In this analysis, we describe outcomes between younger and older adolescents after bariatric surgery in a prospective observational cohort of 242 adolescents at 5 children’s hospitals.
Abstract
OBJECTIVES:
In this report, we compare weight loss, comorbidity resolution, nutritional abnormalities, and quality of life between younger and older adolescents after metabolic and bariatric surgery.
METHODS:
From March 2007 to December 2011, 242 adolescents (≤19 years of age) who underwent bariatric surgery at 5 clinical centers in the United States were enrolled in the prospective, multicenter, long-term outcome study Teen–Longitudinal Assessment of Bariatric Surgery. Outcome data from younger (13–15 years; n = 66) and older (16–19 years; n = 162) study participants were compared. Outcomes included percent BMI change, comorbidity outcomes (hypertension, dyslipidemia, and type 2 diabetes mellitus), nutritional abnormalities, and quality of life over 5 years post surgery.
RESULTS:
Baseline characteristics, except for age, between the 2 cohorts were similar. No significant differences in frequency of remission of hypertension (P = .84) or dyslipidemia (P = .74) were observed between age groups. Remission of type 2 diabetes mellitus was high in both groups, although statistically higher in older adolescents (relative risk 0.86; P = .046). Weight loss and quality of life were similar in the 2 age groups. Younger adolescents were less likely to develop elevated transferrin (prevalence ratio 0.52; P = .048) and low vitamin D levels (prevalence ratio 0.8; P = .034).
CONCLUSIONS:
The differences in outcome of metabolic and bariatric surgery between younger and older adolescents were few. These data suggest that younger adolescents with severe obesity should not be denied consideration for surgical therapy on the basis of age alone and that providers should consider adolescents of all ages for surgical therapy for obesity when clinically indicated.
What’s Known on This Subject:
Bariatric surgery is an effective, durable treatment of severe obesity in adolescents, with results generally similar to or surpassing those for adults. However, little is known about the relative merits and risks of these procedures in younger teenagers compared with older adolescents.
What This Study Adds:
We found that younger and older adolescents had similar weight loss, resolution of hypertension and dyslipidemia, nutritional deficiencies, and improvement in quality of life after surgery. Age alone should not dissuade providers and patients from pursuing surgery when medically indicated.
Over recent decades, the global health and economic burden of obesity has continued to rise rapidly in both developing and developed nations.1,2 In the United States, obesity affects 13.7 million children, of whom 6% meet the definition of having severe obesity.3,4 Obesity has been linked to numerous complications involving every body system.5,6 The rise of childhood obesity has brought an emergence of chronic diseases once only present in adulthood (eg, hypertension, dyslipidemia, and type 2 diabetes mellitus) and associated with a decreased life expectancy of 5 to 20 years.7,8 Intensive medical and lifestyle interventions for the treatment of obesity have demonstrated an average of 5% to 15% weight loss, with variable results in sustainability.9–11 Alternatively, metabolic and bariatric surgery (MBS) has been shown to be an effective and durable treatment for obesity and complications of obesity in adolescents, with weight loss and health improvements far surpassing those achieved with nonsurgical treatments.12–17 The most recent update of the pediatric American Society for Metabolic and Bariatric Surgery guidelines removed the recommendation of achieving adult height or pubertal maturity before pursuing MBS, thereby eliminating younger age restrictions.12
In a recent policy statement, the American Academy of Pediatrics advocates for increased use of and access to MBS for pediatric patients and acknowledges insufficient evidence to support age-based limitations for eligibility.18,19 Evidence of the safety and effectiveness of MBS in young adolescents and children is increasing, but it is estimated that <1% of eligible pediatric patients undergo MBS for the treatment of severe obesity.14,16–22 Hesitancy to refer due to concerns of safety, impact on growth, ethical concerns with patient assent, and compliance with postoperative instructions is a critical barrier to treatment of severe obesity, resulting in less than half of primary care physicians considering referral for pediatric patients.23,24 Data on long-term outcomes after MBS in younger adolescent or preadolescent patients are limited, and outcomes in the youngest children have only been reported in case reports from outside the United States.21,25–28 Comparative studies of adult and adolescent outcomes after MBS reveal similar weight loss and superior comorbidity resolution in the adolescent population. To explore potential age-related differences in MBS outcomes between older and younger adolescents, we divided the Teen–Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study cohort into a younger and older group and compare weight loss, comorbidity change, nutritional deficiencies, and quality of life over 5 years.13,29
Methods
Teen-LABS is a multicenter prospective observational study in which 242 adolescents (≤19 years of age) were enrolled between 2007 and 2011. Participants underwent MBS between 2007 and 2012. Study details, research methods, diagnostic criteria, and data collection have previously been described and are available at ClinicalTrials.gov (identifier NCT00474318).17,30 Baseline data were collected within 30 days of MBS. Participants were then evaluated at 6 months, 12 months, and then annually, and outcomes were evaluated up to 5 years after surgery. During this time frame, 230 of the 242 (95%) participants remained as active study participants, completing 86% (1254 of 1452) of all postoperative research visits. Baseline demographic, anthropometric, and comorbidity data were obtained by trained study staff. Specifically, changes in 3 major comorbidities (hypertension, dyslipidemia, and type 2 diabetes), micronutrient status31 (ferritin, folate, vitamin B12, vitamin D, vitamin A, and parathyroid hormone), and quality-of-life metrics (Impact of Weight on Quality of Life [IWQOL] total score and 36-Item Short Form Health Survey (SF-36) physical and mental component scores) were evaluated. Because of the small number of participants who underwent laparoscopic adjustable gastric banding (n = 14), only those who underwent Roux-en-Y gastric bypass (RYGB) (n = 161) or vertical sleeve gastrectomy (n = 67) were analyzed.
The clinical care of participants was at the discretion of the clinicians at the centers where enrollment occurred and was not dictated by research protocol. Clinical care generally followed national guideline recommendations. Informed, written consent was obtained from caregivers and/or participants as appropriate on the basis of age at the time of enrollment. Reconsenting was done when each participant reached the age of 18. Study protocols, consents, and data and safety monitoring were approved by all institutional sites (Nationwide Children’s Hospital in Columbus, OH; Cincinnati Children’s Hospital Medical Center in Cincinnati, OH; Texas Children’s Hospital in Houston, TX; University of Pittsburgh Medical Center in Pittsburgh, PA; and Children’s of Alabama in Birmingham, AL) and an independent data and safety monitoring board before study initiation. Ongoing review of study performance and safety has been done by the data and safety monitoring board from 2007 to present day.
For this analysis, the Teen-LABS cohort was arbitrarily divided into 2 age groups: age 13 to 15 years and age 16 to 19 years at the time of surgery. Age group–specific categorical measures were presented as frequencies and percentages and were compared by using χ2 tests. Continuous variables were summarized by using means and SDs, and t tests were used to compare by age group. Baseline BMI was calculated as kilograms divided by height (meters) squared. Linear mixed models were used to compare the following outcomes by age group: percentage weight change from baseline, IWQOL total score, SF-36 physical composite score, and SF-36 mental composite score. Poisson mixed modeling with robust error variance was used to compare comorbidity (type 2 diabetes, hypertension, and dyslipidemia) remission and prevalence of micronutrient abnormalities by study cohort. For all models, the following variables were considered for inclusion in the final model: age group, study visit, age group by visit interaction, sex, race, and baseline caregiver education. Percentage BMI change, quality-of-life metrics, and comorbidity prevalence were adjusted for BMI. Remission of hypertension was adjusted for percentage BMI change, baseline hypertension medications, and baseline blood pressure. Remission of dyslipidemia was adjusted for percentage BMI change, baseline lipid medications, and baseline lipid levels. Remission of type 2 diabetes was adjusted for percentage BMI change, baseline diabetes medications, baseline hemoglobin A1c levels, and duration of disease. For each outcome, modeled estimates and 95% confidence intervals (CIs) were calculated by age group and study visit. These models addressed missing values by maximum likelihood estimation under the data missing at random assumption. Weight values from peripartum female participants were omitted from analyses if obtained between the second trimester and 6 months post partum. All statistical analyses were conducted by using SAS version 9.4 (SAS Institute, Inc, Cary, NC); all reported P values were 2 sided and considered statistically significant when <.05. No adjustments were made for multiple comparisons.
Results
Baseline Description of Participants
The overall cohort was predominantly female (75%) and white (72%) and had a mean baseline BMI of 52.6. Most participants underwent RYGB, as shown in Table 1. The younger cohort (n = 66) was 13 to 15 years of age at the time of surgery and had an average age of 15.1 ± 0.81 years and an average BMI of 53.1 ± 11. The older cohort (n = 162) was 16 to 19 years of age at the time of surgery and had an average age of 17.7 ± 1 years and an average BMI of 52.4 ± 9. In Table 1, we present baseline demographic and clinical characteristics by age grouping.
TABLE 1.
Baseline Characteristics by Age at Surgery
| Ages 13–15 y (n = 66) | Ages 16–19 y (n = 162) | P | |
|---|---|---|---|
| Age at surgery, mean (SD), y | 15.1 (0.81) | 17.7 (1.04) | <.001 |
| Female sex, % (n) | 72.7 (48) | 75.9 (123) | .61 |
| White, % (n) | 72.7 (48) | 71.6 (116) | .86 |
| Non-Hispanic, % (n) | 97.0 (64) | 91.4 (148) | .16 |
| Surgical procedure, % (n) | .14 | ||
| RYGB | 63.6 (42) | 73.5 (119) | — |
| VSG | 36.4 (24) | 26.5 (43) | — |
| Household income, % (n) | .31 | ||
| <$25 000 | 34.4 (22) | 39.6 (61) | — |
| $25 000–$74 999 | 34.4 (22) | 39.0 (60) | — |
| $75 000+ | 31.3 (20) | 21.4 (33) | — |
| Caregiver education attained | .98 | ||
| Less than high school | 10.8% (7) | 10.3% (16) | — |
| High school graduate | 30.8% (20) | 30.8% (48) | — |
| Some college | 41.5% (27) | 39.7% (62) | — |
| College graduate or higher | 16.9% (11) | 19.2% (30) | — |
| Wt, mean (SD), kg | 148.6 (31.88) | 148.9 (31.05) | .95 |
| BMI, mean (SD) | 53.1 (10.75) | 52.4 (8.79) | .66 |
| Systolic BP, mean (SD), mm Hg | 121.5 (12.53) | 126.8 (13.31) | .007 |
| Diastolic BP, mean (SD), mm Hg | 71.2 (8.96) | 75.4 (10.27) | .004 |
| Type 2 diabetes, % (n) | 10.6 (7) | 13.6 (22) | .54 |
| Hypertension, % (n) | 27.3 (18) | 37.1 (59) | .16 |
| Dyslipidemia, % (n) | 73.4 (47) | 77.0 (124) | .57 |
| IWQOL total score, mean (SD) | 64.1 (17.75) | 61.7 (18.16) | .37 |
| SF-36 physical composite score, mean (SD) | 44.8 (9.47) | 43.9 (8.12) | .49 |
| SF-36 mental composite score, mean (SD) | 50.0 (10.33) | 49.2 (10.34) | .59 |
Baseline demographics, anthropometrics, comorbidity status, and quality-of-life metrics for both age groups were similar, with the exception of age and BP, which were significantly different at P < .05. BP, blood pressure; VSG, vertical sleeve gastrectomy; —, not applicable.
Percentage Weight Loss Over 5 Years After Surgery
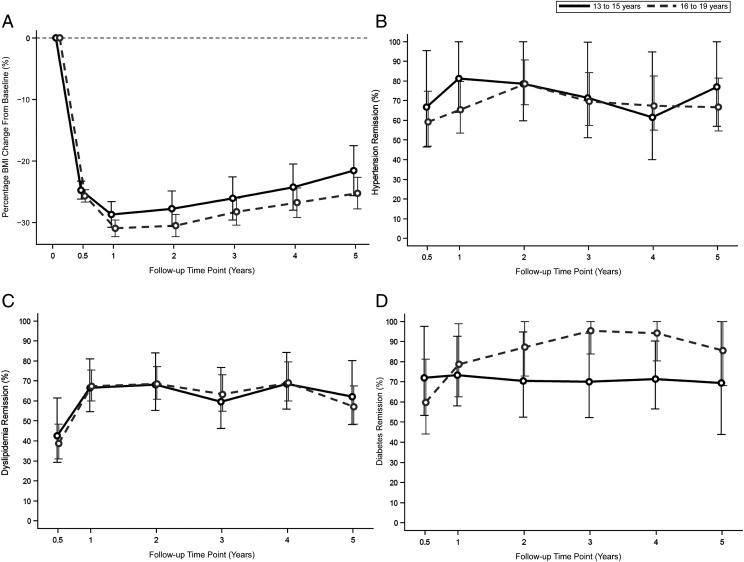
BMI significantly declined from baseline until 1 year post surgery (group by time interaction, P = .60; Fig 1), remained stable between years 1 and 2 (P = .16), and then similarly, but significantly, increased in both groups thereafter (P < .01). Five years after surgery, the percentage BMI change from baseline was similar between younger (−22.2%; 95% CI: −26.2% to −18.2%) and older (−24.6%; 95% CI: −27.7% to −22.5%) adolescents (P = .59).
FIGURE 1.
Percentage BMI change and comorbidity resolution over 5 years after MBS. A, Percentage BMI change from baseline. B, Remission of hypertension. C, Remission of dyslipidemia. D, Remission of type 2 diabetes mellitus. Younger adolescents were less likely to achieve remission of diabetes (P = .046). Error bars indicate 95% CIs.
Hypertension
Baseline prevalence of hypertension was 27% (n = 18) in younger adolescents and 37% (n = 59) in older adolescents (P = .16). At 5 years, remission of hypertension was achieved by 77% (95% CI: 57.1% to 100.0%) of younger adolescents and 67% (95% CI: 54.5% to 81.5%) of older adolescents (Fig 1). After adjustment, postoperative hypertension remission was similar by age group (P = .84). Five years after surgery, the incidence of hypertension in the younger group was 8% (n = 5), compared with 2% (n = 4) in the older group.
Dyslipidemia
Baseline prevalence of dyslipidemia was 73% (n = 47) in younger adolescents and 77% (n = 124) in older adolescents (P = .57). Five-year dyslipidemia remission was 61% (95% CI: 46.3% to 81.1%) among younger adolescents and 58% (95% CI: 48.0% to 68.9%) among older adolescents (Fig 1). Over the 5-year postoperative period, there was no significant difference in remission between age groups (P = .74). Incidence of dyslipidemia at 5 years occurred in 2% (n = 1) of younger adolescents and 4% (n = 6) of older adolescents.
Type 2 Diabetes Mellitus
Baseline prevalence of type 2 diabetes was 11% (n = 7) in younger adolescents and 14% (n = 22) in older adolescents (P = .54). Remission of type 2 diabetes was achieved by 83% (n = 6) of young adolescents and 87% (n = 15) of older adolescents by 5 years. Adjusted analyses revealed that over the 5-year postoperative period, younger adolescents were less likely to achieve remission compared with older adolescents (relative risk 0.86 [95% CI: 0.74 to 0.99]; P = .046). Five years after surgery, incidence type 2 diabetes was rare and present in 4.5% (n = 1) of older adolescents and 0% (n = 0) of younger adolescents.
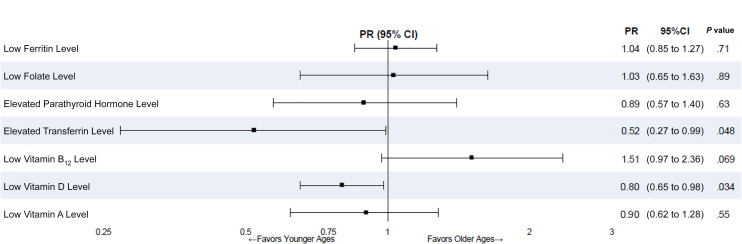
Nutritional Abnormalities
Compared with baseline, prevalence of abnormal ferritin, transferrin, vitamin B12, and vitamin A levels significantly increased by 5 years post surgery. However, over 5 years post surgery, only prevalence of elevated transferrin levels and vitamin D deficiency was significantly different by age group (Fig 2). Specifically, younger adolescents were less likely to have elevated transferrin levels (prevalence ratio [PR] 0.52 [95% CI 0.27 to 0.99]; P = .048) and low vitamin D levels (PR 0.80 [95% CI 0.65 to 0.98]; P = .034) compared with older adolescents.
FIGURE 2.
Micronutrient status over 5 years after MBS and age group associations with micronutrient abnormalities. Micronutrient status was similar over 5 years after surgery. Younger adolescents were less likely to have elevated transferrin (P = .048) or low vitamin D (P = .034) levels compared with older adolescents. Bars represent 95% CIs. Box symbols represent PRs.
Quality of Life
Quality-of-life measures evaluated in this study included the IWQOL total score by visit, the SF-36 physical composite score by visit, and the SF-36 mental composite score by visit (Supplemental Figs 3–5). For each measure, values significantly improved by 6 months (each P < .05) and remained similar thereafter. The trajectory of change for each measure did not differ by age group, nor were there any group differences observed.
Discussion
In this analysis, we identified similar improvement to percentage BMI loss, resolution of hypertension and dyslipidemia, and quality of life over 5 years after MBS between younger and older adolescents. We found a lower likelihood of nutritional deficiencies in younger adolescents. Diabetes remission was slightly higher, although statistically significant, in older youth. These results appear promising for the treatment of severe obesity in young patients; however, further controlled studies are needed to fully evaluate the timing of surgery and extended long-term durability.32 Alqahtani et al21 retrospectively reviewed MBS outcomes at a single center in patients aged >14 and ≤14 years and noted that although the older cohort had superior weight loss at 1 year after surgery, there were no significant differences at years 2 to 5 after surgery, and the groups had similar rates of comorbidity resolution, complications, and mortality. Therefore, this analysis confirms similar findings, in a slightly older age range, to those by Alqahtani et al21 within a multicenter prospective observational data set.
In this study, differences in percentage BMI loss were not identified between the older and younger groups. The average BMI in each cohort was 53, indicating late referral in both age groups. Alqahtani et al21 also had similar percentage BMI loss; however participants had a lower baseline BMI. Ability to achieve a normal BMI after MBS is related to baseline BMI; therefore, what may be more important for postoperative success is intervening at a lower BMI rather than a specific age.33,34 Surgical intervention at a lower BMI is particularly important because current medical treatments have limited success in resulting in weight loss or stabilization in youth with severe obesity.35 Increased duration of obesity is linked to increased mortality, cardiovascular risk, and risk of type 2 diabetes; therefore, the BMI requirements for pediatric patients with complications of obesity have been reduced to 120% of the 95th percentile for age, allowing for earlier intervention before surgery alone may become ineffective.36–39
Using MBS for the purpose of resolving or improving complications of obesity carries significant positive health benefits. This study revealed remission rates for hypertension and dyslipidemia in younger and older adolescents similar to those found by Alqahtani et al.21 Michalsky et al40 previously explored cardiovascular risk prevalence and reduction after MBS in the Teen-LABS cohort. Comparative studies between adults and adolescent patients reveal higher rates of comorbidity resolution after surgery in adolescents, likely due to shorter duration of disease.13,41–43 Resolution of cardiac risk factors with MBS is compelling because authors of previous studies have warned that childhood hypertension generally persists in adulthood and leads to a significant increase in cardiovascular disease.36,44,45 Extended long-term studies are needed to evaluate the full potential benefit of early cardiovascular risk reduction with MBS in pediatric patients; however, in adult studies, researchers estimate a near 50% risk reduction of myocardial infarction, 33% to 50% risk reduction of stroke, and >50% risk reduction of death after a cardiovascular event.46–48
The difference in remission of type 2 diabetes in younger and older adolescents was small, and with a sample size of only 7 individuals in the younger adolescent group, it would not be appropriate to provide any recommendation on the basis of these preliminary data. Patients with type 2 diabetes in the Teen-LABS study had an overall remission rate of 86% (95% CI: 85 to 100), comparable to similar MBS studies in adolescents.17,20,21,42,49 In a similar-sized group, Alqahtani et al21 found a higher baseline prevalence but similar rates of remission of type 2 diabetes in older adolescents compared with younger adolescents. The average age at type 2 diabetes diagnosis is 13.6 years in pediatric patients, presenting a significant long-term health burden and risk of early mortality.50,51 Despite more rapidly progressive β-cell failure in youth with type 2 diabetes, youth with type 2 diabetes have higher remission rates after MBS than adult patients undergoing bariatric surgery or those receiving maximum medical therapy.13,15,38,41,43,52,53 Improvement in glycemic control and insulin sensitivity after MBS leads to the reversal or stabilization of many diabetes complications.53 Patients with obesity and type 1 diabetes also benefit from MBS by reducing insulin requirements and improving complications of diabetes; however, glycemic control remains challenging in these individuals. Furthermore, MBS appears to prevent incident type 2 diabetes.54,55 As such, the authors do not feel that the minor statistical differences in this analysis warrant hesitation to pursue MBS in adolescents for the treatment of type 2 diabetes.
In addition to the medical complications of obesity, obesity also has detrimental effects on quality of life. In the current study, we identified a significant improvement in quality of life in both age groups after MBS. Long-term potential learning, earnings, and opportunity can be impacted by obesity bias, obesity-related cognitive or mental health impairments, and absenteeism secondary to chronic obesity-related health problems.56–63 Although no extended long-term studies decades after MBS in the pediatric populations exist to evaluate the impact of increased quality of life on school performance, occupational opportunity, earnings, and overall psychosocial health, it could be theorized that early intervention may limit the exposure risk of obesity and provide early referral to trained pediatric psychologists integrated into pediatric MBS programs and access to long-term mental health follow-up.64
As MBS has become recognized as the safest and most effective treatment of severe obesity in adults, it initially was recommended that surgery be delayed until patients could reach physical maturity, were of an age to consent to lifelong altering procedures, and to avoid the risk of a surgery or potential nutritional deficiencies. Here we demonstrate lower rates of the 2 most common nutritional deficiencies (vitamin D and iron) in younger adolescents and no differences in other nutritional deficiencies between groups. Thus, the risk of nutritional deficiency is not a reason to delay surgery in young adolescents. More recent studies have refuted common concerns regarding the safety of MBS in pediatric patients and have revealed increased linear growth after MBS.11,12,21,29,65,66 Arguably, the youngest patients are unable to fully understand the risks, benefits, and lifelong lifestyle changes that come with MBS. However, the alternative of delaying MBS and managing severe obesity with less effective therapies, exposing pediatric patients to insufficiently studied medications for obesity and to comorbidities, and increasing the exposure risk of obesity to the child’s health may provide an even greater ethical challenge.64,67 The morbidity and mortality of MBS has improved with time and is now comparable to that of a laparoscopic cholecystectomy.11 Early comparisons between adult and adolescent patients initially revealed a potentially higher complication rate in adolescents that may have been attributed to surgeon experience, case volume, or increased use of RYGB rather than patient age.13,43 However, more recent adolescent studies reveal lower rates of complications in comparison with adults and comparable complication rates in both younger and older pediatric patients.21,25,41,42 Family involvement is more common and necessary in the youngest patients to ensure compliance with supplements and access to healthy foods. Older adolescents are at risk for noncompliance as they transition to adulthood and self-independence, emphasizing the need for long-term follow-up for this unique population.31,68 It is possible that earlier initiation of postoperative habits and increased supervision in the younger cohort lead to the pattern of nutritional deficiencies seen in this study and may support a role for earlier intervention.
Limitations of this study include the observational nature of the study, lack of randomization, and potentially subtle differences in care or technique across the 5 participating institutions, although center effect was accounted for in the statistical models. However, Teen-LABS data were collected prospectively by using rigorous data collection methods. Some analyses were based on small sample sizes (eg, type 2 diabetes), and results should be interpreted with caution. Additionally, many studies of MBS, including this one, have a greater percentage of white and female patients, which limits the generalizability. Furthermore, lack of extended longitudinal studies decades after MBS in this population limits our current ability for fully assess the long-term risk/benefit ratio.
Conclusions
Obesity and duration of obesity are significant risk factors for early mortality, type 2 diabetes, cardiovascular events, multiple cancers, end-stage renal disease, end-stage liver disease, and decreased quality of life.5–8,46,69 MBS currently represents the most effective and durable, yet underused, treatment of severe obesity and complications of obesity in thoughtfully selected children.12,22,65,70 The findings in this study support the use of early intervention based on clinical indication rather than age alone, thereby providing the patient with the best opportunity to reach a normal BMI after surgery, promoting resolution of complications of obesity, and reducing the number of obese years in a child’s lifetime.
Acknowledgments
We thank the Teen-LABS study coordinators and data coordinating center staff for their ongoing dedication and expertise. We also thank the study participants for their long-term contributions, which have made this research project successful.
Glossary
- CI
confidence interval
- IWQOL
Impact of Weight on Quality of Life
- MBS
metabolic and bariatric surgery
- PR
prevalence ratio
- RYGB
Roux-en-Y gastric bypass
- SF-36
36-Item Short Form Health Survey
- Teen-LABS
Teen–Longitudinal Assessment of Bariatric Surgery
Footnotes
Dr Ogle conceptualized and designed the study, contributed to data interpretation, drafted the initial manuscript, and critically reviewed the manuscript for important intellectual content; Dr Dewberry conceptualized and designed the study and reviewed and revised the manuscript; Dr Jenkins designed the data collection instruments, collected data, conducted the analyses, contributed to data interpretation, and reviewed and revised the manuscript; Dr Inge conceptualized and designed the study, contributed to data interpretation, obtained funding for the study, and critically reviewed the manuscript for important intellectual content; Dr Kelsey contributed to data interpretation and critically reviewed the manuscript for important intellectual content; Dr Bruzoni conceptualized and designed the study, contributed to data interpretation, and reviewed and revised the manuscript; Dr Pratt conceptualized and designed the study, contributed to data interpretation, and critically reviewed the manuscript for important intellectual content; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Deidentified individual participant data (including data dictionaries) are available via the National Institute of Diabetes and Digestive and Kidney Diseases Central Repository (https://repository.niddk.nih.gov/home).
This trial has been registered at www.clinicaltrials.gov (identifier NCT00474318).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Funded by National Institute of Diabetes and Digestive and Kidney Diseases grants UM1DK072493 (University of Colorado) and UM1DK095710 (University of Cincinnati). The study was also supported by grants UL1TR000077-04 (Cincinnati Children’s Hospital Medical Center), UL1RR025755 (Nationwide Children’s Hospital), M01-RR00188 (Texas Children’s Hospital and Baylor College of Medicine), UL1RR024153 and UL1TR000005 (University of Pittsburgh), and UL1TR000165 (University of Alabama at Birmingham). The sponsors did not participate in the work presented. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Inge has served as a consultant for Zafgen Corporation, Biomedical Insights, and L&E Research and has received honoraria from Standard Bariatrics, UpToDate, and Independent Medical Expert Consulting Services, all unrelated to this project; the other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Milken Institute . Economic impact of excess weight now exceeds $1.7 trillion: costs include $1.24 trillion in lost productivity, according to study documenting role of obesity and overweight in chronic diseases. 2018. Available at: www.sciencedaily.com/releases/2018/10/181030163458.htm. Accessed May 10, 2020
- 2.World Health Organization. Obesity and overweight. 2020. Available at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed March 10, 2020
- 3.Centers for Disease Control and Prevention. Childhood obesity facts. 2019. Available at: https://www.cdc.gov/obesity/data/childhood.html. Accessed March 3, 2020
- 4.Fryar CD, Carroll MD, Ogden CL. Prevalence of overweight and obesity among children and adolescents aged 2–19 years: United States, 1963–1965 through 2013–2014. 2016. Available at: https://www.cdc.gov/nchs/data/hestat/obesity_child_13_14/obesity_child_13_14.htm. Accessed March 16, 2020
- 5.Browne AF, Inge T. How young for bariatric surgery in children? Semin Pediatr Surg. 2009;18(3):176–185 [DOI] [PubMed] [Google Scholar]
- 6.Inge TH, King WC, Jenkins TM, et al. The effect of obesity in adolescence on adult health status. Pediatrics. 2013;132(6):1098–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. 2003;289(2):187–193 [DOI] [PubMed] [Google Scholar]
- 8.Greenberg JA. Obesity and early mortality in the United States. Obesity (Silver Spring). 2013;21(2):405–412 [DOI] [PubMed] [Google Scholar]
- 9.Svetkey LP, Stevens VJ, Brantley PJ, et al.; Weight Loss Maintenance Collaborative Research Group . Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299(10):1139–1148 [DOI] [PubMed] [Google Scholar]
- 10.Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005;293(1):43–53 [DOI] [PubMed] [Google Scholar]
- 11.Beamish AJ, Reinehr T. Should bariatric surgery be performed in adolescents? Eur J Endocrinol. 2017;176(4):D1–D15 [DOI] [PubMed] [Google Scholar]
- 12.Pratt JSA, Browne A, Browne NT, et al. ASMBS pediatric metabolic and bariatric surgery guidelines, 2018. Surg Obes Relat Dis. 2018;14(7):882–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inge TH, Courcoulas AP, Jenkins TM, et al.; Teen–LABS Consortium . Five-year outcomes of gastric bypass in adolescents as compared with adults. N Engl J Med. 2019;380(22):2136–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inge TH, Coley RY, Bazzano LA, et al.; PCORnet Bariatric Study Collaborative . Comparative effectiveness of bariatric procedures among adolescents: the PCORnet bariatric study. Surg Obes Relat Dis. 2018;14(9):1374–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inge TH, Laffel LM, Jenkins TM, et al.; Teen–Longitudinal Assessment of Bariatric Surgery (Teen-LABS) and Treatment Options of Type 2 Diabetes in Adolescents and Youth (TODAY) Consortia . Comparison of surgical and medical therapy for type 2 diabetes in severely obese adolescents. JAMA Pediatr. 2018;172(5):452–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inge TH, Jenkins TM, Xanthakos SA, et al. Long-term outcomes of bariatric surgery in adolescents with severe obesity (FABS-5+): a prospective follow-up analysis. Lancet Diabetes Endocrinol. 2017;5(3):165–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inge TH, Courcoulas AP, Jenkins TM, et al.; Teen-LABS Consortium . Weight loss and health status 3 years after bariatric surgery in adolescents. N Engl J Med. 2016;374(2):113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armstrong SC, Bolling CF, Michalsky MP, Reichard KW; Section on Obesity; Section on Surgery . Pediatric metabolic and bariatric surgery: evidence, barriers, and best practices. Pediatrics. 2019;144(6):e20193223. [DOI] [PubMed] [Google Scholar]
- 19.Bolling CF, Armstrong SC, Reichard KW, Michalsky MP; Section on Obesity; Section on Surgery . Metabolic and bariatric surgery for pediatric patients with severe obesity. Pediatrics. 2019;144(6):e20193224. [DOI] [PubMed] [Google Scholar]
- 20.Olbers T, Beamish AJ, Gronowitz E, et al. Laparoscopic Roux-en-Y gastric bypass in adolescents with severe obesity (AMOS): a prospective, 5-year, Swedish nationwide study. [published correction appears in Lancet Diabetes Endocrinol. 2017;5(5):e3]. Lancet Diabetes Endocrinol. 2017;5(3):174–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alqahtani A, Elahmedi M, Qahtani AR. Laparoscopic sleeve gastrectomy in children younger than 14 years: refuting the concerns. Ann Surg. 2016;263(2):312–319 [DOI] [PubMed] [Google Scholar]
- 22.Perez NP, Westfal ML, Stapleton SM, et al. Beyond insurance: race-based disparities in the use of metabolic and bariatric surgery for the management of severe pediatric obesity. Surg Obes Relat Dis. 2020;16(3):414–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woolford SJ, Clark SJ, Gebremariam A, Davis MM, Freed GL. To cut or not to cut: physicians’ perspectives on referring adolescents for bariatric surgery. Obes Surg. 2010;20(7):937–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roebroek YGM, Talib A, Muris JWM, van Dielen FMH, Bouvy ND, van Heurn LWE. Hurdles to take for adequate treatment of morbidly obese children and adolescents: attitudes of general practitioners towards conservative and surgical treatment of paediatric morbid obesity. World J Surg. 2019;43(4):1173–1181 [DOI] [PubMed] [Google Scholar]
- 25.Alqahtani AR, Antonisamy B, Alamri H, Elahmedi M, Zimmerman VA. Laparoscopic sleeve gastrectomy in 108 obese children and adolescents aged 5 to 21 years. Ann Surg. 2012;256(2):266–273 [DOI] [PubMed] [Google Scholar]
- 26.Dan D, Harnanan D, Seetahal S, Naraynsingh V, Teelucksingh S. Bariatric surgery in the management of childhood obesity: should there be an age limit? Obes Surg. 2010;20(1):114–117 [DOI] [PubMed] [Google Scholar]
- 27.Baltasar A, Serra C, Bou R, Bengochea M, Andreo L. Sleeve gastrectomy in a 10-year-old child. Obes Surg. 2008;18(6):733–736 [DOI] [PubMed] [Google Scholar]
- 28.Muensterer OJ, Agha RA. Laparoscopic sleeve gastrectomy for a two-and a half year old morbidly obese child - a leap into the unknown. Int J Surg Case Rep. 2013;4(11):1055–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alqahtani AR, Elahmedi MO, Al Qahtani A. Co-morbidity resolution in morbidly obese children and adolescents undergoing sleeve gastrectomy. Surg Obes Relat Dis. 2014;10(5):842–850 [DOI] [PubMed] [Google Scholar]
- 30.Inge TH, Zeller MH, Jenkins TM, et al.; Teen-LABS Consortium . Perioperative outcomes of adolescents undergoing bariatric surgery: the Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study. JAMA Pediatr. 2014;168(1):47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xanthakos SA, Khoury JC, Inge TH, et al.; Teen Longitudinal Assessment of Bariatric Surgery Consortium . Nutritional risks in adolescents after bariatric surgery. Clin Gastroenterol Hepatol. 2020;18(5):1070–1081.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janson A, Järvholm K, Gronowitz E, et al. A randomized controlled trial comparing intensive non-surgical treatment with bariatric surgery in adolescents aged 13-16 years (AMOS2): rationale, study design, and patient recruitment. Contemp Clin Trials Commun. 2020;19:100592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inge TH, Jenkins TM, Zeller M, et al. Baseline BMI is a strong predictor of nadir BMI after adolescent gastric bypass. J Pediatr. 2010;156(1):103–108.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nickel F, de la Garza JR, Werthmann FS, et al. Predictors of risk and success of obesity surgery. Obes Facts. 2019;12(4):427–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryder JR, Fox CK, Kelly AS. Treatment options for severe obesity in the pediatric population: current limitations and future opportunities. Obesity (Silver Spring). 2018;26(6):951–960 [DOI] [PubMed] [Google Scholar]
- 36.Juonala M, Magnussen CG, Berenson GS, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365(20):1876–1885 [DOI] [PubMed] [Google Scholar]
- 37.Tirosh A, Shai I, Afek A, et al. Adolescent BMI trajectory and risk of diabetes versus coronary disease. N Engl J Med. 2011;364(14):1315–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdullah A, Stoelwinder J, Shortreed S, et al. The duration of obesity and the risk of type 2 diabetes. Public Health Nutr. 2011;14(1):119–126 [DOI] [PubMed] [Google Scholar]
- 39.Lee JM, Gebremariam A, Vijan S, Gurney JG. Excess body mass index-years, a measure of degree and duration of excess weight, and risk for incident diabetes. Arch Pediatr Adolesc Med. 2012;166(1):42–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michalsky MP, Inge TH, Jenkins TM, et al.; Teen-LABS Consortium . Cardiovascular risk factors after adolescent bariatric surgery. Pediatrics. 2018;141(2):e20172485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khidir N, El-Matbouly MA, Sargsyan D, Al-Kuwari M, Bashah M, Gagner M. Five-year outcomes of laparoscopic sleeve gastrectomy: a comparison between adults and adolescents. Obes Surg. 2018;28(7):2040–2045 [DOI] [PubMed] [Google Scholar]
- 42.Alqahtani A, Alamri H, Elahmedi M, Mohammed R. Laparoscopic sleeve gastrectomy in adult and pediatric obese patients: a comparative study. Surg Endosc. 2012;26(11):3094–3100 [DOI] [PubMed] [Google Scholar]
- 43.Benedix F, Krause T, Adolf D, et al.; Obesity Surgery Working Group, Competence Network Obesity . Perioperative course, weight loss and resolution of comorbidities after primary sleeve gastrectomy for morbid obesity: are there differences between adolescents and adults? Obes Surg. 2017;27(9):2388–2397 [DOI] [PubMed] [Google Scholar]
- 44.Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117(25):3171–3180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gunnell DJ, Frankel SJ, Nanchahal K, Peters TJ, Davey Smith G. Childhood obesity and adult cardiovascular mortality: a 57-y follow-up study based on the Boyd Orr cohort. Am J Clin Nutr. 1998;67(6):1111–1118 [DOI] [PubMed] [Google Scholar]
- 46.Kwok CS, Pradhan A, Khan MA, et al. Bariatric surgery and its impact on cardiovascular disease and mortality: a systematic review and meta-analysis. Int J Cardiol. 2014;173(1):20–28 [DOI] [PubMed] [Google Scholar]
- 47.Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307(1):56–65 [DOI] [PubMed] [Google Scholar]
- 48.Beamish AJ, Olbers T, Kelly AS, Inge TH. Cardiovascular effects of bariatric surgery. Nat Rev Cardiol. 2016;13(12):730–743 [DOI] [PubMed] [Google Scholar]
- 49.Vilallonga R, Himpens J, van de Vrande S. Long-term (7 years) follow-up of Roux-en-Y gastric bypass on obese adolescent patients (<18 years). Obes Facts. 2016;9(2):91–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosenbloom AL, Silverstein JH, Amemiya S, Zeitler P, Klingensmith GJ; International Society for Pediatric and Adolescent Diabetes . ISPAD clinical practice consensus guidelines 2006-2007. Type 2 diabetes mellitus in the child and adolescent. Pediatr Diabetes. 2008;9(5):512–526 [DOI] [PubMed] [Google Scholar]
- 51.Imperatore G, Boyle JP, Thompson TJ, et al.; SEARCH for Diabetes in Youth Study Group . Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes Care. 2012;35(12):2515–2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barrett T, Jalaludin MY, Turan S, Hafez M, Shehadeh N; Novo Nordisk Pediatric Type 2 Diabetes Global Expert Panel . Rapid progression of type 2 diabetes and related complications in children and young people-a literature review. Pediatr Diabetes. 2020;21(2):158–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stefater MA, Inge TH. Bariatric surgery for adolescents with type 2 diabetes: an emerging therapeutic strategy. Curr Diab Rep. 2017;17(8):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.El-Matbouly MA, Khidir N, Touny HA, El Ansari W, Al-Kuwari M, Bashah M. A 5-year follow-up study of laparoscopic sleeve gastrectomy among morbidly obese adolescents: does it improve body image and prevent and treat diabetes? Obes Surg. 2018;28(2):513–519 [DOI] [PubMed] [Google Scholar]
- 55.Carlsson LM, Peltonen M, Ahlin S, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med. 2012;367(8):695–704 [DOI] [PubMed] [Google Scholar]
- 56.Gortmaker SL, Must A, Perrin JM, Sobol AM, Dietz WH. Social and economic consequences of overweight in adolescence and young adulthood. N Engl J Med. 1993;329(14):1008–1012 [DOI] [PubMed] [Google Scholar]
- 57.Janssen I, Craig WM, Boyce WF, Pickett W. Associations between overweight and obesity with bullying behaviors in school-aged children. Pediatrics. 2004;113(5):1187–1194 [DOI] [PubMed] [Google Scholar]
- 58.Puhl R, Brownell KD. Bias, discrimination, and obesity. Obes Res. 2001;9(12):788–805 [DOI] [PubMed] [Google Scholar]
- 59.Gates DM, Succop P, Brehm BJ, Gillespie GL, Sommers BD. Obesity and presenteeism: the impact of body mass index on workplace productivity. J Occup Environ Med. 2008;50(1):39–45 [DOI] [PubMed] [Google Scholar]
- 60.Agranat-Meged AN, Deitcher C, Goldzweig G, Leibenson L, Stein M, Galili-Weisstub E. Childhood obesity and attention deficit/hyperactivity disorder: a newly described comorbidity in obese hospitalized children. Int J Eat Disord. 2005;37(4):357–359 [DOI] [PubMed] [Google Scholar]
- 61.Loux TJ, Haricharan RN, Clements RH, et al. Health-related quality of life before and after bariatric surgery in adolescents. J Pediatr Surg. 2008;43(7):1275–1279 [DOI] [PubMed] [Google Scholar]
- 62.Zeller MH, Roehrig HR, Modi AC, Daniels SR, Inge TH. Health-related quality of life and depressive symptoms in adolescents with extreme obesity presenting for bariatric surgery. Pediatrics. 2006;117(4):1155–1161 [DOI] [PubMed] [Google Scholar]
- 63.Pearce AL, Mackey E, Cherry JBC, et al. Effect of adolescent bariatric surgery on the brain and cognition: a pilot study. Obesity (Silver Spring). 2017;25(11):1852–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stroud AM, Parker D, Croitoru DP. Timing of bariatric surgery for severely obese adolescents: a Markov decision-analysis. J Pediatr Surg. 2016;51(5):853–858 [DOI] [PubMed] [Google Scholar]
- 65.Michalsky M, Reichard K, Inge T, Pratt J, Lenders C; American Society for Metabolic and Bariatric Surgery . ASMBS pediatric committee best practice guidelines. Surg Obes Relat Dis. 2012;8(1):1–7 [DOI] [PubMed] [Google Scholar]
- 66.Poliakin L, Roberts A, Thompson KJ, Raheem E, McKillop IH, Nimeri A. Outcomes of adolescents compared with young adults after bariatric surgery: an analysis of 227,671 patients using the MBSAQIP data registry. Surg Obes Relat Dis. 2020;16(10):1463–1473 [DOI] [PubMed] [Google Scholar]
- 67.Caniano DA. Ethical issues in pediatric bariatric surgery. Semin Pediatr Surg. 2009;18(3):186–192 [DOI] [PubMed] [Google Scholar]
- 68.Sawhney P, Modi AC, Jenkins TM, et al. Predictors and outcomes of adolescent bariatric support group attendance. Surg Obes Relat Dis. 2013;9(5):773–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Upadhyay J, Farr O, Perakakis N, Ghaly W, Mantzoros C. Obesity as a disease. Med Clin North Am. 2018;102(1):13–33 [DOI] [PubMed] [Google Scholar]
- 70.Griggs CL, Perez NP Jr., Goldstone RN, et al. National trends in the use of metabolic and bariatric surgery among pediatric patients with severe obesity. JAMA Pediatr. 2018;172(12):1191–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]