Abstract
Despite the improvements in life expectancy, neurodegenerative conditions have arguably become the most dreaded maladies of older people. The neuroprotective and anti-ageing potentials of essential oils (EOs) are widely evaluated around the globe. The objective of this review is to analyse the effectiveness of EOs as neuroprotective remedies among the four common age-related neurodegenerative diseases. The literature was extracted from three databases (PubMed, Web of Science and Google Scholar) between the years of 2010 to 2020 using the medical subject heading (MeSH) terms “essential oil”, crossed with “Alzheimer’s disease (AD)”, “Huntington’s disease (HD)”, “Parkinson’s disease (PD)” or “amyotrophic lateral sclerosis (ALS)”. Eighty three percent (83%) of the studies were focused on AD, while another 12% focused on PD. No classifiable study was recorded on HD or ALS. EO from Salvia officinalis has been recorded as one of the most effective acetylcholinesterase and butyrylcholinesterase inhibitors. However, only Cinnamomum sp. has been assessed for its effectiveness in both AD and PD. Our review provided useful evidence on EOs as potential neuroprotective remedies for age-related neurodegenerative diseases.
Keywords: essential oils, neurodegenerative, Alzheimer’s disease, Huntington’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, in vitro, in vivo
1. Introduction
Aromatic plants consist of a wide and diverse array of organic compounds with significant ecological and physiological functions. One of the most vital components synthesised by aromatic plants are essential oils (EOs), along with its secondary metabolites and phenolic compounds [1]. EOs can be extracted and obtained from various parts of plants, such as the flower, bark, leaf, root, or peel [2,3,4]. Generally, monoterpenes and sesquiterpenes are the main constituents of EOs. Phenolic compounds are generated via biochemical synthesis and consist of a chemically heterogeneous group. Phenolic acids, simple phenols, coumarins, flavonoids, stilbenes, lignans, lignins, as well as hydrolysable and condensed tannins are among the well-established phenolic compounds [5,6]. EOs are volatile and they may play a role in cognitive improvement through olfactory pathways [7]. EOs are well-known for various benefits that include its antiviral, antibacterial, antifungal, memory enhancement, medicinal remedy, food preservation, cosmetic preservative, aromatherapy, and many other applications. For example, EO sourced from Salvia sp., which is one of the most common medicinal plant species, was reported for its notable remedy in cough, bronchitis, herpes, thrush wounds, as well as in impaired concentration. The EO of this species is also applied in the food industry and cosmetic industry, for ranges of perfume products [8].
The brain’s central nervous system (CNS) is composed of diverse neurons responsible for the organisation of neuronal and non-neuronal cells, as well as handling various motor, sensory, regulatory, behavioural, and cognitive functions. The neuronal cells are diverse in their morphology and function, suggesting that each neuronal type may indicate its own genomic profile despite having identical genetic codes. Within the CNS, specific regions were noticed to exhibit different vulnerabilities to ageing and various age-related neurodegenerative diseases [9].
Neurodegenerative disorders are often characterised by strong evidence of oxidative stress in their pathogenesis, as a consequence of unregulated synthesis of reactive oxygen species (ROS) [10]. Disparity observed in pro-oxidant and antioxidant cellular mechanisms inter-related with mitochondrial dysfunction, lipid peroxidation, neuroinflammatory processes, and endogenous dopamine metabolism are among the contributing factors to deregulation [10,11]. Many researchers have searched for molecules that activate blocking pathways or minimise the effects of ROS [12,13]. In an effort to overcome the limitations of current therapeutics available for neurodegenerative disorders, substantial research is being undertaken to explore and identify the availability of other possible natural drugs that are equally effective and without any side effects. As such, natural components composed of various polyphenolic phytochemicals have gained notable insight for this purpose [14].
Neurodegenerative disorders are currently incurable, and the available therapies only control symptoms or prolong the disease’s growth. EOs have been proposed as an underlying preventive and treatment strategies for anti-ageing and neurodegenerative disorders [15]. Many studies have reported the potential of various EOs and their components to exhibit neuroprotective effects [16,17]. Cinnamomum sp. [18,19], Salvia sp. [20,21], Polygonum sp. [22], Lavandula sp. [23,24], Citrus sp. [25], Artemisia sp. [26,27], and Zingiber sp. [28] are among the most widely explored species for evaluating the effectiveness of EOs and its respective components in age-related neurodegenerative disorders. The four most commonly studied age-related neurodegenerative diseases are Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD) and amyotrophic lateral sclerosis (ALS).
AD is the most common cause of dementia in the elderly and is classified as a slow yet progressive neurodegenerative disorder. The highest prevalence rates are reported in North America and Western Europe followed by Latin America, China, and the Western Pacific. In general, it is highlighted that large number of AD cases are noticed among elderly people aged over 75, however early-onset of AD can also develop as early as 30 up to 60 years [29,30]. The direct cost entailed to AD diagnosis covers medical treatment or social services where a caregiver is needed, while a patient’s or family members’ income loss is referred to as indirect cost [31].
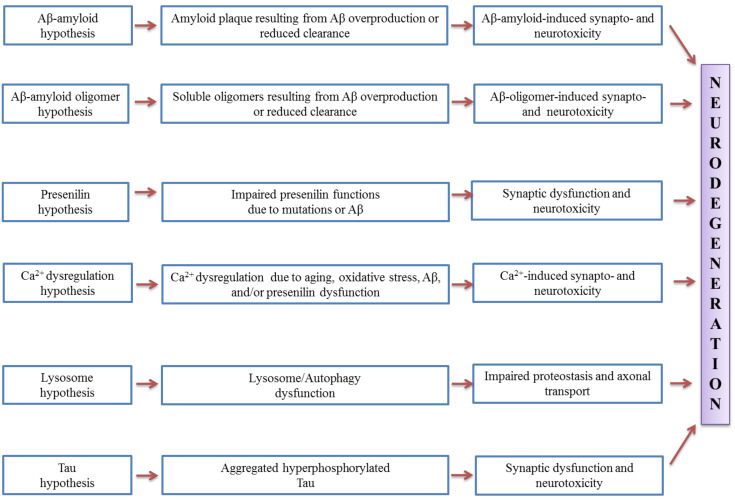
There are several AD profiles, which include deficits in episodic memory, language, semantic knowledge, visuospatial abilities, executive functions in terms of planning and organisation as well as apraxia [32]. Apart from neuronal loss, amyloid plaques and neurofibrillary tangles are inter-related to the presence of reactive astrocytes and activated microglial cells [33,34,35]. Aβ is the most widely studied component of AD pathogenesis, where it can induce neuronal toxicity and activate microglia leading to the indirect damage of neurons [36]. Proteolytic cleavage from the type I cell-surface protein amyloid precursor protein (APP) was known to yield several forms of Aβ [37,38]. The pathogenic hypotheses for synaptic and neuronal toxicity in Alzheimer’s disease is shown in Figure 1.
Figure 1.
Pathogenic hypotheses for synaptic and neuronal toxicity in Alzheimer’s disease (adapted from [39]).
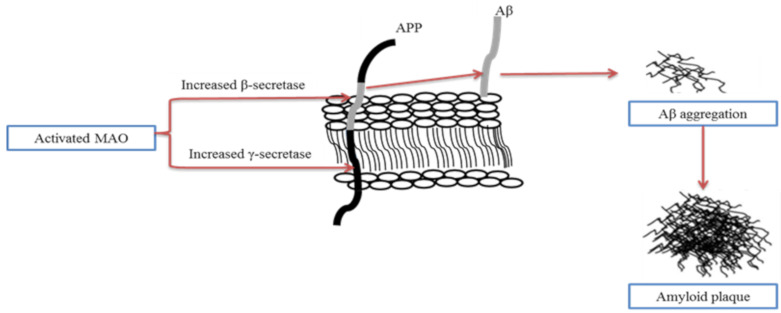
A significant change that correlates to the age-related loss of substantia nigra (SN) dopaminergic cells is the loss of neostriatum dopaminergic innervation. Compelling studies have shown that the involvement of monoamine oxidase (MAO) in AD and neurodegenerative diseases is an important factor in many major pathophysiological pathways [40,41]. MAO-B has been suggested as a biomarker, and its activated form leads to cognitive dysfunction, kills cholinergic neurons, induces cholinergic disorders, and contributes to the development of amyloid plaques. Studies in molecular biology have demonstrated the critical role of Aβ generation through the modulation of the processing of APP by MAO [42,43,44,45]. The mechanism of Aβ generation through modulation of APP processing by activated MAO is shown in Figure 2.
Figure 2.
The mechanism of Aβ generation through modulation of amyloid precursor protein (APP) processing by activated monoamine oxidase (MAO). Activated MAO increases the expression of β-secretase and γ-secretase, improves Aβ generation, and contributes to the formation of amyloid plaques (adapted from [46]).
PD is the second most prevalent condition after AD, and also develops slowly over time [47]. PD can be identified and clinically characterised via motor impairment, which includes bradykinesia, rigidity, resting tremor and postural instability [48]. PD cases can be divided into sporadic (sPD) and familial (fPD), the latter of which represents approximately 20–25% of all PD cases. A common hallmark of sPD and fPD is the presence of intracellular inclusions, termed Lewy bodies [49,50].
α-Synuclein (α-Syn) has been identified as a major component of Lewy bodies in sporadic and familial cases, and is believed to be the central player in PD aetiology [51]. It is worthwhile to note that research conducted on PD has mainly focused on protein aggregation, neurotoxicity, increased oxidative stress, and mitochondrial dysfunction, as well as defects in the protein degradation machinery [52].
Apart from the role caused by α-Syn, the presence of neurotoxins, in particular 6-hydroxydopamine (6-OHDA) and 1-methyl-4-phenylpyridinium (MPP+), are widely accepted to induce neurotoxicity in PD patients. Both neurotoxins are thought to induce dopaminergic toxicity by intra- and extracellular oxidation, hydrogen peroxide formation, and direct inhibition of the mitochondrial respiratory chain [53].
The next common neurodegenerative disease is HD; caused by the recurrent development of cytosine–adenine–guanine (CAG) in the huntingtin (HTT) gene and involves a network of complex pathogenic mechanisms. HD is a profoundly penetrating, autosomal dominant, progressive neurodegenerative movement and neurobehavioural disorder associated with a variety of motor signs, psychological symptoms and cognitive dysfunction that progress with dementia. Knowledge of HD’s causal mutation allows the detection of an ever-expanding number of HD phenotypes and phenocopies. The mean starting age is around 40 years, with a recorded range of 2 to 79+ years [54,55,56,57].
Progressive physical impairment of HD could be contributed to by various movement aspects such as hyperkinetic movements (dystonia, myoclonus, tics) and other motor manifestations (bradykinesia, incoordination, oculomotor function changes, gait impairment) along with chorea as the most distinct involuntary motion. As the disease progresses over time, dystonia becomes more prevalent and replaces chorea.
On the other hand, ALS is categorised as a heterogeneous neurodegenerative condition clinically, genetically, and pathologically [58,59,60]. The degeneration of cortical motor neurons and anterior horn cells of the spinal cord is characterised by ALS, also known as Charcot’s or Lou Gehrig’s disease. This contributes, usually within 3–5 years of diagnosis, to muscle atrophy, loss of muscle function, and death resulting from respiratory failure. The diverse clinical variability in ALS is believed to be due to differences in upper motor neuron (UMN) and lower motor neuron (LMN) involvement, extra-motor symptoms, onset age, survival, and progression rates. Heterogeneity of the disease prevents biomarker production which hinders the accurate evaluation of candidate drugs in clinical trials [59,60,61]. Various studies have shown that oxidative stress plays a major role in this disease’s pathogenesis, identified as an unusual family type that often exhibits superoxide dismutase 1 (SOD1) gene mutations [62,63].
EOs are now commonly used and their advantages in all facets of life are investigated. The attractiveness of EO potential and possible mechanism of actions are ventured on a continual basis around the globe. In this review, we focus on and analyse the effectiveness of EOs as neuroprotective remedies among the four selected age-related neurodegenerative disorders mentioned above. We strongly believe that this review will be beneficial to many researchers and academicians that have great interest in EOs and their extensive applications.
2. Materials and Methods
Search Strategy
Original articles were searched in three databases (PubMed, Web of Science and Google Scholar) from the year 2010 to 2020, using the medical subject heading (MeSH) terms “essential oils”, crossed with the term “Alzheimer’s disease”, “Parkinson’s disease”, “Huntington’s disease” or “amyotrophic lateral sclerosis”. Publications with available abstracts were reviewed and limited to studies published in the English and Malay languages. Papers on human and animal studies, clinical trials, and related to plant-based neurodegenerative medication were included. However, review articles and letters to the editor were excluded. Duplicate articles were eliminated.
3. Results
All the related articles were printed out for further evidence-based assessment to explore the effectiveness of EOs as a neuroprotective remedy for age-related neurodegenerative diseases. After conducting the comprehensive literature review, the articles were selected and divided into several types of neurodegenerative diseases (Table 1). A total of 103 articles were included in this review. Eighty-six articles on AD were found, which consisted of 53 articles on in vitro studies, 20 articles on in vivo studies, 11 articles on the combination of in vitro and in vivo studies, and 2 articles on a combination of in vitro and ex vivo studies. Thirteen articles on PD were found, which consisted of four articles on in vitro studies, six articles on in vivo studies, one article on the combination of in vitro and in vivo studies, and two articles on a combination of in vivo and ex vivo studies. Four articles were categorised as a combination of diseases because several neurodegenerative diseases were mentioned in the articles at once. Unfortunately, no classifiable study was recorded on HD and ALS. Besides the standard biochemical assay, some studies also reported on the chemical composition of the selected EOs.
Table 1.
Effects of essential oils (EOs) on neurodegenerative diseases.
| No. | Disease | Study | Source of Essential Oils | Ref | Method | Findings | Conclusions and/or Recommendations |
|---|---|---|---|---|---|---|---|
| 1. | ALZHEIMER’S DISEASE | In vitro | Ajuga chamaecistus subsp scoparia (Boiss.) Rech.f. | [64] |
|
|
|
| 2. | Allium tuncelianum (Amaryllidaceae) | [65] |
|
|
|
||
| 3. | Aloysia citrodora Palau | [66] |
|
|
|
||
| 4. | ALZHEIMER’S DISEASE | In vitro | Artemisia macrocephala | [26] |
|
|
|
| 5. | Artemisia maderaspatana | [67] |
|
|
|
||
| 6. | Artemisia absinthium | [27] |
|
|
|
||
| 7. | ALZHEIMER’S DISEASE | In vitro | Boswellia dalzielii | [68] |
|
|
|
| 8. |
Chamaecyparis obtusa
Cryptomeria japonica |
[69] |
|
|
|
||
| 9. | Cinnamomum tamala (Buch.-Ham.) | [18] |
|
|
|
||
| 10. | ALZHEIMER’S DISEASE | In vitro | Cinnamomum zeylanicum | [19] |
|
|
|
| 11. | Citrus Sinensis [L.] Osbeck | [70] |
|
|
|
||
| 12. | ALZHEIMER’S DISEASE | In vitro |
Citrus aurantifolia Swingle,
C. aurantium L. C. bergamia Risso and Poit. |
[71] |
|
|
|
| 13. |
Cistus creticus
Cistus salvifolius Cistus libanotis Cistus monspeliensis Cistus villo-sus |
[72] |
|
|
|
||
| 14. | Clinopodium serpyllifolium (M.Bieb.) Kuntze | [73] |
|
|
|
||
| 15. | Daucus aristidis Coss. | [74] |
|
|
|
||
| 16. | ALZHEIMER’S DISEASE | In vitro | Hedychium gardnerianum,Sheppard ex Ker-Gawl | [75] |
|
|
|
| 17. | Hymenocrater bituminous | [76] |
|
|
|
||
| 18. | Illicium verum Hook.f | [77] |
|
|
|
||
| 19. | ALZHEIMER’S DISEASE | In vitro | Lavandula luisieri | [78] |
|
|
|
| 20. | Lavandula angustifolia | [24] |
|
|
|
||
| 21. | Lavandula pubescens | [23] |
|
|
|
||
| 22. | Lavandula viridis L’Her | [79] |
|
|
|
||
| 23. | ALZHEIMER’S DISEASE | In vitro | Mentha spicata L. | [80] |
|
|
|
| 24. | Neomitranthes obscura (DC.) N. Silveira | [81] |
|
|
|
||
| 25. | ALZHEIMER’S DISEASE | In vitro | Origanum rotundifolium Boiss. | [82] |
|
|
|
| 26. |
Panax ginseng
Panax japonicus P. notoginseng P. quinquefolius |
[83] |
|
|
|
||
| 27. | Pinus sp. | [84] |
|
|
|
||
| 28. | ALZHEIMER’S DISEASE | In vitro | Piper divaricatum | [85] |
|
|
|
| 29. | Polygonum hydropiper L. | [22] |
|
|
|
||
| 30. | Prangos gaubae | [86] |
|
|
|
||
| 31. | ALZHEIMER’S DISEASE | In vitro | Rumex hastatus D. Don | [87] |
|
|
|
| 32. | Salvia officinalis | [88] |
|
|
|
||
| 33. | Salvia chionantha | [89] |
|
|
|
||
| 34. | Salvia chrysophylla Staph | [90] |
|
|
|
||
| 35. | ALZHEIMER’S DISEASE | In vitro | Salvia urmiensis | [20] |
|
|
|
| 36. | Salvia nemorosa L. | [91] |
|
|
|
||
| 37. | Salvia officinalis L. | [21] |
|
|
|
||
| 38. | ALZHEIMER’S DISEASE | In vitro | Salvia syriaca L. | [92] |
|
|
|
| 39. | Satureja thymbra L. | [93] |
|
|
|
||
| 40. |
Stachys inflata,
S. lavandulifolia, S. byzantina |
[94] |
|
|
|
||
| 41. | ALZHEIMER’S DISEASE | In vitro | Sideritis galatica Bornm. | [95] |
|
|
|
| 42. |
S. ballsiana
S. cyanescens S. divaricata S. hydrangea S. kronenburgii S. macrochlamys S. nydeggeri S. pachystachys S. pseudeuphratica S. rusellii |
[96] |
|
|
|
||
| 43. | Thymus mastichina L. | [97] |
|
|
|
||
| 44. | ALZHEIMER’S DISEASE | In vitro | Thymus haussknechtii Velen. | [98] |
|
|
|
| 45. | Thymus lotocephalus | [99] |
|
|
|
||
| 46. | ALZHEIMER’S DISEASE | In vitro | Zingiber cassumunar | [100] |
|
|
|
| 47. | Zosima absinthifolia Link | [14] |
|
|
|
||
| 48. | ALZHEIMER’S DISEASE | In vitro | Calamintha nepeta, Foeniculum vulgare, Mentha spicata and Thymus mastichina | [101] |
|
|
|
| 49. | Cymbopogon citratus (Gramineae), Citrus hystrix (Rutaceae) and Zingiber cassumunar (Zingiberaceae) | [102] |
|
|
|
||
| 50. |
Alpinia galanga Linn., Centella asiatica Urban., Cinnamomum bejolghota (Buch. Ham.) Sweet, Citrus aurantifolia Swing, Citrus hystrix DC., Citrus maxima (Burm.) Merr., Citrus reticulata Blanco cv. Shogun, Citrus reticulata var. Fremont, Cymbopogon citratus Stapf., Eupatorium odoratum Linn,. Melissa officinalis Linn., Ocimum basilicum Linn., Ocimum canum Sims., Ocimum gratissimum Linn., Ocimum sanctum Linn., Piper sarmentosum Roxb., Polygonum odoratum Lour., Polyscias fruticosa, Harms.Zingiber cassumunar Roxb,. Zingiber officinale Rosco |
[25] |
|
|
|
||
| 51. | ALZHEIMER’S DISEASE | In vitro |
Lavandula angustifolia
Coriandrum sativum |
[103] |
|
|
|
| 52. |
Artemisia annua L. (Asteraceae) Glycyrrhiza glabra L. (Fabaceae) |
[104] |
|
|
|
||
| 53. | ALZHEIMER’S DISEASE | In vitro |
Angelica sinensis (Oliv) Diels. Ligusticum Chuanxiong Hort |
[105] |
|
|
|
| 54. | In vivo | Achillea biebersteinii (Asteraceae) | [106] |
|
|
|
|
| 55. | ALZHEIMER’S DISEASE | In vivo |
Boswellia serrata (Burseraceae) |
[107] |
|
|
|
| 56. | Chamaecyparis obtuse | [108] |
|
|
|
||
| 57. | ALZHEIMER’S DISEASE | In vivo | Citrus limon | [109] |
|
|
|
| 58. | ALZHEIMER’S DISEASE | In vivo |
Cymbopogon giganteus
Illicium pachyphyllum Carum carvi |
[110] |
|
|
|
| 59. | Ferulago angulata | [111] |
|
|
|
||
| 60. | ALZHEIMER’S DISEASE | In vivo |
Lavandula angustifolia ssp. angustifolia Mill. (Lamiaceae) Lavandula hybrida Rev. (Lamiaceae) |
[112] |
|
|
|
| 61. | ALZHEIMER’S DISEASE | In vivo | Ocimum basilicum | [113] |
|
|
|
| 62. |
Ocimum sanctum L.
Ocimum basilicum L. |
[114] |
|
|
|
||
| 63. | ALZHEIMER’S DISEASE | In vivo | Pimpinella peregrina | [115] |
|
|
|
| 64. | Pinus halepensis | [116] |
|
|
|
||
| 65. | ALZHEIMER’S DISEASE | In vivo | Kushui rose (Rosa setate × Rosa rugosa) | [117] |
|
|
|
| 66. | ALZHEIMER’S DISEASE | In vivo | Rosmarinus officinalis | [118] |
|
|
|
| 67. | Salvia miltiorrhiza Bge | [119] |
|
|
|
||
| 68. | ALZHEIMER’S DISEASE | In vivo | Schisandra chinensis | [120] |
|
|
|
| 69. | SuHeXiang Wan (SHXW) | [121] |
|
|
|
||
| 70. | ALZHEIMER’S DISEASE | In vivo | Tetraclinis articulata | [122] |
|
|
|
| 71. | Zataria multiflora Boiss. | [123] |
|
|
|
||
| 72. | ALZHEIMER’S DISEASE | In vivo | Z. multiflora Boiss. | [124] |
|
|
|
| 73. |
Origanum vulgare
Thymus vulgaris |
[125] |
|
|
|
||
| 74. | ALZHEIMER’S DISEASE | In vitro and In vivo | Acori graminei | [126] |
|
|
|
| 75. | ALZHEIMER’S DISEASE | In vitro and In vivo | Foeniculi vulgare aetheroleum | [127] |
|
|
|
| 76. | Lavandula angustifolia Mill. | [128] |
|
|
|
||
| 77. | ALZHEIMER’S DISEASE | In vitro and In vivo | Lavandula angustifolia Mill. | [129] |
|
|
|
| 78. | Lavandula luisieri | [130] |
|
|
|
||
| 79. | ALZHEIMER’S DISEASE | In vitro and In vivo | Mentha longifolia | [131] |
|
|
|
| 80. | Salviae aetheroleum | [8] |
|
|
|
||
| 81. | SHXW | [132] |
|
|
|
||
| 82. | ALZHEIMER’S DISEASE | In vitro and In vivo | Zelkova serrata | [133] |
|
|
|
| 83. | ALZHEIMER’S DISEASE | In vitro and In vivo | Zingiber officinale | [134] |
|
|
|
| 84. |
Pistacia khinjuk
Allium sativum |
[135] |
|
|
|
||
| 85. | ALZHEIMER’S DISEASE | In vitro and Ex vivo | Salvia sp | [136] |
|
|
|
| 86. |
Syzygium aromaticum
Xylopia aethiopica |
[137] |
|
|
|
||
| 87. | PARKINSON’S DISEASE | In vitro |
Cinnamomum verum
Cinnamomum cassia |
[138] |
|
|
|
| 88. | Eryngium sp. | [139] |
|
|
|
||
| 89. | PARKINSON’S DISEASE | In vitro |
Myrtus communis
Ferula gummosa Eucalyptus globulus Satureja hortensis Rosmarinus officinalis Mentha spicata Mentha piperita Cuminum cyminum Artemisia dracunculus Citrus sinensis Citrus limonum Thymus vulgaris Lavandula officinalis Mentha pulegium Foeniculum vulgare |
[140] |
|
|
|
| 90. | Cuminum cyminum | [141] |
|
|
|
||
| 91. | PARKINSON’S DISEASE | In vivo | Acorus tatarinowii Schott (Shi Chang Pu) | [142] |
|
|
|
| 92. | Acorus tatarinowii Schott (Shi Chang Pu) | [143] |
|
|
|
||
| 93. | PARKINSON’S DISEASE | In vivo | Eplingiella fruticosa | [144] |
|
|
|
| 94. | Foeniculum vulgare | [145] |
|
|
|
||
| 95. | PARKINSON’S DISEASE | In vivo | Pulicaria undulata | [146] |
|
|
|
| 96. |
Zingiber officinale
Syzygium aromaticum |
[28] |
|
|
|
||
| 97. | PARKINSON’S DISEASE | In vitro and In vivo | SHXW | [147] |
|
|
|
| 98. | PARKINSON’S DISEASE | In vivo and ex vivo |
Rosa damascena Mill.
Lavandula angustifolia Mill, |
[148] |
|
|
|
| 99. | PARKINSON’S DISEASE | In vivo and ex vivo | Rosa Damascena Mill. | [149] |
|
|
|
| 100. | OTHER NEURODEGENERATIVE DISEASE | In vitro | Lavandula pedunculata subsp lusitanica (Chaytor) Franco | [150] |
|
|
|
| 101. | Pelargonium graveolens | [151] |
|
|
|
||
| 102. | OTHER NEURODEGENERATIVE DISEASE | In vitro |
Myrcianthes myrsinoides (Kunth) Grifo Myrcia mollis (Kunth) DC. (Myrtaceae) |
[152] |
|
|
|
| 103. | OTHER NEURODEGENERATIVE DISEASE | In vivo | Anthriscus nemorosa | [153] |
|
|
|
4. Discussion
EOs contain the essence of different scents and the properties from their originating plants. These volatile oils display various biological activities [154]. They are mainly used in the beverage, fruit, cosmetic, and fragrance industries [155]. EOs derived from steam distillation process are mainly used in pharmacological activities and food products, while the extracts from lipophilic solvents are utilised in the fragrance industry [156]. Several EOs have been well-known for their usage in fragrances and flavours for hundreds of years. EO usage in the fragrance industry is mainly due to by their attractive odour. The extensive benefits offered by EOs signify the continuous demand that is seen to be increasing steadily.
4.1. The Source of EOs
As mentioned before, EOs can be extracted and obtained from various parts of plants. Clove’s EO derived from the Syzygium aromaticum tree’s aromatic flower buds with origin from Maluku, Indonesia contains the powerful scent used in spiced foods [154,155]. Eucalyptus globulus oil is mint-like, with properties such as a decongestant, pain relievers, antimicrobial agent, immunostimulant, flu and cold/cough treatment, as well as for mental clarity in aromatherapy [157,158]. One of the most influential EO is from Lavandula angustifolia, which is also known as English lavender. Lavender oil possesses strong antioxidant, anti-inflammatory, antibacterial, and antimicrobial properties, and can be used to treat various skin diseases (e.g., eczema, ringworm, acne), improve digestive system, minimise sore muscle swelling, and additionally, alleviate pain [157,158]. Citrus limon EOs are used as antimicrobial and antifungal agents, pain relievers, aids in weight loss, and to reduce extreme nausea as well as for usage as soaps, hair shampoo, furniture polishes, and fresheners [157,158]. Oregano (Origanum vulgare) EOs are often used for skin care, menstrual problems, stomach problems, and to control flu and cold infections [157,158]. Rosemary’s EO originates from the Rosmarinus officinalis evergreen shrub with characteristics of a crisp woody, herbal and balsamic odour, similar to camphor. The usage of rosemary oil ranges from various treatments of skin care, dandruff, and scalp health, as well as to cold prevention and boosting the immune system [157,158]. EO from Mentha piperita is called peppermint oil, which is mainly used in the prevention of flu and colds, reduces headache symptoms, and also in relieving muscle and joint pains [157,158].
Apart from the general usage of EOs, their extensive benefits have also been noticed and reported in relevance to age-related neurodegenerative disorders. Based on available studies, EOs have been proposed as an effective preventive and treatment approach for anti-ageing and neurodegenerative disorders. Therefore, we attempted to describe and highlight the various EOs, and the effectiveness of their components with respect to the four common neurodegenerative diseases (AD, PD, HD, and ALS), as mentioned above. The different parameters that are commonly used for the evaluation of each disease are explained, accordingly.
Based on Table 1, a total of sixty-nine types of EOs from different genera of plants were evaluated for their effectiveness against neurodegenerative diseases among studies conducted between 2010 and 2020. In reference to all the compiled literature, we observed that the IC50 results were presented in several formats, in particular to the units of measurement. Among the units that were reported included mg gallic acid equivalents/g [64,76], percentage [65,74], mg/mL or mg/L [26,67,68,73,75], μm [69], and µg/mL [18,70,71,72]. This diversity, however, was considered troublesome because direct comparison among studies with different measurement units is not possible without conversion.
4.2. Major Component of EOs
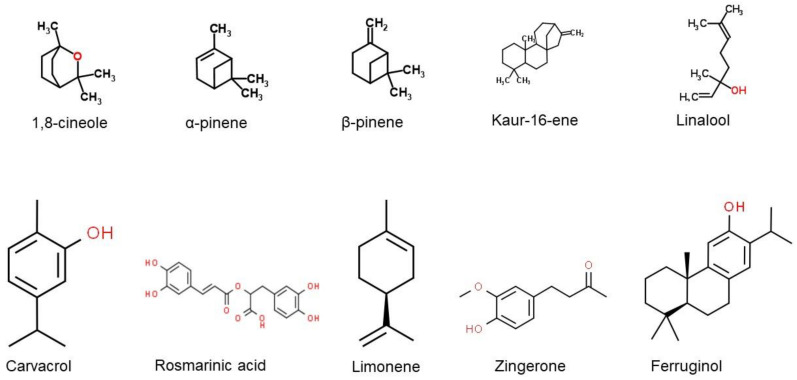
The chemical structures of several major components commonly found in EOs that have been reported to have anti-neurodegenerative properties are presented in Figure 3. Based on our review, 1,8-cineole has been identified as one of the major components found from various types of EOs. The compound 1,8-cineole is a saturated monoterpene that can originate from several plant species (e.g., Eucalyptus, Rosmarinus, and Salvia), with Eucalyptus leaves recognised as the key source [159]. Sometimes called eucalyptol due to its natural source, 1,8-cineole should not be confused with eucalyptus oil, a combination of many other components [160]. Due to its excellent aroma and taste, 1,8-cineole is mostly used in fruit, fragrances, and cosmetics. Furthermore, pure monoterpene 1,8-cineole is used as an alternative sinusitis remedy for respiratory tract infections, such as common cold or bronchitis [161]. It was indicated as one of the most potent free radical scavengers that may influence anticholinesterase activity based on a study reported by El Euch and colleagues [88] The antioxidant activity was measured using the free radical 1,1-diphenyl-2-picrylhydrazyl (DPPH) test. Essential oil concentration providing 50% inhibition (IC50) of the initial DPPH concentration was calculated using the linear relationship between the compound concentration and the percentage of DPPH inhibition. Ascorbic acid was used as a standard. In the study by Abuhamdah et al. [66], EO extracted from the leaves of Aloysia citrodora Palau showed neuroprotective activity and a higher presence of 1,8-cineole was reported (23.66%).
Figure 3.
Chemical structures of the major EO components that have been reported to have anti-neurodegenerative properties. (obtained from [162,163,164,165,166,167,168,169,170,171]).
In another study performed by Cutillas and his team [97] on EO from Thymus mastichina L., it was asserted that among all four compounds (α-pinene, β-pinene, limonene and 1,8-cineole), 1,8-cineole was the best AChE inhibitor with an IC50 of 35.2 ± 1.5 μg/mL. They tested the antioxidant activity using five different methods such as the oxygen radical absorbance capacity (ORAC) assay, the 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) antioxidant method, the 2,2-diphenyl-1-picrylhydrazyl (DPPH) method, the thiobarbituric acid reactive substances (TBARS) method, and the chelating power (ChP) method. All the methods recorded that 1,8-cineole was one of the compounds highest in antioxidant capacity. This finding was similar to several other types of research, which suggested the efficacy of 1,8-cineole as the dominant anticholinesterase agent from EOs of different sources [8,21,25,152].
4.3. Cholinesterase Activity in EOs
The most common criteria used in the determination of AD is related to anti-cholinesterase activity. Cholinesterases (ChEs) are specialised carboxylic ester hydrolases that catalyse the hydrolysis of choline esters. Two types of ChE activity have been identified in mammalian blood and tissues, which are distinguished according to their substrate specificity and sensitivity to selective inhibitors. The first is acetylcholinesterase (AChE), which is systematically known as acetylcholine acetylhydrolase [172]. The second is butyrylcholinesterase (BChE), which is systematically referred to as acetylcholine acyl hydrolase [173,174,175,176]. The preferred substrate for AChE is acetylcholine (ACh), while butyrylcholine (BCh) and propionylcholine (PCh) are ideal for BChE [175,176,177]. AChE activity is known to be inhibited by several compounds, with toxins and drugs as the major inhibitors [178]. AChE activity is used in verifying treatment effects, especially in AD [177].
Both AChE and BChE possess active sites at the bottom of 20 Å-deep gorges with 50% identical amino acid sequence, whereas the gorge entrance locates the peripheral site [179]. The active site for both enzymes comprises a catalytic triad, acyl-binding pocket, and choline binding site [180]. A total of 14 aromatic amino acids are found in the active site of AChE, whereas six of these are substituted by aliphatic amino acids for BChE [181]. Binding and hydrolysis processes of bulky ligands are restricted in AChE due to presence of phenylalanine residues in the acyl binding pocket. In contrast, these residues are substituted with two flexible amino acids that are selective for BChE and allow the binding of bulkier ligands [182]. The different mechanisms involved in relevance to the active gorge site specific for each enzymes have been investigated via molecular modelling, structure-based virtual screening, or even crystallographic studies [181,182,183,184].
Generally, traditional Ellman assay is used with some modifications, applied for the determination of anti-cholinesterase activities [185,186]. This technique is a simple, accurate, and rapid method of measuring ChE activity, that is based on the reaction between thiocholine with the sulfhydryl group of a chromogen such as 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB or Ellman’s reagent). The shift of electrons to sulphur atoms yields a yellow substance called 5-thio-2-nitrobenzoic acid (TNB), which is measured by monitoring absorbance at 410 nm [187,188,189,190]. DTNB is a water-soluble compound and is useful for its fast reaction with thiocholine and minor side effects at neutral pH [185,187,188,189,190,191]. This technique, however, is also subject to certain limitations; it is restricted for testing antidots against organophosphorus AChE inhibitors or for measuring AChE activity in samples of such treated individuals [190]. In addition to the Ellman assay, another method that can also be used for measuring ChE activities is the electrometric method of Michel [192]. This technique is applied based on pH changes that arise from H+ synthesis via cholinester hydrolysis [175,176,193].
4.4. Extracellular Plaque Deposits
Extracellular plaque deposits of the Aβ-peptide and flame-shaped neurofibrillary tangles of the microtubule-binding protein tau are the two hallmark pathologies required for AD patients. Familial early-onset forms of AD are associated with mutations either in the precursor protein for Aβ (APP) or in presenilin-1 (PS1) or presenilin-2 (PS2). Peptide generation pathways synthesise γ-secretase with either PS1 or PS2 as the catalytic subunit. APP is sequentially cleaved, where β-secretase first cleaves APP to release a large, secreted derivative, sAPPβ, followed by γ-secretase that cleaves a fragment of 99 amino acids (CTFβ) to generate Aβ. The process of γ-secretase cleaving can be inaccurate, leading to C-terminal heterogeneity of the resulting peptide population that generates numerous Aβ species, with Aβ1–-40 of the highest abundance followed by Aβ42. The slightly longer forms of Aβ, particularly Aβ1–42, are the principal species deposited in the brain that are more hydrophobic and fibrillogenic [193]. In view of their vital role in Aβ synthesis, both β- and γ-secretase are considered as key components in anti-AD pharmaceuticals developments [193,194]. Normal pathology tests refer to the density in the affected brain regions of neuritic amyloid plaques and neurofibrillary tangles of tau protein. AD diagnosis involves the presence of large neuritic plaque portions, consisting of highly insoluble Aβ in the brain parenchyma. There are also deposits of tau protein, although they occur among less common neurodegenerative disorders, especially in the absence of neuritic plaques. There are some distinctive morphological features of the neurofibrillary tangles in the various diseases, and may exhibit a distinct composition of tau isoforms that vary from AD [195].
It is not only humans which have amyloid beta; non-human primates (NHPs) have the same Aβ sequences as humans, an almost identical APP sequence, and they overlap with related human biochemical pathways in many aspects, however surprisingly with ageing, they develop relatively few AD-like neuropathologies. Aged canines also develop severe amyloid deposition; canines tend to demonstrate extensive amyloid deposition from about ten years of age, unlike in aged NHPs, where it could take several decades [196]. Amyloid deposition in canines is also interrelated with age-related cognitive dysfunction [197], although little neuronal loss is detected. Due to a poor understanding of AD and the human brain complexity, it has been deduced that there is no natural animal model of the disease [198]. For the past 25 years, pharmacological and genetic AD-models, as well as various animal species (primates, dogs, rodents, etc.), have been used in AD research activities [199,200]. The resurgence of interest in rats as the appropriate animal model of AD led to the usage of various types of rat models. As of current practice, transgenic mice have been extensively used in studies on AD. In any selected models, all need the introduction of some combined familial AD mutation into APP or PS1, or even in both [200].
4.5. Current EOs on AD
Salvia is the largest genus of plants in the family Lamiaceae, with the number of species estimated to range from 700 to nearly 1000. Fifteen species of Salvia (namely, officinalis L., chionantha, chrysophylla Staph, urmiensis, nemorosa L., syriaca, ballsiana, cyanescens, divaricate, hydrangea, kronenburgii, macrochlamys, nydeggeri, pachystachys, pseudeuphratica, and rusellii) were studied for cholinesterase inhibition assay, and happen to be the most widely studied source of EOs. Most of the researchers found that Salvia spp. were weak AChE and BChE inhibitors, except for EO from S. pseudeuphratica, which demonstrated the highest inhibitory activity against AChE, at IC50 = 26.00 ± 2.00 μg/mL compared to S. cyanescens and S. pachystachys. In contrast, BChE activity did not show 50% inhibition even at the highest concentration, where IC50 was reported to be above 80 μg/mL for S. pseudeuphratica and S. hydrangea [96]. Salvia officinalis was studied twice, in 2017 and 2019. Based on GC–MS and GC–FID analysis, the main components of S. officinalis were α-thujone, camphor, 1,8-cineole, and β-thujone. Salvia has also been used in ex vivo-based research, using the isolated guinea pig ileum method where the major molecule, rosmarinic acid, showed significant contraction responses on an isolated guinea pig ileum. Docking results of rosmarinic acid also showed a high affinity to the selected target, AChE [136]. The author suggested the potential of rosmarinic acid to become a novel therapeutic candidate for the treatment of AD.
Other than Salvia spp., EOs from Lavandula spp. have also been studied for the treatment of AD. L. luisieri has been found to comprise high contents of oxygen-containing monoterpenes, mainly necrodane derivatives, which are absent from any other oil. This oil was tested on the endogenous beta-site APP-cleaving enzyme 1 (BACE-1) in cultured cells, being responsible for a reduction in Aβ production, with no significant toxicity. Although the study was conducted in vitro, the low molecular weight and high hydrophobicity of terpenoids are properties that provide a good chance for them to cross cellular membranes and the blood–brain barrier, an essential attribute for BACE-1 inhibition in vivo [78]. However, EO from L. angustifolia did not give a required finding because it enhanced the Aβ aggregation based on the thioflavin T method; this effect was further confirmed by atomic force microscope (AFM) imaging. EO of L. angustifolia was also showed to counteract the increase in intracellular reactive oxygen species production and the activation of the pro-apoptotic enzyme caspase-3 induced by Aβ1–42 oligomers [23,24]. Meanwhile, EO from L. pubescens exhibited strong anti-AChE and anti-BChE effects at IC50 of 0.9 μL/mL and 6.82 μL/mL, respectively. Carvacrol (CAR, 2-methyl-5-isopropylphenol) was also found to be higher in L. pubescens. Carvacrol was found to be abundant among EOs of the Lamiaceae family, and is known for various benefits including antibacterial, antifungal, antioxidant, antinociceptive, anti-inflammatory, anti-apoptosis, and anti-cancer activities [201]. Several studies on EOs have reported that carvacrol exerts some actions on the neuronal system, including AChE inhibition [104,202], anxiolytic [203], and antidepressant [204] properties. In addition, carvacrol has the ability to modulate central neurotransmitter pathways, such as dopaminergic, serotonergic and γ-aminobutyric acid (GABA)-ergic systems [201].
Only two types of cell lines, SH-SY5Y and PC-12, were reported to have been used in AD research. The in vitro toxic effects of amyloid peptides are usually examined using the human neuroblastoma-derived SH-SY5Y cell line, because differentiated neuron-like SH-SY5Y cells are extra-sensitive to amyloid peptides compared to non-differentiated cells, because the latter lack long neurites [205]. Z-ligustilide (Z-LIG) EOs effectively protect against fibrillar aggregates of Aβ25–35- and Aβ1–42-induced toxicity in SH-SY5Y and differentiated PC12 cells, possibly through the concurrent activation of the PI3-K/Akt pathway and inhibition of the p38 pathway [105]. Aβ25–35 represents a neurotoxic fragment of Aβ1–40 or Aβ1–42, and retains the toxicity of the full-length peptide [206]. Aβ25–35 is often selected as a model for full-length peptides because it retains both its physical and biological properties [207]. In general, declining levels of PI3K subunits as well as blunted Akt kinase phosphorylation have been observed in the AD brain, which is characterised by Aβ and tau pathologies [208].
There was also a study on the potential therapeutic effect of hybrid EO from Kushui roses. Kushui rose (R. setate × R. rugosa) refers to a natural hybrid of cog rose and traditional Chinese rose that has been cultivated for more than 200 years [209]. In this study, transgenic worm strains purchased from the Caenorhabditis Genetics Center (CGC) were used instead of rat or mice models. They found that rose EO (REO) significantly inhibited AD-like symptoms of worm paralysis and hypersensitivity to exogenous serotonin (5-HT) in a dose-dependent manner. Although the GC–MS analysis revealed the presence of 40 components, the major components, β-citronellol and geraniol, were found to act less effectively than the oil itself. Intriguingly, REO significantly suppressed Aβ deposits and reduced the Aβ oligomers to alleviate the toxicity induced by Aβ overexpression [209].
Su He Xiang Wan (SHXW) has also been studied for its neurodegenerative remedy potential. SHXW is a distinct EO, and is a patent medicine comprising borneol, styrax resin, musk, aquilaria, frankincense, piper, benzoin, saussurea, cyperus, sandalwood, clove, terminallia, aristolachia fruit, rhino horn, and cinnabar. This ancient prescription was recorded in the He Ji Ju Fang of the Song Dynasty [210]. For this plant, the researchers evaluated the effects of a modified SHXW (KSOP1009 formulation) intake on the AD-like phenotypes of Drosophila AD models, which express human Aβ1–42 in their developing eyes or neurons. They found that Aβ1–42-induced eye degeneration, apoptosis, and locomotive dysfunctions were strongly suppressed. However, Aβ1–42 fibril deposits in the Aβ1–42 overexpressing model were not affected by treatment with KSOP1009 extract. Conversely, KSOP1009 extract intake significantly suppressed the constitutive active form of hemipterous, a c-Jun N-terminal kinase (JNK) activator, while it induced eye degeneration and JNK activation. In Drosophila, flies with mutations that augment JNK signalling accumulate less oxidative damage and live dramatically longer than wild-type flies [211,212].
Cinnamomum zeylanicum consisting of (E)-cinnamaldehyde (CAL) (81.39%) and (E)-cinnamyl acetate (CAS) (4.20%) as the main compounds showed over 78.0% inhibitory activity in cholinesterase. In MAO-A and MAO-B inhibition assays, EOs from C. zeylanicum (96.44%, 95.96%) and CAL (96.32%, 96.29%) demonstrated comparable activity to rasagiline (97.42%, 97.38%, respectively). Research by Murata and co-workers [69] found that kaur-16-ene, nezukol, and ferruginol isolated from plants had anti-AChE (IC50) activity at 640, 300 and 95 μm, respectively. Even though ferruginol activity has already been highlighted before, by Gulacti et al. [213], this study documented the activities of kaur-16-ene and nezukol for the first time.
Meanwhile, Citrus limon has been found to significantly lower AChE brain depression in APP/PS1 and wild-type C57BL/6L (WT) mice. PSD95/synaptophysin, the synaptic density index, was substantially improved in histopathological shifts [109]. Based on the previous analysis by other researchers, nobiletin 3′,4′,5,6,7,8-hexamethoxyflavone was found to be the major component of polymethoxylated flavones in citrus peels, such as C. depressa, C. reticulata, C. sinensis, and C. limon [214,215]. Thus, nobiletin may potentially be the compound that substantially alters the development of these diseases. Other than that, Acori graminei, which was found to be rich in β-asarone, enhanced cognitive function of AβPP/PS1 mice and decreased neuronal apoptosis in the AβPP/PS1 mouse cortex. In addition, a substantial increase in the expression of CaMKII/CREB/Bcl-2 was observed in the cortex of AβPP/PS1 mice treated with β-asarone.
In a study conducted by Ayuob et al. [113], Ocimum basilicum up-regulated the serum corticosterone level, the hippocampal protein glucocorticoid receptor, and the brain-derived neurotropic factor (BDNF); however, it down-regulated the neurodegenerative and atrophic changes induced in the hippocampus, which decreased after exposure to chronic unpredictable mild stress (CUMS). According to the data collected by Avetisyan and co-workers [216], the major components of O. basil includes methyl chavicol and linalool. Interestingly, many linalool-producing plants are commonly used in folk medicine and aromatherapy to alleviate symptoms and treat multiple acute and chronic diseases [217,218]. Linalool is frequently used in the manufacture of fragrances for shampoos, soaps, detergents, and in pharmaceutical formulations [219,220]. Research conducted by Sabogal-Guáqueta et al. [221] found that oral administration of monoterpene linalool to elderly mice (21–24 months old) with a triple transgenic form of AD (3x Tg-AD) at 25 mg/kg for three months at an interval of 48 h resulted in enhanced learning and spatial memory and increased risk assessment activity in the elevated plus maze. Hippocampi and amygdalae from 3x Tg-AD linalool-treated mice also showed a large reduction in extracellular β-amyloidosis, tauopathy, astrogliosis, and microgliosis, as well as pro-inflammatory marker levels of p38 MAPK, NOS2, COX2 and IL-1β. Thus, linalool is suitable as an AD prevention candidate for pre-clinical studies. Based on the articles that we have selected, linalool is a major volatile component of EOs in a number of aromatic plant species, such as L. angustifolia Mill., M. officinalis L., R. officinalis L., and C. citrate DC. The presence of linalool can also help to reduce the deposits of Aβ, based on a study conducted by Gradinariu et al. [114] where Aβ1–42-treated rats exhibited the following: a decrease in exploratory activity (crossing number); smaller percentage of time spent and fewer entries in the open arm in the elevated plus-maze test; increase in swim time; and decrease in the immobility time within the forced swimming test.
4.6. Current EOs on PD
In terms of PD, the current therapy in practice is applied as a combination of gold-standard dopaminergic reposition with 3-(3,4-dihydroxyphenyl)-l-alanine (l-dopa), along with other agents such as MAO-B, catechol O-methyltransferase (COMT) inhibitors, dopaminergic agonists, and cholinergic blockers [222]. However, the available treatments are subject to consequences of motor and non-motor side effects, which leads to poor efficacy in advanced stages of PD [144]. These arising phenomena are the main reasons that suggest and emphasise the necessity for the synthesis of anti-PD drugs that could delay the progression of neurodegeneration [144]. As mentioned in the results, PD studies included in this review comprise in vitro and in vivo, as well as combinations of in vitro with in vivo or ex vivo research. Cinnamomum sp., Eryngium sp., Myrtus sp., Acorus sp., Eplingiella sp., Foeniculum sp., Pulicaria sp., Rosa sp., Zingiber sp., and Lavandula sp. were among the identified EOs based on the respective included PD studies.
Four of the included studies on PD were based on in vitro approaches, with various EOs. The first study was focused on the evaluation of protective effects of EOs extracted from Cinnamomum sp. (C. verum and C. cassia) and cinnamaldehyde, in comparison to hydroalcoholic extracts using 6-OHDA-induced PC12 cytotoxicity as the representative model of PD [138]. Cinnamomum sp., or more commonly known as cinnamon, belongs to the Lauraceae family that is composed of almost 250 species and has been acknowledged for extensive health benefits [223,224]. Among the various species, C. verum and C. cassia were the two main species that have been widely applied for their medicinal and culinary applications, especially in Iran [225,226,227]. It is important to note that cinnamaldehyde represents one of the key components of both species, and EOs were reported to exhibit strong antioxidant properties [223]. The findings of this study indicated that 6-OHDA led to cell death, cell apoptosis, and suppression of the p44/42 pathway. On the whole, the study concluded that synergistic effects of cinnamaldehyde and EOs as well as other extract components could promote cinnamon’s roles as neuroprotective agents, specifically for PD treatment [138].
Eryngium sp. belong to the Apiaceae family, and are recognised for their EOs’ potentials in MAO inhibition [139]. MAO is available in two forms (A, B) where MAO-A inhibition is linked to antidepressant effects, while MAO-B is correlated with PD treatment [228,229]. An in vitro study conducted by Klein-Júnior et al. demonstrated the assessment of Eryngium sp. (E. floribundum: EP, E. horridum: EH, E. pandanifolium: EP, E. eriophorum: EE and E. nudicaule: EN) EOs for their MAO inhibitory effect. Intriguingly, the findings of this study indicated that MAO-A activity was not inhibited by any EOs, while EPEO and EHEO resulted in MAO-B inhibition. The literature search has also highlighted that PD patients usually represent elevated levels of MAO-B which arise due to gliosis, and hence contribute towards the collapse of the dopaminergic system [229]. Thus, this study puts forward the claim that Eryngium sp. could have potential applications as CNS bioactive secondary metabolites, particularly for neurodegenerative disease, in relevance to characteristics exhibited by EHEO [139].
Another important aspect that is usually associated with studies on PD is on α-Syn fibrillation. It is presumed that protein structural modifications resulting in amyloid fibril formation progresses towards neurodegenerative disorders [140]. However, the exact factors of α-Syn actions of misfolding and aggregation in the brain are still scarce. In addition, it should also be noted that preventive measures against α-Syn fibrillation are yet to be available; hence, the acceleration of the fibrillation process via certain factors should be avoided. Among some of the common factors are metal ions, small molecules, nanoparticles, and, particularly, toxins that could intensify the aggregation process [140,230,231,232,233]. The next two in vitro studies were performed by the same research team, where they highlighted the effects of 15 various Iranian EOs against α-Syn fibrillation [140,141].
Among all the 15 oils tested, it was shown that M. communis demonstrated potential benefits because it elevated the fibrillation in a concentration-dependent manner. However, it is necessary to understand that the major components of this oil are not responsible for the observed changes, suggesting complexity of both extract and synergistic effects of the available compounds, regardless of their amount [140]. In the second study, the investigation on C. cyminum EO signified the presence of cuminaldehyde as the major active compound that plays its role in the inhibition of α-Syn fibrillation. In addition, cytotoxicity assays on PC12 cells indicated the absence of toxic effects with cuminaldehyde treatment throughout α-Syn fibrillation [141].
Apart from in vitro studies, PD studies are also extensively performed under in vivo conditions. EOs of Acorus sp. cover two of the in vivo studies in this review. Both of these studies were conducted by the same research team, and focused on the regulation effect of β-asarone that was isolated from A. tatarinowii Schott on 6-OHDA-induced parkinsonian rats via two distinct endoplasmic reticulum (ER) stress pathways [142,143]. ER is known for its role in protein folding, where the build-up of protein unfolding/misfolding could initiate a phenomenon called ER stress that further activates the cellular process of unfolded protein response (UPR) [234]. ER stress has been noticed in a number of PD experimental models, and is also provoked by an increase in wild-type α-Syn [235,236,237]. Three main pathways that are categorised as UPR are inositol requiring enzyme 1 (IRE1), protein kinase RNA (PKR)-like ER kinase (PERK), and activating transcription factor 6 (ATF6) [238].
In general, GRP78 functions in the regulation of ER stress, where it binds the three proteins of the UPR pathway and maintains them as inactive when the cells are not exposed to stress conditions. However, under conditions of accumulated protein unfolding or misfolding, GRP78 will bind to the proteins and release them [239,240,241]. In terms of autophagy, the latest research has claimed that it could be induced due to ER stress [242,243]. Beclin-1 is known for its role in forming autophagosomes and is an essential part of the initial autophagy process. The pro-autophagic role of Beclin-1 could be inhibited via its reaction with Bcl-2, however this interaction is also subject to disruption caused by Bcl-2 phosphorylation that leads to Beclin-1 release and accelerates autophagy [244]. As per the second pathway study, it was proven that β-asarone leads to Beclin-1 downregulation, which highlights that Bcl-2 could possibly be the main linkage between autophagy and ER stress. The findings of both studies lead us to conclude that diminishing ER stress via β-asarone regulation is proven to be useful in the impairment of PD pathological progression [142,143].
Another study that also applies the use of 6-OHDA-induced PD mouse models focused on the investigation of the effects of zingerone and eugenol on dopamine concentration, behavioural changes, and antioxidant activities upon 6-OHDA administration and treatment of l-dopa [28]. Zingerone is extracted from the ginger root, while eugenol originates from cloves and was reported to be protective against 6-OHDA-induced depletion of striatal dopamine via increases in SOD activity and elevation of reduced glutathione (GSH) and L-Ascorbate (Asc) concentration, respectively [245,246]. Although these groups of researchers previously reported positive findings where pre-treatment with zingerone or eugenol inhibited 6-OHDA-induced dopamine depression by preventing lipid peroxidation, the current study, which involved post-treatment with similar compounds, resulted in contradictory findings, where dopamine decrease was more pronounced [28,246]. Despite the availability of other findings that propose the benefits of consuming these compounds, Kabuto and Yamanushi [28] suggested that intake of these specific substances upon the onset of PD symptoms should be more carefully monitored to prevent further injury aggravation.
In addition to studies among 6-OHDA-induced Parkinson’s rat models, the possibility to achieve positive effects of EOs when complexed with β-cyclodextrin (βCD) was evaluated by Filho and colleagues using reserpine-induced progressive models for PD in mice [144]. Cyclodextrins are cyclic oligosaccharides that could form host–guest complexes with hydrophobic molecules and were also reported to protect EOs from heat, evaporation, moisture, oxidation and light effects along with facilitating easy solubility [247,248,249]. Complexation effects of cyclodextrins with EOs were shown to be more prominent in exerting positive effects, especially in the treatment of chronic diseases, as published by several studies [250,251]. In this particular study, the same approach was applied using leaf EO extracted from Eplingiella fruticosa (EPL), where one of the key components is 1,8-cineole. Eplingiella sp. belongs to the Lamiaceae family, and was reported for its benefits as anti-inflammatory and antioxidant effects [252,253]. This research demonstrated and proved the hypothesis whereby both treated groups of EPL and EPL-βCD deferred reserpine effects on catalepsy time. However, this effect was noticed to be more remarkable with EPL-βCD treated mice groups.
Another study also applied induction with reserpine, with a different PD model of ovariectomized and non-ovariectomized rats [145]. Reserpine is known as an irreversible inhibitor of the vesicular monoamine transporter 2 (VMAT-2). The approach of reserpine injection to rats as a mode of PD model was proposed in response to its action on the depletion of monoamine and locomotor activities [254]. Ovariectomized rats are subjected to oestrogen deficits, similar to surgically menopaused women, where cognitive damage is highly possible [255]. Lower oestrogen levels are correlated with many side effects, such as mental disorders, memory defects, emotional issues, and other cognitive failures [256]. These incidences have led to much attraction towards phytoestrogens for its protective nature against certain diseases. Fennel plant (Foeniculum vulgare) is classified as in the Apiaceae family, and is known for its phytoestrogen compounds; it showed promising results in the treatment of cognitive disorders, such as dementia and AD [145,257,258,259]. The evaluation of this study indicated that protective oestrogen effects against neurodegenerative disorders were significantly decreased among reserpine-induced ovariectomized rats. Injection of reserpine resulted in a more remarkable observation on limb movement disorder among ovariectomized rats. Fennel treatment at various doses for both groups gave better results on the motor activity, which stressed the importance of oestrogens and phytoestrogens as a protective measure of dopaminergic neurons and improved PD symptoms [145].
Rotenone administration to rats, as induction of a PD model, is an alternative approach where it induces nigrostriatal dopaminergic neuron degeneration that is associated with α-Syn Lewy bodies [260]. Rotenone is an insecticide with high lipophilic nature, and is known to inhibit mitochondrial complex-1 along with causing oxidative stress [261,262]. This rotenone-induced model was reported in the study by Issa and colleagues, based on the neuroprotective effects of Pulicaria undulata EO in male Wistar rats [146]. P. undulata belongs to the Asteraceae family, which is commonly distributed in Asia, Europe, and North Africa [263]. From this study, it was shown that EO of P. undulata could exert its neuroprotective effects via anti-inflammatory and antioxidant properties. The mechanisms involved in neuroinflammation suppression include downregulation of induced nitric oxide synthase (iNOS) expression, followed by lower gene expression of α-Syn [146].
Compared to individual studies, there are also several approaches that examine combined effects that could incorporate in vitro, in vivo, and also ex vivo applications. One such attractive research is on the combined in vitro/in vivo evaluation of SHXW EO with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD mice and SH-SY5Y cell lines [147]. In this study, SHXW was a Chinese herbal formulation that consisted of 15 crude herbs called KSOP1009, composed of eight medicinal plants of different families (Hamamelidaceae, Myristicaceae, Umbelliferae, Santalaceae, Piperaceae, Myrtaceae, Typhaceae, and Lamiaceae). MPTP is known to cause fast degeneration of dopaminergic neurons, and as such, it was believed that the use of this specific model could assist in explaining certain aspects of PD disease mechanisms [147,264]. Positive findings of the study demonstrated that ingestion of KSOP1009 was successful in the protection of MPTP toxicity, where this could be correlated with dopamine reduction that also decreases ROS and restores mitochondrial roles [147].
In terms of in vivo and ex vivo combinatorial approaches, two articles were highlighted in this review, with several authors originating from the same team [148,149]. Both studies explored the approach of L-dopa induction of oxidative toxicity. l-dopa has been recognised as the most effective symptomatic treatment of PD for more than 30 years; however, toxicity issues that were raised via in vitro studies seem to be an unresolved challenge [148]. It was also mentioned that prolonged L-dopa treatment is often associated with side effects that often result in a delay in its administration [148]. Past studies have claimed that l-dopa therapy in combination with antioxidants could lessen the possible side effects. As such, efforts were taken to evaluate the effects; this research team investigated the combined effects of EOs from Lavandula angustifolia, Rosa damascena, vitamin C and Trolox in the initial study [148], followed by another study in 2019 on the combined effects of pre-treatment with Rosa damascena and vitamin C [149].
For the first study, the obtained results indicated that both EOs from herbal plants showed noticeable radical scavenging and antioxidant properties against l-dopa toxicity. Similarly, the second study also put forward equivalent claims with R. damascena characteristics where it was in parallel to vitamin C, and exhibited a significant role of rose oil in its interference against the acute oxidative toxicity of l-dopa [148,149]. Based on all the collective studies, it could be observed that PD treatments remain centralised among several parameters that include mainly α-Syn fibrillation, MAO-B, β-asarone regulation of ER stress pathways, toxicity-induced models with 6-OHDA, MPTP, l-dopa, reserpine, and rotenone with common animal models of rats and mice. Although the regulation mechanisms involved in each of the parameters may differ, the main focus remains towards effective and improved treatments for PD patients.
4.7. Current EOs on Other Neurodegenerative Diseases
Our review findings also revealed that several studies did not specify the studied disease, and instead examined general neurodegenerative disorders. The in vitro study conducted by Costa et al. [150] using Lavandula pedunculata subsp. lusitanica (Chaytor) Franco EO suggested the ability of L. pedunculata as a suitable choice to prevent neurodegenerative disease. A study performed by Elmann et al. [151] with Pelargonium graveolens EO showed that some constituents may depend on synergistic interactions to function. Geranium oil from Pelargonium graveolens inhibited nitric oxide (NO) production, as well as the expression of the proinflammatory enzymes cyclooxygenase-2 (COX-2) and induced nitric oxide synthase (iNOS) in primary cultures of activated microglial cells. The finding showed that none of the major constituents could inhibit NO production when examined at natural relative oil concentrations, with excellent inhibitory activity of citronellol at higher concentrations. The findings indicated that the presence of synergistic interactions between these components are of considerable importance. Thus, geranium oil can be useful in neurodegenerative disease prevention where neuroinflammation is part of pathophysiology [151].
EOs could also be applied as inhalation-based treatment, which could signify a natural way to heal one’s mind, body, and soul [265]. In Bagci et al.’s study [153], it was investigated whether inhalation of the Anthriscus nemorosa EO leads to behavioural changes that indicated significant memory improvement and exhibited both anxiolytic- and antidepressant-like effects in dual-treated rats. The results suggested that A. nemorosa EO inhalation can prevent scopolamine-induced memory impairment, anxiety, and depression [153].
In the in vivo research conducted by Satou et al. [118], mice were administered Rosmarinus officinalis EO (EORO) by inhalation and it was concluded that the rate of spontaneous alternation activity was significantly improved. The key components (1,8-cineole, α-pinene and β-pinene) were detected in the brain in a concentration-dependent manner upon EORO inhalation, which indicated its possible exerted effects. However, in several other interpretations, it was proposed that 1,8-cineole might require synergistic interaction effects. This was highlighted by Costa et al. [150], where α-pinene and 1,8-cineole from Lavandula pedunculata subsp. Lusitanica were shown to be effective cholinesterase inhibitors even at low concentrations and are likely to contribute to this behaviour. The synergistic and antagonistic interactions between certain terpenes that could result in combined effects should also be taken into consideration. Although this compound was also mentioned in a study conducted by Hritcu et al. [112], the study focused more on the positive effect of linalool in the improvement of spatial memory deficit in a scopolamine-induced dementia rat model instead of the 1,8-cineole effect on neurodegeneration. In addition, a study by Kaufmann and colleagues [104] signified the potential of myrtenal as an effective AChE inhibitor, compared to 1,8-cineole with EOs of Artemisia annua L. (Asteraceae) or Glycyrrhiza glabra L. (Fabaceae).
5. Conclusions
In conclusion, our review highlighted that EOs provide many other benefits apart from its common usage as fragrances and flavours, especially for its significant role in neurodegenerative disease therapy. Their roles in reducing disease severity are exerted by different mechanisms that vary respective of their origin. Although human studies were not obtained as part of our search, we strongly believe that the presence of essential components such as 1,8-cineole, carvacrol, or β-asarone could play significant roles in efforts towards the prevention and treatment of neurodegenerative disorders. Therefore, it is vital that the search for novel species of EOs are continued, to explore oils which could be applied towards the application of EO-based therapies or treatment strategies intended for age-related neurodegenerative disorders.
Acknowledgments
The authors thank the Director General of Health Malaysia and the Director of Institute for Medical Research (IMR), Malaysia, for giving permission to publish this article. We also thank the staff of the Nutrition, Metabolism and Cardiovascular Centre, Institute for Medical Research, NIH, for their continuous support.
List of Abbreviations
| Aβ | Amyloid-beta |
| ACh | Acetylcholine |
| AChE | Acetylcholinesterase |
| AD | Alzheimer’s disease |
| AFM | Atomic force microscope |
| ALS | Amyotrophic lateral sclerosis |
| AOPP | Advanced oxidation protein product |
| APP | Amyloid precursor protein |
| Asc | l-Ascorbate |
| α-Syn | α-Synuclein |
| ATF6 | Activating transcription factor 6 |
| BACE-1 | Beta-site APP-cleaving enzyme 1 |
| BCh | Butyrylcholine |
| BChE | Butyrylcholinesterase |
| βCD | β-cyclodextrin |
| BDNF | Brain-derived neurotropic factor |
| CA1 | Cornu ammonis |
| CAG | Cytosine–adenine–guanine |
| CAL | (E)-cinnamaldehyde |
| CAS | (E)-cinnamyl acetate |
| CAT | Catalase |
| CGC | Caenorhabditis Genetics Center |
| ChE | Cholinesterases |
| CNS | Central nervous system (human) |
| COMT | Catechol O-methyltransferase |
| COX-2 | Cyclooxygenase-2 |
| CUMS | Chronic unpredictable mild stress |
| DA | Dopamine |
| DAT | Dark avoidance test |
| DOPAC DSPE-PEG2000 |
3,4-dihydroxyphenylacetic acid Distearoylphosphatidylethanolamine with covalently linked polyethylene glycol of molecular weight 2000 |
| DTNB ELISA |
5,5′-dithiobis-(2-nitrobenzoic acid) Enzyme-linked immunosorbent assay |
| ER | Endoplasmic reticulum |
| EO | Essential oil |
| fPD FITC |
Familial PD Fluorescein isothiocyanate |
| GABA | γ-aminobutyric acid |
| GC–FID | Gas chromatography–flame ionisation detector |
| GC–MS GC–ITMS |
Gas chromatography–mass spectrometry Gas Chromatography/Ion Trap Mass Spectrometry |
| GC–O | Gas chromatography–olfactometry |
| GPx | Gluthathione peroxidase |
| GRP78 | Glucose regulated protein 78 |
| GSH | Reduced glutathione |
| HD HPLC–DAD HPLC–PDA |
Huntington’s disease High-performance liquid chromatography–diode-array detection High-performance liquid chromatography–photodiode array detection |
| HTT | Huntingtin gene |
| HVA | Homovanillic acid |
| iNOS | Induced nitric oxide synthase |
| IRE1 | Inositol requiring enzyme 1 |
| IRE1/XBP1 | IRE1/X-Box Binding Protein 1 |
| JNK | c-Jun N-terminal kinase |
| LDH | Lactate dehydrogenase |
| L-dopa | Levodopa |
| LMN LOX |
Lower motor neuron Lipoxygenase |
| MAO | Monoamine oxidase |
| MDA | Malondialdehyde |
| ME | Microemulsion |
| mHTT | Mutant HTT |
| MMP | Mitochondrial membrane potential |
| MPP+ | 1-methyl-4-phenylpyridinium |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| MWM NA–FB |
Morris water maze test New micro-well plate AChE activity |
| NHPs NMR |
Non-human primates Nuclear magnetic resonance |
| NO OS |
Nitric oxide Oxidative stress |
| PCC | Protein carbonyl content |
| PCh | Propionylcholine |
| PD | Parkinson’s disease |
| PDI | Polydispersity index |
| PERK | Protein kinase RNA (PKR)-like ER kinase |
| p-PERK | PERK phosphorylation |
| PI3K | Phosphatidylinositol-4,5-bisphosphate 3-kinase |
| PKR | Protein kinase RNA |
| PS | Presenilin |
| RNAi | RNA interference |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| SDT | Step-down test |
| SHXW SFE SKN-1 SN |
Su He Xiang Wan Supercritical fluid extraction Skinhead-1 Substantia nigra |
| SNpc | Substantia nigra pars compacta |
| SOD | Superoxide dismutase |
| sPD | Sporadic PD |
| TBARS TLC |
Thiobarbituric acid-reactive substances Thin-layer chromatography |
| TNB | 5-thio-2-nitrobenzoic acid |
| TNF TTC TUNEL |
Tumor necrosis factor 2,3,5-triphenyltetrazolium chloride Terminal deoxynucleotidyl transferase dUTP nick end labeling |
| UMN | Upper motor neuron |
| UPR) | Unfolded protein response |
| VMAT-2 | Vesicular monoamine transporter 2 |
| VOCs WST |
Volatile organic constituents Water-soluble tetrazolium salt |
| Z-LIG | Z-ligustilide |
| 5-HT | Serotonin |
| 6-OHDA | 6-hydroxydopamine |
| 8-OHdG | Urinary 8-hydroxyguanosine |
Author Contributions
Conceptualization, all authors; literature review: all authors; tables: all authors, writing and review all authors; editing: all authors; revisions and final editing: all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Osbourn A.E., Lanzotti V. Plant-Derived Natural Products: Synthesis, Function, and Application. Springer Science & Business Media; New York, NY, USA: 2009. p. 612. [Google Scholar]
- 2.Esposito E.R., Bystrek M.V., Klein J.S. An Elective Course in Aromatherapy Science. Am. J. Pharm. Edu. 2014;78:79. doi: 10.5688/ajpe78479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robins J.L.W. The Science and Art of Aromatherapy. J. Holist. Nurs. 1999;17:5–17. doi: 10.1177/089801019901700102. [DOI] [PubMed] [Google Scholar]
- 4.Smith C.A., Collins C.T., Crowther C.A. Aromatherapy for pain management in labour. Cochrane Database Syst. Rev. 2011 doi: 10.1002/14651858.CD009215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Salas P., Morales-Soto A., Segura-Carretero A., Fernández-Gutiérrez A. Phenolic-compound-extraction systems for fruit and vegetable samples. Molecules. 2010;15:8813–8826. doi: 10.3390/molecules15128813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singer A.C., Crowley D.E., Thompson I.P. Secondary plant metabolites in phytoremediation and biotransformation. Trends Biotechnol. 2003;21:123–130. doi: 10.1016/S0167-7799(02)00041-0. [DOI] [PubMed] [Google Scholar]
- 7.Hsieh M.T., Peng W.H., Wu C.R., Wang W.H. The ameliorating effects of the cognitive-enhancing Chinese herbs on scopolamine-induced amnesia in rats. Phytother. Res. 2000;14:375–377. doi: 10.1002/1099-1573(200008)14:5<375::AID-PTR593>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 8.Cioanca O., Mircea C., Hritcu L., Trifan A., Marius M., Aprotosoaie A.C., Robu S., Gille E., Hancianu M. In vitro—In vivo correlation of the antioxidant capacity of Salviae aetheroleum essential oil. Farmacia. 2015;63:34–39. [Google Scholar]
- 9.Jové M., Portero-Otín M., Naudí A., Ferrer I., Pamplona R. Metabolomics of Human Brain Aging and Age-Related Neurodegenerative Diseases. J. Neuropath. Exp. Neur. 2014;73:640–657. doi: 10.1097/NEN.0000000000000091. [DOI] [PubMed] [Google Scholar]
- 10.Yan M.H., Wang X., Zhu X. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radic. Biol. Med. 2013;62:90–101. doi: 10.1016/j.freeradbiomed.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarrafchi A., Bahmani M., Shirzad H., Rafieian-Kopaei M. Oxidative stress and Parkinson’s disease: New hopes in treatment with herbal antioxidants. Curr. Pharm. Des. 2016;22:238–246. doi: 10.2174/1381612822666151112151653. [DOI] [PubMed] [Google Scholar]
- 12.González-Burgos E., Gómez-Serranillos M.P. Terpene compounds in nature: A review of their potential antioxidant activity. Curr. Med. Chem. 2012;19:5319–5341. doi: 10.2174/092986712803833335. [DOI] [PubMed] [Google Scholar]
- 13.Essa M., Braidy N., Bridge W., Subash S., Manivasagam T., Vijayan R., Al-Adawi S., Guillemin G. Review of natural products on Parkinson’s disease pathology. J. Aging Res. Clin. Pract. 2014;3:1–8. [Google Scholar]
- 14.Karakaya S., Koca M., Yılmaz S.V., Yıldırım K., Pınar N.M., Demirci B., Brestic M., Sytar O. Molecular Docking Studies of Coumarins Isolated from Extracts and Essential Oils of Zosima absinthifolia Link as Potential Inhibitors for Alzheimer’s Disease. Molecules. 2019;24:722. doi: 10.3390/molecules24040722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forrester L.T., Maayan N., Orrell M., Spector A.E., Buchan L.D., Soares-Weiser K. Aromatherapy for dementia. Cochrane Database Syst. Rev. 2014;CD003150:1–56. doi: 10.1002/14651858.CD003150.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Xu P., Wang K., Lu C., Dong L., Gao L., Yan M., Aibai S., Yang Y., Liu X. Protective effects of linalool against amyloid beta-induced cognitive deficits and damages in mice. Life Sci. 2017;174:21–27. doi: 10.1016/j.lfs.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Bansal A., Kirschner M., Zu L., Cai D., Zhang L. Coconut oil decreases expression of amyloid precursor protein (APP) and secretion of amyloid peptides through inhibition of ADP-ribosylation factor 1 (ARF1) Brain Res. 2019;1704:78–84. doi: 10.1016/j.brainres.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Dalai M., Bhadra S., Chaudhary S., Bandyopadhyay A., Mukherjee P. Anti-cholinesterase potential of Cinnamomum tamala (Buch.-Ham.) T.Nees & Eberm. leaves. Indian J. Tradit. Know. 2014;13:691–697. [Google Scholar]
- 19.Sihoglu Tepe A., Ozaslan M. Anti-Alzheimer, anti-diabetic, skin-whitening, and antioxidant activities of the essential oil of Cinnamomum zeylanicum. Ind. Crop. Prod. 2020;145:112069. doi: 10.1016/j.indcrop.2019.112069. [DOI] [Google Scholar]
- 20.Bahadori M.B., Salehi P., Sonboli A. Comparative study of the essential oil composition of Salvia urmiensis and its enzyme inhibitory activities linked to diabetes mellitus and Alzheimer’s disease. Int. J. Food Prop. 2017;20:2974–2981. doi: 10.1080/10942912.2016.1263862. [DOI] [Google Scholar]
- 21.Cutillas A.B., Carrasco A., Martinez-Gutierrez R., Tomas V., Tudela J. Salvia officinalis L. Essential Oils from Spain: Determination of Composition, Antioxidant Capacity, Antienzymatic, and Antimicrobial Bioactivities. Chem. Biodivers. 2017;14 doi: 10.1002/cbdv.201700102. [DOI] [PubMed] [Google Scholar]
- 22.Ayaz M., Junaid M., Ullah F., Sadiq A., Khan M.A., Ahmad W., Shah M.R., Imran M., Ahmad S. Comparative chemical profiling, cholinesterase inhibitions and anti-radicals properties of essential oils from Polygonum hydropiper L: A Preliminary anti- Alzheimer’s study. Lipids Health Dis. 2015;14:141. doi: 10.1186/s12944-015-0145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali-Shtayeh M.S., Abu-Zaitoun S.Y., Dudai N., Jamous R.M. Downy Lavender Oil: A Promising Source of Antimicrobial, Antiobesity, and Anti-Alzheimer’s Disease Agents. Evid-Based Complement. Altern. Med. 2020;2020:5679408. doi: 10.1155/2020/5679408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soheili M., Khalaji F., Mirhashemi M., Salami M. The Effect of Essential Oil of Lavandula Angustifolia on Amyloid Beta Polymerization: An In Vitro Study. Iran. J. Chem. Chem. Eng. 2018;37:201–207. [Google Scholar]
- 25.Chaiyana W., Okonogi S. Inhibition of cholinesterase by essential oil from food plant. Phytomedicine. 2012;19:836–839. doi: 10.1016/j.phymed.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Shoaib M., Shah I., Ali N., Ali W. In vitro acetylcholinesterase and butyrylcholinesterase inhibitory potentials of essential oil of Artemisia macrocephala. Bangladesh J. Pharm. 2015;10:87–91. doi: 10.3329/bjp.v10i1.21171. [DOI] [Google Scholar]
- 27.Ansari M., Eslami H. Preparation and study of the inhibitory effect of nano-niosomes containing essential oil from artemisia absinthium on amyloid fibril formation. Nanomed. J. 2020;7:243–250. doi: 10.22038/nmj.2020.07.0009. [DOI] [Google Scholar]
- 28.Kabuto H., Yamanushi T.T. Effects of zingerone [4-(4-hydroxy-3-methoxyphenyl)-2-butanone] and eugenol [2-methoxy-4-(2-propenyl) phenol] on the pathological progress in the 6-hydroxydopamine-induced parkinson’s disease mouse model. Neurochem. Res. 2011;36:2244. doi: 10.1007/s11064-011-0548-5. [DOI] [PubMed] [Google Scholar]
- 29.Alzheimer’s Association 2019 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2019;15:321–387. doi: 10.1016/j.jalz.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 30.Reitz C., Mayeux R. Alzheimer disease: Epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem. Pharm. 2014;88:640–651. doi: 10.1016/j.bcp.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferretti C., Sarti F.M., Nitrini R., Ferreira F.F., Brucki S.M.D. An assessment of direct and indirect costs of dementia in Brazil. PLoS ONE. 2018;13:e0193209. doi: 10.1371/journal.pone.0193209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weintraub S., Wicklund A.H., Salmon D.P. The neuropsychological profile of Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012;2:a006171. doi: 10.1101/cshperspect.a006171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aliev G., Palacios H.H., Walrafen B., Lipsitt A.E., Obrenovich M.E., Morales L. Brain mitochondria as a primary target in the development of treatment strategies for Alzheimer disease. Int. J. Biochem. Cell Biol. 2009;41:1989–2004. doi: 10.1016/j.biocel.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 34.Serrano-Pozo A., Mielke M.L., Gómez-Isla T., Betensky R.A., Growdon J.H., Frosch M.P., Hyman B.T. Reactive glia not only associates with plaques but also parallels tangles in Alzheimer’s disease. Am. J. Pathol. 2011;179:1373–1384. doi: 10.1016/j.ajpath.2011.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villa V., Thellung S., Bajetto A., Gatta E., Robello M., Novelli F., Tasso B., Tonelli M., Florio T. Novel celecoxib analogues inhibit glial production of prostaglandin E2, nitric oxide, and oxygen radicals reverting the neuroinflammatory responses induced by misfolded prion protein fragment 90–231 or lipopolysaccharide. Pharm. Res. 2016;113:500–514. doi: 10.1016/j.phrs.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 36.Masters C.L., Selkoe D.J. Biochemistry of amyloid β-protein and amyloid deposits in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012;2:a006262. doi: 10.1101/cshperspect.a006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nhan H.S., Chiang K., Koo E.H. The multifaceted nature of amyloid precursor protein and its proteolytic fragments: Friends and foes. Acta. Neuropathol. 2015;129:1–19. doi: 10.1007/s00401-014-1347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campora M., Francesconi V., Schenone S., Tasso B., Tonelli M. Journey on Naphthoquinone and Anthraquinone Derivatives: New Insights in Alzheimer’s Disease. Pharmaceuticals. 2021;14:33. doi: 10.3390/ph14010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kocahan S., Doğan Z. Mechanisms of Alzheimer’s Disease Pathogenesis and Prevention: The Brain, Neural Pathology, N-methyl-D-aspartate Receptors, Tau Protein and Other Risk Factors. Clin. Psychopharmacol. Neurosci. 2017;15:1–8. doi: 10.9758/cpn.2017.15.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng H., Fridkin M., Youdim M.B. From antioxidant chelators to site-activated multi-target chelators targeting hypoxia inducing factor, beta-amyloid, acetylcholinesterase and monoamine oxidase A/B. Mini Rev. Med. Chem. 2012;12:364–370. doi: 10.2174/138955712800493898. [DOI] [PubMed] [Google Scholar]
- 41.Huang L., Lu C., Sun Y., Mao F., Luo Z., Su T., Jiang H., Shan W., Li X. Multitarget-directed benzylideneindanone derivatives: Anti-β-amyloid (Aβ) aggregation, antioxidant, metal chelation, and monoamine oxidase B (MAO-B) inhibition properties against Alzheimer’s disease. J. Med. Chem. 2012;55:8483–8492. doi: 10.1021/jm300978h. [DOI] [PubMed] [Google Scholar]
- 42.Youdim M.B.H., Amit T., Bar-Am O., Weinreb O., Yogev-Falach M. Implications of co-morbidity for etiology and treatment of neurodegenerative diseases with multifunctional neuroprotective-neurorescue drugs; ladostigil. Neurotox. Res. 2006;10:181–192. doi: 10.1007/BF03033355. [DOI] [PubMed] [Google Scholar]
- 43.Bar-Am O., Amit T., Weinreb O., Youdim M.B., Mandel S. Propargylamine containing compounds as modulators of proteolytic cleavage of amyloid-beta protein precursor: Involvement of MAPK and PKC activation. J. Alzheimers Dis. 2010;21:361–371. doi: 10.3233/JAD-2010-100150. [DOI] [PubMed] [Google Scholar]
- 44.Yogev-Falach M., Bar-Am O., Amit T., Weinreb O., Youdim M.B. A multifunctional, neuroprotective drug, ladostigil (TV3326), regulates holo-APP translation and processing. FASEB J. 2006;20:2177–2179. doi: 10.1096/fj.05-4910fje. [DOI] [PubMed] [Google Scholar]
- 45.Weinreb O., Amit T., Bar-Am O., Sagi Y., Mandel S., Youdim M.B.H. Parkinson’s Disease and Related Disorders. 2nd ed. Volume 70. Springer; Vienna, Austria: 2006. Involvement of multiple survival signal transduction pathways in the neuroprotective, neurorescue and APP processing activity of rasagiline and its propargyl moiety; pp. 457–465. [DOI] [PubMed] [Google Scholar]
- 46.Cai Z. Monoamine oxidase inhibitors: Promising therapeutic agents for Alzheimer’s disease (Review) Mol. Med. Rep. 2014;9:1533–1541. doi: 10.3892/mmr.2014.2040. [DOI] [PubMed] [Google Scholar]
- 47.Manouchehrabadi M., Farhadi M., Azizi Z., Torkaman-Boutorabi A. Carvacrol Protects Against 6-Hydroxydopamine-Induced Neurotoxicity in In Vivo and In Vitro Models of Parkinson’s Disease. Neurotox Res. 2020;37:156–170. doi: 10.1007/s12640-019-00088-w. [DOI] [PubMed] [Google Scholar]
- 48.Fahn S. Description of Parkinson’s disease as a clinical syndrome. Ann. N. Y. Acad. Sci. 2003;991:1–14. doi: 10.1111/j.1749-6632.2003.tb07458.x. [DOI] [PubMed] [Google Scholar]
- 49.Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat. Rev. Neurosci. 2001;2:492–501. doi: 10.1038/35081564. [DOI] [PubMed] [Google Scholar]
- 50.Jellinger K.A. The pathology of Parkinson’s disease. Adv. Neurol. 2001;86:55–72. doi: 10.1007/BF03159935. [DOI] [PubMed] [Google Scholar]
- 51.Spillantini M.G., Crowther R.A., Jakes R., Hasegawa M., Goedert M. α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc. Natl. Acad. Sci. USA. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schulz J.B. Update on the pathogenesis of Parkinson’s disease. J. Neurol. 2008;255:3–7. doi: 10.1007/s00415-008-5011-4. [DOI] [PubMed] [Google Scholar]
- 53.Blum D., Torch S., Lambeng N., Nissou M., Benabid A.L., Sadoul R., Verna J.M. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: Contribution to the apoptotic theory in Parkinson’s disease. Prog. Neurobiol. 2001;65:135–172. doi: 10.1016/S0301-0082(01)00003-X. [DOI] [PubMed] [Google Scholar]
- 54.Wexler N.S. Venezuelan kindreds reveal that genetic and environmental factors modulate Huntington’s disease age of onset. Proc. Natl. Acad. Sci. USA. 2004;101:3498–3503. doi: 10.1073/pnas.0308679101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quarrell O.W., Nance M.A., Nopoulos P., Paulsen J.S., Smith J.A., Squitieri F. Managing juvenile Huntington’s disease. Neurodegener. Dis. Manag. 2013;3:267–276. doi: 10.2217/nmt.13.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee J.M., Ramos E.M., Lee J.H., Gillis T., Mysore J.S., Hayden M.R., Warby S.C., Morrison P., Nance M., Ross C.A., et al. CAG repeat expansion in Huntington disease determines age at onset in a fully dominant fashion. Neurology. 2012;78:690–695. doi: 10.1212/WNL.0b013e318249f683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koutsis G., Karadima G., Kladi A., Panas M. Late-onset Huntington’s disease: Diagnostic and prognostic considerations. Parkinsonism Relat. D. 2014;20:726–730. doi: 10.1016/j.parkreldis.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 58.Miller R.G., Mitchell J.D., Lyon M., Moore D.H. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND) Cochrane Database Syst. Rev. 2007;7:1–26. doi: 10.1002/14651858.CD001447.pub2. [DOI] [PubMed] [Google Scholar]
- 59.Katyal N., Govindarajan R. Shortcomings in the current amyotrophic lateral sclerosis trials and potential solutions for improvement. Front. Neurol. 2017;8:521. doi: 10.3389/fneur.2017.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Al-Chalabi A., Jones A., Troakes C., King A., Al-Sarraj S., van den Berg L.H. The genetics and neuropathology of amyotrophic lateral sclerosis. Acta. Neuropathol. 2012;124:339–352. doi: 10.1007/s00401-012-1022-4. [DOI] [PubMed] [Google Scholar]
- 61.Menke R.A., Körner S., Filippini N., Douaud G., Knight S., Talbot K., Turner M.R. Widespread grey matter pathology dominates the longitudinal cerebral MRI and clinical landscape of amyotrophic lateral sclerosis. Brain. 2014;137:2546–2555. doi: 10.1093/brain/awu162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hadzhieva M., Kirches E., Wilisch-Neumann A., Pachow D., Wallesch M., Schoenfeld P., Paege I., Vielhaber S., Petri S., Keilhoff G. Dysregulation of iron protein expression in the G93A model of amyotrophic lateral sclerosis. Neuroscience. 2013;230:94–101. doi: 10.1016/j.neuroscience.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 63.De Assis Lima I.V., Bastos L.F.S., Limborço-Filho M., Fiebich B.L., de Oliveira A.C.P. Role of prostaglandins in neuroinflammatory and neurodegenerative diseases. Mediat. Inflamm. 2012;2012:1–13. doi: 10.1155/2012/946813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Movahhedin N., Zengin G., Bahadori M.B., Sarikurkcu C., Bahadori S., Dinparast L. Ajuga chamaecistus subsp. scoparia (Boiss.) Rech.f.: A new source of phytochemicals for antidiabetic, skin-care, and neuroprotective uses. Ind. Crop. Prod. 2016;94:89–96. doi: 10.1016/j.indcrop.2016.08.028. [DOI] [Google Scholar]
- 65.Karakaya S., Eksi G., Koca M., Demirci B., Kaymak H.C., Kaplan M.E., Aksakal O. Chemical and morphological characterization of Allium tuncelianum (Amaryllidaceae) and its antioxidant and anticholinesterase potentials. Jard. Bot. Madr. 2019;76:85. doi: 10.3989/ajbm.2523. [DOI] [Google Scholar]
- 66.Abuhamdah S., Abuhamdah R., Howes M.J., Al-Olimat S., Ennaceur A., Chazot P.L. Pharmacological and neuroprotective profile of an essential oil derived from leaves of Aloysia citrodora Palau. J. Pharm. Pharm. 2015;67:1306–1315. doi: 10.1111/jphp.12424. [DOI] [PubMed] [Google Scholar]
- 67.Jyotshna, Srivastava N., Singh B., Chanda D., Shanker K. Chemical composition and acetylcholinesterase inhibitory activity of Artemisia maderaspatana essential oil. Pharm. Biol. 2015;53:1677–1683. doi: 10.3109/13880209.2014.1001405. [DOI] [PubMed] [Google Scholar]
- 68.Kohoude M.J., Gbaguidi F., Agbani P., Ayedoun M.A., Cazaux S., Bouajila J. Chemical composition and biological activities of extracts and essential oil of Boswellia dalzielii leaves. Pharm. Biol. 2017;55:33–42. doi: 10.1080/13880209.2016.1226356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murata K., Tanaka K., Akiyama R., Noro I., Nishio A., Nakagawa S., Matsumura S., Matsuda H. Anti-cholinesterase Activity of Crude Drugs Selected from the Ingredients of Incense Sticks and Heartwood of Chamaecyparis obtusa. Nat. Prod. Commun. 2018;13 doi: 10.1177/1934578X1801300704. [DOI] [Google Scholar]
- 70.Ademosun A.O., Oboh G., Olupona A.J., Oyeleye S.I., Adewuni T.M., Nwanna E.E. Comparative Study of Chemical Composition, In Vitro Inhibition of Cholinergic and Monoaminergic Enzymes, and Antioxidant Potentials of Essential Oil from Peels and Seeds of Sweet Orange (Citrus Sinensis [L.] Osbeck) Fruits. J. Food Biochem. 2016;40:53–60. doi: 10.1111/jfbc.12187. [DOI] [Google Scholar]
- 71.Tundis R., Loizzo M.R., Bonesi M., Menichini F., Mastellone V., Colica C., Menichini F. Comparative Study on the Antioxidant Capacity and Cholinesterase Inhibitory Activity of Citrus aurantifolia Swingle, C. aurantium L., and C. bergamia Risso and Poit. Peel Essential Oils. J. Food Sci. 2012;77:H40–H46. doi: 10.1111/j.1750-3841.2011.02511.x. [DOI] [PubMed] [Google Scholar]
- 72.Loizzo M., Ben Jemia M., Senatore F., Bruno M., Menichini F., Tundis R. Chemistry and functional properties in prevention of neurodegenerative disorders of five Cistus species essential oils. Food Chem. Toxicol. 2013;59 doi: 10.1016/j.fct.2013.06.040. [DOI] [PubMed] [Google Scholar]
- 73.Ali-Shtayeh M.S., Jamous R.M., Abu-Zaitoun S.Y., Akkawi R.J., Kalbouneh S.R., Bernstein N., Dudai N. Chemical profile and bioactive properties of the essential oil isolated from Clinopodium serpyllifolium (M.Bieb.) Kuntze growing in Palestine. Ind. Crop. Prod. 2018;124:617–625. doi: 10.1016/j.indcrop.2018.08.038. [DOI] [Google Scholar]
- 74.Lamamra M., Laouer H., Amira S., Erdogan Orhan I., Senol F., Demirci B., Akkal S. Chemical Composition and Cholinesterase Inhibitory Activity of Different Parts of Daucus aristidis Coss. Essential Oils from Two Locations in Algeria. Rec. Nat. Prod. 2017;11:147–156. [Google Scholar]
- 75.Arruda M., Viana H., Rainha N., Neng N.R., Rosa J.S., Nogueira J.M.F., Barreto M.d.C. Anti-acetylcholinesterase and antioxidant activity of essential oils from Hedychium gardnerianum Sheppard ex Ker-Gawl. Molecules. 2012;17:3082–3092. doi: 10.3390/molecules17033082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bahadori S., Bahadori M.B., Zengin G., Maggi F., Dinparast L., Aktumsek A. Chemical composition profile of the essential oil from Hymenocrater bituminous and its health functionality. Int. J. Food Prop. 2017;20:S972–S980. doi: 10.1080/10942912.2017.1325901. [DOI] [Google Scholar]
- 77.Bhadra S., Mukherjee P.K., Kumar N.S., Bandyopadhyay A. Anticholinesterase activity of standardized extract of Illicium verum Hook. f. fruits. Fitoterapia. 2011;82:342–346. doi: 10.1016/j.fitote.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 78.Videira R., Castanheira P., Grãos M., Salgueiro L., Faro C., Cavaleiro C. A necrodane monoterpenoid from Lavandula luisieri essential oil as a cell-permeable inhibitor of BACE-1, the β-secretase in Alzheimer’s disease. Flavour Frag J. 2013;28:380–388. doi: 10.1002/ffj.3156. [DOI] [Google Scholar]
- 79.Costa P., Grosso C., Gonçalves S., Andrade P.B., Valentão P., Gabriela Bernardo-Gil M., Romano A. Supercritical fluid extraction and hydrodistillation for the recovery of bioactive compounds from Lavandula viridis L’Hér. Food Chem. 2012;135:112–121. doi: 10.1016/j.foodchem.2012.04.108. [DOI] [Google Scholar]
- 80.Ali-Shtayeh M.S., Jamous R.M., Abu-Zaitoun S.Y., Khasati A.I., Kalbouneh S.R. Biological Properties and Bioactive Components of Mentha spicata L. Essential Oil: Focus on Potential Benefits in the Treatment of Obesity, Alzheimer’s Disease, Dermatophytosis, and Drug-Resistant Infections. Evid-Based Complement. Altern Med. 2019;2019:3834265. doi: 10.1155/2019/3834265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Amaral R., Fernandes C., Botas G., Cruz R., Santos M., Rocha L. Anticholinesterase Activity of Essential oil from Leaves of Neomitranthes obscura (DC.) N. Silveira. Lat. Am. J. Pharm. 2014;33:860–863. [Google Scholar]
- 82.Özbek H., Güvenalp Z., Ozek T., Sevindik H., Yuca H., Yerdelen K., Demirezer L.Ö. Chemical Composition, Antioxidant and Anticholinesterase Activities of the Essential oil of Origanum rotundifolium Boiss. from Turkey. Rec. Nat. Prod. 2017;11:485–490. doi: 10.25135/rnp.62.16.05.040. [DOI] [Google Scholar]
- 83.Kawamoto H., Takeshita F., Murata K. Inhibitory Effects of Essential Oil Extracts From Panax Plants Against β-Secretase, Cholinesterase, and Amyloid Aggregation. Nat. Prod. Commun. 2019;14 doi: 10.1177/1934578X19881549. [DOI] [Google Scholar]
- 84.Bonesi M., Menichini F., Tundis R., Loizzo M., Conforti F., Passalacqua N., Statti G., Menichini F. Acetylcholinesterase and butyrylcholinesterase inhibitory activity of Pinus species essential oils and their constituents. J. Enzym Inhib. Med. Ch. 2010;25:622–628. doi: 10.3109/14756360903389856. [DOI] [PubMed] [Google Scholar]
- 85.De Oliveira M.S., da Cruz J.N., Gomes Silva S., da Costa W.A., de Sousa S.H.B., Bezerra F.W.F., Teixeira E., da Silva N.J.N., de Aguiar Andrade E.H., de Jesus Chaves Neto A.M., et al. Phytochemical profile, antioxidant activity, inhibition of acetylcholinesterase and interaction mechanism of the major components of the Piper divaricatum essential oil obtained by supercritical CO2. J. Supercrit Fluid. 2019;145:74–84. doi: 10.1016/j.supflu.2018.12.003. [DOI] [Google Scholar]
- 86.Bahadori M.B., Zengin G., Bahadori S., Maggi F., Dinparast L. Chemical Composition of Essential Oil, Antioxidant, Antidiabetic, Anti-obesity, and Neuroprotective Properties of Prangos gaubae. Nat. Prod. Commun. 2017;12 doi: 10.1177/1934578X1701201233. [DOI] [Google Scholar]
- 87.Ahmad S., Ullah F., Sadiq A., Ayaz M., Imran M., Ali I., Zeb A., Ullah F., Shah M.R. Chemical composition, antioxidant and anticholinesterase potentials of essential oil of Rumex hastatus D. Don collected from the North West of Pakistan. BMC Complement. Altern. Med. 2016;16:29. doi: 10.1186/s12906-016-0998-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.El Euch S.K., Hassine D.B., Cazaux S., Bouzouita N., Bouajila J. Salvia officinalis essential oil: Chemical analysis and evaluation of anti-enzymatic and antioxidant bioactivities. S. Afr. J. Bot. 2019;120:253–260. doi: 10.1016/j.sajb.2018.07.010. [DOI] [Google Scholar]
- 89.Tel G., Oztürk M., Duru M.E., Harmandar M., Topçu G. Chemical composition of the essential oil and hexane extract of Salvia chionantha and their antioxidant and anticholinesterase activities. Food Chem. Toxicol. 2010;48:3189–3193. doi: 10.1016/j.fct.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 90.Duru M., Tel Çayan G., Öztürk M., Harmandar M. Chemical Composition, Antioxidant and Anticholinesterase Activities of the Essential Oil of Salvia chrysophylla Staph. Rec. Nat. Prod. 2012;6:175. [Google Scholar]
- 91.Bahadori M.B., Asghari B., Dinparast L., Zengin G., Sarikurkcu C., Abbas-Mohammadi M., Bahadori S. Salvia nemorosa L.: A novel source of bioactive agents with functional connections. LWT. 2017;75:42–50. doi: 10.1016/j.lwt.2016.08.048. [DOI] [Google Scholar]
- 92.Bahadori M.B., Dinparast L., Zengin G., Sarikurkcu C., Bahadori S., Asghari B., Movahhedin N. Functional components, antidiabetic, anti-Alzheimer’s disease, and antioxidant activities of Salvia syriaca L. Int. J. Food Prop. 2017;20:1761–1772. doi: 10.1080/10942912.2016.1218893. [DOI] [Google Scholar]
- 93.Öztürk M. Anticholinesterase and antioxidant activities of Savoury (Satureja thymbra L.) with identified major terpenes of the essential oil. Food Chem. 2012;134:48–54. doi: 10.1016/j.foodchem.2012.02.054. [DOI] [Google Scholar]
- 94.Bahadori M.B., Maggi F., Zengin G., Asghari B., Eskandani M. Essential oils of hedgenettles (Stachys inflata, S. lavandulifolia, and S. byzantina) have antioxidant, anti-Alzheimer, antidiabetic, and anti-obesity potential: A comparative study. Ind. Crop. Prod. 2020;145:112089. doi: 10.1016/j.indcrop.2020.112089. [DOI] [Google Scholar]
- 95.Zengin G., Aktumsek A., Ceylan R. Antioxidant Potential and Inhibition of Key Enzymes Linked to Alzheimer’s Diseases and Diabetes Mellitus by Monoterpene-Rich Essential Oil from Sideritis galatica Bornm. Endemic to Turkey. Rec. Nat. Prod. 2016;75:42–50. [Google Scholar]
- 96.Temel H.E., Demirci B., Demirci F., Celep F., Kahraman A., Doğan M., Hüsnü Can Başer K. Chemical characterization and anticholinesterase effects of essential oils derived from Salvia species. J. Essent Oil Res. 2016;28:322–331. doi: 10.1080/10412905.2016.1159257. [DOI] [Google Scholar]
- 97.Cutillas A.B., Carrasco A., Martinez-Gutierrez R., Tomas V., Tudela J. Thymus mastichina L. essential oils from Murcia (Spain): Composition and antioxidant, antienzymatic and antimicrobial bioactivities. PLoS ONE. 2018;13:e0190790. doi: 10.1371/journal.pone.0190790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sevindik H., Ozek T., Yerdelen K., Önal M., Özbek H., Güvenalp Z., Demirezer L.Ö. Chemical Composition, Antioxidant Capacity, Acetyl- and Butyrylcholinesterase Inhibitory Activities of the Essential Oil of Thymus haussknechtii Velen. Rec. Nat. Prod. 2016;10:503–507. [Google Scholar]
- 99.Costa P., Gonçalves S., Grosso C., Andrade P.B., Valentão P., Bernardo-Gil M.G., Romano A. Chemical profiling and biological screening of Thymus lotocephalus extracts obtained by supercritical fluid extraction and hydrodistillation. Ind. Crop. Prod. 2012;36:246–256. doi: 10.1016/j.indcrop.2011.09.014. [DOI] [Google Scholar]
- 100.Okonogi S., Chaiyana W. Enhancement of anti-cholinesterase activity of Zingiber cassumunar essential oil using a microemulsion technique. Drug Discov. 2012;6:249–255. doi: 10.5582/ddt.2012.v6.5.249. [DOI] [PubMed] [Google Scholar]
- 101.Arantes S., Piçarra A., Candeias F., Teixeira D., Caldeira A.T., Martins M.R. Antioxidant activity and cholinesterase inhibition studies of four flavouring herbs from Alentejo. Nat. Prod. Res. 2017;31:2183–2187. doi: 10.1080/14786419.2017.1278598. [DOI] [PubMed] [Google Scholar]
- 102.Chaiyana W., Saeio K., Hennink W.E., Okonogi S. Characterization of potent anticholinesterase plant oil based microemulsion. Int. J. Pharm. 2010;401:32–40. doi: 10.1016/j.ijpharm.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 103.Caputo L., Piccialli I., Ciccone R., de Caprariis P., Massa A., de Feo V., Pannaccione A. Lavender and coriander essential oils and their main component linalool exert a protective effect against amyloid-β neurotoxicity. Phytother. Res. 2020 doi: 10.1002/ptr.6827. [DOI] [PubMed] [Google Scholar]
- 104.Kaufmann D., Dogra A., Wink M. Myrtenal inhibits acetylcholinesterase, a known Alzheimer target. J. Pharm. Pharm. 2011;63:1368–1371. doi: 10.1111/j.2042-7158.2011.01344.x. [DOI] [PubMed] [Google Scholar]
- 105.Xu W., Yang L., Li J. Protection against β-amyloid-induced neurotoxicity by naturally occurring Z-ligustilide through the concurrent regulation of p38 and PI3-K/Akt pathways. Neurochem. Int. 2016;100:44–51. doi: 10.1016/j.neuint.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 106.Akbaba E., Hassan S., Sur T., Bagci E. Memory Enhancing, Anxiolytic and Antidepressant Effects of Achillea biebersteinii (Asteraceae) Essential Oil on Scopolamine-Induced Rats. J. Essent Oil-Bear Plants. 2018;21:825–839. doi: 10.1080/0972060X.2018.1483741. [DOI] [Google Scholar]
- 107.Zhang Z., Chen W., Luan J., Chen D., Liu L., Feng X. Ameliorative effects of olibanum essential oil on learning and memory in Aβ1-42-induced Alzheimer’s disease mouse model. Trop. J. Pharm. Res. 2020;19:1643–1651. [Google Scholar]
- 108.Bae D., Seol H., Yoon H.G., Na J.R., Oh K., Choi C.Y., Lee D.W., Jun W., Youl Lee K., Lee J., et al. Inhaled essential oil from Chamaecyparis obtuse ameliorates the impairments of cognitive function induced by injection of β-amyloid in rats. Pharm. Biol. 2012;50:900–910. doi: 10.3109/13880209.2011.642886. [DOI] [PubMed] [Google Scholar]
- 109.Liu B., Kou J., Li F., Huo D., Xu J., Zhou X., Meng D., Ghulam M., Artyom B., Gao X., et al. Lemon essential oil ameliorates age-associated cognitive dysfunction via modulating hippocampal synaptic density and inhibiting acetylcholinesterase. Aging. 2020;12:8622–8639. doi: 10.18632/aging.103179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hritcu L., Boiangiu R.S., de Morais M.C., de Sousa D.P. (-)cis-Carveol, a Natural Compound, Improves β-Amyloid-Peptide 1-42-Induced Memory Impairment and Oxidative Stress in the Rat Hippocampus. Biomed. Res. Int. 2020;2020:1–9. doi: 10.1155/2020/8082560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hritcu L., Bagci E., Aydin E., Mihasan M. Antiamnesic and Antioxidants Effects of Ferulago angulata Essential Oil Against Scopolamine-Induced Memory Impairment in Laboratory Rats. Neurochem. Res. 2015;40:1799–1809. doi: 10.1007/s11064-015-1662-6. [DOI] [PubMed] [Google Scholar]
- 112.Hritcu L., Cioanca O., Hancianu M. Effects of lavender oil inhalation on improving scopolamine-induced spatial memory impairment in laboratory rats. Phytomedicine. 2012;19:529–534. doi: 10.1016/j.phymed.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 113.Ayuob N.N., El Wahab M.G.A., Ali S.S., Abdel-Tawab H.S. Ocimum basilicum improve chronic stress-induced neurodegenerative changes in mice hippocampus. Metab. Brain Dis. 2018;33:795–804. doi: 10.1007/s11011-017-0173-3. [DOI] [PubMed] [Google Scholar]
- 114.Gradinariu V., Cioanca O., Hritcu L., Trifan A., Gille E., Hancianu M. Comparative efficacy of Ocimum sanctum L. and Ocimum basilicum L. essential oils against amyloid beta (1–42)-induced anxiety and depression in laboratory rats. PhytoChem. Rev. 2014;14:1–9. doi: 10.1007/s11101-014-9389-6. [DOI] [Google Scholar]
- 115.Aydin E., Hritcu L., Dogan G., Hayta S., Bagci E. The Effects of Inhaled Pimpinella peregrina Essential Oil on Scopolamine-Induced Memory Impairment, Anxiety, and Depression in Laboratory Rats. Mol. Neurobiol. 2016;53:6557–6567. doi: 10.1007/s12035-016-9693-9. [DOI] [PubMed] [Google Scholar]
- 116.Postu P.A., Sadiki F.Z., El Idrissi M., Cioanca O., Trifan A., Hancianu M., Hritcu L. Pinus halepensis essential oil attenuates the toxic Alzheimer’s amyloid beta (1-42)-induced memory impairment and oxidative stress in the rat hippocampus. Biomed. Pharm. 2019;112:1–8. doi: 10.1016/j.biopha.2019.108673. [DOI] [PubMed] [Google Scholar]
- 117.Zhu S., Li H., Dong J., Yang W., Liu T., Wang Y., Wang X., Wang M., Zhi D. Rose Essential Oil Delayed Alzheimer’s Disease-Like Symptoms by SKN-1 Pathway in C. elegans. J. Agric. Food Chem. 2017;65:8855–8865. doi: 10.1021/acs.jafc.7b03224. [DOI] [PubMed] [Google Scholar]
- 118.Satou T., Hanashima Y., Mizutani I., Koike K. The effect of inhalation of essential oil from Rosmarinus officinalis on scopolamine-induced Alzheimer’s type dementia model mice. Flavour Frag J. 2018;33:230–234. doi: 10.1002/ffj.3435. [DOI] [Google Scholar]
- 119.Chen Y., Hu J.-h., Zhang Y., Han C., Li J. Anti-Alzheimer’s disease effect of essential oil from aerial parts of Salvia miltiorrhiza Bge. Int. J. Clin. Exp. Med. 2018;11:641–652. [Google Scholar]
- 120.Xu M., Zhang X., Ren F., Yan T., Wu B., Bi K., Bi W., Jia Y. Essential oil of Schisandra chinensis ameliorates cognitive decline in mice by alleviating inflammation. Food Funct. 2019;10:5827–5842. doi: 10.1039/C9FO00058E. [DOI] [PubMed] [Google Scholar]
- 121.Hong Y.K., Park S.H., Lee S., Hwang S., Lee M.J., Kim D., Lee J.H., Han S.Y., Kim S.T., Kim Y.K., et al. Neuroprotective effect of SuHeXiang Wan in Drosophila models of Alzheimer’s disease. J. Ethnopharmacol. 2011;134:1028–1032. doi: 10.1016/j.jep.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 122.Sadiki F.Z., Idrissi M.E., Cioanca O., Trifan A., Hancianu M., Hritcu L., Postu P.A. Tetraclinis articulata essential oil mitigates cognitive deficits and brain oxidative stress in an Alzheimer’s disease amyloidosis model. Phytomedicine. 2019;56:57–63. doi: 10.1016/j.phymed.2018.10.032. [DOI] [PubMed] [Google Scholar]
- 123.Eskandari-Roozbahani N., Shomali T., Taherianfard M. Neuroprotective Effect of Zataria Multiflora Essential Oil on Rats With Alzheimer Disease: A Mechanistic Study. Basic Clin. Neurosci. 2019;10:85–97. doi: 10.32598/bcn.9.10.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ahmadi M., Taherianfard M., Shomali T. Zataria multiflora could improve hippocampal tau protein and TNF(α) levels and cognitive behavior defects in a rat model of Alzheimer’s disease. Avicenna J. Phytomed. 2019;9:465–473. [PMC free article] [PubMed] [Google Scholar]
- 125.Medhat D., El-Mezayen H.A., El-Naggar M.E., Farrag A.R., Abdelgawad M.E., Hussein J., Kamal M.H. Evaluation of urinary 8-hydroxy-2-deoxyguanosine level in experimental Alzheimer’s disease: Impact of carvacrol nanoparticles. Mol. Biol. Rep. 2019;46:4517–4527. doi: 10.1007/s11033-019-04907-3. [DOI] [PubMed] [Google Scholar]
- 126.Wei G., Chen Y.B., Chen D.F., Lai X.P., Liu D.H., Deng R.D., Zhou J.H., Zhang S.X., Li Y.W., Lii H., et al. β-Asarone inhibits neuronal apoptosis via the CaMKII/CREB/Bcl-2 signaling pathway in an in vitro model and AβPP/PS1 mice. J. Alzheimers Dis. 2013;33:863–880. doi: 10.3233/JAD-2012-120865. [DOI] [PubMed] [Google Scholar]
- 127.Cioanca O., Hancianu M., Mircea C., Trifan A., Hritcu L. Essential oils from Apiaceae as valuable resources in neurological disorders: Foeniculi vulgare aetheroleum. Ind. Crop. Prod. 2016:88. doi: 10.1016/j.indcrop.2016.02.064. [DOI] [Google Scholar]
- 128.Xu P., Wang K., Lu C., Dong L., Gao L., Yan M., Aibai S., Liu X. Protective effect of lavender oil on scopolamine induced cognitive deficits in mice and H2O2 induced cytotoxicity in PC12 cells. J. Ethnopharmacol. 2016;193:408–415. doi: 10.1016/j.jep.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 129.Xu P., Wang K., Lu C., Dong L., Gao L., Yan M., Aibai S., Yang Y., Liu X. The Protective Effect of Lavender Essential Oil and Its Main Component Linalool against the Cognitive Deficits Induced by D-Galactose and Aluminum Trichloride in Mice. Evid-Based Complement. Altern. Med. 2017;2017:7426538. doi: 10.1155/2017/7426538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Videira R., Castanheira P., Grãos M., Resende R., Salgueiro L., Faro C., Cavaleiro C. Dose-dependent inhibition of BACE-1 by the monoterpenoid 2,3,4,4-tetramethyl-5-methylenecyclopent-2-enone in cellular and mouse models of Alzheimer’s disease. J. Nat. Prod. 2014;77:1275–1279. doi: 10.1021/np400903w. [DOI] [PubMed] [Google Scholar]
- 131.Zouari-Bouassida K., Trigui M., Makni S., Jlaiel L., Tounsi S. Seasonal Variation in Essential Oils Composition and the Biological and Pharmaceutical Protective Effects of Mentha longifolia Leaves Grown in Tunisia. Biomed. Res. Int. 2018;2018:7856517. doi: 10.1155/2018/7856517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jeon S., Hur J., Jeong H.J., Koo B.S., Pak S.C. SuHeXiang Wan essential oil alleviates amyloid beta induced memory impairment through inhibition of tau protein phosphorylation in mice. Am. J. Chin. Med. 2011;39:917–932. doi: 10.1142/S0192415X11009305. [DOI] [PubMed] [Google Scholar]
- 133.Yen P.L., Cheng S.S., Wei C.C., Lina H.Y., Liao V.H., Chang S.T. Antioxidant Activities and Reduced Amyloid-β Toxicity of 7-Hydroxycalamenene Isolated from the Essential Oil of Zelkova serrata Heartwood. Nat. Prod. Commun. 2016;11:1357–1362. doi: 10.1177/1934578X1601100943. [DOI] [PubMed] [Google Scholar]
- 134.Fathy M.M., Eid H., Hussein M.A., Ahmed H.H., Hussein A. The role of Zingiber officinale in the treatment of Alzheimer’s disease: In-vitro and in-vivo evidences. Res. J. Pharm. Biol. Chem. Sci. 2015;6:735–749. [Google Scholar]
- 135.Ghajarbeygi P., Hajhoseini A., Hosseini M.S., Sharifan A. An In Vitro and In Vivo Cholinesterase Inhibitory Activity of Pistacia khinjuk and Allium sativum Essential Oils. J. Pharm. 2019;22:231–238. doi: 10.3831/kpi.2019.22.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Demirezer L., Gürbüz P., Kelicen Uğur E.P., Bodur M., Özenver N., Uz A., Güvenalp Z. Molecular docking and ex vivo and in vitro anticholinesterase activity studies of Salvia sp. and highlighted rosmarinic acid. Turk. J. Med. Sci. 2015;45:1141–1148. doi: 10.3906/sag-1404-42. [DOI] [PubMed] [Google Scholar]
- 137.Adefegha A., Oboh G., Odubanjo O., Ogunsuyi O. Comparative study on the antioxidative activities, anticholinesterase properties and essential oil composition of Clove (Syzygium aromaticum) bud and Ethiopian pepper (Xylopia aethiopica) La Rivista Italiana Delle Sostanze Grasse. 2016;92:257–268. [Google Scholar]
- 138.Ramazani E., YazdFazeli M., Emami S.A., Mohtashami L., Javadi B., Asili J., Tayarani-Najaran Z. Protective effects of Cinnamomum verum, Cinnamomum cassia and cinnamaldehyde against 6-OHDA-induced apoptosis in PC12 cells. Mol. Biol. Rep. 2020;7:2437–2445. doi: 10.1007/s11033-020-05284-y. [DOI] [PubMed] [Google Scholar]
- 139.Klein-Júnior L.C., dos Santos Passos C., Tasso de Souza T.J., Gobbi de Bitencourt F., Salton J., de Loreto Bordignon S.A., Henriques A.T. The monoamine oxidase inhibitory activity of essential oils obtained from Eryngium species and their chemical composition. Pharm. Biol. 2016;54:1071–1076. doi: 10.3109/13880209.2015.1102949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Morshedi D., Nasouti M. Essential Oils May Lead α-Synuclein towards Toxic Fibrils Formation. Parkinsons Dis. 2016;2016:6219249. doi: 10.1155/2016/6219249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Morshedi D., Aliakbari F., Tayaranian-Marvian A., Fassihi A., Pan-Montojo F., Pérez-Sánchez H. Cuminaldehyde as the major component of Cuminum cyminum, a natural aldehyde with inhibitory effect on alpha-synuclein fibrillation and cytotoxicity. J. Food Sci. 2015;80:H2336–H2345. doi: 10.1111/1750-3841.13016. [DOI] [PubMed] [Google Scholar]
- 142.Ning B., Deng M., Zhang Q., Wang N., Fang Y. β-Asarone inhibits IRE1/XBP1 endoplasmic reticulum stress pathway in 6-OHDA-induced parkinsonian rats. Neurochem. Res. 2016;41:2097–2101. doi: 10.1007/s11064-016-1922-0. [DOI] [PubMed] [Google Scholar]
- 143.Ning B., Zhang Q., Wang N., Deng M., Fang Y. β-Asarone regulates ER stress and autophagy via inhibition of the PERK/CHOP/Bcl-2/Beclin-1 Pathway in 6-OHDA-induced parkinsonian rats. Neurochem. Res. 2019;44:1159–1166. doi: 10.1007/s11064-019-02757-w. [DOI] [PubMed] [Google Scholar]
- 144.Beserra-Filho J.I., de Macêdo A.M., Leão A.H., Bispo J.M.M., Santos J.R., de Oliveira-Melo A.J., Menezes P.D.P., Duarte M.C., de Souza Araújo A.A., Silva R.H. Eplingiella fruticosa leaf essential oil complexed with β-cyclodextrin produces a superior neuroprotective and behavioral profile in a mice model of Parkinson’s disease. Food Chem. Toxicol. 2019;124:17–29. doi: 10.1016/j.fct.2018.11.056. [DOI] [PubMed] [Google Scholar]
- 145.Nemati M., Hemmati A.A., Najafzadeh H., Mansouri M.T., Khodayar M.J. Evaluation of the Effects of Foeniculum vulgare Essence on Behavioral-Motor Disorders of Parkinson’s Disease induced by Reserpine in Ovariectomized and Non Ovariectomized Rats. Jundishapur J. Nat. Pharm. Prod. 2018;13:e67391. doi: 10.5812/jjnpp.67391. [DOI] [Google Scholar]
- 146.Issa M.Y., Ezzat M.I., Sayed R.H., Elbaz E.M., Omar F.A., Mohsen E. Neuroprotective effects of Pulicaria undulata essential oil in rotenone model of parkinson’s disease in rats: Insights into its anti-inflammatory and anti-oxidant effects. S. Afr. J. Bot. 2020;132:289–298. doi: 10.1016/j.sajb.2020.04.032. [DOI] [Google Scholar]
- 147.Liu Q., Sang Heon K., Yung-Wei S., Sok Cheon P., Wonwoong L., Jongki H., Jaehwan J., Kyoung Sang C., Songhee J., Byung-Soo K. Neuroprotective effects of Suhexiang Wan on the in vitro and in vivo models of Parkinson’s disease. J. Tradit. Chin. Med. 2019;39:800–808. [PubMed] [Google Scholar]
- 148.Nikolova G., Karamalakova Y., Kovacheva N., Stanev S., Zheleva A., Gadjeva V. Protective effect of two essential oils isolated from Rosa damascena Mill. and Lavandula angustifolia Mill, and two classic antioxidants against L-dopa oxidative toxicity induced in healthy mice. Regul. Toxicol. Pharm. 2016;81:1–7. doi: 10.1016/j.yrtph.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 149.Nikolova G., Karamalakova Y., Gadjeva V. Reducing oxidative toxicity of L-dopa in combination with two different antioxidants: An essential oil isolated from Rosa Damascena Mill., and vitamin C. Toxicol. Rep. 2019;6:267–271. doi: 10.1016/j.toxrep.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Costa P., Gonçalves S., Valentão P., Andrade P.B., Almeida C., Nogueira J.M., Romano A. Metabolic profile and biological activities of Lavandula pedunculata subsp. lusitanica (Chaytor) Franco: Studies on the essential oil and polar extracts. Food Chem. 2013;141:2501–2506. doi: 10.1016/j.foodchem.2013.05.055. [DOI] [PubMed] [Google Scholar]
- 151.Elmann A., Mordechay S., Rindner M., Ravid U. Anti-neuroinflammatory effects of geranium oil in microglial cells. J. Funct. Foods. 2010;2:17–22. doi: 10.1016/j.jff.2009.12.001. [DOI] [Google Scholar]
- 152.Montalván M., Peñafiel M.A., Ramírez J., Cumbicus N., Bec N., Larroque C., Bicchi C., Gilardoni G. Chemical Composition, Enantiomeric Distribution, and Sensory Evaluation of the Essential Oils Distilled from the Ecuadorian Species Myrcianthes myrsinoides (Kunth) Grifo and Myrcia mollis (Kunth) DC. (Myrtaceae) Plants. 2019;8:511. doi: 10.3390/plants8110511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bagci E., Aydin E., Ungureanu E., Hritcu L. Anthriscus nemorosa essential oil inhalation prevents memory impairment, anxiety and depression in scopolamine-treated rats. Biomed. Pharm. 2016;84:1313–1320. doi: 10.1016/j.biopha.2016.10.075. [DOI] [PubMed] [Google Scholar]
- 154.Bassam A.-S. Antibacterial activities of some plant extracts utilized in popular medicine in Palestine. Turk. J. Biol. 2004;28:99–102. [Google Scholar]
- 155.Rios J.-L., Recio M. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005;100:80–84. doi: 10.1016/j.jep.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 156.Donelian A., Carlson L.H.C., Lopes T.J., Machado R.A.F. Comparison of extraction of patchouli (Pogostemon cablin) essential oil with supercritical CO2 and by steam distillation. J. Supercrit. Fluids. 2009;48:15–20. doi: 10.1016/j.supflu.2008.09.020. [DOI] [Google Scholar]
- 157.Irshad M., Subhani M.A., Ali S., Hussain A. Biological Importance of Essential Oils. In: El-Shemy H.A., editor. Essential Oils-Oils of Nature. IntechOpen; London, UK: 2020. [DOI] [Google Scholar]
- 158.Natural Living Ideas The 10 Most Popular Essential Oils & 174 Magical Ways To Use Them. [(accessed on 12 December 2020)]; Available online: https://www.naturallivingideas.com/most-popular-essential-oils/
- 159.Sadlon A.E., Lamson D.W. Immune-modifying and antimicrobial effects of Eucalyptus oil and simple inhalation devices. Altern. Med. Rev. 2010;15:33–47. [PubMed] [Google Scholar]
- 160.ESCOP Alchemillae Herba, The Scientific Foundation for Herbal Medicinal Products. [(accessed on 12 December 2020)]; Available online: www.escop.com.
- 161.Juergens U.R. Anti-inflammatory properties of the monoterpene 1.8-cineole: Current evidence for co-medication in inflammatory airway diseases. Drug Res. 2014;64:638–646. doi: 10.1055/s-0034-1372609. [DOI] [PubMed] [Google Scholar]
- 162.CSID:2656. 1,8-cineole. [(accessed on 15 January 2021)]; Available online: http://www.chemspider.com/Chemical-Structure.2656.html.
- 163.CSID:6402. α-pinene. [(accessed on 15 January 2021)]; Available online: http://www.chemspider.com/Chemical-Structure.6402.html.
- 164.CSID:14198. β-pinene. [(accessed on 15 January 2021)]; Available online: http://www.chemspider.com/Chemical-Structure.14198.html.
- 165.CSID:28952. Zingerone. [(accessed on 15 January 2021)]; Available online: http://www.chemspider.com/Chemical-Structure.28952.html.
- 166.CSID:388386. Limonene. [(accessed on 15 January 2021)]; Available online: http://www.chemspider.com/Chemical-Structure.388386.html.
- 167.CSID:390582. Ferruginol. [(accessed on 15 January 2021)]; Available online: http://www.chemspider.com/Chemical-Structure.390582.html.
- 168.CSID:454172. Kaur-16-ene. [(accessed on 15 January 2021)]; Available online: http://www.chemspider.com/Chemical-Structure.454172.html.
- 169.CSID:4474888. Rosmarinic acid. [(accessed on 15 January 2021)]; Available online: http://www.chemspider.com/Chemical-Structure.4474888.html.
- 170.CSID:13849981. Linalool. [(accessed on 15 January 2021)]; Available online: http://www.chemspider.com/Chemical-Structure.13849981.html.
- 171.CSID:21105867. Carvacrol. [(accessed on 15 January 2021)]; Available online: http://www.chemspider.com/Chemical-Structure.21105867.html.
- 172.Dhanasekaran S., Perumal P., Palayan M. In-vitro Screening for acetylcholinesterase enzyme inhibition potential and antioxidant activity of extracts of Ipomoea aquatica Forsk: Therapeutic lead for Alzheimer’s disease. J. Appl. Pharm. Sci. 2015;5:012–016. doi: 10.7324/JAPS.2015.50203. [DOI] [Google Scholar]
- 173.Silver A. The Biology of Cholinesterases. North-Holland Publishing Company; Amsterdam, The Netherlands: 1974. pp. 1–596. [Google Scholar]
- 174.Wilson B., Philip W. Encyclopedia of Toxicology. Elsevier; New York, NY, USA: 2005. Cholinesterase inhibition; pp. 588–599. [Google Scholar]
- 175.Wilson B.W. CHAPTER 48-Cholinesterases. In: Krieger R.I., Krieger W.C., editors. Handbook of Pesticide Toxicology. 2nd ed. Academic Press; San Diego, CA, USA: 2001. pp. 967–985. [DOI] [Google Scholar]
- 176.Chatonnet A., Lockridge O. Comparison of butyrylcholinesterase and acetylcholinesterase. Biochem. J. 1989;260:625–634. doi: 10.1042/bj2600625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Schwarz M., Glick D., Loewenstein Y., Soreq H. Engineering of human cholinesterases explains and predicts diverse consequences of administration of various drugs and poisons. Pharm. Ther. 1995;67:283–322. doi: 10.1016/0163-7258(95)00019-D. [DOI] [PubMed] [Google Scholar]
- 178.Tecles F., Ceron J. Determination of whole blood cholinesterase in different animal species using specific substrates. Res. Vet. Sci. 2001;70:233–238. doi: 10.1053/rvsc.2001.0465. [DOI] [PubMed] [Google Scholar]
- 179.Sramek J.J., Cutler N.R. RBC cholinesterase inhibition: A useful surrogate marker for cholinesterase inhibitor activity in Alzheimer disease therapy? Alzheimer Dis. Assoc. Disord. 2000;14:216–227. doi: 10.1097/00002093-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 180.Nicolet Y., Lockridge O., Masson P., Fontecilla-Camps J.C., Nachon F. Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products. J. Biol. Chem. 2003;278:41141–41147. doi: 10.1074/jbc.M210241200. [DOI] [PubMed] [Google Scholar]
- 181.Saxena A., Redman A.M., Jiang X., Lockridge O., Doctor B.P. Differences in active site gorge dimensions of cholinesterases revealed by binding of inhibitors to human butyrylcholinesterase. Biochemistry. 1997;36:14642–14651. doi: 10.1021/bi971425+. [DOI] [PubMed] [Google Scholar]
- 182.Xu Y., Colletier J.-P., Weik M., Jiang H., Moult J., Silman I., Sussman J.L. Flexibility of aromatic residues in the active-site gorge of acetylcholinesterase: X-ray versus molecular dynamics. Biophys. J. 2008;95:2500–2511. doi: 10.1529/biophysj.108.129601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dighe S.N., Deora G.S., de la Mora E., Nachon F., Chan S., Parat M.-O., Brazzolotto X., Ross B.P. Discovery and Structure–Activity Relationships of a Highly Selective Butyrylcholinesterase Inhibitor by Structure-Based Virtual Screening. J. Med. Chem. 2016;59:7683–7689. doi: 10.1021/acs.jmedchem.6b00356. [DOI] [PubMed] [Google Scholar]
- 184.Zhou Y., Lu X., Du C., Liu Y., Wang Y., Hong K.H., Chen Y., Sun H. Novel BuChE-IDO1 inhibitors from sertaconazole: Virtual screening, chemical optimization and molecular modeling studies. Bioorg. Med. Chem. Lett. 2021;34:127756. doi: 10.1016/j.bmcl.2020.127756. [DOI] [PubMed] [Google Scholar]
- 185.Miles J.A., Kapure J.S., Deora G.S., Courageux C., Igert A., Dias J., McGeary R.P., Brazzolotto X., Ross B.P. Rapid discovery of a selective butyrylcholinesterase inhibitor using structure-based virtual screening. Bioorg. Med. Chem. Lett. 2020;30:127609. doi: 10.1016/j.bmcl.2020.127609. [DOI] [PubMed] [Google Scholar]
- 186.Ellman G.L., Courtney K.D., Andres V., Jr., Featherstone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharm. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 187.Pohanka M., Jun D., Kuca K. Improvement of acetylcholinesterase-based assay for organophosphates in way of identification by reactivators. Talanta. 2008;77:451–454. doi: 10.1016/j.talanta.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 188.Morizono M., Akinaga Y. Studies on Tissue Cholinesterase in Domestic Animals II. Detection of Tissue Cholinesterase Isoenzymes. Mem. Fac. Agr. Kagoshima. Univ. 1981;17:219–234. [Google Scholar]
- 189.Frasco M., Fournier D., Carvalho F., Guilhermino L. Do metals inhibit acetylcholinesterase (AChE)? Implementation of assay conditions for the use of AChE activity as a biomarker of metal toxicity. Biomarkers. 2005;10:360–375. doi: 10.1080/13547500500264660. [DOI] [PubMed] [Google Scholar]
- 190.Šinko G., Čalić M., Bosak A., Kovarik Z. Limitation of the Ellman method: Cholinesterase activity measurement in the presence of oximes. Anal. Biochem. 2007;370:223–227. doi: 10.1016/j.ab.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 191.Marrs T.C. Organophosphates and Health. Imperial College Press; London, UK: 2001. Organophosphates: History, chemistry, pharmacology; pp. 1–36. [Google Scholar]
- 192.Michel H.O. An electrometric method for the determination of red blood cell and plasma cholinesterase activity. J. Lab. Clin. Med. 1949;34:1564–1568. [Google Scholar]
- 193.Selkoe D.J. Alzheimer’s disease: Genes, proteins, and therapy. Physiol. Rev. 2001;81:742–760. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 194.Rochette M., Murphy M. Gamma-secretase: Substrates and inhibitors. Mol. Neurobiol. 2002;26:81–95. doi: 10.1385/MN:26:1:081. [DOI] [PubMed] [Google Scholar]
- 195.Lee V.M., Goedert M., Trojanowski J.Q. Neurodegenerative tauopathies. Annu Rev. Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 196.Satou T., Cummings B.J., Head E., Nielson K.A., Hahn F.F., Milgram N.W., Velazquez P., Cribbs D.H., Tenner A.J., Cotman C.W. The progression of β-amyloid deposition in the frontal cortex of the aged canine. Brain Res. 1997;774:35–43. doi: 10.1016/S0006-8993(97)81684-8. [DOI] [PubMed] [Google Scholar]
- 197.Cummings B.J., Head E., Afagh A.J., Milgram N.W., Cotman C.W. β-amyloid accumulation correlates with cognitive dysfunction in the aged canine. Neurobiol. Learn. Mem. 1996;66:11–23. doi: 10.1006/nlme.1996.0039. [DOI] [PubMed] [Google Scholar]
- 198.De Felice F.G., Munoz D.P. Opportunities and challenges in developing relevant animal models for Alzheimer’s disease. Ageing Res. Rev. 2016;26:112–114. doi: 10.1016/j.arr.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 199.Casadesus G. Handbook of Animal Models in Alzheimer’s Disease. 1st ed. Volume 1. IOS Press; Amsterdam, The Netherlands: 2011. p. 340. [Google Scholar]
- 200.Lecanu L., Papadopoulos V. Modeling Alzheimer’s disease with non-transgenic rat models. Alzheimer’s Res. 2013;5:17. doi: 10.1186/alzrt171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Baser K.H. Biological and pharmacological activities of carvacrol and carvacrol bearing essential oils. Curr Pharm. Des. 2008;14:3106–3119. doi: 10.2174/138161208786404227. [DOI] [PubMed] [Google Scholar]
- 202.Jukic M., Politeo O., Maksimovic M., Milos M., Milos M. In vitro acetylcholinesterase inhibitory properties of thymol, carvacrol and their derivatives thymoquinone and thymohydroquinone. Phytother. Res. 2007;21:259–261. doi: 10.1002/ptr.2063. [DOI] [PubMed] [Google Scholar]
- 203.Melo F.H., Venâncio E.T., de Sousa D.P., de França Fonteles M.M., de Vasconcelos S.M., Viana G.S., de Sousa F.C. Anxiolytic-like effect of Carvacrol (5-isopropyl-2-methylphenol) in mice: Involvement with GABAergic transmission. Fundam. Clin. Pharm. 2010;24:437–443. doi: 10.1111/j.1472-8206.2009.00788.x. [DOI] [PubMed] [Google Scholar]
- 204.Melo F.H., Moura B.A., de Sousa D.P., de Vasconcelos S.M., Macedo D.S., Fonteles M.M., Viana G.S., de Sousa F.C. Antidepressant-like effect of carvacrol (5-Isopropyl-2-methylphenol) in mice: Involvement of dopaminergic system. Fundam. Clin. Pharm. 2011;25:362–367. doi: 10.1111/j.1472-8206.2010.00850.x. [DOI] [PubMed] [Google Scholar]
- 205.Krishtal J., Bragina O., Metsla K., Palumaa P., Tõugu V. In situ fibrillizing amyloid-beta 1-42 induces neurite degeneration and apoptosis of differentiated SH-SY5Y cells. PLoS ONE. 2017;12:e0186636. doi: 10.1371/journal.pone.0186636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Pike C.J., Walencewicz-Wasserman A.J., Kosmoski J., Cribbs D.H., Glabe C.G., Cotman C.W. Structure-activity analyses of β-amyloid peptides: Contributions of the β25–35 region to aggregation and neurotoxicity. J. Neurochem. 1995;64:253–265. doi: 10.1046/j.1471-4159.1995.64010253.x. [DOI] [PubMed] [Google Scholar]
- 207.Kaminsky Y.G., Marlatt M.W., Smith M.A., Kosenko E.A. Subcellular and metabolic examination of amyloid-β peptides in Alzheimer disease pathogenesis: Evidence for Aβ25–35. Exp. Neurol. 2010;221:26–37. doi: 10.1016/j.expneurol.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 208.Gabbouj S., Ryhänen S., Marttinen M., Wittrahm R., Takalo M., Kemppainen S., Martiskainen H., Tanila H., Haapasalo A., Hiltunen M., et al. Altered Insulin Signaling in Alzheimer’s Disease Brain—Special Emphasis on PI3K-Akt Pathway. Front. Neurosci. 2019;13 doi: 10.3389/fnins.2019.00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wu Y., Han X., Yuan W., Wang X., Meng D., Hu J., Lv Z. Salt intervention for the diversities of essential oil composition, aroma and antioxidant activities of Kushui rose (R. setate× R. rugosa) Ind. Crop. Prod. 2020;150:112417. doi: 10.1016/j.indcrop.2020.112417. [DOI] [Google Scholar]
- 210.Hsu H., Hsu C. Commonly used Chinese herb formulas with illustrations. 2nd ed. Volume 1. Oriental Healing Arts Institute; Long Beach, CA, USA: 1980. p. 671. [Google Scholar]
- 211.Wang M.C., Bohmann D., Jasper H. JNK signaling confers tolerance to oxidative stress and extends lifespan in Drosophila. Dev. Cell. 2003;5:811–816. doi: 10.1016/S1534-5807(03)00323-X. [DOI] [PubMed] [Google Scholar]
- 212.Wang M.C., Bohmann D., Jasper H. JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell. 2005;121:115–125. doi: 10.1016/j.cell.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 213.Gulactı T., Ufuk K., Mehmet O., Mehmet B., Seda Damla H., Fatemeh B., Burcu C., Tuncay D. Investigation of Anticholinesterase Activity of a Series of Salvia Extracts and the Constituents of Salvia staminea. Nat. Prod. J. 2013;3:3–9. doi: 10.2174/2210315511303010003. [DOI] [Google Scholar]
- 214.Chen J., Montanari A.M., Widmer W.W. Two New Polymethoxylated Flavones, a Class of Compounds with Potential Anticancer Activity, Isolated from Cold Pressed Dancy Tangerine Peel Oil Solids. J. Agric. Food Chem. 1997;45:364–368. doi: 10.1021/jf960110i. [DOI] [Google Scholar]
- 215.Nogata Y., Sakamoto K., Shiratsuchi H., Ishii T., Yano M., Ohta H. Flavonoid Composition of Fruit Tissues of Citrus Species. Biosci. Biotechnol. Biochem. 2006;70:178–192. doi: 10.1271/bbb.70.178. [DOI] [PubMed] [Google Scholar]
- 216.Avetisyan A., Markosian A., Petrosyan M., Sahakyan N., Babayan A., Aloyan S., Trchounian A. Chemical composition and some biological activities of the essential oils from basil Ocimum different cultivars. BMC Complement. Altern. Med. 2017;17:60. doi: 10.1186/s12906-017-1587-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Batista P.A., Werner M.F., Oliveira E.C., Burgos L., Pereira P., Brum L.F., Story G.M., Santos A.R. The antinociceptive effect of (-)-linalool in models of chronic inflammatory and neuropathic hypersensitivity in mice. J. Pain. 2010;11:1222–1229. doi: 10.1016/j.jpain.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 218.Elisabetsky E., Marschner J., Souza D.O. Effects of Linalool on glutamatergic system in the rat cerebral cortex. Neurochem. Res. 1995;20:461–465. doi: 10.1007/BF00973103. [DOI] [PubMed] [Google Scholar]
- 219.Letizia C.S., Cocchiara J., Lalko J., Api A.M. Fragrance material review on linalool. Food Chem. Toxicol. 2003;41:943–964. doi: 10.1016/S0278-6915(03)00015-2. [DOI] [PubMed] [Google Scholar]
- 220.Mitić-Culafić D., Zegura B., Nikolić B., Vuković-Gacić B., Knezević-Vukcević J., Filipic M. Protective effect of linalool, myrcene and eucalyptol against t-butyl hydroperoxide induced genotoxicity in bacteria and cultured human cells. Food Chem. Toxicol. 2009;47:260–266. doi: 10.1016/j.fct.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 221.Sabogal-Guáqueta A.M., Osorio E., Cardona-Gómez G.P. Linalool reverses neuropathological and behavioral impairments in old triple transgenic Alzheimer’s mice. Neuropharmacology. 2016;102:111–120. doi: 10.1016/j.neuropharm.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Jankovic J., Aguilar L.G. Current approaches to the treatment of Parkinson’s disease. Neuropsych. Dis. Treat. 2008;4:743–757. doi: 10.2147/NDT.S2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Rao P.V., Gan S.H. Cinnamon: A multifaceted medicinal plant. Evid-Based Complement. Altern. Med. 2014;2014:1–2. doi: 10.1155/2014/642942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Brahmachari S., Jana A., Pahan K. Sodium benzoate, a metabolite of cinnamon and a food additive, reduces microglial and astroglial inflammatory responses. J. Immunol. 2009;183:5917–5927. doi: 10.4049/jimmunol.0803336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Mollazadeh H., Hosseinzadeh H. Cinnamon effects on metabolic syndrome: A review based on its mechanisms. Iran. J. Basic Med. Sci. 2016;19:1258. doi: 10.22038/ijbms.2016.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Liu X.C., Cheng J., Zhao N.N., Liu Z.L. Insecticidal activity of essential oil of Cinnamomum cassia and its main constituent, trans-cinnamaldehyde, against the booklice, Liposcelis bostrychophila. Trop. J. Pharm. Res. 2014;13:1697–1702. doi: 10.4314/tjpr.v13i10.18. [DOI] [Google Scholar]
- 227.Nabavi S.F., di Lorenzo A., Izadi M., Sobarzo-Sánchez E., Daglia M., Nabavi S.M. Antibacterial effects of cinnamon: From farm to food, cosmetic and pharmaceutical industries. Nutrients. 2015;7:7729–7748. doi: 10.3390/nu7095359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Youdim M.B., Bakhle Y. Monoamine oxidase: Isoforms and inhibitors in Parkinson’s disease and depressive illness. Br. J. Pharm. 2006;147:S287–S296. doi: 10.1038/sj.bjp.0706464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Youdim M.B., Edmondson D., Tipton K.F. The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neurosci. 2006;7:295–309. doi: 10.1038/nrn1883. [DOI] [PubMed] [Google Scholar]
- 230.McCormack A.L., Mak S.K., Shenasa M., Langston W.J., Forno L.S., di Monte D.A. Pathologic modifications of α-synuclein in 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP)-treated squirrel monkeys. J. Neuropath Exp. Neur. 2008;67:793–802. doi: 10.1097/NEN.0b013e318180f0bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ahmad A., Burns C.S., Fink A.L., Uversky V.N. Peculiarities of copper binding to α-synuclein. J. Biomol. Struct. Dyn. 2012;29:825–842. doi: 10.1080/073911012010525023. [DOI] [PubMed] [Google Scholar]
- 232.Mohammad-Beigi H., Shojaosadati S.A., Marvian A.T., Pedersen J.N., Klausen L.H., Christiansen G., Pedersen J.S., Dong M., Morshedi D., Otzen D.E. Strong interactions with polyethylenimine-coated human serum albumin nanoparticles (PEI-HSA NPs) alter α-synuclein conformation and aggregation kinetics. Nanoscale. 2015;7:19627–19640. doi: 10.1039/C5NR05663B. [DOI] [PubMed] [Google Scholar]
- 233.Silva B.A., Breydo L., Fink A.L., Uversky V.N. Agrochemicals, α-synuclein, and Parkinson’s disease. Mol. Neurobiol. 2013;47:598–612. doi: 10.1007/s12035-012-8333-2. [DOI] [PubMed] [Google Scholar]
- 234.Schröder M., Kaufman R.J. ER stress and the unfolded protein response. Mutat Res./Fund Mol. M. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 235.Ryu E.J., Harding H.P., Angelastro J.M., Vitolo O.V., Ron D., Greene L.A. Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson’s disease. J. Neurosci. 2002;22:10690–10698. doi: 10.1523/JNEUROSCI.22-24-10690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Hoozemans J., van Haastert E., Eikelenboom P., de Vos R., Rozemuller J., Scheper W. Activation of the unfolded protein response in Parkinson’s disease. Biochem. Biophys. Res. Commun. 2007;354:707–711. doi: 10.1016/j.bbrc.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 237.Jiang P., Gan M., Ebrahim A.S., Lin W.-L., Melrose H.L., Yen S.-H.C. ER stress response plays an important role in aggregation of α-synuclein. Mol. Neurodegener. 2010;5:1–15. doi: 10.1186/1750-1326-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Gardner B.M., Pincus D., Gotthardt K., Gallagher C.M., Walter P. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb Perspect. Biol. 2013;5:a013169. doi: 10.1101/cshperspect.a013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Hotamisligil G.S. Endoplasmic reticulum stress and atherosclerosis. Nat. Med. 2010;16:396–399. doi: 10.1038/nm0410-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hotamisligil G.S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Todd D.J., Lee A.-H., Glimcher L.H. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat. Rev. Immunol. 2008;8:663–674. doi: 10.1038/nri2359. [DOI] [PubMed] [Google Scholar]
- 242.Senft D., Ze’ev A.R. UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem. Sci. 2015;40:141–148. doi: 10.1016/j.tibs.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Yorimitsu T., Nair U., Yang Z., Klionsky D.J. Endoplasmic reticulum stress triggers autophagy. J. Biol. Chem. 2006;281:30299–30304. doi: 10.1074/jbc.M607007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Wei Y., Pattingre S., Sinha S., Bassik M., Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol. Cell. 2008;30:678–688. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kabuto H., Nishizawa M., Tada M., Higashio C., Shishibori T., Kohno M. Zingerone [4-(4-hydroxy-3-methoxyphenyl)-2-butanone] prevents 6-hydroxydopamine-induced dopamine depression in mouse striatum and increases superoxide scavenging activity in serum. Neurochem. Res. 2005;30:325–332. doi: 10.1007/s11064-005-2606-3. [DOI] [PubMed] [Google Scholar]
- 246.Kabuto H., Tada M., Kohno M. Eugenol [2-methoxy-4-(2-propenyl) phenol] prevents 6-hydroxydopamine-induced dopamine depression and lipid peroxidation inductivity in mouse striatum. Biol. Pharm. Bull. 2007;30:423–427. doi: 10.1248/bpb.30.423. [DOI] [PubMed] [Google Scholar]
- 247.Siqueira-Lima P.S., Brito R.G., Araújo-Filho H.G., Santos P.L., Lucchesi A., Araújo A.A., Menezes P.P., Scotti L., Scotti M.T., Menezes I.R. Anti-hyperalgesic effect of Lippia grata leaf essential oil complexed with β-cyclodextrin in a chronic musculoskeletal pain animal model: Complemented with a molecular docking and antioxidant screening. Biomed. Pharm. 2017;91:739–747. doi: 10.1016/j.biopha.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 248.Oliveira M.A., Guimarães A.G., Araújo A.A., Quintans-Júnior L.J., Quintans J.S. New drugs or alternative therapy to blurring the symptoms of fibromyalgia—a patent review. Expert Opin. Pat. 2017;27:1147–1157. doi: 10.1080/13543776.2017.1349105. [DOI] [PubMed] [Google Scholar]
- 249.Araújo-Filho H.G., Pereira E.W., Rezende M.M., Menezes P.P., Araújo A.A., Barreto R.S., Martins A.O., Albuquerque T.R., Silva B.A., Alcantara I.S. D-limonene exhibits superior antihyperalgesic effects in a β-cyclodextrin-complexed form in chronic musculoskeletal pain reducing Fos protein expression on spinal cord in mice. Neuroscience. 2017;358:158–169. doi: 10.1016/j.neuroscience.2017.06.037. [DOI] [PubMed] [Google Scholar]
- 250.Santos P.L., Brito R.G., Quintans J.S.S., Araujo A.A.S., Menezes I.R.A., Brogden N.K., Quintans-Junior L.J. Cyclodextrins as complexation agents to improve the anti-inflammatory drugs profile: A systematic review and meta-analysis. Curr. Pharm. Des. 2017;23:2096–2107. doi: 10.2174/1381612823666170126121926. [DOI] [PubMed] [Google Scholar]
- 251.Brito R.G., Araujo A.A., Quintans J.S., Sluka K.A., Quintans-Junior L.J. Enhanced analgesic activity by cyclodextrins–a systematic review and meta-analysis. Expert Opin Drug Del. 2015;12:1677–1688. doi: 10.1517/17425247.2015.1046835. [DOI] [PubMed] [Google Scholar]
- 252.Andrade A.M., Oliveira J.P., Santos A.L., Franco C.R., Antoniolli Â.R., Estevam C.S., Thomazzi S.M. Preliminary study on the anti-inflammatory and antioxidant activities of the leave extract of Hyptis fruticosa Salzm. ex Benth., Lamiaceae. Rev. Bras. Farm. 2010;20:962–968. doi: 10.1590/S0102-695X2010005000034. [DOI] [Google Scholar]
- 253.De Lima A.C.B., Paixão M.S., Melo M., de Santana M.T., Damascena N.P., Dias A.S., Porto Y.C.B.S., Fernandes X.A., Santos C.C.S., Lima C.A., et al. Orofacial antinociceptive effect and antioxidant properties of the hydroethanol extract of Hyptis fruticosa salmz ex Benth. J. Ethnopharmacol. 2013;146:192–197. doi: 10.1016/j.jep.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 254.Tadaiesky M.T., Andreatini R., Vital M.A. Different effects of 7-nitroindazole in reserpine-induced hypolocomotion in two strains of mice. Eur. J. Pharm. 2006;535:199–207. doi: 10.1016/j.ejphar.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 255.Ben J., Soares F.M., Scherer E.B., Cechetti F., Netto C.A., Wyse A.T. Running exercise effects on spatial and avoidance tasks in ovariectomized rats. Neurobiol. Learn. Mem. 2010;94:312–317. doi: 10.1016/j.nlm.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 256.Scott E.L., Zhang Q.G., Han D., Desai B.N., Brann D.W. Long-term estrogen deprivation leads to elevation of Dickkopf-1 and dysregulation of Wnt/β-Catenin signaling in hippocampal CA1 neurons. Steroids. 2013;78:624–632. doi: 10.1016/j.steroids.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Sarkaki A., Badavi M., Hoseiny N., Gharibnaseri M.K., Rahim F. Postmenopausal effects of intrastriatal estrogen on catalepsy and pallidal electroencephalogram in an animal model of Parkinson’s disease. Neuroscience. 2008;154:940–945. doi: 10.1016/j.neuroscience.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 258.Namavar Jahromi B., Tartifizadeh A., Khabnadideh S. Comparison of fennel and mefenamic acid for the treatment of primary dysmenorrhea. Int. J. Gynaecol. Obs. 2003;80:153–157. doi: 10.1016/S0020-7292(02)00372-7. [DOI] [PubMed] [Google Scholar]
- 259.Modaress Nejad V., Asadipour M. Comparison of the effectiveness of fennel and mefenamic acid on pain intensity in dysmenorrhoea. East. Mediterr. Health J. 2006;12:423–427. [PubMed] [Google Scholar]
- 260.Betarbet R., Sherer T.B., MacKenzie G., Garcia-Osuna M., Panov A.V., Greenamyre J.T. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat. Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 261.Talpade D.J., Greene J.G., Higgins D.S., Jr., Greenamyre J.T. In vivo labeling of mitochondrial complex I (NADH:ubiquinone oxidoreductase) in rat brain using [(3)H]dihydrorotenone. J. Neurochem. 2000;75:2611–2621. doi: 10.1046/j.1471-4159.2000.0752611.x. [DOI] [PubMed] [Google Scholar]
- 262.Sherer T.B., Richardson J.R., Testa C.M., Seo B.B., Panov A.V., Yagi T., Matsuno-Yagi A., Miller G.W., Greenamyre J.T. Mechanism of toxicity of pesticides acting at complex I: Relevance to environmental etiologies of Parkinson’s disease. J. Neurochem. 2007;100:1469–1479. doi: 10.1111/j.1471-4159.2006.04333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Liu L.-L., Yang J.-L., Shi Y.-P. Phytochemicals and biological activities of Pulicaria species. Chem. Biodivers. 2010;7:327–349. doi: 10.1002/cbdv.200900014. [DOI] [PubMed] [Google Scholar]
- 264.Lee K.S., Lee J.K., Kim H.G., Kim H.R. Differential Effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine on Motor Behavior and Dopamine Levels at Brain Regions in Three Different Mouse Strains. Korean J. Physiol. Pharm. 2013;17:89–97. doi: 10.4196/kjpp.2013.17.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Worwood V.A. Aromatherapy for the Healthy Child.: More Than 300 Natural, Nontoxic, and Fragrant Essential Oil Blends. 3rd ed. New World Library; Novato, CA, USA: 2000. p. 384. [Google Scholar]