Abstract
Relationships between µ-opioid receptor (MOR) efficacy and effects of mitragynine and 7-hydroxymitragynine are not fully established. We assessed in vitro binding affinity and efficacy and discriminative stimulus effects together with antinociception in rats. The binding affinities of mitragynine and 7-hydroxymitragynine at MOR (Ki values 77.9 and 709 nM, respectively) were higher than their binding affinities at κ-opioid receptor (KOR) or δ-opioid receptor (DOR). [35S]guanosine 5′-O-[γ-thio]triphosphate stimulation at MOR demonstrated that mitragynine was an antagonist, whereas 7-hydroxymitragynine was a partial agonist (Emax = 41.3%). In separate groups of rats discriminating either morphine (3.2 mg/kg) or mitragynine (32 mg/kg), mitragynine produced a maximum of 72.3% morphine-lever responding, and morphine produced a maximum of 65.4% mitragynine-lever responding. Other MOR agonists produced high percentages of drug-lever responding in the morphine and mitragynine discrimination assays: 7-hydroxymitragynine (99.7% and 98.1%, respectively), fentanyl (99.7% and 80.1%, respectively), buprenorphine (99.8% and 79.4%, respectively), and nalbuphine (99.4% and 98.3%, respectively). In the morphine and mitragynine discrimination assays, the KOR agonist U69,593 produced maximums of 72.3% and 22.3%, respectively, and the DOR agonist SNC 80 produced maximums of 34.3% and 23.0%, respectively. 7-Hydroxymitragynine produced antinociception; mitragynine did not. Naltrexone antagonized all of the effects of morphine and 7-hydroxymitragynine; naltrexone antagonized the discriminative stimulus effects of mitragynine but not its rate-decreasing effects. Mitragynine increased the potency of the morphine discrimination yet decreased morphine antinociception. Here we illustrate striking differences in MOR efficacy, with mitragynine having less than 7-hydroxymitragynine.
SIGNIFICANCE STATEMENT
At human µ-opioid receptor (MOR) in vitro, mitragynine has low affinity and is an antagonist, whereas 7-hydroxymitragynine has 9-fold higher affinity than mitragynine and is an MOR partial agonist. In rats, intraperitoneal mitragynine exhibits a complex pharmacology including MOR agonism; 7-hydroxymitragynine has higher MOR potency and efficacy than mitragynine. These results are consistent with 7-hydroxymitragynine being a highly selective MOR agonist and with mitragynine having a complex pharmacology that combines low efficacy MOR agonism with activity at nonopioid receptors.
Introduction
Opioid overdose, a leading cause of death for people under age 50 in the Unites States, has resulted in decreased life expectancy (Crimmins and Zhang, 2019; Melton and Melton, 2019). Current Food and Drug Administration–approved medications to treat opioid use disorder include methadone, buprenorphine, and naltrexone. However, 40%–60% of patients relapse while being maintained on the currently approved treatments (NIDA, 2018). Thus, there is a need for more effective medications to achieve higher levels of abstinence than those associated with the Food and Drug Administration–approved medications.
Kratom (Mitragyna speciosa), a plant native to Southeast Asia, has been used in Malaysia and Thailand to mitigate opioid withdrawal symptoms (Vicknasingam et al., 2010). Kratom use has increased significantly in the West where kratom products are used for the treatment of pain and opioid dependence as well as for recreational purposes. More than 40 alkaloids have been identified in kratom leaves, with mitragynine being the most abundant and accounting for 40%–60% of the total alkaloid content (Dargan and Wood, 2013; Hassan et al., 2013). Three diastereomers of mitragynine present in kratom (speciogynine, speciocilliatine, and mitracilliatine) account for an additional 5%–10% of the total alkaloid content (Dargan and Wood, 2013; Hassan et al., 2013; Gogineni et al., 2014). The successful isolation of mitragynine from its diastereomers and related alkaloids has been an overlooked pitfall in the separation process due to the similar physicochemical properties of kratom alkaloids which might compromise the purity of the individual alkaloids (Sharma et al., 2019).
Mitragynine has received much attention because of its µ-opioid receptor (MOR) pharmacology. For example, mitragynine was a partial agonist at mouse MORs but inactive up to 1.0 µM at δ-opioid receptor (DOR) or κ-opioid receptor (KOR) using a guanosine 5′-O-[γ-thio]triphosphate (GTPγS) functional assay (Váradi et al., 2016). In mice, mitragynine was 2.6-fold less potent than codeine, a prodrug of the MOR agonist morphine, at producing antinociception using a hot-plate test (Macko et al., 1972). The antinociceptive effects of mitragynine were blocked by the nonselective opioid antagonist naloxone in mice and were absent in mice lacking MORs and with δ- and κ-opioid receptors intact (Matsumoto et al., 1996b; Kruegel et al., 2019). Results from additional ex vivo and in vivo studies indicate that the activity of mitragynine may extend beyond MOR. For example, the discriminative stimulus effects of mitragynine in rats were not blocked by naloxone (Harun et al., 2015), whereas the inhibitory effects of mitragynine on the contraction elicited by electrical stimulation in the guinea pig ileum were blocked by naloxone (Watanabe et al., 1997), naltrindole (DOR antagonist), and norbinaltorphimine (KOR antagonist) but not by naloxonazine (MOR antagonist) (Shamima et al., 2012). Several studies have reported that mitragynine is a G-protein–biased agonist at human MORs (hMORs) (Kruegel et al., 2016) and is not self-administered at rates above vehicle when it is substituted for methamphetamine or morphine in rats (Yue et al., 2018; Hemby et al., 2019). Collectively, these findings suggest that mitragynine may be unique among other opioid agonists, whereas 7-hydroxymitragynine appears to be a more consistent opioid agonist (Váradi et al., 2016). However, it is not known to what extent the behavioral effects of mitragynine and 7-hydroxymitragynine reflect differences in MOR efficacy (i.e., intrinsic activity), evidenced by differences in maximum effects and antagonism of higher efficacy MOR agonists.
The present study assessed the in vitro and in vivo opioid receptor pharmacology of mitragynine extracted from a kratom product at greater than 98% purity (Hiranita et al., 2019), and 7-hydroxymitragynine synthesized from mitragynine as previously described (Obeng et al., 2020). Binding affinity was assessed through displacement of radioligand binding at human opioid receptor subtypes, and MOR efficacy was assessed with a [35S]GTPγS assay. Whereas prior studies have used male subjects to evaluate the in vivo pharmacology of kratom alkaloids, both males and females were studied here to address potential sex differences in opioid pharmacology as previously described (e.g., Craft et al., 1996). Female and male rats were trained to discriminate either mitragynine or morphine from vehicle; these discrimination assays were used to assess substitution profiles with various opioid agonists [high- (fentanyl) and low-efficacy (buprenorphine and nalbuphine) µ-, κ- (U69,593), and δ-opioid receptor (SNC 80) agonists]. A hot-plate assay was further employed to compare antinociceptive effects. Reversibility of the behavioral effects of mitragynine and 7-hydroxymitragynine was assessed with naltrexone.
Materials and Methods
Compounds.
The following salt and enantiomeric forms of the drugs were used: [3H][D-Ala2, D-Leu5]-enkephalin ([3H]DADLE) (PerkinElmer, Boston, MA), [3H][D-Ala2, N-MePhe4, Gly-ol]-enkephalin ([3H]DAMGO) (PerkinElmer), [3H]U69,593 (PerkinElmer), buprenorphine hydrochloride (National Institute on Drug Abuse, Drug Supply Program, Rockville, MD), DAMGO (Tocris Bioscience, Bristol, UK), DADLE (Tocris Bioscience), fentanyl hydrochloride (National Institute on Drug Abuse), (-)-mitragynine hydrochloride [extracted as described in Hiranita et al. (2019)] and (-)-7-hydroxymitragynine [semisynthesized from mitragynine as in Obeng et al. (2020)], (-)-morphine sulfate pentahydrate (National Institute on Drug Abuse), (-)-naltrexone hydrochloride (Sigma-Aldrich Co., St. Louis, MO), nalbuphine (Sigma-Aldrich Co.), U69,593 (Sigma-Aldrich Co.), and SNC 80 (Tocris Bioscience). Dose/concentration is expressed as the weight of the salt form listed above or as base if no salt form is noted. For in vitro studies, compounds were dissolved in dimethyl sulfoxide (Sigma-Aldrich Co.) to form stock concentrations of 10 mM. For behavioral studies, a vehicle consisting of sterile water containing 5% Tween 80 (polyoxyethylenesorbitanmonooleate; Sigma-Aldrich Co.) and 5% propylene glycol (Sigma-Aldrich Co.) was used. Each solution was filtered with a 0.2-µm pore size syringe filter (Millex-LG, 0.20 µm, SLLG025SS; Cole-Parmer, Vernon Hills, IL), and compounds and vehicle were administered intraperitoneally in a volume of 1.0 ml/kg of body weight except mitragynine, 7-hydroxymitragynine, and SNC 80, which were prepared in volumes of 1.0–10 ml/kg because of limited solubility. Mitragynine was tested up to 56 mg/kg; a dose of 100 mg/kg of mitragynine was lethal. Mitragynine and naltrexone were administered 30 minutes prior to sessions; other compounds were administered 15 minutes prior to sessions. The dose and pretreatment time ranges of the compounds studied were based on our preliminary data and literature (Hiranita et al., 2014; Harun et al., 2015; Tanda et al., 2016; Obeng et al., 2020).
Receptor-Binding Assay.
[3H]DADLE, [3H]U69,593, and [3H]DAMGO were used to label the δ-, κ-, and µ-opioid receptors, respectively (Barrett and Vaught, 1983; Lahti et al., 1985; Onogi et al., 1995). The Kd and Bmax values for the radioligands were determined using a saturation assay (Table 1). Monoclonal human opioid receptors were stably expressed in Chinese hamster ovary cell lines for δ- (provided by Dr. Stephen J. Cutler, University of South Carolina) and µ-opioid receptors (PerkinElmer) and in human embryonic kidney (HEK) cells for KOR (Dr. Stephen J. Cutler, University of South Carolina). The Bradford protein assay was used to determine and adjust the concentration of protein required for the assay (Tal et al., 1985). Ten micrograms of each membrane protein was separately incubated with the corresponding radioligand in the presence of different concentrations of test compounds in TME buffer [50 mM Tris (Sigma-Aldrich), 3 mM MgCl2 (Sigma-Aldrich), and 0.2 mM EGTA (Sigma-Aldrich), pH 7.7] for 60 minutes at room temperature. The bound radioligand was separated by filtration using the Connectorate filtermat harvester for 96-well microplates (Dietikon, Switzerland) and counted for radioactivity using a Hidex sense β microplate reader (Hidex, Turku, Finland). Specific binding at δ-, κ-, and µ-opioid receptors was determined as the difference in binding obtained in the absence and presence of 10 µM SNC 80; 10 µM U69,593; and 10 µM naltrexone, respectively.
TABLE 1.
Summary of scintillation counting conditions employed for assessing affinity at various binding sites in competition for the radioligands labeling human opioid receptor subtypes
Kd and Bmax values in parentheses are 95% CIs.
| Receptor | Source (Cell) | Radioligand | Radioligand Concentration (nM), (Mean ± S.E.M.) | Nonspecific Binding (10 μM) | Incubation Buffer | Incubation Time (Room Temperature) | Kd (nM) (95% CI) | Bmax (pmol/mg) (95% CI) |
|---|---|---|---|---|---|---|---|---|
| DOR | CHO | [3H]DADLE | 0.864 ± 0.035 | SNC 80 | TME buffer | 60 min | 0.426 (0.272–0.580) | 5.04 (4.54–5.53) |
| KOR | HEK-293 | [3H]U69,593 | 1.60 ± 0.139 | U69,593 | TME buffer | 60 min | 1.44 (0.453–2.42) | 4.98 (4.13–5.83) |
| MOR | CHO | [3H]DAMGO | 1.18 ± 0.211 | Naltrexone | TME buffer | 60 min | 1.72 (0.652–2.79) | 6.41 (5.07–7.74) |
[35S]GTPγS Functional Assay.
MOR efficacy was assessed with the [35S]GTPγS functional assay (Harrison and Traynor, 2003). Twenty micrograms of hMOR-CHO membrane protein was incubated with 10 μM GDP, 0.1 nM [35S]GTPγS, and varying concentrations of the compound under investigation for 1.5 hours at 25°C. In the test for antagonism, a 10-fold higher concentration of the Ki values (Smith et al., 2020) was used for 7-hydroxymitragynine (779 nM), buprenorphine (9.03 nM), mitragynine (7.06 μM), nalbuphine (110 nM), and naltrexone (18.4 nM); these were incubated with increasing concentrations of DAMGO to surmount antagonism. Nonspecific binding was determined with 40 μM unlabeled GTPγS. TME buffer (50 mM Tris-HCl, 9.0 mM MgCl2, 0.2 mM EGTA, pH 7.4) with 150 mM NaCl and 0.14% bovine serum albumin was used to increase agonist-stimulated binding; the final volume in each well was 300 μl. Ten micromolars of DAMGO was included in the assay as the maximum effective concentration at MOR. After the incubation, the bound radioactive ligand was separated from the free radioligand by filtration through a GF/B glass fiber filter paper and rinsed three times with ice-cold wash buffer (50 mM Tris-HCl, pH 7.2) using the Connectorate harvester. Radioactivity was measured with the Hidex sense β microplate reader scintillation counter. All assays were determined in triplicate and repeated at least three times.
Animals.
Adult female and male Sprague Dawley rats (Taconics, Germantown, NY; N = 8 per sex) weighing approximately 250 and 300 g upon arrival, respectively, were singly acclimated for at least 3 days to a temperature- (21.9 ± 1.9°C) and humidity-controlled (53% ± 14%) vivarium with a 12-hour light/dark cycle (lights on at 0700 hours). Food (2918 Teklad global 18% protein rodent diets; Envigo, Frenchtown, NJ) and reverse-osmosis water were available in the home cage. After the acclimation period, individual body weights were maintained at 90% of the free-feeding weight as determined by normative growth curves by adjusting daily amounts of food (Dustless Precision Pellets Grain-Based Rodent Diet; Bio-Serv, Frenchtown, NJ) that were provided 30 minutes after daily experimental sessions in addition to 45-mg sucrose pellets (Dustless Precision Pellets 45 mg, Sucrose; Bio-Serv) available during experimental sessions. Behavioral protocols were approved by the Institutional Animal Care and Use Committee at the University of Florida, which is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International, and were written in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All experiments were conducted in the light cycle at the same time each day 7 days per week. The body weight of each subject was measured daily before experiments.
Apparatus.
For antinociception testing, a clear acrylic cage surrounded the Hot Plate Analgesia Meter (1440 Analgesia Hot Plate with RS-232 Port and Software; Columbus Instruments, Columbus, OH) to confine the animal during experimental sessions. Temperature on the plate surface was stably maintained at 52 ± 0.1°C for at least 30 minutes prior to each use. For drug discrimination testing, 16 operant-conditioning chambers (Model ENV-008; Med Associates Inc., Fairfax, VT) were each enclosed within a sound-attenuating cubicle equipped with a fan for ventilation and white noise to mask extraneous sounds. On the front wall of each chamber were two retractable, 5-cm-long response levers that were 5 cm from the midline and 9 cm above the grid floor. A downward displacement of each lever with a force approximating 0.20 N defined a response. Two amber light-emitting diodes (LEDs) were positioned above each lever (one LED per lever). Sucrose pellets (Dustless Precision Pellets 45 mg, Sucrose; Bio-Serv) were delivered via a dispenser (Model ENV-203-20; Med Associates Inc.) to a receptacle mounted on the midline of the front wall between the two levers and 2 cm above the floor. A house light was mounted on the wall opposite to the levers. Each operant conditioning chamber was connected to a Dell desktop computer (Intel Core i7-7700 3.60 GHz processor, 16.0 GB of RAM; Microsoft Windows 10) through an interface (MED-SYST-8; Med Associates Inc.). Med-PC software version V (Med Associates Inc.) controlled experimental events and provided a record of responses. Each rat was assigned to an operant conditioning chamber, and that assignment remained the same throughout the study.
Antinociception.
Each rat was manually placed on the heated plate; baseline response latency was determined manually using a stopwatch (Martin Stopwatch; Martin Sports, Carlstadt, NJ) by trained and experimentally blinded raters. Latency was defined as the interval that elapsed between placing the rat onto the heated surface and observation of one of the following responses: jumping, paw licking, and paw shaking; the maximum latency was 60 seconds. Immediately after a response or 60 seconds, whichever occurred first, the animal was removed from the apparatus. After the measurement of hot-plate baseline latency, each subject received an intraperitoneal injection and was returned to their home cage. Hot-plate response latency was measured a second time immediately after the drug discrimination test session.
Drug Discrimination Training.
Each daily experimental session commenced by placing an experimental subject in the operant conditioning chamber; the initial session duration was 120 minutes. Both retractable levers were presented, and the LED above each lever was illuminated. Each downward deflection of the lever turned off the LEDs and activated the pellet dispenser for 0.1 seconds [fixed-ratio (FR) 1 schedule] followed by a 0.1-second timeout during which the LEDs were turned off, the house light was illuminated, and responding had no scheduled consequences; the retractable levers remained present during the timeout. The correct lever (left vs. right) was alternated daily, and the ratio value was systematically increased each session. After 50 reinforcers per session were delivered within 20 minutes for two consecutive sessions under the FR10 schedule of reinforcement, drug discrimination training was initiated.
Experimental subjects were divided into two groups: one trained to discriminate morphine (3.2 mg/kg, i.p., administered 15 minutes prior to sessions) and a second group trained to discriminate mitragynine (32 mg/kg, i.p., administered 30 minutes prior to sessions). Immediately after an injection of either the training dose or vehicle, each subject was returned to their home cage for the duration of the pretreatment interval and was then placed into the operant conditioning chamber. Each training session started with the presentation of both levers and the illumination of the LEDs above each lever. The correct lever was determined by the presession injection (i.e., right lever correct after training dose; left lever correct after vehicle). The lever assignments remained the same for that subject for the duration of the study and were counterbalanced among subjects. Each downward deflection the correct lever activated the pellet dispenser; responses on the injection-inappropriate lever had no programmed consequence. Each training session lasted for up to 15 minutes or until a maximum of 50 pellets was delivered, whichever occurred first. The FR value was increased systematically to 10 (i.e., 10 responses on the correct lever were required for pellet delivery). The order of drug and vehicle training followed a double-alternation sequence (i.e., right–left–left–right) with periods of single alternation (i.e., right-left-right-left) irregularly interposed to ensure that drug and vehicle were exerting control over choice behavior.
Drug Discrimination Testing.
Test sessions commenced when the following criteria were met individually per rat for four consecutive sessions under the FR10 schedule of reinforcement: 1) a minimum of 80% of the total responses was correct and 2) the total of incorrect responses made prior to delivery of the first reinforcer was less than 10. All rats in both groups satisfied the test criteria. After the first test session, these criteria needed to be satisfied for one vehicle and one drug training session prior to the next test. The order of training (i.e., drug and vehicle) varied nonsystematically between test sessions. Test sessions were identical to training sessions, except that 10 responses in either lever resulted in delivery of food, and various doses of drugs were administered. Dose-effect assessments were conducted first for each training drug in all subjects, and they were followed in a nonsystematic order by substitution of various compounds for each training drug and pretreatment tests. Doses of test compounds were administered from doses that produced less than group averages of 20% drug-appropriate responding up to doses that produced greater than or equal to group averages of 80% drug-appropriate responding, decreased response rate to less than 20% of the vehicle control per subject, or were deemed potentially toxic or could not be increased further because of limitations in solubility. The following drugs were administered in doses increasing by 0.25 log unit 15 minutes prior to test sessions: morphine (0.32–56 mg/kg), 7-hydroxymitragynine (0.1–17.8 mg/kg), fentanyl (0.0032–0.32 mg/kg), buprenorphine (0.0178–0.56 mg/kg), nalbuphine (1.0–178 mg/kg), U69,593 (0.32–5.6 mg/kg), and SNC 80 (32–100 mg/kg). Mitragynine (3.2–56 mg/kg) and naltrexone (0.032 mg/kg) were administered 30 minutes prior to sessions. Naltrexone (0.032 mg/kg) was administered alone and in combination with morphine (3.2–56 mg/kg), mitragynine (17.8–56 mg/kg), and 7-hydroxymitragynine (0.32–17.8 mg/kg). The largest dose of mitragynine was 56 mg/kg; 100 mg/kg was lethal even in the presence of 10 mg/kg naltrexone. At the end of the study, the dose-effect functions of each training drug were individually redetermined in all subjects.
Data Analyses.
To calculate binding affinity, the IC50 values were determined using average values from at least three experiments conducted in triplicate and calculated using a nonlinear, least-squares regression analysis (Prism 8; GraphPad Software, Inc., San Diego, CA). IC50 values were converted to Ki values using the Cheng-Prusoff equation (Cheng and Prusoff, 1973). Table 1 shows a summary of the present scintillation counting conditions described above. Percent DAMGO-stimulated [35S]GTPγS binding was defined as [(net-stimulated binding by a test compound)/(net-stimulated binding by 10 μM DAMGO)] × 100%. For behavioral testing, a within-subjects design and total sample size of 8 (four rats per sex) were used for every experiment. All data are shown as mean values (±S.E.M.) as a function of dose. Statistical analyses were conducted using GraphPad Prism version 8 for Windows (San Diego, CA) and SigmaPlot version 14.0 (Systat Software Inc., San Jose, CA). Comparisons were considered significantly different when P < 0.05. One- and two-way repeated-measures ANOVAs followed by post hoc Bonferroni t tests were used to analyze the effects of dose, sex, training drug, intertest session, or assessment order (first vs. second dose-effect determination for each training drug). Potencies for morphine and mitragynine are calculated for each sex. For all other drugs, when there was no significant main effect of sex, males and females were combined to calculate potencies and potency ratios. Significant dose × sex interactions were reported and further assessed with post hoc tests; significant differences are noted by asterisks on the abscissae of figures.
The hot-plate latencies were normalized to the percentage of the maximum possible antinociceptive effect (MPE) using the following formula: %MPE = 100 × (postinjection latency − preinjection baseline latency)/(maximum latency 60 seconds − preinjection baseline latency). The percentage of drug-appropriate responding was calculated by dividing the total number of responses on the drug-appropriate lever by the total number of responses on both the drug- and vehicle-appropriate levers. The rate of responding was calculated per animal by dividing the total number of responses by the session time in seconds. Values were considered a potentially unreliable indication of lever selection and were not plotted or analyzed when the rate of responding was less than 20% of the control rate of responding for any given subject. When greater than half of the sample size was unreliable as defined in this way, the group average percentage of drug-appropriate responding was not plotted or analyzed. However, all data on response rate and MPEs were plotted and analyzed.
Standard linear regression on the linear portion of the dose-effect function (Snedecor and Cochran, 1967) was used to calculate the ED50 value and 95% confidence intervals (CIs) when the mean effect (percentages of drug-appropriate responding, MPE, and reductions in response rate) crossed 50% (e.g., more than 50% of drug-appropriate responding and MPE). To compare potency, potency ratios and corresponding 95% CIs were calculated (Tallarida, 2002). If the 95% CIs of the ED50 values did not overlap or the 95% CIs of the potency ratio of the drug alone or in combination with a pretreated compound (i.e., 0.032 mg/kg naltrexone) did not include 1, then the drugs were considered to have significantly different potencies.
Results
Receptor Binding.
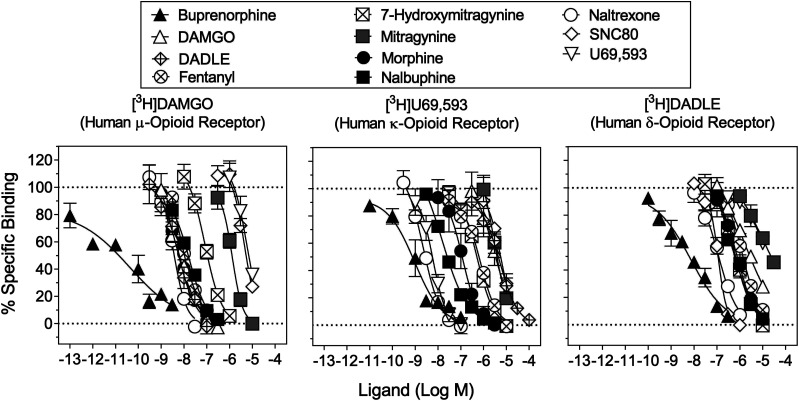
The binding affinities of mitragynine and 7-hydroxymitragynine at the opioid receptor subtypes were compared with those of reference opioid receptor ligands (Fig. 1). The results obtained here were also compared with published values (Table 2). The δ- and κ-opioid receptor agonists, SNC 80 and U69,593, respectively, were not tested beyond 10 μM because of solubility. DADLE and SNC 80 were more potent to displace bound [3H]DADLE than [3H]U69,593 and [3H]DAMGO, whereas U69,593 and DAMGO were selective for the κ- and µ-opioid receptors, respectively. DADLE had high affinity at MOR [Ki value = 3.29 (95% CIs: 1.96–6.77) nM], whereas SNC 80 had relatively low affinity at MOR [Ki value = 2760 (1190–6930) nM] (Table 2).
Fig. 1.
Displacement of radioligands for opioid receptor subtypes. Ordinates: percentage of specific radiotracer bound to membrane preparations. Abscissae: concentrations of each competing compound (log scale). Left: displacement of [3H]DAMGO labeling MORs. Middle: displacement of [3H]U69,593 labeling KORs. Right: displacement of [3H]DADLE labeling DORs. Each data point represents the mean results of three repeated experiments; vertical bars represent S.E.M. (N ≥ 3) from at least three independent triplicate replications per sample. Ki and 95% CI values from curve-fitting analyses of these data are shown in Table 2.
TABLE 2.
Inhibition of binding of the radioligands labeling opioid receptor subtypes
Values are Ki values except as indicated for displacement of the listed radioligands. Values in parentheses are 95% CIs. Values listed from previous studies were also added as reference. EC50 and Emax values from curve-fitting analyses of these data are shown in Fig. 1.
| Compound | µ Ki (95% CIs) nM | δ Ki (95% CIs) nM | κ Ki (95% CIs) nM | κ/µ | δ/µ | δ/κ |
|---|---|---|---|---|---|---|
| Buprenorphine | 0.903 (0.71–1.21) | 1.51 (0.975–2.35) | 1.29 (0.980–2.09) | 1.43 | 1.67 | 1.17 |
| DADLE | 3.29 (1.96–6.77) | 0.426 (0.272–0.580)a | 3050 (2020–4650) | 927 | 0.129 | 0.000140 |
| DAMGO | 4.15a (1.85–13.1) | 880 (442–1930) | 1200 (556–2770) | 289 | 212 | 0.733 |
| Fentanyl | 7.96 (6.19–10.3) | 539 (300–987) | 202 (128–349) | 25.4 | 67.7 | 2.66 |
| 7-Hydroxymitragynine | 77.9 (45.8–152) | 243 (168–355) | 220 (162–302) | 2.82 | 3.12 | 1.15 |
| 37 (S.E.M.: 4, mouse)b | 91 (S.E.M.: 8, mouse)b | 132 (S.E.M.: 7, mouse)b | 3.57 (mouse)b | 2.46 (mouse)b | 0.69 (mouse)b | |
| 47 (S.E.M.: 18, human)c | 219 (S.E.M.: 41, human)c | 188 (S.E.M.: 38, human)c | 4 (human)c | 4.66 (human)c | 1.16 (human)c | |
| 7.16 (S.E.M.: 0.94, human)d | 236 (S.E.M.: 6, human)d | 74.1 (S.E.M.: 7.8, human)d | 10.4 (human)d | 33.0 (human)d | 3.2 (human)d | |
| 70 (human)e | 470 (human)e | 320 (human)e | 4.57 (human)e | 6.71 (human)e | 1.47 (human)e | |
| Mitragynine | 709 (451–1130) | 6800 (2980–15,900) | 1700 (1090–2710) | 2.40 | 9.60 | 4.00 |
| 230 (S.E.M.: 47, mouse)b | 1010 (S.E.M.: 50, mouse)b | 231 (S.E.M.: 21, mouse)b | 1.00 (mouse)b | 4.39 (mouse)b | 4.37 (mouse)b | |
| 233 (S.E.M.: 48, human)c | >10,000 (human)c | 772 (S.E.M.: 207, human)c | 3.31c (mouse) | Not determined (human)c | Not determined (human)c | |
| 502 (S.E.M.: 19.4, rat)f | 7910 (S.E.M.: 1,140, rat)f | 1200 (S.E.M.: 79.7, rat)f | 2.39 (human)c | 15.8 (rat)c | 6.59 (rat)c | |
| 7.24 (S.E.M.: 3.44, guinea pig)g | 60.3 (S.E.M.: 23.1, guinea pig)g | 1100 (S.E.M.: 436, guinea pig)g | 152 (guinea pig)f | 8.33 (guinea pig)f | 0.0548 (guinea pig)f | |
| 740 (human)e | 6500 (human)e | 1300 (human)e | 1.76 (human)e | 8.78 (human)e | 5 (human)e | |
| Morphine | 4.19 (2.03–11.1) | 250 (177–346) | 40.4 (23.7–70.9) | 9.64 | 59.6 | 6.19 |
| Nalbuphine | 11.0 (9.11–13.3) | 146 (88.3–242) | 13.0 (10.6–16.1) | 1.18 | 13.2 | 11.2 |
| Naltrexone | 1.84 (1.14–3.03) | 37.2 (26.3–53.0) | 1.19 (0.803–1.79) | 0.65 | 20.2 | 31.3 |
| SNC 80 | 2760 (1190–6930) | 34.6 (26.5–45.5) | 2020 (1050–3950) | 0.73 | 0.013 | 0.018 |
| U69,593 | 3180 (1050–11,600) | 6700 (2160–28,000) | 1.62a (1.02–2.64) | 0.0005 | 2.11 | 4140 |
Kd values obtained by homologous competition experiments.
[125I]BNtxA for all three opioid receptor subtypes was used in CHO cells expressing mouse opioid receptors (Váradi et al., 2016).
[125I]BNtxA for all three opioid receptor subtypes was used in CHO cells expressing human opioid receptors (Kruegel et al., 2016).
The same radioligands as the present study were used, but the cell lines used were human HEK-293 cells for MOR and rat basophilic leukemia cells for other receptor subtypes (Obeng et al., 2020).
[3H]DAMGO; [3H]U69,593; and [3H]DADLE were used in HEK-293 cells expressing human µ-, κ-, and δ-opioid receptors, respectively (Ellis et al., 2020).
[3H]DAMGO; [3H]U69,593; and [3H]DADLE were used in rat whole brain tissue excluding the cerebellum (Yue et al., 2018).
[3H]DAMGO; [3H]U69,593; and [3H]DPDPE were used in guinea pig whole brain tissue excluding the cerebellum (Takayama et al., 2002).
Mitragynine displaced bound [3H]DAMGO, [3H]U69,593, and [3H]DADLE in a concentration-dependent manner (Fig. 1). The binding affinity of mitragynine at MOR [Ki value = 709 (451–1130) nM] was at least 89-fold higher than those of fentanyl [Ki value = 7.96 (6.19–10.3) nM], morphine [Ki value = 4.19 (2.03–11.1) nM], and naltrexone [Ki value = 1.84 (1.14–3.03) nM] (Table 2). Mitragynine had the lowest affinity at δ- and κ-opioid receptors among all compounds tested. Mitragynine had 2.4- and 9.6-fold higher binding affinity at µ- than κ- [Ki value = 1700 (1090–2710) nM] and δ-opioid [Ki value = 6800 (2980–15,900) nM] receptors, respectively (Table 2).
7-Hydroxymitragynine displaced bound [3H]DAMGO, [3H]U69,593, and [3H]DADLE in a concentration-dependent manner (Fig. 1). The binding affinity of 7-hydroxymitragynine at MOR [Ki value = 77.9 (45.8–152) nM] was at least 7.1-fold lower than the affinities of other reference MOR ligands (Table 2). Among the three opioid receptor subtypes, 7-hydroxymitragynine had 2.8- and 3.1-fold higher affinity at µ-opioid receptor than at κ- [Ki value = 220 (162–302) nM] and δ-opioid receptors [Ki value = 243 (168–355) nM], respectively (Table 2). 7-Hydroxymitragynine had 9.1-, 7.7-, and 28-fold higher binding affinity than mitragynine at µ-, κ-, and δ-opioid receptors, respectively.
[35S]GTPγS Binding.
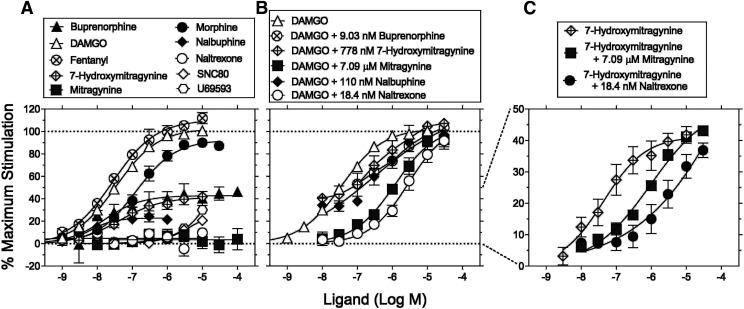
The [35S]GTPγS functional assay at hMOR was used to compare the efficacy and potency of mitragynine and 7-hydroxymitragynine with those of DAMGO; fentanyl; morphine; buprenorphine; nalbuphine; naltrexone; U69,593; and SNC 80 (Fig. 2A). DAMGO, fentanyl, and morphine were full agonists with % maximum stimulation of 103%, 110%, and 92.6%, respectively, with fentanyl being the most potent followed by DAMGO then morphine (Fig. 2A). Buprenorphine and nalbuphine were partial agonists [i.e., % maximum stimulation of 42.8% and 22.8%, respectively (Fig. 2A)]. Naltrexone produced no agonism up to 10 µM (Fig. 2A). The % maximum stimulation values of DAMGO, fentanyl, morphine, buprenorphine, nalbuphine, and naltrexone were similar to reported literature values (Emmerson et al., 1996; Selley et al., 1997). The δ- (SNC 80) and κ-opioid receptor agonists (U69,593) produced 21% and 30% stimulation of MOR at 10 μM, respectively. Higher concentrations were not tested because of solubility limitations. Mitragynine did not produce significant agonism up to 100 µM (Fig. 2A). 7-Hydroxymitragynine was a partial agonist [i.e., % maximum stimulation of 41.3% (Fig. 2A; Table 3)]. Because the lack of MOR activity of mitragynine was not expected, [35S]GTPγS binding was independently tested at Eurofins Cerep (Celle l'Evescault, France). Mitragynine did not produce agonism up to 30 µM at κ- and µ-opioid receptors and up to 200 µM at δ-opioid receptor (Supplemental Fig. 1).
Fig. 2.
[35S]GTPγS stimulation in CHO cell lines stably expressing the hMORs. Ordinates: percentage of maximum stimulation of [35S]GTPγS binding normalized to maximum DAMGO response as 100%. Abscissae: concentrations of each test compound (log scale). (A) Effects of test compounds alone. (B) Effects of DAMGO in combination with buffer, 110 nM (10 × Ki value at hMOR) nalbuphine, 18.4 nM (10 × Ki value at hMOR) naltrexone, 7.09 μM (10 × Ki value at hMOR) mitragynine, 779 nM (10 × Ki value at hMOR) 7-hydroxymitragynine, and 9.03 nM (10 × Ki value at hMOR) buprenorphine. (C) Effects of 7-hydroxymitragynine in the presence of buffer, 18.4 nM (10 × Ki value at hMOR) naltrexone, and 7.09 μM (10 × Ki value at hMOR) mitragynine. Data are percentages of the mean ± S.E.M. (N ≥ 3 per data point) of net stimulated [35S]GTPγS binding divided by stimulation produced by 10 μM DAMGO. The results were selected from at least three independent triplicate replications per sample for all panels. EC50 and Emax values from curve-fitting analyses of these data are shown in Table 3.
TABLE 3.
In vitro functional results from the [35S]GTPγS functional assay in transfected CHO cells expressing cloned hMORs
EC50 and Emax values in parentheses are 95% CIs (unless noted) from curve-fitting analyses of these data shown in Fig. 2.
| Compound | EC50 (95% CI) nM | Emax [%DAMGO] (95% CI) |
|---|---|---|
| Buprenorphine | 16.1 (6.53–39.6) | 42.8 (37.2–48.4) |
| DAMGO | 34.8 (24.9–48.6) | 103 (96.4–109) |
| Fentanyl | 27.8 (22.6–34.2) | 110 (106–115) |
| 7-Hydroxymitragynine | 43.4 (25.5–73.8) | 41.3 (37.1–45.6) |
| 53 (S.E.M.: 4)a | 77 (S.E.M.: 5)a | |
| 7.65 (S.E.M.: 0.884)b | 96.8 (S.E.M.: 1.8)b | |
| Mitragynine | Not determined in the present study | 3.46 ((−0.047 to 6.97) |
| 203 (S.E.M.: 13)a | 65 (S.E.M.: 2.8)a | |
| 320 (S.E.M.: 14.7)b | 44.1 (S.E.M.: 0.62)b | |
| Morphine | 125 (84.8–184) | 92.6 (85.8–99.4) |
| Nalbuphine | 5.87 (4.18–8.23) | 22.8 (21.1–24.6) |
| 9.0 (S.E.M.: 1.6)c | 16 (S.E.M.: 0.4)c | |
| 1.86 (SE: 0.1)d | 12d | |
| Naltrexone | Not determined in the present study | 4.69 (0.162–9.22) |
| SNC 80 | Not determined | 20.7 (S.E.M.: 3.01) at 10 μM |
| U69,593 | Not determined | 30.1 (S.E.M.: 2.88) at 10 μM |
[35S]GTPγS functional assay in transfected CHO cells stably expressing cloned mouse MORs (Váradi et al., 2016).
Homogeneous time-resolved fluorescence cAMP functional assay in transfected CHO cells stably expressing cloned hMORs (Obeng et al., 2020).
[35S]GTPγS functional assay in transfected CHO cells stably expressing cloned mouse MORs (Selley et al., 1998).
[35S]GTPγS functional assay in transfected C6 Glioma cells stably expressing cloned rat MORs (Emmerson et al., 1996).
The effects of naltrexone, nalbuphine, buprenorphine, mitragynine, and 7-hydroxymitragynine on DAMGO-stimulated [35S]GTPγS binding and the effects of naltrexone and mitragynine on 7-hydroxymitragynine–stimulated [35S]GTPγS binding were assessed to explore possible antagonism (Fig. 2, B and C). Naltrexone at a 10-fold greater concentration than its Ki value at MOR (18.4 nM, Table 2) produced an 81-fold rightward shift in the DAMGO concentration-effect curve (Fig. 2B; Table 4). Mitragynine at 10× its MOR Ki value (7.09 µM, Table 2) produced 33-fold rightward shift in the concentration-effect curve of DAMGO (Fig. 2B). Buprenorphine, 7-hydroxymitragynine, and nalbuphine at 10× their MOR Ki values antagonized DAMGO 10-, 7-, and 8-fold, respectively (Fig. 2B; Table 4). Mitragynine (7.09 µM) and naltrexone (18.4 nM) produced 22- and 69-fold rightward shifts in the 7-hydroxymitragynine concentration-effect curve, respectively (Fig. 2C; Table 4). The antagonist effects of mitragynine at the human δ-, κ-, and µ-opioid receptors were further tested at Eurofins Cerep using the [35S]GTPγS functional assay. The IC50 values of mitragynine in the presence of a fixed concentration of DPDPE; U69,593; and DAMGO at δ-, κ-, and µ-opioid receptors were 75.7, 4.73, and 10.8 μM, respectively (Supplemental Figs. 2–4).
TABLE 4.
In vitro functional results for pretreatment with antagonists or partial agonists using the [35S]GTPγS functional assay in transfected CHO cells expressing cloned hMORs
EC50 and Emax values in parentheses are 95% CIs from curve-fitting analyses of these data shown in Fig. 2.
| Compound | EC50 (95% CI) nM | Emax [%DAMGO] (95% CI) | Potency Ratio (vs. DAMGO or 7-Hydroxymitragynine) |
|---|---|---|---|
| DAMGO | 34.8 (24.9–48.6) | 103 (96.4–109) | |
| DAMGO + naltrexone | 2820 (1650–4820) | 107 (90.7–123) | 81.0 (34.0–194) |
| DAMGO + mitragynine | 1160 (656–2050) | 105 (90.0–119) | 33.3 (13.5–82.3) |
| DAMGO + nalbuphine | 289 (16.6–5030) | 118 (66.3–171) | 8.30 (0.34–202) |
| DAMGO + 7-hydroxymitragynine | 235 (27.1–2030) | 134 (94.2–173) | 6.75 (0.56–81.5) |
| DAMGO + buprenorphine | 360 (52.9–2450) | 131 (94.0–168) | 10.3 (1.09–98.4) |
| 7-Hydroxymitragynine | 43.4 (25.5–73.8) | 41.3 (37.1–45.6) | |
| 7-Hydroxymitragynine + naltrexone | 3010 (933–97,30) | 46.1 (32.4–59.8) | 69.4 (12.6–381) |
| 7-Hydroxymitragynine + mitragynine | 960 (287–3180) | 51.9 (39.6–64.1) | 22.1 (3.89–125) |
Control Performance.
Baseline hot-plate response latency determined in animals discriminating either morphine [mean 8.0 (range 6.8–8.8) seconds] or mitragynine [9.6 (7.4–17.3) seconds] did not significantly differ nor was there a difference by sex (P values ≥ 0.130). The mean (range) number of sessions required to satisfy the testing criteria were 44 (40–73) in morphine-trained rats and 44 (34–56) in mitragynine-trained rats. There was no significant effect of the training drug, sex, or training drug × sex interaction (P values ≥ 0.306). Mean (S.E.M.) response rates (responses/second) were 1.0 (0.06) in morphine-trained rats and 0.90 (0.09) in mitragynine-trained rats; there was no significant effect of training drug, sex, or their interaction (P values ≥ 0.133 and 0.362, respectively).
Effects of Training Compounds.
The ED50 values, potency ratios, and corresponding 95% CIs for discriminative-stimulus, rate-decreasing, and antinociceptive effects of all drugs are summarized in Supplemental Table 3 and Tables 5 and 6. In female and male rats discriminating morphine (3.2 mg/kg), vehicle produced a mean (S.E.M.) of 0.25% (0.12%) and 0.15% (0.15%) morphine-appropriate responding, respectively; mean (S.E.M.) response rates normalized to vehicle control were 120% (8.1%) and 110% (1.1%); and hot-plate response latencies expressed as MPE were −4.0% (2.4%) and 0.76% (5.0%), respectively (Supplemental Figs. 5 and 6, left, filled circle and open square above vehicle). Morphine dose-dependently increased drug-lever responding, decreased response rate, and increased %MPE (Supplemental Figs. 5 and 6). The ED50 values (95% CIs) for the discriminative-stimulus effects of morphine were 1.6 (0.88–2.1) in females and 2.1 (1.8–2.6) mg/kg in males (Supplemental Table 3; Tables 5 and 6). Corresponding values in females and males to decrease response rates were 9.8 (5.0–23) and 5.7 (2.6–9.4), respectively; for antinociceptive effects, the values were 38 (36–41) and 35 (29–42) mg/kg, respectively (Supplemental Table 3; Tables 5 and 6).
TABLE 5.
ED50 values (95% CIs) for the discriminative-stimulus, rate-decreasing, and antinociceptive effects of various compounds in rats trained to discriminate 3.2 mg/kg morphine as shown in Figs. 3–10 and Supplemental Figs. 5–7
The sample sizes are described in each figure legend. Each value is a combination of females and males unless otherwise noted. For each training drug, potency ratios (95% CIs) are calculated by dividing the ED50 values for producing rate-decreasing or antinociceptive effects by the ED50 values for producing discriminative-stimulus effects.
| Test Drug | ED50 (95% CIs) | Potency Ratio | |||
|---|---|---|---|---|---|
| Discrimination | Response Rate | Maximum Possible Effect | Rate-Decreasing/Discrimination | Antinociceptive/Discrimination | |
| 7-Hydroxymitragynine | 0.275 (0.0768–0.411) | 4.73 (3.25–6.35) | 10.6 (9.26–12.3) | 17.2 (7.91–82.7) | 38.5 (22.5–160) |
| 7-Hydroxymitragynine + 0.032 mg/kg naltrexone | 0.670 (0.602–0.744) | 6.09 (4.78–7.53) | ND [≤ −2.81% (2.03%) at 32 mg/kg]a | 9.09 (6.42–12.5) | Not applicable |
| Buprenorphine | 0.0844 (0.0456–0.122) | 0.343 (0.249–0.533) | ND [≤ −2.81% (2.03%) at 0.56 mg/kg]a | 4.06 (2.04–11.7) | Not applicable |
| Fentanyl | 0.0220 (0.0111–0.0318) | 0.173 (0.150–0.202) | 0.139 (0.112–0.166) | 7.86 (4.72–18.2) | 6.32 (3.52–15.0) |
| Mitragynine | 29.6 (18.8–55.9) | 45.7 (36.4–64.1) | ND [≤7.01% (6.50%) at 5.6 mg/kg]a | 1.54 (0.651–3.41) | Not applicable |
| Morphine | 1.85 (1.54–2.20)b, 1.60 (1.30–1.91)c | 13.7 (10.6–16.8)b, 21.3 (16.8–26.2)c | 35.2 (32.6–38.1)b, 33.4 (30.9–36.4)c | 7.41 (4.82–10.9)b, 13.3 (8.80–20.2)c | 19.0 (21.2–24.7)b, 20.9 (16.2–28.0)c |
| Morphine + 0.032 mg/kg naltrexone | 15.2 (12.7–17.9) | 35.4 (28.2–47.2) | ND [≤3.35% (1.92%) at 56 mg/kg]d | 2.33 (1.58–3.72) | Not applicable |
| Morphine + 1.78 mg/kg Nalbuphine | 0.335 (0.202–0.444) | 26.0 (22.4–30.3) | ND [≤27.4% (4.95%) at 56 mg/kg]d | 77.6 (50.5–150) | Not applicable |
| Morphine + 5.6 mg/kg Mitragynine | 0.478 (0.265–0.503) | 11.7 (9.33–14.2) | ND [≤40.6% (6.92%) at 56 mg/kg]d | 24.5 (18.6–53.6) | Not applicable |
| Nalbuphine | 7.29 (2.33–11.0) | 81.3 (68.9–96.8) | ND [≤2.86% (5.49%) at 178 mg/kg]e | 11.2 (6.26–41.5) | Not applicable |
| SNC 80 | ND [≤34.3% (11.3%) at 56 mg/kg]e | ND [≤51.2% (13.2%) at 100 mg/kg]e | ND [≤8.63% (5.47%) at 100 mg/kg]e | Not applicable | Not applicable |
| U69,593 | 2.29 (1.64–4.37) | 2.30 (1.85–2.77) | 5.05 (4.12–6.76) | 1.00 (0.423–0.634) | 2.21 (0.943–4.12) |
Due to lethality.
First assessment.
Reassessment.
Due to an adverse reaction (scratching behavior).
Due to insolubility in the chosen vehicle.
TABLE 6.
ED50 values (95% CIs) for the discriminative-stimulus, rate-decreasing, and antinociceptive effects of various compounds in rats trained to discriminate 32 mg/kg mitragynine as shown in Figs. 3–10 and Supplemental Figs. 5–7
The sample sizes are described in each figure legend. Each value is a combination of females and males unless otherwise noted. For each training drug, potency ratios (95% CIs) are calculated by dividing the ED50 values for producing rate-decreasing or antinociceptive effects by the ED50 values for producing discriminative-stimulus effects.
| Test Drug | ED50 (95% CIs) | Potency Ratio | |||
|---|---|---|---|---|---|
| Discrimination | Response Rate | Maximum Possible Effect | Rate-Decreasing/Discrimination | Antinociceptive/Discrimination | |
| 7-Hydroxymitragynine | 0.415 (0.0832–0.656) | 5.54 (3.92–7.35) | 16.4 (13.9–20.7) | 13.3 (5.98–88.3) | 39.5 (21.2–249) |
| Buprenorphine | 0.186 (0.0672–0.311) | 0.400 (0.322–0.534) | ND [≤1.40% (6.09%) at 0.056 mg/kg]a | 2.15 (1.04–7.95) | Not applicable |
| Fentanyl | 0.0507 (0.0124–0.0906) | 0.171 (0.140–0.214) | 0.118 (0.0928–0.143) | 3.37 (1.55–17.3) | 2.33 (1.02–11.5) |
| Mitragynine | 15.1 (12.7–17.6)b, 12.8 (9.31–16.0)c | 46.2 (38.1–60.6)b, 47.2 (38.2–65.5)c | ND [up to 10.0% (3.32%)b and 8.01% (4.80%) at 5.6 mg/kgc]d | 3.06 (2.16–4.77)b, 3.69 (2.39–7.04)c | Not applicable |
| Mitragynine + 0.032 mg/kg naltrexone | ND [≤27.2% (17.1%) at 56 mg/kg]d | ND [≤53.2% (10.8%) at 56 mg/kg]d | ND [≤ −3.91% (4.56%) at 56 mg/kg]d | Not applicable | Not applicable |
| Morphine | 15.7 (10.4–36.3) | 24.8 (19.3–30.5) | 35.6 (31.3–40.8) | 1.58 (0.532–2.93) | 2.27 (0.862–3.92) |
| Nalbuphine | 6.65 (4.30–9.02) | 110 (92.4–137) | ND [≤2.20% (5.09%) at 178 mg/kg]e | 16.5 (10.2–31.9) | Not applicable |
| SNC 80 | ND [≤23.0% (11.1%) at 100 mg/kg]e | ND [≤58.3% (11.9%) at 100 mg/kg]e | ND [≤2.19% (3.71%) at 100 mg/kg]e | Not applicable | Not applicable |
| U69,593 | ND[≤22.4% (14.2%) at 1.78 mg/kg]d | 2.49 (2.07–2.95) | 5.73 (5.07–6.82) | Not applicable | Not applicable |
ND, Not determined.
Due to an adverse reaction (skin ulcer).
First assessment.
Reassessment.
Due to lethality.
Due to insolubility in the chosen vehicle.
In female and male rats discriminating mitragynine (32 mg/kg), mean (S.E.M.) drug-lever responding after vehicle was 2.0% (1.3%) and 2.0% (0.93%), respectively; mean (S.E.M.) response rates normalized to vehicle control were 98% (9.4%) and 91% (4.8%), respectively; and mean (S.E.M.) hot-plate response latencies expressed as MPE were 4.1% (5.8%) and 5.1% (3.8%), respectively (Supplemental Figs. 5 and 6, right, filled circle and open square above vehicle). Mitragynine dose-dependently increased drug-lever responding and decreased response rate; however, no dose of mitragynine was significantly different from vehicle in the hot-plate assay (Supplemental Figs. 5 and 6). The ED50 values (95% CIs) for the discriminative-stimulus effects of mitragynine were 14 (9.0–18) mg/kg in females and 17 (14–20) mg/kg in males (Supplemental Table 3; Tables 5 and 6). Corresponding values to decrease response rates were 36 (30–46) and 65 (43–575) mg/kg, respectively (Supplemental Table 3; Tables 5 and 6). The potencies of morphine and mitragynine to produce discriminative-stimulus, rate-decreasing, and antinociceptive effects did not differ significantly between the first and second determinations (Supplemental Table 3; Tables 5 and 6).
When sex was analyzed as a main effect, there were no significant differences for discriminative-stimulus, rate-decreasing, and antinociceptive effects for morphine (F values ≤ 3.37; P values ≥ 0.116) and mitragynine (F values ≤ 2.03; P values ≥ 0.205, Supplemental Table 2).
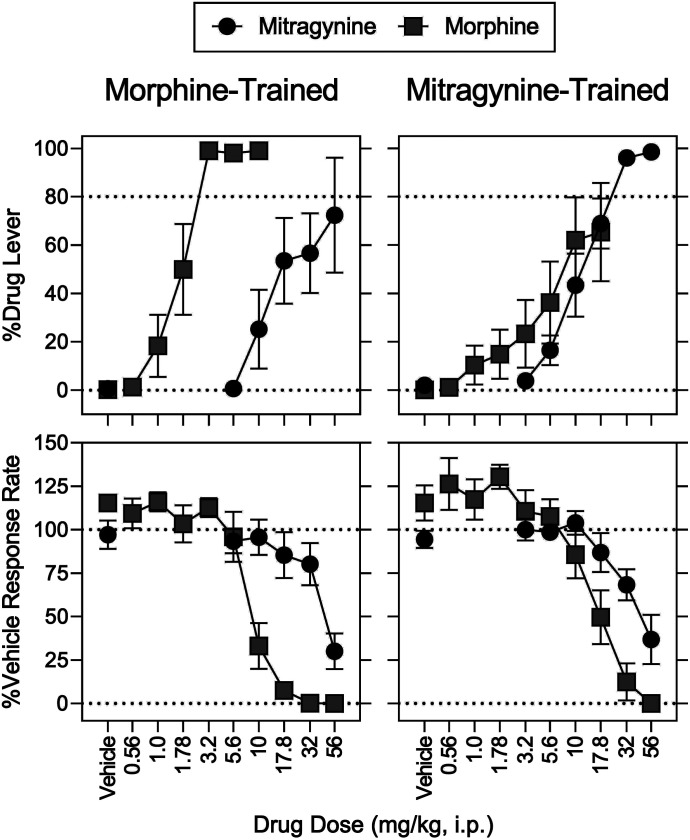
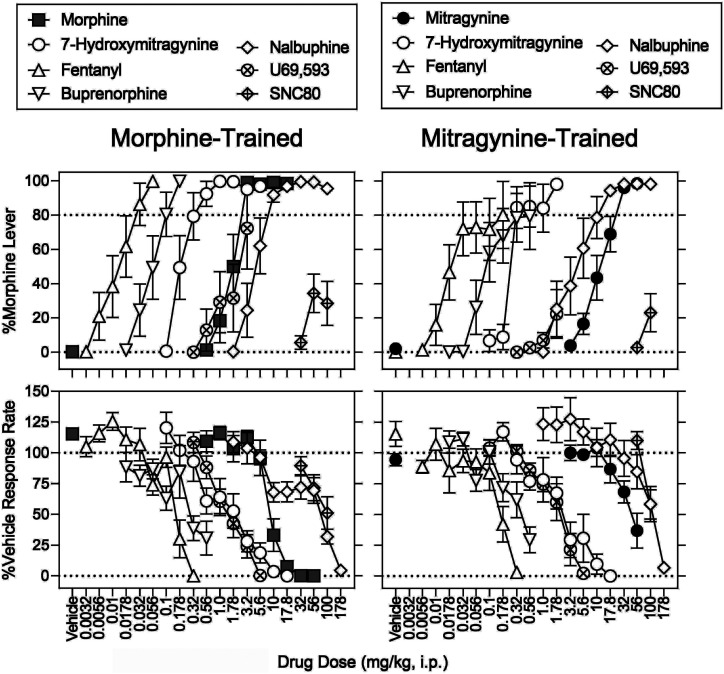
Cross-Substitution.
In rats discriminating morphine, 56 mg/kg of mitragynine produced a maximum of 72% (S.E.M.: 24%) drug-appropriate responding and decreased operant response rates to 30% of vehicle control; %MPE was not increased above 7% by any dose of mitragynine (Figs. 3 and 4, left, filled circles). The mitragynine ED50 values (95% CIs) to increase morphine-lever responding and to decrease response rates were 30 (19–60) and 46 (36–64) mg/kg (Supplemental Table 3; Tables 5 and 6). In rats discriminating mitragynine, there was no significant effect of sex and no morphine dose × sex interaction for the discriminative-stimulus, rate-decreasing, and antinociceptive effects (Supplemental Table 2). Morphine produced a maximum of 65% (S.E.M.: 20%) mitragynine-lever responding at 17.8 mg/kg; the 56 mg/kg dose of morphine eliminated responding and increased MPE to 90% (S.E.M.: 4.3%) (Figs. 3 and 4, right, gray squares). The morphine ED50 values to increase mitragynine-lever responding, to decrease response rates, and to increase MPE were 16 (10–36), 25 (19–31), and 36 (31–41) mg/kg, respectively (Supplemental Table 3; Tables 5 and 6). There was no significant effect of sex and its interaction with morphine dose on mitragynine-lever responding and MPE (Supplemental Table 2). For effects on response rate, there was no significant effect of sex (P = 0.620, Supplemental Table 2); however, there was a significant morphine dose × sex interaction (P = 0.002, Supplemental Table 2). Post hoc testing indicated significant differences that are shown in Supplemental Fig. 6.
Fig. 3.
Mitragynine substitution in rats trained to discriminate morphine (left) and morphine substitution in rats trained to discriminate mitragynine (right). Abscissae: vehicle and drug dose in milligrams per kilogram (intraperitoneal, log scale). Ordinates: top, percentage of responses on the training drug–appropriate lever. Bottom, mean rates of responding expressed as a percentage of vehicle control. Morphine and mitragynine were administered intraperitoneally, respectively, at 15 and 30 minutes before sessions. Each point represents the mean ± S.E.M. (N = 8) except for % Drug Lever at 56 mg/kg mitragynine in the morphine discrimination (N = 4) and 17.8 mg/kg morphine in the mitragynine discrimination (N = 6) mg/kg. Details for statistical analyses are shown in Supplemental Tables 2–4 and Tables 5 and 6.
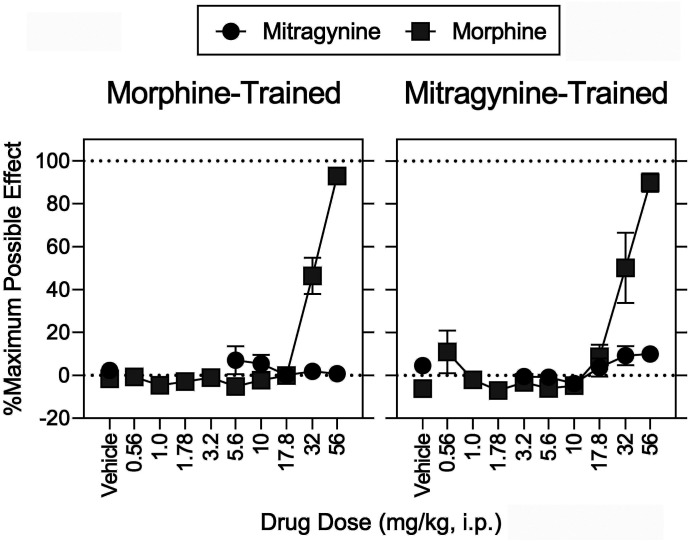
Fig. 4.
Antinociceptive effects determined in conjunction with the discrimination tests shown in Fig. 5. Abscissae: vehicle and drug dose in mg/kg (intraperitoneal, log scale) in separate groups of rats discriminating either morphine (left) or mitragynine (right). Ordinates: percentage of maximum possible antinociceptive effects. Each point represents the mean ± S.E.M. (N = 8). Morphine and mitragynine were administered intraperitoneally, respectively, at 15 and 30 minutes before sessions. Details for statistical analyses are shown in Supplemental Tables 2–4 and Tables 5 and 6.
Table 7 shows potency ratios comparing morphine and mitragynine between the two training drugs. For all three variables (discriminative-stimulus, rate-decreasing, and antinociceptive effects), morphine was at least 8.5-fold more potent in morphine-trained rats than in mitragynine-trained rats. In contrast, the potencies of mitragynine to produce discriminative-stimulus and rate-decreasing effects did not differ between training drugs (Supplemental Table 4).
TABLE 7.
Potency ratios of morphine and mitragynine alone in the presence of various compounds to produce discriminative-stimulus, rate-decreasing, and antinociceptive effects in either morphine- or mitragynine-trained rats
Each potency ratio (95% CIs) is a combination of females and males unless otherwise noted. The ED50 values of morphine and mitragynine alone are shown in Table 4. The sample sizes are described in each figure legend (Figs. 7–10). Significant differences are italicized.
| Rats Trained with Morphine | |||
|---|---|---|---|
| Test Compound | Discriminative Stimulus | Response Rate | Antinociception |
| 0.032 mg/kg Naltrexone + morphine vs. morphine alone | First: 8.22 (5.77–11.6). Reassessment: 9.50 (6.65–13.8) | First: 2.58 (1.68–4.45). Reassessment: 1.66 (1.08–2.81) | Not applicable |
| 0.032 mg/kg Naltrexone + 7-hydroxymitragynine vs. 7-hydroxymitragynine alone | 2.44 (1.46–9.69) | 1.29 (0.753–2.32) | Not applicable |
| 1.78 mg/kg Nalbuphine + morphine vs. morphine alone | First: 0.181 (0.0918–0.288). Reassessment: 0.209 (0.106–0.342) | First: 1.90 (1.33–2.86). Reassessment: 1.22 (0.855–1.80) | Not applicable |
| 5.6 mg/kg Mitragynine + morphine vs. morphine alone | First: 0.258 (0.120–0.327). Reassessment: 0.299 (0.139–0.387) | First: 0.854 (0.555–1.34). Reassessment: 0.549 (0.356–0.845) | Not applicable |
| Rats trained with mitragynine | |||
| 0.032 mg/kg Naltrexone + mitragynine vs. mitragynine alone | Not applicable | Not applicable | Not applicable |
Effects of 7-Hydroxymitragynine.
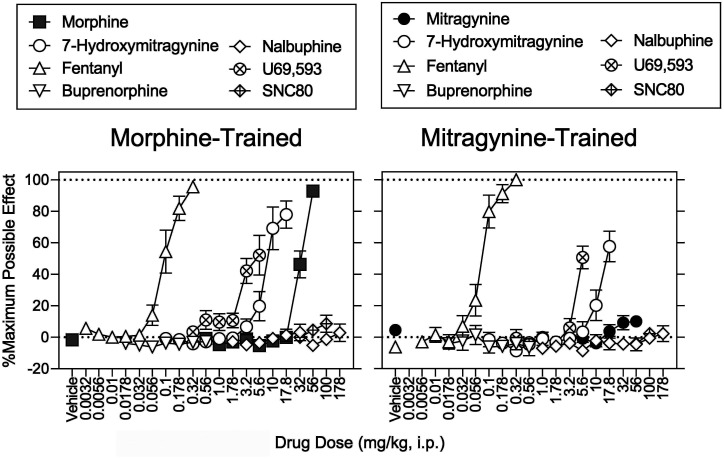
In morphine-trained rats, 7-hydroxymitragynine at 1.0 mg/kg produced a maximum of 100% (0.1%) drug-lever responding; 17.8 mg/kg eliminated responding and produced 78% (8.7%) MPE (Figs. 5 and 6, left, open circles). The ED50 values of 7-hydroxymitragynine to increase morphine-lever responding, decrease response rates, and increase MPE were 0.28 (0.077–0.41), 4.7 (3.3–6.4), and 11 (9.3–12) mg/kg, respectively (Supplemental Table 3; Tables 5 and 6). In mitragynine-trained rats, 7-hydroxymitragynine produced a maximum of 98% (1.2%) drug-lever responding at 1.78 mg/kg; 17.8 mg/kg decreased response rates to 0.041% of vehicle control and produced 58% (9.7%) MPE (Figs. 5 and 6, right, open circles). The ED50 values of 7-hydroxymitragynine to increase mitragynine-lever responding, decrease response rates, and increase %MPE were 0.42 (0.083–0.66), 5.5 (3.9–7.4), and 16 (14–21) mg/kg, respectively (Supplemental Table 3; Tables 5 and 6).
Fig. 5.
Substitution tests in separate groups of rats discriminating either morphine (left) or mitragynine (right). Abscissae: vehicle and drug dose in mg/kg (intraperitoneal, log scale). Ordinates: top, percentage of responses on the training drug-appropriate lever. Bottom, mean rates of responding expressed as a percentage of vehicle control. All compounds were administered intraperitoneally 15 minutes before sessions except mitragynine (30 minutes prior to sessions). The training drug dose-effect functions are replotted from Fig. 5. Each point represents the mean ± S.E.M. (N = 8) except in the morphine discrimination [7-hydroxymitragynine at 0.56, 1.0, and 1.78 mg/kg (N = 7) and 3.2 mg/kg (N = 5); buprenorphine at 0.178 mg/kg (N = 5); nalbuphine at 100 mg/kg (N = 7); U69,593 at 1.0 mg/kg (N = 7), 1.78 mg/kg (N = 6), and 3.2 mg/kg (N = 4); and SNC 80 at 100 mg/kg (N = 7) mg/kg] and in the mitragynine discrimination [7-hydroxymitragynine at 1.0 and 1.78 mg/kg (N = 7); fentanyl at 0.1 mg/kg (N = 7) and 0.178 mg/kg (N = 5); buprenorphine at 0.32 mg/kg (N = 7) and 0.56 mg/kg (N = 5); nalbuphine at 100 mg/kg (N = 6); and U69,593 at 1.0 and 1.78 mg/kg (N = 7)]. Details for statistical analyses are shown in Supplemental Tables 2–4 and Tables 5 and 6.
Fig. 6.
Antinociceptive effects determined in conjunction with the discrimination tests shown in Fig. 7. Abscissae: vehicle and drug dose in mg/kg (intraperitoneal, log scale). Ordinates: percentage of maximum possible antinociceptive effects. Each point represents the mean ± S.E.M. (N = 8). All compounds were administered intraperitoneally 15 minutes before sessions except mitragynine (30 minutes prior to sessions). The morphine and mitragynine dose-effect functions are replotted from Fig. 4 left and right, respectively. Details for statistical analyses are shown in Supplemental Table 3 and Tables 4–7.
Effects of Fentanyl.
In morphine-trained rats, fentanyl dose-dependently increased drug-lever responding to 98% (0.15%) at 0.056 mg/kg; 0.32 mg/kg decreased response rates to 0.018% of vehicle control and increased MPE to 96% (3.0%) (Figs. 5 and 6, left, open upward triangles). The ED50 values of fentanyl to increase morphine-lever responding, decrease response rates, and increase MPE were 0.022 (0.011–0.032), 0.17 (0.15–0.20), and 0.14 (0.11–0.17) mg/kg, respectively (Supplemental Table 3; Tables 5 and 6). In mitragynine-trained rats, fentanyl produced a maximum of 81% (S.E.M.: 20%) drug-lever responding at 0.178 mg/kg; 0.32 mg/kg decreased responding to 3.0% of vehicle control and increased MPE to 100% (Figs. 5 and 6, right, open upward triangles). The ED50 values of fentanyl to increase mitragynine-lever responding, decrease response rates, and increase MPE were 0.051 (0.012–0.091), 0.17 (0.14–0.21), and 0.12 (0.093–0.14) mg/kg, respectively (Supplemental Table 3; Tables 5 and 6). There was a significant fentanyl dose × sex interaction on mitragynine-appropriate responding (Supplemental Table 2). Post hoc testing suggested that fentanyl was more potent in females than males (Supplemental Fig. 6; Supplemental Table 2). None of the effects of fentanyl significantly differed as a function of training drug (Supplemental Table 4).
Effects of U69,593 and SNC 80.
In morphine-trained rats, U69,593 produced a maximum of 72% (24%) drug-lever responding at 3.2 mg/kg; 5.6 mg/kg suppressed responding and increased MPE to 52% (13%) (Figs. 5 and 6, left, crosshatch circles). The U69,593 ED50 values to increase morphine-lever responding, decrease response rates, and increase MPE were 2.3 (1.6–4.4), 2.3 (1.9–2.8), and 5.1 (4.1–6.8) mg/kg (Supplemental Table 3; Tables 5 and 6). There was a significant U69,593 dose × sex interaction for U69,593-induced antinociception. Post hoc testing indicated MPE at 5.6 mg/kg was greater in females than males (Supplemental Table 2). In mitragynine-trained rats, U69,593 produced a maximum of 22% (S.E.M.: 14%) drug-lever responding at 1.78 mg/kg [40% (S.E.M.: 34%) drug-lever responding at 3.2 mg/kg (N = 1 per sex)]; 5.6 mg/kg dose of U69,593 markedly decreased response rates and produced a 51% (7.3%) MPE (Figs. 7 and 8, right, circles with cross hatch). The ED50 values of U69,593 to produce the rate-decreasing and antinociceptive effects were 2.5 (2.1–3.0) and 5.7 (5.1–6.8) mg/kg, respectively (Supplemental Table 3; Tables 5 and 6). In morphine-trained rats, SNC 80 produced a maximum of 34% (11%) drug-lever responding at 56 mg/kg; 100 mg/kg decreased response rates to 51% (13%) of vehicle control and increased MPE to 8.6% (5.5%) (Figs. 5 and 6, left, diamonds with cross hatch). Doses higher than 100 mg/kg were insoluble in the chosen vehicle. In mitragynine-trained rats, SNC 80 produced a maximum of 23% (S.E.M.: 11%) drug-lever responding; 100 mg/kg decreased response rates to 58% (12%) of control and did not significantly increase MPE (Figs. 5 and 6, right, diamonds with cross hatch). The effects of U69,593 and SNC 80 did not significantly differ as a function of training drug (Supplemental Table 4).
Fig. 7.
Naltrexone antagonism of the effects of morphine and 7-hydroxymitragynine in rats discriminating morphine and mitragynine in rats discriminating mitragynine. Abscissae: vehicle and dose in mg/kg (intraperitoneal, log scale) for mitragynine (left), 7-hydroxymitragynine (middle), and mitragynine (right). Ordinates: top, percentage of responses on the training drug-appropriate lever. Bottom, mean rates of responding expressed as a percentage of vehicle control. Morphine and 7-hydroxymitragynine were administered intraperitoneally 15 minutes before sessions, and mitragynine and naltrexone (0.032 mg/kg) were administered intraperitoneally 30 minutes before sessions. The dose-effect functions for the training drugs morphine and mitragynine are replotted from Fig. 5, and the dose-effect function for mitragynine is replotted from Fig. 7. Each point represents the mean ± S.E.M. (N = 8) except for naltrexone + morphine at 17.8 mg/kg (N = 7) and 32 mg/kg (N = 5), naltrexone + 7-hydroxymitragynine at 1.78 mg/kg (N = 6), and 3.2 mg/kg (N = 4) mg/kg, naltrexone + mitragynine at 56 mg/kg (N = 7). Details for statistical analyses are shown in Supplemental Tables 2–4 and Tables 5 and 6.
Fig. 8.
Antinociceptive effects determined in conjunction with the discrimination tests shown in Fig. 9. Abscissae: vehicle and drug dose in mg/kg (intraperitoneal, log scale). Ordinates: percentage of maximum possible antinociceptive effects. Each point represents the mean ± S.E.M. (N = 8). Morphine and 7-hydroxymitragynine were administered intraperitoneally 15 minutes before sessions, and mitragynine and naltrexone (0.032 mg/kg) were administered intraperitoneally 30 minutes before sessions. The morphine and mitragynine dose-effect functions are replotted from Fig. 6 left and right, respectively, and the 7-hydroxymitragynine dose-effect function is replotted from Fig. 8. Details for statistical analyses are shown in Supplemental Tables 2–4 and Tables 5 and 6.
Effects of Buprenorphine and Nalbuphine.
In morphine-trained rats, buprenorphine produced a maximum of 100% (0.09%) drug-lever responding at 0.178 mg/kg; 0.56 mg/kg decreased response rates to 31% (14%) of vehicle control and did not significantly increase MPE (Figs. 5 and 6, right, open downward triangles). The ED50 values of buprenorphine to increase morphine-lever responding and decrease response rates were 0.084 (0.046–0.12) and 0.34 (0.25–0.53) mg/kg, respectively (Supplemental Table 3; Tables 5 and 6). In mitragynine-trained rats, the 0.56 mg/kg dose of buprenorphine produced a maximum of 79% (S.E.M.: 20%) drug-lever responding, decreased response rates to 29% (11%) of control, and did not significantly change MPE relative to vehicle in the hot-plate assay (Figs. 5 and 6, right, open downward triangles). The buprenorphine ED50 values for increasing mitragynine-lever responding and decreasing response rates were 0.19 (0.067–0.31) and 0.40 (0.32–0.53) mg/kg, respectively (Supplemental 3; Tables 5 and 6). Buprenorphine did not show any antinociceptive activity here; however, the antinociceptive effects of 0.32 mg/kg buprenorphine at a lower temperature (50°C) were robust (100%) in naïve female and male rats in a preliminary study (unpublished data).
In morphine-trained rats, nalbuphine produced a maximum of 99% (0.3%) drug-lever responding at 32 mg/kg; 178 mg/kg decreased response rates to 4.4% of control and produced a 2.9% (5.5%) MPE (Figs. 5 and 6, left, open diamonds). The ED50 values of nalbuphine to increase morphine-lever responding and decrease response rates were 7.3 (2.3–11) and 81 (69–97) mg/kg, respectively (Supplemental Table 3; Tables 5 and 6). In mitragynine-trained rats, nalbuphine produced a maximum of 98% (S.E.M.: 0.9%) drug-lever responding at 56 mg/kg; 178 mg/kg markedly decreased rates and produced a 2.2% (5.1%) MPE (Figs. 5 and 6, right, open diamonds). The ED50 values of nalbuphine to increase mitragynine-lever responding and to decrease response rates were 6.7 (4.3–9.0) and 110 (92–137) mg/kg, respectively (Supplemental Table 3; Tables 5 and 6). Nalbuphine did not significantly increase MPE (Figs. 5 and 6, right, diamonds with cross hatch). The effects of buprenorphine and nalbuphine did not significantly differ as a function of training drug (Supplemental Table 4).
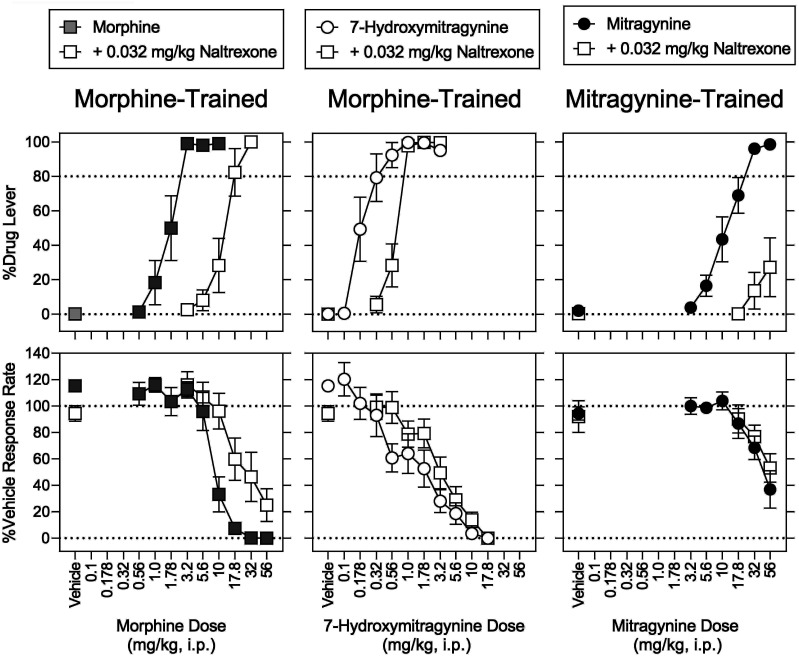
Effects of Naltrexone Combined with Morphine, Mitragynine, and 7-Hydroxymitragynine.
In morphine-trained rats, naltrexone (0.032 mg/kg) resulted in 0.1% (0.1%) drug-lever responding, 94% (5.9%) of vehicle-control response rates, and 2.5% (4.3%) MPE (Figs. 7 and 8, left, open squares above vehicle). When combined with morphine, naltrexone (0.032 mg/kg) produced significant rightward shifts in the dose-response function for the discriminative-stimulus effects of morphine when the control was determined first (8.2-fold) and then redetermined (9.5-fold) (Fig. 7, top left, open squares; Table 7). In the presence of naltrexone, there was a significant main effect of sex (P = 0.049) but not of its interaction with morphine dose (P = 0.372) (Supplemental Table 2). Post hoc tests indicated that drug-lever responding at 10 mg/kg of morphine was greater in females than males (Supplemental Table 2). Naltrexone (0.032 mg/kg) produced a smaller (2.0-fold) rightward shift in the morphine dose-effect function for rate-decreasing effects (Fig. 7, bottom left, open squares; Table 7). In the presence of naltrexone, there was a significant effect of sex (P = 0.037) and no significant interaction of morphine dose (P = 0.657) × sex for rate-decreasing effects (Supplemental Table 2). Post hoc testing indicated response rates at 32 mg/kg of morphine were greater in females than males (Supplemental Table 2). For antinociceptive effects, there was no significant main effect of sex for naltrexone in combination with morphine (P = 0.370); there was a significant morphine dose × sex interaction (P = 0.008), with post hoc testing indicating significantly greater MPE at 56 mg/kg of morphine in females than males (Supplemental Table 2).
In mitragynine-trained rats, naltrexone (0.032 mg/kg, i.p.) resulted in 0.32% (0.21%) drug-lever responding, 92% (12%) of vehicle control response rates, and −6.7% (3.3%) MPE (Figs. 7 and 8, right, open squares above vehicle). In the presence of naltrexone, 56 mg/kg of mitragynine produced a maximum of 27% (S.E.M.: 17%) drug-lever responding, decreased response rates to 53% (S.E.M.: 11%) of vehicle controls, and produced −3.9% (4.6%) MPE (Figs. 7 and 8, right, open squares). The magnitude of the significant antagonism produced by naltrexone on the discriminative-stimulus of mitragynine could not be calculated because of lack of antagonism of the rate-decreasing effects of mitragynine.
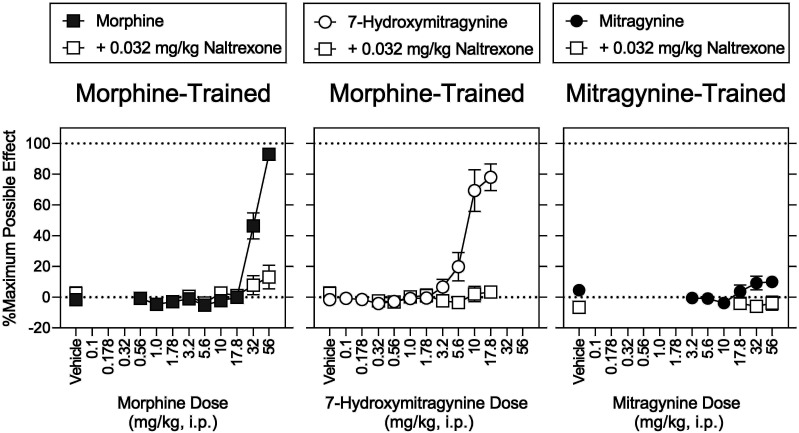
In morphine-trained rats, 0.032 mg/kg naltrexone significantly antagonized the ability of 7-hydroxymitragynine to substitute for morphine, shifting its dose-effect function 2.4-fold rightward (Fig. 7, top middle, open squares; Table 7). Naltrexone, at 0.032 mg/kg, did not significantly antagonize the rate-decreasing effects of 7-hydroxymitragynine (Fig. 7, bottom middle, open squares; Table 7). Naltrexone (0.032 mg/kg) significantly antagonized the antinociceptive effects of 7-hydroxymitragynine, reducing the MPE of 7-hydroxymitragynine (17.8 mg/kg) alone from 78% (8.7%) to 3.4% (1.9%) (Fig. 8, middle, open squares).
Effects of Nalbuphine and Mitragynine Combined with Morphine.
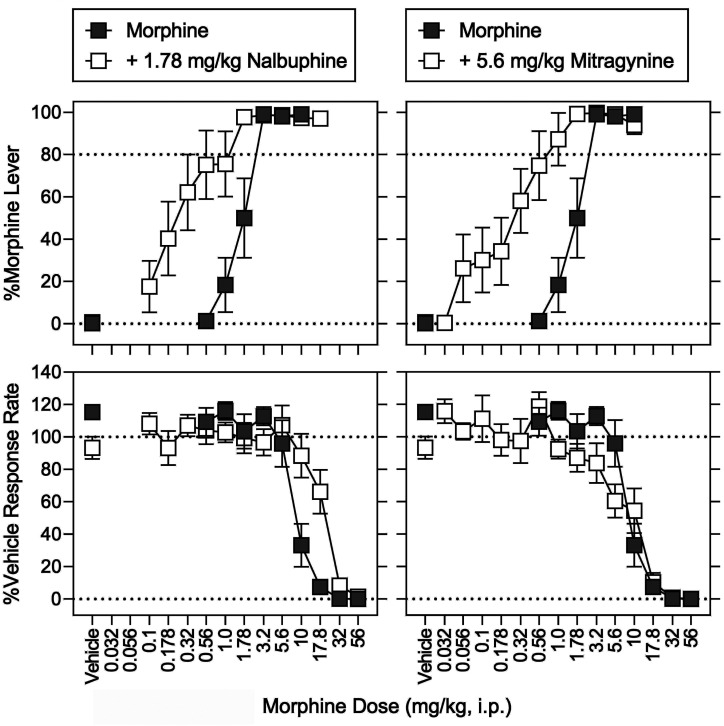
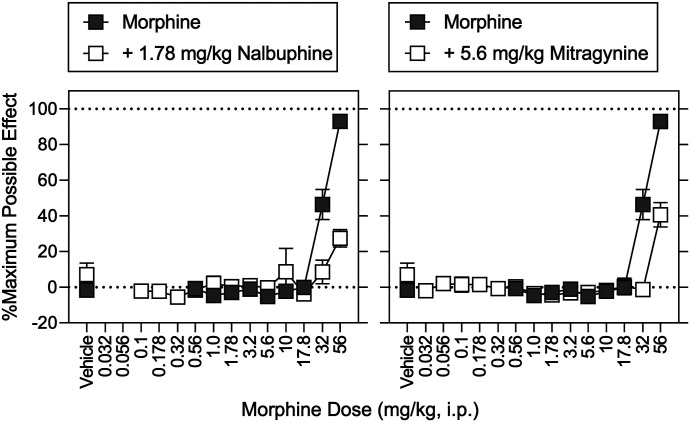
In morphine-trained rats, nalbuphine (1.78 mg/kg) produced 0.32% (0.23%) morphine-appropriate responding, 109% (8.5%) of vehicle-control response rates, and a −2.9% (1.7%) MPE (Figs. 9 and 10, left, open squares above vehicle). The same dose of nalbuphine significantly increased the potency of morphine to produce discriminative-stimulus effects, evidenced by a 4.7-fold leftward in the morphine discrimination dose-effect function (Fig. 9, top left, open squares; Table 7). In contrast, in the same animals and during the same experimental sessions, nalbuphine significantly decreased the potency of morphine to decrease response rates and increase MPE; the morphine dose-effect functions were shifted rightward 1.9-fold and greater than 1.6-fold, respectively (Fig. 9, bottom left and Fig. 10, left, open squares; Table 7). Mitragynine (5.6 mg/kg) produced 0.64% (0.37%) morphine-lever responding, 93% (6.9%) of vehicle-control response rates, and 7.0% (6.5%) MPE (Figs. 9 and 10, right, open squares above vehicle). The same dose of mitragynine significantly increased the potency of morphine 3.3-fold (Fig. 9, top right, open squares; Table 6). In contrast, and in the same animals during the same experimental sessions, mitragynine (5.6 mg/kg) antagonized the antinociceptive effects of morphine (Fig. 10, right, open squares) and did not significantly modify the morphine dose-effect function for rate-decreasing effects (Fig. 9, bottom right, open squares; Table 7).
Fig. 9.
Discriminative-stimulus effects of morphine in combination with nalbuphine (left) and mitragynine (right). Abscissae: vehicle and morphine dose in milligrams per kilogram (intraperitoneal, log scale). Ordinates: top, percentage of responses on the morphine-appropriate lever. Bottom, mean rates of responding expressed as a percentage of vehicle control. Morphine and nalbuphine (1.78 mg/kg) were administered intraperitoneally 15 minutes before sessions, whereas mitragynine (5.6 mg/kg) was administered intraperitoneally 30 minutes before sessions. The morphine dose-effect function is replotted from Fig. 5. Each point represents the mean ± S.E.M. (N = 8) except for nalbuphine + morphine at 17.8 mg/kg (N = 7) and mitragynine + morphine at 10 mg/kg (N = 5). Details for statistical analyses are shown in Supplemental Tables 2–4 and Tables 5 and 6.
Fig. 10.
Antinociceptive effects determined in conjunction with the discrimination tests shown in Fig. 9. Abscissae: vehicle and drug dose in mg/kg (intraperitoneal, log scale). Ordinates: percentage of maximum possible antinociceptive effects. Each point represents the mean ± S.E.M. (N = 8). Morphine and nalbuphine were administered intraperitoneally 15 minutes before sessions, and mitragynine was administered intraperitoneally 30 minutes before sessions. The morphine dose-effect function is replotted from Fig. 6. Details for statistical analyses are shown in Supplemental Tables 2–4 and Tables 5 and 6.
Discussion
Pharmacological mechanisms of two kratom alkaloids, mitragynine and 7-hydroxymitragynine, were assessed 1) in cell membranes expressing hMOR and 2) in behavioral assays sensitive to MOR agonism in rats (i.e., morphine discrimination and antinociception). Mitragynine had lower MOR-binding affinity than 7-hydroxymitragynine; mitragynine lacked intrinsic activity (efficacy) (i.e., was an MOR antagonist, whereas 7-hydroxymitragynine was an MOR partial agonist). In rats, the same apparent rank order of efficacy was evident (i.e., mitragynine < 7-hydroxymitragynine); behavioral effects were apparently more sensitive to MOR agonism than the [35S]GTPγS assay. Mitragynine was established as a discriminative stimulus, naltrexone antagonized the discriminative-stimulus effects of both mitragynine and 7-hydroxymitragynine, and mitragynine functioned as both an agonist and antagonist depending on the efficacy required [i.e., when the efficacy requirement was low (drug discrimination) mitragynine was an agonist, and when the efficacy requirement was high (antinociception) mitragynine was an antagonist].
Mitragynine is typically the most-abundant alkaloid in kratom products, and 7-hydroxymitragynine is a common mitragynine metabolite. The extent to which potential differences in their MOR efficacy translate to behavioral effects is currently unknown. MOR efficacy is a critical determinant of the therapeutic and adverse effects of MOR agonists, and low-efficacy MOR agonists are clinically safer than higher-efficacy MOR agonists. Using [35S]GTPγS stimulation at hMOR to assess efficacy, we found that mitragynine was an MOR antagonist, and 7-hydroxymitragynine was an MOR partial agonist. Both mitragynine and 7-hydroxymitragynine were MOR partial agonists in previous studies (Kruegel et al., 2016; Váradi et al., 2016; Obeng et al., 2020). Differences in the efficacy of mitragynine across studies could reflect differences in receptor reserve and MOR/G-protein–coupling efficiency (Niedernberg et al., 2003). Previous studies used different species, such as hMOR (Kruegel et al., 2016; Obeng et al., 2020) versus rodent MOR (Váradi et al., 2016). The experimental techniques have differed (e.g., bioluminescence resonance energy transfer and homogeneous time-resolved fluorescence (Kruegel et al., 2016; Váradi et al., 2016) versus [35S]GTPγS stimulation).
In contrast to mitragynine exhibiting no efficacy at hMOR in the current study, its behavioral effects were largely consistent with MOR agonism. Mitragynine and morphine exhibited numerous similarities in our drug discrimination assays. In both assays, MOR agonists (buprenorphine, nalbuphine, 7-hydroxymitragynine, and fentanyl) produced high levels of drug-lever responding. The rank order of hMOR-binding affinities of fentanyl, 7-hydroxymitragynine, morphine, nalbuphine, and mitragynine were similar to their ED50 values in the discrimination assays. Buprenorphine was an exception (i.e., exhibited highest affinity and the second-lowest ED50 value). The KOR agonist U69,593 and the DOR agonist SNC 80 produced less drug-lever responding than the MOR agonists, further underscoring predominant actions of the training drugs at MOR. The incomplete substitution of U69,593 and SNC 80 for morphine may reflect very low MOR efficacy at relatively high doses and the high sensitivity of the relatively low training dose of morphine to MOR agonism (see Picker et al., 1990). SNC 80 doses greater than 100 mg/kg could not be dissolved in our chosen vehicle; however, this appears to be a behaviorally active dose as evidenced by antinociceptive effects in drug-naive rats (Craft et al., 2001). The vast majority of morphine discrimination assays are selectively mediated by MOR agonism (Picker et al., 1990; Walker and Young, 2001), and it appears MOR agonism is the predominant mechanism by which mitragynine produces discriminative-stimulus effects. The qualitatively distinct effects of mitragynine in vitro (no agonism in our study) and in vivo (agonism) might be due to metabolism of mitragynine to 7-hydroxymitragynine in vivo (Kruegel et al., 2019; Hiranita et al., 2020; Kamble et al., 2020).
Mitragynine did not completely substitute for the morphine discriminative stimulus and vice versa. The discriminative-stimulus effects of mitragynine may not be solely mediated by MOR, and substitution may have been limited by rate-decreasing effects stemming from this additional pharmacological activity. When two receptor types differentially mediate the discriminative-stimulus effects of a training drug (i.e., due to higher binding affinity and/or efficacy at one site vs. another), actions at the lower affinity site can be detected by systematically increasing the training dose (e.g., nicotine; Jutkiewicz et al., 2011). By contrast, training drugs acting selectively at a receptor type will generally exhibit discriminative-stimulus effects that are mediated predominantly if not exclusively by that receptor type across a range of training doses. Morphine discriminations are selectively mediated by MOR regardless of training dose, and MOR efficacy is a key determinant of substitution; as the training dose of morphine is increased, higher MOR efficacy is required for substitution (Young et al., 1992). It is presently unclear whether multiple receptor sites of action might be detected by varying the training dose of mitragynine. Mitragynine was trained as a discriminative stimulus at a training dose of 15 mg/kg (Harun et al., 2015), which is lower than the current training dose (32 mg/kg). In comparison with the current results, morphine and mitragynine more completely cross-substituted for one another in the previous study (Harun et al.; 2015), perhaps reflecting greater selectivity of the lower training dose at MOR. Mitragynine binds with moderate affinity at several targets (e.g., α-adrenergic receptors; Boyer et al., 2008; Obeng et al., 2020), and activity at these or other nonopioid receptors could limit the degree of cross-substitution between mitragynine and morphine.
Morphine, fentanyl, 7-hydroxymitragynine, and U69,593 produced antinociception, whereas mitragynine, buprenorphine, nalbuphine, and SNC 80 did not. Mitragynine was also previously ineffective against acutely applied noxious heat in rats [see also Hiranita et al. (2019)]. In contrast, mitragynine produced antinociceptive effects in mice (Matsumoto et al., 1996a,b; Shamima et al., 2012; Kruegel et al., 2019). The rats in our study had a history of repeated MOR agonist treatment; we predict this conferred tolerance, a loss of receptor reserve, an increase in the MOR efficacy required for agonism, the greatest degree of tolerance to lower efficacy MOR agonists, and the greatest loss of sensitivity to effects requiring high efficacy, such as antinociception (Allen and Dykstra, 2000; Barrett et al., 2001; Walker and Young, 2001). In contrast, the efficacy required for drug discrimination is low [e.g., low training doses of morphine (3.2 mg/kg) are sensitive to low-efficacy agonists (e.g., Young et al., 1991; present results)]. Here we extended these findings to include substitution of mitragynine for morphine. Doses of nalbuphine and mitragynine that did not substitute for morphine increased the potency of morphine to produce discriminative-stimulus effects. However, in the same animal at the same time the same doses of nalbuphine and mitragynine antagonized the antinociceptive effects of morphine. These simultaneous and opposing pharmacological effects of low-efficacy MOR agonists are striking: they are agonists when low efficacy is required and antagonists when high efficacy is required. This highlights their therapeutic value: they are not only effective opioid substitution therapies, but they also antagonize the effects of higher-efficacy agonists, such as respiratory depression and abuse liability. Our results strongly suggest that mitragynine exhibits this profile.
Both nalbuphine and mitragynine produced a rightward shift in the concentration-effect curve of DAMGO in the [35S]GTPγS assay. Partial agonists can produce a rightward shift in the concentration-effect curve of high-efficacy MOR agonists; here the partial agonist 7-hydroxymitragynine antagonized DAMGO-induced stimulation of [35S]GTPγS. The small difference in the substitution of mitragynine and nalbuphine in morphine discriminative stimulus may reflect greater involvement of nonopioid receptors in the actions of mitragynine (Boyer et al., 2008; Ellis et al., 2020; Obeng et al., 2020) as compared with nalbuphine.
An MOR-preferential dose (0.032 mg/kg) of naltrexone (Millan, 1989) antagonized the discriminative-stimulus effects of mitragynine and morphine as well as substitution of 7-hydroxymitragynine for morphine. In a previous study, naloxone reportedly did not antagonize the discriminative-stimulus effects of mitragynine (Harun et al., 2015). In mice, however, naloxone blocked the antinociceptive effects of mitragynine (Matsumoto et al., 1996b). Both naltrexone and naloxone typically antagonize the effects of MOR agonists (Tanda et al., 2016). In the present study, the magnitude of the naltrexone-induced shift in the mitragynine dose-effect function could not be calculated because 100 mg/kg of mitragynine disrupted behavior and was lethal in a subset of animals even in the presence of naltrexone. The failure of naltrexone to antagonize the rate-decreasing effects of mitragynine has been reported previously (Hiranita et al., 2019). The differential antagonism of discriminative-stimulus and rate-decreasing effects evident for not only mitragynine but also 7-hydroxymitragynine and morphine suggests that MOR agonists engage naltrexone-insensitive receptors to disrupt operant responding.
In summary, mitragynine was demonstrated to be a low-affinity MOR ligand that exerted both agonist and antagonist activity in a predictable manner depending on the efficacy requirements of each assay. Mitragynine binds with moderate affinity to additional receptor types, such as α2-adrenergic receptors (Boyer et al., 2008; Obeng et al., 2020), and it functioned as an α2-adrenergic agonist in mice using a hot-plate assay (Matsumoto et al., 1996a). The present results strongly suggest that mitragynine is a low-efficacy MOR agonist in vivo with additional actions at nonopioid receptors. Low efficacy at MOR combined with additional pharmacological mechanism(s) appears to distinguish mitragynine as a unique molecule with considerable potential as an effective therapeutic.
Acknowledgments
The authors would like to thank Dr. Stephen J. Cutler (University of South Carolina) for providing the human DOR-CHO and human KOR-HEK cell lines. The authors would like to thank Dr. Aidan J. Hampson (Division of Therapeutics and Medical Consequences, National Institute on Drug Abuse, National Institutes of Health) and Tammy Tucker and Samantha N. Hart (College of Pharmacy, University of Florida) for administrative assistance.
Abbreviations
- CI
confidence interval
- DADLE
[D-Ala2, D-Leu5]-enkephalin
- DAMGO
[D-Ala2, N-MePhe4, Gly-ol]-enkephalin
- DOR
δ-opioid receptor
- Emax
maximum effect achievable
- FR
fixed ratio
- GTPγS
guanosine 5′-O-[γ-thio]triphosphate
- HEK
human embryonic kidney
- hMOR
human MOR
- IBNtxA
3-iodobenzoyl naltrexamine
- KOR
κ-opioid receptor
- LED
light-emitting diode
- MOR
µ-opioid receptor
- MPE
maximum possible effect TME Tris MGCl2 EGTA
- TME
Tris MGCl2 EGTA
Authorship Contributions
Participated in research design: Obeng, Wilkerson, McMahon, Hiranita.
Conducted experiments: Obeng, Reeves, Restrepo, Gamez-Jimenez, Patel, Pennington, Taylor, Ho, Braun, Williamson, Pallares, Mottinelli.
Contributed new reagents or analytic tools: León, Fortner, Lopera-Londoño, McCurdy.
Performed data analysis: Obeng, Crowley, Hiranita.
Wrote or contributed to the writing of the manuscript: Obeng, Wilkerson, León, Restrepo, Crowley, Mottinelli, Lopera-Londoño, McCurdy, McMahon, Hiranita.
Footnotes
The present study was supported by National Institutes of Health National Institute on Drug Abuse [Grants DA25267, UG3-DA048353 01, and R01 DA047855 01] and University of Florida Foundation and University of Florida Department of Pharmacodynamics funding.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Allen RM, Dykstra LA (2000) Attenuation of μ-opioid tolerance and cross-tolerance by the competitive N-methyl-D-aspartate receptor antagonist LY235959 is related to tolerance and cross-tolerance magnitude. J Pharmacol Exp Ther 295:1012–1021. [PubMed] [Google Scholar]
- Barrett AC, Cook CD, Terner JM, Craft RM, Picker MJ (2001) Importance of sex and relative efficacy at the mu opioid receptor in the development of tolerance and cross-tolerance to the antinociceptive effects of opioids. Psychopharmacology (Berl) 158:154–164. [DOI] [PubMed] [Google Scholar]
- Barrett RW, Vaught JL (1983) Evaluation of the interactions of mu and delta selective ligands with [3H]D-Ala2-D-Leu5-enkephalin binding to mouse brain membranes. Life Sci 33:2439–2448. [DOI] [PubMed] [Google Scholar]
- Boyer EW, Babu KM, Adkins JE, McCurdy CR, Halpern JH (2008) Self-treatment of opioid withdrawal using kratom (Mitragynia speciosa korth). Addiction 103:1048–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22:3099–3108. [DOI] [PubMed] [Google Scholar]
- Craft RM, Kalivas PW, Stratmann JA (1996) Sex differences in discriminative stimulus effects of morphine in the rat. Behav Pharmacol 7:764–778. [PubMed] [Google Scholar]
- Craft RM, Tseng AH, McNiel DM, Furness MS, Rice KC (2001) Receptor-selective antagonism of opioid antinociception in female versus male rats. Behav Pharmacol 12:591–602. [DOI] [PubMed] [Google Scholar]
- Crimmins EM, Zhang YS (2019) Aging populations, mortality, and life expectancy. Annu Rev Sociol 45:69–89. [Google Scholar]
- Dargan P, Wood D (2013) Novel Psychoactive Substances: Classification, Pharmacology and Toxicology, Academic Press, Amsterdam. [Google Scholar]
- Ellis CR, Racz R, Kruhlak NL, Kim MT, Zakharov AV, Southall N, Hawkins EG, Burkhart K, Strauss DG, Stavitskaya L (2020) Evaluating kratom alkaloids using PHASE. PLoS One 15:e0229646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerson PJ, Clark MJ, Mansour A, Akil H, Woods JH, Medzihradsky F (1996) Characterization of opioid agonist efficacy in a C6 glioma cell line expressing the mu opioid receptor. J Pharmacol Exp Ther 278:1121–1127. [PubMed] [Google Scholar]
- Gogineni V, León F, Avery B, McCurdy C, Cutler S (2014) Phytochemistry of Mitragyna speciosa, in Kratom and Other Mitragynines: The Chemistry and Pharmacology of Opioids from a Non-Opium Source (Raffa R ed) pp 77–94, CRC press, New York. [Google Scholar]
- Harrison C, Traynor JR (2003) The [35S]GTPgammaS binding assay: approaches and applications in pharmacology. Life Sci 74:489–508. [DOI] [PubMed] [Google Scholar]
- Harun N, Hassan Z, Navaratnam V, Mansor SM, Shoaib M (2015) Discriminative stimulus properties of mitragynine (kratom) in rats. Psychopharmacology (Berl) 232:2227–2238. [DOI] [PubMed] [Google Scholar]
- Hassan Z, Muzaimi M, Navaratnam V, Yusoff NH, Suhaimi FW, Vadivelu R, Vicknasingam BK, Amato D, von Hörsten S, Ismail NI, et al. (2013) From Kratom to mitragynine and its derivatives: physiological and behavioural effects related to use, abuse, and addiction. Neurosci Biobehav Rev 37:138–151. [DOI] [PubMed] [Google Scholar]
- Hemby SE, McIntosh S, Leon F, Cutler SJ, McCurdy CR (2019) Abuse liability and therapeutic potential of the Mitragyna speciosa (kratom) alkaloids mitragynine and 7-hydroxymitragynine. Addict Biol 24:874–885. [DOI] [PubMed] [Google Scholar]
- Hiranita T, Kohut SJ, Soto PL, Tanda G, Kopajtic TA, Katz JL (2014) Preclinical efficacy of N-substituted benztropine analogs as antagonists of methamphetamine self-administration in rats. J Pharmacol Exp Ther 348:174–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Leon F, Felix JS, Restrepo LF, Reeves ME, Pennington AE, Obeng S, Avery BA, McCurdy CR, McMahon LR, et al. (2019) The effects of mitragynine and morphine on schedule-controlled responding and antinociception in rats. Psychopharmacology (Berl) 236:2725–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Sharma A, Oyola FL, Obeng S, Reeves ME, Restrepo LF, Patel A, Behnke M, Ho NP, Williamson MR (2020) Potential contribution of 7‐hydroxymitragynine, a metabolite of the primary kratom (Mitragyna speciosa) alkaloid mitragynine, to the μ‐opioid activity of mitragynine in rats. FASEB J 34:1. [Google Scholar]
- Jutkiewicz EM, Brooks EA, Kynaston AD, Rice KC, Woods JH (2011) Patterns of nicotinic receptor antagonism: nicotine discrimination studies. J Pharmacol Exp Ther 339:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamble SH, León F, King TI, Berthold EC, Lopera-Londoño C, Siva Rama Raju K, Hampson AJ, Sharma A, Avery BA, McMahon LR, et al. (2020) Metabolism of a kratom alkaloid metabolite in human plasma increases its opioid potency and efficacy. ACS Pharmacol Transl Sci 3:1063–1068 DOI: 10.1021/acsptsci.0c00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruegel AC, Gassaway MM, Kapoor A, Váradi A, Majumdar S, Filizola M, Javitch JA, Sames D (2016) Synthetic and receptor signaling explorations of the mitragyna alkaloids: mitragynine as an atypical molecular framework for opioid receptor modulators. J Am Chem Soc 138:6754–6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruegel AC, Uprety R, Grinnell SG, Langreck C, Pekarskaya EA, Le Rouzic V, Ansonoff M, Gassaway MM, Pintar JE, Pasternak GW, et al. (2019) 7-Hydroxymitragynine is an active metabolite of mitragynine and a key mediator of its analgesic effects. ACS Cent Sci 5:992–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti RA, Mickelson MM, McCall JM, Von Voigtlander PF (1985) [3H]U-69593 a highly selective ligand for the opioid κ receptor. Eur J Pharmacol 109:281–284. [DOI] [PubMed] [Google Scholar]
- Macko E, Weisbach JA, Douglas B (1972) Some observations on the pharmacology of mitragynine. Arch Int Pharmacodyn Ther 198:145–161. [PubMed] [Google Scholar]
- Matsumoto K, Mizowaki M, Suchitra T, Murakami Y, Takayama H, Sakai S, Aimi N, Watanabe H (1996a) Central antinociceptive effects of mitragynine in mice: contribution of descending noradrenergic and serotonergic systems. Eur J Pharmacol 317:75–81. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Mizowaki M, Suchitra T, Takayama H, Sakai S, Aimi N, Watanabe H (1996b) Antinociceptive action of mitragynine in mice: evidence for the involvement of supraspinal opioid receptors. Life Sci 59:1149–1155. [DOI] [PubMed] [Google Scholar]
- Melton SH, Melton ST (2019) Current state of the problem: opioid overdose rates and deaths. Curr Treat Options Psychiatry 6:164–177. [Google Scholar]
- Millan MJ (1989) Kappa-opioid receptor-mediated antinociception in the rat. I. Comparative actions of mu- and kappa-opioids against noxious thermal, pressure and electrical stimuli. J Pharmacol Exp Ther 251:334–341. [PubMed] [Google Scholar]
- NIDA (2018) Principles of Drug Addiction Treatment: A Research-Based Guide, U.S. Government Printing Office, Washington, DC. [Google Scholar]
- Niedernberg A, Tunaru S, Blaukat A, Harris B, Kostenis E (2003) Comparative analysis of functional assays for characterization of agonist ligands at G protein-coupled receptors. J Biomol Screen 8:500–510. [DOI] [PubMed] [Google Scholar]
- Obeng S, Kamble SH, Reeves ME, Restrepo LF, Patel A, Behnke M, Chear NJY, Ramanathan S, Sharma A, León F, et al. (2020) Investigation of the adrenergic and opioid binding affinities, metabolic stability, plasma protein binding properties, and functional effects of selected indole-based kratom alkaloids. J Med Chem 63:433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onogi T, Minami M, Katao Y, Nakagawa T, Aoki Y, Toya T, Katsumata S, Satoh M (1995) DAMGO, a μ-opioid receptor selective agonist, distinguishes between μ- and δ-opioid receptors around their first extracellular loops. FEBS Lett 357:93–97. [DOI] [PubMed] [Google Scholar]
- Picker MJ, Doty P, Negus SS, Mattox SR, Dykstra LA (1990) Discriminative stimulus properties of U50,488 and morphine: effects of training dose on stimulus substitution patterns produced by mu and kappa opioid agonists. J Pharmacol Exp Ther 254:13–22. [PubMed] [Google Scholar]
- Selley DE, Liu Q, Childers SR (1998) Signal transduction correlates of mu opioid agonist intrinsic efficacy: receptor-stimulated [35S]GTP γ S binding in mMOR-CHO cells and rat thalamus. J Pharmacol Exp Ther 285:496–505. [PubMed] [Google Scholar]
- Selley DE, Sim LJ, Xiao R, Liu Q, Childers SR (1997) μ-Opioid receptor-stimulated guanosine-5′-O-(γ-thio)-triphosphate binding in rat thalamus and cultured cell lines: signal transduction mechanisms underlying agonist efficacy. Mol Pharmacol 51:87–96. [DOI] [PubMed] [Google Scholar]
- Shamima AR, Fakurazi S, Hidayat MT, Hairuszah I, Moklas MAM, Arulselvan P (2012) Antinociceptive action of isolated mitragynine from Mitragyna Speciosa through activation of opioid receptor system. Int J Mol Sci 13:11427–11442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Kamble SH, León F, Chear NJ, King TI, Berthold EC, Ramanathan S, McCurdy CR, Avery BA (2019) Simultaneous quantification of ten key Kratom alkaloids in Mitragyna speciosa leaf extracts and commercial products by ultra-performance liquid chromatography-tandem mass spectrometry. Drug Test Anal 11:1162–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HR, Zamora JC, Chavera TS, Jennings EM, Winger GD, Woods JH, Clarke WP, Berg KA (2020) Agonist‐dependent modulation by the long‐acting mu opioid receptor antagonist, methocinnamox (MCAM). FASEB J 34:1. [Google Scholar]
- Snedecor G, Cochran W, editors (1967) Statistical Methods, 6th ed, Iowa State College, Ames, IA. [Google Scholar]
- Takayama H, Ishikawa H, Kurihara M, Kitajima M, Aimi N, Ponglux D, Koyama F, Matsumoto K, Moriyama T, Yamamoto LT, et al. (2002) Studies on the synthesis and opioid agonistic activities of mitragynine-related indole alkaloids: discovery of opioid agonists structurally different from other opioid ligands. J Med Chem 45:1949–1956. [DOI] [PubMed] [Google Scholar]
- Tal M, Silberstein A, Nusser E (1985) Why does Coomassie Brilliant Blue R interact differently with different proteins? A partial answer. J Biol Chem 260:9976–9980. [PubMed] [Google Scholar]
- Tallarida RJ (2002) The interaction index: a measure of drug synergism. Pain 98:163–168. [DOI] [PubMed] [Google Scholar]
- Tanda G, Mereu M, Hiranita T, Quarterman JC, Coggiano M, Katz JL (2016) Lack of specific involvement of (+)-naloxone and (+)-naltrexone on the reinforcing and neurochemical effects of cocaine and opioids. Neuropsychopharmacology 41:2772–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Váradi A, Marrone GF, Palmer TC, Narayan A, Szabó MR, Le Rouzic V, Grinnell SG, Subrath JJ, Warner E, Kalra S, et al. (2016) Mitragynine/corynantheidine pseudoindoxyls as opioid analgesics with mu agonism and delta antagonism, which do not recruit β-arrestin-2. J Med Chem 59:8381–8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicknasingam B, Narayanan S, Beng GT, Mansor SM (2010) The informal use of ketum (Mitragyna speciosa) for opioid withdrawal in the northern states of peninsular Malaysia and implications for drug substitution therapy. Int J Drug Policy 21:283–288. [DOI] [PubMed] [Google Scholar]
- Walker EA, Young AM (2001) Differential tolerance to antinociceptive effects of mu opioids during repeated treatment with etonitazene, morphine, or buprenorphine in rats. Psychopharmacology (Berl) 154:131–142. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Yano S, Horie S, Yamamoto LT (1997) Inhibitory effect of mitragynine, an alkaloid with analgesic effect from Thai medicinal plant Mitragyna speciosa, on electrically stimulated contraction of isolated Guinea-pig ileum through the opioid receptor. Life Sci 60:933–942. [DOI] [PubMed] [Google Scholar]
- Young A, Masaki M, Geula C (1992) Discriminative stimulus effects of morphine: effects of training dose on agonist and antagonist effects of mu opioids. J Pharmacol Exp Ther 261 (1):246–257 . [PubMed] [Google Scholar]
- Yue K, Kopajtic TA, Katz JL (2018) Abuse liability of mitragynine assessed with a self-administration procedure in rats. Psychopharmacology (Berl) 235:2823–2829. [DOI] [PubMed] [Google Scholar]