Abstract
The neonatal Fc receptor (FcRn) binds endogenous IgG and protects it from lysosomal degradation by transporting it back to the cell surface to re-enter the circulation, extending the serum IgG life span. FcRn plays a role in the function of IVIg because the supraphysiological IgG levels derived from IVIg administrations saturate the FcRn allowing the endogenous IgG to be degraded, instead of being recycled, resulting in high levels of infused IgG ensuring IVIg efficiency. New data in myasthenia gravis patients suggest that the that the Variable Number of Tandem 3/2 (VNTR3/2) polymorphisms in FCGRT, the gene that encodes FcRn, may affect the duration of infused IgG in the circulation and IVIg effectiveness. This review addresses these implications in the context of whether the FCGRT genotype, by affecting the half-life of IVIg, may also play a role in up to 30% of patients with autoimmune neurological diseases, such as Guillain–Barré syndrome, CIDP or Multifocal Motor Neuropathy, who did not respond to IVIg in controlled trials. The concern is of practical significance because in such patient subsets super-high IVIg doses may be needed to achieve high IgG levels and ensure efficacy. Whether FCGRT polymorphisms affect the efficacy of other therapeutic monoclonal antibodies by influencing their distribution clearance and pharmacokinetics, explaining their variable effectiveness, is also addressed. Finally, the very promising effect of monoclonal antibodies that inhibit FcRn, such as efgartigimod, rozanolixizumab and nipocalimab, in treating antibody-mediated neurological diseases is discussed along with their efficacy in the IgG4 subclass of pathogenic antibodies and their role in the blood–brain barrier endothelium, that abundantly expresses FcRn.
Keywords: autoantibodies, Autoimmune neurology, FCGRT gene polymorphisms, FcRn, IgG catabolism IVIg, Neuro-mmunotherapies
The neonatal Fc receptor (FcRn), is a widely expressed Major Histocompatibilty Complex class I-like receptor that binds at acidic pH endogenous IgG and albumin and protects them from lysosomal degradation by transporting them back to the cell surface to re-enter the circulation (Figure 1A).1–4 The protective recycling role of FcRn extends the life span of endogenous serum IgG and albumin and ensures their abundance among all plasma proteins. Although this concept dates back to more than 50 years ago,5 it is now gaining major attention. Stimulated by the contribution of Su et al.6 in this issue of Therapeutic Advances in Neurological Disorders, this timely mini-review intends to highlight the role of FcRn in IgG homeostasis, the influence of FcRn genotypes on the efficacy of IVIg and monoclonal antibodies in neurological diseases, and the emerging role of the new neuro-immunotherapeutics that inhibit the capacity of FcRn to affect IgG autoantibodies.
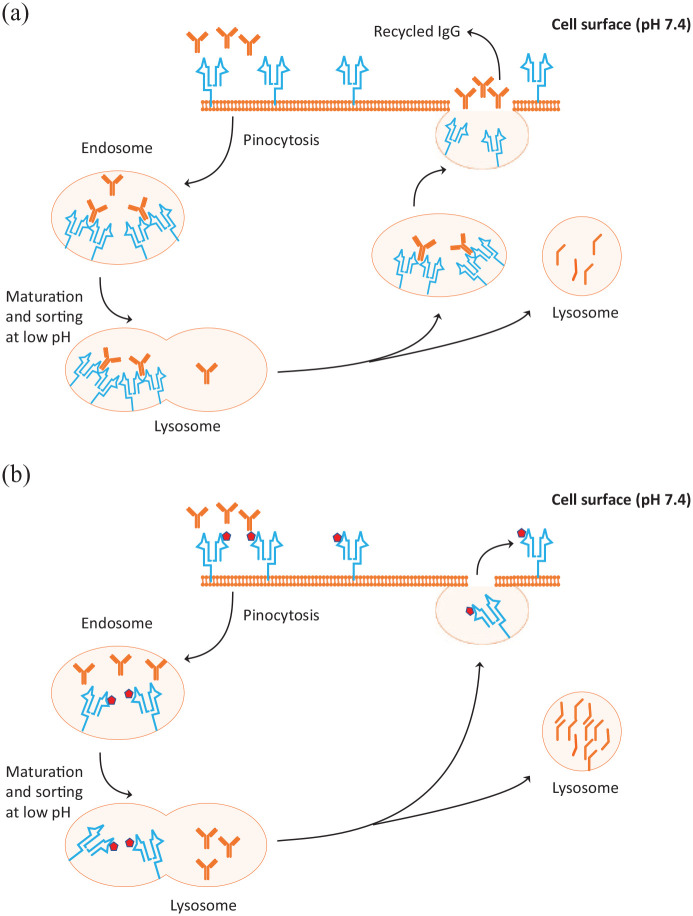
Figure 1.
Mechanism of action of the neonatal Fc receptor (FcRn) in protecting IgG from degradation (A) and enhancement of IgG catabolism by FcRn inhibitors (B). (A) When IgG molecules are ingested by pinocytosis, the pinocytic vesicles fuse with endosomes, which during their maturation become increasingly acidic, allowing binding of IgG with FcRn (shown in blue) sorting out what is not bound. The IgGs bound to FcRn are then retained and recycled to the cell surface where they are released to the circulation by exocytosis. The excess unbound IgGs enter the lysosomes for degradation. (B) FcRn inhibitors are monoclonal antibodies (projected in dark red cubes) engineered to increase binding to FcRn at neutral and acidic pH. When this happens, the ingested IgGs cannot bind to FcRn and remain unbound entering the lysosomes where they are degraded. The process results in reduced IgG levels in the circulation with reduction of IgG including the pathogenic IgG antibodies.
IgG: 
FcRn: 
FcRn inhibitor: 
The FcRn-mediated recycling of IgG is, based on mice studies, about 42% greater than the rate of IgG production,7 highlighting a remarkable economy of nature to prolong the half-life of IgG and maintain a high IgG steady-state level in the circulation. This process is facilitated by the diverse expression of FcRn in several tissues throughout the body, including epithelia, endothelia, hematopoietic cells and antigen-presenting cells, like dendritic cells and macrophages, important for T-cell-mediated immunity.1–3 While in antibody deficiency syndromes the role of FcRn is protective to ensure a higher level of circulating IgG, in antibody-mediated diseases FcRn may theoretically prolong the life even of pathological autoantibodies. Targeting FcRn, therefore, either by reducing its availability, inhibiting its function or flooding the system with IgG as done with IVIg infusions (where 1 g/kg of IVIg is equivalent to the total body IgG),2 offers novel therapeutic opportunities in autoimmune neurological diseases.
FcRn is encoded by FCGRT, a 14 kb gene located on the long arm of chromosome 19.8 Five different alleles with a variable number of tandem repeat (VNTR) polymorphisms in the FCGRT gene, from VNTR1 to VNTR5, result in different expression levels of FcRn mRNA and protein levels.8,9 VNTR3, the most common allele, is associated with an increase in promoter activity which in turn increases FcRn level in homozygous VNTR3/3 individuals, compared to the VNTR2 allele or heterozygous VNTR3/2 individuals. On this basis, it has been argued that polymorphisms in the FCGRT gene may play a role in patients treated with IgG biologicals, such as IVIg and therapeutic monoclonal antibodies, by affecting their efficiency via IgG pharmacokinetics (PKs).8,9 But, how much do these alleles matter in autoimmune neurological therapeutics and whether the variable degrees of FcRn saturation, expression or inhibition are sufficient to influence the IgG catabolism dynamics and the circulating pathogenic autoantibodies or treatment efficacy, remain unsettled or under-recognized.
VNTR polymorphisms and their relation to efficacy of IVIg
A relation between VNTR polymorphisms and the outcome of IVIG therapy has been shown in common variable immunodeficiency (CVID), in which homozygous VNTR3/3 patients have higher FcRn expression resulting in higher protection by slowing the degradation of the administered IgG, compared to patients with the VNTR3/2 polymorphism.9,10 In contrast, in patients with autoimmune or antibody-mediated autoimmune diseases the supraphysiological levels of IgG derived from IVIg administrations saturate the FcRn so the endogenously produced IgGs can be degraded rather than recycled and high serum levels of infused IgG are ensured.11 Pharmacokinetic data from 174 patients with Guillain–Barré syndrome (GBS) have confirmed that 2 weeks after one IVIg administration the patients with a higher increase in circulating IgG had a significantly better outcome.12 In a retrospective analysis, however, there was no relation between the VNTR3/3 polymorphism, the pharmacokinetics of IVIg, or the patients’ clinical course and outcome.13 Although in this study the IgG data were limited and efficacy was assessed only up to 2 weeks,13 similar results were also observed in 23 patients with multifocal motor neuropathy,14 generating uncertainties as to the clinical significance of VNTR polymorphisms in IVIg therapeutics.
In this issue of the journal, Su et al.6 explored whether VNTR3/3 and VNTR2/3 genotypes affected the efficacy of IVIg in 334 patients with myasthenia gravis (MG). They found that those with the VNTR2/3 genotype had significantly lower endogenous IgG levels and poor response to IVIg compared to patients with VNTR3/3 homozygosity. Four VNTR2/3 patients who did not respond to IVIg, had a short duration of infused IVIg presumably due to fast IgG catabolism that shortened IVIg retention and reduced its efficacy. It was concluded that in MG patients VNTR polymorphisms do matter and the VNTR2 allele may be an indicator of lower IVIg efficacy. The results are interesting but challenging because the study did not assess if the VNTR polymorphisms influenced the catabolism of pathogenic AChR antibodies, or whether the VNTR3/2 alleles were less capable of protecting the infused IgG and affected the IgG pharmacokinetics resulting in IVIg ineffectiveness. In spite of these shortcomings, the paper is a stimulus for new prospective studies to assess whether VNTR genotypes affect the levels and sustainability not only of the endogenous IgG but also of the infused IgG, and possibly answer the fundamental question as to why in all effective randomized controlled studies in patients with Chronic Inflammatory Demyelinating Polyneuropathy (CIDP), GBS, multifocal motor neuropathy, MG, Dermatomyositis, or Stiff Person Syndrome,15–18 20–30% of the patients did not respond to IVIg even if their disease status was identical to that of responders. IVIg has multiple functions via complement, cytokines or idiotypic antibodies19 but whether the different VNTR genotypes also play a role will be of clinical importance even in retrospectively collected data. Because VTNR3/3 individuals protect and prolong the half-life of IgG more effectively than VTNR3/2 individuals, they may also protect pathogenic autoantibodies, generating complexities as to the specific role – if any – of VTNR polymorphisms in immunotherapies with IVIg or subcutaneous IgG. Such a clarification is important because if among the 20–30% of patients who do not respond to IVIg in controlled studies the ineffectiveness in some patients is due to VNTR genotypes that affect the half-life or the infused IgG levels, higher IVIg doses or more frequent administration will be needed. We have all seen patients infused with 3 g/kg per month to achieve effectiveness, with a recent series of six CIDP patients responding only to super-high IVIg doses, up 4–6 g/kg per month.20
Effect of VNTR polymorphisms on efficacy of therapeutic monoclonal antibodies
Whether these polymorphisms have also an effect on therapeutic monoclonal antibodies (mAbs) has not been systematically explored. There is evidence, however, that VNTR3 homozygous patients with normal circulating IgG levels have a lower mAb distribution clearance compared to VNTR2/VNTR3 and VNTR3/VNTR4 patients. The passage of cetuximab, a mAb against epidermal growth factor receptor, from the circulation to central compartments was shown to be slower in VNTR3 homozygous patients compared to heterozygous patients, probably due to a higher expression of FcRn and higher recirculating capacity that retained cetuximab more efficiently in the circulation.10 Similar results were observed with two anti-TNF-α agents, infliximab and adalimumab, in inflammatory bowel disease,21 and with farletuzumab, a mAb against α-folate receptor in ovarian cancer patients.22 The expression of FcRn in relevance to VNTR polymorphisms may have therefore been an overlooked factor in understanding the PKs of therapeutic mAbs and explain their variable efficacy. It will also be of interest to examine whether efgartigimod as used in MG patients,23 has a greater reduction in AChR antibody titers and better efficacy in VNTR3 homozygous patients, especially compared to IVIg.6
Role of FcRn in lowering serum IgG: current therapies with FcRn inhibitors
Lowering pathogenic IgG antibodies, either alone or in conjunction with other immune mediators, is a fundamental target in neurological therapeutics. This can now be achieved by: (a) Plasmapheresis, which non-specifically reduces the level not only of pathogenic antibodies (i.e. AChR in MG), but also of large plasma proteins including albumin, clotting factors or the IgG titers of various protective antibodies;2 (b) Immunoadsorption, a therapeutic filtration procedure that removes specific physiological and pathological immunoglobulins; (c) IVIg, which may potentially shorten the half-life of pathogenic autoantibodies by saturating the FcRn;2,11 and (d) FcRn inhibitors, a remarkable group of agents, very promising in neurotherapeutics of antibody-mediated diseases.
FcRn inhibitors bind FcRn with extremely high affinity at low and neutral pH enhancing catabolism of all IgG subclasses leading to selective reduction of serum IgG (Figure 1B). Inhibition of FcRn reduces the circulation of both pathogenic and non-pathogenic IgG by up to 85% from baseline in a dose-dependent manner,24,25 and at levels as low as plasmapheresis does, but without affecting the turnover, production or quality of IgG autoantibodies. Of importance, FcRn inhibition does not affect other components of the innate or adaptive immune systems and does not exert a rebound effect on the total IgG levels.2,3 The IgG reduction caused by FcRn inhibitors is, however, transient and reversible, returning to near-baseline levels 50–57 days after a single dose, and 50–80 days after multiple doses2 indicating that the function of memory B-cells and plasma cells remains unaffected.3 The immunogenicity of vaccines, especially important in this period of the COVID-19 pandemic, is not therefore compromised by the FcRn inhibitors when vaccines are administered 4–8 weeks before treatment begins, allowing time for adaptive immunity to develop. Most importantly, based on present evidence, patients receiving a FcRn inhibitor have a limited risk of increased infection.2,3 The main FcRn inhibitors, currently in trials in neurology showing promise especially in patients with MG in phase II–III studies, include:
(a) Efgartigimod (Argenx BVBA), a humanized IgG1 Fc fragment engineered to increase FcRn binding at neutral and acidic pH. A single dose reduces the total IgG level by 50%, while repeated dosing lowers IgG by 75%.23 The maximum IgG lowering effect is seen with 10 mg/kg which is the dose used in clinical studies. Early results in a placebo controlled, phase II study in 24 MG patients were impressive.23,26,27 Twelve patients received four doses over a 3-week period of 10 mg/kg IV efgartigimod and 12 placebo. All the efgartigimod-receiving patients showed a rapid, within 2 weeks, decrease in the total IgG and anti-AChR autoantibody levels; 75% of patients showed a rapid and long-lasting improvement in four efficacy scales coinciding with the maximal IgG lowering and reduction of AChR antibody levels. The AChR antibody levels returned to normal within 8 weeks. Efgartigimod was not only effective but also safe and well tolerated leading to a phase III ADAPT study which solidified these results meeting the primary endpoint.27 In 167 generalized MG patients (129 AChR-Ab-positive and 38 AChR-Ab-negative), efgartigimod showed clinically meaningful improvement in MG-ADL in 44 of 65 (67.7%) AChR-positive patients compared to 19 of 64 (29.7%) placebo-treated group (p < 0.0001) at 8 weeks. Slightly more than half of the efgartigimod-treated patients significantly improved 2 weeks after starting treatment. The results are remarkable especially considering that this novel therapeutic approach does not cause widespread immunosuppression but a seemingly safe and meaningful reduction of endogenous IgG and AChR antibody levels.
(b) Rozanolixizumab is a high affinity human anti-FcRn IgG4 monoclonal antibody with marked decreases in plasma IgG concentrations (75–90% from baseline) at 50 and 150 mg/kg doses with maximal effects achieved by day 10. In a randomized but not robustly designed trial involving 43 MG patients, no statistically significant change from baseline in QMG, the study’s primary endpoint, was observed but, in spite of the negative results, the authors concluded that concluded that efficacy measures continued to improve leading to an ongoing phase III study (NCT03971422).28
(c) Nipocalimab is a human deglycosylated IgG1 anti-FcRn monoclonal antibody that binds with picomolar affinity to FcRn at both endosomal pH 6.0 and extracellular pH 7.6 allowing occupancy of FcRn throughout the recycling pathway. Greater than 90% FcRn receptor occupancy was achieved with 3 mg/kg doses within 2 h of dosing.3 Following one dose of 30 or 60 mg/kg, maximum IgG reductions of 74–80% were observed; 50% reductions in IgG levels were maintained for 18–27 days for the 30–60 mg/kg dose, respectively. During multiple doses, IgG levels were reduced by 85% below baseline by day 14. The drug is currently undergoing a phase II trial in MG (NCT03772587).
FcRn inhibitors and IgG subclasses
Although the FcRn inhibitors lower all IgG subclasses, the IgG3 seems more affected compared to the other three subclasses.3 Any differential effect on reducing IgG subclasses can, however, be of great relevance to the treatment of IgG4-related neurological diseases that do not respond to IVIg, such as 7% of MG patients with IgG4 anti-MuSK antibodies26 and 10% of CIDP patients with nodal antibodies against neurofascin-155, contacin or Caspr-1.29 Following the administration of both single and multiple doses of efgartigimod, reductions in IgG1-3 were equally reduced but with slightly smaller reductions for IgG4,30 suggesting perhaps less efficient FcRn blockade for IgG4. In contrast, studies of rozanolixizumab and orilanolimab reported dose-dependent reductions in all four IgG subclasses, although most pronounced for IgG3.3,31 More information is therefore needed on their effectiveness in IgG4-mediated autoimmunity, and whether they provide sustained benefits as good as seen with rituximab which is currently the preferred therapy.32
Significance of FcRn in the central nervous system
FcRn is highly expressed in the brain microvascular endothelium and choroid plexus epithelium. Because the endothelial cells of the brain capillaries are joined by tight junctions preventing the passive diffusion of signaling molecules, antibodies, and immune cells across the blood–brain barrier (BBB), FcRn plays a key role in transporting IgG across the BBB preventing serum IgG from entering the Cerebrospinal Fluid (CSF) and central nervous system (CNS) tissue. FcRn in the brain may, however, have a reverse role; instead of transporting IgG into the CNS, it may remove IgG from the CNS transporting it back to the circulation by reverse transcytosis across the BBB, protecting the brain by limiting CNS inflammation in pathological conditions such as bacteremia.1,2 On the other hand, when the BBB is disrupted by inflammatory mediators or injuries, such as during COVID-19 infection in comatose and stuporous patients,33 FcRn facilitates the entry of IgG antibodies from the circulation into the CNS.1,2 Accordingly, FcRn inhibitors may play a major role in treating CNS neuroinflammatory autoimmune diseases characterized by impaired BBB integrity, as seen in SARS-CoV-2.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Marinos C. Dalakas  https://orcid.org/0000-0001-7070-1134
https://orcid.org/0000-0001-7070-1134
Contributor Information
Marinos C. Dalakas, Thomas Jefferson University, 900 Walnut Street, Philadelphia, PA 19107, USA; Neuroimmunology Unit, National and Kapodistrian University of Athens, Athens, Greece.
Peter J. Spaeth, Institute of Pharmacology, University of Bern, Bern, Switzerland
References
- 1. Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol 2007; 7: 715–725. [DOI] [PubMed] [Google Scholar]
- 2. Patel DD, Bussel JB. Neonatal Fc receptor in human immunity: function and role in therapeutic intervention. J Allergy Clin Immunol 2020; 146: 467–478. [DOI] [PubMed] [Google Scholar]
- 3. Peter H-H, Ochs HD, Cunningham-Rundles C, et al. Targeting FcRn for immunomodulation: benefits, risks, and practical considerations. J Allergy Clin Immunol 2020; 146: 479–491.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wani MA, Haynes LD, Kim J, et al. Familial hypercatabolic hypoproteinemia caused by deficiency of the neonatal Fc receptor, FcRn, due to a mutant beta2-microglobulin gene. Proc Natl Acad Sci U S A 2006; 103: 5084–5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brambell FW, Hemmings WA, Morris IG. A theoretical model of gamma-globulin catabolism. Nature 1964; 203: 1352–1354. [DOI] [PubMed] [Google Scholar]
- 6. Su S, Liu Q, Zhang X, et al. VNTR2/VNTR3 genotype in the FCGRT gene associates with the reduced effectiveness of intravenous immunoglobulin treatment in patients with myasthenia gravis. Ther Adv Neurol Disord 2021; In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Passot C, Azzopardi N, Renault S, et al. Influence of FCGRT gene polymorphisms on pharmacokinetics of therapeutic antibodies. MAbs 2013; 5: 614–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sachs UJ, Socher I, Braeunlich CG, et al. A variable number of tandem repeats polymorphism influences the transcriptional activity of the neonatal Fc receptor alpha-chain promoter. Immunology 2006; 119: 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gouilleux-Gruart V, Chapel H, Chevret S, et al. ; DEFI study group. Efficiency of immunoglobulin G replacement therapy in common variable immunodeficiency: correlations with clinical phenotype and polymorphism of the neonatal Fc receptor. Clin Exp Immunol 2013; 171: 186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Freiberger T, Grodecká L, Ravčuková B, et al. Association of FcRn expression with lung abnormalities and IVIG catabolism in patients with common variable immunodeficiency. Clin Immunol 2010; 136: 419–425. [DOI] [PubMed] [Google Scholar]
- 11. Yu Z, Lennon VA. Mechanism of intravenous immune globulin therapy in antibody-mediated autoimmune diseases. N Engl J Med 1999; 340: 227–228. [DOI] [PubMed] [Google Scholar]
- 12. Kuitwaard K, de Gelder J, Tio-Gillen AP, et al. Pharmacokinetics of intravenous immunoglobulin and outcome in Guillain-Barré syndrome. Ann Neurol 2009; 66: 597–603. [DOI] [PubMed] [Google Scholar]
- 13. Fokkink W-J, Haarman AE, Tio-Gillen AP, et al. Neonatal Fc receptor promoter gene polymorphism does not predict pharmacokinetics of IVIg or the clinical course of GBS. Ann Clin Transl Neurol 2016; 3: 547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vlam L, Cats EA, Willemse E, et al. Pharmacokinetics of intravenous immunoglobulin in multifocal motor neuropathy. J Neurol Neurosurg Psychiatry 2014; 85: 1145–1148. [DOI] [PubMed] [Google Scholar]
- 15. Hughes RAC, Swan AV, van Doorn PA. Intravenous immunoglobulin for Guillain–Barré syndrome. Cochrane Database Syst Rev 2012; 7: CD002063. [DOI] [PubMed] [Google Scholar]
- 16. Hughes RAC, Donofrio P, Bril V, et al. Intravenous immune globulin (10% caprylate-chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): a randomized placebo-controlled trial. Lancet Neurol 2008; 7: 136–144. [DOI] [PubMed] [Google Scholar]
- 17. Dalakas MC, Illa I, Dambrosia JM, et al. A controlled trial of high-dose intravenous immune globulin infusions as treatment for dermatomyositis. N Engl J Med 1993; 329: 1993–2000. [DOI] [PubMed] [Google Scholar]
- 18. Dalakas MC, Fujii M, Li M, et al. High-dose intravenous immune globulin for stiff-person syndrome. N Engl J Med 2001; 345: 1870–1876. [DOI] [PubMed] [Google Scholar]
- 19. Lunemann JD, Quast I, Dalakas MC. Efficacy of intravenous immunoglobulin in therapeutic advances in neurological disorders neurological diseases. Neurotherapeutics 2016; 13: 34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kapoor M, Reilly MM, Manji H, et al. Dramatic clinical response to ultra-high dose IVIg in otherwise treatment resistant inflammatory neuropathies. Int J Neurosci. Epub ahead of print 3 September 2020. DOI: 10.1080/00207454.2020.1815733 [DOI] [PubMed] [Google Scholar]
- 21. Billiet T, Dreesen E, Cleynen I, et al. A genetic variation in the neonatal Fc-receptor affects anti-TNF drug concentrations in inflammatory bowel disease. Am J Gastroenterol 2016; 111: 1438–1445. [DOI] [PubMed] [Google Scholar]
- 22. O’Shannessy DJ, Bendas K, Schweizer C, et al. Correlation of FCGRT genomic structure with serum immunoglobulin, albumin and farletuzumab pharmacokinetics in patients with first elapsed ovarian cancer. Genomics 2017; 109: 251–257. [DOI] [PubMed] [Google Scholar]
- 23. Howard JJ, Bril V, Burns TM, et al. Randomized phase 2 study of FcRn antagonist efgartigimod in generalized myasthenia gravis. Neurology 2019; 92: e2661–e2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Newland AC, Sánchez-González B, Rejtö L, et al. Phase 2 study of efgartigimod, a novel FcRn antagonist, in adult patients with primary immune thrombocytopenia. Am J Hematol 2020; 95: 178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Robak T, Kaźmierczak M, Jarque I, et al. Phase 2 multiple dose study of an FcRn inhibitor, rozanolixizumab, in patients with primary immune thrombocytopenia (ITP). Blood Adv 2020; 4: 4136–4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dalakas MC. Progress in the therapy of myasthenia gravis: getting closer to effective targeted immunotherapies. Curr Opin Neurol 2020; 33: 545–552. [DOI] [PubMed] [Google Scholar]
- 27. Howard JF, Bril V, Mantegazza R, et al. Efficacy, safety, and tolerability of efgartigimod in patients with generalized myasthenia gravis: analysis of the phase 3 ADAPT study AAN 2021 Abstract. Also Argenx announcement of ADAPTMG. https://www.argenx.com/news/argenx-announces-positivetopline-phase-3-adapt-trial-results2020 (accessed 26 May 2020). [Google Scholar]
- 28. Bril V, Benatar M, Benatar M, et al. Efficacy and safety of rozanolixizumab in moderate-to-severe generalised myasthenia gravis: a phase 2 RCT. Neurology. Epub ahead of print 20 November 2020. DOI: 10.1212/WNL.0000000000011108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stathopoulos P, Alexopoulos H, Dalakas MC. Autoimmune antigenic targets at the node of Ranvier in demyelinating disorders. Nat Rev Neurol 2015; 11: 143–156. [DOI] [PubMed] [Google Scholar]
- 30. Ulrichts P, Guglietta A, Dreier T, et al. Neonatal Fc receptor antagonist efgartigimod safely and sustainably reduces IgGs in humans. J Clin Invest 2018; 128: 4372–4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kiessling P, Lledo-Garcia R, Watanabe S, et al. The FcRn inhibitor rozanolixizumab reduces human serum IgG concentration: a randomized phase 1 study. Sci Transl Med 2017; 9: eaan1208. [DOI] [PubMed] [Google Scholar]
- 32. Bayry J, Kaveri SV. Kill ’Em All: efgatigimod immunotherapy for autoimmune diseases. Trends Pharmacol Sci 2108; 39: 919–922. [DOI] [PubMed] [Google Scholar]
- 33. Alexopoulos H, Magira E, Bitzogli K, et al. Anti-SARS-CoV-2 antibodies in the CSF, blood–brain barrier dysfunction, and neurological outcome: studies in 8 comatose patients. Neurol Neuroimmunol Neuroinflamm 2020; 7: e893. [DOI] [PMC free article] [PubMed] [Google Scholar]