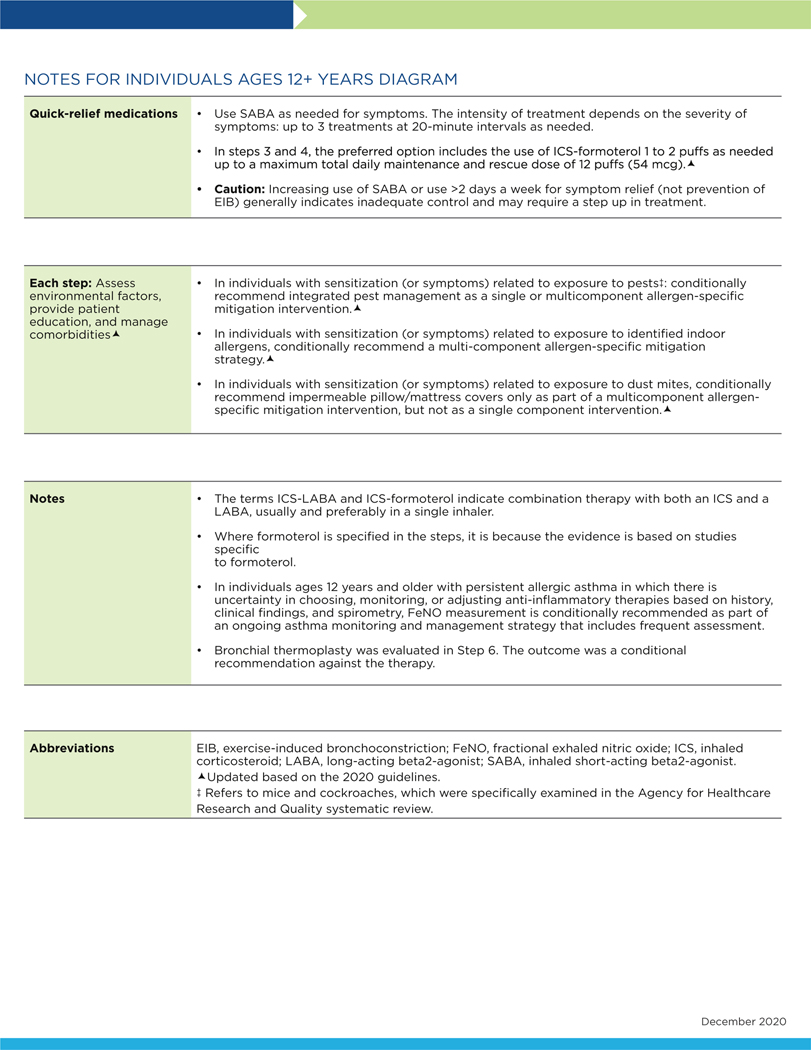
Abstract
The 2020 Focused Updates to the Asthma Management Guidelines: A Report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group was coordinated and supported by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health. It is designed to improve patient care and support informed decision making about asthma management in the clinical setting. This update addresses six priority topic areas as determined by the state of the science at the time of a needs assessment, and input from multiple stakeholders:
Fractional Exhaled Nitric Oxide Testing
Indoor Allergen Mitigation
Intermittent Inhaled Corticosteroids
Long-Acting Muscarinic Antagonists
Immunotherapy in the Treatment of Allergic Asthma
Bronchial Thermoplasty
A rigorous process was undertaken to develop these evidence-based guidelines. The Agency for Healthcare Research and Quality’s (AHRQ) Evidence-Based Practice Centers conducted systematic reviews on these topics, which were used by the Expert Panel Working Group as a basis for developing recommendations and guidance. The Expert Panel used GRADE (Grading of Recommendations, Assessment, Development and Evaluation), an internationally accepted framework, in consultation with an experienced methodology team for determining the certainty of evidence and the direction and strength of recommendations based on the evidence. Practical implementation guidance for each recommendation incorporates findings from NHLBI-led patient, caregiver, and clinician focus groups. To assist clincians in implementing these recommendations into patient care, the new recommendations have been integrated into the existing Expert Panel Report-3 (EPR-3) asthma management step diagram format.
Keywords: NHLBI, Asthma Guideline, asthma, fractional exhaled nitric oxide, allergen mitigation, inhaled corticosteroids, long-acting muscarinic antagonist, bronchial thermoplasty, immunotherapy
PREFACE
This report was developed by an Expert Panel Working Group (hereafter referred to as the “Expert Panel”) of the National Asthma Education and Prevention Program (NAEPP) Coordinating Committee (NAEPPCC), presented to the NAEPPCC for the full committee’s consideration, and adopted by the NAEPPCC during a public meeting. The NAEPPCC is coordinated by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health.
The NHLBI is pleased to present this update, in which several changes to the approaches used in prior NAEPPCC expert panel reports (EPRs) have been implemented. Specifically:
The decision to update Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma (EPR-3) and the selection of topics to update were initiated by engaging the public with a request for information, rather than relying solely on the NAEPP for these decisions.
To use the most rigorous methods for gathering information for the focused update, the Agency for Healthcare Research and Quality (AHRQ) conducted systematic reviews.
A consultant with expertise in GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) methodology guided the Expert Panel members in their deliberations and development of the recommendations based on the systematic review reports.
In this report, which was adopted by the NAEPPCC, the Expert Panel has included practical implementation guidance for each recommendation that incorporates findings from NHLBI-led focus groups. These focus groups included people with asthma, caregivers, and providers. To assist providers in integrating these recommendations into the care of patients, the new recommendations have been integrated into the EPR-3 step diagram format. Overall, a highly rigorous process was undertaken to facilitate the development of the evidence-based recommendations and supporting information in this report for use by stakeholders to improve asthma management.
This report was developed under the leadership of Dr Michelle Cloutier, Expert Panel chair. The NHLBI is grateful for the tremendous dedication of time and outstanding work of all members of the Expert Panel in developing this report. Appreciation is also extended to the NAEPPCC as well as other stakeholder groups (professional societies, health care organizations, government agencies, consumer and patient advocacy organizations, and companies) for their invaluable comments during the public review period. These comments helped enhance the scientific credibility and practical utility of this document.
Ultimately, broad change in clinical practice depends on the uptake, adoption, and implementation of clinical practice recommendations by primary care providers with input from people who have asthma and their families, as well as support from health care systems. This update can serve as a basis to disseminate and facilitate adoption of the asthma recommendations at all levels and to ensure optimal care and equitable outcomes for all individuals with asthma. We ask for the assistance of every stakeholder in reaching our goal: improving asthma care and the quality of life of every person with asthma.
| James P. Kiley, MS, PhD Director Division of Lung Diseases NHLBI |
George A. Mensah, MD Director Center for Translation Research and Implementation Science NHLBI |
FOREWORD
It has been 13 years since the last revision of the asthma recommendations, and substantial progress has been made since that time in understanding the origins of asthma as well as its pathophysiology and treatment. As members of the pulmonary and allergy provider community and the primary care community that provide more than half of all asthma care in the United States, we now recognize that asthma is not one disease, but it is a syndrome composed of multiple phenotypes. Asthma is much more complex than indicated in the Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma (EPR-1),1 released in 1991, which characterized asthma as an inflammatory disease that is responsive to corticosteroids.
This document updates selected topics that were identified as high priority by an NHLBI Advisory Council Asthma Expert Working Group based on input from previous guideline developers, NAEPP participant organizations, and the public. The list of these priority topics was published in 2015.2
Seventeen topics were suggested initially for updating, and six topics were found to have sufficient new information to warrant an update. Key questions were drafted by the Advisory Council and used by AHRQ Evidence-Based Practice Centers (EPCs) to conduct systematic reviews that were published between October 2017 and March 2018.3–7 The Expert Panel was then assembled in July 2018 and charged with using these systematic reviews to develop recommendations on these six previously chosen topics.
The Expert Panel updated the literature for the systematic reviews through October 2018 and then developed its recommendations. These recommendations differ from other guidelines in several important ways:
The key questions were developed a priori and not after a review of the current literature.
The Expert Panel was composed of diverse individuals not only from the asthma specialty community (adult and pediatric pulmonary and allergy specialists) but also from the general medical community (pediatric, internal medicine, family medicine, and emergency medicine providers). Expert Panel members also included health policy and dissemination and implementation experts, and the panel received input from patients and families.
The Expert Panel members abided by strict standards for conflicts of interest (COIs) developed by the Institute of Medicine (now the National Academy of Medicine)8 and in the spirit of the more recently released recommendations from the American College of Physicians (ACP).9 Individuals with any conflict of interest related to the updated topics recused themselves from discussions of those topics.
This was the first time that the NAEPP used the GRADE methodology (discussed later) to provide transparency in the decision-making process.
Lastly, but not insignificantly, the Expert Panel sought comments from external groups and individuals, including from the NAEPP Coordinating Committee (whose members represent a diverse group of stakeholders), the public, and federal agencies. Although the panel that developed the Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma (EPR-3) also sought external input, this approach has rarely been used by other asthma guideline committees. The Expert Panel considered this input when it developed the final recommendations and this document.
The methodology framework used for this update, GRADE, is the internationally recommended approach for developing recommendations that clinicians can trust. This framework endorses a systematic and transparent approach to decision making, uses established criteria to rate the certainty of evidence, and determines the strength of the recommendations. Recommendations developed using GRADE combine certainty of evidence with patient values and preferences and weigh the benefits and harms of making treatment recommendations. Importantly, the recommendations are based on the key questions that clinicians, both generalists and specialists, wanted to be answered.
Users of these recommendations may be disappointed by the absence of many strong recommendations—that is, recommendations that clinicians should adhere to for almost all individuals with asthma as the standard of care. This is not, however, surprising given the variations in asthma phenotypes and endotypes and in the outcomes used in the studies reviewed to develop the recommendations. When the GRADE framework is used, randomized controlled trials (RCTs) are initially rated as offering a high certainty of evidence, but issues with study designs (eg, lack of blinding or of a placebo control), heterogeneity of study results, or small numbers of events may result in downgrading the certainty of evidence. For most of the asthma recommendations, the overall certainty of the evidence was downgraded because of inconsistencies in study results, risk of bias, or absence of critical standardized outcome measures. The need to downgrade the evidence should be a clarion call to investigators to use standardized and validated outcome measures that were outlined in the Asthma Outcomes Workshop (2012).10 This single activity will create more robust evidence to support recommendations in the future.
The working group that identified the six priority topics for this update based its recommendations on information available at that time. This information did not include the subsequent explosion of research and US Food and Drug Administration (FDA) approval of multiple drugs classified as asthma biologics. Any attempt to include biologic agents in this report at the start of this effort would have delayed the release of these recommendations for another 1 to 2 years, and this was felt to be unacceptable. This update also is not a complete revision of EPR-3. Important aspects of care, such as asthma education (including inhaler technique) and assessment tools for asthma control, adherence, and other factors, are not covered. Reasons for these limitations included lack of time, lack of resources, and, for some topics, insufficient new evidence.
Finally, several new features in this update were designed to aid providers and clinicians in addressing these topics with their patients. The biggest of these changes is the addition of an Implementation Guidance section for each recommendation. Each Implementation Guidance section begins with a clinician summary—an expanded statement of the recommendation to quickly assist clinicians in better understanding the recommendation from a user’s perspective. The Implementation Guidance section also provides further clarification of the population to which the recommendation applies, exceptions, and practical aspects of how to use the recommendation in patient care. At the end of each Implementation Guidance section is a list of issues suggested by the Expert Panel to communicate to patients as part of shared decision making about whether to use the therapy or intervention mentioned in the recommendation. Amended step diagrams for asthma management are also provided for the topics being updated. Many of the updated interventions in these diagrams are now preferred first-line treatments.
Moving forward, the process of guideline development needs to be more agile. Creating an ongoing process for developing recommendations that includes individuals with varied expertise and from multiple organizations may facilitate this process. In addition, the structure of the recommendations may need to change. The step diagrams, although useful, are a one-size-fits-all approach. The current recommendations use a patient-centered approach that is critical but not sufficient. In the emerging era of personalized medicine, tailored interventions and treatments customized to particular individuals with specific characteristics will be needed. Discussions about how to address individualized approaches to asthma care and how to incorporate these approaches into the standard of care are needed now so that future recommendations can integrate these new approaches.
Finally, I thank the members of the Expert Panel who voluntarily gave their time and expertise to complete this work. The amount of work that was needed in a compressed period of time from each member was very high. To them, to Drs Kiley and Mensah, whose support was unwavering, and to the NHLBI and Westat staff, thank you.
Michelle M. Cloutier, MD
Chair, Expert Panel
SECTION I: INTRODUCTION
Background and rationale for focused updates
In 1989, the NHLBI created a program, now known as the NAEPP, to address asthma issues in the United States. The NAEPP focuses on raising awareness and ensuring appropriate diagnosis and management of asthma to reduce asthma-related morbidity and mortality and to improve the quality of life of individuals with asthma. To that end, the NAEPP published its first EPR on the diagnosis and management of asthma in 1991.1 A comprehensive revision, EPR-2, was published in 1997,11 followed by an update of selected topics in 2002 and then a third EPR, EPR-3, in 2007.12
In 2014, the Asthma Expert Working Group of the National Heart, Lung, and Blood Advisory Council (NHLBAC) completed an assessment of the need to revise NAEPP’s Expert Panel Report-3: Guidelines for the Diagnosis and Management of Asthma (EPR-3)12 and the content of such a revision. After a discussion and review of the responses to a public request for information on the need for and potential content of an update, the NHLBAC Asthma Expert Working Group (which included members of the EPR-3 expert panel) determined that a focused update on six priority topics was warranted. For each of the six priority topics, the NHLBAC Asthma Expert Working Group determined the key questions to address in the systematic reviews. For each key question, the working group of the NHLBAC identified the patient population, intervention, relevant comparators, and outcomes of interest.
The six priority topics identified for systematic review were as follows:
Fractional exhaled nitric oxide (Feno) in diagnosis, medication selection, and monitoring of treatment response in asthma
Remediation of indoor allergens (eg, house-dust mites/pets) in asthma management
Adjustable medication dosing in recurrent wheezing and asthma
Long-acting antimuscarinic agents in asthma management as add-ons to inhaled corticosteroids (ICSs)
Immunotherapy and the management of asthma
Bronchial thermoplasty (BT) in adult severe asthma
The NHLBAC Asthma Expert Working Group recommended that another 11 topics be acknowledged in the update but that no recommendations be developed for these topics because of the lack of sufficient new data for a systematic review of these topics at that time.12 These emerging topics are as follows:
Adherence
Asthma action plans
Asthma heterogeneity
Biologic agents
Biomarkers (other than Feno)
Classification of asthma severity
Long-acting beta2-agonist (LABA) safety
Physiological assessments
Prevention of asthma onset
Role of community health workers in asthma management
Step down from maintenance therapy
The AHRQ EPCs conducted systematic reviews of the six priority topics and published the findings from these reviews online between October 2017 and March 2018.3–7 These systematic reviews provided the evidence used to update the priority topics for this report.
In 2015, the NAEPPCC, which is a federal advisory committee, was created to continue the work of the NAEPP. In 2018, after the systematic reviews on the priority topics were completed, the NAEPPCC established the “Expert Panel,” which was charged with using the published systematic review reports to make recommendations on the key questions that could be implemented by health care providers and people with asthma.
The Expert Panel, composed of 18 members and a chair, included asthma content experts (pediatric and adult pulmonologists and allergists, an emergency room physician, and a pharmacist), primary care clinicians (pediatric, internal medicine, and family medicine providers), health policy experts, and implementation and dissemination experts. The Expert Panel received support from individuals who had experience using the GRADE approach.13
While the Expert Panel considered its recommendations, the NHLBI convened focus groups made up of diverse asthma management stakeholders, including individuals with asthma, caregivers, and health care providers. These focus groups provided input on participants’ preferences and valuations of various asthma outcomes and interventions. The Expert Panel used summaries of these focus group discussions to inform its recommendations.
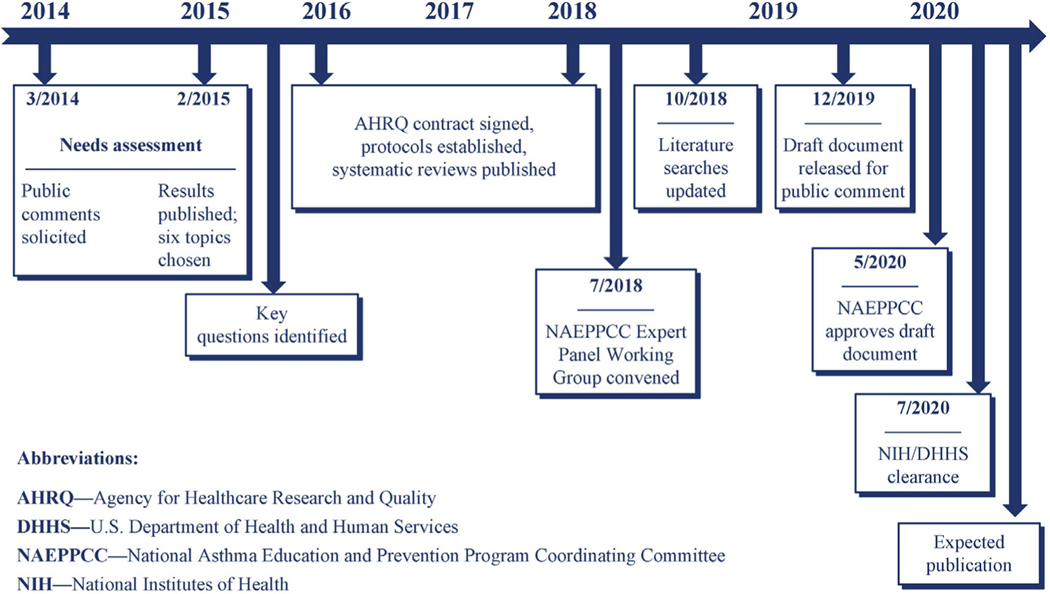
The Expert Panel initially presented its draft recommendations for comment and review to the NAEPPCC. The draft recommendations were also issued for public comment as well as for input from federal agencies. The Expert Panel considered all comments received and incorporated many of them into this final report. The NAEPPCC adopted the Expert Panel’s report during a public meeting and recommended the updated guidelines to the US Department of Health and Human Services. Following review and clearance, the US Department of Health and Human Services approved the updated guidelines, which were subsequently published in the Journal of Allergy and Clinical Immunology (JACI). A timeline of the steps completed to produce this report, beginning with the needs assessment, is shown in Fig 1.
FIG 1.
Timeline for 2020 Asthma Guideline Update.
Methods
Four AHRQ EPCs conducted and published systematic review reports on the key questions for the six priority topics. The pharmacologic topics (adjustable medication dosing and long-acting muscarinic antagonists [LAMAs]) were combined into a single systematic review; therefore, five systematic review reports were prepared on the six priority topics:
The Clinical Utility of Fractional Exhaled Nitric Oxide (FeNO) in Asthma Management (https://doi.org/10.23970/AHRQEPCCER197)
Effectiveness of Indoor Allergen Reduction in Management of Asthma (https://doi.org/10.23970/AHRQEPCCER201)
Intermittent Inhaled Corticosteroids and Long-Acting Muscarinic Antagonists for Asthma (https://doi.org/10.23970/AHRQEPCCER194)
Role of Immunotherapy in the Treatment of Asthma (https://effectivehealthcare.ahrq.gov/products/asthma-immunotherapy/research)
Effectiveness and Safety of Bronchial Thermoplasty in Management of Asthma (https://doi.org/10.23970/AHRQEPCCER202)
Systematic reviews of the literature
The protocols3–7 that the EPCs used in their systematic reviews describe the prespecified key questions that they addressed (listed in Table IA), the methods they used, and the overall analytic framework.
TABLE IA.
Systematic review key questions
| Topic | Key question |
|---|---|
| Feno | What is the diagnostic accuracy of Feno measurement(s) for making the diagnosis of asthma in individuals aged 5 y and older? |
| What is the clinical utility of Feno measurements in monitoring disease activity and asthma outcomes in individuals with asthma aged 5 y and older? | |
| What is the clinical utility of Feno measurements to select medication options (including steroids) for individuals aged 5 y and older? | |
| What is the clinical utility of Feno measurements to monitor response to treatment in individuals aged 5 y and older? | |
| In children aged 0–4 years with recurrent wheezing, how accurate is Feno testing in predicting the future development of asthma at age 5 y and above? | |
| Allergen mitigation | Among individuals with asthma, what is the effectiveness of interventions to reduce or remove exposures to indoor inhalant allergens on asthma control, exacerbations, quality of life, and other relevant outcomes? |
| ICS | What is the comparative effectiveness of intermittent ICS compared to no treatment, pharmacologic therapy, or nonpharmacologic therapy in children aged 0–4 y with recurrent wheezing? |
| What is the comparative effectiveness of intermittent ICS compared to ICS controller therapy in individuals 5 y and older with persistent asthma? | |
| What is the comparative effectiveness of ICS with LABA used as both controller and quick-relief therapy compared to ICS with or without LABA used as controller therapy in individuals 5 y and older with persistent asthma? | |
| LAMA | What is the comparative effectiveness of LAMA compared to other controller therapy as add-on to ICS in individuals aged 12 y and older with uncontrolled, persistent asthma? |
| What is the comparative effectiveness of LAMA as add-on to ICS controller therapy compared to placebo or increased ICS dose in individuals aged 12 y and older with uncontrolled, persistent asthma? | |
| What is the comparative effectiveness of LAMA as add-on to ICS-LABA compared to ICS-LABA as controller therapy in individuals aged 12 y and older with uncontrolled, persistent asthma? | |
| Immunotherapy | What is the evidence for the efficacy of SCIT in the treatment of asthma? |
| What is the evidence for the safety of SCIT in the treatment of asthma? | |
| What is the evidence for the efficacy of SLIT, in tablet and aqueous form, for the treatment of asthma? | |
| What is the evidence for the safety of SLIT, in tablet and aqueous form, for the treatment of asthma? | |
| BT | What are the benefits and harms of using BT in addition to standard treatment for the treatment of individuals aged 18 y and older with asthma? |
When conducting the systematic reviews, the EPCs sought studies that included the prespecified target population(s) and settings and that used the prespecified interventions, comparators, and outcomes. The EPCs excluded articles about studies that did not meet the inclusion criteria listed in the protocols for each systematic review. These inclusion criteria were summarized in the published systematic review reports. (Appendices to the systematic review reports documented the rationales for excluding published articles identified by a broad search of the literature.) The systematic review reports also included the EPCs’ assessments of the risk of bias of each included article and of the strength of evidence for each key question using methods described in the protocols and systematic review reports. The EPCs were not required to use the GRADE methodology to conduct the systematic reviews, but they used a similar framework. After peer review and posting for public comment, the systematic review reports were finalized and published between late 2017 and early 2018.
Updated reviews of the literature
Westat (contract #HHSN268201700020B) conducted a literature search to identify any new articles published between the completion of the EPC’s systematic review literature searches and October 2018, when the Expert Panel began its work. The search strategies and the inclusion and exclusion criteria used in the updated literature searches were as similar as possible to those used in the initial systematic reviews. After reviewing the results of the updated literature searches, the Expert Panel determined that 15 additional articles addressing specific aspects of the key questions should be included in the focused update. The new articles were assessed for risk of bias. The Expert Panel considered the new evidence in conjunction with the evidence from the systematic review reports, but the new evidence was not incorporated into the pooled estimates in the evidence to decision (EtD) tables.
Expert Panel processes
Team structure.
The Expert Panel met both in person and via webinar. In addition to their collective efforts, each panel member was assigned to one of six teams to address the topic-specific key questions identified by the NHLBAC Asthma Expert Working Group. Each topic team consisted of at least one content expert, primary care clinician, and individual with implementation expertise; some topic team members had multiple areas of expertise. The Integration and Implementation Team, composed of one representative from each of the topic teams, was tasked with integrating the new recommendations into the step diagrams from EPR-3 to create visual summaries of these steps. The NHLBI assembled and coordinated the Expert Panel. Westat provided technical and support services, including a methodology team with expertise in GRADE.
Disclosure of COIs and conflict management.
To identify and manage potential COIs, the Expert Panel complied with the Institute of Medicine (now National Academy of Medicine) recommendations and standards for using systematic, evidence-based reviews to develop trustworthy guidelines.8,14 The Expert Panel also followed the spirit of the recommendations for guideline panels that the ACP published in August 2019, midway through the development of these asthma guidelines.9 Where possible, the Expert Panel implemented many of the new ACP guideline panel recommendations.
All Expert Panel members made financial disclosures and reported COIs using the standard author disclosure procedures described by the International Committee of Medical Journal Editors for manuscripts submitted to the JACI; the JACI editors reviewed these COI reports.15 Expert Panel members disclosed all personal fees, grant support, and nonfinancial support received, including support from entities that could be perceived to have influenced or could potentially have influenced the work of the Expert Panel for the past 36 months. They reported these COIs in writing before the Expert Panel initially convened, before each face-to-face meeting, and at the completion of the guidelines. In keeping with JACI requirements, these disclosure reports did not include sources of research funding, such as government agencies, charitable foundations, or academic institutions.
The Expert Panel chair and JACI editors rated each COI as high, moderate, or low and used a modified version of the ACP recommendations to develop a plan to manage each level of COI. For the Expert Panel, a high COI was defined as multiple interactions with biomedical entities (drug, biotechnology, or medical device companies) and could include interactions that were related or not related to the six priority topics. Participation in any speakers’ bureau of any biomedical entity was also considered a high COI. Individuals with a high COI were excluded from the Expert Panel unless they were able to reduce their level of COI. Expert Panel members who reduced the level of a high COI were then subject to the requirements, including recusals, associated with lower levels of COI.
Interactions related to a specific priority topic with a single biomedical entity were considered moderate COIs. Expert Panel members with a moderate COI related to any of the six priority topics were recused from participating in the writing, discussion, and voting on the recommendations or guideline section for that topic. This recusal process was implemented at the start of the Expert Panel’s work, and the Expert Panel formally recognized these COIs as moderate after the release of the ACP recommendations. Resolution of a moderate COI resulted in reinstatement to full participation in all activities related to that topic. Any report of a previously unreported moderate COI resulted in recusal of the member from activities related to that topic. In addition, members who had no COI discussed the topic again and voted again on the associated recommendations. A low COI was defined as no more than two interactions with a biomedical entity not related to asthma or to the topics under discussion.
As new COIs arose during the guideline-development process, Expert Panel members reported these COIs to the Expert Panel chair, and the chair and the JACI editors reviewed these new COIs and developed a plan to manage them. All Expert Panel members were notified when a member reported a new COI. After the release of the ACP recommendations, Expert Panel members with any new COI were recused from the Expert Panel. All Expert Panel members agreed not to undertake any activities that could result in a new COI for 12 months after the guidelines were released.
GRADE methodology
Overview.
GRADE is an internationally accepted framework for determining the quality or certainty of evidence and the direction and strength of recommendations based on this evidence.16,17 A guideline methodologist not involved in the development of the systematic reviews for this update provided training on GRADE methodology to the Expert Panel and ongoing support and consultation throughout the project. The Expert Panel used the GRADE approach to review the evidence, create evidence profiles for critical and important outcomes, develop EtD tables, and write recommendation statements.
Prioritization and rating of asthma outcomes.
The Expert Panel discussed asthma outcomes of potential interest and rated the relative importance of each outcome for clinical decision making using the GRADE approach.18 During this process, the Expert Panel reviewed the definitions of the outcomes in each of the systematic review reports. The outcomes deemed critical to assess for making recommendations across all topic areas were asthma exacerbations, asthma control, and asthma-related quality of life.
The Expert Panel assessed additional outcomes for specific key questions when these outcomes were relevant to the topic or when data for the three critical outcomes were not available. For example, in some instances, the systematic review reports identified limited or not adequate data on the effect of the interventions listed in the key questions on specific critical outcomes (eg, asthma control). In such cases, the Expert Panel considered available data on a related outcome (eg, asthma symptoms), even though validated outcome instruments were not used in studies or were not available. In this example, the Expert Panel confirmed asthma symptoms as an important outcome based on responses from the focus groups. The Expert Panel then used data on this important outcome to create the evidence profiles and EtD tables for the intervention, based on the available evidence.
After prioritizing the outcomes, the Expert Panel used established thresholds for determining significant improvement, also known as the minimally important difference (MID), for asthma control and asthma-related quality-of-life measures. These MID criteria are listed in Table IB.19–27 For outcomes with no MID established in the literature, such as exacerbations, the Expert Panel reached consensuson clinically important differences that were based in part on a review of effect sizes in RCTs in the literature and on their judgments regarding the clinical relevance of a given change. In keeping with the recommendations from the Asthma Outcomes Workshop (2012),10 treatment with systemic (oral and parenteral) corticosteroids, asthma-specific emergency department visits, and hospitalizations were included as core outcome measures for exacerbations. The Expert Panel also included studies that used composite measures of systemic corticosteroids, emergency department visits, and hospitalizations.28
TABLE IB.
| Outcome measure | Range (points) | Score interpretation | MID |
|---|---|---|---|
| Asthma control | |||
| ACT | 5–25 | Well controlled: ≥20 Not well controlled: ≤19 |
≥12 y: MID ≥3 points |
| Asthma Control Questionnaire-5 (ACQ-5) Asthma Control Questionnaire-6 (ACQ-6) |
0–6 | Uncontrolled: ≥1.5 Well controlled: <0.75 |
≥18 y: MID ≥0.5 points |
| Asthma Control Questionnaire-7 (ACQ-7) | 0–6 | Uncontrolled: ≥1.5 Well controlled: <0.75 |
≥6 y: MID ≥0.5 points |
| Asthma-related quality of life | |||
| Asthma Quality of Life Questionnaire Asthma Quality of Life Questionnaire Mini (AQLQ-mini) |
1–7 | Severe impairment = 1 No impairment = 7 |
≥18 y: MID ≥0.5 points |
| Pediatric Asthma Quality of Life Questionnaire | 1–7 | Severe impairment = 1 No impairment = 7 |
7–17 y: MID ≥0.5 points |
| Other | |||
| Rescue medication use (daytime or nighttime) | Continuous measure of puffs per unit of time | NA | ≥18 y: MID = –0.81 puffs/d |
NA, Not applicable/available.
EtD framework.
The EtD framework provides a systematic and transparent approach for moving from evidence to recommendations by guideline panels.29 The topic teams developed EtD tables for each key question using the evidence in the systematic review reports and the GRADEpro Guideline Development Tool.30 New articles found in the updated literature review were noted in the new evidence sections of the EtD tables, but their data were not incorporated into the pooled estimates. See Table IC for the template used for EtD tables. The EtD tables provided a framework for the Expert Panel to use for assessing the evidence and providing rationales for their judgments on a range of factors that influenced there commendations, as described in the next section, “Contextualization of judgments.”31,32
TABLE IC.
EtD table template
| Content area | Question | Judgment (pick one) | Research evidence | Additional considerations |
|---|---|---|---|---|
| Desirable effects | How substantial are the desirable anticipated effects? | Trivial, small, moderate, large, vary, don’t know | ||
| Undesirable effects | How substantial are the undesirable anticipated effects? | Large, moderate, small, trivial, vary, don’t know | ||
| Certainty of evidence | What is the overall certainty of the evidence of the effects? | Very low, low, moderate, high, no included studies | ||
| Values | Is there important uncertainty about or variability in how much people value the main outcomes? |
Important uncertainty or variability, possibly important uncertainty or variability, probably no important uncertainty or variability, no important uncertainty or variability | ||
| Balance of effects | Does the balance between desirable and undesirable effects favor the intervention or the comparison? | Favors the comparison, probably favors the comparison, does not favor either the intervention or the comparison, probably favors the intervention, favors the intervention, varies, don’t know | ||
| Acceptability | Is the intervention acceptable to key stakeholders? | No, probably no, probably yes, yes, varies, don’t know | ||
| Feasibility | Is the intervention feasible to implement? | No, probably no, probably yes, yes, varies, don’t know | ||
| Equity | What would be the impact on health equity? | Reduced, probably reduced, probably no impact, probably increased, increased, varies, don’t know |
Contextualization of judgments.
The Expert Panel members reviewed the summary-of-findings tables in the AHRQ systematic review reports and recorded their judgments about the certainty of the evidence regarding each intervention. See Table ID for explanations of the levels of certainty in the evidence. For each key question, the Expert Panel reviewed the EPCs’ judgments about the risk of bias reported in the systematic review reports. The Expert Panel modified the judgments about the directness or indirectness of, consistency or inconsistency of, precision or imprecision of, and publication bias in the evidence when appropriate to reflect the panel’s contextualized judgments about the certainty of the evidence in the context of clinical practice guidelines.32 Footnotes in the EtD tables in Appendix B (see this article’s Online Repository at www.jacionline.org) provide detailed explanations of these judgments. When the Expert Panel made a contextualized judgment for a specific outcome (and the opinion of the Expert Panel differed from the judgment of the EPC in the AHRQ systematic review report), the Expert Panel used the following words: “The Expert Panel rated this outcome down for…” Otherwise, the certainty of evidence and risk of bias ratings reflected the EPCs’ judgments from the published systematic review reports, and the Expert Panel identified these ratings by statements that began with “The AHRQ systematic review report rated this outcome down for…”
TABLE ID.
Certainty of evidence of effects
| High | We are very confident that the true effect lies close to that of the estimate of the effect. |
| Moderate | We are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. |
| Low | Our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. |
| Very low | We have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of the effect. |
Each EtD table includes a summary of the pooled results from the evidence syntheses (in addition to results from any new studies) in relative and absolute terms. The tables also describe any assumptions or evidence on variability in patient values and preferences regarding the intervention; the overall certainty of the evidence; the intervention’s net benefit based on the desirable and undesirable effects; and judgments about the resource requirements, acceptability, feasibility, and equity issues related to that intervention. The Expert Panel members made judgments within these domains and developed clinical recommendations based on the evidence summarized in the EtD tables. Discussions to make these judgments and develop the recommendations took place during online, telephone, and face-to-face meetings. For each recommendation, the Expert Panel indicated its direction (for or against the intervention) and strength, provided accompanying technical remarks and implementation considerations, and identified relevant evidence gaps.
Framing recommendations and coming to consensus.
In GRADE, each recommendation has a direction, meaning that the recommendation is either for or against the use of an intervention. Each recommendation is also either strong or conditional, as explained in Table IE. Strong recommendations are those for which, in the judgment of the Expert Panel after it has reviewed all of the evidence and individual judgments, all or almost all people would choose the recommended course of action. Conditional recommendations are those for which, after reviewing all of the evidence and individual judgments, the Expert Panel believes that many informed people are likely to make different decisions about whether to take the recommended course of action. A conditional recommendation implies that engaging in a shared decision-making process is essential for individuals with asthma and their health care providers.31–33
TABLE IE.
Implications of strong and conditional recommendations*
| Implications | Strong recommendation | Conditional recommendation |
|---|---|---|
| For individuals with asthma | Most individuals in this situation would want the recommended course of action and only a small proportion would not. | Most individuals in this situation would want the suggested course of action, but many would not. |
| For clinicians | Most individuals should receive the intervention. Formal decision aids are not likely to be needed to help individuals make decisions consistent with their values and preferences. | Different choices will be appropriate for individuals consistent with their values and preferences. Use shared decision making. Decision aids may be useful in helping individuals make decisions consistent with their risks, values, and preferences. |
| For policymakers | The recommendation can be adapted as policy or performance measure in most situations. Adherence to this recommendation according to the guideline could be used as a quality criterion or performance indicator. | Policy making will require substantial debate and involvement of various stakeholders. Performance measures should assess whether decision making is documented. |
| For researchers | The recommendation is supported by credible research or other convincing judgments that make additional research unlikely to alter the recommendation. On occasion, a strong recommendation is based on low or very low certainty in the evidence. In such instances, further research may provide important information that alters the recommendations. | The recommendation is likely to be strengthened (for future updates or adaptation) by additional research. An evaluation of the conditions and criteria (and the related judgments, research evidence, and additional considerations) that determined the conditional (rather than strong) recommendation will help identify possible research gaps. |
Strong recommendations are indicated by statements that lead with “We recommend,” whereas conditional recommendations are indicated by statements that lead with “We conditionally recommend.”
The Expert Panel drafted, discussed, and revised the recommendations multiple times before all eligible members (those who did not have a COI for the topic) voted on each recommendation. The Expert Panel achieved consensus when more than 90% of the Expert Panel members voted in favor of a recommendation. If less than 90% of members voted in favor of a recommendation, the relevant topic team continued to revise the recommendation until it achieved consensus approval according to these criteria.
Focus groups with individuals with asthma and their caregivers
The NHLBI sponsored focus groups with individuals with asthma and their caregivers to:
Identify the types of information and tools that individuals with asthma, their caregivers, and their health care providers would find most helpful in their ongoing efforts to effectively manage asthma and adhere to the new guidelines
Ensure that the new asthma guidelines reflect the voices of individuals with asthma and their caregivers
Identify potential barriers to uptake by individuals with asthma and their caregivers
Using virtual data-collection methods (ie, telephone and online platforms), the NHLBI conducted 11 in-depth interviews with health care providers who treat individuals with asthma and 10 online focus groups with English- and Spanish-speaking adults with asthma and adult caregivers of children with asthma with household incomes lower than $50,000 per year. In accordance with best practices, both the health care provider in-depth interviews and consumer focus group sessions lasted 75 minutes or less to minimize burden and facilitate engagement. Findings were analyzed using a notes- and transcript-based analysis process similar to that recommended by Krueger34 and Patton.35
The focus groups provided insight into outcomes that individuals with asthma and their caregivers considered most important; factors that affected their treatment choices; preferences for medication type and dosing frequency; and opinions about immunotherapy, allergen reduction, and BT. The Expert Panel considered these insights when developing its recommendations and EtD tables.
Findings of interviews and focus groups.
Among both adults with asthma and caregivers of children with asthma, the most desired outcome was relief from symptoms that limit what people with asthma can do. In particular, participants valued symptom relief that would allow individuals with asthma to be more physically active. Caregivers also wanted to reduce the number of hospital visits for individuals with asthma, and Spanish-speaking caregivers sought control of nighttime symptoms. These individuals with asthma and caregiver preferences support the use of asthma symptom relief as an outcome measure when studies did not use validated outcome measurement tools.
Participants stated that cost and insurance coverage, safety, side effects, benefits, success rates, and asthma severity influenced their decisions about asthma treatment. Some participants were concerned that they might become dependent on or addicted to asthma medications (in particular, to pills), and participants with comorbidities expressed concern about drug interactions and contraindications, especially for oral medications.
Individuals with asthma indicated that they preferred inhaled medications over pills or liquids because they perceived inhaled medications to be easier to take or administer, faster acting, and more effective (because the medication is delivered directly to the site where it is needed). Individuals with asthma and caregivers also preferred taking one medication daily at most and viewed a need to take more than two to three medications a day as excessive. Caregivers were concerned about the administration of more medications or more frequent administration of medications to children while they are in school.
Taking medication on a set schedule instead of as needed drew mixed reactions. Perceived benefits of a set schedule included easier adherence, greater effectiveness, and a greater ability to prevent exacerbations (for those with severe asthma). In contrast, taking medication as needed was believed to offer flexibility and potentially reduce side effects. As-needed medications were also described as more appealing to those with mild to moderate asthma and to Spanish-speaking caregivers. Adults with asthma and caregivers were generally receptive to the use of one inhaler to both treat asthma and prevent exacerbations, although they wondered whether medications could do both effectively.
Levels of awareness of immunotherapy were low to moderate in individuals with asthma and caregivers. Some stated that they would consider this type of treatment if it were shown to be effective; others remained skeptical about the value of immunotherapy because of concerns about associated pain, inconvenience, and side effects.
Many participants reported taking action to reduce allergens at home. Most participants said that they used mattress and pillow covers, removed curtains or mold, controlled pests and dust, and vacuumed floors regularly. Some participants who had pets said that the pets were outside most of the time or they vacuumed their floors frequently. Participants also reported keeping windows closed during pollen and wildfire season to reduce the level of allergens and irritants in their home. Very few stated that they would stop their current allergen reduction efforts even if these efforts were proven to be ineffective. Most participants wanted information on cost and level of effort involved to consider making a change.
Spanish-speaking adults with asthma were more receptive to BT than their English-speaking counterparts. However, most participants thought that the procedure was too risky and expressed concerns about the need for anesthesia, multiple hospital visits, and heating of muscle tissue as well as the treatment’s impact on other health conditions. They wanted more information on the therapy’s side effects, risks, complications, and success rates as well as how the procedure is done.
2020 focused updates to the 2007 Asthma Guidelines
After the Expert Panel reached consensus on the recommendations, each topic team drafted a narrative to provide further information on each recommendation. These narratives form the body of this report. Each topic narrative has the following sections:
A brief background section that includes definitions of the terms used in the recommendations
The key questions addressed
The recommendations
An Implementation Guidance section that explains the recommendation in greater detail and provides Expert Panel opinion about how to implement the recommendation in clinical practice
A summary of the evidence
The rationale for the recommendation
A discussion of the evidence supporting the recommendation
A list of topic-specific research gaps and questions
Differences (if any) between the new recommendations and the recommendations in EPR-3 are discussed in Appendix A (in this article’s Online Repository at www.jacionline.org).
The Implementation Guidance sections are for practicing clinicians, and they contain the following information:
Clinician’s summary (more detailed explanation of the recommendation)
Population most likely to benefit from the recommendation
Any populations to which the recommendation does not apply
Topic-specific considerations
Issues that clinicians should discuss with their patients as part of the shared decision-making process
Review and public comment
The NAEPPCC reviewed an initial draft report. The NHLBI subsequently made the draft report available for public review and comment from December 2, 2019, to January 17, 2020. Interested stakeholders—including health professionals; representatives of the scientific community, academic institutions, the private sector, professional societies, advocacy groups, and patient communities; and other interested members of the public—were invited to submit comments. The Expert Panel received and reviewed approximately 500 comments from almost 100 individuals and organizations, and the panel used this input to revise the draft report.
One or more individuals and organizational representatives who submitted public comments mentioned almost all of the emerging topics. Of the 11 emerging topics (see list toward the beginning of Section I of this report), biologic agents received the most attention. The first biologic agent for asthma received approval from the US FDA in 2003, but the second biologic agent did not receive approval until November 2015. Between November 2015 and November 2017, four biologic agents received approval, but several others were not shown to be effective in clinical trials. Thus, at the time that the priority topics and key questions were developed, the only biologic agent available for use in the United States was omalizumab, which EPR-3 had addressed. The NHLBAC Asthma Expert Working Group did not believe that this single available biologic agent warranted inclusion in the update and included biologic agents as an emerging topic.
Limitations and research gaps
The Expert Panel identified several limitations in the process it used to identify topics and develop recommendations, including the following:
A better mechanism is needed to identify topics that need updating and to decrease the time between updates.
The process would benefit from a discussion and development of a plan about how to tailor guideline recommendations in the emerging era of personalized medicine.
Expanding engagement with professional societies might benefit both the development and the implementation of new recommendations.
The Expert Panel also identified several overarching research gaps listed below. Research gaps that are specific to individual topics are listed at the end of each topic section.
Research studies need to use the core outcome measures identified in the 2012 Asthma Outcomes Workshop.10 Federal agencies that contributed to the 2012 Asthma Outcomes Workshop report should require the studies they fund to measure outcomes as recommended in that report. Because new information on asthma outcomes is now available, the workshop report should be reexamined to determine whether it needs to be revised.
The clinical relevance of changes in outcome measures should be formally established to provide MIDs for all asthma outcomes (eg, exacerbations and asthma symptoms) and the cutoffs for tests (eg, Feno). Clinical relevance should be established using a wide range of stakeholder input, especially from individuals with asthma, who should also be included as members of the Expert Panel.
Updates are needed to the definitions of asthma severity that incorporate asthma phenotypes and endotypes. The definitions of low-, medium-, and high-dose ICSs also need to be updated.
Biologically appropriate subpopulations with asthma should be established and standardized. Although the populations of interest for the focused updates were defined for the systematic reviews, the characterizations of study participants did not reflect current understanding of relevant phenotypes and endotypes (eg, based on asthma severity, allergen-specific sensitization, or airway inflammatory type).
Standard reporting of results stratified by race and ethnicity as well as by age groups (0–4 years, 5–11 years, and 12 years and older) is needed to combine results across studies.
The vast majority of studies used to inform the guidelines were designed as efficacy studies,36 which evaluate treatment effects in relatively homogeneous populations and conditions in which fidelity to study protocols is actively promoted. Applicability to real-world clinical and community contexts requires studies with comparative effectiveness designs. Such research would benefit from the use of validated outcome measures and definitions of biologically appropriate subpopulations.
Studies need to use measures and outcomes that are important to individuals with asthma. The GRADE methodology gives highest priority to patient-centered outcomes. However, the studies that the Expert Panel used to develop the recommendations often did not measure outcomes that are most relevant or important to individuals with asthma. Research is needed to understand how preferred outcomes vary by race or ethnicity, asthma severity, age (eg, children or older adults), and socioeconomic status.
All measures and outcomes relevant to making judgments need to be included in the systematic reviews. For example, although cost-effectiveness data are available for some asthma interventions, the systematic review reports used for the updates did not include these data. Moreover, data regarding the safety of all interventions should be explicitly reported in publications on clinical trials.
Recommendations
In Table IF, all of the Expert Panel’s recommendations are grouped by the six priority topics. Please refer to the topic-specific sections in this report for full discussions of each recommendation, including implementation guidance and a clinician’s summary.
TABLE IF.
Expert Panel recommendations
| Topic | Recommendation number* | Recommendation | Strength of recommendationy† | Certainty of evidence‡ |
|---|---|---|---|---|
| Feno | 1 | In individuals aged 5 y and older for whom the diagnosis of asthma is uncertain using history, clinical findings, clinical course, and spirometry, including bronchodilator responsiveness testing, or in whom spirometry cannot be performed, the Expert Panel conditionally recommends the addition of Feno measurement as an adjunct to the evaluation process. | Conditional | Moderate |
| 2 | In individuals aged 5 y and older with persistent allergic asthma, for whom there is uncertainty in choosing, monitoring, or adjusting anti-inflammatory therapies based on history, clinical findings, and spirometry, the Expert Panel conditionally recommends the addition of Feno measurement as part of an ongoing asthma monitoring and management strategy that includes frequent assessments. | Conditional | Low | |
| 3 | In individuals aged 5 y and older with asthma, the Expert Panel recommends against the use of Feno measurements in isolation to assess asthma control, predict future exacerbations, or assess exacerbation severity. If used, it should be as part of an ongoing monitoring and management strategy. | Strong | Low | |
| 4 | In children aged 0–4 y with recurrent wheezing, the Expert Panel recommends against Feno measurement to predict the future development of asthma. | Strong | Low | |
| Allergen mitigation | 5 | In individuals with asthma who do not have sensitization to specific indoor mitigation allergens or who do not have symptoms related to exposure to specific indoor allergens, the Expert Panel conditionally recommends against allergen mitigation interventions as part of routine asthma management. | Conditional | Low |
| 6 | In individuals with asthma who have symptoms related to exposure to identified indoor allergens, confirmed by history taking or allergy testing, the Expert Panel conditionally recommends a multicomponent allergen-specific mitigation intervention. | Conditional | Low | |
| 7 | In individuals with asthma who have sensitization or symptoms related to exposure to pests (cockroaches and rodents), the Expert Panel conditionally recommends the use of integrated pest management alone, or as part of a multicomponent allergen-specific mitigation intervention. | Conditional | Low | |
| 8 | In individuals with asthma who have sensitization or symptoms related to exposure to dust mites, the Expert Panel conditionally recommends impermeable pillow/mattress covers only as part of a multicomponent allergen mitigation intervention, not as a single-component intervention. | Conditional | Moderate | |
| ICS | 9 | In children aged 0–4 y with recurrent wheezing triggered by respiratory tract infections and no wheezing between infections, the Expert Panel conditionally recommends starting a short course of daily ICS at the onset of a respiratory tract infection with as-needed SABA for quick-relief therapy compared to as-needed SABA for quick-relief therapy only. | Conditional | High |
| 10 | In individuals aged 12 y and older with mild persistent asthma, the Expert Panel conditionally recommends either daily low-dose ICS and as-needed SABA for quick-relief therapy or as-needed ICS and SABA used concomitantly. | Conditional | Moderate | |
| 11 | In individuals aged 4 y and older with mild to moderate persistent asthma who are likely to be adherent to daily ICS treatment, the Expert Panel conditionally recommends against a short-term increase in the ICS dose for increased symptoms or decreased peak flow. | Conditional | Low | |
| 12 | In individuals aged 4 y and older with moderate to severe persistent asthma, the Expert Panel recommends ICS-formoterol in a single inhaler used as both daily controller and reliever therapy compared to either: • Higher-dose ICS as daily controller therapy and SABA for quick-relief therapy or • Same-dose ICS-LABA as daily controller therapy and SABA for quick-relief therapy. |
Strong | High (ages ≥ 12 y) Moderate (ages 4–11 y) |
|
| 13 | In individuals aged 12 y and older with moderate to severe persistent asthma, the Expert Panel conditionally recommends ICS-formoterol in a single inhaler used as both daily controller and reliever therapy compared to higher-dose ICS-LABA as daily controller therapy and SABA for quick-relief therapy. | Conditional | High | |
| LAMA | 14 | In individuals aged 12 y and older with uncontrolled persistent asthma, the Expert Panel conditionally recommends against adding LAMA to ICS compared to adding LABA to ICS. | Conditional | Moderate |
| 15 | If LABA is not used, in individuals aged 12 y and older with uncontrolled persistent asthma, the Expert Panel conditionally recommends adding LAMA to ICS controller therapy compared to continuing the same dose of ICS alone. | Conditional | Moderate | |
| 16 | In individuals aged 12 y and older with uncontrolled persistent asthma, the Expert Panel conditionally recommends adding LAMA to ICS-LABA compared to continuing the same dose of ICS-LABA. | Conditional | Moderate | |
| Immunotherapy | 17 | In individuals aged 5 y and older with mild to moderate allergic asthma, the Expert Panel conditionally recommends the use of SCIT as an adjunct treatment to standard pharmacotherapy in those individuals whose asthma is controlled at the initiation, build-up, and maintenance phases of immunotherapy. | Conditional | Moderate |
| 18 | In individuals with persistent allergic asthma, the Expert Panel conditionally recommends against the use of SLIT in asthma treatment. | Conditional | Moderate | |
| BT | 19 | In individuals aged 18 y and older with persistent asthma, the Expert Panel conditionally recommends against BT. Individuals aged 18 y and older with persistent asthma who place a low value on harms (short-term worsening symptoms and unknown long-term side effects) and a high value on potential benefits (improvement in quality of life, a small reduction in exacerbations) might consider BT. | Conditional | Low |
Integration of the new recommendations into asthma care
The Expert Panel that produced this 2020 Asthma Guideline Update was asked to address specific questions about six priority topics rather than revise all of EPR-3. The Expert Panel, however, recognized the need to integrate the new evidence-based recommendations into a comprehensive approach to asthma care using the EPR-3 step diagrams.
Stepwise approach for managing asthma.
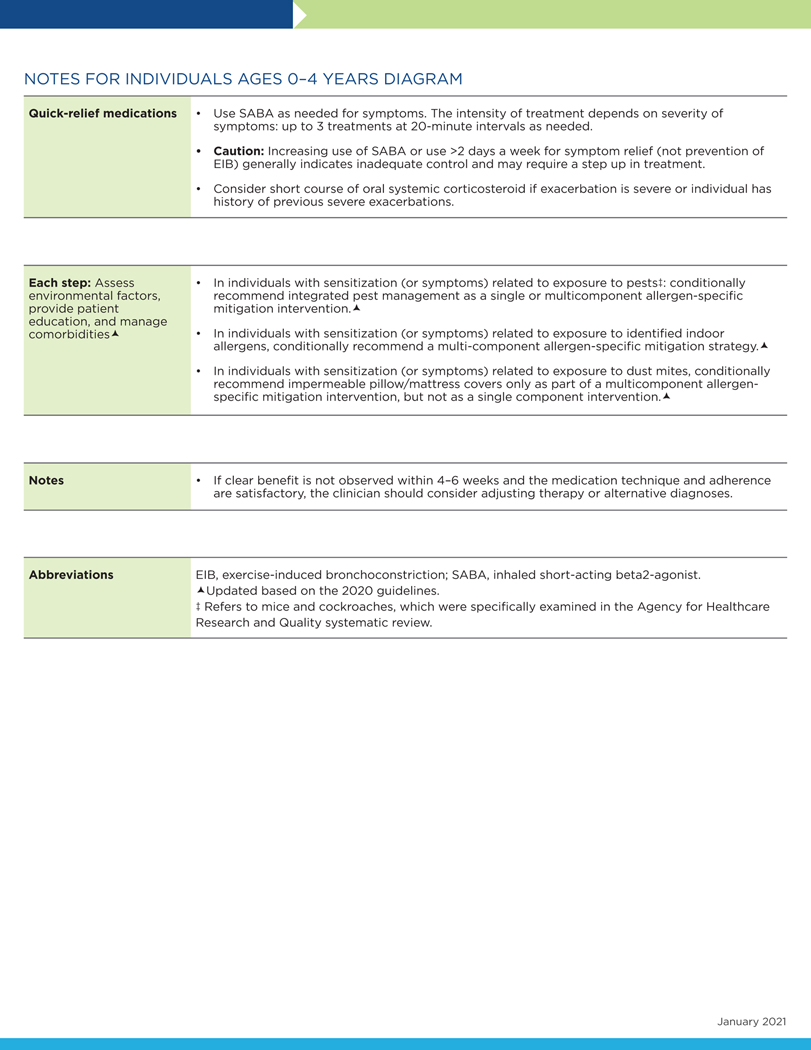
In preparing the step diagrams (Figs 2–4), the Expert Panel used some of the definitions and assumptions from EPR-3. The step diagrams that follow this section retain the EPR-3 recommendations that the Expert Panel did not address in the current report. The Expert Panel encourages readers to review the footnotes in the step diagrams because they offer important information about the use of these diagrams.
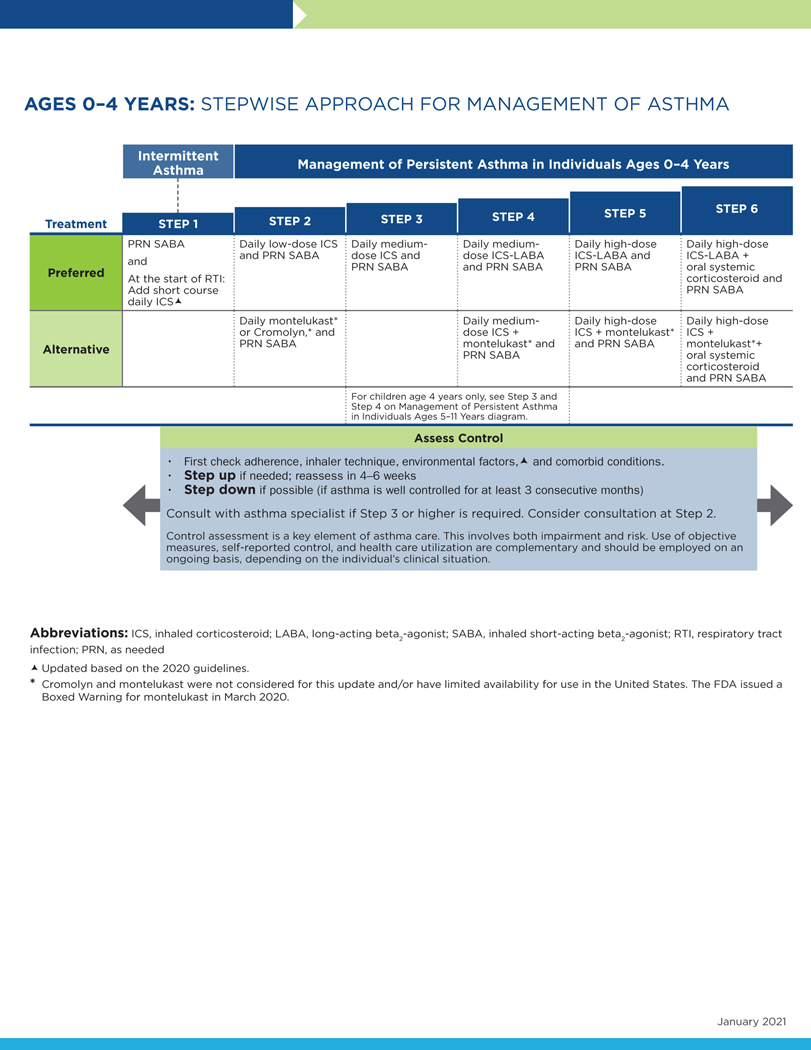
FIG 2.
Stepwise approach for management of asthma in individuals aged 0 to 4 years.
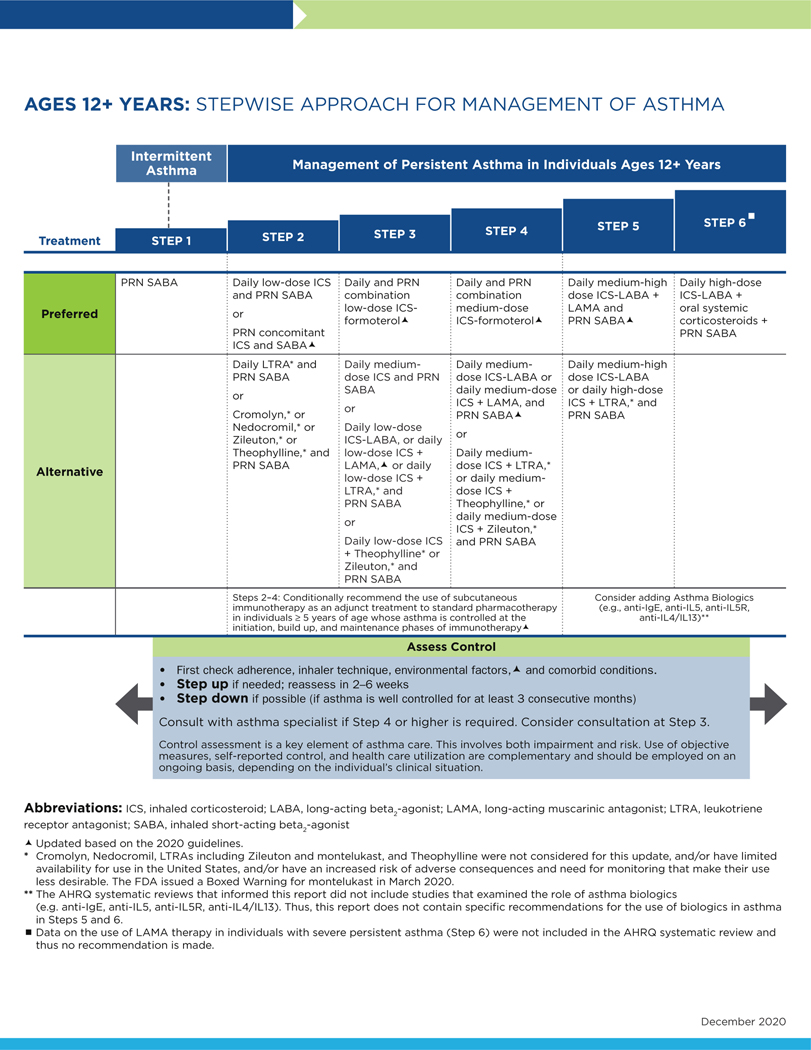
FIG 4.
Stepwise approach for management of asthma in individuals aged 12 years and older.
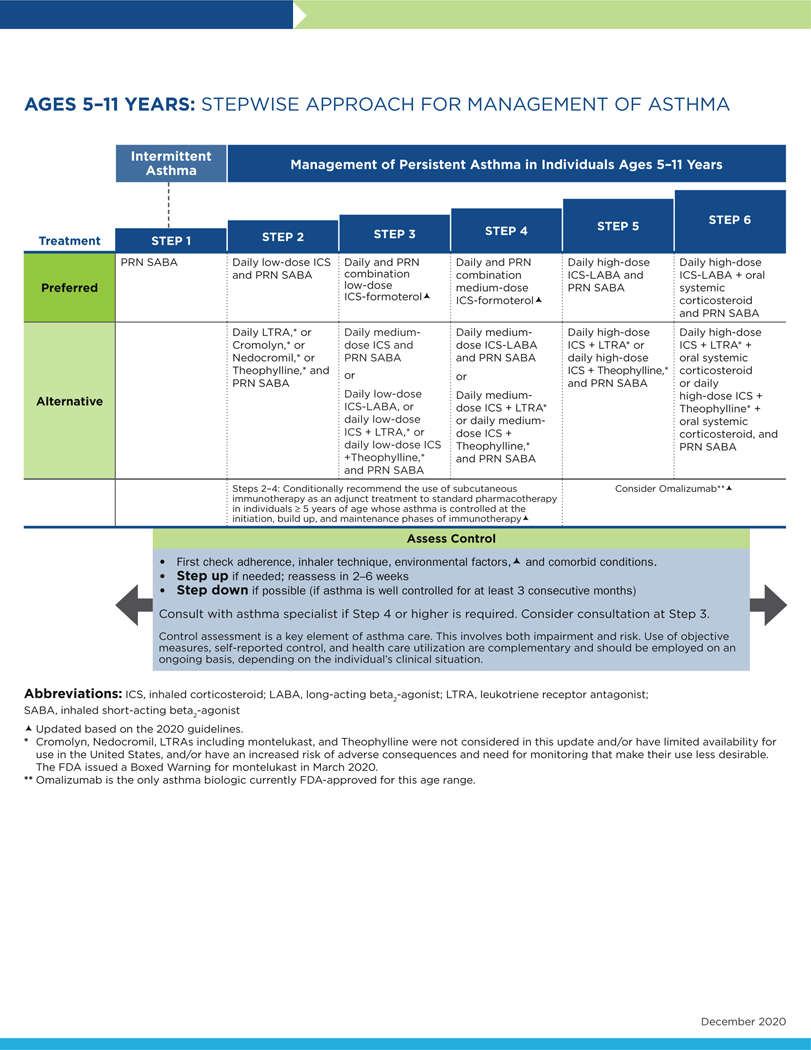
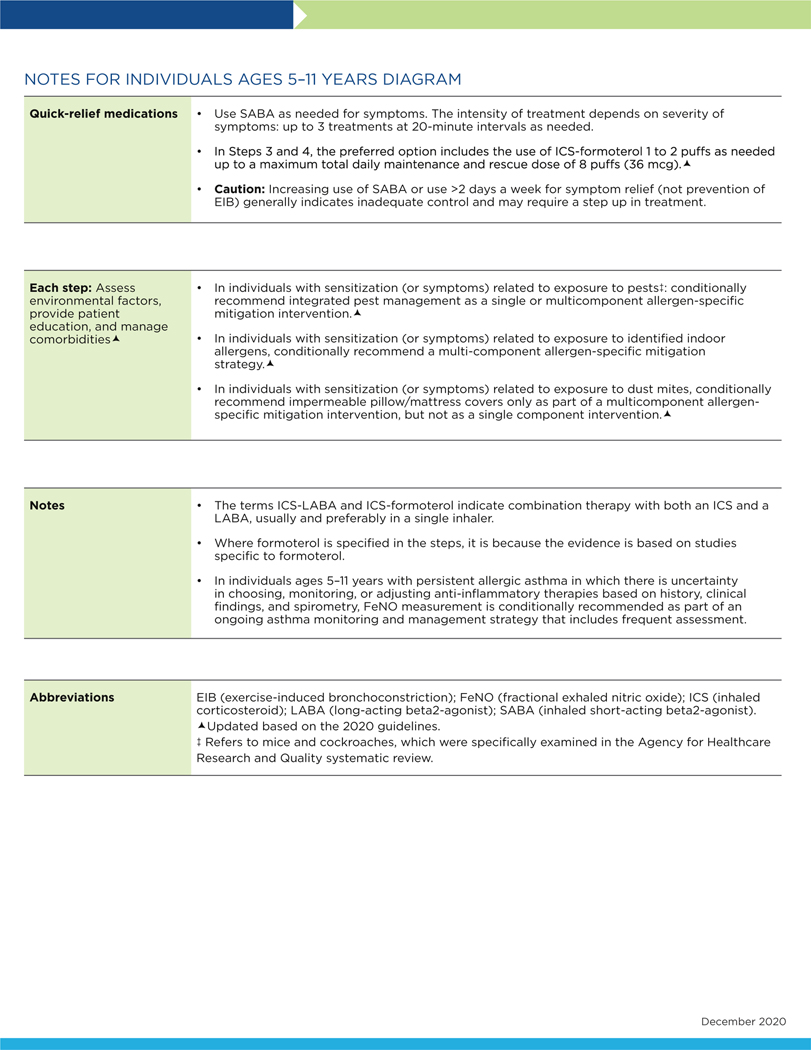
The following conventions apply to Figs 2 to 4:
- Clinicians decide which step of care is appropriate depending on whether the individual is newly diagnosed (ie, is treatment naive) or whether the clinician is adjusting the individual’s therapy to achieve asthma control.
- For newly diagnosed or treatment-naive individuals, clinicians should first choose the appropriate step diagram for the person’s age and then consider both the individual’s level of asthma impairment and risk when selecting the initial step and treatment.
- Within a given step, the preferred options are the best management choices supported by the evidence that the Expert Panel reviewed. When the available evidence is insufficient or does not change a previous recommendation, the step diagrams list preferred options from the EPR-3 step diagrams.
- Within a given step, an alternative option(s) is management strategies that are less effective or have more limited evidence than the preferred options. Clinicians and patients may choose the alternative treatments if individuals with asthma are currently receiving this therapy and their asthma is under control, if the preferred treatments are not available or too costly, or if the individuals with asthma prefer an alternative treatment.
- Preferred and alternative treatments within a step category are listed alphabetically unless the Expert Panel has established a rank order of preference for the preferred or alternative treatments. A lack of rank order is indicated by “or” between treatment options.
- In the stepwise approach to therapy for asthma, the clinician escalates treatment as needed (by moving to a higher step) or, if possible, deescalates treatment (by moving to a lower step) once the individual’s asthma is well controlled for at least three consecutive months.
- For individuals with persistent asthma (ie, who require treatment at Step 2 or above), clinicians should be guided by the current step of treatment and the individual’s response to therapy (in terms of both asthma control and adverse effects) both currently and in the past to decide whether to step up, step down, or continue the current therapy.
- For individuals with persistent asthma who are using an alternative treatment and have an unsatisfactory or inadequate response to that therapy, the Expert Panel suggests replacing the alternative treatment with the preferred treatment within the same step before stepping up therapy.
The Expert Panel did not add management options that the panel recommends against, or for which the evidence is insufficient to determine harms and benefits, to the step diagrams. Instead, these options are listed in Table IF.
The guidance provided in the step diagrams is meant to assist and not replace the clinical decision making required for individual patient management12 and the input from individuals with asthma about their preferences.
FIG 3.
Stepwise approach for management of asthma in individuals aged 5 to 11 years.
SECTION II: RECOMMENDATIONS ON THE USE OF Feno TESTING IN THE DIAGNOSIS AND MANAGEMENT OF ASTHMA
Background
Nitric oxidecan be measured in exhaled breath and can serve as a measure of the level of airway inflammation. In individuals with asthma, Feno may be a useful indicator of type 2 (T2) bronchial or eosinophilic inflammation in the airway. Feno testing requires an expiratory maneuver into a device designed for this purpose.
The Expert Panel addressed key questions on the utility of Feno measurement for asthma diagnosis, management, and prognosis. In this section, the panel discusses factors that confound Feno measurement or the interpretation of Feno test results in the context of the key questions. The evidence in all of these areas reveals important limitations that affect the strength of the recommendations and limit the ability to determine the optimal strategies for Feno measurement. A discussion of the equipment used to measure Feno and how to perform the test is beyond the scope of this update.
Definitions of terms used in this section
Children and adults have allergic asthma if they become symptomatic after acute exposure to something to which they are allergic (eg, a pet) or during a specific season of the year (eg, in the spring, due to tree pollen, or in the fall, due to ragweed pollen).
“Recurrent wheezing” is defined as clinically significant periods of bronchial or respiratory tract wheezing that is reversible or that is consistent with the clinical picture of bronchospasm.
Question 2.1
What is the diagnostic accuracy of Feno measurement(s) for making the diagnosis of asthma in individuals aged 5 years and older?
Recommendation 1: In individuals aged 5 years and older for whom the diagnosis of asthma is uncertain using history, clinical findings, clinical course, and spirometry, including bronchodilator responsiveness testing, or in whom spirometry cannot be performed, the Expert Panel conditionally recommends the addition of Feno measurement as an adjunct to the evaluation process.
Conditional recommendation, moderate certainty of evidence
Implementation guidance
Clinician’s Summary:
The role of an increased level of Feno in the diagnosis of asthma is still evolving, and no definitive test exists for diagnosing asthma. Feno measurement may support a diagnosis of asthma in individuals for whom the diagnosis is uncertain even after a complete history, physical examination, and spirometry testing including bronchodilator responsiveness. Recognition of allergen sensitivity is extremely important for interpreting Feno levels. Allergic rhinitis and atopy, which can be present in individuals with and without asthma, are associated with increased Feno levels, and taking these factors into consideration is critical for accurately interpreting Feno test results.
On the basis of current data on Feno measurement in clinical settings, Feno testing has a supportive role in evaluation when the diagnosis of asthma is uncertain. The Expert Panel makes the following suggestions for use of Feno testing in asthma diagnosis:
- Individuals in whom a diagnosis of asthma is being considered who may benefit from Feno measurement as part of the evaluation process include the following:
- Those aged 5 years and older who have an uncertain diagnosis of asthma
- Those in whom spirometry testing cannot be performed accurately
Because the data on the diagnostic accuracy of Feno measurement in children younger than 4 years are not conclusive, Feno measurement in this age group should not be used.
Feno test results should not be used alone to diagnose asthma. Feno measurements can serve as an adjunct test that may aid in diagnosing asthma in the appropriate setting. After clinicians consider other conditions that may influence Feno levels, they should perform the test when the results of a thorough clinical assessment, including other appropriate tests, are inconclusive.
- Clinicians should use the cutoff levels or ranges listed in Table II for Feno measurement when evaluating persons for asthma. The likelihood that individuals aged 5 years and older have asthma increases by 2.8 to 7.0 times when the Feno test result is high. Clinicians who use Feno testing for asthma diagnosis should keep the following considerations in mind:
- Feno levels of less than 25 parts per billion (ppb) (or <20 ppb in children aged 5–12 years) are inconsistent with T2 inflammation and suggest a diagnosis other than asthma (or that the individual has asthma but their T2 inflammation has been managed with corticosteroids or they have non-T2 inflammation or noneosinophilic asthma).
- Feno levels greater than 50 ppb (or >35 ppb in children aged 5–12 years) are consistent with elevated T2 inflammation and support a diagnosis of asthma. Individuals who have T2 inflammation are more likely to respond to corticosteroid treatment.
- Feno levels of 25 ppb to 50 ppb (or 20–35 ppb in children aged 5–12 years) provide little information on the diagnosis of asthma and should be interpreted with caution and attention to the clinical context.
- The specificity and sensitivity of the Feno testing process depend on the clinical situation. However, in corticosteroid-naive individuals with asthma, Feno measurement is most accurate for ruling out the diagnosis of asthma when the result is less than 20 ppb. In this situation, the test has a sensitivity of 0.79, a specificity of 0.77, and a diagnostic odds ratio (OR) of 12.25.
- ICS treatment should not be withheld solely based on low Feno levels.
Feno measurements should be performed by appropriately trained personnel who have extensive experience in interpreting the result or who consult experienced clinicians who can interpret the findings accurately. Feno testing can be performed in primary or specialty care settings. However, the costs of testing (ie, for equipment and expendable supplies) may prohibit the test’s adoption in the primary care office setting. Cost and the need for reproducible maneuvers will need to be addressed before home testing can become feasible.
- What clinicians should discuss with their patients and families: Clinicians should share the following information about Feno testing with individuals suspected of having asthma and caregivers:
- The Feno measurement process is safe for almost everyone.
- Feno testing may be helpful in determining whether an individual has asthma, but it cannot be used to diagnose asthma.
- Clinicians should inform individuals with asthma who have conditions or behaviors (such as smoking) that could affect the interpretation of the Feno test results that these issues could limit the accuracy of diagnostic attempts.
- Feno test results cannot be used in isolation. Their interpretation must take into account other clinical factors and traditional measures.
- The evidence favors the use of Feno measurement as an adjunct to other diagnostic methods (including a structured history, clinical findings, and pulmonary function testing) when the results from these other measures are not conclusive.
- Decisions about treatment with an ICS are not dependent on Feno measurements, but such measurements may help direct stepwise therapeutic choices.
TABLE II.
Interpretations of Feno test results for asthma diagnosis in nonsmoking individuals not taking corticosteroids*
| Feno level | ||
|---|---|---|
| <25 ppb (<20 in children aged 5–12 y) | 25–50 ppb (20–35 in children aged 5–12 y) | >50 ppb (>35 in children aged 5–12 y) |
| • Recent or current corticosteroid use | • Evaluate in clinical context | • Eosinophilic airways inflammation likely |
| • Alternative diagnoses | • Consider other diagnoses | • Phenotype more likely to respond to ICS |
| • Phenotype less likely to benefit from ICS | • Consider other factors influencing result | • Allergic asthma |
| • Noneosinophilic asthma | • Eosinophilic asthma less likely | • Eosinophilic bronchitis |
| • COPD | ||
| • Bronchiectasis | ||
| • CF | ||
| • Vocal cord dysfunction | ||
| • Rhinosinusitis | ||
| • Smoking | ||
| • Obesity | ||
CF, Cystic fibrosis; COPD, chronic obstructive pulmonary disease.
Reprinted with permission of the American Thoracic Society, ©2019 American Thoracic Society. Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FeNO) for clinical applications. Am J Respir Crit Care Med 2011;184:602–15. The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society.
Summary of the evidence
No RCTs could be found to address Question 2.1 (see Appendix B EtD Table I).
More than 50 studies have been conducted, and some of these studies included healthy and symptomatic individuals, smokers and nonsmokers, atopic and nonatopic individuals, and individuals with and without a prior diagnosis of asthma. The protocols for diagnostic Feno assessments varied, and conclusions about the optimal testing protocol remain uncertain.
Based on the Expert Panel’s interpretation of the literature and the systematic review report findings, the overall certainty of evidence for this recommendation is moderate. The Expert Panel considers implementation of the recommendation in a broad population to be appropriate based on the diversity of the populations included in the systematic review report. The imprecision in the studies on the utility of Feno measurement in asthma diagnosis is notable.
Rationale and discussion
In the Expert Panel’s opinion, an additional tool to aid in diagnosing asthma could be beneficial, especially when that tool may help identify specific asthma phenotypes. The Expert Panel considered many facets of harm, risk, opportunity, and benefits in making its recommendation.
The acceptability of Feno measurement to individuals with a potential diagnosis of asthma is likely to be high, given that the test involves minimal effort and does not incur discomfort or side effects. Publications on studies that used Feno testing did not report any overt harms. The Expert Panel noted that most studies conducted Feno measurements only in specialty care research settings, and few data are available on the use of Feno measurement in primary care settings. As with many innovations, the cost of Feno equipment and testing may limit its broader use. These barriers to broader dissemination could have a negative impact on the availability of Feno testing and lead to less equitable care for populations with limited resources.
Questions 2.2 and 2.3
What is the clinical utility of Feno measurements to select medication options (including corticosteroids) for individuals aged 5 years and older?
What is the clinical utility of Feno measurements to monitor response to treatment in individuals aged 5 years and older?
Recommendation 2: In individuals aged 5 years and older with persistent allergic asthma, for whom there is uncertainty in choosing, monitoring, or adjusting anti-inflammatory therapies based on history, clinical findings, and spirometry, the Expert Panel conditionally recommends the addition of Feno measurement as part of an ongoing asthma monitoring and management strategy that includes frequent assessments.
Conditional recommendation, low certainty of evidence
Implementation guidance
Clinician’s Summary:
This recommendation is specific to using Feno levels when selecting therapy for individuals with asthma and when monitoring the response to and adjusting the dosage of anti-inflammatory therapies. This recommendation does not apply to individuals taking biologic agents, with the exception of omalizumab, because the systematic review literature searches conducted until October 2018 did not include data on biologic agents other than omalizumab. Clinicians must interpret Feno levels in conjunction with other clinical data because these levels are affected by comorbid conditions, including allergic rhinitis and atopy. The weight of the evidence suggests that when used as part of an asthma management strategy, Feno monitoring is effective in preventing exacerbations only when used frequently (such as every 2–3 months), but even frequent monitoring does not improve asthma control or quality of life in individuals with asthma.
The Expert Panel offers the following suggestions on how to use Feno testing to monitor asthma:
- Individuals for whom Feno testing may be useful to monitor asthma include the following:
- Individuals aged 5 years and older with uncontrolled persistent asthma who are currently taking an ICS or an ICS with a LABA, montelukast, or omalizumab
- Individuals whose symptoms indicate that they might require additional anti-inflammatory therapy
- Individuals with atopy, especially children
- Individuals with asthma being treated by providers who agree that frequent (every 2–3 months) assessments of asthma control over the course of a year are warranted
Feno levels must be interpreted in conjunction with other clinical data. Current evidence suggests that Feno can prevent exacerbations only when testing is used frequently (eg, every 2–3 months). Cutoff points for adjusting therapy to reduce the risk of exacerbation have not been established.
The Expert Panel does not recommend using Feno testing to assess adherence to treatment (mostly for ICSs) because the strength of this evidence is low. Moreover, although Feno levels were associated with adherence to ICSs as measured by electronic or dose counters in two observational studies37,38 and one RCT39 in 1035 children and adolescents, no studies have evaluated Feno monitoring to assess adherence in adults.
Feno levels are not well correlated with other asthma outcomes (eg, symptoms or control measured by such tools as the Asthma Control Test [ACT] or the Asthma Control Questionnaire [ACQ], prior or subsequent exacerbations, or exacerbation severity; see Recommendation 3). Therefore, clinicians should not use Feno measurement as a substitute for these measures or in isolation. Rather, Feno testing is best used as part of an ongoing asthma monitoring and management strategy that includes frequent assessments.
- What clinicians should discuss with their patients and families: The Expert Panel suggests that clinicians consider conveying the following information to their patients with asthma as part of shared decision making:
- Feno measurement is safe for almost everyone.
- Feno-based asthma monitoring and management strategies are associated with significant reductions in exacerbation frequency, but not with improvements in control (based on ACT or ACQ results) or on quality-of-life measures.
- To undergo Feno testing, individuals with asthma might need to be referred to a specialty clinic.
- Feno measurements are used in addition to other evaluations of asthma control, such as lung function testing, symptom assessments, and questions about medication adherence.
- Feno levels may be affected by multiple conditions in addition to asthma.
Summary of the evidence
The Expert Panel specified three critical outcomes (exacerbations, asthma control, and quality of life). The summary of evidence for Recommendation 2 can be found in Appendix B (EtD Table II).
In the Expert Panel’s judgment, the benefit of Feno monitoring is moderate. Feno testing to monitor responses to asthma anti inflammatory therapies was associated with a meaningful decrease in exacerbations, whereas the average benefit of Feno monitoring for asthma control and quality of life did not achieve the MID (see EtD Table II). The certainty of evidence (for ACT, Pediatric Asthma Quality of Life Questionnaire, or Asthma Quality of Life Questionnaire) is low. The strategies for adjusting anti-inflammatory therapies using Feno test results in conjunction with other assessments varied widely.39–53 For this reason, no evidence-based Feno cutoff points are available for choosing, monitoring, or adjusting anti-inflammatory therapies, and the Expert Panel has not provided an algorithm to use for this purpose. Most algorithms that have been used in studies involved strict protocols and may not be relevant to typical clinical practices.
The certainty of evidence for the effect of Feno monitoring on exacerbations depends on the definition of an asthma exacerbation. For exacerbations that were defined in terms of a composite end point, the certainty of evidence is high. The composite exacerbation end point used in these studies was defined as any of the following: unscheduled visits to the provider’s office, emergency department visits, hospitalizations, oral corticosteroid use, reductions in FEV1 or in peak expiratory flow, symptom-associated lung function decline, or Global INitiative for Asthma guideline definitions. The studies that compared an asthma management strategy that includes Feno monitoring to one that does not include 6 RCTs in 1536 adults (OR, 0.62; 95% CI, 0.45–0.86) and 7 RCTs in 733 children (OR, 0.50; 95% CI, 0.31–0.82). Strategies that include Feno monitoring in adults result in an absolute risk reduction of 71 exacerbations per 1000 individuals with asthma (range of 108 to 25 fewer exacerbations). Feno monitoring is also associated with 116 fewer exacerbations per 1000 children with asthma. When only those exacerbations that result in oral corticosteroid use are used (based on 10 RCTs in 1664 adults and children), the certainty of evidence is moderate (OR, 0.67; 95% CI, 0.51–0.90). The absolute risk difference is 67 fewer exacerbations per 1000 individuals with asthma (range of 104 to 19 fewer exacerbations). For exacerbations that result in hospitalization (9 RCTs in 1598 adults and children), the certainty of evidence is low (OR, 0.70; 95% CI, 0.32–1.55). The absolute risk difference is 11 fewer exacerbations per 1000 individuals with asthma (range of 25 fewer to 19 more exacerbations).
The certainty of evidence is low for Feno monitoring to exert a change of at least the established MID using the ACT (MID, 3), Pediatric Asthma Quality of Life Questionnaire (MID, 0.5), or Asthma Quality of Life Questionnaire (MID, 0.5). For each of these outcomes, the mean difference in scores between groups with and without Feno monitoring was less than 0.1.
It is not known whether the recommendation applies to children who do not have allergic asthma because atopy (defined based on a positive skin prick test result or elevated aero-allergen-specific IgE)and allergic asthma were inclusion criteria in most of the pediatric studies, or allergic asthma was highly prevalent in the study populations.39,41,42,45–48,53–55 For the studies of adults, the presence of atopy was less consistently reported43,52,56 or was assessed as part of the study.40,44,49–51,57 Therefore, the evidence supporting this recommendation comes from mixed populations of allergic and nonallergic adults.
Studies evaluating the use of Feno to help select or monitor responses to biologic agents, with the exception of omalizumab, were not available for assessment. Therefore, whether this recommendation applies to other biologic agents is not known.
Rationale and discussion
In making this recommendation, the Expert Panel considered the desirable and undesirable effects of Feno monitoring, including the acceptability of this testing to both individuals with asthma and their providers, the feasibility of testing, and the impact of the use of Feno testing to monitor asthma on health equity. Potential benefits of Feno testing include reducing exacerbations, which is a critical outcome from both the patient and provider perspectives. The undesirable direct effects of Feno testing are expected to be minimal. However, the Expert Panel had concerns about the impact of Feno testing for asthma monitoring on accessibility and equity, as noted below.
Feno levels have been shown to be responsive to changes in anti-inflammatory medications, including ICSs, montelukast, and omalizumab. The Expert Panel did not review the effects on Feno levels of newly available anti-inflammatory biologic therapies for this update.
In the Expert Panel’s judgment, individual preferences and values have an important role in the decision to use Feno monitoring. This monitoring can affect quality of life and exacerbation frequency, and different individuals are likely to place different values on these effects. In addition, the burden (cost, time for appointments, and availability of testing) of frequent monitoring will likely influence an individual’s willingness to undergo regular testing. Therefore, a therapeutic monitoring plan that includes frequent Feno testing requires discussion and agreement between the individual with asthma and the clinician.
The Expert Panel was concerned that if Feno testing is not widely available and its use is restricted by insurance coverage policies, some individuals with asthma might not have the benefit of exacerbation reduction using Feno-based monitoring and management algorithms. As a result, disparities in asthma outcomes would widen. Most of the Feno monitoring studies with cost-effectiveness data were conducted outside the United States44,58–61 and were therefore of limited value for this update. The Expert Panel recommends cost-effectiveness analyses conducted in the United States.
Question 2.4
What is the clinical utility of Feno measurements in monitoring disease activity and asthma outcomes in individuals with asthma aged 5 years and older?
Recommendation 3: In individuals aged 5 years and older with asthma, the Expert Panel recommends against the use of Feno measurements in isolation to assess asthma control, predict future exacerbations, or assess exacerbation severity. Feno should only be used as part of an ongoing monitoring and management strategy.
Strong recommendation, low certainty of evidence
Implementation guidance
Clinician’s Summary:
The Expert Panel does not recommend Feno testing on its own to assess asthma control, predict a future asthma exacerbation, or assess the severity of an exacerbation. Feno levels are not well correlated with standard measures of asthma symptoms or control, such as the ACT, ACQ, prior or subsequent exacerbations, or exacerbation severity. Therefore, Feno testing is not a substitute for standard measures and should not be used in isolation to monitor disease activity. Feno measurement, however, may be used in conjunction with an individual’s history, clinical findings, and spirometry as part of an ongoing asthma monitoring and management strategy, which includes frequent assessments as described in Recommendation 2.
The Expert Panel recommends against the use of isolated Feno measurement for asthma management and monitoring.
Feno measurement should only be used as a part of an ongoing monitoring and management strategy to predict future exacerbations and assess exacerbation severity.
Summary of the evidence
The Expert Panel specified three critical outcomes (exacerbations, asthma control, and quality of life).
The Expert Panel considered the use of Feno measurement in adults aged 18 years or older and children aged 5 to 18 years to monitor current asthma control, subsequent and prior exacerbations, and the severity of an ongoing exacerbation. The evidence for these issues comes primarily from correlational studies.
Among adults, Feno levels are weakly associated with asthma control as measured by the ACT and the ACQ.62–65 This association is even weaker among individuals who smoke, are pregnant, or are taking an ICS. The association between Feno levels and prior or subsequent exacerbations is mixed—depending on the study, this association is strong66 or weak,67 or no such association62 exists. Among children and adolescents aged 5 to 18 years, the results are also mixed. For example, two studies showed an association between recent symptoms or uncontrolled asthma and elevated Feno levels.68,69 However, another study showed that Feno levels did not correlate with nasal or asthma symptoms.70
The evidence on the utility of Feno testing to predict exacerbations is inconclusive. These studies assessed different populations and used Feno levels alone as predictors or as part of a strategy that included other tests. For example, two studies showed that Feno levels were moderate predictors of exacerbations.42,71 In contrast, other studies showed that Feno levels, in conjunction with inflammatory markers and clinical characteristics, did not predict exacerbations72 and that Feno levels did not predict future exacerbations among high-risk urban children from minority populations.73
Among children and adults, Feno levels did not correlate with exacerbation severity.74,75 Feno testing was also difficult to perform in children in the acute setting, the results did not correlate with other measures of acute severity,76 and the results were poorly reproducible for individual patients during an exacerbation.77
Rationale and discussion
Based on the evidence summarized above, the Expert Panel recommends against the use of Feno measurement to assess asthma control, predict future exacerbations, or assess exacerbation severity unless these measurements are used as part of an ongoing asthma monitoring and management strategy as described in Recommendation 2. Further research is needed to assess the use of Feno as a marker for medication adherence, as well as its impact on asthma outcomes, acceptability, and cost-effectiveness.
Question 2.5
In children aged 0 to 4 years with recurrent wheezing, how accurate is Feno testing in predicting the future development of asthma at ages 5 years and above?
Recommendation 4:Inchildren aged0 to4 years with recurrent wheezing, the Expert Panel recommends against Feno measurement to predict the future development of asthma.
Strong recommendation, low certainty of evidence
Implementation guidance
Clinician’s Summary:
In children aged 4 years and younger who have recurrent episodes of wheezing, Feno measurement does not reliably predict the future development of asthma. Feno test results in this population should be interpreted with caution until more data are available. The Expert Panel recommends against using Feno testing to predict future development of asthma in this age group until additional research and clinical practice determinations are available.
Summary of the evidence
The summary of evidence for Recommendation 4 can be found in EtD Table III in Appendix B.
Ten studies addressed the ability of Feno measures in children younger than 5 years to predict the subsequent development of asthma in children aged 5 years and older.78–87 None of these studies were RCTs; seven studies were nonrandomized longitudinal studies and three were cross-sectional studies. Only four studies investigated the use of Feno measures to predict the diagnosis of asthma (and not wheezing or Asthma Predictive Index score). In one study in children,86 a Feno level indicating an increased risk of asthma had a positive predictive value of 58.0% on a composite measure of wheezing, diagnosis of asthma, or use of an ICS at age 7 years, where as the negative predictive value was 78.2%. This result was similar to that for the Asthma Predictive Index score without the use of Feno levels. Therefore, although Feno levels appear to reflect eosinophilic bronchial inflammation early in life, the current evidence is in sufficient to justify the conclusion that Feno testing in children aged 0 to 4 years reliably predicts a diagnosis of asthma at ages 5 years and above. Future studies may, however, demonstrate otherwise.
Although Feno levels appear to reflect T2 inflammation early in life, T2 inflammation is not specific to asthma. Feno levels in early childhood (ages 0–4 years) strongly correlate with Asthma Predictive Index scores. This correlation is not surprising because of the relationship between atopy and Feno levels and the fact that this index is heavily predicated on an atopic constitution. Feno levels are higher in children with wheezing than in children without a recent history of wheezing and in children with persistent wheezing than in those with transient wheezing. Because most children with transient wheezing stop wheezing by age 3 years,88,89 young children who continue to wheeze after age 3 years are more likely to develop asthma in the future. Four studies ascertained whether elevated Feno levels in children younger than 5 years predicted a future diagnosis of asthma. The studies, which used Feno and other clinical measures in different models, had mixed results (see EtD Table III). One longitudinal study87 is ongoing and may provide new information on this issue.
Rationale and discussion
Feno can be measured in young children who have normal resting breathing, and normal reference values for Feno have been published for children aged 1 to 5 years.90 Evidence shows that in some preschool children with recurrent coughing and wheezing, an elevated Feno level more than 4 weeks after an upper respiratory tract infection may help predict physician-diagnosed asthma at school age, independently of clinical history or presence of IgE.78–87 However, the studies reviewed for this update had conflicting results, and in the opinion of the Expert Panel, they provided low to moderate certainty for an asthma diagnosis.
A single Feno measurement to predict future asthma is not likely to be physically harmful and is not burdensome. However, unreliable prediction models risk jeopardizing future insurability and could lead to treatment decisions that might rely on inadequate measures. Until better data on the predictive ability of Feno measurement are available for children aged 0 to 4 years, clinicians should inform parents that the data are limited to support the use of Feno measurement for this purpose.
The Expert Panel appreciates the potential value of a noninvasive tool to predict asthma onset, but such testing may cause worry and adversely affect care and treatment if the findings are inaccurate. In the Expert Panel’s judgment, therefore, the acceptability of Feno measurement for predictive purposes is low. Use of this testing is unlikely to change current treatment standards and could actually misdirect care. The feasibility of implementing Feno measurement in this population seems challenging for several reasons, including the likely need for a specialist, not a primary care provider, to do the measuring because of the difficulty of ensuring proper technique and accurate results. In addition, the cost and maintenance requirements of Feno equipment may limit the test’s use.
Given that the Expert Panel recommends against the use of Feno measurement to predict future asthma diagnoses in this population, equity issues are not expected to arise. However, if the test is marketed to patients who have private insurance or who pay for health care out of pocket, it could adversely impact those individuals. Therefore, the Expert Panel believes that the balance of effects does not favor the use of Feno for predicting future asthma diagnoses in young children.
Future research opportunities
The value and potential are clearly high for new methods to evaluate individuals with wheezing, correctly identify those with asthma, select appropriate asthma therapy, and monitor responses to asthma therapy. Research on Feno measurement and its use in asthma has advanced since the Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma was published. To expand this research, further clarify the role of Feno measurement for asthma diagnosis in individuals with wheezing, and use Feno measurement to support the care of individuals with asthma, topics for future research include the following:
Use of Feno measurement in the diagnostic process (eg, to determine the point at which Feno testing should be used in relation to other diagnostic tools and which individuals with asthma aged 5 years and older should be tested)
Prevalence of asthma in the settings in which the Expert Panel recommends Feno measurement (eg, specialty care settings) to better understand the performance of Feno testing as a diagnostic tool
Use of Feno testing to monitor adherence of children and adults to ICSs and other anti-inflammatory treatments
Role of Feno measurements in children aged 0 to 5 years who have wheezing or asthma-like symptoms to predict subsequent asthma diagnoses
Role of point-of-care Feno measurement to identify children who do not require oral corticosteroid therapy
Feno-based asthma management in people with moderate to severe persistent asthma
Potential uses of Feno measurement for asthma management in primary care
Impact on asthma health disparities of differential access to Feno measurement because of lack of health care coverage
Cost-effectiveness of Feno measurement in diverse populations and clinical settings
Role of Feno testing in individuals with uncontrolled asthma to predict the benefit of adding T2-directed biologic therapies
Refinement and validation of Feno cutoff levels for diagnostic purposes (eg, by determining variations in Feno levels in individuals with different comorbid conditions, physiological determinants of Feno levels, and Feno levels in different ethnic and racial groups)
Identification of algorithms for the most useful combination of, and cutoff levels for, objective measures (eg, Feno levels, blood eosinophil levels, spirometry test results, short-acting beta2-agonist [SABA] use, and symptom scores) for choosing, monitoring, or adjusting anti-inflammatory therapy
Refinement of ongoing management strategies that incorporate Feno measurement to better understand the optimal timing and interpretation of Feno levels in a range of asthma phenotypes (eg, eosinophilic vs noneosinophilic asthma)
Identification of the populations most likely to benefit from Feno-guided treatment and the optimal frequency of Feno monitoring
SECTION III: RECOMMENDATIONS FOR INDOOR ALLERGEN MITIGATION IN MANAGEMENT OF ASTHMA
Background
Environmental control is one of the four cornerstones of asthma management in Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma.12 The Expert Panel was tasked with examining the effectiveness of single-component and multicomponent allergen mitigation strategies directed at common, indoor aeroallergens, with the goal of improving asthma outcomes for individuals with asthma. The key questions for this priority topic and the recommendations by the Expert Panel are provided for single-component and multicomponent allergen mitigation strategies.
Not included in the scope of work for this priority topic is an examination of the utility of clinical testing for sensitivity to allergens (eg, using skin prick tests or tests of allergen-specific IgE), mitigation strategies for out door allergens, and mitigation of environmental irritants (eg, tobacco smoke). Specific occupational exposures were also outside the scope of work, although the indoor allergens addressed in these recommendations can be encountered in work settings.
Definitions of terms used in this section
An allergen mitigation intervention aims to decrease an individual’s exposure to allergens. The intervention can have a single component or multiple components.
A single-component intervention is an individual mitigation strategy targeted at one or more specific allergens to which an individual is both exposed and sensitized. Single-component allergen mitigation interventions examined in this report include the following:
Acaricide: a house-dust mite pesticide that can be applied to carpets, mattresses, and furniture.
Air filtration systems and air purifiers, including those with high-efficiency particulate air (HEPA) filters: devices that filter indoor air and remove solid particulates, such as dust, pollen, mold, and bacteria, from the air.
Carpet removal: removal of wall-to-wall or area rugs from one or more rooms.
Cleaning products: including application of bleach or similar products.
HEPA vacuum cleaners: vacuum cleaners that have a HEPA filter.
Impermeable pillow and mattress covers: covers placed on mattresses and pillows that are impermeable to dust mites.
Integrated pest management: a comprehensive approach to removing and controlling common indoor pests (eg, cockroaches and mice) using, for example, traps, poison, and barriers to influx. The Expert Panel considered integrated pest management to be a single-component intervention even though it may include prevention, mitigation, and removal strategies.
Mold mitigation: professional removal, cleaning, sanitization, demolition, or other treatment to remove or prevent mold. The Expert Panel considered mold mitigation to be a single-component intervention even though it may include prevention, mitigation, and removal strategies.
Pet removal: complete removal or confinement of furry pets (eg, dogs and cats) to specific rooms in a house.
A “multicomponent intervention” is defined as the use of two or more of the aforementioned single-component interventions at the same time as part of a bundled approach targeted at one or more allergens to which the individual is both sensitized and exposed. An example of a multicomponent intervention is the use of three single-component interventions (eg, air purifiers, impermeable pillow and mattress covers, and HEPA vacuum cleaners) for individuals sensitized and exposed to dust mites and mold.
“Sensitization” is defined in this section as the production of a specific IgE to an aeroallergen whose presence can be confirmed by skin prick testing or assays for a specific IgE.
Question 3.1
Among individuals with asthma, what is the effectiveness of interventions (eg, pesticides, air filters/purifiers, mattress covers, and pest control) to reduce or remove indoor inhalant allergens on asthma control, exacerbations, quality of life, and other relevant outcomes?
In some individuals, asthma can have an allergic component. Therefore, clinicians should take a history of the individual’s environmental allergen exposure and pursue testing for specific allergen sensitization, when appropriate. The Expert Panel has several recommendations for this question:
Recommendation 5: In individuals with asthma who do not have sensitization to specific indoor allergens or who do not have symptoms related to exposure to specific indoor allergens, the Expert Panel conditionally recommends against allergen mitigation interventions as part of routine asthma management.
Conditional recommendation, low certainty of evidence
Recommendation 6: In individuals with asthma who have symptoms related to exposure to identified indoor allergens, confirmed by history taking or allergy testing, the Expert Panel conditionally recommends a multicomponent allergen-specific mitigation intervention.
Conditional recommendation, low certainty of evidence
Recommendation 7: In individuals with asthma who have sensitization or symptoms related to exposure to pests (cockroaches and rodents), the Expert Panel conditionally recommends the use of integrated pest management alone, or as part of a multicomponent allergen-specific mitigation intervention.
Conditional recommendation, low certainty of evidence
Recommendation 8: In individuals with asthma who have sensitization or symptoms related to exposure to dust mites, the Expert Panel conditionally recommends impermeable pillow/mattress covers only as part of a multicomponent allergen mitigation intervention, not as a single-component intervention.
Conditional recommendation, moderate certainty of evidence
Implementation guidance
Clinician’s Summary:
For individuals with asthma who do not exhibit any allergy symptoms or for whom testing has not suggested that they have an allergy to certain indoor substances (eg, dust mites or cat dander), the Expert Panel recommends no specific environmental interventions to reduce these allergens within the home.
For individuals with asthma who are exposed to an allergen within the home and who have allergy symptoms or a positive test result suggesting that they have an allergy to certain indoor substances (eg, dust mites or cat dander), the Expert Panel recommends using a multicomponent intervention to try to control the indoor allergen in question. Single-component interventions often do not work.
For individuals with asthma who are exposed to cockroaches or rodents (eg, mice) in the home and who have allergy symptoms or sensitization to these allergens demonstrated by allergy skin testing or a specific IgE, the Expert Panel recommends using integrated pest management to improve asthma outcomes. Integrated pest management can be used alone or with other interventions to reduce exposure to pest-related allergens in the home.
For individuals with asthma who have allergy symptoms or a test result suggesting that they are allergic to dust mites, the Expert Panel recommends using multicomponent interventions to reduce dust mite levels in the home and improve asthma outcomes. Use of pillow and mattress covers alone does not improve asthma outcomes.
Overall, the studies of allergen mitigation strategies provide low certainty of evidence that these strategies are beneficial for key asthma outcomes. Therefore, the Expert Panel recommends tailored allergen intervention strategies only for individuals with asthma who are exposed to these specific allergens and have either symptoms based on clinical history or an allergy to these substances based on allergy testing.
Based on current data on the use of a variety of single-component and multicomponent strategies to reduce exposure to allergens, the Expert Panel makes the following suggestions for implementing allergen exposure reduction strategies:
Allergen mitigation strategies can be used in individuals of all ages with asthma of all levels of severity.
Clinicians need to tailor mitigation strategies to the individual based on their allergy symptoms, sensitization, and exposures. Clinicians should consider allergen testing when appropriate, before committing individuals to specific allergen mitigation strategies that may be burdensome. See Table IIIA for allergen-specific mitigation interventions addressed in the systematic review report. Table IIIB summarizes the certainty of evidence on various allergen mitigation interventions.
The Expert Panel recognizes the existing inequities in access to specialists and allergen testing. The panel therefore advises clinicians to, at a minimum, take a clinical history of symptoms and exposures for all individuals with asthma to help determine the need for allergen mitigation.
Allergy testing (with a skin prick or allergen-specific IgE test) may have false-positive and false-negative results, and certain allergens (eg, dust) may also act as irritants. For an individual whose symptoms worsen on exposure to specific aeroallergens, the Expert Panel recommends that the clinician consider mitigating that aeroallergen even if the individual’s test result is negative.
Some of the interventions examined provide no or low certainty of evidence about their efficacy in improving asthma outcomes (including exacerbations, quality of life, asthma control, and symptoms). The Expert Panel recognizes that some of the interventions, especially integrated pest management and mold mitigation, may have broader public health benefits. However, these interventions do not replace routine good practices, including regular and frequent house cleaning and laundering of bedding materials.
Some people are allergic to dander (flakes of skin) or saliva from pets. The few studies on pet removal have had inconclusive results. However, if an individual with asthma experiences symptoms around a pet, the individual should consider removing the pet from the home, keeping the pet outdoors, or, if neither of these options is feasible, keeping the pet out of commonly used rooms. Testing for sensitization to pets may be particularly worthwhile for those with chronic or uncontrolled symptoms and might help support what can be a difficult decision to remove a pet from the home.
Some cleaning and integrated pest management interventions may trigger asthma and/or be hazardous. Individuals with asthma need to balance the potential benefits and harms of interventions before implementing them.
If an individual with asthma has sensitization to an allergen on skin prick testing and is exposed to that allergen but has no objective evidence of worsened disease control and denies having symptoms, chronic exposure could have led to the development of clinical tolerance to that allergen in that environment. Allergen-specific mitigation strategies could adversely modify this established balanced relationship between the individual and the environment.
- What clinicians should discuss with their patients and families:
- Clinicians need to consider the complexity of the patient population and the limitations of the evidence identified. Clinicians may also find it helpful to consider the severity of a patient’s asthma, the small benefit, and the extent of previous symptoms and exacerbations when recommending allergen mitigation interventions.
- Allergen mitigation interventions may be expensive or difficult for patients to use or maintain. Clinicians should consider the cost implications of certain interventions, especially among those with limited financial resources, and assess the magnitude of the potential value of an intervention in improving an individual’s asthma outcomes.
TABLE IIIA.
Examples of allergen mitigation interventions and their targeted allergens
| Intervention assessed in studies in the SR | Allergen |
|||
|---|---|---|---|---|
| Animal dander | Dust mites | Cockroaches | Mold | |
| Acaricide | ++ | |||
| Air filtration systems and air purifiers | ++ | + | + | ++ |
| Carpet removal | ++ | ++ | + | |
| Cleaning products (eg, bleach) | ++ | |||
| HEPA vacuum cleaners | ++ | + | + | ++ |
| Impermeable pillow and mattress covers | ++ | |||
| Integrated pest management | +* | ++ | ||
| Mold mitigation | ++ | |||
| Pet removal | ++ | |||
SR, Systematic review.
Primary target allergen(s) for the intervention.
Secondary target allergen(s) for the intervention.
Dander from rodents.
TABLE IIIB.
Summary of certainty of evidence on allergen mitigation interventions
| Intervention assessed in studies in the SR | EtD table number | Evidence on use as a single-component strategy for allergen mitigation (certainty of evidence) | Evidence on use as part of a multicomponent strategy for allergen mitigation (certainty of evidence)* |
|---|---|---|---|
| Acaricide | IV | † | Intervention makes no difference (moderate certainty of evidence) |
| Impermeable pillow and mattress covers | V | Intervention makes no difference (moderate certainty of evidence) | Evidence favors intervention (moderate certainty of evidence) |
| Carpet removal | VI | † | Intervention makes no difference (low certainty of evidence) |
| Integrated pest management (for cockroaches and mice) | VII | Evidence favors intervention (low certainty of evidence) | Evidence favors intervention (low certainty of evidence) |
| Air filtration systems and air purifiers | VIII | Intervention makes no difference (low certainty of evidence) | Intervention makes no difference (moderate certainty of evidence) |
| HEPA vacuum cleaners | IX | † | Evidence favors intervention (among children only; moderate certainty of evidence) |
| Cleaning products | X | † | † |
| Mold mitigation | XI | † | Evidence favors intervention (low certainty of evidence) |
| Pet removal | XII | † | † |
SR, Systematic review.
Combination of interventions used in the multicomponent studies varied, and the Expert Panel cannot identify or recommend any particular combination of strategies as optimal at this time.
Evidence was insufficient for the Expert Panel to assess the intervention.
Summary of the evidence
The Expert Panel specified four outcomes (exacerbations, asthma quality of life, asthma control, and asthma symptoms) as critical outcomes when it reviewed the evidence. The panel considered outcomes related to health care utilization to be important outcomes. The Expert Panel gave higher priority to outcomes measured in studies that used validated outcome instruments than those assessed with nonvalidated outcome measures. When data on validated outcome measures were not available, the Expert Panel used data from nonvalidated outcome measures, such as asthma symptoms. Table IIIB summarizes the Expert Panel’s assessments of the certainty of evidence for each of the allergen mitigation interventions examined, when used as a single-component intervention or as part of a multicomponent intervention. The table also lists the EtD tables for each of the interventions.
Single-component allergen mitigation interventions
For the majority of single-component allergen mitigation interventions, studies to assess the effectiveness of the interventions were limited. For the single-component interventions with enough studies to assess their impact on critical outcomes, the certainty of the evidence was either low or very low, or the results were limited to one or two critical outcomes on which results were inconclusive or that did not improve. The studies included mixed populations, which made it difficult to determine whether better-defined populations might benefit from the intervention. Certainty of evidence was often downgraded because of the limitations of several studies, including those of single-component interventions with acaricides91,92 and air purifiers.93–96 These limitations included insufficient descriptions of the randomization scheme, absence of a placebo intervention, and imprecision related to small sample size. No single-component intervention studies examining HEPA vacuum cleaners, carpet removal, or mold mitigation were available for review. The evidence was insufficient to allow the Expert Panel to examine the use of cleaning products.97 In contrast, dust mite mitigation using impermeable mattress and pillow covers as a single intervention was the subject of many RCTs, which yielded moderate certainty of evidence of no benefit for the critical outcomes, including asthma symptoms.98–109 Results for pet removal were inconclusive.110
Based on these studies, the Expert Panel made a conditional recommendation against most single-component allergen mitigation interventions as part of routine asthma management for individuals without specific identified triggers or exposure. The Expert Panel also included in the recommendation a conditional recommendation against impermeable pillow and mattress covers as a single-component allergen mitigation intervention.
One RCT and one pre- and postintervention study suggested that integrated pest management for cockroaches and rodents reduces the number of asthma exacerbations but has no effect on asthma control.111,112 As a result, the Expert Panel made a conditional recommendation in favor of using integrated pest management as a single-component allergen mitigation strategy based on the evidence showing a reduction in asthma symptoms (low certainty of evidence). The Expert Panel also noted the importance of pest control as an established public health principle and practice.
Multicomponent allergen mitigation interventions
The effectiveness of multicomponent mitigation interventions was difficult to evaluate because of inconsistencies in the designs used in different studies. Studies on most multicomponent interventions demonstrated minimal or no improvement in critical outcomes. Some studies did, however, demonstrate a reduction in asthma symptoms. The systematic review, using a qualitative comparative analysis, was unable to determine whether specific combinations of interventions were necessary or sufficient to improve the outcomes of interest.4
For multicomponent interventions that included integrated pest management, results were mixed. These studies provided high certainty of evidence of no reduction in exacerbations, although the same studies provided moderate to low certainty of evidence of a reduction in asthma symptoms and exacerbations when a composite measure was used. When examined in the context of a multicomponent intervention, acaricides had no effect on asthma symptoms (high certainty of evidence) and had inconclusive results for exacerbations (very low certainty of evidence).113–117 Multicomponent intervention studies that included the use of HEPA vacuum cleaner shad mixed results; some RCTs demonstrated changes in exacerbations, asthma-related quality of life, or asthma symptoms.118–123 Most of the studies that demonstrated improvements in critical outcomes using HEPA vacuum cleaners were conducted in children.
In multicomponent studies that included air filtration systems and air purifiers (three of the four studies used devices with HEPA filters), the results showed no decrease in exacerbations or improvement in quality of life (high certainty of evidence). The results were mixed for asthma control (no benefit, low certainty of evidence) and asthma symptoms (decreased severity or number of reported symptoms in children but not in mixed populations, low certainty of evidence).118,121,124,125
Studies on the use of impermeable pillow and mattress covers as part of a multicomponent intervention strategy provided high certainty of evidence of a decrease in the number of asthma symptom days but did not show other benefits for any of the critical outcomes examined.121,122,124–126 Studies using a composite score for asthma symptoms or cough and wheeze frequency provided very low to moderate certainty of no benefit of impermeable pillow and mattress covers, depending on the outcome examined.113,114,116–118,121,122,127,128
Some but not all study findings suggested that multicomponent interventions that included mold mitigation reduce symptoms to an extent.129,130 The results of studies of multicomponent interventions that included pet removal were inconclusive.115,130
Most studies did not examine harms, and none reported any important harms from the various allergen mitigation strategies studied. Because of the lack of benefits identified and the potential harms from applications of chemicals, the Expert Panel does not recommend the use of acaricides.
Rationale and discussion
Overall approach for developing allergen mitigation recommendations.
When developing each of the four recommendations in this section, the Expert Panel considered the benefits and harms of each of the allergen mitigation interventions and the level of evidence available for assessing the interventions. In addition, the Expert Panel considered the acceptability of the interventions to individuals with asthma and their providers as well as the ease of use, costs, and impact on health equity of each intervention.
Potential harms.
Although the identified harms from most of the interventions were minimal, studies rarely examined harms. Therefore, the Expert Panel considered theoretical harms, patient burden, and initial and ongoing costs in its recommendations. For example, the Expert Panel’s judgment was that interventions for mold mitigation and carpet removal could be associated with risks or be costly or difficult to complete. Another Expert Panel determination was that impermeable pillow and mattress covers are low-risk interventions with limited costs but are likely to require frequent cleaning of the bedding above the covers to be effective.
Prioritization of outcomes.
Furthermore, the Expert Panel considered the impact of the interventions on asthma symptoms as a critical outcome. The Expert Panel recognized that none of the studies used a validated outcome measure of asthma symptoms, and the definition of asthma symptoms was not standardized across studies. However, asthma symptoms are a relevant patient-centered outcome that was important to individuals with asthma in focus groups and that could be particularly relevant to assess for low-risk interventions.
Heterogeneity of studies.
The Expert Panel found the heterogeneity of available studies to be challenging. As outlined in the allergen reduction systematic review report,4 participants’ baseline clinical characteristics were variable, and the findings from these studies suggested that participants were not equally likely to benefit from the interventions reviewed.
In addition, the Expert Panel preserved the systematic review report authors’ distinction between single-component interventions designed to mitigate a single allergen (eg, an acaricide for house-dust mite allergens); single-component interventions that address multiple allergens (eg, air purifiers to control mold and animal dander); and multicomponent interventions, which usually target more than one allergen (see Table IIIA).
Many of the studies available to the Expert Panel examined multicomponent interventions in mixed populations of patients with varying severities of asthma and sensitizations to allergens. Moreover, the combinations of components examined in each study were rarely the same across studies, and most studies did not assess adherence to or use of the interventions. The Expert Panel concurred with the systematic review report authors’ assessment that the interplay between allergen type, intervention type, and individual patient characteristics could have strongly modified the effects of these interventions in these studies.
Targeting recommendations to individuals who are both exposed and allergic to specific allergens.
It was the Expert Panel’s judgment that individuals with asthma should not burden themselves with allergen mitigation interventions if they are both not regularly exposed to an allergen and not allergic to a specific allergen. Given that certain populations might not have ready access to allergy specialists and allergen skin prick or IgE testing, the Expert Panel noted that patient histories (eg, symptoms related to exposure to specific indoor allergens) to assess patient sensitivities could suffice. Therefore, the Expert Panel is not recommending allergen mitigation interventions for all individuals with asthma. Instead, the panel is recommending basing decisions about allergen mitigation interventions on a combination of the exposures, symptoms, and sensitization of individuals with asthma.
Single-component interventions are rarely effective.
Of the single-component allergen mitigation interventions evaluated in enough studies to assess their impact on critical outcomes, the certainty of the evidence was either low or very low, or the results were limited to one or two critical outcomes, were inconclusive, or demonstrated no improvement. As summarized in Table IIIB, the Expert Panel considered integrated pest management to be a single-component intervention, and it was the only single-component approach with beneficial effects. Single-component dust mite interventions using pillow and mattress covers demonstrated no benefit for any of the critical outcomes, including asthma symptoms. Based on these findings, it was the Expert Panel’s judgment that single-component approaches to mitigating an allergen are rarely effective.
Evidence for multicomponent interventions varies.
Across the allergen mitigation interventions examined in this report, it was the Expert Panel’s judgment that mattress and pillow covers, integrated pest management, HEPA vacuum cleaners, and mold mitigation are potentially beneficial when used as part of a multicomponent allergen mitigation strategy, but the benefits are small. Mattress and pillow covers as part of a multicomponent allergen mitigation strategy did not show improvements when validated outcome measures (eg, exacerbations, ACT, or Asthma Quality of Life Questionnaire) were used. The strength of evidence from the studies demonstrating small reductions in symptom days (anon validated outcome measure) and the low risk and relative cost of impermeable pillow and mattress covers resulted in the Expert Panel’s conditional recommendation for use of this intervention only as part of a multicomponent allergen mitigation strategy.
The evidence was stronger on improvements across asthma outcomes for both integrated pest management and HEPA vacuum cleaners used as part of a multicomponent strategy than the evidence on impermeable mattress and pillow covers.
Only three studies examined multicomponent interventions that included mold mitigation.129–131 The Expert Panel considered the reduction in health care utilization with mold mitigation as well as the broader public health benefit of supporting its use as part of a multicomponent allergen mitigation strategy in making its conditional recommendation.
Additional considerations.
For most of these interventions, the certainty of evidence is low, and the benefits are small. It is not the Expert Panel’s intent to suggest that all four of these interventions (mattress and pillow covers, integrated pest management, HEPA vacuum cleaners, and mold mitigation), when used as part of a multicomponent strategy, serve as the optimal allergen mitigation package. Instead, the Expert Panel is indicating that individuals who have symptoms related to exposure to specific allergens should consider using these interventions when appropriate.129
The Expert Panel recognizes that patients, providers, and other stakeholders generally find mattress and pillow covers to be an acceptable, noninvasive strategy to reduce exposure to dust mites. However, the Expert Panel cautions individuals with asthma not to use these covers as the sole strategy for mitigating dust mites. Studies that applied mattress and pillow covers solely either showed no effect on asthma outcomes or had inconclusive results. It was the Expert Panel’s judgment that mattress and pillow covers should only be applied as part of a multicomponent intervention targeting dust mites.
In summary, the studies of allergen mitigation strategies provided lower certainty of evidence of effectiveness for key asthma outcomes than studies of asthma controller medications. For these reasons, the Expert Panel recommends only tailored allergen intervention strategies for individuals with asthma who have symptoms related to exposure confirmed by allergy testing or clinical history for identified indoor allergens.
Future research opportunities
The Expert Panel has identified the following topics related to allergen mitigation interventions (eg, acaricides, air purifiers, HEPA vacuum cleaners, carpet removal, pet removal, cleaning products, and mold mitigation) that require additional research:
Effectiveness of allergen mitigation interventions that use the validated outcome measures recommended by the Asthma Outcomes Workshop10
Effectiveness of allergen mitigation interventions in individuals with asthma who have demonstrated exposure and/or sensitization to these allergens at home, school, or work
Multicomponent interventions targeted to specific allergens in study populations consisting only of people with demonstrated sensitization and exposure to those allergens
Comparisons of different combinations of multicomponent interventions to determine the optimal combination(s) of allergen-specific mitigation strategies that improve outcomes
Studies to determine the allergen reduction thresholds for symptoms
Interactions and necessity of exposure, sensitization, and symptoms to determine which individuals with asthma will benefit most from allergen mitigation strategies (eg, whether an allergen-specific mitigation strategy is beneficial for an individual with asthma who has sensitization on skin prick testing to an allergen, is exposed to that allergen, and denies having symptoms)
In addition, reports of studies on the effectiveness of allergen mitigation interventions must include details on the intervention studied (eg, the models of air purifiers used) and the protocols for using the intervention (eg, how often the air purifier was turned on, where it was located, and how often the filter was changed). These aspects of the intervention need to be measured, and levels of adherence to the protocol need to be reported.
SECTION IV: RECOMMENDATIONS FOR THE USE OF INTERMITTENT ICS IN THE TREATMENT OF ASTHMA
Background
Scheduled, daily ICS treatment is the currently preferred pharmacologic controller therapy for persistent asthma in individuals of all ages.12 Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma (EPR-3), published in 2007, suggested that intermittent ICS dosing schedules may be useful in some settings, but the evidence at that time was insufficient to support a recommendation in favor of this treatment beyond a recommendation based on expert consensus.12
Definitions of terms used in this section
“Intermittent” ICS dosing in this section includes courses of ICS treatment used for brief periods, usually in response to symptoms or as an add-on with or without a LABA. “Intermittent ICS dosing” does not refer to a single regimen, and its definition is specified in each of the recommendations. Intermittent ICS dosing allows providers to prescribe specific doses, frequencies, and durations of ICS use. When to use intermittent ICS dosing could depend on an individual’s decision (based on need, which is also known as “as-needed” or “pro re nata” dosing), a predefined index showing worsening asthma, or some other predefined criterion.
“Controller therapy” refers to medications that are taken daily on a long-term basis to achieve and maintain control of persistent asthma.12 Both controller therapy and intermittent dosing may involve daily use of a specific dose of an ICS. The terms “ICS-LABA” (inhaled corticosteroid and long-acting beta2-agonist combination, typically in a single device) and “ICS-formoterol” indicate combination therapy with both an ICS and a LABA, usually and preferably in a single inhaler.
“Quick-relief” therapy refers to medications (eg, an inhaled SABA) used to treat acute symptoms or exacerbations.132 In this section, “as-needed” dosing (eg, of a SABA) is intermittent and is based on the patient’s decision (Figs 2–4).
The definitions of “low-,” “medium-,” and “high-dose” ICS are based on the recommendations from EPR-3.12
The term “puff” refers to a single actuation and inhalation of a medication delivered through any type of inhaler.
“Recurrent wheezing” as used for the studies included in this section is defined as three or more episodes of wheezing triggered by apparent respiratory tract infections in a child’s lifetime or two episodes in the past year.
Overview of key questions and recommendations for intermittent ICS use
Given the range of options for intermittent ICS dosing and the number of comparisons embedded in the three key questions for this priority topic, the Expert Panel made five recommendations for intermittent ICS use to address these key questions. The majority of the studies in the systematic review report6 on this topic used comparative efficacy designs as opposed to comparative effectiveness designs.
Table IV provides an overview of the questions on this topic, interventions and comparators that the Expert Panel considered, and resulting recommendations. As shown, in the opinion of the Expert Panel, the evidence was insufficient to support recommendations for all of the comparators in the questions.
TABLE IV.
ICS key questions and recommendations
| Question | Intervention | Comparator | Recommendation | Certainty Of evidence |
|---|---|---|---|---|
| 4.1 | Short-course daily ICS + as-needed SABA at start of RTI (Step 1) | As-needed SABA alone | Recommendation 9: Conditional, in favor of the intervention for ages 0–4 y | High |
| Daily ICS | No recommendation* | |||
| No therapy | No recommendation* | |||
| 4.2 | As-needed, concomitantly administered ICS + SABA | Daily ICS + as-needed SABA (Step 2) | Recommendation 10: Conditional, in favor of either the intervention or the comparator for ages 12 y and above | Moderate |
| No recommendation* for ages 4–11 y | ||||
| Intermittent, higher-dose ICS | Recommendation 11: Conditional, against the intervention for ages 4 y and above | Low | ||
| 4.3 | Daily and as-needed ICS- formoterol (Steps 3 and 4) | Daily same-dose ICS + as-needed SABA | No recommendation* for ages 4 y and above | |
| Daily higher-dose ICS + as-needed SABA | Recommendation 12: Strong, in favor of the intervention for ages 4 y and above | Moderate for ages 4–11 y | ||
| High for ages 12 y and above | ||||
| Daily same-dose ICS-LABA + as-needed SABA | Recommendation 12: Strong, in favor of the intervention for ages 4 y and above | Moderate for ages 4–11 y | ||
| Daily higher-dose ICS-LABA + as-needed SABA | No recommendation* for ages 4–11 y | High for ages 12 y and above | ||
| Recommendation 13: Conditional, in favor of the intervention for ages 12 y and above | High for ages 12 y and above |
RTI, Respiratory tract infection.
Insufficient evidence.
In the remainder of this section, each key question is followed by recommendations that are relevant to the question, the evidence that supports the recommendation, and guidance for implementing each recommendation. The Expert Panel did not address the efficacy and safety of the following types of intermittent ICS treatment because they were not mentioned in the key questions:
Question 4.1
What is the comparative effectiveness of intermittent ICS compared to no treatment, pharmacologic therapy, or nonpharmacologic therapy in children aged 0 to 4 years with recurrent wheezing?
Recommendation 9: In children aged 0 to 4 years with recurrent wheezing triggered by respiratory tract infections and no wheezing between infections, the Expert Panel conditionally recommends starting a short course of daily ICS at the onset of a respiratory tract infection with as-needed SABA for quick-relief therapy compared to as-needed SABA for quick-relief therapy only.
Conditional recommendation, high certainty of evidence
Implementation guidance
Clinician’s Summary:
This recommendation is for children aged 0 to 4 years who have had three or more episodes of wheezing triggered by apparent respiratory tract infections in their lifetime or who have had two such episodes in the past year and are asymptomatic between respiratory tract infections. For this population, the Expert Panel recommends a short (7–10-day) course of ICS daily along with as-needed SABA for quick-relief therapy starting at the onset of signs and symptoms indicating a respiratory tract infection. Respiratory tract infections were not confirmed by culture or PCR in the studies, and no further details on wheezing were provided.
The Expert Panel makes the following suggestions for implementation of intermittent ICS dosing in children aged 0 to 4 years:
One regimen used in two studies133,134 is budesonide inhalation suspension, 1 mg, twice daily for 7 days at the first sign of respiratory tract infection–associated symptoms.
Although the efficacy of intermittent ICS dosing has high certainty of evidence, data regarding effects on growth are conflicting. Clinicians should carefully monitor length or height in children treated with the recommended regimen.
Caregivers can initiate intermittent ICS treatment at home without a visit to a health care provider when they have clear instructions. Clinicians should give caregivers written instructions on how to implement the recommended action plan at the onset of a respiratory infection. In addition, clinicians should review the plan with the caregiver at regular intervals.
Clinicians should consider this intervention in children who are not taking daily asthma treatment at the first sign of respiratory tract infection–associated symptoms.
- What clinicians should discuss with caregivers:
- Caregivers should be confident in the use of the asthma action plan because they will need to decide when to start treatment (ie, at the onset of a respiratory tract infection).
- The main potential benefit of intermittent ICS use during respiratory tract infections is the reduction in exacerbations requiring systemic corticosteroids. Clinicians should inform caregivers that this treatment could affect growth, and they should carefully monitor growth in children who use this recommended treatment. Clinicians should reconsider implementing this recommended treatment if any evidence shows a reduced growth rate that cannot be attributed to other factors (eg, oral corticosteroid treatment). As part of shared decision making, some parents may weigh the potential benefits and harms differently and may not choose this therapy because of concerns related to their child’s growth.
Summary of the evidence
The Expert Panel specified three critical outcomes (exacerbations, asthma control, and quality of life) and one important outcome (rescue medication use) for this question. The summary of evidence for Recommendation 9 is in EtD Table XIII in Appendix B.
Three RCTs with high certainty of evidence133,135,136 compared SABA alone to intermittent ICS with SABA for quick relief. This treatment resulted in a 33% relative risk (RR) reduction in exacerbations requiring systemic corticosteroids. Two of these three trials assessed growth but found different effects on this outcome. Ducharme et al135 found a 5% lower gain in height and weight in study participants receiving intermittent fluticasone (750 μg twice daily at onset of a respiratory tract infection for up to 10 days) than in participants receiving a placebo.135 The authors noted a significant correlation between the cumulative dose of fluticasone and changes in height. In contrast, Bacharier et al133 did not find an effect on linear growth of budesonide inhalation suspension (1 mg twice daily for 7 days) in comparison with placebo in children with an “identified respiratory tract illness.” Whether these differences in growth effects were due to differences in drugs, doses, duration of treatment, or other factors is not clear.
Rationale and discussion
The main comparator for which data are available is SABA-only therapy. The demonstrated efficacy but conflicting data regarding the effect of a short course of a daily ICS with SABA for quick-relief therapy on growth led the Expert Panel to develop a conditional recommendation for this therapy starting at the on set of an apparent respiratory tract infection for children aged 0 to 4 years with recurrent wheezing. Although one study that compared short ICS courses with regular daily ICS treatment showed no differences in exacerbations requiring systemic corticosteroids with moderate certainty of evidence, the Expert Panel made no recommendation based on this comparison because this study was not adequately powered to demonstrate equivalence.134 No studies produced robust data on comparisons of intermittent ICS use with no treatment or a nonpharmacologic therapy.
Question 4.2
What is the comparative effectiveness of intermittent ICS compared to ICS controller therapy in individuals aged 5 years and older with persistent asthma?
Recommendation 10: In individuals aged 12 years and older with mild persistent asthma, the Expert Panel conditionally recommends either daily low-dose ICS and as needed SABA for quick-relief therapy or as-needed ICS and SABA used concomitantly.
Conditional recommendation, moderate certainty of evidence
Implementation guidance
Clinician’s Summary:
For individuals aged 12years and older with mild persistent asthma, the Expert Panel recommends either of the following two treatments as part of Step 2 therapy: a daily low-dose ICS and as-needed SABA for quick-relief therapy or intermittent as-needed SABA and an ICS used concomitantly (ie, one after the other) for worsening asthma. In this recommendation, “intermittent” ICS dosing is defined as the temporary use of an ICS in response to worsening asthma in an individual with asthma who is not taking ICS controller therapy regularly. This recommendation does not apply to ages 5 to 11 years because this therapy has not been adequately studied in this age group.
The Expert Panel makes the following suggestions for implementation of intermittent ICS dosing in individuals aged 12 years and older:
Individuals aged 12 years and older with mild persistent asthma who are not taking asthma treatment may benefit from this therapy. The Expert Panel has made no recommendation for children aged 0 to 4 years or 5 to 11 years with mild persistent asthma because of insufficient evidence.
Individuals aged 12 years and older with asthma and a low or high perception of symptoms may not be good candidates for as-needed ICS therapy. Regular low-dose ICS with SABA for quick-relief therapy may be preferred for such patients to avoid ICS undertreatment (low symptom perception) or overtreatment (high symptom perception).
Based on the regimen assessed in three of the four studies on intermittent ICS dosing,40,137,138 one approach to intermittent therapy is two to four puffs of albuterol followed by 80 to 250 μg of beclomethasone equivalent every 4 hours as needed for asthma symptoms. In these studies, the clinician determined the dosing a priori. Currently, these medications need to be administered sequentially in two separate inhalers, but combination inhalers with albuterol and an ICS may be available in the United States in the future.
Individuals who use this type of therapy can initiate intermittent therapy at home. However, they should receive regular follow-up to ensure that the intermittent regimen is still appropriate.
- What clinicians should discuss with patients and families:
- Clinicians should inform individuals that the two treatment options do not have different effects on asthma control, asthma quality of life, or the frequency of asthma exacerbations when studied in large groups of people. Similarly, side effects are equally infrequent with daily and intermittent use.
- Shared decision making will allow the best choice to be made for a particular individual.
Summary of the evidence
The Expert Panel specified three critical outcomes (exacerbations, asthma control, and quality of life) and one important outcome (rescue medication use) for this question. The summary of evidence for Recommendation 10 can be found in EtD Table XIV in Appendix B.
The studies showed no differences in asthma control, quality of life, or use of rescue therapy with the two types of intermittent ICS therapy (ICS paired with albuterol in two studies and ICS for worsening asthma symptoms in one study) and daily ICS treatment in three studies with high certainty of evidence in individuals aged 12 years and older.40,138,139 The three studies also showed no differences in numbers of exacerbations between groups, but the strength of evidence on exacerbations was low. However, none of these studies was powered as an equivalence study, so the Expert Panel issued a conditional recommendation.
The Expert Panel made no recommendation for children aged 4 to 11 years because only low certainty of evidence was available from one small study by Martinez et al140 that addressed this question in this age group (EtD Table XV). Although the systematic review report6 included one study in children aged 5 to 10 years, this study was not included in the EtD tables. In that study, all children received regular ICS treatment for 6 months. For the next 12 months, children were randomized to receive either intermittent ICS treatment or continued daily low-dose ICS treatment. Children in the continuous ICS group experienced significantly fewer exacerbations per individual (0.97) than those in the intermittent group (1.69; P = .008). However, the intermittent group had a greater increase in height after 6 months than the group that maintained regular therapy during months 6 to 18.141 The Expert Panel concluded that the use of regular ICS therapy for 6 months before intermittent therapy made this study’s results difficult to interpret in the context of the key question.
Rationale and discussion
Outcomes did not differ in the groups treated with the two alternate regimens in the three studies40,138,139 in individuals aged 12 years and older. However, because none of these studies was powered as an equivalence study, the Expert Panel made a conditional recommendation. Although the studies had high certainty of evidence for asthma control and quality of life, they had low certainty of evidence for exacerbations and, taken together, resulted in over all low certainty for the recommendation statement. The Expert Panel made no recommendation based on this comparison for children aged 4to 11 years because the only small included study in this population had low certainty of evidence, and one additional study had a study design that precluded evaluation for this key question.
Recommendation 11: In individuals aged 4 years and older with mild to moderate persistent asthma who are likely to be adherent to daily ICS treatment, the Expert Panel conditionally recommends against a short-term increase in the ICS dose for increased symptoms or decreased peak flow.
Conditional recommendation, low certainty of evidence
Implementation guidance
Clinician’s Summary:
This recommendation addresses temporary increases in the dose of an ICS that is otherwise taken as controller therapy in response to worsening asthma. For this recommendation, a short-term increase in ICS dose refers to a doubling, quadrupling, or quintupling of the regular daily dose. For individuals aged 4 years and older with mild to moderate persistent asthma who are likely to adhere to their daily ICS treatment, the Expert Panel does not recommend doubling, quadrupling, or quintupling the ICS dose for increased symptoms or decreased peak flow. Clinicians can consider quadrupling the regular daily dose for individuals aged 16 years and older whose adherence to daily therapy is not assured (see the Discussion section below).
Summary of the evidence
The Expert Panel specified three critical outcomes (exacerbations, asthma control, and quality of life) and one important outcome (rescue medication use) for this question. The summary of evidence for Recommendation 11 can be found in EtD Table XVI in Appendix B.
In children aged 4 to 11 years, increasing the ICS dose temporarily in response to worsening symptoms did not significantly reduce the rate of exacerbations or improve asthma quality of life in one study by Martinez et al.140 The overall certainty of evidence ranged from low for exacerbations to moderate for quality of life. A more recent study in 254 children by Jackson et al142 also found no difference in the rate of exacerbations treated with systemic corticosteroids with a quintupling of the ICS dose at early signs of loss of asthma control. In this 48-week study, the growth rate in the intervention group was reduced, although this difference did not reach statistical significance (P =.06). The potential for growth suppression by the intervention and the absence of demonstrated efficacy of the intervention in the articles that the Expert Panel reviewed led to a recommendation against using this intervention in this age group. The Expert Panel rated the recommendation as conditional because of the limited number of studies available in this age group.
In individuals aged 12 years and older (EtD Table XVII), the intervention as implemented did not significantly reduce exacerbations or asthma hospitalizations. The certainty of evidence is low for both outcomes of exacerbations and asthma hospitalizations in the systematic review report. A large, more recent study by McKeever et al143 showed a modest but significant reduction in time to severe exacerbation and in the rate of use of systemic corticosteroids in individuals with asthma whose action plan included a quadrupling of the ICS dose.143 However, unlike the studies in the systematic review report, this study did not include a placebo group or use blinding, and the baseline adherence rate was low. Specifically, only50% of participants in the quadruple-dose group and 42% in the non–quadruple-dose group had good adherence, according to the investigators. Because of the low adherence rate, it was not clear whether the increased ICS dose was effective or whether the initiation of ICS treatment in nonadherent participants influenced the results. Thus, based on the lack of efficacy in the studies in the systematic review report and the possible growth effects, the Expert Panel made a recommendation against a short-term increase in the ICS dose.
In the reviewed studies, the indication for increasing the ICS dose was decreased peak flow and/or increased symptoms. When increased, the ICS dose was doubled, quadrupled, or quintupled.142–146
Rationale and discussion
In children aged 4 to 11 years, the intervention did not significantly reduce exacerbations or improve asthma quality of life in one study140 in the systematic review report. The intervention’s potential to suppress growth in a more recent study142 and the lack of demonstrated efficacy of the intervention in either of the reviewed articles led to the Expert Panel’s recommendation against this intervention in this age group.
In individuals aged 12 years and older, the intervention as implemented also did not significantly reduce exacerbations in three studies144–146 in the evidence summary, but the certainty of evidence is low. The more recent study by McKeever et al143 showed modest but significant reductions in time to severe exacerbation and rate of ICS use in individuals whose action plan included a quadrupling of the ICS dose. However, unlike the studies in the AHRQ systematic review report, this study did not include a placebo group or use blinding, and the baseline adherence rate was low (42%−50%). The adherence rate in the McKeever et al study might be more similar to the adherence rates in routine clinical practice, whereas adherence rates in the RCTs144–146 were probably higher than in most real-world settings.
Thus, the Expert Panel believes that this recommendation applies most specifically to individuals who are likely to adhere to their daily ICS regimen. An increase in the ICS dose might be a reasonable strategy to include in the action plans of individuals whose adherence rates are less certain. How to assess adherence or the threshold for adequate adherence for this recommendation cannot be determined from the reviewed studies. Based on the study of McKeever et al143 in individuals aged 12 years and older described in the previous paragraph, the ICS dose could be quadrupled in the short-term in individuals aged 16 years and older in response to an increased need for reliever therapy, greater interference of asthma with sleep, or a peak flow of less than 80% of the individual’s normal level. The potential discrepancy between the efficacy and effectiveness studies described above and the overall low certainty of evidence led to a conditional recommendation for this age group as well.
Question 4.3
What is the comparative effectiveness of ICS with LABA used as both controller and quick-relief therapy compared to ICS with or without LABA used as controller therapy in individuals aged 5 years and older with persistent asthma?
Recommendation 12: In individuals aged 4 years and older with moderate to severe persistent asthma, the Expert Panel recommends ICS-formoterol in a single inhaler used as both daily controller and reliever therapy compared to either a higher-dose ICS as daily controller therapy and SABA for quick-relief therapy or the same-dose ICS-LABA as daily controller therapy and SABA for quick-relief therapy.
Strong recommendation, high certainty of evidence for ages 12 years and above, moderate certainty of evidence for ages 4 to 11 years
Implementation guidance
Clinician’s Summary:
In individuals aged 4 years and older, the preferred Step 3 (low-dose ICS) and Step 4 (medium-dose ICS) therapy is single-inhaler ICS-formoterol both daily and as needed. In the literature, inhaled ICS-formoterol is referred to as “single maintenance and reliever therapy (SMART).” This form of therapy has only been used with formoterol as the LABA. Formoterol has a rapid onset and a maximum total daily dose that allows it to be used more than twice daily.147 The maximum total daily dose of formoterol should not exceed 8 puffs (36 μg) for ages4 to11 years and12puffs (54 μg) for ages12 years and above. SMART is administered with a single inhaler containing both formoterol and an ICS (primarily budesonide in the reviewed studies, but one study used beclomethasone). The regimens compared to address this key question required two inhalers: the controller (ICS or ICS-LABA) and the reliever (SABA). The recommended alternate therapy of maintenance ICS-LABA with SABA as quick-relief therapy does not need to be changed if it is providing adequate control. However, patients whose asthma is uncontrolled on such therapy should receive the preferred SMART if possible before moving to a higher step of therapy.
The Expert Panel makes the following suggestions for implementation of daily and intermittent combination ICS-formoterol in individuals aged 4 years and older:
No patient characteristics exclude consideration of this option in individuals aged 4 years and older with asthma.
The studies demonstrating reduced exacerbations (see below) enrolled individuals with a severe exacerbation in the prior year. The results suggest that such individuals are particularly good candidates for SMART to reduce exacerbations.
SMART might not be necessary for individuals whose asthma is well controlled on alternate treatments, such as conventional maintenance ICS-LABA with SABA as quick-relief therapy.
SMART is appropriate for Step 3 (low-dose ICS-formoterol) and Step 4 (medium-dose ICS-formoterol) treatment.
ICS-formoterol should be administered as maintenance therapy with one to two puffs once to twice daily (depending on age, asthma severity, and ICS dose in the ICS formoterol preparation) and one to two puffs as needed for asthma symptoms. The maximum number of puffs per day is 12 (54 μg formoterol) for individuals aged 12 years and older and 8 (36 μg formoterol) for children aged 4 to 11 years. Clinicians should advise individuals with asthma or their caregivers to contact their physician if they need to use more than these amounts.
The calculation of the dose of formoterol was based on 4.5 μg/inhalation, the most common preparation used in the RCTs reviewed.
ICS-formoterol should not be used as quick-relief therapy in individuals taking ICS-salmeterol as maintenance therapy.
- What clinicians should discuss with their patients and families:
- Clinicians should inform individuals with asthma and their caregivers that in studies, this intervention consistently reduced asthma exacerbations requiring unscheduled medical visits or systemic corticosteroids. In addition, this intervention improved asthma control and quality of life in some studies.
- No differences have been documented in harms between this type of therapy and the comparators (ICS or ICS-LABA) in individuals aged 12 years and older. The reductions in exposure to oral corticosteroids and to ICS treatment in most studies suggest that the intervention might reduce future corticosteroid associated harms.
- In children aged 4 to 11 years, there may be a lower risk of growth suppression among those taking SMART versus daily higher-dose ICS treatment.
- This recommendation might not be appropriate for some individuals with asthma for such reasons as cost, formulary considerations, or medication intolerance. However, the additional cost of the medication may be offset by the decrease in exacerbations and the associated improvement in quality of life and reduction in costs to both the patient and the payer.
- A 1-month supply of ICS-formoterol medication that is sufficient for maintenance therapy may not last a month if the inhaler is used for reliever therapy as well. Providers, individuals with asthma, pharmacists, and payers need to be aware of this possibility and prescribe, plan, dispense, or provide coverage accordingly.
Summary of the evidence
The Expert Panel specified three critical outcomes (exacerbations, asthma control, and quality of life) and one important outcome (asthma symptoms) for this question. The summary of evidence for Recommendation 12 can be found in EtD Tables XVIII and XIX in Appendix B.
SMART versus higher-dose ICS treatment in ages 4 years and older (EtD Table XVIII).
Three large RCTs148–150 (total N = 4662) enrolled individuals aged 12 years and older who were being treated with a low- to medium-dose or medium- to high-dose ICS. Study participants treated with SMART used daily budesonide-formoterol, 160/9 to 320/9 μg, via a dry-powder inhaler. They took up to 10 rescue puffs of budesonide-formoterol (total daily dose of 12 puffs or 54 μg formoterol). The investigators compared this intervention to daily budesonide, 320 to 640 μg, along with SABA for quick-relief therapy. Rabe et al149 showed a 51% RR reduction in exacerbations, whereas the rates were 35% and 43% RR reduction in Scicchitano et al150 and O’Byrne et al,148 respectively. The latter two studies used a composite exacerbation score that included systemic corticosteroid use, hospitalizations, emergency department visits, increase in ICS or other medication doses, and peak expiratory flow less than 70%.148–150 Collectively, these RCTs found an RR of 0.6 (range, 0.53–0.68) favoring SMART for asthma exacerbations (high certainty of evidence). The investigators of these studies did not report results from validated outcome measures of quality of life or asthma control. However, results for individual asthma control measures—including total asthma symptom scores, nighttime awakenings, symptom-free days, and asthma control days—significantly favored SMART. The overall doses of inhaled and oral corticosteroids were significantly lower with SMART (two- to fourfold less for inhaled ICS treatments).
Jenkins et al151 conducted a post hoc analysis of these three studies in 1239 participants aged 12 years and older with milder asthma (daily maintenance ICS dose equal to 400 μg or less budesonide equivalent). The authors confirmed that SMART reduced exacerbations overall. However, in subgroup analyses, participants with the mildest asthma at enrollment (based on rescue SABA use of <1 inhalation/d) showed a marginal and statistically nonsignificant benefit.
Another post hoc analysis of one of the three RCTs (O’Byrne et al148) included 224 children aged 4 to 11 years who used medium to high ICS doses (any brand, 200–500 μg daily). The 118 participants in the SMART group were instructed to take budesonide-formoterol, 80/4.5 μg once daily, as their baseline therapy, with up to seven additional rescue puffs (total daily dose of 36 μg formoterol). The other 106 participants took budesonide, 320 μg daily, with rescue SABA. In the SMART group, the RR for a composite exacerbation measure comprised of systemic corticosteroids, hospitalization, emergency department visits, and increase in ICS or other medication dose dropped by 57% (moderate certainty of evidence). The authors did not report on validated outcome measures of quality of life or asthma control, but nighttime awakenings declined significantly with SMART. SMART participants used a lower daily ICS dose (average 127 vs 320 μg/d in the fixed-dose budesonide group) and demonstrated significantly improved growth rates (adjusted mean difference of 1 cm compared with fixed-dose budesonide).152
SMART versus same-dose ICS-LABA controller therapy for ages 4 years and above (EtD Table XIX).
For ages 12 years and above, the Expert Panel considered four blinded RCTs148,153–155 and two unblinded RCTs156,157 for this question. Collectively, these RCTs demonstrated a 32% reduction in exacerbations with SMART148,153–157 (high certainty of evidence). Two of the studies used validated asthma control measures (ACQ-5), and both demonstrated clinically significant improvements with SMART (high certainty of evidence).155,157
Three of the blinded studies enrolled a total of 7555 participants with mild to severe persistent asthma. Participants were treated with 160/9 or 320/9 μg budesonide-formoterol daily with up to 10 rescue puffs (total daily dose of 12 puffs or 54 μg formoterol) of budesonide-formoterol (SMART) or rescue SABA.148,153,155 In these three blinded studies, SMART significantly reduced exacerbations.
One of these three studies153 demonstrated a statistically significant improvement in asthma control (based on ACQ-5). A second blinded study (N = 1748) enrolled participants aged 18 years or older with poorly controlled asthma who took a moderate to high dose of an ICS or ICS-LABA. The SMART group took two puffs daily of beclomethasone-formoterol, 100/6 μg, and up to six puffs of rescue beclomethasone-formoterol per day (total daily dose of 48 μg formoterol). The comparison group used rescue SABA. The investigators actively managed both arms with dose titration. Although severe exacerbations and systemic corticosteroid use were significantly lower with SMART, asthma control scores (ACQ-7) did not differ significantly between groups.154
An unblinded study, Vogelmeier et al,157 enrolled 2143 participants from Europe and Asia with poorly controlled asthma taking moderate to high ICS or ICS-LABA doses (500 μg or more of budesonide, fluticasone, or equivalent). They received either daily budesonide-formoterol, 640/18 μg, with budesonide-formoterol rescue (SMART group) or daily fluticasone/salmeterol, 500/100 μg, with SABA for quick-relief therapy. The investigators actively managed both arms with dose titration, and the study was unblinded. With SMART, the RR declined by 20% for exacerbations, defined as emergency department visits, oral corticosteroid days, and hospitalization. SMART also improved asthma control (measured by ACQ-5) and quality of life (measured by Asthma-Related Quality of Life Questionnaire), but these changes were not statistically significant. A reanalysis of these data in 404 participants in China, Korea, Taiwan, and Thailand had similar results; the RR reduction in exacerbation rates was 38%.158
Another blinded study, Patel et al,156 enrolled 303 participants in New Zealand who were at risk of severe exacerbations. Participants were treated with budesonide-formoterol, 800/24 μg (by metered-dose inhaler), with one rescue puff of budesonide-formoterol (SMART) or SABA for quick-relief therapy. SMART reduced exacerbations and oral corticosteroid use but increased the use of ICS, and the associated improvement in asthma control (measured by ACQ-7) was not significant.156
For ages 4 to 11 years, one blinded RCT152 used budesonide-formoterol, 80/4.5 μg, with up to seven rescue puffs of budesonide-formoterol, 80/4.5 μg (36 μg total daily dose of formoterol; SMART), or SABA as quick-relief therapy. SMART reduced the RR for exacerbations by 72% (moderate certainty of evidence) and showed superiority in one unvalidated outcome measure of asthma control (nighttime awakenings). Growth rates and other safety measures did not differ between treatment groups.
Rationale and discussion
Because the only SMART studied has included formoterol, the Expert Panel’s recommendation favors the use of ICS-LABA combinations containing formoterol rather than those that contain ICS-salmeterol. Daily ICS-salmeterol remains an appropriate therapeutic option for individuals with moderate to severe persistent asthma, but the reviewed data suggest that the use of ICS-formoterol for maintenance and reliever therapy has superior efficacy, ease of use (because it is administered in a single inhaler rather than two separate inhalers), and perhaps safety as a result of reduced corticosteroid exposure. Other LABAs, including newer agents with a rapid onset, may be effective and safe to use for both maintenance and reliever therapy, but their efficacy and safety will need to be demonstrated in clinical studies. The number of studies available and the consistency of the evidence led the Expert Panel to make a strong recommendation to use ICS-formoterol in a single inhaler as both daily controller and reliever therapy.
Data were insufficient to compare ICS-formoterol as SMART with same-dose ICS for daily controller therapy along with SABA for quick-relief therapy in individuals aged 4 years and older. However, multiple studies have demonstrated that adding any LABA to the same ICS dose is more effective than ICS therapy alone.12 Thus, the lack of comparisons data on ICS-formoterol as SMART versus same-dose ICS and SABA for quick-relief therapy is of minimal clinical importance.
Recommendation 13: In individuals aged 12 years and older with moderate to severe persistent asthma, the Expert Panel conditionally recommends ICS-formoterol in a single inhaler used as both daily controller and reliever therapy compared to higher-dose ICS-LABA as daily controller therapy and SABA for quick-relief therapy.
Conditional recommendation, high certainty of evidence
Implementation guidance
Clinician’s Summary:
In individuals aged 12 years and older, the preferred Step 4 therapy is single-inhaler ICS-formoterol used both daily and as needed. The maximum total daily dose of formoterol should not exceed 12 puffs (54 μg) for those aged 12 years and older. The recommended alternate therapy of maintenance ICS-LABA along with SABA as quick-relief therapy does not need to be changed if it is providing adequate control. However, individuals whose asthma is uncontrolled on such therapy should receive the preferred SMART if possible before stepping up their treatment to a higher step of therapy.
In individuals aged 12 years and older with moderate to severe persistent asthma, combination ICS-formoterol used daily and intermittently is more beneficial than an increase in the daily ICS dose if they are already taking combination ICS-LABA (and as needed SABA). The Expert Panel makes the following suggestions for implementation of daily and intermittent combination ICS-formoterol for individuals aged 12 years and older:
This recommendation applies to all individuals with asthma aged 12 years and older.
Individuals with asthma should use ICS-formoterol as maintenance therapy with one to two puffs once or twice daily (depending on asthma severity and ICS dose in the ICS-formoterol preparation). The additional rescue dose is 1 to 2 puffs as needed for asthma symptoms, up to a maximum of 12 puffs (54 μg formoterol) per day. Clinicians should advise individuals with asthma to contact their clinician if they need to use more than these amounts.
The calculation of the dose of formoterol was based on 4.5 μg/inhalation, the most common preparation used in the RCTs reviewed.
Clinicians managing asthma should regularly assess individuals using this therapy.
This therapy is appropriate for Step 4.
Individuals with asthma should not use ICS-formoterol as reliever therapy if they are taking ICS-salmeterol as maintenance therapy.
SMART might not be necessary for individuals whose asthma is well controlled with alternate treatments, such as conventional maintenance ICS-LABA with SABA as quick-relief therapy.
For individuals aged 5 to 11 years, the evidence was insufficient to make a recommendation regarding SMART compared to higher-dose ICS-LABA. SMART with low- or medium-dose ICS therapy is preferred for children aged 5 to 11 years as opposed to same-, low-, or medium-dose ICS-LABA plus as-needed SABA as part of Step 3 and Step 4 therapy (Recommendation 12).
- What clinicians should discuss with their patients and families:
- Clinicians should inform individuals with asthma and their caregivers that the major demonstrated benefits of combination ICS-formoterol used daily and as needed are reductions in asthma exacerbations requiring unscheduled medical visits and in use of systemic corticosteroids.
- Clinicians should also inform individuals with asthma that studies found no difference in documented harms between this type of therapy and daily ICS-LABA.
- Studies showed that combination ICS-formoterol reduces exposure to corticosteroids, suggesting that the intervention might reduce future corticosteroid associated harms.
- This recommendation might not be appropriate for some individuals for such reasons as cost, formulary considerations, or medication intolerance.
Summary of the evidence
The Expert Panel specified three critical outcomes (exacerbations, asthma control, and quality of life) for this question. The summary of evidence for Recommendation 13 can be found in EtD Table XIX in Appendix B.
Two blinded RCTs (N = 5481) compared SMART to higher-dose ICS-LABA159,160 in individuals with asthma aged 12 years and older. SMART reduced the RR by 25% for exacerbations (high certainty of evidence). SMART also resulted in statistically significant reductions in corticosteroid use but had no significant effect on asthma quality of life or asthma control. As a result, the recommendation was conditional.159,160
Rationale and discussion
Bousquet et al159 compared daily budesonide-formoterol (640/ 18 μg) plus budesonide-formoterol reliever therapy (SMART) in participants aged 12 years and older with daily fluticasone-salmeterol (1000/100 μg) plus SABA for quick-relief therapy, while Kuna et al160 compared daily budesonide-formoterol (320/ 9 μg) plus budesonide-formoterol reliever therapy (SMART) with either daily budesonide-formoterol (640/18 μg) or daily fluticasone-salmeterol (500/100 μg) plus SABA for quick-relief therapy. These two studies showed significant reductions in exacerbations in the SMART groups in comparison with maintenance ICS-LABA along with SABA for quick-relief therapy. However, the studies found no differences between groups in asthma control or quality of life, and the lack of differences in these outcomes led to the Expert Panel’s conditional recommendation. Data were insufficient to make a recommendation regarding whether SMART is superior to daily higher-dose ICS-LABA with SABA for quick-relief therapy in children aged 4 to 11 years.
The systematic review report for this topic also included five open-label, real-world clinical trials that compared daily budesonide-formoterol (160–320/4.5–9 μg) plus budesonide-formoterol reliever therapy (SMART) with conventional best practice treatment (total N = 5056).6,161–164 Active management levels varied in these studies. Because of the heterogeneity of the studies and lack of information regarding the type of therapy prescribed and used in the conventional best practice arms, the formal systematic review report did not include these studies. However, the Expert Panel decided to review these studies to compare the potential benefits of SMART with those of diverse approaches in real-world settings. In general, the real-world studies confirmed the results from the RCTs that used SMART.
Future research opportunities
The Expert Panel identified the following topics that would benefit from additional research:
Differences by race and ethnicity in benefits and risks of the ICS recommendations
Cost-effectiveness of the ICS recommendations
Effects on growth of short ICS courses starting at the onset of an apparent respiratory tract infection in children aged 0 to 4 years who have recurrent wheezing triggered only by such infections
Optimal short-course ICS regimen to use—on the basis of efficacy, effectiveness, and safety—at the onset of an apparent respiratory tract infection in children aged 0 to 4 years whose recurrent wheezing is triggered by respiratory tract infections
Efficacy, effectiveness, and safety of a short ICS course starting at the onset of an apparent respiratory tract infection compared with daily ICS treatment in children aged 0 to 4 years with recurrent wheezing triggered by respiratory tract infections
Daily low-dose ICS treatment with SABA for quick relief versus as-needed ICS plus SABA administered concomitantly in children aged 4 to 11 years with mild persistent asthma
Optimal dose of albuterol and ICS used for as-needed concomitant therapy in individuals with mild persistent asthma
Effectiveness and safety of other rapid-onset LABAs in combination medications used for both daily controller and quick-relief therapy
Combination ICS-formoterol as both daily controller and reliever therapy compared with higher-dose ICS-LABA as daily controller therapy and SABA for quick-relief therapy in children aged 4 to 11 years
Other recommended types of research included the following:
Confirmation of the efficacy data supporting the ICS recommendations using additional real-world effectiveness studies in clearly defined populations using clearly defined treatment regimens
Additional studies powered as equivalence studies to confirm the finding that daily low-dose ICS therapy with SABA for quick relief and concomitant as-needed ICS therapy plus SABA lead to similar outcomes in individuals with mild persistent asthma
Real-world studies that monitor growth in children and adherence to evaluate the effectiveness and safety of quadrupling the ICS dose in individuals with mild to moderate persistent asthma taking daily ICS controller therapy who experience early signs of loss of asthma control
SECTION V: RECOMMENDATIONS FOR THE USE OF LAMAs FOR ASTHMA
Background
LAMAs comprise a pharmacologic class of long-acting bronchodilators. The role of LAMAs in the management of asthma was not addressed in Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma.12 Since that report’s publication in 2007, several trials have investigated LAMAs as controller therapy for individuals with asthma.
The Expert Panel examined the harms and benefits of LAMAs in individuals aged 12 years and older with uncontrolled persistent asthma and addressed three key questions.165 The Expert Panel did not examine the role of LAMA treatment in children aged 6 to 11 years because the key questions and systematic reviews did not address this age group. With the exception of one study that examined the LAMA umeclidinium,166 the RCTs reviewed by the Expert Panel used tiotropium bromide as the LAMA. At the time this report was written, tiotropium bromide (Respimat) was the only formulation of LAMA with US FDA approval for asthma treatment. The majority of LAMA studies used a comparative efficacy design, and not an effectiveness design, but the key questions were about effectiveness. Therefore, the clinical impact of LAMA treatment in real-world settings is not well understood. Table V provides an overview of the key questions and recommendations on LAMAs.
TABLE V.
LAMA key questions and recommendations
| Question | Intervention | Comparator | Recommendation | Certainty of evidence |
|---|---|---|---|---|
| 5.1 | LAMA as an add-on to ICS controller therapy* | LABA as an add-on to same-dose ICS controller therapy* | 14: Conditional, against intervention | Moderate |
| Montelukast as an add-on to same-dose ICS controller therapy* | No recommendation† | |||
| 5.2 | LAMA as an add-on to ICS controller therapy* | Same-dose ICS controller therapy* + placebo | 15: Conditional, in favor of the intervention | Moderate |
| Increased ICS dose | No recommendation† | |||
| 5.3 | LAMA as an add-on to ICS-LABA | Same-dose ICS-LABA as controller therapy* | 16: Conditional, in favor of the intervention | Moderate |
| Doubled ICS dose + LABA | No recommendation† |
ICS controller therapy used daily.
Insufficient evidence.
Definitions of terms used in this section
In this section, “controller therapy” refers to medications that are taken daily on a long-term basis to achieve and maintain control of persistent asthma.12 The term “ICS-LABA” indicates therapy with both an ICS and a LABA, usually (and preferably) in a single inhaler.
Question 5.1
What is the comparative effectiveness of LAMA compared with other controller therapy as add-on therapy to ICSs in individuals aged 12 years and older with uncontrolled persistent asthma?
Question 5.2
What is the comparative effectiveness of LAMA as add-on therapy to ICS controller therapy compared with placebo or increased ICS dose in individuals aged 12 years and older with uncontrolled persistent asthma?
Recommendation 14: In individuals aged 12 years and older with uncontrolled persistent asthma, the Expert Panel conditionally recommends against adding LAMA to ICS compared to adding LABA to ICS.
Conditional recommendation, moderate certainty of evidence
Recommendation 15: If LABA is not used, in individuals aged 12 years and older with uncontrolled persistent asthma, the Expert Panel conditionally recommends adding LAMA to ICS controller therapy compared to continuing the same dose of ICS alone.
Conditional recommendation, moderate certainty of evidence
Implementation guidance
Clinician’s Summary:
In individuals with asthma that is not controlled by ICS therapy alone, the Expert Panel recommends adding a LABA rather than a LAMA to an ICS. However, if the individual is not using or cannot use LABA therapy, adding a LAMA to an ICS is an acceptable alternative. Adding a LAMA to ICS controller therapy is more effective than using ICS controller therapy alone in individuals aged 12 years and older with uncontrolled persistent asthma. However, adding a LAMA to ICS controller therapy is not more efficacious than adding a LABA to ICS controller therapy, and adding a LAMA may increase the risk of harm, based on a single real-world study in Blacks.167 Therefore, the panel recommends preferentially adding LABA over LAMA to ICS. A LABA should not be used when the individual cannot tolerate it, the medication is contraindicated, the device that delivers the LABA is unsuitable for the individual, or the LABA is unavailable for insurance or supply reasons.
The Expert Panel makes the following suggestions on the use of LAMA therapy:
A LAMA can be used as an add-on to ICS therapy in individuals aged 12 years and older with uncontrolled asthma therapy as part of Step 4 therapy, but add-on LABA therapy has a more favorable benefit-harm profile.
Individuals at risk of urinary retention and those who have glaucoma should not receive LAMA therapy.
The small increase in the potential risk of harms from a LAMA may outweigh its benefits in some individuals, particularly in Blacks.
LAMA treatment requires appropriate use of specific inhaler devices. Clinicians should teach individuals with asthma how to use these devices appropriately.
When clinicians prescribe LAMA therapy, they should prescribe this medication for long-term asthma control in the ambulatory setting. LAMA therapy does not have a role in the management of acute exacerbations of asthma in the ambulatory, emergency department, or inpatient settings.
Clinicians should confirm the asthma diagnosis and address factors that often contribute to uncontrolled asthma before they consider intensifying therapy by adding a LAMA. For example, clinicians should identify and suggest ways to mitigate occupational and environmental triggers and ensure that individuals with asthma are using currently prescribed asthma controller therapy appropriately.
- What clinicians should discuss with their patients about LAMA therapy
- When discussing the addition of a LAMA versus a LABA for individuals already taking an ICS, clinicians should explain that the LABA is likely to be preferable.
- Adding a LAMA to ICS controller therapy provides no more benefit than adding a LABA to ICS controller therapy, and may increase the risk of harm, based on a single real-world study in Blacks.
- Clinicians should tell individuals with asthma that adding a LAMA to an ICS provides a small benefit compared to continuing the same ICS dose if the individual cannot use a LABA for any reason.
- Individuals with asthma and glaucoma and those at risk of urinary retention should not use LAMA therapy.
Summary of the evidence
The Expert Panel prespecified three critical outcomes (exacerbations, asthma control, and quality of life) and three important outcomes (rescue medication use, adverse events [harms], and mortality). The Expert Panel did not consider lung function (eg, based on spirometry testing) to be a critical or important outcome for the LAMA studies that it reviewed.
The summary of evidence for Recommendation 14 can be found in EtD Table XX in Appendix B. The Expert Panel examined the efficacy of adding a LAMA to ICS therapy in comparison with adding a LABA to ICS therapy in seven RCTs.166–172 Five RCTs166,168–170 that had a total of 2574 participants found no difference in the exacerbation rate in individuals treated with a LAMA compared with those treated with a LABA (RR = 0.87; 95% CI, 0.53–1.42) as an add-on to an ICS. The exacerbation rate was 4.9% (75 of 1533) in the LAMA group and 5.4% (56 of 1041) in the LABA group (absolute risk difference of 7 fewer per 1000; 95% CI, from 25 fewer to 23 more). The certainty of evidence is moderate for the effect on exacerbations.
Two RCTs170 in 1577 patients detected no differences in asthma control between those treated with a LAMA and those treated with a LABA. The certainty of evidence is high for the lack of improvement in asthma control.
Four RCTs168–170 in 1982 patients found no differences in asthma-related quality of life between those treated with a LAMA and those treated with a LABA. The certainty of evidence is high for the lack of effect on asthma-related quality of life.
Six RCTs166,167,169–172 in 2450 patients found no between-group differences in use of rescue medications. The certainty of evidence is low for the effect on rescue medication use.
Finally, four RCTs166,167,170 showed no between-group differences in all-cause mortality rates (OR = 7.50; 95% CI, 0.78–72.27). The mortality rates were 0.2% (3 of 1835) in the LAMA group and 0% (0 of 1135) in the LABA group. The certainty of evidence is low for the effect on mortality.
With respect to harms, data from double-blinded, placebo-controlled RCTs suggest a similar rate of undesirable side effects in individuals treated with ICS-LABA and those treated with an ICS plus a LAMA.166,168–170 However, a real-world comparative effectiveness study167 that compared the two treatments, the Blacks and Exacerbations on LABA vs Tiotropium (BELT) study, found a 2.6-fold higher rate of asthma-related hospitalizations in the ICS plus LAMA group than in the ICS-LABA group. In addition, the number of hospitalizations in the ICS plus LAMA group in the BELT study (3.6 per 100 hospitalizations/person/y) was higher than in the ICS-LABA groups in the FDA-required safety studies (0.66 per 100 hospitalizations/person/y).173 While few asthma-related deaths occurred in the BELT study (2 of 1070 participants), both deaths occurred in the ICS plus LAMA group (2 of 532 [0.38%]). The proportion of asthma-related deaths in the ICS plus LAMA group in the BELT study was 38 times higher than the proportion in an ICS-LABA group in the FDA-required safety studies.173 Because of its real-world effectiveness design, the BELT study might better reflect the harms and benefits likely to occur in clinical practice than efficacy studies of the combination of LAMA and ICS therapy. The BELT study included only Blacks, and no similar data are available from real-world trials that assessed harms in other populations. Therefore, the Expert Panel was unable to determine whether these harms are a concern only in Blacks or whether they might occur in other populations.
The summary of evidence for Recommendation 15 can be found in Appendix B (EtD Table XXI). The Expert Panel compared the harms and benefits of adding a LAMA to ICS therapy with adding a placebo to continued ICS therapy in five RCTs (total N = 3036).166,169,170,174,175 These trials showed that adding a LAMA to ICS therapy resulted in a slightly smaller rate of exacerbations, 4.2%, than the addition of a placebo to continued ICS therapy, 7.4% (absolute risk difference = 24 fewer per 1000; 95% CI, from 38 fewer to 6 fewer; RR = 0.67; 95% CI, 0.48–0.92). According to these results, 42 patients (95% CI, 26–167) would need treatment to prevent one exacerbation. This effect on exacerbations has moderate certainty of evidence. However, adding a LAMA to ICS therapy did not improve asthma control (measured by the ACQ [ACQ-7, moderate certainty of evidence]). 166,170,174–176 The proportion of responders (those with a ≥0.5-point decrease in score) was 67% in the group treated with ICS plus LAMA and was 61% in the group treated with placebo added to continued ICS therapy (RR = 1.08; 95% CI, 0.96–1.21). In addition, adding a LAMA to an ICS did not improve asthma related quality of life (measured by the Asthma-Related Quality of Life Questionnaire, high certainty of evidence)169,170 and had no effect on rescue medication use (high certainty of evidence).166,170,174–176
Harms data are available from six studies that compared the efficacy of adding a LAMA to ICS therapy with adding a placebo to ICS therapy.166,170,174–176 In these studies, the rate of serious adverse events for the addition of a LAMA to ICS therapy was low and was similar to that for the addition of a placebo to ICS therapy. No deaths were reported for any of these studies (see EtD Table XXI). All studies excluded participants with a history of glaucoma or urinary retention. Therefore, whether adding LAMA to ICS therapy is safe in individuals with these conditions is not known.
Rationale and discussion
Outcomes from seven RCTs166–172 showed no significant differences between groups. This evidence therefore provides no basis, based on benefits, for recommending the addition of a LAMA to ICS therapy as opposed to the addition of a LABA to ICS therapy in adults with uncontrolled persistent asthma.
The Expert Panel considered the serious adverse events in African-American adults assigned to the ICS plus LAMA group in the BELT study.167 The number of asthma-related deaths in this group was higher than expected in African-American adults, and the adjusted rate of asthma-related hospitalizations was statistically higher in the ICS plus LAMA group than in the ICS-LABA group. Although it is difficult for the Expert Panel to draw firm conclusions, in the opinion of the Expert Panel, the balance of the evidence argues against adding a LAMA to an ICS compared with adding a LABA to an ICS because the benefits of added LAMA are trivial, and there is a small concern about the safety of LAMA combined with ICS alone.
In the studies that compared the addition of a LAMA to an ICS with ICS therapy alone, adding a LAMA to an ICS slightly reduced the number of exacerbations166,169,170,174,175 but did not improve asthma control166,170,174–176 or asthma-related quality of life.169,170 The Expert Panel’s judgment about the degree of benefit was subjective because no established standards are available for the MID in exacerbations. In addition, individuals with asthma who place a higher value on asthma control and quality of life than on exacerbations may not perceive any benefit from this intervention.
After considerable discussion about the harms found in the BELT study,167 the Expert Panel concluded that the BELT study did not address the harms of adding a LAMA to an ICS compared with adding placebo to ICS therapy.167 However, because the BELT study showed a higher adverse event rate in participants assigned to ICS plus LAMA than in those treated with ICS-LABA, the Expert Panel recommends first considering the addition of a LABA to an ICS and considering the addition of a LAMA to an ICS as an alternate approach. This prioritization of therapies may be particularly important in Black adults. The balance of evidence demonstrates that the addition of a LAMA to an ICS offers a small benefit compared with ICS therapy alone, but there is a small concern related to harm.
In addition to the studies described above, the systematic review report compared the efficacy of the addition of a LAMA to ICS controller therapy in individuals aged 12 years and older and adults with uncontrolled, persistent asthma with the efficacy of the addition of montelukast to ICS therapy (EtD Table XXII) and with a doubled ICS dose (EtD Table XXIII).6 A single small RCT171,172 produced findings in participants aged 18 to 60 years after 6 months of treatment in a four-arm, parallel-group, unmasked, active-comparator trial (N = 72 for ICS plus LAMA, N = 68 for ICS plus LABA [formoterol], N = 81 for ICS plus montelukast, and N = 76 for ICS plus doxofylline). A total of 297 of the original 362 participants completed the 6-month study. The study report provided no data on critical outcomes designated by the Expert Panel. The authors reported on only one of the important outcomes (rescue medication use, reported as the difference at day 90 compared with at baseline), and results for this outcome did not differ between groups. In addition, the rate of undesirable effects was similar with both treatments.
After reviewing the available evidence and finding the effect on one noncritical outcome to be inconclusive, the Expert Panel concluded that the data were insufficient to address this question. Therefore, the Expert Panel refrained from making any recommendation regarding the addition of a LAMA to an ICS versus adding montelukast to ICS.
Only one study compared the addition of a LAMA to an ICS with doubling the dose of the ICS. This study found no differences in rates of exacerbations, asthma control, or serious adverse events as well as no differences in asthma-related quality of life between the two groups; no deaths occurred in either group.168 Although this study showed an improvement in the proportion of control days and in symptom scores of participants assigned to added LAMA treatment, this outcome measure was not validated, and the Expert Panel could not determine the significance of these differences. Therefore, the Expert Panel concluded that the data were insufficient to make a recommendation regarding the addition of a LAMA to an ICS versus doubling the ICS dose.
The Expert Panel also did not make any recommendation regarding the addition of a LAMA to an ICS versus the addition of doxofylline to an ICS because doxofylline is not available in the United States.
Question 5.3
What is the comparative effectiveness of LAMA as add-on therapy to ICS plus LABA compared with ICS plus LABA as controller therapy in individuals aged 12 years and older with uncontrolled persistent asthma?
Recommendation 16: In individuals aged 12 years and older with uncontrolled persistent asthma, the Expert Panel conditionally recommends adding LAMA to ICS-LABA compared to continuing the same dose of ICS-LABA.
Conditional recommendation, moderate certainty of evidence
Implementation guidance
Clinician’s Summary:
For individuals whose asthma is not controlled with ICS-LABA, the Expert Panel recommends the addition of a LAMA for many individuals.
Based on the studies available, the addition of a LAMA to ICS-LABA in individuals aged 12 years and older with uncontrolled persistent asthma offers a small benefit.
This therapy is recommended for individuals aged 12 years and older whose asthma is uncontrolled even though they are using ICS-LABA therapy.
LAMA therapy should not be used in individuals with glaucoma or urinary retention.
Adding a LAMA to ICS-LABA for individuals with uncontrolled asthma who are already taking ICS-LABA improves asthma control and quality of life but has no effect on asthma exacerbations that require systemic corticosteroids or rescue medication.
- What clinicians should discuss with their patients about adding LAMA therapy to ICS-LABA:
- Adding LAMA therapy to ICS-LABA requires the use of an additional and different type of inhaler.
- The addition of a LAMA may improve asthma control and quality of life but may not decrease the frequency of asthma exacerbations, use of oral corticosteroids, or use of rescue medications.
- Individuals with glaucoma and those at risk of urinary retention should not use LAMA therapy.
Summary of the evidence
The Expert Panel specified three critical outcomes (exacerbations, asthma control, and quality of life) and two important outcomes (rescue medication use and mortality). The summary of evidence for Recommendation 16 can be found in EtD Table XXIV in Appendix B.
Two trials (total N = 912) found that the proportion of adults who achieved the MID of 0.5 points on the ACQ-7 for asthma control was higher when tiotropium was added to ICS-LABA than when placebo was added (RR = 1.28; 95% CI, 1.13–1.46); these studies provided moderate certainty of evidence.177 The single study (N = 388) in youth aged 12 to 17 years found no difference in the proportion whose ACQ-7 scores improved (RR = 1.01; 95% CI, 0.89–1.14).178 These three studies (total N = 1301)177,178 found similar decreases in mean ACQ-7 scores in youths and adults treated with tiotropium and ICS-LABA and in those treated with placebo added to ICS-LABA (mean difference = 0.07 points lower; 95% CI, from 0.31 lower to 0.17 higher); the certainty of evidence is moderate.
Similarly, a higher proportion of adults showed an MID of at least 0.5 points for improved asthma quality of life, as measured by the Asthma-Related Quality of Life Questionnaire, with the addition of a LAMA to ICS-LABA than with the addition of a placebo to continued ICS-LABA (RR = 1.62; 95% CI, 1.34–1.96); the certainty of evidence is high.177 However, the study did not show a between-group difference in the mean Asthma-Related Quality of Life Questionnaire score (high certainty of evidence). In addition, three trials (total N = 1299)177,178 showed no difference in asthma exacerbations requiring treatment with systemic corticosteroids (RR = 0.84; 95% CI, 0.57–1.22; moderate certainty of evidence) or in two trials (N = 907),177 in exacerbations requiring hospitalization (RR = 0.80; 95% CI, 0.42–1.52; moderate certainty of evidence). The findings showed no between-group difference in the mean number of puffs of rescue medication in 24 hours (95% CI, 0.37/d less to 0.18/d more; moderate certainty of evidence) or mortality rates (no deaths in either group; very low certainty of evidence).
Rationale and discussion
In the studies described above, the desirable effects on asthma control and quality of life of the addition of a LAMA to ICS-LABA compared with the addition of placebo were small, and the risks of asthma exacerbations and of adverse events did not differ between the added LAMA and placebo groups. The Expert Panel believes that the balance of outcomes probably favors adding a LAMA to ICS-LABA instead of continuing the same dose of ICS-LABA alone (moderate certainty of evidence). In addition, the Expert Panel does not believe that the extent to which individuals with asthma value the critical outcomes varies or is uncertain. Thus, the addition of a LAMA to ICS-LABA is probably acceptable. However, individuals with asthma and other stakeholders who place less value on asthma control and quality life than on exacerbations may not find the addition of a LAMA acceptable. Using a LAMA as an add-on therapy is feasible but requires teaching individuals with asthma how to appropriately use devices that deliver the LAMA. The Expert Panel concludes that the use of a LAMA as add-on therapy to ICS-LABA would probably improve health equity because asthma disproportionately affects disadvantaged populations.
The Expert Panel also compared the use of a LAMA as add-on therapy to ICS-LABA with doubling the dose of ICS and continuing the same dose of LABA in individuals aged 12 years and older with uncontrolled persistent asthma (EtD Table XXV). A single, small, open-label RCT randomized 94 individuals who continued to take LABA on a 1:1:1 basis to add-on, once-daily tiotropium bromide 18 μg, montelukast 10 mg, or double-dose ICS.179 The data were insufficient to support a judgment about the balance of desirable and undesirable effects. The Expert Panel therefore did not find sufficient data to formulate recommendations about the use of a LAMA as add-on therapy to ICS compared with increasing the dose of ICS and continuing the LABA.
Future research opportunities
The Expert Panel offers the following suggestions for future research:
Comparative effectiveness studies of LAMA therapy for asthma. Because the majority of LAMA studies were efficacy studies, the clinical impact of LAMA treatment in real-world settings is not well understood.
Comparative effectiveness and safety of ICS plus LAMA versus ICS-LABA in ethnically diverse population in studies that are adequately powered to examine the harms and benefits of these two treatment options.
Systematic reviews in children with asthma aged 6 to 11 years to inform future guidelines.
Comparisons of a LAMA to a leukotriene inhibitor as add-on therapy to ICS-LABA in individuals with uncontrolled persistent asthma.
Role of LAMAs other than tiotropium as add-on therapy to ICS therapy in individuals aged 12 years and older with uncontrolled persistent asthma.
SECTION VI: THE ROLE OF SUBCUTANEOUS AND SUBLINGUAL IMMUNOTHERAPY IN THE TREATMENT OF ALLERGIC ASTHMA
Background
This section addresses immunotherapy in individuals with allergic asthma. Immunotherapy is the administration of an aeroallergen either subcutaneously (subcutaneous immunotherapy [SCIT]) or sublingually (sublingual immunotherapy [SLIT] in the form of aqueous drops or tablets). The Expert Panel explored the efficacy and safety of the use of both SCIT and SLIT for the treatment of allergic asthma and made two recommendations.
Definition of terms used in this section
“Allergic asthma” refers to asthma that becomes symptomatic after acute exposure to something to which the individual is allergic (eg, a pet) or during a specific season (eg, in the spring, when trees shed pollen, or in the fall, when ragweed pollen disperses through the air). In contrast, the term “allergic asthma” is used in many clinical trials to describe a population of children and adults with asthma who show evidence of allergic sensitization based on immediate hypersensitivity skin testing or in vitro serum IgE testing, regardless of whether they have documented symptoms after relevant exposures. However, more recent trials of immunotherapy have more clearly documented the presence of sensitization and relevant symptoms on exposure to allergens.
“Immunotherapy” (both subcutaneous and sublingual) in this report refers to treatments used to reduce the IgE-mediated allergic clinical response that is associated with asthma. Immunotherapy consists of the therapeutic administration of exogenous aeroallergens to which a person has demonstrable sensitization with the goal of attenuating that individual’s asthmatic response on subsequent exposure to these aeroallergens. Immunotherapy can be administered in two ways: subcutaneously by injection (in individuals aged 5 years or older) or sublingually in either liquid or tablet form. The US FDA has not approved the use of liquid SLIT or tablet forms of immunotherapy for the specific treatment of asthma, but tablet forms do have FDA approval for treatment of allergic rhinitis and conjunctivitis in individuals aged 5 years and older who have sensitization to northern grass and those aged 18 years and older with sensitization to a short ragweed and dust mite mixture.
Before receiving immunotherapy, individuals with asthma must demonstrate allergic sensitization using one of two methods:
Immediate hypersensitivity skin testing followed by an assessment 15 to 20 minutes later for a wheal and flare reaction to the allergens tested
Laboratory testing to measure the level of (aeroallergen) antigen-specific IgE antibody in a blood sample
Question 6.1
What is the efficacy and safety of SCIT?
Recommendation 17: In individuals aged 5 years and older with mild to moderate allergic asthma, the Expert Panel conditionally recommends the use of SCIT as an adjunct treatment to standard pharmacotherapy in those individuals whose asthma is controlled at the initiation, build-up, and maintenance phases of immunotherapy.
Conditional recommendation, moderate certainty of evidence
Implementation guidance
Clinician’s Summary:
The Expert Panel conditionally recommends SCIT as an adjunctive treatment for individuals who have demonstrated allergic sensitization and evidence of worsening asthma symptoms after exposure to the relevant antigen or antigens either acutely (eg, allergy to pets) or on a seasonal basis (eg, allergy to grass or ragweed) or a chronic basis (eg, allergy to dust mites). Individuals who place a high value on possible small improvements in quality of life, symptom control, and a reduction in long-term and/or quick-relief medication use and a lower value on the risk of systemic reactions of wide-ranging severity might consider SCIT as adjunct therapy.
For individuals with allergic asthma, the Expert Panel makes the following suggestions to implement SCIT:
Clinicians can consider SCIT for adults and children (at a developmental stage at which allergic sensitization can be demonstrated) with allergic asthma, a history compatible with a temporal association of worsening symptoms with exposure to aeroallergens, and testing (as described previously) that confirms this sensitization.
Clinicians can consider SCIT for individuals whose asthma is not well controlled by their current medical therapy and the treating clinician considers allergen exposure to be a significant contributor to this lack of asthma control. However, clinicians should attempt to optimize asthma control before initiating SCIT to reduce the potential for harm.
Clinicians can consider SCIT for individuals whose asthma is well controlled by their current therapy when these individuals and/or their clinicians want to reduce the individuals’ medication burden.
In addition to assessing whether an individual with allergic asthma has an appropriate history before considering SCIT, clinicians must formally assess allergic sensitization using either immediate hypersensitivity skin testing or in vitro antigen-specific IgE antibody testing. This evaluation needs to be performed by a trained health care professional skilled in proper testing and result interpretation. The need for these types of specialty evaluations, as with the need for many diagnostic tests and therapeutic interventions, may limit access to care, depending on local availability of these tests and the patient’s health insurance coverage of testing.
Clinicians should not administer SCIT in individuals with severe asthma. Furthermore, clinicians should not initiate, increase, or administer maintenance SCIT doses while individuals have asthma symptoms. These individuals should achieve optimal asthma control before beginning SCIT to minimize the harms (systemic reactions) associated with SCIT, which tend to intensify as baseline asthma severity increases.
The presence of allergic sensitization is necessary but not sufficient to define the allergic asthma phenotype. A positive test result may not be associated with asthma control over time but might, instead, reflect sensitivity in a different organ (eg, the nose in allergic rhinitis).
Allergen exposure could be the only triggering mechanism for allergic asthma symptoms, or it could be just one triggering factor for an individual, and another factor or factors (eg, respiratory tract infections, irritant exposure, or exercise) might also play a role in triggering allergic asthma symptoms. Because of the heterogeneous nature of allergic asthma, determining the precise efficacy of immunotherapy in reducing the allergic component of an individual’s asthma can be difficult.
Clinicians should administer SCIT in their offices and provide direct supervision because of the risk of systemic reactions. Such reactions can include a range of anaphylactic symptoms involving the skin (urticaria), respiratory tract (rhinitis and asthma), gastrointestinal tract (nausea, diarrhea, and vomiting), and the cardiovascular system (hypotension and arrhythmias). Although rare, deaths after injections have been reported.
Individuals with asthma should not administer SCIT at home.
Because clinicians should administer SCIT with direct supervision, personnel with appropriate training should prepare and administer injections for each individual’s dosing schedule, from the build-up to the maintenance phase. Equipment and personnel should be available to treat serious anaphylactic reactions.
One of the potential benefits of SCIT is its immunomodulatory effects, which can reduce the allergic inflammatory response in various tissues.180,181 Thus, SCIT has the potential to be disease-modifying and to reduce the clinical expression or severity of asthma over time.181,182
Before administering each SCIT injection, clinicians should assess individuals with asthma for worsened asthma symptoms that suggest recent loss of asthma control. Physicians should consider withholding SCIT injections temporarily in patients whose asthma symptoms have worsened until their asthma control is restored.
- What clinicians should discuss with their patients:
- Clinicians should inform individuals with asthma who are considering SCIT that this treatment has the potential to reduce asthma symptoms and the severity of disease over time.
- Individuals need to come to their doctor’s office for SCIT because of the associated risk of systemic reactions.
- Local and systemic reactions of SCIT include a range of anaphylactic symptoms involving the skin (urticaria), respiratory tract (rhinitis and asthma), gastrointestinal tract (nausea, diarrhea, and vomiting),and the cardiovascular system (hypotension and arrhythmias). Although rare, deaths after injections have been reported.
- Individuals with asthma should not administer SCIT at home.
- Before initiating immunotherapy, clinicians must review with the individual who has asthma the travel arrangements and time needed to travel to and from the clinic as well as the requirement for at least a 30minute observational period after each injection. These requirements may complicate compliance. Missed appointments due to scheduling problems are a safety and an efficacy concern because they may increase the likelihood of local and systemic reactions. Missed appointments can also complicate the ability to reach a maintenance dosing regimen that maximizes therapeutic benefit.
- Delayed systemic reactions (those occurring more than 30 minutes after injection) occur in approximately 15% of individuals after injection.183
- The Expert Panel recommends that individuals who have had previous clinically significant reactions to immunotherapy ideally should have injectable epinephrine and carry it on their person to and from the clinic on the day of their injection.
Summary of the evidence
The Expert Panel specified three critical outcomes (exacerbations, asthma control, and quality of life) and three important outcomes (use of quick-relief medication, adverse events [harms], and long-term medication use). Because none of the SCIT studies used validated asthma control outcome measures, the Expert Panel used nonvalidated outcome measures (eg, symptom diaries) as surrogate measures of asthma control when it evaluated 44 studies, but only if the studies used a placebo injection as the comparator.184–226
The summary of evidence for Recommendation 17 can be found in EtD Table XXVI in Appendix B. Most studies included in the systematic review report evaluated individuals with mild to moderate asthma. The status of asthma control in the studies varied and is classified as controlled, not reported, or uncontrolled. The Expert Panel judged the certainty of evidence for SCIT as low for a small benefit with respect to the critical outcomes of exacerbations, quality of life, and asthma control. Studies on exacerbations were limited. One very small study (N = 29) suggested a decrease in exacerbations (very low certainty of evidence).227 Two studies (N = 119) reported an improvement in quality of life (low certainty of evidence).187,200 Both studies used a validated outcome measure but scored the individual domains separately. Two other small studies (N = 57) found no difference in quality of life in individuals treated with SCIT or the comparator.228,229 In the judgment of the Expert Panel, the evidence overall favors SCIT for an improvement in quality of life. Using asthma symptom diaries as a surrogate measure of asthma control, 26 of 44 studies (59%) found reductions in severity of symptoms with SCIT in comparison with the placebo group.185–189,191,194,199–203,205,207,210–215,217,218,222,223,225,226 Based on these data from studies that used surrogate measures, in the judgment of the Expert Panel, the evidence favors SCIT for an improvement in asthma control (low certainty of evidence).
The Expert Panel noted that when asthma is treated with SCIT, the symptoms of comorbid conditions, such as allergic rhinitis and allergic conjunctivitis, may improve and have a beneficial effect on quality of life.
For the important outcomes, SCIT may reduce use of quick-relief medications214 (low certainty of evidence) and reduce long-term medicationuse199,200,214 (moderate certainty of evidence). Reported harms related to SCIT were highly variable, and local reactions around the injection site occurred with 7 % to 11 % of the SCIT doses given.5 Studies5 have found systemic reactions with up to 12% of total injections, during 0.1% of injection visits, and in 80% to 85% of practices. These systemic reactions include pruritus, urticaria, eczema, atopic dermatitis and other forms of eczema, rhinitis, conjunctivitis, nasal congestion, cough, bronchospasm, wheezing, dyspnea, abdominal pain, diarrhea, and hypotension.5 Rates of systemic allergic reactions consistent with anaphylaxis also varied greatly, and RCTs5 did not have the statistical power to assess such effects. Poorly controlled asthma is a major risk factor for fatal allergic reactions from SCIT. The incidence of fatal and near-fatal anaphylactic reactions ranges from 1 in 20,000 to 1 in 200,000 injections.183,230 The incidence of fatal anaphylactic reactions ranges from 1 in 2 million to 1 in 9 million injections230 (low certainty of evidence because of imprecision).
Rationale and discussion
Considering the overall balance between benefits and harms, in the judgment of the Expert Panel, the SCIT recommendation is conditional because individuals may consider SCIT as adjunct therapy if they have the following characteristics:
Place a high value on small improvements in quality of life and symptom control
Place a high value on reductions in long-term and/or quick-relief medication use
Place a lower value on the potential for systemic reactions of wide-ranging severity
The studies available for evaluation tended to have small samples, and study reports did not characterize the races of participants or the social determinants of health that they experienced.5 Studies of SCIT used different protocols and did not use standardized formulations or have a uniform or standardized duration of follow-up. The efficacy of SCIT, which has an acceptable burden of harms, is based on its impact on asthma quality of life and asthma-related symptoms, with low certainty of evidence. Whether to use SCIT should be a shared decision between the individual and the health care provider, and this decision should consider the individual’s asthma severity and willingness to accept the potential harms related to SCIT. Clinicians should administer SCIT in a clinical setting that has the capacity to monitor and treat reactions.
The enthusiasm of the Expert Panel for recommending SCIT for allergic asthma management is reduced by the slight risk of harms and variability in access (because of costs and geographical location); this variability in access can promote health inequities.
Question 6.2
What is the efficacy and safety of SLIT?
Recommendation 18: In individuals with persistent allergic asthma, the Expert Panel conditionally recommends against the use of SLIT in asthma treatment.
Conditional recommendation, moderate certainty of evidence
Implementation guidance
Clinician’s Summary:
The evidence that the Expert Panel reviewed did not support the use of SLIT specifically for the treatment of allergic asthma. However, the FDA has approved SLIT tablets (but not aqueous preparations) for the treatment of allergic rhino conjunctivitis. Individuals with this condition who also have asthma might benefit from SLIT and, if so, this benefit is most likely to be in the form of a reduction in the use of quick-relief and/or long-term control medications.
On the basis of the currently available data, the Expert Panel does not recommend SLIT for allergic asthma. SLIT is beneficial for allergic rhinoconjunctivitis.231 In an individual with comorbid allergic asthma, SLIT for allergic rhino conjunctivitis might reduce the symptoms of allergic asthma as well (and this potential provides the rationale for making the recommendation conditional). For individuals whose allergic asthma symptoms benefit from SLIT for allergic rhino conjunctivitis, the Expert Panel offers the following suggestions.
The clinician should administer the first dose of SLIT in the office, and the individual with asthma should wait in the office for at least 30 minutes after receiving the dose. If no problems develop, the individual may continue the SLIT dosing at home. Individuals receiving SLIT should ideally have an injectable epinephrine prescription and receive education on how to administer this medication.
Currently, only tablet SLIT formulations for short ragweed and dust mite mixture and for northern grass have FDA approval for treatment of allergic rhinitis with and without conjunctivitis. SLIT is not FDA approved specifically for asthma treatment.
- What clinicians should discuss with their patients:
- The Expert Panel does not recommend SLIT for the treatment of allergic asthma, but this treatment may benefit individuals with certain comorbid conditions, such as allergic rhinitis with or without conjunctivitis.
- The FDA has approved the use of SLIT to treat allergic rhinitis and conjunctivitis in response to only a few allergens at this time for individuals aged 5 years and older (for sensitization to northern grass) and in individuals aged 18 years and older (for sensitization to a short ragweed and dust mite mixture).
Summary of the evidence
The Expert Panel specified three critical outcomes (exacerbations, asthma control, and quality of life) and three important outcomes (quick-relief medication, adverse events [harms], and long-term medication use). The summary of evidence for Recommendation 18 can be found in EtD Table XXVII in Appendix B.
The evidence shows that SLIT provides a trivial benefit for the critical outcomes of exacerbations,232,233 asthma control,234–239 and quality of life232–234,237,238 (moderate certainty of evidence). No studies assessed the impact of SLIT on emergency department visits, clinic visits, or hospitalizations. Three studies evaluated exacerbations using different end points. One study did not report the number of exacerbations, but it did report on the time to first exacerbation.233 SLIT decreased the severity of the first moderate exacerbation, but it did not increase the time to first severe exacerbations requiring systemic corticosteroids. Another study did not provide any raw data or rates of the critical outcomes, and the authors only noted that the results showed no statistically significant improvement in asthma exacerbations.234,237,238 The third study, which enrolled only 60 participants, found a significantly lower number of exacerbations in the treatment group.232 Four studies (N = 1193) that evaluated asthma control using validated outcome tools (three used the ACQ, and one used the ACT) found no consistent improvement after treatment.233–239 Finally, multiple studies showed no difference in quality of life in those treated with SLIT or placebo233–235,237–239 (high certainty of evidence).
For important outcomes, SLIT reduced the use of quick-relief medications232,236,240–242 and doses of ICSs,234,235,242,243 with moderate certainty of evidence.
The harms were difficult for the Expert Panel to evaluate. Local reactions were frequent and occurred in up to 80% of individuals treated with SLIT, but adverse local reactions were also common in those receiving placebo. The rate of side effects did not differ by the setting of administration (home, clinic, or other), and the relationship between the risk of side effects and the strength of the dose administered was not consistent across studies. None of the RCTs (N = 1772)233,234,243–246 reported episodes of anaphylaxis. The Expert Panel found no reports of death that was secondary to SLIT.
Rationale and discussion
The 2014–2015 needs assessment report by the NHLBAC Asthma Expert Working Group2 included both aqueous and tablet formulations in the research questions on the efficacy and safety of SLIT. For these questions, the systematic review report combined studies of the two types of SLIT, thereby increasing the sample sizes and precision of results for many of the outcomes evaluated.12 However, the designs and methodologies of RCTs that used aqueous and drop preparations of SLIT were not as rigorous or standardized as they were for studies that used tablet formulations. In evaluating the data on aqueous or drop and tablet formulations combined, the Expert Panel did not find that SLIT reduced asthma symptoms or improved asthma control or asthma quality of life. Although systemic side effects were common (80% of participants), they were also common in the placebo groups.5 In addition, the limited number of FDA-approved antigens, the costs of SLIT, and the variability in access to this treatment promote health inequities.
Overall summary for SCIT and SLIT
The Expert Panel conditionally recommends SCIT as an adjunct treatment to standard pharmacotherapy for individuals aged 5 years and older with mild to moderate persistent asthma who show clear evidence of a relationship between symptoms and exposure to an allergen to which the individual is sensitive.12 The Expert Panel conditionally recommends against the use of SLIT as a treatment specifically for asthma.
The Expert Panel’s immunotherapy recommendations call for shared decision making between the clinician and the individual with asthma. The recommendations also highlight SLIT’s potential to reduce the symptoms of comorbid conditions, such as allergic rhinitis and allergic conjunctivitis, and this potential improvement may be an important consideration for individuals with allergic asthma.5
Future research opportunities
The Expert Panel identified the following opportunities for additional research:
Investigate the safety and efficacy of immunotherapy in individuals with severe asthma, particularly those whose asthma is under control but who want to reduce their medication burden
Include only children aged 5 to 11 years in studies of children, or, if a study includes a broader age group, report findings separately for children aged 5 to 11 years and those aged 12 years and older
Study more diverse populations to determine whether race or ethnicity influences the efficacy and safety of immunotherapy
Study the efficacy and safety of multiple-allergen SCIT or SLIT regimens to assess compliance, adherence, and the effect of these factors on asthma management
Standardize methods to report SCIT and SLIT doses used in studies and use validated outcome measurement instruments, such as asthma symptoms and adverse events
SECTION VII: RECOMMENDATIONS FOR THE USE OF BT TO IMPROVE ASTHMA OUTCOMES
Background
The Expert Panel examined studies that compared BT to multicomponent, standard-of-care, medical management, and sham bronchoscopy plus multicomponent medical management. BT is an asthma intervention that was developed over the last decade and was not addressed in previous versions of the asthma guidelines. The Expert Panel made one recommendation on the use of BT for asthma treatment.
Definitions of terms used in this section
Multicomponent medical therapy consists of medium to high doses of ICS treatment, LABAs, omalizumab (in one study), and/ or oral corticosteroids. Available studies of BT did not include individuals treated with LAMAs, environmental interventions, and/or newer biologic agents.247–249
“Life-threatening asthma” is defined as asthma that has resulted in hospitalization in an intensive care unit and/or has been treated with noninvasive ventilation or intubation in the past 5 years.
Question 7.1
What are the benefits and harms of using BT in addition to standard treatment for the treatment of individuals aged 18 years and older with asthma?
Recommendation 19: In individuals aged 18 years and older with persistent asthma, the Expert Panel conditionally recommends against BT.
Conditional recommendation, low certainty of evidence
Individuals aged 18 years and older with persistent asthma who place a low value on harms (ie, short-term worsening of symptoms and unknown long-term side effects) and a high value on potential benefits (ie, improvement in quality of life and a small reduction in number of exacerbations) might consider BT.
Implementation guidance
Clinician’s Summary:
Most individuals aged 18 years and older with uncontrolled, moderate to severe, persistent asthma should not undergo BT to treat asthma because the benefits are small, the risks are moderate, and the long-term outcomes are uncertain. Some individuals with moderate to severe persistent asthma who have trouble some symptoms may be willing to accept the risks of BT and, therefore, might choose this intervention after shared decision making with their health care provider. Clinicians should offer the procedure in the setting of a clinical trial or a registry study to enable the collection of long-term data on the use of BT for asthma.
The Expert Panel does not recommend BT for individuals aged 18 years and older as part of routine asthma care, even if these individuals have uncontrolled asthma despite using multicomponent medical therapy, because of the small benefit-to-risk ratio. The risks of BT include asthma exacerbations, hemoptysis, and atelectasis during the treatment period. Recognizing, however, that BT is currently being used, the Expert Panel offers the following suggestions for its safe use:
BT should not be used in individuals with low lung function (FEV1 that is <50% or 60% predicted) and life threatening asthma.
BT has not been studied in individuals younger than age 18 years.
In the opinion of the Expert Panel, when BT is implemented, it should be used in settings that enroll participants in registries, ongoing clinical trials, or studies that track BT’s long-term safety and effectiveness.
For individuals who decide to undergo BT, an experienced specialist (eg, a pulmonologist with training in BT administration) should provide this treatment in a center that has appropriate expertise.
Clinicians should optimize asthma treatment and address comorbidities, and they should assess and optimize adherence to existing therapy, before considering BT.
In some individuals, BT may provide a small benefit that might last 5 years or longer.250,251
BT may reduce severe asthma exacerbations in comparison to standard care after treatment.
Risks associated with BT include worsening of asthma, respiratory infections, hemoptysis, bronchiectasis, and pulmonary artery complications.252–254
Severe latent or delayed-onset complications have not been reported with BT, but the number of individuals with asthma included in long-term follow-up assessments is very small (fewer than 250 people at the time the systematic review report3 on this topic was completed).
- What clinicians should discuss with their patients about BT:
- This procedure may reduce severe asthma exacerbations compared with standard care after treatment. Although the benefits could last 5 years or more, only limited data demonstrate that this treatment improves long-term asthma outcomes.
- The risks associated with BT include worsening of asthma, respiratory infections, hemoptysis, bronchiectasis, and pulmonary artery complications.252–254 In addition, severe, delayed-onset complications could occur that have not yet been recognized because of the small numbers of individuals who have undergone the procedure.
- Individuals aged 18 years and older with persistent asthma who place a low value on the harms (short term worsening symptoms and unknown long-term side effects) and a high value on the potential benefits (improvement in asthma quality of life, small reduction in exacerbations) of BT might consider this treatment.
Summary of the evidence
The Expert Panel specified three critical outcomes (exacerbations, asthma control, and quality of life) and one important outcome (use of rescue medication) for this question. The summary of evidence for Recommendation 19 can be found in Appendix B (EtD Table XXVIII).
The conditional recommendation against the use of BT in individuals aged 18 years and older with poorly controlled asthma after medium- to high-dose ICS treatment paired with a LABA (with or without oral corticosteroids) is based on three RCTs.247–249 All of these trials were funded by the company that markets the BT device.
Two of the studies compared BT with standard care.248,249 The Research In Severe Asthma (RISA)study (N = 32)249 enrolled individuals treated with a high-dose ICS (>750 μg fluticasone or equivalent) and a LABA (100 μg salmeterol equivalent) with or without daily oral corticosteroids (<30 mg/d prednisone equivalent). The Asthma Intervention Research (AIR)248 study (N = 112) enrolled individuals taking an ICS (>200 μg/d beclomethasone equivalent) and a LABA (100 μg salmeterol or equivalent). These two studies found improvements in critical outcomes, including decreases in numbers of mild exacerbations not requiring oral or parenteral corticosteroids and in numbers of emergency department visits. The results also showed improved asthma control based on ACQ scores and less rescue medication use (an important outcome).248,249
A third study, AIR 2 (N = 288), compared BT with sham bronchoscopy plus standard care.247 This study enrolled individuals treated with high-dose ICS (>1000 μg betamethasone or equivalent) plus a LABA. Participants could also continue using leukotriene modifiers and omalizumab if they had used these treatments for at least 1 year. This study found reductions in severe exacerbations requiring oral or parenteral corticosteroid treatment over 12 months in participants treated with BT. Other critical outcomes—such as asthma control, mean asthma quality-of-life scores (measured with the Asthma Quality of Life Questionnaire), and rescue medication use (an important outcome)—did not improve. The percentage of participants with Asthma Quality of Life Questionnaire scores of 0.5 or higher (MID) in the BT group (79%) was significantly different from the corresponding proportion (64%) in the control (sham bronchoscopy) group. The strength of evidence was low for all of these outcomes across the three studies. None of the studies found that BT reduced the number of hospitalizations for asthma over 12 months.247–249
The AIR extension study followed 69 individuals (45 treated with BT and 24 with control treatment) for 3 years.250 The results did not demonstrate any differences in rates of asthma-related events between the two groups over the additional 24 months.
The RISA249 and AIR248 studies found increased rates of bronchial irritation, chest discomfort, cough, discolored sputum, dyspnea, night awakenings, and wheezing during the 12-week BT treatment period. The AIR 2 extension study followed 162 of 190 participants treated with BT for up to 5 years after BT treatment.251 Long-term results from the RISA extension255 and AIR extension250 showed ongoing or new dyspnea (9.5% of participants), chest discomfort (4.8%−8.3%), bronchial irritation (2.4%), wheezing (4.8%−8.3%), and cough (4.8%) at the end of the 5-year study period. Hospitalizations during and after the treatment period were more frequent in patients treated with BT in all three studies.247–249 In the AIR 2 study, 16 of 190 patients treated with BT and 2 of 98 patients in the control group were hospitalized during the treatment period. Ten of the 16 patient hospitalizations in patients treated with BT and both of the hospitalizations of patients in the control group were for worsening asthma. In the RISA study, 4 of 15 patients were hospitalized seven times during the 12 months after treatment, whereas none of the 17 patients in the standard care arm was hospitalized.248 In addition to being hospitalized for worsening asthma, participants in the BT arms of the three studies were hospitalized for segmental atelectasis, lower respiratory tract infections, low FEV1, hemoptysis, and an aspirated prosthetic tooth.247–249
Twelve case reports and small case series reports252–254,256–264 also described adverse events, including hemoptysis in seven patients, atelectasis in six patients, and lower respiratory tract infections in three patients. One individual in these reports developed a mediastinal hematoma and bloody pleural effusion while on anticoagulation therapy for a pulmonary embolism. The authors of this case report believed that this effect resulted from a pseudoaneurysm of the pulmonary artery caused by the BT. Complications from case reports with one reported occurrence included a lung abscess, an inflammatory bronchial polyp, a pulmonary cyst, and a case of bronchiectasis.252–254,256–264
None of the 15 studies reviewed (3 RCTs and 12 case reports and case series) attributed any deaths to BT.
Rationale and discussion
The data on the benefits and harms of BT derive primarily from three RCTs that enrolled a total of 432 patients in both the intervention and treatment arms. Overall, the improvements after BT were small, and the harms of BT were moderate. Long-term follow-up of a sufficient number of patients to fully assess clinical benefits and harms is lacking. The therapy may offer an acceptable benefit-to-harm ratio for some patients after careful shared decision making. Further research that includes randomized trials as well as long-term registry outcomes are desirable.
Future research opportunities
The Expert Panel identified the following research gaps:
Identify the population most likely to benefit from BT, such as individuals who have been treated unsuccessfully with different biologic agents.
Develop a registry to determine the risk of significant but rare long-term harms, such as bronchiectasis, vascular damage, and other lung complications. Follow both treated and untreated individuals over the long-term to determine whether side effects reported at 5 years in the AIR 2 study247 are more common in individuals treated with BT than in a control group.
Conduct RCTs and long-term registry studies of BT for asthma treatment, with appropriate controls and a sufficient number of patients, to fully assess the clinical benefits and harms of BT.
Supplementary Material
Abbreviations used
- ACP
American College of Physicians
- ACQ
Asthma Control Questionnaire
- ACQ-5
5-question Asthma Control Questionnaire
- ACQ-6
6-question Asthma Control Questionnaire
- ACQ-7:
7-question Asthma Control Questionnaire
- ACT
Asthma Control Test
- AHRQ
Agency for Healthcare Research and Quality
- AIR
Asthma Intervention Research
- BELT
Blacks and Exacerbations on LABA vs Tiotropium
- BT
Bronchial thermoplasty
- COI
Conflict of interest
- EPC
Evidence-Based Practice Center
- EPR
Expert Panel Report
- EtD
Evidence to decision
- FDA
US Food and Drug Administration
- Feno
Fractional exhaled nitric oxide
- GRADE
Grading of Recommendations, Assessment, Development, and Evaluation
- HEPA
High-efficiency particulate air (a type of filter)
- ICS
Inhaled corticosteroid
- ICS-LABA
Inhaled corticosteroid and long-acting beta2-agonist combination, typically in a single device
- JACI
Journal of Allergy and Clinical Immunology
- LABA
Long-acting beta2-agonist
- LAMA
Long-acting muscarinic antagonist
- MID
Minimally important difference
- NAEPP
National Asthma Education and Prevention Program
- NAEPPCC
National Asthma Education and Prevention Program Coordinating Committee
- NHLBAC
National Heart, Lung, and Blood Advisory Council
- NHLBI
National Heart, Lung, and Blood Institute
- OR
Odds ratio
- ppb
Parts per billion
- RCT
Randomized controlled trial
- RISA
Research In Severe Asthma
- RR:
Relative risk
- SABA
Short-acting beta2-agonist
- SCIT
Subcutaneous immunotherapy
- SLIT
Sublingual immunotherapy
- SMART
Single maintenance and reliever therapy
- T2
Type 2
National Asthma Education and Prevention Program Coordinating Committee
Joseph Kofi Berko Jr, PhD
US Department of Housing and Urban Development
Sheila Brown
US Environmental Protection Agency
Kurtis S. Elward, MD
American Academy of Family Physicians
Anne Mentro Fitzpatrick, PhD, RN
Emory University School of Medicine
Lynn B. Gerald, PhD
University of Arizona
Fernando Holguin, MD, MPH
American Thoracic Society
Joy Hsu, MD
Centers for Disease Control and Prevention
Elliot Israel, MD
Harvard Medical School
Robert F. Lemanske Jr, MD
University of Wisconsin
Kenneth Mendez, MBA
Asthma and Allergy Foundation of America
Giselle Sarah Mosnaim, MD
American Academy of Allergy, Asthma & Immunology
Gary S. Rachelefsky, MD
American Academy of Pediatrics
Lisa M. Wheatley, MD, MPH
National Institute of Allergy and Infectious Disease
Juan P. Wisniesky, DPh, MD
Icahn School of Medicine at Mount Sinai
Darryl C. Zeldin, MD
National Institute of Environmental Health Sciences
National Heart, Lung, and Blood Institute staff
James P. Kiley, PhD
Director
Division of Lung Diseases
Cochair, NAEPPCC
George A. Mensah, MD, FACC
Director
Center for Translation Research and Implementation Science
Cochair, NAEPPCC
Cheryl A. Boyce, PhD
Chief, Implementation Science Branch
Center for Translation Research and Implementation Science
Jennifer Curry, MPH
Program Analyst
Center for Translation Research and Implementation Science
Lenora E. Johnson, DrPH, MPH
Director
Office of Science Policy, Engagement, Education, and Communications
Michelle M. Freemer, MD, MPH
Program Director
Division of Lung Diseases
Susan T. Shero, RN, BSN, MS
Program Officer
Implementation Science Branch
Center for Translation Research and Implementation Science
Executive Secretary, NAEPPCC
Westat staff
Russell E. Mardon, PhD
Project Director
Marguerite Campbell
Meeting and Logistical Support Manager
Sean Chickery, DHSc, MBA, MT(ASCP)
Topic Team Liaison
Susan Hassell, MPH
Project Manager
Nataly Johanson Tello
Topic Team Liaison
Maurice C. Johnson Jr, MPH
Topic Team Liaison
Karen Kaplan
Editorial Manager
Westat subcontractors
Eric Linskens
Health Science Specialist
Roderick MacDonald
Health Science Specialist
Judith Orvos, ELS
Orvos Communications, Senior Editor
Shahnaz Sultan, MD, MHSc
Guideline Methodologist
From the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda.
*Members of the Expert Panel Working Group: Michelle M. Cloutier, MD (Chair of the Expert Panel Working Group; UCONN Health, Farmington, Conn), Alan P. Baptist, MD, MPH (University of Michigan, Ann Arbor, Mich), Kathryn V. Blake, PharmD (Nemours Children’s Health System, Jacksonville, Fla), Edward G. Brooks, MD (University of Texas Health Science Center at San Antonio, San Antonio, Texas), Tyra Bryant-Stephens, MD (Children’s Hospital of Philadelphia, Philadelphia, Pa), Emily DiMango, MD (Columbia University Irving Medical Center, New York, NY), Anne E. Dixon, MA, BM, BCh (University of Vermont, Burlington, Vt), Kurtis S. Elward, MD, MPH, FAAFP (Virginia Commonwealth University, Charlottesville, Va), Tina Hartert, MD, MPH (Vanderbilt University School of Medicine, Nashville, Tenn), Jerry A. Krishnan, MD, PhD (University of Illinois Hospital and Health Sciences System, Chicago, Ill), Robert F. Lemanske Jr, MD (University of Wisconsin Madison, Madison, Wis), Daniel R. Ouellette, MD, FCCP (Henry Ford Health System, Detroit, Mich), Wilson D. Pace, MD, FAAFP (University of Colorado, Aurora, Colo), Michael Schatz, MD, MS (Kaiser Permanente, San Diego, Calif), Neil S. Skolnik, MD (Dr Skolnik withdrew from the Expert Panel on October 1, 2019; Abington - Jefferson Health, Jenkintown, Pa), James W. Stout, MD, MPH (University of Washington, Seattle, Wash), Stephen J. Teach, MD, MPH (George Washington University, Washington, DC), Craig A. Umscheid, MD, MSCE (University of Chicago, Chicago, Ill), and Colin G. Walsh, MD, MA (Dr Walsh withdrew from the Expert Panel on October 29, 2019; Vanderbilt University Medical Center, Nashville, Tenn).
The 2020 Focused Updates to the Asthma Management Guidelines: A Report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group was funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health. No additional funding was received. All authors volunteered their time.
Reported disclosure of potential conflict of interest: Development of this report was funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health. Members of the Expert Panel Working Group (“Expert Panel”) of the National Asthma Education and Prevention Program Coordinating Committee (NAEPPCC) completed financial disclosure forms and disclosed relevant financial interests described as conflicts of interest to each other before their discussions. Members of the Expert Panel were volunteers and received compensation only for travel expenses related to the panel’s in-person meetings. M. M. Cloutier reported that a family member was employed by Regeneron. The Expert Panel did not discuss any Regeneron or related products. M. M. Cloutier was not recused from any Expert Panel discussions. A. Baptist received grant funding from Novartis, AstraZeneca, Teva Pharmaceuticals Industries Ltd (Teva), and Takeda and was recused from drafting, discussing, or voting on sections of the report related to the inhaled corticosteroid recommendations. K. Blake received personal fees from Teva for participation on its Digital Technology Advisory Board and study drugs from Boehringer Ingelheim and GlaxoSmithKline for federally funded clinical trials and was not recused from any Expert Panel discussions. E. G. Brooks served on the Scientific Advisory Board for United Allergy Services until December 2019 and was recused from the initial drafting, discussions, and voting related to the immunotherapy recommendations. However, he participated in the final voting, discussions, and writing of this section of the report. T. Bryant-Stephens received mattress covers and air purifiers from the Asthma and Allergy Foundation of America and was not recused from any Expert Panel discussions. E. DiMango participated in an advisory board meeting for AstraZeneca, reported that a family member was formerly employed by Regeneron, and was not recused from any Expert Panel discussions. A. Dixon received a study medication from MitoQ, also received assistance from a medical writer from Boehringer Ingelheim for a manuscript published in 2019 that was not related to the update topics, and was not recused from any Expert Panel discussions. K. S. Elward had no conflicts of interest and was not recused from any Expert Panel discussions. T. Hartert participated in a Pfizeradvisory board for a maternal respiratory syncytialvirus vaccine and was not recused from any Expert Panel discussions. J. A. Krishnan participated in a data and safety monitoring committee for Sanofi, received funding from Inogen and ResMed for a study of portable oxygen concentrators, received personal fees from the Critical Path Institute, and was not recused from any Expert Panel discussions. R. F. Lemanske received personal fees from Siolta Therapeutics for a microbiome intervention and from the Food Allergy Research & Education Network and was not recused from any Expert Panel discussions. D. R. Ouellette had no conflicts of interest and was not recused from any Expert Panel discussions. W. Pace participated in a meeting sponsored by Mylan to discuss long-acting muscarinic antagonist (LAMA) therapy, received a study drug from Teva for a Patient-Centered Outcomes Research Institute–funded pragmatic clinical study, received a grant from Boehringer Ingelheim through Optimal Patient Care concerning Chronic Obstructive Pulmonary Disease, and was recused from participation in the final discussion, voting, and section writing related to LAMA therapy. M. Schatz received grant funding from Merck, Teva, and ALK-Abello, Inc, and was recused from the writing, discussion, and voting related to the immunotherapy recommendations. N. S. Skolnik received personal fees and nonfinancial support from AstraZeneca; personal fees from Teva, Eli Lilly and Company, Boehringer Ingelheim, Merck, Sanofi, Janssen Pharmaceuticals, Intarcia Therapeutics, GlaxoSmithKline, and Mylan; was recused from the writing, discussion, and voting related to the inhaled corticosteroids and LAMA recommendations; and withdrew from the Expert Panelon October 1, 2019. J. W. Stout received a training grant from the Patient-Centered Outcomes Research Institute, which distributed pillow and mattress dust covers; nonfinancial support for a new quality review design for the EasyOne Air spirometer (the EasyOne Air build was done with a combination of license revenue from the Spirometry 360 course, and his personal research and training account through his division); nonfinancial support for a smartphone spirometer application from Google, and development of an asynchronous and an abridged asynchronous online spirometer training course (asynchronous training was built with a combination of funding from AHRQ, the National Asthma Control Initiative [NACI], Spirometry 360 license revenue, and unrestricted funding from Astra-Zeneca); and was not recused from any Expert Panel discussions. S. Teach had no conflicts of interest and was not recused from any Expert Panel discussions. C. A. Umscheid received funding from the Agency for Healthcare Research and Quality for systematic reviews on allergen reduction and bronchial thermoplasty and was not recused from any Expert Panel discussions. C. G. Walsh had no conflicts of interest, was not recused from any Expert Panel discussions, and withdrew from the Expert Panel on October 29, 2019.
REFERENCES
- 1.National Asthma Education and Prevention Program. Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma. Bethesda, Md: National Heart, Lung, and Blood Institute, National Institutes of Health; 1991. [Google Scholar]
- 2.Heart National, Lung, and Blood Advisory Council Asthma Expert Working Group. Needs Assessment Report for Potential Update of the Expert Panel Report-3 (2007): Guidelines for the Diagnosis and Management of Asthma. Bethesda, Md: National Institutes of Health, National Heart, Lung, and Blood Institute; February 2015. Available at: https://www.nhlbi.nih.gov/sites/default/files/media/docs/NHLBAC-Asthma-WG-Report-2-2015.pdf. [Google Scholar]
- 3.D’Anci KE, Lynch MP, Leas BF, Apter AJ, Bryant-Stephens T, Kaczmarek JL, et al. Effectiveness and safety of bronchial thermoplasty in management of asthma. Comparative Effective Review No. 202. (Prepared by the ECRI Institute–Penn Medicine Evidence-based Practice Center under Contract No. 290–2015-00005-I). AHRQ Publication No. 18-EHC0003-EF. Rockville, Md: Agency for Healthcare Research and Quality; December 2017. Available at: 10.23970/AHRQEPCCER202. [DOI] [PubMed] [Google Scholar]
- 4.Leas BF, D’Anci KE, Apter AJ, Bryant-Stephens T, Schoelles K, Umscheid C. Effectiveness of indoor allergen reduction in management of asthma. Comparative Effectiveness Review No. 201. (Prepared by the ECRI Institute–Penn Medicine Evidence-based Practice Center under Contract No. 290-2015-0005-I). AHRQ Publication No. 18-EHC002-EF. Rockville, Md: Agency for Healthcare Research and Quality; February 2018. Posted final reports are located on the Effective Health Care Program search page. [PubMed] [Google Scholar]
- 5.Lin SY, Azar A, Suarez-Cuervo C, Diette GB, Brigham E, Rice J, et al. The role of immunotherapy in the treatment of asthma. Comparative Effectiveness Review No. 196. (Prepared by the Johns Hopkins University Evidence-based Practice Center under Contract No.290–2015-00006-I). AHRQ Publication No. 17(18) EHC029-EF. Rockville, Md: Agency for Healthcare Research and Quality; March 2018. Posted final reports are located on the Effective Health Care Program search page. [Google Scholar]
- 6.Sobieraj DM, Baker WL, Weeda ER, Nguyen E, Coleman CI, White CM, et al. Intermittent inhaled corticosteroids and long-acting muscarinic antagonists for asthma. Comparative Effectiveness Review No. 194. (Prepared by the University of Connecticut Evidence-based Practice Center under Contract No. 290–201500012-I). AHRQ Publication No. 17(18)-EHC027-EF. Rockville, Md: Agency for Healthcare Research and Quality; March 2018. Available at: https://www.ncbi.nlm.nih.gov/pubmed/29741837. Posted final reports are located on the Effective Health Care Program search page. [PubMed] [Google Scholar]
- 7.Wang Z, Pianosi P, Keogh K, Zaiem F, Alsawas M, Alahdab F, et al. The clinical utility of fractional exhaled nitric oxide (FeNO) in asthma management. Comparative Effectiveness Review No. 197. (Prepared by the Mayo Clinic Evidence based Practice Center under Contract No. 290–2015-00013-I). AHRQ Publication No. 17(18)-EHC030-EF. Rockville, Md: Agency for Healthcare Research and Quality; December 2017. Available at: 10.23970/AHRQEPCCER197 [DOI] [PubMed] [Google Scholar]
- 8.Institute of Medicine Committee on Standards for Developing Trust worthy Clinical Practice Guidelines. In: Graham R, Mancher M, Miller Wolman D, Greenfield S, Steinburg E, eds. Clinical practice guidelines we can trust. Washington, DC: National Academies Press; 2011; Available at: https://www.ncbi.nlm.nih.gov/books/NBK209539/. [PubMed] [Google Scholar]
- 9.Qaseem A, Wilt TJ. Disclosure of interests and management of conflicts of interest in clinical guidelines and guidance statements: methods from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med 2019;171:354–61. [DOI] [PubMed] [Google Scholar]
- 10.Busse WW, Morgan WJ, Taggart V, Togias A. Asthma outcomes workshop: overview. J Allergy Clin Immunol 2012;129:S1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Asthma Education and Prevention Program. Expert Panel Report 2: Guidelines for the Diagnosis and Management of Asthma. Bethesda, Md: National Heart, Lung, and Blood Institute, National Institutes of Health; 1997. [Google Scholar]
- 12.National Asthma Education and Prevention Program. Third Expert Panel on the Diagnosis and Management of Asthma. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, Md: National Heart, Lung, and Blood Institute, National Institutes of Health; August 2007. 440 pp. Available at: https://www.ncbi.nlm.nih.gov/books/NBK7232/. [Google Scholar]
- 13.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines, 1: Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383–94. [DOI] [PubMed] [Google Scholar]
- 14.Institute of Medicine. Conflict of interest in medical research education and practice. Washington, DC: National Academies Press; 2009. [PubMed] [Google Scholar]
- 15.Journal of Allergy and Clinical Immunology. Information for Authors. 2018. [Google Scholar]
- 16.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schunemann H, Brozek J, Guyatt G, Oxman A, eds. Handbook for grading the quality of evidence and strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group; 2013; Available at: gdt.guidelinedevelopment.org/app/handbook/handbook.html. Accessed September 4, 2019. [Google Scholar]
- 18.Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines, 2: framing the question and deciding on important outcomes. J Clin Epidemiol 2011;64:395–400. [DOI] [PubMed] [Google Scholar]
- 19.Juniper EF, Buist AS, Cox FM, Ferrie PJ, King DR. Validation of a standardized version of the Asthma Quality of Life Questionnaire. Chest 1999;115:1265–70. [DOI] [PubMed] [Google Scholar]
- 20.Juniper EF, Gruffydd-Jones K, Ward S, Svensson K. Asthma Control Questionnaire in children: validation, measurement properties, interpretation. Eur Respir J 2010;36:1410–6. [DOI] [PubMed] [Google Scholar]
- 21.Juniper EF, Guyatt GH, Cox FM, Ferrie PJ, King DR. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J 1999;14: 32–8. [DOI] [PubMed] [Google Scholar]
- 22.Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend M. Measuring quality of life in the parents of children with asthma. Qual Life Res 1996;5:27–34. [DOI] [PubMed] [Google Scholar]
- 23.Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend M. Measuring quality of life in children with asthma. Qual Life Res 1996;5:35–46. [DOI] [PubMed] [Google Scholar]
- 24.Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease-specific Quality of Life Questionnaire. J Clin Epidemiol 1994;47:81–7. [DOI] [PubMed] [Google Scholar]
- 25.Juniper EF, Svensson K, Mork AC, Stahl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med 2005;99:553–8. [DOI] [PubMed] [Google Scholar]
- 26.Santanello NC, Zhang J, Seidenberg B, Reiss TF, Barber BL. What are minimal important changes for asthma measures in a clinical trial? Eur Respir J 1999;14: 23–7. [DOI] [PubMed] [Google Scholar]
- 27.Schatz M, Kosinski M, Yarlas AS, Hanlon J, Watson ME, Jhingran P. The minimally important difference of the Asthma Control Test. J Allergy Clin Immunol 2009;124:719–23.e1. [DOI] [PubMed] [Google Scholar]
- 28.Fuhlbrigge A, Peden D, Apter AJ, Boushey HA, Camargo CA Jr, Gern J, et al. Asthma outcomes: exacerbations. J Allergy Clin Immunol 2012;129:S34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, et al. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol 2017;87:4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brozek J, Nowak A, Kunstman P, Sch unemann H. GRADE pro Guideline Development Tool (G2DT). Available at: www.guidelinedevelopment.org. Accessed September 4, 2019.
- 31.Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, et al. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices, 2: clinical practice guidelines. BMJ 2016;353:i2089. [DOI] [PubMed] [Google Scholar]
- 32.Alonso-Coello P, Schunemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, et al. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices, 1: introduction. BMJ 2016;353:i2016. [DOI] [PubMed] [Google Scholar]
- 33.Guyatt GH, Oxman AD, Kunz R, Falck-Ytter Y, Vist GE, Liberati A, et al. Going from evidence to recommendations. BMJ 2008;336:1049–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krueger RA. Designing and conducting focus group interviews. University of Minnesota; October 2002. 18 pp. Available at: https://www.eiu.edu/ihec/Krueger-FocusGroupInterviews.pdf. Accessed November 13, 2019.
- 35.Patton MQ. Qualitative research and evaluation methods. Thousand Oaks, Calif: Sage; 2002. [Google Scholar]
- 36.Lieu TA, Au D, Krishnan JA, Moss M, Selker H, Harabin A, et al. Comparative effectiveness research in lung diseases and sleep disorders: recommendations from the National Heart, Lung, and Blood Institute workshop. Am J Respir Crit Care Med 2011;184:848–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beck-Ripp J, Griese M, Arenz S, Koring C, Pasqualoni B, Bufler P. Changes of exhaled nitric oxide during steroid treatment of childhood asthma. Eur Respir J 2002;19:1015–9. [DOI] [PubMed] [Google Scholar]
- 38.Vijverberg SJ, Koster ES, Koenderman L, Arets HG, van der Ent CK, Postma DS, et al. Exhaled NO is a poor marker of asthma control in children with a reported use of asthma medication: a pharmacy-based study. Pediatr Allergy Immunol 2012;23:529–36. [DOI] [PubMed] [Google Scholar]
- 39.Szefler SJ, Mitchell H, Sorkness CA, Gergen PJ, O’Connor GT, Morgan WJ, et al. Management of asthma based on exhaled nitric oxide in addition to guideline based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet 2008;372:1065–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calhoun WJ, Ameredes BT, King TS, Icitovic N, Bleecker ER, Castro M, et al. Comparison of physician-, biomarker-, and symptom-based strategies for adjustment of inhaled corticosteroid therapy in adults with asthma: the BASALT randomized controlled trial. JAMA 2012;308:987–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Jongste JC, Carraro S, Hop WC, Baraldi E. Daily telemonitoring of exhaled nitric oxide and symptoms in the treatment of childhood asthma. Am J Respir Crit Care Med 2009;179:93–7. [DOI] [PubMed] [Google Scholar]
- 42.Fritsch M, Uxa S, Horak F Jr, Putschoegl B, Dehlink E, Szepfalusi Z, et al. Exhaled nitric oxide in the management of childhood asthma: a prospective 6months study. Pediatr Pulmonol 2006;41:855–62. [DOI] [PubMed] [Google Scholar]
- 43.Garg Y, Kakria N, Katoch CDS, Bhattacharyya D. Exhaled nitric oxide as a guiding tool for bronchial asthma: a randomised controlled trial. Armed Forces Med J India 2018;76:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Honkoop PJ, Loijmans RJ, Termeer EH, Snoeck-Stroband JB, van den Hout WB, Bakker MJ, et al. Symptom- and fraction of exhaled nitric oxide-driven strategies for asthma control: a cluster-randomized trial in primary care. J Allergy Clin Immunol 2015;135:682–8.e11. [DOI] [PubMed] [Google Scholar]
- 45.Peirsman EJ, Carvelli TJ, Hage PY, Hanssens LS, Pattyn L, Raes MM, et al. Exhaled nitric oxide in childhood allergic asthma management: a randomised controlled trial. Pediatr Pulmonol 2014;49:624–31. [DOI] [PubMed] [Google Scholar]
- 46.Petsky HL, Li AM, Au CT, Kynaston JA, Turner C, Chang AB. Management based on exhaled nitric oxide levels adjusted for atopy reduces asthma exacerbations in children: a dual centre randomized controlled trial. Pediatr Pulmonol 2015;50:535–43. [DOI] [PubMed] [Google Scholar]
- 47.Pijnenburg MW, Bakker EM, Hop WC, De Jongste JC. Titrating steroids on exhaled nitric oxide in children with asthma: a randomized controlled trial. Am J Respir Crit Care Med 2005;172:831–6. [DOI] [PubMed] [Google Scholar]
- 48.Pike K, Selby A, Price S, Warner J, Connett G, Legg J, et al. Exhaled nitric oxide monitoring does not reduce exacerbation frequency or inhaled corticosteroid dose in paediatric asthma: a randomised controlled trial. Clin Respir J 2013;7:204–13. [DOI] [PubMed] [Google Scholar]
- 49.Powell H, Murphy VE, Taylor DR, Hensley MJ, McCaffery K, Giles W, et al. Management of asthma in pregnancy guided by measurement of fraction of exhaled nitric oxide: a double-blind, randomised controlled trial. Lancet 2011; 378:983–90. [DOI] [PubMed] [Google Scholar]
- 50.Shaw DE, Berry MA, Thomas M, Green RH, Brightling CE, Wardlaw AJ, et al. The use of exhaled nitric oxide to guide asthma management: a randomized controlled trial. Am J Respir Crit Care Med 2007;176:231–7. [DOI] [PubMed] [Google Scholar]
- 51.Smith AD, Cowan JO, Brassett KP, Filsell S, McLachlan C, Monti-Sheehan G, et al. Exhaled nitric oxide: a predictor of steroid response. Am J Respir Crit Care Med 2005;172:453–9. [DOI] [PubMed] [Google Scholar]
- 52.Syk J, Malinovschi A, Johansson G, Unden AL, Andreasson A, Lekander M, et al. Anti-inflammatory treatment of atopic asthma guided by exhaled nitric oxide: a randomized, controlled trial. J Allergy Clin Immunol Pract 2013;1:639–48.e1–8. [DOI] [PubMed] [Google Scholar]
- 53.Voorend-van Bergen S, Vaessen-Verberne AA, Brackel HJ, Landstra AM, van den Berg NJ, Hop WC, et al. Monitoring strategies in children with asthma: a randomised controlled trial. Thorax 2015;70:543–50. [DOI] [PubMed] [Google Scholar]
- 54.Verini M, Consilvio NP, Di Pillo S, Cingolani A, Spagnuolo C, Rapino D, et al. FeNO as a marker of airways inflammation: the possible implications in childhood asthma management. J Allergy (Cairo) 2010;691425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zeiger RS, Szefler SJ, Phillips BR, Schatz M, Martinez FD, Chinchilli VM, et al. Response profiles to fluticasone and montelukast in mild-to-moderate persistent childhood asthma. J Allergy Clin Immunol 2006;117:45–52. [DOI] [PubMed] [Google Scholar]
- 56.Malerba M, Radaeli A, Olivini A, Ragnoli B, Ricciardolo F, Montuschi P. The combined impact of exhaled nitric oxide and sputum eosinophils monitoring in asthma treatment: a prospective cohort study. Curr Pharm Des 2015;21:4752–62. [DOI] [PubMed] [Google Scholar]
- 57.Hashimoto S, Brinke AT, Roldaan AC, van Veen IH, Moller GM, Sont JK, et al. Internet-based tapering of oral corticosteroids in severe asthma: a pragmatic randomised controlled trial. Thorax 2011;66:514–20. [DOI] [PubMed] [Google Scholar]
- 58.Beerthuizen T, Voorend-van Bergen S, van den Hout WB, Vaessen-Verberne AA, Brackel HJ, Landstra AM, et al. Cost-effectiveness of FENO-based and web based monitoring in paediatric asthma management: a randomised controlled trial. Thorax 2016;71:607–13. [DOI] [PubMed] [Google Scholar]
- 59.Berg J, Lindgren P. Economic evaluation of FE(NO) measurement in diagnosis and 1-year management of asthma in Germany. Respir Med 2008;102:219–31. [DOI] [PubMed] [Google Scholar]
- 60.LaForce C, Brooks E, Herje N, Dorinsky P, Rickard K. Impact of exhaled nitric oxide measurements on treatment decisions in an asthma specialty clinic. Ann Allergy Asthma Immunol 2014;113:619–23. [DOI] [PubMed] [Google Scholar]
- 61.Sabatelli L, Seppala U, Sastre J, Crater G. Cost-effectiveness and budget impact of routine use of fractional exhaled nitric oxide monitoring for the management of adult asthma patients in Spain. J Investig Allergol Clin Immunol 2017;27:89–97. [DOI] [PubMed] [Google Scholar]
- 62.Ko FW, Hui DS, Leung TF, Chu HY, Wong GW, Tung AH, et al. Evaluation of the asthma control test: a reliable determinant of disease stability and a predictor of future exacerbations. Respirology 2012;17:370–8. [DOI] [PubMed] [Google Scholar]
- 63.Michils A, Louis R, Peche R, Baldassarre S, Van Muylem A. Exhaled nitric oxide as a marker of asthma control in smoking patients. Eur Respir J 2009;33: 1295–301. [DOI] [PubMed] [Google Scholar]
- 64.Quaedvlieg V, Sele J, Henket M, Louis R. Association between asthma control and bronchial hyperresponsiveness and airways inflammation: a cross-sectional study in daily practice. Clin Exp Allergy 2009;39:1822–9. [DOI] [PubMed] [Google Scholar]
- 65.Zeiger RS, Schatz M, Zhang F, Crawford WW, Kaplan MS, Roth RM, et al. Association of exhaled nitric oxide to asthma burden in asthmatics on inhaled corticosteroids. J Asthma 2011;48:8–17. [DOI] [PubMed] [Google Scholar]
- 66.Harkins MS, Fiato KL, Iwamoto GK. Exhaled nitric oxide predicts asthma exacerbation. J Asthma 2004;41:471–6. [DOI] [PubMed] [Google Scholar]
- 67.Menzies D, Jackson C, Mistry C, Houston R, Lipworth BJ. Symptoms, spirometry, exhaled nitric oxide, and asthma exacerbations in clinical practice. Ann Allergy Asthma Immunol 2008;101:248–55. [DOI] [PubMed] [Google Scholar]
- 68.Meyts I, Proesmans M, De Boeck K. Exhaled nitric oxide corresponds with office evaluation of asthma control. Pediatr Pulmonol 2003;36:283–9. [DOI] [PubMed] [Google Scholar]
- 69.Warke TJ, Mairs V, Fitch PS, McGovern V, Ennis M, Shields MD. Exhaled nitric oxide in relation to the clinical features of childhood asthma. J Asthma 2004;41: 751–7. [DOI] [PubMed] [Google Scholar]
- 70.de Bot CM, Moed H, Bindels PJ, van Wijk RG, Berger MY, de Groot H, et al. Exhaled nitric oxide measures allergy not symptoms in children with allergic rhinitis in primary care: a prospective cross-sectional and longitudinal cohort study. Prim Care Respir J 2013;22:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Visitsunthorn N, Mahawichit N, Maneechotesuwan K. Association between levels of fractional exhaled nitric oxide and asthma exacerbations in Thai children. Respirology 2017;22:71–7. [DOI] [PubMed] [Google Scholar]
- 72.van Vliet D, Alonso A, Rijkers G, Heynens J, Rosias P, Muris J, et al. Prediction of asthma exacerbations in children by innovative exhaled inflammatory markers: results of a longitudinal study. PLoS One 2015;10:e0119434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McCormack MC, Aloe C, Curtin-Brosnan J, Diette GB, Breysse PN, Matsui EC. Guideline-recommended fractional exhaled nitric oxide is a poor predictor of health-care use among inner-city children and adolescents receiving usual asthma care. Chest 2013;144:923–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Raj D, Lodha R, Mukherjee A, Sethi T, Agrawal A, Kabra SK. Fractional exhaled nitric oxide in children with acute exacerbation of asthma. Indian Pediatr 2014; 51:105–11. [DOI] [PubMed] [Google Scholar]
- 75.Salmeron S, Liard R, Elkharrat D, Muir J, Neukirch F, Ellrodt A. Asthma severity and adequacy of management in accident and emergency departments in France: a prospective study. Lancet 2001;358:629–35. [DOI] [PubMed] [Google Scholar]
- 76.Kwok MY, Walsh-Kelly CM, Gorelick MH. The role of exhaled nitric oxide in evaluation of acute asthma in a pediatric emergency department. Acad Emerg Med 2009;16:21–8. [DOI] [PubMed] [Google Scholar]
- 77.Gill M, Walker S, Khan A, Green SM, Kim L, Gray S, et al. Exhaled nitric oxide levels during acute asthma exacerbation. Acad Emerg Med 2005;12:579–86. [DOI] [PubMed] [Google Scholar]
- 78.Balinotti JE, Colom A, Kofman C, Teper A. Association between the Asthma Predictive Index and levels of exhaled nitric oxide in infants and toddlers with recurrent wheezing. Arch Argent Pediatr 2013;111:191–5. [DOI] [PubMed] [Google Scholar]
- 79.Bloemen K, Van Den Heuvel R, Govarts E, Hooyberghs J, Nelen V, Witters E, et al. A new approach to study exhaled proteins as potential biomarkers for asthma. Clin Exp Allergy 2011;41:346–56. [DOI] [PubMed] [Google Scholar]
- 80.Castro-Rodríguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med 2000;162:1403–6. [DOI] [PubMed] [Google Scholar]
- 81.Caudri D, Wijga AH, Hoekstra MO, Kerkhof M, Koppelman GH, Brunekreef B, et al. Prediction of asthma in symptomatic preschool children using exhaled nitric oxide, Rint and specific IgE. Thorax 2010;65:801–7. [DOI] [PubMed] [Google Scholar]
- 82.Chang D, Yao W, Tiller CJ, Kisling J, Slaven JE, Yu Z, et al. Exhaled nitric oxide during infancy as a risk factor for asthma and airway hyperreactivity. Eur Respir J 2015;45:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Elliott M, Heltshe SL, Stamey DC, Cochrane ES, Redding GJ, Debley JS. Exhaled nitric oxide predicts persistence of wheezing, exacerbations, and decline in lung function in wheezy infants and toddlers. Clin Exp Allergy 2013;43:1351–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Klaassen EM, van de Kant KD, Jobsis Q, Hovig ST, van Schayck CP, Rijkers GT, et al. Symptoms, but not a biomarker response to inhaled corticosteroids, predict asthma in preschool children with recurrent wheeze. Mediators Inflamm 2012; 2012:162571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Prado CM, Martins MA, Tiberio IFLC. Nitric oxide in asthma physiopathology. ISRN Allergy 2011;2011:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singer F, Luchsinger I, Inci D, Knauer N, Latzin P, Wildhaber JH, et al. Exhaled nitric oxide in symptomatic children at preschool age predicts later asthma. Allergy 2013;68:531–8. [DOI] [PubMed] [Google Scholar]
- 87.van Wonderen KE, van der Mark LB, Mohrs J, Geskus RB, van der Wal WM, van Aalderen WM, et al. Prediction and treatment of asthma in preschool children at risk: study design and baseline data of a prospective cohort study in general practice (ARCADE). BMC Pulm Med 2009;9:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Taussig LM, Wright AL, Holberg CJ, Halonen M, Morgan WJ, Martinez FD. Tucson Children’s Respiratory Study: 1980 to present. J Allergy Clin Immunol 2003;111:661–75; quiz 76. [DOI] [PubMed] [Google Scholar]
- 89.Tenero L, Piazza M, Piacentini G. Recurrent wheezing in children. Transl Pediatr 2016;5:31–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cho HJ, Jung YH, Yang SI, Lee E, Kim HY, Seo JH, et al. Reference values and determinants of fractional concentration of exhaled nitric oxide in healthy children. Allergy Asthma Immunol Res 2014;6:169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bahir A, Goldberg A, Mekori YA, Confino-Cohen R, Morag H, Rosen Y, et al. Continuous avoidance measures with or without acaricide in dust mite-allergic asthmatic children. Ann Allergy Asthma Immunol 1997;78: 506–12. [DOI] [PubMed] [Google Scholar]
- 92.Geller-Bernstein C, Pibourdin JM, Dornelas A, Fondarai J. Efficacy of the acaricide: acardust for the prevention of asthma and rhinitis due to dust mite allergy, in children. Allerg Immunol (Paris) 1995;27:147–54. [PubMed] [Google Scholar]
- 93.Francis H, Fletcher G, Anthony C, Pickering C, Oldham L, Hadley E, et al. Clinical effects of air filters in homes of asthmatic adults sensitized and exposed to pet allergens. Clin Exp Allergy 2003;33:101–5. [DOI] [PubMed] [Google Scholar]
- 94.Pedroletti C, Millinger E, Dahlen B, Soderman P, Zetterstrom O. Clinical effects of purified air administered to the breathing zone in allergic asthma: a double-blind randomized cross-over trial. Respir Med 2009;103:1313–9. [DOI] [PubMed] [Google Scholar]
- 95.Warner JA, Marchant JL, Warner JO. Double blind trial of ionisers in children with asthma sensitive to the house dust mite. Thorax 1993;48:330–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wright GR, Howieson S, McSharry C, McMahon AD, Chaudhuri R, Thompson J, et al. Effect of improved home ventilation on asthma control and house dust mite allergen levels. Allergy 2009;64:1671–80. [DOI] [PubMed] [Google Scholar]
- 97.Barnes CS, Kennedy K, Gard L, Forrest E, Johnson L, Pacheco F, et al. The impact of home cleaning on quality of life for homes with asthmatic children. Allergy Asthma Proc 2008;29:197–204. [DOI] [PubMed] [Google Scholar]
- 98.de Vries MP, van den Bemt L, Aretz K, Thoonen BP, Muris JW, Kester AD, et al. House dust mite allergen avoidance and self-management in allergic patients with asthma: randomised controlled trial. Br J Gen Pract 2007;57:184–90. [PMC free article] [PubMed] [Google Scholar]
- 99.Dharmage S, Walters EH, Thien F, Bailey M, Raven J, Wharton C, et al. Encasement of bedding does not improve asthma in atopic adult asthmatics. Int Arch Allergy Immunol 2006;139:132–8. [DOI] [PubMed] [Google Scholar]
- 100.Frederick JM, Warner JO, Jessop WJ, Enander I, Warner JA. Effect of a bed covering system in children with asthma and house dust mite hypersensitivity. Eur Respir J 1997;10:361–6. [DOI] [PubMed] [Google Scholar]
- 101.Halken S, Host A, Niklassen U, Hansen LG, Nielsen F, Pedersen S, et al. Effect of mattress and pillow encasings on children with asthma and house dust mite allergy. J Allergy Clin Immunol 2003;111:169–76. [DOI] [PubMed] [Google Scholar]
- 102.Lee IS. Effect of bedding control on amount of house dust mite allergens, asthma symptoms, and peak expiratory flow rate. Yonsei Med J 2003;44:313–22. [DOI] [PubMed] [Google Scholar]
- 103.Luczynska C, Tredwell E, Smeeton N, Burney P. A randomized controlled trial of mite allergen-impermeable bed covers in adult mite-sensitized asthmatics. Clin Exp Allergy 2003;33:1648–53. [DOI] [PubMed] [Google Scholar]
- 104.Murray CS, Foden P, Sumner H, Shepley E, Custovic A, Simpson A. Preventing severe asthma exacerbations in children: a randomized trial of mite-impermeable bedcovers. Am J Respir Crit Care Med 2017;196:150–8. [DOI] [PubMed] [Google Scholar]
- 105.Nambu M, Shirai H, Sakaguchi M, Aihara M, Takatori K. Effect of house dust mite-free pillow on clinical course of asthma and IgE level – a randomized, double-blind, controlled study. Pediatr Asthma Allergy Immunol 2008;21:137–44. [Google Scholar]
- 106.Rijssenbeek-Nouwens LH, Oosting AJ, de Bruin-Weller MS, Bregman I, de Monchy JG, Postma DS. Clinical evaluation of the effect of anti-allergic mattress covers in patients with moderate to severe asthma and house dust mite allergy: a randomised double blind placebo controlled study. Thorax 2002;57:784–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sheikh A, Hurwitz B, Sibbald B, Barnes G, Howe M, Durham S. House dust mite barrier bedding for childhood asthma: randomised placebo controlled trial in primary care [ISRCTN63308372]. BMC Fam Pract 2002;3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tsurikisawa N, Saito A, Oshikata C, Nakazawa T, Yasueda H, Akiyama K. Encasing bedding in covers made of microfine fibers reduces exposure to house mite allergens and improves disease management in adult atopic asthmatics. Allergy Asthma Clin Immunol 2013;9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Woodcock A, Forster L, Matthews E, Martin J, Letley L, Vickers M, et al. Control of exposure to mite allergen and allergen-impermeable bed covers for adults with asthma. N Engl J Med 2003;349:225–36. [DOI] [PubMed] [Google Scholar]
- 110.Shirai T, Matsui T, Suzuki K, Chida K. Effect of pet removal on pet allergic asthma. Chest 2005;127:1565–71. [DOI] [PubMed] [Google Scholar]
- 111.Levy JI, Brugge D, Peters JL, Clougherty JE, Saddler SS. A community-based participatory research study of multifaceted inhome environmental interventions for pediatric asthmatics in public housing. Soc Sci Med 2006;63:2191–203. [DOI] [PubMed] [Google Scholar]
- 112.Rabito FA, Carlson JC, He H, Werthmann D, Schal C. A single intervention for cockroach control reduces cockroach exposure and asthma morbidity in children. J Allergy Clin Immunol 2017;140:565–70. [DOI] [PubMed] [Google Scholar]
- 113.Carswell F, Birmingham K, Oliver J, Crewes A, Weeks J. The respiratory effects of reduction of mite allergen in the bedrooms of asthmatic children–a double-blind controlled trial. Clin Exp Allergy 1996;26:386–96. [PubMed] [Google Scholar]
- 114.Cloosterman SG, Schermer TR, Bijl-Hofland ID, Van Der Heide S, Brunekreef B, Van Den Elshout FJ, et al. Effects of house dust mite avoidance measures on Der p 1 concentrations and clinical condition of mild adult house dust mite-allergic asthmatic patients, using no inhaled steroids. Clin Exp Allergy 1999; 29:1336–46. [DOI] [PubMed] [Google Scholar]
- 115.El-Ghitany EM, Abd El-Salam MM. Environmental intervention for house dust mite control in childhood bronchial asthma. Environ Health Prev Med 2012;17: 377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Marks GB, Tovey ER, Green W, Shearer M, Salome CM, Woolcock AJ. House dust mite allergen avoidance: a randomized controlled trial of surface chemical treatment and encasement of bedding. Clin Exp Allergy 1994;24:1078–83. [DOI] [PubMed] [Google Scholar]
- 117.Shapiro GG, Wighton TG, Chinn T, Zuckrman J, Eliassen AH, Picciano JF, et al. House dust mite avoidance for children with asthma in homes of low-income families. J Allergy Clin Immunol 1999;103:1069–74. [DOI] [PubMed] [Google Scholar]
- 118.DiMango E, Serebrisky D, Narula S, Shim C, Keating C, Sheares B, et al. Individualized household allergen intervention lowers allergen level but not asthma medication use: a randomized controlled trial. J Allergy Clin Immunol Pract 2016;4:671–9.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Krieger J, Takaro TK, Song L, Beaudet N, Edwards K. A randomized controlled trial of asthma self-management support comparing clinic-based nurses and inhome community health workers: the Seattle-King County Healthy Homes II Project. Arch Pediatr Adolesc Med 2009;163:141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Krieger JW, Takaro TK, Song L, Weaver M. The Seattle-King County Healthy Homes Project: a randomized, controlled trial of a community health worker intervention to decrease exposure to indoor asthma triggers. Am J Public Health 2005;95:652–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Morgan WJ, Crain EF, Gruchalla RS, O’Connor GT, Kattan M, Evans R III, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med 2004;351:1068–80. [DOI] [PubMed] [Google Scholar]
- 122.Parker EA, Israel BA, Robins TG, Mentz G, Xihong L, Brakefield-Caldwell W, et al. Evaluation of Community Action Against Asthma: a community health worker intervention to improve children’s asthma-related health by reducing household environmental triggers for asthma. Health Educ Behav 2008;35: 376–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Warner JA, Frederick JM, Bryant TN, Weich C, Raw GJ, Hunter C, et al. Mechanical ventilation and high-efficiency vacuum cleaning: a combined strategy of mite and mite allergen reduction in the control of mite-sensitive asthma. J Allergy Clin Immunol 2000;105:75–82. [DOI] [PubMed] [Google Scholar]
- 124.Eggleston PA, Butz A, Rand C, Curtin-Brosnan J, Kanchanaraksa S, Swartz L, et al. Home environmental intervention in inner-city asthma: a randomized controlled clinical trial. Ann Allergy Asthma Immunol 2005;95:518–24. [DOI] [PubMed] [Google Scholar]
- 125.Matsui EC, Perzanowski M, Peng RD, Wise RA, Balcer-Whaley S, Newman M, et al. Effect of an integrated pest management intervention on asthma symptoms among mouse-sensitized children and adolescents with asthma: a randomized clinical trial. JAMA 2017;317:1027–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Evans R III, Gergen PJ, Mitchell H, Kattan M, Kercsmar C, Crain E, et al. A randomized clinical trial to reduce asthma morbidity among inner-city children: results of the National Cooperative Inner-City Asthma Study. J Pediatr 1999; 135:332–8. [DOI] [PubMed] [Google Scholar]
- 127.Bryant-Stephens T, Kurian C, Guo R, Zhao H. Impact of a household environmental intervention delivered by lay health workers on asthma symptom control in urban, disadvantaged children with asthma. Am J Public Health 2009;99: S657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bryant-Stephens T, Li Y. Outcomes of a home-based environmental remediation for urban children with asthma. J Natl Med Assoc 2008;100:306–16. [DOI] [PubMed] [Google Scholar]
- 129.Burr ML, Matthews IP, Arthur RA, Watson HL, Gregory CJ, Dunstan FD, et al. Effects on patients with asthma of eradicating visible indoor mould: a randomised controlled trial. Thorax 2007;62:767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Williams SG, Brown CM, Falter KH, Alverson CJ, Gotway-Crawford C, Homa D, et al. Does a multifaceted environmental intervention alter the impact of asthma on inner-city children? J Natl Med Assoc 2006;98:249–60. [PMC free article] [PubMed] [Google Scholar]
- 131.Kercsmar CM, Dearborn DG, Schluchter M, Xue L, Kirchner HL, Sobolewski J, et al. Reduction in asthma morbidity in children as a result of home remediation aimed at moisture sources. Environ Health Perspect 2006;114:1574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.National Asthma Education and Prevention Program. Third Expert Panel on theDiagnosis and Management of Asthma. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, Md: National Heart, Lung, and Blood Institute; Aug 2007:213, Available at: https://www.ncbi.nlm.nih.gov/books/NBK7232/. [Google Scholar]
- 133.Bacharier LB, Phillips BR, Zeiger RS, Szefler SJ, Martinez FD, Lemanske RF Jr, et al. Episodic use of an inhaled corticosteroid or leukotriene receptor antagonist in preschool children with moderate-to-severe intermittent wheezing. J Allergy Clin Immunol 2008;122:1127–35.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zeiger RS, Mauger D, Bacharier LB, Guilbert TW, Martinez FD, Lemanske RF Jr, et al. Daily or intermittent budesonide in preschool children with recurrent wheezing. N Engl J Med 2011;365:1990–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ducharme FM, Lemire C, Noya FJ, Davis GM, Alos N, Leblond H, et al. Preemptive use of high-dose fluticasone for virus-induced wheezing in young children. N Engl J Med 2009;360:339–53. [DOI] [PubMed] [Google Scholar]
- 136.Svedmyr J, Nyberg E, Thunqvist P, Asbrink-Nilsson E, Hedlin G. Prophylactic intermittent treatment with inhaled corticosteroids of asthma exacerbations due to airway infections in toddlers. Acta Paediatr 1999;88:42–7. [DOI] [PubMed] [Google Scholar]
- 137.Camargos P, Affonso A, Calazans G, Ramalho L, Ribeiro ML, Jentzsch N, et al. On-demand intermittent beclomethasone is effective for mild asthma in Brazil. Clin Transl Allergy 2018;8:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Papi A, Canonica GW, Maestrelli P, Paggiaro P, Olivieri D, Pozzi E, et al. Rescue use of beclomethasone and albuterol in a single inhaler for mild asthma. N Engl J Med 2007;356:2040–52. [DOI] [PubMed] [Google Scholar]
- 139.Boushey HA, Sorkness CA, King TS, Sullivan SD, Fahy JV, Lazarus SC, et al. Daily versus as-needed corticosteroids for mild persistent asthma. N Engl J Med 2005;352:1519–28. [DOI] [PubMed] [Google Scholar]
- 140.Martinez FD, Chinchilli VM, Morgan WJ, Boehmer SJ, Lemanske RF Jr, Mauger DT, et al. Use of beclomethasone dipropionate as rescue treatment for children with mild persistent asthma (TREXA): a randomised, double-blind, placebo controlled trial. Lancet 2011;377:650–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Turpeinen M, Nikander K, Pelkonen AS, Syvanen P, Sorva R, Raitio H, et al. Daily versus as-needed inhaled corticosteroid for mild persistent asthma (The Helsinki early intervention childhood asthma study). Arch Dis Child 2008;93: 654–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jackson DJ, Bacharier LB, Mauger DT, Boehmer S, Beigelman A, Chmiel JF, et al. Quintupling inhaled glucocorticoids to prevent childhood asthma exacerbations. N Engl J Med 2018;378:891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McKeever T, Mortimer K, Wilson A, Walker S, Brightling C, Skeggs A, et al. Quadrupling inhaled glucocorticoid dose to abort asthma exacerbations. N Engl J Med 2018;378:902–10. [DOI] [PubMed] [Google Scholar]
- 144.Harrison TW, Oborne J, Newton S, Tatters field AE. Doubling the dose of inhaled corticosteroid to prevent asthma exacerbations: randomised controlled trial. Lancet 2004;363:271–5. [DOI] [PubMed] [Google Scholar]
- 145.Lahdensuo A, Haahtela T, Herrala J, Kava T, Kiviranta K, Kuusisto P, et al. Randomised comparison of guided self management and traditional treatment of asthma over one year. BMJ 1996;312:748–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Oborne J, Mortimer K, Hubbard RB, Tatters field AE, Harrison TW. Quadrupling the dose of inhaled corticosteroid to prevent asthma exacerbations: a randomized, double-blind, placebo-controlled, parallel-group clinical trial. Am J Respir Crit Care Med 2009;180:598–602. [DOI] [PubMed] [Google Scholar]
- 147.Peters M. Single-inhaler combination therapy for maintenance and relief of asthma: a new strategy in disease management. Drugs 2009;69:137–50. [DOI] [PubMed] [Google Scholar]
- 148.O’Byrne PM, Bisgaard H, Godard PP, Pistolesi M, Palmqvist M, Zhu Y, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. Am J Respir Crit Care Med 2005;171:129–36. [DOI] [PubMed] [Google Scholar]
- 149.Rabe KF, Pizzichini E, Stallberg B, Romero S, Balanzat AM, Atienza T, et al. Bu desonide/formoterol in a single inhaler for maintenance and relief in mild-to-moderate asthma: a randomized, double-blind trial. Chest 2006;129:246–56. [DOI] [PubMed] [Google Scholar]
- 150.Scicchitano R, Aalbers R, Ukena D, Manjra A, Fouquert L, Centanni S, et al. Efficacy and safety of budesonide/formoterol single inhaler therapy versus a higher dose of budesonide in moderate to severe asthma. Curr Med Res Opin 2004;20: 1403–18. [DOI] [PubMed] [Google Scholar]
- 151.Jenkins CR, Eriksson G, Bateman ED, Reddel HK, Sears MR, Lindberg M, et al. Efficacy of budesonide/formoterol maintenance and reliever therapy compared with higher-dose budesonide as step-up from low-dose inhaled corticosteroid treatment. BMC Pulm Med 2017;17:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bisgaard H, Le Roux P, Bjamer D, Dymek A, Vermeulen JH, Hultquist C. Budesonide/formoterol maintenance plus reliever therapy: a new strategy in pediatric asthma. Chest 2006;130:1733–43. [DOI] [PubMed] [Google Scholar]
- 153.Atienza T, Aquino T, Fernandez M, Boonsawat W, Kawai M, Kudo T, et al. Budesonide/formoterol maintenance and reliever therapy via Turbuhaler versus fixed-dose budesonide/formoterol plus terbutaline in patients with asthma: phase III study results. Respirology 2013;18:354–63. [DOI] [PubMed] [Google Scholar]
- 154.Papi A, Corradi M, Pigeon-Francisco C, Baronio R, Siergiejko Z, Petruzzelli S, et al. Beclometasone-formoterol as maintenance and reliever treatment in patients with asthma: a double-blind, randomised controlled trial. Lancet Respir Med 2013;1:23–31. [DOI] [PubMed] [Google Scholar]
- 155.Rabe KF, Atienza T, Magyar P, Larsson P, Jorup C, Lalloo UG. Effect of budesonide in combination with formoterol for reliever therapy in asthma exacerbations: a randomised controlled, double-blind study. Lancet 2006;368:744–53. [DOI] [PubMed] [Google Scholar]
- 156.Patel M, Pilcher J, Pritchard A, Perrin K, Travers J, Shaw D, et al. Efficacy and safety of maintenance and reliever combination budesonide-formoterol inhaler in patients with asthma at risk of severe exacerbations: a randomised controlled trial. Lancet Respir Med 2013;1:32–42. [DOI] [PubMed] [Google Scholar]
- 157.Vogelmeier C, D’Urzo A, Pauwels R, Merino JM, Jaspal M, Boutet S, et al. Budesonide/formoterol maintenance and reliever therapy: an effective asthma treatment option? Eur Respir J 2005;26:819–28. [DOI] [PubMed] [Google Scholar]
- 158.Vogelmeier C, Naya I, Ekelund J. Budesonide/formoterol maintenance and reliever therapy in Asian patients (aged >/516 years) with asthma: a sub-analysis of the COSMOS study. Clin Drug Investig 2012;32:439–49. [DOI] [PubMed] [Google Scholar]
- 159.Bousquet J, Boulet LP, Peters MJ, Magnussen H, Quiralte J, Martinez-Aguilar NE, et al. Budesonide/formoterol for maintenance and relief in uncontrolled asthma vs. high-dose salmeterol/fluticasone. Respir Med 2007;101:2437–46. [DOI] [PubMed] [Google Scholar]
- 160.Kuna P, Peters MJ, Manjra AI, Jorup C, Naya IP, Martinez-Jimenez NE, et al. Effect of budesonide/formoterol maintenance and reliever therapy on asthma exacerbations. Int J Clin Pract 2007;61:725–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Louis R, Joos G, Michils A, Vandenhoven G. A comparison of budesonide/formoterol maintenance and reliever therapy vs. conventional best practice in asthma management. Int J Clin Pract 2009;63:1479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Quirce S, Barcina C, Plaza V, Calvo E, Munoz M, Ampudia R, et al. A comparison of budesonide/formoterol maintenance and reliever therapy versus conventional best practice in asthma management in Spain. J Asthma 2011; 48:839–47. [DOI] [PubMed] [Google Scholar]
- 163.Riemersma RA, Postma D, van der Molen T. Budesonide/formoterol maintenance and reliever therapy in primary care asthma management: effects on bronchial hyperresponsiveness and asthma control. Prim Care Respir J 2012;21:50–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sears MR, Boulet LP, Laviolette M, Fitzgerald JM, Bai TR, Kaplan A, et al. Budesonide/formoterol maintenance and reliever therapy: impact on airway inflammation in asthma. Eur Respir J 2008;31:982–9. [DOI] [PubMed] [Google Scholar]
- 165.Mensah GA, Kiley JP, Gibbons GH. Generating evidence to inform an update of asthma clinical practice guidelines: perspectives from the National Heart, Lung, and Blood Institute. J Allergy Clin Immunol 2018;142:744–8. [DOI] [PubMed] [Google Scholar]
- 166.Lee LA, Yang S, Kerwin E, Trivedi R, Edwards LD, Pascoe S. The effect of fluticasone furoate/umeclidinium in adult patients with asthma: a randomized, doseranging study. Respir Med 2015;109:54–62. [DOI] [PubMed] [Google Scholar]
- 167.Wechsler ME, Yawn BP, Fuhlbrigge AL, Pace WD, Pencina MJ, Doros G, et al. Anticholinergic vs long-acting beta-agonist in combination with inhaled corticosteroids in black adults with asthma: the BELT randomized clinical trial. JAMA 2015;314:1720–30. [DOI] [PubMed] [Google Scholar]
- 168.Peters SP, Kunselman SJ, Icitovic N, Moore WC, Pascual R, Ameredes BT, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med 2010;363:1715–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bateman ED, Kornmann O, Schmidt P, Pivovarova A, Engel M, Fabbri LM. Tiotropium is noninferior to salmeterol in maintaining improved lung function in B16-Arg/Arg patients with asthma. J Allergy Clin Immunol 2011;128:315–22. [DOI] [PubMed] [Google Scholar]
- 170.Kerstjens HA, Casale TB, Bleecker ER, Meltzer EO, Pizzichini E, Schmidt O, et al. Tiotropium or salmeterol as add-on therapy to inhaled corticosteroids for patients with moderate symptomatic asthma: two replicate, double-blind, placebo-controlled, parallel-group, active-comparator, randomised trials. Lancet Respir Med 2015;3:367–76. [DOI] [PubMed] [Google Scholar]
- 171.Rajanandh MG, Nageswari AD, Ilango K. Pulmonary function assessment in mild to moderate persistent asthma patients receiving montelukast, doxofylline, and tiotropium with budesonide: a randomized controlled study. Clin Ther 2014;36: 526–33. [DOI] [PubMed] [Google Scholar]
- 172.Rajanandh MG, Nageswari AD, Ilango K. Assessment of montelukast, doxofylline, and tiotropium with budesonide for the treatment of asthma: which is the best among the second-line treatment? A randomized trial. Clin Ther 2015;37: 418–26. [DOI] [PubMed] [Google Scholar]
- 173.Busse WW, Bateman ED, Caplan AL, Kelly HW, O’Byrne PM, Rabe KF, et al. Combined analysis of asthma safety trials of long-acting beta2-agonists. N Engl J Med 2018;378:2497–505. [DOI] [PubMed] [Google Scholar]
- 174.Paggiaro P, Halpin DM, Buhl R, Engel M, Zubek VB, Blahova Z, et al. The effect of tiotropium in symptomatic asthma despite low- to medium-dose inhaled corticosteroids: a randomized controlled trial. J Allergy Clin Immunol Pract 2016;4: 104–13.e2. [DOI] [PubMed] [Google Scholar]
- 175.Hamelmann E, Bateman ED, Vogelberg C, Szefler SJ, Vandewalker M, Moroni-Zentgraf P, et al. Tiotropium add-on therapy in adolescents with moderate asthma: a 1-year randomized controlled trial. J Allergy Clin Immunol 2016;138: 441–50.e8. [DOI] [PubMed] [Google Scholar]
- 176.Ohta K, Ichinose M, Tohda Y, Engel M, Moroni-Zentgraf P, Kunimitsu S, et al. Long-term once-daily tiotropium respimat(R) is well tolerated and maintains efficacy over 52 weeks in patients with symptomatic asthma in Japan: a randomised, placebo-controlled study. PLoS One 2015;10:e0124109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kerstjens HA, Engel M, Dahl R, Paggiaro P, Beck E, Vandewalker M, et al. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med 2012;367:1198–207. [DOI] [PubMed] [Google Scholar]
- 178.Hamelmann E, Bernstein JA, Vandewalker M, Moroni-Zentgraf P, Verri D, Unseld A, et al. A randomised controlled trial of tiotropium in adolescents with severe symptomatic asthma. Eur Respir J 2017;49:1601100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang K, Tian P, Fan Y, Wang Y, Liu C. Assessment of second-line treatments for patients with uncontrolled moderate asthma. Int J Clin Exp Med 2015;8: 19476–80. [PMC free article] [PubMed] [Google Scholar]
- 180.Nelson HS. Injection immunotherapy for inhalant allergens. In: Adkinson NF Jr, Bochner BS, Burks AW, Busse WW, Holgate ST, Lemanske RF Jr, et al. , eds. Middleton’s allergy principles and practice. Philadelphia: Elsevier; 2014. pp. 1416–37. [Google Scholar]
- 181.O’Hehir RE, Sandrini A, Frew AJ. Sublingual immunotherapy for inhalant allergens. In: Adkinson NF Jr, Bochner BS, Burks AW, Busse WW, Holgate ST, Lemanske RF Jr, et al. , eds. Middleton’s allergy principles and practice. Philadelphia: Elsevier; 2014. pp. 1438–46. [Google Scholar]
- 182.Johnstone DE, Dutton A. The value of hyposensitization therapy for bronchial asthma in children–a 14-year study. Pediatrics 1968;42:793–802. [PubMed] [Google Scholar]
- 183.Epstein TG, Liss GM, Berendts KM, Bernstein DI. AAAAI/ACAAI Subcutaneous Immunotherapy Surveillance Study (2013–2017): fatalities, infections, delayed reactions, and use of epinephrine autoinjectors. J Allergy Clin Immunol Pract 2019;7:1996–2003.e1. [DOI] [PubMed] [Google Scholar]
- 184.Adkinson NF Jr, Eggleston PA, Eney D, Goldstein EO, Schuberth KC, Bacon JR, et al. A controlled trial of immunotherapy for asthma in allergic children. N Engl J Med 1997;336:324–31. [DOI] [PubMed] [Google Scholar]
- 185.Altintas D, Akmanlar N, Guneser S, Burgut R, Yilmaz M, Bugdayci R, et al. Comparison between the use of adsorbed and aqueous immunotherapy material in Dermatophagoides pteronyssinus sensitive asthmatic children. Allergol Immunopathol (Madr) 1999;27:309–17. [PubMed] [Google Scholar]
- 186.Alvarez-Cuesta E, Cuesta-Herranz J, Puyana-Ruiz J, Cuesta-Herranz C, BlancoQuiros A. Monoclonal antibody-standardized cat extract immunotherapy: risk benefit effects from a double-blind placebo study. J Allergy Clin Immunol 1994;93:556–66. [DOI] [PubMed] [Google Scholar]
- 187.Ameal A, Vega-Chicote JM, Fernandez S, Miranda A, Carmona MJ, Rondon MC, et al. Double-blind and placebo-controlled study to assess efficacy and safety of a modified allergen extract of Dermatophagoides pteronyssinus in allergic asthma. Allergy 2005;60:1178–83. [DOI] [PubMed] [Google Scholar]
- 188.Arvidsson MB, Lowhagen O, Rak S. Allergen specific immunotherapy attenuates early and late phase reactions in lower airways of birch pollen asthmatic patients: a double blind placebo-controlled study. Allergy 2004;59:74–80. [DOI] [PubMed] [Google Scholar]
- 189.Basomba A, Tabar AI, de Rojas DH, Garcia BE, Alamar R, Olaguibel JM, et al. Allergen vaccination with a liposome-encapsulated extract of Dermatophagoides pteronyssinus: a randomized, double-blind, placebo-controlled trial in asthmatic patients. J Allergy Clin Immunol 2002;109:943–8. [DOI] [PubMed] [Google Scholar]
- 190.Blumberga G, Groes L, Haugaard L, Dahl R. Steroid-sparing effect of subcutaneous SQ-standardised specific immunotherapy in moderate and severe house dust mite allergic asthmatics. Allergy 2006;61:843–8. [DOI] [PubMed] [Google Scholar]
- 191.Bousquet J, Hejjaoui A, Soussana M, Michel FB. Double-blind, placebo controlled immunotherapy with mixed grass-pollen allergoids, IV: comparison of the safety and efficacy of two dosages of a high-molecular-weight allergoid. J Allergy Clin Immunol 1990;85:490–7. [DOI] [PubMed] [Google Scholar]
- 192.Bousquet J, Maasch HJ, Hejjaoui A, Skassa-Brociek W, Wahl R, Dhivert H, et al. Double-blind, placebo-controlled immunotherapy with mixed grass-pollen allergoids, III: efficacy and safety of unfractionated and high-molecular-weight preparations in rhinoconjunctivitis and asthma. J Allergy Clin Immunol 1989;84: 546–56. [DOI] [PubMed] [Google Scholar]
- 193.Bruce CA, Norman PS, Rosenthal RR, Lichtenstein LM. The role of ragweed pollen in autumnal asthma. J Allergy Clin Immunol 1977;59:449–59. [DOI] [PubMed] [Google Scholar]
- 194.Chakraborty P, Roy I, Chatterjee S, Chanda S, Gupta-Bharracharya S. Phoenix sylvestris Roxb pollen allergy: a 2-year randomized controlled trial and follow-up study of immunotherapy in patients with seasonal allergy in an agricultural area of West Bengal, India. J Investig Allergol Clin Immunol 2006;16:377–84. [PubMed] [Google Scholar]
- 195.Creticos PS, Reed CE, Norman PS, Khoury J, Adkinson NF Jr, Buncher CR, et al. Ragweed immunotherapy in adult asthma. N Engl J Med 1996;334:501–6. [DOI] [PubMed] [Google Scholar]
- 196.Dolz I, Martinez-Cocera C, Bartolome JM, Cimarra M. A double-blind, placebo controlled study of immunotherapy with grass-pollen extract Alutard SQ during a 3-year period with initial rush immunotherapy. Allergy 1996;51:489–500. [DOI] [PubMed] [Google Scholar]
- 197.Dreborg S, Agrell B, Foucard T, Kjellman NI, Koivikko A, Nilsson S. A double blind, multicenter immunotherapy trial in children, using a purified and standardized Cladosporium herbarum preparation, I: clinical results. Allergy 1986;41: 131–40. [DOI] [PubMed] [Google Scholar]
- 198.Franco C, Barbadori S, Freshwater LL, Kordash TR. A double-blind, placebo controlled study of Alpare mite D. pteronyssinus immunotherapy in asthmatic patients. Allergol Immunopathol (Madr) 1995;23:58–66. [PubMed] [Google Scholar]
- 199.Gallego MT, Iraola V, Himly M, Robinson DS, Badiola C, Garcia-Robaina JC, et al. Depigmented and polymerised house dust mite allergoid: allergen content, induction of IgG4 and clinical response. Int Arch Allergy Immunol 2010;153: 61–9. [DOI] [PubMed] [Google Scholar]
- 200.Garcia-Robaina JC, Sanchez I, de la Torre F, Fernandez-Caldas E, Casanovas M. Successful management of mite-allergic asthma with modified extracts of Dermatophagoides pteronyssinus and Dermatophagoides farinae in a double-blind, placebo-controlled study. J Allergy Clin Immunol 2006;118:1026–32. [DOI] [PubMed] [Google Scholar]
- 201.Hill DJ, Hosking CS, Shelton MJ, Turner MW. Failure of hyposensitisation in treatment of children with grass-pollen asthma. BMJ 1982;284:306–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Horst M, Hejjaoui A, Horst V, Michel FB, Bousquet J. Double-blind, placebo controlled rush immunotherapy with a standardized Alternaria extract. J Allergy Clin Immunol 1990;85:460–72. [DOI] [PubMed] [Google Scholar]
- 203.Hui Y, Li L, Qian J, Guo Y, Zhang X, Zhang X. Efficacy analysis of three-year subcutaneous SQ-standardized specific immunotherapy in house dust mite allergic children with asthma. Exp Ther Med 2014;7:630–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kuna P, Alam R, Kuzminska B, Rozniecki J. The effect of preseasonal immunotherapy on the production of histamine-releasing factor (HRF) by mononuclear cells from patients with seasonal asthma: results of a double-blind, placebo controlled, randomized study. J Allergy Clin Immunol 1989;83:816–24. [DOI] [PubMed] [Google Scholar]
- 205.Kuna P, Kaczmarek J, Kupczyk M. Efficacy and safety of immunotherapy for allergies to Alternaria alternata in children. J Allergy Clin Immunol 2011;127: 502–8.e1–6. [DOI] [PubMed] [Google Scholar]
- 206.Leynadier F, Herman D, Vervloet D, Andre C. Specific immunotherapy with a standardized latex extract versus placebo in allergic healthcare workers. J Allergy Clin Immunol 2000;106:585–90. [DOI] [PubMed] [Google Scholar]
- 207.Machiels JJ, Somville MA, Lebrun PM, Lebecque SJ, Jacquemin MG, Saint Remy JM. Allergic bronchial asthma due to Dermatophagoides pteronyssinus hypersensitivity can be efficiently treated by inoculation of allergen-antibody complexes. J Clin Invest 1990;85:1024–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Maestrelli P, Zanolla L, Pozzan M, Fabbri LM. Effect of specific immunotherapy added to pharmacologic treatment and allergen avoidance in asthmatic patients allergic to house dust mite. J Allergy Clin Immunol 2004;113:643–9. [DOI] [PubMed] [Google Scholar]
- 209.Malling HJ, Dreborg S, Weeke B. Diagnosis and immunotherapy of mould allergy, V: clinical efficacy and side effects of immunotherapy with Cladosporium herbarum. Allergy 1986;41:507–19. [DOI] [PubMed] [Google Scholar]
- 210.Mirone C, Albert F, Tosi A, Mocchetti F, Mosca S, Giorgino M, et al. Efficacy and safety of subcutaneous immunotherapy with a biologically standardized extract of Ambrosia artemisiifolia pollen: a double-blind, placebo-controlled study. Clin Exp Allergy 2004;34:1408–14. [DOI] [PubMed] [Google Scholar]
- 211.Nouri-Aria KT, Pilette C, Jacobson MR, Watanabe H, Durham SR. IL-9 and cKit1 mast cells in allergic rhinitis during seasonal allergen exposure: effect of immunotherapy. J Allergy Clin Immunol 2005;116:73–9. [DOI] [PubMed] [Google Scholar]
- 212.Nouri-Aria KT, Wachholz PA, Francis JN, Jacobson MR, Walker SM, Wilcock LK, et al. Grass pollen immunotherapy induces mucosal and peripheral IL-10 responses and blocking IgG activity. J Immunol 2004;172:3252–9. [DOI] [PubMed] [Google Scholar]
- 213.Ohman JL Jr, Findlay SR, Leitermann KM. Immunotherapy in cat-induced asthma: double-blind trial with evaluation of in vivo and in vitro responses. J Allergy Clin Immunol 1984;74:230–9. [DOI] [PubMed] [Google Scholar]
- 214.Olsen OT, Larsen KR, Jacobsan L, Svendsen UG. A 1-year, placebo-controlled, double-blind house-dust-mite immunotherapy study in asthmatic adults. Allergy 1997;52:853–9. [DOI] [PubMed] [Google Scholar]
- 215.Ortolani C, Pastorello E, Moss RB, Hsu YP, Restuccia M, Joppolo G, et al. Grass pollen immunotherapy: a single year double-blind, placebo-controlled study in patients with grass pollen-induced asthma and rhinitis. J Allergy Clin Immunol 1984;73:283–90. [DOI] [PubMed] [Google Scholar]
- 216.Pichler CE, Marquardsen A, Sparholt S, Lowenstein H, Bircher A, Bischof M, et al. Specific immunotherapy with Dermatophagoides pteronyssinus and D. farinae results in decreased bronchial hyperreactivity. Allergy 1997;52:274–83. [DOI] [PubMed] [Google Scholar]
- 217.Reid MJ, Moss RB, Hsu YP, Kwasnicki JM, Commerford TM, Nelson BL. Seasonal asthma in northern California: allergic causes and efficacy of immunotherapy. J Allergy Clin Immunol 1986;78:590–600. [DOI] [PubMed] [Google Scholar]
- 218.Roberts G, Hurley C, Turcanu V, Lack G. Grass pollen immunotherapy as an effective therapy for childhood seasonal allergic asthma. J Allergy Clin Immunol 2006;117:263–8. [DOI] [PubMed] [Google Scholar]
- 219.Sin B, Misirligil Z, Aybay C, Gurbuz L, Imir T. Effect of allergen specific immunotherapy (IT) on natural killer cell activity (NK), IgE, IFN-gamma levels and clinical response in patients with allergic rhinitis and asthma. J Investig Allergol Clin Immunol 1996;6:341–7. [PubMed] [Google Scholar]
- 220.Sykora T, Tamele L, Zemanova M, Petras M. Efficacy and safety of specific allergen immunotherapy with standardized allergen H-Al depot (pollens). Cze Alergie 2004;6:170–8. [Google Scholar]
- 221.Valovirta E, Koivikko A, Vanto T, Viander M, Ingeman L. Immunotherapy in allergy to dog: a double-blind clinical study. Ann Allergy 1984;53:85–8. [PubMed] [Google Scholar]
- 222.Varney VA, Edwards J, Tabbah K, Brewster H, Mavroleon G, Frew AJ. Clinical efficacy of specific immunotherapy to cat dander: a double-blind placebo controlled trial. Clin Exp Allergy 1997;27:860–7. [PubMed] [Google Scholar]
- 223.Varney VA, Tabbah K, Mavroleon G, Frew AJ. Usefulness of specific immunotherapy in patients with severe perennial allergic rhinitis induced by house dust mite: a double-blind, randomized, placebo-controlled trial. Clin Exp Allergy 2003;33:1076–82. [DOI] [PubMed] [Google Scholar]
- 224.Walker SM, Pajno GB, Lima MT, Wilson DR, Durham SR. Grass pollen immunotherapy for seasonal rhinitis and asthma: a randomized, controlled trial. J Allergy Clin Immunol 2001;107:87–93. [DOI] [PubMed] [Google Scholar]
- 225.Wang H, Lin X, Hao C, Zhang C, Sun B, Zheng J, et al. A double-blind, placebo controlled study of house dust mite immunotherapy in Chinese asthmatic patients. Allergy 2006;61:191–7. [DOI] [PubMed] [Google Scholar]
- 226.Yukselen A, Kendirli SG, Yilmaz M, Altintas DU, Karakoc GB. Effect of one year subcutaneous and sublingual immunotherapy on clinical and laboratory parameters in children with rhinitis and asthma: a randomized, placebo-controlled, double-blind, double-dummy study. Int Arch Allergy Immunol 2012;157: 288–98. [DOI] [PubMed] [Google Scholar]
- 227.Pifferi M, Baldini G, Marrazzini G, Baldini M, Ragazzo V, Pietrobelli A, et al. Benefits of immunotherapy with a standardized Dermatophagoides pteronyssinus extract in asthmatic children: a three-year prospective study. Allergy 2002;57: 785–90. [DOI] [PubMed] [Google Scholar]
- 228.Kilic M, Altintas DU, Yilmaz M, Bingol-Karakoc G, Burgut R, Guneser-Kendirli S. Evaluation of efficacy of immunotherapy in children with asthma mono sensitized to Alternaria. Turk J Pediatr 2011;53:285–94. [PubMed] [Google Scholar]
- 229.Lozano J, Cruz MJ, Piquer M, Giner MT, Plaza AM. Assessing the efficacy of immunotherapy with a glutaraldehyde-modified house dust mite extract in children by monitoring changes in clinical parameters and inflammatory markers in exhaled breath. Int Arch Allergy Immunol 2014;165:140–7. [DOI] [PubMed] [Google Scholar]
- 230.Lieberman P. The risk and management of anaphylaxis in the setting of immunotherapy. Am J Rhinol Allergy 2012;26:469–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Lin SY, Erekosima N, Suarez-Cuervo C, Ramanathan M, Kim JM, Ward D, et al. Allergen-specific immunotherapy for the treatment of allergic rhino conjunctivitis and/or asthma: comparative effectiveness review. Rockville, Md: Agency for Healthcare Research and Quality (AHRQ). Prepared by the Johns Hopkins University Evidence-based Practice Center under Contract No. 290–2007-10061-I. 2013 Mar (Errata added May and August 2013). Available at: www.effectivehealthcare.ahrq.gov/reports/final.cfm. [PubMed] [Google Scholar]
- 232.Gomez Vera J, Flores Sandoval G, Orea Solano M, Lopez Tiro J, Jimenez Saab N. [Safety and efficacy of specific sublingual immunotherapy in patients with asthma and allergy to Dermatophagoides pteronyssinus]. Rev Alerg Mex 2005; 52:231–6. [PubMed] [Google Scholar]
- 233.Virchow JC, Backer V, Kuna P, Prieto L, Nolte H, Villesen HH, et al. Efficacy of a house dust mite sublingual allergen immunotherapy tablet in adults with allergic asthma: a randomized clinical trial. JAMA 2016;315:1715–25. [DOI] [PubMed] [Google Scholar]
- 234.de Blay F, Kuna P, Prieto L, Ginko T, Seitzberg D, Riis B, et al. SQ HDM SLIT tablet (ALK) in treatment of asthma–post hoc results from a randomised trial. Respir Med 2014;108:1430–7. [DOI] [PubMed] [Google Scholar]
- 235.Devillier P, Fadel R, de Beaumont O. House dust mite sublingual immunotherapy is safe in patients with mild-to-moderate, persistent asthma: a clinical trial. Allergy 2016;71:249–57. [DOI] [PubMed] [Google Scholar]
- 236.Marogna M, Braidi C, Bruno ME, Colombo C, Colombo F, Massolo A, et al. The contribution of sublingual immunotherapy to the achievement of control in birchrelated mild persistent asthma: a real-life randomised trial. Allergol Immunopathol (Madr) 2013;41:216–24. [DOI] [PubMed] [Google Scholar]
- 237.Mosbech H, Canonica GW, Backer V, de Blay F, Klimek L, Broge L, et al. SQ house dust mite sublingually administered immunotherapy tablet (ALK) improves allergic rhinitis in patients with house dust mite allergic asthma and rhinitis symptoms. Ann Allergy Asthma Immunol 2015;114:134–40. [DOI] [PubMed] [Google Scholar]
- 238.Mosbech H, Deckelmann R, de Blay F, Pastorello EA, Trebas-Pietras E, Andres LP, et al. Standardized quality (SQ) house dust mite sublingual immunotherapy tablet (ALK) reduces inhaled corticosteroid use while maintaining asthma control: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol 2014;134:568–75.e7. [DOI] [PubMed] [Google Scholar]
- 239.Wang L, Yin J, Fadel R, Montagut A, de Beaumont O, Devillier P. House dust mite sublingual immunotherapy is safe and appears to be effective in moderate, persistent asthma. Allergy 2014;69:1181–8. [DOI] [PubMed] [Google Scholar]
- 240.Marogna M, Colombo F, Spadolini I, Massolo A, Berra D, Zanon P, et al. Randomized open comparison of montelukast and sublingual immunotherapy as add-on treatment in moderate persistent asthma due to birch pollen. J Investig Allergol Clin Immunol 2010;20:146–52. [PubMed] [Google Scholar]
- 241.Marogna M, Spadolini I, Massolo A, Berra D, Zanon P, Chiodini E, et al. Long-term comparison of sublingual immunotherapy vs inhaled budesonide in patients with mild persistent asthma due to grass pollen. Ann Allergy Asthma Immunol 2009;102:69–75. [DOI] [PubMed] [Google Scholar]
- 242.Niu CK, Chen WY, Huang JL, Lue KH, Wang JY. Efficacy of sublingual immunotherapy with high-dose mite extracts in asthma: a multi-center, double-blind, randomized, and placebo-controlled study in Taiwan. Respir Med 2006;100: 1374–83. [DOI] [PubMed] [Google Scholar]
- 243.Pham-Thi N, Scheinmann P, Fadel R, Combebias A, Andre C. Assessment of sublingual immunotherapy efficacy in children with house dust mite-induced allergic asthma optimally controlled by pharmacologic treatment and mite-avoidance measures. Pediatr Allergy Immunol 2007;18:47–57. [DOI] [PubMed] [Google Scholar]
- 244.Maloney J, Prenner BM, Bernstein DI, Lu S, Gawchik S, Berman G, et al. Safety of house dust mite sublingual immunotherapy standardized quality tablet in children allergic to house dust mites. Ann Allergy Asthma Immunol 2016; 116:59–65. [DOI] [PubMed] [Google Scholar]
- 245.Mosges R, Graute V, Christ H, Sieber HJ, Wahn U, Niggemann B. Safety of ultra rush titration of sublingual immunotherapy in asthmatic children with tree-pollen allergy. Pediatr Allergy Immunol 2010;21:1135–8. [DOI] [PubMed] [Google Scholar]
- 246.Shao J, Cui YX, Zheng YF, Peng HF, Zheng ZL, Chen JY, et al. Efficacy and safety of sublingual immunotherapy in children aged 3–13 years with allergic rhinitis. Am J Rhinol Allergy 2014;28:131–9. [DOI] [PubMed] [Google Scholar]
- 247.Castro M, Rubin AS, Laviolette M, Fiterman J, De Andrade Lima M, Shah PL, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med 2010;181:116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Cox G, Thomson NC, Rubin AS, Niven RM, Corris PA, Siersted HC, et al. Asthma control during the year after bronchial thermoplasty. N Engl J Med 2007;356:1327–37. [DOI] [PubMed] [Google Scholar]
- 249.Pavord ID, Cox G, Thomson NC, Rubin AS, Corris PA, Niven RM, et al. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med 2007;176:1185–91. [DOI] [PubMed] [Google Scholar]
- 250.Thomson NC, Rubin AS, Niven RM, Corris PA, Siersted HC, Olivenstein R, et al. Long-term (5 year) safety of bronchial thermoplasty: Asthma Intervention Research (AIR) trial. BMC Pulm Med 2011;11:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Wechsler ME, Laviolette M, Rubin AS, Fiterman J, Lapa e Silva JR, Shah PL, et al. Bronchial thermoplasty: long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol 2013;132: 1295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Balu A, Ryan D, Niven R. Lung abscess as a complication of bronchial thermoplasty. J Asthma 2015;52:740–2. [DOI] [PubMed] [Google Scholar]
- 253.Facciolongo N, Menzella F, Lusuardi M, Piro R, Galeone C, Castagnetti C, et al. Recurrent lung atelectasis from fibrin plugs as a very early complication of bronchial thermoplasty: a case report. Multidiscip Respir Med 2015;10:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Nguyen DV, Murin S. Bronchial artery pseudoaneurysm with major hemorrhage after bronchial thermoplasty. Chest 2016;149:e95–7. [DOI] [PubMed] [Google Scholar]
- 255.Pavord ID, Thomson NC, Niven RM, Corris PA, Chung KF, Cox G, et al. Safety of bronchial thermoplasty in patients with severe refractory asthma. Ann Allergy Asthma Immunol 2013;111:402–7. [DOI] [PubMed] [Google Scholar]
- 256.Cox G, Miller JD, McWilliams A, Fitzgerald JM, Lam S. Bronchial thermoplasty for asthma. Am J Respir Crit Care Med 2006;173:965–9. [DOI] [PubMed] [Google Scholar]
- 257.Doeing DC, Husain AN, Naureckas ET, White SR, Hogarth DK. Bronchial thermoplasty failure in severe persistent asthma: a case report. J Asthma 2013;50: 799–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Doeing DC, Mahajan AK, White SR, Naureckas ET, Krishnan JA, Hogarth DK. Safety and feasibility of bronchial thermoplasty in asthma patients with very severe fixed airflow obstruction: a case series. J Asthma 2013;50:215–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Funatsu A, Kobayashi K, Iikura M, Ishii S, Izumi S, Sugiyama H. A case of pulmonary cyst and pneumothorax after bronchial thermoplasty. Respirol Case Rep 2018;6:e00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Han X, Zhang S, Zhao W, Wei D, Wang Y, Hogarth DK, et al. A successful bronchial thermoplasty procedure in a “very severe” asthma patient with rare complications: a case report. J Asthma 2019;56:1004–7. [DOI] [PubMed] [Google Scholar]
- 261.Mahajan AK, Hogarth DK. Bronchial thermoplasty: therapeutic success in severe asthma associated with persistent airflow obstruction. J Asthma 2012;49: 527–9. [DOI] [PubMed] [Google Scholar]
- 262.Menzella F, Lusuardi M, Galeone C, Montanari G, Cavazza A, Facciolongo N. Heat-induced necrosis after bronchial thermoplasty: a new concern? Allergy Asthma Clin Immunol 2018;14:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Qiu M, Lai Z, Wei S, Jiang Q, Xie J, Qiu R, et al. Bronchiectasis after bronchial thermoplasty. J Thorac Dis 2018;10:E721–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Takeuchi A, Kanemitsu Y, Takakuwa O, Ito K, Kitamura Y, Inoue Y, et al. A suspected case of inflammatory bronchial polyp induced by bronchial thermoplasty but resolved spontaneously. J Thorac Dis 2018;10:E678–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.