Summary
Background:
Timely diagnosis and treatment of pediatric tuberculosis (TB) is critical to reducing mortality but remains challenging in the absence of adequate diagnostic tools. Even once a TB diagnosis is made, delays in treatment initiation are common, but for reasons that are not well understood.
Methods:
To examine reasons for delay post-diagnosis, we conducted semi-structured interviews with Ministry of Health (MoH) physicians and field workers affiliated with a pediatric TB diagnostic study, and caregivers of children aged 0-14 years who were diagnosed with pulmonary TB in Lima, Peru. Interviews were analyzed using systematic comparative and descriptive content analysis.
Results:
We interviewed five physicians, five field workers and 26 caregivers with children who initiated TB treatment < 7 days after diagnosis (n=15) or who experienced a delay of ≥7 days (n=11). Median time in delay from diagnosis to treatment initiation was 26 days (range, 7-117). Reasons for delay included: health systems challenges (administrative hurdles, medication stock, clinic hours), burden of care on families and caregiver perceptions of disease severity.
Conclusions:
Reasons for delay in treatment initiation are complex. Interventions to streamline administrative processes and tools to identify and support families at risk for delays in treatment initiation are urgently needed.
Keywords: treatment delay, pediatric tuberculosis, barriers, qualitative research
Introduction
Globally, an estimated 400 children die each day of tuberculosis (TB).1 Timely diagnosis and treatment are critical for children, especially infants, because they are susceptible to severe forms of TB.2,3 However, diagnosing pediatric TB is notoriously difficult; children can rarely expectorate sputum required for conventional testing and pediatric TB is often paucibacillary, complicating microbiological confirmation even when a sputum sample is available.4,5 Once a TB diagnosis is made, prompt treatment initiation is vital to limiting morbidity and mortality.
Diagnostic delays in pediatric TB can be explained by lack of adequate tests, health provider shortages, and limited awareness of TB.4,5,6 While studies have examined treatment delays from initial symptom presentation to treatment initiation, delays occurring post-TB diagnosis are not well-documented. For example, a systematic review of barriers to treatment initiation in Sub-Saharan Africa cited health service delays, limited TB literacy, high cost and limited access to care as important contributors to delays but did not distinguish between delays occurring pre vs. post-diagnosis.16 The reasons for pre- and post-diagnosis delay may be different, with lack of an adequate diagnostic tool contributing prominently to pre-diagnosis, but not post-diagnosis delays. Developing targeted strategies to ensure prompt treatment initiation requires an understanding of specific drivers of post-diagnosis delay. To address this gap, we conducted a qualitative study on reasons for treatment delays following a TB diagnosis among children in Lima, Peru.
Methods
Setting:
In 2018, Peru had a TB incidence of 123 per 100,000, and an estimated 5% of new cases occurred in children.17 TB treatment and care is provided free of charge by the Ministry of Health (MoH) through a network of health centers and tertiary care hospitals. Under the MoH, each city district has several primary healthcare centers where general practitioners, nurses and nursing assistants from the National TB Program (NTP) provide TB care. Pulmonologists provide consultation at health centers on a rotating schedule and may refer patients to specialized pediatric pulmonologists at larger hospitals for diagnosis and case management. Treatment plans for complex cases and very young patients are managed by a committee of pediatricians meeting bimonthly. For clinically stable TB cases requiring any second-line drugs, another expert committee approves regimens prior to treatment initiation. Treatment of pediatric TB is initiated upon paperwork completion, which includes diagnostic test results, a physician’s letter confirming diagnosis, prescribed regimen, and, for cases with drug resistant TB, an approval letter from the expert committee.
Study population:
From December 2016 to February 2018, we recruited caregivers of children (0- 14 years old) with TB disease who participated in a TB diagnostic study (the “parent study”)18,19,20. Children in the parent study were diagnosed and treated for TB following Peruvian NTP treatment guidelines.21
We purposively sampled 26 caregivers of children enrolled in the parent study; 11 were caregivers of children who experienced delays of 1 ≥ week before TB treatment initiation and 15 were caregivers of children initiating TB treatment within one week of diagnosis (i.e., no delay). We also interviewed four MoH pediatric pulmonologists and one general practitioner who routinely diagnosed and treated children with TB. These physicians worked in a variety of settings and had 8 to35 years of professional experience. We also interviewed five field workers (nurses and nursing assistants) with varying levels of professional experience who were employed by the parent study. We sought to capture the field workers’ unique perspective gained from working with families navigating the TB diagnosis and treatment process.
Data collection:
A female Peruvian researcher (MW), not involved in the parent study, invited caregivers to participate and conducted all interviews using a semi-structured interview guide exploring the experience of initiating TB treatment. Interviews lasted ~60 minutes, were conducted in a mutually agreed upon location (e.g., participants’ homes, health facilities) and were audio recorded and transcribed verbatim. Dates of diagnosis and treatment initiation, regimen types and sociodemographic data were abstracted from medical charts. Diagnosis date was recorded by the diagnosing physician, and treatment initiation date was the day the first dose was administered, as indicated by the daily treatment log. Identifying participant information was changed to protect confidentiality.
Analysis:
Transcripts were uploaded into Dedoose Version 8.0.35 (2018).22 JC and MW read transcripts and listened to audio files to assure transcript fidelity, and to become familiar with the data to construct preliminary codebooks. Separate codebooks were created for caregivers, clinicians and staff. Next, a systematic, comparative and descriptive content analysis was performed where codes were applied to thematically similar passages of text. To ensure inter-coder reliability, JC and MW met to reach consensus on all emerging themes, and discussed occasional coding discrepancies, through resolution. Text was then grouped by code, and recurring themes emerging from the narratives were identified. We followed the Consolidated Criteria for Reporting Qualitative Research [COREQ] checklist; see Annex 1.
Ethics approval and consent to participate:
The study was approved by the Ethics Committee of Peru’s National Institute of Health and the Office of Human Research Administration at Harvard Medical School. Participants provided written informed consent for all study procedures.
Results
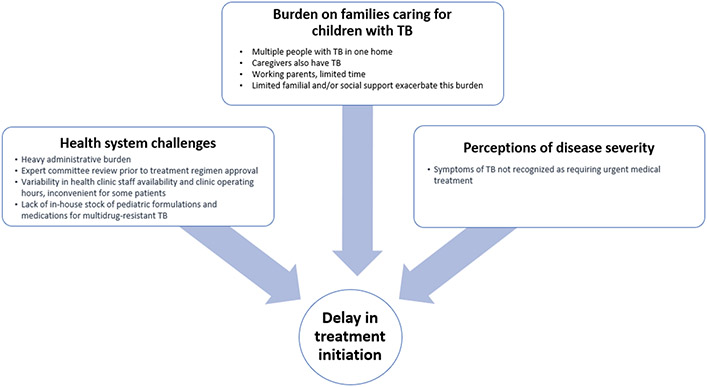
Among 628 children enrolled in the parent study, 136 (22%) were diagnosed with TB and initiated treatment. Among them, 116 (85%) started treatment within one week of diagnosis, and 20 (15%) experienced a delay of a week or more before their first dose (range: 7-117 days). Among those with a delay, five children had MDR-TB and experienced delays of 18, 23, 29, 50 and 117 days. The median delay among those treated with first line medications was 13 days (range: 7-49). All 26 caregivers approached for participation provided consent and were interviewed. Descriptive characteristics and time from diagnosis to treatment start for the children whose caregivers we interviewed are described in Table 1. Three major themes related to pediatric TB treatment delays emerged from the interviews: (1) health systems challenges; (2) burden of care assumed by families when a child is diagnosed with TB; and (3) caregiver perceptions of disease severity (Figure 1). Each theme is presented below with supporting, illustrative quotes (Table 2).
Table 1:
Demographic and TB-related characteristics of children with and without treatment delays
| Children with delay* |
Children without delay |
|
|---|---|---|
| n=11 | n=15 | |
| Age, median [range] | 2.5 [0.9-14.9] | 5.3 [0.6-14.8] |
| Female sex, n (%) | 5 (45%) | 10 (67%) |
| Days from diagnosis to treatment initiation, median [range]** | 26 [7-117] | 1 [0-6] |
| Case status, n (%) | ||
| Culture-confirmed TB | 4 (36%) | 6 (40%) |
| Clinically-diagnosed, unconfirmed TB | 7 (64%) | 9 (60%) |
| Received a regimen other than HREZ, n (%)** | 5 (42%) | 1 (7%) |
| Other regimen received n (%) | ||
| PAS/AMK/CS/ETO/LFX | 0 (0%) | 1 (7%)*** |
| AMK/CS/ETO/LFX/PZA | 1 (9%) | 0 (0%) |
| EMB/LFX/PZA/RIF | 2 (18%) | 0 (0%) |
| CS/EMB/ETO/KM/LFX/PZA | 2 (18%) | 0 (0%) |
Delay defined as time lapse of seven or more days between diagnosis to treatment start, as documented in the child’s medical record
N=25. One child with a TB treatment delay did not start treatment until after study closure. We were therefore unable to obtain data on date of treatment initiation and regimen.
This case represents an exception to committee review for drug resistant cases: due to known MDR-TB in both parents, that drugs happened to be available at the clinic and the attending physician was a member of the national committee of experts, the child was able to start treatment quickly
Figure 1:
Theoretical framework highlighting major themes contributing to delay in treatment start
Table 2:
Representative quotes, by theme
| Theme | Quote | Respondent |
|---|---|---|
| Health systems challenges | ||
| 1 | …for example, if they come at the end of the month, families have to wait 15 or so days for the … committee to meet. It meets the second and 4th Tuesday of each month. So that’s 15 days, plus the dosing sheet, so 22-25 days until they can start treatment. | Doctor |
| 2 | …the letter didn’t come…he felt hot so I took him to the ER with a friend, and they asked me what's wrong? I said he has a fever of 39°C, and they said since when? I told her well, my son has TB and that's when they started calling me out. 'You're so irresponsible’ they said, in front of everyone who was there…‘There are other kids who could be at risk now because of you, he needs a mask. Go get a mask!’…my friend told me I never should have told them he has TB. I left, and we went to the pharmacy for some cough syrup. | Mother of 2-year-old boy, 50-day delay |
| 3 | I came on a Friday…but they said I had to come on Saturday, they said I had to come with my papers so I did, then they said no, it has to be on Monday to start treatment…I went on Monday immediately so he could get in, and then they told me the doctors rotate, there isn’t one at this health center all the time and he’s not in the office on Mondays, to come back on Tuesday…so Tuesday my son got seen by the doctor and started treatment. | Mother of 14-year-old boy, 13-day delay |
| 4 | I think it depends a lot on the doctors because there’s not always a doctor at the health center, and when we get to the health post they say come tomorrow, come tomorrow, it depends on the health center and also on the mom because sometimes there are moms who say I don’t have time, but the health center wants them to be there right then, you know? I think those are two things that conflict with each other. | Study staff member |
| 5 | … it’s a little more difficult for the MDR cases that need second-line drugs, so we talk to the nurse to see if we can borrow some from another patient. Or, we request them from the pharmacy, or from another health center if they have stock… | Doctor |
| 6 | For drug-sensitive cases, they start treatment the same day. But, for the MDR cases we have to wait at least two weeks, because not everyone has the right medications on hand, and we have to send them out to be prepared in the right doses [weight-based dosing in milligrams per kilogram]. | Doctor |
| Burden on families | ||
| 7 | …[treatment] was delayed because I was working, I had to work because otherwise there was no money. I didn’t know if I should stop working or not, I was between a rock and a hard place, like what do I do? ‘If I stop working, what are these kids going to eat’…that’s how it was. And I had to pay the rent on this place, you know? | Mother of 9-year-old girl, >8 months delay |
| 8 | We had a case where a little girl got diagnosed with TB and the only guardian she had was her dad. Her mom had died of tuberculosis. We would schedule visits with the dad, call him and call him to get him to come to the health center, we’d even coordinate with the nurse in charge at the health post. However, he wouldn’t come, he’d say he had to work. That was his major difficulty, there wasn’t anyone else who could bring the little girl so she could start treatment. | Study staff member |
| Perceptions of disease severity | ||
| 9 | In one health center we had three kids diagnosed with TB, all siblings. Once the Dad found out they were diagnosed, he didn’t want them starting treatment. He said he’d take them somewhere else, that they were fine. As far as I know, a month and a half, nearly two months had gone by and the Dad just didn’t want any of it. People went to try to convince him, but he wouldn't budge; he said his kids seemed stable, he didn’t agree with the doctor’s recommendations. | Study staff member |
| 10 | …it’s just that they don’t trust, or they don’t care and like I said the kid isn’t as symptomatic as the adult so that’s another reason they don’t believe it. | Doctor |
| 11 | Until something bad happens to the child, they [parents] aren’t worried about it, they don’t believe it even when you tell them the child has [TB] contacts…[they believe it] even less if the child doesn’t have symptoms. | Doctor |
Health systems challenges
Physicians, caregivers and study staff described healthcare system complexities leading to treatment delays including (1) heavy administrative burden; (2) expert committee review prior to treatment regimen approval; (3) variability in staff availability and inconvenient clinic hours; and (4) limited stock of pediatric formulations and medications for multidrug-resistant (MDR)-TB.
For children with drug-resistant TB, paperwork required prior to starting treatment sometimes took weeks to complete. Screening tests for drug-resistant TB were often not available at one central location and caregivers had to compile results before returning to the health center to finalize paperwork, which included a physician-generated letter indicating diagnosis date and regimen.
The requisite review of some pediatric TB cases by a national committee of experts prior to treatment initiation also contributed to delays. Following review of clinically stable cases where at least one second-line drug was indicated, the committee would send a formal regimen approval letter to the health center. Ideally, recommendations were communicated quickly to local health centers, but a doctor explained that backlogged cases resulted in delays for some children (Table 2, quote 1). One parent described taking her child to the emergency room for an unremitting fever during this wait period because her local health center would not release medications for the child without the letter (quote 2). In this instance, cumbersome administrative processes contributed to treatment delays, and consequently the worsening of symptoms. And, the stigma she experienced led her to leave the hospital without having her child evaluated, resulting in a secondary reason for delay.
Additional health systems challenges resulted from busy schedules and competing priorities (e.g., off-site meetings and trainings) among providers, contributing to inconsistent staffing at health centers. Also, because Peru has a limited number of pediatric pulmonologists, they rotate through primary care facilities and their availability was not always effectively communicated to caregivers. These issues, coupled with inconvenient clinic operating hours caused some families to make multiple trips before receiving care (quotes 3 and 4).
We found that local health centers did not always have medications available to treat pediatric drug-resistant TB. When this occurred, physicians requested medications from a pharmacy, which entailed a waiting period for medication preparation and delivery. Additionally, for children requiring weight-based dosing, pediatric formulations such as syrups and smaller pills were not widely available. Providers often instructed caregivers to take medications to privately-owned pharmacies to prepare age-appropriate formulations, but this was at the caregiver’s discretion, and involved out-of-pocket costs. Alternatively, providers would crush and split adult pills for each dose (quotes 5 and 6).
Burden on families
Health systems challenges exacerbated the burden experienced by families when a child was diagnosed with TB. This burden was particularly acute in households where multiple children had TB and among caregivers who were single, working heads of household and/or sick with TB themselves. The responsibility of multiple clinic visits, while managing one’s own illness and providing financial resources for the household in the absence of familial or social support contributed to delays.
Competing responsibilities in the absence of support was a notable difference between caregivers whose children did and did not experience delay. For example, one caregiver was a mother of five children between the ages of 2-14, three diagnosed with TB. She was diagnosed with TB after severe symptoms, was in a coma for 18 days and hospitalized for nearly two months. Unable to care for her children, she sought support from the children’s grandmother, who assumed responsibility of ensuring the children’s treatment. She understood the 10-day treatment delay, considering the challenges the grandmother faced in managing the logistics and care for three children with TB.
Participants also reported the difficult decisions they faced with two competing priorities; work commitments and helping their child start treatment, a process that required in-person visits to the clinic during operating hours (quotes 7 and 8).
Perceptions of disease severity
Providers and study staff relayed examples of families whose children had been diagnosed with TB, but who reported they did not think their child seemed perceptibly unwell. Respondents perceived that for these parents, the mild nature or absence of symptoms, common in childhood pulmonary TB, led to a lack of urgency in seeking treatment (quotes 9, 10, and 11).
Discussion
We found that health system challenges, the burden on families and perceptions that symptoms were non-severe contributed to delays in treatment initiation in this setting. These findings align with results from studies of the pediatric TB diagnostic process in Peru whichcite similar factors as contributors to diagnostic delays.23, 24 Taken together, we conclude that interventions that address these barriers may accelerate treatment initiation by facilitating multiple steps along the care continuum.
Our findings suggest that administrative processes hindered treatment initiation=. In order to facilitate implementation of national TB treatment guidelines, which indicate that drug sensitive and MDR-TB treatment should start within 24 hours and 14 days of diagnosis, respectively,21 the NTP recently streamlined the case review process. In May 2017the NTP commissioned two additional panels to review challenging cases and provide recommendations. Close monitoring of treatment delays will allow assessment of the impact of these additional committees on delays.
Navigating administrative processes was especially challenging for families where the primary caregivers were single heads of household, sick with TB themselves, and were caring for multiple children. These families were often those that experienced treatment delays. Caregivers voiced their struggles to simultaneously manage their child’s and their own illness, while juggling work commitments that conflicted with clinic operating hours. These caregivers reported time-consuming, expensive, and sometimes futile travel to clinics in pursuit of care. Some families were not able to make the trips at all, citing work as the overarching priority. Conversely, caregivers with family support were often able to take time off work or delegate care responsibilities. Providing health centers with a toolkit to identify families where caregivers have TB, are under or unemployed, single heads of household or rely on limited or no familial support could enable them to provide targeted support, including assistance with childcare, work-friendly operating hours, home-based treatment administration and transportation to the clinic.
Another approach to mitigating delays may be to address caregiver perceptions of disease. Physicians and field workers reported that caregivers deprioritized their child’s treatment initiation because they underestimated the severity of disease. It is unclear whether this was also true for caregivers or whether it represented a more general lack of trust in the healthcare system, or structural and socioeconomic barriers to care that families routinely face. Assigning blame to caregivers is not uncommon,25 however, evidence from other qualitative studies from this setting found that symptoms indicative of TB were not immediately recognized as suggestive of serious illness.23,26 This may be due to limited TB literacy, a high prevalence of other respiratory tract infections with similar non-specific symptoms, and ineffective provider communication. Another potential explanation is TB-related stigma, which could lead to an initial denial of the diagnosis or minimizing of symptom severity.
While we only found one example of TB-related stigma that contributed to treatment delay, caregivers reported encountering stigma along the TB care continuum. Stigmatizing behaviors by providers and stigma internalized by caregivers may have contributed to delays not captured by our study. Prior work from Lima on delays in the diagnostic process found that TB-related stigma and discrimination were important contributors to delay.27 Stigma is also widely recognized as a barrier to effective TB elimination strategies, globally.28 Successful efforts to eliminate TB-related stigma will likely enable timely treatment initiation and improve outcomes in children and adults alike.
Our study captured responses from caregivers of children who participated in a TB diagnostic study. There, caregivers received support beyond what is routinely provided, including accompaniment to medical visits and financial support for costs not supported by the TB program, such as transportation to and from visits, computed tomography (CT) scans, and paperwork processing fees. This financial support may explain why out-of-pocket costs did not emerge as an important barrier, although found to be an important factor in other studies.29,30 It is also possible that support provided in the diagnostic study mitigated the effect of structural barriers; without this, children could have faced even longer delays. Although we interviewed caregivers of children with and without treatment delays, caregivers whose children initiated treatment promptly rarely identified factors that facilitated this outcome, instead highlighting hurdles they faced in other parts of the TB care continuum. This reflected a common cognitive tendency to remember negative events in more detail than positive experiences.
Conclusion
This study highlights the constellation of factors that prevent prompt treatment initiation among children with TB. Burdensome administrative processes, household level challenges faced by families in caring for children with TB, and perceptions that symptoms were non-severe were all contributors. Comprehensive efforts that aim to streamline administrative practices and provide healthcare personnel with tools to identify and support families at risk for delay, may help close the gap between diagnosis and treatment start, ultimately reducing TB-related deaths among children.
Footnotes
Conflict of interest statement: The authors have no conflicts of interest to report.
References
- 1.World Health Organization. Global Tuberculosis Report. 2018.
- 2.Cruz AT, Starke JR. Pediatric Tuberculosis. Pediatr Rev. 2010. January;31(1):13–25 [DOI] [PubMed] [Google Scholar]
- 3.Nityananda M, Anand PK, Gautam S, Das S, Hussain T. Diagnosis and treatment of paediatric tuberculosis: An insight review. Critical Reviews in Microbiology. 2017; 43:4,466–480 [DOI] [PubMed] [Google Scholar]
- 4.Starke JR. Pediatric tuberculosis: Time for a new approach. Tuberculosis. 2003;83(1–3):208–12. [DOI] [PubMed] [Google Scholar]
- 5.Newton S, Brent A, Anderson S, Whittaker E, Kampmann B. Paediatric Tuberculosis. Lancet Infect Dis. 2008;8(8):498–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiang SS, Roche S, Contreras C, Alarcon V, Del Castillo H, Becerra M, et al. Barriers to the diagnosis of childhood tuberculosis: a qualitative study. Int J Tuberc Lung Dis. 2015; 19(10):1144–1152 [DOI] [PubMed] [Google Scholar]
- 7.Lin Y, Enarson DA, Chiang C-Y, Rusen ID, Qiu LX, Kan XH, et al. Patient delay in the diagnosis and treatment of tuberculosis in China: findings of case detection projects. Public Health Action. 2015;5(1):65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Storla DG, Yimer S, Bjune GA. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health. 2008;8:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai J, Wang X, Ma A, Wang Q, Han X, Li Y. Factors associated with patient and provider delays for tuberculosis diagnosis and treatment in Asia: a systematic review and meta-analysis. PLoS One. 2015;10(3):e0120088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sreeramareddy CT, Qin ZZ, Satyanarayana S, Subbaraman R, Pai M. Delays in diagnosis and treatment of pulmonary tuberculosis in India: a systematic review. Int J Tuberc Lung Dis. 2014;18(3):255–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alavi SM, Bakhtiyariniya P, Albagi A. Factors associated with delay in diagnosis and treatment of pulmonary tuberculosis. Jundishapur J Microbiol. 2015;8(3):e19238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beyers N1, Gie RP, Schaaf HS, van Zyl S, Nel ED, Talent JM, et al. Delay in the diagnosis, notification and initiation of treatment and compliance in children with tuberculosis. Tuber Lung Dis. 1994;75(4):260–5. [DOI] [PubMed] [Google Scholar]
- 13.Farah MG, Rygh JH, Steen TW, Selmer R, Heldal E, Bjune G. Patient and health care system delays in the start of tuberculosis treatment in Norway. BMC Infect Dis. 2006;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rifat M, Hall J, Oldmeadow C, Husain A, Milton AH. Health system delay in treatment of multidrug resistant tuberculosis patients in Bangladesh. BMC Infect Dis. 2015;15(1):526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen C-C, Chiang C-Y, Pan S-C, Wang J-Y, Lin H-H. Health system delay among patients with tuberculosis in Taiwan: 2003-2010. BMC Infect Dis. 2015;15:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sullivan BJ, Esmaili BE, Cunningham CK. Barriers to initiating tuberculosis treatment in sub-Saharan Africa: a systematic review focused on children and youth, Global Health Action. 2017; 10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Tuberculosis profile: Peru. 2018. https://extranet.who.int/sree/Reports?op=Replet&name=/WHO_HQ_Reports/G2/PROD/EXT/TBCountryProfile&ISO2=PE&outtype=html
- 18.Coit J, Mendoza M, Pinedo C, Marin H, Chiang S, Lecca L, et al. Performance of a household tuberculosis exposure survey among children in a Latin American setting. Int J Tuberc Lung Dis. 2019; 23:1223–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mesman A, Soto M, Coit J, Calderon R, Aliaga J, Pollock N, Mendoza M, Mestanza F, Mendoza C, Murray M, Lecca L, Holmberg R, Franke MF. Detection of Mycobacterium tuberculosis in pediatric stool samples using TruTip technology. BMC Infect Dis. 2019; 19:563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tafur K, Coit J, Leon SR, Pinedo C, Chiang S, Contreras C, Calderon R, Mendoza M, Lecca L, Franke MF. Feasibility of the string test for tuberculosis diagnosis in children between 4 and 14 years old. BMC Infect Dis. 2018; 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MINSA. Norma técnica de la salud para el control de la Tuberculosis. Peru: Ministerio de Salud. 2018 [Google Scholar]
- 22.Dedoose Version 8.0.35, web application for managing, analyzing, and presenting qualitative and mixed method research data (2018). Los Angeles, CA: SocioCultural Research Consultants, LLC. [Google Scholar]
- 23.Paz-Soldán VA, Alban RE, Jones CD, Oberhelman RA. The provision of and need for social support among adult and pediatric patients with tuberculosis in Lima, Peru: a qualitative study. BMC Health Serv Res 2013;13:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paz-Soldán VA, Alban RE, Jones D, Powell AR, Oberhelman RA. Patient reported delays in seeking treatment for tuberculosis among adult and pediatric TB patients and TB patients co-infected with HIV in Lima, Peru: a qualitative study. Front Public Health. 2014; 2:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaramillo J, Yadav R, Herrera R. Why every word counts: towards patient- and people-centered tuberculosis care. Int J Tuberc Lung Dis. 2019;23(5):547–551 [DOI] [PubMed] [Google Scholar]
- 26.Bonadonna LV, Saunders MJ, Zegarra R, Evans C, Alegria-Flores K, Guio H. Why wait? The social determinants underlying tuberculosis diagnostic delay. PLoS ONE 12(9): e0185018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marais BJ, Schaaf HS. Tuberculosis in children. Cold Spring Harb Perspect Med. 2014; 4: a017855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daftary A, Frick M, Venkatesan N, et al. Fighting TB stigma: we need to apply lessons learnt from HIV activism. BMJ Glob Health. 2017;2: e000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asres A, Jerene D, Deressa W. Pre- and post-diagnosis cost of tuberculosis to patients on Directly Observed Treatment course in districts of southwestern Ethiopia: a longitudinal study. J Health Popul Nutr. 2018; 37:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wingfield T, Tovar M, Huff D, Boccia D, Saunders M, et al. Beyond pills and tests: addressing the social determinants of tuberculosis. Clin Med (Lond). 2016; 16(6): s79–s91 [DOI] [PMC free article] [PubMed] [Google Scholar]