Abstract
Intervertebral disc (IVD) degeneration can cause chronic lower back pain (LBP), leading to disability. Despite significant advances in the treatment of discogenic LBP, the limitations of current treatments have sparked interest in biological approaches, including growth factor and stem cell injection, as new treatment options for patients with chronic LBP due to IVD degeneration (IVDD). Gene therapy represents exciting new possibilities for IVDD treatment, but treatment is still in its infancy. Literature searches were conducted using PubMed and Google Scholar to provide an overview of the principles and current state of gene therapy for IVDD. Gene transfer to degenerated disc cells in vitro and in animal models is reviewed. In addition, this review describes the use of gene silencing by RNA interference (RNAi) and gene editing by the clustered regularly interspaced short palindromic repeats (CRISPR) system, as well as the mammalian target of rapamycin (mTOR) signaling in vitro and in animal models. Significant technological advances in recent years have opened the door to a new generation of intradiscal gene therapy for the treatment of chronic discogenic LBP.
Keywords: genetic therapy, intervertebral disc degeneration, RNAi, mTOR signaling, CRISPR-Cas9, vector
1. Introduction
1.1. Basic Anatomy of Intervertebral Disc
The intervertebral disc (IVD) is made of fibrous cartilage and is the most important functional part of the spine, presenting in between the vertebral bones providing flexibility to the vertebrae. It consists of the nucleus pulposus (NP), surrounded by the annulus fibrosus (AF), and the cartilaginous endplate [1,2,3,4,5,6,7]. NP cells are similar to chondrocytes, which consist of collagen fibers embedded with the gel-like aggrecan. AF also consists of collagen fibers lying parallel with the lamellae, whereas the cartilaginous endplate is made up of a thin layer of hyaline. All these tissues being sandwiched together yields important functional properties to the disc [4,8,9]. IVD acts as a shock absorber, helps in the movement of the vertebrae, and holds the vertebrae together. IVD degeneration (IVDD) results in chronic lower back pain (LBP), which is now a global concern, and causes more dysfunction than any other medical situation [10].
1.2. Pathophysiology of IVD Degeneration
IVDD is the main cause of chronic LBP, which can lead to severe disabilities. It is a crucial factor in the degradation of the structure and function of the IVD. IVDD could alter the structure of the extracellular matrix (ECM), leading to the weakening of tissues and changes in the cells. These changes produce a functional effect on the IVD and ECM composition. This is a crucial factor in the breaking down of the structure and function of the IVD [1,2,4,8]. The main reasons associated with IVDD include nutritional, environmental, and genetic factors [11,12]. These factors lead to changes in cell morphology, inflammation, an increase in senescence cells, apoptosis, and autophagy [13,14,15,16,17,18]. IVDD is characterized by a loss of NP cells (more rounded chondrocyte-like cells) and their replacement by cells with a fibroblast-like phenotype [19]. Inflammatory responses exacerbated by pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF‑α) and interleukin-1β (IL‑1β), are known as major events that occur during IVDD and associated chronic LBP [20]. Senescent cells secrete a senescence-associated secretory phenotype (SASP), which increases the secretion of pro-inflammatory cytokines, chemokines, and tissue-damaging proteases. Increased amounts of senescent cells have been found in the degenerated disc [21]. Moreover, apoptosis and autophagy are known for two patterns of programmed cell death and play important roles in IVDD [22]. In a study, change by age or by heredity shows significant role for the defect in endplate of disc in association with IVDD [23]. IVDD is usually associated with high catabolism and less anabolism in the ECM. One of the main causes of IVDD is a change in the nutrition levels in the discs, which progresses in the presence of a reduced supply of oxygen and low pH; conditions that are harmful to the ECM of the IVD [16]. The possible environmental risk factors for IVDD are smoking, lack of exercise, an unsanitary lifestyle, trauma, and mechanical load. Moreover, with aging, an alteration in the disc ECM reduces the aggrecan level in the NP of the disc, and a decrease in the level of hydration along with functional changes of the matrix also occur. As a result, dehydrated NPs are unable to properly balance the forces in adjacent vertebrae. Thus, the forces eventually become dispersed in the AF region leading to mechanical and gradual structural destruction, which results in a radial annular tear and herniation of the NP region [15].
Some macroscopic changes include annulus tear, decreased disc hydration, loss of disc height, lamella disorganization of the annulus, and osteophytes formation [24,25]. These are generally common in the composition of the ECM. Molecular changes include alterations in genes, protein expression, matrix synthesis, catabolic mechanisms, growth factors, and cytokines [26]. Matrix changes are related to the anabolic mechanism; collagens and proteoglycans (PG) are the two main matrices involved in the degeneration process. A reduction in PG content in the NP is the starting process for the deterioration of the IVD [15]. Because the disc is structurally devoid of blood vessels, it is a hypoxic environment in which oxygen and nutrients are supplied through capillaries in the endplate. Therefore, as disc degeneration progresses, inflammation leads to more hypoxic and acidic conditions [4]. However, NP cells must proliferate and differentiate with a low metabolism, so the catabolic activity must be downregulated to maintain balance. Gene therapy, growth factors, and cell therapy could promote matrix formation. To induce disc regeneration by anabolism, high energy is required for matrix formation. On the other hand, with less energy and resources, different approaches, such as RNA interference (RNAi), can be used to promote disc regeneration. This helps to maintain homeostasis by upregulating anabolism and downregulating catabolism [27] (Figure 1).
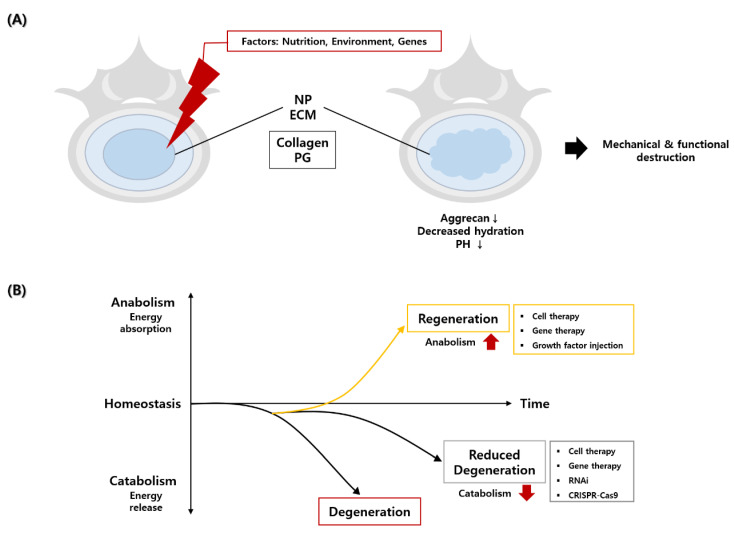
Figure 1.
A schematic diagram of the cause of intervertebral disc degeneration and strategies to reduce disc degeneration and promote disc regeneration. (A) Nutritional, environmental, and genetic factors have caused changes in collagen and proteoglycan (PG) and the composition of the extracellular matrix (ECM) in nucleus pulposus (NP), resulting in a decrease in aggrecan and pH and loss of hydration, which systematically and functionally destroyed NP. (B) Regeneration of degenerated disc could be induced by an increase in anabolism and a decrease in disc degeneration could be induced by a decrease in catabolism.
1.3. Discogenic Low Back Pain (LBP)
Discogenic LBP is a multifactorial and complex disease and is the most common type of chronic LBP, accounting for 39% of cases. Discogenic LBP is caused by an inflammatory response during IVDD [28]. The increase in pro-inflammatory cytokines produced by degenerated and senescent disc cells has been considered as a nociceptive and noxious trigger of the painful condition [29].
1.4. Current Treatments for Chronic LBP due to IVD Degeneration
Current treatments for chronic LBP caused by IVDD include physiotherapy, medication, and surgery [30,31]. If there is no response to conservative treatments, surgery can be done. Surgery involves partial resection of the herniated disc that compresses the nerve roots and a fusion surgery, in which the intervertebral cage is inserted after the disc is completely removed [32,33]. However, many patients suffer from post spinal surgery syndrome (PSSS), that is, persistent or recurrent LBP after surgery [1,4]. The prevalence of PSSS has been reported to be approximately 30% [34,35]. There is a need for the development of new biological treatments that can be clinically applied for the purpose of inhibiting underlying disc degeneration, protecting degenerated discs, or regenerating the discs. This may include injection of various growth factors with or without carriers, use of cells with or without scaffolds, and genetic modification of gene expression through gene therapy. To date, these treatments have been studied in an animal model, but there are no biological agents for IVD regeneration [27,36].
2. Search Strategy
We searched the keywords of “Intervertebral disc”, “Gene therapy”, “RNA interference”, “CRISPR”, “autophagy”, and “mTOR signaling” in PubMed (https://pubmed.ncbi.nlm.nih.gov/) and Google Scholar from January 1997 and December 2020 and reviewed full-text articles (CRISPR: gene editing by the clustered regularly interspaced short palindromic repeats; mTOR: mammalian target of rapamycin).
3. Biological Approaches
As there are still many patients with chronic discogenic LBP who require refractory to conservative (medication and physiotherapy) and surgical treatments, there is an increasing unmet need for biological approaches including growth factor injection, cell-based therapy, tissue engineering, and gene therapy [27].
3.1. Growth Factor Injection
This is a method in which growth factors are injected directly into the degenerated disc by inducing matrix synthesis and preventing inflammation in the degenerated disc. A representative example is growth differentiation factor (GDF)-5, which plays an important role in the formation of the core of bones and joints, and in cell growth and differentiation in embryonic development [9,37]. One study showed the recovery effect of GDF-5 injection in the rabbit disc degeneration model [38]. However, when only the growth factor itself is injected, the long-term persistence within the disc is significantly lower [39]. In order to overcome these shortcomings, treatments such as gene therapy and tissue engineering are gradually being developed. In a recent study, GDF-5 injection was performed through tissue engineering in the form of injecting scaffolds or stem cells in a rat model [40].
In addition, numerous growth factors, including bone morphogenetic proteins (BMPs), insulin-like growth factor-1 (IGF-1), and transforming growth factor-β (TGF-β), have been reported to have therapeutic effects that slow or reverse IVDD [15]. In cartilage, TGF-β has been shown to participate in processes including cartilage formation, metalloproteinase production, and inflammatory responses. Gene expression of TGF-β was found to be promoted in osteoarthritis cells similar to those observed in degenerative disc cells. TGF-β and TGF-β type II receptors are generally present in herniated disc cells and in specimens of non-herniated human discs [15,41,42].
3.2. Cell Therapy
Among the various biological approaches, cell-based therapy appears to be the most promising technology for the treatment of IVDD. In this process, mesenchymal stem cells (MSCs) isolated from bone marrow, adipose tissue, umbilical cord Wharton’s jelly, and synovial membrane have been used due to their self-renewal capacity, anti-inflammatory properties, and their ability to regenerate degenerated disc cells [43,44,45]. The mechanism of stem cell therapy has been reported to have a paracrine effect (anti-inflammatory, anti-apoptotic), be capable of the restoration of degenerated discs, and initiate differentiation of implanted stem cells into NP cells [3,4,5,6,7,8,46].
3.3. Tissue Engineering
Cell therapy has shown some limitations in the repair of IVDD during studies in vitro, in vivo, and in many clinical trials [47]. From this point of view, the tissue engineering approach (the combination of growth factors, stem cells, and a scaffold) is more important because of the positive results of using different types of functional polymers such as chitosan, collagen, gelatin, alginate, hyaluronic acid, polyethylene glycol, polyurethane, polylactic acid, and polyglycolic acid. Based on cells and scaffolds, this strategy is an effective treatment for IVDD [48]. Because of its advantages, many researchers have developed various tissue engineering techniques for the replacement of degenerated discs in animal models [49,50,51]. For mild and moderate IVDD, stem cell transplantation may be an effective treatment. However, for severe IVDD that requires structural support, multifunctional treatment is possible through tissue engineering [4,5,6,7,8,46].
4. Gene Therapy
IVDD involves significant genetic risk factors. It has been reported that an imbalance in the synthesis and catabolism of certain important ECM components can be alleviated by transferring genes to IVD cells that encode factors that regulate matrix synthesis and catabolism [52]. Moreover, successful gene transfer to target cells within IVD in clinically relevant animal models of IVDD represents exciting new possibilities for the treatment of IVDD [52]. Here, we briefly summarize gene transfer to disc cells in in vitro and animal models, gene silencing via RNAi, gene editing via the clustered regularly interspaced short palindromic repeats (CRISPR) system, and the mammalian target of rapamycin (mTOR) signaling [53,54] (Table 1).
Table 1.
Recent studies of gene therapy associated with intervertebral disc degeneration.
| Gene Therapy | Target Gene | Target Cell | Reference | |
|---|---|---|---|---|
| Viral vector | Retro | Bacterial lacZ, Human IL-1 receptor antagonist | Bovine chondrocytic cell | [55] |
| Adeno | Bacterial lacZ | RabbitdiscNP cell | [56] | |
| Adeno-associated | AP-2α, TGF-β1 | RatdiscNP cell | [59] | |
| Baculo | GFP | RabbitdiscNP cell | [62] | |
| Lenti | MMP3, Sox9 | RabbitdiscNP cell | [63] | |
| Nonviral Vector |
RNAi | Klotho, TLR4 | RatdiscNP cell | [64] |
| UTMD | GFP, Firefly luciferase | RatdiscNP cell | [67] | |
| Polyplex micelle | miRNA-25-3p, Firefly luciferase | HumanandRatdiscNP cell | [68] | |
| CRISPR | Cas9 | TRPV4 | HumandiscAF cell | [79] |
| mTOR pathway |
PI3K/AKT | GSK-3β, NF-kappaB, caspase3, mTOR | RatdiscNP cell | [87] |
Abbreviations: AF, annulus fibrosus; AP, activator protein; GFP, green fluorescence protein; IL, interleukin; NP, nucleus pulposus; miRNA, MicroRNA; MMP, matrixmetalloproteinase; mTOR, mammalian target of rapamycin; PI3K, phosphoinositide 3-kinase; Sox, SRY-box transcription factor; TGF, Transforming Growth Factor; TLR, Toll-like receptor; TRP, transient receptor potential; UTMD, ultrasound targeted microbubble destruction.
4.1. Gene Transfer to Target Disc Cells In Vitro andIn Vivo
One of the technologies required for gene therapy is a method of delivering the desired gene to the target. As an essential vehicle, a vector facilitates translocation into the nucleus along with the expression of the transgene. This is useful for transferring the gene of interest, i.e., complementary DNA (cDNA), a reverse-transcribed DNA molecule of the mRNA, to the host cells. Viral vectors have been widely used because they can efficiently rearrange their genetic material; however, non-viral mediated gene transfer to disc cells has been developed because of the potential risks associated with viral gene therapy [2,27].
4.1.1. Virus Vector-Mediated Gene Transfer to Disc Cells
Retrovirus
In 1997, Wehling et al. [55] first introduced the idea of using in vitro gene transfer to reverse IVDD and successfully transfected the target gene into the in vitro-cultured chondrocyte cells isolated from bovine coccygeal vertebral endplates, using a retrovirus vector. The bacterial β-galactosidase (lacZ) gene and the cDNA of the human interleukin-1 (IL-1) receptor antagonist, were introduced into the chondrocyte cells. Transfer of the β-galactosidase (lacZ) gene to cultured cells produced approximately 1% lacZ positive cells, and transfer of the IL-1 receptor antagonist cDNA produced 24 ng/mL/106 cells of the IL-1 receptor antagonist protein. This study showed the potential of ex vivo gene therapy for the degenerated disc [2].
Adenovirus
In 1998, Nishida et al. [56] reported the transfer of the lacZ gene to the disc in both in vitro and in vivo rabbit models using an adenovirus vector. This report was considered to be the first example of gene therapy that targeted NP cells in vitro and in vivo. For the in vitro model, disc NP cells isolated from female New Zealand white rabbits were cultured and transfected with an adenovirus construct encoding the lacZ gene. For the in vivo model, an adenovirus construct encoding the lacZ gene was injected directly into the NP of the rabbit’s lumbar IVD. X-Gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside) staining in vitro and in vivo showed the efficient transduction of disc NP cells, and reporter gene expression in vivo lasted for at least 12 weeks. This study showed that the exogenous gene was successfully delivered to the disc via an adenovirus vector in vitro and in vivo, demonstrating the potential of direct gene therapy for IVDD treatment [57,58].
Adeno-Associated Virus (AAV)
The activatorprotein-2α (AP-2α) has been reported to be involved in IVDD by regulating the expression of TGF-β1 and Smad3 [59]. AP-2α and TGF-β1 were upregulated in NP tissues of patients and rats with IVDD. Injection of low-expressing AP-2α and high-expressing TGF-β1 adeno-associated viral vectors into the IVD of rats reduced the expression of matrix metalloproteinase (MMP)-2, MMP-9, and Smad3 in NP tissue and increased the expression of aggrecan and Col-2. This study confirmed that knockdown of AP-2α restricted the expression of TGF-β1 and Smad3 to promote proliferation and reduce the senescence and apoptosis of NP cells in rats with IVDD [59]. AAVs could be effective tools for gene therapy [60,61].
Baculovirus
Liu et al. [62] treated rabbit IVD cells with baculovirus carrying the green fluorescence protein gene (Ac-CMV-GFP). As a result of confirming the expression of GFP in NP tissue by injection into the intervertebral discs of rabbits on days 7, 13, 20, and 28, Ac-CMV-GFP was expressed for a long time without toxicity to cells, showing the highest transduction rate, and GFP expression level at 7 days after transduction. This study indicates that baculovirus with exogenous genes can be safely used with high efficiency in rabbit NP cells, suggesting that it is a useful tool as a gene therapy vector for IVDD.
Lentivirus
In 2020, Zhao et al. [63] injected lentiviral vectors (LV-MMP3-shRNA and/or LV-Sox9) into rabbit lumbar discs to confirm that lentivirus-mediated MMP3 knockdown could attenuate IVDD. Magnetic resonance imaging (MRI) scans after 8, 12, and 24 weeks showed that IVDD was observed in animals injected with phosphate-buffered saline or 10^7 viral particles of the control virus. In contrast, IVDD was suppressed by 10^7 viral particles of LV-MMP3-shRNA or LV-Sox9. In addition, MMP3 knockdown or Sox9 overexpression stimulated collagen type II, aggrecan, and proteoglycan synthesis. In addition, the injection of a cocktail of LV-MMP3-shRNA and LV-Sox9 (5 × 106viral particles, respectively) significantly delayed the development of IVDD and induced the production of the highest levels of collagen type II and proteoglycan. This study suggests that gene therapy targeting MMP3 is an effective way to delay IVDD in NP tissue.
4.1.2. Non-Virus Vector-Mediated Gene Transfer to Disc Cells
RNA Interference (RNAi)
In 2020, Bi et al. [64] attenuated H₂O₂ induced acute inflammation by overexpressing the Klotho gene using RNAi using the IVDD rat model and inhibiting Toll-like receptor 4 (TLR4). As a result, Klotho inhibition and elevated TLR4 levels showed pro-inflammatory nuclear factor kappa B (NF-κB) signaling and cytokine expression in NP cells of all animal groups. When TLR4 overexpression reduced Klotho expression and interfered with TLR4 expression, the inhibitory effect of H₂O₂ was reduced in NP cells. Klotho knockdown by RNAi reduced the anti-inflammatory and IVD protective effects of the IVD degeneration model. Therefore, this study suggests that the expression of Klotho regulates TLR4-NF-κB signaling transduction [2,65,66].
Ultrasound Targeted Microbubble Destruction
In 2006, Nishida et al. [67] injected plasmid DNA encoding Green Fluorescence Protein (GFP)and luciferase into the IVD in a rat model. After being observed for 1, 3, 6, 12, and 24 weeks, the results showed that expression of the GFP transgene—visualized using ultrasonic target microbubble destruction—was clearly observed in NP cells. Luciferase analysis also revealed an 11-fold increase in luciferase activity in this group compared to the plasmid DNA group. In this study, using the ultrasonic-targeted microbubble destruction transfection method significantly improved the transfection efficiency of plasmid DNA in NP cells, which retained gene expression for up to 24 weeks.
Polyplex Micelle
In 2020, Huang et al. [68] observed the effect of mixed cationic block copolymers (MCBC) PNIPAM-b-PAsp (DET) and PEG-b-PAsp (DET) polyplex micelles using an miRNA-25-3p vector as the therapeutic agent in an IVDD rat model. It has been observed that IL-1β, ZIP8, and MTF1 increased and miRNA-25-3p decreased in degenerated tissue compared to normal tissue. The treatment of miRNA-25-3p inhibited ECM degrading enzyme expression and restored ECM protein expression. MCBC was able to effectively deliver miRNA-25-3p to NP cells, which delayed the progression of IVDD. In this study, polyplex micelles made from a vector that delivers miRNA-25-3p could be applied as a therapeutic agent [2,69,70,71,72].
To date, approximately 40% of adenovirus and retroviral vectors are used in clinical trials. However, research on non-viral vectors that can replace viral vectors is also underway due to various side effects and risks associated with viral vectors. Research and development to overcome the problems of viral vectors and nonviral vectors is actively being conducted, and many studies are being conducted. For the most promising gene therapy in terms of the structural properties and function of IVD cells, it is important to invasively target only IVD cells. Vectors that have been developed to date that are required for gene targeting still need further development. If such challenges can be overcome, safe and efficient clinical applications in IVDD treatment will be possible.
4.2. Clustered Regulatory Interspaced Short Palindromic Repeats-Associated Cas9 (CRISPR/Cas9)
CRISPR/Cas9 is a convenient and versatile tool for modifying the genome. This is one of the more accurate and efficient treatments and is easier to use compared to other genome editing technologies [73]. The working principle of CRISPR-Cas9 is detailed in Figure 2 [74]. In 1987, Ishino et al. originally discovered CRISPR and subsequently observed CRISPR-associated Cas gene proteins. Later, the CRISPR/Cas9 editing system was first described by Cong et al. in 2013 [75]. The system has been used in both viral and non-viral delivery methods [76]. For the gene therapy strategy of IVDD, the regulation of cytokine receptors, TNF receptor 1(TNFR1) and IL-1 receptor 1 (IL1R1) signaling by a lentiviral CRISPR epigenome editing system was tested in human IVD cells to downregulate TNFR1 and IL1R1 pro-inflammatory signals [2,69]. They also showed that TNFR1 expression was downregulated by increasing aggrecan and decreasing MMP3 levels in TGFR1 genome editing. However, in the case of IL1R1 genome editing, IL1R1 expression was not downregulated and did not show any changes in aggrecan and MMP3 [77,78]. Thus the ability of the CRISPR/Cas9 genome editing system can be demonstrated by regulation of TNFR1 but not by IL1R1. This shows that regulation of these genes requires a finite perspective [2,77,78].
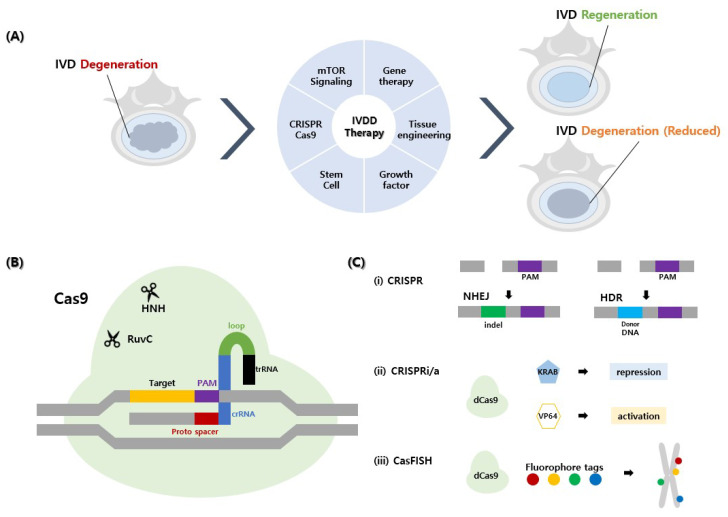
Figure 2.
Schematic illustration of novel biological approaches and CRISPR-Cas9 as a gene therapy for the treatment of intervertebral disc degeneration (IVDD). Representative newly discovered biological approaches for the treatment of IVDD are shown as follows. (A) A simple diagram illustrating regeneration of IVDD using gene therapy, tissue engineering, growth factor, stem cell, CRISPR-Cas9, and mTOR signaling. (B) The form in which sgRNA, Cas9, and DNA are attached is called CRISPR-Cas9. sgRNA consisting of proto-spacer, crRNA, loop, and trRNA is cut and attached to the DNA proto spacer adjacent motif (PAM) sequence through Cas9 using HNH and Ruvc scissors. (C) The recovery mechanism of CRISPR-Cas9 in the intervertebral disc is as follows: (i) non-homologous end joining (NHEJ) of CRISPR inserts or deletes indels, and homology-directed repair (HDR) inserts new segments of DNA. (ii) CRISPR interference (CRISPRi) and CRISPR activation (CRISPRa) use dead Cas9 (dCas9) as a technology to mediate transcription site gene expression but have no cleavage effect and guide-target DNA at the start site of transcription. When combined with the KRAB domain, it inhibits transcription, and when combined with VP64, it activates the transcription of target DNA. (iii) Cas9-mediated fluorescence in situ hybridization (CASFISH) is dCas9, which enables DNA observation using fluorophore tags (multicolor). CRISPR: clustered regularly interspaced short palindromic repeats.
Similarly, Cambria et al. [79] reported that the transient receptor potential vanilloid type 4 (TRPV4) gene was successfully knocked out in vitro by the CRISPR/Cas9 gene-editing system in AF-injured patients with chronic LBP. By using CRISPR, they investigated the role of the TNFR4 gene in the regulation of IL6 and IL8 using CRISPR and the reduction in inflammation induced by hyper physiological stretching of AF cells. They also suggested that in the future studies, this could be used for targeting other genes for treatment of IVDD. Stover et al. [80] investigated changes in dorsal root ganglion (DRG) neuron activity in a rat model and demonstrated a CRISPR epigenetic editing system for pain-based neuromodulatory therapy in IVDD. They used the lentiviral CRISPR epigenome editing vectors to target the AKAP150 gene in peripheral neurons and demonstrated the effects of target promoter histone methylation on gene expression. After delivery to DRG neurons, epigenome editing vectors induced increased neuronal activity [69,80]. Krupkova et al. suggested that CRISPR/Cas9 can be applied to gene knockout, gene editing, inhibition, and gene expression activation, suggesting that these methods could be promising new tools that may play a role in IVDD treatment [53,81].
4.3. Correlation between IVD Degeneration and mTOR Signaling
mTOR is a serine/threonine protein kinase, considered as a necessary mediator for cellular metabolism, which determines whether catabolic and anabolic effects occur depending on nutritional levels. The ability to regulate anabolism and catabolism in vivo may reduce IVDD and increase disc regeneration [82]. mTOR contains serine and threonine proteins, which are necessary for processes related to mammalian survival, such as cell growth, proliferation, motility, survival, and autophagy.
Studies have shown that mTOR signaling regulates cells during metabolic processes. It induces anti-apoptosis, proautophagy, anti-matrix catabolism, and anti-senescence in disc cells. Studies on the Akt-dependent effect of inhibition of mTOR complex 1 (mTORC1) on mTOR have confirmed that mTOR signaling plays an important regulatory role in disc cells [3,83]. In addition, the roles and involvement of autophagy and mTOR signaling in IVDD were confirmed through both in vitro and in vivo studies using human and rabbit disc cells, with increased autophagy, increased disc cell death, apoptosis, metabolism, and increased aging also being noted [54]. Cell loss is a major feature of IVDD and is caused by cell apoptosis. In humans and rodents, the incidence of cell apoptosis is high with disc aging or degeneration [84]. Based on the results of the study, autophagy and mTOR signaling have been demonstrated to be used to combat harsh disc environments such as low glucose, low oxygen, acidic pH, and limited nutrient availability under an inflammatory milieu [83,85]. Selective RNAi-mediated and pharmacological inhibition of mTORC1 protects against inflammation-induced apoptosis, senescence, and ECM catabolism in NP cells [54]. In addition, studies have shown protective effects of rapamycin (mTORC1 inhibitor) against inflammation-induced apoptosis and catabolic gene expression in human chondrocytes [86]. Activation of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway protects against IVDD through multiple mechanisms, including down regulation of MMP-3 and MMP-13 expression and upregulation of Sox9 expression, reduction in caspase-3 activity, and inhibition of apoptosis through activating mTOR [87]. It has been reported that resveratrol—in combination with 17β-estradiol—increases levels of activated phosphorylated mTOR (P-mTOR) and phosphorylated glycogen synthase kinase-3β (P-GSK-3β) leading to the down-regulation of caspase-3, inhibition of IL-1β-induced NP cell apoptosis and recovery of cell viability [88].
The importance of mTOR in IVDD has recently received a great deal of attention due to the high levels of mTOR signaling molecule expression and phosphorylation in human intermediate degenerated discs [54]. Therefore, an accurate understanding of the underlying molecular mechanisms is important to directly guide the development of biological therapies that target the mTOR signaling pathway for treating IVDD.
5. Future Perspectives
IVDD is a multifactorial disease involving the occurrence of both environmental and genetic effects at the same time. The mechanisms of IVDD have been clarified to some extent, but they have not been completely elucidated. Recent advances in science and research have improved our understanding of the mechanisms involved. Genetic mutations of genes involved in matrix formation could affect disc function or biochemical processes, which could lead to IVDD. Gene therapy, as a promising field of study, has the potential to exert therapeutic effects on the treatment of IVDD.
For gene therapy, gene editing technology CRISPR-Cas9 is an innovative technology in the scientific and medical fields that can modify target genes, which could potentially lead to accurate and efficient therapeutic effects. However, for gene editing technology, there are many concerns related to its clinical application, such as off-target effects, low editing efficiency, and immunogenicity. Gene editing technology can be clinically applied if off-target effects can be resolved. Immunogenicity and mutational variability are problems that arise when using CRISPR-Cas9 with viral vectors. If these technologies are to be applied to treat diseases, safer non-viral vectors need to be optimized and improved. In order for CRISPR-Cas9 to be applied for clinical use, it is necessary to focus on solving the problems presented. In addition, new research efforts are essential for enabling future clinical applications [74,88]. mTOR signaling is associated with IVDD in animal models. With this in mind, future therapeutic strategies for IVD regeneration based on gene therapy could target the destruction of mTORC1 and Regulatory-associated protein with mTORRAPTOR. Safety, high cost, and transfection are challenges that must be overcome for the development of IVDD treatments.
A direction for future studies could be to focus specifically on prophylactic and long-term regenerative treatments. Additionally, it is important to investigate more genetic defects. Advances in molecular and cellular biology, including genetic research, have enabled targeted therapies, and studies of intracellular signaling pathways need to move in the direction of providing targeted therapeutic pathways. More innovative technologies will be needed to overcome the obstacles encountered in gene therapy. As research related to gene therapy grows and gradually expands, IVDD may effectively overcome be in the future [2,9,89].
Abbreviations
| AAV | Adeno-associated virus |
| Ac-CMV-GFP | ArecombinantBaculovirusVector expression GFP |
| AF | Annulus Fibrosus |
| AP-2α | ActivatorProtein-2α |
| BMP | BoneMorphogeneticProtein |
| CASFISH | Cas9-mediated Fluorescence In situ Hybridization |
| CRISPR | Clustered Regulatory Interspaced Short Palindromic Repeats |
| CRISPRi | CRISPR interference |
| CRISPRa | CRISPR activation |
| crRNA | CRISPR RNA |
| DRG | DorsalRootGanglion |
| ECM | Extracellular matrix |
| GDF | GrowthDifferentiationFactor |
| GFP | GreenFluorescenceProtein |
| HDR | Homology directed repair |
| IGF-1 | Insulin-likeGrowthFactor-1 |
| IL | Interleukin |
| IL‑1β | Interleukin-1β |
| IL‑1α | Interleukin-1α |
| IVD | Intervertebral disc |
| IVDD | Intervertebral disc degeneration |
| LBP | LowBackPain |
| MCBC | MixedCationicBlockCopolymers |
| miRNA | MicroRNA |
| MMP | MatrixMetalloproteinase |
| MRI | Magnetic Resonance Imaging |
| RNAi | RNA Interference |
| MSCs | Mesenchymal stem cells |
| mTOR | Mechanistic target of rapamycin |
| mTORC1 | mTOR complex 1 |
| NHEJ | Non-homologous end joining |
| NP | Nucleus pulposus |
| SASP | Senescence-AssociatedSecretoryPhenotype |
| PG | Proteoglycan |
| P-GSK-3β | phosphorylated glycogen synthase kinase-3 β |
| PI3K | Phospoinositide3-Kinase |
| P-mTOR | Phosphorylated-mTOR |
| PSSS | PostSpinalSurgerySyndrome |
| RAPTOR | Regulatory-associated protein with mTOR |
| RICTOR | Rapamycin-insensitive companion of mTOR |
| RSV | Resveratrol |
| sgRNA | Single-guide RNA |
| Sox | SRY-Boxtranscriptionfactor |
| TGF-β | Transforming growth factor-β |
| TLR4 | Toll-likeReceptor4 |
| TNF‑α | TumorNecrosisFactor- α |
| trRNA | trans-activating crRNA (tracrRNA) |
| UTMD | Ultrasound-targeted microbubble destruction |
| VP | ViralParticle |
Author Contributions
Conceptualization and methodology, I.H.; writing; E.J.R., A.D.; data acquisition, J.W.K., H.C., S.Y.K., B.B., K.T.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Korea Health Technology Research and Development Project, Ministry for Health and Welfare Affairs (HR16C0002, HI20C0579).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dowdell J., Erwin M., Choma T., Vaccaro A., Iatridis J., Cho S.K. Intervertebral Disk Degeneration and Repair. Neurosurgery. 2017;80:S46–S54. doi: 10.1093/neuros/nyw078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takeoka Y., Yurube T., Nishida K. Gene Therapy Approach for Intervertebral Disc Degeneration: An Update. Neuro-spine. 2020;17:3–14. doi: 10.14245/ns.2040042.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han I. Moving Forward: Gene Therapy for Intervertebral Disc Degeneration. Neurospine. 2020;17:17–18. doi: 10.14245/ns.2040108.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han I., Ropper A.E., Konya D., Kabataş S., Toktas Z., Aljuboori Z., Zeng X., Chi J.H., Zafonte R., Teng Y.D. Biological Approaches to Treating Intervertebral Disk Degeneration: Devising Stem Cell Therapies. Cell Transplant. 2015;24:2197–2208. doi: 10.3727/096368915X688650. [DOI] [PubMed] [Google Scholar]
- 5.Choi U., Joshi H.P., Payne S.L., Kim K.-T., Kyung J.W., Choi H., Cooke M.J., Kwon S.Y., Roh E.J., Sohn S., et al. An Injectable Hyaluronan–Methylcellulose (HAMC) Hydrogel Combined with Wharton’s Jelly-Derived Mesenchymal Stromal Cells (WJ-MSCs) Promotes Degenerative Disc Repair. Int. J. Mol. Sci. 2020;21:7391. doi: 10.3390/ijms21197391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muttigi M.S., Kim B.J., Choi B., Han I., Park H., Lee S.-H. Matrilin-3-Primed Adipose-Derived Mesenchymal Stromal Cell Spheroids Prevent Mesenchymal Stromal-Cell-Derived Chondrocyte Hypertrophy. Int. J. Mol. Sci. 2020;21:8911. doi: 10.3390/ijms21238911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muttigi M.S., Kim B.J., Kumar H., Park S., Choi U.Y., Han I., Park H., Lee S.-H. Efficacy of matrilin-3-primed adi-pose-derived mesenchymal stem cell spheroids in a rabbit model of disc degeneration. Stem Cell Res. Ther. 2020;11:1–12. doi: 10.1186/s13287-020-01862-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahn J., Park E.M., Kim B.J., Kim J.S., Choi B., Lee S.H., Han I. Transplantation of human Wharton’s jelly-derived mesen-chymal stem cells highly expressing TGFbeta receptors in a rabbit model of disc degeneration. Stem Cell Res.Ther. 2015;6:190. doi: 10.1186/s13287-015-0183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kennon J.C., Awad M.E., Chutkan N., DeVine J., Fulzele S. Current insights on use of growth factors as therapy for in-tervertebral disc degeneration. Biomol. Concepts. 2018;9:43–52. doi: 10.1515/bmc-2018-0003. [DOI] [PubMed] [Google Scholar]
- 10.Kos N., Gradisnik L., Velnar T. A Brief Review of the Degenerative Intervertebral Disc Disease. Med Arch. 2019;73:421–424. doi: 10.5455/medarh.2019.73.421-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakai D., Grad S. Advancing the cellular and molecular therapy for intervertebral disc disease. Adv. Drug Deliv. Rev. 2015;84:159–171. doi: 10.1016/j.addr.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Vo N.V., Hartman R.A., Patil P.R., Risbud M.V., Kletsas D., Iatridis J.C., Hoyland J.A., Le Maitre C.L., Sowa G.A., Kang J.D. Molecular mechanisms of biological aging in intervertebral discs. J. Orthop. Res. 2016;34:1289–1306. doi: 10.1002/jor.23195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kepler C.K., Ponnappan R.K., Tannoury C.A., Risbud M.V., Anderson D.G. The molecular basis of intervertebral disc degeneration. Spine J. 2013;13:318–330. doi: 10.1016/j.spinee.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Risbud M.V., Shapiro I.M. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat. Rev. Rheumatol. 2014;10:44–56. doi: 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rizvi M. Novel treatment strategies for intervertebral disc degeneration. Saudi J. Heal. Sci. 2015;4:5. doi: 10.4103/2278-0521.151403. [DOI] [Google Scholar]
- 16.Sampara P., Banala R.R., Vemuri S.K., Av G.R., Gpv S. Understanding the molecular biology of intervertebral disc de-generation and potential gene therapy strategies for regeneration: A review. Gene Ther. 2018;25:67–82. doi: 10.1038/s41434-018-0004-0. [DOI] [PubMed] [Google Scholar]
- 17.Zhang S., Hu B., Liu W., Wang P., Lv X., Chen S., Shao Z. The role of structure and function changes of sensory nervous system in intervertebral disc-related low back pain. Osteoarthr. Cartil. 2021;29:17–27. doi: 10.1016/j.joca.2020.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Zhang F., Zhao X., Shen H., Zhang C. Molecular mechanisms of cell death in intervertebral disc degeneration (Review) Int. J. Mol. Med. 2016;37:1439–1448. doi: 10.3892/ijmm.2016.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Uden S., Silva-Correia J., Oliveira J.M., Reis R.L. Current strategies for treatment of intervertebral disc degeneration: Substitution and regeneration possibilities. Biomater. Res. 2017;21:22. doi: 10.1186/s40824-017-0106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson Z.I., Schoepflin Z.R., Choi H., Shapiro I.M., Risbud M.V. Disc in flames: Roles of TNF-alpha and IL-1beta in in-tervertebral disc degeneration. Eur. Cell Mater. 2015;30:104–117. doi: 10.22203/eCM.v030a08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patil P., Niedernhofer L.J., Robbins P.D., Lee J., Sowa G., Vo N. Cellular senescence in intervertebral disc aging and de-generation. Curr. Mol. Biol. Rep. 2018;4:180–190. doi: 10.1007/s40610-018-0108-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J., Ni B.-B., Li B., Yang Y.-H., Jiang S.-D., Jiang L.-S. The Responses of Autophagy and Apoptosis to Oxidative Stress in Nucleus Pulposus Cells: Implications for Disc Degeneration. Cell. Physiol. Biochem. 2014;34:1175–1189. doi: 10.1159/000366330. [DOI] [PubMed] [Google Scholar]
- 23.Maatta J.H., Kraatari M., Wolber L., Niinimaki J., Wadge S., Karppinen J., Williams F.M. Vertebral endplate change as a feature of intervertebral disc degeneration: A heritability study. Eur. Spine J. 2014;23:1856–1862. doi: 10.1007/s00586-014-3333-8. [DOI] [PubMed] [Google Scholar]
- 24.Marchand F., Ahmed A.M. Investigation of the laminate structure of lumbar disc anulusfibrosus. Spine. 1990;15:402–410. doi: 10.1097/00007632-199005000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Urban J.P., Roberts S. Degeneration of the intervertebral disc. Arthritis Res. Ther. 2003;5:120–130. doi: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoogendoorn R., Zandieh-Doulabi B., Huang C.L., Wuisman P.I., Bank R.A., Helder M.N. Molecular Changes in the Degenerated Goat Intervertebral Disc. Spine. 2008;33:1714–1721. doi: 10.1097/BRS.0b013e31817d2468. [DOI] [PubMed] [Google Scholar]
- 27.Nishida K., Suzuki T., Kakutani K., Yurube T., Maeno K., Kurosaka M., Doita M. Gene therapy approach for disc degen-eration and associated spinal disorders. Eur. Spine J. 2008;17:459–466. doi: 10.1007/s00586-008-0751-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mosley G.E., Evashwick-Rogler T.W., Lai A., Iatridis J.C. Looking beyond the intervertebral disc: The need for behavioral assays in models of discogenic pain. Ann. New York Acad. Sci. 2017;1409:51–66. doi: 10.1111/nyas.13429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujii K., Yamazaki M., Kang J.D., Risbud M.V., Cho S.K., Qureshi S.A., Hecht A.C., Iatridis J.C. Discogenic Back Pain: Literature Review of Definition, Diagnosis, and Treatment. JBMR Plus. 2019;3:e10180. doi: 10.1002/jbm4.10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knezevic N.N., Mandalia S., Raasch J., Knezevic I., Candido K.D. Treatment of chronic low back pain–new approaches on the horizon. J. Pain Res. 2017;10:1111–1123. doi: 10.2147/JPR.S132769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levine J.M., Levine G.J., Johnson S.I., Kerwin S.C., Hettlich B.F., Fosgate G.T. Evaluation of the success of medical man-agement for presumptive thoracolumbar intervertebral disk herniation in dogs. Vet. Surg. 2007;36:482–491. doi: 10.1111/j.1532-950X.2007.00295.x. [DOI] [PubMed] [Google Scholar]
- 32.Ahsan M.K., Hossain M.R., Khan M.S.I., Zaman N., Ahmed N., Montemurro N., Chaurasia B. Lumbar revision micro-discectomy in patients with recurrent lumbar disc herniation: A single-center prospective series. Surg. Neurol. Int. 2020;11:404–407. doi: 10.25259/SNI_540_2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perrini P., Gambacciani C., Martini C., Montemurro N., Lepori P. Anterior cervical corpectomy for cervical spondylotic myelopathy: Reconstruction with expandable cylindrical cage versus iliac crest autograft. A retrospective study. Clin. Neurol. Neurosurg. 2015;139:258–263. doi: 10.1016/j.clineuro.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 34.Li W., Liu T., Wu L., Chen C., Jia Z., Bai X., Ruan D. Blocking the Function of Inflammatory Cytokines and Mediators by Using IL-10 and TGF-β: A Potential Biological Immunotherapy for Intervertebral Disc Degeneration in a Beagle Model. Int. J. Mol. Sci. 2014;15:17270–17283. doi: 10.3390/ijms151017270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palmer N., Guan Z., Chai N.C. Spinal Cord Stimulation for Failed Back Surgery Syndrome--Patient Selection Considerations. Transl. Perioper. Pain Med. 2019;6:81–90. [PMC free article] [PubMed] [Google Scholar]
- 36.Ju D.G., Kanim L.E., Bae H.W. Intervertebral Disc Repair: Current Concepts. Global. Spine J. 2020;10:130S–136S. doi: 10.1177/2192568219872460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng C., Liu H., Yang Y., Huang B., Zhou Y. Growth and Differentiation Factor-5 Contributes to the Structural and Functional Maintenance of the Intervertebral Disc. Cell. Physiol. Biochem. 2015;35:1–16. doi: 10.1159/000369670. [DOI] [PubMed] [Google Scholar]
- 38.Chujo T., An H.S., Akeda K., Miyamoto K., Muehleman C., Attawia M., Andersson G., Masuda K. Effects of growth dif-ferentiation factor-5 on the intervertebral disc—in vitro bovine study and in vivo rabbit disc degeneration model study. Spine. 2006;31:2909–2917. doi: 10.1097/01.brs.0000248428.22823.86. [DOI] [PubMed] [Google Scholar]
- 39.Tendulkar G., Chen T., Ehnert S., Kaps H.-P., Nussler A.K. Intervertebral Disc Nucleus Repair: Hype or Hope? Int. J. Mol. Sci. 2019;20:3622. doi: 10.3390/ijms20153622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu J., Xia K., Yu W., Wang Y., Hua J., Liu B., Gong Z., Wang J., Xu A., You Z., et al. Sustained release of GDF5 from a designed coacervate attenuates disc degeneration in a rat model. Acta Biomater. 2019;86:300–311. doi: 10.1016/j.actbio.2019.01.028. [DOI] [PubMed] [Google Scholar]
- 41.Cho H., Lee S., Park S.-H., Huang J., Hasty K.A., Kim S.-J. Synergistic effect of combined growth factors in porcine inter-vertebral disc degeneration. Connect. Tissue Res. 2013;54:181–186. doi: 10.3109/03008207.2013.775258. [DOI] [PubMed] [Google Scholar]
- 42.Masuda K. Biological repair of the degenerated intervertebral disc by the injection of growth factors. Eur. Spine J. 2008;17:441–451. doi: 10.1007/s00586-008-0749-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grad S., Andersson G., Iatridis J.C., Sakai D., Härtl R., Ito K., Grad S. Cell therapy for intervertebral disc repair: Advancing cell therapy from bench to clinics. Eur Cell Mater. 2014;27:5–11. doi: 10.22203/eCM.v027sa02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teng Y.D., Yu D., Ropper A.E., Li J., Kabatas S., Wakeman D.R., Wang J., Sullivan M.P., Redmond E., Jr., Langer R. Functional multipotency of stem cells: A conceptual review of neurotrophic factor-based evidence and its role in translational research. Curr. Neuropharmacolol. 2011;9:574–585. doi: 10.2174/157015911798376299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jandial R., Aryan H.E., Park J., Taylor W.T., Snyder E.Y. Stem cell–mediated regeneration of the intervertebral disc: Cellular and molecular challenges. Neurosurg. Focus. 2008;24:E21. doi: 10.3171/FOC/2008/24/3-4/E20. [DOI] [PubMed] [Google Scholar]
- 46.Kumar H., Ha D.-H., Lee E.-J., Park J.H., Shim J.H., Ahn T.-K., Kim K.-T., Ropper A.E., Sohn S., Kim C.-H., et al. Safety and tolerability of intradiscal implantation of combined autologous adipose-derived mesenchymal stem cells and hyaluronic acid in patients with chronic discogenic low back pain: 1-year follow-up of a phase I study. Stem Cell Res. Ther. 2017;8:1–14. doi: 10.1186/s13287-017-0710-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kandel R., Roberts S., Urban J.P.G. Tissue engineering and the intervertebral disc: The challenges. Eur. Spine J. 2008;17:480–491. doi: 10.1007/s00586-008-0746-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi Y., Park M.H., Lee K. Tissue Engineering Strategies for Intervertebral Disc Treatment Using Functional Polymers. Polym. 2019;11:872. doi: 10.3390/polym11050872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gullbrand S.E., Kim D.H., Bonnevie E., Ashinsky B.G., Smith L.J., Elliott D.M., Mauck R.L., Smith H.E. Towards the scale up of tissue engineered intervertebral discs for clinical application. Acta Biomater. 2018;70:154–164. doi: 10.1016/j.actbio.2018.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iatridis J.C., Nicoll S.B., Michalek A.J., Walter B.A., Gupta M.S. Role of biomechanics in intervertebral disc degeneration and regenerative therapies: What needs repairing in the disc and what are promising biomaterials for its repair? Spine J. 2013;13:243–262. doi: 10.1016/j.spinee.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moriguchi Y., Mojica-Santiago J., Grunert P., Pennicooke B., Berlin C., Khair T., Navarro-Ramirez R., Arbona R.R., Nguyen J., Härtl R., et al. Total disc replacement using tissue-engineered intervertebral discs in the canine cervical spine. PLOS ONE. 2017;12:e0185716. doi: 10.1371/journal.pone.0185716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sobajima S., Kim J.S., Gilbertson L.G., Kang J.D. Gene therapy for degenerative disc disease. Gene Ther. 2004;11:390–401. doi: 10.1038/sj.gt.3302200. [DOI] [PubMed] [Google Scholar]
- 53.Krupkova O., Cambria E., Bešše L., Besse A., Bowles R., Wuertz-Kozak K. The potential of CRISPR/Cas9 genome editing for the study and treatment of intervertebral disc pathologies. JOR Spine. 2018;1:e1003. doi: 10.1002/jsp2.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yurube T., Ito M., Kakiuchi Y., Kuroda R., Kakutani K. Autophagy and mTOR signaling during intervertebral disc aging and degeneration. JOR Spine. 2020;3:e1082. doi: 10.1002/jsp2.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wehling P., Schulitz K.-P., Robbins P.D., Evans C.H., Reinecke J.A. Transfer of genes to chondrocytic cells of the lumbar spine: Proposal for a treatment strategy of spinal disorders by local gene therapy. Spine. 1997;22:1092–1097. doi: 10.1097/00007632-199705150-00008. [DOI] [PubMed] [Google Scholar]
- 56.Nishida K., Kang J.D., Suh J.K., Robbins P.D., Evans C.H., Gilbertson L.G. Adenovirus-mediated gene transfer to nucleus pulposus cells. Implications for the treatment of intervertebral disc degeneration. Spine. 1998;23:2437–2442. doi: 10.1097/00007632-199811150-00016. [DOI] [PubMed] [Google Scholar]
- 57.High K.A., Roncarolo M.G. Gene Therapy. N. Engl. J. Med. 2019;381:455–464. doi: 10.1056/NEJMra1706910. [DOI] [PubMed] [Google Scholar]
- 58.Naso M.F., Tomkowicz B., Perry W.L., 3rd, Strohl W.R. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs. 2017;31:317–334. doi: 10.1007/s40259-017-0234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li H., Li W., Liang B., Wei J., Yin D., Fan Q. Role of AP-2alpha/TGF-beta1/Smad3 axis in rats with intervertebral disc degeneration. Life Sci. 2020;263:118567. doi: 10.1016/j.lfs.2020.118567. [DOI] [PubMed] [Google Scholar]
- 60.Jiao Y., Xia Z.L., Ze L.J., Jing H., Xin B., Fu S. Research Progress of nucleic acid delivery vectors for gene therapy. Biomed. Microdevices. 2020;22:16. doi: 10.1007/s10544-020-0469-7. [DOI] [PubMed] [Google Scholar]
- 61.Kotterman M.A., Chalberg T.W., Schaffer D.V. Viral Vectors for Gene Therapy: Translational and Clinical Outlook. Annu. Rev. Biomed. Eng. 2015;17:63–89. doi: 10.1146/annurev-bioeng-071813-104938. [DOI] [PubMed] [Google Scholar]
- 62.Liu X., Li K., Song J., Liang C., Wang X., Chen X. Efficient and Stable Gene Expression in Rabbit Intervertebral Disc Cells Transduced With a Recombinant Baculovirus Vector. Spine. 2006;31:732–735. doi: 10.1097/01.brs.0000206977.61305.43. [DOI] [PubMed] [Google Scholar]
- 63.Zhao Z., Li S., Huang H., Fang J., Wei H., Xi Y. In vivo delivery of MMP3-shRNA and Sox9 lentivirus cocktail enhances matrix synthesis to prevent lumbar disc degeneration. Adv. Clin. Exp. Med. 2020;29:639–647. doi: 10.17219/acem/121509. [DOI] [PubMed] [Google Scholar]
- 64.Bi F., Liu W., Wu Z., Ji C., Chang C. Antiaging Factor Klotho Retards the Progress of Intervertebral Disc Degeneration through the Toll-Like Receptor 4-NF-κB Pathway. Int. J. Cell Biol. 2020;8:11. doi: 10.1155/2020/8319516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seki S., Asanuma-Abe Y., Masuda K., Kawaguchi Y., Asanuma K., Muehleman C., Iwai A., Kimura T. Effect of small interference RNA (siRNA) for ADAMTS5 on intervertebral disc degeneration in the rabbit anular needle-puncture model. Arthritis Res. Ther. 2009;11:R166. doi: 10.1186/ar2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Castanotto D., Rossi J.J. The promises and pitfalls of RNA-interference-based therapeutics. Nat. Cell Biol. 2009;457:426–433. doi: 10.1038/nature07758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nishida K., Doita M., Takada T., Kakutani K.-I., Miyamoto H., Shimomura T., Maeno K., Kurosaka M. Sustained Transgene Expression in Intervertebral Disc Cells In Vivo Mediated by Microbubble-Enhanced Ultrasound Gene Therapy. Spine. 2006;31:1415–1419. doi: 10.1097/01.brs.0000219945.70675.dd. [DOI] [PubMed] [Google Scholar]
- 68.Huang Y., Huang L., Li L., Ge Z., Feng G., Liu L., Song Y. MicroRNA-25-3p therapy for intervertebral disc degeneration by targeting the IL-1β/ZIP8/MTF1 signaling pathway with a novel thermo-responsive vector. Ann. Transl. Med. 2020;8:1500. doi: 10.21037/atm-20-6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen Z.-Y., Lin Y., Yang F., Jiang L., Ge S.P. Gene therapy for cardiovascular disease mediated by ultrasound and microbubbles. Cardiovasc. Ultrasound. 2013;11:11. doi: 10.1186/1476-7120-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lentacker I., Wang N., Vandenbroucke R.E., Demeester J., De Smedt S.C., Sanders N.N. Ultrasound Exposure of Lipoplex Loaded Microbubbles Facilitates Direct Cytoplasmic Entry of the Lipoplexes. Mol. Pharm. 2009;6:457–467. doi: 10.1021/mp800154s. [DOI] [PubMed] [Google Scholar]
- 71.Fujii H., Sun Z., Li S.-H., Wu J., Fazel S., Weisel R.D., Rakowski H., Lindner J., Li R.-K. Ultrasound-Targeted Gene Delivery Induces Angiogenesis After a Myocardial Infarction in Mice. JACC: Cardiovasc. Imaging. 2009;2:869–879. doi: 10.1016/j.jcmg.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 72.Feng G., Zha Z., Huang Y., Li J., Wang Y., Ke W., Chen H., Liu L., Song Y., Ge Z. Sustained and Bioresponsive Two-Stage Delivery of Therapeutic miRNA via Polyplex Micelle-Loaded Injectable Hydrogels for Inhibition of Intervertebral Disc Fibrosis. Adv. Heal. Mater. 2018;7:e1800623. doi: 10.1002/adhm.201800623. [DOI] [PubMed] [Google Scholar]
- 73.Luther D., Lee Y., Nagaraj H., Scaletti F., Rotello V. Delivery approaches for CRISPR/Cas9 therapeutics in vivo: Advances and challenges. Expert Opin. Drug Deliv. 2018;15:905–913. doi: 10.1080/17425247.2018.1517746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu X., Wan T., Xin H., Li D., Pan H., Wu J., Ping Y. Delivery of CRISPR/Cas9 for therapeutic genome editing. J. Gene Med. 2019;21:e3107. doi: 10.1002/jgm.3107. [DOI] [PubMed] [Google Scholar]
- 75.Cong L., Ran F.A., Cox D., Lin S., Barretto R., Habib N., Hsu P.D., Wu X., Jiang W., Marraffini L.A., et al. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lino C.A., Harper J.C., Carney J.P., Timlin J.A. Delivering CRISPR: A review of the challenges and approaches. Drug Deliv. 2018;25:1234–1257. doi: 10.1080/10717544.2018.1474964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Farhang N., Brunger J.M., Stover J.D., Thakore P.I., Lawrence B., Guilak F., Gersbach C.A., Setton L.A., Bowles R.D. CRISPR-Based Epigenome Editing of Cytokine Receptors for the Promotion of Cell Survival and Tissue Deposition in Inflammatory Environments. Tissue Eng. Part A. 2017;23:738–749. doi: 10.1089/ten.tea.2016.0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Farhang N., Ginley-Hidinger M., Berrett K.C., Gertz J., Lawrence B., Bowles R.D., Bowles R.D. Lentiviral CRISPR Epigenome Editing of Inflammatory Receptors as a Gene Therapy Strategy for Disc Degeneration. Hum. Gene Ther. 2019;30:1161–1175. doi: 10.1089/hum.2019.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cambria E., Arlt M.J., Wandel S., Krupkova O., Hitzl W., Passini F.S., Hausmann O., Snedeker J.G., Ferguson S.J., Wuertz-Kozak K. TRPV4 Inhibition and CRISPR-Cas9 Knockout Reduce Inflammation Induced by Hyperphysiological Stretching in Human Annulus Fibrosus Cells. Cells. 2020;9:1736. doi: 10.3390/cells9071736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stover J.D., Farhang N., Berrett K.C., Gertz J., Lawrence B., Bowles R.D. CRISPR Epigenome Editing of AKAP150 in DRG Neurons Abolishes Degenerative IVD-Induced Neuronal Activation. Mol. Ther. 2017;25:2014–2027. doi: 10.1016/j.ymthe.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Piazza N., Dehghani M., Gaborski T.R., Wuertz-Kozak K. Therapeutic Potential of Extracellular Vesicles in Degenerative Diseases of the Intervertebral Disc. Front. Bioeng. Biotechnol. 2020;8:311. doi: 10.3389/fbioe.2020.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fontana G., See E., Pandit A. Current trends in biologics delivery to restore intervertebral disc anabolism. Adv. Drug Deliv. Rev. 2015;84:146–158. doi: 10.1016/j.addr.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 83.Ito M., Yurube T., Kakutani K., Maeno K., Takada T., Terashima Y., Kakiuchi Y., Takeoka Y., Miyazaki S., Kuroda R., et al. Selective interference of mTORC1/RAPTOR protects against human disc cellular apoptosis, senescence, and extracellular matrix catabolism with Akt and autophagy induction. Osteoarthr. Cartil. 2017;25:2134–2146. doi: 10.1016/j.joca.2017.08.019. [DOI] [PubMed] [Google Scholar]
- 84.Kamali A., Ziadlou R., Lang G., Pfannkuche J., Cui S., Li Z., Richards R.G., Alini M., Grad S. Small molecule-based treatment approaches for intervertebral disc degeneration: Current options and future directions. Theranostics. 2021;11:27–47. doi: 10.7150/thno.48987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Feng C., Liu H., Yang M., Zhang Y., Huang B., Zhou Y. Disc cell senescence in intervertebral disc degeneration: Causes and molecular pathways. Cell Cycle. 2016;15:1674–1684. doi: 10.1080/15384101.2016.1152433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Harrison D.E., Strong R., Sharp Z.D., Nelson J.F., Astle C.M., Flurkey K., Nadon N.L., Wilkinson J.E., Frenkel K., Carter C.S., et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ouyang Z.-H., Wang W.-J., Yan Y.-G., Wang B., Lv G.-H. The PI3K/Akt pathway: A critical player in intervertebral disc degeneration. Oncotarget. 2017;8:57870–57881. doi: 10.18632/oncotarget.18628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bai X., Guo X., Zhang F., Zheng L., Ding W., Yang S. Resveratrol Combined with 17β-Estradiol Prevents IL-1β Induced Apoptosis in Human Nucleus Pulposus Via The PI3K/AKT/Mtor and PI3K/AKT/GSK-3β Pathway. J. Investig. Surg. 2020:1–8. doi: 10.1080/08941939.2019.1705941. [DOI] [PubMed] [Google Scholar]
- 89.Jacinto F.V., Link W., Ferreira B.I. CRISPR/Cas9-mediated genome editing: From basic research to translational medicine. J. Cell. Mol. Med. 2020;24:3766–3778. doi: 10.1111/jcmm.14916. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.