Abstract
Rationale: Active immunization is needed to protect infants and young children against respiratory syncytial virus (RSV). Rationally designed live-attenuated RSV vaccines are in clinical development.
Objectives: Develop preliminary estimates of vaccine efficacy, assess durability of antibody responses to vaccination and “booster” responses after natural RSV infection, and determine sample sizes needed for more precise estimates of vaccine efficacy.
Methods: We analyzed data from seven phase 1 trials of live-attenuated RSV vaccines in 6- to 24-month-old children (n = 239).
Measurements and Main Results: The five vaccine regimens that induced neutralizing antibody responses in ≥80% of vaccinees (defined post hoc as “more promising”) protected against RSV-associated medically attended acute respiratory illness (RSV-MAARI) and medically attended acute lower respiratory illness (RSV-MAALRI) and primed for potent anamnestic responses upon natural exposure to wild-type RSV. Among recipients of “more promising” RSV vaccines, efficacy against RSV-MAARI was 67% (95% confidence interval [CI], 24 to 85; P = 0.008) and against RSV-MAALRI was 88% (95% CI, −9 to 99; P = 0.04). A greater than or equal to fourfold increase in RSV serum neutralizing antibody following vaccination was strongly associated with protection against RSV-MAARI (odds ratio, 0.26; 95% CI, 0.09 to 0.75; P = 0.014) and RSV-MAALRI; no child with a greater than or equal to fourfold increase developed RSV-MAALRI. Rates of RSV-MAARI and RSV-MAALRI in placebo recipients were 21% and 7%, respectively. Given these rates, a study of 540 RSV-naive children would have 90% power to demonstrate ≥55% efficacy against RSV-MAARI and ≥80% efficacy against RSV-MAALRI; if rates were 10% and 3%, a study of 1,300 RSV-naive children would be needed.
Conclusions: Rapid development of a live-attenuated RSV vaccine could contribute substantially to reducing the global burden of RSV disease.
Keywords: RSV, pediatric, vaccine, immunity, efficacy
At a Glance Commentary
Scientific Knowledge on the Subject
A number of respiratory syncytial virus (RSV) vaccines for immunization of infants and children are in development, including live-attenuated vaccines, but the efficacy of all of these vaccines in the prevention of RSV-associated illness is unknown.
What This Study Adds to the Field
This analysis of data from seven previous clinical trials demonstrates that live-attenuated RSV vaccines can protect against RSV-associated medically attended respiratory illness and lower respiratory illness. To our knowledge, this is the first demonstration that active immunization of infants and young children against RSV can protect against RSV disease.
Respiratory syncytial virus (RSV) is the leading global cause of acute lower respiratory illness (LRI) in infants and children (1, 2), and products designed to provide passive or active immunity in young children against RSV are in clinical development (3, 4). Passive immunoprophylaxis, through either maternal immunization with RSV fusion (F) glycoprotein vaccines or infant immunization with long-acting RSV monoclonal antibodies, may provide substantial short-term protection against severe RSV disease in the earliest months of life. However, >80% of RSV-associated LRI and more than half of RSV deaths occur in children ages 6 months and older (1, 5). Therefore, RSV vaccines that can be safely administered to infants and that provide active durable immunity are needed to address the burden of RSV disease in childhood (1, 5).
Live-attenuated intranasal RSV vaccines are critical for RSV disease prevention because they are expected to induce innate immunity and durable local and systemic immunity (6–9). In addition, live-attenuated RSV vaccines are not associated with enhanced RSV disease that occurred when RSV-naive children received formalin-inactivated RSV and experienced community-acquired RSV infection (10, 11), whereas RSV subunit vaccines may prime for enhanced RSV disease (12, 13). However, immune responses can be inefficient in early life, and it has not been known whether a pediatric RSV vaccine could protect against RSV disease.
Advances in understanding RSV gene function, and the ability to produce engineered live viruses using reverse genetics, have led to development of promising new-generation live-attenuated RSV vaccine candidates, including those with deletions of proteins that regulate viral RNA synthesis or suppress host responses. These vaccines are highly restricted in replication and appear to be well tolerated yet induce serum RSV neutralizing antibody (neutAb) responses in RSV-naive infants and children comparable to those following infection with wild-type (wt) RSV (6) and prime for potent anamnestic antibody responses upon natural exposure to wt RSV (6–9). Although live-attenuated RSV vaccines with these characteristics might prevent RSV LRI, individual early-stage vaccine trials have been neither large enough nor designed to assess this outcome. However, pooled data from recent phase 1 trials of live-attenuated vaccine candidates might begin to address this question.
Postvaccination surveillance for RSV-associated medically attended acute respiratory illness (RSV-MAARI) and RSV-associated medically attended acute lower respiratory illness (RSV-MAALRI, a subset of RSV-MAARI) has been a standard component of clinical trials of live-attenuated RSV vaccine candidates (6–9, 14–17). Surveillance includes weekly contact during the RSV season following immunization, and clinical assessment and nasal wash for viral testing in the event of MAARI. At the end of RSV surveillance, sera are obtained to measure serum RSV neutAb titers. Although postvaccination surveillance was originally designed to assess enhanced RSV disease among vaccinees, it also provides opportunities to learn about 1) magnitude and durability of primary RSV antibody responses in vaccinees; 2) ability of these vaccines to prime for anamnestic (memory) antibody responses upon natural exposure to wt RSV; 3) rates of RSV-MAARI and RSV-MAALRI in placebo recipients to inform sample size estimates for late-stage vaccine trials; 4) incidence of exposure to wt RSV; and 5) comparative rates of RSV-MAARI and RSV-MAALRI in vaccinees and placebo recipients, which may allow for preliminary assessments of vaccine efficacy.
In this report, we analyze combined data from pediatric phase 1 trials of seven different live-attenuated RSV vaccine candidates. We show that the more promising of these candidates induced substantial, durable serum RSV neutAb responses and potent memory responses. We use data on rates of RSV-MAARI and RSV-MAALRI in placebo recipients to develop sample size estimates for future vaccine trials. Additionally, we provide preliminary evidence of the efficacy of these live-attenuated vaccines against RSV-MAARI and RSV-MAALRI.
Methods
Clinical Trials
We analyzed data from previous studies of seven live-attenuated RSV vaccines (Figure 1) (6–9, 17). All trials were conducted in the same manner, with identical methods for assessment of MAARI and MAALRI during postvaccination surveillance and identical viral detection and antibody assays performed in a single laboratory (15). Data on clinical outcomes, detection of RSV-MAARI or MAALRI, and antibody responses were pooled across studies. One hundred sixty-one vaccinees and 80 placebo recipients were enrolled (Figure 1). Participants were followed for safety and clinical outcomes through surveillance (Figure 1 and Table 1). We analyzed efficacy against RSV-MAARI and RSV-MAALRI for 160 vaccinees and 79 placebo recipients (6) (Figure 1, blue boxes). Data from 72 placebo recipients were used for determining background rates of RSV-MAARI and RSV-MAALRI (see results and Figures 1 and 2). We defined serologic evidence of infection with wt RSV as greater than or equal to fourfold increase in serum 60% complement-enhanced plaque reduction–neutralizing Ab titers (PRNT) between presurveillance and postsurveillance specimens. Presurveillance sera were collected in October of the immunization year, and postsurveillance sera were collected the following April. Durability of the antibody response in vaccinees was assessed from day 56 following vaccination through the end of the surveillance period, which ranged from ∼5.5 to 13 months. Signs and symptoms of RSV illness were assessed as previously described (14).
Figure 1.
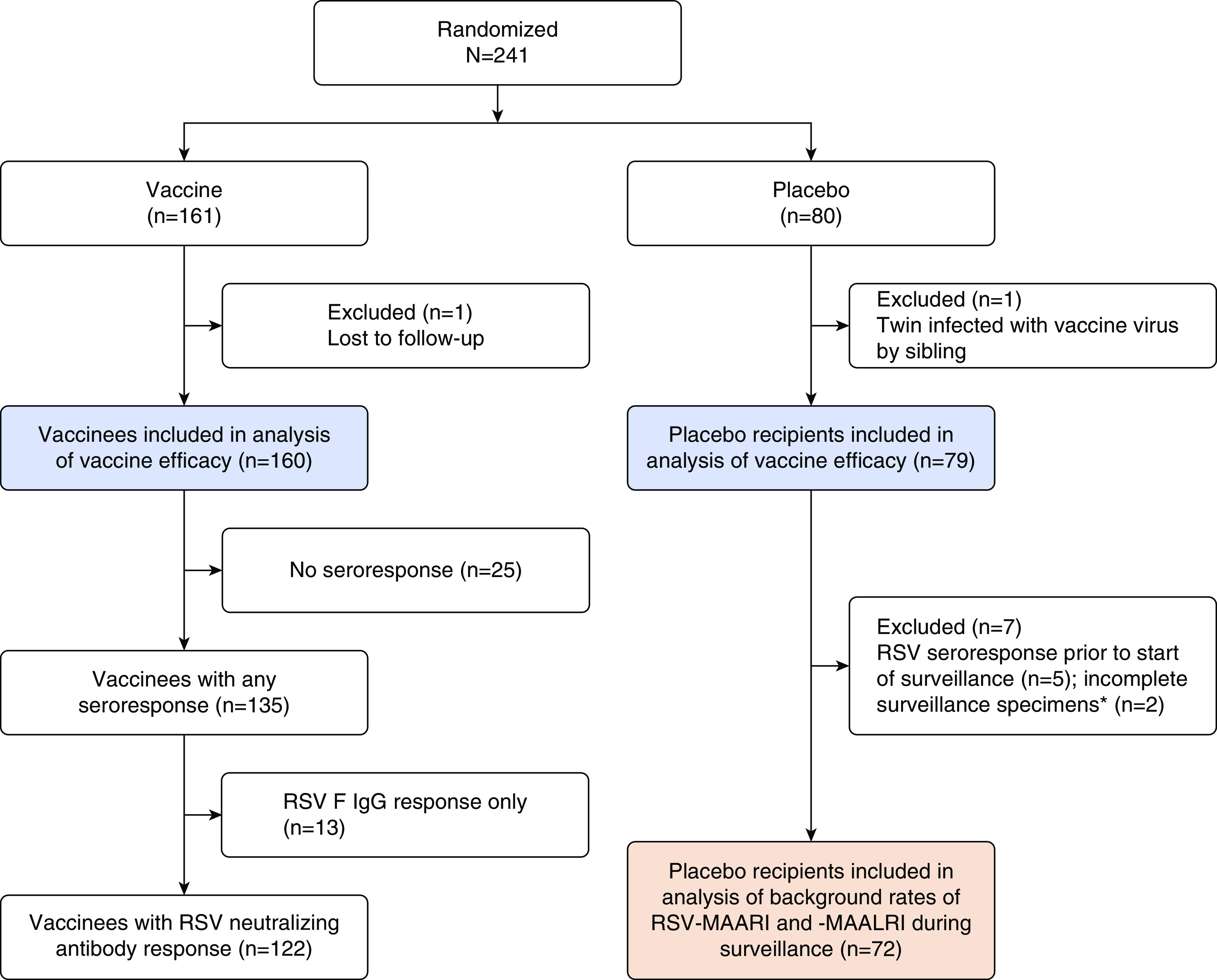
Allocation by study arm and distribution of vaccinees according to immune response. Two hundred forty-one children ages 6–24 months were randomized in seven separate studies of live-attenuated respiratory syncytial virus (RSV) vaccines to receive vaccine (161 children) or placebo (80 children), including RSV ΔNS2/Δ1313/I1314L, RSVcps2, LID/cp/ΔM2–2, MEDIΔM2–2, RSV LIDΔM2–2, RSV LIDΔM2–2/1030s, and D46/NS2/N/ΔM2–2-HindIII (registered in clinicaltrials.gov as NCT01893554, NCT01852266/NCT01968083, NCT02890381/NCT02948127, NCT01459198, NCT02040831/NCT02237209, NCT02794870/NCT02952339, and NCT03099291/NCT03102034; certain vaccines were evaluated in more than one clinical trial). Subset analyses and losses to follow-up are as described in results. RSV F IgG ELISA titers were determined by endpoint titration. *Two placebo recipients were missing one or more surveillance serum specimens. F = fusion; MAALRI = medically attended acute lower respiratory illness; MAARI = medically attended acute respiratory illness.
Table 1.
Vaccinees and Placebo Recipients Enrolled in Phase 1 Clinical Trials of Seven Live-attenuated RSV Vaccines
| Vaccine | Study Years | Vaccinees |
Placebo Recipients |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n) | Evaluable for VE* (n) | Infected with Vaccine Virus† [n (%)] | Any Antibody Response‡ [n (%)] | RSV PRNT Response§ [n (%)] | Total (n) | Evaluable for VE (n) | Evaluable for MAARI Rates‖ (n) | ||||
| Less promising¶ | |||||||||||
| ΔNS2/Δ1313/I1314L (105) | 2014–15 | 15 | 15 | 12 (80) | 10 (67) | 8 (53) | 7 | 7 | 6 | ||
| RSVcps2 | 2013–15 | 34 | 34 | 29 (85) | 23 (68) | 20 (59) | 16 | 16 | 14 | ||
| LID/cp/ΔM2-2 | 2016–17 | 11 | 11 | 6 (55) | 6 (55) | 5 (45) | 6 | 6 | 3 | ||
| Total, less promising | 2013–17 | 60 | 60 | 47 (78) | 39 (65) | 33 (55) | 29 | 29 | 23 | ||
| More promising** | |||||||||||
| MEDIΔM2-2 | 2011–14 | 20 | 20 | 20 (100) | 19 (95) | 19 (95) | 10 | 9 | 9 | ||
| ΔNS2/Δ1313/I1314L (106) | 2015–17 | 20 | 20 | 20 (100) | 19 (95) | 16 (80) | 10 | 10 | 10 | ||
| LIDΔM2-2 | 2014–15 | 20 | 20 | 19 (95) | 18 (90) | 18 (90) | 9 | 9 | 9 | ||
| LIDΔM2-2/1030s | 2016–17 | 20 | 20 | 20 (100) | 20 (100) | 17 (85) | 11 | 11 | 10 | ||
| D46/NS2/N/ΔM2-2-HindIII | 2017–18 | 21 | 20 | 20 (100) | 20 (100) | 19 (95) | 11 | 11 | 11 | ||
| Total, more promising | 2011–18 | 101 | 100 | 99 (99) | 96 (96) | 89 (89) | 51 | 50 | 49 | ||
| Total (all vaccines) | 2011–18 | 161†† | 160 | 146 | 135†† | 122 | 80 | 79 | 72 | ||
Definition of abbreviations: F = fusion; MAALRI = medically attended acute lower respiratory illness; MAARI = medically attended acute respiratory illness; PRNT = plaque reduction–neutralizing antibody titer; RSV = respiratory syncytial virus; VE = vaccine efficacy.
Evaluable for VE: vaccinees included in the analysis of VE.
Infected with vaccine virus: defined as detection of vaccine virus by culture or PCR and/or a greater than or equal to fourfold rise in serum RSV PRNT and/or a greater than or equal to fourfold rise in serum ELISA IgG antibody titer to the RSV F glycoprotein as measured by endpoint titration. Note that for consistency between all studies, ELISA IgG titers measured by endpoint titration were used for these analyses, whereas interpolated ELISA titers were previously reported for several studies (7–9, 17).
With any antibody response: greater than or equal to fourfold rise in RSV PRNT or RSV F IgG titers between Study Days 0 and 56.
With RSV PRNT response: greater than or equal to fourfold rise in RSV PRNT between Study Days 0 and 56.
Placebo recipients evaluable for serosurveillance: placebo recipients with surveillance serum specimens available who did not have a greater than or equal to fourfold rise in RSV PRNT between Days 0 and 56 and could therefore be included in analyses of background rates of MAARI and MAALRI.
“Less promising”: PRNT response ≤59%.
“More promising”: PRNT response ≥80%. Each of these definitions is described in more detail in the text.
Of 161 vaccinees, 1 was lost to follow-up; thus, data from 160 vaccinees in total and 135 vaccinees with any antibody response were evaluable for vaccine efficacy.
Figure 2.
Percentage of the placebo recipients in clinical trials of live-attenuated respiratory syncytial virus (RSV) vaccines who experienced RSV-MAARI (red bars) and percentage with greater than or equal to fourfold increase in RSV plaque reduction–neutralizing antibody titer (PRNT) when presurveillance season and postsurveillance season sera were compared (“RSV neutralizing Ab response,” blue bars). Data are from 2011 through 2018; enrollment varied by year. In all, 72 placebo recipients were included in this analysis. Eight placebo recipients were excluded: in addition to the one placebo recipient who was infected with vaccine virus (see methods), five placebo recipients had a rise in RSV antibody titer between Days 0 and 56 after receipt of study product, presumably reflecting natural infection with wild-type (wt) RSV, and two placebo recipients, although assessed for medically attended respiratory outcomes (and therefore still eligible for the analysis of vaccine efficacy), were missing one or more surveillance serum specimens (see Figure 1). Wt RSV infection was defined as occurring in any participant who had a greater than or equal to fourfold increase in RSV PRNT between the pre- and postsurveillance serum specimens, detection of wt RSV in a nasal wash specimen during surveillance, or both. Ab = antibody; MAARI = medically attended acute respiratory illness.
Written informed consent was obtained from parents or guardians before enrollment. Studies were conducted in accordance with the principles of the Declaration of Helsinki and Standards of Good Clinical Practice under National Institute of Allergy and Infectious Diseases (NIAID)-held Investigational New Drug applications and reviewed by the U.S. Food and Drug Administration. Studies were approved by each site’s institutional review board and monitored by the Independent Data Safety and Monitoring Board of the NIAID Division of Clinical Research.
Statistical Analysis
RSV-associated MAARIs and MAALRIs (6), peak titers of vaccine virus shed, serum RSV F IgG titers measured by endpoint titration, and RSV PRNT were pooled across studies and analyzed for vaccinees and placebo recipients. We divided the pooled data into subsets of “more promising” and “less promising” vaccines, based upon rates of RSV neutAb response (Table 1).
Analyses (including power and sample size) were performed with Stata 15 (StataCorp), using unpaired t tests to compare means of continuous outcomes, Spearman’s correlation coefficients to evaluate associations, and paired t tests to compare differences between paired serum specimens. Logistic regression, χ2, and Fisher’s exact tests were performed to evaluate proportions and dichotomous outcomes. We calculated odds ratios (ORs) of RSV-MAARI and RSV-MAALRI for recipients of each vaccine, group of vaccines, and placebo and used 1 – OR to determine vaccine efficacy. α was set at 0.05.
Results
Study Participants
Two hundred forty-one RSV-seronegative children ages 6–24 months were enrolled: 161 children received vaccine and 80 received placebo (Table 1 and Figure 1). Two children were excluded from all analyses: one vaccinee who received D46/NS2/N/ΔM2–2-HindIII was lost to follow-up before the end of surveillance, and one placebo recipient who participated in the study of RSV MEDIΔM2–2 was infected with vaccine virus transmitted by his sibling (6). One hundred sixty vaccinees and 79 placebo recipients were eligible for the vaccine efficacy analyses (Figure 1; blue boxes).
Of the 160 vaccinees, 135 (84%) had “any seroresponse” (greater than or equal to fourfold rise in serum RSV PRNT and/or a greater than or equal to fourfold rise in serum RSV F IgG) at Day 56 following vaccination (Figure 1). Of these 135 vaccinees, 122 (90%) had serum RSV neutAb responses (“RSV neutAb response”) and 13 vaccinees (10%) had RSV F IgG responses without neutAb responses (Figure 1 and Table 1).
We categorized vaccines according to immune response, and generated subsets of “more promising” and “less promising” vaccines (Table 1). Based on substantial differences in rates of neutAb responses, “more promising” vaccines were those that induced greater than or equal to fourfold increase in RSV PRNT at Day 56 after vaccination in ≥80% of vaccinees. Conversely, “less promising” vaccines were those that induced greater than or equal to fourfold increase in RSV PRNT at Day 56 after vaccination in ≤59% of vaccinees (Table 1). Using these criteria, LIDΔM2–2/1030s, LIDΔM2–2, MEDIΔM2–2, D46/NS2/N/ΔM2–2-HindIII, and the 106.0 plaque-forming unit dose of RSV ΔNS2/Δ1313/I1314L were considered more promising, whereas LID/cp/ΔM2–2, RSVcps2, and the 105.0 plaque-forming unit dose of RSV ΔNS2/Δ1313/I1314L were considered less promising (Table 1). Of the 241 children, 152 (101 vaccinees) were enrolled in studies of more promising vaccines and 89 (60 vaccinees) were enrolled in studies of less promising vaccines (Table 1).
Rates of RSV-MAARI, RSV-MAALRI, and wt RSV Infection: Data from Placebo Recipients
We assessed attack rates (cumulative incidence) of RSV-MAARI, RSV-MAALRI, and wt RSV infection in 72 placebo recipients (Figures 1 and 2). Proportions with RSV-MAARI and RSV-MAALRI varied by year; overall, 21% experienced RSV-MAARI and 7% experienced RSV-MAALRI (Figure 2). The percentage of RSV-infected placebo recipients based upon seroresponse was significantly greater than the rate of RSV-MAARI (56% vs. 21%, P < 0.001), indicating that many children experienced mild RSV infections that did not require medical attention.
Vaccine Efficacy against RSV-MAARI and RSV-MAALRI
We calculated efficacy against RSV-MAARI and RSV-MAALRI for all vaccines combined, for less promising vaccines, and for more promising vaccines. Within the categories of all vaccines and more promising vaccines, we also assessed efficacy among the subset of vaccinees with serum RSV neutAb responses to vaccination (greater than or equal to fourfold increase in RSV PRNT).
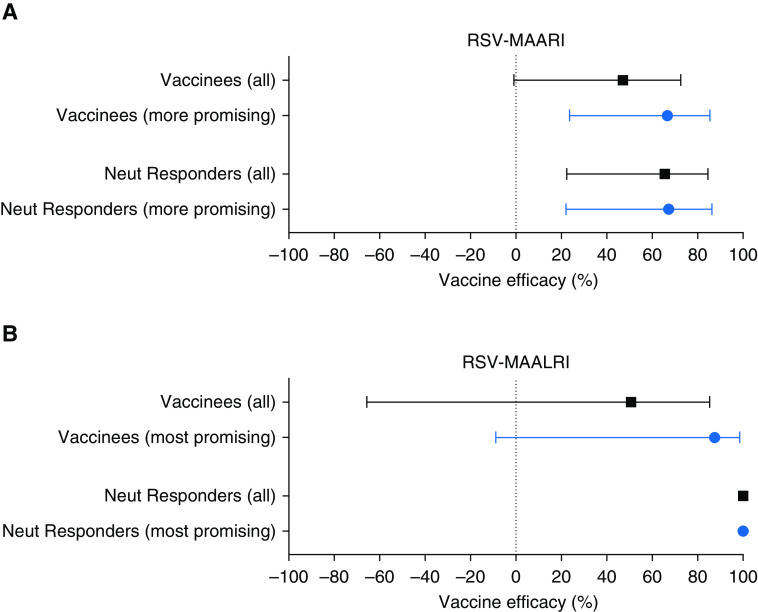
We first assessed data from all vaccinees (Figures 3A and 3B, “all,” black lines; n = 160; Table 1). Vaccine efficacy was 47% against RSV-MAARI (Figure 3A; 95% confidence interval [CI], −0.9 to 73; P = 0.04) and 51% against RSV-MAALRI (Figure 3B; 95% CI, −66 to 85; P = 0.2). However, when analysis was restricted to RSV neutAb responders (n = 122; Table 1 and Figure 1), efficacy was 66% against RSV-MAARI (Figure 3A; 95% CI, 21 to 85; P = 0.007) and 100% against RSV-MAALRI, as no cases of RSV-MAALRI were detected (Figure 3B, P = 0.009).
Figure 3.
(A and B) Vaccine efficacy against respiratory syncytial virus (RSV)-associated medically attended acute respiratory illness (MAARI) (A) and RSV-associated medically attended acute lower respiratory illness (MAALRI) (B), with point estimates and 95% confidence intervals. In both panels, the black bars show the efficacy estimates for all 160 vaccinees (top black bars), and for the 122 vaccinees with RSV neutralizing antibody responses by Day 56 after vaccination “neut responders” (bottom black bars). Similarly, the blue bars in each grouping show the analyses for the 100 children who received the more promising vaccines (top blue bars), and further subset of 90 with neutralizing antibody responses to vaccine (bottom blue bars). Among all 160 vaccinees and 79 placebo recipients, there were 31 cases of RSV-MAARI (16 in vaccinees and 15 in placebo recipients) and 10 cases of RSV-MAALRI (5 in vaccinees and 5 in placebo recipients).
For the subset of children who received more promising vaccines (Figures 3A and 3B, blue lines, n = 100, Table 1), efficacy was 67% against RSV-MAARI (Figure 3A; 95% CI, 24 to 85; P = 0.008) and 88% against RSV-MAALRI (Figure 3B [95% CI, −9 to 99; P = 0.04], with small numbers likely accounting for the wide CI). When analysis was restricted to RSV neutAb responders (n = 89; Table 1) efficacy was 67% against RSV-MAARI (Figure 3A; 95% CI, 22 to 86; P = 0.008) and 100% against RSV-MAALRI (Figure 3B; P = 0.02). For the 60 children who received less promising vaccines, we could not demonstrate any evidence of efficacy against RSV-MAARI or RSV-MAALRI (data not shown).
Predictors of Vaccine Efficacy
We found that development of a greater than or equal to fourfold rise in serum RSV neutAb after vaccination appeared to be an important determinant of vaccine efficacy. To further explore the relationships between efficacy, antibody response, and vaccine virus replication in all 160 vaccinees, we assessed the associations between the occurrence of RSV-MAARI and RSV-MAALRI and 1) greater than or equal to fourfold rise in RSV PRNT after vaccination, 2) magnitude of RSV PRNT, 3) greater than or equal to fourfold rise in RSV F IgG titer, 4) magnitude of RSV F IgG titer, 5) presence or absence of vaccine virus shedding determined by culture or qRT-PCR, and 6) peak titer of vaccine virus shed determined by culture or qRT-PCR. The associations between RSV F IgG and efficacy (3 and 4 above) are presented in the online supplement; others are described below.
In univariable logistic regression, greater than or equal to fourfold rise in RSV PRNT was strongly associated with protection against both RSV-MAARI (OR, 0.26; 95% CI, 0.09–0.75; P = 0.014]) and RSV-MAALRI (OR not applicable, as no child with RSV-MAALRI had a greater than or equal to fourfold rise in PRNT). When assessed as a continuous variable, increases in RSV PRNT were associated with decreased risk of both RSV-MAARI and RSV-MAALRI, but evaluation of several a priori cut points (reciprocal titers of 60, 70, 80, 90, and 100) indicated that no single PRNT could be established as a correlate of protection.
Detection of vaccine virus by culture was not associated with subsequent protection against RSV-MAARI (OR, 0.64; 95% CI, 0.23–1.83; P = 0.41) or RSV-MAALRI (OR, 0.12; 95% CI, 0.01–1.13; P = 0.06). We observed no significant difference in mean peak titers of vaccine virus shed among vaccinees who had serologic evidence of exposure to wt RSV during surveillance without RSV-MAARI (“RSV-MAARI−”; orange dots, Figure 4A) and vaccinees who experienced RSV-MAARI (“RSV-MAARI+”; blue dots, Figure 4A), 1.56 log10 versus 1.62 log10, P = 0.86. This was also the case when restricting the analysis to recipients of more promising vaccines (Figure 4B; “RSV-MAARI−”; 2.10 log10 [orange] vs. “RSV-MAARI+”; 2.28 log10 [blue]). Detection of vaccine virus by qRT-PCR was associated with protection against RSV-MAALRI (OR, 0.13; 95% CI, 0.02–0.84; P = 0.032), but protection was incomplete as two vaccinees who had vaccine virus detected by qRT-PCR experienced RSV-MAALRI. We did not observe an association between detection of vaccine virus by qRT-PCR and protection against RSV-MAARI or an association between the magnitude of vaccine virus shed as measured by qRT-PCR and protection against RSV-MAARI or RSV-MAALRI.
Figure 4.
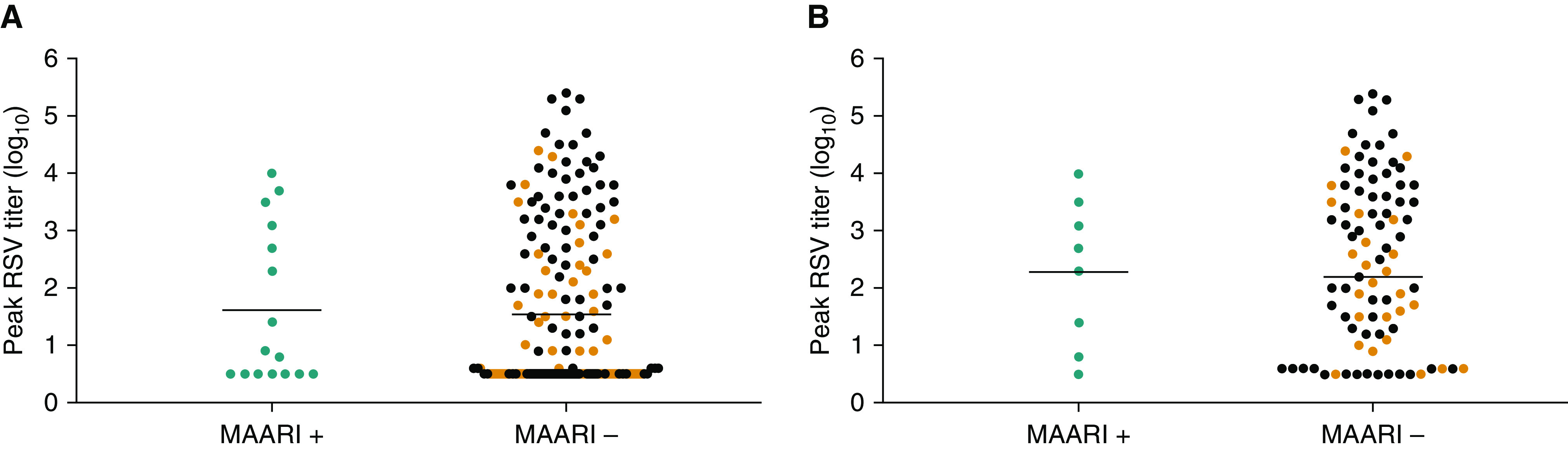
Peak vaccine titers do not appear to be predictive of vaccine efficacy. Peak log10 titers of vaccine virus shed among vaccinees who experienced respiratory syncytial virus (RSV)-associated medically attended acute respiratory illness (MAARI) (blue dots), vaccinees who did not experience RSV-MAARI but had serologic evidence of wild-type (wt) RSV exposure during the RSV season (orange dots), and vaccinees who did not experience RSV-MAARI and who also had no evidence of wt RSV exposure (black dots) during the subsequent RSV surveillance season. Means were calculated for RSV-MAARI+ (blue dots) and for RSV-MAARI− who had evidence of exposure to RSV during surveillance (orange dots). A shows all vaccinees and B shows recipients of the more promising vaccines.
Neutralizing Antibody Responses to wt RSV Infection
We first compared the magnitude of the RSV PRNT at Day 56 after vaccination and following RSV surveillance among 36 vaccinees who developed a primary neutAb response to RSV vaccine and also had serologic evidence of wt RSV infection during surveillance (Figure 5, red scatterplots). The geometric mean titer (GMT) of RSV neutAb in the postsurveillance serum specimens was 14-fold higher than in Day 56 specimens (1,176 [10.2 log2] vs. 84 [6.4 log2], P < 0.0001), indicative of strong anamnestic responses. We next compared the magnitude of this postsurveillance response with the primary neutAb response among the 41 placebo recipients who had serologic evidence of wt RSV infection during surveillance (Figure 5, black scatterplot). Again, the magnitude of the anamnestic neutAb response among vaccinees was nearly 10-fold higher than the primary response among placebo recipients (1,176 [10.2 log2] vs. 128 [7.0 log2], P < 0.0001). Of note, the primary response to wt RSV among placebo recipients was not significantly different from the primary response to RSV vaccine among vaccinees with neutAb responses (128 [7.0 log2] vs. 104 [6.7 log2], Figure 5, black scatterplot versus orange, P = 0.21).
Figure 5.

Comparisons of respiratory syncytial virus (RSV) plaque reduction–neutralizing antibody titer (PRNT) after immunization (Day 56) and after the surveillance period, with titers expressed as reciprocal log2. Dots of the same color represent identical participants but at different time points. Open circles are postimmunization PRNTs; closed circles are postsurveillance PRNTs. Lines indicate the mean PRNT. Red scatterplots: anamnestic antibody responses following exposure to wild-type (wt) RSV in 36 vaccinees who developed a greater than or equal to fourfold PRNT response after immunization, and also had a greater than or equal to fourfold PRNT response during the surveillance period, indicating exposure to wt RSV. PRNTs after immunization (red open circles, mean 6.4 log2, geometric mean titer = 84) are compared with PRNTs following surveillance (red closed circles, mean 10.2 log2, 1,176). Blue scatterplots: durability of neutralizing antibody responses following vaccination in 83 vaccinees who developed a greater than or equal to fourfold PRNT response after immunization but did not have a greater than or equal to fourfold PRNT response during the surveillance period, indicating they were not exposed to wt RSV. PRNTs after immunization (open blue circles, mean 6.8 log2, 111) are compared with PRNTs after surveillance (closed blue circles, mean 6.3 log2, 79). Orange and black scatterplots: PRNTs induced by vaccination are comparable to those induced by wt RSV infection. The mean PRNT on Day 56 following vaccination among all 122 vaccinees was 6.7 log2 (orange scatterplot), and the postsurveillance PRNT among 41 placebo recipients exposed to wt RSV during surveillance was 7.0 log2 (black scatterplot).
Durability of Vaccine-induced Immunity
To assess the durability of vaccine-induced immunity, we compared RSV PRNT at Day 56 and at the end of surveillance among the 83 vaccinees who developed an initial RSV PRNT response following vaccination but did not have a postsurveillance serologic response indicative of infection with wt RSV. Although the GMTs of PRNT were statistically significantly different between these two time points (P < 0.001), the magnitude of the difference was not large: 111 (6.8 log2) after immunization versus 79 (6.3 log2) after surveillance (Figure 5, blue scatterplots).
Sample size estimates for future RSV vaccine trials
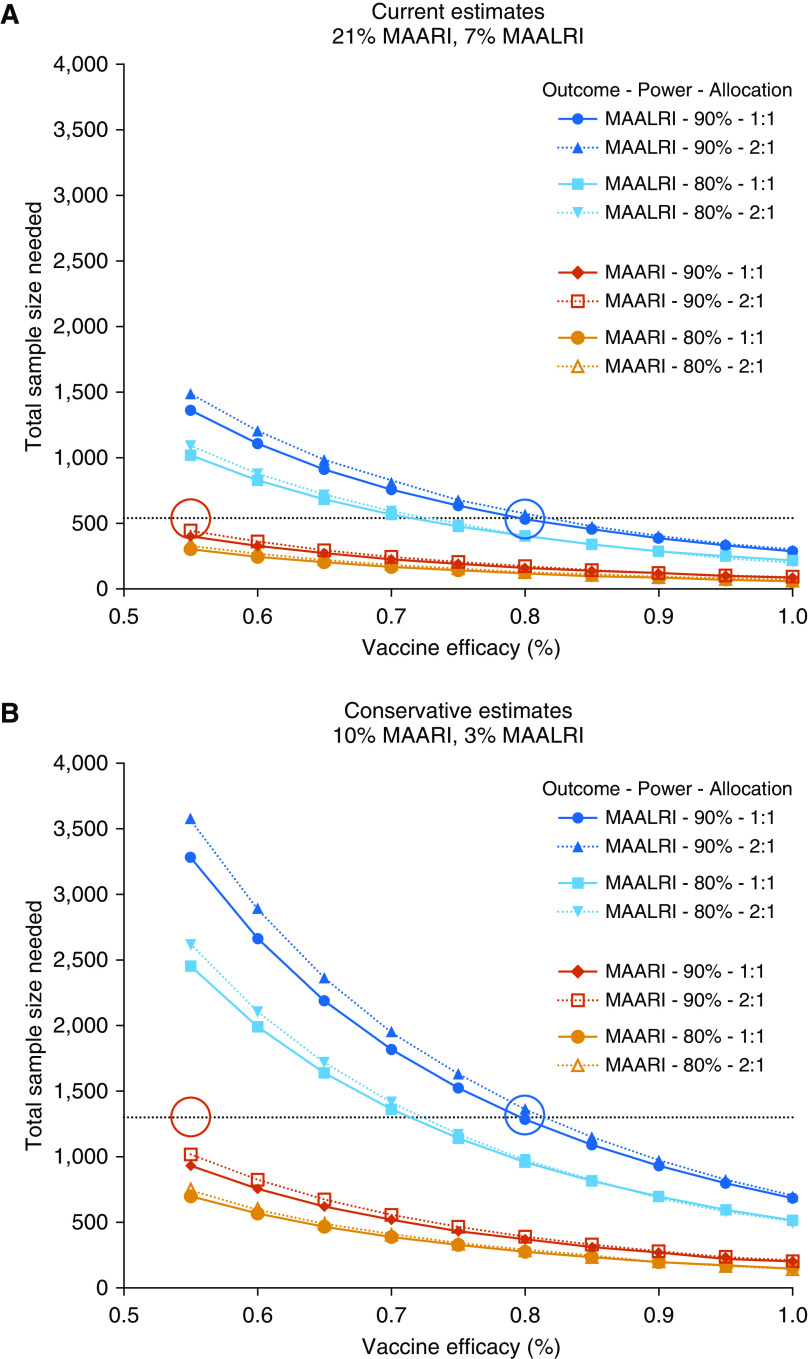
Based upon rates of detection of RSV-MAARI and RSV-MAALRI in placebo recipients, we calculated the numbers of RSV-naive infants and children that would provide adequate power to assess efficacy against RSV-MAARI and RSV-MAALRI in future trials of live-attenuated RSV vaccines, across a range of efficacy estimates. We used observed rates of 21% RSV-MAARI and 7% RSV-MAALRI (Figure 2) as “Current Estimates” (Figure 6A) and rates of 10% RSV-MAARI and 3% RSV-MAALRI as “Conservative Estimates” (Figure 6B), and considered 1:1 and 2:1 randomization of vaccinees to placebo recipients. We calculated sample sizes needed to demonstrate 55% efficacy against RSV-MAARI and 80% efficacy against RSV-MAALRI, which are well within the ranges demonstrated by the more promising vaccines (Figure 3). We found that a study of 540 RSV-naive children (with 1:1 or 2:1 randomization) would be sufficient to demonstrate ≥80% efficacy (blue circle) against RSV-MAALRI and to demonstrate ≥55% (red circle) efficacy against RSV-MAARI (Figure 6A). If conservative estimates of RSV-MAARI and RSV-MAALRI are used, then 1,300 RSV-naive children would be needed (Figure 6B). As the observed rate of RSV-MAARI in our population is ∼threefold greater than RSV-MAALRI, a study powered for efficacy against RSV-MAALRI should be able to detect efficacy against RSV-MAARI (Figure 6).
Figure 6.
Power curves showing estimates of sample sizes needed to detect varying levels of vaccine efficacy against respiratory syncytial virus (RSV)-associated medically attended acute respiratory illness (MAARI) in RSV-seronegative children (red, 90% power; orange, 80% power) and against RSV-associated medically attended acute lower respiratory illness (MAALRI) (dark blue, 90% power; light blue, 80% power). In each case, the solid lines assume a 1:1 vaccine-to-placebo allocation, whereas dotted lines indicate a 2:1 allocation (note that these lines are very similar). The upper panel uses estimates of RSV-MAARI and -MAALRI attack rates derived from our study data (“current estimate”; 21% and 7%, respectively), whereas the lower panel assumes attack rates that are half as large (“conservative estimate”; 10% and 3%, respectively). Dotted lines indicate a sample size of (A) 540 and (B) 1,300; red circles, 55% efficacy against RSV-MAARI; blue circles, 80% efficacy against RSV-MAALRI.
Discussion
RSV infection causes substantial morbidity and mortality in very young infants, older infants, and young children (1, 18). Passive immunoprophylaxis, whether through maternal immunization or administration of RSV mAb, will not be sufficiently durable to protect these latter groups. For this reason, live-attenuated intranasal RSV vaccines have been in development for decades, with new-generation live-attenuated vaccine candidates produced using rational vaccine design (19–22). This study is the first to demonstrate substantial protection against both RSV-MAARI and RSV-MAALRI by the most promising of these vaccine candidates and strengthens the case for rapid clinical development of live-attenuated RSV vaccines.
Our data show that a greater than or equal to fourfold rise in RSV PRNT in vaccinees is predictive of efficacy against RSV-MAARI and RSV-MAALRI. The predictive value of a greater than or equal to fourfold rise for protection against RSV-MAALRI was striking; no child with this rise in titer experienced RSV-MAALRI.
It seems unlikely that serum-neutralizing antibodies alone would completely account for protection against RSV-MAARI/MAALRI, because serum antibodies do not efficiently transudate into the nasal mucosa (23). It is therefore likely that for RSV-MAARI/MAALRI, a greater than or equal to fourfold rise in PRNT is both a direct mediator of protection and a marker for other protective immune responses, such as mucosal antibody and/or cellular immune responses. Similarly, the substantial serum RSV neutAb anamnestic (memory) response that we detected in postsurveillance serum specimens likely reflects exposure to wt RSV and may be a marker for anamnestic mucosal and cellular immune responses that also restrict RSV replication. In this study, a specific protective titer of serum RSV neutAb could not be determined, which may be a consequence of small numbers or may mean that, for these vaccines, other factors are more important for protection against respiratory disease than the magnitude of the initial serum antibody response, such as priming for a strong anamnestic response, or induction of local and cellular immunity. Notably, we could not demonstrate a correlation between the magnitude of vaccine virus shedding and protection against RSV-MAARI in this relatively small group of children. Thus, although assessment of shedding is critically important for assessment of vaccine safety in early clinical trials, it will likely not be needed for efficacy trials of live-attenuated RSV vaccines.
These findings also underscore the importance of considering type and route of immunization when establishing correlates of protection for RSV immunoprophylaxis. Achieving a particular level of serum RSV neutAb is likely to be critical when protection of an infant is mediated exclusively through passively acquired immunity, as with maternal RSV immunization or administration of an RSV mAb. However, this is likely less important for active intranasal immunization, which primes for anamnestic responses yielding serum PRNT ∼10-fold higher than the primary response and also likely induces local and cellular immune responses that could be boosted with RSV infection. Further studies may help elucidate the relative contributions of these components of the immune response.
Our study also demonstrated that vaccine-induced serum RSV neutAb titers were sustained over time: although serum neutAb GMT measured 6–12 months after vaccination in RSV-uninfected children was lower than at Day 56, the difference was modest (111 vs. 79). This was reassuring: immune responses to RSV have been suggested to be short-lived, which was not evident in this study. These data suggest that a single dose of vaccine may be sufficient to protect children throughout an RSV season and that RSV vaccine could therefore be administered either seasonally or with routine immunizations during the first year of life.
This analysis had several limitations, at least two of which may have lowered our estimates of efficacy. First, our individual trials were neither designed nor powered to assess efficacy; even with pooled data (Figure 1), many efficacy estimates had wide CIs (Figure 3) and may have been sensitive to random variation. Second, our efficacy calculations included five placebo recipients with RSV neutAb at Day 56 (presumably a result of asymptomatic wt RSV infection) and were therefore less susceptible to RSV-MAARI and RSV-MAALRI than RSV-naive children, which would have decreased efficacy estimates (although we cannot exclude asymptomatic wt RSV infection in vaccinees during the same time period). In addition, two vaccinees assessed as experiencing RSV-MAARI received antibiotics for asymptomatic otitis media during well-child visits, and thus, this diagnosis may have been made incidentally.
Finally, there are important limitations related to our sample size estimates. Our sample size calculations were based upon rates of RSV-MAARI and RSV-MAALRI observed in an RSV-naive (seronegative) population, but it is likely that phase 3 efficacy trials will enroll unscreened infants and young children, some of whom will not be RSV naive. Our historical data suggest that the rate of RSV seropositivity in children 6–18 months of age may range from 10% to 15%. Studies not restricted to seronegative children will require larger sample sizes. RSV-experienced (seropositive) children would likely derive little benefit from these highly attenuated vaccines but would also likely contribute many fewer RSV-MAALRI study endpoints. Moreover, the studies described here were conducted exclusively in healthy children living in the United States, during a limited timespan (6). Rates of RSV disease can vary markedly from year to year or by geography. Other ranges of seasonal attack rates should be considered in the more complex sample size calculations that will be necessary for optimal design of future trials, which will need to account for potential differences in rates of RSV-associated illnesses and healthcare-seeking behavior in the settings where trials will be conducted. Despite these considerations (and others beyond the scope of this discussion), these preliminary data suggest that the efficacy of a live-attenuated RSV vaccine that reliably induces neutAb responses could be assessed in a relatively small phase 3 trial.
Conclusions
We have shown that serum RSV neutAb following a single dose of vaccine are comparable to primary responses to wt RSV, that vaccine-induced RSV PRNT are durable, that live-attenuated RSV vaccines that induce greater than or equal to fourfold rises in RSV PRNT provide protection against RSV-MAARI and substantial protection against RSV-MAALRI, and that more precise estimates of efficacy could be made with relatively small clinical trials. Efforts to ensure rapid progress in the clinical development of live-attenuated RSV vaccines could have a substantial global impact on pediatric health.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the children and families who participated in the clinical trials that were analyzed in this study, and the pediatric practices that allowed us to approach families in their offices. They acknowledge members of the protocol teams for their expert contributions, including Devasena Gnanashanmugam, Patrick Jean-Philippe, Paul Sato, Jack Moye, Jr., George Siberry, Oswald Dadson, Alexandria DiPerna, Andee Fox, Barbara Heckman, Rachael Henderson, Benjamin Johnston, Linda Marillo, Heather Sprenger, Ana Martinez, Lynette Purdue, Vivian Rexroad, Paul Harding, Linda Lambrecht, Dale Dayton, Carolyn Yanavich, Emily Barr, Michele Kelly, Amanda Dempsey, Philip LaRussa, Elizabeth Schappell, Lolita Kelley, and Scott Watson. They also thank Shirley Jankelevich, Marc Teitelbaum, Kelly Cahill, Susan Vogel, and John Tierney of the Regulatory Compliance & Human Subjects Protection Branch, Division of Clinical Research, NIAID, NIH, and the NIAID Division of Clinical Research Data and Safety Monitoring Board. Gregory Glenn, Novavax, graciously provided baculovirus-expressed RSV F protein used as ELISA antigen. Finally, the authors thank the dedicated investigators and research professionals at the following institutions: Children’s Hospital of Philadelphia, David Geffen School of Medicine at University of California Los Angeles, Emory University, Jacobi Medical Center, Johns Hopkins University Center for Immunization Research, Lurie Children’s Hospital of Chicago, Rush University/Cook County Hospital Chicago, St. Jude Children’s Research Hospital, SUNY Stony Brook, University of California San Diego, University of Colorado Denver, and University of Southern California.
Footnotes
R.A.K., J.E.A., S.W., and K.W. were funded by National Institute of Allergy and Infectious Diseases (NIAID) contract HHS 272200900010C. P.L.C. and U.J.B. and were funded by the NIAID Intramural Program. This work was also supported in part by a Cooperative Research and Development Agreement between NIAID, NIH, and Sanofi Pasteur, Inc., and by the Duke University Center for AIDS Research, NIH 5P30 AI064518. Support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network was provided by NIAID, the National Institute of Child Health and Human Development, and the National Institute of Mental Health, all components of NIH, under UM1AI068632, UM1AI068616, UM1AI106716, and HHSN275201800001I. The content is the responsibility of the authors and does not represent the official views of the NIH.
Author Contributions: P.L.C. and U.J.B. developed all of the vaccines described in this study except for MEDI∆M2–2. R.A.K., E.J.M., C.K.C., P.M., P.L.C., and U.J.B. designed the clinical trials. R.A.K., E.J.M., C.K.C., P.M., C.P., J.L., S.A.S., R.Y., M.A., S.W., and K.W. participated in the conduct of these studies. R.A.K., J.E.A., S.W., and U.J.B. performed and interpreted the data analysis, and all authors contributed to the final draft of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202005-1660OC on September 1, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Shi T, McAllister DA, O’Brien KL, Simoes EAF, Madhi SA, Gessner BD, et al. RSV Global Epidemiology Network. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390:946–958. doi: 10.1016/S0140-6736(17)30938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pneumonia Etiology Research for Child Health (PERCH) Study Group. Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. Lancet. 2019;394:757–779. doi: 10.1016/S0140-6736(19)30721-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karron RA, Zar HJ. Determining the outcomes of interventions to prevent respiratory syncytial virus disease in children: what to measure? Lancet Respir Med. 2018;6:65–74. doi: 10.1016/S2213-2600(17)30303-X. [DOI] [PubMed] [Google Scholar]
- 4.Mazur NI, Higgins D, Nunes MC, Melero JA, Langedijk AC, Horsley N, et al. Respiratory Syncytial Virus Network (ReSViNET) Foundation. The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. Lancet Infect Dis. 2018;18:e295–e311. doi: 10.1016/S1473-3099(18)30292-5. [DOI] [PubMed] [Google Scholar]
- 5.Karron RA, Black RE. Determining the burden of respiratory syncytial virus disease: the known and the unknown. Lancet. 2017;390:917–918. doi: 10.1016/S0140-6736(17)31476-9. [DOI] [PubMed] [Google Scholar]
- 6.Karron RA, Luongo C, Thumar B, Loehr KM, Englund JA, Collins PL, et al. A gene deletion that up-regulates viral gene expression yields an attenuated RSV vaccine with improved antibody responses in children. Sci Transl Med. 2015;7:312ra175. doi: 10.1126/scitranslmed.aac8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McFarland EJ, Karron RA, Muresan P, Cunningham CK, Valentine ME, Perlowski C, et al. International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) 2000 Study Team. Live-attenuated respiratory syncytial virus vaccine candidate with deletion of RNA synthesis regulatory protein M2-2 is highly immunogenic in children. J Infect Dis. 2018;217:1347–1355. doi: 10.1093/infdis/jiy040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchholz UJ, Cunningham CK, Muresan P, Gnanashanmugam D, Sato P, Siberry GK, et al. International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) P1114 Study Team. Live respiratory syncytial virus (RSV) vaccine candidate containing stabilized temperature-sensitivity mutations is highly attenuated in RSV-seronegative infants and children. J Infect Dis. 2018;217:1338–1346. doi: 10.1093/infdis/jiy066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McFarland EJ, Karron RA, Muresan P, Cunningham CK, Perlowski C, Libous J, et al. Live-attenuated respiratory syncytial virus vaccine with M2-2 deletion and with small hydrophobic noncoding region is highly immunogenic in children. J Infect Dis. 2020;221:2050–2059. doi: 10.1093/infdis/jiaa049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1969;89:405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- 11.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 12.Wright PF, Karron RA, Belshe RB, Shi JR, Randolph VB, Collins PL, et al. The absence of enhanced disease with wild type respiratory syncytial virus infection occurring after receipt of live, attenuated, respiratory syncytial virus vaccines. Vaccine. 2007;25:7372–7378. doi: 10.1016/j.vaccine.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acosta PL, Caballero MT, Polack FP. Brief history and characterization of enhanced respiratory syncytial virus disease. Clin Vaccine Immunol. 2015;23:189–195. doi: 10.1128/CVI.00609-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karron RA, Wright PF, Crowe JE, Jr, Clements-Mann ML, Thompson J, Makhene M, et al. Evaluation of two live, cold-passaged, temperature-sensitive respiratory syncytial virus vaccines in chimpanzees and in human adults, infants, and children. J Infect Dis. 1997;176:1428–1436. doi: 10.1086/514138. [DOI] [PubMed] [Google Scholar]
- 15.Karron RA, Wright PF, Belshe RB, Thumar B, Casey R, Newman F, et al. Identification of a recombinant live attenuated respiratory syncytial virus vaccine candidate that is highly attenuated in infants. J Infect Dis. 2005;191:1093–1104. doi: 10.1086/427813. [DOI] [PubMed] [Google Scholar]
- 16.Wright PF, Karron RA, Madhi SA, Treanor JJ, King JC, O’Shea A, et al. The interferon antagonist NS2 protein of respiratory syncytial virus is an important virulence determinant for humans. J Infect Dis. 2006;193:573–581. doi: 10.1086/499600. [DOI] [PubMed] [Google Scholar]
- 17.Cunningham CK, Karron R, Muresan P, McFarland EJ, Perlowski C, Libous J, et al. International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) 2012 Study Team. Live-attenuated respiratory syncytial virus vaccine with deletion of RNA synthesis regulatory protein M2-2 and cold passage mutations is overattenuated. Open Forum Infect Dis. 2019;6:ofz212. doi: 10.1093/ofid/ofz212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins PL, Melero JA. Progress in understanding and controlling respiratory syncytial virus: still crazy after all these years. Virus Res. 2011;162:80–99. doi: 10.1016/j.virusres.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fields BN, Knipe DM, Howley PM.Respiratory syncytial virus Knipe DM, Howley PM.editors. Fields virology Vol. 2New York: Wolters Kluwer/Lippincott Williams & Wilkins; 20131086–1123. [Google Scholar]
- 21.Liesman RM, Buchholz UJ, Luongo CL, Yang L, Proia AD, DeVincenzo JP, et al. RSV-encoded NS2 promotes epithelial cell shedding and distal airway obstruction. J Clin Invest. 2014;124:2219–2233. doi: 10.1172/JCI72948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luongo C, Winter CC, Collins PL, Buchholz UJ. Respiratory syncytial virus modified by deletions of the NS2 gene and amino acid S1313 of the L polymerase protein is a temperature-sensitive, live-attenuated vaccine candidate that is phenotypically stable at physiological temperature. J Virol. 2013;87:1985–1996. doi: 10.1128/JVI.02769-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siber GR, Leombruno D, Leszczynski J, McIver J, Bodkin D, Gonin R, et al. Comparison of antibody concentrations and protective activity of respiratory syncytial virus immune globulin and conventional immune globulin. J Infect Dis. 1994;169:1368–1373. doi: 10.1093/infdis/169.6.1368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.