Abstract
An expert panel was convened in September 2019 by The International Scientific Association for Probiotics and Prebiotics (ISAPP) to develop a definition for fermented foods and to describe their role in the human diet. Although these foods have been consumed for thousands of years, they are receiving increased attention among biologists, nutritionists, technologists, clinicians and consumers. Despite this interest, inconsistencies related to the use of the term ‘fermented’ led the panel to define fermented foods and beverages as “foods made through desired microbial growth and enzymatic conversions of food components”. This definition, encompassing the many varieties of fermented foods, is intended to clarify what is (and is not) a fermented food. The distinction between fermented foods and probiotics is further clarified. The panel also addressed the current state of knowledge on the safety, risks and health benefits, including an assessment of the nutritional attributes and a mechanistic rationale for how fermented foods could improve gastrointestinal and general health. The latest advancements in our understanding of the microbial ecology and systems biology of these foods were discussed. Finally, the panel reviewed how fermented foods are regulated and discussed efforts to include them as a separate category in national dietary guidelines.
Subject terms: Microbiome, Colon
Although fermented foods have been consumed for thousands of years, a clear definition has been lacking. This Consensus Statement outlines a definition for the term ‘fermented foods’ as determined by an expert panel convened by the International Scientific Association for Probiotics and Prebiotics in September 2019.
Introduction
Fermented foods and beverages accompanied and likely facilitated the transition from hunter-gatherer communities to sessile agricultural communities in the Neolithic revolution about 14,000 years ago1,2. They have remained staples of human diets for centuries and are an increasingly popular food category. Yet, their emergent popularity in the past 20 years has led to numerous misunderstandings and questions. What constitutes fermentation? Do fermented foods necessarily contain live microorganisms? Are fermented foods the same as probiotic foods? Do microorganisms in fermented foods become established in the gut or influence the gut microbiota? Do fermented foods provide health benefits and, if so, how?
Accordingly, the International Scientific Association for Probiotics and Prebiotics (ISAPP) organized a meeting of clinical and scientific experts in family medicine, microbiology, food science and technology, ecology, immunology, and microbial genetics held in September 2019 to develop a consensus report on fermented foods (a category that includes fermented beverages). The main goals of this Consensus Statement are to provide researchers, health-care providers, industry, regulators and consumers with a clear and concise definition of fermented foods, to differentiate between fermented foods and probiotics, and to summarize what is known about the health effects and safety of fermented foods. This Consensus Statement also discusses the mechanistic rationale for how fermented foods could improve gastrointestinal and systemic health, the advancements in knowledge on the microbial ecology and systems biology of those foods, and the current regulatory considerations and position of these foods in dietary guidelines.
Methods
The consensus panel was organized under the auspices of ISAPP, which is a non-profit organization governed by a volunteer board of directors. Although funded by member companies, ISAPP’s activities are not stipulated by industry. The mission is to provide objective, science-based information on probiotics, prebiotics and related health topics. Panel members were identified and invited based on their subject matter expertise and experience. An outline was developed and each expert was asked to address specific topics. The panel discussed each issue until consensus was reached. Following the meeting, each panellist wrote relevant sections and the assembled draft was reviewed and approved by all authors. The authors thank members of the ISAPP board of directors who did not directly participate in this consensus panel but who reviewed, provided comments and approved this manuscript: G. Gibson, E. Quigley, S. Salminen, K. Scott and H. Szajewska.
Historical context
Humans must have learned early in their history that fermentation provided many important advantages for managing precious food resources. Fermentation can improve the functional properties of agricultural crops and transform bland raw materials into nutritious, palatable or intoxicating products. Certainly, fermentation would have been regarded as one of the most effective ways to preserve foods owing, in part, to the formation of organic acids, alcohols, bacteriocins and other antimicrobial end-products as a result of fermentation microorganisms3. Fermentation-associated microorganisms usually out-compete potential pathogenic and spoilage organisms, further enhancing food safety and stability. In the absence of potable water, fermented beverages, such as beer, wine, sour milk and cereal gruels, provided a safe and transportable source of liquids4. These qualities, along with the fermentation-mediated transformation of perishable raw food materials into organoleptically satisfying products, led to their adoption by nearly every culture worldwide.
One particular example of how fermented foods and human culture co-evolved is through dairy fermentations5. The consumption of fermented milk products, including cheese, pre-date human lactase persistence, suggesting that lactose removal might have been one of the initial aims of this process6. Similarly, the human attraction to flavour-potentiating nucleotides and amino acids that are enriched in certain fermented foods, such as soy sauce and miso, could have evolved as a result of the safety and nutritional benefits of those foods in early human diets7. The extended shelf-life of fermented foods and the removal of noxious plant compounds by fermentation still serve critical purposes in regions of the world that have low food security and poor access to refrigeration, electricity and clean water. Even in societies for which sanitation and preservation are not a problem, fermented foods constitute an important part of the human diet. It is estimated that more than 5,000 varieties of fermented foods (and beverages) are currently produced and consumed globally8.
Beyond their importance to public health and food preservation and quality, current epidemiological evidence suggests that diets rich in fermented foods can reduce disease risk and enhance longevity, health, and quality of life9–11. Nonetheless, with the exception of yoghurt and other cultured dairy products, few well-designed, randomized controlled trials (RCTs) on the health benefits of the array of fermented foods have been published. Likewise, hypothesis-driven research describing the mechanisms of how fermented foods affect human physiology is limited. Defining these gaps can provide a basis for future research, including experiments aimed at understanding the potential health benefits of fermented foods.
Defining fermentation
Biochemists define fermentation as “an ATP-generating process in which organic compounds act as both donors and acceptors of electrons”12. Although this definition might be relevant for anaerobic lactic and ethanolic fermentations13 that occur in yoghurt, kimchi or wine, it does not apply to numerous other food fermentations. Fermentation as applied to foods and beverages has a much broader meaning and includes reactions and pathways that do not involve any of the criteria implicit in the strict biochemical definition. For example, aerobic metabolism is used by fungi responsible for koji, the starting material for soy sauce and miso, and in the manufacture of vinegar and kombucha by acetic acid bacteria (AAB)14,15. Accordingly, the panel proposes a broader definition that accounts for these variations in metabolic pathways. Thus, we define fermented foods and beverages as: “foods made through desired microbial growth and enzymatic conversions of food components”.
The definition requires the activity of microorganisms. Although endogenous or exogenous enzymes from plants, animals or other sources might be present, the activities of those enzymes alone are insufficient for a food to be regarded as fermented. This definition is sufficiently broad to include not only the fermentations noted earlier but also to distinguish fermentation from its microbiological converse, namely food spoilage. Whereas both processes occur via microbial growth and enzymatic activity on food constituents, spoilage is clearly unintentional and fermentation is deliberate and controlled to generate the desirable attributes.
What is included or excluded in the fermented foods definition?
This definition of fermented foods and beverages accommodates the many products made globally from diverse starting materials (Box 1). The definition includes foods and beverages that are produced by fermentation but might not have living microorganisms at the time of consumption. Fermented foods, such as leavened breads, are baked after fermentation, effectively killing the fermentation microorganisms. The manufacture of some fermented foods (for example, most beers and wines) includes steps to remove live microorganisms from finished products. Although microbial inactivation or removal is not common to all fermentation processes, these products still qualify as fermented foods.
Some salad dressing, mustard and other condiments might include ingredients made by fermentation such as vinegar or sour cream. In our view, these foods would not satisfy the definition of a fermented food, even if they contained an appreciable amount of a fermented ingredient (Box 2), nor would a non-fermented food supplemented with added microorganisms be considered fermented. Lastly, there are chemically derived versions of fermented foods; these foods are not fermented (Box 2). For example, some soft cheeses can be made by chemical acidification and fruits and vegetables are often preserved by ‘pickling’ processes that do not require the presence of live microorganisms. In some regions, the production of so-called synthetic vinegar and non-brewed soy sauce use chemical processes14,16. Of note, some cured meat products (made with nitrate or nitrite salts) can be fermented or non-fermented.
Box 1 Fermented food classification based on the presence of live microorganisms.
Fermented
Live microorganisms present
Yoghurt
Sour cream
Kefir
Most cheeses
Miso
Natto
Tempeh
Non-heated fermented vegetables
Non-heated salami, pepperoni and other fermented sausages
Boza, bushera and other fermented cereals
Most kombuchas
Some beers
Live microorganisms absent
Bread
Heat-treated or pasteurized fermented vegetables, sausage, soy sauce, vinegar and some kombuchas
Wine, most beers and distilled spirits
Coffee and chocolate beans (after roasting)
Not fermented
Chemically leavened bread
Fresh sausage
Vegetables pickled in brine and/or vinegar
Chemically produced soy sauce
Salted or cured processed meats and fish
Box 2 Key conclusions of this consensus paper.
Fermented foods are defined as foods made through desired microbial growth and enzymatic conversions of food components.
Microorganisms (either autochthonous or intentionally added) determine the course and outcome of fermentation processes and contribute to the development of the characteristic properties of the final fermented food.
Fermented food products should only be labelled as ‘containing probiotics’ when there is evidence that their live microbial components provide health benefits and the precise microbiological content is defined.
A modern understanding of patterns of microbial community succession during the fermentation and ageing of fermented foods is being obtained through the application of metagenomics, metatranscriptomics and metabolomics.
A better understanding of the health effects of fermented foods based on data available from population-based diet and health studies as well as new randomized controlled trials are needed to clarify the role of the consumption of fermented foods and of the live microorganisms they might contain in human health.
When properly made, fermented foods and the bacteria and fungi responsible for their manufacture have a long history of safe use.
Fermented foods could benefit health through the nutritive alteration of the ingredients, modulation of the immune system, the presence of bioactive compounds that affect intestinal and systemic function, or by modulating gut microbiota composition and activity.
What is the difference between fermented foods and probiotics?
Fermented foods and beverages are sometimes characterized or labelled as “probiotic foods” or “contains probiotics”. These declarations might reflect efforts by manufacturers to communicate to consumers that living, health-promoting microorganisms are present in the product. However, as noted in a previous consensus statement17, the term ‘probiotic’ should only be used when there is a demonstrated health benefit conferred by well-defined and characterized live microorganisms. The health benefit must, at least in part, be due to the live microorganisms and must extend beyond any nutritional benefit of the food matrix. For these reasons, the terms ‘fermented food’ and ‘probiotics’ cannot be used interchangeably (Table 1).
Table 1.
Distinctions between probiotics, fermented foods and probiotic fermented foods
| Microbial composition | |||||||
|---|---|---|---|---|---|---|---|
| Probiotic substance | Definition | Format | Evidence for health benefit | Claim that is consistent with categorya | Alive and present in levels demonstrated to provide benefit | Taxonomically defined to strain level | Genome sequence available |
| Probiotic | Live microorganisms that, when administered in adequate amounts, confer a health benefit on the host | No specific format required | Required | “Probiotic” can be used on the label along with a health benefit claim, such as “helps to reinforce the body’s natural defences”, if the claim is supported by evidence | Required | Required | Required |
| Fermented food | Foods made through desired microbial growth and enzymatic conversions of food components | Food | Not required | If live microorganisms are not present: “Foods made by fermentation”; if live microorganisms are present: “Contains live and active cultures” | Not required | Not required | Not required |
| Probiotic fermented food | Food fermented by or containing probiotic(s) with strain-specific evidence | Food | Required | Same as for probiotic | Required for probiotic but not for fermentation microorganisms | Required for probiotic but not for fermentation microorganisms | Required for probiotic but not for fermentation microorganisms |
| Food fermented by or containing probiotic(s) without strain-specific evidence | Food | Required | “Contains probiotics” | Required for probiotic but not for fermentation microorganisms | Required for probiotic but not for fermentation microorganisms | Required for probiotic but not for fermentation microorganisms | |
aAs allowed by local or regional regulations.
To label a product as a probiotic fermented food with an additional stipulated health benefit, evidence of a strain-specific benefit from a well-controlled intervention study is required together with proven safety and confirmation of sufficient numbers of that strain in the final product to confer the claimed benefit (Table 1). For example, traditional, spontaneously fermented sauerkrauts likely contain multiple strains of Lactiplantibacillus plantarum (previously Lactobacillus plantarum), but these uncharacterized and unidentified strains, at unknown doses, would not qualify as probiotics. By contrast, if L. plantarum 299v, a genetically characterized strain with clinically demonstrated probiotic properties18,19, was present at an efficacious dose until the end of shelf-life and there were no indications for inhibitory interactions of the sauerkraut matrix, this sauerkraut would meet the minimum criteria for a probiotic fermented food. Such products could contain an appropriately worded claim, for example, “probiotic sauerkraut containing L. plantarum 299v might improve intestinal well-being”, provided that local regulatory requirements are satisfied (Table 1).
In the absence of strain-specific evidence of a health benefit for the live microorganisms in a fermented food, some fermented foods could be appropriately labelled as “contains probiotics” (Table 1). This statement is only supported if at least one of the strains in the food meets the criteria implicit in the term probiotic and if the strain is a member of a well-studied species known to confer probiotic health benefits via the principle of ‘shared benefits’. This principle is based on the knowledge that certain bacterial species that are consistently active in human studies have conserved, or core, properties associated with improving health20. According to Hill et al.17 and Sanders et al.20, these bacterial species are sufficiently well studied such that most strains of that species can be reasonably expected to confer a health benefit. Consistent with this view, certain jurisdictions recognize several common species for which the term ‘probiotic’ can be used in foods. For example, Health Canada recognizes more than 20 species of the Lactobacillus genus complex and Bifidobacterium provided they are delivered at a minimum of 109 colony-forming units per serving21. In Europe, health claims related to live yoghurt cultures and improved lactose digestion are approved by the European Food and Safety Authority based on the core presence of the lactase enzyme in yoghurt cultures (Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus)22. However, in our view, even if the fermented food contains one or more of those species, the label “contains probiotics” should only be used when the strains in the fermented food are defined to the strain level, the genome sequences are known and the strains are present at an appropriate number during product shelf-life (Table 1).
It is expected that the majority of fermented foods sold commercially today do not belong in the “probiotic fermented food” category. Instead, fermented foods and beverages often contain undefined microbial consortia, usually at variable levels, and their potential health benefits have generally not been demonstrated23,24. Thus, we affirm the suggestion from Hill et al.17 that manufacturers should state only that their product contains “live and active cultures” provided the food is not processed to remove or kill the fermentation microorganisms and that these microorganisms are present at levels that are expected for foods of that type (Table 1). For pasteurized fermented foods without live microorganisms in the final product, it is acceptable to label those foods as “foods made by fermentation” (Table 1). Even when characterized cultures are used to perform fermentations and are understood at the strain level, those microorganisms are mostly selected based on performance characteristics, such as rapid acidification, substrate conversion, and flavour and texture properties, rather than on health-related functions. In the absence of evidence for species-level ‘shared benefits’ and knowledge that the strains are present at an appropriate number during product shelf-life, we suggest that manufacturers consider other labelling options (as noted earlier).
Do fermented foods contain prebiotics?
The presence of prebiotics, substrates selectively utilized by host microorganisms that confer a health benefit25, has been reported for several fermented foods and beverages. These examples would include fermented grains or vegetables26 as well as beer and wine27,28 that contain β-glucans, oligosaccharides and polyphenolic compounds29. Other fermented foods might contain prebiotics synthesized in situ by fermentation-associated microorganisms. For example, exopolysaccharides with prebiotic activity can be formed during dairy and cereal fermentations30. It is also possible that some fermented foods and beverages can contain both live microorganisms and prebiotic substrates. However, such products would not qualify as synbiotic foods31 in the absence of a demonstrated health benefit.
Making fermented foods
Which microorganisms are needed to make fermented foods?
To understand the scope of fermented foods in nutrition and health, it is necessary to acknowledge the wide diversity of microorganisms used for fermented food production. The most common fermented foods and beverages require lactic acid bacteria (LAB), AAB, bacilli or other bacteria, yeasts, or filamentous fungi. These microorganisms were among the very first to be isolated and characterized by Pasteur, Lister and other early microbiologists32–34 and have long served as model organisms in biology35,36 and as a source of industrial chemicals and bioactive molecules37,38. More recently, they were integral to the discovery and application of CRISPR technology39.
LAB are a group of Gram-positive, non-spore forming, aerotolerant bacteria that are phylogenetically positioned within the Firmicutes phylum, predominantly in the order Lactobacillales. They are among the most important and widely used microorganisms in food fermentations, serving essential functions in fermented dairy, meat, cereal and vegetable products40. LAB include the reclassified members of the Lactobacillaceae or Lactobacillus genus complex41 and numerous other taxa, including species of Lactococcus and Tetragenococcus associated with milk and soy sauce fermentations, respectively. Besides LAB, particular species of Bacillus and AAB are solely responsible for some fermented foods (for example, Bacillus subtilis used for natto, made from whole soybeans, and AAB for vinegar) or have important supporting roles as is the case for Staphylococcus, Enterococcus, Brevibacterium and Propionibacterium in sausage and cheese fermentations42,43. Among the fungi, ethanol-producing yeasts, usually species of Saccharomyces, are used for bread, beer, wine and various alcoholic fermentations. Interestingly, the domestication of Saccharomyces cerevisiae strains and their adaptation to a range of fermentation substrates and environments has led to the formation of distinct lineages associated with particular products44,45.
Similar domestication events are also likely responsible for the widespread use of atoxigenic filamentous fungi46. Penicillium, Aspergillus and Rhizopus are among the moulds commonly used for fermented dairy, meat and soy products and include proteinase, lipase and amylase-producing strains47. As described later, many food fermentations involve microbial communities consisting of multiple genera and taxa.
Considerable progress has been made towards understanding the function of individual microorganisms in fermented food production and then using that information to improve products and strains. Phylogenomic analyses have shown that, despite their general biochemical and physiological similarities, wine, beer and bread yeast strains evolved independently based on habitat and geography as well as through human-driven domestication48,49. Since the twentieth century, pure starter cultures have been developed to provide consistency and convenience and to accommodate large-scale industrial fermentations50. Typically, only one or two microbial strains (for example, bread, yoghurt, cheese) are necessary to initiate those fermentations51. Although technological performance properties remain one of the main criteria, the isolation and development of new strains increasingly relies on relevant genomic information and on the application of available molecular tools50,52–54.
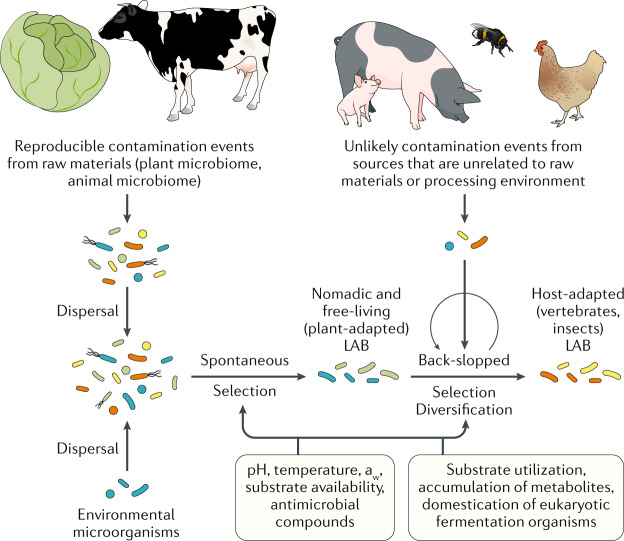
Culture-dependent methods remain the gold standard for the strain-level characterization of fermentation microbiota; however, these methods are increasingly complemented by holistic, meta-omics methods (metagenomics, metatranscriptomics, metaproteomics and metabolomics)55. Molecular approaches have shown that fermented foods are frequently dependent on complex, multi-kingdom, microbial communities functioning in concert via dynamic succession processes56–59. However, despite this complexity, the presence of a so-called core microbiota (defined as widespread microorganisms that are central to the functions of these ecosystems) are often apparent in a wide range of fermented foods60–64. Provided that the starting materials are generally the same, spontaneous fermentations (relying on autochthonous or resident microorganisms present in the ingredients and/or surrounding environment) typically result in products that contain very similar microorganisms (even the same species), regardless of provenance65. For example, fermentations of cabbage and other green leafy vegetables are all initiated by Leuconostoc mesenteroides followed by Lactiplantibacillus species and Levilactobacillus brevis, independent of whether the product is called sauerkraut (Europe and North America), kimchi (Korea), suan-cai (China) or sinki (Nepal)66. This highly reproducible succession of fermentation microbiota in spontaneous vegetable fermentations, whereby the assembly of fermentation microbiota is limited by dispersal, reflects the stable association of these organisms with the raw materials (Fig. 1). Similar reproducible successions occur in fungi-fermented foods67. Collectively, these and other observations suggest that selective and competitive pressures drive microbiome assembly and succession dynamics and provide a basis for predicting the outcome of food fermentations65. Thus, provided that the raw materials and environmental conditions are consistent with the typical practices used for making that food and that salt concentrations, pH, atmosphere or other expected control measures are in place, unpredictable events, which constitute fermentation failure, are relatively rare (Fig. 1). In the absence of those conditions and control measures, food fermentations could result in inferior or unsafe products.
Fig. 1. Processes that determine community assembly in traditional fermented foods.
The conditions established during traditional and industrial fermentations provide a basis for controlling and manipulating autochthonous and allochthonous microorganisms. Microbial communities in spontaneous food fermentations are determined by dispersal and selection. In most spontaneously fermented foods, plant-associated or animal-associated microorganisms are dominant. Back-slopping of fermented foods eliminates dispersal limitation, and selection is the major principle that determines community assembly. Among lactic acid bacteria (LAB), nomadic and free-living species are dominant in spontaneous food fermentations while host-adapted species dominate many back-slopped fermentations184. Speciation and domestication have been demonstrated for eukaryotic food fermenting organisms, including Saccharomyces cerevisiae and Aspergillus oryzae, but not for bacteria49,97. If comparable raw materials and fermentation protocols are employed, community assembly in fermented foods is reproducible at the genus level (spontaneous food fermentations) or even at the species level (back-slopped food fermentations). The assignment of lifestyles to food-fermenting lactobacilli has been previously described185. aw, water activity.
What processes are involved in making fermented foods
The outcome of a food or beverage fermentation process depends on the microorganism-led conversion of substrates into metabolites that support the aroma and taste, appearance, preservation, and nutritional properties of the finished product. These characteristics are time-dependent and determined by the microbiota as well as by a range of physicochemical parameters, including temperature, pH, water activity, oxidation–reduction potential and substrate availability. How these intrinsic and extrinsic environmental parameters are ultimately managed can have profound effects on the final properties and characteristics of fermented foods68.
Systems and evolutionary biology approaches are now providing a rational basis for controlling or managing microbial diversity and community structure to achieve different fermentation processes65,69. Although fermented foods have long been studied as model systems to understand microbial ecology70, these latest efforts integrate broader ecological and evolutionary principles, including dispersal, selection, drift and diversification65,71–73. The contribution of these principles to community assembly in fermented foods is outlined in Fig. 1. The use of these principles enables the control of fermentation microbiota in food independent of whether the fermentation is initiated with starter cultures, spontaneously or by inoculation from a prior successful fermentation of the same type (that is, back-slopping) (Fig. 1). The application of systems biology approaches combined with community reconstructions can identify specific microbial interactions that drive community composition51,74, determine a genetic basis for particular microorganisms to live in a fermented food environment75 and recreate the domestication processes that generated the industrial cultures used in fermentations76,77. Ultimately, findings from those studies will help to address product variation and quality issues that occur even when starter cultures are used. They might also lead to the identification of biomarkers to monitor these foods throughout production and to predict nutritional and health-impacting qualities.
Fermentation and food safety
Does fermentation improve food safety?
Fermented foods that contain appreciable levels of fermentation-produced organic acids (>100 mM), combined with low water activity, salt, nitrite and other antimicrobials, have a long record of food safety78. Likewise, beverages containing 4% or more alcohol and pH values less than 4.5 are also considered microbiologically safe79. Many LAB, whether part of the autochthonous microbiota or added as starter cultures, are known to produce bacteriocins that inhibit undesirable bacteria, including Listeria, Staphylococcus and Clostridium80.
Food fermentations can also enhance food safety and nutritional quality by removing toxic or anti-nutritive compounds from the raw ingredients. For example, the removal of toxic compounds is a prominent feature of cereal, legume and tuber fermentations81. Bitter cassava, for example, contains cyanogenic glycosides that must be removed by fermentation, soaking or other suitable processes to avoid acute toxicity when consumed82. During sourdough fermentations, some LAB facilitate the degradation of phytate, a cereal grain-associated compound that chelates divalent cations and prevents their absorption in the gastrointestinal tract83. Reducing phytate results in enhanced calcium, magnesium, iron and zinc bioavailability from these breads84–86. Sourdough fermentation is also hypothesized to reduce the concentration of other immune-reactive proteins, including the amylase-trypsin inhibitor in wheat, and could therefore be better tolerated than conventional breads by individuals with non-coeliac wheat intolerance or irritable bowel syndrome87.
Do fermented foods have food safety risks?
For any food product, there are safety concerns associated with live pathogenic microorganisms as well as toxins or metabolic products that can produce harmful effects. With few exceptions, food-fermenting LAB, yeasts and filamentous fungi are non-pathogenic and do not produce toxins or harmful end-products88. When properly made from safe and wholesome ingredients, fermented foods are rarely associated with gastroenteritis. Nonetheless, some cheeses and low-acid fermented foods can pose a safety risk if the food is contaminated with Listeria monocytogenes, Salmonella, Clostridium botulinum or other foodborne pathogens89. Although not a direct effect on safety, some microorganisms, including species of the Lactobacillaceae as well as Enterococcus and Staphylococcus associated with long-ripened cheeses, sausages and other fermented foods, can carry transmissible antibiotic-resistance genes90–92.
The microbial metabolites of some fermented foods can, under certain circumstances, also present safety risks. Alcohol (for example, wine, beer and liquor) and salt (for example, soy sauce or kimchi) are inherent constituents of some fermented foods and should be consumed in moderation. Histamine, tyramine and other biogenic amines are formed by some LAB via the decarboxylation of amino acids during the fermentation of cheese, meats, vegetables, soybeans and wine93. In the absence of host-mediated detoxification systems, these amines can cause mild to more severe effects such as migraines94. Several strategies have been adopted to reduce or mitigate biogenic amine formation, including hygiene to minimize the occurrence of microorganisms producing these compounds and using decarboxylase-negative starter cultures95,96.
Mycotoxins are a potential concern for all fermented foods produced with filamentous fungi. However, domestication and careful strain selection have effectively eliminated mycotoxin-producing lineages of Aspergillus and Penicillium from koji, cheese and other fermented foods76,97,98. Other microbial metabolites, including citrulline and reuterin, are precursors of the toxic compounds ethyl carbamate99 and acrolein100, respectively. Both occur in alcoholic beverages as well as in other fermented foods. However, their risks to human health from the exposure to fermented foods have not been established101,102.
Fermented foods and human health
What is the current evidence that fermented foods benefit human health?
Consumer interest in fermented foods has been driven in large part by their suggested nutritional benefits, and this interest has led to renewed popularity of these foods on nearly every continent24,103. However, except for yoghurt and cultured dairy products, few human clinical studies have been performed to verify their benefits23,24,104. Yoghurt consumption is associated with reductions in adiposity factors (BMI, waist circumference)105, type 2 diabetes mellitus and cardiovascular disease (see reviews106,107), among other positive indications108. Although much of this evidence is based on prospective or epidemiological studies, more than 20 RCTs with yoghurt and cultured milk products have been reported for both healthy individuals and patient population groups109. Likewise, milk kefir110, kimchi111, sauerkraut112, natto113, vinegar114 and sourdough bread115 have been investigated in at least one RCT. By contrast, evidence of health promotion for other fermented foods (for example, kombucha) is mostly limited to chemical analyses and animal and cell culture models24.
A better understanding of the health benefits of fermented foods will be obtained from harvesting information from existing population-based diet and health databases as well as with new RCTs. These studies should address the health outcomes arising from the intake of differentiated fermented food categories (including fermented dairy products and other fermented foods with living versus dead microorganisms), food types (such as fermented vegetables, fermented soy and yoghurt), and individual fermented food products with well-characterized strains and nutrient compositions. Large, placebo-controlled RCTs will need to account for the known limitations of these types of nutrition study, including blinding, sample size, diet control, dietary recall and adequate intervention times, as well as the challenges specific to fermented foods (in particular, how to provide relevant placebo treatments). To prevent the foods from being easily distinguished by study participants, placebo controls might need to be made to provide the same sensory attributes expected for the fermented foods being tested. Retrospective cohort or, preferably, prospective cohort studies that meet the Bradford Hill criteria should be used116 and efforts should be made to avoid misleading or unwarranted conclusions117. It should be noted that additional challenges exist for cohort studies because dietary databases do not often include fermented foods as a category and critical aspects of those foods might not be reported (for example, percent fat, percent protein or microbiological content).
What is the mechanistic basis for the health benefits of fermented food?
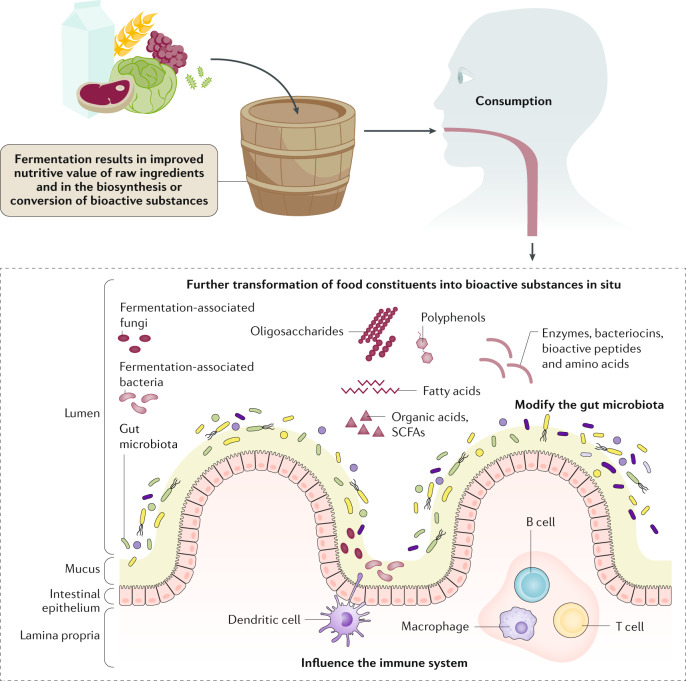
Knowledge on the specific health-promoting properties of fermented foods provides a foundation to evaluate how those properties vary by food type, strain composition and production methods. Several routes for health promotion by fermented foods are proposed (Fig. 2), including nutritive alteration of raw ingredients and the biosynthesis of bioactive compounds, modification of the human gut microbiota, and development and modification of the immune system.
Fig. 2. Mechanistic basis for the health benefits of fermented foods.
Health benefits, beyond the nutritional contributions of the raw ingredients, result from the removal, synthesis and transformation of the food components during fermentation by the activities of fermentation-associated microorganisms. Such actions can result in improved nutritive value of the food (for example, through phytate detoxification or vitamin synthesis) or in the generation of biologically active compounds (for example, bioactive peptides or conjugated linoleic acid). Food constituents and fermentation products, along with any remaining viable fermentation microorganisms, are consumed and enter the intestinal tract. Those microorganisms, along with resident members of the gut microbiota, might further transform food constituents in vivo into bioactive substances such as peptides, bacteriocins, amino acids, conjugated linoleic acid or organic acids. The constituents of fermented foods and fermentation-associated microorganisms and their cell products can interact with gut microbiota, the intestinal epithelium or the host immune system. SCFAs, short-chain fatty acids.
Microbial activity during food fermentations results in the enrichment and/or removal of compounds that affect the nutritional composition of the final food product118. Microorganisms reduce the concentrations of high-calorie monosaccharides and disaccharides (glucose, sucrose and fructose) present in milk, meat and plants via catabolic pathways. Reductions in certain sugars could also reduce the glycaemic index119,120 and improve food tolerability (for example, lactose in dairy foods, fructans in wheat, or raffinose, stachyose and verbascose in soybeans and legumes)121. Fermentation can result in the hydrolysis of polysaccharides, proteins or fats, thereby increasing their digestion122–124. Other enzymatic transformations with important nutritional implications also occur, including detoxification reactions (for example, degradation of linamarin in bitter cassava) and the removal of anti-nutritive factors (for example, inactivation of trypsin inhibitor in soybeans and phytic acid in cereals such as sorghum)125–127. For polyphenol-containing foods, the conversion of phenolic compounds by lactobacilli128 increases the bioavailability of flavonoids, tannins and other bioactive compounds129,130. The biosynthesis of vitamins, amino acid derivatives, organic acids and cofactors can also occur during fermentation23,131,132, with effects at either local gastrointestinal or systemic sites. Some of these compounds are broadly distributed between fermented food types (such as lactic acid133 and acetic acid134), whereas others are common in certain foods (for example, alkyl catechols135) or limited to certain microorganisms with specific enzymatic activities (for example, synthesis of γ-aminobutyric acid, conjugated linoleic acid or angiotensin-converting enzyme inhibitors132).
Multiple studies in humans have shown that microorganisms in fermented foods can survive gastric transit and reach the colon112,136–144. Indeed, many of the LAB that dominate lactic acid-fermented foods possess intrinsic characteristics that promote their ability to survive gastric transit (for example, acid and bile tolerance)145. Depending on individual dietary habits, fermented food-associated LAB can transiently constitute between 0.1% and 1% of the bacteria in the large intestine and a comparable proportion in the small intestine145. This percentage is based on current estimates of autochthonous microbiota in the gastrointestinal tract146 and the presence of up to 1011 LAB cells in a single serving of many fermented foods, such as yoghurt or kefir, that contain live and active microorganisms. Similarly, another study published in 2020 showed that food-associated LAB reached faecal metagenome abundances of >0.1%147. Although these microorganisms are unlikely to maintain long-term residence in the intestine, some fermented food microorganisms are known to be metabolically active in the gastrointestinal tract144,148, and short-term colonization could be sufficient to synthesize bioactive compounds, inhibit intestinal pathogens and mediate epithelial modulatory effects (for example, via interaction with Toll-like receptors149). Such interactions would be augmented by the repeated daily consumption of the fermented food. According to population-based studies and RCTs, fermented foods can also influence the composition of the gut microbiota136,150–153. Modulation of the gut microbiota can result from the living (or inactivated) microorganisms in those foods, the nutritional components and metabolites released as a result of fermentation, and changes these food constituents confer on the host immune system. These effects are likely dependent on inter-individual differences in host physiology and gut microbiota composition154.
As approximately 70% of the human immune system is located in the gastrointestinal tract155, foods and beverages are the major conduit of contact between external antigens and the human body. The gastrointestinal tract is vulnerable to the initial pattern of microbial colonization during the first months of life156, potentially setting a critical window for microbial stimuli effects on the immune system. In one cross-sectional study, fermented food intake (fermented vegetables) during early childhood was associated with a reduced risk of childhood atopy157,158. In another epidemiological study, fermented food consumption combined with common daily-life exposure (for example, hand versus machine dishwashing) also reduced the risk of childhood allergies157,158. The authors from the former study further reported that an anthroposophic lifestyle (low antibiotic use and vaccinations and high intake of fermented vegetables) was associated with differences in infant microbiome structure, including a higher abundance and diversity of LAB, and a higher concentration of acetate compared with infants from a traditional lifestyle159. Fermented food intake is also one of the synergistic factors associated with a farming upbringing, a lifestyle factor that has consistently been associated with reduced allergy and asthma risk (reviewed elsewhere160). These associations could indicate that a lack of fermented foods in modern, industrialized societies constitutes a substantial loss in exposure to non-harmful microorganisms important for immune system development and maintenance.
Although fermented foods such as milk kefir161 have been shown to modulate immune responses in numerous animal models, RCTs or prospective studies on the human immune system have yet to be performed. It is expected that the modulation of the human immune system by fermented foods would be the result of the combined effects of compounds present in the starting ingredients and those formed during fermentation as well as of living and dead or inactivated microorganisms. Those fermentation-associated microorganisms and their cell components (for example, peptidoglycan, surface proteins, exopolysaccharides and lipoteichoic acid) are already known to be immune reactive according to animal model and in vitro studies149,162–164. Knowledge about other immune-modulating compounds, such as d-phenyllactic acid, produced by lactic acid bacteria in situ165, is still emerging. Ultimately, the precise molecular stimuli in fermented foods responsible for immunomodulation probably depend on the total composition of the product133,134,166.
What are the regulatory considerations for fermented foods?
Guidelines that govern food fermentation are covered in international regulations and are mainly concerned with food safety167,168. The use of microbial cultures is also regulated and includes criteria for establishing safety, such as the ‘Generally Recognized As Safe’ designation in the USA or the ‘Qualified Presumption of Safety’ list in Europe. The latter, for example, is a designation assigned by the European Food and Safety Authority to groups of microorganisms that, in general, do not raise safety concerns as components of foods, including fermented foods169. Strains developed by the use of recombinant DNA technology or those that are genetically modified have different regulatory controls. For example, in the USA, genetically modified strains must have a ‘Generally Recognized As Safe’ status, whereas in Europe, such products require Qualified Presumption of Safety status170.
The identification of core microbial components in fermented foods has the potential to lead to new regulations around the labelling of these foods. Regulations could be used to ensure that minimum requirements relating to the involvement of specific microbial taxa in the fermentation process are met. Only a few standards exist, mostly for cultured dairy products. For example, the Codex Alimentarius states that yoghurt should be made using a combination of S. thermophilus and L. delbrueckii subsp. bulgaricus and that kefir is a fermented milk consisting of Lentilactobacillus kefiri and species of the genera Leuconostoc, Lactococcus and Acetobacter, in addition to lactose-fermenting yeasts (Kluyveromyces marxianus) and non-lactose-fermenting yeasts (Saccharomyces unisporus, S. cerevisiae and Saccharomyces exiguus)171. Similar standards could emerge as the microorganisms present in other fermented foods are identified (for example, kombucha and water kefir).
What is the standing of fermented foods in dietary guidelines?
Fermented foods are widely consumed around the world and have been estimated to account for approximately one-third of the human diet172,173. However, with few exceptions, fermented foods are generally absent as a recommended category in dietary guidelines172,174,175. The only country, to our knowledge, that has a specific guideline is India, which encourages pregnant women to consume fermented foods176. Other countries, including the USA and Canada, mention yoghurt and kefir in the dairy products section136,138, but there is no specific emphasis on fermented foods. Owing to the high levels of live, potentially health-promoting microorganisms in many fermented foods, these foods have been advocated for inclusion in dietary recommendations132,172,174. To advance this field, studies that collect dietary information should also track foods that contain live cultures. Adding granularity to dietary intake data so that fermented foods are not subsumed under other categories will enable researchers to better understand the role of these foods in health.
Implications for stakeholders
One of the main goals of this panel was to bring scientific clarity to the rapidly growing field of fermented foods and beverages. We anticipate that the outcomes described in this report (Box 2) could affect a range of stakeholders, including consumers, industry, government, and science communicators.
Consumers
Although consumers have become increasingly interested in fermented foods, it is unfortunate that, in our opinion, much information available on fermented foods in popular press magazines, websites and social media is exaggerated or inaccurate. For example, on the many internet and popular magazine lists of the ‘best super foods’, fermented foods are often ranked at the top. Such labels, while perhaps useful for marketing, do not convey accurate information for consumers regarding nutritional or other specific properties of fermented foods. Furthermore, as discussed earlier, fermented foods are frequently considered as probiotic foods, even when live microorganisms are absent in the final product and the health benefits have not been clinically demonstrated. This report clarifies these points for consumers and communicators.
Industry
As noted previously, fermented foods and beverages were among the first processed foods. Bread, beer, wine and fermented dairy, soy and other products continue to represent a considerable portion of the total processed foods industry. This form of processing remains extremely important in many parts of the world, whereby fermented foods can enhance both food security and sustainability177. Food fermentation can also provide new strategies for industry to address contemporary socioeconomic and health challenges involving ageing, malnutrition and obesity178. Manufacturers who produce and market fermented foods can benefit from clear definitions and criteria for what constitutes probiotic fermented foods. In particular, we reaffirm the statement in Hill et al.17 that fermented foods are not equivalent to probiotic foods. Many fermented food products have no evidence that their live microbial component provides health benefits and the precise microbiological content is rarely defined. Without this level of characterization, they should not be labelled as “containing probiotics”. Some manufacturers supplement fermented foods with microorganisms after a heat treatment, perhaps to satisfy consumer interest in adding live microorganisms to their diet. These products, in our view, do not reflect the expected characteristics of fermented foods containing live microorganisms. In general, there is no expectation that fermented foods must contain live microorganisms. The most notable exception is for yoghurt, where, depending on the jurisdiction, specific requirements can exist. Industry is responsible for producing fermented foods following good manufacturing practices and should practice advertising and labelling that is truthful and informative and should be consistent with the criteria stipulated above.
Government
In most jurisdictions, governments provide regulatory oversight of the safety and marketing of fermented foods, including advertising, product labelling and health benefit claims. In Europe, a broad range of fermented foods are made in accordance with so-called Protected Designation of Origin requirements that impose geographical, manufacturing and quality requirements179. Similar arrangements also exist in other countries. Although the Protected Designation of Origin framework is designed to control product claims about geographical origins and production practices and not microbiological properties of foods, per se, these protections can dictate the type or nature of the cultures used in cheese, sausages, bread, vinegar and other fermented foods. Thus, for these products, governments can indirectly influence how fermented products are produced as well as the safety and quality properties. This process is especially relevant as industrialization and high-throughput production practices have been adopted even by traditional small-scale manufacturers180.
Government agencies are also responsible for providing accurate and informative nutritional labelling and for reviewing and approving health benefit claims. However, as already noted, most regulatory agencies have not considered the potential inclusion of fermented foods in dietary guidance programmes beyond their nutritional contribution to health. Nonetheless, as more clinical and epidemiological studies are reported, such efforts could be warranted.
Conclusions
For more than a century, microbiologists have sought to identify and describe the relevant ‘microbial parts’ within fermented foods and beverages. Only in the past two decades have researchers from multiple scientific disciplines, including systems and molecular biology, microbial ecology, and bioinformatics, begun to understand how those parts are assembled to build microbial communities that are ultimately responsible for the attributes associated with fermented foods and beverages. Collectively, this research provides a rational basis for improving both the functional characteristics and nutritional properties of these foods. It could also be feasible to identify and introduce novel microbial species that can augment desirable traits50. Many spontaneously fermented foods serve as a rich reservoir of potentially valuable strains181–183. Of particular interest is the possibility of predicting the quality attributes of fermented foods and beverages based on the initial microbial composition of the raw materials. Ultimately, the production of fermented foods and beverages with greater quality control will ensure the delivery of products that provide flavour, texture and health-related attributes.
Acknowledgements
The authors thank members of the International Scientific Association for Probiotics and Prebiotics (ISAPP) board of directors who reviewed, edited and approved the manuscript: S. Salminen, K. Scott, G. Gibson, E. Quigley and H. Szajewska. Gratitude is expressed to ISAPP for funding the travel and meeting expenses associated with this panel.
Author contributions
All authors made equal contributions to the discussion of content and reviewing/editing the manuscript before submission. M.L.M., M.E.S., M.C.A., P.D.C., L.D.V., M.G., C.H., W.H., S.L., D.M., G.R., B.W. and R.H. wrote the article. M.L.M. and R.H. collated contributions into the final text.
Competing interests
M.L.M. has been compensated for consulting, speaking fees or service on advisory boards for the Kerry Health and Nutrition Institute and the Icelandic Milk & Skyr Corporation. M.E.S. has been compensated for funding or speaking engagements or for consulting from Associated British Foods, California Dairy Research Foundation, Cargill, Danone Research, Danone USA, Fairlife, General Mills, GlaxoSmithKline, JJ Heimbach, Kellogg, Kerry, Mead Johnson, Medscape, PepsiCo, Pfizer, Probi, Procter & Gamble, Trouw Nutrition, Visalia Dairy Company, Winclove Probiotics and Yakult. P.D.C. has research grants with several industry partners and is the co-founder and Chief Technology Officer of SeqBiome. C.H. serves on the board of International Scientific Association for Probiotics and Prebiotics (ISAPP), is a consultant to Artugen Therapeutics developing a live biotherapeutic and has research grants with several industry partners. W.H. has been compensated for consulting by SymbioPharm, received research grants from industry partners and is co-founder and Chief Technology Officer of HEM. S.L. serves on the board of ISAPP, is chair of the Scientific Advisory Board of Yun, has been compensated for speaking by Yakult and has research grants with several industry partners. D.M. serves on the board of ISAPP and has consulted for Bayer, Pharmavite and Dupont. G.R. serves on the board of ISAPP and has received fees from Seed, Danone, Acerus Pharma and KGK Science. R.H. serves on the board of ISAPP, has received grants or honoraria from Mead Johnson Nutrition, Pharmavite, Danone, Beachbody and PepsiCo, and is a co-owner of Synbiotic Health. M.G.G., M.C.A., L.D.V. and B.E.W. declare no competing interests.
Footnotes
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks F. Carbonero, L. Cocolin and E. Sonnenburg for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arranz-Otaegui A, Gonzalez Carretero L, Ramsey MN, Fuller DQ, Richter T. Archaeobotanical evidence reveals the origins of bread 14,400 years ago in northeastern Jordan. Proc. Natl Acad. Sci. USA. 2018;115:7925–7930. doi: 10.1073/pnas.1801071115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayden B, Canuel N, Shanse J. What was brewing in the natufian? an archaeological assessment of brewing technology in the Epipaleolithic. J. Archeol. Method. Theory. 2013;20:102–150. [Google Scholar]
- 3.Ross RP, Morgan S, Hill C. Preservation and fermentation: past, present and future. Int. J. Food Microbiol. 2002;79:3–16. doi: 10.1016/s0168-1605(02)00174-5. [DOI] [PubMed] [Google Scholar]
- 4.Steinkraus, K. H. In Fermented Food Beverages in Nutrition (ed Gastineau C. F., Darby W. J., Turner T. B.) 36–50 (Academic Press, 1979).
- 5.Segurel L, Bon C. On the evolution of lactase persistence in humans. Annu. Rev. Genomics Hum. Genet. 2017;18:297–319. doi: 10.1146/annurev-genom-091416-035340. [DOI] [PubMed] [Google Scholar]
- 6.Ingram CJ, Mulcare CA, Itan Y, Thomas MG, Swallow DM. Lactose digestion and the evolutionary genetics of lactase persistence. Hum. Genet. 2009;124:579–591. doi: 10.1007/s00439-008-0593-6. [DOI] [PubMed] [Google Scholar]
- 7.Breslin PA. An evolutionary perspective on food and human taste. Curr. Biol. 2013;23:R409–R418. doi: 10.1016/j.cub.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamang JP, Watanabe K, Holzapfel WH. Review: diversity of microorganisms in global fermented foods and beverages. Front. Microbiol. 2016;7:377. doi: 10.3389/fmicb.2016.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez-Gonzalez MA, Gea A, Ruiz-Canela M. The Mediterranean diet and cardiovascular health. Circ. Res. 2019;124:779–798. doi: 10.1161/CIRCRESAHA.118.313348. [DOI] [PubMed] [Google Scholar]
- 10.Pes GM, et al. Male longevity in Sardinia, a review of historical sources supporting a causal link with dietary factors. Eur. J. Clin. Nutr. 2015;69:411–418. doi: 10.1038/ejcn.2014.230. [DOI] [PubMed] [Google Scholar]
- 11.Sofi F, Macchi C, Abbate R, Gensini GF, Casini A. Mediterranean diet and health status: an updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. 2014;17:2769–2782. doi: 10.1017/S1368980013003169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim, B. H. & Gadd, G. M. Prokaryotic Metabolism and Physiology. (Cambridge University Pres., 2019).
- 13.Bamforth, C. W. & Cook, D. J. Food, Fermentation, and Micro-Organisms. (John Wiley & Sons, 2019).
- 14.Luh BS. Industrial production of soy sauce. J. Ind. Microbiol. 1995;14:467–471. [Google Scholar]
- 15.De Roos J, De Vuyst L. Acetic acid bacteria in fermented foods and beverages. Curr. Opin. Biotechnol. 2018;49:115–119. doi: 10.1016/j.copbio.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Ho CW, Lazim AM, Fazry S, Zaki U, Lim SJ. Varieties, production, composition and health benefits of vinegars: a review. Food Chem. 2017;221:1621–1630. doi: 10.1016/j.foodchem.2016.10.128. [DOI] [PubMed] [Google Scholar]
- 17.Hill C, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 18.Lonnermark E, et al. Intake of Lactobacillus plantarum reduces certain gastrointestinal symptoms during treatment with antibiotics. J. Clin. Gastroenterol. 2010;44:106–112. doi: 10.1097/MCG.0b013e3181b2683f. [DOI] [PubMed] [Google Scholar]
- 19.Malik M, et al. Lactobacillus plantarum 299v supplementation improves vascular endothelial function and reduces inflammatory biomarkers in men with stable coronary artery disease. Circ. Res. 2018;123:1091–1102. doi: 10.1161/CIRCRESAHA.118.313565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanders ME, Benson A, Lebeer S, Merenstein DJ, Klaenhammer TR. Shared mechanisms among probiotic taxa: implications for general probiotic claims. Curr. Opin. Biotechnol. 2018;49:207–216. doi: 10.1016/j.copbio.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Health Claims. Probiotic Claims. Summary table of acceptable non-strain specific claims for probiotics and eligible species for the claims. in Section 8.7.3http://www.inspection.gc.ca/english/fssa/labeti/guide/ch8ae.shtml#a8_7 (Canadian Food Inspection Agency, 2013).
- 22.EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) Scientific opinion on the substantiation of health claims related to live yoghurt cultures and improved lactose digestion (ID 1143, 2976) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010;8:1763. [Google Scholar]
- 23.Marco ML, et al. Health benefits of fermented foods: microbiota and beyond. Curr. Opin. Biotechnol. 2017;44:94–102. doi: 10.1016/j.copbio.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Dimidi E, Cox SR, Rossi M, Whelan K. Fermented foods: definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease. Nutrients. 2019;11:1806. doi: 10.3390/nu11081806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibson GR, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 26.Salmerón I. Fermented cereal beverages: from probiotic, prebiotic and synbiotic towards Nanoscience designed healthy drinks. Lett. Appl. Microbiol. 2017;65:114–124. doi: 10.1111/lam.12740. [DOI] [PubMed] [Google Scholar]
- 27.Kanyer AJ, Bornhorst GM, Marco ML, Bamforth CW. Is beer a source of prebiotics? J. Inst. Brew. 2017;123:361–365. [Google Scholar]
- 28.Apolinar-Valiente R, et al. Oligosaccharides of cabernet sauvignon, syrah and monastrell red wines. Food Chem. 2015;179:311–317. doi: 10.1016/j.foodchem.2015.01.139. [DOI] [PubMed] [Google Scholar]
- 29.Dueñas M, et al. Studies on modulation of gut microbiota by wine polyphenols: from isolated cultures to omic approaches. Antioxidants. 2015;4:1–21. doi: 10.3390/antiox4010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salazar N, Gueimonde M, de Los Reyes-Gavilán CG, Ruas-Madiedo P. Exopolysaccharides produced by lactic acid bacteria and bifidobacteria as fermentable substrates by the intestinal microbiota. Crit. Rev. Food Sci. Nutr. 2016;56:1440–1453. doi: 10.1080/10408398.2013.770728. [DOI] [PubMed] [Google Scholar]
- 31.Swanson KS, et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020;17:687–701. doi: 10.1038/s41575-020-0344-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lister JB. On the lactic fermentation and its bearings on pathology. Trans. Pathol. Soc. Lond. 1878;29:425–467. [Google Scholar]
- 33.Pasteur, L. Studies on Fermentation. The Diseases of Beer, their Causes, and the means of Preventing them. A Translation, made with the Author’s Sanction, of “Etudes sur la biere” with Notes, Index, and Original Illustration. (Robb Macmilan and Co., 1879 and Kraus Reprint Co., 1969).
- 34.Hansen, E. C. Practical Studies in Fermentation (E. & F.N. Spon, 1896).
- 35.Botstein D, Chervitz SA, Cherry M. Yeast as a model organism. Science. 1997;277:1259–1260. doi: 10.1126/science.277.5330.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maloney PC, Kashket ER, Wilson TH. A protonmotive force drives ATP synthesis in bacteria. Proc. Natl Acad. Sci. USA. 1974;71:3896–3900. doi: 10.1073/pnas.71.10.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hurst A. Biosynthesis of the antibiotic nisin by whole Streptococcus lactis organisms. J. Gen. Microbiol. 1966;44:209–220. doi: 10.1099/00221287-44-2-209. [DOI] [PubMed] [Google Scholar]
- 38.Sauer M, Russmayer H, Grabherr R, Peterbauer CK, Marx H. The efficient clade: lactic acid bacteria for industrial chemical production. Trends Biotechnol. 2017;35:756–769. doi: 10.1016/j.tibtech.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Barrangou R, et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315:1709–1712. doi: 10.1126/science.1138140. [DOI] [PubMed] [Google Scholar]
- 40.Gänzle MG. Lactic metabolism revisited: metabolism of lactic acid bacteria in food fermentations and food spoilage. Curr. Opin. Food Sci. 2015;2:106–117. [Google Scholar]
- 41.Zheng J, et al. A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Sys. Evol. Microbiol. 2020;70:2782–2858. doi: 10.1099/ijsem.0.004107. [DOI] [PubMed] [Google Scholar]
- 42.Talon R, Leroy S. Diversity and safety hazards of bacteria involved in meat fermentations. Meat Sci. 2011;89:303–309. doi: 10.1016/j.meatsci.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 43.Afshari R, Pillidge CJ, Dias DA, Osborn AM, Gill H. Cheesomics: the future pathway to understanding cheese flavour and quality. Crit. Rev. Food Sci. Nutr. 2020;60:33–47. doi: 10.1080/10408398.2018.1512471. [DOI] [PubMed] [Google Scholar]
- 44.Duan SF, et al. The origin and adaptive evolution of domesticated populations of yeast from Far East Asia. Nat. Commun. 2018;9:2690. doi: 10.1038/s41467-018-05106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hittinger CT, Steele JL, Ryder DS. Diverse yeasts for diverse fermented beverages and foods. Curr. Opin. Biotechnol. 2018;49:199–206. doi: 10.1016/j.copbio.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 46.Gibbons JG. How to train your fungus. mBio. 2019;10:e03031. doi: 10.1128/mBio.03031-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hyde KD, et al. The amazing potential of fungi: 50 ways we can exploit fungi industrially. Fungal Diversity. 2019;97:1–136. [Google Scholar]
- 48.Goncalves M, et al. Distinct domestication trajectories in top-fermenting beer yeasts and wine yeasts. Curr. Biol. 2016;26:2750–2761. doi: 10.1016/j.cub.2016.08.040. [DOI] [PubMed] [Google Scholar]
- 49.Peter J, et al. Genome evolution across 1,011 Saccharomyces cerevisiae isolates. Nature. 2018;556:339–344. doi: 10.1038/s41586-018-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johansen E. Use of natural selection and evolution to develop new starter cultures for fermented foods. Ann. Rev. Food Sci. Technol. 2018;9:411–428. doi: 10.1146/annurev-food-030117-012450. [DOI] [PubMed] [Google Scholar]
- 51.Sieuwerts S, de Bok FA, Hugenholtz J, van Hylckama Vlieg JE. Unraveling microbial interactions in food fermentations: from classical to genomics approaches. Appl. Environ. Microbiol. 2008;74:4997–5007. doi: 10.1128/AEM.00113-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wuyts S, Van Beeck W, Allonsius CN, van den Broek MF, Lebeer S. Applications of plant-based fermented foods and their microbes. Curr. Opin. Biotechnol. 2019;61:45–52. doi: 10.1016/j.copbio.2019.09.023. [DOI] [PubMed] [Google Scholar]
- 53.Langdon QK, et al. Fermentation innovation through complex hybridization of wild and domesticated yeasts. Nat. Ecol. Evol. 2019;3:1576–1586. doi: 10.1038/s41559-019-0998-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krogerus K, Holmstrom S, Gibson B. Enhanced wort fermentation with de novo lager hybrids adapted to high-ethanol environments. Appl. Environ. Microbiol. 2018;84:e02302-17. doi: 10.1128/AEM.02302-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walsh AM, Crispie F, Claesson MJ, Cotter PD. Translating omics to food microbiology. Ann. Rev. Food Sci. Technol. 2017;8:113–134. doi: 10.1146/annurev-food-030216-025729. [DOI] [PubMed] [Google Scholar]
- 56.Einson JE, et al. A vegetable fermentation facility hosts distinct microbiomes reflecting the production environment. Appl. Environ. Microbiol. 2018;84:e01680-18. doi: 10.1128/AEM.01680-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walsh AM, et al. Microbial succession and flavor production in the fermented dairy beverage kefir. mSystems. 2016;1:e00052-16. doi: 10.1128/mSystems.00052-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Illeghems K, De Vuyst L, Papalexandratou Z, Weckx S. Phylogenetic analysis of a spontaneous cocoa bean fermentation metagenome reveals new insights into its bacterial and fungal community diversity. PLoS ONE. 2012;7:e38040. doi: 10.1371/journal.pone.0038040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pswarayi F, Ganzle MG. Composition and origin of the fermentation microbiota of Mahewu, a Zimbabwean fermented cereal beverage. Appl. Environ. Microbiol. 2019;85:e03130-18. doi: 10.1128/AEM.03130-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marsh AJ, O’Sullivan O, Hill C, Ross RP, Cotter PD. Sequencing-based analysis of the bacterial and fungal composition of kefir grains and milks from multiple sources. PLoS ONE. 2013;8:e69371. doi: 10.1371/journal.pone.0069371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ercolini D, et al. Microbial ecology dynamics during rye and wheat sourdough preparation. Appl. Environ. Microbiol. 2013;79:7827–7836. doi: 10.1128/AEM.02955-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bokulich NA, Lewis ZT, Boundy-Mills K, Mills DA. A new perspective on microbial landscapes within food production. Curr. Opin. Biotechnol. 2016;37:182–189. doi: 10.1016/j.copbio.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kergourlay G, Taminiau B, Daube G, Champomier Verges MC. Metagenomic insights into the dynamics of microbial communities in food. Int. J. Food Microbiol. 2015;213:31–39. doi: 10.1016/j.ijfoodmicro.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 64.Vermote L, Verce M, De Vuyst L, Weckx S. Amplicon and shotgun metagenomic sequencing indicates that microbial ecosystems present in cheese brines reflect environmental inoculation during the cheese production process. Int. Dairy J. 2018;87:44–53. [Google Scholar]
- 65.Wolfe BE, Dutton RJ. Fermented foods as experimentally tractable microbial ecosystems. Cell. 2015;161:49–55. doi: 10.1016/j.cell.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 66.Zabat, M. A., Sano, W. H., Wurster, J. I., Cabral, D. J. & Belenky, P. Microbial Community analysis of sauerkraut fermentation reveals a stable and rapidly established community. Foods7, (2018). [DOI] [PMC free article] [PubMed]
- 67.Sternes PR, Lee D, Kutyna DR, Borneman AR. A combined meta-barcoding and shotgun metagenomic analysis of spontaneous wine fermentation. GigaScience. 2017;6:1–10. doi: 10.1093/gigascience/gix040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shiferaw Terefe N, Augustin MA. Fermentation for tailoring the technological and health related functionality of food products. Crit. Rev. Food Sci. Nutr. 2020;60:2887–2913. doi: 10.1080/10408398.2019.1666250. [DOI] [PubMed] [Google Scholar]
- 69.Ganzle M, Ripari V. Composition and function of sourdough microbiota: From ecological theory to bread quality. J. Food Microbiol. 2016;239:19–25. doi: 10.1016/j.ijfoodmicro.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 70.Daeschel MA, Andersson RE, Fleming HP. Microbial ecology of fermenting plant materials. FEMS Microbiol. Lett. 1987;46:357–367. [Google Scholar]
- 71.Pizarro F, Vargas FA, Agosin E. A systems biology perspective of wine fermentations. Yeast. 2007;24:977–991. doi: 10.1002/yea.1545. [DOI] [PubMed] [Google Scholar]
- 72.Blasche S, Kim Y, Oliveira AP, Patil KR. Model microbial communities for ecosystems biology. Curr. Opin. Sys Biol. 2017;6:51–57. [Google Scholar]
- 73.Miller ER, et al. Establishment limitation constrains the abundance of lactic acid bacteria in the napa cabbage phyllosphere. Appl. Environ. Microbiol. 2019;85:e00269-19. doi: 10.1128/AEM.00269-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Irlinger F, Mounier J. Microbial interactions in cheese: implications for cheese quality and safety. Curr. Opin. Biotechnol. 2009;20:142–148. doi: 10.1016/j.copbio.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 75.Morin M, Pierce EC, Dutton RJ. Changes in the genetic requirements for microbial interactions with increasing community complexity. eLife. 2018;7:e37072. doi: 10.7554/eLife.37072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bodinaku I, et al. Rapid phenotypic and metabolomic domestication of wild penicillium molds on cheese. mBio. 2019;10:e02445-19. doi: 10.1128/mBio.02445-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bachmann H, Starrenburg MJ, Molenaar D, Kleerebezem M, van Hylckama Vlieg JE. Microbial domestication signatures of Lactococcus lactis can be reproduced by experimental evolution. Genome Res. 2012;22:115–124. doi: 10.1101/gr.121285.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Adams M, Mitchell R. Fermentation and pathogen control: a risk assessment approach. Int. J. Food Microbiol. 2002;79:75–83. doi: 10.1016/s0168-1605(02)00181-2. [DOI] [PubMed] [Google Scholar]
- 79.Jeon SH, et al. Microbiological diversity and prevalence of spoilage and pathogenic bacteria in commercial fermented alcoholic beverages (beer, fruit wine, refined rice wine, and yakju) J. Food Prot. 2015;78:812–818. doi: 10.4315/0362-028X.JFP-14-431. [DOI] [PubMed] [Google Scholar]
- 80.O’Connor PM, Ross RP, Hill C, Cotter PD. Antimicrobial antagonists against food pathogens: a bacteriocin perspective. Curr. Opin. Food Sci. 2015;2:51–57. [Google Scholar]
- 81.Gänzle MG. Food fermentations for improved digestibility of plant foods – an essential ex situ digestion step in agricultural societies? Curr. Opin. Food Sci. 2020;32:124–132. [Google Scholar]
- 82.Lei V, Amoa-Awua WK, Brimer L. Degradation of cyanogenic glycosides by Lactobacillus plantarum strains from spontaneous cassava fermentation and other microorganisms. Int. J. Food Microbiol. 1999;53:169–184. doi: 10.1016/s0168-1605(99)00156-7. [DOI] [PubMed] [Google Scholar]
- 83.Sharma N, et al. Phytase producing lactic acid bacteria: Cell factories for enhancing micronutrient bioavailability of phytate rich foods. Trends Food Sci. Technol. 2020;96:1–12. [Google Scholar]
- 84.Frontela C, Ros G, Martinez C. Phytic acid content and “in vitro” iron, calcium and zinc bioavailability in bakery products: The effect of processing. J. Cereal Sci. 2011;54:173–179. [Google Scholar]
- 85.Lopez HW, et al. Prolonged fermentation of whole wheat sourdough reduces phytate level and increases soluble magnesium. J. Agric. Food Chem. 2001;49:2657–2662. doi: 10.1021/jf001255z. [DOI] [PubMed] [Google Scholar]
- 86.Gibson RS, Raboy V, King JC. Implications of phytate in plant-based foods for iron and zinc bioavailability, setting dietary requirements, and formulating programs and policies. Nutr. Rev. 2018;76:793–804. doi: 10.1093/nutrit/nuy028. [DOI] [PubMed] [Google Scholar]
- 87.Laatikainen R, et al. Randomised clinical trial: low-FODMAP rye bread vs. regular rye bread to relieve the symptoms of irritable bowel syndrome. Aliment. Pharmacol. Ther. 2016;44:460–470. doi: 10.1111/apt.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cano-Garrido O, Seras-Franzoso J, Garcia-Fruitos E. Lactic acid bacteria: reviewing the potential of a promising delivery live vector for biomedical purposes. Microb. Cell Fact. 2015;14:137. doi: 10.1186/s12934-015-0313-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nout MJR. Fermented foods and food safety. Food Res. Int. 1994;27:291–298. [Google Scholar]
- 90.Campedelli I, et al. Genus-wide assessment of antibiotic resistance in Lactobacillus spp. Appl. Environ. Microbiol. 2018;85:e01738-18. doi: 10.1128/AEM.01738-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hammad AM, Hassan HA, Shimamoto T. Prevalence, antibiotic resistance and virulence of Enterococcus spp. in Egyptian fresh raw milk cheese. Food Control. 2015;50:815–820. [Google Scholar]
- 92.Leroy S, Christieans S, Talon R. Tetracycline gene transfer in Staphylococcus xylosus in situ during sausage fermentation. Front. Microbiol. 2019;10:392. doi: 10.3389/fmicb.2019.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Spano G, et al. Biogenic amines in fermented foods. Eur. J. Clin. Nutr. 2010;64(Suppl. 3):S95–S100. doi: 10.1038/ejcn.2010.218. [DOI] [PubMed] [Google Scholar]
- 94.Alvarez MA, Moreno-Arribas MV. The problem of biogenic amines in fermented foods and the use of potential biogenic amine-degrading microorganisms as a solution. Trends Food Sci. Technol. 2014;39:146–155. [Google Scholar]
- 95.Collins JD, et al. Scientific opinion on risk based control of biogenic amine formation in fermented foods. EFSA J. 2011;9:2393. [Google Scholar]
- 96.Lorenzo, J. M. et al. Controlling biogenic amine formation in food. in Food Chemistry, Function and Analysis (ed Saad, B., Tofalo, R.) (Royal Society of Chemistry, 2019).
- 97.Gibbons JG, et al. The evolutionary imprint of domestication on genome variation and function of the filamentous fungus Aspergillus oryzae. Curr. Biol. 2012;22:1403–1409. doi: 10.1016/j.cub.2012.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ropars J, Lopez-Villavicencio M, Snirc A, Lacoste S, Giraud T. Blue cheese-making has shaped the population genetic structure of the mould Penicillium roqueforti. PLoS ONE. 2017;12:e0171387. doi: 10.1371/journal.pone.0171387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pflaum T, et al. Carcinogenic compounds in alcoholic beverages: an update. Arch. Toxicol. 2016;90:2349–2367. doi: 10.1007/s00204-016-1770-3. [DOI] [PubMed] [Google Scholar]
- 100.Bauer R, Cowan DA, Crouch A. Acrolein in wine: importance of 3-hydroxypropionaldehyde and derivatives in production and detection. J. Agric. Food Chem. 2010;58:3243–3250. doi: 10.1021/jf9041112. [DOI] [PubMed] [Google Scholar]
- 101.Gowd V, Su H, Karlovsky P, Chen W. Ethyl carbamate: an emerging food and environmental toxicant. Food Chem. 2018;248:312–321. doi: 10.1016/j.foodchem.2017.12.072. [DOI] [PubMed] [Google Scholar]
- 102.Henning RJ, Johnson GT, Coyle JP, Harbison RD. Acrolein can cause cardiovascular disease: a review. Cardiovasc. Toxicol. 2017;17:227–236. doi: 10.1007/s12012-016-9396-5. [DOI] [PubMed] [Google Scholar]
- 103.Staudacher HM, Nevin AN. Fermented foods: fad or favourable addition to the diet? Lancet Gastroenterol. Hepatol. 2019;4:19. doi: 10.1016/S2468-1253(18)30392-3. [DOI] [PubMed] [Google Scholar]
- 104.Sanlier N, Gokcen BB, Sezgin AC. Health benefits of fermented foods. Crit. Rev. Food Sci. Nutr. 2019;59:506–527. doi: 10.1080/10408398.2017.1383355. [DOI] [PubMed] [Google Scholar]
- 105.Cormier H, et al. Association between yogurt consumption, dietary patterns, and cardio-metabolic risk factors. Eur. J. Nutr. 2016;55:577–587. doi: 10.1007/s00394-015-0878-1. [DOI] [PubMed] [Google Scholar]
- 106.Fernandez MA, Panahi S, Daniel N, Tremblay A, Marette A. Yogurt and cardiometabolic diseases: a critical review of potential mechanisms. Adv. Nutr. 2017;8:812–829. doi: 10.3945/an.116.013946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guo J, et al. The impact of dairy products in the development of type 2 diabetes: where does the evidence stand in 2019? Adv. Nutr. 2019;10:1066–1075. doi: 10.1093/advances/nmz050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gil Á, Ortega RM. Introduction and executive summary of the supplement, role of milk and dairy products in health and prevention of noncommunicable chronic diseases: a series of systematic reviews. Adv. Nutr. 2019;10:S67–S73. doi: 10.1093/advances/nmz020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Savaiano DA, Hutkins RW. Yogurt, cultured fermented milk and health: a systematic review. Nutr. Rev. 2020 doi: 10.1093/nutrit/nuaa013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Praznikar ZJ, Kenig S, Vardjan T, Bizjak MC, Petelin A. Effects of kefir or milk supplementation on zonulin in overweight subjects. J. Dairy Sci. 2020;103:3961–3970. doi: 10.3168/jds.2019-17696. [DOI] [PubMed] [Google Scholar]
- 111.Kim H-Y, Park K-Y. Clinical trials of kimchi intakes on the regulation of metabolic parameters and colon health in healthy Korean young adults. J. Funct. Foods. 2018;47:325–333. [Google Scholar]
- 112.Nielsen ES, et al. Lacto-fermented sauerkraut improves symptoms in IBS patients independent of product pasteurisation - a pilot study. Food Funct. 2018;9:5323–5335. doi: 10.1039/c8fo00968f. [DOI] [PubMed] [Google Scholar]
- 113.Araki R, et al. The possibility of suppression of increased postprandial blood glucose levels by gamma-polyglutamic acid-rich natto in the early phase after eating: a randomized crossover pilot study. Nutrients. 2020;12:915. doi: 10.3390/nu12040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhu W, et al. Dietary vinegar prevents kidney stone recurrence via epigenetic regulations. EBioMedicine. 2019;45:231–250. doi: 10.1016/j.ebiom.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rizzello CG, et al. Sourdough fermented breads are more digestible than those started with baker’s yeast alone: an in vivo challenge dissecting distinct gastrointestinal responses. Nutrients. 2019;11:2954. doi: 10.3390/nu11122954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hill AB. The environment and disease: association or causation? Proc. R. Soc. Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rossouw JE, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 118.Giraffa G. Studying the dynamics of microbial populations during food fermentation. FEMS Microbiol. Rev. 2004;28:251–260. doi: 10.1016/j.femsre.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 119.Capurso A, Capurso C. The Mediterranean way: why elderly people should eat wholewheat sourdough bread-a little known component of the Mediterranean diet and healthy food for elderly adults. Aging Clin. Exp. Res. 2020;32:1–5. doi: 10.1007/s40520-019-01392-3. [DOI] [PubMed] [Google Scholar]
- 120.Wolever TM. Yogurt is a low-glycemic index food. J. Nutr. 2017;147:1462s–1467s. doi: 10.3945/jn.116.240770. [DOI] [PubMed] [Google Scholar]
- 121.Nyyssölä A, Ellilä S, Nordlund E, Poutanen K. Reduction of FODMAP content by bioprocessing. Trends Food Sci. Technol. 2020;99:257–272. [Google Scholar]
- 122.Joye I. Protein digestibility of cereal products. Foods. 2019;8:e8060199. doi: 10.3390/foods8060199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Poutanen K, Flander L, Katina K. Sourdough and cereal fermentation in a nutritional perspective. Food Microbiol. 2009;26:693–699. doi: 10.1016/j.fm.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 124.Wagenknecht AC, Mattick LR, Lewin LM, Hand DB, Steinkraus KH. Changes in soybean lipids during tempeh fermentation. J. Food Sci. 1961;26:373–376. [Google Scholar]
- 125.Panghal A, Munezero C, Sharma P, Chhikara N. Cassava toxicity, detoxification and its food applications: a review. Toxin Rev. 2019 doi: 10.1080/15569543.2018.1560334. [DOI] [Google Scholar]
- 126.Avilés-Gaxiola S, Chuck-Hernández C, Serna Saldívar SO. Inactivation methods of trypsin inhibitor in legumes: a review. J. Food Sci. 2018;83:17–29. doi: 10.1111/1750-3841.13985. [DOI] [PubMed] [Google Scholar]
- 127.Ojha P, et al. Malting and fermentation effects on antinutritional components and functional characteristics of sorghum flour. Food Sci. Nutr. 2018;6:47–53. doi: 10.1002/fsn3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gaur G, et al. Genetic determinants of hydroxycinnamic acid metabolism in heterofermentative lactobacilli. Appl. Environ. Microbiol. 2020;86:e02461-19. doi: 10.1128/AEM.02461-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Septembre-Malaterre A, Remize F, Poucheret P. Fruits and vegetables, as a source of nutritional compounds and phytochemicals: Changes in bioactive compounds during lactic fermentation. Food Res. Intern. 2018;104:86–99. doi: 10.1016/j.foodres.2017.09.031. [DOI] [PubMed] [Google Scholar]
- 130.Sun B, et al. Evolution of phenolic composition of red wine during vinification and storage and its contribution to wine sensory properties and antioxidant activity. J. Agric. Food Chem. 2011;59:6550–6557. doi: 10.1021/jf201383e. [DOI] [PubMed] [Google Scholar]
- 131.Tarvainen M, Fabritius M, Yang B. Determination of vitamin K composition of fermented food. Food Chem. 2019;275:515–522. doi: 10.1016/j.foodchem.2018.09.136. [DOI] [PubMed] [Google Scholar]
- 132.Melini F, Melini V, Luziatelli F, Ficca AG, Ruzzi M. Health-promoting components in fermented foods: an up-to-date systematic review. Nutrients. 2019;11:1189. doi: 10.3390/nu11051189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Iraporda C, et al. Lactate and short chain fatty acids produced by microbial fermentation downregulate proinflammatory responses in intestinal epithelial cells and myeloid cells. Immunobiology. 2015;220:1161–1169. doi: 10.1016/j.imbio.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 134.Samad A, Azlan A, Ismail A. Therapeutic effects of vinegar: a review. Curr. Opin. Food Sci. 2016;8:56–61. [Google Scholar]
- 135.Senger DR, Li D, Jaminet SC, Cao S. Activation of the Nrf2 cell defense pathway by ancient foods: Disease prevention by important molecules and microbes lost from the modern western diet. PLoS ONE. 2016;11:e0148042. doi: 10.1371/journal.pone.0148042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Milani C, et al. Colonization of the human gut by bovine bacteria present in Parmesan cheese. Nat. Commun. 2019;10:1286. doi: 10.1038/s41467-019-09303-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Oozeer R, et al. Survival of Lactobacillus casei in the human digestive tract after consumption of fermented milk. Appl. Environ. Microbiol. 2006;72:5615–5617. doi: 10.1128/AEM.00722-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vesa T, Pochart P, Marteau P. Pharmacokinetics of Lactobacillus plantarum NCIMB 8826, Lactobacillus fermentum KLD, and Lactococcus lactis MG 1363 in the human gastrointestinal tract. Aliment. Pharmacol. Ther. 2000;14:823–828. doi: 10.1046/j.1365-2036.2000.00763.x. [DOI] [PubMed] [Google Scholar]
- 139.Pochart P, Dewit O, Desjeux JF, Bourlioux P. Viable starter culture, beta-galactosidase activity, and lactose in duodenum after yogurt ingestion in lactase-deficient humans. Am. J. Clin. Nutr. 1989;49:828–831. doi: 10.1093/ajcn/49.5.828. [DOI] [PubMed] [Google Scholar]
- 140.Mater DD, et al. Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus survive gastrointestinal transit of healthy volunteers consuming yogurt. FEMS Microbiol. Lett. 2005;250:185–187. doi: 10.1016/j.femsle.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 141.Elli M, et al. Survival of yogurt bacteria in the human gut. Appl. Environ. Microbiol. 2006;72:5113–5117. doi: 10.1128/AEM.02950-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Han K, et al. Contrasting effects of fresh and fermented kimchi consumption on gut microbiota composition and gene expression related to metabolic syndrome in obese Korean women. Mol. Nutr. Food Res. 2015;59:1004–1008. doi: 10.1002/mnfr.201400780. [DOI] [PubMed] [Google Scholar]
- 143.Lee KE, Choi UH, Ji GE. Effect of kimchi intake on the composition of human large intestinal bacteria. Korean J. Food Sci. Technol. 1996;28:981–986. [Google Scholar]
- 144.David LA, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Plé C, Breton J, Daniel C, Foligné B. Maintaining gut ecosystems for health: Are transitory food bugs stowaways or part of the crew? Int. J. Food Microbiol. 2015;213:139–143. doi: 10.1016/j.ijfoodmicro.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 146.Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14:e1002533. doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pasolli E, et al. Large-scale genome-wide analysis links lactic acid bacteria from food with the gut microbiome. Nat. Commun. 2020;11:2610. doi: 10.1038/s41467-020-16438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lebeer S, Vanderleyden J, De Keersmaecker SCJ. Genes and molecules of lactobacilli supporting probiotic action. Microbiol. Mol. Biol. Rev. 2008;72:728–764. doi: 10.1128/MMBR.00017-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lebeer S, Vanderleyden J, De Keersmaecker SC. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat. Rev. Microbiol. 2010;8:171–184. doi: 10.1038/nrmicro2297. [DOI] [PubMed] [Google Scholar]
- 150.Falony G, et al. Population-level analysis of gut microbiome variation. Science. 2016;352:560–564. doi: 10.1126/science.aad3503. [DOI] [PubMed] [Google Scholar]
- 151.Le Roy CI, et al. Red wine consumption associated with increased gut microbiota α-diversity in 3 independent cohorts. Gastroenterology. 2020;158:270–272. doi: 10.1053/j.gastro.2019.08.024. [DOI] [PubMed] [Google Scholar]
- 152.Gonzalez S, et al. Fermented dairy foods: impact on intestinal microbiota and health-linked biomarkers. Front. Microbiol. 2019;10:1046. doi: 10.3389/fmicb.2019.01046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Taylor BC, et al. Consumption of fermented foods is associated with systematic differences in the gut microbiome and metabolome. mSystems. 2020;5:e00901-19. doi: 10.1128/mSystems.00901-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang C, et al. Ecological robustness of the gut microbiota in response to ingestion of transient food-borne microbes. ISME J. 2016;10:2235–2245. doi: 10.1038/ismej.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vighi G, Marcucci F, Sensi L, Di Cara G, Frati F. Allergy and the gastrointestinal system. Clin. Exp. Immunol. 2008;153(Suppl. 1):3–6. doi: 10.1111/j.1365-2249.2008.03713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Laforest-Lapointe I, Arrieta MC. Patterns of early-life gut microbial colonization during human immune development: an ecological perspective. Front. Immunol. 2017;8:788. doi: 10.3389/fimmu.2017.00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Alm JS, Swartz J, Lilja G, Scheynius A, Pershagen G. Atopy in children of families with an anthroposophic lifestyle. Lancet. 1999;353:1485–1488. doi: 10.1016/S0140-6736(98)09344-1. [DOI] [PubMed] [Google Scholar]
- 158.Hesselmar B, Hicke-Roberts A, Wennergren G. Allergy in children in hand versus machine dishwashing. Pediatrics. 2015;135:e590–e597. doi: 10.1542/peds.2014-2968. [DOI] [PubMed] [Google Scholar]
- 159.Alm JS, et al. An anthroposophic lifestyle and intestinal microflora in infancy. Pediatr. Allergy Immunol. 2002;13:402–411. doi: 10.1034/j.1399-3038.2002.01062.x. [DOI] [PubMed] [Google Scholar]
- 160.Liu AH. Revisiting the hygiene hypothesis for allergy and asthma. J. Allergy Clin. Immunol. 2015;136:860–865. doi: 10.1016/j.jaci.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 161.Bourrie BC, Willing BP, Cotter PD. The microbiota and health promoting characteristics of the fermented beverage kefir. Front. Microbiol. 2016;7:647. doi: 10.3389/fmicb.2016.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Foligne B, et al. Immunomodulation properties of multi-species fermented milks. Food Microbiol. 2016;53:60–69. doi: 10.1016/j.fm.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 163.van Hemert S, et al. Identification of Lactobacillus plantarum genes modulating the cytokine response of human peripheral blood mononuclear cells. BMC Microbiol. 2010;10:293. doi: 10.1186/1471-2180-10-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lee IC, et al. Strain-specific features of extracellular polysaccharides and their impact on Lactobacillus plantarum-host interactions. Appl. Environ. Microbiol. 2016;82:3959–3970. doi: 10.1128/AEM.00306-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Peters A, et al. Metabolites of lactic acid bacteria present in fermented foods are highly potent agonists of human hydroxycarboxylic acid receptor 3. PLoS Genet. 2019;15:e1008145. doi: 10.1371/journal.pgen.1008145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Konig J, et al. Human intestinal barrier function in health and disease. Clin. Transl. Gastroenterol. 2016;7:e196. doi: 10.1038/ctg.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bourdichon F, et al. Food fermentations: microorganisms with technological beneficial use. Int. J. Food Microbiol. 2012;154:87–97. doi: 10.1016/j.ijfoodmicro.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 168.Laulund S, Wind A, Derkx PMF, Zuliani V. Regulatory and safety requirements for food cultures. Microorganisms. 2017;5:28. doi: 10.3390/microorganisms5020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.EFSA Panel on Biological Hazards. Scientific opinion on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA (2017–2019) EFSA J. 2020;18:5966. doi: 10.2903/j.efsa.2020.5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Plavec TV, Berlec A. Safety aspects of genetically modified lactic acid bacteria. Microorganisms. 2020;8:297. doi: 10.3390/microorganisms8020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Food and Agriculture Organization of the United Nations. Codex Alimentarius.Standard for fermented milk (CXS 243-2003) (WHO, 2018).
- 172.Chilton SN, Burton JP, Reid G. Inclusion of fermented foods in food guides around the world. Nutrients. 2015;7:390–404. doi: 10.3390/nu7010390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Campbell-Platt G. Fermented foods — a world perspective. Food Res. Internl. 1994;27:253–257. [Google Scholar]
- 174.Bell V, Ferrao J, Fernandes T. Nutritional guidelines and fermented food frameworks. Foods. 2017;6:e6080065. doi: 10.3390/foods6080065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gille D, Schmid A, Walther B, Vergeres G. Fermented food and non-communicable chronic diseases: a review. Nutrients. 2018;10:448. doi: 10.3390/nu10040448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.National Institute of Nutrition. Dietary guidelines for Indians. National Institute for Nutritionhttps://www.nin.res.in/downloads/DietaryGuidelinesforNINwebsite.pdf (2011)
- 177.Asogwa I, Okoye J, Oni K. Promotion of indigenous food preservation and processing knowledge and the challenge of food security in Africa. J. Food Security. 2017;5:75–87. [Google Scholar]
- 178.Sybesma W, Blank I, Lee Y-K. Sustainable food processing inspired by nature. Trends Biotechnol. 2017;35:279–281. doi: 10.1016/j.tibtech.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 179.Capozzi V, Spano G. Food microbial biodiversity and “microbes of protected origin”. Front. Microbiol. 2011;2:237–237. doi: 10.3389/fmicb.2011.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Leroy F, Geyzen A, Janssens M, De Vuyst L, Scholliers P. Meat fermentation at the crossroads of innovation and tradition: a historical outlook. Trends Food Sci. Technol. 2013;31:130–137. [Google Scholar]
- 181.Franciosa I, Alessandria V, Dolci P, Rantsiou K, Cocolin L. Sausage fermentation and starter cultures in the era of molecular biology methods. Int. J. Food Microbiol. 2018;279:26–32. doi: 10.1016/j.ijfoodmicro.2018.04.038. [DOI] [PubMed] [Google Scholar]
- 182.Garofalo C, et al. Selection of indigenous yeast strains for the production of sparkling wines from native Apulian grape varieties. Int. J. Food Microbiol. 2018;285:7–17. doi: 10.1016/j.ijfoodmicro.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 183.Silva LF, et al. Safety and technological application of autochthonous Streptococcus thermophilus cultures in the buffalo Mozzarella cheese. Food Microbiol. 2020;87:103383. doi: 10.1016/j.fm.2019.103383. [DOI] [PubMed] [Google Scholar]
- 184.Li Q, Gänzle MG. Host-adapted lactobacilli in food fermentations: impact of metabolic traits of host adapted lactobacilli on food quality and human health. Curr. Opin. Food Sci. 2020;31:71–80. [Google Scholar]
- 185.Duar RM, et al. Lifestyles in transition: evolution and natural history of the genus Lactobacillus. FEMS Microbiol. Rev. 2017;41(Suppl. 1):27–48. doi: 10.1093/femsre/fux030. [DOI] [PubMed] [Google Scholar]