The coronavirus disease 2019 (COVID-19) pandemic has caused an extraordinary burden on the healthcare system and has dramatically impacted the delivery of services. Many nonurgent gastrointestinal (GI) endoscopy services and in-person clinic visits have been deferred, and patients have also avoided visiting healthcare facilities because of the risk of exposure to COVID-19.1 Data from the United Kingdom and Hong Kong have shown a drop in the number of patients diagnosed with various GI cancers.2 , 3 However, the overall impact of the COVID-19 pandemic on common GI procedures and cancer diagnoses in the United States has not been thoroughly evaluated.
Methods
We used TriNetX (Cambridge, MA) to retrospectively analyze data from multiple healthcare organizations (HCOs) in the United States. Details of the TriNetX database are described in the Supplementary Methods and in previous studies.4 , 5 We first estimated the change in the number of patients who had healthcare encounters, procedures, and diagnoses of new GI cancers at participating HCOs in the United States between March 15, 2020 and July 15, 2020 (early COVID-19 pandemic), and March 15, 2019 to July 15, 2019. The number of patients with procedures and new diagnoses of GI cancers was calculated per 100,000 patients with healthcare encounters. We further extended our analysis to study the trend later in the pandemic between July 16, 2020 and November 15, 2020, compared with the corresponding duration in 2019. Sensitivity analysis comparing the diagnosis of new GI cancers during the early COVID-19 pandemic with similar times in 2016, 2017, and 2018 was performed. Detailed methodology is provided in the Supplementary Methods.
The study was approved by Charleston Area Medical Center Institutional Review Board, (Charleston, WV). The study qualified as exempt from review. TriNetX data have also been granted a waiver from the Western Institutional Review Board because it is a federated network and only aggregated counts and statistical summaries of the deidentified information without any protected health information is received from member HCOs.
Data are available to member HCOs on the TriNetX research network platform. Data aggregated directly from the electronic health records systems of the member HCOs are provided on the TriNetX cloud-based platform. Codes for creating cohorts of patients are included in the Supplementary Methods.
Results
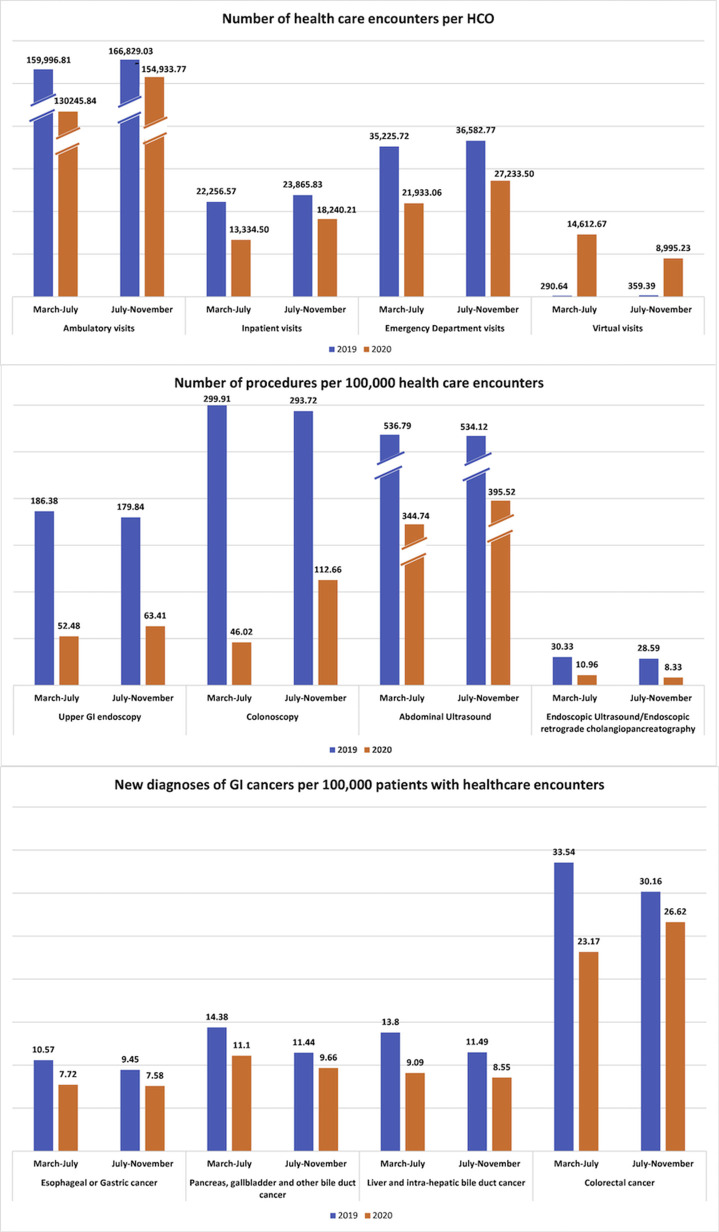
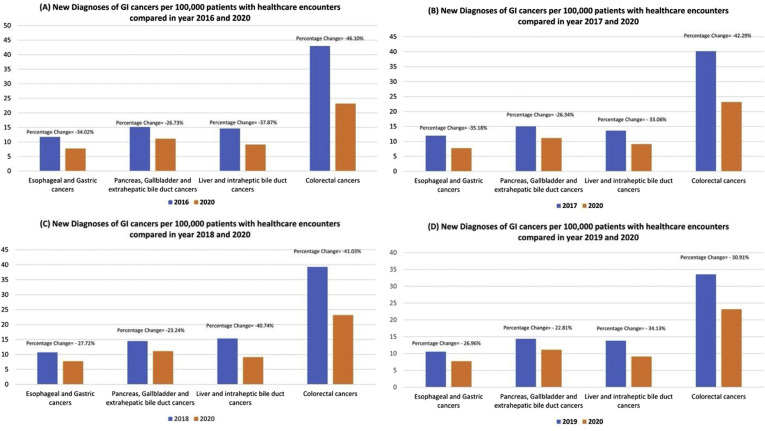
Between March 15, 2019 and July 15, 2019, 8,661,314 adult patients had at least 1 healthcare encounter reported from 41 HCOs. During the early COVID-19 pandemic, 6,264,995 patients had at least 1 healthcare encounter reported from 36 HCOs. Characteristics of these patients are described in the Supplementary Methods. We estimated a decline in inpatient (–42.99%), emergency department (–40.09%), and ambulatory (–22.55%) visits, whereas an increase in virtual (+4465.02%) visits per HCO was seen during the early pandemic phase (March to July 2020) compared with the same time in 2019. Similarly, a decrease in patients who underwent endoscopy (71.84%), colonoscopy (84.66%), abdominal ultrasound (35.78%), endoscopic ultrasound (73.15%), and endoscopic retrograde cholangiopancreatography (48.79%) was noticed. The new diagnoses of malignant liver and intrahepatic (34.13%), colorectal (30.91%), esophageal and gastric (26.96%), and pancreatobiliary (22.81%) cancers per 100,000 patients with healthcare encounters were also decreased during the pandemic (Figure 1 and Supplementary Table 1). The sensitivity analysis also showed a substantial decline in new diagnosis of GI cancers during the early COVID-19 pandemic compared with similar time duration in 2016, 2017, and 2018 (Supplementary Figure 1).
Figure 1.
Healthcare encounters, GI procedures, and new diagnoses of GI cancers during the early phase (March 15, 2020 to July 15, 2020) and later phase (July 16, 2020 to November 15, 2020) of the COVID-19 pandemic compared with corresponding intervals in 2019.
Supplementary Figure 1.
Percentage of change in new diagnoses of GI cancers per 1,000,000 patients with healthcare encounters during the COVID-19 pandemic (between March 15, 2020 and July 15, 2020) compared with same time duration in (A) 2016, (B) 2017, (C) 2018, and (D) 2019.
Similarly, a decrease in the patient visits, procedures, and new diagnoses of cancers were also seen in the later phase of the pandemic (July to November 2020) compared with a similar period in 2019; however, the proportion of the decline was smaller compared with the early pandemic (Figure 1 and Supplementary Table 1). The decrease in new diagnoses of malignant liver and intrahepatic (25.58%), colorectal (11.74%), esophageal and gastric (19.78%), and pancreatobiliary (15.56%) cancers later in the pandemic was considerable but recovered compared with the early COVID-19 pandemic.
Discussion
Our study, including data from multiple HCOs in the United States, showed the collateral damage caused by the COVID-19 pandemic leading to potential delays in the diagnosis of major GI cancers. Patient encounters and new diagnoses of GI cancers and procedures significantly declined during the COVID-19 pandemic compared with a similar period in 2019. Our findings report trends similar to other studies that showed a reduction in all cancer-related patient encounters during the COVID-19 pandemic.2 , 3 These trends may result in an increase in late-stage cancer cases and poor cancer outcomes; moreover, the cancellation of cancer-preventive GI procedures can lead to a rise in the incidence of GI cancers in the future. An increase in virtual visits was seen; however, the volume of these virtual visits was not large enough to account for the decrease in volume of ambulatory visits, and the nature of virtual visits could not replace procedures or imaging needed for cancer diagnosis.
Decreases in GI procedures is a significant disruption of practice patterns and source of revenue for gastroenterologists. Going forward, when GI endoscopy and routine healthcare services are resumed in full capacity, there will be a significant burden and demand for procedures related to cancer screening. An increase in capacity of GI procedures will be needed, and gastroenterologists will also have to carefully triage patient panels to evaluate and schedule persons at risk for malignancy sooner. Similarly, gastroenterologists will need to use all available evidence-based required tools for cancer screening. For instance, in patients needing colon cancer screening, using noninvasive screening methods such as the fecal immunochemical test, fecal-fecal immunochemical test DNA, and computed tomography colonography in addition to colonoscopy will be needed.6 Other experts have also suggested considering weekend and evening endoscopy sessions to meet the backlog of GI endoscopy created during the pandemic.7 However, this will need to be balanced by the burden on the already strained healthcare system and physician burnout. These factors will become especially crucial in a prolonged COVID-19 pandemic. This will further extend these reported trends and delay in new cancer diagnosis.
Our study has several limitations. Data derived from administrative coding systems have the inherent limitations of miscoding or data entry errors. Our study estimated procedures and diagnoses only among the patients with healthcare encounters and did not account for the patient population without healthcare encounters. Differences in cancer staging, histology, treatment modalities, and mortality were not determined. Despite these limitations, our study is the largest study to show a reduction in diagnoses of major GI cancers during the pandemic. This highlights the serious future implications that can result in an increased restraint on healthcare resources and lead to increased morbidity and mortality. Urgent policy and practice interventions will be needed, and HCOs and gastroenterologists will need to use innovative methods to meet the backlog of screening and diagnostic tests for GI cancers.
Acknowledgment
The authors thank the West Virginia Clinical and Translational Science Institute and Charleston Area Medical Center for providing institutional access to the TriNETX global healthcare network (Cambridge, MA) for design assistance for this project. The authors also thank the first-line responders who determinedly worked during the COVID-19 pandemic.
Collaborators: Frank H. Annie; Emad Mansoor, and William Hutson.
CRediT Authorship Contributions
Ahmad Khan, MD, MS (Conceptualization: Lead; Data curation: Equal; Formal analysis: Equal; Methodology: Equal; Writing – original draft: Lead; Writing – review & editing: Equal).
Mohammad Bilal, MD (Conceptualization: Supporting; Methodology: Supporting; Writing – original draft: Equal; Writing – review & editing: Equal).
Vincent Morrow, MSIII (Writing – original draft: Equal). Gregory Cooper, MD (Writing – review & editing: Equal).
Shyam Thakkar, MD (Conceptualization: Equal; Methodology: Equal; Writing – original draft: Equal; Writing – review & editing: Equal).
Shailendra Singh, M.D (Conceptualization: Lead; Data curation: Lead; Methodology: Lead; Supervision: Equal; Writing – original draft: Equal; Writing – review & editing: Equal).
Footnotes
Conflicts of interest The authors disclose no conflicts.
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org and at https://doi.org/10.1053/j.gastro.2021.02.055.
Supplementary Methods
Study Design and Data Source
This was a retrospective cohort study. We used TriNetX (Cambridge, MA), a federated health research network platform, to analyze data from multiple HCOs in the United States. The TriNetX platform allows access to the deidentified longitudinal clinical data aggregated directly from the electronic health record of the participating or member HCOs. A typical HCO is a large academic health center with data coming from most of its affiliates. A single HCO frequently has more than 1 facility, including main and satellite hospitals and outpatient clinics. A typical organization has a complex enterprise architecture where the data flows through several different databases, such as a data warehouse and a research data repository, which is then uploaded to TriNetX.
Based on the analysis run on January 28, 2021, the research network consists of an overall 51,607,816 patients from 45 HCOs in the United States. The mean age of these patients was 51 ± 20 years, and most were women (55%). Fifty-six percent of these patients were white (Hispanic or non-Hispanic), 12% were black (Hispanic or non-Hispanic), 3% were Asian, 1% were Native American (1%), and 25% were race unknown. Geographically, 45% of patients were from the South region, 17% from the Midwest, 14% from the Northeast, and 11% from the West region of United States, whereas location of 13% patients was unknown.
In addition to electronic health record data usually available in a structured fashion (eg, demographics, diagnoses, procedures, medications, lab test results, vital signs), TriNetX can also extract facts of interest from the narrative text of clinical documents using Natural Language Processing. TriNetX ensures HIPAA compliance of the clinical patient data. All statistical analyses were also conducted using the web-based analytical features of TriNetX.
Data Quality Checks
TriNetX maps the data to a standard and controlled set of clinical terminologies. Data are then transformed into a proprietary data schema. This transformation process includes an extensive data quality assessment that includes “data cleaning” that rejects records that do not meet the quality standards. The software checks the basic formatting to ensure, for example, that dates are appropriately represented. It enforces a list of fields that are required (eg, patient identifier) and rejects those records where the required information is missing. Referential integrity checking is done to ensure that data spanning multiple database tables can be successfully joined together. As the data are refreshed, the software monitors changes in volumes of data over time to ensure data validity. TriNetX requires at least 1 nondemographic fact for a patient to be counted in the dataset. Patient records with only demographics information are not included in datasets.
Estimates of Patient Encounters, Procedures, and Diagnoses of GI Cancers
Search queries were performed using specific dates (eg, March 15, 2020 to July 15, 2020) on the TriNetX platform using appropriate codes. We estimated the number of patients who had healthcare encounters, procedures, and diagnoses of new GI cancers at participating HCOs in the United States between March 15, 2020 and July 15, 2020 and March 15, 2019 and July 15, 2019. The first cases of COVID-19 in the United States were reported in January and February 2020 in travelers from China. However, the widespread transmission of COVID-19 occurred by mid-March, leading to disruption of routine healthcare services. Therefore, we considered the period between March 15, 2020 and July 15, 2020 as the early COVID-19 pandemic duration. We further extended the analysis to study the trend later in the pandemic and compared healthcare encounters, procedures, and diagnoses of new GI cancers at participating HCOs in the United States between July 16, 2020 and November 15, 2020 and July 16, 2019 and November 15, 2019.
The healthcare encounters consisted of any inpatient, ambulatory, emergency department, or virtual/telehealth visits identified using Current Procedural Terminology (CPT) and Healthcare Common Procedure Coding System (HCPCS) codes. Ambulatory and virtual/telehealth visits only provided by physicians were included. Counts for patients who underwent upper GI endoscopy, colonoscopy, and abdominal ultrasound were estimated during the specified time period using CPT, International Classification of Diseases, Tenth Revision (ICD-10), and HCPS codes. GI cancers were identified using ICD-10 codes and divided into 4 major groups: (1) Malignant neoplasm of esophagus (C15) or Malignant neoplasm of stomach (C16) (esophageal or gastric cancer), (2) Malignant neoplasm of pancreas (C25) or Malignant neoplasm of gallbladder (C23) or Malignant neoplasm of other and unspecific parts of the biliary tract (C24) (pancreaticobiliary cancer), (3) Malignant neoplasm of liver and intrahepatic parts of the biliary tract (C24) (liver cancer), and (4) Malignant neoplasm of colon (C18) or Malignant neoplasm of rectosigmoid (C19) or Malignant neoplasm of rectum (C20) (colorectal cancer). Counts for a new diagnosis of GI cancers were estimated after excluding patients with any previous diagnosis of malignant neoplasm of the digestive tract. Details of the coding system and CPT, HCPS, and ICD-10 codes to identify patient encounters, procedures, and GI cancers are as follows:
| Codes to identify patient encounters, procedures, and GI cancers | |
|---|---|
| Visits | |
| Emergency department | CPT codes: 99281, 99282, 99283, 99284, 99285 |
| HCPS codes: G0382, G0381, G0383, G0380 | |
| Ambulatory | TNX Visit: Ambulatory |
| CPT codes: 99211, 99212, 99213, 99214, 99215, 99201, 99202, 99203, 99204, 99205, 99024, 99495, 99496 | |
| HCPS codes: G0463, G0438, G0439, T1015, G0466, G0467, G0468, G9050, G9051, G9052, G9054, G9055 | |
| Virtual | TNX Visit: Virtual |
| CPT codes: 99441, 99442, 99443, 99444, 1018510, 98966, 98967, 98968, 99446, 99447, 99448, 99449, 99451, 99452, 1035156,1018515 | |
| HCPS codes: G0406, G0407, G0408, G0509, G0425, G0426, G0427, Q3014 | |
| Inpatient | TNX Inpatient Encounter, Short Stay, Inpatient Acute, Inpatient Non-Acute, Observation Encounter |
| CPT codes: 99251, 99252, 99253, 99254, 99255, 1013659, 1013660, 1013661, 1013699, 1013700, 99217, 1013675, 99231, 99232, 99233, 99234, 99235, 99236, 1013800, 99356, 99221, 99222, 99223, 1019140, 1013729, 1014309, 99291, 99292 | |
| HCPS codes: Q5005, G0408, G0406, G0407 | |
| Procedures | |
| Colonoscopy | CPT codes: 1022231, 45380, 45378, 45385, 45380, 45384, 45390, 45381, 45388, 45386, 44389, 44394, 1007534, 44388, 45391, 44392, |
| HCPCS codes: G0105, G0121, 45393, G0120, G9935, G9936, G9933, G9937 | |
| ICD-10 code: 0DJD8ZZ | |
| Upper endoscopy | CPT codes: 1021431, 1007260, 43239, 43235, 43249, 43259, 1021430, 43248, 1007242, 43242, 43237, 43200, 43251, 43238, 43245, 43253, 43226, 43233, 43252, 43229, 43205, 43197, 43212, 43213, 43217, 43206 |
| ICD 10 codes: 0DJ08ZZ, 0DB68ZX, 0DB98ZX, 0DB58ZX, 0D968ZX, 0D958ZX, 0D998ZX | |
| Abdominal ultrasound | CPT codes: 76705, 1010775, 76700 |
| ICD-10 codes: BD47ZZZ, BF4CZZZ, BF40ZZZ, BF43ZZZ, BF45ZZZ, BF46ZZZ, BF45, BF46 | |
| Endoscopic ultrasound | CPT codes: 43231, 43232, 43237, 43238, 43242, 43253, 43259, 44406, 44407, 45341, 45342, 45391, 45392 |
| Endoscopic retrograde cholangiopancreatography | CPT codes: 1007283, 1021432, 43260, 43261, 43262, 43263, 43264, 43265, 43274, 43275, 43276, 43277, 43278 |
| ICD-10 codes: BF110ZZ, BF111ZZ, BF11YZZ | |
| Diagnoses | |
| GI cancers | Malignant neoplasm of esophagus (C15), Malignant neoplasm of stomach (C16), Malignant neoplasm of pancreas (C25), Malignant neoplasm of gallbladder (C23), Malignant neoplasm of other and unspecific parts of the biliary tract (C24), Malignant neoplasm of liver and intrahepatic parts of the biliary tract (C22), Malignant neoplasm of colon (C18), Malignant neoplasm of rectosigmoid (C19), Malignant neoplasm of rectum (C20) (colorectal cancer). |
Results
Patient Characteristics
Between March 15, 2019 and July 15, 2019, 8,661,314 adult patients had at least 1 healthcare encounter reported from 41 HCOs. The mean age of these patients was 53 ± 19 years, and 59% were women. Most patients were white (69%), followed by black or African American (14%), Asian (3%), and race unknown (14%). Half of these patients (50%) were from HCOs in the South region, 16% from the Midwest, 15% the Northeast, and 13% from the West region. During the COVID-19 pandemic, 6,264,995 patients had at least 1 healthcare encounter reported from 36 HCOs. The mean age was 53 ± 19 years, and 58% were women. Sixty-seven percent of patients were white, 15% were black or African American, 2% were Asian, 1% were Native American, and 15% were race unknown. Geographically, 44% of patients belonged to the South region, followed by an equal distribution of patients in the Northeast and Midwest (18% each), and 17% of the patients were from the West region.
Supplementary Table 1.
Patients with Healthcare Encounters, GI Procedures, and New Diagnoses of GI Cancers During the Early Phase (March 15, 2020 to July 15, 2020) and Later Phase (July 16, 2020 to November 15, 2020) of the COVID-19 Pandemic Compared With Corresponding Intervals in 2019
| Before the Pandemic (March 15 to July 15, 2019) |
During the Pandemic (March 15 to July 15, 2020) |
Before the Pandemic (July 16 to November 15, 2019) |
During the Pandemic (July 16 to November 15, 2020) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Healthcare encounters | ||||||||||||||
| No. of Patients | Total HCOs | Average No. of Patients/HCOs | No. of Patients | Total HCOs | Average No. of Patients/HCOs | Percentage of Change | No. of Patients | Total HCOs | Average No. of Patients/HCOs | No. of Patients | Total HCOs | Average No. of Patients/HCOs | Percentage of Change | |
| Ambulatory visits | 5,919,882 | 35 | 159,996.81 | 4,167,867 | 32 | 130,245.84 | –22.55 | 6,673,161 | 40 | 166,829.03 | 6,042,417 | 39 | 154,933.77 | –7.13 |
| Inpatient visits | 823,493 | 37 | 22,256.57 | 426,704 | 32 | 13,334.50 | –42.99 | 954,633 | 40 | 23,865.83 | 711,368 | 39 | 18,240.21 | –23.57 |
| Emergency department visits | 1,268,126 | 36 | 35,225.72 | 679,925 | 31 | 21,933.06 | –40.92 | 1,426,728 | 39 | 36,582.77 | 1,034,873 | 38 | 27,233.50 | –25.56 |
| Virtual visits | 7266 | 25 | 290.64 | 394,542 | 27 | 14,612.67 | 4465.02 | 10,063 | 28 | 359.39 | 278,852 | 31 | 8,995.23 | 2402.92 |
| Procedures (no. of patients/100,000 patients with healthcare encounters) | ||||||||||||||
| Upper GI endoscopy | 186.38 | 52.48 | –71.84 | 179.84 | 63.41 | –64.74 | ||||||||
| Colonoscopy | 299.91 | 46.02 | –84.66 | 293.72 | 112.66 | –61.64 | ||||||||
| Abdominal ultrasound | 536.79 | 344.74 | –35.78 | 534.12 | 395.52 | –25.95 | ||||||||
| Endoscopic ultrasound | 18.77 | 5.04 | –73.15 | 18.3 | 4.4 | –75.95 | ||||||||
| Endoscopic retrograde cholangiopancreatography | 11.56 | 5.92 | –48.79 | 10.29 | 3.93 | –68.10 | ||||||||
| New diagnoses of cancers (no. of patients/100,000 patients with healthcare encounters) | ||||||||||||||
| Esophageal or gastric cancer | 10.57 | 7.72 | –26.96 | 9.45 | 7.58 | –19.78 | ||||||||
| Pancreas, gallbladder, and other bile duct cancer | 14.38 | 11.1 | –22.81 | 11.44 | 9.66 | –15.56 | ||||||||
| Liver and intrahepatic bile duct cancer | 13.8 | 9.09 | –34.13 | 11.49 | 8.55 | –25.58 | ||||||||
| Colorectal cancer | 33.54 | 23.17 | –30.91 | 30.16 | 26.62 | –11.74 | ||||||||
References
- 1.Repici A., et al. Gastrointest Endosc. 2020;92:192–197. doi: 10.1016/j.gie.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lui T.K.L., et al. Gastroenterology. 2020;159:1164–1166. doi: 10.1053/j.gastro.2020.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rutter M.D., et al. Gut. 2021;70:537–543. doi: 10.1136/gutjnl-2020-322179. [DOI] [PubMed] [Google Scholar]
- 4.Singh S., et al. Gastroenterology. 2020;159:768–771. doi: 10.1053/j.gastro.2020.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh S., et al. Gastroenterology. 2020;159:1575–1578. doi: 10.1053/j.gastro.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaukat A., et al. Lancet Gastroenterol Hepatol. 2020;5:726–727. doi: 10.1016/S2468-1253(20)30191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Issaka R.B., et al. https://jamanetwork.com/channels/health-forum/fullarticle/2766137