Abstract
Lectins are a class of proteins responsible for several biological roles such as cell-cell interactions, signaling pathways, and several innate immune responses against pathogens. Since lectins are able to bind to carbohydrates, they can be a viable target for targeted drug delivery systems. In fact, several lectins were approved by Food and Drug Administration for that purpose. Information about specific carbohydrate recognition by lectin receptors was gathered herein, plus the specific organs where those lectins can be found within the human body.
Keywords: human lectins, carbohydrate specific recognition, biological applications, targeted drug delivery systems, protein expression
1. Introduction
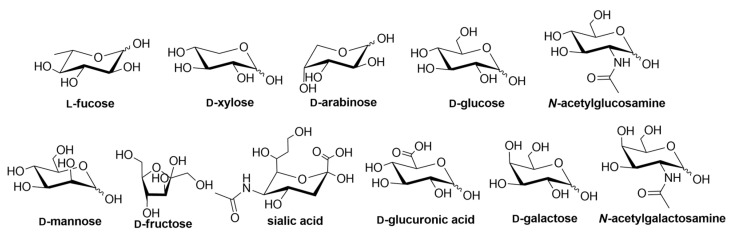
Lectins are an attractive class of proteins of non-immune origin that can either be free or linked to cell surfaces, and are involved in numerous biological processes, such as cell-cell interactions, signaling pathways, cell development, and immune responses [1]. Lectins selectively recognize carbohydrates and reversibly bind to them as long as the ligands are oriented in a specific manner. Some of the commonly occurring carbohydrates that are found in Nature are d-fructose, d-galactose, l-arabinose, d-xylose, d-mannose, d-glucose, d-glucosamine, d-galactosamine, l-fucose, various uronic acids, sialic acid, and their combinations to form other di- and oligosaccharides, or other biomolecules (Figure 1) [2].
Figure 1.
Structures of the carbohydrate building blocks found in Nature.
Lectins in vertebrates can be classified either by their subcellular location, or by their structure. Division based on their location includes integral lectins located in membranes as structural components, or soluble lectins present in intra- and intercellular fluids, which can move freely.
Division according to lectin structure consists of several different types of lectins, such as C-type lectins (binding is Ca2+ dependent), I-type lectins (carbohydrate recognition domain is similar to immunoglobulins), galectin family (or S-type, which are thiol dependent), pentraxins (pentameric lectins) and P-type lectins (specific to glycoproteins containing mannose 6-phosphate) [3].
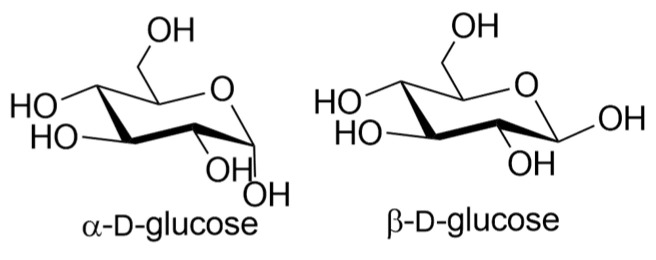
Different lectins have high similarity in the residues that bind to saccharides, most of which coordinate to metal ions, and water molecules. Nearly all animal lectins possess several pockets that recognize molecules other than carbohydrates, meaning that they are multivalent and can present 2 to 12 sites of interaction, allowing the binding of several ligands simultaneously. The specificity and affinity of the lectin-carbohydrate complex depends on the lectin, which can be very sensitive to the structure of the carbohydrate (e.g., mannose versus glucose, Figure 1), or to the orientation of the anomeric substituent (α versus β anomer, e.g., in Figure 2), or both. Lectin-carbohydrate interactions are achieved mainly through hydrogen bonds, van der Waals (steric interactions), and hydrophobic forces (example is given in Figure 3) [3,4].
Figure 2.
Structures of α- and β-d-glucose.
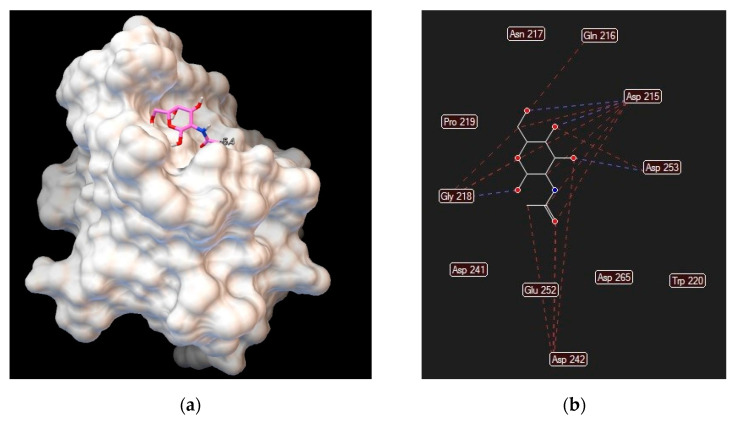
Figure 3.
Asialoglycoprotein receptor (Protein Data Bank entry 1DV8, gene symbol ASGR1) binding interactions with N–acetylgalactosamine: (a) ligand conformation inside the binding site; (b) specific interactions are hydrogen bonds (blue dashed lines) and steric interactions (red dashed lines).
It has been shown that the majority of lectins are conserved through evolution, suggesting that these proteins play a crucial role in the sugar-recognition activities necessary for the living process and development [5,6].
Although lectins are present in animals, plants, lichens, bacteria, and higher fungi [3], this review focuses only on human lectins for targeted drug delivery [7] purposes, their specificity towards carbohydrates and the organs where they are expressed. When referring to gene expression (or RNA expression), one means that those specific organs or cells have that specific gene coded. If active, it produces the respective protein, and one says that the protein is expressed in that organ or cell. In this review, we focus only on protein expression, since that information is the only relevant one for the development of targeted drug delivery systems. More information about carbohydrate-based nanocarriers for targeted drug delivery systems can be found elsewhere [8,9,10]. Since lectins are able to recognize and transport carbohydrates and their derivatives, lectin targeting can be relevant in the research and development of new medicines [7,11,12]. The metabolism of cancer cells, for example, is different from normal cells due to intense glycolytic activity (Warburg effect) [13]. Cancer cells require glutamine and/or glucose for cell growth, and glucose transporter isoforms 1 and 2 (gene symbols GLUT1 and GLUT2, respectively) showed an increase in activity in several tumors (gastrointestinal carcinoma, squamous cell carcinoma of the head and neck, breast carcinoma, renal cell carcinoma, gastric and ovarian cancer) [14,15].
The herein adopted lectin nomenclature is in accordance with the Human Genome Group (HUGO) Gene Nomenclature Committee. However, most common designated aliases (non-standard names) are also included (and appear first). The expression data for all lectin-coding genes was compiled from The Human Protein Atlas [16,17] and GeneCards [18] databases.
2. C-Type Lectins
C-type lectins are involved in the recognition of saccharides in a Ca2+-dependent manner but exhibit low affinities to carbohydrates, requiring multiple valencies of carbohydrate ligands to mediate signaling pathways, such as DC-SIGN2 which gene symbol is CLEC4M (Most genes carry the information to make proteins. The gene name is often used when referring to the corresponding protein). MINCLE (gene symbol CLEC4E), on the other hand, shows high affinity and can detect small numbers of glycolipids on fungal surfaces [19,20]. Most of the lectin-like domains contain some of the conserved residues required to establish the domain fold, but do not present the residues required for carbohydrate recognition [21]. The amino acid residues known to be involved in calcium-dependent sugar-binding are the EPN motif (mannose-binding), the QPD motif (for galactose binding), and the WND motif (for Ca2+binding) [22]. More information about glycan affinity and binding to proteins can be found elsewhere [23]. A comprehensive list of C-type lectins is presented in Table 1, divided by subfamilies that differ in the architecture of the domain [22,24], along with the carbohydrates that they recognize and the human tissues where they are expressed.
Table 1.
C-type superfamily, their carbohydrate ligands and protein expression in human organs.
|
Common Name (HUGO Name if Different) |
Gene Symbol | Carbohydrate Preferential Affinity | Protein Expression in the Organs |
|---|---|---|---|
| Proteoglycans or lecticans | |||
| Aggrecan | ACAN | Hyaluronic acid [25] | Cartilage, soft tissue |
| Brevican | BCAN | Hyaluronic acid [26,27] | Brain |
| Neurocan | NCAN | Hyaluronic acid [28] | Brain |
| Versican | VCAN | Hyaluronic acid [29] | Brain |
| FRAS1 related extracellular matrix 1 | FREM1 | b) | Adrenal gland, appendix, colon, duodenum, epididymis, kidney, lung, pancreas, placenta, rectum, salivary gland, small intestine, stomach, testis, tonsil, thyroid gland |
| Type II transmembrane receptors | |||
| Blood Dendritic Cell Antigen 2 (C-type lectin domain family 4 member C) | CLEC4C | Gal-β-(1-3 or 1-4)-GlcNAc-β-(1-2)-Man trisaccharides [30,31] | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
| DC-SIGN (CD209 molecule) | CD209 | High N-linked d-Mannose- oligosaccharides, and branched l-fucose, both with free OH-3 and OH-4. (N-linked glycans, N-acetyl-d-glucosamine, Lewis a, b, x and y) [32] | Bone marrow, lung |
| DC-SIGN2 | CLEC4M | High N-linked d-Mannose- oligosaccharides, branched l-fucose, N-linked glycans, N-acetyl-d-glucosamine, Lewis a, b and y | Brain, gastrointestinal tract, lung |
| Dectin-2 (C-type lectin domain containing 6A) | CLEC6A | α-(1-2) or α-(1-4) mannans [33] and other high-α-d-mannose carbohydrates [34] | Blood |
| Dendritic cell immunoreceptor (DCIR) (C-type lectin domain family 4 member A) | CLEC4A | Mannose, fucose and weakly interacts with N-acetylglucosamine [35] | Bone marrow, spleen, lung |
| Fc fragment of IgE receptor II | FCER2 | Mannose [36], immunoglobulin E, CD21, galactose [37] | Lymph node, bone marrow, spleen, appendix, tonsil, skin |
| Hepatic Asialoglycoprotein Receptor 1 | ASGR1 | Terminal β-d-galactose and N-acetylgalactosamine units [38] | Stomach, liver, gallbladder |
| Hepatic Asialoglycoprotein Receptor 2 | ASGR2 | Terminal β-d-galactose and N-acetylgalactosamine units [38] | Liver |
| Kupffer Cell receptor (C-type lectin domain family 4 member F) | CLEC4F | Galactose, fucose, and N-acetylgalactosamine [39] | Liver |
| Langerin (CD207 molecule) | CD207 | High-mannose oligosaccharides, mannose, N-acetylglucosamine, fucose. Note that OH-3 and OH-4 should be free for recognition, and preferentially equatorial. N-acetylmannosamine showed less affinity; thereby axial derivatives should be avoided. Sulfated mannosylated glycans, keratan sulfate and β-glucans [40] | Lymph node, tonsil, skin, spleen |
| Liver sinusoidal epithelial cell lectin (LSECtin) (C-type lectin domain family 4 member G) | CLEC4G | Mannose, N-acetylglucosamine and fucose [41] | Lymph node, brain, colon, kidney, liver, testis |
| Macrophage Asialoglycoprotein Receptor | CLEC10A | Terminal galactose and N-acetylgalactosamine residues [42] | Bone marrow, brain, lymph node, oral mucosa, skin, spleen, tonsil |
| Macrophage C-type Lectin (MCL) | CLEC4D | Trehalose 6,6′-dimycolate, α-d-mannans18 (however it was suggested that MCL is not a carbohydrate-binding lectin) [43] | Bone marrow, lung, lymph node, spleen, tonsil |
| MINCLE (C-type lectin domain family 4 member E) | CLEC4E | α-mannose, trehalose-6′6-dimycolate, glucose [19] | a) |
| Collectins | |||
| Collectin-K1 (collectin subfamily member 11) | COLEC11 | High mannose oligosaccharides with at least a mannose-α-(1-2)-mannose residue [44] | a) |
| Collectin-L1 (collectin subfamily member 10) | COLEC10 | Galactose, mannose, fucose, N-acetylglucosamine, N-acetylgalactosamine [45] | a) |
| Mannose-binding lectin 2 | MBL2 | Mannose, fucose, N-acetylglucosamine [46] | Liver |
| Pulmonary surfactant protein 1 (surfactant protein A1) | SFTPA1 | N-acetylmannosamine, l-fucose, mannose, glucose, poorly to galactose. Preferentially oligosaccharides [47] | Lung |
| Pulmonary surfactant protein 2 (surfactant protein A2) | SFTPA2 | N-acetylmannosamine, l-fucose, mannose, glucose, poorly to galactose. Preferentially oligosaccharides [47] | Lung |
| Pulmonary surfactant protein B (surfactant protein B) | SFTPB | b) | Lung |
| Pulmonary surfactant protein C (surfactant protein C) | SFTPC | Lipopolysaccharides [47] | Lung |
| Pulmonary surfactant protein D (surfactant protein D) | SFTPD | Maltose, glucose, mannose, poorly to galactose. Preferentially oligosaccharides [47] | Lung |
| Scavenger receptor with CTLD (SRCL) (collectin subfamily member 12) | COLEC12 | d-galactose, l- and d-fucose, N-acetylgalactosamine (internalizes specifically in nurse-like cells), sialyl Lewis X, or a trisaccharide and asialo-orosomucoid (ASOR). May also play a role in the clearance of amyloid-beta in Alzheimer disease [48] | Brain, lung, placenta |
| Selectins | |||
| Selectin E | SELE | Sialyl Lewis x, a [49] | Bone marrow, colon, nasopharynx |
| Selectin L | SELL | Sialyl Lewis x [50] | Appendix, bone marrow, lymph node, spleen, tonsil |
| Selectin P | SELP | Sialyl Lewis x [49] | Bone marrow, colon |
| Natural Killer (NK) | |||
| C-type lectin domain family 2 member L | CLEC2L | b) | Brain, skeletal muscle |
| C-type lectin domain containing 5A | CLEC5A | Fucose, mannose, N-acetylglucosamine, N-acetylmuramic acid-β(1-4)-N-acetylglucosamine [51] | Blood |
| CD72 molecule | CD72 | b) | Appendix, bone marrow, lymph node, spleen, tonsil |
| Killer cell lectin-like receptor G1 | KLRG1 | Mannose [52] | Appendix, cervix (uterine), colon, duodenum, small intestine, stomach, tonsil |
| Killer cell lectin-like receptor G2 | KLRG2 | b) | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
| CD69 molecule | CD69 | Fucoidan (weak). N-acetylamine was reported but not supported by a second report. Does not bind glucose, galactose, mannose, fucose or N-acetylglucosamine [53] | Appendix, bone marrow, lymph node, spleen, tonsil |
| Killer cell lectin-like receptor F1 | KLRF1 | Predicted to not bind carbohydrates [54] | Blood |
| C-type lectin domain family 2 member B | CLEC2B |
b) Known to bind to KLRF1 |
Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, proximal digestive tract, skin |
| Oxidized low-density lipoprotein receptor 1 | OLR1 | Predicted to not bind to carbohydrates [55] | a) |
| Killer cell lectin-like receptor D1 | KLRD1 | α-(2-3)-linked NeuAc on multi-antennary N-glycan, heparin, sulfate-containing polysaccharides [56] | a) |
| C-type lectin domain family 1 member A | CLEC1A | b) [57] | a) |
| C-type lectin domain family 1 member B | CLEC1B | Predicted to not bind to carbohydrates [58] | a) |
| C-type lectin domain family 12 member B | CLEC12B | b) | a) |
| C-type lectin-like 1 | CLECL1 | Predicted to not bind to carbohydrates [21] | a) |
| C-type lectin domain family 12 member A | CLEC12A | b) | Bone marrow, lung, spleen |
| DNGR (C-type lectin domain containing 9A) | CLEC9A | Specific interactions were not discovered yet, although it is known that this lectin binds to α-actin filaments and β-spectrin [59] | a) |
| C-type lectin domain family 2 member A | CLEC2A | b) | Skin |
| Dectin-1 (C-type lectin domain containing 7A) | CLEC7A | β-(1-3)- and β-(1-6)-d-Glycans (neither mono- or short oligosaccharides/polymers are recognized) [60] | Blood, bone marrow |
| C-type lectin domain family 2 member D | CLEC2D | High molecular weight sulfated glycosaminoglycans [61] | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
| Killer cell lectin-like receptor B1 | KLRB1 | Terminal Gal-α-(1-3)-Gal, N-acetyllactosamine. [62] Sucrose octasulphate [63] | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
| Killer cell lectin-like receptor C1 | KLRC1 | b) | a) |
| Killer cell lectin-like receptor C2 | KLRC2 | b) | a) |
| Killer cell lectin-like receptor C3 | KLRC3 | b) | Colon, duodenum, small intestine, stomach, tonsil |
| Killer cell lectin-like receptor C4 | KLRC4 | b) | a) |
| Killer cell lectin-like receptor K1 | KLRK1 | α-(2-3)-NeuAc-containing N-glycans [64], heparin, heparan sulfate [56] | Appendix, lymph node, spleen, tonsil |
| Macrophage Mannose Receptor (MMR) | |||
| Endo180 (Mannose receptor C type 2) | MRC2 | Mannose, fucose, N-acetylglucosamine [65] | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
| Lymphocyte antigen 75 | LY75 | Predicted to not bind carbohydrates [65] | Appendix, breast, bronchus, cervix (uterine), duodenum, endometrium, fallopian tube, gallbladder, liver, lung, lymph node, nasopharynx, pancreas, placenta, rectum, spleen, stomach, thyroid gland, tonsil, urinary bladder, |
| Mannose receptor C-type 1 c) | MRC1 | Mannose, fucose, glucose, N-acetylglucosamine [66] (C-type) 4-O-sulphated GalNAc (R-type) | Colon, endometrium, kidney, lung, rectum, skin, soft tissue, testis |
| Phospholipase A2 receptor | PLA2R1 | Predicted to not bind carbohydrates [65] but known to bind collagen | Kidney |
| Free C-type Lectin Domains (CTLDs) | |||
| C-type lectin domain containing 19A | CLEC19A | b) | a) |
| Lithostathine-alpha (Regenerating family member 1 alpha) | REG1A | b) | Duodenum, pancreas, small intestine, stomach |
| Lithostathine-beta (Regenerating family member 1 beta) | REG1B | b) | Duodenum, pancreas, small intestine, stomach |
| Regenerating family member 3 alpha | REG3A | Peptidoglycan (binding affinity increases with the length of the carbohydrate moiety) [67] | Appendix, duodenum, skin, small intestine, stomach |
| Regenerating family member 3 gamma | REG3G | Peptidoglycan [67] | a) |
| Regenerating family member 4 | REG4 | Mannans, heparin [67] | Appendix, colon, duodenum, rectum, small intestine |
| Type I receptors | |||
| Chondrolectin | CHODL | b) [68] | Appendix, colon, duodenum, rectum, small intestine, testis |
| Layilin | LAYN | Hyaluronan [69] | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
| Tetranectin | |||
| Cartilage-derived C-type lectin (C-type lectin domain family 3 member A) | CLEC3A | Expected to bind sulfated polysaccharides such as heparin [70] | a) |
| Stem cell growth factor (SCGF) (C-type lectin domain containing 11A) | CLEC11A | b) | Bone marrow, soft tissue |
| Tetranectin (C-type lectin domain family 3 member B) | CLEC3B | Sulfated polysaccharides such as heparin [70] | a) |
| Polycystin | |||
| Polycystin 1 like 3, transient receptor potential channel interacting | PKD1L3 | Predicted to not bind carbohydrates | a) |
| Polycystin 1, transient receptor potential channel interacting | PKD1 | Predicted to bind galactosyl and glucosyl residues. Might bind oligosaccharides with mannosyl moieties [71] | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, pancreas, proximal digestive tract, skin |
| Attractin | |||
| Attractin | ATRN | b) | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, pancreas, proximal digestive tract, skin |
| Attractin-like 1 | ATRNL1 | b) | a) |
| CTLD/acidic neck | |||
| CD302 molecule | CD302 | b) [72] | a) |
| Proteoglycan 2, pro eosinophil major basic protein | PRG2 | Heparin [73] | Bone marrow, placenta |
| Proteoglycan 3, pro eosinophil major basic protein 2 | PRG3 | b) | Bone marrow |
| Endosialin | |||
| CD93 molecule | CD93 | b) | Bone marrow, brain, colon, kidney, lung, spleen |
| C-type lectin domain containing 14A | CLEC14A | b) | Appendix, brain, cervix (uterine), colon, duodenum, esophagus, gallbladder, heart muscle, kidney, lung, pancreas, prostate, rectum, skin, small intestine, stomach, testis |
| Endosialin (CD248 molecule) | CD248 | b) | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, female tissues, gastrointestinal tract, kidney and urinary bladder, muscle tissues, pancreas, skin |
| Thrombomodulin | THBD | b) | Cervix (uterine), colon, esophagus, lymph node, oral mucosa, placenta, skin, tonsil, urinary bladder, vagina |
| Others | |||
| C-type lectin domain family 18 member A | CLEC18A | Fucoidan, β-glucans, β-galactans [74] | a) |
| Prolectin (C-type lectin domain containing 17A) | CLEC17A | Terminal α-d-mannose and fucose residues [75] | Appendix, lymph node, spleen, stomach, tonsil |
| DiGeorge syndrome critical region gene 2 | DGCR2 | b) | Pancreas |
| FRAS1 related extracellular matrix 1 | FREM1 | b) | Adrenal gland, appendix, colon, duodenum, epididymis, kidney, lung, pancreas, placenta, rectum, salivary gland, small intestine, stomach, testis, tonsil, thyroid gland |
3. Chitolectins (or Chilectins)
There are two types of proteins that are able to recognize chitin: chitinases and chitolectins. The first ones are active proteins that bind and hydrolyze oligosaccharides, whereas the latter ones are able to bind oligosaccharides but do not hydrolyze them [76,77] and are presented in Table 2.
Table 2.
Human chitolectins (also called chilectins), their carbohydrate ligands and protein expression in the organs.
|
Common Name (HUGO Name if Different) |
Gene Symbol | Carbohydrate Preferential Affinity | Protein Expression in the Organs |
|---|---|---|---|
| Chitinase 3 like 1 | CHI3L1 | Chitin [78] | a) |
| Chitinase 3 like 2 | CHI3L2 | Chitooligosaccharides ((GlcNAc)5 and (GlcNAc)6 showed the highest affinities) [79] | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, proximal digestive tract |
| Oviductin (Oviductal glycoprotein 1) | OVGP1 | Chitin [80] | Fallopian tube |
| Stabilin-1 interacting chitinase-like protein | SI-CLP | GalNAc, GlcNAc, ribose, mannose. Prefers to bind oligosaccharides with a four-sugar ring core [81] | a) |
4. F-Type Lectins
F-type lectins, also called fucolectins, are characterized by an α-l-fucose recognition domain and display both unique carbohydrate- and calcium-binding sequence motifs [76]. F-type lectins are immune-recognition proteins and are presented in Table 3. Fucose is recognized by specific interactions with O5 (pyranose acetal oxygen), 3-OH and 4-OH [82], the reason why these atoms must be available to form these interactions after the synthesis of fucose derivatives.
Table 3.
Human f-type lectins, their carbohydrate ligands and protein expression in the organs.
|
Common Name (HUGO Name if Different) |
Gene Symbol | Carbohydrate Preferential Affinity | Protein Expression in the Organs |
|---|---|---|---|
| Coagulation factor V a) | F5 | Fucose [83] | b) |
| APC, WNT signalling pathway regulator | APC | c) | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
5. F-Box Lectins
F-box proteins are the substrate-recognition subunits of the SCF (Skp1-Cul1-F-box protein) complex. They have an F-box domain that binds to S-phase kinase-associated protein 1 (Skp1) [84]. The F-box proteins were divided into three different classes: Fbws are those that contains WD-40 domains, Fbls containing leucine-rich repeats, and Fbxs that have either different protein-protein interaction modules or no recognizable motifs [85]. Although F-box proteins are a superfamily of proteins, only five are known to recognize N-linked glycoproteins [84] as presented in Table 4.
Table 4.
Human F-box lectins, their carbohydrate ligands and protein expression in the organs.
|
Common Name (HUGO Name if Different) |
Gene Symbol | Carbohydrate Preferential Affinity | Protein Expression in the Organs |
|---|---|---|---|
| Cyclin F | CCNF | a) | Appendix, bone marrow, lung, lymph node, skin, spleen, tonsil |
| F-box protein 2 | FBXO2 | N-acetylglucosamine disaccharide chitobiose [86] | Breast, ovary, pancreas |
| F-box protein 3 | FBXO3 | a) | b) |
| F-box protein 4 | FBXO4 | a) | b) |
| F-box protein 5 | FBXO5 | a) | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
| F-box protein 6 | FBXO6 | High-mannose glycoproteins [87] | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
| F-box protein 7 | FBXO7 | a) | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
| F-box protein 8 | FBXO8 | a) | Bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, pancreas, proximal digestive tract, skin |
| F-box protein 9 | FBXO9 | a) | b) |
| F-box protein 10 | FBXO10 | a) | Cervix (uterine), colon, duodenum, endometrium, fallopian tube, lung, prostate, rectum, seminal vesicle, small intestine, testis |
| F-box protein 11 | FBXO11 | a) | b) |
| F-box protein 15 | FBXO15 | a) | b) |
| F-box protein 16 | FBXO16 | a) | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
| F-box protein 17 | FBXO17 | Sulfated and galactose-terminated glycoproteins [88] | b) |
| F-box protein, helicase, 18 | FBXO18 | a) | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
| LIM domain 7 | LMO7 | a) | b) |
| F-box protein 21 | FBXO21 | a) | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, proximal digestive tract, skin |
| F-box protein 22 | FBXO22 | a) | b) |
| Tetraspanin 17 | TSPAN17 | a) | b) |
| F-box protein 24 | FBXO24 | a) | b) |
| F-box protein 25 | FBXO25 | a) | b) |
| F-box protein 27 | FBXO27 | a) | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, proximal digestive tract, skin |
| F-box protein 28 | FBXO28 | a) | b) |
| F-box protein 30 | FBXO30 | a) | b) |
| F-box protein 31 | FBXO31 | a) | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, proximal digestive tract, skin |
| F-box protein 32 | FBXO32 | a) | b) |
| F-box protein 33 | FBXO33 | a) | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
| F-box protein 34 | FBXO34 | a) | Adrenal gland, bronchus, colon, epididymis, endometrium, gallbladder, placenta, seminal vesicle, skeletal muscle, skin, stomach, testis, thyroid gland |
| F-box protein 36 | FBXO36 | a) | b) |
| F-box protein 38 | FBXO38 | a) | b) |
| F-box protein 39 | FBXO39 | a) | b) |
| F-box protein 40 | FBXO40 | a) | b) |
| F-box protein 41 | FBXO41 | a) | b) |
| F-box protein 42 | FBXO42 | a) | Bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, pancreas |
| F-box protein 43 | FBXO43 | a) | b) |
| F-box protein 44 | FBXO44 | a) | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
| F-box protein 45 | FBXO45 | a) | b) |
| F-box protein 46 | FBXO46 | a) | b) |
| F-box protein 47 | FBXO47 | a) | b) |
| F-box protein 48 | FBXO48 | a) | Esophagus, kidney, oral mucosa, parathyroid gland, skin, stomach |
6. Ficolins
Ficolins play an important role in innate immunity by recognizing and binding to carbohydrates present on the surface of Gram-positive and Gram-negative bacteria [89]. There are three human ficolins and they are presented in Table 5.
Table 5.
Human ficolins, their carbohydrate ligands and protein expression in the organs.
|
Common Name (HUGO Name if Different) |
Gene Symbol | Carbohydrate Preferential Affinity | Protein Expression in the Organs |
|---|---|---|---|
| Ficolin 1 | FCN1 | GlcNAc, GalNAc; sialic acid [89] | a) |
| Ficolin 2 | FCN2 | GlcNAc (acetyl group); β-(1-3)-d-glucan [89] | a) |
| Ficolin 3 | FCN3 | N-acetylglucose; N-acetylgalactose, fucose, lipopolysaccharides [89] | a) |
7. I-Type Lectins
I-type lectins are a subset of the immunoglobulin superfamily that specifically recognizes sialic acids and other carbohydrate ligands. Most of the members of this group of lectins are siglecs, which are type I transmembrane proteins, and can be divided into two groups: the CD33-related group that includes CD33 (siglec3) siglecs5–11, and siglec14 while the other group includes siglec1, CD22 (siglec2), MAG (siglec4) and Siglec15 [90,91]. CD33-related groups possess between 1 and 4 C-set domains and feature cytoplasmic tyrosine-based motifs involved in signaling and endocytosis. Siglec1 possesses 16 C-set domains, CD22 has 6 C-set domains and MAG presents 4 C-set domains. MAG is the only siglec not found on cells of the immune system. Members of this I-type superfamily are presented in Table 6 along with their carbohydrate ligands and protein expression. An example of a drug delivery system was developed by Spence, Greene and co-workers who developed polymeric nanoparticles of poly(lactic-co-glycolic acid) decorated with sialic acid [92,93].
Table 6.
Human I-type lectins, their carbohydrate ligands and protein expression in the organs.
|
Common Name (HUGO Name if Different) |
Gene Symbol | Carbohydrate Preferential Affinity | Protein Expression in the Organs |
|---|---|---|---|
| Siglecl1 (Sialic acid binding Ig like lectin 1) | SIGLEC1 | α-(2-3)-Sialic acid, α-(2-6)-Sialic acid, α-(2-8)-Sialic acid [94] | Bone marrow, lung |
| Siglec2 (CD22 molecule) a) | CD22 | α-(2-6)-Sialic acid [95,96] | Appendix, lymph node, spleen, tonsil |
| Siglec3 (CD33 molecule) | CD33 | α-(2-6)-Sialic acid, α-(2-3)-Sialic acid [97] | Appendix, bone marrow, lung, lymph node, skin, spleen, tonsil |
| Siglec4a, MAG (Myelin associated glycoprotein) | MAG | α-(2-3)-Sialic acid [98] | Brain |
| Siglec5 (Sialic acid binding Ig like lectin 5) | SIGLEC5 | α-(2-3)-Sialic acid, α-(2-6)-Sialic acid, α-(2-8)-Sialic acid [99] | Bone marrow, lymph node, placenta, spleen, tonsil |
| Siglec6 (Sialic acid binding Ig like lectin 6) | SIGLEC6 | Sialic acid-α-(2-6)-N-acetylgalactosamine (Sialyl-Tn) [100] | Placenta |
| Siglec7 | SIGLEC7 | α-(2-6)-Sialic acid, α-(2-8)-Sialic acid, α-(2-3)-Sialic acid [101] and disialogangliosides [102,103,104] | b) |
| Siglec8 | SIGLEC8 | α-(2-3)-Sialic acid, α-(2-6)-Sialic acid [105] | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
| Siglec9 (Sialic acid binding Ig like lectin 9) | SIGLEC9 | α-(2-3)-Sialic acid, Sialyl Lewis x, α-(2-6)-Sialic acid, α-(2-8)-Sialic acid [106] | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
| Siglec10 (Sialic acid binding Ig like lectin 10) | SIGLEC10 | α-(2-3)-Sialic acid, α-(2-6)-Sialic acid [107] | Appendix, bone marrow, lymph node, soft tissue, spleen, tonsil |
| Siglec11 (Sialic acid binding Ig like lectin 11) | SIGLEC11 | α-(2-8)-Sialic acid [101] | b) |
| Siglec14 (Sialic acid binding Ig like lectin 14) | SIGLEC14 | Sialic acid- α-(2-6)-N-acetylgalactosamine (Sialyl-Tn), N-acetylneuraminic acid [108] | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
| Siglec15 (Sialic acid binding Ig like lectin 15) | SIGLEC15 | Sialyl-Tn [109] | b) |
| CD2 molecule a) | CD2 | N-glycans with fucose [110] | Appendix, lymph node, spleen, tonsil |
| CD83 molecule | CD83 | Sialic acid [111] | Appendix, bone marrow, lung, lymph node, spleen, tonsil |
| Intercellular adhesion molecule 1 | ICAM1 | Hyaluronan [112] | Appendix, bone marrow, brain, endometrium, fallopian tube, kidney, lung, lymph node, spleen, testis, tonsil |
| L1 cell adhesion molecule | L1CAM | α-(2-3)-Sialic acid [113] | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, proximal digestive tract, skin |
| Myelin protein zero | MPZ | SO4– –3GlucA-β-(1-3)-Gal-β-(1–4)-GlcNAc (HNK-1 antigen) [101] | Bronchus, esophagus, fallopian tube, small intestine, soft tissue, stomach, testis |
| Neural cell adhesion molecule 1 | NCAM1 | High N-linked d-mannose [114] | Brain, colon, hearth muscle, pancreas, smooth muscle, soft tissue, thyroid gland |
| Neural cell adhesion molecule 2 | NCAM2 | c) | Brain, bronchus, colon, duodenum, gallbladder, ovary, rectum, small intestine, soft tissue, testis |
8. L-Type Lectins
L-type lectins are distinguished from other lectins on the basis of tertiary structure, not the primary sequence, and are composed of antiparallel β-sheets connected by short loops and β-bends, usually lacking any α-helices [115]. Members of this family of lectins present different glycan-binding specificities as presented in Table 7. L-type superfamily includes Pentraxins [116,117] that require Ca2+ ions for ligand binding. Both LMAN1 and LMAN2 also require Ca2+ ions for their binding activity [115].
Table 7.
Human L-type lectins, their carbohydrate ligands and protein expression in the organs.
|
Common Name (HUGO Name if Different) |
Gene Symbol | Carbohydrate Preferential Affinity | Protein Expression in the Organs |
|---|---|---|---|
| Calnexin | CANX | Non-reducing glucose residues in an oligosaccharide (Glc(Man)9(GlcNAc)2) [118] | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
| Calreticulin | CALR | Non-reducing glucose residues in an oligosaccharide (Glc(Man)9(GlcNAc)2) [119] | Bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, pancreas, skin |
| Calreticulin 3 | CALR3 | a) | Testis |
| Lectin, mannose-binding 1 | LMAN1 | α-(1-2) mannans with free OH-3, OH-4 and OH-6 [120] | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
| Lectin, mannose-binding 1 like | LMAN1L | a) | b) |
| Lectin, mannose-binding 2 | LMAN2 | High α-(1-2) mannans, Low affinity for d-glucose and N-acetylglucosamine [121] | Bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, pancreas |
| Lectin, mannose-binding 2 like | LMAN2L | α-(1-2) trimannose [122] | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
| Adhesion G protein-coupled receptor D1 | ADGRD1 | a) | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
| Adhesion G protein-coupled receptor D2 | ADGRD2 | a) | b) |
| Amyloid P component, serum | APCS | Heparin, dextran sulfate proteoglycans [123] | b) |
| C-reactive protein | CRP | Galactose 6-phosphate, Gal-β-(1-3)-GalNAc, Gal-β-(1-4)-GalNAc, Gal-β-(1-4)-Gal-β-(1-4)-GlcNAc, other phosphate-containing ligands [124,125] | Liver, gallbladder, soft tissue |
| Neuronal pentraxin 1 | NPTX1 | a) | Brain, testis |
| Neuronal pentraxin 2 | NPTX2 | a) | Adrenal gland, brain, pancreas, pituitary gland, testis |
| Neuronal pentraxin receptor | NPTXR | a) | Brain |
| Pentraxin 3 | PTX3 | Heparin [126] | b) |
| Sushi, von Willebrand factor type A, EGF and pentraxin domain containing 1 | SVEP1 | a) | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas |
9. M-Type Lectins
M-type family of lectins consists of α-mannosidases, which are proteins involved in both the maturation and the degradation of Asn-linked oligosaccharides [127]. Members of this family, their binding affinities and protein expression are presented in Table 8.
Table 8.
Human M-type lectins, their carbohydrate ligands and protein expression in the organs.
|
Common Name (HUGO Name if Different) |
Gene Symbol | Carbohydrate Preferential Affinity | Protein Expression in the Organs |
|---|---|---|---|
| Mannosidase alpha class 1A member 1 | MAN1A1 | α-(1-2)-mannans [128,129] | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
| Mannosidase alpha class 1A member 2 | MAN1A2 | α-(1-2)-mannans [128,129] | Bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
| Mannosidase alpha class 1B member 1 | MAN1B1 | α-(1-2)-mannans [128,129] | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
| Mannosidase alpha class 1C member 1 | MAN1C1 | α-(1-2)-mannans [128,129] | Bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas |
10. P-Type Lectins
P-type lectins constitute a two-member family of mannose-6-phosphate receptors (Table 9) that play an essential role in the generation of functional lysosomes. The phosphate group is key to high-affinity ligand recognition by these proteins. Furthermore, optimal ligand-binding ability of M6PR is achieved in the presence of divalent cations, particularly Mn2+ cation [130,131].
Table 9.
Human P-type lectins, their carbohydrate ligands and protein expression in the organs.
|
Common Name (HUGO Name if Different) |
Gene Symbol | Carbohydrate Preferential Affinity | Protein Expression in the Organs |
|---|---|---|---|
| Mannose-6-phosphate receptor, cation dependent a) | M6PR | Mannose-6-phosphate residues [132,133] | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
| Insulin-like growth factor 2 receptor | IGF2R | Mannose-6-phosphate residues (either α or β). Mannose-6-phosphate analogues with carboxylate or malonate groups [134] | b) |
11. R-Type Lectins
R-type lectins are protein-UDP acetylgalactosaminyltransferases that contain an R-type carbohydrate recognition domain, which is conserved between animal and bacterial lectins [135]. Members of this superfamily recognize Gal/GalNAc residues and are expressed in several tissues as presented in Table 10.
Table 10.
Human R-type lectins, their carbohydrate ligands and protein expression in the organs.
|
Common Name (HUGO Name if Different) |
Gene Symbol | Carbohydrate Preferential Affinity | Protein Expression in the Organs |
|---|---|---|---|
| Polypeptide N-acetylgalactosaminyltransferase 1 | GALNT1 | GalNAc [136] | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
| Polypeptide N-acetylgalactosaminyltransferase 2 | GALNT2 | GalNAc [136,137] | Bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
| Polypeptide N-acetylgalactosaminyltransferase 3 | GALNT3 | GalNAc [136] | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
| Polypeptide N-acetylgalactosaminyltransferase 4 | GALNT4 | GalNAc, GalNAc-glycosylated substrates [136,138] | a) |
| Polypeptide N-acetylgalactosaminyltransferase 5 | GALNT5 | GalNAc [136] | Appendix, bronchus, cervix (uterine), colon, duodenum, esophagus, gallbladder, lung, oral mucosa, rectum, salivary gland, small intestine, stomach, tonsil, vagina |
| Polypeptide N-acetylgalactosaminyltransferase 6 | GALNT6 | GalNAc [136] | Bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
| Polypeptide N-acetylgalactosaminyltransferase 7 | GALNT7 | GalNAc, GalNAc-glycosylated substrates [100] | Bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract |
| Polypeptide N-acetylgalactosaminyltransferase 8 b) | GALNT8 | GalNAc [139] | Bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, skin |
| Polypeptide N-acetylgalactosaminyltransferase 9 | GALNT9 | GalNAc [140] | a) |
| Polypeptide N-acetylgalactosaminyltransferase 10 | GALNT10 | GalNAc [141] | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
| Polypeptide N-acetylgalactosaminyltransferase 11 | GALNT11 | GalNAc [142] | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
| Polypeptide N-acetylgalactosaminyltransferase 12 | GALNT12 | GalNAc [143] | Appendix, bone marrow, brain, breast, cervix (uterine), endometrium, fallopian tube, prostate, soft tissue, thyroid gland, tonsil, skin |
| Polypeptide N-acetylgalactosaminyltransferase 13 | GALNT13 | GalNAc [144] | Adrenal gland, lung, salivary gland |
| Polypeptide N-acetylgalactosaminyltransferase 14 | GALNT14 | GalNAc [145] | a) |
| Polypeptide N-acetylgalactosaminyltransferase 15 | GALNT15 | GalNAc [146] | a) |
| Polypeptide N-acetylgalactosaminyltransferase 16 | GALNT16 | GalNAc [147] | Bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
| Polypeptide N-acetylgalactosaminyltransferase 17 | GALNT17 | GalNAc [148] | Brain |
| Polypeptide N-acetylgalactosaminyltransferase 18 | GALNT18 | GalNAc [149] | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
| Polypeptide N-acetylgalactosaminyltransferase like 5 | GALNTL5 | c) [150] | Testis |
12. S-Type Lectins
S-type lectins are known nowadays as galectins and are a superfamily of proteins that show a high affinity for β-galactoside sugars (Table 11). Formerly called S-type lectins because of their sulfhydryl dependency, galectins are the most widely expressed class of lectins in all organisms. Human galectins have been classified into three major groups according to their structure: prototypical, chimeric and tandem-repeat [151,152,153].
Table 11.
Human S-type lectins, their carbohydrate ligands and protein epression in the organs.
|
Common Name (HUGO Name if Different) |
Gene Symbol | Carbohydrate Preferential Affinity | Protein Expression in the Organs |
|---|---|---|---|
| Galectin 1 | |||
| Galectin 1 | LGALS1 | β-d-galactosides, poly-N-acetyllactosamine-enriched glycoconjugates [157,158] | Bone marrow, brain, cervix (uterine), endometrium, lymph node, ovary, parathyroid gland, placenta, smooth muscle, skin, spleen, testis, tonsil, vagina |
| Galectin 2 | LGALS2 | β-d-galactosides, lactose [159] | Appendix, colon, duodenum, gallbladder, kidney, liver, lymph node, pancreas, rectum, small intestine, spleen, tonsil |
| Galectin 3 | |||
| Galectin 3 | LGALS3 | β-d-galactosides, LacNAc [160] | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
| Galectin 3 binding protein | LGALS3BP | β-d-galactosides, lactose [161] | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, proximal digestive tract, skin |
| Galectin 4 | LGALS4 | β-d-galactosides, lactose [162] | Appendix, colon, duodenum, gallbladder, pancreas, rectum, small intestine, stomach |
| Galectin 7 | LGALS7 | Gal, GalNAc, Lac, LacNAc [163] | Cervix (uterine), esophagus, oral mucosa, salivary gland, skin, tonsil, vagina |
| Galectin 8 | LGALS8 | β-d-galactosides. Preferentially binds to 3′-O-sialylated and 3′-O-sulfated glycans [164] | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
| Galectin 9 | LGALS9 | β-d-galactosides. Forssman pentasaccharide, lactose, N-acetyllactosamine [165] | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, skin |
| Galectin 9B | LGALS9B | β-d-galactosides [166] | Appendix, bone marrow, breast, lymph node, spleen, tonsil |
| Galectin 9C | LGALS9C | β-d-galactosides [166] | Appendix, bronchus, colon, duodenum, gallbladder, lung, pancreas, spleen, stomach, tonsil |
| Galectin 10 (Charcot-Leyden crystal galectin, CLC) | LGALS10 | Binds weakly to lactose, N-acetyl-d-glucosamine and d-mannose [167] | Lymph node, spleen, tonsil |
| Galectin 12 | LGALS12 | β-d-galactose and lactose [168,169] | a) |
| Galectin 13 | LGALS13 | N-acetyl-lactosamine, mannose and N-acetyl-galactosamine [170]. Contrary to other galectins, Galectin 13 does not bind β-d-galactosides [171] | Kidney, placenta, spleen, urinary bladder |
| Placental Protein 13 (Galectin 14) | LGALS14 | N-acetyl-lactosamine [172] | Adrenal gland, colon, kidney |
| Galectin 16 | LGALS16 | N-acetyl-lactosamine, β-d-galactose and lactose [172] | Placenta |
Galectins play important roles in immune responses and promoting inflammation. They are also known for having a crucial role in cancer-causing tumor invasion, progression, metastasis and angiogenesis [154,155,156].
13. X-Type Lectins
Intelectins (Table 12) were classified as X-type lectins because they do not have a typical lectin domain, instead, they contain a fibrinogen-like domain and a unique intelectin-specific region [173].
Table 12.
Human X-type lectins, their carbohydrate ligands and protein expression in the organs.
|
Common Name (HUGO Name if Different) |
Gene Symbol | Carbohydrate Preferential Affinity | Protein Expression in the Organs |
|---|---|---|---|
| Intelectin 1 | ITLN1 | Terminal acyclic 1,2-diol-containing structures, including β-d-galactofuranose, d-phosphoglycerol-modified glycans, d-glycero-d-talo-oct-2-ulosonic acid, 3-deoxy-d-manno-oct-2-ulosonic acid [174] | Appendix, colon, duodenum, rectum, small intestine |
| Intelectin 2 | ITLN2 | a) | Appendix, colon, duodenum, rectum, small intestine |
a) Carbohydrate moieties recognized by this protein have not been discovered yet.
14. Orphans
Orphan lectins are those that do not belong to known lectin structural families [175]. Proteins that bind to sulfated glycosaminoglycans are usually not considered as lectins [101], however, the specific binding of these proteins to sulfated glycosaminoglycans can provide a valuable tool to develop targeted drug delivery systems. Glycosaminoglycan binding interactions with proteins were described in detail by Vallet, Clerc and Ricard-Blum [176] which information is outside of the scope of this review.
Acknowledgments
The authors acknowledge Christopher D. Maycock for having reviewed this manuscript.
Author Contributions
Conceptualization, C.D.R.; methodology, C.D.R. and A.B.C.; resources, M.T.B.; writing—original draft preparation, C.D.R.; writing—review and editing, A.B.C. and M.T.B.; visualization, C.D.R.; supervision, M.T.B.; funding acquisition, M.T.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Fundação para a Ciência e a Tecnologia, grant number PD/BD/109680/2015. This work was also supported by the Associate Laboratory for Green Chemistry, LAQV, which is financed by national funds from FCT/MEC (UID/QUI/50006/2013 and UID/QUI/50006/2019) and co-financed by the ERDF under the PT2020 Partnership Agreement (POCI-01-0145-FEDER-007265).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lepenies B., Lang R. Lectins and Their Ligands in Shaping Immune Responses. Front. Immunol. 2019;10:2379. doi: 10.3389/fimmu.2019.02379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stick R. Carbohydrates: The Sweet Molecules of Life. 1st ed. Academic Press; New York, NY, USA: 2001. [Google Scholar]
- 3.Santos A.F.S., Da Silva M.D.C., Napoleão T.H., Paiva P.M.G., Correia M.T.S., Coelho L.C.B.B. Lectins: Function, structure, biological properties and potential applications. Curr. Top. Pept. Protein Res. 2014;15:41–62. [Google Scholar]
- 4.Wang B., Boons G.-J. Carbohydrate Recognition: Biological Problems, Methods and Applications. 1st ed. John Wiley & Sons, Inc.; Danvers, MA, USA: 2011. [Google Scholar]
- 5.Hirabayashi J., Kasai K.I. Evolution of Animal Lectins. In: Jeanteur P., Kuchino Y., Muller W.E.G., Paine P.L., editors. Molecular Evolution: Evidence for Monophyly of Metazoa. Volume 19. Springer; Berlin, Germany: 1998. [Google Scholar]
- 6.Drickamer K. Evolution of Ca2+-dependent Animal Lectins. Prog. Nucleic Acid Res. Mol. Biol. 1993;45:207–232. [PubMed] [Google Scholar]
- 7.Himri I., Guaadaoui A. Cell and organ drug targeting: Types of drug delivery systems and advanced targeting strategies. In: Grumezescu A., editor. Nanostructures for the Engineering of Cells, Tissues and Organs. Elsevier Inc.; Norwich, UK: 2018. pp. 1–66. [Google Scholar]
- 8.Liu K., Jiang X., Hunziker P. Carbohydrate-based amphiphilic nano delivery systems for cancer therapy. Nanoscale. 2016;8:16091–16156. doi: 10.1039/C6NR04489A. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X., Huang G., Huang H. The glyconanoparticle as carrier for drug delivery. Drug Deliv. 2018;25:1840–1845. doi: 10.1080/10717544.2018.1519001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mosaiab T., Farr D.C., Kiefel M.J., Houston T.A. Carbohydrate-based nanocarriers and their application to target macrophages and deliver antimicrobial agents. Adv. Drug Deliv. Rev. 2019;151–152:94–129. doi: 10.1016/j.addr.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Hossain F., Andreana P.R. Developments in Carbohydrate-Based Cancer Therapeutics. Pharmaceuticals. 2019;12:1–18. doi: 10.3390/ph12020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keshavarz-fathi M., Rezaei N. Vaccines, Adjuvants, and Delivery Systems. In: Keshavarz-Fathi M., Rezaei N., editors. Vaccines for Cancer Immunotherapy. Academic Press; Cambridge, MA, USA: 2019. pp. 45–59. [Google Scholar]
- 13.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 14.Chiaradonna F., Moresco R.M., Airoldi C., Gaglio D., Palorini R., Nicotra F., Messa C., Alberghina L. From cancer metabolism to new biomarkers and drug targets. Biotechnol. Adv. 2012;30:30–51. doi: 10.1016/j.biotechadv.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Wesener D.A., Wangkanont K., McBride R., Song X., Kraft M.B., Hodges H.L., Zarling L.C., Splain R.A., Smith D.F., Cummings R.D., et al. Recognition of Microbial Glycans by Human Intelectin. Nat. Struct. Mol. Biol. 2015;22:603–610. doi: 10.1038/nsmb.3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knut & Alice Wallenberg Foundation The Human Protein Atlas. [(accessed on 5 September 2020)]; Available online: https://www.proteinatlas.org/
- 17.Uhlén M., Fagerberg L., Hallström B.M., Lindskog C., Oksvold P., Mardinoglu A., Sivertsson Å., Kampf C., Sjöstedt E., Asplund A., et al. Tissue-based map of the human proteome. Science. 2015;347:394–403. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 18.GeneCards. Weizmann Institute of Science; Rehovot, Israel: 2015. version: 3.12.404. [Google Scholar]
- 19.Furukawa A., Kamishikiryo J., Mori D., Toyonaga K., Okabe Y., Toji A., Kanda R., Miyake Y., Ose T., Yamasaki S., et al. Structural analysis for glycolipid recognition by the C-type lectins Mincle and MCL. Proc. Natl. Acad. Sci. USA. 2013;110:17438–17443. doi: 10.1073/pnas.1312649110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feinberg H., Park-snyder S., Kolatkar A.R., Heise C.T., Taylor M.E., Weis W.I. Structure of a C-type Carbohydrate Recognition Domain from the Macrophage Mannose Receptor. J. Biol. Chem. 2000;275:21539–21548. doi: 10.1074/jbc.M002366200. [DOI] [PubMed] [Google Scholar]
- 21.Ryan E.J., Marshall A.J., Magaletti D., Floyd H., Draves K.E., Olson N.E., Clark E.A. Dendritic Cell-Associated Lectin-1: A Novel Dendritic Cell-Associated, C-Type Lectin-Like Molecule Enhances T Cell Secretion of IL-4. J. Immunol. 2002;169:5638–5648. doi: 10.4049/jimmunol.169.10.5638. [DOI] [PubMed] [Google Scholar]
- 22.Cummings R.D., McEver R.P. C-Type Lectins. In: Varki A., Cummings R.D., Esko J.D., Freeze H.H., Stanley P., Bertozzi C.R., Gerald H.W., Etzler M.E., editors. Essentials of Glycobiology. Cold Spring Harbor Laboratory Press; New York, NY, USA: 2017. [PubMed] [Google Scholar]
- 23.Cummings R.D., Esko J.D. Essentials of Glycobiology. Cold Spring Harbor Laboratory Press; New York, NY, USA: 2009. Principles of Glycan Recognition. [PubMed] [Google Scholar]
- 24.Imperial College Human CTLD Database. [(accessed on 24 December 2020)]; Available online: https://www.imperial.ac.uk/research/animallectins/ctld/mammals/humandataupdated.html.
- 25.Olin A.I., Mörgelin M., Sasaki T., Timpl R., Heinegård D., Aspberg A. The proteoglycans aggrecan and versican form networks with fibulin-2 through their lectin domain binding. J. Biol. Chem. 2001;276:1253–1261. doi: 10.1074/jbc.M006783200. [DOI] [PubMed] [Google Scholar]
- 26.Jaworski D.M., Kelly G.M., Hockfield S. BEHAB, a New Member of the Proteoglycan Tandem Repeat Family of Hyaluronan-binding Proteins That Is Restricted to the Brain. J. Cell Biol. 1994;125:495–509. doi: 10.1083/jcb.125.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamaguchi Y. Brevican: A major proteoglycan in adult brain. Perspect. Dev. Neurobiol. 1996;3:307–317. [PubMed] [Google Scholar]
- 28.Rauch U., Gao P., Janetzko A., Flaccus A., Hilgenberg L., Tekotte H., Margolis R.K., Margolis R.U. Isolation and characterization of developmentally regulated chondroitin sulfate and chondroitin/keratin sulfate proteoglycans of brain identified with monoclonal antibodies. J. Biol. Chem. 1991;266:14785–14801. doi: 10.1016/S0021-9258(18)98755-7. [DOI] [PubMed] [Google Scholar]
- 29.LeBaron R.G., Zimmermann D.R., Ruoslahti E. Hyaluronate binding properties of versican. J. Biol. Chem. 1992;267:10003–10010. doi: 10.1016/S0021-9258(19)50191-0. [DOI] [PubMed] [Google Scholar]
- 30.Riboldi E., Daniele R., Parola C., Inforzato A., Arnold P.L., Bosisio D., Fremont D.H., Bastone A., Colonna M., Sozzani S. Human C-type lectin domain family 4, member C (CLEC4C/BDCA-2/CD303) is a receptor for asialo-galactosyl-oligosaccharides. J. Biol. Chem. 2011;286:35329–35333. doi: 10.1074/jbc.C111.290494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jégouzo S.A.F., Feinberg H., Dungarwalla T., Drickamer K., Weis W.I., Taylor M.E. A novel mechanism for binding of galactose-terminated glycans by the C-type carbohydrate recognition domain in blood dendritic cell antigen 2. J. Biol. Chem. 2015;290:16759–16771. doi: 10.1074/jbc.M115.660613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geurtsen J., Driessen N.N., Appelmelk B.J. Microbial Glycobiology. Elsevier Inc.; Amsterdam, The Netherlands: 2010. Mannose–fucose recognition by DC-SIGN; pp. 673–695. [Google Scholar]
- 33.Feinberg H., Jégouzo S.A.F., Rex M.J., Drickamer K., Weis W.I., Taylor M.E. Mechanism of pathogen recognition by human dectin-2. J. Biol. Chem. 2017;292:13402–13414. doi: 10.1074/jbc.M117.799080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lord A.K., Vyas J.M. Clinical Immunology. Elsevier Ltd.; Amsterdam, The Netherlands: 2019. Host Defenses to Fungal Pathogens; pp. 413–424.e1. [Google Scholar]
- 35.Nagae M., Ikeda A., Hanashima S., Kojima T., Matsumoto N., Yamamoto K., Yamaguchi Y. Crystal structure of human dendritic cell inhibitory receptor C-type lectin domain reveals the binding mode with N-glycan. FEBS Lett. 2016;590:1280–1288. doi: 10.1002/1873-3468.12162. [DOI] [PubMed] [Google Scholar]
- 36.Sun P.D. Human CD23: Is It a Lectin in Disguise? Structure. 2006;14:950–951. doi: 10.1016/j.str.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 37.Kijimoto-Ochiai S., Toshimitsu U. CD23 molecule acts as a galactose-binding lectin in the cell aggregation of EBV-transformed human B-cell lines. Glycobiology. 1995;5:443–448. doi: 10.1093/glycob/5.4.443. [DOI] [PubMed] [Google Scholar]
- 38.Meier M., Bider M.D., Malashkevich V.N., Spiess M., Burkhard P. Crystal structure of the carbohydrate recognition domain of the H1 subunit of the asialoglycoprotein receptor. J. Mol. Biol. 2000;300:857–865. doi: 10.1006/jmbi.2000.3853. [DOI] [PubMed] [Google Scholar]
- 39.Yang C.Y., Chen J.B., Tsai T.F., Tsai Y.C., Tsai C.Y., Liang P.H., Hsu T.L., Wu C.Y., Netea M.G., Wong C.H., et al. CLEC4F Is an Inducible C-Type Lectin in F4/80-Positive Cells and Is Involved in Alpha-Galactosylceramide Presentation in Liver. PLoS ONE. 2013;8:e65070. doi: 10.1371/journal.pone.0065070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stambach N.S., Taylor M.E. Characterization of carbohydrate recognition by langerin, a C-type lectin of Langerhans cell. Glycobiology. 2003;13:401–410. doi: 10.1093/glycob/cwg045. [DOI] [PubMed] [Google Scholar]
- 41.Liu W., Tang L., Zhang G., Wei H., Cui Y., Guo L., Gou Z., Chen X., Jiang D., Zhu Y., et al. Characterization of a novel C-type lectin-like gene, LSECtin: Demonstration of carbohydrate binding and expression in sinusoidal endothelial cells of liver and lymph node. J. Biol. Chem. 2004;279:18748–18758. doi: 10.1074/jbc.M311227200. [DOI] [PubMed] [Google Scholar]
- 42.Nollau P., Wolters-Eisfeld G., Mortezai N., Kurze A.K., Klampe B., Debus A., Bockhorn M., Niendorf A., Wagener C. Protein Domain Histochemistry (PDH): Binding of the Carbohydrate Recognition Domain (CRD) of Recombinant Human Glycoreceptor CLEC10A (CD301) to Formalin-Fixed, Paraffin-Embedded Breast Cancer Tissues. J. Histochem. Cytochem. 2013;61:199–205. doi: 10.1369/0022155412474823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richardson M.B., Williams S.J. MCL and Mincle: C-type lectin receptors that sense damaged self and pathogen-associated molecular patterns. Front. Immunol. 2014;5:1–9. doi: 10.3389/fimmu.2014.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Venkatraman Girija U., Furze C.M., Gingras A.R., Yoshizaki T., Ohtani K., Marshall J.E., Wallis A.K., Schwaeble W.J., El-Mezgueldi M., Mitchell D.A., et al. Molecular basis of sugar recognition by collectin-K1 and the effects of mutations associated with 3MC syndrome. BMC Biol. 2015;13:1–14. doi: 10.1186/s12915-015-0136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohtani K., Suzuki Y., Eda S., Kawai T., Kase T., Yamazaki H., Shimada T., Keshi H., Sakai Y., Fukuoh A., et al. Molecular cloning of a novel human collectin from liver (CL-L1) J. Biol. Chem. 1999;274:13681–13689. doi: 10.1074/jbc.274.19.13681. [DOI] [PubMed] [Google Scholar]
- 46.Muto S., Sakuma K., Taniguchi A., Matsumoto K. Human mannose-binding lectin preferentially binds to human colon adenocarcinoma cell lines expressing high amount of Lewis A and Lewis B antigens. Biol. Pharm. Bull. 1999;22:347–352. doi: 10.1248/bpb.22.347. [DOI] [PubMed] [Google Scholar]
- 47.Wright J.R. Immunoregulatory functions of surfactant proteins. Nat. Rev. Immunol. 2005;5:58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- 48.Coombs P.J., Graham S.A., Drickamert K., Taylor M.E. Selective binding of the scavenger receptor C-type lectin to Lewis x trisaccharide and related glycan ligands. J. Biol. Chem. 2005;280:22993–22999. doi: 10.1074/jbc.M504197200. [DOI] [PubMed] [Google Scholar]
- 49.Erbe D.V., Watson S.R., Presta L.G., Wolitzky B.A., Foxall C., Brandley B.K., Lasky L.A. P- and E-selectin use common sites for carbohydrate ligand recognition and cell adhesion. J. Cell Biol. 1993;120:1227–1236. doi: 10.1083/jcb.120.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ivetic A., Green H.L.H., Hart S.J. L-selectin: A major regulator of leukocyte adhesion, migration and signaling. Front. Immunol. 2019;10:1068. doi: 10.3389/fimmu.2019.01068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sung P.S., Hsieh S.L. CLEC2 and CLEC5A: Pathogenic Host Factors in Acute Viral Infections. Front. Immunol. 2019;10:2867. doi: 10.3389/fimmu.2019.02867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Binsack R., Pecht I. The mast cell function-associated antigen exhibits saccharide binding capacity. Eur. J. Immunol. 1997;27:2557–2561. doi: 10.1002/eji.1830271014. [DOI] [PubMed] [Google Scholar]
- 53.Wong S., Arsequell G. In: Immunobiology of Carbohydrates. Wong S., Arsequell G., editors. Springer; New York, NY, USA: 2003. [Google Scholar]
- 54.Roda-Navarro P., Arce I., Renedo M., Montgomery K., Kucherlapati R., Fernández-Ruiz E. Human KLRF1, a novel member of the killer cell lectin-like receptor gene family: Molecular characterization, genomic structure, physical mapping to the NK gene complex and expression analysis. Eur. J. Immunol. 2000;30:568–576. doi: 10.1002/1521-4141(200002)30:2<568::AID-IMMU568>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 55.Ohki I., Ishigaki T., Oyama T., Matsunaga S., Xie Q., Ohnishi-Kameyama M., Murata T., Tsuchiya D., Machida S., Morikawa K., et al. Crystal structure of human lectin-like, oxidized low-density lipoprotein receptor 1 ligand binding domain and its ligand recognition mode to OxLDL. Structure. 2005;13:905–917. doi: 10.1016/j.str.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 56.Higai K., Imaizumi Y., Suzuki C., Azuma Y., Matsumoto K. NKG2D and CD94 bind to heparin and sulfate-containing polysaccharides. Biochem. Biophys. Res. Commun. 2009;386:709–714. doi: 10.1016/j.bbrc.2009.06.101. [DOI] [PubMed] [Google Scholar]
- 57.Chiffoleau E. C-type lectin-like receptors as emerging orchestrators of sterile inflammation represent potential therapeutic targets. Front. Immunol. 2018;9:227. doi: 10.3389/fimmu.2018.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watson A.A., Brown J., Harlos K., Eble J.A., Walter T.S., O’Callaghan C.A. The crystal structure and mutational binding analysis of the extracellular domain of the platelet-activating receptor CLEC-2. J. Biol. Chem. 2007;282:3165–3172. doi: 10.1074/jbc.M610383200. [DOI] [PubMed] [Google Scholar]
- 59.Zhang J.G., Czabotar P.E., Policheni A.N., Caminschi I., San Wan S., Kitsoulis S., Tullett K.M., Robin A.Y., Brammananth R., van Delft M.F., et al. The Dendritic Cell Receptor Clec9A Binds Damaged Cells via Exposed Actin Filaments. Immunity. 2012;36:646–657. doi: 10.1016/j.immuni.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 60.Schorey J., Lawrence C. The Pattern Recognition Receptor Dectin-1: From Fungi to Mycobacteria. Curr. Drug Targets. 2008;9:123–129. doi: 10.2174/138945008783502430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gange C.T., Quinn J.M.W., Zhou H., Kartsogiannis V., Gillespie M.T., Ng K.W. Characterization of sugar binding by osteoclast inhibitory lectin. J. Biol. Chem. 2004;279:29043–29049. doi: 10.1074/jbc.M312518200. [DOI] [PubMed] [Google Scholar]
- 62.Christiansen D., Mouhtouris E., Milland J., Zingoni A., Santoni A., Sandrin M.S. Recognition of a carbohydrate xenoepitope by human NKRP1A (CD161) Xenotransplantation. 2006;13:440–446. doi: 10.1111/j.1399-3089.2006.00332.x. [DOI] [PubMed] [Google Scholar]
- 63.Kogelberg H., Frenkiel T.A., Birdsall B., Chai W., Muskett F.W. Binding of Sucrose Octasulphate to the C-Type Lectin-Like Domain of the Recombinant Natural Killer Cell Receptor NKR-P1A Observed by NMR Spectroscopy. ChemBioChem. 2002;3:1072–1077. doi: 10.1002/1439-7633(20021104)3:11<1072::AID-CBIC1072>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 64.Imaizumi Y., Higai K., Suzuki C., Azuma Y., Matsumoto K. NKG2D and CD94 bind to multimeric α2,3-linked N-acetylneuraminic acid. Biochem. Biophys. Res. Commun. 2009;382:604–608. doi: 10.1016/j.bbrc.2009.03.081. [DOI] [PubMed] [Google Scholar]
- 65.East L., Rushton S., Taylor M.E., Isacke C.M. Characterization of sugar binding by the mannose receptor family member, Endo180. J. Biol. Chem. 2002;277:50469–50475. doi: 10.1074/jbc.M208985200. [DOI] [PubMed] [Google Scholar]
- 66.Taylor M.E., Bezouska K., Drickamer K. Contribution to ligand binding by multiple carbohydrate-recognition domains in the macrophage mannose receptor. J. Biol. Chem. 1992;267:1719–1726. doi: 10.1016/S0021-9258(18)46005-X. [DOI] [PubMed] [Google Scholar]
- 67.Chen Z., Downing S., Tzanakakis E.S. Four Decades After the Discovery of Regenerating Islet-Derived (Reg) Proteins: Current Understanding and Challenges. Front. Cell Dev. Biol. 2019;7:1–16. doi: 10.3389/fcell.2019.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weng L., Smits P., Wauters J., Merregaert J. Molecular cloning and characterization of human chondrolectin, a novel type I transmembrane protein homologous to C-type lectins. Genomics. 2002;80:62–70. doi: 10.1006/geno.2002.6806. [DOI] [PubMed] [Google Scholar]
- 69.Bono P., Rubin K., Higgins J.M.G., Hynes R.O. Layilin, a novel integral membrane protein, is a hyaluronan receptor. Mol. Biol. Cell. 2001;12:891–900. doi: 10.1091/mbc.12.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Neame P.J., Tapp H., Grimm D.R. The cartilage-derived, C-type lectin (CLECSF1): Structure of the gene and chromosomal location. Biochim. Biophys. Acta Gene Struct. Expr. 1999;1446:193–202. doi: 10.1016/S0167-4781(99)00087-1. [DOI] [PubMed] [Google Scholar]
- 71.Pletnev V., Huether R., Habegger L., Habegger L., Schultz W., Duax W. Rational proteomics of PKD1. I. Modeling the three dimensional structure and ligand specificity of the C_lectin binding domain of Polycystin-1. J. Mol. Model. 2007;13:891–896. doi: 10.1007/s00894-007-0201-z. [DOI] [PubMed] [Google Scholar]
- 72.Lo T.-H., Silveira P.A., Fromm P.D., Verma N.D., Vu P.A., Kupresanin F., Adam R., Kato M., Cogger V.C., Clark G.J., et al. Characterization of the Expression and Function of the C-Type Lectin Receptor CD302 in Mice and Humans Reveals a Role in Dendritic Cell Migration. J. Immunol. 2016;197:885–898. doi: 10.4049/jimmunol.1600259. [DOI] [PubMed] [Google Scholar]
- 73.Swaminathan G.J., Myszka D.G., Katsamba P.S., Ohnuki L.E., Gleich G.J., Acharya K.R. Eosinophil-granule major basic protein, a C-type lectin, binds heparin. Biochemistry. 2005;44:14152–14158. doi: 10.1021/bi051112b. [DOI] [PubMed] [Google Scholar]
- 74.Huang Y.L., Pai F.S., Tsou Y.T., Mon H.C., Hsu T.L., Wu C.Y., Chou T.Y., Yang W.B., Chen C.H., Wong C.H., et al. Human CLEC18 gene cluster contains C-type lectins with differential glycan-binding specificity. J. Biol. Chem. 2015;290:21252–21263. doi: 10.1074/jbc.M115.649814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Graham S.A., Jégouzo S.A.F., Yan S., Powlesland A.S., Brady J.P., Taylor M.E., Drickamer K. Prolectin, a glycan-binding receptor on dividing b cells in germinal centers. J. Biol. Chem. 2009;284:18537–18544. doi: 10.1074/jbc.M109.012807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dalal P., Aronson N.N., Jr., Madura J.D. Family 18 Chitolectins: Comparison of MGP40 and HUMGP39. Bichem. Biophys. Res. Commun. 2007;359:221–226. doi: 10.1016/j.bbrc.2007.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kilpatrick D.C. Animal lectins: A historical introduction and overview. Biochim. Biophys. Acta. 2002;1572:187–197. doi: 10.1016/S0304-4165(02)00308-2. [DOI] [PubMed] [Google Scholar]
- 78.Renkema G.H., Boot R.G., Au F.L., Donker-Koopman W.E., Strijland A., Muijsers A.O., Hrebicek M., Aerts J.M.F.G. Chitotriosidase a chitinase, and the 39-kDa human cartilage glycoprotein, a chitin-binding lectin, are homologues of family 18 glycosyl hydrolases secreted by human macrophages. Eur. J. Biochem. 1998;251:504–509. doi: 10.1046/j.1432-1327.1998.2510504.x. [DOI] [PubMed] [Google Scholar]
- 79.Boot R.G., Blommaart E.F.C., Swart E., Ghauharali-van der Vlugt K., Bijl N., Moe C., Place A., Aerts J.M.F.G. Identification of a Novel Acidic Mammalian Chitinase Distinct from Chitotriosidase. J. Biol. Chem. 2001;276:6770–6778. doi: 10.1074/jbc.M009886200. [DOI] [PubMed] [Google Scholar]
- 80.Fusetti F., Pijning T., Kalk K.H., Bos E., Dijkstra B.W. Crystal structure and carbohydrate-binding properties of the human cartilage glycoprotein-39. J. Biol. Chem. 2003;278:37753–37760. doi: 10.1074/jbc.M303137200. [DOI] [PubMed] [Google Scholar]
- 81.Schimpl M., Rush C.L., Betou M., Eggleston I.M., Recklies A.D., Van Aalten D.M.F. Human YKL-39 is a pseudo-chitinase with retained chitooligosaccharide-binding properties. Biochem. J. 2012;446:149–157. doi: 10.1042/BJ20120377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Malette B., Paquette Y., Merlen Y., Bleau G. Oviductins possess chitinase- and mucin-like domains: A lead in the search for the biological function of these oviduct-specific ZP-associating glycoproteins. Mol. Reprod. Dev. 1995;41:384–397. doi: 10.1002/mrd.1080410315. [DOI] [PubMed] [Google Scholar]
- 83.Aronson N.N., Kuranda M.J. Lysosomal degradation of Asn-linked glycoproteins. FASEB J. 1989;3:2615–2622. doi: 10.1096/fasebj.3.14.2531691. [DOI] [PubMed] [Google Scholar]
- 84.Meng G., Zhao Y., Bai X., Liu Y., Green T.J., Luo M., Zheng X. Structure of human Stabilin-1 Interacting Chitinase-Like Protein (SI-CLP) reveals a saccharide-binding cleft with lower sugar-binding selectivity. J. Biol. Chem. 2010;285:39898–39904. doi: 10.1074/jbc.M110.130781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bianchet M.A., Odom E.W., Vasta G.R., Amzel L.M. A novel fucose recognition fold involved in innate immunity. Nat. Struct. Biol. 2002;9:628–634. doi: 10.1038/nsb817. [DOI] [PubMed] [Google Scholar]
- 86.Vasta G.R., Mario Amzel L., Bianchet M.A., Cammarata M., Feng C., Saito K. F-Type Lectins: A highly diversified family of fucose-binding proteins with a unique sequence motif and structural fold, involved in self/non-self-recognition. Front. Immunol. 2017;8:1648. doi: 10.3389/fimmu.2017.01648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yoshida Y. F-box proteins that contain sugar-binding domains. Biosci. Biotechnol. Biochem. 2007;71:2623–2631. doi: 10.1271/bbb.70074. [DOI] [PubMed] [Google Scholar]
- 88.Cenciarelli C., Chiaur D.S., Guardavaccaro D., Parks W., Vidal M., Pagano M. Identification of a family of human F-box proteins. Curr. Biol. 1999;9:1177–1179. doi: 10.1016/S0960-9822(00)80020-2. [DOI] [PubMed] [Google Scholar]
- 89.Mizushima T., Hirao T., Yoshida Y., Lee S.J., Chiba T., Iwai K., Yamaguchi Y., Kato K., Tsukihara T., Tanaka K. Structural basis of sugar-recognizing ubiquitin ligase. Nat. Struct. Mol. Biol. 2004;11:365–370. doi: 10.1038/nsmb732. [DOI] [PubMed] [Google Scholar]
- 90.Yoshida Y. A novel role for N-glycans in the ERAD system. J. Biochem. 2003;134:183–190. doi: 10.1093/jb/mvg128. [DOI] [PubMed] [Google Scholar]
- 91.Glenn K.A., Nelson R.F., Wen H.M., Mallinger A.J., Paulson H.L. Diversity in tissue expression, substrate binding, and SCF complex formation for a lectin family of ubiquitin ligases. J. Biol. Chem. 2008;283:12717–12729. doi: 10.1074/jbc.M709508200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matsushita M. Ficolins: Complement-activating lectins involved in innate immunity. J. Innate Immun. 2009;2:24–32. doi: 10.1159/000228160. [DOI] [PubMed] [Google Scholar]
- 93.Chen L., Li J., Yang G. A comparative review of intelectins. Scand. J. Immunol. 2020;92:e12882. doi: 10.1111/sji.12882. [DOI] [PubMed] [Google Scholar]
- 94.Crocker P.R., Redelinghuys P. Siglecs as positive and negative regulators of the immune system. Biochem. Soc. Trans. 2008;36:1467–1471. doi: 10.1042/BST0361467. [DOI] [PubMed] [Google Scholar]
- 95.Varki A., Angata T. Siglecs—The major subfamily of I-type lectins. Glycobiology. 2006;16:1–27. doi: 10.1093/glycob/cwj008. [DOI] [PubMed] [Google Scholar]
- 96.Spence S., Greene M.K., Fay F., Hams E., Saunders S.P., Hamid U., Fitzgerald M., Beck J., Bains B.K., Smyth P., et al. Targeting Siglecs with a sialic acid-decorated nanoparticle abrogates inflammation. Sci. Transl. Med. 2015;7:1–13. doi: 10.1126/scitranslmed.aab3459. [DOI] [PubMed] [Google Scholar]
- 97.Crocker P.R., Kelm S., Dubois C., Martin B., McWilliam A.S., Shotton D.M., Paulson J.C., Gordon S. Purification and properties of sialoadhesin, a sialic acid-binding receptor of murine tissue macrophages. EMBO J. 1991;10:1661–1669. doi: 10.1002/j.1460-2075.1991.tb07689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Powell L.D., Varki A. The oligosaccharide binding specificities of CD22β, a sialic acid- specific lectin of B cells. J. Biol. Chem. 1994;269:10628–10636. doi: 10.1016/S0021-9258(17)34106-6. [DOI] [PubMed] [Google Scholar]
- 99.Kelm S., Pelz A., Schauer R., Filbin M.T., Tang S., de Bellard M.E., Schnaar R.L., Mahoney J.A., Hartnell A., Bradfield P., et al. Sialoadhesin, myelin-associated glycoprotein and CD22 define a new family of sialic acid-dependent adhesion molecules of the immunoglobulin superfamily. Curr. Biol. 1994;4:965–972. doi: 10.1016/s0960-9822(00)00220-7. [DOI] [PubMed] [Google Scholar]
- 100.Freeman S.D., Kelm S., Barber E.K., Crocker P.R. Characterization of CD33 as a new member of the sialoadhesin family of cellular interaction molecules. Blood. 1995;85:2005–2012. doi: 10.1182/blood.V85.8.2005.bloodjournal8582005. [DOI] [PubMed] [Google Scholar]
- 101.Collins B.E., Yang L.J.S., Mukhopadhyay G., Filbin M.T., Kiso M., Hasegawa A., Schnaar R.L. Sialic acid specificity of myelin-associated glycoprotein binding. J. Biol. Chem. 1997;272:1248–1255. doi: 10.1074/jbc.272.2.1248. [DOI] [PubMed] [Google Scholar]
- 102.Cornish A.L., Freeman S., Forbes G., Ni J., Zhang M., Cepeda M., Gentz R., Augustus M., Carter K.C., Crocker P.R. Characterization of siglec-5, a novel glycoprotein expressed on myeloid cells related to CD33. Blood. 1998;92:2123–2132. doi: 10.1182/blood.V92.6.2123. [DOI] [PubMed] [Google Scholar]
- 103.Patel N., Der Linden E.C.M.B., Altmann S.W., Gish K., Balasubramanian S., Timans J.C., Peterson D., Bell M.P., Bazan J.F., Varki A., et al. OB-BP1/Siglec-6 A Leptin and Sialic Acid-Binding Protein of The Immunoglobulin Superfamily. J. Biol. 1999;274:22729–22738. doi: 10.1074/jbc.274.32.22729. [DOI] [PubMed] [Google Scholar]
- 104.Angata T, Brinkman-Van der Linden E. I-type lectins. Biochim. Biophys. Acta. 2002;1572:294–316. doi: 10.1016/S0304-4165(02)00316-1. [DOI] [PubMed] [Google Scholar]
- 105.Nicoll G., Ni J., Liu D., Klenermani P., Munday J., Dubock S., Mattei M.G., Crocker P.R., Floyd H., Ni J., et al. Identification and characterization of a novel Siglec, Siglec-7, expressed by human natural killer cells and monocytes. Siglec-8: A novel eosinophil-specific member of the immunoglobulin superfamily. Chemtracts. 2000;13:689–694. [Google Scholar]
- 106.Ito A., Handa K., Withers D.A., Satoh M., Hakomori S. itiroh Binding specificity of siglec7 to disialogangliosides of renal cell carcinoma: Possible role of disialogangliosides in tumor progression. FEBS Lett. 2001;504:82–86. doi: 10.1016/S0014-5793(01)02734-X. [DOI] [PubMed] [Google Scholar]
- 107.Falco M., Biassoni R., Bottino C., Vitale M., Sivori S., Augugliaro R., Moretta L., Moretta A. Identification and molecular cloning of p75/AIRM1, a novel member of the sialoadhesin family that functions as an inhibitory receptor in human natural killer cells. J. Exp. Med. 1999;190:793–801. doi: 10.1084/jem.190.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Floyd H., Ni J., Cornish A.L., Zeng Z., Liu D., Carter K.C., Steel J., Crocker P.R. Siglec-8 A novel Eosinophil-Specific member of The Immunoglobulin Superfamily. J. Biol. Chem. 2000;275:861–866. doi: 10.1074/jbc.275.2.861. [DOI] [PubMed] [Google Scholar]
- 109.Zhang J.Q., Nicoll G., Jones C., Crocker P.R. Siglec-9, a novel sialic acid binding member of the immunoglobulin superfamily expressed broadly on human blood leukocytes. J. Biol. Chem. 2000;275:22121–22126. doi: 10.1074/jbc.M002788200. [DOI] [PubMed] [Google Scholar]
- 110.Munday J., Kerr S., Ni J., Cornish A.L., Zhang J.Q., Nicoll G., Floyd H., Mattei M.G., Moore P., Liu D., et al. Identification, characterization and leucocyte expression of Siglec-10, a novel human sialic acid-binding receptor. Biochem. J. 2001;355:489–497. doi: 10.1042/bj3550489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Angata T., Hayakawa T., Yamanaka M., Varki A., Nakamura M. Discovery of Siglec-14, a novel sialic acid receptor undergoing concerted evolution with Siglec-5 in primates. FASEB J. 2006;20:1964–1973. doi: 10.1096/fj.06-5800com. [DOI] [PubMed] [Google Scholar]
- 112.Angata T., Tabuchi Y., Nakamura K., Nakamura M. Siglec-15: An immune system Siglec conserved throughout vertebrate evolution. Glycobiology. 2007;17:838–846. doi: 10.1093/glycob/cwm049. [DOI] [PubMed] [Google Scholar]
- 113.Warren H.S., Altin J.G., Waldron J.C., Kinnear B.F., Parish C.R. A carbohydrate structure associated with CD15 (Lewisx) on myeloid cells is a novel ligand for human CD2. J. Immunol. 1996;156:2866–2873. [PubMed] [Google Scholar]
- 114.Scholler N., Hayden-Ledbetter M., Hellström K.-E., Hellström I., Ledbetter J.A. CD83 Is a Sialic Acid-Binding Ig-Like Lectin (Siglec) Adhesion Receptor that Binds Monocytes and a Subset of Activated CD8 + T Cells. J. Immunol. 2001;166:3865–3872. doi: 10.4049/jimmunol.166.6.3865. [DOI] [PubMed] [Google Scholar]
- 115.McCourt P.A.G., Ek B., Forsberg N., Gustafson S. Intercellular adhesion molecule-1 is a cell surface receptor for hyaluronan. J. Biol. Chem. 1994;269:30081–30084. doi: 10.1016/S0021-9258(18)43775-1. [DOI] [PubMed] [Google Scholar]
- 116.Kleene R., Yang H., Kutsche M., Schachner M. The Neural Recognition Molecule L1 is a Sialic Acid-binding Lectin for CD24, Which Induces Promotion and Inhibition of Neurite Outgrowth. J. Biol. Chem. 2001;276:21656–21663. doi: 10.1074/jbc.M101790200. [DOI] [PubMed] [Google Scholar]
- 117.Horstkorte R., Schachner M., Magyar J.P., Vorherr T., Schmitz B. The fourth immunoglobulin-like domain of NCAM contains a carbohydrate recognition domain for oligomannosidic glycans implicated in association with L1 and neurite outgrowth. J. Cell Biol. 1993;121:1409–1422. doi: 10.1083/jcb.121.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Etzler M.E., Surolia A., Cummings R.D. Essentials of Glycobiology. Harbor Laboratory Press; New York, NY, USA: 2009. L-Type Lectins. [PubMed] [Google Scholar]
- 119.Bottazzi B., Garlanda C., Teixeira M.M. The Role of Pentraxins: From Inflammation, Tissue Repair and Immunity to Biomarkers. Frontiers Media SA; Lausanne, Switzerland: 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Clos T.W. Du Pentraxins: Structure, Function, and Role in Inflammation. ISRN Inflamm. 2013;2013:1–22. doi: 10.1155/2013/379040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ware F.E., Vassilakos A., Peterson P.A., Jackson M.R., Lehrman M.A., Williams D.B. The molecular chaperone calnexin binds Glc1Man9GlcNAc2 oligosaccharide as an initial step in recognizing unfolded glycoproteins. J. Biol. Chem. 1995;270:4697–4704. doi: 10.1074/jbc.270.9.4697. [DOI] [PubMed] [Google Scholar]
- 122.Spiro R.G., Zhu Q., Bhoyroo V., Söling H.D. Definition of the lectin-like properties of the molecular chaperone, calreticulin, and demonstration of its copurification with endomannosidase from rat liver Golgi. J. Biol. Chem. 1996;271:11588–11594. doi: 10.1074/jbc.271.19.11588. [DOI] [PubMed] [Google Scholar]
- 123.Zheng C., Page R.C., Das V., Nix J.C., Wigren E., Misra S., Zhang B. Structural characterization of carbohydrate binding by LMAN1 protein provides new insight into the endoplasmic reticulum export of factors V (FV) and VIII (FVIII) J. Biol. Chem. 2013;288:20499–20509. doi: 10.1074/jbc.M113.461434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kamiya Y., Yamaguchi Y., Takahashi M., Arata Y., Kasai K.I., Ihara Y., Matsuo I., Ito Y., Yamamoto K., Kato K. Sugar-binding properties of VIP36, an intracellular animal lectin operating as a cargo receptor. J. Biol. Chem. 2005;280:37178–37182. doi: 10.1074/jbc.M505757200. [DOI] [PubMed] [Google Scholar]
- 125.Kamiya Y., Kamiya D., Yamamoto K., Nyfeler B., Hauri H.P., Kato K. Molecular basis of sugar recognition by the human L-type lectins ERGIC-53, VIPL, and VIP36. J. Biol. Chem. 2008;283:1857–1861. doi: 10.1074/jbc.M709384200. [DOI] [PubMed] [Google Scholar]
- 126.Zahedi K. Characterization of the binding of serum amyloid P to Laminin. J. Biol. Chem. 1997;272:2143–2148. [PubMed] [Google Scholar]
- 127.Köttgen E., Hell B., Kage A., Tauber R., Kottgen E. Lectin specificity and binding characteristics of human C-reactive protein. J. Immunol. 1992;149:445–453. [PubMed] [Google Scholar]
- 128.Lee R.T., Lee Y.C. Carbohydrate ligands of human C-reactive protein: Binding of neoglycoproteins containing galactose-6-phosphate and galactose-terminated disaccharide. Glycoconj. J. 2006;23:317–327. doi: 10.1007/s10719-006-6173-x. [DOI] [PubMed] [Google Scholar]
- 129.Deban L., Jarva H., Lehtinen M.J., Bottazzi B., Bastone A., Doni A., Jokiranta T.S., Mantovani A., Meri S. Binding of the Long Pentraxin PTX3 to Factor H: Interacting Domains and Function in the Regulation of Complement Activation. J. Immunol. 2008;181:8433–8440. doi: 10.4049/jimmunol.181.12.8433. [DOI] [PubMed] [Google Scholar]
- 130.Gonzalez D.S., Jordan I.K. The alpha-Mannosidases: Phylogeny and Adaptive Diversification. Mol. Biol. Evol. 2000;17:292–300. doi: 10.1093/oxfordjournals.molbev.a026309. [DOI] [PubMed] [Google Scholar]
- 131.Vallet S.D., Clerc O., Ricard-Blum S. Glycosaminoglycan–Protein Interactions: The First Draft of the Glycosaminoglycan Interactome. J. Histochem. Cytochem. 2020:0022155420946403. doi: 10.1369/0022155420946403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bischoff J., Kornfeld R. The soluble form of rat liver α-mannosidase is immunologically related to the endoplasmic reticulum membrane α-mannosidase. J. Biol. Chem. 1986;261:4758–4765. doi: 10.1016/S0021-9258(17)38566-6. [DOI] [PubMed] [Google Scholar]
- 133.Tremblay L.O., Dyke N.C., Herscovics A. Molecular cloning, chromosomal mapping and tissue-specific expression of a novel human α1,2-mannosidase gene involved in N-glycan maturation. Glycobiology. 1998;8:585–595. doi: 10.1093/glycob/8.6.585. [DOI] [PubMed] [Google Scholar]
- 134.Dahms N.M., Hancock M.K. P-type lectins. Biochim. Biophys. Acta Gen. Subj. 2002;1572:317–340. doi: 10.1016/S0304-4165(02)00317-3. [DOI] [PubMed] [Google Scholar]
- 135.Tong P.Y., Gregory W., Kornfeld S. Ligand interactions of the cation-independent mannose 6-phosphate receptor. The stoichiometry of mannose 6-phosphate binding. J. Biol. Chem. 1989;264:7962–7969. doi: 10.1016/S0021-9258(18)83136-2. [DOI] [PubMed] [Google Scholar]
- 136.Tong P.Y., Kornfeld S. Ligand interactions of the cation-dependent mannose 6-phosphate receptor. Comparison with the cation-independent mannose 6-phosphate receptor. J. Biol. Chem. 1989;264:7970–7975. doi: 10.1016/S0021-9258(18)83137-4. [DOI] [PubMed] [Google Scholar]
- 137.Gary-Bobo M., Nirde P., Jeanjean A., Morere A., Garcia M. Mannose 6-Phosphate Receptor Targeting and its Applications in Human Diseases. Curr. Med. Chem. 2007;14:2945–2953. doi: 10.2174/092986707782794005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cummings R.D., Schnaar R.L. R-Type Lectins. In: Varki A., Cummings R.D., Esko J.D., Stanley P., Hart G.W., Aebi M., Darvill A.G., Kinoshita T., Packer N.H., Prestegard J.H., et al., editors. Essentials of Glycobiology. Cold Spring Harbor; New York, NY, USA: 2015. pp. 401–412. [PubMed] [Google Scholar]
- 139.Clausen H., Bennett E.P. A family of UDP-GalNAc: Polypeptide N-acetylgalactosaminyl-transferases control the initiation of mucin-type O-linked glycosylation. Glycobiology. 1996;6:635–646. doi: 10.1093/glycob/6.6.635. [DOI] [PubMed] [Google Scholar]
- 140.Iwasaki H., Zhang Y., Tachibana K., Gotoh M., Kikuchi N., Kwon Y.D., Togayachi A., Kudo T., Kubota T., Narimatsu H. Initiation of O-glycan synthesis in IgA1 hinge region is determined by a single enzyme, UDP-N-acetyl-α-D-galactosamine: Polypeptide N-acetylgalactosaminyltransferase 2. J. Biol. Chem. 2003;278:5613–5621. doi: 10.1074/jbc.M211097200. [DOI] [PubMed] [Google Scholar]
- 141.Hassan H., Reis C.A., Bennett E.P., Mirgorodskaya E., Roepstorff P., Hollingsworth M.A., Burchell J., Taylor-Papadimitriou J., Clausen H. The lectin domain of UDP-N-acetyl-D-galactosamine: Polypeptide N-acetylgalactosaminyltransferase-T4 directs its glycopeptide specificities. J. Biol. Chem. 2000;275:38197–38205. doi: 10.1074/jbc.M005783200. [DOI] [PubMed] [Google Scholar]
- 142.Bennett E.P., Hassan H., Hollingsworth M.A., Clausen H. A novel human UDP-N-acetyl-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase, GalNAc-T7, with specificity for partial GalNAc-glycosylated acceptor substrates. FEBS Lett. 1999;460:226–230. doi: 10.1016/s0014-5793(99)01268-5. [DOI] [PubMed] [Google Scholar]
- 143.White K.E., Lorenz B., Evans W.E., Meitinger T., Strom T.M., Econs M.J. Molecular cloning of a novel human UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase, GalNAc-T8, and analysis as a candidate autosomal dominant hypophosphatemic rickets (ADHR) gene. Gene. 2000;246:347–356. doi: 10.1016/S0378-1119(00)00050-0. [DOI] [PubMed] [Google Scholar]
- 144.Toba S., Tenno M., Konishi M., Mikami T., Itoh N., Kurosaka A. Brain-specific expression of a novel human UDP-GalNAc: Polypeptide N- acetylgalactosaminyltransferase (GalNAc-T9) Biochim. Biophys. Acta Gene Struct. Expr. 2000;1493:264–268. doi: 10.1016/S0167-4781(00)00180-9. [DOI] [PubMed] [Google Scholar]
- 145.Cheng L., Tachibana K., Zhang Y., Guo J.M., Kahori Tachibana K., Kameyama A., Wang H., Hiruma T., Iwasaki H., Togayachi A., et al. Characterization of a novel human UDP-GalNAc transferase, pp-GalNAc-T10. FEBS Lett. 2002;531:115–121. doi: 10.1016/S0014-5793(02)03399-9. [DOI] [PubMed] [Google Scholar]
- 146.Boskovski M.T., Yuan S., Pedersen N.B., Goth C.K., Makova S., Clausen H., Brueckner M., Khokha M.K. The Heteroataxy gene, GALNT11, glycosylates Notch to orchestrate cilia type and laterality. Nature. 2013;504:456–459. doi: 10.1038/nature12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Guo J.M., Zhang Y., Cheng L., Iwasaki H., Wang H., Kubota T., Tachibana K., Narimatsu H. Molecular cloning and characterization of a novel member of the UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase family, pp-GalNAc-T12. FEBS Lett. 2002;524:211–218. doi: 10.1016/S0014-5793(02)03007-7. [DOI] [PubMed] [Google Scholar]
- 148.Zhang Y., Iwasaki H., Wang H., Kudo T., Kalka T.B., Hennet T., Kubota T., Cheng L., Inaba N., Gotoh M., et al. Cloning and characterization of a new human UDP-N-acetyl-α-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase, designated pp-GalNAc-T13, that is specifically expressed in neurons and synthesizes GalNAc α-serine/threonine antigen. J. Biol. Chem. 2003;278:573–584. doi: 10.1074/jbc.M203094200. [DOI] [PubMed] [Google Scholar]
- 149.Wang H., Tachibana K., Zhang Y., Iwasaki H., Kameyama A., Cheng L., Guo J.M., Hiruma T., Togayachi A., Kudo T., et al. Cloning and characterization of a novel UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase, pp-GalNAc-T14. Biochem. Biophys. Res. Commun. 2003;300:738–744. doi: 10.1016/S0006-291X(02)02908-X. [DOI] [PubMed] [Google Scholar]
- 150.Cheng L., Tachibana K., Iwasaki H., Kameyama A., Zhang Y., Kubota T., Hiruma T., Tachibana K., Kudo T., Guo J.M., et al. Characterization of a novel human UDP-GalNAc transferase, pp-GalNAc-T15. FEBS Lett. 2004;566:17–24. doi: 10.1016/j.febslet.2004.03.108. [DOI] [PubMed] [Google Scholar]
- 151.Raman J., Guan Y., Perrine C.L., Gerken T.A., Tabak L.A. UDP-N-acetyl α-d-galactosamine: Polypeptide N- acetylgalactosaminyltransferases: Completion of the family tree. Glycobiology. 2012;22:768–777. doi: 10.1093/glycob/cwr183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nakayama Y., Nakamura N., Oki S., Wakabayashi M., Ishihama Y., Miyake A., Itoh N., Kurosaka A. A putative polypeptide N-acetylgalactosaminyltransferase/Williams-Beuren syndrome chromosome region 17 (WBSCR17) regulates lamellipodium formation and macropinocytosis. J. Biol. Chem. 2012;287:32222–32235. doi: 10.1074/jbc.M112.370932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li X., Wang J., Li W., Xu Y., Shao D., Xie Y., Xie W., Kubota T., Narimatsu H., Zhang Y. Characterization of ppGalNAc-T18, a member of the vertebrate-specific y subfamily of UDP-N-acetyl-d-galactosamine:polypeptide N- acetylgalactosaminyltransferases. Glycobiology. 2012;22:602–615. doi: 10.1093/glycob/cwr179. [DOI] [PubMed] [Google Scholar]
- 154.Takasaki N., Tachibana K., Ogasawara S., Matsuzaki H., Hagiuda J., Ishikawa H., Mochida K., Inoue K., Ogonuki N., Ogura A., et al. A heterozygous mutation of GALNTL5 affects male infertility with impairment of sperm motility. Proc. Natl. Acad. Sci. USA. 2014;111:1120–1125. doi: 10.1073/pnas.1310777111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cummings R.D., Liu F.-T. Galectins. In: Varki A., Cummings R.D., Esko J.D., Freeze H.H., Stanley P., Bertozzi C.R., Gerald H.W., Etzler M.E., editors. Essentials of Glycobiology. Harbor Laboratory Press; New York, NY, USA: 2009. [PubMed] [Google Scholar]
- 156.Johannes L., Jacob R., Leffler H. Galectins at a glance. J. Cell Sci. 2018;131:1–9. doi: 10.1242/jcs.208884. [DOI] [PubMed] [Google Scholar]
- 157.Barondes S.H., Cooper D.N.W., Gitt M.A., Leffler H. Galectins. Structure and function of a large family of animal lectins. J. Biol. Chem. 1994;269:20807–20810. [PubMed] [Google Scholar]
- 158.Chetry M., Thapa S., Hu X., Song Y., Zhang J., Zhu H., Zhu X. The role of galectins in tumor progression, treatment and prognosis of gynecological cancers. J. Cancer. 2018;9:4742–4755. doi: 10.7150/jca.23628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ebrahim A.H., Alalawi Z., Mirandola L., Rakhshanda R., Dahlbeck S., Nguyen D., Jenkins M., Grizzi F., Cobos E., Figueroa J.A., et al. Galectins in cancer: Carcinogenesis, diagnosis and therapy. Ann. Transl. Med. 2014;2:1–7. doi: 10.3978/j.issn.2305-5839.2014.09.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chou F.C., Chen H.Y., Kuo C.C., Sytwu H.K. Role of galectins in tumors and in clinical immunotherapy. Int. J. Mol. Sci. 2018;19:430. doi: 10.3390/ijms19020430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cho M., Cummings R.D. Galectin-1, a β-galactoside-binding lectin in Chinese hamster ovary cells. I. Physical and chemical characterization. J. Biol. Chem. 1995;270:5198–5206. doi: 10.1074/jbc.270.10.5198. [DOI] [PubMed] [Google Scholar]
- 162.Di Lella S., Ma L., Díaz Ricci J.C., Rabinovich G.A., Asher S.A., Álvarez R.M.S. Critical role of the solvent environment in galectin-1 binding to the disaccharide lactose. Biochemistry. 2009;48:786–791. doi: 10.1021/bi801855g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gitt M.A., Massa S.M., Leffler H., Barondes S.H. Isolation and Expression of a Gene Encoding L-14-II, a New Human Soluble Lactose-binding Lectin. J. Biol. Chem. 1992;267:10601–10606. [PubMed] [Google Scholar]
- 164.Cederfur C., Salomonsson E., Nilsson J., Halim A., Öberg C.T., Larson G., Nilsson U.J., Leffler H. Different affinity of galectins for human serum glycoproteins: Galectin-3 binds many protease inhibitors and acute phase proteins. Glycobiology. 2008;18:384–394. doi: 10.1093/glycob/cwn015. [DOI] [PubMed] [Google Scholar]
- 165.Koths K., Taylor E., Halenbeck R., Casipit C., Wang A. Cloning and characterization of a human Mac-2-binding protein, a new member of the superfamily defined by the macrophage scavenger receptor cysteine-rich domain. J. Biol. Chem. 1993;268:14245–14249. doi: 10.1016/S0021-9258(19)85233-X. [DOI] [PubMed] [Google Scholar]
- 166.Huflejt M.E., Leffler H. Galectin-4 in normal tissues and cancer. Glycoconj. J. 2003;20:247–255. doi: 10.1023/B:GLYC.0000025819.54723.a0. [DOI] [PubMed] [Google Scholar]
- 167.Leonidas D.D., Vatzaki E.H., Vorum H., Celis J.E., Madsen P., Acharya K.R. Structural basis for the recognition of carbohydrates by human galectin- 7. Biochemistry. 1998;37:13930–13940. doi: 10.1021/bi981056x. [DOI] [PubMed] [Google Scholar]
- 168.Ideo H., Matsuzaka T., Nonaka T., Seko A., Yamashita K. Galectin-8-N-Domain Recognition Mechanism for Sialylated and Sulfated Glycans. J. Biol. Chem. 2011;286:11346–11355. doi: 10.1074/jbc.M110.195925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nagae M., Nishi N., Nakamura-Tsuruta S., Hirabayashi J., Wakatsuki S., Kato R. Structural Analysis of the Human Galectin-9 N-terminal Carbohydrate Recognition Domain Reveals Unexpected Properties that Differ from the Mouse Orthologue. J. Mol. Biol. 2008;375:119–135. doi: 10.1016/j.jmb.2007.09.060. [DOI] [PubMed] [Google Scholar]
- 170.Heusschen R., Griffioen A.W., Thijssen V.L. Galectin-9 in tumor biology: A jack of multiple trades. Biochim. Biophys. Acta Rev. Cancer. 2013;1836:177–185. doi: 10.1016/j.bbcan.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 171.Su J. A brief history of Charcot-Leyden crystal protein/galectin-10 research. Molecules. 2018;23:2931. doi: 10.3390/molecules23112931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hotta K., Funahashi T., Matsukawa Y., Takahashi M., Nishizawa H., Kishida K., Matsuda M., Kuriyama H., Kihara S., Nakamura T., et al. Galectin-12, an Adipose-expressed Galectin-like Molecule Possessing Apoptosis-inducing Activity. J. Biol. Chem. 2001;276:34089–34097. doi: 10.1074/jbc.M105097200. [DOI] [PubMed] [Google Scholar]
- 173.Yang R.Y., Hsu D.K., Yu L., Ni J., Liu F.T. Cell Cycle Regulation by Galectin-12, a New Member of the Galectin Superfamily. J. Biol. Chem. 2001;276:20252–20260. doi: 10.1074/jbc.M010914200. [DOI] [PubMed] [Google Scholar]
- 174.Than N.G., Balogh A., Romero R., Kárpáti É., Erez O., Szilágyi A., Kovalszky I., Sammar M., Gizurarson S., Matkó J., et al. Placental Protein 13 (PP13)—A placental immunoregulatory galectin protecting pregnancy. Front. Immunol. 2014;5:348. doi: 10.3389/fimmu.2014.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Su J., Wang Y., Si Y., Gao J., Song C., Cui L., Wu R., Tai G., Zhou Y. Galectin-13, a different prototype galectin, does not bind β-galactosides and forms dimers via intermolecular disulfide bridges between Cys-136 and Cys-138. Sci. Rep. 2018;8:980. doi: 10.1038/s41598-018-19465-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Than N.G., Romero R., Goodman M., Weckle A., Xing J., Dong Z., Xu Y., Tarquini F., Szilagyi A., Gal P., et al. A primate subfamily of galectins expressed at the maternal-fetal interface that promote immune cell death. Proc. Natl. Acad. Sci. USA. 2009;106:9731–9736. doi: 10.1073/pnas.0903568106. [DOI] [PMC free article] [PubMed] [Google Scholar]