Abstract
It was first demonstrated in the late 19th century that human deaths from fever were typically due to infections. As the germ theory gained ground, it replaced the old, unproven theory that deaths from fever reflected a weak personal or even familial constitution. A new enigma emerged at the turn of the 20th century, when it became apparent that only a small proportion of infected individuals die from primary infections with almost any given microbe. Classical genetics studies gradually revealed that severe infectious diseases could be driven by human genetic predisposition. This idea gained ground with the support of molecular genetics, in three successive, overlapping steps. First, many rare inborn errors of immunity were shown, from 1985 onward, to underlie multiple, recurrent infections with Mendelian inheritance. Second, a handful of rare and familial infections also segregating as Mendelian traits but striking humans resistant to other infections were deciphered molecularly from 1996 onward. Third, a growing number of rare or common sporadic infections were shown to result from monogenic, but not Mendelian, inborn errors from 2007 onward. A synthesis of the hitherto mutually exclusive germ and genetic theories is now in view.
Introduction
The continuous threat of human infectious diseases
Fevers killed about half of all humans before the age of 15 years worldwide for about 200,000 years of human evolution, until the late 19th century (1). Following the establishment of the germ theory of disease between 1865 and 1885 (2, 3), it became widely accepted that life-threatening fevers in humans were typically infectious. The conquests that followed the germ theory have gradually prolonged our life expectancy at birth, from about 20 to about 80 years (1). Indeed, hygiene, aseptic surgery, vaccines, serotherapy, and pharmacological treatments have successfully prevented or cured diverse viral, bacterial, fungal, and parasitic infections. However, it may not be sufficient to rely exclusively on these approaches to combat human infections, because (i) they have been unsuccessful for a number of deadly common infectious diseases, (ii) anti-infectious agents (and, to a lesser extent, vaccines) inevitably select resistant microbes by natural selection (at a rate potentially exceeding that at which we can develop new drug treatments and vaccines), (iii) new infectious diseases inevitably emerge (the transmission of which is facilitated by modern lifestyles) through inadvertent exposure to existing pathogens, particularly those present in animals, or the generation of new pathogens through genetic shifts. There are already a number of alarming signs, such as (i) the persistent failure to develop vaccines against HIV, tuberculosis, and malaria (4), (ii) the spread of drug-resistant Mycobacterium tuberculosis and pneumococcal serotypes not covered by current vaccines (5), and (iii) recent pandemics of coronaviruses, Ebola virus, and influenza viruses (6). The current intervention paradigm may therefore need to be upgraded. Microbe-centered approaches may not sustainably ensure that human life expectancy remains at current levels. It would be prudent to consider the arms race between humans and microorganisms as a long-term affair, and not to take our apparent success for granted.
Interindividual variability in the course of infection
The development of alternative approaches for preventing and treating infectious diseases would thus be timely. One promising avenue of research is rooted in the pathogenesis of infectious diseases themselves. Indeed, the key but neglected problem in the field of infectious diseases was posed at the turn of the 20th century, with the discovery of latent (non-replicating) and unapparent (replicating) infections in asymptomatic individuals (7). This led to the recognition that most infectious agents kill only a small proportion (8), and typically only a very small proportion of infected individuals. In this context, what is the root cause of life-threatening infectious diseases? This “infection enigma” is paradoxical (9), as the view that infectious diseases are understood is widespread in both lay and learned circles. The very names of these conditions proclaim their infectious nature, and, as such, they are seen as prototypical environmental conditions. However, the clinical variability between individuals infected with any given human pathogen is enormous, ranging from silent to fatal infections (8). Indeed, the colossal burden of infectious diseases until the late 19th century resulted largely from the diversity of infectious agents rather than from their individual virulence (10, 11). One child would die of smallpox, another of diphtheria, a third of malaria, and so on. Epidemics or pandemics would have modified these proportions only transiently. In a given historical period and geographic area, only a few “emerging” or “re-emerging” pathogens killed a sizeable proportion of infected individuals, such as the European plague in the Middle Ages (12). The proportion of the population wiped out only rarely exceeded 30% (13, 14). If death is a rare outcome of infection with most microbes, how can we explain the pathogenesis of lethal infectious diseases? Infectious agents trigger infectious diseases, and are necessary but not sufficient for their development. As René Dubos put it in 1955, we should have “second thoughts about the germ theory” (15).
History of the genetic theory of infectious diseases
Well-known factors conferring a predisposition to severe infectious diseases include acquired immunodeficiencies, whether caused by immunosuppressive drugs (e.g. in the context of autoimmunity or transplantation) (16), by microbes themselves (e.g. following infection with human immunodeficiency or measles virus) (17, 18), or severe conditions (e.g. cancers or malnutrition) (19, 20). In certain regions of the world, these risk factors account for a sizeable proportion of lethal infections. This observation itself actually suggests that severe infections in other patients without overt acquired immunodeficiency may be favored by other, covert immunodeficiencies. Indeed, any lethal infection, by definition, attests to an immunodeficiency of the particular patient during the specific encounter with the causal infectious agent. In principle, this immunodeficiency in an otherwise healthy human, apparently, but not actually “immunocompetent”, may be a consequence of qualitative or quantitative variation in the pathogen (the microbial theory), variations of the conditions of infection (the ecological theory), or variations of the adaptive immune response to related infectious antigens in the past and present (the immunological theory) (11). Somatic variations in cells other than T and B lymphocytes may also contribute to interindividual variability in host defense (a broad somatic theory). All these explanations have been validated, at least in specific instances, but none of them can fully explain interindividual variability in a given ecosystem, settlement, building, or household. Moreover, they are difficult to test at a large scale. We previously reviewed the history of a fourth theory (i.e. the genetic theory), according to which, severe infectious diseases can be due to inborn errors of immunity (IEI) (11, 21). We have discussed elsewhere the long gestation of this theory, from the turn of the 19th century onward, and the key advances made with classical genetics in the first half of the 20th century (8, 11).
The genetic theory from 1946 onward
In this review, we focus on the modern era (1946 – 2020), which is defined by cells and molecules, analyzing the successive overlapping steps leading to the model discussed here, according to which life-threatening infectious diseases of both children and adults can result from single-gene (monogenic) IEI rarely displaying complete penetrance (i.e. that are rarely Mendelian) (9–11, 21, 22). We will not review Mendelian conditions, such as sickle cell disease and cystic fibrosis, which immunologists do not generally “see” as IEI, although they are evidently inborn errors underlying lethal infections (23). This review will not deal with population-based association studies yielding modest relative risks either, as none of these studies has ever matched the 1954 discovery of the sickle cell trait increasing resistance to severe malaria by a factor of 10 (24). Finally, this review will also not cover the small number of fascinating examples of Mendelian resistance to infectious agents, with inherited deficiencies of DARC, CCR5, and FUT2 underlying resistance to Plasmodium vivax, human immunodeficiency virus, and norovirus, respectively (11, 25). Rightly or wrongly, these three lines of research have not been incorporated into the field of IEI. Instead, we will attempt to understand how gradual paradigm shifts have led to the current model. We discuss here how the seminal reports of the first IEI between 1946–1952 gradually led to the current notion that severe infectious diseases in humans can be due to single-gene IEI with incomplete penetrance. We suggest that this field has progressed toward a synthesis of the infectious (thesis) and genetic (antithesis) nature of severe fevers in three large steps, corresponding to three types of IEI, which we refer to as “primary immunodeficiencies” (PIDs) (the underlying genetic defects of which were discovered from 1985 onward), “Mendelian infections” (1996 onward), and “monogenic but not Mendelian infections” (2007 onward).
Step one: primary immunodeficiencies
The birth of primary immunodeficiencies
The blueprint for conventional IEI, commonly referred to as PIDs, is widely agreed to be the description of Bruton’s X-linked recessive (XR) agammaglobulinemia in 1952 (26–28) (Table 1; Figure 1). Patients with this condition had an immunological phenotype of serum agammaglobulinemia, which had only recently become detectable, with the advent of electrophoresis. Their infectious phenotype consisted of invasive pneumococcal and other pyogenic bacterial infections, which were recurrent and/or multiple, an observation made possible by the recent advent of antibiotics. These two phenotypes cosegregated as an XR trait. Severe congenital neutropenia had been described earlier, in 1950, in children with severe staphylococcal and other bacterial infections and congenital neutropenia, two phenotypes that cosegregated as an autosomal recessive (AR) trait (29, 30). Hereditary agranulocytosis was not listed in the international classification of PIDs until 1999, because neutrophils were studied by hematologists, not immunologists (31). It is telling that the field did not adopt “AR staphylococcal disease” or “XR pneumococcal disease” to describe these conditions; the emphasis was placed on immunological abnormalities, because they were thought to be causal and rarer than the corresponding infections. With hindsight, the first human IEI to be described was actually epidermodysplasia verruciformis, which was defined in 1946 as an AR form of disseminated warts caused by unidentified viruses (32). The lack of a detectable immunological or hematological phenotype prevented this dermatological condition from being seen as an IEI until 2004 (31). The identification of an immunological abnormality has historically been the key to the detection, designation and classification of PIDs, to a much greater extent than the associated clinical phenotype, infectious or otherwise. For example, IgA deficiency, which is common and often asymptomatic, was considered to be a PID, despite its low clinical penetrance, as soon as it was discovered in the late 1960s (33, 34). Moreover, hyper-IgE syndrome (HIES) was named after the high serum IgE levels observed in patients, which do not underlie the patients’ life-threatening infectious phenotype and may not even underlie their severe eczema (35).
Table 1.
Three categories of inborn errors of immunity (IEI) underlying severe infectious diseases
| Characteristics | Primary immunodeficienciesa | Mendelian infectionsb | Monogenic infectionsc |
|---|---|---|---|
| Number of patients | Known (intermediate) | Known (small) | Unknown (large ?) |
| Familial cases | Common | Common | Rare (sporadic) |
| Penetrance | High or complete | High or complete | Low |
| Age at onset | Children >> adults | Children > adults | Children or adults |
| Number of infectious agents | High | Single (or a few) | Single |
| Number of infectious episodes | High (acute or chronic) | Low or high | Low |
| Infectious diseases | Often rare, opportunistic | Rare, idiopathic | Common |
| Immunological abnormalities | Before gene discovery | After gene discovery | After gene discovery |
| Cell types involved | Leukocytes | Leukocytes or other cell types (e.g. keratinocytes and CIB1) | Leukocytes or other cell types (e.g. cortical neurons and TLR3) |
| Other clinical phenotypes | Common (autoimmunity, allergy, autoinflammation, cancer, others) | Rare (syndromic forms) | Very rare |
| Examples (see Figures) | AR SCID and variations in RAG1 XR agammaglobulinemia and BTK AD congenital neutropenia |
AR EV and variations in CIB1
XR EBV disease and SAP AD MSMD and IFNGR1 |
(AR) severe influenza pneumonitis and variations in IRF7 (XR) invasive pneumococcal disease and NEMO (AD) HSE and TLR3 |
Primary immunodeficiencies comprise > 400 monogenic IEI disrupting host defense against various infectious agents. They are also associated with overt immunological abnormalities. They typically display high or complete immunological and clinical penetrance.
Mendelian infections are 5 monogenic IEI that disrupt host defense against one or a few infectious agents. The infections were idiopathic until the discovery of disease-causing genes led to the recognition of immunological abnormalities. Their clinical penetrance is high or complete.
Monogenic infections comprise at least 10 monogenic IEI that disrupt host defense against one or a few infectious agents. These infections also typically remained idiopathic until the discovery of disease-causing genes. Their penetrance is low (hence their mode of inheritance in parenthesis), accounting for these infections being typically sporadic, as opposed to familial.
Figure 1.
Pedigrees for primary immunodeficiencies for the three modes of inheritance: Autosomal recessive (AR), with the example of severe combined immunodeficiency (SCID) due to RAG1 deficiency; X-linked recessive (XR), with the example of agammaglobulinemia due to BTK deficiency; Autosomal dominant (AD), with the example of severe congenital neutropenia due to ELANE deficiency. Primary immunodeficiencies (PIDs) are typically characterized by multiple infections, overt immunological abnormalities and a high penetrance (see Table 1).
Immunological phenotypes of primary immunodeficiencies
Other immunological phenotypes, such as the lack of a spleen (1956) (36), lymphocytes (1958) (37), or thymus (1965) (38), paved the way for the description of many more defects of the development of specific lymphocyte subsets, such as T and B lymphocytes, or CD4+ or CD8+ T lymphocytes (39, 40). This, in turn, led to the description of functional deficiencies of lymphocytes in other patients with normal lymphoid development. Following the discovery of neutropenia, chronic granulomatous disease and other quantitative or qualitative inborn errors of myeloid cells were also progressively discovered. The many Mendelian disorders of complement were also soon considered as PIDs, from 1965 onward, even though most complement proteins are synthesized in the liver by hepatocytes (23). The IUIS classification of PIDs is still structured on the basis of these immunological phenotypes, which have accompanied the development of the field of IEI for 70 years. The successive classifications have also been structured around the innate/adaptive dichotomy. This immunological concept, which holds at the cellular level, with adaptive immunity restricted to T and B cells, does not translate easily to the genetic level, as very few immune system genes can be said to be purely “adaptive” (i.e. expressed and functional only in T or B cells), and only slightly more can be considered purely “innate” (i.e. expressed only in other types of circulating or tissue leukocytes). Most PIDs affect both innate and adaptive leukocytes. It is even harder to adhere to this dichotomy genetically if innate immunity is considered in its broad sense, encompassing both leukocytes and other cell types. Indeed, a growing number of genetic etiologies have been shown to affect both leukocytes and other cell types. Moreover, genetic defects of non-hematopoietic cells may profoundly impair the development of certain leukocytes, as neatly illustrated by inborn errors of the thymic stroma, which prevent the development of T cells (38, 41).
Infectious phenotypes of primary immunodeficiencies
The clinical phenotypes of PIDs are typically infectious, the term “immunodeficiency” indicating an insufficient capacity to fend off microorganisms. They are unusual in their breadth, with multiple and recurrent infections, including viral, bacterial, fungal, and parasitic infections, and also in their depth, with often severe and sometimes rare infections (42). Bruton’s index case had suffered from 19 episodes of invasive pneumococcal disease, each of which had been cured by antibiotics (26). Until antibiotics became available, the first life-threatening bacterial infection in such patients was almost invariably lethal; recovery from these infections revealed a completely new and unsuspected phenotype in a small proportion of cases. Most of the infectious diseases seen in patients with PIDs are not natural phenotypes; they occur both thanks to and despite medical intervention. The infections of PID patients are both recurrent and multiple, whereas most individuals without suspected PID typically suffer from one, or at most two or three life-threatening infections in their lifetime, very rarely caused by the same microbe. The infections of PID patients are also often rare and unusual, sometimes “opportunistic”, i.e. considered to occur preferentially or almost exclusively in patients with a detectable immunological abnormality (whether inherited or acquired) (42). Severe infections that are rare (or opportunistic), recurrent, or multiple are almost universally deemed sufficient to prompt the search for immunological deficits in the patient, and a family history of these deficits leads to a search for Mendelian inheritance (Figure 1). Once infectious and immunological phenotypes have been connected, in sporadic cases, and even more so by Mendelian inheritance in multiplex kindreds, a PID is suspected, and diagnosed or discovered. This field has been extraordinarily successful, with probably more than 300 of the 430 known IEI meeting this definition (39, 40, 43).
Other clinical phenotypes of PIDs
Before moving on to a discussion of the mechanisms of infection in patients with PIDs, it is worth pointing out that the field of IEI in general has also grown horizontally, with the discovery of four other major categories of clinical phenotypes: autoimmunity, autoinflammation, allergy, and cancer. The first autoinflammatory phenotype to be described was probably angioneurotic edema in 1965 (44, 45); the first allergy was probably HIES in 1974 (35, 46); the first autoimmunity phenotype was probably complement deficiency in 1972 (47, 48); and the first cancer was probably ataxia-telangiectasia in 1964 (49). The description of cancers in patients with PIDs did not call into question the status of these conditions as PIDs. Many such cancers are virus-driven, at least those favored by the immunological disorder itself, wheras most of the others are due to DNA repair disorders that compromise both immunity and cell growth. Allergy, autoimmunity, and autoinflammation, occasionally referred to collectively as “immunological dysregulation”, recently led the community to embrace the broader term IEI, in preference to PID (39, 40, 50). Each of these three phenotypic categories comprises an enormous and growing range of phenotypes. For example, autoinflammation encompasses conditions as diverse as type I interferonopathies and the excessive production of IL-1β (51–54). The genetic analysis of “immunological dysregulation” has led to genetic conclusions, in terms of the genetic architecture of clinical phenotypes, similar to those in the field of infectious diseases. The idea has emerged that a severe clinical phenotype related to infection, (virus-driven) cancer, allergy, autoimmunity, or autoinflammation, may be due, at the population level, to rare variants with a strong impact, with individual patients having monogenic lesions displaying high-level, but seldom complete penetrance. In other words, there is often genetic heterogeneity but physiological homogeneity (9). Again, type I interferonopathies neatly illustrate this notion (51, 54). The genetic dissection of IEI other than those underlying infections has had a considerable immunological and clinical impact, but a detailed consideration of these IEI lies beyond the scope of this review.
The pathogenesis of infectious diseases inferred from PIDs
Studies of PIDs can be used to decipher the mechanisms of immunity to infections in natural conditions (55, 56). This approach also provides unique insight into the mechanisms of disease in patients without IEI (e.g. patients infected with human immunodeficiency virus) (57). Many PIDs affect the core antigen-specific reactivity of adaptive immunity, either directly through an impact on T or B cells, or indirectly, by modifying the development or function of subsets of antigen-presenting myeloid cells. This is consistent with the diversity and recurrence of the infectious phenotype, because both the capacity to recognize a broad range of microbial antigens and immunological memory are disrupted. The severity of these infections, some of which are caused by weakly pathogenic microbes, also illustrates the key role played by adaptive immunity. Many PIDs impair both innate and adaptive leukocytes, and sometimes other cell types too, as reported for NEMO mutations (23, 58). Some PIDs, such as severe congenital neutropenia, selectively affect innate leukocytes, elegantly revealing the essential and redundant roles of these cells in host defense (25, 59). We have also recently observed an increase in the number of PIDs affecting predominantly innate (leukocytic) or intrinsic (non-leukocytic) immunity. Collectively, the infections seen in patients with PIDs suggest a role for specific cell subsets or molecular pathways in host defense against a particular type of pathogens. It is, therefore, possible to infer general mechanisms of protective immunity from the list of IEI underlying any given infection. Due to space constraints, we cannot review here all the many human pathogens, and previous reviews have dealt with PIDs disrupting immunity to mycobacteria (60, 61), pneumococcus (62), herpes simplex virus 1 (HSV-1) (63), Epstein-Barr virus (EBV) (64), human papillomaviruses (HPVs) (65), and Candida (66). As an example, studies of the considerable genetic diversity of PIDs underlying invasive pneumococcal disease revealed a common immunological mechanism. The opsonization of pneumococci by Abs or complement, and their destruction by splenic macrophages constitute the essential mechanism of anti-pneumococcal immunity (62, 67).
An immunological lesson from PIDs: redundancy
PIDs have taught us that severe infections can be genetic, and that a given infection can be favored by various genetic deficiencies, which are often immunologically related (9). The other side of the coin is that PIDs have also provided evidence that many genes, even those governing the development of normally abundant leukocyte subsets, display surprisingly high levels of redundancy in host defense, as inferred from the infections not seen in the patients with the corresponding deficiency (25). This aspect was uncertain when only a few patients had been studied, but has become increasingly well-established with studies of large numbers of patients of diverse ancestries living in a multitude of environments. For example, humans without B cells can normally control the vast majority of common viruses, at least in countries with sufficient medical care for the diagnosis of such patients. Patients without autologous T cells suffer from a much broader range of infections, but nevertheless appear to be able to fend off a surprising number of common pathogens, such as herpes simplex virus in the brain and influenza virus in the lungs. Reviewing the infections observed in patients lacking other leukocyte subsets or their functions, or combinations of subsets, would go beyond the scope of this review. Suffice it to say that the genetic etiologies of PIDs have provided unique insight into the redundancy of specific cell types or molecular circuits, when PID-causing genes and their immunological consequences are considered one by one. The genes underlying PIDs display “low redundancy” relative to those underlying Mendelian or monogenic infections discussed below, which display “high redundancy” (25). However, comparisons with mouse models in this respect are already of interest, as there is much greater redundancy even in humans with PIDs infected in natural conditions than in the corresponding mutant inbred mice infected in experimental conditions (25, 68–71).
Identification of the genetic lesions underlying PIDs
It was the identification of molecular genetic lesions underlying PIDs that provided definitive proof that infections can be genetic. It has been known since 1952 that a lack of Ig at birth underlies recurrent, invasive pneumococcal disease, and that Abs are, therefore, required for protective immunity to pneumococcus (26–28). Studies of familial cases strongly suggested that agammaglobulinemia could be inherited as an XR trait, but this was not conclusively proven until the identification of BTK mutations in 1993 (72, 73). We do not have the space here to review all the discoveries of the molecular basis of PIDs to date. Key milestones included the discovery of the first mutated PID gene, ADA, in 1985 (74), and the discovery, by positional cloning, of CYBB in 1986 (75). The annual rate of discovery of PID genes is steadily increasing. These PIDs have provided proof-of-principle, not only that rare, “opportunistic” infections can be genetic (e.g. Pneumocystis pneumonitis), but also that more common infections can be genetic, because patients with these PIDs often suffer from such infections (e.g. invasive pneumococcal disease). Some children with PIDs conferring predispositions to various infections die from a single infection, occasionally also after a late onset, mimicking Mendelian or monogenic infections. For example, children can die in the course of a first episode of invasive pneumococcal disease due to T-cell deficiency (which would have predisposed them to other infections had they survived) or IRAK4 deficiency (which would have predisposed them to recurrences, and perhaps to staphylococcal disease had they survived) (76). It is difficult to overestimate the historical importance of PIDs in the development of the genetic theory of infectious diseases. These conditions have provided proof-of-principle that severe infectious diseases can be due to IEI (8, 21), not only in children with multiple, recurrent, rare infections, but also in children who died from their first common infection.
Step two: Mendelian infections
Idiopathic and Mendelian infections
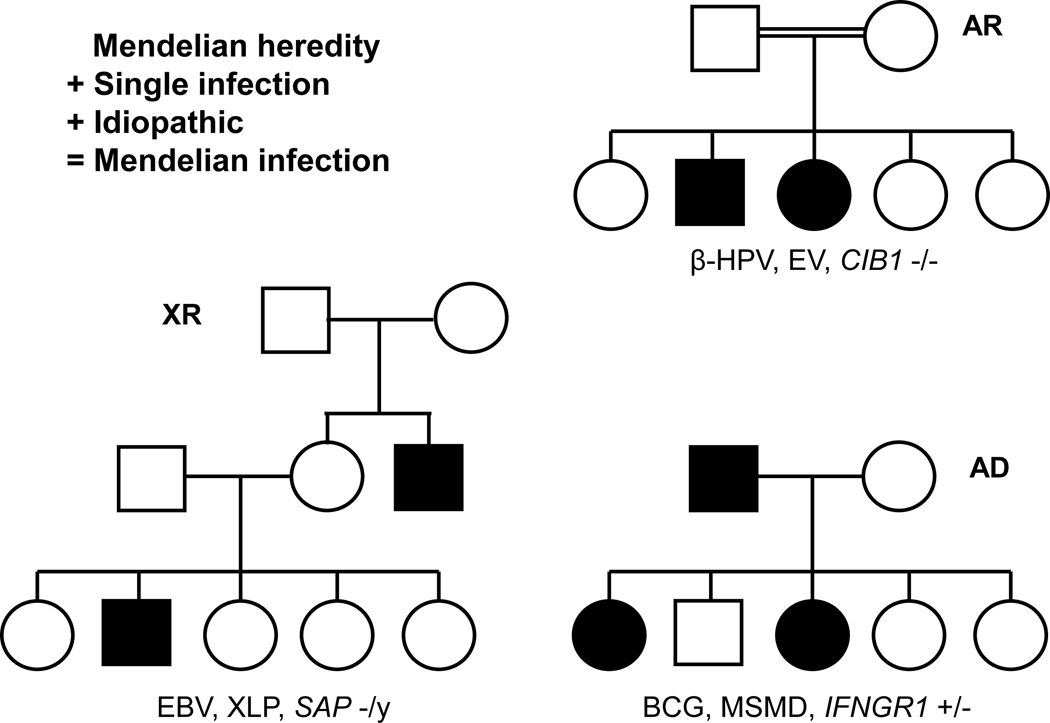
Mendelian inheritance characterizes a handful of infectious diseases that are very rare relative to the large number of infected individuals who remain asymptomatic individuals (e.g. clinical disease caused by weakly virulent BCG vaccines or environmental mycobacteria) (Table 1; Figure 2). This contrasts with the deceptively “Mendelian” occurrence of more common infectious diseases caused by more virulent microbes, which merely coincidentally reflect intrafamilial contagion (e.g. tuberculosis, caused by the more virulent Mycobacterium tuberculosis). The former were long considered “idiopathic” because the lack of an associated immunological abnormality left their pathogenesis unclear. Their frequent familial occurrence, with segregation resembling that of a Mendelian trait, raised the possibility of a genetic etiology. A remarkable feature of these “idiopathic” but Mendelian infections is that they strike otherwise healthy individuals with normal resistance to other infectious agents. The five known “idiopathic” Mendelian infections are (i) epidermodysplasia verruciformis (EV), which was known by 1946 to be an AR predisposition to cutaneous disease caused by viruses (32) identified in 1978 as beta-HPVs (β-HPVs) (77); (ii) Mendelian susceptibility to mycobacterial disease (MSMD), an AR, AD, or XR predisposition to BCG vaccines and environmental mycobacteria, known from 1951 onward (60, 78); (iii) invasive dermatophytic disease, an AR predisposition to severe disease caused by dermatophytic fungi, known from 1957 onward (79, 80); (iv) XR lymphoproliferation and related diseases caused by EBV, which were described from 1974 onward (81, 82); (v) chronic mucocutaneous candidiasis (CMC), an AR or AD condition marked by cutaneous and mucosal lesions due to the fungus Candida albicans, described from 1967 onward (80, 83). Four of these five groups of infectious agents infect almost all humans, typically without clinical consequences, and the remaining agent, EBV, infects more than 90% of individuals worldwide, typically causing self-healing infectious mononucleosis (84). Four of these five infectious diseases have also been described as “opportunistic” in other patients vulnerable to other infections, whereas invasive dermatophytic disease appears to be always isolated and “idiopathic”.
Figure 2.
Pedigrees for Mendelian infections, for the three modes of inheritance: Autosomal recessive (AR), with the example of epidermodysplasia verruciformis (EV) following β-Human papilloma virus (HPV) infection due to CIB1 deficiency; X-linked recessive (XR), with the example of X-lymphoproliferative disease (XLP) following Epstein-Barr virus (EBV) infection due to SAP deficiency; Autosomal dominant (AD), with the example of Mendelian susceptibility to mycobacterial diseases (MSMD) following BCG infection due to IFN-γR1 deficiency. Mendelian infections are typically characterized by severe, “idiopathic” infection with a single, rare pathogen, the absence of classic immunological abnormalities, and a high penetrance (see Table 1).
Epidermodysplasia verruciformis
EV was shown, from 1946 onward, to be an AR predisposition to skin-tropic β-HPVs causing flat warts, pityriasis-like lesions, and non-melanoma skin cancer (32, 85). Keratinocytes are the exclusive hosts of these viruses. The β-HPVs concerned are E5- and E8-deficient viruses, accounting for the lack of clinical manifestations in the general population (85). In its typical form, EV is isolated, with patients developing skin lesions only, beginning in the first two decades of life and persisting or recurring throughout the patient’s lifetime. We will not discuss here the “opportunistic” forms of EV seen in patients with T-cell PIDs before or after hematopoietic stem cell transplantation (86–88). Three genes, TMC6, TMC8, and CIB1, encoding EVER1, EVER2, and CIB1, respectively, are mutated in about 75% of patients with typical EV (89, 90). These genetic defects were discovered by genome-wide approaches from 2002 onward. The products of these genes form a complex in the cytoplasm of keratinocytes, as CIB1 is degraded in EVER-deficient cells (89). This complex probably operates as a restriction factor that can be overcome by HPVs encoding the E5 or E8 virulence factors, which interact with CIB1. Defective β-HPVs, lacking E5 or E8, may therefore cause disease only in rare individuals with genetic defects causing a lack of the EVER-CIB1 restriction factor. The first IEI to be described clinically, EV, was not added to the list of known IEI until 2004, because there was no immunological abnormality associated with these “idiopathic” β-HPV-driven lesions (21). Even now, in 2020, most immunologists do not consider keratinocytes to be immunological cells (23). EV may have provided the first evidence that non-hematopoietic, cell-intrinsic immunity to viruses can be life-saving (23). Interestingly, the EVER-CIB1 complex is not controlled by type I and III interferons (IFNs), consistent with the lack of β-HPV-driven lesions in patients with inborn errors of these IFNs (91–94). The study of EV also provided the first evidence of the oncogenicity of HPVs, before the implication of these viruses in cervical cancer (85). Very few conditions have contributed so much to biomedical knowledge.
X-lymphoproliferative disease and other IEI to Epstein-Barr virus
X-lymphoproliferative disease is another viral disease that can strike otherwise healthy patients upon primary infection. Initially described in the 1970s, this condition manifests as hemophagocytosis during the course of EBV infection, or as hypogammaglobulinemia or B-cell lymphoma at later time points (81, 82). The EBV causing XLP is of similar virulence to the EBV strains causing infection in the general population. The first family described clearly displayed XR inheritance, a finding subsequently confirmed by numerous reports. Patients have no particular clinical history before they encounter the virus. No immunological abnormality has been found in affected male patients diagnosed before EBV infection since the discovery of the causal gene, SAP, in 1998 (95–97). As the three post-infection phenotypes involved leukocytes, each in its own way, and despite the absence of an immunological phenotype before EBV infection, XLP was soon considered to be an IEI. The discovery of SAP defects by genome-wide approaches led to the recognition that SAP-expressing CD8+ T cells were crucial for the control of EBV-infected B cells (98, 99). This line of research turned out to be extremely fruitful. XIAP mutations found in other families presenting XR inheritance can also be pathogenic in the absence of EBV infection, through a different mechanism disrupting B-cell apoptosis (64, 100). AR deficiencies of CD27 (101), CD70 (102, 103), and ITK (104), which are more closely related to XR SAP deficiency, have also been shown to underlie severe EBV disease in otherwise healthy patients. Two of these gene products, CD27 and CD70, interact with each other, CD27 being expressed on the surface of B cells and CD70 on that of CD8+ T cells. ITK operates within T cells, downstream from CD70. These discoveries have shown that isolated, life-threatening EBV disease in otherwise healthy patients can be genetic. They have also revealed a key molecular mechanism by which human T cells control EBV-infected B cells, while suggesting that the SAP-CD70-CD27-ITK circuit is otherwise largely redundant in host defense.
Mendelian susceptibility to mycobacterial disease
The most thoroughly characterized Mendelian infection is caused by weakly pathogenic species of the Mycobacterium genus. The first cases of unexplained disease caused by the BCG vaccine in otherwise healthy children were probably reported in 1951 (105, 106). Affected patients are also vulnerable to environmental mycobacteria. As “idiopathic” BCG-osis and mycobacteriosis are often familial, with AR, AD, or XR modes of inheritance, this condition was designated Mendelian susceptibility to mycobacterial disease (MSMD) (60). However, the patients are also susceptible to a few other intramacrophagic pathogens, such as Salmonella (107). Since 1996, MSMD-causing mutations have been found in various genes encoding proteins involved in the control of IFN-γ immunity (78, 108–111). Some of these genetic defects impair the production of IFN-γ, whereas others impair the response to this cytokine. The IFN-γ response pathway is crucial in mononuclear phagocytes, but the cellular basis of MSMD in poor producers of IFN-γ is unclear, as many innate and adaptive lymphocyte subsets can produce this cytokine (112–114). These findings have had important clinical implications. They have made it possible to obtain a genetic diagnosis, to provide genetic counseling and to prevent or treat disease based on an understanding of its pathogenesis, with injections of IFN-γ in patients with at least a residual IFN-γ response pathway and hematopoietic stem cell transplantation in patients in whom cellular responses to IFN-γ are completely abolished (78, 115, 116). These findings also suggest that “opportunistic” mycobacterial disease in patients with other deficits, whether inherited or acquired, probably results from inadequate IFN-γ immunity. Immunologically, these findings show that IFN-γ acts less as an antiviral IFN and more as a macrophage-activating factor (117). Moreover, IFN-γ appears to be largely redundant for host defense against most intracellular microbes, despite its role as the Th1 signature cytokine in mice (118). This high level of redundancy remains surprising, perhaps more so than for host genes with mutations underlying β-HPV or EBV disease, if only because of the extensive studies of IFN-γ immunity in mice (69).
Chronic mucocutaneous candidiasis
Since 1969, a few patients have been reported to suffer from “idiopathic” CMC, segregating as an AD or AR trait (119, 120). CMC is defined as persistent or recurrent infection of the skin and nails, and oral, esophageal, and genital mucosae, by fungi of the genus Candida (121). It has long been known to occur in patients with acquired or inherited forms of CD4+ T-cell deficiency, who display multiple infections (121). By contrast, inherited forms of “idiopathic” CMC were typically thought to be isolated, although some patients also had staphylococcal lesions of the skin. Studies performed since 2011 have shown that some patients with isolated CMC carry mutations of the gene encoding IL-17F (which can form multimers with IL-17A) or its response pathway, with mutations affecting IL-17RA, IL-17RC, ACT1, or JNK1 (121–125). These discoveries were driven by previous studies identifying STAT3 mutations in a syndromic form of CMC known as hyper-IgE syndrome (HIES), and the identification of auto-Abs directed against IL-17A/F in patients with a monogenic form of autoimmunity with CMC (66, 126, 127). Three other, related forms of syndromic CMC were subsequently reported, due to biallelic loss-of-function mutations of RORC (128) or ZNF341 (129), and monoallelic gain-of-function mutations of STAT1 (130). Overall, the emerging picture is one of IL-17A/F-dependent immunity mediated by IL-17RA/RC being essential for protective mucocutaneous immunity to Candida albicans (and, to a lesser extent, Staphylococcus aureus), but otherwise redundant in host defense. The cellular basis of CMC in these patients remains unclear, as multiple subsets of innate and adaptive lymphocytes can normally produce IL-17A/F, and many non-hematopoietic cell types respond to these cytokines. These studies revealed that “Th17” immunity plays a narrower role in humans infected in natural conditions than in inbred mice challenged by experimental infections (68). These studies also suggest that CMC results from impaired IL-17A/F immunity in patients with human immunodeficiency virus infection or other T-cell deficiencies.
Invasive dermatophytic disease
The fifth and last Mendelian infection considered here is another cutaneous and fungal infection, at least in the early stages of disease. Dermatophytes are fungi that often cause benign, cutaneous infections; in very rare cases, as first reported in 1957, they cause invasive disease, reaching deep organs such as the bones, liver, and brain (79, 80). Such invasive diseases are not seen in patients with PIDs and multiple infections. These fungi have not been reported to be “opportunistic”. They paradoxically strike otherwise healthy patients, who can reach the age of 70 after decades of severe disease caused by one specific microorganism whilst displaying normal resistance to countless other infectious agents. All patients with invasive dermatophytic disease studied carry biallelic mutations of CARD9 (80, 131, 132). This is thus the only example of genetic homogeneity among the five Mendelian infections. Perhaps partly for this reason, the immunological mechanism underlying invasive dermatophytic disease remains unclear, but it is thought to involve the CARD9-dependent responses of phagocytes to the engagement of their receptors by fungi (133). It also remains unclear whether all mutations are loss-of-function and whether they affect all CARD9-dependent pathways equally. Remarkably, many other CARD9-deficient patients have been reported to present with invasive diseases caused by other fungi, including Candida, Aspergillus, Phialophora, Exophiala, Corynesprora, Aureobasidium, and Ochroconis, all of which belong to the phylum Ascomycota (133, 134). Surprisingly, some patients experience their first clinical manifestations late in adulthood, with an entirely unexpected severe fungal infection caused by a common fungus. Intriguingly, each CARD9-deficient patient develops a single fungal disease. The mechanisms governing the nature of the fungal infection do not seem to depend on CARD9 genotype. The search for the cellular and molecular basis of invasive dermatophytic disease and other fungal diseases in CARD9-deficient patients should yield novel immunological and clinical insight.
Lessons from Mendelian infections
The very small group of Mendelian infections has played a crucial role in the history of this field, providing chronological and intellectual bridges to two much larger groups of conditions: the PIDs first described clinically in the 1950s and molecularly from 1985 onward, and the “monogenic infections” first described clinically and molecularly as such from 2007 onward. The disease-causing genes for the few infections known to segregate as Mendelian traits in some families from 1946 onward were discovered from 1996 onward, attracting more attention to these conditions. These five infections were, thus, no longer “idiopathic”. Their Mendelian inheritance had a molecular basis, in turn pointing to an immunological mechanism. The infectious manifestations were seen as a phenotype, and this approach elegantly led to the discovery of a genotype, in turn facilitating the dissection of a molecular, cellular, and immunological mechanism. With the benefit of hindsight, these five Mendelian infections are reminiscent of inborn errors of the terminal components of complement, which selectively underlie meningococcal disease and were characterized clinically and immunologically in the 1970s, with their genetic lesions discovered two decades later (21, 135). These disorders did not usher in an era of research into the genetic basis of isolated infections, because their discovery did not follow the testing of the hypothesis that meningococcal disease could have a monogenic basis (8, 21). The five Mendelian infections reviewed here proved, beyond any reasonable doubt, that a human could be highly, selectively, and genetically vulnerable to a single infectious agent, over the course of an entire lifetime, while successfully fending off countless other pathogens. Admittedly, only five such Mendelian infections have been identified, and they are all relatively rare. Nevertheless, they paved the way for a paradigm shift leading to the discovery of infections that were monogenic but not Mendelian.
Step three: Monogenic infections
From BCG-osis to tuberculosis
Could isolated, severe rare or common infections be monogenic, at least in some patients? As severe infections are more often sporadic than familial, and familial infections may reflect contagion, the underlying monogenic lesions, if any, were predicted to display incomplete penetrance (9, 21). What actually led to the testing of this idea was the discovery of Mendelian infections, and, in particular, the study of MSMD. The first step was the demonstration that AR IL-12Rβ1 deficiency displayed incomplete penetrance for the case-definition phenotype of MSMD (136, 137). Affected patients develop MSMD because the IL-12- and IL-23-dependent induction of IFN-γ is abolished. This surprising and unexplained observation, implying that MSMD is a misnomer for some of its etiologies, led to the discovery that some of these patients had retained vulnerability to the more virulent Mycobacterium tuberculosis (136, 137). Some children, adolescents, and even young adults with IL-12Rβ1 deficiency developed tuberculosis as their sole clinical phenotype (138–143). As IL-12Rβ1 deficiency is rare, found in no more than 1/500,000 births, it cannot be a common cause of human tuberculosis (140). AR TYK2 deficiency is another rare genetic etiology of MSMD, with incomplete penetrance, that also impairs the IL-12- and IL-23-dependent induction of IFN-γ and can underlie tuberculosis (144). We recently found particularly high levels of homozygosity for a common variant of TYK2 (P1104A) in both a heterogeneous cohort of TB patients from non-European countries endemic for TB (112), and in the large European UK Biobank cohort (145). This variant, the P1104A variant, is homozygous in ~1/600 Europeans and ~1/2,500 people from other countries outside East Asia and Subsaharan Africa. Homozygosity for P1104A is associated with a selective impairment of IL-23-dependent IFN-γ production (112). This genetic condition displays low penetrance for MSMD, like IL-23R deficiency (114), but high penetrance for TB (at least 50%) in endemic areas and appears to be responsible for about 1% of TB cases in populations of European descent. It is the first common monogenic cause of TB to be described. The study of BCG-osis has thus led to the discovery of both rare and common monogenic etiologies of TB.
Invasive pyogenic bacterial disease
The study of invasive pneumococcal disease provided a second example of a relatively common infection that turned out to be monogenic in some cases. Pneumococcus (Streptococcus pneumoniae) is a bacterium that infects almost all humans, but causes life-threatening, invasive disease only rarely (67). Known genetic etiologies include disorders of the phagocytosis of opsonized bacteria by splenic macrophages (62, 67). Patients with such defects are prone to multiple infections (PIDs), with the exception of those with isolated congenital asplenia, half of whom carry mutations causing hapoinsufficiency at the RPSA locus (146, 147). Studies of children with a classic PID — XR anhidrotic ectodermal dysplasia with immunodeficiency (148–150)— led to the discovery of mutations of NEMO, which in turn led to the discovery of AR IRAK4 and MyD88 deficiencies in patients with idiopathic pneumococcal disease, often associated with staphylococcal disease (151, 152). Patients with MyD88 or IRAK-4 deficiencies do not respond to TLR agonists (except TLR3), IL-1, IL-18, IL-33, or related cytokines. Remarkably, they suffer from invasive bacterial disease caused by pneumococcus, and/or, more rarely, Staphylococcus aureus (67). The molecular pathogenesis of their pneumococcal disease remains unclear, but AR TIRAP deficiency, which selectively impairs the TLR2- and TLR4-dependent activation of NF-kB, can underlie invasive staphylococcal disease (153). One index case had staphylococcal disease due to an impairment of TLR2-dependent responses to staphylococcal lipotechoic acid (LTA). However, in seven healthy relatives, this genetic defect remained clinically silent, due to the rescue of TLR2-dependent LTA recognition by circulating Abs against LTA. The index case developed no such Abs. An inborn error of TLR2 innate immunity to LTA is, therefore, rescued by adaptive Abs against LTA. Incomplete penetrance for staphylococcal disease in this kindred is explained not by germline modifiers, but by somatic, adaptive, immunological events. Overall, studies of congenital asplenia and of the TLR pathway have also suggested that monogenic but not Mendelian disorders can underlie isolated staphylococcal or pneumococcal disease.
Herpes simplex encephalitis
The question arose as to whether infections that are almost never “opportunistic” and almost always sporadic could be caused by monogenic lesions (Table 1). The first advance was made in 2007, with genetic studies of HSV-1 encephalitis (HSE) (154, 155). Patients with HSE generally develop this disease during primary infection with HSV-1, and are not particularly susceptible to any other infectious disease. This infection is neurotropic, both in terms of the end destination, the central nervous system (CNS), and in terms of the route, with the virus reaching the CNS via the olfactory (to the forebrain) or trigeminal nerves (to the brainstem) (156, 157). We discovered inborn errors of TLR3-mediated and IFNα/β- and IFN-γ-dependent immunity, which includes six forebrain HSE-causing genes (154–156, 158–162). Inherited TLR3 deficiency, which was discovered in 2007, displayed incomplete penetrance, unlike UNC93B deficiency, discovered one year earlier (154, 155). Subsequent studies showed that iPSC-derived cortical neurons from the patients displayed impaired CNS cell-intrinsic immunity to HSV-1, whereas their trigeminal neurons were not affected by TLR3 deficiency (163, 164). Moreover, the TLR3 pathway is redundant in leukocytes, accounting for the lack of dissemination of HSV-1 in the course of HSE (163). AD SNORA31 deficiency underlies forebrain HSE by mechanisms other than disruption of the TLR3 pathway (165). Collectively, these data suggested that childhood HSE could result from a diverse collection of CNS-intrinsic monogenic IEI. The mechanisms of incomplete penetrance are unknown. Finally, a severe incomplete form of AR DBR1 deficiency impairs RNA lariat debranching and underlies brainstem infection by at least three unrelated viruses, including HSV-1 and influenza virus (166). Cell-intrinsic immunity in fibroblasts is impaired, suggesting that the mechanism of disease also involves brainstem cell-intrinsic processes. This experiment of Nature surprisingly connected an RNA metabolism housekeeping gene with viral infections within a very narrow anatomical territory in the CNS. Overall, the study of viral encephalitis has revealed the importance of CNS-intrinsic immunity to viruses (23). These findings also provided proof-of-principle that sporadic, “non-opportunistic” infections can be caused by single-gene inborn errors of immunity.
Severe influenza pneumonitis
Having discovered the first genetic basis of HSE, and a genetic basis of brainstem influenza, we turned our attention to another relatively common viral disease, severe influenza pneumonitis (167). We hypothesized that life-threatening influenza pneumonitis striking otherwise healthy humans might result from a single-gene IEI. We first discovered an AR deficiency of IRF7, a transcription factor that amplifies the production of antiviral IFN-α/β and IFN-γ in both circulating plasmacytoid dendritic cells and iPSC-derived pulmonary epithelial cells (168). AD GATA2 deficiency, which impairs the development of plasmacytoid dendritic cells, is perhaps the only PID that can underlie severe influenza pneumonitis in the context of other infections (167, 169). The study of IRF7 deficiency paved the way for the discovery of AR IRF9 deficiency in another child with severe influenza (170). The patient’s cells did not form STAT1-STAT2-IRF9 trimers, known as ISGF3, in response to stimulation with IFN-α/β or IFN-γ. The penetrance of IRF7 and IRF9 deficiencies cannot be estimated from these families, as both these conditions were found in single patients. However, penetrance is probably incomplete, as another IRF9-deficient patient was subsequently reported to suffer from other viral infections (171). It is unclear whether AR defects of IFNAR1, IFNAR2, and STAT2 have not been implicated in severe influenza because of incomplete penetrance (in which case the description of more patients would reveal such cases) or because of the occurrence of compensatory mechanisms (e.g. the integrity of type III IFN in patients with IFNAR1 or IFNAR2 mutations) (91, 93, 167, 172). Finally, TLR3 mutations have also been found in patients with severe influenza pneumonitis, including the P554S variant, which has been reported in several HSE patients (173). The mechanism of disease probably involves an impairment of the production of anti-viral IFNs in pulmonary epithelial cells. HSE and influenza pneumonitis are, therefore, two severe infections that may be considered allelic at the TLR3 locus, with incomplete penetrance for both phenotypes accounting for the absence or rarity of dual infections.
Other monogenic infections
There are several infectious diseases for which only one genetic etiology has been discovered, often in a single patient. After showing that Kaposi sarcoma can occur in children with certain PIDs and multiple infections (174–177), we identified AR OX40 deficiency as the first genetic etiology of isolated, idiopathic Kaposi sarcoma (178). Other examples of single-gene infections include AR NLRP1 gain-of-function in patients with laryngeal HPV disease (179), AR IL-18BP deficiency in a patient with fulminant viral hepatitis (180), AR NOS2 deficiency in an adult with lethal cytomegalovirus primary infection at the age of 50 years (181), AR APOL1 deficiency in a patient with trypanosomiasis caused by a weakly virulent species (182), AR MDA5 deficiency in a patient with severe respiratory viral illnesses, caused by rhinovirus in particular (183), and MDA5 mutations in patients with other severe respiratory viral infections, caused by respiratory syncytial virus in particular (184), AD combined POLR3A and POLR3C deficiencies in patients with varicella zoster virus encephalitis (185), AD IRF4 deficiency in patients with Whipple’s disease due to Tropheryma whipplei (186), and AR IFNAR1, IFNAR2, or STAT2 deficiency in patients with isolated measles or yellow fever vaccine disease (91, 93, 172, 187). The molecular and cellular bases of disease in these patients are beyond the scope of this review, but the studies concerned provided ample evidence that life-threatening diseases striking otherwise healthy individuals with no relevant familial history in the course of primary infection, that cannot be explained by an overt comorbid condition, may be caused by a single-gene inborn error of immunity. The penetrance of most of these disorders is unknown, but is predicted to be typically incomplete (although exceptions are possible).
From Mendelian to monogenic infections and back again
It has long been known that some rare or even common infections can occur as the first and, sometimes, the last clinical manifestation in patients with PIDs. Such patients may die during this first infection, or be diagnosed and managed appropriately, which would be sufficient to mask the otherwise inevitable development of other infections. This alone could have been sufficient to spark the idea that sporadic, isolated infections, whether rare or common, and particularly those that are opportunistic in other patients, may also have a monogenic basis, but it did not. Even the more directly relevant observation that inborn errors of the terminal components of complement can underlie isolated meningococcal disease did not cause the connection to be made. These observations were reported, but not fully understood. The five Mendelian infections considered here opened up new horizons. Interestingly monogenic infections other than these five Mendelian infections are not always non-Mendelian, as they can show complete penetrance in some kindreds. Conversely, the five Mendelian infections can also present as sporadic infections, for various reasons, such as their occurrence in a single child (for recessive traits) or because a causal variant is de novo (for recessive and dominant traits). Moreover, some genetic etiologies of the five Mendelian infections do not display complete penetrance. For example, AR IFN-γR1 deficiency displays complete penetrance for MSMD by the age of five years, whereas AR IL-12Rβ1 deficiency displays only 50% penetrance in adults, and homozygosity for TYK2 P1104A has a penetrance of less than 5% (112, 136, 137, 145, 188). In some families, both sporadic and familial, rare or common, severe, isolated infections may be caused by Mendelian or non-Mendelian monogenic IEI. The classification of IEI as Mendelian of non-Mendelian monogenic derives from their study at population level, with most monogenic disorders probably showing incomplete penetrance in most, but not all affected families.
Genetic heterogeneity and physiological homogeneity
Different infectious diseases can be allelic at the same human locus. Different defects at the same locus may govern different infections, via different mechanisms. A good example is provided by STAT1, gain-of-function heterozygous mutations of which can underlie fungal infections (because of impaired IL-17 immunity), while loss-of-function heterozygous mutations can underlie mycobacterial infections (because of impaired IFN-γ immunity) (130, 189, 190). Alternatively, two infections can be favored by the same disorder and mechanism, and even the same genotype, as exemplified by HSE and influenza pneumonitis, both of which can be driven by AD TLR3 deficiency and even heterozygosity for the P554S allele (155, 173). In this case, incomplete penetrance accounts for the very rare overlap of the two infections in the same patient (191). Notwithstanding the genetic intersection between some infections (one locus, multiple infections), it is important to stress the apparently high level of genetic heterogeneity underlying the few severe infectious diseases studied to date (one infection, multiple loci). For example, there are already 31 genetic etiologies of MSMD, encompassing mutations of 16 genes (60, 78, 108, 109). This is understandable, as an enormous number of different cells are encountered by the pathogen between its first contact with the host and the host’s death. Another example is provided by the genetic heterogeneity of invasive pneumococcal disease, which is high among both PIDs and monogenic infections. However, children with isolated pneumococcal disease or isolated congenital asplenia display a high level of genetic homogeneity (67, 146, 147). For patients with MSMD or invasive pneumococcal disease, however, the genetic heterogeneity does not mask an equally high level of physiological homogeneity. All genetic etiologies of MSMD disrupt IFN-γ immunity, whereas all known genetic etiologies of pneumococcal disease impair the splenic phagocytosis of opsonized bacteria. Collectively, these findings suggest that life-threatening infectious diseases can each result from a collection of highly diverse and incompletely penetrant monogenic IEI that are physiologically connected (9).
Concluding remarks
Human genetic architecture of severe infectious diseases
If many or most severe infectious diseases of humans manifested as Mendelian traits, their genetic origin would have been discovered long ago. If only they were much more often familial than sporadic, their genetic origin would at least have been suspected, although familial clustering would probably have been attributed to contagion. Their sporadic nature, in the genetic sense of the term, together with their known environmental cause, the microbe, formed formidable obstacles to the conception of a hypothetical genetic architecture that would be both plausible and testable, and to the development of a rigorous experimental approach for testing this model. Severe infectious diseases were shown to contribute to the selection of alleles conferring resistance, as best illustrated by the spread of HbS in regions in which Plasmodium falciparum is endemic, for which candidate and genome-wide association studies based on common variants were unable to explain the human genetic basis of the severe infections tested (8, 145). Death from infection is, indeed, an “extreme” phenotype, and the one that really matters evolutionarily, particularly if it occurs before or during the reproductive years (192). This led to consideration of the idea that severe infections might be caused by monogenic lesions (21). The study of a handful of Mendelian infections connected the genetic study of PIDs with that of sporadic, isolated, idiopathic infections. A small, but growing range of diseases life-threatening in the course of primary infection and caused by various viruses, bacteria, fungi, and parasites, in otherwise healthy children, adolescents, and adults have been shown to be caused by monogenic IEI, mostly with incomplete penetrance (9, 10). Obviously, we are only just beginning to unravel the human genetic architecture of severe infectious diseases.
Upcoming challenges
Only a very small minority of severe infectious diseases are currently explained by monogenic lesions, and only for a very small proportion of cases, with the exception of MSMD, which is understood in about half the patients, and HSE, which is understood in about 5% of cases (9, 78, 156). There are probably novel forms of acquired immunodeficiency to be discovered, associated with aging, for example (8, 21, 193). However, the pursuit of the human genetic study of severe infectious diseases aims to establish a new paradigm. A genetic theory of infectious diseases requires a human genetic architecture of severe infectious diseases. In the jigsaw puzzle of the genetic basis of severe infections, only a very small number of pieces have been assembled as yet, and only a few of them are contiguous. An enormous amount of work lies ahead. At the moment, it seems that we can find monogenic lesions for almost any life-threatening infection studied in humans. As a first step, we will need to test all infections, one by one. We will then need to determine the proportion of life-threatening cases that are monogenic, infection by infection. This is a daunting task, even for an entire generation of scholars. We will then need to analyze the molecular and cellular mechanisms of incomplete penetrance (9, 191). Epistasis may be involved and digenic immunodeficiencies may underlie some infections. Somatic genetics may also be crucial, as shown for TIRAP deficiency (153). The environment itself, through the number of invading pathogens, for example, may influence clinical penetrance (191). These three steps will probably be undertaken in parallel. They will hopefully take us to the summit, i.e. a full understanding of the root cause of severe human infectious diseases. The outlook is certainly worth the effort. If validated, this theory would reconcile the germ theory and the genetic theory, which have structured physiology and pathology across all living species since 1866 and were thought to be mutually exclusive (8, 11, 21).
Immunological and medical implications
The genetic theory of infectious diseases has immunological implications, because it is based on the outcome of primary infection in natural conditions. The genetic studies involved provide an ideal way to define the function of host defense genes in natura, i.e. in the setting of a natural ecosystem (55, 56). In particular, they have revealed a much greater degree of redundancy in outbred humans in natural conditions of infection than in inbred mice in experimental conditions of infection (25). They have also revealed the importance of cell-intrinsic, non-hematopoietic immunity, scaling immunology up from the immune system to the whole organism (23). The clinical implications of this genetic model are also significant, as emerging pathogens and multidrug-resistant pathogens are posing new and challenging threats to humans. The genetic model allows rigorous molecular diagnosis and genetic counseling, based on the identification of disease-causing genetic lesions of known penetrance in families of diverse ancestries worldwide (194, 195). The prognosis of patients and their personalized treatment and follow-up can also be defined on the basis of the genetic lesion. Perhaps more importantly, these studies pave the way for novel therapies in vulnerable individuals with inborn errors (e.g. cytokine therapy, for example, in patients with genetic defects resulting in a deficiency of the cytokine concerned). Prevention or treatment with recombinant cytokines is based on the same principles as govern the use of insulin in diabetic patients. Such treatment is already being used, with children worldwide benefiting from treatment with IFN-γ, G-CSF, or other recombinant molecules. The success of the germ theory has been encouraging, but known infectious diseases have remained formidable killers, while new infectious diseases are emerging. In the long term, a full understanding of the pathogenesis of infectious diseases constitutes our best hope of controlling these diseases. The human genetic approach provides a novel path for developing more personalized strategies for preventing and treating infectious diseases. Archibald Garrod’s seminal concepts of “inborn errors” and “chemical individuality” (196) may find some of their best applications in the study of the individual genetic determinism of infectious diseases, as a complement to traditional strategies targeting the environmental component of infectious diseases.
Implications for non-infectious diseases
The study of IEI also led to the discovery of a growing diversity of monogenic and sometimes Mendelian forms of rare autoinflammatory, autoimmune, malignant, and allergic phenotypes (39, 40). More common autoinflammatory and autoimmune conditions may also result from single-gene IEI. We do not refer here to conditions of the elderly, such as rheumatoid arthritis, but to inflammatory and immunological conditions that strike young individuals and impair reproduction, such as systemic lupus erythematosus. Over the last two decades, various human conditions, rare or common, in fields as diverse as cardiology, neurology, gastroenterology, and nephrology, have been shown to be caused by monogenic lesions (197–202). The discoveries of Mendelian and monogenic infections in this context are not exceptional, but their well-known environmental determinism singles them out. Indeed, the human genetic study of severe infectious diseases can be seen as an exemplary study of host-environment interaction in health and disease. One of the strengths of these conditions is that the environmental trigger, the microbe, is much better known than for the vast majority, if not all other human conditions. There are other genetic conditions for which clinical expression is also dependent on an environmental trigger, including inborn errors of metabolism, in particular. We know, for example, that the clinical expression of phenylketonuria is dependent on dietary intake of phenylalanine (203). Phenylketonuria and HSE are, thus, similar conditions, in that they are intrinsically both genetic and environmental. These similarities raise questions about causality. We classically see phenylketonuria as being caused by tyrosine hydroxylase deficiency. Should we instead consider phenylalanine to be the cause of phenylketonuria, in a manner analogous to the way in which infectious agents are commonly seen in infectious diseases? Or should the human genetic lesions be seen as the cause of infectious diseases? Likewise, are allergens the “causes” of allergy? Is the peanut truly the cause of a child’s death from allergy? The genetic study of immunological conditions, including allergy and infection in particular, will undoubtedly challenge prevailing views about their pathogenesis, and perhaps about human conditions more generally.
Figure 3.
Pedigrees for monogenic infections, for the three modes of inheritance: Autosomal recessive (AR), with the example of severe influenza pneumonitis due to IRF7 deficiency; X-linked recessive (XR), with the example of invasive pneumococcal infection due to NEMO deficiency; Autosomal dominant (AD), with the example of herpes simplex virus-1 (HSV-1) encephalitis due to TLR3 deficiency. Monogenic infections are characterized by severe, unexplained infection with a single, common pathogen, the absence of classic immunological abnormalities, and a low penetrance. Vertical bars indicate healthy carriers of the deleterious genotype (see Table 1).
Acknowledgments
We thank senior administrative and scientific staff, postdoctoral workers, students, and technicians from the two branches of the Laboratory of Human Genetics of Infectious Diseases. The Laboratory of Human Genetics of Infectious Diseases is supported in part by institutional grants from INSERM, Paris Descartes University, St. Giles Foundation, the French Foundation for Medical Research (FRM) (EQU201903007798), the SCOR Corporate Foundation for Science, The Rockefeller University Center for Clinical and Translational Science grant number 8UL1TR001866 from the National Center for Research Resources and the National Center for Advancing Sciences (NCATS), National Institutes of Health grants (R01AI088364, R01NS072381, R37AI095983, P01AI061093, R21AI137371, R01AI127564, and U19AI111143), and grants from the French National Research Agency (ANR) under the “Investments for the future” program (ANR-10-IAHU-01).
References
- 1.Casanova JL, Abel L. 2005. Inborn errors of immunity to infection: the rule rather than the exception. J Exp Med 202: 197–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koch R 1882. Die Aetiologie der Tuberkulose. Berl Klin Wochenschr 19: 221–30 [Google Scholar]
- 3.Pasteur L 1922-1939. Oeuvres complètes de Louis Pasteur, réunies par Pasteur Vallery-Radot. Paris: Masson et Cie [Google Scholar]
- 4.Anonymous. 2019. Failure to vaccinate and vaccine failure. Nat Microbiol 4: 725. [DOI] [PubMed] [Google Scholar]
- 5.de Kraker ME, Stewardson AJ, Harbarth S. 2016. Will 10 Million People Die a Year due to Antimicrobial Resistance by 2050? PLoS Med 13: e1002184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain V, Duse A, Bausch DG. 2018. Planning for large epidemics and pandemics: challenges from a policy perspective. Curr Opin Infect Dis 31: 316–24 [DOI] [PubMed] [Google Scholar]
- 7.Nicolle C 1933. Les infections inapparentes. Scientia: 181–271 [Google Scholar]
- 8.Casanova JL. 2015. Human genetic basis of interindividual variability in the course of infection. Proc Natl Acad Sci U S A 112: E7118–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casanova JL, Abel L. 2020. The human genetic determinism of life-threatening infectious diseases: genetic heterogeneity and physiological homogeneity? Hum Genet: in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alcais A, Quintana-Murci L, Thaler DS, Schurr E, Abel L, Casanova JL. 2010. Life-threatening infectious diseases of childhood: single-gene inborn errors of immunity? Ann N Y Acad Sci 1214: 18–33 [DOI] [PubMed] [Google Scholar]
- 11.Casanova JL, Abel L. 2013. The genetic theory of infectious diseases: a brief history and selected illustrations. Annu Rev Genomics Hum Genet 14: 215–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raoult D, Mouffok N, Bitam I, Piarroux R, Drancourt M. 2013. Plague: history and contemporary analysis. J Infect 66: 18–26 [DOI] [PubMed] [Google Scholar]
- 13.Cairns J 1997. Matters of life and death : perspectives on public health, molecular biology, cancer, and the prospects for the human race. Princeton, N.J.: Princeton University Press. xi, 257 p. pp. [Google Scholar]
- 14.Dobson AP, Carper ER. 1996. Infectious Diseases and Human Population History: Throughout history the establishment of disease has been a side effect of the growth of civilization Bioscience 46: 115–26 [Google Scholar]
- 15.Dubos RJ. 1955. Second thoughts on the germ theory. Scientific American 192: 31–5 [Google Scholar]
- 16.Koo S, Marty FM, Baden LR. 2011. Infectious complications associated with immunomodulating biologic agents. Hematol Oncol Clin North Am 25: 117–38 [DOI] [PubMed] [Google Scholar]
- 17.Naniche D, Oldstone MB. 2000. Generalized immunosuppression: how viruses undermine the immune response. Cell Mol Life Sci 57: 1399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrova VN, Sawatsky B, Han AX, Laksono BM, Walz L, Parker E, Pieper K, Anderson CA, de Vries RD, Lanzavecchia A, Kellam P, von Messling V, de Swart RL, Russell CA. 2019. Incomplete genetic reconstitution of B cell pools contributes to prolonged immunosuppression after measles. Sci Immunol 4 [DOI] [PubMed] [Google Scholar]
- 19.Bourke CD, Berkley JA, Prendergast AJ. 2016. Immune Dysfunction as a Cause and Consequence of Malnutrition. Trends Immunol 37: 386–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yaguchi T, Kawakami Y. 2016. Cancer-induced heterogeneous immunosuppressive tumor microenvironments and their personalized modulation. Int Immunol 28: 393–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casanova JL. 2015. Severe infectious diseases of childhood as monogenic inborn errors of immunity. Proc Natl Acad Sci U S A 112: E7128–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borghesi A, Marzollo A, Michev A, Fellay J. 2020. Susceptibility to infection in early life: a growing role for human genetics. Hum Genet [DOI] [PubMed] [Google Scholar]
- 23.Zhang SY, Jouanguy E, Zhang Q, Abel L, Puel A, Casanova JL. 2019. Human inborn errors of immunity to infection affecting cells other than leukocytes: from the immune system to the whole organism. Curr Opin Immunol 59: 88–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allison AC. 1954. Protection afforded by sickle cell trait against subtertian malarian infection. BMJ 1: 290–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casanova JL, Abel L. 2018. Human genetics of infectious diseases: Unique insights into immunological redundancy. Semin Immunol 36: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruton OC. 1952. Agammaglobulinemia. Pediatrics 9: 722–8 [PubMed] [Google Scholar]
- 27.Bruton OC. 1962. A decade with agammaglobulinemia. J Pediatr 60: 672–6 [DOI] [PubMed] [Google Scholar]
- 28.Janeway CA, Apt L, Gitlin D. 1953. Agammaglobulinemia. Trans Assoc Am Physicians 66: 200–2 [PubMed] [Google Scholar]
- 29.Kostmann R 1950. Hereditär reticulos - en ny systemsjukdom. Svenska Läkartdin 47: 2861 [Google Scholar]
- 30.Kostmann R 1956. Infantile genetic agranulocytosis. Acta Pediatr. Scand. 45: 1–78 [PubMed] [Google Scholar]
- 31.Buckley RH. 2020. Conversations with Founders of the Field of Human Inborn Errors of Immunity. J Clin Immunol [DOI] [PubMed] [Google Scholar]
- 32.Lutz W 1946. A propos de l’épidermodysplasie verruciforme. Dermatologica 92: 30–43 [PubMed] [Google Scholar]
- 33.Crabbe PA, Heremans JF. 1967. Selective IgA deficiency with steatorrhea. A new syndrome. Am J Med 42: 319–26 [DOI] [PubMed] [Google Scholar]
- 34.Wang N, Hammarstrom L. 2012. IgA deficiency: what is new? Curr Opin Allergy Clin Immunol 12: 602–8 [DOI] [PubMed] [Google Scholar]
- 35.Zhang Q, Boisson B, Beziat V, Puel A, Casanova JL. 2018. Human hyper-IgE syndrome: singular or plural? Mamm Genome 29: 603–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Myerson RM, Koelle WA. 1956. Congenital absence of the spleen in an adult; report of a case associated with recurrent Waterhouse-Friderichsen syndrome. N Engl J Med 254: 1131–2 [DOI] [PubMed] [Google Scholar]
- 37.Hitzig WH, Biro Z, Bosch H, Huser HJ. 1958. [Agammaglobulinemia & alymphocytosis with atrophy of lymphatic tissue]. Helvetica paediatrica acta 13: 551–85 [PubMed] [Google Scholar]
- 38.DiGeorge AM. 1965. A new concept of the cellular basis of immunity. J. Pediatr. 67: 907–8 [Google Scholar]
- 39.Bousfiha A, Jeddane L, Picard C, Al-Herz W, Ailal F, Chatila T, Cunningham-Rundles C, Etzioni A, Franco JL, Holland SM, Klein C, Morio T, Ochs HD, Oksenhendler E, Puck J, Torgerson TR, Casanova JL, Sullivan KE, Tangye SG. 2020. Human Inborn Errors of Immunity: 2019 Update of the IUIS Phenotypical Classification. J Clin Immunol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tangye SG, Al-Herz W, Bousfiha A, Chatila T, Cunningham-Rundles C, Etzioni A, Franco JL, Holland SM, Klein C, Morio T, Ochs HD, Oksenhendler E, Picard C, Puck J, Torgerson TR, Casanova JL, Sullivan KE. 2020. Human Inborn Errors of Immunity: 2019 Update on the Classification from the International Union of Immunological Societies Expert Committee. J Clin Immunol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamazaki Y, Urrutia R, Franco LM, Giliani S, Zhang K, Alazami AM, Dobbs AK, Masneri S, Joshi A, Otaizo-Carrasquero F, Myers TG, Ganesan S, Bondioni MP, Ho ML, Marks C, Alajlan H, Mohammed RW, Zou F, Valencia CA, Filipovich AH, Facchetti F, Boisson B, Azzari C, Al-Saud BK, Al-Mousa H, Casanova JL, Abraham RS, Notarangelo LD. 2020. PAX1 is essential for development and function of the human thymus. Sci Immunol 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Casanova JL, Abel L. 2007. Primary immunodeficiencies: a field in its infancy. Science 317: 6179. [DOI] [PubMed] [Google Scholar]
- 43.Meyts I, Bosch B, Bolze A, Boisson B, Itan Y, Belkadi A, Pedergnana V, Moens L, Picard C, Cobat A, Bossuyt X, Abel L, Casanova JL. 2016. Exome and genome sequencing for inborn errors of immunity. J Allergy Clin Immunol 138: 957–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Austen KF, Sheffer AL. 1965. Detection of Hereditary Angioneurotic Edema by Demonstration of a Reduction in the Second Component of Human Complement. N Engl J Med 272: 649–56 [DOI] [PubMed] [Google Scholar]
- 45.Rosen FS, Pensky J, Donaldson V, Charache P. 1965. Hereditary Angioneurotic Edema: Two Genetic Variants. Science 148: 957–8 [DOI] [PubMed] [Google Scholar]
- 46.Hill HR, Quie PG. 1974. Raised serum-IgE levels and defective neutrophil chemotaxis in three children with eczema and recurrent bacterial infections. Lancet 1: 183–7 [DOI] [PubMed] [Google Scholar]
- 47.Agnello V, De Bracco MM, Kunkel HG. 1972. Hereditary C2 deficiency with some manifestations of systemic lupus erythematosus. J Immunol 108: 837–40 [PubMed] [Google Scholar]
- 48.Moncada B, Day NK, Good RA, Windhorst DB. 1972. Lupus-erythematosus-like syndrome with a familial defect of complement. N Engl J Med 286: 689–93 [DOI] [PubMed] [Google Scholar]
- 49.Peterson RD, Kelly WD, Good RA. 1964. Ataxia-Telangiectasia. Its Association with a Defective Thymus, Immunological-Deficiency Disease, and Malignancy. Lancet 1: 1189–93 [DOI] [PubMed] [Google Scholar]
- 50.Bousfiha A, Jeddane L, Picard C, Ailal F, Bobby Gaspar H, Al-Herz W, Chatila T, Crow YJ, Cunningham-Rundles C, Etzioni A, Franco JL, Holland SM, Klein C, Morio T, Ochs HD, Oksenhendler E, Puck J, Tang MLK, Tangye SG, Torgerson TR, Casanova JL, Sullivan KE. 2018. The 2017 IUIS Phenotypic Classification for Primary Immunodeficiencies. J Clin Immunol 38: 129–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crow YJ. 2011. Type I interferonopathies: a novel set of inborn errors of immunity. Ann N Y Acad Sci 1238: 91–8 [DOI] [PubMed] [Google Scholar]
- 52.Manthiram K, Zhou Q, Aksentijevich I, Kastner DL. 2017. The monogenic autoinflammatory diseases define new pathways in human innate immunity and inflammation. Nat Immunol 18: 832–42 [DOI] [PubMed] [Google Scholar]
- 53.Masters SL, Simon A, Aksentijevich I, Kastner DL. 2009. Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease (*). Annu Rev Immunol 27: 621–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodero MP, Crow YJ. 2016. Type I interferon-mediated monogenic autoinflammation: The type I interferonopathies, a conceptual overview. J Exp Med 213: 2527–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Casanova JL, Abel L. 2004. The human model: a genetic dissection of immunity to infection in natural conditions. Nat Rev Immunol 4: 55–66 [DOI] [PubMed] [Google Scholar]
- 56.Quintana-Murci L, Alcais A, Abel L, Casanova JL. 2007. Immunology in natura: clinical, epidemiological and evolutionary genetics of infectious diseases. Nat Immunol 8: 1165–71 [DOI] [PubMed] [Google Scholar]
- 57.Zhang Q, Frange P, Blanche S, Casanova JL. 2017. Pathogenesis of infections in HIV-infected individuals: insights from primary immunodeficiencies. Curr Opin Immunol 48: 122–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Picard C, Casanova JL, Puel A. 2011. Infectious diseases in patients with IRAK-4, MyD88, NEMO, or IkappaBalpha deficiency. Clin Microbiol Rev 24: 490–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Skokowa J, Dale DC, Touw IP, Zeidler C, Welte K. 2017. Severe congenital neutropenias. Nat Rev Dis Primers 3: 17032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Casanova JL, Abel L. 2002. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol 20: 581–620 [DOI] [PubMed] [Google Scholar]
- 61.Reichenbach J, Rosenzweig S, Doffinger R, Dupuis S, Holland SM, Casanova JL. 2001. Mycobacterial diseases in primary immunodeficiencies. Curr Opin Allergy Clin Immunol 1: 503–11 [DOI] [PubMed] [Google Scholar]
- 62.Picard C, Puel A, Bustamante J, Ku CL, Casanova JL. 2003. Primary immunodeficiencies associated with pneumococcal disease. Curr Opin Allergy Clin Immunol 3: 451–9 [DOI] [PubMed] [Google Scholar]
- 63.Sancho-Shimizu V, Zhang SY, Abel L, Tardieu M, Rozenberg F, Jouanguy E, Casanova JL. 2007. Genetic susceptibility to herpes simplex virus 1 encephalitis in mice and humans. Curr Opin Allergy Clin Immunol 7: 495–505 [DOI] [PubMed] [Google Scholar]
- 64.Tangye SG, Latour S. 2020. Primary immunodeficiencies reveal the molecular requirements for effective host defense against EBV infection. Blood [DOI] [PubMed] [Google Scholar]
- 65.Leiding JW, Holland SM. 2012. Warts and all: human papillomavirus in primary immunodeficiencies. J Allergy Clin Immunol 130: 1030–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Puel A, Cypowyj S, Marodi L, Abel L, Picard C, Casanova JL. 2012. Inborn errors of human IL-17 immunity underlie chronic mucocutaneous candidiasis. Curr Opin Allergy Clin Immunol 12: 616–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boisson B 2020. The genetic basis of pneumococcal and staphylococcal infections: inborn errors of human TLR and IL-1 R immunity. Hum Genet [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cypowyj S, Picard C, Marodi L, Casanova JL, Puel A. 2012. Immunity to infection in IL-17-deficient mice and humans. Eur J Immunol 42: 2246–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fortin A, Abel L, Casanova JL, Gros P. 2007. Host genetics of mycobacterial diseases in mice and men: forward genetic studies of BCG-osis and tuberculosis. Annu Rev Genomics Hum Genet 8: 163–92 [DOI] [PubMed] [Google Scholar]
- 70.von Bernuth H, Picard C, Puel A, Casanova JL. 2012. Experimental and natural infections in MyD88- and IRAK-4-deficient mice and humans. Eur J Immunol 42: 3126–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang SY, Herman M, Ciancanelli MJ, Perez de Diego R, Sancho-Shimizu V, Abel L, Casanova JL. 2013. TLR3 immunity to infection in mice and humans. Curr Opin Immunol 25: 19–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsukada S, Saffran DC, Rawlings DJ, Parolini O, Allen RC, Klisak I, Sparkes RS, Kubagawa H, Mohandas T, Quan S, et al. 1993. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell 72: 279–90 [DOI] [PubMed] [Google Scholar]
- 73.Vetrie D, Vorechovsky I, Sideras P, Holland J, Davies A, Flinter F, Hammarstrom L, Kinnon C, Levinsky R, Bobrow M, et al. 1993. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature 361: 226–33 [DOI] [PubMed] [Google Scholar]
- 74.Bonthron DT, Markham AF, Ginsburg D, Orkin SH. 1985. Identification of a point mutation in the adenosine deaminase gene responsible for immunodeficiency. J Clin Invest 76: 894–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Royer-Pokora B, Kunkel LM, Monaco AP, Goff SC, Newburger PE, Baehner RL, Cole FS, Curnutte JT, Orkin SH. 1986. Cloning the gene for an inherited human disorder--chronic granulomatous disease--on the basis of its chromosomal location. Nature 322: 32–8 [DOI] [PubMed] [Google Scholar]
- 76.Picard C, von Bernuth H, Ghandil P, Chrabieh M, Levy O, Arkwright PD, McDonald D, Geha RS, Takada H, Krause JC, Creech CB, Ku CL, Ehl S, Marodi L, Al-Muhsen S, Al-Hajjar S, Al-Ghonaium A, Day-Good NK, Holland SM, Gallin JI, Chapel H, Speert DP, Rodriguez-Gallego C, Colino E, Garty BZ, Roifman C, Hara T, Yoshikawa H, Nonoyama S, Domachowske J, Issekutz AC, Tang M, Smart J, Zitnik SE, Hoarau C, Kumararatne DS, Thrasher AJ, Davies EG, Bethune C, Sirvent N, de Ricaud D, Camcioglu Y, Vasconcelos J, Guedes M, Vitor AB, Rodrigo C, Almazan F, Mendez M, Arostegui JI, Alsina L, Fortuny C, Reichenbach J, Verbsky JW, Bossuyt X, Doffinger R, Abel L, Puel A, Casanova JL. 2010. Clinical features and outcome of patients with IRAK-4 and MyD88 deficiency. Medicine (Baltimore) 89: 403–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Orth G, Jablonska S, Favre M, Croissant O, Jarzabek-Chorzelska M, Rzesa G. 1978. Characterization of two types of human papillomaviruses in lesions of epidermodysplasia verruciformis. Proc Natl Acad Sci U S A 75: 1537–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bustamante J 2020. Mendelian susceptibility to mycobacterial disease: recent discoveries. Hum Genet [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Blank F, Schopflocher P, Poirier P, Riopelle JL. 1957. Extensive Trichophyton infections of about fifty years’ duration in two sisters. Dermatologica 115: 40–51 [DOI] [PubMed] [Google Scholar]
- 80.Li J, Vinh DC, Casanova JL, Puel A. 2017. Inborn errors of immunity underlying fungal diseases in otherwise healthy individuals. Curr Opin Microbiol 40: 46–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Purtilo DT, Cassel C, Yang JP. 1974. Letter: Fatal infectious mononucleosis in familial lymphohistiocytosis. N Engl J Med 291: 736. [DOI] [PubMed] [Google Scholar]
- 82.Purtilo DT, DeFlorio D Jr., Hutt LM, Bhawan J, Yang JP, Otto R, Edwards W. 1977. Variable phenotypic expression of an X-linked recessive lymphoproliferative syndrome. N Engl J Med 297: 1077–80 [DOI] [PubMed] [Google Scholar]
- 83.Chilgren RA, Quie PG, Meuwissen HJ, Hong R. 1967. Chronic mucocutaneous candidiasis, deficiency of delayed hypersensitivity, and selective local antibody defect. Lancet 2: 688–93 [DOI] [PubMed] [Google Scholar]
- 84.Smatti MK, Al-Sadeq DW, Ali NH, Pintus G, Abou-Saleh H, Nasrallah GK. 2018. Epstein-Barr Virus Epidemiology, Serology, and Genetic Variability of LMP-1 Oncogene Among Healthy Population: An Update. Front Oncol 8: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Orth G 2008. Host defenses against human papillomaviruses: lessons from epidermodysplasia verruciformis. Curr Top Microbiol Immunol 321: 59–83 [DOI] [PubMed] [Google Scholar]
- 86.Crequer A, Picard C, Patin E, D’Amico A, Abhyankar A, Munzer M, Debre M, Zhang SY, de Saint-Basile G, Fischer A, Abel L, Orth G, Casanova JL, Jouanguy E. 2012. Inherited MST1 deficiency underlies susceptibility to EV-HPV infections. PLoS One 7: e44010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Crequer A, Troeger A, Patin E, Ma CS, Picard C, Pedergnana V, Fieschi C, Lim A, Abhyankar A, Gineau L, Mueller-Fleckenstein I, Schmidt M, Taieb A, Krueger J, Abel L, Tangye SG, Orth G, Williams DA, Casanova JL, Jouanguy E. 2012. Human RHOH deficiency causes T cell defects and susceptibility to EV-HPV infections. J Clin Invest 122: 3239–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Laffort C, Le Deist F, Favre M, Caillat-Zucman S, Radford-Weiss I, Debre M, Fraitag S, Blanche S, Cavazzana-Calvo M, de Saint Basile G, de Villartay JP, Giliani S, Orth G, Casanova JL, Bodemer C, Fischer A. 2004. Severe cutaneous papillomavirus disease after haemopoietic stem-cell transplantation in patients with severe combined immune deficiency caused by common gammac cytokine receptor subunit or JAK-3 deficiency. Lancet 363: 2051–4 [DOI] [PubMed] [Google Scholar]
- 89.de Jong SJ, Crequer A, Matos I, Hum D, Gunasekharan V, Lorenzo L, Jabot-Hanin F, Imahorn E, Arias AA, Vahidnezhad H, Youssefian L, Markle JG, Patin E, D’Amico A, Wang CQF, Full F, Ensser A, Leisner TM, Parise LV, Bouaziz M, Maya NP, Cadena XR, Saka B, Saeidian AH, Aghazadeh N, Zeinali S, Itin P, Krueger JG, Laimins L, Abel L, Fuchs E, Uitto J, Franco JL, Burger B, Orth G, Jouanguy E, Casanova JL. 2018. The human CIB1-EVER1-EVER2 complex governs keratinocyte-intrinsic immunity to beta-papillomaviruses. J Exp Med 215: 2289–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ramoz N, Rueda LA, Bouadjar B, Montoya LS, Orth G, Favre M. 2002. Mutations in two adjacent novel genes are associated with epidermodysplasia verruciformis. Nat Genet 11: 11. [DOI] [PubMed] [Google Scholar]
- 91.Duncan CJ, Mohamad SM, Young DF, Skelton AJ, Leahy TR, Munday DC, Butler KM, Morfopoulou S, Brown JR, Hubank M, Connell J, Gavin PJ, McMahon C, Dempsey E, Lynch NE, Jacques TS, Valappil M, Cant AJ, Breuer J, Engelhardt KR, Randall RE, Hambleton S. 2015. Human IFNAR2 deficiency: Lessons for antiviral immunity. Sci Transl Med 7: 307ra154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schaffer AA, Noyan F, Perro M, Diestelhorst J, Allroth A, Murugan D, Hatscher N, Pfeifer D, Sykora KW, Sauer M, Kreipe H, Lacher M, Nustede R, Woellner C, Baumann U, Salzer U, Koletzko S, Shah N, Segal AW, Sauerbrey A, Buderus S, Snapper SB, Grimbacher B, Klein C. 2009. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med 361: 2033–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hernandez N, Bucciol G, Moens L, Le Pen J, Shahrooei M, Goudouris E, Shirkani A, Changi-Ashtiani M, Rokni-Zadeh H, Sayar EH, Reisli I, Lefevre-Utile A, Zijlmans D, Jurado A, Pholien R, Drutman S, Belkaya S, Cobat A, Boudewijns R, Jochmans D, Neyts J, Seeleuthner Y, Lorenzo-Diaz L, Enemchukwu C, Tietjen I, Hoffmann HH, Momenilandi M, Poyhonen L, Siqueira MM, de Lima SMB, de Souza Matos DC, Homma A, Maia MLS, da Costa Barros TA, de Oliveira PMN, Mesquita EC, Gijsbers R, Zhang SY, Seligman SJ, Abel L, Hertzog P, Marr N, Martins RM, Meyts I, Zhang Q, MacDonald MR, Rice CM, Casanova JL, Jouanguy E, Bossuyt X. 2019. Inherited IFNAR1 deficiency in otherwise healthy patients with adverse reaction to measles and yellow fever live vaccines. J Exp Med 216: 2057–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huang Z, Peng K, Li X, Zhao R, You J, Cheng X, Wang Z, Wang Y, Wu B, Wang H, Zeng H, Yu Z, Zheng C, Wang Y, Huang Y. 2017. Mutations in Interleukin-10 Receptor and Clinical Phenotypes in Patients with Very Early Onset Inflammatory Bowel Disease: A Chinese VEO-IBD Collaboration Group Survey. Inflamm Bowel Dis 23: 578–90 [DOI] [PubMed] [Google Scholar]
- 95.Coffey AJ, Brooksbank RA, Brandau O, Oohashi T, Howell GR, Bye JM, Cahn AP, Durham J, Heath P, Wray P, Pavitt R, Wilkinson J, Leversha M, Huckle E, Shaw-Smith CJ, Dunham A, Rhodes S, Schuster V, Porta G, Yin L, Serafini P, Sylla B, Zollo M, Franco B, Bolino A, Seri M, Lanyi A, Davis JR, Webster D, Harris A, Lenoir G, de St Basile G, Jones A, Behloradsky BH, Achatz H, Murken J, Fassler R, Sumegi J, Romeo G, Vaudin M, Ross MT, Meindl A, Bentley DR. 1998. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nature Genet 20: 129–35 [DOI] [PubMed] [Google Scholar]
- 96.Nichols KE, Harkin DP, Levitz S, Krainer M, Kolquist KA, Genovese C, Bernard A, Ferguson M, Zuo L, Snyder E, Buckler AJ, Wise C, Ashley J, Lovett M, Valentine MB, Look AT, Gerald W, Housman DE, Haber DA. 1998. Inactivating mutations in an SH2 domain-encoding gene in X-linked lymphoproliferative syndrome. Proc Natl Acad Sci U S A 95: 13765–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sayos J, Wu C, Morra M, Wang N, Zhang X, Allen D, van Schaik S, Notarangelo L, Geha R, Roncarolo MG, Oettgen H, De Vries JE, Aversall G, Terhorst C. 1998. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature 395: 462–9 [DOI] [PubMed] [Google Scholar]
- 98.Palendira U, Low C, Bell AI, Ma CS, Abbott RJ, Phan TG, Riminton DS, Choo S, Smart JM, Lougaris V, Giliani S, Buckley RH, Grimbacher B, Alvaro F, Klion AD, Nichols KE, Adelstein S, Rickinson AB, Tangye SG. 2012. Expansion of somatically reverted memory CD8+ T cells in patients with X-linked lymphoproliferative disease caused by selective pressure from Epstein-Barr virus. J Exp Med 209: 913–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Palendira U, Low C, Chan A, Hislop AD, Ho E, Phan TG, Deenick E, Cook MC, Riminton DS, Choo S, Loh R, Alvaro F, Booth C, Gaspar HB, Moretta A, Khanna R, Rickinson AB, Tangye SG. 2011. Molecular pathogenesis of EBV susceptibility in XLP as revealed by analysis of female carriers with heterozygous expression of SAP. PLoS Biol 9: e1001187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rigaud S, Fondaneche MC, Lambert N, Pasquier B, Mateo V, Soulas P, Galicier L, Le Deist F, Rieux-Laucat F, Revy P, Fischer A, De Saint Basile G, Latour S. 2006. XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature 444: 110–4 [DOI] [PubMed] [Google Scholar]
- 101.van Montfrans JM, Hoepelman AI, Otto S, van Gijn M, van de Corput L, de Weger RA, Monaco-Shawver L, Banerjee PP, Sanders EA, Jol-van der Zijde CM, Betts MR, Orange JS, Bloem AC, Tesselaar K. 2012. CD27 deficiency is associated with combined immunodeficiency and persistent symptomatic EBV viremia. J Allergy Clin Immunol 129: 787–93 e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Abolhassani H, Edwards ES, Ikinciogullari A, Jing H, Borte S, Buggert M, Du L, Matsuda-Lennikov M, Romano R, Caridha R, Bade S, Zhang Y, Frederiksen J, Fang M, Bal SK, Haskologlu S, Dogu F, Tacyildiz N, Matthews HF, McElwee JJ, Gostick E, Price DA, Palendira U, Aghamohammadi A, Boisson B, Rezaei N, Karlsson AC, Lenardo MJ, Casanova JL, Hammarstrom L, Tangye SG, Su HC, Pan-Hammarstrom Q. 2017. Combined immunodeficiency and Epstein-Barr virus-induced B cell malignancy in humans with inherited CD70 deficiency. J Exp Med 214: 91–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Izawa K, Martin E, Soudais C, Bruneau J, Boutboul D, Rodriguez R, Lenoir C, Hislop AD, Besson C, Touzot F, Picard C, Callebaut I, de Villartay JP, Moshous D, Fischer A, Latour S. 2017. Inherited CD70 deficiency in humans reveals a critical role for the CD70-CD27 pathway in immunity to Epstein-Barr virus infection. J Exp Med 214: 73–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huck K, Feyen O, Niehues T, Ruschendorf F, Hubner N, Laws HJ, Telieps T, Knapp S, Wacker HH, Meindl A, Jumaa H, Borkhardt A. 2009. Girls homozygous for an IL-2-inducible T cell kinase mutation that leads to protein deficiency develop fatal EBV-associated lymphoproliferation. J Clin Invest 119: 1350–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Casanova JL, Jouanguy E, Lamhamedi S, Blanche S, Fischer A. 1995. Immunological conditions of children with BCG disseminated infection. Lancet 346: 581. [DOI] [PubMed] [Google Scholar]
- 106.Mimouni J 1951. Notre expérience de trois années de vaccination à Constantine; étude de 25 cas de complications. Alger Médicale 55: 1138–47 [PubMed] [Google Scholar]
- 107.Bustamante J, Boisson-Dupuis S, Abel L, Casanova JL. 2014. Mendelian susceptibility to mycobacterial disease: Genetic, immunological, and clinical features of inborn errors of IFN-gamma immunity. Semin Immunol 26: 454–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kerner G, Rosain J, Guerin A, AlKhabaz A, Oleaga-Quintas C, Rapaport F, Massaad MJ, Ding JY, Khan T, Al Ali F, Rahman M, Deswarte C, Martinez-Barricarte R, Geha RS, Jeanne-Julien V, Garcia DST, Chi CY, Yang R, Roynard M, Fleckenstein B, Rozenberg F, Boisson-Dupuis S, Ku CL, Seeleuthner Y, Beziat V, Marr N, Abel L, Al-Herz W, Casanova JL, Bustamante J. 2020. Inherited human IFNgamma deficiency underlies mycobacterial disease. J Clin Invest [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rosain J, Kong XF, Martinez-Barricarte R, Oleaga-Quintas C, Ramirez-Alejo N, Markle J, Okada S, Boisson-Dupuis S, Casanova JL, Bustamante J. 2019. Mendelian susceptibility to mycobacterial disease: 2014–2018 update. Immunol Cell Biol 97: 360–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rosenzweig SD, Holland SM. 2005. Defects in the interferon-gamma and interleukin-12 pathways. Immunol Rev 203: 38–47 [DOI] [PubMed] [Google Scholar]
- 111.Wu UI, Holland SM. 2015. Host susceptibility to non-tuberculous mycobacterial infections. Lancet Infect Dis 15: 968–80 [DOI] [PubMed] [Google Scholar]
- 112.Boisson-Dupuis S, Ramirez-Alejo N, Li Z, Patin E, Rao G, Kerner G, Lim CK, Krementsov DN, Hernandez N, Ma CS, Zhang Q, Markle J, Martinez-Barricarte R, Payne K, Fisch R, Deswarte C, Halpern J, Bouaziz M, Mulwa J, Sivanesan D, Lazarov T, Naves R, Garcia P, Itan Y, Boisson B, Checchi A, Jabot-Hanin F, Cobat A, Guennoun A, Jackson CC, Pekcan S, Caliskaner Z, Inostroza J, Costa-Carvalho BT, de Albuquerque JAT, Garcia-Ortiz H, Orozco L, Ozcelik T, Abid A, Rhorfi IA, Souhi H, Amrani HN, Zegmout A, Geissmann F, Michnick SW, Muller-Fleckenstein I, Fleckenstein B, Puel A, Ciancanelli MJ, Marr N, Abolhassani H, Balcells ME, Condino-Neto A, Strickler A, Abarca K, Teuscher C, Ochs HD, Reisli I, Sayar EH, El-Baghdadi J, Bustamante J, Hammarstrom L, Tangye SG, Pellegrini S, Quintana-Murci L, Abel L, Casanova JL. 2018. Tuberculosis and impaired IL-23-dependent IFN-gamma immunity in humans homozygous for a common TYK2 missense variant. Sci Immunol 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kong XF, Martinez-Barricarte R, Kennedy J, Mele F, Lazarov T, Deenick EK, Ma CS, Breton G, Lucero KB, Langlais D, Bousfiha A, Aytekin C, Markle J, Trouillet C, Jabot-Hanin F, Arlehamn CSL, Rao G, Picard C, Lasseau T, Latorre D, Hambleton S, Deswarte C, Itan Y, Abarca K, Moraes-Vasconcelos D, Ailal F, Ikinciogullari A, Dogu F, Benhsaien I, Sette A, Abel L, Boisson-Dupuis S, Schroder B, Nussenzweig MC, Liu K, Geissmann F, Tangye SG, Gros P, Sallusto F, Bustamante J, Casanova JL. 2018. Disruption of an antimycobacterial circuit between dendritic and helper T cells in human SPPL2a deficiency. Nat Immunol 19: 973–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Martinez-Barricarte R, Markle JG, Ma CS, Deenick EK, Ramirez-Alejo N, Mele F, Latorre D, Mahdaviani SA, Aytekin C, Mansouri D, Bryant VL, Jabot-Hanin F, Deswarte C, Nieto-Patlan A, Surace L, Kerner G, Itan Y, Jovic S, Avery DT, Wong N, Rao G, Patin E, Okada S, Bigio B, Boisson B, Rapaport F, Seeleuthner Y, Schmidt M, Ikinciogullari A, Dogu F, Tanir G, Tabarsi P, Bloursaz MR, Joseph JK, Heer A, Kong XF, Migaud M, Lazarov T, Geissmann F, Fleckenstein B, Arlehamn CL, Sette A, Puel A, Emile JF, van de Vosse E, Quintana-Murci L, Di Santo JP, Abel L, Boisson-Dupuis S, Bustamante J, Tangye SG, Sallusto F, Casanova JL. 2018. Human IFN-gamma immunity to mycobacteria is governed by both IL-12 and IL-23. Sci Immunol 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Alangari AA, Al-Zamil F, Al-Mazrou A, Al-Muhsen S, Boisson-Dupuis S, Awadallah S, Kambal A, Casanova JL. 2011. Treatment of disseminated mycobacterial infection with high-dose IFN-gamma in a patient with IL-12Rbeta1 deficiency. Clin Dev Immunol 2011: 691956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Holland SM. 2001. Immunotherapy of mycobacterial infections. Semin Respir Infect 16: 47–59 [DOI] [PubMed] [Google Scholar]
- 117.Nathan CF, Murray HW, Wiebe ME, Rubin BY. 1983. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med 158: 670–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schroder K, Hertzog PJ, Ravasi T, Hume DA. 2004. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol 75: 163–89 [DOI] [PubMed] [Google Scholar]
- 119.Canales L, Middlemas RO 3rd, Louro JM, South MA. 1969. Immunological observations in chronic mucocutaneous candidiasis. Lancet 2: 567–71 [DOI] [PubMed] [Google Scholar]
- 120.Wells RS. 1970. Chronic oral candidiasis (autosomal recessive inheritance) (three cases). Proc R Soc Med 63: 890–1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, Migaud M, Israel L, Chrabieh M, Audry M, Gumbleton M, Toulon A, Bodemer C, El-Baghdadi J, Whitters M, Paradis T, Brooks J, Collins M, Wolfman NM, Al-Muhsen S, Galicchio M, Abel L, Picard C, Casanova JL. 2011. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science 332: 65–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Boisson B, Wang C, Pedergnana V, Wu L, Cypowyj S, Rybojad M, Belkadi A, Picard C, Abel L, Fieschi C, Puel A, Li X, Casanova JL. 2013. An ACT1 mutation selectively abolishes interleukin-17 responses in humans with chronic mucocutaneous candidiasis. Immunity 39: 676–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Levy R, Okada S, Beziat V, Moriya K, Liu C, Chai LY, Migaud M, Hauck F, Al Ali A, Cyrus C, Vatte C, Patiroglu T, Unal E, Ferneiny M, Hyakuna N, Nepesov S, Oleastro M, Ikinciogullari A, Dogu F, Asano T, Ohara O, Yun L, Della Mina E, Bronnimann D, Itan Y, Gothe F, Bustamante J, Boisson-Dupuis S, Tahuil N, Aytekin C, Salhi A, Al Muhsen S, Kobayashi M, Toubiana J, Abel L, Li X, Camcioglu Y, Celmeli F, Klein C, AlKhater SA, Casanova JL, Puel A. 2016. Genetic, immunological, and clinical features of patients with bacterial and fungal infections due to inherited IL-17RA deficiency. Proc Natl Acad Sci U S A 113: E8277–E85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li J, Ritelli M, Ma CS, Rao G, Habib T, Corvilain E, Bougarn S, Cypowyj S, Grodecka L, Levy R, Beziat V, Shang L, Payne K, Avery DT, Migaud M, Boucherit S, Boughorbel S, Guennoun A, Chrabieh M, Rapaport F, Bigio B, Itan Y, Boisson B, Cormier-Daire V, Syx D, Malfait F, Zoppi N, Abel L, Freiberger T, Dietz HC, Marr N, Tangye SG, Colombi M, Casanova JL, Puel A. 2019. Chronic mucocutaneous candidiasis and connective tissue disorder in humans with impaired JNK1-dependent responses to IL-17A/F and TGF-beta. Sci Immunol 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ling Y, Cypowyj S, Aytekin C, Galicchio M, Camcioglu Y, Nepesov S, Ikinciogullari A, Dogu F, Belkadi A, Levy R, Migaud M, Boisson B, Bolze A, Itan Y, Goudin N, Cottineau J, Picard C, Abel L, Bustamante J, Casanova JL, Puel A. 2015. Inherited IL-17RC deficiency in patients with chronic mucocutaneous candidiasis. J Exp Med 212: 619–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kisand K, Boe Wolff AS, Podkrajsek KT, Tserel L, Link M, Kisand KV, Ersvaer E, Perheentupa J, Erichsen MM, Bratanic N, Meloni A, Cetani F, Perniola R, Ergun-Longmire B, Maclaren N, Krohn KJ, Pura M, Schalke B, Strobel P, Leite MI, Battelino T, Husebye ES, Peterson P, Willcox N, Meager A. 2010. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med 207: 299–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Puel A, Doffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C, Cobat A, Ouachee-Chardin M, Toulon A, Bustamante J, Al-Muhsen S, Al-Owain M, Arkwright PD, Costigan C, McConnell V, Cant AJ, Abinun M, Polak M, Bougneres PF, Kumararatne D, Marodi L, Nahum A, Roifman C, Blanche S, Fischer A, Bodemer C, Abel L, Lilic D, Casanova JL. 2010. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med 207: 291–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Okada S, Markle JG, Deenick EK, Mele F, Averbuch D, Lagos M, Alzahrani M, Al-Muhsen S, Halwani R, Ma CS, Wong N, Soudais C, Henderson LA, Marzouqa H, Shamma J, Gonzalez M, Martinez-Barricarte R, Okada C, Avery DT, Latorre D, Deswarte C, Jabot-Hanin F, Torrado E, Fountain J, Belkadi A, Itan Y, Boisson B, Migaud M, Arlehamn CS, Sette A, Breton S, McCluskey J, Rossjohn J, de Villartay JP, Moshous D, Hambleton S, Latour S, Arkwright PD, Picard C, Lantz O, Engelhard D, Kobayashi M, Abel L, Cooper AM, Notarangelo LD, Boisson-Dupuis S, Puel A, Sallusto F, Bustamante J, Tangye SG, Casanova JL. 2015. IMMUNODEFICIENCIES. Impairment of immunity to Candida and Mycobacterium in humans with bi-allelic RORC mutations. Science 349: 606–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Beziat V, Li J, Lin JX, Ma CS, Li P, Bousfiha A, Pellier I, Zoghi S, Baris S, Keles S, Gray P, Du N, Wang Y, Zerbib Y, Levy R, Leclercq T, About F, Lim AI, Rao G, Payne K, Pelham SJ, Avery DT, Deenick EK, Pillay B, Chou J, Guery R, Belkadi A, Guerin A, Migaud M, Rattina V, Ailal F, Benhsaien I, Bouaziz M, Habib T, Chaussabel D, Marr N, El-Benna J, Grimbacher B, Wargon O, Bustamante J, Boisson B, Muller-Fleckenstein I, Fleckenstein B, Chandesris MO, Titeux M, Fraitag S, Alyanakian MA, Leruez-Ville M, Picard C, Meyts I, Di Santo JP, Hovnanian A, Somer A, Ozen A, Rezaei N, Chatila TA, Abel L, Leonard WJ, Tangye SG, Puel A, Casanova JL. 2018. A recessive form of hyper-IgE syndrome by disruption of ZNF341-dependent STAT3 transcription and activity. Sci Immunol 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu L, Okada S, Kong XF, Kreins AY, Cypowyj S, Abhyankar A, Toubiana J, Itan Y, Audry M, Nitschke P, Masson C, Toth B, Flatot J, Migaud M, Chrabieh M, Kochetkov T, Bolze A, Borghesi A, Toulon A, Hiller J, Eyerich S, Eyerich K, Gulacsy V, Chernyshova L, Chernyshov V, Bondarenko A, Grimaldo RM, Blancas-Galicia L, Beas IM, Roesler J, Magdorf K, Engelhard D, Thumerelle C, Burgel PR, Hoernes M, Drexel B, Seger R, Kusuma T, Jansson AF, Sawalle-Belohradsky J, Belohradsky B, Jouanguy E, Bustamante J, Bue M, Karin N, Wildbaum G, Bodemer C, Lortholary O, Fischer A, Blanche S, Al-Muhsen S, Reichenbach J, Kobayashi M, Rosales FE, Lozano CT, Kilic SS, Oleastro M, Etzioni A, Traidl-Hoffmann C, Renner ED, Abel L, Picard C, Marodi L, Boisson-Dupuis S, Puel A, Casanova JL. 2011. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med 208: 1635–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Glocker EO, Hennigs A, Nabavi M, Schaffer AA, Woellner C, Salzer U, Pfeifer D, Veelken H, Warnatz K, Tahami F, Jamal S, Manguiat A, Rezaei N, Amirzargar AA, Plebani A, Hannesschlager N, Gross O, Ruland J, Grimbacher B. 2009. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med 361: 1727–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lanternier F, Pathan S, Vincent QB, Liu L, Cypowyj S, Prando C, Migaud M, Taibi L, Ammar-Khodja A, Boudghene Stambouli O, Guellil B, Jacobs F, Goffard JC, Schepers K, del Marmol V, Boussofara L, Denguezli M, Larif M, Bachelez H, Michel L, Lefranc G, Hay R, Jouvion G, Chretien F, Fraitag S, Bougnoux ME, Boudia M, Abel L, Lortholary O, Casanova JL, Picard C, Grimbacher B, Puel A. 2013. Deep dermatophytosis and inherited CARD9 deficiency. N Engl J Med 369: 1704–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Corvilain E, Casanova JL, Puel A. 2018. Inherited CARD9 Deficiency: Invasive Disease Caused by Ascomycete Fungi in Previously Healthy Children and Adults. J Clin Immunol 38: 656–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Drummond RA, Collar AL, Swamydas M, Rodriguez CA, Lim JK, Mendez LM, Fink DL, Hsu AP, Zhai B, Karauzum H, Mikelis CM, Rose SR, Ferre EM, Yockey L, Lemberg K, Kuehn HS, Rosenzweig SD, Lin X, Chittiboina P, Datta SK, Belhorn TH, Weimer ET, Hernandez ML, Hohl TM, Kuhns DB, Lionakis MS. 2015. CARD9-Dependent Neutrophil Recruitment Protects against Fungal Invasion of the Central Nervous System. PLoS Pathog 11: e1005293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hodeib S, Herberg JA, Levin M, Sancho-Shimizu V. 2020. Human genetics of meningococcal infections. Hum Genet [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.de Beaucoudrey L, Samarina A, Bustamante J, Cobat A, Boisson-Dupuis S, et al. 2010. Revisiting human IL-12Rbeta1 deficiency: a survey of 141 patients from 30 countries. Medicine (Baltimore) 89: 381–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fieschi C, Dupuis S, Catherinot E, Feinberg J, Bustamante J, Breiman A, Altare F, Baretto R, Le Deist F, Kayal S, Koch H, Richter D, Brezina M, Aksu G, Wood P, Al-Jumaah S, Raspall M, Da Silva Duarte AJ, Tuerlinckx D, Virelizier JL, Fischer A, Enright A, Bernhoft J, Cleary AM, Vermylen C, Rodriguez-Gallego C, Davies G, Blutters-Sawatzki R, Siegrist CA, Ehlayel MS, Novelli V, Haas WH, Levy J, Freihorst J, Al-Hajjar S, Nadal D, De Moraes Vasconcelos D, Jeppsson O, Kutukculer N, Frecerova K, Caragol I, Lammas D, Kumararatne DS, Abel L, Casanova JL. 2003. Low Penetrance, Broad Resistance, and Favorable Outcome of Interleukin 12 Receptor beta1 Deficiency: Medical and Immunological Implications. J Exp Med 197: 527–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Altare F, Ensser A, Breiman A, Reichenbach J, Baghdadi JE, Fischer A, Emile JF, Gaillard JL, Meinl E, Casanova JL. 2001. Interleukin-12 receptor beta1 deficiency in a patient with abdominal tuberculosis. J Infect Dis 184: 231–6. [DOI] [PubMed] [Google Scholar]
- 139.Boisson-Dupuis S 2020. The monogenic basis of human tuberculosis. Hum Genet [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Boisson-Dupuis S, El Baghdadi J, Parvaneh N, Bousfiha A, Bustamante J, Feinberg J, Samarina A, Grant AV, Janniere L, El Hafidi N, Hassani A, Nolan D, Najib J, Camcioglu Y, Hatipoglu N, Aydogmus C, Tanir G, Aytekin C, Keser M, Somer A, Aksu G, Kutukculer N, Mansouri D, Mahdaviani A, Mamishi S, Alcais A, Abel L, Casanova JL. 2011. IL-12Rbeta1 Deficiency in Two of Fifty Children with Severe Tuberculosis from Iran, Morocco, and Turkey. PLoS One 6: e18524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Caragol I, Raspall M, Fieschi C, Feinberg J, Larrosa MN, Hernandez M, Figueras C, Bertran JM, Casanova JL, Espanol T. 2003. Clinical tuberculosis in 2 of 3 siblings with interleukin-12 receptor beta1 deficiency. Clin Infect Dis 37: 302–6 [DOI] [PubMed] [Google Scholar]
- 142.Ozbek N, Fieschi C, Yilmaz BT, de Beaucoudrey L, Demirhan B, Feinberg J, Bikmaz YE, Casanova JL. 2005. Interleukin-12 receptor beta 1 chain deficiency in a child with disseminated tuberculosis. Clin Infect Dis 40: e55–8 [DOI] [PubMed] [Google Scholar]
- 143.Tabarsi P, Marjani M, Mansouri N, Farnia P, Boisson-Dupuis S, Bustamante J, Abel L, Adimi P, Casanova JL, Mansouri D. 2011. Lethal tuberculosis in a previously healthy adult with IL-12 receptor deficiency. J Clin Immunol 31: 537–9 [DOI] [PubMed] [Google Scholar]
- 144.Kreins AY, Ciancanelli MJ, Okada S, Kong XF, Ramirez-Alejo N, Kilic SS, El Baghdadi J, Nonoyama S, Mahdaviani SA, Ailal F, Bousfiha A, Mansouri D, Nievas E, Ma CS, Rao G, Bernasconi A, Sun Kuehn H, Niemela J, Stoddard J, Deveau P, Cobat A, El Azbaoui S, Sabri A, Lim CK, Sundin M, Avery DT, Halwani R, Grant AV, Boisson B, Bogunovic D, Itan Y, Moncada-Velez M, Martinez-Barricarte R, Migaud M, Deswarte C, Alsina L, Kotlarz D, Klein C, Muller-Fleckenstein I, Fleckenstein B, Cormier-Daire V, Rose-John S, Picard C, Hammarstrom L, Puel A, Al-Muhsen S, Abel L, Chaussabel D, Rosenzweig SD, Minegishi Y, Tangye SG, Bustamante J, Casanova JL, Boisson-Dupuis S. 2015. Human TYK2 deficiency: Mycobacterial and viral infections without hyper-IgE syndrome. J Exp Med 212: 1641–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kerner G, Ramirez-Alejo N, Seeleuthner Y, Yang R, Ogishi M, Cobat A, Patin E, Quintana-Murci L, Boisson-Dupuis S, Casanova JL, Abel L. 2019. Homozygosity for TYK2 P1104A underlies tuberculosis in about 1% of patients in a cohort of European ancestry. Proc Natl Acad Sci U S A 116: 10430–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bolze A, Boisson B, Bosch B, Antipenko A, Bouaziz M, Sackstein P, Chaker-Margot M, Barlogis V, Briggs T, Colino E, Elmore AC, Fischer A, Genel F, Hewlett A, Jedidi M, Kelecic J, Kruger R, Ku CL, Kumararatne D, Lefevre-Utile A, Loughlin S, Mahlaoui N, Markus S, Garcia JM, Nizon M, Oleastro M, Pac M, Picard C, Pollard AJ, Rodriguez-Gallego C, Thomas C, Von Bernuth H, Worth A, Meyts I, Risolino M, Selleri L, Puel A, Klinge S, Abel L, Casanova JL. 2018. Incomplete penetrance for isolated congenital asplenia in humans with mutations in translated and untranslated RPSA exons. Proc Natl Acad Sci U S A 115: E8007–E16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bolze A, Mahlaoui N, Byun M, Turner B, Trede N, Ellis SR, Abhyankar A, Itan Y, Patin E, Brebner S, Sackstein P, Puel A, Picard C, Abel L, Quintana-Murci L, Faust SN, Williams AP, Baretto R, Duddridge M, Kini U, Pollard AJ, Gaud C, Frange P, Orbach D, Emile JF, Stephan JL, Sorensen R, Plebani A, Hammarstrom L, Conley ME, Selleri L, Casanova JL. 2013. Ribosomal protein SA haploinsufficiency in humans with isolated congenital asplenia. Science 340: 976–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Boisson B, Honda Y, Ajiro M, Bustamante J, Bendavid M, Gennery AR, Kawasaki Y, Ichishima J, Osawa M, Nihira H, Shiba T, Tanaka T, Chrabieh M, Bigio B, Hur H, Itan Y, Liang Y, Okada S, Izawa K, Nishikomori R, Ohara O, Heike T, Abel L, Puel A, Saito MK, Casanova JL, Hagiwara M, Yasumi T. 2019. Rescue of recurrent deep intronic mutation underlying cell type-dependent quantitative NEMO deficiency. J Clin Invest 129: 583–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Courtois G, Smahi A, Reichenbach J, Doffinger R, Cancrini C, Bonnet M, Puel A, Chable-Bessia C, Yamaoka S, Feinberg J, Dupuis-Girod S, Bodemer C, Livadiotti S, Novelli F, Rossi P, Fischer A, Israel A, Munnich A, Le Deist F, Casanova JL. 2003. A hypermorphic IkappaBalpha mutation is associated with autosomal dominant anhidrotic ectodermal dysplasia and T cell immunodeficiency. J Clin Invest 112: 1108–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Doffinger R, Smahi A, Bessia C, Geissmann F, Feinberg J, Durandy A, Bodemer C, Kenwrick S, Dupuis-Girod S, Blanche S, Wood P, Rabia SH, Headon DJ, Overbeek PA, Le Deist F, Holland SM, Belani K, Kumararatne DS, Fischer A, Shapiro R, Conley ME, Reimund E, Kalhoff H, Abinun M, Munnich A, Israel A, Courtois G, Casanova JL. 2001. X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF-kappaB signaling. Nat Genet 27: 277–85. [DOI] [PubMed] [Google Scholar]
- 151.Picard C, Puel A, Bonnet M, Ku CL, Bustamante J, Yang K, Soudais C, Dupuis S, Feinberg J, Fieschi C, Elbim C, Hitchcock R, Lammas D, Davies G, Al-Ghonaium A, Al-Rayes H, Al-Jumaah S, Al-Hajjar S, Al-Mohsen IZ, Frayha HH, Rucker R, Hawn TR, Aderem A, Tufenkeji H, Haraguchi S, Day NK, Good RA, Gougerot-Pocidalo MA, Ozinsky A, Casanova JL. 2003. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science 299: 2076–9. [DOI] [PubMed] [Google Scholar]
- 152.von Bernuth H, Picard C, Jin Z, Pankla R, Xiao H, Ku CL, Chrabieh M, Mustapha IB, Ghandil P, Camcioglu Y, Vasconcelos J, Sirvent N, Guedes M, Vitor AB, Herrero-Mata MJ, Arostegui JI, Rodrigo C, Alsina L, Ruiz-Ortiz E, Juan M, Fortuny C, Yague J, Anton J, Pascal M, Chang HH, Janniere L, Rose Y, Garty BZ, Chapel H, Issekutz A, Marodi L, Rodriguez-Gallego C, Banchereau J, Abel L, Li X, Chaussabel D, Puel A, Casanova JL. 2008. Pyogenic bacterial infections in humans with MyD88 deficiency. Science 321: 691–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Israel L, Wang Y, Bulek K, Della Mina E, Zhang Z, Pedergnana V, Chrabieh M, Lemmens NA, Sancho-Shimizu V, Descatoire M, Lasseau T, Israelsson E, Lorenzo L, Yun L, Belkadi A, Moran A, Weisman LE, Vandenesch F, Batteux F, Weller S, Levin M, Herberg J, Abhyankar A, Prando C, Itan Y, van Wamel WJ, Picard C, Abel L, Chaussabel D, Li X, Beutler B, Arkwright PD, Casanova JL, Puel A. 2017. Human Adaptive Immunity Rescues an Inborn Error of Innate Immunity. Cell 168: 789–800 e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Casrouge A, Zhang SY, Eidenschenk C, Jouanguy E, Puel A, Yang K, Alcais A, Picard C, Mahfoufi N, Nicolas N, Lorenzo L, Plancoulaine S, Senechal B, Geissmann F, Tabeta K, Hoebe K, Du X, Miller RL, Heron B, Mignot C, de Villemeur TB, Lebon P, Dulac O, Rozenberg F, Beutler B, Tardieu M, Abel L, Casanova JL. 2006. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science 314: 308–12 [DOI] [PubMed] [Google Scholar]
- 155.Zhang SY, Jouanguy E, Ugolini S, Smahi A, Elain G, Romero P, Segal D, Sancho-Shimizu V, Lorenzo L, Puel A, Picard C, Chapgier A, Plancoulaine S, Titeux M, Cognet C, von Bernuth H, Ku CL, Casrouge A, Zhang XX, Barreiro L, Leonard J, Hamilton C, Lebon P, Heron B, Vallee L, Quintana-Murci L, Hovnanian A, Rozenberg F, Vivier E, Geissmann F, Tardieu M, Abel L, Casanova JL. 2007. TLR3 deficiency in patients with herpes simplex encephalitis. Science 317: 1522–7 [DOI] [PubMed] [Google Scholar]
- 156.Zhang SY. 2020. Herpes simplex virus encephalitis of childhood: inborn errors of central nervous system cell-intrinsic immunity. Hum Genet [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Abel L, Plancoulaine S, Jouanguy E, Zhang SY, Mahfoufi N, Nicolas N, Sancho-Shimizu V, Alcais A, Guo Y, Cardon A, Boucherit S, Obach D, Clozel T, Lorenzo L, Amsallem D, Berquin P, Blanc T, Bost-Bru C, Chabrier S, Chabrol B, Cheuret E, Dulac O, Evrard P, Heron B, Lazaro L, Mancini J, Pedespan JM, Rivier F, Vallee L, Lebon P, Rozenberg F, Casanova JL, Tardieu M. 2010. Age-dependent Mendelian predisposition to herpes simplex virus type 1 encephalitis in childhood. J Pediatr 157: 623–9, 9 e1 [DOI] [PubMed] [Google Scholar]
- 158.Andersen LL, Mork N, Reinert LS, Kofod-Olsen E, Narita R, Jorgensen SE, Skipper KA, Honing K, Gad HH, Ostergaard L, Orntoft TF, Hornung V, Paludan SR, Mikkelsen JG, Fujita T, Christiansen M, Hartmann R, Mogensen TH. 2015. Functional IRF3 deficiency in a patient with herpes simplex encephalitis. J Exp Med 212: 1371–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Guo Y, Audry M, Ciancanelli M, Alsina L, Azevedo J, Herman M, Anguiano E, Sancho-Shimizu V, Lorenzo L, Pauwels E, Philippe PB, Perez de Diego R, Cardon A, Vogt G, Picard C, Andrianirina ZZ, Rozenberg F, Lebon P, Plancoulaine S, Tardieu M, Valerie D, Jouanguy E, Chaussabel D, Geissmann F, Abel L, Casanova JL, Zhang SY. 2011. Herpes simplex virus encephalitis in a patient with complete TLR3 deficiency: TLR3 is otherwise redundant in protective immunity. J Exp Med 208: 2083–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Herman M, Ciancanelli M, Ou YH, Lorenzo L, Klaudel-Dreszler M, Pauwels E, Sancho-Shimizu V, Perez de Diego R, Abhyankar A, Israelsson E, Guo Y, Cardon A, Rozenberg F, Lebon P, Tardieu M, Heropolitanska-Pliszka E, Chaussabel D, White MA, Abel L, Zhang SY, Casanova JL. 2012. Heterozygous TBK1 mutations impair TLR3 immunity and underlie herpes simplex encephalitis of childhood. J Exp Med 209: 1567–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Perez de Diego R, Sancho-Shimizu V, Lorenzo L, Puel A, Plancoulaine S, Picard C, Herman M, Cardon A, Durandy A, Bustamante J, Vallabhapurapu S, Bravo J, Warnatz K, Chaix Y, Cascarrigny F, Lebon P, Rozenberg F, Karin M, Tardieu M, Al-Muhsen S, Jouanguy E, Zhang SY, Abel L, Casanova JL. 2010. Human TRAF3 adaptor molecule deficiency leads to impaired Toll-like receptor 3 response and susceptibility to herpes simplex encephalitis. Immunity 33: 400–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sancho-Shimizu V, Perez de Diego R, Lorenzo L, Halwani R, Alangari A, Israelsson E, Fabrega S, Cardon A, Maluenda J, Tatematsu M, Mahvelati F, Herman M, Ciancanelli M, Guo Y, AlSum Z, Alkhamis N, Al-Makadma AS, Ghadiri A, Boucherit S, Plancoulaine S, Picard C, Rozenberg F, Tardieu M, Lebon P, Jouanguy E, Rezaei N, Seya T, Matsumoto M, Chaussabel D, Puel A, Zhang SY, Abel L, Al-Muhsen S, Casanova JL. 2011. Herpes simplex encephalitis in children with autosomal recessive and dominant TRIF deficiency. J Clin Invest 121: 4889–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lafaille FG, Pessach IM, Zhang SY, Ciancanelli MJ, Herman M, Abhyankar A, Ying SW, Keros S, Goldstein PA, Mostoslavsky G, Ordovas-Montanes J, Jouanguy E, Plancoulaine S, Tu E, Elkabetz Y, Al-Muhsen S, Tardieu M, Schlaeger TM, Daley GQ, Abel L, Casanova JL, Studer L, Notarangelo LD. 2012. Impaired intrinsic immunity to HSV-1 in human iPSC-derived TLR3-deficient CNS cells. Nature 491: 769–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zimmer B, Ewaleifoh O, Harschnitz O, Lee YS, Peneau C, McAlpine JL, Liu B, Tchieu J, Steinbeck JA, Lafaille F, Volpi S, Notarangelo LD, Casanova JL, Zhang SY, Smith GA, Studer L. 2018. Human iPSC-derived trigeminal neurons lack constitutive TLR3-dependent immunity that protects cortical neurons from HSV-1 infection. Proc Natl Acad Sci U S A 115: E8775–E82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lafaille FG, Harschnitz O, Lee YS, Zhang P, Hasek ML, Kerner G, Itan Y, Ewaleifoh O, Rapaport F, Carlile TM, Carter-Timofte ME, Paquet D, Dobbs K, Zimmer B, Gao D, Rojas-Duran MF, Kwart D, Rattina V, Ciancanelli MJ, McAlpine JL, Lorenzo L, Boucherit S, Rozenberg F, Halwani R, Henry B, Amenzoui N, Alsum Z, Marques L, Church JA, Al-Muhsen S, Tardieu M, Bousfiha AA, Paludan SR, Mogensen TH, Quintana-Murci L, Tessier-Lavigne M, Smith GA, Notarangelo LD, Studer L, Gilbert W, Abel L, Casanova JL, Zhang SY. 2019. Human SNORA31 variations impair cortical neuron-intrinsic immunity to HSV-1 and underlie herpes simplex encephalitis. Nat Med 25: 1873–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang SY, Clark NE, Freije CA, Pauwels E, Taggart AJ, Okada S, Mandel H, Garcia P, Ciancanelli MJ, Biran A, Lafaille FG, Tsumura M, Cobat A, Luo J, Volpi S, Zimmer B, Sakata S, Dinis A, Ohara O, Garcia Reino EJ, Dobbs K, Hasek M, Holloway SP, McCammon K, Hussong SA, DeRosa N, Van Skike CE, Katolik A, Lorenzo L, Hyodo M, Faria E, Halwani R, Fukuhara R, Smith GA, Galvan V, Damha MJ, Al-Muhsen S, Itan Y, Boeke JD, Notarangelo LD, Studer L, Kobayashi M, Diogo L, Fairbrother WG, Abel L, Rosenberg BR, Hart PJ, Etzioni A, Casanova JL. 2018. Inborn Errors of RNA Lariat Metabolism in Humans with Brainstem Viral Infection. Cell 172: 952–65 e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang Q 2020. Human genetics of life-threatening influenza pneumonitis. Hum Genet [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ciancanelli MJ, Huang SX, Luthra P, Garner H, Itan Y, Volpi S, Lafaille FG, Trouillet C, Schmolke M, Albrecht RA, Israelsson E, Lim HK, Casadio M, Hermesh T, Lorenzo L, Leung LW, Pedergnana V, Boisson B, Okada S, Picard C, Ringuier B, Troussier F, Chaussabel D, Abel L, Pellier I, Notarangelo LD, Garcia-Sastre A, Basler CF, Geissmann F, Zhang SY, Snoeck HW, Casanova JL. 2015. Life-threatening influenza and impaired interferon amplification in human IRF7 deficiency. Science 348: 448–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sologuren I, Martinez-Saavedra MT, Sole-Violan J, de Borges de Oliveira E Jr., Betancor E, Casas I, Oleaga-Quintas C, Martinez-Gallo M, Zhang SY, Pestano J, Colobran R, Herrera-Ramos E, Perez C, Lopez-Rodriguez M, Ruiz-Hernandez JJ, Franco N, Ferrer JM, Bilbao C, Andujar-Sanchez M, Alvarez Fernandez M, Ciancanelli MJ, Rodriguez de Castro F, Casanova JL, Bustamante J, Rodriguez-Gallego C. 2018. Lethal Influenza in Two Related Adults with Inherited GATA2 Deficiency. J Clin Immunol 38: 513–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hernandez N, Melki I, Jing H, Habib T, Huang SSY, Danielson J, Kula T, Drutman S, Belkaya S, Rattina V, Lorenzo-Diaz L, Boulai A, Rose Y, Kitabayashi N, Rodero MP, Dumaine C, Blanche S, Lebras MN, Leung MC, Mathew LS, Boisson B, Zhang SY, Boisson-Dupuis S, Giliani S, Chaussabel D, Notarangelo LD, Elledge SJ, Ciancanelli MJ, Abel L, Zhang Q, Marr N, Crow YJ, Su HC, Casanova JL. 2018. Life-threatening influenza pneumonitis in a child with inherited IRF9 deficiency. J Exp Med 215: 2567–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bravo Garcia-Morato M, Calvo Apalategi A, Bravo-Gallego LY, Blazquez Moreno A, Simon-Fuentes M, Garmendia JV, Mendez Echevarria A, Del Rosal Rabes T, Dominguez-Soto A, Lopez-Granados E, Reyburn HT, Rodriguez Pena R. 2019. Impaired control of multiple viral infections in a family with complete IRF9 deficiency. J Allergy Clin Immunol 144: 309–12 e10 [DOI] [PubMed] [Google Scholar]
- 172.Hambleton S, Goodbourn S, Young DF, Dickinson P, Mohamad SM, Valappil M, McGovern N, Cant AJ, Hackett SJ, Ghazal P, Morgan NV, Randall RE. 2013. STAT2 deficiency and susceptibility to viral illness in humans. Proc Natl Acad Sci U S A 110: 3053–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lim HK, Huang SXL, Chen J, Kerner G, Gilliaux O, Bastard P, Dobbs K, Hernandez N, Goudin N, Hasek ML, Garcia Reino EJ, Lafaille FG, Lorenzo L, Luthra P, Kochetkov T, Bigio B, Boucherit S, Rozenberg F, Vedrinne C, Keller MD, Itan Y, Garcia-Sastre A, Celard M, Orange JS, Ciancanelli MJ, Meyts I, Zhang Q, Abel L, Notarangelo LD, Snoeck HW, Casanova JL, Zhang SY. 2019. Severe influenza pneumonitis in children with inherited TLR3 deficiency. J Exp Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jackson CC, Dickson MA, Sadjadi M, Gessain A, Abel L, Jouanguy E, Casanova JL. 2016. Kaposi Sarcoma of Childhood: Inborn or Acquired Immunodeficiency to Oncogenic HHV-8. Pediatr Blood Cancer 63: 392–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Camcioglu Y, Picard C, Lacoste V, Dupuis S, Akcakaya N, Cokura H, Kaner G, Demirkesen C, Plancoulaine S, Emile JF, Gessain A, Casanova JL. 2004. HHV-8-associated Kaposi sarcoma in a child with IFNgammaR1 deficiency. J Pediatr 144: 519–23 [DOI] [PubMed] [Google Scholar]
- 176.Picard C, Mellouli F, Duprez R, Chedeville G, Neven B, Fraitag S, Delaunay J, Le Deist F, Fischer A, Blanche S, Bodemer C, Gessain A, Casanova JL, Bejaoui M. 2006. Kaposi’s sarcoma in a child with Wiskott-Aldrich syndrome. Eur J Pediatr 165: 453–7 [DOI] [PubMed] [Google Scholar]
- 177.Byun M, Abhyankar A, Lelarge V, Plancoulaine S, Palanduz A, Telhan L, Boisson B, Picard C, Dewell S, Zhao C, Jouanguy E, Feske S, Abel L, Casanova JL. 2010. Whole-exome sequencing-based discovery of STIM1 deficiency in a child with fatal classic Kaposi sarcoma. J Exp Med 207: 2307–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Byun M, Ma CS, Akcay A, Pedergnana V, Palendira U, Myoung J, Avery DT, Liu Y, Abhyankar A, Lorenzo L, Schmidt M, Lim HK, Cassar O, Migaud M, Rozenberg F, Canpolat N, Aydogan G, Fleckenstein B, Bustamante J, Picard C, Gessain A, Jouanguy E, Cesarman E, Olivier M, Gros P, Abel L, Croft M, Tangye SG, Casanova JL. 2013. Inherited human OX40 deficiency underlying classic Kaposi sarcoma of childhood. J Exp Med 210: 1743–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Drutman SB, Haerynck F, Zhong FL, Hum D, Hernandez NJ, Belkaya S, Rapaport F, de Jong SJ, Creytens D, Tavernier SJ, Bonte K, De Schepper S, van der Werff Ten Bosch J, Lorenzo-Diaz L, Wullaert A, Bossuyt X, Orth G, Bonagura VR, Beziat V, Abel L, Jouanguy E, Reversade B, Casanova JL. 2019. Homozygous NLRP1 gain-of-function mutation in siblings with a syndromic form of recurrent respiratory papillomatosis. Proc Natl Acad Sci U S A 116: 19055–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Belkaya S, Michailidis E, Korol CB, Kabbani M, Cobat A, Bastard P, Lee YS, Hernandez N, Drutman S, de Jong YP, Vivier E, Bruneau J, Beziat V, Boisson B, Lorenzo-Diaz L, Boucherit S, Sebagh M, Jacquemin E, Emile JF, Abel L, Rice CM, Jouanguy E, Casanova JL. 2019. Inherited IL-18BP deficiency in human fulminant viral hepatitis. J Exp Med 216: 1777–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Drutman SB, Mansouri D, Mahdaviani SA, Neehus AL, Hum D, Bryk R, Hernandez N, Belkaya S, Rapaport F, Bigio B, Fisch R, Rahman M, Khan T, Al Ali F, Marjani M, Mansouri N, Lorenzo-Diaz L, Emile JF, Marr N, Jouanguy E, Bustamante J, Abel L, Boisson-Dupuis S, Beziat V, Nathan C, Casanova JL. 2020. Fatal Cytomegalovirus Infection in an Adult with Inherited NOS2 Deficiency. N Engl J Med 382: 437–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Vanhollebeke B, Truc P, Poelvoorde P, Pays A, Joshi PP, Katti R, Jannin JG, Pays E. 2006. Human Trypanosoma evansi infection linked to a lack of apolipoprotein L-I. N Engl J Med 355: 2752–6 [DOI] [PubMed] [Google Scholar]
- 183.Lamborn IT, Jing H, Zhang Y, Drutman SB, Abbott JK, Munir S, Bade S, Murdock HM, Santos CP, Brock LG, Masutani E, Fordjour EY, McElwee JJ, Hughes JD, Nichols DP, Belkadi A, Oler AJ, Happel CS, Matthews HF, Abel L, Collins PL, Subbarao K, Gelfand EW, Ciancanelli MJ, Casanova JL, Su HC. 2017. Recurrent rhinovirus infections in a child with inherited MDA5 deficiency. J Exp Med 214: 1949–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Asgari S, Schlapbach LJ, Anchisi S, Hammer C, Bartha I, Junier T, Mottet-Osman G, Posfay-Barbe KM, Longchamp D, Stocker M, Cordey S, Kaiser L, Riedel T, Kenna T, Long D, Schibler A, Telenti A, Tapparel C, McLaren PJ, Garcin D, Fellay J. 2017. Severe viral respiratory infections in children with IFIH1 loss-of-function mutations. Proc Natl Acad Sci U S A 114: 8342–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ogunjimi B, Zhang SY, Sorensen KB, Skipper KA, Carter-Timofte M, Kerner G, Luecke S, Prabakaran T, Cai Y, Meester J, Bartholomeus E, Bolar NA, Vandeweyer G, Claes C, Sillis Y, Lorenzo L, Fiorenza RA, Boucherit S, Dielman C, Heynderickx S, Elias G, Kurotova A, Auwera AV, Verstraete L, Lagae L, Verhelst H, Jansen A, Ramet J, Suls A, Smits E, Ceulemans B, Van Laer L, Plat Wilson G, Kreth J, Picard C, Von Bernuth H, Fluss J, Chabrier S, Abel L, Mortier G, Fribourg S, Mikkelsen JG, Casanova JL, Paludan SR, Mogensen TH. 2017. Inborn errors in RNA polymerase III underlie severe varicella zoster virus infections. J Clin Invest 127: 3543–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Guerin A, Kerner G, Marr N, Markle JG, Fenollar F, Wong N, Boughorbel S, Avery DT, Ma CS, Bougarn S, Bouaziz M, Beziat V, Della Mina E, Oleaga-Quintas C, Lazarov T, Worley L, Nguyen T, Patin E, Deswarte C, Martinez-Barricarte R, Boucherit S, Ayral X, Edouard S, Boisson-Dupuis S, Rattina V, Bigio B, Vogt G, Geissmann F, Quintana-Murci L, Chaussabel D, Tangye SG, Raoult D, Abel L, Bustamante J, Casanova JL. 2018. IRF4 haploinsufficiency in a family with Whipple’s disease. Elife 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Poyhonen L, Bustamante J, Casanova JL, Jouanguy E, Zhang Q. 2019. Life-Threatening Infections Due to Live-Attenuated Vaccines: Early Manifestations of Inborn Errors of Immunity. J Clin Immunol 39: 376–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dorman SE, Picard C, Lammas D, Heyne K, van Dissel JT, Baretto R, Rosenzweig SD, Newport M, Levin M, Roesler J, Kumararatne D, Casanova JL, Holland SM. 2004. Clinical features of dominant and recessive interferon gamma receptor 1 deficiencies. Lancet 364: 2113–21 [DOI] [PubMed] [Google Scholar]
- 189.Boisson-Dupuis S, Kong XF, Okada S, Cypowyj S, Puel A, Abel L, Casanova JL. 2012. Inborn errors of human STAT1: allelic heterogeneity governs the diversity of immunological and infectious phenotypes. Curr Opin Immunol 24: 364–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Dupuis S, Dargemont C, Fieschi C, Thomassin N, Rosenzweig S, Harris J, Holland SM, Schreiber RD, Casanova JL. 2001. Impairment of mycobacterial but not viral immunity by a germline human STAT1 mutation. Science 293: 300–3. [DOI] [PubMed] [Google Scholar]
- 191.Gruber C, Bogunovic D. 2020. Incomplete penetrance in primary immunodeficiency: a skeleton in the closet. Hum Genet [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Quintana-Murci L 2019. Human Immunology through the Lens of Evolutionary Genetics. Cell 177: 184–99 [DOI] [PubMed] [Google Scholar]
- 193.Brodin P, Jojic V, Gao T, Bhattacharya S, Angel CJ, Furman D, Shen-Orr S, Dekker CL, Swan GE, Butte AJ, Maecker HT, Davis MM. 2015. Variation in the human immune system is largely driven by non-heritable influences. Cell 160: 37–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Delmonte OM, Castagnoli R, Calzoni E, Notarangelo LD. 2019. Inborn Errors of Immunity With Immune Dysregulation: From Bench to Bedside. Front Pediatr 7: 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhang Y, Su HC, Lenardo MJ. 2015. Genomics is rapidly advancing precision medicine for immunological disorders. Nat Immunol 16: 1001–4 [DOI] [PubMed] [Google Scholar]
- 196.Garrod AE. 1931. The Inborn Factors in Disease. Oxford: Clarendon Press [Google Scholar]
- 197.Armstrong ME, Thomas CP. 2019. Diagnosis of monogenic chronic kidney diseases. Curr Opin Nephrol Hypertens 28: 183–94 [DOI] [PubMed] [Google Scholar]
- 198.Chakravarti A, Clark AG, Mootha VK. 2013. Distilling pathophysiology from complex disease genetics. Cell 155: 21–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chong JX, Buckingham KJ, Jhangiani SN, Boehm C, Sobreira N, Smith JD, Harrell TM, McMillin MJ, Wiszniewski W, Gambin T, Coban Akdemir ZH, Doheny K, Scott AF, Avramopoulos D, Chakravarti A, Hoover-Fong J, Mathews D, Witmer PD, Ling H, Hetrick K, Watkins L, Patterson KE, Reinier F, Blue E, Muzny D, Kircher M, Bilguvar K, Lopez-Giraldez F, Sutton VR, Tabor HK, Leal SM, Gunel M, Mane S, Gibbs RA, Boerwinkle E, Hamosh A, Shendure J, Lupski JR, Lifton RP, Valle D, Nickerson DA, Centers for Mendelian G, Bamshad MJ. 2015. The Genetic Basis of Mendelian Phenotypes: Discoveries, Challenges, and Opportunities. Am J Hum Genet 97: 199–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Cirino AL, Harris S, Lakdawala NK, Michels M, Olivotto I, Day SM, Abrams DJ, Charron P, Caleshu C, Semsarian C, Ingles J, Rakowski H, Judge DP, Ho CY. 2017. Role of Genetic Testing in Inherited Cardiovascular Disease: A Review. JAMA Cardiol 2: 1153–60 [DOI] [PubMed] [Google Scholar]
- 201.McGovern DP, Kugathasan S, Cho JH. 2015. Genetics of Inflammatory Bowel Diseases. Gastroenterology 149: 1163–76 e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Rexach J, Lee H, Martinez-Agosto JA, Nemeth AH, Fogel BL. 2019. Clinical application of next-generation sequencing to the practice of neurology. Lancet Neurol 18: 492–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Blau N, van Spronsen FJ, Levy HL. 2010. Phenylketonuria. Lancet 376: 1417–27 [DOI] [PubMed] [Google Scholar]