Abstract
Background: Mast cells (MCs) contain proangiogenic factors, in particular tryptase, associated with increased angiogenesis in several tumours. With special reference to pancreatic cancer, few data have been published on the role of MCs in angiogenesis in both pancreatic ductal adenocarcinoma tissue (PDAT) and adjacent normal tissue (ANT). In this study, density of mast cells positive for c-Kit receptor (MCDP-c-KitR), density of mast cells positive for tryptase (MCDPT), area of mast cells positive for tryptase (MCAPT), and angiogenesis in terms of microvascular density (MVD) and endothelial area (EA) were evaluated in a total of 45 PDAT patients with stage T2–3N0–1M0. Results: For each analysed tissue parameter, the mean ± standard deviation was evaluated in both PDAT and ANT and differences were evaluated by Student’s t-test (p ranged from 0.001 to 0.005). Each analysed tissue parameter was then correlated to each other one by Pearson t-test analysis (p ranged from 0.01 to 0.03). No other correlation among MCDP-c-KitR, MCDPT, MCAPT, MVD, EA and the main clinical–pathological characteristics was found. Conclusions: Our results suggest that tissue parameters increased from ANT to PDAT and that mast cells are strongly associated with angiogenesis in PDAT. On this basis, the inhibition of MCs through tyrosine kinase inhibitors, such as masitinib, or inhibition of tryptase by gabexate mesylate may become potential novel antiangiogenetic approaches in pancreatic cancer therapy.
Keywords: mast cells, c-Kit receptor, tryptase, angiogenesis, microvascular density, endothelial area, pancreatic cancer tissue, adjacent normal tissue
1. Background
Pancreatic cancer (PC) is an infrequent tumour, but at the same time, it corresponds to the fourth most common cause of cancer mortality, with a 5-year survival rate of only 8% and a 10-year survival of just 3%. In its early stages, surgery and adjuvant chemotherapy represent the gold-standard treatment, but nevertheless the relapse rate is 60–70% after 2 years. In the metastatic setting, the FOLFIRINOX regimen is the common first-line treatment for fit patients (Eastern Cooperative Oncology Group Performance Status 0–1 and aged ≤ 75 years), improving median progression-free survival (mPFS) compared to gemcitabine alone (6.4 months vs 3.3 months; p < 0.0001) and median overall survival (mOS) up to 11.6 months (versus 6 months for the gemcitabine-alone group; p = 0.001) [1]. A multicentre, double-blind phase III study evaluated 154 patients with metastatic PC and BRCA 1–2 germline mutation without disease progression during at least 4 months of first-line platinum-based chemotherapy. This study showed that the median PFS was longer in the olaparib group than in the control group (median 7.4 months versus 3.8 months, respectively; p = 0.004) [2].
Computed tomography (CT) diagnoses PC with sensitivity and specificity from 70% up to 100%, and therefore it is always indicated in suspected PC [3]. In patients with clinical and radiological suspicion of PC, the preoperative diagnosis (histological or cytological) should be considered in the absence of clear signs of malignancy and in patients not eligible for surgery. From a pathological point of view, pancreatic ductal adenocarcinoma is the most frequent histotype (85%); it derives from the pancreatic ductal tree and has a glandular differentiation. According to the World Health Organization (WHO), pancreatic ductal adenocarcinoma has been classified into eight variants: colloid carcinoma, signet-ring cell carcinoma, adenosquamous carcinoma, undifferentiated carcinoma with osteoclast-like giant cells, undifferentiated carcinoma, and medullary, rhaboid and hepatoid carcinomas.
Mast cells (MCs) are bone marrow-derived cells, which are found in many human organs and tissues and contain a lot of pre-existing and de-novo-formed secretory granules with peculiar pleiotropic functions [4]. MC activity is mainly regulated by its membrane tyrosine kinase receptor, the c-Kit receptor (c-Kit-R), identified and classified in the cluster of differentiation 117 (CD-117) and binding the stem cell factor (SCF) as its natural ligand [5]. MCs can be activated by several stimuli, including the binding between immunoglobulin E and its antigenic epitope and the interaction between c-Kit-R and SCF [6,7]. After their activation, MCs release their secretory granules in the microenvironment, involved both in allergic reaction and anaphylaxis and in induced immunity [8,9]. In the last decades, several studies have demonstrated that mast cells contain several proangiogenic factors, such as vascular endothelial growth factor (VEGF), fibroblast growth factor-2 (FGF-2) and platelet-derived endothelial cell growth factor/thymidine phosphorylase (PDEC-GF/TP), but their ability to synthesize and secrete a non-classical potent proangiogenic factor called tryptase has only recently become a focus of researchers’ attention [10,11,12]. Tryptase is the most abundant factor stored in mast cell secretory granules. It is a serine protease that has been demonstrated to enhance [13,14,15] endothelial cell (EC) and microvascular proliferation in animal models in several in vitro and in vivo studies [16,17]. Through its binding to protease-activated receptor-2 (PAR-2) on endothelial cells, tryptase can stimulate microvascular formation [18,19,20,21].
Recent preliminary data indicate that mast cells are so involved in pancreatic cancer development that they can even be a therapeutic target, but in this context, very little data have been published so far, as is also the case for the relationship between mast cells density and angiogenesis, including their differences in both primary pancreatic ductal adenocarcinoma tissue (PDAT) and adjacent normal tissue (ANT) [22,23,24,25,26,27,28,29]. MC infiltration is higher in PDAT than in ANT [30,31], and increased MC infiltration directly correlates with higher tumour grade and worse prognosis [32,33]. MCs contribute to the PDAT progression through different mechanisms. For example, MCs express proangiogenic factors such as VEGF, FGF-2 and PDGF, and MC-derived MMPs promote the release of extracellular matrix-bound angiogenic factors. On the other hand, pancreatic cancer cells (PCCs) and stellate pancreatic cells (PSCs) recruit and activate MCs [34]. MCs release IL-13 and tryptase, inducing activation and proliferation of PCCs and PSCs [35]. Moreover, activated PSCs produce several proangiogenic factors and stimulate PCCs [36].
In this exploratory study, through immunohistochemistry and image analysis system, we have examined the density of mast cells positive for c-Kit receptor (MCDP-c-KitR), density of mast cells positive for tryptase (MCDPT), area of mast cells positive for tryptase (MCAPT), microvascular density (MVD) and endothelial area (EA) in both PDAT and ANT, in a series of pancreatic cancer patients who had undergone radical surgery. The correlation between the studied parameters and the main clinical–pathological features has been also investigated.
2. Results
Immunohistochemical staining of c-Kit receptor- and tryptase-positive mast cells, besides MVD in PDAT versus ANT, are reported in Figure 1A, Figure 2A, Figure 3A, Figure 1B, Figure 2B or Figure 3B, respectively (×400 magnification), Figure 4A, Figure 5A, Figure 6A, Figure 4B, Figure 5B and Figure 6B respectively (×1000 magnification).
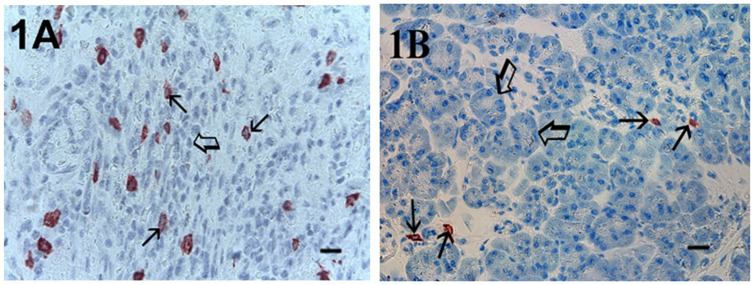
Figure 1.
Magnification ×400, 0.19 mm2 area, immunostaining with the anti-CD117 antibody. (A) High MCDP-c-KitR in primary PDAT section. Small arrows indicate single red-stained MCs. The big arrow indicates tumour epithelium. (B) Low MCDP-c-KitR in ANT section. Small arrows indicate single red-stained MCs. Big arrows indicate normal pancreatic epithelium. Scale bar corresponds to 125 µm. MCDP-c-KitR, density of mast cells positive for c-Kit receptor; PDAT, pancreatic ductal adenocarcinoma tissue; MCs, mast cells; ANT, adjacent normal tissue.
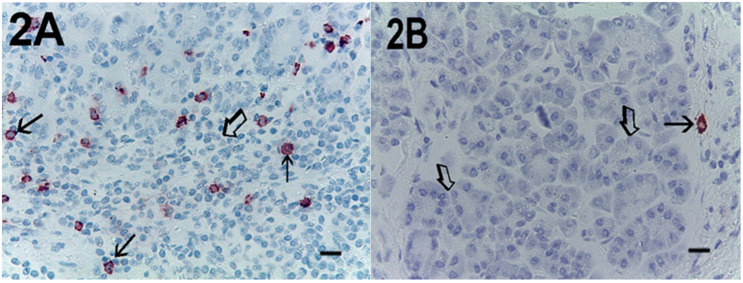
Figure 2.
Magnification ×400, 0.19 mm2 area, immunostaining with the anti-tryptase antibody. (A) High MCDPT in primary PDAT section. Small arrows indicate single red-immunostained MCs. The big arrow indicates tumour epithelium. (B) Low MCDPT in ANT section. The small arrow indicates just one red-stained MC. Big arrows indicate normal pancreatic epithelium. Scale bar corresponds to 125 µm. MCDPT, density of mast cells positive for tryptase.
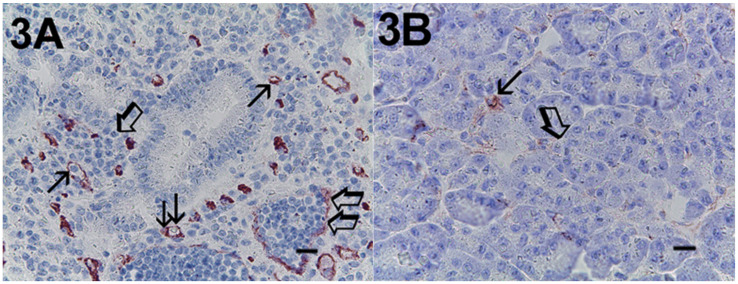
Figure 3.
Magnification ×400, 0.19 mm2 area, immunostaining with the anti-CD31 antibody. (A) High MVD in primary PDAT section. Single small arrows indicate single red-stained microvessels. Double small arrows indicate a single red-stained microvessel with a red blood cell in its lumen as a positive internal control. Single big arrows indicate tumour epithelium. Double big arrows indicate a vessel with a lot of tumour cells in its lumen. (B) Low MVD in ANT section. Small arrows indicate a single red-stained microvessel. The big arrow indicates normal pancreatic epithelium. Scale bar corresponds to 125 µm. MVD, microvascular density.
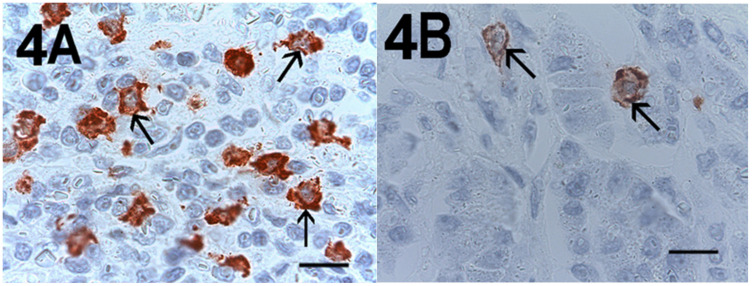
Figure 4.
Magnification ×1000 in oil, 0.06 mm2 area, immunostaining with the anti-CD117 antibody. (A) Very high MCDP-c-KitR in primary PDAT section. Small arrows indicate single red-immunostained MCs. The big arrow indicates tumour epithelium. (B) Low MCDP-c-KitR in ANT section. Small arrows indicate single red-stained mast cells. Please note the filiform membranes in immunostaining. Scale bar corresponds to 150 µm.
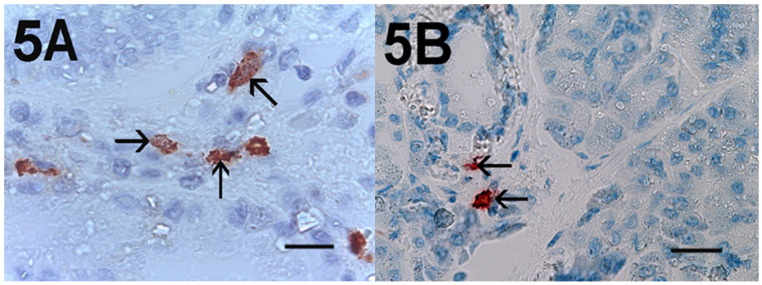
Figure 5.
Magnification ×1000 in oil, 0.06 mm2 area, immunostaining with the anti-tryptase antibody. (A) High MCDPT in primary PDAT section. Small arrows indicate single red-immunostained MCs. Magnification ×1000 in oil. (B) Low MCDPT in ANT section. Small arrows indicate red-stained MCs. Scale bar corresponds to 150 µm.
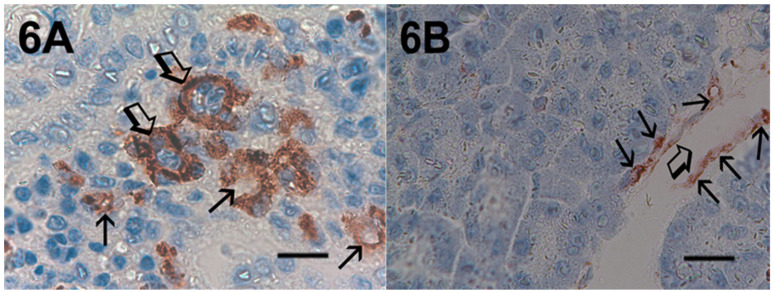
Figure 6.
Magnification ×1000 in oil, 0.06 mm2 area, immunostaining with the anti-CD31 antibody. (A) High MVD in primary PDAT section. Small arrows indicate single red-stained microvessels. Big arrows indicate single microvessels with tumour cells in their lumen. (B) Low MVD in ANT section. Small arrows indicate immunostained endothelium of a large vessel; the big arrow indicates the lumen of the vessel. Scale bar corresponds to 150 µm.
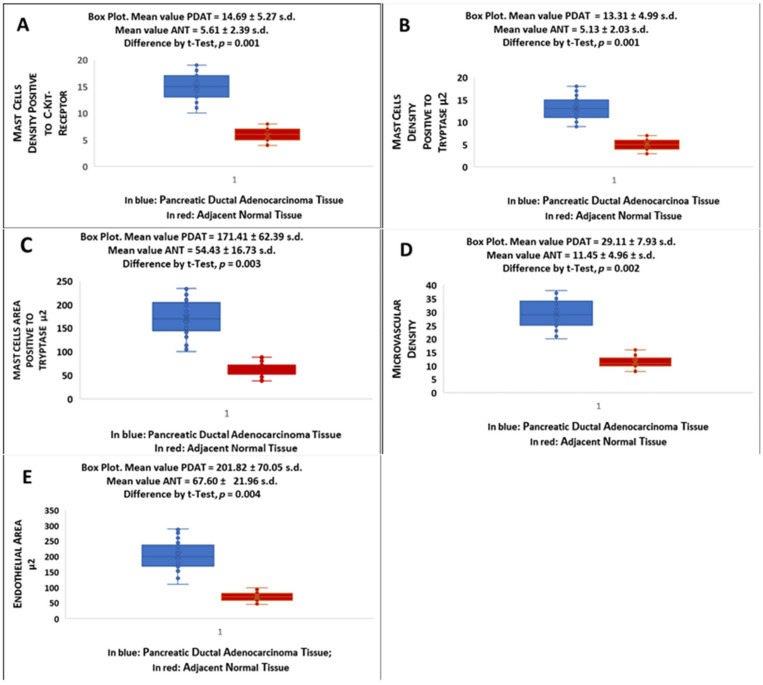
All results are presented in Figure 7 and the acronyms are reported in the below legend. Data demonstrated that MCDP-c-KitR, MCDPT, MCAPT, MVD and EA significantly increased from ANT to PDAT. Differences in terms of mean value ± SD between PDAT and ANT were significant for each analysed tissue biomarker (p ranged from 0.001 to 0.005 by t-test analysis).
Figure 7.
Boxes plot indicate mean ± standard deviation of (A) MCDP-c-KitR, (B) MCDPT, (C) MCAPT, (D) MVD and (E) EA in PDAT and ANT, respectively, and corresponding differences by Student’s t-test in terms of p-value.
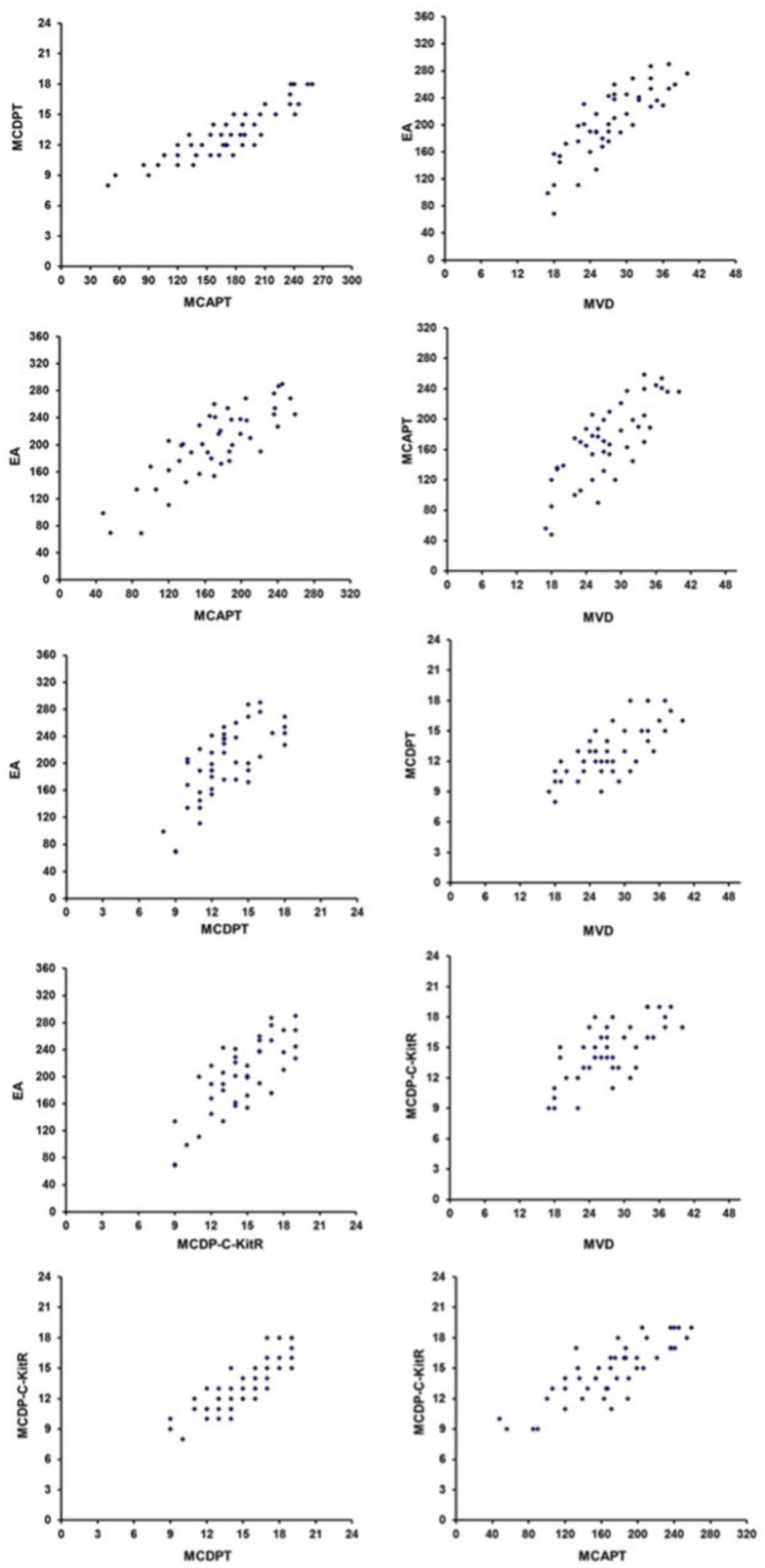
Statistical evaluation by Pearson analysis in PDAT showed a significant correlation between all parameters, as reported in the caption of Figure 8.
Figure 8.
Correlations by Pearson analysis between MCDPT and MCAPT (r = 0.85, p = 0.01), MVD and EA (r = 0.82, p = 0.01), EA and MCAPT (r = 0.66, p = 0.03), MCAPT and MVD (r = 0.76, p = 0.02), EA and MCDPT (r = 0.69, p = 0.03), MCDPT and MVD (r = 0.72, p = 0.02), EA and MCD-c-KitR (r = 0.73, p = 0.02), MCDP-c-KitR and MVD (r = 0.74, p = 0.02), MCDP-c-KitR and MCDPT (r = 0.87, p = 0.01) and MCDP-c-KitR and MCAPT (r = 0.81, p = 0.01).
No other correlation among MCDP-c-KitR, MCDPT, MCAPT, MVD, EA and the main clinical–pathological characteristic was found.
3. Discussion
Several published studies suggest that an increased mast cell density is associated with increased MVD in different animal and human malignancies, but to this regard, very little data have been published for PDAT [37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58]. In the literature, there are some data about the correlation between MVD, evaluated with anti-CD31, and the survival of resected pancreatic cancer patients, demonstrating that high MVD expression is strongly associated with poorer prognosis [59]. At the same time, Strouch et al. [26] showed that the prognosis of resected pancreatic cancer patients with high mast cell count in cancer tissue section, evaluated with anti-tryptase, was significantly worse than those with low mast cell count.
Mast cells migration in the tumour microenvironment is induced by the expression of several growth factors, molecules and proinflammatory cytokines, such as SCF, TGF, TNF, FES kinase, protein kinase D, CXCL12, eicosanoids, chemokines and prostaglandins, and by the activation of subcellular pathways, such as ERβ/CCL2/CCR2, EMT/MMP9 and PI3K/AK. Cells in both the cancer and tumour microenvironment can participate in the production of chemotactic agents for MCs, depending on the tumour type and the specific microenvironment [20,60,61,62,63,64,65]. From a functional point of view, MCs’ activities are mainly regulated by c-Kit-R, known also as CD-117, that binds its ligand SCF, and as a consequence of this interaction, MCs can degranulate [7,25,66,67,68]. It has been well established that MCs can synthesize and then release a plethora of classical proangiogenic factors such as VEGF, FGF-2, sphingosine-1-phosphate (S1P), TNF-α and IL-1, 6 and 8, which may stimulate the proliferation, migration and differentiation of ECs [69,70,71,72]. In particular, S1P synthesis is upregulated upon mast cell activation. Its signalling results in a substantial amount of VEGF-A release and triggers both transcriptional upregulation of VEGF-A and MMP-2 mRNA and protein secretion from mast cells [73]. The proangiogenic effect of MCs is also related to the production of gelatinase A (matrix metalloproteinase-2) and gelatinase B (matrix metalloproteinase-9) that degrade the extracellular matrix, releasing the prestored VEGF that, in turn, stimulates EC proliferation [74,75,76]. MCs also synthesize tryptase, a non-classical potent proangiogenic factor, which represents the most abundant biological substance in MCs’ secretory granules [14,15]. From a biological point of view, tryptase is a trypsin-like neutral serine protease and selective component of the secretory granules of all human MCs, accounting for almost 25% of cell protein (10–35 pg per mast cell) [15]. It consists of four monomers, each of which is stabilized in its active conformation and its tetrameric form by heparin–proteoglycan complexes [20,77,78]. Because of its proteolytic activity, tryptase acts as an agonist of the protease-activated receptor-2 (PAR-2), a G protein expressed on ECs that is involved in their proliferation and whose activation triggers an intracellular signal involving MAPK phosphorylation pathway [79,80].
Tryptase may also convert pro-MMP to active MMP, enhancing the degradation of extracellular matrix, VEGF release and neoangiogenesis. Chymase plays this role too [81,82].
The milestone in vitro data stating mast cell tryptase’s capacity to induce microvessel formation was first presented by Blair in 1997 [16]. In this study, a coculture of the human mast cells-1 (HMC-1) line with their products in the presence of human dermal microvascular ECs led to vessel formation, and the extent of neovascularization was strongly enhanced when HMC-1 MCs were degranulated. The results of this study indicated that tryptase from degranulated MCs was able to induce the genesis of microvessels with an increasing dose–response pattern. To support these findings, the inhibition of tryptase through a specific tryptase inhibitor led to a strong reduction of microvasculature formation. More recently, they demonstrated the angiogenic properties of MC tryptase in an in vivo chorioallantoic membrane assay, showing that the angiogenic potency of tryptase was similar to VEGF [11,17].
With particular reference to pancreatic cancer, preclinical in vivo data suggest that mast cell tryptase plays a role in stimulating angiogenesis and cancer growth also via the activation of the proangiogenic factor angiopoietin-1 [35,83,84]. Based on this biological background, in a previously published pilot study, we evaluated the correlation among MCDPT, MCAPT, MVD and EA in a series of 31 patients with resected primary pancreatic cancer [23]. Results from our study showed an evident association among these parameters, suggesting that tryptase-positive mast cells and the magnitude of tryptase expression correlate well with both MVD and EA in pancreatic cancer angiogenesis. Nevertheless, in this study, no ANT was evaluated and no MCDP-c-KitR was assessed. In a subsequent study conducted in a series of 35 PDAT patients, we observed a significant correlation between MCDP-c-KitR, MCDPT and MVD, and in particular, an increased MCDPT and MVD were found in PDAT when compared to ANT, confirming the role of mast cell tryptase in pancreatic cancer angiogenesis and tumour development [25]. In the latter study, however, no evaluation of MCAPT and EA was performed in normal and cancer tissues.
In the present exploratory research, as innovative technical approaches, immunohistochemistry and an image analysis system allowed us to access MCDP-c-KitR, MCDPT, MCAPT, MVD and EA simultaneously, both in PDAT and ANT, in a series of patients who had undergone radical surgery. Endothelial area represents the immunostained vascular area in a microscopic field, which is independent of their capacity and diameter, and can be interpreted as a surrogate of angiogenic activity in parallel; MCDPT indicates the number of local mast cells and not the area in which the mast cells are acting. We maintain that the identification of a couple of parameters (area and density) is more representative of the real angiogenic (EA and MVD) and enzymatic (MCDPT and MCAPT) activities. Our data indicated not only that MCs accumulate in pancreatic cancer tissue, but also MCAPT is increased in PDAT. The strong correlation between MCDP-c-KitR and MCDPT also suggests that in pancreatic cancer, the majority of MCs are tryptase-expressing cells. As expected, these data strongly correlate to each other and represent internal controls for the pancreatic disease. Nevertheless, to our knowledge, this is the first study that clearly shows these correlations through a new integrated immunohistochemical and image analysis system.
Our data are also supported by Chang’s research involving a transgenic spontaneous PDAT C57BL/6 mouse model, in which an early influx of MCs into the tumour microenvironment was assessed [33]. Even more interestingly, the growth of PDAT was significantly suppressed in mast cell-deficient Kitw-sh/w-sh mice, demonstrating the importance of MCs and their activation by c-Kit receptor in leading to angiogenesis and pancreatic cancer development. Moreover, Gorzalczany et al. showed that cell-to-cell contact interactions by exposing MCs to membranes derived from cancer cells resulted in mast cell activation, leading to increased phosphorylation of the ERK1/2 MAP kinases and Akt through a phosphatidylinositol 3-kinase-dependent pathway [85]. These in vitro results indicated a further mechanism of mast cell activation in tumour stromal microenvironments.
All preclinical data support the results of our investigation, whose message indicates increased tryptase expression parallel to increased angiogenesis in terms of both MVD and higher EA extension in surgically treated PDAT patients.
4. Conclusions
Here, we speculate that MCDPT and MCAPT together could be putative tissue biomarkers of pancreatic cancer angiogenesis status. To this regard, tryptase targeting through tryptase inhibitors, such as gabexate or nafamostat mesylate, could become an interesting strategy as a novel antiangiogenetic intervention in pancreatic cancer patients [86,87,88,89,90]. On the other hand, MC degranulation could be inhibited by c-KitR tyrosine kinase inhibitors, such as masitinib, as first applied in veterinary clinical oncology and then translated to humans for the treatment of PDAT patients, with interesting results, as reported by the only phase 3 clinical trial [91,92,93,94,95]. Finally, MCDPT and MCAPT could become potential predictive biomarkers of response to anti-c-Kit or anti-tryptase therapy, in order to to select patients with a higher risk as assessed by these biomarkers [96]. Confirmatory study and clinical trials are awaited in this context as well as novel anti-angiogenic therapies.
5. Materials and Methods
5.1. Study Population
The clinical–pathological characteristics of the analysed patients are summarized in Table 1. A total of 45 PDAT patients with stage T2–3N0–1M0 amenable to surgery underwent potentially curative resection. Employed surgical procedures were pancreaticoduodenectomy, distal pancreatectomy and total pancreatectomy with lymph node dissection [25]. Patients were staged according to the American Joint Committee on Cancer 7th edition (AJCC-TNM) classification, and the World Health Organization classification (2000 version) was used for pathological grading. All patients had no distant metastases on computed tomography. Full ethical approval and signed consent from individual patients were obtained. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the “Mater Domini” Hospital, “Magna Graecia” University, Catanzaro (No. 242; 22 December 2016).
Table 1.
Clinical–pathological features of patients (n = 45).
| Subgroup | No. of Patients |
|---|---|
| Age | |
| < 65 | 30 (67%) |
| > 65 | 15 (33%) |
| Gender: | |
| Female | 23 (51%) |
| Male | 22 (49%) |
| Tumour site: | |
| Head | 19 (42%) |
| Body-Tail | 26 (58%) |
| TNM by AJCC Stage | |
| T2N0–1M0 | 20 (44%) |
| T3N0–1M0 | 25 (56%) |
| Histologic type | |
| Ductal adenocarcinomas | 45 (100%) |
| Histologic grade | |
| G1–G2 | 34 (77%) |
| G3 | 11 (23%) |
5.2. Immunohistochemistry
Both MCs positive for c-KitR and tryptase and vessels were assessed by immunohistochemistry, employing a three-layer biotin–avidin–peroxidase technique [23,24,25,43]. In summary, 5 μm-thick serial sections of formalin-fixed and paraffin-embedded PDAT and ANT were cut. The obtained slides were processed with a microwave oven at 500 watts for 10 min, and then the endogenous peroxidase enzyme was inhibited with a 3% hydrogen peroxide solution. Soon after, the slides were incubated with the following primary antibodies:
Anti-CD117 to c-KitR (Dako, Glostrup, Denmark) diluted 1:100 at for 1 h at room temperature;
Anti-tryptase (clone AA1; Dako, Glostrup, Denmark) diluted 1:100 for 1 h at room temperature (for MC identification);
Anti-CD31 antibody (QB-END 10; Bio-Optica Milan, Milan, Italy) diluted 1:50 for 1 h at room temperature (as a pan-endothelial marker).
Immunoreactivity was evidenced by employing a biotinylated secondary antibody, the red chromogen avidin–biotin–peroxidase complex (LPS, K0640, Dako, Glostrup, Denmark). Cell nuclei were stained with Gill′s haematoxylin no. 2 (Polysciences, Warrington, PA, USA). No primary antibody was posted in negative controls.
5.3. Morphometrical Assay
A light microscope integrated with an image analysis system (AXIO, Scope A1, ZEISS, Germany) was utilized. For each serial section of PDAT and ANT, the five most heavily immunostained areas (hot spots) were selected at low magnification. Next, MCDP-C-KitR, MCDPT, MCAPT, MVD and EA were assessed at ×400 magnification (0.19 mm2 area) in the five identified hot spot areas for each serial section, respectively (Figure 1A,B, Figure 2A,B, Figure 3A,B). With special reference to MCDP-c-KitR and MCDPT, each immunostained cell was considered in the count, whereas MVD was detected by counting single red-brown-stained endothelial cells, endothelial cell clusters and microvessels, clearly separated from adjacent microvessels, according to the modified Weidner’s method [23,24,25]. Immunostained MCs positive for tryptase and endothelial cells positive for anti-CD31 were also evaluated in terms of immunostained area at ×400 magnification (0.19 mm2 area). Finally, morphological details of MCs positive for c-KitR, MCs positive for tryptase and endothelial cells were observed at ×1000 magnification in oil (Figure 4A,B, Figure 5A,B, Figure 6A,B, respectively).
5.4. Statistical Analysis
Mean value for each section and for the global series was obtained for all evaluated parameters in both PDAT and ANT groups. The differences between the two groups were measured by Student’s t-test. Mean values ± 1 standard deviation (SD) of all evaluated tissue parameters are reported in Figure 7.
Correlations between MCDP-c-KitR, MCDPT, MCAPT, MVD and EA were calculated using Pearson′s (r) analysis (Figure 7). Correlations among all studied parameters and the most important clinical–pathological characteristics, reported in Table 1, were analysed by the chi-square test (χ2). All analyses were considered statistically significant with a p value of < 0.05. Statistical analyses elaboration was performed with the SPSS statistical software package (SPSS, Inc., Chicago, IL, USA).
Author Contributions
Conceptualization, M.A., C.L. and G.R.; methodology, R.M., M.A., N.Z., R.P. and I.U., validation, M.P.; formal analysis, G.N.; investigation, M.P.; resources, L.M. and C.D.G.; data curation, F.L., A.Z. and D.L.; writing—original draft preparation, C.L., M.L. and G.R.; writing—review and ed-iting, M.L.; visualization, G.C., N.Z. and V.Z.; supervision, R.P.; project administration, C.L. and G.R. All authors have read and agreed to the published version of the manuscript.
Funding
There was no funding for this translational research study.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee Regione Calabria Section Area Center A.O.U. Mater Domini Catanzaro (protocol code n° 242, date of approval 22 December 2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All relevant data are included in the article.
Conflicts of Interest
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Conroy T., Desseigne F., Ychou M., Bouché O., Guimbaud R., Bécouarn Y., Adenis A., Raoul J.-L., Gourgou-Bourgade S., De La Fouchardière C., et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 2.Golan T., Hammel P., Reni M., Van Cutsem E., Macarulla T., Hall M.J., Park J.-O., Hochhauser D., Arnold D., Oh D.-Y., et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N. Engl. J. Med. 2019;381:317–327. doi: 10.1056/NEJMoa1903387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bronstein Y.L., Loyer E.M., Kaur H., Choi H., David C., Dubrow R.A., Broemeling L.D., Cleary K.R., Charnsangavej C. Detection of Small Pancreatic Tumors with Multiphasic Helical CT. Am. J. Roentgenol. 2004;182:619–623. doi: 10.2214/ajr.182.3.1820619. [DOI] [PubMed] [Google Scholar]
- 4.Dahlin J.S., Hallgren J. Mast cell progenitors: Origin, development and migration to tissues. Mol. Immunol. 2015;63:9–17. doi: 10.1016/j.molimm.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 5.Ammendola M., Sacco R., Sammarco G., Luposella M., Patruno R., Gadaleta C.D., De Sarro G., Ranieri G. Mast Cell-Targeted Strategies in Cancer Therapy. Transfus. Med. Hemotherapy. 2016;43:109–113. doi: 10.1159/000444942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim W.-J., Cha H.-S., Lee M.-H., Kim S.-Y., Kim S.H., Kim T.-J. Effects ofCymbidiumRoot Ethanol Extract on Atopic Dermatitis. Evid. -Based Complement. Altern. Med. 2016;2016:1–10. doi: 10.1155/2016/5362475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patruno R., Marech I., Zizzo N., Ammendola M., Nardulli P., Gadaleta C., Introna M., Capriuolo G., Rubini R.A., Ribatti D., et al. C-Kit Expression, Angiogenesis, and Grading in Canine Mast Cell Tumour: A Unique Model to Study C-Kit Driven Human Malignancies. BioMed Res. Int. 2014;2014:1–8. doi: 10.1155/2014/730246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.González-De-Olano D., Álvarez-Twose I. Mast Cells as Key Players in Allergy and Inflammation. J. Investig. Allergol. Clin. Immunol. 2018;28:365–378. doi: 10.18176/jiaci.0327. [DOI] [PubMed] [Google Scholar]
- 9.Wasiuk A., De Vries V.C., Hartmann K., Roers A., Noelle R.J. Mast cells as regulators of adaptive immunity to tumours. Clin. Exp. Immunol. 2009;155:140–146. doi: 10.1111/j.1365-2249.2008.03840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patruno R., Arpaia N., Gadaleta C.D., Passantino L., Zizzo N., Misino A., Lucarelli N.M., Catino A., Valerio P., Ribatti D., et al. VEGF concentration from plasma-activated platelets rich correlates with microvascular density and grading in canine mast cell tumour spontaneous model. J. Cell. Mol. Med. 2009;13:555–561. doi: 10.1111/j.1582-4934.2008.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribatti D., Ranieri G. Tryptase, a novel angiogenic factor stored in mast cell granules. Exp. Cell Res. 2015;332:157–162. doi: 10.1016/j.yexcr.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 12.Marone G., Varricchi G., Loffredo S., Granata F. Mast cells and basophils in inflammatory and tumor angiogenesis and lymphangiogenesis. Eur. J. Pharmacol. 2016;778:146–151. doi: 10.1016/j.ejphar.2015.03.088. [DOI] [PubMed] [Google Scholar]
- 13.Ferrara A.L., Piscitelli F., Petraroli A., Parente R., Galdiero M.R., Varricchi G., Marone G., Triggiani M., Di Marzo V., Loffredo S. Altered Metabolism of Phospholipases, Diacylglycerols, Endocannabinoids, and N-Acylethanolamines in Patients with Mastocytosis. J. Immunol. Res. 2019;2019:1–14. doi: 10.1155/2019/5836476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atiakshin D., Buchwalow I., Samoilova V., Tiemann M. Tryptase as a polyfunctional component of mast cells. Histochem. Cell Biol. 2018;149:461–477. doi: 10.1007/s00418-018-1659-8. [DOI] [PubMed] [Google Scholar]
- 15.Fukuoka Y., Schwartz L.B. Active monomers of human β-tryptase have expanded substrate specificities. Int. Immunopharmacol. 2007;7:1900–1908. doi: 10.1016/j.intimp.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blair R.J., Meng H., Marchese M.J., Ren S., Schwartz L.B., Tonnesen M.G., Gruber B.L. Human mast cells stimulate vascular tube formation. Tryptase is a novel, potent angiogenic factor. J. Clin. Investig. 1997;99:2691–2700. doi: 10.1172/JCI119458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribatti M., Ranieri G., Nico B., Benagiano V., Crivellato E. Tryptase and chymase are angiogenic in vivo in the chorioallantoic membrane assay. Int. J. Dev. Biol. 2011;55:99–102. doi: 10.1387/ijdb.103138dr. [DOI] [PubMed] [Google Scholar]
- 18.Itoh Y., Sendo T., Oishi R. Physiology and Pathophysiology of Proteinase-Activated Receptors (PARs): Role of Tryptase/PAR-2 in Vascular Endothelial Barrier Function. J. Pharmacol. Sci. 2005;97:14–19. doi: 10.1254/jphs.FMJ04005X3. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Q., Wang Y.-W., Ni P.-F., Chen Y.-N., Dong H.-Q., Qian Y.-N. Effect of tryptase on mouse brain microvascular endothelial cells via protease-activated receptor 2. J. Neuroinflammation. 2018;15:1–9. doi: 10.1186/s12974-018-1287-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ranieri G., Sacco R., Sammarco G., Piardi T., Zuccalà V., Patruno R., Zullo A., Zizzo N., Nardo B., Marech I., et al. Mast cells positive to tryptase, endothelial cells positive to protease-activated receptor-2, and microvascular density correlate among themselves in hepatocellular carcinoma patients who have undergone surgery. OncoTargets Ther. 2016;9:4465–4471. doi: 10.2147/OTT.S105368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caughey G.H. Mast cell tryptases and chymases in inflammation and host defense. Immunol. Rev. 2007;217:141–154. doi: 10.1111/j.1600-065X.2007.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ammendola M., Sacco R., Vescio G., Zuccalà V., Luposella M., Patruno R., Zizzo N., Gadaleta C., Marech I., Ruggieri R., et al. Tryptase mast cell density, protease-activated receptor-2 microvascular density, and classical microvascular density evaluation in gastric cancer patients undergoing surgery: Possible translational relevance. Ther. Adv. Gastroenterol. 2017;10:353–360. doi: 10.1177/1756283X16673981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ammendola M., Sacco R., Sammarco G., Donato G., Zuccalà V., Luposella M., Patruno R., Marech I., Montemurro S., Zizzo N., et al. Mast Cells Density Positive to Tryptase Correlates with Angiogenesis in Pancreatic Ductal Adenocarcinoma Patients Having Undergone Surgery. Gastroenterol. Res. Pr. 2014;2014:1–7. doi: 10.1155/2014/951957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ammendola M., Sacco R., Marech I., Sammarco G., Zuccalà V., Luposella M., Patruno R., Giordano M., Ruggieri E., Zizzo N., et al. Microvascular density and endothelial area correlate with Ki-67 proliferative index in surgically-treated pancreatic ductal adenocarcinoma patients. Oncol. Lett. 2015;10:967–971. doi: 10.3892/ol.2015.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ammendola M., Gadaleta C.D., Frampton A.E., Piardi T., Memeo R., Zuccalà V., Luposella M., Patruno R., Zizzo N., Gadaleta P., et al. The density of mast cells c-Kit+ and tryptase+ correlates with each other and with angiogenesis in pancreatic cancer patients. Oncotarget. 2017;8:70463–70471. doi: 10.18632/oncotarget.19716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Longo V., Tamma R., Brunetti O., Pisconti S., Argentiero A., Silvestris N., Ribatti D. Mast cells and angiogenesis in pancreatic ductal adenocarcinoma. Clin. Exp. Med. 2018;18:319–323. doi: 10.1007/s10238-018-0493-6. [DOI] [PubMed] [Google Scholar]
- 27.Xu M., Zhou B., Tao M., Liu J., Li W. The Role of Stromal Components in Pancreatic Cancer Progression. Anti-Cancer Agents Med. Chem. 2016;16:1117–1124. doi: 10.2174/1871520616666160404115532. [DOI] [PubMed] [Google Scholar]
- 28.Theoharides T.C. Mast Cells and Pancreatic Cancer. N. Engl. J. Med. 2008;358:1860–1861. doi: 10.1056/NEJMcibr0801519. [DOI] [PubMed] [Google Scholar]
- 29.Soucek L., Lawlor E.R., Soto D., Shchors K., Swigart L.B.I., Evan G. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat. Med. 2007;13:1211–1218. doi: 10.1038/nm1649. [DOI] [PubMed] [Google Scholar]
- 30.Karamitopoulou E., Shoni M., Theoharides T. Increased Number of Non-Degranulated Mast Cells in Pancreatic Ductal Adenocarcinoma but Not in Acute Pancreatitis. Int. J. Immunopathol. Pharmacol. 2014;27:213–220. doi: 10.1177/039463201402700208. [DOI] [PubMed] [Google Scholar]
- 31.Esposito I., Menicagli M., Funel N., Bergmann F., Boggi U., Mosca F., Bevilacqua G., Campani D. Inflammatory cells contribute to the generation of an angiogenic phenotype in pancreatic ductal adenocarcinoma. J. Clin. Pathol. 2004;57:630–636. doi: 10.1136/jcp.2003.014498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strouch M.J., Cheon E.C., Salabat M.R., Krantz S.B., Gounaris E., Melstrom L.G., Dangi-Garimella S., Wang E., Munshi H.G., Khazaie K., et al. Crosstalk between Mast Cells and Pancreatic Cancer Cells Contributes to Pancreatic Tumor Progression. Clin. Cancer Res. 2010;16:2257–2265. doi: 10.1158/1078-0432.CCR-09-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang D.Z., Ma Y., Ji B., Wang H., Deng D., Liu Y., Abbruzzese J.L., Liu Y.-J., Logsdon C.D., Hwu P. Mast Cells in Tumor Microenvironment Promotes the In Vivo Growth of Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. 2011;17:7015–7023. doi: 10.1158/1078-0432.CCR-11-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhan H.-X., Zhou B., Cheng Y.-G., Xu J.-W., Wang L., Zhang G.-Y., Hu S.-Y. Crosstalk between stromal cells and cancer cells in pancreatic cancer: New insights into stromal biology. Cancer Lett. 2017;392:83–93. doi: 10.1016/j.canlet.2017.01.041. [DOI] [PubMed] [Google Scholar]
- 35.Guo X., Zhai L., Xue R., Shi J., Zeng Q., Gao C. Mast Cell Tryptase Contributes to Pancreatic Cancer Growth through Promoting Angiogenesis via Activation of Angiopoietin-1. Int. J. Mol. Sci. 2016;17:834. doi: 10.3390/ijms17060834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haqq J., Howells L.M., Garcea G., Metcalfe M.S., Steward W.P., Dennison A.R. Pancreatic stellate cells and pancreas cancer: Current perspectives and future strategies. Eur. J. Cancer. 2014;50:2570–2582. doi: 10.1016/j.ejca.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 37.Passantino L., Passantino G., Cianciotta A., Ribaud M.R., Presti G.L., Ranieri G., Perillo A. Expression of Proto-Oncogene C-Kit and Correlation with Morphological Evaluations in Canine Cutaneous Mast Cell Tumors. Immunopharmacol. Immunotoxicol. 2008;30:609–621. doi: 10.1080/08923970801949265. [DOI] [PubMed] [Google Scholar]
- 38.Ranieri G., Passantino L., Patruno R., Passantino G., Jirillo F., Catino A., Mattioli V., Gadaleta C., Ribatti M. The dog mast cell tumour as a model to study the relationship between angiogenesis, mast cell density and tumour malignancy. Oncol. Rep. 2003;10:1189–1193. doi: 10.3892/or.10.5.1189. [DOI] [PubMed] [Google Scholar]
- 39.Patruno R., Passantino G., Laface C., Tinelli A., Zito A., Ruggieri R., Luposella F., Gadaleta P., Laforgia M., Lacitignola L., et al. Microvascular density, endothelial area, and Ki-67 proliferative index correlate each other in car post-injection fibrosarcoma. Cells. 2021;10:31. doi: 10.3390/cells10010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goffredo V., Gadaleta C.D., Laterza A., Vacca A., Ranieri G. Tryptase serum levels in patients suffering from hepatocellular carcinoma undergoing intra-arterial chemoembolization: Possible predictive role of response to treatment. Mol. Clin. Oncol. 2013;1:385–389. doi: 10.3892/mco.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ammendola M., Sacco R., Sammarco G., Donato G., Montemurro S., Ruggieri E., Patruno R., Marech I., Cariello M., Vacca A., et al. Correlation between Serum Tryptase, Mast Cells Positive to Tryptase and Microvascular Density in Colo-Rectal Cancer Patients: Possible Biological-Clinical Significance. PLoS ONE. 2014;9:e99512. doi: 10.1371/journal.pone.0099512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marech I., Ammendola M., Sacco R., Capriuolo G.S., Patruno R., Rubini R., Luposella M., Zuccalà V., Savino E., Gadaleta C.D., et al. Serum tryptase, mast cells positive to tryptase and microvascular density evaluation in early breast cancer patients: Possible translational significance. BMC Cancer. 2014;14:1–7. doi: 10.1186/1471-2407-14-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ammendola M., Sacco R., Donato G., Zuccalà V., Russo E., Luposella M., Vescio G., Rizzuto A., Patruno R., De Sarro G., et al. Mast Cell Positivity to Tryptase Correlates with Metastatic Lymph Nodes in Gastrointestinal Cancer Patients Treated Surgically. Oncology. 2013;85:111–116. doi: 10.1159/000351145. [DOI] [PubMed] [Google Scholar]
- 44.Ranieri G., Ammendola M., Patruno R., Celano G., Zito F.A., Montemurro S., Rella A., Di Lecce V., Gadaleta C.D., De Sarro G.B., et al. Tryptase-positive mast cells correlate with angiogenesis in early breast cancer patients. Int. J. Oncol. 2009;35:115–120. doi: 10.3892/ijo_00000319. [DOI] [PubMed] [Google Scholar]
- 45.Ammendola M., Marech I., Sammarco G., Zuccalà V., Luposella M., Zizzo N., Patruno R., Crovace A., Ruggieri E., Zito A.F., et al. Infiltrating Mast Cells Correlate with Angiogenesis in Bone Metastases from Gastric Cancer Patients. Int. J. Mol. Sci. 2015;16:3237–3250. doi: 10.3390/ijms16023237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Albini A., Bruno A., Noonan D.M., Mortara L. Contribution to Tumor Angiogenesis from Innate Immune Cells Within the Tumor Microenvironment: Implications for Immunotherapy. Front. Immunol. 2018;9:527. doi: 10.3389/fimmu.2018.00527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cimpean A.M., Tamma R., Ruggieri S., Nico B., Toma A., Ribatti D. Mast cells in breast cancer angiogenesis. Crit. Rev. Oncol. 2017;115:23–26. doi: 10.1016/j.critrevonc.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 48.Derakhshani A., Vahidian F., Alihasanzadeh M., Mokhtarzadeh A., Nezhad P.L., Baradaran B. Mast cells: A double-edged sword in cancer. Immunol. Lett. 2019;209:28–35. doi: 10.1016/j.imlet.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 49.Gudiseva S., Santosh A.B.R., Chitturi R., Anumula V., Poosarla C., Baddam V.R.R. The role of mast cells in oral squamous cell carcinoma. Współczesna Onkol. 2017;21:21–29. doi: 10.5114/wo.2017.65157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ranieri G., Labriola A., Achille G., Florio G., Zito A.F., Grammatica L., Paradiso A. Microvessel density, mast cell density and thymidine phosphorylase expression in oral squamous carcinoma. Int. J. Oncol. 2002;21:1317–1323. [PubMed] [Google Scholar]
- 51.Komi D.E.A., Redegeld F.A. Role of Mast Cells in Shaping the Tumor Microenvironment. Clin. Rev. Allergy Immunol. 2019;58:313–325. doi: 10.1007/s12016-019-08753-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leporini C., Ammendola M., Marech I., Sammarco G., Sacco R., Gadaleta C.D., Oakley C., Russo E., De Sarro G., Ranieri G. Targeting mast cells in gastric cancer with special reference to bone metastases. World J. Gastroenterol. 2015;21:10493–10501. doi: 10.3748/wjg.v21.i37.10493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Popovici R.A., Raica M., Ribatti D. Mast cells in lymphomas. BioMed Res. Int. 2016;101:207–212. doi: 10.1016/j.critrevonc.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 54.Ribatti D., Tamma R., Vacca A. Mast Cells and Angiogenesis in Human Plasma Cell Malignancies. Int. J. Mol. Sci. 2019;20:481. doi: 10.3390/ijms20030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gaje P.N., Ceausu R.A., Jitariu A., Stratul S.I., Rusu L.-C., Popovici R.A., Raica M. Mast Cells: Key Players in the Shadow in Oral Inflammation and in Squamous Cell Carcinoma of the Oral Cavity. BioMed Res. Int. 2016:9235080. doi: 10.1155/2016/9235080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Visciano C., Prevete N., Liotti F., Marone G. Tumor-Associated Mast Cells in Thyroid Cancer. Int. J. Endocrinol. 2015;2015:1–8. doi: 10.1155/2015/705169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laface C., Ammendola M., Zuccalà V., Luposella F., Zizzo N., Patruno R., Porcelli M., Gadaleta C.D., Tucci M., Ranieri G. Mast cells positive to c-kit receptor and to tryptase in normal to cancer pancreatic tissue and the correlation with angiogenesis. J. Clin. Oncol. 2020;38:e16502. doi: 10.1200/JCO.2020.38.15_suppl.e16502. [DOI] [Google Scholar]
- 58.Ammendola M., Sacco R., Sammarco G., Donato G., Zuccalà V., Romano R., Luposella M., Patruno R., Vallicelli C., Verdecchia G.M., et al. Mast Cells Positive to Tryptase and c-Kit Receptor Expressing Cells Correlates with Angiogenesis in Gastric Cancer Patients Surgically Treated. Gastroenterol. Res. Pr. 2013;2013:1–5. doi: 10.1155/2013/703163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ntellas P., Dadouli K., Perivoliotis K., Sogka E., Pentheroudakis G., Ioannou M., Hadjichristodoulou C., Tepetes K., Mauri D. Microvessel Density and Impact of Angiogenesis on Survival of Resected Pancreatic Cancer Patients. Pancreas. 2019;48:233–241. doi: 10.1097/MPA.0000000000001237. [DOI] [PubMed] [Google Scholar]
- 60.Rao Q., Chen Y., Yeh C.-R., Ding J., Li L., Chang C., Yeh S. Recruited mast cells in the tumor microenvironment enhance bladder cancer metastasis via modulation of ERβ/CCL2/CCR2 EMT/MMP9 signals. Oncotarget. 2015;7:7842–7855. doi: 10.18632/oncotarget.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhong B., Li Y., Liu X., Wang D. Association of mast cell infiltration with gastric cancer progression. Oncol. Lett. 2017;15:755–764. doi: 10.3892/ol.2017.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khan M.W., Keshavarzian A., Gounaris E., Melson J.E., Cheon E.C., Blatner N.R., Chen Z.E., Tsai F.-N., Lee G., Ryu H., et al. PI3K/AKT Signaling Is Essential for Communication between Tissue-Infiltrating Mast Cells, Macrophages, and Epithelial Cells in Colitis-Induced Cancer. Clin. Cancer Res. 2013;19:2342–2354. doi: 10.1158/1078-0432.CCR-12-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang B., Lei Z., Zhang G.-M., Li D., Song C., Li B., Liu Y., Yuan Y., Unkeless J., Xiong H., et al. SCF-mediated mast cell infiltration and activation exacerbate the inflammation and immunosuppression in tumor microenvironment. Blood. 2008;112:1269–1279. doi: 10.1182/blood-2008-03-147033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu W., Qian J., Zeng F., Li S., Guo W., Chen L., Li G., Zhang Z., Wang Q.J., Deng F. Protein kinase Ds promote tumor angiogenesis through mast cell recruitment and expression of angiogenic factors in prostate cancer microenvironment. J. Exp. Clin. Cancer Res. 2019;38:1–13. doi: 10.1186/s13046-019-1118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xiong Y., Liu L., Xia Y., Qi Y., Chen Y., Chen L., Zhang P., Kong Y., Qu Y., Wang Z., et al. Tumor infiltrating mast cells determine oncogenic HIF-2α-conferred immune evasion in clear cell renal cell carcinoma. Cancer Immunol. Immunother. 2019;68:731–741. doi: 10.1007/s00262-019-02314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chataigner L.M.P., Leloup N., Janssen B.J.C. Structural Perspectives on Extracellular Recognition and Conformational Changes of Several Type-I Transmembrane Receptors. Front. Mol. Biosci. 2020;7:129. doi: 10.3389/fmolb.2020.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marech I., Ammendola M., Leporini C., Patruno R., Luposella M., Zizzo N., Passantino G., Sacco R., Farooqi A.A., Zuccalà V., et al. C-Kit receptor and tryptase expressing mast cells correlate with angiogenesis in breast cancer patients. Oncotarget. 2017;9:7918–7927. doi: 10.18632/oncotarget.23722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marech I., Gadaleta C.D., Ranieri G. Possible Prognostic and Therapeutic Significance of c-Kit Expression, Mast Cell Count and Microvessel Density in Renal Cell Carcinoma. Int. J. Mol. Sci. 2014;15:13060–13076. doi: 10.3390/ijms150713060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taracanova A., Tsilioni I., Conti P., Norwitz E.R., Leeman S.E., Theoharides T.C. Substance P and IL-33 administered together stimulate a marked secretion of IL-1β from human mast cells, inhibited by methoxyluteolin. Proc. Natl. Acad. Sci. 2018;115:E9381–E9390. doi: 10.1073/pnas.1810133115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McHale C., Mohammed Z., Deppen J., Gomez G. Interleukin-6 potentiates FcεRI-induced PGD 2 biosynthesis and induces VEGF from human in situ -matured skin mast cells. Biochim. et Biophys. Acta (BBA) -Gen. Subj. 2018;1862:1069–1078. doi: 10.1016/j.bbagen.2018.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim G.-Y., Lee J.-W., Ryu H.-C., Wei J.-D., Seong C.-M., Kim J.-H. Proinflammatory Cytokine IL-1β Stimulates IL-8 Synthesis in Mast Cells via a Leukotriene B4 Receptor 2-Linked Pathway, Contributing to Angiogenesis. J. Immunol. 2010;184:3946–3954. doi: 10.4049/jimmunol.0901735. [DOI] [PubMed] [Google Scholar]
- 72.Chumanevich A., Wedman P., Oskeritzian C.A. Sphingosine-1-Phosphate/Sphingosine-1-Phosphate Receptor 2 Axis Can Promote Mouse and Human Primary Mast Cell Angiogenic Potential through Upregulation of Vascular Endothelial Growth Factor-A and Matrix Metalloproteinase-2. Mediat. Inflamm. 2016;2016:1–8. doi: 10.1155/2016/1503206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gordon J.R., Galli S.J. Mast cells as a source of both preformed and immunologically inducible TNF-α/cachectin. Nat. Cell Biol. 1990;346:274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- 74.Di Girolamo N., Indoh I., Jackson N., Wakefield D., McNeil H.P., Yan W., Geczy C., Arm J.P., Tedla N. Human Mast Cell-Derived Gelatinase B (Matrix Metalloproteinase-9) Is Regulated by Inflammatory Cytokines: Role in Cell Migration. J. Immunol. 2006;177:2638–2650. doi: 10.4049/jimmunol.177.4.2638. [DOI] [PubMed] [Google Scholar]
- 75.Fang K.C., Wolters P.J., Steinhoff M., Bidgol A., Blount J.L., Caughey G.H. Mast cell expression of gelatinases A and B is regulated by kit ligand and TGF-beta. J. Immunol. 1999;162:5528–5535. [PubMed] [Google Scholar]
- 76.Vempati P., Popel A.S., Mac Gabhann F. Extracellular regulation of VEGF: Isoforms, proteolysis, and vascular patterning. Cytokine Growth Factor Rev. 2014;25:1–19. doi: 10.1016/j.cytogfr.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McNeil H.P., Adachi R., Stevens R.L. Mast Cell-restricted Tryptases: Structure and Function in Inflammation and Pathogen Defense. J. Biol. Chem. 2007;282:20785–20789. doi: 10.1074/jbc.R700017200. [DOI] [PubMed] [Google Scholar]
- 78.Maun H.R., Liu P.S., Franke Y., Eigenbrot C., Forrest W.F., Schwartz L.B., Lazarus R.A. Dual functionality of β-tryptase protomers as both proteases and cofactors in the active tetramer. J. Biol. Chem. 2018;293:9614–9628. doi: 10.1074/jbc.M117.812016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rickard A., Portell C., Kell P.J., Vinson S.M., McHowat J. Protease-activated receptor stimulation activates a Ca2+-independent phospholipase A2 in bladder microvascular endothelial cells. Am. J. Physiol. Physiol. 2005;288:F714–F721. doi: 10.1152/ajprenal.00288.2004. [DOI] [PubMed] [Google Scholar]
- 80.Matej R., Mandáková P., Netíková I., Poucková P., Olejár T. Proteinase-activated receptor-2 expression in breast cancer and the role of trypsin on growth and metabolism of breast cancer cell line MDA MB-231. Physiol. Res. 2007;56:475–484. doi: 10.33549/physiolres.930959. [DOI] [PubMed] [Google Scholar]
- 81.Liu Y., Mueller B.M. Protease-activated receptor-2 regulates vascular endothelial growth factor expression in MDA-MB-231 cells via MAPK pathways. Biochem. Biophys. Res. Commun. 2006;344:1263–1270. doi: 10.1016/j.bbrc.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 82.Ranieri G., Laface C., Laforgia M., De Summa S., Porcelli M., Macina F., Ammendola M., Molinari P., Lauletta G., Di Palo A., et al. Bevacizumab Plus FOLFOX-4 Combined with Deep Electro-Hyperthermia as First-line Therapy in Metastatic Colon Cancer: A Pilot Study. Front. Oncol. 2020;10:590707. doi: 10.3389/fonc.2020.590707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ma Y.E., Ullrich S. Intratumoral mast cells promote the growth of pancreatic cancer. OncoImmunology. 2013;2:e25964. doi: 10.4161/onci.25964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ma Y., Hwang R.F., Logsdon C.D., Ullrich S.E. Dynamic Mast Cell–Stromal Cell Interactions Promote Growth of Pancreatic Cancer. Cancer Res. 2013;73:3927–3937. doi: 10.1158/0008-5472.CAN-12-4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gorzalczany Y., Akiva E., Klein O., Merimsky O., Sagi-Eisenberg R. Mast cells are directly activated by contact with cancer cells by a mechanism involving autocrine formation of adenosine and autocrine/paracrine signaling of the adenosine A3 receptor. Cancer Lett. 2017;397:23–32. doi: 10.1016/j.canlet.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 86.Ribatti D., Ranieri G., Basile A., Azzariti A., Paradiso A., Vacca A. Tumor endothelial markers as a target in cancer. Expert Opin. Ther. Targets. 2012;16:1215–1225. doi: 10.1517/14728222.2012.725047. [DOI] [PubMed] [Google Scholar]
- 87.Mori S., Itoh Y., Shinohata R., Sendo T., Oishi R., Nishibori M. Nafamostat Mesilate Is an Extremely Potent Inhibitor of Human Tryptase. J. Pharmacol. Sci. 2003;92:420–423. doi: 10.1254/jphs.92.420. [DOI] [PubMed] [Google Scholar]
- 88.Ammendola M., Leporini C., Marech I., Gadaleta C.D., Scognamillo G., Sacco R., Sammarco G., De Sarro G., Russo E., Ranieri G. Targeting Mast Cells Tryptase in Tumor Microenvironment: A Potential Antiangiogenetic Strategy. BioMed Res. Int. 2014;2014:1–16. doi: 10.1155/2014/154702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ni W.-W., Cao M.-D., Huang W., Meng L., Wei J.-F. Tryptase inhibitors: A patent review. Expert Opin. Ther. Patents. 2017;27:919–928. doi: 10.1080/13543776.2017.1322064. [DOI] [PubMed] [Google Scholar]
- 90.Liang G., Aldous S., Merriman G., Levell J., Pribish J., Cairns J., Chen X., Maignan S., Mathieu M., Tsay J., et al. Structure-based library design and the discovery of a potent and selective mast cell β-tryptase inhibitor as an oral therapeutic agent. Bioorganic Med. Chem. Lett. 2012;22:1049–1054. doi: 10.1016/j.bmcl.2011.11.119. [DOI] [PubMed] [Google Scholar]
- 91.Humbert M., Casteran N., Letard S., Hanssens K., Iovanna J., Finetti P., Bertucci F., Bader T., Mansfield C.D., Moussy A., et al. Masitinib Combined with Standard Gemcitabine Chemotherapy: In Vitro and In Vivo Studies in Human Pancreatic Tumour Cell Lines and Ectopic Mouse Model. PLoS ONE. 2010;5:e9430. doi: 10.1371/journal.pone.0009430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marech I., Patruno R., Zizzo N., Gadaleta C., Introna M., Zito A.F., Gadaleta C.D., Ranieri G. Masitinib (AB1010), from canine tumor model to human clinical development: Where we are? Crit. Rev. Oncol. 2014;91:98–111. doi: 10.1016/j.critrevonc.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 93.Laforgia M., Calabrò C., Scattone A., Laface C., Porcelli M., Gadaleta C.D., Nardulli P., Ranieri G. Pharmacotherapy in Mast Cell Leukemia. Expert Opin. Pharmacother. 2020;21:1059–1069. doi: 10.1080/14656566.2020.1744566. [DOI] [PubMed] [Google Scholar]
- 94.Laforgia M., Marech I., Nardulli P., Calabrò C., Gadaleta C.D., Ranieri G. An evaluation of masitinib for treating systemic mastocytosis. Expert Opin. Pharmacother. 2019;20:1539–1550. doi: 10.1080/14656566.2019.1645121. [DOI] [PubMed] [Google Scholar]
- 95.Waheed A., Purvey S., Saif M.W. Masitinib in treatment of pancreatic cancer. Expert Opin. Pharmacother. 2018;19:759–764. doi: 10.1080/14656566.2018.1459566. [DOI] [PubMed] [Google Scholar]
- 96.Marech I., Ammendola M., Sacco R., Sammarco G., Zuccalà V., Zizzo N., Leporini C., Luposella M., Patruno R., Filippelli G., et al. Tumour-associated macrophages correlate with microvascular bed extension in colorectal cancer patients. J. Cell. Mol. Med. 2016;20:1373–1380. doi: 10.1111/jcmm.12826. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are included in the article.