Abstract
Objectives:
We present a standardized in vitro microfluidic assay and Occlusion Index (OI) for the assessment of red blood cell (RBC)-mediated microcapillary occlusion and its clinical associations in sickle cell disease (SCD).
Methods:
RBC-mediated microcapillary occlusion represented by OI and its clinical associations were assessed for 7 subjects with hemoglobin-SC disease (HbSC), 18 subjects with homozygous SCD (HbSS), and 5 control individuals (HbAA).
Results:
We identified two sub-populations with HbSS based on the OI distribution. HbSS subjects with relatively higher OIs had significantly lower hemoglobin levels, lower fetal hemoglobin (HbF) levels, and lower mean corpuscular volume (MCV), but significantly higher serum lactate dehydrogenase levels and absolute reticulocyte counts, compared to subjects with HbSS and lower OIs. HbSS subjects who had relatively higher OIs were more likely to have had a concomitant diagnosis of intrapulmonary shunting (IPS). Further, lower OI associated with hydroxyurea (HU) responsiveness in subjects with HbSS, as evidenced by significantly elevated HbF levels and MCV.
Conclusions:
We demonstrated that RBC mediated microcapillary occlusion and OI associated with subject clinical phenotype and HU responsiveness in SCD. The presented standardized microfluidic assay may be useful for evaluating clinical phenotype and assessing therapeutic outcomes in SCD, including emerging targeted and curative treatments that aim to improve RBC deformability and microcirculatory health.
Keywords: Erythrocytes, microfluidics, microcirculation, microvascular occlusion, sickle cell disease
INTRODUCTION
Healthy RBCs are biconcave, with an average diameter of 7.5-8.7 μm and a thickness of 1.7-2.2 μm 1, 2. In the human body, RBCs flow through capillaries as small as 4 μm and interendothelial clefts that are less than 1 μm to complete each cycle of circulation 3, 4. Therefore, the cytoskeletal structures of RBCs must undergo extensive and repeated tension/compression cycles during normal blood flow through the microvasculature. The ability of healthy RBCs to extensively deform is due to their large surface area to-volume ratio, low cytoplasmic viscosity, and the intrinsic viscoelastic properties of their membranes 5, 6. Abnormal RBC rheology has been associated with alterations in one or more of these factors 7, 8.
Altered RBC rheology is a major contributor to the clinical manifestations of SCD. SCD is a recessively inherited beta-globin gene disorder 9, in which abnormal hydrophobic hemoglobin, HbS 4, 10, polymerizes into long and stiff intracellular structures under certain physiologic conditions (e.g., dehydration and deoxygenation). Abnormal HbS polymerization ultimately leads to the formation of sickle-shaped and excessively stiff RBCs 11, 12. Stiff and sickle-shaped RBCs are susceptible to mechanical fatigue and to shedding-off microvesicles that contain cell-free hemoglobin or heme, causing disturbed blood flow, vascular inflammation, and endothelial dysfunction, which cumulatively result in VOEs and/or vasculopathy, the principle pathophysiologic manifestations of SCD 13–17. Clinical complications, arising from this abnormal Hb, include acute and chronic pain 18–20, wide-spread organ damage 21, and early mortality 22.
Since SCD arises from a single gene mutation, its clinical heterogeneity is unexpected. The frequency of VOEs, which is the hallmark of SCD and an important index of disease severity, varies considerably from subject to subject, even with identical genotypes. This may be ascribed to variations in clinical management of SCD, such as transfusions or administration of hydroxyurea HU, which have distinct impacts on the pathophysiology of SCD 23–26. Many biorheological and hematological facets of SCD have been extensively studied to associate with disease severity, including RBC adherence 27–32, hemodynamic changes 33–36, HbS fractions 37–39, HbF levels 40, 41, serum LDH levels 42–44, and WBC counts 45, 46. However, the clinical impact of RBC deformability in the context of microcirculation, which denotes the ability of the cell to transverse microcapillary openings without obstructing the flow, is still poorly understood.
The RBC deformability is typically measured using custom AMVNs in research laboratories 47. We have developed a new microfluidic platform, the OcclusionChip 48. Unique features of the OcclusionChip are its architecture that mimics the capillary bed, coupled arteriovenous anastomosis-mimicking conduits to prevent full saturation or upstream clogging of the system, and importantly, an ability to process clinical blood samples at near-physiologic hematocrit in a standardized manner.
We tested RBCs from a clinically well-defined group of subjects with hemoglobin-SC disease (HbSC) or homozygous SCD (HbSS), where the resultant RBC mediated microcapillary occlusion was represented by an intuitive standardized parameter, the OI. Our results show that subjects with HbSC or HbSS exhibit distinct OI profiles. We found that subjects with HbSS who had relatively higher OIs had significantly lower Hb levels, lower HbF levels, and lower MCV, but significantly higher serum LDH levels and ARCs compared to those who had relatively lower OIs. Importantly, we found that HbSS subjects with relatively higher OIs are more likely to have a concomitant diagnosis of IPS. Importantly, we show that lower OI associates with HU responsiveness in subjects with SCD (HbSS), which was evidenced by significantly elevated HbF levels and MCV. Our results suggest that the presented standardized microfluidic assay may be useful for evaluating the clinical phenotype and assessing therapeutic outcomes in SCD, including emerging targeted and curative treatments that aim to improve RBC deformability and microcirculatory health.
METHODS
Microfluidic device and fabrication
The OcclusionChip was fabricated using PDMS under standard soft lithography protocols 48. Initially, a master silicon wafer was coated via SU8 photoresist patterning. PDMS pre-polymer was casted over the master wafer and cured at 80°C overnight. The PDMS block was then peeled off, and inlet and outlet holes were created with a 0.5 mm puncher. Thereafter, the PDMS block was irreversibly bonded to a standard microscope glass slide through oxygen plasma treatment. Tubing was assembled after the microchannel was fabricated (Figure 1A). The microchannel was 4-mm wide, 12-μm tall, and 25-mm long (Figure 1B). Each microchannel consisted of nine micropillar arrays forming uniform microcapillaries within each array but overall finer microcapillaries along the flow direction, from 20 μm down to 4 μm. We designed the microchannel such that RBCs with significantly impaired deformability would be retained by the upstream coarser arrays, while RBCs with moderately impaired deformability would be retained by downstream finer arrays (Figure 1C). Side pathways were incorporated to mimic arteriovenous anastomoses and to prevent saturation or upstream clogging of the microchannel (Figure 1D). A macro view of the OcclusionChip is visible in Figure 1E.
Figure 1. The OcclusionChip microfluidic platform allows standardized assessment of RBC mediated microcapillary occlusion.
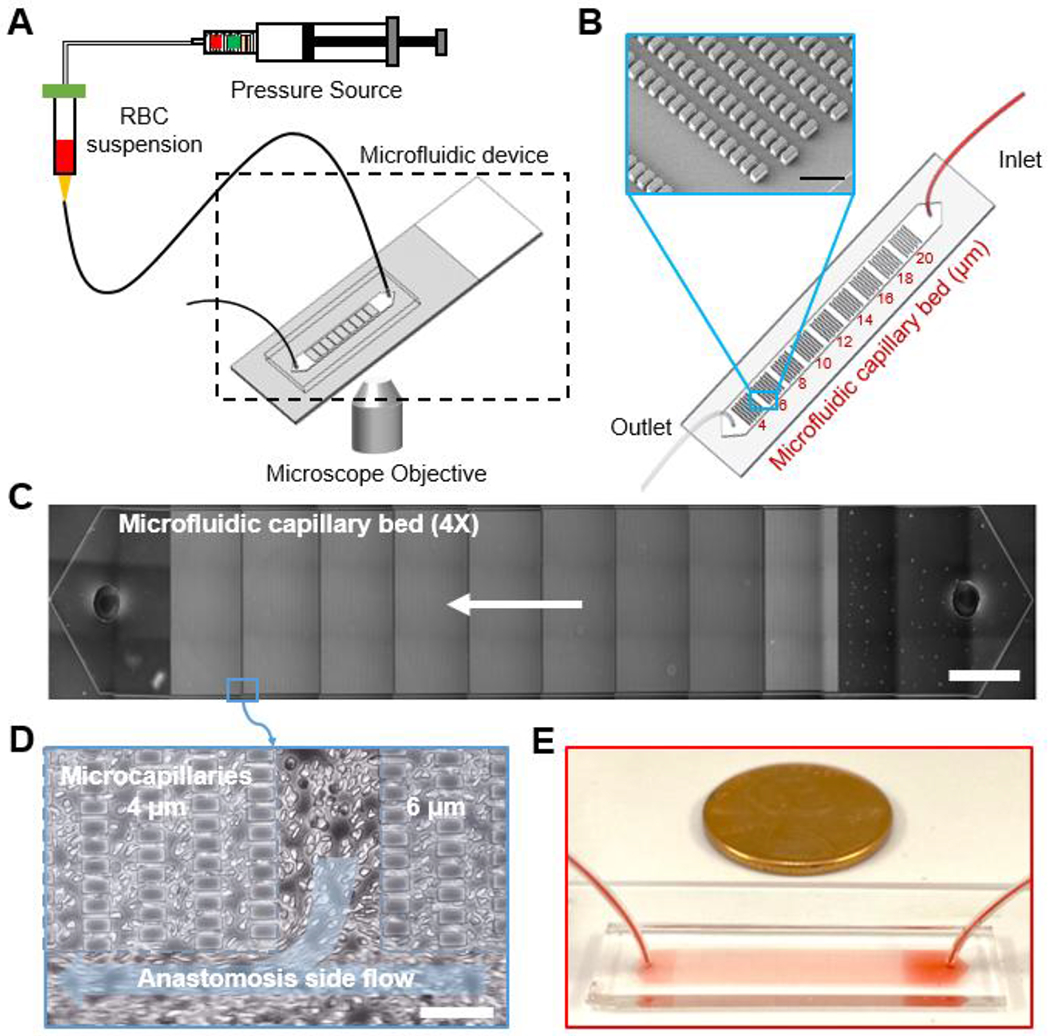
(A) A schematic view of the assay is shown. RBCs diluted in PBS at 20% hematocrit are infused into the microchannel using a manual syringe pump at constant pressure. (B) A schematic of the OcclusionChip design is shown. The OcclusionChip features nine micropillar arrays with microcapillaries ranging from 20 to 4 μm, coupled with two anastomosis-mimicking side pathways that are 60 μm wide. Inset: Scanning electron microscopy image showing the microvascular features. Scale bar represents a length of 50 μm. (C) The entire microchannel is shown at 4X magnification. Arrow indicates flow direction. Scale bar represents a length of 2 mm. (D) Close-up view showing the anastomosis side flow. Scale bar represents a length of 50 μm. (E) Macro view of the OcclusionChip with a blood sample is shown.
Blood sample collection
De-identified samples from non-anemic controls (HbAA) and subjects with HbSS or HbSC were collected in EDTA-containing vacutainers at the adult SCD clinic at UHCMC in Cleveland, Ohio under Institutional Review Board approved protocols, and stored at 4°C. Informed consent was obtained from all study participants. Clinical phenotypes of the subjects with SCD, such as WBC counts, platelet counts, ANCs, ferritin levels, serum LDH levels, and ARCs, were obtained following chart review. Hemoglobin composition was identified through HPLC analysis with the Bio-Rad Variant II Instrument (Bio-Rad, Montreal, QC, Canada) at the Core Laboratory of UHCMC. All microfluidic experiments in this study were performed within 48 hours of venipuncture. A total number of 5 non-anemic control subjects, 18 subjects with homozygous HbSS, and 7 subjects with HbSC were tested. All blood samples were obtained from patients at clinical steady state (non-crisis).
Microfluidic OcclusionChip assay
RBCs were isolated from whole blood samples by centrifuging at 500 × g for 5 min at room temperature. Plasma, buffy coat, and the near-plasma portion of the RBC layer were carefully removed. The isolated RBCs were then washed twice and re-suspended in phosphate-buffered saline (PBS, 1X) at 20% hematocrit. Microchannels were rinsed with pure ethanol and PBS, and incubated with 2% bovine serum albumin (BSA) at 4°C overnight to prevent non-specific cell adhesion to the microchannel walls. A manual syringe pump was designed to maintain a constant pressure of 60 cm H2O at the inlet of the microchannel 49, and the RBC suspension was allowed to flow for 20 minutes. Thereafter, non-retained RBCs were washed away with PBS under the same pressure. In some tests, retained RBCs were fluorescently labeled with anti-glycophorin A antibody (Abcam, Cambridge, MA) under flow for 30 min. An Olympus IX83 inverted motorized microscope with Olympus Cell Sense live-cell imaging and analysis software (excitation/emission wavelength, 488/505–580 for Green Fluorescent Protein, GFP) was used to obtain microscopic phase contrast and fluorescent images. Obtained microscope images were further processed by Adobe Photoshop software (San Jose, CA). Each data point in this study was generated by one OcclusionChip assay on one blood sample, and the OcclusionChip was discarded after one-time use.
Occlusion Index determination
We have previously reported the generalization of an intuitive standardized parameter, OI 48, which is computed based on the distribution of occlusions in a specific microcapillary network, as also shown in the Supporting Information and Figure S1. The OI thereby represents the overall percentage occlusion of the microcapillary network within the area of interest. The area of interest in the OcclusionChip is defined as the 4-μm to 10-μm micropillar arrays, since typical capillary dimension is within 5-10 μm, and no appreciable microcapillary occlusion was observed in the other arrays with larger openings.
Statistical methods
Data were reported as mean ± standard deviation (SD) in this study. Statistical analyses were carried out using Minitab 19 Software (Minitab Inc., State College, PA) and MATLAB (MathWorks, Natick, MA). Data were initially analyzed for normality, which was followed by appropriate multiple comparison tests to compare different groups, parametric one-way ANOVA for normally distributed data and non-parametric Mann-Whitney U test for non-normal data. Statistical significance was defined with P-value less than 0.05 (P < 0.05). To analyze the univariate model, Kernel density estimation was performed based on the OIs of N=18 blood samples for the population with homozygous SCD (HbSS), where the bandwidth was determined according to Silverman’s rule of thumb. A custom written code in MATLAB was used to generate the density distribution of the OI.
RESULTS
Occlusion Index is heterogeneous over a clinically diverse population with SCD
We analyzed OIs of RBCs from HbSS or HbSC subjects as well as from healthy individuals (HbAA). Representative images of RBC mediated microcapillary occlusion of one subject from each of the three cases are shown in Figure 2A–C. The values indicated in Figure 2A–C represent the corresponding OIs of the three shown subjects. Our results indicated that HbSS RBCs had significantly higher OIs than HbSC RBCs or HbAA RBCs (Figure 2D, mean OI ± SD = 2.02 ± 1.22% for HbSS, 0.52 ± 0.15% for HbSC, and 0.20 ± 0.04% for HbAA, P = 0.001 for HbSS vs HbAA and for HbSS vs HbSC, Mann-Whitney). Further, HbSC RBCs had significantly higher OIs relative to HbAA RBCs (Figure 2D, P = 0.001, one-way ANOVA).
Figure 2. Standardized microfluidic assessment of RBC mediated microvascular occlusion reveals a clinically diverse population in subjects with SCD.
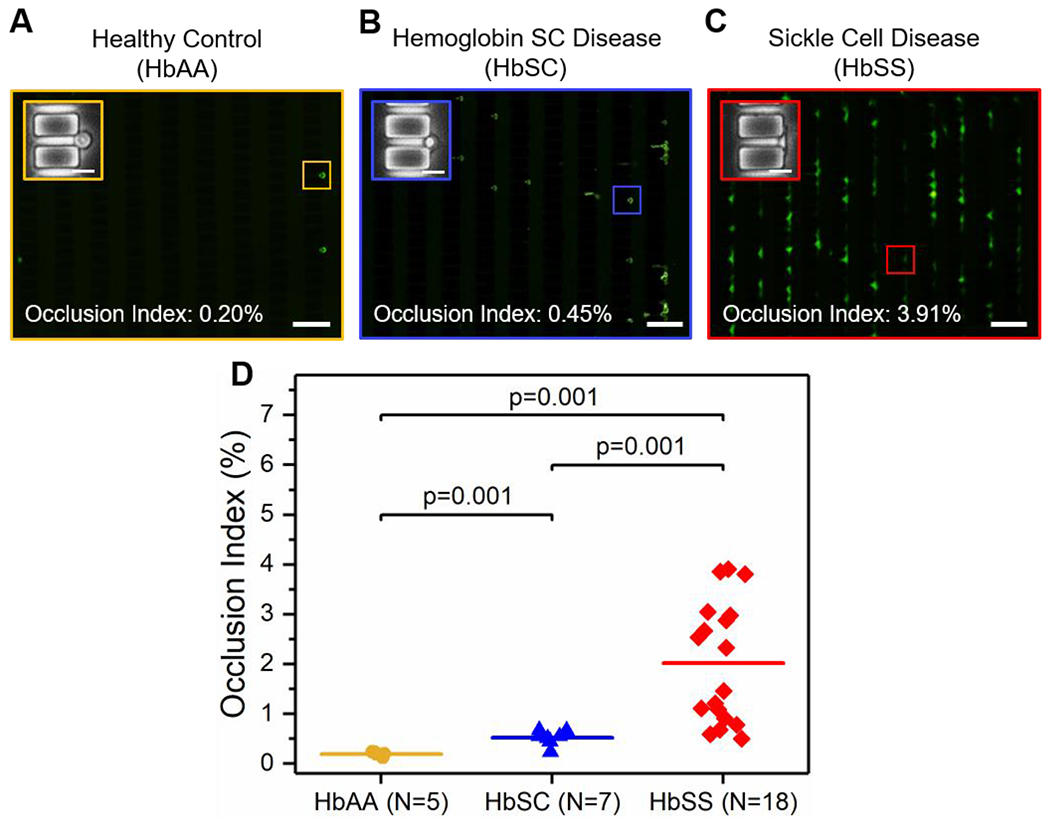
Representative fluorescent microscopy images showing retained RBCs from: (A) healthy individuals (HbAA), (B) subjects with HbSC, and (C) subjects with HbSS. Insets are close-up phase-contrast images showing individual retained cells in 4 μm microcapillaries. Values correspond to the Occlusion Indices of the three shown subjects. Scale bars represent 50 μm and 10 μm, respectively. (D) The Occlusion Index of HbSS RBCs is significantly higher than that of HbSC RBCs or HbAA RBCs, and the OI of HbSC RBCs are significantly higher than that of HbAA RBCs. Colored horizontal lines represent mean of data set. Statistical significance between groups defined by Mann-Whitney U test (P < 0.05).
Occlusion Index reflects the clinical variables of subjects with homozygous SCD
We identified a bimodal distribution of the kernel density of the OI in the population with homozygous SCD, from which two sub-populations were categorized based on the local minimum of 2.18% as a cutoff threshold (Figure 3A&B, Low OI group: mean OI ± SD = 0.93 ± 0.32%, and High OI group: mean OI ± SD = 3.11 ± 0.60%, P < 0.001, one-way ANOVA, N = 9 in each group). Next, we analyzed the clinical variables of the subjects in these two groups and found that subjects in the High OI group, who had significantly higher OIs compared to those in the Low OI group, presented significantly lower Hb levels (Figure 3C, mean ± SD = 8.0 ± 1.3 vs 9.2 ± 0.9 g/dL, P = 0.037 one-way ANOVA), and higher serum LDH levels (Figure 3D, mean ± SD = 451 ± 126 vs 222 ± 33 U/L, P < 0.001 one-way ANOVA) and higher ARCs (Figure 3E, mean ± SD = 356 ± 86 vs 174 ± 86 109/L, P = 0.001, one-way ANOVA). These findings point to an association between an elevated OI (RBC mediated microcapillary occlusion) and increased in vivo hemolysis in these subjects. In addition, subjects in the High OI group also presented significantly lower HbF levels (Figure 3F, mean ± SD = 3.5 ± 2.9 vs 17.6 ± 4.7%, P < 0.001, Mann-Whitney) and MCV (Figure 3G, mean ± SD = 92 ± 8 vs 113 ± 12 fL, P = 0.001, one-way ANOVA) compared to subjects in the Low OI group. No significant difference was observed in WBC counts and ANCs between the two groups (P > 0.05). The clinical variables of the study population with SCD are summarized in Table 1.
Figure 3. Occlusion Index (OI) associates with clinical variables in subjects with homozygous SCD (HbSS).
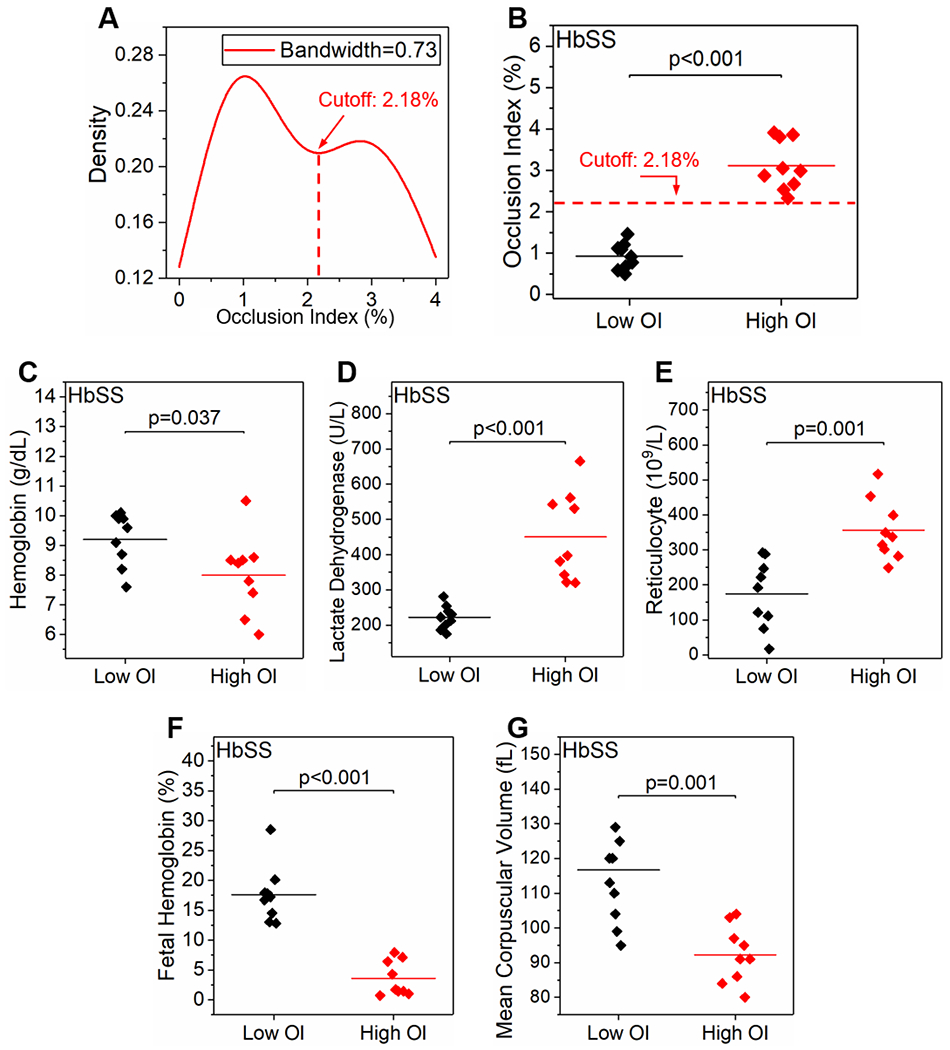
(A&B) A bimodal distribution of the kernel density of the OI among the subjects with homozygous SCD was identified. Subjects were categorized into two groups (Low OI group and High OI group) based on the local minimum of 2.18% as the cutoff threshold. Subjects with higher OIs displayed significantly lower hemoglobin levels (C), significantly higher serum lactate dehydrogenase levels (D) and absolute reticulocyte counts (E), and significantly lower fetal hemoglobin levels (F) and mean corpuscular volume (G), compared to those with lower OIs. Colored horizontal lines represent mean of data set. Statistical significance between groups defined by one-way ANOVA or Mann-Whitney U test depending on data normality (P < 0.05).
Table 1.
Clinical variables of the study population
| Clinical variables | Normal HbAA (Range; N=5) | HbSC (Mean ± SD; N=7) | HbSS Low OI (Mean ± SD; N=9) | HbSS High OI (Mean ± SD; N=9) | P-valueϯ |
|---|---|---|---|---|---|
| Occlusion Index (%) | 0.13–0.23 | 0.52 ± 0.15 | 0.93 ± 0.32 | 3.11 ± 0.60 | <0.001* |
| Age | N/A | 39 ± 7 | 39 ± 10 | 33 ± 9 | 0.233** |
| Hematocrit (%) | 36-50 | 32.3 ± 2.8 | 25.0 ± 3.75 | 23.4 ± 4.8 | 0.446* |
| Hemoglobin (g/dL) | 12–18 | 12 ± 1 | 9.2 ± 0.9 | 8.0 ± 1.3 | 0.037* |
| MCV (fL) | 82–95 | 82 ± 5 | 113 ± 12 | 92 ± 8 | 0.001* |
| WBC Count (109/L) | 4–11 | 11.5 ± 2.4 | 9.0 ± 3.2 | 10.1 ± 1.9 | 0.336* |
| Platelet Count (109/L) | 150–400 | 314 ± 84 | 389 ± 152 | 351 ± 108 | 0.552* |
| ANC (106/L) | 1500–8000 | 6896 ± 2600 | 5354 ± 2347 | 5482 ± 2012 | 0.903* |
| Reticulocyte (109/L) | 20–150 | 183 ± 54 | 174 ± 98 | 356 ± 86 | 0.001* |
| LDH (U/L) | 140–280 | 256 ± 194 | 222 ± 33 | 451 ± 126 | <0.001* |
| Ferritin (μg/L) | 12–300 | 182 ± 159 | 1218 ± 1572 | 544 ± 512 | 0.289** |
| Hemoglobin S (%) | N/A | 43.5 ± 7.3 | 71.3 ± 13.9 | 64.1 ± 30.2 | 0.659** |
| Hemoglobin A (%) | 99–100 | 6.2 ± 13.1 | 6.9 ± 13.5 | 24.9 ± 27.1 | 0.791** |
| Hemoglobin F (%) | 0–0.9 | 1.0 ± 1.1 | 17.6 ± 4.7 | 3.5 ± 2.9 | <0.001** |
| Diagnosis of Intrapulmonary Shunting | N/A | 0% of Subjects (0/7) | 44% of Subjects (4/9) | 89% of Subjects (8/9) | 0.046 (χ2) |
| On-hydroxyurea | N/A | 0% of Subjects (0/7) | 89% of Subjects (8/9) | 44% of Subjects (4/9) | 0.046 (χ2) |
A total of 30 blood samples were obtained from 5 healthy donors (HbAA), 7 subjects with hemoglobin-SC disease (HbSC), and 18 subjects with homozygous SCD (HbSS).
: P-value for comparing the Low OI group and the High OI group.
: Parametric one-way ANOVA.
: Non-parametric Mann-Whitney U test.
χ2: Chi-squared test.
SD: Standard deviation.
Occlusion Index associates with a concomitant diagnosis of intrapulmonary shunting over the study population with homozygous SCD
We have identified two sub-populations (Low OI group vs High OI group) with distinct clinical phenotypes in the study population with homozygous SCD based on their OI profiles. Next, we asked whether the two groups would differ in terms of other co-morbid clinical characteristics, including IPS, stroke, ACS, and PH. Interestingly, we found that 89% of subjects in the High OI group (8 out of 9) had a concomitant diagnosis of IPS, while compared with 44% of subjects in the Low OI group (4 out of 9, Table 1, P = 0.046, chi-squared test). We thereby compared the OI between the IPS and non-IPS subjects among the study HbSS population. The mean OI of the IPS group is higher compared to that of the non-IPS group, but we did not observe a statistically significant difference between the two groups (Table S1, mean OI ± SD = 2.23 ± 1.27% vs 1.60 ± 1.10%, P = NS). Comparisons of OIs relative to other co-morbidities are summarized in Table S1. We are planning to test this association prospectively.
Occlusion Index as a biomarker to evaluate the efficacy of hydroxyurea therapy and identify hydroxyurea non-responders
HU is a FDA-approved drug for the management of SCD, which has been shown to attenuate RBC sickling through an HbF-dependent mechanism 50. We asked whether HU therapy impacted the OI profiles of the study HbSS population. However, we did not observe a significant difference in OIs when comparing the on-HU (N = 12) subjects to those off-HU (N=6, mean OI ± SD = 1.87 ± 1.36% vs 2.32 ± 0.92%, P = NS), Figure 4A). We asked whether this observation could be attributed to HU non-responders. Therefore, we utilized HbF levels and MCV, and categorized the on-HU HbSS subjects into two sub-groups (HU responders vs non-responders) with distinct HbF levels (mean ± SD = 20.2 ± 4.8% vs 9.4 ± 3.9%, P = 0.006, Mann-Whitney) and MCV (mean ± SD = 121 ± 6 vs 100 ± 6 fL, P < 0.001, one-way ANOVA, Figure S2) via K-means clustering analysis. We found that the HU responders (N = 5) had significantly lower OIs compared to the HU non-responders (N = 7, Figure 4B, mean OI ± SD = 0.88 ± 0.24% vs 2.58 ± 1.38%, P = 0.023, one-way ANOVA). We also found that HU responders (N = 5) had relatively lower OIs compared to those subjects with HbSS who were not on HU at all (off-HU HbSS subjects, N = 6, P = 0.083, Mann-Whitney, Figure 4B). We did not observe a significant difference in the OIs when comparing the HU non-responders and the off-HU HbSS subjects (N = 6, P = NS, Figure 4B).
Figure 4. Occlusion Index (OI) associates with hydroxyurea (HU) therapy and can be utilized to identify HU non-response in SCD.
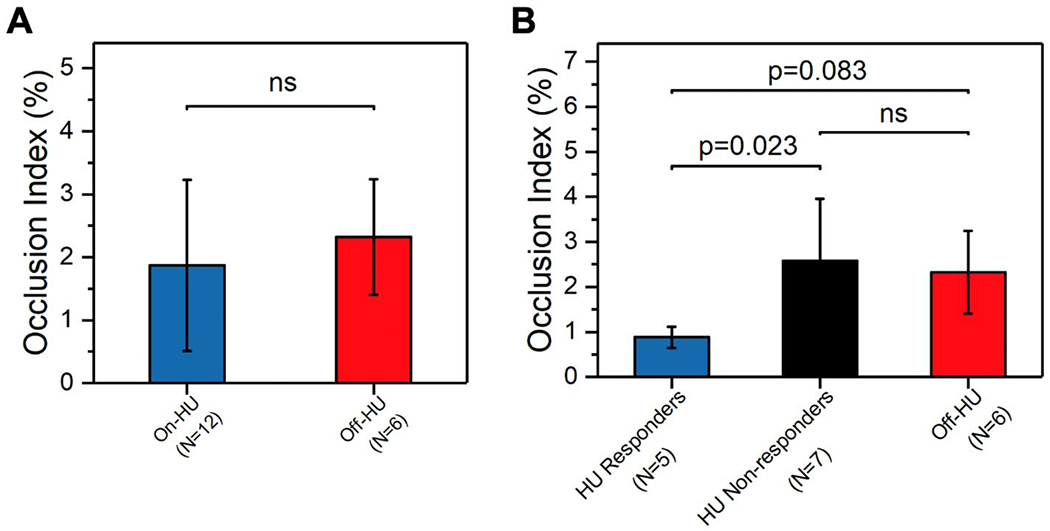
(A) No significant difference was observed in OI when comparing the on-HU subjects (N = 12) to off-HU subjects (N = 6) among the study HbSS population. (B) HU responders (N = 5) had significantly lower OIs compared to HU non-responders (N = 7, P = 0.023, Mann-Whitney). HU responders (N = 5) among the on-HU HbSS subjects had relatively lower OIs compared to off-HU HbSS subjects (N = 6, P = 0.083, Mann-Whitney). No significant difference was observed in the OI between HU non-responders (N = 7) and off-HU HbSS subjects (N = 6). Error bars represent standard deviation (SD). ns, no significance.
DISCUSSION
In this study, we utilized our recently developed OcclusionChip platform to assess microcapillary occlusion mediated by abnormal RBCs using samples from subjects with HbSC or HbSS disease. Washed RBCs were re-suspended in PBS at 20% hematocrit (1:4 v/v) and were then tested. To investigate the contribution that is solely made by the intrinsic RBC rheology on microcapillary occlusion and to standardize the microfluidic assay, we uniformly suspended the RBCs in a standard media at a fixed hematocrit level for all the study subjects. The 20% hematocrit level was chosen since this is within the hematocrit range of most HbSS subjects (Table 1), and this level allows high resolution of cell traversing and occlusion in the microchannel as shown previously 48. Of note, although re-suspending RBCs in patient plasma may better reflect unique patient physiology, this may also introduce other factors, such as external oxidative stress, that may attribute to differential microcapillary occlusion. Further, hematocrit value varies within the study population (Table 1), and could itself alter the OI.
HbSC disease in which RBCs contain approximately 50% HbS and 50% HbC 51, is comparatively neglected in clinical studies, largely due to its milder clinical complications compared to HbSS 52. However, existing studies have shown that serious complications such as acute pain, anemia, organ failure, and increased mortality still occur in HbSC 22, 52–54. From a molecular point of view, the pathogenesis of HbSC disease stems from an activated K+ and Cl− co-transport that results in loss of water and an increased mean corpuscular hemoglobin concentration 55. Such cellular dehydration effect further amplifies the polymerization of the 50% of HbS in the RBC. Here, our results show that even though the OI of HbSC RBCs is relatively lower compared to that of HbSS RBCs, it was still abnormal, and may contribute to pathological microvascular occlusions. These results are consistent with the interpretation that RBC deformability decreases with increasing polymer fraction, as suggested in previous studies 56, 57. Overall, these in vitro observations agree with the clinical observation that HbSC disease is a mild form of SCD with a lower prevalence of vasculopathy.
Low Hb levels, and high serum levels of LDH and ARCs have been associated with intravascular hemolysis and with overall disease severity in SCD 42, 58, 59. Increased ARCs in SCD result from reticulocytes prematurely released from the bone marrow as a consequence of hemolytic and hypoxic stress. Reticulocytes are about 8% larger than normal mature RBCs, and are considered less deformable because of their spherical shape with smaller surface area to-volume ratio, the presence of a mass of chromatin granules in their cytoplasm, and the less optimal organization of the lipids and proteins on their membranes 60–62. However, such abnormality in deformability is insufficient to obstruct the blood flow in the microvasculature, since circulating reticulocytes are also found in other inherited hemolytic anemias, such as pyruvate kinase deficiency, without causing symptomatic microvascular occlusion 63. On the other hand, decreased deformability of sickle, dehydrated RBCs due to abnormal HbS polymerization, is well characterized in SCD 64, 65. However, whether a more severe hemolytic phenotype will be accompanied by decreased RBC deformability and thereby enhanced RBC mediated microcapillary occlusion is still unclear. Here, our results demonstrated that subjects with a more severe hemolytic phenotype presented higher OIs and thereby had increased RBC mediated microcapillary occlusion, providing an additional link between hemolysis and VOEs. Such observation may partially reflect the presence of circulating reticulocytes in the blood samples, but more likely, it could be attributed to the ongoing hemolysis in these subjects, as hemolyzed RBCs release microvesicles from their cell membrane 66, which may lead to reduced surface area-to-volume ratio and consequent decreased deformability.
HU induces HbF production and increased MCV, which has a favorable impact on subjects with HbSS 67. Here, our OI results show that HbSS subjects with relatively lower OIs had significantly higher HbF levels and MCV (Figure 3E&F). We found that 89% (8 out of 9) of the subjects in the Low OI group were on-HU compared to 44% (4 out of 9) of the subjects in the High OI group (Table 1, p=0.046, chi-squared test). However, when comparing on-HU subjects with off-HU subjects among the HbSS population, we did not observe a significant difference in measured OIs (Figure 4A). We hypothesized that such discrepancies arise from the poorly understood molecular mechanism of HU therapy 68. We therefore segregated the HU responders from HU non-responders using HbF levels and MCV via K-means clustering analysis (Figure S2). We found that the OIs of HU responders were significantly lower compared to HU non-responders and were relatively lower compared to the off-HU HbSS subjects (Figure 4B). Altogether, these results were in accordance with previous studies about the benefits of HU; the OcclusionChip assay has the potential to evaluate the efficacy of HU therapy and identify HU non-responders who may require additional management to insure adherence or consideration for alternate therapies.
Pulmonary shunts allow a proportion of blood to bypass the pulmonary capillaries through large-diameter intrapulmonary arteriovenous anastomotic vessels that directly connect pulmonary arteries and veins. Pulmonary capillaries are the site for gas exchange in the lungs, and therefore, poorly oxygenated blood that flows through the shunt vessels does not participate in oxygenation 69. Moreover, pulmonary capillaries also act as natural filters to remove blood clots formed in the venous circulation 70. As a result, abnormal recruitment of IPS under pathological conditions may induce increased chronic hypoxemia and trigger further complications such as ACS 71. Even though the clinical and laboratory associations of IPS are beginning to be outlined, the underlying biophysical mechanisms of IPS have not been elucidated. In SCD, the pulmonary microvascular bypass may be especially detrimental, since intracellular HbS polymerizes under deoxygenated conditions, which significantly impairs the RBC deformability and increases the adhesiveness of the RBC, prominently in high risk patients 28, 39, 72. Here, our results show that HbSS subjects in the High OI group, who had significantly higher levels of pathological microcapillary occlusion, were more likely to have clinically reported IPS compared to those in the Low OI group (Table 1, p=0.046). The mean OI of the IPS group is relatively higher compared to that of the subjects without or not tested for IPS (Table S1), even though we did not observe a statistical significance (p>0.05). Although pathophysiologically plausible, (i.e. hypoxic stress on the RBC leading to a permanent inability of the sickle RBCs to fully re-oxygenate, increasing deformability, contributing to more severe microvascular occlusion in the lungs, resulting in shunting and in a vicious cycle), definitive causality awaits a dedicated prospective effort (e.g., a shunting-on-a-chip microfluidic platform) with carefully curated clinical associations.
A limitation of the study is the relatively small number of subjects, thereby limiting our understanding of treatment associations (transfusion, hydroxyurea, and supportive care i.e. absence of disease modifying therapies, Table S1). Future work will focus on prospectively demonstrating the clinical utility of this assay, as an in vitro therapeutic benchmark in a larger patient population, undergoing a range of treatments, including therapies with curative intent.
In conclusion, we have utilized the OcclusionChip technology to assess RBC mediated microcapillary occlusion in a clinically diverse population with HbSC or HbSS disease. Results suggest that RBC mediated microcapillary occlusion, represented by the OI, associates with subject clinical phenotypes including Hb levels, serum LDH levels, ARCs, HbF levels, and MCV. Notably, we observed that the measured OIs positively correlate with a concomitant diagnosis of IPS, which may have pathophysiological implications in the abnormal recruitment of IPS. Altogether, these findings suggest that RBC mediated microcapillary occlusion reflects clinical severity of SCD. The OcclusionChip and the standardized OI may offer significant benefit in improving effective monitoring and management of emerging targeted and curative therapies in SCD.
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge with gratitude the contributions of patients and clinicians at Seidman Cancer Center (University Hospitals, Cleveland). This work was supported under the grants of National Science Foundation (NSF) CAREER Award 1552782, National Heart, Lung, and Blood Institute (NHLBI) R01HL133574, OT2HL152643, U01HL117659, and T32HL134622.
Funding Information: National Heart, Lung, and Blood Institute Awards: R01HL133574, OT2HL152643, U01HL117659, and T32HL134622
Abbreviations:
- ACS
acute chest syndrome
- AMVNs
artificial microvascular networks
- ANCs
absolute neutrophil counts
- ARCs
absolute reticulocyte counts
- FDA
Food and Drug Administration
- Hb
hemoglobin
- HbF
fetal hemoglobin
- HbS
sickle hemoglobin
- HbSC
hemoglobin-SC disease
- HbSS
homozygous SCD
- HPLC
high-performance liquid chromatography
- HU
hydroxyurea
- IPS
intrapulmonary shunting
- LDH
lactate dehydrogenase
- MCV
mean corpuscular volume
- OI
Occlusion Index
- PDMS
polydimethylsiloxane
- PH
pulmonary hypertension
- RBC
red blood cell
- SCD
sickle cell disease
- UHCMC
University Hospitals Cleveland Medical Center
- VOEs
vaso-occlusive episodes
- WBC
white blood cell
Footnotes
CONFLICT OF INTEREST
None to declare.
REFERENCES
- 1.Sakai T, Hosoyamada Y. Are the precapillary sphincters and metarterioles universal components of the microcirculation? An historical review. The journal of physiological sciences : JPS. 2013; 63(5): 319–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diez-Silva M, Dao M, Han J, Lim CT, Suresh S. Shape and Biomechanical Characteristics of Human Red Blood Cells in Health and Disease. MRS bulletin. 2010; 35(5): 382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Safeukui I, Correas JM, Brousse V, Hirt D, Deplaine G, Mule S, et al. Retention of Plasmodium falciparum ring-infected erythrocytes in the slow, open microcirculation of the human spleen. Blood. 2008; 112(6): 2520–8. [DOI] [PubMed] [Google Scholar]
- 4.Barabino GA, Platt MO, Kaul DK. Sickle cell biomechanics. Annual review of biomedical engineering. 2010; 12: 345–67. [DOI] [PubMed] [Google Scholar]
- 5.Tomaiuolo G Biomechanical properties of red blood cells in health and disease towards microfluidics. Biomicrofluidics. 2014; 8(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuhn V, Diederich L, Keller TCSt, Kramer CM, Luckstadt W, Panknin C, et al. Red Blood Cell Function and Dysfunction: Redox Regulation, Nitric Oxide Metabolism, Anemia. Antioxidants & redox signaling. 2017; 26(13): 718–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LaCelle PL. Alteration of membrane deformability in hemolytic anemias. Seminars in hematology. 1970; 7(4): 355–71. [PubMed] [Google Scholar]
- 8.Mohandas N, Clark MR, Jacobs MS, Shohet SB. Analysis of factors regulating erythrocyte deformability. The Journal of clinical investigation. 1980; 66(3): 563–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weatherall DJ. The inherited diseases of hemoglobin are an emerging global health burden. Blood. 2010; 115(22): 4331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasan MN, Fraiwan A, An R, Alapan Y, Ung R, Akkus A, et al. Paper-based microchip electrophoresis for point-of-care hemoglobin testing. The Analyst. 2020; 145(7): 2525–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noguchi CT, Torchia DA, Schechter AN. Determination of deoxyhemoglobin S polymer in sickle erythrocytes upon deoxygenation. Proceedings of the National Academy of Sciences of the United States of America. 1980; 77(9): 5487–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papageorgiou DP, Abidi SZ, Chang HY, Li X, Kato GJ, Karniadakis GE, et al. Simultaneous polymerization and adhesion under hypoxia in sickle cell disease. P Natl Acad Sci USA. 2018; 115(38): 9473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lizarralde Iragorri MA, El Hoss S, Brousse V, Lefevre SD, Dussiot M, Xu T, et al. A microfluidic approach to study the effect of mechanical stress on erythrocytes in sickle cell disease. Lab Chip. 2018; 18(19): 2975–84. [DOI] [PubMed] [Google Scholar]
- 14.Merle NS, Grunenwald A, Rajaratnam H, Gnemmi V, Frimat M, Figueres ML, et al. Intravascular hemolysis activates complement via cell-free heme and heme-loaded microvesicles. JCI insight. 2018; 3(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Camus SM, De Moraes JA, Bonnin P, Abbyad P, Le Jeune S, Lionnet F, et al. Circulating cell membrane microparticles transfer heme to endothelial cells and trigger vasoocclusions in sickle cell disease. Blood. 2015; 125(24): 3805–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parrow NL, Violet PC, Tu H, Nichols J, Pittman CA, Fitzhugh C, et al. Measuring Deformability and Red Cell Heterogeneity in Blood by Ektacytometry. Journal of visualized experiments : JoVE. 2018; (131): e56910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gladwin MT, Vichinsky E. Pulmonary complications of sickle cell disease. The New England journal of medicine. 2008; 359(21): 2254–65. [DOI] [PubMed] [Google Scholar]
- 18.Tanabe P, Myers R, Zosel A, Brice J, Ansari AH, Evans J, et al. Emergency department management of acute pain episodes in sickle cell disease. Acad Emerg Med. 2007; 14(5): 419–25. [DOI] [PubMed] [Google Scholar]
- 19.Desai PC, Brittain JE, Jones SK, McDonald A, Wilson DR, Dominik R, et al. A pilot study of eptifibatide for treatment of acute pain episodes in sickle cell disease. Thrombosis research. 2013; 132(3): 341–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newcombe P Pathophysiology of sickle cell disease crisis. Emergency nurse : the journal of the RCN Accident and Emergency Nursing Association. 2002; 9(9): 19–22. [DOI] [PubMed] [Google Scholar]
- 21.van Beers EJ, van Tuijn CFJ, Mac Gillavry MR, van der Giessen A, Schnog JJB, Biemond BJ, et al. Sickle cell disease-related organ damage occurs irrespective of pain rate: implications for clinical practice. Haematol-Hematol J. 2008; 93(5): 757–60. [DOI] [PubMed] [Google Scholar]
- 22.Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. The New England journal of medicine. 1994; 330(23): 1639–44. [DOI] [PubMed] [Google Scholar]
- 23.Alexy T, Sangkatumvong S, Connes P, Pais E, Tripette J, Barthelemy JC, et al. Sickle cell disease: selected aspects of pathophysiology. Clinical hemorheology and microcirculation. 2010; 44(3): 155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanter J, Kruse-Jarres R. Management of sickle cell disease from childhood through adulthood. Blood reviews. 2013; 27(6): 279–87. [DOI] [PubMed] [Google Scholar]
- 25.Buchanan G, Vichinsky E, Krishnamurti L, Shenoy S. Severe sickle cell disease--pathophysiology and therapy. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2010; 16(1 Suppl): S64–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Man Y, Goreke U, Kucukal E, Hill A, An R, Liu S, et al. Leukocyte adhesion to P-selectin and the inhibitory role of Crizanlizumab in sickle cell disease: A standardized microfluidic assessment. Blood cells, molecules & diseases. 2020; 83: 102424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alapan Y, Kim C, Adhikari A, Gray KE, Gurkan-Cavusoglu E, Little JA, et al. Sickle cell disease biochip: a functional red blood cell adhesion assay for monitoring sickle cell disease. Translational research : the journal of laboratory and clinical medicine. 2016; 173: 74–91 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim M, Alapan Y, Adhikari A, Little JA, Gurkan UA. Hypoxia-enhanced adhesion of red blood cells in microscale flow. Microcirculation. 2017; 24(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kucukal E, Ilich A, Key NS, Little JA, Gurkan UA. Red blood cell adhesion to heme-activated endothelial cells reflects clinical phenotype in sickle cell disease. Am J Hematol. 2018; 93(8): 1050–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kucukal E, Little JA, Gurkan UA. Shear dependent red blood cell adhesion in microscale flow. Integr Biol-Uk. 2018; 10(4): 194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noomuna P, Risinger M, Zhou S, Sou K, Man Y, An R, et al. Inhibition of Band 3 tyrosine phosphorylation: a new mechanism for treatment of sickle cell disease. Br J Haematol. 2020;190(4): 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan C, Quinn E, Kucukal E, Kapoor S, Gurkan UA, Little JA. Priapism, hemoglobin desaturation, and red blood cell adhesion in men with sickle cell anemia. Blood cells, molecules & diseases. 2019; 79: 102350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nebor D, Bowers A, Hardy-Dessources MD, Knight-Madden J, Romana M, Reid H, et al. Frequency of pain crises in sickle cell anemia and its relationship with the sympatho-vagal balance, blood viscosity and inflammation. Haematol-Hematol J. 2011; 96(11): 1589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Connes P, Alexy T, Detterich J, Romana M, Hardy-Dessources MD, Ballas SK. The role of blood rheology in sickle cell disease. Blood reviews. 2016; 30(2): 111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Du E, Lei H, Tang Y, Dao M, Suresh S, et al. Patient-specific blood rheology in sickle-cell anaemia. Interface Focus. 2016; 6: 20150065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wood DK, Soriano A, Mahadevan L, Higgins JM, Bhatia SN. A biophysical indicator of vaso-occlusive risk in sickle cell disease. Science translational medicine. 2012; 4(123): 123ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brittenham GM, Schechter AN, Noguchi CT. Hemoglobin-S Polymerization - Primary Determinant of the Hemolytic and Clinical Severity of the Sickling Syndromes. Blood. 1985; 65(1): 183–9. [PubMed] [Google Scholar]
- 38.Hebbel RP. Beyond Hemoglobin Polymerization - the Red-Blood-Cell Membrane and Sickle Disease Pathophysiology. Blood. 1991; 77(2): 214–37. [PubMed] [Google Scholar]
- 39.Du E, Diez-Silva M, Kato GJ, Dao M, Suresh S. Kinetics of sickle cell biorheology and implications for painful vasoocclusive crisis. P Natl Acad Sci USA. 2015; 112(5): 1422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Odenheimer DJ, Sarnaik SA, Whitten CF, Rucknagel DL, Sing CF. The Relationship between Fetal Hemoglobin and Disease Severity in Children with Sickle-Cell-Anemia. Am J Med Genet. 1987; 27(3): 525–35. [DOI] [PubMed] [Google Scholar]
- 41.Adeodu OO, Akinlosotu MA, Adegoke SA, Oseni SBA. Foetal Haemoglobin and Disease Severity in Nigerian Children with Sickle Cell Anaemia. Mediterr J Hematol I. 2017; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kato GJ, McGowan V, Machado RF, Little JA, Taylor J, Morris CR, et al. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood. 2006; 107(6): 2279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mikobi TM, Tshilobo PL, Aloni MN, Lelo GM, Akilimali PZ, Muyembe-Tamfum JJ, et al. Correlation between the Lactate Dehydrogenase Levels with Laboratory Variables in the Clinical Severity of Sickle Cell Anemia in Congolese Patients. Plos One. 2015; 10(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Driscoll S, Height SE, Dick MC, Rees DC. Serum lactate dehydrogenase activity as a biomarker in children with sickle cell disease. Brit J Haematol. 2008; 140(2): 206–9. [DOI] [PubMed] [Google Scholar]
- 45.Litos M, Sarris I, Bewley S, Seed P, Okpala L, Oteng-Ntim E. White blood cell count as a predictor of the severity of sickle cell disease during pregnancy. Eur J Obstet Gyn R B. 2007; 133(2): 169–72. [DOI] [PubMed] [Google Scholar]
- 46.Buchanan GR, Glader BE. Leukocyte counts in children with sickle cell disease. Comparative values in the steady state, vaso-occlusive crisis, and bacterial infection. Am J Dis Child. 1978; 132(4): 396–8. [DOI] [PubMed] [Google Scholar]
- 47.Lu M, Rab MA, Shevkoplyas SS, Sheehan VA. Blood rheology biomarkers in sickle cell disease. Exp Biol Med (Maywood). 2020: 1535370219900494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Man Y, Kucukal E, An R, Watson Q, Bosch J, Zimmerman PA, et al. Microfluidic assessment of red blood cell mediated microvascular occlusion. Lab Chip. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lipowsky HH. Microvascular rheology and hemodynamics. Microcirculation. 2005; 12(1): 5–15. [DOI] [PubMed] [Google Scholar]
- 50.Akinsheye I, Alsultan A, Solovieff N, Ngo D, Baldwin CT, Sebastiani P, et al. Fetal hemoglobin in sickle cell anemia. Blood. 2011; 118(1): 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lionnet F, Hammoudi N, Stojanovic KS, Avellino V, Grateau G, Girot R, et al. Hemoglobin sickle cell disease complications: a clinical study of 179 cases. Haematologica. 2012; 97(8): 1136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hannemann A, Weiss E, Rees DC, Dalibalta S, Ellory JC, Gibson JS. The Properties of Red Blood Cells from Patients Heterozygous for HbS and HbC (HbSC Genotype). Anemia. 2011; 2011: 248527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drawz P, Ayyappan S, Nouraie M, Saraf S, Gordeuk V, Hostetter T, et al. Kidney Disease among Patients with Sickle Cell Disease, Hemoglobin SS and SC. Clin J Am Soc Nephro. 2016; 11(2): 207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nagel RL, Lawrence C. The distinct pathobiology of sickle cell-hemoglobin C disease. Therapeutic implications. Hematology/oncology clinics of North America. 1991; 5(3): 433–51. [PubMed] [Google Scholar]
- 55.Nagel RL, Fabry ME, Steinberg MH. The paradox of hemoglobin SC disease. Blood reviews. 2003; 17(3): 167–78. [DOI] [PubMed] [Google Scholar]
- 56.Itoh T, Chien S, Usami S. Effects of Hemoglobin Concentration on Deformability of Individual Sickle Cells after Deoxygenation. Blood. 1995; 85(8): 2245–53. [PubMed] [Google Scholar]
- 57.Itoh T, Chien S, Usami S. Deformability Measurements on Individual Sickle Cells Using a New System with Po2 and Temperature Control. Blood. 1992; 79(8): 2141–7. [PubMed] [Google Scholar]
- 58.Quinn CT. Minireview: Clinical severity in sickle cell disease: the challenges of definition and prognostication. Exp Biol Med (Maywood). 2016; 241(7): 679–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Darbari DS, Onyekwere O, Nouraie M, Minniti CP, Luchtman-Jones L, Rana S, et al. Markers of severe vaso-occlusive painful episode frequency in children and adolescents with sickle cell anemia. The Journal of pediatrics. 2012; 160(2): 286–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xie L, Jiang Y, Yao W, Gu L, Sun D, Ka W, et al. Studies on the biomechanical properties of maturing reticulocytes. Journal of biomechanics. 2006; 39(3): 530–5. [DOI] [PubMed] [Google Scholar]
- 61.Leblond PF, LaCelle PL, Weed RI. Cellular deformability: a possible determinant of the normal release of maturing erythrocytes from the bone marrow. Blood. 1971; 37(1): 40–6. [PubMed] [Google Scholar]
- 62.Walker HK, Hall WD, Hurst JW. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston Butterworths; 1990. [PubMed] [Google Scholar]
- 63.Elion JE, Brun M, Odievre MH, Lapoumeroulie CL, Krishnamoorthy R. Vaso-occlusion in sickle cell anemia: role of interactions between blood cells and endothelium. Hematol J. 2004; 5: S195–S8. [DOI] [PubMed] [Google Scholar]
- 64.Alapan Y, Little JA, Gurkan UA. Heterogeneous red blood cell adhesion and deformability in sickle cell disease. Scientific reports. 2014; 4: 7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huisjes R, Bogdanova A, van Solinge WW, Schiffelers RM, Kaestner L, van Wijk R. Squeezing for Life - Properties of Red Blood Cell Deformability. Front Physiol. 2018; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leal JKF, Adjobo-Hermans MJW, Bosman G. Red Blood Cell Homeostasis: Mechanisms and Effects of Microvesicle Generation in Health and Disease. Front Physiol. 2018; 9: 703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Keikhaei B, Yousefi H, Bahadoram M. Hydroxyurea: Clinical and Hematological Effects in Patients With Sickle Cell Anemia. Global journal of health science. 2015; 8(3): 252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chand AR, Xu H, Wells LG, Clair B, Neunert C, Spellman AE, et al. Are There True Non-Responders to Hydroxyurea in Sickle Cell Disease? a Multiparameter Analysis. Blood; 2014. p. 4073. [Google Scholar]
- 69.Stickland MK, Lovering AT. Exercise-induced intrapulmonary arteriovenous shunting and pulmonary gas exchange. Exerc Sport Sci Rev. 2006; 34(3): 99–106. [DOI] [PubMed] [Google Scholar]
- 70.Joseph D, Puttaswamy RK, Krovvidi H. Non-respiratory functions of the lung. Continuing Education in Anaesthesia Critical Care & Pain. 2013; 13(3): 98–102. [Google Scholar]
- 71.Yale SH, Nagib N, Guthrie T. Acute chest syndrome in sickle cell disease. Crucial considerations in adolescents and adults. Postgraduate medicine. 2000; 107(1): 215–8, 21–2. [DOI] [PubMed] [Google Scholar]
- 72.Poillon WN, Kim BC, Castro O. Intracellular hemoglobin S polymerization and the clinical severity of sickle cell anemia. Blood. 1998; 91(5): 1777–83. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


