Abstract
The differential expression of genes is crucial for plant somatic embryogenesis (SE), and the accurate quantification of gene expression levels relies on choosing appropriate reference genes. To select the most suitable reference genes for SE studies, 10 commonly used reference genes were examined in synchronized somatic embryogenic and subsequent germinative cultures of Liriodendron hybrids by using quantitative real-time reverse transcription PCR. Four popular normalization algorithms: geNorm, NormFinder, Bestkeeper and Delta-Ct were used to select and validate the suitable reference genes. The results showed that elongation factor 1-gamma, histone H1 linker protein, glyceraldehyde-3-phosphate dehydrogenase and α-tubulin were suitable for SE tissues, while elongation factor 1-gamma and actin were best for the germinative organ tissues. Our work will benefit future studies of gene expression and functional analyses of SE in Liriodendron hybrids. It is also serves as a guide of reference gene selection in early embryonic gene expression analyses for other woody plant species.
Subject terms: Developmental biology, Molecular biology, Plant sciences
Introduction
Somatic embryogenesis (SE) is a technique that allows the study of early regulatory and morphogenetic events in regenerative systems of higher plants and is also a tool for mass clonal propagation of genetically improved varieties and germplasm maintenance1–3. Determining the key regulators of important events during cell differentiation and the major morphogenetic transformational stages, which occur during early embryogenesis, will greatly promote plant phylembryogenesis-related research and tissue culture production. In recent years, with the development of the high-throughput sequencing and microarray technologies, several SE-related microRNAs, lncRNAs and genes have been discovered4–8. To elucidate the integrated gene networks of SE processes, the expression levels of these embryo-related genes and their relationships need to be understood. Reverse transcription quantitative real-time PCR (RT-qPCR) has been widely adopted as a standard method for verifying, quantifying and comparing gene expression levels because of its sufficient sensitivity and specificity, as well as being time efficient and technically straightforward compared with other methods. However, not only the biological but also the technological variations, such as the quantity of the initial material, the RNA quality, and the efficiency levels of cDNA synthesis and PCR, may influence the accuracy and reliability of RT-qPCR9–12. Consequently, RT-qPCR results need to be normalized by several parallel internal reference genes that participate in the whole experimental workflow along with the genes of interest but have the least amount of variation in their expression levels under various experimental conditions and in different tissues types. The most stable reference genes vary widely in different species, tissues and developmental stages, as well as under different experimental conditions13–16. Because there are no universal reference genes for all experiments, it is critical to identify the most stable internal control gene or gene combination prior to normalization in different experiments10,17.
Liriodendron is an ancient angiosperm genus that belongs to the order Magnoliales and Liriodendron hybrids (Liriodendron chinense (Hemsl.) Sarg. × L. tulipifera Linn.) is derived from the sexual hybridization between Liriodendron tulipifera and Liriodendron chinense. Since the SE system of Liriodendron hybrids was established in 199318, the origin and development of somatic embryos have been studied and control methods have been improved greatly19. Somatic embryos of Liriodendron hybrids at different developmental stages can be readily obtained on a large scale from very early stages, which have more or less synchronous patterns. Through the development of sequencing technology, genetic information, such as small RNAs4, transcriptomes20,21, proteomes22 and genomes23, for Liriodendron have been investigated, and genetic transformation systems have also been established24. These characteristics make the SE of Liriodendron hybrids a suitable system for investigating the regulation of woody plant SE. Morphological research results and molecular data on SE have been increasingly informative, but limited in gene expression analyses, which are important for studying the molecular regulatory mechanisms. Thus, the establishment of a RT-qPCR detection system is necessary, and suitable internal controls for studying Liriodendron SE are required.
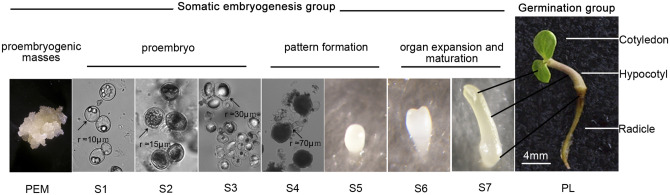
Similar to zygotic embryogenesis, the sequence of SE in an angiosperm species can be divided into three phases: proembryo, specific pattern formation and transition to the cotyledonary stage2,25. To extend the analysis to the early germination period and different organ tissues of somatic embryo-derived seedlings, we established two experimental groups in this study: somatic embryogenesis group and germination group (Fig. 1). In total, 10 candidate genes for the RT-qPCR normalization analysis expressed in the SE of Liriodendron hybrids were compared and evaluated. These included eight widely used reference genes, 18S ribosomal RNA (18S rRNA)26–28, actin (ACT)27,29, elongation factor 1-alpha (EF1a)17,28,29, glyceraldehyde-3-phosphate dehydrogenase (GAPDH)26,29, histone H1 linker protein (HIS1)17,27,28, α-tubulin (TUA)17,29, β-tubulin (TUB)28 and ubiquitin (UBQ)26–28, as well as two other potential candidate genes, ribosomal protein L2 encoding gene (Rpl2) and elongation factor 1-gamma (EF1g).
Figure 1.
Somatic embryogenesis and germination groups of Liriodendron hybrids. The somatic embryogenesis group includes PEM and S1–S7, while the germination group includes cotyledon, hypocotyl and radicle. PEM, proembryogenic masses; S1, proembryogenic single cell stage; S2,embryogenic single cell stage; S3, two to four cell proembryo stage; S4, multicell proembryo stage; S5, globular embryo stage; S6, heart/torpedo -shaped embryo stage; S7, cotyledon embryo stage; PL, plantlet developed from somatic embryo. (The images of PEM and S1 to S7 were provided by the Key Laboratory of Forest Genetics and Biotechnology, Ministry of Education of China, Co-Innovation Center for the Sustainable Forestry in Southern China, Nanjing Forestry University).
Results
Verification of primer specificity and PCR efficiency analysis
To evaluate the expression stability of candidate reference genes in different SE stages and organs, 11 samples of synchronized Liriodendron hybrid embryogenic cultures that were divided into the two test groups were studied. The quality of the total RNA is an important variable factor in RT-qPCR. In our experiment, all the samples were subjected to an on-column DNA removal protocol to eliminate residual genomic DNA. The ratios of A260/A280 and A260/A230 were between 2.01–2.11 and 2.1–2.25, respectively, and the agarose gel electrophoresis also showed good RNA integrity.
The specificity of each primer was verified by dissociation curve analysis, electrophoresis and sequencing. All the primers designed in our experiment showed single peaks in the gradient dilution amplification melt curves (Supplemental data 1). The agarose gel electrophoresis and sequencing results also indicated that the amplicons were of the desired size and were the correct gene sequences (Supplemental data 1). The standard curves analyses revealed slopes between − 3.0 and − 3.5, and the PCR amplification efficiency of each primer pair varied from 93% for Rpl2 to 109% for UBQ. The R2 values of the genes varied from 0.95 to 1 (Table 1). These indexes were all in credible intervals, indicating that the test samples had similar PCR efficiencies and that the RT-qPCR data can be used in the following analyses.
Table 1.
Ranking of the candidate reference genes according to their stability value calculated by NormFinder.
| Rank | Somatic embryogenesis group | Germination group | ||
|---|---|---|---|---|
| Gene name | Stability value | Gene name | Stability value | |
| 1 | EF1g | 0.0024 | EF1g | 0.0013 |
| 2 | HIS1 | 0.0046 | ACT | 0.0016 |
| 3 | GAPDH | 0.0055 | Rpl2 | 0.0030 |
| 4 | TUA | 0.0063 | HIS1 | 0.0035 |
| 5 | Rpl2 | 0.0088 | EF1a | 0.0062 |
| 6 | ACT | 0.0092 | GAPDH | 0.0066 |
| 7 | UBQ | 0.0102 | UBQ | 0.0075 |
| 8 | EF1a | 0.0119 | TUB | 0.0097 |
| 9 | TUB | 0.0126 | TUA | 0.0114 |
| 10 | 18S rRNA | 0.0210 | 18S rRNA | 0.0190 |
The input data for NormFinder are supposed to be on a linear scale. The raw Ct values were transformed to linear scale expression quantities using the standard curve or delta-Ct method.
Expression profiling of reference genes in Liriodendron embryogenesis and germinative tissues
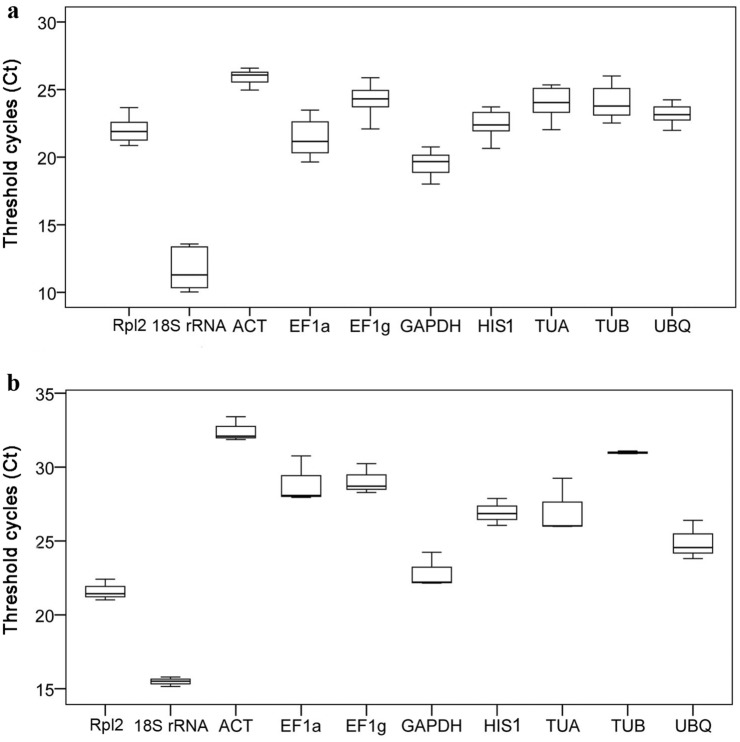
The expression levels of 10 reference genes in two developmental groups were evaluated by comparing the quantification cycle (Cq) values, also known as the threshold cycle (Ct) values, using box-and-whiskers plots. The gene encoding 18S rRNA had the greatest expression levels, with the lowest Ct values, in the 11 tissues because it is one of the most abundant transcripts, as revealed in a study of embryogenesis in longan trees28. The other candidate reference genes’ Ct values in the two test groups were all within moderate boundaries. The median Ct values ranged from 25.92 (ACT) to 19.52 (GAPDH) and from 32.64 (ACT) to 21.88 (Rpl2) in the embryogenesis (Fig. 2a) and germination groups (Fig. 2b), respectively.
Figure 2.
Expression levels of 10 reference genes in Liriodendron hybrids as determined by the quantification cycle values, also known as the threshold cycle (Ct) values, in the somatic embryogenesis (a) and the germination (b) groups. The boxes indicate the 25th and 75th percentiles, the line across each box represents the median, and whisker caps represent the maximum and minimum Ct values.
The range of Ct values in different developmental groups and stages indicated a considerable variability among the 10 candidate reference genes. The least variation in gene expression across the eight embryogenesis samples was found for ACT (1.62 cycles), while the most variable was TUA (4.16 cycles). In the germination group, the least variable were ACT and 18S rRNA (< 2 cycles), while the most variable were EF1a and TUA (> 4 cycles).
Gene expression stability analysis and ranking of Liriodendron reference genes
Four different software programs were employed to assess the reference genes stability: geNorm, NormFinder, BestKeeper and ΔCt.
geNorm analysis
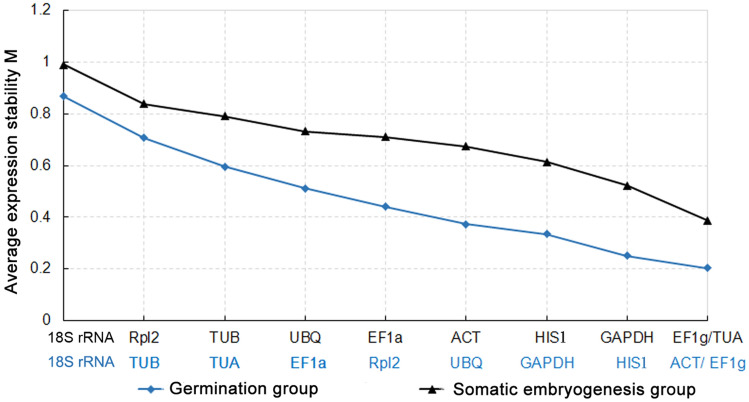
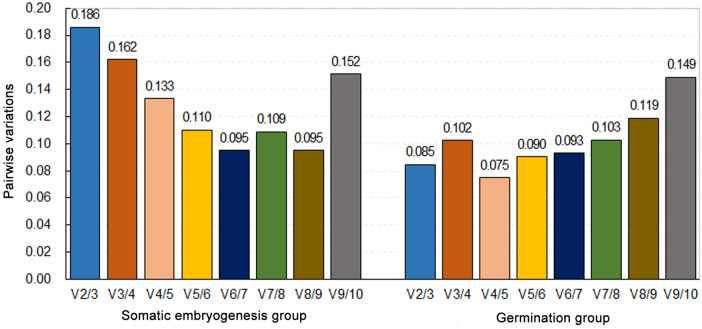
geNorm calculates the expression stability value (M) for each candidate gene on the basis of the mean pair-wise variation between all the genes analyzed. Genes with lower M values are more stably expressed. In this study, we used an M value cut-off value of ⩽ 1 to identify the stably expressed genes. The pairwaise variation of two sequential normalization factors (Vn/n + 1 value) determines the number of control genes for normalization, and a threshold value of 0.15 was adopted, as reported by Vandesompele et al10. In the somatic embryogenesis subset, the stability of genes (from most to least) was ranked as follows: EF1g/TUA > GAPDH > HIS1 > ACT > EF1a > UBQ > TUB > Rpl2 > 18S rRNA (Fig. 3). Because the V4/5 value < 0.15 and V3/4 > 0.15 (Fig. 4), four reference genes with lower M values (EF1g, TUA, GAPDH and HIS1) were required for normalization in the embryogenesis group. For the germination subset, which is not the same as the somatic embryogenesis series, the stability of genes (from most to least) was ranked as follows: EF1g/ACT > HIS1 > GAPDH > UBQ > Rpl2 > EF1a > TUA > TUB > 18S rRNA (Fig. 3). The V2/3 value was 0.085 (Fig. 4), suggesting that the employment of the two best reference genes, ACT and EF1g, was enough for normalization. 18S rRNA was found to be the least stable gene in both groups.
Figure 3.
Expression stability and rankings of reference genes in Liriodendron hybrids as calculated by geNorm in the somatic embryogenesis (black) and the germination (blue) groups. Genes with the most constitutive expression are indicated on the right side of the graph, with the less stably expressed genes on the left.
Figure 4.
Pairwise variation (V) calculated by geNorm to determine the optimal number of reference genes in the somatic embryogenesis (left) and the germination (right) groups of Liriodendron hybrids. A value < 0.15 indicates that the inclusion of an additional reference gene is not required.
NormFinder analysis
The Normfinder software ranked the set of candidate genes according to the stability of their expression in a given sample set. The lowest stability value represented the most stable gene within the examined gene set. The NormFinder analysis of the two groups is summarized in Table 1. EF1g was identified as the most stable gene in both experimental groups. Unlike geNorm, the NormFinder approach takes into account inter- and intra-group variations for normalization factor calculations30. When inter-group variations are considered, the combination of the best two genes is provided. Here, they were EF1g and HIS1. However, the stability value of the two-gene combination was greater than that of the most stable gene, EF1g (0.001). In addition, the correlation coefficient (r) when comparing geNorm and NormFinder was not strong (r = 0.644). When only considering intra-group variation in the NormFinder calculations, the results from both algorithms for each experimental set are well correlated. Both NormFinder and geNorm analyses ranked the top stable genes as EF1g and ACT in the germination series and EF1g, HIS1, GAPDH and TUA in the somatic embryogenesis series, although in a slightly different order, with 18S rRNA ranking last in both groups (Table 1).
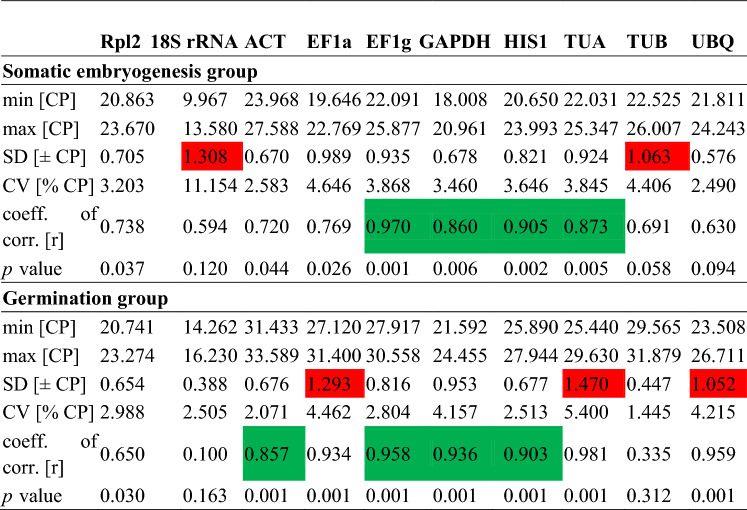
BestKeeper analysis
The results of BestKeeper analysis are shown in Table 2. For the embryogenesis group, 18S rRNA and TUB were eliminated because of their high standard deviation (SD) values of 1.308 and 1.063, respectively, and coefficient of variance values of 11.154 and 4.406, respectively. EF1g was the most stable gene, with the highest correlation coefficient (r) value (0.970, p ˂ 0.01). This was followed by HIS1 (r = 0.905, p ˂ 0.01), TUA (r = 0.873, p ˂ 0.01) and GAPDH (r = 0.860, p ˂ 0.01). These genes were also the four most stable genes identified by geNorm and NormFinder. For the germination group, EF1a, TUA and UBQ were excluded because they had SD values > 1. EF1g, GAPDH, HIS1 and ACT correlated well with each other (0.857 < r < 0.958, p ˂ 0.01). As in the geNorm and NormFinder analyses, in this group, 18S rRNA and TUB ranked last. Because two reference genes were enough for normalization in the germination group, the most reliable gene EF1g combined with one of the other three top genes is acceptable.
Table 2.
BestKeeper software statistics for reference genes based on Ct values in the somatic embryogenesis and the germination groups.
CP equivalent terminology for Ct, standard deviation (SD; ± CP): the SD of the CP (values greater than 1 are highlighted in red); Coefficient of variance (CV; %CP): the CV expressed as a percentage of the CP level. The correlation between each candidate reference gene and the BestKeeper index was calculated using Pearson’s correlation coefficient (r) (values correlated well are highlighted in green) and the P value31.
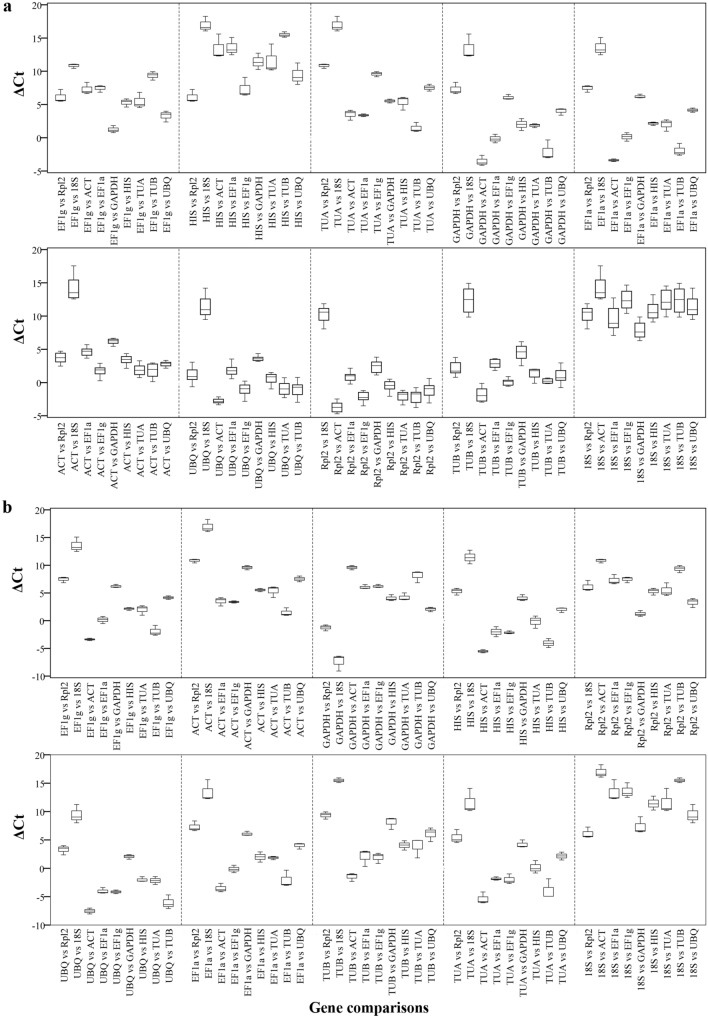
ΔCt analysis
To avoid the influence of the quality of input RNA, we also used the ΔCt approach whereby ‘pairs of genes’ are compared30,31. The stability of the reference genes is ranked according to the average SD index. Each gene was compared against the other nine genes, and the appropriate reference genes with low mean variability levels were selected for the two experimental systems. As shown in Fig. 5 and Supplemental data 2, in the somatic embryogenesis group, EF1g, HIS1, TUA and GAPDH held the top four positions, with mean SD values of 0.782, 0.856, 0.859 and 0.885, respectively. This result corroborated those of the other three applets. In the germination group, EF1g was the most stable genes (mean SD of 0.603), followed by ACT (mean SD of 0.607). In both groups, when 18S rRNA was compared with the other nine genes in the respective developmental groups, it showed the greatest amounts of deviation in ΔCt values, indicating that 18S rRNA was the least stable reference gene in ourtest list. In addition, the range of ΔCt values was relatively wider in the somatic embryogenesis group than in the germination group (Fig. 5), indicating that gene expression during somatic embryogenesis stages was more variable than in embryonic germinative organ tissues.
Figure 5.
ΔCt method for reference gene selection in Liriodendron hybrids. ΔCt variability in reference gene comparisons are shown as medians (lines), 25th percentile to the 75th percentile (boxes) and ranges (whiskers) for all eight samples in the somatic embryogenesis group (a) and three samples in the germination group (b).
Reference gene selection
Each method can introduce bias, and it was assumed that a comparison of the different algorithms would allow a more reliable evaluation32. Consequently, we used three different Visual Basic applets, geNorm, NormFinder and BestKeeper, and the ΔCt method, to evaluate the stability of 10 reference genes in both the Liriodendron SE and germination groups. The gene stability orders of the two experimental groups calculated by four methods are displayed in Table 3. Comparing the outputs obtained from four analytical approaches, as shown in Table 4, the r values indicated that the ranking results of geNorm, NormFinder, and the ΔCt method were significantly correlated at the 0.01 level, and they were inversely associated with the results of BestKeeper at the 0.05 level. In addition, their correlations in the germination group were more relevant than those in the somatic embryogenesis group. The correlation analyses indicated that the results of the four approaches tended to coincidence, although each approach was based on a different algorithm.
Table 3.
Expression stability of reference genes analyzed by geNorm, NormFinder, BestKeeper and delta Ct.
| Stability rank | Somatic embryogenesis group | Germination group | ||||||
|---|---|---|---|---|---|---|---|---|
| geNorm | NormFinder | BestKeeper | ΔCT | geNorm | NormFinder | BestKeeper | ΔCT | |
| 1 | EF1g | EF1g | EF1g | EF1g | EF1g | EF1g | EF1g | EF1g |
| 2 | TUA | HIS1 | HIS1 | HIS1 | ACT | ACT | GAPDH | ACT |
| 3 | GAPDH | GAPDH | TUA | TUA | HIS1 | HIS1 | HIS1 | GAPDH |
| 4 | HIS1 | TUA | GAPDH | GAPDH | GAPDH | Rpl2 | ACT | HIS1 |
| 5 | ACT | Rpl2 | EF1a | EF1a | UBQ | EF1a | EF1a | Rpl2 |
| 6 | EF1a | ACT | Rpl2 | ACT | Rpl2 | GAPDH | UBQ | UBQ |
| 7 | UBQ | UBQ | ACT | UBQ | EF1a | UBQ | TUA | EF1a |
| 8 | TUB | EF1a | TUB | Rpl2 | TUA | TUB | Rpl2 | TUB |
| 9 | Rpl2 | TUB | UBQ | TUB | TUB | TUA | TUB | TUA |
| 10 | 18S rRNA | 18S rRNA | 18S rRNA | 18S rRNA | 18S rRNA | 18S rRNA | 18S rRNA | 18S rRNA |
Table 4.
Correlations of the four algorithms’ results (r values).
| Algorithm | Somatic embryogenesis group | Germination group | ||||||
|---|---|---|---|---|---|---|---|---|
| geNorm | NormFinder | BestKeeper | ΔCT | geNorm | NormFinder | BestKeeper | ΔCT | |
| geNorm | – | 0.876** | − 0.870** | 0.814** | – | 0.910** | − 0.776 | 0.957** |
| NormFinder | 0.876** | – | − 0.870** | 0.916** | 0.91** | – | − 0.689* | 0.939** |
| BestKeeper | − 0.870** | − 0.870** | – | − 0.746* | − 0.776 | − 0.689* | – | − 0.711* |
| ΔCT | 0.814** | 0.916** | − 0.746* | – | 0.957** | 0.939** | − 0.711* | – |
*, **Significantly different at the 0.05 and 0.01 levels, respectively.
The pairwise comparison approach (geNorm) not only produces a gene ranking but provides a rational for determining the minimum number of genes required for accurate normalization. According to the Vn/n + 1 value, four reference genes in the somatic embryogenesis subset and two in the germination subset were needed. Based on the results of all four statistical methods, the most stable combination of reference genes for SE stages was EF1g, HIS1, GAPDH and TUA, while for the germinative organ tissues the most stable reference gene was EF1g and the optimal reference combination was EF1g and ACT.
Discussion
Normalization is crucial for obtaining reliable gene expression data by RT-qPCR. RT-qPCR studies in SE are difficult because, from a single somatic cell to a mature embryo, plant somatic embryos go through cell differentiation, organ formation and maturation, which are accompanied by complex networks containing many dynamic developmental, biochemical and metabolic processes. A large number of genes are variably expressed in space and time, and to different extents. They are also affected by changes in extracellular and external environmental factors1,33. To date, there have been several reports on selecting suitable internal controls for plant SE, such as in maritime pine26, cotton27, longan tree28, citrus34 and conifer species17, but these studies either did not identify stable controls, or the study materials did not cover the complete SE process, lacking especially in the early somatic embryogenesis stages. Additionally, some of them studied gymnosperms which have SE processes that are not quite the same as those of angiosperms2. In this study, 10 candidate genes and four statistical applets (geNorm, NormFinder, BestKeeper and the ΔCt method) were selected to perform expression stability analyses of two test groups at eight developmental stages during SE and in three different tissues in the following germination phase in Liriodendron hybrids. The results suggest that the combination of EF1g, HIS1, GAPDH and TUA is optimal for the normalization of gene expression in the embryo developmental series, while EF1g or EF1g/ACT is a stable reference gene(s) in the germinative tissues.
Elongation factor expression is correlated with the synthesis of proteins during cell cycle and elongation in some cells. EF1a was recommended as a stable reference gene in embryogenic cell suspensions of Coffea arabica35 and in olive mesocarp tissues36. In our research, EF1a ranked in the middle position in both SE and plantlet groups, while EF1g was the most stable reference genes in both groups. Twardowski and Legocki37 and Dell'Aquila et al.38 demonstrated that some elongation factors play important roles in plant cells at early stages of seed germination, and the properties of EF1 are correlated with ageing-related phenomena. We speculated that EF1g and EF1a are expressed differently in different stages and tissues, and EF1a seemed more variable because of some molecular and functional properties.
Histones are the chief protein components of chromatin. They package and order the DNA into nucleosomes. The Histone H1 family is one of the five main Histone families, it is half the size of the other four histones and can be expressed in different or overlapping tissues and developmental stages. HIS1 variants are partially redundant and vary little among different experiments39. Glyceraldehyde -3-phosphate dehydrogenase, as an abundant glycolytic enzyme, participates in the cell metabolism and several non-metabolic processes, including transcriptional activation, cell apoptosis40, endoplasmic reticulum to Golgi vesicle shuttling, and fast axonal or axoplasmic transport41. These cellular energy-associated actions occur consistently during all the developmental stages of Liriodendron SE. Although, in some cases, GAPDH is an inappropriate endogenous control26,35 and it was not the most stable gene in our test, owing to the complex SE-related mechanisms, it is a necessary member of the control combination.
Actin and tubulin are members of cytoskeleton and are ubiquitous in plant cells. Actin fulfils a variety of specific roles, such as in cell division and elongation, cell wall development, cell movement and developmentally regulated programmed cell death42. It has been reported as a housekeeping gene and used as a control in RT-qPCR analyses, but not in the analyses of any tissues. In the non-fiber tissues of cotton, it was the most variable reference gene27. In our study, the stability of ACT in the SE group ranked in the middle position, but it was relatively stable in the germination group. The ACT gene family is large, and different members play different roles in distinct tissues and developmental stage. Schwarzerová et al.42 reported that some actin isoforms could even control the speed and synchrony of development in the SE of spruce. The tubulin genes are similar. Although all the tubulins in the same organism appear able to participate in all the major functions, some tubulins are tissue and stage-specific. α-Tubulin and β-tubulin groups exhibit major differences in their net electric charges, dipole moments and dipole vector orientations, and these differences may influence their functional characteristics43. In our study, in the SE group, the expression of TUA was relatively stable but TUB was variable, while in the germination group, both of them ranked among the lower positions.
During SE in Liriodendron hybrids, 18S rRNA was classified as the least stable gene analyzed by all the applets and, therefore, is not suitable as an internal control. This result is in accordance with other reference gene selection reports related to SE26–28. We speculated that 18S rRNA would not be selected as an internal control gene in this test because of its high content in total RNA, which led to a small Ct value (˂ 15 cycles) in the RT-qPCR.
By accumulating data from transcriptome, genome and microRNA sequencing or Chip technology, superior reference genes might be found, like snoR14 and snoRD25 in the SE of citrus34, SAND, TBP and other expressed genes in brinjal fruit developmental stages44, dlo-miR24 in the SE of longan45, miR167-1_2, miR11-1, miR159-1 and miR168-1 in the seed development of Brassica napus46. Our research group has also carried out microRNA chip and sequencing experiments, but the data did not reveal any microRNAs that were suitable to use as controls in the early stages of Liriodendron hybrid SE4. Further testing of more reference genes should be performed if a more efficient internal control is required, but, at present, the use of more than one reference gene as controls could result in the most reliable gene transcription analyses.
In summary, among the 10 candidate reference genes studied, we recommend the combination of EF1g/HIS1/GAPDH/TUA genes for normalization of qPCR analyses in the somatic embryogenesis group and EF1g or EF1g/ACT for the germination group. The commonly used 18S rRNA should be avoided. The results provide guidelines for the selection of reference genes for the normalization of qPCR in future Liriodendron hybrid transcriptomic and microRNA studies involving somatic embryogenesis and germination-related tissues, and these guidelines may also be useful in SE gene expression studies of other woody plants.
Materials and methods
Plant materials and treatments
In this study, we established two separate experimental groups: somatic embryogenesis and germination. The somatic embryogenesis group started with proembryogenic masses (PEMs) and finished at the cotyledonary stage (S7). It covered the whole embryonic phase prior to germination. In this group, the plant materials were cultured under dark conditions, and each stage had an obvious morphological transformation (Fig. 1). The mature somatic embryos (S7) were then transferred to 16-h (light)/8-h (dark) conditions, and the subsequently developed cotyledon, hypocotyl and radicle were used as the germination group.
Synchronized Liriodendron embryogenic cultures at different developmental stages (Fig. 1) were obtained following previously published methods4,47. The cotyledon, hypocotyl and radicle were harvested from the developed somatic plantlets when the cotyledon just opened (Fig. 1). All stages of somatic embryos and organ tissues were placed in cryostorage vials immersed in liquid nitrogen and then stored at – 80 °C until used.
Total RNA extraction and cDNA synthesis
Total RNA was isolated and purified from each SE stage using a Total RNA Purification kit (Norgen Biotek Corporation, Canada), according to the manufacturer’s instructions with the on-column DNA removal protocol4. The purity and integrity of the extracted RNA were checked using a NanoDrop 2000/2000C spectrophotometer (Thermo Scientific, USA) and 1.5% agarose gel electrophoresis with ethidium bromide staining. First-strand cDNA was synthesized by reverse transcribing 1.5 μg of total RNA with random primers and an oligo dT primer in a final reaction volume of 20 µl using the SuperScript III First-Strand Synthesis System for RT-PCR kit (Invitrogen, USA) according to the manufacturer’s instructions.
Primer design and verification of amplified products
We selected 10 reference genes that are commonly used as controls for plant gene expression studies and had relatively stable expression levels during plant embryogenesis26–28. To identify Liriodendron homologs of these genes, we first searched the Liriodendron protein/nucleotide database in NCBI (Rpl2, 18S rRNA, ACT and EF1a) and ChromDB (HIS1). The other genes were identified in Liriodendron EST databases using TBLASTN with corresponding Arabidopsis protein sequences as the query. Selected Liriodendron ESTs were then used as query for BLASTX searches of Arabidopsis (EF1g, GAPDH, UBQ and TUB) and Populus (TUA) protein databases. The primers were designed with melting temperatures of 58–62 °C using Primer Premier5.0 software (PREMIER Biosoft International, USA) and Oligo 6 (Molecular Biology Insights, USA) according to the obtained mRNA sequences. All the primers were synthesized by Invitrogen.
PCR products obtained using the designed primers were first verified by electrophoresis on 2% agarose gels to have single bands of the expected sizes (Supplemental data 1). To confirm the amplicon sequences, PCR was performed on the cDNAs. Follow the instructions of Ex Taq DNA polymerase (TaKaRa, Japan), reactions were performed with 400 nM of each primer, 1 U Ex Taq DNA polymerase, 200 μM dNTPs (TaKaRa, Japan) and 10 ng of cDNA in a total volume of 20 μl. Amplifications were performed with the universal cycling conditions(95 °C for 5 min, followed by 35 cycles at 94 °C for 30 s, 55 °C for 30 s and 72 °C for 1 min)36. The amplified products were purified using an AxyPrep PCR Cleanup Kit (Axygen Biosciences, USA) according to the manufacturer’s instructions and subsequently cloned into the pMD19-T vector (TaKaRa). Positive colonies for each cDNA were sequenced by Invitrogen. The sequence files were analyzed using DNAMAN and verified by the nucleotide BLAST program at NCBI (http://blast.ncbi.nlm.nih.gov/). All the reference genes’ putative functions, accession numbers, primer sequences and amplicon sizes are provided in Table 5.
Table 5.
Descriptions of Liriodendron hybrids’ candidate reference genes and associated qPCR analyses.
| Symbol | Gene description/function | GenBank no | Primer sequences | Tm (°C) | Amplicon (bp) | Slop | R2 | E (%) |
|---|---|---|---|---|---|---|---|---|
| Rpl2 | Ribosomal protein L2/Protein synthesis | AF123796 | F:5′-ACACCAATCCATCCCGAACT-3′ | 79.73 | 93 | − 3.494 | 0.999 | 93.3 |
| R:5′-TCCTGGCGTCGAGCTATTT-3′ | ||||||||
| 18S rRNA | 18S ribosomal RNA/Constituent of the small ribosomal subunit | FR870008 | F:5′-ATTTCTGCCCTATCAACTTTCG-3′ | 82.42 | 183 | − 3.466 | 0.998 | 94.5 |
| R:5′-TTGTTATTTATTGTCACTACCTCC-3′ | ||||||||
| ACT | Actin/Cytoskeletal structural protein | GQ246181 | F:5′-CTCGGCTGTGGTTGTGAAG-3′ | 82.32 | 160 | − 3.469 | 0.996 | 94.2 |
| R:5′-TGGTGTGATGGTTGGTATGGG-3′ | ||||||||
| EF1a | Elongation factor 1-alpha /Protein synthesis | FR870012 | F:5′-ATCATGAATCACCCAGGACA-3′ | 80.27 | 147 | − 3.017 | 0.954 | 104.5 |
| R:5′-TTCAAGAACTTGGGCTCCTT-3′ | ||||||||
| EF1g | Elongation factor 1-gamma/Protein synthesis | FD496784 | F:5′-ATCCGGGATTCACTTGGATA-3′ | 75.85 | 114 | − 3.349 | 0.998 | 98.9 |
| R:5′-CGTCACCCATAACTTTGCAC-3′ | ||||||||
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase/metabolic function | FD495585 | F:5′-ACAACTAACTGCCTTGCTCCTT-3′ | 83.5 | 274 | − 3.263 | 0.997 | 102.5 |
| R:5′-AGTCAGATCCACCACCGAAA-3′ | ||||||||
| HIS1 | Histone H1 linker protein/Compacting DNA strands and Chromatin regulation | CO995299 | F:5′-AAGCCGAAGAAGGCGAAAGC-3′ | 81.93 | 100 | − 3.137 | 0.986 | 108.3 |
| R:5′-TCTCGAAGTAGGGCGGATGG-3′ | ||||||||
| TUA | a-Tubulin/Cytoskeletal structural protein | FD499140 | F:5′-TGACTGGAGCATAAGATGAAAGC-3′ | 80.46 | 153 | − 3.300 | 0.983 | 100.9 |
| R:5′-CCAATCTCAACCGCCTCG-3′ | ||||||||
| TUB | β-Tubulin/Cytoskeletal structural protein | FD493593 | F:5′-TCTCCGCCTCCGTAAACTC-3′ | 80.64 | 140 | − 3.330 | 0.991 | 99.7 |
| R:5′-ATTCATTGGGAACTCAACATCG-3′ | ||||||||
| UBQ | Ubiquitin/Protein degradation | CK755254 | F:5′-GGCATTCCACCAGACCAGC-3′ | 82.49 | 131 | − 3.118 | 0.998 | 109.3 |
| R:5′-TGCATCCCGCCCCTCAAT-3′ |
F forward primer, R reverse primer, Tm melting temperature, Slop standard curve slope, R2 regression coefficient, E amplification efficiency.
RT-qPCR and statistical analyses
The RT-qPCRs were performed on an ABI 7500 Real-Time PCR System with software (PE Applied Biosystems, USA). Each amplification was performed in a 20 µl final volume that contained 10 μl of Power SYBR Green Master Mix (Applied Biosystems), 0.8 μl of each specific primer pair at 100 nM; 1.0 μl of 5 × diluted cDNA template and 7.4 μl of ddH2O. All the PCRs were performed under the following conditions48: 2 min at 50 °C, 10 min at 95 °C, and 40 cycles of 15 s at 95 °C and 1 min at 60 °C in 96-well optical reaction plates (Applied Biosystems, USA). The specificity of each reaction was verified by a melting curve analysis (65 °C to 95 °C) after 40 cycles and 2% agarose gel electrophoresis (Supplemental data 1). Three biological replicates for each sample were used for RT-qPCR analysis. Three technical replicates and no template controls for every primer pair were performed for each biological replicate.Four-point standard curves of a fivefold dilution series (1:1 to 1:125) from pooled cDNA were used to calculate PCR efficiency levels28 (E) using the following equation: E = (10−1/slope−1) × 100. The calculated slopes of the standard curve, coefficients of determination, R2, and PCR efficiencies are shown in Table 5.
The data obtained were converted into correct input files, according to the requirements of the software, and analyzed using three different Visual Basic applets: geNorm version 3.410, NormFinder (version 0.953)30 and BestKeeper (version 1.0)31. The comparative ΔCt approach49 was also performed using IBM SPSS Statistics Version 19 software (IBM, USA).The geNorm VBA applet can calculate an M-value describing the average pairwise variation of each reference gene in comparison with all the other candidates and ranks the genes according to their expression stability. Lower M values reflect the greater stability of the reference genes. This applet also evaluates the pairwise variation (Vn/Vn+1) to determine the optimal number of genes. If the variation is low (Vn/Vn + 1 < 0.15), then this suggests that the added reference gene is not required for the calculation of the normalization factor and thus can be excluded10,46,50. The InputData of geNorm were the normalized relative quantities transformed from Ct values using the ΔCt method. As in geNorm, the InputData of NormFinder were log transformed into a linear scale. The NormFinder uses an ANOVA-based model to calculate a stability value, and candidate genes with minimal intra- and inter-group variations have the lowest stability values and are, therefore, ranked at the top30,46,51. The BestKeeper algorithm determines the most stably expressed genes based on three variables: SD, coefficient of variance and r value. Genes with SDs greater than 1 are considered unacceptable. BestKeeper also ranks gene stability by estimating the r value, the closer it is to 1, the more stable the gene expression31,52. The ΔCt method calculates the relative expression of ‘pairs of genes’ within each sample to identify useful reference genes. The stability of the reference gene is ranked according to a ‘process of elimination’ technique, in which genes are compared to one another, and the less variability in the ΔCt among different samples, the more stably the reference gene is expressed49,51.
Supplementary Information
Acknowledgements
This work was financially supported by the Cooperation Project of Chinese Academy of Forestry and Zhejiang Academy of Forerstry about Forestry Science and Technology (2018SY08), and the Zhejiang Province Public Welfare Technology Application Research Project (LGN18C030004). We also thank the Zhejiang Hangzhou Urban Forest Ecosystem Research Station for its platform support.
Abbreviations
- SE
Somatic embryogenesis
- Rpl2
Ribosomal protein L2
- 18S rRNA
18S ribosomal RNA
- ACT
Actin
- EF1a
Elongation factor 1-alpha
- EF1g
Elongation factor 1-gamma
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- HIS1
Histone H1 linker protein
- TUA
α-Tubulin
- TUB
β-Tubulin
- UBQ
Ubiquitin
- RT-qPCR
Quantitative real-time reverse transcription PCR
Author contributions
J.S. and T.L. conceived and designed the experiments. T.L. and S.Q. performed the experiments and analysed the data. W.Y. contributed reagents, materials, and analysis tools. T.L. wrote the paper. All authors read and approved the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-84518-w.
References
- 1.Zimmerman JL. Somatic embryogenesis: a model for early development in higher plants. Plant Cell. 1993;5:1411–1423. doi: 10.2307/3869792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Arnold S, Sabala I, Bozhkov P, Dyachok J, Filonova L. Developmental pathways of somatic embryogenesis. Plant Cell Tissue Organ Cult. 2002;69:233–249. doi: 10.1023/a:1015673200621. [DOI] [Google Scholar]
- 3.Winkelmann T. Clonal Propagation of Cyclamen persicum, Via Somatic Embryogenesis. Totowa: Humana Press; 2010. pp. 281–290. [DOI] [PubMed] [Google Scholar]
- 4.Li T, et al. Deep sequencing and microarray hybridization identify conserved and species-specific microRNAs during somatic embryogenesis in hybrid yellow poplar. PLoS ONE. 2012;7:e43451. doi: 10.1371/journal.pone.0043451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang X, et al. Transcript profiling reveals complex auxin signalling pathway and transcription regulation involved in dedifferentiation and redifferentiation during somatic embryogenesis in cotton. BMC Plant Biol. 2012;12:110. doi: 10.1186/1471-2229-12-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X, Wang L, Yuan D, Lindsey K, Zhang X. Small RNA and degradome sequencing reveal complex miRNA regulation during cotton somatic embryogenesis. J. Exp. Bot. 2013;64:1521–1536. doi: 10.1093/jxb/ert013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, et al. Small RNA and Transcriptome Sequencing Reveal a Potential miRNA-Mediated Interaction Network That Functions during Somatic Embryogenesis inLilium pumilumDC. Fisch. Front. Plant Sci. 2017;8:566. doi: 10.3389/fpls.2017.00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, et al. Genome-wide identification and characterization of long non-coding RNAs involved in the early somatic embryogenesis in Dimocarpus longan Lour. BMC Genomics. 2018;19:805. doi: 10.1186/s12864-018-5158-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freeman WM, Walker SJ, Vrana KE. Quantitative RT-PCR: pitfalls and potential. Biotechniques. 1999;26:112–125. doi: 10.2144/99261rv01. [DOI] [PubMed] [Google Scholar]
- 10.Vandesompele J, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:1–12. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Udvardi MK, Tomasz C, Wolf-Rüdiger S. Eleven golden rules of quantitative RT-PCR. Plant Cell. 2008;20:1736–1737. doi: 10.1105/tpc.108.061143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bustin SA, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 13.Chen L, et al. Validation of reference genes for RT-qPCR studies of gene expression in banana fruit under different experimental conditions. Planta. 2011;234:377–390. doi: 10.1007/s00425-011-1410-3. [DOI] [PubMed] [Google Scholar]
- 14.Daifeng C, Zhiling Z, Xiaofang H, Guangwen L. Validation of reference genes in Solenopsis invicta in different developmental stages, castes and tissues. PLoS ONE. 2013;8:e57718. doi: 10.1371/journal.pone.0057718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imai T, Ubi BE, Saito T, Moriguchi T. Evaluation of reference genes for accurate normalization of gene expression for real time-quantitative PCR in Pyrus pyrifolia using different tissue samples and seasonal conditions. PLoS ONE. 2014;9:e86492. doi: 10.1371/journal.pone.0086492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaohua Z, et al. Identification and validation of reference genes for quantitative real-time PCR normalization and its applications in lycium. PLoS ONE. 2014;9:e97039. doi: 10.1371/journal.pone.0097039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Vega-Bartol JJ, Raquen Raissa S, Marta SE, Miguel CM. Normalizing gene expression by quantitative PCR during somatic embryogenesis in two representative conifer species: Pinus pinaster and Picea abies. Plant Cell Rep. 2013;32:715–729. doi: 10.1007/s00299-013-1407-4. [DOI] [PubMed] [Google Scholar]
- 18.Merkle SA, Hoey MT, Watson-Pauley BA, Schlarbaum SE. Propagation of Lirlodendron hybrids via somatic embryogenesis. Plant Cell Tissue Organ Cult. 1993;34:191–198. doi: 10.1007/BF00036101. [DOI] [Google Scholar]
- 19.Chen J, et al. Study on origin and development of somatic embryos of Liriodendron hybrids. J. Nanjing For. Univ. 2012;36:16–20. [Google Scholar]
- 20.Yang Y, Xu M, Luo Q, Wang J, Li H. De novo transcriptome analysis of Liriodendron chinense petals and leaves by Illumina sequencing. Gene. 2014;534:155–162. doi: 10.1016/j.gene.2013.10.073. [DOI] [PubMed] [Google Scholar]
- 21.Shen Y, et al. Population transcriptomes reveal the interspecific adaptive genetic differentiation of liriodendron by landscape genetics. Res. Square. 2020 doi: 10.21203/rs.3.rs-62166/v1. [DOI] [Google Scholar]
- 22.Zhen Y, Li C, Chen J, Chen Q, Shi J. Proteomics of embryogenic and non-embryogenic calli of a Liriodendron hybrid. Acta Physiol. Plant. 2015;37:211. doi: 10.1007/s11738-015-1963-z. [DOI] [Google Scholar]
- 23.Chen J, et al. Liriodendron genome sheds light on angiosperm phylogeny and species-pair differentiation. Nat. Plants. 2019;5:18–25. doi: 10.1038/s41477-018-0323-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huo A, et al. Establishment of transient gene expression systems in protoplasts from Liriodendron hybrid mesophyll cells. PLoS ONE. 2017;12:e0172475. doi: 10.1371/journal.pone.0172475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldberg RB, De Paiva G, Yadegari R. Plant embryogenesis: zygote to seed. Science. 1994;266:605–614. doi: 10.1126/science.266.5185.605. [DOI] [PubMed] [Google Scholar]
- 26.Gonçalves S, Cairney J, Maroco J, Oliveira MM, Miguel C. Evaluation of control transcripts in real-time RT-PCR expression analysis during maritime pine embryogenesis. Planta. 2005;222:556–563. doi: 10.1007/s00425-005-1562-0. [DOI] [PubMed] [Google Scholar]
- 27.Tu L, et al. Suitable internal control genes for qRT-PCR normalization in cotton fiber development and somatic embryogenesis. Chin. Sci. Bull. 2007;52:3110–3117. doi: 10.1007/s11434-007-0461-0. [DOI] [Google Scholar]
- 28.Lin YL, Lai ZX. Reference gene selection for qPCR analysis during somatic embryogenesis in longan tree. Plant Sci. 2010;178:359–365. doi: 10.1016/j.plantsci.2010.02.005. [DOI] [Google Scholar]
- 29.Joseph, et al. Plant reference genes for development and stress response studies. J. Biosci. 2018;43:173–187. doi: 10.1007/s12038-017-9728-z. [DOI] [PubMed] [Google Scholar]
- 30.Andersen CL, Jensen JL, Ørntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Can. Res. 2004;64:5245–5250. doi: 10.1158/0008-5472.can-04-0496. [DOI] [PubMed] [Google Scholar]
- 31.Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper–Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004;26:509–515. doi: 10.1023/B:BILE.0000019559.84305.47. [DOI] [PubMed] [Google Scholar]
- 32.Tong Z, Gao Z, Wang F, Zhou J, Zhang Z. Selection of reliable reference genes for gene expression studies in peach using real-time PCR. BMC Mol. Biol. 2009;10:1–13. doi: 10.1186/1471-2199-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang XY, Zhang XL. Regulation of somatic embryogenesis in higher plants. Crit. Rev. Plant Sci. 2010;29:36–57. doi: 10.1080/07352680903436291. [DOI] [Google Scholar]
- 34.Kou SJ, et al. Selection and validation of suitable reference genes for miRNA expression normalization by quantitative RT-PCR in citrus somatic embryogenic and adult tissues. Plant Cell Rep. 2012;31:2151–2163. doi: 10.1007/s00299-012-1325-x. [DOI] [PubMed] [Google Scholar]
- 35.Freitas NC, et al. Validation of reference genes for qPCR analysis of Coffea arabica L. somatic embryogenesis-related tissues. Plant Cell Tissue Organ Cult. 2016;28:663–678. doi: 10.1007/s11240-016-1147-6. [DOI] [Google Scholar]
- 36.Ray DL, Johnson JC. Validation of reference genes for gene expression analysis in olive (Olea europaea) mesocarp tissue by quantitative real-time RT-PCR. BMC Res. Notes. 2014;7:1–12. doi: 10.1186/1756-0500-7-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Twardowski T, Legocki AB. Elongation factors complex in plant cells. Plant Sci. Lett. 1975;5:89–95. doi: 10.1016/0304-4211(75)90048-6. [DOI] [Google Scholar]
- 38.Dell'Aquila A, Zocchi G, Lanzani GA, Leo PD. Different forms of EF1 and viability in wheat embryos. Phytochemistry. 1976;15:1607–1610. doi: 10.1016/S0031-9422(00)97437-5. [DOI] [Google Scholar]
- 39.Bustin M, Catez F, Lim JH. The dynamics of histone H1 function in chromatin. Mol. Cell. 2005;17:617–620. doi: 10.1016/j.molcel.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 40.Tarze A, et al. GAPDH, a novel regulator of the pro-apoptotic mitochondrial membrane permeabilization. Oncogene. 2007;26:2606–2620. doi: 10.1038/sj.onc.1210074. [DOI] [PubMed] [Google Scholar]
- 41.Diana Z, et al. Vesicular glycolysis provides on-board energy for fast axonal transport. Cell. 2013;152:479–491. doi: 10.1016/j.cell.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 42.Schwarzerová K, et al. The role of actin isoforms in somatic embryogenesis in Norway spruce. BMC Plant Biol. 2010;10:89–89. doi: 10.1186/1471-2229-10-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tuszynski JA, et al. The evolution of the structure of tubulin and its potential consequences for the role and function of microtubules in cells and embryos. Int. J. Dev. Biol. 2006;50:341. doi: 10.1387/ijdb.052063jt. [DOI] [PubMed] [Google Scholar]
- 44.Kanakachari M, et al. Evaluation of suitable reference genes for normalization of qPCR gene expression studies in brinjal (Solanum melongena L.) during fruit developmental stages. Appl. Biochem. Biotechnol. 2015;178:433–450. doi: 10.1007/s12010-015-1884-8. [DOI] [PubMed] [Google Scholar]
- 45.Yu LL, Zhong XL. Evaluation of suitable reference genes for normalization of microRNA expression by real-time reverse transcription PCR analysis during longan somatic embryogenesis. Plant Physiol. Biochem. 2013;66:20–25. doi: 10.1016/j.plaphy.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 46.Machado RD, et al. Comprehensive selection of reference genes for quantitative gene expression analysis during seed development in Brassica napus. Plant Cell Rep. 2015;34:1139–1149. doi: 10.1007/s00299-015-1773-1. [DOI] [PubMed] [Google Scholar]
- 47.Jisen, S., Tingting, L., Jinhui, C. & Wei, L. Method for developmental synchronization of somatic embryogenesis in Liriodendron hybrids. China patent (2011).
- 48.Jain M, Nijhawan A, Tyagi AK, Khurana JP. Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochem. Biophys. Res. Commun. 2006;345:646–651. doi: 10.1016/j.bbrc.2006.04.140. [DOI] [PubMed] [Google Scholar]
- 49.Silver N, Best S, Jiang J, Thein SL. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol. Biol. 2006;7:33. doi: 10.1186/1471-2199-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jin Y, Liu F, Huang W, Sun Q, Huang X. Identification of reliable reference genes for qRT-PCR in the ephemeral plant Arabidopsis pumila based on full-length transcriptome data. Sci. Rep. 2019;9:8408. doi: 10.1038/s41598-019-44849-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang, F. et al. Selection and validation of reference genes for quantitative expression analysis of miRNAs and mRNAs in Poplar. Plant Methods. 10.1186/s13007-019-0420-1 (2019). [DOI] [PMC free article] [PubMed]
- 52.Freitas NC, et al. Validation of reference genes for qPCR analysis of Coffea arabica L. somatic embryogenesis-related tissues. Plant Cell Tissue Organ Cult. 2017;128:663–678. doi: 10.1007/s11240-016-1147-6. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.