Summary
Medicinal plants have been successfully used in the ethno medicine for a wide range of diseases since ancient times. The research on natural products has allowed the discovery of biologically relevant compounds inspired by plant secondary metabolites, what contributed to the development of many chemotherapeutic drugs. Flavonoids represent a group of therapeutically very effective plant secondary metabolites and selected molecules were shown to exert also antiparasitic activity. This work summarizes the recent knowledge generated within past three decades about potential parasitocidal activities of several flavonoids with different chemical structures, particularly on medically important flatworms such as Schistosoma spp., Fasciola spp., Echinococcus spp., Raillietina spp., and model cestode Mesocestoides vogae. Here we focus on curcumin, genistein, quercetin and silymarin complex of flavonolignans. All of them possess a whole spectrum of biological activities on eukaryotic cells which have multi-therapeutic effects in various diseases. In vitro they can induce profound alterations in the tegumental architecture and its functions as well as their activity can significantly modulate or damage worm´s metabolism directly by interaction with enzymes or signaling molecules in dose-dependent manner. Moreover, they seem to differentially regulate the RNA activity in numbers of worm´s genes. This review suggests that examined flavonoids and their derivates are promising molecules for antiparasitic drug research. Due to lack of toxicity, isoflavons could be used directly for therapy, or as adjuvant therapy for diseases caused by medically important cestodes and trematodes.
Keywords: curcumin, genistein, quercetin, silymarin, antiparasitic effects, cestodes, trematodes
Introduction
Natural products are of a great importance due to their unique chemical diversity, what naturally results from diversity in their biological activities (Yuan et al., 2016). A lot of plant-originated drugs used in clinical medicine today were discovered through their previous application in traditional medicine (Fabricant & Farnsworth, 2001; Li-Weber, 2009). The secondary plant metabolites were derived through biodiversity phenomenon in which the interactions among organisms and their environment put together the diverse complex of chemical entities within the plants which further enhance their survival and competitiveness (Lee, 2010).
The great advances in molecular, biochemical and analytical methods allow separation of individual plant phytochemicals and consequently analysis of their chemical structure. In this context, natural products (NPs) are structurally diverse and serve as a valuable source for novel molecular scaffolds in drug development, where the term “molecular scaffold” is used to describe the core structure of a molecule (Schuffenhauer & Varin, 2011). Plant-derived secondary metabolites can be divided on the basis of their molecular formulas into several classes where the most abundant are flavonoids, essential oils, alkaloids, saponins, glycosides, tannins, sesquiterpene lactones with peroxidic structure, amides and proteins with enzymatic activity (Hrčková & Velebný, 2013).
Platyhelminthes known as flatworms are metazoan phylum that includes neodermata lineage composed exclusively of parasitic taxa what include classes Cestoda, Trematoda and Monogenea. The first two classes include the agents of serious human diseases and cause considerable economic losses also in livestock (WHO, 2007; Keiser & Utzinger, 2005; Garcia et al., 2007, Otero et al. 2010). Antiparasitic therapy is the main tool of infection control relying on a few effective and relatively safe drugs, such as benzimidazole carbamates (albendazole, mebendazole, flubendazole), diethylcarbamazine-citrate (DEC) and praziquantel, which are already used for many decades. Drug resistance became an emerging problem for the control of helminth infections in animals. This emerging setback has stimulated research exploiting antiparasitic potential of plant secondary metabolites based on ethno medicine experiences. An excellent example of it is research on plant-derived substances with antiparasitic activity conducted by Prof Y. Tu from China which led to the discovery of artemisinin; a secondary metabolite chemically termed as sesquiterpene lactone in plant Artemisia annua. This compound is highly effective against malaria parasites and some other protozoan parasites for which Prof. Tu was awarded by Nobel Prize in 2015.
Flavonoids are a class of polyphenol secondary metabolites and till now more than 9000 flavonoids have been described (Hasten, 2002; Wang et al., 2015). They can be found in fruits, vegetables, nuts, seeds, herbs, spices, stems, flowers, tea and red wine. The term flavonoid is a collective noun for the plant pigments, mostly derived from benzo-γ-pyrone, which is synonymous with chromone. Different substituents are often bound on chromone what influence structure activity relationship (Middleton et al., 2000). Flavonoids have low molecular weight and are frequently found in glycosylated or esterified forms, consisting of C6-C3-C6 rings (Isoda et al., 2014). Flavonoids attract considerable attention as valuable therapeutic option against a number of diseases and are subdivided according to their substituents into flavones, flavonols, anthocyanidins, flavanols, flavanones, flavanonols, aurones, furan chromones, isoflavones, isoflavanones, biflavones, xanthones, chaocones and dihydrochalcones. Isoflavones represent by far the largest flavonoid subclass (Reynaud et al., 2005; Naguleswaran et al., 2006).
This review focuses on four chemically different flavonoid compounds and summarizes present knowledge about their activities on cestodes and trematodes conducted in vitro and/or in vivo studies.
Selected flavonoids and their effects on flatworms
Curcumin
Curcumin ((1E, 6E)-1, 7-bis (4-hydroxy-3-methoxyphenyl) hepta-1, 6-diene-3, 5-Dione) is lipophilic polyphenol and is the major constituent in rhizome of Curcuma longa, a member of ginger family Zingiberaceae (Hatcher et al., 2008) (Fig. 1).
Fig. 1.
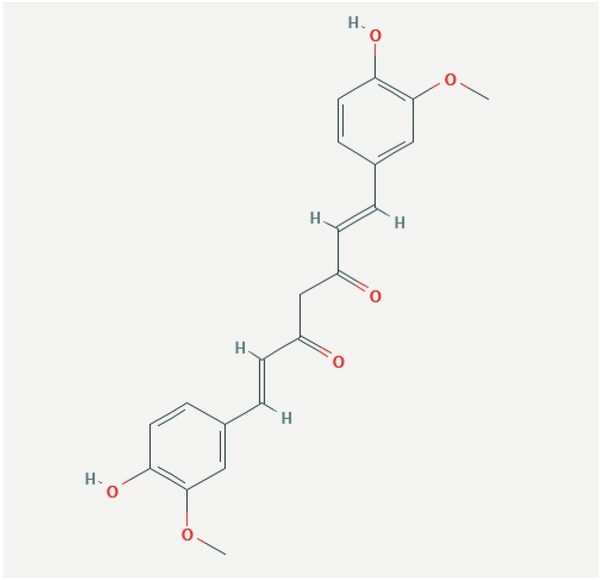
Structure of curcumin.
Following the administration, its decreased bioavailability supports its short half-life and extremely low serum and tissue concentrations. Curcumin undergoes extensive metabolism in liver and intestine (Shehzad et al., 2017). According to the several studies, curcumin is well tolerated at doses up to 8 g/day/kg administered for a short period. However, the other studies showed that doses ranging from 0.9 – 3.6 g/day given from 1 to 4 months can/ may have undesirable effects such as nausea, diarrhea and may increase the level of lactate dehydrogenase and serum alkaline phosphatase. Other side effects in patients have also been recorded after a long-term exposure to higher doses of curcumin. Those include chest tightness, gastrointestinal upset, skin rashes and inflamed skin (Sharma et al., 2004). Curcumin is well known for a wide spectrum of its biological activities. Many clinical studies have confirmed anti-inflammatory effects of curcumin in onset of many diseases (see review of Kahkhaie et al., 2019). Shehzad et al. (2017) concluded that curcumin binds to different molecular targets and affects various cell-signaling pathways in different diseases, including cancers, diabetes, cerebral edema, scleroderma, allergy and bronchial asthma, rheumatoid arthritis, neurodegenerative diseases, renal ischemia, cardiovascular diseases, psoriasis, obesity, and inflammatory bowel disease. Even though different curcumin biological activities are proved, its poor bioavailability due to poor absorption, rapid metabolism and systematic elimination represents a challenge for the optimization of its therapeutic efficacy such as, the utilization of various drug carriers or more soluble derivates (Anand et al., 2007).
The effects of curcumin on flatworms
Curcumin ´s activity on cestodes
Raillietina spp. belongs to the parasitic tapeworms that infect the small intestine of chickens and occasionally other birds such as turkey and guinea fowl. El-Bahy and Bazh (2015) evaluated in vitro and in vivo anthelmintic activity of commercially available ginger root extract containing 5 % gingerols (Now, USA) and curcumin extract, containing 95 % curcuminoids (Earthstream Herbs, USA) against adults’ stage of cestode Raillietina (R.) cesticillus. Both extracts reduced the physical activity (movement) of R. cesticillus in concentration-time-dependent manner and eradicating of 65 – 80 % of worms was observed after 48 h exposures to curcumin at the concentrations of 25 mg/ml or 100 mg/ml, respectively. Antiparasitic activity of extracts in the infected chickens was lower after administration of 1000 mg of curcumin or 500 mg of ginger where 40 – 50 % of worm´s survival was observed. Deleterious effect of both extracts was firstly manifested on worm´s tegument and authors thought that after the extract absorption/penetration interfered with the glucose metabolism in the worms, thus leading finally to their killing. In general, glucose and other simple carbohydrates are the main energy source for the cestodes and trematodes (Roberts, 1983). Lower efficacy in vivo may be due to the differences between pH in culture medium and pH in the animal’s stomach/intestine, what can influence the absorption and pharmacokinetics of these lipophilic compounds (El-Bahy & Bazh, 2015).
Curcumin ´s activity on trematodes
Fasciolosis is an economically important global disease of ruminants in the temperate and tropical regions. It is caused by Fasciola (F.) hepatica and F. gigantica, respectively, and also presents a potential zoonotic threat. Ullah et al. (2017) examined the anthelmintic potential of thymoquinone and curcumin on adult flukes of F. gigantica in vitro. A significant reduction in the worm motility and severe disruption of fluke tegumental surface was observed at the 60 μM concentration for both compounds. Curcumin was more potent in the reduction of activity of fluke´s antioxidant enzymes glutathione-S-transferase and superoxide dismutase as well as in reduction of glutathione (GSH) levels. Results showed that both compounds caused primarily alterations in tegument and tegumental disruption what can also affect the energy dependent Na+ – K+ transport leading probably to the swelling of worm´s syncytium, subsequently reducing parasites motility. The tegumental damage along with trans-tegumental uptake affects excretory/secretory processes, changes the signaling pathways and also impacts the metabolic pathways (Halton, 2004).
Higher plasma levels of insoluble compounds can be achieved after their entrapment in a suitable carrier. Luz et al. (2012) prepared poly(lactic-co-glycolic)acid (PLGA) nanoparticles with incorporated curcumin and demonstrated that 100 μM of curcumin drug formulation caused surface alterations followed by death of all Schistosoma (S.) mansoni adult worms in vitro. In addition, PLGA-curcumin particles reduced also the worm´s motor activity. The effects of polylactic acid (PLA) nanoparticles loaded with curcumin-nisin were examined on ovicidal activity and reproductive capacity of Fasciola spp. in vitro. It was shown that PLA nanoparticles with curcumin at the concentration of 5 mg/ml lead to decrease of percentage in egg hatching to a 41.7 % when compared with the positive control group treated with albendazol only (45.1 %). Aberrations observed in sperm cells were not significantly different between examined groups (Oyeyemi et al., 2018).
The potential in in vitro schistosomicidal effects of pure curcumin was for the first time confirmed by Magalhães et al. (2009) on S. mansoni. After the exposure to 50 μM and 100 μM of the compound, all worms were found dead. Meanwhile lower doses (5 μM and 20 μM) decreased worm viability in comparison with the positive and negative control groups. Moreover, all pairs of coupled adult worms were separated into individual male and female by curcumin at the doses of 20 μM to 100 μM. Significant reduction in egg production by 50 % in comparison with positive control group was found after exposure to 5 μM and 10 μM concentrations. Morais et al. (2013) in their in vitro study on S. mansoni demonstrated that curcumin modulates activity in many genes. More than 2374 genes were significantly and differentially expressed. Those counted were involved in regulating of important signaling pathways which affect embryogenesis and oogenesis, such as Notch and TGF-β (transforming growth factor β) (Sethi & Kang, 2011; Moskowitz & Rothman, 1996).
Abou El Dehab et al. (2019) monitored in vitro effects of curcumin on adult S. mansoni and S. haematobium viability, the tegument ultrastructure and egg hatchability. High doses of curcumin (500 μM) resulted in 100 % irreversible killing of both Schistosoma species after 2 h of incubation and at 50 μM concentration, the all pairs of worms were separated into individual male and female. Curcumin had stronger schistosomicidal effects on S. haematobium than on S. mansoni, and at concentrations 125 – 500 μM, it disrupted the shell wall of parasite’s eggs, thus allowing the untimely escape of the miracidium and leading to its consequent death. Tegumental alterations caused by curcumin exposure probably modulated calcium level in worms by stimulation of Ca2+ uptake and reduction of Ca2+ leakage. Consequently, inhibition of IP3 (inositol triphosphate)-induced Ca2+ release from endoplasmic reticulum affected Ca2+-dependent cellular events (Dyer et al., 2002; Zhang et al., 2014). Moreover, eggs hatchability and viability were also significantly affected by curcumin consequently supporting its potential for use in the therapy of S. mansoni and S. japonicum infections taking the advantage of its increased bioavailability in the gastrointestinal tract.
The ROS mediated apoptosis seems to be an effective strategy to control parasitic infections including the helminth parasites. The in vitro treatment of F. gigantica worms with curcumin at 60 μM, resulted in increased generation of reactive oxygen species (ROS) whereas the level of reduced glutathione, a primary redox regulator, was found to be significantly decreased (p < 0.05) (Rehman et al., 2020). It also inhibited the sigma GST at transcriptional and translational level, which is an important detoxification enzyme and also a key drug/vaccine target. Moreover, curcumin significantly inhibited the activity of antioxidant enzymes glutathione peroxidase and glutathione reductase that are vital in maintenance of redox homeostasis. The oxidative stress along with induction of apoptotic-like events would compromise the survival ability of worms within the host. It was found that two essential antioxidant enzymatic systems thioredoxin and glutathione, which occur in all organisms, differ in parasitic and free-living Platyhelminthes (Otero et al., 2010). Authors found that parasitic Platyhelminthes possess a unique and simplified redox system called thioredoxin glutathione reductase (TGR) for diverse essential processes, which is excellent drug target.
The induction of apoptotic death in parasites by drugs is another important parameter for the reduction of infections. The oxidative stress is harmful to worms as it causes modification in cellular macromolecules and could alter the normal function of key enzymes / proteins, and also promotes cell death. Curcumin was shown to generate oxidative stress, induce apoptosis, DNA damage and fragmentation in adult S. mansoni worms in vitro. DNA fragmentation appears to be the sign of undergoing cell apoptosis. Authors confirmed the increment in expression of SmCASP3/7 transcripts and the activity of caspase 3. Though, the activity of caspase 8 was not affected after curcumin treatment (De Paula Agular et al., 2016). The in vivo schistosomicidal activity of this polyphenol was also confirmed experimentally in mice infected with 80 S. mansoni cercariae which were injected intraperitoneally with curcumin at a total dose of 400 mg/kg body weight (Allam, 2009). Curcumin was effective and responsible for significant worm reduction and tissue-egg burdens, hepatic granuloma volume, liver collagen content, and restored hepatic enzymes activities to the normal levels, and enhanced catalase activity in the liver tissue of infected mice. Moreover, treatment modulated the IL-12 and TNF-α cytokine levels and augmented the production of IgG1 antibodies specific to worm antigens.
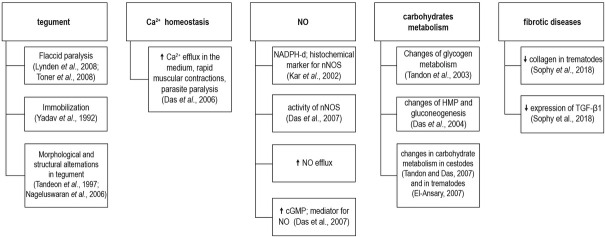
Present reports highlight the potential of curcumin to be used as addition to the primary therapy of cestode and trematode infections utilizing its direct wormicidal activity in conjunction with its health-promoting activity (Fig. 2).
Fig. 2.
Effects of curcumin.
Genistein
Genistein (5, 7-dihydroxy-3-(4-hydroxyphenyl) chromen-4-one) is an isoflavanoid compound, naturally occurring in plants of the soy family, belonging to a group of nutraceuticals (Tandon & Das, 2018) (Fig. 3). However, after the fermentation and digestion it is metabolized from isoflavone glycoside to isoflavone aglycone (Markovits et al., 1989).
Fig. 3.
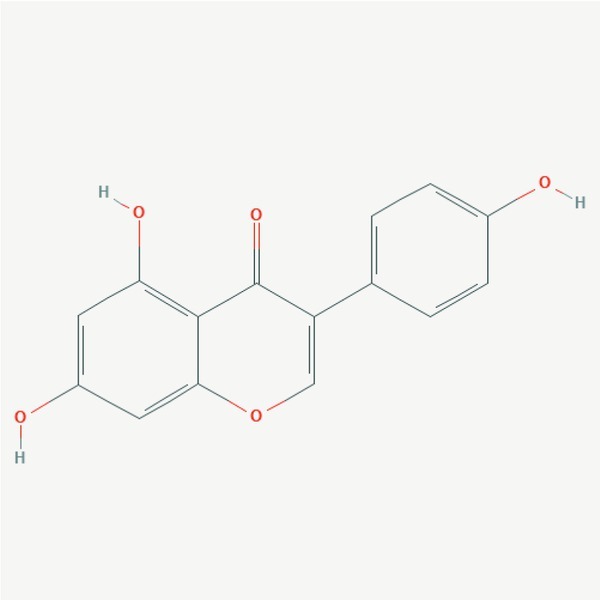
Structure of genistein. (https://pubchem.ncbi.nlm.nih.gov/compound/5280961#section=2D-Structure)
Genistein was for the first time isolated from Genista tinctoria, Fabaceae in 1899 and is present in high concentration in soybean – a high protein legume. Genistein has a similar structure as native estrogens, can act like estrogen-agonist (Brzezinski & Debi, 1999), and can influence a lot of biologically active targets and pathways involved in various metabolic processes. It possesses antioxidant activity, dose-dependent immunomodulatory effects on both the cell-mediated and humoral components of the adaptive immunity, induction of apoptosis and inhibition of proliferation of cancer cells, prevention of DNA damage. Genistein plays an important role in the prophylaxis and treatment of various chronic diseases including protection of cardiovascular diseases, reduction of atherogenic conditions, hypercholesterolemia and allergic diseases (Ganai & Farooqi, 2015). After oral administration of genistein at doses of 6.25, 12.5 and 50 mg/kg-1 to rats the absolute bioavailability of genistein was 21.9 %, 33.5 % and 19.0 %, respectively, what indicates that at high doses absorption, biotransformation and excretion occurs in a non-linear dose-dependent manner (Zhou et al., 2008). Fleminga vestita (Fabaceae) is a common plant in Asia and is traditionally used to treat helminthic diseases in India region. Rao and Reddy (1991) examined the plant’s active ingredients where genistein represented the major component in the ethanolic extract of plant root tuber peel, which was subsequently used for its isolation. According to many in vitro studies (see for review: Tandon & Das, 2018), genistein has been shown to affect several enzymatic systems, localized in the tegument and other worm´s compartments in various species of helminths, thus suggesting its multiple molecular targets.
Genistein´s activity on cestodes
In general, cestodes and trematodes treatment in vitro with genistein causes worm´s immobilization and flaccid paralysis in dose-dependent manner suggesting that genistein acted primarily on the flatworm´s tegument, where it interacts with enzymes and induces irreversible structural alterations (Tandon et al., 1997). The array of its activities on flatworm’s physiology indicates that it can pass through tegument.
The effects of genistein and a number of synthetic genistein derivates on metacestode stage of cestodes Echinococcus multilocullaris and Echinococcus granulosus were investigated in the study of Naguleswaran et al. (2006). In vitro treatment with genistein at the concentrations of 5 and 10 μg/ml for 7 days led to the profound morphological and structural alterations in both Echinococcus species. Moreover, authors showed that two synthetic genistein derivates carrying a modified estrogen receptor binding site were also able to induce dramatic breakdown in the structural integrity of the metacestode germinal layer at the concentration (1 – 10 μg/ml) as it was indicated by concentration dependent release of tegumental enzyme-alkaline phosphatase in vitro. This resulted in decreased viability and subsequent death of parasites. Authors suggest that inhibition of protein kinases which regulate down-stream signaling pathway involving mitogen-activated protein kinase is accounted, at least in part, for cestocidal effects of these compounds.
The multifunctional effect of genistein on flatworm physiology was demonstrated in several in vitro studies utilizing bird’s cestode model of R. echinobothrida. Ca2+ takes part in a muscle contraction during cestodes adult and metacestode stages (Bryant & Behm, 1989) and impacts regulation of several enzymes such as glycogen phosphorylase, glycogen synthase or protein kinase (Bollen et al., 1998; Nelson & Cox, 2000). Das et al. (2006) studied the effect of genistein and root-peel extract of F. vestita on Ca2+ homeostasis in R. echinobothrida in which the significant amount of Ca2+ and several other metal ions was found. The cestodes were incubated with root-peel extract (5 mg/ml), genistein (0.2 mg/ml) or praziquantel (0.001 mg/ml) for 6 h and treatments led to decrease in Ca2+ concentration by 49 %, 39 % and 45 %, respectively. In comparison with the parallel control group an increase in Ca2+ efflux by 100 %, 118 % and 94 % was observed. The results suggest that the changes in the Ca2+ homeostasis can induce rapid muscular contractions leading to the parasite paralysis and may result in anthelmintic stress caused by herbal components. Muscle activity is regulated also by NO (nitric oxide) which is synthetized in the nervous system by nNOS enzyme. NO is a unique neuronal messenger and among other properties it possesses an anthelminthic effect (Mahmoud & Habib, 2003). NO generated from amino acid L-arginine and molecular oxygen by NOS enzymes (nitric oxide synthases) can be divided into neuronal (nNOS), inducible (iNOS) and endothelial (eNOS) (Moncada et al., 1991). It was shown that genistein has a potentiating effect on the nNOS activity, NO efflux and the cGMP concentration (cyclic guanosine monophosphate), which works as a mediator for NO (Das et al., 2007). The activity of nNOS was significantly increased by 35 – 46 % and NO efflux was augmented 2-fold in the incubation medium in a group of worms exposed to genistein (0.2 mg/ml) for various time-periods when compared to the control group. Changes in nNOS activity correlated with the increase of cGMP concentration by 46 – 84 %.
Carbohydrates, stored in the form of glycogen, are the major energy source for cestodes and trematodes (Smyth & McManus, 1989). In R. echinobothrida worms exposed to genistein (0.2 mg/ ml) glycogen concentrations decreased by 15 – 44 % within several hours of incubation. This was associated with an increased activity of the active form of glycogen phosphorylase by 29 – 49 % and decreased activity of glycogen synthase by 36 – 59 %, as compared with controls. Results suggest that genistein may lead to the changes in flatworm’s glycogen metabolism by directing it towards its utilization for higher energy requirements (Tandon et al., 2003). The effect of genistein on glucose metabolism was demonstrated also in the study of Das et al. (2004) showing that genistein in cestodes can influence the HMP (hexose monophosphate pathway) and gluconeogenesis where the HMP converts hexoses to pentoses and yields NADPH (nicotinamide adenine dinucleotide phosphate) as a carrier for chemical energy (Nelson & Cox, 2000). In comparison with praziquantel treatment the in vitro treatment of R. echinobothrida with 0.2 mg/ml of genistein was manifested by decreased activity of glucose 6-phosphate dehydrogenase (G6PDH) by 23 – 31 % and was also associated with elevated activities of enzymes involved in gluconeogenesis where pyruvate carboxylase and phosphoenolpyruvate carboxy-kinase were increased by 32 – 44 % and 44 – 49 %, respectively. These changes in enzyme activities could be a response to higher energy demands of the cestodes under anthelmintic stress caused by exposure to the genistein (Das et al., 2004). The mechanism by which genistein influences metabolism of glucose and glycogen was further investigated in the study of Tandon and Das (2007) on the same cestode model in vitro. Worms exposed to genistein at a concentration of 0.2 mg/ml were able to renew the missing energy from glycogen reserves by GPase activation (glycogen phosphorylase) and GSase (glycogen synthetase) activity inhibition, where deficiency of glucose led to the activation of PEPCK (phosphoenolpyruvate carboxykinase) – malate pathway.
To date, the information are lacking regarding in vivo antiparasitic effect of genistein on the cestode infection and its activity on pathophysiology of the hosts. The anthelmintic effects of genistein in flatworms are summarized in Fig. 4.
Fig. 4.
Anthelmintic effects of genistein on flatworm’s tegument, NO, Ca2+ homeostasis, carbohydrates metabolism and fibrotic diseases.
Genistein´s activity on trematodes
The effect of genistein on the nNOS and NO concentrations was demonstrated also in trematodes in vitro. The NADPH-diaphorase (NADPH-d) is a histochemical marker for the nNOS and Kar et al. (2002) described increased intensity of NADPH-d histochemical reaction in the fluke Fasciolopsis buski treated with the root peel extract of F. vestita and genistein. This histochemical marker was evident in neuronal cell bodies, cerebral ganglia, the brain commissure, main nerve cords, and in the innervation of the pharynx, ventral sucker, terminal genitalia as well as genital parenchyma of the worms. Genistein treatment in vitro resulted in alterations of free amino acid pool and ammonia levels in the same trematode species (Kar et al., 2004).
Reviewing of literature revealed that information is missing about the direct in vitro effects of genistein on Schistosoma spp. The few in vivo studies on S. mansoni and S. japonicum on mouse-models demonstrated its beneficial effect in the therapy as the result of its
direct effect on worms and possibly by modulation of pathological outcomes of infection. In schistosomiasis, the egg deposition in the liver contributes to the formation of hepatic granuloma and fibrosis, which are the most serious clinical pathological features. Sobhy et al. (2018) examined antischistosomal and antifibrotic activity of genistein in acute and chronic experimental S. mansoni infection in comparison to praziquantel treatment. The reduction in the percentage of collagen in both acute and chronic stages and also the reduction in the expression of TGF-β1 in the examined hepatocytes in the both stages were observed. According to the results, genistein, mainly in combination with praziquantel, may protect from S. mansoni-induced liver damage, and reduce the development of fibrosis. It has been proposed that activation of the nuclear factor kappa B (NF-κB) signaling pathways is closely associated with the development of hepatic granuloma and fibrosis. Genistein has been shown to inhibit the activity of NF-κB signaling pathways in many cells. In BALB/c mice infected with S. japonicum the activity of NF-κB signaling and inflammatory markers MCP1 and TNF-α declined sharply after the treatment with genistein what correlated with reduced S. japonicum egg-induced liver granuloma and fibrosis (Wang et al., 2018). This implies that genistein can be a potential natural agent against schistosomiasis.
Quercetin
Quercetin (3, 3’, 4’, 5, 7-pentahydroxyflavone) is natural compound of flavonoid type (Fig. 5) occurring in low amounts in fruits (e.g. cranberries, cherries, grapes) and vegetables (e.g. onion, peppers, asparagus). Its antioxidant, anti-inflammatory, immuno-protective or anticarcinogenic effects are well described (Andres et al., 2018).
Fig. 5.
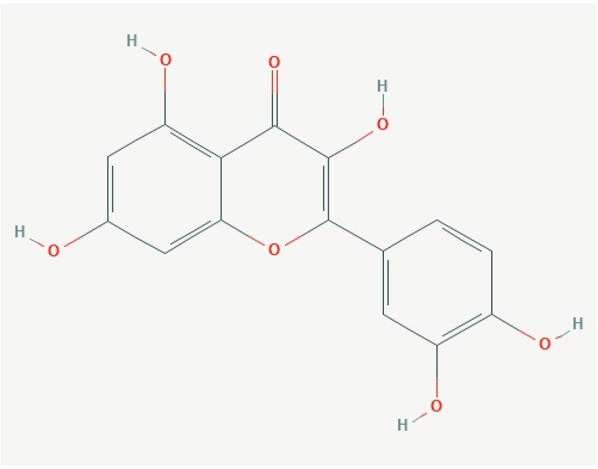
Structure of quercetin. (https://pubchem.ncbi.nlm.nih.gov/compound/5280343)
After the oral administration, quercetin glycosides, mainly quercetin aglycon, may passively permeate through intestinal epithelial barrier and they could also be transported by intestinal sodium/glucose cotransporter-1 (Murota & Terao, 2015; Andres et al., 2018). Quercetin is extensively metabolized in the enterocytes and further in the liver forming a plethora of metabolites (Graefe et al. 1999; Wang et al., 2016). Therefore, it is highly probable that described health-promoting and antiparasitic effects are results of synergistic action of various metabolites. This, however, possesses disadvantages in the evaluation the mechanisms by which quercetin or its metabolites interfere with molecular targets in flatworms. Moreover, the pharmacokinetics can show a high inter-individual variability, depending on, for example, genetic variation, individual antioxidant status and the other factors (Guo & Bruno, 2015).
Quercetin ´s activity on trematodes
In the natural medicine the plants Styrax camporum Pohl and S. pohlii A. DC. (Styracaceae) are used for the treatment of gastrointestinal diseases and fevers, respectively. Braguine et al. (2012) reported for the first time the presence of quercetin and also the other flavonoid kaempferol in ethyl acetate fraction of aerial parts of Styrax camporum. Evaluation of the schistosomicidal activity on S. mansoni adult flukes in vitro revealed that worms incubated with 100 μM of quercetine exhibited moderately reduced parasites motor activity, without tegumental alterations. On the other hand, kaempferol did not show any tegumental alterations and motor activity but was able to completely separate adult worms into males and females. The mechanism by which flavonol derivatives, exert their in vitro schistosomicidal effect is not clear. However, quercetin was identified as a selective inhibitor of the S. mansoni NAD+ catabolizing enzyme (SmNACE), which is localized on the outer surface (tegument) of the adult parasite (Kuhn et al., 2010).
Momordica charantia is considered as important medicinal plant by some African and Asian communities. The presence of quercetin as the major constituent was confirmed in crude extract of this plant by HPLC analysis and subsequent mass-spectrometric analysis (Pereira et al., 2016). Authors examined the effect of the crude extract and sub-fractions on the embryonic development of F. hepatica eggs in vitro. After 12 days no larvae were formed from the eggs incubated with extract at the concentrations above 12.5 mg/ml. Sub-fractions at concentrations between 0.01 and 1000 μg/ml affected differently trematode’s embryonic development, and n-butanol fraction containing the highest proportion of quercetin induced the strongest inhibition of miracidia formation. Authors concluded, that the presence of another compounds beside quercentin in the crude extract of M. charantia are also important, where the synergic effect of all components may lead to the final antiparasitic effect of this extract.
To date, no reports on the activity of quercetin on cestodes or cestodiasis have been published. Anthelmintic effects of quercetin are summarized in Fig. 6.
Fig. 6.
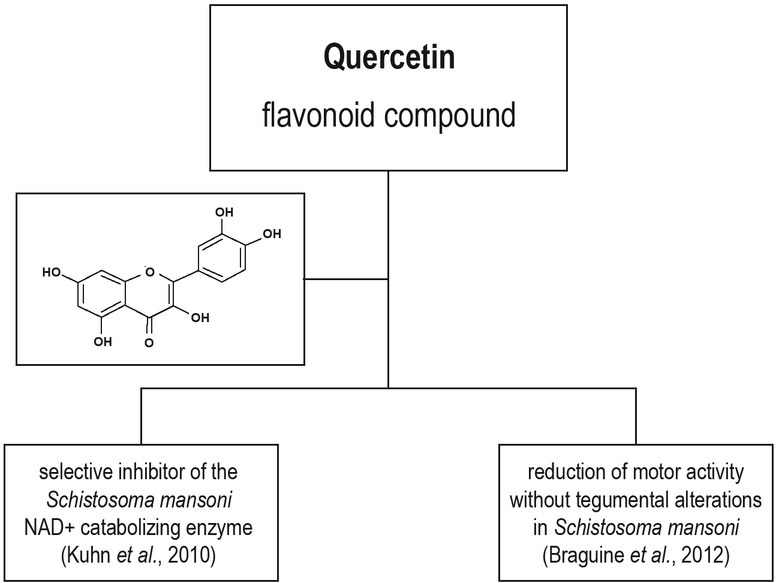
Anthelmintic effects of quercetin.
Silymarin ´s complex of flavonoids
Silybum marianum (Asteraceae) known also as milk thistle is a plant of the Asteraceae family and its seeds are the source of sily
marin - a unique complex of flavonolignans and other polyphenols. The main representatives of this group presented in silymarin are silybin, isosilybin, silychristin, isosylichristin and silydianin (Fig. 7). In addition to the above mentioned flavonolignans, silymarin contains also other constituents (e.g. taxifolin, dihydrosilybin, dihydrokaempherol, kaempherol, naringin, eriodyctol, chrysoeriol), and many other molecules in a very low concentrations (Abenavoli et al., 2018; Anthony & Saleh, 2013). The major bioactive compound in silymarin extract is silybin, which forms up to 40 % of the content and depends on many factors (Lee et al, 2007; Chambers et al., 2017). Silybin is a mixture of two diastereoisomers: A and B what can be separated by high-performance liquid chromatography (HPLC). Due to low water solubility (20 – 50 %) silymarin is after oral administration absorbed from gastro-intestinal tract where undergoes extensive enterohepatic circulation (Saller et al., 2008; Javed et al., 2011). Pharmacokinetic study in rats showed that orally administered silybin (50 mg/kg) had good tissue distribution profile reaching micro molar concentrations, e.g. 8.8 μg/g of livers (Zhao & Agarwal, 1999). The milk thistle is a medicinal plant used for more than 2000 years to treat a wide range of liver and gallbladder disorders, including hepatitis, cirrhosis and jaundice. Protect the liver from poisoning with chemical and environmental toxins. Due to these effects, silymarin was by the WHO in the 1970s classified as an official medicine with hepatoprotective properties (Wesolowska et al., 2007).
Fig. 7.
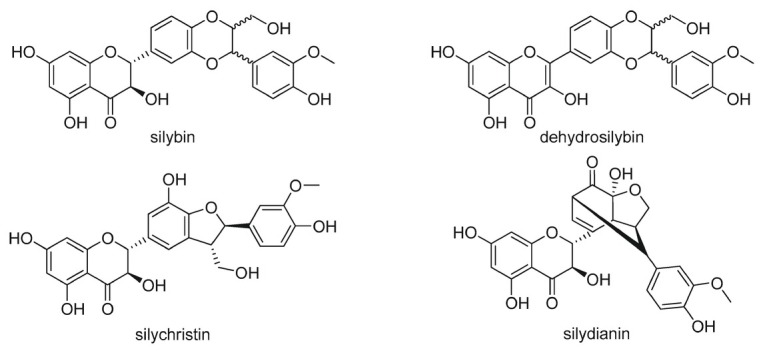
Structures of selected representatives of silymarin complex.
Many scientific teams are interested in multiple effects of silymarin/ silybin of which probably the best characterized is its antioxidant effects. Surai (2015) reviewed various routes and mechanisms of silymarin antioxidant actions in animal models and in human trials. It has been demonstrated that there are strong direct scavengers of some free radicals (Dehmlow et al., 1996). It is well documented, that silymarin/silybin can decrease oxidative stress and has a protective effect on mitochondrial structure (Rolo et al., 2003) and immune cells function (Hrčková et al., 2020a). There are also well documented antioxidant protective properties of silymarin/silybin in the prevention of toxic effects of various chemicals, such as arsenic, carbone tetrachloride, mycotoxins, thioacetamide, cisplatin, manganese, etc. (Surai, 2015). The mechanisms of silymarin hepatoprotective activities are well recognized. For example, Heidarian and Nouri (2019) confirmed the protective effects of silymarin on diclofenac-induced liver toxicity and oxidative stress in male rats. A growing number of studies have shown that silymarin/ silybin exhibit also anticarcinogenic, immunomodulatory and anti-angiogenic activities (Gažák et al., 2007; Esmaeil et al., 2017).
Silymarin/silybin activity on trematodes
To date, the direct in vitro activity of silymarin or individual silymarin´s flavonolignans on trematodes has not been documented. However, there is an increasing number of studies reporting on the effect of silymarin administration alone or in combination with praziquantel on the infections induced by Schistosoma species in vivo (for example: El-Lakkany et al., 2012; El-Sayed et al., 2016; El-Hawary et al., 2018).
Up to now there have been several reports focused on the interactions between silymarin and individual compartments of immune system during schistosomiasis. In particular, a number of immuno-modulatory activities on the infected hosts resulting in amelioration of the parasite induced pathology were reported. Kamel (2016) studied the anti-inflammatory and antifibrotic effects of silymarin alone or when combined with mefloquine during acute mouse model of schistosomiasis. It was confirmed that combined treatment can significantly reduce granulomatous reactions and hepatic fibrosis. Antifibrotic effect of silymarin administration in the livers was documented also in other studies. Silymarin caused a significant reduction in granuloma areas in S. mansoni infected mice when compared to controls (Tousson et al., 2013; Mata-Santos et al., 2010). Initiation of fibrosis and its perpetuation is an immunologically regulated process involving many cell types, cytokines and other mediators.
Tousson et al. (2013) studied the histopathological and immuno-histochemical expression of apoptotic proteins P53 and CD68 marker present on myeloid lineage in the mice livers infected with S. mansoni and examined the protective role of silymarin. A profound increase in P53 and CD68 was found in the liver tissue after the infection in comparison to the control. In contrary a significant decrease in the expression of both pro-apoptotic proteins was observed after silymarin treatment. Cytokines are produced by various cell types and are important fibrosis regulators. The TGF-β1 is the key growth factor involved in fibrosis progression, and is responsible for differentiation of fibroblast to myofibroblasts. The IL-4 induces collagen synthesis and together with IL-13 can drive the differentiation of resident fibroblast and recruited fibrocytes to myofibroblast in a wide range of tissues (Mattey et al., 1997). IFN-γ inhibits fibrosis by antagonizing the pro-fibrotic activity of TGF-β1 whereas the TNF-α is proinflammatory cytokine which activation must be tightly controlled because it can lead to the host-tissue damage (Szekanecz & Koch, 2007). The levels of IL-4, TNF-α, TGF-β1 cytokines were significantly increased in S. mansoni infected group and treatment with silymarin alone or combined with praziquantel resulted in a significant decrease in IL-4, TNF-α and TGF-β1 levels and subsequent significant elevation in serum IFN-γ levels (El-Sayed et al., 2016). Based on the experimental results the treatment with silymarin combined with praziquantel could reduce hepatic fibrosis also by downregulation of profibrotic cytokines due to its immunomodulatory activity on various cell types in murine schistosomiasis. In the study of Mata-Santos et al. (2010) the parasite oviposition capacity was not affected by silymarin treatment. However granulomatous peri-ovular reaction and fibrosis in the liver had been reduced. Results showed that treatment with silymarin in acute phase of schistosomiasis could lead to a mild course of murine schistosomiasis. In the next study (Mata-Santos et al., 2014) authors focused on the changes of profibrogenic cytokines levels in liver and hepatic fibrosis during chronic murine schistosomiasis. Correspondingly, silymarin treatment reduced liver weight, granuloma sizes and fibrosis, what correlated with lower serum levels of ALT (alanine aminotransferase) and AST (aspartate aminotransferase) in the liver: Alongside with reduction of IL-13 cytokine levels and increased levels of IFN-γ.
The strong down-regulation of fibrosis in mice livers infected with S. mansoni after silymarin and praziquantel administration was confirmed also by El-Lakkany et al. (2012). In livers the partial decrease in worm burden, hepatic tissue egg load associated with an increase in percentage of dead eggs, modulation in granuloma size were documented. Moreover, a significant reduction in hepatic hydroxyproline content, the marker of fibrosis, was observed. The alleviated pathology was manifested by decreased expression of MMP-2 (matrix metalloproteinase-2), TGF-β1 and the number of mast cells. The elevation of reduced glutathione (GSH) levels was also detected.
Taken together, all these results suggest that treatment with silymarin in combination with praziquantel could be a safe and more effective treatment possibility for schistosomiasis what resulted in amelioration of liver fibrosis and more effective wormicidal effect. Studies also showed that silymarin therapy has many beneficial immunological effects, what further contributed to the suppression of parasitic infection. Even though the direct parasitocidal effect of individual silymarin´s flavonolignans is also possible, and warrant further examinations.
Activity of silymarin ´s flavonolignans on cestodes
Tetrathyridia of cestode Mesocestoides (M.) vogae represent metacestode stage and are considered as suitable model for the evaluation of larvicidal potential of various compounds. The possibility of axenic cultivation of parasites is a special feature for this model and was used to assess the in vitro effects of three silymarin´s flavonolignans – silybin, 2,3-dehydrosilybin and silychristin given at concentrations of 5 and 50 μM under aerobic and hypoxic conditions for 72 h (Hrčková et al., 2018). Under both sets of conditions, the silybin and silychristin suppressed the metabolic activity, concentration of glucose, lipids and partially motility, but the other hand neutral red uptake was elevated. The dehydrosilybin exerted larvicidal activity and affected the motility and neutral lipid concentrations depending on the cultivation conditions, whereas it decreased glucose concentration. Dehydrosilybin at the 50 μM concentration caused irreversible morphological alterations along with damage to the metacestodes microvillus surface. Authors concluded that silybin and silychristin suppressed mitochondrial functions and energy stores, thus causing a physiological mis-balance. Dehydrosilybin exhibited a direct larvicidal effect due to the tegument damage and triggered complete disruption of larval physiology and metabolism.
The administration of silymarin (30 mg/kg/day) for 10 days as an adjuvant to the primary therapy with praziquantel given at the same doses enhanced the anthelmintic effect of drug in mice infected with the same metacestodes – M. vogae tetrathyridia (Velebný et al., 2008, 2010). Reduced parasite burden in mice livers and peritoneal cavities was significantly higher after combined therapy, and correlated with profoundly decreased ALT (alanine aminotransferase) and AST (aspartate aminotransferase) activities in the serum. Similar association was observed with the hyaluronic acid level, albumin and total protein concentration. Authors showed that silymarin caused higher fibrosis suppression in the liver after combined therapy along with reduction of oxidative stress and inflammation. This was demonstrated by a lesser lipid peroxidation and elevation in GSH content and decreased hydroxyproline content. Similar readouts were observed in mouse trematode infections, where specific silymarin immunomodulatory effect was examined in extended study performed on mice infected with M. vogae (Hrčková et al., 2020b). Co-administration of silymarin modified the effects of praziquantel therapy. The antigenic stimulation of the immune system modulated the levels of Th1/Th2/Tregs cytokines in the serum, and altered gene expression in the livers, what was accompanied with reduced fibrosis.
In summary, silymarin and its main constituent silybin, can be considered as a very effective non-toxic compound for the therapy of flatworm infections where they noticeably potentiate the anthelmintic effect of drug via multiple mechanisms. The reduction of fibrosis other than the direct wormicidal effect, seen already at low concentrations, is also likely.
Discussion
Microbial metabolites and biologically active substances from plants are gaining increasing attention as potential parasiticides. Particularly, after a very successful introduction of commercial treatment with avermectins and milbemycins (Shoop et al., 1995). Discovery of new compounds effective against flatworm infections in both humans and animals, belonging to classes Cestoda and Trematoda is an urgent research goal. At present, due to the difficulties and research costs for discovery of new chemical entities a limited range of anthelmintics is available for the treatment. Basically only benzimidazole carbamates and praziquantel are widely used. Several research groups therefore focus on the evaluation of secondary plant metabolites referring to ethnomedicine experiences using several species of cestodes and trematodes. In this review, we have summarized information about four flavonoids (curcumin, genistein, quercetin and silymarin complex) gained within the period of past three decades. Numerous studies on various natural products including flavonoids revealed that, in general, they can interact with multiple targets in eukaryotic cells, mostly proteins (enzymes, receptors, etc.) in dose-dependent manner. They usually have health beneficial effects against many diseases and interestingly, some of them also possess cestocidal or trematocidal activities in vitro and in vivo. Usually, a significant parasitocidal activity in vitro is achieved in micromolar concentration within the range from 5 up to 500 μM. Though, low bioavailability of water insoluble flavonoids can prevent to achieve its higher concentrations in the tissues of hosts. A group of abundant secondary metabolites in many plant species with reported bioactivity for virtually any biological/pharmaceutical endpoint have received the term “invalid metabolic panaceas – IMPS “ or “pan assay interference compounds – PAINS”, and include also the compounds addressed in present review. The term PAINS is used for compounds which typically interact nonspecifically with proteins in a high percentage of bioassays (Bisson et al., 2016; Courtney, 2017). But, interestingly, over 60 FDA-approved and worldwide drugs contain PAINS chemotypes (Kilchmann et al., 2016). Their multi-target activity may represent a serious problem in the development of a very specific antiparasitic drug according to the standard protocols in drug-discovery research. However, the development of novel chemical structure with high activity against metazoan parasites is a complicated, very costly task and also challenge for both the chemists and biologists. The very important issue is also toxicity of the potent drug to the hosts which is usually neglected in case of plant – derived compounds such as flavonoids (Hewitson et al., 2009).
In case of compounds where anthelmintic activity was demonstrated in vitro at relatively low concentration, the further research is often directed towards preparation of synthetic derivates with modified structure which could bind to a specific target on parasites. For example, synthetic derivate of genistein Rm6423 carries a modified estrogen receptor binding site but retains ability to target epidermal growth factor tyrosine-kinases in E. multilocullaris and E. granulosus protoscoleces at lower concentration (Naguleswaran et al. 2006). The other example are 2, 3-dehydroderivates of flavonolignans silybin, silychristin and silydianin, which have antiradical and cytoprotective activity (Pyszkova et al., 2016). In our previous in vitro study, treatment of other metacestode species M. vogae with silybin, silychristin and dehydrosilybin revealed that dehydrosilybin exhibited a direct larvicidal effect due to tegument damage and complete disruption of larval physiology and metabolism. Silybin and silychristin suppressed mitochondrial functions and energy stores, thus inducing a physiological misbalance (Hrčková et al., 2018).
A number of studies performed on several flatworm species with isoflavone genistein indicate that they can interfere with several parasite enzymatic systems, including enzymes involved in glucose metabolism (Tandon & Das, 2018). Similarly, silymarin flavonolignans at low concentration (5 μM) were able to decrease metabolic activity and glucose content in M. vogae tetrathyridia (Hrčková et al., 2018). This indicates that energy-generating enzymatic systems in mitochondrial respiratory chains in flatworms can be the targets for selected flavonoids. El-Bahy and Bazh (2015) examined effect of curcumin on adult stage of cestode Raillietina (R.) cesticillus in vitro. The authors thought that after absorption/ penetration the extracts itself interfered with worm’s glucose metabolism and thus lead to their killing.
In general, helminths exploit a variety of energy-transducing systems during their adaptation to the peculiar habitats in their hosts, where differences in energy metabolisms between the host and helminths are attractive therapeutic targets. The majority of parasites, including flatworms, do not use the oxygen within the hosts, but employ systems other than oxidative phosphorylation for ATP synthesis (Sakai et al., 2012; Matsumoto et al., 2008). They often live in niche where oxygen tension is low therefore many of them exploit a unique anaerobic respiratory chain, called NADH-fumarate reductase system – complex II. It is a unique enzymatic system for energy generation, not found in normal eukaryotic cells, except for cancer cells (Tomitsuka et al., 2012).
This respiratory chain was studied in detail in Ascaris suum nematode (reviewed in: Kita & Takamiya, 2002). The novel compound nafuredin isolated from Aspergillus niger mold inhibited complex II in this nematode in nM concentrations in vitro (Sakai et al., 2012). Thus differences between parasite and host mitochondria hold great promise as targets for the therapy. However, so far activity of mentioned flavonoids or its derivatives on NADH-fumarate reductase system in flatworms has not been examined. The presence of this system was demonstrated in protoscoleces of Echinococcus species (Matsumoto et al., 2008). Interestingly, complex II is the main site of ROS production, which contributed to the pathology during infections, and was demonstrated in A. suum adult respiratory chain (Paranagama et al., 2010). Taking into consideration the anti-oxidant effects of flavonoids, their co-administration might alleviate pathology directly as scavengers of ROS. Their activities on this specific enzymatic system in flatworms await further research.
Apart of documented in vitro effects on flatworms summarized in this review, in vivo studies on model flatworm infections with Schistosoma species, Echinococcus species, M. vogae infection (and others) using flavonoids curcumin, genistein and mostly silymarin in combination with the anthelmintic drugs refer to their pleiotropic mode of action in the hosts. Moreover, besides the beneficial effects on the hosts, flavonoid co-administration contributed to the increased efficacy of the drugs. Reports cited in present review showed that silymarin contributed to the increased efficacy of praziquantel in S. mansoni and M. vogae infected mice. Collectively authors of all referred studies suggested, that elevated drug´s efficacy is the result of reduced pathological consequences of infection, and stimulation of immunity. Though, the direct interference with enzymatic systems of flavonoids on parasite in vivo is also possible. In general, co-administration of examined flavonoids with anthelmintic drugs seems to be the safe and effective way to potentiate therapy and reduce pathology at infections induced by various developmental stages of cestodes and trematodes.
Conclusion
In recent years, research focused on neglected tropical diseases where the infections caused by flatworm species also belong, has undergone a significant development. Nevertheless, a new chemical entities approved for treatment are still absent. Polyphenols are group of high interests. It is due to their low toxicity on the hosts and multiple mechanisms by which they can modulate pathologically changed processes during infections. Present review based on recently published findings highlights the potential of selected compounds: curcumin, genistein, quercetin and silymarin´s flavonolignans as sources of prospective molecules for drug development. These lipophilic molecules act primarily on soft tegument of flatworms where they can modulate structure and functions of ion channels, receptors and enzymes, and thus lead to the physiological imbalance or death of worms in vitro. Anthelmintic stress induced by transmembrane penetration leads to the disruption of energy metabolism, predominantly affecting glycogen stores and its degradation to the glucose. In addition, interference with other enzymatic systems involved in coordination of muscle activity is also proposed. The clear benefits of selected isoflavones as adjuvants to antiparasitic infection therapy support their potential in reduction of related pathology and modulation of the host immune responses. Despite the fact that multi-target activities of mentioned substances may represent a serious problem in the development of a very specific antiparasitic drugs a detailed discovery of the mode of their action as well as the development of new effective antiparasitic drugs with minimal side effects is a prospective and promising challenge for future research.
Acknowledgements
This study was supported by the EU Structural Fund ITMS 26220220185 (MediPark), bilateral mobility project SAV-AV ČR No. 18–24 and APVV project no. 17-0410 of the Ministry of Education, Science, Research and Sport of the Slovak Republic.
Footnotes
Conflict of Interest
Authors declare no conflict of interest.
References
- Abenavoli L., Izzo A. A., Milić N., Cicala C., Santini A., Capasso R.. Milk thistle (Silybum marianum): A concise overview on its chemistry. pharmacological, and nutraceutical uses in liver diseases, Phytother Res. 2018;32:2202–2213. doi: 10.1002/ptr.6171. [DOI] [PubMed] [Google Scholar]
- Abou El Dehab M.M., Shahat S.M., Mahmoud S.S.M., Mahan N.A.. In vitro effect of curcumin on Schistosoma species viability, tegument ultrastructure and egg hatchability. Exp. Parasitol. 2019;199:1–8. doi: 10.1016/j.exppara.2019.02.010. [DOI] [PubMed] [Google Scholar]
- Allam G.. Immunomodulatory effects of curcumin treatment on murine schistosomiasis mansoni. Immunobiology. 2009;214(8):712–727. doi: 10.1016/j.imbio.2008.11.017. [DOI] [PubMed] [Google Scholar]
- Anand P., Kunnumakkara A.B., Newman R.A., Aggarwal B.B.. Bioavailability of curcumin: problems and promises. Mol. Pharm. 2007;4(6):807–18. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- Andres S., Pevny S., Ziegenhagen R., Bakhiya N., Schäfer B.M., Hirsc h-Ernst K.I., Lam pen A.. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr Food Res. 2018;62(1):1700447. doi: 10.1002/mnfr.201700447. [DOI] [PubMed] [Google Scholar]
- Anthony K. P., Saleh M. A.. Free Radical Scavenging and antioxidant activities of silymarin components. Antioxidants (Basel) 2013;2(4):398–407. doi: 10.3390/antiox2040398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biss on J., Mcalpine J. B., Friesen J. B., Chen S. N., Graham J., Pauli G. F. Can Invalid Bioactives Undermine Natural Product-Based Drug Discovery? J. Med. Chem. 2016;59:1671–1690. doi: 10.1021/acs.jmedchem.5b01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen M., Keppens S., Stalmans W.. Specific features of glycogen metabolism in the liver. Biochem J. 1998;336(Pt 1):19–31. doi: 10.1042/bj3360019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braguine C.G., Bertanha C.S., Goncalves U.O., Magalhaes L.G., Rodrigues V., Gimenes V.M.M., Groppo M., Silva M.L.A.E., Cunha W.R., Januario A.H., Pauletti P.M.. Schistosomicidal evaluation of flavonoids from two species of Styrax against Schistosoma mansoni adult worms. Pharmaceut. Biol. 2012;50(7):925–929. doi: 10.3109/13880209.2011.649857. [DOI] [PubMed] [Google Scholar]
- Bryant C., Behm A.C. Biochemical adaptation in parasites. London, Chapman and Hall: 1989. p. 259. [Google Scholar]
- Brzezinski A., Debi A.. Phytoestrogens: the “natural” selective estrogen receptor modulators? Eur J Obstet Gynecol Reprod Biol. 1999;85(1):47–51. doi: 10.1016/s0301-2115(98)00281-4. [DOI] [PubMed] [Google Scholar]
- Courtney A.. The Ecstasy and Agony of Assay Interference Compounds, ACS Cent. Sci. 2017;3:143–147. doi: 10.1021/acscentsci.7b00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das B., Tandon V., Saha N.. Effects of phytochemicals of Fleminga vestita (Fabaceae) on glucose 6-phosphate dehydrogenase and enyzmes of gluconeogenesis in a cestode (Raillietina echinobothrida) Comp. Biochem. Physiol. 2004;139(part C):141–146. doi: 10.1016/j.cca.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Das B., Tandon V., Saha N.. Effect of isoflavone from Flemingia vestita (Fabaceae) on the Ca2+ homeostasis in Raillietina echinobothrida, the cestode of domestic fowl. Parasitol. Int. 2006;55:17–21. doi: 10.1016/j.parint.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Das B., Tandon V., Saha N.. Genistein from Fleminga vestita (Fabaceae) enhances NO and its mediator (cGMP) production in a cestode parasite, Railietina echinobothrida. Parasitology. 2007;134(10):1457–1463. doi: 10.1017/S003118200700282X. [DOI] [PubMed] [Google Scholar]
- De Paula Agular D., Brunetto Moreira Moscardini M., Rezende Morais E., Graciano De Paula R., Ferreira P.M., Afonso A., Belo S., Tomie Ouchida A., Curti C., Cunha W. R., Rodrigues V., Magal-hães L.G.. Curcumin generates oxidative stress and induces apoptosis in adult Schistosoma mansoni worms. PLoS ONE. 2016;11(11):e0167135. doi: 10.1371/journal.pone.0167135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehmlow C., Erhard J., De Groot H.. Inhibition of Kupffer cell functions as an explanation for the hepatoprotective properties of silibinin. Hepatology. 1996;23(4):749–754. doi: 10.1053/jhep.1996.v23.pm0008666328. [DOI] [PubMed] [Google Scholar]
- Dyer J.I., Khan S.Z., Bilmen J.G., Hawtin S.R., Wheatley M., Javed M.U., Michelangeli F.. Curcumin: a new cell permeant inhibitor of the inositol 1, 4, 5 – triphosphate receptor. Cell calcium. 2002;31(1):45–52. doi: 10.1054/ceca.2001.0259. [DOI] [PubMed] [Google Scholar]
- El-Bahy N.M., Bazh E.K.A.. Anthelmintic activity of ginger, curcumin, and praziquantel against Raillietina cesticillus (in vitro and in vivo) Parasitol. Res. 2015;114(7):2427–2434. doi: 10.1007/s00436-015-4416-0. [DOI] [PubMed] [Google Scholar]
- El-Hawary S.S., Taha K.F., Kirillos F.N., Dahab A.A., El-Mahis A.A., El-Sayed S. H.. Complementary effect of Capparis spinosa L. and silymarin with/without praziquantel on mice experimentally infected with Schistosoma mansoni Helminthologia. 2018;55(1):21–32. doi: 10.1515/helm-2017-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Lakkany N.M., Hammam O.A., El-Maadawy E.H., Badawy A.A., Ain-Shoka A.A., Ebeid F.A.. Anti-inflammatory/anti-fibrotic effects of the hepatoprotective silymarin and the schistosomicide praziquantel against Schistosoma mansoni-induced liver fibrosis. Parasit. Vectors. 2012;5:9. doi: 10.1186/1756-3305-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayed N.M., Fathy G.M., Abdel-Rahman S.A., El-Shafei M.A.. Cytokine patterns in experimental schistosomiasis mansoni infected mice treated with silymarin. J. Parasit. Dis. 2016;40(3):922–929. doi: 10.1007/s12639-014-0606-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmaeil N., Anaraki S.B., Gharagozloo M., Moayedi B.. Silymarin impacts on immune system as an immunomodulator: One key for many locks. Int. Immunopharmacol. 2017;50:194–201. doi: 10.1016/j.intimp.2017.06.030. [DOI] [PubMed] [Google Scholar]
- Fabricant D.S., Farnsworth N.R.. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001;109(1):69–75. doi: 10.1289/ehp.01109s169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganai A.A., Farooqi H.. Bioactivity of genistein: A review of in vitro and iv vivo studies. Biomed pharmacother. 2015;76:30–38. doi: 10.1016/j.biopha.2015.10.026. DOI: 0.1016/j.biopha.2015.10.026. [DOI] [PubMed] [Google Scholar]
- Garcia H. H., Moro P. L., Schantz P. M.. Zoonotic helminth infections of humans:echinococcosis, cysticercosis and fascioliasis. Curr Opin Infect Dis. 2007;20(5):489–494. doi: 10.1097/QCO.0b013e3282a95e39. [DOI] [PubMed] [Google Scholar]
- GažÁk R., Walterova D., Kren V.. Silybin and silymarin - new and emerging applications in medicine. Curr. Med. Chem. 2007;14:315–338. doi: 10.2174/092986707779941159. [DOI] [PubMed] [Google Scholar]
- Graefe E.U., Derendorf H., Veit M.. Pharmacokinetics and bioavailability of the flavonol quercetin in humans. Int. J. Clin Pharmacol. Ther. 1999;37(5):219–233. [PubMed] [Google Scholar]
- Guo Y., Bruno R. S.. Endodenous and exogenous mediators of quercetin bioavailability. J Nutr Biochem. 2015;26(3):201–210. doi: 10.1016/j.jnutbio.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Halton D.. Microscopy and the helminth parasite. Micron. 2004;35(5):361–390. doi: 10.1016/j.micron.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Hatcher H., Planalp R., Cho J., Torti F.M, Torti, S.V.. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65(11):1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havsteen B.H.. The biochemistry and medical significance of flavonoids, Pharmacol. Ther. 2002;96(2–3):67–202. doi: 10.1016/s0163-7258(02)00298-x. [DOI] [PubMed] [Google Scholar]
- Heidarian E., Nouri A.. Hepatoprotective effects of silymarin against diclofenac-induced liver toxicity in male rats based on biochemical parameters and histological study. Arch. Physiol. Biochem. 2019;1–7 doi: 10.1080/13813455.2019.1620785. [DOI] [PubMed] [Google Scholar]
- Hewitson P., Ignatova S., Ye H., Chen L., Sutherland I.. Intermittent counter-current extraction as an alternative approach to purification of Chinese herbal medicine. J chromatogr A. 2009;1219(19):4187–4192. doi: 10.1016/j.chroma.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Hrčková G., Velebný S. Pharmacological Potential of Selected Natural Compounds in the Control of Parasitic Diseases; Springer Science & Business Media; Berlin/Heidelberg, Germany: 2013. p. 115. [Google Scholar]
- Hrčková G., Mačák Kubašková T., Benada O., Kofroňová O., Tumová L., Biedermann D. Molecules. Basel, Switzerland: 2018. Differential effects of the flavonolignans Silybin, Silychristin and 2,3-Dehydrosilybin on Mesocestoides vogae larvae (Cestoda) under hypoxic and aerobic in vitro conditions; p. 2999. 23, articel no. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrčková G., Mačák Kubašková T., Mudroňová D., Bardelčíková A.. Concentration-dependent effect of silymarin on concanavalin A-stimulated mouse spleen cells in vitro. Eur. Pharmaceut. J. 2020a doi: 10.2478/afpuc-2020-0003. (in press) [DOI] [Google Scholar]
- Hrčková G., Mačák Kubašková T., Reiterová K., Biedermann D.. Co-administration of silymarin elevates the therapeutic effect of praziquantel through modulation of specific antibody profiles, Th1/Th2/Tregs cytokines and down-regulation of fibrogenesis in mice with Mesocestoides vogae (Cestoda) infection. Exp. Parasitol. 2020b;213:107888. doi: 10.1016/j.exppara.2020.107888. [DOI] [PubMed] [Google Scholar]
- Chambers Ch. S., Holečková V., Petrásková L., Biedermann D., Valentová K., Buchta M., Křen V.. The silymarin composition. and why does it matter??? Food Res Int. 2017;100:339–353. doi: 10.1016/j.foodres.2017.07.017. [DOI] [PubMed] [Google Scholar]
- Isoda H., Motojima H., Onaga S., Sam et I., Villareal M.O, Han J.. Analysis of the erythroid differentiation effect of flavonoid apigenin on K562 human chronic leukemia cells. Chem. Biol. Interact. 2014;220:269–277. doi: 10.1016/j.cbi.2014.07.00. [DOI] [PubMed] [Google Scholar]
- Javed S., Kohli K., Ali M.. Reassessing Bioavailability of Silymarin. Altern Med Rev. 2011;16(3):239–249. [PubMed] [Google Scholar]
- Kahkhaie K.R., Mirhoseini A., Alibadi A., Mohamadi A., Javad Mosavi M., Haftchesmeh S.M., Sathyapalan T., Sehebkar A.. Curcumin: a modulator of inflammatory signaling pathways in the immune system. Inflammopharmacol. 2019;27:885–900. doi: 10.1007/s10787-019-00607-3. [DOI] [PubMed] [Google Scholar]
- Keiser J., Utzinger J.. Chemotherapy for major food-borne trematodes: a review. Expert Opin Pharmacother. 2005;5(8):1711–1726. doi: 10.1517/14656566.5.8.1711. [DOI] [PubMed] [Google Scholar]
- Kam el R.O.. Interactions between mefloquine and the anti.fibrotic drug silymarin on Schistosoma mansoni infections in mice. J. Helminthol. 2016;90(6):760–765. doi: 10.1017/S0022149X16000018. [DOI] [PubMed] [Google Scholar]
- Kar P.K., Tandon V., Saha N.. Anthelmintic efficacy of Fleminga vestita: genistein-induced effect on the activity of nitric oxide synthase and nitric oxide in trematode parasite, Fasciolopsis buski. Parasitol. Int. 2002;51(3):249–257. doi: 10.1016/s1383-5769(02)00032-6. [DOI] [PubMed] [Google Scholar]
- Kar P.K., Tandon V., Saha N.. Anthelmintic efficacy of genistein, the active principle of Flemingia vestita (Fabaceae): alterations in the free amino acid pool and ammonia levels in the fluke, Fasciolopsis buski. Parasitol. Int. 2004;53:287–291. doi: 10.1016/j.parint.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Kilchman F., Marcaida M. J., Kotak S., Schick T., Boss S. D., Mahendra A., Gonczy P., Reymond J.-L.. Discovery of a selective aurora A Kinase Inhibitor by Virtual Screening. J Med Chem. 2016;59(15):7188–211. doi: 10.1021/acs.jmedchem.6b00709. [DOI] [PubMed] [Google Scholar]
- Kita K., Hirawake H., Miyadera H., Amino H., Takeo S.. Role of complex II in anaerobic respiration of the parasite mitochondria from Ascaris suum and Plasmodium falciparum. Biochim Biophys Acta. 2002;1553:123–139. doi: 10.1016/s0005-2728(01)00237-7. [DOI] [PubMed] [Google Scholar]
- Kuhn I., Kellenberger E., Said-Hassane F., Villa P., Rognan D., Lobstein A., Haiech J., Hibert M., Schuber F., Muller-Steffner H.. Identification by high-throughput screening of inhibitors of Schistosoma mansoni NAD(+) catabolizing enzyme. Bioorg. Med. Chem. 2010;18:7900–7910. doi: 10.1016/j.bmc.2010.09.041. [DOI] [PubMed] [Google Scholar]
- Lee J.I., Narayan M., Barrett J.S.. Analysis and comparison of active constituents in commercial standardized silymarin extracts by liquid chromatography-electrospray ionization mass spectrometry. J. Chromatogr. B. Anal. Technol. Biomed. Life Sci. 2007;845(1):95–103. doi: 10.1016/j.jchromb.2006.07.063. [DOI] [PubMed] [Google Scholar]
- Lee K.H.. Discovery and development of natural product-derived chemotherapeutic agents based on a medicinal chemistry approach, J. Nat. Prod. 2010;73(3):500–513. doi: 10.1021/np900821e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Weber M.. New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Caicalin. Cancer Treat. Rev. 2009;35(1):57–68. doi: 10.1016/j.ctrv.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Luz P.P., Magalhães L.G., Pereira A.C., Cunha W.R., Rodrigues V., Andrade E., Silva M.L.. Curcumin-loaded into PLGA nanoparticles: preparation and in vitro schistosomicidal activity. Parasitol. Res. 2012;110(2):593–598. doi: 10.1007/s00436-011-2527-9. [DOI] [PubMed] [Google Scholar]
- Magalhaes L.G., Mac had o C.B., Morais E.R., Moreira E.B., Soares C.S., Da Silva S.H., Da Silva F.A.A., Rodrigues V.. In vitro schistosomicidal activity of curcumin against Schistosoma mansoni adult worms. Parasitol. Res. 2009;104(5):1197–1201. doi: 10.1007/s00436-008-1311-y. [DOI] [PubMed] [Google Scholar]
- Mahmoud M.S., Habib F.S.. Role of nitric oxide in host defence against Hymenolepis nana infection. J. Egypt. Soc. Parasitol. 2003;33(2):485–496. [PubMed] [Google Scholar]
- Markovits J., Linassier C., Fosse P., Couprie J., Pierre J., Jacquemin-Sablon J., Saucier M., Le Pecq J.B., Larsen A.K.. Inhibitory effects of the tyrosine kinase inhibitor genistein on mammalian DNA topoisomerase II. Cancer Res. 1989;49(18):5111–5117. [PubMed] [Google Scholar]
- Mata-Santos H.A., Dutra F.F., Rocha C.C., Lino F.G., Xavier F.R., Chinalia L.A., Hossy B.H., Castelo-Branco M.T.L., Teodoro A.J., Paiva C.N., Dos Santos Pyrrho A.. Silymarin Reduces Profibrogenic Cytokines and reverses hepatic fibrosis in chronic murine Schistosomiasis. Antimicrob. Agents Chemother. 2014;58(4):2076–2083. doi: 10.1128/AAC.01936-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata-Santos H.A, Lino, F.G., Rocha C.C., Paiva C.N., Castelo Branco M.T., Pyrrho Ados S.. Silymarin treatment reduces granuloma and hepatic fibrosis in experimental schistosomiasis. Parasitol. Res. 2010;107(6):1429–1434. doi: 10.1007/s00436-010-2014-8. [DOI] [PubMed] [Google Scholar]
- Matsumoto J., Sakamoto K., Shinjyo N., Kido Y., Yamamoto N., Yagi K., Miyoshi H., Nonaka N., Katakura K., Kita K., Oku Y.. Anaerobic NADPH-Fumarate Reductase System Is Predominant in the Respiratory Chain of Echinococcus multiocularis. Providing a Novel Target for the Chemotherapy of Alveolar Echinoccosis, Antimicrob Agentes Chemother. 2008;50(1):164–170. doi: 10.1128/AAC.00378-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattey D.L., Dawes P.T., Nixon N.B., Slater H.. Transforming growth factor beta 1 and interleukin 4 induced alpha smooth muscle actin expression and myofibroblast-like differentiation in human sznovial fibroblasts in vitro: modulation by basic fibroblast growth factor. Ann. Rheum. dis. 1997;56(7):426–431. doi: 10.1136/ard.56.7.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton E.Jr., Kandaswami C., Theoharides T.C.. The effects of plant flavonoids on mammalian cells: Implications for Inflamation, Heart disease, and cancer. Pharmacol. Rev. 2000;52(4):673–751. [PubMed] [Google Scholar]
- Moncada S., Palmer R.M.J., Higgs E.A.. Nitric oxide: physiology, pathophysiology and pharmacology. Pharmacol. Rev. 1991;43(2):109–142. [PubMed] [Google Scholar]
- Morais E.R., Oliveira K.C., Magalhaes L.G., Moreira É.B.C., Verjovski-Almeida S., Rodrigues V.. Effects of curcumin on parasite Schistosoma mansoni: A transcriptomic approach. Mol. Biochem. Parasitol. 2013;187:91–97. doi: 10.1016/j.molbiopara.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Moskowitz I.P. Rothman, J.H.. Lin-12 and glp-1 are required zygotically for early embryonic cellular interactions and are regulated by maternal GLP-1 signaling in Caenorhabditis elegans. Development. 1996;122(12):4105–4117. doi: 10.1242/dev.122.12.4105. [DOI] [PubMed] [Google Scholar]
- Murota K., Terao J.. Antioxidative flavonoid quercetin: implication of its intestinal absorption and metabolism. Arch. Biochem. Biophys. 2015;417(1):12–17. doi: 10.1016/s0003-9861(03)00284-4. [DOI] [PubMed] [Google Scholar]
- Naguleswaran A., Spicher M., Vonlaufen N., Ortega-Mora L.M., Torgerson P., Gottstein B., Hemphill A.. In vitro metacestodical activities of genistein and other isoflavones against Echinococcus multilocularis and Echinococcus granulosus. Antimicrob. agents chemother. 2006;50(11):3770–3778. doi: 10.1128/AAC.00578-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D.L., Cox M.M. Lehninger’sprinciples of biochemistry. 3rd Edition. New York: Worth Publications; 2000. p. 1152. [Google Scholar]
- Oyeyemi O., Adegbeyeni O., Oyeyemi I., Meena J., Panda A.. In vitro ovicidal activity of poly lactic acid curcumin-nisin co-entrapped nanoparticle against Fasciola spp. eggs and its reproductive toxicity. J. Basic Clin. Physiol. Pharmacol. 2018;29(1):73–79. doi: 10.1515/jbcpp-2017-0045. [DOI] [PubMed] [Google Scholar]
- Otero L., Bonilla M., Protasio A. V., Fernandéz C., Gladyshev V. N., Salinas G.. Thioredoxin and glutathione systems differ in parasitic and free-living platyhelminths. BMC Genomics. 2010;11:237. doi: 10.1186/1471-2164-11-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranagama P., Sakamoto K., Amino H., Awano M., Miyoshi H., Kita K.. Contribution of the FAD and quinone binding sites to the production of reactive exygen species from Ascaris suum mitochondrial complex II. Mitochondrion. 2010;10(2):158–165. doi: 10.1016/j-mito.2009.12.145. [DOI] [PubMed] [Google Scholar]
- Pereira C.A.J., Oliveira L.L.S., Coaglio A.L., Santos F.S.O., Ceyar R.S.M., Mendes T., Oliveira F.L.P., Conzensa G., Lima W.S.. Anti-helmintic activity of Momordica charantia L. against Fasciola hepatica eggs after twelve days of incubation in vitro. Vet. Parasitol. 2016;228:160–166. doi: 10.1016/j.vetpar.2016.08.025. [DOI] [PubMed] [Google Scholar]
- Pyszková M., Biler M., Biedermann D., Valentová K., Kuzma M., Vrba J., Ulrichová J., Sokolová R., Mojovic M., Popovic-Bijelic A., Kubala M., Trouillas P., Křen V., Vacek J.. Flavonolignan 2,3-dehydroderivates: Preparation. antiradical and cytoprotective activity, Free Radic Biol Med. 2016;90:114–125. doi: 10.1016/j.freeradbiomed.2015.11.01. [DOI] [PubMed] [Google Scholar]
- Rao H.S.P., Reddy K.S.. Isoflavones from Fleminga vestita. Fitoterapia. 1991;63:458. [Google Scholar]
- Rehman A., Ullah R., Gupta D., Khan M. A. H., Rehman L., Beg M. A., Khan A. U., Abidi S. M. A.. Generation of oxidative stress and induction of apoptotic like events in curcumin and thymoquinone treated adult Fasciola gigantica worms. Exp Parasitol. 2020;209:107810. doi: 10.1016/j.exppara.2019.107810. [DOI] [PubMed] [Google Scholar]
- Reynaud J., Guilet D., Terreux R., Lussignol M., Walchshofer N.. Isoflavonoids in non-leguminous families: an update. Nat. Prod. Rep. 2005;22:504–515. doi: 10.1039/b416248j. [DOI] [PubMed] [Google Scholar]
- Roberts L.S. Arme C, Pappas P.W. Biology of the eucestoda. New York, USA: Academic; 1983. Carbohydrate metabolism; pp. 343–390. [Google Scholar]
- Rolo A.P., Oliveira P.J., Moreno A.J., Palmeira C.M.. Protection against post-oschemic mitochondrial injury in rat liver by silymarin or TUDC. Hepatol. Res. 2003;26(3):217–224. doi: 10.1016/s1386-6346(03)00108-6. [DOI] [PubMed] [Google Scholar]
- Sakai Ch., Tomitsuka E., Esumi H., Harada S., Kita K.. Mitochondrial fumarate reductase as a target of chemotherapy: from parasites to cancer cells. Biochim Biophys Acta. 2012;1820:643–651. doi: 10.1016/j.bbagen.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Saller R., Brignoli R., Melzer J., Meier R.. An updated systematic review with meta-analysis for clinical evidence of silymarin. Forsch. Komplement. 2008;15(1):9–20. doi: 10.1159/000113648. [DOI] [PubMed] [Google Scholar]
- Schuffenhauer A., Varin T.. Rule-Based Classification of Chemical Structures by Scaffold. Mol. Inf. 2011;47:646–664. doi: 10.1002/minf.201100078. [DOI] [PubMed] [Google Scholar]
- Sethi N., Kang Y.. Notch signalling in cancer progression and bone metastasis. Br. J. Cancer. 2011;105(12):1805–1810. doi: 10.1038/bjc.2011.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R.A., Euden S.A., Platton S.L., Cooke D.N., Shafayat A., Hewitt H.R., Marczylo T.H., Morgan B., Hemingway D., Plummer S.M., Pirmoham ed M., Gescher A.J., Steward W.P.. Phase I clinical trial of oral curcumin biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004;10(20):6847–6854. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- Shehzad A., Qureshi M., Anwar N.M., Lee Y.S.. Multifunctional curcumin mediate multitherapeutic effects, J. Food Sci. 2017;82(9):2006–2015. doi: 10.1111/1750-3841.13793. [DOI] [PubMed] [Google Scholar]
- Shoop W. L., Mrozik H., Fisher M. H.. Structure and activity of avermectins and milbemycins in animal health. Vet Parasitol. 1995;59(2):139–156. doi: 10.1016/0304-4017(94)00743-v. [DOI] [PubMed] [Google Scholar]
- Smyth J.D., Mcmanus D. P.. The physiology and biochemistry of cestodes. Cambridge University Press, Cambridge. 398 pp. Sobhy, M.M.K., Mahmoud, S.S., El-Sayed, S.H., Rozk, E.M.A., Ra - fat, A., Negm, M.S.I. (2018): Imapact of treatment with a Protein Tyrosine Kinase Inhibitor (Genistein) on acute and chronic experimental Schistosoma mansoni infection. Exp. Parasitol. 1989;185:115–123. doi: 10.1016/j.exppara.2018.01.013. [DOI] [PubMed] [Google Scholar]
- Surai P.F.. Silymarin as a natural antioxidant: an overview of the current evidence and perspectives. Antioxidants. 2015;4(1):204–247. doi: 10.3390/antiox4010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekanecz Z., Koch A.E.. Macrophages and their products in rheumatoid arthritis. Curr. Opin. Rheumatol. 2007;19(3):289–295. doi: 10.1097/BOR.0b013e32805e87ae. [DOI] [PubMed] [Google Scholar]
- Tandon V., Pal P., Roy B., Rao H.S., Reddy K.S.. In vitro anthelmintic activity of root-tuber extract of Flemingia vestita, an indigenous plant in Shillong, India. Parasitol. Res. 1997;83(5):492–498. doi: 10.1007/s004360050286. [DOI] [PubMed] [Google Scholar]
- Tandon V., Bidyadhar D., Saha N.. Anthelmintic efficacy of Fleminga vestita (Fabaceae): effect of genistein on glycogen metabolism in the cestode, Raillietina echinobothrida. Parasitol. Int. 2003;52(2):179–183. doi: 10.1016/s1383-5769(03)00006-0. [DOI] [PubMed] [Google Scholar]
- Tandon V., Das B.. In vitro testing of anthelmintic efficacy of Fleminga vestita (Fabaceae) on carbohydrate metabolism in Raillietina echinobothrida. Methods. 2007;42(4):330–338. doi: 10.1016/j.ymeth.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Tandon V., Das B.. Genistein: is the multifarious botanical a natural anthelmintic too? J. Parasit. Dis. 2018;42(2):151–161. doi: 10.1007/s12639-018-0984-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomitsuka E., Kita K., Esumi H.. An anticancer agent, pyrvinium pamoate inhibits the NADH-fumarate reductase system-a unique mitochondrial energy metabolism in tumor microenvironments. J Biochem. 2012;152(2):171–183. doi: 10.1093/jb/mvs041. [DOI] [PubMed] [Google Scholar]
- Tousson E., Beltagy D.M., Gazia M.A., Al-Behbehani B.. Expressions of P53 and CD68 in mouse liver with Schistosoma mansoni infection and the protective role of silymarin. Toxicol. Ind. Health, 2. 2013;9(8):761. doi: 10.1177/0748233712442733. –. [DOI] [PubMed] [Google Scholar]
- Ullah R., Rehman A., Zafeer M.F., Rehman L., Khan Y.A., Khan M.A.H., Khan S.N., Khan A.U., Abidi S.M.A.. Anthelmintic Potential of thymoquinone and curcumin on Fasciola gigantica. Plos One. 2017;12(2):e0171267. doi: 10.1371/journal.pone.0171267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velebný S., Hrčková G., Kogan G.. Impact of treatment with praziquantel, silymarin and/or beta-glucan on pathophysiological markers of liver damage and fibrosis in mice infected with Mesocestoides vogae (Cestoda) tetrathyridia. J. Helminthol. 2008;82(3):211–219. doi: 10.1017/S0022149X08960776. [DOI] [PubMed] [Google Scholar]
- Velebný S., Hrčková G., Königová A.. Reduction of oxidative stress and liver injury following silymarin and praziquantel treatment in mice with Mesocestoides vogae (Cestoda) infection. Parasitol. Int. 2010;59:524–553. doi: 10.1016/j.parint.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Wang Y., Chen S., Yu O.. Metabolic engineering of flavonoids-rich Chinese bayberry (Mirica rubra Sieb. Et Zucc.) pulp extracts on glucose consumption in human HepG2 cells. J. Funct. Foods. 2015;14:144–153. [Google Scholar]
- Wang W., Sun C., Mao L., Ma P., Liu F., Yang J., Gao Y.. The biological activities, chemical stability, metabolism and delivery systems of quercetin: a review. Trends Food Sci. Tech. 2016;56:21–38. doi: 10.1016/j.tifs.2016.07.004. [DOI] [Google Scholar]
- Wang T.Y., Li Q., Bi K.S.. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. 2018;13(1):12–23. doi: 10.1016/j.ajps.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesolowska O., Lania-Pietrzak B., Kuzdzal M., Stanczak K., Mosiadz D. Dobryszycki, P Ozyhar, A Komorowska, M Hendrich, A.B. Michalak K.. Influence of silybin on biophisical properties of phospholippis bilayers. Acta Pharmacol. Sin. 2007;28(2):296–306. doi: 10.1111/j.1745-7254.2007.00487.x. [DOI] [PubMed] [Google Scholar]
- Yuan H., Ma Q., Ye L., Piao G.. The traditional medicine and modern medicine from natural products. Molecules. 2016;21(5):559. doi: 10.3390/molecules21050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Chen Q., Wang Y., Peng W., Cai H.. Effects of curcumin on ion channels and transporters. Front. Physiol. 2014;5:94. doi: 10.3389/fphys.2014.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Agarwal R.. Tissue distribution of silibinin. the major active consituent of silymarin, in mice and its association with enhancement of phase II enzymes: implications in cancer chemoprevention, Carcinogenesis. 1999;20(11):2101–2108. doi: 10.1093/carcin/20.11.2101. [DOI] [PubMed] [Google Scholar]
- Zhou S., Hu Y., Zhang B., Teng Z., Gan H., Yang Z., Qungwei W., Huan M., Mei Q.. Dose-dependent absorption. metabolism, and excretion of genistein in rats. 2008;56(18):8354–8359. doi: 10.1021/jf801051d. [DOI] [PubMed] [Google Scholar]