Abstract
Cognitive functioning is disrupted during a depressive episode and cognitive dysfunction persists when depression is in remission. A subtype of depressed individuals who exhibit elevated inflammatory biomarkers may be at particular risk for cognitive dysfunction. We examined whether an elevated inflammatory biomarker (C-reactive protein: CRP) in acute and/or remitted depression was associated with specific deficits in executive functioning, episodic memory, and verbal fluency. Data were drawn from a population-based sample of Dutch adolescents (N = 1,066; 46% male) recruited at the age of 11 and followed over the course of eight years. We tested whether adolescents with either, (i) a history of depression (Wave 1 – 3) or (ii) current depression (Wave 4), and elevated levels of C-reactive protein measured in blood at Wave 3 performed worse on cognitive assessments at Wave 4. Eight measures of cognitive functioning were hypothesized to load on to one of three dimensions of cognitive functioning (executive functioning, episodic memory, and verbal fluency) within a structural equation model framework. Higher levels of CRP were associated with worse future executive functioning in adolescents with and without current/prior depression. A current depression diagnosis also was associated with worse future executive functioning. There was consistent evidence linking low socioeconomic status and health-related covariates (high body mass index/sedentary behavior) with worse performance across multiple measures of cognitive functioning and, importantly, the association of depression/CRP and executive functioning was no longer significant when controlling for these covariates. Future studies may benefit from investigating whether specific depressogenic behaviors (e.g., sedentary behavior/substance use) mediate a relationship between depression and worse executive functioning, potentially via a prospective pathway through elevated inflammation.
Keywords: Cognitive functioning, depression, inflammation, adolescence, executive functioning, verbal fluency, episodic memory, youth, CRP, C reactive protein
1. Introduction
Depression is a highly prevalent, recurrent, and burdensome disorder that typically first emerges during adolescence (1, 2). Depression tends to follow a remitting, relapsing course: recurrent episodes occur for 50% of individuals who experience a first depressive episode and 80% of those who experience a second episode (3, 4). The consequences of depression are severe; most depressed individuals (approximately 60%) report that the impact of symptoms on functioning is severe or very severe, particularly for social functioning, and depression is associated with increased risk of adverse outcomes, such as suicidal behavior and cardiovascular disease (4–7). It is the heavy disease burden of depression combined with its high prevalence, early onset, and recurrent course that makes it imperative to better understand the etiology of depression, particularly during adolescence when it typically first emerges.
Cognitive vulnerabilities, that are characterized by negative biases in the way individuals attend to, interpret, and recall information from their environment, have been shown to precede depression and predict its first onset in prospective, longitudinal studies and in high-risk designs (8–12). However, in addition to the existence of negative cognitive biases in depression, there is accumulating evidence that cognitive processes (e.g., memory, attention, and executive functioning) are disrupted in depression. Depressed individuals perform worse across a broad range of cognitive domains (e.g., episodic and working memory, sustained attention, psychomotor speed, and executive function) when compared to healthy controls (13–16). These deficits are observed at first onset (17), in both medicated and unmedicated samples (18), and in both community and in-patient samples (19). Moreover, deficits across a range of cognitive domains persist in remitted depression (most consistently in psychomotor speed, attention, executive functioning, and verbal fluency) (13, 20–22); deficits in episodic memory are typically confined to a current depressive state (13, 21, 23). There is growing interest in understanding why cognitive dysfunction is observed in depression, particularly given its major contribution to functional impairment (24–26).
The association between cognitive functioning and depression may be characterized by four relationships. First, cognitive dysfunction may be caused by the presence of depressive symptoms, with cognitive dysfunction limited to the duration of a depressive episode (16). Second, depression, particularly a more severe and/or chronic course, may lead to neuropsychological scarring, such that cognitive dysfunction persists beyond a depressive episode (27). Third, cognitive dysfunction may play a causal role in the onset of depression (28–31) – for example, cognitive dysfunction may generate stressful life events (e.g., academic failure) precipitating a depressive episode. Finally, depression and cognitive functioning may not be causally related and, instead, observed associations may be due to common underlying causes (e.g., inflammation) (32–34). It should be noted that these relationships are not mutually exclusive, and instead, may exert reciprocal effects. To extend our previous example, cognitive dysfunction generates stressful life events (e.g., academic failure) leading to depression, which, in turn, leads to substance use that further worsens cognitive functioning and, exacerbated by further impairment in cognitive functioning, increases the likelihood of further academic failure, resulting in increasing depressive symptoms…). In fact, Mac Giollabhui et al. (34) have shown in a single cohort that cognitive dysfunction worsens during a depressive episode, persists when depressive symptoms abate, and that stress exposure is longitudinally associated with both increased depressive symptoms and worse cognitive functioning. The lack of firm conclusions about the prospective associations of cognitive functioning and depression may be exacerbated by the relative dearth of longitudinal studies examining cognitive functioning prior to first onset of depression.
Difficulty in accurately characterizing the relationship between cognitive functioning and depression is exacerbated by heterogeneity within depression and variability in how cognitive functioning is assessed. Cognitive deficits in depressed individuals vary based on a wide range of demographic (age, education, socioeconomic status) and clinical characteristics (pre-clinical dementia, severity of depressive symptoms, recurrence of depression, comorbid conditions, and medication status) (14, 16, 35–39). Thus, it may be that group differences observed in cognitive functioning within depressed samples are driven by specific demographic, clinical, and/or biologically-based phenotypes (39, 40). Second, discrepant results may be due to differences in the domains of cognitive functioning assessed in a given study and/or differences in the functional demands of a specific cognitive test; for example, some tasks assessing cogntive flexiblity also capture variability in psychomotor speed while others also capture variability in problem-solving/rule learning. Moreover, most studies examine a relatively small number of cognitive domains, and typically, it is unclear whether cognitive deficits reported are specific to the domain assessed (e.g., differentially affecting episodic memory) or reflect difficulties in cognitive functioning that are more generalized in nature (e.g., affecting episodic memory and multiple other domains).
Meaningful progress toward understanding the relationship between cognitive functioning and depression may require a better understanding of the biological mechanism(s) underpinning cognitive dysfunction in depression, which, in turn, may identify for whom and under which conditions cognitive dysfunction emerges in depression. Multiple, overlapping biological pathways are implicated in the development of cognitive dysfunction in depression (40). In particular, there is convergent evidence for inflammation as a neurobiological mechanism underpinning cognitive dysfunction in depression. Peripheral inflammation can act directly upon the central nervous system (41–44) and disrupt neuronal processes (e.g., long-term potentiation, synaptic plasticity, and neurogenesis) as well as affect brain regions and their respective cognitive associates (e.g., hippocampus: episodic memory; anterior cingulate cortex: executive function). Studies have linked inflammatory biomarkers with impaired cognition in medical (45–47), healthy elderly (48–50), and healthy adult (51–53) samples; however null results also are observed (54). Inflammatory biomarkers also have been prospectively associated with worse cognition in healthy middle-aged samples (55, 56). Inflammation also is implicated in the development of ‘sickness’ behaviors that characterize depression (anhedonia, social withdrawal, psychomotor retardation) (57, 58). Experimental induction of inflammation is associated with the onset of depressive symptoms (59) and it also prospectively predicts depression in community samples (60–62). It is noteworthy that elevated peripheral biomarkers of inflammation are likely present in just a subgroup (approximately 30%) of individuals with MDD (63–67). Thus, there is strong evidence linking inflammatory physiology in both cognitive dysfunction and depression.
A small, emerging body of research has examined the relationship between inflammatory biomarkers and cognitive functioning in major depression (MDD) (68), and there is convergent evidence linking inflammatory biomarkers with structural and functional brain abnormalities observed in MDD as well as cognitive dysfunction (40, 69, 70). Elevated inflammatory biomarkers have been associated with psychomotor retardation, memory deficits, and impaired executive functioning in adults with current MDD (71–74); however, it is notable that, although inflammatory biomarkers are associated with cognitive functioning in the depressed group, they also are associated with cognitive dysfunction in healthy controls (72, 74). Other studies have shown that inflammatory biomarkers may be indirectly associated with worse working memory via higher body mass (53), and longitudinal data indicate that higher body mass prospectively predicts both worse executive functioning and more severe depressive symptoms in a diverse, community sample of adolescents, with interleukin-6 as the mediator of the body mass-executive functioning association (32). These studies provide initial evidence implicating inflammatory biomarkers in the etiology of cognitive dysfunction in depression. However, many outstanding questions exist – two of which will be investigated in this study. First, are depression and inflammatory biomarkers independent risk factors for cognitive dysfunction or do these factors compound so that individuals with elevated depression and inflammatory biomarkers experience greater difficulties than individuals with either depression or an elevated inflammatory biomarker alone? Second, is the association between an elevated inflammatory biomarker and cognitive dysfunction limited to depressive episodes, or might the presence of persistently elevated inflammatory biomarkers explain why cognitive dysfunction is observed outside of depressive episodes?
This study examined these two central questions using four waves of data from a population-based sample of Dutch adolescents (N = 1,066). Specifically, we tested whether adolescents with either (1) a history of depression (self-reported symptoms at Waves 1 – 3 or retrospective diagnosis at W4) or (2) current depression (self-reported symptoms or diagnosis at Wave 4) and elevated levels of an inflammatory biomarker (C-reactive protein: CRP) measured in blood at Wave 3 performed differentially worse on cognitive tests at Wave 4. In addition, we investigated whether CRP and/or depression were associated with specific deficits in executive functioning, episodic memory, and verbal fluency or whether they were associated with a more generalized pattern of cognitive dysfunction. These three dimensions of cognitive functioning were estimated using a structural equation model framework based on eight measures of cognitive functioning: executive functioning, episodic memory, and verbal fluency.
We hypothesized that:
Individuals with current depression at Wave 4 and elevated CRP at Wave 3 will perform worse on tests of executive functioning, episodic memory, and verbal fluency at Wave 4 than individuals with either current depression or elevated CRP alone.
Individuals with a history of depression (Waves 1 – 3) and elevated CRP (Wave 3) will perform worse on tests of executive functioning and verbal fluency (but not episodic memory) at Wave 4 than individuals with either current depression or elevated CRP alone.
These hypotheses are generally in line with previous findings of cognitive deficits in current depression/depression history (13, 14, 20, 21, 75). It should be noted that, because this study only measured CRP and cognitive functioning at a single timepoint, we only investigated a single dimension of the possible associations of CRP, cognitive functioning and depression. For example, it is equally plausible that CRP may lead to difficulties in self-regulatory behavior, which may, in turn, leads to stress generation and depression (76).
2. Materials and methods
2.1. Participants
Data were drawn from the TRacking Adolescents’ Individual Lives Survey (TRAILS), a prospective cohort study examining psychosocial development and mental health during adolescence and early adulthood. Adolescents aged 11 years were recruited and attended regular follow-up assessments every 2–3 years. Two separate cohorts were followed by TRAILS: one population-based and another clinic-based (77). This study is based on data from the TRAILS population-based cohort. Adolescents were recruited from 135 schools in five municipalities in the north of The Netherlands, which included both urban and rural areas. Eligible participants were required to be enrolled in primary school, and of 2935 youth who met this criterion, 2230 (76%) provided informed consent from both parent and child to participate.
The current study utilized data from Waves 1 – 4. From the baseline sample, 1,231 adolescents’ blood was assayed at Wave 3 (W3), of whom 18 participants were excluded because CRP > 10mg/L and they also reported either experiencing illness, injury, or a doctor visit/hospitalization during the prior week. From the 1,213 remaining participants, 1,066 had complete data available on all measures of cognitive functioning. The analytic sample of 1,066 participants had a mean age of 11.09 years (SD = .56) at W1, 13.52 years (SD = .52) at W2, 16.19 years (SD = .65) at W3 and 19.00 years (SD = .57) at W4. In this study, depressive symptoms were measured at each assessment (W1 – W4), depression diagnosis (lifetime and 30-day prevalence) was assessed via a semi-structured interview at W4, a battery of neuropsychological measures were administered at W4 to measure cognitive functioning, and a range of covariates used in this study were assessed at W1 (socio-economic status, age, sex) or W3 (sedentary behavior, body mass index, and substance use).
The analytic sample was less likely than the complete TRAILS sample enrolled at baseline to include individuals of low socioeconomic status [(SES); mean difference = .41, p < .001] and males, χ2(1, 2228) = 7.89, p = .005. No difference in baseline age was observed, (p = .12).
2.2. Measures
2.2.1. Depressive Disorder
Depressive disorders were assessed at W4 using the Composite International Diagnostic Interview, version 3.0 (CIDI) (78). The CIDI is a structured diagnostic interview that uses criteria from the Diagnostic and Statistical Manual of Mental Disorders, fourth edition to identify individuals meeting criteria for a major or dysthymic depressive diagnosis during their lifetime or during the 30 days prior to interview (current). The CIDI is a valid, reliable, and widely used instrument to assess depression (79–81).
2.2.2. Depressive Symptoms
Depressive symptoms were assessed at W1 – W4. During the first three waves, depressive symptoms were measured using the Withdrawn/Depressed scale of the Youth Self-Report (YSR) (82). The scale has demonstrable reliability at W1 (8 items; α = .72), W2 (14 items; α = .74), and W3 (14 items; α = .77). At W4, the Withdrawn/Depressed scale (14 items; α = .76) from the Adult Self-Report form was administered (83).
2.2.3. C-Reactive Protein
C reactive protein (CRP) is a liver-based protein used as an indicator of systemic inflammation. The current study used a high sensitivity CRP assay capable of reliably measuring CRP at lower levels of detection. At Wave 3, 39.5 ml of blood was drawn from fasting participants and breakfast was subsequently provided – 89.9% of participants endorsed fasting at the time of the blood draw. CRP was assayed using an immunonephelometric method, BN2 of Siemens Medical Solutions USA (Malvern, PA, USA) with a lower detection limit of 0.175 mgl−1. Intra-assay coefficients of variance ranged from 2.1 to 4.4 mgl−1, and inter-assay coefficients of variance ranged from 1.1 to 4.0 mgl−1. CRP values were relatively low, as might be expected in a community sample of adolescents, with 90% of participants exhibiting hsCRP values < 3.2mg/L.
2.2.4. Cognitive Functioning
Reliable and valid measures of cognitive functioning were administered at W4. Measures administered are discussed briefly here and thorough descriptions are provided in Supplementary Information. Normative data were not used because Dutch norming data were not available across all measures. Cognitive data are described in Supplementary Table 1. Auditory, verbal working memory was measured using the ‘Digit Span Backwards’ from the Wechsler Adult Intelligence Scale, third edition (WAIS-III), Dutch version (84). Visual working memory was assessed using the Self-Ordered Pointing Task (85); the average number of errors was used as the outcome variable for this task, given that it is the most sensitive outcome (86) and to be consistent with prior TRAILS studies (87). Visual organization, a component of executive functioning, was assessed using the ‘Copy’ trial of the Rey-Osterrieth Complex Figure Test (88), in which participants were asked to draw a complex geometric shape. Visuo-constructional and non-verbal reasoning skills were assessed using the ‘Block Design’ subtest of the WAIS-III (84). Immediate (number of words recalled across five trials) and delayed (number of target words recalled following a delay) verbal episodic memory was assessed using a list learning task (Rey Auditory Verbal Learning Test) (89). Verbal fluency (phonological and semantic) was measured using a modified version of the short test of semantic and phonological fluency
Three latent constructs were hypothesized to underpin these eight measures: executive functioning, episodic memory, and verbal fluency. It should be noted that important components of executive functioning were not available, such as inhibition and cognitive flexibility (90), and less commonly used measures were included, such as visual reasoning and visual organization. This decision was based upon the measures available, and when these four measures were separated into two latent factors of visual/verbal working memory (Self-Ordered Pointing Task/Digit Span Backward) and visual organization/reasoning (Rey-Osterrieth Complex Figure Test/Block Design), the correlation of the latent factors was .83. Although verbal fluency often is considered an index of executive functioning, more recent research has identified it as more closely linked with language skills and psychomotor speed (91, 92).
2.2.5. Covariates
Sociodemographic variables.
Participant sex was measured at W1 (male was coded as ‘1’). Age was assessed at all assessments, as was SES. SES was estimated using five indicators: family income, maternal educational level, paternal educational level, maternal occupational level and paternal occupational level using the International Standard Classification of Occupations and has been consistently used in TRAILS (93, 94). Height and weight were measured at W3 and used to calculate body mass index (kg/m2).
Behavioral variables.
Participant sedentary behavior measured at W3 was calculated as the mean number of hours: sitting at a computer (Monday-Friday); sitting at a computer (Saturday/Sunday); watching television or video (Monday-Friday); and watching television or video (Saturday/Sunday). Substance use was measured at W3 and calculated as the mean of the number of cigarettes smoked in the last week (0 = ‘I don’t smoke’; 1 = ‘I haven’t smoked in the last week’; 2 = ‘< 1 cigarette a day; 3 = ‘1–5 cigarettes a day’; 4 = ‘6–10 cigarettes a day; 5 = ‘11–20 cigarettes a day’; 6 = ‘>20 cigarettes a day’) and number of days of the last week participant drank alcohol.
2.2.6. Analyses
Analyses were conducted in Mplus (Version 7.4) and missing data were handled using Full Information Maximum Likelihood. We used growth mixture modelling to identify the smallest number of classes of individuals exhibiting different intercepts and trajectories of depressive symptoms measured at Waves 1 – 3, as described by Jung and Wickrama (95). Latent class growth analysis, where the variance and covariance estimates of the growth factors are constrained to be zero within each class, was not selected because of the known heterogeneity in depressive symptoms. Estimated models constrained residual variances to be equal and estimation was based on 500 random starts and 200 optimizations in the final stage. Depressive symptoms (W1-W3) do not exhibit evidence of skewness or kurtosis (all estimate < 1.02). We set out with a one-class solution and subsequent models were progressively added. Model identification was based upon theoretical interpretability of the classes estimated (i.e., latent classes being congruent with trajectories of depression in adolescence) and model fit. Model fit was assessed via commonly used indices of fit and likelihood-based statistical tests [Akaike information criterion (AIC), Bayesian information criterion (BIC), entropy, Vuong Lo-Mendell-Rubin likelihood ratio test] (96, 97).
Next, a structural equation model approach identified the model that best fit the cognitive data based on three possible models: single factor model, correlated factors model (three latent factors), or a bifactor model (one general factor/three specific factors). Three factors were generated from eight cognitive tasks thought to measure: executive functioning (verbal/visual working memory and visual organization/visuoconstructional abilities), verbal fluency (phonological/semantic fluency), and episodic memory (immediate/delayed recall). Although, verbal fluency often is considered an executive function, there is strong evidence that it indexes verbal abilities (91) and/or psychomotor speed (92). The model that best fit our data was selected based on the Comparative Fit Index (CFI), Tucker Lewis Index (TLI), Root Mean Square Error of Approximation (RMSEA), AIC and BIC. The Chi-Square test of model fit was reported according to convention, but was not interpreted because it has limited utility in large samples (i.e., N > 200) (98, 99). For the CFI, good fit consisted of a value > .90 and excellent fit by a value > .95. A RMSEA statistic between .05 and .10 was indicative of good fit, whereas a value <.05 was indicative of excellent fit (100). Lower AIC and BIC values were considered indicative of better model fit.
Third, for predictive analyses, two models were fitted separately for the two measures of depression: (i) depressive symptoms measured at Waves 1 – 4 and (ii) lifetime and recent depression diagnosis measured at Wave 4. For both models, we examined whether depression and CRP were associated with cognitive functioning when controlling for demographic and behavioral variables. Subsequently, we included an interactive term of CRP by depression (both current and past). Demographic and behavioral variables were selected that are known correlates of depression, CRP, and/or cognitive functioning (101, 102).
Sensitivity analyses [based on recommendations by Mac Giollabhui et al. (103)] evaluated the generalizability of results based on how extreme CRP values are handled in analyses;. For the main analyses, 18 extreme CRP values were excluded where CRP > 10mg/L and the participant reported experiencing illness, injury, or a doctor visit/hospitalization during the prior week. Sensitivity analyses were reported where all CRP values > 10mg/L (n = 40) were both included and excluded.
3. Results
Bivariate correlations and descriptive statistics for the main study variables are presented in Table 1 for the analytic sample of 1,066 adolescents with complete data on cognitive measures, whose blood was assayed at Wave 3 (W3), and who did not exhibit evidence of acute illness/injury. Notable associations are described below. Identifying as female (W1) was generally associated with higher depression across multiple waves, BMI (W3), CRP (W3), and episodic memory (W4). Higher SES (W1) also was consistently associated with: less severe depression across multiple waves; lower BMI, lower CRP, and sedentary behavior (W3); and better performance on all cognitive measures (W4). Depressive symptoms (W1 –W4) were generally associated with higher BMI, CRP, and sedentary behaviors. Elevated depression was generally associated with worse working memory, but not other aspects of cognitive functioning at W4 and both BMI and CRP were consistently associated with measures of executive functioning.
Table 1.
Correlation Matrix and Descriptive Statistics for Variables of Interest (n = 1,066)
| Variable | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. W1 Age | .03 | .04 | .00 | .00 | .00 | .01 | −.03 | .03 | .04 | .09** | .01 | −.06* | −.04 | .03 | .00 | −.05 | −.03 | −.03 | .03 | .03 | .03 |
| 2. W1 Sex (Male = 1) | – | .03 | −.02 | .15** | .15** | −.04 | −.12** | −.05 | −.17** | −.12** | −.12** | −.03 | .17** | .09** | −.07* | −.07* | −.05 | −.17** | −.19** | −.04 | −.01 |
| 3. W1 SES | – | −.07* | −.05 | −.10** | −.09* | −.07* | −.07* | −.05 | −.15** | −.10** | .06 | −.18** | .26** | .16** | .13** | .24** | .17** | .10** | .22** | .28** | |
| 4. W1 Dep Sxsa | – | .45** | .33** | .26** | .28** | .09** | .18** | .11** | .07* | .03 | .07* | .00 | .02 | .02 | −.10 | −.01 | −.02 | −.01 | −.06 | ||
| 5. W2 Dep. Sxsa | – | .53** | .37** | .52** | .14** | .27** | .06 | .08** | .02 | .04 | −.01 | .00 | .06* | −.05 | .04 | .03 | .01 | −.02 | |||
| 6. W3 Dep. Sxsa | – | .53** | .76** | .14** | .31** | .08* | .01 | −.03 | .13** | −.02 | .02 | .04 | −.07* | .03 | .04 | −.04 | −.05 | ||||
| 7. W4 Dep. Sxsb | – | .44** | .21** | .30** | .04 | .01 | −.02 | .08* | −.01 | −.03 | .04 | −.06 | −.03 | −.02 | −.09* | −.03 | |||||
| 8. W1 – 3 Depression Class | – | .11** | .24** | .06* | .01 | .05 | .07* | −.02 | .02 | .07* | −.07* | .02 | .02 | −.02 | −.02 | ||||||
| 9. W4 Current Dep. Dx. | – | .37** | .03 | .02 | .02 | .02 | −.03 | −.03 | −.05 | −.07* | −.01 | −.02 | −.06 | .00 | |||||||
| 10. W4 Lifetime Dep. Dx. | – | .08** | .04 | −.02 | .06* | −.02 | −.04 | .00 | −.05 | .01 | −.02 | .00 | .00 | ||||||||
| 11. W3 BMI | – | .30** | −.03 | .14** | −.06 * | −.08** | −.07 * | −.12** | −.08** | −.06 | −.06 | −.03 | |||||||||
| 12. W3 CRP | – | −.04 | −.02 | −.09** | −.09** | −.02 | −.07* | −.02 | −.01 | −.05 | −.03 | ||||||||||
| 13. W3 Substance Use | – | .02 | .05 | .10** | .01 | .05 | .01 | −.02 | .03 | .07* | |||||||||||
| 14. W3 Sedentary | – | −.14** | −.11** | −.13** | −.14** | −.13** | −.10** | −.15** | −.14** | ||||||||||||
| 15. W4 Block Design | – | .30** | .21** | .29** | .24** | .17** | .28** | .27** | |||||||||||||
| 16. W4 RCFT: Copy | – | .12** | .23** | .23** | .21** | .13** | .15** | ||||||||||||||
| 17. W4 SOPT | – | .21** | .27** | .23** | .17** | .15** | |||||||||||||||
| 18. W4 DS: Back | – | .35** | .27** | .22** | .34** | ||||||||||||||||
| 19. W4 Rey: Immediate | – | .74** | .31** | .29** | |||||||||||||||||
| 20. W4 Rey: Delay | – | .29** | .22** | ||||||||||||||||||
| 21. W4 Fluency : Seman. | – | .48** | |||||||||||||||||||
| 22. W4 Fluency: Phono. | – | ||||||||||||||||||||
| Mean | .46 | .16 | .35 | .35 | .37 | .23 | .17 | .03 | .17 | 21.20 | 1.16c | 0.87 | 3.17 | 44.43 | 31.47 | 1.56 | 6.75 | 51.14 | 10.53 | 36.45 | 21.98 |
| SD = | .50 | .77 | .29 | .28 | .32 | .27 | .38 | .16 | .38 | 3.08 | 2.47c | 1.08 | 1.29 | 15.11 | 3.06 | 0.78 | 2.06 | 8.65 | 2.64 | 8.37 | 7.38 |
W = Wave; Dep = Depressive; Sxs = Symptoms;
Sxs = Measured using the Withdrawn/Depressed scale of the Youth Self Report;
Sxs = Measured using the Withdrawn/Depressed scale from the Adult Self-Report; BMI = Body Mass Index; CRP = C-Reactive protein; RCFT = Rey-Osterrieth Complex Figure Test; SOPT = Self-ordered Pointing Task; Rey = Rey Verbal Learning Test; Seman. = Semantic; Phono. = Phonological; Fluency: Phono; Descriptive statistics for Age = .11.09, SD = 0.56
= Mean and standard deviation reported for non-transformed raw C-Reactive Protein values (mg/L)
= p <.05
= p <.001
3.1. Latent Class Analysis of Depressive Symptoms
Latent-class analysis identified the best fitting model of depressive symptoms, based on depressive symptoms reported (W1 - W3). Fit statistics, latent class intercept and slope, and latent class size are provided in Table 2 for all models. A two-class solution was selected identifying a group with lower baseline depressive symptoms that decreased across waves 1 – 3 (intercept: .32, p<.001; slope: −.03, p<.05; n = 883) and a group with higher baseline depressive symptoms that increased across waves 1 – 3 (intercept: .49, p<.001; slope: .17, p<.001; n = 183). Complete detail on why a two-, rather than three- or four-, class solution is provided in supplementary material.
Table 2.
Fit Statistics and Statistical Parameters for Four Latent Class Models: a One Class Model, a Two Class Model, a Three Class Model, and a Four Class Model (n = 1,066)
| Fit Statistics | Model 1: One Class Model | Model 2: Two Class Model | Model 3: Tdree Class Model | Model 4: Four Class Model1 | ||||||||
| Log Likelihood | −333.79 | −266.10 | −221.14 | −199.44 | ||||||||
| AIC | 683.59 | 554.20 | 470.28 | 432.89 | ||||||||
| BIC | 723.36 | 608.89 | 539.89 | 517.41 | ||||||||
| Entropy | – | 0.76 | .77 | .79 | ||||||||
| LRT | – | p < .001 | p < .001 | p = .11 | ||||||||
| Model 1: One Class Model | Model 2: Two Class Model | Model 3: Tdree Class Model | Model 4: Four Class Model | |||||||||
| Classes | Intercept | Slope | N | Intercept | Slope | N | Intercept | Slope | N | Intercept | Slope | N |
| Class One | .35** | .01* | 1066 | .32** | −.03* | 883 | .25** | .01 | 800 | .24** | .01 | 783 |
| Class Two | Not estimated – one class model | .49** | .17* | 183 | .47** | .21** | 136 | .28** | .30** | 75 | ||
| Class Tdree | Not estimated – two class model | .81** | −.21** | 130 | .79** | −.25** | 114 | |||||
| Class Four | Not estimated – tdree class model | .71** | .06 | 94 | ||||||||
AIC = Akaike information criterion; BIC = Bayesian information criterion; LRT = adjusted Vuong-Lo-Mendell-Rubin Likelihood Ratio Test(p value)
= p <.05
= p <.001
= Failed to reliably converge on a single solution.
3.2. Factor Models of Cognitive Variables
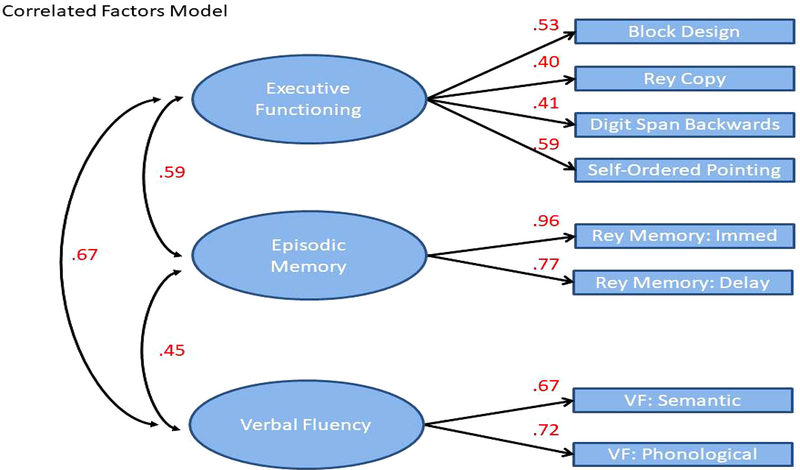
Fit indices for three potential models of the cognitive measures are presented in Table 3. A single factor solution fit the data poorly based on the CFI, TLI, and RMSEA and no reliable solution was found using a bifactor model. Instead, the correlated factors model demonstrated good to excellent fit across all indices. Visual depiction of the correlated factors model including factor loadings and path coefficients is presented in Figure 1. Factor loadings for each latent variable ranged from .40 to .96 and the measure of association ranged from .45 to .67.
Table 3.
Fit Indices for Four Potential Measurement Models underlying Cognitive Measures (n = 1,066).
| Model | χ2 | df | CFI | TLI | SRMR | RMSEA (90% CI) | AIC | BIC |
|---|---|---|---|---|---|---|---|---|
| Single Factor | 442.98 | 20 | .76 | .66 | .086 | .14 (.13−.15) | 22768.02 | 22887.34 |
| Correlated Factors | 78.27 | 17 | .97 | .94 | .032 | .06 (.05−.07) | 22386.23 | 22520.46 |
| Bifactor | Model failed to converge. | |||||||
df = degrees of freedom; CFI = Comparative Fit Index; TLI = Tucker Lewis Index; SRMR = Standardized Root Mean Square Residual; RMSEA = Root Mean Square Error of Approximation; AIC = Akaike Information Criterion; BIC = Bayesian Information Criterion
Figure 1.
Visual depiction of the correlated factors model
3.3. Structural Models Predicting Cognitive Functioning by CRP and prior depression
Following identification of a model that provided a satisfactory fit for the cognitive measures, we subsequently extended the correlated factors model to examine the associations of CRP and depression with domains of cognitive functioning within a structural equation modeling framework. We iteratively examined the following models: depression (current depression and history of depression; Model 1); depression, CRP, and demographic variables (sex, age, SES) (Model 2); depression, CRP, demographic, and biobehavioral variables (sedentary behavior, body mass index, substance use) (Model 3); followed by the introduction of the interaction terms (CRP x current depression: Model 4; CRP x history of depression: Model 5). Fit indices for all models ranged from good to excellent – see Supplementary Table 2 for complete information.
The associations of depressive symptoms [latent trajectory (i.e., history)/current symptoms] and depression diagnostic status [history/current diagnosis (within last 30 days)] and CRP (in addition to important covariates) with cognitive functioning are reported in Table 4. In Model 1, where both current and prior depression were entered, only a current depression diagnosis was associated with worse executive functioning. When adjusting for demographic covariates and depression (past/current), higher CRP was associated with worse executive functioning. Higher SES consistently was associated with higher performance on all measures of cognitive functioning. When additional biobehavioral covariates were introduced in Model 3, the associations of higher CRP and worse executive functioning was no longer statistically significant. Higher levels of sedentary behavior were associated with worse executive functioning and verbal fluency, whereas higher levels of BMI were associated with worse episodic memory. CRP did not interact with trajectories of depressive symptoms or prior depression diagnosis (Model 4) to predict differential performance in any domain of cognitive functioning. It should be noted that, in the case of a current depression diagnosis, all 29 individuals who met criteria for acute depression had previously experienced a depressive episode and consequently it is not possible to disentangle current from recurrent depression.
Table 4.
Depressive Symptoms (Wave 1–3 Trajectories/Wave 4) and Depression Diagnosis (History/Current) Predicting Domains of Cognitive Functioning
| Depressive Sxs – Child Report (n = 1044) | Depression Diagnosis (n = 1058) | |||||
|---|---|---|---|---|---|---|
| Models | Executive Functioning | Episodic Memory | Verbal Fluency | Executive Functioning | Episodic Memory | Verbal Fluency |
| Model 1 | ||||||
| Depression W4 | −.04 | −.05 | −.09 | −.50** | −.10 | −.28 |
| Depression W1 – W3 | −.01 | .11 | .02 | −.08 | .06 | .04 |
| Model 2 | ||||||
| Depression W4 | −.02 | −.03 | −.06 | −.31 | −.04 | −.13 |
| Depression W1 – W3 | .01 | .07 | .07 | −.07 | −.05 | .06 |
| Log-transformed CRP | −.11* | −.03 | −.04 | −.11* | −.04 | −.04 |
| Age | −.06 | −.01 | .07* | −.06 | −.01 | .07* |
| Sex (1 = male) | −.08 | −.40** | −.10 | −.10 | −.42** | −.10 |
| Socio-economic status (Higher status = higher values) | .41** | .17** | .36** | .40** | .16** | .36** |
| Model 3 | ||||||
| Depression W4 | .00 | −.01 | −.04 | −.40 | −.16 | −.13 |
| Depression W1 – W3 | −.01 | .07 | .01 | .07 | .12 | .00 |
| Log-transformed CRP | −.08 | .00 | −.02 | −.07 | −.01 | −.02 |
| Age | −.07 | −.02 | .07 | −.07 | −.01 | .07 |
| Sex (1 = male) | −.04 | −.20** | −.05 | −.03 | −.42** | −.02 |
| Socio-economic status (Higher status = higher values) | .37** | .14** | .33** | .37** | .13** | .33** |
| Sedentary Behavior | −.20** | −.06 | −.13* | −.19** | −.05 | −.14** |
| Body Mass Index | −.08 | −.07* | .03 | −.09 | −.08* | .03 |
| Substance Use | −.07 | −.06 | −.07 | −.05 | −.05 | −.07* |
| Model 4 / Model 3 + Interactions | ||||||
| Log-transformed CRP*Depression W4 | .01 | .05 | .02 | −.01 | −.01 | .00 |
| Log-transformed CRP*Depression W1 – W3 | .01 | .06 | .06 | −.03 | .08 | .01 |
= p <.05
= p <.001
Sxs = Symptoms; Dx = Diagnosis; CRP = C-reactive protein
3.4. Sensitivity analyses and Exploratory Analyses
No meaningful differences were observed in analyses in which all CRP values greater than or equal to 10 mg/L were either removed or included. We conducted additional exploratory analyses to estimate whether the observed association of CRP and executive functioning differed in individuals with current depression/history of depression compared to individuals without depression replicating the analysis presented in Table 4 for depressive symptoms (Depressive symptoms: Model 2). Models were specified separately by group (‘History/current depression diagnosis’ vs. ‘No diagnosis history’) and the parameters for executive functioning regressed on log-transformed CRP did not differ by group (wald test statistic = .541, p = .46).
4. Discussion
In a population-based sample of 1,066 Dutch adolescents who were recruited at age 11 years and assessed on three subsequent occasions, spaced approximately 2.5 years apart, we found that higher levels of CRP at Wave 3 were associated with worse future executive functioning at Wave 4, irrespective of whether they had a history of depression or not (Waves 1 – 4). In addition, adolescents experiencing a current depressive episode exhibited worse executive functioning than non-depressed peers. There was no evidence of an additive effect whereby those with depression and elevated CRP performed worse on measures of cognitive functioning than individuals with depression or elevated CRP alone. There was consistent evidence linking low socioeconomic status and health-related covariates (high body mass index/sedentary behavior) with worse performance across multiple measures of cognitive functioning and, importantly, the association of depression/CRP and executive functioning was no longer significant when controlling for these covariates. These results provide evidence that, in depression, higher CRP is associated with worse executive functioning, but that these associations are not unique to depression.
CRP was prospectively associated with worse future executive functioning independent of age, sex, SES, and depression. This finding is consistent with concurrent and prospective associations observed in middle-aged or elderly samples (48, 49, 104, 105). The strength of the association between CRP and executive functioning did not differ depending on whether adolescents had experienced depression (either a current diagnosis or a prior diagnosis), suggesting that an inflammatory subtype of depression characterized by worse cognitive functioning may exist, but that this association is not unique to depression. This finding is generally consistent with previous work, although few studies have been conducted in community or youth samples. Similar to this study, salivary CRP was concurrently associated with worse performance on some executive functioning tasks in a risk-enriched sample of 107 young adolescents (106), irrespective of the level of internalizing, externalizing, or subclinical psychotic symptoms present. However, CRP was not associated with two measures of cognitive functioning (visual working memory/verbal episodic memory) in a prior TRAILS study investigating the cognitive sequelae of herpes viruses (87) and was not associated with episodic memory or verbal fluency in this study. These results should be considered within a literature where: null results also have been observed, there is marked heterogeneity in the domains of cognition implicated, and effect sizes are frequently attenuated following adjustment for covariates, such as BMI (54, 55, 107–109). Indeed, the magnitude of the association of CRP and future executive functioning was small, although this is generally characteristic of effect sizes in the psychological science (110).
The core hypotheses of this study, namely that individuals with depression and higher levels of CRP would perform worse across multiple measures of cognitive functioning, was not supported. Only one other study has tested a similar hypothesis in a community sample; Cullen et al. (106) examined a risk-enriched sample of 107 children (56% of children had a history of developmental delays; social/emotional/behavioral problems; psychotic-like experiences or a family history of psychosis) and found that children with internalizing symptomology and elevated salivary CRP did not perform worse on measures of cognitive functioning, although CRP was associated with worse performance on verbal fluency and executive functioning tasks – a pattern of results that is broadly in line with the results of this study. A consistent pattern also was observed in clinical studies of adults with a current major depression diagnosis. Across these studies, elevated inflammatory biomarkers were associated with worse cognitive functioning, so that depressed individuals with higher levels of inflammatory biomarkers performed worse across tests of cognitive functioning, including executive functioning (71–74). Significantly and consistent with our results, the two studies that included control groups reported that elevated inflammatory biomarkers also were associated with cognitive dysfunction in the control condition (72, 74). It is important to note, however, that, despite the large sample, this sample may not have been adequately powered to detect a cumulative association of depression and C reactive protein on cognitive functioning given the relative low number of depressed individuals with elevated CRP. If we use diagnostic status as our indicator of depression, only 3 of 29 individuals with a current diagnosis and 22 out of 182 individuals with a lifetime diagnosis have C reactive protein values ≥ 3 mg/L.
When examining the association of depression with individual measures of cognitive functioning, depression, particularly current depression, was consistently associated with visual and verbal working memory and more severe current depressive symptoms (but not current diagnosis) were associated with worse verbal fluency (see Table 1). In primary analyses, the concurrent associations of depression (current and past) with three latent cognitive variables (executive functioning, verbal fluency, and episodic memory) were estimated and current depression alone was associated with worse executive functioning. These findings were generally consistent with prior meta-analyses that have observed worse working memory, and worse executive functioning more broadly, in individuals with acute depression, although such difficulties are also typically observed in remitted depression (13, 22, 75, 111). Depressed adults (14, 35, 75, 112) and youth (113) frequently perform worse on tests of verbal fluency, which also can be observed in remitted depression (22). In this study, consistent verbal fluency differences were not observed and, as a result, these results are more congruent with the considerable heterogeneity in effect size as well as null results that are observed (92). It may be that such deficits are less commonly observed in youth, where relatively fewer studies examining verbal fluency have been carried out (22, 75).
Neither past nor current depression was associated with deficits in episodic memory, measured using a verbal list-learning task, in the current study. Worse episodic memory has long been recognized as impaired in depression (114) and is consistent with reduced hippocampal volumes that are consistently observed in depression (115). However, reduced hippocampal volumes is often associated with recurrent, prolonged or repeated bouts of depression (115–117) and, more importantly, deficits in episodic memory are typically not observed in youth with a history of depression (13, 21, 23). It may be that the type of episodic memory tasks matters when detecting deficits in depression because memory difficulties typically are not observed on list-learning tasks during the first-episode of depression and, instead, are more likely to be observed when participants are asked to recall narratives (75). In addition, deficits in episodic memory are typically not observed in early onset depression but are pronounced in older adults (20). Thus, the null results observed in the current study reflect a broader pattern of findings that episodic memory, at least as assessed using a list-learning task, is not impaired in depressed youth.
The association of CRP and future executive function was substantially attenuated following the inclusion of covariates; however, this does not necessarily imply that CRP is unrelated to cognitive functioning. For example, there is strong theory as well as empirical data to suggest that inflammation may mediate the association between body mass and cognitive functioning (53, 118, 119). For instance, in a longitudinal study of adolescents, Mac Giollabhui et al. found that an inflammatory biomarker (interleukin-6), which is closely related to CRP, mediated the association between BMI and worse future executive functioning, although CRP itself did not (32). It does, however, highlight the need for further studies to disentangle the association of multiple overlapping risk or causal factors, such as SES, BMI, inflammation, stress, diet, with both depression and cognitive functioning (32, 34, 76). For instance, low SES is generally associated with greater risk of depression (120) and risk factors for both depression and cognitive dysfunction, including BMI (32, 118), inflammatory biomarkers (101, 121), and diet (122, 123). An important limitation of this study is that it was only capable of examining the association of depression with future cognition when it is known that the relationship between depression and cognition is more complex (34). Further. a recent meta-analysis by Mac Giollabhui et al. (124) has shown that CRP is associated with future depression and that depression is associated with future CRP, highlighting the multiple potential ways that depression, inflammation and cognitive functioning may be associated. For instance, it also is plausible that, rather than depression leading to cognitive dysfunction, inflammation leads to executive dysfunction, which in turn leads to depression via stress generation (76, 125).
There is compelling evidence that SES, greater body mass, and increased sedentary behaviors are linked with cognitive dysfunction and similar results were observed in this study. In particular, there is a striking difference in youth performance on tests of executive functioning, memory, and language based on SES (126, 127). Strong evidence also exists linking higher body mass (118, 128, 129) and increased sedentary behavior (130, 131) with cognitive dysfunction. Since all of these factors are inter-related (51, 52, 55, 132, 133) and, moreover, also are associated with both depression and inflammatory biomarkers (32, 121, 134), an ongoing challenge for researchers is better characterizing the causal relationships between these variables and better understanding whether, for example, SES is associated with depression and cognitive dysfunction via the impact of SES on inflammation via increased BMI or, alternatively whether other variables, such as diet or genotype, play an important role (122, 123, 135–141). Rather than dissociable risk factors, it may also be that some of these factors represent expressions of shared biological pathways (40).
This study contained a number of notable strengths. Inflammation was assessed in serum using a widely-used inflammatory biomarker (CRP) and three domains of cognitive functioning were estimated from a large battery of reliable, valid, and widely-used behavioral assessments. Moreover, the use of a structural equation modeling approach reduced the risk of type I error caused by the increased number of statistical tests that is a frequent limitation of many studies examining cognitive dysfunction in depression (21). An SEM approach also increased confidence that observed associations were related to the constructs being measured, rather than task-specific variance. Finally, the use of a large, population-based, representative sample of adolescents likely increases the generalizability of these results.
These results also should be considered in the context of important limitations. Cognitive functioning was only assessed at a single occasion, and therefore, we cannot exclude the possibility that, for example, worse cognitive functioning (e.g., verbal fluency) is a risk factor for depression, and consequently, the true temporal relationship is the reverse of that observed in this study (142). Similarly, CRP was only assessed at a single timepoint and the possibility of reverse temporal relations cannot be excluded. Tests of cognitive functioning typically use standardized scores that are age and/or gender-normed; however, norms were not available for all tests of cognitive functioning used in this study. Thus, we cannot exclude the possibility that subtle effects of age or gender bias results, although this possibility is minimized by our same age cohort, a roughly equal number of male and female participants, and by controlling for these variables in all analyses. Finally, biased attrition may limit the generalizability of results because the analytic sample was less likely to retain individuals of low SES and males.
4.1. Conclusions
Depression and CRP are already negatively associated with executive functioning early in development when cognitive abilities are critical for academic and psychosocial functioning. Importantly, these results do provide evidence that in individuals who experience depression are more likely to exhibit executive functioning difficulties, although these associations are unlikely to be unique to depression. Future research is needed to disentangle the effect of multiple, overlapping risk factors for cognitive dysfunction in youth by examining whether inflammatory biomarkers are risk markers or mediators for other deleterious processes, such as adiposity, substance use, sedentary behavior, and/or low socioeconomic status.
Supplementary Material
Highlights.
Higher levels of CRP were associated with worse future executive functioning.
The association of higher CRP with worse future executive functioning was observed in individuals with and without current and prior depression.
A current depression diagnosis was associated with worse executive functioning.
Low socioeconomic status, high body mass index, and increased sedentary behaviors were associated with worse cognitive functioning and, importantly, the associations of depression and CRP with executive functioning were no longer significant when controlling for these covariates.
Acknowledgements
This research was supported by National Institute of Mental Health Grants MH079369 and MH101168 to Lauren Alloy and National Institute of Mental Health National Research Service Award F31MH118808 as well as an American Psychological Foundation grant to Naoise Mac Giollabhui.
Footnotes
Disclosures
The authors have no disclosures or conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hasin DS, Sarvet AL, Meyers JL, Saha TD, Ruan WJ, Stohl M, et al. (2018): Epidemiology of Adult DSM-5 Major Depressive Disorder and Its Specifiers in the United States. JAMA Psychiatry. 75:336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasin DS, Goodwin RD, Stinson FS, Grant BF (2005): Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch Gen Psychiatry. 62:1097–1106. [DOI] [PubMed] [Google Scholar]
- 3.Burcusa SL, Iacono WG (2007): Risk for recurrence in depression. Clin Psychol Rev. 27:959–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. (2003): The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 289:3095–3105. [DOI] [PubMed] [Google Scholar]
- 5.Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D (2006): Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 63:530–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van der Kooy K, van Hout H, Marwijk H, Marten H, Stehouwer C, Beekman A (2007): Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry. 22:613–626. [DOI] [PubMed] [Google Scholar]
- 7.Oquendo MA, Friend JM, Halberstam B, Brodsky BS, Burke AK, Grunebaum MF, et al. (2003): Association of Comorbid Posttraumatic Stress Disorder and Major Depression With Greater Risk for Suicidal Behavior. Am J Psychiatry. 160:580–582. [DOI] [PubMed] [Google Scholar]
- 8.Abramson LY, Metalsky GI, Alloy LB (1989): Hopelessness depression: A theory-based subtype of depression. Psychol Rev. 96:358–372. [Google Scholar]
- 9.Beck AT (1976): Cognitive therapy of depression. New York, NY: International Universities Press. [Google Scholar]
- 10.Gotlib IH, Joormann J (2010): Cognition and depression: current status and future directions. Annu Rev Clin Psychol. 6:285–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mac Giollabhui N, Hamilton JL, Nielsen J, Connolly SL, Stange JP, Varga S, et al. (2018): Negative cognitive style interacts with negative life events to predict first onset of a major depressive episode in adolescence via hopelessness. J Abnorm Psychol. 127:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alloy LB, Abramson LY, Whitehouse WG, Hogan ME, Panzarella C, Rose DT (2006): Prospective incidence of first onsets and recurrences of depression in individuals at high and low cognitive risk for depression. J Abnorm Psychol. 115:145. [DOI] [PubMed] [Google Scholar]
- 13.Rock PL, Roiser JP, Riedel WJ, Blackwell AD (2014): Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med. 44:2029–2040. [DOI] [PubMed] [Google Scholar]
- 14.Snyder HR (2013): Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol Bull. 139:81–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagner, Doering B, Helmreich I, Lieb K, Tadić A (2012): A meta-analysis of executive dysfunctions in unipolar major depressive disorder without psychotic symptoms and their changes during antidepressant treatment. Acta Psychiatr Scand. 125:281–292. [DOI] [PubMed] [Google Scholar]
- 16.McDermott LM, Ebmeier KP (2009): A meta-analysis of depression severity and cognitive function. J Affect Disord. 119:1–8. [DOI] [PubMed] [Google Scholar]
- 17.Lee R, Hermens DF, Porter Ma, Redoblado-Hodge MA (2012): A meta-analysis of cognitive deficits in first-episode Major Depressive Disorder. J Affect Disord. 140:113–124. [DOI] [PubMed] [Google Scholar]
- 18.Porter RJ, Gallagher P, Thompson JM, Young AH (2003): Neurocognitive impairment in drug-free patients with major depressive disorder. Br J Psychiatry. 182:214–220. [DOI] [PubMed] [Google Scholar]
- 19.Porter RJ, Bourke C, Gallagher P (2007): Neuropsychological impairment in major depression: its nature, origin and clinical significance. Aust N Z J Psychiatry. 41:115–128. [DOI] [PubMed] [Google Scholar]
- 20.Bora E, Harrison BJ, Yücel M, Pantelis C (2013): Cognitive impairment in euthymic major depressive disorder: a meta-analysis. Psychol Med. 43:2017–2026. [DOI] [PubMed] [Google Scholar]
- 21.Hasselbalch BJ, Knorr U, Kessing LV (2011): Cognitive impairment in the remitted state of unipolar depressive disorder: a systematic review. J Affect Disord. 134:20–31. [DOI] [PubMed] [Google Scholar]
- 22.Semkovska M, Quinlivan L, O’Grady T, Johnson R, Collins A, O’Connor J, et al. (2019): Cognitive function following a major depressive episode: a systematic review and meta-analysis. The Lancet Psychiatry. 6:851–861. [DOI] [PubMed] [Google Scholar]
- 23.Bora E, Yucel M, Pantelis C (2009): Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. J Affect Disord. 113:1–20. [DOI] [PubMed] [Google Scholar]
- 24.Jaeger J, Berns S, Uzelac S, Davis-Conway S (2006): Neurocognitive deficits and disability in major depressive disorder. Psychiatry Res. 145:39–48. [DOI] [PubMed] [Google Scholar]
- 25.Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, et al. (2013): Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 382:1575–1586. [DOI] [PubMed] [Google Scholar]
- 26.Woo YS, Rosenblat JD, Kakar R, Bahk WM, McIntyre RS (2016): Cognitive Deficits as a Mediator of Poor Occupational Function in Remitted Major Depressive Disorder Patients. Clin Psychopharmacol Neurosci. 14:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allott K, Fisher CA, Amminger GP, Goodall J, Hetrick S (2016): Characterizing neurocognitive impairment in young people with major depression: state, trait, or scar? Brain Behav. 6:e00527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franz CE, Lyons MJ, O’Brien R, Panizzon MS, Kim K, Bhat R, et al. (2011): A 35-year longitudinal assessment of cognition and midlife depression symptoms: the Vietnam era twin study of aging. Am J Geriatr Psychiatry. 19:559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koenen KC, Moffitt TE, Roberts AL, Martin LT, Kubzansky L, Harrington H, et al. (2009): Childhood IQ and adult mental disorders: A test of the cognitive reserve hypothesis. Am J Psychiatry. 166:50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zammit S, Allebeck P, David AS, Dalman C, Hemmingsson T, Lundberg I, et al. (2004): A longitudinal study of premorbid IQ score and risk of developing schizophrenia, bipolar disorder, severe depression, and other nonaffective psychoses. Arch Gen Psychiatry. 61:354–360. [DOI] [PubMed] [Google Scholar]
- 31.Gale CR, Batty GD, Tynelius P, Deary IJ, Rasmussen F (2010): Intelligence in early adulthood and subsequent hospitalization for mental disorders. Epidemiology. 21:70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mac Giollabhui N, Swistun D, Murray S, Moriarity DP, Kautz MM, Ellman LM, et al. (2020): Executive dysfunction in depression in adolescence: the role of inflammation and higher body mass. Psychol Med. 50:683–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mac Giollabhui N, Nielsen J, Seidman S, Olino TM, Abramson LY, Alloy LB (2018): The development of future orientation is associated with faster decline in hopelessness during adolescence. J Youth Adolesc.1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mac Giollabhui N, Olino TM, Nielsen J, Abramson LY, Alloy LB (2019): Is Worse Attention a Risk Factor for or a Consequence of Depression, or Are Worse Attention and Depression Better Accounted for by Stress? A Prospective Test of Three Hypotheses. Clin Psychol Sci. 7:93–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veiel HOF (1997): A preliminary profile of neuropsychological deficits associated with major depression. J Clin Exp Neuropsychol. 19:587–603. [DOI] [PubMed] [Google Scholar]
- 36.Sommerfeldt SL, Cullen KR, Han G, Fryza BJ, Houri AK, Klimes-Dougan B (2016): Executive Attention Impairment in Adolescents With Major Depressive Disorder. J Clin Child Adolesc Psychol. 45:69–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keilp JG, Gorlyn M, Oquendo MA, Burke AK, Mann JJ (2008): Attention deficit in depressed suicide attempters. Psychiatry Res. 159:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Engels AS, Heller W, Spielberg JM, Warren SL, Sutton BP, Banich MT, et al. (2010): Co-occurring anxiety influences patterns of brain activity in depression. Cogn Affect Behav Neurosci. 10:141–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snyder HR, Miyake A, Hankin BL (2015): Advancing understanding of executive function impairments and psychopathology: Bridging the gap between clinical and cognitive approaches. Front Psychol. 6:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carvalho AF, Miskowiak KK, Hyphantis TN, Kohler CA, Alves GS, Bortolato B, et al. (2014): Cognitive dysfunction in depression - pathophysiology and novel targets. CNS Neurol Disord Drug Targets. 13:1819–1835. [DOI] [PubMed] [Google Scholar]
- 41.Hoogland I (2015): Systemic inflammation and microglial activation: systematic review of animal experiments. J Neuroinflammation. 12:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lampa J, Westman M (2012): Peripheral inflammatory disease associated with centrally activated IL-1 system in humans and mice. Proc Natl Acad Sci. 109:12728–12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harrison NA, Doeller CF, Voon V, Burgess N, Critchley HD (2014): Peripheral inflammation acutely impairs human spatial memory via actions on medial temporal lobe glucose metabolism. Biol Psychiatry. 76:585–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Christine MS, Jean-Dominique G, Brian P, Nabeel N, Keunpoong L, Shu-Fei L, et al. (2015): Imaging robust microglial activation after lipopolysaccharide administration in humans with PET. Proc Natl Acad Sci. 112:12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crisan AF, Oancea C, Timar B, Fira-Mladinescu O, Crisan A, Tudorache V (2014): Cognitive impairment in chronic obstructive pulmonary disease. PLoS One. 9:e102468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang YS, Guilleminault C, Hwang FM, Cheng C, Lin CH, Li HY, et al. (2016): Inflammatory cytokines in pediatric obstructive sleep apnea. Medicine. 95:e4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li X, Robertson CM, Yu X, Cheypesh A, Dinu IA, Li J (2014): Early postoperative systemic inflammatory response is an important determinant for adverse 2-year neurodevelopment-associated outcomes after the Norwood procedure. J Thorac Cardiovasc Surg. 148:202–206. [DOI] [PubMed] [Google Scholar]
- 48.Jenny NS, French B, Arnold AM, Strotmeyer ES, Cushman M, Chaves PH, et al. (2012): Long-term assessment of inflammation and healthy aging in late life: the Cardiovascular Health Study All Stars. The Journals of Gerontology: Series A. 67:970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Noble JM, Manly JJ, Schupf N, Tang MX, Mayeux R, Luchsinger JA (2010): Association of C-reactive protein with cognitive impairment. Arch Neurol. 67:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baune B, Ponath G, Golledge J, Varga G (2008): Association between IL-8 cytokine and cognitive performance in an elderly general population—the MEMO-Study. Neurobiol Aging. 29:937–944. [DOI] [PubMed] [Google Scholar]
- 51.Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, et al. (2001): Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 58:445–452. [DOI] [PubMed] [Google Scholar]
- 52.Paine NJ, Bosch Ja, Ring C, Drayson MT, Veldhuijzen van Zanten JJCS (2015): Induced mild systemic inflammation is associated with impaired ability to improve cognitive task performance by practice. Psychophysiology. 52:333–341. [DOI] [PubMed] [Google Scholar]
- 53.Yang Y, Shields GS, Wu Q, Liu Y, Chen H, Guo C (2019): The association between obesity and lower working memory is mediated by inflammation: Findings from a nationally representative dataset of US adults. Brain Behav Immun. [DOI] [PubMed] [Google Scholar]
- 54.Dik M, Jonker C, Hack C, Smit J, Comijs H, Eikelenboom P (2005): Serum inflammatory proteins and cognitive decline in older persons. Neurology. 64:1371–1377. [DOI] [PubMed] [Google Scholar]
- 55.Singh-Manoux A, Dugravot A, Brunner E, Kumari M, Shipley M, Elbaz A, et al. (2014): Interleukin-6 and C-reactive protein as predictors of cognitive decline in late midlife. Neurology. 83:486–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karlamangla AS, Miller-Martinez D, Lachman ME, Tun PA, Koretz BK, Seeman TE (2014): Biological correlates of adult cognition: midlife in the United States (MIDUS). Neurobiol Aging. 35:387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maes M, Meltzer HY, Bosmans E, Bergmans R, Vandoolaeghe E, Ranjan R, et al. (1995): Increased plasma concentrations of interleukin-6, soluble interleukin-6, soluble interleukin-2 and transferrin receptor in major depression. J Affect Disord. 34:301–309. [DOI] [PubMed] [Google Scholar]
- 58.Smith RS (1991): The macrophage theory of depression. Med Hypotheses. 35:298–306. [DOI] [PubMed] [Google Scholar]
- 59.Dantzer R (2001): Cytokine-induced sickness behavior: where do we stand? Brain Behavior and Immunity. 15:7–24. [DOI] [PubMed] [Google Scholar]
- 60.Au B, Smith KJ, Gariepy G, Schmitz N (2015): The longitudinal associations between C-reactive protein and depressive symptoms: evidence from the English Longitudinal Study of Ageing (ELSA). Int J Geriatr Psychiatry. 30:976–984. [DOI] [PubMed] [Google Scholar]
- 61.Gimeno D, Kivimaki M, Brunner EJ, Elovainio M, De Vogli R, Steptoe A, et al. (2009): Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psycholological Medicine. 39:413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB (2014): Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry. 71:1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. (2010): A meta-analysis of cytokines in major depression. Biol Psychiatry. 67:446–457. [DOI] [PubMed] [Google Scholar]
- 64.Howren MB, Lamkin DM, Suls J (2009): Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 71:171–186. [DOI] [PubMed] [Google Scholar]
- 65.Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimäki M (2015): Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behavior and Immunity. 49:206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kohler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, et al. (2017): Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand. 135:373–387. [DOI] [PubMed] [Google Scholar]
- 67.Osimo EF, Baxter LJ, Lewis G, Jones PB, Khandaker GM (2019): Prevalence of low-grade inflammation in depression: a systematic review and meta-analysis of CRP levels. Psychol Med. 49:1958–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Misiak B, Beszlej JA, Kotowicz K, Szewczuk-Boguslawska M, Samochowiec J, Kucharska-Mazur J, et al. (2018): Cytokine alterations and cognitive impairment in major depressive disorder: From putative mechanisms to novel treatment targets. Prog Neuropsychopharmacol Biol Psychiatry. 80:177–188. [DOI] [PubMed] [Google Scholar]
- 69.Drevets WC, Price JL, Furey ML (2008): Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Structure and Function. 213:93–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McAfoose J, Baune BT (2009): Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev. 33:355–366. [DOI] [PubMed] [Google Scholar]
- 71.Chang HH, Lee IH, Gean PW, Lee SY, Chi MH, Yang YK, et al. (2012): Treatment response and cognitive impairment in major depression: association with C-reactive protein. Brain Behavior and Immunity. 26:90–95. [DOI] [PubMed] [Google Scholar]
- 72.Krogh J, Benros ME, Jørgensen MB, Vesterager L, Elfving B, Nordentoft M (2014): The association between depressive symptoms, cognitive function, and inflammation in major depression. Brain Behavior and Immunity. 35:70–76. [DOI] [PubMed] [Google Scholar]
- 73.Goldsmith DR, Haroon E, Woolwine BJ, Jung MY, Wommack EC, Harvey PD, et al. (2016): Inflammatory markers are associated with decreased psychomotor speed in patients with major depressive disorder. Brain Behavior and Immunity. 56:281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grassi-Oliveira R, Bauer ME, Pezzi JC, Teixeira AL, Brietzke E (2011): Interleukin-6 and verbal memory in recurrent major depressive disorder. Neuro Endocrinol Lett. 32:540–544. [PubMed] [Google Scholar]
- 75.Ahern E, Semkovska M (2017): Cognitive functioning in the first-episode of major depressive disorder: A systematic review and meta-analysis. Neuropsychology. 31:52. [DOI] [PubMed] [Google Scholar]
- 76.Shields GS, Moons WG, Slavich GM (2017): Inflammation, self-regulation, and health: an immunologic model of self-regulatory failure. Perspect Psychol Sci. 12:588–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huisman M, Oldehinkel AJ, de Winter A, Minderaa RB, de Bildt A, Huizink AC, et al. (2008): Cohort profile: The dutch ‘TRacking adolescents’ individual lives’ survey’; TRAILS. Int J Epidemiol. 37:1227–1235. [DOI] [PubMed] [Google Scholar]
- 78.Kessler RC, Üstün TB (2004): The world mental health (WMH) survey initiative version of the world health organization (WHO) composite international diagnostic interview (CIDI). Int J Methods Psychiatr Res. 13:93–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haro JM, Arbabzadeh-Bouchez S, Brugha TS, De Girolamo G, Guyer ME, Jin R, et al. (2006): Concordance of the Composite International Diagnostic Interview Version 3.0 (CIDI 3.0) with standardized clinical assessments in the WHO World Mental Health surveys. Int J Methods Psychiatr Res. 15:167–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kessler RC, Avenevoli S, Green J, Gruber MJ, Guyer M, He Y, et al. (2009): National comorbidity survey replication adolescent supplement (NCS-A): III. Concordance of DSM-IV/CIDI diagnoses with clinical reassessments. J Am Acad Child Adolesc Psychiatry. 48:386–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wittchen H-U (1994): Reliability and validity studies of the WHO-Composite International Diagnostic Interview (CIDI): a critical review. J Psychiatr Res. 28:57–84. [DOI] [PubMed] [Google Scholar]
- 82.Achenbach TM (1991): Manual for the youth self-report and 1991 profile. Department of Psychiatry, University of Vermont; Burlington. [Google Scholar]
- 83.Achenbach T, Rescorla L (2003): Manual for the ASEBA adult forms & profiles. Burlington, Vermont: University of Vermont Research Center for Children, Youth, & Families. [Google Scholar]
- 84.Wechsler D (1997): Wechsler Adult Intelligence Scale, third edition, Dutch version. Swets & Zeitlinger. [Google Scholar]
- 85.Petrides M, Milner B (1982): Deficits on subject-ordered tasks after frontal- and temporal-lobe lesions in man. Neuropsychologia. 20:249–262. [DOI] [PubMed] [Google Scholar]
- 86.Ross TP, Hanouskova E, Giarla K, Calhoun E, Tucker M (2007): The reliability and validity of the self-ordered pointing task. Arch Clin Neuropsychol. 22:449–458. [DOI] [PubMed] [Google Scholar]
- 87.Jonker I, Klein HC, Duivis HE, Yolken RH, Rosmalen JG, Schoevers RA (2014): Association between exposure to HSV1 and cognitive functioning in a general population of adolescents. The TRAILS study. PLoS One. 9:e101549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shin M-S, Park S-Y, Park S-R, Seol S-H, Kwon JS (2006): Clinical and empirical applications of the Rey–Osterrieth complex figure test. Nat Protoc. 1:892. [DOI] [PubMed] [Google Scholar]
- 89.Van Der Elst W, Van Boxtel MP, Van Breukelen GJ, Jolles J (2005): Rey’s verbal learning test: normative data for 1855 healthy participants aged 24–81 years and the influence of age, sex, education, and mode of presentation. J Int Neuropsychol Soc. 11:290–302. [DOI] [PubMed] [Google Scholar]
- 90.Miyake A, Friedman NP (2012): The nature and organization of individual differences in executive functions: Four general conclusions. Curr Dir Psychol Sci. 21:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Whiteside DM, Kealey T, Semla M, Luu H, Rice L, Basso MR, et al. (2016): Verbal Fluency: Language or Executive Function Measure? Applied Neuropsychology: Adult. 23:29–34. [DOI] [PubMed] [Google Scholar]
- 92.Henry JD, Crawford JR (2005): A Meta-Analytic Review of Verbal Fluency Deficits in Depression. J Clin Exp Neuropsychol. 27:78–101. [DOI] [PubMed] [Google Scholar]
- 93.Ganzeboom HB, Treiman DJ (1996): Internationally comparable measures of occupational status for the 1988 International Standard Classification of Occupations. Soc Sci Res. 25:201–239. [Google Scholar]
- 94.Jonker I, Rosmalen J, Schoevers R (2017): Childhood life events, immune activation and the development of mood and anxiety disorders: the TRAILS study. Transl Psychiatry. 7:e1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jung T, Wickrama KA (2008): An introduction to latent class growth analysis and growth mixture modeling. Soc Personal Psychol Compass. 2:302–317. [Google Scholar]
- 96.Nylund KL, Asparouhov T, Muthén BO (2007): Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural equation modeling: A multidisciplinary Journal. 14:535–569. [Google Scholar]
- 97.Golden RM (2000): Statistical tests for comparing possibly misspecified and nonnested models. J Math Psychol. 44:153–170. [DOI] [PubMed] [Google Scholar]
- 98.Chen FF (2007): Sensitivity of Goodness of Fit Indexes to Lack of Measurement Invariance. Structural Equation Modeling: A Multidisciplinary Journal. 14:464–504. [Google Scholar]
- 99.Cheung GW, Rensvold RB (2002): Evaluating Goodness-of-Fit Indexes for Testing Measurement Invariance. Structural Equation Modeling: A Multidisciplinary Journal. 9:233–255. [Google Scholar]
- 100.Schermelleh-Engel K, Moosbrugger H, Müller H (2003): Evaluating the fit of structural equation models: Tests of significance and descriptive goodness-of-fit measures. Methods of Psychological Research Online. 8:23–74. [Google Scholar]
- 101.O’Connor MF, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, et al. (2009): To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun. 23:887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nolen-Hoeksema S (2001): Gender differences in depression. Curr Dir Psychol Sci. 10:173–176. [Google Scholar]
- 103.Mac Giollabhui N, Ellman LM, Coe CL, Byrne ML, Abramson LY, Alloy LB (2020): To exclude or not to exclude: Considerations and recommendations for C-reactive protein values higher than 10 mg/L. Brain Behav Immun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Teunissen C, Van Boxtel M, Bosma H, Bosmans E, Delanghe J, De Bruijn C, et al. (2003): Inflammation markers in relation to cognition in a healthy aging population. J Neuroimmunol. 134:142–150. [DOI] [PubMed] [Google Scholar]
- 105.Zheng F, Xie W (2018): High-sensitivity C-reactive protein and cognitive decline: the English Longitudinal Study of Ageing. Psychol Med. 48:1381–1389. [DOI] [PubMed] [Google Scholar]
- 106.Cullen AE, Tappin BM, Zunszain PA, Dickson H, Roberts RE, Nikkheslat N, et al. (2017): The relationship between salivary C-reactive protein and cognitive function in children aged 11–14years: Does psychopathology have a moderating effect? Brain Behav Immun. 66:221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Palta P, Xue Q-L, Deal JA, Fried LP, Walston JD, Carlson MC (2015): Interleukin-6 and C-reactive protein levels and 9-year cognitive decline in community-dwelling older women: the Women’s Health and Aging Study II. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 70:873–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Alley DE, Crimmins EM, Karlamangla A, Hu P, Seeman TE (2008): Inflammation and rate of cognitive change in high-functioning older adults. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 63:50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cohen-Manheim I, Doniger GM, Sinnreich R, Simon ES, Pinchas-Mizrachi R, Otvos JD, et al. (2015): Increase in the Inflammatory Marker GlycA over 13 Years in Young Adults Is Associated with Poorer Cognitive Function in Midlife. PLoS One. 10:e0138036–e0138036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, et al. (2013): Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 14:365–376. [DOI] [PubMed] [Google Scholar]
- 111.Snyder HR (2013): Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol Bull. 139:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zakzanis K, Leach L, Kaplan E (1998): On the nature and pattern of neurocognitive function in major depressive disorder. Cogn Behav Neurol. 11:111–119. [PubMed] [Google Scholar]
- 113.Baune BT, Fuhr M, Air T, Hering C (2014): Neuropsychological functioning in adolescents and young adults with major depressive disorder – A review. Psychiatry Res. 218:261–271. [DOI] [PubMed] [Google Scholar]
- 114.Burt DB, Zembar MJ, Niederehe G (1995): Depression and memory impairment: a meta-analysis of the association, its pattern, and specificity. Psychol Bull. 117:285–305. [DOI] [PubMed] [Google Scholar]
- 115.Videbech P, Ravnkilde B (2004): Hippocampal volume and depression: a meta-analysis of MRI studies. The American journal of psychiatry. 161:1957–1966. [DOI] [PubMed] [Google Scholar]
- 116.Sheline YI, Sanghavi M, Mintun MA, Gado MH (1999): Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 19:5034–5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW (1996): Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci. 93:3908–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang Y, Shields GS, Guo C, Liu Y (2018): Executive function performance in obesity and overweight individuals: A meta-analysis and review. Neurosci Biobehav Rev. 84:225–244. [DOI] [PubMed] [Google Scholar]
- 119.Spyridaki EC, Avgoustinaki PD, Margioris AN (2016): Obesity, inflammation and cognition. Current Opinion in Behavioral Sciences. 9:169–175. [Google Scholar]
- 120.Reiss F (2013): Socioeconomic inequalities and mental health problems in children and adolescents: A systematic review. Soc Sci Med. 90:24–31. [DOI] [PubMed] [Google Scholar]
- 121.Muscatell KA, Brosso SN, Humphreys KL (2018): Socioeconomic status and inflammation: a meta-analysis. Mol Psychiatry.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Darmon N, Drewnowski A (2008): Does social class predict diet quality? Am J Clin Nutr. 87:1107–1117. [DOI] [PubMed] [Google Scholar]
- 123.Nyaradi A, Foster JK, Hickling S, Li J, Ambrosini GL, Jacques A, et al. (2014): Prospective associations between dietary patterns and cognitive performance during adolescence. J Child Psychol Psychiatry. 55:1017–1024. [DOI] [PubMed] [Google Scholar]
- 124.Mac Giollabhui N, Ng TH, Ellman LM, Alloy LB (In Press): The longitudinal associations of inflammatory biomarkers and depression revisited: systematic review, meta-analysis, and meta-regression. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Snyder HR, Hankin BL (2016): Spiraling out of control: Stress generation and subsequent rumination mediate the link between poorer cognitive control and internalizing psychopathology. Clin Psychol Sci. 4:1047–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hackman DA, Gallop R, Evans GW, Farah MJ (2015): Socioeconomic status and executive function: developmental trajectories and mediation. Developmental science. 18:686–702. [DOI] [PubMed] [Google Scholar]
- 127.Duncan GJ, Magnuson K (2012): Socioeconomic status and cognitive functioning: moving from correlation to causation. Wiley Interdiscip Rev Cogn Sci. 3:377–386. [DOI] [PubMed] [Google Scholar]
- 128.Tabriz AA, Sohrabi MR, Parsay S, Abadi A, Kiapour N, Aliyari M, et al. (2015): Relation of intelligence quotient and body mass index in preschool children: a community-based cross-sectional study. Nutr Diabetes. 5:e176–e176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cheke LG, Simons JS, Clayton NS (2016): Higher body mass index is associated with episodic memory deficits in young adults. Q J Exp Psychol (Hove). 69:2305–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fedewa AL, Ahn S (2011): The effects of physical activity and physical fitness on children’s achievement and cognitive outcomes: a meta-analysis. Res Q Exerc Sport. 82:521–535. [DOI] [PubMed] [Google Scholar]
- 131.Carson V, Kuzik N, Hunter S, Wiebe SA, Spence JC, Friedman A, et al. (2015): Systematic review of sedentary behavior and cognitive development in early childhood. Prev Med. 78:115–122. [DOI] [PubMed] [Google Scholar]
- 132.Hanson MD, Chen E (2007): Socioeconomic status, race, and body mass index: the mediating role of physical activity and sedentary behaviors during adolescence. J Pediatr Psychol. 32:250–259. [DOI] [PubMed] [Google Scholar]
- 133.O’Dea JA, Wilson R (2006): Socio-cognitive and nutritional factors associated with body mass index in children and adolescents: possibilities for childhood obesity prevention. Health Educ Res. 21:796–805. [DOI] [PubMed] [Google Scholar]
- 134.Matthews KA, Chang Y, Bromberger JT, Karvonen-Gutierrez CA, Kravitz HM, Thurston RC, et al. (2016): Childhood Socioeconomic Circumstances, Inflammation, and Hemostasis among Midlife Women: Study of Women’s Health across the Nation (SWAN). Psychosom Med. 78:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Francis HM, Stevenson RJ (2011): Higher reported saturated fat and refined sugar intake is associated with reduced hippocampal-dependent memory and sensitivity to interoceptive signals. Behav Neurosci. 125:943–955. [DOI] [PubMed] [Google Scholar]
- 136.Baym CL, Khan NA, Monti JM, Raine LB, Drollette ES, Moore RD, et al. (2014): Dietary lipids are differentially associated with hippocampal-dependent relational memory in prepubescent children. Am J Clin Nutr. 99:1026–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Molteni R, Barnard RJ, Ying Z, Roberts CK, Gomez-Pinilla F (2002): A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. J Neurosci. 112:803–814. [DOI] [PubMed] [Google Scholar]
- 138.Junger M, van Kampen M (2010): Cognitive ability and self-control in relation to dietary habits, physical activity and bodyweight in adolescents. Int J Behav Nutr Phys Act. 7:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Beydoun MA, Gamaldo AA, Beydoun HA, Tanaka T, Tucker KL, Talegawkar SA, et al. (2014): Caffeine and alcohol intakes and overall nutrient adequacy are associated with longitudinal cognitive performance among U.S. adults. J Nutr. 144:890–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Retterstol L, Eikvar L, Berg K (2003): A twin study of C-Reactive Protein compared to other risk factors for coronary heart disease. Atherosclerosis. 169:279–282. [DOI] [PubMed] [Google Scholar]
- 141.Su S, Miller AH, Snieder H, Bremner JD, Ritchie J, Maisano C, et al. (2009): Common genetic contributions to depressive symptoms and inflammatory markers in middle-aged men: the Twins Heart Study. Psychosom Med. 71:152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Scult MA, Paulli AR, Mazure ES, Moffitt TE, Hariri AR, Strauman TJ (2017): The association between cognitive function and subsequent depression: a systematic review and meta-analysis. Psychol Med. 47:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.