Abstract
Chronic inflammation in asthmatics is initiated/exacerbated by many environmental factors, such as bacterial lipopolysaccharide and allergens. Phospholipase A2 and histone acetyltransferase/deacetylases are enzymes involved in inflammatory process, particularly in lipid inflammatory mediators production and control of transcription of many inflammatory genes, respectively. The aim of the study was to identify differences in the inflammatory process in patients with severe and non-severe asthma, taking as a criterion expression of two groups of enzymes: phospholipases A2 and histone acetyltransferases/deacetylases. Thirty-two patients with severe, non-severe atopic to house dust mite asthmatics and 14 healthy volunteers were recruited. Peripheral blood mononuclear cells were stimulated with Dermatophagoides pteronyssinus allergen (nDer p1) and bacterial lipopolysaccharide (LPS). The expression of phospholipases A2 and histone acetyltransferases and deacetylases were assessed using TaqMan Low Density Array Cards. The protein expression was analyzed with immunoblot. Increased expression of phospholipase A2 Group IVC (PLA2G4C) and cytosolic phospholipase A2 gamma (cPLA2γ) protein was observed in peripheral blood mononuclear cells (PBMC) from severe asthmatics in response to LPS and nDer p1, compared to non-severe asthmatics. nDer p1-stimulated PBMC from severe asthmatics exhibit induced expression of HDAC1 and similar trend was observed in protein concentration. Decreased expression of EP300 occurred in PBMC of severe asthmatics. PBMC from non-severe asthmatics showed decreased expression of HDAC2 and PLA2G15 after LPS treatment. In conclusion, in response to LPS and dust mite allergen, PBMC from severe and non-severe asthmatics modulate expression of selected phospholipase A2, histone acetyltransferases and deacetylases, while increased expression of cPLA2γ characterizes PBMC response from severe asthmatics.
Keywords: allergens, asthma, cPLA2, HAT, HDAC
Background
Global Initiative for Asthma (GINA) defines asthma as a heterogeneous disease, usually characterized by chronic airway inflammation. The features of asthma involve a varied severity of clinical symptoms, different hallmarks of the inflammatory process and different responses to commonly used treatments. One of the approaches to classification of asthma phenotypes is research based on cluster analysis. It involves the assessment of a given population (in this case asthmatic patients) for many clinical, cellular and molecular features, and then the division of patients into relatively homogeneous groups (clusters) based on the similarities of the analyzed variables.1 The principal advantage of performing classification numerically is objectivity. Furthermore, this method includes multiple variables that assume equal weighting, which helps minimize a priori bias.2
Phospholipases A2 (PLA2), present in cells involved in inflammation, participate in the production of lipid inflammatory mediators by releasing arachidonic acid from cell membranes.3,4 Both in vitro and in vivo studies indicate secretory phospholipase A2 from group IIA, V, X, XII, and cytosolic PLA2: α, β, γ are involved in asthma pathogenesis.5,6 Moreover, our previous studies showed that cytosolic phospholipase A2 is involved in the pathogenesis of asthma.7,8 We have previously shown that microsatellite fragments (T)n and (CA)n in the promoter region of the cPLA2α gene (PLA2G4A), although highly polymorphic, are significantly shorter in patients with severe asthma than in healthy subjects.7
Bacteria and allergens are the factors that participate most in asthma pathogenesis and exacerbation.9–14 Proteins of house dust mite, and among them, Der p1 and Der p2, are major indoor allergens. Der p1is potent immunomodulator, able to stimulate production of many cytokines in many cells types and might influence the cells phenotype.15,16
Lipopolysaccharide (LPS) is a strong modulator of immunologic reactions – it induces the release of many pro-inflammatory cytokines and mediators responsible for the enhancement of inflammation.17 LPS also regulates the phospholipases expression and enzymatic activity and is also used as an adjuvant.18–20
Gene expression regulation undergoes through control, involving many transcription factors but also by epigenetic mechanisms DNA methylation and histone acetylation. The acetylation of histones involves lysine residue modification in the tails of histone proteins. Histone acetyltransferases (HAT) are the enzymes that participate in the acetylation process In turn, histone deactylases (HDAC), act by removing acetyl residues, resulting in chromatin conformational change and inhibition of transcription.21 An imbalance between HAT and HDAC activities was observed in patients with asthma.22,23 In relation to phospholipases, studies show that p300 acetyltransferase is involved in the regulation of PLA2G4A expression.24,25
In bronchial biopsies from patients with asthma, an increased level of HAT is observed. Therefore, even a small reduction in HDAC activity, in comparison to normal airways, favors accelerated inflammatory gene expression.26,27 HDAC/HAT activities may contribute to glucocorticoid insensitivity which appears in patients with severe steroid–insensitive asthma.
There is evidence that the different HDACs act on different patterns of acetylation, thus regulating the expression of different types of genes. In biopsies from asthmatic patients, an increase in HAT activity and a reduction in HDAC activity occurred, favoring increased inflammatory gene expressions.27,28
The aim of this study was to identify differences among severe, non-severe asthmatic and healthy subjects in the expression of two groups of enzymes – phospholipases A2 and histone acetyltransferases and deacetylases.
Phospholipases A2 play a crucial role in the generation of inflammatory lipid mediators, and a group of enzymes with acetyltransferase and deacetylase activity that regulate gene expression by modifying chromatin structure.
Materials and methods
Reagents
The LoTox natural D. pteronyssinus allergen 1 (nDer p1) was ordered from Indoor Biotechnologies (Cardiff, UK). The LPS from Escherichia coli, serotype R515, was purchased from Enzo Life Sciences (New York, NY). The TaqMan Low Density Array (TLDA) cards, High Capacity cDNA kit and AMI-V medium were obtained from Life Technologies (Carlsbad, CA) and the Histopaque 1077 – from Sigma Aldrich (Saint Louis, MO). The RNeasy Cell Mini Kit with QIAshredder and DNAse set were purchased from Qiagen (Hilden, Germany). BCIP/NBT alkaline phosphatase substrate was purchased from Merck Millipore (Darmstadt, Germany). The BCA protein assay kit was purchased from Pierce Thermo Scientific (Rockford, Ill., USA). The rabbit anti-HDAC1(catalog number: sc-7872), anti-HDAC2 (catalog number: sc-7899) and anti-p300 (catalog number: sc-585) polyclonal antibodies were obtained from Santa Cruz Biotechnology (Dallas, Tex., USA); the rabbit anti-cPLA2γ polyclonal antibody (catalog number: HPA043083) from Sigma Aldrich (Saint Louis, MO); and the rabbit anti-β-actin monoclonal antibody (catalog number: 4970L) from Cell Signaling (Danvers, Mass., USA).
Patients
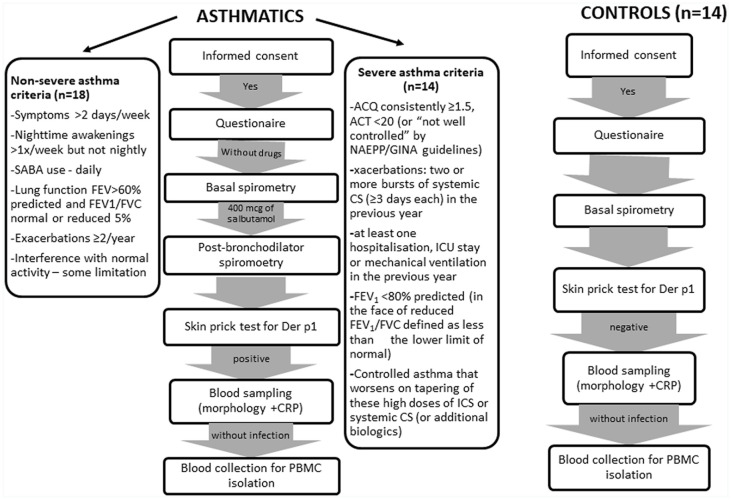
Thirty-two patients with severe and non-severe asthma of atopic origin, as well as 14 healthy volunteers, were enrolled into the study. The project, conducted from May 2014 to February 2015, was approved by the Bioethical Committee for Research Studies of the Medical University of Lodz (ethics approval number: RNN/102/11/KE) and informed written consent was obtained from all the subjects. The subjects were recruited from the Department of Internal Medicine, Asthma and Allergy of the Medical University of Lodz. Asthma had to be diagnosed at least 6 months prior to the study and meet the criteria of the Global Initiative for Asthma29 and severe asthma was defined according to American Thoracic Society (ATS) directions.30 Figure 1 shows the scheme subject enrollment. Each of the participants were asked to fill a validated questionnaire, part of which was completed by the attending physician.31–33 Each of the volunteers had a resting spirometry, skin tests, morphology and CRP (all executed on the same day) and was evaluated by a doctor. All the patients were organized into the appropriate asthma cluster according to Moore’s et al.34 criteria. Patients were asked to abstain from systemic GC (glucocorticoids) – 1 month before the test, unless the patient was on stable low doses (up to 5 mg of prednisone) – 24 h without drugs before the test. AH1 (antihistamine drugs), LTRA (leukotriene receptor agonists), LABA (long acting β2-agonists), LAMA (long acting antimuscarinic) and drugs in combination with inhaled GC – 24 h before blood sampling. SABA, SAMA – 6 h before blood collection. Non-asthma medications – 2 weeks prior to the study, long-acting beta agonists hours and short-acting beta agonists before blood drawing. The period of non-administration of oral glucocorticosteroids by patients was determined with a specialist physician and it was determined by the clinical condition of patients with severe asthma. Moreover, the period of 24 h without the drug is the maximum time allowed by the Bioethics Committee. The issue of drug withdrawal is a common problem in trials involving patients with advanced disease. The wash-out time for a drug is a compromise between the desire to characterize severe asthma and not being able to turn off the treatment for a longer period of time. In the article, we included information on the doses of glucocorticosteroids taken by patients and the period of GCS deprivation to indicate the possibility of the influence of corticosteroids on the observed cell responses. It should be noted that systemic GCs are taken 1 or 2 times a day, so 24 h should be enough not to observe the effect of GCS on peripheral blood leukocytes.
Figure 1.
Scheme of subject enrollment procedure.
To exclude atopy, the following skin tests were executed to healthy volunteers: mites: D. peron, D. farinae. Additionally, Artemisia, birch, hazel, alder, grass, cat hair, dog hair, Cladosporium herba, and Alternaria tenius.
Detailed characteristics of the patients and controls are shown in Table 1.
Table 1.
Parameters and characteristics of patients and controls.
| Severe asthmatics (SA) | Non-severe asthmatics (NSA) | Healthy subjects (HS) | |
|---|---|---|---|
| (n) | 14 | 18 | 14 |
| Age | 47 | 38 | 43 |
| Gender F/M | 8/6 | 10/8 | 8/6 |
| Atopic to Der p1 | 14 | 18 | 0 |
| FEV1 baseline (%) | 47.7*# | 91.6 | 107.1 |
| FEV1 max (%) | 66.4* | 98.7 | – |
| Cluster | 4–5 | 1–2 | – |
| WBC (g/l) | 8.1 | 6.7 | 6.7 |
| NE (%) | 56.3 | 55.6 | 55.7 |
| LIMF (%) | 26.4 | 32.4 | 33.6 |
| MO (%) | 8.4 | 7.7 | 7.5 |
| EOS (%) | 7.9 | 3.6 | 2.6 |
| BASO (%) | 1 | 0.7 | 0.6 |
| HGB (g/dl) | 14.3 | 14.2 | 13.8 |
| PLT (g/l) | 275.9 | 260.8 | 262.7 |
| CRP (mg/l) | 4.3 | 2.5 | 2.2 |
| Glucocorticoids use | High | low | n.a |
FEV1: forced expiratory volume; WBC: white blood cells; NE: neutrophils; LIMF: lymphocytes; MO: monocytes; EOS: eosinophils; BASO: basophils; HGB: hemoglobin; PLT: platelets; CRP: C reactive protein.
P < 0.05 in comparison to non-severe asthmatics.
P < 0.05 in comparison to healthy subjects.
PBMC isolation and stimulation
PBMCs were isolated by centrifugation on Histopaque 1077, a density gradient cell separation medium according to the manufacturer’s instructions. Cells were plated on a 24-well plate and cultured in AIM-V medium overnight. The next day, 6 × 106/ml PBMCs were stimulated in vitro with LoTox nDer p1 (1 µg/ml) or LPS from E.coli serotype R515 (500 ng/ml), or without vehicle (control) for 72 h.
RNA extraction and reverse transcription
Total RNA was isolated from PBMCs using the RNeasy Cell Mini Kit with QIA shredder according to the manufacturer’s instructions. RNA was DNase treated, purified, eluted in 30 μl of RNase-free water and stored at −80°C for further analysis. RNA was then reverse transcribed to cDNA using High Capacity cDNA kit.
TLDA cards
Each card of the low-density array contained eight separate loading ports that fed into 48 separate wells for a total of 384 wells per card. Each 2-μl well contained a lyophilized TaqMan assay to enable a single gene to be detected. In this study, the TLDA card was configured into eight identical gene sets (CREBBP-Hs00231733_m1, EP300-Hs00914223_m1, HDAC1-Hs02621185_s1, HDAC2-Hs00231032_m1, NCOA1-Hs00186661_m1, NCOA2-Hs00896106_m1, PLA2G2A-Hs00179898_m1, PLA2G4A- Hs00233352_m1, PLA2G4C-Hs01003743_m1, PLA2G5-Hs00173472_m1, PLA2G10- Hs00358567_m1, PLA2G12A-Hs00913513_m1, PLA2G15-Hs00952741_m1, SIRT1- Hs01009005_m1). Each set of 14 genes also contained two reference genes: 18S (Hs99999901_s1) and GAPDH (Hs99999905_m1).
Real time PCR
qPCR was performed as described previously.10 Briefly, 300 ng of cDNA was used for each port of TLDA. The array was centrifuged for 1 min twice at 306 × g to distribute the samples from the loading port into each well.
The card was then sealed and quantitative PCR (qPCR) was performed using an ABI Prism 7900HT (Applied Biosystems) sequence detection system. RQ Manager 1.2.1 software was used to analyze raw qPCR data. The results were analyzed in comparison to the expression of the reference gene, using Livak’s method, and represented as the relative expression of mRNA in the form of RQ = 2–∆∆Cq. The untreated sample was used as a calibrator.
The results are presented as relative expressions to the untreated control.
Immunoblotting
Total proteins from the PBMCs of patients with asthma and healthy controls were extracted in RIPA protein extraction buffer supplemented with protease inhibitor, and the protein concentrations were determined by the BCA protein assay kit. NuPAGE Bis-Tris Gels (Life Technologies) were used to separate proteins (200V, from 110 mA – start to 70 mA – end). Subsequently, the samples were transferred onto a nitrocellulose membrane using the eBlot Protein Transfer System. To block non-specific binding, 5% non-fat dry milk diluted in TBST (0.01% Tween 20 in TBS) was used. Then, membranes were washed three times with TBST and incubated for 12 h at 4°C with primary antibodies and subsequently for 1 h with secondary antibodies at room temperature. After incubation with secondary antibodies and triple washing in TBST once again, colorimetric detection of bands by means of BCIP/NBT Alkaline Phosphatase Substrate (Millipore) was performed following the manufacturer’s instructions.
Densitometric analysis of bands was performed with ImageJ 1.34s software (Wayne Rasband, National Institutes of Health, Bethesda, Md., USA) and the results are presented as fold change in optical density.
Statistical analyses
Data were analyzed using Statistica software (v. 10.0; StatSoft, Tulsa, OK). The distribution of data and the equality of variances were checked by Shapiro-Wilk and Levene’s tests, respectively. Significant changes were determined by ANOVA with the appropriate post-hoc tests as multiple comparison procedure. Values of P < 0.05 were considered statistically significant.
Principal component analysis (PCA) was performed using the SigmaPlot 13.0. according to its technical manual, with using protein data. PCA is a statistical technique that analyzes a data set in which observations are described by several inter-correlated quantitative dependent variables. In this study, PCA analysis shows chosen gene expressions in relation to asthma severity.
Results
Blood and spirometry parameters
Complete blood count, spirometry and CRP analyses revealed significantly higher forced expiratory volume baseline in severe asthmatics, comparing to non-severe asthmatics healthy volunteers. Other parameters showed no significant differences (Table 1). Cell viability, assessed by trypan blue, was over 90% before stimulation and over 84% – after LPS stimulation.
Gene expression
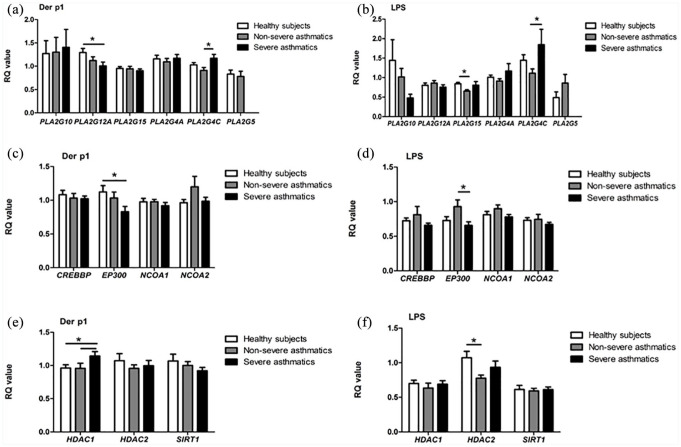
House dust mite allergen and LPS modulated the expression of selected phospholipase A2 genes. An increased expression of PLA2G4C and a decreased expression of PLA2G12 was observed in nDer p1-stimulated PBMC from severe asthmatics (P < 0.05). Similarly, stimulation with LPS caused an increased expression of PLA2G4C gene in severe asthmatics (P < 0.05; Figure 2a and b).
Figure 2.
Results of qPCR. Expression of phospholipase A2 (a, b) histone acetylase (c, d), histone deacetylase (e, f) genes in the PBMCs of healthy subjects (white bar, n = 14) and patients with non-severe (gray bar, n = 18), severe asthma (black bar, n = 14) after stimulation with nDerp1 and LPS have been assessed.
Results are presented as the mean value of the RQ ±SEM. *P < 0.05.
Four histone acetyltransferase gene expressions was assessed in our study. We observed that the stimulation of PBMC in severe asthmatics with nDer p1 – comparing to healthy subjects, and LPS – comaring to non-severe asthmatics, resulted in a decreased expression of EP300 gene (P < 0.05; Figure 2c and d).
Among histone deacetylase genes, a difference in HDAC1 gene expression was observed between severe asthmatics and other groups after PBMC incubation with nDer p1. Also, the expression of HDAC2 significantly changed after LPS stimulation (P < 0.05; Figure 2e and f).
Protein expression
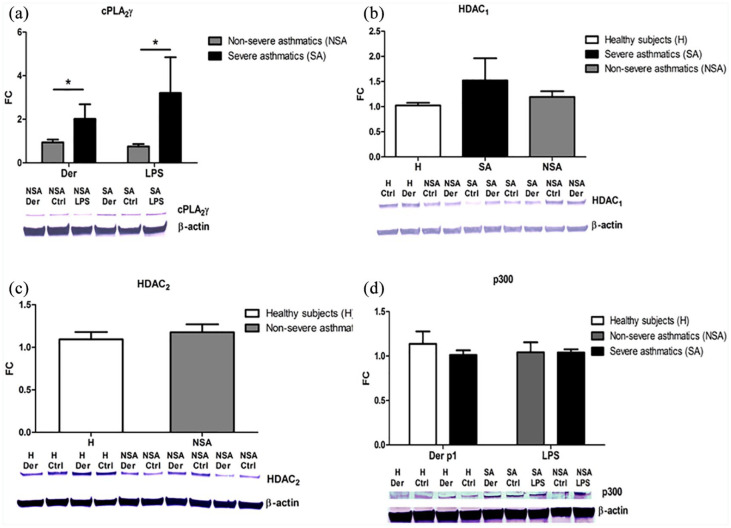
Based on the qPCR results, we evaluated the concentrations of the selected proteins. The immunoblotting analysis showed that among the phospholipases A2, cPLA2γ expression differed between the studied groups and reflected the changes observed on mRNA level (Figure 3a).
Figure 3.
Relative cPLA2γ (a), HDAC1 (b), HDAC2 (c), p300 (d) protein expression. PBMC from severe (black bar), non-severe asthmatics (gray bar) and healthy subjects (white bar) were in vitro stimulated with nDer p1 or LPS. Each sample of all participants was analyzed by Western Blot six times showing similar results, and the blots presented are representatives. The box-plots present densitometry results. Data presented as fold change in comparison to the vehicle-treated cells (control) both normalized to β-actin. Data represent the mean ± SEM.
*P < 0.05 as comparison between studied groups.
The immunoblotting analysis showed a similar trend in the p300 protein concentration (Figure 3d). However, histone deacetylase (HDAC1 and HDAC2) protein concentrations did not differ significantly between the studied groups (Figures 2c and 3b).
Principal component analysis
Results of the PCA of the PBMCs after stimulation with nDer p1 for all subjects revealed that EP300 variable is negatively correlated with the first component. Its factor loading informs that the correlation between the first principal component and the EP300 expression equals –0.71 which constitutes 30.8% of the first component. PLA2G12 has a smaller influence on the first component (25.4%) and is also negatively correlated with it (–0.68). HDAC1 – similarly to the two previous variables but its influence on the first component is smaller. The correlation of the PLA2G4 and the first component is the weakest, and the sign of that correlation is positive. The angle between the vectors illustrating the PLA2G12 and EP300 (similarly EP300 and HADC1) is small, which means those variables are strongly correlated (Figure 4).
Figure 4.
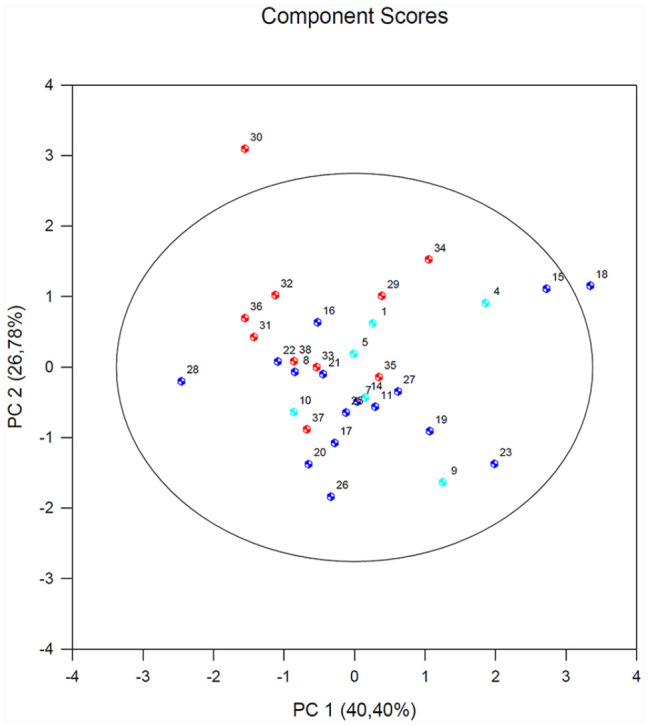
Results of PCA in the PBMCs after stimulation with nDER p1 for all subjects. A biplot of individual subjects according to their scores of the first (PC1) and second (PC2) principal component (with prediction ellipse 95%). The HA group (healthy subjects; 1–13) is marked in turquoise; the NSA group (non-serve asthmatics; 14–28) is marked in navy blue; and the SA group (serve asthmatics; 29–39) is marked in red.
The results of PCA in the PBMCs after stimulation with LPS for all subjects showed that EP300 variable is negatively correlated with the first component. Its factor loading informs that the correlation between the first principal component and the EP300 equals –0.26 which constitutes 3.99% of the first component. PLA2G12 and PLA2G4 constitutes 47% of the first component. The second component represents chiefly the original variable – HDAC1 (51.1%) and EP300 (42.7%) the remaining original variables are reflected in it to a slight degree. The vectors representing original values almost reach the rim of the unit circle which means they are all well represented by the two initial principal components which form the coordinate system. PLA2G4 and PLA2G12 vectors are located close to each other, which suggests that their expression is highly correlated. The vector of the EP300 and HDAC1 point to an entirely different direction. They are only slightly correlated with the remaining original values, which are shown by the inclination angle with respect to the remaining original values (Figure 5).
Figure 5.
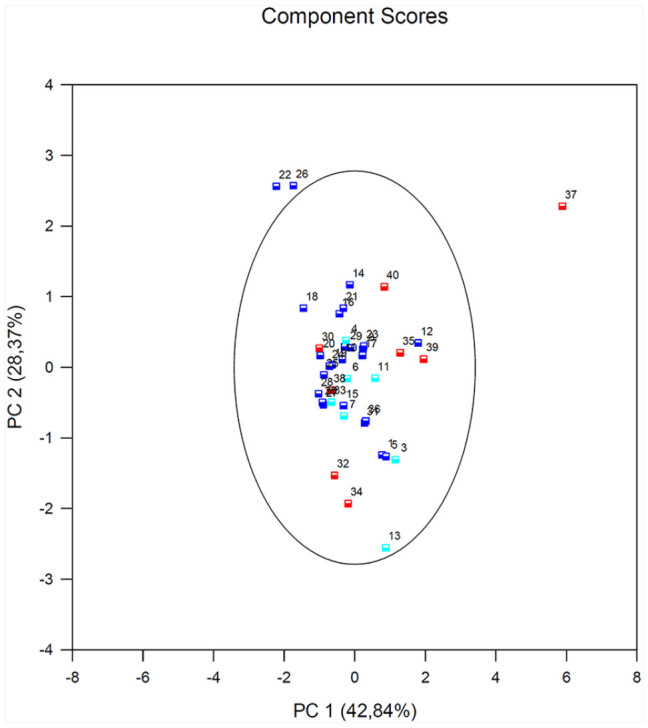
Results of PCA in the PBMCs after stimulation with LPS for all subjects. A biplot of individual subjects according to their scores of the first (PC1) and second (PC2) principal component (with prediction ellipse 95%). The HA group (healthy subjects; 1–13) is marked in turquoise; the NSA group (non-serve asthmatics; 14–28) is marked in navy blue; and the SA group (serve asthmatics; 29–40) is marked in red.
Discussion
In present study we assessed how bacterial lipopolysaccharide and house dust mite allergen influence expression of selected phospholipase A2 and histone acetyltransferase/deacetylase genes and proteins in studied groups. PBMC from severe asthmatics turned out to be more sensitive to stimuli – in this group, allergen treatment resulted in more explicit modulation, comparing to control group. This might be explained by the fact that on the surface of PBMC, there are PAR receptors, which are involved in Der p1-signal transduction.35 We therefore hypothesized that observed changes are specific to this allergen action. However further studies with specific PAR inhibitors or siRNA may be needed to understand the mechanism of observed changes.
LPS and allergen-stimulated PBMC of patients with phenotype of severe asthma characterized increased cPLA2γ gene and protein expression in comparison to non-severe asthmatics. Human cPLA2γ shares 30% homology and the same amino acids in the catalytic center with cPLA2α. cPLA2γ, unlike cPLA2α, lacks the regulatory phosphorylation sites, thus, the C2 domain is Ca2+ –independent and is constantly bound to the cell membrane.36 The enzyme possesses phospholipase A2 and lysophospholipase/transacylase activities.37 The mechanism of this phenomenon might be connected to phospholipids remodeling, which cPLA2γ contributes.38 Bickford et al.39 proved that TNF-α induces the cPLA2γ mRNA and protein expression. This mechanism is important – it highlights the significant role of TNF-α in the pathophysiology of severe asthma and its increased presence in PBMCs and the airway of severe asthmatics.39,40 They also39 revealed an increased expression of cPLA2γ in allergic inflammation in a mouse model of asthma. In our study, the increased expression of cPLA2γ in severe asthmatics may appear by a mechanism of pro-inflammatory cytokines such as TNF or NF-κB, which may be induced by Der p1/LPS and involved in further cellular mechanism.41
House dust mite allergen increased expression of the HDAC1 gene in patients with severe asthma. This might be through the mechanism involving NF-κB, strongly connected with asthma development.42 Decreased activity of the HDAC enzyme is associated with increased intensity of the inflammatory process, thus rendering the histone deacetylase as a potential anti-inflammatory drug. In our study, Der p1 induced expression of HDAC1 in PBMCs. Such action may promote increased airway hyper-responsiveness and bronchi constriction. As severe asthmatics are administrated corticosteroids, which are known to act through HDAC genes, the increased expression of HDAC1 could be partially due to their action.43 Treatment of animal asthma models with HDAC inhibitors helped to reduce airway hypersensitivity and remodeling with debatable impact on airway inflammation.44 These differences are probably due to the action of different histone deacetylase enzymes.
Both LPS and Der p1 decreased the expression of the EP300 gene in severe asthmatics. The mechanism of LPS impact on the expression of histone acetyltransferases may be cell-specific and dose-dependent. It is difficult to pinpoint the result of Der p1 and LPS-EP300 interaction because the significant changes were observed only on the mRNA, not protein, level. However, in THP-1 human promonocytes, HAT enzymes were decreased by LPS and acetylated lysine modification was dose-dependent.45 Gunawardhana et al.46 showed that there were no differences in EP300 and HDACs 1 and 2 expressions in blood monocytes from subjects with asthma.47 However, since the authors did not specify the severity of asthma in their study, it is possible that the dissimilarities result from different study group designs. Moreover, these differences in expression were not reflected in activity; thus factors other than HAT or HDAC transcription may be important in this context. An analysis of a larger panel of HAT and HDAC expression might be useful in future studies, as the selection of HATs, HDACs, and their importance in airway inflammation, was mainly based on existing literature.
The PCA revealed that the PLA2G12 and EP300 (similarly EP300 and HADC1) variables are strongly correlated, whereas the PLA2G4 and the EP300, PLA2G12 are only slightly correlated, in the PBMCs after stimulation with nDer p1; the PLA2G12 and PLA2G4 variables are strongly correlated and the EP300 and HDAC1 are only slightly correlated in the PBMCs after stimulation with LPS. PCA showed differences in allergen and LPS action in relation to different PLA2 isoforms. nDer p1 is more potent in the stimulation of PLA2G4C expression in severe asthmatics, whereas LPS induces PLA2G4C as well as PLA2G12 in similar manners. Data obtained from protein expression in PBMCs from severe, non-severe asthma, and healthy individuals were used for PCA analysis. For this, the expression data of four of the thirteen genes tested was used due to the fact that only four genes showed the expression significantly different between groups. Protein data was used, due to the fact that from the biological point of view, it is more important and additionally, the mRNA data is not always confirmed on the protein level. Despite the observed correlations, studies including more participants are necessary to verify the indicated relationships.
In our study, some data from gene and protein expression is contradictory. The first study indicating the possibility of a correlation between the concentration of mRNA (easy to determine) and protein concentration (difficult to determine) appeared in 2004. However, only approx. 40% of the protein concentration corresponded to the amount of mRNA.48 The lack of correlation between mRNA and protein expression may be a result of the time difference of transcription and translation. Furthermore, the process of gene expression is a multistep process, and each of the stages might be responsible for the observed differences in the expression of mRNA and protein (control of transport of mRNA, mRNA stability, translation process control, control of protein degradation). Our study is limited because it did not separately analyze protein concentration in the nucleus and cytoplasm. As HAT and HDAC proteins are more abundant in the nucleus, this type of analysis may be more valuable in the assessment of mRNA and protein correlation. Another limitation is lack of the statistical power calculation, however the results obtained by statistical analyzes performed, allow us to consider that the size of the groups were sufficient.
Asthma is characterized by the presence of persistent inflammation (higher CRP level), hence the inflammatory cells undergo natural priming. The patients undertake specific therapy to manage this. Since we cannot exclude these aspects during study design, they should be taken into consideration in critical analysis of results from molecular studies. Three strategies were used to decrease the negative impact of these factors in our study: first, we carefully selected patients (without exacerbation, without high doses of GC, drug deprivation); second, after the isolation of PBMCs, we incubated them overnight before stimulation with Der p1; and third, we decided to validate the results from gene expression on the protein level. Only for cPLA2γ, we reported both the elevated gene and protein expression in severe asthmatics. Current literature does not indicate other diseases with cPLA2γ involvement, except for breast and colon cancer. However, more sophisticated studies are needed to confirm specificity of observed changes to severe asthma phenotype.
In the present study, we used PBMC as an experimental model. Though these cells might only partially reflect the changes occurring in targeted organs, the presence of systemic inflammation in chronic diseases also influences the function of PBMC. Additionally, the changes in the PBMC are easily observable, and they might be easily employed for possible therapeutic solutions.
Conclusion
In conclusion, this project provided differences observed in gene expression of selected phospholipases A2 in PBMCs of patients with severe and non-severe asthma, compared to a control of healthy volunteers. Enzymes responsible for epigenetic control of gene expression in the pathogenesis of asthma were also evaluated. This project constituted a complex analysis of LPS/Der p1 and phospholipases A2/histone acetyltransferases/deacetylases interactions and helps to verify existing literature data. This study aimed to explore the molecular responses of bacterial factor (lipopolysaccharide) and house dust mite allergen exposure in those with varying degrees of asthma. The results of the study show that cytosolic phospholipase A2 gamma (cPLA2γ) may be the molecule that differentiates the responses seen between different asthmatics.
Supplemental Material
Supplemental material, sj-pdf-1-iji-10.1177_2058738421990952 for Expression of cPLA2γ mRNA and protein differs the response of PBMC from severe and non-severe asthmatics to bacterial lipopolysaccharide and house dust mite allergen by Ewa Pniewska-Dawidczyk, Izabela Kupryś-Lipińska, Gabriela Turek, Dorota Kacprzak, Joanna Wieczfinska, Paulina Kleniewska, Piotr Kuna and Rafal Pawliczak in International Journal of Immunopathology and Pharmacology
Supplemental material, sj-pdf-2-iji-10.1177_2058738421990952 for Expression of cPLA2γ mRNA and protein differs the response of PBMC from severe and non-severe asthmatics to bacterial lipopolysaccharide and house dust mite allergen by Ewa Pniewska-Dawidczyk, Izabela Kupryś-Lipińska, Gabriela Turek, Dorota Kacprzak, Joanna Wieczfinska, Paulina Kleniewska, Piotr Kuna and Rafal Pawliczak in International Journal of Immunopathology and Pharmacology
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Science Centre [Dec-2012/05/N/NZ5/02630] and Medical University of Lodz [503/0-149-03/503-01-001-19-00].
Ethics approval: Ethical approval for this study was obtained from Bioethical Committee for Research Studies of the Medical University of Lodz (ethics approval number: RNN/102/11/KE).
Informed consent: Written informed consent was obtained from all subjects before the study.
ORCID iDs: Joanna Wieczfinska  https://orcid.org/0000-0002-8830-1507
https://orcid.org/0000-0002-8830-1507
Rafal Pawliczak  https://orcid.org/0000-0001-6784-453X
https://orcid.org/0000-0001-6784-453X
Supplemental material: Supplemental material for this article is available online.
References
- 1. Moore WC, Meyers DA, Wenzel SE, et al. (2010) Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. American Journal of Respiratory and Critical Care Medicine 181(4): 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Haldar P, Pavord ID, Shaw DE, et al. (2008) Cluster analysis and clinical asthma phenotypes. American Journal of Respiratory and Critical Care Medicine 178(3): 218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dennis EA. (2000) Phospholipase A2 in eicosanoid generation. American Journal of Respiratory and Critical Care Medicine 161(2 Pt 2): S32–S35. [DOI] [PubMed] [Google Scholar]
- 4. Funk CD. (2001) Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 294(5548): 1871–1875. [DOI] [PubMed] [Google Scholar]
- 5. Murakami M, Taketomi Y, Girard C, et al. (2010) Emerging roles of secreted phospholipase A2 enzymes: Lessons from transgenic and knockout mice. Biochimie Open 92(6): 561–582. [DOI] [PubMed] [Google Scholar]
- 6. Murakami M, Taketomi Y, Miki Y, et al. (2011) Recent progress in phospholipase A2 research: From cells to animals to humans. Progress in Lipid Research 50(2): 152–192. [DOI] [PubMed] [Google Scholar]
- 7. Sokolowska M, Stefanska J, Wodz-Naskiewicz K, et al. (2010) Cytosolic phospholipase A2 group IVA is overexpressed in patients with persistent asthma and regulated by the promoter microsatellites. Journal of Allergy and Clinical Immunology 125(6): 1393–1395. [DOI] [PubMed] [Google Scholar]
- 8. Sokolowska M, Borowiec M, Ptasinska A, et al. (2007) 85-kDa cytosolic phospholipase A2 group IValpha gene promoter polymorphisms in patients with severe asthma: A gene expression and case-control study. Clinical and Experimental Immunology 150(1): 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jatzlauk G, Bartel S, Heine H, et al. (2017) Influences of environmental bacteria and their metabolites on allergies, asthma, and host microbiota. Allergy 72(12): 1859–1867. [DOI] [PubMed] [Google Scholar]
- 10. Kim CK, Callaway Z, Gern JE. (2018) Viral infections and associated factors that promote acute exacerbations of asthma. Allergy, Asthma & Immunology Research 10(1): 12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Scadding GK. (2015) Allergens, germs and asthma. Clinical Respiratory Journal 9(2): 153–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kloepfer KM, Lee WM, Pappas TE, et al. (2014) Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and asthma exacerbations. Journal of Allergy and Clinical Immunology 133(5): 1301–1307, 7e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Armann J, von Mutius E. (2010) Do bacteria have a role in asthma development? European Respiratory Journal 36(3): 469–471. [DOI] [PubMed] [Google Scholar]
- 14. Gern JE. (2000) Viral and bacterial infections in the development and progression of asthma. Journal of Allergy and Clinical Immunology 105(2 Pt 2): S497–S502. [DOI] [PubMed] [Google Scholar]
- 15. Kauffman HF, Tamm M, Timmerman JA, et al. (2006) House dust mite major allergens Der p 1 and Der p 5 activate human airway-derived epithelial cells by protease-dependent and protease-independent mechanisms. Clinical and Molecular Allergy 4: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Whalen KA, Legault H, Hang C, et al. (2008) In vitro allergen challenge of peripheral blood induces differential gene expression in mononuclear cells of asthmatic patients: Inhibition of cytosolic phospholipase A2alpha overcomes the asthma-associated response. Clinical and Experimental Allergy 38(10): 1590–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Siwiec J, Zaborowski T, Jankowska O, et al. (2009) Evaluation of Th1/Th2 lymphocyte balance and lipopolysaccharide receptor expression in asthma patients. Pneumonologia i Alergologia Polska 77(2): 123–130. [PubMed] [Google Scholar]
- 18. Jiang YJ, Lu B, Choy PC, et al. (2003) Regulation of cytosolic phospholipase A2, cyclooxygenase-1 and -2 expression by PMA, TNFalpha, LPS and M-CSF in human monocytes and macrophages. Molecular and Cellular Biochemistry 246(1–2): 31–38. [PubMed] [Google Scholar]
- 19. Qi HY, Shelhamer JH. (2005) Toll-like receptor 4 signaling regulates cytosolic phospholipase A2 activation and lipid generation in lipopolysaccharide-stimulated macrophages. Journal of Biological Chemistry 280(47): 38969–38975. [DOI] [PubMed] [Google Scholar]
- 20. Tian W, Wijewickrama GT, Kim JH, et al. (2008) Mechanism of regulation of group IVA phospholipase A2 activity by Ser727 phosphorylation. Journal of Biological Chemistry 283(7): 3960–3971. [DOI] [PubMed] [Google Scholar]
- 21. Barnes PJ, Adcock IM, Ito K. (2005) Histone acetylation and deacetylation: Importance in inflammatory lung diseases. European Respiratory Journal 25(3): 552–563. [DOI] [PubMed] [Google Scholar]
- 22. Ghizzoni M, Haisma HJ, Maarsingh H, et al. (2011) Histone acetyltransferases are crucial regulators in NF-κB mediated inflammation. Drug Discovery Today 16(11–12): 504–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Su RC, Becker AB, Kozyrskyj AL, et al. (2009) Altered epigenetic regulation and increasing severity of bronchial hyperresponsiveness in atopic asthmatic children. Journal of Allergy and Clinical Immunology 124(5): 1116–1118. [DOI] [PubMed] [Google Scholar]
- 24. Blaine SA, Wick M, Dessev C, et al. (2001) Induction of cPLA2 in lung epithelial cells and non-small cell lung cancer is mediated by Sp1 and c-Jun. Journal of Biological Chemistry 276(46): 42737–42743. [DOI] [PubMed] [Google Scholar]
- 25. Lee CW, Lin CC, Lee IT, et al. (2011) Activation and induction of cytosolic phospholipase A2 by TNF-α mediated through Nox2, MAPKs, NF-κB, and p300 in human tracheal smooth muscle cells. Journal of Cellular Physiology 226(8): 2103–2114. [DOI] [PubMed] [Google Scholar]
- 26. Barnes PJ, Adcock IM, Ito K. (2005) Histone acetylation and deacetylation: Importance in inflammatory lung diseases. European Respiratory Journal 25(3): 552–563. [DOI] [PubMed] [Google Scholar]
- 27. Ito K, Caramori G, Lim S, et al. (2002) Expression and activity of histone deacetylases in human asthmatic airways. American Journal of Respiratory and Critical Care Medicine 166(3): 392–396. [DOI] [PubMed] [Google Scholar]
- 28. Zhang HP, Fu JJ, Fan T, et al. (2015) Histone deacetylation of memory T lymphocytes by You-Gui-Wan alleviates allergen-induced eosinophilic airway inflammation in asthma. Chinese Medical Journal 10: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sacheck JM, Cannon JG, Hamada K, et al. (2006) Age-related loss of associations between acute exercise-induced IL-6 and oxidative stress. American Journal of Physiology. Endocrinology and Metabolism 291(2): E340–E349. [DOI] [PubMed] [Google Scholar]
- 30. Chung KF, Wenzel SE, Brozek JL, et al. (2014) International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. European Respiratory Journal 43(2): 343–373. [DOI] [PubMed] [Google Scholar]
- 31. Jia CE, Zhang HP, Lv Y, et al. (2013) The Asthma Control Test and Asthma Control Questionnaire for assessing asthma control: Systematic review and meta-analysis. Journal of Allergy and Clinical Immunology 131(3): 695–703. [DOI] [PubMed] [Google Scholar]
- 32. Nkoy FL, Stone BL, Fassl BA, et al. (2013) Longitudinal validation of a tool for asthma self-monitoring. Pediatrics 132(6): e1554–e1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schatz M, Zeiger RS, Yang SJ, et al. (2013) Development and preliminary validation of the Adult Asthma Adherence Questionnaire. Journal of Allergy and Clinical Immunology 1(3): 280–288. [DOI] [PubMed] [Google Scholar]
- 34. Moore WC, Meyers DA, Wenzel SE, et al. (2010) Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. American Journal of Respiratory and Critical Care Medicine 181(4): 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nieuwenhuizen L, Falkenburg WJ, Schutgens RE, et al. (2013) Stimulation of naïve monocytes and PBMCs with coagulation proteases results in thrombin-mediated and PAR-1-dependent cytokine release and cell proliferation in PBMCs only. Scandinavian Journal of Immunology 77(5): 339–349. [DOI] [PubMed] [Google Scholar]
- 36. Ghosh M, Tucker DE, Burchett SA, et al. (2006) Properties of the Group IV phospholipase A2 family. Progress in Lipid Research 45(6): 487–510. [DOI] [PubMed] [Google Scholar]
- 37. Yamashita A, Tanaka K, Kamata R, et al. (2009) Subcellular localization and lysophospholipase/transacylation activities of human group IVC phospholipase A2 (cPLA2gamma). Biochimica et Biophysica Acta 1791(10): 1011–1022. [DOI] [PubMed] [Google Scholar]
- 38. Stewart A, Ghosh M, Spencer DM, et al. (2002) Enzymatic properties of human cytosolic phospholipase A(2)gamma. Journal of Biological Chemistry 277(33): 29526–29536. [DOI] [PubMed] [Google Scholar]
- 39. Bickford JS, Newsom KJ, Herlihy JD, et al. (2012) Induction of group IVC phospholipase A2 in allergic asthma: Transcriptional regulation by TNFα in bronchoepithelial cells. Biochemical Journal 442(1): 127–137. [DOI] [PubMed] [Google Scholar]
- 40. Brightling C, Berry M, Amrani Y. (2008) Targeting TNF-alpha: A novel therapeutic approach for asthma. Journal of Allergy and Clinical Immunology 121(1): 5–10; quiz 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wong CK, Li ML, Wang CB, et al. (2006) House dust mite allergen Der p 1 elevates the release of inflammatory cytokines and expression of adhesion molecules in co-culture of human eosinophils and bronchial epithelial cells. International Immunology 18(8): 1327–1335. [DOI] [PubMed] [Google Scholar]
- 42. van den Bosch T, Kwiatkowski M, Bischoff R, et al. (2017) Targeting transcription factor lysine acetylation in inflammatory airway diseases. Epigenomics 9(7): 1013–1028. [DOI] [PubMed] [Google Scholar]
- 43. Chen Y, Watson AM, Williamson CD, et al. (2012) Glucocorticoid receptor and histone deacetylase-2 mediate dexamethasone-induced repression of MUC5AC gene expression. American Journal of Respiratory Cell and Molecular Biology 47(5): 637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Banerjee A, Trivedi CM, Damera G, et al. (2012) Trichostatin A abrogates airway constriction, but not inflammation, in murine and human asthma models. American Journal of Respiratory Cell and Molecular Biology 46(2): 132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rios E, Soriano F. (2013) Histone acetyltransferase and DNA methyltransferase expression in response to LPS stimulation. Crit Care 17: 89. DOI: 10.1186/cc12988. [DOI] [Google Scholar]
- 46. Gunawardhana LP, Gibson PG, Simpson JL, et al. (2013) Activity and expression of histone acetylases and deacetylases in inflammatory phenotypes of asthma. Clinical and Experimental Allergy 44(1): 47–57. [DOI] [PubMed] [Google Scholar]
- 47. Gunawardhana LP, Gibson PG, Simpson JL, et al. (2014) Activity and expression of histone acetylases and deacetylases in inflammatory phenotypes of asthma. Clinical and Experimental Allergy 44(1): 47–57. [DOI] [PubMed] [Google Scholar]
- 48. Schwanhäusser B, Busse D, Li N, et al. (2011) Global quantification of mammalian gene expression control. Nature 473(7347): 337–342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-iji-10.1177_2058738421990952 for Expression of cPLA2γ mRNA and protein differs the response of PBMC from severe and non-severe asthmatics to bacterial lipopolysaccharide and house dust mite allergen by Ewa Pniewska-Dawidczyk, Izabela Kupryś-Lipińska, Gabriela Turek, Dorota Kacprzak, Joanna Wieczfinska, Paulina Kleniewska, Piotr Kuna and Rafal Pawliczak in International Journal of Immunopathology and Pharmacology
Supplemental material, sj-pdf-2-iji-10.1177_2058738421990952 for Expression of cPLA2γ mRNA and protein differs the response of PBMC from severe and non-severe asthmatics to bacterial lipopolysaccharide and house dust mite allergen by Ewa Pniewska-Dawidczyk, Izabela Kupryś-Lipińska, Gabriela Turek, Dorota Kacprzak, Joanna Wieczfinska, Paulina Kleniewska, Piotr Kuna and Rafal Pawliczak in International Journal of Immunopathology and Pharmacology