Abstract
Background:
Abnormal gaze discrimination in schizophrenia (SZ) is associated with impairment in social functioning, but the neural mechanisms remain unclear. Evidence suggests that local neural oscillations and inter-areal communication through neural synchronization are critical physiological mechanisms supporting basic and complex cognitive processes. The roles of these mechanisms in abnormal gaze processing in SZ have not been investigated. The present study examined local neural oscillations and connectivity between anterior and bilateral posterior brain areas during gaze processing.
Methods:
Twenty-eight SZ and 34 healthy control (HC) participants completed a gaze discrimination task during electroencephalography (EEG) recording. Time-frequency decomposition of EEG data was used to examine neural oscillatory power and inter-trial phase consistency at bilateral posterior and midline anterior scalp sites. In addition, connectivity between these anterior and posterior sites, in terms of cross-frequency coupling between theta phase and gamma amplitude, was examined using the Kullback-Leibler Modulation Index (KLMI).
Results:
SZ showed reduced total power of theta-band activity relative to HC at all sites examined. This group difference could be accounted for by SZ’s reduced inter-trial phase consistency of theta activity, which was related to reduced gaze discrimination accuracy in SZ. In addition, SZ exhibited reduced KLMI indexing both feedforward and feedback connectivity between the posterior and anterior sites.
Conclusions:
These findings suggest abnormal theta phase consistency and dysconnection between posterior face-processing and anterior areas may underlie gaze processing deficits in SZ.
Keywords: schizophrenia, EEG, neural oscillations, social cognition, face processing, cross-frequency coupling
INTRODUCTION
Eye gaze is a ubiquitous social cue that conveys information about the attention and mental states of others. The ability to discriminate others’ gaze direction and use it to guide behavior constitutes a critical building block of social cognition (1). Gaze discrimination is altered in schizophrenia (SZ) (2,3) and such abnormalities are associated with poor functional outcomes (4), but the neural mechanisms remain unclear. Studies have demonstrated abnormal visual processing in SZ (5), suggesting deficits in processing basic social information (e.g., gaze direction) may begin with altered visual processing. At the same time, compromised higher-level cognition, particularly self-referential processing that recruits the medial prefrontal cortex (6,7), is prevalent in SZ and likely contributes to altered gaze perception. To gain insight into the neurobiological basis of abnormal gaze perception in SZ, the present study examined neural oscillations and synchrony during a gaze discrimination task; this allowed us to make inference about the roles of local neural activity at posterior face-processing and anterior brain areas, as well as their inter-areal communications.
Neural oscillations refer to rhythmic fluctuations in the excitability of neurons. When the oscillations of large numbers of neurons synchronize, the collective electrical signals are amplified and detectable with electrocorticogram, electroencephalogram (EEG), magnetoencephalogram, and local field potential recordings (8,9). Synchronization of neural oscillations measurable with EEG mainly arises from interactions between glutamatergic pyramidal (excitatory) cells and GABAergic (inhibitory) interneurons, which produce a balanced alternation between excitation and inhibition of neuronal populations (10–12). Neural oscillations occur in different frequency bands simultaneously, and the activity and coordination of different frequency bands are thought to be critical physiological mechanisms underlying basic and complex cognitive processes. For example, while high-frequency activity (e.g., gamma [30–100 Hz]), especially in sensory cortices, is linked to sensory/perceptual processing (13), low-frequency activity (e.g., theta [4–8 Hz]) is linked to higher-level cognitive processes such as learning/memory (14–16) and mentalizing (17,18). Additionally, animal and human studies using intracranial electrode recording show the phase of low-frequency (e.g., theta or alpha [8–12 Hz]) activity at one region modulates the amplitude of high-frequency (e.g., gamma) activity at another (19,20), suggesting phase-amplitude cross-frequency coupling (CFC) is an important mechanism via which distal neuronal populations communicate and coordinate to give rise to complex cognition (21). Furthermore, evidence indicates theta-gamma CFC is the strongest form of CFC in the human cortex among a range of phase-amplitude CFC pairings (22), making it a prime candidate to elucidate inter-areal communications.
Given that altered glutamatergic and GABAergic functions are well-documented in SZ (23–25), it is possible that abnormal neural oscillations may underlie observed difficulties in gaze perception. Event-related potential (ERP) studies, which examine phase-locked neural activity with respect to stimulus onset, have shown that N170 is sensitive to gaze/face processing in healthy individuals [see (26) for review]. Within SZ, reduced N170 is frequently reported during gaze/face processing (27), although increased in specific contexts (28). Because N170 is within the frequency range of theta, reduced N170 implies abnormalities in the phase aspect of theta activity in SZ. Intracranial studies of psychiatrically healthy individuals suggest increased gamma activity during gaze processing (29,30), but no studies thus far have focused on SZ. However, there have been neural oscillation studies of visual and face processing in SZ that have generally reported reduced low-frequency activity (e.g., theta) and high-frequency activity (e.g., gamma) at midline anterior and bilateral posterior scalp sites (31–35), which were linked to social cognitive deficits (31,36) and clinical symptoms in SZ (32,37). These findings inform the neural oscillatory abnormalities that may also be present during gaze processing. Gaze processing network as revealed in neuroimaging studies includes the medial prefrontal cortex/anterior cingulate cortex (mPFC/ACC), posterior superior temporal sulcus, and fusiform gyrus (26,38,39). Consistent with this general brain network of gaze processing, abnormal spectral features of EEG have been observed at midline anterior and bilateral posterior scalp sites in SZ during visual and face processing, although it cannot be concluded that these effects observed at EEG scalp sites are generated by underlying brain areas. Gaze processing also involves other brain regions such as the amygdala and anterior insula (26,38,39), but their activities are harder to detect with scalp EEG. Taken together, these findings suggest that examining the spectral features of neural activity during gaze processing at bilateral posterior (associated with ventral face processing) and midline anterior areas (associated with higher-level social cognition and top-down control) would provide better understanding of the system-level neurophysiological underpinnings of abnormal gaze perception in SZ.
In addition to local cortical dysfunctions, dysconnection between brain regions is widespread in SZ (40). Such functional disintegration, as suggested by neuroimaging findings of reduced functional connectivity between visual areas and the prefrontal cortex in SZ during visual processing (41), likely contributes to gaze perception abnormalities in SZ. In the case of gaze processing, dysfunction in brain regions implicated in early visual and ventral face processing may lead to impaired information flow to higher-level brain regions, compromising the ability to determine the self-referential nature of others’ gaze. Similarly, altered activity in regions associated with higher-level social cognition and top-down control (e.g., mPFC/ACC) could result in a failure to properly modulate visual and face-processing areas, affecting gaze discrimination accuracy. Although previous face processing studies have demonstrated abnormal early visual and face-processing ERPs in SZ (e.g., P1, N170) (27,42), it is premature to conclude the source of deficits lies solely in early processes, because even “early” ERPs can be modulated by higher-level cognitive processes such as attention and intention (43,44). Therefore, it remains to be elucidated whether abnormal feedforward or feedback connectivity, or both, underlies altered gaze processing in SZ. Examining CFC of theta phase and gamma amplitude between posterior and anterior areas may offer an avenue to address this question.
This study investigated the neural bases of abnormal gaze processing in SZ by examining neural oscillatory activity over bilateral posterior areas (P7/P8) associated with mid-level ventral face processing and midline anterior area (Fz) associated with higher-level cognition. We conducted time-frequency analyses on EEG data collected during a gaze discrimination task in SZ and a healthy comparison (HC) group. Based on previous SZ findings of oscillatory aberrations during visual and face processing, we hypothesized that SZ, relative to HC, would show reduced theta and gamma activity at these posterior and anterior sites. We also investigated the directions of disrupted information flow during gaze processing in SZ by examining CFC between P7/P8 and Fz. Furthermore, the relationship between these EEG measures and clinical symptoms and behavior in SZ were examined. Finally, we explored whether neural oscillatory activity during gaze processing in SZ is modulated by factors with documented influence on gaze perception (gaze direction, face emotion, and head orientation) (3,4,45–47).
METHODS AND MATERIALS
Participants
Twenty-nine volunteers with SZ and 44 HC completed the study. Participants were recruited from the community and monetarily compensated. SZ met criteria for schizophrenia (n=22) or schizoaffective disorder (n=7), confirmed with the Structured Clinical Interview for DSM-IV-TR (48). Detailed inclusion/exclusion criteria are provided in the Supplemental Information. For SZ, clinical symptoms were assessed with the Scale for the Assessment of Positive Symptoms (SAPS) (49) and the Scale for the Assessment of Negative Symptoms (SANS) (50). For all participants, cognition was assessed with the Brief Assessment of Cognition in Schizophrenia (BACS) (51). Participant characteristics are displayed in Table 1.
Table 1:
Demographic, clinical, and behavioral characteristics (N=62)
| HC (n=34) M (SD) |
SZ (n=28) M (SD) |
t / χ2 | p | |
|---|---|---|---|---|
| Age | 41.6 (12.9) | 41.6 (13.3) | −0.01 | 0.989 |
| Sex (male/female) | 21/13 | 20/8 | 0.28 | 0.596 |
| Education in years | 16.5 (2.3) | 13.5 (1.9) | 5.46 | <0.001 |
| Parental education in years | 14.6 (2.7) | 14.5 (3.7) | 0.02 | 0.985 |
| DSM-IV-TR diagnosis | -- | 21 SZ, 7 SA | -- | -- |
| Duration of Illness in years | -- | 21.1 (13.0) | -- | -- |
| SAPS | -- | 0.8 (0.6) | -- | -- |
| SANS | -- | 1.1 (0.8) | -- | -- |
| BACS z-score | 0.00 (1.0) | −1.8 (1.4) | 5.25 | <0.001 |
| Medication | ||||
| Antipsychotic | -- | 25 (92.6%) | -- | -- |
| CPZ | -- | 570.9 (581.0) | -- | -- |
| Anxiolytic | -- | 7 (25.9%) | -- | -- |
| Antidepressant | -- | 9 (33.3%) | -- | -- |
| Mood stabilizer | -- | 7 (25.9%) | -- | -- |
| Gaze discrimination task | ||||
| Accuracy | 81.8% (7.9%) | 77.9% (10.5%) | 1.62 | 0.111 |
| Reaction time (ms) | 681.9 (102.3) | 815.3 (186.5) | −3.39 | 0.002 |
| Trials retained after rejection* | 451.9 (40.9) | 454.6 (41.7) | −0.26 | 0.797 |
Note. HC = Healthy Control. SZ = Schizophrenia. SA = Schizoaffective disorder. Missing education data for 2 HC and parental education data for 5 HC. SAPS = Scale for the Assessment of Positive Symptoms (0–5 range). SANS = Scale for the Assessment of Negative Symptoms (0–5 range). CPZ = daily chlorpromazine equivalent antipsychotic dose. BACS = Brief Assessment of Cognition in Schizophrenia (missing data for 9 HC). BACS z-scores were calculated from HC mean and standard deviation. Medication information missing for 1 SZ.
The task included 512 trials in total.
A subset of this study sample also participated in a behavioral gaze perception study (22 SZ, 22 HC) (4) and/or a basic visual perception study (23 SZ, 22 HC) (52). ERP analysis of EEG data of the current gaze task of all SZ and 32 HC from this sample has been reported elsewhere (28).
Procedure
The study was conducted in accordance with the Declaration of Helsinki and approved by the University of Michigan Medical School Institutional Review Board. Written informed consent was obtained from each participant.
Experimental task.
Participants performed a gaze discrimination task. Details of the task are provided in the Supplemental Information (Procedure and Figure S1) and elsewhere (28).
EEG data acquisition and preprocessing.
Details of data acquisition and preprocessing are included in the Supplemental Information. We used custom MATLAB scripts (R2019a; MathWorks) based on functions from EEGLAB 14.1.2 (53) for all preprocessing steps. Participants were excluded if <70% of trials remained after preprocessing, resulting in the exclusion of 9 HC and 1 SZ. Most of the excluded HC were due to a defective cap that was later replaced during the study.
Time-frequency decomposition.
Time-frequency decomposition was performed on cleaned (i.e., post-ICA and artifact rejection) epochs of −1,500–2,300 ms from P7, P8, and Fz sites. Time-frequency decomposition on such long data epochs was to minimize edge artifacts in the time window where the spectral features would be examined (0–750 ms) in subsequent analyses. P7/P8 were selected due to their proximity to posterior regions associated with face (54) and gaze processing (55). Fz was selected due to its proximity to medial frontal regions associated with higher-level cognition (mPFC/ACC) (56,57). We used a Morlet wavelet convolution with 3 cycles at the lowest frequency (3 Hz) and 15 cycles at the highest (50 Hz) (58).
ERSP and ITPC.
Two spectral features were examined from stimulus onset (0 ms) to 750 ms post-stimulus at each site. First, total power of neural activity 3–50 Hz was measured using event-related spectral perturbation (ERSP) (59), which indicates event-related shifts in power; this was standardized in Decibels, normalized using a baseline of −200 to −50 ms. Then, we measured the consistency of phase angle relative to stimulus onset across trials (inter-trial phase consistency; ITPC) (60).
CFC.
Cross-frequency coupling between theta phase and gamma amplitude, illustrated in Supplemental Information Figure S2, was quantified using the Kullback-Leibler Modulation Index (KLMI) (19) implemented with the PACTools plugin for EEGLAB (61). More details of the computation of KLMI are provided in the Supplemental Information. Although KLMI measures a statistical relationship between activity of two different frequency bands (covariance of phase and amplitude for each latency across all trials) (62), intracranial studies show modulation of amplitude of high-frequency activity by phase of low-frequency activity occurs in many species and brain regions (63), providing biological support for the inference of directed CFC made using measures like KLMI. KLMI was calculated for each site pairing indexing feedforward (P7→Fz; P8→Fz) and feedback connectivity (Fz→P7; Fz→P8).
Statistical Analyses
Task performance.
Independent-samples t-tests were used to assess group differences in accuracy and reaction time on the gaze task.
ERSP and ITPC.
Mean ERSP values of theta, alpha, beta, and gamma activity, as well as mean ITPC values of theta within specific time-frequency windows were extracted from each of the three sites for each participant. See Supplemental Information (Methods and Figure S3) for details of the time-frequency windows selection method. Note that ITPC of only theta activity was analyzed because ITPC is typically only detectable in low-frequency activity, since slight temporal jitter in stimulus presentation can obliterate higher-frequency ITPC (63). Extracted ERSP and ITPC values were then submitted to subsequent analyses to test for group differences between HC and SZ. Specifically, mixed ANOVA, with site as a within-subject factor and group as a between-subjects factor, was performed for ERSP of each of the four frequency bands and for ITPC of theta activity. Significant results were followed up with Tukey HSD to assess pairwise site differences or group difference at each site.
Because significant group differences were observed in both theta ERSP and ITPC, we investigated if group differences in theta ERSP could be accounted for by group differences in ITPC. An ANCOVA with ITPC as covariate was conducted on theta ERSP at each site.
We also conducted exploratory analyses to examine the effects of different factors of the faces (gaze direction, facial emotion, head orientation) on ERSP and ITPC and potential differential group differences (see Supplemental Information for details).
All ANOVA and ANCOVA analyses were performed in RStudio (version 1.2.5003), with an FDR correction of p<0.05.
CFC.
Mean KLMI value within a specific time window of interest (where difference between HC and SZ was consecutively at least 0.2) was calculated for each individual for each of the four site pairings. Permutation-based two-samples t-tests (10,000 permutations) were used to examine group differences.
Clinical and behavioral correlates of EEG measures.
The ten EEG measures of interest were strongly correlated with one another (Supplemental Information, Table S1). To reduce the number of comparisons, principal Components Analysis (PCA) was used to extract a single component of each measure (theta ERSP, theta ITPC, KLMI) for subsequent correlation analyses with clinical symptoms (SAPS, SANS), cognition (BACS), and task behavior (accuracy, reaction time).
RESULTS
Task performance
SZ were as accurate, but slower to respond, on the gaze task, compared with HC (Table 1).
ERSP and ITPC
ERSP and ITPC results are illustrated in Figures 1 and 2, respectively. Full details of the ANOVA results are summarized in Table 2. Overall, post-stimulus theta ERSP was reduced in SZ, compared to HC; post-hoc analysis showed group difference at each site (P7: p=0.007; P8: p<0.001; Fz: p=0.01). Relative to HC, SZ showed less post-stimulus beta suppression; post-hoc analysis showed group difference at posterior sites only (P7: p=0.021; P8: p=0.002).
Figure 1. Event-related spectral perturbation (ERSP) at bilateral posterior and midline anterior sites.
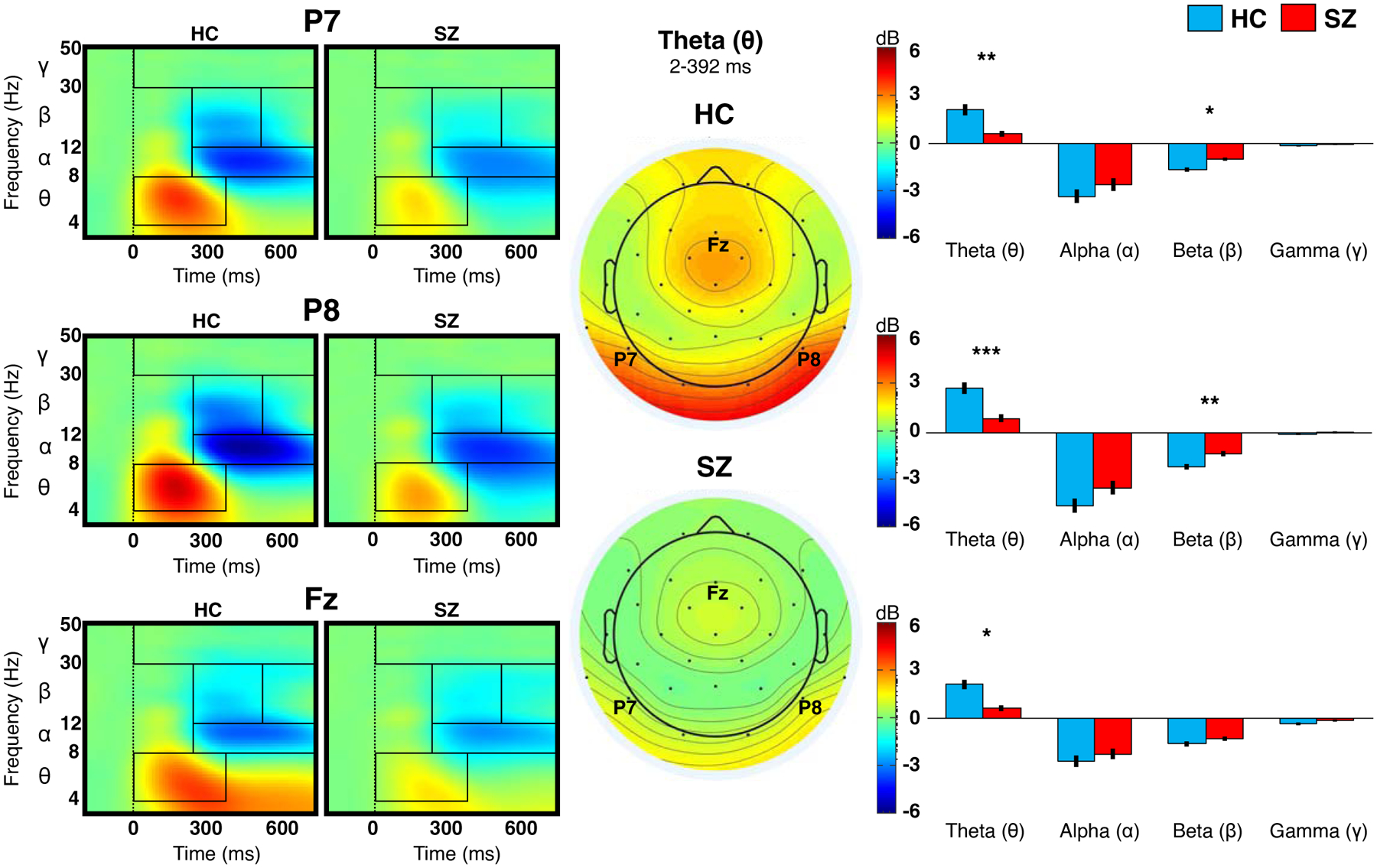
The plots on the left show the amplitude of ERSP at frequency 3–50 Hz for healthy controls (HC) and schizophrenia (SZ). Rectangles inside the plots indicate the time-frequency windows used to extract ERSP for statistical comparisons between the two groups (results displayed in the bar graphs). Topographies in the center indicate mean theta ERSP within the indicated time window. The bar graphs to the right show (post-hoc) group differences in each frequency band at each site; vertical lines indicate standard errors. *p<0.05. **p<0.01. ***p<0.001.
Figure 2. Inter-trial phase consistency (ITPC) at bilateral posterior and midline anterior sites.
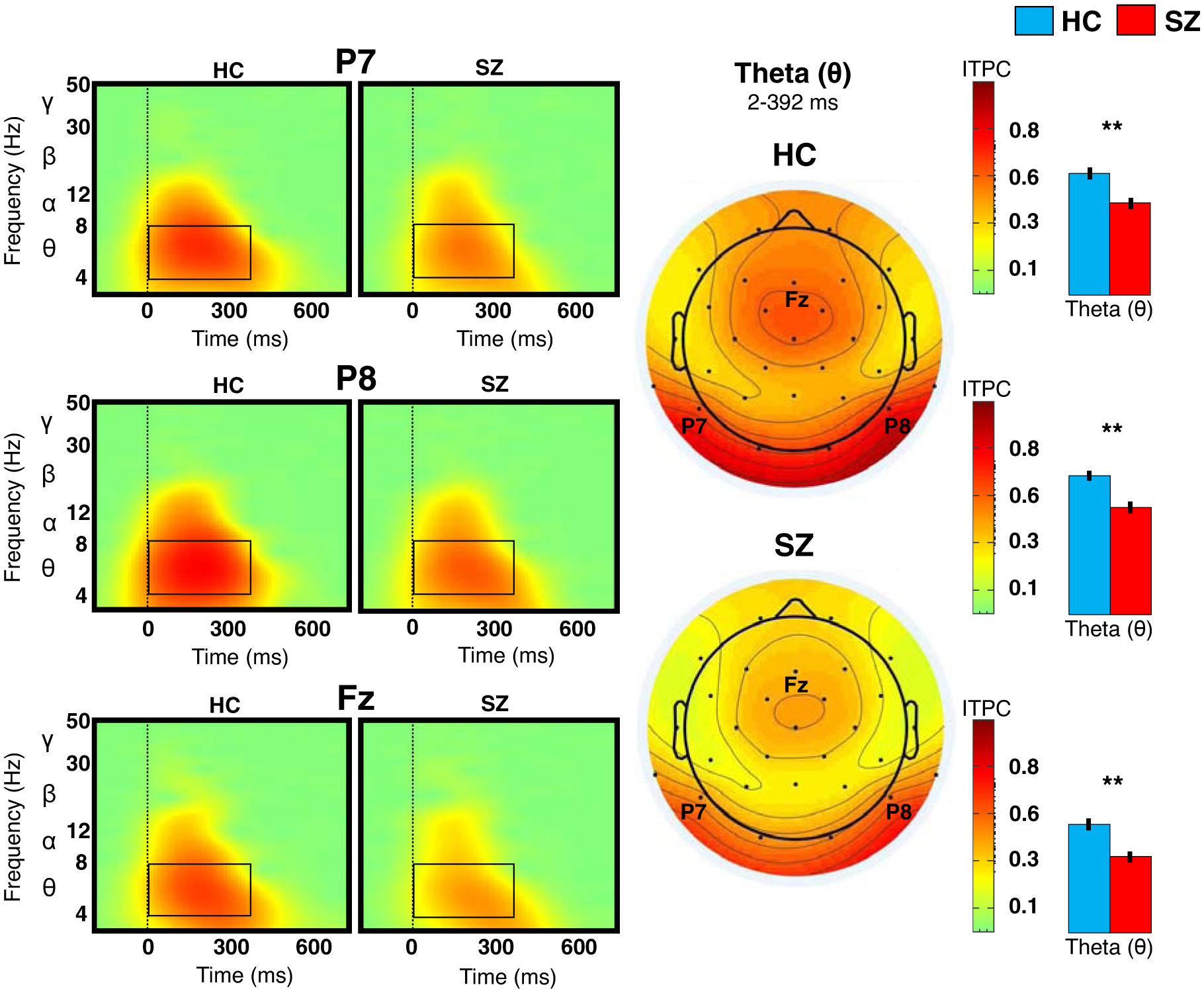
The plots on the left show the ITPC of theta activity at P7, P8, and Fz for healthy controls (HC) and schizophrenia (SZ). Topographies in the center of the figure indicate mean theta ITPC within the indicated time window. The bar graphs to the right show (post-hoc) group differences in theta ITPC at each site; vertical lines indicate standard errors. **p<0.01.
Table 2:
Mixed ANOVA for ERSP of each frequency band and theta ITPC
| df | F | p | ηp2 | Post-hoc | |
|---|---|---|---|---|---|
| ERSP THETA | |||||
| Group (HC, SZ) | 1, 60 | 18.30 | <0.001 | 0.20 | HC > SZ |
| Site (P7, P8, Fz) | 2, 120 | 10.66 | <0.001 | 0.02 | P8 > P7/Fz |
| Group × Site | 2, 120 | 2.10 | 0.127 | 0.00 | -- |
| ERSP ALPHA suppression | |||||
| Group (HC, SZ) | 1, 60 | 1.40 | 0.242 | 0.02 | -- |
| Site (P7, P8, Fz) | 2, 120 | 60.26 | <0.001 | 0.06 | P8 > P7 > Fz |
| Group × Site | 2, 120 | 1.90 | 0.231 | 0.00 | -- |
| ERSP BETA suppression | |||||
| Group (HC, SZ) | 1, 60 | 12.12 | 0.001 | 0.12 | HC > SZ |
| Site (P7, P8, Fz) | 2, 120 | 12.09 | <0.001 | 0.05 | P8 > P7/Fz |
| Group × Site | 2, 120 | 3.29 | 0.041 | 0.01 | HC > SZ* |
| ERSP GAMMA suppression | |||||
| Group (HC, SZ) | 1, 60 | 4.08 | 0.072 | 0.04 | -- |
| Site (P7, P8, Fz) | 2, 120 | 11.60 | <0.001 | 0.07 | Fz > P7/P8 |
| Group × Site | 2, 120 | 0.77 | 0.466 | 0.01 | -- |
| ITPC THETA | |||||
| Group (HC, SZ) | 1, 60 | 18.55 | <0.001 | 0.18 | HC > SZ |
| Site (P7, P8, Fz) | 2, 120 | 50.02 | <0.001 | 0.12 | P8 > P7 > Fz |
| Group × Site | 2, 120 | 0.08 | 0.924 | 0.00 | -- |
Note. Post-hoc tests were conducted using Tukey HSD.
For beta ERSP, there was a significant Group × Site interaction, where HC showed a pattern of greater beta suppression at P8 relative to P7/Fz, but this pattern was weaker in SZ than HC.
ERSP = event-related spectral perturbation. ITPC = inter-trial phase consistency.
HC = healthy control. SZ = schizophrenia.
False Discovery Rate (FDR) correction (p<0.05) was applied to each ANOVA.
ITPC (of theta activity) was significantly reduced in SZ relative to HC. Post-hoc analysis showed a group difference at all sites (P7: p=0.005; P8: p=0.002; Fz: p=0.002).
Results of the ANCOVAs on theta ERSP showed that group differences at all sites were no longer significant after adjusting for ITPC (Table 3; Supplemental Information Figure S4 for scatterplots).
Table 3:
ANCOVA for theta ERSP at each site
| P7 THETA ERSP | P8 THETA ERSP | FZ THETA ERSP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| df | F | p | ηp2 | F | p | ηp2 | F | p | ηp2 | |
| Covariate | ||||||||||
| Site’s Theta ITPCa | 1, 59 | 131.31 | <0.001 | 0.68 | 121.45 | <0.001 | 0.67 | 147.41 | <0.001 | 0.71 |
| Fixed Factor | ||||||||||
| Group (HC, SZ) | 1, 59 | 1.94 | 0.191 | 0.01 | 1.65 | 0.204 | 0.01 | 1.97 | 0.166 | 0.01 |
Note.
For each model, theta ITPC site is the same as theta ERSP site.
ERSP = event-related spectral perturbation. ITPC = inter-trial phase consistency.
HC = healthy control. SZ = schizophrenia.
False Discovery Rate (FDR) correction (p<0.05) was applied to each ANCOVA.
The results of the exploratory analyses overall showed that HC and SZ did not differ in the effects of gaze direction, facial emotion, and head orientation on ERSP and ITPC (shaded results in Supplemental Information, Table S2).
CFC
Group comparisons of CFC as measured with KLMI is illustrated in Figure 3. SZ showed significantly reduced KLMI across all site pairing indicating feedforward (P7→Fz: t(60)=2.93, p=0.008; P8→Fz: t(60)=2.26, p=0.028) and feedback connectivity (Fz→P7: t(60)=2.82, p=0.006; Fz→P8: t(60)=3.05, p=0.006).
Figure 3. Cross-frequency coupling (CFC) between bilateral posterior (P7/P8) and midline anterior (Fz) sites.
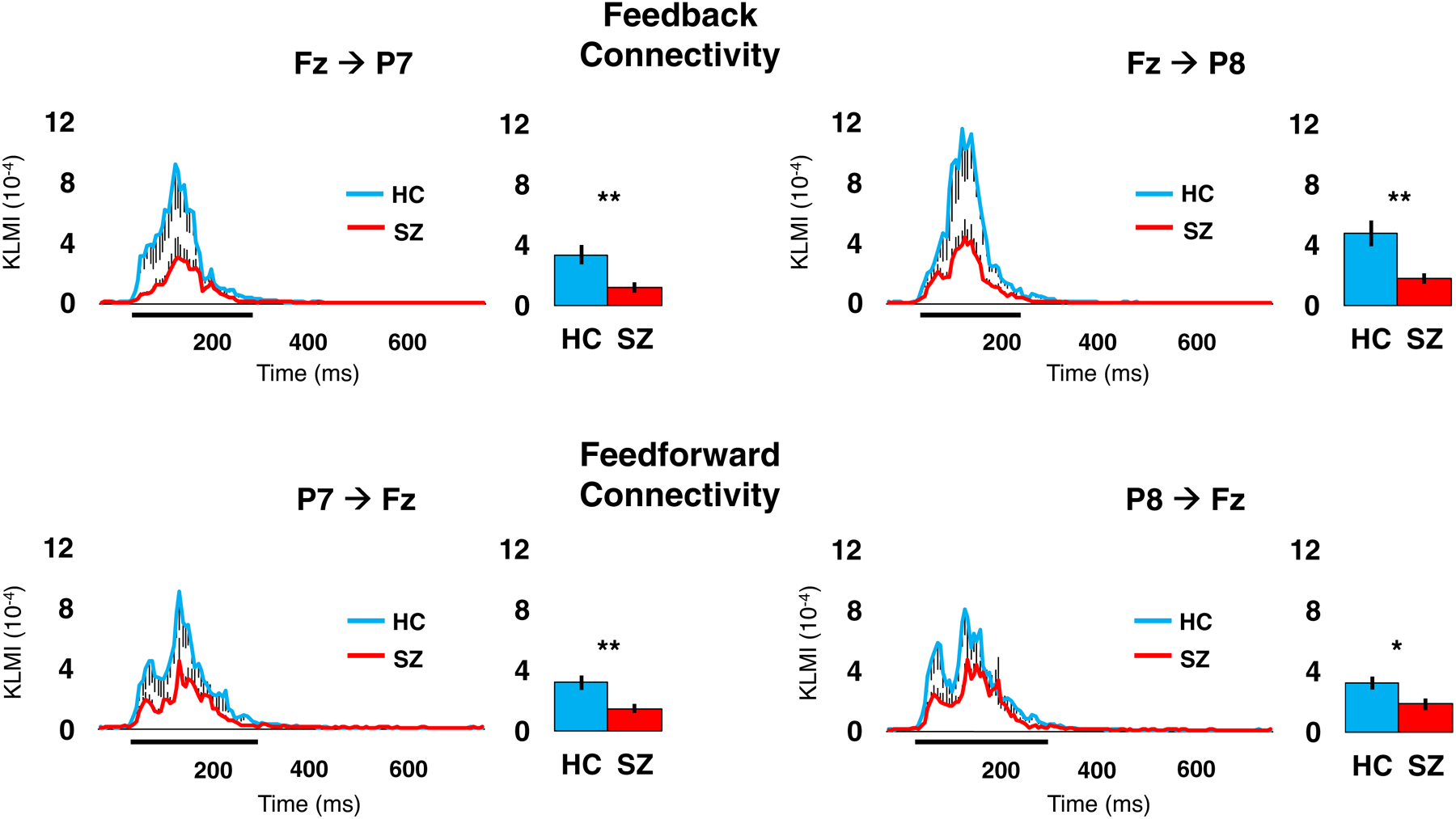
For each site pairing, the line graph illustrates the Kullback-Leibler Modulation Index (KLMI) value at each time point, which indicates the covariance between theta phase at the site of origin and gamma amplitude at the modulated site; black vertical lines indicate standard errors. The thick black horizontal lines directly below the x-axis indicate the time window used to extract KLMI values for group comparisons (results in bar graphs). *p<0.05. ** p<0.01.
Clinical and behavioral correlates of EEG measures
Reduced theta ERSP and ITPC were associated with reduced gaze task accuracy in SZ (Table 4). Reduced theta ERSP and KLMI were associated with poorer BACS performance when all participants were considered, although inspection of the correlations in each group suggests that these correlations were largely driven by the SZ group; these correlations reached statistical significance only when the two groups were combined and provided higher statistical power.
Table 4.
Spearman correlations (rho) between EEG and clinical/behavioral measures
| Theta ERSP | Theta ITPC | KLMI | |
|---|---|---|---|
| SZ only | |||
| SAPS | 0.00 | 0.02 | 0.00 |
| SANS | −0.25 | −0.30 | −0.28 |
| CPZ | −0.19 | −0.30 | −0.07 |
| BACS | |||
| All participants | 0.37** | 0.26 | 0.36** |
| HC | −0.25 | −0.12 | 0.04 |
| SZ | 0.36 | 0.14 | 0.32 |
| Gaze task behavior | |||
| Accuracy | |||
| All participants | 0.17 | 0.21 | 0.24 |
| HC | −0.04 | −0.08 | 0.00 |
| SZ | 0.39* | 0.43* | 0.29 |
| Reaction time | |||
| All participants | −0.23 | −0.12 | −0.23 |
| HC | −0.04 | 0.07 | 0.05 |
| SZ | −0.03 | 0.03 | −0.28 |
Note. Principal component analysis (PCA) scores of EEG measures were used in the computation of the correlations.
SZ = schizophrenia. HC = healthy control. ERSP = event-related spectral perturbation. ITPC = inter-trial phase consistency. KLMI = Kullback-Leibler Modulation Index.
SAPS = Scale for the Assessment of Positive Symptoms.
SANS = Scale for the Assessment of Negative Symptoms.
BACS = Brief Assessment of Cognition in Schizophrenia.
CPZ = daily chlorpromazine equivalent antipsychotic dose.
p<0.05.
p<0.01.
DISCUSSION
This study examined neural oscillations and synchrony during a gaze discrimination task to investigate the roles of local neural activity and their inter-areal communications in altered gaze processing in SZ. We observed widespread abnormalities in theta activity in SZ. First, theta power (ERSP) was reduced in SZ at both bilateral posterior (P7/P8) and midline anterior (Fz) sites. This suggests that altered theta power is pervasive in SZ across mid-level and higher-level cognitive processes that support gaze perception. Additionally, theta activity in SZ demonstrated less inter-trial phase consistency (ITPC) compared with HC at these sites and latency. Because ERSP consists of both phase-locked and non-phase-locked activity (8,64), we conducted additional analyses to isolate phase-locked activity from non-phase-locked activity. We found that group differences in theta power disappeared after controlling for theta phase consistency. This indicates that theta power reductions in SZ during gaze/face processing reported here and elsewhere (31) may be driven by reductions in phase consistency rather than reductions in theta amplitude. Because ERP components consist of phase-locked activity, such altered phase consistency may also explain reduced N170, N250, and P300 (ERP components in the frequency band of theta) amplitude previously observed in SZ during face processing (27,65). It is worth noting there are several theories about what gives rise to post-stimulus phase consistency, with some positing event-related ‘phase-resetting’ and others hypothesizing ‘evoked oscillations’ (66). Regardless of the specific process to which it is ascribed, future studies should clarify whether reductions in phase consistency rather than amplitude of theta activity better account for ERP abnormalities frequently reported in SZ.
In addition to its role in local neural processing, theta phase is thought to play a crucial role in inter-areal communication in the brain (67,68). As discussed above, theta-gamma CFC has been shown to be a neurobiological basis for inter-regional communications in the brain, with theta phase at one region modulating gamma amplitude at another (22). It is possible that reductions in theta phase consistency observed in SZ also disrupt information flow between face-processing areas and higher-level cognitive areas, contributing to gaze perception aberrations in SZ (69). Reduced theta phase consistency was associated with reduced accuracy in gaze perception in the SZ group but not HC, suggesting this relationship may be specific to SZ. Furthermore, theta-gamma CFC between posterior and anterior sites was reduced in SZ, and this occurred in the same time window where reduced theta power and phase consistency was observed. We also observed correlations between CFC and local theta activity in SZ, providing further support for this claim. This finding of dysconnection between posterior and anterior brain areas, as indicated by CFC, is in line with neuroimaging findings showing reduced functional connectivity between regions at different levels of the visual processing hierarchy in SZ (e.g., visual cortex; mPFC) (41). Taken together, these findings suggest theta phase may be a potential target that future studies can look to engage to investigate its therapeutic potential. For example, theta burst stimulation (TBS) (70), a transcranial magnetic stimulation (TMS) sequence that delivers pulses mimicking the coupling of theta phase and gamma amplitude, may be utilized to modulate theta phase and improve neural connectivity, thereby improving social cognition.
Considering that neural oscillations and synchrony arise from balanced interactions between glutamatergic pyramidal (excitatory) cells and GABAergic (inhibitory) interneurons (10–12), aberrant oscillatory activity in SZ found in this study and elsewhere (31) is consistent with the excitatory/inhibitory imbalance theory (71). Somatostatin-type and parvalbumin-type GABAergic interneurons have been respectively implicated in the generation of low-frequency (e.g. theta) and high-frequency activity (e.g. beta/gamma), and their dysfunction has been linked to the pathophysiology of SZ (23,72). Future investigation of the precise mechanisms of GABAergic interneuron abnormalities in SZ would offer more insight into the cellular mechanisms of altered social cognition and identify new treatment targets.
Despite cognitive impairments (as indicated by poorer BACS score) in SZ, both groups performed this gaze task with similar accuracy. This finding does not conflict with previous SZ findings of abnormal gaze perception, because our task included only clearly direct and averted gaze, perception of which was found to be equal in SZ and HC in other studies (2,73). Equal accuracy on the current task helps rule out the possible confound that the observed group differences in oscillatory activity arose from decreased engagement and ability to perform the task among SZ. The absence of group differences in post-stimulus alpha suppression, thought to reflect a shift from a state of ‘idling’ to a task-focused state (74), also supports that SZ were comparable to HC in terms of engagement and attention.
Some findings were less central to our hypotheses, but worthy of note. First, we did not observe group differences in gamma power, despite previous findings of reduced gamma power in SZ during visual processing tasks (33). This may be due to the low signal of such high-frequency activity, which requires larger samples for greater statistical power to detect more subtle group differences. However, visual processing studies with small samples (e.g., ≤25 SZ) have found group differences in gamma power (32,34). This suggests the current lack of group differences in gamma power was likely due to the low difficulty level of our task, since gamma power reductions in SZ are typically seen during more cognitively demanding tasks (e.g., oddball task) but not necessarily during basic visual/face recognition paradigms (75) like the current one. Second, a general pattern of increased right hemisphere activity was observed across participants, including SZ. This pattern suggests that right hemispheric specialization of face processing (76,77) remains at least partially intact in SZ. Third, post-stimulus beta suppression was reduced in SZ relative to HC. Beta abnormalities may be responsible for SZ’s slowing (relative to HC) on our task, as previous studies show impairment in sensory-motor coordination in SZ (78) is associated with reduced beta suppression (79). Fourth, we did not observe correlations between EEG measures and clinical symptoms in SZ. Our patient sample’s clinical stability may have limited the range in clinical symptom scores and the ability to detect such relationships reported in previous studies (32,37). Finally, group differences in theta ERSP and ITPC seem to be about the general gaze processing and were not impacted by the features of the faces (such as gaze direction, facial emotion, and head orientation). However, the lack of significant group differences in the effects of these facial features on theta activity may be due to reduced statistical power when the trials were divided into conditions. Further investigation is needed to clarify whether theta activity is sensitive to these face attributes with documented influence on ERP.
The present results should be interpreted in light of several limitations. First, our analysis was limited by the low spatial resolution of scalp EEG. Replicating our findings with magnetoencephalography (MEG), which offers higher spatial resolution (80), would help to localize brain regions where these theta abnormalities may originate. Second, we did not perform volume conduction correction analyses (e.g., surface Laplacian) due to our low-density (32-channel) data acquisition. Although the concern for volume conduction in the current study was significantly reduced by the large distance between the P7, P8, and Fz scalp sites (> 10 cm), future investigations using high-density EEG (≥ 64 channels) would allow for accurate volume conduction correction and more accurate results (63). Third, we relied on a statistical approach (KLMI) to infer the directionality of inter-areal communications in the brain. Although the biological plausibility of this approach is supported by previous studies (19,20), more studies using other neuroimaging modalities and analytical techniques (e.g., dynamic causal modeling of fMRI or MEG data) (81,82) are needed to provide convergent evidence of our findings. Fourth, we were unable to rule out medication effects, although we observed no correlations between EEG measures and antipsychotic dose in the current SZ sample. There is also evidence that neural oscillation abnormalities in SZ occur independent of antipsychotic use (83,84). Future studies should address this limitation by comparing medicated and unmedicated samples or early-psychosis samples with limited antipsychotic exposure to clarify medication effects. Fifth, we are unable to rule out ‘spurious CFC’, or transient activity that produces cortical CFC in the absence of functional interaction between neural sources (85). To mitigate spurious CFC, we used established methods known to reduce this risk, including a robust CFC measure (KLMI) (19) as well as artifact rejection and ICA to remove non-neural artifacts (86). Sixth, our sample size is small, limiting the generalizability of our findings. Secondary analysis pooling together EEG data collected during different social cognitive paradigms would help to generalize our findings to other critical social cognitive processes in addition to gaze processing. Seventhly, our findings could be affected by reduced visual scan paths over the eyes region documented in SZ, particularly when viewing fearful faces (87). The use of eye tracking in future gaze processing studies would help to address this confound. Finally, although all participants had at least 20/30 vision, we did not record fine-grained information of visual acuity. There may have been group differences in visual acuity driving the results, and this needs to be carefully assessed in future studies.
To conclude, theta abnormalities, including reduced power and inter-trial phase consistency, were observed at posterior as well as anterior scalp sites associated with mid-level face processing and higher-level cognition in SZ during gaze processing. Reduced theta power was likely due to reduced phase consistency. Furthermore, SZ also exhibited reductions in both feedforward and feedback connectivity as suggested by reduced theta-gamma coupling between posterior and anterior sites in both directions. Taken together, these findings support our hypothesis that local theta abnormalities and dysconnection between brain areas underlie gaze processing deficits in SZ.
Supplementary Material
Acknowledgments
The researchers wish to thank all of the participants who volunteered to complete this study.
Funding
This research was supported in part by grants from the National Institutes of Health (5KL2TR000434 to I.F.T.), National Institute of Mental Health (K23MH108823 to I.F.T.), and American Psychological Foundation (Benton-Meier Neuropsychology Scholarship to I.F.T.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Emery NJ (2000): The eyes have it: the neuroethology, function and evolution of social gaze. Neurosci Biobehav Rev. 24: 581–604. [DOI] [PubMed] [Google Scholar]
- 2.Hooker C, Park S (2005): You must be looking at me: the nature of gaze perception in schizophrenia patients. Cogn Neuropsychiatry. 10: 327–345. [DOI] [PubMed] [Google Scholar]
- 3.Rosse R, Kendrick K, Wyatt R, Isaac A, Deutsch S (1994): Gaze discrimination in patients with schizophrenia: preliminary report. Am J Psychiatry. 151: 919–921. [DOI] [PubMed] [Google Scholar]
- 4.Tso IF, Mui ML, Taylor SF, Deldin PJ (2012): Eye-contact perception in schizophrenia: relationship with symptoms and socioemotional functioning. J Abnorm Psychol. 121: 616–627. [DOI] [PubMed] [Google Scholar]
- 5.Butler PD, Silverstein SM, Dakin SC (2008): Visual perception and its impairment in schizophrenia. Biol Psychiatry. 64: 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J (2006): Self-referential processing in our brain-A meta-analysis of imaging studies on the self. Neuroimage. 31: 440–457. [DOI] [PubMed] [Google Scholar]
- 7.D’Argembeau A, Ruby P, Collette F, Degueldre C, Balteau E, Luxen A, et al. (2007): Distinct Regions of the Medial Prefrontal Cortex Are Associated with Self-referential Processing and Perspective Taking. J Cogn Neurosci. 19: 935–944. [DOI] [PubMed] [Google Scholar]
- 8.Roach BJ, Mathalon DH (2008): Event-related EEG time-frequency analysis: An overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophr Bull. 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herreras O (2016): Local field potentials: Myths and misunderstandings. Front Neural Circuits. 10: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X-J (2010): Neurophysiological and computational principles of cortical rhythms in cognition. Physiol Rev. 90: 1195–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Llinás RR, Grace AA, Yarom Y (1991): In vitro neurons in mammalian cortical layer 4 exhibit intrinsic oscillatory activity in the 10- to 50-Hz frequency range. Proc Natl Acad Sci U S A. 88: 897–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buzsáki G, Anastassiou CA, Koch C (2012): The origin of extracellular fields and currents-EEG, ECoG, LFP and spikes. Nat Rev Neurosci. 13: 407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrmann CS, Fründ I, Lenz D (2010): Human gamma-band activity: A review on cognitive and behavioral correlates and network models. Neurosci Biobehav Rev. 34: 981–992. [DOI] [PubMed] [Google Scholar]
- 14.Headley DB, Paré D (2017): Common oscillatory mechanisms across multiple memory systems. npj Sci Learn. 2: 0–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seager MA, Johnson LD, Chabot ES, Asaka Y, Berry SD (2002): Oscillatory brain states and learning: Impact of hippocampal theta-contingent training. Proc Natl Acad Sci U S A. 99: 1616–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colgin LL (2013): Mechanisms and Functions of Theta Rhythms. Annu Rev Neurosci. 36: 295–312. [DOI] [PubMed] [Google Scholar]
- 17.Bögels S, Barr DJ, Garrod S, Kessler K (2015): Conversational interaction in the scanner: Mentalizing during language processing as revealed by MEG. Cereb Cortex. 25: 3219–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seymour RA, Wang H, Rippon G, Kessler K (2018): NeuroImage Oscillatory networks of high-level mental alignment : A perspective-taking MEG study. Neuroimage. 177: 98–107. [DOI] [PubMed] [Google Scholar]
- 19.Tort ABL, Komorowski R, Eichenbaum H, Kopell N (2010): Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J Neurophysiol. 104: 1195–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonnefond M, Kastner S, Jensen O (2017): Communication between brain areas based on nested oscillations. eNeuro. 4. doi: 10.1523/ENEURO.0153-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Márton CD, Fukushima M, Camalier CR, Schultz SR, Averbeck BB (2019): Signature patterns for top-down and bottom-up information processing via cross-frequency coupling in macaque auditory cortex. Eneuro. 6: ENEURO.0467–18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Canolty RTKR (2010): The functional role of cross-frequency coupling. Trends Cogn Sci. 14: 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez-Burgos G, Lewis DA (2008): GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull, 2008/06/26. 34: 944–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferguson BR, Gao W-J (2018): PV Interneurons: Critical Regulators of E/I Balance for Prefrontal Cortex-Dependent Behavior and Psychiatric Disorders. Front Neural Circuits. 12: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foss-Feig JH, Adkinson BD, Ji JL, Yang G, Srihari VH, McPartland JC, et al. (2017): Searching for Cross-Diagnostic Convergence: Neural Mechanisms Governing Excitation and Inhibition Balance in Schizophrenia and Autism Spectrum Disorders. Biol Psychiatry, 2017/03/14. 81: 848–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Itier RJ, Batty M (2009): Neural bases of eye and gaze processing: the core of social cognition. Neurosci Biobehav Rev. 33: 843–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCleery A, Lee J, Joshi A, Wynn JK, Hellemann GS, Green MF (2015): Meta-analysis of face processing event-related potentials in schizophrenia. Biol Psychiatry. 77: 116–126. [DOI] [PubMed] [Google Scholar]
- 28.Tso IF, Calwas AM, Chun J, Mueller SA, Taylor SF, Deldin PJ (2015): Altered attentional and perceptual processes as indexed by N170 during gaze perception in schizophrenia: Relationship with perceived threat and paranoid delusions. J Abnorm Psychol. 124: 519–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato W, Kochiyama T, Uono S, Matsuda K, Usui K, Usui N, et al. (2016): Rapid gamma oscillations in the inferior occipital gyrus in response to eyes. Sci Rep. 6: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato W, Kochiyama T, Uono S, Matsuda K, Usui K, Inoue Y, Toichi M (2011): Rapid amygdala gamma oscillations in response to fearful facial expressions. Neuropsychologia. 49: 612–617. [DOI] [PubMed] [Google Scholar]
- 31.Csukly G, Stefanics G, Komlósi S, Czigler I, Czobor P (2014): Event-related theta synchronization predicts deficit in facial affect recognition in schizophrenia. J Abnorm Psychol. 123: 178–189. [DOI] [PubMed] [Google Scholar]
- 32.Lee SH, Kim DW, Kim EY, Kim S, Im CH (2010): Dysfunctional gamma-band activity during face structural processing in schizophrenia patients. Schizophr Res. 119: 191–197. [DOI] [PubMed] [Google Scholar]
- 33.Tan HRM, Lana L, Uhlhaas PJ (2013): High-frequency neural oscillations and visual processing deficits in schizophrenia. Front Psychol. 4: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grützner C, Wibral M, Sun L, Rivolta D, Singer W, Maurer K, Uhlhaas PJ (2013): Deficits in High- (> 60 Hz) Gamma-Band Oscillations during Visual Processing in Schizophrenia. Front Neuroeng. 7: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramos-Loyo J, González-Garrido AA, Sánchez-Loyo LM, Medina V, Basar-Eroglu C (2009): Event-related potentials and event-related oscillations during identity and facial emotional processing in schizophrenia. Int J Psychophysiol. 71: 84–90. [DOI] [PubMed] [Google Scholar]
- 36.Williams LM, Whitford TJ, Nagy M, Flynn G, Harris AWF, Silverstein SM, Gordon E (2009): Emotion-elicited gamma synchrony in patients with first-episode schizophrenia: A neural correlate of social cognition outcomes. J Psychiatry Neurosci. 34: 303–313. [PMC free article] [PubMed] [Google Scholar]
- 37.Berger B, Minarik T, Griesmayr B, Stelzig-Schoeler R, Aichhorn W, Sauseng P (2016): Brain oscillatory correlates of altered executive functioning in positive and negative symptomatic schizophrenia patients and healthy controls. Front Psychol. 7: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nummenmaa L, Calder AJ (2009): Neural mechanisms of social attention. Trends Cogn Sci. 13: 135–143. [DOI] [PubMed] [Google Scholar]
- 39.Carlin JD, Calder AJ, Kriegeskorte N, Nili H, Rowe JB (2011): A head view-invariant representation of gaze direction in anterior superior temporal sulcus. Curr Biol. 21: 1817–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stephan KE, Friston KJ, Frith CD (2009): Dysconnection in Schizophrenia: From abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 35: 509–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang SS, Sponheim SR, Chafee MV, MacDonald AW (2011): Disrupted functional connectivity for controlled visual processing as a basis for impaired spatial working memory in schizophrenia. Neuropsychologia. 49: 2836–2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Earls HA, Curran T, Mittal V (2016): Deficits in early stages of face processing in schizophrenia: A systematic review of the P100 component. Schizophr Bull. 42: 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luck SJ, Kappenman ES (2012): ERP Components and Selective Attention. Oxford Handb Event-Related Potential Components. 295–327. [Google Scholar]
- 44.Luck SJ, Woodman GF, Vogel EK (2000): Event-related potential studies of attention. Trends Cogn Sci. 4: 432–440. [DOI] [PubMed] [Google Scholar]
- 45.Itier RJ, Alain C, Kovacevic N, McIntosh AR (2007): Explicit versus implicit gaze processing assessed by ERPs. Brain Res. 1177: 79–89. [DOI] [PubMed] [Google Scholar]
- 46.Adams RB Jr., Kleck RE (2005): Effects of Direct and Averted Gaze on the Perception of Facially Communicated Emotion. Emotion. 5. [DOI] [PubMed] [Google Scholar]
- 47.Tipples J (2006): Fear and fearfulness potentiate automatic orienting to eye gaze. Cogn Emot. 20: 309–320. [Google Scholar]
- 48.First MB, Spitzer RL, Gibbon M, Williams JBW (1997): Structured Clinical Interview for DSM-IV Axis I Disorders SCID-I: Clinician Version, Administration Booklet. American Psychiatric Press. Retrieved from http://books.google.co.in/books?id=fuwt2STFnkcC. [Google Scholar]
- 49.Andreasen NC (1984): Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, IA: University of Iowa. [Google Scholar]
- 50.Andreasen NC (1989): Scale for the Assessment of Negative Symptoms (SANS). Br J Psychiatry. 155: 53–58. [PubMed] [Google Scholar]
- 51.Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L (2004): The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 68: 283–297. [DOI] [PubMed] [Google Scholar]
- 52.Tso IF, Carp J, Taylor SF, Deldin PJ (2014): Role of visual integration in gaze perception and emotional intelligence in schizophrenia. Schizophr Bull. 40: 617–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Delorme A, Makeig S (2004): EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 134: 9–21. [DOI] [PubMed] [Google Scholar]
- 54.Gao C, Conte S, Richards JE, Xie W, Hanayik T (2019): The neural sources of N170: Understanding timing of activation in face-selective areas. Psychophysiology, 2019/02/02. 56: e13336–e13336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nguyen VT, Cunnington R (2014): The superior temporal sulcus and the N170 during face processing: Single trial analysis of concurrent EEG-fMRI. Neuroimage. 86: 492–502. [DOI] [PubMed] [Google Scholar]
- 56.Gehring WJ, Willoughby AR (2002): The medial frontal cortex and the rapid processing of monetary gains and losses. Science (80- ). 295: 2279–2282. [DOI] [PubMed] [Google Scholar]
- 57.Reischies FM, Neuhaus AH, Hansen ML, Mientus S, Mulert C, Gallinat J (2005): Electrophysiological and neuropsychological analysis of a delirious state: the role of the anterior cingulate gyrus. Psychiatry Res Neuroimaging. 138: 171–181. [DOI] [PubMed] [Google Scholar]
- 58.Cohen MX (2019): A better way to define and describe Morlet wavelets for time-frequency analysis. Neuroimage. 199: 81–86. [DOI] [PubMed] [Google Scholar]
- 59.Makeig S (1993): Auditory event-related dynamics of the EEG spectrum and effects of exposure to tones. Electroencephalogr Clin Neurophysiol. 86: 283–293. [DOI] [PubMed] [Google Scholar]
- 60.Tallon-Baudry C, Bertrand O, Delpuech C, Pernier J (1996): Stimulus specificity of phase-locked and non-phase-locked 40 Hz visual responses in human. J Neurosci. 16: 4240–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martínez-Cancino R, Heng J, Delorme A, Kreutz-Delgado K, Sotero RC, Makeig S (2019): Measuring transient phase-amplitude coupling using local mutual information. Neuroimage. 185: 361–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Voytek B, D’Esposito M, Crone NE, Knight RT (2013): A method for event-related phase/amplitude coupling. Neuroimage. 64: 416–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cohen MX (2014): Analyzing Neural Time Series Data: Theory and Practice. Cambridge, MA: MIT Press. [Google Scholar]
- 64.Makeig S, Debener S, Onton J, Delorme A (2004): Mining event-related brain dynamics. Trends Cogn Sci. 8: 204–210. [DOI] [PubMed] [Google Scholar]
- 65.Turetsky BI, Kohler CG, Indersmitten T, Bhati MT, Charbonnier D, Gur RC (2007): Facial emotion recognition in schizophrenia: when and why does it go awry? Schizophr Res. 94: 253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mathalon DH, Sohal VS (2015): Neural oscillations and synchrony in brain dysfunction and neuropsychiatric disorders it’s about time. JAMA Psychiatry. 72: 840–844. [DOI] [PubMed] [Google Scholar]
- 67.Jensen O (2001): Networks : Reading the Hippocampal Phase Code. 2761: 2743–2761. [DOI] [PubMed] [Google Scholar]
- 68.Duprez J, Gulbinaite R, Cohen MX (2020): Midfrontal theta phase coordinates behaviorally relevant brain computations during cognitive control. Neuroimage. 207: 116340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yao B, Mueller SA, Grove TB, McLaughlin M, Thakkar K, Ellingrod V, et al. (2018): Eye gaze perception in bipolar disorder: Self-referential bias but intact perceptual sensitivity. Bipolar Disord. 20. doi: 10.1111/bdi.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC (2005): Theta burst stimulation of the human motor cortex. Neuron, 2005/01/25. 45: 201–206. [DOI] [PubMed] [Google Scholar]
- 71.Gao R, Penzes P (2015): Common Mechanisms of Excitatory and Inhibitory Imbalance in Schizophrenia and Autism Spectrum Disorders. Curr Mol Med. 15: 146–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA (2008): Conserved Regional Patterns of GABA-Related Transcript Expression in the Neocortex of Subjects With Schizophrenia. Am J Psychiatry. 165: 479–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Franck N, Montoute T, Labruyere N, Tiberghien G, Marie-Cardine M, Dalery J, et al. (2002): Gaze direction determination in schizophrenia. Schizophr Res. 56: 225–234. [DOI] [PubMed] [Google Scholar]
- 74.Foxe J, Snyder A (2011): The Role of Alpha-Band Brain Oscillations as a Sensory Suppression Mechanism during Selective Attention. Front Psychol. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moran LV, Hong LE (2011): High vs low frequency neural oscillations in schizophrenia. Schizophr Bull. 37: 659–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.de Moraes R, de Sousa BM, Fukusima S (2014): Hemispheric specialization in face recognition: From spatial frequencies to holistic/analytic cognitive processing. Psychol Neurosci. 7: 503–511. [Google Scholar]
- 77.Behrmann M, Plaut DC (2013): Distributed circuits, not circumscribed centers, mediate visual recognition. Trends Cogn Sci. 17: 210–219. [DOI] [PubMed] [Google Scholar]
- 78.Krigolson OE, Cheng D, Binsted G (2015): The role of visual processing in motor learning and control: Insights from electroencephalography. Vision Res. 110: 277–285. [DOI] [PubMed] [Google Scholar]
- 79.Tzagarakis C, Ince NF, Leuthold AC, Pellizzer G (2010): Beta-band activity during motor planning reflects response uncertainty. J Neurosci. 30: 11270–11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Singh SP (2014): Magnetoencephalography: Basic principles. Ann Indian Acad Neurol. 17: S107–S112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Friston KJ, Harrison L, Penny W (2003): Dynamic causal modelling. Neuroimage. 19: 1273–1302. [DOI] [PubMed] [Google Scholar]
- 82.Kiebel SJ, Garrido MI, Moran RJ, Friston KJ (2008): Dynamic causal modelling for EEG and MEG. Cogn Neurodyn. 2: 121–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Minzenberg MJ, Firl AJ, Yoon JH, Gomes GC, Reinking C, Carter CS (2010): Gamma oscillatory power is impaired during cognitive control independent of medication status in first-episode schizophrenia. Neuropsychopharmacology. 35: 2590–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ramyead A, Studerus E, Kometer M, Uttinger M, Gschwandtner U, Fuhr P, Riecher-Rössler A (2016): Prediction of psychosis using neural oscillations and machine learning in neuroleptic-naïve at-risk patients. World J Biol Psychiatry. 17: 285–295. [DOI] [PubMed] [Google Scholar]
- 85.Lozano-Soldevilla D, Huurne N, Oostenveld R (2016): Neuronal oscillations with non-sinusoidal morphology produce spurious phase-to-amplitude coupling and directionality. Front Comput Neurosci. 10: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aru J, Aru J, Priesemann V, Wibral M, Lana L, Pipa G, et al. (2015): Untangling cross-frequency coupling in neuroscience. Curr Opin Neurobiol. 31: 51–61. [DOI] [PubMed] [Google Scholar]
- 87.Lee J, Gosselin F, Wynn JK, Green MF (2011): How do schizophrenia patients use visual information to decode facial emotion? Schizophr Bull. 37: 1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


