Abstract
Hydrogen sulfide (H2S) has a long history as toxic gas and environmental hazard; inhibition of cytochrome c oxidase (mitochondrial Complex IV) is viewed as a primary mode of its cytotoxic action. However, studies conducted over the last two decades unveiled multiple biological regulatory roles of H2S as an endogenously produced mammalian gaseous transmitter. Cystathionine γ-lyase (CSE), cystathionine β-synthase (CBS) and 3-mercaptopyruvate sulfurtransferase (3-MST) are currently viewed as the principal mammalian H2S-generating enzymes. In contrast to its inhibitory (toxicological) mitochondrial effects, at lower (physiological) concentrations, H2S serves as a stimulator of electron transport in mammalian mitochondria, by acting as an electron donor—with sulfide:quinone oxidoreductase (SQR) being the immediate electron acceptor. The mitochondrial roles of H2S are significant in various cancer cells, many of which exhibit high expression and partial mitochondrial localization of various H2S producing enzymes. In addition to the stimulation of mitochondrial ATP production, the roles of endogenous H2S in cancer cells include the maintenance of mitochondrial organization (protection against mitochondrial fission) and the maintenance of mitochondrial DNA repair (via the stimulation of the assembly of mitochondrial DNA repair complexes). The current article overviews the state-of-the-art knowledge regarding the mitochondrial functions of endogenously produced H2S in cancer cells.
Keywords: bioenergetics, ATP, gasotransmitters, DNA repair
1. Hydrogen sulfide (H2S), an Endogenous Mammalian Biological Mediator
Over the majority of the last century hydrogen sulfide (H2S) has been viewed as an environmental toxin and biological hazard. H2S (administered as an inhaled gas, or systemically, in aqueous solutions) exerts various toxicological effects in all mammalian organisms including humans. An extensive body of literature exists which focuses on the toxicological aspects of H2S [1,2,3,4,5]. Relevant for the current article is the fact that the molecular mode of H2S’ toxic action is largely (although not exclusively) attributed to its ability to inhibit mitochondrial Complex IV (cytochrome c oxidase), which, in turn, shuts down mitochondrial electron transport and inhibits aerobic ATP generation. The (largely reversible) binding of H2S to the cytochrome a3 prosthetic group of Complex IV has been extensively characterized [6,7]. Although Complex IV inhibition by H2S is usually viewed in the environmental toxicological context, there are some pathophysiological conditions—for example Down syndrome, where increased levels of endogenously produced H2S can inhibit Complex IV [8,9].
Over the last two decades, the field of H2S has undergone a significant transition, whereby various endogenous H2S-producing enzymes have been recognized and characterized, and a broad spectrum of biological roles of H2S has been identified. The timeline of H2S research and the emergence of H2S as an endogenous mammalian mediator has been covered in specialized review articles [10,11]. Moreover, the diverse biological roles of H2S in mammals in the regulation of the cardiovascular, nervous and immune system, and the biochemistry and pharmacology of various H2S-producing enzymes—a stand-alone field that consists of over 10,000 published papers—is covered in specialized review articles [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30]. Briefly, H2S in mammalian cells and tissues is produced by three principal enzymes: cystathionine-β-synthase (CBS), cystathionine γ-lyase (CSE) and 3-mercaptopyruvate sulfurtransferase (3-MST), with several additional enzymes (as well as non-enzymatic reactions and H2S-producing bacteria in the bacterial microbiota) also contributing. CBS, CSE and (indirectly) 3-MST all utilize sulfur-containing amino acids as their enzymatic substrates; the biochemistry and the molecular regulation of each enzyme in health and disease has been extensively reviewed in specialized articles [31,32,33,34,35]. H2S is a diffusible mediator that reaches various cellular compartments, and can also exit the cell that produces it. Thus, it can exert biological actions in autocrine as well as paracrine manners. H2S can also react with other labile, diffusible small molecules (such as nitric oxide, peroxynitrite, superoxide, hydrogen peroxide and others) to produce various secondary and tertiary reactive species: thus, H2S is now viewed as a component of the “reactive species interactome” [36,37]. While we cannot attempt to outline the complex roles and regulation of H2S and the H2S-producing enzymes in the current article, a brief discussion of the following aspects will be useful in the context of the current, focused review article:
There are important parallels between bacterial sulfur biochemistry and the mitochondrial roles of H2S. This is not at all surprising considering that mitochondria, from an evolutionary standpoint, are, in fact, modified bacteria. Bacteria (similar to present-day mitochondria) are known to produce and/or utilize H2S; these processes involve several evolutionarily conserved enzymes [10,38].
The mammalian H2S-producing enzymes are (partially) mitochondrial. While 3-MST is often referred to as a “mitochondrial enzyme”, it actually has both mitochondrial and cytosolic localization; the other two major enzymes can either have low-level mitochondrial baseline localization, and/or can translocate to mitochondria under various specialized (pathophysiological) conditions (including cancer) [39,40,41]. However, mitochondrial localization of a H2S-producing enzyme is not an absolute requirement for mitochondrial effects of H2S, since this mediator can readily diffuse from one intracellular compartment to another [28].
For the sake of simplicity, one tends to simply refer to “H2S”, when discussing the complex biochemical products of CBS, CSE and 3-MST. However, the reality is that in a cellular environment, due to a complex series of reactions, multiple reactive sulfur species exist, as well as various hybrid (“S/N”) species. These diverse species can have different chemical reactivity and diverse (but often overlapping) sets of molecular targets. Polysulfides (small segments of S-Sn chains) represent a special group of reactive sulfur species; while CBS and CSE is primarily viewed as “H2S synthases”, 3-MST’s principal product is polysulfide (although, each enzyme generates a mixture of these species in the intracellular environment) [28,34,42].
While H2S (and polysulfides) have multiple intra- and extra-cellular molecular targets, one of the principal modes of reactive sulfur species’ action is sulfhydration (a posttranslational modification of protein cysteines), which is primarily catalyzed by polysulfide (rather than H2S) [34,42,43].
“Protein sulfhydromes” (collections of sulfhydrated proteins) have recently been characterized in various cells and tissues; thousands of proteins are subject to this modification [44,45,46,47]. Some of the better-characterized enzymes that are known to be functionally affected by sulfhydration include KATP channels (regulating vascular tone, angiogenesis and many other processes), nuclear factor kB (regulating signal transduction), and Keap 1 (regulating NRF2 activation and thus inducing a generalized cellular antioxidant response, responsible for cytoprotection, preconditioning and other responses) [43,44,45,46,47].
It should be mentioned that a significant reprogramming of the cellular sulfur metabolism (which includes alterations in cysteine catabolism and metabolism, cysteine transport, methionine homeostasis, and many other aspects) occurs in cancer cells [48,49]; the mitochondrial roles of H2S in cancer (reviewed in the current article), therefore, should be viewed in this broader context.
Generally, H2S is known to have a bell-shaped (or biphasic) biological character, with low-to-mid levels exerting one type of biological response (in many cases, regulatory, protective or stimulatory), while higher (toxicological) levels of H2S often exerting the opposite effects (which are, in many cases, related to the inhibition of mitochondrial Complex IV, as discussed above) [28].
Finally—as extensively discussed previously [24,28,31,41]—it should be emphasized that many of the experimental findings discussed in the following sections rely on pharmacological inhibitors of CBS (such as aminooxyacetic acid, AOAA) and of 3-MST (such as 2-[(4-hydroxy-6-methylpyrimidin-2-yl)sulfanyl]-1-(naphthalen-1-yl)ethan-1-one, HMPSNE). These agents have low micromolar potency on their respective enzymatic targets in biochemical assays in vitro, and have acceptable cell uptake. (When cells are incubated with these agents in mid-to-high micromolar concentrations, cellular H2S production is significantly suppressed). However, these compounds can also inhibit other enzymes (for instance, AOAA also inhibits CSE, and indirectly, 3-MST), and their selectivity and specificity, as well as their bioavailability and metabolism are incompletely understood. It is, therefore, always preferable to test the potential reversibility of the biological effects of these inhibitors with H2S donors, and to complement the pharmacological studies with genetic studies (e.g., CBS, CSE or 3-MST silencing).
2. Upregulation of Various H2S-Producing Enzymes in Cancer Cells
In 2013, we have discovered that CBS is upregulated and H2S generation is increased in primary human colon cancer tissues compared to the surrounding (nominally healthy) tissues [50]. Similarly, we have noticed that several human colon cancer cell lines have highly expressed CBS levels [50]. Over the subsequent seven years, these findings have been confirmed and extended to many different tumor types: it is now clear that CBS, and/or CSE and/or 3-MST is overexpressed in many forms of cancer. Table 1 shows an overview of the currently published body of literature in this respect [40,41,51,52].
Table 1.
Changes in H2S-producing enzymes in various types of cancer.
| Cancer Type | Upregulation of H2S-Producing Enzyme(s) |
|---|---|
| Biliary tract carcinoma | CBS ↑ |
| Bladder urothelial cell carcinoma | CSE ↑, CBS ↑, 3-MST ↑ |
| Breast cancer | CBS ↑ |
| Colon cancer | CSE ↑, CBS ↑↑ |
| Gastric cancer | CSE ↑, CBS ↑ |
| Glioma | 3-MST ↑ |
| Hepatocellular carcinoma | CSE ↑, CBS ↑ |
| Leukemia, lymphoma | CBS ↑ |
| Melanoma | CSE ↑, 3-MST ↑ |
| Myeloma | CBS ↑ |
| Ovarian cancer | CBS ↑↑ |
| Oral squamous cell carcinoma | CSE ↑, CBS ↑, 3-MST ↑ |
| Prostate cancer | CSE ↑, CBS ↑↑ |
| Renal cell carcinomas | CSE ↑, CBS ↑, 3-MST ↑ |
| Thyroid carcinoma | CSE ↑, CBS ↑↑ |
What, then, is the functional relevance of this increased H2S production in cancer cells? The functional role of CBS- or 3-MST-derived H2S in colon cancer cells has been studied extensively. Utilizing knockdown of various H2S-producing enzymes and/or pharmacological inhibitors, and/or forced overexpression of H2S-producing enzymes into non-transformed cells, our group as well as several other groups of independent investigators have demonstrated that cancer cells upregulate their H2S-producing capacity to help their bioenergetic function (glycolysis as well as oxidative phosphorylation, see below), to maximize ATP generation in support of their increased (uncontrolled) growth, proliferation and migration [50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71].
Moreover, endogenously produced H2S in colon cancer cells stimulates various cellular signalling pathways, reinforces stemness, provides protection against anticancer chemotherapeutic agents, and helps maintaining cancer cells in a mesenchymal (as opposed to epithelial) state, in support of cellular mobility, invasion and metastasis. H2S is an endogenous vasodilator [72,73,74] and pro-angiogenic factor [75,76,77,78]; tumor-derived H2S, therefore, also exerts local (paracrine) actions in the tumor microenvironment, which are important to increase blood flow and nutrient supply to the growing tumor tissue [39,40,50]. The above-listed roles of H2S are not restricted to colon cancer: similar roles of H2S have also been demonstrated in a variety of other cancer types [40,51,79,80,81,82,83]. Some of the enzymes that are known to be regulated by H2S, and which, in turn, serve the increased metabolic demands of the tumor cell are listed in Table 2 [19,60,66,71,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101].
Table 2.
Selected, H2S-activated enzymatic targets with potential relevance for cancer cell metabolism.
| Target | Effect | Functional Consequence | Reference |
|---|---|---|---|
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) |
Activation via sulfhydration |
Stimulation of glycolysis | [84] |
| Sirtuin 1, Sirtuin 3 (Sirt1, Sirt3) |
Activation via sulfhydration |
Elevation of cellular NAD+ | [85,86] |
| Lactate dehydrogenase A (LDH-A) |
Activation via sulfhydration |
Elevation of cellular NAD+ | [60] |
| Protein phosphatase 2A (PP2A) |
Inhibition via sulfhydration |
Stimulation of AMP kinase | [87] |
| ATP citrate lyase (ACLY) |
Upregulation via promoter activation | Stimulation of acetyl-CoA synthesis | [71] |
| Sulfide quinone oxidoreductase (SQR) | Electron donation | Stimulation of mitochondrial electron transport | [66,88,89,90] |
| F0F1 ATP synthase (Complex V) |
Activation via sulfhydration |
Stimulation of ATP synthesis | [91,92] |
| Mitochondrial cAMP phosphodiesterase (PDE2A) |
Inhibition via sulfhydration and dimerization | Increased mitochondrial cAMP content, stimulation of mitochondrial ATP synthesis | [93] |
| Mitofusin 2 (MFN2) |
Upregulation through inhibition of its proteosomal degradation | Stimulation of mitochondrial biogenesis | [94] |
| Dynamin 1 like protein (Drp1) |
Upregulation via ERK1/2 | Stimulation of mitochondrial biogenesis | [95] |
| Reactive oxygen and reactive nitrogen species (ROS, RNS) |
Neutralization of ROS/RNS via direct interactions and via upregulation of antioxidant systems through NRF2 activation and p66Shc sulfhydration | Protection against mitochondrial oxidative stress | [19,96,97,98,99,100] |
| DJ-1 | Sulfhydration, which prevents its oxidative inactivation | Maintenance of mitochondrial redox balance | [45] |
All of the above actions of H2S can also be viewed, in a broader context, as one of the multitude of mechanisms that cancer cells mobilize, in order to serve their extreme bioenergetic demand. According to the original version of the “Warburg hypothesis”, cancer cells switch to glycolysis from oxidative phosphorylation. However, more recent data indicate that many cancer cells, while upregulating glycolysis (as well as glutaminolysis, and many additional metabolic pathways) can also maintain or even increase their aerobic ATP generation via the stimulation of mitochondrial electron transport [101,102,103,104,105,106]. To anthropomorphize: maximizing ATP generation is the cancer cell’s primary “metabolic goal”: whatever biochemical mechanism serves this goal—even if it is ”wasteful” or perhaps useful in the short-term but detrimental in the longer-term—will be deemed ‘good enough’ to satisfy the “short-term thinking” of the cancer cell.
3. H2S, a Mitochondrial Electron Donor and Stimulatory Bioenergetic Factor in Cancer Cells
Mitochondrial respiration is responsible for the majority of ATP generation in eukaryotes. Electron transport along mitochondrial electron transport chain complexes I, II, III and IV creates an electrochemical proton gradient, which acts as the driving force for ATP generation; the proton gradient is “harvested” by ATP synthase (mitochondrial Complex V). Pioneering work of Bouillaud and colleagues, starting in 2007, demonstrated that H2S can serve as an alternative mitochondrial electron donor; in fact, H2S was characterized as the ”first inorganic substrate for human cells” [88]. The initial studies have been conducted in nominally normal cells (i.e., non-transformed intestinal epithelial cells), and was discussed in a physiological context (i.e., the function of the gut epithelial cells to protect against the H2S generated by the intestinal microbiome, and to utilize it as their own bioenergetic “fuel”) [89,107,108,109,110]. However, further studies demonstrated that the same basic biochemical mechanisms are also operative in cancer cells; in this case the H2S that drives the mitochondrial electron transport is not the consequence of external (i.e., bacterial) sources but is produced internally (through the upregulation of the various H2S-producing enzymes; Table 1). For instance, in colon cancer cells CBS silencing suppresses basal mitochondrial function (oxygen consumption, ATP generation) [50] and similar effects are seen with CBS silencing in ovarian cancer cells [111] and with 3-MST silencing in hepatoma cells [90]. Likewise, AOAA, a pharmacological inhibitor of CBS, or HMPSNE, a pharmacological inhibitor of 3-MST, suppresses electron transport and mitochondrial bioenergetics in various cancer cell types, while supplementation of these enzymes’ respective substrates further stimulates these processes [55,56,67,70,90,111,112,113]. While H2S, on its own, is unable to initiate or maintain mitochondrial electron transport, it balances and enhances the effects of the physiological, glycolysis-derived electron donors such as NADH and FAD2, which physiologically deliver electrons to mitochondrial electron transport Complexes I and II.
The mechanism of H2S-mediated mitochondrial electron donation involves the reaction of H2S with SQR [66,89,90,114,115,116,117]. The SQR mechanism appears to be evolutionary conserved, as various bacteria are also utilizing SQR for H2S “detoxification” [118,119]; as mentioned earlier, such parallels make sense due to the evolutionary bacterial origin of mitochondria. Hypoxia acts as a stimulus to induce an upregulation of SQR expression [66]—perhaps as a potential mechanism by which tumor cells attempt to maximize the bioenergetic stimulatory impact of H2S. However, it must be pointed out that, in a cell-type dependent manner, and especially when cells are exposed to higher [exogenous] H2S concentrations, the electron flow from SQR can also occur in the opposite direction (i.e., reverse electron transport) [89,120]; such a mechanism, in a cancer cell, would not be useful to support electron transport, proton pumping or ATP generation, but rather, would stimulate mitochondrial ROS generation.
In addition to direct electron transport stimulation, H2S can also directly increase the catalytic activity of ATP synthase, via cysteine sulfhydration [91,92]. This effect makes functional sense; increased mitochondrial electron transport would be expected to lead to a consequent increase in the proton gradient between the two sides of the mitochondrial inner membrane; this proton gradient, would, in turn, be better “harvested” if the specific activity of ATP synthase is also increased.
H2S has been shown to act as an inhibitor of various cAMP and cGMP phosphodiesterases; this effect is important in mediating many physiological actions of H2S, such as vasodilatation and angiogenesis [73,121,122,123,124,125,126]. We have demonstrated that H2S is an inhibitor of the mitochondrial form of cAMP phosphodiesterase (PDE2A), which, in turn, elevates mitochondrial cAMP levels and stimulates mitochondrial electron transport [93]. cAMP in mitochondria is known to activate various cAMP-dependent kinases (e.g., protein kinase A), which, in turn, phosphorylates (and consequently activates) various key proteins in the mitochondrial electron transport chain [127,128,129,130,131]. Thus, a H2S-induced elevation in intramitochondrial cAMP may be a further mechanism by which cancer cells may maximize their mitochondrial electron transport and ATP generation.
The above three principal mechanisms by which H2S can enhance mitochondrial electron transport and ATP production in cancer cells are shown in Figure 1. Additional mechanisms whereby H2S may contribute to the stimulation of cancer cell metabolism are summarized in Table 2. Some of these mechanisms relate to the regulation of mitochondrial organization (discussed below). H2S can also stimulate glycolysis (another key energetic process in cancer cells, which, however, is also intimately linked to the support of mitochondrial function, because it supplies electron donors to the mitochondria). Several sulfhydration targets (e.g., LDH-A and various sirtuins) can be activated by H2S, which, in turn, will lead to an increase in cellular NAD+ levels.
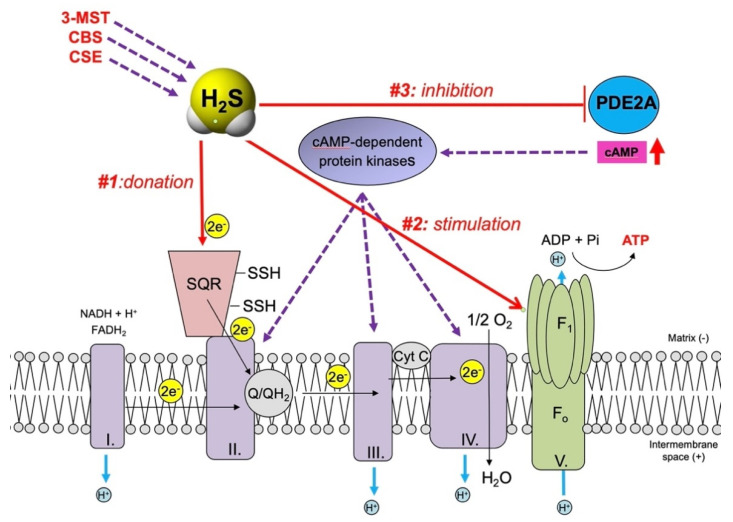
Figure 1.
Mechanisms by which mitochondrial H2S (produced by CBS, CSE or 3-MST) can stimulate mitochondrial electron transport and aerobic ATP generation in cancer cells. #1: H2S acts as a direct electron donor at the level of SQR, which feeds electrons into the electron transport chain at the level of Complex II. #2: H2S inhibits intramitochondrial cAMP phosphodiesterase; this results in an elevation of intramitochondrial cAMP, which, in turn, phosphorylates electron transport chain proteins via the activation of intramitochondrial cAMP-dependent protein kinases. #3: H2S acts as direct stimulator of ATP synthase activity via sulfhydration of the α subunit (ATP5A1) at Cys 244 and Cys 294.
A further, recently discovered mechanism relates to the upregulation of ACLY by H2S [71]. So far, this mechanism has only been investigated in the context of colon epithelial cells’ mesenchymal-epithelial transition process: the H2S-mediated upregulation of ACLY serves to maintain the cells in the mesenchymal state, at least in part through the upregulation of the Wnt pathway [71]. However, given the broad bioenergetic roles of ACLY, and its known upregulation in cancer cells [132,133,134], the H2S-ACLY axis may have further bioenergetic and metabolic implications.
“Broader” (i.e., more generalized) mechanisms by which H2S can protect mitochondria may be related to its antioxidant effects, which, in part, may relate to direct reactions of H2S with various reactive species, and, in part, may be due to a globalized upregulation of antioxidant processes—at least in part via NRF2 and p66Shc activation [19,96,97,98,99,100].
4. H2S, a Regulator of Mitochondrial Dynamics in Cancer Cells
H2S has been shown to regulate all significant aspects of mitochondrial dynamics: mitochondrial fusion, mitochondrial fission, mitochondrial macroautophagy/mitophagy, and mitochondrial biogenesis) [87,94,95,135,136,137,138,139,140,141,142,143,144,145,146]. The majority of the published studies indicate that H2S (especially in lower concentrations) tends to stabilize and preserve mitochondria, and, in many cases, can also stimulate mitochondrial biogenesis. Importantly, most of the studies conducted to date utilized exogenous H2S administration (as opposed to investigating the role of endogenously produced H2S). Moreover, most studies focus on pathophysiological conditions other than cancer. In the current section, primarily the body of evidence that directly relates to cancer will be discussed.
The first study, investigating the role of endogenous CBS on the organization of mitochondria in a cancer cell was conducted in ovarian cancer cells by Bhattacharyya and colleagues in a human ovarian cancer cell line (OvCa). These cells contain predominantly fused, elongated mitochondria. After siRNA-mediated silencing of CBS, the mitochondria exhibited predominantly spherical morphology, with increased individual unbranched populations and impaired mitochondrial network quality (i.e., fewer average branches per network and shorter average network branch length). These data indicate that CBS (via its enzymatic product, H2S) protects ovarian cancer cells against mitochondrial fragmentation; this effect may be important in maintaining mitochondrial function. The molecular mechanism that was implicated in the mitochondrial quality control in OvCa cells was the regulation of mitofusin 2 (MFN2) stability by CBS-derived H2S via a JNK-mediated regulation of MFN2 degradation via the via the ubiquitin-proteasome system [94]. (A similar MFN2-related mechanism has recently also been implicated in the maintenance of mitochondrial integrity in endothelial cells [146]). In an independent study, performed in N2a cells (a murine neuroblastoma cell line), H2S (in this case, administered exogenously to the cells) was found to inhibit mitochondrial fission; in this case the molecular mechanism was attributed to the downregulation of dynamin 1 like protein (Drp1) mRNA and protein expression by H2S, most likely through the modulation of ERK1/2 activity [95].
There are multiple H2S-regulated pathways that can influence mitochondrial quality control in various cell types. For instance, in murine hepatocytes, H2S was shown to upregulate peroxisome proliferator activated receptor-γ coactivator-related protein (PPRC) and peroxisome proliferator activated receptor gamma coactivator-1α (PGC-1α) which, in turn, stimulates mitochondrial biogenesis [141]. In the diabetic heart, ubiquitin specific peptidase 8 (USP8) has been implicated: H2S was found to increase the association of parkin with USP8. In turn, USP8 (a deubiquitination enzyme) was shown to promote the association of parkin to damaged mitochondria to augment mitophagy [145]. In another study focusing on cardiac myocytes, H2S-stimulated mitochondrial biogenesis was shown to involve AMP-activated protein kinase (AMPK) activation and subsequent induction of PGC1α signaling [87]. The various mechanisms by which H2S stimulates mitochondrial DNA repair and maintain mitochondrial DNA integrity (see below) can also play an indirect role in the maintenance of mitochondrial structural integrity. Future studies are needed to test whether the above-mentioned pathways are connected to each other in the regulation of mitochondrial dynamics in cancer cells.
5. H2S, A Stimulator of Mitochondrial DNA Repair in Cancer Cells
The regulation of DNA integrity is another example where the bell-shaped or biphasic effects of H2S are prominently featured. It has been known, for at least two decades, that exposure of high concentrations of H2S can induce DNA damage, while lower concentrations of H2S (i.e., endogenously generated H2S) can stimulate DNA repair. The nature of the H2S-induced DNA damage—predominantly characterized in the context of nuclear, rather than mitochondrial DNA—is, to a significant part, indirect, i.e., related to the intracellular generation of secondary, reactive oxygen species [147,148,149,150,151,152,153]. The molecular mechanisms of H2S-stimulated nuclear DNA repair are complex; multiple pathways and mechanisms (including PARP11 and g-H2AX foci formation, PCNA, CHK2, Ku70, Ku80, and DNA polymerase-d) have been implicated; this topic has been recently covered in a comprehensive review [154]. The subsequent paragraph of the current review will concentrate on the role of H2S in the regulation of mitochondrial DNA repair.
Perhaps the best proof for the bacterial evolutionary origin of mitochondria is the existence, structure and function of the mitochondrial DNA. Similar to bacterial DNA, mitochondrial DNA consists of a small, circular DNA structure which is not protected by histones (and which, therefore, is substantially more sensitive to oxidative damage than the nuclear DNA). The mitochondrial DNA has only approximately 16,500 base pairs, and it only encodes 13 proteins (as well as 22 tRNAs, and 2 rRNAs). The mitochondrially encoded proteins are essential protein components of the mitochondrial electron transport chain complexes. (From an evolutionary standpoint, it appears that many more mitochondrial proteins that were originally encoded on the mitochondrial DNA are now encoded by the nuclear DNA, but a select number of proteins remain mitochondrially encoded, most likely in order to maintain a rapid local control of mitochondrial function). Mutations in mitochondrial DNA disrupt the transcription of mitochondrially encoded proteins, which, in turn, can disrupt mitochondrial protein synthesis (and, consequently, mitochondrial function) in the short term and mitochondrial dynamics and organization in the long term [155,156,157].
The “grand total” of the literature on the role of H2S in the regulation of mitochondrial DNA repair consists of three published articles [99,158,159]. The first report, conducted in endothelial cells, demonstrates that AP39, a mitochondrially targeted H2S donor, attenuates the degree of mitochondrial DNA damage and accelerates the recovery of mitochondrial DNA integrity after oxidative damage [99]. However, this study did not investigate the underlying mechanisms of the H2S donor’s action. The second report, conducted in murine smooth muscle and aorta tissue focused on the role of CSE-derived H2S in the regulation of mitochondrial DNA copy numbers, mitochondrial content, mitochondrial-specific mRNAs (MT-CO1, CytB, and Atp 6), and implicated a role of mitochondrial transcription factor A mRNA and protein expression (TFAM) in these processes. The study concluded that H2S, via the regulation of DNA methyltransferase 3A (Dnmt3a) expression, and the consequent regulation of TFAM promoter methylation, is involved in the stimulation of mitochondrial DNA repair [158]. The most recent study (and, to date, the only published report focusing on the role of H2S in mitochondrial DNA repair in cancer cells) was conducted by our group in A549 lung adenocarcinoma cells [159]. These cells show an increased expression of all 3 H2S-producing enzymes. Oxidative mitochondrial DNA damage in these cells was increased and/or the efficacy of the DNA repair was impaired when the cells’ H2S biosynthesis was suppressed (either by treating of the cells with the pharmacological CBS inhibitor AOAA or after siRNA-mediated silencing any of the three major H2S-producing enzymes). The mechanism of H2S’ action to stimulate mitochondrial DNA repair was linked to the ability of H2S to induce the sulfhydration of the mitochondrial DNA repair enzyme EXOG (on Cys 76), which, in turn, promoted the assembly of a mitochondrial DNA repair complex (including EXOG, APE1 and Lig3) [159].
Clearly, the current body of knowledge on the role of H2S in regulating mitochondrial DNA integrity (or replication) or other mitochondrial DNA-related processes (e.g., the transcription or translation of mitochondrially encoded proteins) in cancer cells is minimal or non-existent: thus, future work will be needed to investigate these processes. Nevertheless, even from the current body of data, an interesting paradox emerges: on one hand, H2S production in cancer cells is upregulated, and mitochondrial DNA repair is activated. On the other hand, there are reports that show that in cancer cells, in fact, the mitochondrial DNA integrity is impaired [160,161]. It is conceivable that the increased DNA repair capacity of the cancer cell is unable to keep up with the extent of DNA damage (which is also increased in cancer cells, due to a multitude of processes including increased cellular ROS/RNS generation). A possible rationalization may be related to the previously mentioned “short-term thinking” of the cancer cell: by maximizing bioenergetic capacity in the short-term, cellular integrity (including DNA integrity) may be “expandable”. Possibly, survivable mutations may even be beneficial to the tumor tissue as a whole (although not necessarily to individual cancer cells), as they might produce clones that are resistant to the body’s own immunological tumor elimination processes or to chemotherapeutic agents [160,161].
6. Anticancer Effects of Pharmacological H2S Donation
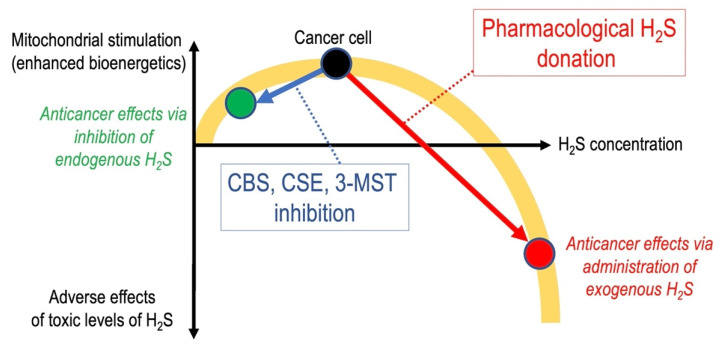
In line with the bell-shaped or biphasic effects of H2S, a significant body of data demonstrates that H2S donor compounds can exert anticancer effects by suppressing cancer cell metabolism and inducing cancer cell death. As previously discussed, [28,39,40,81,162], these cytotoxic H2S effects are do not invalidate the mechanisms and pathways discussed in the current article; while endogenously produced H2S maintains and supports a variety of beneficial (for the cancer cell, that is—i.e., not for the tumor-bearing host) processes (such as DNA repair, mitochondrial ATP generation, etc.), exogenously administered H2S donors reach high local concentrations, which are cytotoxic to cancer cells (but also to any other cell type) (Figure 2). The various chemical classes of anticancer H2S donors, their cellular actions (which, in some cases include the initiation of mitochondrial cell death pathways), and the potential difficulties with testing and developing such compounds (e.g., the theoretical and practical problems around selective targeting of the tumor cells with H2S donors in vivo) are extensively discussed in specialized reviews [163,164,165,166,167,168,169,170] and will not be reiterated in the current article.
Figure 2.
Due to the bell-shaped/biphasic pharmacological character of H2S, pharmacological inhibitors of H2S biosynthesis (via the removal of the stimulatory effects of endogenously produced, CBS, CSE or 3-MST-derived H2S) as well as exogenous, pharmacological H2S donor compounds (via stimulation of multiple cytostatic and cytotoxic mechanisms) can exert anticancer effects.
7. Additional Mitochondrial Roles of H2S in Cancer Cells
It is likely that the stimulation of mitochondrial electron transport, ATP generation, mitochondrial dynamics and mitochondrial DNA repair by endogenous H2S are not stand-alone processes, but, rather, they are part of a coordinated broad metabolic reprogramming process of the cancer cell. In fact, H2S may play an active role in this reprogramming process. For instance, H2S has been reported to sulfhydrate (activate) GAPDH to stimulate glycolysis [84]. An additional, H2S-regulated bioenergetic pathways that may become upregulated in cancer cell include the nicotinamide phosphoribosyltransferase (Nampt) (which has been implicated in the cancer cells’ ability to recover from hypoxic or oxidative damage) [54]. Moreover, metabolomic studies indicate that H2S can stimulate the activity of multiple Krebs cycle enzymes [56,58]. H2S was also found to upregulate glucose uptake in various cell types [171,172,173,174]; this may be very important to support the high glucose utilization of the cancer cell. Multiple metabolomic studies and genome wide gene expression studies [56,58,174] suggest that endogenously produced H2S plays a role in the reprogramming of the pyrimidine and purine metabolism, amino acid metabolism, nicotinate and nicotinamide metabolism, fatty acid metabolism, glutamate metabolism, the urea cycle, and several other pathways. The target enzymes involved in these processes remain to be characterized in the future. The available “sulfhydrome libraries” list thousands of sulfhydrated cellular proteins [44,45,46,47]. Although, for the majority of these proteins, functional follow-up studies remain to be conducted, it is important to point out that many of these sulfhydratable proteins are involved in the regulation of the above-mentioned biochemical and metabolic processes.
Endoplasmic reticulum stress (ER stress) has been implicated in the pathophysiology of many forms of cancer; this process also known to have a close and complex interrelationship with mitochondrial function/dysfunction [175,176,177]. Exogenous and endogenous H2S has been demonstrated to regulate ER stress [178,179,180,181]; deeper mechanistic aspects of this interrelationship remain to be investigated in the future. In addition (and, at least in part, in the context of ER stress) the potential role of cancer-cell-derived H2S in the regulation of mitochondrial KATP channels and mitochondrial aspects of cellular calcium handling should also be investigated in the future, given the fact that several studies implicate a regulatory role of H2S in these processes [182,183,184,185].
Many investigators consider CBS, CSE and 3-MST as the sole sources of H2S in mammalian cells. However, there are additional sources of H2S, the function of which remains largely unexplored in the pathophysiology of cancer. For instance, there are non-enzymatic sources of H2S; however, these are difficult to investigate for practical reasons (e.g., the lack of selective H2S scavengers). Moreover, several additional enzymes have been identified as mammalian sources of H2S; these include D-amino acid oxidase (DAO) in the kidney and gut [185,186], cysteinyl-tRNA synthetases (CARSs) [44], and selenium-binding protein 1 (SBP1) [187,188]. The regulation of DAO, CARSs and SBP1 in cancer and the functional role of the associated H2S/polysulfide production remains to be explored in future studies.
Clearly, many questions remain to be addressed in the context of the above-discussed processes. One of them relates to the mechanism(s) involved in the upregulation of H2S biosynthesis in cancer cells (in general, and with respect to potential translocation of H2S-producing enzymes into the mitochondria). CBS and 3-MST expression may be regulated both at the level of transcription, as well as at the level of degradation/protein stability [31,35]; the importance of these mechanisms in various cancers remain to be further defined. It will be also interesting to assess whether the increased H2S biosynthesis in cancer cells is linked to the well-known global reprogramming of substrate (cysteine, homocysteine) biosynthesis and/or cell uptake in cancer. Clearly, cancer cells undergo a global reprogramming of sulfur metabolism [48,49], and the mechanisms discussed in the current article must be placed into this broader context.
8. Conclusions and Implications
In cancer cells, upregulation of various H2S-producing enzymes occurs in various cellular compartments (including the mitochondria), which raises intracellular (including intramitochondrial) H2S levels. H2S, in turn, stimulates mitochondrial electron transport, ATP generation, regulates mitochondrial dynamics and promotes mitochondrial DNA repair: all of these processes serve the extreme bioenergetic demand of the cancer cell. Pharmacological inhibition of H2S generation, which can impair the cancer cell’s mitochondrial function (and, more broadly, it can disrupt the cancer cell’s bioenergetic supply) emerges as a potential novel anticancer therapeutic concept.
Funding
The work of C.S. in the field of cancer and H2S is funded by the Novartis Foundation (#18N092) and the Swiss Krebsliga (KLS-4504-08-2018).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Beauchamp R.O., Bus J.S., Popp J.A., Boreiko C.J., Andjelkovich D.A., Leber P. A critical review of the literature on hydrogen sulfide toxicity. CRC Crit. Rev. Toxicol. 1984;13:25–97. doi: 10.3109/10408448409029321. [DOI] [PubMed] [Google Scholar]
- 2.Reiffenstein R.J., Hulbert W.C., Roth S.H. Toxicology of hydrogen sulfide. Ann. Rev. Pharmacol. Toxicol. 1992;32:109–134. doi: 10.1146/annurev.pa.32.040192.000545. [DOI] [PubMed] [Google Scholar]
- 3.Milby T.H., Baselt R.C. Hydrogen sulfide poisoning: Clarification of some controversial issues. Am. J. Ind. Med. 1999;35:192–195. doi: 10.1002/(SICI)1097-0274(199902)35:2<192::AID-AJIM11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 4.IRIS . Support of Summary Information on the Integrated Risk Information System. U.S. Environmental Protection Agency; Washington, DC, USA: 2003. Toxicological Review of Hydrogen Sulfide. CAS No. 7783-06-4. [Google Scholar]
- 5.Guidotti T.L. Hydrogen sulfide intoxication. In: Lotti M., Bleecker M.L., editors. Handbook of Clinical Neurology. Elsevier; Amsterdam, The Netherlands: 2015. [DOI] [PubMed] [Google Scholar]
- 6.Nicholls P., Marshall D.C., Cooper C.E., Wilson M.T. Sulfide inhibition of and metabolism by cytochrome c oxidase. Biochem. Soc. Trans. 2013;41:1312–1316. doi: 10.1042/BST20130070. [DOI] [PubMed] [Google Scholar]
- 7.Collman J.P., Ghosh S., Dey A., Decréau R.A. Using a functional enzyme model to understand the chemistry behind hydrogen sulfide induced hibernation. Proc. Natl. Acad. Sci. USA. 2009;106:22090–22095. doi: 10.1073/pnas.0904082106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panagaki T., Randi E.B., Augsburger F., Szabo C. Overproduction of H2S, generated by CBS, inhibits mitochondrial Complex IV and suppresses oxidative phosphorylation in Down syndrome. Proc. Natl. Acad. Sci. USA. 2019;116:18769–18771. doi: 10.1073/pnas.1911895116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szabo C. The re-emerging pathophysiological role of the cystathionine-beta-synthase-hydrogen sulfide system in Down syndrome. FEBS J. 2020;287:3150–3160. doi: 10.1111/febs.15214. [DOI] [PubMed] [Google Scholar]
- 10.Szabo C. A timeline of hydrogen sulfide (H2S) research: From environmental toxin to biological mediator. Biochem. Pharmacol. 2018;149:5–19. doi: 10.1016/j.bcp.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aroca A., Gotor C., Bassham D.C., Romero L.C. Hydrogen sulfide: From a toxic molecule to a key molecule of cell life. Antioxidants. 2020;9:621. doi: 10.3390/antiox9070621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimura H. Hydrogen sulfide as a neuromodulator. Mol. Neurobiol. 2002;26:13–19. doi: 10.1385/MN:26:1:013. [DOI] [PubMed] [Google Scholar]
- 13.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 14.Kimura H. Hydrogen sulfide: Its production, release and functions. Amino Acids. 2011;41:113–121. doi: 10.1007/s00726-010-0510-x. [DOI] [PubMed] [Google Scholar]
- 15.Li L., Rose P., Moore P.K. Hydrogen sulfide and cell signaling. Annu. Rev. Pharmacol. Toxicol. 2011;51:169–187. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- 16.Whiteman M., Winyard P.G. Hydrogen sulfide and inflammation: The good, the bad, the ugly and the promising. Expert Rev. Clin. Pharmacol. 2011;4:13–32. doi: 10.1586/ecp.10.134. [DOI] [PubMed] [Google Scholar]
- 17.Predmore B.L., Lefer D.J., Gojon G. Hydrogen sulfide in biochemistry and medicine. Antioxid. Redox. Signal. 2012;17:119–140. doi: 10.1089/ars.2012.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 2012;92:791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 19.Li Q., Lancaster J.R., Jr. Chemical foundations of hydrogen sulfide biology. Nitric Oxide. 2013;35:21–34. doi: 10.1016/j.niox.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimura H. The physiological role of hydrogen sulfide and beyond. Nitric Oxide. 2014;41:4–10. doi: 10.1016/j.niox.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Bełtowski J. Hydrogen sulfide in pharmacology and medicine—An update. Pharmacol. Rep. 2015;67:647–658. doi: 10.1016/j.pharep.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Yang G., Wang R. H2S and blood vessels: An overview. Handb. Exp. Pharmacol. 2015;230:85–110. doi: 10.1007/978-3-319-18144-8_4. [DOI] [PubMed] [Google Scholar]
- 23.Wang R., Szabo C., Ichinose F., Ahmed A., Whiteman M., Papapetropoulos A. The role of H2S bioavailability in endothelial dysfunction. Trends Pharmacol. Sci. 2015;36:568–578. doi: 10.1016/j.tips.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papapetropoulos A., Whiteman M., Cirino G. Pharmacological tools for hydrogen sulphide research: A brief, introductory guide for beginners. Br. J. Pharmacol. 2015;172:1633–1637. doi: 10.1111/bph.12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanagy N.L., Szabo C., Papapetropoulos A. Vascular biology of hydrogen sulfide. Am. J. Physiol. Cell. Physiol. 2017;312:C537–C549. doi: 10.1152/ajpcell.00329.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rose P., Moore P.K., Zhu Y.Z. H2S biosynthesis and catabolism: New insights from molecular studies. Cell. Mol. Life Sci. 2017;74:1391–1412. doi: 10.1007/s00018-016-2406-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szabo C. Hydrogen sulfide, an enhancer of vascular nitric oxide signaling: Mechanisms and implications. Am. J. Physiol. Cell. Physiol. 2017;312:C3–C15. doi: 10.1152/ajpcell.00282.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szabo C., Papapetropoulos A. International Union of Basic and Clinical Pharmacology. CII: Pharmacological Modulation of H2S Levels: H2S Donors and H2S Biosynthesis Inhibitors. Pharmacol. Rev. 2017;69:497–564. doi: 10.1124/pr.117.014050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blachier F., Andriamihaja M., Larraufie P., Ahn E., Lan A., Kim E. Production of hydrogen sulfide by the intestinal microbiota and epithelial cells and consequences for the colonic and rectal mucosa. Am. J. Physiol. Gastrointest. Liver Physiol. 2020 doi: 10.1152/ajpgi.00261.2020. [DOI] [PubMed] [Google Scholar]
- 30.Dilek N., Papapetropoulos A., Toliver-Kinsky T., Szabo C. Hydrogen sulfide: An endogenous regulator of the immune system. Pharmacol. Res. 2020;161:105119. doi: 10.1016/j.phrs.2020.105119. [DOI] [PubMed] [Google Scholar]
- 31.Zuhra K., Augsburger F., Majtan T., Szabo C. Cystathionine-β-synthase: Molecular regulation and pharmacological inhibition. Biomolecules. 2020;10:697. doi: 10.3390/biom10050697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan S., Shen X., Kevil C.G. Beyond a gasotransmitter: Hydrogen sulfide and polysulfide in cardiovascular health and immune response. Antioxid. Redox. Signal. 2017;27:634–653. doi: 10.1089/ars.2017.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimura H. Signaling by hydrogen sulfide (H2S) and polysulfides (H2Sn) in the central nervous system. Neurochem. Int. 2019;126:118–125. doi: 10.1016/j.neuint.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 34.Kimura H. Signalling by hydrogen sulfide and polysulfides via protein S-sulfuration. Br. J. Pharmacol. 2020;177:720–733. doi: 10.1111/bph.14579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pedre B., Dick T.P. 3-Mercaptopyruvate sulfurtransferase: An enzyme at the crossroads of sulfane sulfur trafficking. Biol. Chem. 2020 doi: 10.1515/hsz-2020-0249. [DOI] [PubMed] [Google Scholar]
- 36.Cortese-Krott M.M., Koning A., Kuhnle G.G.C., Nagy P., Bianco C.L., Pasch A., Wink D.A., Fukuto J.M., Jackson A.A., van Goor H., et al. The reactive species interactome: Evolutionary emergence, biological significance, and opportunities for redox metabolomics and personalized medicine. Antioxid. Redox. Signal. 2017;27:684–712. doi: 10.1089/ars.2017.7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sies H., Jones D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell. Biol. 2020;21:363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 38.Toliver-Kinsky T., Cui W., Törö G., Lee S.J., Shatalin K., Nudler E., Szabo C. H2S, a bacterial defense mechanism against the host immune response. Infect. Immun. 2018;87:e00272-18. doi: 10.1128/IAI.00272-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hellmich M.R., Coletta C., Chao C., Szabo C. The therapeutic potential of cystathionine β-synthetase/hydrogen sulfide inhibition in cancer. Antioxid. Redox. Signal. 2015;22:424–448. doi: 10.1089/ars.2014.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szabo C. Gasotransmitters in cancer: From pathophysiology to experimental therapy. Nat. Rev. Drug Discov. 2016;15:185–203. doi: 10.1038/nrd.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Augsburger F., Szabo C. Potential role of the 3-mercaptopyruvate sulfurtransferase (3-MST)-hydrogen sulfide (H2S) pathway in cancer cells. Pharmacol. Res. 2020;154:104083. doi: 10.1016/j.phrs.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 42.Nagy P. Mechanistic chemical perspective of hydrogen sulfide signaling. Methods Enzymol. 2015;554:3–29. doi: 10.1016/bs.mie.2014.11.036. [DOI] [PubMed] [Google Scholar]
- 43.Paul B.D., Snyder S.H. H2S: A novel gasotransmitter that signals by sulfhydration. Trends Biochem. Sci. 2015;40:687–700. doi: 10.1016/j.tibs.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akaike T., Ida T., Wei F.Y., Nishida M., Kumagai Y., Alam M.M., Ihara H., Sawa T., Matsunaga T., Kasamatsu S., et al. Cysteinyl-tRNA synthetase governs cysteine polysulfidation and mitochondrial bioenergetics. Nat. Commun. 2017;8:1177. doi: 10.1038/s41467-017-01311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zivanovic J., Kouroussis E., Kohl J.B., Adhikari B., Bursac B., Schott-Roux S., Petrovic D., Miljkovic J.L., Thomas-Lopez D., Jung Y., et al. Selective persulfide detection reveals evolutionarily conserved antiaging effects of S-sulfhydration. Cell Metab. 2019;30:1152–1170.e13. doi: 10.1016/j.cmet.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao X.H., Li L., Parisien M., Wu J., Bederman I., Gao Z., Krokowski D., Chirieleison S.M., Abbott D., Wang B., et al. Discovery of a redox thiol switch: Implications for cellular energy metabolism. Mol. Cell. Proteom. 2020;19:852–870. doi: 10.1074/mcp.RA119.001910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bibli S.I., Hu J., Looso M., Weigert A., Ratiu C., Wittig J., Drekolia M.K., Tombor L., Randriamboavonjy V., Leisegang M.S., et al. Mapping the endothelial cell S-sulfhydrome highlights the crucial role of integrin sulfhydration in vascular function. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.051877. [DOI] [PubMed] [Google Scholar]
- 48.Ward N.P., DeNicola G.M. Sulfur metabolism and its contribution to malignancy. Int. Rev. Cell. Mol. Biol. 2019;347:39–103. doi: 10.1016/bs.ircmb.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 49.Serpa J. Cysteine as a carbon source, a hot spot in cancer cells survival. Front. Oncol. 2020;10:947. doi: 10.3389/fonc.2020.00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Szabo C., Coletta C., Chao C., Módis K., Szczesny B., Papapetropoulos A., Hellmich M.R. Tumor-derived hydrogen sulfide, produced by cystathionine-beta-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc. Natl. Acad. Sci. USA. 2013;110:12474–12479. doi: 10.1073/pnas.1306241110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akbari M., Sogutdelen E., Juriasingani S., Sener A. Hydrogen sulfide: Emerging role in bladder, kidney, and prostate malignancies. Oxid. Med. Cell. Longev. 2019;2019:2360945. doi: 10.1155/2019/2360945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giuffrè A., Tomé C.S., Fernandes D.G.F., Zuhra K., Vicente J.B. Hydrogen sulfide metabolism and signaling in the tumor microenvironment. Adv. Exp. Med. Biol. 2020;1219:335–353. doi: 10.1007/978-3-030-34025-4_17. [DOI] [PubMed] [Google Scholar]
- 53.Módis K., Coletta C., Asimakopoulou A., Szczesny B., Chao C., Papapetropoulos A., Hellmich M.R., Szabo C. Effect of S-adenosyl-L-methionine (SAM), an allosteric activator of cystathionine-beta-synthase (CBS) on colorectal cancer cell proliferation and bioenergetics in vitro. Nitric Oxide. 2014;41:146–156. doi: 10.1016/j.niox.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ostrakhovitch E.A., Akakura S., Sanokawa-Akakura R., Goodwin S., Tabibzadeh S. Dedifferentiation of cancer cells following recovery from a potentially lethal damage is mediated by H2S-Nampt. Exp. Cell. Res. 2015;330:135–150. doi: 10.1016/j.yexcr.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 55.Druzhyna N., Szczesny B., Olah G., Módis K., Asimakopoulou A., Pavlidou A., Szoleczky P., Gerö D., Yanagi K., Törö G., et al. Screening of a composite library of clinically used drugs and well-characterized pharmacological compounds for cystathionine beta-synthase inhibition identifies benserazide as a drug potentially suitable for repurposing for the experimental therapy of colon cancer. Pharmacol. Res. 2016;113:18–37. doi: 10.1016/j.phrs.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chao C., Zatarain J.R., Ding Y., Coletta C., Mrazek A.A., Druzhyna N., Johnson P., Chen H., Hellmich J.L., Asimakopoulou A., et al. Cystathionine-beta-synthase inhibition for colon cancer: Enhancement of the efficacy of aminooxyacetic acid via the prodrug approach. Mol. Med. 2016;22:361–379. doi: 10.2119/molmed.2016.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sekiguchi F., Sekimoto T., Ogura A., Kawabata A. Endogenous hydrogen sulfide enhances cell proliferation of human gastric cancer AGS cells. Biol. Pharm. Bull. 2016;39:887–890. doi: 10.1248/bpb.b15-01015. [DOI] [PubMed] [Google Scholar]
- 58.Phillips C.M., Zatarain J.R., Nicholls M.E., Porter C., Widen S.G., Thanki K., Johnson P., Jawad M.U., Moyer M.P., Randall J.W., et al. Upregulation of cystathionine-beta-synthase in colonic epithelia reprograms metabolism and promotes carcinogenesis. Cancer Res. 2017;77:5741–5754. doi: 10.1158/0008-5472.CAN-16-3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oláh G., Módis K., Törö G., Hellmich M.R., Szczesny B., Szabo C. Role of endogenous and exogenous nitric oxide, carbon monoxide and hydrogen sulfide in HCT116 colon cancer cell proliferation. Biochem. Pharmacol. 2018;149:186–204. doi: 10.1016/j.bcp.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Untereiner A.A., Oláh G., Módis K., Hellmich M.R., Szabo C. H2S-induced S-sulfhydration of lactate dehydrogenase a (LDHA) stimulates cellular bioenergetics in HCT116 colon cancer cells. Biochem. Pharmacol. 2017;136:86–98. doi: 10.1016/j.bcp.2017.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jia H., Ye J., You J., Shi X., Kang W., Wang T. Role of the cystathionine beta-synthase/H2S system in liver cancer cells and the inhibitory effect of quinolone-indolone conjugate QIC2 on the system. Oncol. Rep. 2017;37:3001–3009. doi: 10.3892/or.2017.5513. [DOI] [PubMed] [Google Scholar]
- 62.Wang L., Cai H., Hu Y., Liu F., Huang S., Zhou Y., Yu J., Xu J., Wu F. A pharmacological probe identifies cystathionine β-synthase as a new negative regulator for ferroptosis. Cell Death Dis. 2018;9:1005. doi: 10.1038/s41419-018-1063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Untereiner A.A., Pavlidou A., Druzhyna N., Papapetropoulos A., Hellmich M.R., Szabo C. Drug resistance induces the upregulation of H2S-producing enzymes in HCT116 colon cancer cells. Biochem. Pharmacol. 2018;149:174–185. doi: 10.1016/j.bcp.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zuhra K., Tomé C.S., Masi L., Giardina G., Paulini G., Malagrinò F., Forte E., Vicente J.B., Giuffrè A. N-acetylcysteine serves as substrate of 3-mercaptopyruvate sulfurtransferase and stimulates sulfide metabolism in colon cancer cells. Cells. 2019;8:828. doi: 10.3390/cells8080828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Libiad M., Vitvitsky V., Bostelaar T., Bak D.W., Lee H.J., Sakamoto N., Fearon E., Lyssiotis C.A., Weerapana E., Banerjee R. Hydrogen sulfide perturbs mitochondrial bioenergetics and triggers metabolic reprogramming in colon cells. J. Biol. Chem. 2019;294:12077–12090. doi: 10.1074/jbc.RA119.009442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Malagrinò F., Zuhra K., Mascolo L., Mastronicola D., Vicente J.B., Forte E., Giuffrè A. Hydrogen sulfide oxidation: Adaptive changes in mitochondria of SW480 colorectal cancer cells upon exposure to hypoxia. Oxid. Med. Cell. Longev. 2019;2019:8102936. doi: 10.1155/2019/8102936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Augsburger F., Randi E.B., Jendly M., Ascencao K., Dilek N., Szabo C. Role of 3-mercaptopyruvate sulfurtransferase in the regulation of proliferation, migration, and bioenergetics in murine colon cancer cells. Biomolecules. 2020;10:447. doi: 10.3390/biom10030447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yue T., Zuo S., Bu D., Zhu J., Chen S., Ma Y., Ma J., Guo S., Wen L., Zhang X., et al. Aminooxyacetic acid (AOAA) sensitizes colon cancer cells to oxaliplatin via exaggerating apoptosis induced by ROS. J. Cancer. 2020;11:1828–1838. doi: 10.7150/jca.35375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ye F., Li X., Sun K., Xu W., Shi H., Bian J., Lu R., Ye Y. Inhibition of endogenous hydrogen sulfide biosynthesis enhances the anti-cancer effect of 3,3’-diindolylmethane in human gastric cancer cells. Life Sci. 2020;261:118348. doi: 10.1016/j.lfs.2020.118348. [DOI] [PubMed] [Google Scholar]
- 70.Zuhra K., Panagaki T., Randi E.B., Augsburger F., Blondel M., Friocourt G., Herault Y., Szabo C. Mechanism of cystathionine-β-synthase inhibition by disulfiram: The role of bis (N,N-diethyldithiocarbamate)-copper(II) Biochem. Pharmacol. 2020;182:114267. doi: 10.1016/j.bcp.2020.114267. [DOI] [PubMed] [Google Scholar]
- 71.Ascenção K., Dilek N., Augsburger F., Panagaki T., Zuhra K., Szabo C. Pharmacological induction of mesenchymal-epithelial transition via inhibition of H2S biosynthesis and consequent suppression of ACLY activity in colon cancer cells. Pharmacol. Res. 2021 doi: 10.1016/j.phrs.2020.105393. in press. [DOI] [PubMed] [Google Scholar]
- 72.Yang G., Wu L., Jiang B., Yang W., Qi J., Cao K., Meng Q., Mustafa A.K., Mu W., Zhang S., et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Coletta C., Papapetropoulos A., Erdelyi K., Olah G., Módis K., Panopoulos P., Asimakopoulou A., Gerö D., Sharina I., Martin E., et al. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc. Natl. Acad. Sci. USA. 2012;109:9161–9166. doi: 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cirino G., Vellecco V., Bucci M. Nitric oxide and hydrogen sulfide: The gasotransmitter paradigm of the vascular system. Br. J. Pharmacol. 2017;174:4021–4031. doi: 10.1111/bph.13815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cai W.J., Wang M.J., Moore P.K., Jin H.M., Yao T., Zhu Y.C. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc. Res. 2007;76:29–40. doi: 10.1016/j.cardiores.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 76.Papapetropoulos A., Pyriochou A., Altaany Z., Yang G., Marazioti A., Zhou Z., Jeschke M.G., Branski L.K., Herndon D.N., Wang R., et al. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc. Natl. Acad. Sci. USA. 2009;106:21972–21977. doi: 10.1073/pnas.0908047106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Szabo C., Papapetropoulos A. Hydrogen sulphide and angiogenesis: Mechanisms and applications. Br. J. Pharmacol. 2011;164:853–865. doi: 10.1111/j.1476-5381.2010.01191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Katsouda A., Bibli S.I., Pyriochou A., Szabo C., Papapetropoulos A. Regulation and role of endogenously produced hydrogen sulfide in angiogenesis. Pharmacol. Res. 2016;113:175–185. doi: 10.1016/j.phrs.2016.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guo F.F., Yu T.C., Hong J., Fang J.Y. Emerging roles of hydrogen sulfide in inflammatory and neoplastic colonic diseases. Front. Physiol. 2016;7:156. doi: 10.3389/fphys.2016.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu H., Blake S., Chan K.T., Pearson R.B., Kang J. Cystathionine beta-synthase in physiology and cancer. Biomed. Res. Int. 2018;2018:3205125. doi: 10.1155/2018/3205125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kashfi K. The dichotomous role of H2S in cancer cell biology? Deja vu all over again. Biochem. Pharmacol. 2018;149:205–223. doi: 10.1016/j.bcp.2018.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Murphy B., Bhattacharya R., Mukherjee P. Hydrogen sulfide signaling in mitochondria and disease. FASEB J. 2019;33:13098–13125. doi: 10.1096/fj.201901304R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Youness R.A., Gad A.Z., Sanber K., Ahn Y.J., Lee G.J., Khallaf E., Hafez H.M., Motaal A.A., Ahmed N., Gad M.Z. Targeting hydrogen sulphide signaling in breast cancer. J. Adv. Res. 2020;27:177–190. doi: 10.1016/j.jare.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mustafa A.K., Gadalla M.M., Sen N., Kim S., Mu W., Gazi S.K., Barrow R.K., Yang G., Wang R., Snyder S.H. H2S signals through protein S-sulfhydration. Sci. Signal. 2009;2:ra72. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Du C., Lin X., Xu W., Zheng F., Cai J., Yang J., Cui Q., Tang C., Cai J., Xu G., et al. Sulfhydrated sirtuin-1 increasing its deacetylation activity is an essential epigenetics mechanism of anti-atherogenesis by hydrogen sulfide. Antioxid. Redox. Signal. 2019;30:184–197. doi: 10.1089/ars.2017.7195. [DOI] [PubMed] [Google Scholar]
- 86.Yuan Y., Zhu L., Li L., Liu J., Chen Y., Cheng J., Peng T., Lu Y. S-sulfhydration of SIRT3 by hydrogen sulfide attenuates mitochondrial dysfunction in cisplatin-induced acute kidney injury. Antioxid. Redox. Signal. 2019;31:1302–1319. doi: 10.1089/ars.2019.7728. [DOI] [PubMed] [Google Scholar]
- 87.Shimizu Y., Polavarapu R., Eskla K.-L., Nicholson C.K., Koczor C.A., Wang R., Lewis W., Shiva S., Lefer D.J., Calvert J.W. Hydrogen sulfide regulates cardiac mitochondrial biogenesis via the activation of AMPK. J. Mol. Cell. Cardiol. 2018;116:29–40. doi: 10.1016/j.yjmcc.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goubern M., Andriamihaja M., Nübel T., Blachier F., Bouillaud F. Sulfide, the first inorganic substrate for human cells. FASEB J. 2007;21:1699–1706. doi: 10.1096/fj.06-7407com. [DOI] [PubMed] [Google Scholar]
- 89.Lagoutte E., Mimoun S., Andriamihaja M., Chaumontet C., Blachier F., Bouillaud F. Oxidation of hydrogen sulfide remains a priority in mammalian cells and causes reverse electron transfer in colonocytes. Biochim. Biophys. Acta. 2010;1797:1500–1511. doi: 10.1016/j.bbabio.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 90.Módis K., Coletta C., Erdélyi K., Papapetropoulos A., Szabo C. Intramitochondrial hydrogen sulfide production by 3-mercaptopyruvate sulfurtransferase maintains mitochondrial electron flow and supports cellular bioenergetics. FASEB J. 2013;27:601–611. doi: 10.1096/fj.12-216507. [DOI] [PubMed] [Google Scholar]
- 91.Módis K., Ju Y., Ahmad A., Untereiner A.A., Altaany Z., Wu L., Szabo C., Wang R. S-sulfhydration of ATP synthase by hydrogen sulfide stimulates mitochondrial bioenergetics. Pharmacol. Res. 2016;113:116–124. doi: 10.1016/j.phrs.2016.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang C., Du J., Du S., Liu Y., Li D., Zhu X., Ni X. Endogenous H2S resists mitochondria-mediated apoptosis in the adrenal glands via ATP5A1 S-sulfhydration in male mice. Mol. Cell. Endocrinol. 2018;474:65–73. doi: 10.1016/j.mce.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 93.Módis K., Panopoulos P., Coletta C., Papapetropoulos A., Szabo C. Hydrogen sulfide-mediated stimulation of mitochondrial electron transport involves inhibition of the mitochondrial phosphodiesterase 2A, elevation of cAMP and activation of protein kinase A. Biochem. Pharmacol. 2013;86:1311–1319. doi: 10.1016/j.bcp.2013.08.064. [DOI] [PubMed] [Google Scholar]
- 94.Chakraborty P.K., Murphy B., Mustafi S.B., Dey A., Xiong X., Rao G., Naz S., Zhang M., Yang D., Dhanasekaran D.N., et al. Cystathionine beta-synthase regulates mitochondrial morphogenesis in ovarian cancer. FASEB J. 2018;32:4145–4157. doi: 10.1096/fj.201701095R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qiao P., Zhao F., Liu M., Gao D., Zhang H., Yan Y. Hydrogen sulfide inhibits mitochondrial fission in neuroblastoma N2a cells through the Drp1/ERK1/2 signaling pathway. Mol. Med. Rep. 2017;16:971–977. doi: 10.3892/mmr.2017.6627. [DOI] [PubMed] [Google Scholar]
- 96.Calvert J.W., Jha S., Gundewar S., Elrod J.W., Ramachandran A., Pattillo C.B., Kevil C.G., Lefer D.J. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ. Res. 2009;105:365–374. doi: 10.1161/CIRCRESAHA.109.199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kimura Y., Goto Y., Kimura H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid. Redox. Signal. 2010;12:1–13. doi: 10.1089/ars.2008.2282. [DOI] [PubMed] [Google Scholar]
- 98.Suzuki K., Olah G., Modis K., Coletta C., Kulp G., Gerö D., Szoleczky P., Chang T., Zhou Z., Wu L., et al. Hydrogen sulfide replacement therapy protects the vascular endothelium in hyperglycemia by preserving mitochondrial function. Proc. Natl. Acad. Sci. USA. 2011;108:13829–13834. doi: 10.1073/pnas.1105121108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Szczesny B., Módis K., Yanagi K., Coletta C., Le Trionnaire S., Perry A., Wood M.E., Whiteman M., Szabo C. AP39, a novel mitochondria-targeted hydrogen sulfide donor, stimulates cellular bioenergetics, exerts cytoprotective effects and protects against the loss of mitochondrial DNA integrity in oxidatively stressed endothelial cells in vitro. Nitric Oxide. 2014;41:120–130. doi: 10.1016/j.niox.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xie Z.Z., Shi M.M., Xie L., Wu Z.Y., Li G., Hua F., Bian J.S. Sulfhydration of p66Shc at cysteine59 mediates the antioxidant effect of hydrogen sulfide. Antioxid. Redox. Signal. 2014;21:2531–2542. doi: 10.1089/ars.2013.5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cairns R., Harris I., Mak T. Regulation of cancer cell metabolism. Nat. Rev. Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 102.Park J.H., Pyun W.Y., Park H.W. Cancer metabolism: Phenotype, signaling and therapeutic targets. Cells. 2020;9:2308. doi: 10.3390/cells9102308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Farhadi P., Yarani R., Dokaneheifard S., Mansouri K. The emerging role of targeting cancer metabolism for cancer therapy. Tumour Biol. 2020;42:1010428320965284. doi: 10.1177/1010428320965284. [DOI] [PubMed] [Google Scholar]
- 104.Pascale R.M., Calvisi D.F., Simile M.M., Feo C.F., Feo F. The Warburg effect 97 years after its discovery. Cancers (Basel) 2020;12:2819. doi: 10.3390/cancers12102819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yoshida G.J. Beyond the Warburg effect: N-myc contributes to metabolic reprogramming in cancer cells. Front. Oncol. 2020;10:791. doi: 10.3389/fonc.2020.00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Serpa J. Tumor Microenvironment: The Main Driver of Metabolic Adaptation. Springer International Publishing; New York, NY, USA: 2020. [Google Scholar]
- 107.Mimoun S., Andriamihaja M., Chaumontet C., Atanasiu C., Benamouzig R., Blouin J.M., Tomé D., Bouillaud F., Blachier F. Detoxification of H2S by differentiated colonic epithelial cells: Implication of the sulfide oxidizing unit and of the cell respiratory capacity. Antioxid. Redox. Signal. 2012;17:1–10. doi: 10.1089/ars.2011.4186. [DOI] [PubMed] [Google Scholar]
- 108.Szabo C., Ransy C., Módis K., Andriamihaja M., Murghes B., Coletta C., Olah G., Yanagi K., Bouillaud F. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. Br. J. Pharmacol. 2014;171:2099–2122. doi: 10.1111/bph.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Beaumont M., Andriamihaja M., Lan A., Khodorova N., Audebert M., Blouin J.M., Grauso M., Lancha L., Benetti P.H., Benamouzig R., et al. Detrimental effects for colonocytes of an increased exposure to luminal hydrogen sulfide: The adaptive response. Free Radic. Biol. Med. 2016;93:155–164. doi: 10.1016/j.freeradbiomed.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 110.Blachier F., Beaumont M., Kim E. Cysteine-derived hydrogen sulfide and gut health: A matter of endogenous or bacterial origin. Curr. Opin. Clin. Nutr. Metab. Care. 2019;22:68–75. doi: 10.1097/MCO.0000000000000526. [DOI] [PubMed] [Google Scholar]
- 111.Bhattacharyya S., Saha S., Giri K., Lanza I.R., Nair K.S., Jennings N.B., Rodriguez-Aguayo C., Lopez-Berestein G., Basal E., Weaver A.L., et al. Cystathionine beta-synthase (CBS) contributes to advanced ovarian cancer progression and drug resistance. PLoS ONE. 2013;8:e79167. doi: 10.1371/journal.pone.0079167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang C., Chen H., Zhang M., Zhang J., Wei X., Ying W. Malate-aspartate shuttle inhibitor aminooxyacetic acid leads to decreased intracellular ATP levels and altered cell cycle of C6 glioma cells by inhibiting glycolysis. Cancer Lett. 2016;378:1–7. doi: 10.1016/j.canlet.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 113.Lee J.S., Oh S.J., Choi H.J., Kang J.H., Lee S.H., Ha J.S., Woo S.M., Jang H., Lee H., Kim S.Y. ATP production relies on fatty acid oxidation rather than glycolysis in pancreatic ductal adenocarcinoma. Cancers. 2020;12:2477. doi: 10.3390/cancers12092477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ackermann M., Kubitza M., Maier K., Brawanski A., Hauska G., Piña A.L. The vertebrate homolog of sulfide-quinone reductase is expressed in mitochondria of neuronal tissues. Neuroscience. 2011;199:1–12. doi: 10.1016/j.neuroscience.2011.10.044. [DOI] [PubMed] [Google Scholar]
- 115.Linden D.R., Furne J., Stoltz G.J., Abdel-Rehim M.S., Levitt M.D., Szurszewski J.H. Sulphide quinone reductase contributes to hydrogen sulphide metabolism in murine peripheral tissues but not in the CNS. Br. J. Pharmacol. 2012;165:2178–2190. doi: 10.1111/j.1476-5381.2011.01681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mishanina T.V., Yadav P.K., Ballou D.P., Banerjee R. Transient kinetic analysis of hydrogen sulfide oxidation catalyzed by human sulfide quinone oxidoreductase. J. Biol. Chem. 2015;290:25072–25080. doi: 10.1074/jbc.M115.682369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ackermann M., Kubitza M., Hauska G., Piña A.L. The vertebrate homologue of sulfide-quinone reductase in mammalian mitochondria. Cell Tissue Res. 2014;358:779–792. doi: 10.1007/s00441-014-1983-9. [DOI] [PubMed] [Google Scholar]
- 118.Xia Y., Lü C., Hou N., Xin Y., Liu J., Liu H., Xun L. Sulfide production and oxidation by heterotrophic bacteria under aerobic conditions. ISME J. 2017;11:2754–2766. doi: 10.1038/ismej.2017.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu D., Zhang J., Lü C., Xia Y., Liu H., Jiao N., Xun L., Liu J. Synechococcus sp. Strain PCC7002 uses sulfide: Quinone oxidoreductase to detoxify exogenous sulfide and to convert endogenous sulfide to cellular sulfane sulfur. mBio. 2020;11:e03420-19. doi: 10.1128/mBio.03420-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jia J., Wang Z., Zhang M., Huang C., Song Y., Xu F., Zhang J., Li J., He M., Li Y., et al. SQR mediates therapeutic effects of H2S by targeting mitochondrial electron transport to induce mitochondrial uncoupling. Sci. Adv. 2020;6:eaaz5752. doi: 10.1126/sciadv.aaz5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bucci M., Papapetropoulos A., Vellecco V., Zhou Z., Pyriochou A., Roussos C., Roviezzo F., Brancaleone V., Cirino G. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler. Thromb. Vasc. Biol. 2010;30:1998–2004. doi: 10.1161/ATVBAHA.110.209783. [DOI] [PubMed] [Google Scholar]
- 122.Bucci M., Papapetropoulos A., Vellecco V., Zhou Z., Zaid A., Giannogonas P., Cantalupo A., Dhayade S., Karalis K.P., Wang R., et al. cGMP-dependent protein kinase contributes to hydrogen sulfide-stimulated vasorelaxation. PLoS ONE. 2012;7:e53319. doi: 10.1371/journal.pone.0053319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nagpure B.V., Bian J.S. Hydrogen sulfide inhibits A2A adenosine receptor agonist induced β-amyloid production in SH-SY5Y neuroblastoma cells via a cAMP dependent pathway. PLoS ONE. 2014;9:e88508. doi: 10.1371/journal.pone.0088508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sun Y., Huang Y., Yu W., Chen S., Yao Q., Zhang C., Bu D., Tang C., Du J., Jin H. Sulfhydration-associated phosphodiesterase 5A dimerization mediates vasorelaxant effect of hydrogen sulfide. Oncotarget. 2017;8:31888–31900. doi: 10.18632/oncotarget.16649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nalli A.D., Bhattacharya S., Wang H., Kendig D.M., Grider J.R., Murthy K.S. Augmentation of cGMP/PKG pathway and colonic motility by hydrogen sulfide. Am. J. Physiol. Gastrointest. Liver Physiol. 2017;313:G330–G341. doi: 10.1152/ajpgi.00161.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cao X., Wu Z., Xiong S., Cao L., Sethi G., Bian J.S. The role of hydrogen sulfide in cyclic nucleotide signaling. Biochem. Pharmacol. 2018;149:20–28. doi: 10.1016/j.bcp.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 127.Sardanelli A.M., Technikova-Dobrova Z., Scacco S.C., Speranza F., Papa S. Characterization of proteins phosphorylated by the cAMP-dependent protein kinase of bovine heart mitochondria. FEBS Lett. 1995;377:470–474. doi: 10.1016/0014-5793(95)01407-1. [DOI] [PubMed] [Google Scholar]
- 128.Bender E., Kadenbach B. The allosteric ATP-inhibition of cytochrome c oxidase activity is reversibly switched on by cAMP-dependent phosphorylation. FEBS Lett. 2000;466:130–134. doi: 10.1016/S0014-5793(99)01773-1. [DOI] [PubMed] [Google Scholar]
- 129.Technikova-Dobrova Z., Sardanelli A.M., Speranza F., Scacco S., Signorile A., Lorusso V., Papa S. Cyclic adenosine monophosphate-dependent phosphorylation of mammalian mitochondrial proteins: Enzyme and substrate characterization and functional role. Biochemistry. 2001;40:13941–13947. doi: 10.1021/bi011066p. [DOI] [PubMed] [Google Scholar]
- 130.Papa S., Scacco S., De Rasmo D., Signorile A., Papa F., Panelli D., Nicastro A., Scaringi R., Santeramo A., Roca E., et al. cAMP-dependent protein kinase regulates post-translational processing and expression of complex I subunits in mammalian cells. Biochim. Biophys. Acta. 2010;1797:649–658. doi: 10.1016/j.bbabio.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 131.De Rasmo D., Signorile A., Santeramo A., Larizza M., Lattanzio P., Capitanio G., Papa S. Intramitochondrial adenylyl cyclase controls the turnover of nuclear-encoded subunits and activity of mammalian complex I of the respiratory chain. Biochim. Biophys. Acta. 2015;1853:183–191. doi: 10.1016/j.bbamcr.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 132.Furuta E., Okuda H., Kobayashi A., Watabe K. Metabolic genes in cancer: Their roles in tumor progression and clinical implications. Biochim. Biophys. Acta. 2010;1805:141–152. doi: 10.1016/j.bbcan.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zaidi N., Swinnen J.V., Smans K. ATP-citrate lyase: A key player in cancer metabolism. Cancer Res. 2012;72:3709–3714. doi: 10.1158/0008-5472.CAN-11-4112. [DOI] [PubMed] [Google Scholar]
- 134.Granchi C. ATP citrate lyase (ACLY) inhibitors: An anti-cancer strategy at the crossroads of glucose and lipid metabolism. Eur. J. Med. Chem. 2018;157:1276–1291. doi: 10.1016/j.ejmech.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 135.Sen U., Sathnur P.B., Kundu S., Givvimani S., Coley D.M., Mishra P.K., Qipshidze N., Tyagi N., Metreveli N., Tyagi S.C. Increased endogenous H2S generation by CBS, CSE, and 3MST gene therapy improves ex vivo renovascular relaxation in hyperhomocysteinemia. Am. J. Physiol. Cell. Physiol. 2012;303:C41–C51. doi: 10.1152/ajpcell.00398.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pan H., Xie X., Chen D., Zhang J., Zhou Y., Yang G. Protective and biogenesis effects of sodium hydrosulfide on brain mitochondria after cardiac arrest and resuscitation. Eur. J. Pharmacol. 2014;741:74–82. doi: 10.1016/j.ejphar.2014.07.037. [DOI] [PubMed] [Google Scholar]
- 137.Veeranki S., Tyagi S.C. Role of hydrogen sulfide in skeletal muscle biology and metabolism. Nitric Oxide. 2015;46:66–71. doi: 10.1016/j.niox.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sun A., Wang Y., Liu J., Yu X., Sun Y., Yang F., Dong S., Wu J., Zhao Y., Xu C., et al. Exogenous H2S modulates mitochondrial fusion-fission to inhibit vascular smooth muscle cell proliferation in a hyperglycemic state. Cell. Biosci. 2016;6:36. doi: 10.1186/s13578-016-0102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhao F.L., Fang F., Qiao P.F., Yan N., Gao D., Yan Y. AP39, a mitochondria-targeted hydrogen sulfide donor, supports cellular bioenergetics and protects against Alzheimer’s disease by preserving mitochondrial function in APP/PS1 mice and neurons. Oxid. Med. Cell. Longev. 2016;2016:8360738. doi: 10.1155/2016/8360738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Miao L., Shen X., Whiteman M., Xin H., Shen Y., Xin X., Moore P.K., Zhu Y.Z. Hydrogen sulfide mitigates myocardial infarction via promotion of mitochondrial biogenesis-dependent M2 polarization of macrophages. Antioxid. Redox. Signal. 2016;25:268–281. doi: 10.1089/ars.2015.6577. [DOI] [PubMed] [Google Scholar]
- 141.Untereiner A.A., Fu M., Módis K., Wang R., Ju Y.J., Wu L. Stimulatory effect of CSE-generated H2S on hepatic mitochondrial biogenesis and the underlying mechanisms. Nitric Oxide. 2016;58:67–76. doi: 10.1016/j.niox.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 142.Liu N., Wu J., Zhang L., Gao Z., Sun Y., Yu M., Zhao Y., Dong S., Lu F., Zhang W. Hydrogen sulphide modulating mitochondrial morphology to promote mitophagy in endothelial cells under high-glucose and high-palmitate. J. Cell. Mol. Med. 2017;21:3190–3203. doi: 10.1111/jcmm.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang S., Chi Q., Hu X., Cong Y., Li S. Hydrogen sulfide-induced oxidative stress leads to excessive mitochondrial fission to activate apoptosis in broiler myocardia. Ecotoxicol. Environ. Saf. 2019;183:109578. doi: 10.1016/j.ecoenv.2019.109578. [DOI] [PubMed] [Google Scholar]
- 144.Guan R., Cai Z., Wang J., Ding M., Li Z., Xu J., Li Y., Li J., Yao H., Liu W., et al. Hydrogen sulfide attenuates mitochondrial dysfunction-induced cellular senescence and apoptosis in alveolar epithelial cells by upregulating sirtuin 1. Aging (Albany NY) 2019;11:11844–11864. doi: 10.18632/aging.102454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sun Y., Lu F., Yu X., Wang B., Chen J., Lu F., Peng S., Sun X., Yu M., Chen H., et al. Exogenous H2S promoted USP8 sulfhydration to regulate mitophagy in the hearts of db/db mice. Aging Dis. 2020;11:269–285. doi: 10.14336/AD.2019.0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rao G., Murphy B., Dey A., Dwivedi S.K.D., Zhang Y., Roy R.V., Chakraborty P., Bhattacharya R., Mukherjee P. Cystathionine beta synthase regulates mitochondrial dynamics and function in endothelial cells. FASEB J. 2020;34:9372–9392. doi: 10.1096/fj.202000173R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Attene-Ramos M.S., Wagner E.D., Plewa M.J., Gaskins H.R. Evidence that hydrogen sulfide is a genotoxic agent. Mol. Cancer Res. 2006;4:9–14. doi: 10.1158/1541-7786.MCR-05-0126. [DOI] [PubMed] [Google Scholar]
- 148.Attene-Ramos M.S., Wagner E.D., Gaskins H.R., Plewa M.J. Hydrogen sulfide induces direct radical-associated DNA damage. Mol. Cancer Res. 2007;5:455–459. doi: 10.1158/1541-7786.MCR-06-0439. [DOI] [PubMed] [Google Scholar]
- 149.Baskar R., Li L., Moore P.K. Hydrogen sulfide-induces DNA damage and changes in apoptotic gene expression in human lung fibroblast cells. FASEB J. 2007;21:247–255. doi: 10.1096/fj.06-6255com. [DOI] [PubMed] [Google Scholar]
- 150.Attene-Ramos M.S., Nava G.M., Muellner M.G., Wagner E.D., Plewa M.J., Gaskins H.R. DNA damage and toxicogenomic analyses of hydrogen sulfide in human intestinal epithelial FHs 74 Int cells. Environ. Mol. Mutagen. 2010;51:304–314. doi: 10.1002/em.20546. [DOI] [PubMed] [Google Scholar]
- 151.Joyner-Matos J., Predmore B.L., Stein J.R., Leeuwenburgh C., Julian D. Hydrogen sulfide induces oxidative damage to RNA and DNA in a sulfide-tolerant marine invertebrate. Physiol. Biochem. Zool. 2010;83:356–365. doi: 10.1086/597529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hoffman M., Rajapakse A., Shen X., Gates K.S. Generation of DNA-damaging reactive oxygen species via the autoxidation of hydrogen sulfide under physiologically relevant conditions: Chemistry relevant to both the genotoxic and cell signaling properties of H2S. Chem. Res. Toxicol. 2012;25:1609–1615. doi: 10.1021/tx300066z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Xiao A.Y., Maynard M.R., Piett C.G., Nagel Z.D., Alexander J.S., Kevil C.G., Berridge M.V., Pattillo C.B., Rosen L.R., Miriyala S., et al. Sodium sulfide selectively induces oxidative stress, DNA damage, and mitochondrial dysfunction and radiosensitizes glioblastoma (GBM) cells. Redox. Biol. 2019;26:101220. doi: 10.1016/j.redox.2019.101220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shackelford R., Ozluk E., Islam M.Z., Hopper B., Meram A., Ghali G., Kevil C.G. Hydrogen sulfide and DNA repair. Redox. Biol. 2020;38:101675. doi: 10.1016/j.redox.2020.101675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gustafsson C.M., Falkenberg M., Larsson N.G. Maintenance and expression of mammalian mitochondrial DNA. Annu. Rev. Biochem. 2016;85:133–160. doi: 10.1146/annurev-biochem-060815-014402. [DOI] [PubMed] [Google Scholar]
- 156.Sharma P., Sampath H. Mitochondrial DNA integrity: Role in health and disease. Cells. 2019;8:100. doi: 10.3390/cells8020100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mukherjee S., Ghosh A. Molecular mechanism of mitochondrial respiratory chain assembly and its relation to mitochondrial diseases. Mitochondrion. 2020;53:1–20. doi: 10.1016/j.mito.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 158.Li S., Yang G. Hydrogen sulfide maintains mitochondrial DNA replication via demethylation of TFAM. Antioxid. Redox Signal. 2015;23:30–42. doi: 10.1089/ars.2014.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Szczesny B., Marcatti M., Ahmad A., Montalbano M., Brunyánszki A., Bibli S.I., Papapetropoulos A., Szabo C. Mitochondrial DNA damage and subsequent activation of Z-DNA binding protein 1 links oxidative stress to inflammation in epithelial cells. Sci. Rep. 2018;8:914. doi: 10.1038/s41598-018-19216-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Luo Y., Ma J., Lu W. The significance of mitochondrial dysfunction in cancer. Int. J. Mol. Sci. 2020;21:5598. doi: 10.3390/ijms21165598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Maybury B.D. Mitochondrial DNA damage is uncommon in cancer but can promote aggressive behaviour. Anticancer Res. 2013;33:3543–3552. [PubMed] [Google Scholar]
- 162.Herst P.M., Grasso C., Berridge M.V. Metabolic reprogramming of mitochondrial respiration in metastatic cancer. Cancer Metastasis Rev. 2018;37:643–653. doi: 10.1007/s10555-018-9769-2. [DOI] [PubMed] [Google Scholar]
- 163.Cao X., Ding L., Xie Z.Z., Yang Y., Whiteman M., Moore P.K., Bian J.S. A review of hydrogen sulfide synthesis, metabolism, and measurement: Is modulation of hydrogen sulfide a novel therapeutic for cancer? Antioxid. Redox. Signal. 2019;31:1–38. doi: 10.1089/ars.2017.7058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lee Z.W., Deng L.W. Role of H2S donors in cancer biology. Handb. Exp. Pharmacol. 2015;230:243–265. doi: 10.1007/978-3-319-18144-8_13. [DOI] [PubMed] [Google Scholar]
- 165.Wu D., Si W., Wang M., Lv S., Ji A., Li Y. Hydrogen sulfide in cancer: Friend or foe? Nitric Oxide. 2015;50:38–45. doi: 10.1016/j.niox.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 166.Wu D., Hu Q., Zhu Y. Therapeutic application of hydrogen sulfide donors: The potential and challenges. Front. Med. 2016;10:18–27. doi: 10.1007/s11684-015-0427-6. [DOI] [PubMed] [Google Scholar]
- 167.Verma R., Akhtar Y., Singh S. A review of patents on therapeutic potential and delivery of hydrogen sulfide. Recent Pat. Drug Deliv Formul. 2017;11:114–123. doi: 10.2174/1872211311666170911160914. [DOI] [PubMed] [Google Scholar]
- 168.Yang C.T., Chen L., Xu S., Day J.J., Li X., Xian M. Recent development of hydrogen sulfide releasing/stimulating reagents and their potential applications in cancer and glycometabolic disorders. Front. Pharmacol. 2017;8:664. doi: 10.3389/fphar.2017.00664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Reis A.K.C.A., Stern A., Monteiro H.P. S-nitrosothiols and H2S donors: Potential chemo-therapeutic agents in cancer. Redox. Biol. 2019;27:101190. doi: 10.1016/j.redox.2019.101190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Li H., Xu F., Gao G., Gao X., Wu B., Zheng C., Wang P., Li Z., Hua H., Li D. Hydrogen sulfide and its donors: Novel antitumor and antimetastatic therapies for triple-negative breast cancer. Redox. Biol. 2020;34:101564. doi: 10.1016/j.redox.2020.101564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liang M., Jin S., Wu D.D., Wang M.J., Zhu Y.C. Hydrogen sulfide improves glucose metabolism and prevents hypertrophy in cardiomyocytes. Nitric Oxide. 2015;46:114–122. doi: 10.1016/j.niox.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 172.Parsanathan R., Jain S.K. Hydrogen sulfide increases glutathione biosynthesis, and glucose uptake and utilisation in C2C12 mouse myotubes. Free Radic. Res. 2018;52:288–303. doi: 10.1080/10715762.2018.1431626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhang H., Huang Y., Chen S., Tang C., Wang G., Du J., Jin H. Hydrogen sulfide regulates insulin secretion and insulin resistance in diabetes mellitus, a new promising target for diabetes mellitus treatment? A review. J. Adv. Res. 2020;27:19–30. doi: 10.1016/j.jare.2020.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Abdollahi Govar A., Törő G., Szaniszlo P., Pavlidou A., Bibli S.I., Thanki K., Resto V.A., Chao C., Hellmich M.R., Szabo C., et al. 3-Mercaptopyruvate sulfurtransferase supports endothelial cell angiogenesis and bioenergetics. Br. J. Pharmacol. 2020;177:866–883. doi: 10.1111/bph.14574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Krebs J., Agellon L.B., Michalak M. Ca2+ homeostasis and endoplasmic reticulum (ER) stress: An integrated view of calcium signaling. Biochem. Biophys. Res. Commun. 2015;460:114–121. doi: 10.1016/j.bbrc.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 176.Wang J., Yang X., Zhang J. Bridges between mitochondrial oxidative stress, ER stress and mTOR signaling in pancreatic beta cells. Cell. Signal. 2016;28:1099–1104. doi: 10.1016/j.cellsig.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 177.van Vliet A.R., Agostinis P. Mitochondria-associated membranes and ER Stress. Curr. Top Microbiol. Immunol. 2018;414:73–102. doi: 10.1007/82_2017_2. [DOI] [PubMed] [Google Scholar]
- 178.Ying R., Wang X.Q., Yang Y., Gu Z.J., Mai J.T., Qiu Q., Chen Y.X., Wang J.F. Hydrogen sulfide suppresses endoplasmic reticulum stress-induced endothelial-to-mesenchymal transition through Src pathway. Life Sci. 2016;144:208–217. doi: 10.1016/j.lfs.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 179.Wang C.Y., Zou W., Liang X.Y., Jiang Z.S., Li X., Wei H.J., Tang Y.Y., Zhang P., Tang X.Q. Hydrogen sulfide prevents homocysteine-induced endoplasmic reticulum stress in PC12 cells by upregulating SIRT-1. Mol. Med. Rep. 2017;16:3587–3593. doi: 10.3892/mmr.2017.7004. [DOI] [PubMed] [Google Scholar]
- 180.Majumder A., Singh M., Behera J., Theilen N.T., George A.K., Tyagi N., Metreveli N., Tyagi S.C. Hydrogen sulfide alleviates hyperhomocysteinemia-mediated skeletal muscle atrophy via mitigation of oxidative and endoplasmic reticulum stress injury. Am. J. Physiol. Cell. Physiol. 2018;315:C609–C622. doi: 10.1152/ajpcell.00147.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Panagaki T., Randi E.B., Szabo C. Role of hydrogen sulfide and 3-mercaptopyruvate sulfurtransferase in the regulation of the endoplasmic reticulum stress response in hepatocytes. Biomolecules. 2020;10:1692. doi: 10.3390/biom10121692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lu A., Chu C., Mulvihill E., Wang R., Liang W. ATP-sensitive K+ channels and mitochondrial permeability transition pore mediate effects of hydrogen sulfide on cytosolic Ca2+ homeostasis and insulin secretion in beta-cells. Pflugers Arch. 2019;471:1551–1564. doi: 10.1007/s00424-019-02325-9. [DOI] [PubMed] [Google Scholar]
- 183.Lei F., Wang W., Fu Y., Wang J., Zheng Y. Mitochondrial KATP channels contribute to the protective effects of hydrogen sulfide against impairment of central chemoreception of rat offspring exposed to maternal cigarette smoke. PLoS ONE. 2020;15:e0237643. doi: 10.1371/journal.pone.0237643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Faris P., Ferulli F., Vismara M., Tanzi M., Negri S., Rumolo A., Lefkimmiatis K., Maestri M., Shekha M., Pedrazzoli P., et al. Hydrogen sulfide-evoked intracellular Ca2+ signals in primary cultures of metastatic colorectal cancer cells. Cancers (Basel) 2020;12:3338. doi: 10.3390/cancers12113338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shibuya N., Koike S., Tanaka M., Ishigami-Yuasa M., Kimura Y., Ogasawara Y., Fukui K., Nagahara N., Kimura H. A novel pathway for the production of hydrogen sulfide from D-cysteine in mammalian cells. Nat. Commun. 2013;4:1366. doi: 10.1038/ncomms2371. [DOI] [PubMed] [Google Scholar]
- 186.Souza L.K., Araújo T.S., Sousa N.A., Sousa F.B., Nogueira K.M., Nicolau L.A., Medeiros J.V. Evidence that d-cysteine protects mice from gastric damage via hydrogen sulfide produced by d-amino acid oxidase. Nitric Oxide. 2017;64:1–6. doi: 10.1016/j.niox.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 187.Pol A., Renkema G.H., Tangerman A., Winkel E.G., Engelke U.F., de Brouwer A.P.M., Lloyd K.C., Araiza R.S., van den Heuvel L., Omran H., et al. Mutations in SELENBP1, encoding a novel human methanethiol oxidase, cause extraoral halitosis. Nat. Genet. 2018;50:120–129. doi: 10.1038/s41588-017-0006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Elhodaky M., Hong L.K., Kadkol S., Diamond A.M. Selenium-binding protein 1 alters energy metabolism in prostate cancer cells. Prostate. 2020;80:962–976. doi: 10.1002/pros.24028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.