Abstract
i. Background:
Acute infection is a well-established risk factor of cardiovascular inflammation increasing the risk for a cardiovascular complication within the first weeks after infection. However, the nature of the processes underlying such aggravation remains unclear. Lipopolysaccharide (LPS) derived from Gram-negative bacteria is a potent activator of circulating immune cells including neutrophils, which foster inflammation through discharge of neutrophil extracellular traps (NETs). Here we utilize a model of endotoxinemia to link acute infection and subsequent neutrophil activation with acceleration of vascular inflammation.
ii. Methods:
Acute infection was mimicked by injection of a single dose of LPS into hypercholesterolemic mice. Atherosclerosis burden was studied by histomorphometric analysis of the aortic root. Arterial myeloid cell adhesion was quantified by intravital microscopy.
iii. Results:
LPS treatment rapidly enhanced atherosclerotic lesion size by expansion of the lesional myeloid cell accumulation. LPS treatment led to the deposition of NETs along the arterial lumen and inhibition of NET release annulled lesion expansion during endotoxinemia, thus suggesting that NETs regulate myeloid cell recruitment. To study the mechanism of monocyte adhesion to NETs, we employed in vitro adhesion assays and biophysical approaches. In these experiments, NET-resident histone H2a attracted monocytes in a receptor-independent, surface charge-dependent fashion. Therapeutic neutralization of histone H2a by antibodies or by in silico designed cyclical peptides enables us to reduce luminal monocyte adhesion and lesion expansion during endotoxinemia.
iv. Conclusions:
Our study shows, that NET-associated histone H2a mediates charge-dependent monocyte adhesion to NETs and accelerates atherosclerosis during endotoxinemia.
Keywords: neutrophil, inflammation, atherosclerosis, histone, neutrophil extracellular trap, endotoxinemia, sepsis
Introduction
Atherosclerosis is a lipid-driven chronic inflammation of the arterial vessel wall. In its late stages, atherosclerosis is the underlying pathophysiology of myocardial infarction and stroke and hence the leading cause of mortality worldwide. Monocytes and macrophages hold crucial roles throughout all stages of atherosclerosis as they contribute to lipid modification and respond with a pronounced inflammatory response upon uptake of modified lipids1. With only limited numbers of macrophages residing in large arteries, the vast majority of monocytic cells needs to be de novo recruited in a process known as the leukocyte recruitment cascade, which is orchestrated by fine-tuned interactions of selectins, chemokines, and integrins and their respective receptors2. In recent years, studies have provided evidence for the contribution of neutrophils to the arterial monocyte recruitment. Herein, neutrophils deposit preformed chemoattractants on endothelial cells or activate endothelial cells directly to stimulate monocyte adhesion3–7.
Multiple bacterial and viral pathogens have been associated with atherosclerosis by seroepidemiologial studies and identification of the infectious agent in human atherosclerotic tissue. Moreover, there is strong clinical evidence for the acceleration of arterial inflammation by acute infection8. As an example, urinary tract infection and bacteremia associate with an increase in the short-term risk of myocardial infarction9,10. Most striking data, however are available from patients with pneumonia, with the risk of myocardial infarction peaking at the onset of infection and is proportional to the severity of illness11,12. Division of Gram-negative bacteria or their elimination leads to release of lipopolysaccharide (LPS) into the blood stream, i. e. endotoxinemia. Neutrophils are rapidly activated by LPS possibly leading to the release of neutrophil extracellular traps (NETs), a complex structure composed of nuclear chromatin and proteins of nuclear cytoplasmatic and granule origin13. Since proteins reported to attract monocytes are localized within NETs and since NETs have been detected at the luminal side of large arteries14, we here hypothesized that LPS-triggered NET release acts as a link between endotoxinemia and heightened vascular inflammation by triggering monocyte recruitment.
Methods
An expanded method section can be found in online supplement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Animal experiments
We surveyed atheroprogression in Apoe−/− or Apoe−/−Cx3cr1GFP reporter mice on C57Bl/6J background after 4 weeks of high-fat diet (HFD) (21% fat, Ssniff). Endotoxinemia was induced by intraperitoneal (i.p.) injection of lipopolysaccharide (LPS, Escherichia coli, O111:B4, Sigma Aldrich, 1 mg/kg BW, i.p.). Control mice received vehicle (PBS, 100 μl, i.p.). Thereafter, atherosclerotic lesions were analyzed in aortic root sections or cell adhesion was studied by intravital microscopy of the left carotid artery. To assess the effect of NETs we blocked NET-formation with BB Cl-amidine (1 mg/kg BW, Cayman Chemical Company). In additional experiments, mice received antibodies to histone H2a (20 μg/mouse, Biorbyt), its respective IgG isotype control (Jackson ImmunoResearch), or a cyclical histone 2A interference peptide (CHIP, 5 mg/kg BW). All animal experiments were approved by the local ethics committee and performed in accordance with institutional guidelines.
Intravital microscopy
Leukocyte-endothelial interactions along the carotid artery were analyzed in Apoe−/−Cx3cr1GFP reporter mice by intravital microscopy as previously reported3–5. In brief, mice were placed in supine position, and the right jugular vein was cannulated with a catheter for antibody injection. Intravital microscopy was performed after injection of a PE-conjugated antibody to Ly6G (1 μg; clone 1A8; BioLegend) and 4’,6-Diamidino-2-phenylindol (DAPI, Thermo Fischer). Using an Olympus BX51 microscope equipped with a Hamamatsu 9100–02 EMCCD camera, and a 10× saline-immersion objective movies of 30 s were acquired and analyzed offline. In the carotid artery, one field of view was analyzed per mouse. gfp expressing cells were considered monocytes. For identification of NET like structures, the original DAPI image was transformed into a 8 bit gray scale image and subsequently thresholded. Particles in the latter image were quantified and particles above 80 px2 and a circularity below 0.75 were considered NET like structures.
Human samples
All in vitro experiments were performed with peripheral human white blood cells donated from healthy volunteers. Blood sampling was approved by the Klinikum der Univerität München ethics board and participants gave written informed consent.
Plasma samples from previously described IRB-approved biorepositories at Brigham and Women’s Hospital15 were analyzed from patients with Gram-negative rod bacteremia or sepsis with cardiovascular risk factors and compared with those from age-matched controls with cardiovascular risk factors.
Statistics
All statistics analysis was performed by using GraphPad Prism 8 software. Outliers have been determined by Grubbs’ test with α=0.05. To test normal distribution, the Shapiro-Wilk test was used. If normality was passed, data were tested by two-tailed unpaired t-test or one-way ANOVA test. The Mann-Whitney test or Kurskal-Wallis test with Dunn’s correction was performed when data were not normally distributed. In all used tests a 95% confidence interval was utilized with p<0.05 was assumed as significant difference. All data are represented as mean ±SEM.
Results
NETs induce luminal myeloid cell adhesion in atherosclerosis during endotoxinemia
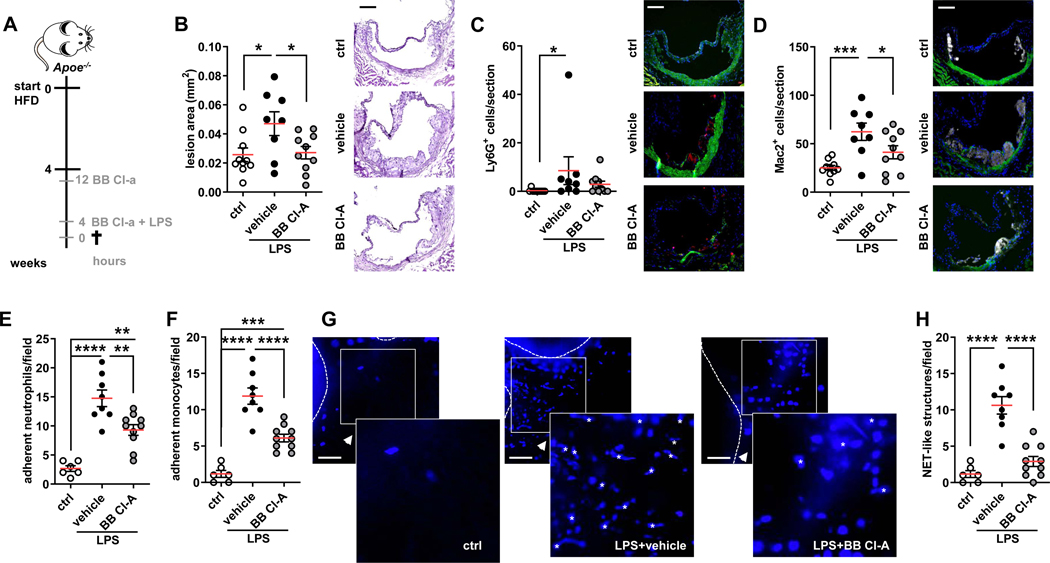
To investigate the effect of endotoxinemia on myeloid cell recruitment during atheroprogression, Apoe−/− mice were fed a high fed diet for 4 weeks and injected with LPS for 4 hours (Figure 1A). Treatment in this way resulted in the striking expansion of atherosclerotic lesion sizes (Figure 1B). While plasma cholesterol and triglyceride levels were not affected by treatment in this way, counts of circulating neutrophils and monocytes were depleted (Figure IA-F in the Data Supplement) likely due to margination and extravasation of activated myeloid cells. Indeed, the number of neutrophils, monocytes and macrophages recruited to atherosclerotic lesions as well as to inflamed aorta was drastically increased in LPS-treated mice (Figure 1C/D, Figure IG/H in the Data Supplement). This notion was confirmed by use of intravital microscopy of carotid arteries performed in Apoe−/−Cx3cr1eGFP mice. There, LPS enhanced adhesion of both GFP+ monocytes as well as of antibody-labeled neutrophils (Figure 1E/F).
Figure 1: Endotoxinemia accelerates atherosclerosis.
(A) Experimental setup. Apoe−/− or Apoe−/−Cx3cr1GFP mice were fed a HFD for 4 weeks and treated with either PBS (ctrl) or with LPS (1mg/kg BW). Another LPS-treated group received a BB Cl-amidine (BB Cl-A, 1mg/kg BW) 12 h before and along with LPS injection. (B) Aortic root lesion size analyzed in HE-stained sections. Representative images, scale bar 50 μm. (C/D) Lesional neutrophils (Ly6G+ cells) (C) and macrophages (Mac2+ cells) (D) quantified in root sections. Representative immunofluorescence images showing lesional neutrophil (red), Mac2+ positive cells (grey) and nuclei (DAPI, blue), scale bar 50 μm. (E/F) Quantification of luminally adherent neutrophils (E) and monocytes (F). (G) Representative intravital microscopy (IVM) images of the carotid artery of Apoe−/−Cx3cr1GFP mice. DNA is stained with DAPI, scale bar 50 μm. NET-like structures as identified by automized image analyzes are marked up with Asterisks. (H) Count of luminal NET-like structures in left common carotid artery. Data are analyzed by one-way ANOVA with Tukey’s multiple comparisons test (B, D, E, F, H) or Kruskal-Wallis test with Dunn’s multiple comparisons test (C); *p≤0.05, **p≤0.01, ***p≤0.001, ****p≤0.0001. All data are presented as mean±SEM.
LPS is known to trigger the release of NETs16 and hence we hypothesized that in our experimental setting NETs may contribute to heightened neutrophil and monocyte recruitment. Intravital microscopy permitted to visualize DAPI+ structures with a NET-like shape in the lumen of mice receiving LPS (Figure 1G/H). Additionally, cell-free DNA as well as DNA-MPO complexes, a plasma marker of NETs, are strikingly increased in the plasma of mice with endotoxinemia (Figure II/J in the Data Supplement). Of note, plasma dsDNA correlated with the number of aortic myeloid cells as well as with plasma DNA-MPO complexes suggesting that plasma dsDNA is a valid surrogate marker of NETs predicting aortic cell infiltration (Figure IK-M in the Data Supplement). Finally, plasma DNA-MPO complexes correlated with plasma endotoxin levels indicating that LPS directly induces NET release (Figure IN in the Data Supplement). To generate a link to the clinical situation we assessed the amount of plasma circulating cell free dsDNA, a surrogate marker of NETs, in cardiovascular risk patients (Table I in the Data supplement) admitted to the hospital with Gram-negative rod bacteremia or sepsis. Here hospitalized patients with confirmed Gram-negative rod bacteremia or sepsis presented with significantly higher DNA levels compared to hospitalized matched controls exhibiting the same cardiovascular risk profile but without Gram-negative rod bacteremia or sepsis (Figure IM in the Data Supplement). Of note, patients with Gram-negative rod bacteremia or sepsis had higher blood neutrophil counts as compared to the respective control group and circulating neutrophil counts across both groups of patients correlated with plasma dsDNA levels.
To test if NETs along the arterial lumen would contribute to the atherosclerosis phenotype observed in endotoxinemic mice, we treated mice with BB Cl-amidine, a potent inhibitor of protein arginine deiminases (PADs) with favorable pharmacokinetics in terms of plasma half-life and EC50. BB Cl-amidine delivery in endotoxinemic mice drastically reduced cell-free DNA in the plasma (Figure II in the Data Supplement), while MPO-DNA complexes were not detectable in this group. Intravital imaging confirmed that BB Cl-amidine treatment abrogated luminal NET release in response to stimulation with LPS for 4 hours (Figure 1G/H). In addition, heightened lesion formation as well as lesional accumulation of myeloid cells in response to LPS was completely abolished (Figure 1B–D, Figure IG/H in the Data Supplement). In agreement herewith, BB Cl-amidine treatment led to a vast reduction of arterial myeloid cell adhesion (Figure 1E/F) suggesting that luminal NETs promote arterial myeloid cell adhesion during endotoxinemia.
NETs promote monocyte adhesion independent of receptor signaling
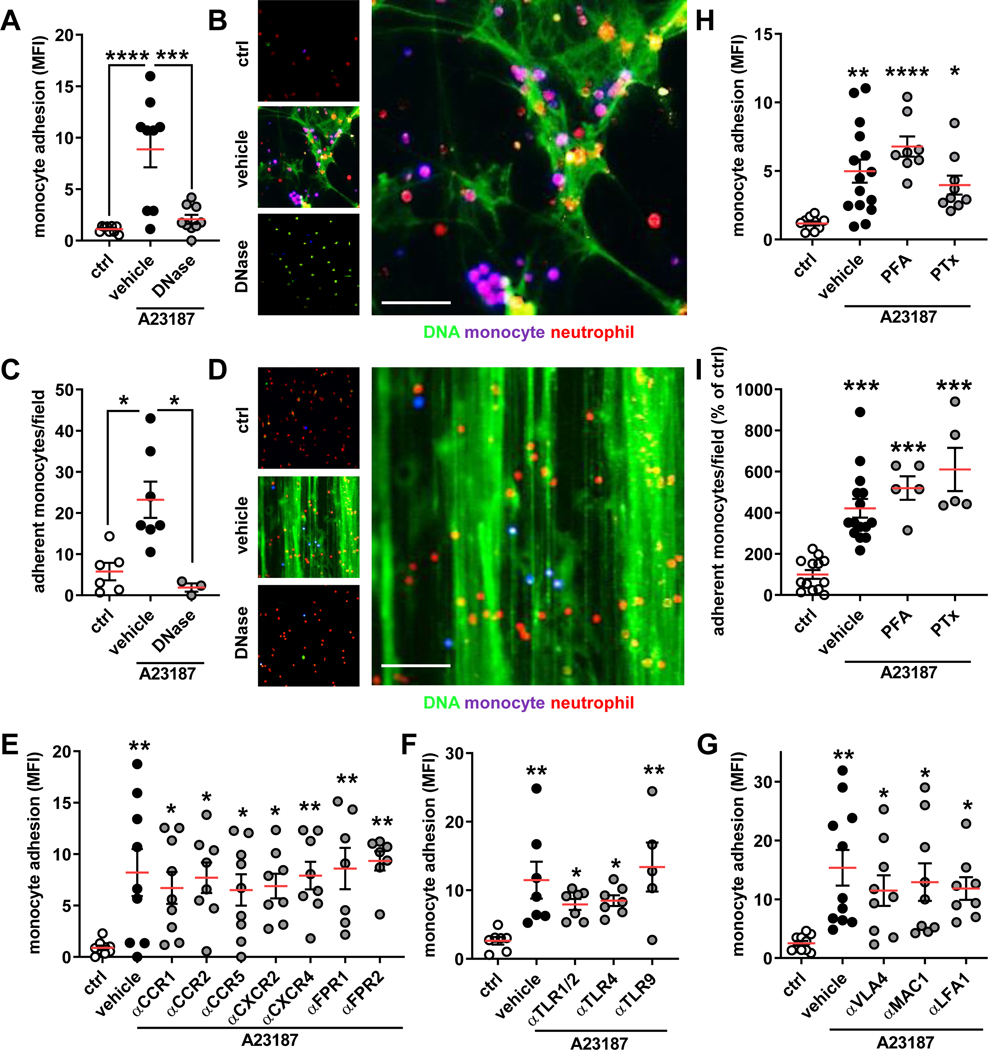
To study how NETs would promote monocyte adhesion, we allowed human classical monocytes, a proatherogenic monocyte subsets17,18, to sediment on NETs in vitro. Indeed, we witnessed a significant adhesion of monocytes to NETting neutrophils (Figure 2A/B). Fluorescence imaging revealed that these classical monocytes predominantly adhered directly to the NET scaffold and inhibition of monocyte adhesion after NET degradation with DNase I confirmed the importance of NETs in static monocyte adhesion (Figure 2A/B). Of note, such finding could also be recapitulated when using CD4+ T cells instead of monocytes (Figure IIA in the Data Supplement). To transfer these findings to a physiologically relevant setting, we initiated NET release in adherent human neutrophils and perfused classical monocytes in a flow chamber assay. As for the static adhesion assay, we found that monocytes primarily bound to DNA fibers and that degradation of NETs abolished adhesion evoked by activated neutrophils (Figure 2C/D). Similar data sets were obtained when NETs were induced on TNF-activated endothelial cells. Here, a higher number of classical monocytes was found to adhere at baseline, likely as a consequence of direct monocyte-endothelial cell interaction. The number of adherent monocytes under static or flow conditions, however, significantly increased when NETs were induced on endothelial cells, an effect fully reversed upon DNase I degradation (Figure IIB/C in the Data Supplement).
Figure 2: Monocyte adhesion to NETs is receptor independent.
In vitro monocyte adhesion to expelled NETs under static or flow conditions. (A/B) Monocytes were added to neutrophils (ctrl) or NETting neutrophils (induced by A23187) and adhesion was quantified by a fluorescence plate reader. NETs were also degraded by DNase I. Representative microscopic image (B) of monocytes (violet) adherent to NETs (green; neutrophils red), scale bar 50 μm. (C/D) Monocytes were perfused at 0.5 dyne/cm2 over neutrophils, NETs, or degraded NETs and their adhesion was quantified manually. Representative microscopic image (D) of monocytes (violet) adherent to NETs (green; neutrophils red), scale bar 100 μm. (E-G) Monocytes where pre-treated with antagonists or antibodies to chemokine receptors (E), Toll-like receptors (F), or integrins (G) prior to incubation with NETs. (H/I) Monocytes fixed with PFA or treated with pertussis toxin (PTx) were used in static (H) or flow (I) adhesion assays. Data are analyzed by one-way ANOVA with Tukey’s multiple comparisons test (A, H, G) or Kruskal-Wallis test with Dunn’s multiple comparisons test (C, E, F, I); *p≤0.05, **p≤0.01, ***p≤0.001, **** p≤0.0001. All data are presented as mean±SEM.
These observations are reminiscent of earlier work showing that proteins typically found in NETs or NETs themselves can increase cell adhesion by engaging chemokine receptor signaling and leukocyte integrin activation2,19–22. Thus, we neutralized chemokine receptors (Figure 2E), receptors of alarmins (Figure 2F, Figure IID in the Data Supplement), or integrins (Figure 2G) prior to incubation with NETs. Much to our surprise, none of these treatments impacted on adhesion evoked by NETs. Thus we suspected that the adhesion evoked by NETs may be signaling independent. Indeed, depletion of calcium by use of a chelator did not affect NET-mediated adhesion (Figure IIE in the Data Supplement). In addition, abrogation of signaling of G-protein coupled receptors by pertussis toxin or even fixation of monocytes with 4% paraformaldehyde (PFA) did not impair adhesion of monocytes to NETs (Figure 2H/I). Taken together, these data indicate that monocyte adhesion to NETs is independent of receptor signaling.
NET-resident histone H2a attracts monocytes electrostatically
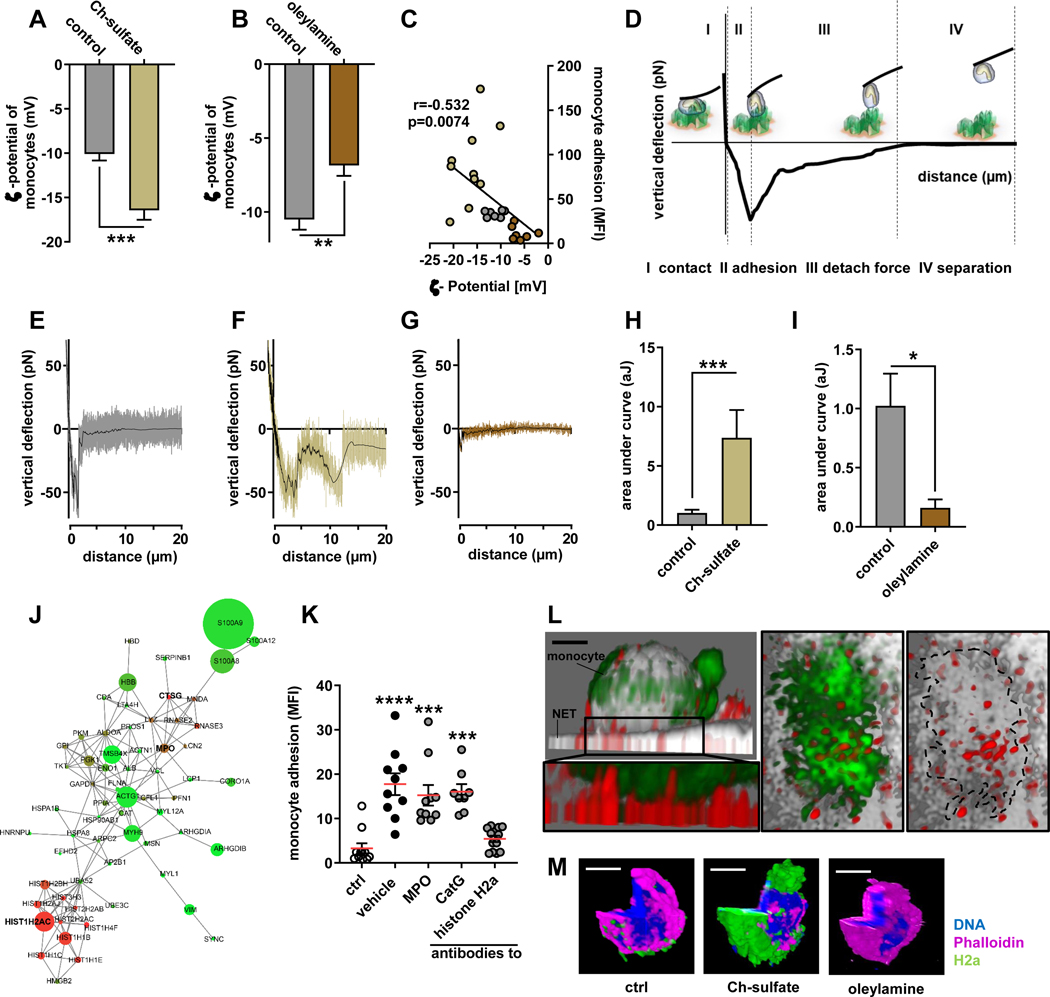
Given the signaling-independent adhesion of monocytes to NETs, we assumed that biophysical interactions such as electrostatic charges could be important in this process. In fact, monocytes present an overall negative surface charge (ζ potential −10.51±0.69 mV), while NETs are decorated with highly cationic proteins. To test the importance of charge interaction in adhesion of classical monocytes to NETs, monocytes were incubated with either cholesterol sulfate or oleylamine. Cholesterol sulfate is a negatively-charged steroid lipid, whereas oleylamine is a positively-charged unsaturated fatty acid. Both lipids integrate with their lipophilic part into the phospholipid bilayer of the cell membrane and hence allow manipulating cell surface charge. In our hands, incubation with cholesterol sulfate or oleyelamine rendered monocyte surface charges more negative or more positive, respectively (Figure 3A/B). To assess the relevance of the monocyte surface charge during adhesion to NETs we allowed human classical monocytes of different surface charges to adhere to NETs. In these experiments we were able to generate a stringent correlation with more negative surface charges resulting in higher monocyte adhesion and vice versa (Figure 3C). To assess the physical properties of charge-dependent adhesion in more depth, we performed atomic force spectroscopy with a monocyte immobilized at the tip of the cantilever. Atomic force microscopy (AFM) is a scanning probe microscope with piezoelectric elements to move the spring like cantilever. The deflecting cantilever is used to directly measure forces acting on the monocyte. A force-distance curve reports on the contact force (I), the force until maximum adhesion (II), the forces needed to fully detach the monocyte from the NET (III) and the distance of this detachment (IV) (Figure 3D). Classical monocytes with different surface charges were immobilized at the tip of the AFM cantilever and in this position probed on NETs. Manipulation of the monocyte surface charges impacted on the maximum adhesion force, the detachment force and the detachment distance to separate monocytes from NETs, the energy required to separate the monocyte from the NET, and the adhesion frequency. Overall, NET-adhesion strength and the ability to adhere to NETs was heightened when monocytes were rendered more negatively charged and opposite effects were found in less negatively charged monocytes (Figure 3E–I, Figure IIIA-H in the Data Supplement), thus confirming the concept of charge-driven monocyte adhesion.
Figure 3: Monocyte adhesion to NETs in regulated by cationic histone H2a.
(A/B) ζ-potential analysis of isolated monocytes treated with Ch-sulfate (A) or oleylamine (B). (C) Pearson correlation of monocyte adhesion to NETs versus monocyte ζ-potential. (D) Scheme of single cell atomic force microscopy (AFM) force spectroscopy. Monocytes were probed on expelled NETs at 200 pN contact force. (E-G) Representative force curves of native monocytes (E) or monocytes treated with Ch-sulfate (F) or oleylamine (G) probed on NETs. (H/I) Quantification of the area under the curve reflecting the energy required to rupture the monocyte-NET interaction. (J) Proteome analysis of NET resident proteins. Circle size reflects protein abundance, while color codes for charge (red cationic, green anionic). (K) Monocyte adhesion to NETs pre-incubated with antibodies to indicated NET-associated proteins. (L) Representative immunofluorescence confocal microscopy image showing the tight interaction between NET resident histone H2a and a NET-bound monocyte, scale bar 3 μm. Displayed is a xy projection (DNA gray, monocyte green, histone H2a red) and a zoom-in underneath (monocyte green, histone H2a red). The right two panels represent a top view (xz) of the zoom-in area thus visualizing the interface between the monocyte (green) and NET-resident histone H2a (red). In the right panel the monocyte is outlined (dashed line). (M) Representative confocal microscopy images of histone H2a binding monocyte in a charge dependent-manner, DNA blue, monocyte purple, histone H2a green, scale bar 5 μm. Data are analyzed by unpaired t-test (A, B, H, I) or Kruskal-Wallis test with Dunn’s multiple comparisons test (K); *p≤0.05, **p≤0.01, ***p≤0.001. All data are presented as mean±SEM. HNP, human neutrophil peptides; MPO, myeloperoxidase; PR3, proteinase 3; NE, neutrophil elastase; CatG, cathepsin G.
Neutrophil extracellular traps are decorated with a large array of granule-derived, cytoplasmatic, and nuclear proteins. Proteomics of NETs allowed the identification of 73 enriched polypeptides of which two clusters exhibited cationic surface charge as deferred from the peptides isoelectric point. One cluster was centered on nuclear histones of which histone H2a is the most abundant one. A second cluster revealed antimicrobial polypeptides with myeloperoxidase (MPO) and cathepsin G being the two candidates characterized by both cationic charge and high abundance (Figure 3J, Table II in the Data Supplement). To assess the contribution of these NET-resident proteins towards monocyte adhesion, we treated NETs with antibodies towards these polypeptides and recorded adhesion of human classical monocytes. In these studies, neutralization of antimicrobial polypeptides abundantly found in NETs (MPO, cathepsin G) and of such previously found to contribute to monocyte adhesion (LL37, elastase, proteinase 3, HNP1–3) yielded no effect (Figure 3K, Figure IIII in the Data Supplement)4,5,23. In addition, neutralization of highly abundant S100A8/9 peptides failed to reduce monocyte adhesion (Figure IIIJ in the Data Supplement). However, neutralization of histone H2a, the most abundant nuclear protein found in NETs, fully abrogated NET-driven monocyte adhesion (Figure 3K). High resolution confocal microscopy revealed that monocytes indeed bound to NET-resident histone H2a (Figure 3L). Finally, histone H2a binds to human classical monocytes in a charge dependent fashion (Figure 3M), thus confirming our understanding of charge-mediated monocyte adhesion. When studying the binding of histone H2a to monocyte subsets we found that a similar percentage of both classical and non-classical monocytes reacted with histone H2a (Figure IIIK in the Data Supplement). However, histone H2a bound in much higher intensity to classical monocytes when compared to their non-classical counterparts irrespective if they expressed Slan or not (Figure IIIL in the Data Supplement).
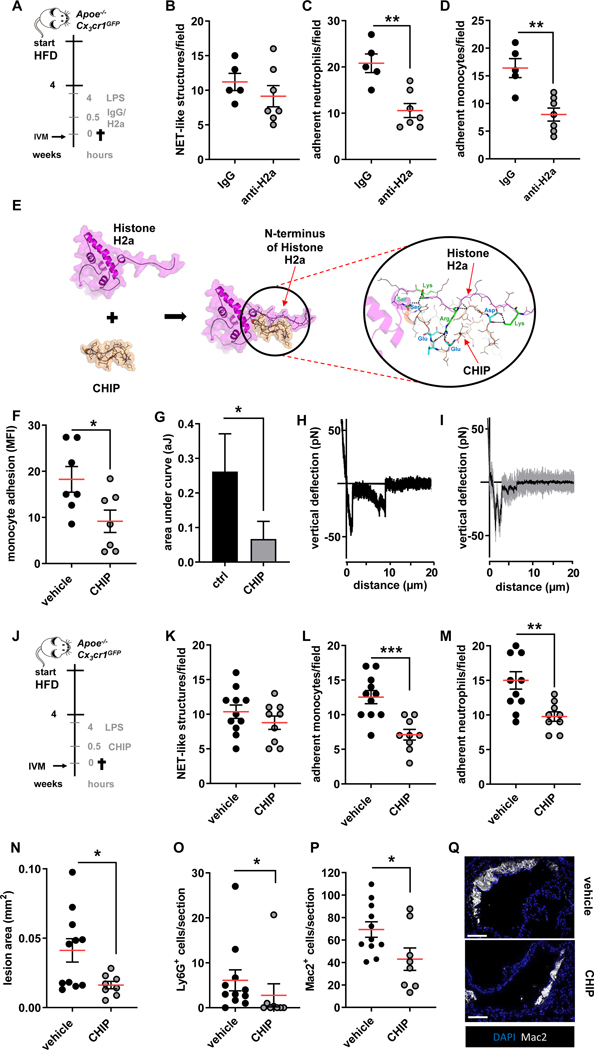
Therapeutic neutralization of histone H2a diminishes arterial monocyte adhesion and atheroprogression during endotoxinemia
Thus far our data suggest that histone H2a presented in NETs released during endotoxinemia causes myeloid cell adhesion, a process dramatically accelerating atheroprogression. Hence, we devised a protocol aimed at halting histone H2a-induced monocyte adhesion in vivo. Herein, a histone H2a neutralizing antibody or an isotype control IgG were administered in hypercholesterolemic mice treated with LPS for 4 hours and luminal adhesive events were studied by intravital microscopy (Figure 4A). While treatment with histone H2a targeting antibodies did not impact on the presence of luminal NET-like structures and the number of circulating leukocytes, adhesion of myeloid cells was significantly reduced (Figure 4B–D, Figure IVA/B in the Data Supplement). Whereas, antibody targeting in vivo may be associated to undesired side effects, we aimed at generating an alternative interference strategy. A resurgence of peptides as therapeutic agents to treat many diseases, especially for cardiovascular disease, has inspired us to develop peptidic inhibitors to neutralize histone H2a. Peptides contain several advantages such as ease of synthesis and optimization to improve pharmacokinetic and binding properties, large-scale and cost-effective production and applicability to conjugate with specific probes for drug delivery or for using as imaging tools24. Moreover, we have previously demonstrated and proven that monocyte adhesion can be interrupted by peptidic inhibitors5,25 and the highly positively charged N-terminus of histone H4 can be neutralized by the cyclic peptide26. By use of the same structure-based approaches as in our previous work we in silico designed and developed peptides targeting histone H2a. A few peptide candidates were selected based on binding free energy and visual inspection of the interactions between the candidates and the N-terminal tail of histone H2a for further synthesis and functional characterization. The most potent peptide called Cyclical Histone 2a Interference Peptide (CHIP) bound with the N-terminal domain of histone 2A.The electrostatic interactions between positively charged residues (Arg and Lys) of histone H2a with negatively charged residues (Asp and Glu) of CHIP and H-bond interactions promoted a stable complex formation (Figure 4E). To test the functionality of this peptide we first performed in vitro experiments. Herein, pretreatment of NETs with CHIP reduced adhesion significantly (Figure 4F). Biophysically, CHIP reduced the interaction strength of monocytes and NETs as shown by atomic force microscopy (Figure 4G–I). Given these encouraging findings, we aimed at testing CHIP in vivo. Delivery of CHIP to hypercholesterolemic mice receiving LPS for 4 hours did not impact on the presence of NET-like structures in the arterial lumen, but significantly diminished arterial adhesion of neutrophils and monocytes (Figure 4J–M). Beyond impacting on luminal events, CHIP allowed to drastically reduce atherosclerotic lesion sizes (Figure 4O). These changes in lesion size were not associated with differences in blood counts or plasma lipid levels (Figure IVC-F in the Data Supplement). The lesions of mice treated with CHIP were characterized by the accumulation of less neutrophils and macrophages (Figure 4P–R). In line herewith, aortic neutrophil and monocyte numbers were strikingly reduced by CHIP (Figure IVG/H in the Data Supplement). Taken together, neutralization of histone H2a by antibodies or peptides allows reducing arterial myeloid cell recruitment and accelerated atherosclerosis evoked by endotoxinemia.
Figure 4: Neutralization of histone H2a inhibits endotoxin-induced arterial myeloid cell recruitment and atheroprogression.
(A) Experimental outline. Apoe−/−Cx3cr1GFP were fed a HFD for 4 weeks, treated with LPS (1 mg/kg, 4h) and injected with isotype respective control (IgG), or a histone H2a-targeting antibody (anti-H2a). (B-D) Intravital microscopy was used to quantify luminal NET-like structures (B) in the left carotid artery as well as adherent neutrophils (C) and monocytes (D).(E) The structure of histone H2a (magenta), CHIP (orange) and the histone H2a-CHIP complex which was derived from protein-protein docking and molecular dynamic simulation. CHIP bound and interacted with the N-terminal part of histone H2a. The electrostatic interactions between Arg or Lys of Histone H2a (green sticks) with Glu or Asp of CHIP (cyan sticks) as well as hydrogen bonds (displayed as dash lines) help to stabilize the complex formation between histone H2a and CHIP. (F-I) Pharmacological interruption of histone H2a monocyte-binding was validated in static adhesion assays (F) as well as by single cell force spectroscopy (G-I). (J-Q) In vivo validation of therapeutic effect of CHIP in endotoxin-accelerated atherosclerosis. (J) Experimental outline. Apoe−/−Cx3cr1GFP were fed a HFD for 4 weeks, treated with LPS (1 mg/kg, 4h) and injected with vehicle or CHIP (5 mg/kg). (K-M) Intravital microscopy was used to quantify luminal NET-like structures (K) in the left carotid artery as well as adherent neutrophils (L) and monocytes (M). (N) Aortic root lesion size analyzed after HE staining. Quantification of Lesional neutrophils (Ly6G+ cells) (O), and macrophages (Mac2+ cells) (P). (Q) Representative immunofluorescence images showing lesional Mac2+ cells (grey) and nuclei (DAPI, blue), scale bar 50 μm. Data are analyzed by Mann-Whitney test (B, C, D, F, G, O) or unpaired t-test (K-M, N, P); *p≤0.05, **p≤0.01, ***p≤0.001. All data are presented as mean ±SEM.
Discussion
Atherosclerosis is the leading cause of mortality in Western society. Excess mortality from cardiovascular disease during influenza epidemics was first recognized early in the 20th century, but the specific association of influenza and other infections with myocardial infarction was not characterized until decades later. In fact, over the last 20 years epidemiological studies have consistently demonstrated the association between acute infection with multiple bacterial and viral pathogens and the short term increase in cardiovascular complications8. As an example, a self-controlled case series involving U.S. veterans showed a remarkable increase in the risk of myocardial infarction during the first 15 days after hospitalization for acute bacterial pneumonia, to a risk that was 48 times higher than that in any 15-day period during the year before or after the onset of infection27. An increase in the short-term risk of myocardial infarction has also been described in association with urinary tract infection and bacteremia9,10. The strength and temporal pattern of the association between acute infections and an increased risk of myocardial infarction suggest a causal relationship. Because the association has been shown with a variety of pathogens, sites of infection, and the association is stronger and lasts longer when the infection is more severe, it is likely that the infection and the host response to infection are major determinants in this relationship. Here we used a model of endotoxinemia to mimic acute, severe infection. In these experiments, administration of endotoxin induces a rapid expansion of atherosclerotic lesions characterized by extension of the lesional myeloid cell compartment. This increase was fully abrogated when release of NETs was pharmacologically inhibited. Our studies identify a mechanism that centers on monocyte adhesion to luminal NETs in general and to NET-resident histone H2a in particular. Neutralization of histone H2a by antibodies or by in silico designed peptides permits inhibition of endotoxin-accelerated monocyte adhesion and lesional myeloid cell accumulation, thus providing a potential strategy for improved care of patients at risk of cardiovascular events and experiencing an acute infection.
Since our study was performed with endotoxin stimulation only, this may be perceived as limitation in the applicability of our study to clinical scenarios. However, an increased risk for cardiovascular complications after an acute infections has been observed for numerous pathogens, including viruses such as influenza virus or respiratory syncytial virus, Gram-positive bacteria including Streptococcus pneumonia and Staphylococcus aureus, as well as Gram-negative bacteria like Escherichia coli and Haemophilus influenza8,10,12,28. Interestingly, NET release is triggered by a variety of stimuli including all of the pathogens listed above29 and hence the NET-centered mechanism identified in our study may in part be applicable to a large variety of pathogen-associated cardiovascular complications although confirmation in additional studies is required.
Neutrophils have previously been reported to pave the way for inflammatory monocytes into developing atherosclerotic lesions. Rendering mice neutropenic during initial stages of atherosclerosis diminishes lesion sizes as well as lesion composition, with lowered macrophage accumulation30. Neutrophils stimulate various mechanisms that promote monocyte recruitment. Among these, secretion of chemotactic proteins stands out as an important mechanism for arterial monocyte recruitment. Cathepsin G, cathelicidin, complexes formed of neutrophil-derived α-defensin and platelet-borne CCL5, and CCL2 released from neutrophils in a circadian fashion were shown to induce firm monocyte adhesion in mouse models of vascular inflammation when immobilized on arterial endothelial cells3–5,31. However, all these ligands bind to specific receptors and increase adhesion through increased integrin affinity and avidity. In our study we identify electrostatic interactions between cationic histone H2a and negatively charged classical monocyte surfaces as a novel mechanism driving infection-associated monocyte recruitment into atherosclerotic lesions. Histone H2a combines abundance within NETs and surface charge and may hence stand out as an important epitope for monocyte adhesion. However, other histone isotypes may engage similar activities as they are comparable in abundance and charge within NETs. Of note, a recent study has shown that activated smooth muscle cells residing in the fibrous cap of advanced atherosclerotic lesions stimulate neutrophils to release NETs. These are rich in cytotoxic histone H4 that can puncture human and murine SMCs leading to their death and, when re-occurring, thinning of the fibrous cap26. Thus, therapeutic neutralization of histone epitopes may exert a dual beneficial effect allowing to limit macrophage burden and fibrous cap thinning32,33.
The mechanism of NET-driven monocyte recruitment is receptor independent and hence lacks specificity. In fact, in unpublished observations we also found that neutrophils adhere to NETs. Consequently, continued inhibition of neutrophil adhesion to NETs may lower the number of neutrophils at sites of inflammation and hereby reduce local NET burden. In addition, surgical removal of tumors (especially colorectal cancer) is frequently associated with bacteremia and NETs triggered in such settings have been suggested to promote immobilization of tumor cells in vascular beds thus promoting formation of metastases34–36. Mechanistically, some studies reported beta1 integrins on tumor cells to mediate adhesion to NETs while other studies found unspecific binding to NETs underlying the process of NET-driven metastasis2,19,20. Consequently, degradation of NETs using DNase I has been found to limit metastases formation in animal models combining infection and tumor development2,19. Observations made in the here presented study suggest that histone H2a can also promote adhesion of tumor cells and promote metastases and perioperative treatment with histone-neutralizing therapies may provide an efficient way to reduce cancer spreading.
Monocyte adhesion to endothelial cells is typically looked upon as a bi-cellular interaction37. However, such simplified model can be modified by accessory cells including platelets and neutrophils through direct interaction or release of secretory products with chemotactic effects38,39. In the context of atherogenesis NET release along the arterial endothelium appears an infrequent event14 making a major contribution of NETs as adhesive substrate for monocytes unlikely. Irrespective of this conclusion, depletion of NETs during early stages of atherosclerosis has repeatedly been shown to limit lesion size and macrophage accumulation40,41. Whether reduced macrophage accumulation in these studies is a consequence of lowered monocyte adhesion and possibly processes described in this study remains to be determined. In an acute inflammatory setting, such as a LPS challenge, however, luminal NET burden increases and herewith the chance for monocytes to adhere to NETs in this location. Based on our in vitro experiments it seems unlikely that histone H2a is deposited on the arterial endothelium as digestion of NETs on endothelial cells prevents monocyte adhesion. Yet another intriguing aspect of NETs in luminal location is their potential contribution to endothelial erosion. Previous reports have pointed to a mechanism by which NETs released at sites of disturbed flow contribute to endothelial cell death and subsequent endothelial denudation42,43. Further studies are required to investigate if NET-resident cytotoxic components such as histones26,44 contribute to this process.
While our study aims at understanding the acute effect of LPS on atherosclerosis development, others have reported the impact of chronic LPS delivery45. In such setting atherosclerotic plaques of mice receiving LPS are infiltrated by NK1.1 cells and T cells together promoting a proinflammatory microenvironment. In addition, activated lymphocytes populated the adventitia in these mice. In a similar disease model, the Apolipoprotein C-I-dependent activation of macrophages was found to be crucial46. Thus far, however, the involvement of neutrophils in models of chronic LPS exposure in the context of atherosclerosis has not been studied. One limiting factor may be the development of neutrophilic myeloid derived suppressor cells in the spleens of mice chronically challenged with LPS47 and future studies need to clarify the importance of neutrophils in such settings.
Infections in cardiovascular risk patients increase the chance of cardiovascular complications several-fold within the first three weeks after an infection several fold. We here identify a mechanism centered on extracellular histone H2a which induces adhesion and recruitment of monocytes. While data from the CANTOS trial reveal the overall positive effects of anti-inflammation therapy in the context of atherosclerosis, neutralization of IL1β was also associated to heightened risk of bacterial infections48. Therapeutic neutralization of histone H2a will likely not be linked to such adverse side effects as the antimicrobial actions of histone H2a can be compensated by the abundance of other antimicrobial polypeptides residing within NETs. In addition, the strict focus on infection-associated cardiovascular complications will be relevant to a very short time window of now more than three weeks after infection and can be combined with standard antibiotic treatment. Thus, targeting histone H2a may stand out as an innovative way to lower arterial monocyte recruitment accelerated by infection while coming with limited intrinsic side effects.
Supplementary Material
Clinical Perspective.
1). What is new?
Neutrophils and specifically neutrophil extracellular traps (NET) control accelerated atherosclerosis during endotoxinemia.
NET-resident histone H2a heightens arterial monocyte recruitment in endotoxinemia in a mechanism involving electrostatic charge interaction.
2). What are the clinical implications?
NET-driven arterial monocyte recruitment is a mechanism operational during endotoxinemia.
Therapeutic neutralization of NET-resident cationic molecules including histone H2a by use of antibodies or peptides may protect cardiovascular risk patients during an acute infection from secondary cardiovascular events.
Acknowledgments
The authors would like to acknowledge the other investigators who made possible the assembly of Registry of Critical Illness (RoCI) and Study of Epigenetics in Medicine (StEM) biorepositories, including Maura Benson and Drs. Sam Ash, Paul Dieffenbach, Laura Fredenburgh, and Andrew Stergachis.
Sources of Funding
The authors receive funding from the Deutsche Forschungsgemeinschaft (SO876/11-1, SFB914 TP B8, SFB1123 TP A6, TP B5, and TP Z2, OR465/1-1), the Vetenskapsrådet (2017-01762), the Else-Kröner-Fresenius Stiftung, and the Leducq foundation. Dr. Libby receives funding support from the National Heart, Lung, and Blood Institute (1R01HL134892), the American Heart Association (18CSA34080399), the RRM Charitable Fund, and the Simard Fund.
Nonstandard abbreviations
- AFM
Atomic force microscopy
- CHIP
Cyclical Histone 2a Interference Peptide
- DAPI
4’,6-Diamidino-2-phenylindol
- LPS
lipopolysaccharide
- MPO
myeloperoxidase
- NET
neutrophil extracellular trap
- PAD
protein arginine deiminases
- PFA
paraformaldehyde
- PTx
pertussis toxin
Footnotes
Disclosures
O.S., K.W. and G.N. hold a patent on targeting histones in cardiovascular inflammation. Dr. Libby is an unpaid consultant to, or involved in clinical trials for Amgen, AstraZeneca, Baim Institute, Beren Therapeutics, Esperion, Therapeutics, Genentech, Kancera, Kowa Pharmaceuticals, Medimmune, Merck, Norvo Nordisk, Merck, Novartis, Pfizer, Sanofi-Regeneron. Dr. Libby is a member of scientific advisory board for Amgen, Corvidia Therapeutics, DalCor Pharmaceuticals, Kowa Pharmaceuticals, Olatec Therapeutics, Medimmune, Novartis, and XBiotech, Inc. Dr. Libby’s laboratory has received research funding in the last 2 years from Novartis. Dr. Libby is on the Board of Directors of XBiotech, Inc. Dr. Libby has a financial interest in Xbiotech, a company developing therapeutic human antibodies. Dr. Libby’s interests were reviewed and are managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies.
References
- 1.Fayad ZA, Swirski FK, Calcagno C, Robbins CS, Mulder W, Kovacic JC. Monocyte and Macrophage Dynamics in the Cardiovascular System: JACC Macrophage in CVD Series (Part 3). J Am Coll Cardiol. 2018;72:2198–2212. doi: 10.1016/j.jacc.2018.08.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park J, Wysocki RW, Amoozgar Z, Maiorino L, Fein MR, Jorns J, Schott AF, Kinugasa-Katayama Y, Lee Y, Won NH, et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med. 2016;8:361ra138. doi: 10.1126/scitranslmed.aag1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winter C, Silvestre-Roig C, Ortega-Gomez A, Lemnitzer P, Poelman H, Schumski A, Winter J, Drechsler M, de Jong R, Immler R, et al. Chrono-pharmacological Targeting of the CCL2-CCR2 Axis Ameliorates Atherosclerosis. Cell Metab. 2018;28:175–182.e5. doi: 10.1016/j.cmet.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Ortega-Gomez A, Salvermoser M, Rossaint J, Pick R, Brauner J, Lemnitzer P, Tilgner J, de Jong RJ, Megens RT, Jamasbi J, et al. Cathepsin G Controls Arterial But Not Venular Myeloid Cell Recruitment. Circulation. 2016;134:1176–1188. doi: 10.1161/CIRCULATIONAHA.116.024790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alard JE, Ortega-Gomez A, Wichapong K, Bongiovanni D, Horckmans M, Megens RT, Leoni G, Ferraro B, Rossaint J, Paulin N, et al. Recruitment of classical monocytes can be inhibited by disturbing heteromers of neutrophil HNP1 and platelet CCL5. Sci Transl Med. 2015;7:317ra196. doi: 10.1126/scitranslmed.aad5330. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Alcaide P, Liu L, Sun J, He A, Luscinskas FW, Shi GP. Regulation of endothelial cell adhesion molecule expression by mast cells, macrophages, and neutrophils. PLoS One. 2011;6:e14525. doi: 10.1371/journal.pone.0014525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silvestre-Roig C, Braster Q, Ortega-Gomez A, Soehnlein O. Neutrophils as regulators of cardiovascular inflammation. Nat Rev Cardiol. 2020;17:327–340. doi: 10.1038/s41569-019-0326-7. [DOI] [PubMed] [Google Scholar]
- 8.Musher DM, Abers MS, Corrales-Medina VF. Acute Infection and Myocardial Infarction. N Engl J Med. 2019;380:171–176. doi: 10.1056/NEJMra1808137. [DOI] [PubMed] [Google Scholar]
- 9.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–8. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 10.Dalager-Pedersen M, Søgaard M, Schønheyder HC, Nielsen H, Thomsen RW. Risk for myocardial infarction and stroke after community-acquired bacteremia: a 20-year population-based cohort study. Circulation. 2014;129:1387–96. doi: 10.1161/CIRCULATIONAHA.113.006699. [DOI] [PubMed] [Google Scholar]
- 11.Ramirez J, Aliberti S, Mirsaeidi M, Peyrani P, Filardo G, Amir A, Moffett B, Gordon J, Blasi F, Bordon J. Acute myocardial infarction in hospitalized patients with community-acquired pneumonia. Clin Infect Dis. 2008;47:182–7. doi: 10.1086/589246. [DOI] [PubMed] [Google Scholar]
- 12.Corrales-Medina VF, Alvarez KN, Weissfeld LA, Angus DC, Chirinos JA, Chang CC, Newman A, Loehr L, Folsom AR, Elkind MS, et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA. 2015;313:264–74. doi: 10.1001/jama.2014.18229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pieterse E, Rother N, Yanginlar C, Hilbrands LB, van der Vlag J. Neutrophils Discriminate between Lipopolysaccharides of Different Bacterial Sources and Selectively Release Neutrophil Extracellular Traps. Front Immunol. 2016;7:484. doi: 10.3389/fimmu.2016.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Megens RT, Vijayan S, Lievens D, Döring Y, van Zandvoort MA, Grommes J, Weber C, Soehnlein O. Presence of luminal neutrophil extracellular traps in atherosclerosis. Thromb Haemost. 2012;107:597–8. doi: 10.1160/TH11-09-0650. [DOI] [PubMed] [Google Scholar]
- 15.Reyes M, Filbin MR, Bhattacharyya RP, Billman K, Eisenhaure T, Hung DT, Levy BD, Baron RM, Blainey PC, Goldberg MB, et al. An immune-cell signature of bacterial sepsis. Nat Med. 2020; 6:333–340. doi: 10.1038/s41591-020-0752-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–9. doi: 10.1038/nm1565 [DOI] [PubMed] [Google Scholar]
- 17.Soehnlein O, Drechsler M, Döring Y, Lievens D, Hartwig H, Kemmerich K, Ortega-Gómez A, Mandl M, Vijayan S, Projahn D, et al. Distinct functions of chemokine receptor axes in the atherogenic mobilization and recruitment of classical monocytes. EMBO Mol Med. 2013;5:471–481. doi: 10.1002/emmm.201201717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P, Ferri L. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013;123:3446–58. doi: 10.1172/JCI67484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Najmeh S, Cools-Lartigue J, Rayes RF, Gowing S, Vourtzoumis P, Bourdeau F, Giannias B, Berube J, Rousseau S, Ferri LE, et al. Neutrophil extracellular traps sequester circulating tumor cells via β1-integrin mediated interactions. Int J Cancer. 2017;140:2321–2330. doi: 10.1002/ijc.30635. [DOI] [PubMed] [Google Scholar]
- 21.Monti M, De Rosa V, Iommelli F, Carriero MV, Terlizzi C, Camerlingo R, Belli S, Fonti R, Di Minno G, Del Vecchio S. Neutrophil Extracellular Traps as an Adhesion Substrate for Different Tumor Cells Expressing RGD-Binding Integrins. Int J Mol Sci. 2018;19:2350. doi: 10.3390/ijms19082350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monti M, Iommelli F, De Rosa V, Carriero MV, Miceli R, Camerlingo R, Di Minno G, Del Vecchio S. Integrin-dependent cell adhesion to neutrophil extracellular traps through engagement of fibronectin in neutrophil-like cells. PLoS One. 2017;12:e0171362. doi: 10.1371/journal.pone.0171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wantha S, Alard JE, Megens RT, van der Does AM, Döring Y, Drechsler M, Pham CT, Wang MW, Wang JM, Gallo RL, et al. Neutrophil-derived cathelicidin promotes adhesion of classical monocytes. Circ Res. 2013;112:792–801. doi: 10.1161/CIRCRESAHA.112.300666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henninot A, Collins JC, Nuss JM. The Current State of Peptide Drug Discovery: Back to the Future? J Med Chem. 2018;61:1382–1414. doi: 10.1021/acs.jmedchem.7b00318. [DOI] [PubMed] [Google Scholar]
- 25.Wichapong K, Alard JE, Ortega-Gomez A, Weber C, Hackeng TM, Soehnlein O, Nicolaes GA. Structure-Based Design of Peptidic Inhibitors of the Interaction between CC Chemokine Ligand 5 (CCL5) and Human Neutrophil Peptides 1 (HNP1). J Med Chem. 2016;59:4289–4301. doi: 10.1021/acs.jmedchem.5b01952. [DOI] [PubMed] [Google Scholar]
- 26.Silvestre-Roig C, Braster Q, Wichapong K, Lee EY, Teulon JM, Berrebeh N, Winter J, Adrover JM, Santos GS, Froese A, et al. Externalized histone H4 orchestrates chronic inflammation by inducing lytic cell death. Nature. 2019; 569:236–240. doi: 10.1038/s41586-019-1167-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corrales-Medina VF, Serpa J, Rueda AM, Giordano TP, Bozkurt B, Madjid M, Tweardy D, Musher DM. Acute bacterial pneumonia is associated with the occurrence of acute coronary syndromes. Medicine (Baltimore). 2009;88:154–159. doi: 10.1097/MD.0b013e3181a692f0. [DOI] [PubMed] [Google Scholar]
- 28.Violi F, Cangemi R, Falcone M, Taliani G, Pieralli F, Vannucchi V, Nozzoli C, Venditti M, Chirinos JA, Corrales-Medina VF; SIXTUS (Thrombosis-Related Extrapulmonary Outcomes in Pneumonia) Study Group. Cardiovascular Complications and Short-term Mortality Risk in Community-Acquired Pneumonia. Clin Infect Dis. 2017;64:1486–1493. doi: 10.1093/cid/cix164. [DOI] [PubMed] [Google Scholar]
- 29.Delgado-Rizo V, Martínez-Guzmán MA, Iñiguez-Gutierrez L, García-Orozco A, Alvarado-Navarro A, Fafutis-Morris M. Neutrophil Extracellular Traps and Its Implications in Inflammation: An Overview. Front Immunol. 2017;8:81. doi: 10.3389/fimmu.2017.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drechsler M, Megens RT, van Zandvoort M, Weber C, Soehnlein O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation. 2010;122:1837–1845. doi: 10.1161/CIRCULATIONAHA.110.961714. [DOI] [PubMed] [Google Scholar]
- 31.Döring Y, Drechsler M, Wantha S, Kemmerich K, Lievens D, Vijayan S, Gallo RL, Weber C, Soehnlein O. Lack of neutrophil-derived CRAMP reduces atherosclerosis in mice. Circ Res. 2012;110:1052–6. doi: 10.1161/CIRCRESAHA.112.265868. [DOI] [PubMed] [Google Scholar]
- 32.Döring Y, Libby P, Soehnlein O. Neutrophil Extracellular Traps Participate in Cardiovascular Diseases: Recent Experimental and Clinical Insights. Circ Res. 2020;126:1228–1241. doi: 10.1161/CIRCRESAHA.120.315931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Avondt K, Maegdefessel L, Soehnlein O. Therapeutic Targeting of Neutrophil Extracellular Traps in Atherogenic Inflammation. Thromb Haemost. 2019;119:542–552. doi: 10.1055/s-0039-1678664. [DOI] [PubMed] [Google Scholar]
- 34.Yang LY, Luo Q, Lu L, Zhu WW, Sun HT, Wei R, Lin ZF, Wang XY, Wang CQ, Lu M, et al. Increased neutrophil extracellular traps promote metastasis potential of hepatocellular carcinoma via provoking tumorous inflammatory response. J Hematol Oncol. 2020;13:3. doi: 10.1186/s13045-019-0836-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tohme S, Yazdani HO, Al-Khafaji AB, Chidi AP, Loughran P, Mowen K, Wang Y, Simmons RL, Huang H, Tsung A. Neutrophil Extracellular Traps Promote the Development and Progression of Liver Metastases after Surgical Stress. Cancer Res. 2016;76:1367–1380. doi: 10.1158/0008-5472.CAN-15-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sawabata N, Okumura M, Utsumi T, Inoue M, Shiono H, Minami M, Nishida T, Sawa Y. Circulating tumor cells in peripheral blood caused by surgical manipulation of non-small-cell lung cancer: pilot study using an immunocytology method. Gen Thorac Cardiovasc Surg. 2007; 55:189–192. doi: 10.1007/s11748-007-0101-2. [DOI] [PubMed] [Google Scholar]
- 37.Gerhardt T, Ley K. Monocyte trafficking across the vessel wall. Cardiovasc Res. 2015;107:321–330. doi: 10.1093/cvr/cvv147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rossaint J, Margraf A, Zarbock A. Role of Platelets in Leukocyte Recruitment and Resolution of Inflammation. Front Immunol. 2018;9:2712. doi: 10.3389/fimmu.2018.02712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soehnlein O, Lindbom L, Weber C. Mechanisms underlying neutrophil-mediated monocyte recruitment. Blood. 2009;114:4613–23. doi: 10.1182/blood-2009-06-221630. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Carmona-Rivera C, Moore E, Seto NL, Knight JS, Pryor M, Yang ZH, Hemmers S, Remaley AT, Mowen KA, et al. Myeloid-Specific Deletion of Peptidylarginine Deiminase 4 Mitigates Atherosclerosis. Front Immunol. 2018;9:1680. doi: 10.3389/fimmu.2018.01680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knight JS, Luo W, O’Dell AA, Yalavarthi S, Zhao W, Subramanian V, Guo C, Grenn RC, Thompson PR, Eitzman DT, et al. Peptidylarginine deiminase inhibition reduces vascular damage and modulates innate immune responses in murine models of atherosclerosis. Circ Res. 2014;114:947–956. doi: 10.1161/CIRCRESAHA.114.303312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Franck G, Mawson TL, Folco EJ, Molinaro R, Ruvkun V, Engelbertsen D, Liu X, Tesmenitsky Y, Shvartz E, Sukhova GK, et al. Roles of PAD4 and NETosis in Experimental Atherosclerosis and Arterial Injury: Implications for Superficial Erosion. Circ Res. 2018;123:33–42. doi: 10.1161/CIRCRESAHA.117.312494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Franck G, Mawson T, Sausen G, Salinas M, Masson GS, Cole A, Beltrami-Moreira M, Chatzizisis Y, Quillard T, Tesmenitsky Y, et al. Flow Perturbation Mediates Neutrophil Recruitment and Potentiates Endothelial Injury via TLR2 in Mice: Implications for Superficial Erosion. Circ Res. 2017;121:31–42. doi: 10.1161/CIRCRESAHA.117.310694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wildhagen KC, García de Frutos P, Reutelingsperger CP, Schrijver R, Aresté C, Ortega-Gómez A, Deckers NM, Hemker HC, Soehnlein O, Nicolaes GA. Nonanticoagulant heparin prevents histone-mediated cytotoxicity in vitro and improves survival in sepsis. Blood. 2014;123:1098–1101. doi: 10.1182/blood-2013-07-514984. [DOI] [PubMed] [Google Scholar]
- 45.Ostos MA, Recalde D, Zakin MM, Scott-Algara D. Implication of natural killer T cells in atherosclerosis development during a LPS-induced chronic inflammation. FEBS Lett. 2002;519:23–29. doi: 10.1016/s0014-5793(02)02692-3. [DOI] [PubMed] [Google Scholar]
- 46.Westerterp M, Berbée JF, Pires NM, van Mierlo GJ, Kleemann R, Romijn JA, Havekes LM, Rensen PC. Apolipoprotein C-I is crucially involved in lipopolysaccharide-induced atherosclerosis development in apolipoprotein E-knockout mice. Circulation. 2007;116:2173–81. doi: 10.1161/CIRCULATIONAHA.107.693382 [DOI] [PubMed] [Google Scholar]
- 47.Greifenberg V, Ribechini E, Rössner S, Lutz MB. Myeloid-derived suppressor cell activation by combined LPS and IFN-gamma treatment impairs DC development. Eur J Immunol. 2009;39:2865–76. doi: 10.1002/eji.200939486. [DOI] [PubMed] [Google Scholar]
- 48.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 49.Maruchi Y, Tsuda M, Mori H, Takenaka N, Gocho T, Huq MA, Takeyama N. Plasma myeloperoxidase-conjugated DNA level predicts outcomes and organ dysfunction in patients with septic shock. Crit Care. 2018;22:176. doi: 10.1186/s13054-018-2109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wichapong K, Poelman H, Ercig B, Hrdinova J, Liu X, Lutgens E, Nicolaes GA. Rational modulator design by exploitation of protein-protein complex structures. Future Med Chem. 2019;11:1015–1033. doi: 10.4155/fmc-2018-0433 [DOI] [PubMed] [Google Scholar]
- 51.van Zundert GCP, Rodrigues JPGLM, Trellet M, Schmitz C, Kastritis PL, Karaca E, Melquiond ASJ, van Dijk M, de Vries SJ, Bonvin AMJJ. The HADDOCK2.2 Web Server: User-Friendly Integrative Modeling of Biomolecular Complexes. J Mol Biol. 2016;428:720–725. doi: 10.1016/j.jmb.2015.09.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.