Abstract
Objectives
Small dense low-density lipoprotein cholesterol (sdLDL-C) might be a better cardiovascular disease (CVD) indicator than low-density lipoprotein cholesterol (LDL-C); however, details regarding its epidemiology remain elusive. The present study aimed at evaluating the association between the demographic factors, such as age, gender and menopausal status, and sdLDL-C levels and sdLDL-C/LDL-C ratio in the Japanese population.
Design
This was a cross-sectional study.
Setting
13 rural districts in Japan, 2010–2017.
Participants
This study included 5208 participants (2397 men and 2811 women), who underwent the health mass screening that was conducted in accordance with the medical care system for the elderly and obtained informed consent for this study.
Results
In total, 517 premenopausal women (mean age ±SD, 45.1±4.2 years), 2294 postmenopausal women (66.5±8.8 years) and 2397 men (64.1±11.2 years) were analysed. In men, the sdLDL-C levels and sdLDL-C/LDL-C ratio increased during younger adulthood, peaked (36.4 mg/dL, 0.35) at 50–54 years, and then decreased. In women, relatively regular increasing trends of sdLDL-C level and sdLDL-C/LDL-C ratio until approximately 65 years (32.7 mg/dL, 0.28), followed by a downward or pleated trend. Given the beta value of age, body mass index, fasting glucose and smoking and drinking status by multiple linear regression analysis, standardised sdLDL-C levels and sdLDL-C/LDL-C ratio in 50-year-old men, premenopausal women and postmenopausal women were 26.6, 22.7 and 27.4 mg/dL and 0.24, 0.15 and 0.23, respectively. The differences between premenopausal and postmenopausal women were significant (p<0.001).
Conclusions
SdLDL-C and sdLDL-C/LDL-C ratios showed different distributions by age, gender and menopausal status. A subgroup-specific approach would be necessary to implement sdLDL-C for CVD prevention strategies, fully considering age-related trends, gender differences and menopausal status.
Keywords: cardiology, heart failure, ischaemic heart disease, internal medicine
Strengths and limitations of this study.
To the best of our knowledge, the present study is the first to demonstrate the association between age, gender and menopausal status on the small dense low-density lipoprotein cholesterol (sdLDL-C) and sdLDL-C/low-density lipoprotein cholesterol ratio.
This study is based on a large representative sample from Japanese general population.
Serum lipid markers were measured by the standardised programme proposed by the Clinical and Laboratory Standards Institute.
It is unclear whether our results of sdLDL-C would be valid for other populations.
This study did not control for several confounding factors, such as diet, life activity, socioeconomic status and genetic factors.
Introduction
Although hypercholesterolaemia is one of the leading causes of cardiovascular disease (CVD), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C) and non-high-density lipoprotein cholesterol (nonHDL-C) have not been good enough to predict risk stratification and the novel target is needed.1–3 Small dense low-density lipoprotein cholesterol (sdLDL-C) easily penetrates into the arterial wall, has a high susceptibility to oxidation and may exacerbate and perpetuate atherosclerosis.4 In fact, patients with metabolic syndrome, which have been found as highly atherogenic conditions without hypercholesterolaemia, have elevated sdLDL-C.5 The sdLDL-C/LDL-C ratio, reflecting the ability to generate sdLDL-C from LDL-C, might increase by the high activity of hepatic lipase, which was associated with higher risk of CVD. Current studies suggest that the sdLDL-C or sdLDL-C/LDL-C ratio might be the better factors for the prediction of CVD than TC or LDL-C in the general population or patients with CVD.6–9
However, almost all of the current analytical strategies might be not able to adjust accurately the interaction between age and sdLDL-C. Few studies have evaluated how age is associated with sdLDL-C and sdLDL-C/LDL-C ratio over a wide age range and distinguished the effects of menopause and gender on sdLDL-C and sdLDL-C fraction from those of ageing.10 11
Diet composition, which is affected by ageing, is associated with blood cholesterol and the absorption, synthesis and metabolism per se of fat and lipoproteins change with age.12 13 Another study showed Asian age-related trends of traditional lipid profiles displayed roughly an increasing trend, followed by a decreasing one at the middle-aged stage.14 15 Meanwhile, sdLDL-C has been regulated by more complex mechanisms than regulating traditional lipids and might be plateaued or increased even at the middle aged by changed metabolic functions with ageing influencing sdLDL-C synthesis.5 7 12 16 17 Furthermore, the detailed multiple mechanisms of metabolising sdLDLs are unknown in the real-world, population-based setting and the age-related trend of sdLDL-C might be different from the sdLDL-C/LDL-C ratio. In other words, the ability to generate sdLDL-C from LDL-C might be different among each generation, gender and menopausal status. Therefore, we evaluated the association between the demographic factors, such as age, gender and menopausal status, and sdLDL-C and sdLDL-C/LDL-C ratio in Japanese general population.
Methods
Population
The present cross-sectional study was conducted as part of the Jichi Medical School-II Cohort Study, a population-based cohort study of the risk factors of atherosclerosis and CVD in the Japanese general population. A total of 6436 individuals participated in this study. Details of the methods of enrolment have been reported previously.18 19 In brief, from April 2010 through December 2017, this study evaluated Japanese individuals who were residents of 13 rural districts in Japan, Shimotsuke, Kakara, Sue, Omori, Kamiichi, Wara, Takasu, Onabi, Nakatsu, Yame, Miwa, Ueno and Saji areas. Local government offices in each community issued invitations to eligible residents for the mass CVD screening, and personal invitations were also sent to all potential participants by mail. All the participants in the present study provided written informed consent prior to inclusion.
We excluded individuals as follows: (1) taking lipid-lowering agents or antihyperglycaemia agents (n=1073); (2) the use of hormone replacement therapy (n=96); and (3) the data such as age, gender status, menopausal status and sdLDL-C were not available (n=73).
Measurements
A central committee, composed of the chief medical officers of all 13 participating districts, developed a detailed manual for data collection. Body weight was recorded with the subjects clothed. Height was measured with stockinged feet. Body mass index (BMI) was calculated as weight (kg)/height (m2). Blood samples were taken after overnight fasting. TC was measured via a cholesterol dehydrogenase-ultraviolet method. Triglycerides (TG) was measured using an enzymatic method. LDL-C and high-density lipoprotein cholesterol (HDL-C) were measured by direct methods using a commercial kit (Cholestest from Sekisui Medical, Tokyo, Japan). SdLDL-C level was directly and selectively measured using a commercial kit (sdLDL-EX from Denka Seiken, Tokyo, Japan). An external laboratory (SRL, Tokyo, Japan) measured the serum lipid markers. The markers were measured by the standardised programme proposed by the Clinical and Laboratory Standards Institute. The nonHDL-C was calculated by subtracting HDL-C from TC. Information about medical history, lifestyle and menopausal status were obtained with a self-reported questionnaire. Smoking status was classified as smoking, former smoking, or never smoking.
Statistical analysis
Baseline characteristics were summarised as mean±SD for normally distributed continuous variables and numbers and percentages for categorical variables. SdLDL-C and TG were highly skewed; these data were expressed as the median and IQR and transformed into natural logarithms before statistical analysis. The participants were divided into three groups (men, premenopausal women and postmenopausal women) according to gender and menopausal status.
The one-way analysis of variance was used for comparison among three groups, and differences were tested via post hoc pairwise comparison (Bonferroni). To explore the age-related trend in sdLDL-C and sdLDL-C/LDL-C ratio with age, geometric means or means and 95% CIs for each variable in 5-year age ranges were derived and plotted against age range in each of the three groups.
Among the three groups, correlations between age and each parameter were assessed using multiple linear regression analysis. Considering the beta value of age, BMI, fasting glucose and smoking and drinking status, we calculated the estimated sdLDL-C and sdLDL-C/LDL-C ratio. The agreement between the estimated sdLDL-C and sdLDL-C/LDL-C ratio and measured ones was assessed by Pearson’s correlation coefficient. To evaluate the effect of menopausal status on sdLDL-C and sdLDL-C/LDL-C ratio, using the beta value of each variable from the analysis in the premenopausal and postmenopausal group, data were standardised to a nominal 50 years of menopausal age, never smoking and zero alcohol for participants with normal weight (BMI 18.5–22.0). All statistical analyses were performed using SPSS V.22 (IBM), and statistical significance was defined as p<0.05.
Patient and public involvement
Participants of this study or members of the public were not directly and personally involved with study design, data provision, analysis and publication of the study.
Results
Baseline characteristics
After exclusions, 517 premenopausal women (mean age ±SD, 45.1±4.2 years), 2294 postmenopausal women (66.5±8.8 years) and 2397 men (64.1±11.2 years) were analysed. Demographic data for the three groups are shown in table 1. Compared with men, premenopausal women had higher HDL-C and postmenopausal women had higher TC, LDL-C, HDL-C and nonHDL-C. Compared with premenopausal women, postmenopausal women had higher fasting glucose, TC, LDL-C, TG, nonHDL-C, TC/LDL-C, sdLDL-C and sdLDL-C/LDL-C. TC and LDL-C did not differ between men and premenopausal women.
Table 1.
Baseline characteristics
| Group 1 (G1) Men (n=2397) |
Group 2 (G2) Premenopausal women (n=517) |
Group 3 (G3) Postmenopausal women (n=2294) |
P value G1 versus G2 |
P value G1 versus G3 |
P value G2 versus G3 |
|
| Age, years | 64.1±11.2 | 45.1±4.2 | 66.5±8.8 | <0.001 | <0.001 | <0.001 |
| BMI, kg/m2 | 23.3±3.0 | 22.3±3.6 | 22.5±3.3 | <0.001 | <0.001 | 0.631 |
| Smoking | ||||||
| Current | 600 (25.1%) | 40 (7.7%) | 67 (2.9%) | <0.001 | <0.001 | 0.007 |
| Ex | 1204 (50.3%) | 73 (14.1%) | 97 (4.2%) | <0.001 | <0.001 | <0.001 |
| Drinker | 1869 (78.2%) | 316 (61.1%) | 866 (37.8%) | <0.001 | <0.001 | <0.001 |
| Glucose, mg/dL | 100.7±17.8 | 90.9±9.4 | 96.3±12.3 | <0.001 | <0.001 | <0.001 |
| TC, mg/dL | 198.7±32.9 | 199.2±31.2 | 215.4±31.6 | 1.000 | <0.001 | <0.001 |
| LDL-C, mg/dL | 115.2±29.6 | 114.2±28.5 | 126.7±28.7 | 1.000 | <0.001 | <0.001 |
| TGs, mg/dL | 100 (71 to 146) | 68 (50 to 94) | 89 (67 to 123) | <0.001 | <0.001 | <0.001 |
| HDL-C, mg/dL | 56.3±13.8 | 67.8±14.7 | 62.8±14.9 | <0.001 | <0.001 | <0.001 |
| Non HDL-C, mg/dL | 142.4±32.6 | 131.4±31.2 | 152.5±31.3 | <0.001 | <0.001 | <0.001 |
| TC/HDL-C | 3.7±1.0 | 3.1±0.8 | 3.6±0.9 | <0.001 | <0.001 | <0.001 |
| SdLDL-C, mg/dL | 34.1 (24.8 to 46.5) | 23.0 (16.8 to 30.5) | 31.2 (23.5 to 41.8) | <0.001 | <0.001 | <0.001 |
| SdLDL-C/LDL-C | 0.32±0.14 | 0.22±0.08 | 0.29±0.12 | <0.001 | <0.001 | <0.001 |
Data are expressed as mean±SD, %, and median (25th percentile, 75th percentile). P values were assessed in one-way analysis of variance and post hoc pairwise comparison (Bonferroni).
BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; non HDL-C, non high-density lipoprotein cholesterol; sdLDL-C, small dense low-density lipoprotein cholesterol; TC, total cholesterol; TGs, triglycerides.
sdLDL-C trends in 5-year age groups
To assess the age-related trend in sdLDL-C levels, a 5-year age stratification was applied, and geometric mean sdLDL-C levels for each age groups were calculated and plotted against gender.
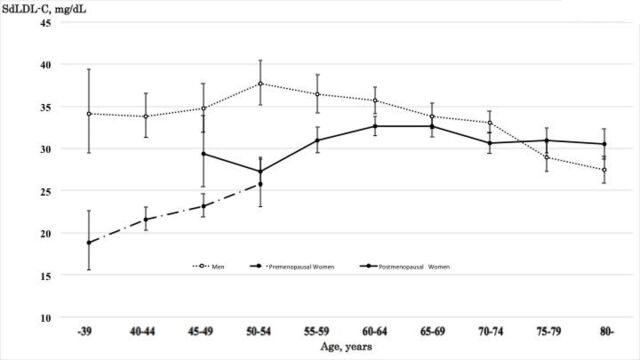
For men, the level of sdLDL-C increased from 34.1 mg/dL in those <39 years to a maximum of 37.7 mg/dL in those of 50–54 years, followed by decreasing from 36.4 mg/dL in those of 55–59 years to 27.4 mg/dL in those of 80≤years (figure 1). For women, a relatively regular increasing trend of the sdLDL-C level was found up to 60–64 year olds. After 65 years, a downward trend was fitted. The maximum of the sdLDL-C level of women was 32.7 mg/dL. Moreover, sdLDL-C levels in men were higher than those in women for all age groups younger than 70–74 year olds but exceeded those in women after the age of 75–79 years.
Figure 1.
Geometric mean and 95% CI of small dense low-density lipoprotein cholesterol (sdLDL-C) for age, gender and menopausal status.
sdLDL-C/LDL-C ratio trends in 5-year age groups
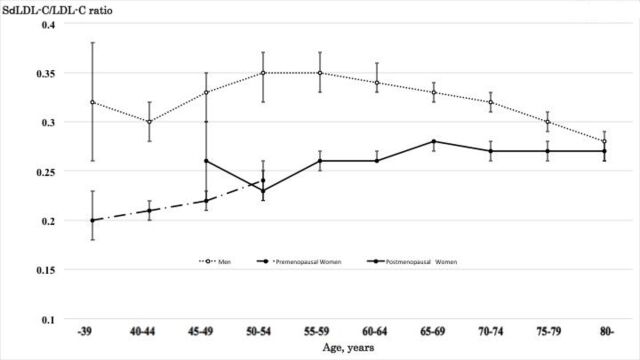
SdLDL-C/LDL-C ratio in men increased from 0.30 in 40–44 year olds to a maximum of 0.35 in 50–54 year olds, plateaued in those of 55–59 years, and then decreased from 0.34 in those of 60–64 years to 0.28 in those of 80 ≤years (figure 2). For women, these values increased from 0.20 in those <39 years to a maximum of 0.28 in those of 65–69 years and plateaued after 70≤years (with mean levels of 0.27). SdLDL-C/LDL-C ratio in men was higher than those in women for all age groups and the crossover of sdLDL-C/LDL-C ratio for the genders did not occur.
Figure 2.
Mean and 95% CI of small dense low-density lipoprotein cholesterol (sdLDL-C)/low-density lipoprotein cholesterol (LDL-C) ratio for age, gender and menopausal status.
Trends in other lipoproteins (LDL-C, TC, TG, HDL-C and TC/HDL-C ratio) in 5-year age groups
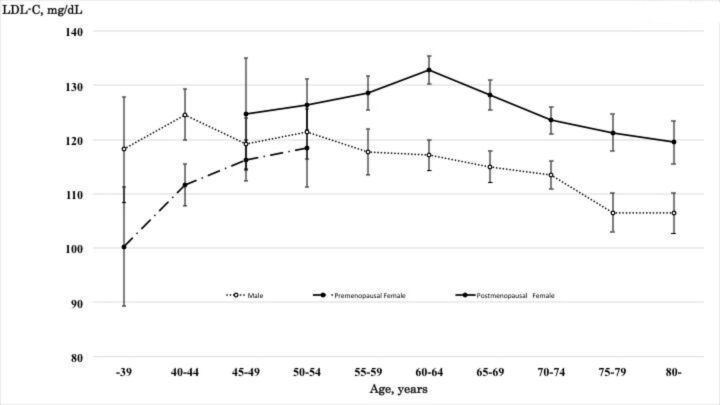
LDL-C level in men decreased almost linearly, while LDL-C level in women rapidly increased from 100.3 mg/dL in those aged <39 years to a maximum of 132.8 mg/dL in 60–64 year olds and decreased from 128.2 mg/dL in those aged 65–69 to 119.5 mg/dL in those 80≤years (figure 3). The level of TC, nonHDL-C and TC/HDL-C ratio revealed a pattern similar to the trend of LDL-C levels (see online supplemental figures 1-3). The TG levels in men decreased almost linearly, while the level in women increased linearly (see online supplemental figure 4). HDL-C in both men and women decreased almost linearly (see online supplemental figure 5).
Figure 3.
Mean and 95% CI of low-density lipoprotein cholesterol (LDL-C) for age, gender and menopausal status.
bmjopen-2020-041613supp001.pdf (334.5KB, pdf)
bmjopen-2020-041613supp002.pdf (328.9KB, pdf)
bmjopen-2020-041613supp003.pdf (327.8KB, pdf)
bmjopen-2020-041613supp004.pdf (344KB, pdf)
bmjopen-2020-041613supp005.pdf (327.7KB, pdf)
sdLDL-C and sdLDL-C/LDL-C ratio in the standardised analysis among the three groups
To standardise sdLDL-C and sdLDL-C/LDL-C ratio among the three groups and validate the above-mentioned turning points, the participants were restratified by age ranges corresponding to increasing, plateau and decreasing phases for each marker by gender and multiple linear regression analysis was then applied.
As shown in table 2, among men, age was positively and negatively associated with log-transformed small dense low-density lipoprotein cholesterol (LNsdLDL-C) levels in those ≤54 years and ≥55 years. Among premenopausal women, postmenopausal women ≤64 years and postmenopausal women 65≥years, age was positively, positively and negatively associated with LNsdLDL-C levels. But the association between LNsdLDL-C and age was not significantly associated with men ≤54 years.
Table 2.
Factors associated with log-transformed small dense low-density lipoprotein cholesterol level in age groups by gender
| Variable | β | SE | P value |
| Men ≤54, n=475; mean±SD, 46.7±4.9 years, Pearson’s r=0.320 (p<0.001) | |||
| Age | 0.006 | 0.004 | 0.169 |
| BMI | 0.033 | 0.006 | <0.001 |
| Fasting glucose | 0.004 | 0.002 | 0.003 |
| Smoker | |||
| Current | 0.018 | 0.054 | 0.747 |
| Ex | 0.050 | 0.053 | 0.342 |
| Drinker | 0.144 | 0.059 | 0.015 |
| Men ≥55, n=1922; 68.4±7.6 years, Pearson’s r=0.316 (p<0.001) | |||
| Age | −0.010 | 0.001 | <0.001 |
| BMI | 0.032 | 0.003 | <0.001 |
| Fasting glucose | 0.002 | 0.001 | <0.001 |
| Smoker | |||
| Current | 0.025 | 0.030 | 0.402 |
| Ex | 0.032 | 0.024 | 0.192 |
| Drinker | 0.076 | 0.024 | 0.001 |
| Women (premenopausal), n=517; 45.1±4.2 years, Pearson’s r=0.330 (p<0.001) | |||
| Age | 0.014 | 0.005 | 0.002 |
| BMI | 0.024 | 0.006 | <0.001 |
| Fasting glucose | 0.008 | 0.002 | <0.001 |
| Smoker | |||
| Current | 0.021 | 0.072 | 0.775 |
| Ex | −0.005 | 0.056 | 0.934 |
| Drinker | 0.033 | 0.039 | 0.398 |
| Women≤64 years (postmenopausal), n=978; 58.3±4.5 years, Pearson’s r=0.261 (p<0.001) | |||
| Age | 0.014 | 0.003 | <0.001 |
| BMI | 0.019 | 0.004 | <0.001 |
| Fasting glucose | 0.004 | 0.001 | <0.001 |
| Smoker | |||
| Current | 0.052 | 0.067 | 0.437 |
| Ex | 0.036 | 0.051 | 0.479 |
| Drinker | 0.007 | 0.026 | 0.792 |
| Women 65≥years (postmenopausal), n=1316; 72.6±5.7 year olds, Pearson’s r=0.228 (p<0.001) | |||
| Age | −0.004 | 0.002 | 0.045 |
| BMI | 0.022 | 0.004 | <0.001 |
| Fasting glucose | 0.003 | 0.001 | 0.001 |
| Smoker | |||
| Current | −0.086 | 0.078 | 0.267 |
| Ex | 0.204 | 0.076 | 0.007 |
| Drinker | −0.007 | 0.024 | 0.760 |
β is a coefficient indicating a one-unit increase in an independent variable in multivariable linear logic regression analyses.
BMI, body mass index.
As shown in table 3, age in men ≤54 years, 55–59 years and 60≥years, was positively, positively and negatively associated with sdLDL-C/LDL-C ratio. In women, age in premenopausal women, postmenopausal women ≤69 years was positively associated with sdLDL-C/LDL-C ratio, whereas age in postmenopausal women 70≥years was not significantly associated sdLDL-C/LDL-C ratio. The association between sdLDL-C/LDL-C and age was not significantly associated with men 55–59 years, premenopausal women and postmenopausal women 70≥years.
Table 3.
Factors associated with small dense low-density lipoprotein cholesterol/low-density lipoprotein cholesterol ratio in age groups by gender
| Variable | β | SE | P value |
| Men ≤54 years, n=475; mean±SD, 46.7±4.9 year olds, Pearson’s r=0.320 (p<0.001) | |||
| Age | 0.003 | 0.001 | 0.020 |
| BMI | 0.005 | 0.002 | 0.012 |
| Fasting glucose | 0.001 | 0.000 | 0.010 |
| Smoker | |||
| Current | 0.029 | 0.016 | 0.081 |
| Ex | 0.011 | 0.016 | 0.501 |
| Drinker | 0.049 | 0.018 | 0.007 |
| Men 55–59 years, n=245; 57.2±1.4 years, Pearson’s r=0.222 (p<0.001) | |||
| Age | 0.004 | 0.007 | 0.589 |
| BMI | 0.003 | 0.003 | 0.385 |
| Fasting glucose | 0.001 | 0.001 | 0.285 |
| Smoker | |||
| Current | 0.049 | 0.032 | 0.125 |
| Ex | 0.062 | 0.030 | 0.042 |
| Drinker | 0.055 | 0.027 | 0.041 |
| Men 60≥years, n=1677; 70.0±6.8 years, Pearson’s r=0.272 (p<0.001) | |||
| Age | −0.002 | 0.000 | <0.001 |
| BMI | 0.005 | 0.001 | <0.001 |
| Fasting glucose | 0.001 | 0.000 | <0.001 |
| Smoker | |||
| Current | 0.029 | 0.009 | 0.001 |
| Ex | 0.009 | 0.007 | 0.235 |
| Drinker | 0.055 | 0.007 | <0.001 |
| Women (premenopausal), n=517; 45.1±4.2 years, Pearson’s r=0.313 (p<0.001) | |||
| Age | 0.001 | 0.001 | 0.147 |
| BMI | 0.003 | 0.001 | 0.002 |
| Fasting glucose | 0.001 | 0.000 | <0.001 |
| Smoker | |||
| Current | 0.010 | 0.012 | 0.413 |
| Ex | 0.000 | 0.010 | 0.988 |
| Drinker | 0.015 | 0.007 | 0.027 |
| Women≤69 years (postmenopausal), n=1434; 61.0±5.5 years, Pearson’s r=0.264 (p<0.001) | |||
| Age | 0.002 | 0.000 | <0.001 |
| BMI | 0.004 | 0.001 | <0.001 |
| Fasting glucose | 0.001 | 0.000 | <0.001 |
| Smoker | |||
| Current | 0.001 | 0.012 | 0.914 |
| Ex | 0.013 | 0.010 | 0.201 |
| Drinker | 0.003 | 0.005 | 0.555 |
| Women 70≥years (postmenopausal), n=860; 75.6±4.6 year olds, Pearson’s r=0.167 (p<0.001) | |||
| Age | 0.000 | 0.001 | 0.704 |
| BMI | 0.004 | 0.001 | <0.001 |
| Fasting glucose | 0.001 | 0.000 | <0.001 |
| Smoker | |||
| Current | −0.049 | 0.025 | 0.052 |
| Ex | 0.034 | 0.021 | 0102 |
| Drinker | −0.004 | 0.006 | 0.501 |
β is a coefficient indicating a one-unit increase in an independent variable in multivariable linear logic regression analyses.
BMI, body mass index.
Considering the beta value of each variable, 50-year-old standardised sdLDL-C levels in men, premenopausal women and postmenopausal women were 26.6 mg/dL (95% CI 26.4 to 26.9 mg/dL), 22.7 mg/dL (95% CI 22.5 to 22.9 mg/dL) and 27.4 mg/dL (95% CI 27.3 to 27.5 mg/dL), respectively. Standardised sdLDL-C/LDL-C ratio in men, premenopausal women and postmenopausal women were 0.24 (95% CI 0.24 to 0.24), 0.15 (95% CI 0.15 to 0.16) and 0.23 (95% CI 0.22 to 0.23), respectively. These differences between premenopausal women and postmenopausal women were significant (Bonferroni analysis, p<0.001).
Discussion
To the best of our knowledge, the present study is the first to demonstrate the association between age, gender, menopausal status and sdLDL-C and sdLDL-C/LDL-C ratio. The age-related sdLDL-C trends showed roughly an increasing phase, followed by a decreasing phase in men and a plateaued phase in middle-aged women. The age-related sdLDL-C trend in men, but not in women, was similar to traditional lipid cholesterol profiles. The reason for this gender difference might be related to the mechanism of hypertriglyceridaemia in postmenopausal women, which induced small LDL particles.20–22 There were age or gender-related differences in sdLDL-C/LDL-C ratio, reflecting the ability to generate sdLDL-C from LDL-C. This ability in men was higher than that in women for all age groups or standardised groups, which is identical to the fact that atherosclerosis is more common in men than in women, considering sdLDL-C is a highly atherogenic factor.
Our study showed three important results. First, age showed partial correlation trends with sdLDL-C levels and sdLDL-C/LDL-C ratio and non-linear trends between age and sdLDL-C and sdLDL-C/LDL-C ratio were found in both men and women. Therefore, using the sdLDL-C and sdLDL-C/LDL-C ratio, the definition of CVD risk assessment and the adaption of the lipid-lowering therapy should fully consider age-related trends and gender differences.
Second, menopausal status was an additional determinant of increasing sdLDL-C and sdLDL-C/LDL-C ratio. Many factors such as excess adiposity, free fatty acids, apo-lipoproteins and action of lipoprotein lipase activity and cholesterol ester transfer protein affected multiple and complex mechanisms regulating sdLDL.12 16 17 In postmenopausal women, the decrease of plasma oestrogen levels plays a significant role in reducing the clearance of LDL particles via LDL receptor and increasing TG and the number of smaller LDL particles.23 This hormone change was related to the process of regulating sdLDL-C but there was little evidence available on the association between menopausal status and sdLDL-C or sdLDL-C/LDL-C ratio in a real-world, population setting.24 Our results showed that sdLDL-C in postmenopausal women was 0.8 or 3.9 mg/dL higher than men or premenopausal women in the standardised analysis.
Finally, the relationships between age-related trends in sdLDL-C and sdLDL-C/LDL-C ratio and gender were different from traditional lipid factors, such as LDL-C. The crossover of LDL-C for the genders occurred in middle-aged patients. On the contrary, the crossover of sdLDL-C occurred between 70 and 74 years and the sdLDL-C/LDL-C ratio did not occur. Rather than LDL-C, the results of the sdLDL-C and sdLDL-C/LDL-C ratio might reflect the fact that, for all age groups, men have more susceptible to CVD than women, even with the narrowing gap of risk for CVD in postmenopausal women.25
Our findings suggest that a subgroup-specific approach is required to develop efficient CVD prevention strategies using the sdLDL-C and sdLDL-C/LDL-C ratio.
Limitations
Our study has several limitations. First, age-related trends and levels of traditional lipid factors were almost similar to National Health and Nutrition Survey in Japan and our age-related trends of these factors were also similar to the trends of the Korean and Chinese Singaporeans population.14 15 But the trends of the US population or healthy Caucasian26 27 were not similar. Especially in healthy Caucasian patients aged ≥70 years, the trends for TC, LDL-C and nonHDL-C differed from our observed trends and continuously increased. Although our results could not identify the mechanism, there might be racial differences. Therefore, it is unclear whether our results of sdLDL-C would be valid for these populations. Second, compared with mean lipid levels of the Korean population from KNHANES, Japanese men showed higher mean TC, LDL-C and HDL levels (TC 199 mg/dL; LDL-C 115 mg/dL; HDL-C 56 mg/dL) compared with Korean men (TC 183 mg/dL; LDL-C 106 mg/dL; HDL 50 mg/dL), and Japanese women also showed higher mean levels (TC 212 mg/dL; LDL-C 124 mg/dL; HDL-C 64 mg/dL) than Korean women (TC 188 mg/dL; LDL-C 111 mg/dL; HDL-C 55 mg/dL). The reason for the difference in the lipoprotein profile between Japanese and Korean populations might be due to genetics and environmental factors. It is also unknown whether these factors might affect sdLDL-C levels and sdLDL-C/LDL-C ratio because sdLDLs are regulated through complex mechanisms. Third, we did not control for the effects of diet, life activity, socioeconomic status and genetic factors, which might be associated with changes in lipid metabolism.28–30 Fourth, there might be several biases. Selection bias might come from potential non-representativeness of the study population, which was rural dwelling. There might be information bias and data misclassification due to error in measurement of the lipid parameters. Fifth, as shown in the online supplemental figures 6 and 7, the results regarding the association between demographic factors and sdLDL-C and sdLDL-C/LDL-C ratio remained the same in 6282 participants including patients taking lipid-lowering therapy. SdLDL-C/LDL-C ratio in men including patients taking lipid-lowering therapy was higher than in men excluding these patients (0.45 vs 0.35). Our assessment was limited in terms of this difference, because data regarding type and dose of medications for dyslipidaemia were not available. We need to validate the association in patients taking lipid-lowering therapy in another cohort. Finally, our study could not evaluate the association between the demographic factors and other lipid markers, such as Lp(a) and oxidised LDL-C. Lp(a) was a significant risk factor for cardiovascular disorders and to be in the spotlight due to a novel therapy using antisense oligonucleotides. These lipid markers should be discussed in further study.31
bmjopen-2020-041613supp006.pdf (1.1MB, pdf)
bmjopen-2020-041613supp007.pdf (1.1MB, pdf)
Conclusion
SdLDL-C and sdLDL-C/LDL-C ratio are differently distributed by age, gender and menopausal status. Our findings suggest that a subgroup-specific approach is required to develop efficient CVD prevention strategies using the sdLDL-C and sdLDL-C/LDL-C ratio.
Supplementary Material
Acknowledgments
The authors thank the public health doctors, nurses, and officers in Shimotsuke, Kakara, Sue, Omori, Kamiichi, Wara, Takasu, Onabi, Nakatsu, Yame, Miwa, Ueno, and Saji areas, Japan, for their help, support, and contributions.
Footnotes
Contributors: All authors have participated in the research and designed the study. TI and SI performed the statistics analysis. TI contributed to the drafting of the manuscript. YN, YS and SI provided feedback on the manuscript, and all authors read and approved the final manuscript.
Funding: This research was supported by the MEXT-Supported Program for the Strategic Research Foundation at Private Universities (S0901032); a Japanese Society for the Promotion of Science KAKENHI grant (No. 16K09141); a Grant-In-Aid from the Ministry of Health, Labour, and Welfare; Health and Labor Sciences and Japan Comprehensive Research on Cardiovascular and Lifestyle-Related Diseases grants (H26-Junkankitou-[Seisaku]-Ippan-001 and H29-Junkankitou-Ippan-003; IRB No. G09-39 [G17-64 revised]); and Jichi Medical University Almuni Association Project Grant 2020 (5-3).
Competing interests: None declared.
Patient consent for publication: All the participants included in the present study provided written informed consent for publication.
Ethics approval and consent to participate: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All the participants included in the present study provided written informed consent prior to inclusion, and this study was approved by the Institutional Review Board of Jichi Medical School (Tochigi, Japan, IRB No. G09-39 (G17-64 revised)).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1. Grundy SM, Stone NJ, Bailey AL, et al. 2018AHA/ACC/ AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/ NLA/PCNA guideline on the management of blood cholesterol. Circulation 2019;139:e1082–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lawler PR, Akinkuolie AO, Harada P, et al. Residual risk of atherosclerotic cardiovascular events in relation to reductions in very‐low‐density lipoproteins. J Am Heart Assoc 2017;6:1–11. 10.1161/JAHA.117.007402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Varbo A, Nordestgaard BG, Cholesterol R. Remnant cholesterol and triglyceride-rich lipoproteins in atherosclerosis progression and cardiovascular disease. Arterioscler Thromb Vasc Biol 2016;36:2133–5. 10.1161/ATVBAHA.116.308305 [DOI] [PubMed] [Google Scholar]
- 4. Ivanova EA, Myasoedova VA, Melnichenko AA, et al. Small dense low-density lipoprotein as biomarker for atherosclerotic diseases. Oxid Med Cell Longev 2017;2017:1–10. 10.1155/2017/1273042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kathiresan S, Otvos JD, Sullivan LM, et al. Increased small low-density lipoprotein particle number. Circulation 2006;113:20–9. 10.1161/CIRCULATIONAHA.105.567107 [DOI] [PubMed] [Google Scholar]
- 6. Arai H, Kokubo Y, Watanabe M, et al. Small dense low-density lipoproteins cholesterol can predict incident cardiovascular disease in an urban Japanese cohort: the Suita study. J Atheroscler Thromb 2013;20:195–203. 10.5551/jat.14936 [DOI] [PubMed] [Google Scholar]
- 7. Hoogeveen RC, Gaubatz JW, Sun W, et al. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease. Arterioscler Thromb Vasc Biol 2014;34:1069–77. 10.1161/ATVBAHA.114.303284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. St-Pierre AC, Cantin B, Dagenais GR, et al. Ber- nard P-M, Després J-P, Lamarche B. low-density lipoprotein subfractions and the long-term risk of ischemic heart disease in men. Arterioscler Thromb Vasc Biol 2005;25:553–9. [DOI] [PubMed] [Google Scholar]
- 9. Nishikura T, Koba S, Yokota Y, et al. Elevated small dense low-density lipoprotein cholesterol as a predictor for future cardiovascular events in patients with stable coronary artery disease. J Atheroscler Thromb 2014;21:755–67. 10.5551/jat.23465 [DOI] [PubMed] [Google Scholar]
- 10. Mogarekar MR, Kulkarni SK. Small dense low density lipoprotein cholesterol, paraoxonase 1 and lipid profile in postmenopausal women: quality or quantity? Arch Med Res 2015;46:534–8. 10.1016/j.arcmed.2015.08.007 [DOI] [PubMed] [Google Scholar]
- 11. Gentile M, Panico S, Mattiello A, et al. Association between small dense LDL and early atherosclerosis in a sample of menopausal women. Clin Chim Acta 2013;426:1–5. 10.1016/j.cca.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 12. Johnson AA, Stolzing A. The role of lipid metabolism in aging, lifespan regulation, and age-related disease. Aging Cell 2019;18:e13048. 10.1111/acel.13048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Toth MJ, Tchernof A. Lipid metabolism in the elderly. Eur J Clin Nutr 2000;54 Suppl 3:S121–5. 10.1038/sj.ejcn.1601033 [DOI] [PubMed] [Google Scholar]
- 14. Park JH, Lee MH, Shim J-S, et al. Effects of age, sex, and menopausal status on blood cholesterol profile in the Korean population. Korean Circ J 2015;45:141–8. 10.4070/kcj.2015.45.2.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goh VHH, Tong TYY, Mok HPP, et al. Differential impact of aging and gender on lipid and lipoprotein profiles in a cohort of healthy Chinese Singaporeans. Asian J Androl 2007;9:787–94. 10.1111/j.1745-7262.2007.00294.x [DOI] [PubMed] [Google Scholar]
- 16. Qamar A, Khetarpal SA, Khera AV, et al. Plasma apolipoprotein C-III levels, triglycerides, and coronary artery calcification in type 2 diabetics. Arterioscler Thromb Vasc Biol 2015;35:1880–8. 10.1161/ATVBAHA.115.305415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pechlaner R, Tsimikas S, Yin X, et al. Very-low-density lipoprotein-associated apolipoproteins predict cardiovascular events and are lowered by inhibition of APOC-III. J Am Coll Cardiol 2017;69:789–800. 10.1016/j.jacc.2016.11.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ishikawa S, Gotoh T, Nago N, et al. The Jichi medical school (JMS) cohort study: design, baseline data and standardized mortality ratios. J Epidemiol 2002;12:408–17. 10.2188/jea.12.408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Izumida T, Nakamura Y, Hino Y, et al. Combined effect of small dense low-density lipoprotein cholesterol (sdLDL-C) and remnant-like particle cholesterol (RLP-C) on low-grade inflammation. J Atheroscler Thromb 2020;27:319–30. 10.5551/jat.49528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cho Y, Lee SG, Jee SH, et al. Hypertriglyceridemia is a major factor associated with elevated levels of small dense LDL cholesterol in patients with metabolic syndrome. Ann Lab Med 2015;35:586–94. 10.3343/alm.2015.35.6.586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hayashi T, Koba S, Ito Y, et al. Method for estimating high sdLDL-C by measuring triglyceride and apolipoprotein B levels. Lipids Health Dis 2017;16:21. 10.1186/s12944-017-0417-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dayspring TD. Understanding hypertriglyceridemia in women: clinical impact and management with prescription omega-3-acid ethyl esters. Int J Womens Health 2011;3:87–97. 10.2147/IJWH.S16702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Campos H, Walsh BW, Judge H, et al. Effect of estrogen on very low density lipoprotein and low density lipoprotein subclass metabolism in postmenopausal women. J Clin Endocrinol Metab 1997;82:3955–63. 10.1210/jc.82.12.3955 [DOI] [PubMed] [Google Scholar]
- 24. Carr MC, Kim KH, Zambon A, et al. Changes in LDL density across the menopausal transition. J Investig Med 2000;48:245–50. [PubMed] [Google Scholar]
- 25. Bhatnagar P, Wickramasinghe K, Williams J, et al. The epidemiology of cardiovascular disease in the UK 2014. Heart 2015;101:1182–9. 10.1136/heartjnl-2015-307516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anagnostis P, Stevenson JC, Crook D, et al. Effects of menopause, gender and age on lipids and high-density lipoprotein cholesterol subfractions. Maturitas 2015;81:62–8. 10.1016/j.maturitas.2015.02.262 [DOI] [PubMed] [Google Scholar]
- 27. Schaefer EJ, Lamon-Fava S, Cohn SD, et al. Effects of age, gender, and menopausal status on plasma low density lipoprotein cholesterol and apolipoprotein B levels in the Framingham offspring study. J Lipid Res 1994;35:779–92. [PubMed] [Google Scholar]
- 28. Tenk J, Mátrai P, Hegyi P, et al. Perceived stress correlates with visceral obesity and lipid parameters of the metabolic syndrome: a systematic review and meta-analysis. Psychoneuroendocrinology 2018;95:63–73. 10.1016/j.psyneuen.2018.05.014 [DOI] [PubMed] [Google Scholar]
- 29. Slopen N, Goodman E, Koenen KC, et al. Socioeconomic and other social stressors and biomarkers of cardiometabolic risk in youth: a systematic review of less studied risk factors. PLoS One 2013;8:e64418. 10.1371/journal.pone.0064418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Garcia CK, Mues G, Liao Y, et al. Sequence diversity in genes of lipid metabolism. Genome Res 2001;11:1043–52. 10.1101/gr.172301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Viney NJ, van Capelleveen JC, Geary RS, et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet 2016;388:2239–53. 10.1016/S0140-6736(16)31009-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-041613supp001.pdf (334.5KB, pdf)
bmjopen-2020-041613supp002.pdf (328.9KB, pdf)
bmjopen-2020-041613supp003.pdf (327.8KB, pdf)
bmjopen-2020-041613supp004.pdf (344KB, pdf)
bmjopen-2020-041613supp005.pdf (327.7KB, pdf)
bmjopen-2020-041613supp006.pdf (1.1MB, pdf)
bmjopen-2020-041613supp007.pdf (1.1MB, pdf)