Abstract
Background
The ability to grow new cartilage remains the standard goal of any treatment strategy directed at cartilage repair. Chondroprogenitors have garnered interest due to their applicability in cell therapy. Pulsed electromagnetic field (PEMF) favors chondrogenesis by possible upregulation of genes belonging to TGFβ superfamily. Since TGFβ is implicated in chondrogenic signalling, the aim of the study was to evaluate the ability of PEMF to induce chondrogenesis via endogenous TGFβ production in chondroprogenitors vs differentiation using chondrogenic medium inclusive of TGFβ.
Methods
Chondroprogenitors were harvested from three non-diseased human knee joints via fibronectin assay. Passage 3 pellets were subjected to four different culture conditions: a) negative control contained chondrogenic medium without TGFβ2, b) positive control contained medium with TGFβ2, c) PEMF 1 contained medium of negative control plus single exposure to PEMF and d) PEMF 2 contained medium of negative control plus multiple exposures to PEMF. Following differentiation (day 21), pellets were assessed for gene expression of ACAN, SOX9, COL2A1, TGFβ1, TGFβ2, and TGFβ3. Alcian blue staining to detect glycosaminoglycan deposition was also performed. Medium supernatant was used to detect endogenous latent TGF-β1 levels using ELISA.
Results
All study arms exhibited comparable gene expression without any significant difference. Although positive control and PEMF study arms demonstrated notably better staining than negative control, the level of latent TGF-β1 was seen to be significantly high in supernatant from positive control (P < 0.05) when compared to other groups.
Conclusion
Our results indicate that PEMF induced chondrogenesis might involve other signalling molecules, which require further evaluation.
Keywords: Chondroprogenitors, Pulsed electromagnetic field, Chondrogenic differentiation
1. Introduction
Articular cartilage, made up of hyaline tissue, plays a vital role in joint mobility and weight bearing.1 Chondrocytes, sparsely distributed within the cartilage, help in the production of collagenous proteins and the dense extracellular matrix. Relative avascularity and aneural microenvironment contribute to poor healing of articular cartilage in disease states.2 Cell-based therapies play a significant role in cartilage repair, although regeneration via cellular implantation requires further optimization, i.e. enhancing the chondrogenic potential and limiting hypertrophy. Cell types most commonly employed are, namely, mesenchymal stem cells (MSCs) and chondrocytes.3 The major limitations with their use remain formation of fibrocartilage or a combination of fibro and hyaline tissue, both of which are suboptimal and result in inferior quality tissue with subpar biomechanical properties.4,5
The discovery of chondroprogenitors has amassed interest due to its potential role in cartilage regeneration. These cells were first identified by Dowthwaite et al. in the superficial zone of articular cartilage,6 while further in-vitro characterization demonstrated that chondroprogenitors conform to minimal criteria for defining MSCs as per International Society for Cellular Therapy 2006.7 Classified as MSCs, they additionally displayed higher SOX9 expression and telomerase activity when compared to chondrocytes.8,9 Further reports show that, when compared to bone marrow (BM) derived MSCs and chondrocytes, chondroprogenitors have better repair tissue with lower expression of hypertrophic markers, thus posing a more significant potential for cartilage repair.10,11
The pulsed electromagnetic field (PEMF) is a non-interventional and non-pharmacological reparative technique that boasts of several clinical applications.12 Use of electromagnetic field bursts has been shown to market proliferation and regeneration of damaged tissue.13,14 Based on this, several clinical studies have demonstrated that PEMF effectively promotes wound healing, accelerates fusion of fractured bones and aids with the healing of osteotomies.14, 15, 16 In cartilage tissue regeneration, PEMF use has been linked to increased chondrocyte proliferation, extracellular matrix synthesis and protection from detrimental effects of inflammatory cytokines in an osteoarthritic environment.17,18 Results from an in-vivo rabbit study showed significant healing of an osteochondral defect, scaffolded with calcium phosphate when exposed to PEMF therapy.19 With regard to lineage differentiation, PEMF has well-documented physiological effects, which importantly include the upregulation of expression in TGFβ superfamily members20,21 demonstrating higher expression TGFβ 1 and TGFβ2, both known transcription factors associated with chondrogenic differentiation.22,23
In this study, our aim was to evaluate the role of PEMF as signal for endogenous TGFβ production during in-vitro chondrogenic differentiation of human articular cartilage derived chondroprogenitors when compared to standard chondrogenic differentiation medium (containing TGFβ).
2. Methods
2.1. Study design
The study was carried out following approval from the Institutional review board and as per guidelines laid down by the Ethics Panel. Three non-diseased human knee joints (were procured from patients undergoing above knee amputation as a part of their treatment). All experiments were conducted only after obtaining written informed consent. Chondrocytes were obtained by enzymatic digestion of cartilage slices and further subjected to fibronectin adhesion assay. Chondroprogenitors retrieved following the assay were further expanded to passage 3. Harvested chondroprogenitors were subjected to flow cytometric analysis for positive MSC markers: CD105, CD73 and CD90, negative MSC markers: CD34 and CD45 and integrin marker: CD49e. Chondroprogenitors grown in 3D pellet cultures were used for chondrogenic differentiation. The pellets were differentiated using four different protocols for 21 days which comprised the four study arms (Fig. 1). We assessed human latent TGFβ1 levels in the supernatant collected from the chondrogenic medium being added to the chondroprogenitors. Post differentiation, the pellets were subjected to Alcian blue staining for evaluation of glycosaminoglycan production and also homogenized to evaluate mRNA expression of markers of chondrogenesis (ACAN, SOX9 and COL2A1) and members of TGFβ superfamily (TGFβ1, TGFβ2 and TGFβ3).
Fig. 1.
Study algorithm outlining the four chondrogenic differentiation conditions used for comparison. Samples were taken from three donors in each group. FACS: flow cytometric analysis, CD: cluster of differentiation, FCS: fetal calf serum, HEPES: 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, TGF: transforming growth factor, PEMF: pulsed electromagnetic field, ACAN: Aggrecan, SOX9: SRY-box9 and COL2A1: Collagen type 2A1.
2.2. Isolation of chondroprogenitors
Shavings of whole depth articular cartilage were obtained from non-diseased human knee joints (n = 3, mean age:22 ± 4 yrs) and minced. The sequential enzymatic digestion was performed using 12IU of Pronase for 3 h, followed by 100IU of collagenase type II for 16 h. The digested tissue was passed through a 40 μm strainer to obtain single cells, and the viability assessed using trypan blue dye exclusion test. Two-thousand cells were loaded on a fibronectin (10 μg/ml) coated plate and allowed to incubate under standard culture conditions for a period of 20min. The non-adherent cells were removed, and the remaining cells cultured to achieve a minimum population doubling of 5 (over 10–12 days). The clonal cells were trypsinized and expanded in culture using basal medium containing GlutaMax DMEM-F12 (Dulbecco Eagles medium) with 10% fetal calf serum (FCS), 10 mM HEPES(4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), 0.1 mM ascorbic acid, 2 mM l-glutamine, 1 ng/ml human TGFβ2 and 5 ng/ml FGF224. The medium was replaced once in 3 days and cells were cultured to sub confluence. Following this, the cells were trypsinized and replated to obtain subsequent passage (sub-culture).
2.3. Flow cytometric analysis (FACS)
Passage 3 chondroprogenitors were washed with phosphate buffer solution (PBS) and incubated with CD105-FITC, CD73-PE, CD90-PE, CD34-PE, CD45-FITC and CD49e-PE as per manufacturers protocol. BD FACS Celesta flow cytometer was used for data acquisition. Unstained control and isotype control were also run for each conjugate. Data obtained was analysed using BD FACS Diva v 5.0.2 software.
2.4. 3D pellet differentiation study arms
Passage 3 chondroprogenitors were added at a concentration of 1 million cells per microcentrifuge tube containing basal medium. The cell suspension was centrifuged and left undisturbed for 48 h to form a pellet. Following this, the medium was replaced with differentiation medium. The basal medium used for differentiation composed of DMEM-GlutaMax, 2% FCS, ascorbic acid (50 μg/ml), d-glucose (1 mg/ml), 10 mM HEPES, 1% Insulin Transferrin Selenium, 0.1 μM dexamethasone, sodium pyruvate (1 mg/ml), penicillin-streptomycin (100IU/ml) and amphotericin-B (2 μg/ml). For the negative control as well as the two PEMF arms, only basal differentiation medium was used. At the same time, TGFβ2 (5 ng/ml) was additionally supplemented to the basal differentiation medium for the positive control. In the PEMF 1 study arm, the pellet was stimulated with a single exposure (2 mT, signal frequency 15 Hz, and sinewave pulse duration 6 msec, Fig. S1) for 10 min at day 0 of differentiation as specified by Parate et al.,25 while for the PEMF 2 study arm, stimulation was performed for 10min during each exposure accompanying the medium change occurring every three days (7 exposures in 21 days). A complete medium change was performed every third day with care taken not to disturb the pellet. At the end of 21 days, the pellets were subjected to Alcian blue staining and RNA isolation.
2.5. Latent TGFβ1 levels
To estimate endogenous production of TGFβ1 levels, supernatant of the medium used was collected 18 h following a medium change, centrifuged, and stored at −70 °C till further evaluation. The collection time point was fixed based on prior timeline analysis (data not shown). The culture supernatant was collected on day 1,4,7,10, 13, 16,19 and 22. The quantitative determination of latent human TGFβ1 was performed using MABTECH ELISA development kit (product code: 3550-1H-6). In brief, 96 well microplates were coated with monoclonal antibody at a concentration of 2 μg/100μl/well of PBS (pH 7.4) at 4 °C for 12 h. The plates were then blocked with 0.05% Tween 20 containing 0.1% bovine serum albumin (incubation buffer) and washed. This was followed by addition of sample or standards, (100μl/well) diluted with incubation buffer for 1 h at room temperature. Following a wash, the microplates were sequentially incubated with Streptavidin-HRP (1:1000) and TMB substrate. After a suitable developing time, the optical density was measured at 450 nm by an ELISA reader.
2.6. Reverse transcriptase-polymerase chain reaction (RT-PCR)
Post differentiation the pellets were homogenized using a pellet pestle, and total RNA was isolated using TRIzol reagent as per manufacturer’s instructions. The isolated RNA was quantified using Nanodrop 2000c spectrophotometer at 260 nm and 280 nm. RNA was reverse transcribed to cDNA using Takara Bio 1st strand DNA synthesis kit. Quantitative RT-PCR was performed using Applied Biosystem QuantStudio 6 K Flex with TakyonTM Low Rox SYBR Master Mix Dttp Blue (Eurogentec). Each sample was run in duplicates. The relative mRNA expression for each target gene was normalized to the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression. Sequences of the primers used for this study are listed in Table S1.
2.7. Alcian Blue
To visualize glycosaminoglycan accumulation following differentiation, the pellets were subjected to fixation, stained with Alcian Blue (pH 2.5) and counterstained with neutral red.
2.8. Statistical analysis
The offline line analysis was performed using Microsoft excel and the graphical data representation using IGOR Pro Version 5.0.4.8 (Wave metrics Inc.). Statistical analysis was done using SPSS software version 17.0. Comparison of study arms was made using one-way ANOVA, post hoc Bonferroni correction. Values were expressed as mean ± standard deviation and a P-value of <0.05 was considered to be significant.
3. Results
3.1. Chondroprogenitor isolation and characterization using FACS
Chondroprogenitors exhibited clonal growth following fibronectin adhesion assay and were expanded to passage 3 (Fig. 2). They showed positive expression of CD105, CD73, CD90, CD49e and negative expression of CD34 and CD45 (Table 1).
Fig. 2.
Representative clonally derived human articular chondroprogenitor cells (Fibronectin differential adhesion). A) A clone at day 4 forming a loose cluster of few cells (40X). B) Clone cluster at day 10 (10X). C) Expanded chondroprogenitors at passage 3 (10X).
Table 1.
Flow cytometric data (percentage expression) of CD markers at passage 3. Data is expressed as percentage mean ± SD (n = 3).
| CD marker | Passage 3 mean ± SD | |
|---|---|---|
| Positive MSC markers | CD105 | 58.1 ± 43.93 |
| CD 73 | 98.7 ± 1.2 | |
| CD 90 | 99.97 ± 0.06 | |
| Negative MSC markers | CD34 | 0.77 ± 0.83 |
| CD45 | 1.3 ± 1.82 | |
| Integrin marker | CD49e | 99.97 ± 0.06 |
3.2. Latent TGFβ1 levels
All groups displayed similar baseline TGFβ1 levels on day 1 of differentiation, following which a gradual decrease was noted up to day 10. The positive control group showed a significant increase in TGFβ1 levels at day 13 (vs negative control, P = 0.026; vs PEMF 1, P = 0.025; vs PEMF 2, P = 0.024), day 16 (vs negative control, PEMF 1 and PEMF 2, P < 0.001) and day 19 (vs negative control, P = 0.040; vs PEMF 1, P = 0.008; vs PEMF 2, P = 0.003) (Fig. 3).
Fig. 3.
Human latent TGFβ1 levels from supernatant medium between the four groups used for comparison at different timepoints. Value expressed in picomoles (pm) as mean ± SD (n = 3). PEMF1: pellet stimulated with a single exposure of pulsed electromagnetic field and PEMF2: pellet stimulated with multiple exposure of pulsed electromagnetic field (7 exposures in 21 days).
3.3. RT-PCR
When gene expression was compared between the four study groups, no significant difference was observed for markers of chondrogenesis and members of TGFβ superfamily assessed. There was high expression of ACAN, SOX9, TGFβ1, TGFβ2 and TGFβ3 among all the groups with low expression of COL2A1 (Fig. 4).
Fig. 4.
Relative expression of Aggrecan (ACAN), SRY-box9(SOX9), Collagen type II(COL2A1) and transforming growth factor β 1–3 (TGFβ1-3) in all groups. ΔCt values normalized to GAPDH are expressed as Mean ± SD. Samples taken from n = 3 donors, each sample(n) was run in duplicate. NC: negative control. PC: positive control.
3.4. Alcian blue stain
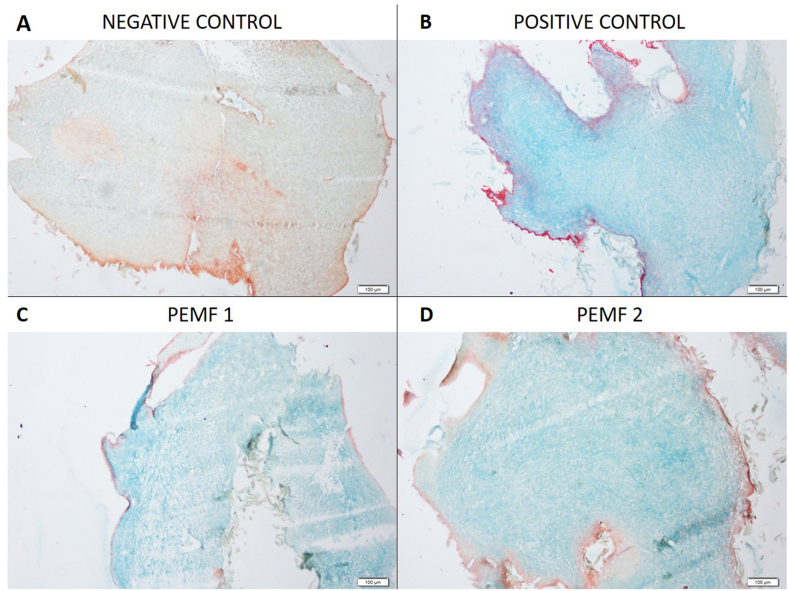
Alcian blue staining indicating glycosaminoglycan deposition showed that positive control, PEMF 1 and PEMF 2 revealed comparable uptake, which was notably better than staining seen in the negative control (Fig. 5).
Fig. 5.
Chondrogenic differentiation of chondroprogenitors. Images of formed pellets post chondrogenic differentiation (A-D). Representative microscopic images of Alcian Blue staining of formed pellets. Magnification: 10×. PEMF1: pellet stimulated with a single exposure of pulsed electromagnetic field and PEMF2: pellet stimulated with multiple exposure of pulsed electromagnetic field (7 exposures in 21 days).
4. Discussion
Focus on the use of chondroprogenitors as a cell-based treatment for cartilage related pathologies has gained popularity due to the inherent potential observed in these cells. Few reports point to the capacity displayed by PEMF, to induce chondrogenesis, by upregulation of genes belonging to TGFβ superfamily, which show involvement in chondrogenic differentiation.20, 21, 22 Thus, in this study, we evaluated the role of PEMF in stimulating endogenous TGFβ production and in differentiating chondroprogenitors towards a chondrogenic lineage. On analysis of gene expression and GAG deposition, equivalent levels were noted between the PEMF study arms and positive control. Interestingly, no difference was observed between the two PEMF arms, where-in the frequency of electromagnetic field pulsed through the pellets differed. This was in contrast to previous literature, reporting optimal chondrogenic outcome in MSC’s subjected to brief and low-intensity exposure (specified by Parate et al.) as compared to prolonged and repetitive exposures, which resulted in decreased collagen type II expression and loss of chondrogenic phenotype.25, 26, 27 Another noteworthy observation was the increase in latent TGFβ1 levels, observed in positive control on day 16 of culture.
As TGFβ1 has been documented as an essential signalling molecule in the chondrogenic cascade, this paracrine release seen during culture warrants further evaluation, as progenitors could be used to facilitate manufacturing of conditioned medium. Although this was the first study to document the endogenous production of latent TGFβ1 by human articular cartilage derived chondroprogenitors in culture; anticipated results were not noted in the PEMF arms. This leads us to believe that differentiation seen due to PEMF induction causes possible stimulation of genes other than TGFβ1, TGFβ2 or TGFβ3. As there are multiple other chemokines implicated in chondrogenic differentiation and only a few genes were assessed by mRNA or ELISA analysis, the study is limited in its capacity to suggest an alternative mechanism. It would also have been beneficial if a larger sample size was available so as to draw specific conclusions. Moreover, inclusion of another study arm to assess the effect of both PEMF and supplemental TGFβ2 in the chondrogenic medium would provide further insight.
To conclude, though PEMF study arms exhibited glycosaminoglycan deposition comparable to positive control, latent TGFβ1 levels were seen to be significantly lower in the PEMF group. The inference from this observation, coupled with results of gene expression, suggest that the physiological basis for chondrogenesis achieved via utilization of PEMF involves activation of signalling factors, other than the members of TGFβ superfamily assessed in the study. However, confirmation of the same requires an in-depth analysis of signalling factors associated with PEMF induced chondrogenesis. Due to its non-invasive nature and potential role in chondrogenic induction, further evaluation of PEMF is warranted, as an adjunct therapy in treatment for cartilage repair and regeneration.
5. Funding and acknowledgement
This project was supported by Institutional Fluid grant, Christian Medical College, Vellore (IRB Min no: 11066 dated December 20, 2017).
The authors would like to acknowledge Madras Institute of Magnetobiology for their assistance with the device and its standardization and Centre for Stem Cell Research (A unit of inStem, Bengaluru), Christian Medical College, Vellore for infrastructural support.
Declaration of competing interest
All authors declare no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcot.2020.09.034.
Contributor Information
Elizabeth Vinod, Email: elsyclarence@cmcvellore.ac.in.
Upasana Kachroo, Email: upasana_k@hotmail.com.
Grace Rebekah, Email: gracerebekah@gmail.com.
Sajo Thomas, Email: sajothomas86@gmail.com.
Boopalan Ramasamy, Email: jpboopy@gmail.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
References
- 1.Sophia Fox A.J., Bedi A., Rodeo S.A. The basic science of articular cartilage. Sport Health. 2009;1(6):461–468. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buckwalter J.A., Mankin H.J. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998;47:487–504. [PubMed] [Google Scholar]
- 3.Mobasheri A., Kalamegam G., Musumeci G., Batt M.E. Chondrocyte and mesenchymal stem cell-based therapies for cartilage repair in osteoarthritis and related orthopaedic conditions. Maturitas. 2014;78(3):188–198. doi: 10.1016/j.maturitas.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 4.Karuppal R. Current concepts in the articular cartilage repair and regeneration. J Orthop. 2017;14(2):A1–A3. doi: 10.1016/j.jor.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Bari C., Roelofs A.J. Stem cell-based therapeutic strategies for cartilage defects and osteoarthritis. Curr Opin Pharmacol. 2018;40:74–80. doi: 10.1016/j.coph.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Dowthwaite G.P., Bishop J.C., Redman S.N. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004;117(Pt 6):889–897. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- 7.Dominici M., Le Blanc K., Mueller I. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 8.Williams R., Khan I.M., Richardson K. Identification and clonal characterisation of a progenitor cell sub-population in normal human articular cartilage. PLoS One. 2010;5(10) doi: 10.1371/journal.pone.0013246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan I.M., Bishop J.C., Gilbert S., Archer C.W. Clonal chondroprogenitors maintain telomerase activity and Sox9 expression during extended monolayer culture and retain chondrogenic potential. Osteoarthritis Cartilage. 2009;17(4):518–528. doi: 10.1016/j.joca.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 10.McCarthy H.E., Bara J.J., Brakspear K., Singhrao S.K., Archer C.W. The comparison of equine articular cartilage progenitor cells and bone marrow-derived stromal cells as potential cell sources for cartilage repair in the horse. Vet J Lond Engl. 2012;192(3):345–351. doi: 10.1016/j.tvjl.2011.08.036. 1997. [DOI] [PubMed] [Google Scholar]
- 11.Kachroo U., Vinod E. Comparative analysis of gene expression between articular cartilage-derived cells to assess suitability of fibronectin adhesion assay to enrich chondroprogenitors. Knee. 2020;27(3):755–759. doi: 10.1016/j.knee.2020.04.015. [DOI] [PubMed] [Google Scholar]
- 12.Galli C., Pedrazzi G., Mattioli-Belmonte M., Guizzardi S. The use of pulsed electromagnetic fields to promote bone responses to biomaterials in vitro and in vivo. Int J Biomater. 2018;2018 doi: 10.1155/2018/8935750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Girolamo L., Stanco D., Galliera E. Low frequency pulsed electromagnetic field affects proliferation, tissue-specific gene expression, and cytokines release of human tendon cells. Cell Biochem Biophys. 2013;66(3):697–708. doi: 10.1007/s12013-013-9514-y. [DOI] [PubMed] [Google Scholar]
- 14.Hannemann P.F.W., van Wezenbeek M.R., Kolkman K.A. CT scan-evaluated outcome of pulsed electromagnetic fields in the treatment of acute scaphoid fractures: a randomised, multicentre, double-blind, placebo-controlled trial. Bone Jt J. 2014;96-B(8):1070–1076. doi: 10.1302/0301-620X.96B8.33767. [DOI] [PubMed] [Google Scholar]
- 15.Kwan R.L.-C., Wong W.-C., Yip S.-L., Chan K.-L., Zheng Y.-P., Cheing G.L.-Y. Pulsed electromagnetic field therapy promotes healing and microcirculation of chronic diabetic foot ulcers: a pilot study. Adv Skin Wound Care. 2015;28(5):212–219. doi: 10.1097/01.ASW.0000462012.58911.53. [DOI] [PubMed] [Google Scholar]
- 16.Midura R.J., Ibiwoye M.O., Powell K.A. Pulsed electromagnetic field treatments enhance the healing of fibular osteotomies. J Orthop Res Off Publ Orthop Res Soc. 2005;23(5):1035–1046. doi: 10.1016/j.orthres.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 17.Chang S.-H., Hsiao Y.-W., Lin H.-Y. Low-frequency electromagnetic field exposure accelerates chondrocytic phenotype expression on chitosan substrate. Orthopedics. 2011;34(1):20. doi: 10.3928/01477447-20101123-10. [DOI] [PubMed] [Google Scholar]
- 18.Veronesi F., Fini M., Giavaresi G. Experimentally induced cartilage degeneration treated by pulsed electromagnetic field stimulation; an in vitro study on bovine cartilage. BMC Muscoskel Disord. 2015;16 doi: 10.1186/s12891-015-0760-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boopalan P.R.J.V.C., Arumugam S., Livingston A., Mohanty M., Chittaranjan S. Pulsed electromagnetic field therapy results in healing of full thickness articular cartilage defect. Int Orthop. 2011;35(1):143–148. doi: 10.1007/s00264-010-0994-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aaron R.K., Wang S., Ciombor D.M. Upregulation of basal TGFbeta1 levels by EMF coincident with chondrogenesis--implications for skeletal repair and tissue engineering. J Orthop Res Off Publ Orthop Res Soc. 2002;20(2):233–240. doi: 10.1016/S0736-0266(01)00084-5. [DOI] [PubMed] [Google Scholar]
- 21.Anbarasan S., Baraneedharan U., Paul S.F., Kaur H., Rangaswami S., Bhaskar E. Low dose short duration pulsed electromagnetic field effects on cultured human chondrocytes: an experimental study. Indian J Orthop. 2016;50(1):87–93. doi: 10.4103/0019-5413.173522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aaron R.K., Ciombor D.M., Keeping H., Wang S., Capuano A., Polk C. Power frequency fields promote cell differentiation coincident with an increase in transforming growth factor-beta(1) expression. Bioelectromagnetics. 1999;20(7):453–458. doi: 10.1002/(sici)1521-186x(199910)20. 7<453::aid-bem7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 23.Boopalan P., Chittaranjan S., Balamurugan R., Nandakumar N.S., Sabareeswaran A., Mohanty M. Pulsed electromagnetic field (PEMF) treatment for fracture healing. Curr Orthop Pract. 2009;20(4):423–428. doi: 10.1097/BCO.0b013e318198e8b2. [DOI] [Google Scholar]
- 24.Nelson L., McCarthy H.E., Fairclough J., Williams R., Archer C.W. Evidence of a viable pool of stem cells within human osteoarthritic cartilage. Cartilage. 2014;5(4):203–214. doi: 10.1177/1947603514544953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parate D., Franco-Obregón A., Fröhlich J. Enhancement of mesenchymal stem cell chondrogenesis with short-term low intensity pulsed electromagnetic fields. Sci Rep. 2017;7(1):9421. doi: 10.1038/s41598-017-09892-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayer-Wagner S., Passberger A., Sievers B. Effects of low frequency electromagnetic fields on the chondrogenic differentiation of human mesenchymal stem cells. Bioelectromagnetics. 2011;32(4):283–290. doi: 10.1002/bem.20633. [DOI] [PubMed] [Google Scholar]
- 27.Wang J., Tang N., Xiao Q. Pulsed electromagnetic field may accelerate in vitro endochondral ossification. Bioelectromagnetics. 2015;36(1):35–44. doi: 10.1002/bem.21882. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.