Abstract
Objectives:
To test the hypothesis that poststimulation block of nerve conduction can be achieved by low-frequency (≤1 kHz) biphasic stimulation (LFBS).
Materials and Methods:
A tripolar cuff electrode was placed around the pudendal nerve in cats to deliver LFBS (1 kHz, 500 Hz, and 100 Hz). Two hook electrodes were placed central and distal to the cuff electrode to induce external urethral sphincter (EUS) contractions. A catheter was inserted into the urethra to record EUS contraction pressure. Pudendal nerve block by LFBS was confirmed by the failure of the central hook electrode stimulation to induce EUS contractions, while the distal hook electrode stimulation still induced contractions.
Results:
Pudendal nerve conduction was completely blocked by LFBS at different frequencies (1 kHz, 500 Hz, and 100 Hz) after terminating LFBS. The post-LFBS block induced at the minimal stimulation intensity and duration was fully reversible within the same time period (10–15 min on average) for the three frequencies. However, the stimulation duration to induce block significantly (p < 0.05) increased from 23 ± 8 sec to 95 ± 14 sec when frequency increased from 100 Hz to 1 kHz.
Conclusion:
This study discovered that LFBS (≤1 kHz), like high-frequency (≥5 kHz) biphasic stimulation (HFBS), can induce poststimulation block. The result provides support for the theory that biphasic stimulation waveforms block axonal conduction by changing intracellular and extracellular ion concentrations. The post-LFBS block provides the opportunity to develop new neuromodulation devices for clinical applications where initial nerve firing is acceptable.
Keywords: Block, cat, low frequency, pudendal nerve
INTRODUCTION
It is well known that high-frequency (≥5 kHz) biphasic stimulation (HFBS) can block nerve conduction (1–3). Currently, HFBS has been used successfully in human subjects to block abdominal vagus nerves to treat obesity or block the sciatic nerve to suppress amputation limb pain (4,5). HFBS has also been used clinically for spinal cord stimulation to treat chronic back and leg pain (6), although whether nerve block is the mechanism of pain suppression by spinal HFBS is still debatable. In addition to the block of nerve conduction during HFBS, animal studies (7,8) have shown that nerve block persists after the end of HFBS, that is, a post-HFBS block. Despite significant progress in both clinical and basic science studies, the mechanism underlying HFBS-induced nerve block is currently still unknown.
Our previous computer simulation studies (9,10) which examined possible mechanisms for the nerve block induced during HFBS revealed that potassium channels are constantly open at HFBS frequencies above 5 kHz. This eliminates the delay between the opening of sodium and potassium channels when a propagating action potential arrives at the site of HFBS, thereby preventing the generation of the action potential and causing a block of nerve conduction (9,10). However, this mechanism cannot explain the discovery in recent animal studies (7,8) that nerve block persists even after the end of HFBS because the potassium channels close quickly after terminating HFBS (10). However, our computer simulation studies (9,10) also revealed that when axonal ion channels remain open during HFBS each electrical pulse during stimulation generates large inward sodium and outward potassium currents. Therefore, during HFBS, the intracellular sodium and extracellular potassium concentrations will increase and at the end of HFBS, the changed ion concentrations can cause a nerve conduction block. This post-HFBS block can reverse with time as the axonal membrane ion pump gradually restores the normal intra- and extra-cellular ion concentrations. This theory can explain the post-HFBS block shown in recent animal studies (7,8). However, based on this hypothesis, it is not necessary to use frequencies ≥5 kHz to produce the post-HFBS block. Low-frequency (≤1 kHz) biphasic stimulation (LFBS) should also be able to repeatedly generate large inward sodium and outward potassium currents with each stimulus pulse and thereby produce a post-LFBS block due to large changes in axonal intra- and extra-cellular ion concentrations that persist after the end of stimulation. To test this hypothesis, we conducted the current study in cats to determine whether LFBS at a frequency between 100 Hz and 1 kHz can produce post-LFBS block of pudendal nerve conduction.
MATERIALS AND METHODS
The experimental protocol and animal use in this study were approved by the Animal Care and Use Committee at the University of Pittsburgh.
Experimental Protocol
A total of ten cats (five females and five males, 2.9–3.7 kg) were used in this study. The animals were anesthetized initially with isoflurane (2–5% in oxygen) during surgery and then switched to alpha-chloralose anesthesia (initial dose 65 mg/kg i.v. followed by supplemental doses as needed) during data collection. The right cephalic vein was catheterized for intravenous administration of fluid and drugs. A midline anterior cervical incision was used to access the airway, which was kept patent via tracheostomy. The right carotid artery was catheterized for monitoring arterial blood pressure. Oxygen saturation and heart rate were measured via a pulse oximeter (9847V; NONIN Medical, Plymouth, MN, USA) attached to the tongue.
Through an abdominal incision, the ureters were isolated, cut, and drained externally. A catheter was inserted into the urethra via a small incision in the proximal urethra. The catheter was connected to a syringe pump (SP200i; World Precision Instruments, Sarasota, FL, USA) and a pressure transducer (BLPR2, World Precision Instruments) via a three-way stopcock to slowly (1 mL/min) perfuse the urethra with saline (Fig. 1). The external urethral sphincter (EUS) is innervated by the pudendal nerve. Pudendal nerve stimulation can cause EUS contraction that can increase the urethral outlet resistance to the saline perfusion resulting in an increase in urethral pressure. Since the urethra was tied around the perfusion catheter (Fig. 1), the bladder was isolated from the urethra and therefore changes in bladder pressure did not influence the measurements of urethral pressure. Each pudendal nerve was exposed via a 3–4 cm incision in the sciatic notch lateral to the tail for implantation of a tripolar cuff electrode (NEC113; MicroProbes Inc, Gaithersburg, MD, USA) to deliver a biphasic stimulation waveform (Stim.B, Fig. 1). Bipolar hook electrodes were placed distal (Stim.D) and central (Stim.C) to the tripolar cuff electrode (Fig. 1). Each pudendal nerve was transected centrally to prevent reflex activation of the EUS. The nerve and electrodes were covered with warm (37°C) mineral oil. Monophasic negative stimulus pulses (30 Hz, 0.2 msec) generated by a stimulator (Grass S88; Grass Technologies, RI, USA) were delivered via a stimulus isolator (SIU5; Grass Technologies) to the hook electrodes (Stim.C or Stim.D) to induce a EUS contraction and >30 cmH2O increase in urethral pressure. The biphasic stimulation waveform generated by a computer running a LabView program (National Instruments, TX, USA) was delivered via a stimulus isolator (A395; World Precision Instruments, FL, USA) to the tripolar cuff electrode to block pudendal nerve conduction and suppress EUS contractions (Fig. 1). It is worth noting that the biphasic stimulation waveform is continuous square pulses without any pulse interval (Fig. 1).
Figure 1.
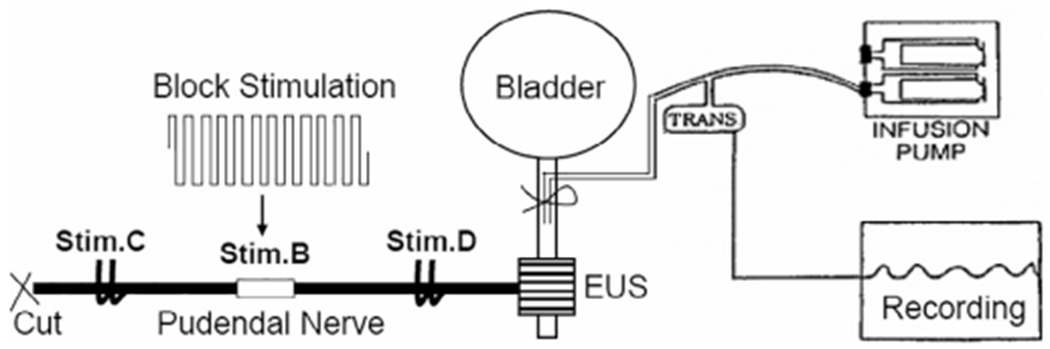
Experimental setup. Block stimulation was applied to the pudendal nerve by a tripolar cuff electrode (Stim.B) to block propagation of the action potentials induced by electrical stimulation at the central site (Stim.C) by a bipolar hook electrode. Responses elicited by electrical stimulation at a distal site (Stim.D) by a bipolar hook electrode confirmed that the external urethral sphincter (EUS) was not fatigued during post-stimulation block induced by Stim.B. The urethra was slowly perfused by an infusion pump. The EUS contraction was recorded by the increase in urethral pressure.
Stimulation Protocol
The intensity threshold to block pudendal nerve conduction was determined at the beginning of every experiment by applying 10 kHz HFBS for 50–60 sec at an increasing intensity starting from 1 mA with 1 mA increments. The minimal intensity for 10 kHz HFBS to completely suppress the urethral pressure induced by stimulation at Stim.C was determined as the block threshold (T) (Fig. 2). This frequency and duration of stimulation was shown in previous experiments to consistently block pudendal nerve conduction during the stimulation without causing a poststimulation block (8). We choose 10 kHz block threshold as the reference intensity in this study because 10 kHz nerve block is well known (1–5) and our recent study shows that 10 kHz requires a very long stimulation duration (10–30 min) to induce a poststimulation block (8). In preliminary studies using the first three cats, LFBS (1 kHz, 500 Hz, or 100 Hz) was applied to the pudendal nerve at different intensities (1 T, 2 T, or 3 T) with different durations (five seconds to three minutes) randomly to determine whether postLFBS block could occur. In every experiment, Stim.B was identified as the site of post-LFBS nerve block by showing that stimulation at the Stim.D site (30 Hz frequency, 0.2 msec pulse width, 5 sec duration) still induced >30 cmH2O EUS contractions when stimulation at the Stim.C site (30 Hz frequency, 0.2 msec pulse width, 5 sec on, and 55 sec off) failed to induce EUS contractions (Fig. 3).
Figure 2.
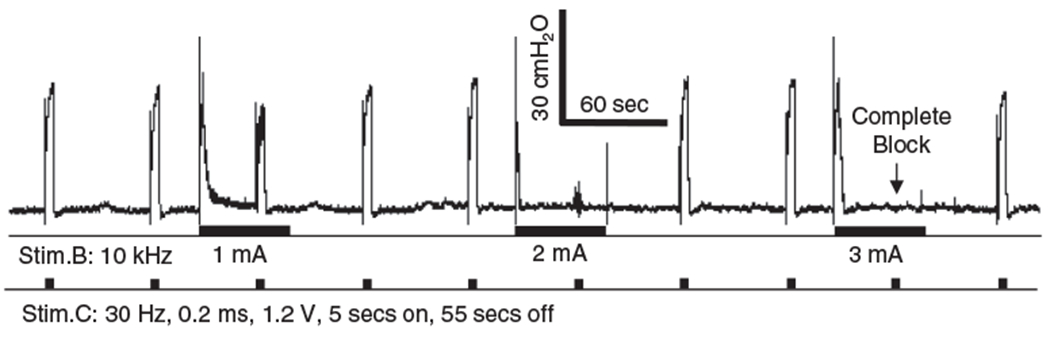
The block threshold (T) is the stimulus intensity at which 10 kHz stimulation (Stim.B) completely blocked the EUS contraction induced by the 30 Hz intermittent (5 sec on, 55 sec off) stimulation at the central site (Stim.C). T = 3 mA as shown in this figure. The thick black bars under the pressure trace indicate the durations of stimulation. Please note that the 10 kHz Stim.B did not induce a poststimulation block. A much longer stimulation duration (10–30 min) was required for 10 kHz to induce a post-stimulation block as reported in our previous study (8).
Figure 3.
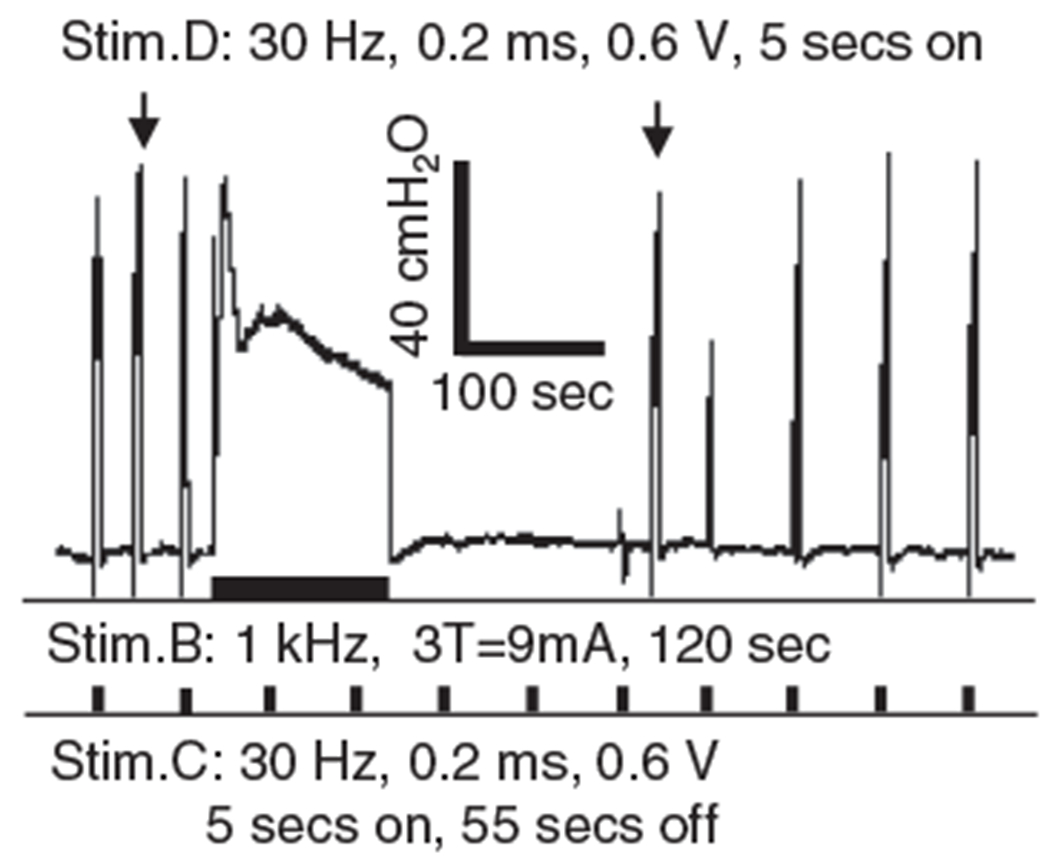
Complete nerve block occurs after the end of the 1 kHz stimulation. The arrows indicate the EUS contractions induced by the 30 Hz stimulation at the distal site (Stim.D). The thick bars under the EUS pressure trace indicate the stimulation durations for Stim.B and Stim.C. After ending the 1 kHz Stim.B, EUS contraction can still be induced by the distal Stim.D but the contractions induced by the central Stim.C are completely blocked initially and then gradually recover, indicating that nerve conduction block occurs locally at Stim.B and the EUS is not fatigued.
In the remaining seven cats, the minimal intensity and duration of LFBS (1 kHz, 500 Hz, or 100 Hz) to induce a complete post-LFBS block was examined in a systematic manner. A complete post-LFBS block was defined as the suppression of at least one of the EUS contractions induced by stimulation at Stim.C (30 Hz frequency, 0.2 msec pulse width, 5 sec on, and 55 sec off) to <5 cmH2O urethral pressure. The testing was started using 1 kHz LFBS of 60 sec duration applied at an increasing intensity of 1 T, 2 T, and 3 T. If a complete post-LFBS block did not occur, then the LFBS intensity was kept at 3 T and the stimulation duration was increased sequentially by 30 sec in repeated tests until a complete post-LFBS was observed (Fig. 4). This was then followed by 500 Hz LFBS at 1 T starting at 5 sec duration. The duration was then increased sequentially in five to ten seconds increments to a maximum of 60 sec. If complete block was not elicited, the intensity was then increased to 2 T at 60 sec and then to 3 T if needed starting at 60 sec followed by sequential 5 sec increases in duration until the complete post-LFBS block was observed. In the final series of tests, 100 Hz LFBS was initially applied at 1 T intensity and 5 sec duration followed by sequential 5–10 sec increases to a maximum of 60 sec. If a complete post-LFBS block was not observed, the intensity was increased to 3 T and 5 sec duration and then duration was increased sequentially in five to ten sec steps until a complete post-LFBS block was observed. At the end of each LFBS test, a rest period (3–30 min) was inserted to allow the EUS contractions to fully recover before applying the next period of LFBS; 1 kHz was tested in 14 nerves (left and right in seven cats), 500 Hz was tested in 9 nerves, and 100 Hz was tested in 6 nerves.
Figure 4.
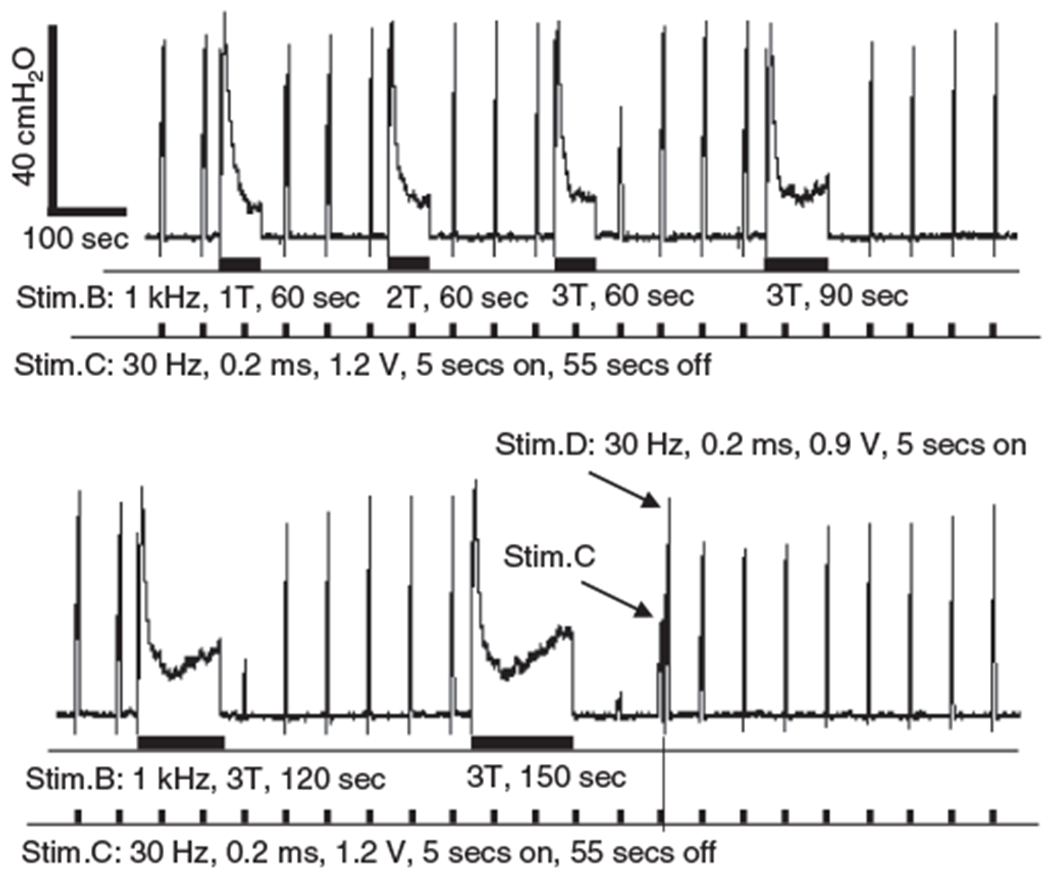
Search protocol to determine the minimal intensity and duration for 1 kHz stimulation to induce a poststimulation block that can reduce EUS contractions to <5 cmH2O urethral pressure, that is, a complete poststimulation block. The bottom trace is the continuation of the top trace. The two arrows indicate the EUS contractions induced by the 30 Hz stimulation at the distal site (Stim.D) and the central site (Stim.C). The thick bars under the EUS pressure trace indicate the stimulation durations for Stim.B and Stim.C. T = 3 mA.
Data Analysis
The recovery period was defined as the time period required for the EUS contraction pressure to reach >90% of the prestimulation pressure (Fig. 5a). The minimal LFBS stimulation durations to induce a complete post-LFBS block at different frequencies (1 kHz, 500 Hz, and 100 Hz) were compared. The data obtained under the same conditions in different animals were averaged and presented as mean ± standard error. Unpaired student t-test was performed to detect significant differences (p < 0.05).
Figure 5.
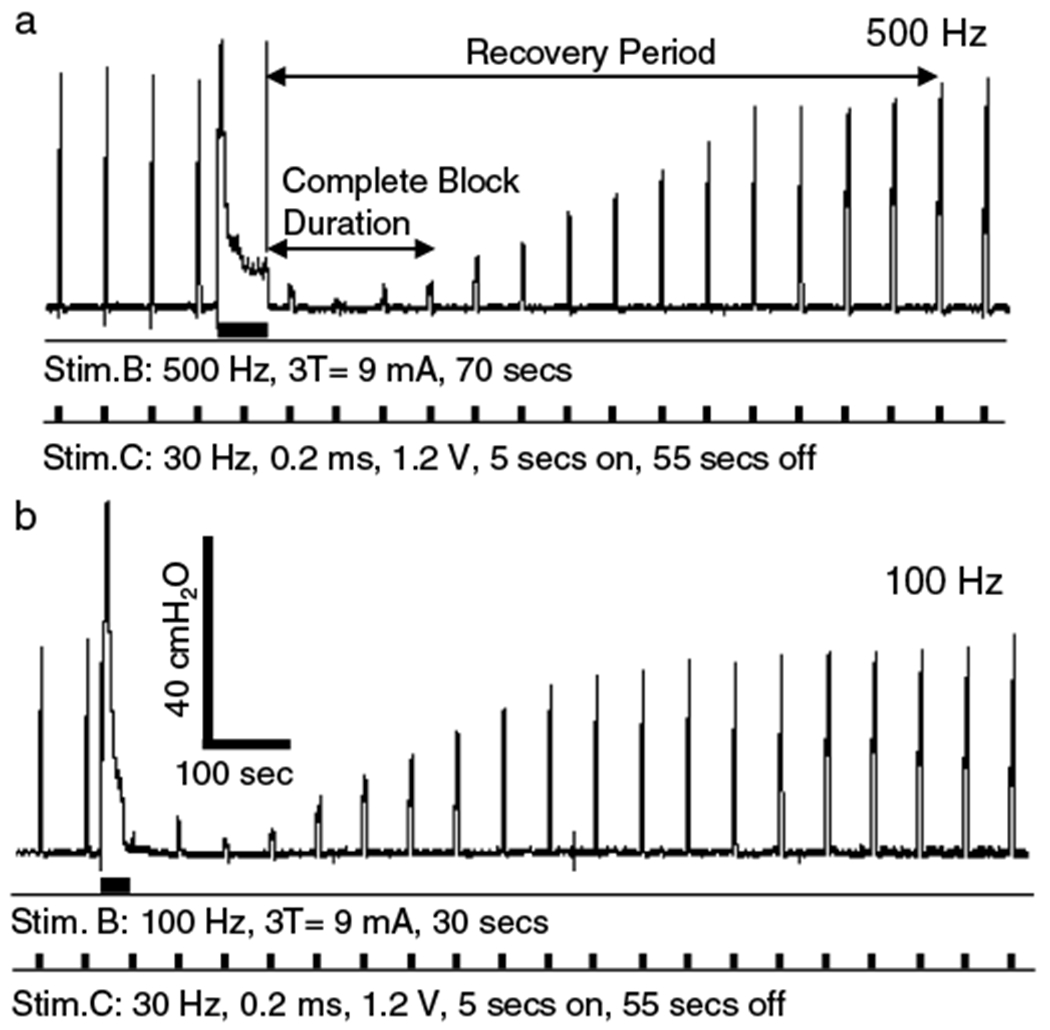
The minimal stimulation intensity and duration to induce a complete poststimulation were also determined for 500 Hz (A) or 100 Hz (B) stimulation. The thick bars under the EUS pressure trace indicate the stimulation durations for Stim.B and Stim.C. The data were obtained in the same experiment as shown in Figure 4. The recovery period is defined as the time period required for the EUS contraction pressure to reach >90% of the prestimulation pressure. The complete block duration is defined as the poststimulation period during which Stim.C can only induce <5 cmH2O urethral pressure.
RESULTS
At the beginning of each experiment, the urethral pressure responses indicating EUS contractions induced by electrical stimulation (30 Hz intermittent, 5 sec on, and 55 sec off) of the pudendal nerve at sites central (Stim.C) and distal (Stim.D) to the blocking electrode (Stim.B) (Fig. 1) were elicited over a range of intensities. Stimulus intensities (0.6–1.2 V, 0.2 msec pulse width) that produced approximately equal increases in urethral pressure >30 cmH2O at both sites were used for the remainder of the experiments to elicit control responses that were tested for sensitivity to LFBS block. Preliminary studies in the first three cats examined the effect of LFBS at different frequencies (1 kHz, 500 Hz, or 100 Hz) and stimulus intensities. Stimulus intensities were normalized in each animal by referencing to the stimulus intensity threshold (T) at 10 kHz that produced a transient increase in urethral pressure followed by a complete block of the urethral pressure response to stimulation at Stim.C (Fig. 2). As shown in Figure 3, the LFBS (Stim.B: 1 kHz, 3 T = 9 mA, 120 sec) induced a tonic EUS contraction during the stimulation and a complete post-LFBS block that fully suppressed EUS contractions induced by electrical stimulation (30 Hz, 0.2 msec, 0.6 V, 5 sec on, and 55 sec off) via the electrode at Stim.C. The EUS contractions gradually recovered in about 5.5 min following the LFBS (Fig. 3). During the post-LFBS block, stimulation at the distal electrode (Stim.D: 30 Hz, 0.2 msec, 0.6 V, 5 sec on) induced a EUS contraction of the same amplitude as that prior LFBS (Fig. 3), indicating that the LFBS blocked the pudendal nerve locally at the Stim.B site but not distally at sites in the nerve, the neuromuscular junction, or due to fatigue of the EUS muscle.
The search protocol to determine the minimal stimulation intensity and duration required to induce a complete post-LFBS block after 1 kHz stimulation is shown in Figure 4. As the 1 kHz LFBS of 60 sec duration increased in intensity from 1 T to 3 T, the post-LFBS block was first observed at 3 T intensity as a 50% reduction in the EUS contraction pressure (the top trace in Fig. 4). At 3 T intensity, further increasing the duration of stimulation from 60 sec to 150 sec produced a complete post-LFBS block (he bottom trace in Fig. 4). Therefore, the minimal stimulation intensity and duration were determined as 3 T and 150 sec for 1 kHz LFBS to induce a complete nerve block (Fig. 4). Using the same nerve in this cat, the minimal stimulation intensity and duration for LFBS of 500 Hz (Fig. 5a) or 100 Hz (Fig. 5b) to induce a complete post-LFBS block were also determined. The recovery from complete block had a similar time course for both frequencies of stimulation (Fig. 5). Although 3 T intensity was required in this cat for 1 kHz, 500 Hz, and 100 Hz LFBS to induce a complete post-LFBS block, the block was also observed at 1 T or 2 T intensity in the other six cats at different frequencies.
On average (N = 7 cats), the minimal stimulation duration to induce a complete block significantly (p < 0.05) increased from 23 ± 8 sec to 95 ± 14 sec when the frequency increased from 100 Hz to 1 kHz (Fig. 6a) although the block occurred at various stimulation intensities (1–3 T). At the minimal stimulation intensity and duration, the complete post-LFBS block induced by stimulation of different frequencies had similar complete block duration (Fig. 6b) and recovery period (Fig. 6c). The post-LFBS block induced at the minimal stimulation intensity and duration was fully reversible (Figs. 3–5) in every experiment within the same time period (10–15 min on average) for the three frequencies (Fig. 6b,c).
Figure 6.
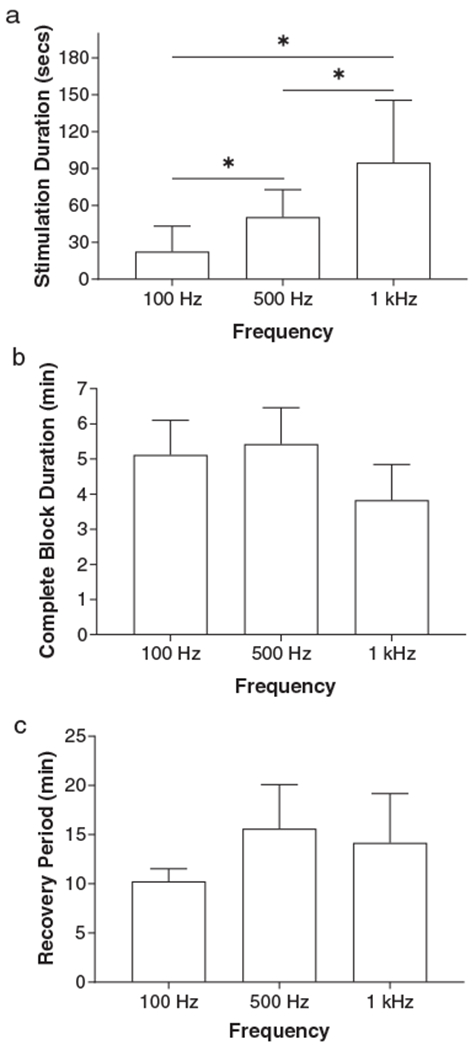
Effects of stimulation frequency on the complete post-LFBS block induced by LFBS at the minimal intensity and duration. A longer stimulation duration is required for higher frequencies to induce a complete post-LFBS block (a). However, complete block duration (b) and recovery period (c) do not change with stimulation frequency (p > 0.05, un-paired t-test). The nerve block can occur at intensities of 1–3 T (3–9 mA) at different frequencies. N = 6 for 100 Hz, N = 9 for 500 Hz, and N = 14 for 1 kHz. * indicates significant difference (p < 0.05, un-paired t-test).
DISCUSSION
This study in cats discovered that poststimulation block of pudendal nerve conduction could be induced by LFBS at a frequency less than 1 kHz (Figs. 3–5) and at a lower frequency, a shorter stimulation duration is required to induce a complete post-LFBS block (Fig. 6a). The block induced by LFBS at the minimal intensity and duration is fully reversible within a similar recovery period for different frequencies (Fig. 6b,c). These results provide valuable information for understanding the possible mechanisms underlying the block of nerve conduction by biphasic stimulation waveforms. This study also opens the opportunity for clinical applications to induce a poststimulation block at a low frequency (≤1 kHz) within 30–60 sec (Fig. 6a), while the traditional 10 kHz HFBS requires a much longer stimulation duration (10–30 min) to achieve a poststimulation block (8).
The post-LFBS block is fully reversible, which indicates that the nerve is likely not damaged by the LFBS. Therefore, it is reasonable to assume that the post-LFBS block is caused by alteration of the ionic mechanisms underlying axonal conduction. Our previous computer simulation studies (9,10) revealed that each stimulus pulse of the biphasic stimulation waveform can generate an inward sodium current and an outward potassium current. Therefore, it is reasonable to expect that the intracellular and extracellular ion concentrations must be changed dramatically as the LFBS continues. When LFBS is terminated, these large changes in ion concentrations must disrupt the normal transmembrane ionic gradients necessary for the generation of the action potential and cause nerve conduction block. As the transmembrane ion pumps gradually restore the normal intracellular and extracellular ion concentrations, the post-LFBS block will slowly disappear and the nerve conduction will be restored. This mechanism revealed by computer simulation studies (9,10) can explain very well the post-LFBS block observed in current study. It is worth noting that the LFBS induced a tonic EUS contraction during the stimulation but blocked the nerve conduction after the stimulation (Figs. 3–5). This is in dramatic contrast to the HFBS (10 kHz) that instead of inducing a tonic EUS contraction produced a nerve block during the stimulation but did not block the nerve after the stimulation (Fig. 2). These results indicate that the mechanisms are very different for post-LFBS block and the block induced by HFBS during the stimulation. In fact, our previous computer simulation studies (9,10) did reveal a different blocking mechanism for HFBS that maintains potassium channels in a constantly open state at stimulation frequencies ≥5 kHz. This causes nerve conduction block during stimulation (Fig. 2) instead of the block that occurs after stimulation that is mediated by changes in transmembrane ionic gradients.
It is also important to note that at a lower frequency, each stimulus pulse of the biphasic stimulation waveform (Fig. 1) has a longer duration that can drive more sodium and potassium ions across the axonal membranes and therefore be more effective in changing the ion concentrations than higher frequencies of stimulation that have a shorter duration stimulus pulse. This may explain why the lower frequency (100 Hz) stimulation requires a shorter stimulation duration than the higher frequencies (500 Hz or 1 kHz) to induce a complete post-LFBS block (Fig. 6a). This finding also raises the question of whether a lower frequency (<100 Hz) would be effective at an even shorter duration? However, our proposed mechanism must have a minimal effective frequency because during a very long stimulus pulse, the sodium and potassium channels will open at the beginning of the pulse but will be closed before the end of the pulse due to the ion channel kinetics. Therefore, for a very low frequency (i.e., a very long stimulus pulse), the LFBS will become less effective as the inward sodium and outward potassium currents during the last phase of the long stimulus pulse will decline and have less impact on the intracellular and extracellular ion concentrations.
Although the minimal stimulation duration for producing post-LFBS block is frequency dependent, the nerve blocks induced by different LFBS frequencies have a similar time course for recovery (Fig. 6b,c). This is expected because complete conduction block at different frequencies is probably occurring after the same change in ion concentrations induced by LFBS at the minimal intensity and duration, whereas recovery occurs after a time required for the membrane pumps to restore normal ion concentrations, which should be frequency independent because the starting point for recovery should be the same after 100 Hz to 1 kHz stimulation. However, based on the ion concentration hypothesis, the duration of post-LFBS block should be increased by LFBS of a high intensity or a long duration. The maximal duration of post-LFBS block induced by a strong LFBS of a long duration without causing nerve tissue damage still needs to be determined. In addition, the intensity-duration relationship for LFBS at a fixed frequency to induce a post-LFBS block also needs to be studied as well as the intensity-frequency relationship for LFBS of a fixed duration. These relationships will provide more information to support or refute the ion concentration hypothesis of nerve block.
The LFBS always produces tonic nerve firing during stimulation before it can induce a post-LFBS block (Figs. 3–5). Therefore, it is less likely that LFBS will be used clinically to block peripheral nerves for treatment of chronic pain. However, the post-LFBS block will be very useful in a clinical condition such as SCI where sensory pathways are disrupted and when the initial tonic afferent nerve firing evoked by stimulation is acceptable. For example, post-LFBS block could be used to facilitate voiding in people with detrusor sphincter dyssynergia (DSD) after complete supra-lumbar spinal cord injury. DSD, which is produced by abnormal reflex activity of the EUS mediated by afferent input from the bladder and motor axons in the pudendal nerves, increases urethral outlet resistance and suppresses bladder emptying. During two to five minutes of complete pudendal nerve block by post-LFBS (Fig. 6b), DSD will be blocked, thereby relaxing the EUS and allowing efficient voiding at a low bladder pressure (11,12).
In summary, this study discovered a novel method to block nerve conduction using a biphasic stimulation waveform of a low (≤1 kHz) frequency. The results confirmed that post-stimulation block can be induced not only by HFBS (≥5 kHz) but also by LFBS (≤1 kHz), which is consistent with the theory that ion concentration changes may play an important role in nerve conduction block by biphasic stimulation waveform. The post-LFBS block could be used in many clinical applications where initial tonic nerve firing is acceptable, providing opportunities to develop new neuromodulation devices.
Acknowledgments
Source(s) of financial support: This study is funded by the National Institute of Neurological Disorders and Stroke under grant R01NS109198 and by the National Institute of Diabetes and Digestive and Kidney Diseases under grants R01DK121698 and R01DK111382.
Footnotes
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to http://www.wiley.com/WileyCDA/Section/id-301854.html
Conflicts of Interest: Dr. Tai is an inventor of a patent application related to this study. The other authors have no conflicts of interest.
REFERENCES
- 1.Gaunt RA, Prochazka A. Transcutaneously coupled, high-frequency electrical stimulation of the pudendal nerve blocks external urethral sphincter contractions. Neurorehabil Neural Repair 2009;23:615–626. [DOI] [PubMed] [Google Scholar]
- 2.Waataja JJ, Tweden KS, Honda CN. Effects of high-frequency alternating current on axonal conduction through the vagus nerve. J Neural Eng 2011;8:056013. [DOI] [PubMed] [Google Scholar]
- 3.Avendano-Coy J, Serrano-Munoz D, Taylor J, Goicoechea-Garcia C, Gomez-Soriano J. Peripheral nerve conduction block by high-frequency alternating currents: a systematic review. IEEE Trans Neural Syst Rehabil Eng 2018;26:1131–1140. [DOI] [PubMed] [Google Scholar]
- 4.Apovian CM, Shah SN, Wolfe BM et al. Two-year outcomes of vagal nerve blocking (vBloc) for the treatment of obesity in the ReCharge trial. Obes Surg 2017;27:169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soin A, Shah NS, Fang ZP. High-frequency electrical nerve block for post-amputation pain: a pilot study. Neuromodulation 2015;18:197–205. [DOI] [PubMed] [Google Scholar]
- 6.Kapural L, Yu C, Doust MW et al. Novel 10-kHz high-frequency therapy (HF10 therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: the SENZA-RCT randomized controlled trial. Anesthesiology 2015;123:851–860. [DOI] [PubMed] [Google Scholar]
- 7.Yang G, Xiao Z, Wang J et al. Post-stimulation block of frog sciatic nerve by high-frequency (kHz) biphasic stimulation. Med Biol Eng Comput 2017;55:585–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Pace N, Cai H et al. Poststimulation block of pudendal nerve conduction by high-frequency (kHz) biphasic stimulation in cats. Neuromodulation 2020, in early view. 10.1111/ner.13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tai C, de Groat WC, Roppolo JR. Simulation of nerve block by high-frequency sinusoidal electrical current based on the Hodgkin-Huxley model. IEEE Trans Neural Syst Rehabil Eng 2005;13:415–422. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Roppolo JR, de Groat WC, Tai C. Mechanism of nerve conduction block induced by high-frequency biphasic electrical currents. IEEE Trans Biomed Eng 2006;53:2445–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang G, Wang J, Shen B, Roppolo JR, de Groat WC, Tai C. Pudendal nerve stimulation and block by a wireless-controlled implantable stimulator in cats. Neuromodulation 2014;17:490–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai H, Morgan T, Pace N et al. Low pressure voiding induced by a novel implantable pudendal nerve stimulator. NeurourolUrodyn 2019;38:1241–1249. [DOI] [PubMed] [Google Scholar]


