Abstract
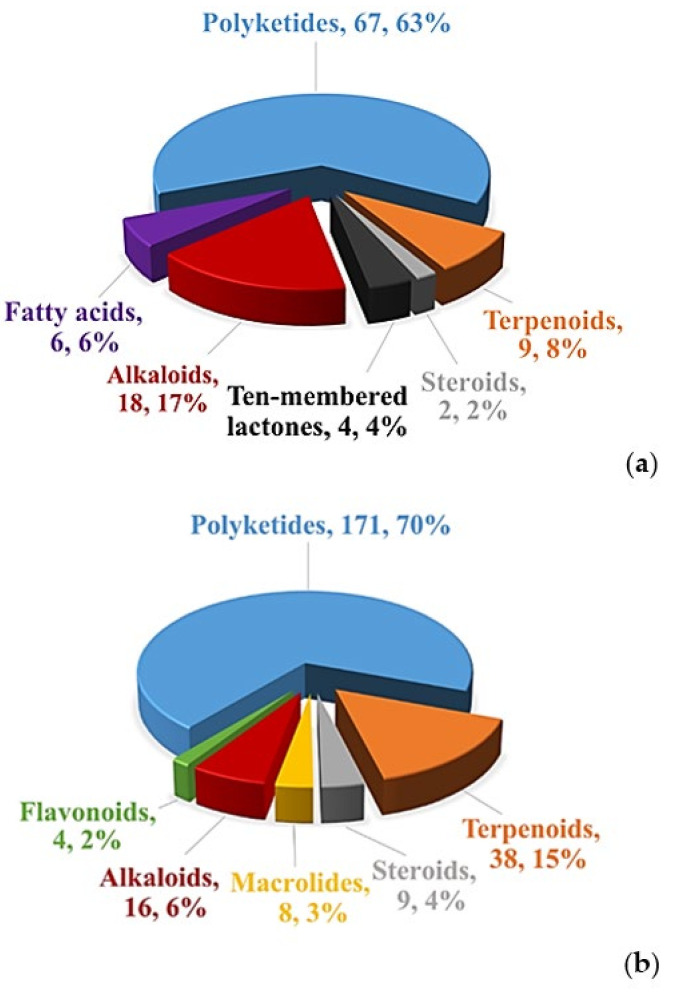
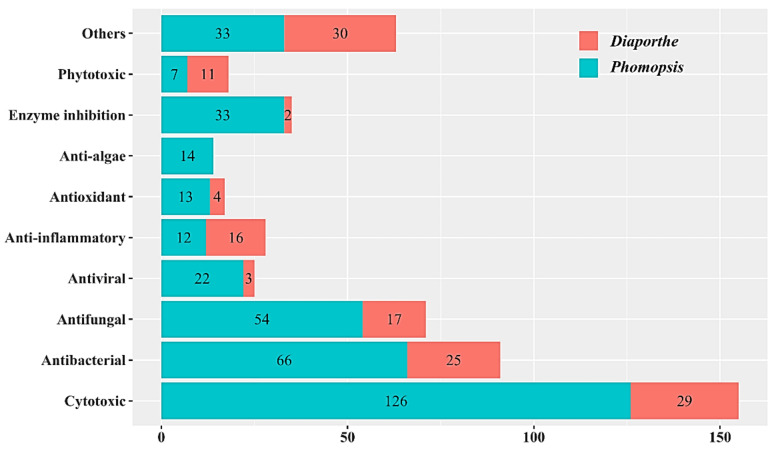
The genus Diaporthe and its anamorph Phomopsis are distributed worldwide in many ecosystems. They are regarded as potential sources for producing diverse bioactive metabolites. Most species are attributed to plant pathogens, non-pathogenic endophytes, or saprobes in terrestrial host plants. They colonize in the early parasitic tissue of plants, provide a variety of nutrients in the cycle of parasitism and saprophytism, and participate in the basic metabolic process of plants. In the past ten years, many studies have been focused on the discovery of new species and biological secondary metabolites from this genus. In this review, we summarize a total of 335 bioactive secondary metabolites isolated from 26 known species and various unidentified species of Diaporthe and Phomopsis during 2010–2019. Overall, there are 106 bioactive compounds derived from Diaporthe and 246 from Phomopsis, while 17 compounds are found in both of them. They are classified into polyketides, terpenoids, steroids, macrolides, ten-membered lactones, alkaloids, flavonoids, and fatty acids. Polyketides constitute the main chemical population, accounting for 64%. Meanwhile, their bioactivities mainly involve cytotoxic, antifungal, antibacterial, antiviral, antioxidant, anti-inflammatory, anti-algae, phytotoxic, and enzyme inhibitory activities. Diaporthe and Phomopsis exhibit their potent talents in the discovery of small molecules for drug candidates.
Keywords: ascomycetes, endophytic fungi, plant pathogens, biological activities, natural products
1. Introduction
Diaporthe is an important fungal genus of plant pathogens [1] belonging to the family Diaporthaceae, order Diaporthales, class Sordariomycetes [2]. It is mainly isolated from various hosts distributed in tropical and temperate zones and can cause diseases to a wide range of plant hosts, as well as humans and other mammals [3,4]. The ascomycetes of Diaporthe Nitschke 1870 and Phomopsis (Sacc.) Bubák 1905 are regarded to form a genus [5,6]. In Index Fungorum (2020), more than 1120 records of Diaporthe and 986 of Phomopsis are listed (http://www.indexfungorum.org/, accessed December 2020). There is a common understanding that, in these ascomycetes, the teleomorph states are named as Diaporthe and the anamorph states called as Phomopsis [7,8,9,10]. For a long time, a dispute has remained concerning whether the generic name should be defined as Diaporthe or Phomopsis. Due to the importance of this genus as plant pathogens, the classification of Diaporthe has been discussed by many researchers. Since Diaporthe was cited earlier and represents most of the species described in nature, more mycologists suggest that the use of Diaporthe as a generic name have more priority and is more suitable for the current study of this fungal group [11,12,13]. In recent years, the previous classification methods based on morphological characteristics are no longer applicable to the genus Diaporthe and advanced molecular techniques will replace them to solve the classification problem of Diaporthe [13,14]. In this review, we use the older name Diaporthe as the generic name.
Based on the existing literature investigations, more secondary metabolites have been separated from Phomopsis than Diaporthe. To date, a large number of compounds have been isolated from endophytic fungi of terrestrial plants in Diaporthe and Phomopsis, some of which originate from the marine environment (mainly mangroves and sediments). Most of compounds are classified as polyketides, which is the main structural type of secondary metabolites in this genus. The reported compounds showed various bioactivities, such as cytotoxic [15], antifungal [16], antibacterial [17], antiviral [18], antioxidant [19], anti-inflammatory [20], phytotoxic [21], and enzyme inhibition [22]. Up to now, there are 26 known species and various unidentified species of Diaporthe and Phomopsis have been studied for their metabolites. Our current review comprehensively summarize a total of 335 bioactive natural products from Diaporthe and Phomopsis between 2010 and 2019, covering their detailed chemical structures with classifications in structural types, as well as their bioactivities and habitats.
2. Bioactive Secondary Metabolites from Phomopsis
The Phomopsis fungi are important resource of bioactive compounds in the field of drug discovery, and have remarkable medical application value. According to the literature reports in recent ten years, a total of 246 bioactive compounds are summarized from Phomopsis herein. These substances have rich and diverse biological activities, such as cytotoxic, antifungal, antibacterial, antiviral, antioxidant, anti-inflammatory, phytotoxic, antimalarial, antialgae, antimigratory, pro-apoptotic, accelerating, and inhibiting the growth of subintestinal vessel plexus (SIV) branches, protecting effects on pancreatic β-cells, motility inhibitory and zoosporicidal potential, and enzyme inhibitory activities (Table 1). Among them, some interesting and promising bioactive compounds might be used in pharmaceutical and agricultural fields. The derived habitats of the Phomopsis strains can also be found in Table 1, which shows that there are 174 (accounting for 71%) and 66 (accounting for 27%) compounds obtained from terrestrial and marine environments, respectively, while six compounds (accounting for 2%) were not mentioned their habitats.
Table 1.
The bioactive secondary metabolites of the anamorph Phomopsis during 2010–2019.
| Number | Structural Types | Compounds | Strains | Habitats (T/M a) |
Activities | Refs. |
|---|---|---|---|---|---|---|
| 1 | Xanthones | 1,5-Dihydroxy-3-hydroxyethyl-6-methoxy-carbonylxanthone | Phomopsis sp. | Paris polyphylla var. yunnanensis (T) | Cytotoxic | [23] |
| 2 | 1-Hydroxy-5-methoxy-3-hydroxyethyl-6-methoxycarbonylxanthone | Phomopsis sp. | P. polyphylla var. yunnanensis (T) | Cytotoxic | [23] | |
| 3 | 1-Hydroxy-3-hydroxyethyl-8-ethoxycarbonyl-xanthone | Phomopsis sp. | P. polyphylla var. yunnanensis (T) | Cytotoxic | [23] | |
| 4 | Pinselin | Phomopsis sp. | P. polyphylla var. yunnanensis (T) | Cytotoxic | [23] | |
| 5 | 1-Hydroxy-8-(hydroxymethyl)-3-methoxy-6-methylxanthone | Phomopsis sp. | P. polyphylla var. yunnanensis (T) | Cytotoxic | [23] | |
| 6 | 2,6-Dihydroxy-3-methyl-9-oxoxanthene-8-carboxylic acid methyl ester | Phomopsis sp. (No. SK7RN3G1) | Sediment (M) | Cytotoxic | [24] | |
| 7 | 4,5-Dihydroxy-3-(2-hydroxyethyl)-1-methoxy-8-methoxy- carbonylxanthone | P. amygdali | Paris axialis (T) | Cytotoxic | [25] | |
| 8 | 1,8-Dihydroxy-4-(2-hydroxyethyl)-3-methoxyxanthone | P. amygdali | P. axialis (T) | Cytotoxic | [25] | |
| 9 | Hydroxyvertixanthone | Phomopsis sp. YM 355364 | Aconitum carmichaelii (T) | Antimicrobial | [26] | |
| 10 | Dalienxanthone A | Phomopsis sp. | Paris daliensis (T) | Cytotoxic | [27] | |
| 11 | Dalienxanthone B | Phomopsis sp. | P. daliensis (T) | Cytotoxic | [27] | |
| 12 | Dalienxanthone C | Phomopsis sp. | P. daliensis (T) | Cytotoxic | [27] | |
| 13 | Paucinervin E | P. amygdali | P. axialis (T) | Cytotoxic | [25] | |
| 14 | 1,3-Dihydroxy-4-(1,3,4-trihydroxybutan-2-yl)-8-methoxy-9H-xanthen-9-one | P. amygdali | P. polyphylla var. yunnanensis (T) | Cytotoxic | [28] | |
| 15 | 3-Methoxy-1,4,8-trihydroxy-5-(1ʹ,3ʹ,4ʹ-trihydroxybutan-2ʹ-yl)-xanthone | P. amygdali | P. axialis (T) | Cytotoxic | [29] | |
| 16 | 8-Methoxy-1,3,4-trihydroxy-5-(1ʹ,3ʹ,4ʹ-trihydroxybutan-2ʹ-yl)-xanthone | P. amygdali | P. axialis (T) | Cytotoxic | [29] | |
| 17 | Secosterigmatocystin |
Phomopsis sp. P. amygdali |
P. polyphylla var. yunnanensis (T) P. axialis (T) |
Cytotoxic Cytotoxic |
[23] [29] |
|
| 18 | 3,8-Dihydroxy-4-(2,3-dihydroxy-1-hydroxymethylpropyl)-1-methoxyxanthone | Phomopsis sp. | P. daliensis (T) | Cytotoxic | [27] | |
| 19 | Oliganthins E | Phomopsis sp. | P. daliensis (T) | Cytotoxic | [27] | |
| 20 | Dihydrosterigmatocystin | P. amygdali | P. axialis(T) | Cytotoxic | [29] | |
| 21 | Vieillardixanthone | P. amygdali | P. axialis (T) | Cytotoxic | [29] | |
| 22 | 1,7-Dihydroxy-2-methoxy-3-(3-methylbut-2-enyl)xanthone | Phomopsis sp. | P. polyphylla var. yunnanensis (T) | Cytotoxic | [23] | |
| 23 | 1-Hydroxy-4,7-dimethoxy-6-(3-oxobutyl)-xanthone | Phomopsis sp. | P. polyphylla var. yunnanensis (T) | Cytotoxic | [23] | |
| 24 | Asperxanthone | Phomopsis sp. | P. polyphylla var. yunnanensis (T) | Cytotoxic | [23] | |
| 25 | 6-O-Methyl-2-deprenylrheediaxanthone B | Phomopsis sp. | P. polyphylla var. yunnanensis (T) | Cytotoxic | [23] | |
| 26 | Cratoxylumxanthone D | Phomopsis sp. | P. daliensis (T) | Cytotoxic | [27] | |
| 27 | 3-O-(6-O-α-L-Arabinopyranosyl)-β-D-glucopyranosyl-1,4-dimethoxyxanthone | Phomopsis sp. (ZH76) | Excoecaria agallocha (M) | Cytotoxic | [30] | |
| 28 | Phomoxanthone A |
P. longicolla Phomopsis sp. IM 41-1 Phomopsis sp. 33# |
Sonneratia caseolaris (M) Rhizhopora mucronata (M) Rhizophora stylosa (M) |
Pro-apoptotic Antimicrobial Inhibiting acetylcholinesterase and α-glucosidase, Antioxidant |
[31] [32] [33] |
|
| 29 | 12-O-Deacetyl-phomoxanthone A | Phomopsis sp. IM 41-1 | R. mucronata (M) | Antimicrobial | [32] | |
| 30 | Dicerandrol A |
P. longicolla S1B4 Phomopsis sp. HNY29-2B |
- b
Acanthus ilicifolius (M) |
Antimicrobial Cytotoxic |
[34] [35] |
|
| 31 | Dicerandrol B |
P. longicolla S1B4 Phomopsis sp. HNY29-2B |
- b
A. ilicifolius (M) |
Antibacterial Cytotoxic |
[34] [35] |
|
| 32 | Dicerandrol C | P. longicolla S1B4 | - b | Antibacterial | [34] | |
| 33 | Deacetylphomoxanthone B |
P. longicolla S1B4 Phomopsis sp. HNY29-2B |
- b
A. ilicifolius(M) |
Antibacterial Cytotoxic |
[34] [35] |
|
| 34 | Penexanthone A | Phomopsis sp. HNY29-2B | A. ilicifolius (M) | Cytotoxic | [35] | |
| 35 | Chromones | (+)-Phomopsichin A | Phomopsis sp. 33# | R. stylosa (M) | Antimicrobial, Antioxidant, Inhibiting acetylcholinesterase and α-glucosidase | [33] |
| 36 | (−)-Phomopsichin B | Phomopsis sp. 33# | R. stylosa (M) | Antimicrobial, Antioxidant, Inhibiting acetylcholinesterase and α-glucosidase | [33] | |
| 37 | Phomopsichin C | Phomopsis sp. 33# | R. stylosa (M) | Antimicrobial, Antioxidant, Inhibiting acetylcholinesterase and α-glucosidase | [33] | |
| 38 | Phomopsichin D | Phomopsis sp. 33# | R. stylosa (M) | Antimicrobial, Antioxidant, Inhibiting acetylcholinesterase and α-glucosidase | [33] | |
| 39 | Chaetocyclinone B | Phomopsis sp. HNY29-2B | A. ilicifolius (M) | Cytotoxic | [36] | |
| 40 | Pestalotiopsone F | Phomopsis sp. IFB-ZS1-S4 | Scaevola hainanensis (M) | Inhibiting neuraminidase | [37] | |
| 41 | Phomoxanthone F | Phomopsis sp. xy21 | Xylocarpus granatum (M) | Anti-HIV | [38] | |
| 42 | 5-Hydroxy-3-hydroxymethyl-2-methyl-7-methoxychromone | Phomopsis sp. (No. Gx-4) | Sediment (M) | Cytotoxic, Inhibiting the growth of SIV branch | [39] | |
| 43 | Phomochromone A | Phomopsis sp. | Cistus monspeliensis (T) | Antimicrobial, Antialgal | [40] | |
| 44 | Phomochromone B | Phomopsis sp. | C. monspeliensis (T) | Antimicrobial, Antialgal | [40] | |
| 45 | Phomochromanone A | Phomopsis sp. CGMCC No. 5416 | Achyranthes bidentata (T) | Cytotoxic, Anti-HIV | [41] | |
| 46 | Phomochromanone B | Phomopsis sp. CGMCC No. 5416 | A. bidentata (T) | Cytotoxic, Anti-HIV | [41] | |
| 47 | 5-Hydroxy-6,8-dimethoxy-2-benzyl-4H-naphtho[2,3-b]-pyran-4-one | Phomopsis sp. ZSU-H26 | E. agallocha (M) | Cytotoxic | [42] | |
| 48 | Phomopsis-H76 A | Phomopsis sp. (#zsu-H76) | E. agallocha (M) | Accelerating the growth of SIV branch | [43] | |
| 49 | Chromanones | (3R,4S)-3,4-Dihydro-4,5,8-trihydroxy-3-methylisocoumarin | Phomopsis sp. (No. ZH-111) | Sediment (M) | Accelerating the growth of SIV branch, Cytotoxic | [44] |
| 50 | (3R,4S)-3,4-Dihydro-8-hydroxy-4-methoxy-3-methylisocoumarin | Phomopsis sp. (No. Gx-4) | Sediment (M) | Cytotoxic, Accelerating the growth of SIV branch | [39] | |
| 51 | 3,4-Dihydro-8-hydroxy-3-methyl-1H-2-benzopyran-1-one-5-carboxylic acid | Phomopsis sp. (No. Gx-4) | Sediment (M) | Cytotoxic, Accelerating the growth of SIV branch | [39] | |
| 52 | 5,8-Dihydroxy-4-methylcoumarin | Phomopsis sp. (No. Gx-4) | Sediment (M) | Cytotoxic, Inhibiting the growth of SIV branch | [39] | |
| 53 | (10S)-Diaporthin | Phomopsis sp. sh917 | Isodon eriocalyx var. laxiflora (T) | Antiangiogenic | [45] | |
| 54 | Cytosporone D | Phomopsis sp. CMU-LMA | Alpinia malacensis (T) | Antimicrobial, Inibiting DnaG primase | [46] | |
| 55 | Alternariol |
Phomopsis sp. A240 Phomopsis sp. CAFT69 Phomopsis sp. |
Taxus chinensis var. mairei (T) Endodesmia calophylloides (T) Senna spectabilis (T) |
Cytotoxic Motility inhibitory and zoosporicidal potential Anti-inflammatory |
[47] [48] [49] |
|
| 56 | Alternariol-5-O-methyl ether | Phomopsis sp. CAFT69 | E. calophylloides (T) | Motility inhibitory and zoosporicidal potential | [48] | |
| 57 | 5ʹ-Hydroxyalternariol |
Phomopsis sp. A240 Phomopsis sp. CAFT69 |
T. chinensis var. mairei (T) E. calophylloides (T) |
Antioxidant Motility inhibitory and zoosporicidal potential |
[47] [48] |
|
| 58 | Phomochromanone C | Phomopsis sp. CGMCC No. 5416 | A. bidentata (T) | Cytotoxic, Pro-apoptotic | [41] | |
| 59 | Benzofuranones | 7-Methoxy-6-methyl-3-oxo-1,3-dihydroisobenzofuran-4-carboxylic acid | Phomopsis sp. A123 | Kandelia candel (M) | Cytotoxic, Antifungal, Antioxidant |
[50] |
| 60 | Diaporthelactone | Phomopsis sp. A123 | K. candel (M) | Cytotoxic, Antifungal, Antioxidant |
[50] | |
| 61 | 7-Hydroxy-4,6-dimethy-3H-isobenzofuran-1-one | Phomopsis sp. A123 | K. candel (M) | Cytotoxic, Antifungal, Antioxidant |
[50] | |
| 62 | 7-Methoxy-4,6-dimethyl-3H-isobenzofuran-1-one | Phomopsis sp. A123 | K. candel (M) | Cytotoxic, Antifungal, Antioxidant |
[50] | |
| 63 | 4-(Hydroxymethyl)-7-methoxy-6-methyl-1(3H)-isobenzofuranone | Phomopsis sp. (No. ZH-111) | Sediment (M) | Inhibiting the growth of SIV branch, Cytotoxic | [44] | |
| 64 | Cytosporone E | Phomopsis sp. BCC 45011 | X. granatum(M) | Cytotoxic, Antimalarial | [51] | |
| 65 | Cytosporone P | Phomopsis sp. BCC 45011 | X. granatum (M) | Antimalarial | [51] | |
| 66 | Phomopsidone A | Phomopsis sp. A123 | K. candel (M) | Cytotoxic, Antifungal, Antioxidant |
[50] | |
| 67 | Excelsione | Phomopsis sp. A123 | K. candel (M) | Cytotoxic, Antifungal, Antioxidant |
[50] | |
| 68 | Excelsional | Phomopsis sp. CAFT69 | E. calophylloides (T) | Motility inhibitory and zoosporicidal potential | [48] | |
| 69 | Lithocarol A | P. lithocarpus FS508 | Sediment (M) | Cytotoxic | [52] | |
| 70 | Lithocarol B | P. lithocarpus FS508 | Sediment (M) | Cytotoxic | [52] | |
| 71 | Lithocarol C | P. lithocarpus FS508 | Sediment (M) | Cytotoxic | [52] | |
| 72 | Lithocarol D | P. lithocarpus FS508 | Sediment (M) | Cytotoxic | [52] | |
| 73 | Lithocarol E | P. lithocarpus FS508 | Sediment (M) | Cytotoxic | [52] | |
| 74 | Lithocarol F | P. lithocarpus FS508 | Sediment (M) | Cytotoxic | [52] | |
| 75 | Isoprenylisobenzofuran A | P. lithocarpus FS508 | Sediment (M) | Cytotoxic | [52] | |
| 76 | 7-Methoxy-2-(4-methoxyphenyl)-3-methyl-5-(3-prenyl)-benzofuran | Phomopsis sp. | P. polyphylla var. yunnanensis (T) | Anti-TMV | [53] | |
| 77 | 2-(4-Methoxyphenyl)-3-methyl-5-(3-prenyl)-benzofuran-7-ol | Phomopsis sp. | P. polyphylla var. yunnanensis (T) | Anti-TMV | [53] | |
| 78 | 2-(4-Hydroxy-3,5-dimethoxyphenyl)-3-methyl-5-(3-prenyl) benzofuran-7-ol | Phomopsis sp. | P. polyphylla var. yunnanensis (T) | Anti-TMV | [53] | |
| 79 | Moracin N | Phomopsis sp. | P. polyphylla var. yunnanensis (T) | Anti-TMV | [53] | |
| 80 | 2-(2′-Methoxy-4′-hydroxy)-aryl-3-methy-6-hydroxybenzofuran | Phomopsis sp. | P. polyphylla var. yunnanensis (T) | Anti-TMV | [53] | |
| 81 | Iteafuranal B | Phomopsis sp. | P. polyphylla var. yunnanensis (T) | Anti-TMV | [53] | |
| 82 | Moracin P | Phomopsis sp. | P. polyphylla var. yunnanensis (T) | Anti-TMV | [53] | |
| 83 | Pyrones | Phomaspyrone A | P. asparagi SWUKJ5.2020 | Kadsura angustifolia (T) | Cytotoxic | [54] |
| 84 | Macommelin-8,9-diol | P. asparagi SWUKJ5.2020 | K. angustifolia (T) | Cytotoxic | [54] | |
| 85 | Phomaspyrone B | P. asparagi SWUKJ5.2020 | K. angustifolia (T) | Cytotoxic | [54] | |
| 86 | Phomaspyrone C | P. asparagi SWUKJ5.2020 | K. angustifolia (T) | Cytotoxic | [54] | |
| 87 | Phomaspyrone D | P. asparagi SWUKJ5.2020 | K. angustifolia (T) | Cytotoxic | [54] | |
| 88 | Phomaspyrone E | P. asparagi SWUKJ5.2020 | K. angustifolia (T) | Cytotoxic | [54] | |
| 89 | Macommelin-9-ol | P. asparagi SWUKJ5.2020 | K. angustifolia (T) | Cytotoxic | [54] | |
| 90 | Macommelin | P. asparagi SWUKJ5.2020 | K. angustifolia (T) | Cytotoxic | [54] | |
| 91 | Pyrenocine J | Phomopsis sp. | Cistus salvifolius (T) | Antifungal, Antibacterial, Algicidal |
[55] | |
| 92 | Pyrenocine K | Phomopsis sp. | C. salvifolius (T) | Antifungal, Antibacterial, Algicidal |
[55] | |
| 93 | Pyrenocine L | Phomopsis sp. | C. salvifolius (T) | Antibacterial, Algicidal | [55] | |
| 94 | Pyrenocine M | Phomopsis sp. | C. salvifolius (T) | Antifungal, Antibacterial, Algicidal |
[55] | |
| 95 | Phomopsis-H76 C | Phomopsis sp. (#zsu-H76) | E. agallocha (M) | Inhibiting the growth of SIV branch | [43] | |
| 96 | Quinones | Anhydrojavanicin | Phomopsis sp. HCCB04730 | Radix Stephaniae Japonicae (T) | Cytotoxic, Anti-HIV | [56] |
| 97 | Dihydroanhydrojavanicin | Phomopsis sp. HCCB04730 | Radix Stephaniae Japonicae (T) | Cytotoxic, Anti-HIV | [56] | |
| 98 | Fusarubin | Phomopsis sp. HCCB04730 | Radix Stephaniae Japonicae (T) | Cytotoxic, Anti-HIV | [56] | |
| 99 | Javanicin | Phomopsis sp. HCCB04730 | Radix Stephaniae Japonicae (T) | Cytotoxic, Anti-HIV | [56] | |
| 100 | 2-Acetonyl-3methyl-5-hydroxy-7-methoxy-naphthazarin | Phomopsis sp. HCCB04730 | Radix Stephaniae Japonicae (T) | Cytotoxic, Anti-HIV | [56] | |
| 101 | Bostrycoidin | Phomopsis sp. HCCB04730 | Radix Stephaniae Japonicae (T) | Cytotoxic, Anti-HIV | [56] | |
| 102 | Altersolanol B | P. longicolla HL-2232 | Bruguiera sexangula var. rhynchopetala (M) | Antibacterial | [57] | |
| 103 | Altersolanol A |
Phomopsis sp. (PM0409092) P. foeniculi |
Nyctanthes arbor-tristis (T) Foeniculum vulgare (T) |
Cytotoxic Phytotoxic |
[58] [59] |
|
| 104 | (2R,3S)-7-Ethyl-1,2,3,4-tetrahydro-2,3,8-trihdroxy-6-methoxy-3-methyl-9,10-anthracenedione | Phomopsis sp. PSU-MA214 | Rhizophora apiculata (M) | Cytotoxic, Antibacterial | [60] | |
| 105 | Altersolanol J | P. foeniculi | F. vulgare (T) | Phytotoxic | [59] | |
| 106 | 2-Hydroxymethyl-4β,5α,6β-trihydroxycyclohex-2-en | Phomopsis sp. | Notobasis syriaca (T) | Antibacterial, Algicidal | [61] | |
| 107 | (−)-Phyllostine | Phomopsis sp. | N. syriaca (T) | Antifungal, Antibacterial, Algicidal | [61] | |
| 108 | (+)-Epiepoxydon | Phomopsis sp. | N. syriaca (T) | Antibacterial, Algicidal | [61] | |
| 109 | (+)-Epoxydon monoacetate | Phomopsis sp. | N. syriaca (T) | Antifungal, Antibacterial, Algicidal | [61] | |
| 110 | Phomonaphthalenone A | Phomopsis sp. HCCB04730 | Radix Stephaniae Japonicae (T) | Cytotoxic, Anti-HIV | [56] | |
| 111 | Ampelanol | Phomopsis sp. HNY29-2B | A. ilicifolius (M) | Antibacterial | [62] | |
| 112 | Phenols | Phomosine K | Phomopsis sp. | N. syriaca (T) | Antibacterial | [61] |
| 113 | Phomosine A | Phomopsis sp. | Ligustrum vulgare (T) | Antifungal, Antibacterial, Inhibiting algae |
[63] | |
| 114 | Phomosine B | Phomopsis sp. | L. vulgare (T) | Antifungal, Antibacterial | [63] | |
| 115 | Phomosine C | Phomopsis sp. | L. vulgare (T) | Antifungal, Antibacterial | [63] | |
| 116 | Phomosine D | Phomopsis sp. | L. vulgare (T) | Antifungal, Inhibiting algae | [63] | |
| 117 | Phomosine I | Phomopsis sp. | L. vulgare (T) | Antifungal, Antibacterial | [63] | |
| 118 | 4-(3-Methoxy-5-methylphenoxy)-2-(2-hydroxyethyl)-6-(hydroxymethyl)phenol | P. asparagi | P. polyphylla var. yunnanensis (T) | Anti-MRSA | [64] | |
| 119 | 4-(3-Hydroxy-5-methylphenoxy)-2-(2-hydroxyethyl)-6-(hydroxymethyl)phenol | P. asparagi | P. polyphylla var. yunnanensis (T) | Anti-MRSA | [64] | |
| 120 | 4-(3-Methoxy-5-methylphenoxy)-2-(2-hydroxyethyl)-6-methylphenol | P. fukushii | P. polyphylla var. yunnanensis (T) | Anti-MRSA | [65] | |
| 121 | 4-(3-Hydroxy-5-methylphenoxy)-2-(2-hydroxyethyl)-6-methylphenol | P. fukushii | P. polyphylla var. yunnanensis (T) | Anti-MRSA | [65] | |
| 122 | 4-(3-Methoxy-5-methylphenoxy)-2-(3-hydroxypropyl)-6-methylphenol | P. fukushii | P. polyphylla var. yunnanensis (T) | Anti-MRSA | [65] | |
| 123 | 1-(4-(3-Methoxy-5-methylphenoxy)-2-methoxy-6-methylphenyl)-3-methylbut-3-en-2-one | P. fukushii | P. polyphylla var. yunnanensis (T) | Anti-MRSA | [66] | |
| 124 | 1-(4-(3-(Hydroxymethyl)-5methoxyphenoxy)-2-methoxy-6-methylphenyl)-3-methylbut-3-en-2-one | P. fukushii | P. polyphylla var. yunnanensis (T) | Anti-MRSA | [66] | |
| 125 | 1-(4-(3-Hydroxy-5(hydroxymethyl)phenoxy)-2-methoxy-6-methylphenyl)-3-methylbut-3-en-2-one | P. fukushii | P. polyphylla var. yunnanensis (T) | Anti-MRSA | [66] | |
| 126 | 1-[2-Methoxy-4-(3-methoxy-5-methylphenoxy)-6-methylphenyl]-ethanone | P. fukushii | P. polyphylla var. yunnanensis (T) | Anti-MRSA | [67] | |
| 127 | 1-[4-(3-(Hydroxymethyl)-5-methoxyphenoxy)-2-methoxy-6-methylphenyl]-ethanone | P. fukushii | P. polyphylla var. yunnanensis (T) | Anti-MRSA | [67] | |
| 128 | 3-Hydroxy-1-(1,8-dihydroxy-3,6-dimethoxynaphthalen-2-yl)propan-1-one | P. fukushii | P. polyphylla var. yunnanensis (T) | Anti-MRSA | [68] | |
| 129 | 3-Hydroxy-1-(1,3,8-trihydroxy-6-methoxynaphthalen-2-yl)propan-1-one | P. fukushii | P. polyphylla var. yunnanensis (T) | Anti-MRSA | [68] | |
| 130 | 3-Hydroxy-1-(1,8-dihydroxy-3,5-dimethoxynaphthalen-2-yl)propan-1-one | P. fukushii | P. polyphylla var. yunnanensis (T) | Anti-MRSA | [68] | |
| 131 | 5-Methoxy-2-methyl-7-(3-methyl-2-oxobut-3-enyl)-1-naphthaldehyde | Phomopsis sp. | P. polyphylla var. yunnanensis (T) | Anti-MRSA | [69] | |
| 132 | 2-(Hydroxymethyl)-5-methoxy-7-(3-methyl-2-oxobut-3-enyl)-1-naphthaldehyde | Phomopsis sp. | P. polyphylla var. yunnanensis (T) | Anti-MRSA | [69] | |
| 133 | Tenellone H | P. lithocarpus FS508 | Sediment (M) | Cytotoxic | [70] | |
| 134 | 16-Acetoxycytosporone B | Phomopsis sp. YM 355364 | A. carmichaeli (T) | Antifungal | [71] | |
| 135 | Cytosporone B |
Phomopsis sp. 0391 Phomopsis sp. PSU-H188 |
P. polyphylla var. yunnanensis (T) Hevea brasiliensis (T) |
Inhibiting lipase Protecting pancreatic β-cells |
[72] [73] |
|
| 136 | Dothiorelone A | Phomopsis sp. 0391 | P. polyphylla var. yunnanensis (T) | Inhibiting lipase | [72] | |
| 137 | Lithocarpinol A | P. lithocarpus FS508 | Sediment (M) | Cytotoxic | [74] | |
| 138 | Lithocarpinol B | P. lithocarpus FS508 | Sediment (M) | Cytotoxic | [74] | |
| 139 | Phomoindene A | Phomopsis sp. (No. GX7-4A) | Sediment (M) | Cytotoxic | [75] | |
| 140 | 4-Hydroxybenzaldehyde | Phomopsis sp. YM 355364 | A. carmichaelii (T) | Antimicrobial | [26] | |
| 141 | 5,5′-Dimethoxybiphenyl-2,2′-diol | P. longicolla HL-2232 | B. sexangula var. rhynchopetala (M) | Antibacterial | [57] | |
| 142 | Phomonitroester | Phomopsis sp. PSU-MA214 | R. apiculate (M) | Cytotoxic | [60] | |
| 143 | Cytosporone U | Phomopsis sp. FJBR-11 | Brucea javanica (T) | Anti-TMV | [76] | |
| 144 | Altenusin | Phomopsis sp. CAFT69 | E. calophylloides (T) | Motility inhibitory and zoosporicidal potential | [48] | |
| 145 | Cosmochlorin D | Phomopsis sp. N-125 | Ficus ampelas (T) | Cytotoxic, Growth-inhibition activity | [77] | |
| 146 | Cosmochlorin E | Phomopsis sp. N-125 | F. ampelas (T) | Cytotoxic, Growth-inhibition activity | [77] | |
| 147 | Oblongolides | Oblongolide Z | Phomopsis sp. BCC 9789 | Musa acuminate (T) | Cytotoxic, Anti-HSV-1 | [78] |
| 148 | Oblongolide Y | Phomopsis sp. BCC 9789 | M. acuminate (T) | Cytotoxic | [78] | |
| 149 | Oblongolide C1 | Phomopsis sp. XZ-01 | Camptotheca acuminate (T) | Cytotoxic | [79] | |
| 150 | Oblongolide P1 | Phomopsis sp. XZ-01 | C. acuminate (T) | Cytotoxic | [79] | |
| 151 | Oblongolide X1 | Phomopsis sp. XZ-01 | C. acuminate (T) | Cytotoxic | [79] | |
| 152 | 6-Hydroxyphomodiol | Phomopsis sp. XZ-01 | C. acuminate (T) | Cytotoxic | [79] | |
| 153 | Oblongolide C | Phomopsis sp. XZ-01 | C. acuminate (T) | Cytotoxic | [79] | |
| 154 | 2-Deoxy-4α-hydroxyoblongolide X | Phomopsis sp. BCC 9789 | M. acuminate (T) | Anti-HSV-1 | [78] | |
| 155 | Unclassified polyketides | Phomoxydiene C | Phomopsis sp. BCC 45011 | X. granatum (M) | Cytotoxic, Antimalarial | [51] |
| 156 | 1893 A | Phomopsis sp. BCC 45011 | X. granatum (M) | Cytotoxic | [51] | |
| 157 | Mycoepoxydiene | Phomopsis sp. BCC 45011 | X. granatum (M) | Cytotoxic, Antimalarial | [51] | |
| 158 | Deacetylmycoepoxydiene | Phomopsis sp. BCC 45011 | X. granatum (M) | Cytotoxic, Antimalarial | [51] | |
| 159 | Phomoxydiene A | Phomopsis sp. BCC 45011 | X. granatum (M) | Cytotoxic, Antimalarial | [51] | |
| 160 | Phomopoxide A | Phomopsis sp. YE3250 | Paeonia delavayi (T) | Cytotoxic, Antifungal, Inhibiting α-glycosidase |
[80] | |
| 161 | Phomopoxide B | Phomopsis sp. YE3250 | P. delavayi (T) | Cytotoxic, Antifungal, Inhibiting α-glycosidase |
[80] | |
| 162 | Phomopoxide C | Phomopsis sp. YE3250 | P. delavayi (T) | Cytotoxic, Antifungal, Inhibiting α-glycosidase |
[80] | |
| 163 | Phomopoxide D | Phomopsis sp. YE3250 | P. delavayi (T) | Cytotoxic, Antifungal, Inhibiting α-glycosidase |
[80] | |
| 164 | Phomopoxide E | Phomopsis sp. YE3250 | P. delavayi (T) | Cytotoxic, Antifungal, Inhibiting α-glycosidase |
[80] | |
| 165 | Phomopoxide F | Phomopsis sp. YE3250 | P. delavayi (T) | Cytotoxic, Antifungal, Inhibiting α-glycosidase |
[80] | |
| 166 | Phomopoxide G | Phomopsis sp. YE3250 | P. delavayi (T) | Cytotoxic, Antifungal, Inhibiting α-glycosidase |
[80] | |
| 167 | Phomentrioloxin | Phomopsis sp. | Carthamus lanatus (T) | Phytotoxic | [81] | |
| 168 | Phomotenone | Phomopsis sp. | C. monspeliensis (T) | Antifungal, Antibacterial, Antialgal | [40] | |
| 169 | Phomopsolide B | Phomopsis sp. DC275 | Vitis vinifera (T) | Antibacterial, Phytotoxic | [82] | |
| 170 | Phomopsolidone A | Phomopsis sp. DC275 | V. vinifera (T) | Antibacterial, Phytotoxic | [82] | |
| 171 | Phomopsolidone B | Phomopsis sp. DC275 | V. vinifera (T) | Antibacterial, Phytotoxic | [82] | |
| 172 | Monoterpenoids | Acropyrone | Phomopsis sp. HNY29-2B | A. ilicifolius (M) | Antibacterial | [62] |
| 173 | Nectriapyrone | P. foeniculi | F. vulgare (T) | Phytotoxic | [59] | |
| 174 | (1S,2S,4S)-Trihydroxy-p-menthane | Phomopsis sp. | C. monspeliensis (T) | Antibacterial, Antialgal | [40] | |
| 175 | Sesquiterpenoids | Phomophyllin A | Phomopsis sp. TJ507A | Phyllanthus glaucus (T) | Inhibiting BACE1 | [83] |
| 176 | Phomophyllin B | Phomopsis sp. TJ507A | P. glaucus (T) | Inhibiting BACE1 | [83] | |
| 177 | Phomophyllin C | Phomopsis sp. TJ507A | P. glaucus (T) | Inhibiting BACE1 | [83] | |
| 178 | Phomophyllin D | Phomopsis sp. TJ507A | P. glaucus (T) | Inhibiting BACE1 | [83] | |
| 179 | Phomophyllin E | Phomopsis sp. TJ507A | P. glaucus (T) | Inhibiting BACE1 | [83] | |
| 180 | Phomophyllin F | Phomopsis sp. TJ507A | P. glaucus (T) | Inhibiting BACE1 | [83] | |
| 181 | Phomophyllin G | Phomopsis sp. TJ507A | P. glaucus (T) | Inhibiting BACE1 | [83] | |
| 182 | Radulone B | Phomopsis sp. TJ507A | P. glaucus (T) | Inhibiting BACE1 | [83] | |
| 183 | Phomophyllin I | Phomopsis sp. TJ507A | P. glaucus (T) | Inhibiting BACE1 | [83] | |
| 184 | Onitin | Phomopsis sp. TJ507A | P. glaucus (T) | Inhibiting BACE1 | [83] | |
| 185 | (7R,9S,10R)-3,9-Di-hidroxicalamenene | P. cassiae | Cassia spectabilis (T) | Inhibiting acetylcholinesterase, Antifungal | [84] | |
| 186 | (7R,9R,10R)-3,9-Di-hidroxicalamenene | P. cassiae | C. spectabilis (T) | Inhibiting acetylcholinesterase, Antifungal | [84] | |
| 187 | (7S,10R)-3-Hidroxicalamen-8-one | P. cassiae | C. spectabilis (T) | Inhibiting acetylcholinesterase, Antifungal | [84] | |
| 188 | Aristelegone-A | P. cassiae | C. spectabilis (T) | Inhibiting acetylcholinesterase, Antifungal | [84] | |
| 189 | Phomoarcherin A | P. archeri | Vanilla albidia (T) | Cytotoxic | [85] | |
| 190 | Phomoarcherin B | P. archeri | V. albidia (T) | Cytotoxic, Antimalarial | [85] | |
| 191 | Phomoarcherin C | P. archeri | V. albidia (T) | Cytotoxic | [85] | |
| 192 | Kampanol A | P. archeri | V. albidia (T) | Cytotoxic | [85] | |
| 193 | (+)-S-1-Methyl-abscisic-6-acid | P. amygdali | Call midge (T) | Antibacterial | [86] | |
| 194 | (+)-S-Abscisic acid | P. amygdali | C. midge (T) | Antibacterial | [86] | |
| 195 | 7-Hydroxy-10-oxodehydrodihydrobotrydial | Phomopsis sp. TJ507A | P. glaucus (T) | Inhibiting BACE1 | [83] | |
| 196 | Curcumol | P. castaneae-mollissimae GQH87 | Artemisia annua (T) | Cytotoxic | [87] | |
| 197 | 9-Hydroxyphomopsidin | Phomopsis sp. CAFT69 | E. calophylloides (T) | Motility inhibitory and zoosporicidal potential | [48] | |
| 198 | Phomopsidin | Phomopsis sp. CAFT69 | E. calophylloides (T) | Motility inhibitory and zoosporicidal potential | [48] | |
| 199 | AA03390 | P. lithocarpus FS508 | Sediment (M) | Cytotoxic | [70] | |
| 200 | Diterpenoids | Libertellenone J | Phomopsis sp. S12 | Illigera rhodantha (T) | Anti-inflammatory | [88] |
| 201 | Libertellenone C | Phomopsis sp. S12 | - b | Anti-inflammatory | [89] | |
| 202 | Libertellenone T | Phomopsis sp. S12 | - b | Anti-inflammatory | [89] | |
| 203 | Pedinophyllol K | Phomopsis sp. S12 | - b | Anti-inflammatory | [89] | |
| 204 | Pedinophyllol L | Phomopsis sp. S12 | - b | Anti-inflammatory | [89] | |
| 205 | Fusicoccin J | P. amygdali | C. midge (T) | Antibacterial | [86] | |
| 206 | 3α-Hydroxyfusicoccin J | P. amygdali | C. midge (T) | Antibacterial | [86] | |
| 207 | Triterpenoids | 3S,22R,26-Trihydroxy-8,24E-euphadien-11-one | P. chimonanthi | Tamarix chinensis (T) | Cytotoxic | [90] |
| 208 | Betulinic acid | Phomopsis sp. SNB-LAP1-7-32 | Diospyros carbonaria (T) | Antiviral, Cytotoxic | [91] | |
| 209 | Oleanolic acid |
P. castaneae-mollissi mae GQH87 |
A. annua (T) | Cytotoxic | [87] | |
| 210 | Steroids | (14β,22E)-9,14-Dihydroxyergosta-4,7,22-triene-3,6-dione | Phomopsis sp. | A. carmichaeli (T) | Antifungal | [92] |
| 211 | (5α,6β,15β,22E)-6-Ethoxy-5,15-dihydroxyergosta-7,22-dien-3-one | Phomopsis sp. | A. carmichaeli (T) | Antifungal | [92] | |
| 212 | Calvasterol A | Phomopsis sp. | A. carmichaeli (T) | Antifungal | [92] | |
| 213 | Calvasterol B | Phomopsis sp. | A. carmichaeli (T) | Antifungal | [92] | |
| 214 | Ganodermaside D | Phomopsis sp. | A. carmichaeli (T) | Antifungal | [92] | |
| 215 | Dankasterone A | Phomopsis sp. YM 355364 | A. carmichaeli (T) | Antifungal, Anti-influenza | [71] | |
| 216 | 3β,5α,9α-Trihydroxy-(22E,24R)-ergosta-7,22-dien-6-one | Phomopsis sp. YM 355364 | A. carmichaeli (T) | Antifungal | [71] | |
| 217 | Phomopsterone B | Phomopsis sp. TJ507A | P. glaucus (T) | Anti-inflammatory | [93] | |
| 218 | Cyathisterol | Phomopsis sp. YM 355364 | A. carmichaelii (T) | Antifungal | [26] | |
| 219 | Macrolides | Sch-642305 | Phomopsis sp. CMU-LMA | Alpinia malaccensis (T) | Cytotoxic, Antimicrobial | [94] |
| 220 | LMA-P1 | Phomopsis sp. CMU-LMA | A. malaccensis (T) | Cytotoxic | [94] | |
| 221 | Benquoine | Phomopsis sp. CMU-LMA | A. malaccensis (T) | Cytotoxic, Antimicrobial | [94] | |
| 222 | Aspergillide C | Phomopsis sp. IFB-ZS1-S4 | S. hainanensis (M) | Inhibiting neuraminidase | [37] | |
| 223 | Lithocarpin A | P. lithocarpus FS508 | Sediment (M) | Cytotoxic | [95] | |
| 224 | Lithocarpin B | P. lithocarpus FS508 | Sediment (M) | Cytotoxic | [95] | |
| 225 | Lithocarpin C | P. lithocarpus FS508 | Sediment (M) | Cytotoxic | [95] | |
| 226 | Lithocarpin D | P. lithocarpus FS508 | Sediment (M) | Cytotoxic | [95] | |
| 227 | Alkaloids | Phomopchalasin B | Phomopsis sp. shj2 | I. eriocalyx var. laxiflora (T) | Antimigratory | [96] |
| 228 | Phomopsichalasin G | P. spp. xy21 and xy22 | X. granatum (M) | Cytotoxic | [97] | |
| 229 | 18-Metoxycytochalasin J | Phomopsis sp. | Garcinia kola (T) | Cytotoxic, Antibacterial | [98] | |
| 230 | Cytochalasin H |
Phomopsis sp. Phomopsis sp. By254 Phomopsis sp. |
G. kola (T) Gossypium hirsutum (T) S. spectabilis (T) |
Cytotoxic, Antibacterial Antifungal Inhibiting acetylcholinesterase, Anti-inflammatory |
[98] [99] [49] |
|
| 231 | Cytochalasin J |
Phomopsis sp. Phomopsis sp. P. asparagi |
G. kola (T) S. spectabilis (T) Peperomia sui (T) |
Cytotoxic, Antibacterial Anti-inflammatory Antiandrogen |
[98] [49] [100] |
|
| 232 | Phomopchalasin C | Phomopsis sp. shj2 | I. eriocalyx var. laxiflora (T) | Cytotoxic, Anti-inflammatory, Antimigratory | [96] | |
| 233 | Cytochalasin N | Phomopsis sp. By254 | G. hirsutum (T) | Antifungal | [99] | |
| 234 | Epoxycytochalasin H | Phomopsis sp. By254 | G. hirsutum (T) | Antifungal | [99] | |
| 235 | Diaporthalasin | Phomopsis sp. PSU-H188 | H. brasiliensis (T) | Anti-MRSA | [73] | |
| 236 | (+)-Tersone E | P. tersa FS441 | Sediment (M) | Antibacterial, Cytotoxic | [101] | |
| 237 | ent-Citridone A | P. tersa FS441 | Sediment (M) | Antibacterial | [101] | |
| 238 | Phochrodine C | Phomopsis sp. 33# | R. stylosa (M) | Anti-inflammatory | [102] | |
| 239 | Phochrodine D | Phomopsis sp. 33# | R. stylosa (M) | Anti-inflammatory, Antioxidant | [102] | |
| 240 | PM181110 | P. glabrae | Pongamia pinnata (T) | Anticancer | [103] | |
| 241 | Fusaristatin A | P. longicolla S1B4 | - b | Antibacterial | [34] | |
| 242 | Exumolide A | Phomopsis sp. (No. ZH-111) | Sediment (M) | Accelerating the growth of SIV branch, Cytotoxic | [44] | |
| 243 | Flavonoids | Quercetin | P. castaneae-mollissimae GQH87 | A. annua (T) | Cytotoxic | [87] |
| 244 | Luteolin | P. castaneae-mollissimae GQH87 | A. annua (T) | Cytotoxic | [87] | |
| 245 | Naringenin | P. castaneae-mollissimae GQH87 | A. annua (T) | Cytotoxic | [87] | |
| 246 | Luteolin-7-O-glucoside | P. castaneae-mollissimae GQH87 | A. annua (T) | Cytotoxic | [87] |
a T: terrestrial environment; M: marine environment; b The habitat was not mentioned.
2.1. Polyketides
Polyketides are a large and diverse family of natural products, containing various chemical structures and biological activities [104]. In this review, 171 polyketides are summarized from Phomopsis, accounting for 70% of the total compounds from Phomopsis. The main bioactivities involve cytotoxic, antibacterial and antifungal activities. Herein, we classify these polyketides into xanthones, chromones, chromanones, benzofuranones, pyrones, quinones, phenols, oblongolides, and unclassified polyketides.
2.1.1. Xanthones
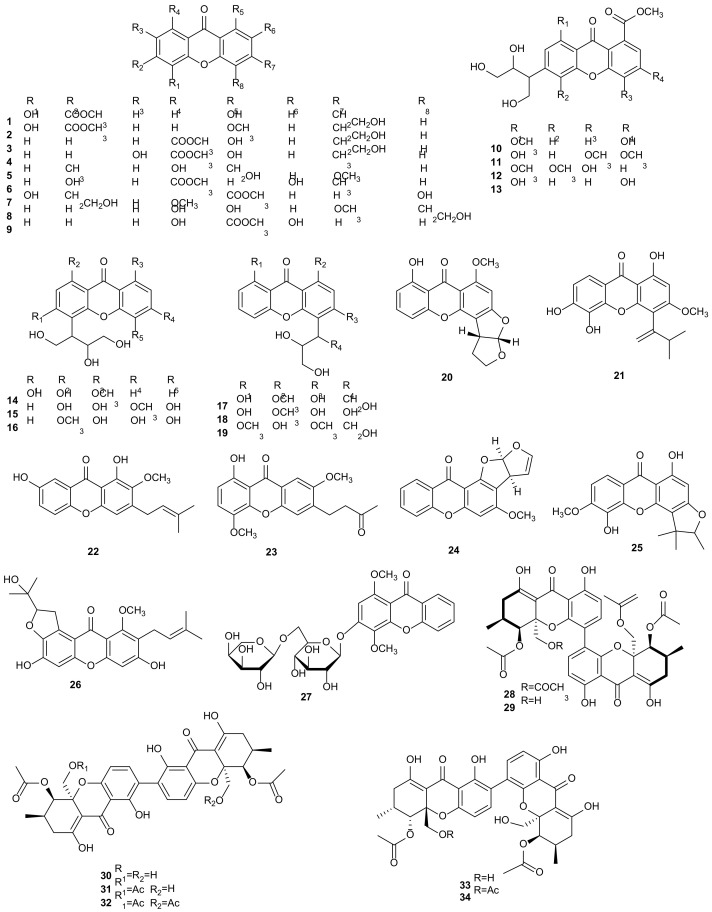
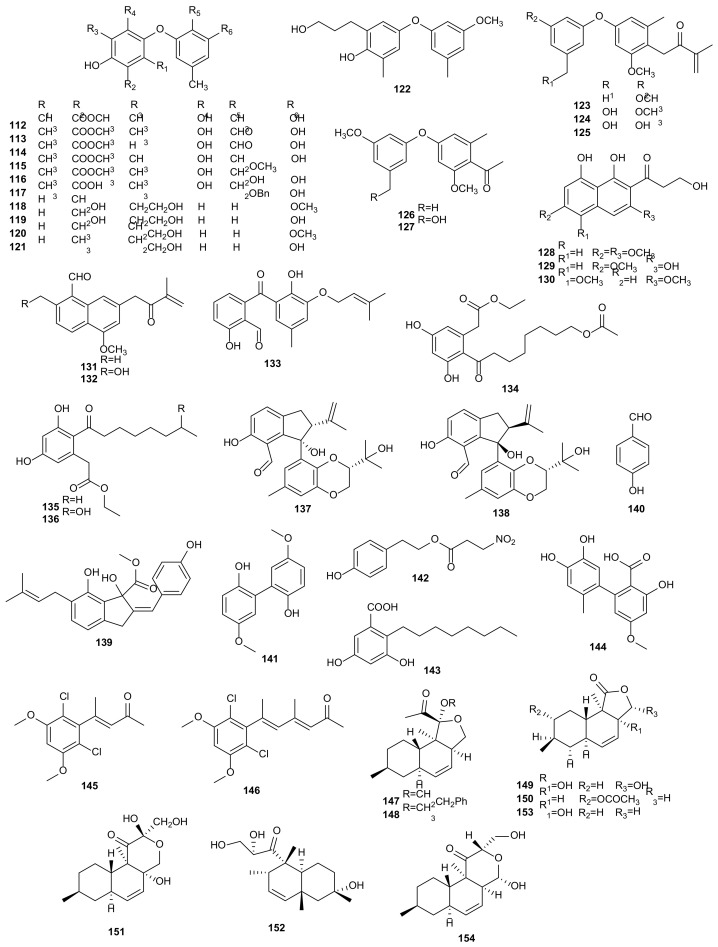
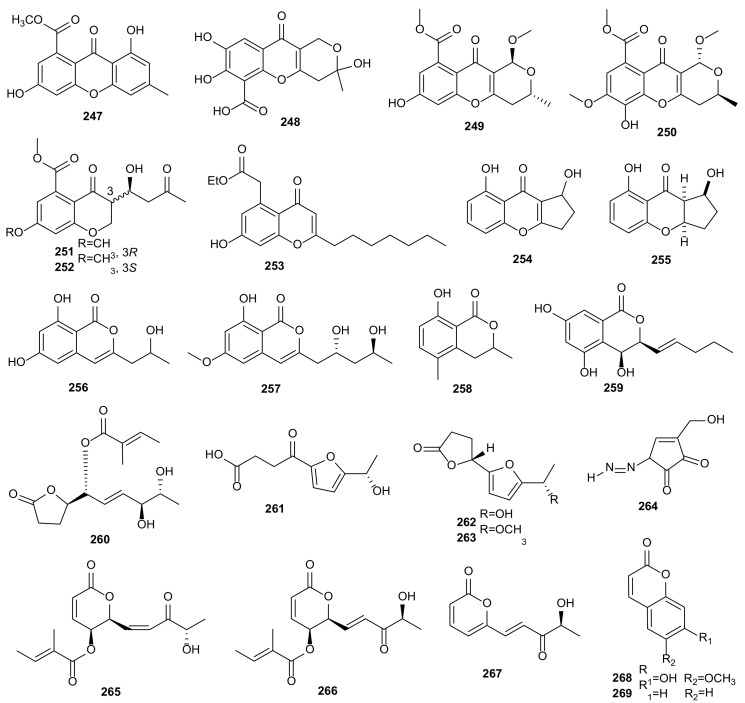
Xanthones are a kind of compounds with the framework of 9H-xanthen-9-one, which mainly have anti-inflammatory, antimicrobial, antioxidant and cytotoxic activities [105]. A series of xanthones were obtained from the fermentation products of Phomopsis sp. isolated from Paris polyphylla var. yunnanensis, including three new compounds, 1,5-dihydroxy-3-hydroxyethyl-6-methoxycarbonylxanthone (1), 1-hydroxy-5-methoxy-3-hydroxyethyl-6-methoxycarbonylxanthone (2), 1-hydroxy-3-hydroxyethyl-8-ethoxy-carbonyl-xanthone (3), and seven known ones, pinselin (4), 1-hydroxy-8-(hydroxymethyl)-3-methoxy-6-methylxanthone (5), secosterigmatocystin (17), 1,7-dihydroxy-2-methoxy-3-(3-methylbut-2-enyl)xanthone (22), 1-hydroxy-4,7-dimethoxy-6-(3-oxobutyl)xanthone (23), asperxanthone (24) and 6-O-methyl-2-deprenylrheediaxanthone B (25). The cytotoxicities of all compounds to five human tumor cells (NB4, A549, SHSY5Y, PC3, and MCF7) were evaluated by using paclitaxel as positive control. The results showed that compounds 1 and 3 displayed cytotoxic activities and provided the IC50 values of 3.6 and 2.5 μM against A549 cells, and 1 gave an IC50 value of 2.7 μM against MCF7 cells. Compounds 22–23 showed weak activities and offered IC50 values greater than 10 μM for five tested cells. The others gave IC50 values between 3.8–10 μM against tested cells [23]. A new compound, 2,6-dihydroxy-3-methyl-9-oxoxanthene-8-carboxylic acid methyl ester (6), was isolated from Phomopsis sp. (No. SK7RN3G1) of mangrove sediment in the Shankou, Hainan, China. It showed cytotoxicity towards HEp-2 (IC50 = 8 μg/mL) and HepG2 (IC50 = 9 μg/mL) cancer cells [24]. Three secondary metabolites were characterized from fermentation products of P. amygdali, isolated from Paris axialis: 4,5-dihydroxy-3-(2-hydroxyethyl)-1-methoxy-8-methoxycarbonylxanthone (7), 1,8-dihydroxy-4-(2-hydroxyethyl)-3-methoxyxanthone (8), and paucinervin E (13). Compound 7 was active against A549 (IC50 = 2.6 μM) and PC3 (IC50 = 2.4 μM) cell lines. Compounds 8 and 13 displayed moderate activities with IC50 values in the range of 5.2–9.2 μM against one or more cell lines of NB4, A549, SHSY5Y, PC3 and MCF7 [25]. Hydroxyvertixanthone (9) was obtained from the endophytic fungus Phomopsis sp. YM 355364, originated from Chinese medicinal plant Aconitum carmichaelii. It showed antimicrobial activity with minimal inhibitory concentration (MIC) values of 256, 256, 128, and 64 μg/mL against Escherichia coli, Bacillus subtilis, Pyricularia oryzae, and Candida albicans, respectively [26]. The fermentation of fungus Phomopsis sp. derived from Paris daliensis, led to the isolation of six xanthones and identified as dalienxanthones A-C (10–12), 3,8-dihydroxy-4-(2,3-dihydroxy-1-hydroxymethylpropyl)-1-methoxyxanthone (18), oliganthins E (19), and cratoxylumxanthone D (26). These compounds were evaluated for cytotoxicities of five cancer cell lines (NB4, A549, SHSY5Y, PC3 and MCF-7). Compounds 12 and 18 were active to SHSY5Y with IC50 values of 3.8 and 3.5 μM, respectively, and the remaining compounds provided IC50 values in the range of 4.6–9.2 μM [27]. An investigation of extracts from fungus P. amygdali derived from the rhizome of Paris polyphylla var. yunnanensis afforded a new xanthone, 1,3-dihydroxy-4-(1,3,4-trihydroxybutan-2-yl)-8-methoxy-9H-xanthen-9-one (14). The bioactive results showed that 14 exhibited significant cytotoxic activity against A549 (IC50 = 5.8 μM) and PC3 (IC50 = 3.6 μM) [28].
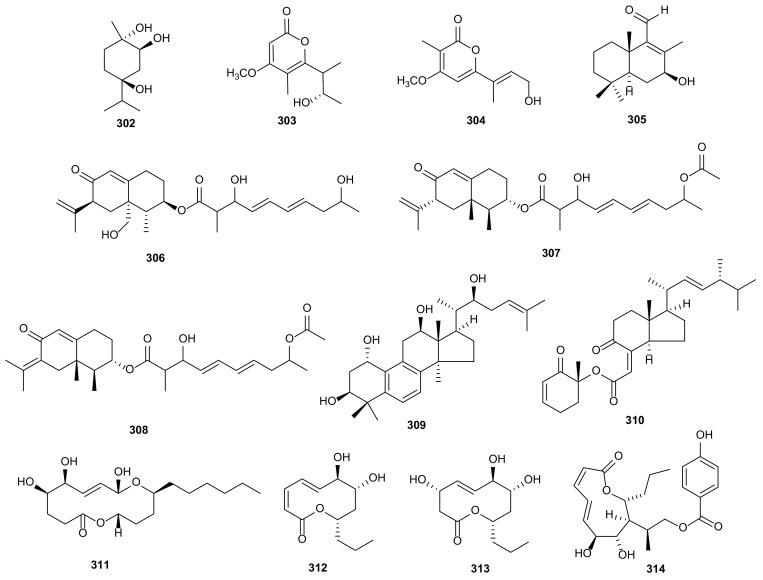
An endophytic fungus P. amygdali associated with the rhizome of Paris axialis was cultured to obtain five xanthones: 3-methoxy-1,4,8-trihydroxy-5-(1ʹ,3ʹ,4ʹ-trihydroxybutan-2ʹ-yl)-xanthone (15), 8-methoxy-1,3,4-trihydroxy-5-(1ʹ,3ʹ,4ʹ-trihydroxybutan-2ʹ-yl)-xanthone (16), secosterigmatocystin (17), dihydrosterigmatocystin (20), and vieillardixanthone (21). The cytotoxic assay for NB4, A549, SHSY5Y, PC3 and MCF7 cancer cells were evaluated. The IC50 values of compound 15 against A549 and 16 against SHSY5Y were 3.6 and 4.2 μM, respectively. Compounds 17 and 20–21 displayed moderate activities with IC50 values in the range of 5.4–8.8 μM [29]. Studies of an endophytic fungus Phomopsis sp. (ZH76) from the stems of the mangrove tree Excoecaria agallocha contained a new O-glycoside compound, 3-O-(6-O-α-L-arabinopyranosyl)-β-D-glucopyranosyl-1,4-dimethoxyxanthone (27). The IC50 values of cytotoxicity for compound 27 on HEp-2 and HepG2 cells were 9 and 16 μmol/mL, respectively [30]. Phomoxanthone A (28), a dimeric tetrahydroxanthone, was extracted from P. longicolla of the mangrove tree Sonneratia caseolaris. Compound 28 had the strongest pro-apoptotic activity on human cancer cell lines and cisplatin-resistant cells, and its activity on healthy blood cells was reduced by more than 100 times. It was the most effective activator of mouse T lymphocytes, NK cells, and macrophages [31]. The study on secondary metabolites from fungus Phomopsis sp. IM 41-1 of mangrove plant Rhizhopora mucronata afforded phomoxanthone A (28) and 12-O-deacetyl-phomoxanthone A (29). When the concentration was 30 μg/ disk, compounds 28 and 29 showed moderate antimicrobial activities against Botrytis cinerea, Sclerotinia sclerotiorum, Diaporthe medusaea, and Staphylococcus aureus, but were inactive against Pseudomonas aeruginosa [32]. Four bioactive metabolites, dicerandrols A-C (30–32) and deacetylphomoxanthone B (33), were derived from P. longicolla S1B4. All compounds exhibited strong antibacterial activities against Xanthomonas oryzae KACC 10331. Dicerandrol A (30) also displayed notable antimicrobial activity against S. aureus, B. subtilis, and C. albicans with MIC values of 0.25, 0.125 and 2 μg/mL [34]. Phomopsis sp. HNY29-2B, isolated from mangrove plant Acanthus ilicifolius, produced four xanthone derivatives, 30–31, 33 and penexanthone A (34). Compounds 30–31 and 33–34 displayed cyctotoxicities and provided IC50 values of 1.76–42.82 μM against MDA-MB-435, HCT-116, Calu-3, Huh7, and MCF-10A human cancer cell lines [35]. The structures of xanthones (1–34) are shown in Figure 1.
Figure 1.
Chemical structures of compounds 1–34 from Phomopsis.
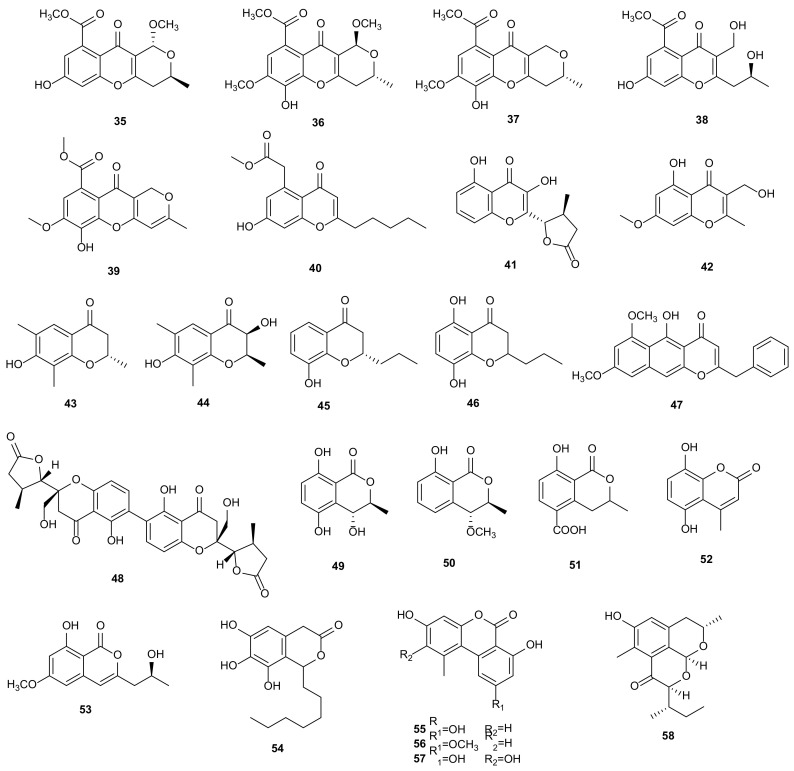
2.1.2. Chromones
Chromones are a class of bioactive compounds with a benzo-γ-pyrone skeleton, which have been reported to have various activities, such as anti-tumor, anti-viral, antimicrobial, anti-inflammatory, and antioxidant [106]. Phomopsis sp. 33#, a mangrove endophytic fungus isolated from the bark of Rhizophora stylosa, produced four new chromone derivatives, (+)-phomopsichin A (35), (−)-phomopsichin B (36), phomopsichins C (37) and D (38), along with a known phomoxanthone A (28). These metabolites displayed low effects on inhibitions of acetylcholinesterase and α-glucosidase, radical scavenging function on DPPH and OH, and antimicrobial activities [33]. A cytotoxic chromone, chaetocyclinone B (39), was characterized from a culture of Phomopsis sp. HNY29-2B, an endophytic fungus obtained from the mangrove plant A. ilicifolius Linn. Compound 39 had cytotoxic activity against PC-3 (IC50 = 8.13 μmol/L) and DU145 (IC50 = 3.59 μmol/L) [36]. The fungus Phomopsis sp. IFB-ZS1-S4 isolated from Scaevola hainanensis Hance extracted a known pestalotiopsone F (40), which showed moderate inhibition on neuraminidase in vitro with IC50 value of 9.90 ± 0.42 μM [37]. Cultivation of Phomopsis sp. xy21 derived from the mangrove Xylocarpus granatum afforded a new xanthone-derived polyketide, phomoxanthone F (41). It showed inhibitory effects on VSV-G pseudotyped viral supernatant (HIV-1) with the inhibitory rate of 16.48 ± 6.67% at a concentration of 20 μM, which was higher than that of the positive control, efavirenz with a rate of 88.54 ± 0.45% [38]. 5-Hydroxy-3-hydroxymethyl-2-methyl-7-methoxychromone (42) was separated from the extracts of Phomopsis sp. (No. Gx-4) derived from mangrove sediment in ZhuHai, Guangdong, China. It showed low cytotoxic activity with IC50 values greater than 50 μmol/mL towards Hep-2 and HepG2. Moreover, it also significantly inhibited the growth of subintestinal vessel plexus (SIV) branches [39]. According to the bioassay-guided fractionation, two new chromones, phomochromones A (43) and B (44) were obtained from an endophytic fungus Phomopsis sp. of Cistus monspeliensis. They displayed remarkable antifungal, antibacterial, and antialgal activities against Microbotryum violaceum, E. coli, Bacillus megaterium, and Chlorella fusca [40]. Chemical investigation of Phomopsis sp. CGMCC No. 5416 isolated from Achyranthes bidentata led to the identification of two novel chromanones, phomochromanones A (45) and B (46). They showed anti-HIV activities with IC50 values of 20.4 and 32.5 μg/mL, and exhibited moderate cytotoxic activities towards A549, MDA-MB-231, and PANC-1 with CC50 values between 62.5–79.3 μg/mL [41]. A new naphtho-γ-pyrone compound, 5-hydroxy-6,8-dimethoxy-2-benzyl-4H-naphtho[2,3-b]-pyran-4-one (47), was obtained from Phomopsis sp. ZSU-H26 of the mangrove tree E. agallocha. This compound showed cytotoxic activity against HEp-2 (IC50 = 10 μg/mL) and HepG2 (IC50 = 8 μg/mL) [42]. The following work on the similar strain Phomopsis sp. (#ZSU-H76) from the same host additionally obtained phomopsis-H76 A (48), which significantly promoted the growth of the branches of SIV [43]. The structures of chromones (35–48) are shown in Figure 2.
Figure 2.
Chemical structures of compounds 35–58 from Phomopsis.
2.1.3. Chromanones
Chromanones have been widely studied due to their structural characteristics. They always have important biological and pharmacological activities, including cytotoxic, antimicrobial, antiviral, antioxidant, etc [107]. The culture of a marine fungus Phomopsis sp. (No. ZH-111) from mangrove sediment of Zhuhai, Guangdong, China, obtained a new isochroman, (3R,4S)-3,4-dihydro-4,5,8-trihydroxy-3-methylisocoumarin (49). It could promote the growth of SIV branches and exhibited low cytotoxic activity against Hep-2 and HepG2 cells with IC50 values above 50 mg/mL [44]. Three compounds were separated from Phomopsis sp. (No. Gx-4), including (3R,4S)-3,4-dihydro-8-hydroxy-4-methoxy-3-methylisocoumarin (50), 3,4-dihydro-8-hydroxy-3-methyl-1H-2-benzopyran-1-one-5-carboxylic acid (51), and 5,8-dihydroxy-4-methylcoumarin (52). All isolated compounds showed weak cytotoxic activities against Hep-2 and HepG2 cells with IC50 values above 50 μmol/mL. In addition, compounds 50 and 51 significantly promoted the growth of SIV branches, while 52 inhibited their growth [39]. The endophytic fungus Phomopsis sp. sh917 found in stems of Isodon eriocalyx var. laxiflora obtained (10S)-diaporthin (53), showing antiangiogenic activity that inhibited the angiogenesis process induced by vascular endothelial growth factor (VEGF) [45]. From agar-supported fermentation culture of Phomopsis sp. CMU-LMA derived from Alpinia malacensis, a trihydroxybenzene lactone, cytosporone D (54) was isolated. It showed antimicrobial activity and inhibited E. coli DnaG primase with an IC50 value of 0.25 mM [46]. Alternariol (55) and 5ʹ-hydroxyalternariol (57) were isolated from the endophytic fungus Phomopsis sp. A240 of Taxus chinensis var. mairei. Compound 55 showed low cytotoxicity against SF-268 (IC50 = 88.1 μM), MCF-7 (IC50 = 94.36 μM), and NCI-H460 (IC50 = 81.35 μM). Moreover, compound 57 had antioxidant activity with IC50 values of 42.83 μM [47]. Three compounds were sourced from Endodesmia calophylloides associated with Phomopsis sp. CAFT69, including alternariol (55), alternariol-5-O-methyl ether (56) and 5ʹ-hydroxyalternariol (57). In the range of 1–10 μg/mL, compounds 55–57 had certain motility inhibition and lytic activities on the zoospores of grapevine downy mildew pathogen P. viticola in dose- and time-dependent manner [48]. Phomochromanone C (58) was extracted from Phomopsis sp. CGMCC No. 5416. The bioactivity assay revealed that compound 58 showed cytotoxicity towards A549, MDA-MB-231, and PANC-1 with CC50 values of 69.4, 53.5, and 36.5 μg/mL, and it induced early apoptosis of PANC-1 cancer cells with the rate of 10.52% [41]. The structures of chromanones (49–58) are shown in Figure 2.
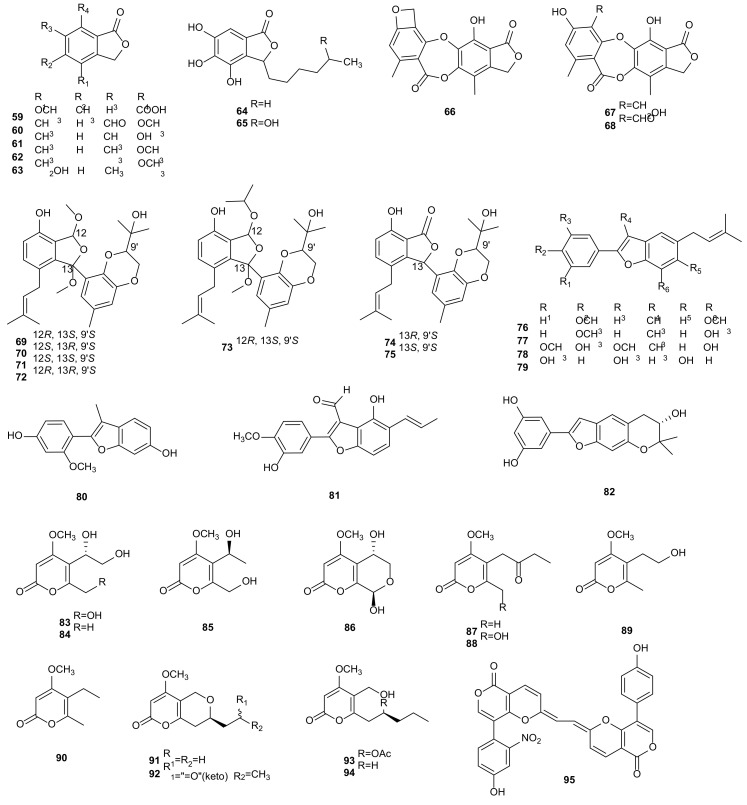
2.1.4. Benzofuranones
Benzofuranones are an important intermediate of pharmacophores and drug molecules in natural products. Due to the furan ring being unstable and easy to open and crack, benzofuranones as a pharmaceutical intermediate have been widely concerned by pharmaceutical chemists [108]. The endophytic fungus Phomopsis sp. A123 isolated from mangrove plant Kandelia candel (L.) Druce, produced a novel depsidone, phomopsidone A (66), a known excelsione (67), and four known isobenzofuranones (59–62). All compounds showed different degrees of cytotoxicities against Raji and MDA-MB-435 tumor cells with IC50 values above 18 μM, displayed low antioxidant activities through DPPH radical scavenging effects, and exhibited antifungal activities [50]. The research on bioactive metabolites of marine fungus Phomopsis sp. (No. ZH-111) led to the isolation of 4-(hydroxymethyl)-7- methoxy-6-methyl-1(3H)-isobenzofuranone (63). Compound 63 inhibited the growth of SIV branches and exhibited low cytotoxic activity with IC50 values above 50 mg/mL against Hep-2 and HepG2 cells [44]. Chemical investigations of secondary metabolites from Phomopsis sp. BCC 45011 of X. granatum resulted in the identification of two known metabolites, cytosporones E (64) and P (65). Compounds 64 and 65 showed antimalarial activities against Plasmodium falciparum K1 with IC50 values of 2.02 and 3.65 μg/mL, and 64 exhibited cytotoxicity against MCF-7, NCI-H187, and Vero cells with IC50 values at 29.66, 5.84, and 4.53 μg/mL, respectively [51]. Cultivation of Phomopsis sp. CAFT69 afforded excelsional (68). In the range of 1–10 μg/mL, compound 68 had certain motility inhibition and lytic activities on the zoospores of grapevine downy mildew pathogen P. viticola in dose- and time-dependent manner [48]. Lithocarols A-F (69–74), with highly-oxygenated isobenzofuran skeleton, and isoprenylisobenzofuran A (75), were derived from P. lithocarpus FS508 isolated from a deep-sea sediment collected from the Indian Ocean. These metabolites were cytotoxic and provided IC50 values between 10.5–87.7 μM against HepG-2, MCF-7, SF-268, and A549 cells [52]. The endophytic fungus Phomopsis sp., separated from Paris polyphylla var. yunnanensis, gave three new arylbenzofurans (76–78) and four known compounds, moracin N (79), 2-(2′-methoxy-4′-hydroxy)-aryl-3-methy-6-hydroxybenzofuran (80), iteafuranal B (81), and moracin P (82). Compounds 76–82 showed inhibitory effects on tobacco mosaic virus (TMV) with inhibition rates of 18.6–35.2% [53]. The structures of benzofuranones (59–82) are shown in Figure 3.
Figure 3.
Chemical structures of compounds 59–95 from Phomopsis.
2.1.5. Pyrones
Pyrones are a kind of polyketides with six membered oxygen-containing heterocycles. As the precursor of many plants, animals, and microorganisms’ biosynthetic reactions, as well as its outstanding anti-tumor and antibacterial activities, researchers have shown strong interest [109]. Eight compounds were identified from the strain P. asparagi SWUKJ5.2020 isolated from medicinal plant Kadsura angustifolia, including five new 2-pyrone compounds, phomaspyrones A-E (83 and 85–88), along with three known metabolites, macommelin-8,9-diol (84), macommelin-9-ol (89), and macommelin (90). All isolated metabolites showed significant cytotoxic activities against six tested tumor cells (A549, Raji, HepG2, MCF-7, HL-60 and K562) with IC50 values of 1.0–26.8 μg/mL. However, phomaspyrone C (86) display better activity than the other compounds with IC50 values of 1.0–2.2 μg/mL against all tested cells [54]. The endophytic fungus Phomopsis sp. isolated from the plant Cistus salvifolius, yielded four new pyrenocines, pyrenocines J-M (91–94). They exhibited antibacterial and algicidal activities against E. coli, B. megaterium, and C. fusca. The antifungal assay showed that 92 and 94 were active against M. violaceum, and compounds 91–92, and 94 were active against Septoria tritici [55]. An unusual pyrone metabolite, phomopsis-H76 C (95), was isolated from Phomopsis sp. (#zsu-H76), which inhibited the growth of SIV branch [43]. The structures of pyrones (83–95) are shown in Figure 3.
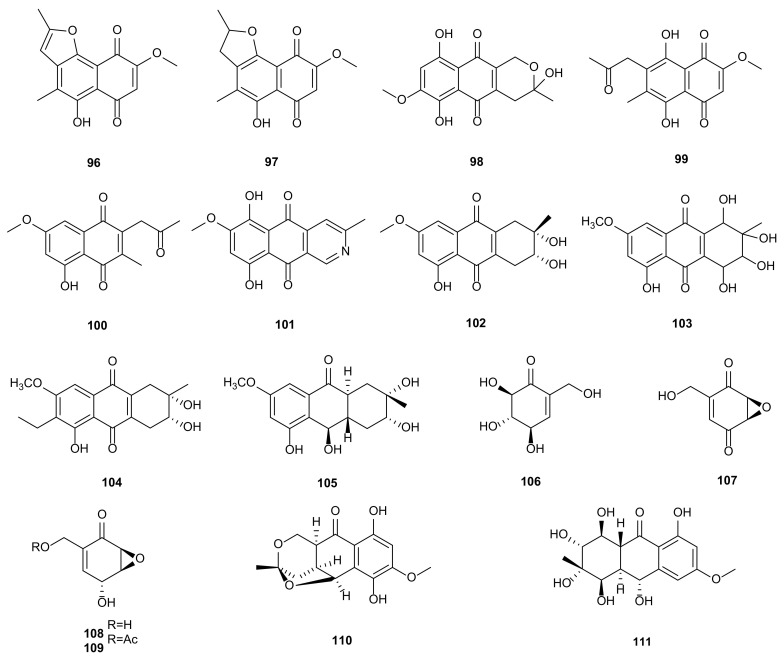
2.1.6. Quinones
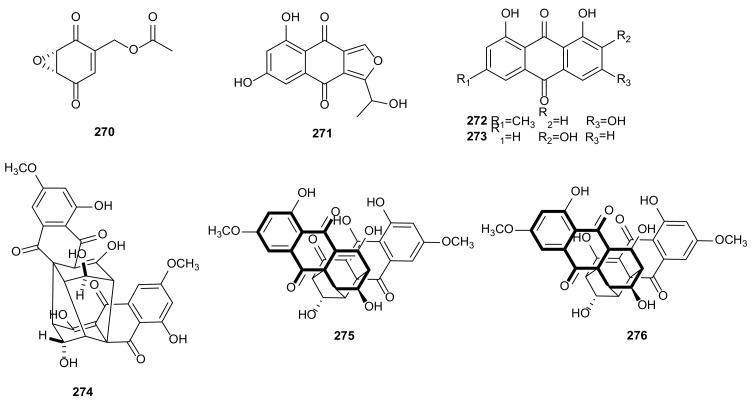
Quinones are natural bioactive molecules with unsaturated cyclic diketones, such as cytotoxic, antimicrobial, antiviral and anti-inflammatory activities. In recent years, the development of new anti-tumor quinones and their derivatives as lead compounds has become a hot topic [110,111]. Studies of the endophytic fungus Phomopsis sp. HCCB04730 associated with stems of Radix Stephaniae Japonicae obtained six known naphthoquinones 96–101. These metabolites showed cytotoxic activities against A549, MDA-MB-231 and PANC-1 cancer cells with IC50 values of 1.1–120.5 μg/mL, and anti-HIV activities with IC50 values between 1.6–26.8 μg/mL [56]. Altersolanol B (102) was separated from P. longicolla HL-2232 of leaves of Bruguiera sexangula var. rhynchopetala collected from the South China Sea. Compound 102 showed antibacterial activity against Vibrio parahaemolyticus (MIC = 2.5 μg/mL) and Vibrio anguillarum (MIC = 5 μg/mL) [57]. A cytotoxic anthraquinone described as altersolanol A (103), was extracted from Phomopsis sp. (PM0409092) isolated from Nyctanthes arbor-tristis. Compound 103 had cytotoxic activity to 34 human cancer cells in vitro and gave the mean IC50 (IC70) value of 0.005 μg/mL (0.024 μg/mL) [58]. A new tetrahydroanthraquinone, named (2R,3S)-7-ethyl-1,2,3,4-tetrahydro-2,3,8-trihydroxy-6-methoxy-3-methyl-9,10-anthracenedione (104), was separated from Phomopsis sp. PSU-MA214 associated with mangrove plant Rhizophora apiculata. Compound 104 was found to have low cytotoxic activity against MCF-7 and antibacterial activity against S. aureus ATCC25923 and methicillin-resistant Staphylococcus aureus SK1 [60]. The extraction of fungus P. foeniculi associated with Foeniculum vulgare in Bulgaria, resulted in the isolation of two octaketides anthracenones, altersolanols A (103) and J (105). They exhibited phytotoxic activities by leaf puncture bioassay [59]. Four known compounds were isolated from Phomopsis sp. derived from Notobasis syriaca, including 2-hydroxymethyl-4β,5α,6β-trihydroxycyclohex-2-en (106), (−)-phyllostine (107), (+)-epiepoxydon (108), and (+)-epoxydon monoacetate (109). All metabolites exhibited antifungal (M. violaceum), antibacterial (E. coli, B. megaterium), and algicidal activities (C. fusca), but 106 and 108 were inactive against M. violaceum [61]. A novel dihydronaphthalenone, phomonaphthalenone A (110), was derived from Phomopsis sp. HCCB04730. In terms of bioactive evaluation, compound 110 showed weak cytotoxic activity and moderate inhibitory activity on HIV with IC50 value of 11.6 μg/mL [56]. Ampelanol (111) was extracted from Phomopsis sp. HNY29-2B isolated from mangrove plant A. ilicifolius. Compound 111 showed antibacterial activity towards B. subtilis and S. aureus with MIC of 25 and 50 μM [62]. The structures of quinones (96–111) are shown in Figure 4.
Figure 4.
Chemical structures of compounds 96–111 from Phomopsis.
2.1.7. Phenols
Phenols are a kind of secondary metabolites which are widely distributed and have important physiological functions. They normally have antioxidant activity and play an important role in food industry [112]. Phomosine K (112) isolated from a Phomopsis strain showed remarkable antibacterial activity against Legionella pneumophila Corby and E. coli K12 [61]. Five known metabolites, phomosines A-D (113–116) and phomosine I (117) were isolated from a Phomopsis strain derived from Ligustrum vulgare. They had antibacterial and antifungal activities against B. megaterium and M. violaceum, except 116 was not active against B. megaterium. Moreover, compounds 113 and 116 inhibited the growth of algae [63]. Two new diphenyl ethers (118–119) were obtained from the culture of P. asparagi isolated from the rhizome of Paris polyphylla var. yunnanensis, collected in Kunming, Yunnan, China. These compounds displayed anti-methicillin-resistant S. aureus (anti-MRSA) activities with inhibition zone diameters (IZD) 10.8 ± 2.0 and 11.4 ± 1.8 mm, respectively [64]. Three new diphenyl ethers, 4-(3-methoxy-5-methylphenoxy)-2-(2-hydroxyethyl)-6-methylphenol (120), 4-(3-hydroxy-5-methylphenoxy)-2-(2-hydroxyethyl)-6-methylphenol (121), and 4-(3-methoxy-5-methylphenoxy)-2-(3-hydroxypropyl)-6-methylphenol (122), were extracted from P. fukushii of Paris polyphylla var. yunnanensis. Compounds 120–122 showed anti-MRSA activities and provided an IZD of 20.2 ± 2.5 mm, 17.9 ± 2.2 mm, and 15.2 ± 1.8 mm, respectively [65]. An endophytic fungus P. fukushii, separated from the rhizome of Paris polyphylla var. yunnanensis, gave three new isopentylated diphenyl ethers (123–125). Compounds (123–125) had notable anti-MRSA activities, and their IZD were 21.8 ± 2.4 mm, 16.8 ± 2.2 mm, and 15.6 ± 2.0 mm, respectively [66]. Two new diphenyl ethers (126–127) were obtained from the fermentation products of P. fukushii isolated from Paris polyphylla var. yunnanensis. The results of the anti-MRSA activities assay revealed that compounds 126 and 127 gave IZD of 13.8 ± 1.5 mm and 14.6 ± 1.6 mm, respectively [67]. Three new napthalene derivatives (128–130) were separated from P. fukushii, an endophytic fungus isolated from Paris polyphylla var. yunnanensis. Compounds 128–130 showed anti-MRSA activities with MCI values of 4, 4 and 6 mg/mL [68]. From fermentation products of the fungus Phomopsis sp. associated with Paris polyphylla var. yunnanensis, two new naphthalene derivatives (131–132) were obtained. Compounds 131–132 displayed anti-MRSA activities with IZD of 14.5 ± 1.2 and 15.2 ± 1.3 mm [69]. A culture of the marine fungus P. lithocarpus FS508 isolated from deep-sea sediment collected from Indian Ocean, obtained a new benzophenone, tenellone H (133). It showed cytotoxicity against HepG-2 (IC50 = 16 μM) and A549 (IC50 = 17.6 μM) [70].
The new metabolite, 16-acetoxycytosporone B (134), was sourced from Phomopsis sp. YM 355364 associated with Aconitum carmichaeli. In the bioassay, compound 134 had remarkable antifungal activity towards C. albicans, Hormodendrum compactum, and Trichophyton gypseum with MIC values of 32, 128, and 512 μg/mL [71]. Cultivation of Phomopsis sp. 0391 isolated from the stems of Paris polyphylla var. yunnanensis afforded cytosporone B (135) and dothiorelone A (136). These two compounds showed notable lipase inhibition and gave IC50 values of 115 and 275 μg/mL with Orlistat (IC50 = 43 μg/mL) as positive control [72]. Cytosporone B (135) was extracted from the cultivation of Phomopsis sp. PSU-H188, an endophytic fungus from Hevea brasiliensis. 135 showed protective effect on INS-1 832/13 pancreatic β-cells (EC50 = 11.08 μM) [73]. Two diastereomeric antineoplastic tenellone derivatives identified as lithocarpinols A (137) and B (138), were isolated from P. lithocarpus FS508, a deep-sea derived fungus derived from a sediment collected in the Indian Ocean. During the cytotoxic assay, compounds 137–138 showed inhibitory effects against HepG-2, MCF-7, SF-268, and A549 cancer cells with IC50 values ranging from 9.4 to 35.9 μmol/L [74]. Phomoindene A (139), a new indene derivative, was produced by Phomopsis sp. (No. GX7-4A) from the mangrove sediment of BeiHai, GuangXi, China. Compound 139 showed weak cytotoxicity againt KB, KBv 200, and MCF-7 cancer cells with IC50 values greater than 50 μmoL/mL [75]. Then, 4-Hydroxybenzaldehyde (140) was extracted from a strain of Phomopsis sp. YM 355364. The antimicrobial activities of 140 provided MIC values at 256 and 128 μg/mL against B. subtilis and P. oryzae [26]. An investigation of the extracts from P. longicolla HL-2232, afforded a new biphenyl derivative, 5,5′-dimethoxybiphenyl-2,2′-diol (141). Compound 141 displayed antibacterial activity against V. parahaemolyticus with MIC value of 10 μg/mL [57]. A known phenylethyl alcohol, phomonitroester (142), was derived from Phomopsis sp. PSU-MA214, exhibiting cytotoxicity with IC50 value of 43 μg/mL against KB [60]. Cytosporone U (143) was isolated from the fermentation products of Phomopsis sp. FJBR-11. This compound displayed inhibitory effect on TMV with IC50 value of 144.6 μg/mL [76]. Altenusin (144) was extracted from Phomopsis sp. CAFT69, possessing a certain motility inhibitory and lytic activity against the zoospores of grapevine downy mildew pathogen P. viticola between 1–10 μg/mL [48]. Cosmochlorins D (145) and E (146) produced by the endophytic fungus Phomopsis sp. N-125 of Ficus ampelas, showed significant cytotoxic activities against HL60 cells with IC50 values of 6.1 and 1.8 μM, and displayed growth-inhibition activities [77]. The structures of phenols (112–146) are shown in Figure 5.
Figure 5.
Chemical structures of compounds 112–154 from Phomopsis.
2.1.8. Oblongolides
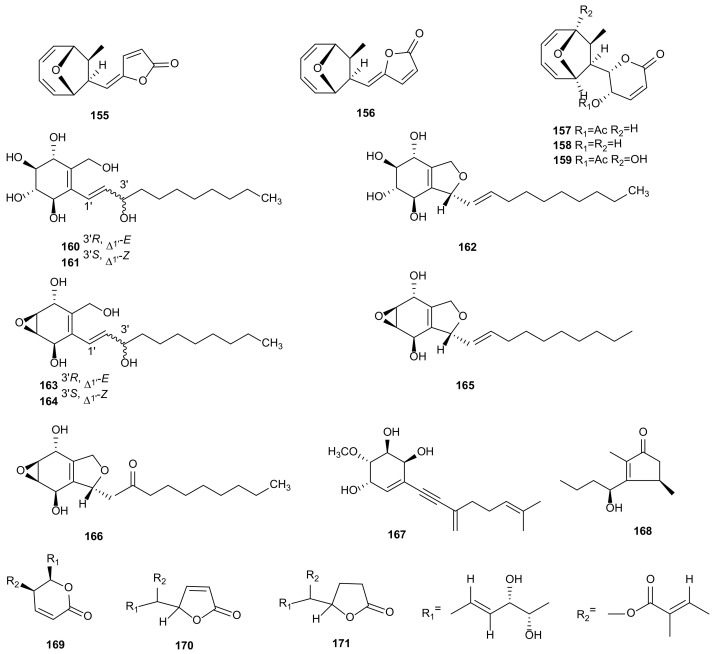
Oblongolides are a kind of natural active products with novel norsesquiterpene γ-lactone. At present, oblongolides are relatively less reported than other kinds of polyketides. Most of them exist in the fungi of Phomopsis, and mainly have cytotoxic activities [113]. Three new oblongolides, oblongolides Z (147) and Y (148) and 2-deoxy-4α-hydroxyoblongolide X (154), were extracted from Phomopsis sp. BCC 9789 isolated from a wild banana (Musa acuminata) leaf. Compound 147 was found to have inhibitory effect on anti-herpes simplex virus type 1 (HSV-1) with IC50 value of 14 μM and showed cytotoxicities with IC50 values at 26–60 μM towards KB, BC, NCI-H187, and Vero cancer cells. Compound 148 was cytotoxic against BC (IC50 = 48 μM) and 154 showed anti-HSV-1 activity with IC50 value of 76 μM [78]. Five metabolites, oblongolides C1 (149), P1 (150), X1 (151), and C (153), along with 6-hydroxyphomodiol (152), were separated from the strain Phomopsis sp. XZ-01, an endophytic fungus of Camptotheca acuminate. Compounds 149–153 displayed different degrees of selective inhibition in cytotoxicities against HepG2 and A549 [79]. The structures of oblongolides (147–154) are shown in Figure 5.
2.1.9. Unclassified Polyketides
Five compounds were obtained from Phomopsis sp. BCC 45011, including phomoxydiene C (155), 1893 A (156), mycoepoxydiene (157), deacetylmycoepoxydiene (158), and phomoxydiene A (159). All metabolites, except 156, showed strong antimalarial activities against P. falciparum K1 with IC50 values at 2.41–3.52 μg/mL and cytotoxicities against KB, MCF-7, NCI-H187, and Vero with IC50 values between 1.49–45.5 μg/mL [51]. Seven new polyoxygenated cyclohexenoids, phomopoxides A-G (160–166) were obtained from the fermentation products of Phomopsis sp. YE3250 isolated from Paeonia delavayi. All compounds exhibited α-glycosidase inhibition with IC50 values from 1.47 to 3.16 mM, cytotoxic activities against Hela, MCF-7, and NCI-H460 cancer cell lines, and moderate antifungal activities against C. albicans, Aspergillus niger, P. oryzae, Fusarium avenaceum, and H. compactum [80]. A new geranylcyclohexenetriol, named phomentrioloxin (167), was obtained from Phomopsis sp. of the plant Carthamus lanatus. This compound showed phytotoxic activity and might be considered a potential mycoherbicide [81]. A new natural cyclopentenone, phomotenone (168) was produced by Phomopsis sp. Compound 168 displayed remarkable antifungal, antibacterial, and antialgal activities against M. violaceum, E. coli, B. megaterium, and C. fusca [40]. The cytotoxicity-guided investigation of the fungus Phomopsis sp. DC275 of Vitis vinifera yielded two new furanones, phomopsolidones A (170) and B (171), and a known phomopsolide B (169). All these metabolites showed weak phytotoxic and antibacterial activities [82]. The structures of unclassified polyketides (155–171) are shown in Figure 6.
Figure 6.
Chemical structures of compounds 155–171 from Phomopsis.
2.2. Terpenoids
Terpenoids are a kind of natural bioactive substances with isoprene as scaffold, which are widely distributed and rich in species [114,115]. Herein, a total of 38 terpenoids, including three monoterpenoids, 25 sesquiterpenoids, seven diterpenoids, and three triterpenoids, were isolated from various Phomopsis strains, accounting for 15% of all the described metabolites, second only to polyketides. It is worth noting that some terpenoids showed interesting bioactivities, such as enzyme inhibitory and anti-inflammatory activities.
2.2.1. Monoterpenoids
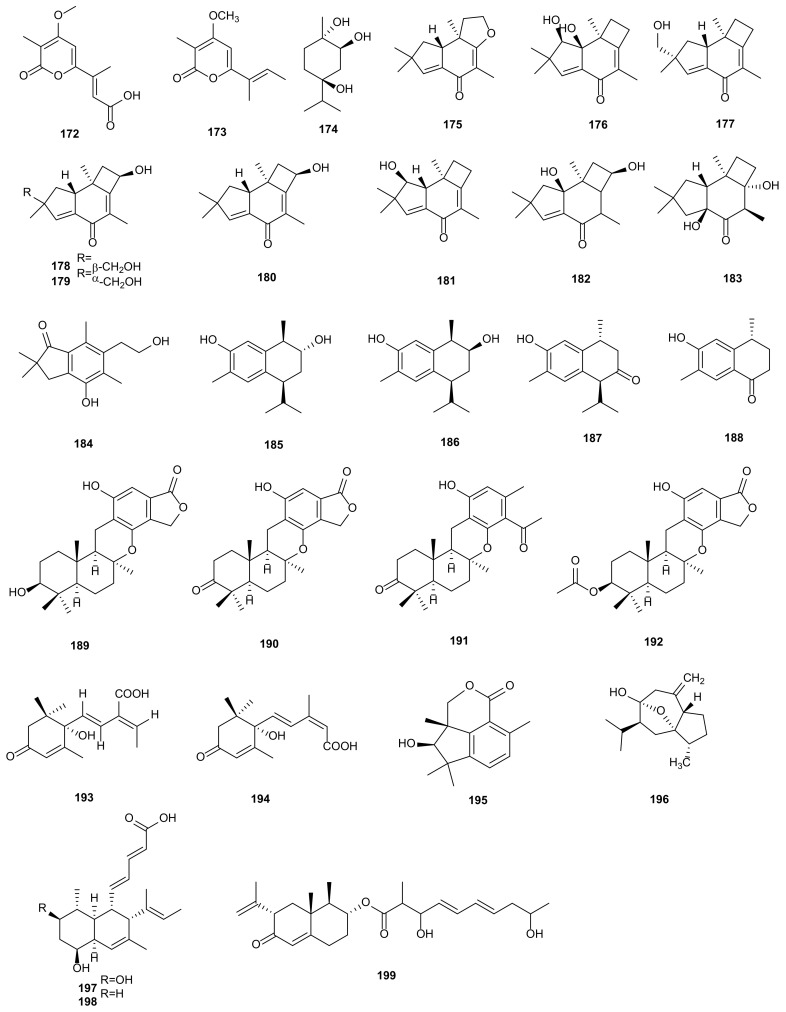
Monoterpenoids and their derivatives have a variety of biological activities, such as cytotoxic, antimicrobial, and anti-inflammatory, which have potential application value in clinical medicine [116]. Acropyrone (172) was extracted from culture of Phomopsis sp. HNY29-2B. Compound 172 showed antibacterial activity towards B. subtilis (MIC = 25 μM) and P. aeruginosa (MIC = 50 μM) [62]. A phytotoxic pentaketide monoterpenoid, nectriapyrone (173), was produced by the fungus P. foeniculi [59]. According to bioassay-guided procedure, a known compound, (1S,2S,4S)-trihydroxy-p-menthane (174) was obtained from Phomopsis sp., displaying antialgal activity against C. fusca and antibacterial activity against E. coli and B. megaterium [40]. The structures of monoterpenoids (172–174) are shown in Figure 7.
Figure 7.
Chemical structures of compounds 172–199 from Phomopsis.
2.2.2. Sesquiterpenoids
Sesquiterpenoids are the most abundant members of natural terpenoids because of their various structures and notable bioactivities. The chemical components of sesquiterpenoids had been found in plants, animals, microorganisms and marine organisms [117,118]. A series of sesquiterpenoids (175–184 and 195) were isolated from a strain of Phomopsis sp. TJ507A obtained from Phyllanthus glaucus. All compounds exhibited the inhibitory rates in the range of 19.4% to 43.8% against β-site amyloid precursor protein cleaving enzyme 1 (BACE1) at the concentration of 40 μM [83]. From the endophytic fungus P. cassia associated with Cassia spectabilis, two new diastereoisomeric cadinanes sesquiterpenes (185–186), (7S,10R)-3-hidroxicalamen-8-one (187), and aristelegone-A (188) were isolated. Compounds 185–188 showed antifungal activities towards Cladosporium cladosporioides and Cladosporium sphaerospermum, and acetylcholinesterase inhibitory activities [84]. Four metabolites were separated from P. archeri of Vanilla albidia, including three new sesquiterpenes, phomoarcherins A-C (189–191), and a known kampanol A (192). The cytotoxic activites of 189–192 provided IC50 values from 0.1 to 19.6 μg/mL against five cholangiocarcinoma cells (KKU-100, KKU-M139, KKU-M156, KKU-M213, and KKU-M214), and 189–190 showed little activities against the KB with IC50 values at 42.1 and 9.4 μg/mL. Compound 190 displayed antimalarial activity against P. falciparum (IC50 = 0.79 μg/mL) [85]. A new sesquiterpene, (+)-S-1-methyl-abscisic-6-acid (193), and a known (+)-S-abscisic acid (194), were extracted from P. amygdali of Call midge. Compounds 193–194 showed antibacterial activities against P. aeruginosa 2033E with MIC at 30 and 58 μg/mL [86]. Curcumol (196), isolated from P. castaneae-mollissimae GQH87 derived from medicinal plant Artemisia annua, showed cytotoxicity against MCF-7, HepG2, and A549 with IC50 values of 25.73, 65.18, and 178.32 μg/mL, respectively [87]. The cultivation of fungus Phomopsis sp. CAFT69, afforded two bioactive compounds, 9-hydroxyphomopsidin (197) and phomopsidin (198). Both of them showed motility inhibition and lytic activities on the zoospores of grapevine downy mildew pathogen P. viticola [48]. AA03390 (199) was isolated from a strain of P. lithocarpus FS508. The compound had low cytotoxicity with IC50 values of 25.5–29.6 μM against HepG-2, MCF-7, SF-268, and A549 [70]. The structures of sesquiterpenoids (175–199) are shown in Figure 7.
2.2.3. Diterpenoids
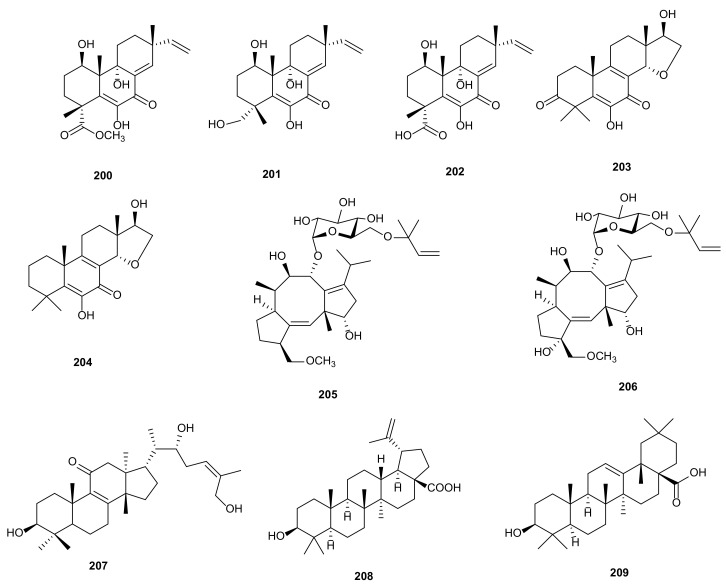
Diterpenoids are a kind of terpenoids with various skeletons. They possess significant pharmacological activities, such as cytotoxic, antimicrobial, and anti-inflammatory activities [119]. A new diterpenes, libertellenone J (200), was derived from fungus Phomopsis sp. S12 isolated from Illigera rhodantha. This compound showed anti-inflammatory activity by reducing the production of NO, IL-1β, IL-6 and TNF-α, and inhibiting MAPKs and NF-κB pathways [88]. Four metabolites were extracted from Phomopsis sp. S12, including three new pimaranes, libertellenone T (202), pedinophyllols K (203) and L (204), together with a known compound, libertellenone C (201). Compounds 201–204 showed different degrees of anti-inflammatory activities against inhibiting the production of inflammatory factors (IL-1β, IL-6) by lipopolysaccharide in macrophages [89]. Secondary metabolites from fungus P. amygdali contained two known compounds, fusicoccin J (205) and 3α-hydroxyfusicoccin J (206). Biologically, compounds 205–206 showed antibacterial activities against P. aeruginosa 2033E with MICs at 26 μg/mL [86]. The structures of diterpenoids (200–206) are shown in Figure 8.
Figure 8.
Chemical structures of compounds 200–209 from Phomopsis.
2.2.4. Triterpenoids
Triterpenoids are a kind of organic compounds widely found in nature. They have attracted the attention of researchers because their structural diversity and rich bioactivities [120]. A new euphane triterpenoid, 3S,22R,26-trihydroxy-8,24E-euphadien-11-one (207), was isolated from P. chimonanthi obtained from medicinal plant Tamarix chinensis in the yellow river delta, Dongying. Compound 207 exhibited cytotoxicity against A549, MDA-MB-231, and PANC-1 cancer cells with IC50 values of 20.32, 19.87 and 30.45 μM, respectively [90]. The fungus Phomopsis sp. SNB-LAP1-7-32, occurring from plant Diospyros carbonaria, produced a first lupane-type triterpenoid, betulinic acid (208). Compound 208 displayed antiviral activity on inhibiting RNA-dependant RNA polymerase with IC50 values of 4.3 μM and cytotoxicity against HCT-116 and MRC-5 [91]. Oleanolic acid (209) was extracted from P. castaneae-mollissimae GQH87, which showed cytotoxicity against MCF-7, HepG2, and A549 with IC50 values of 16.61, 39.53, and 40.08 μg/mL, respectively [87]. The structures of triterpenoids (207–209) are shown in Figure 8.
2.3. Steroids
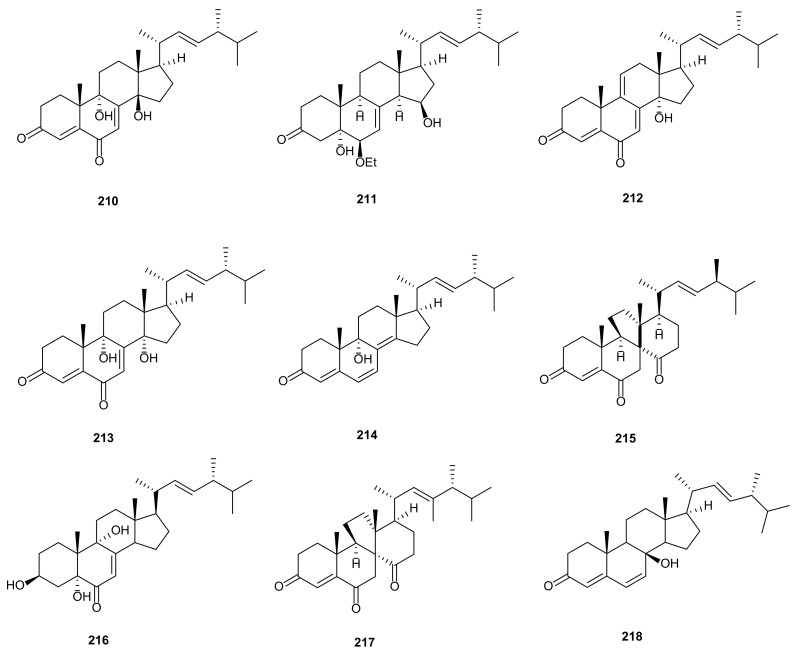
Steroids are secondary metabolites with a variety of chemical structures and biological activities. At present, many researchers try to find steroidal metabolites as potential lead compounds in drug design [121]. Till now, only nine steroids were isolated from Phomopsis and showed antifungal, anti-inflammatory, and antiviral activities. Five steroids were derived from culture of Phomopsis sp., an endophytic fungus separated from A. carmichaeli, including (14β,22E)-9,14-dihydroxyergosta-4,7,22-triene-3,6-dione (210), (5α,6β,15β,22E)-6-ethoxy-5,15-dihydroxyergosta-7,22-dien-3 one (211), calvasterols A (212) and B (213), and ganodermaside D (214). All isolated compounds displayed different degrees of selective antifungal activities against C. albicans, A. niger, P. oryzae, F. avenaceum, H. compactum, and T. gypseum with MIC values between 64–512 μg/mL [92]. Dankasterone A (215) and 3β,5α,9α-trihydroxy-(22E,24R)-ergosta- 7,22-dien-6-one (216) were isolated from Phomopsis sp. YM 355364. Compound 215 showed anti-influenza activity against H5N1pseudovirus (IC50 = 3.56 μM). Compounds 215–216 showed antifungal activities against C. albicans, P. oryzae, H. compactum, and T. gypseum with MIC values of 64–512 μg/mL [71]. A new functionalized ergostane-type steroid, named phomopsterone B (217), was obtained from Phomopsis sp. TJ507A isolated from medicinal plant P. glaucus. Compound 217 showed anti-inflammatory activity by inhibiting iNOS enzyme with an IC50 value of 1.49 μM [93]. Cyathisterol (218) was extracted from Phomopsis sp. YM 355364, displaying moderate antifungal activity toward P. oryzae (MIC = 128 μg/mL) [26]. The structures of steroids (210–218) are shown in Figure 9.
Figure 9.
Chemical structures of compounds 210–218 from Phomopsis.
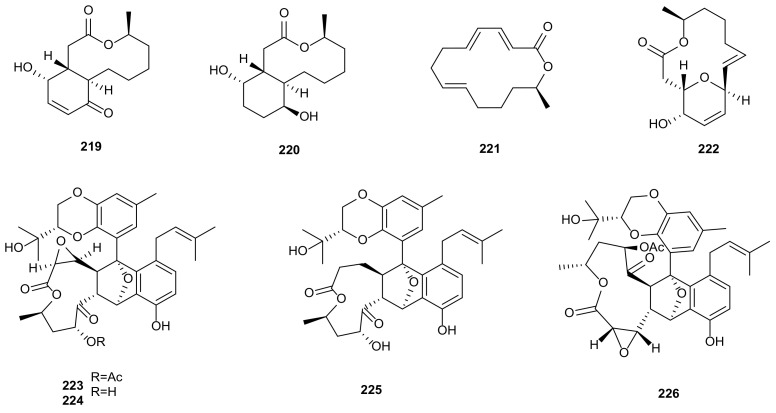
2.4. Macrolides
Macrolides are a class of medicinal compounds containing macrolactone ring structures, many of which are used as antifungal and antibacterial drugs in clinic, such as erythromycins [122]. Nowadays, a large number of macrolide antibiotics are widely used in the treatment of human diseases. Eight secondary metabolites were obtained from Phomopsis and showed cytotoxic, antimicrobial, and enzyme inhibitory activities. Three cytotoxic polyketides, Sch-642305 (219), LMA-P1 (220), and benquoine (221), were found in the endophytic fungus Phomopsis sp. CMU-LMA of Alpinia malaccensis. Compounds 219 and 221 also displayed antimicrobial activities [94]. The endophytic fungus Phomopsis sp. IFB-ZS1-S4 provided a known aspergillide C (222), which had moderate inhibitory effect on neuraminidase in vitro with IC50 value of 5.59 μM [37]. Four highly oxygenated tenellone-macrolide conjugated dimers, lithocarpins A-D (223–226), were obtained from P. lithocarpus FS508 isolated from the deep-sea sediment sample collected in the Indian Ocean. All metabolites (223–226) showed cytotoxic activities against three human tumor cells (SF-268, MCF-7, and HepG-2) with IC50 values in the range of 17.0–52.2 μM [95]. The structures of macrolides (219–226) are shown in Figure 10.
Figure 10.
Chemical structures of compounds 219–226 from Phomopsis.
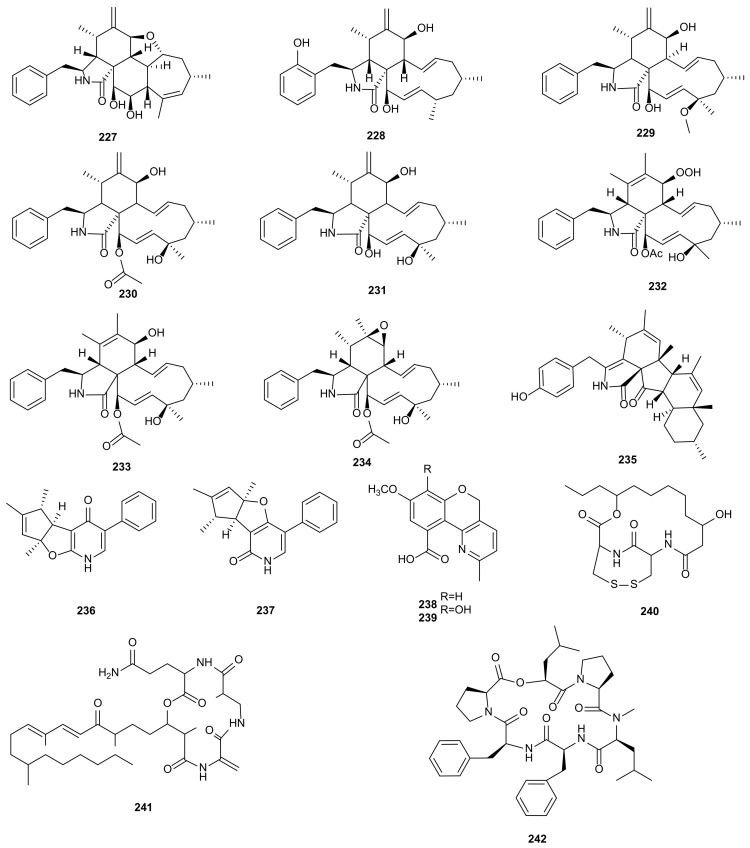
2.5. Alkaloids
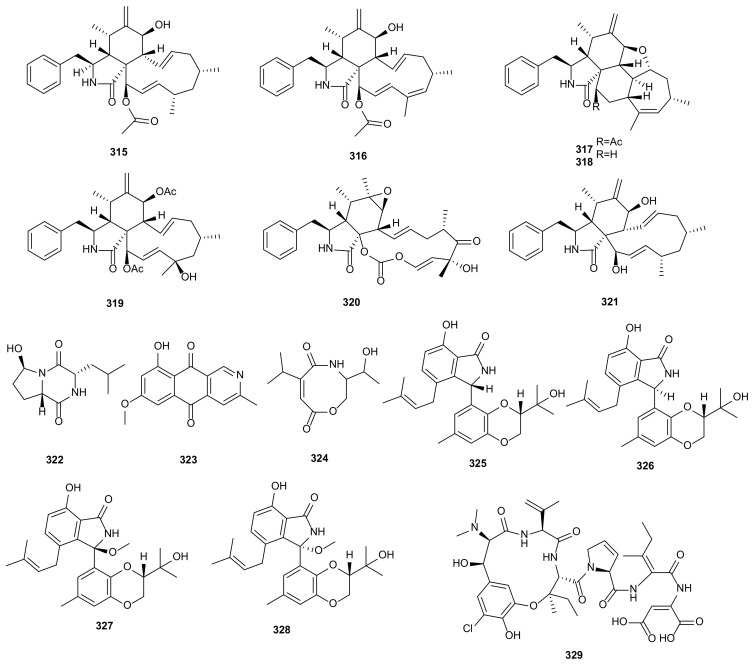
Alkaloids are important nitrogen-containing organic compounds widely existing in microorganisms. At present, some alkaloids have been used to treat human diseases [123]. A total of 16 alkaloids have been isolated from Phomopsis and display various important bioactivities, such as cytotoxic, antibacterial, anti-inflammatory activities. Two compounds with special carbon skeleton, named phomopchalasins B (227) and C (232) were isolated from Phomopsis sp. shj2, an endophytic fungus obtained from the stems of Isodon eriocalyx var. laxiflora. Compound 232 showed cytotoxic activity against HL-60, SMMC-7721, and A-549 with IC50 values of 14.9, 22.7, and 21.1 μM, and displayed anti-inflammatory activity by reducing NO production (IC50= 11.2 μM). In addition, compounds 227 and 232 showed antimigratory activities against MDA-MB-231 with IC50 values of 19.1 and 12.7 μM [96]. Chemical investigation of Phomopsis spp. xy21 and xy22 obtained from leaves of the mangrove tree X. granatum, collected in Trang Province, Thailand, led to the isolation of a new cytochalasin, phomopsichalasin G (228). It showed cytotoxicities against HCT-8, HCT- 8/T, A549, MDA-MB-231, and A2780 cancer cells with IC50 values between 3.4–8.6 μM [97]. Three known compounds, namely 18-metoxycytochalasin J (229), cytochalasins H (230) and J (231), were obtained from Phomopsis sp. isolated from the nut of Garcinia kola. These three compounds exhibited cytotoxicities against HeLa (LC50 = 3.66–35.69 μg/mL) and Vero (LC50 = 73.88–129.10 μg/mL), and different degrees of antibacterial activities against six bacterial pathogens (Vibrio cholera SG24, V. cholera CO6, V. cholera NB2, V. cholera PC2, Shigella flexneri SDINT, and S. aureus ATCC 25923) [98]. The cytochalasins, epoxycytochalasin H (234) and cytochalasin N (233) and H (230), were extracted from Phomopsis sp. By254 derived from the root of Gossypium hirsutum. They showed remarkable antifungal activities with IC50 values between 0.1–50 μg/mL against S. sclerotiorum, Bipolaris maydis, Fusarium oxysporum, B. cinerea, Bipolaris sorokiniana, Gaeumannomyces graminis var. tritici and Rhizoctonia cerealis [99]. Cytochalasins H (230) and J (231), and alternariol (55) were extracted from Phomopsis sp. of Senna spectabilis and showed anti-inflammatory activities by inhibiting the production of reactive oxygen species (ROS). Compound 230 also showed antifungal and acetylcholinesterase enzyme (AChE) inhibitory activities [49]. Cytochalasin J (231) was derived from P. asparagi of plant Peperomia sui and exhibited antiandrogen activity (IC50 = 6.2 μM) [100]. The antibacterial diaporthalasin (235) was extracted from Phomopsis sp. PSU-H188, showing anti-MRSA activity with MIC of 4 μg/mL [73]. A phenylfuropyridone racemate, (+)-tersone E (236), and a known ent-citridone A (237), were separated from P. tersa FS441 derived from deep-sea sediment in the Indian Ocean. Compound 236 showed cytotoxicity with IC50 values at 32.0, 29.5, 39.5 and 33.2 μM towards SF-268, MCF-7, HepG-2, and A549 cancer cells. Compounds 236–237 had antibacterial activities against S. aureus with MIC value of 31.2 and 31.5 μg/mL [101]. Two new chromenopyridine derivatives, phochrodines C (238) and D (239) with 5H-chromeno[4,3-b]pyridine, were isolated from Phomopsis sp. 33# associated with the bark of R. stylosa in the South China Sea. Compounds 238–239 displayed anti-inflammatory activities with IC50 values of 49 and 51 μM by inhibiting nitric oxide production. Moreover, compound 239 also showed antioxidant activity with IC50 value at 34 μM [102]. A novel depsipeptide, PM181110 (240), was obtained from P. glabrae of Pongamia pinnata. It showed anticancer activity towards 40 human cancer cells in vitro (mean IC50 = 0.089 μM) and 24 human tumor xenografts ex vivo (mean IC50 = 0.245 μM) [103]. Fusaristatin A (241) was separated for the first time from P. longicolla S1B4, showing antibacterial activity against X. oryzae [34]. Exumolide A (242) from the strain Phomopsis sp. (No. ZH-111) significantly promoted the growth of SIV branches and showed low cytotoxic activity against Hep-2 and HepG2 [44]. The structures of alkaloids (227–242) are shown in Figure 11.
Figure 11.
Chemical structures of compounds 227–242 from Phomopsis.
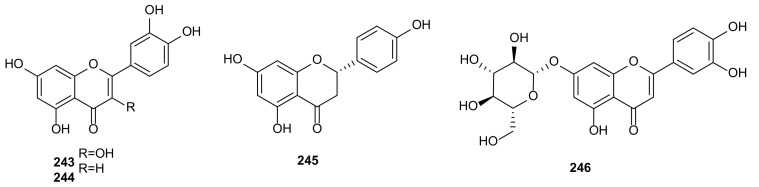
2.6. Flavonoids
Flavonoids are a kind of natural active substances of polyphenols. They are relatively less occurred in fungi [124]. In this review, only four flavonoids, quercetin (243) (Figure 12), luteolin (244), naringenin (245), and luteolin-7-O-glucoside (246) were isolated from P. castaneae-mollissimae GQH87. They displayed cytotoxic activities against MCF-7, HepG2, and A549 with IC50 values between 18.7 and 169.8 μg/mL [87].
Figure 12.
Chemical structures of compounds 243–246 from Phomopsis.
3. Bioactive Secondary Metabolites from Diaporthe spp.
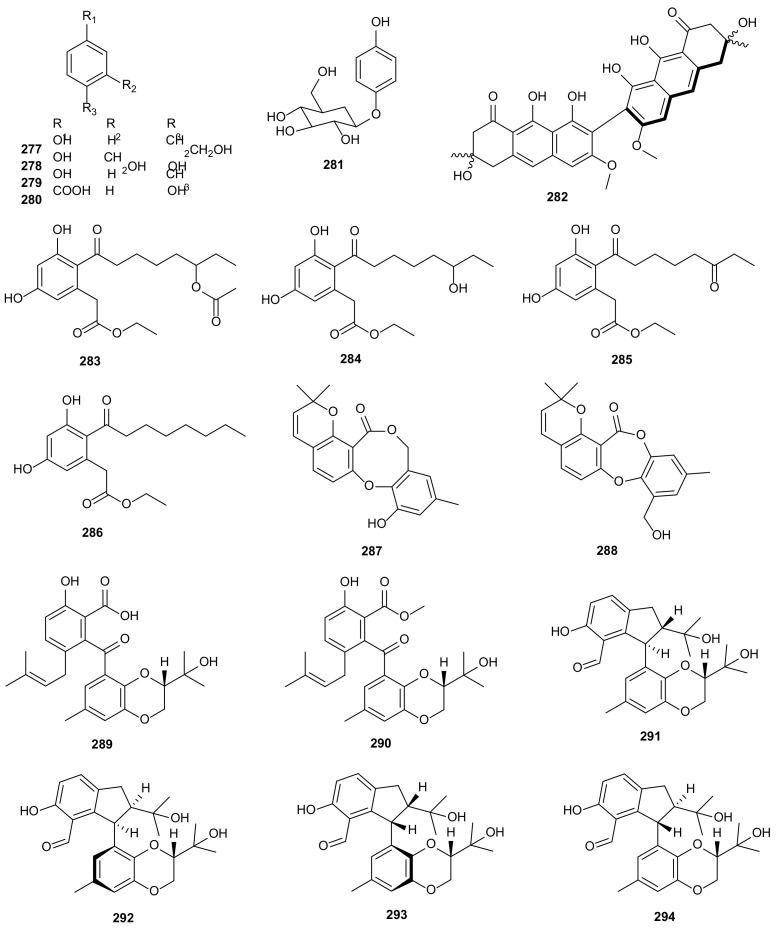
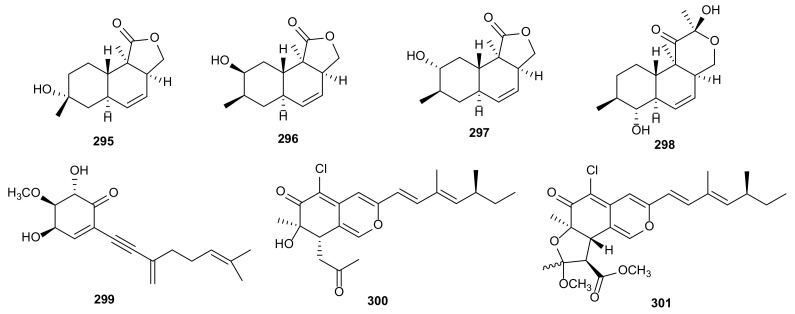
In the last ten years, a total of 106 bioactive secondary metabolites have been isolated from the genus Diaporthe (Table 2). These compounds exhibit various bioactivities, such as cytotoxic, antifungal, antibacterial, antiviral, antioxidant, anti-inflammatory, phytotoxic, antitubercular, antifibrotic, antidiabetic, antimigratory, antiangiogenic, antihyperlipidemic, inhibiting leishmanicidal, activating the NF-κB pathway, enzyme inhibition, inhibitory effects on osteoclastogenesis, antifeedant, contact toxicity, and oviposition deterrent activities. The habitats of the Diaporthe strains were also shown in Table 2, which revealed that there are 73 (accounting for 69%) and 32 (accounting for 30%) compounds isolated from terrestrial and marine environments, respectively, while only one compound (1%) was not mentioned with its habitat.
Table 2.
The bioactive secondary metabolites of the genus Diaporthe during 2010–2019.
| Number | Structural Types | Compounds | Strains | Habitats (T/M a) | Activities | Refs. |
|---|---|---|---|---|---|---|
| 247 | Xanthones | 3,8-Dihydroxy-6-methyl-9-oxo-9H-xanthene-1-carboxylate | Diaporthe sp. SCSIO 41011 | Rhizophora stylosa (M) | Anti-IAV | [125] |
| 28 | Phomoxanthone A |
Diaporthe sp. GZU-1021 D. phaseolorum FS431 |
Chiromanteshae- matochir (M)Sediment (M) |
Anti-inflammatory Cytotoxic |
[126] [127] |
|
| 248 | Chromones | Penialidin A | Diaporthe sp. GZU-1021 |
Chiromanteshae matochir (M) |
Anti-inflammatory | [126] |
| 35 | (+)-Phomopsichin A | D. phaseolorum SKS019 | Acanthus ilicifolius (M) | Inhibitory effects on osteoclastogenesis | [128] | |
| 249 | (−)-Phomopsichin A | D. phaseolorum SKS019 | A. ilicifolius (M) | Inhibitory effects on osteoclastogenesis | [128] | |
| 250 | (+)-Phomopsichin B | D. phaseolorum SKS019 | A. ilicifolius (M) | Inhibitory effects on osteoclastogenesis | [128] | |
| 36 | (−)-Phomopsichin B | D. phaseolorum SKS019Diaporthe sp. GZU-1021 |
A. ilicifolius (M) Chiromateshaem atochir (M) |
Inhibitory effects on osteoclastogenesis Anti-inflammatory |
[128] [126] |
|
| 251 | Diaporchromanone C | D. phaseolorum SKS019 | A. ilicifolius (M) | Inhibitory effects on osteoclastogenesis | [128] | |
| 252 | Diaporchromanone D | D. phaseolorum SKS019 | A. ilicifolius (M) | Inhibitory effects on osteoclastogenesis | [128] | |
| 40 | Pestalotiopsone F | Diaporthe sp. SCSIO 41011 | R. stylosa (M) | Anti-IAV | [125] | |
| 253 | Pestalotiopsone B |
Diaporthe sp. SCSIO 41011 D. pseudomangiferaea |
R. stylosa (M) Tylophora ouata (T) |
Anti-IAV Antifibrotic |
[125] [129] |
|
| 254 | Diaportheone A | Diaporthe sp. P133 | Pandanus amaryllifolius (T) | Antitubercular | [130] | |
| 255 | Diaportheone B | Diaporthe sp. P133 | P. amaryllifolius (T) | Antitubercular | [130] | |
| 53 | Chromanones | (10S)-Diaporthin | D. terebinthifolii LGMF907 | Schinus terebinthifolius (T) | Antibacterial | [131] |
| 256 | Orthosporin | D. terebinthifolii LGMF907 | S. terebinthifolius (T) | Antibacterial | [131] | |
| 54 | Cytosporone D | D. pseudomangiferaea | T. ouata (T) | Cytotoxic, Antioxidant Antidiabetic |
[129] | |
| 257 | Mucorisocoumarin A | D. pseudomangiferaea | T. ouata (T) | Antifibrotic | [129] | |
| 258 | 3,4-Dihydro-8-hydroxy-3,5-dimethyl-isocoumarin | D. eres | Hedera helix (T) | Phytotoxic | [132] | |
| 259 | Diportharine A | Diaporthe sp. | Datura inoxia (T) | Antioxidant | [133] | |
| 260 | Furanones | (1R,2E,4S,5R)-1-[(2R)-5-Oxotetrahydrofuran-2-yl]-4,5-dihydroxy-hex-2-en-1-yl(2E)-2-methylbut-2-enoate | Diaporthe sp. SXZ-19 | Camptotheca acuminate (T) | Cytotoxic | [134] |
| 261 | Butyl 5-[(1R)-1-hydroxyethyl]-γ-oxofuran-2-butanoate | Diaporthe sp. SXZ-19 | C. acuminate (T) | Cytotoxic | [134] | |
| 262 | 3,4-Dihydro-5ʹ-[(1R)-1-hydroxyethyl] [2,2ʹ-bifuran]-5(2H)-one | Diaporthe sp. SXZ-19 | C. acuminate (T) | Cytotoxic | [134] | |
| 263 | 3,4-Dihydro-5ʹ-[(1R)-1-hydroxymethylethyl][2,2ʹ-bifuran]-5(2H)-one | Diaporthe sp. SXZ-19 | C. acuminate (T) | Cytotoxic | [134] | |
| 264 | Kongiidiazadione | D. Kongii | Carthamus lanatus (T) | Phytotoxic, Antibacterial | [135] | |
| 265 | Pyrones | Phomopsolide A | D. maritima |
Picea mariana(T) Picea rubens (T) |
Antifungal, Antibiotic | [136] |
| 169 | Phomopsolide B | D. maritima | P. mariana (T) P. rubens (T) | Antifungal, Antibiotic | [136] | |
| 266 | Phomopsolide C | D. maritima | P. mariana (T)P. rubens (T) | Antifungal, Antibiotic | [136] | |
| 267 | (S,E)-6-(4-Hydroxy-3-oxopent-1-en-1-yl)-2H-pyran-2-one | D. maritima | P. mariana (T) P. rubens (T) | Antifungal, Antibiotic | [136] | |
| 268 | 7-Hydroxy-6-metoxycoumarin | D. lithocarpus | Artocarpus heterophyllus (T) | Antifungal | [137] | |
| 269 | Coumarin | D. lithocarpus | A. heterophyllus (T) | Antibacterial | [137] | |
| 270 | Quinones | Phyllostine acetate | D. miriciae | Cyperus iria (T) | Antifeedant, Contact toxicity, Oviposition deterrent activities | [138] |
| 107 | (−)-Phyllostine | D. miriciae | C. iria (T) | Antifeedant, Contact toxicity, Oviposition deterrent activities | [138] | |
| 271 | Biatriosporin N | Diaporthe sp. GZU-1021 |
Chiromanteshae- matochir (M) |
Anti-inflammatory | [126] | |
| 272 | Emodin | D. lithocarpus | A. heterophyllus (T) | Cytotoxic, Antibacterial | [137] | |
| 273 | 1,2,8-Trihydroxyanthraquinone | D. lithocarpus | A. heterophyllus (T) | Antibacterial | [137] | |
| 274 | (+)-2,2′-Epicytoskyrin A | Diaporthe sp. GNBP-10 | Uncaria gambir Roxb (T) | Antifungal | [139] | |
| 275 | Cytoskyrin C | Diaporthe sp. | Anoectochilus roxburghii (T) | Cytotoxic, Activating the NF-κB pathway | [140] | |
| 276 | (+)-Epicytoskyrin | Diaporthe sp. | A. roxburghii (T) | Cytotoxic, Activating the NF-κB pathway | [140] | |
| 277 | Phenols | Tyrosol |
D. helianthin D. eres |
Luehea divaricate (T) Vitis vinifera (T) |
Antagonistic Phytotoxic |
[141] [142] |
| 278 | 2,5-Dihydroxybenzyl alcohol | D. vochysiae LGMF1583 | Vochysia divergens (T) | Cytotoxic | [143] | |
| 140 | 4-Hydroxybenzaldehyde | D. eres | V. vinifera (T) | Phytotoxic | [142] | |
| 279 | p-Cresol | D. eres | V. vinifera (T) | Phytotoxic | [142] | |
| 280 | 4-Hydroxybenzoic acid | D. eres | V. vinifera (T) | Phytotoxic | [142] | |
| 281 | Arbutin | D. lithocarpus | A. heterophyllus (T) | Cytotoxic | [137] | |
| 113 | Phomosine A | Diaporthe sp. F2934 | Siparuna gesnerioides (T) | Antibacterial | [144] | |
| 115 | Phomosine C | Diaporthe sp. F2934 | S. gesnerioides (T) | Antibacterial | [144] | |
| 282 | Flavomannin-6,6′-di-O-methyl ether | D. melonis | Annona squamosal (T) | Antimicrobial | [145] | |
| 283 | Acetoxydothiorelone B | D. pseudomangiferaea | T. ouata (T) | Antifibrotic | [129] | |
| 284 | Dothiorelone B | D. pseudomangiferaea | T. ouata (T) | Antifibrotic | [129] | |
| 285 | Dothiorelone L | D. pseudomangiferaea | T. ouata (T) | Antifibrotic | [129] | |
| 286 | Dothiorelone G | D. pseudomangiferaea | T. ouata (T) | Antifibrotic | [129] | |
| 287 | Diaporthol A | Diaporthe sp. ECN-137 | Phellodendron amurense (T) | Anti-migration | [146] | |
| 288 | Diaporthol B | Diaporthe sp. ECN-137 | P. amurense (T) | Anti-migration | [146] | |
| 289 | Tenellone C | Diaporthe sp. SYSU-HQ3 | Excoecaria agallocha (M) | MptpB inhibitory | [147] | |
| 290 | Tenellone D | Diaporthe sp. SYSU-HQ3 | E. agallocha (M) | Anti-inflammatory | [148] | |
| 291 | Diaporindene A | Diaporthe sp. SYSU-HQ3 | E. agallocha (M) | Anti-inflammatory | [148] | |
| 292 | Diaporindene B | Diaporthe sp. SYSU-HQ3 | E. agallocha (M) | Anti-inflammatory | [148] | |
| 293 | Diaporindene C | Diaporthe sp. SYSU-HQ3 | E. agallocha (M) | Anti-inflammatory | [148] | |
| 294 | Diaporindene D | Diaporthe sp. SYSU-HQ3 | E. agallocha (M) | Anti-inflammatory | [148] | |
| 75 | Isoprenylisobenzofuran A | Diaporthe sp. SYSU-HQ3 | E. agallocha (M) | Anti-inflammatory | [148] | |
| 295 | Oblongolides | Oblongolide D | Diaporthe sp. SXZ-19 | C. acuminate (T) | Cytotoxic | [134] |
| 296 | Oblongolide H | Diaporthe sp. SXZ-19 | C. acuminate (T) | Cytotoxic | [134] | |
| 297 | Oblongolide P | Diaporthe sp. SXZ-19 | C. acuminate (T) | Cytotoxic | [134] | |
| 298 | Oblongolide V | Diaporthe sp. SXZ-19 | C. acuminate (T) | Cytotoxic | [134] | |
| 299 | Unclassified polyketides | Phomentrioloxin B | D. gulyae | C. lanatus (T) | Phytotoxic | [149] |
| 300 | epi-Isochromophilone II | Diaporthe sp. SCSIO 41011 | R. stylosa (M) | Cytotoxic | [150] | |
| 301 | Isochromophilone D | Diaporthe sp. SCSIO 41011 | R. stylosa (M) | Cytotoxic | [150] | |
| 302 | Monoterpenoids | (1R,2R,4R)-Trihydroxy-p-menthane | Diaporthe sp. SXZ-19 | C. acuminate (T) | Cytotoxic | [134] |
| 303 | Gulypyrone A | D. gulyae | C. lanatus (T) | Phytotoxic | [149] | |
| 304 | Gulypyrone B | D. gulyae | C. lanatus (T) | Phytotoxic | [149] | |
| 173 | Nectriapyrone | D. Kongii | C. lanatus (T) | Phytotoxic | [135] | |
| 305 | Sesquiterpenoids | Diaporol R | Diaporthe sp. | R. stylosa (M) | Cytotoxic | [151] |
| 306 | Eremofortin F | Diaporthe sp. SNB-GSS10 | Sabicea cinerea (T) | Cytotoxic | [152] | |
| 307 | Lithocarin B | D. lithocarpus A740 | Morinda officinalis (T) | Cytotoxic | [153] | |
| 308 | Lithocarin C | D. lithocarpus A740 | M. officinalis (T) | Cytotoxic | [153] | |
| 309 | Triterpenoids | 19-Nor-lanosta-5(10),6,8,24-tetraene-1α,3β,12β,22S-tetraol | Diaporthe sp. LG23 | Mahonia fortunei (T) | Antibacterial | [154] |
| 216 | Steriods | 3β,5α,9α-Trihydroxy-(22E,24R)-ergosta-7,22-dien-6-one | Diaporthe sp. LG23 | M. fortunei (T) | Antibacterial | [154] |
| 310 | Chaxine C | Diaporthe sp. LG23 | M. fortunei (T) | Antibacterial | [154] | |
| 311 | Ten-membered lactones | Phomolide C | Diaporthe sp. | Aucuba japonica var. borealis (T) | Inhibitory of proliferation of human colon adenocarcinoma cells | [155] |
| 312 | Xylarolide | D. terebinthifolii | Glycyrrhiza glabra (T) | Antimicrobial, Cytotoxic | [156] | |
| 313 | Phomolide G | D. terebinthifolii | G. glabra (T) | Antibacterial | [156] | |
| 314 | Xylarolide A | Diaporthe sp. | D. inoxia (T) | Cytotoxic, Antioxidant | [133] | |
| 315 | Alkaloids | 18-Des-hydroxy cytochalasin H | D. phaseolorum-92C | Combretum lanceolatum (T) | Inhibiting leishmanicidal, Antioxidant, Cytotoxic | [157] |
| 316 | 21-Acetoxycytochalasin J2 | Diaporthe sp. GDG-118 | Sophora tonkinensis (T) | Antifungal, Antibacterial | [158] | |
| 317 | 21-Acetoxycytochalasin J3 | Diaporthe sp. GDG-118 | S. tonkinensis (T) | Antifungal, Antibacterial | [158] | |
| 318 | Cytochalasin J3 | Diaporthe sp. GDG-118 | S. tonkinensis (T) | Antifungal, Antibacterial | [158] | |
| 230 | Cytochalasin H |
Diaporthe sp. GDG-118 Diaporthe sp. GZU-1021 |
S. tonkinensis (T) Chiromanteshae matochir (M) |
Antifungal, AntibacterialAnti-inflammatory | [158] [126] |
|
| 319 | 7-Acetoxycytochalasin H | Diaporthe sp. GDG-118 | S. tonkinensis (T) | Antifungal, Antibacterial | [158] | |
| 231 | Cytochalasin J | Diaporthe sp. GDG-118 | S. tonkinensis (T) | Antifungal, Antibacterial | [158] | |
| 320 | Cytochalasin E | Diaporthe sp. GDG-118 | S. tonkinensis (T) | Antifungal, Antibacterial | [158] | |
| 321 | 21-O-Deacetyl-L-696,474 | Diaporthe sp. GZU-1021 |
Chiromanteshae matochir (M) |
Anti-inflammatory | [126] | |
| 322 | Cordysinin A | D. arecae | Kandelia obovate (M) | Anti-angiogenic | [159] | |
| 323 | 5-Deoxybostrycoidin | D. phaseolorum SKS019 | A. ilicifolius (M) | Cytotoxic | [160] | |
| 241 | Fusaristatin A | D. phaseolorum SKS019 | A. ilicifolius (M) | Cytotoxic | [160] | |
| 324 | Vochysiamide B | D. vochysiae LGMF1583 | V. divergens (T) | Antibacterial, Cytotoxic | [143] | |
| 325 | Diaporisoindole A | Diaporthe sp. SYSU-HQ3 | E. agallocha (M) | Anti-inflammatory | [148] | |
| 326 | Diaporisoindole B | Diaporthe sp. SYSU-HQ3 | E. agallocha (M) | Anti-inflammatory | [148] | |
| 327 | Diaporisoindole D |
Diaporthe sp. SYSU-HQ3 Diaporthe sp. SYSU-HQ3 |
E. agallocha (M) E. agallocha (M) |
Anti-inflammatory MptpB inhibitory |
[148] [147] |
|
| 328 | Diaporisoindole E | Diaporthe sp. SYSU-HQ3 | E. agallocha (M) | Anti-inflammatory | [148] | |
| 329 | Phomopsin F | D. toxica | - b | Cytotoxic | [161] | |
| 330 | Fatty acids | 3-Hydroxypropionic acid | D. phaseolorum | Laguncularia racemose (M) | Antimicrobial | [162] |
| 331 | 3-Nitropropionic acid | D. gulyae | C. lanatus (T) | Phytotoxic | [149] | |
| 332 | Diapolic acid A | D. terebinthifolii | G. glabra (T) | Antibacterial | [156] | |
| 333 | Diapolic acid B | D. terebinthifolii | G. glabra (T) | Antibacterial | [156] | |
| 334 | Diaporthsin E | Diaporthe sp. JC-J7 | Dendrobium nobile (T) | Antihyperlipidemic | [163] | |
| 335 | 3-Hydroxy-5-methoxyhex-5-ene-2,4-dione | Diaporthe sp. ED2 | Orthosiphon stamieus (T) | Antifungal | [164] |
a T: terrestrial environment; M: marine environment; b The habitat was not mentioned.
3.1. Polyketides
There are 67 polyketides reviewed from Diaporthe and they exhibit rich biological activities. Here, we classify these polyketides into the following structural types: xanthones, chromones, chromanones, furanones, pyrones, quinones, phenols, oblongolides, and unclassified polyketides.
3.1.1. Xanthones
Chemical investigation of Diaporthe sp. SCSIO 41011 derived from mangrove plant R. stylosa led to identification of a known compound, 3,8-dihydroxy-6-methyl-9-oxo-9H-xanthene-1-carboxylate (247) (Figure 13). It showed influenza A virus (IAV) inhibition against A/Puerto Rico/8/34 H274Y (H1N1), A/FM-1/1/47 (H1N1), and A/Aichi/2/68 (H3N2) with IC50 values of 9.40, 4.80, and 5.12 μM, respectively [125]. Phomoxanthone A (28) with novel carbon skeleton was isolated from the fungus Diaporthe sp. GZU-1021 derived from a red-clawed crab Chiromanteshaematochir and D. phaseolorum FS431 of deep-sea sediment from the Indian Ocean. This compound showed anti-inflammatory activity by inhibiting nitric oxide (NO) production in RAW 264.7 cells with an IC50 value of 6.1 μM [126], and it displayed good cytotoxicity against MCF-7, HepG-2, and A549 with IC50 values of 2.60, 2.55, and 4.64 μM, respectively [127].
Figure 13.
Chemical structures of compounds 247–269 from Diaporthe.
3.1.2. Chromones
Chemical analysis of Diaporthe sp. GZU-1021 associated with Chiromanteshaematochir resulted in the identification of penialidin A (248) and (−)-phomopsichin B (36). They showed inhibitory effects on NO production with IC50 values at 11.9 and 16.5 μM [126]. Six bioactive metabolites were separated from D. phaseolorum SKS019 derived from mangrove plant A. ilicifolius, including four new compounds, (−)-phomopsichin A (249), (+)-phomopsichin B (250), diaporchromanones C (251) and D (252), along with two known compounds, (+)-phomopsichin A (35) and (−)-phomopsichin B (36). These metabolites showed moderate inhibition on osteoclastogenesis by inhibiting RANKL-induced NF-κB activation [128]. Pestalotiopsones F (40) and B (253) were isolated from Diaporthe sp. SCSIO 41011. The two compounds exhibited remarkable anti-IAV activities with IC50 values between 2.52–39.97 μM [125]. Two new benzopyranones, diaportheones A (254) and B (255), were extracted from Diaporthe sp. P133 derived from Pandanus amaryllifolius. They showed moderate antitubercular activities and provided MIC values of 100.9 and 3.5 μM against Mycobacterium tuberculosis H37Rv with Rifampin (MIC = 0.25 μM) as the positive control [130]. The structures of chromones (248–255) are shown in Figure 13.
3.1.3. Chromanones
Two isocoumarins, (10S)-diaporthin (53) and orthosporin (256), were extracted from D. terebinthifolii LGMF907 isolated from Schinus terebinthifolius. They showed antibacterial activities against the methicillin-sensitive Staphylococcus aureus (MSSA) and methicillin-resistant S. aureus (MRSA) [131]. Cytosporone D (54) and mucorisocoumarin A (257) were isolated from the endophytic fungus D. pseudomangiferaea of Tylophora ouata. Compound 257 displayed anti-fibrosis activity with the inhibitory rate of 52.1% on the activation of human lung fibroblasts MRC-5 cells induced by TFG-β at 10 μM. Cytosporone D (54) showed cytotoxicity toward BGC-823 (IC50 = 8.1 μM), antioxidant activity with the inhibition rate of 63.3% by releasing MOA at the concentration of 10 μM, and moderate antidiabetic activity against protein tyrosine phosphatase 1B (PTP1B) [129]. The fungus D. eres derived from pathogen-infected leaf of Hedera helix produced an isocoumarin, 3,4-dihydro-8-hydroxy-3,5-dimethylisocoumarin (258), showing phytotoxic activity in Lemna paucicostata growth [132]. A novel metabolite, diportharine A (259), was obtained from the culture of Diaporthe sp. isolated from Datura inoxia. It showed notable antioxidant activity through DPPH radical scavenging effects (EC50 = 10.3 μM) [133]. The structures of chromanones (256–259) are shown in Figure 13.
3.1.4. Furanones
Furanones are widely used in the field of synthesis, and the synthesized products have important pharmacological activities, such as antiviral, antitumor and antimicrobial [165]. Four bioactive furanones were derived from Diaporthe sp. SXZ-19 of C. acuminate, including the new (1R,2E,4S,5R)-1-[(2R)-5-oxotetrahydrofuran-2-yl]-4,5-dihydroxy-hex-2-en-1-yl(2E)-2-methylbut-2-enoate (260) and three linear furanopolyketides (261–263). These compounds had weak cytotoxicities against HCT 116 cells with the concentration at 10 μM [134]. A new 3-substituted-5-diazenylcyclopentendione, named kongiidiazadione (264), was separated from D. kongii of plant C. lanatus, which was phytotoxic component and showed low antibacterial activity against Bacillus amyloliquefaciens [135]. The structures of furanones (260–264) are shown in Figure 13.
3.1.5. Pyrones
Four secondary metabolites were isolated from D. maritima of healthy Picea mariana and Picea rubens needles collected from the Acadian forest of Eastern Canada, including three dihydropyrones, phomopsolides A (265), B (169), and C (266), and a stable α-pyrone, (S,E)-6-(4-hydroxy-3-oxopent-1-en-1-yl)-2H-pyran-2-one (267). All compounds showed antifungal and antibiotic activities against M. violaceum, Saccharomyces cerevisiae, and B. subtilis [136]. Two known metabolites, 7-hydroxy-6-metoxycoumarin (268) and coumarin (269), were isolated from the endophytic fungus D. lithocarpus obtained from Artocarpus heterophyllus. Compounds 268 showed significant antifungal activity against Sporobolomyces salminocolor with the of 12.2 ± 0.3 mm, and 269 had a diameter inhibition zone of 12.3 ± 0.3 mm against the bacteria B. subtilis [137]. The structures of pyrones (265–269) are shown in Figure 13.
3.1.6. Quinones
Two cyclohexeneoxidedione derivatives, phyllostine acetate (270) and phyllostine (107), showing strong antifeedant activities on Plutella xylostella, were extracted from culture of D. miriciae of plant Cyperus iria. Compounds 270 and 107 had the feeding inhibition of 100% at 50 μg/cm2 and the 50% feeding deterrence (DC50) values of 9 and 4.7 μg/cm2, displayed contact toxicities with the median lethal concentration (LC50) values of 4.38 and 6.54 μg/larva, and exhibited oviposition deterrent activities with the indexes of 100% and 28.6% at 50 μg/cm2, respectively [138]. The new biatriosporin N (271) was isolated from the marine-derived fungus Diaporthe sp. GZU-1021 and displayed anti-inflammatory activity by inhibiting NO production in RAW 264.7 cells with an IC50 value of 11.5 μM [126]. Two anthraquinone derivatives, emodin (272) and 1,2,8-trihydroxyanthraquinone (273), were isolated from an endophytic fungus D. lithocarpus. Emodin (272) exhibited notable cytotoxic activity against murine leukemia P-388 cells (IC50 = 0.41 μg/mL) and antibacterial activity against B. subtilis, M. luteus, Pseudomonas fluorescences, E. coli, and S. cerevisiae with the diameter of inhibition zones of 14.7, 13.2, 13.7, 12.7, and 11.7 mm, respectively. Compound 273 also displayed antibacterial activity against B. subtilis, E. coli, and S. cerevisiae at 14.2, 11.3, and 10.7 mm, respectively [137]. A bis-anthraquinone derivative, named (+)-2,2′-epicytoskyrin A (274), was isolated from Diaporthe sp. GNBP-10 of Uncaria gambir Roxb. It showed antifungal activity against 22 yeast strains and 3 filamentous fungi with MICs between 16–128 μg/mL [139]. Two cytoskyrin type bisanthraquinones, cytoskyrin C (275) and (+)-epicytoskyrin (276), were isolated from Diaporthe sp., an endophytic fungus obtained from Anoectochilus roxburghii. Compounds 275–276 could activate NF-κB pathway and increase the relative activity of luciferase at the concentration of 50 μM, and showed cytotoxicities against SMMC-7721 cells in dose-dependent manner [140]. The structures of quinones (270–276) are shown in Figure 14.
Figure 14.
Chemical structures of compounds 270–276 from Diaporthe.
3.1.7. Phenols
The phenolic metabolite, tyrosol (277), was extracted from D. helianthi isolated from Luehea divaricate. Tyrosol showed significant antagonistic activity against the tested pathogenic bacteria (Enterococcus hirae, E. coli, M. luteus, Salmonella typhi, S. aureus, and Xanthomonas asc. Phaseoli) [141]. 2,5-Dihydroxybenzyl alcohol (278) was derived from D. vochysiae LGMF1583 of medicinal plant Vochysia divergens, which showed cytotoxic activity against A549 (EC50 = 54.8 μM) and PC3 (EC50 = 9.45 μM) [143]. Four phytotoxic compounds, 4-hydroxybenzaldehyde (140), p-cresol (279), 4-hydroxybenzoic acid (280), and tyrosol (277), were isolated from D. eres of grapevine (V. vinifera) wood. In the leaf disk and leaf absorption bioassay, phytotoxicities of all compounds increased with the concentration ranging in 0.1–1 mg/mL [142]. Arbutin (281), obtained from an endophytic fungus D. lithocarpus, had moderate cytotoxicity against murine leukemia P-388 cells and gave an IC50 value at 2.91 μg/mL [137]. Two antibacterial metabolites, phomosines A (113) and C (115), were extracted from Diaporthe sp. F2934 of plant Siparuna gesnerioides. They were active against S. aureus, M. luteus, Streptococcus oralis, Enterococcus fecalis, Enterococcus cloacae, and Bordetella bronchiseptica with inhibition zone diameter from 6 ± 0.62 to 12 ± 1.18 mm at the concentration of 4 μg/μL [144]. Flavomannin-6,6ʹ-di-O-methyl ether (282) was extracted from an endophytic strain of D. melonis from Annona squamosal, which showed antimicrobial activity against S. aureus 25697, S. aureus 29213, and Streptococcus pneumoniae ATCC 49619 with MIC values of 32, 32, and 2 μg/mL, respectively [145]. Four secondary metabolites, acetoxydothiorelone B (283), and dothiorelones B (284), L (285) and G (286), were isolated from D. pseudomangiferaea. All of them displayed antifibrotic activities with the inhibitory rates of 17.4, 62.9, 59.2 and 41.1% on the activation of human lung fibroblasts MRC-5 cells induced by TFG-β at 10 μM, with pirfenidone (53.2%) as positive control at 1 mM [129]. Two diphenyl ether derivatives, diaporthols A (287) and B (288), were extracted from Diaporthe sp. ECN-137 isolated from the leaves of Phellodendron amurense. Compounds 287–288 exhibited anti-migration effects on TGF-β1-elicited MDA-MB-231 breast cancer cells with an concentration at 20 μM [146]. Tenellone C (289) was obtained from Diaporthe sp. SYSU-HQ3 of mangrove plant E. agallocha, displaying inhibitory effect on M. tuberculosis protein tyrosine phosphatase B (MptpB) (IC50 = 5.2 μM) [147]. Six compounds were isolated from endophytic fungus Diaporthe sp. SYSU-HQ3 derived from the branches of E. agallocha, including a new benzophenone derivative, tenellone D (290), four special 2,3-dihydro-1H-indene isomers, diaporindenes A-D (291–294), and isoprenylisobenzofuran A (75). All isolated compounds showed anti-inflammatory activities by LPS-Induced NO production in RAW 264.7 cells with IC50 values of 4.2–18.6 μM [148]. The structures of phenols (277–294) are shown in Figure 15.
Figure 15.
Chemical structures of compounds 277–294 from Diaporthe.
3.1.8. Oblongolides
Four lovastatin analogues, oblongolides D (295), H (296), P (297) and V (298), were obtained from the endophytic fungus Diaporthe sp. SXZ-19. These metabolites showed weak cytotoxic activities against HCT 116 cells with the concentration of 10 μM [134]. The structures of oblongolides (295–298) are shown in Figure 16.
Figure 16.
Chemical structures of compounds 295–301 from Diaporthe.
3.1.9. Unclassified Polyketides
Phomentrioloxin B (299) was obtained from a strain of D. gulyae isolated from C. lanatus, which had low phytotoxic effect to cause small necrosis against several weedy and crop plant species [149]. The fungus Diaporthe sp. SCSIO 41011 derived from mangrove plant R. stylosa, afforded two metabolites, epi-isochromophilone II (300) and isochromophilone D (301). Compound 300 displayed cytotoxicities against ACHN, OS-RC-2, and 786-O cells with IC50 values between 3.0 and 4.4 μM, and 301 had an IC50 of 8.9 μM against 786-O cancer cells [150]. The structures of unclassified polyketides (299–301) are shown in Figure 16.
3.2. Terpenoids
(1R,2R,4R)-Trihydroxy-p-menthane (302) was isolated from Diaporthe sp. SXZ-19, and displayed weak cytotoxicity on HCT 116 cells [134]. Two new α-pyrones, gulypyrones A (303) and B (304), were extracted from D. gulyae. Both of them showed phytotoxic activities and gulypyrone A caused necrosis against Helianthus annuus plantlets [149]. A pentaketide monoterpenoid, nectriapyrone (173), was isolated from culture of D. Kongii, showing phytotoxic activity [135]. A new brasilane-type sesquiterpenoid, diaporol R (305) was produced by an endophytic fungus Diaporthe sp. isolated from leaves of R. stylosa. Diaporol R had moderate cytotoxic effect on SW480 cancer cells and provided an IC50 value at 8.72 ± 1.32 μΜ [151]. Eremofortin F (306) was obtained from endophytic fungus Diaporthe sp. SNB-GSS10 of Sabicea cinerea. It showed cytotoxic activity against KB and MRC5 cells with IC50 values of 13.9 and 12.2 μM [152]. Two new eremophilanes, lithocarins B (307) and C (308), were extracted from D. lithocarpus A740, an endophytic fungus isolated from Morinda officinalis. These compounds displayed low cytotoxicities against SF-268, MCF-7, HepG-2, and A549 tumor cells with IC50 values between 37.68–97.71 μM [153]. The new triterpenoid, 19-nor-lanosta-5(10),6,8,24-tetraene- 1α,3β,12β,22S-tetraol (309), was obtained from Diaporthe sp. LG23 of the Chinese medicinal plant Mahonia fortunei, and displayed antibacterial activity against both Gram-positive and Gram-negative bacteria [154]. The structures of terpenoids (302–309) are shown in Figure 17.
Figure 17.
Chemical structures of compounds 302–314 from Diaporthe.
3.3. Steriods
Only two steroids, 3β,5α,9α-trihydroxy-(22E,24R)-ergosta-7,22-dien-6-one (216) and chaxine C (310) (Figure 17), were isolated from Diaporthe sp. LG23, showing antibacterial activities against B. subtilis with streptomycin as a positive control [154].
3.4. Ten-Membered Lactones
Ten-membered lactones always have anti-tumor, anti-inflammatory, anti-viral, anti-bacterial and other pharmacological activities, exhibiting important medical value in clinical practice [166]. Phomolide C (311) from Diaporthe sp. of Aucuba japonica var. borealis, inhibited the proliferation of human colon adenocarcinoma cells with concentration of 50 μg/mL [155]. The endophytic fungus D. terebinthifolii GG3F6 derived from medicinal plant Glycyrrhiza glabra, afforded two known compounds, xylarolide (312) and phomolide G (313). Compound 312 had cytotoxicity in vitro against cancer cells MIAPaCa-2, HCT-116 and T47D cancer cells with IC50 values of 38, 100, and 7 μM and showed notable antimicrobial activity against C. albicans and Yersinia enterocolitica with IC50 values at 78.8 and 72.1 μM. Moreover, Compound 313 showed an IC50 value of 69.2 μM against Y. enterocolitica [156]. A novel metabolite, named xylarolide A (314), was isolated from the fungus Diaporthe sp. of D. inoxia. Compound 314 had remarkable cytotoxicities against MIAPaCa-2 and PC-3 cancer cells with IC50 values between 14–32 μM, and also showed antioxidant activity on DPPH radical scavenging effect (EC50 = 10.3 μM) [133]. The structures of four ten-membered lactones (311–314) are shown in Figure 17.
3.5. Alkaloids
18-Des-hydroxy cytochalasin H (315) was obtained from endophytic fungus D. phaseolorum-92C of Combretum lanceolatum. This compound inhibited leishmanicidal activity, displayed moderate antioxidant activity, and had cytotoxic activity against the breast cancer cells MDA-MB-231 and MCF-7 [157]. A series of the cytochalasins were extracted from Diaporthe sp. GDG-118 of Sophora tonkinensis, including 21-acetoxycytochalasins J2 (316) and J3 (317), 7-acetoxycytochalasin H (319), and cytochalasins J3 (318), H (230), J (231), and E (320). All isolated metabolites showed different degrees of antifungal activities against Alternaria oleracea, Pestalotiopsis theae, Colletotrichum capsici, and Ceratocystis paradoxa with MIC values of 1.56–100 μg/mL, and antibacterial activities against Gram-positive bacteria (B. subtilis, B. megaterium and Bacillus anthraci) and Gram-negative bacteria (Proteus vuigaris, E. coli and Salmonella paratyphi B) with MIC values in the range of 12.5–100 μg/mL [158]. The fungus Diaporthe sp. GZU-1021 yielded cytochalasin H (230) and 21-O-deacetyl-L-696,474 (321), which showed anti-inflammatory activities by inhibiting NO production in RAW 264.7 cells with IC50 values of 1.94 and 7.35 μM [126]. Cordysinin A (322) was derived from endophytic fungus D. arecae of Kandelia obovate. It showed anti-angiogenic activity against the human endothelial progenitor cells (EPCs) with IC50 value of 15.1 ± 0.2 μg/mL [159]. Further research led to the identification of 5-deoxybostrycoidin (323) and fusaristatin A (241) from D. phaseolorum SKS019 of mangrove plant A. ilicifolius. Compound 323 showed cytotoxic activity against MDA-MB-435 and NCI-H460 with IC50 values at 5.32 and 6.57 μM, and the IC50 value of 241 was 8.15 μM on MDA-MB-435 [160]. A new carboxamide, vochysiamide B (324), was extracted from new species D. vochysiae LGMF1583, which displayed antibacterial activity on the Gram-negative bacterium Klebsiella pneumoniae (KPC) with MIC value at 80 μg/mL and showed cytotoxic activity against A549 (EC50 = 86.4 μM) and PC3 (EC50 = 40.25 μM) [143]. Four compounds, diaporisoindoles A (325), B (326), D (327), and E (328), were obtained from an endophytic fungus Diaporthe sp. SYSU-HQ3. They all showed anti-inflammatory activities by reducing NO production with IC50 values of 22.7, 18.2, 8.9, and 8.3 μM, respectively [148]. Diaporisoindole D (327) also exhibited inhibitory activity towards M. tuberculosis protein tyrosine phosphatase B (MptpB) (IC50 = 4.2 μM) [147]. Phomopsin F (329) was isolated from D. toxica, and showed cytotoxic activity against HepG2 cells [161]. The structures of alkaloids (315–329) are shown in Figure 18.
Figure 18.
Chemical structures of compounds 315–329 from Diaporthe.
3.6. Fatty Acids
Fatty acids are simple linear compounds that play an important role in the synthesis and catabolism of organisms [167]. Over here, six fatty acids are reported from Diaporthe. The fungus D. phaseolorum derived from Laguncularia racemose, afforded 3-hydroxypropionic acid (330), which showed antimicrobial activity against S. aureus and S. typhi [162]. A phytotoxic metabolite, 3-nitropropionic acid (331), was isolated from D. gulyae. Compound 331 was notably active in causing necroses on several weedy and crop plant species [149]. Two new fatty acids, diapolic acids A and B (332 and 333), were isolated from endophytic fungus D. terebinthifolii. They had moderate antibacterial activities against Y. enterocolitica with IC50 values of 78.4 and 73.4 μM [156]. Studies of the strain Diaporthe sp. JC-J7 from stems of Dendrobium nobile led to the isolation of a new compound, diaporthsin E (334). It showed low antihyperlipidemic activity on triglycerides (TG) in steatotic L-02 cells with the inhibition rate of 26% at the concentration of 5 μg/mL [163]. The novel anti-candidal metabolite, 3-hydroxy-5-methoxyhex-5-ene-2,4-dione (335), was derived from Diaporthe sp. ED2 of medicinal herb Orthosiphon stamieus Benth. It showed antifungal activity against C. albicans with MIC value of 3.1 μg/mL [164]. The structures of fatty acids (330–335) are shown in Figure 19.
Figure 19.
Chemical structures of compounds 330–335 from Diaporthe.
4. Characteristics of Bioactive Secondary Metabolites from the Genus Diaporthe and Anamorph Phomopsis
In this paper, a total of 335 bioactive compounds from the genus Diaporthe and Phomopsis are summarized. There are 106 secondary metabolites from Diaporthe and 246 ones from Phomopsis, in which 17 compounds were obtained from both of Diaporthe and Phomopsis. These compounds are classified into polyketides, terpenoids, steroids, macrolides, ten-membered lactones, alkaloids, flavonoids, and fatty acids. As seen in Figure 20, about two thirds of all compounds reported from Diaporthe and Phomopsis are refered to polyketides, accounting for 63% and 70%, respectively. Moreover, terpenoids (8%, 15%), alkaloids (17%, 6%), and steroids (2%, 4%) were also produced by both of Diaporthe and Phomopsis. It is worth noting that fatty acids (6%) and ten-membered lactones (4%) are only reported from Diaporthe, while flavonoids (2%) and macrolides (3%) are only found in Phomopsis. Polyketides, as the largest member of the metabolites, are widely used in the field of medicine and play an important role in the treatment of cancer diseases.
Figure 20.
(a) The proportion of structural types of bioactive compounds from Diaporthe; (b) The proportion of structural types of bioactive compounds from Phomopsis.
The various bioactivities of the compounds isolated from Diaporthe and Phomopsis are presented in Figure 21, mainly containing cytotoxic, antibacterial, antifungal, antiviral, anti-inflammatory, antioxidant, antialgae, enzyme inhibition, and phytotoxic activities. Most of compounds have at least one kind of bioactivities. As seen in Figure 21 and Table 1 and Table 2, secondary metabolites of Diaporthe and Phomopsis mainly exhibit cytotoxic, antibacterial and antifungal activities, accounting for 73% of all compounds, with 56 in Diaporthe and 200 from Phomopsis. Interestingly, in recent years, more and more compounds with anti-inflammatory, antioxidant and enzyme inhibitory activities have been studied in important human diseases.
Figure 21.
The distribution of main bioactivities of compounds isolated from Diaporthe and Phomopsis.
5. Conclusions
This review presents the diverse chemical structures and bioactivities of 335 compounds isolated from 26 known species and various unidentified species of the genus Diaporthe and its anamorph Phomopsis between 2010–2019. Here, we can see from Table 1 and Table 2, among all of the reported compounds, there are 236 (accounting for about 70%) and 92 (about 27%) compounds derived only from terrestrial and marine environments (including mangroves, sediments, deep-sea fungi and marine animals), respectively. In addition, only one compound is obtained from both of terrestrial and marine environments. In contrast, six compounds are not mentioned with their habitats in the literature. Polyketides represent the main chemical population, accounting for 64%. About 73% of all metabolites possess cytotoxic, antibacterial, and antifungal activities. The species named as Phomopsis significantly produce much more compounds than Diaporthe, and most strains have not yet been identified at the species level. In conclusion, these results illustrate that the metabolic resources of Diaporthe and Phomopsis are of great value and deserved to conduct further research. Interestingly, in the past three years, there have been more reports on the secondary metabolites of the fungi in Diaporthe and Phomopsis than before, displaying an increasing trend, which indicates that Diaporthe and Phomopsis are regarded as important sources for discovering new natural bioactive substances.
In the past many years, lots of interesting fungal bioactive metabolites had been widely developed into new drugs, like antibiotics. Although most compounds obtained from Diaporthe and Phomopsis fungi had been studied on their isolation, structures, and activities, the in-depth research on pharmacological mechanisms and development of potent active compounds in drugs are still less. According to current studies, some compounds with remarkable bioactivities may serve as potential drug candidates in the future, such as cytotoxic altersolanol A and PM181110, and antimicrobial dicerandrol A. In order to ascertain the therapeutic potential of these compounds, further studies of pharmacological and producing mechanisms are required.
The fungal species in Diaporthe and Phomopsis have been considered to be important sources that can produce diverse and novel bioactive metabolites, which has attracted many natural product chemists and pharmacologists to study in recent years. The metabolites produced by Diaporthe and Phomopsis have rich biological activities, which is enough to show the importance of its metabolic resources. Nowadays, many fungi produce interesting bioactive metabolites that have been studied for their biosynthesis pathway, while similar studies in Diaporthe and Phomopsis are performed relatively less often. In the following work, the microbial biosynthesis pathway might be considered for further developing valuable products from Diaporthe or Phomopsis, which are hoped to be used as drug molecules for disease treatment. However, it cannot be ignored that Diaporthe or Phomopsis are important plant pathogens which might cause a wide range of plant host diseases and even serious human pathogens. In the future work, we should also focus on the role of metabolites produced by these pathogens, as well as the relationships with their hosts.
Author Contributions
Manuscript preparation, T.-C.X.; prepared figures, tables, and analyzed data, Y.-H.L.; data proofreading and manuscript revision, J.-F.W., Z.-Q.S., Y.-G.H., S.-S.L., and C.-S.L.; manuscript conception and revision, S.-H.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 81860634), Applied Basic Research Key Project of Yunnan Province (No. 202001BB050029), Major Science and Technology Projects of Yunnan Province (Digitalization, development and application of biotic resource, 202002AA100007), and Project of Innovative Research Team of Yunnan Province (202005AE160005).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data in this article is openly available without any restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Guarnaccia V., Groenewald J.Z., Woodhall J., Armengol J., Cinelli T., Eichmeier A., Ezra D., Fontaine F., Gramaje D., Gutierrez-Aguirregabiria A., et al. Diaporthe diversity and pathogenicity revealed from a broad survey of grapevine diseases in Europe. Persoonia. 2018;40:135–153. doi: 10.3767/persoonia.2018.40.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dissanayake A.J., Chen Y.Y., Liu J.K.J. Unravelling Diaporthe species associated with woody hosts from karst formations (Guizhou) in China. J. Fungi. 2020;6:251. doi: 10.3390/jof6040251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomes R.R., Glienke C., Videira S.I.R., Lombard L., Groenewald J.Z., Crous P.W. Diaporthe: A genus of endophytic, saprobic and plant pathogenic fungi. Persoonia. 2013;31:1–41. doi: 10.3767/003158513X666844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Udayanga D., Castlebury L.A., Rossman A.Y., Chukeatirote E., Hyde K.D. Insights into the genus Diaporthe: Phylogenetic species delimitation in the D. eres species complex. Fungal Divers. 2014;67:203–229. doi: 10.1007/s13225-014-0297-2. [DOI] [Google Scholar]
- 5.Gao Y., Liu F., Duan W., Crous P.W., Cai L. Diaporthe is paraphyletic. IMA Fungus. 2017;8:153–187. doi: 10.5598/imafungus.2017.08.01.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santos J.M., Correia V.G., Phillips A.J.L. Primers for mating-type diagnosis in Diaporthe and Phomopsis: Their use in teleomorph induction in vitro and biological species definition. Fungal Biol. 2010;114:255–270. doi: 10.1016/j.funbio.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Gong J.L., Lu Y., Wu W.H., He C.P., Liang Y.Q., Huang X., Zheng J.L., Xi J.G., Tang S.B., Yi K.X. First report of Phomopsis heveicola (anamorph of Diaporthe tulliensis) causing leaf blight of Coffee (Coffea arabica) in China. Plant Dis. 2020;104:570–571. doi: 10.1094/PDIS-09-19-1833-PDN. [DOI] [Google Scholar]
- 8.Rehner S.A., Uecker F.A. Nuclear ribosomal internal transcribed spacer phylogeny and host diversity in the coelomycete Phomopsis. Can. J. Bot. 1994;72:1666–1674. doi: 10.1139/b94-204. [DOI] [Google Scholar]
- 9.Santos J.M., Vrandecic K., Cosic J., Duvnjak T., Phillips A.J.L. Resolving the Diaporthe species occurring on soybean in Croatia. Persoonia. 2011;27:9–19. doi: 10.3767/003158511X603719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou H., Hou C.L. Three new species of Diaporthe from China based on morphological characters and DNA sequence data analyses. Phytotaxa. 2019;422:157–174. doi: 10.11646/phytotaxa.422.2.3. [DOI] [Google Scholar]
- 11.Leon M., Berbegal M., Rodriguez-Reina J.M., Elena G., Abad-Campos P., Ramon-Albalat A., Olmo D., Vicent A., Luque J., Miarnau X., et al. Identification and characterization of Diaporthe spp. associated with twig cankers and shoot blight of almonds in Spain. Agronomy. 2020;10:1062. doi: 10.3390/agronomy10081062. [DOI] [Google Scholar]
- 12.Rossman A.Y., Adams G.C., Cannon P.F., Castlebury L.A., Crous P.W., Gryzenhout M., Jaklitsch W.M., Mejia L.C., Stoykov D., Udayanga D., et al. Recommendations of generic names in Diaporthales competing for protection or use. IMA Fungus. 2015;6:145–154. doi: 10.5598/imafungus.2015.06.01.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Udayanga D., Liu X., McKenzie E.H.C., Chukeatirote E., Bahkali A.H.A., Hyde K.D. The genus Phomopsis: Biology, applications, species concepts and names of common phytopathogens. Fungal Divers. 2011;50:189–225. doi: 10.1007/s13225-011-0126-9. [DOI] [Google Scholar]
- 14.Santos L., Alves A., Alves R. Evaluating multi-locus phylogenies for species boundaries determination in the genus Diaporthe. PeerJ. 2017;5 doi: 10.7717/peerj.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin X., Huang Y.J., Fang M.J., Wang J.F., Zheng Z.H., Su W.J. Cytotoxic and antimicrobial metabolites from marine lignicolous fungi, Diaporthe sp. FEMS Microbiol. Lett. 2005;251:53–58. doi: 10.1016/j.femsle.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 16.Silva G.H., Teles H.L., Zanardi L.M., Marx Young M.C., Eberlin M.N., Hadad R., Pfenning L.H., Costa-Neto C.M., Castro-Gamboa I., Bolzani V.d.S., et al. Cadinane sesquiterpenoids of Phomopsis cassiae, an endophytic fungus associated with Cassia spectabilis (Leguminosae) Phytochemistry. 2006;67:1964–1969. doi: 10.1016/j.phytochem.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Niaz S.I., Khan D., Naz R., Safdar K., Ul Abidin S.Z., Khan I.U., Gul R., Khan W.U., Khan M.A.U., Lan L. Antimicrobial and antioxidant chlorinated azaphilones from mangrove Diaporthe perseae sp. isolated from the stem of Chinese mangrove Pongamia pinnata. J. Asian Nat. Prod. Res. 2020 doi: 10.1080/10286020.2020.1835872. [DOI] [PubMed] [Google Scholar]
- 18.Yang Z.J., Zhang Y.F., Wu K., Xu Y.X., Meng X.G., Jiang Z.T., Ge M., Shao L. New azaphilones, phomopsones A-C with biological activities from an endophytic fungus Phomopsis sp. CGMCC No.5416. Fitoterapia. 2020;145 doi: 10.1016/j.fitote.2020.104573. [DOI] [PubMed] [Google Scholar]
- 19.Da Rosa B.V., Kuhn K.R., Ugalde G.A., Zabot G.L., Kuhn R.C. Antioxidant compounds extracted from Diaporthe schini using supercritical CO2 plus cosolvent. Bioprocess Biosyst. Eng. 2020;43:133–141. doi: 10.1007/s00449-019-02211-9. [DOI] [PubMed] [Google Scholar]
- 20.Fan M.M., Xiang G., Chen J.W., Gao J., Xue W.W., Wang Y.X., Li W.H., Zhou L., Jiao R.H., Shen Y., et al. Libertellenone M, a diterpene derived from an endophytic fungus Phomopsis sp. S12, protects against DSS-induced colitis via inhibiting both nuclear translocation of NF-κB and NLRP3 inflammasome activation. Int. Immunopharmacol. 2020;80 doi: 10.1016/j.intimp.2019.106144. [DOI] [PubMed] [Google Scholar]
- 21.Tsantrizos Y.S., Ogilvie K.K., Watson A.K. Phytotoxic metabolites of Phomopsis convolvulus, a host-specific pathogen of field bindweed. Can. J. Chem. 1992;70:2276–2284. doi: 10.1139/v92-286. [DOI] [Google Scholar]
- 22.Zhang C.W., Ondeyka J.G., Herath K.B., Guan Z.Q., Collado J., Platas G., Pelaez F., Leavitt P.S., Gurnett A., Nare B., et al. Tenellones A and B from a Diaporthe sp.: Two highly substituted benzophenone inhibitors of parasite cGMP-dependent protein kinase activity. J. Nat. Prod. 2005;68:611–613. doi: 10.1021/np049591n. [DOI] [PubMed] [Google Scholar]
- 23.Yang H.Y., Gao Y.H., Niu D.Y., Yang L.Y., Gao X.M., Du G., Hu Q.F. Xanthone derivatives from the fermentation products of an endophytic fungus Phomopsis sp. Fitoterapia. 2013;91:189–193. doi: 10.1016/j.fitote.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Yang J.X., Qiu S.X., She Z.G., Lin Y.C. A new xanthone derivative from the marine fungus Phomopsis sp. (No. SK7RN3G1) Chem. Nat. Compd. 2013;49:31–33. doi: 10.1007/s10600-013-0498-z. [DOI] [Google Scholar]
- 25.Yuan L., Huang W., Du G., Gao X., Yang H., Hu Q., Ma Y. Isolation of xanthones from the fermentation products of the endophytic fungus of Phomopsis amygdali. Chem. Nat. Compd. 2015;51:460–463. doi: 10.1007/s10600-015-1315-7. [DOI] [Google Scholar]
- 26.Huang R., Ma K.X., Xie X.S., Wang T., Wu S.H. Secondary metabolites of an endophytic fungus Phomopsis sp. Chem. Nat. Compd. 2015;51:392–394. doi: 10.1007/s10600-015-1295-7. [DOI] [Google Scholar]
- 27.Yuan L., Huang W.Z., Zhou K., Wang Y.D., Dong W., Lou J., Li L.M., Du G., Yang H.Y., Ma Y.H., et al. Xanthones from the fermentation products of an endophytic fungus Phomopsis sp. Heterocycles. 2015;91:381–387. doi: 10.3987/com-14-13136. [DOI] [Google Scholar]
- 28.Yang Y., Yang H., Li Y., Ye Y., Hu Q., Gao X., Du G. A new xanthone from the fermentation products of endophytic fungus of Phomopsis species. Asian J. Chem. 2014;26:4591–4593. doi: 10.14233/ajchem.2014.16127. [DOI] [Google Scholar]
- 29.Hu Q., Yang Y., Yang S., Cao H., Chunyang M., Yang H., Gao X., Du G. Xanthones from the fermentation products of the endophytic fungus of Phomopsis amygdali. Chem. Nat. Compd. 2015;51:456–459. doi: 10.1007/s10600-015-1314-8. [DOI] [Google Scholar]
- 30.Huang Z., Yang J., Lei F., She Z., Lin Y. A new xanthone O-glycoside from the mangrove endophytic fungus Phomopsis sp. Chem. Nat. Compd. 2013;49:27–30. doi: 10.1007/s10600-013-0497-0. [DOI] [Google Scholar]
- 31.Roensberg D., Debbab A., Mandi A., Vasylyeva V., Boehler P., Stork B., Engelke L., Hamacher A., Sawadogo R., Diederich M., et al. Pro-apoptotic and immunostimulatory tetrahydroxanthone dimers from the endophytic fungus Phomopsis longicolla. J. Org. Chem. 2013;78:12409–12425. doi: 10.1021/jo402066b. [DOI] [PubMed] [Google Scholar]
- 32.Shiono Y., Sasaki T., Shibuya F., Yasuda Y., Koseki T., Supratman U. Isolation of a phomoxanthone A derivative, a new metabolite of tetrahydroxanthone, from a Phomopsis sp. isolated from the mangrove, Rhizhopora mucronata. Nat. Prod. Commun. 2013;8:1735–1737. doi: 10.1177/1934578X1300801220. [DOI] [PubMed] [Google Scholar]
- 33.Meixiang H., Jing L., Lan L., Sheng Y., Jun W., Yongcheng L.J.M.D. Phomopsichin A–D; four new chromone derivatives from mangrove endophytic fungus Phomopsis sp. 33#. Mar. Drugs. 2016;14:215. doi: 10.3390/md14110215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim C., Kim J., Choi J.N., Ponnusamy K., Jeon Y., Kim S.U., Kim J.G., Lee C.H. Identification, fermentation, and bioactivity against xanthomonas oryzae of antimicrobial metabolites isolated from Phomopsis longicolla S1B4. J. Microbiol. Biotechnol. 2010;20:494–500. doi: 10.4014/jmb.0909.09026. [DOI] [PubMed] [Google Scholar]
- 35.Ding B., Yuan J., Huang X., Wen W., Zhu X., Liu Y., Li H., Lu Y., He L., Tan H.J.M.D. New dimeric members of the phomoxanthone family: Phomolactonexanthones A, B and deacetylphomoxanthone C isolated from the fungus Phomopsis sp. Mar. Drugs. 2013;11:4961–4972. doi: 10.3390/md11124961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding B., Wang Z., Xia G., Huang X., Xu F., Chen W., She Z. Three new chromone derivatives produced by Phomopsis sp. HNY29-2B from Acanthus ilicifolius linn. Chin. J. Chem. 2017;35:1889–1893. doi: 10.1002/cjoc.201700375. [DOI] [Google Scholar]
- 37.Wu Q., Guo Y., Guo Z.K., Chu Y.L., Wang T., Tan R.X. Two new cytosporones from the culture of endophytic Phomopsis sp. Chem. Nat. Compd. 2013;48:938–941. doi: 10.1007/s10600-013-0433-3. [DOI] [Google Scholar]
- 38.Hu H.B., Luo Y.F., Wang P., Wang W.J., Wu J. Xanthone-derived polyketides from the Thai mangrove endophytic fungus Phomopsis sp. xy21. Fitoterapia. 2018;131:265–271. doi: 10.1016/j.fitote.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 39.Yang J.X., Qiu S., She Z., Lin Y. A new isochroman derivative from the marine fungus Phomopsis sp. (No. Gx-4) Chem. Nat. Compd. 2014;50:424–426. doi: 10.1007/s10600-014-0976-y. [DOI] [Google Scholar]
- 40.Ahmed I., Hussain H., Schulz B., Draeger S., Padula D., Pescitelli G., van Ree T., Krohn K. Three new antimicrobial metabolites from the endophytic fungus Phomopsis sp. Eur. J. Org. Chem. 2011;2011:2867–2873. doi: 10.1002/ejoc.201100158. [DOI] [Google Scholar]
- 41.Yang Z., Wu K., Xu Y., Xia X., Wang X., Ge M., Shao L. Three novel chromanones with biological activities from the endophytic fungus Phomopsis CGMCC No. 5416. J. Antibiot. 2020;73:194–199. doi: 10.1038/s41429-019-0270-0. [DOI] [PubMed] [Google Scholar]
- 42.Huang Z., Yang R., Guo Z., She Z., Lin Y. A new naphtho-γ-pyrone from mangrove endophytic fungus ZSU-H26. Chem. Nat. Compd. 2010;46:15–18. doi: 10.1007/s10600-010-9514-8. [DOI] [Google Scholar]
- 43.Yang J., Xu F., Huang C., Li J., She Z., Pei Z., Lin Y. Metabolites from the mangrove endophytic fungus Phomopsis sp. (#zsu-H76) Eur. J. Org. Chem. 2010;2010:3692–3695. doi: 10.1002/ejoc.201000329. [DOI] [Google Scholar]
- 44.Yang J.X., Chen Y., Huang C., She Z., Lin Y. A new isochroman derivative from the marine fungus Phomopsis sp. (No. ZH-111) Chem. Nat. Compd. 2011;47:13–16. doi: 10.1007/s10600-011-9820-9. [DOI] [Google Scholar]
- 45.Tang J.W., Wang W.G., Li A., Yan B.C., Chen R., Li X.N., Du X., Sun H.D., Pu J.X. Polyketides from the endophytic fungus Phomopsis sp. sh917 by using the one strain/many compounds strategy. Tetrahedron. 2017;73:3577–3584. doi: 10.1016/j.tet.2017.02.019. [DOI] [Google Scholar]
- 46.Adelin E., Martin M.T., Cortial S., Retailleau P., Lumyong S., Ouazzani J. Bioactive polyketides isolated from agar-supported fermentation of Phomopsis sp. CMU-LMA, taking advantage of the scale-up device, Platotex. Phytochemistry. 2013;93:170–175. doi: 10.1016/j.phytochem.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 47.Tao M.H., Chen Y.C., Wei X.Y., Tan J.W., Zhang W.M. Chemical constituents of the endophytic fungus Phomopsis sp. A240 isolated from Taxus chinensis var. mairei. Helv. Chim. Acta. 2014;97:426–430. doi: 10.1002/hlca.201300367. [DOI] [Google Scholar]
- 48.Talontsi F.M., Islam M.T., Facey P., Douanla-Meli C., von Tiedemann A., Laatsch H. Depsidones and other constituents from Phomopsis sp. CAFT69 and its host plant Endodesmia calophylloides with potent inhibitory effect on motility of zoospores of grapevine pathogen Plasmopara viticola. Phytochem. Lett. 2012;5:657–664. doi: 10.1016/j.phytol.2012.06.017. [DOI] [Google Scholar]
- 49.Chapla V.M., Zeraik M.L., Ximenes V.F., Zanardi L.M., Lopes M.N., Cavalheiro A.J., Silva D.H.S., Young M.C.M., da Fonseca L.M., Bolzani V.S., et al. Bioactive secondary metabolites from Phomopsis sp., an endophytic fungus from Senna spectabilis. Molecules. 2014;19:6597–6608. doi: 10.3390/molecules19056597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang W., Xu L., Yang L., Huang Y., Li S., Shen Y. Phomopsidone A, a novel depsidone metabolite from the mangrove endophytic fungus Phomopsis sp. A123. Fitoterapia. 2014;96:146–151. doi: 10.1016/j.fitote.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 51.Kornsakulkarn J., Somyong W., Supothina S., Boonyuen N., Thongpanchang C. Bioactive oxygen-bridged cyclooctadienes from endophytic fungus Phomopsis sp. BCC 45011. Tetrahedron. 2015;71:9112–9116. doi: 10.1016/j.tet.2015.10.015. [DOI] [Google Scholar]
- 52.Xu J.L., Liu Z.M., Chen Y.C., Tan H.B., Li H.H., Li S.N., Guo H., Huang Z.L., Gao X.X., Liu H.X., et al. Lithocarols A-F, six tenellone derivatives from the deep-sea derived fungus Phomopsis lithocarpus FS508. Bioorg. Chem. 2019;87:728–735. doi: 10.1016/j.bioorg.2019.03.078. [DOI] [PubMed] [Google Scholar]
- 53.Du G., Wang Z.C., Hu W.Y., Yan K.L., Wang X.L., Yang H.M., Yang H.Y., Gao Y.H., Liu Q., Hu Q.F. Three new 3-methyl-2-arylbenzofurans from the fermentation products of an endophytic fungus Phomopsis sp. and their anti-TMV activity. Phytochem. Lett. 2017;21:287–290. doi: 10.1016/j.phytol.2016.04.003. [DOI] [Google Scholar]
- 54.Song H.C., Qin D., Han M.J., Wang L., Zhang K., Dong J.Y. Bioactive 2-pyrone metabolites from an endophytic Phomopsis asparagi SWUKJ5.2020 of Kadsura angustifolia. Phytochem. Lett. 2017;22:235–240. doi: 10.1016/j.phytol.2017.06.020. [DOI] [Google Scholar]
- 55.Hussain H., Ahmed I., Schulz B., Draeger S., Krohn K. Pyrenocines J-M: Four new pyrenocines from the endophytic fungus, Phomopsis sp. Fitoterapia. 2012;83:523–526. doi: 10.1016/j.fitote.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 56.Yang Z., Ding J., Ding K., Chen D., Cen S., Ge M. Phomonaphthalenone A: A novel dihydronaphthalenone with anti-HIV activity from Phomopsis sp. HCCB04730. Phytochem. Lett. 2013;6:257–260. doi: 10.1016/j.phytol.2013.02.003. [DOI] [Google Scholar]
- 57.Li X.B., Chen G.Y., Liu R.J., Zheng C.J., Song X.M., Han C.R. A new biphenyl derivative from the mangrove endophytic fungus Phomopsis longicolla HL-2232. Nat. Prod. Res. 2017;31:2264–2267. doi: 10.1080/14786419.2017.1300799. [DOI] [PubMed] [Google Scholar]
- 58.Mishra P.D., Verekar S.A., Deshmukh S.K., Joshi K.S., Fiebig H.H., Kelter G. Altersolanol A: A selective cytotoxic anthraquinone from a Phomopsis sp. Lett. Appl. Microbiol. 2015;60:387–391. doi: 10.1111/lam.12384. [DOI] [PubMed] [Google Scholar]
- 59.Evidente A., Rodeva R., Andolfi A., Stoyanova Z., Perrone C., Motta A. Phytotoxic polyketides produced by Phomopsis foeniculi, a strain isolated from diseased Bulgarian fennel. Eur. J. Plant Pathol. 2011;130:173–182. doi: 10.1007/s10658-011-9743-0. [DOI] [Google Scholar]
- 60.Klaiklay S., Rukachaisirikul V., Phongpaichit S., Pakawatchai C., Saithong S., Buatong J., Preedanon S., Sakayaroj J. Anthraquinone derivatives from the mangrove-derived fungus Phomopsis sp. PSU-MA214. Phytochem. Lett. 2012;5:738–742. doi: 10.1016/j.phytol.2012.08.003. [DOI] [Google Scholar]
- 61.Hussain H., Tchimene M.K., Ahmed I., Meier K., Steinert M., Draeger S., Schulz B., Krohn K. Antimicrobial chemical constituents from the endophytic fungus Phomopsis sp. from Notobasis syriaca. Nat. Prod. Commun. 2011;6:1905–1906. doi: 10.1177/1934578X1100601228. [DOI] [PubMed] [Google Scholar]
- 62.Cai R., Chen S., Liu Z., Tan C., Huang X., She Z. A new α-pyrone from the mangrove endophytic fungus Phomopsis sp. HNY29-2B. Nat. Prod. Res. 2017;31:124–130. doi: 10.1080/14786419.2016.1214833. [DOI] [PubMed] [Google Scholar]
- 63.Krohn K., Farooq U., Hussain H., Ahmed I., Rheinheimer J., Draeger S., Schulz B., van Ree T. Phomosines H-J, novel highly substituted biaryl ethers, isolated from the endophytic fungus Phomopsis sp. from Ligustrum vulgare. Nat. Prod. Commun. 2011;6:1907–1912. doi: 10.1177/1934578X1100601229. [DOI] [PubMed] [Google Scholar]
- 64.Hu S.S., Liang M.J., Mi Q.L., Chen W., Ling J., Chen X., Li J., Yang G.Y., Hu Q.F., Wang W.G., et al. Two new diphenyl ether derivatives from the fermentation products of the endophytic fungus Phomopsis asparagi. Chem. Nat. Compd. 2019;55:843–846. doi: 10.1007/s10600-019-02828-y. [DOI] [Google Scholar]
- 65.Gao Y.H., Zheng R., Li J., Kong W.S., Liu X., Ye L., Mi Q.L., Kong W.S., Zhou M., Yang G.Y., et al. Three new diphenyl ether derivatives from the fermentation products of an endophytic fungus Phomopsis fukushii. J. Asian Nat. Prod. Res. 2019;21:316–322. doi: 10.1080/10286020.2017.1421177. [DOI] [PubMed] [Google Scholar]
- 66.Li Z.J., Yang H.Y., Li J., Liu X., Ye L., Kong W.S., Tang S.Y., Du G., Liu Z.H., Zhou M., et al. Isopentylated diphenyl ether derivatives from the fermentation products of an endophytic fungus Phomopsis fukushii. J. Antibiot. 2018;71:359–362. doi: 10.1038/s41429-017-0006-y. [DOI] [PubMed] [Google Scholar]
- 67.Yang H.Y., Duan Y.Q., Yang Y.K., Liu X., Ye L., Mi Q.L., Kong W.S., Zhou M., Yang G.Y., Hu Q.F., et al. Two new diphenyl ether derivatives from the fermentation products of an endophytic fungus Phomopsis fukushii. Chem. Nat. Compd. 2019;55:428–431. doi: 10.1007/s10600-019-02706-7. [DOI] [PubMed] [Google Scholar]
- 68.Yang H.Y., Duan Y.Q., Yang Y.K., Li J., Liu X., Ye L., Mi Q.L., Kong W.S., Zhou M., Yang G.Y., et al. Three new napthalene derivatives from the endophytic fungus Phomopsis fukushii. Phytochem. Lett. 2017;22:266–269. doi: 10.1016/j.phytol.2017.10.021. [DOI] [Google Scholar]
- 69.Li X.M., Zeng Y.C., Chen J.H., Yang Y.K., Li J., Ye L., Du G., Zhou M., Hu Q.F., Guangyu Y., et al. Two new naphthalene derivatives from the fermentation products of an endophytic fungus Phomopsis sp. Chem. Nat. Compd. 2019;55:618–621. doi: 10.1007/s10600-019-02762-z. [DOI] [Google Scholar]
- 70.Xu J.L., Liu H.X., Chen Y.C., Tan H.B., Guo H., Xu L.Q., Li S.N., Huang Z.L., Li H.H., Gao X.X., et al. Highly substituted benzophenone aldehydes and eremophilane derivatives from the deep-Sea derived fungus Phomopsis lithocarpus FS508. Mar. Drugs. 2018;16:329. doi: 10.3390/md16090329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ma K.X., Shen X.T., Huang R., Wang T., Xie X.S., Liu S.W., Wu S.H., He J. Bioactiye metabolites produced by the endophytic fungus Phomopsis sp. YM355364. Nat. Prod. Commun. 2014;9:669–670. doi: 10.1177/1934578X1400900521. [DOI] [PubMed] [Google Scholar]
- 72.Sheng S.L., Li Y.P., Xiang H.Y., Liu Y., Wang Y.D., Kong L.P., Du G., Hu Q.F., Chen Y.J., Wang W.G. Histone deacetylase inhibitor induced lipase Inhibitors from endophytic Phomopsis sp. 0391. Rec. Nat. Prod. 2020;14:42–47. doi: 10.25135/rnp.134.19.01.1243. [DOI] [Google Scholar]
- 73.Kongprapan T., Xu X., Rukachaisirikul V., Phongpaichit S., Sakayaroj J., Chen J., Shen X. Cytosporone derivatives from the endophytic fungus Phomopsis sp. PSU-H188. Phytochem. Lett. 2017;22:219–223. doi: 10.1016/j.phytol.2017.10.002. [DOI] [Google Scholar]
- 74.Xu J., Tan H., Chen Y., Li S., Guo H., Huang Z., Li H., Gao X., Liu H., Zhang W. Lithocarpinols A and B, a pair of diastereomeric antineoplastic tenellone derivatives from the deep-sea derived fungus Phomopsis lithocarpus FS508. Chin. Chem. Lett. 2019;30:439–442. doi: 10.1016/j.cclet.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 75.Chen Y.G., Pan J.H., Xu F., Liu F., Yang J.X., Huang C.H., Xu C.L., Lu Y.J., Cai X.L., She Z.G., et al. A new indene derivative from the marine fungus Phomopsis sp. (No. GX7-4A) Chem. Nat. Compd. 2010;46:230–232. doi: 10.1007/s10600-010-9576-7. [DOI] [Google Scholar]
- 76.Tan Q.W., Fang P.H., Ni J.C., Gao F., Chen Q.J. Metabolites Produced by an Endophytic Phomopsis sp. and Their Anti-TMV Activity. Molecules. 2017;22:2073. doi: 10.3390/molecules22122073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shiono Y., Muslihah N.I., Suzuki T., Ariefta N.R., Anwar C., Nurjanto H.H., Aboshi T., Murayama T., Tawaraya K., Koseki T., et al. New eremophilane and dichlororesorcinol derivatives produced by endophytes isolated from Ficus ampelas. J. Antibiot. 2017;70:1133–1137. doi: 10.1038/ja.2017.125. [DOI] [PubMed] [Google Scholar]
- 78.Bunyapaiboonsri T., Yoiprommarat S., Srikitikulchai P., Srichomthong K., Lumyong S. Oblongolides from the endophytic fungus Phomopsis sp. BCC 9789. J. Nat. Prod. 2010;73:55–59. doi: 10.1021/np900650c. [DOI] [PubMed] [Google Scholar]
- 79.Lin T., Wang G.H., Lin X., Hu Z.Y., Chen Q.C., Xu Y., Zhang X.K., Chen H.F. Three new oblongolides from Phomopsis sp. XZ-01, an endophytic fungus from Camptotheca acuminate. Molecules. 2011;16:3351–3359. doi: 10.3390/molecules16043351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang R., Jiang B.G., Li X.N., Wang Y.T., Liu S.S., Zheng K.X., He J., Wu S.H. Polyoxygenated cyclohexenoids with promising α-glycosidase inhibitory activity produced by Phomopsis sp. YE3250, an endophytic fungus derived from Paeonia delavayi. J. Agric. Food Chem. 2018;66:1140–1146. doi: 10.1021/acs.jafc.7b04998. [DOI] [PubMed] [Google Scholar]
- 81.Cimmino A., Andolfi A., Zonno M.C., Troise C., Santini A., Tuzi A., Vurro M., Ash G., Evidente A. Phomentrioloxin: A phytotoxic pentasubstituted geranylcyclohexentriol produced by Phomopsis sp., a potential mycoherbicide for Carthamus lanatus Biocontrol. J. Nat. Prod. 2012;75:1130–1137. doi: 10.1021/np300200j. [DOI] [PubMed] [Google Scholar]
- 82.Goddard M.L., Mottier N., Jeanneret-Gris J., Christen D., Tabacchi R., Abou-Mansour E. Differential production of phytotoxins from Phomopsis sp. from grapevine plants showing esca symptoms. J. Agric. Food Chem. 2014;62:8602–8607. doi: 10.1021/jf501141g. [DOI] [PubMed] [Google Scholar]
- 83.Xie S.S., Wu Y., Qiao Y.B., Guo Y., Wang J.P., Hu Z.X., Zhang Q., Li X.N., Huang J.F., Zhou Q., et al. Protoilludane, illudalane, and botryane sesquiterpenoids from the endophytic fungus Phomopsis sp. TJ507A. J. Nat. Prod. 2018;81:1311–1320. doi: 10.1021/acs.jnatprod.7b00889. [DOI] [PubMed] [Google Scholar]
- 84.Zanardi L.M., Bolzani V.d.S., Cavalheiro A.J., Siqueira Silva D.H., Trevisan H.C., Araujo A.R., Silva G.H., Teles H.L., Young M.C.M. Sesquiterpenes produced by endophytic fungus Phomopsis cassiae with antifungal and acetylcholinesterase inhibition activities. Quim. Nova. 2012;35:2233–2236. doi: 10.1590/S0100-40422012001100026. [DOI] [Google Scholar]
- 85.Hemtasin C., Kanokmedhakul S., Kanokmedhakul K., Hahnvajanawong C., Soytong K., Prabpai S., Kongsaeree P. Cytotoxic pentacyclic and tetracyclic aromatic sesquiterpenes from Phomopsis archeri. J. Nat. Prod. 2011;74:609–613. doi: 10.1021/np100632g. [DOI] [PubMed] [Google Scholar]
- 86.Ma X., Wang W., Li E., Gao F., Guo L., Pei Y. A new sesquiterpene from the entomogenous fungus Phomopsis amygdali. Nat. Prod. Res. 2016;30:276–280. doi: 10.1080/14786419.2015.1055742. [DOI] [PubMed] [Google Scholar]
- 87.Qian Y.X., Kang J.C., Luo Y.K., He J., Wang L., Li Q.R. Secondary metabolites of an endophytic fungus Phomopsis castaneae-mollissimae. Chem. Nat. Compd. 2018;54:346–347. doi: 10.1007/s10600-018-2340-0. [DOI] [Google Scholar]
- 88.Wei W., Gao J., Shen Y., Chu Y.L., Xu Q., Tan R.X. Immunosuppressive diterpenes from Phomopsis sp. S12. Eur. J. Org. Chem. 2014;2014:5728–5734. doi: 10.1002/ejoc.201402491. [DOI] [Google Scholar]
- 89.Xu K., Zhang X., Chen J.W., Shen Y., Jiang N., Tan R.X., Jiao R.H., Ge H.M. Anti-inflammatory diterpenoids from an endophytic fungus Phomopsis sp. S12. Tetrahedron Lett. 2019;60 doi: 10.1016/j.tetlet.2019.151045. [DOI] [Google Scholar]
- 90.Zhang Y., Hao F., Liu N., Xu Y., Jia A., Yang Z., Xia X., Liu C. Stereochemical determination of a new and cytotoxic euphane triterpenoid from the plant endophytic fungus Phomopsis chimonanthi. J. Antibiot. 2013;66:679–682. doi: 10.1038/ja.2013.70. [DOI] [PubMed] [Google Scholar]
- 91.Peyrat L.A., Eparvier V., Eydoux C., Guillemot J.C., Litaudon M., Stien D. Betulinic acid, the first lupane-type triterpenoid isolated from both a Phomopsis sp. and its host plant Diospyros carbonaria benoist. Chem. Biodivers. 2017;14 doi: 10.1002/cbdv.201600171. [DOI] [PubMed] [Google Scholar]
- 92.Wu S.H., Huang R., Miao C.P., Chen Y.W. Two new steroids from an endophytic fungus Phomopsis sp. Chem. Biodivers. 2013;10:1276–1283. doi: 10.1002/cbdv.201200415. [DOI] [PubMed] [Google Scholar]
- 93.Hu Z.X., Wu Y., Xie S.S., Sun W.G., Guo Y., Li X.N., Liu J.J., Li H., Wang J.P., Luo Z.W., et al. Phomopsterones A and B, two functionalized ergostane-type steroids from the endophytic fungus Phomopsis sp. TJ507A. Org. Lett. 2017;19:258–261. doi: 10.1021/acs.orglett.6b03557. [DOI] [PubMed] [Google Scholar]
- 94.Adelin E., Servy C., Cortial S., Levaique H., Martin M.T., Retailleau P., Le Goff G., Bussaban B., Lumyong S., Ouazzani J. Isolation, structure elucidation and biological activity of metabolites from Sch-642305-producing endophytic fungus Phomopsis sp. CMU-LMA. Phytochemistry. 2011;72:2406–2412. doi: 10.1016/j.phytochem.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 95.Xu J., Tan H., Chen Y., Li S., Huang Z., Guo H., Li H., Gao X., Liu H., Zhang W. Lithocarpins A-D: Four tenellone-macrolide conjugated [4 + 2] hetero-adducts from the deep-sea derived fungus Phomopsis lithocarpus FS508. Org. Chem. Front. 2018;5:1792–1797. doi: 10.1039/C8QO00095F. [DOI] [Google Scholar]
- 96.Yan B.C., Wang W.G., Hu D.B., Sun X., Kong L.M., Li X.N., Du X., Luo S.H., Liu Y., Li Y., et al. Phomopchalasins A and B, two cytochalasans with polycyclic-fused skeletons from the endophytic fungus Phomopsis sp. shj2. Org. Lett. 2016;18:1108–1111. doi: 10.1021/acs.orglett.6b00214. [DOI] [PubMed] [Google Scholar]
- 97.Luo Y.F., Zhang M., Dai J.G., Pedpradab P., Wang W.J., Wu J. Cytochalasins from mangrove endophytic fungi Phomopsis spp. xy21 and xy22. Phytochem. Lett. 2016;17:162–166. doi: 10.1016/j.phytol.2016.07.027. [DOI] [Google Scholar]
- 98.Jouda J.B., Tamokou J.D.D., Mbazoa C.D., Douala-Meli C., Sarkar P., Bag P.K., Wandji J. Antibacterial and cytotoxic cytochalasins from the endophytic fungus Phomopsis sp. harbored in Garcinia kola (Heckel) nut. BMC Complement. Altern. Med. 2016;16 doi: 10.1186/s12906-016-1454-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fu J., Zhou Y., Li H.F., Ye Y.H., Guo J.H. Antifungal metabolites from Phomopsis sp. By254, an endophytic fungus in Gossypium hirsutum. Afr. J. Microbiol. Res. 2011;5:1231–1236. doi: 10.1186/1475-2859-10-37. [DOI] [Google Scholar]
- 100.Chang H.S., Peng C.J., Cheng M.J., Wu H.C., Chan H.Y., Hsieh S.Y., Yuan G.F., Chen I.S. Chemical constituents of the endophytic fungus Phomopsis asparagi isolated from the plant Peperomia sui. Chem. Nat. Compd. 2018;54:504–508. doi: 10.1007/s10600-018-2390-3. [DOI] [Google Scholar]
- 101.Chen S.C., Liu Z.M., Tan H.B., Chen Y.C., Li S.N., Li H.H., Guo H., Zhu S., Liu H.X., Zhang W.M. Tersone A-G, new pyridone alkaloids from the deep-sea fungus Phomopsis tersa. Mar. Drugs. 2019;17:394. doi: 10.3390/md17070394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen H., Huang M., Li X., Liu L., Chen B., Wang J., Lin Y. Phochrodines A-D, first naturally occurring new chromenopyridines from mangrove entophytic fungus Phomopsis sp. 33#. Fitoterapia. 2018;124:103–107. doi: 10.1016/j.fitote.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 103.Verekar S.A., Mishra P.D., Sreekumar E.S., Deshmukh S.K., Fiebig H.H., Kelter G., Maier A. Anticancer activity of new depsipeptide compound isolated from an endophytic fungus. J. Antibiot. 2014;67:697–701. doi: 10.1038/ja.2014.58. [DOI] [PubMed] [Google Scholar]
- 104.Katz L. Manipulation of modular polyketide syntheses. Chem. Rev. 1997;97:2557–2575. doi: 10.1021/cr960025+. [DOI] [PubMed] [Google Scholar]
- 105.Le Pogam P., Boustie J. Xanthones of lichen source: A 2016 update. Molecules. 2016;21:294. doi: 10.3390/molecules21030294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Duan Y.D., Jiang Y.Y., Guo F.X., Chen L.X., Xu L.L., Zhang W., Liu B. The antitumor activity of naturally occurring chromones: A review. Fitoterapia. 2019;135:114–129. doi: 10.1016/j.fitote.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 107.Mayuri B., Kavitha P., Basavoju S., Bhargavi G., Reddy K.L. Synthesis, structural characterisation and biological evolution of chromanones. J. Mol. Struct. 2017;1145:1–9. doi: 10.1016/j.molstruc.2017.05.013. [DOI] [Google Scholar]
- 108.Li Y., Li X., Cheng J.P. Catalytic asymmetric synthesis of chiral benzofuranones. Adv. Synth. Catal. 2014;356:1172–1198. doi: 10.1002/adsc.201301038. [DOI] [Google Scholar]
- 109.McGlacken G.P., Fairlamb I.J.S. 2-Pyrone natural products and mimetics: Isolation, characterisation and biological activity. Nat. Prod. Rep. 2005;22:369–385. doi: 10.1039/b416651p. [DOI] [PubMed] [Google Scholar]
- 110.Bolton J.L., Dunlap T. Formation and biological targets of quinones: Cytotoxic versus cytoprotective effects. Chem. Res. Toxicol. 2017;30:13–37. doi: 10.1021/acs.chemrestox.6b00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jones T.J.M., Douglas C.J. The metabolism and toxicity of quinones, quinonimines, quinone methides, and quinone-thioethers. Curr. Drug Metab. 2002;3:425–438. doi: 10.2174/1389200023337388. [DOI] [PubMed] [Google Scholar]
- 112.Gajera H.P., Gevariya S.N., Hirpara D.G., Patel S.V., Golakiya B.A. Antidiabetic and antioxidant functionality associated with phenolic constituents from fruit parts of indigenous black jamun (Syzygium cumini L.) landraces. J. Food Sci. Technol. 2017;54:3180–3191. doi: 10.1007/s13197-017-2756-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shing T.K.M., Yang J. A short synthesis of natural (−)-oblongolide via an intramolecular or a transannular diels-alder reaction. J. Org. Chem. 1995;60:5785–5789. doi: 10.1021/jo00123a011. [DOI] [Google Scholar]
- 114.Huang M., Lu J.J., Huang M.Q., Bao J.L., Chen X.P., Wang Y.T. Terpenoids: Natural products for cancer therapy. Expert Opin. Investig. Drugs. 2012;21:1801–1818. doi: 10.1517/13543784.2012.727395. [DOI] [PubMed] [Google Scholar]
- 115.Thoppil R.J., Bishayee A. Terpenoids as potential chemopreventive and therapeutic agents in liver cancer. World J. Hepatol. 2011;3:228–249. doi: 10.4254/wjh.v3.i9.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zielinska-Blajet M., Feder-Kubis J. Monoterpenes and their derivatives-recent development in biological and medical applications. Int. J. Mol. Sci. 2020;21:7078. doi: 10.3390/ijms21197078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen D.L., Wang B.W., Sun Z.C., Yang J.S., Xu X.D., Ma G.X. Natural nitrogenous sesquiterpenoids and their bioactivity: A review. Molecules. 2020;25:2485. doi: 10.3390/molecules25112485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen L., Lu X., El-Seedi H., Teng H. Recent advances in the development of sesquiterpenoids in the treatment of type 2 diabetes. Trends Food Sci. Technol. 2019;88:46–56. doi: 10.1016/j.tifs.2019.02.003. [DOI] [Google Scholar]
- 119.Su Y.D., Su J.H., Hwang T.L., Wen Z.H., Sheu J.H., Wu Y.C., Sung P.J. Briarane diterpenoids isolated from octocorals between 2014 and 2016. Mar. Drugs. 2017;15:44. doi: 10.3390/md15020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ren Y., Kinghorn A.D. Natural product triterpenoids and their semi-synthetic derivatives with potential anticancer activity. Planta Med. 2019;85:802–814. doi: 10.1055/a-0832-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rahman S.U., Ismail M., Khurram M., Ullah I., Rabbi F., Iriti M. Bioactive steroids and saponins of the genus Trillium. Molecules. 2017;22:2156. doi: 10.3390/molecules22122156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang M., Zhang J., He S., Yan X. A review study on macrolides isolated from cyanobacteria. Mar. Drugs. 2017;15:126. doi: 10.3390/md15050126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mishra S.K., Tripathi G., Kishore N., Singh R.K., Singh A., Tiwari V.K. Drug development against tuberculosis: Impact of alkaloids. Eur. J. Med. Chem. 2017;137:504–544. doi: 10.1016/j.ejmech.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 124.Wang T.Y., Li Q., Bi K.S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. 2018;13:12–23. doi: 10.1016/j.ajps.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Luo X., Yang J., Chen F., Lin X., Chen C., Zhou X., Liu S., Liu Y. Structurally diverse polyketides from the mangrove-derived fungus Diaporthe sp. SCSIO 41011 with their anti-influenza A virus activities. Front. Chem. 2018;6 doi: 10.3389/fchem.2018.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu Y., Ruan Q., Jiang S., Qu Y., Chen J., Zhao M., Yang B., Liu Y., Zhao Z., Cui H. Cytochalasins and polyketides from the fungus Diaporthe sp. GZU-1021 and their anti-inflammatory activity. Fitoterapia. 2019;137 doi: 10.1016/j.fitote.2019.104187. [DOI] [PubMed] [Google Scholar]
- 127.Niu Z., Chen Y., Guo H., Li S.N., Li H.H., Liu H.X., Liu Z., Zhang W. Cytotoxic polyketides from a deep-sea sediment derived fungus Diaporthe phaseolorum FS431. Molecules. 2019;24:3062. doi: 10.3390/molecules24173062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cui H., Ding M., Huang D., Zhang Z., Liu H., Huang H., She Z. Chroman-4-one and pyrano[4,3-b]chromenone derivatives from the mangrove endophytic fungus Diaporthe phaseolorum SKS019. RSC Adv. 2017;7:20128–20134. doi: 10.1039/C7RA03032K. [DOI] [Google Scholar]
- 129.Liu Z., Zhao J., Liang X., Lv X., Li Y., Qu J., Liu Y. Dothiorelone derivatives from an endophyte Diaporthe pseudomangiferaea inhibit the activation of human lung fibroblasts MRC-5 cells. Fitoterapia. 2018;127:7–14. doi: 10.1016/j.fitote.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 130.Bungihan M.E., Tan M.A., Kitajima M., Kogure N., Franzblau S.G., dela Cruz T.E.E., Takayama H., Nonato M.G. Bioactive metabolites of Diaporthe sp. P133, an endophytic fungus isolated from Pandanus amaryllifolius. J. Nat. Med. 2011;65:606–609. doi: 10.1007/s11418-011-0518-x. [DOI] [PubMed] [Google Scholar]
- 131.De Medeiros A.G., Savi D.C., Mitra P., Shaaban K.A., Jha A.K., Thorson J.S., Rohr J., Glienke C. Bioprospecting of Diaporthe terebinthifolii LGMF907 for antimicrobial compounds. Folia Microbiol. 2018;63:499–505. doi: 10.1007/s12223-018-0587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Meepagala K.M., Briscoe W.E., Techen N., Johnson R.D., Clausen B.M., Duke S.O. Isolation of a phytotoxic isocoumarin from Diaporthe eres-infected Hedera helix (English ivy) and synthesis of its phytotoxic analogs. Pest Manag. Sci. 2018;74:37–45. doi: 10.1002/ps.4712. [DOI] [PubMed] [Google Scholar]
- 133.Sharma V., Singamaneni V., Sharma N., Kumar A., Arora D., Kushwaha M., Bhushan S., Jaglan S., Gupta P. Valproic acid induces three novel cytotoxic secondary metabolites in Diaporthe sp., an endophytic fungus from Datura inoxia Mill. Bioorganic Med. Chem. Lett. 2018;28:2217–2221. doi: 10.1016/j.bmcl.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 134.Liu Y., Hu Z., Lin X., Lu C., Shen Y. A new polyketide from Diaporthe sp. SXZ-19, an endophytic fungal strain of Camptotheca acuminate. Nat. Prod. Res. 2013;27:2100–2104. doi: 10.1080/14786419.2013.791819. [DOI] [PubMed] [Google Scholar]
- 135.Evidente M., Boari A., Vergura S., Cimmino A., Vurro M., Ash G., Superchi S., Evidente A. Structure and absolute configuration of kongiidiazadione, a new phytotoxic 3-substituted-5-diazenylcyclopentendione produced by Diaporthe Kongii. Chirality. 2015;27:557–562. doi: 10.1002/chir.22466. [DOI] [PubMed] [Google Scholar]
- 136.Tanney J.B., McMullin D.R., Green B.D., Miller J.D., Seifert K.A. Production of antifungal and antiinsectan metabolites by the Picea endophyte Diaporthe maritima sp. nov. Fungal Biol. 2016;120:1448–1457. doi: 10.1016/j.funbio.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 137.Riga R., Happyana N., Quentmeier A., Zammarelli C., Kayser O., Hakim E.H. Secondary metabolites from Diaporthe lithocarpus isolated from Artocarpus heterophyllus. Nat. Prod. Res. 2019 doi: 10.1080/14786419.2019.1672685. [DOI] [PubMed] [Google Scholar]
- 138.Ratnaweera P.B., Jayasundara J.M.N.M., Herath H.H.M.S.D., Williams D.E., Rajapaksha S.U., Nishantha K.M.D.W.P., de Silva E.D., Andersen R.J. Antifeedant, contact toxicity and oviposition deterrent effects of phyllostine acetate and phyllostine isolated from the endophytic fungus Diaporthe miriciae against Plutella xylostella larvae. Pest Manag. Sci. 2020;76:1541–1548. doi: 10.1002/ps.5673. [DOI] [PubMed] [Google Scholar]
- 139.Wulansari D., Julistiono H., Nurkanto A., Agusta A. Antifungal activity of (+)-2,2′-epicytoskyrin A and its membrane-disruptive action. Makara J. Sci. 2016;20:160–166. doi: 10.7454/mss.v20i4.6703. [DOI] [Google Scholar]
- 140.Tian W., Liao Z., Zhou M., Wang G., Wu Y., Gao S., Qiu D., Liu X., Lin T., Chen H. Cytoskyrin C, an unusual asymmetric bisanthraquinone with cage-like skeleton from the endophytic fungus Diaporthe sp. Fitoterapia. 2018;128:253–257. doi: 10.1016/j.fitote.2018.05.032. [DOI] [PubMed] [Google Scholar]
- 141.Specian V., Sarragiotto M.H., Pamphile J.A., Clemente E. Chemical characterization of bioactive compounds from the endophytic fungus Diaporthe helianthi isolated from Luehea divaricata. Braz. J. Microbiol. 2012;43:1174–1182. doi: 10.1590/S1517-83822012000300045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Reveglia P., Pacetti A., Masi M., Cimmino A., Carella G., Marchi G., Mugnai L., Evidente A. Phytotoxic metabolites produced by Diaporthe eres involved in cane blight of grapevine in Italy. Nat. Prod. Res. 2019 doi: 10.1080/14786419.2019.1679133. [DOI] [PubMed] [Google Scholar]
- 143.Noriler S.A., Savi D.C., Ponomareva L.V., Rodrigues R., Rohr J., Thorson J.S., Glienke C., Shaaban K.A. Vochysiamides A and B: Two new bioactive carboxamides produced by the new species Diaporthe vochysiae. Fitoterapia. 2019;138 doi: 10.1016/j.fitote.2019.104273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sousa J.P.B., Aguilar-Perez M.M., Arnold A.E., Rios N., Coley P.D., Kursar T.A., Cubilla-Rios L. Chemical constituents and their antibacterial activity from the tropical endophytic fungus Diaporthe sp. F2934. J. Appl. Microbiol. 2016;120:1501–1508. doi: 10.1111/jam.13132. [DOI] [PubMed] [Google Scholar]
- 145.Ola A.R.B., Debbab A., Kurtan T., Broetz-Oesterhelt H., Aly A.H., Proksch P. Dihydroanthracenone metabolites from the endophytic fungus Diaporthe melonis isolated from Annona squamosa. Tetrahedron Lett. 2014;55:3147–3150. doi: 10.1016/j.tetlet.2014.03.110. [DOI] [Google Scholar]
- 146.Nakashima K.I., Tomida J., Kamiya T., Hirai T., Morita Y., Hara H., Kawamura Y., Adachi T., Inoue M. Diaporthols A and B: Bioactive diphenyl ether derivatives from an endophytic fungus Diaporthe sp. Tetrahedron Lett. 2018;59:1212–1215. doi: 10.1016/j.tetlet.2018.02.032. [DOI] [Google Scholar]
- 147.Cui H., Lin Y., Luo M., Lu Y., Huang X., She Z. Diaporisoindoles A-C: Three isoprenylisoindole alkaloid derivatives from the mangrove endophytic fungus Diaporthe sp. SYSU-HQ3. Org. Lett. 2017;19:5621–5624. doi: 10.1021/acs.orglett.7b02748. [DOI] [PubMed] [Google Scholar]
- 148.Cui H., Liu Y., Li J., Huang X., Yan T., Cao W., Liu H., Long Y., She Z. Diaporindenes A-D: Four unusual 2,3-dihydro-1H-indene analogues with anti-inflammatory activities from the mangrove endophytic fungus Diaporthe sp. SYSU-HQ3. J. Org. Chem. 2018;83:11804–11813. doi: 10.1021/acs.joc.8b01738. [DOI] [PubMed] [Google Scholar]
- 149.Andolfi A., Boari A., Evidente M., Cimmino A., Vurro M., Ash G., Evidente A. Gulypyrones A and B and phomentrioloxins B and C Produced by Diaporthe gulyae, a potential mycoherbicide for saffron thistle (Carthamus lanatus) J. Nat. Prod. 2015;78:623–629. doi: 10.1021/np500570h. [DOI] [PubMed] [Google Scholar]
- 150.Luo X., Lin X., Tao H., Wang J., Li J., Yang B., Zhou X., Liu Y. Isochromophilones A-F, cytotoxic chloroazaphilones from the marine mangrove endophytic fungus Diaporthe sp. SCSIO 41011. J. Nat. Prod. 2018;81:934–941. doi: 10.1021/acs.jnatprod.7b01053. [DOI] [PubMed] [Google Scholar]
- 151.Chen C.J., Liu X.X., Zhang W.J., Zang L.Y., Wang G., Ng S.W., Tan R.X., Ge H.M. Sesquiterpenoids isolated from an endophyte fungus Diaporthe sp. RSC Adv. 2015;5:17559–17565. doi: 10.1039/C4RA13136C. [DOI] [Google Scholar]
- 152.Mandavid H., Rodrigues A.M.S., Espindola L.S., Eparvier V., Stien D. Secondary metabolites isolated from the amazonian endophytic fungus Diaporthe sp. SNB-GSS10. J. Nat. Prod. 2015;78:1735–1739. doi: 10.1021/np501029s. [DOI] [PubMed] [Google Scholar]
- 153.Liu H., Chen Y., Li H., Li S., Tan H., Liu Z., Li D., Liu H., Zhang W. Four new metabolites from the endophytic fungus Diaporthe lithocarpus A740. Fitoterapia. 2019;137 doi: 10.1016/j.fitote.2019.104260. [DOI] [PubMed] [Google Scholar]
- 154.Li G., Kusari S., Kusari P., Kayser O., Spiteller M. Endophytic Diaporthe sp. LG23 produces a potent antibacterial tetracyclic triterpenoid. J. Nat. Prod. 2015;78:2128–2132. doi: 10.1021/acs.jnatprod.5b00170. [DOI] [PubMed] [Google Scholar]
- 155.Ito A., Maeda H., Tonouchi A., Hashimoto M. Relative and absolute structure of phomolide C. Biosci. Biotechnol. Biochem. 2015;79:1067–1069. doi: 10.1080/09168451.2015.1015953. [DOI] [PubMed] [Google Scholar]
- 156.Yedukondalu N., Arora P., Wadhwa B., Malik F.A., Vishwakarma R.A., Gupta V.K., Riyaz-Ul-Hassan S., Ali A. Diapolic acid A-B from an endophytic fungus, Diaporthe terebinthifolii depicting antimicrobial and cytotoxic activity. J. Antibiot. 2017;70:212–215. doi: 10.1038/ja.2016.109. [DOI] [PubMed] [Google Scholar]
- 157.Brissow E.R., da Silva I.P., de Siqueira K.A., Senabio J.A., Pimenta L.P., Januario A.H., Magalhaes L.G., Furtado R.A., Tavares D.C., Sales Junior P.A., et al. 18-Des-hydroxy cytochalasin: An antiparasitic compound of Diaporthe phaseolorum-92C, an endophytic fungus isolated from Combretum lanceolatum Pohl ex Eichler. Parasitol. Res. 2017;116:1823–1830. doi: 10.1007/s00436-017-5451-9. [DOI] [PubMed] [Google Scholar]
- 158.Huang X., Zhou D., Liang Y., Liu X., Cao F., Qin Y., Mo T., Xu Z., Li J., Yang R. Cytochalasins from endophytic Diaporthe sp. GDG-118. Nat. Prod. Res. 2019 doi: 10.1080/14786419.2019.1700504. [DOI] [PubMed] [Google Scholar]
- 159.Chang F.R., Wang S.W., Li C.Y., Lu Y.Y., Liu S.Y.V., Chen C.Y., Wu Y.C., Cheng Y.B. Natural products from Diaporthe arecae with anti-angiogenic activity. Isr. J. Chem. 2019;59:439–445. doi: 10.1002/ijch.201800158. [DOI] [Google Scholar]
- 160.Cui H., Yu J., Chen S., Ding M., Huang X., Yuan J., She Z. Alkaloids from the mangrove endophytic fungus Diaporthe phaseolorum SKS019. Bioorganic Med. Chem. Lett. 2017;27:803–807. doi: 10.1016/j.bmcl.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 161.Schloss S., Hackl T., Herz C., Lamy E., Koch M., Rohn S., Maul R. Detection of a toxic methylated derivative of phomopsin A produced by the legume-infesting fungus Diaporthe toxica. J. Nat. Prod. 2017;80:1930–1934. doi: 10.1021/acs.jnatprod.6b00662. [DOI] [PubMed] [Google Scholar]
- 162.Sebastianes F.L.S., Cabedo N., El Aouad N., Valente A.M.M.P., Lacava P.T., Azevedo J.L., Pizzirani-Kleiner A.A., Cortes D. 3-Hydroxypropionic acid as an antibacterial agent from endophytic fungi Diaporthe phaseolorum. Curr. Microbiol. 2012;65:622–632. doi: 10.1007/s00284-012-0206-4. [DOI] [PubMed] [Google Scholar]
- 163.Hu M., Yang X.Q., Wan C.P., Wang B.Y., Yin H.Y., Shi L.J., Wu Y.M., Yang Y.B., Zhou H., Ding Z.T. Potential antihyperlipidemic polyketones from endophytic Diaporthe sp. JC-J7 in Dendrobium nobile. RSC Adv. 2018;8:41810–41817. doi: 10.1039/C8RA08822E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yenn T.W., Ring L.C., Nee T.W., Khairuddean M., Zakaria L., Ibrahim D. Endophytic Diaporthe sp. ED2 produces a novel anti-candidal ketone derivative. J. Microbiol. Biotechnol. 2017;27:1065–1070. doi: 10.4014/jmb.1612.12009. [DOI] [PubMed] [Google Scholar]
- 165.El-Helw E.A.E., Hashem A.I. Synthesis and antitumor activity evaluation of some pyrrolone and pyridazinone heterocycles derived from 3-((2-oxo-5-(p-tolyl)furan-3(2H)-ylidene)methyl)quinolin-2(1H)-one. Synth. Commun. 2020;50:1046–1055. doi: 10.1080/00397911.2020.1731549. [DOI] [Google Scholar]
- 166.Zheng C.J., Shao C.L., Chen M., Niu Z.G., Zhao D.L., Wang C.Y. Merosesquiterpenoids and ten-membered macrolides from a soft coral-derived Lophiostoma sp. fungus. Chem. Biodivers. 2015;12:1407–1414. doi: 10.1002/cbdv.201400331. [DOI] [PubMed] [Google Scholar]
- 167.Jozwiak M., Filipowska A., Fiorino F., Struga M. Anticancer activities of fatty acids and their heterocyclic derivatives. Eur. J. Pharmacol. 2020;871 doi: 10.1016/j.ejphar.2020.172937. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data in this article is openly available without any restrictions.