Abstract
Small cell lung cancer (SCLC; accounting for approximately 13%–15% of all lung cancers) is an exceptionally lethal malignancy characterized by rapid doubling time and high propensity to metastasize. In contrast to the increasingly personalized therapies in other types of lung cancer, SCLC is still regarded as a homogeneous disease and the prognosis of SCLC patients remains poor. Recently, however, substantial progress has been made in our understanding of SCLC biology. Advances in genomics and development of new preclinical models have facilitated insights into the intratumoral heterogeneity and specific genetic alterations of this disease. This worldwide resurgence of studies on SCLC has ultimately led to the development of novel subtype-specific classifications primarily based on the neuroendocrine features and distinct molecular profiles of SCLC. Importantly, these biologically distinct subtypes might define unique therapeutic vulnerabilities. Herein, we summarize the current knowledge on the molecular profiles of SCLC subtypes with a focus on their potential clinical implications.
Keywords: small cell lung cancer, heterogeneity, neuroendocrine, molecular profile
Graphical Abstract
Small cell lung cancer is still regarded as a homogeneous disease associated with poor prognosis. Recent analysis, however, has led to the development of novel subtype-specific classifications primarily based on the neuroendocrine features and molecular profiles. The better understanding of these biologically distinct subtypes might help to define unique therapeutic vulnerabilities.
Main text
Lung cancer, the leading cause of cancer-related deaths in the Western world, is classified into two major groups: small cell lung cancer (SCLC) and non-SCLC (NSCLC).1 SCLC accounts for approximately 13%–15% of all lung cancers, and with a 5-year survival rate of less than 7%, it remains one of the most lethal forms of malignant diseases.2,3 It has a very aggressive course and is characterized by genomic instability, almost universal inactivation of the genes TP53 and RB1, rapid tumor growth, increased vascularity, and high metastatic potential.4, 5, 6 Consequently, at the time of diagnosis, most SCLC patients already present with a metastatic spread outside the chest, which often leads to premature death.7,8 Most SCLC patients are current or former heavy smokers resulting in a high tumor mutational burden (TMB) (with C:G>A:T transversions being the most common type of base substitutions).9,10 Early detection strategies are mostly ineffective for SCLC even among high-risk populations, and there have been no significant improvements in survival and therapeutic approaches for more than 30 years, leading SCLC to be categorized as a “recalcitrant” cancer.5,11
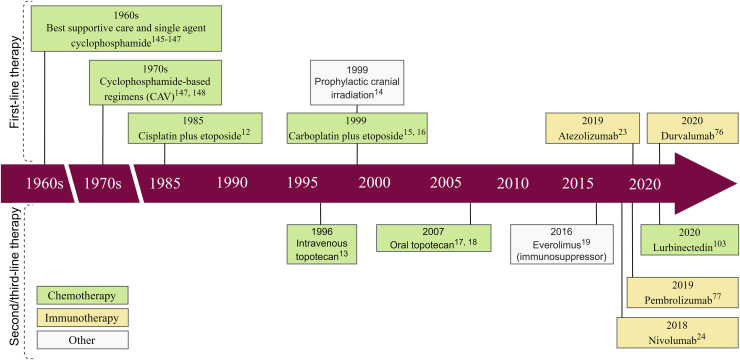
Platinum-based chemotherapy (CHT) in combination with etoposide and/or radiation therapy (RT) has been used in SCLC treatment and still remains the backbone for current combination strategies (Figure 117, 18, 19, 20, 21, 22, 23).16,24 Unlike NSCLC, which has an intrinsic tendency for CHT resistance, SCLC is initially highly sensitive to cytotoxic agents.25 Even with response, however, SCLC frequently recurs within a short time span, and patients are seldom cured.26 The most notable recent clinical progress in SCLC was the approval of the immune-checkpoint inhibitors (ICIs) atezolizumab, pembrolizumab, and nivolumab.27,28 Unfortunately, however, only 12.6% of patients remain progression-free at 1 year, and to date there are no reliable biomarkers predicting response to immune checkpoint blockade.27,29 With regard to other therapeutic approaches, due to the rapid doubling time of tumors and their high propensity to metastasize, surgery is rarely performed.30 Accordingly, limited human SCLC tissue availability has increased the importance of preclinical models.5,31
Figure 1.
Timeline of relevant therapeutic advances for small cell lung cancer (SCLC)
Initial therapeutic strategies for SCLC included surgery or radiotherapy alone. However, given the aggressive behavior and high metastatic potential of SCLC, systemic therapy with cytotoxic agents has rapidly become the cornerstone of management. In the 1940s, alkylating agents (such as nitrogen mustard) were used for the treatment of all bronchogenic carcinomas (including SCLC), resulting in tumor regression in more than 50% of patients. Yet, at that time, the true nature of SCLC was widely unknown, and all lung carcinomas were treated similarly. The first chemotherapeutic agent to show a statistically significant survival benefit for selected SCLC patients was cyclophosphamide, which doubled the survival when compared to best supportive care alone 12, 13, 14. Despite the encouraging results with single-agent therapy, it became obvious early in the 1970s that combination therapy produces superior survival outcomes when compared to single-agent treatment 14, 15. Therefore, during the late 1970s and early 1980s, cyclophosphamide was used in combination with other cytotoxic agents such as doxorubicin and vincristine (CAV). The basis of the currently used platinum-based combination chemotherapy (CHT) was defined during the mid-1980s when Evans et al.16 showed a clear survival benefit for patients treated with cisplatin plus etoposide. Since then, there have been no relevant advances in the standard-of-care CHT regimens, and the backbone for current combination strategies are still the platinum compounds. In more recent years, targeted therapy and immunotherapy have also been actively tested, leading to the approval of several immune-checkpoint inhibitors such as atezolizumab, pembrolizumab, and nivolumab.
Unlike the increasingly personalized approach to clinical care of patients with other types of lung cancer, SCLC is still regarded as a “homogeneous” disease with a single morphological type.32 Consequently, current clinical study protocols for SCLC are generally based on disease stage with no consideration of predefining distinct molecular marker expressions that might have predictive or prognostic significance.5,33 However, recently, there has been a worldwide resurgence of studies on SCLC, including the development of new preclinical models (such as patient-derived xenografts [PDXs] and genetically engineered mouse models [GEMMs]), comprehensive genomic profiling, and the identification of biologically distinct molecular subtypes.34 Exploring the molecular profiles of SCLC subtypes might help to focus and accelerate therapeutic research. In this review, we systematically analyze the molecular heterogeneity of SCLC, mainly focusing on rationally targeted therapeutic implications and new treatment opportunities that may ultimately improve the clinical outcomes for patients with this devastating disease.
Tumor heterogeneity in SCLC
Neuroendocrine (NE) subtypes
Although clinically SCLC is still regarded as a single disease entity, preclinical studies from the past decades identified biologically different SCLC subgroups (Figure 2). Accordingly, SCLC can be classified today into NE-high and NE-low subtypes primarily based on the expression pattern of different NE markers such as chromogranin A (CHGA), synaptophysin (SYP), neural cell adhesion molecule 1 (NCAM1/CD56), and gastrin-releasing peptide (GRP).5,6,33,35 Additionally, some SCLCs lack NE differentiation and are termed as non-NE tumors.33 The NE-high versus NE-low subtypes show major differences in genetic alterations, morphology, growth properties, and immune infiltration.35 NE-low SCLCs are furthermore associated with increased immune cell infiltration and referred to as “immune oasis” tumors, whereas NE-high SCLCs are characterized by low numbers of infiltrating immune cells and, consequently, have an “immune desert” phenotype.36 This categorization has major clinical impact, as the two phenotypes are anticipated to respond differently to targeted therapeutics and ICIs.37,38
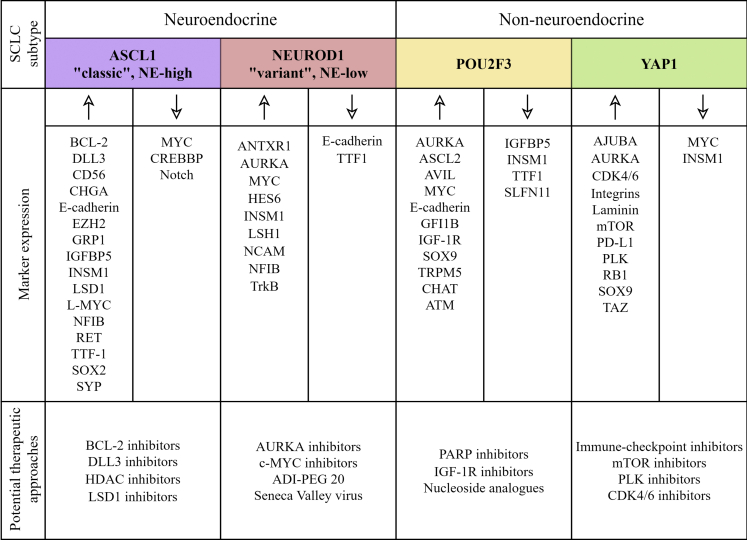
Figure 2.
Tumor heterogeneity in SCLC with regard to neuroendocrine differentiation, molecular subtypes, and gene expression profile
Neuroendocrine (NE) differentiation can be defined by the expression pattern of different NE markers, including chromogranin A, synaptophysin, neural cell adhesion molecule 1, and gastrin-releasing peptide. However, a minority of SCLCs are negative for all standard NE markers. Additionally, SCLC can be subclassified according to the relative expression of four key transcriptional regulators: achaete-scute homolog 1 (ASCL1; also known as ASH1), neurogenic differentiation factor 1 (NEUROD1), yes-associated protein 1 (YAP1), and POU class 2 homeobox 3 (POU2F3). Various genetic alterations, biological properties, and thus potential therapeutic vulnerabilities are associated with these molecular subsets.
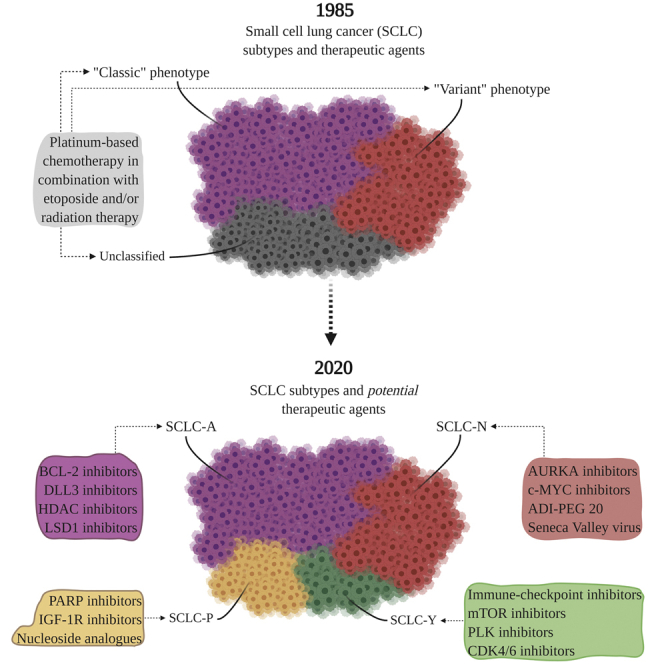
Of note, another example of NE heterogeneity was described by Gazdar et al.39,40 in 1985 based on the in vitro and in vivo behavior of SCLC cells. The “classic” phenotype is associated with typical morphology, high expression of NE markers, and non-adherent growth pattern in cell cultures.5,39 In contrast, the “variant” phenotype is usually characterized by larger cells with prominent nucleoli, low expression of NE features, and an adherent or loosely adherent growth pattern in vitro.5,33,35
Molecular subtypes
Recent SCLC profiling studies on cancer cell lines, PDXs, GEMMs, and primary human tumors suggest a model of distinct subtypes defined by the relative expression of four key transcriptional regulators: achaete-scute homolog 1 (ASCL1; also known as ASH1), neurogenic differentiation factor 1 (NEUROD1), yes-associated protein 1 (YAP1), and POU class 2 homeobox 3 (POU2F3).33,41 The association between these transcription factors (TFs) and the NE expression profile may provide subtype-specific therapeutic vulnerabilities (Figure 2).33 SCLC-A (ASCL1) tumors show high expression of NE markers and classic morphology, compared to the NE-low SCLC-N (NEUROD1) subtype with variant morphology.34 The transcription regulators ASCL1 and NEUROD1 both have been implicated as essential determinants of the developmental maturation of pulmonary NE cells (PNECs).42 In addition, SCLC-A and SCLC-N subtypes preferentially express the TF insulinoma-associated protein 1 (INSM1) as well.33 By inhibiting the Notch signaling pathway, INSM1 also plays a key role in NE differentiation, and thus its expression levels are low in non-NE SCLCs.43, 44, 45 NEUROD1, ASCL1, and INSM1 low-expressing or non-expressing SCLCs are classified either into SCLC-Y (YAP1) or SCLC-P (POU2F3) subtype, depending on their TF expression pattern.33 YAP1 is a transcription regulator activated by the HIPPO signaling pathway.45 Meanwhile, POU2F3 is required for the development of pulmonary tuft cells and chemosensory cells of the gastrointestinal epithelium.46 Accordingly, recent data on high POU2F3 expression patterns detected in certain SCLCs suggest tuft cells as the origin of the SCLC-P subtype.33,46
Genomic landscape of SCLC
SCLCs are likely to gain various genetic alterations as they evolve or as they metastasize outside the chest.47 The metastatic potential of SCLC is often driven by the overexpression of nuclear factor I B (NFIB), which functions as an oncogene and is frequently amplified in metastases.48 Likewise, lymph node (LN) metastases often undergo a change in NE expression patterns compared to the primary cancer, thus enhancing the aggressive course of the disease.6,49 It is important to consider intratumoral heterogeneity in SCLC, as NE cancer cells are also capable of raising non-NE tumor cells that expedite the tumorigenesis and additionally contribute to CHT resistance.47 Meanwhile, cancer stem cells (CSCs) are suspected to promote long-term tumor growth and affect the cellular heterogeneity of SCLC.47 In this context, SOX2, the transcriptional regulator of pluripotent stem cells, is frequently overexpressed, especially in the SCLC-A subtype.50
Notch signaling in SCLC is involved in NE differentiation.51 Tumor cells, which exhibit active Notch signaling, are slowly growing and often chemoresistant. Accordingly, Notch is considered as a tumor suppressor in SCLC.51,52 Based on the inhibitory activity of DLL3 on the Notch pathway or the inactivating mutations in Notch pathway genes, Notch is frequently inactivated in SCLCs with a high NE expression profile.6 Loss of NE differentiation and concomitant activation of Notch signaling is facilitated by the activation of the RE1 silencing transcription (REST) factor, a transcriptional repressor of NE and neuronal differentiation.53 Notably, REST is absent in most NE SCLCs, also resulting in the inhibition of Notch signaling.5
NE-high SCLCs often harbor overexpression in one of the Myc family members, including c-MYC, l-MYC, and n-MYC.54 The SCLC-A subtype is highly associated with the expression of l-MYC, whereas the upregulation of c-MYC is related to the SCLC-N subtype.33 In addition, the SCLC-A subtype is suspected to lose ASCL1 expression as a result of c-MYC overexpression since high levels of c-MYC might contribute to the progression of SCLC cells from the classic phenotype to the NE-low variant phenotype.55
It has also been hypothesized that MYC amplification appears during tumor progression and is connected to CHT resistance.54,56 Aurora kinase A and B (AURKA and AURKB) are serine/threonine kinases and have a main function in the regulation of mitosis.57 The overexpression of AURKA promotes cell proliferation.58 Meanwhile, AURKB phosphorylates RB1 and regulates the postmitotic checkpoints as well as prevents polyploidy after irregular mitosis.55 The overexpression of aurora kinases in SCLC accompanied by Myc family amplification provides a growth advantage and causes polyploidy in SCLC.59
The programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) signaling pathway is a major therapeutic target in SCLC.60 A considerable proportion of SCLCs exhibit aberrant PD-L1 expression on tumor cells that may be fundamental for moderate responses to immunotherapy.61 A subset of SCLCs is associated with the amplification of the fibroblast-growth factor receptor 1 (FGFR1) gene that displays a target for FGFR1 inhibitor therapy.62 Other unique features in SCLC include the expression of TFs SOX9 and ASCL2 and the receptor tyrosine kinase insulin-growth factor receptor 1 (IGF-1R) in POU2F3-expressing tumor cells.46 Important aberrations in the SCLC-A subtype comprise amplifications of BCL2, EZH2, and the decrease of CREBBP, whereas SCLC-Y is associated with mutations in the phosphatidylinositol 3-kinase (PI3K)/AKT/mTOR signaling pathway.5 Conclusively, intertumoral heterogeneity in SCLC has major therapeutic impacts.
Therapeutic implications
CHT
SCLC is initially highly sensitive to CHT and, accordingly, up to 75%–80% of all SCLCs first respond to platinum compounds.63 However, the development of resistance is essentially universal, and patients are rarely cured. Chemorefractory tumor cells may arise due to the extensive TMB in SCLC and the coexisting subpopulations within a tumor.5,47,64. In addition, therapeutic outcomes might also be altered by the growth of resistant cell clones during disease progression.47 Recent data suggest increased intratumoral heterogeneity after the onset of therapeutic resistance in SCLC.34,65 In contrast to NE-high SCLC cells (which are more sensitive to CHT because of their fast proliferation and high mitotic rate), the slower growing SCLC cells such as the previously mentioned Notchactive SCLC cells may be inherently chemoresistant.47 Inactive Notch signaling triggers the NE cell differentiation during lung development, while active Notch signaling controls the non-NE cell fates.66 Notch signaling further mediates the transition of NE to non-NE phenotype.66 In SCLC, Notch signaling delivers context-dependent tumor-suppressive or oncogenic signals through its receptors.66 MYC is also hypothesized to mediate NE plasticity in SCLC by activating Notch signaling.51,67 Furthermore, this interaction controls the dynamic behavior of cancer cells contributing to the co-existence of subtypes within a tumor.51,67
The expression of Schlafen 11 (SLFN11) (a member of the Schlafen family involved in the control of cell proliferation and induction of the immune response) is observed to predict treatment responses to DNA-damaging agents such as cisplatin, etoposide, and poly(ADP-ribose) polymerase (PARP) inhibitors in SCLCs.34,68,69 SLFN11 expression, therefore, correlates with sensitivity to various DNA-damaging chemotherapeutics, but in many cases it is silenced in SCLC by methylation or acetylation.34,70 On the contrary, the upregulation of EZH2 mediates chemoresistance based on SLFN11 downregulation via histone methylation and modification.71 Preclinical data led to the hypothesis that therapeutic targeting of EZH2 might prolong and augment sensitivity to the CHT response.60
Immunotherapy
ICIs have had a major impact on the clinical outcome of several solid tumors, including NSCLC, melanoma, and urothelial cancer.34,64,72,73 Epidemiological, biological, and clinical features of SCLC suggest that immunotherapy might be effective in this malignancy as well since many of the ICI susceptibility features in NSCLC are even more pronounced in SCLC.33,34,74 First, SCLC occurs almost exclusively in heavy smokers, and exposure to cigarette smoking is a predictive factor for responsiveness to ICIs in NSCLC.75,76 Second, compared to NSCLCs, which exhibit a lower TMB of 6.3–9 mutations/Mb, SCLCs display a higher median TMB of 9.9 mutations/Mb and lack the recurrent driver alterations in EGFR or ALK that correlate with poor response to immunotherapy.24,77 Third, SCLCs can also spontaneously provoke a strong immune response based on the segregation of humoral or cellular components that are summarized as paraneoplastic syndromes (PNSs).78 However, despite the above-mentioned susceptibility features, the latest clinical trials regarding the treatment of SCLC with ICIs have shown only modest improvement both in progression-free survival (PFS) and overall survival (OS) (Table 1).74
Table 1.
Completed clinical trials evaluating the safety and efficacy of immune-checkpoint inhibitors in SCLC patients
| Study name | Study phase | Mechanism of action | Agent | Outcomes |
|---|---|---|---|---|
| First-line therapy | ||||
| IMpower13327 | I/III | PD-L1 inhibitor | atezolizumab | safety: well tolerated |
| ORR: 60.2% versus 64.4% | ||||
| PFS:a 5.2 versus 4.3 (p = 0.02) | ||||
| OS:a 12.3 versus 10.3 (p = 0.007) | ||||
| CASPIAN80 | III | PD-L1 inhibitor | durvalumab | safety: well tolerated |
| ORR: 79% versus 70% | ||||
| PFS: 5.1 versus 5.4 (p = ns) | ||||
| OS: 13.0 versus 10.3 (p = 0.004) | ||||
| KEYNOTE-604138 | III | PD-1 inhibitor | pembrolizumab | safety: well tolerated |
| ORR: 70.6% versus 61.8% | ||||
| 12-month PFS: 13.6% versus 3.1% (p = 0.0023) | ||||
| 24-month OS: 22.5% versus 11.2% (p = 0.0164) | ||||
| REACTION139 | II | PD-1 inhibitor | pembrolizumab | safety: well tolerated |
| ORR: 61% | ||||
| PFS: 4.7 versus 5.4 | ||||
| OS: 12.3 versus 10.4 | ||||
| ClinicalTrials.gov: NCT01450761140 |
III |
CTLA-4 inhibitor |
ipilimumab |
safety: well tolerated |
| ORR: 62% versus 62% | ||||
| PFS: 4.6 versus 4.4 (p = 0.016) | ||||
| OS: 11.0 versus 10.9 (p = 0.377) | ||||
| Maintenance | ||||
| CheckMate 451141 | III | PD-1 and CTLA-4 inhibitors | nivolumab plus ipilimumab | non-significant results |
| ClinicalTrials.gov: NCT02359019142 |
II |
PD-1 inhibitor |
Pembrolizumab |
safety: well tolerated |
| ORR: 11.1% | ||||
| PFS: 1.4 | ||||
| OS: 9.6 | ||||
| Recurrent SCLC | ||||
| CheckMate-032143 | I/II | PD-1 and CTLA-4 inhibitors | nivolumab/ nivolumab plus ipilimumabb | safety: manageable safety profile |
| ORR: 10% versus 23% versus 19% | ||||
| PFS: 1.4 versus 2.6 versus 1.4 | ||||
| OS: 4.4 versus 7.7 versus 6.0 | ||||
| BIOLUMAc,144 | II | PD-1 and CTLA-4 inhibitors | nivolumab plus ipilimumab | safety: high toxicity rates |
| ORR: 38.8% | ||||
| CheckMate-331145 | III | PD-1 inhibitor | nivolumab | safety: well tolerated |
| ORR: 14% versus 16% | ||||
| PFS: 1.4 versus 3.8 | ||||
| OS: 7.5 versus 8.4 | ||||
| KEYNOTE-028146 | Ib | PD-1 inhibitor | pembrolizumab | safety: well tolerated |
| ORR: 33.3% | ||||
| PFS: 1.9 | ||||
| OS: 9.7 | ||||
| KEYNOTE-158147 | II | PD-1 inhibitor | pembrolizumab | safety: well tolerated |
| ORR: 18.7% | ||||
| PFS: 2.0 | ||||
| OS: 9.1 | ||||
| ClinicalTrials.gov: NCT02261220148 | I/II | PD-L1 and CTLA-4 inhibitors | durvalumab plus tremelimumab | safety: well tolerated |
| ORR: 13.3% | ||||
| PFS: 1.8 | ||||
| OS: 7.9 | ||||
PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; CTLA-4, cytotoxic T lymphocyte-associated protein 4; ORR, objective response rate; PFS, progression-free survival; OS, overall survival.
In months.
Nivolumab plus ipilimumab combination consisted of nivolumab (1 mg/kg) + ipilimumab (3 mg/kg) OR nivolumab (3 mg/kg) + ipilimumab (1 mg/kg).
Only patients with high tumor mutation burden were included.
The IMpower133 trial evaluated the efficacy and safety of the anti-PD-L1 antibody conjugate atezolizumab in combination with carboplatin and etoposide in treatment-naive patients diagnosed with advanced SCLC.60,79 The investigators found that although the addition of atezolizumab to CHT significantly prolonged the PFS and OS of these patients (versus the placebo group; OS and PFS were 12.3 versus 10.3 months and 5.2 versus 4.3 months, respectively), the gain in survivals was relatively modest compared to those achieved in NSCLC.79 Nevertheless, results of this trial represent the first significant improvement in systemic therapy for untreated SCLC patients in the last 30 years. Accordingly, the IMpower133 study has prompted the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) to approve atezolizumab in this setting.24
Notably, similar results were observed in the CASPIAN study, where first-line durvalumab plus platinum-etoposide resulted in moderately improved OS and overall response rate (ORR) (versus a clinically relevant control group; OS rates were 13 versus 10.3 months, and ORRs were 79% versus 70%, respectively).80 Importantly, however, there were no significant differences in PFS (5.1 versus 5.4 months).80
Pembrolizumab is an anti-PD-1 antibody approved for metastatic SCLC patients with disease progression after platinum-based CHT and at least one other line of therapy.81 Results from the KEYNOTE-028 and KEYNOTE-158 studies revealed antitumor activity in a subset of patients with recurrent SCLC.81 The KEYNOTE-158 study enrolled patients regardless of their PD-L1 status, whereas the KEYNOTE-028 study only included patients with PD-L1-positive SCLCs. Based on the median OS of 9.7 (KEYNOTE-028) and 9.1 (KEYNOTE-158) months in patients treated with pembrolizumab, this agent constitutes a treatment option for recurrent SCLC as third- or subsequent-line therapy.81 The CheckMate 032 clinical trial evaluated the efficacy of nivolumab alone or in combination with ipilimumab.28,82 The patients eligible for the study received platinum-based CHT and at least one other agent, but they still showed a rapid disease progression. In addition, the study also demonstrated an improved cytotoxic activity for combined cytotoxic T lymphocyte-associated protein 4 (CTLA-4) (ipilimumab)- and PD-1 (nivolumab)-targeted antibodies.82 Of note, however, third-line therapy for metastatic SCLC patients with nivolumab monotherapy demonstrated a durable response in a subgroup of SCLC patients regardless of their PD-L1 status.28,82 Accordingly, in 2018, the FDA granted accelerated approval to nivolumab for patients with metastatic SCLC based on the results of the CheckMate 032 clinical trial.28
All in all, although some data suggest that SCLC should be more vulnerable to immunotherapy than NSCLC, the available clinical results are less compelling. Therefore, there is an unmet need to identify the determinants of ICI activity in SCLC that might differ from those in NSCLC and other solid tumors.57 Immune phenotypes as well as the NE and molecular subtypes may provide a better understanding of the underlying properties of SCLC patients. NE-high tumors with the immune desert phenotype are associated with decreased immune cell (CD8+ effector T cells primarily) infiltration compared to NE-low tumors.36,38 Expressions of indoleamine 2,3-dioxygenase (IDO) and poliovirus receptor (PVR), which are both important factors of the SCLC immune microenvironment, are also significantly lower in NE-high tumors (versus NE-low tumors).38,83, 84, 85 In addition, these tumors are associated with low T cell immunoglobulin and mucin domain-3 (TIM3) levels as well.38,86 Of note, TIM3 is a specific marker of lymphocyte exhaustion and is one of the most promising immune-checkpoint targets.86 Altogether, patients with NE-high tumors are less likely to respond to ICIs.36,38 Another possible explanation for the poor response rates to immunotherapy in SCLC may be that PD-L1 expression is much lower in SCLC compared to other solid tumors, and cancer cell PD-L1 expression seemingly does not correlate with ICI efficacy.34,87 Furthermore, due to the suppressed expression of major histocompatibility complex class I (MHC class I) in the tumor microenvironment, antigen presentation might also be defective.88 Thus, the alterations affecting the antigen presentation by MHC molecules may contribute to escape from T cell recognition and destruction.34,87,88 Expressions of HLA-A, HLA-B, HLA-C, and β2-microglobulin are also significantly lower in SCLC cell lines (versus NSCLC), and, consequently, these cell lines are less immunogenic when injected into immunocompetent mice.34,88, 89, 90 Finally, the specific clinical features of SCLC might also affect the beneficial use of ICIs.74 Most SCLC patients often require prolonged steroid therapy due to superior vena cava syndrome or brain metastases.74 Chronic steroids, however, are a known limitation for immunotherapy.74,91
Immunotherapy-related adverse events (irAEs) represent a major concern in SCLC.74 irAEs mostly include inflammatory or autoimmune complications with sometimes severe sequelae for patients.74,92 Although the exact pathophysiological mechanism of irAEs has not been fully uncovered in SCLC, it is suspected that the genetic predisposition and the latent (i.e., clinically asymptomatic) PNSs might play a key role.92 One possible mechanism is a T cell-mediated reaction to shared antigens that are expressed both in tumors and inflammatory lesions.92 Additionally, the development of autoantibodies might also contribute to the appearance of irAEs, just as the overactivation of innate and adaptive immune cells, which lead to increased cytokine secretion.92 Lastly, the role of the gut mucosal immune system and the gut microbiome in irAEs has also been intensively investigated.93,94
Targeted therapy
Several targeted agents are being tested for the treatment of SCLC (Table 2). However, to advance these treatment options, it is necessary to better understand the molecular alterations that have been already described in SCLCs.5,6,108
Table 2.
Summary of clinical trials evaluating the safety and/or efficacy of targeted agents in advanced-stage SCLC patients
| Study name | Study phase | Mechanism of action | Agent | Outcomes |
|---|---|---|---|---|
| ClinicalTrials.gov: NCT0228969095 | I/II | PARP inhibitor | veliparib | safety: well tolerated |
| ClinicalTrials.gov: NCT0128698796 | I | PARP inhibitor | talazoparib | safety: well tolerated |
| ClinicalTrials.gov: NCT0163854697 | II | PARP inhibitor | veliparib | ORR: 39% versus 14% (p = 0.016) |
| PFS:a 3.8 versus 2.0 (p = 0.39) | ||||
| OS:a 82 versus 7.0 (p = 0.50) | ||||
| ECOG-ACRIN 251198 | II | PARP inhibitor | veliparib | ORR: 71.9% versus 65.6% (p = 0.57) |
| PFS: 6.1 versus 5.5 (p = 0.06) | ||||
| OS: 10.3 versus 8.9 (p = 0.17) | ||||
| TAHOE99 | III | DLL3-targeted antibody drug conjugate | Rova-T | non-significant results |
| TRINITY100 | II | DLL3-targeted antibody drug conjugate | Rova-T | non-significant results |
| ALTER 1202101 | II | tyrosine kinase inhibitor | anlotinib | PFS: 4.1 versus 0.7 (p < 0.0001) |
| OS: 7.3 versus 4.9 (p = 0.0210 | ||||
| ClinicalTrials.gov: NCT00154388102 | II | tyrosine kinase inhibitor | imatinib | non-significant results |
| ClinicalTrials.gov: NCT01533181103 | II | IGF-R1 inhibitor | linsitinib | non-significant results |
| ClinicalTrials.gov: NCT00869752104 | I | IGF-R1 inhibitor | dalotuzumab | safety: well tolerated |
| ClinicalTrials.gov: NCT02038647105 | II | AURKA inhibitor | alisertib | ORR: 22% versus 18% (p = 0.406) |
| PFS: 3.32 versus 2.17 (p = 0.113) | ||||
| OS: 6.86 versus 5.58 (p = 0.714) | ||||
| SALUTE106 | II | VEGF inhibitor | bevacizumab | ORR: 58% versus 48% |
| PFS: 5.5 versus 4.4 | ||||
| OS: 9.4 versus 10.9 | ||||
| ClinicalTrials.gov: NCT02454972107 | II | RNA polymerase II inhibitor | lurbinectedin | ORR: 35.2% |
| PFS: 3.5 | ||||
| OS: 9.3 |
PARP, poly(ADP-ribose) polymerase; DLL3, delta-like protein 3; IGF-R1, insulin-like growth factor 1 receptor; AURKA, aurora kinase A; VEGF, vascular endothelial growth factor.
In months.
Compared to other lung cancer subtypes and normal lung epithelial cells, SCLC cells show a high PARP expression profile and are highly sensitive to PARP inhibitors.98,109 In addition, PARP inhibitors also enhance the effects of CHT and ionizing radiation both in vivo and in vitro.109,110 Therefore, the use of PARP inhibitors might be a promising targeted therapeutic approach in SCLC patients. The phase II clinical trial ECOG-ACRIN 2511 investigated the efficacy of the PARP inhibitor veliparib in untreated, advanced-stage SCLC patients.98 The investigators found that although patients treated with veliparib in combination with cisplatin and etoposide doublet had an improved OS compared to the control group (OSs were 10.3 versus 8.9 months), the results were not statistically significant.98 PARP inhibitor combinations might thus be attractive therapeutic approaches for SCLC patients, but predictive biomarkers are required to maximize their clinical efficacy. Of note, expression of the already mentioned SLFN11 strongly correlates with veliparib efficacy and may represent a potential biomarker for these patients.97
The delta-like ligand 3 (DLL3) is highly expressed in a subset of SCLCs with NE origin.24 The antibody-drug conjugate rovalpituzumab tesirine (Rova-T) binds DLL3 on these target-expressing cells to induce cell death.24 Rova-T is the first targeted therapeutic agent in SCLC to use DLL3 as a novel biomarker.100 The phase II TRINITY study investigated the efficacy and safety of Rova-T as a third-line agent in relapsed and refractory SCLC patients.100 The study results revealed modest anti-tumor activity of Rova-T, which also caused toxicity.100 The Rova-T development program with reference to the phase III MERU trial was considered ineffective in case of unselected patients and was terminated by the manufacturer.111 Notably, although the results show a lack of survival benefit, Rova-T may still be a promising therapeutic approach for properly selected SCLC patients.
The phase II ClinicalTrials.gov: NCT01045421 study tested the activity and safety of alisertib in patients with relapsed or refractory SCLC or with other tumors.60,112 Patients eligible for the study had to have undergone two or fewer previous cytotoxic regimens. The phase II clinical trial of alisertib as a monotherapy or in combination with other agents in multiple tumor types showed antitumor activity and provides a therapeutic strategy in relapsed SCLCs.112
Surgery
Several retrospective observational studies and cancer registries have previously provided encouraging long-term results in patients who underwent surgical resection for early stage SCLC.113, 114, 115, 116, 117 Unfortunately, current screening and diagnostic approaches clearly fail to identify early stage SCLC patients who might be eligible for surgery.118 Surgery is thus rarely performed in SCLC and approximately 80%–85% of SCLC patients are being diagnosed with extensive disease.119 Therefore, there is an urgent clinical need for novel biomarkers with the potential of enabling early SCLC diagnosis and of improved selection of surgical candidates. Recent data have demonstrated for example the predominance of the ASCL1 subtype in early SCLC lesions.42,55 Importantly, novel targeted drugs may also find their way to the armamentarium of SCLC therapies in the neoadjuvant setting. The development of such neoadjuvant approaches could even allow to offer surgery for SCLC patients with initially more advanced disease. Thus, a better understanding of the molecular subtypes might revolutionize the role of surgery in SCLC and raise the hope for better outcomes in the future.
Targeted therapy with regard to NE and molecular subtypes
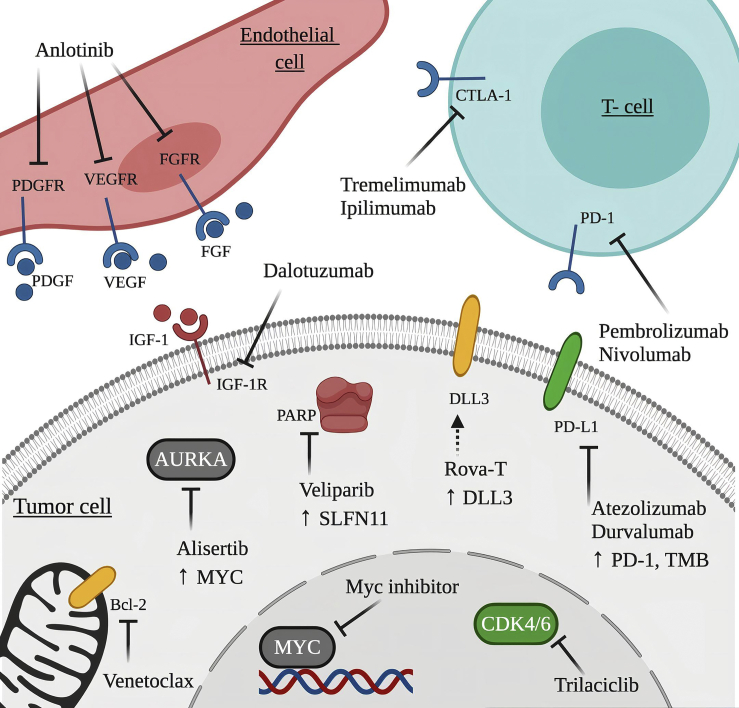
Targeted therapies for SCLC have so far failed, and the success of immunotherapy in NSCLC has not been reflected in SCLC.120 One major reason for these relatively disappointing results lies behind the heterogeneity of SCLC.33,47 Unlike in NSCLC therapy and clinical studies, SCLC patients are still enrolled in clinical trials irrespective of their molecular background.33 Accordingly, identification of subtype-specific molecular profiles and clinically meaningful biomarkers may contribute to novel targeted strategies in SCLC (Figure 3).
Figure 3.
Potential novel therapeutic approaches in SCLC
Subtype-specific potential therapeutic targets for SCLC-A, SCLC-N, SCLC-P, and SCLC-Y subtypes are highlighted in yellow (BCL2 and DLL3), gray (c-MYC and AURKA), red (PARP and IGF-1R), and green (CDK4/6 and PD-L1), respectively. The association between the aforementioned molecular subtypes and the potentially targetable molecules highlighted in blue (PDGFR, VEGFR, FGFR, CTLA-4 and PD-1), is currently unknown. The figure was created with BioRender.
SCLC-A
The SCLC-A subtype is anticipated to respond to DLL3-targeted antibody drug conjugates due to the direct transcriptional interaction of DLL3 with ASCL1 in Notchinactive tumor cells.121,122 Accordingly, treatment with the DLL3-targeted antibody drug conjugate Rova-T defines a subtype-specific therapy for SCLC-A.
BCL2 is another direct transcriptional target of ASCL1, and the high expression levels are suggestive of potential therapeutic benefit from the BCL2 inhibitor venetoclax.
The histone demethylase LSD1 activity has been described to be dependent on the disruption of INSM1, which is linked to the NE subtypes SCLC-A and SCLC-N.123 Recent data also indicate that LSD1 inhibition leads to NOTCH1 activation, resulting in ASCL1 suppression in SCLC.124
In addition, the SCLC-A subtype is also associated with CREBBP inactivation and thus with increased sensitivity to histone deacetylase (HDAC) inhibitors (e.g., pracinostat).125
SCLC-N
The SCLC-N subtype is frequently associated with MYC amplification, which serves as a potential target for therapeutic agents.56 Increased AURKA activity and arginine biosynthesis are also characteristic features of this particular subtype.55 Accordingly, the combination therapy of both AURKA (e.g., alisertib) and c-MYC inhibitors is hypothesized to enhance the therapeutic efficacy.33,126 Additionally, the SCLC-N (i.e., “variant”) subtype exhibits a selective tropism for the oncolytic Seneca Valley virus (SVV),127 which infects and eliminates the NE cancer cells via lysis. Therefore, with appropriate biomarker-guided patient selection, the SVV oncolytic virus may have selective efficacy either as single agent therapy or in combination with immunotherapy.33,127,128 In this context, the NEUROD1-to-ASCL1 ratio may function as a predictive biomarker.127 Finally, based on recent in vivo studies, “NEUROD1-high” tumor cells are also suspected to be sensitive to arginine depletion caused by pegylated arginine deaminase (ADI-PEG 20), which leads to the inhibition of tumor cell growth.33,54
SCLC-P
Based on the results of CRISPR screens, the SCLC-P subtype possesses a unique vulnerability to IGF-1R deficiency.46 This leads to the hypothesis that IGF-1R inhibitors (e.g., dalotuzumab) may serve as potential specific therapeutic agents for these patients.46 PARP inhibitors (e.g., veliparib) are also suspected to be most effective in this molecular subtype, although, to the best of our knowledge, SLFN11 expression does not correlate with the subtype-specific transcriptional regulators.129 Lastly, we mention that the SCLC-P subtype might be sensitive to nucleoside analogues as well.130
SCLC-Y
PD-1 or PD-L1 expressions are not subtype-specific but may preferentially be linked to the SCLC-Y subtype since YAP1 has been shown to upregulate PD-L1 transcripts and induce an immunosuppressive tumor microenvironment.131,132 In addition, SCLC-Y tumor cells have higher expression levels of both CD38 and LAG-3 and, consequently, they show a higher likelihood to respond to ICIs.131 Based on gene expression and recent in silico results, the SCLC-Y subtype is also the most sensitive to mTOR, PLK, and potentially to CDK4/6 inhibitors.45,133
Metabolic pathways may also provide new targets for the treatment of SCLC. Recent data described an iron-dependent type of regulated necrosis called ferroptosis.134 Non-NE SCLCs were demonstrated to be selectively sensitive to induced ferroptosis.134 In contrast, high-NE SCLCs are resistant to induced ferroptosis, but respond exquisitely to thioredoxin (TRX) pathway inhibition.134 A combination of ferroptosis induction and TRX pathway inhibition may thus provide a treatment regimen for intratumoral NE/non-NE heterogeneous SCLC.134
Open questions
What is the clinical relevance of intratumoral and intertumoral heterogeneity in SCLC?
The molecular characterization of SCLC is still of particular interest, although there have been major steps forward in identifying the exact pathogenesis and developing novel therapeutic approaches.6,33,47 SCLC is not a single entity as was thought before, but it displays heterogeneity in multiple ways. Significant intratumoral and intertumoral heterogeneity in SCLC was shown at the level of molecular diversity and NE differentiation. Bronchoscopic examination of the tumor may not be sufficient to determine the histological features and mutational landscape due to a possible presence of multiple subtypes within a tumor.51,135
Do NE and molecular subtypes correlate with different prognosis and clinical implications?
Genetic alterations lead to the development of SCLC subtypes and therapeutic resistance. The classification of NE (NE-high, NE-low, and non-NE) and molecular subtypes (ASCL1, NEUROD1, YAP1, and POU2F3) in recent years improved our understanding of SCLC. Recent clinical data demonstrate that ASCL1 overexpression might be a negative prognostic indicator in early stage resected SCLC patients.122 With regard to therapeutic implications, however, the SCLC-A subtype is suspected to be more chemosensitive compared to the SCLC-N subtype, since most SCLC-A cell lines are derived from treatment-naive patients whereas the SCLC-N cell lines mostly originate from post-treatment patients.40 As for the SCLC-Y subtype, YAP1 expression is linked with poor prognosis and decreased survival plus increased chemoresistance.45 These hypotheses are widely disputed, and the SCLC behavior in a clinical context is still not clarified.
What is the significance of biological plasticity between subtypes?
The presence of multiple subpopulations within a tumor and the biological plasticity between the subtypes might contribute to therapeutic outcomes. Recent preclinical studies suggest a possible hierarchy between subtypes, with SCLC-A being a necessary precursor of SCLC-N.33,42,55 This tumor evolution might greatly influence the response rates, as some tumor subpopulations may escape from therapy.33 Of note, however, the success of targeted therapies in cancer treatment is impaired by other mechanisms of resistance as well. Increased TMB potentially leads to a higher chance of developing drug resistance just as the amplification of the transcriptional regulator NFIB, driving tumor initiation, progression, and metastasis of SCLC.5
Do metabolic pathways in each subtype have therapeutic impact?
Beside the molecular background in SCLC, metabolic pathways in each particular subtype also influence the tumor behavior and therapeutic response. Ferroptosis and arginine depletion have recently been investigated to become targets for subtype-specific therapy.54,134 MYC-driven SCLC cells are dependent on different arginine-regulated pathways.54 Non-NE SCLCs are reported to be specifically sensitive to induced ferroptosis.134 Addressing selective cell death and metabolic pathways in SCLC subtypes may help to identify subtype-specific vulnerabilities for targeted therapies.134
Do SCLC subtypes display different metastatic potential and organotropism?
The NE pattern of LN or organ metastases might not reflect that of the primary tumor.49 Therefore, the resulting discordance between the primary tumor and metastases may result in the partial efficacy of therapeutic agents. Metastases of SCLC are observed to have a preference for certain organs. Very common sites for metastases comprise brain, bone, liver, and adrenal glands.136 Additionally, SCLC cells are suspected to arise from different cells of origin. The definition of the distinct precursor cells may reveal biomarkers, which help to understand the early events of tumorigenesis and predict the tumor evolution.137
Conclusions
In contrast to NSCLC, where genotype-based targeted therapies have dramatically improved the treatment outcomes in patients with advanced stage disease, the therapy options in SCLC are still limited and the survival rates are dismal. No significant progress has been made in the systemic treatment of SCLC in the last three decades, mainly due to the high plasticity of SCLC and also to the non-selected patient groups in clinical trials. Recent research defined the heterogeneity in SCLC, but further exploration of the nature of SCLC subtypes is needed to interpret their similarities, diversities, and respective behavior. Defining the distinct gene expression profiles (ASCL1, NEUROD1, POU2F3, and YAP1) of SCLC patients will be fundamental to choose the most effective therapy. Because immunotherapies, biomarker-directed therapies, and chemotherapies operate on different targets and mechanisms, a combined or synergistic treatment may increase the therapeutic effects. Conclusively, the development of new drugs and a combination of different subtype-specific therapies are substantial to fight this deadly disease.
Acknowledgments
B.D. acknowledges funding from the Hungarian National Research, Development and Innovation Office (KH130356, K129065, and KNN121510). B.D. and V.L. were also supported by the Austrian Science Fund (FWF I3977 and FWF I4677 to B.D. and FWF I3522 to V.L.). V.L. is a recipient of Janos Bolyai Research Scholarship of the Hungarian Academy of Sciences and the New National Excellence Program of the Ministry for Innovation and Technology (UNKP-19-4). Z.M. was supported by the New National Excellence Program of the Ministry for Innovation and Technology (UNKP-20-3).
Author contributions
Conceptualization, A.S., Z.M., V.L., and B.D.; methodology, A.S., Z.M., S.P., A.L., J.F., M.R, G.M.-V., K.B., G.G., F.R.-V., M.A.H., K.H., and W.K.; investigation, A.S., Z.M., N.B., Z.V., E.B., C.L., B.F., V.L., and B.D.; writing – original draft, A.S., Z.M., V.L., and B.D.; writing – review & editing, all authors; funding acquisition, Z.M., V.L., and B.D.; resources, F.R.-V., W.K., V.L., and B.D.; supervision, L.V. and B.D.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Zsolt Megyesfalvi, Email: megyesfalvi.zsolt@semmelweis-univ.hu.
Balazs Dome, Email: balazs.dome@meduniwien.ac.at.
References
- 1.Blandin Knight S., Crosbie P.A., Balata H., Chudziak J., Hussell T., Dive C. Progress and prospects of early detection in lung cancer. Open Biol. 2017;7:170070. doi: 10.1098/rsob.170070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahnert K., Kauffmann-Guerrero D., Huber R.M. SCLC—state of the art and what does the future have in store? Clin. Lung Cancer. 2016;17:325–333. doi: 10.1016/j.cllc.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Karachaliou N., Pilotto S., Lazzari C., Bria E., de Marinis F., Rosell R. Cellular and molecular biology of small cell lung cancer: an overview. Transl. Lung Cancer Res. 2016;5:2–15. doi: 10.3978/j.issn.2218-6751.2016.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 5.Gazdar A.F., Bunn P.A., Minna J.D. Small-cell lung cancer: what we know, what we need to know and the path forward. Nat. Rev. Cancer. 2017;17:725–737. doi: 10.1038/nrc.2017.87. [DOI] [PubMed] [Google Scholar]
- 6.George J., Lim J.S., Jang S.J., Cun Y., Ozretić L., Kong G., Leenders F., Lu X., Fernández-Cuesta L., Bosco G. Comprehensive genomic profiles of small cell lung cancer. Nature. 2015;524:47–53. doi: 10.1038/nature14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matthews M.J., Kanhouwa S., Pickren J., Robinette D. Frequency of residual and metastatic tumor in patients undergoing curative surgical resection for lung cancer. Cancer Chemother. Rep. 3. 1973;4:63–67. [PubMed] [Google Scholar]
- 8.Carter B.W., Glisson B.S., Truong M.T., Erasmus J.J. Small cell lung carcinoma: staging, imaging, and treatment considerations. Radiographics. 2014;34:1707–1721. doi: 10.1148/rg.346140178. [DOI] [PubMed] [Google Scholar]
- 9.Pleasance E.D., Stephens P.J. A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature. 2010;463:184–190. doi: 10.1038/nature08629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexandrov L.B., Ju Y.S., Haase K., Van Loo P., Martincorena I., Nik-Zainal S., Totoki Y., Fujimoto A., Nakagawa H., Shibata T. Mutational signatures associated with tobacco smoking in human cancer. Science. 2016;354:618–622. doi: 10.1126/science.aag0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.US Congress . 2012. H.R.733—Recalcitrant cancer research act of 2012.https://www.congress.gov/bill/112th-congress/house-bill/733 [Google Scholar]
- 12.Green R.A., Humphrey E., Close H., Patno M.E. Alkylating agents in bronchogenic carcinoma. Am. J. Med. 1969;46:516–525. doi: 10.1016/0002-9343(69)90071-0. [DOI] [PubMed] [Google Scholar]
- 13.Chan B.A., Coward J.I.G. Chemotherapy advances in small-cell lung cancer. J. Thorac. Dis. 2013;5(Suppl 5):S565–S578. doi: 10.3978/j.issn.2072-1439.2013.07.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haddadin S., Perry M.C. History of small-cell lung cancer. Clin. Lung Cancer. 2011;12:87–93. doi: 10.1016/j.cllc.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Einhorn L.H., Fee W.H., Farber M.O., Livingston R.B., Gottlieb J.A. Improved chemotherapy for small-cell undifferentiated lung cancer. JAMA. 1976;235:1225–1229. [PubMed] [Google Scholar]
- 16.Evans W.K., Osoba D., Feld R., Shepherd F.A., Bazos M.J., DeBoer G. Etoposide (VP-16) and cisplatin: an effective treatment for relapse in small-cell lung cancer. J. Clin. Oncol. 1985;3:65–71. doi: 10.1200/JCO.1985.3.1.65. [DOI] [PubMed] [Google Scholar]
- 17.Eckardt J., Gralla R., Palmer M.C., Gandara D., Laplante J., Sandler A., Fields S.Z., Fitts D., Broom C. Topotecan (T) as second-line therapy in patients (Pts) with small cell lung cancer (SCLC): a phase II study. Ann. Oncol. 1996;7(Suppl 5):107. [Google Scholar]
- 18.Aupérin A., Arriagada R., Pignon J.P., Le Péchoux C., Gregor A., Stephens R.J., Kristjansen P.E., Johnson B.E., Ueoka H., Wagner H., Aisner J., Prophylactic Cranial Irradiation Overview Collaborative Group Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. N. Engl. J. Med. 1999;341:476–484. doi: 10.1056/NEJM199908123410703. [DOI] [PubMed] [Google Scholar]
- 19.Samantas E., Skarlos D.V., Pectasides D., Nicolaides P., Kalofonos H., Mylonakis N., Vardoulakis T.h., Kosmidis P., Pavlidis N., Fountzilas G. Combination chemotherapy with low doses of weekly carboplatin and oral etoposide in poor risk small cell lung cancer. Lung Cancer. 1999;23:159–168. doi: 10.1016/s0169-5002(98)00095-6. [DOI] [PubMed] [Google Scholar]
- 20.Okamoto H., Watanabe K., Nishiwaki Y., Mori K., Kurita Y., Hayashi I., Masutani M., Nakata K., Tsuchiya S., Isobe H., Saijo N. Phase II study of area under the plasma-concentration-versus-time curve-based carboplatin plus standard-dose intravenous etoposide in elderly patients with small-cell lung cancer. J. Clin. Oncol. 1999;17:3540–3545. doi: 10.1200/JCO.1999.17.11.3540. [DOI] [PubMed] [Google Scholar]
- 21.2007). FDA approves oral topotecan for relapsed small-cell lung cancer. Oncology 21. https://www.cancernetwork.com/view/fda-approves-oral-topotecan-relapsed-small-cell-lung-cancer.
- 22.Eckardt J.R., von Pawel J., Pujol J.L., Papai Z., Quoix E., Ardizzoni A., Poulin R., Preston A.J., Dane G., Ross G. Phase III study of oral compared with intravenous topotecan as second-line therapy in small-cell lung cancer. J. Clin. Oncol. 2007;25:2086–2092. doi: 10.1200/JCO.2006.08.3998. [DOI] [PubMed] [Google Scholar]
- 23.Yao J.C., Fazio N., Singh S., Buzzoni R., Carnaghi C., Wolin E., Tomasek J., Raderer M., Lahner H., Voi M., RAD001 in Advanced Neuroendocrine Tumours, Fourth Trial (RADIANT-4) Study Group Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387:968–977. doi: 10.1016/S0140-6736(15)00817-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang C., Leighl N.B., Wu Y.L., Zhong W.Z. Emerging therapies for non-small cell lung cancer. J. Hematol. Oncol. 2019;12:45. doi: 10.1186/s13045-019-0731-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossi A., Di Maio M., Chiodini P., Rudd R.M., Okamoto H., Skarlos D.V., Früh M., Qian W., Tamura T., Samantas E. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data. J. Clin. Oncol. 2012;30:1692–1698. doi: 10.1200/JCO.2011.40.4905. [DOI] [PubMed] [Google Scholar]
- 26.Gong J., Salgia R. Managing patients with relapsed small-cell lung cancer. J. Oncol. Pract. 2018;14:359–366. doi: 10.1200/JOP.18.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horn L., Mansfield A.S., Szczęsna A., Havel L., Krzakowski M., Hochmair M.J., Huemer F., Losonczy G., Johnson M.L., Nishio M., IMpower133 Study Group First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N. Engl. J. Med. 2018;379:2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 28.Ready N., Farago A.F., de Braud F., Atmaca A., Hellmann M.D., Schneider J.G., Spigel D.R., Moreno V., Chau I., Hann C.L. Third-line nivolumab monotherapy in recurrent SCLC: CheckMate 032. J. Thorac. Oncol. 2019;14:237–244. doi: 10.1016/j.jtho.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iams W.T., Porter J., Horn L. Immunotherapeutic approaches for small-cell lung cancer. Nat. Rev. Clin. Oncol. 2020;17:300–312. doi: 10.1038/s41571-019-0316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inoue M., Sawabata N., Okumura M. Surgical intervention for small-cell lung cancer: what is the surgical role? Gen. Thorac. Cardiovasc. Surg. 2012;60:401–405. doi: 10.1007/s11748-012-0072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao G., Zhao Z., Jia Y. Advances in preclinical models of small cell lung cancer. Med One. 2019;4:e190019. [Google Scholar]
- 32.Travis W.D., Brambilla E., Nicholson A.G., Yatabe Y., Austin J.H.M., Beasley M.B., Chirieac L.R., Dacic S., Duhig E., Flieder D.B. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J. Thorac. Oncol. 2015;10:1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 33.Rudin C.M., Poirier J.T., Byers L.A., Dive C., Dowlati A., George J., Heymach J.V., Johnson J.E., Lehman J.M., MacPherson D. Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat. Rev. Cancer. 2019;19:289–297. doi: 10.1038/s41568-019-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drapkin B.J., Rudin C.M. Advances in small-cell lung cancer (SCLC) translational research. Cold Spring Harb. Perspect. Med. 2020:a038240. doi: 10.1101/cshperspect.a038240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang W., Girard L., Zhang Y.A., Haruki T., Papari-Zareei M., Stastny V., Ghayee H.K., Pacak K., Oliver T.G., Minna J.D., Gazdar A.F. Small cell lung cancer tumors and preclinical models display heterogeneity of neuroendocrine phenotypes. Transl. Lung Cancer Res. 2018;7:32–49. doi: 10.21037/tlcr.2018.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gazdar A.F. Molecular phenotypes of SCLC. J. Thorac. Oncol. 2018;13(10, Suppl) doi: 10.1016/j.jtho.2018.08.211. S309. [DOI] [Google Scholar]
- 37.Saito M., Shiraishi K., Goto A., Suzuki H., Kohno T., Kono K. Development of targeted therapy and immunotherapy for treatment of small cell lung cancer. Jpn. J. Clin. Oncol. 2018;48:603–608. doi: 10.1093/jjco/hyy068. [DOI] [PubMed] [Google Scholar]
- 38.Dora D., Rivard C., Yu H., Bunn P., Suda K., Ren S., Lueke Pickard S., Laszlo V., Harko T., Megyesfalvi Z. Neuroendocrine subtypes of small cell lung cancer differ in terms of immune microenvironment and checkpoint molecule distribution. Mol. Oncol. 2020;14:1947–1965. doi: 10.1002/1878-0261.12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carney D.N., Gazdar A.F., Bepler G., Guccion J.G., Marangos P.J., Moody T.W., Zweig M.H., Minna J.D. Establishment and identification of small cell lung cancer cell lines having classic and variant features. Cancer Res. 1985;45:2913–2923. [PubMed] [Google Scholar]
- 40.Gazdar A.F., Carney D.N., Nau M.M., Minna J.D. Characterization of variant subclasses of cell lines derived from small cell lung cancer having distinctive biochemical, morphological, and growth properties. Cancer Res. 1985;45:2924–2930. [PubMed] [Google Scholar]
- 41.Baine M.K., Hsieh M.S., Lai W.V., Egger J.V., Jungbluth A.A., Daneshbod Y., Beras A., Spencer R., Lopardo J., Bodd F. SCLC subtypes defined by ASCL1, NEUROD1, POU2F3, and YAP1: a comprehensive immunohistochemical and histopathologic characterization. J. Thorac. Oncol. 2020;15:1823–1835. doi: 10.1016/j.jtho.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borromeo M.D., Savage T.K., Kollipara R.K., He M., Augustyn A., Osborne J.K., Girard L., Minna J.D., Gazdar A.F., Cobb M.H., Johnson J.E. ASCL1 and NEUROD1 reveal heterogeneity in pulmonary neuroendocrine tumors and regulate distinct genetic programs. Cell Rep. 2016;16:1259–1272. doi: 10.1016/j.celrep.2016.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neptune E.R., Podowski M., Calvi C., Cho J.H., Garcia J.G., Tuder R., Linnoila R.I., Tsai M.J., Dietz H.C. Targeted disruption of NeuroD, a proneural basic helix-loop-helix factor, impairs distal lung formation and neuroendocrine morphology in the neonatal lung. J. Biol. Chem. 2008;283:21160–21169. doi: 10.1074/jbc.M708692200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borges M., Linnoila R.I., van de Velde H.J., Chen H., Nelkin B.D., Mabry M., Baylin S.B., Ball D.W. An achaete-scute homologue essential for neuroendocrine differentiation in the lung. Nature. 1997;386:852–855. doi: 10.1038/386852a0. [DOI] [PubMed] [Google Scholar]
- 45.McColl K., Wildey G., Sakre N., Lipka M.B., Behtaj M., Kresak A., Chen Y., Yang M., Velcheti V., Fu P., Dowlati A. Reciprocal expression of INSM1 and YAP1 defines subgroups in small cell lung cancer. Oncotarget. 2017;8:73745–73756. doi: 10.18632/oncotarget.20572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang Y.-H., Klingbeil O., He X.-Y., Wu X.S., Arun G., Lu B., Somerville T.D.D., Milazzo J.P., Wilkinson J.E., Demerdash O.E. POU2F3 is a master regulator of a tuft cell-like variant of small cell lung cancer. Genes Dev. 2018;32:915–928. doi: 10.1101/gad.314815.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shue Y.T., Lim J.S., Sage J. Tumor heterogeneity in small cell lung cancer defined and investigated in pre-clinical mouse models. Transl. Lung Cancer Res. 2018;7:21–31. doi: 10.21037/tlcr.2018.01.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Denny S.K., Yang D., Chuang C.H., Brady J.J., Lim J.S., Grüner B.M., Chiou S.H., Schep A.N., Baral J., Hamard C. Nfib promotes metastasis through a widespread increase in chromatin accessibility. Cell. 2016;166:328–342. doi: 10.1016/j.cell.2016.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lohinai Z., Megyesfalvi Z., Suda K., Harko T., Ren S., Moldvay J., Laszlo V., Rivard C., Dome B., Hirsch F.R. Comparative expression analysis in small cell lung carcinoma reveals neuroendocrine pattern change in primary tumor versus lymph node metastases. Transl. Lung Cancer Res. 2019;8:938–950. doi: 10.21037/tlcr.2019.11.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rudin C.M., Durinck S., Stawiski E.W., Poirier J.T., Modrusan Z., Shames D.S., Bergbower E.A., Guan Y., Shin J., Guillory J. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat. Genet. 2012;44:1111–1116. doi: 10.1038/ng.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lim J.S., Ibaseta A., Fischer M.M., Cancilla B., O’Young G., Cristea S., Luca V.C., Yang D., Jahchan N.S., Hamard C. Intratumoural heterogeneity generated by Notch signalling promotes small-cell lung cancer. Nature. 2017;545:360–364. doi: 10.1038/nature22323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ntziachristos P., Lim J.S., Sage J., Aifantis I. From fly wings to targeted cancer therapies: a centennial for notch signaling. Cancer Cell. 2014;25:318–334. doi: 10.1016/j.ccr.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thiel G., Ekici M., Rössler O.G. RE-1 silencing transcription factor (REST): a regulator of neuronal development and neuronal/endocrine function. Cell Tissue Res. 2015;359:99–109. doi: 10.1007/s00441-014-1963-0. [DOI] [PubMed] [Google Scholar]
- 54.Chalishazar M.D., Wait S.J., Huang F., Ireland A.S., Mukhopadhyay A., Lee Y., Schuman S.S., Guthrie M.R., Berrett K.C., Vahrenkamp J.M. MYC-driven small-cell lung cancer is metabolically distinct and vulnerable to arginine depletion. Clin. Cancer Res. 2019;25:5107–5121. doi: 10.1158/1078-0432.CCR-18-4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mollaoglu G., Guthrie M.R., Böhm S., Brägelmann J., Can I., Ballieu P.M., Marx A., George J., Heinen C., Chalishazar M.D. MYC drives progression of small cell lung cancer to a variant neuroendocrine subtype with vulnerability to aurora kinase inhibition. Cancer Cell. 2017;31:270–285. doi: 10.1016/j.ccell.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Massó-Vallés D., Beaulieu M.E., Soucek L. MYC, MYCL, and MYCN as therapeutic targets in lung cancer. Expert Opin. Ther. Targets. 2020;24:101–114. doi: 10.1080/14728222.2020.1723548. [DOI] [PubMed] [Google Scholar]
- 57.Lum C., Alamgeer M. Technological and therapeutic advances in advanced small cell lung cancer. Cancers (Basel) 2019;11:1570. doi: 10.3390/cancers11101570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yan M., Wang C., He B., Yang M., Tong M., Long Z., Liu B., Peng F., Xu L., Zhang Y. Aurora-A kinase: a potent oncogene and target for cancer therapy. Med. Res. Rev. 2016;36:1036–1079. doi: 10.1002/med.21399. [DOI] [PubMed] [Google Scholar]
- 59.Sos M.L., Dietlein F., Peifer M., Schöttle J., Balke-Want H., Müller C., Koker M., Richters A., Heynck S., Malchers F. A framework for identification of actionable cancer genome dependencies in small cell lung cancer. Proc. Natl. Acad. Sci. USA. 2012;109:17034–17039. doi: 10.1073/pnas.1207310109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sabari J.K., Lok B.H., Laird J.H., Poirier J.T., Rudin C.M. Unravelling the biology of SCLC: implications for therapy. Nat. Rev. Clin. Oncol. 2017;14:549–561. doi: 10.1038/nrclinonc.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schultheis A.M., Scheel A.H., Ozretić L., George J., Thomas R.K., Hagemann T., Zander T., Wolf J., Buettner R. PD-L1 expression in small cell neuroendocrine carcinomas. Eur. J. Cancer. 2015;51:421–426. doi: 10.1016/j.ejca.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 62.Schultheis A.M., Bos M., Schmitz K., Wilsberg L., Binot E., Wolf J., Büttner R., Schildhaus H.U. Fibroblast growth factor receptor 1 (FGFR1) amplification is a potential therapeutic target in small-cell lung cancer. Mod. Pathol. 2014;27:214–221. doi: 10.1038/modpathol.2013.141. [DOI] [PubMed] [Google Scholar]
- 63.Karim S.M., Zekri J. Chemotherapy for small cell lung cancer: a comprehensive review. Oncol. Rev. 2012;6:e4. doi: 10.4081/oncol.2012.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boumber Y. Tumor mutational burden (TMB) as a biomarker of response to immunotherapy in small cell lung cancer. J. Thorac. Dis. 2018;10:4689–4693. doi: 10.21037/jtd.2018.07.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stewart C.A., Gay C.M., Xi Y., Sivajothi S., Sivakamasundari V., Fujimoto J., Bolisetty M., Hartsfield P.M., Balasubramaniyan V., Chalishazar M.D. Single-cell analyses reveal increased intratumoral heterogeneity after the onset of therapy resistance in small-cell lung cancer. Nat. Can. 2020;1:423–436. doi: 10.1038/s43018-019-0020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meder L., Büttner R., Odenthal M. Notch signaling triggers the tumor heterogeneity of small cell lung cancer. J. Thorac. Dis. 2017;9:4884–4888. doi: 10.21037/jtd.2017.11.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ng J., Sutherland K.D. NOTCH your usual suspect: MYC charged with controlling neuroendocrine cell-fate in small cell lung cancer. Cancer Cell. 2020;38:17–20. doi: 10.1016/j.ccell.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 68.Zoppoli G., Regairaz M., Leo E., Reinhold W.C., Varma S., Ballestrero A., Doroshow J.H., Pommier Y. Putative DNA/RNA helicase Schlafen-11 (SLFN11) sensitizes cancer cells to DNA-damaging agents. Proc. Natl. Acad. Sci. USA. 2012;109:15030–15035. doi: 10.1073/pnas.1205943109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Allison Stewart C., Tong P., Cardnell R.J., Sen T., Li L., Gay C.M., Masrorpour F., Fan Y., Bara R.O., Feng Y. Dynamic variations in epithelial-to-mesenchymal transition (EMT), ATM, and SLFN11 govern response to PARP inhibitors and cisplatin in small cell lung cancer. Oncotarget. 2017;8:28575–28587. doi: 10.18632/oncotarget.15338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nogales V., Reinhold W.C., Varma S., Martinez-Cardus A., Moutinho C., Moran S., Heyn H., Sebio A., Barnadas A., Pommier Y., Esteller M. Epigenetic inactivation of the putative DNA/RNA helicase SLFN11 in human cancer confers resistance to platinum drugs. Oncotarget. 2016;7:3084–3097. doi: 10.18632/oncotarget.6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gardner E.E., Lok B.H., Schneeberger V.E., Desmeules P., Miles L.A., Arnold P.K., Ni A., Khodos I., de Stanchina E., Nguyen T. Chemosensitive relapse in small cell lung cancer proceeds through an EZH2-SLFN11 axis. Cancer Cell. 2017;31:286–299. doi: 10.1016/j.ccell.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gopalakrishnan D., Koshkin V.S., Ornstein M.C., Papatsoris A., Grivas P. Immune checkpoint inhibitors in urothelial cancer: recent updates and future outlook. Ther. Clin. Risk Manag. 2018;14:1019–1040. doi: 10.2147/TCRM.S158753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Herrscher H., Robert C. Immune checkpoint inhibitors in melanoma in the metastatic, neoadjuvant, and adjuvant setting. Curr. Opin. Oncol. 2020;32:106–113. doi: 10.1097/CCO.0000000000000610. [DOI] [PubMed] [Google Scholar]
- 74.Pavan A., Attili I., Pasello G., Guarneri V., Conte P.F., Bonanno L. Immunotherapy in small-cell lung cancer: from molecular promises to clinical challenges. J. Immunother. Cancer. 2019;7:205. doi: 10.1186/s40425-019-0690-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim J.H., Kim H.S., Kim B.J. Prognostic value of smoking status in non-small-cell lung cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Oncotarget. 2017;8:93149–93155. doi: 10.18632/oncotarget.18703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reck M., Rodríguez-Abreu D., Robinson A.G., Hui R., Csőszi T., Fülöp A., Gottfried M., Peled N., Tafreshi A., Cuffe S., KEYNOTE-024 Investigators Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 77.Chalmers Z.R., Connelly C.F., Fabrizio D., Gay L., Ali S.M., Ennis R., Schrock A., Campbell B., Shlien A., Chmielecki J. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9:34. doi: 10.1186/s13073-017-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sebastian M., Koschade S., Stratmann J.A. SCLC, paraneoplastic syndromes, and the immune system. J. Thorac. Oncol. 2019;14:1878–1880. doi: 10.1016/j.jtho.2019.07.033. [DOI] [PubMed] [Google Scholar]
- 79.Mansfield A.S., Każarnowicz A., Karaseva N., Sánchez A., De Boer R., Andric Z., Reck M., Atagi S., Lee J.S., Garassino M. Safety and patient-reported outcomes of atezolizumab, carboplatin, and etoposide in extensive-stage small-cell lung cancer (IMpower133): a randomized phase I/III trial. Ann. Oncol. 2020;31:310–317. doi: 10.1016/j.annonc.2019.10.021. [DOI] [PubMed] [Google Scholar]
- 80.Paz-Ares L., Dvorkin M., Chen Y., Reinmuth N., Hotta K., Trukhin D., Statsenko G., Hochmair M.J., Özgüroğlu M., Ji J.H., CASPIAN investigators Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394:1929–1939. doi: 10.1016/S0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 81.Chung H.C., Piha-Paul S.A., Lopez-Martin J., Schellens J.H.M., Kao S., Miller W.H., Jr., Delord J.P., Gao B., Planchard D., Gottfried M. Pembrolizumab after two or more lines of previous therapy in patients with recurrent or metastatic SCLC: results from the KEYNOTE-028 and KEYNOTE-158 studies. J. Thorac. Oncol. 2020;15:618–627. doi: 10.1016/j.jtho.2019.12.109. [DOI] [PubMed] [Google Scholar]
- 82.Ready N.E., Ott P.A., Hellmann M.D., Zugazagoitia J., Hann C.L., de Braud F., Antonia S.J., Ascierto P.A., Moreno V., Atmaca A. Nivolumab monotherapy and nivolumab plus ipilimumab in recurrent small cell lung cancer: results from the CheckMate 032 randomized cohort. J. Thorac. Oncol. 2020;15:426–435. doi: 10.1016/j.jtho.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 83.Hornyák L., Dobos N., Koncz G., Karányi Z., Páll D., Szabó Z., Halmos G., Székvölgyi L. The role of indoleamine-2,3-dioxygenase in cancer development, diagnostics, and therapy. Front. Immunol. 2018;9:151. doi: 10.3389/fimmu.2018.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Katz J.B., Muller A.J., Prendergast G.C. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol. Rev. 2008;222:206–221. doi: 10.1111/j.1600-065X.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- 85.Sloan K.E., Eustace B.K., Stewart J.K., Zehetmeier C., Torella C., Simeone M., Roy J.E., Unger C., Louis D.N., Ilag L.L., Jay D.G. CD155/PVR plays a key role in cell motility during tumor cell invasion and migration. BMC Cancer. 2004;4:73. doi: 10.1186/1471-2407-4-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Friedlaender A., Addeo A., Banna G. New emerging targets in cancer immunotherapy: the role of TIM3. ESMO Open. 2019;4(Suppl 3):e000497. doi: 10.1136/esmoopen-2019-000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leclerc M., Mezquita L., Guillebot De Nerville G., Tihy I., Malenica I., Chouaib S., Mami-Chouaib F. Recent advances in lung cancer immunotherapy: input of T-cell epitopes associated with impaired peptide processing. Front. Immunol. 2019;10:1505. doi: 10.3389/fimmu.2019.01505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Doyle A., Martin W.J., Funa K., Gazdar A., Carney D., Martin S.E., Linnoila I., Cuttitta F., Mulshine J., Bunn P. Markedly decreased expression of class I histocompatibility antigens, protein, and mRNA in human small-cell lung cancer. J. Exp. Med. 1985;161:1135–1151. doi: 10.1084/jem.161.5.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Doyle L.A., Cuttitta F., Mulshine J.L., Bunn P.A., Minna J.D. Markedly different antibody responses to immunized small cell and non-small cell lung cancer cells. Cancer Res. 1987;47:5009–5013. [PubMed] [Google Scholar]
- 90.Yazawa T., Kamma H., Fujiwara M., Matsui M., Horiguchi H., Satoh H., Fujimoto M., Yokoyama K., Ogata T. Lack of class II transactivator causes severe deficiency of HLA-DR expression in small cell lung cancer. J. Pathol. 1999;187:191–199. doi: 10.1002/(SICI)1096-9896(199901)187:2<191::AID-PATH206>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 91.Arbour K.C., Mezquita L., Long N., Rizvi H., Auclin E., Ni A., Martínez-Bernal G., Ferrara R., Lai W.V., Hendriks L.E.L. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J. Clin. Oncol. 2018;36:2872–2878. doi: 10.1200/JCO.2018.79.0006. [DOI] [PubMed] [Google Scholar]
- 92.König D., Läubli H. Mechanisms of immune-related complications in cancer patients treated with immune checkpoint inhibitors. Pharmacology. 2020 doi: 10.1159/000509081. Published online July 28, 2020. [DOI] [PubMed] [Google Scholar]
- 93.Mizrahi M., Ilan Y. The gut mucosa as a site for induction of regulatory T-cells. Curr. Pharm. Des. 2009;15:1191–1202. doi: 10.2174/138161209787846784. [DOI] [PubMed] [Google Scholar]
- 94.Anderson R., Theron A.J., Rapoport B.L. Immunopathogenesis of immune checkpoint inhibitor-related adverse events: roles of the intestinal microbiome and Th17 cells. Front. Immunol. 2019;10:2254. doi: 10.3389/fimmu.2019.02254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Atrafi F., Groen H.J.M., Byers L.A., Garralda E., Lolkema M.P., Sangha R.S., Viteri Ramirez S., Chae Y.K., Camidge D.R., Gabrail N.Y. Phase 1/2 study of veliparib (V) combined with carboplatin (Cb) and etoposide (E) in patients (pts) with extensive-stage disease (ED) small cell lung cancer (SCLC) and other solid tumors: phase 1 results. J. Clin. Oncol. 2017;35(15, Suppl):8530. doi: 10.1158/1078-0432.CCR-18-2014. [DOI] [PubMed] [Google Scholar]
- 96.Wainberg Z.A., de Bono J.S., Mina L., Sachdev J., Byers L.A., Chugh R., Zhang C., Henshaw J.W., Dorr A., Glaspy J. Update on first-in-man trial of novel oral PARP inhibitor BMN 673 in patients with solid tumors. Mol. Cancer Ther. 2013;12(11, Suppl):C295. [Google Scholar]
- 97.Pietanza M.C., Waqar S.N., Krug L.M., Dowlati A., Hann C.L., Chiappori A., Owonikoko T.K., Woo K.M., Cardnell R.J., Fujimoto J. Randomized, double-blind, phase II study of temozolomide in combination with either veliparib or placebo in patients with relapsed-sensitive or refractory small-cell lung cancer. J. Clin. Oncol. 2018;36:2386–2394. doi: 10.1200/JCO.2018.77.7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Owonikoko T.K., Dahlberg S.E., Sica G.L., Wagner L.I., Wade J.L., 3rd, Srkalovic G., Lash B.W., Leach J.W., Leal T.B., Aggarwal C., Ramalingam S.S. Randomized phase II trial of cisplatin and etoposide in combination with veliparib or placebo for extensive-stage small-cell lung cancer: ECOG-ACRIN 2511 study. J. Clin. Oncol. 2019;37:222–229. doi: 10.1200/JCO.18.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.AbbVie . 2018. Phase 3 trial of Rova-T as second-line therapy for advanced small-cell lung cancer (TAHOE study)https://news.abbvie.com/news/phase-3-trial-rova-t-as-second-line-therapy-for-advanced-small-cell-lung-cancer-tahoe-study-halted.htm [Google Scholar]
- 100.Morgensztern D., Besse B., Greillier L., Santana-Davila R., Ready N., Hann C.L., Glisson B.S., Farago A.F., Dowlati A., Rudin C.M. Efficacy and safety of rovalpituzumab tesirine in third-line and beyond patients with DLL3-expressing, relapsed/refractory small-cell lung cancer: results from the phase II TRINITY study. Clin. Cancer Res. 2019;25:6958–6966. doi: 10.1158/1078-0432.CCR-19-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cheng Y., Wang Q., Li K., Shi J., Wu L., Han B., Chen G., He J., Wang J., Qin H. Anlotinib as third-line or further-line treatment in relapsed SCLC: a multicentre, randomized, double-blind phase 2 trial. J. Thorac. Oncol. 2018;13(10, Suppl):S351. [Google Scholar]
- 102.Soria J.C., Johnson B.E., Chevalier T.L. Imatinib in small cell lung cancer. Lung Cancer. 2003;41(Suppl 1):S49–S53. doi: 10.1016/s0169-5002(03)00142-9. [DOI] [PubMed] [Google Scholar]
- 103.Chiappori A.A., Otterson G.A., Dowlati A., Traynor A.M., Horn L., Owonikoko T.K., Ross H.J., Hann C.L., Abu Hejleh T., Nieva J. A randomized phase II study of linsitinib (OSI-906) versus topotecan in patients with relapsed small-cell lung cancer. Oncologist. 2016;21:1163–1164. doi: 10.1634/theoncologist.2016-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ellis P.M., Shepherd F.A., Laurie S.A., Goss G.D., Olivo M., Powers J., Seymour L., Bradbury P.A. NCIC CTG IND.190 phase I trial of dalotuzumab (MK-0646) in combination with cisplatin and etoposide in extensive-stage small-cell lung cancer. J. Thorac. Oncol. 2014;9:410–413. doi: 10.1097/JTO.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 105.Owonikoko T.K., Niu H., Nackaerts K., Csoszi T., Ostoros G., Mark Z., Baik C., Joy A.A., Chouaid C., Jaime J.C., C14018 study investigators Randomized phase II study of paclitaxel plus alisertib versus paclitaxel plus placebo as second-line therapy for SCLC: primary and correlative biomarker analyses. J. Thorac. Oncol. 2020;15:274–287. doi: 10.1016/j.jtho.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 106.Spigel D.R., Townley P.M., Waterhouse D.M., Fang L., Adiguzel I., Huang J.E., Karlin D.A., Faoro L., Scappaticci F.A., Socinski M.A. Randomized phase II study of bevacizumab in combination with chemotherapy in previously untreated extensive-stage small-cell lung cancer: results from the SALUTE trial. J. Clin. Oncol. 2011;29:2215–2222. doi: 10.1200/JCO.2010.29.3423. [DOI] [PubMed] [Google Scholar]
- 107.Trigo J., Subbiah V., Besse B., Moreno V., López R., Sala M.A., Peters S., Ponce S., Fernández C., Alfaro V. Lurbinectedin as second-line treatment for patients with small-cell lung cancer: a single-arm, open-label, phase 2 basket trial. Lancet Oncol. 2020;21:645–654. doi: 10.1016/S1470-2045(20)30068-1. [DOI] [PubMed] [Google Scholar]
- 108.Schulze A.B., Evers G., Kerkhoff A., Mohr M., Schliemann C., Berdel W.E., Schmidt L.H. Future options of molecular-targeted therapy in small cell lung cancer. Cancers (Basel) 2019;11:690. doi: 10.3390/cancers11050690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Byers L.A., Wang J., Nilsson M.B., Fujimoto J., Saintigny P., Yordy J., Giri U., Peyton M., Fan Y.H., Diao L. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer Discov. 2012;2:798–811. doi: 10.1158/2159-8290.CD-12-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Calabrese C.R., Almassy R., Barton S., Batey M.A., Calvert A.H., Canan-Koch S., Durkacz B.W., Hostomsky Z., Kumpf R.A., Kyle S. Anticancer chemosensitization and radiosensitization by the novel poly(ADP-ribose) polymerase-1 inhibitor AG14361. J. Natl. Cancer Inst. 2004;96:56–67. doi: 10.1093/jnci/djh005. [DOI] [PubMed] [Google Scholar]
- 111.Tata P.R., Rajagopal J. Plasticity in the lung: making and breaking cell identity. Development. 2017;144:755–766. doi: 10.1242/dev.143784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Melichar B., Adenis A., Lockhart A.C., Bennouna J., Dees E.C., Kayaleh O., Obermannova R., DeMichele A., Zatloukal P., Zhang B. Safety and activity of alisertib, an investigational aurora kinase A inhibitor, in patients with breast cancer, small-cell lung cancer, non-small-cell lung cancer, head and neck squamous-cell carcinoma, and gastro-oesophageal adenocarcinoma: a five-arm phase 2 study. Lancet Oncol. 2015;16:395–405. doi: 10.1016/S1470-2045(15)70051-3. [DOI] [PubMed] [Google Scholar]
- 113.Vallières E., Shepherd F.A., Crowley J., Van Houtte P., Postmus P.E., Carney D., Chansky K., Shaikh Z., Goldstraw P., International Association for the Study of Lung Cancer International Staging Committee and Participating Institutions The IASLC Lung Cancer Staging Project: proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J. Thorac. Oncol. 2009;4:1049–1059. [Google Scholar]
- 114.Schreiber D., Rineer J., Weedon J., Vongtama D., Wortham A., Kim A., Han P., Choi K., Rotman M. Survival outcomes with the use of surgery in limited-stage small cell lung cancer: should its role be re-evaluated? Cancer. 2010;116:1350–1357. doi: 10.1002/cncr.24853. [DOI] [PubMed] [Google Scholar]
- 115.Varlotto J.M., Recht A., Flickinger J.C., Medford-Davis L.N., Dyer A.-M., DeCamp M.M. Lobectomy leads to optimal survival in early-stage small cell lung cancer: a retrospective analysis. J. Thorac. Cardiovasc. Surg. 2011;142:538–546. doi: 10.1016/j.jtcvs.2010.11.062. [DOI] [PubMed] [Google Scholar]
- 116.Takei H., Kondo H., Miyaoka E., Asamura H., Yoshino I., Date H., Okumura M., Tada H., Fujii Y., Nakanishi Y., Japanese Joint Committee of Lung Cancer Registry Surgery for small cell lung cancer: a retrospective analysis of 243 patients from Japanese Lung Cancer Registry in 2004. J. Thorac. Oncol. 2014;9:1140–1145. doi: 10.1097/JTO.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 117.Combs S.E., Hancock J.G., Boffa D.J., Decker R.H., Detterbeck F.C., Kim A.W. Bolstering the case for lobectomy in stages I, II, and IIIA small-cell lung cancer using the National Cancer Data Base. J. Thorac. Oncol. 2015;10:316–323. doi: 10.1097/JTO.0000000000000402. [DOI] [PubMed] [Google Scholar]
- 118.Hoda M.A., Klikovits T., Klepetko W. Controversies in oncology: surgery for small cell lung cancer? It’s time to rethink the case. ESMO Open. 2018;3:e000366. doi: 10.1136/esmoopen-2018-000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dowell J.E. Small cell lung cancer: are we making progress? Am. J. Med. Sci. 2010;339:68–76. doi: 10.1097/MAJ.0b013e3181bccef5. [DOI] [PubMed] [Google Scholar]
- 120.Taniguchi H., Sen T., Rudin C.M. Targeted therapies and biomarkers in small cell lung cancer. Front. Oncol. 2020;10:741. doi: 10.3389/fonc.2020.00741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim D.-W., Kim K.-C., Kim K.-B., Dunn C.T., Park K.-S. Transcriptional deregulation underlying the pathogenesis of small cell lung cancer. Transl. Lung Cancer Res. 2018;7:4–20. doi: 10.21037/tlcr.2017.10.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Furuta M., Sakakibara-Konishi J., Kikuchi H., Yokouchi H., Nishihara H., Minemura H., Harada M., Yamazaki S., Akie K., Fujita Y., Hokkaido Lung Cancer Clinical Study Group Analysis of DLL3 and ASCL1 in surgically resected small cell lung cancer (HOT1702) Oncologist. 2019;24:e1172–e1179. doi: 10.1634/theoncologist.2018-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Takagi S., Ishikawa Y., Mizutani A., Iwasaki S., Matsumoto S., Kamada Y., Nomura T., Nakamura K. LSD1 inhibitor T-3775440 inhibits SCLC cell proliferation by disrupting LSD1 interactions with SNAG domain proteins INSM1 and GFI1B. Cancer Res. 2017;77:4652–4662. doi: 10.1158/0008-5472.CAN-16-3502. [DOI] [PubMed] [Google Scholar]
- 124.Augert A., Eastwood E., Ibrahim A.H., Wu N., Grunblatt E., Basom R., Liggitt D., Eaton K.D., Martins R., Poirier J.T. Targeting NOTCH activation in small cell lung cancer through LSD1 inhibition. Sci. Signal. 2019;12:eaau2922. doi: 10.1126/scisignal.aau2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jia D., Augert A., Kim D.W., Eastwood E., Wu N., Ibrahim A.H., Kim K.B., Dunn C.T., Pillai S.P.S., Gazdar A.F. Crebbp loss drives small cell lung cancer and increases sensitivity to HDAC inhibition. Cancer Discov. 2018;8:1422–1437. doi: 10.1158/2159-8290.CD-18-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tsoukalas N., Aravantinou-Fatorou E., Baxevanos P., Tolia M., Tsapakidis K., Galanopoulos M., Liontos M., Kyrgias G. Advanced small cell lung cancer (SCLC): new challenges and new expectations. Ann. Transl. Med. 2018;6:145. doi: 10.21037/atm.2018.03.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Poirier J.T., Dobromilskaya I., Moriarty W.F., Peacock C.D., Hann C.L., Rudin C.M. Selective tropism of Seneca Valley virus for variant subtype small cell lung cancer. J. Natl. Cancer Inst. 2013;105:1059–1065. doi: 10.1093/jnci/djt130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cao L., Zhang R., Liu T., Sun Z., Hu M., Sun Y., Cheng L., Guo Y., Fu S., Hu J. Seneca Valley virus attachment and uncoating mediated by its receptor anthrax toxin receptor 1. Proc. Natl. Acad. Sci. USA. 2018;115:13087–13092. doi: 10.1073/pnas.1814309115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Inno A., Stagno A., Gori S. Schlafen-11 (SLFN11): a step forward towards personalized medicine in small-cell lung cancer? Transl. Lung Cancer Res. 2018;7(Suppl 4):S341–S345. doi: 10.21037/tlcr.2018.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gay C.M., Diao L., Stewart C.A., Xi Y., Cardnell R.J., Swisher S.G., Roth J.A., Glisson B.S., Wang J., Heymach J.V. Inter- and intra-tumoral variations in ASCL1, NEUROD1, and POU2F3 transcriptional programs underlie three distinct molecular subtypes of small cell lung cancers. Cancer Res. 2019;79(13, Suppl) doi: 10.1158/1538-7445.AM2019-3772. 3772. [DOI] [Google Scholar]
- 131.Shibata M., Ham K., Hoque M.O. A time for YAP1: tumorigenesis, immunosuppression and targeted therapy. Int. J. Cancer. 2018;143:2133–2144. doi: 10.1002/ijc.31561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee B.S., Park D.I., Lee D.H., Lee J.E., Yeo M.K., Park Y.H., Lim D.S., Choi W., Lee D.H., Yoo G. Hippo effector YAP directly regulates the expression of PD-L1 transcripts in EGFR-TKI-resistant lung adenocarcinoma. Biochem. Biophys. Res. Commun. 2017;491:493–499. doi: 10.1016/j.bbrc.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 133.Horie M., Saito A., Ohshima M., Suzuki H.I., Nagase T. YAP and TAZ modulate cell phenotype in a subset of small cell lung cancer. Cancer Sci. 2016;107:1755–1766. doi: 10.1111/cas.13078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bebber C.M., Thomas E.S., Chen Z., Stroh J., Androulidaki A., Schmitt A., Höhne M.N., Stüker L., de Pádua Alves C., Khonsari A. Ferroptosis response segregates small cell lung cancer (SCLC) neuroendocrine subtypes. bioRxiv. 2020 doi: 10.1101/2020.07.11.198408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yang D., Denny S.K., Greenside P.G., Chaikovsky A.C., Brady J.J., Ouadah Y., Granja J.M., Jahchan N.S., Lim J.S., Kwok S. Intertumoral heterogeneity in SCLC is influenced by the cell type of origin. cancer Discov. 2018;8:1316–1331. doi: 10.1158/2159-8290.CD-17-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nakazawa K., Kurishima K., Tamura T., Kagohashi K., Ishikawa H., Satoh H., Hizawa N. Specific organ metastases and survival in small cell lung cancer. Oncol. Lett. 2012;4:617–620. doi: 10.3892/ol.2012.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Park K.S., Liang M.C., Raiser D.M., Zamponi R., Roach R.R., Curtis S.J., Walton Z., Schaffer B.E., Roake C.M., Zmoos A.F. Characterization of the cell of origin for small cell lung cancer. Cell Cycle. 2011;10:2806–2815. doi: 10.4161/cc.10.16.17012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rudin C.M., Awad M.M., Navarro A., Gottfried M., Peters S., Csőszi T., Cheema P.K., Rodriguez-Abreu D., Wollner M., Yang J.C., KEYNOTE-604 Investigators Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J. Clin. Oncol. 2020;38:2369–2379. doi: 10.1200/JCO.20.00793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Besse B., Menis J., Bironzo P., Gervais R., Greillier L., Monnet I., Livi L., Young R., Decroisette C., Cloarec N. LBA85—REACTION: A phase II study of etoposide and cis/carboplatin with or without pembrolizumab in untreated extensive small cell lung cancer. Ann. Oncol. 2020;31(Suppl 4):S1211–S1212. [Google Scholar]
- 140.Reck M., Luft A., Szczesna A., Havel L., Kim S.-W., Akerley W., Pietanza M.C., Wu Y.L., Zielinski C., Thomas M. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J. Clin. Oncol. 2016;34:3740–3748. doi: 10.1200/JCO.2016.67.6601. [DOI] [PubMed] [Google Scholar]
- 141.Owonikoko T.K., Kim H.R., Govindan R., Ready N., Reck M., Peters S., Dakhil S.R., Navarro A., Rodriguez-Cid J., Schenker M. Nivolumab (nivo) plus ipilimumab (ipi), nivo, or placebo (pbo) as maintenance therapy in patients (pts) with extensive disease small cell lung cancer (ED-SCLC) after first-line (1L) platinum-based chemotherapy (chemo): results from the double-blind, randomized phase III CheckMate 451 study. Ann. Oncol. 2019;30(Suppl 2):ii77. [Google Scholar]
- 142.Gadgeel S.M., Pennell N.A., Fidler M.J., Halmos B., Bonomi P., Stevenson J., Schneider B., Sukari A., Ventimiglia J., Chen W. Phase II study of maintenance pembrolizumab in patients with extensive-stage small cell lung cancer (SCLC) J. Thorac. Oncol. 2018;13:1393–1399. doi: 10.1016/j.jtho.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Antonia S.J., López-Martin J.A., Bendell J., Ott P.A., Taylor M., Eder J.P., Jäger D., Pietanza M.C., Le D.T., de Braud F. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17:883–895. doi: 10.1016/S1470-2045(16)30098-5. [DOI] [PubMed] [Google Scholar]
- 144.Fischer R.N., George J., Scheel A.H., Schloesser H.A., Vehreschild M., Brossart P., Engel-Riedel W., Griesinger F., Grohé C., Kambartel K.-O. BIOLUMA: a phase II trial of nivolumab in combination with ipilimumab to evaluate efficacy and safety in lung cancer and to evaluate biomarkers predictive for response—preliminary results from the SCLC cohort. J. Clin. Oncol. 2019;37(15, Suppl):8563. [Google Scholar]
- 145.Reck M., Vicente D., Ciuleanu T., Gettinger S., Peters S., Horn L., Audigier-Valette C., Pardo N., Juan-Vidal O., Cheng Y. Efficacy and safety of nivolumab (nivo) monotherapy versus chemotherapy (chemo) in recurrent small cell lung cancer (SCLC): results from CheckMate 331. Ann. Oncol. 2018;29(Suppl 10):x43. [Google Scholar]
- 146.Ott P.A., Elez E., Hiret S., Kim D.-W., Morosky A., Saraf S., Piperdi B., Mehnert J.M. Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the phase Ib KEYNOTE-028 study. J. Clin. Oncol. 2017;35:3823–3829. doi: 10.1200/JCO.2017.72.5069. [DOI] [PubMed] [Google Scholar]
- 147.Chung H.C., Lopez-Martin J.A., Kao S.C.-H., Miller W.H., Ros W., Gao B., Marabelle A., Gottfried M., Zer A., Delord J.-P. Phase 2 study of pembrolizumab in advanced small-cell lung cancer (SCLC): KEYNOTE-158. J. Clin. Oncol. 2018;36(15, Suppl):8506. [Google Scholar]
- 148.Cho D.C., Mahipal A., Dowlati A., Chow W.A., Segal N.H., Chung K.Y., Schneider B.J., Nemunaitis J.J., Abdul Razak A.R., Tsai F.Y.-C. Safety and clinical activity of durvalumab in combination with tremelimumab in extensive disease small-cell lung cancer (ED-SCLC) J. Clin. Oncol. 2018;36(15, Suppl):8517. [Google Scholar]