Abstract
The placenta is a vital, multi-functional organ that acts as an interface between maternal and fetal circulation during pregnancy. Nutritional deficiencies during pregnancy alter placental development and function, leading to adverse pregnancy outcomes, such as pre-eclampsia, infants with small for gestational age and low birthweight, preterm birth, stillbirths and maternal mortality. Maternal nutritional supplementation may help to mitigate the risks, but the evidence base is difficult to navigate. The primary purpose of this umbrella review is to map the evidence on the effects of maternal nutritional supplements and dietary interventions on pregnancy outcomes related to placental disorders and maternal mortality. A systematic search was performed on seven electronic databases, the PROSPERO register and references lists of identified papers. The results were screened in a three-stage process based on title, abstract and full-text by two independent reviewers. Randomized controlled trial meta-analyses on the efficacy of maternal nutritional supplements or dietary interventions were included. There were 91 meta-analyses included, covering 23 types of supplements and three types of dietary interventions. We found evidence that supports supplementary vitamin D and/or calcium, omega-3, multiple micronutrients, lipid-based nutrients, and balanced protein energy in reducing the risks of adverse maternal and fetal health outcomes. However, these findings are limited by poor quality of evidence. Nutrient combinations show promise and support a paradigm shift to maternal dietary balance, rather than single micronutrient deficiencies, to improve maternal and fetal health. The review is registered at PROSPERO (CRD42020160887).
Keywords: maternal dietary interventions, nutritional supplements, pre-eclampsia, small for gestational age, low birthweight, stillbirths, maternal mortality, umbrella review
1. Introduction
Sustainable Development Goal 2 aims to eradicate world hunger by 2030; however, the 2020 State of Food Security and Nutrition in the World report indicated that 8.9% of people in the world are undernourished, which has impacts on both maternal and fetal well-being [1]. Maternal undernutrition is known to have important impacts on fetal development and early infancy as the sole source of nutrients for a growing infant from conception through exclusive breastfeeding [2]. Undernourished mothers are more likely to have low birthweight (LBW) infants; preconception folate deficiencies are associated with neural tube defects and there is increasing recent understanding of the impact of early nutrition on chronic diseases in later life, through the “developmental origins of health and disease” (DOHaD) research focus [3].
While adverse effects of maternal undernutrition have traditionally focused on reduced maternal nutrient supply to the fetus, a review by Belkacemi et al. [4] highlighted the crucial mediating role of the placenta. The placenta is a multi-functional organ that acts as an interface between the maternal and fetal circulations [4]. Maternal undernutrition alters placental development and function, leading to fetal growth restriction [4]. Studies of the impact of severe maternal undernutrition during the Dutch Famine during the winter of 1944–1945 found associations between placental weight and rates of LBW, preterm births (PTB), stillbirths and neonatal death [5,6]. Additionally, the failure of the proper development of the placenta is associated with pre-eclampsia (PE), the most serious of the hypertensive disorders of pregnancy (HDP) and the second leading direct cause of maternal mortality worldwide [7,8,9]. HDPs are associated with an estimated 46,000 maternal deaths, 416,000 stillbirths and 1.5–2 million neonatal deaths annually [7]. The development of PE involves inadequate placentation, maternal inflammatory response and generalized endothelial dysfunction [8,9]. Nutrition plays an important role in placentation in part due to the clinical antioxidant and anti-inflammatory properties of certain micronutrients [10].
Nutritional supplementation is considered an important part of policies to protect vulnerable populations, such as pregnant and lactating mothers and their infants, from health risks associated with undernutrition [1]. Previous umbrella reviews report that calcium supplementation is promising for reducing the risk of PE, while there was a lack of evidence for supplementary vitamin C, E or D [10,11]. Additionally, zinc or vitamin D supplementation on their own may reduce the risk of PTB [12], and balanced protein energy and multiple micronutrients (MMN) may reduce the risk of small for gestational age (SGA) and vitamin A, calcium, MMN and antenatal nutritional counselling may protect against LBW [13,14]. However, since these umbrella reviews were published, new systematic reviews, as well as updated versions of Cochrane systematic reviews on nutritional supplements, have been published. In addition, while PE, SGA, LBW, PTB, stillbirths and maternal mortality may be interconnected through maternal nutritional influences on placental disorders, evidence of the efficacy of nutritional supplementation and dietary intervention trials has not yet been mapped across outcomes and a gap exists, particularly for stillbirth and maternal mortality. This umbrella review included a systematic review of existing meta-analyses of randomized controlled trials (RCTs) and generated an evidence map for the efficacy of nutritional supplements and dietary interventions for adverse outcomes related to placental disorders and maternal mortality.
2. Materials and Methods
This review was been developed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist (Table S1) [15]. The protocol was registered to PROSPERO (CRD42020160887) prior to conducting the review.
2.1. Objective
The primary objective of the umbrella review was to evaluate the effects of nutritional supplements and dietary interventions on pregnancy outcomes related to placental disorders or maternal mortality. The primary outcomes were PE (as defined by individual study authors), PTB (<37 weeks or as defined by study authors), SGA (<10th centile or SGA/intrauterine growth restriction as defined by study authors), LBW (<2500 g or as defined by study authors), stillbirth and maternal death (42 days postpartum or as defined by study authors). Secondary outcomes were severe PE, gestational hypertension, eclampsia, and HELLP syndrome (hemolysis, elevated liver enzymes and low platelet count; all as defined by study authors). The secondary objective of this review was to break down contributing studies by those conducted in high-income countries (HICs) and low- and middle-income countries (LMICs) and explore whether findings shifted when analyses were restricted to trials conducted in LMICs.
2.2. Study Inclusion and Exclusion
All reviews reporting meta-analyses of RCTs assessing the efficacy of nutritional supplements or dietary interventions on primary outcomes of interest among pregnant women or those planning on becoming pregnant were included. Reviews of observational studies and those that incorporate theoretical studies or published opinion as their primary source of evidence were excluded [16]. Existing umbrella reviews were excluded, but were reviewed for any meta-analyses not captured in searches. Reviews not written in English were excluded due to limited capacity of the review team to comprehensively search non-English databases. There were no restrictions on the date of publication.
2.3. Search Strategy
Searches were conducted on Medline Ovid, EBM Reviews (The Cochrane Database of Systematic Reviews, ACP Journal Club, Clinical Evidence, Evidence-Based Mental Health, Evidence-Based Nursing, Evidence Report/Technology Assessment), JBI Database of Systematic Reviews and Implementation Reports, Web of Science, Cumulative Index to Nursing and Allied Health (CINAHL) and Embase. Additionally, searches were supplemented by reviewing the Database of Abstracts of Reviews of Effects, the PROSPERO register and scanning reference lists. Searches were conducted from database inception to November 2019. Search terms are included in Table S2.
Results were screened in a three-stage process based on title, abstract and then full-text review in duplicate by two independent reviewers (MWK, SO) at each stage. Study selections were compared and discrepancies were resolved by discussion with a third reviewer (K.S.). Duplicates and studies that did not meet the selection criteria were removed at each round. Search results were uploaded in Mendeley (Elsevier, London, UK) to remove duplicates, then the reference list was uploaded to Excel (Microsoft, Redmond, Washington, USA) for study selection.
2.4. Data Extraction
Data were extracted in a two-stage process. From reviews, details were extracted regarding the intervention, outcome, number of RCT studies, number of participants, variation between studies and pooled results with 95% confidence intervals (CIs) on a piloted table in Word (Microsoft, Redmond, Washington, USA). Where there were multiple reviews for an intervention, individual RCTs were extracted from reviews, their full text located, and details extracted on their study design, country, participants, interventions, comparison, co-interventions, and outcomes relevant to the current overview review. Two reviewers independently extracted data from a sample of eligible studies until full agreement was achieved on the details pulled (five studies), with the remainder extracted by one reviewer (M.W.K.).
2.5. Quality and Risk of Bias Assessment
We assessed the quality of reviews using AMSTAR 2, a widely used and validated critical appraisal tool to evaluate systematic reviews of healthcare interventions [16,17,18,19]. The risk of bias (RoB) of individual RCTs was based on their assessment in the most recent, highest quality review, according to AMSTAR 2, and evaluated on random sequence generation, allocation concealment, blinding of participants, personnel and outcome assessment, incomplete outcome data, selective reporting, and any other bias [20]. If there was no existing review with an AMSTAR 2 rating of moderate or above, then RoB was assessed by independent review by two reviewers.
2.6. Data Analysis
Included reviews were narratively synthesized according to included interventions, key findings and quality assessment. Evidence from studies of similar design, data collection methodology, sample and outcomes reported were pooled using Review Manager (RevMan 5) and reported as risk ratios (RRs) with 95% CI. To overcome the challenge that meta-analyses of systematic reviews could repeat individual studies and consequently give too much statistic power and result in a misleading estimate, each of the included reviews were unpicked when there were multiple reviews on a given intervention and the results of the individual included studies combined [17]. Evidence was pooled according to Mantel–Haenszel random-effects model for analyses with substantial heterogeneity (I2 > 50%) and sensitivity analyses were planned a priori on primary outcomes, (i) excluding RCTs with high RoB and (ii) excluding studies conducted in HICs. Estimates of publication bias were considered using funnel plots if there were more than 10 included studies. Certainty of evidence of primary outcomes were mapped using GRADE (Grading of Recommendations Assessment, Development and Evaluation) and classified as high, moderate, low or very low [20]. Strength of association was evaluated according to the Harvard Cancer Index.
3. Results
3.1. Search Results
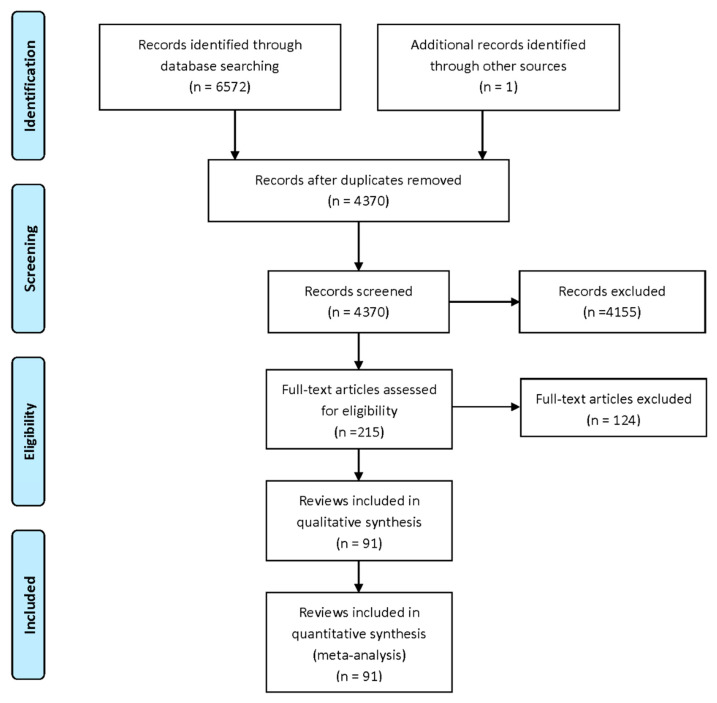
We identified 6572 records from database searches (2310 from Medline, 1268 from EBM reviews, 210 from JBI, 1468 from Web of Science, 665 from CINAHL and 651 from Embase). There were 4370 unique records after the removal of duplicates. After title and abstract screening, 215 records remained for full-text review (Figure 1). In total, 124 records were excluded at full-text review, including a mismatch in populations in a single study (i.e., supplementing infants), three in intervention (dietary interventions not reported separately), three in comparator (compared dosages, thus supplement given to both intervention and control), 12, which did not report outcomes of interest (i.e., infant mortality), 19 in study design (i.e., observational studies, commentary) and 48 duplicates and older versions of Cochrane reviews (Table S3). Nineteen umbrella reviews [10,11,12,13,14,21,22,23,24,25,26,27,28,29,30,31,32,33,34] were excluded and 19 reviews that did not report meta-analyses of RCTs (narrative syntheses only, meta-analysis of observational studies or combined observational and RCTs) (vitamin A [35,36,37], vitamin B6 and/or 12 [35,38], vitamin C and/or E [35,38,39], vitamin D and/or calcium [35,37,40,41,42], iron and/or folic acid [35,37,41,43,44], magnesium [35,37], zinc [45,46,47], MMN [35,37,41,48,49,50], balanced protein energy supplementation [51,52], antenatal dietary counselling [53]) were also excluded. The umbrella reviews found in searches were reviewed for unique meta-analyses; one was considered but was ultimately not included because, while a meta-analysis was planned, their searches found no studies for inclusion [49] (Table S4).
Figure 1.
PRISMA flow diagram.
There were 91 meta-analyses in the umbrella review, including 23 Cochrane systematic reviews (latest versions) (Table S5). The interventions evaluated in the meta-analyses included 23 types of supplements (vitamins A, B6, C and/or E, D and/or calcium, iodine, iron and/or folic acid, magnesium, zinc, antioxidants, garlic, L-arginine, MMN, polyunsaturated omega-3 fatty acids, balanced protein-energy, high protein, calf blood extract, glucose, galactose, lipid-based nutrient supplements (LNS), food and fortified food products) and three types of dietary interventions (salt restriction, caffeine restriction, antenatal dietary counselling). L-arginine [54,55], antioxidants [56,57,58] and food and fortified food products [59] were not reported separately because they covered trials reported in other categories. For example, a study on the impact of L-arginine supplementation also included other vitamins and minerals and was included with multiple micronutrient supplementation [60].
3.2. Quality Assessment
Figure 2 reports the quality assessment, according to the AMSTAR 2 critical domains. Quality assessments by review are included in Table S6. Of the 91 included meta-analyses, 47% registered a review protocol prior to commencing, though 8% lacked detail on a meta-analysis plan and planned sensitivity analyses, and 89% had a fair to comprehensive literature search strategy. Excluded studies were reported in 82%, though 29% lacked referencing and/or justification details. Over half (57%) considered random allocation sequence, unconcealed allocation, blinding of patients and assessors and selective reporting in individual studies, though 82% included some form of RoB assessment. Ninety percent of included meta-analyses had appropriate methods, 81% considered RoB when interpreting the results of the review and 77% described the assessment or planned assessment of publication bias.
Figure 2.
Quality assessment of reviews with meta-analyses.
3.3. Effects of Nutrient Supplementation and Dietary Interventions
A summary of outcomes by review is reported in Table S7. The study characteristics of individual trials covered by reviews are included in Table S8, their individual trial outcomes in Table S9 and RoB in Table S10. Forest plots and funnel plots are included in Figure S1 and sensitivity analyses in Figure S2.
3.3.1. Vitamin A
There were three reviews reporting meta-analyses on SGA, LBW, PTB, stillbirths and maternal deaths, consistently finding no effect of vitamin A and/or beta-carotene (vitamin A precursor) supplementation [61,62,63]. A Cochrane review also found no effect of vitamin A on maternal mortality in areas of high vitamin A deficiency [62]. The reviews covered ten trials published between 1999 and 2011 [64,65,66,67,68,69,70,71,72,73], with a secondary analysis published in 2013 [74]. Pooled outcomes confirmed no significant effect of vitamin A and/or beta-carotene supplementation across outcomes of interest. None of the studies reported PE outcomes, though one study found no significant effect on gestational hypertension [67] or rates of eclampsia [73]. Sensitivity analyses were not applicable as all studies had low or unclear RoB and all were conducted in LMICs.
3.3.2. Vitamin C and/or E
Eight reviews found no significant effects of vitamin C and/or E supplementation [75,76,77,78,79,80,81,82]. The only significant finding was a weak adverse effect on LBW rates among women at risk of PE in the oldest review [79]. Among women with low baseline intake, two Cochrane reviews did not find a significant effect of vitamin C and/or E supplementation [75,82]. Reviews covered 19 trials examining vitamin C and/or E [83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99], including one secondary analysis of smokers [100]. Three trials were published in 2014 or later [84,92,100]. Pooled outcomes confirmed no significant effect of vitamin C and/or E supplementation across all outcomes of interest. Almost half of the trials were conducted in HICs [85,86,89,91,92,94,95,99,100]. Sensitivity analyses restricted to LMIC studies found a marginally significant protective effect on PE (RR 0.86, 95% CI: 0.76–0.99, I2 40%, 11 studies, n = 6883); there were no changes in the direction or significance in other primary outcomes. Sensitivity analyses that excluded four studies considering high RoB [97,98,101,102] also found no changes in the direction or significance in primary outcomes. Vitamin C and/or E supplementation was not significantly associated with any of the secondary outcomes.
3.3.3. Vitamin D
There were 13 reviews that reported meta-analyses for vitamin D supplementation [81,103,104,105,106,107,108,109,110,111,112,113,114]. Approximately half of reviews reported that vitamin D supplementation had a significant protective effect on PE incidence [81,107,109,110,113], SGA [103,104,111], LBW [105,111,113]. There was little evidence that vitamin D supplementation had an effect on PTB or stillbirth. A Cochrane review examined PE, LBW and PTB for studies conducted North of the Tropic of Cancer or South of the Tropic of Capricorn, where there is less sunlight exposure for the synthesis of vitamin D3. All studies reporting on PE included in the Cochrane review were conducted outside of the tropics, a non-significant effect on PTB did not change and there was a potentially stronger effect on LBW (all: RR 0.55, 95% CI: 0.35–0.87; outside tropics: RR 0.39, 95% CI: 0.24–0.65) though CIs overlap [113]. The reviews covered 25 trials [115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139], of which a majority were published in 2014 or later [117,118,119,121,122,123,125,126,127,128,129,130,132,133,137,138,139]. There were ten studies with high RoB [116,120,125,128,129,131,133,135,137,138] and ten were conducted in HICs [116,119,127,130,131,132,133,134,135,136]. Pooled outcomes found a 38% reduced risk of developing PE among pregnant women who received vitamin D supplementation in comparison to those who did not without heterogeneity between studies (RR 0.62 95% CI: 0.43–0.91, I2 0%, 12 studies, n = 1353), which did not change in direction or significance with sensitivity analyses. Pooled outcomes also found that vitamin D supplementation reduced the risk of SGA by 41% (RR 0.59, 95% CI: 0.39–0.88, I2 0%, five studies, n = 726), but the association was not significant after studies with a high RoB were excluded (RR 0.78, 95%CI: 0.42–1.44, I2 0%, two studies, n = 305). Associations with LBW, PTB and stillbirth were non-significant, and no studies reported maternal mortality outcomes. For PTB, the 95% CI reached 1.00 in the fixed effects model but not in the random effects model; evidence for a non-significant association was strengthened in sensitivity analyses, excluding studies with a high RoB. Sensitivity analyses restricted to studies conducted in LMICs found a significantly reduced risk of PTB with vitamin D supplementation (RR 0.54 95% CI: 0.38–0.77, I2 0%, 11 studies, n = 1154). Vitamin D supplementation was not significantly associated with any of the secondary outcomes.
3.3.4. Vitamin D and Calcium
Four moderate-to-high quality reviews reporting meta-analyses on the effect of vitamin D and calcium co-supplementation [110,112,113,140] found a consistently significant effect on reduced rates of PE [110,112,113,140] but higher rates of PTB [113,140]. Results by baseline maternal dietary status were not reported by the two Cochrane reviews [113,141]. The reviews covered eight trials [120,121,142,143,144,145,146,147], of which two were published in 2014 or later [143,146], four had high RoB [120,144,145,147] and all were conducted in LMICs. Pooled outcomes found that vitamin D and calcium co-supplementation was associated with 51% reduced risk of PE [RR 0.49 (0.31–0.77, I2 0%, three studies, n = 1120) and a 53% increased risk of PTB (RR 1.53 (1.02–2.30, I2 0%, six studies, n = 988), though the adverse effect on PTB was not significant after studies with high RoB were excluded. There was no significant effect on SGA or LBW nor severe or gestational hypertension among secondary outcomes. No studies reported on stillbirth or maternal mortality outcomes and sensitivity analyses excluding studies from HICs were not applicable.
3.3.5. Calcium
Calcium supplementation was evaluated in 19 meta-analyses [47,110,140,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163], in which a majority were found to be protective against PE incidence, with the few reviews that reported no effects largely among subgroups, such as those with adequate baseline calcium intake [153,156] or featuring periconceptual supplementation [148]. A majority of reviews also reported significant beneficial effects of calcium supplementation on rates of PTB [47,140,149,150,160,161] but largely no effect on LBW or SGA. When outcomes were considered by baseline dietary calcium in a Cochrane review, only low calcium diet was significantly associated with lower rates of PE [140]. Results for LBW, SGA, PTB and stillbirths were non-significant for both those with low or adequate calcium baseline intake [140]. The reviews covered 23 trials [164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186], of which ten were considered studies with high RoB [164,165,167,170,173,176,179,182,184,186] and two studies were published in 2014 or later [164,185]. The majority of studies were conducted in LMICs with only six that were conducted in HICs [166,174,175,177,181,183]. Pooled outcomes found a 48% reduction in the risk of PE (RR 0.52, 95% CI: 0.41–0.65, I2 67%, 24 studies, n = 27,442) with significant heterogeneity and publication bias suspected. Sensitivity analyses restricted to studies with low or unclear RoB and studies conducted in LMICs reduced the heterogeneity slightly, but this was still substantial (I2 63–69%). Calcium supplementation was associated with a 16% risk reduction in LBW rates (RR 0.84, 95% CI: 0.73–0.96, I2 43%, 11 studies, n = 7800). The effect was strengthened in sensitivity analyses limited to studies conducted in LMICs only (RR 0.58, 95% CI: 0.37–0.90, I2 0%, five studies, n = 1110). Calcium supplementation was associated with a 47% reduced risk of PTB (RR 0.53, 95% CI: 0.33–0.86, I2 95%, 18 studies, n = 14,078) but there was significant heterogeneity and publication bias suspected. Significant heterogeneity remained in sensitivity analyses. Calcium supplementation was not associated with SGA, stillbirth, or maternal mortality. Among secondary outcomes, there was a significant reduction in the risk of developing severe PE (RR 0.78, 95% CI: 0.78–0.93, I2 0%, six studies, n = 14,099) and gestational hypertension (RR 0.75, 95% CI: 0.61–0.93, I2 58%, 10 studies, n = 11,143) but not for eclampsia or HELLP syndrome.
3.3.6. Iron and/or Folic Acid
There were nine reviews that reported meta-analyses for iron and/or folic acid supplementation [47,152,187,188,189,190,191,192,193], which largely found no effect on outcomes of interest. A network meta-analysis found that iron was significantly attributed to lower rates of PTB [47] and another review found that iron with or without folic acid supplementation had a weak effect on reducing rates of LBW [188]. A Cochrane review examined iron alone and iron with folic acid supplementation by baseline anemia status and found that studies were largely conducted with women who were non-anemic at the start of supplementation when reported [189]. The reviews covered 21 trials evaluating iron and/or folic acid supplementation [194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214], including nine trials with high RoB [195,197,200,201,203,206,207,213,214] and ten conducted in HICs [195,198,199,201,203,206,209,211,213,214]. None were published in 2014 or later. Pooled outcomes found that iron and/or folic acid significantly reduced rates of LBW by 13% (RR 0.87, 95% CI: 0.77–0.98, I2 27%, 13 studies, n = 22,946), though the association was not significant after studies with high RoB were excluded. There was no evidence of a significant effect on PE, SGA, PTB, stillbirth, and maternal mortality, which were unchanged in the direction of effect and significance with sensitivity analyses. Sensitivity analyses were not applicable for maternal mortality. Among the secondary outcomes, only eclampsia was reported by one study, which found no effect [196].
3.3.7. Zinc
There were four reviews that reported meta-analyses for zinc supplementation [42,45,46,47], which found evidence of a beneficial effect on PTB [45,46,47], except among adolescent pregnancies [42]. A review covering a single trial found a significant effect on LBW among adolescent pregnancies [42], though two other reviews did not find a significant effect on LBW or SGA [45,46]. A Cochrane review found that significant effects on PTB was only found among those with low baseline zinc, no effect remained for LBW and SGA, whether low or adequate baseline and maternal baseline zinc status were not reported for PE [46]. The reviews covered 15 trials [66,196,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229], including five with high RoB [215,218,222,225,229], almost half of which were conducted in HICs [216,218,220,224,226,228,229] and none were published in 2014 or later. Pooled outcomes found a significant 14% reduction in the risk of PTB with zinc supplementation (RR 0.86, 95% CI: 0.76–0.97, I2 17%, 16 studies, n = 7563), though the association was not significant after studies with high RoB were excluded. The association also lost its significance when restricted to studies in LMICs, although notably this excluded the six oldest studies conducted between 1984–1996. There was no significant effect on other primary outcomes and of the secondary outcomes, only one study reported on gestational hypertension, and the findings were not found to be significant [215]. In sensitivity analyses that excluded studies with high RoB, zinc was adversely associated with risk of PE (RR 3.62, 95% CI: 1.02–12.82, I2 N/A, 1 study, n = 479), though this was based on a single study from the late 1980s. Sensitivity analyses found no changes in the direction or significance in the associations with SGA and LBW and stillbirth and maternal mortality associations were uninterpretable due to the small number of included studies in the original analyses.
3.3.8. Multiple Micronutrients, with or without Lipid-Based Nutrient Supplementation
Eight reviews reporting meta-analyses evaluating the effect of MMN supplements on their own [47,81,230,231,232,233,234,235,236] found beneficial effects on LBW and SGA and mixed findings on PTB and stillbirth. Evidence of a beneficial effect on PE is limited by the report from only a single, poor quality review [81]. A Cochrane review found that the significant effect of MMN supplementation on PTB was only found in women with BMI ≤ 20 [233]. MMN supplementation was not significant for SGA regardless of BMI status [233]. The reviews on MMN supplementation covered 25 trials [60,67,196,198,204,210,237,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255], which included three secondary analyses [256,257,258]. Of these, six were considered studies with a high RoB [60,241,242,243,244,248] and five studies were published in 2014 or later [241,249,252,253,255]. The majority of studies were conducted in LMIC settings, with one study conducted in a HIC [255]. Nine studies used the UNIMMAP multiple micronutrients formulation in their recommended dosages [242,243,245,247,248,249,250,251,254]. Pooled outcomes found a 60% reduced risk of PE (RR 0.40, 95% CI: 0.27–0.59, I2 0%, two studies, n = 510], though the association was not significant after excluding one study with a high RoB in sensitivity analyses. Additionally, a significant reduction in the risks of SGA (RR 0.88, 95% CI: 0.82–0.95, I2 59%, 16 studies, n = 39,696) and LBW (RR 0.87, 95% CI: 0.85–0.90, I2 22%, 20 studies, n = 70,293) were also found with maternal MMN supplementation, with no change in direction or significance in sensitivity analyses. There was a high level of heterogeneity observed for SGA, which increased slightly in sensitivity analyses (I2 62–67%). There was no evidence of a significant effect for PTB, stillbirth, maternal mortality nor severe PE, eclampsia or gestational hypertension among secondary outcomes.
There were two reviews reporting meta-analyses evaluating the effect of LNS [259,260], with one review reporting protective effects on SGA and LBW [260]. The reviews covered five trials [241,252,253,261,262], of which two were considered studies with a high RoB [241,261]. All were conducted in LMICs and four were published in 2014 or later [252,253,262,263]. Pooled outcomes found a significantly reduced risk of SGA (RR 0.93, 95% CI: 0.88–0.98, I2 0%, four studies, n = 4828) and LBW (RR 0.89, 95% CI: 0.82–0.98, I2 0%, five studies, n = 5851) among pregnant women receiving MMN with LNS, with less heterogeneity than MMN supplementation only. There was no evidence of a significant effect for PTB, stillbirth, or maternal mortality. No changes in direction or significance with the exclusion of studies with high RoB for all analyses (not applicable for maternal mortality). None reported on PE or secondary outcomes.
3.3.9. Polyunsaturated Omega-3 Fatty Acids
There were 14 reviews with meta-analyses that evaluated the effect of polyunsaturated omega-3 fatty acid supplementation [264,265,266,267,268,269,270,271,272,273,274,275,276,277], with no largely significant effects, except some mixed findings on PTB. Maternal nutritional status at baseline was not reported by a Cochrane review, though when examining the underlying risk for outcomes, omega-3 supplementation had a significant effect for pre-eclampsia, only among women with high risk [274]. The reviews covered 35 trials [123,278,279,280,281,282,283,284,285,286,287,288,289,290,291,292,293,294,295,296,297,298,299,300,301,302,303,304,305,306,307,308,309,310], including two secondary analyses [311,312]. Nine studies were published in the year 2014 or later [281,283,288,289,290,291,296,306,310]. Twenty-three studies were conducted in HICs [279,280,281,285,286,288,289,290,291,292,293,294,295,298,299,300,302,304,305,307,308,309,310]. There were 14 studies considered to be at high RoB [279,280,281,286,287,290,291,295,298,301,302,304,306,308,310]. Three studies examined omega-3 and omega-6 co-supplementation [302,303,304]. Pooled outcomes found a 13% reduction in LBW (RR 0.87, 95% CI: 0.78–0.96, I2 28%, 16 studies, n = 7940) and SGA (RR 0.85, 95% CI: 0.76–0.95, I2 13%, 28 studies, n = 10,524) with omega-3 supplementation, which remained significant in sensitivity analyses that removed studies with high RoB, but not when studies were restricted to those conducted in LMICs. Additionally, while there was no significant effect on PE, SGA and stillbirths, sensitivity analyses restricting to studies in LMICs only found a significant effect on PE (RR 0.40, 95% CI: 0.21–0.77, I2 0%, six studies, n = 1343). None reported maternal mortality outcomes. There was no significant effect for severe PE, eclampsia or gestational hypertension.
3.3.10. Dietary Interventions
Based on a single Cochrane review for each exposure, there is no current evidence that dietary salt [313] or caffeine restriction [314] reduces rates of PE, SGA or PTB. LBW, stillbirth and maternal mortality were not explored in these reviews. From seven reviews that reported meta-analyses on the effect of antenatal diet and nutritional counselling [59,264,315,316,317,318,319], there is some evidence for a reduced risk of developing PE among normal weight women [264,318] but not among overweight or obese women [317]. Reviews reported mixed results on effects for LBW and PTB, with potentially significant effects for nutritional counselling to increase protein energy intake in particular [316], and consistently no effect across reviews on SGA. The reviews covered 21 trials with antenatal dietary counselling [310,320,321,322,323,324,325,326,327,328,329,330,331,332,333,334,335,336,337,338,339]; all but one [334] was conducted in a HIC. There were eight reviews at high RoB [310,320,323,327,329,331,332,334] and six published in 2014 or later [310,327,328,329,330,337]. Eight studies included diet and nutrition counseling, alongside physical activity promotion [322,323,324,325,326,327,328,329,330]. Pooled outcomes found a 28% reduction in PTB with antenatal dietary counselling (RR 0.72, 95% CI: 0.61–0.86, I2 21%, 14 studies, n = 7612) and sensitivity analyses, excluding studies with high risk, did not change the direction of effect or significance and marginally lowered heterogeneity. Other primary outcomes were not significant and there was no evidence of a significant effect for severe PE or gestational hypertension among secondary outcomes. Almost all studies were conducted in HICs; therefore, sensitivity analyses restricted to studies conducted in LMICs were not applicable for primary outcomes, except in LBW, where antenatal dietary counselling was found to be significantly protective in one study with a high RoB [334].
3.3.11. Other Nutrient Factors
One Cochrane review each reported on balanced protein energy supplementation [316], high protein supplementation [316], calf blood extract supplementation [340], glucose supplementation [340] and galactose supplementation [340]. Balanced protein energy supplementation was found to significantly reduce the risk of SGA (RR 0.79, 95% CI: 0.69–0.90, I2 16%, seven studies, n = 4408) and stillbirth (RR 0.60, 95% CI 0.39–0.94, I2 10%, five studies, n = 3408), while high protein supplementation may be associated with adverse effects on SGA (RR 1.58, 95% CI: 1.03–2.41, I2 N/A, one study, n = 505) [316]. Calf blood, glucose supplementation or galactose supplementation were not significantly associated with SGA [340]. Other primary outcomes were not reported. Additionally, a single Cochrane review each reported on vitamin B6 [341], iodine [342], magnesium [343], or garlic [344]. No significant associations were found (see Table 1).
Table 1.
Evidence map of direction of effect, strength of association and certainty of the evidence.
| PE | SGA | LBW | PTB | Stillbirth | Maternal Mortality | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nutritional supplements | |||||||||||||
| Vitamin A | N/A | RR 1.00 (0.98–1.03, I2 = 0%, 3 studies, n = 14,694) ●●● | RR 0.92 (0.75–1.13, I2 = 51%, 6 studies, n = 16,214) ●● | RR 0.98 (0.94–1.01, I2 = 20%, 6 studies, n = 40,788) ●●● | RR 0.97 (0.92–1.03, I2 = 0%, 4 studies, n = 140,145) ●●● | RR 0.82 (0.56–1.19, I2 = 62%, 5 studies, n = 161,474) ●● | |||||||
| Vitamin B6 | RR 1.71 (0.85–3.45, I2 N/A, 2 studies, n = 1197) ●● | N/A | N/A | N/A | N/A | N/A | |||||||
| Vitamin C and/or E | RR 0.96 (0.89–1.04, I2 33%, 19 studies, n = 24,819) ●● | RR 0.96 (0.89–1.03, I2 24%, 13 studies, n = 21,964) ●●● | RR 0.93 (0.73–1.19, I2 86%, 7 studies, n = 14,356) ●● | RR 1.00 (0.90–1.11, I2 53%,17 studies, n = 23,687) ●● | RR 1.21 (0.92–1.58, I2 0%, 9 studies, n = 19,908) ●●● | RR 0.60 (0.14–2.52, I2 0%, 6 studies, n = 17,574) ●●● | |||||||
| Vitamin D | RR 0.62 (0.43–0.91, I2 0%, 12 studies, n = 1353) ●●●● | RR 0.59 (0.39–0.88, I2 0%, 5 studies, n = 726) ●● | RR 0.76 (0.54–1.06, I2 64%, 6 studies, n = 792) ●● | RR 0.70 (0.49–1.00, I2 39%, 17 studies, n = 4019) ●●● | RR 0.62 (0.19–2.00, I2 0%, 5 studies, n = 860) ●●● | N/A | |||||||
| Vitamin D and calcium | RR 0.49 (0.31–0.77, I2 0%, 3 studies, n = 1120) ●●●● | RR 0.90 (0.58–1.38, I2 NA, 1 study, n = 660) ●● | RR 0.63 (0.12–3.24, I2 16%, 2 studies, n = 107) ●● | RR 1.53 (1.02–2.30, I2 0%, 6 studies, n = 988) ●●● | N/A | N/A | |||||||
| Calcium | RR 0.52 (0.41–0.65, I2 67%, 24 studies, n = 27,442) ●● | RR 1.01 (0.83–1.23, I2 20%, 9 studies, n = 6407) ●●● | RR 0.84 (0.73–0.96, I2 43%, 11 studies, n =7800) ●●● | RR 0.53 (0.33–0.86, I2 95%, 18 studies, n = 14,078) ●● | RR 0.55 (0.24–1.23, I2 75%, 7 studies, n = 10,687) ●● | RR 0.59 (0.18–1.92, I2 40%, 5 studies, n = 10,057) ●●● | |||||||
| Iodine | N/A | RR 1.26 (0.77–2.05, I2 0%, 2 studies, n = 377) ●● | RR 0.56 (0.26–1.20, I2 0%, 2 studies, n = 377) ●● | RR 0.71 (0.30–1.66, I2 32%, 2 studies, n = 376) ●● | N/A | N/A | |||||||
| Iron and/or folic acid | RR 0.99 (0.67–1.47, I2 0%, 6 studies, n = 4454) ●● | RR 0.92 (0.80–1.06, I2 67%, 7 studies, n = 8507 ●● | RR 0.87 (0.77–0.98, I2 27%, 13 studies, n = 22,946) ●●● | RR 0.97 (0.89–1.06, I2 0%, 15 studies, n = 24,420) ●●● | RR 0.88 (0.66–1.17, I2 0%, 7 studies, n = 16,891) ●●● | N/A | |||||||
| Magnesium | RR 0.87 (0.58–1.32, I2 0%, 3 studies, n = 1042) ●●● | RR 0.76 (0.54–1.07, I2 7%, 3 studies, n = 1291) ●●● | RR 0.95 (0.83–1.09, I2 22%, 5 studies, n = 5577) ●●● | RR 0.89 (0.69–1.14, I2 37%, 7 studies, n = 5981) ●●● | RR 0.73 (0.43–1.25, I2 0%, 4 studies, n = 5526) ●● | N/A | |||||||
| Zinc | RR 1.31 (0.82–2.10, I2 39%, 5 studies, n = 2518) ●● | RR 1.03 (0.95–1.11, I2 28%, 8 studies, n =4334) ●●● | RR 1.05 (0.94–1.17, I2 26%, 12 studies, n = 5545) ●● | RR 0.86 (0.76–0.97, I2 17%, 16 studies, n = 7563) ●●● | RR 0.20 (0.01–4.12, I2 NA, 2 studies, n = 376) ●● | RR 0.31 (0.01–7.43, I2 NA, 1 study, n = 85) ●● | |||||||
| Garlic | RR 0.78 (0.31–1.93, I2 N/A, 1 study, n = 100) ●● | N/A | N/A | N/A | N/A | N/A | |||||||
| Multiple micronutrients | RR 0.40 (0.27–0.59, I2 0%, 2 studies, n = 510) ●● | RR 0.88 (0.82–0.95, I2 59%, 16 studies, n = 39,696) ●●● | RR 0.87 (0.85–0.90, I2 22%, 20 studies, n = 70,293) ●●●● | RR 0.93 (0.85–1.02, I2 74%, 17 studies, n = 87,731) ●● | RR 1.09 (0.89–1.34, I2 75%, 20 studies, n = 11,4385) ●● | RR 1.17 (0.82–1.67, I2 0%, 8 studies, n = 84,081) ●●● | |||||||
| Lipid-based nutrients | N/A | RR 0.93 (0.88–0.98, I2 0%, 4 studies, n = 4828) ●●●● | RR 0.89 (0.82–0.98, I2 0%, 5 studies, n = 5851) ●●●● | RR 0.99 (0.86–1.14, I2 0%, 5 studies, n = 6072) ●●● | RR 1.06 (0.60–1.87, I2 55%, 4 studies, n = 6778) ●● | RR 0.52 (0.12–2.28, I2 0%, 3 studies, n = 5628) ●●● | |||||||
| Balanced protein-energy | RR 1.48 (0.82–2.66, I2 N/A, 2 study, n = 463) ●● | RR 0.79 (0.69–0.90, I2 16%, 7 studies, n = 4408) ●●● | N/A | RR 0.96 (0.80–1.16, I2 0%, 5 studies, n = 3374) ●● | RR 0.60 (0.39–0.94, I2 10%, 5 studies, n = 3408) ●●● | N/A | |||||||
| High protein | N/A | RR 1.58 (1.03–2.41, I2 N/A, 1 study, n = 505) ●● | N/A | N/A | RR 0.81 (0.31–2.15, I2 NA, 1 study, n = 529) ●● | N/A | |||||||
| Calf blood extract | N/A | RR 0.54 (0.20–1.47, I2 N/A, 1 study, n = 31) | N/A | N/A | N/A | N/A | |||||||
| Glucose | N/A | RR 1.11 (0.64–1.92, I2 N/A, 1 study, n = 30) ● | N/A | N/A | N/A | N/A | |||||||
| Galactose | N/A | RR 0.78 (0.39–1.54, I2 N/A, 1 study, n = 30) ● | N/A | N/A | N/A | N/A | |||||||
| Omega-3 | RR 0.87 (0.71–1.07, I2 9%, 18 studies, n = 7166) ●●● | RR 1.01 (0.90–1.13, I2 0%, 8 studies, n = 6907) ●●● | RR 0.87 (0.79–0.96, I2 28%, 16 studies, n = 7940) ●●●● | RR 0.85 (0.76–0.95, I2 13%, 28 studies, n = 10,524) ●●●● | RR 0.89 (0.58–1.37, I2 32%, 13 studies, n = 7547) ●● | N/A | |||||||
| Dietary interventions | |||||||||||||
| Salt restriction | RR 1.11 (0.46–2.66, I2 0%, 2 studies, n = 603) ●● | RR 1.50 (0.73–3.07, I2 NA, 1 study, n = 242) ●● | N/A | RR 1.08 (0.46–2.56, I2 NA, 1 study, n = 242) ●● | N/A | N/A | |||||||
| Caffeine restriction | N/A | RR 0.97 (0.57–1.64, I2 NA, 1 study, n = 1150) ●● | N/A | RR 0.81 (0.48–1.37, I2 NA, 1 study, n = 1153) ●● | N/A | N/A | |||||||
| Antenatal dietary counselling | RR 0.97 (0.84–1.14, I2 6%, 15 studies, n = 8087) ●●● | RR 1.15 (0.94–1.41, I2 0%, 10 studies, n = 5529) ●●● | RR 0.54 (0.17–1.71, I2 84%, 4 studies, n = 2271) ●● | RR 0.72 (0.61–0.86 I2 21%, 14 studies, n = 7612) ●●●● | RR 0.63 (0.28–1.40, I2 0%, 6 studies, n = 5631) ●●● | RR 1.08 (0.07–17.32, I2 NA, 1 study, n = 2122) ●● | |||||||
|
LEGEND
Direction of effect and strength of association | |||||||||||||
| Benefit—Strong effect (RR < 0.40) | Benefit—Moderate effect (RR 0.40–0.69) | Benefit—Weak effect (RR 0.70–0.89) | Benefit—Not discernible (RR 0.90–0.99) | No significant effect (95% CI crosses 1.00) | |||||||||
| Harm—Not discernable (RR 1.01–1.09) | Harm—Weak effect (RR 1.10–1.49) | Harm—Moderate effect (RR 1.50–2.99) | Harm—Strong effect (RR ≥ 3.00) | Not available | |||||||||
| Certainty of the evidence | |||||||||||||
| High ●●●● | Moderate ●●● | Low ●● | Very low ● | ||||||||||
PE—pre-eclampsia; SGA—small-for-gestational age; LBW—low birth weight; PTB—preterm birth.
3.4. Evidence Map
Table 1 is an evidence map summarizing the findings for included interventions. Details of the GRADE assessment for each intervention are included in Table S11. The map shows low to moderate certainty of the evidence for almost all interventions and outcomes; however, there was a high certainty of evidence for both vitamin D and combined vitamin D and calcium supplementation on the risk of PE and LNS on SGA. There was a high certainty of evidence for MMN, LNS and omega-3 supplementation on LBW rates. The map shows high certainty of evidence for omega-3 supplementation and antenatal dietary counselling on PTB rates. Evidence was poor in evaluating the effects of vitamin B6, iodine, garlic, balanced protein energy, high protein, calf blood extract, glucose and galactose supplementation, as well as salt and caffeine restriction dietary interventions. The availability of data was especially sparse for the effect of interventions on maternal mortality.
4. Discussion
The purpose of the umbrella review was to evaluate the current evidence on the effect of nutritional supplements and dietary interventions on PE, SGA, LBW, PTB, stillbirth and maternal death. There were few significant effects of interventions, though there were areas where evidence was sparse. Where there were significant associations, effect sizes were largely weak (RR 0.70–0.89) or not discernible (RR 0.90–0.99). Associations with moderate strength included a protective effect of vitamin D supplementation on PE and SGA, combined vitamin D and calcium supplementation on PE, calcium on PE and PTB, MMN on PE and balanced protein energy supplementation on risk of stillbirth. Balanced protein energy supplementation was the only intervention with evidence to reduce stillbirth rates. None were shown to reduce maternal mortality rates.
Considering all significant effects, our findings were largely similar to previous umbrella reviews and the most recent Cochrane reviews. We were able to include more trials in our updated umbrella review and this contributed to the few differences found in comparison with previous reviews. Similar to previous umbrella reviews, calcium supplementation was associated with a reduced risk of PE [10]. In addition, there was evidence to support vitamin D supplementation for PE prevention, which is in line with a network meta-analysis demonstrating that vitamin D is the most promising intervention in comparison to vitamin D and calcium co-supplementation or calcium on its own [110]. MMN supplementation may have been a promising intervention for PE prevention when individual trials were reviewed, which was previously not reported in reviews [10,233]. The findings showed that balanced protein energy supplementation and MMN supplementation was protective against SGA, which is in line with previous reviews [13,14]. Additionally, LNS may be protective against SGA when compared with iron–folic acid supplementation. Similar to a Cochrane review [345], there was a significant association of vitamin D with SGA, but there is some uncertainty as the association was not significant after excluding studies with high RoB. Findings showed that calcium, iron and/or folic acid, MMN, LNS and omega-3 supplementation was protective against LBW; this result builds on previous umbrella reviews where effectiveness was limited to MMN and calcium [13,14]. In contrast to previous umbrella reviews [13,14], but in line with a recent Cochrane review [62], there was no significant effect of vitamin A supplementation on reduced rates of LBW. This review expanded on findings of a previous overview review that zinc or vitamin D supplementation may help to lower rates of PTB [12] and also to suggest that calcium, omega-3 and antenatal dietary counselling may be beneficial. Similar to a previous umbrella review [11] and Cochrane review [113], it was also found that vitamin D and calcium co-supplementation may increase risk of PTB [12,113]. This review suggests uncertainty in the conclusion as the association was not significant after excluding studies with high RoB. This umbrella review adds to the literature by considering multiple interventions on multiple outcomes associated with placental conditions. No single intervention was effective in reducing risk across the six adverse pregnancy outcomes, highlighting that, while all may, in part, be associated with placental disorders, there is heterogeneity in the conditions and different pathways of influence. Interventions associated with protective effects on multiple outcomes included vitamin D (PE, SGA), calcium (PE, LBW, PTB), MMN (PE, SGA, LBW), LNS (SGA, LBW), omega-3 (LBW, PTB) and balanced protein energy supplementation (SGA, stillbirths).
Understanding the pathway to disease and the putative protective role each nutrient may help to explain the heterogeneity of the associations observed. Calcium supplementation may reduce risk of PE through reducing maternal hypertension [10]. Calcium is an important blood pressure regulator; low serum calcium levels increase the secretion of the parathyroid hormone, which increases intracellular calcium in vascular smooth muscle and leads to vasoconstriction [162]. Calcium supplementation decreases parathyroid hormone release and reduces smooth muscle contractility to lower blood pressure as well as potentially preventing preterm labor by reducing the uterine smooth muscle contractility [162]. Vitamin D supports calcium homeostasis by increasing calcium absorption in the intestine, but also helps to regulate the placental immune system and inflammation during placental development [346], which may help explain its effect on SGA, whereas its relationship with calcium may be more apparent in later pregnancy. Omega-3 fatty acids reduce oxidative stress and inflammation associated with PE development, as well as PTB risk factors [274,347,348]. The promising effect of balanced protein energy supplementation on fetal growth and stillbirth supports a shift from single micronutrient deficiencies, which has been much of the focus of the included studies to date, to a broader conceptualization of balanced nutrition and a combination of nutrients. This is also supported by the promising findings of MMN, LNS and vitamin D–calcium co-supplementation, though more research is needed on vitamin D–calcium co-supplementation to understand mechanisms of potential adverse effects on PTB.
A shift to considering diet quality and promoting healthy eating practices is highlighted in the latest WHO guidelines on antenatal care, which recommends counselling on healthy eating and keeping physically active during pregnancy [349]. Daily oral iron and folic acid supplementation, increasing daily energy and protein intake through education or supplementation in undernourished populations, calcium supplementation among women with low baseline intake, vitamin A supplementation among populations with severe deficiency and caffeine restriction among women with high intake were also recommended [349]. While our review did not find direct effects on our outcomes of interest, iron–folic acid supplementation is important in reducing maternal anemia [189], and there is a current gap in the literature on anemia as a potential mediator on the association between supplementation and pregnancy outcomes. Context-specific recommendations also underline the need to understand local malnutrition prevalence and maternal nutritional statuses. While inconsistently reported in trials, recent Cochrane reviews suggest the influence of baseline maternal nutritional status. Calcium supplementation was significantly protective against PE only among women with low calcium diets [140], zinc supplementation was protective against PTB only among those with low baseline zinc [46] and a significant effect of MMN supplementation on PTB was only found in women with BMI ≤ 20 [233]. We found protective effects of omega-3 and vitamin C/E supplementation on PE and vitamin D supplementation on PTB only among studies in LMICs. A significant effect of vitamin C/E supplementation on PE was particularly of note because antioxidant supplementation (i.e., vitamin A, C, E, garlic, etc.) was not associated with significant effects across outcomes, which is in line with existing reviews [9,82,347]. This suggests a need to further look at relationships between supplementation and underlying maternal nutritional deficiencies, which has been reported as common in LMICs [350]. However, the findings with vitamin C/E supplementation in LMICs must be taken with caution as effects were largely driven by two studies with unclear and high RoB. Lastly, the WHO recommendation on counselling on healthy eating during pregnancy is based largely on studies in HICs; whether these recommendations apply in LMIC settings, where food insecurity is common, needs to be explored in detail.
Although this umbrella review covers an impressive body of global research spanning decades, much remains inconclusive. First, some evidence for nutritional interventions are based on old and low-quality studies with small sample sizes, such as with iron and/or folic acid. Secondly, there are gaps in data; earlier studies did not always examine outcomes such as pregnancy hypertension, fetal growth restriction or stillbirths, and there is often a focus on infant outcomes even with recent studies. More studies measure LBW and PTB in almost all interventions and there are less that examine PE, stillbirth and maternal mortality. Pregnant and lactating mothers are targeted for interventions to improve infant health and growth, which represents a missed opportunity to also understand how nutritional interventions can help prevent maternal morbidities and mortality [351]. Third, nutritional interventions can be challenging to evaluate due to insufficient follow-up time in RCTs to detect dietary behavioral change, adequately accounting for underlying dietary deficiencies and routine prenatal supplementation and that RCTs may not always be ethically feasible when existing evidence supports a particular intervention or when a nutritional deficiency is known to be associated with adverse outcomes [352]. Consequently, while conventional systematic reviews value RCTs as the gold standard of study design and undervalue weak strength of associations, well-implemented observational cohort studies may also provide high quality evidence and small effect sizes may have a large impact at a population level [352]. Additionally, effects on outcomes such as stillbirth and maternal death may be confounded by access to quality maternity care [353,354]. The WHO antenatal care guidelines do not recommend MMN supplementation due to potential adverse effects on perinatal mortality [349], which some researchers highlighted that trials reporting adverse effects were conducted in poor rural settings where most mothers had no education and poor access to quality care [232]. They suggest that supplements need to be delivered, together with improving obstetric and postnatal care [232]. This highlights the necessity of nutrition-specific interventions, including nutritional supplementation, alongside nutrition-sensitive interventions, such as those around poverty, water and sanitation, food security, access to care and women’s empowerment working in tandem [355]. However, this also makes for complex interventions and, therefore, interpretation of results.
While this umbrella review comprehensively covered existing reviews to broadly map the evidence, it was limited in capturing the nuances of individual trials. Though most studies had common definitions, such as delivery <37 weeks gestation for PTB, outcome definitions sometimes varied. Maternal nutritional status at baseline and dosages or gestational age of supplementation were not explored, which could influence outcomes. Additionally, the review was limited by the availability of systematic reviews. While most intervention domains had a recent review with moderate to high quality rating, according to the AMSTAR 2 assessment, this may not have captured the most recent individual trials reported or interventions without coverage in a systematic review. For example, the analyses did not cover the FACT trial, a large international, multi-center RCT evaluating folic acid supplementation on pre-eclampsia, published in 2018, because it was not covered in existing systematic reviews [356].
5. Conclusions
In summary, this overview of the efficacy of nutritional supplements and dietary intervention on maternal mortality and placental disorders found evidence that supports vitamin D and/or calcium, omega-3, MMN, LNS and balanced protein energy supplementation to reduce the risks of multiple outcomes. However, more research is needed, particularly around potential adverse effects of vitamin D and calcium co-supplementation and high-protein maternal diets. Overall, these findings are limited by poor quality of evidence but show promise in considering combinations of nutrient factors and a strengthening of policy guidelines that focus on healthy eating and promote dietary balance rather than a focus on trials of single micronutrients.
Acknowledgments
This manuscript is part of the PRECISE (PREgnancy Care Integrating translational Science, Eve-rywhere) Network. The authors would like to express their gratitude to the PRECISE Team for their support. The PRECISE Conceptual Framework Working Group includes: King’s College London (Peter von Dadelszen, Laura A. Magee, Lucilla Poston, Hiten D. Mistry, Marie-Laure Volvert, Cristina Escalona Lopez, Sophie Moore, Rachel Tribe, Andrew Shennan, Tatiana Salis-bury, Lucy Chappell, Rachel Craik); Aga Khan University, Nairobi (Marleen Temmerman, Angela Koech Etyang, Sikolia Wanyonyi, Geoffrey Omuse, Patricia Okiro, Grace Mwashigadi); Centro de Investigação de Saúde de Manhiça (Esperança Sevene, Helena Boene, Corssino Tchavana, Euse-bio Macete, Carla Carillho, Lazaro Quimice, Sonia Maculuve); Donna Russell Consulting (Donna Russell); Imperial College London (Ben Baratt); London School of Hygiene and Tropical Medicine (Joy Lawn, Hannah Blencowe, Veronique Filippi, Matt Silver); Midlands State University (Prestige Tatenda Makanga, Liberty Makacha, Yolisa Dube, Newton Nyapwere, Reason Mlambo); MRC Unit The Gambia at LSHTM (Umberto D’Alessandro, Anna Roca, Melisa Martinez-Alvarez, Ha-wanatu Jah, Brahima Diallo, Abdul Karim Sesay, Fatima Touray, Abdoulie Sillah); University of Oxford (Alison Noble, Aris Papageorghiou); St George’s, University of London (Judith Cart-wright; Guy Whitley, Sanjeev Krishna, Rosemarie Townsend, Asma Khalil); University of British Colombia (Marianne Vidler, Joel Singer, Jing (Larry) Li, Jeffrey Bone, Mai-Lei (Maggie) Woo Kin-shella, Kelly Pickerill, Ash Sandhu, Domena Tu, Rajavel Elango); University of Malawi (William Stones).
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/13/2/472/s1, Table S1: PRISMA checklist, Table S2: Search terms, Table S3: Excluded studies, Table S4: Summary of overview reviews, Table S5: Included reviews by dietary factor, Table S6: Quality assessment for included reviews, Table S7: Summary of outcomes by reviews, Table S8: Study characteristics of individual trials covered in reviews, Table S9: Individual trials’ outcomes, Table S10: Risk of bias assessment, Table S11: GRADE, Figure S1: Primary analyses and funnel plots, Figure S2: Sensitivity analyses.
Author Contributions
Conceptualization, M.-L.W.K.; methodology, M.-L.W.K., S.O. and K.S.; investigation, M.-L.W.K., S.O. and K.S.; formal analysis, M.-L.W.K.; writing—original draft preparation, M.-L.W.K.; writing—review and editing, M.-L.W.K., S.O., K.S., M.V., L.A.M., P.v.D., S.E.M. and R.E.; supervision, M.V., S.E.M. and R.E.; funding acquisition, P.v.D. and R.E. All authors have read and agreed to the published version of the manuscript.
Funding
The PRECISE Network is funded by the UK Research and Innovation Grand Challenges Research Fund GROW Award scheme (grant number: MR/P027938/1). MWK is supported by the Vanier Canada Graduate Scholarship funded by the Government of Canada through the Canadian Institutes of Health Research (CIHR); and Canadian Institute of Health Research (FRN 10321) to R.E.
Institutional Review Board Statement
No ethical approval was needed because data was retrieved from previous published studies in which informed consent was obtained by primary investigators.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in supplementary materials.
Conflicts of Interest
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.FAO. IFAD. UNICEF. WFP. WHO . The State of Food Security and Nutrition in the World 2020: Transforming Food Systems for Affordable Healthy Diets. FAO; Rome, Italy: 2020. [Google Scholar]
- 2.Mason J.B., Shrimpton R., Saldanha L.S., Ramakrishnan U., Victora C.G., Girard A.W., McFarland D.A., Martorell R. The first 500 days of life: Policies to support maternal nutrition. Glob. Health Action. 2015;8:23623. doi: 10.3402/gha.v7.23623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King J.C. A Summary of Pathways or Mechanisms Linking Preconception Maternal Nutrition with Birth Outcomes. J. Nutr. 2016;146:1437S–1444S. doi: 10.3945/jn.115.223479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belkacemi L., Nelson D.M., Desai M., Ross M.G. Maternal Undernutrition Influences Placental-Fetal Development. Biol. Reprod. 2010;83:325–331. doi: 10.1095/biolreprod.110.084517. [DOI] [PubMed] [Google Scholar]
- 5.Lumey L.H. Compensatory placental growth after restricted maternal nutrition in early pregnancy. Placenta. 1998;19:105–111. doi: 10.1016/s0143-4004(98)90105-9. [DOI] [PubMed] [Google Scholar]
- 6.Susser M., Stein Z. Timing in Prenatal Nutrition: A Reprise of the Dutch Famine Study. Nutr. Rev. 2009;52:84–94. doi: 10.1111/j.1753-4887.1994.tb01395.x. [DOI] [PubMed] [Google Scholar]
- 7.Von Dadelszen P., Magee L.A. Preventing deaths due to the hypertensive disorders of pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2016;36:83–102. doi: 10.1016/j.bpobgyn.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Von Dadelszen P., Ayres de Campos D., Barivalala W. Classification of the hypertensive disorders of pregnancy. In: Magee L., von Dadelszen P., Stones W., Mathai M., editors. The FIGO Textbook of Pregnancy Hypertension. The Global Library of Women’s Medicine; London, UK: 2016. pp. 33–61. [Google Scholar]
- 9.Salam R.A., Qureshi R.N., Sheikh S., Khowaja A.R., Sawchuck D., Vidler M., von Dadelszen P., Zaidi S., Bhutta Z. Potential for task-sharing to Lady Health Workers for identification and emergency management of pre-eclampsia at community level in Pakistan. Reprod. Health. 2016;13:107. doi: 10.1186/s12978-016-0214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Achamrah N., Ditisheim A. Nutritional approach to preeclampsia prevention. Curr. Opin. Clin. Nutr. Metab. Care. 2018;21:168–173. doi: 10.1097/MCO.0000000000000462. [DOI] [PubMed] [Google Scholar]
- 11.Mateussi M.V., Latorraca C.D.O.C., Daou J.P., Martimbianco A.L.C., Riera R., Pacheco R.L., Pachito D.V. What do Cochrane systematic reviews say about interventions for vitamin D supplementation? Sao Paulo Med. J. 2017;135:497–507. doi: 10.1590/1516-3180.2017.0230150817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medley N., Vogel J.P., Care A., Alfirevic Z. Interventions during pregnancy to prevent preterm birth: An overview of Cochrane systematic reviews. Cochrane Database Syst. Rev. 2018;11:CD012505. doi: 10.1002/14651858.CD012505.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merialdi M., Carroli G., Villar J., Abalos E., Gulmezoglu A.M., Kulier R., de Onis M. Nutritional interventions during pregnancy for the prevention or treatment of impaired fetal growth: An overview of randomized controlled trials. J. Nutr. 2003;133:1626S. doi: 10.1093/jn/133.5.1626S. [DOI] [PubMed] [Google Scholar]
- 14.Da Silva Lopes K., Ota E., Shakya P., Dagvadorj A., Balogun O.O., Pena-Rosas J.P., De-Regil L.M., Mori R. Effects of nutrition interventions during pregnancy on low birth weight: An overview of systematic reviews. BMJ Glob. Health. 2017;2:e000389. doi: 10.1136/bmjgh-2017-000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.A., Clarke M., Devereaux P.J., Kleinjen J., Moher D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aromataris E., Fernandez R., Godfrey C.M., Holly C., Khalil H., Tungpunkom P. Summarizing systematic reviews. Int. J. Evid. Based Healthc. 2015;13:132–140. doi: 10.1097/XEB.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 17.Smith V., Devane D., Begley C.M., Clarke M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med. Res. Methodol. 2011;11:15. doi: 10.1186/1471-2288-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shea B.J., Reeves B.C., Wells G., Thuku M., Hamel C., Moran J., Moher D., Tugwell P., Welch V., Kristjansson E., et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pieper D., Puljak L., Gonzalez L.M., Minozzi S. Oral Presentation at Cochrane Colloquium. Cochrane; Edinburgh, UK: 2018. Comparison of AMSTAR 2 with ROBIS in systematic reviews including randomized and non-randomized studies. [Google Scholar]
- 20.Khan S.U., Khan M.S.U., Riaz H., Valavoor S., Zhao D., Vaughan L., Okunrintemi V., Riaz I.B., Khan M.B., Kaluski E., et al. Effects of Nutritional Supplements and Dietary Interventions on Cardiovascular Outcomes. Ann. Intern. Med. 2019;171:1–10. doi: 10.7326/M19-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iqbal S., Ekmekcioglu C. Maternal and neonatal outcomes related to iron supplementation or iron status: A summary of meta-analyses. J. Matern. Neonatal. Med. 2019;32:1528–1540. doi: 10.1080/14767058.2017.1406915. [DOI] [PubMed] [Google Scholar]
- 22.Kiely M., Hemmingway A., O’Callaghan K.M. Vitamin D in pregnancy: Current perspectives and future directions. Ther. Adv. Musculoskelet. Dis. 2017;9:145–154. doi: 10.1177/1759720X17706453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matei A., Saccone G., Vogel J.P., Armson A.B. Primary and secondary prevention of preterm birth: A review of systematic reviews and ongoing randomized controlled trials. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019;236:224–239. doi: 10.1016/j.ejogrb.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 24.Villar J., Merialdi M., Gulmezoglu A.M., Abalos E., Carroli G., Kulier R., De Onis M. Characteristics of randomized controlled trials included in systematic reviews of nutritional interventions reporting maternal morbidity, mortality, preterm delivery, intrauterine growth restriction and small for gestational age and birth weight outcomes. J. Nutr. 2003;133:1632S. doi: 10.1093/jn/133.5.1632S. [DOI] [PubMed] [Google Scholar]
- 25.Moutquin J.M., Garner P.R., Burrows R.F., Rey E., Helewa M.E., Lange I.R., Rabkin S.W. Report of the Canadian Hypertension Society Consensus Conference: 2. Nonpharmacologic management and prevention of hypertensive disorders in pregnancy. CMAJ. 1997;157:907–919. [PMC free article] [PubMed] [Google Scholar]
- 26.Mwangi M.N., Prentice A.M., Verhoef H. Safety and benefits of antenatal oral iron supplementation in low-income countries: A review. Br. J. Haematol. 2017;177:884–895. doi: 10.1111/bjh.14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Callaghan K.M., Kiely M. Systematic Review of Vitamin D and Hypertensive Disorders of Pregnancy. Nutrients. 2018;10:294. doi: 10.3390/nu10030294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Secher N.J. Does fish oil prevent preterm birth? J. Perinat. Med. 2007;35:S25–S27. doi: 10.1515/JPM.2007.033. [DOI] [PubMed] [Google Scholar]
- 29.Villar J., De Onis M., Gulmezoglu A.M. Nutritional and antimicrobial interventions to prevent preterm birth: An overview of randomized controlled trials. Obstet. Gynecol. Surv. 1998;53:575. doi: 10.1097/00006254-199809000-00025. [DOI] [PubMed] [Google Scholar]
- 30.Bourassa M.W., Osendarp S.J.M., Adu-Afarwuah S., Ahmed S., Ajello C., Bergeron G., Black R., Christian P., Cousens S., De Pee S., et al. Review of the evidence regarding the use of antenatal multiple micronutrient supplementation in low- and middle-income countries. Ann. N. Y. Acad. Sci. 2019;1444:6–21. doi: 10.1111/nyas.14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eddib A., Yeh J. Prevention of preeclampsia: Is it still a disappointment? Clin. Med. Women’s Health. 2009;2:9–15. doi: 10.4137/CMWH.S2385. [DOI] [Google Scholar]
- 32.Grieger J.A., Clifton V.L. A review of the impact of dietary intakes in human pregnancy on infant birthweight. Nutrients. 2014;7:153–178. doi: 10.3390/nu7010153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gulmezoglu M., Villar J., De Onis M. Effectiveness of Interventions to Prevent or Treat Impaired Fetal Growth. Obstet. Gynecol. Surv. 1997;52:139–148. doi: 10.1097/00006254-199702000-00023. [DOI] [PubMed] [Google Scholar]
- 34.Hosli I., Zanetti-Daellenbach R., Holzgreve W., Lapaire O. Role of omega 3-fatty acids and multivitamins in gestation. J. Perinat. Med. 2007;35:S19–S24. doi: 10.1515/JPM.2007.032. [DOI] [PubMed] [Google Scholar]
- 35.Hovdenak N., Haram K. Influence of mineral and vitamin supplements on pregnancy outcome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012;164:127–132. doi: 10.1016/j.ejogrb.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 36.Ross L., Simkhada P., Smith W.C.S. Evaluating effectiveness of complex interventions aimed at reducing maternal mortality in developing countries. J. Public Health. 2005;27:331–337. doi: 10.1093/pubmed/fdi058. [DOI] [PubMed] [Google Scholar]
- 37.Samuel T.M., Sakwinska O., Makinen K., Burdge G.C., Godfrey K.M., Silva-Zolezzi I. Preterm Birth: A Narrative Review of the Current Evidence on Nutritional and Bioactive Solutions for Risk Reduction. Nutrients. 2019;11:1811. doi: 10.3390/nu11081811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dror D.K., Allen L.H. Interventions with Vitamins B6, B12 and C in Pregnancy. Paediatr. Perinat. Epidemiol. 2012;26:55–74. doi: 10.1111/j.1365-3016.2012.01277.x. [DOI] [PubMed] [Google Scholar]
- 39.Swaney P., Thorp J., Allen I. Vitamin C Supplementation in Pregnancy--Does It Decrease Rates of Preterm Birth? A Systematic Review. Am. J. Perinatol. 2014;31:91–97. doi: 10.1055/s-0033-1338171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agarwal S., Kovilam O., Agrawal D.K. Vitamin D and its impact on maternal-fetal outcomes in pregnancy: A critical review. Crit. Rev. Food Sci. Nutr. 2018;58:755–769. doi: 10.1080/10408398.2016.1220915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zerfu T.A., Ayele H.T. Micronutrients and pregnancy; effect of supplementation on pregnancy and pregnancy outcomes: A systematic review. Nutr. J. 2013;12:20. doi: 10.1186/1475-2891-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soltani H., Duxbury A.M.S., Rundle R., Chan L.-N. A systematic review of the effects of dietary interventions on neonatal outcomes in adolescent pregnancy. Evid. Based Midwifery. 2015;13:29–34. [Google Scholar]
- 43.Hodgetts V., Morris R., Francis A., Gardosi J., Ismail K. Effectiveness of folic acid supplementation in pregnancy on reducing the risk of small-for-gestational age neonates: A population study, systematic review and meta-analysis. BJOG An. Int. J. Obstet. Gynaecol. 2015;122:478–490. doi: 10.1111/1471-0528.13202. [DOI] [PubMed] [Google Scholar]
- 44.Imdad A., Bhutta Z.A. Routine iron/folate supplementation during pregnancy: Effect on maternal anaemia and birth outcomes. Paediatr. Perinat. Epidemiol. 2012;26:168–177. doi: 10.1111/j.1365-3016.2012.01312.x. [DOI] [PubMed] [Google Scholar]
- 45.Chaffee B.W., King J.C. Effect of zinc supplementation on pregnancy and infant outcomes: A systematic review. Paediatr. Perinat. Epidemiol. 2012;26:118–137. doi: 10.1111/j.1365-3016.2012.01289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ota E., Mori R., Middleton P., Tobe-Gai R., Mahomed K., Miyazaki C., Bhutta Z.A. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst Rev. 2015:CD000230. doi: 10.1002/14651858.CD000230.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park J.J.H., Fang M.L., Harari O., Dron L., Siden E.G., Majzoub R., Jeziorska V., Thorlund K., Mills E.D., Bhutta Z.A. Association of Early Interventions With Birth Outcomes and Child Linear Growth in Low-Income and Middle-Income Countries: Bayesian Network Meta-analyses of Randomized Clinical Trials. JAMA Netw. Open. 2019;2:e197871. doi: 10.1001/jamanetworkopen.2019.7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramakrishnan U., Grant F., Goldenberg T., Zongrone A., Martorell R. Effect of Women’s Nutrition Before and During Early Pregnancy on Maternal and Infant Outcomes: A Systematic review. Volume 26. John Wiley & Sons, Ltd.; Hoboken, NJ, USA: 2012. pp. 285–301. Paediatric and Perinatal Epidemiology. [DOI] [PubMed] [Google Scholar]
- 49.Suchdev P.S., Pena-Rosas J.P., De-Regil L.M., PenaRosas P.J., DeRegil M.L. Multiple micronutrient powders for home (point-of-use) fortification of foods in pregnant women. Cochrane database Syst. Rev. 2015:CD011158. doi: 10.1002/14651858.CD011158.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolf H.T., Hegaard H.K., Huusom L.D., Pinborg A.B. Multivitamin use and adverse birth outcomes in high-income countries: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2017;217:e1–e404. doi: 10.1016/j.ajog.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 51.Imdad A., Bhutta Z.A. Maternal nutrition and birth outcomes: Effect of balanced protein-energy supplementation. Paediatr. Perinat. Epidemiol. 2012;26:178–190. doi: 10.1111/j.1365-3016.2012.01308.x. [DOI] [PubMed] [Google Scholar]
- 52.Liberato S.C., Singh G., Mulholland K. Effects of protein energy supplementation during pregnancy on fetal growth: A review of the literature focusing on contextual factors. Food. Nutr. Res. 2013;57:20499. doi: 10.3402/fnr.v57i0.20499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dodd J.M., Crowther C.A., Robinson J.S. Dietary and lifestyle interventions to limit weight gain during pregnancy for obese or overweight women: A systematic review. Acta Obstet. Gynecol. Scand. 2008;87:702–706. doi: 10.1080/00016340802061111. [DOI] [PubMed] [Google Scholar]
- 54.Gui S., Jia J., Niu X., Bai Y., Zou H., Deng J., Zhou R. Arginine supplementation for improving maternal and neonatal outcomes in hypertensive disorder of pregnancy: A systematic review. J. Renin. Angiotensin Aldosterone Syst. 2014;15:88–96. doi: 10.1177/1470320313475910. [DOI] [PubMed] [Google Scholar]
- 55.Dorniak-Wall T., Grivell R.M., Dekker G.A., Hague W., Dodd J.M. The role of L-Arginine in the Prevention and Treatment of Pre-eclampsia: A Systematic Review of Randomised Trials. J Hum Hypertens. 2014;28:230–235. doi: 10.1038/jhh.2013.100. [DOI] [PubMed] [Google Scholar]
- 56.Rumbold A., Duley L., Crowther C.A., Haslam R.R. Antioxidants for preventing pre-eclampsia. Cochrane Database Syst. Rev. 2008:CD004227. doi: 10.1002/14651858.CD004227.pub3. [DOI] [PubMed] [Google Scholar]
- 57.Ribeiro Salles A.M., Galvao T.F., Silva M.T., Domingues Motta L.C., Pereira M.G. Antioxidants for Preventing Preeclampsia: A Systematic Review. Sci. World J. 2012:243476. doi: 10.1100/2012/243476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tenório M.B., Ferreira R.C., Moura F.A., Bueno N.B., Goulart M.O.F., Oliveira A.C.M. Oral antioxidant therapy for prevention and treatment of preeclampsia: Meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2018;28:865–876. doi: 10.1016/j.numecd.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 59.Gresham E., Bisquera A., Byles J.E., Hure A.J. Effects of dietary interventions on pregnancy outcomes: A systematic review and meta-analysis. Matern. Child Nutr. 2016;12:5–23. doi: 10.1111/mcn.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vadillo-Ortega F., Perichart-Perera O., Espino S., Avila-Vergara M.A., Ibarra I., Ahued R., Godines M., Parry S., George Macones G., Jerome F., et al. Effect of supplementation during pregnancy with L-arginine and antioxidant vitamins in medical food on pre-eclampsia in high risk population: Randomised controlled trial. BMJ. 2011;342:d2901. doi: 10.1136/bmj.d2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kongnyuy E.J., Wiysonge C.S., Shey M.S. A systematic review of randomized controlled trials of prenatal and postnatal vitamin A supplementation of HIV-infected women. Int. J. Gynecol. Obstet. 2009;104:5–8. doi: 10.1016/j.ijgo.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 62.McCauley M.E., van den Broek N., Dou L., Othman M. Vitamin A supplementation during pregnancy for maternal and newborn outcomes. Cochrane Database Syst Rev. 2015:CD008666. doi: 10.1002/14651858.CD008666.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thorne-Lyman A.L., Fawzi W.W. Vitamin A and carotenoids during pregnancy and maternal, neonatal and infant health outcomes: A systematic review and meta-analysis. Paediatr. Perinat. Epidemiol. 2012;26:36–54. doi: 10.1111/j.1365-3016.2012.01284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coutsoudis A., Pillay K., Spooner E., Kuhn L., Coovadia H.M. Randomized trial testing the effect of vitamin A supplementation on pregnancy outcomes and early mother-to-child HIV-1 transmission in Durban, South Africa. AIDS. 1999;13:1517–1524. doi: 10.1097/00002030-199908200-00012. [DOI] [PubMed] [Google Scholar]
- 65.Cox S.E., Staalsoe T., Arthur P., Bulmer J.N., Tagbor H., Hviid L., Frost C., Riley E.M., Kirkwood B.R. Maternal vitamin A supplementation and immunity to malaria in pregnancy in Ghanaian primigravids. Trop. Med. Int. Health. 2005;10:1286–1297. doi: 10.1111/j.1365-3156.2005.01515.x. [DOI] [PubMed] [Google Scholar]
- 66.Dijkhuizen M.A., Wieringa F.T., West C.E. Zinc plus β-carotene supplementation of pregnant women is superior to β-carotene supplementation alone in improving vitamin A status in both mothers and infants. Am. J. Clin. Nutr. 2004;80:1299–1307. doi: 10.1093/ajcn/80.5.1299. [DOI] [PubMed] [Google Scholar]
- 67.Fawzi W.W., Msamanga G.I., Spiegelman D., Urassa E.J.N., McGrath N., Mwakagile D., Antelman G., Mbise R., Hererra G., Kapiga S., et al. Randomised trial of effects of vitamin supplements on pregnancy outcomes and T cell counts in HIV-1-infected women in Tanzania. Lancet. 1998;351:1477–1482. doi: 10.1016/S0140-6736(98)04197-X. [DOI] [PubMed] [Google Scholar]
- 68.Kirkwood B.R., Hurt L., Amenga-Etego S., Tawiah C., Zandoh C., Danso S., Hurt C., Edmond K., Hill Z., Asbroek G.T., et al. Effect of vitamin A supplementation in women of reproductive age on maternal survival in Ghana (ObaapaVitA): A cluster-randomised, placebo-controlled trial. Lancet. 2010;375:1640–1649. doi: 10.1016/S0140-6736(10)60311-X. [DOI] [PubMed] [Google Scholar]
- 69.Kumwenda N., Miotti P.G., Taha T.E., Broadhead R., Biggar R.J., Jackson J.B., Melikian G., Richard D., Semba R.D. Antenatal vitamin A supplementation increases birth weight and decreases anemia among infants born to human immunodeficiency virus-infected women in Malawi. Clin. Infect. Dis. 2002;35:618–624. doi: 10.1086/342297. [DOI] [PubMed] [Google Scholar]
- 70.Radhika M.S., Bhaskaram P., Balakrishna N., Ramalakshmi B.A. Red palm oil supplementation: A feasible diet-based approach to improve the vitamin A status of pregnant women and their infants. Food Nutr. Bull. 2003;24:208–217. doi: 10.1177/156482650302400214. [DOI] [PubMed] [Google Scholar]
- 71.Van Den Broek N., White S., Flowers C., Cook J., Letsky E., Tanumihardjo S., Mhango C., Molyneux M., Neilson J.P. Randomised trial of vitamin A supplementation in pregnant women in rural Malawi found to be anaemic on screening by HemoCue. BJOG An. Int. J. Obstet. Gynaecol. 2006;113:569–576. doi: 10.1111/j.1471-0528.2006.00891.x. [DOI] [PubMed] [Google Scholar]
- 72.West K.P., Katz J., Khatry S.K., Leclerq S.C., Pradhan E.K., Shrestha S.R., Connor P.B., Dali S.M., Christian P., Pokhrel R.P., et al. Double blind, cluster randomised trial of low dose supplementation with vitamin A or βcarotene on mortality related to pregnancy in Nepal. BMJ. 1999;318:570–575. doi: 10.1136/bmj.318.7183.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.West K.P., Christian P., Labrique A.B., Rashid M., Shamim A.A., Klemm R.D.W., Massie A.B., Mehdra S., Schulze K.J., Ali H., et al. Effects of vitamin A or beta carotene supplementation on pregnancy-related mortality and infant mortality in rural Bangladesh: A cluster randomized trial. JAMA J Am Med Assoc. 2011;305:1986–1995. doi: 10.1001/jama.2011.656. [DOI] [PubMed] [Google Scholar]
- 74.Christian P., Klemm R., Shamim A.A., Ali H., Rashid M., Shaikh S., Wu L., Mehra S., Labrique A., Katz J., et al. Effects of vitamin A and β-carotene supplementation on birth size and length of gestation in rural Bangladesh: A cluster-randomized trial. Am. J. Clin. Nutr. 2013;97:188–194. doi: 10.3945/ajcn.112.042275. [DOI] [PubMed] [Google Scholar]
- 75.Rumbold A., Ota E., Nagata C., Shahrook S., Crowther C.A. Vitamin C supplementation in pregnancy. Cochrane Database Syst. Rev. 2015:CD004072. doi: 10.1002/14651858.CD004072.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Basaran A., Basaran M., Topatan B. Combined vitamin C and E supplementation for the prevention of preeclampsia: A systematic review and meta-analysis. Obstet. Gynecol. Surv. 2010;65:653–667. doi: 10.1097/OGX.0b013e3182095366. [DOI] [PubMed] [Google Scholar]
- 77.Conde-Agudelo A., Romero R., Kusanovic J.P., Hassan S.S. Supplementation with vitamins C and E during pregnancy for the prevention of preeclampsia and other adverse maternal and perinatal outcomes: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2011;204:503.e1–503.e12. doi: 10.1016/j.ajog.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Polyzos N., Mauri D., Tsappi M., Tzioras S., Kamposioras K., Cortinovis I., Casazza G. Combined vitamin C and E supplementation during pregnancy for preeclampsia prevention: A systematic review. Obstet. Gynecol. Surv. 2007;62:202–206. doi: 10.1097/01.ogx.0000256787.04807.da. [DOI] [PubMed] [Google Scholar]
- 79.Rahimi R., Nikfar S., Rezaie A., Abdollahi M. A Meta-Analysis on the Efficacy and Safety of Combined Vitamin C and E Supplementation in Preeclamptic Women. Hypertens Pregnancy. 2009;28:417–434. doi: 10.3109/10641950802629667. [DOI] [PubMed] [Google Scholar]
- 80.Rossi A.C., Mullin P.M. Prevention of pre-eclampsia with low-dose aspirin or vitamins C and E in women at high or low risk: A systematic review with meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011;158:9–16. doi: 10.1016/j.ejogrb.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 81.Fu Z., Ma Z., Liu G., Wang L., Guo Y. Vitamins supplementation affects the onset of preeclampsia. J. Formos. Med. Assoc. 2018;117:6–13. doi: 10.1016/j.jfma.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 82.Rumbold A., Ota E., Hori H., Miyazaki C., Crowther C.A. Vitamin E supplementation in pregnancy. Cochrane Database Syst. Rev. 2015:CD004069. doi: 10.1002/14651858.CD004069.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Casanueva E., Ripoll C., Tolentino M., Morales R.M., Pfeffer F., Vilchis P., Vadillo-Ortega F. Vitamin C supplementation to prevent premature rupture of the chorioamniotic membranes: A randomized trial. Am. J. Clin. Nutr. 2005;81:859–863. doi: 10.1093/ajcn/81.4.859. [DOI] [PubMed] [Google Scholar]
- 84.Kiondo P., Wamuyu-Maina G., Wandabwa J., Bimenya G.S., Tumwesigye N.M., Okong P. The effects of vitamin C supplementation on pre-eclampsia in Mulago Hospital, Kampala, Uganda: A randomized placebo controlled clinical trial. BMC Pregnancy Childbirth. 2014;14:283. doi: 10.1186/1471-2393-14-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Roberts J.M., Myatt L., Spong C.Y., Thom E.A., Hauth J.C., Leveno K.J., Pearson G.D., Wapner R.J., Varner M.W., Thorp Jr J.M., et al. Vitamins C and E to Prevent Complications of Pregnancy-Associated Hypertension. N. Engl. J. Med. 2010;362:1282–1291. doi: 10.1056/NEJMoa0908056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rumbold A.R., Crowther C.A., Haslam R.R., Dekker G.A., Robinson J.S. Vitamins C and E and the Risks of Preeclampsia and Perinatal Complications. N. Engl. J. Med. 2006;354:1796–1806. doi: 10.1056/NEJMoa054186. [DOI] [PubMed] [Google Scholar]
- 87.Spinnato J.A., Freire S., Pinto e Silva J.L., Cunha Rudge M.V., Martins-Costa S., Koch M.A., Goco N., de Barros Santos C., Cecatti J.G., Costa R., et al. Antioxidant Therapy to Prevent Preeclampsia. Obstet. Gynecol. 2007;110:1311–1318. doi: 10.1097/01.AOG.0000289576.43441.1f. [DOI] [PubMed] [Google Scholar]
- 88.Villar J., Purwar M., Merialdi M., Zavaleta N., Thi Nhu Ngoc N., Anthony J., De Greeff A., Poston L., Shennan A. WHO Vitamin C and Vitamin E trial group. World Health Organisation multicentre randomised trial of supplementation with vitamins C and E among pregnant women at high risk for pre-eclampsia in populations of low nutritional status from developing countries. BJOG An. Int. J. Obstet. Gynaecol. 2009;116:780–788. doi: 10.1111/j.1471-0528.2009.02158.x. [DOI] [PubMed] [Google Scholar]
- 89.Xu H., Perez-Cuevas R., Xiong X., Reyes H., Roy C., Julien P., Smith G., von Dadelszen P., Leduc L., Audibert F., et al. An international trial of antioxidants in the prevention of preeclampsia (INTAPP) Am. J. Obstet. Gynecol. 2010;202:239.e1–239.e10. doi: 10.1016/j.ajog.2010.01.050. [DOI] [PubMed] [Google Scholar]
- 90.Taghriri A., Danesh A. Effects of vitamins E and C in reduction of preeclampsia blood pressure incidence in primigravids. J. Shahrekord Univ. Med. Sci. 2007;9:50–54. [Google Scholar]
- 91.McCance D.R., Holmes V.A., Maresh M.J.A., Patterson C.C., Walker J.D., Pearson D.W.M., Young I.S. Vitamins C and E for prevention of pre-eclampsia in women with type 1 diabetes (DAPIT): A randomised placebo-controlled trial. Lancet. 2010;376:259–266. doi: 10.1016/S0140-6736(10)60630-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McEvoy C.T., Schilling D., Clay N., Jackson K., Go M.D., Spitale P., Bunten C., Leiva M., Gonzales D., Hollister-Smith J., et al. Vitamin C supplementation for pregnant smoking women and pulmonary function in their newborn infants: A randomized clinical trial. JAMA. 2014;311:2074–2082. doi: 10.1001/jama.2014.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Steyn P.S., Odendaal H.J., Schoeman J., Stander C., Fanie N., Grové D. A randomised, double-blind placebo-controlled trial of ascorbic acid supplementation for the prevention of preterm labour. J. Obstet. Gynaecol. 2003;23:150–155. doi: 10.1080/014436103000074673. [DOI] [PubMed] [Google Scholar]
- 94.Beazley D., Ahokas R., Livingston J., Griggs M., Sibai B.M. Vitamin C and E supplementation in women at high risk for preeclampsia: A double-blind, placebo-controlled trial. Am. J. Obstet. Gynecol. 2005;192:520–521. doi: 10.1016/j.ajog.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 95.Chappell L.C., Seed P.T., Briley A.L., Kelly F.J., Lee R., Hunt B.J., Parmar K., Bewley S.J., Shennan A.H., Steer P.J., et al. Effect of antioxidants on the occurrence of pre-eclampsia in women at increased risk: A randomised trial. Lancet. 1999;354:810–816. doi: 10.1016/S0140-6736(99)80010-5. [DOI] [PubMed] [Google Scholar]
- 96.Huria A., Gupta P., Kumar D., Sharma M. Vitamin C and Vitamin E Supplementation in Pregnant Women at Risk for Pre Eclampsia: A Randomized Controlled Trial. Internet J. Health. 2009:10. doi: 10.1016/S0140-6736(06)68433-X. [DOI] [Google Scholar]
- 97.Kalpdev A., Saha S.C., Dhawan V. Vitamin C and e supplementation does not reduce the risk of superimposed PE in pregnancy. Hypertens Pregnancy. 2011;30:447–456. doi: 10.3109/10641955.2010.507840. [DOI] [PubMed] [Google Scholar]
- 98.Nasrolahi S., Alimohammady S., Zamani M. The effect of antioxidants (vitamin C and E) on preeclampsia in primiparious women. J. Gorgan. Univ. Med. Sci. 2006;8:17–21. [Google Scholar]
- 99.Poston L., Briley A., Seed P., Kelly F., Shennan A. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): Randomised placebo-controlled trial. Lancet. 2006;367:1145–1154. doi: 10.1016/S0140-6736(06)68433-X. [DOI] [PubMed] [Google Scholar]
- 100.Abramovici A., Gandley R.E., Clifton R.G., Leveno K.J., Myatt L., Wapner R.J., Thorp J.M., Jr., Mercer B.M., Peaceman A.M., Samuels P., et al. Prenatal vitamin C and E supplementation in smokers is associated with reduced placental abruption and preterm birth: A secondary analysis. BJOG An. Int. J. Obstet. Gynaecol. 2015;122:1740–1747. doi: 10.1111/1471-0528.13201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bastani P., Hamdi K., Abasalizadeh F., Navali N. Effects of vitamin E supplementation on some pregnancy health indices: A randomized clinical trial. Int. J. Gen. Med. 2011;4:461. doi: 10.2147/IJGM.S20107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mahdy Z.A., Siraj H.H., Khaza’ai H., Mutalib M.S.A., Azwar M.H., Wahab M.A., Dali A.Z.H.M., Jaafar R., Ismail N.A.M., Jamil M.A., et al. Does palm oil vitamin E reduce the risk of pregnancy induced hypertension? Acta Medica. 2013;56:104–109. doi: 10.14712/18059694.2014.17. [DOI] [PubMed] [Google Scholar]
- 103.Bi W.G., Nuyt A.M., Weiler H., Leduc L., Santamaria C., Wei S.Q. Association Between Vitamin D Supplementation During Pregnancy and Offspring Growth, Morbidity, and Mortality: A Systematic Review and Meta-analysis. JAMA Pediatr. 2018;172:635–645. doi: 10.1001/jamapediatrics.2018.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Roth D.E., Leung M., Mesfin E., Qamar H., Watterworth J., Papp E. Vitamin D supplementation during pregnancy: State of the evidence from a systematic review of randomised trials. BMJ. 2017;359:j5237. doi: 10.1136/bmj.j5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thorne-Lyman A., Fawzi W.W. Vitamin D during pregnancy and maternal, neonatal and infant health outcomes: A systematic review and meta-analysis. Paediatr. Perinat. Epidemiol. 2012;26:75–90. doi: 10.1111/j.1365-3016.2012.01283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhou S., Tao Y., Huang K., Zhu B., Tao F. Vitamin D and risk of preterm birth: Up-to-date meta-analysis of randomized controlled trials and observational studies. J. Obstet. Gynaecol. Res. 2017;43:247–256. doi: 10.1111/jog.13239. [DOI] [PubMed] [Google Scholar]
- 107.Fogacci S., Fogacci F., Banach M., Michos E.D., Hernandez A.V., Lip G.Y.H., Blaha M.J., Toth P.P., Borghi C., Cicero A.F. Vitamin D supplementation and incident preeclampsia: A systematic review and meta-analysis of randomized clinical trials. Clin. Nutr. 2019;39:1742–1752. doi: 10.1016/j.clnu.2019.08.015. [DOI] [PubMed] [Google Scholar]
- 108.Gallo S., McDermid J.M., Al-Nimr R.I., Hakeem R., Moreschi J.M., Pari-Keener M., Stahnke B., Papoutsakis C., Handu D., Cheng F.W. Vitamin D Supplementation during Pregnancy: An Evidence Analysis Center Systematic Review and Meta-Analysis. J. Acad. Nutr. Diet. 2019;120:898–924. doi: 10.1016/j.jand.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 109.Hypponen E., Cavadino A., Williams D., Hypponen E., Williams D.C.A. Vitamin D and Pre-Eclampsia: A Systematic Review and Meta-Analysis. Ann. Nutr. Metab. 2012;60:137–138. doi: 10.1159/000358338. [DOI] [PubMed] [Google Scholar]
- 110.Vallibhakara S.A.-O., Rattanasiri S., Thakkinstian A., Khaing W., Tantrakul V., Vallibhakara O., Attia J., Thakkinstian A. Calcium and Vitamin D Supplementation for Prevention of Preeclampsia: A Systematic Review and Network Meta-Analysis. Nutrients. 2017;9:1141. doi: 10.3390/nu9101141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maugeri A., Barchitta M., Blanco I., Agodi A. Effects of Vitamin D Supplementation during Pregnancy on Birth Size: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2019;11:442. doi: 10.3390/nu11020442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Palacios C., De-Regil L.M., Lombardo L.K., Pena-Rosas J.P. Vitamin D supplementation during pregnancy: Updated meta-analysis on maternal outcomes. J. Steroid Biochem. Mol. Biol. 2016;164:148–155. doi: 10.1016/j.jsbmb.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Palacios C., Kostiuk L.K., Pena-Rosas P.J. Vitamin D supplementation for women during pregnancy. 2019;1:CD008873. doi: 10.1002/14651858.CD008873.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Perez-Lopez F.R., Pasupuleti V., Mezones-Holguin E., Benites-Zapata V.A., Thota P., Deshpande A., Hernandez A.V. Effect of vitamin D supplementation during pregnancy on maternal and neonatal outcomes: A systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2015;103:1278–1288.e4. doi: 10.1016/j.fertnstert.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 115.Asemi Z., Samimi M., Tabassi Z., Shakeri H., Esmaillzadeh A. Vitamin D supplementation affects serum high-sensitivity C-reactive protein, insulin resistance, and biomarkers of oxidative stress in pregnant women. J. Nutr. 2013;143:1432–1438. doi: 10.3945/jn.113.177550. [DOI] [PubMed] [Google Scholar]
- 116.Brooke O.G., Carter N.D., Brown I.R.F., Cleeve H.J.W., Robinson V.P., Bone C.D.M., Robinson V.P., Winder S.M. Vitamin D supplements in pregnant Asian women: Effects on calcium status and fetal growth. Br. Med. J. 1980;280:751. doi: 10.1136/bmj.280.6216.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Karamali M., Beihaghi E., Mohammadi A.A., Asemi Z. Effects of high-dose Vitamin D supplementation on metabolic status and pregnancy outcomes in pregnant women at risk for pre-eclampsia. Horm. Metab. Res. 2015;47:867–872. doi: 10.1055/s-0035-1548835. [DOI] [PubMed] [Google Scholar]
- 118.Khan F.R., Ahmad T., Hussain R., Bhutta Z.A. A Randomized Controlled Trial of Oral Vitamin D Supplementation in Pregnancy to Improve Maternal Periodontal Health and Birth Weight. J. Int. Oral. Health. 2016;8:657–665. [Google Scholar]
- 119.Litonjua A.A., Carey V.J., Laranjo N., Harshfield B.J., McElrath T.F., O’Connor G.T., Sandel M., Iverson R.E., Lee-Paritz A., Strunk R.C., et al. Effect of prenatal supplementation with Vitamin D on asthma or recurrent wheezing in offspring by age 3 years: The VDAART randomized clinical trial. JAMA J. Am. Med. Assoc. 2016;315:362–370. doi: 10.1001/jama.2015.18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Marya R.K., Ratjee S., Dua V., Sangwan K. Effect of vitamin D supplementation during pregnancy on foetal growth. Indian. J. Med. Res. 1988;88:488–492. [PubMed] [Google Scholar]
- 121.Mohammad-Alizadeh-Charandabi S., Mirghafourvand M., Mansouri A., Najafi M., Khodabande F. The Effect of Vitamin D and Calcium plus Vitamin D during Pregnancy on Pregnancy and Birth Outcomes: A Randomized Controlled Trial. J Caring Sci. 2015;4:35–44. doi: 10.5681/jcs.2015.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Naghshineh E., Sheikhaliyan S. Effect of vitamin D supplementation in the reduce risk of preeclampsia in nulliparous women. Adv. Biomed. Res. 2016;5:7. doi: 10.4103/2277-9175.175239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Razavi M., Jamilian M., Samimi M., Ebrahimi F.A., Taghizadeh M., Bekhradi R., Hosseini E.S., Kashani H.H., Karamali M., Asemi Z. The effects of vitamin D and omega-3 fatty acids co-supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in patients with gestational diabetes. Nutr Metab. 2017;14:1–9. doi: 10.1186/s12986-017-0236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Roth D.E., Al Mahmud A., Raqib R., Akhtar E., Perumal N., Pezzack B., Baqui A.H. Randomized placebo-controlled trial of high-dose prenatal third-trimester vitamin D3 supplementation in Bangladesh: The AViDD trial. Nutr. J. 2013:12. doi: 10.1186/1475-2891-12-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sablok A., Batra A., Thariani K., Batra A., Bharti R., Aggarwal A.R., Kabi B.C., Chellani H. Supplementation of vitamin D in pregnancy and its correlation with feto-maternal outcome. Clin. Endocrinol. 2015;83:536–541. doi: 10.1111/cen.12751. [DOI] [PubMed] [Google Scholar]
- 126.Sasan S.B., Zandvakili F., Soufizadeh N., Baybordi E. The Effects of Vitamin D Supplement on Prevention of Recurrence of Preeclampsia in Pregnant Women with a History of Preeclampsia. Obstet. Gynecol. Int. 2017;2017:1–5. doi: 10.1155/2017/8249264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chawes B.L., Bønnelykke K., Stokholm J., Vissing N.H., Bjarnadóttir E., Schoos A.M.M., Wolsk H.M., Pedersen T.M., Vinding R.K., Thorsteinsdóttir S., et al. Effect of Vitamin D3 supplementation during pregnancy on risk of persistent wheeze in the offspring: A randomized clinical trial. JAMA J. Am. Med. Assoc. 2016;315:353–361. doi: 10.1001/jama.2015.18318. [DOI] [PubMed] [Google Scholar]
- 128.Singh G., Hariharan C., Bhaumik D. Role of vitamin D in reducing the risk of preterm labour. Int. J. Reprod. Contracept. Obs. Gynecol. 2015;1:86–93. doi: 10.5455/2320-1770.ijrcog20150217. [DOI] [Google Scholar]
- 129.Valizadeh M., Piri Z., Mohammadian F., Kamali K., Reza H., Moghadami A. The Impact of Vitamin D Supplementation on Post-Partum Glucose Tolerance and Insulin Resistance in Gestational Diabetes: A Randomized Controlled Trial. Int. J. Endocrinol. Metab. 2016;14:34312. doi: 10.5812/ijem.34312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yap C., Cheung N.W., Gunton J.E., Athayde N., Munns C.F., Duke A., McLean M. Vitamin D supplementation and the effects on glucose metabolism during pregnancy: A randomized controlled trial. Diabetes Care. 2014;37:1837–1844. doi: 10.2337/dc14-0155. [DOI] [PubMed] [Google Scholar]
- 131.Yu C.K.H., Sykes L., Sethi M., Teoh T.G., Robinson S. Vitamin D deficiency and supplementation during pregnancy. Clin. Endocrinol. 2009;70:685–690. doi: 10.1111/j.1365-2265.2008.03403.x. [DOI] [PubMed] [Google Scholar]
- 132.Zerofsky M.S., Jacoby B.N., Pedersen T.L., Stephensen C.B. Daily Cholecalciferol Supplementation during Pregnancy Alters Markers of Regulatory Immunity, Inflammation, and Clinical Outcomes in a Randomized Controlled Trial. J. Nutr. 2016;146:2388–2397. doi: 10.3945/jn.116.231480. [DOI] [PubMed] [Google Scholar]
- 133.Cooper C., Harvey N.C., Bishop N.J., Kennedy S., Papageorghiou A.T., Schoenmakers I., Fraser R., Gandhi S.V., Carr A., D’Angelo S., et al. Maternal gestational vitamin D supplementation and offspring bone health (MAVIDOS): A multicentre, double-blind, randomised placebo-controlled trial. Lancet Diabetes Endocrinol. 2016;4:393–402. doi: 10.1016/S2213-8587(16)00044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dawodu A., Saadi H.F., Bekdache G., Javed Y., Altaye M., Hollis B.W. Randomized Controlled Trial (RCT) of Vitamin D Supplementation in Pregnancy in a Population With Endemic Vitamin D Deficiency. J. Clin. Endocrinol. Metab. 2013;98:2337–2346. doi: 10.1210/jc.2013-1154. [DOI] [PubMed] [Google Scholar]
- 135.Delvin E.E., Salle B.L., Glorieux F.H., Adeleine P., David L.S. Vitamin D supplementation during pregnancy: Effect on neonatal calcium homeostasis. J. Pediatr. 1986;109:328–334. doi: 10.1016/S0022-3476(86)80396-1. [DOI] [PubMed] [Google Scholar]
- 136.Grant C.C., Stewart A.W., Scragg R., Milne T., Rowden J., Ekeroma A., Wall C., Mitchell E.A., Crengle S., Trenholme A., et al. Vitamin D during pregnancy and infancy and infant serum 25-hydroxyvitamin D concentration. Pediatrics. 2014;133:e143–e153. doi: 10.1542/peds.2013-2602. [DOI] [PubMed] [Google Scholar]
- 137.Hashemipour S., Ziaee A., Javadi A., Movahed F., Elmizadeh K., Javadi E.H., Lalooha F. Effect of treatment of vitamin D deficiency and insufficiency during pregnancy on fetal growth indices and maternal weight gain: A randomized clinical trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014;172:15–19. doi: 10.1016/j.ejogrb.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 138.Hossain N., Kanani F.H., Ramzan S., Kausar R., Ayaz S., Khanani R., Pal L. Obstetric and neonatal outcomes of maternal vitamin D supplementation: Results of an open-label, randomized controlled trial of antenatal vitamin D supplementation in Pakistani women. J. Clin. Endocrinol. Metab. 2014;99:2448–2455. doi: 10.1210/jc.2013-3491. [DOI] [PubMed] [Google Scholar]
- 139.Jamilian M., Amirani E., Asemi Z. The effects of vitamin D and probiotic co-supplementation on glucose homeostasis, inflammation, oxidative stress and pregnancy outcomes in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019;38:2098–2105. doi: 10.1016/j.clnu.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 140.Hofmeyr G.J., Lawrie T.A., Atallah A.N., Duley L., Torloni M.R. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst. Rev. 2018;10:CD001059. doi: 10.1002/14651858.CD001059.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ponzetto A., Figura N., Riva P. Prepregnancy Calcium Supplementation and Pre-eclampsia. Vol. 394. Department of Medical Sciences, University of Turin; Turin, Italy: 2019. p. e6. [DOI] [PubMed] [Google Scholar]
- 142.Asemi Z., Tabassi Z., Heidarzadeh Z., Khorammian H., Sabihi S.S., Samimi M. Effect of calcium-vitamin D supplementation on metabolic profiles in pregnant women at risk for pre-eclampsia: A randomized placebo-controlled trial. Pakistan J. Biol. Sci. 2012;15:316–324. doi: 10.3923/pjbs.2012.316.324. [DOI] [PubMed] [Google Scholar]
- 143.Asemi Z., Samimi M., Siavashani M., Mazloomi M., Tabassi Z., Karamali M., Jamilian M., Esmaillzadeh A. Calcium-Vitamin D co-supplementation affects metabolic profiles, but not pregnancy outcomes, in healthy pregnant women. Int. J. Prev. Med. 2016;7:49. doi: 10.4103/2008-7802.177895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Diogenes M.E.L., Bezerra F.F., Rezende E.P., Taveira M.F., Pinhal I., Donangelo C.M. Effect of calcium plus vitamin D supplementation during pregnancy in Brazilian adolescent mothers: A randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2013;98:82–91. doi: 10.3945/ajcn.112.056275. [DOI] [PubMed] [Google Scholar]
- 145.Li X., Gou W. Study on prevention of pregnancy induced hypertension and effect of platelet intracellular free ca(2+) by calcium supplementation. J. Xi’an Med. Univ. 2000;21:46–48. [Google Scholar]
- 146.Samimi M., Kashi M., Foroozanfard F., Karamali M., Bahmani F., Asemi Z., Hamidian Y., Talari H.R., Esmaillzadeh A. The effects of vitamin D plus calcium supplementation on metabolic profiles, biomarkers of inflammation, oxidative stress and pregnancy outcomes in pregnant women at risk for pre-eclampsia. J. Hum. Nutr. Diet. 2016;29:505–515. doi: 10.1111/jhn.12339. [DOI] [PubMed] [Google Scholar]
- 147.Taherian A.-A., Taherian A., Shirvani A. Prevention of Preeclampsia with low-dose aspirin or calcium supplementation. Arch. Iran Med. 2002;5:151–156. [Google Scholar]
- 148.Hofmeyr J.G., Manyame S., Medley N., Williams M.J. Calcium supplementation commencing before or early in pregnancy, for preventing hypertensive disorders of pregnancy. Cochrane Database Syst. Rev. 2019;9:CD011192. doi: 10.1002/14651858.CD011192.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Imdad A., Bhutta Z.A. Effects of calcium supplementation during pregnancy on maternal, fetal and birth outcomes. Paediatr. Perinat. Epidemiol. 2012;26:138–152. doi: 10.1111/j.1365-3016.2012.01274.x. [DOI] [PubMed] [Google Scholar]
- 150.Imdad A., Jabeen A., Bhutta Z.A. Role of calcium supplementation during pregnancy in reducing risk of developing gestational hypertensive disorders: A meta-analysis of studies from developing countries. BMC Public Health. 2011;11:S18. doi: 10.1186/1471-2458-11-S3-S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jabeen M., Yakoob M.Y., Imdad A., Bhutta Z.A. Impact of interventions to prevent and manage preeclampsia and eclampsia on stillbirths. BMC Public Health. 2011;11:S6. doi: 10.1186/1471-2458-11-S3-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kulier R., de Onis M., Gulmezoglu A.M., Villar J. Nutritional interventions for the prevention of maternal morbidity. Int. J. Obstet. Gynecol. 1998;63:231–246. doi: 10.1016/S0020-7292(98)00163-5. [DOI] [PubMed] [Google Scholar]
- 153.Patrelli T., Dall’Asta A., Gizzo S., Pedrazzi G., Piantelli G., Jasonni V.M., Modena A.B. Calcium supplementation and prevention of preeclampsia: A meta-analysis. J. Matern. Neonatal Med. 2012;25:2570–2574. doi: 10.3109/14767058.2012.715220. [DOI] [PubMed] [Google Scholar]
- 154.Sun X., Li H., He X., Li M., Yan P., Xun Y., Lu C., Yang K., Zhang X. The association between calcium supplement and preeclampsia and gestational hypertension: A systematic review and meta-analysis of randomized trials. Hypertens Pregnancy. 2019;38:129–139. doi: 10.1080/10641955.2019.1593445. [DOI] [PubMed] [Google Scholar]
- 155.Tang R., Tang I.C., Henry A., Welsh A. Limited evidence for calcium supplementation in preeclampsia prevention: A meta-analysis and systematic review. Hypertens Pregnancy. 2015;34:181–203. doi: 10.3109/10641955.2014.988353. [DOI] [PubMed] [Google Scholar]
- 156.Villar J.B.J.M., Villar J., Belizan J.M., Villar J.B.J.M. Same nutrient, different hypotheses: Disparities in trials of calcium supplementation during pregnancy. Am. J. Clin. Nutr. 2000;71:1375S. doi: 10.1093/ajcn/71.5.1375s. [DOI] [PubMed] [Google Scholar]
- 157.An L., Li W., Xie T., Peng X., Li B., Xie S., Xu J., Zhou X.H., Guo S.N. Calcium supplementation reducing the risk of hypertensive disorders of pregnancy and related problems: A meta-analysis of multicentre randomized controlled trials. Int. J. Nurs. Pract. 2015;21:19–31. doi: 10.1111/ijn.12171. [DOI] [PubMed] [Google Scholar]
- 158.Bucher H., Guyatt G.H., Cook R., Hatala R., Cook D., Lang J., Hunt D. Effect of calcium supplementation on pregnancy-induced hypertension and preeclampsia: A meta-analysis of randomized controlled trials. JAMA. 1996;275:1113–1117. doi: 10.1001/jama.1996.03530380055031. [DOI] [PubMed] [Google Scholar]
- 159.Buppasiri P., Lumbiganon P., Thinkhamrop J., Ngamjarus C., Laopaiboon M. Calcium supplementation (other than for preventing or treating hypertension) for improving pregnancy and infant outcomes. Cochrane Database Syst. Rev. 2011:CD007079. doi: 10.1002/14651858.CD007079.pub3. [DOI] [PubMed] [Google Scholar]
- 160.Carroli G., Duley L., Belizan J.M., Villar J. Calcium supplementation during pregnancy: A systematic review of randomised controlled trials. Br. J. Obstet. Gynaecol. 1994;101:753–758. doi: 10.1111/j.1471-0528.1994.tb11940.x. [DOI] [PubMed] [Google Scholar]
- 161.Hofmeyr GJAtallah A.N., Duley L.R.A. Calcium supplementation to prevent pre-eclampsia-A systematic review. South Afr. Med. J. 2003;93:224. [PubMed] [Google Scholar]
- 162.Hofmeyr G., Duley L., Atallah A. Dietary calcium supplementation for prevention of pre-eclampsia and related problems: A systematic review and commentary. BJOG. 2007;114:933–943. doi: 10.1111/j.1471-0528.2007.01389.x. [DOI] [PubMed] [Google Scholar]
- 163.Hofmeyr G.J., Belizan J.M., von Dadelszen P. Study CPCAP. Low-dose calcium supplementation for preventing pre-eclampsia: A systematic review and commentary. BJOG AN Int. J. Obstet. Gynaecol. 2014;121:951–957. doi: 10.1111/1471-0528.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Aghamohammadi A., Zafari M. Calcium supplementation in pregnancy and prevention of hypertensive disorders in elderly women. ScienceAsia. 2015;41:259–262. doi: 10.2306/scienceasia1513-1874.2015.41.259. [DOI] [Google Scholar]
- 165.Almirante C. Calcium supplementation during pregnancy in the prevention of EPH gestosis. Prenat. Neonatal Med. 1998;3:24. [Google Scholar]
- 166.Levine R.J., Hauth J.C., Curet L.B., Sibai B.M., Catalano P.M., Morris C.D., DerSimonian R., Esterlitz J.R., Raymond E.G., Bild D.E., et al. Trial of Calcium to Prevent Preeclampsia. N. Engl. J. Med. 1997;337:69–77. doi: 10.1056/NEJM199707103370201. [DOI] [PubMed] [Google Scholar]
- 167.Lopez-Jaramillo P., Narvaez M., Wetgel R., Yepez R. Calcium supplementation reduces the risk of pregnancy-induced hypertension in an Andes population. BJOG. 1989;96:648–655. doi: 10.1111/j.1471-0528.1989.tb03278.x. [DOI] [PubMed] [Google Scholar]
- 168.Lopez-Jaramillo P., Narvaez M., Felix C., Lopez A. Dietary calcium supplementation and prevention of pregnancy hypertension. Lancet. 1990;335:293. doi: 10.1016/0140-6736(90)90112-I. [DOI] [PubMed] [Google Scholar]
- 169.López-Jaramillo P., Delgado F., Jácome P., Terán E., Ruano C., Rivera J. Calcium supplementation and the risk of preeclampsia in Ecuadorian pregnant teenagers. Obstet. Gynecol. 1997;90:162–167. doi: 10.1016/S0029-7844(97)00254-8. [DOI] [PubMed] [Google Scholar]
- 170.Nenad S., Olivera K.-V., Goran R., Ljiljana S. Did calcium management prevent preeclampsia? Pregnancy Hypertens. An. Int. J. Women’s Cardiovasc. Health. 2011;1:287. doi: 10.1016/j.preghy.2011.08.093. [DOI] [PubMed] [Google Scholar]
- 171.Niromanesh S., Laghaii S., Mosavi-Jarrahi A. Supplementary calcium in prevention of pre-eclampsia. Int. J. Gynecol. Obstet. 2001;74:17–21. doi: 10.1016/S0020-7292(01)00374-5. [DOI] [PubMed] [Google Scholar]
- 172.Purwar M., Kulkarni H., Motghare V., Dhole S. Calcium Supplementation and Prevention of Pregnancy Induced Hypertension. J. Obstet. Gynaecol. Res. 1996;22:425–430. doi: 10.1111/j.1447-0756.1996.tb01052.x. [DOI] [PubMed] [Google Scholar]
- 173.Rogers M.S., Fung H.Y.M., Hung C.Y. Calcium and low-dose aspirin prophylaxis in women at high risk of pregnancy-induced hypertension. Hypertens. Pregnancy. 1999;18:165–172. doi: 10.3109/10641959909023076. [DOI] [PubMed] [Google Scholar]
- 174.Sanchez-Ramos L., Briones D., Kaunitz A., Delvalle G., Gaudier F., Walker C. Prevention of pregnancy-induced hypertension by calcium supplementation in angiotensin II-sensitive patients. Obs. Gynecol. 1994;84:349–353. [PubMed] [Google Scholar]
- 175.Villar J., Repke J., Belizan J., Pareja G. Calcium supplementation reduces blood pressure during pregnancy: Results of a randomized controlled clinical trial. Obstet. Gynecol. 1987;70:317–322. [PubMed] [Google Scholar]
- 176.Bassaw B., Roopnarinesingh S., Roopnarinesingh A., Homer H. Prevention of hypertensive disorders of pregnancy. J. Obstet. Gynaecol. 1998;18:123–126. doi: 10.1080/01443619867830. [DOI] [PubMed] [Google Scholar]
- 177.Villar J., Repke J.T. Calcium supplementation during pregnancy may reduce preterm delivery in high-risk populations. Am. J. Obstet. Gynecol. 1990;163:1124–1131. doi: 10.1016/0002-9378(90)90669-X. [DOI] [PubMed] [Google Scholar]
- 178.Villar J., Abdel-Aleem H., Merialdi M., Mathai M., Ali M.M., Zavaleta N., Purwar M., Hofmeyr J., Campódonico L., Landoulsi S., et al. World Health Organization randomized trial of calcium supplementation among low calcium intake pregnant women. Am. J. Obstet. Gynecol. 2006;194:639–649. doi: 10.1016/j.ajog.2006.01.068. [DOI] [PubMed] [Google Scholar]
- 179.Wanchu M., Malhotra S., Khulla M. Calcium supplementation in pre-eclampsia. J. Assoc. Physicians India. 2001;49:795–798. [PubMed] [Google Scholar]
- 180.Belizán J.M., Villar J., Gonzalez L., Campodonico L., Bergel E. Calcium Supplementation to Prevent Hypertensive Disorders of Pregnancy. N. Engl. J. Med. 1991;325:1399–1405. doi: 10.1056/NEJM199111143252002. [DOI] [PubMed] [Google Scholar]
- 181.Boggess K.A., Samuel L., Schmucker B.C., Waters J., Easterling T.R. A randomized controlled trial of the effect of third-trimester calcium supplementation on maternal hemodynamic function. Obstet. Gynecol. 1997;90:157–161. doi: 10.1016/S0029-7844(97)00248-2. [DOI] [PubMed] [Google Scholar]
- 182.Cong K., Chi S., Liu G. Calcium supplementation during pregnancy for reducing pregnancy induced hypertension. Chin. Med. J. 1995;108:57–59. [PubMed] [Google Scholar]
- 183.Crowther C.A., Hiller J.E., Pridmore B., Bryce R., Duggan P., Hague W.M., Robinson J.S. Calcium Supplementation In Nulliparous Women for the Prevention of Pregnancy-Induced Hypertension, Preeclampsia and Preterm Birth: An Australian Randomized Trial. Aust. New Zeal. J. Obstet. Gynaecol. 1999;39:12–18. doi: 10.1111/j.1479-828X.1999.tb03434.x. [DOI] [PubMed] [Google Scholar]
- 184.Goldberg G.R., Jarjou M.A., Cole T.J., Prentice A. Randomized, Placebo-Controlled, Calcium Supplementation Trial in Pregnant Gambian Women Accustomed to a Low Calcium Intake: Effects On maternal Blood Pressure and Infant Growth 1–4. [(accessed on 30 August 2020)]; doi: 10.3945/ajcn.113.059923. Available online: https://academic.oup.com/ajcn/article/98/4/972/4577249. [DOI] [PMC free article] [PubMed]
- 185.Hofmeyr G.J., Betrán A.P., Singata-Madliki M., Cormick G., Munjanja S.P., Fawcus S.S., Mose S., Hall D., Ciganda A., Seuc A.H., et al. Prepregnancy and early pregnancy calcium supplementation among women at high risk of pre-eclampsia: A multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2019;393:330–339. doi: 10.1016/S0140-6736(18)31818-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kumar A., Devi S.G., Batra S., Singh C., Shukla D.K. Calcium supplementation for the prevention of pre-eclampsia. Int. J. Gynecol. Obstet. 2009;104:32–36. doi: 10.1016/j.ijgo.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 187.Cantor A.G., Bougatsos C., Dana T., Blazina I., McDonagh M. Routine iron supplementation and screening for iron deficiency anemia in pregnancy: A systematic review for the U. S. Preventive Services Task Force. Ann. Intern. Med. 2015;162:566–576. doi: 10.7326/M14-2932. [DOI] [PubMed] [Google Scholar]
- 188.Haider B.A., Olofin I., Wang M., Spiegelman D., Ezzati M., Fawzi W.W. Anaemia, prenatal iron use, and risk of adverse pregnancy outcomes: Systematic review and meta-analysis. BMJ. 2013;346:f3443. doi: 10.1136/bmj.f3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.PenaRosas P.J., DeRegil M.L., GarciaCasal M.N., Dowswell T. Daily oral iron supplementation during pregnancy. Cochrane Database Syst. Rev. 2015;7:CD004736. doi: 10.1002/14651858.CD004736.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.De-Regil L.M., Pena-Rosas J.P., Fernandez-Gaxiola A.C., Rayco-Solon P. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst. Rev. 2015:CD007950. doi: 10.1002/14651858.CD007950.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hua X., Zhang J., Guo Y., Shen M., Gaudet L., Janoudi G., Walker M., Wen S.W. Effect of folic acid supplementation during pregnancy on gestational hypertension/preeclampsia: A systematic review and meta-analysis. Hypertens. Pregnancy. 2016;35:447–460. doi: 10.1080/10641955.2016.1183673. [DOI] [PubMed] [Google Scholar]
- 192.Lassi Z.S., Salam R.A., Haider B.A., Bhutta Z.A. Folic acid supplementation during pregnancy for maternal health and pregnancy outcomes. Cochrane Database Syst. Rev. 2013:CD006896. doi: 10.1002/14651858.CD006896.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Saccone G., Berghella V. Folic acid supplementation in pregnancy to prevent preterm birth: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016;199:76–81. doi: 10.1016/j.ejogrb.2016.01.042. [DOI] [PubMed] [Google Scholar]
- 194.Chan K.K.L., Chan B.C.P., Lam K.F., Tam S., Lao T.T. Iron supplement in pregnancy and development of gestational diabetes-A randomised placebo-controlled trial. BJOG. 2009;116:789–798. doi: 10.1111/j.1471-0528.2008.02014.x. [DOI] [PubMed] [Google Scholar]
- 195.Cogswell M.E., Parvanta I., Ickes L., Yip R., Brittenham G.M. Iron supplementation during pregnancy, anemia, and birth weight: A randomized controlled trial. Am. J. Clin. Nutr. 2003;78:773–781. doi: 10.1093/ajcn/78.4.773. [DOI] [PubMed] [Google Scholar]
- 196.Christian P., Khatry S.K., Katz J., Pradhan E.K., LeClerq S.C., Shrestha S.R., Adhikari R.K., Sommer A., Keith P.W. Effects of alternative maternal micronutrient supplements on low birth weight in rural nepal: Double blind randomised community trial. BMJ. 2003;326:571–574. doi: 10.1136/bmj.326.7389.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Fleming A.F., De J.P., Allan N.C. The prevention of megaloblastic anaemia in pregnancy in Nigeria. BJOG. 1968;75:425–432. doi: 10.1111/j.1471-0528.1968.tb00139.x. [DOI] [PubMed] [Google Scholar]
- 198.Kirke P.N., Daly L.E., Elwood J.H. A randomised trial of low dose folic acid to prevent neural tube defects the Irish Vitamin Study Group. Arch. Dis. Child. 1992;67:1442–1446. doi: 10.1136/adc.67.12.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wald N., Sneddon J.D., Frost C., Stone R., MRC Vitamin Study Research Group Prevention of neural tube defects: Results of the Medical Research Council Vitamin Study. Lancet. 1991;338:131–137. doi: 10.1016/0020-7292(92)90076-U. [DOI] [PubMed] [Google Scholar]
- 200.Lee J.I., Lee J.A., Lim H.S. Effect of time of initiation and dose of prenatal iron and folic acid supplementation on iron and folate nutriture of Korean women during pregnancy. Am. J. Clin. Nutr. 2005;82:843–849. doi: 10.1093/ajcn/82.4.843. [DOI] [PubMed] [Google Scholar]
- 201.Meier P.R., Nickerson H.J., Olson K.A., Berg R.L., Meyer J.A. Prevention of iron deficiency anemia in adolescent and adult pregnancies. Clin. Med. Res. 2003;1:29–36. doi: 10.3121/cmr.1.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ndyomugyenyi R., Magnussen P. Chloroquine prophylaxis, iron-folie acid supplementation or case management of malaria attacks in primigravidae in western Uganda: Effects on maternal parasitaemia and haemoglobin levels and on birthweight. Trans. R. Soc. Trop. Med. Hyg. 2000;94:413–418. doi: 10.1016/S0035-9203(00)90125-1. [DOI] [PubMed] [Google Scholar]
- 203.Taylor D., Mallen C., McDougall N., Lind T. Effect of iron supplementation on serum ferritin levels during and after pregnancy. BJOG. 1982;89:1011–1017. doi: 10.1111/j.1471-0528.1982.tb04656.x. [DOI] [PubMed] [Google Scholar]
- 204.Zeng L., Cheng Y., Dang S., Yan H., Dibley M.J., Chang S., Kong L. Impact of micronutrient supplementation during pregnancy on birth weight, duration of gestation, and perinatal mortality in rural western China: Double blind cluster randomised controlled trial. BMJ. 2008;337:1211–1215. doi: 10.1136/bmj.a2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ziaei S., Norrozi M., Faghihzadeh S., Jafarbegloo E. A randomised placebo-controlled trial to determine the effect of iron supplementation on pregnancy outcome in pregnant women with haemoglobin ≥ 13.2 g/dL. BJOG. 2007;114:684–688. doi: 10.1111/j.1471-0528.2007.01325.x. [DOI] [PubMed] [Google Scholar]
- 206.Eskeland B., Malterud K., Ulvik R.J., Hunskaar S. Iron supplementation in pregnancy: Is less enough? Acta Obstet. Gynecol. Scand. 1997;76:822–828. doi: 10.3109/00016349709024359. [DOI] [PubMed] [Google Scholar]
- 207.Menendez C., Todd J., Alonso P.L.L., Francis N., Lulat S., Ceesay S., M’boge B., Greenwood B.M. The effects of iron supplementation during pregnancy, given by traditional birth attendants, on the prevalence of anaemia and malaria. Trans. R. Soc. Trop. Med. Hyg. 1994;88:590–593. doi: 10.1016/0035-9203(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 208.Falahi E., Akbari S., Ebrahimzade F., Gargari B.P. Impact of prophylactic iron supplementation in healthy pregnant women on maternal iron status and birth outcome. Food Nutr. Bull. 2011;32:213–217. doi: 10.1177/156482651103200305. [DOI] [PubMed] [Google Scholar]
- 209.Harvey L.J., Dainty J.R., Hollands W.J., Bull V.J., Hoogewerff J.A., Foxall R.J., McAnena L., Strain J.J., Fairweather-Tait S.J. Effect of high-dose iron supplements on fractional zinc absorption and status in pregnant women. Am. J. Clin. Nutr. 2007;85:131–136. doi: 10.1093/ajcn/85.1.131. [DOI] [PubMed] [Google Scholar]
- 210.Liu J.M., Mei Z., Ye R., Serdula M.K., Ren A., Cogswell M.E. Micronutrient supplementation and pregnancy outcomes: Double-blind randomized controlled trial in China. JAMA Intern. Med. 2013;173:276–282. doi: 10.1001/jamainternmed.2013.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Makrides M., Crowther C.A., Gibson R.A., Gibson R.S., Skeaff C.M. Efficacy and tolerability of low-dose iron supplements during pregnancy: A randomized controlled trial. Am. J. Clin. Nutr. 2003;78:145–153. doi: 10.1093/ajcn/78.1.145. [DOI] [PubMed] [Google Scholar]
- 212.Ouladsahebmadarek E., Sayyah-Melli M., Taghavi S., Abbasalizadeh S., Seyedhejazie M. The effect of supplemental iron elimination on pregnancy outcome. Pakistan J. Med. Sci. 2011;27:641–645. [Google Scholar]
- 213.Siega-Riz A.M., Hartzema A.G., Turnbull C., Thorp J., McDonald T., Cogswell M.E. The effects of prophylactic iron given in prenatal supplements on iron status and birth outcomes: A randomized controlled trial. Am. J. Obstet. Gynecol. 2006;194:512–519. doi: 10.1016/j.ajog.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 214.Charles D.H.M., Ness A.R., Campbell D., Smith G.D., Whitley E., Hall M.H. Folic acid supplements in pregnancy and birth outcome: Re-analysis of a large randomised controlled trial and update of Cochrane review. Paediatr. Perinat. Epidemiol. 2005;19:112–124. doi: 10.1111/j.1365-3016.2005.00633.x. [DOI] [PubMed] [Google Scholar]
- 215.Merialdi M., Caulfield L.E., Zavaleta N., Figueroa A., Dominici F., Dipietro J.A. Randomized controlled trial of prenatal zinc supplementation and the development of fetal heart rate. Am. J. Obstet. Gynecol. 2004;190:1106–1112. doi: 10.1016/j.ajog.2003.09.072. [DOI] [PubMed] [Google Scholar]
- 216.Mahomed K., James D.K., Golding J., McCabe R. Zinc supplementation during pregnancy: A double blind randomised controlled trial. Br. Med. J. 1989;299:826–830. doi: 10.1136/bmj.299.6703.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Osendarp S.J.M., Van Raaij J.M.A., Darmstadt G.L., Baqui A.H., Hautvast J.G.A.J., Fuchs G.J. Zinc supplementation during pregnancy and effects on growth and morbidity in low birthweight infants: A randomised placebo controlled trial. Lancet. 2001;357:1080–1085. doi: 10.1016/S0140-6736(00)04260-4. [DOI] [PubMed] [Google Scholar]
- 218.Robertson J.S., Heywood B., Atkinson S.M. Zinc supplementation during pregnancy. J. Public Health. 1991;13:227–229. doi: 10.1093/oxfordjournals.pubmed.a042625. [DOI] [PubMed] [Google Scholar]
- 219.Saaka M., Oosthuizen J., Beatty S. Effect of prenatal zinc supplementation on birthweight. J. Health Popul. Nutr. 2009;27:619–631. doi: 10.3329/jhpn.v27i5.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Simmer K., Lort-Phillips L., James C., Thompson R.P.H. A double-blind trial of zinc supplementation in pregnancy. Eur. J. Clin. Nutr. 1991;45:139–144. [PubMed] [Google Scholar]
- 221.Xie L., Chen X., Pan J. The effects of zinc supplementation to Chinese rural pregnant women and their pregnancy outcome. J. Shanghai Second. Med. Univ. 2001;13:119–124. [Google Scholar]
- 222.Castillo-Durán C., Marín V.B., Alcázar L.S., Iturralde H., Ruz M.O. Controlled trial of zinc supplementation in Chilean pregnant adolescents. Nutr. Res. 2001;21:715–724. doi: 10.1016/S0271-5317(01)00285-8. [DOI] [Google Scholar]
- 223.Caulfield L.E., Zavaleta N., Figueroa A. Adding zinc to prenatal iron and folate supplements improves maternal and neonatal zinc status in a Peruvian population. Am. J. Clin. Nutr. 1999;69:1257–1263. doi: 10.1093/ajcn/69.6.1257. [DOI] [PubMed] [Google Scholar]
- 224.Cherry F.F., Sandstead H.H., Rojas P., Johnson L.A.K., Batson H.K., Wang X.B. Adolescent pregnancy: Associations among body weight, zinc nutriture, and pregnancy outcome. Am. J. Clin. Nutr. 1989;50:945–954. doi: 10.1093/ajcn/50.5.945. [DOI] [PubMed] [Google Scholar]
- 225.Danesh A., Janghorbani M., Mohammadi B. Effects of zinc supplementation during pregnancy on pregnancy outcome in women with history of preterm delivery: A double-blind randomized, placebo-controlled trial. J. Matern. Neonatal. Med. 2010;23:403–408. doi: 10.3109/14767050903165214. [DOI] [PubMed] [Google Scholar]
- 226.Goldenberg R.L., Copper R.L., Dubard M.B., Hauth J.C., Tamura T., Johnston K.E., Hauth J.C. The Effect of Zinc Supplementation on Pregnancy Outcome. JAMA J. Am. Med. Assoc. 1995;274:463–468. doi: 10.1001/jama.1995.03530060037030. [DOI] [PubMed] [Google Scholar]
- 227.Hafeez A., Mehmood G., Mazhar F. Oral zinc supplementation in pregnant women and its effect on birth weight: A randomised controlled trial. Arch. Dis. Child Fetal Neonatal Ed. 2005;90:F170–F171. doi: 10.1136/adc.2004.063008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hunt I.F., Murphy N.J., Cleaver A.C. Zinc supplementation during pregnancy: Effects on selected blood constituents and on progress and outcome of pregnancy in low-income women of Mexican descent. Am J Clin Nutr. 1984;40:508–521. doi: 10.1093/ajcn/40.3.508. [DOI] [PubMed] [Google Scholar]
- 229.Jønsson B., Hauge B., Larsen M.F., Hald F. Zinc supplementation during pregnancy: A double blind randomised controlled trial. Acta Obstet. Gynecol. Scand. 1996;75:725–729. doi: 10.3109/00016349609065735. [DOI] [PubMed] [Google Scholar]
- 230.Fall C.H.D., Fisher D.J., Osmond C., Margetts B.M. Multiple micronutrient supplementation during pregnancy in low-income countries: A meta-analysis of effects on birth size and length of gestation. Food Nutr. Bull. 2009;30:S533–S546. doi: 10.1177/15648265090304S408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Haider B.A., Yakoob M.Y., Bhutta Z.A. Effect of multiple micronutrient supplementation during pregnancy on maternal and birth outcomes. BMC Public Health. 2011;11:1–9. doi: 10.1186/1471-2458-11-S3-S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kawai K., Spiegelman D., Shankar A.H., Fawzi W.W. Maternal multiple micronutrient supplementation and pregnancy outcomes in developing countries: Meta-analysis and meta-regression. Bull. World Health Organ. 2011;89:402B–411B. doi: 10.2471/BLT.10.083758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Keats E.C., Haider B.A., Tam E., Bhutta Z.A. Multiple-micronutrient supplementation for women during pregnancy. Cochrane database Syst Rev. 2019;3:CD004905. doi: 10.1002/14651858.CD004905.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ronsmans C., Fisher D.J., Osmond C., Margetts B.M., Fall C.H. Multiple micronutrient supplementation during pregnancy in low-income countries: A meta-analysis of effects on stillbirths and on early and late neonatal mortality. Food Nutr. Bull. 2009;30:S547. doi: 10.1177/15648265090304S409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Shah P.S., Ohlsson A. Effects of prenatal multimicronutrient supplementation on pregnancy outcomes: A meta-analysis. CMAJ. 2009;180:E99–E108. doi: 10.1503/cmaj.081777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Smith E.R., Shankar A.H., Wu L.S.-F., Aboud S., Adu-Afarwuah S., Ali H., Agustina R., Arifeen S., Ashorn P., Bhutta Z.A., et al. Modifiers of the effect of maternal multiple micronutrient supplementation on stillbirth, birth outcomes, and infant mortality: A meta-analysis of individual patient data from 17 randomised trials in low-income and middle-income countries. Lancet Glob. Health. 2017;5:E1090–E1100. doi: 10.1016/S2214-109X(17)30371-6. [DOI] [PubMed] [Google Scholar]
- 237.Fawzi W.W., Msamanga G.I., Urassa W., Hertzmark E., Petraro P., Willett W.C., Spiegelman D. Vitamins and Perinatal Outcomes among HIV-Negative Women in Tanzania. N. Engl. J. Med. 2007;356:1423–1431. doi: 10.1056/NEJMoa064868. [DOI] [PubMed] [Google Scholar]
- 238.Friis H., Gomo E., Nyazema N., Ndhlovu P., Krarup H., Kæstel P., Michaelsen K.F. Effect of multimicronutrient supplementation on gestational length and birth size: A randomized, placebo-controlled, double-blind effectiveness trial in Zimbabwe. Am. J. Clin. Nutr. 2004;80:178–184. doi: 10.1093/ajcn/80.1.178. [DOI] [PubMed] [Google Scholar]
- 239.Gupta P., Ray M., Dua T., Radhakrishnan G., Kumar R., Sachdev H.P.S. Multimicronutrient supplementation for undernourished pregnant women and the birth size of their offspring: A double-blind, randomized, placebo-controlled trial. Arch Pediatr. Adolesc. Med. 2007;161:58–64. doi: 10.1001/archpedi.161.1.58. [DOI] [PubMed] [Google Scholar]
- 240.Delhi I.N. Multicentric study of efficacy of periconceptional folic acid containing vitamin supplementation in prevention of open neural tube defects in India. Indian, J. Med. Res. 2000;112:206–211. [PubMed] [Google Scholar]
- 241.Johnson W., Darboe M.K., Sosseh F., Nshe P., Prentice A.M., Moore S.E. Association of prenatal lipid-based nutritional supplementation with fetal growth in rural Gambia. Matern. Child Nutr. 2017;13:e12367. doi: 10.1111/mcn.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kæstel P., Michaelsen K.F., Aaby P., Friis H. Effects of prenatal multimicronutrient supplements on birth weight and perinatal mortality: A randomised, controlled trial in Guinea-Bissau. Eur. J. Clin. Nutr. 2005;59:1081–1089. doi: 10.1038/sj.ejcn.1602215. [DOI] [PubMed] [Google Scholar]
- 243.Osrin D., Vaidya A., Shrestha Y., Baniya R.B., Manandhar D.S., Adhikari R.K., Filteau S., Tomkins A., Anthony M.D.L. Effects of antenatal multiple micronutrient supplementation on birthweight and gestational duration in Nepal: Double-blind, randomised controlled trial. Lancet. 2005;365:955–962. doi: 10.1016/S0140-6736(05)71084-9. [DOI] [PubMed] [Google Scholar]
- 244.Ramakrishnan U., González-Cossío T., Neufeld L.M., Rivera J., Martorell R. Multiple micronutrient supplementation during pregnancy does not lead to greater infant birth size than does iron-only supplementation: A randomized controlled trial in a semirural community in Mexico. Am. J. Clin. Nutr. 2003;77:720–725. doi: 10.1093/ajcn/77.3.720. [DOI] [PubMed] [Google Scholar]
- 245.Roberfroid D., Huybregts L., Lanou H., Henry M.C., Meda N., Menten J., Kolsteren P., The MISAME Study Group Effects of maternal multiple micronutrient supplementation on fetal growth: A double-blind randomized controlled trial in rural Burkina Faso. Am. J. Clin. Nutr. 2008;88:1330–1340. doi: 10.3945/ajcn.2008.26296. [DOI] [PubMed] [Google Scholar]
- 246.Rumiris D., Purwosunu Y., Wibowo N., Farina A., Sekizawa A. Lower rate of preeclampsia after antioxidant supplementation in pregnant women with low antioxidant status. Hypertens Pregnancy. 2006;25:241–253. doi: 10.1080/10641950600913016. [DOI] [PubMed] [Google Scholar]
- 247.Sunawang Utomo B., Hidayat A., Kusharisupeni S. Preventing low birthweight through maternal multiple micronutrient supplementation: A cluster-randomized, controlled trial in lndramayu, West Java. Food Nutr. Bull. 2009;30:S488–S495. doi: 10.1177/15648265090304S403. [DOI] [PubMed] [Google Scholar]
- 248.Tofail F., Persson L.Å., Arifeen SEl Hamadani J.D., Mehrin F., Ridout D., Ekström E.C., Huda S.N., Grantham-McGregor S.M. Effects of prenatal food and micronutrient supplementation on infant development: A randomized trial from the Maternal and Infant Nutrition Interventions, Matlab (MINIMat) study. Am. J. Clin. Nutr. 2008;87:704–711. doi: 10.1093/ajcn/87.3.704. [DOI] [PubMed] [Google Scholar]
- 249.West K.P., Shamim A.A., Mehra S., Labrique A.B., Ali H., Shaikh S., Klemm R.D., Wu L.S., Mitra M., Haque R., et al. Effect of Maternal multiple micronutrient vs iron-folic acid supplementation on infant mortality and adverse birth outcomes in rural Bangladesh: The JiVitA-3 randomized trial. JAMA. 2014;312:2649–2658. doi: 10.1001/jama.2014.16819. [DOI] [PubMed] [Google Scholar]
- 250.Zagré N.M., Desplats G., Adou P., Mamadoultaibou A., Aguayo V.M. Prenatal multiple micronutrient supplementation has greater impact on birthweight than supplementation with iron and folic acid: A cluster-randomized, double-blind, controlled programmatic study in rural Niger. Food Nutr. Bull. 2007;28:317–327. doi: 10.1177/156482650702800308. [DOI] [PubMed] [Google Scholar]
- 251.Supplementation with Multiple Micronutrients Intervention Trial (SUMMIT) Study Group Effect of maternal multiple micronutrient supplementation on fetal loss and infant death in Indonesia: A double-blind cluster-randomised trial. Lancet. 2008;371:215–227. doi: 10.1016/S0140-6736(08)60133-6. [DOI] [PubMed] [Google Scholar]
- 252.Adu-Afarwuah S., Lartey A., Okronipa H., Ashorn P., Zeilani M., Peerson J.M., Arimond M., Vosti S., Dewey K.G. Lipid-based nutrient supplement increases the birth size of infants of primiparous women in Ghana. Am. J. Clin. Nutr. 2015;101:835–846. doi: 10.3945/ajcn.114.091546. [DOI] [PubMed] [Google Scholar]
- 253.Ashorn P., Alho L., Ashorn U., Cheung Y.B., Dewey K.G., Harjunmaa U., Lartey A., Nkhoma M., Phiri N., Phuka J., et al. The impact of lipid-based nutrient supplement provision to pregnant women on newborn size in rural Malawi: A randomized controlled trial. Am. J. Clin. Nutr. 2015;101:387–397. doi: 10.3945/ajcn.114.088617. [DOI] [PubMed] [Google Scholar]
- 254.Bhutta Z.A., Rizvi A., Raza F., Hotwani S., Zaidi S., Hossain S.M., Soofi S., Bhutta S. A comparative evaluation of multiple micronutrient and iron-folic acid supplementation during pregnancy in Pakistan: Impact on pregnancy outcomes. Food Nutr. Bull. 2009;30:S496–S505. doi: 10.1177/15648265090304S404. [DOI] [PubMed] [Google Scholar]
- 255.Brough L., Rees G.A., Crawford M.A., Morton R.H., Dorman E.K. Effect of multiple-micronutrient supplementation on maternal nutrient status, infant birth weight and gestational age at birth in a low-income, multi-ethnic population. Br J Nutr. 2010;104:437–445. doi: 10.1017/S0007114510000747. [DOI] [PubMed] [Google Scholar]
- 256.Merchant A.T., Msamanga G., Villamor E., Saathoff E., O’Brien M., Hertzmark E., Hunter D.J., Fawzi W.W. Multivitamin supplementation of HIV-positive women during pregnancy reduces hypertension. J. Nutr. 2005;135:1776–1781. doi: 10.1093/jn/135.7.1776. [DOI] [PubMed] [Google Scholar]
- 257.Persson L.Å., Arifeen S., Ekström E.C., Rasmussen K.M., Frongillo E.A., Yunus M. Effects of prenatal micronutrient and early food supplementation on maternal hemoglobin, birth weight, and infant mortality among children in Bangladesh: The MINIMat randomized trial. JAMA. 2012;307:2050–2059. doi: 10.1001/jama.2012.4061. [DOI] [PubMed] [Google Scholar]
- 258.Christian P., Khatry S.K., LeClerq S.C., Dali S.M. Effects of prenatal micronutrient supplementation on complications of labor and delivery and puerperal morbidity in rural Nepal. Int. J. Gynecol. Obstet. 2009;106:3–7. doi: 10.1016/j.ijgo.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 259.Das J.K., Hoodbhoy Z., Salam R.A., Bhutta A.Z., Valenzuela-Rubio N.G., Weise Prinzo Z., Bhutta Z.A. Lipid-based nutrient supplements for maternal, birth, and infant developmental outcomes. Cochrane Database Syst. Rev. 2018:CD012610. doi: 10.1002/14651858.CD012610.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Goto E. Effectiveness of Prenatal Lipid-Based Nutrient Supplementation to Improve Birth Outcomes: A Meta-analysis. Am. J. Trop. Med. Hyg. 2019;101:994–999. doi: 10.4269/ajtmh.19-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Huybregts L., Roberfroid D., Lanou H., Menten J., Meda N., Van Camp J., Kolsteren P. Prenatal food supplementation fortified with multiple micronutrients increases birth length: A randomized controlled trial in rural Burkina Faso. Am. J. Clin. Nutr. 2009;90:1593–1600. doi: 10.3945/ajcn.2009.28253. [DOI] [PubMed] [Google Scholar]
- 262.Mridha M.K., Matias S.L., Chaparro C.M., Paul R.R., Hussain S., Vosti S.A., Harding K.L., Cummins J.R., Day L.T., Saha S.L., et al. Lipid-based nutrient supplements for pregnant women reduce newborn stunting in a cluster-randomized controlled effectiveness trial in Bangladesh. Am. J. Clin. Nutr. 2016;103:236–249. doi: 10.3945/ajcn.115.111336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Graft-Johnson J., de Vesel L., Rosen H.E., Rawlins B., Abwao S., Mazia G., Bozsa R., Mwebesa W., Khadka N., Kamunya R., et al. Cross-sectional observational assessment of quality of newborn care immediately after birth in health facilities across six sub-Saharan African countries. BMJ Open. 2017;7:e014680. doi: 10.1136/bmjopen-2016-014680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Allen R., Rogozinska E., Sivarajasingam P., Khan K.S., Thangaratinam S. Effect of diet- And lifestyle-based metabolic risk-modifying interventions on preeclampsia: A meta-analysis. Acta Obstet. Gynecol. Scand. 2014;93:973–985. doi: 10.1111/aogs.12467. [DOI] [PubMed] [Google Scholar]
- 265.Chen B., Ji X., Zhang L., Hou Z., Li C., Tong Y. Fish Oil Supplementation does not Reduce Risks of Gestational Diabetes Mellitus, Pregnancy-Induced Hypertension, or Pre-Eclampsia: A Meta-Analysis of Randomized Controlled Trials. Med. Sci. Monit. 2015;21:2322–2330. doi: 10.12659/MSM.894033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Saccone G., Saccone I., Berghella V. Omega-3 long-chain polyunsaturated fatty acids and fish oil supplementation during pregnancy: Which evidence? J. Matern. Neonatal. Med. 2016;29:2389–2397. doi: 10.3109/14767058.2015.1086742. [DOI] [PubMed] [Google Scholar]
- 267.Szajewska H., Horvath A., Koletzko B. Effect of n-3 long-chain polyunsaturated fatty acid supplementation of women with low-risk pregnancies on pregnancy outcomes and growth measures at birth: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2006;83:1337–1344. doi: 10.1093/ajcn/83.6.1337. [DOI] [PubMed] [Google Scholar]
- 268.Newberry S.J., Chung M., Booth M., Maglione M.A., Tang A.M., O’Hanlon C.E., Wang D.D., Okunogbe A., Huang C., Motala A., et al. Omega-3 Fatty Acids and Maternal and Child Health: An Updated Systematic Review. Evid. Rep. Technol Assess. 2016;224:1–826. doi: 10.23970/AHRQEPCERTA224. [DOI] [PubMed] [Google Scholar]
- 269.Salvig J.D., Lamont R.F. Evidence regarding an effect of marine n-3 fatty acids on preterm birth: A systematic review and meta-analysis. ACTA Obstet. Gynecol. Scand. 2011;90:825–838. doi: 10.1111/j.1600-0412.2011.01171.x. [DOI] [PubMed] [Google Scholar]
- 270.Chen B., Ji X., Zhang L., Hou Z., Li C., Tong Y. Fish oil supplementation improves pregnancy outcomes and size of the newborn: A meta-analysis of 21 randomized controlled trials. J. Matern Neonatal Med. 2016;29:2017–2027. doi: 10.3109/14767058.2015.1072163. [DOI] [PubMed] [Google Scholar]
- 271.Horvath A., Koletzko B., Szajewska H. Effect of supplementation of women in high-risk pregnancies with long-chain polyunsaturated fatty acids on pregnancy outcomes and growth measures at birth: A meta-analysis of randomized controlled trials. Br. J. Nutr. 2007;98:253–259. doi: 10.1017/S0007114507709078. [DOI] [PubMed] [Google Scholar]
- 272.Imhoff-Kunsch B., Briggs V., Goldenberg T., Ramakrishnan U. Effect of n-3 long-chain polyunsaturated fatty acid intake during pregnancy on maternal, infant, and child health outcomes: A systematic review. Paediatr. Perinat. Epidemiol. 2012;26:91–107. doi: 10.1111/j.1365-3016.2012.01292.x. [DOI] [PubMed] [Google Scholar]
- 273.Kar S., Wong M., Rogozinska E., Thangaratinam S. Effects of omega-3 fatty acids in prevention of early preterm delivery: A systematic review and meta-analysis of randomized studies. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016;198:40–46. doi: 10.1016/j.ejogrb.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 274.Middleton P., Gomersall J.C., Gould J.F., Shepherd E., Olsen S.F., Makrides M. Omega-3 fatty acid addition during pregnancy. Cochrane Database Syst. Rev. 2018;11:CD003402. doi: 10.1002/14651858.CD003402.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Saccone G., Berghella V., Maruotti G.M., Sarno L., Martinelli P. Omega-3 supplementation during pregnancy to prevent recurrent intrauterine growth restriction: Systematic review and meta-analysis of randomized controlled trials. Ultrasound Obstet. Gynecol. 2015;46:659–664. doi: 10.1002/uog.14910. [DOI] [PubMed] [Google Scholar]
- 276.Saccone G., Berghella V. Omega-3 long chain polyunsaturated fatty acids to prevent preterm birth: A systematic review and meta-analysis. Obstet Gynecol. 2015;125:663–672. doi: 10.1097/AOG.0000000000000668. [DOI] [PubMed] [Google Scholar]
- 277.Saccone G., Berghella V. Omega-3 supplementation to prevent recurrent preterm birth: A systematic review and metaanalysis of randomized controlled trials. Am. J. Obstet. Gynecol. 2015;213:135–140. doi: 10.1016/j.ajog.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 278.Lucia Bergmann R., Bergmann K.E., Haschke-Becher E., Richter R., Dudenhausen J.W., Barclay D., Haschke F. Does maternal docosahexaenoic acid supplementation during pregnancy and lactation lower BMI in late infancy? J. Perinat. Med. 2007;35:295–300. doi: 10.1515/JPM.2007.085. [DOI] [PubMed] [Google Scholar]
- 279.Hauner H., Much D., Vollhardt C., Brunner S., Schmid D., Sedlmeier E.M., Heimberg E., Schuster T., Zimmermann A., Schneider K.T.M., et al. Effect of reducing the n-6:n-3 long-chain PUFA ratio during pregnancy and lactation on infant adipose tissue growth within the first year of life: An open-label randomized controlled trial. Am. J. Clin. Nutr. 2012;95:383–394. doi: 10.3945/ajcn.111.022590. [DOI] [PubMed] [Google Scholar]
- 280.Helland I.B., Saugstad O.D., Smith L., Saarem K., Solvoll K., Ganes T., Drevon C.A. Similar effects on infants of n-3 and n-6 fatty acids supplementation to pregnant and lactating women. Pediatrics. 2001;108:e82. doi: 10.1542/peds.108.5.e82. [DOI] [PubMed] [Google Scholar]
- 281.Horvaticek M., Djelmis J., Ivanisevic M., Oreskovic S., Herman M. Effect of eicosapentaenoic acid and docosahexaenoic acid supplementation on C-peptide preservation in pregnant women with type-1 diabetes: Randomized placebo controlled clinical trial. Eur. J. Clin. Nutr. 2017;71:968–972. doi: 10.1038/ejcn.2017.46. [DOI] [PubMed] [Google Scholar]
- 282.Jamilian M., Samimi M., Kolahdooz F., Khalaji F., Razavi M., Asemi Z. Omega-3 fatty acid supplementation affects pregnancy outcomes in gestational diabetes: A randomized, double-blind, placebo-controlled trial. J. Matern Neonatal Med. 2016;29:669–675. doi: 10.3109/14767058.2015.1015980. [DOI] [PubMed] [Google Scholar]
- 283.Jamilian M., Hashemi Dizaji S., Bahmani F., Taghizadeh M., Memarzadeh M.R., Karamali M., Akbari M., Asemi Z. A Randomized Controlled Clinical Trial Investigating the Effects of Omega-3 Fatty Acids and Vitamin E Co-Supplementation on Biomarkers of Oxidative Stress, Inflammation and Pregnancy Outcomes in Gestational Diabetes. Can. J. Diabetes. 2017;41:143–149. doi: 10.1016/j.jcjd.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 284.Lalooha F. Evaluation of the effect of omega-3 supplements in the prevention of preeclampsia among high risk women. Afr. J. Pharm. Pharmacol. 2012;6:2580–2583. doi: 10.5897/AJPP11.836. [DOI] [Google Scholar]
- 285.Makrides M., Gibson R.A., McPhee A.J., Yelland L., Quinlivan J., Ryan P. Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: A randomized controlled trial. JAMA. 2010;304:1675–1683. doi: 10.1001/jama.2010.1507. [DOI] [PubMed] [Google Scholar]
- 286.Malcolm C.A., Hamilton R., McCulloch D.L., Montgomery C., Weaver L.T. Scotopic electroretinogram in term infants born of mothers supplemented with docosahexaenoic acid during pregnancy. Investig. Ophthalmol. Vis. Sci. 2003;44:3685–3691. doi: 10.1167/iovs.02-0767. [DOI] [PubMed] [Google Scholar]
- 287.Mardones F., Urrutia M.T., Villarroel L., Rioseco A., Castillo O., Rozowski J., Tapia J.L., Bastias G., Bacallao J., Rojas I. Effects of a dairy product fortified with multiple micronutrients and omega-3 fatty acids on birth weight and gestation duration in pregnant Chilean women. Public Health Nutr. 2008;11:30–40. doi: 10.1017/S1368980007000110. [DOI] [PubMed] [Google Scholar]
- 288.Harris M.A., Miller S., Harris M., Baker S., Davalos D., Clark A., McGirr K.A. Intake of Total Omega-3 Docosahexaenoic Acid Associated with Increased Gestational Length and Improved Cognitive Performance at 1 Year of Age. J. Nutr. Health Food. Eng. 2016;5:642–651. doi: 10.15406/jnhfe.2016.05.00176. [DOI] [Google Scholar]
- 289.Bisgaard H., Stokholm J., Chawes B., Vissing N., Bjarnadóttir E., Schoos A., Wolsk H.M., Pedersen T.M., Vinding R.K., Thorsteinsdóttir S., et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. Acta Pediatr. Esp. 2016;375:25. doi: 10.1056/NEJMoa1503734. [DOI] [PubMed] [Google Scholar]
- 290.Min Y., Djahanbakhch O., Hutchinson J., Bhullar A.S., Raveendran M., Hallot A., Eram S., Namugere I., Nateghian S., Ghebremeskel K. Effect of docosahexaenoic acid-enriched fish oil supplementation in pregnant women with type 2 diabetes on membrane fatty acids and fetal body composition-double-blinded randomized placebo-controlled trial. Diabet Med. 2014;31:1331–1340. doi: 10.1111/dme.12524. [DOI] [PubMed] [Google Scholar]
- 291.Min Y., Djahanbakhch O., Hutchinson J., Eram S., Bhullar A.S., Namugere I., Ghebremeskel K. Efficacy of docosahexaenoic acid-enriched formula to enhance maternal and fetal blood docosahexaenoic acid levels: Randomized double-blinded placebo-controlled trial of pregnant women with gestational diabetes mellitus. Clin. Nutr. 2016;35:608–614. doi: 10.1016/j.clnu.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 292.Mozurkewich E.L., Clinton C.M., Chilimigras J.L., Hamilton S.E., Allbaugh L.J., Berman D.R., Marcus S.M., Romero V.C., Treadwell M.C., Keeton K.L., et al. The Mothers, Omega-3, and Mental Health Study: A double-blind, randomized controlled trial. Am. J. Obstet. Gynecol. 2013;208:313.e1–313.e9. doi: 10.1016/j.ajog.2013.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Olsen S.F., Dalby Sorensen J., Secher N.J., Hedegaard M., Brink Henriksen T., Hansen H.S., Grant A. Randomised controlled trial of effect of fish-oil supplementation on pregnancy duration. Lancet. 1992;339:1003–1007. doi: 10.1016/0140-6736(92)90533-9. [DOI] [PubMed] [Google Scholar]
- 294.Olsen S.F., Secher N.J., Tabor A., Weber T., Walker J.J., Gluud C. Randomised clinical trials of fish oil supplementation in high risk pregnancies. BJOG An. Int. J. Obstet. Gynaecol. 2000;107:382–395. doi: 10.1111/j.1471-0528.2000.tb13235.x. [DOI] [PubMed] [Google Scholar]
- 295.Onwude J.L., Lilford R.J., Hjartardottir H., Staines A., Tuffnell D. A randomised double blind placebo controlled trial of fish oil in high risk pregnancy. BJOG. 1995;102:95–100. doi: 10.1111/j.1471-0528.1995.tb09059.x. [DOI] [PubMed] [Google Scholar]
- 296.Ostadrahimi A., Mohammad-Alizadeh S., Mirghafourvand M., Farshbaf-Khalili S., Jafarilar-Agdam N., Farshbaf-Khalili A. The effect of fish oil supplementation on maternal and neonatal outcomes: A triple-blind, randomized controlled trial. J. Perinat. Med. 2017;45:1069–1077. doi: 10.1515/jpm-2016-0037. [DOI] [PubMed] [Google Scholar]
- 297.Ramakrishnan U., Stein A.D., Parra-Cabrera S., Wang M., Imhoff-Kunsch B., Juárez-Márquez S., Rivera J., Martorell R. Effects of docosahexaenoic acid supplementation during pregnancy on gestational age and size at birth: Randomized, double-blind, placebo-controlled trial in Mexico. Food Nutr. Bull. 2010;31:S108–S116. doi: 10.1177/15648265100312S203. [DOI] [PubMed] [Google Scholar]
- 298.Smuts C.M., Borod E., Peeples J.M., Carlson S.E. High-DHA eggs: Feasibility as a means to enhance circulating DHA in mother and infant. Lipids. 2003;38:407–414. doi: 10.1007/s11745-003-1076-y. [DOI] [PubMed] [Google Scholar]
- 299.Smuts C.M., Huang M., Mundy D., Plasse T., Major S., Carlson S.E. A randomized trial of docosahexaenoic acid supplementation during the third trimester of pregnancy. Obstet. Gynecol. 2003;101:469–479. doi: 10.1016/s0029-7844(02)02585-1. [DOI] [PubMed] [Google Scholar]
- 300.Bulstra-Ramakers M.T.E.W., Huisjes H.J., Visser G.H.A. The effects of 3g eicosapentaenoic acid daily on recurrence of intrauterine growth retardation and pregnancy induced hypertension. BJOG. 1995;102:123–126. doi: 10.1111/j.1471-0528.1995.tb09064.x. [DOI] [PubMed] [Google Scholar]
- 301.Tofail F., Kabir I., Hamadani J.D., Chowdhury F., Yesmin S., Mehreen F., Huda S.N. Supplement of fish-oil and soy-oil during pregnancy and psychomotor development of infants. J. Health Popul. Nutr. 2006;24:48–56. [PubMed] [Google Scholar]
- 302.Van Goor S.A., Dijck-Brouwer D.A.J., Hadders-Algra M., Doornbos B., Erwich J.J.H.M., Schaafsma A., Muskiet F.A. Human milk arachidonic acid and docosahexaenoic acid contents increase following supplementation during pregnancy and lactation. Prostaglandins Leukot. Essent. Fat. Acids. 2009;80:65–69. doi: 10.1016/j.plefa.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 303.D’Almeida A., Carter J.P., Anatol A., Prost C. Effects of a combination of evening primrose oil (Gamma linolenic acid) and fish oil (eicosapentaenoic + docahexaenoic acid) versus magnesium, and versus placebo in preventing pre-eclampsia. Women Health. 1992;19:117–131. doi: 10.1300/J013v19n02_07. [DOI] [PubMed] [Google Scholar]
- 304.De Groot R.H.M., Hornstra G., Van Houwelingen A.C., Roumen F. Effect of α-linolenic acid supplementation during pregnancy on maternal and neonatal polyunsaturated fatty acid status and pregnancy outcome. Am. J. Clin. Nutr. 2004;79:251–260. doi: 10.1093/ajcn/79.2.251. [DOI] [PubMed] [Google Scholar]
- 305.Carlson S.E., Colombo J., Gajewski B.J., Gustafson K.M., Mundy D., Yeast J., Georgieff M.K., Markley L.A., Kerling E.H., Shaddy D.J. DHA supplementation and pregnancy outcomes. Am. J. Clin. Nutr. 2013;97:808–815. doi: 10.3945/ajcn.112.050021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Dilli D., Doğan N.N., İpek M.Ş., Çavuş Y., Ceylaner S., Doğan H., Dursun A., Küçüközkan T., Zenciroğlu A. MaFOS-GDM trial: Maternal fish oil supplementation in women with gestational diabetes and cord blood DNA methylation at insulin like growth factor-1 (IGF-1) gene. Clin. Nutr. ESPEN. 2018;23:73–78. doi: 10.1016/j.clnesp.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 307.Dunstan J.A., Mori T.A., Barden A., Beilin L.J., Taylor A.L., Holt P.G., Prescott S.L. Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: A randomized, controlled trial. J. Allergy Clin. Immunol. 2003;112:1178–1184. doi: 10.1016/j.jaci.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 308.Haghiac M., Yang X.H., Presley L., Smith S., Dettelback S., Minium J., Belury M.A., Catalano P.M., Hauguel-de Mouzon S. Dietary omega-3 fatty acid supplementation reduces inflammation in obese pregnant women: A randomized double-blind controlled clinical trial. PLoS ONE. 2015;10:e0137309. doi: 10.1371/journal.pone.0137309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Harper M., Thom E., Klebanoff M.A., Thorp J., Sorokin Y., Varner M.W., Wapner R.J., Caritis S.N., Iams J.D., Carpenter M.W., et al. Omega-3 Fatty Acid Supplementation to Prevent Recurrent Preterm Birth. Obstet. Gynecol. 2010;115:234–242. doi: 10.1097/AOG.0b013e3181cbd60e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Harris M.A., Reece M.S., McGregor J.A., Wilson J.W., Burke S.M., Wheeler M., Anderson J.E., Auld G.W., French J.I., Allen K.G. The Effect of Omega-3 Docosahexaenoic Acid Supplementation on Gestational Length: Randomized Trial of Supplementation Compared to Nutrition Education for Increasing n-3 Intake from Foods. Biomed. Res. Int. 2015;2015:123078. doi: 10.1155/2015/123078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Tofail F., Hamadani J.D., Ahmed A.Z.T., Mehrin F., Hakim M., Huda S.N. The mental development and behavior of low-birth-weight Bangladeshi infants from an urban low-income community. Eur. J. Clin. Nutr. 2012;66:237–243. doi: 10.1038/ejcn.2011.165. [DOI] [PubMed] [Google Scholar]
- 312.Zhou S.J., Yelland L., Mcphee A.J., Quinlivan J., Gibson R.A., Makrides M. Fish-oil supplementation in pregnancy does not reduce the risk of gestational diabetes or preeclampsia 1-3. Am. J. Clin. Nutr. 2012;95:1378–1384. doi: 10.3945/ajcn.111.033217. [DOI] [PubMed] [Google Scholar]
- 313.Duley L., Henderson‐Smart D.J., Meher S. Altered dietary salt for preventing pre-eclampsia, and its complications. Cochrane Database Syst. Rev. 2005;4:CD005548. doi: 10.1002/14651858.CD005548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Jahanfar S., Jaafar H.S. Effects of restricted caffeine intake by mother on fetal, neonatal and pregnancy outcomes. Cochrane Database Syst. Rev. 2015:CD006965. doi: 10.1002/14651858.CD006965.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Gresham E., Byles J.E., Bisquera A., Hure A.J. Effects of dietary interventions on neonatal and infant outcomes: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2014;100:1298–1321. doi: 10.3945/ajcn.113.080655. [DOI] [PubMed] [Google Scholar]
- 316.Ota E., Tobe-Gai R., Mori R., Farrar D. Antenatal dietary education and supplementation to increase energy and protein intake. Cochrane Database Syst. Rev. 2015:CD000032. doi: 10.1002/14651858.CD000032.pub3. [DOI] [PubMed] [Google Scholar]
- 317.Syngelaki A., Sequeira Campos M., Roberge S., Andrade W., Nicolaides K.H. Diet and exercise for preeclampsia prevention in overweight and obese pregnant women: Systematic review and meta-analysis. J. Matern. Neonatal Med. 2019;32:3495–3501. doi: 10.1080/14767058.2018.1481037. [DOI] [PubMed] [Google Scholar]
- 318.Thangaratinam S., Rogoziska E., Jolly K., Glinkowski S., Roseboom T., Tomlinson J.W., Kunz R., Mol B.W., Coomarasamy A., Khan K.S. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: Meta-analysis of randomised evidence. BMJ. 2012;344:12. doi: 10.1136/bmj.e2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Zhang R., Han S., Chen G.-C., Li Z.-N., Silva-Zolezzi I., Parés G.V., Wang Y., Qin L.Q. Effects of low-glycemic-index diets in pregnancy on maternal and newborn outcomes in pregnant women: A meta-analysis of randomized controlled trials. Eur. J. Nutr. 2018;57:167–177. doi: 10.1007/s00394-016-1306-x. [DOI] [PubMed] [Google Scholar]
- 320.Bonomo M., Corica D., Mion E., Gonçalves D., Mottat G., Merati R., Ragusa A., Morabito A. Evaluating the therapeutic approach in pregnancies complicated by borderline glucose intolerance: A randomized clinical trial. Diabet Med. 2005;22:1536–1541. doi: 10.1111/j.1464-5491.2005.01690.x. [DOI] [PubMed] [Google Scholar]
- 321.Wolff S., Legarth J., Vangsgaard K., Toubro S., Astrup A. A randomized trial of the effects of dietary counseling on gestational weight gain and glucose metabolism in obese pregnant women. Int. J. Obes. 2008;32:495–501. doi: 10.1038/sj.ijo.0803710. [DOI] [PubMed] [Google Scholar]
- 322.Bogaerts A.F.L., Devlieger R., Nuyts E., Witters I., Gyselaers W., Van Den Bergh B.R.H. Effects of lifestyle intervention in obese pregnant women on gestational weight gain and mental health: A randomized controlled trial. Int. J. Obes. 2013;37:814–821. doi: 10.1038/ijo.2012.162. [DOI] [PubMed] [Google Scholar]
- 323.Guelinckx I., Devlieger R., Mullie P., Vansant G. Effect of lifestyle intervention on dietary habits, physical activity, and gestational weight gain in obese pregnant women: A randomized controlled trial. Am. J. Clin. Nutr. 2010;91:373–380. doi: 10.3945/ajcn.2009.28166. [DOI] [PubMed] [Google Scholar]
- 324.Luoto R., Kinnunen T.I., Aittasalo M., Kolu P., Raitanen J., Ojala K., Mansikkamäki K., Lamberg S., Vasankari T., Komulainen T., et al. Primary Prevention of Gestational Diabetes Mellitus and Large-for-Gestational-Age Newborns by Lifestyle Counseling: A Cluster-Randomized Controlled Trial. PLoS Med. 2011;8:e1001036. doi: 10.1371/journal.pmed.1001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Phelan S., Phipps M.G., Abrams B., Darroch F., Schaffner A., Wing R.R. Randomized trial of a behavioral intervention to prevent excessive gestational weight gain: The Fit for delivery study. Am. J. Clin. Nutr. 2011;93:772–779. doi: 10.3945/ajcn.110.005306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Polley B.A., Wing R.R., Sims C.J. Randomized controlled trial to prevent excessive weight gain in pregnant women. Int. J. Obes. 2002;26:1494–1502. doi: 10.1038/sj.ijo.0802130. [DOI] [PubMed] [Google Scholar]
- 327.Poston L., Bell R., Croker H., Flynn A.C., Godfrey K.M., Goff L., Hayes L., Khazaezadeh N., Nelson S.M., Oteng-Ntim E., et al. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): A multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2015;3:767–777. doi: 10.1016/S2213-8587(15)00227-2. [DOI] [PubMed] [Google Scholar]
- 328.Renault K.M., Nørgaard K., Nilas L., Carlsen E.M., Cortes D., Pryds O., Secher N.J. The Treatment of Obese Pregnant Women (TOP) study: A randomized controlled trial of the effect of physical activity intervention assessed by pedometer with or without dietary intervention in obese pregnant women. Am. J. Obstet. Gynecol. 2014;210:134.e1–134.e9. doi: 10.1016/j.ajog.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 329.Bruno R., Petrella E., Bertarini V., Pedrielli G., Neri I., Facchinetti F. Adherence to a lifestyle programme in overweight/obese pregnant women and effect on gestational diabetes mellitus: A randomized controlled trial. Matern. Child Nutr. 2017;13:e12333. doi: 10.1111/mcn.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Dodd J.M., Newman A., Moran L.J., Deussen A.R., Grivell R.M., Yelland L.N., Crowther C.A., McPhee A.J., Wittert G., Owens J.A., et al. The effect of antenatal dietary and lifestyle advice for women who are overweight or obese on emotional well-being: The LIMIT randomized trial. Acta Obstet. Gynecol. Scand. 2016;95:309–318. doi: 10.1111/aogs.12832. [DOI] [PubMed] [Google Scholar]
- 331.Briley C., Flanagan N.L., Lewis N.M. In-home prenatal nutrition intervention increased dietary iron intakes and reduced low birthweight in low-income African-American women. J. Am. Diet. Assoc. 2002;102:984–987. doi: 10.1016/S0002-8223(02)90225-7. [DOI] [PubMed] [Google Scholar]
- 332.Kafatos A.G., Vlachonikolis I.G., Codrington C.A. Nutrition during pregnancy: The effects of an educational intervention program in Greece. Am. J. Clin. Nutr. 1989;50:970–979. doi: 10.1093/ajcn/50.5.970. [DOI] [PubMed] [Google Scholar]
- 333.Crowther C.A., Hiller J.E., Moss J.R., McPhee A.J., Jeffries W.S., Robinson J.S. Effect of Treatment of Gestational Diabetes Mellitus on Pregnancy Outcomes. N. Engl. J. Med. 2005;352:2477–2486. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 334.Jahan K., Roy S.K., Mihrshahi S., Sultana N., Khatoon S., Roy H., Datta L.R., Roy A., Jahan S., Khatun W., et al. Short-Term nutrition education reduces low birthweight and improves pregnancy outcomes among urban poor women in Bangladesh. Food Nutr. Bull. 2014;35:414–421. doi: 10.1177/156482651403500403. [DOI] [PubMed] [Google Scholar]
- 335.Khoury J., Henriksen T., Christophersen B., Tonstad S. Effect of a cholesterol-lowering diet on maternal, cord, and neonatal lipids, and pregnancy outcome: A randomized clinical trial. Am. J. Obstet. Gynecol. 2005;193:1292–1301. doi: 10.1016/j.ajog.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 336.Landon M.B., Spong C.Y., Thom E., Carpenter M.W., Ramin S.M., Casey B., Wapner R.J., Varner M.W., Rouse D.J., Thorp Jr J.M., et al. A Multicenter, Randomized Trial of Treatment for Mild Gestational Diabetes. N. Engl. J. Med. 2009;361:1339–1348. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Peccei A., Blake-Lamb T., Rahilly D., Hatoum I., Bryant A. Intensive Prenatal Nutrition Counseling in a Community Health Setting. Obstet. Gynecol. 2017;130:423–432. doi: 10.1097/AOG.0000000000002134. [DOI] [PubMed] [Google Scholar]
- 338.Thornton Y.S., Smarkola C., Kopacz S.M., Ishoof S.B. Perinatal outcomes in nutritionally monitored obese pregnant women: A randomized clinical trial. J. Natl. Med. Assoc. 2009;101:569–577. doi: 10.1016/S0027-9684(15)30942-1. [DOI] [PubMed] [Google Scholar]
- 339.Walsh J.M., McGowan C.A., Mahony R., Foley M.E., McAuliffe F.M. Low glycaemic index diet in pregnancy to prevent macrosomia (ROLO study): Randomised control trial. BMJ. 2012;345:e5605. doi: 10.1136/bmj.e5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Say L., Gülmezoglu A.M., Hofmeyr G.J. Maternal nutrient supplementation for suspected impaired fetal growth. Cochrane Database Syst. Rev. 2003:CD000148. doi: 10.1002/14651858.CD000148. [DOI] [PubMed] [Google Scholar]
- 341.Salam R.A., Zuberi N.F., Bhutta Z.A. Pyridoxine (vitamin B6) supplementation during pregnancy or labour for maternal and neonatal outcomes. Cochrane database Syst Rev. 2015:CD000179. doi: 10.1002/14651858.CD000179.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Harding K.B., Pena-Rosas J.P., Webster A.C., Yap C.M., Payne B.A., Ota E., De-Regil L.M. Iodine supplementation for women during the preconception, pregnancy and postpartum period. Cochrane Database Syst. Rev. 2017;3:CD011761. doi: 10.1002/14651858.CD011761.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Makrides M., Crosby D.D., Shepherd E., Crowther C.A. Magnesium supplementation in pregnancy. Cochrane Database Syst. Rev. 2014:CD000937. doi: 10.1002/14651858.CD000937.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Meher S., Duley L. Garlic for preventing pre-eclampsia and its complications. Cochrane Database Syst. Rev. 2010:CD006065. doi: 10.1002/14651858.CD006065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Palacios C., Trak-Fellermeier M.A., Martinez R.X., Lopez-Perez L., Lips P., Salisi J.A., John J.C., Peña-Rosas J.P. Regimens of vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2019:CD013446. doi: 10.1002/14651858.CD013446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Olmos-Ortiz A., Avila E., Durand-Carbajal M., Díaz L. Regulation of calcitriol biosynthesis and activity: Focus on gestational vitamin D deficiency and adverse pregnancy outcomes. Nutrients. 2015;7:443–480. doi: 10.3390/nu7010443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Wilson N.A., Mantzioris E., Middleton P.T., Muhlhausler B.S. Gestational age and maternal status of DHA and other polyunsaturated fatty acids in pregnancy: A systematic review. Prostaglandins Leukot. Essent. Fatty Acids. 2019;144:16–31. doi: 10.1016/j.plefa.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 348.Goldenberg R.L., Culhane J.F., Iams J.D., Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.World Health Organization . WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience. WHO; Geneva, Switzerland: 2016. [PubMed] [Google Scholar]
- 350.Lee S.E., Talegawkar S.A., Merialdi M., Caulfield L.E. Dietary intakes of women during pregnancy in low- and middle-income countries. Public Health Nutr. 2013;16:1340–1353. doi: 10.1017/S1368980012004417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Kinshella M.-L.W., Moore S.E., Elango R. The missing focus on women’s health in the First 1,000 Days approach to nutrition. Public Health Nutr. 2020;7:1–15. doi: 10.1017/S1368980020003894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Neuhouser M.L. Red and processed meat: More with less? Am. J. Clin. Nutr. 2020;111:252–255. doi: 10.1093/ajcn/nqz294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Pacagnella R.C., Cecatti J.G., Osis M.J., Souza J.P. The role of delays in severe maternal morbidity and mortality: Expanding the conceptual framework. Reprod. Health Matters. 2012;20:155–163. doi: 10.1016/S0968-8080(12)39601-8. [DOI] [PubMed] [Google Scholar]
- 354.Thaddeus S., Maine D. Too far to walk: Maternal mortality in context. Soc. Sci. Med. 1994;38:1091–1110. doi: 10.1016/0277-9536(94)90226-7. [DOI] [PubMed] [Google Scholar]
- 355.Ruel M.T., Alderman H. Nutrition-Sensitive Interventions and Programmes: How can They Help to Cccelerate Progress in Improving Maternal and Child Nutrition? Lancet. 2013;382:536–551. doi: 10.1016/S0140-6736(13)60843-0. [DOI] [PubMed] [Google Scholar]
- 356.Wen S.W., White R.R., Rybak N., Gaudet L.M., Robson S., Hague W., Simms-Stewart D., Carroli G., Smith G., Fraser W.D., et al. Effect of high dose folic acid supplementation in pregnancy on pre-eclampsia (FACT): Double blind, phase III, randomised controlled, international, multicentre trial. BMJ. 2018;362:18. doi: 10.1136/bmj.k3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in supplementary materials.