Abstract
Rationale
After alcohol ingestion, the brain partly switches from consumption of glucose to consumption of the alcohol metabolite acetate. In heavy drinkers, the switch persists after abrupt abstinence, leading to the hypothesis that the resting brain may be “starved” when acetate levels suddenly drop during abstinence, despite normal blood glucose, contributing to withdrawal symptoms. We hypothesized that ketone bodies, like acetate, could act as alternative fuels in the brain and alleviate withdrawal symptoms.
Objectives
We previously reported that a ketogenic diet during alcohol exposure reduced acute withdrawal symptoms in rats. Here, our goals were to test whether 1) we could reproduce our findings, in mice and with longer alcohol exposure, 2) ketone bodies alone are sufficient to reduce withdrawal symptoms (clarifying mechanism), 3) introduction of ketogenic diets at abstinence (a clinically more practical implementation) would also be effective.
Methods
Male C57BL/6NTac mice had intermittent alcohol exposure for three weeks using liquid diet. Somatic alcohol withdrawal symptoms were measured as handling-induced convulsions, anxiety-like behavior was measured using the light-dark transition test. We tested a ketogenic diet, and a ketone monoester supplement with a regular carbohydrate-containing diet.
Results
The regular diet with ketone monoester was sufficient to reduce handling-induced convulsions and anxiety-like behaviors in early withdrawal. Only the ketone monoester reduced handling-induced convulsions when given during abstinence, consistent with faster elevation of blood ketones, relative to ketogenic diet.
Conclusions
These findings support the potential utility of therapeutic ketosis as an adjunctive treatment in early detoxification in alcohol-dependent patients seeking to become abstinent.
Keywords: alcohol withdrawal, alcohol dependence, ethanol, alcoholism, ketone bodies, anxiety-like behavior, mice, detoxification, ketone monoester
Introduction
Worldwide, harmful use of alcohol kills more than 3 million people annually, more than diabetes, hypertension, or road injuries ((WHO) 2018). Alcohol use disorder (AUD) exacts a tremendous personal and economic toll worldwide. A challenge of treatment is the withdrawal symptoms that occur upon cessation of prolonged heavy drinking. Acutely, severe alcohol withdrawal can result in life-threatening seizures and brain damage, classically attributed to increased neuronal excitability (Becker and Mulholland 2014; Finn and Crabbe 1997). Withdrawal symptoms are alleviated by alcohol consumption and thus contribute to relapse to alcohol use (Brower 2003; Engel et al. 2016; Heilig et al. 2010). During a life-time, as many as 80% of abstinent alcohol users relapse (Barrick and Connors 2002; Connor et al. 2016; Jin et al. 1998). Benzodiazepines, the most common treatment, are effective against withdrawal symptoms but have side effects including addictive properties. Indeed, patients with AUD have a high prevalence of non-medical benzodiazepine use and benzodiazepine dependence, with estimates ranging from 14% current to 78% lifetime (Johansson 2003; Kan et al. 2001; Morel et al. 2016; Votaw et al. 2019). While few studies directly compared benzodiazepine dependence in AUD vs. general population, one study showed 15% and 1%, respectively (Johansson 2003). The disease remains severely undertreated: a recent US survey reported that only 4.2% of patients diagnosed with AUD received specialty treatment ((SAMHSA) 2019). There exists a clear need for new treatment options based on a better understanding of the effects that heavy drinking and withdrawal have on the brain.
Brain imaging studies show that acute alcohol administration lowers glucose metabolism in the human brain (de Wit et al. 1990; Volkow et al. 1990). Because the decrease also happens at low alcohol doses that have minimal behavioral effects (Volkow et al. 2006), it was hypothesized that the reduction in glucose metabolism reflects utilization of an alternate energy substrate by the brain, e.g., the alcohol metabolite acetate (Pawlosky et al. 2010; Volkow et al. 1993). Acetate is readily taken up into the brain via the monocarboxylic acid transporter 1 and is used as an energy substrate by astrocytes (Cruz et al. 2005; Lebon et al. 2002; Minchin and Beart 1975; Waniewski and Martin 1998). Brain acetate metabolism is increased while glucose metabolism is decreased during alcohol intoxication, an effect more pronounced in heavy drinkers than in control subjects (Jiang et al. 2013; Volkow et al. 2013; Volkow et al. 2015). The findings led to the hypothesis that the brain may be in a state of reduced energy turnover when acetate availability drops upon abrupt cessation of sustained alcohol drinking, despite normal plasma glucose, contributing to the neurotoxicity and symptoms observed in acute withdrawal. Providing the brain with a non-glucose energy substrate like acetate may then alleviate withdrawal symptoms.
The ketone bodies acetoacetate, acetone, and β-hydroxybutyrate (BHB) also rise during ethanol consumption (Baraona and Lieber 1979; Lefèvre et al. 1970; Lukivaskaya and Buko 1993). Ketone bodies are readily consumed by the brain, whether in starvation (Hasselbalch et al. 1995; Hawkins et al. 1986) or by exogenous administration (Gjedde 1983; Jiang et al. 2011b; Pan et al. 2002). Therefore, alcohol consumption has may promote consumption of both acetate and ketone bodies in the brain. Ketone bodies are also produced in the liver when glucose is not available, such as during fasting or adherence to a ketogenic diet (KD; very high fat, moderate protein, very low carbohydrate). Similar to acetate, ketone bodies are taken up into the brain via monocarboxylic acid transporters, and can serve as energy substrate for all brain cell types, with oxidation distributed in neurons vs. astrocytes in proportions similar to glucose utilization (Achanta and Rae 2017; Jiang et al. 2011a; Pan et al. 2002).
Thus, we hypothesized that ketone bodies could replace ethanol-derived acetate and ketones as energy substrates during alcohol withdrawal. Indeed, we previously reported that rats maintained on a KD during alcohol exposure had reduced withdrawal symptoms when alcohol was discontinued, relative to control diet (Dencker et al. 2018). In practice, adherence to a strict KD would be challenging for alcohol-dependent patients before initiation of sobriety, because the KD is difficult to maintain, requires medical supervision and adequate micronutrient supplementation, and its effects on long-term health are debated. However, ketosis (defined as an increase in blood ketone concentration >0.5 mM) can also be achieved by intake of exogenous ketones, typically as ketone salts or esters.
Here, we replicated our findings from rats, in mice with longer alcohol exposure, and we extended the studies by asking two main questions to clarify both mechanism of action and clinical potential. First, we asked whether ketone bodies alone are sufficient to reduce withdrawal symptoms. We tested this hypothesis using a regular diet supplemented with a ketone monoester (KME) that produces acute elevations of blood BHB (Clarke et al. 2012a). Second, we asked whether diet-induced ketosis could reduce alcohol withdrawal symptoms when introduced at the start of abstinence rather than during alcohol exposure, i.e., in a manner more suitable for clinical application.
Material and methods
Subjects
Male C57BL/6NTac (Taconic, Lille Skensved, Denmark) were used because they are not prone to spontaneous convulsions and to facilitate comparisons with planned studies involving alcohol drinking. Mice were acquired at 5–6 weeks of age and acclimated at least one week to the facilities before testing. Mice were group-housed four per cage (not necessarily litter mates) in type III cages (42.5 × 26.6 × 15.5 cm) with aspen bedding (Tapvei) and with hiding devices, nesting material, and wooden chewing blocks as enrichment, in a room maintained under a reversed light-dark cycle (light on 19:00 – 07:00), temperature maintained at 22 ± 2°C and relative humidity at 55 ± 10%. A total of 118 mice were used. Three mice were euthanized during the studies and no data were included: two due to fighting during the habituation period, and one due to sudden weight loss (control diet alcohol group). Procedures were approved by the Animal Experiments Inspectorate under the Danish Ministry of Food, Agriculture, and Fisheries in accordance with the EU directive 2010/63/EU (approval number 2017-15-0201-01334). Method of euthanasia was cervical dislocation. Tap water was available freely throughout. Standard rodent chow (Altromin 1310, Brogaarden, Denmark) was available freely at arrival, specialty diets were provided during experiments as described below.
Experiments and diets
Alcohol dependence and withdrawal were induced using a liquid diet in three cycles of five days on 6.7% v/v alcohol, two days forced abstinence, a common liquid-diet approach in the literature and a concentration used in commercially available alcohol liquid diets [Figure 1A; (Overstreet et al. 2002; Snell et al. 1996)]. After acclimation, mice were gradually switched to liquid diets over 2 days (liquid diet with 3% alcohol + 3g chow/mouse; then liquid diet with 4.5% alcohol + 1.5g chow/mouse). For feasibility, mice were tested in two consecutive cohorts in each experiment, each cohort comprising all experimental groups, i.e., each experiment followed a randomized block design. In each block, cages were allocated randomly to experimental group. The experimenter was blinded to experimental group for all tests.
Fig. 1. Experimental setup and diets.
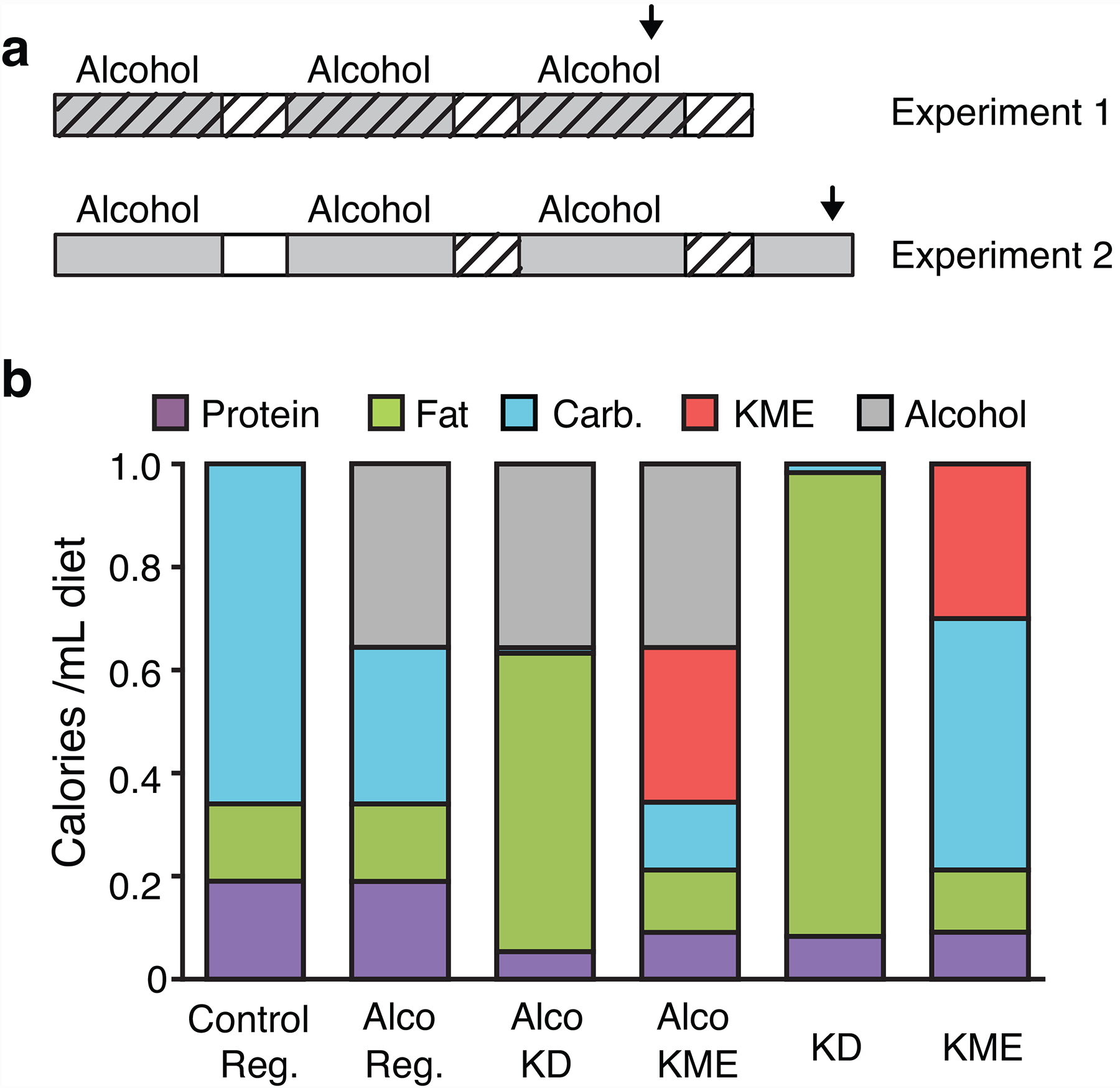
(a) Timeline of alcohol exposure (grey) and forced abstinence (open), with test times as hours since alcohol removal shown for the last cycle. Periods with test diets are indicated by diagonal hatching. Arrows indicate blood alcohol sampling (pointing down) or light-dark transition test (pointing up). (b) Composition of diets as calories provided by each nutrient, per mL diet. Reg.: regular diet. Alco: alcohol-containing. All diets also contained vitamins, minerals, and 5.5–5.7% fiber.
In Experiment 1 “Keto Throughout”, in service of rigor/reproducibility of our previous report (Dencker et al. 2018), we tested the effects of diet-induced ketosis started during alcohol exposure: mice were fed alcohol-containing regular, KD, or KME diet, plus a regular-control (no alcohol) group. On the test days, food was replaced by the corresponding (regular, KD, KME) no-alcohol diet at 13:00 to start forced abstinence. Due to a technical error, no data could be collected from the last cycle in Experiment 1 first cohort, including light-dark transition test and blood measures, resulting in a lower sample size.
In Experiment 2 “Keto After”, we tested the effects of ketosis induced after alcohol exposure. Mice were fed alcohol-regular diet, then no-alcohol regular, KD, or KME diet during abstinence. To ensure fast and uniform onset of ketosis in the KME group, a bolus dose of KME (3g/kg by gavage, 7.8 mL/kg) was administered once at abstinence start, in addition to the available KME diet.
To mix alcohol into a KD without evaporation (e.g., provided in closed bottles), and be able to compare KD, KME, and regular diets as directly as possible, we developed our own diets. The diets were isocaloric, closely based on commercial formulations to meet both nutritional and scientific needs, and were prepared fresh daily. The regular-control and regular-alcohol diets were based on the commonly used open source formulations of diet AIN-76 liquid, the alcohol diets providing 35.6% of calories from alcohol. The KD had a caloric distribution (before addition of alcohol) comparable to the commercial diet used in our previous study (Dencker et al. 2018). The KME diet was based on the regular diet but provided 30% of calories from the ketone ester (D-BHB ester; HVMN ketone ester, HVMN Inc., San Francisco, CA), a dose previously shown to be well tolerated and produce ketosis in rodents (Clarke et al. 2012b; Murray et al. 2016; Srivastava et al. 2012). The KME is converted to BHB and acetoacetate (Clarke et al. 2012c; Desrochers et al. 1995; Tate et al. 1971). Figure 1B shows the nutritional composition of each diet. Unshelled peanuts were offered weekly (never during abstinence) in all groups as enrichment and to limit weight loss (Anji and Kumari 2008).
Apparatus
Light-dark transition test was conducted in open field activity chambers fitted with beam-break movement detection systems (OFA-510, Med Associates, St Albans, VT, USA). A partition of dark red plastic (not transparent for mice but allowing beams through) was used to create two compartments each measuring 27 × 13.5 cm, 30 cm tall. Walls and lid were clear in one compartment, opaque black in the other. The light side had low illumination (~40 lux; Fisherbrand Traceable dual-range light meter) not anxiogenic alone (Hascoet et al. 2001), allowing detection of anxiogenic-like effects of alcohol withdrawal (McGinnis et al. 2020; Vranjkovic et al. 2018). The partition had a 4 × 4 cm opening allowing free movement between compartments. Operant procedures used mouse operant-conditioning chambers (Med Associates ENV-307A) each containing two nose-poke holes fitted with a photocell and a yellow cue light, and a steel dish into which reinforcers were delivered from a syringe pump (Thomsen and Caine 2005; Thomsen et al. 2017). All chambers were individually enclosed in sound-attenuating cubicles equipped with a light and a ventilation fan.
Alcohol withdrawal testing: somatic symptoms
Handling-induced convulsions (HIC) were assessed after 1, 2, 3, 4, 6, and 8 hours of abstinence in the “Keto Throughout” experiment, and after 1, 2, 3, 5, 7, 9, 11, 20, and 28 hours in the “Keto After” experiment to allow more time for ketosis to develop from the KD. Alcohol diets were again provided at 08:00 the next day, i.e., after 43h of abstinence, starting the next 5-day alcohol access cycle. HIC scoring was adapted from Crabbe et al. (Crabbe et al. 1991) with possible scores 0–7, and is described in detail in Supplemental methods. HIC scores were averaged over test cycles, then, area under the curve (AUC) was calculated in each mouse for the first three hours (3h-AUC), i.e., the maximum time predicted to produce ketosis after KME administration, and for the entire observation period.
Alcohol withdrawal testing: anxiety-like behavior
Mice were tested in the light-dark transition test 6h after abstinence onset (19:00), in the last cycle only to avoid habituation. Mice were placed in the lit side of the apparatus and activity was recorded for 10 min. Primary measures were light-dark crossings and voluntary time on lit side (Hascoet et al. 2001; Kliethermes 2005). Data from no-alcohol regular diet controls tested in different experiments did not differ significantly (p=0.10 and p=0.38) and were pooled for data presentation and analysis.
Blood measures
Blood BHB and glucose levels were assessed from needle sticks to the tail using strips and FreeStyle Precision Neo analyzer (Abbott). In the “Keto Throughout” experiment, in which test diet exposure was continuous, blood levels were measured 24h before HIC testing in each cycle, to minimize stress on test days. In the “Keto After” experiment, BHB levels were assessed in cycle 2 and 3, at 24h before and after HIC testing at 1, 2, 7.5, and 24h after withdrawal start in the KME group, and in regular and KD groups, at −24 and 2h. Blood glucose was tested at −24, 2, and 24h. Blood alcohol levels were determined using a GL6 analyzer, Analox Instruments (Stokesley, UK). In the “Keto Throughout” experiment, blood samples were taken 24h before the last cycle of HIC testing by retro-orbital bleeding under brief sevoflurane gas anesthesia. In the “Keto After” experiment, to minimize stress and provide better measurements, mice were maintained four days on their respective alcohol diets after ended testing, and were euthanized by decapitation and trunk blood collected. Liquid diet intake (by bottle weight) and bodyweights were recorded.
Control experiments
Effects of diet-induced ketosis per se in the light-dark transition test were tested in a separate cohort (n=6) after six days on alcohol-free regular, KD, or KME diet – admittedly a shorter diet exposure than the three weeks used in Experiment 1. The next day, the same mice were tested for effects of diet on alcohol clearance and intoxication (anesthetic/sedative effect). To this end, mice were administered 3.5 g/kg alcohol intraperitoneally (22% in saline, 20 mL/kg), and loss of righting reflex was tested as described in Supplemental methods (Fee et al. 2004; Lynch et al. 2013). The mice were euthanized 180 min after alcohol injection and trunk blood was collected for blood alcohol analysis.
Because we observed lower blood alcohol levels in the KME group in the “Keto Throughout”, we added a control experiment to assess whether KME affects voluntary intake of alcohol. In two separate cohorts of mice maintained on regular chow (n=7, 9), we tested the effect of KME pretreatment on voluntary intake of, and operant behavior maintained by 30μl of, oral alcohol (20% in water, never sweetened) and liquid food (vanilla flavor nutridrink, Nutricia, Denmark), respectively. Mice were trained to nose-poke reinforced under a fixed-ratio 1 timeout 20s schedule of reinforcement in 2h-sessions as previously described (Bornebusch et al. 2019; Thomsen et al. 2009), see Supplemental methods for details. Mice were administered 3 g/kg KME by gavage 60 min before session start. As a control for effect of satiety, an isocaloric amount of oil emulsion (Calogen unflavored, Nutricia) was tested, within-subjects, in a counterbalanced order with KME. Chow was removed 2h before gavage.
Data analysis
Light-dark transition test data, HIC AUC, and blood alcohol were analyzed for each experiment by one-way ANOVA with diet as between-subjects factor and planned posthoc comparisons (KD and KME vs. alcohol-regular) adjusted for false discovery rate (Benjamini, Krieger and Yekutieli procedure, limit 5%). Food intake, bodyweight, blood BHB and glucose were analyzed by two-way ANOVA with time as a repeated measure and diet as between-subjects measure, Greenhouse-Geisser corrected where appropriate and with false discovery rate-adjusted posthoc comparisons. Food intake was averaged per cycle component (alcohol/abstinence); bodyweight was averaged per cycle. Loss of righting reflex duration was analyzed by Logrank test. Reinforcers earned per session were analyzed by two-tailed paired-sample t-test with false discovery rate correction. No data were excluded based on statistical outlier values or other criteria. Analyses were performed using GraphPad Prism version 8. A priori power calculations were not performed for these studies.
Results
Both KD and KME increased blood BHB levels
Blood BHB levels reflected the diet ([F3,94=116.1, p<0.0001], [F1,21=18.5, p=0.0003]) and time ([F1.4,64.3=11.4, p=0.0004], [F2.1,35.8=16.9, p<0.0001]) in the “Keto Throughout” and “Keto After” experiment, respectively, with an interaction in “Keto After” [F6,94=11.8, p<0.0001]. In the “Keto Throughout” Experiment (test diets provided during alcohol exposure and abstinence), both KD and KME produced BHB levels higher than the regular diets (p<0.0001; Figure 2A). In the “Keto After” Experiment, KME produced higher BHB than KD at all time points (p<0.05; Figure 2B). Blood glucose was measured in the “Keto After” experiment and was related to diet only [F2,30=13.6, p<0.0001], with KD and KME producing lower glucose levels than regular diet (p<0.001; Figure 2C).
Fig. 2. Blood ketone and glucose levels.
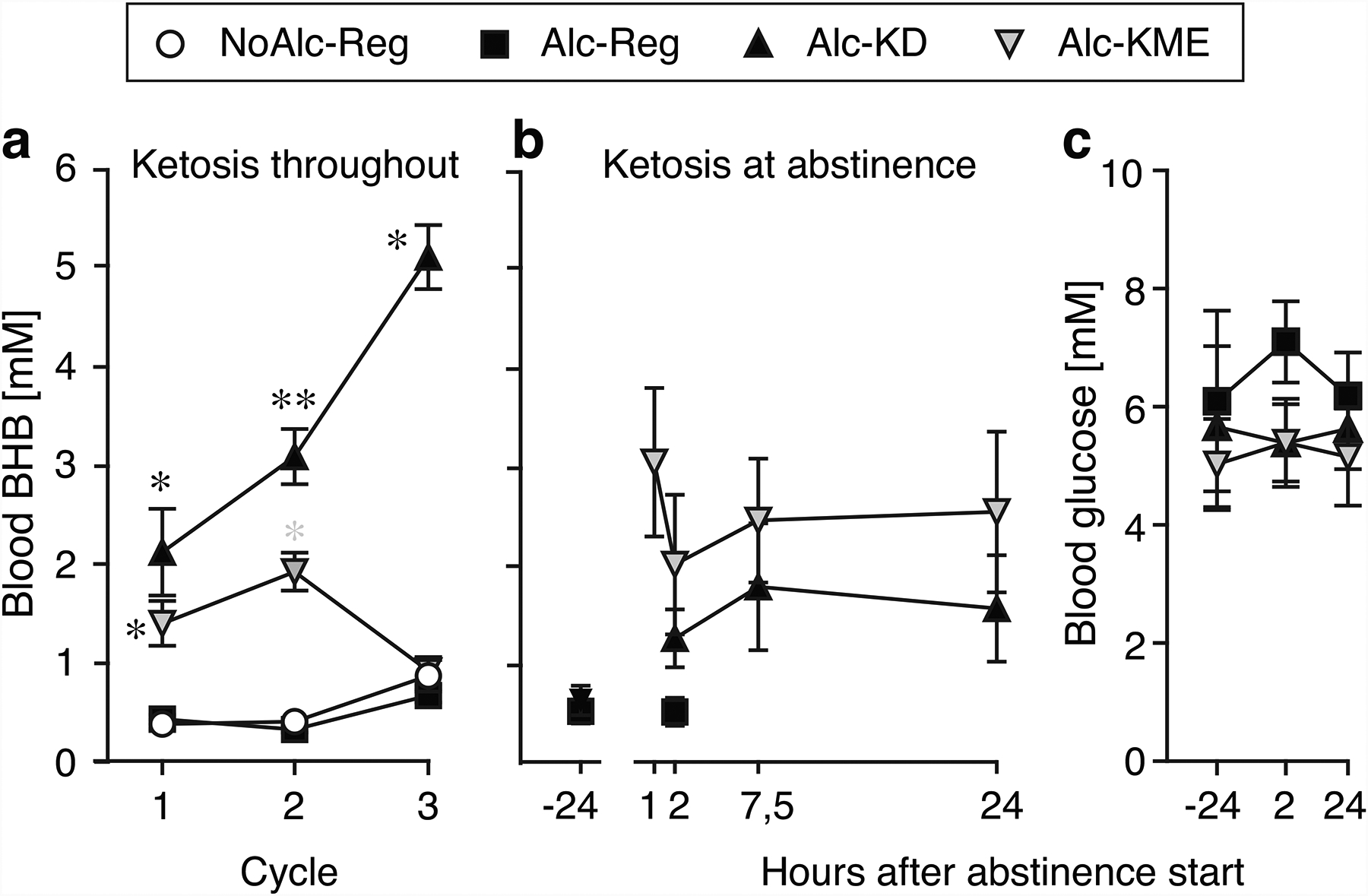
Blood BHB as a function of time in the “Keto Throughout” experiment (a, n=12) and the “Keto After” experiment (b, n=10–12), blood glucose as a function of time in “Keto After” (c, n=10–12). *p<0.05, **p<0.01 vs. alcohol-regular diet. Data are group means ± s.e.m.
Alcohol withdrawal testing: somatic symptoms
In both experiments, alcohol-regular diet mice showed HIC scores consistent with previous reports from this strain (Anji and Kumari 2008; Metten and Crabbe 2005), reaching peak scores of around 3 from 3h to 8–10h after alcohol removal, declining by 20–28h. The use of a relatively long observation period with fewer time points in the last half may have resulted in higher calculated AUC relative to studies with shorter observation or higher temporal resolution. In the “Keto Throughout” Experiment, HIC score was related to diet group as 3h-AUC [F2,33=3.74, p=0.03] and as 8h-AUC [F2,33=26.1, p<0.0001]. Consistent with our previous report, mice in KD group had lower scores relative to alcohol-regular diet for 3h-AUC (p=0.03; Figure 3A) and 8h-AUC (p<0.0001; Figure 3B), while the KME group had reduced score only for 3h-AUC (p=0.047). In the “Keto After” Experiment, HIC score was related to diet group as 3h-AUC [F2,30=6.85, p<0.004] but not as total AUC; only KME-fed mice had lower scores relative to regular diet (p=0.007; Figure 3A). Scrutiny of the cage averages suggested a consistent effect rather than random cage differences; for instance all three cages in the KME group had lower average 3h-AUC than the alcohol-regular diet cages in “Keto After”, and similarly for the 8h-AUC in alcohol-KD vs. alcohol-regular in “Ketosis Throughout”. HIC score as total AUC was negatively correlated with blood BHB levels in the KD group only, in both “Keto Throughout” (p=0.004) and “Keto After” (p=0.0006; Figure S1). Thus with the KD, higher BHB was associated with lower withdrawal symptoms, while with KME, the symptoms were improved at all BHB elevations.
Fig. 3. Effects of diets on handling-induced convulsions.
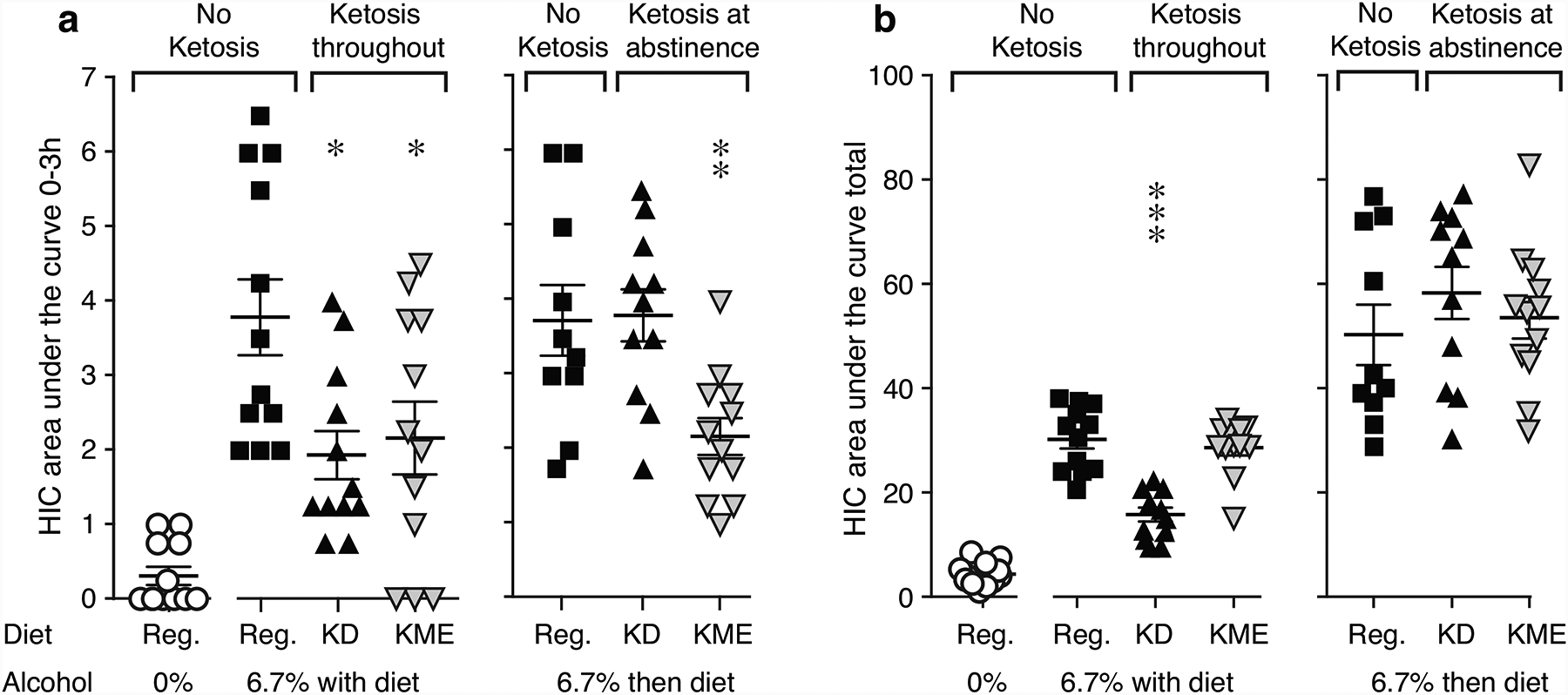
HIC score as AUC for the first three hours of abstinence (a) and total observation period (b) for no-alcohol controls, in “Keto Throughout” (n=12), and “Keto After” (n=10–12). **p<0.01, ***p<0.001 vs. alcohol-regular diet. Data are shown as individual subjects, with bars representing group means ± s.e.m. Note different ordinate scales for 0–3h and for total time.
Alcohol withdrawal testing: anxiety-like behavior
Number of light-dark compartment crossings was related to treatment group in the “Keto Throughout” Experiment [F2,15=4.38, p=0.03]. KD-fed mice had more crossings relative to alcohol-regular diet (p=0.01; Figure 4A), interpreted as reduced anxiety-like behavior. A similar trend was seen for KME-fed mice (p=0.08). Voluntary time in the lit compartment was also affected by treatment condition in “Keto Throughout” [F2,15=11.1, p=0.001]. KME-fed mice spent more time in the lit side relative to alcohol-regular diet (p=0.0005; Figure 4D), interpreted as reduced anxiety-like behavior. Neither measure was affected by diet in the “Keto After” experiment (Figure 4B,E), or by the diets per se (no alcohol, Figure 4C,F). Anxiety-like behaviors were not correlated with blood BHB levels (Figure S2).
Fig. 4. Effects of diets in the light-dark transition test.
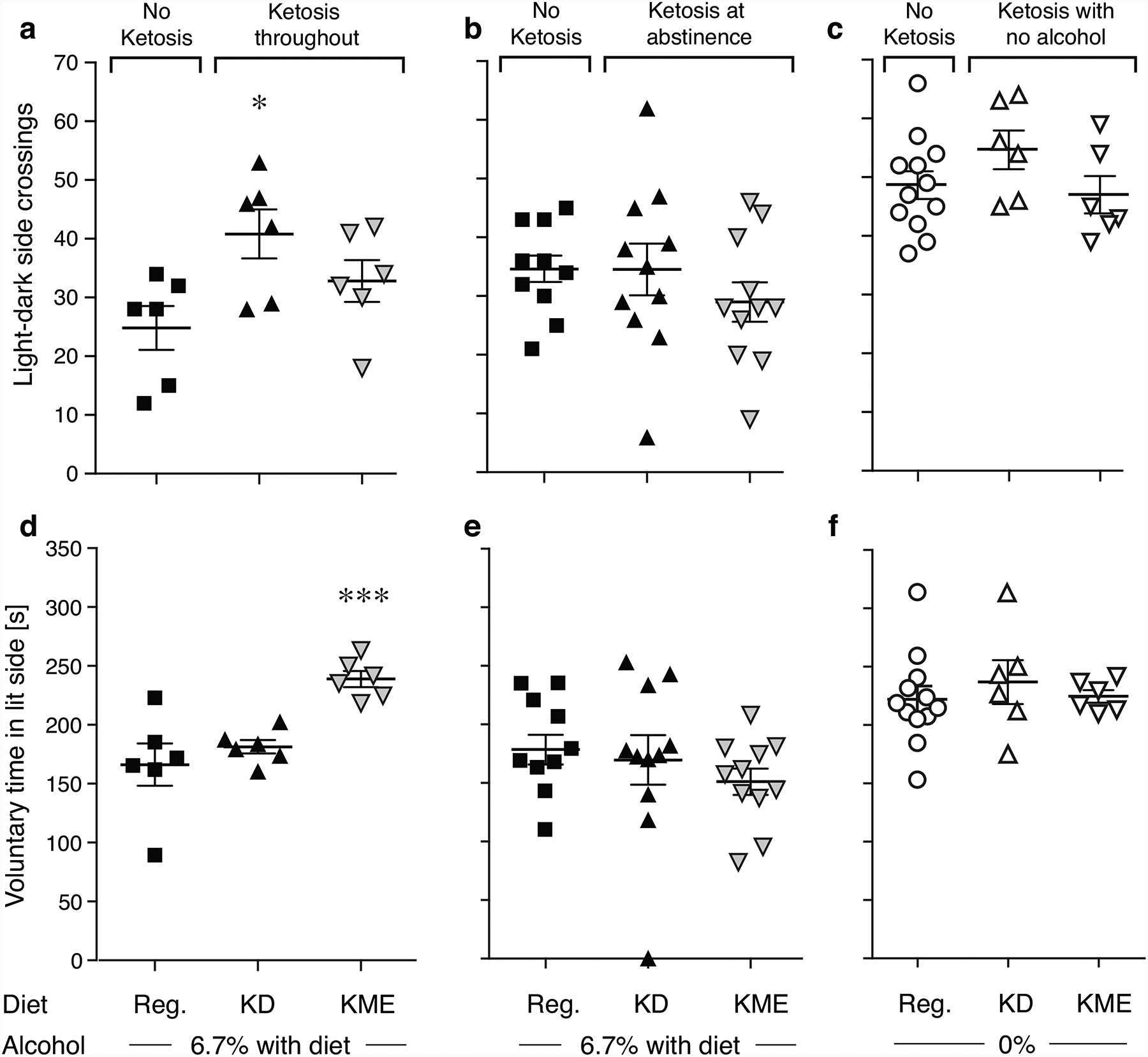
Light-dark side crossings (a-c) and voluntary time in the lit side (d-f) in the “Keto Throughout” experiment (a,d, n=6), the “Keto After” experiment (b,e, n=10–11), and no-alcohol controls (c,f, n=6–12). *p<0.05, ***p<0.001 vs. alcohol-regular diet. Data are shown as individual subjects, with bars representing group means ± s.e.m.
Blood alcohol, food intake, and bodyweight
Food intake was significantly related to time in the “Keto Throughout” experiment [F1.7,6.69=9.37, p=0.01], less so in the “Keto After” experiment (p=0.09), but in both there was an interaction of diet and time ([F18,24=2.29, p=0.03], [F12,36=2.24, p=0.03]), with no main effect of diet (Figure S3A,B). No post-hoc comparisons were significant. Bodyweight changed over time in both experiments ([F1.7,61.7=12.2, p<0.0001], [F1.4,41.6=7.40, p=0.005]; Figure S3C,D), with a diet by time interaction in both ([F9,108=17.4, p<0.0001], [F6,90=2.57, p=0.02]) and a main effect of diet in the “Keto Throughout” experiment only [F3,44=14.0, p<0.0001]. In the “Keto Throughout” experiment, all alcohol groups weighed less than no-alcohol (p<0.01); KD and KME groups also weighed less than alcohol-regular (p<0.0001). In the “Keto After” experiment, the KD group weighed more than the other groups overall (p<0.05) but this seemed to reflect a preexisting difference between the cages.
Blood alcohol levels were related to diet in the “Keto Throughout” experiment [F2,15=5.67, p=0.01], with mice consuming alcohol-KME (but not KD) diet showing lower blood alcohol levels relative to alcohol-regular diet (p=0.005; Figure 5A). In the “Keto After” experiment, in which all mice consumed the same alcohol-regular diet during alcohol exposure, alcohol levels did not differ between alcohol-exposed groups.
Fig. 5. Blood alcohol levels and control experiments.
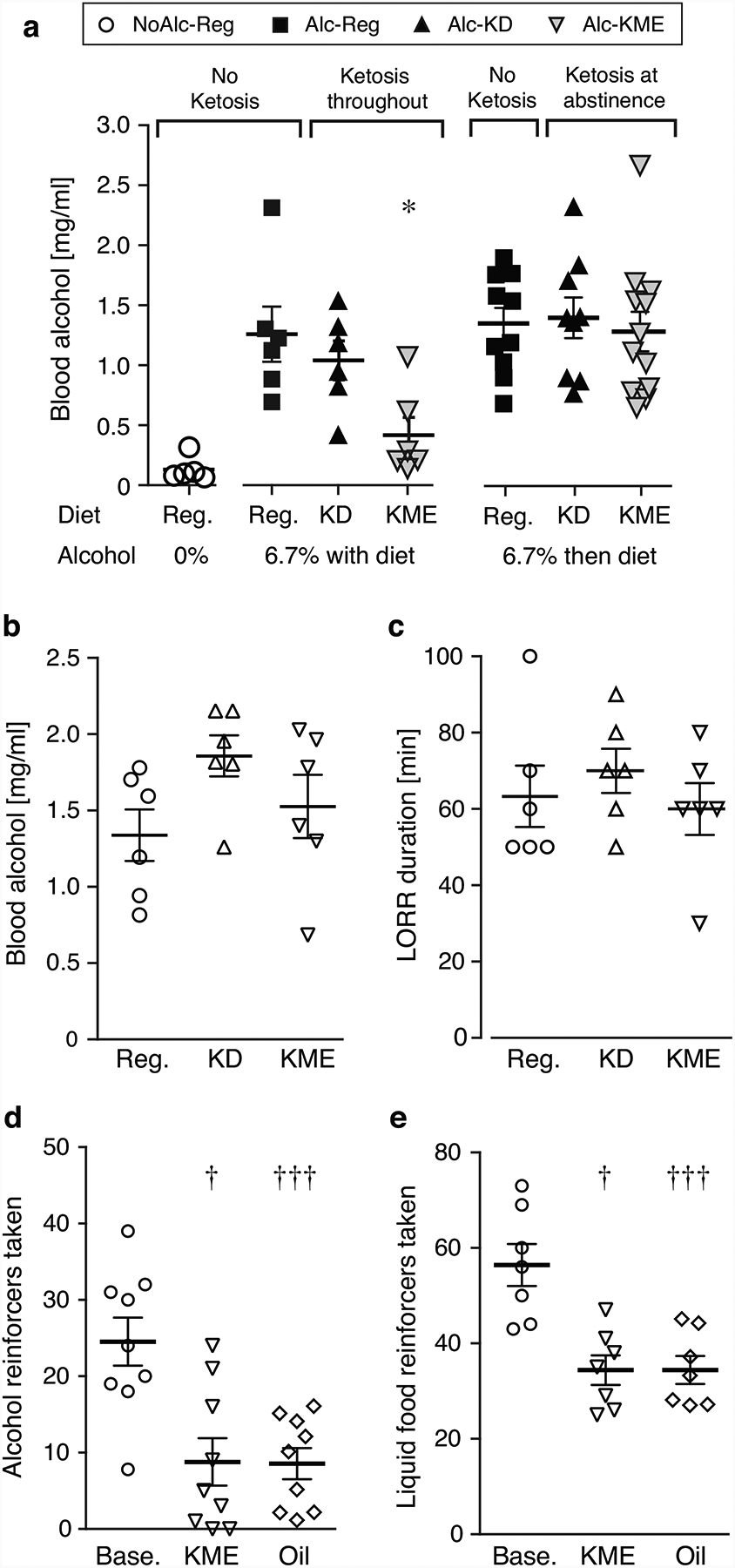
Blood alcohol in “Keto Throughout” and “Keto After” (a, n=5–6). Blood alcohol (b) and loss of righting reflex (c, LORR) after acute 3.5 g/kg alcohol administration, in mice fed no-alcohol regular, KD, or KME diet; n=6. Number of alcohol (20% in water, d) or liquid food (e) reinforcers taken per 2h-session at baseline and after intragastric administration of 3 g/kg KME or isocaloric oil control, n=7–9. Data are shown as individual subjects, with bars representing group means ± s.e.m.
Control experiment: alcohol clearance, loss of righting reflex, and voluntary alcohol and food intake
Because we observed lower blood alcohol levels in the KME group in the “Keto Throughout” experiment, we tested whether ketosis affected alcohol clearance or acute intoxication, by measuring blood alcohol and loss of righting reflex, respectively. Diet did not significantly affect blood alcohol 180 min after administration of 3.5 g/kg alcohol (Figure 5B) or loss of righting reflex duration (Figure 5C). Bodyweight remained comparable between groups over the week on experimental diets (Figure S3E). Blood BHB levels at righting reflex test start were: control 0.28±0.03, KD 1.63±0.30, KME 2.35±0.60 mM.
We then tested the hypothesis that the difference in blood alcohol in “Keto Throughout” was due to KME decreasing voluntary alcohol intake or food intake. Acute KME administration decreased both food- and alcohol-reinforced operant responding and did so to the same degree as an isocaloric oil emulsion control (Figure 5D,E). The basis behind the impact of KME and oil remains an open and interesting question to be explored in future studies.
Discussion
When test diets were given throughout, the reduction in HIC was briefer for KME than for KD, consistent with less sustained blood BHB levels by KME ingestion relative to endogenous production. While BHB levels under the KD is likely relatively stable, the measure in the KME-fed mice would have fluctuated depending on meal patterns, and harder to “capture” a good measure of. As opposed to effect duration, the effect size in the first 3 hours was comparable between KD and KME, perhaps suggesting that once a certain threshold is reached, additional BHB may not provide further HIC reduction, although there was some correlation between blood BHB level and effect. When ketosis was not induced until abstinence, only KME reduced HIC. Again, the effect lasted only a few hours, consistent with the high blood BHB level recorded after the intragastric loading dose, followed by a lower level maintained by the diet. Blood BHB increases gradually over days after onset of KD. Here, it did not surpass 1.8 mM within the HIC testing period in the “Keto After” experiment, although in all groups, total ketone levels (BHB, acetoacetate and acetone) were probably higher than recorded, as we only measured BHB. We confirmed the expected increased effect of KME relative to KD when administered at abstinence start. The effect of KME on HIC is consistent with studies using different formulations of exogenous ketones showing reduced epileptic-like brain activity or seizures (D’Agostino et al. 2013; Kovacs et al. 2017). Taken together, the present study indicates that ketone bodies alone, including exogenous, are sufficient to reduce somatic seizure-like alcohol withdrawal symptoms in rodents, including administered at abstinence start.
Both KD and KME produced effects consistent with decreased anxiety-like behavior (i.e., normalization towards behavior in no-alcohol controls), when administered throughout the experiment. The fact that voluntary time spent in the lit compartment was normalized in the KME group at a time when any effect on HIC had ceased suggests some lasting effects, or effect on the development of anxiety-like behavior. The latter would be consistent with the lack of significant effect of KD or KME when given at abstinence, perhaps suggesting, speculatively, that ketosis can reduce the development but not expression of anxiety-like behaviors. The lack of effect could also be due to too low blood ketone levels. In the previous study in rats (Dencker et al. 2018), an anxiolytic-like effect of the KD using the common anxiety test elevated plus-maze failed to reach statistical significance. This may represent a greater sensitivity of the light-dark transition test for detecting anxiogenic-like effects of alcohol withdrawal in rodents, which is consistent with an overview of the literature (Kliethermes 2005). Also, consistent with our present results using two measures in the light-dark transition test, KD and KME may produce subtly different improvements in anxiety-like behaviors that are detected using different behavioral endpoints. Alternatively, the effects of ketosis on anxiety-like behavior may not be robust or consistent. Further investigation is needed to determine whether therapeutic ketosis can provide clinically relevant improvement in anxiety or other affective symptoms of alcohol withdrawal, which are associated with risk of relapse in alcohol-dependent patients (Brower 2003; Engel et al. 2016; Heilig et al. 2010). Nevertheless, other studies have reported moderately reduced anxiety-like behaviors in rodents fed exogenous ketone supplements (Ari et al. 2016; Kashiwaya et al. 2013), lending support to the potential utility of the approach.
The brain uptake and utilization of ketone bodies increases as a function of blood ketone concentration in rodents and humans (Courchesne-Loyer et al. 2017; Hasselbalch et al. 1996; Zivin and Snarr 1972). However, transport is a saturable and inducible process with a high KM, and it has been shown to increase after KD or fasting in rodents and humans, concordant with increased brain monocarboxylic acid transporter 1 expression (Gjedde and Crone 1975; Leino et al. 2001; Morris 2005; Pifferi et al. 2011; Zivin and Snarr 1972). This likely explains why a higher blood BHB was apparently needed to achieve measurable HIC reduction in the “ketosis at abstinence” condition relative to the “ketosis throughout” condition: in the latter, diets had time to cause monocarboxylic acid transporter upregulation and increase brain ketone uptake. We also measured lower blood BHB levels in the KME-consuming mice in the last week of “Keto Throughout” relative to the earlier weeks. It is possible that increased brain uptake and utilization of ketone bodies contributed to this, but it is unclear why the effect should be restricted to KME, while ketone levels in the KD group increased over weeks. Interestingly, chronic intermittent alcohol exposure was recently shown to increase brain expression of monocarboxylic acid transporters in mice (Lindberg et al. 2019); it is not known whether effects of alcohol and ketosis on monocarboxylic acid transporter expression are additive. If also the case in humans, alcohol-induced monocarboxylic acid transporter upregulation would help brain uptake of ketones in detoxification in patients with AUD. Further, brain uptake of ketones may be higher in humans than in rodents (Morris 2005). Taken together, those findings support the feasibility of inducing therapeutic levels of ketosis in patients, but also caution that KME doses needed may be relatively high and/or frequent to produce clinically relevant effects.
The mice drinking the KME-containing alcohol diet had lower blood alcohol relative to alcohol-regular or alcohol-KD, at the time of testing. While that introduces the possibility that reduced withdrawal symptoms stem simply from lower alcohol exposure, the fact that total-time HIC in the KME group was comparable to regular-alcohol group makes it unlikely. Control studies indicate that KME did not alter alcohol metabolism, and our preliminary measurements of voluntary intake showed similar effects of KME and oil (i.e., likely attributable to satiety). Therefore, the apparent difference may be an artifact due to measuring time-varying blood alcohol levels at a single time point. It would be worthwhile testing whether KD or KME can reduce voluntary alcohol intake in alcohol-dependent rodents, and in particular whether it can reduce abstinence-induced escalation in alcohol intake. It would also be useful to repeat the studies using alcohol vapor exposure (Rogers et al. 1979) to obtain tighter control over blood alcohol levels. Oral exposure, the typical route of alcohol taking in humans, was chosen because changes in gut microbiota may underlie some effects of diet-induced ketosis (Ma et al. 2018; Newell et al. 2016; Olson et al. 2018), alcohol (Dubinkina et al. 2017; Peterson et al. 2017; Temko et al. 2017; Xiao et al. 2018), or their interaction, and it is unclear whether these factors are affected similarly by vapor exposure. Although alcohol permeates the body water and fat, it may also be that flow of alcohol directly through the digestive track has a different impact. The immediate effect of KME on HIC argues against this hypothesis, although it cannot be excluded that effects on anxiety-like behaviors were absent in the “Keto After” experiment because the intervention did not allow time for diet to modify gut microbiota. A potential limitation to be addressed in our future studies is a need for an oil placebo bolus to balance the KME bolus administration. Finally, it will be important to extend these studies to female subjects, as well as strains showing more pronounced withdrawal symptoms. These studies used male mice because a main focus was the acute somatic withdrawal signs, which have been more consistent in male mice in the literature, and acute withdrawal severity does not appear to be gender-biased in humans (Goodson et al. 2014). However, women may experience more prolonged and/or severe later withdrawal symptoms (Petit et al. 2017), and future studies should include both sexes (see (Kliethermes et al. 2004).
KD and BHB lower seizures and produce neuroprotective and anti-inflammatory effects via multiple mechanisms, including modulation of glutamate and GABA systems (Boison 2017; Calderon et al. 2017; Kashiwaya et al. 2010; Olson et al. 2018; Pflanz et al. 2019). For instance, KD was shown to increase hippocampal GABA/glutamate ratio in rodents (Calderon et al. 2017; Olson et al. 2018). Glutamate and GABA, among other systems, are altered by chronic alcohol intake and sudden abstinence, and increased extracellular glutamate is thought to be an important contributor to acute withdrawal symptoms and neurotoxicity (Fliegel et al. 2013; Hermann et al. 2012; Roberto and Varodayan 2017). Thus, ketone bodies might also affect withdrawal symptoms by balancing glutamate and/or GABA transmission. Unlike KD, fasting, acetone, or acetoacetate, BHB generally has not shown antiepileptic effects in (adult) laboratory animals, at least not as acute dosing (Simeone et al. 2018; Wood et al. 2018). In a pilot study, both KD and KME failed to attenuate morphine withdrawal in mice (data not shown), a syndrome that also features increased neuronal excitability (Brown and Russell 2004). Taken together, reduced neuronal excitability is less likely to be a major mechanism of action by which ketogenic manipulations reduce alcohol withdrawal symptoms.
Conclusions, implications, and future directions
First, we replicated our previous findings that a KD decreased alcohol withdrawal symptoms in rats, extending the findings to mice and to longer alcohol exposure. Second, the present study showed that ketone bodies alone, including exogenous BHB in a regular carbohydrate-containing diet, are sufficient to reduce alcohol withdrawal symptoms in rodents. This is important not only for understanding mechanism, but also in terms of potential clinical application. Taking a ketone product as medication is arguably easier than implementing a KD. Further, the use of KD may be particularly challenging in patients with AUD who often present with low bodyweight and poor nutritional status (Addolorato et al. 1998; Jeynes and Gibson 2017). Because heavy alcoholic consumption can itself induce ketoacidosis, a pathological state of profound ketosis (McGuire et al. 2006), it would nevertheless be important to ascertain ketosis and nutritional status in patients before any ketogenic intervention. Third, our findings indicate that diet-induced ketosis can decrease at least some withdrawal symptoms when administered at abstinence start rather than throughout alcohol exposure, e.g., when a patient with AUD is admitted for detoxification. Thrice-daily intake of KME for 28 days was safe and well tolerated in healthy volunteers (Soto-Mota et al. 2019), and we believe that therapeutic ketosis warrants evaluation as an adjunctive treatment to early detoxification in alcohol-dependent patients seeking to become abstinent. Clinical trials are currently ongoing (clinicaltrials.gov NCT03878225, NCT03255031). Whether KD or ketone supplements can also reduce later alcohol withdrawal symptoms such anxiety, sleep disorders, or other affective symptoms linked to relapse, should be evaluated further. Finally, mechanisms of action largely remain to be elucidated.
Supplementary Material
Acknowledgements
We thank Saiy Kiasari, Lisa Højkilde, and Anne-Marie Paulsen for technical assistance.
Funding and disclosure
The research was funded by the Mental Health Services - Capital Region of Denmark (AFJ), the Research Foundation Mental Health Services in the Capital Region of Denmark (MT), NIH/NIAAA grant R01AA025071 (MT), Independent Research Fund Denmark grant 0134-00044B (MT), and the Ivan Nielsen Foundation (MT). Dr. Mason was supported by NIH/NIDDK grant R01DK108283 and NIH/NIAAA grant R01AA021984. Funding agencies had no role in data interpretation or the decision to publish.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
The authors report no conflict of interest.
References
- (SAMHSA) SAaMHSA (2019) Behavioral Health Barometer: United States, Volume 5: Indicators as measured through the 2017 National Survey on Drug Use and Health and the National Survey of Substance Abuse Treatment Services. HHS Publication No SMA–19–Baro-17-US Rockville, MD: Substance Abuse and Mental Health Services Administration, 2019. [PubMed] [Google Scholar]
- (WHO) WHO (2018) Global status report on alcohol and health 2018. WHO Press, Geneva, Switzerland: Licence: CC BY-NC-SA 30 IGO ISBN 978-92-4-156563-9. [Google Scholar]
- Achanta LB, Rae CD (2017) beta-Hydroxybutyrate in the Brain: One Molecule, Multiple Mechanisms. Neurochem Res 42: 35–49. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Capristo E, Greco AV, Stefanini GF, Gasbarrini G (1998) Influence of chronic alcohol abuse on body weight and energy metabolism: is excess ethanol consumption a risk factor for obesity or malnutrition? J Intern Med 244: 387–95. [DOI] [PubMed] [Google Scholar]
- Anji A, Kumari M (2008) Supplementing the liquid alcohol diet with chow enhances alcohol intake in C57 BL/6 mice. Drug Alcohol Depend 97: 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ari C, Kovacs Z, Juhasz G, Murdun C, Goldhagen CR, Koutnik AP, Poff AM, Kesl SL, D’Agostino DP (2016) Exogenous Ketone Supplements Reduce Anxiety-Related Behavior in Sprague-Dawley and Wistar Albino Glaxo/Rijswijk Rats. Front Mol Neurosci 9: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraona E, Lieber CS (1979) Effects of ethanol on lipid metabolism. Journal of lipid research 20: 289–315. [PubMed] [Google Scholar]
- Barrick C, Connors GJ (2002) Relapse prevention and maintaining abstinence in older adults with alcohol-use disorders. Drugs Aging 19: 583–94. [DOI] [PubMed] [Google Scholar]
- Becker HC, Mulholland PJ (2014) Neurochemical mechanisms of alcohol withdrawal. Handb Clin Neurol 125: 133–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D (2017) New insights into the mechanisms of the ketogenic diet. Curr Opin Neurol 30: 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornebusch AB, Fink-Jensen A, Wortwein G, Seeley RJ, Thomsen M (2019) Glucagon-Like Peptide-1 Receptor Agonist Treatment Does Not Reduce Abuse-Related Effects of Opioid Drugs. eNeuro 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower KJ (2003) Insomnia, alcoholism and relapse. Sleep Med Rev 7: 523–39. [DOI] [PubMed] [Google Scholar]
- Brown CH, Russell JA (2004) Cellular mechanisms underlying neuronal excitability during morphine withdrawal in physical dependence: lessons from the magnocellular oxytocin system. Stress 7: 97–107. [DOI] [PubMed] [Google Scholar]
- Calderon N, Betancourt L, Hernandez L, Rada P (2017) A ketogenic diet modifies glutamate, gamma-aminobutyric acid and agmatine levels in the hippocampus of rats: A microdialysis study. Neurosci Lett 642: 158–162. [DOI] [PubMed] [Google Scholar]
- Clarke K, Tchabanenko K, Pawlosky R, Carter E, Knight NS, Murray AJ, Cochlin LE, King MT, Wong AW, Roberts A (2012a) Oral 28-day and developmental toxicity studies of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate. Regulatory Toxicology and Pharmacology 63: 196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke K, Tchabanenko K, Pawlosky R, Carter E, Knight NS, Murray AJ, Cochlin LE, King MT, Wong AW, Roberts A, Robertson J, Veech RL (2012b) Oral 28-day and developmental toxicity studies of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate. Regul Toxicol Pharmacol 63: 196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke K, Tchabanenko K, Pawlosky R, Carter E, Todd King M, Musa-Veloso K, Ho M, Roberts A, Robertson J, Vanitallie TB, Veech RL (2012c) Kinetics, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate in healthy adult subjects. Regul Toxicol Pharmacol 63: 401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JP, Haber PS, Hall WD (2016) Alcohol use disorders. Lancet 387: 988–998. [DOI] [PubMed] [Google Scholar]
- Courchesne-Loyer A, Croteau E, Castellano CA, St-Pierre V, Hennebelle M, Cunnane SC (2017) Inverse relationship between brain glucose and ketone metabolism in adults during short-term moderate dietary ketosis: A dual tracer quantitative positron emission tomography study. J Cereb Blood Flow Metab 37: 2485–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Merrill CD, Belknap JK (1991) Effects of convulsants on handling-induced convulsions in mice selected for ethanol withdrawal severity. Brain Res 550: 1–6. [DOI] [PubMed] [Google Scholar]
- Cruz NF, Lasater A, Zielke HR, Dienel GA (2005) Activation of astrocytes in brain of conscious rats during acoustic stimulation: acetate utilization in working brain. J Neurochem 92: 934–47. [DOI] [PubMed] [Google Scholar]
- D’Agostino DP, Pilla R, Held HE, Landon CS, Puchowicz M, Brunengraber H, Ari C, Arnold P, Dean JB (2013) Therapeutic ketosis with ketone ester delays central nervous system oxygen toxicity seizures in rats. Am J Physiol Regul Integr Comp Physiol 304: R829–36. [DOI] [PubMed] [Google Scholar]
- de Wit H, Metz J, Wagner N, Cooper M (1990) Behavioral and subjective effects of ethanol: relationship to cerebral metabolism using PET. Alcohol Clin Exp Res 14: 482–9. [DOI] [PubMed] [Google Scholar]
- Dencker D, Molander A, Thomsen M, Schlumberger C, Wortwein G, Weikop P, Benveniste H, Volkow ND, Fink-Jensen A (2018) Ketogenic Diet Suppresses Alcohol Withdrawal Syndrome in Rats. Alcohol Clin Exp Res 42: 270–277. [DOI] [PubMed] [Google Scholar]
- Desrochers S, Dubreuil P, Brunet J, Jette M, David F, Landau BR, Brunengraber H (1995) Metabolism of (R,S)-1,3-butanediol acetoacetate esters, potential parenteral and enteral nutrients in conscious pigs. Am J Physiol 268: E660–7. [DOI] [PubMed] [Google Scholar]
- Dubinkina VB, Tyakht AV, Odintsova VY, Yarygin KS, Kovarsky BA, Pavlenko AV, Ischenko DS, Popenko AS, Alexeev DG, Taraskina AY, Nasyrova RF, Krupitsky EM, Shalikiani NV, Bakulin IG, Shcherbakov PL, Skorodumova LO, Larin AK, Kostryukova ES, Abdulkhakov RA, Abdulkhakov SR, Malanin SY, Ismagilova RK, Grigoryeva TV, Ilina EN, Govorun VM (2017) Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome 5: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel K, Schaefer M, Stickel A, Binder H, Heinz A, Richter C (2016) The Role of Psychological Distress in Relapse Prevention of Alcohol Addiction. Can High Scores on the SCL-90-R Predict Alcohol Relapse? Alcohol Alcohol 51: 27–31. [DOI] [PubMed] [Google Scholar]
- Fee JR, Sparta DR, Knapp DJ, Breese GR, Picker MJ, Thiele TE (2004) Predictors of high ethanol consumption in RIIbeta knock-out mice: assessment of anxiety and ethanol-induced sedation. Alcohol Clin Exp Res 28: 1459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Crabbe JC (1997) Exploring alcohol withdrawal syndrome. Alcohol Health Res World 21: 149–56. [PMC free article] [PubMed] [Google Scholar]
- Fliegel S, Brand I, Spanagel R, Noori HR (2013) Ethanol-induced alterations of amino acids measured by in vivo microdialysis in rats: a meta-analysis. In Silico Pharmacol 1: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjedde A (1983) Modulation of substrate transport to the brain. Acta Neurol Scand 67: 3–25. [DOI] [PubMed] [Google Scholar]
- Gjedde A, Crone C (1975) Induction processes in blood-brain transfer of ketone bodies during starvation. Am J Physiol 229: 1165–9. [DOI] [PubMed] [Google Scholar]
- Goodson CM, Clark BJ, Douglas IS (2014) Predictors of severe alcohol withdrawal syndrome: a systematic review and meta-analysis. Alcohol Clin Exp Res 38: 2664–77. [DOI] [PubMed] [Google Scholar]
- Hascoet M, Bourin M, Nic Dhonnchadha BA (2001) The mouse light-dark paradigm: a review. Prog Neuropsychopharmacol Biol Psychiatry 25: 141–66. [DOI] [PubMed] [Google Scholar]
- Hasselbalch SG, Knudsen GM, Jakobsen J, Hageman LP, Holm S, Paulson O (1995) Blood-brain barrier permeability of glucose and ketone bodies during short-term starvation in humans. American Journal of Physiology-Endocrinology and Metabolism 268: E1161–E1166. [DOI] [PubMed] [Google Scholar]
- Hasselbalch SG, Madsen PL, Hageman LP, Olsen KS, Justesen N, Holm S, Paulson OB (1996) Changes in cerebral blood flow and carbohydrate metabolism during acute hyperketonemia. Am J Physiol 270: E746–51. [DOI] [PubMed] [Google Scholar]
- Hawkins RA, Mans AM, Davis DW (1986) Regional ketone body utilization by rat brain in starvation and diabetes. American Journal of Physiology-Endocrinology and Metabolism 250: E169–E178. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, Becker HC (2010) Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol 15: 169–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann D, Weber-Fahr W, Sartorius A, Hoerst M, Frischknecht U, Tunc-Skarka N, Perreau-Lenz S, Hansson AC, Krumm B, Kiefer F, Spanagel R, Mann K, Ende G, Sommer WH (2012) Translational magnetic resonance spectroscopy reveals excessive central glutamate levels during alcohol withdrawal in humans and rats. Biol Psychiatry 71: 1015–21. [DOI] [PubMed] [Google Scholar]
- Jeynes KD, Gibson EL (2017) The importance of nutrition in aiding recovery from substance use disorders: A review. Drug Alcohol Depend 179: 229–239. [DOI] [PubMed] [Google Scholar]
- Jiang L, Gulanski BI, De Feyter HM, Weinzimer SA, Pittman B, Guidone E, Koretski J, Harman S, Petrakis IL, Krystal JH, Mason GF (2013) Increased brain uptake and oxidation of acetate in heavy drinkers. J Clin Invest 123: 1605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Mason GF, Rothman DL, de Graaf RA, Behar KL (2011a) Cortical substrate oxidation during hyperketonemia in the fasted anesthetized rat in vivo. J Cereb Blood Flow Metab 31: 2313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Mason GF, Rothman DL, De Graaf RA, Behar KL (2011b) Cortical substrate oxidation during hyperketonemia in the fasted anesthetized rat in vivo. Journal of Cerebral Blood Flow & Metabolism 31: 2313–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Rourke SB, Patterson TL, Taylor MJ, Grant I (1998) Predictors of relapse in long-term abstinent alcoholics. J Stud Alcohol 59: 640–6. [DOI] [PubMed] [Google Scholar]
- Johansson BA (2003) Dependence on Legal Psychotropic Drugs among Alcoholics. Alcohol and Alcoholism 38: 613–618. [DOI] [PubMed] [Google Scholar]
- Kan CC, Breteler MHM, van der Ven AHGS, Timmermans MAY, Zitman FG (2001) Assessment of benzodiazepine dependence in alcohol and drug dependent outpatients-A research report. Substance use and misuse 36: 1085–1109. [DOI] [PubMed] [Google Scholar]
- Kashiwaya Y, Bergman C, Lee JH, Wan R, King MT, Mughal MR, Okun E, Clarke K, Mattson MP, Veech RL (2013) A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer’s disease. Neurobiol Aging 34: 1530–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwaya Y, Pawlosky R, Markis W, King MT, Bergman C, Srivastava S, Murray A, Clarke K, Veech RL (2010) A ketone ester diet increases brain malonyl-CoA and Uncoupling proteins 4 and 5 while decreasing food intake in the normal Wistar Rat. J Biol Chem 285: 25950–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliethermes CL (2005) Anxiety-like behaviors following chronic ethanol exposure. Neurosci Biobehav Rev 28: 837–50. [DOI] [PubMed] [Google Scholar]
- Kliethermes CL, Cronise K, Crabbe JC (2004) Anxiety-like behavior in mice in two apparatuses during withdrawal from chronic ethanol vapor inhalation. Alcohol Clin Exp Res 28: 1012–9. [DOI] [PubMed] [Google Scholar]
- Kovacs Z, D’Agostino DP, Dobolyi A, Ari C (2017) Adenosine A1 Receptor Antagonism Abolished the Anti-seizure Effects of Exogenous Ketone Supplementation in Wistar Albino Glaxo Rijswijk Rats. Front Mol Neurosci 10: 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebon V, Petersen KF, Cline GW, Shen J, Mason GF, Dufour S, Behar KL, Shulman GI, Rothman DL (2002) Astroglial contribution to brain energy metabolism in humans revealed by 13C nuclear magnetic resonance spectroscopy: elucidation of the dominant pathway for neurotransmitter glutamate repletion and measurement of astrocytic oxidative metabolism. Journal of Neuroscience 22: 1523–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefèvre A, Adler H, Lieber CS (1970) Effect of ethanol on ketone metabolism. The Journal of clinical investigation 49: 1775–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leino RL, Gerhart DZ, Duelli R, Enerson BE, Drewes LR (2001) Diet-induced ketosis increases monocarboxylate transporter (MCT1) levels in rat brain. Neurochem Int 38: 519–27. [DOI] [PubMed] [Google Scholar]
- Lindberg D, Ho AMC, Peyton L, Choi DS (2019) Chronic Ethanol Exposure Disrupts Lactate and Glucose Homeostasis and Induces Dysfunction of the Astrocyte-Neuron Lactate Shuttle in the Brain. Alcohol Clin Exp Res 43: 1838–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukivaskaya OY, Buko VU (1993) Utilization of ketone bodies by the rat liver, brain and heart in chronic alcohol intoxication. Alcohol and alcoholism 28: 431–436. [PubMed] [Google Scholar]
- Lynch LJ, Sullivan KA, Vallender EJ, Rowlett JK, Platt DM, Miller GM (2013) Trace amine associated receptor 1 modulates behavioral effects of ethanol. Subst Abuse 7: 117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Wang AC, Parikh I, Green SJ, Hoffman JD, Chlipala G, Murphy MP, Sokola BS, Bauer B, Hartz AMS, Lin AL (2018) Ketogenic diet enhances neurovascular function with altered gut microbiome in young healthy mice. Sci Rep 8: 6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis MM, Parrish BC, McCool BA (2020) Withdrawal from chronic ethanol exposure increases postsynaptic glutamate function of insular cortex projections to the rat basolateral amygdala. Neuropharmacology 172: 108129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire LC, Cruickshank AM, Munro PT (2006) Alcoholic ketoacidosis. Emergency Medicine Journal 23: 417–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metten P, Crabbe JC (2005) Alcohol withdrawal severity in inbred mouse (Mus musculus) strains. Behav Neurosci 119: 911–25. [DOI] [PubMed] [Google Scholar]
- Minchin M, Beart P (1975) Compartmentation of amino acid metabolism in the rat dorsal root ganglion; a metabolic and autoradiographic study. Brain research 83: 437–449. [DOI] [PubMed] [Google Scholar]
- Morel A, Grall-Bronnec M, Bulteau S, Chauvin-Grelier P, Gailledrat L, Pinot ML, Jolliet P, Victorri-Vigneau C (2016) Benzodiazepine dependence in subjects with alcohol use disorders: what prevalence? Expert Opinion on Drug Safety 15: 1313–1319. [DOI] [PubMed] [Google Scholar]
- Morris AA (2005) Cerebral ketone body metabolism. J Inherit Metab Dis 28: 109–21. [DOI] [PubMed] [Google Scholar]
- Murray AJ, Knight NS, Cole MA, Cochlin LE, Carter E, Tchabanenko K, Pichulik T, Gulston MK, Atherton HJ, Schroeder MA, Deacon RM, Kashiwaya Y, King MT, Pawlosky R, Rawlins JN, Tyler DJ, Griffin JL, Robertson J, Veech RL, Clarke K (2016) Novel ketone diet enhances physical and cognitive performance. FASEB J 30: 4021–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell C, Bomhof MR, Reimer RA, Hittel DS, Rho JM, Shearer J (2016) Ketogenic diet modifies the gut microbiota in a murine model of autism spectrum disorder. Mol Autism 7: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, Hsiao EY (2018) The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell 174: 497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR (2002) Accentuated decrease in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol Clin Exp Res 26: 1259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan JW, de Graaf RA, Petersen KF, Shulman GI, Hetherington HP, Rothman DL (2002) [2, 4–13C2]-β-Hydroxybutyrate metabolism in human brain. Journal of Cerebral Blood Flow & Metabolism 22: 890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlosky RJ, Kashiwaya Y, Srivastava S, King MT, Crutchfield C, Volkow N, Kunos G, Li TK, Veech RL (2010) Alterations in brain glucose utilization accompanying elevations in blood ethanol and acetate concentrations in the rat. Alcohol Clin Exp Res 34: 375–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson VL, Jury NJ, Cabrera-Rubio R, Draper LA, Crispie F, Cotter PD, Dinan TG, Holmes A, Cryan JF (2017) Drunk bugs: Chronic vapour alcohol exposure induces marked changes in the gut microbiome in mice. Behav Brain Res 323: 172–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit G, Luminet O, Cordovil de Sousa Uva M, Monhonval P, Leclercq S, Spilliaert Q, Zammit F, Maurage P, de Timary P (2017) Gender Differences in Affects and Craving in Alcohol-Dependence: A Study During Alcohol Detoxification. Alcohol Clin Exp Res 41: 421–431. [DOI] [PubMed] [Google Scholar]
- Pflanz NC, Daszkowski AW, James KA, Mihic SJ (2019) Ketone body modulation of ligand-gated ion channels. Neuropharmacology 148: 21–30. [DOI] [PubMed] [Google Scholar]
- Pifferi F, Tremblay S, Croteau E, Fortier M, Tremblay-Mercier J, Lecomte R, Cunnane SC (2011) Mild experimental ketosis increases brain uptake of 11C-acetoacetate and 18F-fluorodeoxyglucose: a dual-tracer PET imaging study in rats. Nutr Neurosci 14: 51–8. [DOI] [PubMed] [Google Scholar]
- Roberto M, Varodayan FP (2017) Synaptic targets: Chronic alcohol actions. Neuropharmacology 122: 85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Wiener SG, Bloom FE (1979) Long-term ethanol administration methods for rats: advantages of inhalation over intubation or liquid diets. Behav Neural Biol 27: 466–86. [DOI] [PubMed] [Google Scholar]
- Simeone TA, Simeone KA, Stafstrom CE, Rho JM (2018) Do ketone bodies mediate the anti-seizure effects of the ketogenic diet? Neuropharmacology 133: 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell LD, Szabo G, Tabakoff B, Hoffman PL (1996) Gangliosides reduce the development of ethanol dependence without affecting ethanol tolerance. J Pharmacol Exp Ther 279: 128–36. [PubMed] [Google Scholar]
- Soto-Mota A, Vansant H, Evans RD, Clarke K (2019) Safety and tolerability of sustained exogenous ketosis using ketone monoester drinks for 28 days in healthy adults. Regul Toxicol Pharmacol 109: 104506. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Kashiwaya Y, King MT, Baxa U, Tam J, Niu G, Chen X, Clarke K, Veech RL (2012) Mitochondrial biogenesis and increased uncoupling protein 1 in brown adipose tissue of mice fed a ketone ester diet. FASEB J 26: 2351–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate RL, Mehlman MA, Tobin RB (1971) Metabolic fate of 1,3-butanediol in the rat: conversion to - hydroxybutyrate. J Nutr 101: 1719–26. [DOI] [PubMed] [Google Scholar]
- Temko JE, Bouhlal S, Farokhnia M, Lee MR, Cryan JF, Leggio L (2017) The Microbiota, the Gut and the Brain in Eating and Alcohol Use Disorders: A ‘Menage a Trois’? Alcohol Alcohol 52: 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Caine SB (2005) Chronic intravenous drug self-administration in rats and mice. Curr Protoc Neurosci Chapter 9: Unit 9 20. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Dencker D, Wortwein G, Weikop P, Egecioglu E, Jerlhag E, Fink-Jensen A, Molander A (2017) The glucagon-like peptide 1 receptor agonist Exendin-4 decreases relapse-like drinking in socially housed mice. Pharmacol Biochem Behav 160: 14–20. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Hall FS, Uhl GR, Caine SB (2009) Dramatically decreased cocaine self-administration in dopamine but not serotonin transporter knock-out mice. J Neurosci 29: 1087–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wolf AP, Logan J, Fowler JS, Christman D, Dewey SL, Schlyer D, Burr G, Vitkun S, et al. (1990) Acute effects of ethanol on regional brain glucose metabolism and transport. Psychiatry Res 35: 39–48. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Kim SW, Wang GJ, Alexoff D, Logan J, Muench L, Shea C, Telang F, Fowler JS, Wong C, Benveniste H, Tomasi D (2013) Acute alcohol intoxication decreases glucose metabolism but increases acetate uptake in the human brain. Neuroimage 64: 277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Franceschi D, Fowler JS, Thanos PP, Maynard L, Gatley SJ, Wong C, Veech RL, Kunos G, Kai Li T (2006) Low doses of alcohol substantially decrease glucose metabolism in the human brain. Neuroimage 29: 295–301. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Hitzemann R, Fowler JS, Wolf AP, Pappas N, Biegon A, Dewey SL (1993) Decreased cerebral response to inhibitory neurotransmission in alcoholics. Am J Psychiatry 150: 417–22. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Shokri Kojori E, Fowler JS, Benveniste H, Tomasi D (2015) Alcohol decreases baseline brain glucose metabolism more in heavy drinkers than controls but has no effect on stimulation-induced metabolic increases. J Neurosci 35: 3248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votaw VR, Witkiewitz K, Valeri L, Bogunovic O, McHugh RK (2019) Nonmedical prescription sedative/tranquilizer use in alcohol and opioid use disorders. Addictive Behaviors 88: 48–55. [DOI] [PubMed] [Google Scholar]
- Vranjkovic O, Winkler G, Winder DG (2018) Ketamine administration during a critical period after forced ethanol abstinence inhibits the development of time-dependent affective disturbances. Neuropsychopharmacology 43: 1915–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waniewski RA, Martin DL (1998) Preferential utilization of acetate by astrocytes is attributable to transport. Journal of Neuroscience 18: 5225–5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood TR, Stubbs BJ, Juul SE (2018) Exogenous Ketone Bodies as Promising Neuroprotective Agents for Developmental Brain Injury. Dev Neurosci 40: 451–462. [DOI] [PubMed] [Google Scholar]
- Xiao HW, Ge C, Feng GX, Li Y, Luo D, Dong JL, Li H, Wang H, Cui M, Fan SJ (2018) Gut microbiota modulates alcohol withdrawal-induced anxiety in mice. Toxicol Lett 287: 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivin JA, Snarr JF (1972) Glucose and D(−)-3-hydroxybutyrate uptake by isolated perfused rat brain. J Appl Physiol 32: 664–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


