Abstract
Summary
Background
Chronic wounds, a common morbidity in recessive dystrophic epidermolysis bullosa (RDEB), lack definitive therapies.
Objective
To assess allogeneic epidermal skin grafts wound healing and durability over time.
Methods
In a prospective, open-label clinical trial for post-allogeneic hematopoietic cell transplantation (post-alloHCT) RDEB patients, up to 9 chronic wounds per patient were grafted over 1 year. Epidermal grafts measuring 5 cm2 were obtained from related alloHCT donors in the outpatient setting using the CELLUTOME™ Epidermal Harvesting System. Wounds were photographed and symptom inventories completed at baseline, 6, 12, and 52 weeks after grafting.
Results
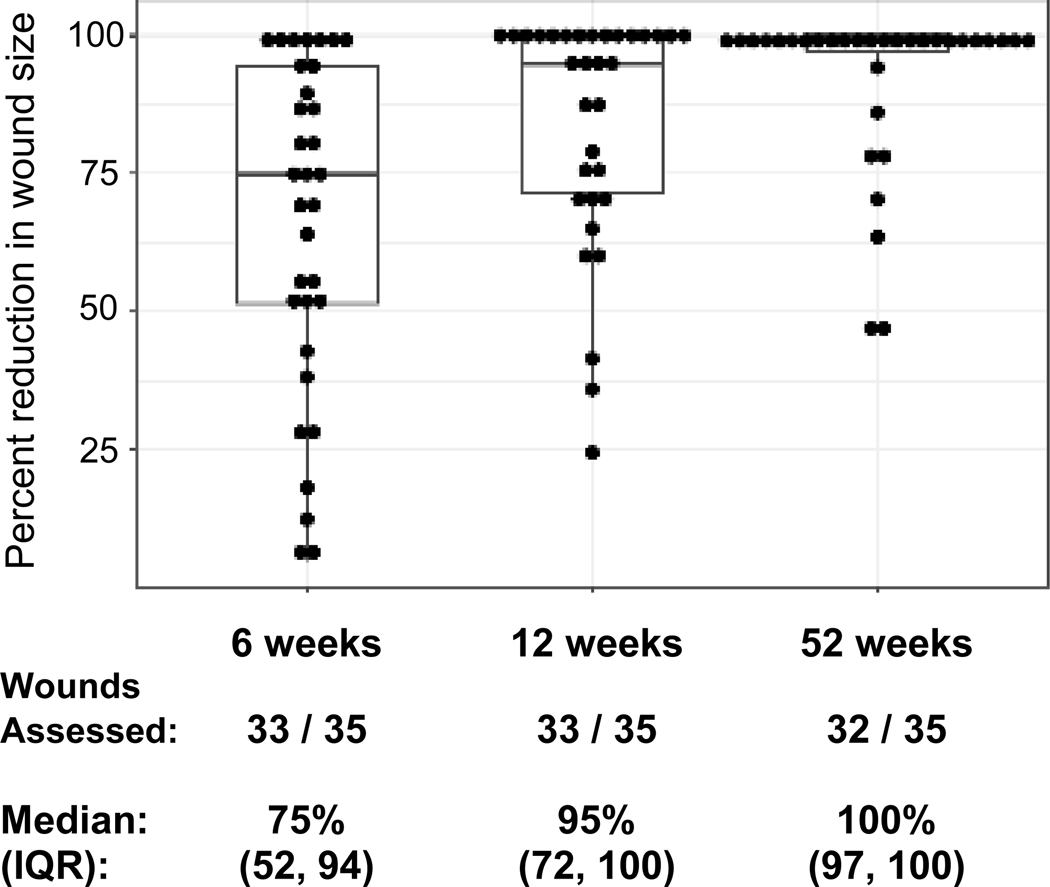
Between August 2016 and January 2019, eight RDEB patients received a total of 35 epidermal allografts at a median of 1157 days (range 548–2884) post-alloHCT. The median (IQR) percent reduction in wound surface area was 75% (52–94), 95% (72–100), and 100% (97–100), at 6, 12, and 52 weeks post-graft, respectively, each significantly reduced from baseline (P<0.0001). Donor harvest sites healed quickly without scarring. Biopsy evaluation at 1 year of an epidermal allograft site revealed wild-type type VII collagen (immunofluorescence), anchoring fibrils (electron microscopy), and full-thickness skin whole DNA donor chimerism of 42% (compared to 16% in concurrently biopsied native skin). This strategy subsequently supported RDEB pseudosyndactyly release.
Conclusions
The immune tolerance established by alloHCT supports successful adoptive transfer of donor epidermal grafts. Persistence of donor grafts in a single patient beyond 1 year and observed migration of donor-grafted cells into adjacent wound suggest epidermal allografts include non-terminally differentiated cells and/or trigger recruitment of donor bone marrow-derived cells to mediate wound healing.
Trial registration
ClinicalTrials.gov Identifier: NCT02670837
Sustained transplantation of skin grafts from one unrelated individual to another without systemic immune suppression has remained an unfulfilled aspiration. Studies of immune-mediated tissue tolerance stemmed from the field of animal husbandry in the mid-1900s1,2 and have evolved into modern clinical trials of donor hematopoietic mixed- or micro-chimerism to support human allogeneic solid organ transplantation3–5. In the interim, investigations of allogeneic hematopoietic cell transplantation (alloHCT) and the contributions of the donor inoculum revealed that, in addition to providing cellular foundations for immune tolerance, some bone marrow-derived stem and progenitor populations are capable of engrafting in the skin and contributing directly to tissue repair6–9.
Chronic, non-healing wounds contribute greatly to the morbidity of recessive dystrophic epidermolysis bullosa (RDEB), an inherited blistering disorder resulting from biallelic mutations in COL7A1 encoding type VII collagen (C7). C7 polymerizes to form the anchoring fibrils responsible for binding together the dermal-epidermal junction (DEJ). With absent, reduced, or abnormal C7, the integrity of the DEJ is compromised, and minor trauma results in mechanical separation, blister formation, and wounding. In addition to causing pain and itching, skin wounds compromise the barrier function critical for thermoregulation and protection from infection, as well as lead to functionally debilitating scarring. Chronic wounds are also common sites for development of lethal cutaneous squamous cell carcinoma10. Despite their significance, current management of chronic wounds in EB is limited to cleansing and bandaging.
Various methods of skin grafting to heal chronic wounds have been reported, each with limitations, such as invasiveness, size limitation, and uneven effectiveness11. Isolated epidermal grafting evolved from “pinch grafting” in the 1800s12 to suction blister methods in the 1960s13, to a variety of syringe or suction-cup-based techniques14. While effective, such epidermal grafting methods are labor- and time-intensive operating room procedures, limiting broad use. Recent use of a minimally invasive, automated device to harvest epidermal micrografts (CELLUTOME™, Acelity, San Antonio, TX) has reinvigorated interest in epidermal grafting. The CELLUTOME device provides uniform negative pressure suction and heat to efficiently separate epidermis from dermis without need for anesthesia or surgical skills15. Results with autologous epidermal grafting are excellent, but do not translate to the RDEB patient population due to the intrinsic DEJ defect16. An exception is autografts of revertant mosaic skin patches, where acquired gene mutations restore DEJ protein production; however, skin for grafting is often limited in area and recurrence of EB pathology is high17,18.
Grafting of healthy allogeneic skin provides a transient barrier function19 and stimulates endogenous wound-healing cytokine production14, but in the absence of donor immune tolerance, such grafts are ultimately rejected. Allogeneic skin grafts have only shown sustained benefit in the setting of established bi-directional immune tolerance. Following alloHCT, skin allografts from the same donor have been successfully used to heal cutaneous defects from necrotizing skin infections or graft-versus-host disease (n=20 case reviews compiled/reviewed) 20.
We hypothesized that prior alloHCT would support successful epidermal allografting of chronic RDEB wounds. Our primary endpoint was reduction in wound surface area measured by photography. Secondary objectives included assessments of longevity of grafted skin, subjective quality-of-life measures, and healing of donor harvest sites. We herein describe a targeted boost to RDEB skin function by serial allogeneic bone marrow transplantation and skin grafting, achieved by leveraging established donor immune tolerance and the potential for epithelial recruitment of pluripotent donor stem and progenitor cells. With minimally invasive epidermal grafting, donor skin not only expanded into adjacent wound space but persisted beyond the typical lifespan of the epidermal layer and beyond one year of follow-up. This unique combination of alloHCT and epidermal allografting warrants further investigation.
METHODS
Patients
Eligible patients were at least six months post-alloHCT for treatment of generalized, severe RDEB (primary diagnosis by mutational analysis, characteristic immunofluorescence, and electron microscopy skin biopsy findings) with at least one chronic wound and an eligible alloHCT donor. Patient had to demonstrate stable donor chimerism within 21 days of skin grafting, be off immune suppressive therapy, and without pre-alloHCT history of immune-mediated cytopenias. Stable donor chimerism was defined as peripheral blood cells of donor origin equal to or exceeding prior values and ≥5%. An eligible chronic wound was visibly free of infection and present for at least six weeks. An alloHCT donor was eligible to be an epidermal graft donor if >2 years of age, in good general health, and with negative blood tests for communicable infectious diseases including hepatitis B and C, HIV, and HTLV1/2 within 30 days of grafting. Voluntary consent (and assent for minors) was obtained from all participating graft recipients and donors on an Institutional Review Board-approved informed consent in accordance with the Declaration of Helsinki. Study is registered as NCT02670837 at ClinicalTrials.gov.
Trial procedures
In a prospective, open-label clinical trial between August 2016 and January 2019, up to nine chronic wounds were grafted with epidermal skin grafts measuring 5 cm2, obtained from the patient’s original alloHCT donor in the outpatient setting using CELLUTOME (Figure 1). A maximum of three grafting sessions with up to three wounds each was permitted within one year of enrollment, each session approximately 12 weeks apart. The harvest procedure has been previously described21, but briefly, up to three donor graft sites were selected (inner thigh) and cleaned with 70% isopropyl alcohol. The CELLUTOME sterile vacuum head was attached and engaged, providing −400 to −500 mmHg negative pressure and 37–41°C heat, forming 128 epidermal micrografts spaced 2 mm apart over the 5×5 cm2 array. After the epidermal micrografts were visually mature (30–40 minutes), the vacuum head was removed, a 2”×3” (SI 5.08×7.62 cm) non-adherent silicone dressing applied (APAPTIC™; Acelity), and the harvester’s internal blade released. Concurrently, the patient’s chronic wound graft sites were undressed and cleaned per family routine, but not otherwise debrided or prepped. The allogeneic donor epidermal micrografts were then applied as a living bandage to the chronic wound, secured with silicone tape, and left as undisturbed as possible for the following two weeks.
Figure 1. AlloHCT establishes immune tolerance for donor epidermal graft.
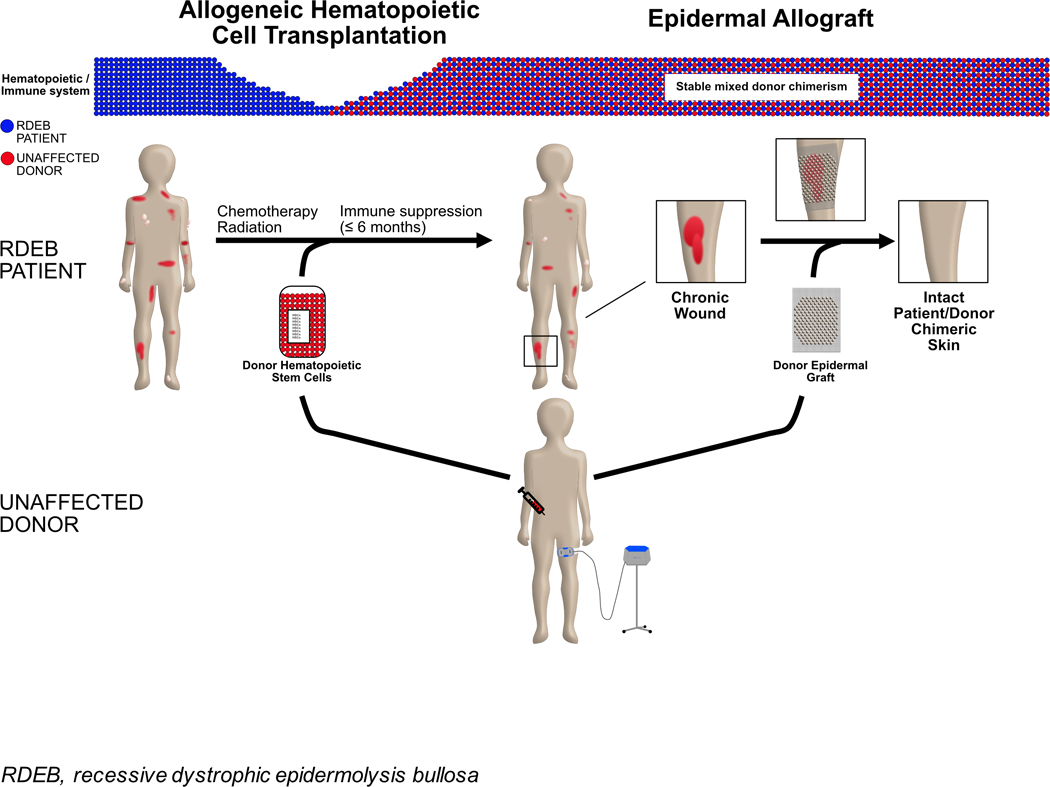
With alloHCT, the RDEB patient immune system transitions to that of the unaffected donor. This establishes immune tolerance allowing for subsequent skin grafts from the same donor to heal chronic wounds of the patient with RDEB.
Outcomes
Wounds were photographed and surface area assessed at baseline (before and after grafting), 6 (±1), 12 (±1), and 52 (±12) weeks. When possible, follow-up photography was completed in clinic by staff (S.R.), alternatively sent electronically from home. Wound surface area was outlined at baseline. While reduction of 3-dimensional body surfaces to 2-dimensional photographs made determining absolute wound size challenging, changes in wound size over time were assessed using ImageJ (NIH; measurements completed by C.L.E.).
Care providers (S.R. and C.L.E) and parents/guardians additionally completed their sections of the Instrument for Scoring Clinical Outcomes of Research for EB (iscorEB) 22, a validated measure of dynamic wound healing including quality-of-life and disease-symptom measures such as pain, skin pain, and pruritus at these three timepoints. Up to two additional sessions of epidermal grafting were permitted within one year of trial enrollment, at ≥12 week intervals. Donor sites were similarly photographed with follow-up questions about scarring, pain or pigmentation changes at the same intervals.
To better interrogate the composition of a healed wound after epidermal allografting, the protocol was amended to permit skin biopsies. To date, one patient (Patient 4) had 3–4 mm punch skin biopsies of a right forearm epidermal graft (one year post-grafting) and native thigh skin (two years post-alloHCT) completed concurrently for assessment of C7 expression by immunofluorescence (IF, using 4 different C7 stains and 2 controls), anchoring fibrils and C7 presence by immune electron microscopy (IEM), and full-thickness DNA donor chimerism (as described in Ebens CL, et al. 201923).
Case report of epidermal allografting in pseudosyndactyly release
Separate from this clinical trial, CELLUTOME epidermal allografting was used to close skin defects created on the palms and phalanges when a post-alloHCT RDEB patient underwent concurrent bilateral pseudosyndactyly release (Patient 6 in the clinical trial cohort). Silicone dressings with epidermal micrografts attached were cut and applied over open dermis and sutured in place.
Statistical analysis
Statistical analysis was on observed data and primarily descriptive, with variable distributions summarized using medians and interquartile ranges. Differences in wound reduction by wound location were assessed using Kruskal-Wallis tests. Trial size was determined by the number of eligible patients who agreed to participate over a three-year period. Analysis was performed using R software, version 3.4.
Reduction in wound size was analyzed per wound, not per patient, as wounds from the same patient could have different responses. This assumption was mostly true in our data with intraclass correlation coefficients for wound reduction of 0.28 (week 6) and 0.26 (week 12), indicating considerable within-patient variability. At week 52, intraclass correlation increased to 0.77; however, by that time a majority of wounds were 100% reduced.
RESULTS
Patient characteristics
Eight RDEB patients received a total of 35 epidermal allografts, with initial grafting at a median of 1157 days (range 267–3111) post-alloHCT (Tables 1 and S1). Wound distribution included head and neck (n=7), trunk (n=13), and extremities (n=15). Peripheral-blood donor chimerism at the time of epidermal allografting exceeded the eligibility threshold of 5% in both lymphoid and myeloid compartments for all patients. AlloHCT and subsequent epidermal allograft donors were human leukocyte antigen (HLA)-matched siblings in 6 of 8, with the remaining two being haploidentical (one sibling, one maternal).
TABLE 1.
Epidermal allograft recipient demographics upon enrollment
| Patient | Sex | Donor* | Donor chimerism (%): | Age (years) | Days post-alloHCT | ||
|---|---|---|---|---|---|---|---|
| Lymphoid PB (CD3+) | Myeloid PB (CD33+) | Skin | |||||
| 1 | M | MSD | 100 | 100 | 16 | 11.4 | 2236 |
| 2 | M | MSD | 34 | 43 | 5 | 2.5 | 743 |
| 3 | M | MSD | 35 | 35 | 17 | 6.6 | 1207 |
| 4 | M | Maternal haplo | 100 | 100 | 18 | 4.4 | 245 |
| 5 | F | Sibling haplo | 100 | 100 | 18 | 9.5 | 483 |
| 6 | F | MSD | 32 | 24 | 13 | 7.9 | 1107 |
| 7 | F | MSD | 100 | 100 | ND | 15.4 | 3111 |
| 8 | F | MSD | 100 | 100 | ND | 23 | 3100 |
| Median: | 100% | 100% | 16.5% | 8.7 years | 1157 days | ||
| (IQR): | (34–100) | (37–100) | (11–18) | (5–14.4) | (548–2884) | ||
AlloHCT and epidermal graft
RDEB, recessive dystrophic epidermolysis bullosa; alloHCT, allogeneic hematopoietic cell transplantation; M, male; MSD, human leukocyte antigen-matched sibling donor; haplo, human leukocyte antigen haploidentical donor; F, female; ND, not done.
Epidermal allograft induced wound closure
The median (IQR) percent reduction in wound surface area achieved was 75% (52–94), 95% (25–100), and 100% (97–100), at 6, 12, and 52 weeks, respectively; each timepoint reduced compared to baseline (P<0.0001; Figure 2b). Figure 2a demonstrates wound closure measurement over time in a selected patient with a large chronic back wound (Patient 5 from Table 1; all wounds in Figure S1). Exploratory analyses of factors impacting wound closure at 6, 12, and 52 weeks revealed no association with time since alloHCT (data not shown), but trend toward greater percentage reduction in head and neck wounds (median 94.6%, 100%) compared to trunk (median 74.9%, 93.9%) or extremity (median 55.7%, 79%) wounds at 6 and 12 weeks, respectively (P=0.14, P=0.04). Given the small number of observations, these findings are not conclusive.
Figure 2. Wound closure with epidermal allografting over one year.
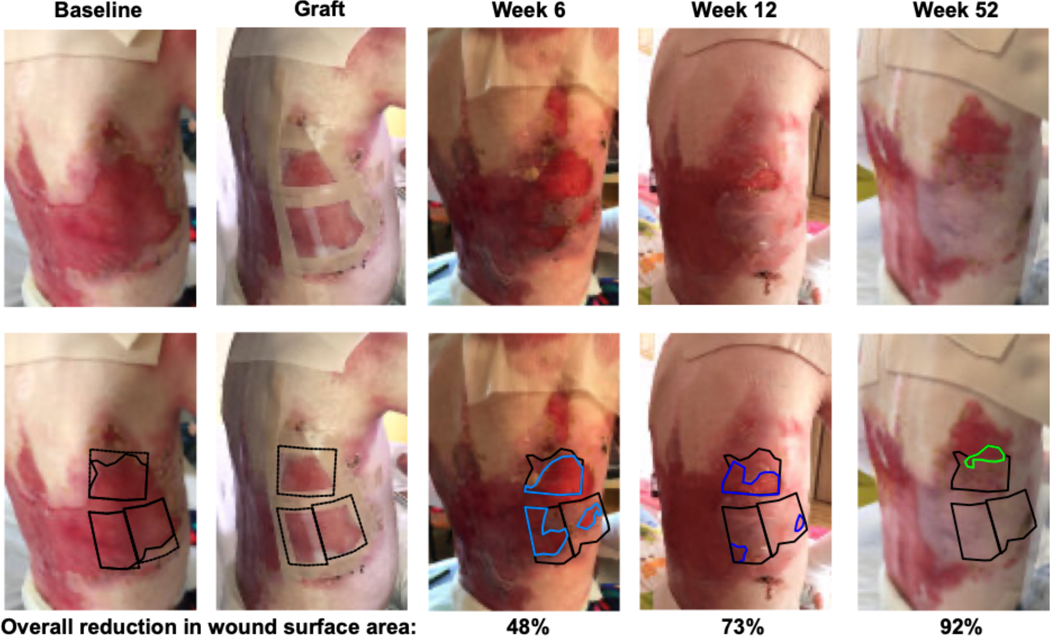
Figure 2a shows a selected example of epidermal allograft placement and wound closure (large back wound of Patient 5), with quantification of wound surface area on replicate images. Black outlines the epidermal graft at baseline/graft placement and open wound available for wound closure beneath the epidermal allograft at all timepoints. Residual wound is outlined in light blue at week 6, dark blue at week 12 and green at week 52. For individual wounds, the percent reduction in wound size over one year following epidermal allografting is displayed in Figure 2b (median and IQR boxplot), statistically significantly reduced at all timepoints compared to baseline, p<0.0001.
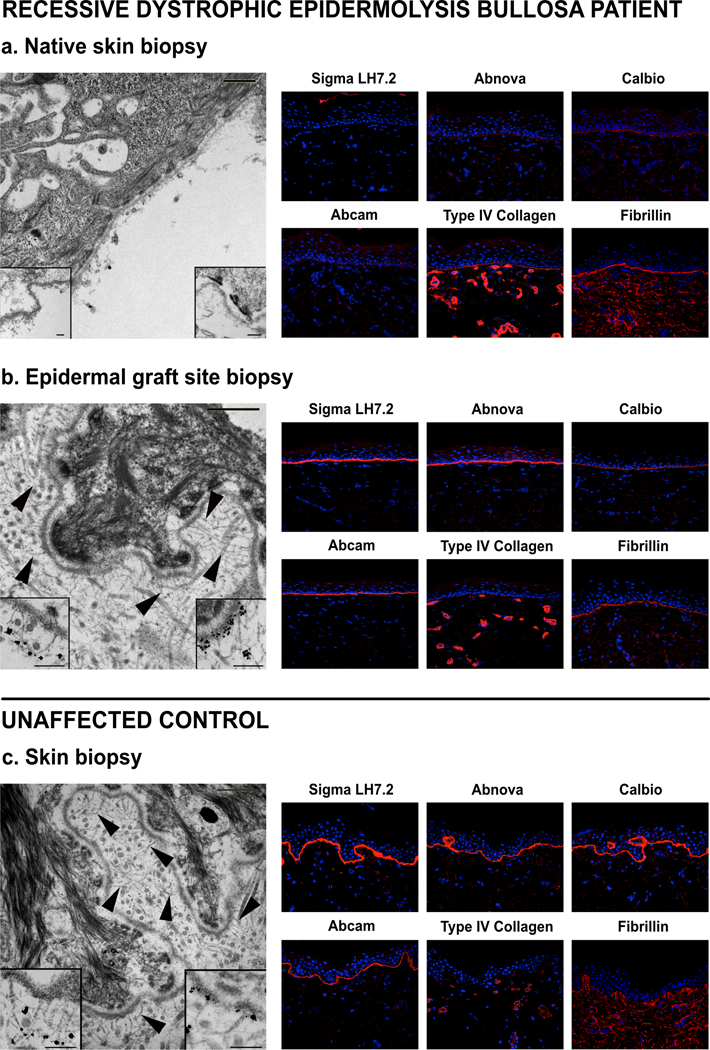
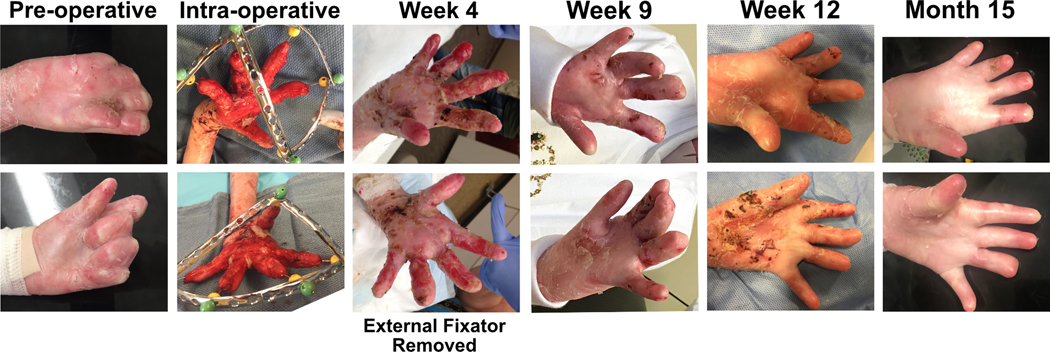
Patient 4 underwent biopsy evaluation of a right forearm epidermal graft at one year, revealing wild-type C7 expression by immunofluorescence, normal C7 and anchoring fibrils by immune electron microscopy, and full-thickness skin whole-DNA donor chimerism of 42% (compared to 16% in concurrently biopsied native skin; Figure 3). Application of epidermal allografts to the palms and proximal phalanges (not thumbs) of Patient 5 to aid in closing epidermal defects created by concurrent bilateral pseudosyndactyly release showed excellent early take and stability over time, demonstrated to 15 months post-procedure in Figure 4.
Figure 3. Epidermal allograft microarchitecture resembles control skin with intact C7 and anchoring fibrils at 1-year post-epidermal allograft (two years post-alloHCT).
Skin biopsies of Patient 4 native skin (a) and epidermal graft site (b), compared to an unaffected control (c). Immune electron microscopy images on the left. Main images without immune label, arrows highlight anchoring fibrils, scale bar = 500 nm. Inserts with C7 immunolabels, left lower insert with LH24, right lower insert with mAb185, scale bar = 200 nm. Immunofluorescence images on the right with four C7 antibodies and two control antibodies, Type IV collagen and fibrillin, shown in red and DAPI nuclear staining in blue (40× magnification).
Figure 4. Epidermal allografts aid bilateral pseudosyndactyly release.
Serial photographs of the palmar surfaces of Patient 5’s hands following use of epidermal allografts for closure of skin defects during pseudosyndactyly release.
Systemic clinical impact
Systemic clinical status did not change over the course of the trial. The median overall iscoreEB scores were 32, 35, and 33, at baseline, week 6, and week 12, respectively, with an insignificant increase to 41 at week 52. The variance of scores also increased over time, with interquartile range rising from 5 at baseline to 19 at week 52. Subscale scores for pain, skin pain, and pruritus followed a similar pattern.
Safety
Donor harvest sites healed quickly with no scarring (mild transient hypopigmentation was noted in 4 of 8; all resolved by 52-week follow-up). While one patient developed subsequent metastatic cutaneous squamous cell carcinoma, no lesions were in areas of epidermal allografting.
DISCUSSION
Epidermal grafting of chronic RDEB wounds with alloHCT donor tissue was simple, safe, and successful. The serial transplantation of a hematopoietic system followed by epidermis provided a lasting improvement to RDEB skin function. Systemic impact on quality of life iscorEB measures was unremarkable, likely secondary to the overall limited body surface area addressed in this trial. Study limitations included the small size of epidermal grafts, which required repeated sessions to adequately cover larger wounds, and lack of comparison control chronic wounds. Broader clinical implementation is limited by the risks associated with prior alloHCT to establish donor immune tolerance. The application of alloHCT donor epidermal grafts to repair skin defects introduced in pseudosyndactyly release is a promising extension of this work, allowing delivery of healthy epidermis to the most frequently scarred region of the body in RDEB, restoring critical hand function with greater and longer lasting integrity compared to autografts. Long-term follow-up is necessary to assess the impact of epidermal allografting on preventing pseudosyndactyly as well as squamous cell carcinoma risk.
Sustained acceptance of non-self, or allogeneic, tissue grafts without need for chronic immune suppression requires bi-directional immune tolerance. The earliest literature highlighting the critical role of the hematopoietic system in such transplant immunology appeared in the mid-twentieth century. Geneticist Ray Owen described detectable contribution of two individuals to hematopoiesis, or mixed chimerism, in non-identical twin cattle during gestation1. Nobel prize winning zoologist Peter Medawar then demonstrated acceptance of skin grafts between such non-identical twin mice2. With discovery of human leukocyte antigens (HLA), and improved understanding of central and peripheral immune tolerance, the field of alloHCT rapidly expanded beyond fully HLA-matched sibling to HLA-mismatched alternative donor alloHCT for both malignant and non-malignant diseases. Further, establishment of mixed donor chimerism proved possible with less toxic conditioning chemotherapy and/or radiation regimens, achieved therapeutic goals in many diseases, and showed stability over time without graft-versus-host disease or graft rejection despite discontinuation of immune suppression. Using pre-clinical canine alloHCT models, Rainer Storb and colleagues demonstrated that establishing donor hematopoietic chimerism, even just transiently, allows for either subsequent24 or concurrent25 acceptance of donor vascularized skin allografts.
Leveraging knowledge of immune tolerance and mixed hematopoietic donor chimerism, several leading solid organ transplant programs have explored combination hematopoietic/immune and kidney transplants. By inducing donor immune tolerance, researchers permit long-term survival of transplanted kidneys without need for life-long pharmacologic immune suppression, demonstrating success in both HLA-matched and mismatched donor-recipient pairs3–5,26–28. This work parallels our combined alloHCT/epidermal grafting approach and may be further expanded to other tissues in the burgeoning field of regenerative medicine.
Intriguing characteristics of the epidermal allografts in this study—including horizontal outgrowth (as visualized in Patient 5, Figure 2a), persistence beyond the expected 48-day epidermal lifespan, and incorporation of recipient dermal adnexa—reveal a skin graft functionally superior to any previously reported. The discontinuous array of epidermal micrografts seems to support concurrent growth of donor and recipient skin structures, as patients subjectively report reduced or absent blistering at grafted sites, normal temperature regulation, and tactile sensation equivalent to native skin. However, we suspect the hybrid skin includes more than a simple combination of donor epidermis and recipient dermis. Biopsy of a graft site on Patient 4 after one year yielded a much higher-than-expected donor contribution of 42%, nearly 3× a concurrent native skin biopsy.
An extensive body of research describes contribution of bone marrow-derived epithelial cells to normal tissue repair29, cutaneous wound healing6, and epithelial neoplasms in mice30 and post-alloHCT humans31. Collectively, these data describe environments permissive to incorporation of bone-marrow derived epithelial cells as a result of acute inflammatory or chronic damage signals. We suspect our epidermal allograft donor contribution over time will include both donor cells applied with the graft as well as donor bone marrow-derived epithelial cells recruited to support healing. The epidermal allograft is avascular and, upon application, likely creates a hypoxic signaling gradient, recruiting recipient cells to support epithelial regeneration. High-mobility group box 1 (HMGB1), a non-histone nuclear protein released from necrotic keratinocytes, is the only described serum biomarker correlating positively with RDEB disease severity32. Functional analysis in mice by Tamai and colleagues showed HMGB1 mobilized bone marrow-derived platelet-derived growth factor receptor alpha-positive mesenchymal cells to sites of skin injury or inflammation via upregulation of chemokine receptor CXCR433. Upon arrival, these mesenchymal cells promoted tissue repair, gave rise to keratinocytes in damaged skin, and attenuated inflammation in adjacent skin34. Unique to our post-alloHCT patient group, the mesenchymal cells recruited to support epidermal regeneration may be of donor origin.
Additionally, epidermal grafts may include non-terminally differentiated cells contributing to graft outgrowth and persistence. The epithelium is comprised of layers of keratinocytes, with differing self-renewal and replicative potentials, including epidermal stem cells that give rise to holoclones in culture35,36. Three stem cell niches in the skin have been characterized by lineage tracing and flow cytometry in mice, including cells at hair follicle bulge, the upper pilosebaceous gland, and the basal layer of the interfollicular epidermis37. When genetically corrected, autografts derived from holoclones have been shown to sustain functional epidermis in junctional epidermolysis bullosa38–40. Presence of such progenitor cell populations in an epidermal allograft is feasible and an area of active investigation.
One substantial limitation of this study is lack of control ungrafted or alternatively grafted chronic wounds. Many enrolled patients had all chronic wounds addresses in one grafting session and preferred not to leave one ungrafted for comparison. Grafting of all available chronic wounds was permitted to optimize the clinical impact. Alternative grafting methods were considered but deferred given the small cohort and few wounds available for assessment. While non-HLA-matched cadaveric grafts would be rejected within 1–2 weeks19, we could anticipate some improvement in wound healing with the combination of barrier function of the graft as well as induction of wound healing cytokines as described above. Focusing on clinical outcomes and desire to maintain the integrity of the grafted areas, we failed to initially incorporate skin biopsies for IF, IEM, and donor chimerism analyses, to date completed for only 1 subject followed amendment of the protocol. Finally, while the technical feasibility of the epidermal allografting procedure is far superior to other grafting approaches – with no need for wound-site preparation, anesthesia, immobilization or pain control, as well as no residual scar or wound closure difficulty for the donor – establishing immune tolerance in advance of the procedure, here with alloHCT, is a barrier to broader use of this exact approach.
Epidermal allografting in RDEB provided both excellent functional wound repair and intriguing evidence of stimulated local epidermal proliferation. Future directions include investigations for non-terminally differentiated cell components of the epidermal graft and local regulatory factors or cytokines to optimize graft take and persistence, investigation of donor-versus-recipient components in the full-thickness skin at epidermal graft sites, and investigation of alternative methods of establishing immune tolerance, such as micro-tolerance induction, to expand eligibility of epidermal allografting beyond alloHCT recipients.
Supplementary Material
What’s already known about this topic?
Chronic non-healing wounds cause great morbidity in recessive dystrophic epidermolysis bullosa (RDEB) including pain, itch, loss of local barrier/thermoregulatory skin function, and serve as a nidus for infection and squamous cell carcinoma development
The standard of care for chronic RDEB wounds is supportive, with local cleansing, emollient and dressing application
Skin grafts from healthy allogeneic (non-self) donors provide only transient benefit as genetic difference drive immune rejection of transplanted tissue
What does this study add?
Demonstrates that successful allogeneic hematopoietic cell transplant establishes immune tolerance for secondary tissue grafts from the same donor, herein demonstrated with skin grafts
Persistence of epidermal allografts beyond the keratinocyte lifespan and outgrowth into adjacent wound suggest inclusion of non-terminally differentiated cells in the graft
Epidermal allografting can be completed in a minimally invasive manner in the outpatient setting, with tolerability to the donor allowing for repeat sessions over time
Acknowledgments
Funding: This research was conducted with funding support from EB Charities, the Richard M. Schulze Family Foundation, the Zona Family Foundation for EB Research, and NIH grant P30 CA77598, the latter supporting the Biostatistics and Bioinformatics Core shared resource of the Masonic Cancer Center, University of Minnesota, and by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR002494 and KL2TR002492. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funding sources were not involved in study design, data collection, data analysis, manuscript preparation or publication decisions.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Owen RD. Immunogenetic Consequences of Vascular Anastomoses between Bovine Twins. Science 1945;102:400–1. [DOI] [PubMed] [Google Scholar]
- 2.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature 1953;172:603–6. [DOI] [PubMed] [Google Scholar]
- 3.Millan MT, Shizuru JA, Hoffmann P, et al. Mixed chimerism and immunosuppressive drug withdrawal after HLA-mismatched kidney and hematopoietic progenitor transplantation. Transplantation 2002;73:1386–91. [DOI] [PubMed] [Google Scholar]
- 4.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. The New England journal of medicine 2008;358:353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leventhal J, Abecassis M, Miller J, et al. Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Sci Transl Med 2012;4:124ra28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borue X, Lee S, Grove J, et al. Bone marrow-derived cells contribute to epithelial engraftment during wound healing. Am J Pathol 2004;165:1767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Arnam JS, Herzog E, Grove J, et al. Engraftment of bone marrow-derived epithelial cells. Stem Cell Rev 2005;1:21–7. [DOI] [PubMed] [Google Scholar]
- 8.Murata H, Janin A, Leboeuf C, et al. Donor-derived cells and human graft-versus-host disease of the skin. Blood 2007;109:2663–5. [DOI] [PubMed] [Google Scholar]
- 9.Tolar J, Ishida-Yamamoto A, Riddle M, et al. Amelioration of epidermolysis bullosa by transfer of wild-type bone marrow cells. Blood 2009;113:1167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montaudie H, Chiaverini C, Sbidian E, Charlesworth A, Lacour JP. Inherited epidermolysis bullosa and squamous cell carcinoma: a systematic review of 117 cases. Orphanet J Rare Dis 2016;11:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andreassi A, Bilenchi R, Biagioli M, D’Aniello C. Classification and pathophysiology of skin grafts. Clin Dermatol 2005;23:332–7. [DOI] [PubMed] [Google Scholar]
- 12.Biswas A, Bharara M, Hurst C, Armstrong DG, Rilo H. The micrograft concept for wound healing: strategies and applications. J Diabetes Sci Technol 2010;4:808–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiistala U, Mustakallio KK. In-Vivo Separation of Epidermis by Production of Suction Blisters. Lancet 1964;2:1444–5. [DOI] [PubMed] [Google Scholar]
- 14.Kanapathy M, Hachach-Haram N, Bystrzonowski N, et al. Epidermal grafting for wound healing: a review on the harvesting systems, the ultrastructure of the graft and the mechanism of wound healing. Int Wound J 2017;14:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osborne SN, Schmidt MA, Harper JR. An Automated and Minimally Invasive Tool for Generating Autologous Viable Epidermal Micrografts. Adv Skin Wound Care 2016;29:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Claude O, Binder JP, Bustamante K, et al. [Role of cutaneous pinch grafts in the healing of patients with dystrophic epidermolysis bullosa wounds: report of four cases]. Ann Chir Plast Esthet 2005;50:189–96. [DOI] [PubMed] [Google Scholar]
- 17.Yuen WY, Huizinga J, Jonkman MF. Punch grafting of chronic ulcers in patients with laminin-332-deficient, non-Herlitz junctional epidermolysis bullosa. J Am Acad Dermatol 2013;68:93–7, 7 e1–2. [DOI] [PubMed] [Google Scholar]
- 18.Gostynski A, Pasmooij AM, Jonkman MF. Successful therapeutic transplantation of revertant skin in epidermolysis bullosa. J Am Acad Dermatol 2014;70:98–101. [DOI] [PubMed] [Google Scholar]
- 19.Buonocore SD, Ariyan S. Cadaveric allograft for wound closure after resection of squamous cell carcinoma in patients with recessive dystrophic epidermolysis bullosa: a report of 32 resections and repairs in 2 patients. Ann Plast Surg 2009;63:297–9. [DOI] [PubMed] [Google Scholar]
- 20.Nunez-Villaveiran T, Feasel P, Keenan S, et al. Donor skin allograft survival after bone marrow transplantation: Case report and systematic review of the literature. J Plast Reconstr Aesthet Surg 2019;72:23–34. [DOI] [PubMed] [Google Scholar]
- 21.Osborne SN, Schmidt MA, Derrick K, Harper JR. Epidermal micrografts produced via an automated and minimally invasive tool form at the dermal/epidermal junction and contain proliferative cells that secrete wound healing growth factors. Adv Skin Wound Care 2015;28:397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruckner AL, Fairclough DL, Feinstein JA, et al. Reliability and validity of the instrument for scoring clinical outcomes of research for epidermolysis bullosa (iscorEB). Br J Dermatol 2018;178:1128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ebens CL, McGrath JA, Tamai K, et al. Bone marrow transplant with post-transplant cyclophosphamide for recessive dystrophic epidermolysis bullosa expands the related donor pool and permits tolerance of nonhaematopoietic cellular grafts. Br J Dermatol 2019;181:1238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathes DW, Hwang B, Graves SS, et al. Tolerance to vascularized composite allografts in canine mixed hematopoietic chimeras. Transplantation 2011;92:1301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathes DW, Chang J, Hwang B, et al. Simultaneous transplantation of hematopoietic stem cells and a vascularized composite allograft leads to tolerance. Transplantation 2014;98:131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spitzer TR, Sykes M, Tolkoff-Rubin N, et al. Long-term follow-up of recipients of combined human leukocyte antigen-matched bone marrow and kidney transplantation for multiple myeloma with end-stage renal disease. Transplantation 2011;91:672–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawai T, Sachs DH, Sprangers B, et al. Long-term results in recipients of combined HLA-mismatched kidney and bone marrow transplantation without maintenance immunosuppression. Am J Transplant 2014;14:1599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scandling JD, Busque S, Shizuru JA, et al. Chimerism, graft survival, and withdrawal of immunosuppressive drugs in HLA matched and mismatched patients after living donor kidney and hematopoietic cell transplantation. Am J Transplant 2015;15:695–704. [DOI] [PubMed] [Google Scholar]
- 29.Krause DS, Theise ND, Collector MI, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 2001;105:369–77. [DOI] [PubMed] [Google Scholar]
- 30.Park H, Lad S, Boland K, et al. Bone marrow-derived epithelial cells and hair follicle stem cells contribute to development of chronic cutaneous neoplasms. Nat Commun 2018;9:5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janin A, Murata H, Leboeuf C, et al. Donor-derived oral squamous cell carcinoma after allogeneic bone marrow transplantation. Blood 2009;113:1834–40. [DOI] [PubMed] [Google Scholar]
- 32.Petrof G, Abdul-Wahab A, Proudfoot L, Pramanik R, Mellerio JE, McGrath JA. Serum levels of high mobility group box 1 correlate with disease severity in recessive dystrophic epidermolysis bullosa. Exp Dermatol 2013;22:433–5. [DOI] [PubMed] [Google Scholar]
- 33.Aikawa E, Fujita R, Kikuchi Y, Kaneda Y, Tamai K. Systemic high-mobility group box 1 administration suppresses skin inflammation by inducing an accumulation of PDGFRalpha(+) mesenchymal cells from bone marrow. Sci Rep 2015;5:11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamai K, Yamazaki T, Chino T, et al. PDGFRalpha-positive cells in bone marrow are mobilized by high mobility group box 1 (HMGB1) to regenerate injured epithelia. Proc Natl Acad Sci U S A 2011;108:6609–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barrandon Y, Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci U S A 1987;84:2302–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beaver CM, Ahmed A, Masters JR. Clonogenicity: holoclones and meroclones contain stem cells. PLoS One 2014;9:e89834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Page ME, Lombard P, Ng F, Gottgens B, Jensen KB. The epidermis comprises autonomous compartments maintained by distinct stem cell populations. Cell Stem Cell 2013;13:471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mavilio F, Pellegrini G, Ferrari S, et al. Correction of junctional epidermolysis bullosa by transplantation of genetically modified epidermal stem cells. Nat Med 2006;12:1397–402. [DOI] [PubMed] [Google Scholar]
- 39.Bauer JW, Koller J, Murauer EM, et al. Closure of a Large Chronic Wound through Transplantation of Gene-Corrected Epidermal Stem Cells. J Invest Dermatol 2017;137:778–81. [DOI] [PubMed] [Google Scholar]
- 40.Hirsch T, Rothoeft T, Teig N, et al. Regeneration of the entire human epidermis using transgenic stem cells. Nature 2017;551:327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.