Abstract
Background: Ferula ammoniacum (D. Don) is one of the endemic medicinal plants that is traditionally used to treat a number of diseases. Although the plant has been used to enhance memory, the investigational evidence supporting the nootropic effect was unsubstantial. Hence, the rationale for this study was to assess the potential beneficial effect of F. ammoniacum seed extracts on learning and memory in mice. Methods: The powdered plant samples (aerial parts) were subjected to extraction ad fractionation. Among the extracts, crude and ethyl acetate extracts were screened for major phytochemicals through HPLC analysis. All the extracts were evaluated for the in vitro anticholinesterase (AChE and BChE) and antioxidant potentials. Among the extracts the active fraction was further assessed for improving learning and memory in mice using behavioural tests like Y-maze and novel object recognition test (NORT) using standard protocols. After behavioural tests, all the animals were sacrificed and brains tissues were assessed for the ex vivo anticholinesterase and antioxidant potentials. Results: Phytochemicals like chlorogenic acid, quercetin, mandelic acid, phloroglucinol, hydroxy benzoic acid, malic acid, epigallocatechin gallate, ellagic acid, rutin, and pyrogallol were identified in crude methanolic extract (Fa.Met) and ethyl acetate fraction (Fa.EtAc) through HPLC. Fa.EtAc and Fa.Chf extracts more potently inhibited AChE and BChE with IC50 values of 40 and 43 µg/mL, and 41 and 42 µg/mL, respectively. Similarly highest free radical scavenging potential was exhibited by Fa.EtAc fraction against DPPH (IC50 = 100 µg/mL) and ABTS (IC50 = 120 µg/mL). The extract doses, 100 and 200 mg/kg body weight significantly (p < 0.01) improved the short-term memory by increasing the percent spontaneous alternation in the Y-maze test along with increasing discrimination index in the NORT that clearly indicated the enhancement in the recognition memory of mice. Conclusion: The extracts more potently scavenged the tested free radicals, exhibited anticholinesterase activities, improved the learning abilities and reduced the memory impairment induced by scopolamine in mice model thus suggesting that these extracts could be effectively used for the management of oxidative stress, neurodegenerative diseases and memory loss.
Keywords: Ferula ammoniacum (D. Don), Dorema ammoniacum (D. Don), HPLC, cholinesterases (AChE and BChE), DPPH, ABTS, Y-Maze, NORT, Alzheimer’s disease
1. Introduction
According to a 2001 World Health Organization (WHO) report, 25% of the world population is suffering from neurological disorders like epilepsy, headache, migraine, madness, insomnia, stress, depression, Alzheimer’s (AD), and Parkinson’s diseases [1]. AD is a chronic neurodegenerative disease where the deposition of neuronal amyloid along with a deficit of neurotransmitters; acetylcholine (ACh) level occurs, leading to memory dysfunction [2]. Oxidative stress has been pointed to be main factor involved in the pathogenesis of AD. Another predominant reason is the reduction of acetylcholine level in the brain, which is the most notable biochemical change in AD. Acetyl cholinesterase (AChE) and butyryl cholinesterase (BChE) are the key enzymes that causes the degradation of ACh resulting in cholinergic deficits and termination of cholinergic neurotransmission. Thus, the inhibition of cholinesterases is considered to be the base while treating AD and this promising strategy is clinically applied everywhere for treating neurodegenerative diseases and is a rational approach. Inhibition of brain cholinesterases (AChE and BChE) by potential inhibitors reinstates the level of ACh and, thus, plays an important role in the treatment of AD, Dementia, and Parkinson’s disease [3] and the consequences of cholinesterases inhibition are assessed by observing cholinergically mediated processes such as cognition [4]. Acetylcholine is a key neurotransmitter in the nervous system that interacts with receptors associated with processes of learning and memory. It has been proved that cholinesterase inhibition is a preferable direct receptor agonist therapy that relives ACh deficit and stimulates either nicotinic or muscarinic ACh receptors. Cholinesterases inhibitors like Donepezil are approved by the Food and Drug Administration for the symptomatic treatment of AD. These drugs improve cognitive and neuropsychiatric symptoms. Donepezil is a second-generation cholinesterases inhibitor with a high selectivity in the central nervous system, can reversibly inhibit cholinesterases (AChE and BChE) whereby reducing ACh degradation, thus increase neurotransmitter concentration in the synaptic cleft that prolongs its duration of action, ultimately elevating the central cholinergic activity to improve cognitive function. The use of donepezil seems to have a positive effect on the functioning and enhancements in cognition [5].
Researchers these days are in search of plants-based cholinesterase inhibitors and antioxidants which would significantly recovers cognitive decline. Although synthetic antioxidants are utilized in the food industry, but they have exhibited adverse side effects including liver damage and carcinogenesis. Natural sources, especially plants, provide a miscellaneous and largely unexploited reservoir of bioactive phytochemicals for drug discovery and for the development of new cholinesterase inhibitors and antioxidants. Previous studies have highlighted the potential beneficial effects of plants as vital sources for cholinesterase inhibitors [6]. Individuals suffering from neurological disorders in the developing and underdeveloped countries primarily rely on traditional herbal medicines [7]. The folkloric knowledge about plants have helped in isolation of a large number of bioactive compounds from various medicinal plants for example Galantamine, a potential inhibitor of acetylcholinesterase that have been isolated from Galanthus nivalis and is frequently used to treat AD [8].
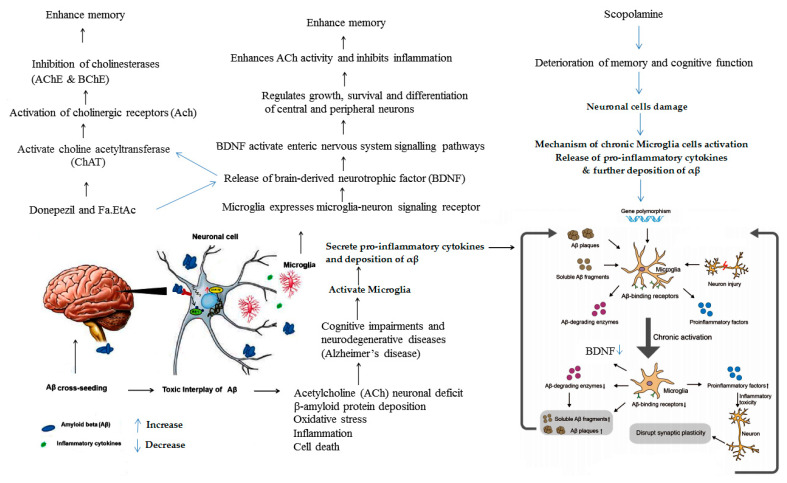
The association of neurodegenerative diseases like AD with some pathological conditions, such as impaired mitochondrial function, amyloids–beta (αβ) deposition, neuroinflammation, cholinergic deficit, and oxidative stress has been pointed out by many researchers [9,10]. In patients with AD, intense AChE, BChE and free radical activities have been found in the area of amyloid plaques and neurofibrillary tangles. Amyloids-beta (αβ) and cholinesterase activities may act as a physiological modulator of cholinergic function and induce neurotoxicity, alongside inhibiting the synthesis and release of ACh, resulting in cholinergic hypofunction, reduced neural efficiency, and cognitive impairment. These effects seem to appear in the cortex and hippocampus regions but not in other brain areas. αβ activates microglial cells to act as pro-inflammatory cells that secrete pro-inflammatory cytokines, which induces further αβ deposition. Microglia cells are the resident immune cells of the central nervous system which express surface receptors that activate or amplify the innate immune response. During cellular damage microglial cells respond quickly by inducing a protective immune response, which result in the up regulation of inflammatory molecules as well as neurotrophic factors. However, in case of chronic inflammation the prolonged activation of microglia leads to the production of a wide array of neurotoxicins, proinflammatory cytokines such as interleukin-1 (IL-1β), interleukin-6 (IL-6), tumour necrosis factor alpha (TNFα), and cholinesterases (AChE and BChE) in the extracellular spaces [9,10]. It has been proposed that the increase microglial cells activation in brain may be one of the early events that leads to oxidative damage and is considered to be the most abundant source of free radicals in the brain (such as superoxide and nitric oxide). Microglial cell derived radicals, as well as their reaction products such as hydrogen peroxide and peroxynitrite have been shown to be involved in oxidative damage and neuronal cell death in neurological diseases such as AD. Studies have shown that microglia cells play a role in supporting cognitive processes and homeostasis in the healthy adult brain while their absence results in cognitive and learning deficits in rodents during development. It also should be noted that microglial cells have efficient antioxidative defence mechanisms but when the production of reactive oxygen species (ROS) is prolonged, the endogenous reserves of antioxidants become exhausted and result in cellular damage [9].
ROS are constantly generated in redox processes during metabolism that attacks the biomolecules like enzymes, lipids, proteins, DNA and RNA resulting in an irreversible damage to these important commodities of life. Luckily, the human body can manage to cope with oxidative stress, through a number of defensive systems involving oxidant enzymes, and non-enzymatic compounds. However, in pathological conditions where excessive amounts of ROS encounters the defensive systems cannot properly manage the situations leading to chronic diseases like cancer, atherosclerosis, nephritis, diabetes mellitus, rheumatism, ischemic, cardiovascular disease, and aging. Oxidative stress also plays a key role in the development of neurodegenerative disorders like AD and Parkinson’s diseases [11]. The accumulation of ROS has been found in several chronic diseases, including AD suggesting that ROS may contribute to the pathogenesis of these diseases by inducing oxidative stress. Medicinal plants counteract the potential damaging effects of oxidative stress by the production of antioxidants. Previous studies have revealed that antioxidants have significant potential to reduce the symptoms and prevalence of AD [12].
Antioxidant like phenolics and flavonoids are commonly found in various fruits and vegetables and they have been shown to provide a fruitful defence against oxidative stress from oxidizing agents/free radicals. Oxidative stress is often defined as an imbalance between free radicals and antioxidant defence system. These radicals contribute to the development of many diseases including AD and can be neutralized by antioxidants. The consumption of fruits and vegetables are beneficial for health as they contain various secondary metabolites and other nutrients capable of curing a number of health complications [13]. Due to toxic effects of synthetic antioxidants; recent studies are mainly focused on their replacement with naturally occurring antioxidants from medicinal plants. Therefore, it is important to discover and isolate novel bioactive compounds from natural sources and evaluate their various biological potentials. Secondary metabolites like flavonoid and phenolics have great importance as antioxidants as they have high potentials of scavenging free radicals like ROS [14].
Ferula ammoniacum (D. Don) Spalik, M. panahi, Piwczynski and Puchalka, is an important members of family Apiaceae also known as Dorema ammoniacum (D. Don) [15], has a wide variety of traditional uses, for example, it produces gum which exude from stem, roots, and petioles that is medicinally used to treat a number of diseases. This gum resin is traditionally used as a carminative, diaphoretic, mild diuretic, expectorant, stimulant, cardiovascular, anthelmintic, antiepileptic, asthmatic, and antitussive. D. ammoniacum gum resin has anti-helminthic potentials and has been used to treat gastrointestinal disorders and as anti-inflammatory agent in Iranian traditional medicine [16]. Its latex has also medicinal uses like expectorant, bronchitis, and stomach-ache [17]. Ghasemi et al. (2018) elucidates the electrophysiological mechanism of the effect of D. ammoniacum gum on a cellular model of epilepsy, using intracellular recording method [18]. D. ammoniacum gum resin has shown a significant anticonvulsant activity in pentylentetrazole model (in mice) in a dose dependent manner via involvement of GABAergic and opioid systems [19]. The gum resin has exhibited significant acetylcholine esterase inhibitory potential [20] and antimicrobial activities [21]. Apart from the mentioned potentials, D. ammoniacum have also exhibited other pharmacological effects, including anxiolytic, anti-inflammatory, anti-coagulation, anti-epileptogenic and anticonvulsant [17]. In Persian traditional medicine textbooks, D. ammoniacum is one of the materia medica that is frequently used for stroke and paralysis as it opens the blood vessels and is also considered as a thrombolytic agent. In traditional medicines, it is used as a remedy by itself and sometimes it is used in combination with other drugs to treat health complications like stroke and paralysis [22]. The essential oil of D. ammoniacum leaf, fruit, and stem have already been reported to have antioxidant, antimicrobial, and low cytotoxicity activities [23,24]. Chemical composition of essential oil and toxicological studies like acute and sub-acute toxicity of D. ammoniacum oleo-gum-resin has also been reported [25]. Antibacterial and vasodilatory effects of this herbal plant have also been reported [26]. D. ammoniacum aerial part extract has been used as a mediator in the synthesis of silver nanoparticles that have displayed antimicrobial activities [27]. The Dorema other species are also traditionally used as anticancer, antimicrobial, insecticidal, and as antioxidant agents [28,29,30]. However, its effect as nootropic agent has not been investigated yet.
Therefore, the current investigational study was designed to evaluate the phytochemical composition and evaluate in in vitro its anticholinesterase and antioxidant potential along with in vivo nootropic activities in mice.
2. Material and Methods
2.1. Drugs and Chemicals
AChE (Electron eel type-VI-S) and Aquine BChE along with their substrates acetylthiocholine iodide and butyrylthiocholine iodide, and potassium phosphate buffer (pH 8.0) were purchased from Sigma Aldrich St. Louis, MO, USA. Antioxidant chemicals: DPPH, ABTS, ascorbic acid, malic acid, morin, epigallocatechin gallate, pyrogallol, rutin, quercetin, chlorogenic acid, mandelic acid, hydroxy benzoic acid, and gallic acid along with Folin–Ciocalteu regent were acquired from Sigma Aldrich, Darmstadt, Germany. Donepezil and scopolamine were also purchased from Sigma Aldrich (USA). All these chemicals used were of analytical grade; however, the HPLC solvents were of HPLC grade.
2.2. Plant Material Collection and Identification
Ferula ammoniacum aerial parts were collected from the hilly areas of village Yar Khan Banda (Kondoly), Timergara Dir (Lower), Pakistan in 2019. The plant was identified by plant taxonomist Prof. Mehboob-ur-Rahman, PGC, Swat, Khyber Pakhtunkhwa, Pakistan. The plant specimens were deposited with voucher number BGH.UOM.163 in the Botanical Garden Herbarium, University of Malakand, Pakistan.
2.3. Preparation of F. ammoniacum Aerial Parts Extract/Fractions
The collected samples were cleaned and kept on a clean paper to shade dried for 20 days. The dried aerial parts sample were crushed through mechanical grinder. Approximately 3 kg of powder sample were subjected to maceration in 80% methanol for 14 days with periodical shaking [13]. Filtration was conducted through muslin cloth followed by filtration through Whattman filter paper. The filtrate was concentrated into a semisolid mass using rotary evaporator (Heidolph Laborota 4000, Schwabach, Germany) under reduced pressure and then completely dried through lyophiliser. The semisolid mass was then solidified (280 g end yield product) in open air. The crude methanolic extract (Fa.Met) 250 g was subjected to fractionation using solvent-solvent extraction procedure starting from a low polarity solvent to highly polar one. The final yield of n-hexane (Fa.Hex), chloroform (Fa.Chf), ethyl acetate (Fa.EtAc), n-butanol (Fa.Bn), and aqueous (Fa.Aq) fraction were 35, 22, 45, 22 and 113 g, respectively.
2.4. HPLC-UV Characterization of Phytochemicals
To prepare the sample for HPLC analysis, about 1 g of extract was mixed with 20 mL of methanol and water (1:1 v/v) and heated on a water bath at 70 °C for 1 h. Centrifugation of sample was conducted at 4000 rpm for 10 min after cooling. Then, 2 mL from sample was filtered with Whattman filter paper into HPLC vials and labelled with proper codes.
An Agilent 1260 infinity High-performance liquid chromatography (HPLC) system was used to analyse the samples while separation was achieved via Agilent Zorbax Eclipse XDB-C18 column with gradients system comprising of solvent A (methanol:acetic acid:deionized water, 100:20:180, v/v) and solvent B (methanol:acetic acid:deionized water, 900:20:80, v/v) used to elute the bioactive compounds [31]. The concentration of a given phytochemicals was determined using following single point calibration formula:
| (1) |
where: Cx = Sample concentration; As = Standard peak area; Ax = Sample peak area; Cs = Standard concentration (0.09 µg/mL).
2.5. Assessment of Total Phenolic Contents
Total phenolic content (TPC) in the extracts was determined using previously reported method [32]. In this test, extract/fractions samples (100 µL), distilled water (500 µL), Folin–Ciocalteu reagent (100 µL), and 7% sodium carbonate (1000 µL) were mixed together and allowed to stand for 90 min. Finally, absorbance was measured at 760 nm using UV-Spectrophotometer. Gallic acid standard curve was obtained using the dilutions: 1000, 500, 250, 125, 62.5, and 31.05 µg/mL. The TPC was expressed as mg of gallic acid equivalent per gram (mg GAE/g) of dry sample (averaged from three parallel measurements).
2.6. Assessment of Total Flavonoid Contents
Total flavonoids contents (TFC) in F. ammoniacum aerial parts extract/fractions were calculated using previously reported method [32]. Quercetin was used as a standard and TFC was determined as mg of Quercetin equivalent (mg QE/g) per gram of dry sample of extract/fractions. A calibration curve for Quercetin was obtained using their various dilutions (1000, 500, 250, 125, 62.5, and 31.05 µg/mL). about 100 µL from each sample dilutions were taken and mixed with distilled water (500 µL), 5% sodium nitrate (100 µL), 10% aluminum chloride (150 µL) solution, and 1 M sodium hydroxide (200 µL), then allowed to stand for 5 min and its absorbance was recorded at 510 nm using UV-Spectrophotometer. All results were taken in triplicate.
2.7. In Vitro Cholinesterase Inhibition Potential of Extracts
Ellman assay [33] was used to assess the F. ammoniacum aerial parts methanolic extract/fractions for their cholinesterase inhibition potentials. About 205 µL extract/fractions and 5 µL of AChE (0.03 U/mL)/BChE (0.01 U/mL) along with 5 µL DTNB were taken in a cuvette and incubated at 30 °C for 15 min. After incubation, 5 µL acetylthiocholine iodide or butyrylthiocholine iodide (substrate) were added to the mixture that resulted in yellow coloration due to formation of 5-Thio-2-nitro benzoate anion. Then, the absorbance of the resulting mixture was measured at 412 nm through double beam spectrophotometer (Thermo electron-corporation, Waltham, MA, USA). As a negative control, a solution containing all the above-mentioned components except plant extracts/fractions were mixed together. The same procedure mentioned above was used to constitute the reaction mixture of positive control galantamine and absorbance was measured at 412 nm. For each sample, absorbance was recorded for 4 min. Percent enzyme activity and inhibition potential of both enzymes were measured using the following formulae:
| (2) |
| (3) |
| (4) |
where: V shows the rate of reaction in the presence of inhibitor and Vmax, the rate of reaction in its absence.
2.8. DPPH (2,2-Diphenyl-1-picrylhydrazyl) Free Radical Scavenging Potential of Extracts
Brand-Williams assay [34] was used to determine the free radical scavenging potential of F. ammoniacum aerial parts methanolic extract/fractions against DPPH. The synthetic free radical, DPPH solution was prepared by dissolving 24 mg of it in 100 mL of methanol. The plant samples (1 mg/mL) were also made in methanol and working solutions were prepared using serial dilutions in the concentration range of 31.05–1000 µg/mL. About 0.1 mL of each working dilution was mixed with 3 mL of DPPH solution and incubated for 30 min at 25 °C. Absorbance was measured at 517 nm using UV-spectrophotometer (Thermo Electron Corporation, Beverly, MA, USA). Ascorbic acid was used as a positive control. All results were taken in triplicates and are presented as mean SEM. Percent radical scavenging activity was calculated using the following equation:
| (5) |
2.9. ABTS (2,2′-Azinobis-3-ethylbenzothiazoline-6-sulfonic Acid) Free Radical Scavenging Potential of Extracts
According to the previously reported Re et al. [35] method antioxidant activity of the selected plant methanolic extract/fractions were determined against ABTS free radical. ABTS (7 mM) and Potassium per sulphate (2.45 mM) solutions were prepared and mixed thoroughly. For the production of ABTS free radicals in the solution, the mixture was kept in dark for overnight at room temperature. After incubation absorption of a 3 mL volume was adjusted 0.7 (noted at 745 nm) by adding methanol. To determine the free radical scavenging ability about 300 µL of extract/fractions working dilutions and 3 mL of ABTS solution were mixed thoroughly and incubated for about 6 min. Finally, absorbance of the resulting mixtures was recorded at 745 nm using a double-beam spectrophotometer. Ascorbic acid was used as a positive control. Results were taken in triplicates and % ABTS scavenging potential was calculated using Equation (5).
2.10. In Vivo Studies
2.10.1. Experimental Animals
The animals used in the in vivo study were Swiss male albino mice (26–31 g body weight) that were obtained from National Institute of Health Islamabad, Pakistan. All the animals were maintained in the animal house of the Department of Pharmacy, University of Malakand. All the mice were divided into six groups and housed in individual cages. All the animals were kept under normal laboratory conditions of light/dark cycle (12/12 h) and were provided water and normal pellet diet. All the animal procedures were conducted according ARRIVE guidelines and also the approval was taken from the Departmental Animal Ethical Committee (DAEC/2019/1), University of Malakand, Pakistan.
2.10.2. Acute Toxicity Studies of the Fa.EtAc Fraction
The acute oral toxicity study on the plant extract (Fa.EtAc) was investigated according to the Organization for Economic Co-operation and Development (OECD) guideline 423. All animals (n = 6) were treated orally with a single dose of 2000 mg/kg body weight of Fa.EtAc (the most active fraction) to 3 animals, and the other 3 animals receiving distilled water at 10 mL/kg to evaluate the toxic effects in any in the experimental animals. Immediately after dosing, the mice were observed continuously for 2 h for any symptoms of toxicity such as convulsions, loss of righting reflex, motor activity, muscle spasm, tremors, lacrimation, sedation, hypnosis, diarrhoea, and salivation. Mice were then kept under observation up to 14 days for any signs of toxicity or mortality. The Fa.EtAc remained safe and nontoxic up to the dose range of 2000 mg/kg body weight. The toxicity assessment was accomplished following guidelines of the Organization for Economic Cooperation and Development (OECD). The animals were administered Fa.EtAc 200 mg/kg body weight as 1/10th of the highest dose for multiple dose investigation to assess the in vivo neuroprotective and nootropic activity [36].
2.10.3. Experimental Design
After the acclimatization period, in the scopolamine-induced amnesia test the Albino male mice were randomly divided into six groups (n = 6), of eight animals each for administration of Fa.EtAc fraction and were given doses as per following details:
Group I was used as normal control group and were given normal saline (8 mL/kg, p.o.) solution.
Groups II (negative control) received scopolamine 1 mg/kg body weight intraperitoneally.
Group III was used as positive control, and were given donepezil (2 mg/kg, p.o) and scopolamine (1 mg/kg, i.p.).
Group IV, V, and VI were used as treatment groups and were treated with Fa.EtAc fraction with different doses. Group IV were treated with 50 mg/kg body weight (b.w) Fa.EtAc (p.o) and scopolamine (1 mg/kg, i.p.). Group V were treated with 100 mg/kg Fa.EtAc (p.o) and scopolamine (1 mg/kg, i.p.), while Group VI were given with 200 mg/kg Fa.EtAc (p.o) and scopolamine (1 mg/kg, i.p.).
The dosing details are also given in Table 1. The volume of oral (p.o.) and intraperitoneal (i.p.) administrations was 1 mL/100 g b.w of mice. The treatments were continued for 8 days. In the behavioural Y-maze and Novel object recognition tests, scopolamine (1 mg/kg, i.p.) was given to these groups only on day 8th, and according to the standard procedure, 1 h after the administration of drugs all the animals were subjected to Y-maze and Novel object recognition test.
Table 1.
Experimental design showing dosing details.
| Group | Group Category | Treatment Given | Route |
|---|---|---|---|
| I | DW + Sal | Sal (8 mL/kg) | p.o. |
| II | DW + Scop | Scop (1 mg/kg) | i.p. |
| III | DZP + Scop | DZP (2 mg/kg) + Scope (1 mg/kg) | p.o., i.p. |
| IV | Fa.EtAc 50 + Scop | Fa.EtAc (50 mg/kg) + Scop (1 mg/kg) | p.o. |
| V | Fa.EtAc 100 + Scop | Fa.EtAc (100 mg/kg) + Scop (1 mg/kg) | p.o |
| VI | Fa.EtAc 200 + Scop | Fa.EtAc (200 mg/kg) + Scop (1 mg/kg) | p.o. |
Fa.EtAc, Ferula ammoniacum ethyl acetate fraction; DW, Distilled water; Sal, Normal Saline; Scop, scopolamine; DZP, Donepezil; p.o., Per oral; i.p., Intraperitoneal.
2.11. Behavioural Assessment
2.11.1. Y-Maze Spontaneous Alternation Behaviour Test
Y-Maze spontaneous alternation behaviour test was used to evaluate the short-term memory potentials (STM) of mice by recording spontaneous alternation in a single session on day 10 [36]. The Y-maze apparatus is comprised of three arms of equivalent size, labelled as A, B, and C, respectively. Each arm is 20 cm long, 6 cm wide and 15.5 cm high and is oriented at an angle of 120° from the other two. One hour after the last treatment and 30 min after scopolamine injection (except for the distilled water group), all the mice were allowed to move freely through the maze for 8 min. The numbers of arm entries were recorded for each mouse. An arm entry was considered to be completed when the hind paws of the mouse were completely placed in an arm. Spontaneous alternation was defined as consecutive entries in a specific sequence of arm transitions (ABC, BCA, or CAB but not BAB or CAC or CBC) by a mouse into the three different arms that reflects short-term memory. The total number of arm entries reveals an overall locomotor activity. The arms of the maze were cleaned using 70% v/v ethanol between trials to avoid olfactory cues. The number of arm entries, same arm returns (SAR), and alternate arm returns (AAR) were measured. The percentage of spontaneous alternation performance (% SAP) was determined using the following equation.
| (6) |
2.11.2. The Novel Object Recognition Test and Novel Object Location Tests
The novel object recognition test (NORT) was conducted in order to evaluate recognition memory in mice [36]. The apparatus was comprised of a white coloured plywood box (40 cm × 40 cm × 66 cm) with a network floor that was carefully cleaned with 70% v/v ethanol after every trial. The apparatus was illuminated with a 60 W light suspended 50 cm over the crate. The arena of the apparatus and objects were cleaned with 70% v/v ethanol between trials to avoid olfactory cues. NORT consisted of habituation, sample, and test phases. For behavioural assessment, 1 h after dosing the mice were habituated to the experimental apparatus in the absence of objects twice a day (with 5 h interval) in a 10 min session for three consecutive days to explore the objects. On the fourth day, two similar objects were placed in two opposite places of the box and exploration was recorded for 15 min session called sample phase (first trial: T1). After this session the animals were kept for a retention break of 1 h. Exploration was considered when an animal touches the object or it directs its nose at a distance less than 2 cm to the object. The mouse was then returned to its home cage after test. At the 2nd day of the test (day 10th of drug treatment), 30 min after scopolamine injection (except for the distilled water group) called test phase (second trial: T2), each mouse was placed again in the open field in which a novel object (plastic square) was replaced by one of the objects placed in the 1st day trial and mice were left individually in the box for 5 min. The location of the object was counterbalanced so that one half of the mice in each group saw the novel object on the left side of the box arena, and the other half saw the novel object on the right side of the box arena to eliminate bias of sides. The time spent by the animal for exploring the novel object (N) and the familiar (F) objects was recorded during 5 min. A mouse was scored as exploring when its head was oriented towards the object within a distance of 2 cm or when the nose was in contact with the object. Parameters including the time (seconds) spend in exploring familiar (F) object, time (in seconds) spend on exploring the novel (N) object, and total time (in seconds) spend on exploring both objects (N + F) were measured separately. Percentage of discrimination index (DI) was calculated by the following equation:
| (7) |
The same apparatus was used for the assessment of novel object location task in experimental animals. In the sample phase, animals were exposed to two similar objects in the same location (initial location) for 15 min. After 1 h retention phase, one object is changed to a novel position while the other was kept on its initial position. Exploration time for assessing the novel location (NL) and familiar location (FL) was determined for 15 min in the test phase [37]. Percent exploration time was determined by the following formula:
| (8) |
2.11.3. Isolation of Frontal Cortex and Hippocampus
Immediately after the Y-Maze test and NORT, all the animals were sacrificed by cervical dislocation before decapitation to provide each animal with a quick and painless death according to the procedure illustrated in schedule-1 of UK (animal scientific procedure act 1986) and the brain of each animal was carefully isolated. The frontal cortex (FC) and hippocampus (HC) were dissected out in ice cold phosphate buffer saline (0.1 M; pH 8.0). The tissues were weighed and 20 mg tissue/mL homogenate of brain samples was prepared by homogenizing the isolated parts in phosphate buffer (pH 8.0). The homogenates were centrifuged at 10,000 rpm for 10 min at 4 °C, and finally supernatants were collected at a standardized protein content of 5 mg/mL that was used for the estimation of ex vivo cholinesterase activity following Elman’s assay [33] and antioxidants activity using Brand William’s assay [34].
2.12. Statistical Analysis
All the in vitro and in vivo experiments were performed in three replicate and results are presented as Mean ± SEM. The Student’s t-test and one-way ANOVA followed by Dunnett’s post hoc multiple comparison test have been used. p ≤ 0.05 were considered as significant. Linear regression was used to calculate IC50 for % DPPH, ABTS, AChE, and BChE inhibition against the different concentration of test samples by means of Excel program 2007.
3. Results
3.1. Identification and Quantification of Phytochemicals Compounds
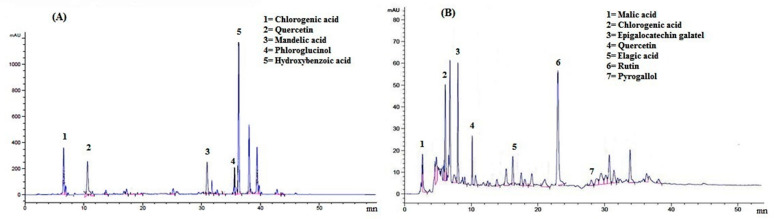
A typical HPL-UV chromatogram of F. ammoniacum aerial parts methanolic and ethyl acetate extracts have been shown in Figure 1. A total of five phytochemicals were identified in methanolic extract while in ethyl acetate fraction. The detailed identification of each antioxidant with their respective peak position in chromatogram and retention time (Rt) is given in Table 2. Chlorogenic acid, quercetin, mandelic acid, phloroglucinol, and hydroxy benzoic acid eluted at retention times of 6.5, 10.5, 30.5, 35.5, and 36.3 min were present in Fa.Met, while malic acid, chlorogenic acid, epigallocatechin gallate, quercetin, ellagic acid, rutin, pyrogallol were identified in Fa.EtAc fraction at retention times of 3.1, 6.5, 8.0, 10.5, 16.6, 22.7 and 28.1 min. These compounds are the possible compounds as on same retention time different compounds can come out from column even under the same HPLC method. The concentrations of possible phytochemicals in crude extracts were: 9.203, 0.008, 0.014, 14.953, and 93.825 µg/mL (chlorogenic acid, quercetin, mandelic acid, phloroglucinol, and hydroxy benzoic acid, respectively) as presented in Table 2.
Figure 1.
HPLC chromatogram of Ferula ammoniacum (D. Don) aerial parts extract/fraction. (A) crude methanolic extract (Fa.Met) and (B) ethyl acetate fraction (Fa.EtAc).
Table 2.
Possible compounds identified in Ferula ammoniacum (D. Don) aerial parts extracts.
| Extract | Peak No | Retention Time (min) | Detected Phenolic Compounds | Sample Peak Area | Standard Peak Area | Concentration (µg/mL) |
|---|---|---|---|---|---|---|
| Fa.Met | 1 | 6.5 | Chlorogenic acid | 132.221 | 12.9 | 9.20 |
| 2 | 10.5 | Quercetin | 64.97 | 90.9 | 7.14 | |
| 3 | 30.5 | Mandelic acid | 110.82 | 72.0 | 15.39 | |
| 4 | 35.5 | Phloroglucinol | 415.64 | 25.02 | 14.95 | |
| 5 | 36.3 | Hydroxy benzoic acid | 4190.44 | 40.19 | 93.83 | |
| Fa.EtAc | 1 | 3.1 | Malic acid | 50.88 | 40.32 | 12.63 |
| 2 | 6.5 | Chlorogenic acid | 180.83 | 12.9 | 140.17 | |
| 3 | 8.0 | Epigallocatechin gallate | 90.70 | 7261.47 | 69.52 | |
| 4 | 10.5 | Quercetin | 27.82 | 90.9 | 3.05 | |
| 5 | 16.6 | Ellagic acid | 24.46 | 319.24 | 0.76 | |
| 6 | 22.7 | Rutin | 107.12 | 2241.2 | 47.82 | |
| 7 | 28.1 | Pyrogallol | 9.1 | 1.014 | 91.0 |
3.2. Total Phenolic Content
Results of TPC in the crude methanolic extract and various fractions of F. ammoniacum aerial parts are presented in Table 3. A standard gallic acid curve was constructed by preparing the dilutions 20, 40, 60, 80 and 100 mg/mL to estimate the TPC using regression equation. The phenolic contents of Fa.Met, Fa.Hex, Fa.Chf, Fa.EtAc, Fa.Bn and Fa.Aq were 68.25 ± 1.14, 65.55 ± 0.84, 75.12 ± 1.58, 88.23 ± 1.13, 55.18 ± 0.68, and 51.75 ± 1.87 mg GAE/g of dry sample, respectively. Out of them Fa.EtAc, Fa.Bn, and Fa.Chf fractions were rich in total phenolic contents.
Table 3.
Total Phenolic Content and Flavonoids Content in the extracts/fractions of Ferula ammoniacum (D. Don).
| S. No | Extract/Fractions | TPC (mg GAE/g) | TFC (mg QE/g) |
|---|---|---|---|
| 1 | Fa.Met | 68.25 ± 1.14 | 66.97 ± 0.99 |
| 2 | Fa.Hex | 65.55 ± 0.84 | 68.01 ± 1.17 |
| 3 | Fa.Chf | 75.12 ± 1.58 | 77.83 ± 2.16 |
| 4 | Fa.EtAc | 88.23 ± 1.13 | 85.93 ± 0.67 |
| 5 | Fa.Bn | 55.18 ± 0.68 | 45.51 ± 1.99 |
| 6 | Fa.Aq | 51.75 ± 1.87 | 59.79 ± 1.39 |
Fa, Ferula ammoniacum; TPC, Total Phenolic Content; TFC, Total Flavonoid Content; Fa.Cr, Crude methanolic extract; Fa.Hex, n-hexane fraction; Fa.Chf, Chloroform fraction; Fa.EtAc, Ethyl acetate fraction; Fa.Bn, n-Butanol; Fa.Aq, Aqueous fraction; GAE, Gallic acid equivalent; QE, Quercetin equivalent.
3.3. Total Flavonoid Contents
To estimate the TFC in F. ammoniacum aerial parts, the quercetin calibration standard curve was used for which dilutions 20, 40, 60, 80 and 100 mg/mL were prepared. TFC were: Fa.Met, 66.97 ± 0.99, 68.01 ± 1.17, 77.83 ± 2.16, 85.93 ± 0.67, 45.51 ± 1.99 and 59.79 ± 1.39, respectively, in Fa.Hex, Fa.Chf, Fa.EtAc, Fa.Bn and Fa.Aq (Table 3). Highest total flavonoid content were calculated in Fa.EtAc, Fa.Bn, and Fa.Chf fractions.
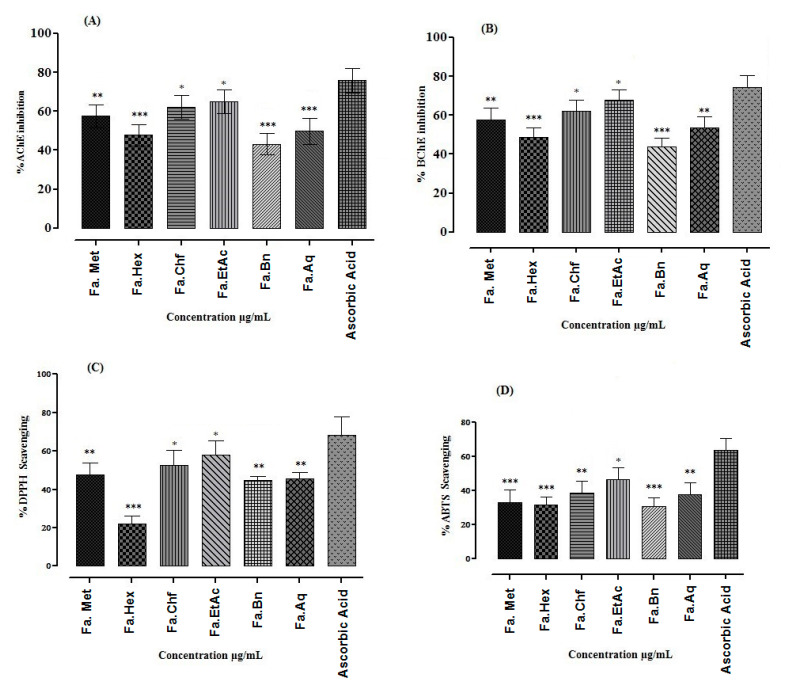
3.4. In Vitro Cholinesterase Inhibitory Potential of F. ammoniacum Aerial Parts Methanolic Extract/Fractions
Cholinesterase (AChE and BChE) inhibitions of F. ammoniacum methanolic extract/fractions were determined at various concentrations and are presented in Figure 2A,B. Among different fractions of F. ammoniacum, Fa.EtAc and Fa.Chf showed prominent inhibition against AChE (89.22 ± 1.74 and 88.44 ± 0.80) at highest concentration (1000 µg/mL) with IC50 values 40 and 43 µg/mL, respectively. Galantamine was used as a standard and showed percent inhibition of 96.56 ± 1.08 against AChE (IC50 = 30 µg/mL) at highest concentration 1000 µg/mL (Figure 2A). Similarly, Fa.EtAc and Fa.Chf fractions also showed highest inhibition against, BChE which were 86.37 ± 0.61, and 85.31 ± 0.49 at highest concentration of 1000 µg/mL with IC50 values of 41 and 42 µg/mL, respectively (Table S1). The positive control galantamine showed percent inhibition of 95.17 ± 071 against BChE at 1000 µg/mL concentration with IC50 value of 32 µg/mL. Other extracts of F. ammoniacum also showed a concentration dependent response against the selected enzymes.
Figure 2.
Percent anticholinesterase and antioxidant potential of extract/fractions of Ferula ammoniacum (D. Don) aerial parts. {(A) Percent inhibition potential of extract/fractions for AChE and (B) BChE; (C) DPPH and (D) ABTS free radical. The data are expressed as Mean ± SEM, (n = 3). One-way ANOVA followed by Dunnett’s post hoc multiple comparison test to determine the values of p. Values are significantly different as compare to positive control, * p < 0.05, ** p < 0.01, *** p < 0.001}.
3.5. In Vitro DPPH and ABTS Free Radicals Scavenging Potential of Extracts
DPPH and ABTS free radical inhibitory potential of Fa.Met, Fa.Hex, Fa.Chf, Fa.EtAc, Fa.Bn and Fa.Aq are presented in Figure 2C,D. The results revealed that highest percent free radical scavenging potential was exhibited by Fa.EtAc fraction against DPPH and ABTS with lowest IC50 values of 100 and 120 µg/mL, respectively (Table S2). Ascorbic acid was used as a positive control that caused 91.32 ± 0.34 and 95.31 ± 0.75 % inhibition at 1000 µg/mL against DPPH and ABTS with IC50 value 30 and 45 µg/mL.
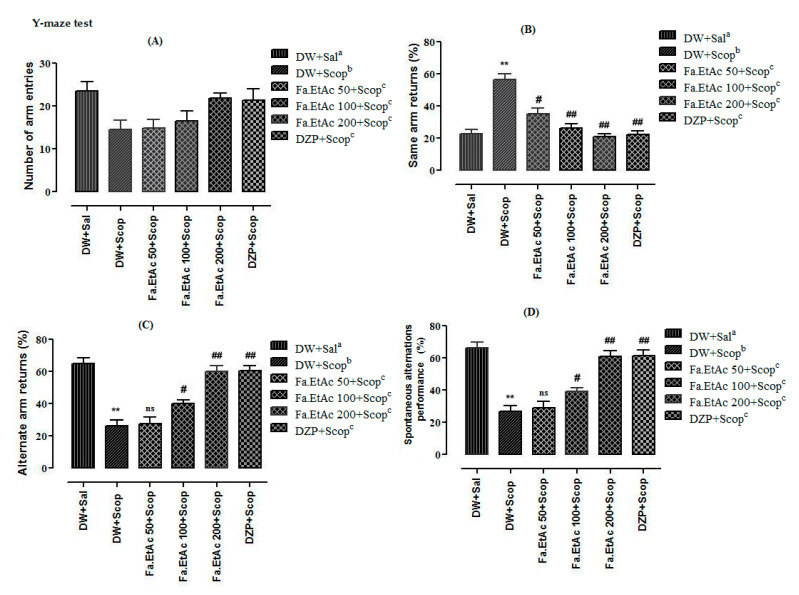
3.6. Nootropic Effect of the Extracts in Y-Maze Test
Y-maze test results are presented in Figure 3. Results indicated that no significant (p > 0.05) reduction were observed in the total numbers of arm entries in both scopolamine and treatment groups (Fa.EtAc and DZP) as compared to normal saline group (Normal control) (Figure 3A). Same returns percentages were recorded significantly (p < 0.01) high in scopolamine-treated group as compared to normal saline group. Fa.EtAc (100 and 200 mg/kg body weight), and DZP (2 mg/kg) exhibited significant (p < 0.01) reduction in the percentage of returns to the same arm as compared to DW + Scop-treated group (Figure 3B). Similarly, Fa.EtAc (100 and 200 mg/kg body weight), and standard drug DZP (2 mg/kg body weight) significantly reverse the effect of scopolamine and have displayed a significant (p ˂ 0.05; p < 0.01) rise in the percentage of alternate arm returns comparable to scopolamine. Although, DW + Scop-treated group significantly (p ˂ 0.01) decreased the number of returns to alternate arm at the percentage of 26.2% as compared to normal saline group (65%) (Figure 3C).
Figure 3.
Effect of Fa.EtAc fraction of Ferula ammoniacum (D. Don) on mice in the behavioural Y-maze test. (A) Number of arm entries (B) Same arm returns (C) alternate arm returns (D) % Spontaneous alternation performance. The data are expressed as Mean ± SEM; each value corresponds to a mean of eight animals. One-way ANOVA followed by Dunnett’s post hoc multiple comparison test to determine the values of p. ** p < 0.01; comparison of DW + Sala (Normal control) vs. DW + Scopb (Scopolamine treated), # p < 0.05 and ## p < 0.01; comparison of (DW + Scop)b vs. DZP + Scopc (Donepezil treated)- and Fa.EtAcc (50, 100 and 200 mg/kg)-treated groups), ns: values not significantly different in comparison to (DW + Scop)b-treated group using one-way ANOVA followed by Dunnett’s post hoc multiple comparison test.
The results of spontaneous alternation performance shows that there was a significant difference observed among all the treatments groups. Scopolamine significantly reduced (p ˂ 0.01) the mice spontaneous alternation percentage compared to normal saline group. Fa.EtAc at dose of 100 and 200 mg/kg body weight significantly reversed the effect of scopolamine and increased the spontaneous alternation performance percentage (p ˂ 0.05; p < 0.01) when compared to scopolamine-treated group. Standard drug Donepezil (2 mg/kg body weight) also significantly (p < 0.01) reversed the effects of scopolamine with a percentage of 60.4 (Figure 3D).
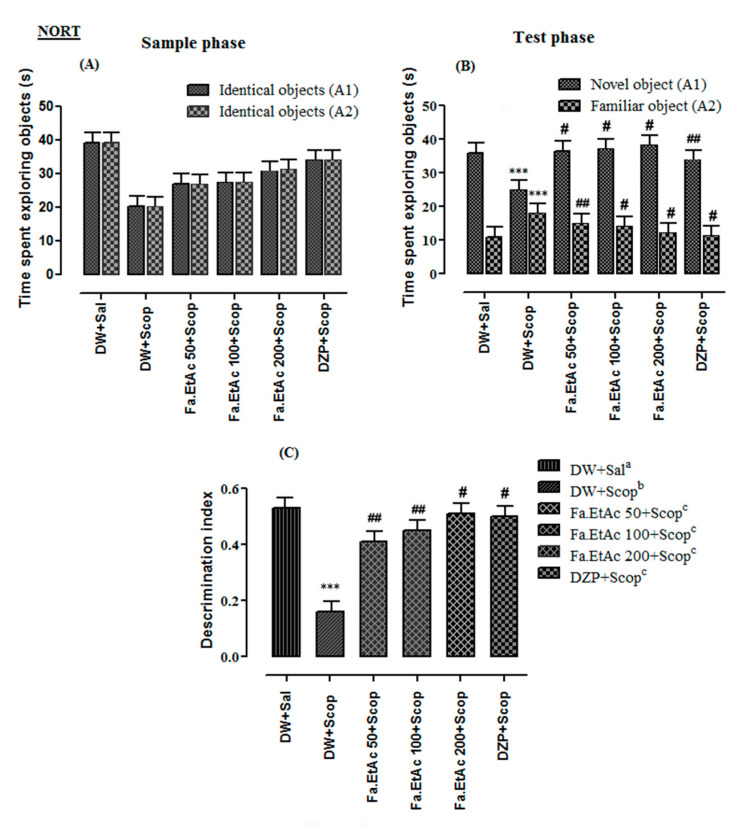
3.7. Nootropic Effect of Extracts in Novel Object Recognition Test
The NORT was used to assess the recognition memory of mice after a single injection of scopolamine. The results obtained with the NORT are shown in Figure 4 and Table S3 (Supplementary File). There were no significant differences in the time spent in exploring the two identical objects between Fa.EtAc- and DZP-treated groups and scopolamine-treated group (Figure 4A). However, in the test phase Fa.EtAc (100 and 200 mg/kg), and donepezil (2 mg/kg) groups spent more time with novel object as compared to scopolamine-treated group (p < 0.05, p < 0.01). The administration of scopolamine before the retention phase of the test resulted in a decrease of the exploration time of the novel object in comparison with the familiar object (p < 0.01) (Figure 4B). The discrimination index (DI) which is 0.46 + 0.03 in DW + Sal (Distilled water plus normal saline) group was significantly reduced (p < 0.001) to a value of 0.07 + 0.05 in the scopolamine-treated group. The DI was significantly high for Fa.EtAc (50, 100 and 200 mg/kg) and donepezil (p < 0.01; p < 0.001) groups in comparison to scopolamine group (Figure 4C). The discrimination index of the standard drug donepezil group was 0.50 + 0.02. All groups showed %DI above 50% while scopolamine group has shown significantly low value as compared to vehicle control. The F. ammoniacum extracts significantly increased the discrimination index from 0.16 in the scopolamine-treated group to 0.45 and 0.51 (p < 0.001) in mice treated with Fa.EtAc at the doses 100 and 200 mg/kg, respectively. The exploration time of the novel object was also significantly increased by F. ammoniacum extracts from 25.0 ± 1.6 in the scopolamine-treated group to 37.3 ± 3.5 and 38.5 ± 2.2 in mice treated with Fa.EtAc at the doses of 100 and 200 mg/kg, respectively (p < 0.05, p < 0.01). The Fa.EtAc (100 and 200 mg/kg) also significantly decreased the exploration time of the familiar object (p < 0.01). The donepezil (2 mg/kg) groups significantly increased the discrimination index and the exploration time of the novel object (34.2 ± 1.2) and decreased the exploration time of the familiar object (11.30 ± 1.5) (p < 0.001). Here also, the effect of F. ammoniacum extracts at the dose 200 mg/kg was greater than the effect of donepezil.
Figure 4.
Effect of Fa.EtAc fraction of Ferula ammoniacum (D. Don) on mice in behavioural NORT (A) Time spent in the sample phase (B) Time spent in the test phase (C) % Discrimination index were recorded in Fa.EtAc (50, 100 and 200 mg/kg)-treated groups versus scopolamine (Scop. 1 mg/kg)-treated group for assessment of recognition memory in mice model in behavioural NORT. The data are expressed as Mean ± SEM; each value corresponds to a mean of eight animals. One-way ANOVA followed by Dunnett’s post hoc multiple comparison test to determine the values of p. *** p < 0.001; comparison of DW + Sala (Normal control) vs. DW + Scopb (Scopolamine-treated group), # p < 0.05 and ## p < 0.01; comparison of (DW + Scop)b vs. DZP + Scopc (Donepezil treated) and Fa.EtAcc (50, 100 and 200 mg/kg)-treated groups using one-way ANOVA followed by Dunnett’s post hoc multiple comparison test.
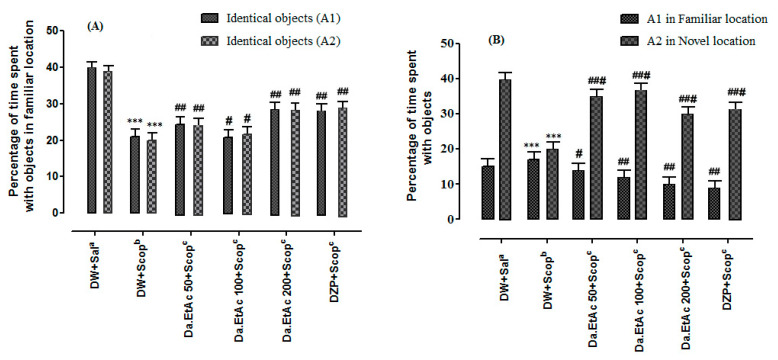
In the NOL task, the % exploration time for each object was recorded both in the familiar location and novel location. In the sample phase, no difference was observed in exploration time between objects A1 and B2 (Figure 5A). However, in the test phase the animal gives preference to explore the novel location object as compared to the object in familiar location (Figure 5B).
Figure 5.
Effect of Fa.EtAc fraction of Ferula ammoniacum (D. Don) on mice in behavioural NOL task (A) Percent exploration time between the objects A1 and A2 in familiar location in the sample phase (B) Percent exploration time between the objects A1 and A2 in novel location in the test phase. The data are expressed as Mean ± SEM; each value corresponds to a mean of eight animals. One-way ANOVA followed by Dunnett’s post hoc multiple comparison test to determine the values of p. *** p < 0.001, # p < 0.05, and ## p < 0.01 and ### p < 0.001; comparison of A1 (Object in familiar location) vs. A2 (Object in novel location).
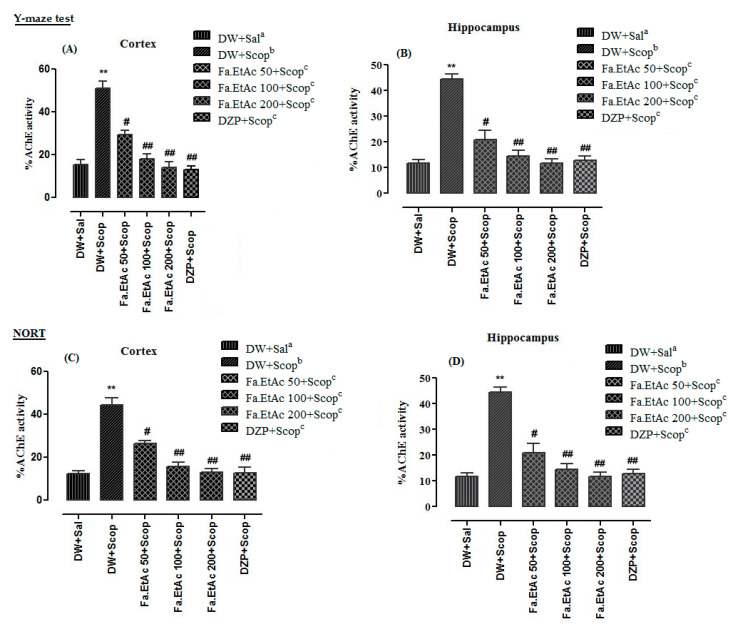
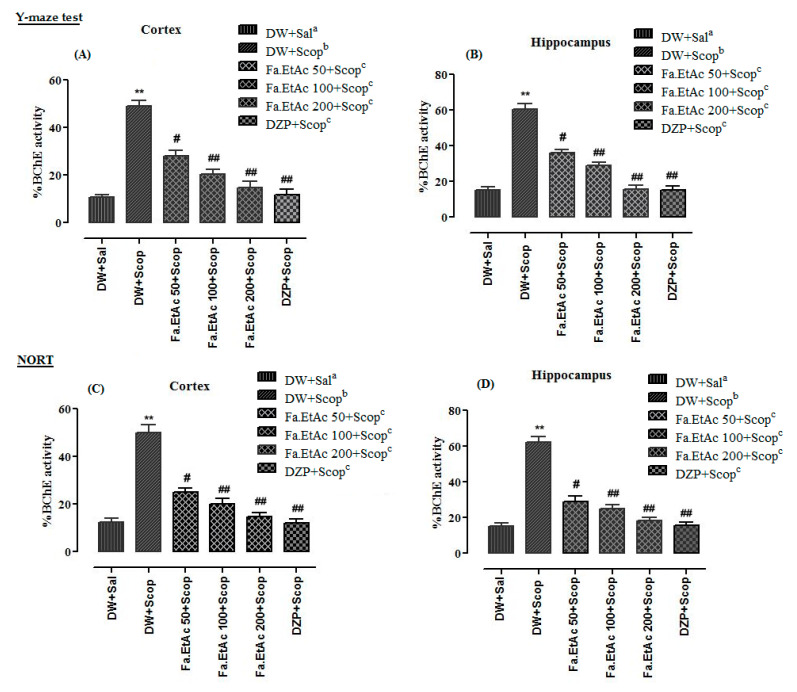
3.8. Effect of Extracts on Brain Cholinesterases (AChE and BChE) Activity in Y-Maze Test in Mice
Ex vivo analysis of cholinesterase (AChE and BChE) inhibitory potentials was measured using Fa.EtAc extract to assess the impairment in cholinergic functions in mice model. Percent AChE activity of frontal cortex and hippocampus tissues of different animal groups in Y-Maze test are given in Figure 6A,B, while %BChE activity are presented in Figure 7A,B. %AChE activity in the frontal cortex and hippocampus of scopolamine-treated groups were 51.2 + 0.2 and 44.4 + 0.4, while %BChE activities were 49.0 + 0.4 and 60.4 + 0.2 which were significantly (p < 0.01) ** higher than that of the normal saline group. In Fa.EtAc + Scop (50mg/kg, p.o.), Fa.EtAc +Scop (100 mg/kg, p.o.), and Fa.EtAc + Scop (200 mg/kg, p.o.)-treated groups, and DZP + Scop (20 mg/kg; (i.p.) a standard drug-treated group, a significant decline (p < 0.01) ##; (p < 0.001) ### in %AChE and %BChE activities were observed in frontal cortex and hippocampus tissues which were lower than that of the scopolamine-treated group.
Figure 6.
Ex vivo % AChE activity in the (A) frontal cortex and (B) hippocampus of different animal groups in Y-maze behavioural test and (C,D) NORT. The data are expressed as Mean ± SEM; each value corresponds to a mean of eight animals. One-way ANOVA followed by Dunnett’s post hoc multiple comparison test to determine the values of p. ** p < 0.01; comparison of DW + Sala (Normal control) vs. DW + Scopb (Scopolamine treated), # p < 0.05 and ## p < 0.01; comparison of (DW + Scop)b vs. DZP + Scopc (Donepezil treated) and Fa.EtAcc (50, 100 and 200 mg/kg)-treated groups), ns: values not significantly different in comparison to (DW + Scop)b-treated group using one-way ANOVA followed by Dunnett’s post hoc multiple comparison test.
Figure 7.
Ex vivo % BChE activity in the (A) frontal cortex and (B) hippocampus of different animal in Y-maze behavioural task and (C,D) NORT. The data are expressed as Mean ± SEM; each value corresponds to a mean of eight animals. One-way ANOVA followed by Dunnett’s post hoc multiple comparison test to determine the values of p. ** p < 0.01; comparison of DW + Sala (Normal control) vs. DW + Scopb (Scopolamine treated), # p < 0.05 and ## p < 0.01; comparison of (DW + Scop)b vs. DZP + Scopc (Donepezil treated) and Fa.EtAcc (50, 100 and 200 mg/kg)-treated groups), ns: values not significantly different in comparison to (DW + Scop)b-treated group using one-way ANOVA followed by Dunnett’s post hoc multiple comparison test.
3.9. Effect of Extracts on Brain Cholinesterases (AChE and BChE) Activity in NORT in Mice
Results of percent AChE and BChE activity in frontal cortex and hippocampus of different animal groups in NORT are given in Figure 6C,D and Figure 7C,D. Percent AChE and BChE activities in the cortex and hippocampus of scopolamine-treated groups were significantly (p < 0.001 ***) higher than that of the normal saline group. %AChE and BChE activities in the cortex and hippocampus of Fa.EtAc + Scop (100 mg/kg, p.o.) and Fa.EtAc + Scop (200 mg/kg, p.o.)-treated groups, and standard DZP + Scop (20 mg/kg; (i.p.)-treated groups were significantly (p < 0.01) ##; lower as compared to scopolamine-treated group.
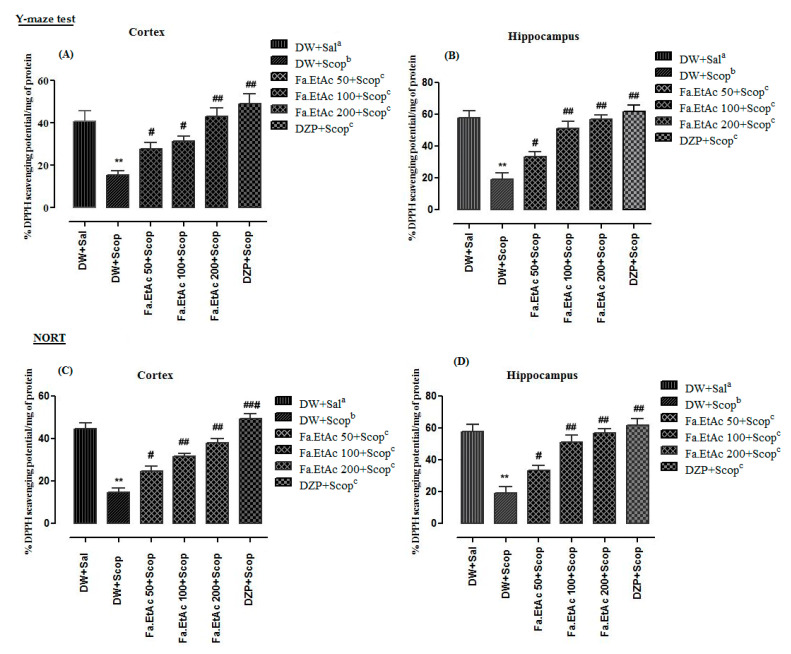
3.10. Effect of Extracts on Brain Antioxidant Activity in Y-Maze Test
Percent ex vivo antiradicals activity in cortex and hippocampus of different animal groups in Y-Maze test are presented in Figure 8A,B. Scopolamine-treated group significantly (p < 0.01 **) reduced the %DPPH free radicals scavenging activity/mg protein compared to the normal saline-treated group. Pre-treatment of mice with F. ammoniacum extract fractions like Fa.EtAc + Scop (50, 100, 200mg/kg, p.o.), and standard donepezil (20 mg/kg; (i.p.) significantly ((p < 0.05) #; p < 0.01) ## reversed the effect of scopolamine.
Figure 8.
Ex vivo %DPPH free radical scavenging effects in (A) frontal cortex and (B) hippocampus of different animal groups in Y-maze behavioural task and (C,D) NORT. The data are expressed as Mean ± SEM; each value corresponds to a mean of eight animals. One-way ANOVA followed by Dunnett’s post hoc multiple comparison test to determine the values of p. ** p < 0.01; comparison of DW + Sala (Normal control) vs. DW + Scopb (Scopolamine treated), # p < 0.05, ## p < 0.01, and ### p < 0.001; comparison of (DW + Scop)b vs. DZP + Scopc (Donepezil treated) and Fa.EtAcc (50, 100 and 200 mg/kg)-treated groups), ns: values not significantly different in comparison to (DW + Scop)b-treated group using one-way ANOVA followed by Dunnett’s post hoc multiple comparison test.
Furthermore results regarding NORT of ex vivo %DPPH free radicals scavenging activity in cortex and hippocampus of different animals groups are presented in Figure 8C,D. %DPPH inhibition activity in the cortex and hippocampus of scopolamine-treated groups were significantly (**: p < 0.01) lower (14.6 + 0.2 and 19.4 + 0.5) as compared to normal saline group (44.6 + 0.3 and 58.0 + 0.4.). Fa.EtAc + Scop (50 mg/kg, p.o.), Fa.EtAc + Scop (100 mg/kg, p.o.), and Fa.EtAc + Scop (200 mg/kg, p.o.) and standard donepezil-treated groups showed significantly higher %anti-radicals activities of 24.6 + 0.1 #, 31.6 + 0.5 ##, 38.2 + 0.4 ##, and 49.4 + 0.1 ### in cortex and 33.6 + 0.5 #, 51.4 + 0.5 ##, 56.8 + 0.2 ##, and 62.0 + 0.3 ## in the hippocampus, respectively.
4. Discussion
Different Dorema species are in use in the Middle East countries as folk remedies of asthma, bronchitis, diabetes and other infections [38]. Reported studies revealed that people of Iran are well-known to the resin-gum of D. ammoniacum from centuries ago and its collection was started there from nearly 4000 years ago. Being rich in ammoniacum, the medicinal gum-resin has been used for the treatment of various disorders, such as upper respiratory tract, gastrointestinal and central nervous system complications. Several chemical compounds (terpenes, coumarins and phenolic compounds) with a wide range of pharmacological activities including antioxidant, anti-microbial, cytotoxicity, anti-inflammatory, anti-diabetic, anticonvulsant, and hypolipidemic activities have been reported from this genus and are used in modern medicines [26,38]. Regardless of an extensive preclinical and clinical study to assess the safety and toxicity efficacy of such medicinal plants very few investigational studies are available. However, in recent times, there has been an increasing concern related to their safety and toxicity assessment [39]. Such toxicity studies will enhance the public reliabilities on the herbal medicinal plants which would increase their traditional and beneficial uses. Due to the extensive use D. ammoniacum in Persian traditional medicine, this study was designed to assess its nootropic effect in mice model. Before in vivo assessment, the chemical composition of F. ammoniacum (D. ammoniacum) methanolic extract and ethyl acetate fraction (active fraction) was determined through HPLC and in vitro antioxidant and anticholinesterase activities were performed along with determination of total phenolic and flavonoid contents. Presence of phenolic compounds such as sesquiterpene coumarins, phenols, flavonoids and phloroacetophenone glycosides have also been reported from D. ammoniacum and other Dorema species previously [38,40].
Phenolic compounds present in F. ammoniacum and other plants serve as antioxidants due to its hydroxyl groups, which have exhibited strong antioxidant potential and have been reported to cause cholinesterase inhibition as well as they are capable of reacting with active oxygen radicals such as hydroxyl radicals. Flavonoids are polyphenolic compounds found in fruits and vegetables, and are highly effective scavengers of most oxidizing molecules, including singlet oxygen, and various free radicals implicated in several diseases including AD. Flavonoids can provide protection to the brain cells by modulating intracellular signals and promoting cellular survival [41]. In the current study highest contents of total phenolic (88.23 ± 1.13) and flavonoids (85.93 ± 0.67) were detected in Fa.EtAc followed by Fa.Chf extract (TPC: 75.12 ± 1.58 and TFC: 77.83 ± 2.16). Phenolics and flavonoids are free radical scavengers responsible for the observed high antioxidant and anticholinesterase activities, thus having protective effects in many neuro degenerative diseases such AD. The inhibition of cholinesterase enzymes is considered as a promising strategy in the management of neurological and neurodegenerative disorders such as AD, where a deficit in cholinergic neurotransmission is often observed. Several pharmacological effects of the plant extract such as antioxidant, anticholinesterase, free radical scavenging, antidiabetic, and hypolipidemic activities regarding the essential oil and crude extract of roots and aerial parts of Dorema species have also been associated with the presence of phenolic and flavonoid compounds [23,28,29,42].
The cholinesterase inhibitory activity of several medicinal plants has been reported in the literature [43,44]. Additionally, antioxidants such as vitamin E and vitamin C low level are associated with incidences and prevalence of AD. AD patients administered with high doses of antioxidants were reported to have a slower rate of cognitive deterioration. Thus, the good antioxidant and anticholinesterase activities of the F. ammoniacum extract/fractions in this study suggest that these extract/fractions are good sources of phenolic and flavonoid compounds, with potential cholinesterase inhibitory and antioxidant properties that may find usefulness in the management of AD [43,44]. Furthermore, antioxidants and neuroprotective activities have been reported for the identified possible compounds in crude (chlorogenic acid, quercetin, mandelic acid, phloroglucinol, and hydroxy benzoic acid) and ethyl acetate extract (malic acid, chlorogenic acid, epigallocatechin gallate, quercetin, ellagic acid, rutin, pyrogallol), identified in other plant extracts. Among the identified phytochemicals; quercetin, chlorogenic acid, mandelic acid, epigallocatechin gallate, ellagic acid, and rutin has been reported as a potent antioxidant and neuroprotective agents [36,45,46,47,48]. In this study Fa.EtAc fraction showed free radical scavenging activity and strong inhibitions of cholinesterases (AChE and BChE), with low IC50 values. Reported studies also revealed that ethyl acetate extract F. ammoniacum has shown the highest antioxidant activity in both DPPH and FRAP assays [23]. Fa.EtAc fraction was found rich in phenolics and flavonoids that might thus be capable of reducing the risk of various degenerative diseases including AD, by prompting antioxidative activities. Previous studies also revealed that phenolics and flavonoids act as free radical scavengers of many oxidizing species and are inhibitors of cholinesterases (AChE and BChE) [49]. Further studies are needed to isolate and characterize the bioactive phytochemical compounds to be used as a promising candidate drug in management of AD.
The key clinical symptom of AD is a progressive deterioration in learning and memory function due to deficiency of ACh levels in the brain. Ach is an essential neurotransmitter present in the synapses. Its low level can reduce cognitive functions. Cholinesterase inhibitors are employed to reduce ACh hydrolysis, thereby increasing the concentration of ACh at the synapses and presynaptic receptors that prevents the death of cholinergic neurons [50]. Hence, treatment with plant extract (Fa.EtAc) might prevent the breakdown of ACh and increases cholinergic transmission, leading to the amelioration of symptoms [3] like that of donepezil which is a standard inhibitor of AChE and BChE [5]. AChE and BChE appear to be simultaneously active in the synaptic hydrolysis of ACh, terminating its neurotransmitter action, and co-regulating levels of ACh which is considered to be the most essential neurotransmitter that controls the regulation of cognitive functions. Increased AChE activity cause the loss of cholinergic neurotransmissions leading to Dementia and Alzheimer’s disease. Inhibitions of AChE decrease the hydrolysis of ACh in the brain and increase cholinergic neurotransmissions which might be helpful in treating mild to moderate levels of Alzheimer’s disease. In order to search out effective inhibitors of AChE and BChE from natural sources particular attention is needed in adapting an effective strategy to treat AD and that is by enhancing the level of ACh in the synapses of neurons [50]. The selected plant extracts provide a considerable source of secondary metabolites like phenolic acids and flavonoids which plays a role as antioxidant and as cholinesterase inhibitors. These phytochemicals are less toxic due to its origin from medicinal plants as compared to synthetic drugs and if isolated would be safer alternatives of the drugs to treat the neurodegenerative disorders. Present study revealed that Fa.EtAc fraction showed an excellent inhibition of AChE and BChE that might be due to the presence of anticholinesterase compounds (quercetin, chlorogenic acid, epigallocatechin gallate, ellagic acid, and rutin). Other plant in form of extracts have also shown anticholinesterase potentials for example, Ginkgo biloba extract, which has been used as an efficient cholinesterase inhibitor, memory enhancer, and provide benefits for cognition and treatment of mild-to-moderate Alzheimer’s disease [51]. Ammoniacum gum has also been used to treat disorders like epilepsy and to reduce joint pain. The cytotoxic, and acetylcholinesterase inhibitory activities about them have already been reported [38].
Inhibition of acetyl cholinesterase enzyme can restore cholinergic functions and allow more retention of acetylcholine in the brain, which is essential for enhancing cognitive functions, learning, and memory. The present study validates the useful effect of ethyl acetate fraction of F. ammoniacum on scopolamine-induced memory impairments. Scopolamine significantly decreased memory in rodent’s model, indicating impairment in learning and memory in the behavioural Y-maze test. Our results were in line with the previous reported studies of other authors [36,48]. Scopolamine is a muscarinic cholinergic receptor antagonist which significantly causes memory impairments in mice especially in the progressions of learning acquisition and short-term memory. Scopolamine induces oxidative stress and inflammatory responses in the brain tissue which can cause chronic activation of microglia that disrupt synaptic plasticity and finally neuronal death. The ROS directly damage the neuron by increasing intracellular Ca2+ level, while inflammatory cytokines are produced due to the activation of microglia that inhibit the production of BDNF and decrease the level of ACh [52].
First the extract was evaluated for in vitro antioxidant and cholinesterase inhibitory potentials then behavioural tests like Y-maze and NORT were performed in in vivo model of mice and after getting promising results the mentioned potentials were checked in the frontal cortex and hippocampus. High AChE and BChE activities were observed in the frontal cortex and hippocampus tissues of the diseased control group (measured from supernatant part of homogenized brain tissues at a standardized protein content of 5 mg/mL). In standard control group (Donepezil) and test control (F. ammoniacum extract) group a significant decline in both AChE and BChE activities (Figure 6; Figure 7), and DPPH free radical scavenging activity (Figure 8) in the cortex and hippocampus, respectively, were observed which were also in line with previous reported studies [36,52]. The pre-treatment with Fa.EtAc significantly increased the learning and memory in rodent’s model. Reported studies also revealed that some plant extract significantly reversed the memory loss in mice model justifying its neuroprotective potential [36,52]. In the current study of NORT, the increase in the percent DI and exploration time with novel object compared to familiar object in mice treated with Fa.EtAc suggested improvement in learning and memory which is in agreement with the reported study [53]. On the other hand, scopolamine significantly decreased the DI, representing impairment in learning and memory. This effect was overturned by F. ammoniacum ethyl acetate fraction, due to their memory enhancing activity and nootropic effect. Nootropic agents or cognitive enhancers have already been reported in traditional medicinal system to improve mental functions such as cognition, memory, or attention [53].
Behavioural study was further confirmed by biochemical assessment of brain cholinesterases (AChE and BChE) enzymes activity and DPPH free radicals scavenging activity in mice. F. ammoniacum has clearly demonstrated anticholinesterase and antiradical activity with strong neuroprotective and nootropic potential. F. ammoniacum inhibited brain cholinesterases (AChE and BChE) enzymes levels suggesting that its nootropic effects may strongly be due to the antioxidant action and, therefore, might play an effective role as antioxidant and free radical scavenger. Reported studies revealed that F. ammoniacum gum resin also shows AChE inhibition due to presence of bioactive compounds [23]. This study is further supported by the possible involvement of brain-derived neurotropic factor (BDNF; a neuronal growth factor) found in the brain, majorly found in the hippocampus, amygdala, cerebellum and cerebral cortex in both rodents and humans. BDNF activate enteric nervous system signalling pathways and synaptic communication which is released by the microglia mediated microglia-neuron signalling receptor. BDNF activates the neurotransmitter system and choline acetyltransferase (ChAT) enzymes which synthesize the neurotransmitter acetylcholine (ACh). Acetylcholine is implicated in cognitive functions and is a treatment target for many psychiatric and neurological disorders [54]. Inhibitions of cholinesterases (AChE and BChE) probably enhance the levels of neurotransmitter (ACh) that might increase BDNF level. The donepezil and Fa.EtAc extract enhanced the Ach level while scopolamine a muscarinic cholinergic receptor antagonist suppressed its level in the cortex and hippocampus due to blockade of cholinergic signalling pathways, therefore, it is suggested that enhancement of neurogenesis by activating ACh is involved in the activation of muscarinic acetylcholine receptors. Although currently available therapies for AD are cholinesterase inhibitors such as donepezil and rivastigmine, which only reduces the disease progression and provide symptomatic relief. Efforts are still being made to find out better alternative and better therapeutic options. The probable mechanistic overview of major factors involved in the cognitive and memory impairments are presented in Figure 9. Moreover, based on the phytochemical, behavioural, and biochemical results, we hypothesize that F. ammoniacum could possibly act directly as a free radical scavenger or regulator to inhibit cholinesterases (AChE and BChE), oxidative stress, and memory impairments induced by scopolamine. No memory impairments in the scopolamine-treated mice exposed to ethyl acetate fraction of F. ammoniacum, were observed suggesting that F. ammoniacum possess nootropic and neuroprotective activities.
Figure 9.
Mechanistic overview of Fa.EtAc as nootropic on the responsible factors involved in the aetiology of neurodegenerative diseases.
5. Conclusions
In conclusion, the fraction Fa.EtAc having highest level of total phenolic/flavonoid contents resulted in good inhibition of AChE and BChE enzymes with low IC50 values that also exhibited highest % free radical scavenging potential against DPPH and ABTS could be used as alternative drug to treat oxidative stress and neurodegenerative diseases. This extract has also exhibited nootropic and memory enhancing effect in mice model. In in vivo studies, it has exhibited strong antioxidant and anticholinesterase potential and was capable of reversing scopolamine-induced learning and memory impairments through reduction of brain cholinesterases (AChE and BChE) enzymes level. These results confirm the common use of F. ammoniacum as neuroprotective and nootropic medicinal plant in traditional medicine. However, further work to isolate the novel and safe nootropic agents present in F. ammoniacum and to understand the mechanisms of action responsible for these effects is needed. The findings of current study demonstrate the role of Fa.EtAc extract that probably activate the enteric nervous system signalling, increased the level of ACh and enhance neuronal activity that might be involved in memory processing.
Acknowledgments
Authors wish to thanks and acknowledge the support and APC from Deanship of Scientific Research under Research Group Number (RG-1440-100), King Saud University, Riyadh, Saudi Arabia. M.Z. is thankful to the Higher Education Commission of Pakistan for providing support in HPLC analysis under project number HEC-NRPU-20-2515.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3425/11/2/259/s1, Table S1: Percent AChE and BChE inhibition potential of Ferula ammoniacum (D. Don) seed extracts at various concentrations, Table S2: Percent DPPH and ABTS free radical scavenging activity of Ferula ammoniacum (D. Don) seed extracts at various concentrations. Table S3: Nootropic effect of Ferula ammoniacum (D. Don) seeds extracts in Novel Object Recognition Test.
Author Contributions
N.N. conducted in vitro and in vivo experimental work, and data collection, literature search and manuscript preparation. M.N. conducted identification of plant. F.U., R.U., S.U., and S.A.A. helps in statistical analysis. H.M.M., A.B., and A.A. helps in formal analysis. Finally, N.N. and M.Z. refined the manuscript for publication. All authors have read and agreed to the published version of the manuscript.
Funding
Deanship of Scientific Research, King Saud University Riyadh Saudi Arabia; under Research Group Number (RG-1440-100).
Institutional Review Board Statement
All procedures related to the animal activities have been approved by the Departmental Animal Ethical Committee (DAEC/2019/1), University of Malakand and were conducted according to the ARRIVE guidelines. These guidelines were in accordance with the internationally documented principles for laboratory used and care.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization . The World Health Report 2001: Mental Health: New Understanding, New Hope. WHO; Geneva, Switzerland: 2001. [Google Scholar]
- 2.Thakur A.K., Kamboj P., Goswami K., Ahuja K. Pathophysiology and management of Alzheimer’s disease: An overview. J. Anal. Pharm. Res. 2018;7 doi: 10.15406/japlr.2018.07.00230. [DOI] [Google Scholar]
- 3.Ortner M., Stange M., Schneider H., Schroeder C., Buerger K., Müller C., Dorn B., Goldhardt O., Diehl-Schmid J., Förstl H., et al. Serum Concentrations of Cholinesterase Inhibitors in Patients with Alzheimer’s Dementia Are Frequently Below the Recommended Levels. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.00691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanciu G.D., Luca A., Rusu R.N., Bild V., Beschea Chiriac S.I., Solcan C., Bild W., Ababei D.C. Alzheimer’s Disease Pharmacotherapy in Relation to Cholinergic System Involvement. Biomolecules. 2020;10:40. doi: 10.3390/biom10010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim G.W., Kim B.C., Park K.S., Jeong G.W. A pilot study of brain morphometry following donepezil treatment in mild cognitive impairment: Volume changes of cortical/subcortical regions and hippocampal subfields. Sci. Rep. 2020;10:10912. doi: 10.1038/s41598-020-67873-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okello E.J., Mather J. Comparative Kinetics of Acetyl- and Butyryl-Cholinesterase Inhibition by Green Tea Catechins|Relevance to the Symptomatic Treatment of Alzheimer’s Disease. Nutrients. 2020;12:1090. doi: 10.3390/nu12041090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uddin M.J., Zidorn C. Traditional herbal medicines against CNS disorders from Bangladesh. Nat. Prod. Bioprospect. 2020;10:377–410. doi: 10.1007/s13659-020-00269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heinrich M. Galanthamine from Galanthus and other Amaryllidaceae—Chemistry and biology based on traditional use. Alkaloids Chem. Biol. 2010;68:157–165. doi: 10.1016/s1099-4831(10)06804-5. [DOI] [PubMed] [Google Scholar]
- 9.Fleiss B., Van Steenwinckel J., Bokobza C.K., Shearer I., Ross-Munro E., Gressens P. Microglia-Mediated Neurodegeneration in Perinatal Brain Injuries. Biomolecules. 2021;11:99. doi: 10.3390/biom11010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitz T.W., Soreq H., Poirier J., Spreng R.N. Longitudinal Basal Forebrain Degeneration Interacts with TREM2/C3 Biomarkers of Inflammation in Presymptomatic Alzheimer’s Disease. J. Neurosci. 2020;40:1931–1942. doi: 10.1523/JNEUROSCI.1184-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simpson D.S.A., Oliver P.L. ROS generation in microglia: Understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants. 2020;9:743. doi: 10.3390/antiox9080743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bobadilla M., García-Sanmartín J., Martínez A. Natural Food Supplements Reduce Oxidative Stress in Primary Neurons and in the Mouse Brain, Suggesting Applications in the Prevention of Neurodegenerative Diseases. Antioxidants. 2021;10:46. doi: 10.3390/antiox10010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nazir N., Zahoor M., Nisar M., Khan I., Karim N., Abdel-Halim H., Ali A. Phytochemical analysis and antidiabetic potential of Elaeagnus umbellata (Thunb.) in streptozotocin-induced diabetic rats: Pharmacological and computational approach. BMC Complement. Altern. Med. 2018;18:1–16. doi: 10.1186/s12906-018-2381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nazir N., Karim N., Abdel-Halim H., Khan I., Wadood S.F., Nisar M. Phytochemical analysis, molecular docking and antiamnesic effects of methanolic extract of Silybum marianum (L.) Gaertn seeds in scopolamine induced memory impairment in mice. J. Ethnopharmcol. 2018;210:198–208. doi: 10.1016/j.jep.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 15.Rahimi R., Bahramsoltani R. Traditional Persian Medicinal Plants Database. Department of Traditional Pharmacy, School of Persian Medicine, Tehran University of Medical Sciences; Tehran, Iran: 2018. [Google Scholar]
- 16.Pandpazir M., Kiani A., Fakhri S., Mousavi Z. Anti-Inflammatory Effect and Skin Toxicity of Aqueous Extract of Dorema ammoniacum Gum in Experimental Animals. Res. J. Pharmacogn. 2018;5:1–8. [Google Scholar]
- 17.Abizadeh M., Heysieattalab S., Saeedi N., Hosseinmardi N., Janahmadi M., Salari F., Golpayegani S.M., Shojaii A. Ameliorating Effects of Dorema ammoniacum on PTZ-Induced Seizures and Epileptiform Brain Activity in Rats. Planta Med. 2020;86:1353–1362. doi: 10.1055/a-1229-4436. [DOI] [PubMed] [Google Scholar]
- 18.Ghasemi F., Tamadon H., Hosseinmardi N., Janahmadi M. Effects of Dorema ammoniacum Gum on Neuronal Epileptiform Activity-Induced by Pentylenetetrazole. Iran. J. Pharm. Res. 2018;17:735–742. [PMC free article] [PubMed] [Google Scholar]
- 19.Motevalian M., Mehrzadi S., Ahadi S., Shojaii A. Anticonvulsant activity of Dorema ammoniacum gum: Evidence for the involvement of benzodiazepines and opioid receptors. Res. Pharm. Sci. 2017;12:53–59. doi: 10.4103/1735-5362.199047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mazaheritehrani M., Hosseinzadeh R., Mohadjerani M., Tajbakhsh M., Ebrahimi S.N. Acetylcholinesterase inhibitory activity of Dorema ammoniacum gum extracts and molecular docking studies. Int. J. Pharm. Sci. Res. 2020;11:637–644. [Google Scholar]
- 21.Rajani M., Saxena N., Ravishankara M.N., Desai N., Padh H. Evaluation of the Antimicrobial Activity of Ammoniacum Gum from Dorema ammoniacum. Pharm. Biol. 2002;40:534–541. doi: 10.1076/phbi.40.7.534.14686. [DOI] [Google Scholar]
- 22.Mehrpour M., Yousefi M., AfzalAghaee M., Rakhshandeh H., Azizi H., Hadianfar A., Mehrpur O., Ghandehari K., Bahrami-Taghanaki H. Evaluation of Dorema ammoniacum and Acupuncture’s Therapeutic Effects in Patients with Ischemic Stroke: A Randomized Controlled Clinical Trial. Res. Sq. 2020 doi: 10.21203/rs.3.rs-45701/v1. [DOI] [Google Scholar]
- 23.Masoudi S., Kakavand S. Volatile constituents of the aerial parts of Terataenium lasiopentalum (Boiss.) Manden., stems and leaves of Dorema ammoniacum D. Don. and leaves, fruits and stems of Leutea petiolare (DC.) M.pimen from Iran. J. Chil. Chem. Soc. 2017;62:3311–3314. doi: 10.4067/S0717-97072017000100001. [DOI] [Google Scholar]
- 24.Zandpour F., Vahabi M., Allafchian A., Farhang H. Phytochemical investigation of the essential oils from the leaf and stem of Dorema ammoniacum D. Don. (Apiaceae) in Central Zagros, Iran. J. Herb Drug. 2016;7:109–116. [Google Scholar]
- 25.Raeesdana A., Farzaei M., Amini M., Rahimi R. Chemical composition of essential oil and evaluation of acute and sub-acute toxicity of Dorema ammoniacum d. Don. Oleo-gum-resin in rats. Afr. J. Tradit. Complement. Altern. Med. 2018;15:26–33. doi: 10.21010/ajtcam.v15i1.3. [DOI] [Google Scholar]
- 26.Zibaee E., Amiri M.S., Boghrati Z., Farhadi F., Ramezani M., Emami S.A., Sahebkar A. Ethnomedicinal Uses, Phytochemistry and Pharmacology of Dorema Species (Apiaceae): A Review. J. Pharmacopunct. 2020;23:91–123. doi: 10.3831/KPI.2020.23.3.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zandpour F., Allafchian A., Vahabi M., Jalali S.A.H. The green synthesis of silver nanoparticles by Arial part of Dorema ammoniacum D. extract with antimicrobial analysis. IET Nanobiotechnol. 2018;12:491–495. doi: 10.1049/iet-nbt.2017.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dadizadeh E., Talebpour A., Eskandani M., Nazemiyeh H. Free radical scavenging potential and essential oil composition of the Dorema glabrum Fisch. & C.A. Mey. roots from Iran. Bioimpacts. 2011;1:241–244. doi: 10.5681/bi.2011.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hadjiakhoondia A., Delazarb A., Ajania Y., Tavakolia S., Yassaa N. Phytochemical and Antioxidant Investigation of the Aerial Parts of Dorema glabrum Fisch. & C.A. Mey. Iran. J. Pharma. Res. 2015;14:925–931. [PMC free article] [PubMed] [Google Scholar]
- 30.Mirzaei A., Mirzaei N., Ghavamizadeh M. Antioxidant activity and cytotoxicity of Dorema aucheri by Artemia urmiana: A brine shrimp lethality test. Life Sci. J. 2013;10:8–12. [Google Scholar]
- 31.Zeb A. A reversed phase HPLC-DAD method for the determination of phenolic compounds in plant leaves. Anal. Methods. 2015;7:7753–7757. doi: 10.1039/C5AY01402F. [DOI] [Google Scholar]
- 32.Kim D., Jeond S., Lee C. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003;81:321–326. doi: 10.1016/S0308-8146(02)00423-5. [DOI] [Google Scholar]
- 33.Ellman G.L., Courtney K., Andres V., Featherstone R. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 34.Brand-Williams W., Cuvelier M., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 35.Re R., Pellegrini N., Proteggente A., Pannala A., Yong M.R.E. Antioxidant activity applying an improved FBTS radical cation decolorization assay. Free Rad. Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 36.Nazir N., Zahoor M., Nisar M., Karim N., Latif A., Ahmad S., Uddin Z. Evaluation of neuroprotective and anti-amnesic effects of Elaeagnus umbellata Thunb. On scopolamine-induced memory impairment in mice. BMC Complement. Med. Ther. 2020;20:1–17. doi: 10.1186/s12906-020-02942-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaspary K.V., Reolon G.K., Gusso D., Bonan C. Novel object recognition and object location tasks in zebrafish: Influence of habituation and NMDA receptor antagonism. Neurobiol. Learn. Mem. 2018;155:249–260. doi: 10.1016/j.nlm.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Adhami H.R., Lutz J., Kahlig H., Zehl M., Krenn L. Compounds from Gum Ammoniacum with Acetylcholinesterase Inhibitory Activity. Sci. Pharm. 2013;81:793–806. doi: 10.3797/scipharm.1306-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mlozi S.H., Mmongoyo J.A., Chacha M. The in vivo toxicity evaluation of leaf and root methanolic extracts of Tephrosia vogelii Hook.f using animal model. Clin. Phytosci. 2020;6:1–9. doi: 10.1186/s40816-020-00216-6. [DOI] [Google Scholar]
- 40.Iranshahi M., Shaki F., Mashlab A., Porzel A., Wessjohann L.A., Kopetdaghins A.E. Sesquiterpene Derivatives from the Aerial Parts and the Roots of Dorema kopetdaghense. J. Nat. Prod. 2007;70:1240–1243. doi: 10.1021/np070043u. [DOI] [PubMed] [Google Scholar]
- 41.Cichon N., Saluk-Bijak J., Gorniak L., Przyslo L., Bijak M. Flavonoids as a Natural Enhancer of Neuroplasticity—An Overview of the Mechanism of Neurorestorative Action. Antioxidants. 2020;9:1035. doi: 10.3390/antiox9111035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahangarpour A., Zamaneh H.T., Jabari A., Nia H.M., Heidari H. Antidiabetic and hypolipidemic effects of Dorema aucheri hydroalcoholic leave extract in streptozotocin-nicotinamide induced type 2 diabetes in male rats. Iran. J. Basic. Med. Sci. 2014;17:808–814. [PMC free article] [PubMed] [Google Scholar]
- 43.Lopa S.S., Al-Amin M.Y., Hasan M.K., Ahammed M.S., Islam K.M.M., Alam A.H.M.K., Tanaka T., Sadik M.G. Phytochemical Analysis and Cholinesterase Inhibitory and Antioxidant Activities of Enhydra fluctuans Relevant in the Management of Alzheimer’s Disease. Int. J. Food Sci. 2021;2021 doi: 10.1155/2021/8862025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saeedi M., Rashidy-Pour A. Association between chronic stress and Alzheimer’s disease: Therapeutic effects of Saffron. Biomed. Pharmacother. 2021;133:110995. doi: 10.1016/j.biopha.2020.110995. [DOI] [PubMed] [Google Scholar]
- 45.Nazir N., Khalil A.A.K., Nisar M., Zahoor M., Ahmad S. HPLC-UV characterization, anticholinesterase, and free radical-scavenging activities of Rosa moschata Herrm. leaves and fruits methanolic extracts. Braz. J. Bot. 2020;43:523–530. doi: 10.1007/s40415-020-00635-2. [DOI] [Google Scholar]
- 46.Sharma S., Raj K., Singh S. Neuroprotective Effect of Quercetin in Combination with Piperine Against Rotenone- and Iron Supplement–Induced Parkinson’s Disease in Experimental Rats. Neurotox. Res. 2020;37:198–209. doi: 10.1007/s12640-019-00120-z. [DOI] [PubMed] [Google Scholar]
- 47.Arafa M.H., Atteia H.H. Protective Role of Epigallocatechin Gallate in a Rat Model of Cisplatin-Induced Cerebral Inflammation and Oxidative Damage: Impact of Modulating NF-κB and Nrf2. Neurotox. Res. 2020;37:380–396. doi: 10.1007/s12640-019-00095-x. [DOI] [PubMed] [Google Scholar]
- 48.Rebas E., Rzajew J., Radzik T., Zylinska L. Neuroprotective Polyphenols: A Modulatory Action on Neurotransmitter Pathways. Curr. Neuropharmacol. 2020;18:431–445. doi: 10.2174/1570159X18666200106155127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adedayo B.C., Oyeleye S.I., Okeke B.M., Oboh G. Anti-cholinesterase and antioxidant properties of alkaloid and phenolic-rich extracts from pawpaw (Carica papaya) leaf: A comparative study. Flavour. Fragr. J. 2021;36:47–54. doi: 10.1002/ffj.3615. [DOI] [Google Scholar]
- 50.Bekdash R.A. The Cholinergic System, the Adrenergic System and the Neuropathology of Alzheimer’s Disease. Int. J. Mol. Sci. 2021;22:1273. doi: 10.3390/ijms22031273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thancharoen O., Limwattananon C., Waleekhachonloet O., Rattanachotphanit T., Limwattananon P., Limpawattana P. Ginkgo biloba Extract (EGb761), Cholinesterase Inhibitors, and Memantine for the Treatment of Mild-to-Moderate Alzheimer’s Disease: A Network Meta-Analysis. Drugs Aging. 2019;36:435–452. doi: 10.1007/s40266-019-00648-x. [DOI] [PubMed] [Google Scholar]
- 52.Wang X., Zhang D., Song W., Cai C.F., Zhou Z., Fu Q., Yan X., Cao Y., Fang M. Neuroprotective effects of the aerial parts of Polygala tenuifolia Wild extract on scopolamine-induced learning and memory impairments in mice. Biomed. Rep. 2020;13 doi: 10.3892/br.2020.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakamichi N., Nakao S., Nishiyama M., Takeda Y., Ishimoto T., Masuo Y., Matsumoto S., Suzuki M., Kato Y. Oral administration of the food derived hydrophilic antioxidant ergothioneine enhances object recognition memory in mice. Curr. Mol. Pharmacol. 2020;14:220–233. doi: 10.2174/1874467213666200212102710. [DOI] [PubMed] [Google Scholar]
- 54.Miranda M., Morici J.F., Zanoni M.B., Bekinschtein P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell. Neurosci. 2019;13:363. doi: 10.3389/fncel.2019.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.