Abstract
Background
SARS-CoV-2 aerosolization during noninvasive positive-pressure ventilation may endanger health care professionals. Various circuit setups have been described to reduce virus aerosolization. However, these setups may alter ventilator performance.
Research Question
What are the consequences of the various suggested circuit setups on ventilator efficacy during CPAP and noninvasive ventilation (NIV)?
Study Design and Methods
Eight circuit setups were evaluated on a bench test model that consisted of a three-dimensional printed head and an artificial lung. Setups included a dual-limb circuit with an oronasal mask, a dual-limb circuit with a helmet interface, a single-limb circuit with a passive exhalation valve, three single-limb circuits with custom-made additional leaks, and two single-limb circuits with active exhalation valves. All setups were evaluated during NIV and CPAP. The following variables were recorded: the inspiratory flow preceding triggering of the ventilator, the inspiratory effort required to trigger the ventilator, the triggering delay, the maximal inspiratory pressure delivered by the ventilator, the tidal volume generated to the artificial lung, the total work of breathing, and the pressure-time product needed to trigger the ventilator.
Results
With NIV, the type of circuit setup had a significant impact on inspiratory flow preceding triggering of the ventilator (P < .0001), the inspiratory effort required to trigger the ventilator (P < .0001), the triggering delay (P < .0001), the maximal inspiratory pressure (P < .0001), the tidal volume (P = .0008), the work of breathing (P < .0001), and the pressure-time product needed to trigger the ventilator (P < .0001). Similar differences and consequences were seen with CPAP as well as with the addition of bacterial filters. Best performance was achieved with a dual-limb circuit with an oronasal mask. Worst performance was achieved with a dual-limb circuit with a helmet interface.
Interpretation
Ventilator performance is significantly impacted by the circuit setup. A dual-limb circuit with oronasal mask should be used preferentially.
Key Words: aerosolization, COVID-19, CPAP, filter, noninvasive ventilation
FOR EDITORIAL COMMENT, SEE PAGE 13
Patients with severe SARS-CoV-2 infection can receive respiratory support using high-flow nasal therapy, CPAP,1 or noninvasive ventilation (NIV).2 The use of these treatments is associated with virus aerosolization,3 which may endanger caregivers.4 , 5 For high-flow therapy, surgical masks worn by patients can limit aerosolization.6 , 7 During NIV or CPAP, surgical masks cannot be worn. NIV and CPAP are usually delivered to patients using an interface with a built-in intentional leak to avoid CO2 rebreathing. Various strategies have been suggested by experts to minimize the risk of aerosolization.5 , 8 For CPAP and NIV, the use of nonvented masks with the addition of another bacterial filter on the circuit has been suggested9 to limit aerosolization during expiration. These changes have been implemented using various circuit setups.
If reducing the risk of aerosolization is a priority in the treatment of patients with SARS-CoV-2 infection, we still need to deliver the best care possible to patients. The addition of a second bacterial filter in an NIV/CPAP circuit is not the standard of care except when dual-limb circuits are used. The addition of these filters may impact on the resistance of the circuit and increase the patient’s work of breathing. It may also alter ventilator performance and generate patient-ventilator asynchrony, which have a deleterious impact on acute respiratory failure.10 , 11
Our hypothesis was that the use of these modifications on NIV/CPAP circuits altered ventilator performance. Our aim was to assess the consequences of the various suggested circuit setups for the treatment of SARS-CoV-2-infected patients on ventilator efficacy during CPAP and NIV.
Study Design and Methods
Experimental Model
We used a three-dimensional (3D) printed head mimicking human upper airways and trachea (e-Fig 1). The 3D printed head was designed with ZBrush 2019 (Pixologic) by Phoenix Effect Studio. The model was then printed using 3D printers Pro2 and Pro2 Plus (Raise3D). The model had a dead space of 152 mL and a resistance of 2.4 cm H2O.
We applied a nonvented orobuccal mask (Quattro FX; ResMed) on the head and verified adequate fitting of the mask before each maneuver. We assessed one setup using a helmet interface. In that case, we used a NIV Zip Helmet mask (Dimar). Circuit setups were evaluated with heat and moisture exchange (HME) filters (Inter-Therm; Intersurgical), with low-resistance bacterial filters (Gibeck ISO-Gard; Teleflex), or without any filter. Eight circuit setups were evaluated during NIV and CPAP (Fig 1 ). Setup 4 used a 3D printed piece designed by M. P., E. F., and J. G.-B. for this purpose and freely available (e-Fig 2).12 With CPAP, we analyzed a ninth setup using Boussignac CPAP (Vygon). The Boussignac setup was assessed with only one circuit setup but with the two-filter configuration.
Figure 1.
Setups evaluated in the experiments. (1) Mask, filter, and right-angle connector in which a 4-mm hole has been made (courtesy of C. R.); (2) mask, filter, and a whisper swivel exhalation valve; (3) mask, T-connector, filter, and whisper swivel exhalation valve; (4) mask, 3D-printed piece with a 4-mm leak (provided courtesy of M. P., E. F., and J. G.-B., and shown in more detail in e-Fig 2; see KerNel Biomedical12 for availability), and bacterial filter; (5) mask, dual-limb circuit with filter on the inspiratory and expiratory circuits; (6) mask, active expiratory valve, and bacterial filter; (7) mask, filter, and active expiratory valve; (8) helmet interface, bacterial filter on the inspiratory and expiratory circuits; (9) Boussignac CPAP montage, bacterial filter between valve and mask.
The trachea was connected to an artificial lung (ASL-5000; Ingmar Medical). Respiratory effort was simulated, with a drop in airway pressure at 100 milliseconds (or P0.1) of 5 cm H2O and a breathing frequency of 30 breaths/min. This setting was chosen to match the respiratory mechanics seen during SARS-CoV-2 infections. The shape of the effort curve was a double exponential. This effort was combined with three different lung mechanics conditions, reflecting the pulmonary function of the simulated patients by modulating resistance (R) and compliance (C) parameters. We simulated a normal lung condition with R = 5 cm H2O/L × s and C = 60 mL/cm H2O, during 20 cycles according to measurements performed in patients with severe SARS-CoV-2 infection13; a restrictive lung condition with R = 5 cm H2O/L × s and C = 30 mL/cm H2O, during 15 cycles; and an obstructive lung condition with R = 25 cm H2O/L × s and C = 60 mL/cm H2O, during 15 cycles.
All experiments were conducted with an Astral 150 ventilator version 0601 (ResMed) without active humidification. Ventilator pretests were conducted before each experiment. The ventilator was set as follows: inspiratory pressure, 16 cm H2O; expiratory pressure, 8 cm H2O; inspiratory time window, 0.8 to 1.4 s; rise time, 100 milliseconds; trigger sensitivity, high; cycling, 50% of the peak inspiratory flow. With CPAP, the expiratory pressure was set at 8 cm H2O. These settings were chosen according to the clinical experience of the authors in the treatment of patients with severe SARS-CoV-2 infection. When CPAP was provided with Boussignac CPAP (Vygon), pressure was generated with O2 at 30 L/min.
Measurements
Measurements were performed on the basis of the flow and pressure curves provided by the artificial lung (e-Fig 3).
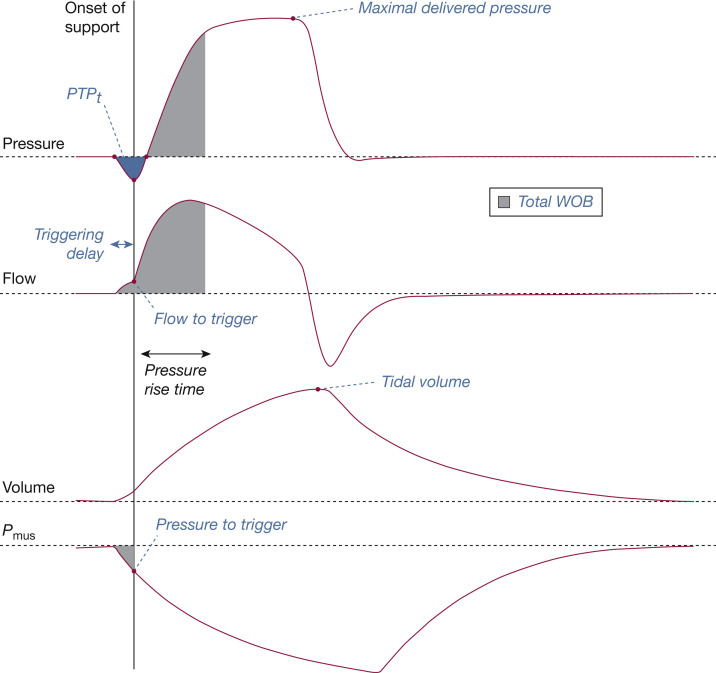
For each cycle labeled as synchronized during NIV, we computed seven indicators (Fig 2 ). We used four indicators to characterize inspiratory trigger. The indicators were as follows: triggering delay (ms), which measured the time lag between the beginning of simulated effort and the onset of pressure support; flow to trigger (L/min), defined as the value of patient flow measured at the onset of support pressure; pressure to trigger (cm H2O), defined as the value of muscular pressure (Pmus) measured at the onset of support pressure; and inspiratory pressure-time product (PTPt) (cm H2O × s), defined as the area under the pressure-time curve between the onset of inspiratory effort and the return to the set positive end-expiratory pressure as described.14 We used three indicators to characterize pressurization. The indicators were as follows: delivered inspiratory pressure (cm H2O), defined as the peak pressure reached during the inspiratory pressurization phase; tidal volume (Vt; mL), defined as the difference between the maximal volume delivered within the current cycle to the mechanical lung and the residual volume; and total work of breathing of the system (WOB) (mJ), defined as the sum of the patient WOB (integral of muscular pressure × flow product preceding the onset of ventilatory support) and ventilator WOB (integral of airway pressure × flow product between onset of support and instant when 95% of the inspiratory positive airway pressure level is reached during pressure rise time).
Figure 2.
Description of how ventilator performance was assessed during noninvasive ventilation. The onset of pressure support allows measurement of the triggering delay, the flow to trigger, and the pressure to trigger, and calculation of the inspiratory pressure-time product (PTPt). The maximal delivered pressure and the tidal volume were measured from the ASL-5000 airway pressure and piston volume. The total work of breathing (WOB) corresponds to the checkerboard area (combination of patient and ventilatory work). Pmus = muscular pressure.
For each cycle labeled as asynchronized during NIV, we characterized the simulated patient-ventilator asynchrony (sPVA) events according to the framework proposed by the SomnoNIV group.15 , 16 We distinguished rate asynchronies from intracycle asynchronies. Rate asynchronies were defined as a mismatch between ventilator and patient rates. We identified the following: ineffective efforts when an inspiratory effort was not assisted by the ventilator (ie, a drop in airway pressure associated with an increase or decrease in airflow occurring during the expiratory or inspiratory phase, respectively), double triggering when two mechanical cycles were triggered by the patient, separated by a very short expiratory time (< 30% of mean inspiratory time) and auto-triggering when mechanical cycles were unrelated to the patient’s spontaneous breathing. Rate intracycle asynchronies were defined as a distortion of the flow and pressure curves during inspiration and/or expiration. We identified premature cycling when the end of the mechanical insufflation preceded the end of the patient’s inspiration and delayed cycling mechanical insufflation exceeded the patient’s own neural expiration. Each asynchrony event was expressed as a percentage by dividing the number of asynchronous cycles by the total of simulated respiratory cycles.
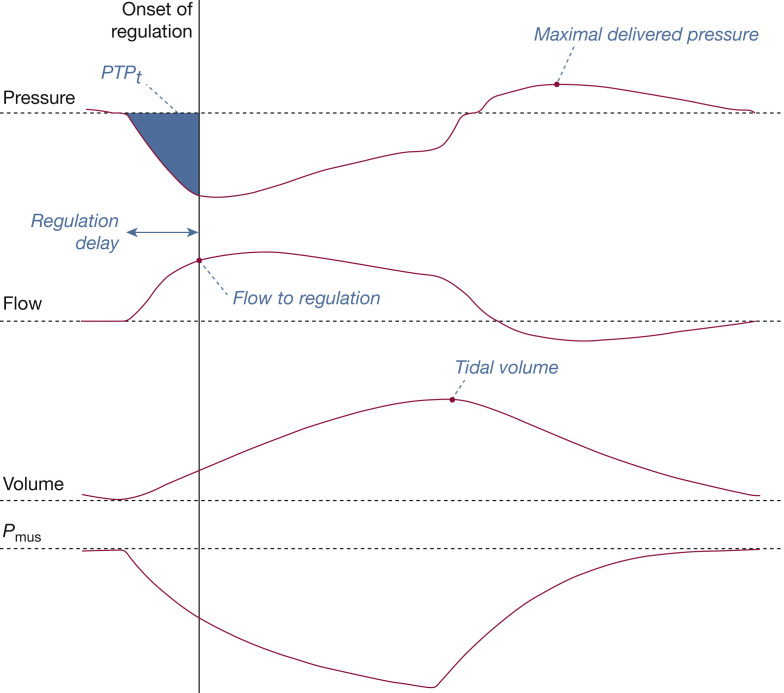
During CPAP, the depression generated by the patient’s inspiratory efforts is detected by the ventilator, which regulates the pressure delivered accordingly. For each breathing cycle, the following indicators were computed (Fig 3 ): regulation delay (ms), which measures the time lag between the start of simulated effort and the onset of pressure regulation; flow to regulation (L/min), corresponding to the flow preceding the ventilator pressurization response; PTPt (cm H2O × s), defined as the area under the pressure-time curve between the onset of inspiratory effort and the onset of pressure regulation; the maximal delivered pressure (cm H2O), defined as the peak pressure reached during the current cycle; and the tidal volume (mL), defined as the difference between the maximal volume delivered within the current cycle to the mechanical lung and the residual volume.
Figure 3.
Description of how ventilator performance was assessed during CPAP. The onset of pressure regulation allows measurement of the regulation delay and the flow to regulation, and calculation of the equivalent PTPt. The maximal delivered pressure and the tidal volume were measured from the ASL 5000 airway pressure and piston volume. Pmus = muscular pressure; PTPt = inspiratory pressure-time product.
Statistical Analysis
Results are expressed as median and interquartile range. χ2 tests were used to compare categorical variables. Kruskal-Wallis tests were used to compare continuous variables. Dunn’s correction was applied for multiple comparisons. When assessing the impact of a filter, we compared the absence of a filter with each filter type, as well as the low-resistance filter with the HME filter. When assessing the impact of circuits, setup 5 was used as reference. All tests were two-sided. For all tests, the significance level was set at .05. Statistical analysis was performed with Prism 9.0.0 (GraphPad Software).
Results
For each setup, 135 respiratory cycles were analyzed with NIV, and 150 with CPAP. Each experiment was conducted with three different filter configurations: no filter, low-pressure filter, or HME filter. In total, 2,430 respiratory cycles were analyzed: 810 (33%) with a normal compliance and resistance profile, 810 (33%) with a low compliance and normal resistance profile, and 810 (33%) with a normal compliance and increased resistance profile.
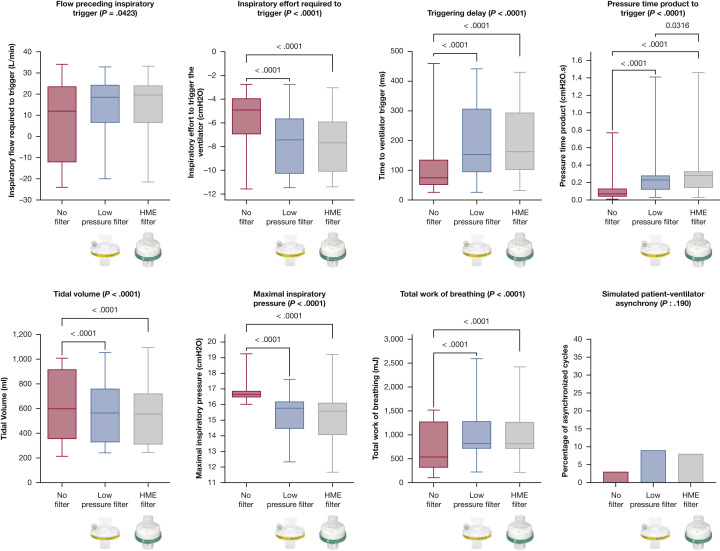
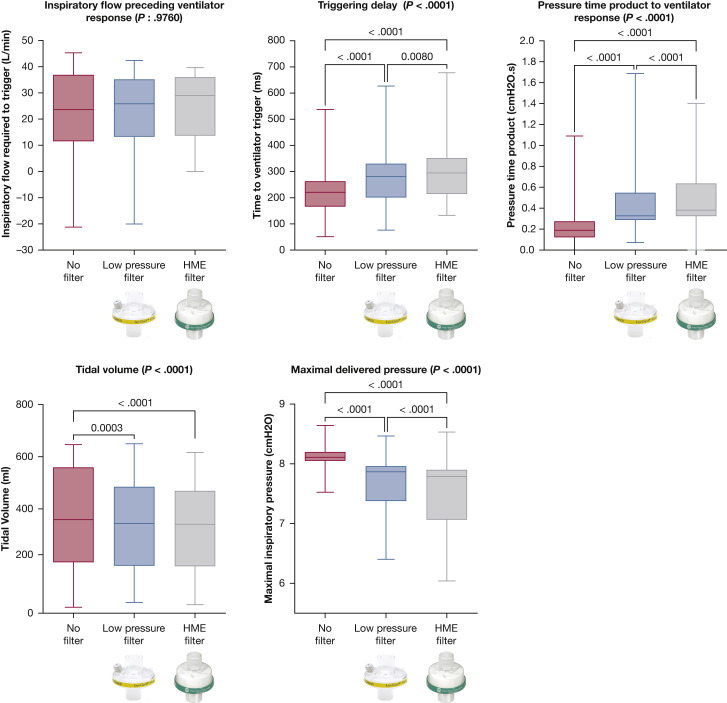
With NIV, the addition of a low-pressure or a HME filter had a significant impact on ventilator performance. The addition of a filter (low pressure or HME) was associated with an increase in flow preceding triggering (P = .0423), inspiratory effort to trigger the ventilator (P < .0001), triggering delay (P < .0001), WOB (P < .0001), and PTPt (P < .001) and a decrease in maximal inspiratory pressure (P < .0001) and Vt (P < .0001). Percentage of sPVA was the only parameter for which adding a filter did not cause a significant difference (P = .190) (e-Table 1). No difference was seen between low-pressure and HME filters except for PTPt, which was higher when using an HME filter (P = .0316) (Table 1 , Fig 4 ). Similar results were seen with CPAP: albeit for inspiratory flow preceding trigger, all parameters were significantly influenced by the addition of filters. Except for tidal volume, HME filters performed significantly worse than low-pressure filters (Table 1, Fig 5 ).
Table 1.
Impact of Filters on Ventilator Performance During Noninvasive Ventilation and CPAP
| Variables | No Filter |
Low-Resistance Filter |
HME Filter |
|---|---|---|---|
| [Median (IQR)] | [Median (IQR)] | [Median (IQR)] | |
| Noninvasive ventilation | |||
| Flow to trigger, L/min | 11.9 (–12.1 to 23.6) | 18.6 (6.5 to 24.3) | 19.6 (6.5 to 24) |
| Inspiratory effort, cm H2O | –4.91 (–6.95 to –3.94) | –7.43 (–10.27 to –5.64)a | –7.68 (–10.1 to –5.9)a |
| Time to trigger, ms | 75 (51 to 135) | 153 (94 to 307)a | 163 (102 to 294)a |
| Delivered pressure, cm H2O | 16.7 (16.5 to 16.9) | 15.8 (14.5 to 16.2)a | 15.6 (14.1 to 16.1)a |
| Work of breathing, mJ | 537 (317 to 1274) | 822 (714 to 1282)a | 814 (712 to 1268)a |
| Tidal volume, mL | 598 (354 to 917) | 564 (328 to 760)a | 555 (310 to 721)a |
| Patient-ventilator asynchrony, No. (%) | 3 (3%) | 9 (9%) | 8 (8%) |
| PTPt, cm H2O × s | 0.070 (0.040 to 0.130) | 0.230 (0.120 to 0.280)a,b | 0.280 (0.140 to 0.328)a,b |
| CPAP | |||
| Flow to trigger, L/min | 23.6 (11.5 to 36.9) | 25.9 (13.3 to 35.1) | 29 (13.7 to 36) |
| Time to trigger, ms | 220.7 (166 to 262.7) | 281.3 (201.2 to 330.1)a,b | 294.9 (214.8 to 351.6)a,b |
| Delivered pressure, cm H2O | 8.1 (8.1 to 8.2) | 7.9 (7.4 to 8)a,b | 7.8 (7.1 to 7.9)a,b |
| Tidal volume, mL | 359 (196 to 559) | 344 (182 to 485)a,b | 341 (180 to 469)a,b |
| PTPt, cm H2O × s | 0.189 (0.122 to 0.275) | 0.328 (0.289 to 0.548)a,b | 0.382 (0.325 to 0.637)a,b |
Values represent median (IQR) unless otherwise indicated. HME = heat and moisture exchange; IQR = interquartile range; PTPt = inspiratory pressure-time product.
P < .05 after correction for multiple comparisons when compared with no filter.
P < .05 after correction for multiple comparisons when comparing low-resistance filter and HME filter.
Figure 4.
Ventilator performance according to the type of filter used during noninvasive ventilation. P values are reported if there is a significant difference after correction for multiple comparisons when compared with no filter. HME = heat and moisture exchange.
Figure 5.
Ventilator performance according to the type of filter used during CPAP. P values are reported if there is a significant difference after correction for multiple comparisons when compared with no filter. HME = heat and moisture exchange.
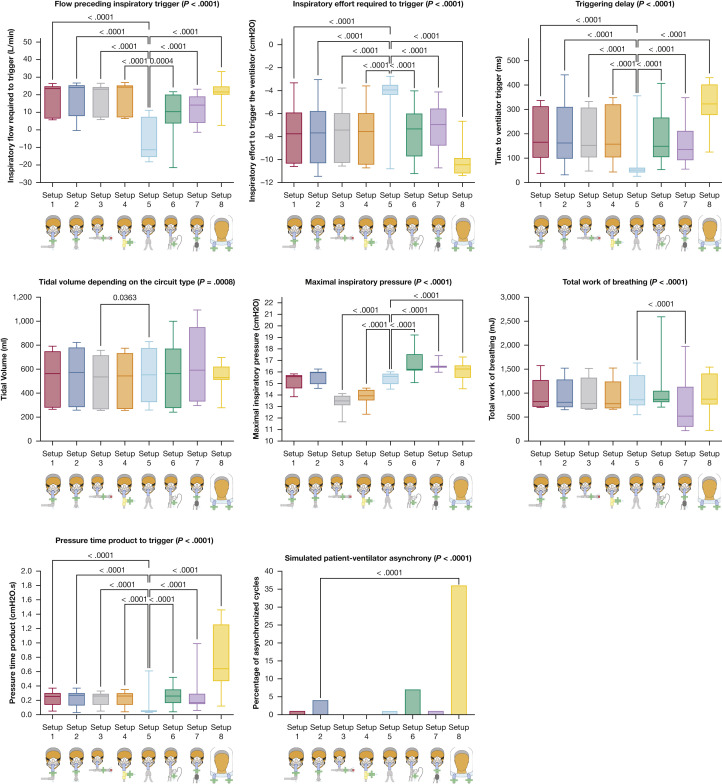
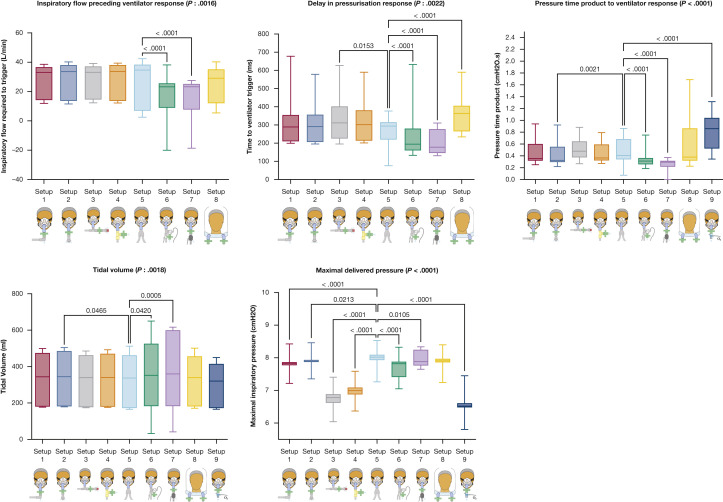
With NIV, the type of circuit setup had a significant impact on flow preceding triggering (P < .0001), inspiratory effort to trigger the ventilator (P < .0001), triggering delay (P < .0001), maximal inspiratory pressure (P < .0001), Vt (P = .0008), WOB (P < .0001), PTPt (P < .0001), and sPVA (P < .0001) (Table 2 , Fig 6 ). The type of sPVA varied significantly between circuit setups (P < .0001) (e-Table 2). Setup 5, using a dual-limb circuit, was the best setup as flow preceding triggering, inspiratory effort to trigger the ventilator, triggering delay, and PTPt were lower than in other setups with similar Vt delivered pressure and sPVA. Setup 8, using a helmet interface, had the poorest performance regarding triggering delay, PTPt, inspiratory effort to trigger the ventilator, and sPVA. Similar results were obtained with CPAP (Table 2, Fig 7 ). With CPAP, the use of setup 8 (helmet) was associated with a significant delay in pressurization, and the use of setup 9 (Boussignac CPAP) was associated with higher PTPt values. The maximal delivered inspiratory pressure was significantly lower with the Boussignac setup compared with that delivered by ventilators (P < .0001).
Table 2.
Impact of Circuit Setup on Ventilator Performance During Noninvasive Ventilation and CPAP
| Variables | Setup 1 |
Setup 2 |
Setup 3 |
Setup 4 |
Setup 5 |
Setup 6 |
Setup 7 |
Setup 8 |
Setup 9 |
|---|---|---|---|---|---|---|---|---|---|
| [Median (IQR)] | [Median (IQR)] | [Median (IQR)] | [Median (IQR)] | [Median (IQR)] | [Median (IQR)] | [Median (IQR)] | [Median (IQR)] | [Median (IQR)] | |
| Noninvasive ventilation | |||||||||
| Flow to trigger, L/min | 23.5 [6.5 to 24.7]a | 24.1 [7.9 to 25.3]a | 23.2 [7.2 to 24.3]a | 24.3 [7.2 to 25.2]a | –11.3 [–15.4 to 7.4] | 10.4 [3.7 to 20]a | 14.1 [4.1 to 19]a | 21.6 [20.1 to 24.7]a | NA |
| Inspiratory effort, cm H2O | –7.8 [–10.3 to –5.9]a | –7.7 [–10.3 to –5.8]a | –7.4 [–10.3 to –6.0]a | –7.6 [–10.4 to –6.0]a | –3.9 [–4.4 to –3.5] | –7.3 [–9.7 to –6.0]a | –7.0 [–8.8 to –5.57]a | –10.5 [–11.2 to –9.9]a | NA |
| Time to trigger, ms | 165 [102 to 312]a | 163 [99 to 310]a | 153 [104 to 307]a | 158 [104 to 320]a | 51 [41 to 61] | 149 [105 to 266]a | 135 [92 to 211]a | 323 [278 to 402]a | NA |
| Delivered pressure, cm H2O | 15.6 [14.6 to 15.7] | 15.9 [15 to 16] | 13.5 [13.1 to 13.9]a | 13.9 [13.6 to 14.4]a | 15.6 [15 to 15.9] | 16.2 [16.1 to 17.5]a | 16.5 [16.4 to 16.5]a | 16.3 [15.5 to 16.6]a | NA |
| Work of breathing, mJ | 823 [717 to 1,270] | 809 [709 to 1,281] | 783 [675 to 1,320] | 780 [686 to 1,240] | 861 [747 to 1,373] | 872 [815 to 1,046] | 520 [301 to 1,129]a | 875 [766 to 1,406] | NA |
| Tidal volume, mL | 563 [280 to 748] | 573 [285 to 780] | 535 [267 to 715]a | 544 [270 to 732] | 553 [325 to 776] | 563 [277 to 770] | 592 [331 to 949] | 529 [512 to 620] | NA |
| Patient-ventilator asynchrony, No. (%) | 2 (2%) | 6 (7%) | 2 (2%) | 2 (2%) | 2 (2%) | 37 (41%) | 11 (12%) | 38 (42%) | NA |
| PTPt, cm H2O × s | 0.255 [0.140 to 0.300]a | 0.270 [0.130 to 0.300]a | 0.260 [0.140 to 0.290]a | 0.260 [0.140 to 0.298]a | 0.050 [0.040 to 0.060] | 0.260 [0.165 to 0.350]a | 0.170 [0.150 to 0.290]a | 0.640 [0.470 to 1.255]a | NA |
| CPAP | |||||||||
| Flow to trigger, L/min | 33.0 [14.3 to 36.6] | 33.6 [13.7 to 37.9] | 33.2 [14.6 to 37] | 33.7 [13.7 to 38] | 34.6 [7 to 38.3] | 23.2 [8.9 to 25.6]a | 23.3 [7.8 to 25.1]a | 29.1 [12.2 to 35.1] | NA |
| Time to trigger, ms | 289.1 [210.9 to 354] | 291 [207 to 356] | 311.5 [226.1 to 401.4]a | 301.8 [214.4 to 379.9] | 293.9 [220.2 to 314.5] | 194.3 [160.2 to 279.8]a | 177.7 [146.5 to 276.4]a | 363.3 [265.6 to 404.8]a | NA |
| Delivered pressure, cm H2O | 7.8 [7.8 to 7.9]a | 7.9 [7.9 to 7.9]a | 6.8 [6.6 to 6.9]a | 7.0 [6.9 to 7.1]a | 8.0 [7.9 to 8.1] | 7.8 [7.4 to 7.9]a | 7.9 [7.8 to 8.2]a | 7.9 [7.9 to 8]a | 6.5 [6.5 to 6.6]a |
| Tidal volume, mL | 344 [181 to 474] | 345 [182 to 485]a | 339 [178 to 461] | 340 [180 to 470] | 337 [173 to 461] | 352 [183 to 526]a | 360 [183 to 599]a | 339 [182 to 455] | 320 [173 to 414] |
| PTPt, cm H2O × s | 0.353 [0.321 to 0.599] | 0.325 [0.297 to 0.552]a | 0.478 [0.376 to 0.643] | 0.365 [0.325 to 0.59] | 0.405 [0.343 to 0.68] | 0.312 [0.257 to 0.364]a | 0.293 [0.219 to 0.314]a | 0.380 [0.322 to 0.863] | 0.860 [0.526 to 1.039]a |
Values represent median (IQR) unless otherwise indicated. IQR = interquartile range; NA = not applicable; PTPt = inspiratory pressure-time product.
P < .05 after correction for multiple comparisons when compared with setup 5.
Figure 6.
Ventilator performances during noninvasive ventilation according to the type of circuit setup used. P values are reported if there is a significant difference after correction for multiple comparisons when compared with setup 2.
Figure 7.
Ventilator performances during CPAP according to the type of circuit setup used. P values are reported if there is a significant difference after correction for multiple comparisons when compared with setup 2.
Discussion
In this bench study of various setups proposed for delivering NIV and CPAP during the SARS-CoV-2 pandemic, we have shown that modifying the circuit of a ventilator can impair ventilator triggering, pressurization, and performance, and affect work of breathing.
In our study, the use of a dual-limb circuit achieved the best performance. Its use was associated with the lowest inspiratory effort to trigger the ventilator. Therefore, the use of ventilators that allow the use of dual-limb tubing for ventilation should be preferred. Unfortunately, given the burden that the pandemic has put on ventilator supplies, physicians are frequently obliged to use home noninvasive ventilators to set up intermediate-care facilities.15 Most of these ventilators can be used only with single-limb circuits. In this case, the addition of intentional leaks (setups 1, 3, and 4) led to a lower maximal pressure without a significant impact on the work of breathing and without increasing sPVA. With CPAP, the use of active expiratory valves (setups 6 and 7) achieved better performance than setups with intentional leaks.
Use of a helmet interface was associated with the worst ventilator performance in this study. This may be explained by the fact that we did not change the ventilator settings. Indeed, helmet interfaces usually require higher pressures than do facial or nasal masks.17 , 18 Unless the team has expertise in the use of helmets,19 , 20 we suggest limiting its use to patients who do not tolerate oronasal or facial masks or to those for whom adequate fitting of oronasal or facial masks cannot be achieved.21 In this situation, in addition to using higher pressures, we recommend increasing the sensitivity of trigger and cycling settings and performing close monitoring for patient-ventilator asynchronies. Indeed, in our simulations, ineffective triggering and late cycling were the most common sPVAs identified with the helmet setup (e-Table 2).
Since the beginning of the SARS-CoV-2 pandemic, NIV and CPAP have been used for the management of acute respiratory failure outside ICUs.1 , 4 In these units, physicians and health care-associated professionals may be less experienced in the delivery of acute NIV and/or CPAP, which may further increase the risk of nosocomial transmission.4 In this context, in order to limit aerosol generation during NIV/CPAP, we would recommend using the simplest available setup in each organization. This setup may vary between centers. Indeed, this choice needs to take into account the availability of ventilators, the availability of additional pieces required for the setup, as well as the use of prone positioning outside of ICU.19 The availability of trained staff to detect and adjust ventilator settings in case of asynchronies is essential to manage patients initiated on NIV or CPAP.22 We believe that a trained staff, when available, may overcome the limits of circuit setups identified in our bench tests by personalizing NIV or CPAP settings to patient requirements.
With the use of single-limb circuits, we did not assess CO2 rebreathing. However, CO2 rebreathing is proportional to the dead-space volume between the patient and the exhalation port. Therefore, the exhalation port is usually placed as close as possible to the mask. Given the SARS-CoV-2 pandemic and risk of droplet aerosolization, it has been suggested to connect the filter directly to the mask. Such a strategy increases the dead-space volume. In setups 4 and 6, the filter was placed after the leak. This may therefore limit CO2 rebreathing with limited droplet aerosolization.
Given the lack of available ventilators during the critical phase of the pandemic, the use of Boussignac CPAP has been suggested as an alternative. In our study, Boussignac CPAP achieved lower pressures, and a lower tidal volume for a higher patient inspiratory effort than ventilator-based CPAP. Because of technical limitations, we were unable to increase the flow above 30 L/min; a higher flow may have helped to achieve a pressure similar to ventilator-based CPAP.
The level of intentional leakage of each circuit setup may be different; this could have had an impact on ventilator performance. However, in setups 2 and 3, the level of leaks was identical but, on NIV, setup 3 performed better than setup 2. Hence, we hypothesize that the resistance added on the circuit by the second filter is one of the main drivers of the differences seen.
Our results suggest that the use of low-pressure filters has a less deleterious impact than that of HME filters. However, these results need to be interpreted with caution as we ran our tests for a limited period of time and without the impact of humidification coming from air exhaled by the patient. Therefore, in a nonsimulated environment, humidity may increase the resistance of low-pressure filters more rapidly compared with HME filters. This may lead to an increase in the work of breathing as well as a decrease in the delivered pressure.
There are a few limitations of our study. First, we did not assess aerosol dispersion. This would have been difficult to replicate using a bench model. However, with the use of filters, the only meaningful aerosol dispersion that can occur would be related to unintentional mask leaks caused by mask displacement or malposition. Therefore, in addition to the use of dedicated circuit setups, physicians should carefully choose their CPAP/NIV interface while initiating patients with SARS-CoV-2 infection on a ventilator. Second, this is a bench model study. We identified significant differences between setups, but we were not able to assess their clinical relevance. However, assessing eight different setups would have been extremely difficult in clinical practice, even using a crossover design. Third, we did not assess the impact of the circuit setup for each of the three lung mechanics conditions simulated (normal, obstructive, and restrictive). Fourth, we could not assess the impact of the various circuit setups on the comfort of patients. As an example, setup 3 may add significant weight to the mask; this may contribute to unintentional leaks and require further tightening of the mask straps. This setup may also make prone positioning of patients more difficult.
Interpretation
Ventilator performance is affected by the various circuit setups that have been proposed to minimize aerosolization of viral particles during care for SARS-CoV-2-infected patients. The use of dual-limb circuits should be preferred by physicians to maintain ventilator performance. If dual-limb circuit ventilators are not available, we suggest using the single-limb setup that is the easiest to provide and monitor in their institution.
Take-home Points.
Study Question: Is ventilator performance altered by circuit setups used to limit viral aerosolization of virus?
Results: Circuit setups and the use of a filter significantly impact the performance of ventilators during noninvasive ventilation and CPAP.
Interpretation: Modifying the circuit of a ventilator can impair ventilator triggering, pressurization, and performance, and affect work of breathing.
Acknowledgments
Author contributions: M. P. is the guarantor of the content of the manuscript, including the data and analysis. M. P., E. F.: conception, acquisition, analysis, interpretation, drafting of the work, critical revision. L. R.: acquisition, interpretation. J.-P. J., C. R., A. Carlucci, A. K., H. N., Y. T.-L., A. Cuvelier, J.-F. M., C. L., B. L., J. S., J. G.-B., M. L.: conception, interpretation, critical revision.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: M. P. reports personal fees from ResMed, Philips Respironics, grants and nonfinancial support from Fisher & Paykel, nonfinancial support and personal fees from Asten, research grants from B&D Electromedical and Fisher & Paykel, and personal fees and nonfinancial support from Chiesi outside the submitted work. In the last 2 years J. G.-B. received fees from Philips, ResMed, Breas, Lowenstein, and Air Liquide for expertise, and a grant from BREAS for a trial, outside the submitted work. J. S. reports advisory and teaching payments from ResMed, Philips, Chiesi, and Menarini outside the submitted work. In the last two years, C. R. received fees from Philips, ResMed, Lowenstein, and Fisher & Paykel for expertise, outside the submitted work. None declared (E. F., A. K., L. R., A. Carlucci, C. L., B. L., M. L., A. Cuvelier, J.-F. M., H. N., Y. T.-L.).
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
∗SomnoNIV Group Collaborators: Annalisa Carlucci, MD, PhD, Bruno Langevin, MD, Javier Sayas-Catalan, MD, PhD, Manuel Lujan-Torné, MD, PhD, Frederic Lofaso, MD, PhD, Claudio Rabec, MD, Joao Winck Carlo, MD, PhD, Cristina Lamolda, physiotherapist.
Other contributions: The authors thank Asten Santé for providing the ventilator, filters, masks, and circuits used in this trial. The authors also thank Alexandre Nicol and Phoenix Effect for prototyping the 3D model used in setup 4 and for sharing it.
Footnotes
FUNDING/SUPPORT: Funding was provided by the ADIR Association. Asten Santé provided ventilator, filters, masks, and circuits used in the trial.
Contributor Information
SomnoNIV Group:
Annalisa Carlucci, Bruno Langevin, Javier Sayas-Catalan, Manuel Lujan-Torné, Frederic Lofaso, Claudio Rabec, Joao Winck Carlo, and Cristina Lamolda
Supplementary Data
References
- 1.Oranger M., Gonzalez-Bermejo J., Dacosta-Noble P., et al. Continuous positive airway pressure to avoid intubation in SARS-CoV-2 pneumonia: a two-period retrospective case-control study. Eur Respir J. 2020;56(2):2001692. doi: 10.1183/13993003.01692-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raoof S., Nava S., Carpati C., Hill N.S. High-flow, noninvasive ventilation and awake (nonintubation) proning in patients with coronavirus disease 2019 with respiratory failure. Chest. 2020;158(5):1992–2002. doi: 10.1016/j.chest.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn J.Y., An S., Sohn Y., et al. Environmental contamination in the isolation rooms of COVID-19 patients with severe pneumonia requiring mechanical ventilation or high-flow oxygen therapy. J Hosp Infect. 2020;106(3):570–576. doi: 10.1016/j.jhin.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franco C., Facciolongo N., Tonelli R., et al. Feasibility and clinical impact of out-of-ICU noninvasive respiratory support in patients with COVID-19-related pneumonia. Eur Respir J. 2020;56(5):2002130. doi: 10.1183/13993003.02130-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simonds A.K., Hanak A., Chatwin M., et al. Evaluation of droplet dispersion during non-invasive ventilation, oxygen therapy, nebuliser treatment and chest physiotherapy in clinical practice: implications for management of pandemic influenza and other airborne infections. Health Technol Assess. 2010;14(46):131–172. doi: 10.3310/hta14460-02. [DOI] [PubMed] [Google Scholar]
- 6.Leonard S., Atwood C.W., Jr., Walsh B.K., et al. Preliminary findings of control of dispersion of aerosols and droplets during high velocity nasal insufflation therapy using a simple surgical mask: implications for high flow nasal cannula. Chest. 2020;158(3):1046–1049. doi: 10.1016/j.chest.2020.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leonard S., Strasser W., Whittle J.S., et al. Reducing aerosol dispersion by high flow therapy in COVID-19: high resolution computational fluid dynamics simulations of particle behavior during high velocity nasal insufflation with a simple surgical mask. J Am Coll Emerg Physicians Open. 2020;1(4):578–591. doi: 10.1002/emp2.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adir Y., Segol O., Kompaniets D., et al. COVID-19: minimising risk to healthcare workers during aerosol-producing respiratory therapy using an innovative constant flow canopy. Eur Respir J. 2020;55(5):2001017. doi: 10.1183/13993003.01017-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rabec C., Gonzalez-Bermejo J., Respiratory Support Chronic Care Group AVO2 of the French Society of Respiratory Diseases SPLF; GAVO2 Collaborators Respiratory support in patients with COVID-19 (outside intensive care unit): a position paper of the Respiratory Support and Chronic Care Group of the French Society of Respiratory Diseases. Respir Med Res. 2020;78:100768. doi: 10.1016/j.resmer.2020.100768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thille A.W., Rodriguez P., Cabello B., Lellouche F., Brochard L. Patient-ventilator asynchrony during assisted mechanical ventilation. Intensive Care Med. 2006;32(10):1515–1522. doi: 10.1007/s00134-006-0301-8. [DOI] [PubMed] [Google Scholar]
- 11.Tonnelier A., Lellouche F., Bouchard P.A., L’Her E. Impact of humidification and nebulization during expiratory limb protection: an experimental bench study. Respir Care. 2013;58(8):1315–1322. doi: 10.4187/respcare.01785. [DOI] [PubMed] [Google Scholar]
- 12.KerNel Biomedical. T-piece connector with integrated leakage. https://3dleak.kernelbiomedical.com
- 13.Gattinoni L., Coppola S., Cressoni M., Busana M., Rossi S., Chiumello D. Covid-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201(10):1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Battisti A., Tassaux D., Janssens J.-P., Michotte J.-B., Jaber S., Jolliet P. Performance characteristics of 10 home mechanical ventilators in pressure-support mode: a comparative bench study. Chest. 2005;127(5):1784–1792. doi: 10.1378/chest.127.5.1784. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez-Bermejo J., Perrin C., Janssens J.P., et al. SomnoNIV Group Proposal for a systematic analysis of polygraphy or polysomnography for identifying and scoring abnormal events occurring during non-invasive ventilation. Thorax. 2012;67(6):546–552. doi: 10.1136/thx.2010.142653. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Bermejo J., Janssens J.-P., Rabec C., et al. SomnoNIV Group Framework for patient-ventilator asynchrony during long-term non-invasive ventilation. Thorax. 2019;74(7):715–717. doi: 10.1136/thoraxjnl-2018-213022. [DOI] [PubMed] [Google Scholar]
- 17.Vargas F., Thille A., Lyazidi A., Campo F.R., Brochard L. Helmet with specific settings versus facemask for noninvasive ventilation. Crit Care Med. 2009;37(6):1921–1928. doi: 10.1097/CCM.0b013e31819fff93. [DOI] [PubMed] [Google Scholar]
- 18.Pisani L., Mega C., Vaschetto R., et al. Oronasal mask versus helmet in acute hypercapnic respiratory failure. Eur Respir J. 2015;45(3):691–699. doi: 10.1183/09031936.00053814. [DOI] [PubMed] [Google Scholar]
- 19.Retucci M., Aliberti S., Ceruti C., et al. Prone and lateral positioning in spontaneously breathing patients with COVID-19 pneumonia undergoing noninvasive helmet CPAP treatment. Chest. 2020;158(6):2431–2435. doi: 10.1016/j.chest.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aliberti S., Radovanovic D., Billi F., et al. Helmet CPAP treatment in patients with COVID-19 pneumonia: a multicentre cohort study. Eur Respir J. 2020;56(4):2001935. doi: 10.1183/13993003.01935-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hui D.S., Chow B.K., Lo T., et al. Exhaled air dispersion during high-flow nasal cannula therapy versus CPAP via different masks. Eur Respir J. 2019;53(4):1802339. doi: 10.1183/13993003.02339-2018. [DOI] [PubMed] [Google Scholar]
- 22.Elliott M.W. Non-invasive ventilation: essential requirements and clinical skills for successful practice. Respirology. 2019;24(12):1156–1164. doi: 10.1111/resp.13445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.