Abstract
It is currently understood that osteoarthritis (OA) is a major chronic inflammatory musculoskeletal disease. While this disease has long been attributed to biomechanical trauma, recent evidence establishes a significant correlation between osteoarthritic progression and unbridled oxidative stress, responsible for prolonged inflammation. Research describes this as a disturbance in the balanced production of reactive oxygen species (ROS) and antioxidant defenses, generating macromolecular damage and disrupted redox signaling and control. Since ROS pathways are being considered new targets for OA treatment, the development of antioxidant therapy to counteract exacerbated oxidative stress is being continuously researched and enhanced in order to fortify the cellular defenses. Experiments with glutathione and its precursor molecule, N-acetylcysteine (NAC), have shown interesting results in the literature for the management of OA, where they have demonstrated efficacy in reducing cartilage degradation and inflammation markers as well as significant improvements in pain and functional outcomes. Glutathione remains a safe, effective and overall cheap treatment alternative in comparison to other current therapeutic solutions and, for these reasons, it may prove to be comparably superior under particular circumstances.
Methods
Literature was reviewed using PubMed and Google Scholar in order to bring up significant evidence and illustrate the defensive mechanisms of antioxidant compounds against oxidative damage in the onset of musculoskeletal diseases. The investigation included a combination of keywords such as: oxidative stress, oxidative damage, inflammation, osteoarthritis, antioxidant, glutathione, n-acetylcysteine, redox, and cell signaling.
Conclusion
Based on the numerous studies included in this literature review, glutathione and its precursor N-acetylcysteine have demonstrated significant protective effects in events of prolonged, exacerbated oxidative stress as seen in chronic inflammatory musculoskeletal disorders such as osteoarthritis.
Keywords: Oxidative stress, Inflammation, Osteoarthritis, Antioxidant, Glutathione
1. Introduction
Osteoarthritis (OA) is a major painful chronic joint disease affecting various anatomical sites including the hip, knee and hand, being responsible for loss of function and disability in adults.1,2 This complex multifactorial orthopedic condition, commonly attributed to aging and obesity, is known to affect more than one-third of the elderly population above the age of 65 and has doubled in prevalence since the mid-20th century.3,4
It is well characterized by morphological, biomechanical and biochemical alterations in the microenvironment which ultimately result in detrimental effects. Commonly observed features include: progressive catabolism of articular cartilage, subchondral bone sclerosis, and formation of osteophytes and subchondral cysts. Furthermore, there is also degeneration of ligaments and menisci of the knee, as well as overall inflammation and hypertrophy of the joint capsule.4 OA is a multifactorial disorder and its pathogenesis is influenced by several intrinsic and extrinsic factors which may activate signaling of molecular pathways associated with articular injury.5
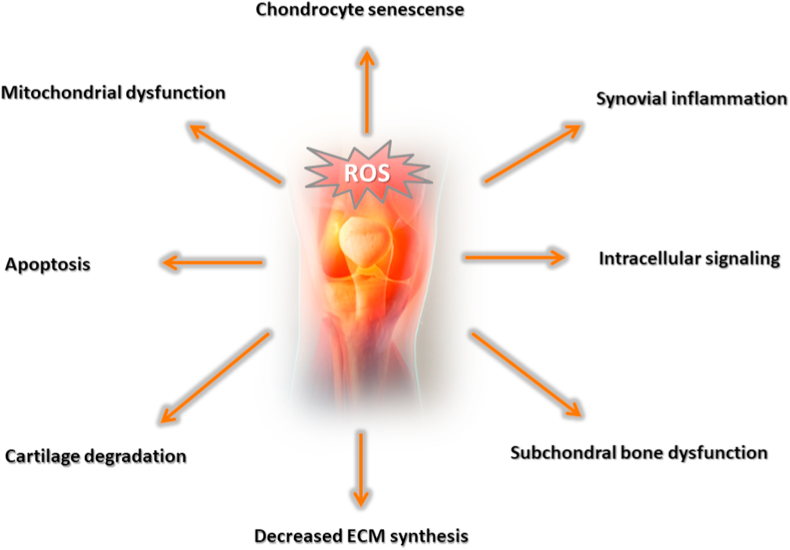
Prescription of ordinary therapeutics such as corticosteroids for the temporary alleviation of pain is one of the common management strategies. However, this alternative does not reverse the progression of OA. Actually, the prolonged administration of these pharmacological compounds may generate additional complications, bringing undesirable side effects upon the patient.1 OA pathogenesis appears to be closely linked to exasperated oxidative stress and the overproduction of reactive oxygen species (ROS). These are known to be involved in biological events such as cell signaling, chondrocyte senescence and apoptosis, extracellular matrix (ECM) synthesis and degradation as well as synovial inflammation and dysfunction of the subchondral bone,6 as illustrated by Fig. 1. Indeed, previous studies have concluded that OA progression is significantly attributed to oxidative stress and ROS.7, 8, 9, 10, 11 Taking that into consideration, evidence supporting the administration of antioxidants to mitigate OA aggressiveness is slowly beginning to rise, yet additional clarification of their protective mechanisms in joint tissues is still desirable in order to further reveal potential benefit to patients.
Fig. 1.
The deleterious effects of the exacerbated production of ROS.
Therefore, this review aims to evaluate the effects of glutathione and the potential benefits it may provide against exacerbated oxidative stress in osteoarthritis.
2. Oxidative stress in OA pathophysiology
It has long been suggested that OA arises from a cluster of multiple factors including genetic predisposition, obesity, trauma, aging and even the presence of other systemic diseases.12 It is understood that OA is a disease affecting the entire joint compartment and previous investigations suggest that the degenerative process is given in two stages. Firstly, in the biosynthetic phase, local chondrocytes repeatedly attempt to repair damaged ECM. Secondly, in the degradative phase, increased catabolic enzyme activity results in matrix digestion and subsequent matrix synthesis inhibition.4 The combination of unresolved biomechanical and biochemical stress promotes secondary alterations which lead to a predominant catabolic microenvironment. Collectively, these biological processes lead to erosion of cartilage as well as lesions of the subchondral bone and peripheral structures, all of which aggravate physical pain and debilitation.13
More recently, researchers found a significant correlation between OA progression and oxidative stress. This has been described as a disturbance in the balanced production of ROS and antioxidant defenses, generating macromolecular damage and disrupted redox signaling and control.14 ROS are unstable oxygen-containing molecules that easily react with other molecules in a cell due to the presence of one or more unpaired electrons (free radicals) or the absence of unpaired electrons. Either way, both are reactive enough to the point of conversion into ROS (non-radical).7 Well known examples of radical ROS include nitric oxide (NO), superoxide, and hydroxyl (OH) groups, whilst non-radical ROS encompasses molecules such as ozone, peroxide, hydroxide and peroxynitrite.7 These reactive molecules are mainly produced by the mitochondria through oxidative phosphorylation,15 which also produce other intracellular ROS such as nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, the main source of ROS in phagocytes, for example. Stimulation of phagocytes triggers migration of cytosolic components towards the internal compartment of plasma membrane, forming an active enzyme complex with NADPH oxidase activity.16
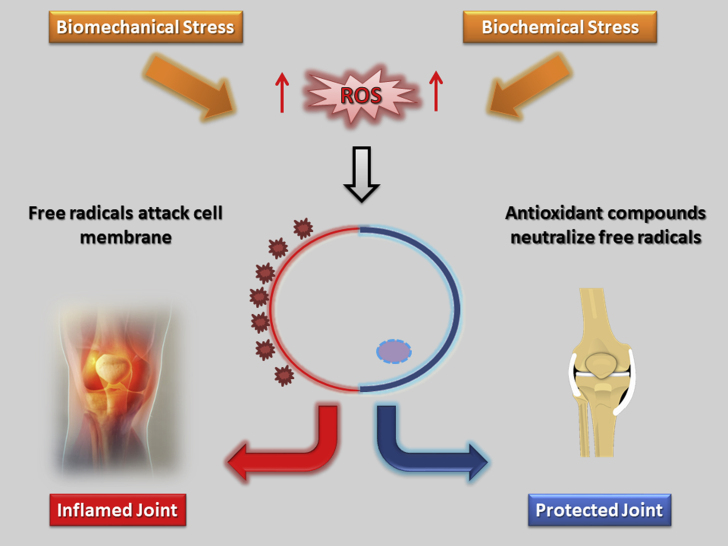
In order to defend itself from the disruptive nature of ROS, the organism relies on endogenous ROS scavengers such as glutathione, for example. These antioxidant molecules react and scavenge the atomically unstable molecules, protecting the intracellular redox milieu.17 While ROS are immediately associated with inflammation and degradation, it is worthy to note that they are a “necessary evil” because they play an essential role in natural redox signaling for the maintenance of physiological functions.18,19 ROS have long been recognized as signaling molecules that regulate a wide variety of physiological processes. It has been shown that hydrogen peroxide (H2O2), for example, is required for cytokine, insulin, growth factor, AP-1, and NF-κB signaling.19 In addition, other reports have also demonstrated that H2O2 is able to promote phosphatase inactivation by cysteine oxidation, serving as a feasible biochemical mechanism by which ROS can affect signaling pathways.20 In current redox biology concepts, low levels of ROS trigger the activation of signaling pathways, initiating various biological events. Conversely, oxidative stress is attributed to unbridled production of ROS, surpassing the amount of antioxidant defenses and aggravating damage to cells, proteins, lipids and DNA,18 a process which is also indicated by Fig. 2. In this sense, it appears that the delicate equilibrium in redox biology might dictate the shift towards physiological or pathological conditions.
Fig. 2.
Oxidative versus antioxidative fate: a cellular perspective.
Relating back to osteoarthritic progression, ROS assumes a pivotal role in its pathogenesis since it becomes partially responsible for cartilage destruction and chronic inflammation. Under prolonged oxidative stress, the cartilaginous tissue synthesizes large amounts of proteolytic enzymes, which in turn favors the shift towards catabolic reactions.21 Additionally, there is also concern with the involvement of the NO free radical in OA. Nitric oxide ultimately leads to the formation of RNS (reactive nitrogen species) which is known to cause mitochondrial damage and is therefore suggested to be a critical agent in chondrocyte function and apoptosis.22 Furthermore, NO may also harm nuclear DNA and ECM, a process which appears to be regulated by NO synthase (NOS). The inducible isoform of this enzyme is present in osteoarthritic cartilage and appears to be induced as response to proinflammatory stimuli in macrophages as a defensive mechanism through its oxidative potential.23,24
The synovial space is another site that is greatly affected by ROS in the onset of OA. Synoviocytes produce synovial fluid, which functions as a natural biological lubricant and a biochemical pool that allows nutrients and regulatory cytokines to traverse between the joint, thus bearing benevolent properties.25 The primary function of synoviocytes is to continuously secrete high-molar-mass hyaluronans (HA) into the synovial fluid. In the joint, hyaluronans display vital roles associated with transport of nutrients and protection of cartilage since they have been found to promote an active anti-inflammatory effect.25,26 Inflammation mediated by oxidative stress increases the rate of hyaluronan degradation, thereby contributing to the development of several joint disorders, namely, osteoarthritis.25
3. Current therapeutic alternatives for OA
The management of OA still remains a challenge since its pathophysiology proves to be a complex process. Currently, the disease may be approached either pharmacologically or non-pharmacologically. Some examples of popular non-pharmacological strategies include physical therapy and education, exercise, weight loss, physical aids, genicular nerve radiofrequency ablation and, in more severe cases, surgical joint replacement.27, 28, 29 Pharmacological alternatives, on the other hand, only bring temporary alleviation of pain but do not protect the microenvironment from the detrimental conditions.1 A usual strategy is to apply a combination of drugs at different stages of OA with the sole objective of blocking the inflammatory processes. Non-steroidal anti-inflammatory drugs (NSAIDs), analgesics and steroid drugs, for example, are commonly indicated for this purpose.30,31 The administration of NSAIDs is particularly concerning because, although they effectively mitigate pain associated with severe cases of OA, their use is known to be associated with potentially serious dose-dependent gastrointestinal (GI) complications, such as upper GI bleeding.31
Given these complicated circumstances, there is a great need for suitable interventional therapies capable of counteracting the degenerative joint microenvironment safely and efficaciously.32 This, in turn, would alleviate pain and improve the patient’s quality of life. Since ROS pathways are being considered new targets for OA treatment, the development of antioxidant therapy to counteract exacerbated oxidative stress is being continuously researched and enhanced in order to fortify the cellular defenses.
Dietary supplementation of natural antioxidant compounds derived from plants has been much discussed in the literature. For instance, the inclusion of vegetable extracts such as curcumin has shown the potential to increase the amount of serum glutathione in human osteoarthritic knees.33
Despite known therapeutic effects of these natural chemicals found in plants, there are certain inconveniences. Most of them happen to be unstable and susceptible to degradation or oxidation, while others prove to be of poor water solubility which therefore implies poor bioavailability to act on the target site.34,35 Research has indicated that gender may also influence pharmacokinetics of certain compounds to some extent.36 Other biologically active components may be highly soluble in water or possess high molecular weights, which also hinders absorption since they are unable to cross lipid membranes.30 In addition to that, there is yet another molecular challenge. It should be noted that articular cartilage is an avascular structure and relatively acellular with a dense and highly charged ECM,37 which also contributes to the difficult delivery of bioactive components into the target site. For these reasons, their potential becomes quite limited. Systemic administration of high doses of antiarthritic drugs may then be required in order to promote intended effects in the target tissues that are already facing advanced deterioration.
In light of this, clinicians and health practitioners may resort to a more idealistic treatment of OA, relying on the direct and easy introduction of antioxidant molecules into the synovial capsule, envisaging long term effects.38 This is where intra-articular delivery methods arise as a suitable alternative, given the localized nature of OA. Logically, local administration of antioxidant agents into the target site may be comparably superior, especially considering the increase in bioavailability and accurate targeting of the joint, bypassing the pharmacokinetic challenges and reducing exposure to inappropriate sites.39, 40, 41 However, the molecules are still susceptible to the rapid clearance from the synovial cavity and reduced tissular penetration.42 In order to amend such inconvenience, increasing the frequency of injections may prove to be an intelligent strategy to ensure a sustained delivery of therapeutic agents.
4. Glutathione protective mechanisms
Recently, studies have further demonstrated the negative effects of oxidative stress and impaired redox signaling in the onset of OA, aggravating significant oxidative damage of cellular structures and vital molecules in cartilage as well as other tissues.43, 44, 45 Evidence supporting glutathione as a suitable candidate for oxidative stress resistance is gradually being accumulated. Previous studies have already illustrated some of its protective roles in organisms by lowering levels of oxidative stress and oxidant damage.46, 47, 48, 49
This section will describe glutathione in more detail and will also shed light on its mechanisms of action.
Glutathione (GSH) is a tripeptide antioxidant composed of three amino acids - cysteine, glycine, and glutamate. It is released in two forms: reduced glutathione (GSH) and oxidized glutathione (GSSG).50 The tripeptide γ-l-glutamyl-l-cysteinyl-glycine (GSH) is the most abundant, low-molecular weight thiol-containing peptide found in most living cells, from simple prokaryotes to more complex, eukaryotic organisms.51,52 It is synthesized from three amino acids in two ATP-dependent steps: formation of γ-glutamylcysteine from glutamate and cysteine, followed by the subsequent formation of GSH from γ-glutamylcysteine and glycine.53 GSH plays a critical role in protecting cells from oxidative damage and toxicity of xenobiotic electrophiles, therefore establishing a redox equilibrium.50 The thiol group acts as a proton donor and is responsible for the biological activity of GSH.54 Furthermore, GSH has also been found to be a key determinant of important biologic processes such as cell proliferation, apoptosis, immune function and fibrogenesis.53
4.1. Antioxidant mechanisms
The antioxidant property of GSH is mostly attributed to GSH peroxidase-catalyzed reactions, where hydrogen peroxide and lipid peroxide are reduced while GSH is oxidized to GSSG. GSSG is then reduced back to GSH by GSSG reductase via the utilization of nicotinamide adenine dinucleotide phosphate (NADPH), establishing a redox cycle.55 This makes GSH a vital agent in shielding mitochondria from both physiologically and pathologically generated oxidative stress.56 It is important to note that the intracellular redox potential is largely determined by the GSH:GSSG ratio and oxidative stress overcomes the ability of the cell to reduce GSSG to GSH. Therefore, to prevent a significant shift in the redox equilibrium, GSSG can either be actively exported out of the cell or react with a protein sulfhydryl group, which leads to the formation of a mixed disulfide.55 This is how severe oxidative stress can deplete the cellular reserve of GSH, which would facilitate the progression of certain disorders associated with aging.
4.2. Redox signaling
GSH regulates redox signaling in cells by modifying the oxidation state of cysteine residues.57 GSH can bind to sulfhydryl groups of protein cysteinyl residues by a reversible process called glutathionylation. This generates glutathionylated proteins, which can activate or inactive the protein57. This mechanism is a rather beneficial strategy since it protects sensitive thiols which would be exposed to irreversible oxidation. Additionally, this may also prevent the loss of GSH under oxidative stress. To reverse glutathionylation, glutaredoxin and sulfiredoxin-catalyzed reactions occur, using GSH as a reductant.58 Numerous signaling molecules and transcription factors, for instance, contain redox-sensitive cysteine residues and undergo reversible oxidative modifications once they are stimulated by growth factors or oxidants.59, 60, 61, 62 The reversible oxidative modifications of cysteine residues imply an important mechanism whereby ROS and RNS can readily interact with these molecules, dictating protein function and cell signaling that can be regulated by GSH.58
4.3. Cellular growth and death
The increased level of GSH has been associated with proliferative responses and is known to be essential for cell cycle progression in eukaryotic cells.63 The key role of GSH in DNA synthesis is attributed to the maintenance of reduced glutaredoxin or thioredoxin, which are required for the proper function of ribonucleotide reductase, the rate-limiting enzyme in DNA synthesis.53 Additionally, the GSH redox status can influence the expression and activity of certain factors which are critical for the progression of the cell cycle. To elaborate, GSH co-localizes to the nucleus in proliferative events, which indicates that, via redox changes, it can affect the activity of many nuclear proteins, especially histones.63,64 These observations suggest that a reducing condition in the nucleus proves to be essential in maintaining a stable cell cycle, especially considering the fact that oxidative stress harms lipids, proteins and DNA.18,43, 44, 45 Glutathione is also known to regulate the death of cells. Apoptosis (chromatin condensation, fragmentation and internucleosomal DNA cleavage) and necrosis (rupture or fragmentation of the plasma membrane and ATP depletion) can coexist and share common pathways. Both types of cell death can be regulated by GSH and share the involvement of mitochondria.65,66 The levels of GSH can influence the expression of caspases and signaling molecules that regulate cell death.67 To elaborate, the levels of GSH fall during apoptotic events in many cell types as a consequence of ROS, enhanced GSH efflux and decreased glutamate-cysteine ligase (GCL) activity.68 Considering an apoptotic event, GSH efflux seems to be a logical and important mechanism to avoid the regular protective role of GSH, ensuring that the programmed cell death proceeds as intended.67 On the other hand, if GSH levels are dramatically lowered, apoptosis may shift towards necrosis, indicating that elevated ROS levels may then disrupt the standard cellular process. Critical depletion of GSH in mitochondria culminates in increased levels of ROS and RNS, mitochondrial dysfunction and subsequent ATP depletion, which are responsible for the shift from apoptosis towards necrosis and, therefore, inflammation.66
5. The role of glutathione in osteoarthritis
The intra-articular administration of viscosupplements into diseased joints has demonstrated alleviation of OA symptoms.54 Hyaluronic acid (HA), for example, is commonly used as a treatment alternative due to its ability to restore viscoelastic properties of joints with satisfactory results in the early stages of the disease.69,70 HA is a major component present in synovial fluid and its main function is to provide lubrication and absorb shock from physical impacts.71 Additionally, this component also promotes an increase in the synthesis of vital biomolecules such as sulfated glycosaminoglycan (GAG), hydroxyproline, chondroitin-6-sulfate as well as a boost in the deposition type II collagen in chondrocytes.72 Chondrocytes, fibroblast-like synoviocytes and infiltrating leukocytes are acknowledged as key players in the pathogenesis of OA. Chondrocytes, more specifically, produce cytokines that promote autocrine effects, inducing synthesis of catabolic enzymes and inflammatory mediators, for instance73 According to reports, inflammatory cytokines interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α) disrupt cartilage homeostasis and promote degenerative alterations due to the catabolic effects of secreted matrix metalloproteinases (MMPs) which digest cartilage matrix.73 Mitochondria, once again, become fragile targets in the pathogenesis of OA. IL-1β and TNF-α cause damage to mitochondrial DNA, decreased energy production and mitochondrial transcription, therefore triggering apoptosis in chondrocytes.74 Furthermore, ROS and RNS are also major culprits in the progression of OA, as previously introduced. In addition to attacking proteins, lipids, nucleic acids and matrix components, these pro-oxidative agents also serve as intracellular signaling molecules that escalate the inflammatory response in osteoarthritic joints.7,75,76 Elaborating further, in the synovial capsule these oxidants coordinate synergistic effects on chondrocytes because they initiate inflammatory reactions which dysregulate the ECM turnover, ultimately destroying cartilage.77
Since OA is in great part an oxidative stress disease, the antioxidant levels of OA patients tend to decrease in the synovial fluid, eventually.10 This is a particularly noteworthy hallmark because GSH depletion is reflective of a decrease in the synthesis of proteoglycan and type II collagen in chondrocytes.54 Furthermore, the enzymes responsible for GSH transformation have their activities increased in the progression of OA.76
To circumvent these complicated set of conditions, many researchers have experimented with joint viscosupplementation and evaluated various effects.
A study conducted by Yang KC54 aimed to investigate the effects of GSH administration to HA on FLS retrieved from OA patients. At first glance, cell morphology and GAG production suffered no alteration under treatment and, interestingly, the HA + 20% GSH group showed a significant decrease in cell survival in comparison to other groups. Naturally, IL-1β stimulation promoted expressive decreases in the total antioxidant capacity of cells. At the lowest concentration (HA + 5% GSH), antioxidant capacity was restored and intracellular ROS/RNS was decreased. Real-time PCR analysis revealed that in HA or HA + GSH cell cultures, mRNA levels of inflammatory markers (cytokines and catabolic enzymes) were significantly down-regulated. It would appear that GSH greatly enhances the antioxidant potential and attenuates pro-inflammatory expression in osteoarthritic samples when combined with HA in low concentrations.
N-acetylcysteine (NAC), a glutathione precursor, is another suitable interventional alternative discussed in the literature. This precursor molecule can scavenge ROS in both direct and indirect manners by penetrating the cell membrane and reacting with glutamic acid and glycine to form glutathione, intracellularly.6 A previous in vivo study78 aimed to evaluate the effects of NAC in a murine experimental model of osteoarthritis. Nakagawa et al. extracted and isolated rat chondrocytes and administered sodium nitroprusside dehydrate (SNP), producing NO and therefore promoting apoptosis. The cells were then treated with various concentrations of NAC (0–2 mM) in order to determine the nature of the results. After running multiple biochemical assays, the authors learned that NAC was able to prevent NO-induced apoptosis, overproduction of ROS, p53 up-regulation and caspase-3 activation. Additionally, the authors report that the protective effects of NAC are significantly impeded by buthionine sulfoximine, a glutathione synthetase inhibitor. This also indicates that the ability of NAC to prevent apoptosis is heavily dependent upon glutathione availability. In this particular setting, NAC yielded optimistic results in preventing chondrocyte apoptosis and cartilage degeneration. On a side note, it appears that NAC diffuses rather quickly into joint tissue following in vivo administration. Designing drug delivery systems for sustained release of bioactive compounds in order to reduce the number of injections may therefore prove to be a strategy of great benefit to clinicians.78
Additional investigations further reinforced the protective roles of NAC in inflammatory conditions. This antioxidant also appears to suppress the deleterious effects of other pro-oxidant agents such as oxidized low-density lipoprotein (Ox-LDL) and hydrogen peroxide (H2O2) on collagen type X expression, which is linked to hypertrophy-like changes in chondrocytes.79 NAC administration can block the hypertrophic differentiation of articular chondrocytes triggered by H2O2 and Ox-LDL-mediated upregulation of Runx2, one of the genes that contribute to OA pathogenesis via promotion of chondrocyte hypertrophy and matrix breakdown.80 In cartilage and synovia derived from human OA patients, NAC may reduce the secretion of PGE2 and the expression of COX-2 and MMP-13 protein in synoviocytes stimulated by IL-1β.81 In stimulated chondrocytes, however, NAC does not appear to significantly affect the synthesis of the aforementioned inflammatory mediators, indicating that this supplement might display a more symptomatic effect on the synovium instead of a structural effect on cartilage.81
A recent pilot study,82 however, aimed to establish the relative effectiveness of intra-articular injection of NAC and HA on pain, function and cartilage degradation markers in individuals suffering from mild to moderate knee OA. Ozcamdalli and colleagues recruited 20 patients who were diagnosed with Kellgren-Lawrence grade 2–3 knee OA, randomly allocating the participants to the HA or NAC treatment groups to receive 3 mL single shots of either HA or NAC. Functional status and pain were recorded before and after the treatment administration, based on the Western Ontario and McMaster Universities Arthritis Index (WOMAC) and the visual analogue scale (VAS) scores. Inflammatory marker concentrations were recorded pre- and post-treatment as well. After studying the results, the authors learned that intra-articular injections of both HA and NAC generated expressive reduction in cartilage degradation and inflammation markers. However, NAC decreased total oxidant status concentration to a greater extent than HA, indicative of the antioxidant capacity of NAC. Additionally, the glutathione precursor appears to be more effective in reducing synovial fluid degradation markers MMP-3, chondroitin-6-sulfate and cross-linked C-terminal telopeptide of type II collagen. No effects on total antioxidant concentration were perceived in this particular scenario. Regarding pain and functional improvements, significant changes in both the VAS and WOMAC scores were identified in both groups. Overall, NAC does seem to be effective in reducing some but not all cartilage degradation and inflammation markers when compared to HA, considering significant improvements in pain and functional outcomes. However, due to the fact that NAC is comparably cheaper than HA, it could provide a more financially intelligent alternative in many cases.82
6. Conclusion
In this manuscript we discuss and shed light on the known protective mechanisms of glutathione, shielding vital molecules and cells from oxidative damage and stabilizing impaired redox equilibrium. The recent demonstration of the importance of oxidative stress in osteoarthritis places the use of intra-articular antioxidants, namely glutathione and its precursor, as an important interventional strategy for both joint preparation for biologic therapies as well as a powerful ally in viscosupplementation. Multiple studies have presented the numerous beneficial effects and safety of the administration of glutathione for the management of debilitating musculoskeletal disorders, especially OA. These effects include: reestablishment of the redox equilibrium; attenuation of the exacerbated oxidative stress and inflammation, which is harmful to cellular structures and biological components; and an appropriate regulation of cellular growth and death. In addition to being safe and effective, the administration of glutathione is comparably cheaper to other alternatives, offering a financially viable solution for many patients. There is still an insufficient amount of publications specifically evaluating the effects of intra-articular administration of antioxidants for the management of human OA. As such, additional investigations are highly warranted in order to further illustrate the potential benefits of the intra-articular delivery of antioxidants in diseased joints.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgements
We would like to thank Instituto do Osso e da Cartilagem for the support and José Fábio Lana for his remarkable academic contributions towards the production of this manuscript.
Contributor Information
Thiago Setti, Email: thiagosetti@hotmail.com.
Miguel Gustavo Luz Arab, Email: mglarab@hotmail.com.
Gabriel Silva Santos, Email: gabriel1_silva@hotmail.com.
Natasha Alkass, Email: natasha.alkass@outlook.com.
Marco Antonio Percope Andrade, Email: mapa.bhz@terra.com.br.
José Fábio Santos Duarte Lana, Email: josefabiolana@gmail.com.
References
- 1.Hafsi K., McKay J., Li J. Nutritional, metabolic and genetic considerations to optimise regenerative medicine outcome for knee osteoarthritis. J Clin Orthop Trauma. 2019;10(1) doi: 10.1016/j.jcot.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grover A.K., Samson S.E. Benefits of antioxidant supplements for knee osteoarthritis: Rationale and reality. Nutr J. 2016 doi: 10.1186/s12937-015-0115-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wallace I.J., Worthington S., Felson D.T. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc Natl Acad Sci U S A. 2017 doi: 10.1073/pnas.1703856114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lana J.F., Macedo A., Ingrao I.L.G., Huber S.C., Santos G.S., Santana M.H.A. Leukocyte-rich PRP for knee osteoarthritis: current concepts. J Clin Orthop Trauma. 2019 doi: 10.1016/j.jcot.2019.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia B., Chen Di, Zhang J., Hu S., Jin H., Tong P. Osteoarthritis pathogenesis: a review of molecular mechanisms. Calcif Tissue Int. 2014 doi: 10.1007/s00223-014-9917-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lepetsos P., Papavassiliou A.G. ROS/oxidative stress signaling in osteoarthritis. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2016 doi: 10.1016/j.bbadis.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Henrotin Y., Kurz B., Aigner T. 2005. Oxygen and Reactive Oxygen Species in Cartilage Degradation: Friends or Foes? Osteoarthritis and Cartilage. [DOI] [PubMed] [Google Scholar]
- 8.Li D., Xie G., Wang W. Reactive oxygen species: the 2-edged sword of osteoarthritis. Am J Med Sci. 2012 doi: 10.1097/MAJ.0b013e3182579dc6. [DOI] [PubMed] [Google Scholar]
- 9.Zhuang C., Wang Y., Zhang Y., Xu N. Oxidative stress in osteoarthritis and antioxidant effect of polysaccharide from angelica sinensis. Int J Biol Macromol. 2018 doi: 10.1016/j.ijbiomac.2018.04.083. [DOI] [PubMed] [Google Scholar]
- 10.Regan E.A., Bowler R.P., Crapo J.D. Joint fluid antioxidants are decreased in osteoarthritic joints compared to joints with macroscopically intact cartilage and subacute injury. Osteoarthritis Cartilage. 2008 doi: 10.1016/j.joca.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Altindag O., Erel O., Aksoy N., Selek S., Celik H., Karaoglanoglu M. Increased oxidative stress and its relation with collagen metabolism in knee osteoarthritis. Rheumatol Int. 2007 doi: 10.1007/s00296-006-0247-8. [DOI] [PubMed] [Google Scholar]
- 12.Wojdasiewicz P., Poniatowski Ł.A., Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat Inflamm. 2014 doi: 10.1155/2014/561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Man G.S., Mologhianu G. Osteoarthritis pathogenesis - a complex process that involves the entire joint. Journal of medicine and life. 2014 [PMC free article] [PubMed] [Google Scholar]
- 14.Poulet B., Beier F. Targeting oxidative stress to reduce osteoarthritis. Arthritis Res Ther. 2016 doi: 10.1186/s13075-015-0908-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farnaghi S., Prasadam I., Cai G. Protective effects of mitochondria-targeted antioxidants and statins on cholesterolinduced osteoarthritis. Faseb J. 2017 doi: 10.1096/fj.201600600R. [DOI] [PubMed] [Google Scholar]
- 16.Dupré-Crochet S., Erard M., Nüβe O. ROS production in phagocytes: why, when, and where? J Leukoc Biol. 2013 doi: 10.1189/jlb.1012544. [DOI] [PubMed] [Google Scholar]
- 17.Das K., Roychoudhury A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Frontiers in Environmental Science. 2014 [Google Scholar]
- 18.Schieber M., Chandel N.S. ROS function in redox signaling and oxidative stress. Curr Biol. 2014 doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sena L.A., Chandel N.S. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012 doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rhee S.G., Bae Y.S., Lee S.R., Kwon J. Hydrogen peroxide: A key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE : Signal Transduct Knowl Environ. 2000 doi: 10.1126/stke.2000.53.pe1. [DOI] [PubMed] [Google Scholar]
- 21.Hui W., Young D.A., Rowan A.D., Xu X., Cawston T.E., Proctor C.J. Oxidative changes and signalling pathways are pivotal in initiating age-related changes in articular cartilage. Ann Rheum Dis. 2016 doi: 10.1136/annrheumdis-2014-206295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hwang H.S., Kim H.A. Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int J Mol Sci. 2015 doi: 10.3390/ijms161125943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birben E., Sahiner U.M., Sackesen C., Erzurum S., Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organization Journal. 2012 doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNeill E., Crabtree M.J., Sahgal N. Regulation of iNOS function and cellular redox state by macrophage Gch1 reveals specific requirements for tetrahydrobiopterin in NRF2 activation. Free Radic Biol Med. 2015 doi: 10.1016/j.freeradbiomed.2014.10.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamer T.M. Hyaluronan and synovial joint: function, distribution and healing. Interdiscipl Toxicol. 2013 doi: 10.2478/intox-2013-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masuko K., Murata M., Yudoh K., Kato T., Nakamura H. Anti-inflammatory effects of hyaluronan in arthritis therapy: not just for viscosity. Int J Gen Med. 2009 doi: 10.2147/ijgm.s5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foster N.E., Healey E.L., Holden M.A. A multicentre, pragmatic, parallel group, randomised controlled trial to compare the clinical and cost-effectiveness of three physiotherapy-led exercise interventions for knee osteoarthritis in older adults: the BEEP trial protocol (ISRCTN: 93634563) BMC Muscoskel Disord. 2014 doi: 10.1186/1471-2474-15-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salli A., Sahin N., Baskent A., Ugurlu H. The effect of two exercise programs on various functional outcome measures in patients with osteoarthritis of the knee: a randomized controlled clinical trial. Isokinet Exerc Sci. 2010 [Google Scholar]
- 29.Kidd V.D., Strum S.R., Strum D.S., Shah J. Genicular nerve radiofrequency ablation for painful knee arthritis: the why and the how. JBJS Essent Surg Tech. 2019 doi: 10.2106/JBJS.ST.18.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.González-Rodríguez M.L., Fernández-Romero A.M., Rabasco A.M. Towards the antioxidant therapy in Osteoarthritis: Contribution of nanotechnology. J Drug Deliv Sci Technol. 2017 [Google Scholar]
- 31.Goldstein J.L., Cryer B. Gastrointestinal injury associated with NSAID use: a case study and review of risk factors and preventative strategies. Drug Healthc Patient Saf. 2014 doi: 10.2147/DHPS.S71976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qvist P., Bay-Jensen A.C., Christiansen C., Dam E.B., Pastoureau P., Karsdal M.A. The disease modifying osteoarthritis drug (DMOAD): is it in the horizon? Pharmacol Res. 2008 doi: 10.1016/j.phrs.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 33.Panahi Y., Alishiri G.H., Parvin S., Sahebkar A. Mitigation of systemic oxidative stress by curcuminoids in osteoarthritis: results of a randomized controlled trial. J Diet Suppl. 2016 doi: 10.3109/19390211.2015.1008611. [DOI] [PubMed] [Google Scholar]
- 34.Anand P., Kunnumakkara A.B., Newman R.A., Aggarwal B.B. Bioavailability of curcumin: Problems and promises. Mol Pharm. 2007 doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 35.Nelson K.M., Dahlin J.L., Bisson J., Graham J., Pauli G.F., Walters M.A. The essential medicinal Chemistry of curcumin. J Med Chem. 2017 doi: 10.1021/acs.jmedchem.6b00975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schiborr C., Kocher A., Behnam D., Jandasek J., Toelstede S., Frank J. The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes. Mol Nutr Food Res. 2014 doi: 10.1002/mnfr.201300724. [DOI] [PubMed] [Google Scholar]
- 37.Sophia Fox A.J., Bedi A., Rodeo S.A. The basic science of articular cartilage: structure, composition, and function. Sport Health. 2009 doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pradal J., Jordan O., Allémann E. Intra-articular drug delivery for arthritis diseases: the value of extended release and targeting strategies. J Drug Deliv Sci Technol. 2012 [Google Scholar]
- 39.Maheu E., Rannou F., Reginster J.Y. Efficacy and safety of hyaluronic acid in the management of osteoarthritis: evidence from real-life setting trials and surveys. Semin Arthritis Rheum. 2016 doi: 10.1016/j.semarthrit.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 40.Oliveira M.Z., Albano M.B., Stirma G.A., Namba M.M., Vidigal L., Cunha LAM da. Intra-articular viscosupplementation of hyaluronic acids in an experimental osteoarthritis model. Rev Bras Ortop. 2018 doi: 10.1016/j.rboe.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bowman S., Awad M.E., Hamrick M.W., Hunter M., Fulzele S. Recent advances in hyaluronic acid based therapy for osteoarthritis. Clin Transl Med. 2018 doi: 10.1186/s40169-017-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu S.P., Hunter D.J. Intra-articular therapies for osteoarthritis. Expet Opin Pharmacother. 2016 doi: 10.1080/14656566.2016.1232396. [DOI] [PubMed] [Google Scholar]
- 43.Bolduc J.A., Collins J.A., Loeser R.F. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic Biol Med. 2019 doi: 10.1016/j.freeradbiomed.2018.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loeser R.F., Collins J.A., Diekman B.O. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016 doi: 10.1038/nrrheum.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drevet S., Gavazzi G., Grange L., Dupuy C., Lardy B. Reactive oxygen species and NADPH oxidase 4 involvement in osteoarthritis. Exp Gerontol. 2018 doi: 10.1016/j.exger.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 46.Kimura Y., Goto Y.I., Kimura H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxidants Redox Signal. 2010 doi: 10.1089/ars.2008.2282. [DOI] [PubMed] [Google Scholar]
- 47.Sekhar R.V., Patel S.G., Guthikonda A.P. Deficient synthesis of glutathione underlies oxidative stress in aging and can be corrected by dietary cysteine and glycine supplementation. Am J Clin Nutr. 2011 doi: 10.3945/ajcn.110.003483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jozefczak M., Remans T., Vangronsveld J., Cuypers A. Glutathione is a key player in metal-induced oxidative stress defenses. Int J Mol Sci. 2012 doi: 10.3390/ijms13033145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diaz-Vivancos P., De Simone A., Kiddle G., Foyer C.H. Glutathione - Linking cell proliferation to oxidative stress. Free Radic Biol Med. 2015 doi: 10.1016/j.freeradbiomed.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 50.Forman H.J., Zhang H., Rinna A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol Aspect Med. 2009 doi: 10.1016/j.mam.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masip L., Veeravalli K., Georgiou G. The many faces of glutathione in bacteria. Antioxidants Redox Signal. 2006 doi: 10.1089/ars.2006.8.753. [DOI] [PubMed] [Google Scholar]
- 52.Gaucher C., Boudier A., Bonetti J., Clarot I., Leroy P., Parent M. Glutathione: antioxidant properties dedicated to nanotechnologies. Antioxidants. 2018 doi: 10.3390/antiox7050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu S.C. Glutathione synthesis. Biochim Biophys Acta Gen Subj. 2013 doi: 10.1016/j.bbagen.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang K.C., Wu C.C., Chen W.Y., Sumi S., Huang T Le. l-Glutathione enhances antioxidant capacity of hyaluronic acid and modulates expression of pro-inflammatory cytokines in human fibroblast-like synoviocytes. J Biomed Mater Res. 2016 doi: 10.1002/jbm.a.35729. [DOI] [PubMed] [Google Scholar]
- 55.Lu S.C. Regulation of glutathione synthesis. Mol Aspect Med. 2009 doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garcia-Ruiz C., Fernandez-Checa J.C. Journal of Gastroenterology and Hepatology (Australia) 2006. Mitochondrial glutathione: Hepatocellular survival-death switch. [DOI] [PubMed] [Google Scholar]
- 57.Dalle-Donne I., Rossi R., Colombo G., Giustarini D., Milzani A. Protein S-glutathionylation: a regulatory device from bacteria to humans. Trends Biochem Sci. 2009 doi: 10.1016/j.tibs.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 58.Liu R.M., Gaston Pravia K.A. Oxidative stress and glutathione in TGF-β-mediated fibrogenesis. Free Radic Biol Med. 2010 doi: 10.1016/j.freeradbiomed.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rahman I., Biswas S.K., Jimenez L.A., Torres M., Forman H.J. Glutathione, stress responses, and redox signaling in lung inflammation. Antioxidants Redox Signal. 2005 doi: 10.1089/ars.2005.7.42. [DOI] [PubMed] [Google Scholar]
- 60.Foley T.D., Kintner M.E. Brain PP2A is modified by thiol-disulfide exchange and intermolecular disulfide formation. Biochem Biophys Res Commun. 2005 doi: 10.1016/j.bbrc.2005.03.108. [DOI] [PubMed] [Google Scholar]
- 61.Kamata H., Honda S.I., Maeda S., Chang L., Hirata H., Karin M. Reactive oxygen species promote TNFα-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005 doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 62.Rao R.K., Clayton L.W. Regulation of protein phosphatase 2A by hydrogen peroxide and glutathionylation. Biochem Biophys Res Commun. 2002 doi: 10.1016/S0006-291X(02)00268-1. [DOI] [PubMed] [Google Scholar]
- 63.Pallardó F.V., Markovic J., García J.L., Viña J. Role of nuclear glutathione as a key regulator of cell proliferation. Mol Aspect Med. 2009 doi: 10.1016/j.mam.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 64.Diaz Vivancos P., Wolff T., Markovic J., Pallardó F.V., Foyer C.H. A nuclear glutathione cycle within the cell cycle. Biochem J. 2010 doi: 10.1042/BJ20100409. [DOI] [PubMed] [Google Scholar]
- 65.Lemasters J.J. Dying a thousand deaths: Redundant pathways from different organelles to apoptosis and necrosis. Gastroenterology. 2005 doi: 10.1053/j.gastro.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 66.Garcia-Ruiz C., Fernández-Checa J.C. Journal of Gastroenterology and Hepatology (Australia) 2007. Redox regulation of hepatocyte apoptosis. [DOI] [PubMed] [Google Scholar]
- 67.Ballatori N., Krance S.M., Notenboom S., Shi S., Tieu K., Hammond C.L. Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem. 2009 doi: 10.1515/BC.2009.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Franklin C.C., Krejsa C.M., Pierce R.H., White C.C., Fausto N., Kavanagh T.J. Caspase-3-dependent cleavage of the glutamate-L-cysteine ligase catalytic subunit during apoptotic cell death. Am J Pathol. 2002 doi: 10.1016/S0002-9440(10)61135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arrich J., Piribauer F., Mad P., Schmid D., Klaushofer K., Müllner M. Intra-articular hyaluronic acid for the treatment of osteoarthritis of the knee: systemic review and meta-analysis. CMAJ (Can Med Assoc J) 2005 doi: 10.1503/cmaj.1041203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Printz J.O., Lee J.J., Knesek M., Urquhart A.G. Conflict of interest in the assessment of hyaluronic acid injections for osteoarthritis of the knee: an updated systematic review. J Arthroplasty. 2013 doi: 10.1016/j.arth.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 71.Fam H., Bryant J.T., Kontopoulou M. Rheological properties of synovial fluids. Biorheology. 2007 [PubMed] [Google Scholar]
- 72.Akmal M., Singh A., Anand A. The effects of hyaluronic acid on articular chondrocytes. J Bone Jt Surg - Ser B. 2005 doi: 10.1302/0301-620X.87B8.15083. [DOI] [PubMed] [Google Scholar]
- 73.Zwerina J., Hayer S., Tohidast-Akrad M. Single and combined inhibition of tumor necrosis factor, interleukin-1, and RANKL pathways in tumor necrosis factor-induced arthritis: effects on synovial inflammation, bone erosion, and cartilage destruction. Arthritis Rheum. 2004 doi: 10.1002/art.11487. [DOI] [PubMed] [Google Scholar]
- 74.Kim J., Xu M., Xo R. Mitochondrial DNA damage is involved in apoptosis caused by pro-inflammatory cytokines in human OA chondrocytes. Osteoarthritis Cartilage. 2010 doi: 10.1016/j.joca.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 75.Bubici C., Papa S., Dean K., Franzoso G. Mutual cross-talk between reactive oxygen species and nuclear factor-kappa B: molecular basis and biological significance. Oncogene. 2006 doi: 10.1038/sj.onc.1209936. [DOI] [PubMed] [Google Scholar]
- 76.Ostalowska A., Birkner E., Wiecha M. Lipid peroxidation and antioxidant enzymes in synovial fluid of patients with primary and secondary osteoarthritis of the knee joint. Osteoarthritis Cartilage. 2006 doi: 10.1016/j.joca.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 77.Hiramitsu T., Yasuda T., Ito H. Intercellular adhesion molecule-1 mediates the inhibitory effects of hyaluronan on interleukin-1β-induced matrix metalloproteinase production in rheumatoid synovial fibroblasts via down-regulation of NF-κB and p38. Rheumatology. 2006 doi: 10.1093/rheumatology/kel026. [DOI] [PubMed] [Google Scholar]
- 78.Nakagawa S., Arai Y., Mazda O. N-acetylcysteine prevents nitric oxide-induced chondrocyte apoptosis and cartilage degeneration in an experimental model of osteoarthritis. J Orthop Res. 2010 doi: 10.1002/jor.20976. [DOI] [PubMed] [Google Scholar]
- 79.He Y., Siebuhr A.S., Brandt-Hansen N.U. Type X collagen levels are elevated in serum from human osteoarthritis patients and associated with biomarkers of cartilage degradation and inflammation. BMC Muscoskel Disord. 2014 doi: 10.1186/1471-2474-15-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kishimoto H., Akagi M., Zushi S. Induction of hypertrophic chondrocyte-like phenotypes by oxidized LDL in cultured bovine articular chondrocytes through increase in oxidative stress. Osteoarthritis Cartilage. 2010 doi: 10.1016/j.joca.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 81.Roman-Blas J.A., Contreras-Blasco M.A., Largo R., Álvarez-Soria M.A., Castañeda S., Herrero-Beaumont G. Differential effects of the antioxidant n-acetylcysteine on the production of catabolic mediators in IL-1β-stimulated human osteoarthritic synoviocytes and chondrocytes. Eur J Pharmacol. 2009 doi: 10.1016/j.ejphar.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 82.Ozcamdalli M., Misir A., Kizkapan T.B. Cartilage; 2017. Comparison of Intra-articular Injection of Hyaluronic Acid and N-Acetyl Cysteine in the Treatment of Knee Osteoarthritis: A Pilot Study. [DOI] [PMC free article] [PubMed] [Google Scholar]