although mechanisms of endocytosis have been extensively identified in mammalian and plant systems along with model systems including budding yeast, relatively little is known about endocytosis in protozoan parasites including those belonging to the phylum Apicomplexa. Whereas it has been long established that the apicomplexan agents of malaria (Plasmodium spp.) internalize and degrade hemoglobin from infected red blood cells to acquire amino acids for growth, that the related and pervasive parasite Toxoplasma gondii has a functional and active endocytic system was only recently discovered. Here we discuss emerging and hypothesized mechanisms of endocytosis in Toxoplasma gondii with reference to model systems and malaria parasites. Establishing a framework for potential mechanisms of endocytosis in Toxoplasma gondii will help guide future research aimed at defining the molecular basis and biological relevance of endocytosis in this tractable and versatile parasite.
Keywords: Toxoplasma gondii, Plasmodium, malaria, endocytosis, ingestion, intracellular trafficking
1. Introduction
Endocytosis of extracellular material is fundamental to eukaryotic organisms. Eukaryotic cells require endocytosis to maintain membrane homeostasis, turnover membrane proteins, downregulate receptors, and acquire exogenous nutrients, among other functions [1]. Although it is generally expected that all eukaryotes are capable of endocytosis, the existence of a functional endocytic pathway in the protozoan parasite Toxoplasma gondii remained unclear until recently. There were several reasons for this. First, Toxoplasma gondii resides in a membrane-bound intracellular compartment called the parasitophorous vacuole (PV) that in most cell types does not receive host material via vesicular fusion. Therefore, it was not clear whether Toxoplasma gondii could access host resources via endocytosis. Second, the existence of a complete set of endocytic organelles in Toxoplasma gondii was not appreciated until the discovery of the parasite lysosome termed the Plant-Like Vacuole or Vacuolar Compartment (VAC, the term used hereafter) [2,3]. Finally, while Toxoplasma gondii has the basic elements for endocytosis including endosome-like compartments (ELCs) marked by Rab5 or Rab7, several studies indicated that ELCs were repurposed as a conduit for exocytic trafficking en route to regulated secretory organelles (micronemes and rhoptries) involved in parasite invasion [4,5,6,7,8]. Together with earlier suggestive studies [9,10], more recent work has established that Toxoplasma gondii undergoes active endocytosis of host cytosolic material during intracellular infection via the so-called ingestion pathway [11].
During invasion, Toxoplasma gondii burrows into the host cell, pushing the host plasma membrane in toward the cytosol, and pinching it off to form its intracellular niche, the PV. This cloak of host-derived membrane provides a protective barrier that resists fusion with host lysosomes and limits autophagic destruction of the parasite [12,13,14,15]. However, the PV also represents a potential impediment to accessing nutrients in the host cell cytosol including amino acids, lipids and nucleotides [16,17]. This potential barrier is overcome in several different ways including the permeability of the PV to small molecules [18,19], sequestration of host organelles within the PV [20,21,22], and by endocytosis [11,23], which is the main focus of this review. Toxoplasma gondii endocytosis can be broken down into two main steps. First, material from the host cell cytosol is taken up across the PV membrane (PVM) and parasite plasma membrane into endocytic vesicles in the parasite cytosol. Second, endocytic cargo is delivered via the endosomal system to the VAC for degradation. This review will compare emerging insights into endocytosis in Toxoplasma gondii with established paradigms in model organisms and malaria parasites to illustrate the vast potential for discovery in the field. We will also discuss the prospects for discovering novel mechanisms of endocytosis given the peculiarities of Toxoplasma gondii. For an additional perspective on the topic, readers are referred to another recent review comparing endocytosis in malaria parasites and Toxoplasma gondii [24].
2. Endocytosis and Endolysosomal Trafficking in Model Organisms
2.1. Clathrin-Dependent Mechanisms of Endocytosis in Model Organisms
Endocytosis involves bending of the plasma membrane in toward the cytosol and scission of the plasma membrane to form endocytic vesicles. Mechanisms underlying this process in model systems can be divided into three categories—clathrin-dependent, clathrin-independent/dynamin-dependent, and clathrin- and dynamin-independent (Figure 1). These pathways will be discussed in turn, beginning with clathrin-mediated endocytosis (see also [1,25,26,27] for comprehensive reviews).
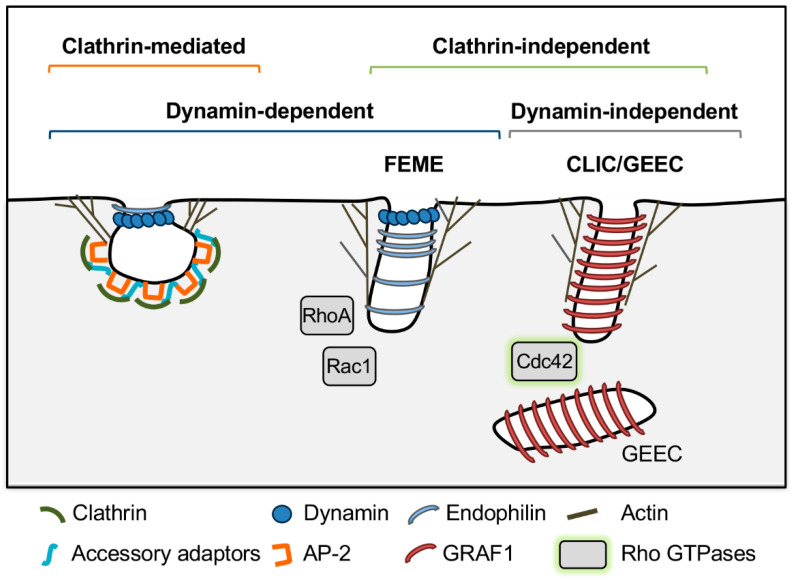
Figure 1.
Mechanisms of endocytosis in model systems. Note that these models are simplified from the text to depict key differentiating features of each pathway. See text for full description of endocytic mechanisms. Fast endophilin-mediated endocytosis (FEME), clathrin-independent carriers/glycosylphosphatidylinositol (GPI)-anchored protein enriched early endosomal compartments (CLIC/GEEC). Caveolin-mediated endocytosis was omitted because it is not relevant to Toxoplasma gondii and other apicomplexan parasites, which lack caveolin.
In mammalian cells, clathrin-mediated endocytosis facilitates internalization of transmembrane receptors. First, a nucleation complex, consisting of the F-BAR (Fes/CIP4 homology Bin-Amphiphysin-Rvs) domain proteins FCHO1/2 (Fer/Cip4 homology domain only 1 and 2), the clathrin adaptor complex for endocytosis adaptor protein 2 (AP-2), and the scaffolding proteins epidermal growth factor receptor substrate 15 (EPS15), EPS15-related protein (EPS15R) and intersectins 1 and 2 are recruited to phosphatidylinositol 4,5 bisphosphate (PI(4,5)P2 or PIP2) at the plasma membrane [1,27]. AP-2 serves as a major hub that binds to cytosolic domains of transmembrane cargoes, recruits accessory adaptors such as AP180/CALM (adaptor protein 180/clathrin assembly lymphoid myeloid), epsins and HIP1R (Huntingtin-interacting protein 1 related) and their associated cargoes and, together with the accessory adaptors, recruits clathrin to form the clathrin coat [1,27]. Membrane bending is driven by binding of BAR domains in FCHO1/2, insertion of amphipathic helices in membrane-binding ENTH (Epsin N-Terminal Homology Domain) and ANTH (AP180 N-Terminal Homology Domain) domains of epsin and CALM, respectively, and polymerization of a curved clathrin lattice that encases the forming vesicle, which may drive and/or stabilize membrane curvature [1,27]. Actin nucleation and branching-promoting proteins N-WASP (Neural Wiskott-Aldrich syndrome protein) and actin related protein 2//3 complex (Arp2/3) are also recruited, and drive polymerization of actin coupled to the clathrin coat through interactions with epsin and HIP1R. This is thought to push against the plasma membrane, elongating the neck of the forming vesicle and further promote membrane bending through tensile pressure [27,28,29]. Vesicle scission is mediated by recruitment of the N-BAR proteins endophilin and amphiphysin, which are thought to restrict the vesicle neck and also recruit the guanosine triphosphatase (GTPase) dynamin [27,30]. Dynamin is thought to act as a “pinchase” that forms a coil around vesicle necks and hydrolyzes guanosine triphosphate (GTP) to squeeze and sever the membrane to form endocytic vesicles [31]. Finally, the vesicle is uncoated and clathrin coat components are recycled back to the cytosol by HSC70 (heat shock cognate protein 70) and its cofactor auxillin/GAK (cyclin G-associated kinase) [1,27].
Although clathrin-mediated endocytosis is generally a highly conserved process, the requirement of key players in mammalian cells such as dynamin, AP-2 and other components of the nucleation complex varies among other eukaryotes. For example, the protozoan parasite Trypanosoma brucei undergoes clathrin-mediated endocytosis despite lacking AP-2 and having a single dynamin that is devoted to mitochondrial maintenance, but not endocytosis [32,33]. Plants lack FCHO1/2 and are not dependent on AP-2, but they have evolved a unique nucleator complex for clathrin-mediated endocytosis called the TPLATE complex, which is recruited to sites of clathrin-mediated endocytosis prior to AP-2 [34,35]. Similarly, AP-2 is not essential for yeast clathrin-mediated endocytosis; its only demonstrated role in clathrin-mediated endocytosis is for uptake of the yeast toxin K28 [1,36,37,38]. Finally, although the yeast dynamin Vps1 (vacuolar protein sorting 1) is required for efficient endocytic vesicle scission, its role in endocytosis is controversial. On the one hand, one study showed that Vps1 is recruited to sites of clathrin-mediated endocytosis, a dominant-negative Vps1 mutant severely inhibits endocytosis, and that Vps1 is able to tubulate liposomes in vitro, suggesting that dynamin may act as a pinchase, as in mammalian cells [39]. On the other hand, 22% of clathrin-mediated endocytic sites in this study lacked Vps1, and other studies have suggested that dynamin indirectly mediates membrane scission through its role in actin organization [39,40,41]. Accordingly, in less well understood systems, it should not be assumed that dynamin is necessary for clathrin-mediated endocytosis.
2.2. Clathrin-Independent Mechanisms of Endocytosis in Model Organisms
Mechanisms of clathrin-independent endocytosis are generally less understood and have been characterized mostly in mammalian cells. These pathways are diverse, but share common features, which include local production of phosphatidylinositols, and recruitment of Rho family GTPases (RhoA, Rac1 and Cdc42), actin nucleation proteins, and in some cases BAR domain proteins to promote membrane bending and scission for endocytic vesicle formation (see [42,43] for comprehensive reviews). For the purposes of this review, the FEME and CLIC-GEEC pathways will be described as representative examples of clathrin-independent endocytic pathways that are dynamin dependent or dynamin independent, respectively.
Fast endophilin-mediated endocytosis (FEME) mediates endocytosis of activated G protein-coupled receptors. Lamellipodin binds to phosphatidylinositol 3,4 bisphosphate (PI(3,4)P2) at the plasma membrane and recruits the N-BAR protein endophilin. Endophilin is thought to induce membrane curvature and recruits N-WASP, Arp2/3, and dynamin to drive actin polymerization and endocytic vesicle formation [43]. This process is regulated by the GTPases Rac1 and RhoA and the kinase Pak1, which also likely mediate N-WASP and Arp2/3 recruitment [44]. Finally, dynamin is required for scission of endocytic vesicles in FEME [44].
CLIC/GEEC, on the other hand, is a dynamin-independent pathway that mediates uptake of GPI-anchored proteins, transmembrane proteins and bulk uptake of fluid-phase markers into GPI-anchored protein enriched early endosomal compartments (GEEC) derived from fusion of uncoated clathrin-independent carriers (CLICs) [45,46]. In CLIC/GEEC, cycling of Cdc42 activity is required for productive endocytosis. GTPases cycle between active GTP-bound and inactive guanosine diphosphate (GDP)-bound states that are regulated by GTPase activating proteins (GAPs), which stimulate GTP hydrolysis, and guanine nucleotide exchange factors (GEFs), which exchange GDP for GTP [47]. Both Cdc42 activation and inactivation are required for productive actin polymerization, and the small GTPase Arf1 recruits the Cdc42 GAP protein ARHGAP10/21 to mediate Cdc42 inactivation [42,43]. Activated Cdc42 also recruits the GAP GRAF1, which regulates Cdc42 and also has a BAR domain that is thought to contribute to membrane curvature and work together with forces generated by actin polymerization to drive membrane fission in a dynamin-independent manner [42,43,48].
2.3. Endocytic Trafficking to the Lysosome in Model Organisms
In yeast and mammals, endocytosed cargoes, whether they are derived from clathrin-mediated or clathrin-independent endocytosis, are trafficked sequentially through the Rab5 endosome, the Rab7 endosome and then the lysosome [42,49]. These trafficking events are coordinated by the Rab GTPases Rab5 and Rab7. Rab5 activity is maintained by its guanine exchange factor (GEF), RABGEF1/Rabex5, and recruits the class C core vacuole/endosome tethering factor (CORVET) complex, which drives homotypic fusion of Rab5 endosomes [49,50,51,52]. Then the Rab7 GEF, SAND1/Mon1-Ccz1 is recruited, leading to dissociation of RABGEF1/Rabex5 and Rab5 and recruitment of Rab7 [53,54]. Finally, CORVET (composed of Vps3, 8, 11, 16, 18, and 33) is converted to the homotypic fusion and vacuole protein sorting (HOPS) complex by switching out Vps3 and 8 for Vps39 and 41 [53,54,55].
Plants employ the same conserved machinery as yeast and mammalian cells for trafficking to the lysosome but have several distinguishing features. First, plants deliver endocytosed cargoes initially to the trans Golgi network (TGN), and emerging evidence suggests that cargoes may be delivered to the plant vacuole by at least three different pathways [56,57]. The SAND1/Mon1-Ccz1 complex acts as a Rab7 GEF and mediates conversion from Rab5 to Rab7 on endosomes in a Rab5 and Rab7-dependent pathway similar to yeast and mammalian cells. However, plants also have pathways that are Rab5- and Rab7-independent and Rab5-dependent but Rab7-independent [57,58]. At least some Rab5 endosomes are derived by maturation of the TGN [59]. Homologs of CORVET and HOPS are also present in plants and promote trafficking to the plant vacuole [60]. Studies investigating shared core CORVET/HOPS subunits, support a role in trafficking to the plant vacuole. Vps16 interacts with Vps11 and Vps33 at late endosomal and lysosomal membranes and regulates biogenesis and fusion of the plant vacuole [61,62,63]. However, a recent study suggests that CORVET and HOPS do not act sequentially in plants but regulate distinct trafficking pathways to the plant vacuole. Plant CORVET subunit Vps3 interacts with Rab5 and is thought to mediate fusion of endosomes to the plant vacuole in the Rab5-dependent, Rab7-independent pathway, while HOPS subunits Vps39 and Vps41 interact with Rab7 and regulate homotypic fusion of vacuoles for the Rab5- and Rab7-dependent pathway [64,65].
3. Mechanisms of Endocytosis in Plasmodium spp.
Similar to Toxoplasma gondii, Plasmodium spp. parasites also replicate in a PV during acute infection, but they do so by infecting red blood cells (RBCs) and consuming RBC cytosol from within this niche. Electron microscopy studies show that RBC cytosol is taken up across the PVM and parasite plasma membrane simultaneously [66,67]. The PVM extends in toward a mouth-like structure at the parasite plasma membrane called the cytostome. Subsequent pinching off of both membranes results in a bite of the RBC cytosol encased in two layers of membrane derived from parasite plasma membrane and PVM.
Most attempts to define the molecular mechanisms involved in host cytosol uptake and delivery to the digestive vacuole have included small-molecule inhibitors. A role for dynamin was proposed after inhibition of Plasmodium falciparum dynamin-like protein 1 (PfDYN1) with the small-molecule dynamin inhibitor dynasore led to a reduction in the amount of hemoglobin internalized by the parasite [68]. Efforts to deplete the parasites of PfDYN1 to further elucidate its role in the uptake of host cytosol have not been fruitful, and analysis of its localization did not identify PfDYN1 at the cystosomal collar where actin was observed in some cases [69,70].
A study using actin inhibitors with different mechanisms of action, cytochalasin D (CytoD) and jasplakinolide (JAS), reported a decrease in the number of cystostomes following a short treatment [69]. Treatment also led to an increase in double membrane vesicles that were designated as part of a convoluted cystostome and further identified as cytostomal sections. This is comparable with the higher number of hemoglobin containing vesicles observed by other groups following actin inhibition [70,71]. Transport of hemoglobin to the food vacuole (FV) is negatively affected by actin polymerization, as observed by the inhibition of actin depolymerization using JAS and was further shown to be dependent on the actin-myosin motor [69,70]. Although these studies suggest a role for actin dynamics in endocytosis by malaria parasites in a manner akin to yeast [29,72], the precise role of actin in this process remain to be determined.
Rab5a was among the first proteins to be implicated in hemoglobin uptake by Plasmodium falciparum through genetic modification, in this case via expression of a constitutively active mutant [73]. However, more recent work using conditional mislocalization or conditional knockout of Rab5a resulted in growth arrest in the schizont stage [74]. The same study showed that conditional mislocalization of a Mon1 protein (a putative Rab7 GEF) was also growth arrested at the schizont stage. These findings are inconsistent with PfRab5a or PfMon1 playing a role in hemoglobin uptake, which principally occurs in the trophozoite stage. The first definitive genetic evidence of a protein’s role in hemoglobin uptake was reported for the vacuolar sorting protein 45, PfVps45. PfVps45 is a member of the Sec1/Munc-18 family, which in model systems is critical for activating SNAP receptor (SNARE) complexes for vesicular fusion. Conditional inactivation of PfVps45 led to an increase in vesicles containing host cytosolic material, thus impairing delivery of endocytosed material to the food vacuole [75]. Recent studies have provided additional insight into the molecular mechanisms involved in this process. The localization of the Kelch domain-containing protein (K13) at the cell periphery, forming a ring-like structure [76] and interacting with EPS15 have led to the identification of an endocytic compartment complex required for the uptake of hemoglobin [77]. Interestingly, the AP-2μ subunit, but not clathrin, localized with the K13-EPS15 complex, suggesting a potential role for clathrin-independent endocytosis that still requires the AP-2 complex. Several additional proteins were identified as interactors of K13 based on proximity biotinylation. Disruption of these interactors also blocked hemoglobin uptake, suggesting that the K13-EPS15 complex plays a crucial role in the early stages of uptake, possible during the formation of endocytic vesicles. Further in-depth characterization of the K13-EPS15 complex components should provide important new insight into endocytosis at the cytostome.
4. Endocytosis and Endocytic Trafficking in Toxoplasma gondii
4.1. Endocytic Trafficking across the PVM and Parasite Plasma Membrane
For the Toxoplasma gondii ingestion pathway, host cytosol must be taken up across the PVM and parasite plasma membrane before trafficking to the VAC, where it is degraded by cathepsin protease L (CPL) and other lysosomal hydrolases [11] (Figure 2). To track this process, fluorescent proteins such as GFP or mCherry are expressed in the host cytosol as non-specific tracers that can be visualized by microscopy. How these trafficking events are accomplished is not well established, but life inside a PV adds an extra layer of complexity. Possible mechanisms for endocytosis of host cytosolic fluorescent proteins into Toxoplasma gondii will involve trafficking across both the PVM and parasite plasma membrane to enter the parasite rather than just the plasma membrane in model organisms.
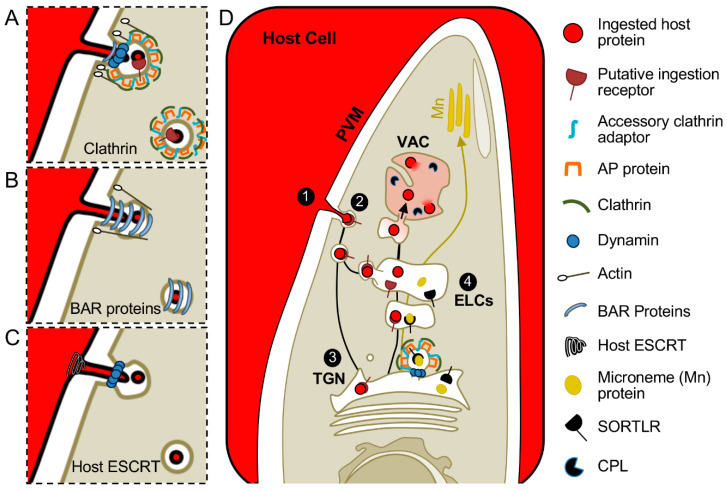
Figure 2.
Hypothetical models for Toxoplasma gondii endocytosis and trafficking of ingested protein. Ingested proteins are proposed to traverse the PVM in internally budding vesicles and enter the parasite at a discrete site. Mechanisms could resemble model systems and depend on coat proteins, dynamin, and actin. Examples of (A) clathrin-mediated or (B) clathrin-independent processes, represented by CLIC-GEEC, are depicted. (C) Scission of endocytic vesicles may also require host proteins for pinching of the PVM as depicted by possible recruitment of host Endosomal Sorting Complex Required for Transport (ESCRT) machinery and garroting at the parasite plasma membrane by a dynamin-related protein. (D) After scission from the PVM (step 1) and entering the parasite (step 2), guided by a putative ingestion receptor, the ingested material is proposed to traverse the TGN (step 3) before trafficking through the ELCs (step 4) en route to the VAC for degradation by proteases including CPL. Mn, microneme; Rop, rhoptry.
Similar to Plasmodium spp., Toxoplasma gondii has a mouth-like structure at the plasma membrane called the micropore, which was proposed to be a site of endocytosis in Toxoplasma gondii [9]. Electron microscopy studies show vesicles occupying the micropore that were proposed to be derived from the PVM and thus would contain material derived from the host cytosol [9]. However, it is impossible to say whether the vesicle-like structures in the Toxoplasma gondii micropore are free vesicles or continuous with the PVM, or whether or being trafficked in or out of the parasite. Further, although micropores are present in acute and chronic stages of infection, micropores occupied by these vesicle-like structures have only been reported in the chronic cyst stage [9]. Toxoplasma gondii and Plasmodium spp. might employ similar mechanisms for endocytic trafficking across the PVM and parasite plasma membrane, but currently there is limited evidence to support a common mechanism. Further, putative endocytic vesicles have been observed in Toxoplasma gondii at sites that are distinct from the micropore upon treatment of infected cells with excess lipids [78] or tracking of endocytosis in extracellular parasites [79]. Such sites lack the hallmark collar of the micropore and are often seen in a more posterior site than the micropore. These observations raise the possibility of multiple and mechanistically distinctive sites of endocytosis in Toxoplasma gondii.
4.2. Components of Clathrin-Mediated Endocytosis in Toxoplasma gondii
Clathrin-mediated endocytosis is a major route of endocytic uptake that is thought to be used by all eukaryotic organisms [1]. Although it has not been directly tested, there is some evidence to support that Toxoplasma gondii undergoes clathrin-mediated endocytosis. The micropore sometimes appears to have a proteinaceous coat detectable by electron microscopy [9]. There are two forms of endocytosis with characteristic coats visible by electron microscopy, clathrin-mediated endocytosis and caveolar endocytosis. Toxoplasma gondii does not express caveolin/cavin proteins required for caveolar endocytosis, and thus it is reasonable to consider clathrin as a prime candidate for a coat protein [80]. Consistent with a potential role for clathrin, many classical endocytic players are conserved in Toxoplasma gondii (e.g., dynamin-related proteins, epsin-like proteins, and actin) [8,81,82,83,84,85].
However, neither clathrin nor actin, dynamin-related proteins, or epsin-like proteins have been localized to sites of endocytosis at the parasite plasma membrane [8,81,82,83,84,85]. These proteins have been implicated in other roles including parasite motility and organelle dynamics (actin), maintenance of the chloroplast-like organelle called the apicoplast (the dynamin-related protein, DrpA), exocytic trafficking to the parasite secretory organelles called micronemes and rhoptries (clathrin, DrpB and potentially the epsin-like protein TgEpsL) [8,81,82,83,84,85] or daughter cell formation and organelle maintenance including fission of the parasite’s single mitochondrion (DrpC) [81,86]. Further, a bioinformatic search for homologs of genes in the yeast clathrin interactome revealed a paucity of effectors required for clathrin-mediated endocytosis [81]. While Pieperhoff et al. [81] found homologs for the effectors mentioned above, the scaffolding protein EPS15 and the clathrin adaptor complex AP-2, they did not find homologs for other crucial players in yeast and mammalian cells such as the nucleator intersectin, adaptors AP180 or epsin, actin polymerizing and branching proteins N-WASP and Arp2/3, Hip1R, which links actin to the clathrin coat, or uncoating proteins related to auxillin [1,27,81].
Regardless, missing key effectors may not be problematic. It is possible that Toxoplasma gondii does not require components of the canonical clathrin-mediated endocytosis machinery or may have evolved novel effector proteins. Protein BLAST (basic local alignment search tool) searches did not reveal obvious homologs of any TPLATE complex members in the Toxoplasma gondii genome, but other yet to be discovered parasite-specific effectors could exist and coordinate clathrin-mediated endocytosis. For example, Toxoplasma gondii does encode two hypothetical BAR domain proteins (TGME49_259720 and TGME49_320760) and a hypothetical ENTH-domain containing protein (TGME49_216030) that could participate in membrane bending or nucleation or endocytosis like FCHO1/2 (Table 1).
Table 1.
Toxoplasma gondii endocytic proteins.
| GeneID | Protein Name |
Domain/Complex | Known or Prospective Function | Phenotype Score [87] |
|---|---|---|---|---|
| TGME49_290950 | Clathrin | Heavy chain | Interacts with AP-1 mu1 at TGN [8], promotes microneme and rhoptry trafficking at the TGN [81] | −4.77 |
| TGME49_221940 | AP2 | Alpha subunit | Clathrin adaptor for endocytosis? [88] | −4.32 |
| TGME49_240870 | Beta2 subunit | −2.83 | ||
| TGME49_230920 | Mu2 subunit | −1.60 | ||
| TGME49_313450 | sigma-1 subunit | −2.88 | ||
| TGME49_320760 | Hypothetical | BAR/IRSp53 and MIM homology domain (IMD)-like domain | Promote membrane curvature? | 0.56 |
| TGME49_259720 | Hypothetical | BAR/IMD-like domain | Promote membrane curvature? | −4.55 |
| TGME49_216030 | Hypothetical | ENTH/ Vps-27, Hrs and STAM (VHS) domain | Promote membrane curvature, clathrin adaptor protein? | −0.67 |
| TGME49_227800 | TgEPS15 | Eps15 homology domain | Clathrin-mediated endocytosis nucleation/scaffold protein? | −3.19 |
| TGME49_214180 | TgEpsL | ENTH domain | Interacts with TgAP-1 at TGN but likely not AP-2, formation of clathrin coated vesicles in secretory trafficking [8] | −0.52 |
| TGME49_213370 | Frm3 | Actin binding | Actin nucleation during endocytosis? [89] | −2.79 |
| TGME49_267045 | Hypothetical | Ras superfamily/Rho GTPase | Regulation of actin during endocytosis? | 0.81 |
| TGME49_249170 | Hypothetical | Ras superfamily/Rho GTPase | Regulation of actin during endocytosis? | −0.79 |
| TGME49_233300 | Hypothetical | Rho GTPase activating protein (RhoGAP) | Regulation of actin during endocytosis? | 1.52 |
| TGME49_321620 | DrpB | GTPase | Microneme and rhoptry trafficking at the TGN | −4.91 |
| TGME49_270690 | DrpC | GTPase | Mitochondrial maintenance? [81] | −4.54 |
| TGME49_267810 | Rab5A | GTPase | Microneme and rhoptry trafficking at the TGN/ELCs [5] | −4.48 |
| TGME49_207460 | Rab5B | GTPase | Undefined [5], recycling to plasma membrane? | 0.55/−1.35 |
| TGME49_219720 | Rab5C | GTPase | Microneme and rhoptry trafficking at the TGN/ELCs [5] | −4.24 |
| TGME49_248880 | Rab7 | GTPase | Undefined [5], fusion with the lysosome? | −2.67 |
| TGME49_230220 | Vps11 | CORVET/HOPS | Exocytic trafficking to the micronemes and rhoptries [6], biogenesis of the VAC [6], endocytic trafficking through Rab5 and Rab7 compartments to the VAC? | −4.09 |
| TGME49_320670 | Vps16 | CORVET/HOPS | Exocytic trafficking to the micronemes and rhoptries? Endocytic trafficking through Rab5 and Rab7 compartments to the VAC? | −4.82 |
| TGME49_289730 | Vps18 | CORVET/HOPS | Exocytic trafficking to the micronemes and rhoptries [6], endocytic trafficking through Rab5 and Rab7 compartments to the VAC? | −3.24 |
| TGME49_295000 | Vps33 | CORVET/HOPS | Exocytic trafficking to the micronemes and rhoptries? Endocytic trafficking through Rab5 and Rab7 compartments to the VAC? | −4.78 |
| TGME49_224270 | Vps41 | HOPS | Exocytic trafficking to the micronemes and rhoptries? Endocytic trafficking through Rab5 and Rab7 compartments to the VAC? | −3.75 |
| TGME49_315530 | Vps39 | HOPS | Exocytic trafficking to the micronemes and rhoptries [6], endocytic trafficking through Rab5 and Rab7 compartments to the VAC? | −4.18 |
| TGME49_291120 | Mon1 | SAND1/Mon1-Ccz1 | Rab7 GEF? Exocytic trafficking to the micronemes and rhoptries [6], endocytic trafficking through Rab5 and Rab7 compartments to the VAC? | −4.42 |
| TGME49_207960 | Ccz1 | SAND1/Mon1-Ccz1 | Rab7Gef? Exocytic trafficking to the micronemes and rhoptries? Endocytic trafficking through Rab5 and Rab7 compartments to the VAC? | −3.96 |
| TGME49_271060 | Vps45 | Sec1 family protein | Vesicular trafficking of endocytosed material to the VAC [90] | −5.04 |
| TGME49_224710 | VSR1 | PA domain | Undefined, cargo recognition for sorting to the VAC? | −0.90 |
| TGME49_312860 | VSR2 | PA domain | Undefined cargo recognition for sorting to the VAC? | −0.09 |
It is important to note that while many of the canonical clathrin-mediated endocytosis effectors have established roles in exocytic trafficking in Toxoplasma gondii, only DrpB has been functionally tested in the context of host protein endocytosis. DrpB is not required for Toxoplasma gondii endocytosis, suggesting that it is dedicated to exocytic trafficking [23]. However, this should not be interpreted to mean that Toxoplasma gondii endocytosis is independent of dynamin. As noted above, Toxoplasma gondii expresses two other dynamin-related proteins, DrpA and DrpC. DrpA is responsible for the maintenance of the apicoplast [84]. DrpC localizes to cytoplasmic puncta and is associated with formation of daughter parasites and mitochondria constriction; however, it physically interacts with components of the AP-2 complex, a homolog of intersectin-1, and a homolog of the malaria K13 protein [86]. How its subcellular localization is tied to its physical interaction with components of endocytosis and vesicular trafficking remains unknown. Since the roles of DrpA and DrpC have not been tested in the context of host protein endocytosis, it is still possible that a dynamin-related protein could drive endocytic vesicle fission. Regardless, testing the role of clathrin itself in host protein endocytosis will ultimately determine whether clathrin-mediated endocytosis exists in Toxoplasma gondii. This should be a priority in the field.
4.3. Components of Clathrin-Independent Endocytosis into Toxoplasma gondii
It is also possible that ingestion of host proteins by Toxoplasma gondii could resemble clathrin-independent mechanisms and be coordinated by actin, regulatory Rho family GTPases, and/or BAR-domain containing proteins [42,43,91]. In the absence of obvious N-WASP and Arp2/3 homologs, actin could be nucleated by formins similar to the Rho1-dependent pathway in yeast. This pathway is dependent on Rho1 and formin, but independent of clathrin and Arp2/3 [92]. Toxoplasma gondii expresses three formin proteins capable of polymerizing actin (Frm1, Frm2 and Frm3). Whereas Toxoplasma gondii Frm1 and Frm2 promote parasite motility during invasion, Frm3 is not required for invasion and localizes to a discrete, unidentified structure at the apical end of the parasite [89,93]. Toxoplasma gondii also has two hypothetical Rho-like GTPases and a hypothetical RhoGAP-like protein, TGME49_267045, TGME49_249170 and TGME49_233300, respectively (Table 1). Alternatively, proteins with N-BAR or ENTH domains that have amphipathic helices may act independently in membrane scission. The N-BAR domains of endophilin and amphiphysin and the ENTH domain of epsin have amphipathic helices and are capable of vesiculating liposomes in vitro [94,95]. Further, overexpression of epsin in mammalian cells can rescue scission of clathrin-coated vesicles when dynamin is knocked down [95].
4.4. Potential Contribution of Parasite Proteins Associated with the PVM and PV Lumen
While parasite cytosolic proteins discussed above putatively coordinate invagination and scission of the parasite plasma membrane, there is no evidence that such proteins are secreted. Thus, other factors must be involved in creating vesicles at the PVM. Parasite proteins secreted into the PV lumen from secretory organelles called the dense granules modify the PV and are thought to facilitate acquisition of nutrients from the host cell. GRA2, 4 and 6 promote formation of a system of membrane tubules invaginated from the PVM called the intravacuolar network (IVN), which is thought to act like villi, increasing surface area of the PVM for maximum nutrient exchange [96,97,98]. GRA7 has been implicated in scavenging cholesterol from the host cell via structures called Host Organelle Sequestering Tubulo-structures (H.O.S.T.s) [20]. In H.O.S.T.s, host lysosomes are recruited to the PV on microtubules, engulfed in PV membrane invaginations and pinched off, presumably by GRA7, to form lysosome-containing vesicles in the PV lumen. The ability of Toxoplasma gondii to ingest host proteins is reduced by 40% in parasites lacking GRA2 and unaffected in the absence of GRA7 [11]. This precludes a H.O.S.T.-like mechanism and suggests that the IVN contributes to host protein ingestion. In addition to its suggested role in nutrient exchange, the IVN also acts as a structural component to organize replicated parasites into a characteristic rosette pattern within the PV [99]. It remains to be determined whether the IVN plays a direct role in the ingestion pathway by acting as vesicular conduit or an indirect role as a structural organizer of parasites in the PV.
4.5. Potential Contribution of Host Proteins for Trafficking across the PVM
Host proteins also have the potential to contribute to Toxoplasma gondii endocytosis across the PVM. The host Endosomal Sorting Complex Required for Transport (ESCRT) is a rational candidate for several reasons. First, ESCRT protein complexes (ESCRT-0, -I, -II, -III and the ATPase Vps4) are expressed in the host cell cytosol and are sequentially recruited to endosomal compartments to invaginate (ESCRT 0–II) and pinch off endosomal membranes (Vps4) to release vesicles into the lumen of the endosomal compartment [100]. The topology of this reaction matches the requirement for Toxoplasma gondii endocytosis, membrane bending and scission away from the cytosol to create vesicles in the PV lumen.
Second, a recent study found that host ESCRT machinery is hijacked by Toxoplasma gondii for invasion. PT/SAP, YPXL, and PPXY amino acid motifs were recently found in the Toxoplasma gondii rhoptry neck proteins RON2, 4 and 5 [101]. These same motifs are found in the Gag proteins of retroviruses such as Human Immunodeficiency Virus (HIV) and Rous Sarcoma Virus and are well-documented motifs for recruitment of the ESCRT complex for budding from the plasma membrane [102,103]. PT/SAP and YPXL motifs in the cytosolic portion of HIV Gag directly recruit Tsg101 of ESCRT-I and the ESCRT-associated protein ALIX [103]. ALIX binds to HIV Gag, Tsg101 and CHMP4 of ESCRT-III, and facilitates recruitment of Vps4 to mediate scission of HIV particles out of the cell [103]. PPXY motifs in Rous Sarcoma Virus Gag recruit Nedd4 family ubiquitin ligases, which may ubiquitinate Gag or recruit arrestin-related trafficking proteins (ARTs) to provide a scaffold for ESCRT assembly at the viral budding site [100,103,104,105,106,107]. Similarly, these motifs in RON4 and 5 facilitated recruitment of ALIX and Tsg101 to the moving junction for host cell invasion [101]. PX(P/A)XPR motifs were also identified in RON2, 4 and 5 and contributed to recruitment of the ALIX binding partner CIN85 and its homolog CD2AP to the moving junction [101].
Interestingly, host protein endocytosis is initiated during or immediately following invasion and is active through at least the first 6 h post-invasion [23]. RON2, 4 and 5 are in the moving junction complex during host cell invasion, and at least part of this complex remains on the PVM for several hours following invasion, as evidenced by staining of RON4 and RON5 [108,109]. It will be interesting to know if RON4 and 5 remain competent to recruit ESCRT to the PVM following invasion and if ESCRT or other host proteins contribute to Toxoplasma gondii endocytosis.
4.6. Does Endocytic Trafficking in Toxoplasma gondii Resemble Yeast/Mammals or Plants?
Toxoplasma gondii belongs to the phylum Apicomplexa, which has evolutionary origins that are thought to predate the split between plants and animals and share features of both [110]. In relation to plants in particular, Toxoplasma gondii possesses a vacuolar pyrophosphatase proton pump (VP1) and a Rab5 isoform (Rab5B) specific to plants that are associated with its endolysosomal system [2,5]. This suggests endocytic trafficking to the VAC could resemble either yeast/mammals or plants. A recent study demonstrated that endocytic trafficking proceeds through the ELCs, but whether endocytic trafficking also proceeds through the TGN as in plants remains unclear, since current markers used to identify the TGN also label the ELCs to some extent [23]. That fluorescent phospholipids endocytosed by extracellular parasites colocalized with Vps53, a putative TGN marker, provides further support for the potential involvement of the parasite TGN as a staging site for endocytic material [79]. Plant-like features of Toxoplasma gondii endocytosis should be of particular interest for future work given their therapeutic potential and identifying specific and validated markers of the TGN will be a crucial first step.
4.7. Endocytic Trafficking to the VAC Intersects with Exocytic Trafficking to the Micronemes
Toxoplasma gondii also expresses key players for trafficking of endocytosed material to the lysosome including three Rab5 proteins, the Rab5 GEF Vps9, Rab7, the Rab7 GEFs Mon1 and Ccz1, the core subunits shared by the CORVET and HOPS complexes (Vps11, Vps16, Vps18, Vps33) and the HOPS-specific subunits Vps39 and Vps41 [5,6,111]. Based on model systems, trafficking to the VAC would be predicted to involve a Rab5 to Rab7 switch mediated by recruitment of the Rab7 GEF SAND1/Mon1-Ccz1 and conversion of the CORVET complex to the HOPS complex [26,53,54]. However, exocytic trafficking to the parasite’s unique secretory organelles called the micronemes and rhoptries proceeds through the TGN and ELCs and requires Rab5A, Rab5C, Vps9, and the HOPS complex [6]. This could indicate that these proteins, which are typically involved in endocytic trafficking, have been uniquely adapted for exocytic trafficking and secretion. Alternatively, endocytic and exocytic trafficking could share at least some common trafficking mechanisms. Recent evidence suggests that endocytic trafficking of ingested host proteins intersects with exocytic trafficking of microneme proteins but not rhoptry proteins [23]. Similar findings of an intersection of the endocytic and exocytic systems were reported from tracing the uptake and trafficking of fluorescent phospholipids and nano-gold particles in extracellular parasites [79]. The merging of endocytic and exocytic trafficking is intriguing given that ingested host proteins are sent to the VAC for degradation whereas microneme proteins must arrive intact at the microneme organelles to mediate their essential roles in parasite invasion and egress [3,5,6,8,81,111,112]. Therefore, in addition to possibly sharing trafficking mechanisms, especially where trafficking is merged, there will need to be distinct sorting mechanisms to ensure that endocytic and exocytic cargoes are sorted to their appropriate destinations.
Sorting of microneme and rhoptry proteins is partially understood. The sorting receptor sortillin (TgSORTLR) and transmembrane microneme and rhoptry proteins bind to soluble microneme and rhoptry proteins in the TGN [113,114,115]. The clathrin adaptor protein complex AP-1 is thought to recognize tyrosine-based and acidic sorting signals in the cytoplasmic portion of TgSORTLR or transmembrane microneme and rhoptry proteins [4,8]. AP-1 also interacts with the epsin-like protein TgEpsL, and together they are thought to recruit clathrin for sorting from the TGN to the ELCs via clathrin-coated vesicles that are pinched off by DrpB [8,86]. How microneme and rhoptry proteins are sorted from ELCs is not known.
Whether ingested host proteins utilize sorting mechanisms that overlap with those of microneme and rhoptry is also unknown and should be a focus of future studies. Further, it is important to remember that if the Plasmodium model of endocytosis is correct, then what is actually being trafficked to the VAC are host protein-containing vesicles. These endocytosed vesicles could be delivered to the VAC for degradation via a bulk flow pathway as demonstrated for endocytosed red fluorescent protein, RFP, delivered to the TGN in plants without the help of any sorting signals or sorting receptors [49,116,117]. On the other hand, sorting receptors could recognize parasite proteins embedded in the PVM-derived vesicle membrane. Recognition by such receptors could begin as early as the plasma membrane and could guide trafficking anywhere along the pathway to the VAC.
It will also be interesting to understand other mechanisms that distinguish endocytic and exocytic trafficking in Toxoplasma gondii. That disruption of TgVps45 affects the trafficking of ingested host proteins to the VAC, but not the biogenesis or discharge of microneme or rhoptry proteins [90], suggests distinct mechanisms. Further, DrpB is required for trafficking of microneme and rhoptry proteins from the TGN to their respective organelles, but DrpB is not required for trafficking of ingested host proteins to the VAC [23]. The roles of Rab5 proteins, Rab7, their associated GEFs, CORVET and HOPS in Toxoplasma gondii endocytosis have not been tested. Like plants and Plasmodium spp., Toxoplasma gondii expresses two C-terminally geranylated, conventional Rab5 isoforms, Rab5A and Rab5C, and the aforementioned plant-specific isoform that is N-terminally myristoylated, Rab5B [5,118,119]. The conventional Rab5A and Rab5C show nearly perfect colocalization on endosomal membranes, and expression of dominant negative mutants of Rab5A, Rab5C and the Rab5A GEF Vps9 disrupts trafficking of a subset of microneme and rhoptry proteins to their respective organelles [5,111]. The plant-like Rab5B colocalizes partially with RabA and Rab5C and also to the parasite plasma membrane [5]. Rab5B has no defined function since expression of dominant negative Rab5B had no effect on exocytic trafficking to the microneme and rhoptry organelles [5]. Similarly, expression of dominant negative Rab7 also did not interrupt microneme and rhoptry biogenesis, suggesting that Rab7 is not required for exocytic trafficking in Toxoplasma gondii. Yet, the putative Rab7 GEF Mon1 is required for the biogenesis of micronemes and rhoptries [5,6], thus clouding the role of Rab7 in exocytic trafficking. The possibility that Rab5A and Rab5C are dedicated to exocytic trafficking and Rab5B, Rab7, and a second putative Rab7 GEF, Ccz1, are devoted to endocytic trafficking is worthy of additional investigation.
4.8. The Function and Importance of Toxoplasma gondii Endocytosis
Although the exact function and importance of Toxoplasma gondii ingestion are not known, there is reason to believe that endocytosis could be central to parasite survival. Endocytosis is essential for nutrient acquisition, intracellular signaling, plasma membrane homeostasis, and receptor turnover in eukaryotes [1]. Further, host cytosol ingestion in Plasmodium spp. parasites is essential for acquisition of amino acids [120]. Targeting this pathway has been a successful therapeutic strategy [121,122].
Beyond nutrient acquisition from the host cell, endocytosis could be important for cell signaling, membrane turnover, or specifically in the case of Toxoplasma gondii evading the immune response or sensing the host cell environment [1]. Accordingly, the virulence defect of CPL knockout parasites is completely rescued in the absence of interferon gamma receptor, suggesting that protein degradation in the VAC is somehow involved in immune evasion [11]. CPL was also recently identified in a genetic screen as conferring fitness in interferon gamma activated macrophages [123]. As for sensing, although ingestion of host cytosolic mCherry across the PVM is expected to occur by a bulk flow-like mechanism, the parasite could have receptors on the PVM that bind to and internalize components of cell intrinsic defense systems as an immune evasion strategy or it could use PVM receptors as a sensory system to monitor the health of the cells it infects.
Finally, although host cytosol ingestion has been recently reported to also occur in chronic stage Toxoplasma gondii bradyzoites [124], the precise role of this pathway in bradyzoite biology remains to be determined. Parasites lacking CPL can differentiate into cysts normally, but eventually accumulate undigested autophagosomes and die [125]. Whether autophagosomes accumulate solely because they are not being turned over or are induced in response to starvation for host-derived nutrients such as amino acids, including those derived from the degradation of ingested host cytosolic proteins, is unclear. Future dissection of these possibilities will help clarify how the parasite uses its endolysosomal system to persist indefinitely in its hosts.
5. Conclusions
How, when, and why Toxoplasma gondii ingests proteins from the host cell cytosol largely remain a mystery. Endocytosis in model systems was heavily relied upon for envisioning how endocytosis works in Toxoplasma gondii due to the infancy of the field, but what is most exciting are the peculiarities of Toxoplasma gondii and potential parasite-specific aspects that cannot be predicted. The paucity of conserved endocytic players and the fact that Toxoplasma gondii performs endocytosis across two membranes presents an exciting opportunity to discover potentially novel aspects of endocytosis for eukaryotes. Toxoplasma gondii may also serve as a useful model of endocytosis in other parasitic pathogens. The analogous endocytic pathway for ingestion of red blood cell cytosol in Plasmodium spp. has been a successful therapeutic strategy, but drug resistance to nearly every antimalarial treatment is emerging and yet there remains very little known about how this pathway works [120,121,122,126]. It is also not known whether other apicomplexan parasites have analogous ingestion pathways, including Cryptosporidium which causes serious diarrheal illness that is often not curable in immunocompromised individuals [127]. Although nothing is known about ingestion by Toxoplasma gondii sexual stages, Eimeria macrogametes were reported to internalize nanoparticles [128], and thus further interrogation of apicomplexan sexual stages is warranted. Future studies of Toxoplasma gondii endocytosis have the potential to deliver exciting and impactful results that could help explain the remarkable success and promiscuity of this intracellular pathogen.
Author Contributions
O.L.M. conceptualized and drafted this manuscript; Y.R.-C. and V.B.C. wrote parts of this manuscript; O.L.M., Y.R.-C. and V.B.C. reviewed and edited this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the US National Institutes of Health, grant number R01AI120607 (to V.B.C.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McMahon H.T., Boucrot E. Molecular Mechanism and Physiological Functions of Clathrin-mediated Endocytosis. Nat. Rev. Mol. Cell Biol. 2011;12:517–533. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- 2.Miranda K., Pace D.A., Cintron R., Rodrigues J.C., Fang J., Smith A., Rohloff P., Coelho E., de Haas F., de Souza W., et al. Characterization of a Novel Organelle in Toxoplasma Gondii with Similar Composition and Function to the Plant Vacuole. Mol. Microbiol. 2010;76:1358–1375. doi: 10.1111/j.1365-2958.2010.07165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parussini F., Coppens I., Shah P.P., Diamond S.L., Carruthers V.B. Cathepsin L Occupies a Vacuolar Compartment and Is a Protein Maturase within the Endo/Exocytic System of Toxoplasma Gondii. Mol. Microbiol. 2010;76:1340–1357. doi: 10.1111/j.1365-2958.2010.07181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sloves P.J., Delhaye S., Mouveaux T., Werkmeister E., Slomianny C., Hovasse A., Alayi T.D., Callebaut I., Gaji R.Y., Schaeffer-Reiss C., et al. Toxoplasma Sortilin-like Receptor Regulates Protein Transport and Is Essential for Apical Secretory Organelle Biogenesis and Host Infection. Cell Host Microbe. 2012;11:515–527. doi: 10.1016/j.chom.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Kremer K., Kamin D., Rittweger E., Wilkes J., Flammer H., Mahler S., Heng J., Tonkin C.J., Langsley G., Hell S.W., et al. An Overexpression Screen of Toxoplasma Gondii Rab-GTPases Reveals Distinct Transport Routes to the Micronemes. PLoS Pathog. 2013;9:e1003213. doi: 10.1371/journal.ppat.1003213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morlon-Guyot J., Pastore S., Berry L., Lebrun M., Daher W. Toxoplasma Gondii Vps11, a Subunit of HOPS and CORVET Tethering Complexes, Is Essential for the Biogenesis of Secretory Organelles. Cell. Microbiol. 2015;17:1157–1178. doi: 10.1111/cmi.12426. [DOI] [PubMed] [Google Scholar]
- 7.Sangare L.O., Alayi T.D., Westermann B., Hovasse A., Sindikubwabo F., Callebaut I., Werkmeister E., Lafont F., Slomianny C., Hakimi M.A., et al. Unconventional Endosome-like Compartment and Retromer Complex in Toxoplasma Gondii Govern Parasite Integrity and Host Infection. Nat. Commun. 2016;7:11191. doi: 10.1038/ncomms11191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venugopal K., Werkmeister E., Barois N., Saliou J.M., Poncet A., Huot L., Sindikubwabo F., Hakimi M.A., Langsley G., Lafont F., et al. Dual Role of the Toxoplasma Gondii Clathrin Adaptor AP1 in the Sorting of Rhoptry and Microneme Proteins and in Parasite Division. PLoS Pathog. 2017;13:e1006331. doi: 10.1371/journal.ppat.1006331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nichols B.A., Chiappino M.L., Pavesio C.E. Endocytosis at the Micropore of Toxoplasma Gondii. Parasitol. Res. 1994;80:91–98. doi: 10.1007/BF00933773. [DOI] [PubMed] [Google Scholar]
- 10.Botero-Kleiven S., Fernández V., Lindh J., Richter-Dahlfors A., von Euler A., Wahlgren M. Receptor-Mediated Endocytosis in an Apicomplexan Parasite (Toxoplasma Gondii) Exp. Parasitol. 2001;98:134–144. doi: 10.1006/expr.2001.4624. [DOI] [PubMed] [Google Scholar]
- 11.Dou Z., McGovern O.L., Cristina M.D., Carruthers V.B. Toxoplasma Gondii Ingests and Digests Host Cytosolic Proteins. mBio. 2014;5:e01188-14. doi: 10.1128/mBio.01188-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mordue D.G., Sibley L.D. Intracellular Fate of Vacuoles Containing Toxoplasma Gondii Is Determined at the Time of Formation and Depends on the Mechanism of Entry. J. Immunol. 1997;159:4452–4459. [PubMed] [Google Scholar]
- 13.Mordue D.G., Desai N., Dustin M., Sibley L.D. Invasion by Toxoplasma Gondii Establishes a Moving Junction That Selectively Excludes Host Cell Plasma Membrane Proteins on the Basis of Their Membrane Anchoring. J. Exp. Med. 1999;190:1783–1792. doi: 10.1084/jem.190.12.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mordue D., Hakansson S., Niesman I.R., Sibley L.D. Toxoplasma Gondii Resides in a Vacuole That Resists Fusion with Host Cell Endocytic and Exocytic Vesicular Trafficking Pathways. Exp. Parasitol. 1999;92:87–99. doi: 10.1006/expr.1999.4412. [DOI] [PubMed] [Google Scholar]
- 15.Ling Y.M., Shaw M.H., Ayala C., Coppens I., Taylor G.A., Ferguson D.J., Yap G.S. Vacuolar and Plasma Membrane Stripping and Autophagic Elimination of Toxoplasma Gondii in Primed Effector Macrophages. J. Exp. Med. 2006;203:2063–2071. doi: 10.1084/jem.20061318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blader I.J., Koshy A.A. Toxoplasma Gondii Development of Its Replicative Niche: In Its Host Cell and Beyond. Eukaryotic Cell. 2014;13:965–976. doi: 10.1128/EC.00081-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marino N.D., Boothroyd J.C. Toxoplasma Growth in Vitro Is Dependent on Exogenous Tyrosine and Is Independent of AAH2 Even in Tyrosine-Limiting Conditions. Exp. Parasitol. 2017;176:52–58. doi: 10.1016/j.exppara.2017.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joiner K.A., Beckers C.J., Bermudes D., Ossorio P.N., Schwab J.C., Dubremetz J.F. Structure and Function of the Parasitophorous Vacuole Membrane Surrounding Toxoplasma Gondii. Ann. N. Y. Acad. Sci. 1994;730:1–6. doi: 10.1111/j.1749-6632.1994.tb44233.x. [DOI] [PubMed] [Google Scholar]
- 19.Gold D.A., Kaplan A.D., Lis A., Bett G.C., Rosowski E.E., Cirelli K.M., Bougdour A., Sidik S.M., Beck J.R., Lourido S., et al. The Toxoplasma Dense Granule Proteins GRA17 and GRA23 Mediate the Movement of Small Molecules between the Host and the Parasitophorous Vacuole. Cell Host Microbe. 2015;17:642–652. doi: 10.1016/j.chom.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coppens I., Dunn J.D., Romano J.D., Pypaert M., Zhang H., Boothroyd J.C., Joiner K.A. Toxoplasma Gondii Sequesters Lysosomes from Mammalian Hosts in the Vacuolar Space. Cell. 2006;125:261–274. doi: 10.1016/j.cell.2006.01.056. [DOI] [PubMed] [Google Scholar]
- 21.Romano J.D., Sonda S., Bergbower E., Smith M.E., Coppens I. Toxoplasma Gondii Salvages Sphingolipids from the Host Golgi through the Rerouting of Selected Rab Vesicles to the Parasitophorous Vacuole. Mol. Biol. Cell. 2013;24:1974–1995. doi: 10.1091/mbc.e12-11-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romano J.D., Nolan S.J., Porter C., Ehrenman K., Hartman E.J., Hsia R.C., Coppens I. The Parasite Toxoplasma Sequesters Diverse Rab Host Vesicles within an Intravacuolar Network. J. Cell Biol. 2017;216:4235–4254. doi: 10.1083/jcb.201701108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGovern O.L., Rivera-Cuevas Y., Kannan G., Jr A.N., Carruthers V.B. Intersection of Endocytic and Exocytic Systems in Toxoplasma Gondii. Traffic. 2018;19:336–353. doi: 10.1111/tra.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spielmann T., Gras S., Sabitzki R., Meissner M. Endocytosis in Plasmodium and Toxoplasma Parasites. Trends Parasitol. 2020;36:520–532. doi: 10.1016/j.pt.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Weinberg J., Drubin D.G. Clathrin-Mediated Endocytosis in Budding Yeast. Trends Cell Biol. 2012;22:1–13. doi: 10.1016/j.tcb.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valencia J.P., Goodman K., Otegui M.S. Endocytosis and Endosomal Trafficking in Plants. Annu. Rev. Plant Biol. 2016;67:309–335. doi: 10.1146/annurev-arplant-043015-112242. [DOI] [PubMed] [Google Scholar]
- 27.Kaksonen M., Roux A. Mechanisms of Clathrin-Mediated Endocytosis. Nat. Rev. Mol. Cell Biol. 2018;19:313–326. doi: 10.1038/nrm.2017.132. [DOI] [PubMed] [Google Scholar]
- 28.Mooren O.L., Galletta B.J., Cooper J.A. Roles for Actin Assembly in Endocytosis. Annu. Rev. Biochem. 2012;81:661–686. doi: 10.1146/annurev-biochem-060910-094416. [DOI] [PubMed] [Google Scholar]
- 29.Goode B.L., Eskin J.A., Wendland B. Actin and Endocytosis in Budding Yeast. Genetics. 2015;199:315–358. doi: 10.1534/genetics.112.145540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dawson J.C., Legg J.A., Machesky L.M. Bar Domain Proteins: A Role in Tubulation, Scission and Actin Assembly in Clathrin-Mediated Endocytosis. Trends Cell Biol. 2006;16:493–498. doi: 10.1016/j.tcb.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Ferguson S.M., Camilli P.D. Dynamin, a Membrane-Remodelling GTPase. Nat. Rev. Mol. Cell Biol. 2012;13:75–88. doi: 10.1038/nrm3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allen C.L., Goulding D., Field M.C. Clathrin-Mediated Endocytosis Is Essential in Trypanosoma Brucei. EMBO J. 2003;22:4991–5002. doi: 10.1093/emboj/cdg481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gabernet-Castello C., Dacks J.B., Field M.C. The Single ENTH-Domain Protein of Trypanosomes; Endocytic Functions and Evolutionary Relationship with Epsin. Traffic. 2009;10:894–911. doi: 10.1111/j.1600-0854.2009.00910.x. [DOI] [PubMed] [Google Scholar]
- 34.Damme D.V., Coutuer S., Rycke R.D., Bouget F.Y., Inze D., Geelen D. Somatic Cytokinesis and Pollen Maturation in Arabidopsis Depend on TPLATE, Which Has Domains Similar to Coat Proteins. Plant Cell. 2006;18:3502–3518. doi: 10.1105/tpc.106.040923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gadeyne A., Sanchez-Rodriguez C., Vanneste S., Rubbo S.D., Zauber H., Vanneste K., Leene J.V., Winne N.D., Eeckhout D., Persiau G., et al. The TPLATE Adaptor Complex Drives Clathrin-Mediated Endocytosis in Plants. Cell. 2014;156:691–704. doi: 10.1016/j.cell.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 36.Huang K.M., D’Hondt K., Riezman H., Lemmon S.K. Clathrin Functions in the Absence of Heterotetrameric Adaptors and AP180-Related Proteins in Yeast. EMBO J. 1999;18:3897–3908. doi: 10.1093/emboj/18.14.3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carroll S.Y., Stirling P.C., Stimpson H.E., Giesselmann E., Schmitt M.J., Drubin D.G. A Yeast Killer Toxin Screen Provides Insights into a/b Toxin Entry, Trafficking, and Killing Mechanisms. Dev. Cell. 2009;17:552–560. doi: 10.1016/j.devcel.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Motley A., Bright N.A., Seaman M.N., Robinson M.S. Clathrin-Mediated Endocytosis in AP-2-Depleted Cells. J. Cell Biol. 2003;162:909–918. doi: 10.1083/jcb.200305145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rooij I.I.S.-D., Allwood E.G., Aghamohammadzadeh S., Hettema E.H., Goldberg M.W., Ayscough K.R. A Role for the Dynamin-like Protein Vps1 during Endocytosis in Yeast. J. Cell Sci. 2010;123:3496–3506. doi: 10.1242/jcs.070508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu X., Cai M. The Yeast Dynamin-Related GTPase Vps1p Functions in the Organization of the Actin Cytoskeleton via Interaction with Sla1p. J. Cell Sci. 2004;117:3839–3853. doi: 10.1242/jcs.01239. [DOI] [PubMed] [Google Scholar]
- 41.Palmer S.E., II R.S., Marklew C.J., Allwood E.G., Mishra R., Johnson S., Goldberg M.W., Ayscough K.R. A Dynamin-Actin Interaction Is Required for Vesicle Scission during Endocytosis in Yeast. Curr. Biol. 2015;25:868–878. doi: 10.1016/j.cub.2015.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mayor S., Parton R.G., Donaldson J.G. Clathrin-Independent Pathways of Endocytosis. Cold Spring Harb. Perspect. Biol. 2014;6:a016758. doi: 10.1101/cshperspect.a016758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferreira A.P.A., Boucrot E. Mechanisms of Carrier Formation during Clathrin-Independent Endocytosis. Trends Cell Biol. 2018;28:188–200. doi: 10.1016/j.tcb.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Boucrot E., Ferreira A.P., Almeida-Souza L., Debard S., Vallis Y., Howard G., Bertot L., Sauvonnet N., McMahon H.T. Endophilin Marks and Controls a Clathrin-Independent Endocytic Pathway. Nature. 2015;517:460–465. doi: 10.1038/nature14067. [DOI] [PubMed] [Google Scholar]
- 45.Kirkham M., Fujita A., Chadda R., Nixon S.J., Kurzchalia T.V., Sharma D.K., Pagano R.E., Hancock J.F., Mayor S., Parton R.G. Ultrastructural Identification of Uncoated Caveolin-Independent Early Endocytic Vehicles. J. Cell Boil. 2005;168:465–476. doi: 10.1083/jcb.200407078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Howes M.T., Kirkham M., Riches J., Cortese K., Walser P.J., Simpson F., Hill M.M., Jones A., Lundmark R., Lindsay M.R., et al. Clathrin-Independent Carriers Form a High Capacity Endocytic Sorting System at the Leading Edge of Migrating Cells. J. Cell Biol. 2005;168:465–476. doi: 10.1083/jcb.201002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bos J.L., Rehmann H., Wittinghofer A. GEFs and GAPs: Critical Elements in the Control of Small G Proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 48.Lundmark R., Doherty G.J., Howes M.T., Cortese K., Vallis Y., Parton R.G., McMahon H.T. The GTPase-Activating Protein GRAF1 Regulates the CLIC/GEEC Endocytic Pathway. Curr. Biol. 2008;18:1802–1808. doi: 10.1016/j.cub.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scott C.C., Vacca F., Gruenberg J. Endosome Maturation, Transport and Functions. Semin. Cell Dev. Biol. 2014;31:2–10. doi: 10.1016/j.semcdb.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 50.Balderhaar H.J., Lachmann J., Yavavli E., Brocker C., Lurick A., Ungermann C. The CORVET Complex Promotes Tethering and Fusion of Rab5/Vps21-Positive Membranes. Proc. Natl. Acad. Sci. USA. 2013;110:3823–3828. doi: 10.1073/pnas.1221785110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perini E.D., Schaefer R., Stoter M., Kalaidzidis Y., Zerial M. Mammalian CORVET Is Required for Fusion and Conversion of Distinct Early Endosome Subpopulations. Traffic. 2014;15:1366–1389. doi: 10.1111/tra.12232. [DOI] [PubMed] [Google Scholar]
- 52.Kant R.V.D., Jonker C.T., Wijdeven R.H., Bakker J., Janssen L., Klumperman J., Neefjes J. Characterization of the Mammalian CORVET and HOPS Complexes and Their Modular Restructuring for Endosome Specificity. J. Biol. Chem. 2015;290:30280–30290. doi: 10.1074/jbc.M115.688440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mizuno-Yamasaki E., Rivera-Molina F., Novick P. GTPase Networks in Membrane Traffic. Annu. Rev. Biochem. 2012;81:637–659. doi: 10.1146/annurev-biochem-052810-093700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Numrich J., Ungermann C. Endocytic Rabs in Membrane Trafficking and Signaling. Biol. Chem. 2014;395:327–333. doi: 10.1515/hsz-2013-0258. [DOI] [PubMed] [Google Scholar]
- 55.Solinger J.A., Spang A. Tethering Complexes in the Endocytic Pathway: CORVET and HOPS. FEBS J. 2013;280:2743–2757. doi: 10.1111/febs.12151. [DOI] [PubMed] [Google Scholar]
- 56.Dettmer J., Hong-Hermesdorf A., Stierhof Y.D., Schumacher K. Vacuolar H+-ATPase Activity Is Required for Endocytic and Secretory Trafficking in Arabidopsis. Plant Cell. 2006;18:715–730. doi: 10.1105/tpc.105.037978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ebine K., Inoue T., Ito J., Ito E., Uemura T., Goh T., Abe H., Sato K., Nakano A., Ueda T. Plant Vacuolar Trafficking Occurs through Distinctly Regulated Pathways. Curr. Biol. 2014;24:1375–1382. doi: 10.1016/j.cub.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 58.Singh M.K., Kruger F., Beckmann H., Brumm S., Vermeer J.E.M., Munnik T., Mayer U., Stierhof Y.D., Grefen C., Schumacher K., et al. Protein Delivery to Vacuole Requires SAND Protein-Dependent Rab GTPase Conversion for MVB-Vacuole Fusion. Curr. Biol. 2014;24:1383–1389. doi: 10.1016/j.cub.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 59.Scheuring D., Viotti C., Kruger F., Kunzl F., Sturm S., Bubeck J., Hillmer S., Frigerio L., Robinson D.G., Pimpl P., et al. Multivesicular Bodies Mature from the Trans-Golgi Network/Early Endosome in Arabidopsis. Plant Cell. 2011;23:3463–3481. doi: 10.1105/tpc.111.086918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klinger C.M., Klute M.J., Dacks J.B. Comparative Genomic Analysis of Multi-Subunit Tethering Complexes Demonstrates an Ancient Pan-Eukaryotic Complement and Sculpting in Apicomplexa. PLoS ONE. 2013;8:e76278. doi: 10.1371/journal.pone.0076278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rojo E., Zouhar J., Kovaleva V., Hong S., Raikhel N.V. The AtC-VPS Protein Complex Is Localized to the Tonoplast and the Prevacuolar Compartment in Arabidopsis. Mol. Biol. Cell. 2003;14:361–369. doi: 10.1091/mbc.e02-08-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ahmed S.U., Rojo E., Kovaleva V., Venkataraman S., Dombrowski J.E., Matsuoka K., Raikhel N.V. The Plant Vacuolar Sorting Receptor AtELP Is Involved in Transport of NH(2)-Terminal Propeptide-Containing Vacuolar Proteins in Arabidopsis Thaliana. J. Cell Biol. 2000;149:1335–1344. doi: 10.1083/jcb.149.7.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ebine K., Okatani Y., Uemura T., Goh T., Shoda K., Niihama M., Morita M.T., Spitzer C., Otegui M.S., Nakano A., et al. A SNARE Complex Unique to Seed Plants Is Required for Protein Storage Vacuole Biogenesis and Seed Development of Arabidopsis Thaliana. Plant Cell. 2008;20:3006–3021. doi: 10.1105/tpc.107.057711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hao L., Liu J., Zhong S., Gu H., Qu L.J. AtVPS41-Mediated Endocytic Pathway Is Essential for Pollen Tube-Stigma Interaction in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2016;113:6307–6312. doi: 10.1073/pnas.1602757113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takemoto K., Ebine K., Askani J.C., Kruger F., Gonzalez Z.A., Ito E., Goh T., Schumacher K., Nakano A., Ueda T. Distinct Sets of Tethering Complexes, SNARE Complexes, and Rab GTPases Mediate Membrane Fusion at the Vacuole in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2018;115:E2457–E2466. doi: 10.1073/pnas.1717839115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aikawa M., Hepler P.K., Huff C.G., Sprinz H. The Feeding Mechanism of Avian Malarial Parasites. J. Cell Biol. 1966;28:355–373. doi: 10.1083/jcb.28.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Slomianny C. Three-Dimensional Reconstruction of the Feeding Process of the Malaria Parasite. Blood Cells. 1990;16:369–378. [PubMed] [Google Scholar]
- 68.Zhou H., Gao Y., Zhong X., Wang H. Dynamin like Protein 1 Participated in the Hemoglobin Uptake Pathway of Plasmodium Falciparum. Chin. Med. J. 2009;122:1686–1691. [PubMed] [Google Scholar]
- 69.Lazarus M.D., Schneider T.G., Taraschi T.F. A New Model for Hemoglobin Ingestion and Transport by the Human Malaria Parasite Plasmodium Falciparum. J. Cell Sci. 2008;121:1937–1949. doi: 10.1242/jcs.023150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Milani K.J., Schneider T.G., Taraschi T.F. Defining the Morphology and Mechanism of the Hemoglobin Transport Pathway in Plasmodium Falciparum Infected Erythrocytes. Eukaryotic Cell. 2015;14:415–426. doi: 10.1128/EC.00267-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smythe W.A., Joiner K.A., Hoppe H.C. Actin Is Required for Endocytic Trafficking in the Malaria Parasite Plasmodium Falciparum. Cell. Microbiol. 2007;10:452–464. doi: 10.1111/j.1462-5822.2007.01058.x. [DOI] [PubMed] [Google Scholar]
- 72.Lacy M.M., Baddeley D., Berro J. Single-Molecule Turnover Dynamics of Actin and Membrane Coat Proteins in Clathrin-Mediated Endocytosis. eLife. 2019;8:8. doi: 10.7554/eLife.52355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Elliott D.A., McIntosh M.T., Hosgood H.D., 3rd, Chen S., Zhang G., Baevova P., Joiner K.A. Four Distinct Pathways of Hemoglobin Uptake in the Malaria Parasite Plasmodium Falciparum. Proc. Natl. Acad. Sci. USA. 2008;105:2463–2468. doi: 10.1073/pnas.0711067105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Birnbaum J., Flemming S., Reichard N., Soares A.B., Mesén-Ramírez P., Jonscher E., Bergmann B., Spielmann T. A Genetic System to Study Plasmodium Falciparum Protein Function. Nat. Methods. 2017;14:450–456. doi: 10.1038/nmeth.4223. [DOI] [PubMed] [Google Scholar]
- 75.Jonscher E., Flemming S., Schmitt M., Sabitzki R., Reichard N., Birnbaum J., Bergmann B., Höhn K., Spielmann T. PfVPS45 Is Required for Host Cell Cytosol Uptake by Malaria Blood Stage Parasites. Cell Host Microbe. 2019;25:166–173.e5. doi: 10.1016/j.chom.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 76.Yang T., Yeoh L.M., Tutor M.V., Dixon M.W., McMillan P.J., Xie S.C., Bridgford J.L., Gillett D.L., Duffy M.F., Ralph S.A., et al. Decreased K13 Abundance Reduces Hemoglobin Catabolism and Proteotoxic Stress, Underpinning Artemisinin Resistance. Cell Rep. 2019;29:2917–2928.e5. doi: 10.1016/j.celrep.2019.10.095. [DOI] [PubMed] [Google Scholar]
- 77.Birnbaum J., Scharf S., Schmidt S., Jonscher E., Hoeijmakers W.A.M., Flemming S., Toenhake C.G., Schmitt M., Sabitzki R., Bergmann B., et al. A Kelch13-Defined Endocytosis Pathway Mediates Artemisinin Resistance in Malaria Parasites. Science. 2020;367:51–59. doi: 10.1126/science.aax4735. [DOI] [PubMed] [Google Scholar]
- 78.Nolan S.J., Romano J.D., Coppens I. Host Lipid Droplets: An Important Source of Lipids Salvaged by the Intracellular Parasite Toxoplasma Gondii. PLoS Pathog. 2017;13:e1006362. doi: 10.1371/journal.ppat.1006362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gras S., Jimenez-Ruiz E., Klinger C.M., Schneider K., Klingl A., Lemgruber L., Meissner M. An Endocytic-Secretory Cycle Participates in Toxoplasma Gondii in Motility. PLoS Biol. 2019;17:e3000060. doi: 10.1371/journal.pbio.3000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lige B., Romano J.D., Sampels V., Sonda S., Joiner K.A., Coppens I. Introduction of Caveolae Structural Proteins into the Protozoan Toxoplasma Results in the Formation of Heterologous Caveolae but Not Caveolar Endocytosis. PLoS ONE. 2012;7:e51773. doi: 10.1371/journal.pone.0051773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pieperhoff M.S., Schmitt M., Ferguson D.J., Meissner M. The Role of Clathrin in Post-Golgi Trafficking in Toxoplasma Gondii. PLoS ONE. 2013;8:e77620. doi: 10.1371/journal.pone.0077620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wetzel D.M., Hakansson S., Hu K., Roos D., Sibley L.D. Actin Filament Polymerization Regulates Gliding Motility by Apicomplexan Parasites. Mol. Biol. Cell. 2003;14:396–406. doi: 10.1091/mbc.e02-08-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bargieri D., Lagal V., Andenmatten N., Tardieux I., Meissner M., Ménard R. Host Cell Invasion by Apicomplexan Parasites: The Junction Conundrum. PLoS Pathog. 2014;10:e1004273. doi: 10.1371/journal.ppat.1004273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Breinich M.S., Ferguson D.J., Foth B.J., Dooren G.G.V., Lebrun M., Quon D.V., Striepen B., Bradley P.J., Frischknecht F., Carruthers V.B., et al. A Dynamin Is Required for the Biogenesis of Secretory Organelles in Toxoplasma Gondii. Curr. Biol. 2009;19:277–286. doi: 10.1016/j.cub.2009.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van Dooren G.G., Reiff S.B., Tomova C., Meissner M., Humbel B.M., Striepen B. A Novel Dynamin-Related Protein Has Been Recruited for Apicoplast Fission in Toxoplasma Gondii. Curr. Biol. 2009;19:267–276. doi: 10.1016/j.cub.2008.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Heredero-Bermejo I., Varberg J.M., Charvat R., Jacobs K., Garbuz T., Sullivan W.J.J., Arrizabalaga G. TgDrpC, an Atypical Dynamin-Related Protein in Toxoplasma Gondii, Is Associated with Vesicular Transport Factors and Parasite Division. Mol. Microbiol. 2019;111:46–64. doi: 10.1111/mmi.14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sidik S.M., Huet D., Ganesan S.M., Huynh M.H., Wang T., Nasamu A.S., Thiru P., Saeij J.P., Carruthers V.B., Niles J.C., et al. A Genome-Wide CRISPR Screen in Toxoplasma Identifies Essential Apicomplexan Genes. Cell. 2016;166:1423–1435.e12. doi: 10.1016/j.cell.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tomavo S., Slomianny C., Meissner M., Carruthers V.B. Protein Trafficking through the Endosomal System Prepares Intracellular Parasites for a Home Invasion. PLoS Pathog. 2013;9:e1003629. doi: 10.1371/journal.ppat.1003629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Daher W., Klages N., Carlier M.F., Soldati-Favre D. Molecular Characterization of Toxoplasma Gondii Formin 3, an Actin Nucleator Dispensable for Tachyzoite Growth and Motility. Eukaryotic Cell. 2012;11:343–352. doi: 10.1128/EC.05192-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bisio H., Chaabene R.B., Sabitzki R., Maco B., Marq J.B., Gilberger T.-W., Spielmann T., Soldati-Favre D. The ZIP Code of Vesicle Trafficking in Apicomplexa: SEC1/Munc18 and SNARE Proteins. mBio. 2020;11 doi: 10.1128/mBio.02092-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marques P.E., Grinstein S., Freeman S.A. SnapShot: Macropinocytosis. Cell. 2017;169:766–766.e1. doi: 10.1016/j.cell.2017.04.031. [DOI] [PubMed] [Google Scholar]
- 92.Prosser D.C., Drivas T.G., Maldonado-Baez L., Wendland B. Existence of a Novel Clathrin-Independent Endocytic Pathway in Yeast That Depends on Rho1 and Formin. J. Cell Biol. 2011;195:657–671. doi: 10.1083/jcb.201104045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Daher W., Plattner F., Carlier M.F., Soldati-Favre D. Concerted Action of Two Formins in Gliding Motility and Host Cell Invasion by Toxoplasma Gondii. PLoS Pathog. 2010;6:e1001132. doi: 10.1371/journal.ppat.1001132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ford M.G., Mills I.G., Peter B.J., Vallis Y., Praefcke G.J., Evans P.R., McMahon H.T. Curvature of Clathrin-Coated Pits Driven by Epsin. Nature. 2002;419:361–366. doi: 10.1038/nature01020. [DOI] [PubMed] [Google Scholar]
- 95.Boucrot E., Pick A., Camdere G., Liska N., Evergren E., McMahon H.T., Kozlov M.M. Membrane Fission Is Promoted by Insertion of Amphipathic Helices and Is Restricted by Crescent BAR Domains. Cell. 2012;149:124–136. doi: 10.1016/j.cell.2012.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mercier C., Dubremetz J.F., Rauscher B., Lecordier L., Sibley L.D., Cesbron-Delauw M.F. Biogenesis of Nanotubular Network in Toxoplasma Parasitophorous Vacuole Induced by Parasite Proteins. Mol. Biol. Cell. 2002;13:2397–2409. doi: 10.1091/mbc.e02-01-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sibley L.D., Niesman I.R., Parmley S.F., Cesbron-Delauw M.-F. Regulated Secretion of Multi-Lamellar Vesicles Leads to Formation of a Tubulo-Vesicular Network in Host-Cell Vacuoles Occupied by Toxoplasma Gondii. J. Cell Sci. 1995;108:1669–1677. doi: 10.1242/jcs.108.4.1669. [DOI] [PubMed] [Google Scholar]
- 98.Labruyere E., Lingnau M., Mercier C., Sibley L.D. Differential Membrane Targeting of the Secretory Proteins GRA4 and GRA6 within the Parasitophorous Vacuole Formed by Toxoplasma Gondii. Mol. Biochem. Parasitol. 1999;102:311–324. doi: 10.1016/S0166-6851(99)00092-4. [DOI] [PubMed] [Google Scholar]
- 99.Travier L., Mondragon R., Dubremetz J.F., Musset K., Mondragon M., Gonzalez S., Cesbron-Delauw M.F., Mercier C. Functional Domains of the Toxoplasma GRA2 Protein in the Formation of the Membranous Nanotubular Network of the Parasitophorous Vacuole. Int. J. Parasitol. 2008;38:757–773. doi: 10.1016/j.ijpara.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 100.Schmidt O., Teis D. The ESCRT Machinery. Curr. Biol. 2012;22:R116–R120. doi: 10.1016/j.cub.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guerin A., Corrales R.M., Parker M.L., Lamarque M.H., Jacot D., Hajj H.E., Soldati-Favre D., Boulanger M.J., Lebrun M. Efficient Invasion by Toxoplasma Depends on the Subversion of Host Protein Networks. Nat. Microbiol. 2017;2:1358–1366. doi: 10.1038/s41564-017-0018-1. [DOI] [PubMed] [Google Scholar]
- 102.Wollert T., Yang D., Ren X., Lee H.H., Im Y.J., Hurley J.H. The ESCRT Machinery at a Glance. J. Cell Sci. 2009;122:2163–2166. doi: 10.1242/jcs.029884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fujii K., Hurley J.H., Freed E.O. Beyond Tsg101: The Role of Alix in “ESCRTing” HIV-1. Nat. Rev. Genet. 2007;5:912–916. doi: 10.1038/nrmicro1790. [DOI] [PubMed] [Google Scholar]
- 104.Votteler J., Sundquist W.I. Virus Budding and the ESCRT Pathway. Cell Host Microbe. 2013;14:232–241. doi: 10.1016/j.chom.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dowlatshahi D.P., Sandrin V., Vivona S., Shaler T.A., Kaiser S.E., Melandri F., Sundquist W.I., Kopito R.R. ALIX Is a Lys63-Specific Polyubiquitin Binding Protein That Functions in Retrovirus Budding. Dev. Cell. 2012;23:1247–1254. doi: 10.1016/j.devcel.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Joshi A., Munshi U., Ablan S.D., Nagashima K., Freed E.O. Functional Replacement of a Retroviral Late Domain by Ubiquitin Fusion. Traffic. 2008;9:1972–1983. doi: 10.1111/j.1600-0854.2008.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rauch S., Martin-Serrano J. Multiple Interactions between the ESCRT Machinery and Arrestin-Related Proteins: Implications for PPXY-Dependent Budding. J. Virol. 2010;85:3546–3556. doi: 10.1128/JVI.02045-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lebrun M., Michelin A., Hajj H.E., Poncet J., Bradley P.J., Vial H., Dubremetz J.F. The Rhoptry Neck Protein RON4 Re-Localizes at the Moving Junction during Toxoplasma Gondii Invasion. Cell. Microbiol. 2005;7:1823–1833. doi: 10.1111/j.1462-5822.2005.00646.x. [DOI] [PubMed] [Google Scholar]
- 109.Besteiro S., Michelin A., Poncet J., Dubremetz J., Lebrun M. Export of a Toxoplasma Gondii Rhoptry Neck Protein Complex at the Host Cell Membrane to Form the Moving Junction during Invasion. PLoS Pathog. 2009;5:e1000309. doi: 10.1371/journal.ppat.1000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Baldauf S.L. The Deep Roots of Eukaryotes. Science. 2003;300:1703–1706. doi: 10.1126/science.1085544. [DOI] [PubMed] [Google Scholar]
- 111.Sakura T., Sindikubwabo F., Oesterlin L.K., Bousquet H., Slomianny C., Hakimi M.A., Langsley G., Tomavo S. A Critical Role for Toxoplasma Gondii Vacuolar Protein Sorting VPS9 in Secretory Organelle Biogenesis and Host Infection. Sci. Rep. 2016;6:38842. doi: 10.1038/srep38842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Harper J.M., Huynh M.H., Coppens I., Parussini F., Moreno S., Carruthers V.B. A Cleavable Propeptide Influences Toxoplasma Infection by Facilitating the Trafficking and Secretion of the TgMIC2-M2AP Invasion Complex. Mol. Biol. Cell. 2006;17:4551–4563. doi: 10.1091/mbc.e06-01-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Reiss M., Viebig N., Brecht S., Fourmaux M.N., Soete M., Cristina M.D., Dubremetz J.F., Soldati D. Identification and Characterization of an Escorter for Two Secretory Adhesins in Toxoplasma Gondii. J. Cell Biol. 2001;152:563–578. doi: 10.1083/jcb.152.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Meissner M., Reiss M., Viebig N., Carruthers V.B., Toursel C., Tomavo S., Ajioka J., Soldati D. A Family of Transmembrane Microneme Proteins of Toxoplasma Gondii Contain EGF-like Domains and Function as Escorters. J. Cell Sci. 2002;115:563–574. doi: 10.1242/jcs.115.3.563. [DOI] [PubMed] [Google Scholar]
- 115.Huynh M.H., Carruthers V.B. Toxoplasma MIC2 Is a Major Determinant of Invasion and Virulence. PLoS Pathog. 2006;2:e84. doi: 10.1371/journal.ppat.0020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brillada C., Rojas-Pierce M. Vacuolar Trafficking and Biogenesis: A Maturation in the Field. Curr. Opin. Plant Biol. 2017;40:77–81. doi: 10.1016/j.pbi.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 117.Kunzl F., Fruholz S., Fassler F., Li B., Pimpl P. Receptor-Mediated Sorting of Soluble Vacuolar Proteins Ends at the Trans-Golgi Network/Early Endosome. Nat. Plants. 2016;2:16017. doi: 10.1038/nplants.2016.17. [DOI] [PubMed] [Google Scholar]
- 118.Ezougou C.N., Ben-Rached F., Moss D.K., Lin J.W., Black S., Knuepfer E., Green J.L., Khan S.M., Mukhopadhyay A., Janse C.J., et al. Plasmodium Falciparum Rab5B Is an N-Terminally Myristoylated Rab GTPase That Is Targeted to the Parasite’s Plasma and Food Vacuole Membranes. PLoS ONE. 2014;9:e87695. doi: 10.1371/journal.pone.0087695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ebine K., Miyakawa N., Fujimoto M., Uemura T., Nakano A., Ueda T. Endosomal Trafficking Pathway Regulated by ARA6, a RAB5 GTPase Unique to Plants. Small GTPases. 2012;3:23–27. doi: 10.4161/sgtp.18299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu J., Istvan E.S., Gluzman I.Y., Gross J., Goldberg D.E. Plasmodium Falciparum Ensures Its Amino Acid Supply with Multiple Acquisition Pathways and Redundant Proteolytic Enzyme Systems. Proc. Natl. Acad. Sci. USA. 2006;103:8840–8845. doi: 10.1073/pnas.0601876103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Meshnick S.R. Artemisinin: Mechanisms of Action, Resistance and Toxicity. Int. J. Parasitol. 2002;32:1655–1660. doi: 10.1016/S0020-7519(02)00194-7. [DOI] [PubMed] [Google Scholar]
- 122.Slater A.F. Chloroquine: Mechanism of Drug Action and Resistance in Plasmodium Falciparum. Pharmacol. Ther. 1993;57:203–235. doi: 10.1016/0163-7258(93)90056-J. [DOI] [PubMed] [Google Scholar]
- 123.Wang Y., Sangaré L.O., Paredes-Santos T.C., Hassan M.A., Krishnamurthy S., Furuta A.M., Markus B.M., Lourido S., Saeij J.P.J. Genome-Wide Screens Identify Toxoplasma Gondii Determinants of Parasite Fitness in IFNγ-Activated Murine Macrophages. Nat. Commun. 2020;11:5258. doi: 10.1038/s41467-020-18991-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kannan G., Thaprawat P., Schultz T.L., Carruthers V.B. Acquisition of Host Cytosolic Protein by Toxoplasma Gondii Bradyzoites. bioRxiv. 2020;2020:293225. doi: 10.1101/2020.09.11.293225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Di Cristina M., Dou Z., Lunghi M., Kannan G., Huynh M.H., McGovern O.L., Schultz T.L., Schultz A.J., Miller A.J., Hayes B.M., et al. Toxoplasma Depends on Lysosomal Consumption of Autophagosomes for Persistent Infection. Nat. Microbiol. 2017;2:1–7. doi: 10.1038/nmicrobiol.2017.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cui L., Mharakurwa S., Ndiaye D., Rathod P.K., Rosenthal P.J. Antimalarial Drug Resistance: Literature Review and Activities and Findings of the ICEMR Network. Am. J. Trop. Med. Hyg. 2015;93:57–68. doi: 10.4269/ajtmh.15-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chalmers R.M., Davies A.P. Minireview: Clinical Cryptosporidiosis. Exp. Parasitol. 2010;124:138–146. doi: 10.1016/j.exppara.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 128.Frölich S., Wallach M. Use of Fluorescent Nanoparticles to Investigate Nutrient Acquisition by Developing Eimeria Maxima Macrogametocytes. Sci. Rep. 2016;6:29030. doi: 10.1038/srep29030. [DOI] [PMC free article] [PubMed] [Google Scholar]