Abstract
Background:
Recent clinical guidelines support intensive blood pressure (BP) treatment targets. However, observational data suggest that excessive diastolic BP (DBP) lowering might increase the risk of myocardial infarction (MI); reflecting a J- or U-shaped relationship.
Methods:
We analyzed 47,407 participants from 5 cohorts (median age 60 years). First, to corroborate prior observational analyses, we used traditional statistical methods to test the shape of association between DBP and CVD. Second, we created polygenic risk scores (PRS) of DBP and SBP and generated linear Mendelian randomization (MR) estimates for the effect of DBP on CVD. Third, using novel non-linear MR approaches, we evaluated for non-linearity in the genetic relationship between DBP and CVD. Comprehensive MR interrogation of DBP required us to also model SBP, given the two are strongly correlated.
Results:
Traditional observational analysis of our cohorts suggested a J-shaped association between DBP and MI. By contrast, linear MR analyses demonstrated an adverse effect of increasing DBP increments on CVD outcomes, including MI (MI Hazard ratio = 1.07 per unit mmHg increase in DBP, p<0.001). Furthermore, non-linear MR analyses found no evidence for a J-shaped relationship, instead confirming that MI risk decreases consistently per unit decrease in DBP, even among individuals with low values of baseline DBP.
Conclusions:
In this analysis of the genetic effect of DBP, we found no evidence for a non-linear J- or U-shaped relationship between DBP and adverse CVD outcomes; including MI.
Keywords: Diastole, blood pressure, Mendelian randomization
Introduction
Hypertension is a widely prevalent disease that is associated with significant morbidity and mortality worldwide1. Although the adverse associations of hypertension with cardiovascular disease (CVD) morbidity and mortality are undeniable, the optimal blood pressure (BP) treatment target has been a matter of heated debate. Some experts caution against overly intensive BP lowering due to the presence of a non-linear, J- or U-shaped, association between BP and CVD events in many observational analyses (with excess risk as both the low and high extremes of BP)2,3. When considering the risk of myocardial infarction (MI), this specific concern is particularly operative for diastolic BP (DBP) since it is the main driver of coronary artery filling. Multiple observational cohort studies have reported increased coronary heart disease and MI risk in individuals with very low diastolic blood pressure4-6.
The 2015 Systolic Blood Pressure Intervention (SPRINT) trial challenged that view by demonstrating a reduction in CVD and all-cause mortality among participants randomized to a systolic BP (SBP) target of 120 mmHg compared to 140 mmHg7. While SPRINT specifically targeted systolic BP, a post hoc analysis of the trial revealed that there was no statistical interaction between baseline DBP and the benefit seen in the intensive treatment arm8. Thus, even though SPRINT investigators observed a U-shaped association between DBP and CVD in both arms of the trial, a statistically similar benefit from more intensive BP treatment was seen among SPRINT participants who had low baseline DBP as was seen among those with higher baseline DBPs. These findings do not support a J- or U-shaped causal relationship between low DBP and CVD and instead implicate potential confounding factors in this association9. However, the SPRINT trial was not designed to lower DBP specifically and did not titrate therapy to reach target DBP levels. To further address the question of whether excessive DBP lowering might have a causal effect resulting in increased risk of cardiovascular events, we performed a Mendelian randomization (MR) study and used a polygenetic risk score (PRS) to examine the shape of the association between genetically driven variability in DBP and cardiovascular outcomes.
Methods
Samples, Genotype Quality Control and Imputation
The data and study materials used in this project are part of dbGaP and are available with appropriate request to dbGaP. The analytic methods are described in detail below and in Methods in the Supplement.
We analyzed individual-level data from five cohorts that study incident CVD events (Framingham Heart Study, Cardiovascular Health Study, Atherosclerosis Risk in Communities Study, Multi-Ethnic Study of Atherosclerosis, and Women’s Health Initiative). For our primary analysis, we excluded study participants with a known history of prevalent MI at baseline. Raw genotype and phenotype data were downloaded from dbGAP (accession numbers phs000007.v29.p11, phs000287.v6.p1, phs000209.v13.p3, phs000280.v4.p1, phs000200.v11.p3, phs000888.v1.p1). For each of the five cohorts, extensive genotype quality control procedures were followed according to the current standard for genomic studies10 (Methods in the Supplement).
Ethical Considerations
Our study was approved by the local Johns Hopkins School of Medicine IRB (IRB # 00196536). All subjects enrolled in each of the included cohorts provided written informed consent for their inclusion in the corresponding cohorts.
Exposure and Outcomes
We evaluated the genetic effect of DBP and SBP on a series of outcomes. To facilitate a comprehensive investigation of DBP it was necessary to also study SBP, given the two parameters are so strongly correlated. In particular, the inclusion of SBP as an exposure in this investigation was necessary because the vast majority of genetic polymorphisms that influence DBP also influence SBP. Our primary outcome of interest was MI. Secondary CVD outcomes analyzed included coronary artery disease events, coronary revascularization, ischemic stroke, heart failure, fatal coronary disease, and all-cause mortality. All outcomes were evaluated in a time-to-event manner. More complete definitions of the exposure and outcomes are provided in Methods and Table I in the Supplement.
Statistical Analysis
Observational association evaluations
As a baseline evaluation and to corroborate prior observational analyses, we first assessed the relationship between SBP, DBP and CVD outcomes using traditional statistical approaches. We applied a proportional hazards time-to-event Cox model to assess the influence of DBP or SBP on each of our primary and secondary outcomes, after controlling for age, sex and a dummy categorical variable for each individual substudy (Methods in the Supplement). Statistical significance was calculated based on the Wald test. In addition, we evaluated for potential non-linear observational relationships between either DBP or SBP and each outcome using a multivariable meta-analytic approach based on fractional polynomials11 (Methods in the Supplement).
Instrumental Variable
To test the genetic effect of DBP variability on outcomes, we generated a polygenic risk score (PRS) for DBP, consisting of 718 single nucleotide polymorphisms (SNPs), to use as an instrumental variable in Mendelian randomization (MR) analyses12 taking a standard pruning and thresholding approach (Methods in the Supplement). We additionally calculated a PRS for SBP following the same procedure.
For sensitivity analyses, we generated PRS for DBP independent of body mass index (BMI) or heart rate by excluding all variants in the original DBP PRS that were also associated with BMI or heart rate in recent large BMI12 or heart rate13 GWAS at a Bonferroni-adjusted p-value < 0.05. These analyses were conducted to reduce the likelihood of BMI or heart rate introducing pleiotropy in any association between DBP and CVD. For our multivariate MR analysis, we also generated a combined PRS for both DBP and SBP, consisting of 1015 SNPs in total (Methods in the Supplement).
Linear Mendelian Randomization
We performed a two-stage MR analysis to model the linear effect of DBP as follows. After merging together all the individual sub-studies in a common design matrix, we first regressed DBP on the PRS via linear regression to generate an estimate of the effect of the PRS on the exposure. This estimate was the denominator of our MR estimate. We then regressed the effect of the PRS on the outcome using a proportional hazard (Cox) regression analysis for the time-to-event data to generate an estimate of the PRS on the outcome. This estimate (logarithm of the hazard ratio (HR)) was the numerator of our MR estimate. To calculate the MR hazard ratio of the outcome per unit increase in DBP we exponentiated the ratio of the numerator over the denominator. 95% confidence intervals (CI) of that estimate were generated using a first order Taylor series approximation (standard error (SE) of MR = SEoutcome/betaexposure). Both regressions included a binary dummy covariate to control for each participant’s membership in each substudy, which accounts for intra-study differences including race group, genotyping array, and differences in outcome definitions. Both regressions also included as covariates participant age, participant sex, and 2 genotype principal components (PCs) for each substudy to account for residual population stratification (ie. 36 total PC covariates in addition to controlling for self-reported race based on the aforementioned substudy dummy covariate) (see Methods in the Supplement for details). The above MR analysis was then repeated, but with SBP as the exposure of interest.
We performed a number of sensitivity analyses to evaluate how our DBP MR estimates may be affected by confounding from SBP, BMI, medication use, or other pleiotropic effects. We additionally performed a sensitivity analysis including all participants with and without prevalent MI at study enrollment to evaluate how prevalent events before enrollment in the study might affect our results (Methods in the Supplement).
Non-linear Mendelian Randomization
Mendelian randomization analyses to assess for potential non-linear J- or U-shape effects of DBP or SBP on the study outcomes were performed with state-of-the-art methodologies14,15, using the strategy of conditioning on quantiles of instrumental variable (IV)-free exposure and generating localized average causal effect (LACE) estimates (Methods in the Supplement).
We also performed several sensitivity analyses to assess how the shape of the relationship between DBP and outcomes may change in response to different potential pleiotropic factors and confounders. Specifically, we evaluated the shape of the aforementioned associations using DBP PRS that were independent of BMI or heart rate. In addition, following the same non-linear MR approach, we visualized the shape of the association between DBP and outcomes separately in males vs. females, and in individuals on no BP medications at baseline. Lastly, since it proved difficult in multivariate MR to disentangle the individual effect of DBP independent of SBP, we performed an additional analysis in which we generated non-linear LACE estimates of DBP on MI using IV-free exposures in which we adjusted for the linear genetic effects of both SBP and DBP in combination (Methods in the Supplement). We then repeated the same sensitivity analyses for SBP.
Finally, a simulation study was performed to assess power for MR detection of non-linear effects (Methods in the Supplement). We used two techniques to reduce the impact of extreme DBP outliers in our MR analyses, (a) we excluded outliers outside the range of the mean ±3 standard deviations of the DBP distribution or (b) we winsorized these values. Genotype quality control was performed using PLINK versions 1.9 and 2.0, while all statistical analyses were conducted using R version 3.5.1, we considered a p-value (2-sided) of <0.05 to be statistically significant.
Results
Demographics and baseline information
In aggregate, 48,918 individuals passed all genotype filters and had sufficiently reliable BP estimates to satisfy inclusion in our study. A total of 1,400 (2.9%) participants had a documented MI before baseline study enrollment and 111 (0.2%) had incomplete data on time to MI and were excluded in our primary analysis leaving 47,407 participants. Of those, 77% were females. The proportion of females in our study sample was 55% when the all-female WHI was not included in the calculation. Median age at enrollment was 60 years (Q1-Q3 interquartile interval 52-68 years). Average follow up for events was 16.5 years. During the follow up period, 7.3% of participants (n=3,456) had an incident MI and average age at MI during follow-up was 73 years (Q1-Q3 interval 66-80 years). Median age of death among the participants who died during follow up (n=12,072) was 78 years (Q1-Q3 interval: 71-84 years). Summary demographic and outcome information is provided in Table 1, whereas a break-down of the demographics and outcomes in each individual cohort is provided in Table II in the Supplement.
Table 1.
Summary demographic and outcome information (N= 47,407 combined participants from Framingham Heart Study, Cardiovascular Health Study, Atherosclerosis Risk in Communities Study, Multi-Ethnic Study of Atherosclerosis, and Women’s Health Initiative)
| Parameter | Median | Q1-Q3 |
|---|---|---|
| Age at enrollment (years) | 60 | 52-68 |
| Female sex (%) | 77 | |
| BMI (Kg/m2) | 27.7 | 24.6-31.7 |
| European ancestry (%) | 58 | |
| SBP (mmHg) | 125 | 114-139 |
| DBP (mmHg) | 75 | 68-81 |
| MI during follow-up (%) | 7.3 | |
| Age at MI event (years) | 73 | 66-80 |
| HF during follow-up (%) | 7.8 | |
| Age at HF event (years) | 74 | 68-80 |
| Fatal CHD during follow-up (%) | 3.7 | |
| Ischemic stroke during follow-up (%) | 5.5 | |
| Age at ischemic stroke (years) | 76 | 69-81 |
| Coronary revascularization during follow-up (%) | 8.3 | |
| Age at coronary revascularization (years) | 71 | 65-77 |
| Death during follow-up (%) | 25.4 | |
| Age at death (years) | 78 | 71-84 |
BMI: Body mass index, CHD: Coronary heart disease, DBP: Diastolic blood pressure, HF: Heart failure, MI: Myocardial infarction, SBP: Systolic blood pressure
Traditional observational analysis of the association between BP and cardiovascular events
To verify that our source data were consistent with prior observational reports, we used traditional statistics to evaluate the association of DBP (per 1 mmHg increase) with our primary and secondary outcomes. The hazard ratios per 1 mmHg increase in DBP for MI (HR (95%CI) = 1.012 (1.009-1.016), p<0.001), coronary disease events (HR (95% CI) = 1.011 (1.008-1.015), p<0.001), fatal coronary disease (HR (95% CI) = 1.008 (1.003-1.013), p<0.001), coronary revascularization (HR (95% CI) = 1.010 (1.007-1.014), p<0.001), heart failure (HR (95% CI) = 1.013 (1.010-1.017), p<0.001), ischemic stroke (HR (95% CI) = 1.017 (1.013-1.021), p<0.001) and all-cause mortality (HR (95% CI) = 1.004 (1.003-1.007), p<0.001) are all significantly > 1, consistent with expectation. Similar results were observed for SBP.
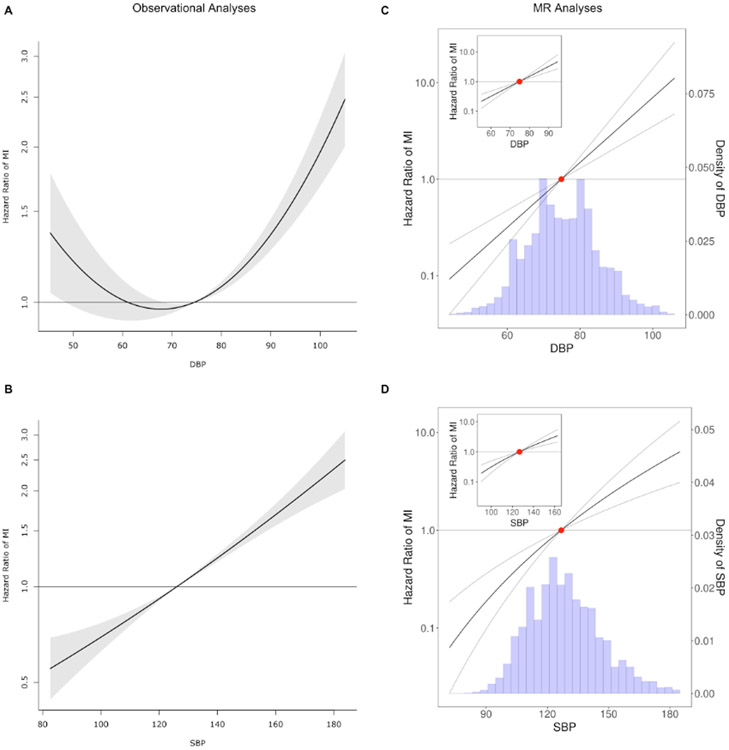
We subsequently assessed for non-linear associations of DBP with CVD events in our observational data. Fitting a fractional polynomial to model the shape of association between DBP and MI revealed a J- or U-shaped association (Figure 1A). A similar J-shape was observed in the association between DBP and all secondary outcomes with the exception of ischemic stroke (Figure I in the Supplement). A sensitivity analysis of individuals with DBP < 70 mmHg confirmed that the hazard of MI trends lower with an increase in DBP within that subset after controlling for age, sex, and substudy (HR=0.99, p=0.06) (consistent with an observational J- or U- shape). This result persisted after controlling additionally for SBP and BMI (HR=0.98, p=0.001) and in the subset of participants on no BP medications (Figure II in the Supplement). Notably, a similar U-shape was not observed in the association between SBP and MI (Figure 1B).
Figure 1.
Analysis of the shape of the relationship between blood pressure and cardiovascular outcomes. A. Shape of the relationship between diastolic blood pressure and myocardial infarction based on observational data that reveal a U- or J-curve. B. Shape of the relationship between systolic blood pressure and myocardial infarction. C. Shape of the genetic relationship between diastolic blood pressure and myocardial infarction based on the fractional polynomial non-linear MR approach. D. Shape of the genetic relationship between systolic blood pressure and myocardial infarction based on the fractional polynomial non-linear MR approach. The hazard ratio of the mean DBP (panel C) or mean SBP (panel D) is centered at 1 (red dot). Note that the hazard ratios presented in the MR figures represent a meta-regression of hazard ratios for each quantile of BP compared to the mean BP in an effort to approximate hazard ratio per unit increase in the exposure. The y-axes are in log-scale.
Linear Mendelian Randomization of DBP and SBP on incident cardiovascular events
Since the instrumental variable was generated based on summary statistics from an independent study, as a check before proceeding with the MR analysis we evaluated whether the instrumental variable does in fact predict DBP in our included cohorts. Linear regression of DBP on the PRS shows a highly significant positive correlation between the two (beta=0.572, p-value<0.001) (Figure III in the Supplement). The association was present across different ancestries, although we did observe some cross-ancestry differences in the strength of the relationship (Figure IV in the Supplement). The PRS for SBP was similarly validated (Figure V in the Supplement).
To obtain an estimate of the causal relationship between either DBP or SBP and our outcomes of interest we then performed linear MR. This MR analysis showed a causal adverse effect of DBP increments on cardiovascular events. Nevertheless, in contrast to SBP, we did not observe a significant increase in risk of all-cause mortality per unit increase in DBP (HR (95% CI) = 1.01 (0.996-1.02)). Results are summarized in Figure 2.
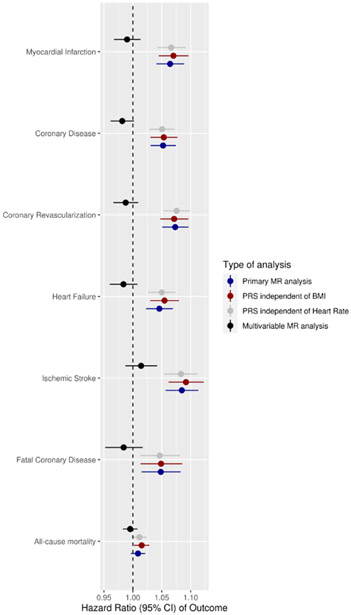
Figure 2.
Linear association between blood pressure and cardiovascular outcomes based on MR data. The figure shows a Forest plot of the association between diastolic blood pressure (per unit increase in mmHg) and a series of cardiovascular events analyzed based on linear MR in the primary analysis (dark blue color), sensitivity analysis using the PRS that is independent of BMI (dark red color), sensitivity analysis using the PRS that is independent of heart rate (gray color) and multivariable MR analysis of the effect of diastolic blood pressure after controlling for systolic BP (black color).
Sensitivity analysis with two-stage linear MR estimates using summary statistics from an MI GWAS of the UK Biobank were consistent with the estimates from the individual level MR for the effect of DBP on MI (Odds ratio (OR) = 1.08, p <0.001), thereby providing independent confirmation of our primary MR estimate. Further, the estimates remain similar in size and significance when using MR methods that are known to account for pleiotropy, like MR Egger (OR=1.10, p<0.001) and MR median (OR=1.08, p<0.001) (Figure VI in the Supplement). The linear MR estimates between DBP and cardiovascular events using PRS that are independent of BMI or heart rate are provided in Figure 2 and are consistent with the estimates in our primary analysis.
To disentangle the independent roles of SBP and DBP, we performed multivariate linear MR, also summarized in Figure 2 and Table 2. This analysis confirmed the causal influence of SBP on MI but did not show evidence of statistically significant influence of DBP on MI after adjustment for SBP. To further examine whether this lack of an effect of DBP in multivariate MR adjusting for SBP was because of lack of power, we repeated these estimates in a two-stage summary level multivariate MR using large GWA meta-analyses of the UK Biobank. This analysis revealed a linear effect of both SBP (HR=1.03, p<0.001) and DBP (HR=1.03, p=0.002) on the risk of MI (albeit with reduced effect size estimates compared to the univariable analyses and with the effect of DBP being of nominal statistical significance, Table 2). In contrast to SBP, we did not find evidence in multivariate linear MR of an independent effect of DBP on coronary disease, heart failure or ischemic stroke (Figure 2, Table 2, Figure VII in the Supplement).
Table 2.
Sensitivity analyses of linear MR estimates using data from the primary analytic sample (N= 47,407 from 5 cohorts) or from two-sample estimates from different genome-wide association studies.
| Outcome Tested | Univariable MR of SBP |
Multivariable** MR of SBP (independent of DBP) |
Two-sample$ Multivariable MR of SBP (independent of DBP) |
Two-sample$ Multivariable MR of DBP (independent of SBP) |
||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) |
P-value | HR (95% CI) |
P-value | OR (95% CI) |
P-value | OR (95% CI) |
P-value | |
| MI | 1.04 (1.03-1.05) |
<0.0001* | 1.03 (1.02-1.05) |
<0.0001* | 1.03 (1.02-1.04) |
<0.0001* | 1.03 (1.01-1.05) |
0.002 |
| Coronary artery disease | 1.04 (1.02-1.05) |
<0.0001* | 1.04 (1.02-1.05) |
<0.0001* | 1.03 (1.03-1.04) |
<0.0001* | 1.01 (1.00-1.02) |
0.2 |
| Fatal CHD | 1.02 (1.00-1.04) |
0.04 | 1.04 (1.02-1.06) |
<0.0001* | NA | NA | ||
| Heart failure | 1.03 (1.01-1.04) |
<0.0001* | 1.04 (1.03-1.05) |
<0.0001* | 1.02 (1.01-1.02) |
<0.0001* | 1.01 (1.00-1.02) |
0.3 |
| Ischemic stroke | 1.05 (1.03-1.06) |
<0.0001* | 1.03 (1.02-1.05) |
<0.0001* | 1.03 (1.02-1.04) |
<0.0001* | 1.01 (0.99-1.02) |
0.3 |
| Coronary revascularization | 1.04 (1.03-1.05) |
<0.0001* | 1.03 (1.02-1.05) |
<0.0001* | NA | NA | ||
| All-cause mortality | 1.01 (1.00-1.02) |
0.002 | 1.01 (1.01-1.02) |
0.0004* | NA | NA | ||
CHD: Coronary heart disease, CI: Confidence intervals, HR: Hazard ratio, MI: Myocardial infarction, MR: Mendelian randomization, NA: Not applicable, OR: Odds Ratio
Significant after Bonferroni adjustment for multiple comparisons
Adjusted for age, sex, two genotype principal components, the dummy variable for each substudy, and DBP
Adjusted for age, sex, two genotype principal components, the dummy variable for each substudy, and SBP
Two-sample multivariable MR analysis based on summary statistics from different GWAS of the exposure and the outcome
Non-linear MR to test the shape of the relationship between DBP and SBP and cardiovascular outcomes
To further evaluate the shape of the genetic association between DBP and cardiovascular events, we performed a non-linear MR analyses of DBP and cardiovascular events by obtaining LACE estimates in centiles of instrumental variable-free exposure, finding and visualizing the best fit second degree polynomial in these estimates. The results for our primary outcome are summarized in Figure 1C and Figures VIII and IX in the Supplement, whereas results for MI in each cohort are shown in Figure X in the Supplement and results for secondary outcomes are presented in Figure XI in the Supplement. We observed no evidence of a non-linear relationship in the MR analysis (p-value for non-linearity in all associations between DBP and our primary/secondary outcomes was > 0.1). Indeed, in the subset of individuals with instrumental variable-free DBP of < 70 mmHg we still observed a significantly positive adverse association between DBP increments and MI (HR 1.07, 95% CI 1.02-1.11). A sensitivity analysis including individuals with prevalent MI (Figure XII in the Supplement) did not alter the main findings. Further, these findings were consistent with results from similar analyses conducted on PRS that were independent of BMI (Figure XIII in the Supplement) and heart rate (Figure XIV in the Supplement) and in individuals not on baseline blood pressure medications (Figure XV in the Supplement).
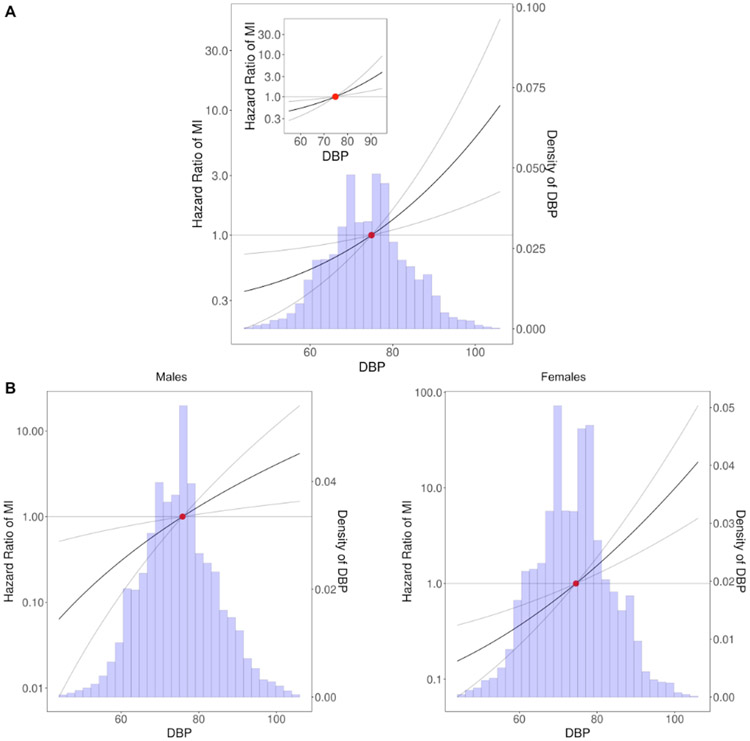
The genetic association between SBP and MI is illustrated in Figure 1D, while the associations between SBP and other CVD outcomes are presented in Figure XVI in the Supplement and all analyses show no evidence of non-linearity (p-value for non-linearity > 0.1). Further, we did not observe any evidence for non-linearity in the DBP-MI association in a LACE analysis accounting for the linear effect of DBP and SBP combined (Figure 3A), nor did we find evidence of non-linearity in a similar analysis for SBP (Figure XVII in the Supplement). It is also important to note that we also did not observe evidence of deviations from a monotonically increasing linear association between DBP and MI in LACE analyses stratified by participants’ sex (Figure 3B) (non-linearity p-value > 0.1 for all comparisons). A simulation study revealed sufficient power to detect non-linear effects in our dataset (Figure XVIII in the Supplement), whereas an analysis of the strength of the instrumental variable demonstrated that the overlap in samples between the GWAS used for generation of the PRS and our primary cohorts is not expected to lead to considerable bias16 (Methods in the Supplement).
Figure 3.
Sensitivity analysis of the shape between DBP and MI based on non-linear MR. A. Shape of the relationship between DBP and MI in an analysis of IV-free exposure quantiles accounting for the linear genetic effects of both SBP and DBP. B. Shape of the relationship between DBP and MI conditioning on participants’ sex. The y-axes are in log-scale.
Discussion
Blood pressure treatment targets are constantly changing, driven by a dynamic evidence base that is sometimes conflicting and difficult to interpret. Despite the promising findings of the SPRINT trial7, which advocates for a lower is better BP treatment paradigm, the full implications of achieving very low BP with therapy are still incompletely understood17. By demonstrating a J-curve association, observational data suggest that low DBP is a high-risk state. However, these observational analyses are often plagued by confounding factors and their findings often do not replicate when subjected to the rigors of a randomized trial8. This is particularly true for BP, where low baseline levels may reflect a poor baseline health status (inclusive of stiff and diseased vasculature that influece low DBP in particular5,18,19); leading to reverse causation as one possible explanation for the link between low BP and increased risk for events. Indeed, some observational analyses that account for baseline comorbidities have shown an attenuation of the J- or U-shaped association between BP and outcomes20,21. Mendelian randomization studies exploit the natural randomization provided by the genotypes of each individual to investigate causal relationships, and are therefore known to be minimally affected by confounders22. In our study, we performed a large-scale MR analysis using time-to-event data from five large population cohorts to more definitively establish the causal direction and shape of the relationship between DBP and CVD, in particular MI. We found that the effect of DBP on CVD is linear and there is no genetic evidence for a J- or U-shaped association between DBP and CVD outcomes. There was also no evidence of a non-linear genetic effect of SBP on CVD.
Our study followed a stepwise approach in which we first tested for linear associations between BP (both diastolic and systolic) and outcomes using traditional MR design, followed by a state-of-the-art non-linear MR to evaluate the shape of that relationship. In linear MR, we found that BP has a strong, significant effect in increasing the risk of all major cardiovascular outcomes tested. In fact, when modeled as a genetically determined exposure, the causal influence of BP on events appears to be more pronounced than the corresponding observational data would suggest. This phenomenon is supported by our summary-level MR sensitivity analyses in the UK Biobank and corroborated by a recent independent MR study that showed that a 2.9 mmHg increase in SBP corresponds to an Odds Ratio for major coronary events of 1.22 (which translates to an odds ratio of 1.07 per unit change in SBP)23 – which is higher than observational HR estimates20. This observation likely implies that cumulative risk of high BP as determined by genetic factors throughout a person’s lifetime may be more strongly linked to cardiovascular events than a single measurement of high BP in a clinical setting. Other MR analyses have also reported stronger effect sizes for the genotype exposure compared to more traditional phenotypic exposures (typically measured at one point in time)23-25.
We subsequently evaluated the shape of the genetically determined relationship between BP and cardiovascular events using non-linear MR analyses; focusing particularly on DBP. These showed no evidence of non-linearity in the estimates for DBP (or for SBP). Further, a number of sensitivity analyses on DBP confirmed the lack of evidence for non-linearity, including analyses stratified by sex and analyses accounting for the combined linear effects of SBP and DBP when both are modeled together. Our results are consistent with those from the post hoc analysis of the SPRINT trial8 and effectively challenge the notion that the J- or U-shape in the association between DBP and MI reflects a causal relationship. Although the U- or J-shape association in the observational data is not in doubt and is also observed in our study, our results suggest that this association is likely the result of underappreciated confounders. Low levels of DBP can therefore be considered as a marker of increased risk, but, because this relationship does not appear to be causal, our analysis and the post-hoc analysis from SPRINT both suggest that further BP control, when necessary, is not expected to increase the risk of persons with low baseline diastolic BP. It is worth noting that the casual relationship between DBP and MI may differ from its relationship with other outcomes, which is why we also tested the shape of the relationship between DBP and a series of secondary outcomes (coronary disease, ischemic stroke, heart failure, fatal coronary disease and all-cause mortality), confirming no causal evidence of a J-shape.
Our analysis has certain strengths. First, compared with traditional observational studies, MR analyses are less prone to bias by confounding factors and reverse causation. Indeed, observational studies even when expertly performed, often reveal associations that do not hold when submitted to the rigorous test of a randomized trial. This is a consequence of the fact that it is often impossible to measure and account for every possible confounder in an observational association, whereas MR is able to largely overcome that limitation, since the genetic variants are randomly allocated at conception and in general cannot be affected by environmental factors acting throughout life26. Second, we used a state-of-the-art method to probe the non-linearity in the relationship between DBP and CVD, which allows us to evaluate the shape of the relationship between exposure and the outcome in a range of DBP well beyond the range of the predicted values of DBP based on the instrumental variable14 (noted in Figure III in the Supplement). Further, although power is a common concern when presenting negative data, our simulation study for power analysis coupled with the fact that we still observed a statistically significant and directionally robust detrimental effect of increasing DBP increments on MI among individuals with IV-free DBP < 70 mmHg demonstrate that power is not the reason for the lack of a U-shape in our MR analysis. We should note however, that our analysis cannot exclude a U-shape in the association between the exposure and outcomes with an inflection point in extremes of the exposure distribution (ie. a DBP value much lower than 70mmHg). Last but not least, the large sample size of the most recent GWAS for BP allowed us to create a robust instrumental variable that is predicted to give unbiased MR estimates even in the presence of some inevitable overlap between the GWAS and our estimation cohorts (Methods in the Supplement).
Our analysis also has certain limitations. First, as others have pointed out15, MR is not equivalent to a randomized trial and MR estimates can only be interpreted as representative of what would occur in a randomized trial if the genetic variants in DBP influence outcomes in the same way as the effect of therapeutic interventions15. It is indeed possible that medication effects will be different than effects of genetic variation in lowering DBP. Mitigating this concern is a lack of evidence that reducing BP with treatment imparts favorable prognostic effects on CVD in ways that differ substantially from genetic lowering of BP. Indeed, MR analyses have been consistently shown to accurately predict the effects of drug therapy in prior similar studies23,27,28 and several known blood pressure variants have been successfully used as proxies for medication effects in a recent study by Gill et al.29. Furthermore, all other things being equal, one would anticipate persons who are ‘randomized’ to low genetic DBP to be more susceptible to risk for MI from other variables that further lower DBP and occur in mid- to later- life (like vascular stiffness or medications). We did not find any such susceptibility. Second, since the majority of participants enrolled did not have known clinical CVD at baseline, a possible limitation of the results of these 5 cohorts may not fully apply to all patients with known obstructive CAD or to individuals who are hospitalized or have severe comorbidities that cause substantial BP lowering. However, it is worth highlighting that the observational analyses of the 5 cohorts included in our report nonetheless found a J- or U-shaped relationship with MI. Therefore, at least in our cohorts, we found evidence of a J- or U-curve in traditional analyses that was not replicated in more causally rigorous MR analyses- pointing to residual confounding or reverse causation as an explanation for the J- or U-shaped relationship between DBP and MI seen in observational reports. In addition, the mean age of participants enrolled in the 5 cohorts studied was 60 and therefore there was probably a notable proportion of these individuals with asymptomatic (subclinical) CAD. Third, due to lack of information about prevalent events for some of our secondary outcomes in certain cohorts, we were unable to fully account for prevalent cases in our analyses of some of the secondary outcomes. However, we were able to exclude individuals with prevalent events in our primary outcome of MI. Last, our study is limited by the inherent difficulties in disentangling the influence of DBP in isolation and independent of SBP. These two aspects of BP are tightly linked and highly correlated even in their genetic underpinnings, which makes it difficult to study the role of one independent of the other20. Indeed, because of this collinearity, the power of evaluating the genetic influence of DBP is substantially reduced in a multivariable MR approach that completely adjusts for SBP, which precludes complete evaluation of the independent DBP relationship with CVD outcomes. To overcome this limitation, we performed a sensitivity analysis of our non-linear MR by generating quantiles of IV-free SBP and DBP based on the genetic instruments for both BP parameters combined. The persistent lack of evidence for non-linearity between both DBP and SBP and MI in this analysis suggests that a genetically determined decrease in BP consistently leads to better outcomes even in individuals with baseline low DBP and regardless of their SBP levels.
In summary, we performed a large-scale Mendelian randomization analysis of the effects of BP on cardiovascular outcomes. We confirmed a linear causal influence of genetically determined DBP on cardiovascular events, finding no evidence of non-linearity in the shape of that effect even in individuals with baseline low DBP. This was despite traditional observational analysis of the same data demonstrating a J-curve association between DBP and MI. Taken together, these results suggest two conclusions; (1) the J curve relationship between DBP and MI is confirmed and low DBP represents a high-risk state for MI; however, (2) this relationship between low DBP and MI does not appear to be due to causal influence of low DBP on MI risk and instead appears to be due to unmeasured confounders. As such, when considered alongside post-hoc analyses of SPRINT, our data suggest that treatment of BP among persons with low baseline DBP (for example when pulse pressure is high and systolic BP is consequently above target) is not expected to cause further increased risk for MI.
Supplementary Material
Clinical Perspective.
What is new?
Prior observational studies (and our present observational analyses) demonstrate a J- or U-shape observational relationship between diastolic BP and myocardial infarction (implicating low diastolic BP as a high-risk clinical phenotype); however, the Mendelian Randomization analyses presented here show no evidence of a J-curve in the relationship between genetically-driven diastolic BP and MI.
Thus, while the observational diastolic BP J-curve is confirmed, our genetic data suggest that this relationship does not appear to represent a causal phenomenon and may; therefore, be the result of reverse causation or unobserved residual confounding.
What are the clinical implications?
This genetic analysis suggests that, when necessary, reducing blood pressure among individuals with baseline low diastolic blood pressure (for example when concurrent elevations in systolic BP and pulse pressure are present) may not cause a further increase in risk for adverse CVD outcomes or myocardial infarction and, in fact, might reduce that baseline risk; a finding that is concordant with post-hoc analyses of the randomized SPRINT trial.
Acknowledgments
Sources of Funding
Dr. Arvanitis was supported by NIH T32-HL007227 for this work.
Drs. Chatterjeee, and Qi were supported by NIH R01-HG010480-01.
Non-standard Abbreviations and Acronyms
- BMI
Body mass index
- BP
Blood pressure
- CAD
Coronary artery disease
- CHD
Coronary heart disease
- CI
Confidence interval
- CVD
Cardiovascular disease
- DBP
Diastolic blood pressure
- GWAS
Genome-wide association study
- HR
Hazard ratio
- IRB
Institutional review board
- IV
Instrumental variable
- LACE
Localized average causal effects
- MI
Myocardial infarction
- MR
Mendelian Randomization
- PC
Principal component
- PRS
Polygenic risk score
- SBP
Systolic blood pressure
- SNP
Single nucleotide polymorphism
- SE
Standard error
Footnotes
Disclosures
Dr. Deepak L. Bhatt discloses the following relationships - Advisory Board: Cardax, Cereno Scientific, Elsevier Practice Update Cardiology, Medscape Cardiology, PhaseBio, PLx Pharma, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Medtelligence/ReachMD (CME steering committees), Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Idorsia, Ironwood, Ischemix, Lexicon, Lilly, Medtronic, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, Novo Nordisk, Takeda.
References
- 1.Qamar A, Braunwald E. Treatment of Hypertension: Addressing a Global Health Problem. JAMA. 2018; 320: 1751–1752. [DOI] [PubMed] [Google Scholar]
- 2.Vidal-Petiot E, Ford I, Greenlaw N, Ferrari R, Fox KM, Tardif JC, Tendera M, Tavazzi L, Bhatt DL, Steg PG et al. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet. 2016; 388: 2142–2152. [DOI] [PubMed] [Google Scholar]
- 3.Basu S, Sussman JB, Rigdon J, Steimle L, Denton BT, Hayward RA. Benefit and harm of intensive blood pressure treatment: Derivation and validation of risk models using data from the SPRINT and ACCORD trials. PLoS Med. 2017; 14: e1002410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohm M, Schumacher H, Teo KK, Lonn EM, Mahfoud F, Mann JFE, Mancia G, Redon J, Schmieder RE, Sliwa K, et al. Achieved blood pressure and cardiovascular outcomes in high-risk patients: results from ONTARGET and TRANSCEND trials. Lancet. 2017; 389: 2226–2237. [DOI] [PubMed] [Google Scholar]
- 5.McEvoy JW, Chen Y, Rawlings A, Hoogeveen RC, Ballantyne CM, Blumenthal RS, Coresh J, Selvin E. Diastolic Blood Pressure, Subclinical Myocardial Damage, and Cardiac Events: Implications for Blood Pressure Control. J Am Coll Cardiol. 2016; 68: 1713–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatt DL. Troponin and the J-Curve of Diastolic Blood Pressure: When Lower Is Not Better. J Am Coll Cardiol. 2016; 68: 1723–1726. [DOI] [PubMed] [Google Scholar]
- 7.SPRINT Research Group, Wright JT, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015; 373: 2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beddhu S, Chertow GM, Cheung AK, Cushman WC, Rahman M, Greene T, Wei G, Campbell RC, Conroy M, Freedman BI, et al. Influence of Baseline Diastolic Blood Pressure on Effects of Intensive Compared With Standard Blood Pressure Control. Circulation. 2018; 137: 134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ilkun OL, Greene T, Cheung AK, Whelton PK, Wei G, Boucher RE, Ambrosius W, Chertow GM, Beddhu S. The Influence of Baseline Diastolic Blood Pressure on the Effects of Intensive Blood Pressure Lowering on Cardiovascular Outcomes and All-Cause Mortality in Type 2 Diabetes. Diabetes Care. 2020; 43: 1878–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson CA, Pettersson FH, Clarke GM, Cardon LR, Morris AP, Zondervan KT. Data quality control in genetic case-control association studies. Nat Protoc. 2010; 5: 1564–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White IR, Kaptoge S, Royston P, Sauerbrei W, Emerging Risk Factors Collaboration. Meta-analysis of non-linear exposure-outcome relationships using individual participant data: A comparison of two methods. Stat Med. 2019; 38: 326–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pulit SL, Stoneman C, Morris AP, Wood AR, Glastonbury CA, Tyrrell J, Yengo L, Ferreira T, Marouli E, Ji Y, et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum Mol Genet. 2019; 28: 166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eppinga RN, Hagemeijer Y, Burgess S, Hinds DA, Stefansson K, Gudbjartsson DF, van Veldhuisen DJ, Munroe PB, Verweij N, van der Harst P. Identification of genomic loci associated with resting heart rate and shared genetic predictors with all-cause mortality. Nat Genet. 2016; 48: 1557–1563. [DOI] [PubMed] [Google Scholar]
- 14.Burgess S, Davies NM, Thompson SG, EPIC-InterAct Consortium. Instrumental variable analysis with a nonlinear exposure-outcome relationship. Epidemiology. 2014; 25: 877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun YQ, Burgess S, Staley JR, Wood AM, Bell S, Kaptoge SK, Guo Q, Bolton TR, Mason AM, Butterworth AS, et al. Body mass index and all cause mortality in HUNT and UK Biobank studies: linear and non-linear mendelian randomisation analyses. BMJ. 2019; 364: l1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burgess S, Davies NM, Thompson SG. Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol. 2016; 40: 597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahman F, McEvoy JW. The J-shaped Curve for Blood Pressure and Cardiovascular Disease Risk: Historical Context and Recent Updates. Curr Atheroscler Rep. 2017; 19: 34–41. [DOI] [PubMed] [Google Scholar]
- 18.Wan EYF, Yu EYT, Chin WY, Fong DYT, Choi EPH, Lam CLK. Association of Blood Pressure and Risk of Cardiovascular and Chronic Kidney Disease in Hong Kong Hypertensive Patients. Hypertension. 2019; 74: 331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selvaraj S, Steg PG, Elbez Y, Sorbets E, Feldman LJ, Eagle KA, Ohman EM, Blacher J, Bhatt DL, REACH Registry Investigators. Pulse Pressure and Risk for Cardiovascular Events in Patients With Atherothrombosis: From the REACH Registry. J Am Coll Cardiol. 2016; 67: 392–403. [DOI] [PubMed] [Google Scholar]
- 20.Flint AC, Conell C, Ren X, Banki NM, Chan SL, Rao VA, Melles RB, Bhatt DL. Effect of Systolic and Diastolic Blood Pressure on Cardiovascular Outcomes. N Engl J Med. 2019; 381: 243–251. [DOI] [PubMed] [Google Scholar]
- 21.Boshuizen HC, Izaks GJ, van Buuren S, Ligthart GJ. Blood pressure and mortality in elderly people aged 85 and older: community based study. BMJ. 1998; 316: 1780–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018; 362: k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ference BA, Bhatt DL, Catapano AL, Packard CJ, Graham I, Kaptoge S, Ference TB, Guo Q, Laufs U, Ruff CT, et al. Association of Genetic Variants Related to Combined Exposure to Lower Low-Density Lipoproteins and Lower Systolic Blood Pressure With Lifetime Risk of Cardiovascular Disease. JAMA. 2019; 322: 1381–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ference BA, Julius S, Mahajan N, Levy PD, Williams KA S, Flack JM. Clinical effect of naturally random allocation to lower systolic blood pressure beginning before the development of hypertension. Hypertension. 2014; 63: 1182–1188. [DOI] [PubMed] [Google Scholar]
- 25.Kaltoft M, Langsted A, Nordestgaard BG. Obesity as a Causal Risk Factor for Aortic Valve Stenosis. J Am Coll Cardiol. 2020; 75: 163–176. [DOI] [PubMed] [Google Scholar]
- 26.Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018; 362: k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myocardial Infarction Genetics Consortium Investigators, Stitziel NO, Won HH, Morrison AC, Peloso GM, Do R, Lange LA, Fontanillas P, Gupta N, Duga S, et al. Inactivating mutations in NPC1L1 and protection from coronary heart disease. N Engl J Med. 2014; 371: 2072–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med. 2015; 372: 2387–2397. [DOI] [PubMed] [Google Scholar]
- 29.Gill D, Georgakis MK, Koskeridis F, Jiang L, Feng Q, Wei WQ, Theodoratou E, Elliott P, Denny JC, Malik R, et al. Use of Genetic Variants Related to Antihypertensive Drugs to Inform on Efficacy and Side Effects. Circulation. 2019; 140: 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Das S, Forer L, Schonherr S, Sidore C, Locke AE, Kwong A, Vrieze SI, Chew EY, Levy S, McGue M, et al. Next-generation genotype imputation service and methods. Nat Genet. 2016; 48: 1284–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loh PR, Danecek P, Palamara PF, Fuchsberger C, A Reshef Y, K Finucane H, Schoenherr S, Forer L, McCarthy S, Abecasis GR, et al. Reference-based phasing using the Haplotype Reference Consortium panel. Nat Genet. 2016; 48: 1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evangelou E, Warren HR, Mosen-Ansorena D, Mifsud B, Pazoki R, Gao H, Ntritsos G, Dimou N, Cabrera CP, Karaman I, et al. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat Genet. 2018; 50: 1412–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vilhjalmsson BJ, Yang J, Finucane HK, Gusev A, Lindstrom S, Ripke S, Genovese G, Loh PR, Bhatia G, Do R, et al. Modeling Linkage Disequilibrium Increases Accuracy of Polygenic Risk Scores. Am J Hum Genet. 2015; 97: 576–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burgess S, Thompson SG, CRP CHD Genetics Collaboration. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. 2011; 40: 755–764. [DOI] [PubMed] [Google Scholar]
- 35.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015; 44: 512–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol. 2016; 40: 304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burgess S, Thompson SG. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol. 2015; 181: 251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanderson E, Davey Smith G, Windmeijer F, Bowden J. An examination of multivariable Mendelian randomization in the single-sample and two-sample summary data settings. Int J Epidemiol. 2019; 48: 713–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou W, Nielsen JB, Fritsche LG, Dey R, Gabrielsen ME, Wolford BN, LeFaive J, VandeHaar P, Gagliano SA, Gifford A, et al. Efficiently controlling for case-control imbalance and sample relatedness in large-scale genetic association studies. Nat Genet. 2018; 50: 1335–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Harst P, Verweij N. Identification of 64 Novel Genetic Loci Provides an Expanded View on the Genetic Architecture of Coronary Artery Disease. Circ Res. 2018; 122: 433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arvanitis M, Tampakakis E, Zhang Y, Wang W, Auton A, 23andMe Research Team, Dutta D, Glavaris S, Keramati A, Chatterjee N, et al. Genome-wide association and multi-omic analyses reveal ACTN2 as a gene linked to heart failure. Nat Commun. 2020; 11: 1122–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A, Rutten-Jacobs L, Giese AK, van der Laan SW, Gretarsdottir S, et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet. 2018; 50: 524–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Staley JR, Burgess S. Semiparametric methods for estimation of a nonlinear exposure-outcome relationship using instrumental variables with application to Mendelian randomization. Genet Epidemiol. 2017; 41: 341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.