Abstract
Rationale: Many lung transplant centers prescribe antifungal medications after transplantation to prevent invasive fungal infections (IFIs); however, the effectiveness of antifungal prophylaxis at reducing the risk of all-cause mortality or IFI has not been established.
Objectives: We aimed to evaluate the effect of antifungal prophylaxis on all-cause mortality and IFI in lung transplant patients.
Methods: Using administrative claims data, we identified adult patients who underwent lung transplantation between January 1, 2005, and December 31, 2018. Propensity score analysis using inverse probability treatment–weighting approach was used to balance the differences in baseline characteristics between those receiving antifungal prophylaxis and those not receiving antifungal prophylaxis. Cox proportional hazards regression was used to compare rates of all-cause mortality and IFI in both groups.
Results: We identified 662 lung transplant recipients (LTRs) (387 received prophylaxis and 275 did not). All-cause mortality was significantly lower in those receiving antifungal prophylaxis compared with those not receiving antifungal prophylaxis (event rate per 100 person-years, 8.36 vs. 19.49; hazard ratio, 0.43; 95% confidence interval, 0.26–0.71; P = 0.003). Patients receiving antifungal prophylaxis had a lower rate of IFI compared with those not receiving prophylaxis (event rate per 100 person-years, 14.94 vs. 22.37; hazard ratio, 0.68; 95% confidence interval, 0.44–1.05; P = 0.079), but did not reach statistical significance.
Conclusions: In this real-world analysis, antifungal prophylaxis in LTRs was associated with reduced all-cause mortality compared with those not receiving antifungal prophylaxis. Rates of IFI were also lower in those receiving prophylaxis, but this was not statistically significant in our primary analysis.
Keywords: lung transplant, antifungal prophylaxis, fungal infections, Aspergillus, triazole
Lung transplant recipients (LTRs) are particularly susceptible to invasive fungal infections (IFIs) secondary to reduced mucociliary clearance and altered alveolar macrophage phagocytic function (1). Despite appropriate treatment, IFIs in LTRs are associated with a nearly threefold increase in all-cause mortality (2–4). Therefore, prevention of IFIs using antifungal prophylaxis is an increasingly common practice. The proposed benefit of antifungal prophylaxis in LTRs is largely extrapolated from studies involving patients with hematologic malignancies (5–7). No prospective studies have been conducted to establish a benefit of antifungal prophylaxis in LTRs. Although many transplant centers have reported their experiences with various prophylactic strategies in the form of observational cohort studies (8–16), a recent comprehensive systematic review and meta-analysis of these studies cited insufficient evidence to establish a benefit or to endorse a specific prophylactic strategy, antifungal medication, or duration of prophylaxis (17). Protracted use of antifungal medications introduces the possibility of severe adverse consequences, including cardiomyopathy, prolonged QTc, skin cancer, periostitis, and liver dysfunction, among others (18, 19). Moreover, triazole antifungal medications are expensive and interact with immunosuppressive agents that have a narrow therapeutic index, necessitating close, serial monitoring.
Despite ongoing clinical equipoise in this population, surveys of lung transplant practices demonstrate an increasing endorsement of universal antifungal prophylaxis (20, 21). In a survey published by Neoh and colleagues in 2011, 58.6% of transplant centers surveyed (worldwide) endorsed universal prophylaxis (21). In our study published in 2019, 90% of United States’ transplant centers routinely prescribed antifungal medications after lung transplant to prevent IFI (20). However, real-world confirmation of universal antifungal prophylaxis uptake is lacking. In addition, prophylactic strategies vary considerably regarding specific antifungal medication and duration of prophylaxis (20).
Given the practice variation, potential for toxicity, excessive costs, and drug interactions, it is imperative to establish whether or not antifungal prophylaxis is beneficial in LTRs. These studies have been historically limited by feasibility issues. One tool that has been used to assess pharmacologic effectiveness questions in other clinical domains is large claims databases, such as OptumLabs Data Warehouse (OLDW) (22). To date, real-world U.S. claims data have not been routinely used to answer clinically important questions in lung transplant. However, the ability to identify a large cohort of LTRs, control for potential confounders, identify medication fills, and follow the cohort longitudinally makes this approach particularly attractive to attempt to address the question of utility of antifungal prophylaxis. In this study, we aim to assess the risk of all-cause mortality and IFI in LTRs receiving antifungal prophylaxis compared with those not receiving antifungal prophylaxis using individuals enrolled in commercial and Medicare Advantage health plans in the United States.
Methods
Data Source
We performed a retrospective cohort study using deidentified administrative claims data with demographic and death information from OLDW, which contains medical and pharmacy claims and enrollment records for commercial and Medicare Advantage enrollees. The database contains longitudinal health information on enrollees and patients, representing a diverse mixture of ages, ethnicities, and geographical regions across the United States (23). The advantage of the OLDW is that it provides data on prescriptions that were actually filled and outcomes for LTR in geographically and demographically diverse populations. In accordance with the Health Insurance Portability and Accountability Act, the use of preexisting, deidentified claims data is exempted from institutional review board review.
Study Population
Inclusion criteria
The cohort included adult patients (≥18 yr) in OLDW who underwent single or double lung transplant or concurrent heart–lung transplant between January 1, 2005, and December 31, 2018, as defined by the presence of a lung transplant procedure code. Eligible procedure codes using the International Classification of Diseases, Ninth Revision (ICD-9); International Classification of Diseases, Tenth Revision (ICD-10); and current procedural terminology (CPT) included ICD-9 33.5, 33.6; ICD-10 0BYxxxx; and CPT 32851, 32852, 32853, 32854, and S2060. Patients were required to have at least 90 days of continuous health plan coverage before lung transplant surgery to capture each patient’s medical history.
Exclusion criteria
Retransplantations were excluded by removing patients who had multiple hospital encounters with transplant procedure codes. Patients with same-day hospital admission and discharge dates were considered to be aborted transplant procedures (so called “dry runs”) and were therefore not included. Patients with pre–lung transplant claims that included a code for IFI (in any diagnosis position, as defined below), indicating a patient eligible for antifungal treatment in lieu of prophylaxis, were excluded. Patients with antifungal medication fills 90 days before transplant surgery were excluded.
Independent Variables
Variables to describe medical history were defined by the presence of a claim with eligible diagnosis codes, procedure codes, or prescription fills. The absence of these claims was interpreted as the absence of the condition or prescription.
Independent variables of interest included sex, age, race and ethnicity, census region, single versus double lung transplant, indication for transplant, and comorbidities. ICD-9 and ICD-10 codes defined the specific chronic respiratory diseases to identify the indication for transplant and could be found in any diagnosis position on a claim during the lung transplant stay. The indication categories were defined using a combination of clinical groupings from the Agency for Healthcare Research and Quality Clinical Classification Software (CCS) tool (https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp) and individual ICD-9/ICD-10 codes. In the event that a patient had codes for more than one indication category, a hierarchy was applied, whereby the most probable diagnosis was considered the primary reason for transplant. The hierarchy was as follows, from most probable to least probable: 1) cystic fibrosis (CCS 56), 2) idiopathic pulmonary fibrosis (IPF) (ICD-9 516.31; ICD-10 J84.112), 3) non-IPF interstitial lung disease (ILD) (CCS 132, 211, and 234; ICD-9 516.3 and 515), 4) chronic obstructive pulmonary disease and bronchiectasis (CCS 127), 5) pulmonary hypertension, respiratory distress syndrome, and other (CCS 2,602; ICD-9 416.0; ICD-10 I27.0 and I27.21). Comorbidities were captured by ICD-9 and ICD-10 codes on claims in any position in the 90 days before the admission date for the transplant procedure. Elixhauser sum of conditions was used to assess comorbidity burden (24).
Exposure
Antifungal prescription fills within 7 days of post-transplant hospital discharge were identified as prophylactic treatment to accurately capture prophylaxis, minimize prescription fills to treat fungal infections, and preserve sample size. Antifungal medications included fluconazole, itraconazole, posaconazole, voriconazole, isavuconazonium, amphotericin B deoxycholate, and liposomal amphotericin. In the absence of any IFI diagnosis codes, prescription fills for amphotericin B deoxycholate and liposomal amphotericin were assumed to be nebulized. The end of supply for the last consecutive antifungal prescription filled was considered the end of prophylaxis, defining consecutive fills as those occurring within 30 days of each prescription fill’s supply days. Patients were categorized by the dominant antifungal prescribed. The dominant antifungal was defined as the antifungal prescription that the patient received for the longest duration.
Outcomes
The primary outcome was 1-year all-cause mortality with secondary outcomes of IFI and antifungal prophylaxis uptake. Mortality was identified based on the Social Security Death Master File and supplemented by hospital discharge status to augment capture rates of inpatient deaths (22, 25). IFI was defined as any diagnosis of posthospital lung transplant IFI during a clinic encounter, emergency department visit, or inpatient stay. Relevant posthospital lung transplant IFI included aspergillosis (ICD-9 117.3 and 484.6; ICD-10 B44.0, B44.1, B44.7, and B44.9), cryptococcosis (ICD-9 117.5; ICD-10 B45.0, B45.7, and B45.9), zygomycosis (ICD-9 117.7; ICD-10 B46.0, B46.4, B46.5, B46.8, and B46.9), coccidioidomycosis (ICD-9 114.0, 114.1, 114.2, and 114.3; ICD-10 B38.0, B38.3, B38.4, and B38.7), and histoplasmosis (ICD-9 115.xx; ICD-10 B39.0, B39.2, B39.3, B39.4, B39.5, and B39.9). Follow-up started from the day after lung transplantation and continued until the end of the study period (March 31, 2019), the end of enrollment in health insurance plans, or death (whichever occurred first).
Statistical Analysis
To protect patient confidentiality and in accordance with OptumLabs restrictions, any event occurring in 11 or fewer participants was masked. Primary analysis was conducted using propensity score weighting (an overlap weight). Specifically, we used weight of 1/propensity score for patients receiving prophylaxis and 1/(1 − propensity score) for patients not receiving prophylaxis (26). A propensity score was estimated using logistic regression based on sex, age, race/ethnicity, census region, year of admission for transplant encounter, single versus double lung transplant, Elixhauser comorbidity index score, and indication for transplant. Combined heart–lung transplant was considered equivalent to double lung transplant for propensity scoring.
Event rates are reported as events per 100 person-years. Cox proportional hazards regression was then used to compare patients receiving and not receiving antifungal prophylaxis for IFI and mortality in the propensity-matched cohort with robust sandwich estimates to account for clustering within matched sets (27). Using the same weighted cohort and statistical methods, we performed a subgroup analysis of patients receiving mold-active triazole (i.e., itraconazole, voriconazole, posaconazole, or isavuconazole) for prophylaxis versus no prophylaxis. P < 0.05 was considered statistically significant. All analyses were conducted using SAS Enterprise Guide 7.1 (SAS Institute Inc.) and Stata version 15.1 (StataCorp).
Sensitivity Analysis
Analysis was repeated with propensity score matching using 1:1 nearest neighbor caliper matching to match patients on the basis of the logit of the propensity score using a caliper equal to 0.2 of the standard deviation of the logit propensity (28). The balance of covariates after matching was assessed using standardized difference, and a standardized difference of <20% was acceptable (29).
We performed the analysis by testing two falsification endpoints (myocardial infarction and fracture) to test for residual confounding (30). These endpoints were chosen because they are unlikely to be related to antifungal prophylaxis exposure.
Results
Baseline Characteristics
A total of 662 LTRs met the inclusion criteria. Table 1 summarizes cohort characteristics. Of these, 387 (58.5%) received antifungal prophylaxis, and 275 (41.5%) did not. The mean follow-up was 283.6 (standard deviation 111.4) days after lung transplantation. The indication for transplant was similar for those receiving antifungal prophylaxis and those who did not. Over half of the cohort had ILD; approximately one-third had non-IPF ILD and one-quarter had IPF. Most patients received bilateral lung transplants.
Table 1.
Baseline characteristics of the preweighted and prematched cohort
| No Prophylaxis (N = 275) | Prophylaxis (N = 387) | Standardized Difference | |
|---|---|---|---|
| Age, yr | 55.3 (13.4) | 55.0 (12.7) | 0.026 |
| Sex | |||
| F | 115 (41.8) | 162 (41.9) | 0.001 |
| M | 160 (58.2) | 225 (58.1) | 0.001 |
| Region | |||
| Midwest | 77 (28.0) | 85 (22.0) | 0.140 |
| Northeast | 41 (14.9) | 31 (8.0) | 0.218 |
| South | 140 (50.9) | 208 (53.7) | 0.057 |
| West | 17 (6.2) | 63 (16.3) | 0.324 |
| Race/ethnicity | |||
| Black | 15 (5.5) | 21 (5.4) | 0.001 |
| Hispanic | 25 (9.1) | 23 (5.9) | 0.120 |
| Other/unknown | 52 (18.9) | 68 (17.6) | 0.035 |
| White | 183 (66.5) | 275 (71.1) | 0.098 |
| Elixhauser score | 3.6 (2.3) | 3.6 (2.4) | 0.014 |
| Indication for transplant | |||
| COPD and bronchiectasis | 50 (18.2) | 82 (21.2) | 0.076 |
| Cystic fibrosis | 45 (16.4) | 51 (13.2) | 0.090 |
| IPF | 64 (23.3) | 95 (24.5) | 0.030 |
| Non-IPF interstitial lung disease | 101 (36.7) | 142 (36.7) | 0.001 |
| Other | 15 (5.5) | 17 (4.4) | 0.049 |
| Single vs. double transplant | |||
| Single transplant | 84 (30.5) | 118 (30.5) | 0.001 |
| Double transplant | 191 (69.5) | 269 (69.5) | 0.001 |
| Year of admission for transplant | |||
| 2005–2007 | 53 (19.3) | 54 (14.0) | 0.143 |
| 2008–2010 | 55 (20.0) | 55 (14.2) | 0.154 |
| 2011–2014 | 78 (28.4) | 101 (26.1) | 0.051 |
| 2015–2018 | 89 (32.4) | 177 (45.7) | 0.277 |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; IPF = idiopathic pulmonary fibrosis.
Age and Elixhauser score are presented as means with standard deviation parenthetically. The remainder of data are presented as absolute n (%).
Antifungal Medications
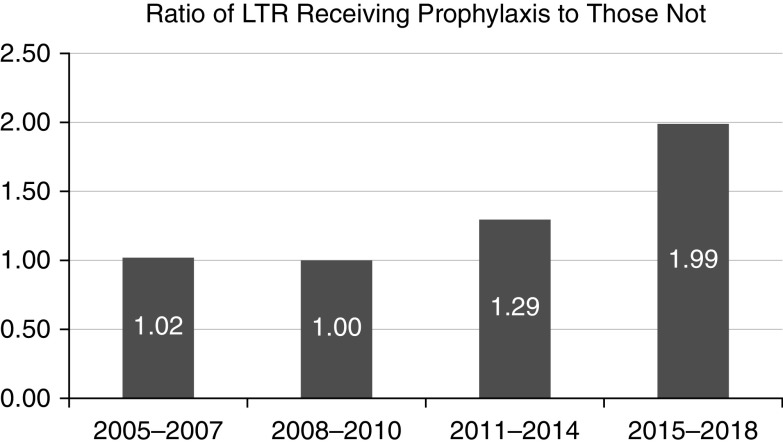
The most common medications used for prophylaxis were voriconazole (35.47%) and itraconazole (32.8%) (Table 2). Nebulized amphotericin, fluconazole, and either isavuconazole or posaconazole were used in approximately 10% of patients each. The ratio of LTRs receiving antifungal prophylaxis compared with those receiving no prophylaxis increased over time (1.02 in 2005–2007 compared with 1.99 in 2015–2018) (Figure 1). The median duration of antifungal prophylaxis was 133.0 (interquartile range, 63.0–246.0) days.
Table 2.
Antifungal medications used for prophylaxis in the unmatched and matched cohorts presented as the absolute number with percentage of cohort indicated parenthetically
| Unmatched Cohort (n = 387) | Matched Cohort (n = 232) | |
|---|---|---|
| Amphotericin | 38 (9.8) | 29 (12.5) |
| Fluconazole | 43 (11.1) | 22 (9.5) |
| Isavuconazole and posaconazole | 41 (10.6) | 16 (6.9) |
| Itraconazole | 127 (32.8) | 77 (33.2) |
| Voriconazole | 138 (35.7) | 88 (37.9) |
Amphotericin represents nebulized amphotericin B deoxycholate and liposomal amphotericin. Isavuconazole and posaconazole are presented together to mask cohort sizes <11.
Data are presented as n (%).
Figure 1.
Ratio of lung transplant recipients receiving antifungal prophylaxis to those not receiving prophylaxis over time. LTR = lung transplant recipient.
All-Cause Mortality
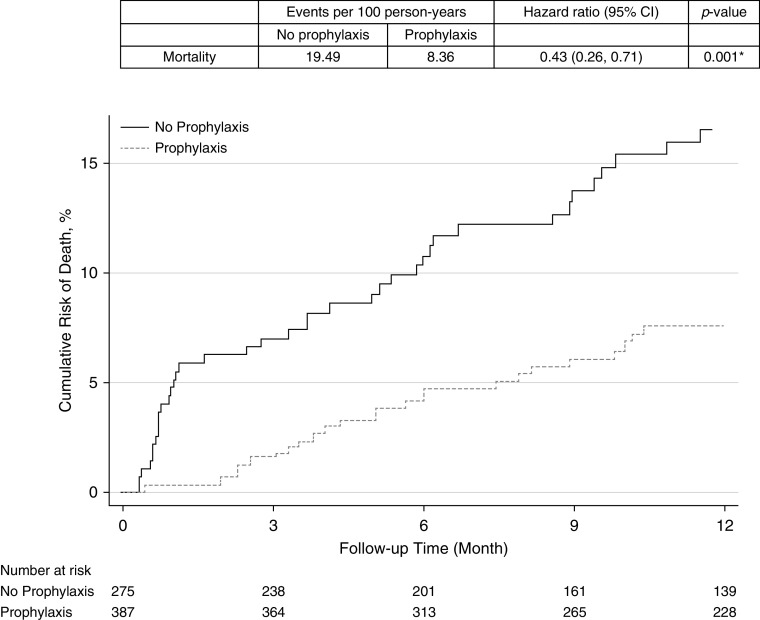
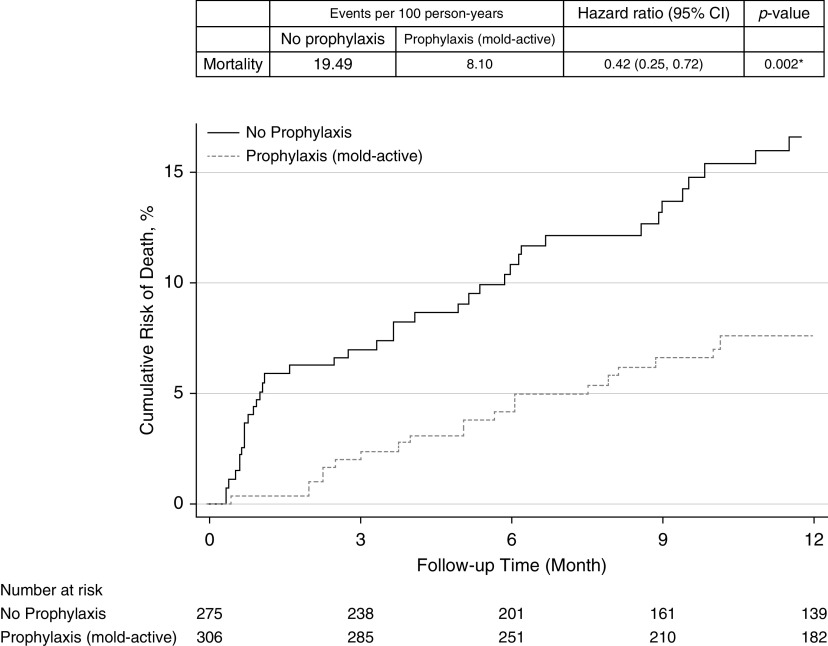
There were 65 deaths in our cohort. All-cause 1-year mortality was significantly lower in those receiving antifungal prophylaxis compared with those not receiving prophylaxis (event rate per 100 person-years 8.36 vs. 19.49; hazard ratio [HR], 0.48; 95% confidence interval [CI], 0.26–0.71; P = 0.001) (Figure 2). All-cause mortality was also significantly lower in those specifically receiving mold-active triazole prophylaxis compared with those not receiving it (event rate per 100 person-years 8.10 vs. 19.49; HR, 0.42; 95% CI, 0.25–0.72; P = 0.002) (Figure 3).
Figure 2.
Cumulative risk for mortality in lung transplant recipients receiving antifungal prophylaxis compared with those receiving no antifungal prophylaxis. CI = confidence interval.
Figure 3.
Cumulative risk for mortality in lung transplant recipients receiving antifungal prophylaxis with mold-active triazole compared with those receiving no antifungal prophylaxis. CI = confidence interval.
IFIs
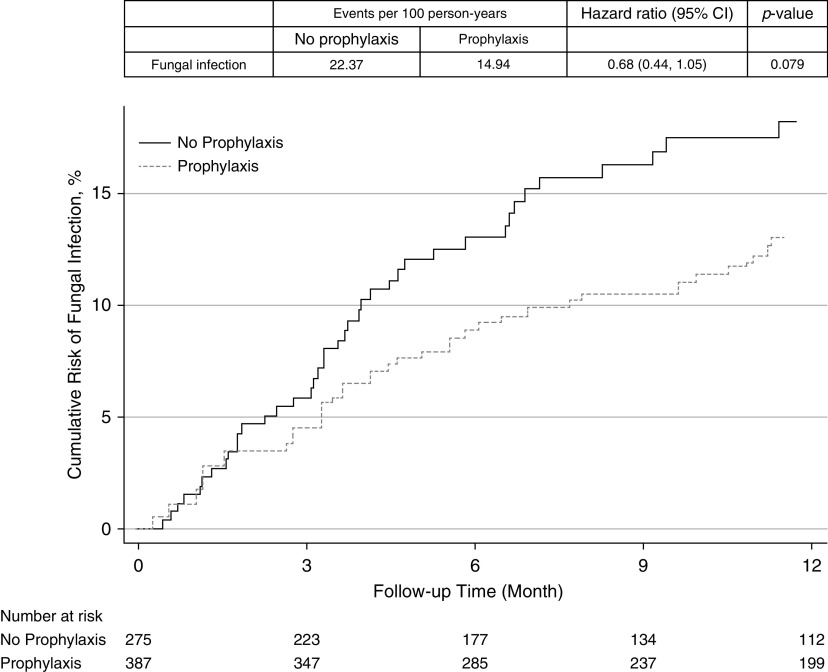
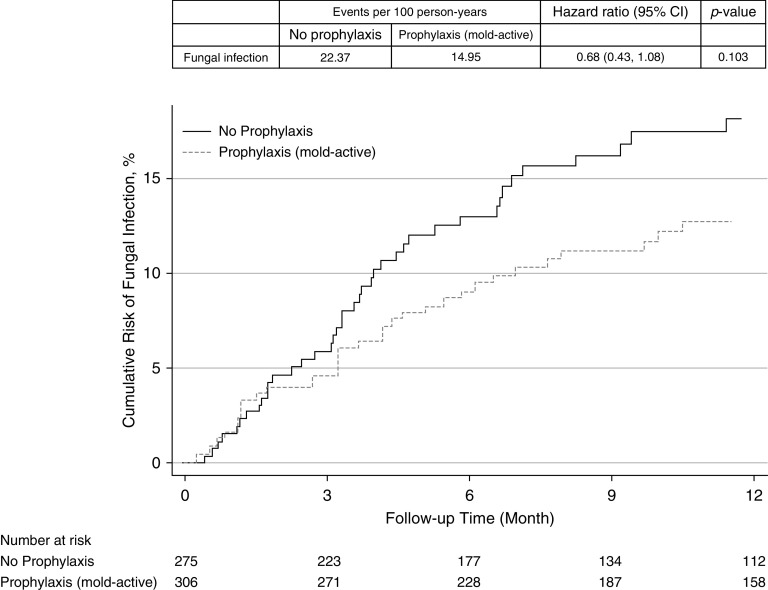
Patients receiving antifungal prophylaxis had a lower rate of IFI compared with those not receiving prophylaxis (event rate per 100 person-years 14.94 vs. 22.37; HR, 0.68; 95% CI, 0.45–1.05; P = 0.079), but this only reached statistical significance (Figure 4). Aspergillus species infections accounted for 92.0% and 82.0% of the infections in the nonprophylaxis and prophylaxis groups, respectively. Those specifically receiving mold-active triazole prophylaxis also had a lower incidence of IFI compared with those not receiving mold-active triazole prophylaxis (event rate per 100 person-years 14.95 vs. 22.37; HR, 0.68; 95% CI, 0.43–1.08; P = 0.103), but this did not reach statistical significance (Figure 5). When evaluating mold infections as the outcome, those on mold-active prophylaxis had a lower incidence of IFI compared with those not receiving mold-active triazole prophylaxis (event rate per 100 person-years 20.53 vs. 13.05; HR, 0.65; 95% CI, 0.40–1.05; P = 0.080), but this also did not reach statistical significance (Figure E1 in the online supplement).
Figure 4.
Cumulative risk for fungal infection in lung transplant recipients receiving antifungal prophylaxis compared with those receiving no antifungal prophylaxis. CI = confidence interval.
Figure 5.
Cumulative risk for fungal infections in lung transplant recipients receiving antifungal prophylaxis with a mold-active triazole agent compared with those receiving no antifungal prophylaxis. CI = confidence interval.
Sensitivity Analysis
The propensity-matched cohort included 232 matched pairs of LTRs receiving antifungal prophylaxis or not receiving antifungal prophylaxis. Baseline characteristics were balanced between the two groups, with standardized differences of <20% (Table E1). The results of the analysis were consistent using propensity score 1:1 matching for both outcomes of overall mortality (event rate per 100 person-years prophylaxis cohort 9.65 vs. nonprophylaxis cohort 18.14; HR, 0.54; 95% CI, 0.30–0.95; P = 0.034) and IFI (event rate per 100 person-years prophylaxis cohort 13.16 vs. nonprophylaxis cohort 23.81; HR, 0.56; 95% CI, 0.33–0.94; P = 0.028) (Table E2).
Falsification Endpoint Analysis
There was no evidence of residual confounding by our falsification endpoint analysis. There was no significant relationship between antifungal prophylaxis and myocardial infarction (event rate per 100 person-years prophylaxis cohort 0.58 vs. nonprophylaxis cohort 1.31; HR, 0.43; 95% CI, 0.12–1.56; P = 0.201) or fracture (event rate per 100 person-years prophylaxis cohort 3.20 vs. nonprophylaxis cohort 3.98; HR, 0.77; 95% CI, 0.41–1.47; P = 0.432) (Table E3).
Discussion
This is the first study using administrative claims data to evaluate outcomes in lung transplant and is the largest study, to date, evaluating the effectiveness of antifungal prophylaxis in LTRs. We found a significant reduction in all-cause mortality in LTRs receiving any antifungal prophylaxis compared with no prophylaxis. LTR snot receiving antifungal prophylaxis had a mortality event rate more than twice that of the group receiving antifungal prophylaxis (19.5 vs. 8.4). Prior systematic reviews and meta-analyses of single-center cohort studies have reported the current literature insufficient to demonstrate antifungal prophylaxis benefit in LTRs (17, 31). Our findings are consistent with several prior studies in hematologic malignancies and bone marrow transplant (BMT) populations that suggest a reduction in mortality with antifungal prophylaxis (5–7).
Prior survey studies have demonstrated widespread use of antifungal prophylaxis in the United States. Although a recent U.S. practice survey suggested 90% of transplant centers use universal antifungal prophylaxis in their practices (20), the uptake in the current study was lower at ∼58% and more consistent with the survey conducted by Neoh and colleagues a decade ago (21). This could be because we saw an increase in the proportion of patients receiving antifungal prophylaxis over time, nearly doubling during the course of the study. It is interesting, however, that the uptake of ∼66% in the most recent years of the study is still far below the reported rates from the surveyed transplant centers. The reason for this discrepancy could be response bias on the survey (i.e., providers giving the perceived desirable response regardless of practice habits). However, further examination of patient factors (e.g., nonadherence) and provider factors (e.g., knowledge gaps) possibly contributing to this discrepancy should be explored.
The tendency toward more universal prophylaxis and broader-spectrum agents has also been described on survey studies (20, 21). This may be secondary to the increased availability of oral antifungal medications. The types of medications used for antifungal prophylaxis in our cohort were similar to those reported by transplant centers, with the most common medications being itraconazole and voriconazole and a minority of patients receiving fluconazole, posaconazole, or isavuconazole (20). Whereas itraconazole and voriconazole were available for the duration of the study, agents such as isavuconazole and posaconazole are relatively newer. The U.S. Food and Drug Administration approved posaconazole in 2006, but the delayed-release tablets became available in 2013. Isavuconazole was not approved until 2015. Recent data from our practice demonstrated that voriconazole was more likely to be discontinued because of side effects, whereas itraconazole was more likely to be discontinued because of breakthrough infections or lack of absorption (19). It will be interesting to follow the uptake trends of these newer agents in future studies.
Because invasive Aspergillus species infections are the most common IFI in LTRs (3, 8, 12), as demonstrated in our cohort, and because there has been concern with systemic anti-Aspergillus prophylaxis selecting for resistant organisms (32), we completed a subgroup analysis to evaluate the effect of anti-Aspergillus prophylaxis on mortality. All-cause mortality remained significantly lower in LTRs receiving systemic anti-Aspergillus medications (i.e., itraconazole, voriconazole, posaconazole, and isavuconazole). Our study cannot definitively endorse one antifungal prophylaxis agent or regimen over another regimen; however, given that use of a mold-active triazole was associated with a 50% reduction in mortality, this is the strongest evidence to date for practice standardization to include at least a mold-active triazole after transplant. The duration of antifungal prophylaxis cannot be delineated from our study; however, the median duration in this study was a little less than 5 months.
In contrast to hematologic malignancy and BMT populations, LTRs have both immunologic and anatomic reasons for increased susceptibility to IFIs. Moreover, although the increased susceptibility to IFIs is temporary in hematologic malignancies and BMT, LTRs remain at increased risk for IFIs (4, 8). In our study, IFI was lower in LTR receiving prophylaxis, but this did not reach statistical significance (likely secondary to insufficient statistical power) in our primary analysis. However, this reduction in IFI was statistically significant in the propensity score–matched analysis. It is unknown whether some IFI events were missed in our study because of physicians using less-specific diagnostic coding during the IFI or because of cases discovered postmortem. However, it is reassuring that the cumulative incidence of IFI in LTRs in our cohort was consistent with that reported in previous cohorts (2, 8, 12). Moreover, Aspergillus species infection was the most common IFI in our cohort. This is consistent with the experiences described in the literature (3, 8, 12). Interestingly, the most common IFI did not vary between those receiving antifungal prophylaxis and those who did not.
Limitations
The primary limitation of any observational study is the possibility for uncontrolled confounding. Because of the lack of relevant data in the deidentified cohort, we could not account for additional risk factors such as intensification of immunosuppressive regimens, pretransplant colonization, or high-risk occupations. We did control for known confounding variables such as sex, age, race/ethnicity, region of the country, year of transplant, primary diagnosis, type of transplant (single vs. double or heart/lung), and comorbidities. We also tested for the presence of uncontrolled confounding using falsification endpoint analysis. Therefore, we are reasonably confident we have adequately controlled for confounding variables, but we are unable to report on the effects of induction immunosuppression and immunosuppression intensification.
An advantage of the OLDW is the enhanced racial, ethnic, and geographic diversity of the included cohort compared with single-center or even limited multicenter observational studies. Compared with commonly used datasets such as the Scientific Registry of Transplant Recipients, the lung transplant cohort is smaller, but it adds granularity to data not available in the Scientific Registry of Transplant Recipients, such as medication-prescribing and filling practices necessary to conduct comparative effectiveness studies. The disadvantage to the dataset, however, is that it is confined to patients enrolled in commercial and Medicare Advantage health plans with pharmaceutical benefits, which may limit the generalizability of this study. Uninsured patients or patients enrolled in other government health plans were not captured in our cohort.
Finally, the realities of the deidentified dataset can lead to additional limitations. For example, we can only identify a cohort that filled a prescription; we acknowledge that filling a prescription does not mean that the prescription was actually used as prescribed. Another limitation is the reliance on the Social Security Death Master File to obtain the primary outcome of mortality. Since 2011, this database has been limited—losing capture in up to one-third of deaths (33). Because most deaths occur in the hospital setting, we attempted to minimize this limitation by using discharge status to supplement the Social Security Death Master File and insurance discontinuation because of death (insurance knowledge of death). We acknowledge that a small proportion of patients who died outside the hospital setting could be missing; however, we would expect this to be similar between the groups that we are comparing. Lastly, billing codes were used to identify IFIs. Billing codes require correct diagnosis and coding by the treating provider. Given the possibility for misdiagnosis or miscoding, we used an absolute endpoint (death) as the primary outcome.
Future Directions
We have provided evidence that antifungal prophylaxis is protective against death in LTRs in the first year after transplant. We cannot definitively recommend a specific agent; however, when antifungal prophylaxis was narrowed to mold-active triazoles, the protective effect was similarly significant. Further delineation would require a larger cohort than can be performed currently. Although antifungal prophylaxis was associated with an overall survival benefit, there may be a subset of LTRs that derive a greater benefit from antifungal prophylaxis, such as patients with fungal airway colonization, high-risk occupations, or certain pretransplant diagnoses. We were unable to evaluate these subsets with our cohort. In addition, the comparative efficacy of the various antifungal medications for prophylaxis in LTR should be explored in future studies.
Conclusions
Itraconazole and voriconazole were the primary agents used for antifungal prophylaxis in this LTR cohort spanning from 2005 to 2018. Use of antifungal prophylaxis was associated with an ∼50% decrease in all-cause mortality compared with LTRs not receiving antifungal prophylaxis. This held true even when evaluating individuals specifically receiving systemic mold-active antifungal agents compared with those receiving no prophylaxis.
Supplementary Material
Footnotes
Supported by the Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, Mayo Clinic, Rochester, MN (K.M.P.). Over the last 36 months, N.D.S. has received research support through Mayo Clinic from the Centers of Medicare and Medicaid Innovation, from the Food and Drug Administration (U01FD004585), from the Agency for Healthcare Research and Quality (R01HS025164, R01HS025402, R03HS025517; and U19HS024075), from the National Heart, Lung and Blood Institute of the U.S. National Institutes of Health (R56HL130496 and R01HL131535), from the Medical Devices Innovation Consortium/National Evaluation System for Health Technology, from the National Science Foundation, and from the Patient-Centered Outcomes Research Institute. C.C.K. is supported by a National Heart, Lung, and Blood Institute grant (K23 HL128859) from the National Institutes of Health. The manuscript’s contents are solely the responsibility of the authors and do not necessarily represent the official view of the National Institutes of Health.
Author Contributions: K.M.P., S.G.P., J.N.B., R.R.R., and C.C.K. developed the concept and study design. H.J.D., X.Y., L.R.S., and N.D.S. acquired and evaluated the claims data. K.M.P. drafted the initial manuscript. All authors reviewed and approved the final manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Chan KM, Allen SA. Infectious pulmonary complications in lung transplant recipients. Semin Respir Infect. 2002;17:291–302. doi: 10.1053/srin.2002.36444. [DOI] [PubMed] [Google Scholar]
- 2.Arthurs SK, Eid AJ, Deziel PJ, Marshall WF, Cassivi SD, Walker RC, et al. The impact of invasive fungal diseases on survival after lung transplantation. Clin Transplant. 2010;24:341–348. doi: 10.1111/j.1399-0012.2009.01076.x. [DOI] [PubMed] [Google Scholar]
- 3.Neofytos D, Treadway S, Ostrander D, Alonso CD, Dierberg KL, Nussenblatt V, et al. Epidemiology, outcomes, and mortality predictors of invasive mold infections among transplant recipients: a 10-year, single-center experience. Transpl Infect Dis. 2013;15:233–242. doi: 10.1111/tid.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pappas PG, Alexander BD, Andes DR, Hadley S, Kauffman CA, Freifeld A, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET) Clin Infect Dis. 2010;50:1101–1111. doi: 10.1086/651262. [DOI] [PubMed] [Google Scholar]
- 5.Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356:348–359. doi: 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- 6.Ullmann AJ, Lipton JH, Vesole DH, Chandrasekar P, Langston A, Tarantolo SR, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007;356:335–347. doi: 10.1056/NEJMoa061098. [DOI] [PubMed] [Google Scholar]
- 7.Wang JF, Xue Y, Zhu XB, Fan H. Efficacy and safety of echinocandins versus triazoles for the prophylaxis and treatment of fungal infections: a meta-analysis of RCTs. Eur J Clin Microbiol Infect Dis. 2015;34:651–659. doi: 10.1007/s10096-014-2287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minari A, Husni R, Avery RK, Longworth DL, DeCamp M, Bertin M, et al. The incidence of invasive aspergillosis among solid organ transplant recipients and implications for prophylaxis in lung transplants. Transpl Infect Dis. 2002;4:195–200. doi: 10.1034/j.1399-3062.2002.t01-2-02002.x. [DOI] [PubMed] [Google Scholar]
- 9.Borro JM, Solé A, de la Torre M, Pastor A, Fernandez R, Saura A, et al. Efficiency and safety of inhaled amphotericin B lipid complex (Abelcet) in the prophylaxis of invasive fungal infections following lung transplantation. Transplant Proc. 2008;40:3090–3093. doi: 10.1016/j.transproceed.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Brett J, Chong O, Graham GG, Ray JE, Marriott D, Williams KM, et al. Antifungal use and therapeutic monitoring of plasma concentrations of itraconazole in heart and lung transplantation patients. Ther Drug Monit. 2013;35:133–136. doi: 10.1097/FTD.0b013e318275fe69. [DOI] [PubMed] [Google Scholar]
- 11.Cadena J, Levine DJ, Angel LF, Maxwell PR, Brady R, Sanchez JF, et al. Antifungal prophylaxis with voriconazole or itraconazole in lung transplant recipients: hepatotoxicity and effectiveness. Am J Transplant. 2009;9:2085–2091. doi: 10.1111/j.1600-6143.2009.02734.x. [DOI] [PubMed] [Google Scholar]
- 12.Chong PP, Kennedy CC, Hathcock MA, Kremers WK, Razonable RR. Epidemiology of invasive fungal infections in lung transplant recipients on long-term azole antifungal prophylaxis. Clin Transplant. 2015;29:311–318. doi: 10.1111/ctr.12516. [DOI] [PubMed] [Google Scholar]
- 13.Drew RH, Dodds Ashley E, Benjamin DK, Jr, Duane Davis R, Palmer SM, Perfect JR. Comparative safety of amphotericin B lipid complex and amphotericin B deoxycholate as aerosolized antifungal prophylaxis in lung-transplant recipients. Transplantation. 2004;77:232–237. doi: 10.1097/01.TP.0000101516.08327.A9. [DOI] [PubMed] [Google Scholar]
- 14.Hayes D, Jr, Ball AM, Mansour HM, Martin CA, Flynn JD. Fungal infection in heart-lung transplant recipients receiving single-agent prophylaxis with itraconazole. Exp Clin Transplant. 2011;9:399–404. [PubMed] [Google Scholar]
- 15.Husain S, Paterson DL, Studer S, Pilewski J, Crespo M, Zaldonis D, et al. Voriconazole prophylaxis in lung transplant recipients. Am J Transplant. 2006;6:3008–3016. doi: 10.1111/j.1600-6143.2006.01548.x. [DOI] [PubMed] [Google Scholar]
- 16.Koo S, Kubiak DW, Issa NC, Dietzek A, Boukedes S, Camp PC, et al. A targeted peritransplant antifungal strategy for the prevention of invasive fungal disease after lung transplantation: a sequential cohort analysis. Transplantation. 2012;94:281–286. doi: 10.1097/TP.0b013e318255f864. [DOI] [PubMed] [Google Scholar]
- 17.Pennington KM, Baqir M, Erwin PJ, Razonable RR, Murad MH, Kennedy CC. Antifungal prophylaxis in lung transplant recipients: a systematic review and meta-analysis. Transpl Infect Dis. 2020;22:e13333. doi: 10.1111/tid.13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis RE. Current concepts in antifungal pharmacology. Mayo Clin Proc. 2011;86:805–817. doi: 10.4065/mcp.2011.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pennington KM, Razonable RR, Peters S, Scott JP, Wylam M, Daly RC, et al. Why do lung transplant patients discontinue triazole prophylaxis? Transpl Infect Dis. 2019;21:e13067. doi: 10.1111/tid.13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pennington KM, Yost KJ, Escalante P, Razonable RR, Kennedy CC. Antifungal prophylaxis in lung transplant: a survey of United States’ transplant centers. Clin Transplant. 2019;33:e13630. doi: 10.1111/ctr.13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neoh CF, Snell GI, Kotsimbos T, Levvey B, Morrissey CO, Slavin MA, et al. Antifungal prophylaxis in lung transplantation--a world-wide survey. Am J Transplant. 2011;11:361–366. doi: 10.1111/j.1600-6143.2010.03375.x. [DOI] [PubMed] [Google Scholar]
- 22.Dempsey TM, Sangaralingham LR, Yao X, Sanghavi D, Shah ND, Limper AH. Clinical effectiveness of antifibrotic medications for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2019;200:168–174. doi: 10.1164/rccm.201902-0456OC. [DOI] [PubMed] [Google Scholar]
- 23.Wallace PJ, Shah ND, Dennen T, Bleicher PA, Crown WH. Optum Labs: building a novel node in the learning health care system. Health Aff (Millwood) 2014;33:1187–1194. doi: 10.1377/hlthaff.2014.0038. [Published erratum appears in Health Aff (Millwood) 33:1703.] [DOI] [PubMed] [Google Scholar]
- 24.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 25.Yao X, Noseworthy PA. Left atrial appendage occlusion and surgical ablation for atrial fibrillation during cardiac surgery-reply. JAMA. 2018;320:1602–1603. doi: 10.1001/jama.2018.11341. [DOI] [PubMed] [Google Scholar]
- 26.Li F, Morgan KL, Zaslavsky AM. Balancing covariates via propensity score weighting. J Am Stat Assoc. 2018;113:390–400. [Google Scholar]
- 27.Gayat E, Resche-Rigon M, Mary JY, Porcher R. Propensity score applied to survival data analysis through proportional hazards models: a Monte Carlo study. Pharm Stat. 2012;11:222–229. doi: 10.1002/pst.537. [DOI] [PubMed] [Google Scholar]
- 28.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–161. doi: 10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prasad V, Jena AB. Prespecified falsification end points: can they validate true observational associations? JAMA. 2013;309:241–242. doi: 10.1001/jama.2012.96867. [DOI] [PubMed] [Google Scholar]
- 31.Bhaskaran A, Mumtaz K, Husain S. Anti-aspergillus prophylaxis in lung transplantation: a systematic review and meta-analysis. Curr Infect Dis Rep. 2013;15:514–525. doi: 10.1007/s11908-013-0380-y. [DOI] [PubMed] [Google Scholar]
- 32.Castagnola E, Machetti M, Bucci B, Viscoli C. Antifungal prophylaxis with azole derivatives. Clin Microbiol Infect. 2004;10:86–95. doi: 10.1111/j.1470-9465.2004.00847.x. [DOI] [PubMed] [Google Scholar]
- 33.da Graca B, Filardo G, Nicewander D. Consequences for healthcare quality and research of the exclusion of records from the death master file. Circ Cardiovasc Qual Outcomes. 2013;6:124–128. doi: 10.1161/CIRCOUTCOMES.112.968826. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.