Abstract
Cardiac ATP production primarily depends on oxidative phosphorylation in mitochondria and is dynamically regulated by Ca2+ levels in the mitochondrial matrix as well as cytosolic ADP. Here we discuss mitochondrial Ca2+ signaling and its dysfunction which has recently been linked to cardiac pathology including arrhythmia, and heart failure. Similar dysfunction in other excitable and long-lived cells like neurons is associated with neurodegenerative diseases such as Alzheimer’s disease, ALS and Parkinson’s disease. Central to this new understanding is the critical regulation by Ca2+ of both mitochondrial quality control and ATP production. Mitochondrial associated membrane (MAM) signaling from sarcoplasmic and endoplasmic reticulum to mitochondria is discussed. We propose future research directions that emphasize a need to define quantitatively the physiological roles of MAMs, and both mitochondrial quality control and ATP production.
Keywords: Mitochondrial Ca2+ signaling, Mitochondrial quality control, mitophagy, mitochondrial associated membranes, heart failure, neurodegeneration
Mitochondrial Ca2+ signaling and quality control
Mitochondria are central to the generation of ATP from diverse carbon-based sources of energy ranging from carbohydrates to amino acids and lipids. Ca2+ plays critical signaling roles in regulating ATP production by mitochondria in heart and has been put forward as a primary signal of energy consumption in other tissues as well [1, 2]. Ca2+ can do this because intracellular Ca2+ ([Ca2+]i) elevation ties together cardiac electrical activity with cardiac contraction and is responsible for promoting Ca2+ flux into the mitochondria. The cardiac [Ca2+]i transient is triggered by the cardiac action potential (AP) and underlies the activation of contraction. This same [Ca2+]i transient also controls the levels of mitochondrial matrix Ca2+ ([Ca2+]m) to stimulate mitochondrial ATP production. Here we review the dependence of ATP production on Ca2+ which has been recently updated with respect to the mitochondrial processes that are Ca2+ -dependent and those that are not under physiological conditions [2]. In addition, cytosolic and matrix [Ca2+] also play other critical roles in mitochondrial biology, including regulation of mitochondrial quality control (see Glossary), the term used here to describe processes by which proper mitochondrial function is regulated. It is also used to identify the processes by which mitophagy is controlled and specific quality control proteins are produced and removed during normal physiologic functioning and when mitochondrial and endoplasmic reticulum stress occurs. Our current understanding of Ca2+ -dependent mitochondrial quality control will be assessed. The relevance of regulated mitochondrial ATP production and quality control cannot be overstated. These processes may contribute not only to healthy excitation-contraction coupling in heart and normal neuronal and brain function but, when defective, contribute to many diseases including heart failure [3–6] and neurodegenerative diseases such as Alzheimer’s Disease [7], Parkinson’s Disease [8], Amyotrophic Lateral Sclerosis [9], Charcot-Marie-Tooth peripheral neuropathy [10] and many others.
Physiological regulation of mitochondrial ATP production
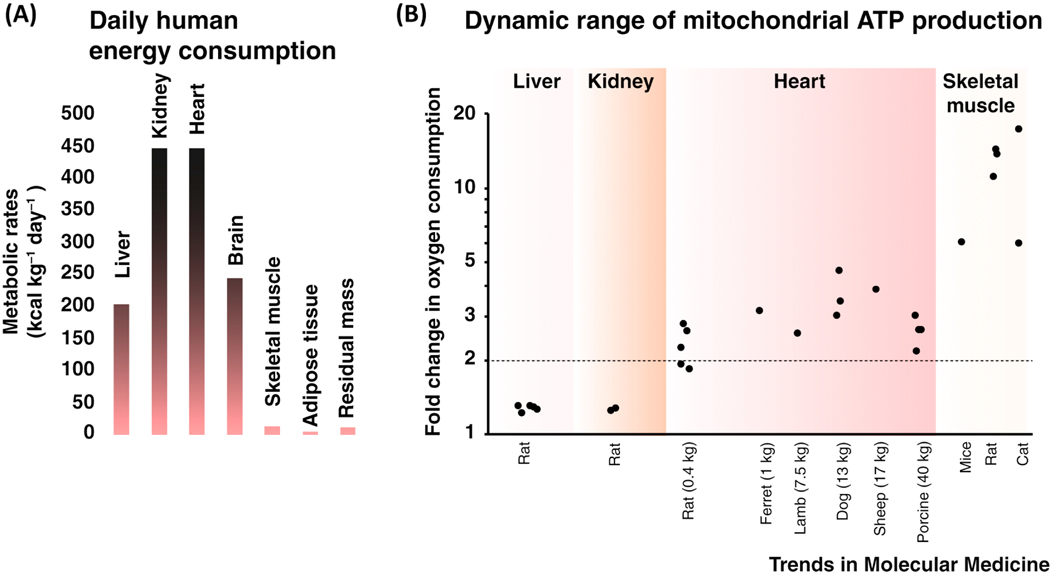
In high energy-consuming tissues such as the liver, kidney, heart, and brain, the mitochondria are the major source of cellular ATP. It is thus not surprising that mitochondria encompass roughly 22 percent of the cellular volume in the kidney and liver [11–13], and over 30 percent of the cellular volume in heart muscle cells [14]. In these tissues, the rate of mitochondrial ATP production is also dynamic, leading to rapid and significant increase in oxygen consumption when the rate of mitochondrial ATP synthesis ramps up (Figure. 1). This increase in oxygen consumption in the liver and the kidneys is rather modest, rising from the basal O2-consumption levels of the tissue to levels that are about 25 percent higher [15–22]. On the other hand, in heart, with increased adrenergic tone and increased circulatory demand, the cardiac workload becomes much higher and oxygen consumption can rise by 2.5-fold or upward of 4.5-fold [23–32]. Even larger increases in oxygen consumption occurs in the skeletal muscle ranging from 6-fold to 17-fold [33–36]. Increased mitochondrial ATP production depends in part on the vascular supply of oxygen and carbon-based sources of energy. Thus, the capacity of the circulatory system to provide the blood flow determines how well the tissue can function. The regulatory mechanisms intrinsic to the mitochondria also contribute to the management of ATP production. Such components within the energy-producing pathways have long been suggested to be Ca2+-sensitive with the evidence in support of this view dating back to the 1980’s. In these early studies, the partially-purified pyruvate dehydrogenase activity and the activity of other matrix-localized dehydrogenases of the Krebs cycle were found to be Ca2+ sensitive [37, 38]. Together with subsequent studies [39–41] these results suggest that [Ca2+]m may act as a signal that tunes mitochondrial metabolism. Recent important advances [2] complement active work on the molecular and quantitative aspects of [Ca2+]m signaling and its importance in ATP production among other matrix signaling pathways [42–45].. Here, the recent molecular and quantitative advances are discussed within the physiological context of the heart muscle cell.
Figure 1. Energy consumption and its dynamic range in different tissues.
A. Daily metabolic rates of major organs and tissues. Shown values are from human subjects. Data is from Wang et al., 2010 [215]. B. Fold changes in oxygen consumption rates of different tissues in different species. Each data point shows the fold change in oxygen consumption determined by distinct studies performed in liver [15–20], kidney [21, 22], heart [23–32], and skeletal muscle [33–36]. All shown measurements were carried out with healthy tissues of wild-type animals under similar, physiologically relevant basal and strenuous activities. The indicated weight (in Kg) is the total body weight.
Mitochondrial Ca2+ signaling in heart
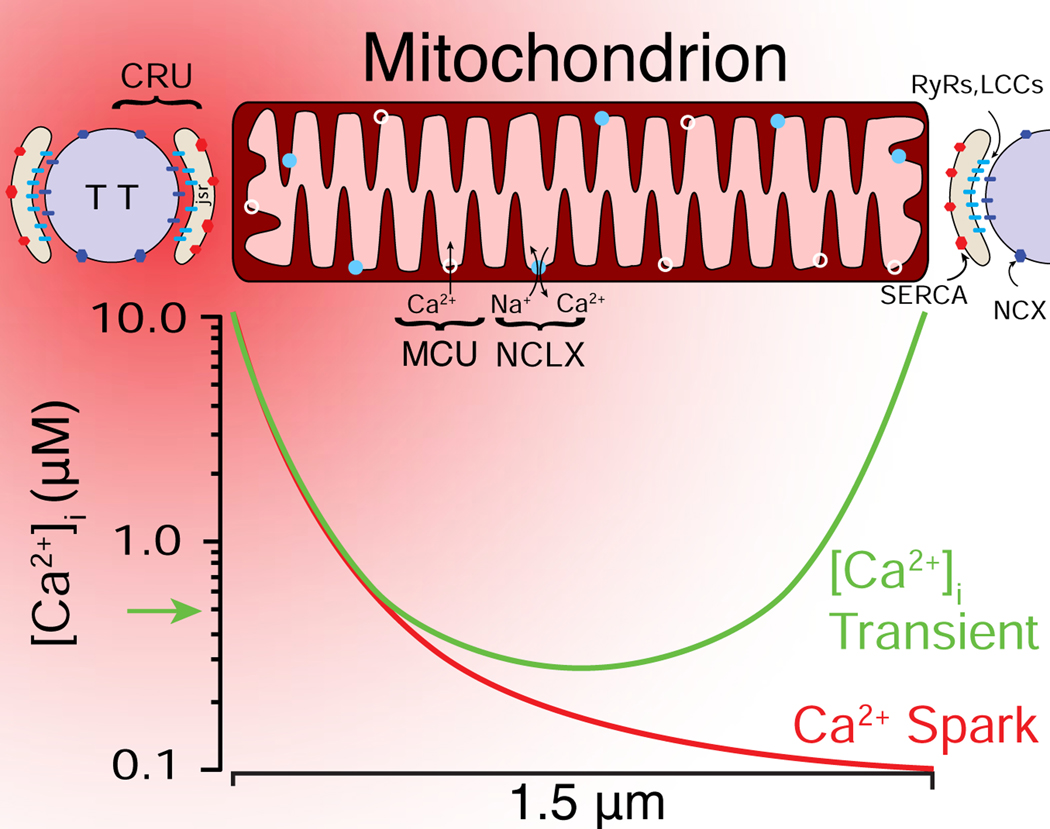
Cardiac ventricular myocytes are ideal to study Ca2+ flux across the inner mitochondrial membrane (IMM) leading to the regulation of mitochondrial functions. In the mammalian heart, mitochondria are the predominant source of ATP, and more abundant per unit volume than in any other tissue. They account for approximately one-third of the cell volume. The mitochondria in myocytes experience regular, repetitive elevations in extra mitochondrial Ca2+ (i.e., local elevations of cytosolic [Ca2+]i). The abundant intermyofibrillar mitochondria (IFM) are in close proximity to the sarcoplasmic reticulum (SR), the primary intracellular Ca2+ storage organelle, which releases Ca2+ with every heartbeat (Fig. 2). Although the cell-wide (global) [Ca2+]i increases from 100 nM to ∼500 nM with each heartbeat, the microdomain [Ca2+]i near the ends of the IFM may transiently rise to 5–10 μM during the release phase [46, 47]. This high local [Ca2+]i occurs in the close proximity of the mitochondrial ends to the SR Ca2+ release units (CRUs). [Ca2+]i can traverse the IMM thought the mitochondrial calcium uniporter (MCU), the only known route for Ca2+ entry into the mitochondrial matrix. This Ca2+ entry is driven by the large negative voltage within the matrix that establishes the voltage gradient across the IMM (−150 to −160 mV) known as ΔΨM. The entry of Ca2+ into the matrix is increased further when cytosolic [Ca2+]i is elevated as it is during the peak of the [Ca2+]i transient. Ca2+ is pumped out of the matrix by the mitochondrial Na+/Ca2+ exchanger NCLX [5, 48, 49]. The amounts of Ca2+ that move in and out of the mitochondria are rather modest when compared to the large Ca2+ fluxes across the sarcolemmal (SL) membrane or the SR [46]. Thus, normal physiological mitochondrial Ca2+ movement does not have a significant effect on cytosolic [Ca2+]i signals and the mitochondria in heart do not act as a significant dynamic buffer for [Ca2+]i [46, 50–52]. This mitochondrial Ca2+ movement however, is important for the physiological regulation of cardiac mitochondrial ATP production [2], and if [Ca2+]m is pathologically high, it can contribute to the development of heart disease [3–5]. It is also clear that the findings are complex [4, 40, 53–58] and quantitative and specific experiments are needed to resolve the contradictions and complexities [3].
Figure 2. spatial distribution of cardiac mitochondrial Ca2+ signaling components.
A spatial representation of a Ca2+ spark (red gradient) initiated at the Ca2+ release unit (CRU), which is located between the transverse-tubule (TT) and the junctional SR (JSR) membranes. At the peak of a Ca2+ spark, [Ca2+]i briefly (10 ms) bathes the end of a mitochondrion with high [Ca2+]i (5–10 μM). During a [Ca2+]i transient, multiple CRUs release Ca2+, bathing both ends of the mitochondrion with high [Ca2+]i. LCCs, L-type Ca2+ channels; RyR2s (ryanodine receptor type 2); SERCA: sarcoplasmic reticulum and endoplasmic reticulum Ca2+ ATPase; NCX: Na+-Ca2+ exchanger; MCU: mitochondrial Ca2+ uniporter; NCLX: mitochondrial NCX. Adapted from [46].
The physiological and pathophysiological roles of the mitochondrial calcium uniporter
[Ca2+]i enters the mitochondrial matrix through the set of highly-selective MCU Ca2+ channels in the IMM [59], termed the mitochondrial calcium uniporters (MCUs). Only a few MCUs are open, and are of very low conductance (~0.1 fS) at physiological [Ca2+]i (~100–500 nM) [46, 59, 60]. Cytosolic Ca2+ can enter the mitochondrial matrix through the open MCU channels to stimulate ATP production under physiological conditions [2]. In contrast, under experimental conditions abnormally high entry of Ca2+ through the MCU can be detrimental to mitochondrial function. The mitochondrial dysfunction occurs due to the very high levels of [Ca2+]m and the possible opening of one or more non-selective large-conductance “pores” that effectively permeabilize the IMM [61]. These huge pores or “mega-channels” are in stark contrast to the miniscule conductance (fS) of the MCUs. Even a single mega-channel can disrupt mitochondrial osmotic regulation and ΔΨM and has been labeled the “mitochondrial permeability transition pore” (mPTP) [62–64]. The mechanistic details of how the mPTPs contribute to heart cell function remain unclear [65]. Additionally, mPTP investigations should include calibrated quantitative experiments that seek information on how reactive oxygen species [ROS]m, and [Ca2+]m, and other factors interact to open mPTPs. There is an additional uncertainty due to the contentious identification of the channel components that comprise mPTP [64, 66–68].
The MCU was identified by two groups in 2011 as a 40-kD protein of the IMM [69, 70]. In MCU knockout (KO) mice, cardiac mitochondria are incapable of rapidly taking up Ca2+. Surprisingly, conditional MCU knockout in the adult heart and complete MCU KO mice display no apparent phenotype [51, 71–74]. Thus, in all of these mouse models, mitochondrial ATP was produced at sufficiently high rates so that the basic energetic needs of the contracting heart were met. However, when fight-or-flight responses were demanded by acute β-adrenergic stimulation, some of the models performed poorly. Notably, these findings included sinoatrial nodal pacemaker cells that failed to increase their AP frequency, a heart-rate that only modestly would rise in response to large systemic boluses of β-adrenergic agonists, and impaired β-adrenergic hemodynamic responses [51, 73–75]. A closer examination of MCU deficient mice also revealed broad transcriptional reprogramming [74], profound changes of cytosolic Ca2+ signaling [73, 74], and other effects which in some models were partially explained as secondary compensatory responses to cytosolic ATP insufficiencies [74].
While transgenic studies provided evidence linking MCU Ca2+ flux to peak cardiac performances, the pathophysiological role of MCU was not as clear. In some genetic studies the lack of MCU appears to have reduced the extent of cardiac ischemia-reperfusion (IR) injury [51, 73], but in other models it did not [71, 72, 74]. The reduction in IR injury was taken to suggest that in these models possibly less Ca2+ entered the mitochondria via the MCU to underlie the opening of the mPTP but this hypothesis was not tested quantitatively. The absence of this effect in other MCU KO mouse models was thought to be related to the specific array of compensatory mechanisms that are currently under investigation [76].
Molecular regulation of the MCU channel
The MCU is thought to be composed of 2 types of pore-forming subunits, regulated by several auxiliary proteins including MICU1 [77–79] (see Fig 3). MCU and MICU1 strongly interact and are co-expressed in many eukaryotes [69, 80] as well as in every tissue tested [81]. Knockouts of MICU1 or its homologue MICU2 cause severe disease with cardiac implications and premature death [82–84], indicating the importance of these proteins. On the other hand, MCU KO itself is not lethal and mitochondrial ATP continues to be produced. Thus, it is important to study MICU1 and its paralogues to determine how they affect mitochondrial functions via MCUs and MCU-independent pathways [85].
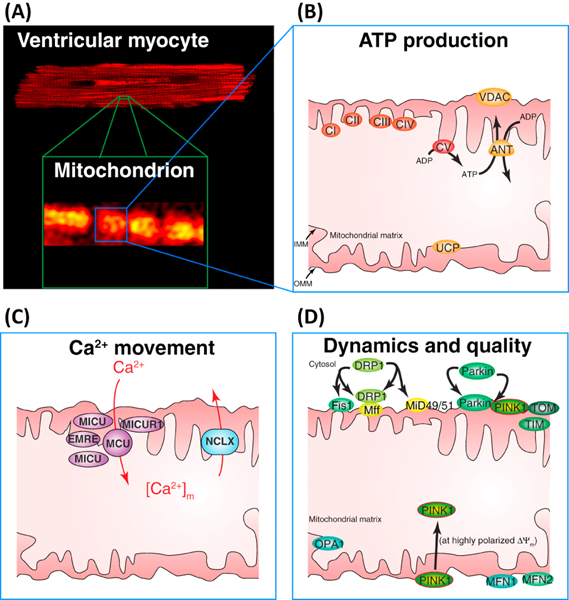
Figure 3. ATP production, Ca2+ movement, and mitochondrial dynamics in the inner (IMM) and outer mitochondrial membranes (OMM) in ventricular myocytes.
A. Top, confocal image of a ventricular cardiomyocyte showing the fluorescence of TMRM in mitochondria. Bottom, zoomed-in view showing a small region of the cell with 4–6 mitochondria. B. Schematic diagram of one of the intermyofibrillar mitochondria. The electron transport chain (ECT) is composed of five complexes: CI CII, CIII, CIV and CV. CI-CIV extrude protons to hyperpolarize IMM to approximately −160 mV. CV (the ATP synthase), uses the energy in ΔΨM to phosphorylate ADP to produce ATP. The adenine nucleotide translocase (ANT) exchanges ADP for ATP across the IMM. The voltage dependent anion channel is permeant to ATP, ADP, Ca2+, etc. The uncoupling proteins (UCPs) on the IMM regulates proton movement from the intermembrane space to the matrix in the production of heat. C. Mitochondrial Ca2+ entry and exit. MCU responsible for Ca2+ entry across the IMM is composed of the MCU, EMRE and MICUR1 subunits and is probably associated with one or more MICU1 (or MICU2 or MICU3) proteins. Ca2+ is extruded from the matrix by the Na+ dependent Ca2+ transporter, NCLX (also called the mitochondrial NCX, Na+-Ca2+ exchanger). D. Proteins involved with mitochondrial dynamics and quality control: OMM: Drp1 which associates with Fis1, Miff, MiD49/51; Parkin and PINK1; translocase of the OMM (Tom) complex, Mfn1, Mfn2. IMM: Opa1, translocase of the IMM (Tim) complex. Note: Tom and Tim proteins form a mitochondrial protein import path.
Measuring how auxiliary subunits of the MCU regulate its function quantitatively under physiological conditions is particularly challenging. The MCU channel is expressed in the IMM, a contiguous membrane that in each mitochondrion has a surface area of about 70–100 μm2 [59, 60], which is relatively small compared to the vast sarcolemma membrane in heart cells that is 2 to 3 orders of magnitude larger. In addition, the density of MCU channels in this membrane is rather low and under physiological conditions the MCU has low unitary conductance (~fS) [46, 59, 60], making it difficult to study with electrophysiological approaches, compared to other ion channels. Nevertheless, several studies explored the role of MICU1 in the regulation of MCU in a qualitative manner but with no broad agreement reached to date. The subcellular localization of MICU1 remains debated, with studies concluding that MICU1 localizes in the intermembrane space, and thus interacts with cytosolic Ca2+, while other studies conclude that it is a soluble protein of the mitochondrial matrix that interacts with matrix Ca2+ [77–79]. Furthermore, regulatory functions with stringent quantitative definition in electrophysiological studies such as gating functions and ion-conduction threshold have been particularly difficult to clearly define in the context of the MCU. Quantitative investigations are needed to better understand the molecular regulation of MCU under physiological and pathophysiological conditions. Apart from better understanding the intrinsic biophysical features of the MCU channel, it is also critical to better understand the signaling micro environment that surrounds each mitochondrion in the cell, and therefore the localized levels of [Ca2+]i that each MCU channel is exposed to. These localized signaling [Ca2+]i levels depends on the proximity of each mitochondrion to the Ca2+ release units on the “junctional” SR (jSR) in heart, or sites of ER Ca2+ release via IP3R in other cell types. Potentially important aspects of cellular physiology may arise from this signaling organization which has been hypothesized to arise from interactions between the outer mitochondrial membrane (OMM) and nearby SR or ER membranes (Mitochondrial Associated Membranes, MAMs).
Mitochondrial associated membranes (MAMs)
Mitochondria within cardiac ventricular myocytes occupy approximately a third of the cell volume [3, 46, 50, 86]. The remaining cellular volume is filled with membrane networks (e.g. SR, ER, Golgi, etc.), contractile filaments, a dense cytoskeleton with diverse motors and cargos, transverse and axial tubules (TATs), multiple nuclei, other invaginations (e.g. caveolae) and much more. Virtually all of these elements are dynamic and are able to move, grow, retreat, and undergo other activities including quality control [87]. It is thus not surprising that with such density and activity, diverse membranes come close to each other and the OMM. To identify a subset of those membranes that are associated with mitochondria with a unique and deliberate purpose that demands a specific long-lasting organization and specific engaged signaling provoked the need for a clearly defined physiological role. Currently, the presentation of spatial proximity and temporal coincidence is the primary argument in support of existence of the molecularly defined mitochondria:ER domains. Current efforts in mammalian cells examine the spatial proximity with emphasis on identifying certain proteins with potential inter-organelle tethering functions, such as mitofusin 2 (Mfn2) [88], PDZD8 [89] and others. However, the importance of quantitative approaches is exemplified by the fact that using similar cell models, some groups proposed that Mfn2 (Tables 1 and 2) serves as a mitochondrion:ER tethering protein [88, 90], while others concluded that Mfn2 drives the ER membranes away from the mitochondria [91–93]. Given the molecular uncertainties and unclear physiological role of such domains, there is a crucial need for quantitative evidence that will constitute a clear framework for future research.
Table 1:
Some of the proteins that are involved in mitochondrial quality control and mitophagy.
| Protein | Function | Calcium sensitivity | Bioenergetics/ ATP generation | Role in mitochondrial quality control |
|---|---|---|---|---|
| Drp1 | Dynamin family GTPase; essential for mitochondrial fission [137]. | Yes | Reduced ATP generation in deficient cells [180]. Normal intracellular ATP levels in Drp1-deficient MEFs [181]. | Controls Parkin-dependent and -independent mitophagy [151, 177, 182]. |
| Mff | Mitochondrial receptor of Drp1 [183]. | NA | NA | NA |
| MiD49/MiD51 | Mitochondrial receptor of Drp1[184]. | NA | NA | Degraded via the UPS upon mitochondrial stress [185]. |
| Fis1 | Controls stress-induced mitochondrial fission [186] | NA | NA | Facilitates Parkin-mediated degradation of dysfunctional mitochondria [186]. |
| Opa1 | Dynamin family GTPase; essential for mitochondrial fusion [187]. | Yes | Reduced ATP generation in deficient cells [156, 188]. | No direct role. Proteolytic processing suppresses mitochondrial fusion and facilitate removal of dysfunctional mitochondria. |
| Mfn1/Mfn2 | Dynamin family GTPases; mitochondrial fusion [189]. | Yes | Reduced ATP generation in deficient cells [190]. Deficiency in axonal transport of mitochondria in DRG neurons from Mfn2−/− embryos [191]. | Major targets of Parkin-mediated ubiquitination; degradation facilitates removal of dysfunctional mitochondria [166]. |
| Parkin | E3 Ub ligase; ubiquitination of the OMM proteins of dysfunctional mitochondria, [159] and mitochondrial structure [158]. | NA | NA | Regulates mitophagy of dysfunctional mitochondria [159]. |
| Pink1 | Kinase; phosphorylation of ubiquitin in dysfunctional mitochondria [192, 193]. | NA | Reduced bioenergetic activity of the mitochondria and increased oxidative stress [142, 143]. | Essential for Parkin-mediated degradation of dysfunctional mitochondria [168, 192, 193]. |
| MARCH5 | The integral OMM E3 Ub ligase; regulates mitochondrial fission [185, 194], also proposed to control mitochondrial fusion [195] and ER MAMs [90]. | NA | Normal OXPHOS activity in knockout cells [185]. | Removes misfolded proteins from the OMM [196], implicated in Parkin- [197] and FUNDC1-[198] dependent mitophagy. |
| VCP/p97 | AAA-ATPase; many cellular functions. In mitochondria facilitates extraction of ubiquitinated proteins from the OMM [165]. | Yes | Reduced ATP generation and OXPHOS in deficient/ mutant cells [199]. | Controls Parkin-dependent mitophagy [166]. |
Table 2:
Neuronal and cardiac phenotypes in transgenic murine models of mitochondrial quality control proteins and their genetic association with human disease.
| Protein | Neuronal phenotype in murine KO models | Cardiac phenotype in murine KO models | Human disease |
|---|---|---|---|
| Drp1 | Brain-specific Drp1 knockout: developmental defects of the cerebellum in which Purkinje cells contained few giant mitochondria instead of the many tubular mitochondria observed in control cells [181]. | Cardiac-specific conditional Drp1 knockout: fibrosis, left ventricular dysfunction, preceded by mitochondrial dysfunction and inhibition of autophagy. Lethal within 13 wk. [151]. Drp1 overexpression does not affect heart function in mouse [200]. | Mutations linked to several developmental deficiencies; early lethality[201]. Reduced expression in AD patients [202]. |
| Mff | Whole body Mff knockout aggravates neurological phenotypes in a Huntington’s disease mouse model [203]. | Whole body Mff knockout mice die at 13 wk. as a result of severe dilated cardiomyopathy and heart failure. Reduced mitochondrial density and respiratory chain activity and increased mitophagy [149]. | Mutations linked to Leigh-like encephalopathy, optic atrophy and peripheral neuropathy [204]. |
| MiD49/51 | NA | NA | NA |
| Fis1 | NA | NA | Increased expression in Alzheimer’s disease patient-derived fibroblasts [202, 205]. |
| Opa1 | NA | Late onset cardiomyopathy in OPA1+/− mouse hearts. Decreased fractional shortening, cardiac output and myocyte contraction [206]. | Mutations linked to a dominant optic atrophy (DOA) [155, 156]. Low expression levels in HF [207]. Reduced expression in AD patients [202]. |
| Mfn1/Mfn2 | Ablation of Mfn1 or Mfn2 causes embryonic lethality in mice [189]. | Cardiac-specific conditional Mfn1/Mfn2 knockout model; protection against acute myocardial infraction [120]. Mfn2−/− mice display modest cardiac hypertrophy, accompanied by slight functional deterioration, delay in mitochondrial permeability transition downstream of Ca2+ stimulation or due to local generation of ROS [121]. | Mutations linked to a Charcot-Marie-Tooth type 2A peripheral neuropathy [154]. Reduced expression in AD patients [202]. |
| Parkin | Whole body Parkin knockout: no evidence for nigrostriatal, cognitive, or noradrenergic dysfunction [208]. Parkin knockout in -mito-PstI background (double-strand breaks of mtDNA only in dopaminergic neurons) exacerbated the neurodegenerative process [209]. | No apparent defects in Parkin knockout mouse [210]. | Mutations linked to an early onset Parkinson’s disease [157]. |
| Pink1 | Whole body PINK1 knockout enhances neurodegeneration in dOTC overexpression model of Parkinson’s disease [211]. Impairment in limb motor skills in the absence of nigrostriatal dopamine loss [212]. | Whole body PINK1 knockout mice develop fibrosis, left ventricular dysfunction and cardiac hypertrophy. Oxidative stress and impaired mitochondrial function in cardiac myocytes are also observed [141]. | Mutations linked to an early onset Parkinson’s disease [157]. |
| MARCH5 | Homozygous knockout mouse displays embryonic lethality http://www.informatics.jax.org/marker/MGI:1915207. Oxidative stress in MARCH5-/brain slices [213]. | NA | NA |
| p97/VCP | Targeted deletion of p97 in mouse results in early embryonic lethality [214]. | NA | Mutations linked to many diseases, including ALS [164], CMT2 [163] and Inclusion bodies myopathy with Paget’s disease of bone and frontotemporal dementia (IBMPFD) [162]. |
Gold standard.
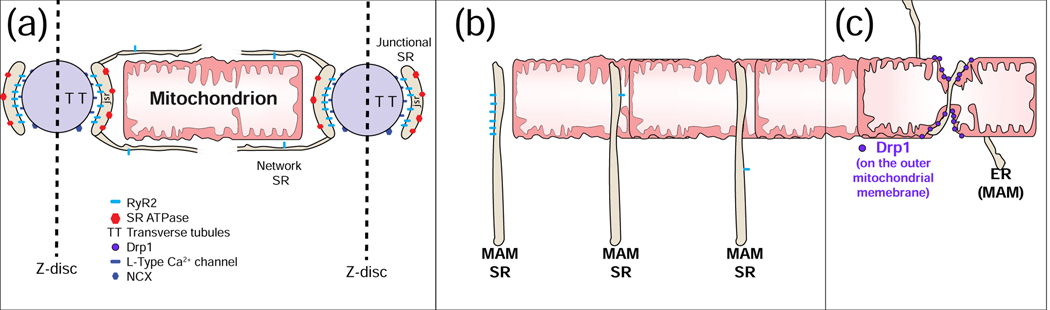
One of the best examples of intimate membrane interactions is “excitation-contraction coupling” (ECC) in heart (Fig. 4A). This process starts when a local Ca2+ signal is generated in a narrow space (15 nm) when an L-type Ca2+ channels in the sarcolemmal membrane (SL) is opened by the SL depolarization due to an action potential (AP). A cluster of Ryanodine Receptor type 2 (RyR2) Ca2+ channels in the cardiac jSR protrude into that narrow space and are activated by the local elevation of [Ca2+]i. When the RyR2 channels are activated, a Ca2+ spark is produced. There are a huge number of these structures within each cardiac ventricular myocyte. They are found along the SL and along SL invaginations called the transverse-axial tubular (TAT) system.
Figure 4. Mitochondrial Associated Membranes (MAMs) and Ca2+ signals.
A. Excitation-contraction coupling (ECC) and MAMs. The sarcoplasmic reticulum (SR) is contiguous with the endoplasmic reticulum (ER) and contribute membranes to MAMs. In cardiac myocytes, [Ca2+]i transient is triggered electrically by action potentials (AP). In ventricular myocytes, there are a very large number of Ca2+ spark sites (~20,000 per cell). Each site includes an array of ~50 SR Ca2+-release channels (ryanodine receptors type 2; RyR2) in the junctional SR that faces a transverse tubule (TT) or the sarcolemma (SL) that become activated upon Ca2+ elevation in the narrow ~15 nm subspace. During cardiac AP, the L-type Ca2+ channels in the TT and SL membranes are depolarization and conduct Ca2+ into the subspace. This high subspace Ca2+ activates the RyR2s enabling Ca2+ release from the SR to produce a Ca2+ spark. B. Three common arrangements of RyR2s in MAMs. Left: A cluster of RyR2s facing away from the mitochondrion, 20 to 100 nm away, similar to those of a Ca2+ spark site. Middle: Isolated RyR2 channel in a MAM facing an OMM at ~15 to 50 nm. These channels are not normally triggered to open. Under quiescent conditions, the opening rate is ~10−4 s−1. Right: Isolated RyR2 in a MAM more than 100 nm away from the mitochondrion. C. Garrote-assisted fission. It has been suggested that mitochondrial fission, normally attributed to the action of Drp1, may be assisted by the encircling action of ER or SR.
In heart, when only a single jSR cluster of RyR2 Ca2+ channels is activated, an isolated Ca2+ spark (Fig. 2 and Fig. 4A) is produced. There are proteins in heart that facilitate the ECC signaling by linking the SL or TT membranes to the SR membranes [94, 95] and are thus responsible for spatially organizing signals [96–99]. At the membrane junctions that produce Ca2+ sparks, the TT and SR membranes are separated by about 15 nm narrow gap called the “subspace” or diadic space. At those locations the SR contains many identical proteins that form a para-crystalline array of RyR2 Ca2+ channels [100]. The details of this behavior have been extensively studied enabling us to develop mathematical models [101, 102] that permit us to test quantitatively how the system works [47, 103]. These models test how excitation-linked Ca2+ entry through L-type Ca2+ channels increases subspace [Ca2+] within the small volume of the 15 nm wide gap (the [Ca2+]subspace is around 10 μM [104, 105] ). The models help us to know if this signal is sufficient to activate the RyR2 Ca2+ channels. This modeling further tests many other features of the system. When Ca2+ spark activation is synchronized within the cell, a cell-wide [Ca2+]i transient is produced which in turn underlies contraction. Many aspects of this process can be investigated with the model. An important discovery is that each huge RyR2 channel also serves as an protein signaling scaffold [106] which is a local home for kinases and phosphatases and other relevant proteins. In this manner the RyR2-dependent Ca2+ signals may be modulated by local PKA, CaMKII [107], X-ROS signaling [108–111], and other signaling pathways. These investigations, while rich and productive, all involve significant quantitative investigations in the same kind of space within the ventricular myocyte as exhibited between MAMs and mitochondria. These are indeed technically challenging investigations, but conceivably feasible, and when carried out rigorously and quantitatively could profoundly advance our understanding of MAMs signaling pathways if properly applied.
Skepticism with hope.
The preponderance of links between mitochondrial function and MAMs are primarily the associations of proximity and do not generally involve a specific quantitative experiment that address a sharply stated physiological hypothesis [112, 113]. However, a recent report examining PDZ domain containing protein 8 (PDZD8) involved in ER-mitochondrial Ca2+ signaling in neurons [5, 89] and Mfn2 in cardiomyocytes are steps in the right direction [114, 115]. For example, PDZD8 is presented as an orthologue of the yeast ER–mitochondria encounter structure (ERMES) complex and is suggested to play an important role in ER-mitochondrial signaling. However, quantitative (i.e., calibrated) measurements of [Ca2+]m or spatially resolved [Ca2+]i to clarify the function have yet to be reported. Furthermore, it is also important to note that currently available genetic in-vivo studies do not provide evidence to support the proposed physiological role of augmented Ca2+ movement into the mitochondria in tissues and cells where the ER releases Ca2+ through IP3 receptors, such as in neurons and epithelial cells. Genetic deletion of MCU in such tissues and cells has not been reported to cause an apparent change of tissue function [72, 116]. On the other hand, the available evidence is in support of an important physiological role for augmented entry of Ca2+ through the MCU into the mitochondria in heart and skeletal muscle [51, 52, 71–74], two tissues in which intracellular Ca2+ release occurs via RyR2 and RyR1 channels, respectively.
The Ca2+ signaling between SR and ER and nuclear envelope membranes and the mitochondria is a broadly proposed MAMs function. In ventricular myocytes, for example, the SR, ER and nuclear envelope all contain Ca2+ at a high level (mM) compared to cytosol and mitochondrial matrix (100 nM under quiescent condition to μM levels with significant activity). The three high Ca2+ stores (SR, ER, and nuclear membranes) constitute a contiguous network that shares Ca2+ content across all elements [117]. To date, there are generally three types of simple interactions between the MAMs and mitochondria that involve Ca2+ signaling (Fig. 4B). (1) Cluster of Ca2+ channels that are organized at distances 50 to 100 nm away from a mitochondrion (e.g. the RyR2 cluster at the SR-TT junction; left panel of Fig. 4B and also in Fig. 2). The other two types of MAM arrangements have one or a very few RyR2 channels or IP3R channels within 10–20 nm of a mitochondrion (middle panel in Fig. 4B) or one or a very few Ca2+ channels hundreds of nm away (Fig. 4B right panel). Mathematical modeling and a few direct measurements [50, 95] in ventricular myocytes suggests that very little Ca2+ enters the mitochondria via MCUs by any of these sources, given the brief openings of the Ca2+ release channels, the dilution with distance of the para-mitochondrial [Ca2+], the very small conductance of the MCU’s (fS) [118], and the low number of MCU’s in the IMM [2, 118]. Thus, there is uncertainty about the rigor of MAM-linked functional conclusions, especially in cells that lack RyR channels. Signaling between the MAMs and the mitochondria need to be measured quantitatively and not simply inferred by the proximity of the two structures. Importantly, the absence of rigorous measurements does not itself disprove a linkage between MAMs and the mitochondria. Many associations link MAMs to disease and critical mitochondrial features including heart failure [119–123], mitochondrial quality control[124], diabetic cardiomyopathy [125, 126], and various neurodegenerative diseases including Alzheimer’s disease, Parkinson’s disease, and ALS [127]. At the present time, however, quantitative and functional links between MAMs and mitochondrial signaling needs much work.
Another role of MAMs has been proposed in which a tight association of the ER and OMM occurs and an ER tubule “garrote” facilitates mitochondrial fission. It is proposed that an ER MAM encircles the mitochondrion and, working with dynamin-related protein1 (Drp1) proteins on the OMM somehow triggers mitochondrial fission [128]. This mechanism [90, 128, 129] implies that the “standard mechanism” that involves Drp1 without assistance of the ER tubule garrote (Fig. 4C [128]) does not function. Like the other MAM hypotheses, quantitative methods are needed to validate and extend the role of ER MAMs in mitochondrial fission, if indeed there is physiological requirement for this feature.
Ca2+ signaling in mitochondrial quality control
In addition to the above-discussed direct role of Ca2+ in mitochondrial ATP generation, several lines of evidence suggest that Ca2+ could also control this process indirectly, through regulation of various mitochondrial quality control pathways. Distinct quality control mechanisms ensure mitochondrial function, including ROS detoxification and a network of proteolytic systems that degrade mitochondrial proteins that are damaged, misfolded or mislocalized and control the activities of stress-responsive mitochondrial factors. The ubiquitin (Ub)/proteasome system (UPS), through degradation/control of OMM-associated proteins, is also vital for the maintenance of mitochondrial function. Upon irreversible mitochondrial damage, mitochondria-specific autophagy (mitophagy) is activated to remove dysfunctional mitochondria. While these mechanisms were extensively reviewed elsewhere [87, 130–133], here we focus on recent evidence linking these processes with Ca2+ signaling.
Mitochondrial fission and the ubiquitin (Ub)-proteasome system (UPS)-mediated processes
Mitochondrial fission is a mechanism by which cells remove dysfunctional mitochondria from the system. Fission separates damaged mitochondria from functional mitochondrial networks [134, 135], and thereby facilitates their subsequent removal by E3 Ub ligase Parkin-, and PTEN-induced kinase 1 (PINK1)-regulated mitochondria-specific autophagy (mitophagy) (see BOX1 for mitochondrial quality control overview). The role of Ca2+ in mitochondrial fission was originally revealed by data showing that mitochondrial fragmentation in cells subjected to apoptosis inducer C2-ceramide was inhibited by extracellular Ca2+ or chelating intracellular [Ca2+]i [136]. Subsequent studies proposed that the role of [Ca2+]i in mitochondrial fission was to control the localization Drp1 [137]. Specifically, calcineurin-mediated Drp1 dephosphorylation induced accumulation of Drp1 on the OMM and thereby increased mitochondrial fission rates [138]. It appears therefore that changes in [Ca2+]i control mitochondrial association of Drp1 and thereby mitochondrial fission rates. Complexity of Ca2+-dependent control of mitochondrial fission was added, however, by data that suggest that “inverted formin 2” (INF2) can mediate actin polymerization at the mitochondria:ER contact sites. It is thought that ER Ca2+ release can stimulate a rise in [Ca2+]m through MCU [129] that will recruit the IMM in the fission process. Earlier works by Higgs and colleagues showed that INF2-dependent local actin polymerization control mitochondrial assembly of Drp1 [139]. The new data indicate that OMM-associated actin controls MCU-mediated uptake of Ca2+ and thereby facilitate fission of the IMM [129]. A similar function for MCU in mitochondrial fission has been proposed by Cho et al. [134]. The authors found that Drp1 can transiently interact with the OMM-associated Zn2+ transporter Zip1 that in turn may facilitate Zip1:MCU interactions and results in loss of mitochondrial membrane potential in a subset of mitochondria within each cell. It has been concluded that this mechanism facilitates mitochondrial translocation of E3 Ub ligase Parkin and mitophagy-mediated removal of dysfunctional mitochondria (see BOX1 and Tables 1 & 2) and therefore restores a population of “healthy” mitochondria. The role of MAMs in this model has not been investigated. Interestingly, Parkin may also regulate MCU activity through control of the Ub-mediated degradation of MICU1 [140] (See also discussion above on MCU and MICU1). MICU1 is a relatively short-lived protein (~4 hr half-life) and its fast turnover could be applied by the cell to tune the mitochondrial Ca2+ handling. As an extension, MICU1 degradation could also serve as a feedback mechanism regulating MCU-dependent mitochondrial fission and Parkin-mediated elimination of dysfunctional mitochondria. While the role of mitochondrial Ca2+ and MCU complex in mitochondrial quality control has just begun to be understood, the work discussed above already point to the high significance of this signaling axis.
BOX1: The basics of mitochondrial quality control.
Ubiquitin (Parkin/PINK1)-mediated mitophagy
Mitochondria-specific autophagy (mitophagy) is instigated by irreversible damage of mitochondria [131, 167, 168]. Early steps in mitophagy rely on the accumulation of PTEN-induced putative kinase 1 (PINK1) on the OMM of mitochondria with low (depolarized) membrane potential (ΔΨm). The E3 Ub ligase Parkin translocates to damaged mitochondria and, through massive ubiquitination of the OMM proteins, facilitates mitophagy activation [168, 169]. Specifically, Ub chains added by Parkin to various OMM-localized proteins are phosphorylated by PINK1, which serves as autophagy adaptor proteins, such as Optineurin, NBR2 or p62, recruitment signal, that subsequently facilitate selective degradation of damaged mitochondria. Consistent with this model, mitochondria-acting deubiquitinases (DUBs), including Usp30 and Usp15 slow down mitophagy by removing Ub chains from the OMM proteins [170–173], while the mitochondrial E3 Ub ligase MUL1 partially rescue mitophagy in mutant Parkin-expressing cells [174]. This model has been established in cultured cell lines and primary neurons. One report suggested that PINK1 phosphorylated mitochondrial fusion protein Mfn2, which serves as a signal recruiting Parkin to dysfunctional mitochondria in cardiac myocytes [175]. This unique mechanism in the heart has not been broadly supported yet by additional studies.
Mitochondrial membrane dynamics (fusion and fission)
Mitochondrial fission requires a large GTPase of the dynamin family, dynamin-related protein 1 (Drp1). Drp1 is recruited to the mitochondria through interaction with the OMM localized Drp1 receptors, mitochondrial fission factor (Mff) and/or mitochondrial division (MiD49 and MiD51) complex. Upon recruitment, Drp1 oligomerizes on the OMM and initiates GTP hydrolysis-dependent mitochondrial scission. Mitochondrial fission is highly regulated and was proposed to require ER tubules, and transient assembly of actin to occur. These processes are regulated by Ca2+. Drp1 is a prerequisite for mitophagy [135, 176–178]. Mitochondrial fusion is also regulated by the large GTPases, the OMM localized mitofusins 1 and 2 (Mfn1 and Mfn2) and the IMM/IMS Opa1. These proteins coordinate the fusion of the IMM and the OMM. Selective inhibition of mitochondrial fusion by low (depolarized) ΔΨm, a characteristic of dysfunctional mitochondria, was proposed to facilitate their removal from the system [177].
Mitochondrial quality control in heart
Most of the data on mitochondrial quality control mechanisms was generated using model cells (e.g. HeLa cells) or studies focusing on understanding mitochondrial degeneration linked to various neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, and peripheral neuropathies. Relatively little is known about mechanisms protecting heart mitochondria from functional decline.
Mouse knockouts of the critical mitophagy regulator PINK1 (See BOX1 and Tables 1 and 2) showed strong alterations in heart, including left ventricular dysfunction and pathological cardiac hypertrophy as early as 2 months of age [141]. This study and experiments performed in other model systems [142, 143] revealed reduced bioenergetic competence of the mitochondria and increased oxidative stress. However, since the heart shows the strongest phenotype in PINK1 knockout animals, one can assume that this mitochondria-rich organ may especially rely on PINK1. Indeed, significantly reduced levels of PINK1 were found in tissue samples obtained from patients with end-stage heart failure [141]. Through phosphorylation of putative IMM-localized Ca2+/H+ antiporter leucine zipper-EF-hand containing transmembrane protein 1 (LETM1) [144], PINK1 regulates mitochondrial Ca2+ [145]. Furthermore, a strong reduction of the mitochondrial Ca2+ efflux observed in PINK1 knockdown cells suggests that this protein could also regulate NCLX [146]. Whether, like in other tissues, PINK1 is implicated in Parkin-mediated degradation of mitochondria in the heart remains to be tested. Further studies will be necessary to determine the degree to which PINK1 controls mitochondrial quality through its role in Ca2+ signaling.
The role of Drp1-mediated mitochondrial fission in the elimination of dysfunctional mitochondria in heart is not well understood. In contrast to earlier findings showing slow mitochondrial fusion rates [147], recent whole heart and freshly dissociated cardiomyocyte imaging experiments showed that mitochondrial fusion in rat heart happens more frequently than reported before [148]. Given these new findings, and since mitochondrial size is maintained by opposing activities of fusion and fission, one can assume that mitochondrial fission in cardiac cells must also be more robust then thought before. Connecting mitochondrial fusion to Ca2+ signaling, the increase in mitochondrial fusion rates were noted after spontaneous contraction or short-term field stimulation [148]. Further supporting the critical role of mitochondrial fission in heart function, and perhaps in mitochondrial quality control in cardiac myocytes, knock out of mitochondrial fission factor (Mff), a mitochondrial receptor of Drp1, result in early, at 13-week, lethality attributed to dilated cardiomyopathy leading to heart failure [149]. Mff-deficient hearts showed reduced oxidative phosphorylation activity and ATP levels and increased mitochondrial ubiquitination and mitophagy (see Table 2). These data support the physiological role of Mff in cardiac mitochondria function, and in general, these data also support the physiological role of mitochondrial fission in cardiac function. However, another possibility is that Mff directly controls mitochondrial ubiquitination, and subsequent mitophagy, which has been shown in non-cardiac cells [150], but has not yet been demonstrated in the heart. The downregulation of Drp1 in cardiac myocytes resulted in suppressed autophagosome formation and autophagic flux in response to glucose deprivation [151]. This suggests that, like model cells, Drp1 and perhaps Drp1-mediated mitochondrial fission, is a vital quality control mechanism in the heart. Similar to Mff knockout mice [149], cardiac-specific Drp1 knockout mice develop left ventricular dysfunction, preceded by mitochondrial enlargement with reduction in cellular ATP levels and followed by death within 13 weeks [151]. To our knowledge, however, no specific data on Ca2+ regulation of mitochondrial fission in the heart has been reported. In addition to mitochondrial fission, Drp1-independent mitochondrial shape changes, including mitochondrial shape transition (MiST) [152] or formation of mitochondrial nanotubes [153] also contribute to Ca2+-regulated mitochondrial quality control. These processes, regulated by Ca2+-binding EF hand proteins Miro1 and Miro2 (mitochondrial Rho GTPases), facilitate mitophagy and in consequence provide protection of cardiac cells from mitochondrial decline.
The growing molecular understanding of mitochondrial quality control suggest that apart from its important roles in cardiac health and disease, these processes are also critical for normal neuronal function, and when altered, play a major role in neurodegenerative diseases.
The roles of mitochondrial dysfunction in neurodegenerative disease
Many neurodegenerative diseases, such as Alzheimer’s Disease (AD), Parkinson’s Disease (PD), and Amyotrophic Lateral Sclerosis (ALS) display functional deficiencies that are often associated with abnormal mitochondrial structure (Table 2). However, specific genetic causes leading to neuronal degeneration in AD, PD, and ALS are not as well understood as they are in other neurodegenerative disease such as peripheral neuropathy Charcot- Marie-Tooth disease (CMT) or dominant optic atrophy (DOA), which were linked to mutations in genes encoding mitochondrial fusion factors, Mfn2 [154] and Opa1 [155, 156], respectively (Table 2). Nevertheless, in all cases it is still uncertain how the mitochondrial alterations contribute to the course of the disease progression and how they influence the stages of neuronal dysfunction and death. The findings that PARK7, PARK6/PINK1 and PARK2/ PARKIN genes are mutated in early onset PD [157] along with the fact that these genes encode proteins that directly control mitochondrial function [157–160] suggest that mitochondrial dysfunction may underlie PD (Table 1 and 2). At this time however, the specific role of mitochondrial matrix/IMS-localized deglycase DJ-1 (PARK7) [161] in mitochondrial function has not been fully established. As discussed, above (see BOX1), the E3 ubiquitin ligase Parkin and kinase Pink1 work together to remove accumulation of abnormal mitochondria and this is likely important in their contribution to PD “prevention”. These observations support the hypothesis that they do this by maintaining the fidelity of oxidative phosphorylation and mitochondrial ATP generation. Furthermore, among other functions, the familial ALS-, and CMT 2-linked AAA ATPase, p97/VCP [162–164], controls mitochondrial ubiquitination [165] and Parkin/PINK1 mediated mitophagy [166]. Taken together, there is a body of evidence implicating mitochondria in the origins and progression of diverse neurodegenerative diseases.
Concluding Remarks
Here we provide a brief overview of Ca2+ dependent ATP production by mitochondria and issues related to mitochondrial protein quality control. This area is moving very quickly with many recent reports on MCU, MICU1, NCLX and control of ATP production. The quality control research field is also very dynamic, resulting in almost daily reports of important discoveries and insights. We have provided physiological and pathophysiological context for this understanding. We also discuss a topic of considerable interest to many of us working in the field: mitochondrial associated membranes or “MAMs”. As exciting as MAMs may be, we hold the position that each of us working on mitochondrial biology and mitochondrial signaling have an obligation to make our work more rigorous and more quantitative and more testable.
There are four “take-home” messages from this brief review:
Ca2+ signaling plays an important role in regulating ATP production in heart and in other tissues.
Ca2+ signaling contributes to the regulation of mitochondrial quality control.
Mitochondrial dysfunction contributes importantly to the molecular causes and progression of both heart failure and neurodegenerative diseases (“brain or neuron failure”).
Focused quantitative investigations are needed to improve our understanding of mitochondrial contributions to health maintenance and disease origin and progression.
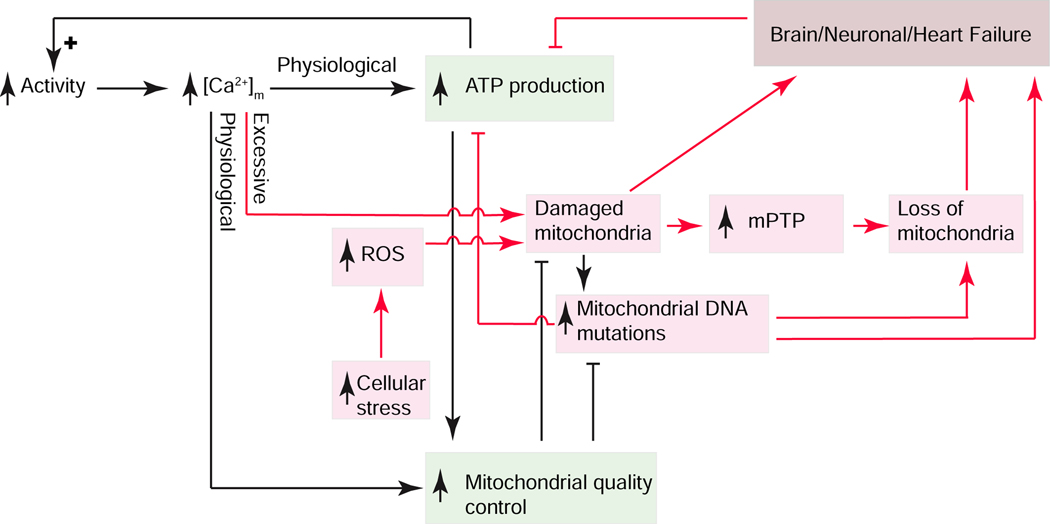
Figure 5. Signaling elements linking ATP production, mitochondrial quality control and Brain/Neuronal/Heart failure.
Cellular activity controls ATP production and mitochondrial quality control (green boxes) through Ca2+ signaling. Excessive [Ca2+]m and stress-dependent reactive oxygen species (ROS) production (red arrows) appear to damage mitochondria which can lead to manifestations of functional failure of heart and neuronal tissues with positive feedback amplification. Mitochondrial permeability transition pore (mPTP).
Box 2: Mitochondrial Matrix Ca2+ excess: Neurodegeneration versus Death - role of NCLX.
Under quiescent conditions in heart, matrix Ca2+ concentration, [Ca2+]m, is moderate (about 150 nM) and rises with activity (up to about 1 μM). This [Ca2+]m links EC coupling activity to ATP production and is central to the mitochondrial response to physiologic activity [2]. Diverse examples, however, of cardiac dysfunction arise when the RyR2 SR Ca2+ release channels become “leaky” and it is thought this leakiness of Ca2+ out of the SR in close proximity to intermyofibrillar mitochondria that increases matrix Ca2+ and contributes to heart failure [4]. While careful quantitative experiments to understand the nature of the hypothesized Ca2+ overload are required [3], a clear experimental test of the critical role of matrix Ca2+ was done in genetically engineered mice. Here tamoxifen-induced deletion of NCLX in adult mouse hearts caused sudden death to develop in the mice that correlated with severe myocardial dysfunction and rapid onset heart failure [5]. This finding is consistent with the suggestion that mitochondrial-linked diseases and pathology may benefit from increased extrusion of Ca2+ from the mitochondrial matrix. An experimental example from Parkinson disease “model neurons” showed the benefit of increasing NCLX extrusion of Ca2+ from the mitochondrial matrix [146]. A recent compelling example of how triple-transgenic mouse model (3X) of Alzheimer’s Disease was associated with the down regulation of NCLX in cortical neurons and that was associated with matrix Ca2+ overload [179]. Additional experiments suggested that these same 3X mice had gained improved cognitive function and performance in a Y-Maze test when neuronally targeted NCLX overexpression was imposed. These data suggest that when mitochondrial matrix Ca2+ is excessive in cortical neurons, there is increased likelihood of neurodegeneration and when this occurs in heart it leads to death.
Clinician’s Corner
In heart failure (HF), cardiac myocytes are unable to contract with enough force to pump blood at an appropriate pressure. There are three basic categories of HF: stunning (quickly reversible), hibernation (slowly reversible) and irreversible failure. One possible cause of HF is inadequate ATP production by damaged mitochondria and the differences in degree of HF may reflect the extent of the damage.
Analogous to heart failure, neuronal mitochondrial damage may lead to inadequate ATP production. A major job of the neuronal ATP is to enable pumping ions out of or into the neuron. With ATP insufficiency neurons may be damaged by inadequate transport of ions or other intracellular cargos. Recent work suggests that mitochondrial Ca2+ overload is associated with damage and neurodegenerative diseases.
Ca2+ is a signal that can dynamically regulate ATP production. Within the physiological range of Ca2+, increases in matrix Ca2+ stimulates ATP production. In contrast, Ca2+ overload in the mitochondrial matrix can lead to mitochondria dysfunction or failure.
Highlights.
ATP production in the heart is primarily dependent on oxidative phosphorylation in mitochondria and is dynamically regulated by the level of Ca2+ in the mitochondrial matrix ([Ca2+]m) and the concentration of ADP in the cytosol.
Mitochondrial Ca2+ signaling dysfunction in heart has been linked to arrhythmia, heart failure and death while in neurons it is associated with neurodegenerative diseases such as Alzheimer’s disease, ALS and Parkinson’s disease.
Tight mitochondrial “quality control” in heart and brain mitochondria is regulated in part by Ca2+.
Although mitochondrial associated membranes (MAMs) from SR and ER are hypothesized to have distinct signaling modalities, often involving Ca2+, quantitatively physiologic investigations are needed to characterize them.
Outstanding Questions.
How does high matrix Ca2+ degrade mitochondrial function? As noted in the text, this is an important but unanswered question for heart muscle and neuronal biology.
What are the possible molecular causes of irreversible heart failure? Are they due to excessive mitochondrial ROS damage leading to mutations of mitochondrial DNA? The 13 key proteins coded by the mitochondrial DNA are crucial for ATP generation. The mitochondrial DNA is “naked” and exposed to ROS and thus can be readily damaged. The approximate ten copies of mitochondrial DNA in each mitochondrion and modest repair capabilities can be “used up” and thus lead to reduced function of the mitochondria.
What are the MCU-independent mitochondrial pathways regulated by MICU1 and MICU2? Do they directly contribute to mitochondrial Ca2+ signaling?
While Parkin is a key component of machinery that removes dysfunctional mitochondria in model cell systems such as HeLa cells, Parkin deficiency does not affect mitochondrial activity in mice hearts. Why not?
How tightly coordinated is the linkage between mitochondrial DNA mutations and the reduced ability of mitochondria to produce ATP?
Acknowledgement and Funding
This work was supported by American Heart Association grants SDG 15SDG22100002 (to L.B.); By 1R01HL142290, U01 HL116321, 1 R01 HL140934, 1R01 AR071618 (to W.J.L.); By NIH R01 GM 129584 (to M.K).
Glossary
- Diadic space
the cytosolic region between the T tubule membrane and the junctional SR membrane, also known as the subspace
- Excitation contraction (EC) coupling
the process by which the electrical signal in the heart, the action potential, triggers the cellular [Ca2+]i transient that underlies contraction
- Mitochondria associated membranes (MAMs)
the membranes, usually from the SR or ER, that are close to the OMM - usually within 15 to 100 nm
- Mitochondrial calcium uniporter (MCU) complex
the MCU is a transmembrane channel protein of the IMM that conducts Ca2+ from the intermembrane space (IMS) to the matrix with high selectivity and low conductance. Since Ca2+ freely moves from the cytosol into the IMS through VDAC in the OMM, the MCU permits Ca2+ to move from the cytosol into the mitochondrial matrix. The MCU complex is thought to composed of the MCU channel along with accessory proteins (EMRE, MCUB, MICUR1) as well as a special additional component, the “mitochondrial calcium uptake 1 and 2” proteins (MICU1 and 2)
- Mitochondrial quality control
a number of processes, including the regulation of mitochondrial protein turnover, the management of mitochondrial fusion and mitochondrial fission and the control of mitophagy. Taken together, these processes protect the mitochondria from damage and protect the cell from accumulation of defective mitochondria
- Mitochondrial permeability transition pore (mPTP)
a large conductance pathway in the IMM that is known to be activated by various pathological conditions, including Ca2+ overload and reactive oxygen species (ROS) or other kinds of oxidative damage. The increased permeability of the IMM during mPTP opening or “activation” often leads to a large-scale mitochondrial swelling and mitochondrial death
- Mitochondrial sodium-calcium exchanger (NCLX)
NCLX is a protein that works as the mitochondrial matrix Ca2+ extrusion “pump”. This mechanism of transport is powered by the influx of Na+ ions across the IMM on the NCLX in exchange for Ca2+ extruded from the matrix into the IMS
- Oxidative phosphorylation
the mechanism by which mitochondria phosphorylate ADP to make ATP. NADH (or FADH2), made by the Krebs cycle proteins within the matrix, donates an electron to the ETC (electron transport chain). The ETC is a series of specialized proteins in the IMM and linked functionally together to extract an electron from NADH to power the pumping of protons out of the matrix. The extrusion of protons establishes a negative matrix potential and a relatively proton-poor environment in the matrix. The electron is used to reduce oxygen. The ETC thus creates a proton motive force across the IMM that powers the ATP synthase to make the ATP
- X-ROS signaling
NADPH oxidase is activated by mechanotransduction to produce ROS that can oxidize local protein targets to enhance Ca2+ signaling. This signaling mechanism is activated during physiologic stretch of cardiomyocytes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McCormack JG and Denton RM (1993) The role of intramitochondrial Ca2+ in the regulation of oxidative phosphorylation in mammalian tissues. Biochem Soc Trans 21 ( Pt 3) (3), 793–9. [DOI] [PubMed] [Google Scholar]
- 2.Wescott AP et al. (2019) Voltage-energized Calcium-sensitive ATP Production by Mitochondria. Nature Metabolism In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyman L. et al. (2015) The growing importance of mitochondrial calcium in health and disease. Proc Natl Acad Sci U S A 112 (36), 11150–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santulli G. et al. (2015) Mitochondrial calcium overload is a key determinant in heart failure. Proc Natl Acad Sci U S A 112 (36), 11389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luongo TS et al. (2017) The mitochondrial Na(+)/Ca(2+) exchanger is essential for Ca(2+) homeostasis and viability. Nature 545 (7652), 93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy E. et al. (2016) Mitochondrial Function, Biology, and Role in Disease: A Scientific Statement From the American Heart Association. Circ Res 118 (12), 1960–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Q. et al. (2019) Role of PGC-1alpha in Mitochondrial Quality Control in Neurodegenerative Diseases. Neurochem Res. [DOI] [PubMed] [Google Scholar]
- 8.Wu Y. et al. (2019) Mitochondrial dysfunction in neurodegenerative diseases and drug targets via apoptotic signaling. Mitochondrion 49, 35–45. [DOI] [PubMed] [Google Scholar]
- 9.Panchal K. and Tiwari AK (2019) Mitochondrial dynamics, a key executioner in neurodegenerative diseases. Mitochondrion 47, 151–173. [DOI] [PubMed] [Google Scholar]
- 10.Stuppia G. et al. (2015) MFN2-related neuropathies: Clinical features, molecular pathogenesis and therapeutic perspectives. J Neurol Sci 356 (1–2), 7–18. [DOI] [PubMed] [Google Scholar]
- 11.Weibel ER et al. (1969) Correlated morphometric and biochemical studies on the liver cell. I. Morphometric model, stereologic methods, and normal morphometric data for rat liver. J Cell Biol 42 (1), 68–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veltri KL et al. (1990) Distinct genomic copy number in mitochondria of different mammalian organs. J Cell Physiol 143 (1), 160–4. [DOI] [PubMed] [Google Scholar]
- 13.Blouin A. et al. (1977) Distribution of organelles and membranes between hepatocytes and nonhepatocytes in the rat liver parenchyma. A stereological study. J Cell Biol 72 (2), 441–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barth E. et al. (1992) Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. J Mol Cell Cardiol 24 (7), 669–81. [DOI] [PubMed] [Google Scholar]
- 15.van Dyke RW et al. (1983) Oxygen consumption by rat liver: effects of taurocholate and sulfobromophthalein transport, glucagon, and cation substitution. Am J Physiol 244 (5), G523–31. [DOI] [PubMed] [Google Scholar]
- 16.Bracht L. et al. (2016) Effect of the Combination of Ezetimibe and Simvastatin on Gluconeogenesis and Oxygen Consumption in the Rat Liver. Basic Clin Pharmacol Toxicol 118 (6), 415–20. [DOI] [PubMed] [Google Scholar]
- 17.Shadrin KV et al. (2015) Characteristics of oxygen transport through the surface of the isolated perfused rat liver. Dokl Biochem Biophys 464, 298–300. [DOI] [PubMed] [Google Scholar]
- 18.do Nascimento GS et al. (2018) The acute effects of citrus flavanones on the metabolism of glycogen and monosaccharides in the isolated perfused rat liver. Toxicol Lett 291, 158–172. [DOI] [PubMed] [Google Scholar]
- 19.de Medeiros HC et al. (2015) Effect of fipronil on energy metabolism in the perfused rat liver. Toxicol Lett 236 (1), 34–42. [DOI] [PubMed] [Google Scholar]
- 20.Colturato CP et al. (2012) Metabolic effects of silibinin in the rat liver. Chem Biol Interact 195 (2), 119–32. [DOI] [PubMed] [Google Scholar]
- 21.Cohen JJ et al. (1980) Relation of Na+ reabsorption to utilization of O2 and lactate in the perfused rat kidney. Am J Physiol 238 (5), F415–27. [DOI] [PubMed] [Google Scholar]
- 22.Silva P. et al. (1982) Relationship among gluconeogenesis, QO2, and Na+ transport in the perfused rat kidney. Am J Physiol 242 (5), F508–13. [DOI] [PubMed] [Google Scholar]
- 23.From AH et al. (1986) 31P-NMR studies of respiratory regulation in the intact myocardium. FEBS Lett 206 (2), 257–61. [DOI] [PubMed] [Google Scholar]
- 24.From AH et al. (1990) Regulation of the oxidative phosphorylation rate in the intact cell. Biochemistry 29 (15), 3731–43. [DOI] [PubMed] [Google Scholar]
- 25.Katz LA et al. (1989) Relation between phosphate metabolites and oxygen consumption of heart in vivo. Am J Physiol 256 (1 Pt 2), H265–74. [DOI] [PubMed] [Google Scholar]
- 26.Portman MA et al. (1989) Developmental changes in the relation between phosphate metabolites and oxygen consumption in the sheep heart in vivo. J Clin Invest 83 (2), 456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J. et al. (1995) Transmural bioenergetic responses of normal myocardium to high workstates. Am J Physiol 268 (5 Pt 2), H1891–905. [DOI] [PubMed] [Google Scholar]
- 28.Elliott AC et al. (1994) The metabolic consequences of an increase in the frequency of stimulation in isolated ferret hearts. J Physiol 474 (1), 147–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matthews PM et al. (1981) The steady-state rate of ATP synthesis in the perfused rat heart measured by 31P NMR saturation transfer. Biochem Biophys Res Commun 103 (3), 1052–9. [DOI] [PubMed] [Google Scholar]
- 30.Massie BM et al. (1994) Myocardial metabolism during increased work states in the porcine left ventricle in vivo. Circ Res 74 (1), 64–73. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz GG et al. (1994) Relation among regional O2 consumption, high-energy phosphates, and substrate uptake in porcine right ventricle. Am J Physiol 266 (2 Pt 2), H521–30. [DOI] [PubMed] [Google Scholar]
- 32.Xu Y. et al. (1998) beta-adrenergic stimulation induces transient imbalance between myocardial substrate uptake and metabolism in vivo. Am J Physiol 275 (6), H2181–90. [DOI] [PubMed] [Google Scholar]
- 33.Hoppeler H. et al. (1987) Relationship between mitochondria and oxygen consumption in isolated cat muscles. J Physiol 385, 661–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrara PJ et al. (2018) Hypothermia Decreases O2 Cost for Ex Vivo Contraction in Mouse Skeletal Muscle. Med Sci Sports Exerc 50 (10), 2015–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McAllister RM et al. (1990) Impact of reduced cytochrome oxidase activity on peak oxygen consumption of muscle. J Appl Physiol (1985) 69 (1), 384–9. [DOI] [PubMed] [Google Scholar]
- 36.McAllister RM and Terjung RL (1990) Acute inhibition of respiratory capacity of muscle reduces peak oxygen consumption. Am J Physiol 259 (6 Pt 1), C889–96. [DOI] [PubMed] [Google Scholar]
- 37.Denton RM et al. (1972) Stimulation by calcium ions of pyruvate dehydrogenase phosphate phosphatase. Biochem J 128 (1), 161–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCormack JG and Denton RM (1979) The effects of calcium ions and adenine nucleotides on the activity of pig heart 2-oxoglutarate dehydrogenase complex. Biochem J 180 (3), 533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brandes R. and Bers DM (1996) Increased work in cardiac trabeculae causes decreased mitochondrial NADH fluorescence followed by slow recovery. Biophys J 71 (2), 1024–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maack C. et al. (2006) Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circ Res 99 (2), 172–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glancy B. and Balaban RS (2012) Role of mitochondrial Ca2+ in the regulation of cellular energetics. Biochemistry 51 (14), 2959–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Benedetto G. et al. (2013) Mitochondrial Ca(2)(+) uptake induces cyclic AMP generation in the matrix and modulates organelle ATP levels. Cell Metab 17 (6), 965–75. [DOI] [PubMed] [Google Scholar]
- 43.Burdyga A. et al. (2018) Phosphatases control PKA-dependent functional microdomains at the outer mitochondrial membrane. Proc Natl Acad Sci U S A 115 (28), E6497–E6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lefkimmiatis K. et al. (2013) The inner and outer compartments of mitochondria are sites of distinct cAMP/PKA signaling dynamics. J Cell Biol 202 (3), 453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Covian R. et al. (2014) Stimulation of oxidative phosphorylation by calcium in cardiac mitochondria is not influenced by cAMP and PKA activity. Biochim Biophys Acta 1837 (12), 1913–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams GS et al. (2013) Mitochondrial calcium uptake. Proc Natl Acad Sci U S A 110 (26), 10479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wescott AP et al. (2016) Ryanodine receptor sensitivity governs the stability and synchrony of local calcium release during cardiac excitation-contraction coupling. J Mol Cell Cardiol 92, 82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palty R. et al. (2010) NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc Natl Acad Sci U S A 107 (1), 436–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boyman L. et al. (2013) NCLX: The mitochondrial sodium calcium exchanger. J Mol Cell Cardiol 59, 205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boyman L. et al. (2014) Calcium movement in cardiac mitochondria. Biophys J 107 (6), 1289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kwong JQ et al. (2015) The Mitochondrial Calcium Uniporter Selectively Matches Metabolic Output to Acute Contractile Stress in the Heart. Cell Rep 12 (1), 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kwong JQ et al. (2018) The mitochondrial calcium uniporter underlies metabolic fuel preference in skeletal muscle. JCI Insight 3 (22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu T. and O’Rourke B. (2009) Regulation of mitochondrial Ca(2+) and its effects on energetics and redox balance in normal and failing heart. J.Bioenerg.Biomembr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Y. and O’Rourke B. (2001) Opening of mitochondrial K(ATP) channels triggers cardioprotection. Are reactive oxygen species involved? Circulation Research 88 (8), 750–752. [DOI] [PubMed] [Google Scholar]
- 55.Kohlhaas M. and Maack C. (2010) Adverse bioenergetic consequences of Na+-Ca2+ exchanger-mediated Ca2+ influx in cardiac myocytes. Circulation 122 (22), 2273–80. [DOI] [PubMed] [Google Scholar]
- 56.Bertero E. and Maack C. (2018) Metabolic remodelling in heart failure. Nat Rev Cardiol 15 (8), 457–470. [DOI] [PubMed] [Google Scholar]
- 57.Bertero E. and Maack C. (2018) Calcium Signaling and Reactive Oxygen Species in Mitochondria. Circ Res 122 (10), 1460–1478. [DOI] [PubMed] [Google Scholar]
- 58.Michels G. et al. (2009) Regulation of the human cardiac mitochondrial Ca2+ uptake by 2 different voltage-gated Ca2+ channels. Circulation 119 (18), 2435–43. [DOI] [PubMed] [Google Scholar]
- 59.Kirichok Y. et al. (2004) The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427 (6972), 360–4. [DOI] [PubMed] [Google Scholar]
- 60.Fieni F. et al. (2012) Activity of the mitochondrial calcium uniporter varies greatly between tissues. Nat Commun 3, 1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bernardi P. et al. (1992) Modulation of the mitochondrial permeability transition pore. Effect of protons and divalent cations. J Biol Chem 267 (5), 2934–9. [PubMed] [Google Scholar]
- 62.Halestrap AP (2010) A pore way to die: the role of mitochondria in reperfusion injury and cardioprotection. Biochem Soc Trans 38 (4), 841–60. [DOI] [PubMed] [Google Scholar]
- 63.Briston T. et al. (2019) Mitochondrial Permeability Transition: A Molecular Lesion with Multiple Drug Targets. Trends Pharmacol Sci 40 (1), 50–70. [DOI] [PubMed] [Google Scholar]
- 64.Carraro M. et al. (2019) F-ATP synthase and the permeability transition pore: fewer doubts, more certainties. FEBS Lett 593 (13), 1542–1553. [DOI] [PubMed] [Google Scholar]
- 65.Boyman L. et al. (2019) Dynamics of the mitochondrial permeability transition pore: Transient and permanent opening events. Arch Biochem Biophys 666, 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Halestrap AP (2014) The C Ring of the F1Fo ATP Synthase Forms the Mitochondrial Permeability Transition Pore: A Critical Appraisal. Front Oncol 4, 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karch J. and Molkentin JD (2014) Identifying the components of the elusive mitochondrial permeability transition pore. Proc Natl Acad Sci U S A 111 (29), 10396–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.He J. et al. (2017) Permeability transition in human mitochondria persists in the absence of peripheral stalk subunits of ATP synthase. Proc Natl Acad Sci U S A 114 (34), 9086–9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baughman JM et al. (2011) Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476 (7360), 341–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Stefani D. et al. (2011) A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476 (7360), 336–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Holmstrom KM et al. (2015) Assessment of cardiac function in mice lacking the mitochondrial calcium uniporter. J Mol Cell Cardiol 85, 178–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pan X. et al. (2013) The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat Cell Biol 15 (12), 1464–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Luongo TS et al. (2015) The Mitochondrial Calcium Uniporter Matches Energetic Supply with Cardiac Workload during Stress and Modulates Permeability Transition. Cell Rep 12 (1), 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rasmussen TP et al. (2015) Inhibition of MCU forces extramitochondrial adaptations governing physiological and pathological stress responses in heart. Proc Natl Acad Sci U S A 112 (29), 9129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu Y. et al. (2015) The mitochondrial uniporter controls fight or flight heart rate increases. Nat Commun 6, 6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Murphy E. et al. (2014) Unresolved questions from the analysis of mice lacking MCU expression. Biochem Biophys Res Commun 449 (4), 384–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pallafacchina G. et al. (2018) Recent advances in the molecular mechanism of mitochondrial calcium uptake. F1000Res 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nemani N. et al. (2018) Molecular regulation of MCU: Implications in physiology and disease. Cell Calcium 74, 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kamer KJ and Mootha VK (2015) The molecular era of the mitochondrial calcium uniporter. Nat Rev Mol Cell Biol 16 (9), 545–53. [DOI] [PubMed] [Google Scholar]
- 80.Bick AG et al. (2012) Evolutionary diversity of the mitochondrial calcium uniporter. Science 336 (6083), 886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vecellio Reane D. et al. (2016) A MICU1 Splice Variant Confers High Sensitivity to the Mitochondrial Ca2+ Uptake Machinery of Skeletal Muscle. Mol Cell 64 (4), 760–773. [DOI] [PubMed] [Google Scholar]
- 82.Liu JC et al. (2016) MICU1 Serves as a Molecular Gatekeeper to Prevent In Vivo Mitochondrial Calcium Overload. Cell Rep 16 (6), 1561–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Antony AN et al. (2016) MICU1 regulation of mitochondrial Ca(2+) uptake dictates survival and tissue regeneration. Nat Commun 7, 10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bick AG et al. (2017) Cardiovascular homeostasis dependence on MICU2, a regulatory subunit of the mitochondrial calcium uniporter. Proc Natl Acad Sci U S A 114 (43), E9096–E9104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tufi R. et al. (2019) Comprehensive Genetic Characterization of Mitochondrial Ca(2+) Uniporter Components Reveals Their Different Physiological Requirements In Vivo. Cell Rep 27 (5), 1541–1550 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Williams GS et al. (2015) Mitochondrial calcium and the regulation of metabolism in the heart. J Mol Cell Cardiol 78c, 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Karbowski M. and Youle RJ. (2011) Regulating mitochondrial outer membrane proteins by ubiquitination and proteasomal degradation. Curr Opin Cell Biol 23 (4), 476–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Brito OM and Scorrano L. (2008) Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456 (7222), 605–10. [DOI] [PubMed] [Google Scholar]
- 89.Hirabayashi Y et al. (2017) ER-mitochondria tethering by PDZD8 regulates Ca(2+) dynamics in mammalian neurons. Science 358 (6363), 623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sugiura A. et al. (2013) MITOL regulates endoplasmic reticulum-mitochondria contacts via Mitofusin2. Mol Cell 51 (1), 20–34. [DOI] [PubMed] [Google Scholar]
- 91.Filadi R. et al. (2015) Mitofusin 2 ablation increases endoplasmic reticulum-mitochondria coupling. Proc Natl Acad Sci U S A 112 (17), E2174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Filadi R. et al. (2017) On the role of Mitofusin 2 in endoplasmic reticulum-mitochondria tethering. Proc Natl Acad Sci U S A 114 (12), E2266–E2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Leal NS et al. (2016) Mitofusin-2 knockdown increases ER-mitochondria contact and decreases amyloid beta-peptide production. J Cell Mol Med 20 (9), 1686–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen W. et al. (2014) The ryanodine receptor store-sensing gate controls Ca2+ waves and Ca2+-triggered arrhythmias. Nat Med 20 (2), 184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ather S. et al. (2013) Inhibition of CaMKII phosphorylation of RyR2 prevents inducible ventricular arrhythmias in mice with Duchenne muscular dystrophy. Heart Rhythm 10 (4), 592–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Niggli E. and Lederer WJ (1990) Voltage-independent calcium release in heart muscle. Science 250 (4980), 565–568. [DOI] [PubMed] [Google Scholar]
- 97.Cheng H. et al. (1993) Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science 262 (5134), 740–744. [DOI] [PubMed] [Google Scholar]
- 98.Brochet DX et al. (2005) Ca2+ blinks: rapid nanoscopic store calcium signaling. Proc Natl Acad Sci U S A 102 (8), 3099–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cannell MB et al. (1994) Spatial non-uniformities in [Ca2+]i during excitation-contraction coupling in cardiac myocytes. Biophys J 67 (5), 1942–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Franzini-Armstrong C. et al. (1999) Shape, size, and distribution of Ca 2+ release units and couplons in skeletal and cardiac muscles. Biophys.J 77 (3), 1528–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Walker MA et al. (2014) Superresolution modeling of calcium release in the heart. Biophys J 107 (12), 3018–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chikando AC et al. (2011) Ca2+ dynamics in the mitochondria - state of the art. J Mol Cell Cardiol 51 (5), 627–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Santana LF et al. (1996) Relation between the sarcolemmal Ca2+ current and Ca2+ sparks and local control theories for cardiac excitation-contraction coupling. Circulation Research 78 (1), 166–171. [DOI] [PubMed] [Google Scholar]
- 104.Cannell MB and Soeller C. (1997) Numerical analysis of ryanodine receptor activation by L-type channel activity in the cardiac muscle diad. Biophysical Journal 73 (1), 112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Soeller C. and Cannell MB (1997) Numerical simulation of local calcium movements during L-type calcium channel gating in the cardiac diad. Biophysical Journal 73 (1), 97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Marx SO et al. (2000) PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101 (4), 365–76. [DOI] [PubMed] [Google Scholar]
- 107.Anderson ME (2015) Oxidant stress promotes disease by activating CaMKII. J Mol Cell Cardiol 89 (Pt B), 160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ward CW et al. (2014) Mechanical Stretch-Induced Activation of ROS/RNS Signaling in Striated Muscle. Antioxid Redox Signal 20 (6), 929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Prosser BL et al. (2013) X-ROS signalling is enhanced and graded by cyclic cardiomyocyte stretch. Cardiovasc Res 98 (2), 307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Prosser BL et al. (2013) X-ROS signaling in the heart and skeletal muscle: Stretch-dependent local ROS regulates [Ca2+]i. J Mol Cell Cardiol 58, 172–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Prosser BL et al. (2011) X-ROS signaling: rapid mechano-chemo transduction in heart. Science 333 (6048), 1440–5. [DOI] [PubMed] [Google Scholar]
- 112.Csordas G. et al. (2018) Endoplasmic Reticulum-Mitochondrial Contactology: Structure and Signaling Functions. Trends Cell Biol 28 (7), 523–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Picard M. et al. (2015) Trans-mitochondrial coordination of cristae at regulated membrane junctions. Nat Commun 6, 6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen Y. et al. (2012) Mitofusin 2-containing mitochondrial-reticular microdomains direct rapid cardiomyocyte bioenergetic responses via interorganelle Ca(2+) crosstalk. Circ Res 111 (7), 863–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Seidlmayer LK et al. (2019) Mitofusin 2 Is Essential for IP3-Mediated SR/Mitochondria Metabolic Feedback in Ventricular Myocytes. Front Physiol 10, 733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Flicker D. et al. (2019) Exploring the In Vivo Role of the Mitochondrial Calcium Uniporter in Brown Fat Bioenergetics. Cell Rep 27 (5), 1364–1375 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wu X. and Bers DM (2006) Sarcoplasmic reticulum and nuclear envelope are one highly interconnected Ca2+ store throughout cardiac myocyte. Circ Res 99 (3), 283–91. [DOI] [PubMed] [Google Scholar]
- 118.Fieni F. et al. (2014) Mitochondrial Ca2+ uniporter and CaMKII in heart. Nature 513 (7519), E1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang K. et al. (2018) Mitochondria-associated endoplasmic reticulum membranes (MAMs) involve in the regulation of mitochondrial dysfunction and heart failure. Acta Biochim Biophys Sin (Shanghai) 50 (6), 618–619. [DOI] [PubMed] [Google Scholar]
- 120.Hall AR et al. (2016) Hearts deficient in both Mfn1 and Mfn2 are protected against acute myocardial infarction. Cell Death Dis 7, e2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Papanicolaou KN et al. (2011) Mitofusin-2 maintains mitochondrial structure and contributes to stress-induced permeability transition in cardiac myocytes. Mol Cell Biol 31 (6), 1309–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.De la Fuente S. and Sheu SS (2019) SR-mitochondria communication in adult cardiomyocytes: A close relationship where the Ca(2+) has a lot to say. Arch Biochem Biophys 663, 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dorn GW 2nd, et al. (2015) Functional implications of mitofusin 2-mediated mitochondrial-SR tethering. J Mol Cell Cardiol 78, 123–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu S. et al. (2017) Binding of FUN14 Domain Containing 1 With Inositol 1,4,5-Trisphosphate Receptor in Mitochondria-Associated Endoplasmic Reticulum Membranes Maintains Mitochondrial Dynamics and Function in Hearts in Vivo. Circulation 136 (23), 2248–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu ZW et al. (2013) Protein kinase RNA-like endoplasmic reticulum kinase (PERK) signaling pathway plays a major role in reactive oxygen species (ROS)-mediated endoplasmic reticulum stress-induced apoptosis in diabetic cardiomyopathy. Cardiovasc Diabetol 12, 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wu S. et al. (2019) Hyperglycemia-Driven Inhibition of AMP-Activated Protein Kinase alpha2 Induces Diabetic Cardiomyopathy by Promoting Mitochondria-Associated Endoplasmic Reticulum Membranes In Vivo. Circulation 139 (16), 1913–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Janikiewicz J. et al. (2018) Mitochondria-associated membranes in aging and senescence: structure, function, and dynamics. Cell Death Dis 9 (3), 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Friedman JR et al. (2011) ER tubules mark sites of mitochondrial division. Science 334 (6054), 358–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chakrabarti R. et al. (2018) INF2-mediated actin polymerization at the ER stimulates mitochondrial calcium uptake, inner membrane constriction, and division. J Cell Biol 217 (1), 251–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bonomini F. et al. (2015) Metabolic syndrome, aging and involvement of oxidative stress. Aging Dis 6 (2), 109–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Eiyama A. and Okamoto K. (2015) PINK1/Parkin-mediated mitophagy in mammalian cells. Curr Opin Cell Biol 33, 95–101. [DOI] [PubMed] [Google Scholar]
- 132.Friedman JR and Nunnari J. (2014) Mitochondrial form and function. Nature 505 (7483), 335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Youle RJ and van der Bliek AM (2012) Mitochondrial fission, fusion, and stress. Science 337 (6098), 1062–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cho HM et al. (2019) Drp1-Zip1 Interaction Regulates Mitochondrial Quality Surveillance System. Mol Cell 73 (2), 364–376 e8. [DOI] [PubMed] [Google Scholar]
- 135.Twig G. et al. (2008) Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 27 (2), 433–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pinton P. et al. (2001) The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. Embo j 20 (11), 2690–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Smirnova E. et al. (2001) Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell 12 (8), 2245–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cereghetti GM et al. (2008) Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci U S A 105 (41), 15803–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Korobova F. et al. (2013) An actin-dependent step in mitochondrial fission mediated by the ER-associated formin INF2. Science 339 (6118), 464–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Matteucci A. et al. (2018) Parkin-dependent regulation of the MCU complex component MICU1. Sci Rep 8 (1), 14199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Billia F. et al. (2011) PTEN-inducible kinase 1 (PINK1)/Park6 is indispensable for normal heart function. Proc Natl Acad Sci U S A 108 (23), 9572–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gautier CA et al. (2008) Loss of PINK1 causes mitochondrial functional defects and increased sensitivity to oxidative stress. Proc Natl Acad Sci U S A 105 (32), 11364–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Heeman B. et al. (2011) Depletion of PINK1 affects mitochondrial metabolism, calcium homeostasis and energy maintenance. J Cell Sci 124 (Pt 7), 1115–25. [DOI] [PubMed] [Google Scholar]
- 144.Jiang D. et al. (2009) Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science 326 (5949), 144–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Huang E. et al. (2017) PINK1-mediated phosphorylation of LETM1 regulates mitochondrial calcium transport and protects neurons against mitochondrial stress. Nat Commun 8 (1), 1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kostic M. et al. (2015) PKA Phosphorylation of NCLX Reverses Mitochondrial Calcium Overload and Depolarization, Promoting Survival of PINK1-Deficient Dopaminergic Neurons. Cell Rep 13 (2), 376–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Huang X. et al. (2013) Kissing and nanotunneling mediate intermitochondrial communication in the heart. Proc Natl Acad Sci U S A 110 (8), 2846–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Eisner V. et al. (2017) Mitochondrial fusion dynamics is robust in the heart and depends on calcium oscillations and contractile activity. Proc Natl Acad Sci U S A 114 (5), E859–e868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chen H. et al. (2015) Titration of mitochondrial fusion rescues Mff-deficient cardiomyopathy. J Cell Biol 211 (4), 795–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cherok E. et al. (2017) Novel regulatory roles of Mff and Drp1 in E3 ubiquitin ligase MARCH5-dependent degradation of MiD49 and Mcl1 and control of mitochondrial dynamics. Mol Biol Cell 28 (3), 396–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ikeda Y. et al. (2015) Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ Res 116 (2), 264–78. [DOI] [PubMed] [Google Scholar]
- 152.Nemani N. et al. (2018) MIRO-1 Determines Mitochondrial Shape Transition upon GPCR Activation and Ca(2+) Stress. Cell Rep 23 (4), 1005–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cao Y. et al. (2019) Miro2 Regulates Inter-Mitochondrial Communication in the Heart and Protects Against TAC-Induced Cardiac Dysfunction. Circ Res 125 (8), 728–743. [DOI] [PubMed] [Google Scholar]
- 154.Zuchner S. et al. (2004) Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat Genet 36 (5), 449–451. [DOI] [PubMed] [Google Scholar]
- 155.Alexander C. et al. (2000) OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet 26 (2), 211–5. [DOI] [PubMed] [Google Scholar]
- 156.Zanna C. et al. (2008) OPA1 mutations associated with dominant optic atrophy impair oxidative phosphorylation and mitochondrial fusion. Brain 131 (Pt 2), 352–367. [DOI] [PubMed] [Google Scholar]
- 157.Dodson MW and Guo M. (2007) Pink1, Parkin, DJ-1 and mitochondrial dysfunction in Parkinson’s disease. Curr Opin Neurobiol 17 (3), 331–7. [DOI] [PubMed] [Google Scholar]
- 158.Poole AC et al. (2008) The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci U S A 105 (5), 1638–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Narendra D. et al. (2008) Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 183 (5), 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Canet-Aviles RM et al. (2004) The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci U S A 101 (24), 9103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang L. et al. (2005) Mitochondrial localization of the Parkinson’s disease related protein DJ-1: implications for pathogenesis. Hum Mol Genet 14 (14), 2063–73. [DOI] [PubMed] [Google Scholar]
- 162.Benatar M. et al. (2013) Motor neuron involvement in multisystem proteinopathy: implications for ALS. Neurology 80 (20), 1874–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gonzalez MA et al. (2014) A novel mutation in VCP causes Charcot-Marie-Tooth Type 2 disease. Brain 137 (Pt 11), 2897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Johnson JO et al. (2010) Exome sequencing reveals VCP mutations as a cause of familial ALS. Neuron 68 (5), 857–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Xu S. et al. (2011) The AAA-ATPase p97 is essential for outer mitochondrial membrane protein turnover. Mol Biol Cell 22 (3), 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tanaka A. et al. (2010) Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J Cell Biol 191 (7), 1367–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hasson SA et al. (2013) High-content genome-wide RNAi screens identify regulators of parkin upstream of mitophagy. Nature 504 (7479), 291–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lazarou M. et al. (2015) The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 524 (7565), 309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ordureau A. et al. (2015) Defining roles of PARKIN and ubiquitin phosphorylation by PINK1 in mitochondrial quality control using a ubiquitin replacement strategy. Proc Natl Acad Sci U S A 112 (21), 6637–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bingol B. et al. (2014) The mitochondrial deubiquitinase USP30 opposes parkin-mediated mitophagy. Nature 510 (7505), 370–5. [DOI] [PubMed] [Google Scholar]
- 171.Liang JR et al. (2015) USP30 deubiquitylates mitochondrial Parkin substrates and restricts apoptotic cell death. EMBO Rep 16 (5), 618–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Thobois S. (2015) USP30: a new promising target for Parkinson’s disease? Mov Disord 30 (3), 340. [DOI] [PubMed] [Google Scholar]
- 173.Cornelissen T. et al. (2014) The deubiquitinase USP15 antagonizes Parkin-mediated mitochondrial ubiquitination and mitophagy. Hum Mol Genet 23 (19), 5227–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yun J. et al. (2014) MUL1 acts in parallel to the PINK1/parkin pathway in regulating mitofusin and compensates for loss of PINK1/parkin. Elife 3, e01958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chen Y. and Dorn GW 2nd (2013) PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science 340 (6131), 471–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kageyama Y. et al. (2014) Parkin-independent mitophagy requires Drp1 and maintains the integrity of mammalian heart and brain. Embo j 33 (23), 2798–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Twig G. and Shirihai OS (2011) The interplay between mitochondrial dynamics and mitophagy. Antioxid Redox Signal 14 (10), 1939–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Narendra DP et al. (2010) PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol 8 (1), e1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jadiya P. et al. (2019) Impaired mitochondrial calcium efflux contributes to disease progression in models of Alzheimer’s disease. Nat Commun 10 (1), 3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Parone PA et al. (2008) Preventing mitochondrial fission impairs mitochondrial function and leads to loss of mitochondrial DNA. PLoS One 3 (9), e3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wakabayashi J. et al. (2009) The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J Cell Biol 186 (6), 805–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Burman JL et al. (2017) Mitochondrial fission facilitates the selective mitophagy of protein aggregates. J Cell Biol 216 (10), 3231–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Otera H. et al. (2010) Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J Cell Biol 191 (6), 1141–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Palmer CS et al. (2011) MiD49 and MiD51, new components of the mitochondrial fission machinery. EMBO Rep 12 (6), 565–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Xu S. et al. (2016) Mitochondrial E3 ubiquitin ligase MARCH5 controls mitochondrial fission and cell sensitivity to stress-induced apoptosis through regulation of MiD49 protein. Mol Biol Cell 27 (2), 349–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Shen Q. et al. (2014) Mutations in Fis1 disrupt orderly disposal of defective mitochondria. Mol Biol Cell 25 (1), 145–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Anand R. et al. (2014) The i-AAA protease YME1L and OMA1 cleave OPA1 to balance mitochondrial fusion and fission. J Cell Biol 204 (6), 919–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.MacVicar T. and Langer T. (2016) OPA1 processing in cell death and disease - the long and short of it. J Cell Sci 129 (12), 2297–306. [DOI] [PubMed] [Google Scholar]
- 189.Chen H. et al. (2003) Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 160 (2), 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chen H. et al. (2005) Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem 280 (28), 26185–92. [DOI] [PubMed] [Google Scholar]
- 191.Misko A. et al. (2010) Mitofusin 2 is necessary for transport of axonal mitochondria and interacts with the Miro/Milton complex. J Neurosci 30 (12), 4232–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Koyano F. et al. (2014) Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature 510 (7503), 162–6. [DOI] [PubMed] [Google Scholar]
- 193.Kane LA et al. (2014) PINK1 phosphorylates ubiquitin to activate Parkin E3 ubiquitin ligase activity. J Cell Biol 205 (2), 143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Karbowski M. et al. (2007) The mitochondrial E3 ubiquitin ligase MARCH5 is required for Drp1 dependent mitochondrial division. J Cell Biol 178 (1), 71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yonashiro R. et al. (2006) A novel mitochondrial ubiquitin ligase plays a critical role in mitochondrial dynamics. EMBO J 25 (15), 3618–3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sugiura A. et al. (2011) A mitochondrial ubiquitin ligase MITOL controls cell toxicity of polyglutamine-expanded protein. Mitochondrion 11 (1), 139–46. [DOI] [PubMed] [Google Scholar]
- 197.Koyano F. et al. (2019) Parkin recruitment to impaired mitochondria for nonselective ubiquitylation is facilitated by MITOL. J Biol Chem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Chen Z. et al. (2017) Mitochondrial E3 ligase MARCH5 regulates FUNDC1 to fine-tune hypoxic mitophagy. EMBO Rep 18 (3), 495–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bartolome F. et al. (2013) Pathogenic VCP mutations induce mitochondrial uncoupling and reduced ATP levels. Neuron 78 (1), 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Song M. et al. (2017) Abrogating Mitochondrial Dynamics in Mouse Hearts Accelerates Mitochondrial Senescence. Cell Metab 26 (6), 872–883 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Waterham HR et al. (2007) A lethal defect of mitochondrial and peroxisomal fission. N Engl J Med 356 (17), 1736–1741. [DOI] [PubMed] [Google Scholar]
- 202.Wang X. et al. (2009) Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J Neurosci 29 (28), 9090–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cha MY et al. (2018) Removal of the Mitochondrial Fission Factor Mff Exacerbates Neuronal Loss and Neurological Phenotypes in a Huntington’s Disease Mouse Model. PLoS Curr 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Koch J. et al. (2016) Disturbed mitochondrial and peroxisomal dynamics due to loss of MFF causes Leigh-like encephalopathy, optic atrophy and peripheral neuropathy. J Med Genet 53 (4), 270–8. [DOI] [PubMed] [Google Scholar]
- 205.Joshi AU et al. (2018) Drp1/Fis1 interaction mediates mitochondrial dysfunction, bioenergetic failure and cognitive decline in Alzheimer’s disease. Oncotarget 9 (5), 6128–6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Chen L. et al. (2012) OPA1 mutation and late-onset cardiomyopathy: mitochondrial dysfunction and mtDNA instability. J Am Heart Assoc 1 (5), e003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chen L. et al. (2009) Mitochondrial OPA1, apoptosis, and heart failure. Cardiovasc Res 84 (1), 91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Perez FA and Palmiter RD (2005) Parkin-deficient mice are not a robust model of parkinsonism. Proc Natl Acad Sci U S A 102 (6), 2174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Pinto M. et al. (2018) Lack of Parkin Anticipates the Phenotype and Affects Mitochondrial Morphology and mtDNA Levels in a Mouse Model of Parkinson’s Disease. J Neurosci 38 (4), 1042–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Song M. et al. (2015) Interdependence of Parkin-Mediated Mitophagy and Mitochondrial Fission in Adult Mouse Hearts. Circ Res 117 (4), 346–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Moisoi N. et al. (2014) Loss of PINK1 enhances neurodegeneration in a mouse model of Parkinson’s disease triggered by mitochondrial stress. Neuropharmacology 77, 350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kelm-Nelson CA et al. (2018) Characterization of early-onset motor deficits in the Pink1−/− mouse model of Parkinson disease. Brain Res 1680, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Nagashima S. et al. (2019) MITOL deletion in the brain impairs mitochondrial structure and ER tethering leading to oxidative stress. Life Sci Alliance 2 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Muller JM et al. (2007) Targeted deletion of p97 (VCP/CDC48) in mouse results in early embryonic lethality. Biochem Biophys Res Commun 354 (2), 459–65. [DOI] [PubMed] [Google Scholar]
- 215.Wang Z. et al. (2010) Specific metabolic rates of major organs and tissues across adulthood: evaluation by mechanistic model of resting energy expenditure. Am J Clin Nutr 92 (6), 1369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]