Summary
A growing need exists for reliable in vivo measurement of neuroinflammation to better characterize the inflammatory processes underlying various diseases and to inform the development of novel therapeutics that target deleterious glial activity. Positron emission tomography (PET) is well suited to quantify neuroinflammation and has the potential to discriminate components of the neuroimmune response. However, PET imaging is not currently used to measure neuroinflammation in clinical practice, in part because of the complexity of the brain’s varied immune responses and the technical challenges associated with reliable quantification. Despite these challenges, PET studies have consistently identified associations between neuroimmune response and pathophysiology in Alzheimer’s disease and chronic traumatic encephalopathy. Recently, positive results have been observed with second-generation radioligands as markers of immune response in immune-mediated diseases, such as multiple sclerosis and HIV-related cognitive impairment, as well as in neurodegenerative disorders, epilepsy, and stroke. A small but growing number of studies have similarly suggested that PET imaging of neuroinflammation could play a role in drug discovery. However, interpreting neuroinflammation PET studies remains somewhat controversial because of limited understanding regarding the cellular mechanisms that underlie changes in PET signal. Future studies are needed to improve our knowledge of how immune response contributes to neurological disease and how it might be therapeutically modified.
1.0. Introduction
Recent discovery of glial-expressed risk variants associated with neurodegenerative diseases1, coupled with increasing evidence supporting neuroimmune modulation as a strategy for drug development, underscore the critical need for reliable in vivo measurement of neuroinflammation, which would enable research into the neuroimmune mechanisms that contribute to neurological disease and inform clinical trial design. Due to its ability to measure select proteins at low concentrations, positron emission tomography (PET) is particularly well-suited to quantify neuroinflammation and has the potential to discriminate components of the neuroimmune response (Figure 1). However, obstacles to reliable PET measurement of neuroinflammation include misperceptions and limitations regarding 18 kDa translocator protein (TSPO; the most common neuroinflammatory target), such as high nonspecific binding and sensitivity to a genetic polymorphism that affects binding affinity of early TSPO radioligands, as well as a paucity of non-TSPO targets with validated radioligands. In addition, the only validated non-TSPO target in recent use is the astrocyte-expressed protein monoamine oxidase B (MAO-B), which has its own limitations (e.g., expression by neurons).
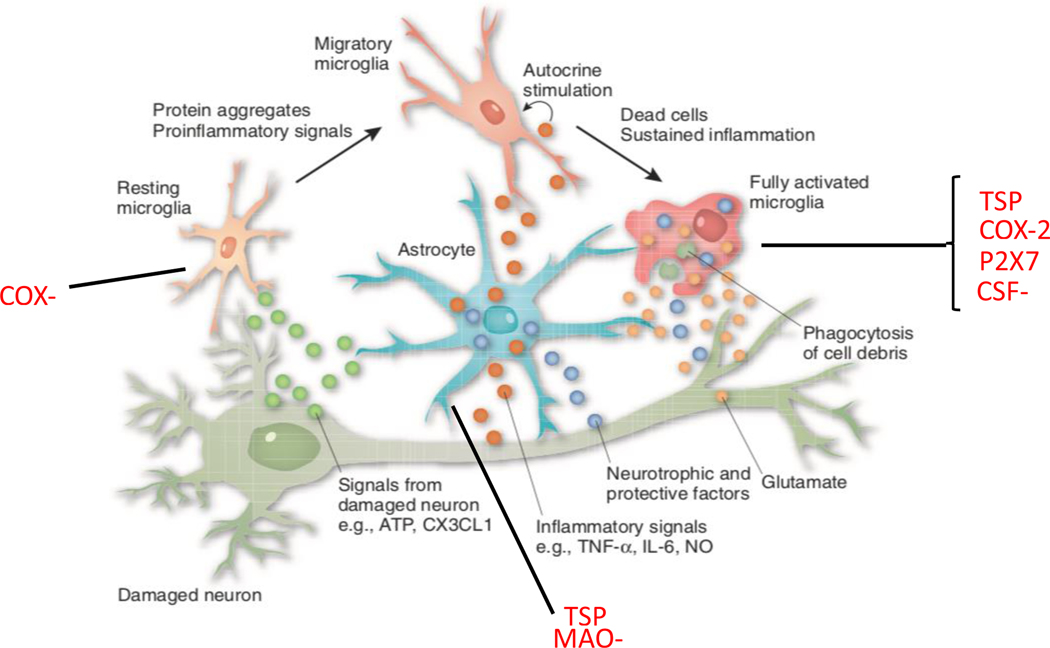
Figure 1. Neuroinflammation.
Relationship between neurons and glial cells (microglia and astrocytes). Microglia become activated in response to several immunological signals, including from cytokines and aggregated proteins. This activation may be either protective or toxic for the surrounding glial cells and neurons. Proteins expressed by glial cells are established and proposed targets for positron emission tomography (PET) imaging to quantify neuroinflammation (red text).
Abbreviations: ATP: adenosine triphosphate; CX3CL1: CX3C chemokine ligand 1; TNF-alpha: tumor necrosis factor alpha; IL-6: interleukin-6; NO: nitric oxide; TSPO: 18 kDa translocator protein; COX: cyclooxygenase; P2X7R: P2X purinergic receptor 7; CSF-1R: colony stimulating factor 1 receptor; MAO-B: monoamine oxidase B.
Modified with permission from.128
Fortunately, there are several reasons for optimism. First, combining TSPO PET with other biomarkers has provided insights into the temporal and spatial relationships between neuroinflammation and the canonical pathologies underlying brain disorders such as Alzheimer’s disease. Second, tissue studies have begun to clarify the meaning of increased TSPO PET signal in certain diseases. Third, improved TSPO radioligands, particularly [11C]ER176, have overcome some of the major disadvantages of earlier tracers. Finally, novel non-TSPO targets are under investigation, and several radioligands are in various stages of early development.
This review provides a critical assessment of the role of PET imaging of neuroinflammation in neurological disorders. Rather than provide an exhaustive, historical list of all published PET studies, the review only discusses disorders for which at least one positive study was conducted using a second-generation TSPO radioligand. These include multiple sclerosis (MS), HIV-associated cognitive impairment, Alzheimer’s disease (AD), frontotemporal dementia, chronic traumatic encephalopathy (CTE), Huntington’s disease, amyotrophic lateral sclerosis, epilepsy, and stroke. Other disorders such as corticobasal degeneration, progressive supranuclear palsy, and dementia with Lewy bodies were excluded because they have not yet been studied with second-generation TSPO radioligands. In addition, only negative studies using second-generation radioligands in Parkinson’s disease (PD) have been published; however, the possible role of TSPO PET in drug discovery for PD is discussed. Disorders are discussed based on similarities in terms of proposed relatedness to neuroinflammatory response. Classic immune-related disorders are discussed first, followed by neurodegenerative disorders, and then epilepsy and stroke.
2.0. Overcoming obstacles to identifying neuroinflammation using PET imaging
In our experience, the major obstacles to reliable PET measurement of neuroinflammation include misperceptions regarding TSPO as a biomarker, the limitations associated with early TSPO radioligands, and the paucity of non-TSPO targets with validated radioligands. Fortunately, recent multimodal imaging and tissue studies have increased our understanding of the meaning of the TSPO PET signal, and radioligand development has improved the ability to measure TSPO in vivo.
Although widely used as an inflammatory biomarker, TSPO has important caveats that require consideration to avoid misinterpreting PET imaging results. First, while TSPO is predominantly expressed in brain by microglia,2 expression by other cell types must be considered. TSPO was originally found in peripheral tissue,3 and is also expressed in brain by astrocytes4 and vascular endothelium.5 Migration of peripheral myeloid cells into brain can also contribute to the TSPO signal.6 Therefore, the relative contribution of TSPO radioligand binding by microglia versus other cells depends on the disease studied. For example, some,7, 8 though not all,9 autopsy studies have shown that microglia are the predominant TSPO-positive cells in the brain of individuals with AD. Similarly, TSPO in human MS lesions is mostly expressed in microglia but also in astrocytes, though to a lesser extent.5 However, disease stage can, in some cases, also influence cellular expression of TSPO. For instance, in an experimental stroke models in rats, TSPO-expressing microglia were first found in the ischemic lesion; days later, TSPO-expressing astrocytes were found in the surrounding area.10 In addition, the meaning of increased TSPO binding remains controversial, even within the proportion of signal due to microglia. Rodent studies found that increased TSPO expression signals a shift from resting to activated morphology in microglia, resulting in the widely held view that increased TSPO binding equates to microglial activation.11 However, human tissue studies do not necessarily support this view. Pro-inflammatory conditions did not increase TSPO expression in a study using human microglia, suggesting that radioligand binding may reflect microglial density rather than microglial phenotype.12 Furthermore, while human autopsy studies identified TSPO-expressing microglia proximal to neuritic plaques in the AD brain,7, 8 one AD study demonstrated that area fraction of TSPO immunoreactivity did not correlate with that of microglial activation (defined as CD68 immunoreactivity).9 In addition, while increased TSPO immunoreactivity was detected in brain and spinal cord tissue from individuals with MS, TSPO was found in both pro-inflammatory and anti-inflammatory microglia (defined by co-expression of CD40 or CD206, respectively).5 Nevertheless, immunohistochemistry results do not always agree with PET results. TSPO antibodies attach to the C terminus of the target protein, while radioligands bind to its active site. Furthermore, autoradiography and PET represent the available number of binding sites, not just the total amount of protein, and both techniques are inherently more quantifiable than immunostaining. Therefore, while TSPO binding should not be broadly assumed to reflect the extent of microglial activation, disease- and species-specific tissue studies should guide the interpretation of increased TSPO signal.
Another challenge intrinsic to TSPO PET is determining which radioligand to use, given the tracers available and their varying limitations. The prototypical radioligand [11C]-(R)-PK11195 has low signal-to-noise ratio, which limits its ability to detect subtle changes in TSPO density.13 While second-generation radioligands have improved ratios of specific-to-nonspecific binding, they are sensitive to a common polymorphism (rs6971) in the TSPO gene.14 Individuals with two copies of the rare allele (low affinity binders) bind these radioligands with a lower affinity than those with two copies of the major allele (high affinity binders), and heterozygotes (mixed affinity binders) express both high and low binding sites in similar proportions. Thus, individuals with the same TSPO density but different genotypes will produce different PET signals. This obstacle has been addressed by performing TSPO genotyping prior to imaging (to exclude low affinity binders) and by including binding affinity as a statistical covariate.15, 16 One study compared four carbon-11-labeled TSPO radioligands ([11C]-(R)-PK11195, [11C]PBR28, [11C]DPA-713, and [11C]ER176) and found that [11C]DPA-713 had the greatest signal-to-noise ratio in human brain.17 However, [11C]ER176 also had a high signal-to-noise ratio, was least likely to generate brain-penetrant radiometabolites, and was sufficiently insensitive to the rs6971 polymorphism to allow reliable TSPO measurement in low affinity binders.17 Thus, current evidence suggests that [11C]ER176 is the best available TSPO radioligand. While [18F]GE180 has been described as a “third-generation” TSPO radioligand, this tracer has unfavorable kinetics for human brain imaging due to low penetration into brain from the vascular compartment;18 thus, we do not advise use of this tracer.
Another potential obstacle for TSPO PET is lack of a true reference region. TSPO is diffusely expressed throughout the brain, and accurate measurement of its density relies on kinetic modeling using the metabolite-corrected arterial input function (AIF). Different methods have attempted to circumvent the need for arterial sampling, including cluster sampling techniques19 and the use of “pseudo-reference” regions.20, 21 Although AIF-free methods inherently introduce bias, several such studies have detected increased TSPO in various neurological disorders, with colocalization to abnormalities in other biomarkers.22, 23 In addition, AIF-free methods often reduce variance caused by inter-subject differences in physiological TSPO expression, thus improving power for statistical analysis.21 Because binding behavior may differ among different radioligands and diseases, we recommend validating pseudo-reference methods against arterial sampling prior to their application. The diffuse nature of TSPO is less of an issue in focal disorders such as stroke, where contralateral tissue of the same volume can be used for comparison. However, Wallerian degeneration or diaschisis may affect regions distant to focal injury,24 potentially influencing results.
Finally, although [11C]ER176 has emerged as the preferred TSPO radioligand, TSPO appears to reflect a broad spectrum of immune responses; thus, more precise targeting of inflammatory mechanisms will require novel radioligands for novel biomarkers. The only non-TSPO radioligand in recent usage is [11C]deuterium-L-deprenyl, a radioligand for MAO-B. However, while MAO-B is expressed by astrocytes, it is also expressed by pyramidal neurons in AD brain,25 and physiological signal in basal ganglia—due to its high dopamine turnover—may limit sensitivity to detect changes in this region.26 Novel inflammatory targets include cyclooxygenase-1 (COX-1) and COX-2, colony stimulating factor 1 receptor (CSF1-R), and the P2X purinergic receptor 7 (P2X7R). To date, no radioligand for any of these targets has been fully validated in human disease although each shows promise for more precisely measuring neuroimmune response. Discussion of these emerging targets can be found in our companion review in The Lancet Psychiatry.27 Table 1 summarizes the neuroinflammatory radioligand studies performed in humans in the last five years, noting those using non-TSPO radioligands, arterial sampling, or autopsy tissue, as well as studies in which neuroinflammation PET has been incorporated into clinical trials.
Table 1.
Human studies using neuroinflammatory radioligands 2015–2020
| Disorder | Radioligands used | Studies using absolute quantification | Autopsy studies | Use in clinical trials† | Key findings |
|---|---|---|---|---|---|
| Multiple sclerosis | • [11C]-(R)-PK11195 • [11C]PBR28 • [18F]PBR-111 |
• [18F]GE-180117, 118 • [18F]DPA-714119 • [11C]PBR28120 |
___ | • [11C]-(R)-PK1119535, 37, 38 | • TSPO decreased after disease modifying treatment35, 37, 38 • TSPO predicts new lesions20, 28 |
| HIV-associated cognitive impairment | • [11C]-(R)-PK11195 • [11C]PBR28 • [11C]DPA-713 |
• [11C]PBR2845 • [11C]DPA-71346 |
___ | ___ | • Regional TSPO binding associated with domain-specific cognitive impairment40, 45 |
| Alzheimer’s disease | • [11C]-(R)-PK11195 • [11C]PBR28 • [18F]FEPPA • [11C]DAA1106 • [18F]DPA-714 • [11C]DPA-713 • [11C]deuterium-L-deprenyl* |
• [11C]PBR2821, 59, 121, 122 • [18F]FEPPA63, 123, 124 • [18F]DPA-714**125 |
• [11C]-(R)-PK11195126 | ___ | • MAO-B increased in presymptomatic AD48 • TSPO increased in amyloid-positive controls23, 51 • TSPO correlates with tau pathology22, 23, 54 |
| Frontotemporal dementia | • [11C]-(R)-PK11195 • [11C]PBR28 |
• [11C]PBR2872 | • [11C]-(R)-PK1119573 | ___ | • Patterns of TSPO binding discriminate FTD subtypes73 |
| Chronic traumatic encephalopathy / traumatic brain injury | • [11C]PBR28 • [11C]DPA-713 |
• [11C]PBR2878 • [11C]DPA-71380, 81 |
___ | • [11C]PBR2878 | TSPO increased in medial temporal cortex and supramarginal gyrus in active and former NFL players80, 81 |
| Huntington’s disease | • [11C]-(R)-PK11195 • [11C]PBR28 |
___ | ___ | ___ | • TSPO increased in presymptomatic mutation carriers86 |
| Amyotrophic lateral sclerosis | • [11C]PBR28 • [18F]DPA-714 • [11C]deuterium-L-deprenyl* • [11C]JNJ717* |
• [18F]DPA-71496 • [11C]JNJ717*96 • [11C]PBR28127 |
• [18F]DPA-71496 • [11C]JNJ717*96 |
• [11C]PBR2895 | • TSPO correlates with changes in white matter integrity and cortical atrophy91–93 |
| Epilepsy | • [11C]-(R)-PK11195 • [11C]PBR28 • [11C]DPA-713 |
• [11C]PBR28101, 102 • [11C]DPA-713101, 102 |
___ | ___ | • TSPO increased ipsilateral to seizure foci in temporal lobe epilepsy101, 102 |
| Stroke‡ | • [11C]-(R)-PK11195 | ___ | ___ | ___ | • TSPO increased around hematoma in two-fifths of patients with acute intracerebral hemorrhage108 |
non-TSPO radioligand; binds to monoamine oxidase B (MAO-B), a proposed marker of astrocytosis.
Second-generation TSPO radioligand study where TSPO genotype correction was not performed.
Jucaite and colleagues110 found reduced [11C]PBR28 binding in individuals with Parkinson’s disease after treatment with a novel myeloperoxidase inhibitor; however, PET studies published after 2015 using either [11C]PBR28 or [18F]FEPPA111, 112 failed to show increased TSPO in individuals with Parkinson’s disease.113
3.0. Neuroimmunological and infectious disorders
3.1. Multiple Sclerosis
Most PET studies quantifying neuroinflammation in MS have used TSPO as the PET target. Numerous studies have found increased TSPO expression in MRI-defined white matter lesions in individuals with relapsing-remitting MS (RRMS) or secondary progressive MS (SPMS).28–31 The fact that white matter lesions, which are indistinguishable on MRI, have different patterns with TSPO PET suggests that TSPO PET can detect pathophysiological heterogeneity to which MRI is insensitive.28, 29 As may be expected, the molecular changes detected by TSPO PET precede the structural changes detected by MRI.31 In addition to the higher signal in lesions, non-lesional white matter in MS also shows greater TSPO signal than in age-matched controls.28, 32, 33 This increase is associated with greater brain atrophy and worse disability.20, 29, 34 Higher signal also predicts the appearance of new lesions, worsening brain atrophy, and a more severe trajectory of disability worsening over the subsequent 12 months.20, 28 Increased signal in cortical grey matter has also been detected, which again correlates with disability and cognitive impairment.32, 34 The effect of standard medications (including glatiramer acetate, fingolimod, and atalizumab) on TSPO signal has been assessed in a handful of studies, all of which have shown modest reductions in the signal in either lesional or non-lesional white matter.35–38
While previous neuropathology studies identified activated microglia as the source of TSPO in lesions,8 a recent comprehensive assessment of post-mortem MS brain demonstrated that TSPO is not preferentially expressed on activated microglia but, rather, is found equally across all microglial phenotypes.5 Furthermore, although microglia are the main contributors to TSPO signal in white matter lesions, a substantial contribution (~25%) is made from astrocytes and, to a lesser extent, endothelial cells.5 It should also be noted that TSPO PET studies in MS participants relative to controls identified much smaller differences than might be expected from pathological investigations. This could be due to the relatively small size of the lesions or could reflect differences in the configuration of TSPO between in vitro and in vivo states.
3.2. HIV Cognitive Impairment
Cognitive impairment remains prevalent among individuals infected with human immunodeficiency virus (HIV), including those using effective antiretroviral therapy.39, 40 Microglial activation and reactive astrocytosis are among the posited contributing factors to cognitive impairment in HIV. One [11C]-(R)-PK11195 PET study found higher regional binding in HIV+ participants (cognitively impaired and unimpaired) relative to uninfected controls, with the highest binding observed in those with HIV-associated dementia.41 A second [11C]-(R)-PK11195 PET study found higher binding in cognitively intact HIV+ participants compared to uninfected controls,42 while a third study found no difference between HIV+ individuals (cognitively impaired or unimpaired) and controls.43 For studies using the second-generation radiotracers [11C]DPA-71344 or [11C]PBR28,45 higher regional binding was found in virally-suppressed HIV+ individuals compared to uninfected controls. Those with HIV-associated dementia had higher [11C]DPA-713 binding in frontal cortex,44 and subsequent analyses using data from HIV+ individuals revealed inverse correlations between regional [11C]DPA-713 binding and performance in particular cognitive domains.46 In HIV+ individuals, [11C]PBR28 PET also revealed region-specific (hippocampus, thalamus) associations between higher binding and lower performance in memory and verbal learning.45 Inconsistencies between these studies may stem from differences between radioligands, clinical characteristics within patient and control groups, and analytic methods. Nevertheless, taken together the data suggest that TSPO may be regionally elevated and linked to domain-specific cognitive impairment in treated HIV.
4.0. Neurodegenerative disorders
4.1. Alzheimer’s disease (AD)
Both human and preclinical PET studies have linked neuroinflammation with AD pathology. Most TSPO PET studies have shown increased binding in AD participants compared to controls, particularly in fronto-temporal regions, with more modest increases observed in neocortical regions in individuals with mild cognitive impairment (MCI) (see47 for a meta-analysis). Presymptomatic carriers of autosomal dominant AD mutations48 showed increased binding as assessed using [11C]deuterium-L-deprenyl, a radioligand for MAO-B. While these studies suggest astrocytosis as a pathological entity in early-stage AD, MAO-B is also expressed by pyramidal neurons in AD brain.25 Transgenic AD mouse studies found increased TSPO and MAO-B binding on PET, often confirmed with autoradiography.49, 50
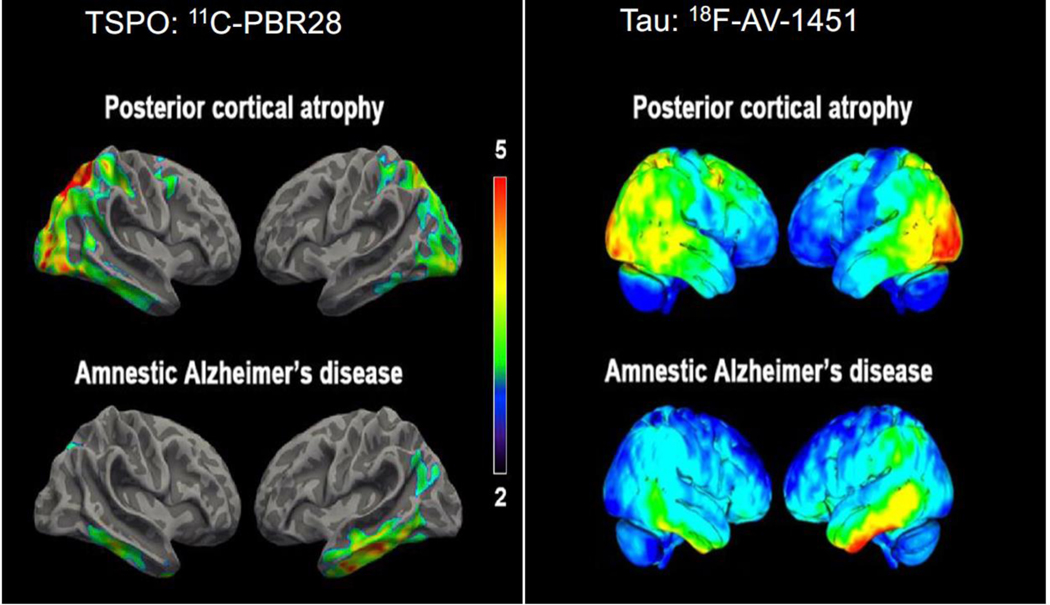
The exact pathological stimulus for increased TSPO in AD remains unclear. Studies have observed increased TSPO binding in asymptomatic individuals with incidental amyloid positivity23, 51 and in participants meeting clinical criteria for amnestic MCI or mild AD with absence of amyloid binding on PET.23, 52 This suggests that TSPO may increase in response to both amyloid deposition and amyloid-independent neurodegeneration. Multimodal PET studies have looked for spatial correlations between TSPO binding and both amyloid plaque and neurofibrillary tau burden. Studies comparing TSPO and amyloid binding have been inconsistent, showing no correlation,15, 53 positive correlations,51, 54–56 and negative correlations.57 Nevertheless, three of four studies found positive correlations between TSPO and tau binding.22, 23, 54, 58 One study identified distinct patterns of TSPO binding in different clinical variants of AD,59 similar to previously reported patterns of tau pathology60 (Figure 2). Notably, both TSPO and tau binding were increased in younger AD participants than in older ones.61, 62 Therefore, TSPO may have a stronger relationship with tau than with amyloid, at least during the clinical stages of AD.
Figure 2. TSPO and tau imaging: Alzheimer’s Disease (AD) versus Posterior Cortical Atrophy (PCA).
Topographical distribution of translocator protein (TSPO) resembles that of tau pathology in different clinical subtypes of Alzheimer’s disease (AD). Left panel: Surface-based projection maps showing differences in [11C]PBR28 binding (measured as standardized uptake value ratio (SUVR), cerebellar reference) between individuals with AD and age-matched controls for posterior cortical atrophy (PCA, a visual variant of AD, top) and typical amnestic presentation of AD (bottom). Contrast threshold is P < 0.05 after family-wise correction for multiple comparisons and TSPO genotype, age, and education as covariates. Color bars denote T-values. Right panel: Single-subject PET SUVR images from a separate study in which [18F]AV-1451 was used to label neurofibrillary tau deposits. Representative participants with PCA (top) or amnestic AD (bottom) are shown. [11C]PBR28 images adapted from59 and [18F]AV-1451 images adapted from.60 The [18F]AV-1451 images are printed and adapted by permission of Oxford University Press on behalf of the Guarantors of Brain. OUP and the Guarantors of Brain are not responsible or in any way liable for the accuracy of the adaptation.
Whether increased TSPO binding in the early stages of AD represents a beneficial or maladaptive glial response remains controversial. Cross-sectional studies in AD found that TSPO binding is associated with worse cognitive impairment.15, 63 In addition, the first longitudinal TSPO PET studies in AD showed overall increased binding as the disease advanced.61, 64 However, amyloid-positive individuals with MCI had greater [18F]DPA-714 binding than individuals with AD, and greater [18F]DPA-714 binding was associated with higher Mini-Mental State Exam (MMSE) score.51 Another study found that six of eight individuals with MCI (four of whom were amyloid-negative) showed a mean reduction in [11C]-(R)-PK11195 binding at follow-up.65 The authors interpreted these results as evidence for a bimodal pattern of neuroimmune activation in AD, with a beneficial phase of glial behavior occurring prior to dementia onset followed by a detrimental phase of pro-inflammatory glial activity that worsened throughout the dementia stage.66 In another study, [11C]deuterium-L-deprenyl binding decreased over time in individuals with autosomal dominant AD, although no change was seen in individuals with sporadic MCI.67 That MCI participants with higher [11C]PBR28 binding had less cortical atrophy on MRI68 supports the notion that TSPO-expressing microglia may have a beneficial effect early in AD. However, the results from that study could be interpreted another way, given that larger cortical volume is a marker of “brain reserve”—the ability to retain cognitive function despite increasing pathology.69 In that context, MCI participants with less atrophy could be more resilient to the damaging effects of microglial and/or astrocyte activation, allowing a similar degree of cognitive impairment as those with more atrophy despite greater amounts of TSPO binding. Alternatively, inflammation-induced neuronal and glial swelling could result in increased cell volume, as posited by one [11C]deuterium-L-deprenyl study showing that MAO-B binding was associated with greater cortical thickness.70 The bimodal hypothesis of TSPO binding in AD progression has not been consistently supported in the literature, given that [18F]DPA-714 binding was found to increase in both MCI and AD participants over time,55 and several studies have observed a more or less linear increase in PBR28 binding across the clinical AD spectrum.15, 21, 23
4.2. Frontotemporal dementia
PET studies have consistently observed increased TSPO binding in individuals with clinically-diagnosed frontotemporal dementia (FTD). Using a reference region method, the first study found that five FTD participants showed an average increase in [11C]-(R)-PK11195 binding in left dorsolateral prefrontal cortex, right hippocampus, right parahippocampus, and bilateral putamen compared to eight controls.71 An [11C]PBR28 study that used arterial sampling found increased binding in four individuals with FTD and further confirmed the lack of comorbid AD pathology with amyloid PET (Figure 3).72 While participants lacked genetic or neuropathological determination of underlying histopathology, the pattern of [11C]PBR28 binding mirrored that of FDG hypometabolism. In both studies, the patients had varied clinical presentations and patterns of atrophy on MRI. In a larger study, the topographic pattern of [11C]-(R)-PK11195 binding discriminated FTD subtypes from each other and from controls.73 That study also found that, in autopsy tissue, the density of microglia, particularly in those with activated morphology, correlated with the extent of abnormal protein aggregation (phosphorylated tau or TAR DNA-binding protein 43).
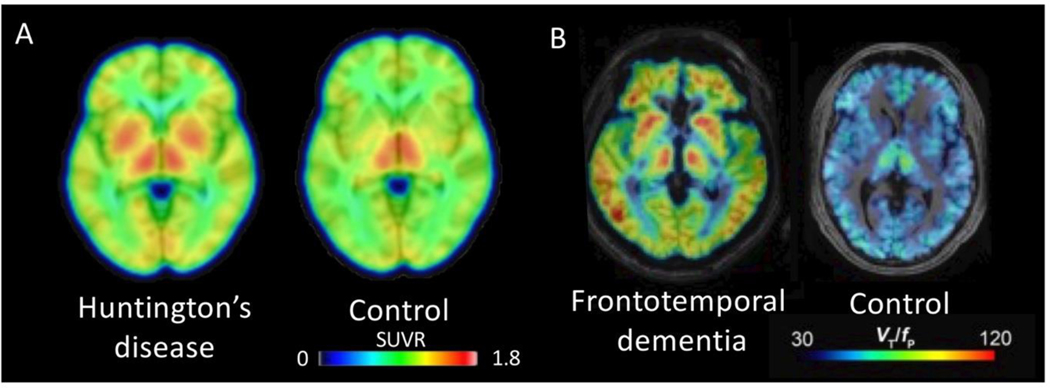
Figure 3. TSPO imaging in Huntington’s Disease (HD) and Frontotemporal Dementia (FTD).
(A) Averaged [11C]PBR28 PET images from three controls and seven individuals with Huntington’s disease (HD). PET images represent standardized uptake value ratio (SUVR; normalized to whole brain activity) using images acquired 60–90 minutes post-injection. Increased binding in bilateral basal ganglia was found in the HD participants. (B) Representative [11C]PBR28 PET images from an individual with frontotemporal dementia (FTD) and an age-matched control, both high affinity binders. Images represent total distribution volume, corrected for free fraction of radioligand in plasma (VT/fP). Increased binding was most notable in frontal and temporal lobes. Adapted from.72, 87 Reprinted with permission from,87 copyright 2018 American Chemical Society.
4.3. Chronic Traumatic Encephalopathy
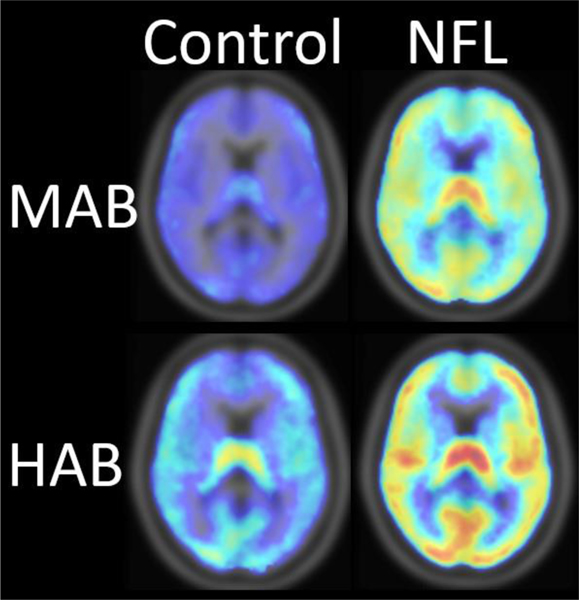
Chronic traumatic encephalopathy (CTE) is pathologically defined by deposits of phosphorylated tau in a perivascular distribution, particularly in the depths of cortical sulci.74 CTE has been found in human brain following traumatic brain injury (TBI), including sports-related, repetitive concussion incurred through American football.74, 75 Prolonged microglial activation after repeated TBI has been hypothesized to contribute to CTE.76 Indeed, increased [11C]-(R)-PK11195 or [11C]PBR28 binding has been reported in the brains of TBI participants,77, 78 even years after injury.79 In addition, higher [11C]DPA-713 binding was found in young NFL players in medial temporal cortex and supramarginal gyrus (Figure 4).80 Relatively high levels of [11C]DPA-713 binding were also observed in the supermarginal gyrus in a study of older players decades after their last NFL play.81 Whole brain images included in each [11C]DPA-713 PET study80, 81 suggested widespread distribution of high TSPO beyond the examined regions of interest. However, the clinical implications of high TSPO signal in former NFL players remain elusive. No cognitive deficits were found in the cross-sectional population of active and recently former NFL players with high [11C]DPA-713 binding.80 Longitudinal investigation of the relationship between neuroimmune activation marked by high TSPO and TBI-associated behavioral decline is needed.
Figure 4. TSPO imaging of Chronic Traumatic Encephalopathy (CTE).
Compared to demographically- and genotype-matched controls, binding of [11C]DPA-713 in gray matter was 53% higher in the brains of former National Football League (NFL) players with the mixed affinity binding (MAB) genotype and 34% higher in NFL players with the high affinity binding (HAB) genotype. Comparative mean [11C]DPA-713 binding [total distribution volume (VT)] is displayed for individuals with the MAB genotype (upper panel, six controls, five NFL players) and those with the HAB genotype (lower panel, five controls, seven NFL players). Adapted from.80
4.4. Huntington’s Disease
The neurodegenerative disorder Huntington’s disease (HD) is associated with increased gliosis and expression of glial fibrillary acidic protein (GFAP) and complement proteins, particularly in the striatum.82 Increased pro-inflammatory cytokines are found in HD gene carriers.83
Early PET studies showed that asymptomatic gene carriers and HD participants had increased [11C]-(R)-PK11195 binding.84, 85 In a more recent study, pre-manifest HD carriers had greater [11C]-(R)-PK11195 binding in cortical, basal ganglia, and thalamic brain regions;86 radioligand binding in somatosensory cortex also correlated with plasma concentrations of interleukin-1β (IL-1β), IL-6, IL-8, and tumor necrosis factor (TNF)-α. Second-generation TSPO radioligands have shown similar results. For instance, one study found that HD participants had greater [11C]PBR28 binding in putamen and pallidum than controls (Figure 3);87 however, arterial sampling was not performed, and only relative [11C]PBR28 binding was reported (using whole brain as a reference region). While a similar approach has been used in [11C]PBR28 studies of chronic pain88 and amyotrophic lateral sclerosis (ALS),89 this methodology has not been validated against a “gold-standard” kinetic modeling approach.
4.5. Amyotrophic Lateral Sclerosis
An [11C]-(R)-PK11195 study first identified increased TSPO binding in motor cortex and associated brain regions of individuals with ALS,90 and the finding has since been reproduced using second-generation TSPO radioligands. Interestingly, increased TSPO binding in motor cortex significantly correlated with severity of upper motor neuron symptoms with both [11C]-(R)-PK11195 and [11C]PBR28.89, 90 Furthermore, studies using larger populations of individuals with either ALS or primary lateral sclerosis (PLS) reported that increased [11C]PBR28 binding in motor cortex also correlated with other MR image parameters such as diffusion tensor imaging and cortical thickness measured both cross-sectionally and longitudinally.91–93 [18F]DPA714 studies also found increased TSPO binding in the motor cortex, although these did not evaluate correlation with clinical severity.94 As an extension of these findings, a therapeutic trial using [11C]PBR28 PET as a biomarker of neuroinflammation was conducted in individuals with ALS, but the results identified no difference between pre- and post-treatment TSPO uptake, possibly due to low statistical power.95 With regard to radioligands targeting other neuroinflammatory mediators than TSPO, a preliminary result with [11C]JNJ717, a P2X7 radioligand, found no such increase in ALS participants.96
5.0. Epilepsy
Several PET studies targeting TSPO support the role of neuroinflammation in epileptogenesis or ictogenesis, although most of these studies were based on small sample sizes. Initial case reports with [11C]-(R)-PK11195 PET imaging demonstrated a focal increase of TSPO uptake co-localized with the seizure focus;97, 98 a subsequent study revealed that this uptake had even greater intensity and spatial extent in post-seizure status (~36 hours) compared to the seizure-free period, perhaps due to transient seizure-induced inflammation.99
In more extensive studies conducted with second-generation TSPO radioligands, [11C]PBR28 uptake was found to be higher ipsilateral to the seizure focus in 16 participants with unilateral temporal lobe epilepsy (TLE).100 Using full quantitation of TSPO binding, the same group demonstrated that [11C]PBR28 binding was higher in TLE participants than healthy controls for all ipsilateral as well as some contralateral temporal regions.101 When the same evaluation was done in participants with neocortical seizure foci, nine of 11 participants had significant asymmetry in seizure foci, although the absolute binding levels in patients did not significantly differ from those in healthy controls.102
PET imaging of neuroinflammation in epilepsy has thus far investigated pathophysiology without correlating it with clinical severity or prognosis, suggesting that additional studies with full quantitative methods are needed before clinical application.
6.0. Stroke
Studies using [11C]-(R)-PK11195 in acute ischemic stroke observed widespread TSPO binding at the primary infarct site and in the peri-infarct lesions.103 During the chronic phase, increased TSPO binding also involved sites distant from the primary stroke lesion, perhaps as a result of Wallerian degeneration of neuronal tracts.103, 104 One case report found increased binding in a subacute lacunar infarction as assessed via [11C]PBR28.105 Using [11C]vinpocetine, a radioligand that binds with moderate affinity to TSPO but has favorable brain penetration, another study also demonstrated increased TSPO binding in the peri-stroke region for several weeks after ischemic stroke.106 An [18F]DPA714 study in nine individuals with recent ischemic stroke found co-localized uptake within areas of ischemic infarction as well as extension beyond the region corresponding to blood-brain barrier damage.107
In contrast to such well-reproduced findings in acute or subacute ischemic stroke, hemorrhagic stroke has rarely been investigated with PET imaging of neuroinflammation. One [11C]-(R)-PK11195 study reported low uptake in hematomas, with two of five participants showing widespread increases in TSPO binding in the perihematomal region compared to the contralateral hemisphere.108 However, it remains unknown whether these peri-lesional or distant increases of TSPO are associated with any favorable or unfavorable clinical outcomes.
7.0. Neuroinflammation PET in drug development
Although TSPO PET does not have an established clinical application, evidence supports potential use in drug development. First, TSPO density may predict treatment response in some instances. For example, greater TSPO binding in major depressive episodes was associated with greater reduction of symptoms after treatment with celecoxib,109 suggesting that TSPO PET may play a potential role in participant stratification, similar to how amyloid PET is currently employed to select participants for AD trials. Second, TSPO has served as a surrogate biomarker in proof-of-concept studies, although some results have been difficult to interpret. For instance, while minocycline treatment led to reduced [11C]PBR28 binding in individuals with brain trauma, it also increased plasma concentrations of neurofilament light chains, raising the question of whether reduced TSPO signal is necessarily beneficial.78 In another example, after treatment with a myeloperoxidase inhibitor, individuals with PD showed mean reductions in [11C]PBR28 binding in nigro-striatal regions, with a 13·2–15·7% decrease in distribution volume (VT) from baseline.110 However, similar decreases were found in all other measured brain regions, indicating a global effect on TSPO binding and raising the question of whether the treatment resulted in a change in microglial (or astrocyte) function or just depletion of TSPO protein. Notably, despite three well-designed studies using [11C]PBR28 or [18F]FEPPA, no second-generation TSPO study has shown increased binding in individuals with PD.111–113 Conversely, the same myeloperoxidase inhibitor failed to reduce [11C]PBR28 binding in individuals with multiple system atrophy (MSA)114. Phase 3 studies are needed to determine whether these changes in TSPO binding equate to clinical efficacy. Emerging non-TSPO biomarkers are also expected to be useful in evaluating novel treatments, particularly those targeting the same protein as the radioligand. For example, CSF1R radioligands could be used for target engagement studies of CSF1R antagonists.
8.0. Conclusions and future directions
While neuroinflammatory PET has largely been limited to targeting TSPO, this imaging modality has far-reaching potential. Although emerging non-TSPO radioligands may soon allow more precise investigation of the mechanisms underlying specific immune response, for the time being TSPO PET remains the most extensively studied method for spatial measurement of neuroinflammation.
In neurology, the most consistent TSPO imaging results have arguably been in: 1) AD, where inflammation may more closely reflect the distribution of tau than of amyloid and where neuroinflammation may have a meaningful—though not necessarily linear—relationship to disease progression; and 2) CTE, where five of five studies of recent or remote TBI found elevated TSPO levels. Yet, discrepancies in the TSPO literature remain, the most striking being in MS, in which a prominent in vitro TSPO signal is nevertheless much less able to be imaged. This and other in vitro/in vivo discrepancies for TSPO could reflect disruption of the in vivo multimeric complex during tissue preparation, as occurs for other multimeric complexes. This phenomenon could be present in other disorders and could explain the subtle genotype effect on in vivo binding to peripheral organs seen with [11C]-(R)-PK11195 and [11C]ER176 despite these two radioligands having similar in vitro affinity between high and low affinity binders.115, 116
As noted above, TSPO PET has potential for drug development in select instances. For instance, TSPO binding changes over time in AD, is modifiable by minocycline and myeloperoxidase inhibition, predicts response to COX inhibition during major depressive episodes, and is increased in presymptomatic HD mutation carriers. Thus, this imaging modality may have important clinical applications in determining which individuals are most likely to respond to novel drugs and which stage of disease is optimal for treatment.
Overall, our view is that this is a promising time for neuroinflammation PET, in that improved TSPO radioligands may be used as broad markers of immune response. Depending on their success, emerging biomarkers may allow more precise targeting of specific proteins involved in immune response; these could be used alone or in combination to delineate the mechanisms underlying neurological disease. Finally, neuroinflammation PET may be most useful in the context of clinical trials, in terms of both predicting and monitoring response to treatment in early drug discovery.
Acknowledgments
Funding and Role of Funding Source
This study was partly funded by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health (ZIAMH002852) (to MJK, IDH, and RBI). WCK and JMC received funding from the National Institutes of Health (K23AG052633 to WCK and R01NS100847 to JMC). DRO received funding from the Medical Research Council (MR/N008219/1). These institutions had no further role in study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Declaration of Interests
Disclosures
All authors have no conflict of interest to disclose, financial or otherwise.
Search Strategies and Selection Criteria
References for this review were identified by searching PubMed between 2015 and 2020 and by further examining the reference lists from relevant articles. Combinations of the following search terms were used: “PET”, “TSPO”, “translocator protein”, “peripheral benzodiazepine receptor”, “inflammation PET”, “microglia PET”, “Alzheimer’s disease”, “progressive supranuclear palsy”, “corticobasal degeneration”, “dementia”, “Huntington’s disease”, “multiple sclerosis”, “TBI”, “CTE”, “HIV”, “HAND”, “epilepsy”, “stroke”, “Parkinson’s disease”, “dementia with Lewy bodies,” and “amyotrophic lateral sclerosis”. There were no language restrictions. Only references published within the last five years were included, except for key or landmark studies in the field. In addition, we limited disorders to those for which there was at least one positive study using a second-generation TSPO radioligand. The final reference list was generated on the basis of relevance to the theme of this review.
References
- 1.Colonna M, Wang Y. TREM2 variants: new keys to decipher Alzheimer disease pathogenesis. Nat Rev Neurosci 2016; 17: 201–7. [DOI] [PubMed] [Google Scholar]
- 2.Horti AG, Naik R, Foss CA, et al. PET imaging of microglia by targeting macrophage colony-stimulating factor 1 receptor (CSF1R). Proc Natl Acad Sci U S A 2019; 116(5): 1686–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braestrup C, Squires RF. Specific benzodiazepine receptors in rat brain characterized by high-affinity (3H)diazepam binding. Proc Natl Acad Sci U S A 1977; 74: 3805–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tournier BB, Tsartsalis S, Ceyzériat K, et al. Fluorescence-activated cell sorting to reveal the cell origin of radioligand binding. J Cereb Blood Flow Metab 2019; 40: 1242–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nutma E, Stephenson JA, Gorter RP, et al. A quantitative neuropathological assessment of translocator protein expression in multiple sclerosis. Brain 2019; 142: 3440–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taniguchi T, Wang JK, Spector S. Properties of [3H] diazepam binding to rat peritoneal mast cells. Life Sci 1980; 27: 171–8. [DOI] [PubMed] [Google Scholar]
- 7.Maeda J, Zhang MR, Okauchi T, et al. In vivo positron emission tomographic imaging of glial responses to amyloid-beta and tau pathologies in mouse models of Alzheimer’s disease and related disorders. J Neurosci 2011; 31: 4720–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cosenza-Nashat M, Zhao ML, Suh HS, et al. Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathol Appl Neurobiol 2009; 35: 306–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gui Y, Marks JD, Das S, Hyman BT, Serrano-Pozo A. Characterization of the 18 kDa translocator protein (TSPO) expression in post-mortem normal and Alzheimer’s disease brains. Brain Pathol 2020; 30: 151–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martín A, Boisgard R, Thézé B, et al. Evaluation of the PBR/TSPO radioligand [(18)F]DPA-714 in a rat model of focal cerebral ischemia. J Cereb Blood Flow Metab 2010; 30: 230–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang M, Wang X, Zhao L, et al. Macroglia-microglia interactions via TSPO signaling regulates microglial activation in the mouse retina. J Neurosci 2014; 34: 3793–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Owen DR, Narayan N, Wells L, et al. Pro-inflammatory activation of primary microglia and macrophages increases 18 kDa translocator protein expression in rodents but not humans. J Cereb Blood Flow Metab 2017; 37: 2679–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parente A, Feltes PK, Vállez Garcia D, et al. Pharmacokinetic analysis of 11C-PBR28 in the rat model of herpes encephalitis: comparison with (R)-11C-PK11195. J Nucl Med 2016; 57: 785–91. [DOI] [PubMed] [Google Scholar]
- 14.Owen DR, Yeo AJ, Gunn RN, et al. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab 2012; 32: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kreisl WC, Jenko KJ, Hines CS, et al. A genetic polymorphism for translocator protein 18 kDa affects both in vitro and in vivo radioligand binding in human brain to this putative biomarker of neuroinflammation. . J Cereb Blood Flow Metab 2013; 33: 53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kreisl WC, Lyoo CH, McGwier M, et al. In vivo radioligand binding to translocator protein correlates with severity of Alzheimer’s disease. Brain 2013; 136: 2228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujita M, Kobayashi M, Ikawa M, et al. Comparison of four 11C-labeled PET ligands to quantify translocator protein 18 kDa (TSPO) in human brain: (R)-PK11195, PBR28, DPA-713, and ER176-based on recent publications that measured specific-to-non-displaceable ratios. EJNMMI Res 2017; 7: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zanotti-Fregonara P, Pascual B, Rostomily RC, et al. Anatomy of 18F-GE180, a failed radioligand for the TSPO protein. Eur J Nucl Med Mol Imaging 2020; February 22 [epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 19.Turkheimer FE, Edison P, Pavese N, et al. Reference and target region modeling of [11C]-(R)-PK11195 brain studies. J Nucl Med 2007; 48: 158–67. [PubMed] [Google Scholar]
- 20.Datta D, Colasanti A, Rabiner EA, et al. Neuroinflammation and its relationship to changes in brain volume and white matter lesions in multiple sclerosis. Brain 2017; 140: 2927–38. [DOI] [PubMed] [Google Scholar]
- 21.Lyoo CH, Ikawa M, Liow JS, et al. Cerebellum can serve as a pseudo-reference region in Alzheimer Disease to detect neuroinflammation measured with PET radioligand binding to translocator protein. J Nucl Med 2015; 56: 701–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terada T, Yokokura M, Obi T, et al. In vivo direct relation of tau pathology with neuroinflammation in early Alzheimer’s disease. J Neurol 2019; 266: 2186–96. [DOI] [PubMed] [Google Scholar]
- 23.Zou J, Tao S, Johnson A, et al. Microglial activation, but not tau pathology, is independently associated with amyloid positivity and memory impairment. Neurobiol Aging 2020; 85: 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arlicot N, Petit E, Katsifis A, et al. Detection and quantification of remote microglial activation in rodent models of focal ischaemia using the TSPO radioligand CLINDE. Eur J Nucl Med Mol Imaging 2010; 37: 2371–80. [DOI] [PubMed] [Google Scholar]
- 25.Schedin-Weiss S, Inoue M, Hromadkova L, et al. Monoamine oxidase B is elevated in Alzheimer disease neurons, is associated with γ-secretase and regulates neuronal amyloid β-peptide levels. Alzheimers Res Ther 2017; 9: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tong J, Meyer JH, Furukawa Y, et al. Distribution of monoamine oxidase proteins in human brain: implications for brain imaging studies. J Cereb Blood Flow Metab 2013; 33(6): 863–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer JH, Cervenka S, Kim MJ, Kreisl WC, Henter ID, Innis RB. Neuroinflammation in psychiatric disorders: PET imaging and promising new targets. Lancet Psychiatry in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bodini B, Poirion E, Tonietto M, et al. Individual mapping of innate immune cell activation is a candidate marker of patient-specific trajectories of disability worsening in multiple sclerosis. J Nucl Med 2020; 61: 1043–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Datta D, Colasanti A, Kalk N, et al. 11C-PBR28 and 18F-PBR111 detect white matter inflammatory heterogeneity in multiple sclerosis. J Nucl Med 2017; 58: 1477–82. [DOI] [PubMed] [Google Scholar]
- 30.Debruyne JC, Versijpt J, Van Laere KJ, et al. PET visualization of microglia in multiple sclerosis patients using [11C]PK11195. Eur J Neurol 2003; 10: 257–64. [DOI] [PubMed] [Google Scholar]
- 31.Oh U, Fujita M, Ikonomidou VN, et al. Translocator protein PET imaging for glial activation in multiple sclerosis. J Neuroimmune Pharmacol 2011; 6: 354–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herranz E, Gianni C, Louapre C, et al. Neuroinflammatory component of gray matter pathology in multiple sclerosis. Ann Neurol 2016; 80: 776–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rissanen E, Tuisku J, Rokka J, et al. In vivo detection of diffuse inflammation in secondary progressive multiple sclerosis using PET imaging and the radioligand 11C-PK11195. J Nucl Med 2014; 55: 939–44. [DOI] [PubMed] [Google Scholar]
- 34.Colasanti A, Guo Q, Muhlert N, et al. In vivo assessment of brain white matter inflammation in multiple sclerosis with (18)F-PBR111 PET. J Nucl Med 2014; 55: 1112–8. [DOI] [PubMed] [Google Scholar]
- 35.Kauzner UW, Kang Y, Monohan E, et al. Reduction of PK11195 uptake observed in multiple sclerosis lesions after natalizumab initiation. Mult Scler Relat Disord 2017; 15: 27–33. [DOI] [PubMed] [Google Scholar]
- 36.Ratchford JN, Endres CJ, Hammoud DA, et al. Decreased microglial activation in MS patients treated with glatiramer acetate. J Neurol 2012; 259: 1199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sucksdorff M, Rissanen E, Tuisku J, et al. Evaluation of the effect of fingolimod treatment on microglial activation using serial PET imaging in multiple sclerosis. J Nucl Med 2017; 58: 1646–51. [DOI] [PubMed] [Google Scholar]
- 38.Sucksdorff M, Tuisku J, Matilainen M, et al. Natalizumab treatment reduces microglial activation in the white matter of the MS brain. Neurol Neuroimmunol Neuroinflamm 2019; 6: e574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simioni S, Cavassini M, Annoni J- M, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS (London, England) 2010; 24(9): 1243–50. [DOI] [PubMed] [Google Scholar]
- 40.Rubin LH, Maki PM, Springer G, et al. Cognitive trajectories over 4 years among HIV-infected women with optimal viral suppression. Neurology 2017; 89(15): 1594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hammoud DA, Endres CJ, Chander AR, et al. Imaging glial cell activation with [11C]-R-PK11195 in patients with AIDS. J Neurovirol 2005; 11(4): 346–55. [DOI] [PubMed] [Google Scholar]
- 42.Garvey LJ, Pavese N, Politis M, et al. Increased microglia activation in neurologically asymptomatic HIV-infected patients receiving effective ART. AIDS (London, England) 2014; 28(1): 67–72. [DOI] [PubMed] [Google Scholar]
- 43.Wiley CA, Lopresti BJ, Becker JT, et al. Positron emission tomography imaging of peripheral benzodiazepine receptor binding in human immunodeficiency virus-infected subjects with and without cognitive impairment. J Neurovirol 2006; 12(4): 262–71. [DOI] [PubMed] [Google Scholar]
- 44.Coughlin JM, Wang Y, Ma S, et al. Regional brain distribution of translocator protein using [(11)C]DPA-713 PET in individuals infected with HIV. J Neurovirol 2014; 20(3): 219–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vera JH, Guo Q, Cole JH, et al. Neuroinflammation in treated HIV-positive individuals: A TSPO PET study. Neurology 2016; 86(15): 1425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rubin LH, Sacktor N, Creighton J, et al. Microglial activation is inversely associated with cognition in individuals living with HIV on effective antiretroviral therapy. Aids 2018; 32(12): 1661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bradburn S, Murgatroyd C, Ray N. Neuroinflammation in mild cognitive impairment and Alzheimer’s disease: A meta-analysis. Ageing Res Rev 2019; 50: 1–8. [DOI] [PubMed] [Google Scholar]
- 48.Schöll M, Carter SF, Westman E, et al. Early astrocytosis in autosomal dominant Alzheimer’s disease measured in vivo by multi-tracer positron emission tomography. Sci Rep 2015; 5: 16404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olsen M, Aguilar X, Sehlin D, et al. Astroglial responses to amyloid-beta progression in a mouse model of Alzheimer’s Disease. Mol Imaging Biol 2018; 20: 605–14. [DOI] [PubMed] [Google Scholar]
- 50.Mirzaei N, Tang SP, Ashworth S, et al. In vivo imaging of microglial activation by positron emission tomography with [(11)C]PBR28 in the 5XFAD model of Alzheimer’s disease. Glia 2016; 64: 993–1006. [DOI] [PubMed] [Google Scholar]
- 51.Hamelin L, Lagarde J, Dorothee G, et al. Early and protective microglial activation in Alzheimer’s disease: a prospective study using 18F-DPA-714 PET imaging. Brain 2016; 139: 1252–64. [DOI] [PubMed] [Google Scholar]
- 52.Okello A, Edison P, Archer HA, et al. Microglial activation and amyloid deposition in mild cognitive impairment: a PET study. Neurology 2009; 72: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiley CA, Lopresti BJ, Venneti S, et al. Carbon 11-labeled Pittsburgh Compound B and carbon 11-labeled (R)-PK11195 positron emission tomographic imaging in Alzheimer disease. Arch Neurol 2009; 66: 60–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dani M, Wood M, Mizoguchi R, et al. Microglial activation correlates in vivo with both tau and amyloid in Alzheimer’s disease. Brain 2018; 141: 2740–54. [DOI] [PubMed] [Google Scholar]
- 55.Hamelin L, Lagarde J, Dorothee G, et al. Distinct dynamic profiles of microglial activation are associated with progression of Alzheimer’s disease. Brain 2018; 141: 1855–70. [DOI] [PubMed] [Google Scholar]
- 56.Parbo P, Ismail R, Hansen KV, et al. Brain inflammation accompanies amyloid in the majority of mild cognitive impairment cases due to Alzheimer’s disease. Brain 2017; 140: 2002–11. [DOI] [PubMed] [Google Scholar]
- 57.Yokokura M, Mori N, Yagi S, et al. In vivo changes in microglial activation and amyloid deposits in brain regions with hypometabolism in Alzheimer’s disease. Eur J Nucl Med Mol Imaging 2011; 38: 343–51. [DOI] [PubMed] [Google Scholar]
- 58.Parbo P, Ismail R, Sommerauer M, et al. Does inflammation precede tau aggregation in early Alzheimer’s disease? A PET study. Neurobiol Dis 2018; 117: 211–6. [DOI] [PubMed] [Google Scholar]
- 59.Kreisl WC, Lyoo CH, Liow JS, et al. Distinct patterns of increased translocator protein in posterior cortical atrophy and amnestic Alzheimer’s disease. Neurobiol Aging 2017; 51: 132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ossenkoppele R, Schonhaut DR, Scholl M, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain 2016; 139: 1551–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kreisl WC, Lyoo CH, Liow JS, et al. (11)C-PBR28 binding to translocator protein increases with progression of Alzheimer’s disease. Neurobiol Aging 2016; 44: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sintini I, Martin PR, Graff-Radford J, et al. Longitudinal tau-PET uptake and atrophy in atypical Alzheimer’s disease. Neuroimage Clin 2019; 23: 101823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suridjan I, Pollock BG, Verhoeff NP, et al. In-vivo imaging of grey and white matter neuroinflammation in Alzheimer’s disease: a positron emission tomography study with a novel radioligand, [18F]-FEPPA. Mol Psychiatry 2015; 20: 1579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fan Z, Okello AA, Brooks DJ, Edison P. Longitudinal influence of microglial activation and amyloid on neuronal function in Alzheimer’s disease. Brain 2015; 138: 3685–98. [DOI] [PubMed] [Google Scholar]
- 65.Fan Z, Brooks DJ, Okello A, Edison P. An early and late peak in microglial activation in Alzheimer’s disease trajectory. Brain 2017; 140: 792–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Calsolaro V, Edison P. Neuroinflammation in Alzheimer’s disease: current evidence and future directions. Alzheimers Dement 2016; 12: 719–32. [DOI] [PubMed] [Google Scholar]
- 67.Rodriguez-Vieitez E, Saint-Aubert L, Carter SF, et al. Divering longitudinal changes in astrocytosis and amyloid PET in autosomal dominant Alzheimer’s disease. Brain 2016; 139: 922–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Femminella GD, Dani M, Wood M, et al. Microglial activation in early Alzheimer trajectory is associated with higher gray matter volume. Neurology 2019; 92: e1331–e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Negash S, Xie S, Davatzikos C, et al. Cognitive and functional resilience despite molecular evidence of Alzheimer’s disease pathology. Alzheimers Dement 2013; 9: e89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vilaplana E, Rodriguez-Vieitez E, Ferreira D, et al. Cortical microstructural correlates of astrocytosis in autosomal-dominant Alzheimer disease. Neurology 2020; 94: e2026–e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cagnin A, Rossor M, Sampson EL, Mackinnon T, Banati RB. In vivo detection of microglial activation in frontotemporal dementia. Ann Neurol 2004; 56: 894–7. [DOI] [PubMed] [Google Scholar]
- 72.Kim MJ, McGwier M, Jenko KJ, et al. Neuroinflammation in frontotemporal lobar degeneration revealed by 11 C-PBR28 PET. Ann Clin Transl Neurol 2019; 6: 1327–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bevan-Jones RW, Cope TE, Jones PS, et al. Neuroinflammation and protein aggregation co-localize across the frontotemporal dementia spectrum. Brain 2020; 143: 1010–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain 2013; 136(Pt 1): 43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mez J, Daneshvar DH, Kiernan PT, et al. Clinicopathological Evaluation of Chronic Traumatic Encephalopathy in Players of American Football. JAMA 2017; 318(4): 360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cherry JD, Tripodis Y, Alvarez VE, et al. Microglial neuroinflammation contributes to tau accumulation in chronic traumatic encephalopathy. Acta neuropathologica communications 2016; 4(1): 112-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Folkersma H, Boellaard R, Yaqub M, et al. Widespread and prolonged increase in (R)-(11)C-PK11195 binding after traumatic brain injury. J Nucl Med 2011; 52(8): 1235–9. [DOI] [PubMed] [Google Scholar]
- 78.Scott G, Zetterberg H, Jolly A, et al. Minocycline reduces chronic microglial activation after brain trauma but increases neurodegeneration. Brain 2018; 141(2): 459–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ramlackhansingh AF, Brooks DJ, Greenwood RJ, et al. Inflammation after trauma: microglial activation and traumatic brain injury. Ann Neurol 2011; 70(3): 374–83. [DOI] [PubMed] [Google Scholar]
- 80.Coughlin JM, Wang Y, Minn I, et al. Imaging of glial cell activation and white matter integrity in brains of active and recently retired national football league players. JAMA Neurol 2017; 74(1): 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Coughlin JM, Wang Y, Munro CA, et al. Neuroinflammation and brain atrophy in former NFL players: An in vivo multimodal imaging pilot study. Neurobiol Dis 2015; 74: 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hodges A, Strand AD, Aragaki AK, et al. Regional and cellular gene expression changes in human Huntington’s disease brain. Hum Mol Genet 2006; 15: 965–77. [DOI] [PubMed] [Google Scholar]
- 83.Björkqvist M, Wild EJ, Thiele J, et al. A novel pathogenic pathway of immune activation detectable before clinical onset in Huntington’s disease. J Exp Med 2008; 205: 1869–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pavese N, Gerhard A, Tai YF, et al. Microglial activation correlates with severity in Huntington disease: a clinical and PET study. Neurology 2006; 66: 1638–43. [DOI] [PubMed] [Google Scholar]
- 85.Tai YF, Pavese N, Gerhard A, et al. Microglial activation in presymptomatic Huntington’s disease gene carriers. Brain 2007; 130 (Pt 7): 1759–66. [DOI] [PubMed] [Google Scholar]
- 86.Politis M, Lahiri N, Niccolini F, et al. Increased central microglial activation associated with peripheral cytokine levels in premanifest Huntington’s disease gene carriers. Neurobiol Dis 2015; 83: 115–21. [DOI] [PubMed] [Google Scholar]
- 87.Lois C, González I, Izquierdo Garcia D, et al. Neuroinflammation in Huntington’s Disease: new insights with 11C-PBR28 PET/MRI. ACS Chem Neurosci 2018; 9: 2563–71. [DOI] [PubMed] [Google Scholar]
- 88.Loggia ML, Chonde DB, Akeju O, et al. Evidence for brain glial activation in chronic pain patients. Brain 2015; 138 (Pt 3): 604–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zurcher NR, Loggia ML, Lawson R, et al. Increased in vivo glial activation in patients with amyotrophic lateral sclerosis: assessed with [(11)C]-PBR28. Neuroimage Clin 2015; 7: 409–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Turner MR, Cagnin A, Turkheimer FE, et al. Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: an [11C](R)-PK11195 positron emission tomography study. Neurobiol Dis 2004; 15(3): 601–9. [DOI] [PubMed] [Google Scholar]
- 91.Alshikho MJ, Zurcher NR, Loggia ML, et al. Glial activation colocalizes with structural abnormalities in amyotrophic lateral sclerosis. Neurology 2016; 87(24): 2554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alshikho MJ, Zurcher NR, Loggia ML, et al. Integrated magnetic resonance imaging and [(11) C]-PBR28 positron emission tomographic imaging in amyotrophic lateral sclerosis. Ann Neurol 2018; 83(6): 1186–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Paganoni S, Alshikho MJ, Zurcher NR, et al. Imaging of glia activation in people with primary lateral sclerosis. Neuroimage Clin 2018; 17: 347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Corcia P, Tauber C, Vercoullie J, et al. Molecular imaging of microglial activation in amyotrophic lateral sclerosis. PLoS One 2012; 7(12): e52941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Paganoni S, Alshikho MJ, Luppino S, et al. A pilot trial of RNS60 in amyotrophic lateral sclerosis. Muscle Nerve 2019; 59(3): 303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Van Weehaeghe D, Van Schoor E, De Vocht J, et al. TSPO versus P2X7 as target for neuroinflammation - an in vitro and in vivo study. J Nucl Med 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kumar A, Chugani HT, Luat A, Asano E, Sood S. Epilepsy surgery in a case of encephalitis: use of 11C-PK11195 positron emission tomography. Pediatr Neurol 2008; 38(6): 439–42. [DOI] [PubMed] [Google Scholar]
- 98.Butler T, Ichise M, Teich AF, et al. Imaging inflammation in a patient with epilepsy due to focal cortical dysplasia. J Neuroimaging 2013; 23(1): 129–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Butler T, Li Y, Tsui W, et al. Transient and chronic seizure-induced inflammation in human focal epilepsy. Epilepsia 2016; 57(9): e191–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hirvonen J, Kreisl WC, Fujita M, et al. Increased in vivo expression of an inflammatory marker in temporal lobe epilepsy. J Nucl Med 2012; 53(2): 234–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gershen LD, Zanotti-Fregonara P, Dustin IH, et al. Neuroinflammation in temporal lobe epilepsy measured using positron emission tomographic imaging of translocator protein. JAMA Neurol 2015; 72(8): 882–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dickstein LP, Liow JS, Austermuehle A, et al. Neuroinflammation in neocortical epilepsy measured by PET imaging of translocator protein. Epilepsia 2019; 60(6): 1248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Price CJ, Wang D, Menon DK, et al. Intrinsic activated microglia map to the peri-infarct zone in the subacute phase of ischemic stroke. Stroke 2006; 37(7): 1749–53. [DOI] [PubMed] [Google Scholar]
- 104.Thiel A, Radlinska BA, Paquette C, et al. The temporal dynamics of poststroke neuroinflammation: a longitudinal diffusion tensor imaging-guided PET study with 11C-PK11195 in acute subcortical stroke. J Nucl Med 2010; 51(9): 1404–12. [DOI] [PubMed] [Google Scholar]
- 105.Kreisl WC, Mbeo G, Fujita M, et al. Stroke incidentally identified using improved positron emission tomography for microglial activation. Arch Neurol 2009; 66(10): 1288–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gulyas B, Toth M, Schain M, et al. Evolution of microglial activation in ischaemic core and peri-infarct regions after stroke: a PET study with the TSPO molecular imaging biomarker [((11))C]vinpocetine. J Neurol Sci 2012; 320(1–2): 110–7. [DOI] [PubMed] [Google Scholar]
- 107.Ribeiro MJ, Vercouillie J, Debiais S, et al. Could (18) F-DPA-714 PET imaging be interesting to use in the early post-stroke period? EJNMMI Res 2014; 4: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Abid KA, Sobowale OA, Parkes LM, et al. Assessing inflammation in acute intracerebral hemorrhage with PK11195 PET and dynamic contrast-enhanced MRI. J Neuroimaging 2018; 28(2): 158–61. [DOI] [PubMed] [Google Scholar]
- 109.Attwells S, Setiawan E, Rusjan P, et al. Translocator protein distribution volume predicts reduction of symptoms during open-label trial of celecoxib in major depressive disorder. Biol Psychiatry 2020; March 29 [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jucaite A, Svenningsson P, Rinne JO, et al. Effect of the myeloperoxidase inhibitor AZD3241 on microglia: a PET study in Parkinson’s disease. Brain 2015; 138: 2867–700. [DOI] [PubMed] [Google Scholar]
- 111.Ghadery C, Koshimori Y, Coakeley S, et al. Microglial activation in Parkinson’s disease using [18F]-FEPPA. J Neuroinflammation 2017; 14: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Koshimori Y, Ko JH, Mizrahi R, et al. Imaging striatal microglial activation in patients with Parkinson’s Disease. PLoS One 2015; 10: e0138721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Varnäs K, Cselényi Z, Jucaite A, et al. PET imaging of [11C]PBR28 in Parkinson’s disease patients does not indicate increased binding to TSPO despite reduced dopamine transporter binding. Eur J Nucl Med Mol Imaging 2019; 46: 367–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.AstraZeneca. AZD3241 PET MSA trial, Phase 2, randomized, 12 week safety and tolerability trial with PET in MSA patients. NCT02388295. Retrieved from: https://clinicaltrials.gov/ct2/show/results/NCT02388295?view=results. 2017. [Google Scholar]
- 115.Kreisl WC, Fujita M, Fujimara Y, et al. Comparison of [(11)C]-(R)-PK 11195 and [(11)C]PBR28, two radioligands for translocator protein (18 kDa) in human and monkey: Implications for positron emission tomographic imaging of this inflammation biomarker. Neuroimage 2010; 49: 2924–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ikawa M, Lohith TG, Shrestha S, et al. 11C-ER176, a radioligand for 18-kDa translocator protein, has adequate sensitivity to robustly image all three affinity genotypes in human brain. J Nucl Med 2017; 58: 320–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Feeney C, Scott G, Raffel J, et al. Kinetic analysis of the translocator protein positron emission tomography ligand [18F]GE-180 in the human brain. Eur J Nucl Med Mol Imaging 2016; 43: 2201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sridharan S, Raffel J, Nandoskar A, et al. Confirmation of specific binding of the 18-kDa translocator protein (TSPO) radioligand [18F]GE-180: a blocking study using XBD173 in multiple sclerosis normal appearing white and grey matter. Mol Imaging Biol 2019; 21: 935–44. [DOI] [PubMed] [Google Scholar]
- 119.Hagens MHJ, Golla SV, Wijburg MT, et al. In vivo assessment of neuroinflammation in progressive multiple sclerosis: a proof of concept study with [18F]DPA714 PET. J Neuroinflammation 2018; 15: 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Park E, Gallezot JD, Delgadillo A, et al. (11)C-PBR28 imaging in multiple sclerosis patients and healthy controls: test-retest reproducibility and focal visualization of active white matter areas. Eur J Nucl Med Mol Imaging 2015; 42: 1081–92. [DOI] [PubMed] [Google Scholar]
- 121.Zanotti-Fregonara P, Pascual B, Rizzo G, et al. Head-to-head comparison of 11C-PBR28 and 18F-GE180 for quantification of the translocator protein in the human brain. J Nucl Med 2018; 59: 1260–6. [DOI] [PubMed] [Google Scholar]
- 122.Schain M, Zanderigo F, Ogden RT, Kreisl WC. Non-invasive estimation of [11C]PBR28 binding potential. Neuroimage 2018; 169: 278–85. [DOI] [PubMed] [Google Scholar]
- 123.Knezevic D, Verhoeff NP, Hafizi S, et al. Imaging microglial activation and amyloid burden in amnestic mild cognitive impairment. J Cereb Blood Flow Metab 2018; 38: 1885–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mabrouk R, Strafella AP, Knezevic D, et al. Feasibility study of TSPO quantification with [18F]FEPPA using population-based input function. PLoS One 2017; 12: e0177785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Golla SS, Boellaard R, Oikonen V, et al. Quantification of [18F]DPA-714 binding in the human brain: initial studies in healthy controls and Alzheimer’s disease patients. J Cereb Blood Flow Metab 2015; 35: 766–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Metaxas A, Thygesen C, Briting SRR, Landau AM, Darvesh S, Finsen B. Increased inflammation and unchanged density of synaptic vesicle glycoprotein 2A (SV2A) in the postmortem frontal cortex of Alzheimer’s disease patients. Front Cell Neurosci 2019; 13: 538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Albrecht DS, Normandin MD, Shcherbinin S, et al. Pseudoreference Regions for Glial Imaging with (11)C-PBR28: Investigation in 2 Clinical Cohorts. J Nucl Med 2018; 59(1): 107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Monk PN, Shaw PJ. ALS: Life and death in a bad neighborhood. Nat Med 2006; 12: 885–7. [DOI] [PubMed] [Google Scholar]