Abstract
In the past few decades, obesity has reached pandemic proportions. Obesity is among the main risk factors for cardiovascular diseases, since chronic fat accumulation leads to dysfunction in vascular endothelium and to a precocious arterial stiffness. So far, not all the mechanisms linking adipose tissue and vascular reactivity have been explained. Recently, novel findings reported interesting pathological link between endothelial dysfunction with gut hormones and gut microbiota and energy homeostasis. These findings suggest an active role of gut secretome in regulating the mediators of vascular function, such as nitric oxide (NO) and endothelin-1 (ET-1) that need to be further investigated. Moreover, a central role of brain has been suggested as a main player in the regulation of the different factors and hormones beyond these complex mechanisms. The aim of the present review is to discuss the state of the art in this field, by focusing on the processes leading to endothelial dysfunction mediated by obesity and metabolic diseases, such as insulin resistance. The role of perivascular adipose tissue (PVAT), gut hormones, gut microbiota dysbiosis, and the CNS function in controlling satiety have been considered. Further understanding the crosstalk between these complex mechanisms will allow us to better design novel strategies for the prevention of obesity and its complications.
Keywords: obesity 1, endothelial dysfunction 2, metabolic syndrome 3, gut microbiota 4, gut hormones 5, Brain-Gut-Microbiome Axis 6
1. Introduction
Obesity is a major public health challenge of the 21st century, especially in urban settings, and in developing countries [1], where its incidence rises every year [2].
In 2016, the WHO estimated that more than 650 million adults were obese, and obese children and adolescent accounted for more than 340 million around the world. Since obesity related comorbidities, like diabetes and cardiovascular diseases, represent the leading causes of morbidity and mortality, there is an urgent global need to address the increase in obesity rate and its complications [3].
Even though several studies have been performed to understand all the factors underlying obesity and its related complication, so far, not all mechanisms are well understood. Novel, interesting theories highlight a pivotal role of gut flora and gut hormones in maintain the homeostasis of the central and peripheral organism axes controlling the metabolic function and accumulation of adipose tissue. Moreover, the same mechanisms have been also proposed to regulate the circulating factors involved in vascular homeostasis, further suggesting a multiple potential link with obesity and its complications.
Therefore, based on these considerations, investigating novel mechanisms involved in the regulation of fat accumulation, energy gain/expenditure, and appetite/satiety may be useful for the development of new treatments able to decelerate this emerging pandemic and its complications.
This review aims to describe some of the mechanisms involved in the pathophysiology of obesity related vascular dysfunction, especially the pathological link between gut hormones, food intake, microbiome environment, and autosomal nervous system in the development of obesity related complications.
2. Methods
The current literature investigating obesity, endothelial dysfunction, gut hormones, and gut microbiota are analyzed and contextualized in this review. Specifically, research has been conducted on Medline (Pubmed). This review article analyzes studies (both in vivo and in vitro studies) published up to 2020.
3. Obesity and Vascular Complications
Obesity is a multifactorial disease, classified as a chronic and non-communicable disease and is characterized by an imbalance between calories consumed and energy expenditure [4]. The main risk factors associated to obesity are insulin resistance, age, sex, smoking, sedentary, genetics, among others [4]. All these factors predispose to an increase of chronic inflammatory state per se, which is typical of obesity, and to an increase of oxidative stress organic damage.
Moreover, the chronic accumulation of fat leads to the deregulation of energetic storage homeostasis that in turn increase the circulating levels of saturated fatty acid and then glucose and lipids blood levels [5,6]. These events chronically lead to classical complications of obesity, which are dyslipidemia, cardiovascular disease, hypertension, atherosclerosis, and diabetes mellitus (T2DM) [7].
All these chronic diseases linked with obesity recognize a “fil rouge” in the double way of pathogenesis recognized as the lesion on the vascular bed that may become evident as the stiffness in arterial vessel or endothelial dysfunction. When these are evident as phenotypes of damage, they are already subclinical markers of cardiovascular disease, and major complications of obesity are already ahead. Understanding all the mechanisms and steps, even the less investigated, between interactions linking obesity, vascular risk factors, and associated vascular damage, is pivotal to prevent this pathological loop. It is important to also understand all the mechanisms leading to vascular damage that are endothelial independent.
4. Obesity, Metabolic Diseases, and Endothelial Dysfunction
The endothelium is an inner lining of the vessels composed by a singular layer of endothelial cells surrounded by smooth vascular muscle cells, interacting with each other to develop vascular response. In physiological conditions, the endothelium develops a tight control balance between vasodilation, through nitric oxide (NO) and prostacyclin production, and vasoconstriction by the regulation of endothelin 1 (ET-1) and thromboxane A2 activity [8,9,10].
The endothelium has either homeostatic and metabolic adaptation ability by regulating vascular response to several physiological stimuli like acetylcholine, bradykinin, catecholamines, and insulin or to injury factors, such as shear stress, temperature change, ischemia, reactive oxygen species (ROS), and Oxidized low-density lipoprotein (OxLDL), among others [11]. The vasoactive substances produced by the endothelium, like vascular endothelial growth factor (VEGF), play also a role in regulation of vascular growth and development [12].
As mentioned below, endothelial dysfunction is manly defined as an imbalance between NO bioavailability and increase in ET-1 vascular activity [13], and it may be triggered by several factors, such as aging, inflammation, oxidative stress, hypertension, and hyperglycemia, all typical of obesity and metabolic alteration [14]. Moreover, since endothelium is an active organ, besides NO and ET-1, in response to alteration in metabolic homeostasis (e.g., high circulating insulin levels), other factors are released to the final development of its dysfunction [15]. These factors are extracellular matrix proteins, hormones, growth factors, and several enzymes, such as prostacyclines [15].
Pathological conditions particularly cause several alterations of endothelial signaling leading to endothelial nitric oxide synthase (eNOS) uncoupling, soluble guanylyl cyclase desensitization (sGS), inactivation of prostacyclin synthase (CYP8A1), oxidative activation of the endothelin-1 system, and inactivation of NO by superoxide [16,17,18]. The final results of all these signaling pathway dysfunctions are an impairment in the endothelium-dependent vasodilation and increased proinflammatory and procoagulant activity [13]. This process is considered among the most important initial step of atherosclerosis towards cardiovascular diseases, and it is a fingerprint of obese-insulin resistant-diabetic subjects.
However, it is important to highlight that vascular dysfunction related to obesity and metabolic diseases, involves not only vasoreactivity in an endothelium-dependent manner (e.g., ach-NO mediated) but also other factors, such as co-impairment on vascular smooth muscle responsiveness to NO, as demonstrated by a reduced blood flow response to sodium nitroprusside, NO donor, in obese patients [19,20]. Interestingly, vascular improvement has been demonstrated to be independent of change in weight but correlates strongly with glucose levels. This suggests that obesity related endothelial dysfunction is manly associated to glucometabolic alterations rather than adipose tissue mass accumulation [21].
On the other hand, vascular homeostasis in obese subjects can be also altered by an increased activity of ET-1 system, as described following. ET-1 is considered the most powerful vasoconstrictor mediator, produced by endothelium; it is involved in pathophysiology mechanisms of endothelial vasomotor dysfunction and atherosclerotic plaque formation and progression [22]. Leg vascular responses to intra-arterial infusions of an ETA receptor antagonist (BQ123) restored impaired endothelial depend vasodilation in obese subjects, suggesting the important role of ET-1 in obesity related vascular dysfunction [23].
Furthermore, an enhanced ET-1 vasoconstriction tone has been shown in patients with metabolic syndrome. A significant higher vasodilator response to ETA antagonist accompanied with an impaired vasoconstriction response to the LMNA (NG-monomethyl-L-arginine), a NO synthase inhibitor, consistent with impaired NO bioavailability in obese vasculature, were present in these subjects [24]. Nevertheless, it is important to remark that ET-1 has also vasodilator effect. Its double effect is mediated by its receptors at vascular levels. In fact, through the activation of ETB Receptor, ET-1 induces the release of NO and other vascular release factors in the endothelium while by activating ETA Receptor it induces vasoconstriction [25].
We previously reported as obesity was associated with vascular damage independently by metabolic abnormalities underlying metabolic syndrome; indeed, patients with obesity but without abnormalities that define metabolic syndrome, have abnormal vascular reactivity, although their endothelial dysfunction is less pronounced than in patients with metabolic syndrome [19].
Metabolic syndrome is defined as a cluster of conditions that occur together, increasing the risk of cardiovascular diseases and T2DM [26]. These conditions include increased blood pressure, abnormal fasting blood glucose, excess body fat around the waist, and atherogenic dyslipidemia [27]. Several pathophysiological mechanisms may account for the development of this metabolic phenotype but insulin resistance and hyperinsulinemia seem to be the main players in this process [28]. Arterial endothelial dysfunction is the common denominator between insulin resistance, hypertension, and vascular damage [29]. ET-1 dependent vasoconstrictor tone has been shown to increase in patients affected by hypertension [30], hypercholesterolemia [31], T2DM [32,33], and metabolic syndrome, coexisting with an impaired NO bioactivity in the vasculature of these patients [24].
In the recent past, the insulin activity on endothelial cells has been widely studied [34,35]. At this moment, it is well known the role of insulin on activation of both vasodilator and vasoconstrictor pathways through the endothelial insulin receptor by different intracellular mediators, leading to vasodilation prevalence under insulin stimuli [36,37,38]. Physiologically, insulin induced-NO release in the skeletal muscle circulation could lead to an expansion of the capillary surface area in periphery in order to improve the delivery of nutrients, insulin, glucose and other metabolites to active tissues, thereby increasing insulin sensitivity [39]. Furthermore, insulin, besides anabolic function, to induce vasodilatation and reduce oxidative stress, promotes angiogenesis and proliferation of vascular smooth muscle cells [40]. An important role in the regulation of L-arginine transport has been also suggested as the pathway involved in insulin-mediated vasodilatation [41].
Interestingly, the vascular effect of insulin and impairment in its signaling have been studied in several vascular bed, such as carotids, renal vessels, brain cerebral arteries, and coronaries [37]. Specifically, coronary arteries show diminished effects of the vasoconstrictor insulin-dependent MAPK pathway in order to protect this vital vascular bed, as reported in a study by [42]
During insulin resistance, the PI3K/AKT pathway downstream ISR is impaired while the MAPK signaling is functional [43]. As a consequence of this, the compensatory hyperinsulinemia related to the status of insulin resistance induces an overstimulation of the MAPK pathway, resulting in the hyperactivity of the ET-1 system, while the NO bioavailability is impaired and cannot balance the increase in vascular tone [44]. Conditions associated with impaired endothelial vasodilator response to insulin may, therefore, decrease the delivery of nutrients to peripheral tissue, enhancing in turn insulin resistance. There are many evidences on the role of ET-1 in decrease insulin sensitivity in muscle cells [45], and the use of ETA receptor blockers in increasing the glucose utilization by peripheral insulin target cells [46,47].
Other mechanisms that could be involved in vasodilator dysfunction linked with insulin resistance are the reduced endothelium derived hyperpolarization (K channel dysfunction) and altered Rho kinase inhibition in the smooth muscle cells [48,49].
Taken together, these findings indicate that vascular dysfunction in obesity results in an imbalance between NO vasodilator pathway and ET-1 vasoconstrictor activity towards the latter, where ET-1 contribution to the endothelial function is powerful and is associated with impaired in NO- bioavailability.
5. Perivascular Adipose Tissue (PVAT) and Fat Accumulation Mechanisms Linked with Endothelial Dysfunction and Insulin Resistance
The last but not the least in order of relevance is the impact of perivascular adipose tissue (PVAT) on the protection and development of vascular insulin resistance [50]. The adipose tissue is an endocrine organ with immune functions, beyond its well-known role as an energy storage depot.
In general, adipose tissue synthetizes several adipokines that exert different functions on glucose and lipid metabolism, on body weight control, and in organs or tissues insulin sensitivity, including regulation of vascular tone by local autocrine/paracrine actions and systemic endocrine effect [51]. Among those: adiponectin, whose plasma concentration is reduced in obesity and T2DM [52], and inversely correlates with BMI and visceral fat [53,54], enhances insulin- mediated glucose uptake in skeletal muscle, increases insulin sensitivity in the liver, and decreases glucose production by gluconeogenesis [55]. It improves endothelium vasoactivity stimulating NO production via the PI3K pathway [56], and the endothelium redox state under oxidative stress stimuli [57]. Leptin is involved in the regulation of energy intake and appetite and serves as a mediator of the adaptation to fasting [58]. Plasma leptin levels correlate with fat stores and respond to changes in energy balance. The increased circulating leptin in obese patients is related to a kind of systemic leptin resistance [59]. On endothelial cells and smooth muscle cells, leptin acts to stimulate the NO system [60], but long exposure to leptin induces a reduction in NO bioavailability likely increasing oxidative cellular stress [61]. This shows that hyperleptinaemia in obese patients is correlate with increased adverse cardiovascular outcomes, and by itself it is considered an independent risk factor for coronary artery disease and a predictor of acute myocardial infarction [62]. A summary of adipokines functions is listed in Table 1.
Table 1.
Adipokines, release and function.
| Adipokines | Site of Release | Stimulated by | Function | Reference |
|---|---|---|---|---|
| Adiponectin | PVAT | Plasma concentration is inversely correlated with BMI and visceral fat | ↑ insulin-mediated glucose uptake in skeletal muscle ↑ liver insulin sensitivity ↑ endothelium vasoactivity ↑ glucose production by gluconeogenesis |
[52,53,54,55] |
| Leptin | PVAT | Plasma levels ↑ with fat stores | Regulator of energy intake Adaptation to fasting ↑ oxidative cellular stress by long exposure ↓ NO endothelial availability by long exposure |
[58,59,60,61] |
Abbreviation: PVAT, perivascular adipose tissue.
The expansion of visceral adipose tissue occurring during obesity correlates with an abnormal synthesis of vasoactive adipokines [63], increased NEFA, and proinflammatory cytokines release into the bloodstream that lead to a systemic low grade inflammation and impair vascular homeostasis towards an increased ET-1 system activity [64,65,66].
It is well known that obese adipose tissue is characterized by adipocytes hyperplasia and hypertrophy and by macrophage infiltration due to preadipocytes and endothelial cells secretion of monocyte chemoattractant protein-1 (MCP-1) in response to cytokines (TNF alfa, and IL6) [67,68,69].
Thus, fat tissue becomes an important source of inflammatory cytokines, which are involved in the development of insulin resistance (by inhibition of insulin dependent glucose uptake) [70], and endothelial dysfunction (impairing vasodilation endothelium NO dependent [71], and endothelium independent, likely the one associated to increased oxidative stress [72].
Recent findings recognize in PVAT an important regulator of vascular tone and functions [73]. Thus, the PVAT results to have vasodilator, anti-contractile, and anti-proliferative functions under physiologic conditions mediated by adipokines, like adiponectin or molecules with vasodilator activity like angiotensin-(1–7) acting in an autocrine or paracrine manner [74,75]. Studies on PVAT human small arteries demonstrated that adipocytes secrete factors (e.g., adiponectin) modulate vasodilation by influencing NO bioavailability. This function of local vascular tone modulator is lost in obesity related metabolic syndrome with evidence of fat hypertrophy, and increased production of inflammatory cytokines (TNF alfa, and IL6) resulting in increased oxidative stress and hypoxia (reversible by use of cytokines blockers, and free radical scavengers) [76,77]. PVAT adipocytes exhibit a different phenotype compared to visceral and subcutaneous depot, with a different reaction to fat intake, since modifying their genes expression and cytokines production towards a proinflammatory state leads to perivascular dysfunction [78].
On the other hand, insulin resistance induced by local inflammation is the link between proinflammatory cytokines and vascular dysfunction mediated by perivascular adipocytes and, which results in impaired NO synthesis and vasoconstrictor tone [79] TNF alfa produced by adipose perivascular tissue is the major mediator involved in this dysfunction [73].
The mechanism by which TNF alfa induces insulin resistance in endothelial cells has been well characterized. It acts through IRS-1 and interferes downstream with the PI3K Akt/eNOS pathway via a p38 MAPK-dependent mechanism. This leads to impairment in the vascular production of NO and enhancement in ERK1/2 and AMPK phosphorylation independently by the p38 MAPK pathway [80,81] (Figure 1). In clinical studies, vascular insulin resistance induced by obesity was ameliorated following the use of TNF alfa blockers, likely in relation to a decrease in oxidative stress [72]. In obese patients, the impairment of vascular insulin signaling is also associated to the excess of FFA in adipose tissue. NEFA interfere with insulin signaling through the same pathways of TNF alfa, downstream the IRS1–2, reducing PI3K activation, and at the end resulting in impaired NO production [82,83]. Taken together, all these findings suggest that adipose tissues around the arteries play an important role in the pathogenesis of vascular dysfunction in the obese-metabolic syndrome condition. In obesity, PVAT loses its dilator and anti-inflammatory actions, leading to a proinflammatory state which promotes vasculature insulin resistance, impairs insulin vasodilation NO dependent, and leads to vascular dysfunction (Figure 1).
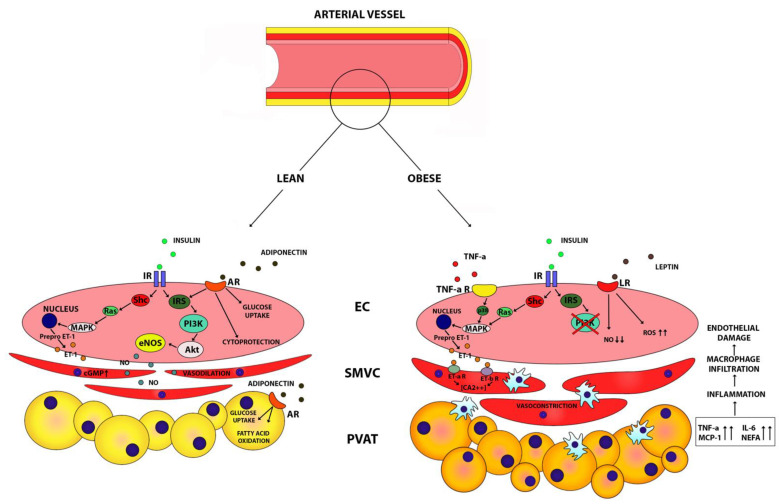
Figure 1.
Interplay mechanisms of action between vascular endothelial cells and perivascular adipose tissue in lean and obese subjects. In lean subjects, insulin and adiponectin stimulate endothelial cells to secrete nitric oxide and endothelin 1. This mechanism induces homeostasis between vasodilation and vasoconstriction, promotes glucose uptake and cytoprotection, and the oxidation of fatty acids in adipose tissue. Instead, in obese subjects, insulin resistance, the increased release of leptin and TNF-alpha stimulate vasoconstriction and reduction of NO bioavailability. Insulin resistance, Leptin, and TNF-alpha induce ROS production, the release of pro-inflammatory cytokines with inflammation, macrophage infiltration of PVAT and endothelial damage. Abbreviations: IR, insulin receptor; IRS, insulin receptor substrate; PI3K, phosphoinositide 3-kinase; Akt, protein kinase B; MAPK, mitogen-activated protein kinase; cGMP, cyclic guanosine monophosphate; AR, adiponectin receptor; LR, leptin receptor; TNF-a R, tumor necrosis factor-alpha receptor; NO, nitric oxide; eNOS, endothelial nitric oxide synthase; ET-1, endothelin-1; Prepro ET-1, Prepro endothelin-1; ET-a R, endothelin 1-a receptor; ET-b R, endothelin 1-b receptor; ROS, radical oxygen species; EC, endothelial cell; SMVC, smooth muscle vascular cell; PVAT, perivascular adipose tissue; TNF-a, tumor necrosis factor-alpha; IL-6, interleukin-6; MCP-1, monocyte chemoattractant protein-1; NEFA, non-esterified fatty acids.
Similarly, it has been hypothesized that deposits of fat tissue on the origin of arterioles supplying the skeletal muscle have vasoactive and “vasocrine” role, interfering with post prandial insulin signaling in over feed setting. Insulin induces vasodilation and increases nutritive capillary recruitment in order to allow glucose upload after a meal, through a vasodilator NO dependent action. In overfeeding, as well as in all insulin resistance settings, the PVAT arterioles modulates the insulin signaling through adipocytokines production and secretion in the lumen in paracrine fashion (TNF alfa), inducing vasoconstriction and blood flow redistribution toward adipose tissue, in order to rescue the muscle from the excess of the substrate delivery [73].
In conditions of obesity, DMT2, and insulin resistance, vasoconstriction seems to also involve the adipose tissue, resulting in blunted post prandial adipose tissue perfusion, low blood flow, hypoxia, and increased inflammation. In healthy conditions, the mechanism by which insulin induces adipose tissue vasodilation seems to be through an indirect systemic effect due to increased sympathetic hyperactivity either through the central nervous system [84] (via paraventricular nucleus of the hypothalamus) [85] than through downstream beta adrenergic stimulation [86,87,88]. A down regulation of these adrenergic receptors, due to a sympathetic continuous overstimulation as effect of hyperinsulinemia, may account for the lack of post prandial adipose tissue perfusion in case of insulin resistance [89].
These effects result in hypoxia, reduced glucose uptake from target organs, an increase in insulin resistance, and increase ectopic lipid accumulation (liver, muscles) [90].
6. Gut Hormones
In the last decade, mounting evidence demonstrated the central role of gut hormones in the regulation of glucose homeostasis, insulin secretion, appetite, body weight, regulation of immune system, and several other physiological functions [91]. Gut hormones are molecules produced by enteroendocrine cells (EECs), specialized secretory cells scattered throughout the mucosal epithelium of the gastrointestinal tract, in response to several stimuli from luminal ingested food [92,93,94,95]. Gut hormones achieve their effects acting on different targets within and outside the gastrointestinal tract. Currently, about 20 different gut hormones have been discovered, some of which exert multiple physiological functions and some with overlapping physiological roles. They recently have attracted more attention because of their role in controlling metabolism in healthy and disease, as implicated in the regulation of insulin secretion and appetite control. Their role has a particular translational interest for future therapeutic approaches on metabolic disorders [96].
6.1. Gut Hormones in Metabolic Disease and Obesity
As we discussed previously in this article, obesity and insulin resistance associated to central fat distribution are closely related to an increase in cardiovascular risk and diabetes [26,28].
Achieving glycemic control in diabetes patients is associated with reduction of cardiovascular outcomes [97] as well as weight loss by itself can ameliorate diabetic hyperglycemia [98]. However, the experience obtained through bariatric surgery indicates that beside insulin action, other hormones secreted as consequence of food intake may influence glucose metabolism [99]. Indeed, insulin release after an oral glucose load results higher than after isoglycemic intravenous glucose administration, suggesting a role of gastroenteric tract in stimulating insulin secretion after food ingestion [100].
A number of gut hormones called incretin system, which include Glucagone like peptide 1 (GLP1), Glucose-dependent insulinotropic hormone (GIP), and Oxyntomodulin, are central to this function. They are secreted rapidly a few minutes after a meal by EECs, historically identified as L cells in the distal small intestine (GLP-1, and Oxymodulin), and K cells in the proximal gut (GIP) [101,102].
The half-life of incretins is very short, since they are subjected to a rapid degradation by the dipeptidyl peptidase IV (DPP4) enzyme [103,104].
The incretin hormones stimulate glucose dependent insulin secretion and sensitizing pancreatic beta cells to glucose, which result in a modulation of post prandial glucose excursion [105]. A summary of incretins functions is listed in Table 2.
Table 2.
Gut hormones, release and function.
| Gut Hormones | Site of release | Stimulated by | Function | Reference |
|---|---|---|---|---|
| GLP-1 | Intestine L cells | Secreted after meal | ↑ insulin secretion ↑ pancreatic beta cells survival and proliferation ↑ pancreatic alfa cells inhibition of glucagon secretion ↓ lipid secretion ↓ glucose production by liver ↓ gastric motility ↑ sense of satiety ↓ food ingestion ↓ oxidative stress/platelet aggregation ↑ insulin stimulated vasodilator reactivity |
[106,107] |
| GIP | Intestine K cells | Secreted after meal | ↑ insulin secretion ↑ beta cells proliferation ↓ beta cell apoptosis |
[107] |
| OMX | Intestine L cells | Secreted after meal | ↑ insulin secretion ↓ food ingestion |
[108,109,110] |
| Ghrelin | Gastric P/D1 cells | Secreted during fasting | ↑ food ingestion ↑ GLP-1 secretory ↑ NO endothelial availability |
[111,112,113] |
| Obestatin | Gastric P/D1 cells | Secreted during fasting | ↑ food ingestion ↑ NO endothelial availability ↑ pancreatic beta cells survival and proliferation ↓ lipolysis in adipose tissue |
[114,115,116,117,118] |
| PYY | Intestine L cells | Secreted after meal | ↓ gastric motility/↓ intestinal motility ↑ sense of satiety ↑ pancreatic secretion |
[119,120,121,122] |
| INSL-5 | Intestine L cells | Secreted during fasting | ↑ food ingestion | [123,124] |
| CKK | Intestine I cells | Secreted after meal | ↑ gallbladder contraction inhibition of gastric acid secretion ↑ insulin secretion ↑ pancreatic beta cells survival and proliferation ↑ sense of satiety |
[95,125,126,127,128,129] |
Abbreviations: GLP-1, glucagon like peptide-1; GIP, glucose-dependent insulinotropic peptide; OMX, oxyntomodulin; PYY, peptide tyrosine tyrosine; INSL-5, insulin-like peptide 5; CKK, cholecystokinin.
6.2. GLP-1, GIP, and Oxyntomodulin
GLP-1 has generated great interest over time because of its multiple metabolic effects mediated by GLP-1 receptors that are expressed in many different tissues [130]. GLP-1 metabolic actions include the influence on pancreatic beta cells survival and proliferation, pancreatic alfa cells inhibition of glucagon secretion, the reduction of lipid secretion, and glucose production by the liver. On the other hand, GLP-1 inhibits gastric motility resulting in the delay of nutrient absorption; it increases the sense of satiety, reduces food ingestion, and promotes weight loss (Figure 2). GLP-1 exerts other systemic roles directly or indirectly modulating inflammatory response in multiple sites, including heart and blood vessels, reducing oxidative stress and platelet aggregation, improving plaque stability, left ventricular function, and vascular vasodilation [106].
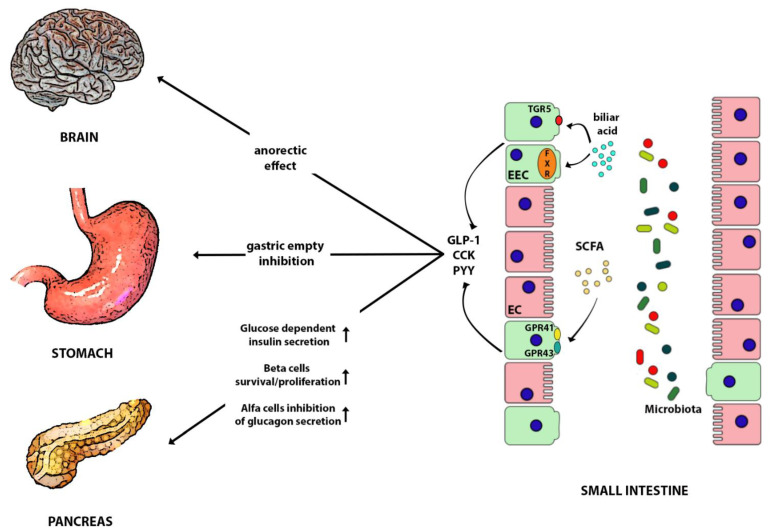
Figure 2.
Effect of Gut microbiota in regulating the incretin secretion and their anorectic effects in peripheral organs. Gut microbiotas release chemical mediators, including SCFA which, together with bile acids, stimulate enterochromaffin cells to secrete GLP-1, CCK, and PYY. GLP-1, CCK and PYY induce an anorectic effect, gastric empty inhibition, glucose dependent insulin secretion, beta cells survival/proliferation, and alfa cells inhibition of glucagon secretion. Abbreviations: SCFA, short chain fatty acid; GLP-1, glucagon-like peptide 1; CCK, cholecystokinin; PYY, peptide YY; EEC, enterochromaffin cell; EC, enteric cell; GPR 41, G protein-coupled receptor 41; GPR 43, G protein-coupled receptor 43; FXR, farnesoid x receptor; TGR5, G-protein bile acid receptor.
GLP-1 restores insulin stimulated vasodilator reactivity reducing vascular oxidative stress. Clinical studies have shown an improvement of insulin mediated endothelium dependent and independent vasodilation in patients with metabolic syndrome [131].
Recent observations pointed the attention on incretin-based therapy for diabetes in addiction with the traditional care regimen. The use of degradation-resistant GLP-1 analogues (liraglutide in the LEADER trial, and semaglutide in the SUSTAIN-6 trial) in patients with T2DM showed a reduction of cardiovascular mortality, non-fatal acute myocardial infarction (AMI), and stroke compared with the placebo group. All end points achieved by incretins were independent by the little improvement of glycemic control obtained [132,133,134].
The most translation relevance of GLP-1 is due to its insulinotropic effect which results preserved in patients with T2DM, unlike GIP, with significant consequence on developing GLP-1 based therapy to improve insulin resistance.
Another clinical use of this therapy is to enhance weight loss in obese subjects, because of the anorexigenic effect on satiety mediated by GLP-1 receptors located at different sites in the brain [135,136] It is important to make note of the various neural substrates through which GLP-1 and its analogs act in reducing food intake, including the hypothalamic area of the brain (arcuate nucleus of the hypothalamus, periventricular hypothalamus, lateral hypothalamic areas) [137]. These evidence allow us to consider this hormone as a pivotal target for controlling both central and peripheral mechanisms underlying obesity.
GIP is secreted by the proximal small intestine K cells in response to nutrients and acts by binding GIP receptors expressed on pancreatic beta cells, adipocytes, bone, and CNS [138,139,140]. GIP, together with GLP-1, under glycemic stimuli, increase insulin secretion covering 70% of post prandial necessity, promote beta cells proliferation, and suppress beta cell apoptosis [107] (Figure 2). However, since the GIP insulinotropic effect is impaired in T2DM patients, and is reduced in first degree relatives [141,142], while an enhanced glucanotropic effect is seen in the same conditions, less attention has been given to this molecule as a therapeutic target [143]. GIP shows also anabolic proprieties as it promotes adipocytes fat storage and inhibits lipolysis [144], although its obesogenic effect cannot be confirmed in all setting. For instance, mice overexpressing GIP are leaner than wild type [145] because more factors are involved in the control of energy balance, and this process seems to be mediated by GIP modulation of adipokines and other gut hormones secretion.
Oxyntomodulin (OMX) is a peptide that contains glucagon’s amino acid sequence (29 aa) plus an octapeptide tail [108], co-secreted from intestine L cells with GLP-1 in response to nutrient intake. Like GLP-1, OXM is an incretin, which directly induces insulin release from pancreatic islet cells. OXM exerts anorectic effect and increase energy expenditure by different mechanisms that have been incompletely characterized. To date, no specific receptors for OXM have been identified, but it is known that it can activate glucagon (GSIS) and GLP-1 receptors (GLP1R) even whether with less potency than native agonists [109,110]. Most likely OXM plays its anorexigenic effect through GLP-1 R, as confirmed by knockout (K/O) GLP-1R mice studies and pharmacological blockers [146,147,148,149]. These evidences led to development of ‘dual or tri agonist’ analogues (GLP-1R/GCGR/GIP) to obtain anti-obesity effects [150].
6.3. Ghrelin and Obestatin
Ghrelin is secreted by the P/D1 closed type cells in the gastric fundus as a prohormone, which requires cleavage and post translational acylation, by the enzyme ghrelin O acyltrasferase (GOAT), to be functional on its own receptors, the growth hormone secretagogue receptor (GHSR 1a) [114]. GHSR 1a is expressed mainly in the central nervous system (CNS) and in the small intestine and pancreatic islets [151]. Ghrelin has multiple effects and exerts its orexigenic action in the hypothalamus by stimulating the Agouti related peptide/neuropeptide Y (AgRP/NPY) neurons and inhibiting the anorectic Proopiomelanocortin/Cocaine and amphetamine-regulated transcript (POMC/CART) neurons in the arcuate nucleus (ARC) [111,112]. Moreover, as it has been demonstrated in pre-clinical studies, the stimulus of AgRP/NPY neurons by different mechanisms, such as circulating levels of prostaglandins, may induce orexigenic and anorexigenic effects with a significant role in central energy metabolism [152,153]
Ghrelin plasma levels are elevated during fasting and in conditions related to malnutrition like cachexia and anorexia nervosa [154]. By contrast plasmatic Ghrelin is reduced in obesity and in insulin resistance, T2DM, and hypertension [155]. Ghrelin secretion is controlled by cholinergic stimuli [156] and even if in vitro, is stimulated by noradrenaline and can be suppressed by a b1 blocker, atenolol [157].
Ghrelin is considered a key regulator of glycemic homeostasis by inducing the enhancement of GLP-1 secretory response to nutrients. Since plasmatic Ghrelin peaks during fasting, it acts by preparing the target cells to release GLP-1 following food ingestion. Evidence in animal models showed an enhanced glucose-stimulated GLP-1 release after Ghrelin treatment, and an improvement of glucose tolerance, which are blunted in presence of GLP-1 receptor blockers or in case of GLP-1R KO mice [158].
In addition, Ghrelin is involved in the modulation of lipid metabolism and body composition [159], and it has been linked to anti-inflammatory [160,161] and antiapoptotic effects [162].
Ghrelin exerts relevant effects on cardiovascular systems as GHS receptors are found in the heart, where they induce positive inotropic effects, and in the vasculature, where it leads to an improvement of blood pressure by lowering peripheral resistance [163]. Clinical studies showed that intra-arterial Ghrelin administration in obese patients with metabolic syndrome restores endothelial NO dependent vasodilation [113].
Ghrelin acts not only by enhancing NO bioavailability in endothelial cells but also by reducing the ET-1 imbalance vasoconstrictor action in obese patients. Exogenous Ghrelin blunts the vasodilator action of ET-1 blocker BQ123 in obese but not in lean subjects and increases vasoconstriction reactivity to the LMNA (NG-monomethyl-L-arginine), and to the NO synthase inhibitor. These evidence confirm that Ghrelin can normalize the balance between vasoconstrictor (ET-1) and vasodilatation (NO) mediators by increasing NO production [24]. Same results were obtained by using Ghrelin in hypertensive patients modulating oxidative stress [164].
Obestatin results from an alternative splicing of the Ghrelin gene, as a 23 amino-acid peptide derived from the common precursor prepro-ghrelin [115].
The precise identity of the cognate receptor for obestatin has still not been determined. Some findings suggest that obestatin may signal thought the GLP-1 receptor, since the same effect on some target cells, but current knowledges are insufficient to draw any conclusion. Obestatin’s half-life is very short because it is degraded by enzymatic proteases. Obestatin has anorectic effects and can modulate metabolic homeostasis, improving insulin sensitivity and glycemic control by glucose uptake, promoting pancreatic beta cell proliferation and survival, inhibiting lipolysis in adipose tissue. Obestatin also appears to be involved in blood pressure regulation, promotes cardioprotective actions [116], and exerts beneficial effects on endothelial function by increasing NO production [117].
The intra-arterial infusion of increasing doses of obestatin results in augmented vasodilation in obese and lean subjects; this effect is blunted following infusion of L-NMMA, an NO synthase inhibitor, confirming that obestatin acts by enhancing NO-dependent vasodilation in the human circulation. This effect is preserved in obese patients, where it is accompanied by reduced ET-1-mediated vasoconstriction as shown by the use of BQ 123, ET-1 blocker whose effect in obesity does not enhanced by obestatin [118].
6.4. PYY and Insulin like Peptide 5
Peptide tyrosine tyrosine (PYY) is secreted after meal stimulation by L enteroendocrine cells together with GLP-1 [119], along the proximal and distal intestinal tract. Whereas GLP-1 is more present in the proximal tract, PYY is produced in a large amount in the distal ileum toward the colon [165], and its release is triggered under neurohormonal control [166], and responses to metabolites of the gut microbiota [167] [168]. The same gene encodes for two proteins, PYY 1–36 and PYY 3–36, resulting from DPP4 cleavage of the entire form [169]. Both have a considerable role in the “ileal brake” function (together with cholecystokinin, GLP1), a local feedback signal which inhibits gastric emptying, pancreatic secretion, and intestinal mobility induced by food intake [120,121] in order to slow down the transit and increase the nutrients absorption in the upper gut. PYY has anorectic effect [122], acting through the Neuropeptide Y receptors (NPYs) located on enterocytes, myenteric, and submucosal neurons, afferent fiber, and in CNS [166] (Figure 2). PYY in high doses induces nausea and anorexia [170], which is why this peptide has not been considered a good therapeutic target for obesity. In fasting humans PYY is inversely related to BMI. Because of the distal location of enteroendocrine cells secreting PYY, a high amount of this hormone is produced in case of bariatric gastric surgery (tenfold higher), when increased flow of nutrients is delivered to the distal gut and directly stimulates L cells to release the hormone [171,172]
In physiological conditions, PYY is secreted likely by paracrine and neural mechanisms. PYY does not have clear metabolic effect to date, and PYY infusion in human does not modify glycemic control, insulin, or glucagon levels [173,174].
Insulin like Peptide 5 (INSL-5) is co-secreted with GLP-1 and PYY by the colonic L enteroendocrine cells where they are co-stored into separate vesicular, [123], and represents the major enterohormone produced from large intestine. In contrast with the GLP-1 and PPY function, INSL-5 is considered an orexigenic hormone; indeed, intraperitoneal administration increase food intake in mice, acting through the Relaxin/Insulin-like family peptide receptor 4 (RXFP4) [124], behavior lost in K/O RXFP4 mice [175]. The INSL-5 secretion by Colonic enteroendocrine L cells most likely is modulated by several factors. Fasting or restricted caloric diet increases INSL-5 colonic and plasma levels and are normalized after food intake. On the other hand, germ free mice have shown an increased INSL-5 expression, and a high concentration in the lumen of short chain fatty acid, bile acids, produced by intestinal bacteria reduces INLS-5 secretion. These last evidence suggest the role of some microbial metabolites in signaling for gut hormones secretion and INSL5 as a link between metabolism, microbiota and host [176]. Other systemic factors such as angiotensin II (Ang II) and arginine vasopressin (AVP) could modulate INSL-5 secretion [123].
The effect of INSL-5 on metabolic homeostasis is less clear, and the role of INSL5 in glucose control and in insulin sensitivity is still debated.
6.5. Cholecystokinin
Cholecystokinin (CKK) is one of the first gut peptides discovered with the ability to regulate the digestion through stimulation of gallbladder contraction. Today many other functions have been assigned to CCK including antinflammatory action [177], the modulation of cardiovascular functions [178], the inhibition of gastric acid secretion [125], insulin secretion [126], and the sense of satiety [127].
CCK is secreted by the enteroendocrine cell historically known as I-cells, located closely to the K-cells GIP releasing in the duodenum and proximal jejunum, and close to L-cells GLP-1 releasing in the distal part of the enteric [179]. As previously reported, it is not surprising that K-cells secret CCK [95], and I-cells can release GIP or other gut hormones [180]. CCK is secreted after meal in response of fat and protein nutrients [128], and plasma level are enhanced of 10–20 folds. Most likely, CCK secretion from the EECs is triggered by contact of ingested food with the secretory cells in the duodenum. The immediate effect is on gastric empty control but also on signal by the vagal afferents to the central brain in order to inhibit appetite.
The encoded form of the CCK gene is a 115 amino acid polypeptides, which eventually will be differentially cleaved in many truncated circulation forms known as CCK-8, CCK-33, CCK-58 peptides [181]. CCK acts through CCK1 (located in peripheral tissue), and CCK2 receptors (located centrally). Most of its actions occur by the CCK1 receptors, which are also found in visceral afferent fiber vagal. When CCK1 receptors are stimulated they may signal satiety to hypothalamus [129].
Beside the regulation of energy control, CCK may also exert incretin effects, thereby enhancing insulin secretion by beta pancreatic cells [126], promote beta cells survive, and suppress apoptosis [182,183]. CCK represents an interesting target for the development of obesity-diabetes therapy, as shown by in vitro studies and animal models based on the administration of CCK peptide analogues [184].
7. Obesity and Gut Microbiota
The gastrointestinal tract is colonized by the gut microbiota which consist of more than 100 trillion microbial cells, spread based on a concentration gradient along the gastrointestinal tract, with the highest density in the colon [185].
The gut microbiota collaborates with the host to accomplish many physiological activities including the improvement of enteric digestion and absorption of nutrients, the defense against colonization by pathogen microbes, the maintenance of the gut barrier, the modulation of inflammation and of the entero-hormones secretion by the ECCs, the promotion of immune system maturation, and the influence on the formation of capillary networks in the small intestinal mucosa [186,187,188,189].
In physiologic conditions, host and microbiome cohabit in symbiosis, so that the microbiome contributes to health maintenance and the host provides a favorable environment for its survival. Mounting evidence shows that quantitative and qualitative alterations of gut microbiota composition, called “dysbiosis”, may represent a new risk factor for developing future diseases, including obesity.
It has been shown that the intestinal microbiome can be responsible for the efficiency of expenditure or harvest energy from food intake and has a role in the development of obesity by altering host energy storage and harvest [190,191,192]. The microbiome also seems to affect the susceptibility to insulin resistance and promotes the development of non-alcoholic fatty liver disease [193].
Despite intraindividual variability, 5 phyla are prevailing in the intestinal community: Actinobacteria, Bacteroidetes, Firmicutes, Proteobacteria, and Verrucomicrobia. Environmental factors govern the distribution along the gastrointestinal tract, especially the pH and the oxygen content (mainly in the stomach and duodenum). Thus, the proximal trait is enriched of species that can survive at low pH and high oxygen like Firmicutes (Lactobacillaceae) and Proteobacteria (Enterobacteriaceae), while the large intestine presents an increasing of diversity with several obligate anaerobic bacteria as Bacteroidetes (Bacteroidaceae, Prevotellaceae, Rikenellaceae), and Firmicutes (Lachnospiraceae, Ruminococcaceae), and Akkermansia muciniphila, (as representative of Verrucomicrobia) [194].
Most of the functional consequences of alterations of the gut microbiota into changes in health and susceptibility to disease derived from pre-clinical animal studies [195]. For instance, mice have been very helpful to evaluate mechanisms influencing microbiota composition, and there is significant interest in mouse gastrointestinal microbiota as an easily manipulable model for research studies. Moreover, animal models were of great interest to investigate the potential of fecal microbiota transplantation (FMT), which has been proposed as a therapeutic approach to directly introduce intestinal flora. The FMT in mice has been already demonstrated to increased proportion of Firmicutes/Bacteroidetes and then to reduce the level of vascular inflammation and cardiovascular disease in this animal [196].
In obese subjects, the bacterial diversity in large intestine is decreased, with difference in microbial composition [197], resulting in microbial enzymatic alterations [198]. Obesity is associated with a reduction in Firmicutes/Bacteroidetes ratio [186], which increases after weight loss following dietary restrictions and the Roux-en-Y gastric bypass intervention [199,200]. Unfortunately the reproducibility of these studies in humans is limited by differences in race-ethnicities, diet, and metabolic diseases, and other investigations have found conflicting findings [201].
On the other hand, it is known that gut microbiota is influenced by an interplay of genetic, immunologic innate, and environmental factors. Among them, diet is a major factor driving the composition and the metabolism of gut microbiota and has been associated more than the others to obesity [202,203]. Indeed, the dietary intake of processed foods, beverages with sugar, and red meat has been correlated to obesity [204] as well as to altered microbiota composition. In contrast, intake of vegetables, fibers, and yogurt are associated to weight loos and to a different fecal microbial composition [187,205].
8. Gut Microbiota, Energy Harvest, and Storage
The mechanisms by which gut microbiota may drive obesity have not been elucidated yet. However, there is evidence supporting a correlation between microbial active metabolites with energy harvest. Bacteria fermentation of food can influence the nutritional intake of the host up to 10% of energy. Fiber digestion through bacterial fermentation in the colon leads to the production of short chain fatty acids (SCFA) as acetate, propionate and butyrate. Studies from germ free models have demonstrated that SCFA are almost undetectable in the absence of gut microbiome [206]. Interestingly, germ free mice appear to be resistant to diet-induced obesity (High Fat Diet) and to have low levels of inflammatory cytokines (TNF alfa) and improved insulin sensitivity [207]. Butyrate promotes gut barrier by reducing luminal pH. However, it is also a source of energy for colonic cells and for bacteria [208]. The importance of this pathway in human increases in patients with sugar malabsorption when delivery of unabsorbed sugar is increased to the colonic lumen. Acetate and propionate are absorbed rapidly and used as energy source for hepatic cells. Further SCFA released in small amount in circulation, participate to different metabolic effects and brain related host-signaling mechanism [209]. In animal models, SCFA have been shown to act on liver and muscle cells through the energy sensor AMP-activated protein kinase stimulating glucose uptake and fatty acids oxidation with a regulation of gluconeogenesis, lipogenesis and energy metabolism [210]. Moreover, propionate can directly initiate a gut-brain neural circuit acting as agonist of FFAR3 (Free Fatty Acid Receptor 3) in the periportal afferent neural system to induce intestinal gluconeogenesis with beneficial effects on host physiology [211].
In addition, gut microbiota influences energy homeostasis and energy harvest through bile acids metabolism. Intestinal bacteria can modify bile acids through a deconjugation process. Furthermore, some specific strains from the clostridium genus can also dehydroxylate the unconjugated molecules in the colon. It is known that bile acids are endogenous ligands for the nuclear receptor, Farnesoid X receptor (FXR) [212] and for membrane G-protein bile acid receptor (TGR5) [213]. FXR is a ubiquitously expressed nuclear receptor found in different tissues, such as liver, intestine, kidney, adipose tissues and immune cells. FXR-mediated bile acid signaling plays a role in the maintenance of lipid and glucose homeostasis, as shown by the evidence in FXR-null mice of impaired insulin signaling with dysregulated glucose homeostasis [214,215] and elevated blood cholesterol and triglyceride levels. The G protein coupled bile acid receptor 1 (TGR5) is a member of GPCR membrane proteins and is ubiquitously expressed in diverse tissues, including muscle, adipose tissue, immune cells, endocrine organs, and the intestinal tract [216] with a known role in thermogenesis, energy expenditure and energy homeostasis [214]. TGR5- deficient mice show severe metabolic syndromes including obesity, insulin resistance and impaired glucose and lipid homeostasis [217]. TGR5 has been reported to promote incretin secretion like GLP-1 from the intestinal L enteroendocrine cells, therefore bile acids secreted after meal control the release of insulinotropic hormones [218]. Gut microbiota-dehydroxylated bile acids are more hydrophobic, with more efficacy to bind both nuclear receptors amplifying the signals downstream.
Bile acid signaling is an important mediator of the beneficial effects of sleeve gastrectomy surgery in rodent models [219]. Fecal transplant from mice who have undergone to Roux-en-Y gastric bypass (RYGB) surgery to germ free mice leads to a significant weight loss and decreased fat mass compared with sham controls, supporting the role of gut microbial composition in weight loss and the reduction of adiposity after RYGB surgery [220]. These evidences in animals, could explain the beneficial effects in human of the RYGB surgery, whose metabolic improvements and mortality reduction rates are not justified by only weight loss and by lowering caloric intake [221].
The gut microbiota not only increases energy uptake from food intake, processing undigested molecules and modifying endogenous metabolites, but also promotes the harvest of calories in adipose tissue by signaling involved in energy storage. Indeed, the lipoprotein lipase inhibitor Angiopoietin-like 4 (Angpl-4) is downregulated by the gut microbiota, resulting in increased LPL activity and in enhanced hydrolysis of triglycerides with uptake of fatty acid in adipose tissue. In GF mice higher level of Angpl4 and lower LPL activity with less energy storage [190] is present. In humans, levels of Angpl-4 are higher in twins with lower BMI as compared to their obese counterpart [222].
Another signaling influenced by gut microbiota is the AMP-activated protein kinase (AMPK) sensor [223], which regulates energy producing pathways. Evidences show that SCFA bacterial production is a possible direct AMPK activator [224]. In mice, AMPK is activated by both venous infusion and oral administration of SCFAs, explaining the regulatory effect of gut microbiota on AMPK activity [225]. The activation of AMPK develops the phosphorylation of acetyl-CoA carboxylase, and the reduction of malonyl-CoA inducing utilization of fatty acid by its oxidation, as energy source [226].
9. Gut Microbiota and Satiety Signaling
Gut bacteria may have a specific role in the regulation of host appetite. Indeed the symbiotic interactions between intestinal microbiota and their host influence brain functions and behavior [227].
Different metabolic phenotypes are associated with similar gut microbial composition (in obese there is a reduction of relative proportion of Bacteroidetes/ Firmicutes) [186] and transplant of microbiota from obese phenotype to germ free mice results in to increase total body fat, suggesting a role of microbiota in energy harvest [191].
Following those evidences and in line with the recent findings that gut bacterial proteins expressed in their different growth times can modify the host appetite control [228], it is possible to hypothesize a bacterial-host homeostatic model of appetite control that integrates gut bacterial growth dynamics and host molecular pathways controlling energy homeostasis [229].
Gut bacterial growth is constantly influenced by nutrient supply [230] and by the host chemical (digestive gastric and bile acid juices, digestive enzymes), and physical (intestinal peristalsis, colon contractions preparatory to bacteria elimination by defecation) factors.
Regular daily meals trigger bacterial growth (from exponential growth to stationary phase in 20’ after nutrients supply), and according with the different growth phases induced by nutrient supply (exponential or stationary phases), gut bacteria express different molecules [231], which may act locally in a paracrine way through intestinal mucosa, or in an endocrine/systemic way to the brain, involving the short term and long term regulation of feeding behavior.
Studies carried out on E. Coli, the most abundant facultative anaerobe gastrointestinal bacteria, have shown as E. Coli proteome profile in stationary (Stat) growing phase is more abundant and is different than the one in exponential phase (Exp). Between all molecules, E. Coli expresses and secretes the caseinolytic peptidase B (ClpB), a bacterial protein mimetic of alfa MSH that can activate POMC/ARC neurons, signaling anorexigenic message (which induce satiety in the host). Intraperitoneal injection of Stat E. Coli proteins activates anorexigenic neurons in the brain [228]. The role of ClpB protein in the physiological and pathological regulation of eating behavior is shown by its higher level in the plasma of patients with eating disorders than healthy subjects.
The intestinal infusion of E. Coli proteins from different growth phases correlate with plasma increased GLP1 (exp) and PYY (Stat) levels. [232,233]. Of note, the bacterial protein release, in Exp bacterial growth phase stimulates GLP-1 secretion improving glucose metabolism, and similarly the one released in Stat phase can stimulate PYY, 20’ min after started meal, developing satiety signal. Those mediators produced by intestinal enterocytes under influences of gut microbiota and the ClpB, expressed by the bacteria, are responsible of short and long term appetite control respectively [229].
All this evidence supports an integrative model in which the appetite regulatory centers are affected by host signals, and bacteria derived signals, in the different phases of growth after nutrient supply and in response to the homeostatic needs.
10. Gut Microbiota, Insulin Resistance, Vascular Disfunction, and Cardiovascular Disease
Microbial metabolites, which results as product from diet nutrients and different kind of microbial population, can interfere with host metabolic functions and have a role in pathophysiology of metabolic disease. SCFA, indole, bile acids, and LPS are among the most important microbial products with known bioactivity in stimulating EECs secretion of gut hormones [234] (Figure 2). Moreover, it is recently reported that aromatic amino acid metabolism mediated by microbiota is associated to toxin production, involved in endothelial disfunction and cardiovascular disease [235]. Acetate, propionate, and butyrate are SCFA, resulting from the anaerobic fermentation of undigested dietary starch, fiber and other polysaccharides reached the colon, which can be absorbed by the colonic mucosa and have systemic effects. SCFA provide from 5 to 10% of host energy intake, but their role is much broader and they seem to have a function on inflammatory responses, modulation of autonomic system and interfere on many cellular functions included chemotaxis, phagocytosis, ROS stimulation, cell proliferation, histone deacetylases inhibition, and intestinal barrier integrity [236]. It has been identified some G protein coupled receptors as target of SCFA, spread on intestinal epithelial cells, adipocytes, immune cells, smooth muscle cells of small vessels (for tensive control), renal juxtaglomerular apparatus (for renin secretion), that justify the broad spectrum of effects associated with SCFA [237,238,239]. Not only products of bacterial metabolism or proteins secreted by them, but also molecules produced by the breakdown of the bacterial wall play a role in the modulation of host metabolism: polysaccharides, peptidoglycans, and lipoproteins are bacterial derived molecules (from intestinal gram-negative bacteria) that can activate the Toll Like receptors (TLR) expressed by enteroendocrine cells on gut epithelium [240].
TLRs are specialized pattern recognition receptors (PRR) which stimulate host immune response when activated by pathogen-associated microbial products (PAMPs) like LPS [241]. Lipopolysaccharide (LPS) and peptidoglycans might act on gut epithelial cells, but they can also translocate through the intestine and reach several target tissues triggering pro-inflammatory response. It has been shown that mice under high feed diet (HFD) underwent induced obesity (DIO) and insulin resistance associated with finding of plasma high levels of LPS, TNF alfa, IL1 and IL6, which are all proinflammatory markers [242]. This evidence links the low grade inflammation found in obesity and diabetes related insulin resistance with gut microbiota and diet [243]. Indeed, many studies have confirmed the association between high levels of LPS or proinflammatory markers and inhibition of insulin pathways on target organs, justifying the found insulin resistance [244,245]. Tlr4 knock out mice show low expression of inflammatory cytokines and do not develop insulin resistance [246]. Primum movens of this mechanism is the LPS/bacterial translocation from the intestinal lumen to other tissues locations where they can induce inflammation. Translocation must occur before the beginning of metabolic disorders and explains their manifestation [246]. Bacterial translocation might occur for enterocytes internalization by phagocytosis [247], or through the innate immune cell phagocytosis and mesenteric lymph nodes dissemination [248]. Therefore, all conditions which impair the gut wall barrier function facilitate the transition, and it is known that HFD mice and obese mice have an increased gut permeability and higher metabolic endotoxemia [249]>.
Gut microbiota can also affect the host through bioactive metabolites that may contribute to development of diseases. A very important observation comes from metabolomics studies which have identified in the trimethylamine N-oxide (TMAO) a gut microbe-derived metabolite, a strong predictor of plaque atherosclerosis and coronary artery disease [250,251,252]. TMAO is an oxidative hepatic product from trimethylamine (TMA), a gas generated by intestinal microbes from the choline, phosphatidylcholine, and L-carnitine, in the colon tract. Red meat, eggs, milk, liver, shellfish, and fish are major sources of lipid phosphatidylcholine in human plasma. TMAO increases 4–8 h after a rich meal and is normalized in 24 h in preserved renal function. TMAO acts on platelets and further enhances susceptibility to thrombosis risk [253]. Mice fed with carnitine or L choline rich diet have shown an increased atherosclerotic plaques development together with higher plasma levels of TMAO, macrophage cholesterol accumulation and foam cell formation. According with the proatherogenic contribution of the TMA/TMAO gut microbiota generation, studies from germ free mice have shown the suppression of TMAO production even under specific diet while the use of a broad-spectrum antibiotic for short term has shown suppression of diet related atherosclerotic plaque development [252]. However, the TMA/TMAO pathway is just one of the many known and not yet known microbiome-dependent pathways that could be involved to diseases pathogenesis.
In the last decade, gut microbiota metabolism of amino acids and nitrogen metabolites gained a role in endothelial impairment and increased development of cardiovascular disease [254]. The aromatic amino acids in proteins can be metabolized by the gut microbiota [255,256], and host liver [257] to toxins such as indoxyl sulfate, indoxyl glucuronide, indoleacetic acid, p-cresyl sulfate, p-cresyl, phenyl sulfate, and others [258].
Further some bacterial products are involved in the communication between the gut nervous system and the central nervous system through sympathetic activation and involvement of immune system [259,260].
About heart failure, many evidences support the gut hypothesis of heart failure that implies together with systemic congestion, intestinal mucosa ischemia and wall edema which promotes intestinal permeability, bacterial translocation, increased endotoxemia and systemic inflammation [261,262] [263,264]. However, even if the association with vascular compliance and function is complex, at the best of our knowledge we did not find any direct association between gut microbiota and endothelial factors other than NO that may regulate these mechanisms.
All these and other findings demonstrated the important role of the microbiota to promote health and diseases and even if until now the major works have been focused on discover the community of bacteria that are associated with enhanced disease susceptibilities, now it seems to be more relevant the interaction between host diet and microbiota generated metabolites biologically active in order to test novel therapy targeting microbiome or the enzymatic pathway triggered by it.
11. The Autonomic Nervous System (ANS)
In the complex mechanisms that regulate food intake, the autonomic nervous system (ANS) has a major role because is involved in appetite/satiety signal and energy storage/expenditure. ANS plays a role through the short-term regulation of body weight, which is affected by the local gut environment. On the other hand, the long-term regulation developed by ANS depends by the CNS response to endocrine signal as resulting of the nutrients absorption. Through long term and short-term regulation, ANS allows the communication between CNS and gastrointestinal system in both ways.
The short term regulation of body weight is mediated by afferent sensorial nerves which control the sense of satiety, by gastric distension and gut hormones release (like GLP1-GIP, CKK) [265]. The afferent sensorial nerves are activated by gastric mechanoreceptors triggered by wall distension and specific nutrients like SCFA and others stimulating gut hormones release [266]. All these afferent signals reach the solitary tract/area postrema complex in the brain, where are elaborated with other peripheral message. After this afference integration process, it generates a new signal that reach the gut via vagovagal autonomic reflexes and control the gastroenteric secretory, motility, and absorption functions [267,268].
The ANS long term regulation of body weight involves the homeostatic system of energy expenditure and storage. ANS acts on energy expenditure by activation of sympathetic nervous system, responsible for fat mobilization from the adipose tissue, and for thermogenesis from the brown adipose tissue [266]. Sympathetic hyperactivity has been found in obese subjects; it is not generalized but is selective to specific systems like the muscle vasculature and kidneys [269,270,271]. Sympathetic hyperactivity in obesity has negative cardiovascular effect including develop of obesity related hypertension and it does not have any favorable effect in enhancing energy expenditure and weigh loss. Therefore, the increased SNS activity might be an ineffective adaptive mechanism to weight gain, according with initial studies [272].
The assumed mechanisms responsible for the sympathetic hyperactivity in obesity related hypertension involve OSAS, impaired baroreflex, increased leptin with central mechanism, and hyperinsulinemia and insulin resistance, among peripheral mechanisms of sympathetic activation.
In conclusion the afferent vagal signals seem to be the major link between gut and the CNS in controlling food intake and body weight regulation.
Recent discoveries brought additional light to this concept, indeed among the ECCs have been identified a type of sensory epithelial cells that synapses directly with vagal neurons. As kind of neuropod cells, they form a neuroepithelial circuit which connect the gut lumen to the brainstem, transducing sensory stimuli from nutrients (sugar) in milliseconds, using glutamate as neurotransmitter [273].
12. The Brain-Gut-Microbiome Axis (BGM)
The term “Brain-Gut-Microbiome Axis” refers to the established bidirectional communication and interaction that involves nervous system, the gastrointestinal organ and gut microbiome [274]. Perturbations in the communication between the parts or alterations at any level, seem to have a role in the pathogenesis of some neurological (Parkinson’s Disease, multiple sclerosis), gastrointestinal and metabolic disorder (IBD, obesity and food addiction) or socio-affective behavior (autism spectrum disorders, depression and anxiety).
The important role of microbiota on healthy development and maintenance of the central nervous system is confirmed by preclinical evidence, involving germ free animal models, broad spectrum antibiotic short-term use, fecal microbial transplantation, colonization of human or synthetic microbiota and probiotic therapy administration [275].
However, the clinical studies performed, mostly based on the identification of central nervous system effects by probiotic or prebiotics treatment, need to be expanded. The new technologies are a good opportunity to determine the impact of all microbial community (transient and resident) on diseases [275].
Gut microbiotas send signals to brain through the neuroendocrine and neuroimmune circuits and by the vagus nerve [276] The communication occurs through microbial molecules or their derived metabolites which stimulate enterochromaffin, enteroendocrine or immune systems target cells. SCFA, secondary bile acids (2BAs) tryptophan (Trp) metabolites are the most used messengers in this communication [277,278,279,280] (Figure 3).
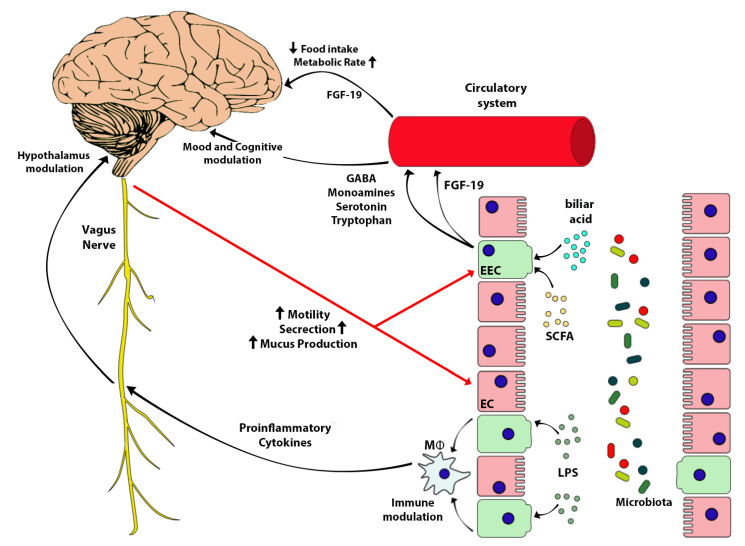
Figure 3.
Brain/Gut axis as interaction between microbiota, gut cells, and peripheral and central nervous system in controlling food intake, metabolism, and the cognitive and circulatory systems. Gut microbiota releases chemical mediators and metabolites, such as SCFA and bile acids that stimulate enterochromaffin cells to secrete FGF-19, GABA, monoamines, serotonin and tryptophan. FGF-19 reduces food intake and increases metabolic rate. GABA, monoamines, fibroblast growth factor 19; EEC, enterochromaffin cell; EC, enteric cell; SCFA, short chain fatty acid; Mϕ, macrophage.
The major signaling molecules are SCFA. They are important for the host as energy resource, the regulation of water and electrolytes absorption, and mucosal proliferation. Further they activate target receptors (GPR41 and GPR43) on EEC (L cells) to stimulate GLP1 and PYY secretion which regulate metabolic functions and induce satiety [277,278] (Figure 2).
Bile acid are products of cholesterol metabolism in the liver, after undergoing a secondary metabolism by the gut microbiota [281]. Secondary bile acids interact through ileal nuclear receptor Farnesoid X Factor (FXR) and TGR5 leading to GLP1 release and regulation of glucose homeostasis [218]. In add, secondary bile acids stimulate fibroblast grow factor (FGF-19) production in small intestine. The FGF-19, crossing the blood–brain barrier (BBB), sends signals to the arcuate nucleus for regulation of energy intake [282]. Evidence shows as in mice, intracerebroventricular injection of FGF-19 directly into the brain increases metabolic rate and decreases circulating glucose and insulin concentrations [283,284,285,286] (Figure 3).
SCFAs and 2 BA act on enterochromaffin cell stimulating the synthesis and release of serotonin (5HT) in the lumen. 5HT is important for regulation GI motility and secretion. 5HT is produced by the EEC and stored there and in enteric neurons for the 95% of all body. It derives from tryptophan (Trp), an essential amino acid available only through the diet, and made accessible by gut microbiota [280]. According with the amount of Trp in the diet, bacteria produce theirs signal molecules which stimulate EEC to produce 5HT. ANS modulates the 5HT secretion on the gut lumen directly acting on EEC, and EEC signals to afferent fiber through connections similar to synapsis and neuropods [287].
The bacterial structural molecules (LPS and peptidoglycan) can activate enteric nervous cell signaling locally in the gut (through TLRs family) [288] or systemically. Microbiota structural molecules and metabolites can translocate through the gut barrier and reach the central nervous system by crossing the blood–brain barrier (BBB) [289], interacting with FXR, TGR5, GPR41 expressed in different location of central nervous system [290,291,292]. Gut microbiota can also produce itself neuroactive molecules like gamma aminobutyric acid, 5HT, norepinephrine and dopamine, and stimulate sensitive targets [293,294,295,296].
Noteworthy, SCFAs are indispensable for the correct development of blood-brain barrier [297]: GF mice show an increased in BBB permeability since intrauterine life due to a reduced expression of some proteins belonging to the tight junctions. This evidence confirm how important is the interaction between microbiota and host for organ health and the development of the systems.
The brain signals to gut microbiota first modulating the gut environment through the ANS, which is responsible for the gut microbiota habitat, the community structure, composition, and activity. Indeed, the ANS regulates the GI mobility and establishes the intestinal transit times. This influences water absorption, nutrient availability, and microbial clearance rates [298,299], promotes the intestinal mucus layer integrity, and contributes to gut barrier healthy by maintenance of permeability (Figure 3). Stressing stimuli are associated with increased intestinal permeability (and enhanced inflammation induced by bacterial molecules) [300,301] while hyperactivity of sympathetic signal (catecholamines mediated) may affect the goblet cells, impairing the quality and quantity mucus production [302,303]. Moreover the ANS influences microbiota, modulating the secretion of gastrointestinal juices (which modifies the pH: gastric juice and bicarbonate), and interferes with antimicrobial peptides production and mucosal immune response [304].
Brain can signal directly to the gut through activation of ECC, neurons, immune cells, which release 5 HT, catecholamines, dynorphin and cytokines [305,306]. These molecules recognize microbial receptors and modulate bacterial behavior sometime increasing virulence [307,308,309,310,311].
In summary, we can affirm that microbiota act as an organ to be considered an indispensable integral part for human health and wellbeing. The microbiota depends on the regular functioning of the intestine (especially in relation to nutrients supply), and itself collaborates in intestinal maintenance. A healthy microbiota affects the wellbeing of the whole organism and the greatest confirmation is the constant double direction dialogue with the brain. Brain function is modulated by the messages sent through directed mediators by the microbiota, and the microbiota in turn depends by CNS activity, for most of its life cycle. Improving the health of the microbiota, with a healthy diet, reducing stress-inducing stimuli, and hopefully by intervening on the microbial flora through pharmacological treatments, could be therapeutic equivalents for diseases associated to dysbiosis and altered colonization. Further studies must be developed to better understand how intervene on microbiota in order to cure related diseases.
13. Conclusions
Obesity is one of the major public health challenges of the current century, which has reached the proportions of a global pandemic. Obesity is the main risk factor for developing type 2 diabetes, cardiovascular disease, and specific types of cancers.
The pathogenesis of obesity is complex and implies many pathophysiological mechanisms involved in the control systems of energy harvest and storage, satiety and appetite, the homeostasis of nutrient metabolism, and regulatory hormones and microbiota. This last one acts as an organ and contributes to gastrointestinal functions by intervening in a crosstalk between the intestine and the brain through many types of cells (ECC, EEC, immune system, neurons) and many mediators (SCFA, 2 BA, LPS, 5HT, catecholamine, cytokines). This crosstalk is further exacerbated by the evidence that the negative modulation of vascular function, and then the predisposition to cardiovascular diseases that is characteristic of the obese subjects, is mediated by both gut hormones effect and adipose tissue accumulation and their second products. In fact, as described, excessive adipose deposition increases adipokine release leading to increases in inflammation and recruitment of immune cells [312].
Understanding all these complex mechanisms and intervening in their dysfunctions could represent a novel strategy to reduce this major menace of the 21st century.
Author Contributions
Conceptualization V.R. and M.T.; writing—original draft preparation V.R., G.R., F.D.D., A.N., and E.C.; writing—review and editing V.R., D.D.-M., G.R. and M.T.; supervision V.R., C.C., U.C., D.D.-M. and M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by fondi d’ateneo grant from the University of Rome Tor Vergata (Beyond Borders 2019) to Manfredi Tesauro.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ng M., Fleming T., Robinson M., Thomson B., Graetz N., Margono C., Mullany E.C., Biryukov S., Abbafati C., Abera S.F., et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finucane M.M., Stevens G.A., Cowan M.J., Danaei G., Lin J.K., Paciorek C.J., Singh G.M., Gutierrez H.R., Lu Y., Bahalim A.N., et al. National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahima R.S., Lazar M.A. Physiology. The health risk of obesity—Better metrics imperative. Science. 2013;341:856–858. doi: 10.1126/science.1241244. [DOI] [PubMed] [Google Scholar]
- 4.Purnell J.Q. Definitions, Classification, and Epidemiology of Obesity. In: Feingold K.R., Anawalt B., Boyce A., Chrousos G., de Herder W.W., Dungan K., Grossman A., Hershman J.M., Hofland J., Kaltsas G., et al., editors. Endotext. Endotext; South Dartmouth, MA, USA: 2000. [Google Scholar]
- 5.Candi E., Tesauro M., Cardillo C., Lena A.M., Schinzari F., Rodia G., Sica G., Gentileschi P., Rovella V., Annicchiarico-Petruzzelli M., et al. Metabolic profiling of visceral adipose tissue from obese subjects with or without metabolic syndrome. Biochem. J. 2018;475:1019–1035. doi: 10.1042/BCJ20170604. [DOI] [PubMed] [Google Scholar]
- 6.Piro M.C., Tesauro M., Lena A.M., Gentileschi P., Sica G., Rodia G., Annicchiarico-Petruzzelli M., Rovella V., Cardillo C., Melino G., et al. Free-amino acid metabolic profiling of visceral adipose tissue from obese subjects. Amino Acids. 2020;52:1125–1137. doi: 10.1007/s00726-020-02877-6. [DOI] [PubMed] [Google Scholar]
- 7.Cercato C., Fonseca F.A. Cardiovascular risk and obesity. Diabetol. Metab. Syndr. 2019;11:74. doi: 10.1186/s13098-019-0468-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esper R.J., Nordaby R.A., Vilariño J.O., Paragano A., Cacharrón J.L., Machado R.A. Endothelial dysfunction: A comprehensive appraisal. Cardiovasc. Diabetol. 2006;5:4. doi: 10.1186/1475-2840-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Versari D., Daghini E., Virdis A., Ghiadoni L., Taddei S. Endothelial dysfunction as a target for prevention of cardiovascular disease. Diabetes Care. 2009;32(Suppl. 2):S314–S321. doi: 10.2337/dc09-S330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vita J.A., Keaney J.F., Jr. Endothelial function: A barometer for cardiovascular risk? Circulation. 2002;106:640–642. doi: 10.1161/01.CIR.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- 11.Marti C.N., Gheorghiade M., Kalogeropoulos A.P., Georgiopoulou V.V., Quyyumi A.A., Butler J. Endothelial dysfunction, arterial stiffness, and heart failure. J. Am. Coll. Cardiol. 2012;60:1455–1469. doi: 10.1016/j.jacc.2011.11.082. [DOI] [PubMed] [Google Scholar]
- 12.Rajendran P., Rengarajan T., Thangavel J., Nishigaki Y., Sakthisekaran D., Sethi G., Nishigaki I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013;9:1057–1069. doi: 10.7150/ijbs.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deanfield J.E., Halcox J.P., Rabelink T.J. Endothelial function and dysfunction: Testing and clinical relevance. Circulation. 2007;115:1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 14.Daiber A., Steven S., Weber A., Shuvaev V.V., Muzykantov V.R., Laher I., Li H., Lamas S., Münzel T. Targeting vascular (endothelial) dysfunction. Br. J. Pharmacol. 2017;174:1591–1619. doi: 10.1111/bph.13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pi X., Xie L., Patterson C. Emerging Roles of Vascular Endothelium in Metabolic Homeostasis. Circ. Res. 2018;123:477–494. doi: 10.1161/CIRCRESAHA.118.313237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daiber A., Di Lisa F., Oelze M., Kröller-Schön S., Steven S., Schulz E., Münzel T. Crosstalk of mitochondria with NADPH oxidase via reactive oxygen and nitrogen species signalling and its role for vascular function. Br. J. Pharmacol. 2017;174:1670–1689. doi: 10.1111/bph.13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daiber A., Oelze M., Daub S., Steven S., Schuff A., Kröller-Schön S., Hausding M., Wenzel P., Schulz E., Gori T., et al. Vascular Redox Signaling, Redox Switches in Endothelial Nitric Oxide Synthase (eNOS Uncoupling), and Endothelial Dysfunction. In: Laher I., editor. Systems Biology of Free Radicals and Antioxidants. Springer; Berlin/Heidelberg, Germany: 2014. pp. 1177–1211. [DOI] [Google Scholar]
- 18.Förstermann U., Münzel T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 19.Schinzari F., Iantorno M., Campia U., Mores N., Rovella V., Tesauro M., Di Daniele N., Cardillo C. Vasodilator responses and endothelin-dependent vasoconstriction in metabolically healthy obesity and the metabolic syndrome. Am. J. Physiol. Endocrinol. Metab. 2015;309:E787–E792. doi: 10.1152/ajpendo.00278.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campia U., Tesauro M., Di Daniele N., Cardillo C. The vascular endothelin system in obesity and type 2 diabetes: Pathophysiology and therapeutic implications. Life Sci. 2014;118:149–155. doi: 10.1016/j.lfs.2014.02.028. [DOI] [PubMed] [Google Scholar]
- 21.Bigornia S.J., Mott M.M., Hess D.T., Apovian C.M., McDonnell M.E., Duess M.A., Kluge M.A., Fiscale A.J., Vita J.A., Gokce N. Long-term successful weight loss improves vascular endothelial function in severely obese individuals. Obesity (Silver Spring) 2010;18:754–759. doi: 10.1038/oby.2009.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levin E.R. Endothelins. N. Engl. J. Med. 1995;333:356–363. doi: 10.1056/NEJM199508103330607. [DOI] [PubMed] [Google Scholar]
- 23.Mather K.J., Lteif A., Steinberg H.O., Baron A.D. Interactions between endothelin and nitric oxide in the regulation of vascular tone in obesity and diabetes. Diabetes. 2004;53:2060–2066. doi: 10.2337/diabetes.53.8.2060. [DOI] [PubMed] [Google Scholar]
- 24.Tesauro M., Schinzari F., Rovella V., Di Daniele N., Lauro D., Mores N., Veneziani A., Cardillo C. Ghrelin restores the endothelin 1/nitric oxide balance in patients with obesity-related metabolic syndrome. Hypertension. 2009;54:995–1000. doi: 10.1161/HYPERTENSIONAHA.109.137729. [DOI] [PubMed] [Google Scholar]
- 25.Mazzuca M.Q., Khalil R.A. Vascular endothelin receptor type B: Structure, function and dysregulation in vascular disease. Biochem. Pharmacol. 2012;84:147–162. doi: 10.1016/j.bcp.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alberti K.G., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A., Fruchart J.C., James W.P., Loria C.M., Smith S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/circulationaha.109.192644. [DOI] [PubMed] [Google Scholar]
- 27.Grundy S.M., Brewer H.B., Jr., Cleeman J.I., Smith S.C., Jr., Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 28.Kahn B.B., Flier J.S. Obesity and insulin resistance. J. Clin. Investig. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barton M., Yanagisawa M. Endothelin: 20 years from discovery to therapy. Can. J. Physiol. Pharmacol. 2008;86:485–498. doi: 10.1139/Y08-059. [DOI] [PubMed] [Google Scholar]
- 30.Cardillo C., Kilcoyne C.M., Waclawiw M., Cannon R.O., 3rd, Panza J.A. Role of endothelin in the increased vascular tone of patients with essential hypertension. Hypertension. 1999;33:753–758. doi: 10.1161/01.HYP.33.2.753. [DOI] [PubMed] [Google Scholar]
- 31.Cardillo C., Kilcoyne C.M., Cannon R.O., 3rd, Panza J.A. Increased activity of endogenous endothelin in patients with hypercholesterolemia. J. Am. Coll. Cardiol. 2000;36:1483–1488. doi: 10.1016/S0735-1097(00)00910-4. [DOI] [PubMed] [Google Scholar]
- 32.Cardillo C., Campia U., Bryant M.B., Panza J.A. Increased activity of endogenous endothelin in patients with type II diabetes mellitus. Circulation. 2002;106:1783–1787. doi: 10.1161/01.CIR.0000032260.01569.64. [DOI] [PubMed] [Google Scholar]
- 33.Mather K.J., Mirzamohammadi B., Lteif A., Steinberg H.O., Baron A.D. Endothelin contributes to basal vascular tone and endothelial dysfunction in human obesity and type 2 diabetes. Diabetes. 2002;51:3517–3523. doi: 10.2337/diabetes.51.12.3517. [DOI] [PubMed] [Google Scholar]
- 34.Ohkita M., Takaoka M., Shiota Y., Nojiri R., Matsumura Y. Nitric oxide inhibits endothelin-1 production through the suppression of nuclear factor kappa B. Clin. Sci. 2002;103(Suppl. 48):68s–71s. doi: 10.1042/CS103S068S. [DOI] [PubMed] [Google Scholar]
- 35.Vicent D., Ilany J., Kondo T., Naruse K., Fisher S.J., Kisanuki Y.Y., Bursell S., Yanagisawa M., King G.L., Kahn C.R. The role of endothelial insulin signaling in the regulation of vascular tone and insulin resistance. J. Clin. Investig. 2003;111:1373–1380. doi: 10.1172/JCI15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cardillo C., Nambi S.S., Kilcoyne C.M., Choucair W.K., Katz A., Quon M.J., Panza J.A. Insulin stimulates both endothelin and nitric oxide activity in the human forearm. Circulation. 1999;100:820–825. doi: 10.1161/01.CIR.100.8.820. [DOI] [PubMed] [Google Scholar]
- 37.Muniyappa R., Montagnani M., Koh K.K., Quon M.J. Cardiovascular actions of insulin. Endocr. Rev. 2007;28:463–491. doi: 10.1210/er.2007-0006. [DOI] [PubMed] [Google Scholar]
- 38.Zeng G., Nystrom F.H., Ravichandran L.V., Cong L.N., Kirby M., Mostowski H., Quon M.J. Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation. 2000;101:1539–1545. doi: 10.1161/01.CIR.101.13.1539. [DOI] [PubMed] [Google Scholar]
- 39.Clark M.G., Wallis M.G., Barrett E.J., Vincent M.A., Richards S.M., Clerk L.H., Rattigan S. Blood flow and muscle metabolism: A focus on insulin action. Am. J. Physiol. Endocrinol. Metab. 2003;284:E241–E258. doi: 10.1152/ajpendo.00408.2002. [DOI] [PubMed] [Google Scholar]
- 40.Anfossi G., Russo I., Doronzo G., Trovati M. Relevance of the vascular effects of insulin in the rationale of its therapeutical use. Cardiovasc. Hematol. Disord. Drug Targets. 2007;7:228–249. doi: 10.2174/187152907782793581. [DOI] [PubMed] [Google Scholar]
- 41.Rajapakse N.W., Chong A.L., Zhang W.Z., Kaye D.M. Insulin-mediated activation of the L-arginine nitric oxide pathway in man, and its impairment in diabetes. PLoS ONE. 2013;8:e61840. doi: 10.1371/journal.pone.0061840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Contreras C., Sanchez A., Garcia-Sacristan A., Martinez M.C., Andriantsitohaina R., Prieto D. Preserved insulin vasorelaxation and up-regulation of the Akt/eNOS pathway in coronary arteries from insulin resistant obese Zucker rats. Atherosclerosis. 2011;217:331–339. doi: 10.1016/j.atherosclerosis.2011.03.036. [DOI] [PubMed] [Google Scholar]
- 43.Cusi K., Maezono K., Osman A., Pendergrass M., Patti M.E., Pratipanawatr T., DeFronzo R.A., Kahn C.R., Mandarino L.J. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J. Clin. Investig. 2000;105:311–320. doi: 10.1172/JCI7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim J.A., Montagnani M., Koh K.K., Quon M.J. Reciprocal relationships between insulin resistance and endothelial dysfunction: Molecular and pathophysiological mechanisms. Circulation. 2006;113:1888–1904. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- 45.Ottosson-Seeberger A., Lundberg J.M., Alvestrand A., Ahlborg G. Exogenous endothelin-1 causes peripheral insulin resistance in healthy humans. Acta Physiol. Scand. 1997;161:211–220. doi: 10.1046/j.1365-201X.1997.00212.x. [DOI] [PubMed] [Google Scholar]
- 46.Lteif A., Vaishnava P., Baron A.D., Mather K.J. Endothelin limits insulin action in obese/insulin-resistant humans. Diabetes. 2007;56:728–734. doi: 10.2337/db06-1406. [DOI] [PubMed] [Google Scholar]
- 47.Shemyakin A., Salehzadeh F., Böhm F., Al-Khalili L., Gonon A., Wagner H., Efendic S., Krook A., Pernow J. Regulation of glucose uptake by endothelin-1 in human skeletal muscle in vivo and in vitro. J. Clin. Endocrinol. Metab. 2010;95:2359–2366. doi: 10.1210/jc.2009-1506. [DOI] [PubMed] [Google Scholar]
- 48.Busija D.W., Miller A.W., Katakam P., Erdos B. Adverse effects of reactive oxygen species on vascular reactivity in insulin resistance. Antioxid. Redox Signal. 2006;8:1131–1140. doi: 10.1089/ars.2006.8.1131. [DOI] [PubMed] [Google Scholar]
- 49.Sandu O.A., Ito M., Begum N. Selected contribution: Insulin utilizes NO/cGMP pathway to activate myosin phosphatase via Rho inhibition in vascular smooth muscle. J. Appl. Physiol. 2001;91:1475–1482. doi: 10.1152/jappl.2001.91.3.1475. [DOI] [PubMed] [Google Scholar]
- 50.Baltieri N., Guizoni D.M., Victorio J.A., Davel A.P. Protective Role of Perivascular Adipose Tissue in Endothelial Dysfunction and Insulin-Induced Vasodilatation of Hypercholesterolemic LDL Receptor-Deficient Mice. Front. Physiol. 2018;9:229. doi: 10.3389/fphys.2018.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mohamed-Ali V., Pinkney J.H., Coppack S.W. Adipose tissue as an endocrine and paracrine organ. Int. J. Obes. Relat. Metab. Disord. 1998;22:1145–1158. doi: 10.1038/sj.ijo.0800770. [DOI] [PubMed] [Google Scholar]
- 52.Weyer C., Funahashi T., Tanaka S., Hotta K., Matsuzawa Y., Pratley R.E., Tataranni P.A. Hypoadiponectinemia in obesity and type 2 diabetes: Close association with insulin resistance and hyperinsulinemia. J. Clin. Endocrinol. Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 53.Lara-Castro C., Luo N., Wallace P., Klein R.L., Garvey W.T. Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes. 2006;55:249–259. doi: 10.2337/diabetes.55.01.06.db05-1105. [DOI] [PubMed] [Google Scholar]
- 54.Matsubara M., Maruoka S., Katayose S. Inverse relationship between plasma adiponectin and leptin concentrations in normal-weight and obese women. Eur. J. Endocrinol. 2002;147:173–180. doi: 10.1530/eje.0.1470173. [DOI] [PubMed] [Google Scholar]
- 55.Yamauchi T., Kamon J., Minokoshi Y., Ito Y., Waki H., Uchida S., Yamashita S., Noda M., Kita S., Ueki K., et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 56.Chen H., Montagnani M., Funahashi T., Shimomura I., Quon M.J. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J. Biol. Chem. 2003;278:45021–45026. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- 57.Motoshima H., Wu X., Mahadev K., Goldstein B.J. Adiponectin suppresses proliferation and superoxide generation and enhances eNOS activity in endothelial cells treated with oxidized LDL. Biochem. Biophys. Res. Commun. 2004;315:264–271. doi: 10.1016/j.bbrc.2004.01.049. [DOI] [PubMed] [Google Scholar]
- 58.Ahima R.S., Flier J.S. Leptin. Annu. Rev. Physiol. 2000;62:413–437. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- 59.Martin S.S., Qasim A., Reilly M.P. Leptin resistance: A possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J. Am. Coll. Cardiol. 2008;52:1201–1210. doi: 10.1016/j.jacc.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vecchione C., Maffei A., Colella S., Aretini A., Poulet R., Frati G., Gentile M.T., Fratta L., Trimarco V., Trimarco B., et al. Leptin effect on endothelial nitric oxide is mediated through Akt-endothelial nitric oxide synthase phosphorylation pathway. Diabetes. 2002;51:168–173. doi: 10.2337/diabetes.51.1.168. [DOI] [PubMed] [Google Scholar]
- 61.Korda M., Kubant R., Patton S., Malinski T. Leptin-induced endothelial dysfunction in obesity. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H1514–H1521. doi: 10.1152/ajpheart.00479.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Söderberg S., Ahrén B., Jansson J.H., Johnson O., Hallmans G., Asplund K., Olsson T. Leptin is associated with increased risk of myocardial infarction. J. Intern. Med. 1999;246:409–418. doi: 10.1046/j.1365-2796.1999.00571.x. [DOI] [PubMed] [Google Scholar]
- 63.Hajer G.R., van Haeften T.W., Visseren F.L. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur. Heart J. 2008;29:2959–2971. doi: 10.1093/eurheartj/ehn387. [DOI] [PubMed] [Google Scholar]
- 64.Piatti P.M., Monti L.D., Conti M., Baruffaldi L., Galli L., Phan C.V., Guazzini B., Pontiroli A.E., Pozza G. Hypertriglyceridemia and hyperinsulinemia are potent inducers of endothelin-1 release in humans. Diabetes. 1996;45:316–321. doi: 10.2337/diab.45.3.316. [DOI] [PubMed] [Google Scholar]
- 65.Schinzari F., Tesauro M., Cardillo C. Increased endothelin-1-mediated vasoconstrictor tone in human obesity: Effects of gut hormones. Physiol. Res. 2018;67:S69–S81. doi: 10.33549/physiolres.933821. [DOI] [PubMed] [Google Scholar]
- 66.Stow L.R., Jacobs M.E., Wingo C.S., Cain B.D. Endothelin-1 gene regulation. FASEB J. 2011;25:16–28. doi: 10.1096/fj.10-161612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003;112:1796–1808. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wellen K.E., Hotamisligil G.S. Obesity-induced inflammatory changes in adipose tissue. J. Clin. Investig. 2003;112:1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu H., Barnes G.T., Yang Q., Tan G., Yang D., Chou C.J., Sole J., Nichols A., Ross J.S., Tartaglia L.A., et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003;112:1821–1830. doi: 10.1172/JCI200319451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rask-Madsen C., Domínguez H., Ihlemann N., Hermann T., Køber L., Torp-Pedersen C. Tumor necrosis factor-alpha inhibits insulin’s stimulating effect on glucose uptake and endothelium-dependent vasodilation in humans. Circulation. 2003;108:1815–1821. doi: 10.1161/01.CIR.0000091406.72832.11. [DOI] [PubMed] [Google Scholar]
- 71.Pittas A.G., Joseph N.A., Greenberg A.S. Adipocytokines and insulin resistance. J. Clin. Endocrinol. Metab. 2004;89:447–452. doi: 10.1210/jc.2003-031005. [DOI] [PubMed] [Google Scholar]
- 72.Tesauro M., Schinzari F., Rovella V., Melina D., Mores N., Barini A., Mettimano M., Lauro D., Iantorno M., Quon M.J., et al. Tumor necrosis factor-alpha antagonism improves vasodilation during hyperinsulinemia in metabolic syndrome. Diabetes Care. 2008;31:1439–1441. doi: 10.2337/dc08-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yudkin J.S., Eringa E., Stehouwer C.D. “Vasocrine” signalling from perivascular fat: A mechanism linking insulin resistance to vascular disease. Lancet. 2005;365:1817–1820. doi: 10.1016/S0140-6736(05)66585-3. [DOI] [PubMed] [Google Scholar]
- 74.Schinzari F., Tesauro M., Veneziani A., Mores N., Di Daniele N., Cardillo C. Favorable Vascular Actions of Angiotensin-(1-7) in Human Obesity. Hypertension. 2018;71:185–191. doi: 10.1161/HYPERTENSIONAHA.117.10280. [DOI] [PubMed] [Google Scholar]
- 75.Aghamohammadzadeh R., Withers S., Lynch F., Greenstein A., Malik R., Heagerty A. Perivascular adipose tissue from human systemic and coronary vessels: The emergence of a new pharmacotherapeutic target. Br. J. Pharmacol. 2012;165:670–682. doi: 10.1111/j.1476-5381.2011.01479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Greenstein A.S., Khavandi K., Withers S.B., Sonoyama K., Clancy O., Jeziorska M., Laing I., Yates A.P., Pemberton P.W., Malik R.A., et al. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation. 2009;119:1661–1670. doi: 10.1161/CIRCULATIONAHA.108.821181. [DOI] [PubMed] [Google Scholar]
- 77.Schinzari F., Tesauro M., Cardillo C. Endothelial and Perivascular Adipose Tissue Abnormalities in Obesity-Related Vascular Dysfunction: Novel Targets for Treatment. J. Cardiovasc. Pharmacol. 2017;69:360–368. doi: 10.1097/FJC.0000000000000469. [DOI] [PubMed] [Google Scholar]
- 78.Chatterjee T.K., Stoll L.L., Denning G.M., Harrelson A., Blomkalns A.L., Idelman G., Rothenberg F.G., Neltner B., Romig-Martin S.A., Dickson E.W., et al. Proinflammatory phenotype of perivascular adipocytes: Influence of high-fat feeding. Circ. Res. 2009;104:541–549. doi: 10.1161/CIRCRESAHA.108.182998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xia N., Horke S., Habermeier A., Closs E.I., Reifenberg G., Gericke A., Mikhed Y., Munzel T., Daiber A., Forstermann U., et al. Uncoupling of Endothelial Nitric Oxide Synthase in Perivascular Adipose Tissue of Diet-Induced Obese Mice. Arterioscler. Thromb. Vasc. Biol. 2016;36:78–85. doi: 10.1161/ATVBAHA.115.306263. [DOI] [PubMed] [Google Scholar]
- 80.Li G., Barrett E.J., Barrett M.O., Cao W., Liu Z. Tumor necrosis factor-alpha induces insulin resistance in endothelial cells via a p38 mitogen-activated protein kinase-dependent pathway. Endocrinology. 2007;148:3356–3363. doi: 10.1210/en.2006-1441. [DOI] [PubMed] [Google Scholar]
- 81.Steinberg G.R., Michell B.J., van Denderen B.J., Watt M.J., Carey A.L., Fam B.C., Andrikopoulos S., Proietto J., Görgün C.Z., Carling D., et al. Tumor necrosis factor alpha-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab. 2006;4:465–474. doi: 10.1016/j.cmet.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 82.Shulman G.I. Cellular mechanisms of insulin resistance. J. Clin. Investig. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Steinberg H.O., Baron A.D. Vascular function, insulin resistance and fatty acids. Diabetologia. 2002;45:623–634. doi: 10.1007/s00125-002-0800-2. [DOI] [PubMed] [Google Scholar]
- 84.Rahmouni K., Morgan D.A., Morgan G.M., Liu X., Sigmund C.D., Mark A.L., Haynes W.G. Hypothalamic PI3K and MAPK differentially mediate regional sympathetic activation to insulin. J. Clin. Investig. 2004;114:652–658. doi: 10.1172/JCI21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cassaglia P.A., Hermes S.M., Aicher S.A., Brooks V.L. Insulin acts in the arcuate nucleus to increase lumbar sympathetic nerve activity and baroreflex function in rats. J. Physiol. 2011;589:1643–1662. doi: 10.1113/jphysiol.2011.205575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Anderson E.A., Hoffman R.P., Balon T.W., Sinkey C.A., Mark A.L. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J. Clin. Investig. 1991;87:2246–2252. doi: 10.1172/JCI115260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rowe J.W., Young J.B., Minaker K.L., Stevens A.L., Pallotta J., Landsberg L. Effect of insulin and glucose infusions on sympathetic nervous system activity in normal man. Diabetes. 1981;30:219–225. doi: 10.2337/diab.30.3.219. [DOI] [PubMed] [Google Scholar]
- 88.Young C.N., Deo S.H., Chaudhary K., Thyfault J.P., Fadel P.J. Insulin enhances the gain of arterial baroreflex control of muscle sympathetic nerve activity in humans. J. Physiol. 2010;588:3593–3603. doi: 10.1113/jphysiol.2010.191866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ardilouze J.L., Sotorník R., Dennis L.A., Fielding B.A., Frayn K.N., Karpe F. Failure to increase postprandial blood flow in subcutaneous adipose tissue is associated with tissue resistance to adrenergic stimulation. Diabetes Metab. 2012;38:27–33. doi: 10.1016/j.diabet.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 90.Emanuel A.L., Meijer R.I., Muskiet M.H., van Raalte D.H., Eringa E.C., Serné E.H. Role of Insulin-Stimulated Adipose Tissue Perfusion in the Development of Whole-Body Insulin Resistance. Arterioscler. Thromb. Vasc. Biol. 2017;37:411–418. doi: 10.1161/ATVBAHA.116.308670. [DOI] [PubMed] [Google Scholar]
- 91.Gribble F.M. The gut endocrine system as a coordinator of postprandial nutrient homoeostasis. Proc. Nutr. Soc. 2012;71:456–462. doi: 10.1017/S0029665112000705. [DOI] [PubMed] [Google Scholar]
- 92.Cho H.J., Robinson E.S., Rivera L.R., McMillan P.J., Testro A., Nikfarjam M., Bravo D.M., Furness J.B. Glucagon-like peptide 1 and peptide YY are in separate storage organelles in enteroendocrine cells. Cell Tissue Res. 2014;357:63–69. doi: 10.1007/s00441-014-1886-9. [DOI] [PubMed] [Google Scholar]
- 93.Egerod K.L., Engelstoft M.S., Grunddal K.V., Nøhr M.K., Secher A., Sakata I., Pedersen J., Windeløv J.A., Füchtbauer E.M., Olsen J., et al. A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology. 2012;153:5782–5795. doi: 10.1210/en.2012-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fothergill L.J., Callaghan B., Hunne B., Bravo D.M., Furness J.B. Costorage of Enteroendocrine Hormones Evaluated at the Cell and Subcellular Levels in Male Mice. Endocrinology. 2017;158:2113–2123. doi: 10.1210/en.2017-00243. [DOI] [PubMed] [Google Scholar]
- 95.Habib A.M., Richards P., Cairns L.S., Rogers G.J., Bannon C.A., Parker H.E., Morley T.C., Yeo G.S., Reimann F., Gribble F.M. Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology. 2012;153:3054–3065. doi: 10.1210/en.2011-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Frias J.P., Nauck M.A., Van J., Kutner M.E., Cui X., Benson C., Urva S., Gimeno R.E., Milicevic Z., Robins D., et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: A randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet. 2018;392:2180–2193. doi: 10.1016/S0140-6736(18)32260-8. [DOI] [PubMed] [Google Scholar]
- 97.Holman R.R., Paul S.K., Bethel M.A., Matthews D.R., Neil H.A. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 98.Schwartz S.S., Kohl B.A. Glycemic control and weight reduction without causing hypoglycemia: The case for continued safe aggressive care of patients with type 2 diabetes mellitus and avoidance of therapeutic inertia. Mayo Clin. Proc. 2010;85:S15–S26. doi: 10.4065/mcp.2010.0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Buchwald H., Estok R., Fahrbach K., Banel D., Jensen M.D., Pories W.J., Bantle J.P., Sledge I. Weight and type 2 diabetes after bariatric surgery: Systematic review and meta-analysis. Am. J. Med. 2009;122:248–256.e245. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 100.Nauck M.A., Homberger E., Siegel E.G., Allen R.C., Eaton R.P., Ebert R., Creutzfeldt W. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J. Clin. Endocrinol. Metab. 1986;63:492–498. doi: 10.1210/jcem-63-2-492. [DOI] [PubMed] [Google Scholar]
- 101.Drucker D.J. Biologic actions and therapeutic potential of the proglucagon-derived peptides. Nat. Clin. Pract. Endocrinol. Metab. 2005;1:22–31. doi: 10.1038/ncpendmet0017. [DOI] [PubMed] [Google Scholar]
- 102.Fehmann H.C., Göke R., Göke B. Cell and molecular biology of the incretin hormones glucagon-like peptide-I and glucose-dependent insulin releasing polypeptide. Endocr. Rev. 1995;16:390–410. doi: 10.1210/edrv-16-3-390. [DOI] [PubMed] [Google Scholar]
- 103.Deacon C.F., Nauck M.A., Toft-Nielsen M., Pridal L., Willms B., Holst J.J. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes. 1995;44:1126–1131. doi: 10.2337/diab.44.9.1126. [DOI] [PubMed] [Google Scholar]
- 104.Zhu L., Tamvakopoulos C., Xie D., Dragovic J., Shen X., Fenyk-Melody J.E., Schmidt K., Bagchi A., Griffin P.R., Thornberry N.A., et al. The role of dipeptidyl peptidase IV in the cleavage of glucagon family peptides: In vivo metabolism of pituitary adenylate cyclase activating polypeptide-(1–38) J. Biol. Chem. 2003;278:22418–22423. doi: 10.1074/jbc.M212355200. [DOI] [PubMed] [Google Scholar]
- 105.Nauck M.A., Kleine N., Orskov C., Holst J.J., Willms B., Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7–36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36:741–744. doi: 10.1007/BF00401145. [DOI] [PubMed] [Google Scholar]
- 106.Drucker D.J. The Cardiovascular Biology of Glucagon-like Peptide-1. Cell Metab. 2016;24:15–30. doi: 10.1016/j.cmet.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 107.Widenmaier S.B., Ao Z., Kim S.J., Warnock G., McIntosh C.H. Suppression of p38 MAPK and JNK via Akt-mediated inhibition of apoptosis signal-regulating kinase 1 constitutes a core component of the beta-cell pro-survival effects of glucose-dependent insulinotropic polypeptide. J. Biol. Chem. 2009;284:30372–30382. doi: 10.1074/jbc.M109.060178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bataille D., Gespach C., Tatemoto K., Marie J.C., Coudray A.M., Rosselin G., Mutt V. Bioactive enteroglucagon (oxyntomodulin): Present knowledge on its chemical structure and its biological activities. Peptides. 1981;2(Suppl. 2):41–44. doi: 10.1016/0196-9781(81)90008-5. [DOI] [PubMed] [Google Scholar]
- 109.Baldissera F.G., Holst J.J., Knuhtsen S., Hilsted L., Nielsen O.V. Oxyntomodulin (glicentin-(33–69)): Pharmacokinetics, binding to liver cell membranes, effects on isolated perfused pig pancreas, and secretion from isolated perfused lower small intestine of pigs. Regul. Pept. 1988;21:151–166. doi: 10.1016/0167-0115(88)90099-7. [DOI] [PubMed] [Google Scholar]
- 110.Gros L., Thorens B., Bataille D., Kervran A. Glucagon-like peptide-1-(7–36) amide, oxyntomodulin, and glucagon interact with a common receptor in a somatostatin-secreting cell line. Endocrinology. 1993;133:631–638. doi: 10.1210/endo.133.2.8102095. [DOI] [PubMed] [Google Scholar]
- 111.Chen H.Y., Trumbauer M.E., Chen A.S., Weingarth D.T., Adams J.R., Frazier E.G., Shen Z., Marsh D.J., Feighner S.D., Guan X.M., et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 2004;145:2607–2612. doi: 10.1210/en.2003-1596. [DOI] [PubMed] [Google Scholar]
- 112.Wang Q., Liu C., Uchida A., Chuang J.C., Walker A., Liu T., Osborne-Lawrence S., Mason B.L., Mosher C., Berglund E.D., et al. Arcuate AgRP neurons mediate orexigenic and glucoregulatory actions of ghrelin. Mol. Metab. 2014;3:64–72. doi: 10.1016/j.molmet.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tesauro M., Schinzari F., Iantorno M., Rizza S., Melina D., Lauro D., Cardillo C. Ghrelin improves endothelial function in patients with metabolic syndrome. Circulation. 2005;112:2986–2992. doi: 10.1161/CIRCULATIONAHA.105.553883. [DOI] [PubMed] [Google Scholar]
- 114.Müller T.D., Nogueiras R., Andermann M.L., Andrews Z.B., Anker S.D., Argente J., Batterham R.L., Benoit S.C., Bowers C.Y., Broglio F., et al. Ghrelin. Mol. Metab. 2015;4:437–460. doi: 10.1016/j.molmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang J.V., Ren P.G., Avsian-Kretchmer O., Luo C.W., Rauch R., Klein C., Hsueh A.J. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake. Science. 2005;310:996–999. doi: 10.1126/science.1117255. [DOI] [PubMed] [Google Scholar]
- 116.Cowan E., Burch K.J., Green B.D., Grieve D.J. Obestatin as a key regulator of metabolism and cardiovascular function with emerging therapeutic potential for diabetes. Br. J. Pharmacol. 2016;173:2165–2181. doi: 10.1111/bph.13502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Agnew A.J., Robinson E., McVicar C.M., Harvey A.P., Ali I.H., Lindsay J.E., McDonald D.M., Green B.D., Grieve D.J. The gastrointestinal peptide obestatin induces vascular relaxation via specific activation of endothelium-dependent NO signalling. Br. J. Pharmacol. 2012;166:327–338. doi: 10.1111/j.1476-5381.2011.01761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schinzari F., Veneziani A., Mores N., Barini A., Di Daniele N., Cardillo C., Tesauro M. Vascular Effects of Obestatin in Lean and Obese Subjects. Diabetes. 2017;66:1214–1221. doi: 10.2337/db16-1067. [DOI] [PubMed] [Google Scholar]
- 119.Habib A.M., Richards P., Rogers G.J., Reimann F., Gribble F.M. Co-localisation and secretion of glucagon-like peptide 1 and peptide YY from primary cultured human L cells. Diabetologia. 2013;56:1413–1416. doi: 10.1007/s00125-013-2887-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Savage A.P., Adrian T.E., Carolan G., Chatterjee V.K., Bloom S.R. Effects of peptide YY (PYY) on mouth to caecum intestinal transit time and on the rate of gastric emptying in healthy volunteers. Gut. 1987;28:166–170. doi: 10.1136/gut.28.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu T., Rayner C.K., Young R.L., Horowitz M. Gut motility and enteroendocrine secretion. Curr. Opin. Pharmacol. 2013;13:928–934. doi: 10.1016/j.coph.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 122.Batterham R.L., Cohen M.A., Ellis S.M., Le Roux C.W., Withers D.J., Frost G.S., Ghatei M.A., Bloom S.R. Inhibition of food intake in obese subjects by peptide YY3-36. N. Engl. J. Med. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 123.Billing L.J., Smith C.A., Larraufie P., Goldspink D.A., Galvin S., Kay R.G., Howe J.D., Walker R., Pruna M., Glass L., et al. Co-storage and release of insulin-like peptide-5, glucagon-like peptide-1 and peptideYY from murine and human colonic enteroendocrine cells. Mol. Metab. 2018;16:65–75. doi: 10.1016/j.molmet.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ang S.Y., Hutchinson D.S., Patil N., Evans B.A., Bathgate R.A.D., Halls M.L., Hossain M.A., Summers R.J., Kocan M. Signal transduction pathways activated by insulin-like peptide 5 at the relaxin family peptide RXFP4 receptor. Br. J. Pharmacol. 2017;174:1077–1089. doi: 10.1111/bph.13522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lloyd K.C., Raybould H.E., Walsh J.H. Cholecystokinin inhibits gastric acid secretion through type “A” cholecystokinin receptors and somatostatin in rats. Am. J. Physiol. 1992;263:G287–G292. doi: 10.1152/ajpgi.1992.263.3.G287. [DOI] [PubMed] [Google Scholar]
- 126.Ahrén B., Hedner P., Lundquist I. Interaction of gastric inhibitory polypeptide (GIP) and cholecystokinin (CCK-8) with basal and stimulated insulin secretion in mice. Acta Endocrinol. 1983;102:96–102. doi: 10.1530/acta.0.1020096. [DOI] [PubMed] [Google Scholar]
- 127.Rehfeld J.F. Cholecystokinin as satiety signal. Int. J. Obes. 1981;5:465–469. [PubMed] [Google Scholar]
- 128.Koop I., Schindler M., Bosshammer A., Scheibner J., Stange E., Koop H. Physiological control of cholecystokinin release and pancreatic enzyme secretion by intraduodenal bile acids. Gut. 1996;39:661–667. doi: 10.1136/gut.39.5.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rogers R.C., Hermann G.E. Mechanisms of action of CCK to activate central vagal afferent terminals. Peptides. 2008;29:1716–1725. doi: 10.1016/j.peptides.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Holst J.J. The physiology of glucagon-like peptide 1. Physiol. Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 131.Tesauro M., Schinzari F., Adamo A., Rovella V., Martini F., Mores N., Barini A., Pitocco D., Ghirlanda G., Lauro D., et al. Effects of GLP-1 on forearm vasodilator function and glucose disposal during hyperinsulinemia in the metabolic syndrome. Diabetes Care. 2013;36:683–689. doi: 10.2337/dc12-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Marso S.P., Bain S.C., Consoli A., Eliaschewitz F.G., Jódar E., Leiter L.A., Lingvay I., Rosenstock J., Seufert J., Warren M.L., et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 133.Marso S.P., Daniels G.H., Brown-Frandsen K., Kristensen P., Mann J.F., Nauck M.A., Nissen S.E., Pocock S., Poulter N.R., Ravn L.S., et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zinman B., Wanner C., Lachin J.M., Fitchett D., Bluhmki E., Hantel S., Mattheus M., Devins T., Johansen O.E., Woerle H.J., et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 135.Alhadeff A.L., Grill H.J. Hindbrain nucleus tractus solitarius glucagon-like peptide-1 receptor signaling reduces appetitive and motivational aspects of feeding. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014;307:R465–R470. doi: 10.1152/ajpregu.00179.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dickson S.L., Shirazi R.H., Hansson C., Bergquist F., Nissbrandt H., Skibicka K.P. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: A new role for mesolimbic GLP-1 receptors. J. Neurosci. 2012;32:4812–4820. doi: 10.1523/JNEUROSCI.6326-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kanoski S.E., Hayes M.R., Skibicka K.P. GLP-1 and weight loss: Unraveling the diverse neural circuitry. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016;310:R885–R895. doi: 10.1152/ajpregu.00520.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bollag R.J., Zhong Q., Phillips P., Min L., Zhong L., Cameron R., Mulloy A.L., Rasmussen H., Qin F., Ding K.H., et al. Osteoblast-derived cells express functional glucose-dependent insulinotropic peptide receptors. Endocrinology. 2000;141:1228–1235. doi: 10.1210/endo.141.3.7366. [DOI] [PubMed] [Google Scholar]
- 139.Faivre E., Gault V.A., Thorens B., Hölscher C. Glucose-dependent insulinotropic polypeptide receptor knockout mice are impaired in learning, synaptic plasticity, and neurogenesis. J. Neurophysiol. 2011;105:1574–1580. doi: 10.1152/jn.00866.2010. [DOI] [PubMed] [Google Scholar]
- 140.Yip R.G., Boylan M.O., Kieffer T.J., Wolfe M.M. Functional GIP receptors are present on adipocytes. Endocrinology. 1998;139:4004–4007. doi: 10.1210/endo.139.9.6288. [DOI] [PubMed] [Google Scholar]
- 141.Meier J.J., Hücking K., Holst J.J., Deacon C.F., Schmiegel W.H., Nauck M.A. Reduced insulinotropic effect of gastric inhibitory polypeptide in first-degree relatives of patients with type 2 diabetes. Diabetes. 2001;50:2497–2504. doi: 10.2337/diabetes.50.11.2497. [DOI] [PubMed] [Google Scholar]
- 142.Mentis N., Vardarli I., Köthe L.D., Holst J.J., Deacon C.F., Theodorakis M., Meier J.J., Nauck M.A. GIP does not potentiate the antidiabetic effects of GLP-1 in hyperglycemic patients with type 2 diabetes. Diabetes. 2011;60:1270–1276. doi: 10.2337/db10-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lund A., Vilsbøll T., Bagger J.I., Holst J.J., Knop F.K. The separate and combined impact of the intestinal hormones, GIP, GLP-1, and GLP-2, on glucagon secretion in type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2011;300:E1038–E1046. doi: 10.1152/ajpendo.00665.2010. [DOI] [PubMed] [Google Scholar]
- 144.Gögebakan Ö., Andres J., Biedasek K., Mai K., Kühnen P., Krude H., Isken F., Rudovich N., Osterhoff M.A., Kintscher U., et al. Glucose-dependent insulinotropic polypeptide reduces fat-specific expression and activity of 11β-hydroxysteroid dehydrogenase type 1 and inhibits release of free fatty acids. Diabetes. 2012;61:292–300. doi: 10.2337/db10-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kim S.J., Nian C., Karunakaran S., Clee S.M., Isales C.M., McIntosh C.H. GIP-overexpressing mice demonstrate reduced diet-induced obesity and steatosis, and improved glucose homeostasis. PLoS ONE. 2012;7:e40156. doi: 10.1371/journal.pone.0040156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Baggio L.L., Huang Q., Brown T.J., Drucker D.J. Oxyntomodulin and glucagon-like peptide-1 differentially regulate murine food intake and energy expenditure. Gastroenterology. 2004;127:546–558. doi: 10.1053/j.gastro.2004.04.063. [DOI] [PubMed] [Google Scholar]
- 147.Dakin C.L., Small C.J., Batterham R.L., Neary N.M., Cohen M.A., Patterson M., Ghatei M.A., Bloom S.R. Peripheral oxyntomodulin reduces food intake and body weight gain in rats. Endocrinology. 2004;145:2687–2695. doi: 10.1210/en.2003-1338. [DOI] [PubMed] [Google Scholar]
- 148.Scott R., Minnion J., Tan T., Bloom S.R. Oxyntomodulin analogue increases energy expenditure via the glucagon receptor. Peptides. 2018;104:70–77. doi: 10.1016/j.peptides.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wynne K., Park A.J., Small C.J., Meeran K., Ghatei M.A., Frost G.S., Bloom S.R. Oxyntomodulin increases energy expenditure in addition to decreasing energy intake in overweight and obese humans: A randomised controlled trial. Int. J. Obes. 2006;30:1729–1736. doi: 10.1038/sj.ijo.0803344. [DOI] [PubMed] [Google Scholar]
- 150.Finan B., Yang B., Ottaway N., Smiley D.L., Ma T., Clemmensen C., Chabenne J., Zhang L., Habegger K.M., Fischer K., et al. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat. Med. 2015;21:27–36. doi: 10.1038/nm.3761. [DOI] [PubMed] [Google Scholar]
- 151.Sun Y., Garcia J.M., Smith R.G. Ghrelin and growth hormone secretagogue receptor expression in mice during aging. Endocrinology. 2007;148:1323–1329. doi: 10.1210/en.2006-0782. [DOI] [PubMed] [Google Scholar]
- 152.Kaneko K., Yoshikawa M., Ohinata K. Novel orexigenic pathway prostaglandin D2-NPY system—Involvement in orally active orexigenic delta opioid peptide. Neuropeptides. 2012;46:353–357. doi: 10.1016/j.npep.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 153.Sohn J.W. Network of hypothalamic neurons that control appetite. BMB Rep. 2015;48:229–233. doi: 10.5483/BMBRep.2015.48.4.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ghigo E., Broglio F., Arvat E., Maccario M., Papotti M., Muccioli G. Ghrelin: More than a natural GH secretagogue and/or an orexigenic factor. Clin. Endocrinol. 2005;62:1–17. doi: 10.1111/j.1365-2265.2004.02160.x. [DOI] [PubMed] [Google Scholar]
- 155.Pöykkö S.M., Kellokoski E., Hörkkö S., Kauma H., Kesäniemi Y.A., Ukkola O. Low plasma ghrelin is associated with insulin resistance, hypertension, and the prevalence of type 2 diabetes. Diabetes. 2003;52:2546–2553. doi: 10.2337/diabetes.52.10.2546. [DOI] [PubMed] [Google Scholar]
- 156.Korbonits M., Goldstone A.P., Gueorguiev M., Grossman A.B. Ghrelin—A hormone with multiple functions. Front. Neuroendocrinol. 2004;25:27–68. doi: 10.1016/j.yfrne.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 157.Zhao T.J., Sakata I., Li R.L., Liang G., Richardson J.A., Brown M.S., Goldstein J.L., Zigman J.M. Ghrelin secretion stimulated by {beta}1-adrenergic receptors in cultured ghrelinoma cells and in fasted mice. Proc. Natl. Acad. Sci. USA. 2010;107:15868–15873. doi: 10.1073/pnas.1011116107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gagnon J., Baggio L.L., Drucker D.J., Brubaker P.L. Ghrelin Is a Novel Regulator of GLP-1 Secretion. Diabetes. 2015;64:1513–1521. doi: 10.2337/db14-1176. [DOI] [PubMed] [Google Scholar]
- 159.Tschöp M., Smiley D.L., Heiman M.L. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 160.Dixit V.D., Schaffer E.M., Pyle R.S., Collins G.D., Sakthivel S.K., Palaniappan R., Lillard J.W., Jr., Taub D.D. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J. Clin. Investig. 2004;114:57–66. doi: 10.1172/JCI200421134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li W.G., Gavrila D., Liu X., Wang L., Gunnlaugsson S., Stoll L.L., McCormick M.L., Sigmund C.D., Tang C., Weintraub N.L. Ghrelin inhibits proinflammatory responses and nuclear factor-kappaB activation in human endothelial cells. Circulation. 2004;109:2221–2226. doi: 10.1161/01.CIR.0000127956.43874.F2. [DOI] [PubMed] [Google Scholar]
- 162.Baldanzi G., Filigheddu N., Cutrupi S., Catapano F., Bonissoni S., Fubini A., Malan D., Baj G., Granata R., Broglio F., et al. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J. Cell Biol. 2002;159:1029–1037. doi: 10.1083/jcb.200207165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Isgaard J., Granata R. Ghrelin in cardiovascular disease and atherogenesis. Mol. Cell. Endocrinol. 2011;340:59–64. doi: 10.1016/j.mce.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 164.Virdis A., Duranti E., Colucci R., Ippolito C., Tirotta E., Lorenzini G., Bernardini N., Blandizzi C., Taddei S. Ghrelin restores nitric oxide availability in resistance circulation of essential hypertensive patients: Role of NAD(P)H oxidase. Eur. Heart J. 2015;36:3023–3030. doi: 10.1093/eurheartj/ehv365. [DOI] [PubMed] [Google Scholar]
- 165.Cho H.J., Kosari S., Hunne B., Callaghan B., Rivera L.R., Bravo D.M., Furness J.B. Differences in hormone localisation patterns of K and L type enteroendocrine cells in the mouse and pig small intestine and colon. Cell Tissue Res. 2015;359:693–698. doi: 10.1007/s00441-014-2033-3. [DOI] [PubMed] [Google Scholar]
- 166.Holzer P., Reichmann F., Farzi A. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut-brain axis. Neuropeptides. 2012;46:261–274. doi: 10.1016/j.npep.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Greiner T.U., Backhed F. Microbial regulation of GLP-1 and L-cell biology. Mol. Metab. 2016;5:753–758. doi: 10.1016/j.molmet.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gribble F.M., Reimann F. Enteroendocrine Cells: Chemosensors in the Intestinal Epithelium. Annu. Rev. Physiol. 2016;78:277–299. doi: 10.1146/annurev-physiol-021115-105439. [DOI] [PubMed] [Google Scholar]
- 169.Mentlein R., Dahms P., Grandt D., Krüger R. Proteolytic processing of neuropeptide Y and peptide YY by dipeptidyl peptidase IV. Regul. Pept. 1993;49:133–144. doi: 10.1016/0167-0115(93)90435-B. [DOI] [PubMed] [Google Scholar]
- 170.Le Roux C.W., Borg C.M., Murphy K.G., Vincent R.P., Ghatei M.A., Bloom S.R. Supraphysiological doses of intravenous PYY3-36 cause nausea, but no additional reduction in food intake. Ann. Clin. Biochem. 2008;45:93–95. doi: 10.1258/acb.2007.007068. [DOI] [PubMed] [Google Scholar]
- 171.Dirksen C., Damgaard M., Bojsen-Møller K.N., Jørgensen N.B., Kielgast U., Jacobsen S.H., Naver L.S., Worm D., Holst J.J., Madsbad S., et al. Fast pouch emptying, delayed small intestinal transit, and exaggerated gut hormone responses after Roux-en-Y gastric bypass. Neurogastroenterol. Motil. 2013;25:346-e255. doi: 10.1111/nmo.12087. [DOI] [PubMed] [Google Scholar]
- 172.Pournaras D.J., Aasheim E.T., Bueter M., Ahmed A.R., Welbourn R., Olbers T., le Roux C.W. Effect of bypassing the proximal gut on gut hormones involved with glycemic control and weight loss. Surg. Obes. Relat. Dis. 2012;8:371–374. doi: 10.1016/j.soard.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 173.Adrian T.E., Sagor G.R., Savage A.P., Bacarese-Hamilton A.J., Hall G.M., Bloom S.R. Peptide YY kinetics and effects on blood pressure and circulating pancreatic and gastrointestinal hormones and metabolites in man. J. Clin. Endocrinol. Metab. 1986;63:803–807. doi: 10.1210/jcem-63-4-803. [DOI] [PubMed] [Google Scholar]
- 174.Ahren B., Larsson H. Peptide YY does not inhibit glucose-stimulated insulin secretion in humans. Eur. J. Endocrinol. 1996;134:362–365. doi: 10.1530/eje.0.1340362. [DOI] [PubMed] [Google Scholar]
- 175.Grosse J., Heffron H., Burling K., Akhter Hossain M., Habib A.M., Rogers G.J., Richards P., Larder R., Rimmington D., Adriaenssens A.A., et al. Insulin-like peptide 5 is an orexigenic gastrointestinal hormone. Proc. Natl. Acad. Sci. USA. 2014;111:11133–11138. doi: 10.1073/pnas.1411413111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lee Y.S., De Vadder F., Tremaroli V., Wichmann A., Mithieux G., Bäckhed F. Insulin-like peptide 5 is a microbially regulated peptide that promotes hepatic glucose production. Mol. Metab. 2016;5:263–270. doi: 10.1016/j.molmet.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Meng A.H., Ling Y.L., Zhang X.P., Zhang J.L. Anti-inflammatory effect of cholecystokinin and its signal transduction mechanism in endotoxic shock rat. World J. Gastroenterol. 2002;8:712–717. doi: 10.3748/wjg.v8.i4.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lovick T.A. CCK as a modulator of cardiovascular function. J. Chem. Neuroanat. 2009;38:176–184. doi: 10.1016/j.jchemneu.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 179.Spreckley E., Murphy K.G. The L-Cell in Nutritional Sensing and the Regulation of Appetite. Front. Nutr. 2015;2:23. doi: 10.3389/fnut.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Verhoeckx K., Cotter P., López-Expósito I., Kleiveland C., Lea T., Mackie A., Requena T., Swiatecka D., Wichers H., editors. The Impact of Food Bioactives on Health: In vitro and Ex Vivo Models. Springer; Cham, Switzerland: 2015. [DOI] [PubMed] [Google Scholar]
- 181.Noble F., Wank S.A., Crawley J.N., Bradwejn J., Seroogy K.B., Hamon M., Roques B.P. International Union of Pharmacology. XXI. Structure, distribution, and functions of cholecystokinin receptors. Pharmacol. Rev. 1999;51:745–781. [PubMed] [Google Scholar]
- 182.Lavine J.A., Raess P.W., Stapleton D.S., Rabaglia M.E., Suhonen J.I., Schueler K.L., Koltes J.E., Dawson J.A., Yandell B.S., Samuelson L.C., et al. Cholecystokinin is up-regulated in obese mouse islets and expands beta-cell mass by increasing beta-cell survival. Endocrinology. 2010;151:3577–3588. doi: 10.1210/en.2010-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sanger G.J., Lee K. Hormones of the gut-brain axis as targets for the treatment of upper gastrointestinal disorders. Nat. Rev. Drug Discov. 2008;7:241–254. doi: 10.1038/nrd2444. [DOI] [PubMed] [Google Scholar]
- 184.Pathak V., Flatt P.R., Irwin N. Cholecystokinin (CCK) and related adjunct peptide therapies for the treatment of obesity and type 2 diabetes. Peptides. 2018;100:229–235. doi: 10.1016/j.peptides.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 185.Bäckhed F., Ley R.E., Sonnenburg J.L., Peterson D.A., Gordon J.I. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 186.Ley R.E., Turnbaugh P.J., Klein S., Gordon J.I. Microbial ecology: Human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 187.Noce A., Marrone G., Di Daniele F., Ottaviani E., Wilson Jones G., Bernini R., Romani A., Rovella V. Impact of Gut Microbiota Composition on Onset and Progression of Chronic Non-Communicable Diseases. Nutrients. 2019;11:1073. doi: 10.3390/nu11051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tang W.H., Hazen S.L. The Gut Microbiome and Its Role in Cardiovascular Diseases. Circulation. 2017;135:1008–1010. doi: 10.1161/CIRCULATIONAHA.116.024251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Reinhardt C., Bergentall M., Greiner T.U., Schaffner F., Ostergren-Lunden G., Petersen L.C., Ruf W., Backhed F. Tissue factor and PAR1 promote microbiota-induced intestinal vascular remodelling. Nature. 2012;483:627–631. doi: 10.1038/nature10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bäckhed F., Ding H., Wang T., Hooper L.V., Koh G.Y., Nagy A., Semenkovich C.F., Gordon J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 192.Annalisa N., Alessio T., Claudette T.D., Erald V., Antonino de L., Nicola D.D. Gut microbioma population: An indicator really sensible to any change in age, diet, metabolic syndrome, and life-style. Mediat. Inflamm. 2014;2014:901308. doi: 10.1155/2014/901308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Dumas M.E., Barton R.H., Toye A., Cloarec O., Blancher C., Rothwell A., Fearnside J., Tatoud R., Blanc V., Lindon J.C., et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc. Natl. Acad. Sci. USA. 2006;103:12511–12516. doi: 10.1073/pnas.0601056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Belzer C., de Vos W.M. Microbes inside—From diversity to function: The case of Akkermansia. ISME J. 2012;6:1449–1458. doi: 10.1038/ismej.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tang W.H., Kitai T., Hazen S.L. Gut Microbiota in Cardiovascular Health and Disease. Circ. Res. 2017;120:1183–1196. doi: 10.1161/CIRCRESAHA.117.309715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kim T.T., Parajuli N., Sung M.M., Bairwa S.C., Levasseur J., Soltys C.M., Wishart D.S., Madsen K., Schertzer J.D., Dyck J.R.B. Fecal transplant from resveratrol-fed donors improves glycaemia and cardiovascular features of the metabolic syndrome in mice. Am. J. Physiol. Endocrinol. Metab. 2018;315:E511–E519. doi: 10.1152/ajpendo.00471.2017. [DOI] [PubMed] [Google Scholar]
- 197.Turnbaugh P.J., Hamady M., Yatsunenko T., Cantarel B.L., Duncan A., Ley R.E., Sogin M.L., Jones W.J., Roe B.A., Affourtit J.P., et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Le Chatelier E., Nielsen T., Qin J., Prifti E., Hildebrand F., Falony G., Almeida M., Arumugam M., Batto J.M., Kennedy S., et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 199.Furet J.P., Kong L.C., Tap J., Poitou C., Basdevant A., Bouillot J.L., Mariat D., Corthier G., Doré J., Henegar C., et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: Links with metabolic and low-grade inflammation markers. Diabetes. 2010;59:3049–3057. doi: 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kong L.C., Tap J., Aron-Wisnewsky J., Pelloux V., Basdevant A., Bouillot J.L., Zucker J.D., Doré J., Clément K. Gut microbiota after gastric bypass in human obesity: Increased richness and associations of bacterial genera with adipose tissue genes. Am. J. Clin. Nutr. 2013;98:16–24. doi: 10.3945/ajcn.113.058743. [DOI] [PubMed] [Google Scholar]
- 201.Finucane M.M., Sharpton T.J., Laurent T.J., Pollard K.S. A taxonomic signature of obesity in the microbiome? Getting to the guts of the matter. PLoS ONE. 2014;9:e84689. doi: 10.1371/journal.pone.0084689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Scott K.P., Gratz S.W., Sheridan P.O., Flint H.J., Duncan S.H. The influence of diet on the gut microbiota. Pharmacol. Res. 2013;69:52–60. doi: 10.1016/j.phrs.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 203.Zhernakova A., Kurilshikov A., Bonder M.J., Tigchelaar E.F., Schirmer M., Vatanen T., Mujagic Z., Vila A.V., Falony G., Vieira-Silva S., et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science. 2016;352:565–569. doi: 10.1126/science.aad3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Mozaffarian D., Hao T., Rimm E.B., Willett W.C., Hu F.B. Changes in diet and lifestyle and long-term weight gain in women and men. N. Engl. J. Med. 2011;364:2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.De Filippis F., Pellegrini N., Vannini L., Jeffery I.B., La Storia A., Laghi L., Serrazanetti D.I., Di Cagno R., Ferrocino I., Lazzi C., et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65:1812–1821. doi: 10.1136/gutjnl-2015-309957. [DOI] [PubMed] [Google Scholar]
- 206.Høverstad T., Midtvedt T. Short-chain fatty acids in germfree mice and rats. J. Nutr. 1986;116:1772–1776. doi: 10.1093/jn/116.9.1772. [DOI] [PubMed] [Google Scholar]
- 207.Rabot S., Membrez M., Bruneau A., Gérard P., Harach T., Moser M., Raymond F., Mansourian R., Chou C.J. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J. 2010;24:4948–4959. doi: 10.1096/fj.10-164921. [DOI] [PubMed] [Google Scholar]
- 208.Peng L., He Z., Chen W., Holzman I.R., Lin J. Effects of butyrate on intestinal barrier function in a Caco-2 cell monolayer model of intestinal barrier. Pediatr. Res. 2007;61:37–41. doi: 10.1203/01.pdr.0000250014.92242.f3. [DOI] [PubMed] [Google Scholar]
- 209.Holmes E., Li J.V., Marchesi J.R., Nicholson J.K. Gut microbiota composition and activity in relation to host metabolic phenotype and disease risk. Cell Metab. 2012;16:559–564. doi: 10.1016/j.cmet.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 210.Den Besten G., Lange K., Havinga R., van Dijk T.H., Gerding A., van Eunen K., Müller M., Groen A.K., Hooiveld G.J., Bakker B.M., et al. Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids. Am. J. Physiol. Gastrointest. Liver Physiol. 2013;305:G900–G910. doi: 10.1152/ajpgi.00265.2013. [DOI] [PubMed] [Google Scholar]
- 211.De Vadder F., Kovatcheva-Datchary P., Goncalves D., Vinera J., Zitoun C., Duchampt A., Bäckhed F., Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156:84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 212.Makishima M., Okamoto A.Y., Repa J.J., Tu H., Learned R.M., Luk A., Hull M.V., Lustig K.D., Mangelsdorf D.J., Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 213.Watanabe M., Houten S.M., Mataki C., Christoffolete M.A., Kim B.W., Sato H., Messaddeq N., Harney J.W., Ezaki O., Kodama T., et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 214.Ma K., Saha P.K., Chan L., Moore D.D. Farnesoid X receptor is essential for normal glucose homeostasis. J. Clin. Investig. 2006;116:1102–1109. doi: 10.1172/JCI25604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zhang Y., Lee F.Y., Barrera G., Lee H., Vales C., Gonzalez F.J., Willson T.M., Edwards P.A. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc. Natl. Acad. Sci. USA. 2006;103:1006–1011. doi: 10.1073/pnas.0506982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Duboc H., Taché Y., Hofmann A.F. The bile acid TGR5 membrane receptor: From basic research to clinical application. Dig. Liver Dis. 2014;46:302–312. doi: 10.1016/j.dld.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Velazquez-Villegas L.A., Perino A., Lemos V., Zietak M., Nomura M., Pols T.W.H., Schoonjans K. TGR5 signalling promotes mitochondrial fission and beige remodelling of white adipose tissue. Nat. Commun. 2018;9:245. doi: 10.1038/s41467-017-02068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Thomas C., Gioiello A., Noriega L., Strehle A., Oury J., Rizzo G., Macchiarulo A., Yamamoto H., Mataki C., Pruzanski M., et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–177. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ryan K.K., Tremaroli V., Clemmensen C., Kovatcheva-Datchary P., Myronovych A., Karns R., Wilson-Pérez H.E., Sandoval D.A., Kohli R., Bäckhed F., et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183–188. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Liou A.P., Paziuk M., Luevano J.M., Jr., Machineni S., Turnbaugh P.J., Kaplan L.M. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci. Transl. Med. 2013;5:178ra141. doi: 10.1126/scitranslmed.3005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Buchwald H., Oien D.M. Metabolic/bariatric surgery Worldwide 2008. Obes. Surg. 2009;19:1605–1611. doi: 10.1007/s11695-009-0014-5. [DOI] [PubMed] [Google Scholar]
- 222.Robciuc M.R., Naukkarinen J., Ortega-Alonso A., Tyynismaa H., Raivio T., Rissanen A., Kaprio J., Ehnholm C., Jauhiainen M., Pietiläinen K.H. Serum angiopoietin-like 4 protein levels and expression in adipose tissue are inversely correlated with obesity in monozygotic twins. J. Lipid Res. 2011;52:1575–1582. doi: 10.1194/jlr.P015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Bäckhed F., Manchester J.K., Semenkovich C.F., Gordon J.I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. USA. 2007;104:979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hu G.X., Chen G.R., Xu H., Ge R.S., Lin J. Activation of the AMP activated protein kinase by short-chain fatty acids is the main mechanism underlying the beneficial effect of a high fiber diet on the metabolic syndrome. Med. Hypotheses. 2010;74:123–126. doi: 10.1016/j.mehy.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 225.Carvalho B.M., Guadagnini D., Tsukumo D.M.L., Schenka A.A., Latuf-Filho P., Vassallo J., Dias J.C., Kubota L.T., Carvalheira J.B.C., Saad M.J.A. Modulation of gut microbiota by antibiotics improves insulin signalling in high-fat fed mice. Diabetologia. 2012;55:2823–2834. doi: 10.1007/s00125-012-2648-4. [DOI] [PubMed] [Google Scholar]
- 226.Hardie D.G., Carling D. The AMP-activated protein kinase—Fuel gauge of the mammalian cell? Eur. J. Biochem. 1997;246:259–273. doi: 10.1111/j.1432-1033.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- 227.Cryan J.F., Dinan T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 228.Breton J., Tennoune N., Lucas N., Francois M., Legrand R., Jacquemot J., Goichon A., Guérin C., Peltier J., Pestel-Caron M., et al. Gut Commensal, E. coli Proteins Activate Host Satiety Pathways following Nutrient-Induced Bacterial Growth. Cell Metab. 2016;23:324–334. doi: 10.1016/j.cmet.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 229.Fetissov S.O. Role of the gut microbiota in host appetite control: Bacterial growth to animal feeding behaviour. Nat. Rev. Endocrinol. 2017;13:11–25. doi: 10.1038/nrendo.2016.150. [DOI] [PubMed] [Google Scholar]
- 230.Martchenko S.E., Martchenko A., Cox B.J., Naismith K., Waller A., Gurges P., Sweeney M.E., Philpott D.J., Brubaker P.L. Circadian GLP-1 Secretion in Mice Is Dependent on the Intestinal Microbiome for Maintenance of Diurnal Metabolic Homeostasis. Diabetes. 2020;69:2589–2602. doi: 10.2337/db20-0262. [DOI] [PubMed] [Google Scholar]
- 231.Wick L.M., Quadroni M., Egli T. Short- and long-term changes in proteome composition and kinetic properties in a culture of Escherichia coli during transition from glucose-excess to glucose-limited growth conditions in continuous culture and vice versa. Environ. Microbiol. 2001;3:588–599. doi: 10.1046/j.1462-2920.2001.00231.x. [DOI] [PubMed] [Google Scholar]
- 232.Breton J., Legrand R., Akkermann K., Järv A., Harro J., Déchelotte P., Fetissov S.O. Elevated plasma concentrations of bacterial ClpB protein in patients with eating disorders. Int. J. Eat. Disord. 2016;49:805–808. doi: 10.1002/eat.22531. [DOI] [PubMed] [Google Scholar]
- 233.Wichmann A., Allahyar A., Greiner T.U., Plovier H., Lunden G.O., Larsson T., Drucker D.J., Delzenne N.M., Cani P.D., Backhed F. Microbial modulation of energy availability in the colon regulates intestinal transit. Cell Host Microbe. 2013;14:582–590. doi: 10.1016/j.chom.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 234.Gribble F.M., Reimann F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat. Rev. Endocrinol. 2019;15:226–237. doi: 10.1038/s41574-019-0168-8. [DOI] [PubMed] [Google Scholar]
- 235.Liu X., Xu X., Shang R., Chen Y. Asymmetric dimethylarginine (ADMA) as an important risk factor for the increased cardiovascular diseases and heart failure in chronic kidney disease. Nitric Oxide. 2018;78:113–120. doi: 10.1016/j.niox.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ohira H., Tsutsui W., Fujioka Y. Are Short Chain Fatty Acids in Gut Microbiota Defensive Players for Inflammation and Atherosclerosis? J. Atheroscler. Thromb. 2017;24:660–672. doi: 10.5551/jat.RV17006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Natarajan N., Hori D., Flavahan S., Steppan J., Flavahan N.A., Berkowitz D.E., Pluznick J.L. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol. Genom. 2016;48:826–834. doi: 10.1152/physiolgenomics.00089.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Pluznick J.L. Renal and cardiovascular sensory receptors and blood pressure regulation. Am. J. Physiol. Ren. Physiol. 2013;305:F439–F444. doi: 10.1152/ajprenal.00252.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Pluznick J.L., Protzko R.J., Gevorgyan H., Peterlin Z., Sipos A., Han J., Brunet I., Wan L.X., Rey F., Wang T., et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc. Natl. Acad. Sci. USA. 2013;110:4410–4415. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Palazzo M., Balsari A., Rossini A., Selleri S., Calcaterra C., Gariboldi S., Zanobbio L., Arnaboldi F., Shirai Y.F., Serrao G., et al. Activation of enteroendocrine cells via TLRs induces hormone, chemokine, and defensin secretion. J. Immunol. 2007;178:4296–4303. doi: 10.4049/jimmunol.178.7.4296. [DOI] [PubMed] [Google Scholar]
- 241.Brown J.M., Hazen S.L. The gut microbial endocrine organ: Bacterially derived signals driving cardiometabolic diseases. Annu. Rev. Med. 2015;66:343–359. doi: 10.1146/annurev-med-060513-093205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Cani P.D., Amar J., Iglesias M.A., Poggi M., Knauf C., Bastelica D., Neyrinck A.M., Fava F., Tuohy K.M., Chabo C., et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 243.Caesar R., Reigstad C.S., Backhed H.K., Reinhardt C., Ketonen M., Lunden G.O., Cani P.D., Backhed F. Gut-derived lipopolysaccharide augments adipose macrophage accumulation but is not essential for impaired glucose or insulin tolerance in mice. Gut. 2012;61:1701–1707. doi: 10.1136/gutjnl-2011-301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Lackey D.E., Olefsky J.M. Regulation of metabolism by the innate immune system. Nat. Rev. Endocrinol. 2016;12:15–28. doi: 10.1038/nrendo.2015.189. [DOI] [PubMed] [Google Scholar]
- 245.Liang H., Hussey S.E., Sanchez-Avila A., Tantiwong P., Musi N. Effect of lipopolysaccharide on inflammation and insulin action in human muscle. PLoS ONE. 2013;8:e63983. doi: 10.1371/journal.pone.0063983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Shi H., Kokoeva M.V., Inouye K., Tzameli I., Yin H., Flier J.S. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Investig. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Neal M.D., Leaphart C., Levy R., Prince J., Billiar T.R., Watkins S., Li J., Cetin S., Ford H., Schreiber A., et al. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J. Immunol. 2006;176:3070–3079. doi: 10.4049/jimmunol.176.5.3070. [DOI] [PubMed] [Google Scholar]
- 248.Amar J., Chabo C., Waget A., Klopp P., Vachoux C., Bermúdez-Humarán L.G., Smirnova N., Bergé M., Sulpice T., Lahtinen S., et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: Molecular mechanisms and probiotic treatment. EMBO Mol. Med. 2011;3:559–572. doi: 10.1002/emmm.201100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Cani P.D., Possemiers S., Van de Wiele T., Guiot Y., Everard A., Rottier O., Geurts L., Naslain D., Neyrinck A., Lambert D.M., et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Koeth R.A., Wang Z., Levison B.S., Buffa J.A., Org E., Sheehy B.T., Britt E.B., Fu X., Wu Y., Li L., et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Tang W.H., Wang Z., Levison B.S., Koeth R.A., Britt E.B., Fu X., Wu Y., Hazen S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Wang Z., Klipfell E., Bennett B.J., Koeth R., Levison B.S., Dugar B., Feldstein A.E., Britt E.B., Fu X., Chung Y.M., et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zhu W., Gregory J.C., Org E., Buffa J.A., Gupta N., Wang Z., Li L., Fu X., Wu Y., Mehrabian M., et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell. 2016;165:111–124. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Karbach S.H., Schonfelder T., Brandao I., Wilms E., Hormann N., Jackel S., Schuler R., Finger S., Knorr M., Lagrange J., et al. Gut Microbiota Promote Angiotensin II-Induced Arterial Hypertension and Vascular Dysfunction. J. Am. Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Nallu A., Sharma S., Ramezani A., Muralidharan J., Raj D. Gut microbiome in chronic kidney disease: Challenges and opportunities. Transl. Res. 2017;179:24–37. doi: 10.1016/j.trsl.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Pereira-Fantini P.M., Byars S.G., Pitt J., Lapthorne S., Fouhy F., Cotter P.D., Bines J.E. Unravelling the metabolic impact of SBS-associated microbial dysbiosis: Insights from the piglet short bowel syndrome model. Sci. Rep. 2017;7:43326. doi: 10.1038/srep43326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Webster L.T., Siddiqui U.A., Lucas S.V., Strong J.M., Mieyal J.J. Identification of separate acyl-CoA:glycine and acyl-CoA:L-glutamine N-acyltransferase activities in mitochondrial fractions from liver of rhesus monkey and man. J. Biol. Chem. 1976;251:3352–3358. doi: 10.1016/S0021-9258(17)33444-0. [DOI] [PubMed] [Google Scholar]
- 258.Amedei A., Morbidelli L. Circulating Metabolites Originating from Gut Microbiota Control Endothelial Cell Function. Molecules. 2019;24:3992. doi: 10.3390/molecules24213992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Pålsson J., Ricksten S.E., Delle M., Lundin S. Changes in renal sympathetic nerve activity during experimental septic and endotoxin shock in conscious rats. Circ. Shock. 1988;24:133–141. [PubMed] [Google Scholar]
- 260.Santisteban M.M., Qi Y., Zubcevic J., Kim S., Yang T., Shenoy V., Cole-Jeffrey C.T., Lobaton G.O., Stewart D.C., Rubiano A., et al. Hypertension-Linked Pathophysiological Alterations in the Gut. Circ. Res. 2017;120:312–323. doi: 10.1161/CIRCRESAHA.116.309006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Kiouptsi K., Pontarollo G., Todorov H., Braun J., Jackel S., Koeck T., Bayer F., Karwot C., Karpi A., Gerber S., et al. Germ-free housing conditions do not affect aortic root and aortic arch lesion size of late atherosclerotic low-density lipoprotein receptor-deficient mice. Gut Microbes. 2020;11:1809–1823. doi: 10.1080/19490976.2020.1767463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Kiouptsi K., Jackel S., Pontarollo G., Grill A., Kuijpers M.J.E., Wilms E., Weber C., Sommer F., Nagy M., Neideck C., et al. The Microbiota Promotes Arterial Thrombosis in Low-Density Lipoprotein Receptor-Deficient Mice. mBio. 2019;10 doi: 10.1128/mBio.02298-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Niebauer J., Volk H.D., Kemp M., Dominguez M., Schumann R.R., Rauchhaus M., Poole-Wilson P.A., Coats A.J., Anker S.D. Endotoxin and immune activation in chronic heart failure: A prospective cohort study. Lancet. 1999;353:1838–1842. doi: 10.1016/S0140-6736(98)09286-1. [DOI] [PubMed] [Google Scholar]
- 264.Sandek A., Swidsinski A., Schroedl W., Watson A., Valentova M., Herrmann R., Scherbakov N., Cramer L., Rauchhaus M., Grosse-Herrenthey A., et al. Intestinal blood flow in patients with chronic heart failure: A link with bacterial growth, gastrointestinal symptoms, and cachexia. J. Am. Coll. Cardiol. 2014;64:1092–1102. doi: 10.1016/j.jacc.2014.06.1179. [DOI] [PubMed] [Google Scholar]
- 265.Dockray G.J. Gastrointestinal hormones and the dialogue between gut and brain. J. Physiol. 2014;592:2927–2941. doi: 10.1113/jphysiol.2014.270850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Guarino D., Nannipieri M., Iervasi G., Taddei S., Bruno R.M. The Role of the Autonomic Nervous System in the Pathophysiology of Obesity. Front. Physiol. 2017;8:665. doi: 10.3389/fphys.2017.00665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Berthoud H.R. The vagus nerve, food intake and obesity. Regul. Pept. 2008;149:15–25. doi: 10.1016/j.regpep.2007.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Li Y., Owyang C. Endogenous cholecystokinin stimulates pancreatic enzyme secretion via vagal afferent pathway in rats. Gastroenterology. 1994;107:525–531. doi: 10.1016/0016-5085(94)90180-5. [DOI] [PubMed] [Google Scholar]
- 269.Alvarez G.E., Ballard T.P., Beske S.D., Davy K.P. Subcutaneous obesity is not associated with sympathetic neural activation. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H414–H418. doi: 10.1152/ajpheart.01046.2003. [DOI] [PubMed] [Google Scholar]
- 270.Esler M., Straznicky N., Eikelis N., Masuo K., Lambert G., Lambert E. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension. 2006;48:787–796. doi: 10.1161/01.HYP.0000242642.42177.49. [DOI] [PubMed] [Google Scholar]
- 271.Grassi G., Seravalle G., Cattaneo B.M., Bolla G.B., Lanfranchi A., Colombo M., Giannattasio C., Brunani A., Cavagnini F., Mancia G. Sympathetic activation in obese normotensive subjects. Hypertension. 1995;25:560–563. doi: 10.1161/01.HYP.25.4.560. [DOI] [PubMed] [Google Scholar]
- 272.Landsberg L. Diet, obesity and hypertension: An hypothesis involving insulin, the sympathetic nervous system, and adaptive thermogenesis. Q. J. Med. 1986;61:1081–1090. [PubMed] [Google Scholar]
- 273.Kaelberer M.M., Buchanan K.L., Klein M.E., Barth B.B., Montoya M.M., Shen X., Bohórquez D.V. A gut-brain neural circuit for nutrient sensory transduction. Science. 2018;361 doi: 10.1126/science.aat5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Grasset E., Burcelin R. The gut microbiota to the brain axis in the metabolic control. Rev. Endocr. Metab. Disord. 2019;20:427–438. doi: 10.1007/s11154-019-09511-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Martin C.R., Osadchiy V., Kalani A., Mayer E.A. The Brain-Gut-Microbiome Axis. Cell. Mol. Gastroenterol. Hepatol. 2018;6:133–148. doi: 10.1016/j.jcmgh.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., Bienenstock J., Cryan J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Samuel B.S., Shaito A., Motoike T., Rey F.E., Backhed F., Manchester J.K., Hammer R.E., Williams S.C., Crowley J., Yanagisawa M., et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl. Acad. Sci. USA. 2008;105:16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Tolhurst G., Heffron H., Lam Y.S., Parker H.E., Habib A.M., Diakogiannaki E., Cameron J., Grosse J., Reimann F., Gribble F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Wikoff W.R., Anfora A.T., Liu J., Schultz P.G., Lesley S.A., Peters E.C., Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Yano J.M., Yu K., Donaldson G.P., Shastri G.G., Ann P., Ma L., Nagler C.R., Ismagilov R.F., Mazmanian S.K., Hsiao E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Wahlström A., Sayin S.I., Marschall H.U., Bäckhed F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016;24:41–50. doi: 10.1016/j.cmet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 282.Hsuchou H., Pan W., Kastin A.J. Fibroblast growth factor 19 entry into brain. Fluids Barriers CNS. 2013;10:32. doi: 10.1186/2045-8118-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Fu L., John L.M., Adams S.H., Yu X.X., Tomlinson E., Renz M., Williams P.M., Soriano R., Corpuz R., Moffat B., et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology. 2004;145:2594–2603. doi: 10.1210/en.2003-1671. [DOI] [PubMed] [Google Scholar]
- 284.Marcelin G., Jo Y.H., Li X., Schwartz G.J., Zhang Y., Dun N.J., Lyu R.M., Blouet C., Chang J.K., Chua S., Jr. Central action of FGF19 reduces hypothalamic AGRP/NPY neuron activity and improves glucose metabolism. Mol. Metab. 2014;3:19–28. doi: 10.1016/j.molmet.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Morton G.J., Matsen M.E., Bracy D.P., Meek T.H., Nguyen H.T., Stefanovski D., Bergman R.N., Wasserman D.H., Schwartz M.W. FGF19 action in the brain induces insulin-independent glucose lowering. J. Clin. Investig. 2013;123:4799–4808. doi: 10.1172/JCI70710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Ryan K.K., Kohli R., Gutierrez-Aguilar R., Gaitonde S.G., Woods S.C., Seeley R.J. Fibroblast growth factor-19 action in the brain reduces food intake and body weight and improves glucose tolerance in male rats. Endocrinology. 2013;154:9–15. doi: 10.1210/en.2012-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Bohórquez D.V., Liddle R.A. The gut connectome: Making sense of what you eat. J. Clin. Investig. 2015;125:888–890. doi: 10.1172/JCI81121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Barajon I., Serrao G., Arnaboldi F., Opizzi E., Ripamonti G., Balsari A., Rumio C. Toll-like receptors 3, 4, and 7 are expressed in the enteric nervous system and dorsal root ganglia. J. Histochem. Cytochem. 2009;57:1013–1023. doi: 10.1369/jhc.2009.953539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Haghikia A., Jörg S., Duscha A., Berg J., Manzel A., Waschbisch A., Hammer A., Lee D.H., May C., Wilck N., et al. Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity. 2015;43:817–829. doi: 10.1016/j.immuni.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 290.Huang C., Wang J., Hu W., Wang C., Lu X., Tong L., Wu F., Zhang W. Identification of functional farnesoid X receptors in brain neurons. FEBS Lett. 2016;590:3233–3242. doi: 10.1002/1873-3468.12373. [DOI] [PubMed] [Google Scholar]
- 291.Keitel V., Görg B., Bidmon H.J., Zemtsova I., Spomer L., Zilles K., Häussinger D. The bile acid receptor TGR5 (Gpbar-1) acts as a neurosteroid receptor in brain. Glia. 2010;58:1794–1805. doi: 10.1002/glia.21049. [DOI] [PubMed] [Google Scholar]
- 292.Kimura I., Inoue D., Maeda T., Hara T., Ichimura A., Miyauchi S., Kobayashi M., Hirasawa A., Tsujimoto G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41) Proc. Natl. Acad. Sci. USA. 2011;108:8030–8035. doi: 10.1073/pnas.1016088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Asano Y., Hiramoto T., Nishino R., Aiba Y., Kimura T., Yoshihara K., Koga Y., Sudo N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;303:G1288–G1295. doi: 10.1152/ajpgi.00341.2012. [DOI] [PubMed] [Google Scholar]
- 294.Barrett E., Ross R.P., O’Toole P.W., Fitzgerald G.F., Stanton C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012;113:411–417. doi: 10.1111/j.1365-2672.2012.05344.x. [DOI] [PubMed] [Google Scholar]
- 295.Özogul F. Effects of specific lactic acid bacteria species on biogenic amine production by foodborne pathogen. Int. J. Food Sci. Technol. 2011;46:478–484. doi: 10.1111/j.1365-2621.2010.02511.x. [DOI] [Google Scholar]
- 296.Shishov V.A., Kirovskaia T.A., Kudrin V.S., Oleskin A.V. Amine neuromediators, their precursors, and oxidation products in the culture of Escherichia coli K-12. Prikl. Biokhim. Mikrobiol. 2009;45:550–554. doi: 10.1134/S0003683809050068. [DOI] [PubMed] [Google Scholar]
- 297.Braniste V., Al-Asmakh M., Kowal C., Anuar F., Abbaspour A., Tóth M., Korecka A., Bakocevic N., Ng L.G., Kundu P., et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014;6:263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Van Felius I.D., Akkermans L.M., Bosscha K., Verheem A., Harmsen W., Visser M.R., Gooszen H.G. Interdigestive small bowel motility and duodenal bacterial overgrowth in experimental acute pancreatitis. Neurogastroenterol. Motil. 2003;15:267–276. doi: 10.1046/j.1365-2982.2003.00410.x. [DOI] [PubMed] [Google Scholar]
- 299.Vandeputte D., Falony G., Vieira-Silva S., Tito R.Y., Joossens M., Raes J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut. 2016;65:57–62. doi: 10.1136/gutjnl-2015-309618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Meddings J.B., Swain M.G. Environmental stress-induced gastrointestinal permeability is mediated by endogenous glucocorticoids in the rat. Gastroenterology. 2000;119:1019–1028. doi: 10.1053/gast.2000.18152. [DOI] [PubMed] [Google Scholar]
- 301.Saunders P.R., Santos J., Hanssen N.P., Yates D., Groot J.A., Perdue M.H. Physical and psychological stress in rats enhances colonic epithelial permeability via peripheral CRH. Dig. Dis Sci. 2002;47:208–215. doi: 10.1023/A:1013204612762. [DOI] [PubMed] [Google Scholar]
- 302.Kim Y.S., Ho S.B. Intestinal goblet cells and mucins in health and disease: Recent insights and progress. Curr. Gastroenterol. Rep. 2010;12:319–330. doi: 10.1007/s11894-010-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Rubio C.A., Huang C.B. Quantification of the sulphomucin-producing cell population of the colonic mucosa during protracted stress in rats. In vivo. 1992;6:81–84. [PubMed] [Google Scholar]
- 304.Mayer E.A. Gut feelings: The emerging biology of gut-brain communication. Nat. Rev. Neurosci. 2011;12:453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Lyte M. The role of microbial endocrinology in infectious disease. J. Endocrinol. 1993;137:343–345. doi: 10.1677/joe.0.1370343. [DOI] [PubMed] [Google Scholar]
- 306.Mayer E.A., Savidge T., Shulman R.J. Brain-gut microbiome interactions and functional bowel disorders. Gastroenterology. 2014;146:1500–1512. doi: 10.1053/j.gastro.2014.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Alverdy J., Holbrook C., Rocha F., Seiden L., Wu R.L., Musch M., Chang E., Ohman D., Suh S. Gut-derived sepsis occurs when the right pathogen with the right virulence genes meets the right host: Evidence for in vivo virulence expression in Pseudomonas aeruginosa. Ann. Surg. 2000;232:480–489. doi: 10.1097/00000658-200010000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Clarke M.B., Hughes D.T., Zhu C., Boedeker E.C., Sperandio V. The QseC sensor kinase: A bacterial adrenergic receptor. Proc. Natl. Acad. Sci. USA. 2006;103:10420–10425. doi: 10.1073/pnas.0604343103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Cogan T.A., Thomas A.O., Rees L.E., Taylor A.H., Jepson M.A., Williams P.H., Ketley J., Humphrey T.J. Norepinephrine increases the pathogenic potential of Campylobacter jejuni. Gut. 2007;56:1060–1065. doi: 10.1136/gut.2006.114926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Hughes D.T., Sperandio V. Inter-kingdom signalling: Communication between bacteria and their hosts. Nat. Rev. Microbiol. 2008;6:111–120. doi: 10.1038/nrmicro1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Lyte M. The role of catecholamines in gram-negative sepsis. Med. Hypotheses. 1992;37:255–258. doi: 10.1016/0306-9877(92)90197-K. [DOI] [PubMed] [Google Scholar]
- 312.Booth A., Magnuson A., Fouts J., Foster M.T. Adipose tissue: An endocrine organ playing a role in metabolic regulation. Horm. Mol. Biol. Clin. Investig. 2016;26:25–42. doi: 10.1515/hmbci-2015-0073. [DOI] [PubMed] [Google Scholar]